BSI-201_160003-66-7_DataSheet_MedChemExpress
CEMEDINE施敏打硬产品型号

弹性接着剂
一液变性矽利康聚合物:Super x、SX8008、SX720W、SX720WH、SX720B、SX720BH、AX-018、AX-019、AX-096、AX-039、AX-040、AX-041、AX-045、AX-047
二液变性有机硅树脂(EP001、PM200)
反应形(热固)树脂系接着剂
一液环氧树脂(EP138、EP170、EP171)
二液丙烯酸树脂(YF-610、Y-600、Y-600H)
二液环氧树脂(EP330、EP008、1500)
瞬间接着剂
一液氰基丙烯酸脂瞬间胶(3000RX、3000DX、3000RXP、3000-Super、3000 Jelly)
乳胶
EV A、SBR、Acryl一液水基胶
热熔胶
EV A、P.O、合成橡胶热熔胶
合成橡胶系溶剂形接着剂
CR溶剂形胶水(575、575F)
NBR溶剂形胶水(540、545N)
热塑性树脂系溶剂形接着剂
醋酸乙烯酯、聚氯乙烯、聚胺脂胶水(201)
台湾施敏打硬产扬声器专用胶水
AB胶-磁回路胶(Y-358AB、Y-450AB、Y-480AB)
中心胶(CS-4501、CS-4503、CS-4505)
音膜胶-柔软胶-制动剂(638、223B、223BL、610B)
封边胶(525L、634、645A、606C、CS-700、5000、CT-850)
引线胶(366L、366LW)深圳市岩濑贸易有限公司。
微生物CLSI文件集锦(你想要的都在这里)

微生物CLSI文件集锦(你想要的都在这里)说起临床微生物的CLSI文件,大家首先想到的就是CLSI M100S——《抗微生物药物敏感性试验的执行标准》。
但是其实,与临床微生物相关的CLSI文件很多,截至2017年7月,已多达43个。
下面,小编就给您简单介绍一下吧。
1、M02-A12:Approved Standard中文:抗菌药敏试验的性能标准英文:Performance Standards for Antimicrobial Disk Susceptibility Tests内容与解释:介绍药物纸片扩散法的质量控制标准和最新折点标准。
2、M06-A2: Approved Standard中文:脱水MH琼脂的评估程序英文:Protocols for Evaluating Dehydrated Mueller-Hinton Agar内容与解释:略3、M07-A10:Approved Standard中文:需氧菌稀释法抗菌药物敏感性试验英文:Methods for Dilution Antimicrobial Susceptibility T ests for Bacteria That Grow Aerobically内容与解释:描述了肉汤稀释法和琼脂稀释法,而且还包含这些方法的标准化操作流程和CLSI推荐方法的性能,局限性,适应性。
4、M11-A8:Approved Standard中文:厌氧菌抗菌药物敏感性试验英文:Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria内容与解释:在过去的几年内,大部分厌氧菌的耐药表型都发生了很大的改变,导致了许多菌种的经验用药的面临很大的挑战。
对于厌氧菌,琼脂稀释法仍然是参考方法,对于调查研究和科研同样都适用。
而且其他的方法的对比标准也进行了说明,肉汤稀释法也应用于临床实验室,但是现在对脆弱拟杆菌和一些抗生素没有标准。
氨苄青霉素 LB琼脂产品说明书

化学品安全技术说明书第一部分化学品及企业标识产品中文名称:氨苄青霉素LB琼脂产品英文名称:LB Agar with Ampicillin产品编号:028335企业名称:广东环凯微生物科技有限公司地址:广东省广州市黄埔区广州开发区科学城神舟路788号邮编:510663公司网址电子邮件地址:*********************传真号码:************销售热线:************-8602技术热线:************-8877/8876推荐用途和限制用途:生化研究/分析第二部分危险性概述GSH危害性类别非危险物质或混合物GSH标签要素非危险物质或混合物其它危害(健康危害、环境危害)未见报道第三部分成分/组成信息混合物化学品成分:参考培养基使用说明。
有害物质成分:无第四部分急救措施一般信息:无特殊的措施要求。
皮肤接触:立即用清水彻底清洗。
眼睛接触:立即提起眼睑,用大量流动清水冲洗15min。
如不适就医。
吸入:如果吸入,将人员移动到新鲜空气处,如果没有呼吸,进行人工呼吸操作,并联系医生。
食入:如误食,用水冲洗口腔,如不适就医。
就医信息:出示产品使用说明或者此MSDS。
第六部分 泄露应急处理个人防护: 穿个人实验服,佩戴手套和口罩,避免吸入干粉。
环境保护措施: 用湿布和地拖擦拭干净。
清洁/收集措施: 保持干燥。
迅速清洗弄脏的区域。
第七部分 操作处置与储存安全操作注意事项: 防止粉尘扬起,应提供通风设备。
储存注意事项: 贮存于避光、干燥处,用后立即旋紧瓶盖。
第八部分 接触控制/个人防护职业接触限值 没有已知的国家规定的暴露极限。
工程控制: 提供安全淋浴和洗眼设备个人保护措施 呼吸系统防护: 在通风橱里称取产品,佩戴口罩。
眼睛防护: 佩戴安全眼镜。
身体防护: 穿实验室服。
手防护: 戴防化学品手套。
其他防护: 常规的工业卫生操作,工作后及时清洗双手。
第九部分 理化特性外观: 粉末 pH 值: 7.4±0.2(25℃) 颜色: 淡黄色 气味: 特征性 熔点: 无数据资料 沸点: 无数据资料 燃点: 无数据资料闪点:无数据资料爆炸限度 下限: 无数据资料 上限: 无数据资料 热分解:无数据资料第五部分 消防措施危险特性: 不助燃。
荧光素@UiO-66金属有机框架材料荧光检测曲利苯蓝

第41卷第6期2023年12月沈阳师范大学学报(自然科学版)J o u r n a l o f S h e n y a n g N o r m a lU n i v e r s i t y(N a t u r a l S c i e n c eE d i t i o n)V o l.41N o.6D e c.2023文章编号:16735862(2023)06048806荧光素@U i O-66金属有机框架材料荧光检测曲利苯蓝刘丽艳1,乔丹1,刘珂帆1,2,刘笑言1,史建军1,于湛1(1.沈阳师范大学化学化工学院,沈阳110034;2.大连市一一七中学,辽宁大连116100)摘要:采用水热法制备了荧光素@U i O-66(F N@U i O-66)金属有机框架材料,借助X射线衍射和红外光谱技术确定了其物相结构,并利用扫描电子显微镜㊁动态光散射等技术表征了其形貌特征㊂结果表明,F N@U i O-66大小均匀,平均水合粒径为380.4n m㊂随后进行了F N@U i O-66对典型染料曲利苯蓝的荧光检测研究,实验结果表明,曲利苯蓝可以猝灭F N@U i O-66的荧光发射,其S t e r n-V o l m e r系数K S V为3593L㊃m o l-1,说明F N@U i O-66材料对曲利苯蓝具有良好的选择性,且曲利苯蓝的检出限为0.06μm o l㊃L-1㊂由此可见,F N@U i O-66可以高效地识别曲利苯蓝,且具有良好的选择性和检测灵敏度,可实现对曲利苯蓝的快速荧光检测㊂关键词:F N@U i O-66;合成;曲利苯蓝;荧光探针中图分类号:O657.3文献标志码:Ad o i:10.3969/j.i s s n.16735862.2023.06.002F l u o r e s c e n c e d e t e c t i o n o f t r y p a nb l u e b a s e d o n t h e f l u o r e s c e i n@U i O-66m e t a l-o r g a n i c f r a m e w o r km a t e r i a lL I U L i y a n1,Q I A O D a n1,L I U K e f a n1,2,L I U X i a o y a n1,S H I J i a n j u n1,Y UZ h a n1(1.C o l l e g e o fC h e m i s t r y a n dC h e m i c a lE n g i n e e r i n g,S h e n y a n g N o r m a lU n i v e r s i t y,S h e n y a n g110034,C h i n a;2.D a l i a nN o.117M i d d l eS c h o o l,D a l i a n116100,C h i n a)A b s t r a c t:I nt h i s w o r k,t h ec o m p o s i t ef l u o r e s c e i n@U i O-66(F N@U i O-66)m e t a l-o r g a n i cf r a m e w o r km a t e r i a lw a s s y n t h e s i z e db y ah y d r o t h e r m a l p r o t o c o l.T h es t r u c t u r a l c h a r a c t e r i s t i c so fF N@U i O-66w e r ed e t e r m i n e dw i t h t h eh e l p o fX-r a y d i f f r a c t i o na n d i n f r a r e ds p e c t r o s c o p y a sw e l la s t h em o r p h o l o g i c a l c h a r a c t e r i s t i c sw e r e i n v e s t i g a t e db y sc a n n i n g e l e c t r o nm i c r o s c o p y(S E M)a n dd y n a m i c l i g h t s c a t te r i n g(D L S).E x p e r i m e n t a l r e s u l t s s h o wt h a tF N@U i O-66i su n if o r mi ns i z e,w i t h a na v e r a g eh y d r o d y n a m i cd i a m e t e ro f380.4n m.S u b s e q u e n t l y,af l u o r e s c e n c es t u d y o fF N@U i O-66o n t r y p a nb l u e(T B)w a s c a r r i e do u t,a n d f u r t h e r e x p e r i m e n t s s h o wt h a t t h eS t e r n-V o l m e r c o e f f i c i e n t o fT B,K S V,i s3593L㊃m o l-1,w h i c h i n d i c a t e s t h a t t h eF N@U i O-66h a sag o o de f f e c t o nT B.T h e s e l e c t i v i t y o f F N@U i O-66f o rT B i s g o o d,a n d t h e d e t e c t i o n l i m i t o fT B i s0.06μm o l㊃L-1,s h o w i n g t h a t F N@U i O-66c a n r e c o g n i z eT Be f f i c i e n t l y.F N@U i O-66h a s a g o o ds e l e c t i v i t y a n dd e t e c t i o n s e n s i t i v i t y t oT B,a n d i t c a n r e a l i z e t h e r a p i d f l u o r e s c e n c e d e t e c t i o no fT B.K e y w o r d s:F N@U i O-66;s y n t h e s i s;t r y p a nb l u e;f l u o r e s c e n t p r o b e金属有机框架(m e t a l-o r g a n i c f r a m e w o r k s,MO F s)材料因其在结构上具有规律性㊁刚性㊁多变性㊁收稿日期:20230920基金项目:辽宁省教育厅高等学校基本科研项目(L J C202009)㊂作者简介:刘丽艳(1977 ),女,辽宁沈阳人,沈阳师范大学副教授,博士㊂可设计性,成为一类具有广泛应用前景的新型材料[1]㊂MO F s 具有可调控的多孔通道,可以容纳多种极性㊁体积不同的客体分子,因而常作为探针载体用于荧光传感中[2]㊂2008年,挪威奥斯陆大学的C a v k a 研究组[3]首次报道了一类以金属Z r 为中心㊁对苯二甲酸(H 2B D C )为有机配体的刚性金属有机框架材料,命名为U i O -66㊂与其他MO F s 材料相比,U i O -66具有特别的热稳定性和化学稳定性[4],晶体结构可在500ħ下保持稳定,其框架结构可承受1.0M P a 的机械压力㊂U i O -66在水㊁苯或丙酮等溶剂中可以保持结构稳定,并且还具有很强的耐酸性和一定的耐碱性[5]㊂曲利苯蓝(t r y pa nb l u e ,T B )是一种常见的偶氮类染料,可用于给棉㊁麻㊁蚕丝㊁化纤制品染色,并可作为纸张㊁皮革等材料的染色剂[6]㊂由于死细胞的细胞膜不完整,短时间T B 染色可将其染成蓝色,而具有完整细胞膜的活细胞会排斥染料,因而在生物学实验中,T B 也常用于区分正常细胞与死亡细胞㊂但是有文献[7]证实,经过5m i n 染色,T B 就会对细胞产生毒性,5~30m i n 染色会导致T B 染料渗透健康细胞的细胞膜,细胞会随着时间的推移而死亡,最终健康细胞也会被T B 染色,因而使用T B 进行细胞染色的最终结果是降低细胞活性[8]㊂本文成功制备了一种荧光素(f l u o r e s c e i n ,F N )与U i O -66的复合材料F N@U i O -66,表征了其结构并研究了其在水溶液中对T B 的荧光检测性能㊂实验研究表明,F N@U i O -66对T B 具有良好的选择性和灵敏度,可用于荧光检测T B ㊂1 材料与方法1.1 试剂与仪器四氯化锆㊁荧光素(F N )㊁曲利苯蓝(T B )㊁对苯二甲酸(H 2B D C )㊁N ,N -二甲基甲酰胺(D M F )㊁盐酸㊁甲醇㊁冰乙酸㊁醋酸钠㊁乙腈㊁乙酸乙酯㊁三氯甲烷㊁二甲基亚砜(D M S O )等试剂均为分析纯或更高纯度,实验中未进行纯化而直接使用;实验用水为超纯水(18.2MΩ㊃c m )㊂采用日本日立公司的S U 8010型扫描电子显微镜(s c a n n i n g e l e c t r o n m i c r o s c o p e ,S E M )对样品的表面形貌特征进行检测,工作距离为3.8mm ,加速电压为3.0k V ;采用日本理学公司的X 射线粉末衍射仪测定样品的X 射线衍射(X -r a y d i f f r a c t i o n ,X R D )图谱,铜靶K α线波长为0.15405n m ,扫描速度为10ʎ㊃m i n -1,扫描范围为5ʎ~50ʎ,管电压为40k V ,管电流为40m A ;采用英国马尔文公司的N a n o -Z S 90型纳米粒度分析仪测试样品的平均粒度;采用美国赛默飞公司的N i c o l e t i S5型傅里叶变换红外光谱仪测试样品的红外光谱;采用美国瓦里安公司的C a r y E c l i p s e 型荧光光谱仪对悬浮液样品进行荧光测试㊂1.2 F N @U i O -66的制备F N@U i O -66的合成方法参照文献[9]㊂首先准确称取0.4514g Z r C l 4,0.3289g H 2B D C ,0.0338g F N ,34.0m LD M F 和0.34m LH C l 于250m L 烧杯中,混合搅拌1h 后使全部反应物完全溶解在D M F 中,得到均匀的反应液㊂随后将反应液转移至装有50m L 聚四氟乙烯内衬的不锈钢反应釜中,在120ħ烘箱中连续加热24h ,待其自然冷却后,将得到的黄色粉末用D M F 和甲醇溶液洗涤4次并抽滤,得到粗产物㊂将粗产物在80ħ下烘干12h 后,自然冷却并研磨,得到黄色固体粉末,即为F N@U i O -66㊂1.3 荧光实验将F N@U i O -66粉末(30m g)加入30m L 超纯水中,室温下静置24h ,然后将此样品进行30m i n 超声处理后得到稳定的悬浮液㊂分别准确移取多份2.5m L 的悬浮液,加入不同体积的5ˑ10-4m o l ㊃L -1T B 水溶液,并测试其光致发光(ph o t o l u m i n e s c e n c e ,P L )谱图㊂进行重复性实验时,将F N@U i O -66粉末从悬浮液中高速离心出,经无水乙醇充分洗涤后晾干即可重复使用㊂2 结果与讨论2.1 F N @U i O -66的表征图1分别给出了文献[10]报道的U i O -66单晶数据模拟㊁本文合成的F N@U i O -66及在T B 溶液984第6期 刘丽艳,等:荧光素@U i O -66金属有机框架材料荧光检测曲利苯蓝图1 U i O -66单晶模拟㊁F N @U i O -66及在T B 溶液中浸泡24h 后的F N @U i O -66的X R D 图F i g .1 S i m u l a t e da n de x pe r i m e n t a l (b ef o r ea n da f t e r s o a k i ng i n t r y pa nb l u es o l u t i o n f o r 24h )X R D pa t t e r n s o f F N @U i O -66中浸泡24h 后的F N@U i O -66的X R D 谱图㊂由图1可以看出,U i O -66在2θ为7.36ʎ和8.50ʎ处有明显的特征衍射峰,在14.76ʎ,17.06ʎ,25.72ʎ,30.72ʎ等处也有相对较强的衍射峰㊂本文合成的F N@U i O -66材料衍射峰与单晶数据模拟的U i O -66特征峰峰位相同且强度较高,未出现其他杂峰,这表明已经成功获得F N@U i O -66材料,并且其纯度和结晶度良好㊂当F N@U i O -66在T B 溶液中浸泡24h 后,其峰位和峰强并没有发生变化,也没有出现明显的杂峰,表明在荧光检测T B过程中,F N@U i O -66材料结构稳固,没有发生变化㊂图2给出30000倍和60000倍放大倍率下的F N@U i O -66样品的S E M 照片㊂可以看出,F N@U i O -66样品晶化程度良好,晶体颗粒大小均匀,晶粒呈正四面体,粒径范围约为150~300n m ,与文献[10]报道相一致,表明成功地合成出F N@U i O -66样品,样品纯度比较高,并且F N 的引入并不会导致U i O -66的结构发生变化㊂(a )放大倍率30000倍(b )放大倍率60000倍图2 F N @U i O -66的S E M 照片F i g .2 S E Mi m a ge s of F N @U i O -66图3为纯水介质中U i O -66与F N@U i O -66的水合粒径分布图㊂由图3可见,U i O -66与F N@U i O -66的粒径分布较为均匀,平均粒径分别为334.5n m 和380.4n m ,尤其是当F N 与U i O -66形成F N@U i O -66复合材料后,复合材料的平均粒径稍稍增大,证明了F N@U i O -66复合材料的成功合成㊂图3 (a )U i O -66与(b )F N @U i O -66的粒径分布图F i g .3 A v e r a ge p a r t i c l es i z ed i s t r i b u t i o n p l o t of (a )U i O -66a n d (b )F N @U i O -66图4为U i O -66,F N 与F N@U i O -66的红外光谱图㊂如图4(a )所示,3430c m -1处的宽峰可归属为O H 键伸缩振动,1398c m -1处强吸收峰可归属为配体中羧基伸缩振动,1505c m -1和1583c m -1094沈阳师范大学学报(自然科学版) 第41卷图4 (a )U i O -66,(b )F N ,(c )F N @U i O -66的红外光谱图F i g .4 I Rs pe c t r aof (a )U i O -66,(b )F Na n d (c )F N @U i O -66处的特征峰是配体分子中苯环的骨架振动引起的,550c m -1处的吸收峰对应Z r O C 键,这个吸收峰的存在证明了金属有机框架结构的建立,表明成功地合成了U i O -66㊂图4(c )给出F N@U i O -66材料的红外光谱图,由图4(c )可见,复合F N 后U i O -66的红外谱图变化不明显,一些强吸收峰出现几个波数的红移,例如,1398c m -1处吸收峰红移至1401c m -1处,1583c m -1处吸收峰红移至1584c m -1处,但并未出现F N 如1598c m -1,1112c m -1处的特征吸收峰,推测其原因可能是U i O -66中复合的F N 量较少㊂由图5(a )可以看出,U i O -66及F N@U i O -66的氮气吸附脱附等温曲线均呈现典型的Ⅰ类吸附等温线特点,即在P /P 0比较低时吸附量快速上升,随着P /P 0的增加,吸附量达到一个饱和值,当接近饱和压力(P /P 0接近1.0)时,曲线上扬㊂这表明U i O -66及F N@U i O -66都是典型的微孔结构㊂复合F N 后,U i O -66的氮气吸附量明显下降,比表面积由1005.3452m 2㊃g -1下降至873.5886m 2㊃g -1,这也从另一方面证明了F N@U i O -66的成功合成㊂图5(b )给出U i O -66及F N@U i O -66的孔分布情况,复合F N 后U i O -66的孔体积显著减小,只有0.86n m 大小的孔体积变大,本文推测这可能是复合F N后,U i O -66表面部分孔的孔径发生改变所致㊂(a )(b)图5 U i O -66与F N @U i O -66的(a )氮气吸附脱附等温曲线和(b)孔分布曲线F i g .5 (a )N 2a d s o r p t i o n /d e s o r p t i o na n d (b )po r es i z ed i s t r i b u t i o no f U i O -66a n dF N @U i O -66图6 F N @U i O -66的荧光发射光谱(λe x =320n m )F i g .6 F l u o r e s c e n c es pe c t r aof U i O -66a n d F N @U i O -66(λe x=320n m )图6为U i O -66及F N@U i O -66悬浊液的荧光光谱图㊂由图6可见,U i O -66在369n m 处有发射峰,对应B D C 配体的π-π*跃迁[11]㊂而与之相比,F N@U i O -66复合材料除了369n m 处峰外,在523n m 处存在强度更大的发射峰,此峰对应于F N 分子的特征发射㊂2.2 F N @U i O -66对T B 的荧光检测图7(a )为不同浓度T B 存在下F N@U i O -66悬浊液的发光情况㊂可以看出,随着T B 浓度的增加,体系的发光强度逐渐降低,发生荧光猝灭现象,并且主发射峰发生蓝移,由523n m 变为520n m ,表明T B 的194第6期 刘丽艳,等:荧光素@U i O -66金属有机框架材料荧光检测曲利苯蓝引入降低了体系F N 分子周围的极性,增加了环境的疏水性㊂一般来说,荧光猝灭包括静态猝灭和动态猝灭,可使用S t e r n -V o l m e r 方程(式(1))分析㊂F 0F=1+K S V [Q ](1)其中:F 与F 0分别为有无猝灭剂时体系的荧光发射强度;[Q ]是猝灭剂浓度;K S V 是S t e r n -V o l m e r 常数㊂利用式(1)对图7(a )中荧光发射数据进行计算[1213],可以看出,T B 浓度在0~3.0ˑ10-4m o l㊃L -1时F 0/F 与T B 浓度呈现良好的线性关系,线性方程为y =1.02+3593x ,R 2为0.9973㊂根据3δ/S(δ为空白样品标准偏差,S 为线性方程斜率)计算可知,T B 检出限为0.06μm o l ㊃L -1,表明F N@U i O -66能够较灵敏地检测T B㊂(a )(b)(从1到8,T B 浓度分别为0.0,0.25ˑ10-4,0.5ˑ10-4,1.0ˑ10-4,1.5ˑ10-4,2.0ˑ10-4,2.5ˑ10-4和3.0ˑ10-4m o l㊃L -1)图7 (a )不同浓度T B 存在条件下F N @U i O -66荧光发射光谱(λe x =320n m )和(b )S t e r n -V o l m e r 图F i g .7 (a )F l u o r e s c e n c ee m i s s i o n s p e c t r ao f U i O -66@F l u o r e s c ew i t hd i f f e r e n t c o n c e n t r a t i o n s o f T B (λe x =320n m )a n d (b )S t e r n -V o l m e r p l o t (a 归一化的T B 可见吸收光谱;b 归一化的F N@U i O -66荧光发射光谱)图8 光谱重叠谱图F i g .8 Sc h e m a t i z ed s pe c t r a l o v e r l a ps 根据F ör s t e r 共振能量传递理论[14],当荧光体与物质距离较近时,如果荧光体的发射光谱与物质的吸收光谱之间存在重叠,二者之间会发生能量传递,这是引起荧光猝灭的原因之一㊂图8给出了室温下F N@U i O -66荧光发射光谱与T B 紫外可见光谱的重叠谱图㊂由图8可见,二者之间存在较大程度的重叠,导致F N@U i O -66荧光发射能量向T B 转移,F N@U i O -66则出现明显的猝灭现象㊂本文还考察了F N@U i O -66识别T B 的重复性㊂设第1次实验中F N@U i O -66的发光强度为100%,则第2次至第5次实验中F N @U i O -66的发光强度分别为98.83%,94.12%,90.86%和90.82%㊂可以看出,经过5次循环,F N@U i O -66的荧光强度仍旧维持在较高水平,F N@U i O -66的使用重复性较好㊂3 结 论本文采用水热法成功制备了金属有机框架材料F N@U i O -66,并通过X 射线衍射㊁红外光谱等手段表征了材料的结构㊂同时,将F N@U i O -66用作传感器,使用荧光猝灭方法识别检测T B ㊂实验结果显示,T B 可以有效地猝灭F N@U i O -66发光,并且随着T B 加入量的增加,F N@U i O -66的猝灭越来越显294沈阳师范大学学报(自然科学版) 第41卷著,F N@U i O -66对T B 的检出限为0.06μm o l ㊃L -1㊂因此,F N@U i O -66对T B 具有良好的选择性和灵敏度,可实现T B 的快速发光检测㊂参考文献:[1]F R E U N DR ,Z A R E M B A O ,A R N A U T S G ,e ta l .T h ec u r r e n ts t a t u so f MO Fa n dC O Fa p p l i c a t i o n s [J ].A n g e w C h e mI n tE d ,2021,60(45):2397524001.[2]WA N G G D ,L IY Z ,S H I W J ,e t a l .Ar o b u s t c l u s t e r -b a s e dE u -MO Fa s m u l t i -f u n c t i o n a l f l u o r e s c e n c es e n s o r f o r d e t e c t i o no f a n t i b i o t i c s a n d p e s t i c i d e s i nw a t e r [J ].S e n sA c t u a t o r sBC h e m ,2021,331:129377.[3]C A V K A J ,J A K O B S E N S ,O L S B Y E U ,e ta l .A n e w z i r c o n i u m i n o r g a n i cb u i l d i n g b r i c kf o r m i n g m e t a lo r g a n i c f r a m e w o r k sw i t he x c e p t i o n a l s t a b i l i t y [J ].JA m C h e mS o c ,2008,130(42):1385013851.[4]K A N D I A H M ,N I L S E N M H ,U S S E G L I O S ,e t a l .S y n t h e s i sa n ds t a b i l i t y o f t a g g e d U i O -66Z r -MO F s [J ].C h e m M a t e r ,2010,22(24):66326640.[5]P I S C O P O CG ,P O L Y Z O I D I SA ,S C HWA R Z E R M ,e t a l .S t a b i l i t y o fU i O -66u n d e r a c i d i c t r e a t m e n t :O p p o r t u n i t i e s a n d l i m i t a t i o n s f o r p o s t -s y n t h e t i cm o d i f i c a t i o n s [J ].M i c r o p o rM e s o p o rM a t ,2015,208:3035.[6]R A V I S HA N K A R T N ,MA N J U N A T HA K ,R AMA K R I S HN A P P A T ,e ta l .C o m p a r i s o no ft h e p h o t o c a t a l yt i c d e g r a d a t i o n o f t r y p a n b l u e b y u n d o p e d a n d s i l v e r -d o pe d z i n c o x i d e n a n o p a r t i c l e s [J ].M a t S c i S e m i c o nP r o c ,2014,26:717.[7]T S A O U S I S K T ,K O P S A C H I L I S N ,T S I N O P O U L O SIT ,e ta l .T i m e -d e p e n d e n t m o r p h o l o g i c a la l t e r a t i o n sa n d v i a b i l i t y o f c u l t u r e dh u m a n t r a b e c u l a r c e l l s a f t e r e x p o s u r e t o t r y p a nb l u e [J ].C l i nE x p O p h t h a l m o l ,2013,41(5):484490.[8]KWO K A K H ,Y E U N GCK ,L A I TYY ,e t a l .E f f e c t s o f t r y p a n b l u e o n c e l l v i a b i l i t y a n d g e n e e x p r e s s i o n i n h u m a n r e t i n a l p i g m e n t e p i t h e l i a l c e l l s [J ].B r i t JO ph t h a l m o l ,2004,88(12):15901594.[9]S HA N A HA NJ ,K I S S E L D S ,S U L L I V A N E .P A N I @U i O -66a n d P A N I @U i O -66-N H 2p o l y m e r -MO F h y b r i d c o m p o s i t e s a s t u n a b l e s e m i c o n d u c t i n g m a t e r i a l s [J ].A C SO m e g a ,2020,5(12):63956404.[10]L IJ ,D A IY ,C U IJ ,e ta l .D y e -e n c a p s u l a t e dZ r -b a s e d MO F sc o m p o s i t e sa sas e n s i t i v e p l a t f o r m f o rr a t i o m e t r i c l u m i n e s c e n t s e n s i n g of a n t i b i o t i c s i nw a t e r [J ].T a l a n t a ,2023,251:123817.[11]S U N Z ,L IJ ,WA N G X ,e ta l .R h B -e n c a p s u l a t e d MO F -b a s e dc o m p o s i t ea ss e l f -c a l i b r a t i ng s e n s o rf o rs e l e c t i v e d e t e c t i o no f 4-n i t r o a n i l i n e [J ].JL u m i n ,2022,241:118480.[12]Z HA OD ,L I U X H ,Z HA O Y ,e t a l .L u m i n e s c e n t C d (Ⅱ)-o r g a n i c f r a m e w o r k sw i t h c h e l a t i n g N H 2s i t e s f o r s e l e c t i v e d e t e c t i o no fF e (Ⅲ)a n da n t i b i o t i c s [J ].JM a t e rC h e m A ,2017,5(30):1579715807.[13]L I UZQ ,Z HA O Y ,Z HA N GXD ,e t a l .M e t a l -o r g a n i c f r a m e w o r k sw i t h 1,4-d i (1H -i m i d a z o l -4-y l )b e n z e n e a n d v a r i e d c a r b o x y l a t e l i g a n d sf o rs e l e c t i v e l y s e n s i n g Fe (Ⅲ)i o n sa n dk e t o n e m o l e c u l e s [J ].D a l t o n T r a n s ,2017,46(40):1394313951.[14]R OW L A N DC E ,B R OWN C W ,M E D I N T ZIL ,e t a l .I n t r a c e l l u l a rF R E T -b a s e d p r o b e s :Ar e v i e w [J ].M e t h o d sA p pl F l u o r e s c ,2015,3(4):042006.394第6期 刘丽艳,等:荧光素@U i O -66金属有机框架材料荧光检测曲利苯蓝。
BSI-201_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :BSI-201Catalog No. :HY-12015CAS No. :160003-66-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Eye irritation (Category 2A), H3192.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H319 Causes serious eye irritation.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P280 Wear protective gloves/ eye protection/ face protection.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.P330 Rinse mouth.P337 + P313 If eye irritation persists: Get medical advice/ attention.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Iniparib; NSC⁻746045; IND⁻71677; BSI 201; BSI201Formula:C7H5IN2O3Molecular Weight:292.03CAS No. :160003-66-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Trigonox 311 3,3,5,7,7-Pentamethyl-1,2,4-trioxepan
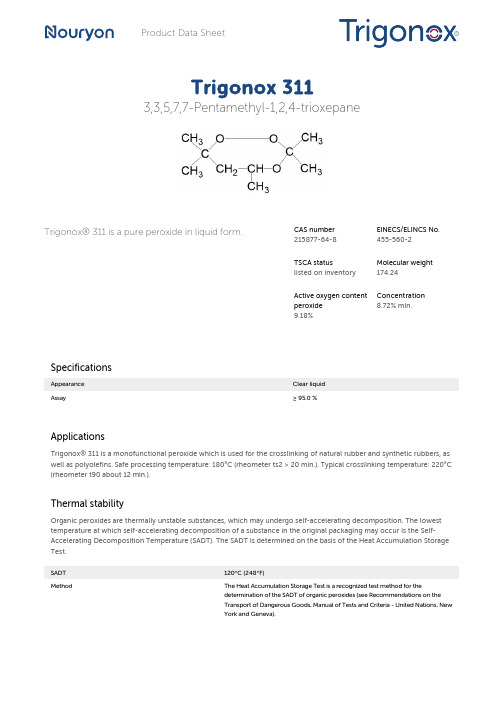
Product Data SheetTrigonox 3113,3,5,7,7-Pentamethyl-1,2,4-trioxepaneTrigonox® 311 is a pure peroxide in liquid form.CAS number215877-64-8EINECS/ELINCS No. 455-560-2TSCA statuslisted on inventory Molecular weight 174.24Active oxygen content peroxide9.18%Concentration 8.72% min.SpecificationsAppearance Clear liquidAssay≥ 95.0 %ApplicationsTrigonox® 311 is a monofunctional peroxide which is used for the crosslinking of natural rubber and synthetic rubbers, as well as polyolefins. Safe processing temperature: 180°C (rheometer ts2 > 20 min.). Typical crosslinking temperature: 220°C (rheometer t90 about 12 min.).Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT120°C (248°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperatureTs Max.40°C (104°F)Ts Min.15°C (59°F) to prevent crystallizationNote When stored under these recommended storage conditions, Trigonox® 311 willremain within the Nouryon specifications for a period of at least 12 months afterdelivery.Packaging and transportThe standard packaging is a 30-liter HDPE can (Nourytainer®) for 25 kg peroxide solution. Both packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Trigonox® 311 is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® 311 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 311. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsMethane, Acetone, Isopropyl acetate, 3-Hydroxy-1,3-dimethylbutyl acetate, 3-Methoxy-1,3-dimethylbutyl acetateAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox 311。
感控相关英语单词短语及缩写中英对照

抗生素相关腹泻 鲍曼不动杆菌 免疫实践咨询委员会 主动筛查 腺病毒 主动检测和隔离 全自动内镜清洗消毒机 产生气溶胶的操作 抗酸杆菌 美国医院协会 速干手消毒剂 经空气传播疾病 急性白血病 氨基青霉素 耐药性 抗菌缝线 抗菌药物管理团队 抗菌剂 美国感染控制与流行病学专业协会 成人型呼吸窘迫综合征 耐药性微生物 耐药性病原体 抗菌药物管理项目 黑曲霉菌 抗菌药物敏感性试验 抗菌药物应用和耐药性 苯扎氯铵 菌血症 细菌性气溶胶 细菌性腹膜炎 血源性病原体 床旁隔离 生物膜 血琼脂平板 呼吸回路 布里斯托分型表 广谱抗生素 布鲁氏菌 血流感染
临时性血管通路 终末消毒 清洗效果测试物 四环素 替加环素 全髋关节置换术 全肠外营养 阿萨希毛孢子菌 甲氧苄氨嘧啶 万古霉素 呼吸机相关肺炎 汽化过氧化氢 霍乱弧菌 创伤弧菌 耐万古霉素肠球菌 耐万古霉素屎肠球菌 水痘-带状疱疹病毒 白细胞计数 西尼罗病毒 泛耐药鲍曼不动杆菌 泛耐药
感控相关英语单词、短语及缩写
Buffer room Burkholderia cepacia CA-BSI (catheter-associated blood stream infections) Campylobacter jejuni CA-MRSA (community associated MRSA ) Candida parapsilosis Carbapenems CASS(continuous aspiration of subglottic secretions) Candida albicans CAUTI(Catherter—associated urinary tract infection) CBIC(Certification Board of Infection Control and Epidemiology) CCNA(cell cytotoxin neutralization assay) CCT( Controlled clinical trail ) CDAD (Clostridium difficile–associated disease) CDI(Clostridium difficile infection) Cephalosporins CHA(Chlorhexidine acetate) CHG(Chlorhexidine gluconate) Chlamydia trachomatis Chlorine product CJD(Creutzfedt-Jekob disease) CLABSI(central line-associated bloodstream infections) CLAD(closed Luer access device) Clindamycin Clostridium perfringens Colistin colonisation CoNS(coagulase-negative staphylococci) Contact isolation CoV(coronavirus) CoxA16(Coxsackievirus) CPE(carbapenemase-producing Enterobacteriaceae) CPIS(clinical pulmonary infection score) CPS(coagulase-positive staphylococci) CRAB(Carbapenem-resistant Acinetobacter) CRE(carbapenem-resistant Enterobacteriaceae) Critical dental instruments CRKP(carbapenem-resistant Klebsiella pneumoniae) CRV(community respiratory viruses) CSSD(Central sterile supply department) Decontamination area Dental scaler Di间 洋葱伯克霍尔德菌 导管相关血流感染 空肠弯曲杆菌 社区相关甲氧西林耐药金葡菌 近平滑假丝酵母菌 碳青霉烯类 持续声门下吸引 白色念珠菌 导尿管相关尿路感染 感染控制与流行病学认证委员会 细胞毒素中和试验 临床对照试验 艰难梭菌相关性腹泻 艰难梭菌感染 头孢菌素类 醋酸氯已定 葡萄糖酸氯已定 沙眼衣原体 含氯消毒剂 克雅病 中心静脉导管相关血流感染 密封的鲁尔接着装置 克林霉素 产气荚膜梭菌 黏菌素 定植 凝固酶阴性葡萄球菌 接触隔离 冠状病毒 柯萨奇A16型病毒 产碳青霉烯酶肠杆菌 临床肺部感染评分 凝固酶阳性葡萄球菌 耐碳青霉烯鲍曼不动杆菌 耐碳青霉烯类肠杆菌 高危口腔器械 耐碳青霉烯肺炎克雷伯菌 社区呼吸道病毒 消毒供应中心 去污区 牙洁治器 透析
Immersion Cooler NeoCool Dip Model BE201 201F 301

Immersion CoolerNeoCool DipModel BE201/201F/301Instruction ManualFirst Edition●Thank you for choosing BE series immersion NeoCool Dip coolers from Yamato Scientific Co., Ltd.●For proper equipment operation, please read thisinstruction manual thoroughly before use. Alwayskeep equipment documentation safe and close athand for convenient future reference.Warning: Read instruction manual warnings andcautions carefully and completely beforeproceeding.Yamato Scientific America Inc.Santa Clara,CAPrinted on recycled paper1. SAFETY PRECAUTIONS (1)Explanation of Safety Symbols (1)Symbol Glossary (2)Warnings & Cautions (3)2. PRE-OPERATION PROCEDURES (4)Placement Precautions & Procedures (4)3. COMPONENT NAMES & FUNCTIONS (10)Main Unit Overview (10)4. OPERATION PROCEDURE (12)Main Operation (12)Cooling Capacity Curves (Reference Data) (13)Choosing Coolant for Low-T emp Applications (Reference Data) (15)5. HANDLING PRECAUTIONS (16)6. INSPECTION & MAINTENANCE (17)7. STORAGE & DISPOSAL (18)Extended Storage / Unit Disposal (18)Disposal Considerations (18)8. TROUBLESHOOTING (19)Troubleshooting Guide (19)9. SERVICE & REPAIR (20)10. SPECIFICATIONS (21)11. WIRING DIAGRAM (22)12. LIST OF HAZARDOUS SUBSTANCES (23)1.Explanation of Safety SymbolsSymbol GlossaryGeneral WarningDanger!: HighVoltageDanger!:Extremely HotDanger!: MovingPartsDanger!: BlastHazardGeneral CautionCaution:Shock Hazard!Caution: BurnHazard!Caution: Do NotHeat WithoutWater!Caution: MayLeak Water!Caution: WaterOnlyCaution: T oxicChemicalsGeneralRestrictionNo Open FlameDo NotDisassembleDo Not T ouchGeneral ActionRequiredConnect GroundWireLevel InstallationRequiredDisconnect PowerInspectRegularly1.Warnings & CautionsWarning Do not install or operate CF301 unit near flammable or explosive gases/fumes. Unit is NOT fire orSubstances” (PAlways ground this unit properly to avoid electric shock.Damagingshock to the operator.NeverHazardous Substances” (PBe sure there is adequate ventilation when working with certain flammable substances (such as ethanol, etc.), which evaporate quickly at or below room temperature, and emit flammable fumes. Insufficient ventilation may cause a fire or explosion.Caution2. Placement Precautions & ProceduresWarningNever connect ground wire to rods. Doing so may result in fire or electrical shock. 2. Place in suitable locations.Whit Black GreenPlacement Precautions & ProceduresWarning4. DO NOT disassemble or modify.Attempting to disassemble or modifiy this unit in any way may result in malfunction, electric shock.Placement Precautions & ProceduresWarningCaution 6. Place on level surfaces.Place unit on a level and even surface. Failure to do so may result in abnormal vibrations ornoise and damage to the refrigeration system.Placement Precautions & ProceduresCautionPlacement Precautions & ProceduresCautionPlacement Precautions & Procedures3. Main Unit OverviewBE201 front viewBE201 rear viewPower Switch (10A)Operation LampCooling CoilPower Cable(with plug)Cooling Coil SheathHandleHeat VentMain Unit OverviewBE201F/301 front viewBE201F/301 rear viewPower Switch (10A)Operation LampHandle Flexible Cooling Tube BE201F :500mm BE301 :1000mmMain OperationImmerse cooling coil in fluid as shown in diagram below. Turn power switch (ELB) “ON” to start operation.Wait at least five minutes to resume after halting an operation (e.g. turning power off). DONOT turn power switch “ON” and “OFF” repeatedly within a short timeframe. Damage tothe refrigeration system may result.Avoid operating unit continuously at fluid temperatures abive 35°C.Cooling Capacity Curves (Reference Data)Analysis provisions・External temperature:23°C・Power:220V AC・CPM:50Hz・Antifreeze fluid:60% Naiburain solution (covered, stirred and heat insulated)・Fluid quantity:BE201/201F: 5 ℓ BE301: 5 ℓ /10ℓ・T emp sensor placement:container centerBE201(5L)BE301(10L)BE201F(5L)BE301(5L)Cooling Capacity Curves (Reference Data) Analysis provisions・Room temperature:20°C・Power supply:220V AC・CPM:50Hz・Antifreeze fluid:60% Naiburain solution (covered, stirred and heat insulated)・Fluid quantity:BE201/201F: 5 ℓ BE301:10ℓ・Temp sensor placement:container centerChoosing Coolant for Low-T emp Applications (Reference Data)An anti-freeze coolant solution is required for applications below 10°C. Select a Naiburain® product with freezing point of 10°C or more below objective temperature. See “select coolant based on operating temperature ” on P .10.0-10-20-30-40-500102030405060708090100-10-20-30-40-50Concentration (wt%) Naiburain ® Freezing PointsT e m p e r a t u r e (°C )Warning2. DO NOT operate equipment when abnormalities are detected.Caution2. DO NOT place objects on equipment.3. DO NOT operate equipment during thunderstorms.4. DO NOT operate dry.5. Operate equipment as directed.6. Keep upright.6.Daily general maintenance and inspection is recommended to ensure optimal performance.WarningCaution◆ Contact local dealer or Yamato sales office for further assistance.7.Extended Storage / Unit DisposalCaution WarningDisposal Considerations Dispose of or recycle this unit in a responsible and environmentally friendly manner.Yamato Scientific Co., Ltd. strongly recommends disassembling unit, as far as is possible, in order to separate parts and recycle them in contribution to preserving the global environment.Troubleshooting Guide◆If problem persists, turn off power immediately, disconnect power cable and call for service.9.When a problem occurs, terminate operation immediately, turn off main power switch (ELB) and disconnect power cable.Contact a local dealer or Yamato sales office for assistance.The following information is required for all repairs. ● Model name ● Serial Number● Date (year/month/day) of purchase● Description of problem in as much detail as possibleGuaranteed maximum supply period for repair parts is 7 (seven) years from date of discontinuation for BE series NeoCool Dip immersion coolers .“Repair parts” is defined as components which, when installed, allow for continued unit operation.See production/rating label on unit. Refer to P .11 & 12 for location.⌘1 Performance based on AC220V power supply; 20°C±5°C external temperature; 65%RH±20% humidity; no load. Performance will vary depending on operation conditions..⌘2 Protrusions excluded.11. BE201/201F/30112.Never process explosive substances, flammable substances or substances that containexplosives or flammables.Excerpt from T able 1, Hazardous Substances, of Cabinet Order of the Occupational Safety and Health Law (substances related to Articles 1, 6, and 9)Limited liabilityAlways operate equipment in strict compliance to the handling and operation procedures set forth by this instruction manual.Yamato Scientific Co., Ltd. assumes no responsibility for malfunction, damage, injury or death resulting from negligent equipment use.Never attempt to disassemble, repair or perform any procedure on BE series unit which is not expressly mandated by this manual. Doing so may result in equipment malfunction, serious personal injury or death.Notice●Instruction manual descriptions and specifications are subject to change withoutnotice.●Yamato Scientific Co., Ltd. will replace flawed instruction manuals (pages missing,pages out of order, etc.) upon request.Instruction ManualImmersion Cooler NeoCool DipBE201/201F/301First Edition:June 6, 2017Yamato Scientific America Inc.925 Walsh Avenue, Santa Clara, CA 95050Phone: 800.292.6286 / 408.235.7725。
英国药典BP标准品氯二甲苯酚盐酸扑尔敏头孢洛宁甲磺酸二氢麦角碱杂质A丙氧基苯甲酸阿莫西林杂质

英国药典于 1864年在英国成立,是英国制药标准的重要来源。
该药典由三卷本组成。
其中两卷为英国药典、一卷为英国兽药典(兽医药品部分)。
各条目均以药品名称字母顺序排列,内容包括药品性质、制法、血产品、免疫产品、电磁药品制法及外科材料等部分.公司一直在为药学方面提供权威的官方标准品,它在药品和保健品方面做出了重大贡献,英国药典不仅为我们提供了药用和成药配方标准以及公式配药标准,而且也向我们展示了许多明确分类并可参照的欧洲药典专著。
广州优瓦仪器有限公司是从事提供实验室分析领域内产品的专业公司;目前公司已是集研发、生产与销售为一体的综合性企业;在行业内具有良好的声誉;主要产品包括色谱产品、化学试剂、标准品、实验室用品、分析仪器配件及耗材等,总部位于香港。
我们代理的品牌有:USP、EP、BP、TLC、TRC、Molcan、LGC、Wellington、Chiron、Witega、NRC、ERM、Irmm、MBH、Fluorochem、TCI、Chemservice、Accstandard 、 GmbH 、CIL 、C/D/N ISOTopes 、InorganicVentures 、ULTRA Scientific、NSI 、KeyOrganics、LC-Laboratoies、Wibby 、APSC、SPEX CertiPrep 、NIST 、ACROS、Fluka、Matrix、Sigma、TCI、Strem、BP、Cerilliant 、Chromadex 、Frontier、Echelon、Serva 、MedicalIsotope 、ChemBridge 、AMRESCO、SGE Analytical Science、Brand BRAND、VITLAB、ISO 、Hamilton、ISOLAB我们专注进口标准品。
详情请与我司联系。
Code中文名称Product001萜烯苯酚的合成树脂Acepromazinemaleate904乙酰唑胺分析标准品Acetazolamide Assay Standard6814-乙酰 2-氟联苯4-Acetyl2-fluorobiphenyl907乙酰半胱氨酸分析标准品AcetylcysteineAssay Standard438阿昔洛韦分析标准品Aciclovir AssayStandard003肾上腺素酸式酒石酸盐分析标准品Adrenaline (epinephrine) acid tartrate Assay Standard763阿苯达唑分析标准品Albendazole AssayStandard436亚历山大番泻叶世果粉分析标准品Alexandrian senna fruit powder Assay Standard006阿法多龙三醋酸分析标准品Alfadoloneacetate Assay Standard007硫酸丁胺卡那霉素分析标准品AlfaxaloneAssay Standard825盐酸阿夫唑嗪分析标准品Alfuzosinhydrochloride Assay Standard415异丁嗪分析标准品Alimemazine tartrateAssay Standard831别嘌呤醇杂质AAllopurinol ImpurityA832别嘌呤醇杂质BAllopurinol ImpurityB843别嘌呤醇杂质CAllopurinol ImpurityC844别嘌呤醇杂质DAllopurinol ImpurityD845别嘌呤醇杂质EAllopurinol ImpurityE560溴苄铵托西酸盐9-Allyl-2-chlorothioxanthen-9-ol725枸橼酸阿尔维林杂质分析标准品Alverinecitrate Assay Standard724枸橼酸阿尔维林杂质标准品Alverine citrate impurity standard solution530盐酸金刚烷胺分析标准品Amantadinehydrochloride Assay Standard853硫酸丁胺卡那霉素分析标准品AmikacinSulphate Assay Standard008盐酸阿米洛利分析标准品Amiloridehydrochloride Assay Standard010头孢洛宁4-Amino-6-chlorobenzene-1-3-disulphonamide374头孢呋辛酯3-Amino-6-chloro-1-methyl-4-phenylquinolin-2-ol839苯丁酸氮芥3-amino-4-(2-chlorophenyl)-6-nitroquinolin-2(1H)-one813氯二甲苯酚2-Amino-5-chloropyridine012盐酸扑尔敏2-Amino-4,6-Dichlorophenolhydrochloride011顺铂7-Aminodesacetoxycephalosporanic acid602盐酸富马酸氯马斯汀(E)-4-Amino-2-ethylidenebutyricacid hydrochloride684氯美噻唑乙二磺酸盐分析标准品AminoglutethimideAssay Standard881甲磺酸二氢麦角碱杂质AAminoglutethimideimpurity A4172-Amino-1-(4-nitrophenyl)propane-1,3-diol2-Amino-1-(4-nitrophenyl)propane-1,3 -diol6013-Aminopent-4-ene-1,1-dicarboxylicacid3-Aminopent-4-ene-1,1-dicarboxylic acid5363-Amino-4-phenoxy-5-sulphamoylbenzoicacid3-Amino-4-phenoxy-5-sulphamoylbenzoicacid014丙氧基苯甲酸3-Amino-4-propoxybenzoicacid532盐酸丙氧基苯甲酸分析标准品Amiodaronehydrochloride Assay Standard785无水的氨苄西林分析标准品AnhydrousAmpicillin Assay Standard021氨苄青霉素Ampicillintrihydrate015双甲脒分析标准品Amitraz AssayStandard016盐酸阿米替林分析标准品Amitriptylinehydrochloride Assay Standard019阿莫西林三水物分析标准品AmoxicillinTrihydrate Assay Standard748阿莫西林杂质标准品Amoxicillinimpurity standard546阿泊拉霉素Apramycin694盐酸马来酸麦角新碱分析标准品Argininehydrochloride Assay Standard461酒石酸麦角胺Ascorbic acid617阿匹司林分析标准品Aspirin AssayStandard492阿替洛尔分析标准品Atenolol AssayStandard370阿替洛尔杂质标准品Atenolol impuritystandard023雌二醇半水合物分析标准品Atropinesulphate Assay Standard366依他尼酸1-(3-Azabicyclo[]oct-3-yl)-3-o-tolylsulphonylurea 024氮杂羟基嘌呤2-Azahypoxanthine025阿扎哌隆分析标准品Azaperone AssayStandard534阿扎丙宗分析标准品AAzapropazone AssayStandard515阿扎丙宗分析标准品BAzapropazoneimpurity A516阿扎丙宗分析标准品CAzapropazoneimpurity B517阿扎丙宗分析标准品Azapropazoneimpurity C527阿扎丙宗分析标准品Azapropazoneimpurity standard028氟比洛芬钠分析标准品Baclofen AssayStandard535膦甲酸钠Baclofen lactam030二丙酸倍氯米松分析标准品Beclometasonedipropionate Assay Standard760灰黄霉素Beclometasone17-propionate761胍乙啶Beclometasone21-propionate685盐酸氢溴酸后马托品分析标准品Benserazide036甲磺酸苯扎托品分析标准品Benzatropinemesilate Assay Standard610盐酸羟丙甲纤维素分析标准品Benzydaminehydrochloride Assay Standard426苯甲酸苄酯分析标准品Benzyl benzoateAssay Standard611单硝酸异山梨酯1-Benzyl-3-(3-diethylamino-propoxy)-1H-indazole037伊维菌素(1S,2R)-1-benzyl-3-dimethylamino-2-methyl-1-phenylpropyl acetate609左薄荷脑1-Benzyl-1-H-indazol-3-ol824盐酸左炔诺孕酮分析标准品Betahistinedihydrochloride Assay Standard575倍他米松分析标准品Betamethasone AssayStandard041倍他米松磷酸钠Betamethasone sodiumphosphate042倍他米松戊酸分析标准品Betamethasonevalerate Assay Standard043倍他米松Betamethasone21-valerate686盐酸倍他洛尔分析标准品Betaxololhydrochloride Assay Standard755苯扎贝特分析标准品Bezafibrate AssayStandard046莫美他松糠酸酯2-(Biphenyl-4-yl)propionicacid047比沙可啶分析标准品Bisacodyl AssayStandard612溴苄铵托西酸盐分析标准品Bretyliumtosilate Assay Standard613盐酸诺氟沙星2-Bromobenzyldimethylaminehydrochloride050甲磺酸溴隐亭分析标准品BromocriptineMesilate Assay Standard440盐酸芍药苷杂质标准品Buclizinehydrochloride impurity standard793布地奈德分析标准品Budesonide AssayStandard537布美他尼分析标准品Bumetanide AssayStandard479盐酸布比卡因定分析标准品Bupivacaine403白消安分析标准品Busulfan AssayStandard192强的松2-tert-Butylamino-1-(4-hydroxy-3-methylphenyl)ethanolsulphate531氢化泼尼松磷酸钠2-Butyl-3-(4-hydroxy-3,5-di-iodobenzoyl)benzofuran766咖啡因分析标准品Caffeine AssayStandard355降血钙素分析标准品Calcitonin (salmon)Assay Standard059硫酸卷曲霉素Capreomycinsulphate538卡托普利分析标准品Captopril AssayStandard500柳氮磺胺吡啶Captoprildisulphide477盐酸舒必利2-Carbamoyl-1-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride523甲萘威分析标准品Carbaryl AssayStandard060卡比多巴分析标准品Carbidopa AssayStandard749卡比马唑分析标准品Carbimazole AssayStandard711卡帕分析标准品Carboplatin AssayStandard767羧甲基纤维素钠分析标准品Carmellosesodium Assay Standard567盐酸卡替洛尔分析标准品Carteololhydrochloride Assay Standard792头孢克洛分析标准品Cefaclor AssayStandard784头孢羟氨苄分析标准品Cefadroxil AssayStandard061头孢氨苄分析标准品Cefalexin AssayStandard562头孢洛宁分析标准品Cefalonium AssayStandard819头孢唑林钠Cefazolin Sodium820头孢西丁钠分析标准品Cefoxitin SodiumAssay Standard063头孢拉定分析标准品Cefradine AssayStandard976头孢曲松钠分析标准品Ceftriaxone sodiumAssay Standard977头孢曲松钠异构体Ceftriaxone sodiumE-isomer502头孢呋辛酯分析标准品Cefuroxime axetilAssay Standard925头孢呋辛钠分析标准品Cefuroxime sodiumAssay Standard757盐酸塞利洛尔分析标准品Celiprololhydrochloride Assay Standard926鹅去氧胆酸Chenodeoxycholicacid064苯丁酸氮芥分析标准品ChlorambucilAssay Standard854氯霉素分析标准品ChloramphenicolAssay Standard067盐酸氯环嗪Chlorcyclizinehydrochloride068醋酸氯己定分析标准品Chlorhexidineacetate Assay Standard4411,4-bis(4-Chlorobenzhydryl)piperazine1,4-bis(4-Chlorobenzhydryl)piperazine756N-p-chlorobenzoyltyramineN-p-chlorobenzoyltyramine6044-Chlorobenzylphthalazinone4-Chlorobenzylphthalazinone3766-氯-4-(2-氯苯基)喹啉-2-甲醛6-Chloro-4-(2-chlorophenyl)quinazoline-2-carboxaldehyde4426-Chloro-1,4-dihydro-1-methyl-4-phenylquinazolin-4-ol6-Chloro-1,4-dihydro-1-m ethyl-4-phenylquinazolin-4-ol4707-Chloro-1-5-dihydro-5-phenyl-1,5-benzodiazepine-2,4(3H)-dione7-Chloro-1-5-di hydro-5-phenyl-1,5-benzodiazepine-2,4(3H)-dione04917β,17'β-bis(3-[bis-(2-chloroethyl)carbamoyloxy]estra-1,3,5,(10)-trienyl)pyrophosphate17β,17'β-bis(3-[bis-(2-chloroethyl)carbamoyloxy]estra-1,3,5,(10)-trienyl)pyrophosphate4465-(2-Chloroethyl)-4-methyl-3-[2-(4-methylthiazol-5-yl)ethyl]thiazolium chloride5-(2-Chloroethyl)-4-methyl-3-[2-(4-methylthiazol-5-yl)ethyl]thiazolium chloride0714-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulphonamide4-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulphonamide9655-氯-1-甲基-4-硝基咪唑5-Chloro-1-methyl-4-nitroimidazole3785-Chloro-2-methylaminobenzophenone5-Chloro-2-methylaminobenzophenone4436-(2-Chlorophenyl)-2,4-dihydro-2-[(dimethylamino)methylene]8-nitroimidazo[1,2-a][1,4]benzodiazepin-1-one6-(2-Chlorophenyl)-2,4-dihydro-2-[( dimethylamino)methylene]8-nitroimidazo[1,2-a][1,4]benzodiazepin-1-one0744-Chloro-5-sulphamoylanthranilicacid4-Chloro-5-sulphamoylanthranilic acid075二苯甲酸2-(4-Chloro-3-sulphamoylbenzoyl)benzoicacid076氯噻嗪Chlorothiazide5592-氯噻吨酮2-Chlorothioxanthone0772-(6-Chlorothymoxy)ethyldimethylaminehydrochloride2-(6-Chlorothymoxy)ethyldimethylaminehydrochloride0792-Chlorotritanol2-Chlorotritanol080氯二甲苯酚分析标准品ChloroxylenolAssay Standard081扑尔敏分析标准品Chlorphenaminemaleate Assay Standard856盐酸氯丙嗪Chlorpromazinehydrochloride467氧氯丙嗪亚砜Chlorpromazinesulphoxide491氯塞酮分析标准品Chlortalidone AssayStandard783盐酸金霉素分析标准品Chlortetracyclinehydrochloride Assay Standard618胆酸分析标准品Cholic acid AssayStandard475西米替汀分析标准品Cimetidine AssayStandard809顺铂分析标准品Cisplatin AssayStandard833克拉霉素分析标准品ClarithromycinAssay Standard834克拉霉素杂质EClarithromycinimpurity E525富马酸氯马斯汀分析标准品Clemastinefumarate Assay Standard084氯碘羟喹分析标准品Clioquinol AssayStandard507氯巴占分析标准品受控药物Clobazam Assay Standard Controlled Substance522氯倍他索杂质AClobetasol impurityA521丙酸氯倍他索分析标准品Clobetasolpropionate Assay Standard482丁酸氯倍他松分析标准品Clobetasonebutyrate Assay Standard406氯可托龙己酸盐Clocortolonehexanoate663氯苯吩嗪分析标准品Clofazimine Assay Standard543氯美噻唑乙二磺酸盐分析标准品Clomethiazole edisilate Assay Standard980盐酸氯米帕明Clomipraminehydrochloride983氯丙咪嗪杂质FClomipramine ImpurityF981氯丙咪嗪杂质CClomipramine impurityC484氯安定分析标准品受控药物Clonazepam Assay Standard Controlled Substance085盐酸可乐定分析标准品Clonidinehydrochloride Assay Standard565二盐酸化物反式氯哌噻吨醋酸trans-Clopenthixol acetate dihydrochloride566二盐酸化物反氯哌噻吨癸酸酯trans-Clopenthixol decanoate dihydrochloride561盐酸反氯哌噻吨trans-Clopenthixol hydrochloride628氯前列烯醇钠分析标准品Cloprostenolsodium Assay Standard379克霉唑分析标准品Clotrimazole AssayStandard088苄星邻氯青霉素分析标准品Cloxacillin benzathine Assay Standard905氯氮平分析标准品Clozapine AssayStandard091甲磺酸二氢麦角碱分析标准品Co-dergocrine mesilate Assay Standard715盐酸薄片分析标准品受控药物Cocainehydrochloride Assay Standard Controlled Substance514盐酸可待因分析标准品受控药物Codeinehydrochloride Assay Standard Controlled Substance090磷酸可待因分析标准品受控药物CodeinePhosphate Assay Standard Controlled Substance787维生素D3分析标准品ColecalciferolAssay Standard614盐酸考来替泊Colestipolhydrochloride551考来烯胺分析标准品ColestyramineAssay Standard585醋酸可的松分析标准品Cortisone acetateAssay Standard094克罗米同分析标准品Crotamiton AssayStandard466维生素B12分析标准品CyanocobalaminAssay Standard4782-Cyano-1-methyl-3-[2-(5-methylimidazol-4-yl-methylsulphinyl)ethyl]guanidine2 -Cyano-1-methyl-3-[2-(5-methylimidazol-4-yl-methylsulphinyl)ethyl]guanidine096盐酸苯甲嗪分析标准品Cyclizinehydrochloride Assay Standard886盐酸环苯扎林Cyclobenzaprinehydrochloride098环戊噻嗪Cyclopenthiazide380盐酸环喷托酯分析标准品Cyclopentolatehydrochloride Assay Standard688醋酸环丙氯地孕酮分析标准品Cyproteroneacetate Assay Standard383阿糖胞苷分析标准品Cytarabine AssayStandard100达卡巴嗪Dacarbazine771丹曲林分析标准品Dantrolene AssayStandard428二羟蒽醌Dantron429二羟蒽醌杂质标准品Dantron impuritystandard102氨苯砜分析标准品Dapsone AssayStandard103硫酸异喹胍Debrisoquinesulphate104癸氧喹酯Decoquinate791Delta-3-CefaclorDelta-3-Cefaclor572DeltamedraneDeltamedrane631溴氰菊酯分析标准品Deltamethrin AssayStandard632溴氰菊酯杂质标准品Deltamethrinimpurity standard789盐酸去甲金霉素分析标准品Demeclocyclinehydrochloride Assay Standard889N-DemethylerythromycinAN-Demethylerythromycin A504去乙酰韦替洛尔Desacetylmetipranolol730N-DesalkylflurazepamN-Desalkylflurazepam987云铁胺甲磺酸酯Desferrioxaminemesilate931盐酸去郁敏Desipraminehydrochloride689盐酸去甲苯扎托品Desmethylbenzatropine hydrochloride568去氧孕烯分析标准品Desogestrel AssayStandard816去氧孕烯杂质DDesogestrel impurityD817去氧孕烯杂质EDesogestrel impurityE594DesogestrelΔ3-isomerDesogestrel Δ3-isomer578地塞米松分析标准品Dexamethasone AssayStandard108地塞米松磷酸钠Dexamethasone sodiumphosphate646磷酸地塞米松分析标准品Dexamethasonephosphate Assay Standard811地塞米松杂质标准品Dexamethasoneimpurity standard465右旋丙氧芬分析标准品受控药物DextropropoxypheneHCl Assay Standard Controlled Substance549荧光素二乙酸盐分析标准品DiacetylfluoresceinAssay Standard887 石斛 dongding;盐酸脉石中的矿石,填充料,物料分析标准品受控药物DiamorphineHydrochloride Assay Standard Controlled Substance111地西泮受控药物Diazepam ControlledSubstance1146,11-二氢二苯并[b,e]硫杂卓-11-酮Dibenzo[b,e]thiepin-11(6H)-one1243-(Dibenzo[b,e]thiepin-11(6H)-ylidene)-N,N-dimethylaminopropan-1-amine-S-oxid ehydrochloride3-(Dibenzo[b,e]thiepin-11(6H)-ylidene)-N,N-dimethylaminopropan-1-am ine-S-oxidehydrochloride85810,11-二氢二苯并[a,b]环庚烯-5-酮Dibenzosuberone6083-(1,5-Dibenzyl-1H-indazole-3-yl)oxypropyldimethylaminehydrochloride3-(1,5-Dibenzyl-1H-indazole-3-yl)oxypropyldimethylamine hydrochloride116双氯酚分析标准品Dichlorophen AssayStandard420二氯芬杂质分析标准品Dichlorophenimpurity standard Assay Standard7211-(2,5-Dichlorophenyl)-5-isopropylbiguanidehydrochloride1-(2,5-Dichlorophenyl)-5-isopropylbiguanidehydrochloride619双氯芬酸钠分析标准品Diclofenac sodiumAssay Standard598双氯芬酸二乙胺Diclofenacdiethylamine828双氯芬酸杂质ADiclofenac impurityA810双氯西林钠Dicloxacillinsodium118褐霉酸二乙醇胺分析标准品Diethanolaminefusidate Assay Standard3612-Diethylaminoethyl-3-(1-naphthyl)-2-(1-naphthylmethyl)propionateoxalate2-Diethylaminoethyl-3-(1-naphthyl)-2-(1-naphthylmethyl)propionate oxalate737枸橼酸二乙碳酰嗪Diethylcarbamazinecitrate119Diethyl-4-decyloxy-3-ethoxyanilinomethylenemalonateDiethyl-4-decyloxy-3-ethox yanilinomethylenemalonate859己烯雌酚分析标准品DiethylstilbestrolAssay Standard677DiethylstilbestrolmonophosphateDiethylstilbestrolmonophosphate401戊酸双氟可龙分析标准品Diflucortolonevalerate Assay Standard397戊酸双氟可龙杂质标准品Diflucortolonevalerate impurity standard855洋地黄毒甙分析标准品Digitoxin AssayStandard431酒石酸双氢可待因分析标准品受控药物DihydrocodeineTartrate Assay Standard Controlled Substance120甲磺酸双氢麦角汀Dihydroergocristinemesilate1222,3-二氢-6-苯基咪唑噻唑盐酸盐2,3-Dihydro-6-phenylimidazo[2,1-b] thiazole815bis-(1,7-dihydro-6H-purine)-6,6-disulphidebis-(1,7-dihydro-6H-purine)-6,6-dis ulphide726硫氮酮Diltiazem HCl899地尔硫卓杂质标准品Diltiazem ImpurityStandard5826,6-Dimethoxy-2,2'-binaphthyl6,6-Dimethoxy-2,2'-binaphthyl1954-Dimethylamino-3-methyl-1,2-diphenylbutan-2-olhydrochloride4-Dimethylamino-3-methyl-1,2-diphenylbutan-2-olhydrochloride6073-Dimethylaminopropyl 2-benzylaminobenzoatehydrochloride3-Dimethylaminopropyl2-benzylaminobenzoate hydrochloride4561,5-二甲基己胺1,5-Dimethylhexyl(methyl)amine865Dimethyl-2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylateDimethyl-2,6 -dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate866Dimethyl-2,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylateDimethyl-2 ,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylate511Dimethyl{5-[2-(1-methylamino-2-nitrovinylamino)ethylsulphinylmethyl]furfuryl}amineDimethyl{5-[2-(1-methylamino-2-nitrovinylamin o)ethylsulphinylmethyl]furfuryl}amine3642,2-Dimethyl-5(2,4-xylyloxy)valericacid2,2-Dimethyl-5(2,4-xylyloxy)valericacid690敌匹硫磷分析标准品Dimpylate AssayStandard691Dimpylate forchromatographyDimpylate for chromatography902地诺前列酮分析标准品Dinoprostone AssayStandard896盐酸苯海拉明Diphenhydraminehydrochloride128盐酸地匹哌酮分析标准品受控药物Dipipanonehydrochloride Assay Standard Controlled Substance673盐酸地匹福林分析标准品Dipivefrinehydrochloride Assay Standard659地匹福林杂质标准品Dipivefrineimpurity standard589利巴韦林杂质标准品分析标准品DiprenorphineAssay Standard131双嘧达莫分析标准品Dipyridamole AssayStandard384N1,N2-Diquinoxalin-2-ylsulphanilamideN1,N2-Diquinoxalin-2-ylsulphanilamide615帕米膦酸钠分析标准品Disodiumpamidronate Assay Standard132二硫化四乙基秋兰姆分析标准品DisulfiramAssay Standard385蒽三酚分析标准品Dithranol AssayStandard674盐酸多巴酚丁胺分析标准品Dobutaminehydrochloride Assay Standard133多库酯钠分析标准品Docusate sodiumAssay Standard669马来酸多潘立酮分析标准品Domperidonemaleate Assay Standard468盐酸多巴胺分析标准品Dopaminehydrochloride Assay Standard134盐酸度琉平分析标准品Dosulepinhydrochloride Assay Standard135盐酸多沙普仑分析标准品Doxapramhydrochloride Assay Standard136盐酸多虑平分析标准品Doxepinhydrochloride Assay Standard823多塞平杂质标准品Doxepin ImpurityStandard990盐酸阿霉素分析标准品Doxorubicinhydrochloride Assay Standard780盐酸强力霉素分析标准品Doxycyclinehyclate Assay Standard675氟哌利多分析标准品Droperidol Assay Standard138去氢孕酮Dydrogesterone139硝酸益康唑分析标准品Econazole nitrate Assay Standard741马来酸依那普利Enalaprilmaleate742依那普利拉Enalaprilat743依那普利双酮Enalaprildiketopiperazine387盐酸麻黄碱分析标准品毒品Ephedrine hydrochloride Assay Standard Drug Precursor 992盐酸表阿霉素Epirubicinhydrochloride788alpha-骨化醇分析标准品Ergocalciferol Assay Standard405马来酸麦角新碱分析标准品Ergometrine maleate Assay Standard Drug Precursor141酒石酸麦角胺分析标准品Ergotamine tartrate Assay Standard Drug Precursor781红霉素Erythromycin794红霉素A分析标准品Erythromycin AAssay Standard795红霉素B分析标准品Erythromycin BAssay Standard796红霉素C分析标准品Erythromycin CAssay Standard790依托红霉素Erythromycinestolate798琥乙红霉素Erythromycin ethylsuccinate488硬脂酸红霉素分析标准品Erythromycin stearate Assay Standard396苯甲酸雌二醇分析标准品Estradiol benzoate Assay Standard729雌二醇半水合物分析标准品Estradiol hemihydrate Assay Standard142雌莫司汀Estramustine752雌三醇分析标准品Estriol AssayStandard753雌三醇杂质标准品Estriol impurity standard860雌酚酮Estrone616雌酮硫酸酯哌嗪分析标准品EstropipateAssay Standard143依他尼酸分析标准品Etacrynic acidAssay Standard421炔雌醇分析标准品EthinylestradiolAssay Standard145乙氧酰胺苯甲酯分析标准品Ethopabate Assay Standard146乙琥胺分析标准品Ethosuximide AssayStandard150Ethyldimethyl[2-(2-methylbenzhydryloxy)ethyl]ammonium chlorideEthyldimethyl[2-(2-methylbenzhydryloxy)ethyl]ammonium chloride 437N-EthylglucaminehydrochlorideN-Ethylglucamine hydrochloride149甲氯芬那酸Ethylmeclofenamate529依托度酸分析标准品Etodolac AssayStandard533依托度酸二聚体Etodolac aciddimer541依托度酸Etodolac 1-methylanalogue542依托度酸Etodolac 8-methylanalogue885依托泊苷分析标准品Etoposide AssayStandard653法莫替丁分析标准品Famotidine AssayStandard655法莫替丁降解杂质1Famotidinedegradation impurity 1656法莫替丁降解杂质2Famotidinedegradation impurity 2654法莫替丁杂质CFamotidine impurityC6204-联苯乙酸分析标准品Felbinac AssayStandard777非洛地平分析标准品Felodipine AssayStandard758非洛地平杂质标准品Felodipine impurity standard692苯硫咪唑Fenbendazole693达虫净杂质CFenbendazole impurityC {5-(phenylthio)-2-aminobenzimidazole}354联苯丁酮酸分析标准品Fenbufen Assay Standard661非诺特罗降解杂质AFenoteroldegradation impurity A660氢溴酸芬忒醇分析标准品Fenoterol hydrobromide Assay Standard665枸橼酸芬太尼分析标准品受控药物Fentanyl citrate Assay Standard Controlled Substance 666芬太尼杂质AFentanyl impurityA156倍硫磷分析标准品Fenthion AssayStandard740非那雄胺分析标准品Finasteride Assay Standard571盐酸黄酮哌酯分析标准品Flavoxate hydrochloride Assay Standard676醋酸氟卡胺分析标准品Flecainide acetate Assay Standard159醋酸氟氢可的松分析标准品Fludrocortisone acetate Assay Standard840氟硝西泮受控药物FlunitrazepamControlled Substance160醋酸肤轻松分析标准品Fluocinolone acetonide Assay Standard489醋酸氟轻松分析标准品Fluocinonide Assay Standard161氟可龙己酸分析标准品Fluocortolone hexanoate Assay Standard162氟可龙三甲基乙酸分析标准品Fluocortolone pivalate Assay Standard163氯苯丁酮4'-Fluoro-4-chlorobutyrophenone573氟米龙分析标准品FluorometholoneAssay Standard797盐酸氟西汀分析标准品Fluoxetine hydrochloride Assay Standard164氟甲睾酮Fluoxymesterone738氟哌噻吨cis-Flupentixol554二盐酸化物噻吨癸酸酯分析标准品Flupentixol decanoate dihydrochloride Assay Standard556盐酸反噻吨癸酸酯trans-Flupentixol decanoate dihydrochloride597盐酸顺式三氟噻吨丙酸cis-Flupenthixol propionate dihydrochloride167盐酸氟奋乃静分析标准品Fluphenazinehydrochloride Assay Standard574氟比洛芬钠分析标准品Flurbiprofensodium Assay Standard587丙酸氟替卡松分析标准品Fluticasonepropionate Assay Standard588氟及卡松丙酸酯杂质FluticasoneS-methyl impurity600马来酸氟伏沙明分析标准品Fluvoxaminemaleate Assay Standard671氟伏沙明杂质标准品Fluvoxamine maleateimpurity standard627甲酰基利福霉素 SV3-FormylrifamycinSV639Form-2',4'-xylidideForm-2',4'-xylidide623膦甲酸钠分析标准品Foscarnet sodiumAssay Standard678磷雌酚钠Fosfestrol sodium547呋噻米分析标准品Furosemide AssayStandard643加拉明杂质标准品Gallamine impuritystandard363吉非罗齐分析标准品Gemfibrozil AssayStandard303二甲苯氧庚酸杂质AGemfibrozilimpurity A 2,2-dimethyl-5-(4-propen-1-yl)-2,5-xylyloxy)valeric acid365吉非罗齐甲酯Gemfibrozil methylester174硫酸庆大霉素Gentamicinsulphate175格列本脲分析标准品Glibenclamide AssayStandard368格列齐特分析标准品Gliclazide AssayStandard580格列喹酮分析标准品Gliquidone AssayStandard581硫胺格列喹酮Gliquidonesulphonamide652硝酸甘油溶液分析标准品Glyceryltrinitrate solution Assay Standard814戈舍瑞林六胜肽GoserelinHexapeptide180灰黄霉素分析标准品Griseofulvin AssayStandard181胍乙啶分析标准品Guanethidinemonosulphate Assay Standard879鸟嘌呤Guanine407氟哌啶醇分析标准品Haloperidol AssayStandard185氢溴酸后马托品分析标准品Homatropinehydrobromide Assay Standard186双氢氯噻嗪分析标准品HydrochlorothiazideAssay Standard576氢化可的松分析标准品HydrocortisoneAssay Standard584醋酸氢化可的松分析标准品Hydrocortisoneacetate Assay Standard187氢化可的松半琥酯HydrocortisoneHydrogen Succinate188氢化可的松磷酸钠Hydrocortisonesodium phosphate190氢氟噻嗪Hydroflumethiazide770对羟基苯乙酮4'Hydroxyacetophenone197羟基脲分析标准品HydroxycarbamideAssay Standard526N-甲基-2-(2-羟乙基)吡咯烷2-(2-Hydroxyethyl)-1-methylpyrrolidine1965-[1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl]salicylicacidhydrochloride5-[1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl]salicylicacid hydrochloride829D-alpha-(4-hydroxyphenyl)glycineD-alpha-(4-hydroxyphenyl)glycine888(5RS)-3-(2-hydroxyphenyl)-5-phenylcyclohex-2-enone(5RS)-3-(2-hydroxyphenyl)-5 -phenylcyclohex-2-enone1942-(6-Hydroxythymoxy)ethyldimethylaminehydrochloride2-(6-Hydroxythymoxy)ethyldimethylaminehydrochloride198丁溴东莨菪碱分析标准品Hyoscinebutylbromide Assay Standard199东莨菪碱氢溴酸盐分析标准品HyoscineHydrobromide Assay Standard774羟丙甲纤维素分析标准品HypromelloseAssay Standard539布洛芬分析标准品Ibuprofen AssayStandard2025-碘-2'-脱氧尿苷分析标准品IdoxuridineAssay Standard759异环磷酰胺分析标准品Ifosfamide AssayStandard664IminophenazineIminophenazine932盐酸丙咪嗪Imipraminehydrochloride999吲达帕胺分析标准品Indapamide AssayStandard1000吲达帕胺杂质BIndapamide impurityB625异丙托溴铵分析标准品Ipratropiumbromide Assay Standard557异丁基苯乙酮4'-Isobutylacetophenone550硝酸异康唑分析标准品Isoconazolenitrate Assay Standard205盐酸异丙肾上腺素分析标准品Isoprenalinehydrochloride Assay Standard778盐酸异丙美沙嗪Isopromethazinehydrochloride206硝酸异山梨酯Isosorbidedinitrate799异脱二水山梨醇-2-硝酸酯Isosorbide2-nitrate800单硝酸异山梨酯分析标准品Isosorbidemononitrate Assay Standard4994'-(2-Isopropylaminoethyl)methanesulphonanilide hydrochloride4'-(2-Isopropylaminoethyl)methanesulphonanilide hydrochloride626苯酰甲硝唑8s-Isopropyl-3β-hydroxytropaniumbromide621萜烯苯酚的合成树脂分析标准品IsradipineAssay Standard622伊拉地平杂质BIsradipine impurityB624伊拉地平杂质DIsradipine impurityD864伊维菌素分析标准品Ivermectin AssayStandard861KanamycinmonosulpateKanamycin monosulpate736盐酸氯胺酮受控药物KetamineHydrochloride Controlled Substance6681-(2-甲氧苯基)哌嗪分析标准品Ketoprofen Assay Standard667酮基布洛芬乙酯Ketoprofen ethylester707甲基多巴分析标准品Lacidipine Assay Standard647拉西地平杂质标准品Lacidipineimpurity Standard802拉米夫定分析标准品Lamivudine Assay Standard872兰索拉唑分析标准品Lansoprazole Assay Standard873兰索拉唑杂质标准品Lansoprazoleimpurity standard212盐酸左旋咪唑Levamisolehydrochloride356盐酸甲基苯巴比妥分析标准品Levobunolol hydrochloride Assay Standard213甲基强的松龙分析标准品Levodopa Assay Standard772左薄荷脑分析标准品Levomenthol Assay Standard723甲基泼尼松龙醋酸酯分析标准品Levomepromazine maleate Assay Standard634左美丙嗪亚砜Levomepromazinesulphoxide501左炔诺孕酮分析标准品LevonorgestrelAssay Standard215盐酸林可霉素分析标准品Lincomycin hydrochloride Assay Standard727利多卡因分析标准品Lidocaine Assay Standard214盐酸利多卡因Lidocaine (Lignocaine) hydrochloride733亚麻油Linseed oil695赖诺普利分析标准品Lisinoprildihydrate Assay Standard696赖诺普利二酮哌嗪Lisinoprildiketopiperazine433乳酸锂分析标准品Lithium lactateAssay Standard927石胆酸Lithocholic acid697盐酸洛非帕明分析标准品Lofepramine hydrochloride Assay Standard635盐酸米赛林分析标准品Loperamide hydrochloride Assay Standard636洛派丁胺 N-氧化物LoperamideN-oxide874扑米酮分析标准品Loratadine Assay Standard875兰索拉唑杂质标准品LoratadineImpurity Standard447甲磺酸氯普唑仑受控药物Loprazolammesilate Controlled Substance434普鲁卡因青霉素分析标准品受控药物Lorazepam Assay Standard Controlled Substance528丙氯拉嗪分析标准品受控药物Lormetazepam Assay Standard Controlled Substance867环苯咯丙醇分析标准品Losartan Potassium Assay Standard218赖甲环素Lymecycline776利奈孕酮Lynestrenol1008甲苯咪唑Mebendazole220盐酸美克洛嗪片Meclozinehydrochloride221醋酸甲羟孕酮分析标准品Medroxyprogesterone acetate Assay Standard222甲地孕酮Megestrol223醋酸甲地孕酮分析标准品Megestrol acetate Assay Standard629美洛昔康分析标准品Meloxicam Assay Standard630美洛昔康杂质标准品Meloxicam impurity standard391马法兰分析标准品Melphalan AssayStandard699盐酸吡多素分析标准品Mepivacaine hydrochloride Assay Standard583盐酸酒石酸美托洛尔分析标准品Meptazinol hydrochloride Assay Standard7732-羟基丙酸单锂盐分析标准品Mercaptopurine Assay Standard762巯嘌呤杂质标准品Mercaptopurineimpurity standard496美索达嗪苯磺酸Mesoridazinebesilate226酒石酸美拉明Metaraminoltartrate227盐酸伊拉地平分析标准品Metforminhydrochloride Assay Standard552盐酸美沙酮分析标准品受控药物Methadonehydrochloride Assay Standard Controlled Substance232酮基布洛芬1-(2-Methoxyphenyl)piperazine644对乙酰氨基-邻甲氧基苯甲酸甲酯 Methyl4-acetamido-2-hydroxybenzoate6061-Methylazepan-4-onehydrochloride1-Methylazepan-4-onehydrochloride6051-Methyl-4-(2-benzoylhydrazino)azepanhydrochloride1-Methyl-4-(2-benzoylhydrazino)azepanhydrochloride2393-Methyl-2,2-diphenyl-4-piperidinobutyronitrile3-Methyl-2,2-diphenyl-4-piperi dinobutyronitrile240甲基多巴分析标准品Methyldopa AssayStandard241盐酸甲基多巴乙酯分析标准品Methyldopatehydrochloride Assay Standard569拉米夫定3-Methylflavone-8-carboxylicacid5703-Methylflavone-8-carboxylic acid ethylester3-Methylflavone-8-carboxylic acid ethylester4444-methyl-5-(2-hydroxyethyl)thiazole4-methyl-5-(2-hydroxyethyl)thiazole4572-Methyl-6-methylaminoheptan-2-ol2-Methyl-6-methylaminoheptan-2-ol4761-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride1-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride237MethylN-4[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulphonylcarbamateMethylN-4[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulphonylcarbamate244兰索拉唑2-Methyl-5-Nitroimidazole245(1-Methyl-5-nitroimidazol-2-yl)methanol(1-Methyl-5-nitroimidazol-2-yl)methano l641N-Methyl-N'-(2,4-xylyl)formamidinehydrochlorideN-Methyl-N'-(2,4-xylyl)formamidinehydrochloride662甲苯比妥分析标准品受控药物MethylphenobarbitalAssay Standard Controlled Substance248甲基强的松龙分析标准品MethylprednisoloneAssay Standard249左美丙嗪马来酸盐分析标准品Methylprednisoloneacetate Assay Standard846三盐酸化物左美丙嗪马来酸盐N-methyl-bis[beta-(2-pyridyl)ethyl]amine trihydrochloride4454-Methyl-5-vinylthiazoleedisilate4-Methyl-5-vinylthiazole edisilate409马来酸美西麦角分析标准品Methysergidemaleate Assay Standard642美替洛尔分析标准品Metipranolol AssayStandard357盐酸胃复安分析标准品Metoclopramidehydrochloride Assay Standard540酒石酸美托洛尔分析标准品Metoprololtartrate Assay Standard603甲硝唑分析标准品Metronidazole AssayStandard735苯酰甲硝唑Metronidazolebenzoate251美克西酮Mexenone252盐酸米安色林分析标准品Mianserinhydrochloride Assay Standard253硝酸咪康分析标准品Miconazole nitrateAssay Standard884咪康唑杂质AMiconazole impurityA722咪达唑仑分析标准品受控药物Midazolam AssayStandard Controlled Substance394盐酸米诺环素分析标准品Minocyclinehydrochloride Assay Standard637米诺地尔分析标准品Minoxidil AssayStandard842米氮平分析标准品Mirtazapine AssayStandard768莫美他松糠酸酯分析标准品Mometasone。
WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA

WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
VersaFlow Coriolis 6000 Model Selection Guide.pdf_

_5
*
_A
*
0_
*
1_
*
R_
*
T_
*
U_
*
V_
*
_0
*
_N
*
The minimum value of orders acceptable for Honeywell is USD 500. Handling fee is the amount of the difference between USD 500 and the actual purchase price.
(Tube material C-22 only)
Flange connection size 1/2" ANSI 150 lb
1/2" ANSI 300 lb
1/2" ANSI 600 lb
1/2" ASME 1500 lb
(Tube material C-22 only)
10 A JIS 20 K
Selection Availability
0___
*
1___
*
2___
*
_0__
*
_1__
*
_3__
*
_4__
*
_A__
*
_B__
*
_D__
*
_E__
*
_R__
*
_S__
*
__0_
*
__1_
*
__C_
g
__D_
g
__T_
h
___0
*
___1
*
___G
*
TABLE VII No Selection
抗菌标准大全(产品版)

抗菌标准大全(产品版)
备注:
备注一:其他相关法规、规范和标准(19项)
美国药典USP39(51)微生物防腐功效测试
欧洲药典EP9.0(5.1.3)微生物防腐功效测试
英国药典BP(2016)附录XVIC微生物防腐功效测试
中国药典2015(第二部)附录XIX N 抑菌剂效力检查法指导原则
卫生部《消毒技术规范》(2002版)
《化妆品安全技术规范》(2015年版)
GB 4806.1-2016 食品安全国家标准食品接触材料及制品通用安全要求
GB 9685-2016 食品安全国家标准食品接触材料及制品用添加剂使用标准GB 4806.10-2016 食品安全国家标准食品接触用涂料及涂层
GB/T 17219-1998《生活饮用水输配水设备及防护材料安全性评价标准》
GB/T 18260-2000 木材防腐剂对白蚁毒效实验室试验方法
GB/T 29399-2012 木材防虫( 蚁) 技术规范
NY/T 1151.1-2015 农药登记用卫生杀虫剂室内药效试验及评价第1 部分:防蛀剂
NY/T 1151.2-2006 农药登记用卫生杀虫剂室内药效试验及评价第2 部分: 灭螨和驱螨剂
NY/T 1151.4-2012 农药登记卫生用杀虫剂室内药效试验及评价第4 部分: 驱蚊帐
NY/T 1151.6-2016 农药登记用卫生杀虫剂室内药效试验及评价第6 部分: 服装面料用驱避剂
NY/T 1153.4-2013 农药登记用白蚁防治剂药效试验方法及评价第4 部分: 农药木材处理预防白蚁
QB/T 4367-2012 衣物防蛀剂出入境检验检疫
SN/T 3229-2012 食品消毒剂和防腐剂杀菌效果评价方法备注二:本体系涉及标准类别说明。
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
BSI-201_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:BSI–201 (Iniparib; NSC–746045) is a PARP1 inhibitor with demonstrated effectiveness in triple–negative breast cancer (TNBC).IC50 Value:Target:PARP1in vitro: BSI–201 inhibits the growth of E–ras 20 cells, the effect of which can be augmented 4–fold when BOS is added. RecentlyBSI–201 shows no ability to inhibit PARP enzymatic or cellular activity, but can non–selectively modify cysteine–containing proteins in tumor cells, suggesting the mechanism of action for BSI–201 is likely not via inhibition of PARP activity. BSI–201 (100 μM) inhibits ionizing radiation–induced single–strand breaks (SSBs) repair in human lymphoid cell lines based on large endogenous Epstein–Barr virus (EBV) circular episomes assay, resulting in 55% repair by 2 hours, which can be reversed surprisingly by knockdown of PARP1,indicating that the mechanism of inhibition does not involve trapping PARP at SSBs. BSI–201 is not able to selectively kill homologous recombination (HR)–deficient cells between BRCA2–deficient PEO1 and BRCA2–revertant PEO4, or ATM–deficient GM16666 and ATM–restored GM16667 fibroblasts. BSI–201 is cytotoxic to a variety of cell lines at concentrations above 40 μM reflecting a mechanism independent of PARP.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [5]Cells plated at 5 × 104 cells/60 mm dish were allowed to adhere for 24 h, then treated with veliparib, olaparib, or Iniparib in 0.1% (v/v)DMSO for 4 days (olaparib) or 6 days (veliparib, Iniparib) based on preliminary time course experiments. At the end of treatment,adherent cells were recovered by trypsinization, combined with cells in the supernatant, sedimented at 150 × g for 10 min, washed in ice–cold calcium– and magnesium–free Dulbecco's phosphate–buffered saline (PBS), and fixed at 4°C by drop–wise addition ofethanol to a final concentration of 50% (v/v). Cells were subsequently rehydrated in cold PBS, sedimented, resuspended in 300 μl 0.1%(w/v) sodium citrate containing 1 mg/ml RNase A, incubated for 15 min at 37°C, diluted with 300 μl 0.1% sodium citrate containing 100 μg/ml PI, incubated in the dark at 20°C for 15 min, and analyzed (20,000 events) on a FACSCanto II flow cytometer using excitation and emission wavelengths of 488 and 617 nm, respectively. After data were analyzed using Becton Dickinson CellQuest software, normalized apoptosis was calculated as (observed – baseline)/(100 – baseline) to correct for differences in basal apoptosis rates between cell lines. Results are representative of at least 3 independent experiments.References:[1]. Domagala P, Lubinski J, Domagala W.Iniparib in metastatic triple–negative breast cancer.N Engl J Med. 2011 May 5;364(18):1780; author reply 1781.[2]. Fogelman DR, Wolff RA, Kopetz S, Javle M, Bradley C, Mok I, Cabanillas F, Abbruzzese JL.Evidence for the efficacy of Iniparib, a PARP–1 inhibitor, in BRCA2–associated pancreatic cancer.Anticancer Res. 2011 Apr;31(4):1417–20.Product Name:BSI–201Cat. No.:HY-12015CAS No.:160003-66-7Molecular Formula:C 7H 5IN 2O 3Molecular Weight:292.03Target:PARP; PARP Pathway:Epigenetics; Cell Cycle/DNA Damage Solubility:10 mM in DMSO[3]. Fojo T, Amiri–Kordestani L, Bates SE.Potential pitfalls of crossover and thoughts on iniparib in triple–negative breast cancer.J Natl Cancer Inst. 2011 Dec 7; 103(23):1738–40. Epub 2011 Nov 1.[4]. Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH.Failure of iniparib to inhibit poly(ADP–Ribose) polymerase in vitro.Clin Cancer Res. 2012 Mar 15;18(6):1655–62. Epub 2012 Jan 30.[5]. BiPar Sciences presents interim phase 2 results for PARP inhibitor BSI–201 at San Antonio Breast Cancer Symposium. Cancer Biol Ther. 2009 Jan;8(1):2–3.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
有源医疗器械超温试验检测研究

Research on the Detectionof Overtemperature Test for Active Medical DevicesYANG Yan 1*,SHENG Meina 2,LUO Zhenhua 3,ZHAO Xu 1(1.Liaoning Inspection,Examination &Certification Centre 〔Liaoning Medical Device Test Institute 〕,Shenyang 110036,China;2.Alphavita Bio-scientific 〔Dalian 〕Co.,Ltd.,Dalian 116000,China;3.Yisheng Dahua Petrochemical Co.,Ltd.,Dalian 116600,China)Abstract :This article refers to the standard of GB 9706.1—2020Medical electrical equipment —Part 1:General requirements for basic safety and essential performance and the standard of GB 4793.1—2007Safety requirements for electrical equipment for measurement,control,and laboratory use —Part1:General requirements ,combined with the characteristics of overtemperature test,the standard clauses were combed,and the key points of overtemperature test for active medical devices were summarized for reference.Keywords :active medical devices;overtemperature test;detection有源医疗器械超温试验检测研究杨艳1*,盛美娜2,罗振华3,赵旭1(1.辽宁省检验检测认证中心〔辽宁省医疗器械检验检测院〕,辽宁沈阳110036;2.冰山松洋生物科技〔大连〕有限公司,辽宁大连116000;3.逸盛大化石化有限公司,辽宁大连116600)【摘要】本文参照GB 9706.1—2020《医用电气设备第1部分:基本安全和基本性能的通用要求》和GB 4793.1—2007《测量、控制和实验室用电气设备的安全要求第1部分:通用要求》,结合超温试验自身特点,对标准条款进行了梳理,总结出有源医疗器械超温试验的检测重点,以供相关人员参考。
离子色谱法测定南京市道路灰尘中的四种无机阴离子

Vol. 11, No. 29 〜14第11卷第2期2 0 21年4月中国无机分析化学ChineseJournalofInorganicAnalyticalChemistrydoi :10. 3969". iisn. 2095-1035. 2021. 02. 003离子色谱法测定南京市道路灰尘中的四种无机阴离子周 悦,周曼菲,沈禹,如柯耶•居麦,曹修玉,蔡越,高 蓉,余静"(南京医科大学公共卫生学院卫生检验与检疫系,南京211166)摘 要 采用离子色谱法测定南京市道路灰尘中无机阴离子F 、Cl 、SOZ 、NO3的含量。
道路灰尘样品过150 %m 孔径的筛网,经3次涡旋离心提取,再取上清液高速离心,0.22 %m 滤膜过滤后上离子色谱测定。
离心后的样品经IonPac TM AS 11-HCC250 mmX4 mm)色谱柱分离,以NaOH 溶液(25 mmol/L )为淋洗液,采用电导检测器检测$ 4种阴离子均具有较宽的线性范围(0. 01〜0.20 mg/L )其线性相关系数r $0.999$样品的加标回收率在87. 4%〜95. 2%,仪器相对标准偏差(RSD)均%3. 5%,方法相对标准偏差(RSD)均%7.7%$方法具有分析速度快,精密度好等优点,可用于评价南京市城区道路灰尘中的四种无机阴离子水平。
关键词 离子色谱法;道路灰尘;无机阴离子中图分类号:O657. 7+5;TH833文献标志码:A文章编号:2 0 95-1 0 35(2 021) 0 2-00 0 9-0 6Determination of Four Inorganic Anions in RoadDust#nNan*ngbyIonChromatographyZHOU Yue,ZHOU Manfei,SHEN Yu,RU KEYE ・ Jumai ,CAO Xiuyu,CAI Yue,GAO Rong,YU Jing *(Department of Health Inspection and Quarantine , School of Public Health ,Nanjing Medical University , Nanjing , Jiangsu 211166 , China )Abstract F - , Cl - , SO 42_ , NO 3 - in road dust of Nanjing city were determined by ionchromatography.Sampleswerea l owedtobeanalyzedbyionchromatographyafterpassingthrougha15 %m sieveextracted by vortex centrifugation three times takingthesupernatantby high-speed centrifugation andfiltering by a 0 . 22 %m filter membrane. Samples after centrfugtion were separated with IonPac TM AS 11-HC25 mmX4 mm )chromatographiccolumn.25 mmol /L NaOH wasselectedas mobilephaseandconductivity detectorwasadoptedasthedetectionsensor.For4anionsthemethodshowedgoodlinearityoverawideanalysisrange(0. 01—0. 20 mg/L) with correlation coefficients greater than 0. 999. The addition recoveries were in the range of between 87. 4%—95. 2%. This method provides as fast analysis speed and good precision. It is suitable forevaluatingthelevelsoffourinorganicanionsinroaddustofNanjingurbanarea.Keywords ion chromatography ; road dust ; inorganic anion收稿日期20200709修回日期:2020-10-11基金项目:江苏省大学生创新创业训练计划省级重点项目(201910312005Z)作者简介:周悦,女,本科在读$ E-mail :1614805401@qq. com*通信作者:余静,女,博士 ,讲师,主要从事卫生检验与检疫研究$ E-mail :yujingfei@126. com引用格式:周悦,周曼菲,沈禹,等•离子色谱法测定南京市道路灰尘中的四种无机阴离子中国无机分析化学,2021 ,112):9-14FZHOU Yue ZHOU Manfei SHEN Yu etalFDeterminationofFourInorganic Anionsin Road Dustin NanjingbyIon Chromatography [J ]. Chinese Journal of Inorganic Analytical Chemistry , 2021,11(2) : 9-14.10中国无机分析化学2021年■>I—1—刖_随着城市化进程的加快,城市的生态环境受到了化工企业、交通运输和居民生活的多重影响城市道路灰尘主要来源于人类活动,如化工业生产排放、机动车尾气排放、建筑施工以及商业活动排放等是有机及无机污染物扩散的载体3$灰尘中含量较多的重金属和水溶性离子成分在一定条件下能通过呼吸和皮肤接触等各种暴露途径对人体造成危害$水溶性无机阴离子是道路灰尘的重要化学成分之一,包括F_、Cl_、NO厂、SO/等,具有较强的亲水性与吸湿性,是城市雾霾的形成、大气的能见度降低主因之一;同时灰尘中水溶性离子会增加多环芳'等有毒有害有机物质的溶解性,可对人体健康产生不可忽视的影响&5'$NO厂和SO/主要是人为排放的氮氧化物和二氧化硫通过光化学反应生成的二次污染物,是大气中含量较高的水溶性离子氟的安全范围很窄,缺乏会影响牙齿的发育,过量则会造成氟中毒,且氟化物在大气中扩散距离远,容易引起农作物和水源污染,对人体健康造成潜在风险$因此,研究常见无机阴离子在道路灰尘中的分布具有重要的意义$离子色谱法(IC)是无机阴离子分析的首选方法可快速同时测定多种离子,准确度高已广泛应用于公共卫生、环境等多个领域[1012]$本文采用离子色谱法对南京市道路灰尘中的主要无机阴离子含量进行了分析与评价,对于控制城市污染源、改善城市人群健康状况具有重大意义,并为南京市政府的环境防控提供参考$1实验部分1.1主要仪器及试剂戴安公司ICS-5000离子色谱仪,配美国戴安公司Chromeleon型色谱工作站;电导检测器(ICS-DC CD);IonPac™AS11-HC(250mmX4mm)色谱柱;KH5200DE型数控超声波清洗器(昆山禾剑超声仪器有限公司),DJY-2500多管漩涡混合仪(北京佳源兴业科技有限公司),台式低速大容量离心机L550(湖南湘仪实验室仪器开发有限公司),贝克曼台式高速冷冻离心机Allegra64R(Beckman Coulter,Inc.)$超纯水(电阻率>18.0M#-cm),NaOH溶液(中国赛默飞世尔科技有限公司,批号:SS254-500mL, 50%),F_、C1_、NO厂、SO/标准溶液(浓度均为1000mg/L,国家标准物质认证中心)。
2019年多粘菌素最佳使用方法的国际共识指南

2019年多粘菌素最佳使用方法的国际共识指南前言本临床指南的目的在于为多粘菌素类抗生素(包括多粘菌素E和多粘菌素B)的临床应用提供专家共识和推荐意见。
多粘菌素类抗生素在上世纪50年的就已面世,因而没有经历过现代药物开发应该经历的流程。
多粘菌素的独特作用机制在于它可破坏革兰阴性杆菌的外膜的完整性,除此之外还具有快速的杀菌活性,并和其他类抗生素具有协同作用。
它们的临床价值在近些年又重新被予以重视,并认为在其他药物治疗无效的革兰阴性杆菌感染的挽救性治疗方案中扮演了关键的角色,主要针对多重耐药细菌(MDR)和泛耐药细菌(XDR)如:假单胞菌属、鲍曼不动杆菌、肠杆菌属。
从1980年代开始重新进入到临床,直到现在,由于剂型的问题,多粘菌素的使用一直存在着相当的困惑。
粘菌素本身为一种不具备活性的前体药物,然而多粘菌素B则具有药物活性。
除此之外,多粘菌素类药物的剂量也存在多种不同的使用习惯,尤其是粘菌素,产品信息已经过时,而且其敏感性的测试仍存在不确定性。
因此,粘菌素和多粘菌素B最佳使用方法仍不太清楚。
不幸的是,多粘菌素存在严重的肾毒性,传统的使用方法容易诱发AKI。
鉴于目前多粘菌素治疗窗较窄(低治疗指数),因此本篇指南将为临床工作者提供使用多粘菌治疗多重耐药或泛耐药的革兰阴性杆菌造成的感染的实践框架。
方法共识指定组成人员包含各国际专家,他们代表的组织包括美国临床药学院(ACCP),欧洲临床微生物学及感染性疾病学会(ESCMID),美国感染病学会(IDSA),国际抗感染病药理学会(ISAP),美国重症医学会(SCCM)和感染性疾病药剂师协会(SIDP)。
评价过程是根据建议评价的等级划分进行开发和评估(GRADE),部分话题由于涉及非临床证据,无法采用GRADE分级。
临床问题和推荐意见敏感性和药代动力学/药效学1.如何测定敏感性和指导多粘菌素治疗的最小抑菌浓度(MIC)的折点是多少?推荐意见1:欧洲抗菌素敏感性试验联合委员会(EUCAST)和临床及实验室标准协会(CLSI)的多粘菌素敏感性折点联合工作小组推荐使用标准的肉稀微量释法ISO-74 20776作为粘菌素MIC测定的参考方法,并在经阳离子校对的穆勒辛顿(Mueller Hinton)肉汤中完成,且必须在无添加剂(例如聚山梨醇酯-80)的普通聚苯乙烯托盘中添加硫酸盐粘菌素。
【脉铂医药】MedBio160003-66-7Iniparib(BSI-201)技术资料

292.031
密度
2.1±0.1 g/cm3
沸点
344.8±32.0 °C at 760 mmHg
分子式
C7H5IN2O3
熔点
无资料
闪点
162.3±25.1 °C
2、Iniparib (BSI-201)物理化学性质:
密度
2.1±0.1 g/cm3
沸点
344.8±32.0 °C at 760 mmHg
BI-9564
BI-9564
1883429-22-8
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11849
2',3',5'-triacetyl-5-Azacytidine
2',3',5'-triacetyl-5-Azacytidine
10302-78-0
5mg
≥98%
品牌
货号
AZ505 ditrifluoroacetate
1035227-44-1
2mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11707
UNC 926 hydrochloride
UNC 926 hydrochloride
1184136-10-4
25mg
≥98%
品牌
货号
中文名称
英文名称
分子式
C7H5IN2O3
分子量
292.031
闪点
162.3±25.1 °C
精确质量
醋酸纤维素山梨酸酯抗菌纤维的制备及其性能

醋酸纤维素山梨酸酯抗菌纤维的制备及其性能宋俊;隗立颖;李俊男;侯源富【摘要】为了提高醋酸纤维的抗菌性能,首先将醋酸纤维素和山梨酰氯在N,N-二甲基乙酰胺(DMAc)中进行接枝反应,合成醋酸纤维素山梨酸酯(CASA),将合成的CASA溶解在DMAc/LiCl中,并加入少量纤维素,制成CASA/cellulose/(DMAc/LiCl)纺丝溶液;基于溶液的流变性能,通过干湿法纺丝技术纺制出CASA纤维,并对其形貌、结晶性、力学性能、热性能、耐化学腐蚀性及抗菌性进行分析.结果表明:CASA/cellulose/(DMAc/LiCl)溶液体系为切力变稀流体;取代度为1.13的CASA纤维断裂强度可达2.02 cN/dtex,且本文实验条件下在HCl和NaOH溶液中的失重率分别为4.5%和10.3%,取代度为0.81的CASA纤维对大肠杆菌和金黄色葡萄球菌的抑菌率分别为76.5%和90.2%;说明CASA纤维具有良好的抗菌性,并具有较好的耐化学腐蚀性,拓宽了醋酸纤维在医疗卫生领域的应用.【期刊名称】《天津工业大学学报》【年(卷),期】2018(037)006【总页数】6页(P24-29)【关键词】醋酸纤维素;山梨酰氯;干湿法纺丝;抗菌性;耐化学腐蚀性【作者】宋俊;隗立颖;李俊男;侯源富【作者单位】天津工业大学材料科学与工程学院,天津 300387;天津工业大学材料科学与工程学院,天津 300387;天津工业大学材料科学与工程学院,天津 300387;天津工业大学材料科学与工程学院,天津 300387【正文语种】中文【中图分类】TS102.51醋酸纤维素(CA)来源于纤维素的乙酰化反应,是一种重要的纤维素有机酯类材料[1].它具有高光泽性、透明性、热塑性、生物降解性[2-4]等优点,在纺织、膜材料、香烟滤嘴等领域具有广泛的应用[5-7].虽然它的优点很多,但由于醋酸纤维素属于典型的多聚葡萄糖结构[8-9],使其不耐微生物降解,因此限制了CA的实际应用.如果能够研究出具有良好抗菌性能的醋酸纤维素材料并纺制成纤维,便可以很好的应用于医疗卫生领域,如婴儿纸尿裤、一次性口罩等.但目前有关抗菌性醋酸纤维素纤维制备的报道比较少.山梨酸分子中的山梨酰基为α,β-不饱和羰基结构,是典型的抗菌试剂[10-11],已被广泛应用于食品保鲜、动物饲料等领域[12-13],将其引入材料中可以提高其抗菌性能[14-15].基于此,本文首先通过酰氯酯化法合成醋酸纤维素山梨酸酯,然后以DMAc/LiCl为溶剂,通过干湿法纺丝技术制备出醋酸纤维素山梨酸酯纤维,并对纺丝溶液进行流变分析,最后对纤维的微观形貌、结晶性、力学性能、热性能、耐化学腐蚀性及抗菌性进行考察.1 实验部分1.1 材料与仪器材料:二醋酸纤维素(CDA,取代度 DS=2.45),南通醋酸纤维有限公司产品;山梨酰氯,上海玉博生物科技有限公司产品;大肠杆菌(ATCC 8739)、金黄色葡萄球菌(ATCC 6538),天津医科大学产品;牛肉膏、蛋白胨、琼脂粉,北京奥博星生物技术有限责任公司产品;N,N-二甲基乙酰胺(DMAc)、无水LiCl、HCl、NaOH、Na2HPO4·12H2O、KH2PO4、无水乙醇、去离子水、吡啶,均为分析纯,天津科密欧化学试剂有限公司产品.仪器:MARSⅢ型哈克流变仪,美国Fisher公司产品;D8 DISCOVER with GADOS型X-射线衍射仪,德国Bruker公司产品;204F1 Phoenix ®型差示扫描量热仪(DSC),德国Netzsh公司产品;S4800型扫描电子显微镜(SEM),日本Hitachi公司产品;LLY-06E/PC型电子单纤维强力仪,山东莱州电子仪器有限公司产品;BSA124S型紫外分光光度仪,北京赛多利斯科学仪器有限公司产品;BXM-30R型立式压力蒸汽灭菌器,上海博迅实业有限公司医疗设备厂产品;HNY-200B型恒温培养振荡器,天津市欧诺仪器仪表有限公司产品;SW-CJ-2F型双人双面净化工作台,江苏通净净化设备有限公司产品;DHP-600型电热恒温培养箱,北京市永光明医疗仪器厂产品.1.2 醋酸纤维素山梨酸酯的合成对CDA进行酸水解反应制备CA.将1 g CA加入到20 mL DMAc中,在60℃油浴及机械搅拌下使其完全溶解,滴加缚酸剂吡啶;然后按比例在冰水浴条件下缓慢滴加山梨酰氯,20 min后将体系移入60℃油浴下搅拌反应24 h;反应结束后,所得产物用去离子水和无水乙醇洗涤数遍至中性,得到醋酸纤维素山梨酸酯(CASA),如图1所示.图1 醋酸纤维素山梨酸酯的合成Fig.1 Synthesis of CASA对CDA进行酸水解反应可制备出不同取代度的CA,通过改变反应物摩尔比及CA 的取代度,可得到不同取代度的 CASA,记为 CASA1、CASA2和 CASA3.用核磁计算出其取代度(DS,CASA)分别为 0.48、0.81 和1.13.1.3 醋酸纤维素山梨酸酯纤维的制备以DMAc/LiCl为溶剂,并加入一定量的纤维素以增加溶液的可纺性,配制质量分数为10%(7%CASA和3%纤维素)的纺丝溶液.将上述配制好的溶液真空脱泡至无气泡.安装纺丝装置,以水作为凝固浴,在常温下开始纺丝.在0.2 MPa的氮气压力下,溶液细流以1.2 m/min的挤出速度从喷丝头中挤出,经过空气层后进入凝固浴中,溶液细流中的溶剂向水中扩散,同时水中的凝固剂向溶液细流内部扩散,CASA纤维从水中析出成型.本文还采用同样方法纺制了CDA纤维进行性能对比.1.4 测试与表征(1)流变性能测试.采用MARSⅢ型哈克流变仪对纺丝溶液在不同温度下的表观黏度和剪切速率的关系进行分析,所用椎板为C 35TiL,测试温度为20~60℃;剪切速率范围为30~120 s-1.(2)扫描电镜测试.采用S4800型扫描电子显微镜观察CASA纤维的表面及断面形貌,纤维的断面结构用液氮淬断后再进行观察.(3)XRD测试.采用X-射线衍射仪分析CASA的结晶情况,Cu Kα射线,加速电压和电流分别为40 kV和150 mA,扫描速率为5°/min,扫描范围为2θ=5°~45°.根据公式(1)计算 CASA 的结晶百分数[16-17]:式中:Xc为结晶百分数;Ac为结晶区域的面积;Aa为无定形区域的面积. (4)力学性能测试.采用LLY-06E/PC型电子单纤维强力仪测试CASA纤维的力学强度,测试条件为隔距10 mm、速率10 mm/min、湿度50%、温度25℃,每个样品测试10次,取其平均值.(5)热性能测试.采用204F1 Phoenix ®型差示扫描量热仪测试产物熔点,测试温度范围为25~300℃,N2氛围,升温速率为10℃/min.(6)耐化学腐蚀性测试.将称重后的纯CDA纤维及CASA纤维分别浸泡在5 mol/L HCl溶液中6 h及1 mol/L NaOH溶液中1 h后,室温下重新称重,计算失重率:式中:Wi为纤维浸入溶剂之前的质量;Wc为纤维浸入溶剂之后的质量.(7)抗菌性能测试.采用接触震荡法对CASA纤维进行抗菌性测试.向灭菌后的培养液(10 g蛋白胨、10 g NaCl、5 g牛肉膏、1 000 mL去离子水)中加入一块细菌,置于恒温培养振荡器中在37℃下培养24 h.完成后对其进行稀释接种二代菌,同样条件下培养12 h,然后用纯培养液和PBS缓冲液对培养好的菌液进行稀释,并分别加入经提前灭菌处理的样品0.2 g.将其放入恒温培养振荡器中在(24±1)℃下振荡处理18~24 h,完成后对其进行梯度稀释.将100 μL稀释液和原菌液分别加入到事先配制好的琼脂固体培养基中,并用涂布器将其涂布均匀,后将其放入恒温培养箱中,在37℃下培养24~48 h,最后拍照统计菌落,每个试样重复3次,取平均值.抑菌率用公式(3)[18]计算:式中:NB和NS分别为对照组和样品组上的菌落数.2 结果与讨论2.1 纺丝溶液的流变性能图2所示为CASA1/cellulose/(DMAc/LiCl)纺丝溶液在不同温度下的表观黏度和剪切速率的关系.图2 CASA1/cellulose/(DMAc/LiCl)在不同温度下的流动曲线Fig.2Flow curves of CASA1/cellulose/(DMAc/LiCl)at different temperatures由图2可以看出:不同温度下纺丝溶液的表观黏度随着剪切速率的增大而减小,呈现出切力变稀行为,表明此溶液为非线性流体.这是由于在热运动和剪切作用下,溶液中形成的交联点处于不断拆散和重建的动态平衡中,随着剪切作用力的提高,分子链间的缠结逐渐被拉开,分子间作用力减弱,纺丝液流动性能提高,表观黏度下降.而且随着温度的升高,溶液的表观黏度也逐渐下降,尤其到40℃时,下降速度更快.这是由于温度升高,分子运动的能量增加,导致溶液自由体积增加,分子间作用力降低,从而使溶液的流动性能增强.2.2 形貌分析图3为CASA纤维的电镜图.图3 CASA纤维的SEM图Fig.3 SEM images of CASA fibers由图3可以看出,3种纤维都具有较为光滑的表面形貌,断面中则存在一些微孔,这可能是由溶剂和水的双扩散作用所引起的.相比之下,CASA3纤维断面的微孔相对较少,比较致密,这可能是由于CASA3的取代度比较高,空间位阻相对较大,减慢了溶剂和水的双扩散作用,从而断面较为致密.2.3 XRD谱图图4为CASA纤维的XRD谱图.图4 CASA纤维的XRD谱图Fig.4 XRD spectrum of CASA fibers由图4可以看出,2θ为17°处为典型的纤维素结晶峰,22.4°处的衍射峰为非晶相的弥散特征峰[19],CASA2与CASA1相比,衍射峰形状变得尖而窄,这表明山梨酰基的增加导致分子链规整性降低.然而CASA3中山梨酰基的比例大大增加,分子链规整度有所上升,此处衍射峰的变化程度略有减弱.经计算可得,CASA1、CASA2、CASA33种纤维的结晶百分数分别为16.0%、13.4%、16.6%,这也说明随着取代度增大,三者的相对结晶度呈现先下降后上升的趋势.2.4 力学性能表1所示为3种CASA纤维的力学性能.表1 CASA纤维的力学性能Tab.1 Mechanical properties of CASA fibers种类断裂强度/(cN·dtex-1)断裂伸长率/%CASA1纤维 1.62 01.75 CASA2纤维1.70 04.34 CASA3纤维 2.02 12.83由表1可知,随着CASA取代度的增大,纤维的断裂强度呈现逐渐升高的趋势.CASA3纤维的取代度为1.13,其断裂强度达到2.02 cN/dtex,断裂伸长率也有所增加,这可能是由于该条件下纤维结构的规整性较高,所以强度较大.2.5 热性能图5为CASA纤维的DSC谱图.图5 CASA纤维的DSC谱图Fig.5 DSC spectrum of CASA fibers由图5可以看出:CASA纤维具有较高的熔点,CASA1纤维的熔点在220℃附近,随着取代度的增大,CASA2纤维的熔点呈现下降的趋势,这是因为原料中山梨酰氯的加入破坏了CA分子间和分子内的氢键作用,使得熔点下降;而CASA3纤维的熔点升高,这可能是由于CASA3的取代度增大,分子中含有的山梨酰基增多,分子链规整度增大,导致其熔点升高.这一结果与XRD所显示结果一致.2.6 耐化学腐蚀性图6所示为CASA纤维的耐化学腐蚀性.酯类物质不耐酸碱的主要原因是酸的质子化作用和碱的皂化反应.由图6可以看出,纯CDA纤维在酸溶液和碱溶液中的失重率分别为28.1%和63.6%;取代度越大,CASA纤维的失重率越小,耐酸碱性越高.其中,CASA3纤维在酸和碱溶液中的失重率仅为4.5%和10.3%,这说明山梨酰基的空间屏蔽效应能有效阻止酸碱质子对酯键的攻击.CASA纤维的这一优异性能拓宽了醋酸纤维素材料的应用领域.图6 CASA纤维分别在5 mol/L HCl和1 mol/L NaOH溶液中浸泡一定时间后的失重率Fig.6 Mass loss of CASA fibers soaked in 5 mol/L HCl and 1 mol/L NaOH solutions for some times2.7 抗菌性能图7为所制备纤维经接触震荡法测试所得2种细菌菌液中残留菌落的生长分布实物图.图7 CASA纤维抗菌实验中的菌落分布Fig.7 Colony distribution in antibacterial test of CASA fibers由图7可以看出,与CDA纤维接触的琼脂培养基表面细菌生长较为明显,存在着大量菌落.相比之下,与CASA纤维接触的培养基表面菌落数明显减少.图8所示为CASA纤维对2种细菌的抑菌率.图8 CASA纤维的抑菌率Fig.8 Bacteriostatic rate of CASA fibers由图7和图8可见,随着取代度的增大,CASA纤维对大肠杆菌和金黄色葡萄球菌的抑菌率均呈现先上升后下降的趋势,最高分别达到76.5%和90.2%.这是由于随着取代度的增加,CA骨架上更多的亲水性基团羟基被疏水性物质山梨酸酯化,CASA可以自由通过细胞膜进入细胞,并与生命物质功能域发生作用,抑制酶体系,达到抑菌作用.然而,当取代度增大到1.13时,高取代度会产生一定的空间位阻效应,从而降低了反应活动中心的空间活动自由度,与微生物功能结构域的有效碰撞降低,最终影响抗菌活性的发挥[20].另外,同种纤维对金黄色葡萄球菌的抑制作用要高于大肠杆菌,这可能与两者的细胞壁结构差异有关[21].3 结论将醋酸纤维素和山梨酰氯在DMAc中进行接枝反应,制备出CASA,并利用干湿法纺丝技术制备出CASA纤维,对纤维性能进行分析,结果表明:(1)CASA1/cellulose/(DMAc/LiCl)纺丝溶液为切力变稀体系,体系的黏度随着剪切速率的增加以及温度的提高呈下降趋势.(2)CASA纤维具有较好的表面形貌,且具有较高的熔点.随着取代度增大,CASA纤维的断面变得更为致密,结晶度和熔点先下降后上升.(3)随着取代度的增大,CASA纤维的断裂强度逐渐增大,取代度为1.13的CASA纤维断裂强度达到2.02 cN/dtex.(4)CASA纤维具有良好的耐化学腐蚀性,取代度为1.13的CASA纤维在5mol/LHCl和1mol/L NaOH溶液中分别浸泡6 h和1 h后失重率分别为4.5%和10.3%.(5)CASA纤维具有良好的抗菌效果,取代度为0.81的CASA纤维对大肠杆菌和金黄色葡萄球菌的抑菌率分别为76.5%和90.2%.【相关文献】[1] 许冬生.纤维素衍生物[M].北京:化学工业出版社,2001.XU D S.CelluloseDerivatives[M].Beijing:Chemical Industry Press,2001(in Chinese).[2]BHATTACHARYAA,MISRABN.Grafting:Aversatilemeans to modify polymers:Techniques,factors and applications[J].Progress in Polymer Science,2004,29(8):767-814.[3]SAKAI K,YAMAUCHI T,NAKASU F,et al.Biodegradation of cellulose acetate by neisseria sicca[J].Bioscience Biotechnology&Biochemistry,1996,60(10):1617. [4]MORIYOSHI K,OHMOTO T,OHE T,et al.Purification and characterization of an esterase involved in cellulose acetate degradation by neisseria sicca SB[J].Bioscience Biotechnology&Biochemistry,1999,63(10):1708-1713.[5]WILBERT M C,PELLEGRINO J,ZYDNEY A.Bench-scale testing of surfactant-modified reverse osmosis/nanofiltration membranes[J].Desalination,1998,115(1):15-32. [6]SIVAKUMAR M,MALAISAMY R,SAJITHA C J,et al.Ultrafiltration application of cellulose acetate-polyurethane blend membranes[J].European Polymer Journal,1999,35(9):1647-1651.[7] RUSTEMEYER P.History of CA and evolution of the markets[J].Macromolecular Symposia,2004,208(1):1-6.[8]VOICU S I,CONDRUZ R M,MITRAN V,et al.Sericin covalent immobilization onto cellulose acetate membrane for biomedical applications[J].ACS SustainableChemistry&Engineering,2016.DOI:10.1021/acssuschemeng.5b01756.[9]陈婧.新型纤维素衍生物的结构设计、合成及气体分离性能研究[D].北京:中国科学院大学,2013.CHEN J.Structural design,synthesis and gas separation properties of novel cellulose derivatives[D].Beijing:University of Chinese Academy of Sciences,2013(in Chinese).[10]宁正祥,谭龙飞,张德聪,等.α,β-不饱和羰基化合物的分子结构特性与抗菌活性间的关系[J].应用化学,1996(1):38-42.NING Z X,TAN L F,ZHANG D C,et al.Relationship between molecular structure and antibacterial activity of α,βunsaturated carbonyl compounds[J].Chinese Journal of Applied Chemistry,1996(1):38-42(in Chinese).[11]徐雅琴,李凤芝.山梨酸及其应用[J].化学工程师,1997(5):35-36.XU Y Q,LI F Z.Sorbic acid and its application[J].Chemical Engineer,1997(5):35-36(in Chinese).[12]陈彦玲,高丽娟,王敬平,等.山梨酸的应用与制取[J].长春师范大学学报,2002(5):31-34.CHEN Y L,GAO L J,WANG J P,et al.Application and preparation of sorbicacid[J].Journal of Changchun Normal University,2002(5):31-34(in Chinese).[13]颜廷良.山梨酸的制备及发展前景[J].贵州化工,1999(3):28-29.YAN T L.Preparation and prospects of sorbic acid[J].Guizhou Chemical Industry,1999(3):28-29(in Chinese). [14]JIPA I M,STOICAGUZUN A,STROESCU M.Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films[J].LWT-Food Scienceand Technology,2012,47(2):400-406.[15]薛苏生.山梨酸的生产应用及发展前景[J].江苏化工,1998(1):51-54.XUE S S.Production and application of sorbic acid and its development prospects[J].Jiangsu Chemical Industry,1998(1):51-54(in Chinese).[16]NARA S,KOMIYA T.Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch[J].Starch-Stärke,2010,35(12):407-410.[17]VINODHINI P A,SANGEETHA K,THANDAPANI G,et al.FTIR,XRD and DSC studiesof nanochitosan,cellulose acetate and polyethylene glycol blend ultrafiltration membranes[J].International Journal of Biological Macromolecules,2017,104:1721-1729.[18]LIU C X,ZHANG D R,HE Y,et al.Modification of membrane surface for anti-biofouling performance:Effect of antiadhesion and anti-bacteria approaches[J].Journal of Membrane Science,2010,346(1):121-130.[19]FAN G,WANG M,LIAO C,et al.Isolation of cellulose from rice straw and its conversion into cellulose acetate catalyzed by phosphotungstic acid[J].Carbohydrate Polymers,2013,94(1):71-76.[20]黄志良.山梨酸衍生物的合成与抗菌活性研究[D].广州:华南理工大学,2002.HUANG ZL.Synthesis of sorbic acid derivatives and antibacterial activity[D].Guangzhou:South China University of Technology,2002(in Chinese).[21]BRUL S,COOTE P.Preservative agents in foods:Mode of action and microbial resistance mechanisms[J].International Journal of Food Microbiology,1999,50(1):1-2.。
