ACR与EULAR2010年RA新标准解读
RA新标准解读

RA新标准解读:2009年10月美国风湿病年会上,美国风湿病学会(ACR)和欧洲抗风湿联盟(EULAR)共同发布了新的类风湿关节炎(RA)分类标准,这是20多年来对RA分类标准的再次更新,也是近年来风湿学界最重要的事件之一。
一石激起千层浪,此标准一经出现,迅速引起了广泛的社会关注和深入讨论。
新标准“出炉”颇费周折近十年来,随着抗环瓜氨酸多肽(CCP)抗体等诊断新手段的出现,以及治疗观念的进步,对RA患者及早采用改善病情的抗风湿药物(DMARDs)、减少骨破坏和关节功能丧失已经成为共识。
由于1987年版的RA分类标准识别早期RA患者的能力较差,其指导临床工作的意义受到越来越多的置疑。
近年来,曾有多个工作组尝试对其进行改进,但均未得到公认。
ACR和EULAR耗时3年,联合开展了修订新标准的研究,研究流程非常严谨,业内认同度高。
该项研究综合了近年来RA临床研究的成果和专家意见,致力于早期识别需要治疗的慢性致畸性关节炎人群,及早应用改善病情的抗风湿药物,以防止患者发展成为符合1987年标准的较晚期RA人群,从而改善患者预后、减少整体医疗费用。
新标准的出炉经历了两个主要步骤。
首先,ACR/EULAR联合专家组以DMARDs治疗需求为导向,通过9个中心3115例早期关节炎(病程<3年)患者的数据库筛选出判断慢性持续性(或致畸性)关节炎的关键指标,通过统计学方法确定各个判断指标的权重。
随后,由ACR推举出的11名专家和EULAR推举的11名共同分析30个病例,在前期工作的基础上进一步筛选能判断出早期RA的核心指标。
此项目在去年EULAR会议上报告后,工作组根据反馈意见进一步细化,并在2009年10月的ACR会议上发布。
新标准特点分析这一新的分类标准将患者的临床表现分为受累关节情况、血清学检查、滑膜炎的病程和急性时相反应物等4个方面进行评分,总分在6分以上即诊为确定RA。
ACR/EULAR联合专家组下一步的工作是制定甄别“可能RA”和“不太可能RA”的判断标准,其临界分点可能被定为3或4分(见表)。
ACR2010诊断RA标准
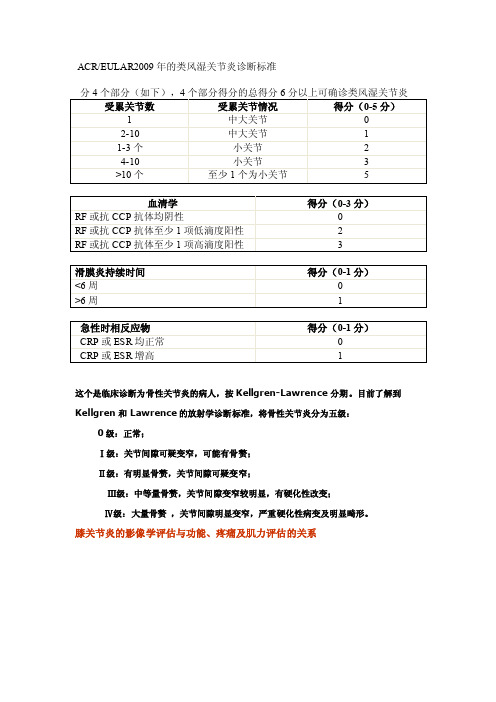
ACR/EULAR2009年的类风湿关节炎诊断标准这个是临床诊断为骨性关节炎的病人,按Kellgren-Lawrence分期。
目前了解到Kellgren和Lawrence的放射学诊断标准,将骨性关节炎分为五级:0级:正常;Ⅰ级:关节间隙可疑变窄,可能有骨赘;Ⅱ级:有明显骨赘,关节间隙可疑变窄;Ⅲ级:中等量骨赘,关节间隙变窄较明显,有硬化性改变;Ⅳ级:大量骨赘,关节间隙明显变窄,严重硬化性病变及明显畸形。
膝关节炎的影像学评估与功能、疼痛及肌力评估的关系Para Español haga clic aquí.FAST FACTS∙OA is the most common form of joint disease in humans and is a leading cause of disability among the elderly.∙It typically occurs in the hand joints, spine, hips and knees.∙It is caused by cartilage breakdown and subsequent bony changes of the joints.∙Although the joint changes are irreversible, only a small number of patients will progress to the point that requires joint replacement surgery.∙OA symptoms can vary greatly among patients. Y our rheumatologist can make the diagnosis and prescribe appropriate treatment recommendations for you.In osteoarthritis, the cartilage between the bones in the joint breaks down, and bony enlargement occurs.WHA T IS OSTEOARTHRITIS?Osteoarthritis (OA) is a slowly progressive joint disease typically seen in middle-age to elderly people. It occurs when the joint cartilage breaks down, causing the underlying bone to fail. OA symptoms include joint pain, stiffness, knobby swelling, cracking noises with joint movements and decreased function. It typically affects the joints of the hands and spine and weight-bearing joints such as the hips and knees.WHO GETS OSTEOARTHRITIS?OA typically occurs in patients age 40 and above. However, some risk factors might cause it to occur sooner (see below). It affects people of all races and gender.WHA T ARE THE RISK FACTORS FOR OSTEOARTHRITIS?∙Older age∙Family members with OA∙Obesity∙Joint trauma or repetitive use of jointsHOW IS OSTEOARTHRITIS DIAGNOSED?OA usually is diagnosed by having typical symptoms and physical examination as described above. In some cases, imaging studies may be useful to tell the extent of disease or to help rule out other joint problems.The circles on this figure indicate joints that are commonly affected by osteoarthritis.HOW IS OSTEOARTHRITIS TREATED?The goal of treatment is to reduce pain and improve function of the affected joints. This can be achieved with a combination of physical measures, drug therapy and, sometimes, surgery.Physical measures– Exercise, support devices and thermal therapy are useful in OA. Some forms of unproven alternative treatment such as spa, massage, acupuncture and chiropractic manipulation can help relieve pain for a short duration, but usually are costly and require repeated treatments.Drug Therapy– Available forms of drug therapy include topical and oral agents. Topical drugs, which include capsaicin cream, lidocaine, and diclofenac gel, can be applied directly on the skin overlying the affected joints. Oral pain relievers such as acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used as first-line treatment. For more serious pain, stronger medications, such as narcotics, may be required. Joint injections with corticosteroids or a form of lubricant called hyaluronic acid (HA) derivatives have proven effective for some patients.Surgery– Arthroscopy and/or joint replacement is considered when the joint is seriously damaged, or the patient is in intractable pain and experiencing significant loss of function.Supplements– Many over-the-counter nutritional supplements have been used for treatment of OA, but most lack good research data to support their effectiveness and safety. Among the most widely used areglucosamine/chondroitin sulfate, calcium and vitamin D, and omega-3 fatty acids. To ensure safety and avoid drug interaction, consult your doctor or pharmacist before using any of these agents, especially in combination with prescribed drugs.LIVING WITH OSTEOARTHRITISThere is no cure for OA, but you can manage how it impacts your lifestyle. For instance, giving proper positioning and support to the neck and back during sitting or sleeping; adjusting furniture, such as raising a chair or toilet seat; and avoiding trauma and repetitive motions of the joint, especially frequent bending, are great starts.Adding regular exercise to your daily activities will improve muscle strength. Exercises that increase strength of the quadriceps muscles (the front thigh muscles) also can help decrease knee pain and reduce subsequent disability associated with osteoarthritis. Working with a physical or occupational therapist can help you learn the best exercises and choose appropriate assistive devices for your joints.Weight loss in obese people can reduce pain and progression of OA. Achieving and keeping an ideal weight will make a difference in your overall comfort levels.POINTS TO REMEMBER∙OA is the most common form of arthritis and can occur together with other types of arthritis.∙Evaluation by your doctor will help confirm the diagnosis and develop an appropriate treatment plan for your condition.∙The goal of treatment in OA is to reduce pain and improve function.∙At present, there is no available therapy that can reverse the damage of OA in the joint, but many studies are underway.TO FIND A RHEUMA TOLOGISTFor more information about rheumatologists, click here.Learn more about rheumatologists and rheumatology health professionals.FOR MORE INFORMATIONThe American College of Rheumatology has compiled this list to give you a starting point for your own additional research. The ACR does not endorse or maintain these Web sites, and is not responsible for any information or claims provided on them. It is always best to talk with your rheumatologist for more information and before making any decisions about your care.Arthritis FoundationUpdated August 2009Written by Thitinan Srikulmontree, MD, and reviewed by the American College of Rheumatology Patient Education Task Force.This patient fact sheet is provided for general education only. Individuals should consult a qualified health care provider for professional medical advice, diagnoses and treatment of a medical or health condition.© 2010 American College of Rheumatology5次坐-立试验在膝骨关节炎患者功能评估中的价值。
ACREULAR类风湿关节炎分类标准

2010 ACR/EULAR 类风湿关节炎分类标准发表时间:2010-09-18 发表者:张昌丽 (访问人次:310)目标人群:有下列表现的患者:1、有至少一个关节具有明确的临床滑膜炎(肿胀)【新标准目的在于给新症患者分类。
另外,具有侵蚀性疾病如类风湿关节炎典型表现,其病史符合2010标准的,应该分类为类风湿关节炎。
患者有长期疾病,包括那些疾病处于非活动期(经过治疗或未经治疗),基于回顾性的可以获得的资料,先前符合2010年标准的,也应分类为类风湿关节炎。
】2、具有滑膜炎,用其他疾病不能得到更好解释的【不同表现的患者鉴别诊断也不同,但是系统性红斑狼疮,银屑病关节炎和痛风应该可以包括在内。
如果不清楚应该考虑哪些相关的鉴别诊断,应该咨询风湿病学专家】RA分类标准(评分算法:A-D的项目评分相加;患者如果按下列标准评分≥6/10,明确诊断为类风湿性关节炎)【虽然患者评分不足6分的不能分类为类风湿关节炎,但是他们的状态可以再次评价,随着时间推移,可能会符合标准。
】A:受累关节【指的是查体时发现的任何肿胀或触痛的关节,可通过滑膜炎的影像学证据证实。
在评估中,远端指间关节,第一腕掌和第一跖趾关节除外。
关节分布的分类根据受累关节的位置和数量,划入最可能受累关节类目。
】- 1个大关节(0分)【大关节指的是肩关节,肘关节,髋关节,膝关节和踝关节】- 2-10大关节(1分)- 1-3小关节(有或没有大关节)(2分)【小关节指的是掌指关节,近端指间关节,2-5跖趾关节,拇指指间关节和腕关节】- 4-10小关节(有或没有大关节)(3分)-超过10个小关节(至少一个小关节)(5分)【在这一条中,至少一个受累关节必须是小关节;其他关节可以包括任何大的或额外的小关节的组合,如其他别处未特别列出的关节(颞颌关节,肩峰锁骨关节,胸锁关节)】B:血清学(至少需要1项结果)【阴性指的是低于或等于当地实验室正常值的上限。
低滴度阳性指的是国际单位值高于正常值上限,但是低于正常值上限3倍。
2010年美国风湿病学会联合欧洲抗风湿病联盟的类风湿关节炎分类标准解读

1987年RA分类标准的可靠性曾被进一
步验证。Emery等在1999年的研究结果显示,关节 炎病史小于12周者只有51%满足ACR 1987年 RA分类标准。12周以内的早期患者往往达不到分 类标准.难以作出诊断。许多患者关节炎病史大于 12周甚至1年才符合ACRl987年的RA分类标准 的条件。众所周知,所有的医师都希望在3个月以 内而不是在1年才作出诊断。1995至1997年法国 的Brittany等7所医院对病史小于1年的272例新 发病的关节炎患者进行前瞻性研究.应用这个标准 每6个月观察1次.在第2年末时评价分类标准的 准确性。在最后1次随访时,由5名专家作出诊断。 272例新发病例共有98例确诊为RA。其中初次符 合ACR 1987年RA分类标准的有65例f66%),而 非RA有31/172(18%)达到了诊断标准。观察2年, 85例符合分类标准。灵敏度为87%。172例非RA 中有43例f25%)达到ACR 1987年RA分类标准的 4条标准。因而特异度只有75%,其原因是ACR 1987年RA分类标准中没有排除标准。对于病史小 于1年的关节炎患者.该标准无法预测是否会发展 为RA。有些患者初次就诊符合标准,但2年后证实 并非RA。在另一项486例炎症性关节病的前瞻性 研究中发现,ACR标准的灵敏度较好(77%~87%)。 但是在预测骨侵蚀的特异性方面很差。1990年在 英国Norfolk的Norwich卫生专署机构调查了该地 RA的发病率。结果显示。第5年符合ACR标准的 患病率与第1年相比,女性高出45%,男性高出 36%。这说明ACR 1987年的RA分类标准对于1
ACR
年内发病的关节炎灵敏度不够理想。人们希望在疾 病的早期作出诊断并给予早期治疗。以避免致残。 显然ACRl987年的RA分类标准不能满足这一要 求。对RA早期诊断、早期治疗、预防残废并保护关 节功能是努力争取的目标。但并非所有的RA患者 都需要联合使用DMARDs和生物制剂。RA的治疗 应个体化,因此需对预后有效的评估。有少数RA 患者预后好,这些患者可能并不是真正的RA.不需 要用DMARDsl蝴。 新标准的背景、作用及内容 为发展新的RA分类标准,EULAR组织了“RA 早期疾病队列研究”(I期研究)。该研究针对分类 不明的炎性关节炎中那些高风险的慢性损伤。并且 迅速使用改善病程药物。参加单位有阿姆斯特丹、 维也纳、曼彻斯特、奥斯陆、利兹、荷兰莱顿、多伦多 等共9个团队,录入患者3 115例,条件是:①有炎
ACR及EULAR 2010RA新标准解读

目的是足够早的作出
诊断
EULAR2009
专家共识
有滑膜炎表现并且有放射影像学骨侵蚀证 据者应视为RA
专家推荐
以下两种病人应分类为RA: 符合两项强制标准的病人(至少一个关节 的滑膜炎和临床表现,并排除其它疾病) 有典型RA骨侵蚀的放射学证据的病人
ACR/EULAR 2010RA 新标准解读
嘉兴市第一医院风湿科 王宏智
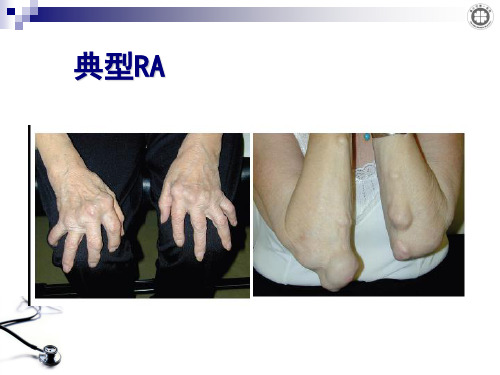
典型RA
难的是……
早期RA 未分化关节炎(UA) 炎性关节炎(IA) 特发性关节炎 ……
?
RA
RA分类标准的发展
1956/8 1961 1966 1987
ARA
(美国风湿病 学会)
罗马 标准
纽约 标准
纽约修订 标准
及GPI等
3、CRP
早期诊断成为可能!!
×
ACR/EULAR2010年RA新分类标准
关节炎以排除其它疾病为前提
强调CCP抗体和RF,增加急性相反应物 保留受累关节数和滑膜炎持续时间 废除晨僵、皮下结节、对称性关节炎三个 条目
Ⅰ期总体目标
从表现为未分化炎性关节炎的病人中, 发现那些
血清学 (0-3)
RF和ACPA均阴性 ≥1项低滴度阳性 ≥1项高滴度阳性
总分≥6分 即明确的RA
滑膜炎持续时间 (0-1)
<6周 ≥6周
急性相反应物 (0-1)
CRP和ESR均正常 ≥1项异常
谢谢
滑膜炎 由风湿病学专家体检或影像学证实的≥1个 关节受累 (不包括远端指间关节、第一跖趾 关节、第一腕掌关节(CMC),但包括拇指 掌指关节)
《美国风湿病学会欧洲抗风湿联盟痛风分类标准》解读

3、病理学标准
2、对于疑似痛风患者,医生可以根据该标准进行初步诊断。如果患者的临床 表现符合标准,则可以进一步进行影像学和病理学检查以明确诊断。如果患者的 临床表现不符合标准,则可能需要进一步检查以排除其他疾病的可能性。
Hale Waihona Puke 3、病理学标准3、在治疗方面,医生可以根据该标准对痛风患者进行分期治疗。对于急性发 作期患者,医生可以采取非甾体抗炎药、糖皮质激素等药物治疗以缓解疼痛和炎 症。对于慢性期患者,医生可以采取降尿酸治疗以降低血尿酸水平,预防痛风发 作。同时,医生还需要对患者进行生活方式的干预,如控制饮食、增加运动等, 以降低痛风复发的风险。
痛风概述
痛风概述
痛风是一种由于嘌呤代谢紊乱或尿酸排泄障碍导致的慢性代谢性疾病。根据 国家卫健委2019年的数据,我国痛风患病率呈逐年上升趋势,且发病年龄日趋年 轻化。痛风的主要病理生理机制是尿酸盐结晶沉积,导致急性痛风性关节炎和痛 风石形成。痛风患者常伴发高血压、糖尿病、肥胖、高血脂等代谢性疾病。
指南解读
要点分析
要点分析
1、分类:该指南将痛风分为急性发作期、间歇期和慢性期三个阶段,有助于 更好地理解患者的病情和制定相应的治疗方案。
要点分析
2、诊断:指南强调了病史采集、体格检查、实验室检查和影像学检查的重要 性,有助于提高诊断的准确性。
要点分析
3、治疗:该指南提出了针对不同阶段的治疗方案,包括抗炎、降尿酸和尿酸 盐溶解等治疗措施,具有很强的实操性。
在影像学方面,有以下两个标准: (1)关节超声:超声检查发现关节内有结晶沉积或痛风石;
2、影像学标准
(2)关节X线检查:X线平片显示关节周围有骨质破坏或囊性变。
3、病理学标准
3、病理学标准
2010ACR EULAR 类风湿诊断指南

ARTHRITIS&RHEUMATISMVol.62,No.9,September2010,pp2569–2581DOI10.1002/art.27584©2010,American College of Rheumatology2010Rheumatoid Arthritis Classification CriteriaAn American College of Rheumatology/European League Against RheumatismCollaborative InitiativeDaniel Aletaha,1Tuhina Neogi,2Alan J.Silman,3Julia Funovits,1David T.Felson,2Clifton O.Bingham,III,4Neal S.Birnbaum,5Gerd R.Burmester,6Vivian P.Bykerk,7Marc D.Cohen,8Bernard Combe,9Karen H.Costenbader,10Maxime Dougados,11Paul Emery,12Gianfranco Ferraccioli,13Johanna M.W.Hazes,14Kathryn Hobbs,15Tom W.J.Huizinga,16Arthur Kavanaugh,17Jonathan Kay,18Tore K.Kvien,19Timothy Laing,20 Philip Mease,21Henri A.Ménard,22Larry W.Moreland,23Raymond L.Naden,24 Theodore Pincus,25Josef S.Smolen,1Ewa Stanislawska-Biernat,26Deborah Symmons,27 Paul P.Tak,28Katherine S.Upchurch,18JirˇíVencovsky´,29Frederick Wolfe,30and Gillian Hawker31This criteria set has been approved by the American College of Rheumatology(ACR)Board of Directors and the Euro-pean League Against Rheumatism(EULAR)Executive Committee.This signifies that the criteria set has been quantita-tively validated using patient data,and it has undergone validation based on an external data set.All ACR/EULAR-approved criteria sets are expected to undergo intermittent updates.The American College of Rheumatology is an independent,professional,medical and scientific society which does not guarantee,warrant,or endorse any commercial product or service.Objective.The1987American College of Rheuma-tology(ACR;formerly,the American Rheumatism As-sociation)classification criteria for rheumatoid arthri-tis(RA)have been criticized for their lack of sensitivity in early disease.This work was undertaken to develop new classification criteria for RA.Methods.A joint working group from the ACR and the European League Against Rheumatism devel-This article is published simultaneously in the September 2010issue of Annals of the Rheumatic Diseases.Supported by the American College of Rheumatology and the European League Against Rheumatism.1Daniel Aletaha,MD,MSc,Julia Funovits,Dipl.Ing,Josef S. Smolen,MD:Medical University of Vienna,Vienna,Austria;2Tuhina Neogi,MD,PhD,FRCPC,David T.Felson,MD,MPH:Boston University School of Medicine,Boston,Massachusetts;3Alan J.Sil-man,FRCP,FmedSci,DSc(Hons):Arthritis Research UK,Chester-field,UK;4Clifton O.Bingham,III,MD:Johns Hopkins University,Baltimore,Maryland;5Neal S.Birnbaum,MD:California PacificMedical Center and University of California,San Francisco;6Gerd R.Burmester,MD:Charite´Hospital–University Medicine Berlin,FreeUniversity and Humboldt University Berlin,Berlin,Germany;7VivianP.Bykerk,MD,FRCPC:Mount Sinai Hospital and University ofToronto,Toronto,Ontario,Canada;8Marc D.Cohen,MD:NationalJewish Medical and Research Center,Denver,Colorado;9BernardCombe,MD,PhD:Lapeyronie Hospital and Montpellier I University,Montpellier,France;10Karen H.Costenbader,MD,MPH:Brighamand Women’s Hospital and Harvard University,Boston,Massachu-setts;11Maxime Dougados,MD:Cochin Hospital,Assistance PubliqueHoˆpitaux de Paris,and Paris-Descartes University,Paris,France; 12Paul Emery,MD,MA,FRCP:University of Leeds and NIHR Leeds Musculoskeletal Biomedical Research Unit,Leeds,UK;13GianfrancoFerraccioli,MD:School of Medicine,Catholic University of theSacred Heart,Rome,Italy;14Johanna M.W.Hazes,MD,PhD:Erasmus Medical Center,University Medical Center Rotterdam,andUniversity of Rotterdam,Rotterdam,The Netherlands;15KathrynHobbs,MD:University of Colorado School of Medicine,Denver;Arthritis&Rheumatism An Official Journal of the American College of Rheumatology and 2569oped,in3phases,a new approach to classifying RA.The work focused on identifying,among patients newly presenting with undifferentiated inflammatory synovi-tis,factors that best discriminated between those who were and those who were not at high risk for persistent and/or erosive disease—this being the appropriate cur-rent paradigm underlying the disease construct“rheu-matoid arthritis.”Results.In the new criteria set,classification as “definite RA”is based on the confirmed presence of synovitis in at least1joint,absence of an alternative diagnosis that better explains the synovitis,and achieve-ment of a total score of6or greater(of a possible10) from the individual scores in4domains:number and site of involved joints(score range0–5),serologic abnormality(score range0–3),elevated acute-phase response(score range0–1),and symptom duration(2 levels;range0–1).Conclusion.This new classification system rede-fines the current paradigm of RA by focusing on fea-tures at earlier stages of disease that are associated with persistent and/or erosive disease,rather than defining the disease by its late-stage features.This will refocus attention on the important need for earlier diagnosis and institution of effective disease-suppressing therapy to prevent or minimize the occurrence of the undesir-able sequelae that currently comprise the paradigm underlying the disease construct“rheumatoid arthri-tis.”IntroductionRheumatoid arthritis(RA)is a chronic inflam-matory disease characterized by joint swelling,joint tenderness,and destruction of synovial joints,leading to severe disability and premature mortality(1–5).Given16Tom W.J.Huizinga,MD,PhD:Leiden University Medical Centre, Leiden,The Netherlands;17Arthur Kavanaugh,MD:University of California,San Diego;18Jonathan Kay,MD,Katherine S.Upchurch, MD:UMassMemorial Medical Center and University of Massachu-setts Medical School,Worcester;19Tore K.Kvien,MD,PhD:Diakon-hjemmet Hospital,Oslo,Norway;20Timothy Laing,MD:University of Michigan,Ann Arbor;21Philip Mease,MD:Swedish Medical Center and University of Washington,Seattle;22Henri A.Me´nard,MD: McGill University Health Centre and McGill University,Montreal, Quebec,Canada;23Larry W.Moreland,MD:University of Pittsburgh, Pittsburgh,Pennsylvania;24Raymond L.Naden,MB ChB,FRACP: Ministry of Health,Auckland,New Zealand;25Theodore Pincus,MD: New York University Hospital for Joint Diseases,New York,New York;26Ewa Stanislawska-Biernat,MD,PhD:Institute of Rheumatol-ogy,Warsaw,Poland;27Deborah Symmons,MD,FFPH,FRCP: University of Manchester,Manchester,UK;28Paul P.Tak,MD,PhD: Academic Medical Center,University of Amsterdam,Amsterdam, The Netherlands;29Jirˇı´Vencovsky´,MD,DSc:Institute of Rheumatol-ogy,Prague,Czech Republic;30Frederick Wolfe,MD:National Data Bank for Rheumatic Diseases and University of Kansas,Wichita; 31Gillian Hawker,MD,MSc,FRCPC:Women’s College Hospital and University of Toronto,Toronto,Ontario,Canada.Dr.Aletaha has received consulting fees,speaking fees, and/or honoraria from Abbott,Bristol-Myers Squibb,UCB,Schering-Plough,Wyeth,and Roche(less than$10,000each).Dr.Bingham has received consulting fees,speaking fees,and/or honoraria from UCB, Roche,Genentech,Celgene,and Merck Serono(less than$10,000 each);he has received research and/or educational grant support from Bristol-Myers Squibb,Genentech,UCB,Centocor,Abbott,and Am-gen.Dr.Birnbaum has received consulting fees,speaking fees,and/or honoraria from Amgen,Pfizer,Centocor,Abbott,and UCB(less than $10,000each).Dr.Burmester has received consulting fees,speaking fees,and/or honoraria from Abbott,Bristol-Myers Squibb,Pfizer, UCB,and Roche(less than$10,000each).Dr.Bykerk has received consulting fees,speaking fees,and/or honoraria from Amgen,Wyeth, Abbott,Schering-Plough,Roche,Bristol-Myers Squibb,and UCB(less than$10,000each);her spouse is employed by Genzyme and owns stock in the company.Dr.Cohen has received consulting fees, speaking fees,and/or honoraria from UCB,Genentech,Bristol-Myers Squibb,and Human Genome Sciences(less than$10,000each).Dr. Combe has received consulting fees,speaking fees,and/or honoraria from Abbott,Bristol-Myers Squibb,Pfizer,Roche,Schering-Plough, and Merck,Sharpe,and Dohme(less than$10,000each).Dr.Emeryhas received consulting fees,speaking fees,and/or honoraria from Pfizer,Abbott,Centocor,UCB,Roche,Bristol-Myers Squibb,and Merck,Sharpe,and Dohme(less than$10,000each).Dr.Ferraccioli holds a patent for T cell receptor clonotype analysis(PCT/IB2008/ 053152NP).Dr.Huizinga has received consulting fees,speaking fees, and/or honoraria from Schering-Plough,Bristol-Myers Squibb,UCB, Biotest AG,Wyeth/Pfizer,Novartis,Roche,Sanofi-Aventis,Abbott, and Axis-Shield(less than$10,000each).Dr.Kavanaugh has con-ducted clinical research for Amgen,Abbott,Bristol-Myers Squibb, UCB,Roche,Centocor,Genentech,and Sanofi-Aventis.Dr.Kay has received consulting fees from Array BioPharma,Bristol-Myers Squibb, Celgene,Centocor,Genentech,Roche,UCB,and Sanofi-Aventis(less than$10,000each).Dr.Mease has received consulting fees,speaking fees,and/or honoraria from Abbott,Amgen,Biogen Idec,Bristol-Myers Squibb,Centocor,Roche,Genentech,UCB,Pfizer,Novartis, and Eli Lilly(less than$10,000each).Dr.Me´nard has received un-restricted educational and research grants as well as consulting and speaking fees from Abbott,Amgen,Inova,Merck,Pfizer,Roche, Schering-Plough,UCB,and Wyeth(less than$10,000each)and investigator-initiated research grants from Bristol-Myers Squibb,Euro-Immun AG,and Roche(more than$10,000each);he owns stock or stock options in Merck;and he has a license agreement with Euro-Immun AG for an anti-Sa enzyme-linked immunosorbent assay.Dr. Moreland has received consulting fees,speaking fees,and/or honoraria from Biogen Idec,Centocor,Pfizer,Takeda,KaloBios,ChemoCen-tryx,UCB,Genentech,Incyte,and Eli Lilly(less than$10,000each). Dr.Naden has received consulting fees from the American College of Rheumatology in regard to the methodology of developing weighted scoring systems(more than$10,000).Dr.Pincus has received consult-ing fees,speaking fees,and/or honoraria from Amgen,Abbott,Bristol-Myers Squibb,Centocor,UCB,Wyeth,and Genentech(less than $10,000each)and investigator-initiated research grants from Amgen, Bristol-Myers Squibb,UCB,and Centocor.Dr.Stanislawska-Biernat has received speaking fees from Abbott and Pfizer(less than$10,000 each).Dr.Vencovsky´has received speaking fees from Pfizer,UCB, Abbott,Roche,and Merck,Sharpe,and Dohme(less than$10,000 each).Address correspondence and reprint requests to Alan J. Silman,MD,FRCP,Arthritis Research UK,Copeman House,Ches-terfield S417TD,UK.E-mail:a.silman@.Submitted for publication January22,2010;accepted in revised form May20,2010.2570ALETAHA ET ALthe presence of autoantibodies,such as rheumatoid factor(RF)and anti–citrullinated protein antibody (ACPA)(tested as anti–cyclic citrullinated peptide[anti-CCP]),which can precede the clinical manifestation of RA by many years(6–9),RA is considered an auto-immune disease(10,11).Autoimmunity and the overall systemic and articular inflammatory load drive the de-structive progression of the disease.However,although structural changes,which can be visualized by conven-tional radiography or other imaging techniques,best distinguish RA from other arthritic disorders(12),joint damage is rarely apparent in the very early stages of disease,but rather accumulates consistently over time (13–16).Over the last decade,the optimal use of disease-modifying antirheumatic drugs(DMARDs),in particu-lar the anchor DMARD methotrexate(MTX)(17–19), and the availability of new biologic agents(11,20),have dramatically enhanced the success of RA management. Moreover,it has been recognized that early therapeutic intervention improves clinical outcomes and reduces the accrual of joint damage and disability(21–23).Undoubt-edly,treating patients at a stage at which evolution of joint destruction can still be prevented would be ideal. However,at present,clinical trials of RA treatments are hampered by lack of criteria allowing for study enroll-ment of patients at early stages of disease.Thus,to date it has not been possible to effectively investigate the efficacy of early interventions in terms of their ability to prevent later-stage RA,since there are no validated or accepted uniform criteria to classify such individuals with early disease.The standard and accepted means of defining RA is by use of classification criteria.Classification criteria enable the stratification of groups of individuals into those with and those without RA in order to standardize recruitment into clinical trials and related studies,and provide the basis for a common approach to disease definition that can be used to compare across studies and centers.The classification criteria set that is in widespread international use to define RA is the1987 American College of Rheumatology(ACR;formerly the American Rheumatism Association)criteria(24).These criteria are well accepted as providing the benchmark for disease definition,but have a significant limitation in that they were derived by trying to discriminate patients with established RA from those with a combination of other definite rheumatologic diagnoses.They are there-fore not helpful in achieving the goal of identifying patients who would benefit from early effective interven-tion,as discussed above.Indeed,with modern therapies,the goal is to prevent individuals from reaching the chronic,erosive disease state that is exemplified in the 1987criteria for RA.A joint working group of the ACR and the European League Against Rheumatism(EULAR)was therefore formed to develop a new approach for classi-fication of RA.While classification criteria are poten-tially adopted for use as aids for diagnosis,the focus of this endeavor was not on developing diagnostic criteria or providing a referral tool for primary care physicians. Indeed,a separate body of work is needed to develop such tools,which may be informed by classification criteria.Thus,the specific charge was to develop new classification criteria for RA to facilitate the study of persons at earlier stages of the disease.It was with this framework in mind that the working group developed the2010ACR/EULAR classification criteria for RA. Overview on hypothesis and methods of Phases1and2A priori,the working group focused on develop-ing an approach that would be appropriate for newly presenting patients with undifferentiated inflammatory synovitis,in order to identify that subset of patients who are at sufficiently high risk of persistent and/or erosive disease—this being the appropriate current paradigm underlying the disease construct“rheumatoid arthritis”—to be classified as having RA.It was recog-nized that such a scheme should not be developed using existing criteria sets as the“gold standard,”because of the inherent circularity.The goal set forth was to develop a set of rules to be applied to newly presenting patients with undifferentiated synovitis that would1) identify the subset at high risk of chronicity and erosive damage;2)be used as a basis for initiating disease-modifying therapy;and3)not exclude the capture of patients later in the disease course.To achieve these goals,the working group de-vised a3-phase program.Phase1was a data-driven approach based on cohorts of real-world patients with early arthritis,to identify factors,and their relative weights,that were associated with the subsequent deci-sion by a physician to start MTX treatment.Phase2was a consensus-driven,decision science–based approach, informed by the data from Phase1,to refine these factors and their weights using a series of“paper pa-tients,”as well as to identify any other factors that may be of relevance based on current clinical thinking.Phase 3,which is the focus of this report,describes the derivation,from the previous2phases,of the finalACR/EULAR CLASSIFICATION CRITERIA FOR RA2571classification criteria set.The details of the methods and results from Phases1and2are provided elsewhere (25,26),and are briefly summarized below.Phase1.The aim of Phase1was to identify the contributions of clinical and laboratory variables that in practice were the most predictive of the decision to initiate DMARD therapy in a population of patients with early undifferentiated synovitis.Initiation of DMARD therapy was used as an indicator of the physician’s opinion that the patient was at risk of developing persistent and/or erosive arthritis that we would currently consider to be RA.Data on3,115 patients from9early arthritis cohorts who were consid-ered not to have evidence of another possible diagnosis explaining their presentation were obtained.Between July2007and November2008an expert working group developed an analysis strategy that related an agreed-upon list of standardized clinical and laboratory vari-ables collected at baseline to the initiation of DMARD treatment within the next12months.MTX initiation was used as the gold standard for this purpose.The analytical process aimed to identify the independent contribution of each variable on this list and included univariate regression modeling,a subsequent principal components analysis,and a multivariate regression model that included all identified components(25).The resulting list of informative variables identified during that process and the weights based on the odds ratios are shown in Table1.Phase2.Phase2consisted of a consensus-based, decision science–informed approach,which took place between November2008and June2009.The purpose of this phase was to derive a clinician-based judgment on the relative contribution of clinical and laboratory fac-tors deemed to be important in influencing the proba-bility of developing“persistent inflammatory and/or erosive arthritis that is currently considered to be RA”(hereinafter referred to as“developing RA”).An expert panel was assembled,comprising12 rheumatologists from Europe and12from North Amer-ica with extensive experience in the diagnosis and man-agement of RA.They provided real-life case scenarios of patients with early undifferentiated inflammatory arthri-tis representing low to high probability of developing RA.A2-day workshop was held in May2009in which domains(factors)and categories within those domains that were important in determining the probability of developing RA were identified.When appropriate, these judgments were informed by the results of Phase1 and other available literature.The relative importance or weights of these domains and their categories were determined by means of decision science theory and conjoint adaptive technology,using the computerized 1000Minds program()in an inter-active and iterative process(26).This analysis permitted the calculation of an individual’s score of the likelihood of developing RA from0to100,where a higher score indicated greater likelihood of RA development.The domains,categories,and weights derived during that initial process are shown in Table2.Objectives,methods,and results of Phase3Objectives of Phase3.In Phase3the working group integrated the findings of the first2phases, refined the scoring system,and determined the optimal cut point to define“definite RA.”The goal of this final phase was to utilize the results of Phases1and2to develop a scoring system that would be applicable to newly presenting patients with undifferentiated inflam-matory arthritis to permit identification of those with a high probability of developing persistent and/or erosive RA.Being intended for use with newly presenting patients,the scoring system should be robust enough that it could be applied repeatedly during the early course of disease,such that a patient identified as not classifiable as having definite RA at initial presentation might be classified as having definite RA at a subsequent time point.The work was not aimed at classifying subjects with established disease,either active or inac-tive.However,the working group recognized that pa-tients may present for the first time with disease that is at a later stage and being treated.Thus,although it was not the explicit charge of the working group to provideTable1.Summary of Phase1results*Variable Comparison Relative weight†Swollen MCP joint Present vs.absent 1.5Swollen PIP joint Present vs.absent 1.5Swollen wrist Present vs.absent 1.6Hand tenderness Present vs.absent 1.8Acute-phase response Low-level abnormal vs.normal 1.2Highly abnormal vs.normal 1.7Serology Low-positive vs.negative 2.2(RF or ACPA)High-positive vs.negative 3.9*MCPϭmetacarpophalangeal;RFϭrheumatoid factor;ACPAϭantiϪcitrullinated protein antibody.†Derived from odds ratios from the multivariate regression model,and interpreted as the increase in the odds of having rheumatoidarthritis(RA)with as opposed to without the respective feature(e.g.,weight of1.5for swelling of proximal interphalangeal[PIP]jointsmeans that the odds of having RA is1.5-fold in patients with asopposed to patients without swelling of a PIP joint).2572ALETAHA ET ALrules for the classification of such patients,it is appro-priate to have a single criteria system that could be applied to all patients;these issues were addressed by the expert panel during Phase3.Determination of the optimal cut point for defi-nite rheumatoid arthritis.Determination of the optimal cut point to classify an individual as having definite RA was achieved using2complementary approaches,mir-roring the approaches used in the first2phases:data-informed and consensus-based.From the consensus-based approach,the expert panel was asked to examine the rankings of case scenarios based on the new scoring system and to indicate,in their opinion,the point at which the cases changed from“probable”to“definite”RA.Four cases were excluded due to missing domain information(nϭ2)or ineligibility(2cases were more likely another diagnosis).For the remaining50cases,the mean cut point defining definite RA was65.7(median66.1;range60.0–70.3)of a total possible score of100.A data-driven verification of that cut point was then attempted,in which the new scoring system was applied to3of the existing cohorts used for Phase1(the Etude et Suivi des Polyarthrites Indifferenciees Re-centes data set from France,the Norwegian data set,and the Rotterdam Early Arthritis Cohort data set from Rotterdam)(25).These cohorts were chosen based on the completeness of data and the collected variables, enabling calculation of the patients’probability scores at baseline.The disease characteristics of these cohorts were not substantively different from those of the re-maining cohorts(data not shown).The area under the curve(AUC)for the3 receiver operating characteristic(ROC)curves(which plot sensitivity against1Ϫspecificity for the range of scores)indicated good discrimination of those who did versus those who did not receive MTX(or another DMARD/biologic agent)within a year(AUC0.82for Norway,0.66for France,and AUC0.69for Rotterdam; PϽ0.0001for all).The probability scores similarly discriminated between those who fulfilled the1987ACR criteria at12months and those who did not(AUC for the ROC curves0.88[Norway],0.67[France],and0.72 [Rotterdam]).Visual inspection of the diagnostic test parameters associated with curves that used MTX initi-ation as the outcome showed a maximum slope for both the positive and negative likelihood ratios between a score of60/100and70/100,with flattening thereafter(67 in the Norway cohort,66in the French cohort,and66in the Rotterdam cohort).The cut point of60–70that was derived from expert consensus was therefore supported by these data.Given the consistency with the consensus-based approach,and to maximize sensitivity of the criteria,a cut point of60was deemed to be most appropriate.Rationale for the composition and weight of the final criteria.For development of the final criteria set, the results and weights from the comprehensive Phase2 process(26)were used as a starting point.Based onTable2.Summary of Phase2results and subsequent modificationsExact (0Ϫ100)Rescaled(0Ϫ10)Rounded to0.5(0Ϫ10)Joint involvement*1large000Ͼ1Ϫ10large,asymmetric10.2 1.021Ͼ1Ϫ10large,symmetric16.1 1.61 1.51Ϫ3small21.2 2.1224Ϫ10small28.8 2.883Ͼ10,including at least1small joint50.8 5.085Serology†Negative RF andnegative ACPA000Low-positive RF orlow-positive ACPA22.0 2.202High-positive RF orhigh-positive ACPA33.9 3.39 3.5Acute-phase reactants‡Normal CRP andnormal ESR000Abnormal CRP orabnormal ESR5.90.590.5Duration of symptoms§Ͻ6weeks000Ն6weeks9.30.931*Joint involvement refers to any swollen or tender joint on examina-tion.Distal interphalangeal joints,first carpometacarpal joints,andfirst metatarsophalangeal joints are excluded from assessment.Catego-ries of joint distribution are classified according to the location andnumber of the involved joints,with placement into the highest categorypossible based on the pattern of joint involvement.“Large joints”refers to shoulders,elbows,hips,knees,and ankles.“Small joints”refers to the metacarpophalangeal joints,proximal interphalangealjoints,second through fifth metatarsophalangeal joints,thumb inter-phalangeal joints,and wrists.“Symmetric”is defined as bilateralinvolvement of at least1region.In the category“Ͼ10joints,”at least1of the involved joints must be a small joint;the other joints caninclude any combination of large and additional small joints,as well asother joints not specifically listed elsewhere(e.g.,temporomandibular,acromioclavicular,sternoclavicular,etc.).†Negative refers to IU values that are less than or equal to the upperlimit of normal(ULN)for the laboratory and assay;low-positive refersto IU values that are higher than the ULN butՅ3times the ULN forthe laboratory and assay;high-positive refers to IU values that areϾ3times the ULN for the laboratory and assay.Where rheumatoid factor(RF)information is only available as positive or negative,a positiveresult should be scored as low-positive for RF.ACPAϭantiϪcitrullinated protein antibody.‡Normal/abnormal is determined by local laboratory standards.CRPϭC-reactive protein;ESRϭerythrocyte sedimentation rate.§Duration of symptoms refers to patient self-report of the duration ofsigns or symptoms of synovitis(e.g.,pain,swelling,tenderness)ofjoints that are clinically involved at the time of assessment,regardlessof treatment status.ACR/EULAR CLASSIFICATION CRITERIA FOR RA2573these categories and weights,we aimed in the final steps of the project to simplify the criteria in order to ensure that they were user friendly.We used the results of the data-driven Phase1as a guide for these adaptations,and verified at each step that the main properties of the criteria were not altered and that classification of pa-tients remained unchanged.The general steps toward simplification are shownTable3.The2010American College of Rheumatology/European League Against Rheumatism classi-fication criteria for rheumatoid arthritisScoreTarget population(Who should be tested?):Patients who1)have at least1joint with definite clinical synovitis(swelling)*2)with the synovitis not better explained by another disease†Classification criteria for RA(score-based algorithm:add score of categories A–D;a score ofՆ6/10is needed for classification of a patient as having definite RA)‡A.Joint involvement§1large joint¶02Ϫ10large joints11Ϫ3small joints(with or without involvement of large joints)#24Ϫ10small joints(with or without involvement of large joints)3Ͼ10joints(at least1small joint)**5B.Serology(at least1test result is needed for classification)††Negative RF and negative ACPA0Low-positive RF or low-positive ACPA2High-positive RF or high-positive ACPA3C.Acute-phase reactants(at least1test result is needed for classification)‡‡Normal CRP and normal ESR0Abnormal CRP or abnormal ESR1D.Duration of symptoms§§Ͻ6weeks0Ն6weeks1*The criteria are aimed at classification of newly presenting patients.In addition,patients with erosivedisease typical of rheumatoid arthritis(RA)with a history compatible with prior fulfillment of the2010criteria should be classified as having RA.Patients with longstanding disease,including those whosedisease is inactive(with or without treatment)who,based on retrospectively available data,havepreviously fulfilled the2010criteria should be classified as having RA.†Differential diagnoses vary among patients with different presentations,but may include conditions suchas systemic lupus erythematosus,psoriatic arthritis,and gout.If it is unclear about the relevant differentialdiagnoses to consider,an expert rheumatologist should be consulted.‡Although patients with a score ofϽ6/10are not classifiable as having RA,their status can be reassessedand the criteria might be fulfilled cumulatively over time.§Joint involvement refers to any swollen or tender joint on examination,which may be confirmed byimaging evidence of synovitis.Distal interphalangeal joints,first carpometacarpal joints,and firstmetatarsophalangeal joints are excluded from assessment.Categories of joint distribution are classifiedaccording to the location and number of involved joints,with placement into the highest category possiblebased on the pattern of joint involvement.¶“Large joints”refers to shoulders,elbows,hips,knees,and ankles.#“Small joints”refers to the metacarpophalangeal joints,proximal interphalangeal joints,second throughfifth metatarsophalangeal joints,thumb interphalangeal joints,and wrists.**In this category,at least1of the involved joints must be a small joint;the other joints can include anycombination of large and additional small joints,as well as other joints not specifically listed elsewhere(e.g.,temporomandibular,acromioclavicular,sternoclavicular,etc.).††Negative refers to IU values that are less than or equal to the upper limit of normal(ULN)for thelaboratory and assay;low-positive refers to IU values that are higher than the ULN butՅ3times the ULNfor the laboratory and assay;high-positive refers to IU values that areϾ3times the ULN for thelaboratory and assay.Where rheumatoid factor(RF)information is only available as positive or negative,a positive result should be scored as low-positive for RF.ACPAϭantiϪcitrullinated protein antibody.‡‡Normal/abnormal is determined by local laboratory standards.CRPϭC-reactive protein;ESRϭerythrocyte sedimentation rate.§§Duration of symptoms refers to patient self-report of the duration of signs or symptoms of synovitis(e.g.,pain,swelling,tenderness)of joints that are clinically involved at the time of assessment,regardlessof treatment status.2574ALETAHA ET AL。
acreular狼疮性肾炎指南解读

相似。
第15页/共37页
糖皮质激素(GC)
• 诱导缓解推荐静脉冲击(500-1000mg甲强×3天) 是专家意见
• 随后口服0.5-1.0mg/kg/d,再减量至最小有效 剂量
• 因LN和肾外表现不同,无固定的减量方法 • 每月1次甲强冲击+每月1次CYC冲击未达成一致意
但孕妇禁用
第9页/共37页
辅助用药
✓合并高血压患者,血压控制在≤130/80mmHg, 可显著延缓肾脏疾病进展。
✓患者LDL>100mg/dl(2.58mmol/L),应予 他汀类药物治疗
✓注意,GFR<60ml/min或肌酐>133umol/L可 加速动脉粥样硬化。
✓SLE本身是脉粥样硬化的独立危险因素。
第2页/共37页
狼疮性肾炎(LN)定义
✓ 持续蛋白尿>0.5g/24h 或>3+ 尿蛋白/肌酐>0.5 ✓ 细胞管型包括:红细胞、血红蛋白、颗粒型、管型、
混合型
尿沉渣>5个RBC或5个WBC/hpf缺乏感染情况下 或细胞管型仅限于RBC或WBC管型
✓ 最佳标准是肾病理证实免疫复合物介导的肾小球肾炎 符合LN。
Ⅴ型(纯膜性)+肾病范畴的LN治疗
单纯激素,激 素+CsA提到, 但未推荐
Figure 3. Treatment of class V without proliferative changes and with nephrotic range proteinuria (>3g/24h)
第18页/共37页
第14页/共37页
CYC治疗两种方案
类风湿关节炎诊断及评价标准

类风湿关节炎诊断与评价标准一、诊断标准:1. ACR(美国风湿病学会,1987)诊断标准:(1)晨僵,持续至少1小时。
(2)至少三个关节区的关节炎:关节肿痛涉及双侧近端指间关节、掌指关节、腕关节、肘关节、跖趾关节、踝关节、膝关节共14个关节区中至少3个。
(3)手关节炎。
关节肿胀累及近端指间关节,或掌指关节,或腕关节。
(4)对称性关节炎。
同时出现左、右两侧的对称性关节炎(近端指间关节、掌指关节及跖趾关节不要求完全对称)。
(5)皮下结节。
(6)RF阳性(所用方法在正常人的检出率<5%。
(7)手和腕关节X线片显示骨侵蚀或骨质疏松。
注:表中1-4项必须持续超过6周,符合表中7项中至少4项者可诊断为RA 但是,不除外符合标准者合并另一种疾病的可能性。
2. 国内诊断标准(全国中西医结合风湿类疾病学术会议修订,1988):①症状:以小关节为主,多为多发性关节肿痛或小关节对称性肿痛(单发者须认真与其他鉴别,关节症状至少持续6周以上),晨僵。
②体征:受累关节肿胀压痛,活动功能受限,或畸形,或强直,部分病例可有皮下结节。
③实验室检查:RF(类风湿因子)阳性,ESR血沉)多增快。
④X线检查:重点受累关节具有典型类风湿性关节炎X线所见。
对具备上述症状及体征的患者,或兼有RF阳性,或兼有典型X线表现者均可诊断。
并有如下分期:①早期:绝大多数受累关节有肿痛及活动受限,但X线仅显示软组织肿胀及骨质疏松。
②中期:部分受累关节功能活动明显受限,X线片显示关节间隙变窄及不同程度骨质腐蚀。
③晚期:多数受累关节出现各种畸形或强直,活动困难,X线片显示关节严重破坏、脱位或融合。
3. ACR/EULAR美国风湿病学会/欧洲风湿病防治联合会,2010)新标准:冀节受累血淸学【至少需要1采》(0-3^)0RF和ACPA均阴性02-1M大关节1FJF和/缺(:册底漓度阳件2小天廿{椁成车伴太黄节受累)2RF和/或ACPA高橋度處过正常垃3儕以上)阳性34 7叶小黄节佛敢甲侔大黄节曼JS)3冋0牛关节【至少一节小天节哽累)ZA5益性时相反应物(至少需要1条}需裁(0-1^)龊狀持读时间CRPM ES RHjiEB0<6ffl0CRP 或ESRJfl 高11总得分6分以上可确诊RA注:名词解释:①受累关节数:指评价时压痛和肿胀的关节数但不包括DIP、第一腕掌关节、第一跖趾关节;②关节大小的定义:中大关节指肩、肘、膝、髋、踝;小关节指MCP PIP、第一指间关节、跖趾关节2-5及腕;③滴度的定义:高滴度阳性指RF或抗CCP K体中至少1项高于正常上线3倍或以上;低滴度阳性指RF 或抗CCP抗体中至少1项高于正常上线但不超过正常上线3倍.二、评价标准:目前并没有统一的评估方案,因此可以说对疾病的活动性也没有统一的标准来加以衡量,但是总的原则是一致的,即炎症程度、骨侵蚀的加剧和功能的快速减低均提示病情处于活动状态。
2010 acreular 类风湿关节炎分类标准

2010 acreular 类风湿关节炎分类标准
2010年Acreular(亦称为ACR/EULAR)类风湿关节炎分类标准是由美国类风湿病学会(American College of Rheumatology,ACR)和欧洲类风湿病学会(European League Against Rheumatism,EULAR)共同制定的一套用于诊断和分类类风湿关节炎(rheumatoid arthritis,RA)的标准。
这一标准在2010年被提出,旨在将RA患者划分为四个不同的分类。
以下是2010 Acreular类风湿关节炎分类标准所涵盖的四个主要方面:
1. 关节炎的持续时间:根据关节炎症状持续的时间进行评估。
如果症状已持续至少6周,则符合这个条件。
2. 关节受累部位:根据关节受累的部位确定是否满足标准。
关节包括手、腕、肘、肩、髋、膝和踝。
3. 类风湿因子(RF)和抗环瓜氨酸肽(anti-CCP)抗体检测:针对RF和anti-CCP抗体进行血清检测,如果其中至少一个阳性,则符合这个条件。
4. 综合评分:根据以上三个方面的结果,给予不同权重进行计算,以判断是否满足RA的分类标准。
综合评分可用于确定患者是否被归类为无RA、可能有RA或明确有RA。
需要注意的是,这些分类标准仅作为辅助诊断工具,且在临床实践中会结合其他症状、体征和医生的临床判断来综合考虑。
如果你有任何关于自己的健康问题,请咨询专业医生以获取准确的诊断和治疗建议。
ACR及EULAR RA新标准解读
概率,采集相关影响因素并达成共识
必要条件
RA的诊断必须满足至少有一个关节滑膜炎的证
据(临床表现,US或MRI证据),并排除其它疾 病
主要标准
必测CCP和RF
关节受累的多少和分布,关节肿胀和压痛。由
于重叠DIP、第一腕掌关节、第一掌跖关节的 OA除外,计算受累关节个数
滑膜炎持续时间
类风湿 关节炎
人群
类风湿关节炎病程演进
Access To cohorts
大型人群 数据????
抗CCP抗体 初级医疗
临床试 验
YEAR 临床试 验 生物制 剂
生物标记 CCP RF 细胞因子 遗传学 临床数据
数据采集
Emery P. ACR 2009.
RA的早期诊断是否可行?
以下指标对于诊断RA意义很大
HAQ对疾病预后有意义 主因素分析显示 掌指关节肿、腕关节肿、近端指间关节肿 掌指关节和腕关节压痛 异常的CRP和血沉 RF、CCP阳性
结
果
年龄和性别无意义 除MCP和腕关节外,关节受累的对称性无意
义
晨僵对诊断无意义
II期总体目标
针对临床医生如何估计疾病将演变为持续炎 症或侵蚀性关节炎,即极有可能是”RA”的
希望在3个月以内而不是在1年才作出诊断
ACR1987年标准存在的问题
ACR1987年标准是分类标准而非诊断标准 部分患者达到完全缓解但是关节的结构仍在
破坏
目前ACR1987年标准仍然是正在使用的指南
定义类风湿关节炎的表型
临床表现
正常/ 无症状
临床前
可能是 未分化 未分化 关节炎 关节炎
类风湿关节炎分类标准2010

类风湿关节炎分类标准20101. 引言类风湿关节炎(rheumatoid arthritis,RA)是一种常见的自身免疫性疾病,主要影响关节,导致关节炎和关节功能障碍。
为了更好地理解和诊断RA,国际类风湿学会(ACR)和欧洲风湿学会(EULAR)于2010年联合制定了一套RA分类标准。
本文将详细介绍这套分类标准的内容及其在临床实践中的应用。
2. RA分类标准的制定背景2.1 RA的临床表现和诊断难点RA是一种多系统、多器官受累的复杂疾病,其临床表现多样化且缺乏特异性。
早期RA常常被误诊为其他类型的关节炎或其他系统性自身免疫性疾病。
因此,制定一套具有高度敏感性和特异性的分类标准对于早期诊断和治疗至关重要。
2.2 既往版本中存在的问题在此之前,ACR曾于1958年、1987年和1991年分别发布了三个版本的RA分类标准。
然而,这些版本存在着一些问题,如对早期RA的敏感性较低,对于非关节表现的RA的诊断标准不明确等。
因此,制定新版的RA分类标准成为必要。
3. RA分类标准的制定过程3.1 参与制定的专家团队ACR和EULAR联合成立了一个专家团队,由风湿学、流行病学和疾病分类学等领域的专家组成。
他们通过系统性文献回顾、临床经验和多中心临床研究等方式,共同制定了这套新版分类标准。
3.2 标准制定过程在制定过程中,专家团队首先明确了RA诊断和分类的目标,并确定了关键问题。
随后进行了一系列系统性文献回顾和多中心临床研究,并通过德尔菲法进行多轮征求意见。
最终,在广泛讨论和修改后,确定了这套新版RA分类标准。
4. RA分类标准的内容4.1 关节受累特征新版RA分类标准将关节受累特征作为首要因素进行评估。
关节受累特征包括关节肿胀、压痛、早晨僵硬等,其中关节肿胀是关键指标。
根据关节受累的数量和分布,将RA分为单关节受累型、多关节受累型和弥漫型。
4.2 血清标志物血清标志物在RA的诊断和分类中起到了重要的作用。
新版分类标准将类风湿因子(RF)和抗环瓜氨酸肽抗体(anti-CCP)作为血清标志物进行评估。
2010-ACREULAR-类风湿关节炎分类标准

2010 ACR/EULAR 类风湿关节炎分类标准发表时间:2010-09-18 发表者:张昌丽 (访问人次:310)目标人群:有下列表现的患者:1、有至少一个关节具有明确的临床滑膜炎(肿胀)【新标准目的在于给新症患者分类。
另外,具有侵蚀性疾病如类风湿关节炎典型表现,其病史符合2010标准的,应该分类为类风湿关节炎。
患者有长期疾病,包括那些疾病处于非活动期(经过治疗或未经治疗),基于回顾性的可以获得的资料,先前符合2010年标准的,也应分类为类风湿关节炎。
】2、具有滑膜炎,用其他疾病不能得到更好解释的【不同表现的患者鉴别诊断也不同,但是系统性红斑狼疮,银屑病关节炎和痛风应该可以包括在内。
如果不清楚应该考虑哪些相关的鉴别诊断,应该咨询风湿病学专家】RA分类标准(评分算法:A-D的项目评分相加;患者如果按下列标准评分≥6/10,明确诊断为类风湿性关节炎)【虽然患者评分不足6分的不能分类为类风湿关节炎,但是他们的状态可以再次评价,随着时间推移,可能会符合标准。
】A:受累关节【指的是查体时发现的任何肿胀或触痛的关节,可通过滑膜炎的影像学证据证实。
在评估中,远端指间关节,第一腕掌和第一跖趾关节除外。
关节分布的分类根据受累关节的位置和数量,划入最可能受累关节类目。
】- 1个大关节(0分)【大关节指的是肩关节,肘关节,髋关节,膝关节和踝关节】- 2-10大关节(1分)- 1-3小关节(有或没有大关节)(2分)【小关节指的是掌指关节,近端指间关节,2-5跖趾关节,拇指指间关节和腕关节】- 4-10小关节(有或没有大关节)(3分)-超过10个小关节(至少一个小关节)(5分)【在这一条中,至少一个受累关节必须是小关节;其他关节可以包括任何大的或额外的小关节的组合,如其他别处未特别列出的关节(颞颌关节,肩峰锁骨关节,胸锁关节)】B:血清学(至少需要1项结果)【阴性指的是低于或等于当地实验室正常值的上限。
低滴度阳性指的是国际单位值高于正常值上限,但是低于正常值上限3倍。
ACR与EULAR2010年RA新标准解读

Access To cohorts 人群 临床表现
定义类风湿关节炎的表型
正常/ 无症状
临床前
可能是 未分化 未分化 关节炎 关节炎
类风湿 关节炎
类风湿关节炎病程演进
大型人群 抗CCP抗体 数据???? 初级医疗
生物标记 CCP RF 细胞因子 遗传学 临床数据
观察指标:
关节肿(28个关节) 关节压痛(28个关节) 血沉 CRP HAQ CCP RF
结果
寡关节(2-6个关节)肿胀和压痛OR值分别为2.9和2.0 多关节(7-28个关节)肿胀和压痛OR值分别为:5.2和 3.3 C反应蛋白的意义大于血沉 血清学CCP和RF有价值
HAQ对疾病预后有意义
新的诊断措施 1、MRI、超声 2、CCP抗体阳性率达82%,特异性>90%以
及GPI等 3、CRP 早期诊断成为可能!!
×
ACR/EULAR2010年RA新分类标准
关节炎以排除其它疾病为前提 强调CCP抗体和RF,增加急性相反应物 保留受累关节数和滑膜炎持续时间 废除晨僵、皮下结节、对称性关节炎三个
ACR1987年标准的特异性和敏感性
观察结果:
98例确诊为RA,其中初次符合ACR1987年标准的有65例 (66%)
非RA的31/172(18%)达到了ACR1987年标准 2年后有85例符合ACR1987年标准,敏感性为87% 2年后172例非RA中有43例(25%)达到2年后标准,特异性
EULAR2009
专家共识
有滑膜炎表现并且有放射影像学骨侵蚀证 据者应视为RA
专家推荐
以下两种病人应分类为RA: 符合两项强制标准的病人(至少一个关节
2010+ACR+EULAR+类风湿关节炎分类标准

Phase 2 Consensus process
Determinants of high probability of RA
Phase 3 Integration of 1 and 2
Increase feasibility
Final Criteria
5 (0.99)
.094 .117 .004 -.042 .062 .526 .149 .852 .055 .022 .384 .108 .710 .001 .163 .194
6 (0.94)
.878 .878 .055 .121 -.074 .125 .158 .176 .102 .127 .047 .094 .098 .048 .062 -.037
Phase 1
Data Driven Approach
Phase 1: Patients and Methods
• Patients – EARLY ARTHRITIS COHORTS
– 3115 patients from 9 cohorts – Inflammatory arthritis (no other definite diagnosis) of <3 years – No previous DMARD/MTX treatment
Phase 1 data
+
Positive factors
-
Negative factors
Specify target population Identifying domains and categories
Phase 2: Overview
Loadings:
acr标准和eular标准

acr标准和eular标准ACR标准和Euler标准是两种常用的计算机辅助工程(CAE)软件中的数学方法和计算模型。
本文将介绍ACR标准和Euler标准的基本概念、应用领域和差异,并以此为基础分析两种方法的优缺点与适用场景。
一、ACR标准ACR标准是一种基于流体动力学的计算方法,主要应用于CFD(计算流体动力学)领域。
它是由流体动力学原理推导出的一套数学模型和精确解算方法,可以用于模拟流体的流动、传热、传质等现象。
ACR标准的核心概念是控制方程,即动量守恒定律、质量守恒定律和能量守恒定律。
通过对这些方程进行离散化和数值解算,可以得到流体的速度、温度、压力等场变量的数值解。
ACR标准的计算结果可以为工程设计提供重要的参考数据,如汽车的气动性能、航空器的气动布局等。
二、Euler标准Euler标准是一种计算流体力学(CFM)方法,主要应用于模拟流体的流动行为和流动特性。
它是基于欧拉方程组及其所得的数学模型,通过数值解算来探索流体运动的规律和特性。
Euler方程组是由质量守恒、动量守恒和能量守恒三个基本方程组成的非线性偏微分方程组。
这些方程描述了流体在流动过程中的力学行为,通过对这些方程进行离散化和数值解算,可以得到流体的速度、压力、密度等场变量的数值解。
Euler标准的计算结果可以为工程设计提供重要的流动参数,如空气动力学特性、流体声学行为等。
三、ACR标准和Euler标准的应用领域ACR标准主要应用于流体力学领域,如航空航天工程、汽车工程、石化工程等。
它可以帮助工程师分析和优化流体的流动特性,从而改善产品的性能和安全性。
例如,在航空航天工程中,可以使用ACR标准来模拟飞机的空气动力学特性,以便优化飞机的设计和改进其飞行性能。
Euler标准主要应用于计算流体力学领域,如风力发电、水利工程、环境工程等。
它可以帮助工程师了解流体在不同条件下的流动规律,优化工程设计和改善工程施工质量。
例如,在风力发电领域,可以使用Euler标准来模拟风机的叶轮及整机的流动特性,以评估风机的性能和优化装配方式。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ACR1987年标准的特异性和敏感性
观察结果:
98例确诊为RA,其中初次符合ACR1987年标准的有65例 (66%)
非RA的31/172(18%)达到了ACR1987年标准 2年后有85例符合ACR1987年标准,敏感性为87% 2年后172例非RA中有43例(25%)达到2年后标准,特异性
EULAR2009
专家共识
有滑膜炎表现并且有放射影像学骨侵蚀证 据者应视为RA
1990年在英国Norfolk的Norwich 卫生专署机构 调查了该地RA的患病率。结果显示,第5年符合 ACR标准的患病率与第1年相比,女性高45%,男 性高36%。这说明ACR1987年的标准对于1年内发 病的关节炎敏感性不够理想。
Harrison et al.J Rheum 1998;25: 2324-30
Emery P. ACR 2009.
数据采集
RA的早期诊断是否可行?
以下指标对于诊断RA意义很大
掌指、跖趾关节受累或3个 以上关节受累
晨僵大于30分钟 掌指关节握挤(Squeeze
Test)试验阳性
早期治疗能明显的提高疗效,改善预后
1、新的传统DMARDs,如来氟米特、米诺霉 素 2、生物制剂,特别是TNF-a抑制剂
Analytical approach Comparing disease in patients
1987年ACR分类标准产生的过程
由9个亚委员会委员和另外32个来自美国大 学或私人诊所的风湿病学专家对262例RA和 等量其它疾病的对照组进行平均7年的研究
ACR1987年RA分类标准
1、晨僵: 至少1小时 (≥6周) 2、多关节炎: 14个关节区中≥3个同时肿胀或积液 (≥6周) 3、手关节炎: 腕或掌指或近端指间关节肿胀(≥6周) 4、对称性关节炎(≥6周) 5、皮下结节: 6、X线:手和腕关节的X线改变 7、类风湿因子: 类风湿因子阳性(滴度正常人阳性率 <5%) *符合≥4条, 可诊断为RA
观察指标:
关节肿(28个关节) 关节压痛(28个关节) 血沉 CRP HAQ CCP RF
结果
寡关节(2-6个关节)肿胀和压痛OR值分别为2.9和2.0 多关节(7-28个关节)肿胀和压痛OR值分别为:5.2和 3.3 C反应蛋白的意义大于血沉 血清学CCP和RF有价值
Acces s To cohorts 人群 临床表现
定义类风湿关节炎的表型
正常/ 无症状
临床前
可能是 未分化 未分化 关节炎 关节炎
类风湿 关节炎
类风湿关节炎病程演进
大型人群 抗CCP抗体 数据???? 初级医疗
生物标记 CCP RF 细胞因子 遗传学 临床数据
临床试 验
YEAR 临床试 验
生物制 剂
必要条件
RA的诊断必须满足至少有一个关节滑膜炎的证 据(临床表现,US或MRI证据),并排除其它疾 病
主要标准
必测CCP和RF 关节受累的多少和分布,关节肿胀和压痛。由
于重叠DIP、第一腕掌关节、第一掌跖关节的 OA除外,计算受累关节个数 滑膜炎持续时间 血沉和CRP 通过电脑系统进行分析, 目的是足够早的作出 诊断
为75%
ACR1987年标准的特异性和敏感性
原因:
ACR1987年标准中没有排除标准 对于病史小于1年的关节炎患者,此标准无
法预测是否会发展为RA 有些病人初次就诊符合标准,但2年后证实
并非RA
1987年ACR标准的敏感性较好(77-87%),但是在 预测骨侵蚀的特异性方面很差。
HAQ对疾病预后有意义
主因素分析显示
掌指关节肿、腕关节肿、近端指间关节肿
掌指关节和腕关节压痛
异常的CRP和血沉
RF、CCP阳性
结果
年龄和性别无意义 除MCP和腕关节外,关节受累的对称性无意 义 晨僵对诊断无意义
II期总体目标
针对临床医生如何估计疾病将演变为持续炎 症或侵蚀性关节炎,即极有可能是”RA”的 概率,采集相关影响因素并达成共识
ACR/EULAR 的是……
早期RA
? 未分化关节炎(UA)
炎性关节炎(IA)
RA
特发性关节炎
……
RA分类标准的发展
1956/8 1961 1966
1987
ARA
(美国风湿病 学会)
罗马 标准
From consensus
纽约 标准
纽约修订 标准
关节炎﹤12周者只有51%满足1987年标准
许多病史大于12周甚至1年才能符合1987年标 准
希望在3个月以内而不是在1年才作出诊断
ACR1987年标准存在的问题
ACR1987年标准是分类标准而非诊断标准 部分患者达到完全缓解但是关节的结构仍在
破坏 目前ACR1987年标准仍然是正在使用的指南
ACR1987年标准的特异性和敏感性
观察方法: 前瞻性研究 1995-1997年,法国Brittany等七所医院 对病史小于1年的272例新发关节炎,每6个月观察一次 在第2年末时评价1987年分类标准的准确性 在第一次和最后一次随访时,5位专家做出诊断
Saraux et al. Arth Rheum 2001; 44: 2485-91
条目
Ⅰ期总体目标
从表现为未分化炎性关节炎的病人中, 发现那些
将发展为慢性和/或关节破坏的高风险病人 需要迅速启用DMARDs的病人
找出阐释“类风湿关节炎”疾病的所
有概念和特征
Ⅰ期研究
参加单位:阿姆斯特丹等9个团队 录入病例:3115例 入组条件:
炎性关节炎 病史少于3年 此前未用过DMARDs或MTX治疗 2000年以后的病人 关节炎没有其它确定诊断
新的诊断措施 1、MRI、超声 2、CCP抗体阳性率达82%,特异性>90%以
及GPI等 3、CRP 早期诊断成为可能!!
×
ACR/EULAR2010年RA新分类标准
关节炎以排除其它疾病为前提 强调CCP抗体和RF,增加急性相反应物 保留受累关节数和滑膜炎持续时间 废除晨僵、皮下结节、对称性关节炎三个
