胃癌术后输血与预后
胃癌的病理分期与预后评估指标

胃癌的病理分期与预后评估指标胃癌是一种常见的消化系统恶性肿瘤,它的病理分期和预后评估是临床治疗和预后判断的重要依据。
本文将介绍胃癌病理分期的相关指标以及与之关联的预后评估指标。
一、胃癌病理分期胃癌病理分期是根据肿瘤的深度侵犯、淋巴结转移情况和远处转移情况进行划分。
根据国际癌症分期联盟(UICC)和美国癌症学会(AJCC)的TNM分期分类系统,我们可以将胃癌分为以下几个阶段。
1. T分期:T分期是根据原发肿瘤的深度和侵犯范围进行评估的,主要包括以下几类。
- T1期:肿瘤侵犯黏膜层和黏膜下层。
- T2期:肿瘤侵犯肌层。
- T3期:肿瘤侵犯浆膜层。
- T4期:肿瘤直接侵犯周围器官。
2. N分期:N分期是根据淋巴结转移的情况进行评估的。
- N0期:无淋巴结转移。
- N1期:转移至胃癌区域的1-2个淋巴结。
- N2期:转移至胃癌区域的3-6个淋巴结。
- N3期:转移至远离胃的远处淋巴结或大量转移至胃癌区域的淋巴结。
3. M分期:M分期是根据远处转移的情况进行评估的。
- M0期:无远处转移。
- M1期:有远处器官的转移,如肝脏、肺部等。
根据T、N、M三个指标的不同组合,可以确定胃癌的具体分期。
二、预后评估指标胃癌的预后评估指标是评估患者术后生存期和复发情况的依据。
以下是一些常用的预后评估指标。
1. 淋巴结转移:淋巴结转移是影响胃癌预后的重要因素。
病理分期中N分期的不同组合可以反映淋巴结转移的程度,一般来说,淋巴结转移越少,预后越好。
2. 肿瘤大小:肿瘤大小与患者的预后有关,一般来说,肿瘤越小,预后越好。
3. 侵犯范围:肿瘤侵犯范围也是预后的重要指标之一。
T分期中,属于T1和T2期的预后较好,而属于T3和T4期的预后较差。
4. 分子标志物:某些分子标志物的过度表达或缺失也与胃癌的预后相关。
例如,HER2阳性与预后较差有关,而CDH1(E-钙粘蛋白)的突变则与预后较好有关。
5. 年龄与整体健康状况:年龄和整体健康状况也会对胃癌预后产生影响,年轻且身体状况良好的患者,通常有更好的预后。
119例胃癌术后预后的多因素分析

关键词 :胃癌 ; 响因素 ;C x回归分析 ; 影 o 免疫组织化学 中图分类号 : 3 . R752 文献标志码 : A 文章编号 : 6 2 3 3 2 1 )4—0 4 1 7 —2 5 (0 12 10—0 3
随访 时 间 5 9个 月 , ~8 中位 随访 时 间为 4 6个 月 , 平 均随访 时 间为 4 . 。 7 7月
22 影 响 胃癌预后 的单 因素分析 .
单 因素分 析表 明 : 最 大直径 、 肿瘤 病理 组织 分 级 、 or n 分 型 、 Br ma n TNM 分期 、 瘤 的浸润 深度 、 肿 手 术方 式 、 术根 治 度 、 巴结 转 移情 况 、 无 远 手 淋 有
1 资料 与方法
20 年 0 02 1月至 20 04年 1 2月期 间 经病 理 确 诊, 并在 盐城市 第 一 人 民医 院进 行 手术 治 疗 的有
完整病历资料 、 随访资料和术后病理资料的 19 1 例病例 , 访 时 间均 在 6 随 0个 月 以 上 。本 组 资 料
中: 7 男 3例 , 4 女 6例 。年 龄分 布在 3 ~8 2 8岁 , 肿
影响胃癌 的预后的因素可以分为临床的 , 组
MMP一3 MMP一9 V】 、 、 GF和 Ki 7多 克 隆抗 一6
织病理的及细胞分子生物学等多方面。近年部分
生物标志物在肿瘤的诊断 、 治疗及预测复发等方 面发挥了重要作用 , 本研究应用免疫组织化学方 法检 测 MMP一3 MMP一9 VE F和 Ki 7蛋 、 、 G 一6 白在 胃癌组织 中的表达 , 为判断 胃癌预后提供客
6 个淋巴结转移的 3 8例 , ~1 7 5个淋巴结转移 2 例 ,1 以上 淋 巴结转 移 7例 。T 5 5个 NM 分 期 : Ia 1 期 3例 , 期 1 b 9例 , Ⅱ期 2 3例 , 期 1 ma 9例 ,
输血指征及标准

输血指征及标准输血是现代医学中常见的治疗方法之一,当人体失血或因其他原因造成贫血时,输血可以及时地提供氧气和营养物质,帮助患者恢复健康。
然而,对于不同的病人,输血指征和标准并不相同。
本篇文章将从以下几个方面分析输血的指征和标准:一、输血的指征1.急性及慢性失血:在手术和创伤中,可能会导致大量的失血,导致患者出现失血性休克等紧急情况。
在这些情况下,输血是必要的。
2.贫血:对于严重贫血的病人,无论贫血的原因是由于营养不良还是由于其他疾病引起的,输血都有可能是必要的。
3.手术前后:在进行某些大型手术时,可能需要提前为患者进行输血,以确保手术过程中患者的生命安全。
手术后,如果患者失血过多,也需要进行输血。
4.器官移植:在进行某些器官移植手术时,由于手术本身的复杂性,患者有可能需要进行输血来维持身体机能。
二、输血的标准1.血红蛋白水平:通常,若患者的血红蛋白水平低于70-80g/L,就需要进行输血。
对于某些特殊情况,如急性失血性休克时,此标准可以适当调低。
2.血容量:血容量指血液循环中血液的总量,包括血红细胞、血小板、血浆等,通常情况下,血容量低于体积的1/3需要进行输血。
3.病情及病人的身体状况:除了血红蛋白水平和血容量外,还需考虑患者的年龄、体重、病情及病人的身体状况等多种因素。
例如,老年人和儿童需要进行输血的标准可能略有不同。
总之,输血是一项非常严谨的医疗行为,需要在鉴别诊断后才能进行。
同时,为了减少输血的风险和副作用,在进行输血前需要进行全面的评估,并严格遵守输血的指征和标准,从而确保患者的生命安全和康复。
胃肠外科手术中的贫血管理与输血策略

胃肠外科手术中的贫血管理与输血策略贫血是临床上常见的一个病症,它指的是血液中的红细胞数量或血红蛋白浓度低于正常范围。
在胃肠外科手术中,患者可能会出现不同程度的贫血,因此贫血管理和输血策略显得尤为重要。
本文将从贫血的定义和分类、贫血在胃肠外科手术中的原因和影响、贫血管理的基本原则和输血策略等方面进行论述,以期为胃肠外科手术中的贫血管理提供一定的参考。
一、贫血的定义和分类贫血是指血液中的红细胞数量不足或者血红蛋白浓度低于正常范围所导致的病症。
根据红细胞指标,贫血分为以下几类:①红细胞数目减少型贫血,即缺铁性贫血、再生障碍性贫血等;②红细胞体积减小型贫血,如碳酸氢盐积累症;③红细胞色素含量减少型贫血,如营养性贫血。
二、胃肠外科手术中贫血的原因和影响胃肠外科手术中,贫血的发生原因主要包括手术相关因素、术前贫血和术中出血等。
首先,手术相关因素如开腹、肿瘤切除等会导致术中的血液流失增加,从而容易造成贫血。
其次,术前贫血是指患者在手术前已经存在的贫血,可能是由于慢性失血、营养不良等原因造成的。
最后,术中出血是最主要的原因之一,特别是对于一些大型切除手术,如胃癌根治术、结直肠癌根治术等,术中是不可避免地会出现大量的出血。
贫血对于胃肠外科手术的影响主要表现在以下几个方面。
首先,贫血会导致组织供氧不足,使手术后恢复的时间延长,容易导致术后并发症的发生。
其次,贫血还会影响免疫功能,增加感染的风险。
再次,贫血还会对心肺功能产生影响,特别是在术后对机械通气的患者中,贫血会加重肺功能不全。
最后,贫血还会延长住院时间和增加医疗费用。
三、贫血管理的基本原则在胃肠外科手术中,贫血的管理应根据患者的具体情况制定个体化的治疗方案。
具体的管理原则如下:1. 术前评估:术前应对患者的贫血情况进行评估,包括贫血的原因、程度和可能产生的并发症等。
同时,需评估患者的心肺功能等其他指标,以确定患者的手术风险。
2. 术中积极控制出血:在手术过程中,应积极控制出血,减少术中的血液损失。
胃癌根治术后常见并发症的护理

胃癌根治术后常见并发症的护理【摘要】胃癌根治术是治疗胃癌最有效的主要措施,但其术后并发症的发生,直接影响到患者术后愈合。
故术后严密的观察和规范精心的护理,可以大大减少或避免术后并发症的发生,从而提高胃癌根治术的成功率。
【关键词】胃癌根治术后;并发症;护理胃癌是我国常见的恶性肿瘤之一,发病率高,死亡率居全部恶性肿瘤首位。
胃癌是一个严重危害我国人民健康的常见病,应引起重视。
胃癌根治术是外科治疗胃癌的主要措施之一,由于创伤大,时间长,术后易发生并发症,影响到患者的愈后,甚至危及生命,因此术后并发症的预防和护理质量对患者的恢复是关键环节之一[1]。
胃癌根治术后的早期并发症主要包括:术后出血、感染、吻合口瘘和梗阻等。
1 术后出血的观察和护理1.1 严密观察生命体征的变化,包括血压、脉搏、心率和呼吸、神志和体温的变化。
1.2 禁食和胃肠减压保持胃管通畅,持续胃肠减压。
术后妥善固定胃管,避免胃管受压、扭曲、或堵塞,定时挤压胃管,保持胃管通畅,注意观察胃液的颜色、性质和量。
一旦胃管发生堵塞,及时报告医生,必要时,遵医嘱用生理盐水低压冲洗胃管。
一般术后24 h内胃管内引流出咖啡色液100~300 ml,第3~4天逐渐减少,如发现在短时间内从胃管不断引流出新鲜血液,甚至出现呕血和黑便,提示术后出血,一般术后胃出血多为吻合口出血,多为术中止血不彻底,及时报告医生处理。
术后4~6 d发生的出血,常为吻合口黏膜坏死脱落所致;术后10~20 d发生的出血,与吻合口缝线处感染、腐蚀血管有关。
1.3 接通腹腔引流管,妥善固定,定时挤压,保持通畅,避免受压、扭曲或抻出。
两根以上引流管,要用标识标明,注意观察引流液的颜色性质和量。
若术后持续从腹腔引流管引出大量新鲜血性液体,应怀疑有腹腔出血,要及时报告医生处理。
1.4 一旦发生出血,应遵医嘱给予止血药物应用,并积极做好配血输血准备等。
并积极准备好急救物品和药品,做好术前准备工作。
2 感染2.1 预防术后肺部感染和肺不张2.1.1 护士在术前应做好相应的术前指导工作。
输血在肿瘤外科手术中应用的相关问题研究进展

体输血可加速肿瘤 生长 , 使肿瘤患者预后变差 。贾文焯等 [] 9 对接 种肉瘤细胞的大 鼠行手 术 , 分别行 同种异系不 同成分输 血及 同种 同系输血 , 以输生理盐水组为 阴性对照组 。结果术
中输入 同种异系大 鼠全血及 白细胞 组大 鼠的肿瘤生长速度 明
起的不 良后果往往没有得 到重视 。而 自体输 血早在 1 8 8 6年
1 对肿瘤 患者预后 的影响 . 2
异体输 血除 了有上述 的并发
首次被报道 ,后来 在人们 的不断研究和技术发展下 , 到了 得 广泛地应用。本文对输血在肿瘤外科手术 中相关问题的研究 进展综述如下 。
症 ,更严重 的是异 体输血可导致肿 瘤患者预后 变差 。oia j m 等 … 研究 了 8 6例行根 治性 胃切 除术患者异体 输j 与长期 5 血 l 预后的关 系 , 现异体输 血是独 立的预后 不 良因素 , 发 且在 Ⅱ
免疫功 能抑制 , 不利于患者预后 。王小军等 _ ] 1研究也发现异 3
和异体输血对照组 , 发现稀释组患者术 中输入 的异体 血量 明
显减少 , 血流 动力 学维持稳定 。以上 的研究都充分说 明了贮 存式 自体输血 和稀释式 自体输血应用于肿瘤 患者 ,对患者的 免疫功能 、 预后情 况 、 环稳定 等临床状况没有 明显 的影 响。 循 这是异体输血所不能及 的。 22 回收式 自体 输血应 用效果 . 那 么 回收式 自体输 血在肿
瘤外科手术 中应用 如何 ?对于肿瘤手术 , 由于惧怕肿瘤细胞
污染 血液致肿瘤 细胞转 移至其他部位 , 一直是 自体血液 回收
的禁忌症 。如何对这部分患者实施血液 回收成为现实 ,国 内
去白细胞输血和未去白细胞输血的临床效果比较

去白细胞输血和未去白细胞输血的临床效果比较【摘要】目的观察比较去白细胞输血和未去白细胞输血的临床效果。
方法选取我院2015年1月-2016年12月收治的124例恶性肿瘤患者作为本次的研究对象,随机分为去白细胞组和未去白细胞组,每组62例。
未去白细胞组采用未去白细胞输血治疗,去白细胞组采用去白细胞输血治疗,比较两组患者的输血不良反应发生率、术后感染发生率以及转移复发率。
结果去白细胞组的输血不良反应发生率、术后感染发生率以及转移复发率分别为4.84%、6.45%以及24.19%,均明显低于未去白细胞组的20.97%、19.35%以及24.19%,差异有统计学意义(P<0.05)。
结论采用去白细胞输血能够有效的降低恶性肿瘤患者的输血不良反应、术后感染以及转移复发,具有在临床上进行大力推广应用的价值。
【关键词】去白细胞输血;未去白细胞输血;临床效果【中图分类号】R973+.4【文献标识码】A【文章编号】1276-7808(2017)03-055-01输血是临床上治疗危急重症患者的常用方法,安全、有效的输血治疗可以挽救患者的生命。
但是,随着近年来输血治疗不良反应发生率的不断上升,也给患者的治疗带来了严重的影响。
恶性肿瘤患者由于自身的免疫能力比较差,输血可能会加重病情,从而导致肿瘤转移和复发[1]。
研究发现,输血不良反应的发生机制与血液中的白细胞存在密切关系,白细胞可在血液中产生大量细胞因子,异体输血治疗后,可与患者自体血发生反应,释放炎性因子,导致患者发生输血不良反应,因此建议对患者实施去白细胞输血治疗[2]。
本研究比较了去白细胞输血和未去白细胞输血的临床疗效,现报告如下。
1 资料与方法1.1一般资料选取我院2015年1月-2016年12月收治的124例恶性肿瘤患者作为本次的研究对象,随机分为去白细胞组和未去白细胞组,每组62例。
其中,去白细胞组男性患者35例,女性患者27例,年龄17-75岁,平均年龄为(58.6±12.7)岁;其中肺癌18例,胃癌17例,肝癌10例,乳腺癌8例,直肠癌5例,其他4例。
胃癌护理常规

胃癌护理【观察要点】1、病人的心理状况。
2、病人的生命征。
3、疼痛的部位、性质、程度。
4、病人对手术的耐受力如营养状态、有无并发病及纠正情况。
5、观察术后伤口敷料和引流管引流情况。
6、并发症的观察:出血、感染、十二指肠残端破裂、吻合口瘘、梗阻、倾倒综合症、低血糖反应等。
【护理措施】术前护理1、心理护理:鼓励患者树立战胜疾病的信心,对晚期癌肿患者,尽量设法减轻疾病的痛苦。
2、饮食:给予高蛋白、高热量、高维生素易消化的饮食,术前1天进流质,术前12小时禁食、6小时禁饮。
营养状况较差者,如贫血、低蛋白血症,术前应予以纠正,必要时做好输血的各项准备工作,以提高病人手术耐受力。
3、幽门梗阻者,术前3日进行持续胃肠减压及用生理盐水洗胃,使胃体积缩小。
4、肠道准备:包括术前口服肠道不吸收抗菌素和清洁灌肠等,以减少术中污染机会。
5、手术日晨放置胃管及尿管。
术后护理1、严密观察生命征变化。
2、术后体位:全身麻醉清醒后生命征平稳后6—8小时应采用半卧位或低坡半卧位。
3、预防肺部并发症:鼓励深呼吸、有效咳嗽、咳痰,定时翻身拍背,必要时给予超声雾化。
4、输液:禁食期间应静脉补充液体,每日输量应在24小时内比较均匀输入,必要时给予血浆、全血等营养支持治疗,改善营养状况促进吻合口及切口愈合。
5、术后饮食:禁食,持续胃肠减压,术后肠蠕动恢复,肛门排气后,可拔除胃管,拔管后当日可少量(20ml左右)饮水或进米汤;如无不适,第二天进半量流质饮食,每次50—80ml,第三天进全量流质,每次100—150ml,进食后无不适可进半流质饮食,食物宜温、软、易消化、少量多餐,开始时每日5—6餐,以后逐渐减少进餐次数并增加每次进餐量,最后逐步恢复正常饮食,全胃切除术者饮食时间应适当延长。
6、鼓励患者早期活动,除年老体弱或病情较重者,术后第1天坐起轻微活动,第2天协助患者下床,进行床边活动,第3天可在病室内活动,第5天可到室外活动。
7、并发症的观察和护理(1)出血:多发生在术后48小时内,表现为短期内从胃管内流出大量鲜血,甚至呕血或黑便,持续不止者可趋向休克。
胃癌围手术期输血与预后关系的探讨
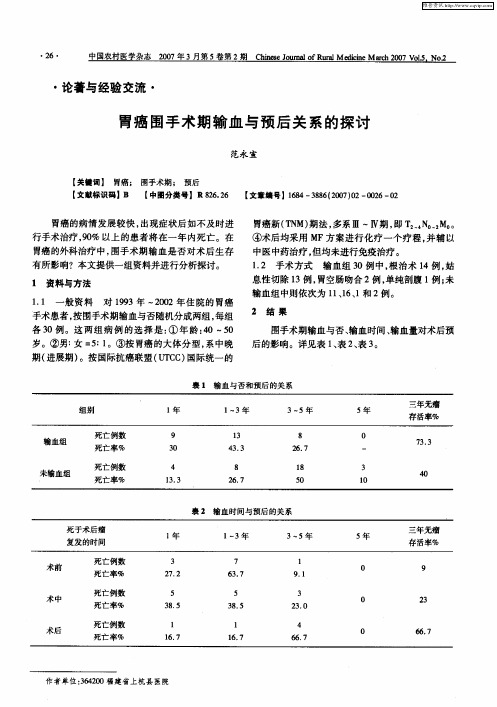
与术后输血在 5年生存率方 面, 并无很大 的差 异。 但在 E ni 的研究 材料 中, ac s 则认 为术 中输血 比术 前、 术后输血具有更大的危险性。本组资料中, 输血
在术前 l 例 、 中 1 、 1 术 3例 术后 6例 , 术后 3年 的无 输血较易促进癌的复发转移 , 缩短术后的生存期限。 Cra o n曾研究指出: m 围手术期输血量是 与术后
胃癌新( N 期法 , T M) 多系 Ⅲ ~Ⅳ期 , T N M 。 即 :. 。: 。
④术后均采用 MF方案进行 化疗 一个疗程。 并辅以 中医中药治疗 , 但均未进行免疫治疗 。 12 手术 方式 . 输血组 3 O例 中, 根治术 1 , 4例 姑
胃癌 的外科治疗中, 围手术期输血是否对术后生存 有所影响?本文提供一组资料并进行分析探讨。
33 对“ . 随意输血” 的商榷 部分癌症病者或其亲 友受既往陈旧观点 的影响, 为输血能补给人体必 认 需的营养物质 , 增强抗病能力 , 从而延长其有限的生
肾、 乳腺等部位的各类癌症也得出类似的结论 , 认为
围手术期输血将使癌症的术后复发率增高或远期生
存率下降。本文资料在诸如年龄 、 性别 、 肿瘤分期 、
这将是 “ 福兮 祸所伏” 终归带来 , 瘤生存率依次为9 、3 和5 .% , % 2% 67 似能提示术前 尤为多见。其实,
的预后呈负相关 的。由于癌症病者常伴有不同程度
的贫血 , 在外科 治疗 中, 围手术期输血是 难 以避免 的。在本文输血组 3 0例中, 输血量的多少 , 除依据 度等指征外 , 有些是单纯为了满足病者及其亲友 的 要求而输 的。因此 , 有少数患者曾多次输血 , 中输 其
异性免疫抑制 , 导致肿瘤生长和复发。以后 , 、 肺 胃、
围手术期输血对胃癌患者预后的影响

s e t ey r ve d a d c u tr d it w r u s i r w t o tbo d t n f so . Unv r t a d mu t a a e a ay e ft e ic — p ci l e iwe n l s e no t o go p :w t o i u lo r su in v e h h a iai e n l v r t n ls so h n i a i i
显 示年 龄 、 浸润 深 度 、 巴结 转 移 、N 淋 T M分 期 和 围手 术 期输 血 均 是 影 响 胃癌 患 者 预 后 的独 立 因素 。 结 论 : 围手 术 期 输 血 是 影 响 胃癌患 者 预 后 的 独 立 因子 , 中对 Ⅱ和 Ⅲ期 胃癌 患 者 的影 响 更 大 , 其 而且 其 影 响 力 度 不 受 肿 瘤 大 小 和 患 者 的整 体 状 况 的 影 响 ,
这为临床上合理输血及适度治疗提供 了一定 的参考。
【 关键词 】 胃癌 ; 围手术期输血 ; 生存分析
中 图分 类 号 :7 05 R 3.1 文献 标 识 码 : A 文章 编 号 :0 9— 4 0 2 0 )8— 79—ቤተ መጻሕፍቲ ባይዱ6 10 06 (0 8 0 00 0
Efe t fpe i pe a i e blod t a su i nso g src c n e f cs o ro r tv o r n f so n a ti a c r
Y.M e ho t ds: x h d e sa d s v ny—i aint Si un r d n e e t sx p t swhoun e we tc r tv a te tmy frg src c n e r m 0 o 2 0 r er — e d r n u aie g sr co o a t a c rfo 2 00 t 0 4 we er to i
胃癌根治术后患者预后的多因素分析

胃癌根治术后患者预后的多因素分析目的:探讨影响胃癌患者根治术预后的因素。
方法:回顾性收集289例行胃癌根治性手术患者的临床和病理资料,随访5年,Cox比例风险回归进行生存分析。
结果:(1)中位生存时间(45.23±7.58)个月,1、3和5年生存率分别是77.98%、53.64%和41.26%。
(2)单因素分析:病理类型、浸润深度、组织分化、TNM分期、淋巴转移、综合治疗等是影响胃癌根治术预后的主要因素(P<0.05);性别、年龄、瘤体部位、瘤体大小、手术方式等与胃癌根治术预后无关(P>0.05)。
(3)多因素分析:淋巴转移(RR=3.284)、组织分化(RR=3.168)、浸润深度(RR=3.174)和TNM分期(RR=3.152)是影响预后的独立因素。
结论:淋巴转移、组织分化、浸润深度和TNM分期是预后的独立危险因素,可为临床判断胃癌预后提供依据。
标签:胃癌;根治术;预后因素;生存分析【Abstract】Objective:To explore the prognostic factors of gastric cancer patients after radical resection. Method:The clinical and pathological and 5 years of follow-up data for 289 patients with gastric cancer who underwent radical resection were retrospectively collected. The prognostic factors of gastric cancer patients after radical resection were analyzed by Cox proportional hazards regression model. Result:(1)The median survival time was (45.23±7.58)months,and the 1,3 and 5 year survival rates were 77.98%,53.64% and 41.26%. (2)Univariate analysis showed that pathological type,depth of invasion,tissue differentiation,TNM stage,lymph node metastasis and comprehensive treatment were the main prognosis factors in these patients (P<0.05). However,gender,age,tumor location,tumor size,surgical methods and prognosis of gastric resection were not significant predictors of the prognosis in these patients (P>0.05). (3)Multivariate analysis showed that lymph node metastasis (RR=3.284),tissue differentiation (RR=3.168),depth of invasion (RR=3.174),and TNM stage (RR=3.152)were independent prognostic factors in these patients. Conclusion:Lymph node metastasis,tissue differentiation,depth of invasion,and TNM stage are independent risk factors,and can be helpful for prognosis of gastric cancer patients who received radical resection.【Key words】Gastric cancer;Radical operation;Prognosis factors;Survival analysis胃癌是最常见的恶性肿瘤之一,多数患者胃癌被确诊时已到中、晚期,其高发病率和病死率严重威胁着人们的身体健康。
肿瘤患者的输血

(二)预防措施
据国外资料报道, 据国外资料报道,择期手术不必要 的输血达25%。健康报 的输血达 。健康报1996年7月2 年 月 日报道, 日报道,我国如果严格控制输血指 临床总用血量可减少将近一半。 征,临床总用血量可减少将近一半。
13
Friedman等对 多万例手术患者的 等对50多万例手术患者的 等对 用血情况进行了回顾性调查, 用血情况进行了回顾性调查,发现 女性患者术中用血量明显高于男性。 女性患者术中用血量明显高于男性。 作者推测女患者用血量较多的原因 是这些患者的红细胞压积( 是这些患者的红细胞压积(HCT) ) 低于某一固定值, 低于某一固定值,而未考虑妇女 HCT的基线本来就低。 的基线本来就低。 的基线本来就低
1981年 Gantt首先提出肿瘤抗原 1981年,Gantt首先提出肿瘤抗原 在许多方面与组织相容性抗原相似, 在许多方面与组织相容性抗原相似, 输血有可能像作用于移植器官那样 作用于肿瘤组织, 作用于肿瘤组织,有利于肿瘤组织 在体内的存活。 在体内的存活。
3
此后, 此后,越来越多的动物实验和临床 研究提示输血有可能影响肿瘤患者 的免疫功能,导致一定程度的免疫 的免疫功能, 抑制, 抑制,机体免疫功能的抑制可能会 促进肿瘤细胞的生长,对预后不利。 促进肿瘤细胞的生长,对预后不利。
19
提倡自体输血 贮存式自体输血 这种输血方式就 是把自己本身的血液预先贮存起来, 是把自己本身的血液预先贮存起来, 以备将来自己需要时应用。 以备将来自己需要时应用。主要适 用于稀有血型或曾经配血发生困难 以及曾有严重输血不良反应的肿瘤 患者。 患者。
20
某些肿瘤或恶性血液病患者, 某些肿瘤或恶性血液病患者,也可 在化疗或放疗后的缓解期预存自体 血液成分(如冰冻红细胞、 血液成分(如冰冻红细胞、冰冻血 小板),再次化疗或放疗时回输。 ),再次化疗或放疗时回输 小板),再次化疗或放疗时回输。
胃癌手术患者术前贫血的情况及其与术后并发症的关系

181中西医结合心血管病电子杂志Cardiovascular Disease Electronic Journal of integratedtraditional Chinese and Western Medicine2019 年 9月 B 第 7 卷第 26期Sept. B 2019 V ol. 7 No. 26胃癌手术患者术前贫血的情况及其与术后并发症的关系陆烟生(无锡市锡山人民医院,江苏 无锡 214000)【摘要】目的 探讨胃癌手术患者术前贫血的情况及其与术后并发症的关系进行分析。
方法 选取了2016年~2018年收治的患者80例,分为贫血组38例和非贫血组42例,作为此次观察对象。
结果 在术中贫血组患者输血的有4例10.53%,术后输血的有8例18.42%均高于非贫血组的2.38%、4.76%,P <0.05;住院时间对比,贫血组患者均比非贫血组患者的时间要长,P <0.05;在术后并发症情况对比,贫血组患者在术后发生伤口感染、呼吸系统感染、心血管疾病等并发症情况明显高于非贫血组的患者,P <0.05,呼吸系统受到感染的情况较多。
结论 贫血组患者的住院时间长以及术后产生并发症情况较多。
【关键词】胃癌手术;术前贫血;术后并发症;关系【中图分类号】R735.2 【文献标识码】A 【文章编号】ISSN.2095.6681.2019.26.181.02贫血是比较常见的问题,贫血会引起组织、器官长期供血不足影响各组织器官的功能,可能造成严重后果。
特别是患者在围手术其时出现贫血,会直接导致影响患者在手术后出现并发症。
胃癌手术的患者在手术后胃酸减少,不利于食物游离和吸收,血红蛋白下降,影响患者的康复[1]。
而在术前患者输血本身就及其的危险,因此要及时在患者术前纠正其贫血,避免在术后产生严重的并发症。
本文叙述了2016年~2018年收治的胃肠手术患者80例,探讨胃癌手术患者术前贫血的情况及其与术后并发症的关系进行分析,现分析如下。
癌症病人应合理输血

癌症病人的输血
输血促使癌症复发?
输血后受血者的血液中,淋巴细胞数量下降,功 能显著抑制,自然杀伤细胞活性降低。两种免疫细 胞的数量以及功能的下降,致使受血者的免疫功能 降低,使残余的癌细胞迅速生长和转移,增加了癌 症的复发率。
癌症病人应合理输血
尽管输血可以抑制癌症病人的免疫功能,增加癌 症的复发率,但是对于围手术期的癌症病人而言, 失血、输血在很多情况下是不可避免的。 围手术期癌症病人的输血不能绝对禁止,但应持 谨慎、保守的态度,尽可能减少不必要的输血,如 果确有输血指征,应大力推广成分输血和自体输血。
癌症病人手术中输血
当失血量达全身血容量的80%或以上时,除输 注上述各种制剂外,还需根据病情补充血小板或血 浆成分。 与此同时,癌症病人术中失血亦可采取稀释式自 体输血,即对于术前血红蛋白大于或等于120g/L 的病人,在手术前麻醉前后,先采1500~2000mL, 用血液保存液常温保存待用;随即给病人输注等量 的胶体溶液,并在术中补充晶体液和胶体液,待手 术完成后再将保存的自身血液回输给病人。 该方法在临床实验中证明安全有效,但贫血、心 肺功能差或肝病是稀释式自体输血的禁忌症。
癌症病人手术前输血
对于年轻、肿瘤分化好、早期癌症的低危病人, 血红蛋白应维持在70~90g/L为宜; 对于高龄、肿瘤分化差、晚期癌症的病人,血红 蛋白应维持在100~120g/L为宜; 尽可能输注少含或不含白细胞和血浆的浓缩红细 胞或洗涤红细胞。
癌症病人手术输血
当术中失血量达全身血容量的20%时,需要补 充血容量以防休克。这时首先考虑输注晶体液和胶 体液; 当失血量达全身血容量的30%时,除及时采取 上述措施外,还应输注浓缩红细胞或洗涤红细胞; 如果失血量达全身血容量的50%时,除及时输 注晶体液和胶体液外,还需要输注浓缩红细胞或洗 涤红细胞以及白蛋白;
胃癌术后护理课件

情绪波动
术后患者的情绪状态可能变得不 稳定,包括情绪低落、易怒、失
眠等。
对生活失去信心
面对可能的康复期和后续治疗, 患者可能对生活失去信心,产生
消极情绪。
心理护理方法
建立良好的护患关系
提供心理支持
护士应与患者建立信任和亲近的关系,以 更好地了解患者的心理状态。
护士应倾听患者的担忧和问题,提供安慰 、支持和鼓励,帮助患者建立信心。
术后1-2周
可逐渐过渡到软食和普通 饮食,但仍需注意少食多 餐,避免暴饮暴食。
饮食注意事项
避免刺激性食物
如辛辣、油腻、烟酒等,以免刺激胃肠道。
保持口腔卫生
饭后漱口,保持口腔清洁,防止口腔感染。
避免过硬、过粗的食物
以免造成胃肠道机械性损伤。
定期随访
定期随访医生,了解饮食情况及胃肠道功能 恢复情况,及时调整饮食方案。
随访Байду номын сангаас复查的重要性
及时发现术后并发症
通过随访和复查,可以及时发现并处理术后可能出现的并发症, 如感染、吻合口漏等。
监测康复情况
随访和复查有助于了解患者的康复情况,包括营养状况、生活质量 等,从而及时调整护理方案。
评估治疗效果
通过随访和复查,可以评估治疗效果,为后续治疗提供参考。
随访与复查的时间安排
药物治疗
根据患者疼痛程度遵医嘱给予 镇痛药,如非甾体抗炎药、阿
片类镇痛药等。
心理疏导
与患者沟通,了解其心理状态 ,给予安慰和鼓励,减轻其焦 虑和紧张情绪。
改变体位
术后根据患者情况协助其采取 舒适的体位,如半卧位、侧卧 位等,减轻伤口张力。
物理治疗
可采用冷敷、热敷、按摩等方 法缓解疼痛。
肿瘤患者大量输血后临床检验指标变化的特点分析

•医学检验.肿瘤患者大量输血后临床检验指标变化的特点分析张静路蔓做好输血后各项指标监测,有助于及时发现大量输血患者的异常状况。
为确定大量输血对肿瘤患者的影响,本研究主要以72例肿瘤患者为研究对象,探讨患者输血前后的凝血功能、肾功能、血清电解质及炎症指标变化。
1资料与方法1.1一般资料:选择2018年5月至2019年11月于我院就诊的72例肿瘤患者为研究对象。
所有患 者均大量输血。
入选标准:①经磁共振成像(MRI&检查确诊为肿瘤;②有手术适应证;③签署知情;④均输、血,24h输量在10U以上;⑤术大量输血。
标准:①用可影响血常规、凝血功能等指标药物者;②不合手术、检者。
其中,39例,女性33例;平均年(49士15);输量(13.1±1.8)U;血输注量(1426±180)mL o1.2$72例肿瘤患者均于术中大量输血前、后,检:①血标本。
于清肿瘤患者5mL血,做标后,于用。
为的准确,尽量肿瘤患者的血标本时于24h 。
②。
用化、血等,以肿瘤患者的血标本功能(AST)、(ALT)、(TP)、肾功能(UA)、(BUN)、(Cr)、血(PLT)以及凝血功能凝血时(PT)、化凝血时间(FIB)、(FIB)、血时(TT)。
彳:各,对输血前、后所血标本的对。
1.3:用SPSS24.0。
用及LSDT,以!<0.05为异有。
2结果2.1功能:肿瘤患者大量输血后,TP水平明显下降,输血后1h TP(42.8±1.6)g/L,于大量输血前(!<0.05),输血后1h AST及ALT指标升高,输血前后对异有(!<0.05)。
随着肿瘤患者输血后隔时的延长,患者的功能指标变化幅度逐渐缩小,但与输血前较差异有(!<0.05)。
见表l o表1不同时间段肝功能检验结果("#$)项目例AST(U/L)ALT(U/L)TP(g/L)大量输血前7251.0±2.441.1±2.660.6±2.1大量输血1h后72132.2±3.31)119.3±7.01)42.8±1.61)大量输血12h后72105.2±3.11)87.1±5.71)46.7±1.81)大量输血24h后7272.2±2.71)54.6±5.11)53.2±1.91) 1)与大量输血前对!<0.05。
137例胃癌术后并发症的处理

137例胃癌术后并发症的处理发表时间:2012-11-27T09:45:57.497Z 来源:《医药前沿》2012年第25期供稿作者:倪铭施少兵[导读] 目的探讨胃癌患者胃大部分切除术术后并发症的处理。
方法回顾性分析2010年9月~2012年9月在安徽省无为县人民医院外一科行胃大部分切除术治疗137例胃癌患者的临床资料,并探讨手术治疗术后并发症的处理。
倪铭施少兵 (安徽省无为县人民医院外一科 238300)【摘要】目的探讨胃癌患者胃大部分切除术术后并发症的处理。
方法回顾性分析2010年9月~2012年9月在安徽省无为县人民医院外一科行胃大部分切除术治疗137例胃癌患者的临床资料,并探讨手术治疗术后并发症的处理。
结果全组手术无死亡病例。
137例胃癌胃大部分切除术患者术后发生胃瘫综合征2例,术后再出血16例,术后残胃排空障碍13例,吻合口瘘5例。
结论胃癌患者目前仍以胃大部分切除术为主,但同时如并发症,应早期预防,及时治疗,最大限度的减轻患者痛苦是我们追求的目标。
【关键词】胃癌手术临床效果术后并发症【中图分类号】R730.5 【文献标识码】A 【文章编号】2095-1752(2012)25-0115-01137 Cases of Gastric Cancer Postoperative ComplicationsNi Ming,Shi ShaobingFirst department Surgery of Wuwei People's Hospital,Anhui province【Abstract】 Objective To explore the treatment of post-operative complications of gastric cancer patients with gastrectomy.Method A retrospective analysis of the treatment of the clinical data of 137 patients with gastric cancer in Wuwei County in Anhui Province People's Hospital Division One gastrectomy, from September 2010 to September 2012 and to explore the treatment of post-operative complications of surgical treatment.Result The whole group were no deaths. 137 cases of gastric gastrectomy patients five cases of postoperative gastroparesis syndrome, two cases of postoperative hemorrhage in 16 cases, 13 cases of postoperative residual delayed gastric emptying, anastomotic leakage.Conclusion Gastric cancer patients is still partial gastrectomy based, but such complications, should be early prevention and timely treatment, to maximize reduce the suffering of patients is our goal.【Keywords】 Gastric surgery Clinical effect Postoperative complications胃癌是消化系统最常见的恶性肿瘤之一,临床上较为常见且多发,在全世界的所有国家中,中国的发病率和病死率最高,居各类恶性肿瘤之首[1]。
围术期输血与肿瘤患者预后

二、输血影响癌肿病人预后可能机理
Penn认为癌肿是机体免疫机能受到严重抑制的一种 并发症, 并在某些癌肿复发病人中已观测到存在有 免疫参数的衰退。 Haffejee 等还在乳腺癌手术后早期复发的病人血清 中发现免疫抑制物水平明显升高。 输血能增加免疫抑制代谢物, 抑制细胞免疫是促进 癌肿生长和增加其复发率的可能机理。
国内胡谱绵等对手术治疗的538 例Ⅱ期胃癌病人进行了回 顾性调查, 其中围手术期输血组术后3年和5年生存率分别 为46.5%和 41.8%, 而未输血组则分别为 81.6%和58.7%, 统计学处理的结果表明两组间的差异有意义, 同时提示病 人的生存期与输血量成反比。
7、宫颈癌
有人报道130 例宫颈癌病人中, 输血患者有早期肿瘤复发 的趋势。另外研究发现输血和病死率之间具有明显的相关 性。
2.乳腺癌:
Tartter等回顾了1964年-1972年间I、Ⅱ、Ⅲ期乳腺癌169 例患者, 根据围手术期是否输血、结果发现未输血的病人 , 其累积五年无瘤生存率前者为65 %, 后者为51 % , 各 期乳腺癌患者均有类似倾向, 因此他认为因手术期输血对 乳腺癌病人来说是一个重要的影响预后因素。
一、输血对肿瘤病人预后的影响
一、输血对肿瘤病人预后的影响
• 其他意见:
• 围手术期输血与术后病人生存之间并无紧密联系 • Foster等对226例乳癌切除病人(其中65 例接受围手术期输血) 进行 了随访, 经统计学分析, 认为输血并不增加病死率。 • Nathanson 也对366例接受结直肠癌切除病人进行了随访,其中119 例 曾在围手术期输血。结果不输血病人生存率为5 6.5% , 输血病人为4 3%, 但经统计学处理,调整其它干扰因素, 则显示此种差异无意义。
胃癌术后出血的应急预案

胃癌术后出血的应急预案1. 简介胃癌手术是治疗胃癌的一种常见方法,但术后出血是一个常见的并发症。
为了应对胃癌术后出血的紧急情况,制定一份应急预案对于确保患者的安全至关重要。
2. 预防措施- 术前准备:严格按照医嘱进行术前准备,包括血液检查、止血药物的使用、禁止使用抗凝剂等。
-手术规范:尽量选择有经验的外科医生进行手术,并严格按照手术操作规范进行操作。
3. 出血的判断术后出血可通过以下表现进行判断:- 不正常的术后伤口渗血- 术后出现全身性症状,如心率加速、血压下降、皮肤苍白等- 视觉观察出现消化道出血的现象,如呕血、黑便等4. 应急预案步骤- 步骤一:及时发现出血迹象若发现术后有出血的可能,包括伤口渗血、患者出现明显的全身不适,医护人员应立即引起重视,并及时通知主治医师。
- 步骤二:保持患者平卧在等待医生或护士的到来期间,将患者保持平卧位,减少出血的风险。
- 步骤三:处理伤口若出现伤口渗血,切勿随意处理,应在医生或护士的指导下进行处理,避免加重出血的情况。
- 步骤四:输血止血如果患者出现大量出血的情况,需要紧急采取输血止血的措施,需立即联系血液科医生,跟进输血的流程。
- 步骤五:通知主治医师在采取紧急措施后,及时通知术后主治医师,并将具体情况做好记录,以便医生参考和进一步处理。
5. 注意事项- 在处理术后出血时,应遵循无菌操作,避免感染。
- 出血是一种危急情况,应尽快通知主治医师,以便及时处理。
6. 总结胃癌术后出血是一种常见的并发症,出血的判断和应急预案的制定对于保证患者的安全至关重要。
医护人员应具备快速判断和处理术后出血的能力,并及时通知主治医师进行进一步处理。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ORIGINAL ARTICLE –GASTROINTESTINAL ONCOLOGYDo Perioperative Blood Transfusions Influence Prognosis of Gastric Cancer Patients?Analysis of 927Patients and Interactions with SplenectomyFabio Pacelli,MD 1,Fausto Rosa,MD 1,Daniele Marrelli,MD 2,Corrado Pedrazzani,MD 2,Maurizio Bossola,MD 1,Marco Zoccali,MD 3,Alberto Marchet,MD 4,Mariantonietta Di Cosmo,MD 5,Claudia Roata,MD 6,Luigina Graziosi,MD 7,Emanuel Cavazzoni,MD 7,Marcello Covino,MD 8,Domenico D’Ugo,MD 3,Franco Roviello,MD 2,Donato Nitti,MD 4,and Giovanni Battista Doglietto,MD 11Department of Digestive Surgery,Catholic University of Rome,Rome,Italy;2Institute of Surgical Sciences,University of Siena,Seina,Italy;31st General Surgery,Catholic University of Rome,Rome,Italy;4Clinica Chirurgica Generale 2,University of Padova,Padova,Italy;51st Division of Surgery,University of Verona,Verona,Italy;6Servizio Trasfusionale,Borgo Trento Hospital,Verona,Italy;7Section of General and Emergency Surgery,University of Perugia,Perugia,Italy;8Department of Emergency Medicine,Catholic University of Rome,Rome,ItalyABSTRACTBackground.This study was to assess the influence of perioperative blood transfusions on the prognosis of patients undergoing a potentially curative resection for gastric cancer and to investigate the interaction between transfusions and splenectomy.Materials and Methods.Between January 1990and December 2005,927patients from 6Italian tertiary referral centers underwent curative resections for gastric cancer.Clinical and pathologic variables were prospectively col-lected.The influence of perioperative blood transfusions on survival were evaluated by univariate and multivariate analysis.Moreover,the influence of splenectomy both in transfused and nontransfused patients undergoing total gastrectomy was also evaluated.Results.The overall 5-year survival was 54.6%.The 5-year survival rate in transfused patients (n =327)was 50.6%compared with 56.6%in nontransfused patients (n =600)(P =.094).In the subgroup of patients who underwent total gastrectomy with spleen preservation(n =209),5-year survival rate was 46%and 51.4%in transfused and nontransfused patients,respectively (P =.418);those who underwent total gastrectomy with splenectomy (n =199)presented a 5-year survival rate of 45%in transfused group compared with 39.1%in non-transfused patients (P =.571).Conclusions.Our study indicates a slightly,but not sig-nificantly,negative effect of allogeneic blood transfusion on prognosis of gastric cancer patients.In the subgroup of patients who underwent total gastrectomy,splenectomy seems to invert this mild effect,with a positive influence on overall survival.The negative effects of transfusion-induced immuno-suppression on cancer recurrence have been debated in the literature for more than a decade,with contradictory results,although most recent issues on gastric cancer conclude that transfused patients experience more tumor recurrences and less overall survival.1–6Interactions between splenectomy and blood transfusion were also hypothesized,and evidence that the immuno-suppressive effects of transfusion could be abrogated by splenectomy was first shown in organ-transplantation experiments.7–9The issue takes particular importance in gastric cancer surgery as splenectomy may or may not be added to gastric resection;in actual fact,although most authors recommend splenic preservation in the surgical treatment of gastric cancer,splenectomy is still considered for proximal gastric and gastroesophageal junction (GEJ)This study is conducted on behalf of the Italian Research Group for Gastric Cancer—IRGGC.ÓSociety of Surgical Oncology 2011First Received:26June 2010;Published Online:15January 2011F.Pacelli,MDe-mail:fpacelli@rm.unicatt.itAnn Surg Oncol (2011)18:1615–1623DOI10.1245/s10434-010-1543-9cancers(type II and III),because the incidence of lymph node metastases in the splenic hilum is thought to be higher in these tumors.10–12The aim of this study was to assess,in a large multi-centric national series,the prognostic effect of perioperative blood transfusion in patients undergoing a potentially curative resection for gastric cancer and to investigate the interaction between transfusions and splenectomy in patients undergoing total gastrectomy.PATIENTS AND METHODSPatients and TreatmentIn this retrospective multicentric study,data were col-lected from the medical records of986patients who underwent radical resection(R0)for histologically con-firmed gastric carcinoma from January1990through December2005.Patients were operated on at6Italian centers experienced in gastric cancer treatment:Digestive Surgery,Catholic University of Rome(n=393);Institute of Surgical Sciences,University of Siena(n=255);1st General Surgery,Catholic University of Rome(n=112); 1st Division of General Surgery,University of Verona (n=101);Clinica Chirurgica Generale2,University of Padova(n=99);Section of General and Emergency Surgery,University of Perugia(n=26).Eligibility criteria included histologically confirmed R0 gastric resection(i.e.,negative resection margins,en bloc resection of adherent organs,and en bloc resection of greater and lesser omentum)and pathologic evaluation of the total number of resected lymph nodes(at least15). Patients with distant metastases(e.g.,hepatic,lung,peri-toneal dissemination,or extraregional lymph nodes—superior mesenteric artery,middle colic artery,and para-aortic lymph nodes),those with fewer than15lymph nodes dissected,previous neoplastic diseases,and hematological pathologies were excluded from the study.In addition,patients dead in the perioperative period (n=34)and lost at follow-up(n=25)were excluded from the analysis.As a result,927patients who had undergone curative resections for gastric cancer were enrolled for analysis.Patients who underwent curative surgery with patho-logicfindings of serosal involvement or nodal metastases were potentially eligible for adjuvant chemotherapy.The total number of patients who received adjuvant chemo-therapy was460(49.6%).The most used regimens were epirubicin hydrochloride,cisplatin,andfluorouracil(ECF) for a mean of3cycles after surgery,depending on clinical response or the occurrence of adverse effects.The following operative procedures were performed: total gastrectomies and distal subtotal gastrectomies.D2or more extended lymph node dissection was performed on all patients,according to the rules of the Japanese Research Society for Gastric Cancer.13The definitions as to the extent of the lymphadenectomy are as follows.The regional lymph nodes of the stomach were classified into4 partment1consisted of the perigastric lymph partment2consisted of lymph nodes along the left gastric artery,along the common hepatic artery,around the celiac axis,and along the splenic artery. Compartment3consisted of lymph nodes in the hepa-toduodenal ligament,at the posterior aspect of the head of the pancreas,and at the root of the mesenterium.When the cancer was located in the lower third of the stomach,lymph nodes along the splenic artery were classified as compart-ment3.The anatomical level of D2lymphadenectomy included complete dissection of compartments1and2;D3 lymphadenectomy included that of compartments1,2,and 3.Extensive surgery was defined as combined resection of adjacent organs(gallbladder,left pancreas,liver,or colon).Splenectomy was performed for the purpose of lymph node dissection or because of suspicion of direct tumor invasion to the spleen or because of intraoperative iatro-genic reasons.Perioperative blood transfusion was defined as either whole blood or packed red blood cells administered within 14days before surgery,during surgery,or14days after surgery at the discretion of the surgeon and anesthesiolo-gist.Amount and timing of transfusion were also recorded. Usually,a hemoglobin level of8–10g/dL or hemody-namically significant intraoperative blood loss was used as thresholds for transfusion.The mean amount of blood transfusion in the transfused patients was4.1±2.7units (range,1–25units).Hospital morbidity and mortality were recorded.Post-operative septic complications were classified according to:(1)major septic complications:pneumonia,requires radiographic evidence and documentation of a pathologic organism in sputum or pleuralfluid;abdominal abscess, requires clinical or instrumental(ultrasound or computed tomographic scan)evidence of an abdominal purulent collection and spontaneous or operative drainage;and septicemia,requires a temperature of38.5°C or higher and at least1blood culture yielding a pathogenic organism; and(2)minor septic complications:wound infection, requires purulent exudate in the wound with or without culture growth;and urinary tract infection,requires bac-teriologic confirmation of more than100.000organisms per1mL of urine.1616 F.Pacelli et al.Tumor stage was classified according to the6th edition of the International Union Against Cancer/American Joint Committee on Cancer guidelines.14Patients were fol-lowed-up until death or until December31,2008.Patient demographics,clinicopathological features,type of surgery,and survival were evaluated in all population and in patients undergoing total gastrectomy.Statistical AnalysisAll statistical analyses were conducted by using SPSS 10.0for Windows(SPSS,Chicago,IL).Intergroup com-parisons were made by using a2-tailed chi-square test and the t test.Risk factors associated with tumor recurrence were determined by using logistic regression analysis. Survival curves were conducted according to the Kaplan–Meier method and compared by means of the log-rank test. Multivariate analysis to identify the independent prognos-tic factors was performed by using the Cox regression model with backward stepwise procedure.Differences were considered significant at the P\.05level. RESULTSThe characteristics of the927patients included in this study,according to transfusion and nontransfusion group, are shown in Table1.Of the927patients,327patients (35.3%)had received perioperative blood transfusion and 600patients(64.7%)had not.The majority of transfused patients(211patients,64.5%)received2U of blood or less,whereas50patients(15.3%)received more than4U. Most patients(81.1%)were transfused intraoperatively, postoperatively,or both(Table2).Among927patients,246patients(26.5%)had under-gone splenectomy and681patients(73.5%)had not. Indications for splenectomy in246patients(26.5%) included:lymph node dissection and/or extent of tumor in 207patients,bleeding in20patients,and ischemia of the spleen in5patients.The basis for splenectomy could not be determined in14patients.In the207patients who underwent splenectomy with the purpose of lymph node dissection and/or extent of tumor, the recurrence rate from lymph nodes in the splenic hilum was8.7%.The median follow-up was97months(range1–193).At last follow-up,423patients had died.For all patients of the study group,overall5-year sur-vival was54.6%.According to cancer stage was89.2%for stage IA disease,74.1%for stage IB disease,66.5%for stage II disease,41.4%for stage IIIA disease,19.0% for stage IIIB disease,and10.7%for stage IV disease (P\.0001)(Fig.1a).The5-year survival was50.6%in TABLE1Characteristics of all patients(927)according to transfu-sion and nontransfusion groupPatient variable Nontransfusiongroup(600)Transfusiongroup(327)P valueAge(years).0014 \65293124C65307203Sex.231 Male352205Female248122Tumor location.058 Lower third312134Middle third193129Upper third9564Tumor size(cm)\.0001 \6341133C6254199Lauren classification.138 Diffuse526228Intestinal6340Undetermined1951Type of operation.125 Total gastrectomy a253155Subtotal distal-gastrectomy347172Extended operation\.0001 No510245Yes9082Type of lymphadenectomy.032 D15134D2321191D3228102 Splenectomy.066 No429252Yes17175Cancer stage.155 IA3453IB9764II10661IIIA10358IIIB4836IV11255Preoperative hemoglobin(Hb)value(g/dL,mean±SD)12.0±2.111.8±7.0.566Major postoperativecomplications.0089 No493245Yes10782a Includes20total resections of gastric stump and9upper polar resectionsPrognostic Factors in Gastric Cancer1617patients who had received perioperative blood transfusion and56.6%in those who had not(P=.094)(Fig.1b).Results of the univariate and multivariate analysis of prognostic factors for all population are shown in detail in Table3.Considering all patients,the5-year survival rate was significantly affected by age,tumor location,tumor size,type of operation,need for extensive operation, splenectomy,cancer stage,and major postoperative com-plications;cancer stage and age were independent predictors of lower survival rate according to multivariate analysis.Among927patients,408patients(44%)had undergone total gastrectomy,among them155patients(38%)had received perioperative blood transfusion and253patients (62%)had not;199patients(48.8%)had undergone sple-nectomy and209patients(51.2%)had not.For75patients who both had received perioperative blood transfusion and had undergone splenectomy,the mean amount of blood transfusion was3.8±2.1units (range1–13units).For81patients who had received per-ioperative blood transfusion,without splenectomy,the mean amount of blood transfusion was4.3±2.9units (range1–25units).A total of110patients did not receive either transfusion or splenectomy.Results of the univariate and multivariate analysis of prognostic factors for patients undergoing total gastrec-tomy are shown in detail in Table4.Considering all patients,the5-year survival rate was significantly affected by age,tumor location,need for extensive operation,type of lymphadenectomy,splenectomy,and cancer stage;cancer stage,age,tumor location,and type of lymphade-nectomy were independent predictors of lower survival rate according to multivariate analysis.The interaction between perioperative blood transfu-sions and splenectomy on cumulative survival was analyzed in patients undergoing total gastrectomy,where splenectomy was mostly performed to achieve an R0 resection and is shown in Fig.2.In patients(n=209)who underwent splenic preservation(Fig.2a),5-year survival rate was46%and51.4%in transfused and nontransfused patients,respectively(P=.418);those who underwent total gastrectomy with splenectomy(n=199)presented a 5-year survival rate of45%in transfused group compared with39.1%in nontransfused patients(P=.571)(Fig.2b).TABLE2Incidence,timing,and amount of transfusionVariable No.%Incidence of transfusionsNontransfused60035.3 Transfused32764.7 Amount of transfusions(U)1–221164.5 3–46620.2 [45015.3 Mean±SD 4.1±2.7 Median2Range1–25TimingPreoperative transfusions only3410.4 Intraoperative transfusions only13942.5 Postoperative transfusions only9529.1 Preoperative and intraoperative transfusions18 5.5 Intraoperative and postoperative transfusions319.5Preoperative,intraoperative,and postoperative transfusions 1031618 F.Pacelli et al.Among the460patients(49.6%)who received adjuvant chemotherapy,207patients(45%)had been transfused and 253patients(55%)had not;292patients(63.5%)had undergone splenectomy and168(36.5%)had not.No heavy,statistically significant influence on prognosis of gastric cancer patients resulted by adjuvant chemotherapy.TABLE3Factors affecting5-year overall survival according to univariate and multivariate analysis in all927 patientsNS not statistically significant, OR odd ratioa Includes29total resections of gastric stump Patient variable No.5-yearsurvival(%)Univariateanalysis P valueMultivariateanalysisP value OR Age(years)\6541763.7\.0001\.0001 1.617 C6551047.1SexMale37051.9Female55758.7.0534NSTumor locationLower third and middle third76858.2Upper third15937.2\.0001NSTumor size(cm)\647460.4C645349.2.0061NSLauren classificationDiffuse40855.4Intestinal44964.8Undetermined7054.4.122NSType of operationTotal gastrectomy a40845.6Subtotal distal-gastrectomy51961.3\.0001NSExtended operationNo75557.0.00026NSYes17243.1Type of lymphadenectomyD18749.4D250056.6.232NSD334053.0SplenectomyNo68159.4\.0001NSYes24640.1Cancer stageIA18789.2IB16174.1II16766.5\.0001IIIA16141.4\.0001 1.753 IIIB8419.0IV16710.7Perioperative transfusionNo60056.6.094NSYes32750.6Major postoperative complicationsNo73857.4.00034NSYes18943.3Prognostic Factors in Gastric Cancer1619DISCUSSIONBlood transfusion therapy may result in immunologic changes that may be harmful in neoplastic patients.As far as gastric cancer in particular is considered,although both in retrospective and prospective randomized studies many attempts have been made to determine the effects of perioperative transfusion on survival,theirfindings resulted to be contradictory and even confusing.3,15–21 Some reports have demonstrated the adverse effect of transfusion on the prognosis of patients with gastric can-cer.3,15–18,21Decreased helper/suppressor T-cell ratios, decreased natural-killer cell activity,decreased macro-phage antigen presentation,suppression of lymphocyteTABLE4Factors affecting5-year overall survival according to univariate and multivariate analysis in408 patients undergoing total gastrectomy(includes20total resections of gastric stump and 9upper polar resections)NS not statistically significant, OR odd ratio Patient variable No.5-yearsurvival(%)Univariateanalysis P v alueMultivariateanalysisP value OR Age(years)\6521552.4.032\.05 1.478 C6519338.0.032SexMale27645.7.451NSFemale13242.8Tumor locationLower third?middle third24950.1.00124\.05 1.39 Upper third15935.7Tumor size(cm)\621753.0.055NSC619038.7Lauren classificationDiffuse21441.7Intestinal15744.2.235NS Undetermined3749.4Extended operationNo28648.2.0051NSYes12236.4Type of lymphadenectomyD15638.6D230471.8.0012\.05.807 D34852.8SplenectomyNo20949.3\.0081NSYes19940.4Cancer stageIA6189.1IB5366.6II6561.9\.0001\.0001 1.717 IIIA8334.7IIIB5319.7IV9311.2Perioperative transfusionNo25344.1.0982NSYes15545.8Major postoperative complicationsNo30747.2.108NSYes10137.51620 F.Pacelli et al.blastogenesis,and decreased delayed-type hypersensitivity have been observed after transfusion,demonstrating a relative immunosuppression.22The mechanism of this transfusion effect is not well defined.Different hypothe-ses,such as overload of the reticuloendothelial system, alterations in interleukin-2and prostaglandin metabolism, and immunosuppression by clonal deletion or generation of active suppressor factors have been proposed.Active suppressor factors include anti-idiotypic antibodies or suppressor T lymphocytes that are generated after trans-fusion.22The latter effect is strictly related to the results of this study,as splenectomy precludes the development of suppressor T-lymphocytes,explaining a possible interaction between the effects of splenectomy and blood transfusion on the immune system.23By contrast,other studies have shown that allogeneic blood transfusions do not seem to have deleterious effects on the5-year survival rates of patients who undergo curative surgery for gastric carcinoma.19,20Hyung et al.retrospectively reported that among the treatment factors in gastric cancer,total gastrectomy, splenectomy,D3or more extended lymphadenectomy,and postoperative adjuvant chemotherapy were associated with transfusion.6In their study,they showed a significantly poor survival rate in the transfused group,and a dose–response relationship between the amount of transfused blood and prognosis was evident.Heiss et al.have reported that the prognostic effect of transfusion is mediated through its effect on minimal residual disease in resected-cancer patients.24They have observed that transfusion-associated immune modulation affects minimal residual disease after curative tumor resection.They have also witnessed that quantitative assessment of tumor cells in bone marrow during follow-up demonstrated a significant quantitative increase of tumor cells in transfused patients only.24The further the stage advances,the greater the possibility of minimal residual disease,even when curative surgery is performed.In advanced stages,immunosuppression may cause progres-sion of metastatic foci and failure to remove circulating cancer cells and cells in bone marrow.Sa´nchez-Bueno et al.analyzed the influence of packet red blood cell transfusions on the prognosis of163 patients with gastric adenocarcinoma undergoing subtotal gastrectomy with a curative intention,and they con-cluded that blood transfusion per se does not influence the long-term survival of resected patients,although it does have an influence when associated with other prognostic factors and has a statistically significant influence on prognosis only in patients with tumor stage III(P=.0051).19These contradictory results,however,may arise through inadequate study design,inadequate eligibility criteria, small sample sizes,or inadequate statistical analysis.The present study,which is one of the largest conducted on a population of western patients assessing this specific issue,aims to overcome some of these methodological problems.Thefirstfinding is that the need for transfusion in our population of gastric cancer patients was related to some demographic,pathologic,and surgical variables (patient’s age,tumor location,need for extensive surgery, number of major postoperative complications)but not to preoperative hemoglobin values.Secondly,univariate and multivariate survival analysis clearly show that transfu-sions have no significant influence on prognosis of gastricPrognostic Factors in Gastric Cancer1621cancer patients.The only variables independently associ-ated with survival are patient’s age and cancer stage.The additional aim of our study was to assess the prognostic interaction between spelenectomy and transfu-sion.It is well known that splenectomy might facilitate a more complete lymphadenectomy by thorough clearance of the lymph nodes in the splenic hilum;however,numerous retrospective as well as prospective randomized trials have not demonstrated a prognostic benefit for splenectomy or extended lymphadenectomy.25Moreover,in gastric cancer patients,some retrospective studies even demonstrated a worse survival with splenectomy.26–32Evidence for an interaction between splenectomy and blood transfusion was first demonstrated in animal organ transplantation experi-ments.Immunosuppressive effects of transfusion were abrogated by splenectomy in these experiments;animals that underwent splenectomy and blood transfusion showed an increased rate of rejection of the transplanted organs compared with animals that only received blood transfu-sions before transplantation.7–9,33Weitz et al.were thefirst to clinically investigate the interaction between perioperative allogeneic blood trans-fusions and splenectomy on the prognosis of patients undergoing resection of proximal gastric or GEJ cancer.34 The most strikingfinding of their study was that periop-erative allogeneic blood transfusion and splenectomy showed an interaction in their effect on survival.Patients who received a perioperative blood transfusion showed a shorter relapse-free survival only if the spleen was pre-served.They concluded that the adverse effect of allogeneic blood transfusion on prognosis in patients with gastric cancer seemed to be associated with the presence of an intact spleen and is abrogated by its absence.By contrast,Shen et al.,in patients undergoing total gastrectomy,showed that splenectomy was associated with a higher rate of tumor recurrence among transfused patients with stage III gastric cancer but not among nontransfused patients.35In addition,splenectomy was not associated with survival among either transfused or nontransfused patients. Theyfinally concluded that splenectomy did not appear to abrogate the adverse effect of perioperative transfusion on prognosis in gastric cancer patients.Moreover,it may increase postoperative recurrence in transfused patients.Results of the present study show that splenectomy, aiming at a superior lymphadenectomy,negatively influ-ences survival both in all patients,and in those undergoing total gastrectomy,although this variable turned out to be not statistically significant at multivariate survival analysis.Moreover,in the subgroup of patients who underwent total gastrectomy among those who received splenic pres-ervation,perioperative blood transfusion had a slightly negative effect,even if not significant,whereas,in patients who underwent splenectomy,this effect on survival turned out to be inverted.Our data do not statistically support the notion of a routine splenectomy in patients who had undergone total gastrectomy,for this reason we cannot recommend a rou-tine‘‘prophylactic’’splenectomy in patients undergoing perioperative blood transfusion.Although the immunological understanding of the trans-fusion effect in gastric cancer patients was beyond the aim of the present study,these results suggest an evident role of the spleen in this context.Immune regulation is the mechanism responsible for outcomes in patients undergoing both trans-fusion and splenectomy.34,35However,no retrospective study could adequately address these mechanism issues. Further investigation after splenectomy in patients receiving perioperative blood transfusion are needed.In conclusion,the mainfindings of this study are that allogeneic blood transfusions have a slightly,but not sig-nificantly,negative effect on prognosis of gastric cancer patients,and splenectomy counteracts this effect,with a positive influence on overall survival. ACKNOWLEDGMENT Prof.Fabio Pacelli had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.REFERENCES1.Vamvakas EC,More SB.Blood transfusion and postoperativeseptic complications.Transfusion.1994;34:714–27.2.Blumberg N,Heal J,Chuang C,Murphy P,Agarwal M.Furtherevidence supporting a cause and effect relationship between blood transfusion and earlier cancer recurrence.Ann Surg.1988;207:410–5.3.Maeta M,Shimizu N,Oka A,Kondo A,Yamashiro H,TsujitaniS,et al.Perioperative blood transfusion exacerbates surgical stress-induced postoperative immunosuppression and has a neg-ative effect on prognosis in patients with gastric carcinoma.J Surg Oncol.1994;55:149–53.4.Bellantone R,Sitges-Serra A,Bossola M,Doglietto GB,MalerbaM,Franch G,et al.Transfusion timing and postoperative septic complications after gastric cancer surgery.A retrospective study of179consecutive patients.Arch Surg.1998;133:988–92.5.Yamashita K,Sakuramoto S,Kikuchi S,Katada N,Kobayashi N,Watanabe M.Transfusion alert for patients with curable cancer.World J Surg.2007;31:2315–22.6.Hyung WJ,Noh SH,Shin DW,Huh J,Huh BJ,Choi SH,et al.Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer.Ann Surg Oncol.2002;9:5–12.7.Marquet RL,Heineman E,Tank B,Obertop H,Niessen GJ,Bijnen AB,et al.Abrogation of the beneficial blood transfusion effect in dogs by splenectomy.World J Surg.1984;8:408–13.8.Shelby J,Wakely E,Corry RJ.Suppressor cell induction in donorspecific transfused mouse heart recipients.Surgery.1984;96: 296–301.9.Shelby J,Wakley E,Corry RJ.Splenectomy abrogates theimproved graft survival achieved by donor-specific transfusion.Transplant Proc.1985;17:1083–6.1622 F.Pacelli et al.。
