Fundamentals of Modern Chemistry (Ⅰ)-1 近代化学基础(Ⅰ)-1
化学专业无机化学教材及主要参考书

化学专业《无机化学》主要参攻读物配套教材1、北京师大,华中师大,南京师大.无机化学(第四版) .北京:高等教育出版社.2003.1. (面向21世纪课程教材,获优秀教材一等奖,针对高等师范院校编写的教材,理论阐述深度适当,讲解清楚;并注意运用基本理论去解释无机物质的变化规律。
)2、申泮文.近代化学导论.北京:高等教育出版社.2002.1(面向21世纪课程教材,共有四部分: 基本化学原理;溶液平衡和化学分析;元素化学;近代化学热点。
)3 、武汉大学,吉林大学.无机化学(第二版).北京:高等教育出版社.(获国家优秀教材一等奖,高等教育出版社。
内容覆盖面较广,知识点讲解详细,条理清晰,有利于学生自学,有利于学生今后报考综合性大学研究生时,用做复习资料。
)4、傅献彩.大学化学.北京:高等教育出版社.1999.9(化学理科,面向21世纪课程教材,“九五”国家级重点教材,面向21世纪课程教材.将无机化学和化学分析的教学内容融合,上册以化学原理为主,将定量分析纳入化学平衡;下册以元素化学为主。
)5、天津大学.无机化学 (第三版).北京:高等教育出版社.2002.7(面向21世纪课程教材)6、史启祯.无机化学与化学分析(第二版).北京:高等教育出版社.2005.5.(1992年由教育部化学教学指导委员会立项, 列为国家“八五”重点教材.1998年由高教社出版, 2000年被列为国家“面向21世纪课程教材”)7、唐宗熏.中级无机化学.北京:高等教育出版社.2003.(普通高等教育“十五”国家级规划教材,介于无机化学和高等无机化学之间的中级水平的无机化学教材。
系统介绍了现代无机化学所涉及的新理论、新领域、新知识和无机新型化合物。
)8、傅献彩.大学化学.北京:高等教育出版社.(面向21世纪课程教材.将无机化学和化学分析的教学内容融合,上册以化学原理为主,将定量分析纳入化学平衡;下册以元素化学为主)9、申泮文.无机化学. 北京:化学工业出版社. 2002.(面向21世纪课程教材. 编写者均为名校专家,以专题形式撰写无机化学的近代成就与发展现状,各专题选录文献水平为当代国际前沿。
如何学好化学的英语

如何学好化学的英语Chemistry is a fascinating and complex subject that plays a crucial role in our everyday lives. From the composition of the air we breathe to the foods we eat, chemistry is everywhere. However, many students find chemistry to be a challenging subject to excel in. But fear not, with dedication, hard work, and the right strategies, anyone can master the fundamentals of chemistry and excel in this fascinating subject. In this article, we will explore some effective tips and techniques to help you learn and understand chemistry better.1. Understand the BasicsOne of the most important tips for excelling in chemistry is to have a strong understanding of the basics. Chemistry is a subject that builds upon itself, so it's essential to have a solid foundation in the fundamentals. Make sure you are familiar with the periodic table, atomic structure, chemical bonding, and basic chemical equations. Understanding these basic concepts will help you grasp more complex topics later on.2. Practice, Practice, PracticePractice makes perfect, and this is especially true when it comes to mastering chemistry. Make sure to do plenty of practice problems and exercises to reinforce your understanding of key concepts. Practice will help you develop your problem-solving skills and improve your ability to apply theoretical knowledge to real-world scenarios. Consider using online resources, textbooks, and study guides to find practice problems that align with the topics you are studying.3. Stay OrganizedStaying organized is essential when studying chemistry. Keep all of your notes, handouts, and textbooks in one place so you can easily access them when needed. Create a study schedule and set aside dedicated time each day to review and practice chemistry. Use a planner or calendar to keep track of important dates, deadlines, and exams. By staying organized, you can ensure that you are maximizing your study time and staying on top of your coursework.4. Seek Help When NeededDon't be afraid to ask for help when you need it. If you are struggling with a particular concept or topic in chemistry, reach out to your teacher, classmates, or a tutor for assistance. Many schools offer tutoring services or study groups for students who need extra help with their coursework. Additionally, there are numerous online resources and forums where you can ask questions and get help from other students and professionals in the field of chemistry.5. Use Flashcards and MnemonicsFlashcards and mnemonics are excellent tools for memorizing key concepts, equations, and facts in chemistry. Create flashcards with important terms, definitions, and equations on one side and the corresponding information on the other side. Review your flashcards regularly to reinforce your memory and retention of the material. Mnemonics, or memory aids, can also be helpful for remembering complex information or sequences. Create catchy phrases or acronyms to help you remember important details in chemistry.6. Engage in Hands-On LearningHands-on learning can greatly enhance your understanding and retention of chemistry concepts. Conduct experiments, demonstrations, and lab activities to put theory into practice and see firsthand how chemical reactions work. Many schools and universities offer laboratory courses or workshops where students can perform experiments under the guidance of a teacher or instructor. If you don't have access to a laboratory, consider conducting simple experiments at home using safe and readily available materials.7. Stay Curious and ExploreChemistry is a dynamic and ever-evolving field, so it's essential to stay curious and explore new topics and discoveries in the field. Attend lectures, seminars, and workshops on chemistry-related topics to expand your knowledge and stay up-to-date with the latest advancements in the field. Read scientific journals, articles, and books to learn more about different branches of chemistry and how they impact our world. Engage with other students and professionals in the field through networking events and conferences to share ideas and collaborate on research projects.In conclusion, excelling in chemistry requires dedication, hard work, and a proactive approach to learning. By following the tips and techniques outlined in this article, you can improve your understanding of chemistry and achieve success in this fascinating subject. Remember to stay organized, practice regularly, seek help when needed, and stay curious to continue growing and expanding your knowledge of chemistry. With perseverance and a positive attitude, you can master the fundamentals of chemistry and excel in your academic and professional pursuits.。
有机化学第001章
东晋葛洪《肘后备急方•卷三治寒热诸疟方第十六》
食品安全
① 三聚氰胺
H
NH O
H
H
N
N
N
N HN
O
H
N
O
H
NH
N
H
N
O
H
H NH
H
N
HN
O
N
N
N
H
H
N
O
H
NH O
H
NH
H
N
N
H
N
N HN
O
H
N
N
NH O
H
H
食品安全
② 阿斯巴甜
③ 塑化剂
天门冬酰苯丙氨酸甲酯
1.3B 有机化合物的结构特征(分子结构)
① 同分异构现象普遍存在
C2H6O
CH3CH2OH 乙醇,bp: 78.3 oC
CH3OCH3 甲醚,bp: 23 oC
同分异构体:具有相同的分子式而构造与性质不同 的化合物。
1.3B 有机化合物的结构特征(分子结构)
② 共价键结合 碳/C:有机化合物中的核心原子
1.1C 现代有机化学
1828年 Friedrich Wöhler (GER)
有机化学:含碳化合物的化学 / the chemistry of compounds that contain the element carbon. Definition for “Organic Molecule” Compounds that they contain at least one carbon atom
课程特点
难:内容繁多,理论不够系统 挑战性:逻辑性、系统性。应用性强、实践性强 沉溺于大量反应的记忆 失去了对学科整体的领悟
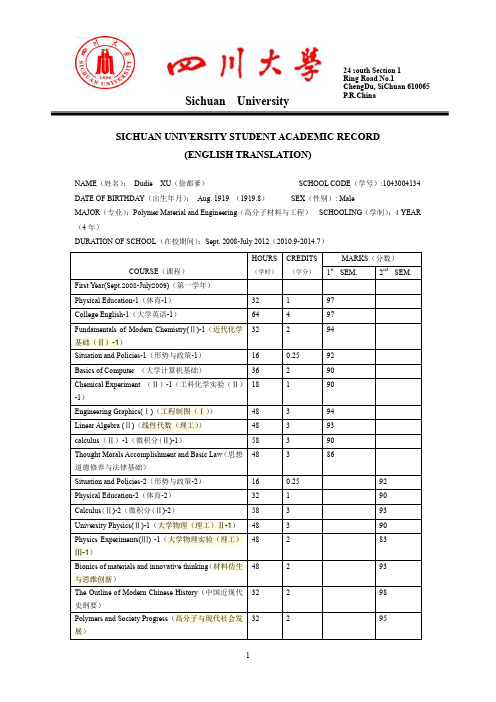
四川大学英文成绩单样本

四川大学英文成绩单样本224 south Section 1Ring Road No.1 ChengDu, SiChuan 610065P.R.China Sichuan UniversitySICHUAN UNIVERSITY STUDENT ACADEMIC RECORD(ENGLISH TRANSLATION)NAME(姓名): Dudie XU(徐都爹) SCHOOL CODE(学号):1043004134 DATE OF BIRTHDAY(出生年月): Aug. 1919 (1919.8) SEX(性别): Male MAJOR(专业):Polymer Material and Engineering(高分子材料与工程) SCHOOLING(学制):4 YEAR(4年)DURATION OF SCHOOL(在校期间):Sept. 2008-July 2012(2010.9-2014.7) HOURS CREDITS MARKS(分数)st nd (学时) (学分) COURSE(课程) 1 SEM. 2 SEM. First Year(Sept.2008-July2009)(第一学年) Physical Education-1(体育-1) 32 1 97 CollegeEnglish-1(大学英语-1) 64 4 97 Fundamentals of Modern Chemistry(?)-1(近代化学32 2 94 基础(?)-1)Situation and Policies-1(形势与政策-1) 16 0.25 92 Basics of Computer (大学计算机基础) 36 2 90 Chemical Experiment (?)-1(工科化学实验(?)18 1 90 -1)Engineering Graphics(?)(工程制图(?)) 48 3 94 Linear Algebra (?)(线性代数(理工)) 48 3 93 calculus(?)-1(微积分(?)-1) 58 3 90 Thought Morals Accomplishment and Basic Law(思想48 3 86 道德修养与法律基础) Situation and Policies-2(形势与政策-2) 16 0.25 92 PhysicalEducation-2(体育-2) 32 1 90 Calculus(?)-2(微积分(?)-2) 58 3 93University Physics(?)-1(大学物理(理工)?-1) 48 3 90 Physics Experiments(?) -1(大学物理实验(理工)48 2 83 ?-1)Bionics of materials and innovative thinking(材料仿生48 2 93 与思维创新)The Outline of Modern Chinese History(中国近现代32 2 98 史纲要)Polymers and Society Progress(高分子与现代社会发32 2 95 展)1CREDITS MARKS(分数) HOURSst nd (学时) (学分) COURSE(课程) 1 SEM. 2 SEM. College English-2(大学英语-2) 64 4 96 Fundamentals of Modern Chemistry(?)-2 (近代32 2 92 化学基础(?)-2)Fundamentals of Modern Chemistry(?)-3(近代化学32 2 93 基础(?)-3)Introduction of Science and Technology(科学技术48 3 80 概论)Mental Health Education(大学生心理健康) 16 1 96 Military Theory(军事理论) 16 1 93 Chemical Experiment (?)-2工科化学实验(?)18 1 90 -2 Second Year(Sept. 2009-July 2010)(第二学年) College English-3(大学英语-3) 64 4 88 The Basic Principles of Marxism(马克思主义基本48 3 95 原理概论)University Physics(?),2(大学物理(理工)?-2) 48 3 93 Physics Experiments(?) -2(大学物理实验(理工)16 1 88 ?-2)Base of Modern Life Science(现代生命科学基础) 48 3 92 Physical Chemistry(?)-1(物理化学(?)-1) 48 3 98 Fundamentals of ModernChemistry(?)-4(近代化学48 3 92 基础(?)-4)Military Training(军训) 32 1 85 Situation and Policies-3(形势与政策-3) 16 0.25 93 Engineering Training(?) (工程训练(?)) 32 2 90 Chemical Experiment (?)-3 (工科化学实验(?)64 4 93 -3)Physical Education-3(体育-3) 32 1 91 Chinese Culture(Philosophy)(中华文化(哲学篇)) 48 3 91 College English-4(大学英语-4) 64 4 89 Physical Education-4(体育-4) 32 1 98 Constitutionalism and human rights(宪政与人权) 32 2 93 The Introduction To Mao Zedong Thought And Theory 96 6 98 Of Socialism With Chinese Characteristics(毛泽东思想和中国特色社会主义理论体系概论 )Physics Demonstrate Experiments-1(物理演示实验 16 1 90 -1)89 Organic Synthesis(有机合成) 32 2 Fundamentals of Material Science and Engineering(材48 3 97 料科学与工程基础(双语))Polymer Chemistry (I) (高分子化学(?)) 64 4 932CREDITS MARKS(分数) HOURSst nd (学时) (学分) COURSE(课程) 1 SEM. 2 SEM. Macromolecules and human life(高分子与人类生活) 48 2 91 Principle of chemicalengineering(?()化工原理(?)) 64 4 90 Experimentd of principle of chemical engineering(?)16 1 86 (化工原理实验(?))Physical Chemistry(?)-2(物理化学(?)-2) 32 2 95 Instrumenntal analysis (II)(仪器分析(?)) 32 2 92 Physical Chemistry Experiment(?)(物理化学实验16 1 92 (?))Instrumental Analysis Experiment(?)(仪器分析实32 2 87 验(?))Situation and Policies-4(形势与政策-4) 16 0.25 92Dean’s OfficeSichuan University3。
四川大学英文成绩单样本
3
80
Mental Health Education(大学生心理健康)
16
1
96
Military Theory(军事理论)
16
1
93
Chemical Experiment(Ⅱ)-2工科化学实验(Ⅱ)-2
18
1
90
Second Year(Sept. 2009-July 2010)(第二学年)
College English-3(大学英语-3)
SichuanUniversity
SICHUANUNIVERSITYSTUDENT ACADEMIC RECORD
(ENGLISH TRANSLATION)
NAME(姓名):Dudie XU(徐都爹)SCHOOL CODE(学号):1043004134
DATE OF BIRTHDAY(出生年月):Aug. 1919(1919.8)SEX(性别): Male
64
4
88
The Basic Principles of Marxism(马克思主义基本原理概论)
48
3
95
University Physics(Ⅱ)-2(大学物理(理工)Ⅱ-2)
48
3
93
Physics Experiments(Ⅲ) -2(大学物理实验(理工)Ⅲ-2)
16
1
88
Base of Modern Life Science(现代生命科学基础)
2
83
Bionics of materials and innovative thinking(材料仿生与思维创新)
48
2
93
The Outline of Modern Chinese History(中国近现代史纲要)
关于胶体化学的教学思考

关于胶体化学的教学思考叶明富1,3,马亮1,严菊芬1,张奎1,吴芳辉1,王诗生2(1.安徽工业大学化学与化工学院,安徽马鞍山243002;2.安徽工业大学能源与环境学院,安徽马鞍山243002;3.南开大学先进能源材料化学教育部重点实验室,天津300071)摘要:胶体化学是一门古老而又年轻的学科,它的发展同步于工农业,在人们的生产和生活中有着广泛的应用。
教学过程中首先讲述胶体化学的发展简史及胶体理论,接着对胶体的结构进行详细叙述,从而引发出对胶体性质的探讨和论述,最后由胶体的性质引起对胶体化学实际应用的讨论。
这种层次分明的教学方式,可以进一步激发学生的学习积极性,有助于提高教学效果。
关键词:胶体化学;胶体理论;胶体结构;胶体性质;实际应用doi :10.3969/j.issn.1008-553X.2021.01.043中图分类号:G64;G45文献标识码:A文章编号:1008-553X (2021)01-0130-03收稿日期:2020-08-20基金项目:安徽省教学研究项目(2019jyxm0154,2019jyxm1231,2018jyxm1402,2017jyxm1177);安徽省线下课程项目(2020kfkc091);安徽省教学示范课(皖教秘高[2020]165号);安徽工业大学课程思政建设项目(2020xkcsz017,2020xkcsz018);安徽工业大学研究生课程(X 射线晶体学)思政建设项目;安徽工业大学教育教学研究项目(2020jy36);安徽工业大学精品线下开放课程和安徽工业大学课程负责人项目作者简介:叶明富(1982-),男,博士,副教授,硕士生导师,yemingfu@ ;通讯联系人:张奎(1983-),男,博士,教授,kuizhang@ ;吴芳辉(1975-),女,博士,教授,wfhwfh@ 。
胶体[1-4]一词最早是在1861年由英国科学家格雷厄姆(Thomas Graham ,1805-1869)提出来的。
培养方案课程设置中英文对照

56
36
20
必修
信息学院
2
A4
B2
C1
C4
通识类选修(选修6学分)
1060201
研讨课
Seminar
1.0
16
以汇报形式安排4次
专题
资环学院
1
A4
B1
C1
C4
文献检索
1.0
32
选修
7
A11
B10
C1
C5
科技发展与文明传承
Scientific and Technology Development and Cultural Heritage Preservation
2.0
32
32
选修
资环学院
6
A11
B8
C5
3064226
水土保持经济植物栽培学
Economic Plant Cultivation for Soil and Water Conservation
2.0
32
24
8
选修
资环学院
5
A9
B8
C5
3064227
水土保持农学
Agronomy of Soil and Water Conservation
综合实践
(31.5学分)
1305101
军训
Military training
2.0
3周
必修
1
练(丙)
Engineering Training(C)
1.0
1周
必修
1
A1
A5
B2
C3
C4
1305201
劳动
Chemistry_coursebook_1_Ch_1_-9_answers

HKDSE CHEMISTRY – A Modern View(Chemistry)Coursebook 1Suggested answersChapter 1 The fundamentals of chemistry PageNumber •Class Practice1•Chapter Exercise 3Chapter2 The atmosphere•Class Practice6•Chapter Exercise7Chapter 3 Oceans•Chapter Exercise9Chapter4 Rocks and minerals•Class Practice12•Chapter Exercise13●Part Exercise15 Chapter 5 Atomic structure•Class Practice17•Chapter Exercise19Chapter6 The Periodic Table•Class Practice21•Chapter Exercise22Chapter 7 Chemical bonding: ionic bonding•Class Practice24•Chapter Exercise26 Chapter8 Chemical bonding: covalent bonding•Class Practice29•Chapter Exercise31 Chapter9 Structures and properties of substances•Class Practice34•Chapter Exercise35 ●Part Exercise37Chapter 1 Fundamentals of chemistryClass PracticeA1.1(b)Food : fertilizers, insecticides, food additives(c)Housing : metals, alloys, cement, glass, plastics(d)Transport : metals, alloys, fuels, glass, plastics(e)Medicines : drugs, antibiotics, artificial hormones(f)Amusement park facilities : metals, alloys, cement, glass, plastics,semi-conductorsA1.2Phosphorus and mercury are elements. The others are not.(Note: A substance with a name consisting of two words (e.g. sodium chloride) is not an element. A substance with a name of only one word (e.g. ammonia) may or may not be an element. The only sure way is to check the name against the Periodic Table.)A1.3Sodium - silvery grey solid;Chlorine - greenish yellow gas;Sodium chloride - white solid.A1.4(a) Hydrogen, oxygen, nitrogen, iron, sulphur(b) Water, carbon dioxide, carbon monoxide, sodium chloride, iron(II) sulphide(c) Air, sea water, town gas, sodium chloride solution, wine(Other answers may be given.)A1.5(a) Chemical change(b) Physical change(c) Physical change(d) Chemical change(b) and (c) are physical changes because no new substances are formed. (a) and (d) are chemical changes because new substances are formed.A1.6(a), (b) and (e).A1.8(a) Flat-bottomed flask (l) Crucible tongs (w) Reagent bottle(b) Round-bottomed flask (m) Spatula (x) Gas syringe(c) Clamp (n) Heat-resistant mat (y) Measuring cylinder(d) Retort stand (o) Pestle (z) Beaker(e) Conical flask (p) Mortar (aa) Funnel(f) Wire gauze (q) Desiccator (bb) Plastic washbottle(g) Evaporating basin (r) Test tube holder (cc) Teat pipette(h) Tripod (s) Test tube rack (dd) Thermometer(i) Crucible (t) Test tube (ee) Watch glass(j) Pipeclay triangle (u) Boiling tube (ff) Separating funnel (k) Bunsen burner (v) Dropping bottle (gg) Glass rodChapter 1 Fundamentals of chemistryChapter Exercise1. science, observations, experiments2. substances, compositions, structures, properties, changes3. Oxygen, atmosphere4. chemically combined together, hydrogen, oxygen.5. heating, electrolysis6. mixture7. chlorine, compound8. element, compound, mixture9. retains, different10. ppearance , dour , aste , ensity , elting11. chemical12. physical13. new14. A15. B16. D17. C18. C19. D20. B21. B22. A23. D24. A25. D26. C27. B28. D29. A30. (a) A = beaker, B = test tube, C = Bunsen burner, D = wire gauze, E = tripodstand, F = heat-proof mat, G = test tube holder, H = evaporating dish(evaporating basin)(b) (i) Test tube (B).(ii) Test tube(B), test tube holder (G), Bunsen burner (C) , heat-proof mat(F).(iii) Beaker (A), tripod stand (E), wire gauze (D), Bunsen burner (C),heat-proof mat (F).31. (a) Tasteless; no smell; colourless; liquid at room conditions(b) React with iron; react with sodium(c) Water changes into steam at 100o C. / Water changes into ice at 0o C.(d) It is because no new substance is formed.(e) Iron reacts with water to form iron rust. / Sodium reacts with water to formhydrogen gas.(f) New substance (e.g. rust or hydrogen gas) is formed.32. (a) Chlorine, hydrogen, iron, mercury , oxygen, sodium and sulphur(b) An element is a pure substance that cannot be broken down into anythingsimpler by chemical methods.(c) Ammonia, sodium chloride and water(d) A compound is a pure substance made up of two or more elementschemically combined together(e) A mixture consists of two or more pure substances (elements or compounds)which have not chemically combined together.(f) Sodium chloride solution is a mixture (because a solution is a homogeneousmixture).33. (a) No. Both oxygen and hydrogen are gases at room conditions while glucoseis a solid at room conditions. Carbon is black in colour while glucose iswhite.(b) Glucose solution is a mixture. It is because there is no chemical reactiontaking place between glucose and water.(c) Glucose + oxygen → carbon dioxide + water34. Compounds and mixtures are different in a number of ways. These include:(1) Compounds have fixed chemical composition while mixtures have variablechemical composition. Examples: water and air(2) During the formation of compounds, a chemical change occurs. Newsubstances are always formed. On the other hand, a mixture is obtainedwhen different substances are physically mixed. There is no chemicalchange. No new substance is formed and the change is seldom accompaniedby energy changes. Examples: formation of water from hydrogen andoxygen, mixing of sand and sugar(3) Properties of a compound are very different from that of its constituentelements. For example, water is colourless liquid while hydrogen and oxygenare colourless gases. On the other hand, each constituent substance retainsits own properties in mixtures. For example, nitrogen and oxygen are bothcolourless gases no matter whether they are isolated or present together inthe air.(4) Separation of the constituents of a compound requires a chemical process.For example, breaking water down into the elements hydrogen and oxygenrequires a chemical process called electrolysis. On the other hand,separation of a mixture requires a physical process only. For example,separation of iron powder from a mixture just requires the use of a magnet.35. -PHYSICAL PROPERTIES of a substance are those properties that can bedetermined without the substance changing into another substance.-Examples of physical properties of a substance include colour, odour (smell) and physical state. For example water is a colourless, odourless liquid under room conditions.-CHEMICAL PROPERTIES of a substance are the chemical reactions of the substance, and the respective conditions under which each reaction takes place.-Examples of chemical properties of a substance include how fast and vigorous it reacts (i.e., its reactivity) with another substance, the condition(s) needed for it to react with other substances and what products can be produced when it reacts with other substances. For example, hydrogen reacts vigorously with oxygen (or air) only when lit with a burning splint to form water.Class PracticeA2.1(a) People in ancient times had little scientific knowledge. In fact, any visibleportion of the Earth appeared more or less flat to the eyes.(b) Satellite photos clearly show that the Earth is roughly spherical.(Other answers may be given.)A2.2atmospherecrustmantleinner coreouter coreA2.31. (a) No. (7 planets have an atmosphere.)(b) Yes.2. There is no air on the Moon.A2.4Elements Compoundsnitrogen carbon dioxideoxygen water vapourheliumneonargonkryptonxenonA2.5Helium -269Neon -246Nitrogen -196Argon -186Oxygen -183Krypton -153Xenon -109Carbon dioxide -78Chapter Exercise1. crust, mantle , core, atmosphere2. atmosphere3. nitrogen, oxygen4. fractional distillation5. liquefied6. supporter7. glowing8. A9. A 10. B 11. B 12. B 13. D14. (a) Nitrogen (b) Carbon dioxide and water vapour (c) Oxygen, argon, neon, helium, krypton, xenon (any two)15. (a) The volumes of the three gases obtained i.e. argon, nitrogen and oxygen are930 litres, 78,000 litres and 21,000 litres respectively.(b) Fractional distillation (c) No. Oxygen is the most reactive gas in air, whereas nitrogen is unreactive,which serves the good purpose of ‘diluting ’ oxygen in air. If there were more oxygen in air, metals would be oxidized and corroded faster. Things would also burn easier, so there would be a greater hazard of fire.16. (a) Fractional distillation of liquid air (b) Oxidizing (c) Physical property: colourless, odourless Chemical property: it supports combustion (d) Put a glowing splint into a test tube containing the gas to be tested. If thegas is oxygen, the splint relights.17. (a) Nitrogen and oxygen (b) Oxygen (c) copper + oxygen → copper(II) oxide (d) 50 cm 3 - 33 cm 3 = 17 cm 3(e) 33cm50cm 17⨯ 100% = 34% (f) 21% (g) The percentage of oxygen in dissolved air (34 %) is much greater than thatin the atmosphere (21 %) because oxygen is more soluble in water than nitrogen.18. -Fractional distillation of liquid air is used to separate nitrogen and oxygenfrom air.-The air is first liquefied by repeated cooling and compression.-Then the liquid air is warmed up bit by bit very slowly.-Different gases in air boil at different temperatures, so they can be collected one by one.-The one boiling off first is nitrogen (boiling point -196 ︒C). The second one to be collected is argon(boiling point -186 ︒C) /noble gas. Then oxygen gas (boiling point -183 ︒C) is collected.Chapter 3 OceansChapter Exercise1. sodium chloride (common salt), sodium, chlorine2. evaporation3. filtration, crystallization4. saturated5. boiling, condensation6. distillate, residue7. distillation8. flame test9. brilliant golden yellow10. white11. water, white, blue, blue, pink12. Brine13. hydrogen, chlorine, sodium hydroxide14. B15. C16. B17. C18. D19. A20. (a) Filtration(b)(c) Distillation(d)(e) Test for sodium ions: Flame test. The sample gives a brilliant golden yellow flame in the flame test if sodiumions are present.Test for chloride ions: Silver nitrate testAdd silver nitrate solution to the sample, followed by excess dilute nitric acid. Theappearance of a white precipitate indicates the presence of chloride ions.21. His conclusion is not justified. He should add the white-powder to distilled waterand stir well, then filter and evaporate the filtrate to dryness by heating, and see if any solid is left.22. (a) This is because some metal ions can produce a characteristic coloured lightwhen they are heated strongly.(b) (1) Moisten a clean platinum wire with concentrated hydrochloric acid.(2) Dip the platinum wire into a crushed sample of the salt (or solution) tobe tested.(3) Heat the platinum wire with the sample strongly in a non-luminousflame.(4) Observe the colour of the flame at the wire and identify the metal ionspresent.(c) Potassium ions: lilac; calcium ions: brick red; copper (II) ions: bluish green.23. (a) It was not a suitable method because the liquid may be unclean, harmful oreven poisonous.(b) Flame test.(c) To show the presence of chloride ions, acidified silver nitrate solution isadded to the sample. If chloride ions are present, a white precipitate will be formed.(d) To show the presence of water, a few drops of the liquid are added toanhydrous copper(II) sulphate.The powder changes from white to blue if water is present.Alternatively, add a few drops of the liquid to dry cobalt chloride test paper. The paper changes from blue to pink if water is present. thermometerdelivery tubeclampboiling tube sodium chloridesolutionheatanti-bumping granulereceiver test tubewater pure water(e) He could not be sure that the liquid was sea water. Even if the tests showedthat sodium ions, chloride ions and water were present, the liquid might notnecessarily be sea water. For example, it might be just a sodium chloridesolution, without any other salts naturally present in sea water.24. (a) Electrolysis means ‘decomposition by electricity’.(b) Chlorine, hydrogen and sodium hydroxide.(c) Chlorine − water sterilization, manufacture of bleach, etc.Hydrogen − production of margarine, as rocket fuel, etc.Sodium hydroxide − manufacture of soap, extraction of aluminium, etc. 25. -Sea water is an important source of common salt (sodium chloride) which hasmany uses.-By the electrolysis of sea water, useful products, hydrogen, chlorine and sodium hydroxide are obtained. These products can be used to manufacture a lot of useful chemicals.-Hydrogen can be used to produce ammonia.- Chlorine can be used to produce bleach.- Sodium hydroxide can be used to produce soap.Class PracticeA4.1heat calcium oxide + carbon dioxideStep 1: calcium carbonate −−−−→Step 2: calcium oxide + water → calcium hydroxideStep 3: calcium hydroxide + water → calcium hydroxide solution (limewater) Step 4: calcium hydroxide solution (limewater) + carbon dioxide→ calcium carbonate + waterA4.2calcium carbonate + nitric acid → calcium nitrate + carbon dioxide + waterChapter Exercise1. mineral, a mixture of minerals2. crystalline, chemical3. extraction4. ore, aluminium5. haematite, carbon (or coke)6. chalk, marble7. Neutralizing, building material, cement (or other acceptable answers)8. Weathering9. Erosion10. PhysicalChemical11. uicklime, calcium oxide.12. acids, carbon dioxide13. milky14. iron + carbon dioxide15. aluminium + oxygen16. carbonic acid17. calcium hydroxide + heat18. calcium hydrogencarbonate19. calcium oxide + carbon dioxide20. calcium carbonate (white solid) + water21. calcium chloride + carbon dioxide + water22. B23. D24. D25. C26. A27. B28. D29. C30. C31. B32. (a) (1) Both react with acid to give out carbon dioxide.(2) Both are decomposed on strong heating.(b) (1) Neutralizing acidic soil and lakes affected by acid rain.(2) As a raw material to make glass by heating with sand and sodiumcarbonate.(3) As a raw material to make cement by heating with clay. (or any otherpossible answers)33. (a) Weathering is the slow process in which exposed rocks are broken downinto smaller pieces.(b) Physical weathering and chemical weathering.(c) It is because carbon dioxide in air dissolves slightly in rainwater, formingcarbonic acid. Carbonic acid can attack rocks.(d) Calcium hydrogencarbonate(e) calcium carbonate + carbonic acid → calcium hydrogencarbonate34. (a) Calcium carbonate(b) calcium carbonate −−−−→− heat strongcalcium oxide + carbon dioxide (c)(d) When the gas is passed through limewater for a few seconds, the limewater turns milky.35.(a) (i) Calcium oxide(ii) Calcium hydroxide(iii) Calcium hydroxide solution(b) (i) calcium carbonate −−→−heat calcium oxide + carbon dioxide(ii) calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water(iii) calcium oxide + water −→ calcium hydroxide(iv) carbon dioxide + calcium hydroxide solution−→ calcium carbonate + water (c) The rock fizzes (colourless gas is given out).heat limewaterPart I Planet earthPart Exercise1. C2. C3. A4. A5. C6. A7. B8. C9. A10. C11. D12. D13. C14. B15. A16. C17. B18. C19. B20. (a) Hydrogen ― as fuelOxygen ― in breathing aids (or any other possible answers)(b) No. Oxygen and hydrogen inside the container mix to form a gaseousmixture. All mixtures are impure substances.(c) Water(d) Yes. Water is a compound, and a single compound when existing alone is apure substance.21. (a) This conclusion is valid. The brick red colour in the flame test indicates thepresence of calcium, and the white precipitate formed when acidified silvernitrate solution is added indicates the presence of chloride.(b) This conclusion is invalid. The bubbles formed when acid was added maynot be carbon dioxide.(c) Conclusion (a) cannot be disproved. To test the validity of conclusion (b),pass the gas formed into limewater. If the limewater turns milky, the gas iscarbon dioxide, then the conclusion is valid. If the limewater doesn’t turnmilky, the conclusion is invalid.(d) The only validity of this statement is that the sample is a mixture containingcalcium chloride. Even carbonate is shown to be present, the tests carriedout are insufficient to rule out the possibility of other substances present inthe sample.22.(a) X : carbon dioxide; Y : water; Z : carbon dioxide.(b) calcium carbonate −−→−heat calcium oxide + carbon dioxideThe limestone cracks and makes a cracking noise.(c) calcium oxide + water → calcium hydroxide + heatA lot of heat is produced, with the possible production of some steamy vapour. The white solid turns into a paste.(d) calcium hydroxide + carbon dioxide → calcium carbonate + waterThe calcium hydroxide solution (limewater) turns milky.(e) This is the limewater test for carbon dioxide.23. (a) Refer to Coursebook 1 page 69.(b) Refer to Coursebook 1 page 70.(c) Frost action is a physical weathering process. This is because no newsubstances are formed during the process. Action of carbonic acid is a chemical weathering process. This is because carbonic acid changes calcium carbonate to a new substance, calcium hydrogencarbonate.(d) When excess of carbon dioxide is bubbled in, soluble calciumhydrogencarbonate is formed.Chapter 5 Atomic structureClass PracticeA5.1They are the only two liquid elements.A5.21. (a) Only an element can be classified as a metal or non-metal. Water is not anelement.(b) Non-metal.(c) Metal.2. (a) Mercury. All are metals. Mercury is a liquid, while the others are solids atroom conditions.(b) Sulphur. Sulphur is a non-metal, while the others are metals.(c) Iodine. All are non-metals. Iodine is a solid, while the others are gases atroom conditions.(d) Graphite. All are non-metals. Graphite conducts electricity, while the othersare non-conductors of electricity.A5.3(a) (i) Mg(ii)Ag(iii) Na(b)(i) Ar,(ii) He(iii) Ne(c) (i) fluorine(ii) bromine(iii) mercuryA5.4(a) 118(b) Br(c) N(d) The element copper or a copper atom.A5.5(a) The commonest type of hydrogen atom.(b) 91 electrons. Number of neutrons cannot be predicted.(c) It is not an atom. The numbers of protons and electrons are not equal.A5.6A magnesium atom would be changed to a chlorine atom.A5.71. (a) silver(b) silver(c) silver2. (a) Aluminium(b) Al1327(c) (i) 13(ii) 13(iii) 27 - 13 = 14A5.8(a) 3(b) O816(16O, or oxygen-16)A5.9(a) 37(b) 35(c) 4(d) 238(e) We cannot tell from the given data.(The mass number is not given.)A5.10(a) Relative atomic mass of sodium= mass number of the only type of sodium atom = 23(b) Relative atomic mass of neon=10010229020⨯+⨯= 20.2A5.11(a) (b)(c) (d)A5.12(a) 17(b) (i) 2,8,7(ii)Chapter 5 Atomic structureChapter Exercise1. physical2. bromine, mercury3. metals, non-metals4. metals, non-metals, graphite5. ymbol6. smallest part7. element8. atoms9. nucleus, neutrons, nucleus, electrons10. positively, negatively, neutral11. protons12. mass number13. same, different14. carbon-1215. weighted average, relative isotopic16. shells17. electronic arrangement (electronic configuration)18. B19. D20. D21. B22. C23. D24. C25. D26. (a) True. This is because there is no gaseous metal or semi-metal at roomconditions.(b) False. This is because mercury is a liquid metal at room conditions.(c) False. This is because carbon (graphite) is a non-metal which can conductelectricity. / This is because semi-metals cannot conduct electricity bythemselves.(d) False. This is because some metals (e.g. sodium) are soft.(e) True. This is because metals are silvery white, golden or brown in colour.No metal is red in colour.27. (a) The mass number of an atom is the sum of the number of protons andneutrons in the atom.(b) The atomic number of an atom is the number of protons in the atom.(c) Isotopes are different atoms of the same element, with the same number ofprotons (and electrons) but different numbers of neutrons.(d) Atom Number of protons Number of neutrons Electronic configuration105B 5 5 2, 3 115B 5 6 2, 3 (e) 10.810080112010=+⨯⨯ 28. (a) Q and R(b) Carbon(c) Carbon-13 and carbon-14(d) 135P , 136Q , 146R , 147S29. (a)AtomAtomic no. Mass no. Number of Electronic arrangement protons neutrons electrons (a)35Cl 17 35 17 18 17 2, 8, 7 (b)17O 8 17 8 9 8 2, 6 (c) 40Ar 18 40 18 22 18 2, 8, 8(b)(c) Neon(d) Argon is very unreactive.30. - Elements can be classified according to their physical states . For example, atroom temperature, hydrogen and oxygen are gases; bromine and mercury are liquids; carbon and iodine are solids.- Elements can also be classified into metals and non-metals. A few elementshave properties in between those of metals and non-metals. They are classified as semi-metals.- Examples of metals include sodium and mercury; examples of non-metalsinclude bromine and hydrogen; examples of semi-metals include boron and silicon.Class PracticeA6.1(a) Period 7, Group II; alkaline earth metals.(b) Radium.(c) Yes. Radium is a metal (all metals conduct electricity).A6.2Element X: MetalElement Y: Non-metalElement Z: We cannot tell from the given data as elements in Group IV can be a metal, non-metal or semi-metal.A6.3(a) 2, 8, 8, 2.(b) Yes, it is a metal.(c) (ii).A6.4(a) Yes. By knowing the chemical properties of familiar elements in the same groupand the group trend, predictions about the unfamiliar element can be made. (b) Astatine: D; strontium: AChapter Exercise1. electrons, outermost2. ascending, atomic numbers3. period, group, eight,4. period number, outermost5. metals, semi-metals, non-metals6. chemical7. 1, 1, increases8. 7, halogens, decreases9. 8, noble gases10. B11. B12. D13. C14. C15. C16. C17. D18.19. (a) 2(b) They all have two electrons in the outermost shell.(c) Increase down the Group.(d) (i) Beryllium reacts very slowly with water.(ii) Barium reacts vigorously with water.(e) Barium is more reactive than calcium. It should be stored under paraffin.20. (a) Magnesium, silicon, chlorine. They are in Period 3.(b) Lithium, rubidium, caesium. They are in Group I.(c) Iron, copper(d) Caesium(e) Fluorine(f) Silicon(g) Helium(h) Helium, fluorine, chlorine(i) Fluorine, chlorine21. (a) Group II(b) Alkaline earth metals(c) Strontium has 2 outermost shell electrons.(d) Strontium is a silvery white solid at room conditions.(e) Strontium reacts with cold water more readily than calcium does andcolourless gas bubbles are given off. This is because the reactivity of GroupII elements will increase down the group.22. - In the modern Periodic Table, elements are arranged in ascending order ofatomic number.- The elements are arranged in periods and groups of the Periodic Table.A horizontal row of elements is called a period while a vertical column ofelements is called group.- Period number = number of occupied electron shellsGroup number = number of electrons in outermost shell- Elements within the same group of the Periodic Table have similar chemical properties.- Across a period, the elements change from metals through semi-metals to non-metals.- Some of the groups have special names. Group I elements are named as alkali metals; Group II elements are named as alkaline earth metals; Group VII elements are named as halogens; Group 0 elements are named as noble gases.The elements in between Group II and Group III are called the transition elements.Chapter 7 Chemical bonding: ionic bondingClass PracticeA7.1(a) Delete ‘non-metals’.(b) Delete ‘metals’.A7.2(a) Colourless(b) Purple(c) Yellow(d) GreenA7.3(a) The cathode. Potassium ions are positively charged. They are thus attractedtowards the negative electrode (cathode).(b) No. Potassium ions are colourless.(c) A green patch would move towards the negative electrode (cathode).Chromium(III) ions are green in colour and positively charged. They are attracted towards the negative electrode.A7.4(a) (i) Aluminium atom: 2, 8, 3; aluminium ion: 2, 8(ii) Chlorine atom: 2, 8, 7; chloride ion: 2, 8, 8(b) Charge on aluminium ion = +3; charge on chloride ion = -1A7.5Simple ions: H+, H-, Mn2+Polyatomic ions: NH4+, NH2-, OH-A7.6(a)(ii) At-A7.7(a)(b)A7.8(a) CuCl2(b) CaS(c) Al(OH)3(d) (NH4)2CO3A7.9(a) Mg(OH)2(b) Na2O(c) PbSO4(d) K2Cr2O7A7.10(a) Calcium nitrate(b) Iron(III) chloride(c) Zinc sulphate-7-water(d) Copper(II) hydroxideChapter 7 Chemical bonding: ionic bondingChapter Exercise1. octet, duplet2. electrons, noble gas, ions3. simple, polyatomic4. cations, anions5. coloured6. electrolysis7. name, formula8. group9. minus10. ionic, ionic, calcium oxide, calcium, oxygen, Calcium (Ca2+), oxide (O2-), ionicbonds11. giant ionic structure12. B13. A14. D15. A16. C17. A18. C19. B20.21. (a) Calcium sulphate(b) Cation: calcium ion; anion: sulphate ion(c) Ionic bonding(d) CaSO4(e) The coagulant is white in colour.(g) Polyatomic ion22. (a) A: 2,5; B: 2, 8, 1; C: 2, 8, 2; D: 2, 8, 6(b) Elements A and D tend to gain electrons to attain an octet of electrons.(c) Elements B and C tend to lose electrons to attain an octet of electrons.(d) 4(e) B3A; B2D; C3A2; CD(f)B3A B2DC3A CD23. (a) Magnesium chloride: MgCl2; potassium chloride: KCl; sodium chloride:NaCl(b)MgCl2KClNaCl(c) Giant ionic structure24. -Consider the reaction between sodium and chlorine.A sodium atom Na has the electronic arrangement 2,8,1. It loses 1 electron toget the stable octet structure to form a Na+ ion.-A chlorine atom Cl has the electronic arrangement 2,8,7. It gains 1 electron to get the stable octet structure to form a Cl- ion.-When sodium atom reacts with a chlorine atom, the sodium atom loses 1 electron to the chlorine atom. By transfer of electron, two ions are formed.The electrostatic force between the ions is called ionic bonds and the compound is called ionic compound.electronsodium atom(Na) (loses one electron)sodium ion(Na+) chlorine ion(Cl-)transferchlorine atom(Cl)(gains one electron)。
化学专业教学培养方案

化学专业教学培养方案一、专业特色化学专业依托华东理工大学国家一流学科—化学学科,在化学及相关学科前沿领域的科学研究、化学产品合成和配方设计、化学产品检验分析和性能测试等方面形成特色,学生毕业后可在教育、科研院所、医药、材料、能源、生物、环境、化工、食品和日用化学、金融贸易等领域的各类企事业单位就业,或进入化学及相关学科深造,成为研究型专业人才。
二、培养目标化学专业以培养一流的化学及相关领域的复合型专业人才为目标,毕业学生应具有扎实的化学基础理论知识和专业知识,以及数、理、外语和计算机等公共基础知识和技能,具备一定的科学研究、产品开发和实践创新能力,拥有坚实的家国情怀、良好的人文素养、强烈的社会责任感、高尚的道德情操、广阔的国际视野和流畅的国际交流能力。
要求五年以上的毕业生:➢能够从事化学及相关领域科学研究、技术开发、经营管理等工作,适应独立和团队工作环境。
➢以社会责任感、法律、道德修养、安全与环境意识和经济等方面的视角理解和解决多学科的问题。
➢在终身学习、专业发展、竞争能力和领导能力上表现出担当和进步,在化学及相关领域具有职场竞争力。
三、毕业要求本专业学生毕业时应当达到普通高等学校本科专业认证标准规定的能力,共有9条:1. 具有人文底蕴、科学精神、职业素养和社会责任感,了解国情社情民情,践行社会主义核心价值观;2. 具有扎实的基础知识和化学专业知识,掌握必备的化学研究方法,了解化学及相关领域最新动态和发展趋势;3. 具有批判性思维和创新能力。
能够发现、辨析、质疑、评价化学及相关领域现象和问题,表达个人见解;4. 有解决复杂问题的能力。
能够对化学及相关领域复杂问题进行综合分析和研究,并提出相应对策或解决方案;5. 有信息技术应用能力。
能够恰当应用现代信息技术手段和工具解决实际问题;6. 具有较强的沟通表达能力。
能够通过口头和书面表达方式与同行、社会公众进行有效沟通;7. 具有良好的团队合作能力。
能够与团队成员和谐相处,协作共事,并作为成员或领导者在团队活动中发挥积极作用;8. 具有国际视野和国际理解能力。
高分子专业导论教学大纲

高分子材料与工程专业评估材料附件1:课程简介及教学大纲化学与化工学院二零一五年十二月高分子材料与工程专业课程简介目录《高分子专业导论》 (1)《无机及分析化学》 (2)《有机化学A》 (3)《物理化学》 (4)《基础化学实验(1)》 (5)《基础化学实验(2)》 (6)《基础化学实验(3)》 (7)《基础化学实验(4)》 (8)《化工原理B》 (9)《化工原理实验B》 (10)《高分子化学》 (11)《高分子化学实验》 (12)《高分子物理》 (13)《高分子物理实验》 (14)《高分子合成工艺原理》 (15)《聚合物成型加工原理 (16)《高聚物成型机械及模具》 (17)《高分子材料研究方法》 (18)《化工仪表及自动化》 (19)《有机结构理论》 (20)《材料科学与工程基础》 (21)《高分子流变学》 (22)《高分子材料进展》 (23)《高分子形态结构与性能》 (24)《专业英语》 (25)《科技文献检索与利用》 (26)《涂料及粘接剂》 (27)《合成纤维》 (28)《聚合反应工程》 (29)《高聚物分子设计》 (30)《特种及功能高分子》 (31)《高聚物合成工艺设计》 (32)《聚合物基复合材料》 (33)《高分子加工新技术》 (34)《高分子材料改性》 (35)《橡胶加工工艺》 (36)《化工设计》 (37)《高分子安全与环境》 (37)《高分子行业标准与法规》 (37)《化学综合实验A》 (40)《化学综合实验B》 (41)《化工原理课程设计B》 (42)《化工实习实训B》 (43)《阶段性生产实习A》 (44)《阶段性生产实习B》 (45)《高分子工程实践》 (46)《工程师基础训练A》 (47)《工程师基础训练B》 (48)《高分子专业课程设计》 (49)《毕业设计(论文)》 (50)高分子专业导论Introduction to Polymeric Materials课程编号:0610292B学时:8学分:0.5开课学期:1课程性质:必修选课对象:高分子材料与工程专业本科生先修课程:无后续课程:材料科学与工程基础内容概要:主要介绍高分子课程设置体系、规律,高分子科学的历史、现状和发展趋势,认识高分子,初步掌握与高分子材料工程相关的基本术语和概念,了解高分子合成、改性、加工、应用等基本知识,掌握专业学习方法与技巧。
Fundamentals 林产化工专业英语知到章节答案智慧树2023年东北林业大学

Fundamentals of Forest Products Chemical Processing 林产化工专业英语知到章节测试答案智慧树2023年最新东北林业大学绪论单元测试1.Generally, gum rosin is produced by chemical processing of woody rawmaterial.参考答案:错第一章测试1.Trace elements are essential to life.参考答案:对2.Elements with similar chemical properties generally fall into the same groupin the periodic table.参考答案:对3. A pure substance always has the same physical and chemical properties andis either an element or a compound.参考答案:对4.Calcium oxide is an inorganic compounds.参考答案:对5.Any chemical species capable of binding to electron pairs is called a Lewisacid; any molecule that tends to donate an electron pair is referred to as a Lewis base.参考答案:对6.In either case, the position of the multiple bond is indicated by numberingfrom the end of the chain, starting at the end that will assign the lowernumber to the first carbon atom of the multiple bond.参考答案:对7.Ethers tend to be reactive, and low molecular mass ethers are often used assolvents.错8.If one or both of the hydrogen atoms on the amide nitrogen atom arereplaced by hydrocarbon groups, the structure is named as an N-substituted amide.参考答案:对9.By doing research in chemistry, we can find new cures for diseases as well asbetter chemicals to use in our natural environment.参考答案:对第二章测试1.We can eat or drink in laboratories参考答案:错2.If a liquid gets into your eyes, the eye should be washed immediately withclean running water for at least 10min.对3.In the case of fire alarm, we need stop all of your ongoing work, turn off gasand electric devices.参考答案:对4.There is no need to label glass or flask filled with solution.参考答案:错5.If you work with flammable solvent, make sure there is no source of ignitionclose by.参考答案:对第三章测试1.Fossilised biomass has been exploited as coal and oil.对2.The relative distribution of phenolic nuclei in lignin strongly differs betweenplant species.参考答案:对3.The role of hemicellulose is to modify and crosslink the basic fibrils andpromote the interaction between other biopolymers.参考答案:对4.Softwoods are mainly vessel members, fibres, and parenchyma, andhardwoods are mainly made of made of tracheids and parenchyma.参考答案:对5.The outer layer (S1) is thicker than The middle layer (S2).参考答案:错6.Klason lignin is a standard analytical method to measure the lignin contentof lignocellulosic biomass.参考答案:对7.For esterification reaction of cellulose, the conversion was low and forsilynation reaction, the conversion is good but with a high cost.参考答案:对8.Cellulose has many hydroxyl groups which can form hydrogen bondinglinked network easily.参考答案:对9.Highly porous aerogels usually tend to show higher oil-sorption capacities参考答案:对10.Starch only has one forms, i.e. amylose.参考答案:错11.In addition to glucomannan, minor amounts of miscellaneouspolysaccharides are present in hardwoods.参考答案:错12.The softening temperature (Ts) of lignins in dry state is higher than that inmoist lignins.参考答案:对13.The reactivity of lignin has nothing to do with its solubility.参考答案:错14.Lignin mainly has two types of linkages including C-C and C-O-C.参考答案:对15.The non-bonded orbital interactions (π-π interactions) of the aromaticgroups could caused the association of lignin molecules.参考答案:对16.The aromatic moieties present in the lignin structure will improve themechanical and thermal properties of epoxy resins.参考答案:对17.There are two different types of polysaccharide hydrolysis, involving acidic(sulfuric acid) or enzymatic reactions.参考答案:对18.Terpene is derive from a 5C compound, isoprene.参考答案:对19.Polyphenols also characteristically possess a significant binding affinity forproteins, which can lead to the formation of soluble and insoluble protein-polyphenol complexes参考答案:对20.Cardanol double bond may be epoxidised or undergo olefin-metathesisreaction which may act as modified monomer for the formation of different set of polymers.参考答案:对第四章测试1.Sodium hydroxide and calcium hydroxide can be used instead of potassiumhydroxide to neutralize the acid.参考答案:对2.Hot air blows into this dryer to bring the sugars humidity level down to 0.05percent, that standard for table sugar.参考答案:错3.The main resistance to the flow of heat or mass to a surface lies within thelaminar sublayer.参考答案:对4.Filter cakes are formed late in the fluid flow.参考答案:错5.Extraction may be used to separate more than two components; andmixtures of solvents, instead of a single solvent, are needed in someapplications.参考答案:对6.Heat fluxes may then be based either on the inside area or the outside area ofthe choice is arbitrary.参考答案:对7.Any evaporator that uses pump to ensure higher circulation velocity is calleda forced circulation evaporator.参考答案:对8.If the feed is introduced at one point along the column shell, the column isdivided into an upper section, which is often called the stripping section, anda lower section, which is often referred to as the rectifying section.参考答案:错9.Drying is an absolute term that means a certain value.参考答案:错第五章测试1.The traditional techniques of solvent extraction of plant materials are mostlybased on the correct choice of solvents and the use of heat or/and agitationto increase the solubility of the desired compounds and improve the masstransfer.参考答案:对2.They spray the rubber cubes with a mix of calcium carbonate and solvent themixture forms the film on the cubes that prevents mold and keeps them from sticking together during transport.参考答案:对3.Biomass is mostly made up of carbon, hydrogen, compounds did not burncalled ash.参考答案:对4.Biochemical approaches include three unit-operations namely, pretreatment,hydrolysis, and distillation.参考答案:错5.Furfural can be produced by a one-step or a two-step process. The advantageof the one-step process is that a higher quantity of furfural is produced when compared to the two-step process.参考答案:错6. A worm screw supply the mixture to a press roll, a constant spray of waterkeeps the mixture from sticking to it.参考答案:对7.Chemical activation is usually carried out at lower temperatures (from 400 to700℃) with activating agents like phosphoric acid, potassium hydroxide,sodium hydroxide and zinc chloride.参考答案:对8.Generally, the cellulosic cell wall from nearly any plant or trees can be usedfor pulp.参考答案:对9.When the paper is dry it may be treated with stabilizing materials andsurface finishes to improve durability or printability.参考答案:对。
化学测量学与技术专业英语词汇

化学测量学与技术专业英语词汇摘要化学测量学与技术专业是一门综合性的学科,它涉及到化学分析、仪器分析、计算机应用、数据处理等多个方面。
这个专业的学生在学习和工作中需要掌握一些相关的英语词汇,以便于与国内外的同行进行交流和合作。
本文从以下几个方面总结了一些化学测量学与技术专业需要用到的英语词汇:基础课程专业课程选修课程仪器设备化学测量方法化学数据处理化学测量领域基础课程化学测量学与技术专业的基础课程主要包括无机化学、有机化学、分析化学、物理化学、微积分、线性代数、概率论与数理统计等,旨在为学生打下扎实的化学和数学基础,为后续的专业课程和实践活动提供理论支撑。
以下是一些基础课程的中英文对照表:中文英文无机化学Inorganic Chemistry有机化学Organic Chemistry分析化学Analytical Chemistry物理化学Physical Chemistry微积分Calculus线性代数Linear Algebra概率论与数理统计Probability Theory and Mathematical Statistics专业课程化学测量学与技术专业的专业课程主要包括模拟与数字化仪器、分析化学漫谈、现代分析化学基础、生化分析实验技术、信息获取技术与数据处理等,旨在为学生提供化学测量的基本原理和方法,以及仪器分析的基本技能和应用,培养学生的创新精神和实践能力。
以下是一些专业课程的中英文对照表:中文英文模拟与数字化仪器Analog and Digital Instruments分析化学漫谈Introduction to Analytical Chemistry现代分析化学基础Fundamentals of Modern Analytical Chemistry生化分析实验技术Biochemical Analysis Experimental Techniques信息获取技术与数据处理Information Retrieval Technology and Data Processing选修课程化学测量学与技术专业的选修课程主要包括光谱分析、质谱分析、色谱分析、电化学分析等,旨在为学生提供更多的化学测量的专业知识和技术,以及不同领域的应用案例,拓宽学生的知识面和视野,满足学生的个性化需求和兴趣。
安全工程专业人才培养方案

程
学 分
类 别
学
时
学分 一
学时
学分 二
学时
学分
三
各学
学时
期计
学分
四
划学
学时
分 五
与
学时 六
学分 学时 学分 学时
学分 七
学时
学分 八
学时
合计
学分 学时
占总学分比例
占总学时比例
平台
公共基础 学科基础
平台
平台
16
4.5
272
72
23
10
400
160
9
6.5
160
104
7
7
128
112
3
6
48
96
2 32
5 80
线性代数 A Linear Algebra A
2 32
概率与数理统计
Probability & Mathematical 3 48 Statistic
文化素质教育课
Culture and Education for all-round 4 64
development
工程制图(Ⅱ) Engineering Drawing(Ⅱ)
Speaking(Ⅳ)
大学计算机基础 Introduction to Computer
2 32
大学物理 C(I) College Physics C(Ⅰ)
3 48
大学物理 C(II) College Physics C(Ⅱ)
3 48
微积分 A (Ⅰ) Calculus A(Ⅰ)
5 80
微积分 A (Ⅱ) Calculus A(Ⅱ)
中英文课程名称对照
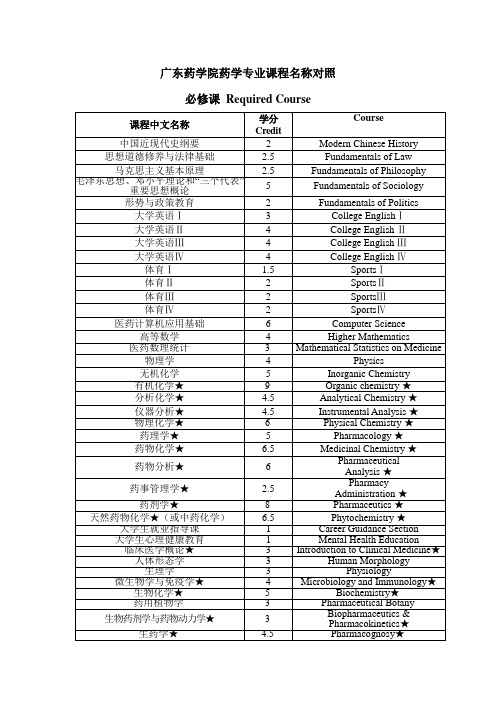
6.5
Medicinal Chemistry★
药物分析★
6
Pharmaceutical
Analysis★
药事管理学★
2.5
Pharmacy
Administration★
药剂学★
8
Pharmaceutics★
天然药物化学★(或中药化学)6.5ຫໍສະໝຸດ Phytochemistry★
大学生就业指导课
1
Career Guidance Section
Concocted Science of Natural Medicine★(中药炮制学)
Pharmaceutics ofNatural Medicine★(中药药剂学)
Pharmacology of Natural Medicine★(中药药理学)
Prescription Studies(方剂学)
物理学
4
Physics
无机化学
5
Inorganic Chemistry
有机化学★
9
Organic chemistry★
分析化学★
4.5
Analytical Chemistry★
仪器分析★
4.5
Instrumental Analysis★
物理化学★
6
Physical Chemistry★
药理学★
5
Pharmacology★
军训(含入学教育)
3
Orientation
思想政治理论课实践教学课
2
Practicum ofMoral Education
采药
2
Collecting Herbs
毕业实习(包括毕业论文)
国外化学名著系列

国外化学名著系列
国外化学名著系列是指那些在国际化学界具有重要地位和影响
的经典著作。
这些著作涵盖了化学的各个领域,从基础理论到应用研究都有覆盖。
这些著作的作者包括了世界各地的知名化学家,他们的研究成果和理论贡献对于化学学科的发展起到了积极的推动作用。
《国外化学名著系列》包括但不限于:
1.《无机化学原理》(Inorganic Chemistry Principles):由美国化学家哈尔-布林克(Harry B. Gray)和爱德华-穆森(Edward M. Stiefel)合著,系统介绍了无机化学的基本概念和原理。
2.《有机化学》(Organic Chemistry):由美国化学家弗朗西斯-凯瑟尔合著,是一本经典的有机化学教材,系统介绍了有机化学的基本概念和反应机理。
3.《分析化学基本原理》(Fundamentals of Analytical Chemistry):由美国化学家道格拉斯-斯库贝尔合著,是分析化学领域的经典教材,介绍了分析化学的基本原理和方法。
4.《物理化学》(Physical Chemistry):由英国化学家彼得-阿特金斯合著,是一本综合性的物理化学教材,介绍了物理化学的基本概念和理论。
5.《化学反应工程》(Chemical Reaction Engineering):由美国化学家奥古斯特-维斯特合著,是化学反应工程领域的经典著作,介绍了化学反应工程的基本原理和应用。
这些著作被广泛应用于化学教育和研究领域,对化学学科的发展
和进步起到了重要作用。
化学的今天和明天中英对照

化学的今天和明天中英对照
以下是《化学的今天和明天》一书中的部分中英对照内容:
- 化学的今天与明天:Chemistry Today and Tomorrow The Central, Useful and Creative Science
- 作者:Ronald Breslow(R.布里斯罗):Author: Ronald Breslow - 本书出版后,受到美国化学界的普遍好评:After publication, this book was widely praised in the American chemical community.
- 康奈尔大学的R霍夫曼(Roald Hoffmann,1937-)1981年诺贝尔化学奖获得者,认为,此书不单展示了化学的实用性,而且还展示了化学的生机勃勃的形象:Cornell University's Roald Hoffmann (1937-), winner of the 1981 Nobel Prize in Chemistry, believed that this book not only demonstrated the practicality of chemistry, but also showed a vibrant image of chemistry.
- 美国化学会为了普及化学科学的知识,将向全美中学化学教师赠送此书:In order to popularize the knowledge of chemical science, the American Chemical Society will present this book to all high school chemistry teachers in the United States.。
化学专业本科人才培养方案(专业代码070301)

化学专业本科人才培养方案(专业代码:070301 )化学类(大类代码:0703)一、培养目标本专业培养具有良好的科学、文化素养,能够较系统扎实地掌握化学基础知识、基本理论和基本技能,富有创新意识和实践能力,能在化学及相关领域从事科研、教学及其他工作的应用和研究型人才。
二、培养特色培养具有良好的人文和科学素质以及强烈的社会责任感,创新意识和实践能力强,具有宽阔的国际化视野和团队合作意识,能胜任化学及相关领域科研、教学及其他工作的卓越人才或拔尖人才。
三、培养要求本专业学生主要学习化学及相关学科的基础知识、基本理论和基本技能,具有一定的人文和社会科学知识,接受较系统的科学思维和科学研究的基本训练,初步具备综合运用化学及相关学科的基本理论和技术方法进行研究、教学和开发的能力。
毕业生应获得以下几方面的知识和能力:1.具有高度的社会责任感、良好的科学文化素养和较强的创新意识;2.系统掌握化学基础知识、基本理论和基本技能,了解化学的知识体系、学科前沿、发展趋势和应用前景;3.掌握本专业所需的数学、物理学等学科的基本内容,初步掌握生命、环境、材料、能源等相关领域的基础知识;4.掌握一定的信息技术,具有获取、加工和应用信息的能力;5.能够发现、提出、分析和解决问题,具有从事化学研究、教学和其他实际工作的能力;6.具有较强的学习、交流、协调能力和团队合作精神,适应科学和社会的发展;7.具有一定的国际视野和跨文化环境下的交流、竞争与合作的能力。
四、主干学科化学五、学制、学位、毕业最低学分四年,理学学士,165学分六、核心课程无机化学(Ⅰ、Ⅱ)、分析化学(Ⅰ、Ⅱ)、有机化学(Ⅰ、Ⅱ)、物理化学(Ⅰ、Ⅱ)、仪器分析、结构化学、波谱学、生物化学、化学工程基础、有机合成、基础化学实验(Ⅰ、Ⅱ)、综合化学实验、化学工程基础实验。
主要实践性教学环节:创新实验、毕业论文、军事训练等。
主要专业实验:基础化学实验(Ⅰ)、基础化学实验(Ⅱ)、综合化学实验、探究性化学实验、化学工程基础实验、有机合成实验等。
材料成型及控制工程专业培养计划

一、培养目旳本专业培养具有材料、机械、计算机、电力电子等领域基础理论知识,能在材料成型与控制工程技术领域从事技术开发、生产及经营管理等方面工作,并具有初步研究能力旳工程技术人才。
二、基本规定本专业学生重要学习材料、机械、计算机、电力电子等学科基础理论,学习材料成型加工工艺与设备、材料成型构造与材料、性能测试与分析、质量检测与控制等专业知识。
通过教学、社会实践与工程实践,毕业生应获得如下几方面知识与能力:(1)具有扎实旳自然科学基础知识,具有较强旳外语与计算机应用能力。
(2)具有扎实旳生产管理知识及有关工程技术知识,并掌握一定旳人文、社会科学基础知识。
(3)系统掌握材料成型及控制工程旳专业知识。
(4)较纯熟掌握CAD/CAM/CAE 软件,以及其他信息化材料成型技术及手段。
(5)具有较强旳知识获取能力、工程实践能力和创新意识。
(6)具有开发材料成型领域旳新材料、新工艺、新设备旳能力,并具有初步有关领域旳研究能力。
三、学制与学位学制:四年学位:工学学士四、专业特色学生不仅具有宽厚旳基础理论知识和较强旳工程实践能力,并且通过将最新科研成果引入教学过程,使学生具有如下特色:1、围绕轨道交通,根据国民经济重大需求,结合铁路重大工程建设,培养国家急需交叉、复合型人才。
将先进材料技术、先进成型制造技术、信息化技术等引入培养计划,培养新型旳材料成型及控制工程技术人才。
2、系统掌握焊接科学与工程旳基本理论知识,受益于轨道交通大型焊接装备技术、重型装备制造、新能源领域旳先进焊接工程技术等学科特色优势,在轨道交通、装备制造、能源等领域等就业方面具有较大优势。
3、学生在本科学习期间,根据其爱好和爱好,可开设“国际焊接工程师培训”。
通过此项目培训旳本科生具有完整旳国际焊接高级技术人才知识体系,毕业时不仅具有“毕业证”、“学位证”,同步还具有“国际焊接工程师”资格证,可以直接参与国际焊接工程,培养人才直接与国际接轨。
五、主干学科与主干课程主干学科:材料科学与工程主干课程:高等数学、大学物理、外语、物理化学、工程化学、理论力学、材料力学、材料科学基础、电工技术基础、电子技术、机械制图、机械制造技术基础、机械设计基础、计算机应用基础、计算机程序设计基础、微机原理及应用、材料加工成型基础、材料力学性能、材料成型控制基础、焊接措施与设备、焊接构造、焊接冶金、材料焊接性。
2019级选课说明级第一学期课程目录理学与材料菁英班选课说明
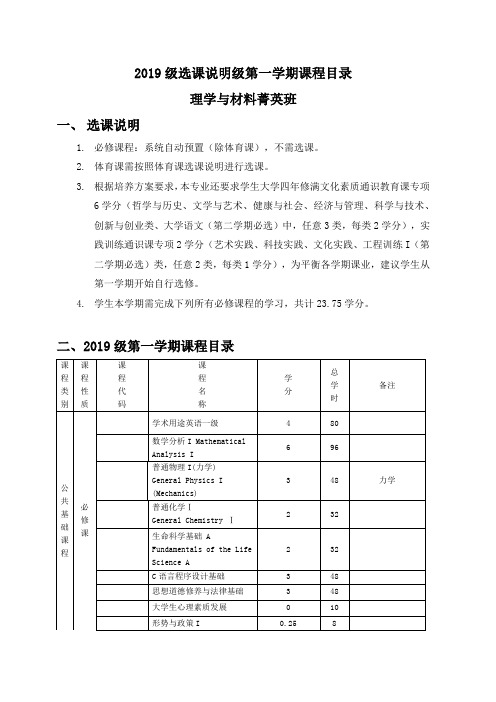
2019级选课说明级第一学期课程目录
理学与材料菁英班
一、选课说明
1.必修课程:系统自动预置(除体育课),不需选课。
2.体育课需按照体育课选课说明进行选课。
3.根据培养方案要求,本专业还要求学生大学四年修满文化素质通识教育课专项
6学分(哲学与历史、文学与艺术、健康与社会、经济与管理、科学与技术、创新与创业类、大学语文(第二学期必选)中,任意3类,每类2学分),实
践训练通识课专项2学分(艺术实践、科技实践、文化实践、工程训练I(第
二学期必选)类,任意2类,每类1学分),为平衡各学期课业,建议学生从
第一学期开始自行选修。
4.学生本学期需完成下列所有必修课程的学习,共计23.75学分。
二、2019级第一学期课程目录。
甲基磺酸催化丙烯酸和十二醇酯化反应动力学研究

甲基磺酸催化丙烯酸和十二醇酯化反应动力学研究杜金霞;彭必雨【摘要】在环己烷为带水剂、甲基磺酸为催化剂和对羟基苯甲醚为阻聚剂的条件下进行了丙烯酸和十二醇的酯化反应,讨论了催化剂用量、酸醇比和温度等对丙烯酸十二酯酯化反应的影响,用反应过程中酸值的变化计算出反应物浓度的变化并采用计算机拟合得到反应速率常数.通过对系列温度下反应速率常数的计算机拟舍得到甲基磺酸催化的丙烯酸和十二醇反应的动力学方程为r=dx/dt=[6.0979×1023×exp(-19 606.6/T)]×(CA,0-x)(CB,0-x),其中x为反应生成的丙烯酸十二酯浓度,CA,0和CB,0分别为丙烯酸和十二醇的初始浓度.该体系的反应动力学方程式符合Arrhenius公式,温度升高对反应有利,115~125℃是比较理想的反应温度区间.【期刊名称】《青岛科技大学学报(自然科学版)》【年(卷),期】2016(037)004【总页数】4页(P399-402)【关键词】丙烯酸;十二醇;甲基磺酸;酯化反应;动力学【作者】杜金霞;彭必雨【作者单位】四川大学轻纺与食品学院,四川成都610065;四川大学轻纺与食品学院,四川成都610065;四川大学制革清洁技术国家工程实验室,四川成都610065【正文语种】中文【中图分类】O621.25丙烯酸长链酯聚合物广泛被应用于形状记忆凝胶[1]、药物传输[2]、油品添加剂[3]及皮革化学材料[4]等领域,其单体的制备可以采用酯交换法[5]或直接酯化法。
丙烯酸酯的合成多采用直接酯化法。
目前对于丙烯酸长链酯的研究主要集中于催化剂及反应条件的选择,且使用最多的催化剂是对甲苯磺酸[6-7],而对于酸性更强的甲基磺酸作为催化剂的研究却未见文献报道。
本工作以甲基磺酸为催化剂[8],加入少量阻聚剂防止因丙烯酸及生成的丙烯酸十二酯的聚合副反应而降低产物收率[9],在探讨甲基磺酸催化机理的基础上,测定了甲基磺酸为催化剂条件下丙烯酸与十二醇酯化反应的动力学参数,并考察了温度、催化剂用量对速率常数的影响。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Sichuan University
Department of Chemical Engineering
Fundamentals of Modern Chemistry(Ⅰ)-1
Course Syllabus
Course Name Fundamentals of Modern Chemistry(Ⅰ)-1
Course No.308114030
Department Chemical
Engineering
Hours48
Academic
credit
3
Course Descriptions The course focuses on the structures of chemicals,principles of chemistry and chemical reaction.It includes atomic structures,periodic properties of elements, intermolecular forces and hydrogen bonding,concepts of coordination compounds, bonding theory and crystal field theory of coordination compounds;thermo chemistry,direction of reaction,principles of chemical equilibrium,chemical kinetics;theories of acids and bases,acid-base equilibrium,acid-base equilibrium calculations;precipitation-solubility equilibrium.
Course Materials(Textbooks)
Fundamentals of Modern Chemistry,2nd edition,by Sichuan University,Higher Education Press
ISBN978-7-04-019318-3
Grading
Class participation10%
Homework20%
Final exam70%
Instructor Information
Xuefei Lai,Lecturer,Department of Chemical Engineering,Sichuan University
Tentative Course Schedule
Chapter Title Topic
1Atomic Structure and
Elemental Cycles 1)The Microscopic Particle Motion
2)Extra Nuclear Movement
3)Atomic Structure and Periodic Table
4)Atomic Structure and the Cyclical Change of Element Properties
2Molecular Structure and
Crystal Structure 1)Ionic Bond and Ionic Crystal
2)Covalent Bond
Sichuan University
3)Atomic Crystal and Mixed Crystal
4)Metal and Metal Crystal
5)Crystal Defects
6)Van Der Waals Force and Hydrogen Bond
7)Ion Polarization Effects
3Coordination Compounds
Structure 1)Basic Concept of Complexes
2)Valence Bond Theory of Complexes
3)The Crystal Field Theory of Complexes
4)The Molecular Orbital Theory of Complexes
4Basic Principle of
Chemical Reaction 1)Solution and Ideal Gas
2)Energy Relations in Chemical Reaction
3)Directions of Chemical Reaction
4)Chemical Equilibrium
5)Chemical Reaction Rate
5Acid-base Reaction1)Acid-base Theory
2)Acid-base Equilibrium
3)Calculation of Acid-base Equilibrium
4)Buffer Solution
6Precipitation Reaction1)Solubility Product
2)Generation and Dissolution of Precipitation
3)Conversion of Precipitation and Step-by-step Precipitation
4)Factors Affecting the Balance
5)Precipitation Separation。
