Salmeterol_xinafoate_HNMR_11611_MedChemExpress
美国FDA批准一种用于阿尔茨海默病早期诊断的检测仪

美国FDA批准一种用于阿尔茨海默病早期诊断的检测仪夏训明(编译)
【期刊名称】《广东药科大学学报》
【年(卷),期】2022(38)3
【摘要】美国FDA于2022年5月4日批准Fujirebio诊断公司(Fujirebio Diagnostics,Inc.)研发的一种名为“The Lumipulse G β-Amyloid Ratio(1-42/1-40)test”的体外诊断检测仪,用于检测与阿尔茨海默病(Alzheimer’s Disease)相关的淀粉样斑块(amyloid plaques),可有效改进阿尔茨海默病的早期诊断。
【总页数】1页(P48-48)
【作者】夏训明(编译)
【作者单位】不详
【正文语种】中文
【中图分类】R74
【相关文献】
1.美国FDA批准一种试剂用于辅助诊断白血病和淋巴瘤
2.美国FDA批准一种试剂用于辅助诊断白血病和淋巴瘤
3.美国FDA批准一种试剂用于检测急性淋巴细胞白血病及多发性骨髓瘤微小残留病
4.美国FDA批准一种检测试剂盒用于检测耐甲氧西林金黄色葡萄球菌(MRSA)
5.美国FDA批准Aduhelm(aducanumab)用于治疗阿尔茨海默病
因版权原因,仅展示原文概要,查看原文内容请购买。
阿米三嗪萝巴新片商品名都可喜-国家药品不良反应监测中心

阿米三嗪萝巴新片临床疗效再评价相关情况介绍1.阿米三嗪萝巴新片的基本情况如何?阿米三嗪萝巴新片由法国施维雅公司研制,商品名为“都可喜(Duxil)”,于1978年在法国首次注册,随后在全球55个国家上市。
阿米三嗪萝巴新片于1988年8月在我国首次获得进口许可。
2005年5月,施维雅(天津)制药有限公司获准在国内生产阿米三嗪萝巴新片(国药准字H20054931)。
阿米三嗪萝巴新片的适应症为“治疗老年人认知和慢性感觉神经损害的有关症状(不包括阿尔茨海默病和其他类型的痴呆);血管源性视觉损害和视野障碍的辅助治疗;血管源性听觉损害、眩晕和/或耳鸣的辅助治疗。
”该药品的不良反应有:体重减轻,周围神经病变;恶心、上腹部沉闷或烧灼感、消化不良、排空障碍;失眠、嗜睡、激动、焦虑、头晕;心悸。
另外,我国有两家企业生产复方阿米三嗪片,其有效成分组成与阿米三嗪萝巴新片相同,这两家企业分别为常州制药厂有限公司和南阳普康集团衡淯制药有限公司,首次获批时间分别为1997年2月(国药准字H10970117)和2000年1月(国药准字H20000060)。
2.阿米三嗪萝巴新片为何在法国撤市?法国卫生安全和健康产品局(AFSSAPS)在重新评价血管扩张剂的风险效益比后,认为阿米三嗪萝巴新片在治疗已批准的三个适应症方面疗效证据不充分,该药有少见的外周神经病变及体重减轻的副作用,要求法国施维雅药厂按照新的疗效评价标准提交相关临床研究证据。
法国施维雅药厂从企业发展策略及经济角度考虑,不愿再对都可喜进行新的临床试验,随后主动在法国市场撤出该产品。
基于法国施维雅药厂的决定,AFSSAPS撤销阿米三嗪萝巴新片的法国市场授权许可证,该决定于2005年9月28日生效。
3.目前阿米三嗪萝巴新片在国际市场的生产与销售情况如何?法国施维雅药厂主动在法国市场撤出阿米三嗪萝巴新片后,也停止了向海外其它国家的出口,因此从法国进口该药品的国家陆续停止了阿米三嗪萝巴新片的销售。
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
医学英语写作与翻译

第三部分医学英语的写作任务一标题的写作(Title)标题的结构1. 名词+介词Blindness(视觉缺失)after Treatment for Malignant Hypertension 2. 名词+分词Unilateral Neurogenic Pruritus Following Stroke中风后单侧神经性瘙痒3. 名词+不定式Suggestion to Abolish Icterus Index Determination(黄疸指数测定)where Quantitative Bilirubin Assay(胆红素定量)is Available建议能做胆红素定量的化验室不再做黄疸指数测定4. 名词+同位语Gentamicine, a Selelctive Agent for the isolation of Betahemolytic Streptocc ociβ-溶血性链球菌庆大霉素是分离β-溶血性链球菌的选择性药物5. 名词+从句Evidence that the V-sis Gene Product Transforms by Interaction with the Receptor for Platelet-derived Growth Factor血小板源性生长因子.V-sis 基因产物由血小板生成因子受体相互作用而转化的依据6. 动名词短语Preventing Stroke in patients with Atrial Fibrillation心房纤维性颤动心旁纤颤患者中风预防Detecting Acute Myocardial Infarction(急性心肌梗死)byRadio-immunoassay for Creative Kinase(酐激酶)用放射免疫法测定酐激酶诊断急性心肌梗死7. 介词短语On Controlling Rectal Cancer8. 陈述句Dietary Cholesterol is Co-carcinogenic协同致癌因素for Human Colon Cancer9. 疑问句Home or Hospital BirthsIs Treatment of Borderline Hypertension Good or Bad?注意副标题的作用1.数目:Endoluminal Stent-graft 带支架腔内搭桥for Aortic Aneurysms动脉瘤: A report of 6 cases带支架腔内搭桥治疗动脉瘤的六例报告2.重点:Aorto-arteritis 大动脉炎Chest X-ray Appearance and Its Clinical Significance大动脉炎胸部X线表现及临床意义3.方法:Gallstone Ileus(胆结石梗阻): A Retrospective Study 4.作用:Carcinoembryonic Antigen in Breast-cancer Tissue: A useful prognostic indictor乳腺癌组织中癌胚抗原——一种有用的预后指示5.疑问:Unresolved—Do drinkers have less coronary heart disease? 6.连载顺序:Physical and Chemical Studies of Human Blood Serum: II. A study of miscellaneous Disease conditions人类血清的理论研究:II. 多种病例的研究7.时间:A Collaborative 综合Study of Burn Nursing in China: 1995-1999常见标题句式举例1. 讨论型:Discussion of/ on; An approach to; A probe into; Investigation of; Evaluation of / on汉语中的“初步体会”、“试论”、“浅析”之类的谦辞可以不译。
Non-InvasiveandInvasiveCardiacImagingIntroduction

Non-Invasive and Invasive Cardiac ImagingIntroduction:Coronary artery disease is the most common cause of patient hospitalization and mortality in many industrialized countries. The current standard of assessment is coronary angiography (CA) in conjunction with interventional therapeutic procedures. While CA and other invasive cardiac imaging procedures have become relatively safe, the inconvenience to the patient is extensive. This has caused a popularization throughout the past decades of potential, non-invasive cardiac imaging devices. Different devices such as magnetic resonance and electron beam computed tomography have been explored. In this monograph various imaging technologies are discussed.The Problem:The current cardiac imaging devices provide an inconvenience to the patient in many forms including hospitalization and higher economic burden. Various technologies have been developed to ease patient burden in the form of non-invasive cardiac imaging devices. However, these new technologies are not without their unique challenges. Such challenges as cardiac motion and calcium deposits often render scans inadequate. The characterization of atherosclerotic plaque is another major challenge for non-invasive imaging as the rupture of such plaques can cause acute vessel occlusion. There is a need to find a suitable procedure that minimizes or eliminates rupturing the plaque. In addition, conventional imaging techniques expose the patient to ionizing radiation. These are some of the various challenges surrounding the design of a non-invasive device.Invasive ImagingModern IVUS systems have presented some difficulties that necessitate further exploration. One such difficulty with IVUS is total obstruction when a transducer is brought in close proximity to a severely stenosed vessel. Volcano Corporation recently launched the VH ™ IVUS system. This is the first technology to enable real-time compositional assessment of atherosclerotic plaque in coronary arteries. It provides automated measurement tools to simplify image interpretation. It also uses a color key to better display plaque composition. This technology allows for colorized VH images of four plaque component types. This is the first IVUS system capable of providing information about plaque composition.Optical Coherence Tomography (OCT) is another popular technology used for cardiac imaging. This non-contact, light-based imaging modality providesin situ tissue images at near histological resolution. This technique allows for the identification of mural as well as luminal morphologies. When comparedto IVUS, studies showed that OCT provides additional morphologic information which is helpful in the characterization of plaque. Optical frequencydomain imaging (OFDI) is a new technology in this area of imaging. OFDI is used in the diagnosis and management of coronary artery disease. It uses infrared light delivered to the imaging site through a single optical fiber incorporated within a catheter. Advanced algorithms are used to remove thereflected signals from the infrared light. This provides the clinician with real-time cross sectional and 3-dimensional images.Non-invasive Imaging:Nuclear cardiac imaging is a popular non-invasive imaging method which uses technetium Tc 99m sestamibi (MIBI), a radionuclide, in its process. MIBI is a technetium imaging agent that is used to reveal blood-starved tissue, usually during a heart attack. It has been used for more than a decade as an imaging agent. MIBI concentrates in tissues in proportion to desmoplastic and metabolic activity and blood flow. This technique has been considered a reliable method of assessing myocardial salvage in patients with acute myocardial infarction in addition to evaluating and diagnosing a heart attack.(Nuclear cardiac illustration from Google Images)Cardiovascular Magnetic Resonance (CMR) is another technique used for non-invasive imaging. This device was developed to quantify calcium deposits and coronary morphology and flow. It is based upon the magnetic characteristics of tissues and molecules within a magnetic field. CMR is superior to other methods for use on those patients with complex congenital heart disease. CMR provides excellent visualization of extracardiac venous structures and intracardiac baffles as well. However, it is not feasible for use on patients with pacemakers or other metallic implants or on those who suffer from claustrophobia.Most recently, the technique of spiral balanced steady-state free precession cardiac imaging (SSFP) has been brought to the forefront of CMR. These sequences are useful in cardiac imaging because they have the ability to achieve high signal efficiency and blood-myocardium contrast. This procedure enables efficient acquisition with reduced blood flow and motion artifacts. SSPF has been combined with spiral imaging allowing for real-time interactive cardiac CMR. In contrast to conventional echo imaging, this method yields an intrinsic blood-myocardium contrast independent of inflow and has become extremely useful in cardiac imaging.Computed Tomography (CT) is another well-known device that is being used for non-invasive cardiac imaging. Like CMR, electron beam computed tomography (EBCT) was designed with the goal of measuring calcium deposits and coronary artery morphology and flow. CT of the heart throughout the last decade has been the exclusive realm of EBCT. The most popular use for this technology has been assessment of myocardial perfusion and function as well visualization of the coronary arteries.In recent years, multidetector-row computed tomography (MDCT) has become available. This technology has become the preferred method because it rectifies some of the limitations of the EBCT such as low special resolution and pronounced noise. The ability of the MDCT scanners to acquisition multiple slices has considerably improved cardiac imaging. The image quality produced by MDCT favorably compares to that of coronary angiography. In addition, it provides assessment of coronary calcium and plaque characterization while also producing a high image quality.Non-invasive echocardiography is an optional ultrasonic method of cardiac imaging. Recently, live three dimensional echocardiography (L3DE) has broken though into the field of medical ultrasound. This non-invasive system is easy to operate and images rapidly and clearly. In L3DE ultrasonic beams are generated in a phased array manner which gives the clinician the ability to evaluate the cardiac structures from every direction. The ability of the operator to improve temporal and special resolution of the image during acquisition is one benefit of this procedure.References and Bibliography:World Wide Web•http://www.mediguide.co.il/MediGuide-Medical Positioning System•Health Center Laboratories•Echocardiography (Ultrasound of the Heart)•Cardiac Perfusion Scan•Technetium tc 99m sestamibi-definition from •Google Images•/cgi/news/release?id=146453Press Release: Volcano Corp. Announces Commercial Release of VH™ IVUS SystemJournal articles and Reviews•_________. Terumo Initiates Vulnerable Plaque Program with Exclusive License from Massachusetts General Hospital. Press Release December 15, 2004.•Coover LR. The Role of Technetium Tc 99m Sestamibi in the Early Detection of Breast Carcinoma. Hospital Physician 1999: 16-21.•Dong J, Ndrepepa G, Schmitt C, et. al. Early Resolution of ST-Segment Elevation Correlates With Myocardial Salvage Assessed by Tc-99m Sestamibi Scintigraphy in Patients With Acute Myocardial Infarction After Mechanical or Thrombolytic Reperfusion Therapy. Circulation 2002,105: 2946-2949.•Gaudio C, Mirabelli F, Di Michele S, et. al. Multidetector Computed Tomography of the Coronary Arteries. Ital Heart J 2004, 5: 423-430.•Gerckens U, Buellesfeld L, McNamara E, Grube E. Optical Coherence Tomography (OCT): Potential of a new high-resolution intracoronary imaging technique. Herz 2003, 28(6): 496-500.•Kiaffas MG, Davlourous P, Tsertos F, et. al. Cardiovascular Magnetic Resonance Evaluation of Patients with Transposition of the Great Arteries Following Atrial Switch Surgical Correction 2005, 46: 69-73.•Light ED and Smith SW. Two Dimensional Arrays for Real Time 3D Intravascular Ultrasound 2004, 26(2): 115-128.•Nayak KS, Hargreaves BA, HU SB, et. al. Spiral Balanced Steady-State Free Precession Cardiac Imaging. Magnetic Resonance Medicine 2005, 53: 1468-1473.•Nieman K, van Genus RM, Wieloposki, P, et. al. Noninvasive Coronary Imaging: A comparison of computed tomography and magnetic resonance techniques. Reviews in Cardiovascular Medicine 2002, 3(2): 77-84.•Wang XF, Deng YB, Nanda NC, et. al. Live Three-Dimensional Echocardiography: Imaging principles and clinical application.Echocardiography: A journal of CV Ultrasound & Allied Technologies 2003, 20(7): 593-604.。
美索巴莫注射液标准

美索巴莫注射液标准美索巴莫注射液是一种中枢性肌肉松弛剂,主要用于治疗急性骨骼肌疼痛或不适症状。
以下是关于美索巴莫注射液的一些标准信息:1. 中文通用名:美索巴莫注射液2. 英文通用名:Methocarbamol Injection3. 标准号:ws-10001-(hd-0269)-20024. 药品名称:美索巴莫注射液5. 药品英文名:Methocarbamol Injection6. 主要成分:本品为美索巴莫的灭菌聚乙二醇,400水溶液。
含美索巴莫(c11h15no5)应为标示量的95.0%~105.%。
7. 处方:具体处方应根据医生建议和患者病情来确定。
8. 性状:本品为无色或几乎无色略带黏稠的澄明液体。
9. 鉴别:通过化学分析和物理测试等方法来鉴别美索巴莫注射液的真伪和质量。
10. 作用类别:中枢性肌肉松弛剂11. 药理毒理:本品对中枢神经系统有选择作用,特别对脊椎中神经元作用明显。
抑制与骨骼肌痉挛有关的神经突触反射,有抗士的宁和电刺激所致惊厥的作用,并有解痉、镇痛和抗炎作用。
其作用机制主要是阻断脊髓内中枢神经元从而使骨骼肌松弛。
12. 药代动力学:美索巴莫注射液在体内的吸收、分布、代谢和排泄过程。
13. 适应症:主要用于急性骨骼肌疼痛或不适症状的辅助治疗。
14. 用法用量:美索巴莫注射液的用法主要是采用静脉滴注或者是静脉推注的方式给药。
如果是用于静脉推注时,患者在静卧的条件下缓慢推注,给药的速度每分钟不可以超过3ml,注射之后应该至少休息10~15分钟。
如果是用于静脉滴注时,将药品配在9%的氯化钠注射液或者是5%的葡萄糖注射液当中,滴注的速度不宜过快。
使用剂量和次数根据病情和治疗效果来决定。
成人一次使用剂量为1.0g,一日最大剂量为3.0g,连续使用不得超过3天。
轻度病例静注后应改为口服给药以维持治疗。
15. 不良反应:使用美索巴莫注射液可能出现的不良反应,如头痛、恶心、呕吐、皮疹等。
16. 禁忌症:对美索巴莫过敏者、肝肾功能不全者、哺乳期妇女禁用。
商品名-圣马尔定医院
Vimpat 100mg/tab
維帕特膜衣錠
Lacosamide
1.十六歲以上有或無次發性全身作的局部癲癇發作患者的單一藥物治療。2.十六歲以上之(1)複雜性局部癲癇發作(complex partial seizure)與(2)單純或複雜性局部發作之合併有次發性全身發作(simple or complex partial seizure with secondary generalization)癲癇患者之輔助治療(add-on therapy)
適用於4歲以上(含4)患者Lennox-Gastaut症候群相關癲癇發作之輔助治療
39.4
粉紅色橢圓形錠
262
參閱健保規範
DC(DIO4){160mg}Diovan得安穩
輕度至中度潰瘍性直腸炎。
38.1
Asacol 0.5g/supp廠商缺貨,缺貨期間暫代
參閱健保規範
DIC1
Dicetel 50mg/Tab
得舒特膜衣錠
Pinaverium
胃、十二指腸潰瘍、過敏性結腸炎、膽管運動困難
9
橘色
圓形錠50
NEU5
Neuquinon 10mg/tab
能淤奇朗糖衣錠
Ubidecarenone
心衰竭之輔助療法
3.36
SOVP
Sovenor 5ug/hr/patch
舒免疼穿皮貼片劑
Buprenorphine
在長期需要類鴉片藥物之止痛效果時,用於中度非癌症疼痛的治療。
0
舒免疼不適用於治療急性疼痛
屬第3級管制藥品
自費價700元
INO2
Inovelon 200mg/tab
克雷葛膜衣錠
Rufinamide
中国痴呆与认知障碍诊治指南(三)_神经心理评估的量表选择

万方数据
万方数据
万方数据
万方数据
万方数据
万方数据
万方数据
中国痴呆与认知障碍诊治指南(三):神经心理评估的量表选择
作者:贾建平, 王荫华, 张振馨, 肖世富, 周爱红, 汪凯, 丁新生, 张晓君, 张朝东,李焰生, 杨莘, 陈晓春, 罗本燕, 唐牟尼, 徐江涛, 章军建, 彭丹涛, 蔡晓杰,
魏翠柏
作者单位:贾建平,周爱红,魏翠柏(首都医科大学宣武医院神经科,北京,100053), 王荫华(北京大学第一医院神经科), 张振馨(北京协和医学院北京协和医院神经内科), 肖世富(上海市精神卫
生中心), 汪凯(安徽医科大学第一附属医院神经科), 丁新生(南京医科大学第一附属医院
神经内科), 张晓君(北京同仁医院神经内科), 张朝东(中国医科大学第一临床医学院神经
内科), 李焰生(上海交通大学医学院附属仁济医院神经内科), 杨莘(首都医科大学宣武医
院护理部,北京,100053), 陈晓春(福建医科大学附属协和医院神经内科), 罗本燕(浙江大
学医学院附属第一医院神经内科), 唐牟尼(广州脑科医院精神科), 徐江涛(兰州军区乌鲁
木齐总医院神经内科), 章军建(武汉大学中南医院神经科), 彭丹涛,蔡晓杰(卫生部北京
医院神经内科)
刊名:
中华医学杂志
英文刊名:NATIONAL MEDICAL JOURNAL OF CHINA
年,卷(期):2011,91(11)
本文链接:/Periodical_zhyx201111007.aspx。
Helena 0-0-21 植物滋养剂说明书

0-0-21CONCENTRATED POTASH SOLUTION AND OXIDIZED SULFURGUARANTEED ANALYSIS :Soluble Potash (K 2O) . . . . . . . . . . . . . . . . . . . . . . ……………………………….21.00% Sulfur (S)………………………………………………………………………………13.00% 13.00% Combined Sulfur (S)Derived from potassium thiosulfate. KEEP OUT OF REACH OF CHILDREN WARNINGMay be harmful if swallowedMay be harmful in contact with skin Causes serious eye irritation Causes skin irritation May be harmful if inhaledWEIGHT PER GALLON: 11.47 lbs. (5.20 kg) @ 68°F SN 041415/0815G FREEZING TEMPERATURE:.30°F NET CONTENTS: □ 5 gal (18.93 L) □ 30 gal (113.56 L)□ 250 gal (946.25 L) □ 275 gal (1040.99 L) □ Bulk ____________Information about the components of this lot of fertilizer may be obtained by writing to Helena Chemical Company, 225 Schilling Boulevard, Suite 300, Collierville, TN 38017 and giving the lot number which is found on the container.Information regarding the contents and levels of metals in this product is available on the Internet at /metals.htm F224MANUFACTURED FORHELENA CHEMICAL COMPANY225 SCHILLING BOULEVARD, SUITE 300 COLLIERVILLE, TN 38017 901-761-0050PRECAUTIONARY STATEMENTSHAZARDS TO HUMANS AND DOMESTIC ANIMALSWARNINGBEFORE USING THIS PRODUCT, READ ALL PRECAUTIONS, DIRECTIONS FOR USE, CONDITIONS OF SALE–LIMITED WARRANTY AND LIMITATIONS OF LIABILITY AND REMEDIES.May be harmful if swallowed. May be harmful in contact with skin. Causes serious eye irritation. Causes skin irritation. May be harmful if inhaled. Keep product locked up and out of the reach of children. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum or smoking tobacco. Remove and wash contaminated clothing before reuse. Do not take internally. Avoid contact with or inhalation of spray application mist if present. Do not apply this product in such a manner as to directly expose workers or other persons. If product is being mixed with pesticides and/or spray adjuvants, follow all precautionary statements on the accompanying product(s) labeling. Not for human or animal consumption.Personal Protective Equipment (PPE): Wear protective eyewear (goggles or face shield), chemical-resistant gloves, long-sleeved shirt and long pants, and shoes plus socks when using this product. Take off any contaminated clothing and wash before reuse. FIRST AID IF IN EYES: ∙ Rinse cautiously with water for several minutes. Removecontact lenses, if present and easy to do. Continue rinsing. ∙ Call a poison control center or doctor for treatmentadvice.IF SWALLOWED: ∙ Call a POISON CENTER or doctor immediately.∙ Rinse mouth. Do NOT induce vomiting. ∙ Do not give anything by mouth to an unconscious person.∙ Immediately call a POISON CENTER or doctor.IF INHALED:∙ Move person to fresh air and keep at rest in a position comfortable for breathing if they feel unwell.∙ If not breathing, call 911 or an ambulance, then give artificial respiration, preferably mouth-to-mouth if possible.∙ Call a POISON CENTER or doctor for treatment advice. IF ON SKIN ORHAIR:∙ Take off immediately all contaminated clothing. Rinse skin with water or shower.∙ Get medical attention if irritation develops or persists. ∙Wash contaminated clothing before reuse.HOT LINE NUMBERHave the product container or label with you when calling a poison control center or doctor, or going for treatment. You may also contact 1-800-424-9300 for emergency medical treatment information.STORAGE AND DISPOSALKeep container tightly closed and do not allow water to be introduced into it. Store in a dry place. Temperatures below 25°F may result in product crystallization. The product will readily reconstitute, however, with warmer temperatures and gentle agitation of the container.Do not contaminate water sources by cleaning of equipment or disposal of spray waste.Dispose of empty containers by triple rinsing with detergent solution or puncture and discard empty containers in a landfill in accordance with current local, state, and federal regulations. GENERAL INFORMATIONNUCLEUS® 0-0-21 is a highly concentrated water-based solution of potash and oxidized sulfur useful in the correction of nutritional deficiencies in plants. The unique formulation of NUCLEUS® 0-0-21 provides a non-corrosive liquid that is stable at cold temperatures. Applications of NUCLEUS® 0-0-21 through mixing, application, and irrigation equipment may reduce the rate and degree of corrosion that occurs when this equipment is exposed to fertilizer solutions. When used as directed this product does not supply all nutrients required by plants and is to supplement a soil fertility program based on soil tests.APPLICATION AND MIXING GUIDEApplication rates for general use are 2-6 gallons per acre.TURF: Apply 2 to 10 fl. ounces per 1,000 sq. ft. using 2 to 5 gallons of water per 1,000 sq. ft. Use lower rate during summer applications. Apply as needed.DO NOT APPLY NEAR WATER, STORM DRAINS, OR DRAINAGE DITCHES. DO NOT APPLY IF HEAVY RAIN IS EXPECTED. APPLY THIS PRODUCT ONLY TO YOUR LAWN/GARDEN. MIXING:NUCLEUS® 0-0-21 provides a neutral or slightly acidic pH value when diluted with water. This characteristic makes it compatible with most pesticides. In any mixing operation, NUCLEUS® 0-0-21 should be introduced in the following sequence: 1. Water2. NUCLEUS® 0-0-213. Other Fertilizer Products4. Pesticides5. Spray AdjuvantsCompatibility tests are always recommended prior to preparing a spray mixture of NUCLEUS® 0-0-21 with other products.CONDITIONS OF SALE–LIMITED WARRANTY AND LIMITATIONS OF LIABILITY AND REMEDIES Read the Conditions of Sale–Warranty and Limitations of Liability and Remedies before using this product. If the terms are not acceptable, return the product, unopened, and the full purchase price will be refunded.This label is believed to be reliable and must be followed carefully. Injury to the crop to which the product is applied may result from the occurrence of extraordinary or unusual weather conditions or the failure to follow the label directions or goodapplication practices, all of which are beyond the control of Helena Chemical Company (the "Company") or seller. In addition, failure to follow label directions may cause injury to crops, animals, man or the environment. The Company warrants that this product conforms to the chemical description on the label and is reasonably fit for the purpose referred to subject to the factors noted above which are beyond the control of the Company. The Company makes no other warranties or representations of any kind, express or implied, concerning the product, including no implied warranty of merchantability or fitness for any particular purpose, and no such warranty shall be implied by law.The exclusive remedy against the Company for any cause of action relating to the handling or use ofthis product shall be limited to, at Helena Chemical Company’s election, one of the following:1.Refund of the purchase price paid by buyer or user for product bought, or2.Replacement of the product usedTo the extent allowed by law, the Company shall not be liable and any and all claims against the Company are waived for special, indirect, incidental, or consequential damages or expense of any nature, including, but not limited to, loss of profits or income. The Company and the seller offer this product and the buyer and user accept it, subject to the foregoing conditions of sale and limitation of warranty, liability and remedies.© Copyright Helena Holding Company, 2015NUCLEUS® is a registered trademark of Helena Holding Company.。
Salmeterol xinafoate_94749-08-3_DataSheet_MedChemExpress
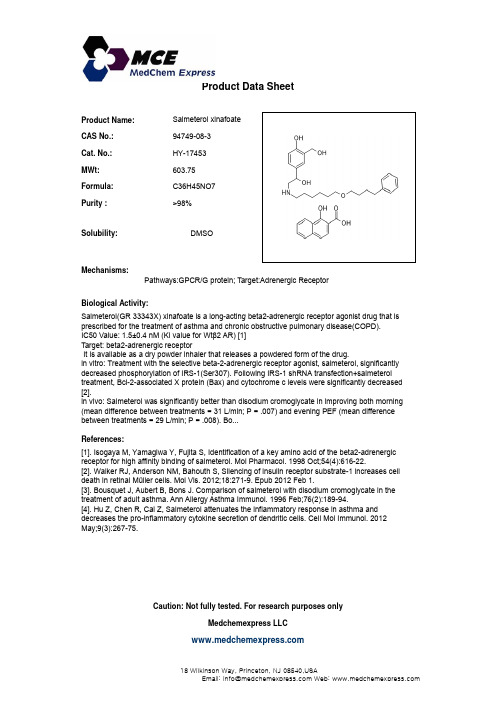
Product Name:Salmeterol xinafoate CAS No.:94749-08-3Cat. No.:HY-17453Product Data SheetMWt:603.75Formula:C36H45NO7Purity :>98%Solubility:DMSOy Mechanisms:Biological Activity:Salmeterol(GR 33343X)xinafoate is a long acting beta2adrenergic receptor agonist drug that is Pathways:GPCR/G protein; Target:Adrenergic Receptor Salmeterol(GR 33343X) xinafoate is a long-acting beta2-adrenergic receptor agonist drug that is prescribed for the treatment of asthma and chronic obstructive pulmonary disease(COPD).IC50 Value: 1.5±0.4 nM (Ki value for Wt β2 AR) [1]Target: beta2-adrenergic receptorIt is available as a dry powder inhaler that releases a powdered form of the drug.in vitro: Treatment with the selective beta-2-adrenergic receptor agonist, salmeterol, significantly decreased phosphorylation of IRS-1(Ser307). Following IRS-1 shRNA transfection+salmeterol treatment, Bcl-2-associated X protein (Bax) and cytochrome c levels were significantly decreased[2].References:[1]. Isogaya M, Yamagiwa Y, Fujita S, Identification of a key amino acid of the beta2-adrenergicreceptor for high affinity binding of salmeterol. Mol Pharmacol. 1998 Oct;54(4):616-22.[2]. Walker RJ, Anderson NM, Bahouth S, Silencing of insulin receptor substrate-1 increases cell[]in vivo: Salmeterol was significantly better than disodium cromoglycate in improving both morning (mean difference between treatments = 31 L/min; P = .007) and evening PEF (mean difference between treatments = 29 L/min; P = .008). Bo...[]g pdeath in retinal Müller cells. Mol Vis. 2012;18:271-9. Epub 2012 Feb 1.[3]. Bousquet J, Aubert B, Bons J. Comparison of salmeterol with disodium cromoglycate in thetreatment of adult asthma. Ann Allergy Asthma Immunol. 1996 Feb;76(2):189-94.[4]. Hu Z, Chen R, Cai Z, Salmeterol attenuates the inflammatory response in asthma and decreases the pro-inflammatory cytokine secretion of dendritic cells. Cell Mol Immunol. 2012May;9(3):267-75.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
司美格鲁肽联合二甲双胍恩格列净治疗2_型糖尿病合并非酒精性脂肪性肝病患者疗效及安全分析

DOI:10.16658/ki.1672-4062.2024.02.012司美格鲁肽联合二甲双胍恩格列净治疗2型糖尿病合并非酒精性脂肪性肝病患者疗效及安全分析戚玉琴1,杨浩2,盖显英11.上海市松江区泗泾医院内分泌科,上海201600;2.上海市松江区泗泾医院心血管内科,上海201600[摘要]目的观察司美格鲁肽与二甲双胍恩格列净联合对2型糖尿病(Type 2 Diabetes Mellitus, T2DM)合并非酒精性脂肪性肝病(Non-alcoholic Fatty Liver Disease, NAFLD)患者治疗的临床应用价值。
方法选取2022年5月—2023年5月上海市松江区泗泾医院收治的100例T2DM合并NAFLD患者为研究对象,通过抽签法分为两组,各50例。
对照组给予二甲双胍恩格列净,观察组在对照组的基础上加用司美格鲁肽。
比较两组的血糖、肝功能、血脂和不良反应。
结果观察组治疗后空腹血糖、餐后2 h血糖、丙氨酸氨基转移酶、门冬氨酸氨基转移酶、谷氨酰转肽酶、总胆固醇、三酰甘油水平低于对照组,差异有统计学意义(P均<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
结论二甲双胍恩格列净联合司美格鲁肽治疗T2DM合并NAFLD患者,能调节糖脂代谢异常情况,改善其肝功能,安全性可靠。
[关键词] 2型糖尿病;非酒精性脂肪性肝病;二甲双胍恩格列净;司美格鲁肽[中图分类号] R4 [文献标识码] A [文章编号] 1672-4062(2024)01(b)-0012-04Analysis of the Efficacy and Safety of Semaglutide Combined with Metfor⁃min and Empagliflozin in the Treatment of Patients with Type 2 Diabetes Combined with Non-alcoholic Fatty Liver DiseaseQI Yuqin1, YANG Hao2, GAI Xianying11.Department of Endocrinology, Sijing Hospital, Songjiang District, Shanghai, 201600 China;2.Department of Cardio⁃vascular Medicine, Sijing Hospital, Songjiang District, Shanghai, 201600 China[Abstract] Objective To observe the clinical application value of semaglutide combined with metformin and empa⁃gliflozin in the treatment of patients with Type 2 Diabetes Mellitus (T2DM) combined with Non-Alcoholic Fatty Liver Disease (NAFLD). Methods A total of 100 patients with T2DM complicated with NAFLD admitted to Sijing Hospital of Songjiang District of Shanghai from May 2022 to May 2023 were selected as the study objects and divided into two groups with 50 cases each by drawing lots. The control group was given metformin empagliflozin, and the observation group was given semaglutide on the basis of the control group. The blood glucose, liver function, blood lipid and ad⁃verse reactions were compared between the two groups. Results The levels of fasting blood glucose, 2-hour postpran⁃dial blood glucose, alanine aminotransferase, aspartate aminotransferase, glutamyl transpeptidase, total cholesterol and triglyceride in the observation group were lower than those in the control group, and the differences were statistically significant (all P<0.05). There was no statistically significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion Metformin empagliflozin combined with semaglutide can regulate abnormal glu⁃[基金项目]上海市松江区科学技术攻关项目(22SJKJGG82);上海市松江区新一轮医学重点学科(ZK2019B02)[作者简介]戚玉琴(1995-),女,硕士,住院医师,研究方向为内分泌及代谢病学。
曲安奈德鼻喷雾剂联合地氯雷他定治疗过敏性鼻炎的临床效果

·五官医学·中国当代医药2021年1月第28卷第2期CHINA MODERN MEDICINE Vol.28No.2January 2021过敏性鼻炎为耳鼻喉科发生率较高的一种变态反应疾病,主要是由于机体在接触变应原后导致鼻粘膜非感染性炎性病变。
目前,临床对于该病的治疗尚未研究出有效根治的方法,多以避免接触过敏原、免疫治疗、手术以及药物等治疗手段进行控制[1]。
而药物治疗则是临床应用较为广泛的一种治疗方式,多数患者在药物治疗后,鼻塞、流涕等症状能够得到较好的控制[2]。
但不同药物临床治疗效果也存在较大的差异,地氯雷他定是临床治疗该病的常用药物,其在抗炎上具有较好的效果,但单独运用疗效有限。
而曲安奈德鼻喷雾剂直接作用于患部,具有清炎、抗过敏的功效。
故本次研究选取122例过敏性鼻炎患者为研究对象,旨在探究曲安奈德鼻喷雾剂联合地氯雷他定治疗的效果。
1资料与方法1.1一般资料选取2018年12月~2019年12月于辽宁省鞍山市长大医院就诊的122例过敏性鼻炎患者作为研究对象,按随机数字表法分为对照组与研究组,每组61例。
对照组中,男39例,女22例;年龄20~70岁,平曲安奈德鼻喷雾剂联合地氯雷他定治疗过敏性鼻炎的临床效果周桂锋辽宁省鞍山市长大医院耳鼻喉科,辽宁鞍山114005[摘要]目的探讨曲安奈德鼻喷雾剂联合地氯雷他定治疗过敏性鼻炎的临床效果。
方法选取2018年12月~2019年12月于辽宁省鞍山市长大医院就诊的122例过敏性鼻炎患者作为研究对象,按随机数字表法分为对照组与研究组,每组61例。
对照组接受单一地氯雷他定治疗,研究组则在对照组基础上联合曲安奈德鼻喷剂治疗。
比较两组的临床疗效,白细胞介素-4(IL-4)及白细胞介素-10(IL-10)的水平变化,症状改善情况。
结果研究组临床总有效率(91.80%)高于对照组(78.69%),差异有统计学意义(P <0.05)。
治疗后研究组的IL-4、IL-10水平均低于对照组,差异有统计学意义(P <0.05)。
维派特说明书

核准日期:2018年11月21日修订日期:2019年8月19日;2020年3月09日;2020年9月01日拉考沙胺片说明书请仔细阅读说明书并在医师指导下使用。
【药品名称】通用名称:拉考沙胺片商品名称:维派特®(英文:VIMPAT®)英文名称:Lacosamide tablets汉语拼音:Lakaosha’an Pian【成份】本品主要成份为拉考沙胺。
化学名称:(R)-2-(乙酰基氨基)-N-苯甲基-3-甲氧基丙酰胺化学结构式:分子式:C13H18N2O3分子量:250.30【性状】本品为薄膜衣片,除去包衣后显白色至类白色。
【适应症】本品适用于4岁及以上癫痫患者部分性发作的单药治疗和联合治疗。
【规格】50mg、100mg、150mg和200mg【用法用量】1.剂量信息成人和4岁~17岁儿科患者的推荐剂量见表1。
在4岁~17岁的儿科患者中,推荐的剂量方案取决于体重。
应根据临床反应和耐受性增加剂量,增加频率不超过每周一次。
剂量调整增量不应超过表1所示。
表1:成人和4岁及以上儿科癫痫患者部分性发作的单药治疗和联合治疗的推荐剂量**未说明时,癫痫患者部分性发作的单药治疗与癫痫患者部分性发作的联合治疗的剂量相同在成人癫痫患者部分性发作的联合治疗临床试验中,剂量高于每次200mg、每日二次(每日400mg)并非更有效,并且引起不良反应发生率明显升高(见【不良反应】和【临床试验】)。
成人患者(17岁及以上)的负荷剂量在成人患者中,可以按200mg单次负荷剂量开始拉考沙胺治疗,之后在约12小时后,采用每次100mg,每日二次(每日200mg)剂量。
该维持剂量方案应持续一周。
然后可以按表1中的推荐调整拉考沙胺剂量。
由于中枢神经系统不良反应发生率升高,成人负荷剂量应在医学监督下给药(见【不良反应】和【临床试验】)。
尚未对儿科患者使用负荷剂量进行研究。
2.肾功能受损患者的剂量信息轻至中度肾功能受损患者不需要调整剂量。
益生菌对阿尔茨海默病作用的研究进展

益生菌对阿尔茨海默病作用的研究进展发布时间:2021-12-14T06:08:15.523Z 来源:《中国结合医学杂志》2021年12期作者:宋鑫萍1,2,李盛钰2,金清1[导读] 阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
宋鑫萍1,2,李盛钰2,金清11.延边大学农学院,吉林延吉 1330022.吉林省农业科学院农产品加工研究所,吉林长春 130033摘要:阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
本文综述了近几年来国内外益生菌对阿尔茨海默病的作用进展,以及其预防和治疗阿尔茨海默病的潜在作用机制。
关键词:益生菌;阿尔茨海默病;肠道菌群;机制Recent Progress in Research on Probiotics Effect on Alzheimer’s DiseaseSONG Xinping1,2,LI Shengyu2,JI Qing1*(1.College of Agricultural, Yanbian University, Yanji 133002,China)(2.Institute of Agro-food Technology, Jilin Academy of Agricultural Sciences, Chanchun 130033, China)Abstract:Alzheimer’s disease has become one of the major diseases threatening the life and health of the global elderly. The number of patients is increasing year by year, and the economic cost of nursing is high, which poses a major challenge to the global economy. In recent years, studies have shown that probiotics, as microorganisms beneficial to the health of the host, have a positive impact on the prevention and treatment of Alzheimer’s disease. Its mechanism may be through regulating intestinal flora, affecting the nervous immune system, regulating the neuroactive substances and metabolites, and affecting the occurrence and development of the disease through thegut- brain axis. This paper reviews the progress of probiotics on Alzheimer’s disease at home and abroad in recent years, as well as its potential mechanism of prevention and treatment.Key words:probiotics; Alzheimer’s disease; gut microbiota; mechanism阿尔茨海默病(Alzheimer’s disease, AD),系中枢神经系统退行性疾病,属于老年期痴呆常见类型,临床特征主要包括:记忆力减退、认知功能障碍、行为改变、焦虑和抑郁等。
世界卫生组织儿童标准处方集

WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。
老年脓毒症患者血清氨基末端脑钠肽前体升高的影响因素分析

论著DOI:10.16662/ki.1674-0742.2023.17.001老年脓毒症患者血清氨基末端脑钠肽前体升高的影响因素分析李程锦,石松菁,陈湘平,陈凤朱福建医科大学教学医院福建省老年医院重症医学科,福建福州350000[摘要]目的探讨影响老年脓毒症患者NT-proBNP升高的影响因素。
方法采用回顾性研究分析2017年10月—2019年12月福建省老年医院收治的老年脓毒症患者114例的临床资料。
根据患者确诊入院时的血清NT-proBNP水平是否升高,将其分为观察组(NT-proBNP升高)60例和对照组(NT-proBNP正常)54例。
收集患者的人口学特征以及既往病史、BMI、是否有脓毒性休克及SOFA评分、APACHE Ⅱ评分等。
入院时采集患者外周静脉血,送检血清肌酐、NT-proBNP、心肌损伤标志物、炎症指标、心肌抑制因子、心肌自身免疫抗体等指标。
以单因素和多因素分析老年脓毒症患者NT-proBNP的影响因素。
结果与对照组比较,观察组的脓毒性休克占比、SOFA评分、APACHEⅡ评分、CK-MB、cTnI、PCT、CRP、IL-1β、TNF-α、β1-AAB、M2-AA显著较高,Ccr水平更低,差异有统计学意义(P<0.05)。
Logistic多因素回归分析结果表明老年脓毒症患者NT-proBNP水平的独立影响因素为PCT、IL-1β、BMI、Ccr(P<0.05)。
结论老年脓毒症患者NT-proBNP的升高与BMI、PCT、IL-1β、Ccr指标相关,上述指标是老年脓毒症患者NT-proBNP升高的影响因素,但这些发现需要样本量更大的前瞻性研究进一步验证。
[关键词]脓毒症;氨基末端脑钠肽前体;炎症因子;心肌损伤标志物;心肌自身免疫抗体[中图分类号]R5 [文献标识码]A [文章编号]1674-0742(2023)06(b)-0001-07Analysis of Factors Affecting Elevated Serum Amino-terminal Brain Natri⁃uretic Peptide Precursors in Elderly Patients with SepsisLI Chengjin, SHI Songjing, CHEN Xiangping, CHEN FengzhuDepartment of Critical Care Medicine, Fujian Provincial Geriatric Hospital, Fujian Medical University Teaching Hos⁃pital, Fuzhou, Fujian Province, 350000 China[Abstract] Objective To investigate the influencing factors affecting the elevation of NT-proBNP in elderly patients with sepsis. Methods A retrospective study was used to analyze the clinical data of 114 cases of geriatric sepsis pa⁃tients admitted to Fujian Provincial Geriatric Hospital from October 2017 to December 2019. The patients were di⁃vided into 60 cases in the observation group (elevated NT-proBNP) and 54 cases in the control group (normal NT-proBNP) according to whether their serum NT-proBNP levels were elevated at the time of confirmed admission. The patients' demographic characteristics as well as past medical history, BMI, presence of septic shock and SOFA score and APACHE Ⅱ score were collected. Peripheral venous blood was collected from patients on admission and sent for serum creatinine, NT-proBNP, myocardial injury markers, inflammatory indexes, myocardial inhibitory factors, and myocardial autoimmune antibodies. Analyzed the influencing factors of NT proBNP levels in elderly sepsis patients us⁃ing single and multiple factors. Results Compared with the control group, septic shock percentage, SOFA score, APACHEⅡ score, CK-MB, cTnI, PCT, CRP, IL-1β, TNF-α, β1-AAB, and M2-AA in the observation group were significantly higher, Ccr levels was lower, the difference was statistically significant (P<0.05). Logistic multi-factor re⁃[基金项目]福建省卫生计生科研人才培养项目青年科研课题(2017/2/19)。
西那卡塞和依特卡肽不良事件信号的挖掘与分析

西那卡塞和依特卡肽不良事件信号的挖掘与分析Δ王红力 1*,钟贵遵 1,李东炫 2,沈正泽 1 #(1.重庆医科大学附属永川医院药学部,重庆 402100;2.重庆医科大学 附属第三医院药剂科,重庆 401100)中图分类号 R 969.3 文献标志码 A 文章编号 1001-0408(2024)08-0986-05DOI 10.6039/j.issn.1001-0408.2024.08.15摘要 目的 挖掘并分析西那卡塞和依特卡肽的不良事件(ADE )信号,为临床安全用药提供参考。
方法 利用OpenVigil 在线工具,收集美国FDA 不良事件报告系统数据库2004年1月1日至2023年6月30日的西那卡塞和依特卡肽相关ADE 报告,采用贝叶斯置信区间递进神经网络法对重点系统的ADE 信号进行检测,并根据《国际医学用语词典》(26.0版)ADE 术语集中的首选术语进行编码。
结果 分别检索到西那卡塞、依特卡肽ADE 报告41 709、1 710份,重点系统阳性信号29、45个,未被药品说明书收录的阳性信号20、36个。
低钙血症/血钙降低、血清甲状旁腺素(PTH )异常/血清PTH 升高或降低是两药常见的ADE ,均被检出;在未被药品说明书收录的信号中,检出了西那卡塞致钙化防御(代谢及营养类疾病)、骨饥饿综合征和高转换型骨病(肌肉骨骼及结缔组织类疾病)等新的中强、强信号,以及依特卡肽致猝死、坏死及治疗无反应(全身及给药部位反应),不稳定型心绞痛、心肌缺血(心脏器官疾病),肠穿孔、胃窦血管扩张、胃溃疡(胃肠道疾病)等新的中强、强信号。
结论 临床应用两药时,除需关注低钙血症、血清PTH 异常等常见ADE 外,还应监测一些新的潜在的ADE 信号,如西那卡塞致骨饥饿综合征、钙化防御、心室疾病等,依特卡肽致猝死、心肌缺血、不稳定型心绞痛、肠穿孔及胃溃疡等;当出现新的ADE 时,临床应及时评估患者的治疗获益与风险,更新治疗方案和药学监护计划,以保障患者用药安全。
脑心浸液肉汤培养基配制使用

脑心浸液肉汤(B HI )培养基 Brain He ar t In fusion M ediu m配方:(g/L ) 蛋白胨脱水小牛脑浸粉 脱水牛心浸粉 氯化钠 葡萄糖 磷酸氢二钠 pH 值7.4 ± 0.210.0 12.5 5.0 5.0 2.0 2.5 25℃原理:蛋白胨、脱水小牛脑浸粉、脱水牛心浸粉提供氮源、维生素和生长因子;葡萄糖可为多种细菌提供能源;氯化钠维持均衡的渗透压;磷酸氢二钠为缓冲剂。
用法:称取本品 38.5g ,加热搅拌溶解于1000ml 蒸馏水中,121℃高压灭菌15 分钟,备用。
质控菌株接种后于35±0.5℃培养24h,结果如下:哥伦比亚CNA 琼脂基 Columbia CNA Agar Base 用于革兰氏阳性球菌的分离培养,也可用于制备哥伦比亚CNA 血琼脂(需另加无菌脱纤维羊血)改良Giolitti-Cantoni 肉汤基础 Modified Giolitti-Cantoni Broth Base 用于金黄色葡萄球菌的增菌培养(ISO 标准)卵黄甘露醇高盐琼脂基础Egg-Yolk Mannitol Salt Agar Base 用于金黄色葡萄球菌的选择性分离培养(需另加50%卵黄液)甲苯胺蓝 -DNA 酶琼脂 Toluidine Blue-O-DNase Agar用于脱氧核糖核酸酶试验(SN 标准)葡萄球菌选择性琼脂 Steptococcus Selective Agar用于凝固酶阳性葡萄球菌的选择性分离培养葡萄球菌增菌肉汤Steptococcus Enrichment Broth用于金黄色葡萄球菌的增菌培养亚碲酸钠肉汤基础Sodium Tellurite BrothBase用于金黄色葡萄球菌的增菌培养普通肉汤培养基 GeneralBroth Medium 用于金黄色葡萄球菌的培养(SN 标准)7.5% 氯化钠肉汤 7.5%Sodium ChlorideBroth用于金黄色葡萄球菌的增菌培养(GB 标准) DNA 酶琼脂 DNase Agar 用于金黄色葡萄球菌 DNA 酶试验冻干血浆 Freeze-Dried Plasma 用于血浆凝固酶试验Baird-Parker 琼脂基础Baird-Par kerAgar Base 用于金黄色葡萄球菌的选择性分离培养(ISO、FDA BAM、 GB、SN 标准)10% 氯化钠胰酪胨大豆肉汤(TSB) 10% Sodium ChlorideTryptic Soy Broth 用于金黄色葡萄球菌的选择性增菌培养(GB 标准)胰蛋白胨大豆肉汤(TSB) Tryptic Soy Broth 用于金黄色葡萄球菌MPN法测定的增菌培养,NaCl浓度可根据需要而调整(FDA BAM、SN标准);用于β溶血性链球菌的增菌培养(GB标准); 也用于一般细菌的培养肠毒素产毒培养基(不含琼脂)Enterotoxin-Producing Medium(Agar-Free)用于葡萄球菌肠毒素检验(GB 标准)肉浸液肉汤 Meat Infusion Broth 用于金黄色葡萄球菌、溶血性链球菌和蜡样芽胞杆菌的培养(GB标准)。
依达拉奉右莰醇治疗缺血性脑卒中的研究进展

- 179 -①滨州医学院附属医院神经内科 山东 滨州 256600通信作者:鹿树军依达拉奉右莰醇治疗缺血性脑卒中的研究进展席娅琳① 汪临华① 鹿树军① 【摘要】 缺血性脑卒中是脑血管疾病中的常见病,严重可导致高级认知及运动障碍,甚至死亡。
缺血性脑卒中的治疗方法主要包括早期溶栓和保护神经细胞等治疗,然而目前神经保护剂的临床疗效有待考证,大多数神经保护剂仍未得出有益的证据。
新型双靶点复合型神经保护剂依达拉奉右莰醇(ED)可抑制诱导型一氧化氮合酶(iNOS)和肿瘤坏死因子-α(TNF-α)的表达,降低自由基过氧化亚硝基阴离子(ONOO -)水平,从而改善缺血性脑卒中所致的神经损伤症状、功能障碍及活动障碍,本文将对ED 的作用机制及其应用发展做一综述,并对ED 的临床应用进行展望,为后续的用药提供指导。
【关键词】 缺血性脑卒中 自由基清除剂 神经保护剂 依达拉奉右莰醇 Research Progress of Edaravone Dexborneol in the Treatment of Ischemic Stroke/XI Yalin, WANG Linhua, LU Shujun. //Medical Innovation of China, 2024, 21(10): 179-183 [Abstract] Ischemic stroke is a common type of cerebrovascular disease that can lead to advanced cognitive and motor deficits and even death. The treatment of ischemic stroke mainly includes early thrombolysis and neuroprotection. However, the clinical efficacy of neuroprotective agents remains to be verified, and most neuroprotective agents have not yet received useful evidence. Edaravone Dexborneol (ED), a new dual-target neuroprotective agent, can inhibit the expression of inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α(TNF-α), reduce the level of peroxynitrite anion (ONOO -), and improve the symptoms of nerve injury, dysfunction, and activity disorder caused by ischemic stroke. This article will review the mechanism of ED and its application development, and prospect the clinical application of ED, so as to provide guidance for subsequent medication. [Key words] Ischemic stroke Free radical scavenger Neuroprotective agent Edaravone Dextrogenol First-author's address: Department of Neurology, Binzhou Medical University Hospital, Binzhou 256600, China doi:10.3969/j.issn.1674-4985.2024.10.041 脑卒中已成为我国居民寿命的“第一杀手”,其中,急性缺血性脑卒中(acute ischemic stroke,AIS)约占我国脑卒中的70%,为最常见的卒中类型[1-2]。
