Cyproterone acetate_427-51-0_DataSheet_MedChemExpress
克龄蒙-mims

制造商
拜耳医药保健 (Bayer)
成份
11片白色片戊酸雌二醇 Estradiol valerate 2mg,10片浅橙红色复方片戊酸雌二醇 Estradiol valerate 2mg, 醋酸环丙孕酮 Cyproterone acetate 1mg
注意事项
本品不能用于避孕。有血栓形成或其倾向者,头痛、高血压、肝功能异常或肝脏肿瘤、糖尿病、子宫/乳腺疾患患者慎用。
不良反应
罕见 :心血管意外和栓塞 ;胆汁积郁性黄疸,肝腺瘤 ;阴道出血类型改变,痛经,生殖系统和乳腺疾病 ;胃肠道疾病 ;皮肤和皮下组织疾病 ;神经系统疾病 ;心悸,水肿,肌肉痉挛,体重改变,食欲增加,性欲改变,视觉异常,不能耐受隐形眼镜,过敏反应。
药物相互作用
长期使用肝酶诱导药物可能降低性激素的疗效。一些经过牢固结合的物质(如扑热息痛)可能增加雌二醇的生物利用度。可能需调整同时使用的口服降糖药或胰岛素的用量。
查看克龄蒙[Climen]详细药物相互作用信息
MIMS药物分类
雌激素、孕激素及其相关合成药 (Oestrogens & Progesterones & Related Synthetic Drugs)
ATC编码
G
克龄蒙 片剂
(日历型包装) 21 片 ( ¥93 )
制造商:
拜耳医药保健 (Bayer)
适应症
与绝经相关的雌激素缺乏 :血管舒缩性疾病(潮热),生殖泌尿道营养性疾病(外阴阴道萎缩、性交困难、尿失禁),精神性疾病(睡眠障碍、衰弱)。预防雌激素缺乏所致的骨质丢失。
用量
1片/次/日,共服21日,停药7日后继续服用下一个周期的药物。(参阅产品说明书)
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
Invitrogen TrueCut Cas9 Proteins 说明书
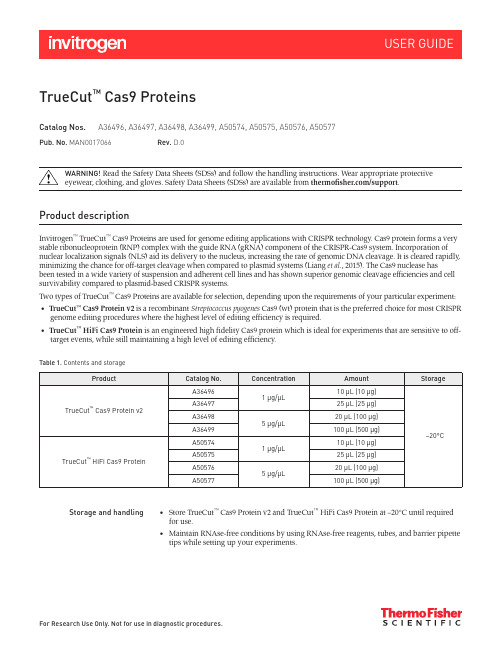
For Research Use Only. Not for use in diagnostic procedures.TrueCut ™ Cas9 ProteinsCatalog Nos.A36496, A36497, A36498, A36499, A50574, A50575, A50576, A50577WARNING! Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective eyewear, clothing, and gloves. Safety Data Sheets (SDSs) are available from thermofi/support .Product descriptionInvitrogen ™ TrueCut ™ Cas9 Proteins are used for genome editing applications with CRISPR technology. Cas9 protein forms a very stable ribonucleoprotein (RNP) complex with the guide RNA (gRNA) component of the CRISPR-Cas9 system. Incorporation of nuclear localization signals (NLS) aid its delivery to the nucleus, increasing the rate of genomic DNA cleavage. It is cleared rapidly, minimizing the chance for off-target cleavage when compared to plasmid systems (Liang et al ., 2015). The Cas9 nuclease hasbeen tested in a wide variety of suspension and adherent cell lines and has shown superior genomic cleavage efficiencies and cell survivability compared to plasmid-based CRISPR systems.Two types of TrueCut ™ Cas9 Proteins are available for selection, depending upon the requirements of your particular experiment:• TrueCut ™ Cas9 Protein v2 is a recombinant Streptococcus pyogenes Cas9 (wt) protein that is the preferred choice for most CRISPR genome editing procedures where the highest level of editing efficiency is required. • TrueCut ™ HiFi Cas9 Protein is an engineered high fidelity Cas9 protein which is ideal for experiments that are sensitive to off-target events, while still maintaining a high level of editing efficiency.Table 1.Contents and storagePub. No. MAN0017066Rev.D.0Storage and handling• Store TrueCut ™ Cas9 Protein v2 and TrueCut ™ HiFi Cas9 Protein at –20°C until required for use. • Maintain RNAse-free conditions by using RNAse-free reagents, tubes, and barrier pipette tips while setting up your experiments.Before you beginMaterials required but notprovided• TrueGuide™ Synthetic gRNAs (see /trueguide)orGeneArt™ Precision gRNA Synthesis Kit (Cat. No. A29377)• Lipofectamine™ CRISPRMAX™ Cas9 Transfection Kit (Cat. Nos. CMAX00001,CMAX00003, CMAX00008, CMAX00015, CMAX00030) (for most cell lines)orNeon™ Transfection System (Cat. Nos. MPK5000, MPK1025, MPK1096) (for highesttransfection efficiency in challenging cell types including suspension cell lines)• GeneArt™ Genomic Cleavage Detection Kit (Cat. No. A24372)• Opti-MEM™ I Reduced Serum Medium (Cat. No. 31985-062)• 1X TE buffer, pH 8.0 (Cat. No. AM9849) and nuclease-free water (Cat. No. AM9914G)Prepare working stock ofTrueGuide™ Synthetic gRNA If TrueGuide™ Synthetic gRNA is being used, resuspend the gRNA (sgRNA, crRNA, ortracrRNA) in1X TE buffer to prepare 100 μM (100 pmol/μL) stock solutions.Before opening, centrifuge each TrueGuide™ Synthetic gRNA tube at low speed (maximum1.RCF 4,000 × g) to collect the contents at the bottom of the tube, then remove the cap from thetube carefully.Using a pipette and sterile tips, add the required volume of 1X TE buffer to prepare 100 μM2.(100 pmol/μL) stock solutions.3.Vortex the tube to resuspend the oligos, briefly centrifuge to collect the contents at thebottom of the tube, then incubate at room temperature for 15–30 minutes to allow the gRNAoligos to dissolve.Vortex the tube again to ensure that all the contents of the tube are resuspended, then briefly4.centrifuge to collect the contents at the bottom of the tube.(Optional) Check the concentration of the resuspended oligos using the NanoDrop™5.Spectrophotometer (or equivalent) or a UV-base plate reader.6.(Optional) Aliquot the working stock into one or more tubes for storage.Use working stocks immediately or freeze at –20°C until needed for use.7.(Optional) Generate gRNA byin vitro transcription If using in vitro transcribed gRNA with TrueCut™ Cas9 Protein v2 or TrueCut™ HiFi Cas9Protein in CRISPR-Cas9-mediated genome editing, the GeneArt™ Precision gRNA SynthesisKit is recommended for preparation of the gRNA. For detailed instructions on how togenerate full length gRNA, see the GeneArt™ Precision gRNA Synthesis Kit User Guide (Pub.No. MAN0014538), at .Transfection guidelinesGeneral CRISPR/gRNAtransfection guidelines• The efficiency with which mammalian cells are transfected with gRNA varies accordingto cell type and the transfection reagent used. See Table 2 (page 3) for delivery reagentrecommendations.• For gene editing (including gene knockout) editing efficiency is highest with a 1:1 molarratio of gRNA to TrueCut™ Cas9 Protein v2 or TrueCut™ HiFi Cas9 Protein. In some celltypes such as iPSC and THP1, we have used up to 2 μg TrueCut™ Cas9 Protein v2 and400 ng gRNA per well in 24-well format.• For HDR knock-in editing, a 1.5:1 molar ratio of donor ssODN to gRNA or TrueCut™ Cas9Protein v2 or TrueCut™ HiFi Cas9 Protein is recommend for highest knock-in efficiency.The donor can be added directly to RNPs (a premixed gRNA-Cas9 protein). If using adsDNA donor, further optimization may be necessary to determine the appropriate donoramount, since the toxicity level is dependent on the length and format of the donor DNAand cell type.• The optimal cell density for transfection varies depending on cell size and growthcharacteristics. In general, use cells at 30–70% confluence on the day of transfectionwith lipid-mediated delivery, or 70–90% confluence for electroporation using the Neon™Transfection System.• After the optimal cell number and dosage of Cas9/gRNA and/or donor that providesmaximal gene editing efficiency is determined for a given cell type, do not varyconditions across experiments to ensure consistency.For an overview of the factors that influence transfection efficiency, see the “TransfectionBasics” chapter of the Gibco™ Cell Culture Basic Handbook, available at /cellculturebasics.• Use the TrueGuide™ Positive Controls (human AVVS1, CDK4, HPRT1, or mouse Rosa 26)and negative control gRNA (non-coding) to determine gRNA amount and transfectionconditions that give the optimal gene editing efficiency with highest cell viability. TheTrueGuide™ Positive and Negative sgRNA and crRNA Controls are available separatelyfrom Thermo Fisher Scientific. For more information, refer to /trueguide.• The cell number and other recommendations provided in the following proceduresare starting point guidelines based on the cell types we have tested. For multiplewells, prepare a master mix of components to minimize pipetting error, then dispense theappropriate volumes into each reaction well. When making a master mix for replicate wells,we recommend preparing extra volume to account for any pipetting variations.Recommended deliveryoptions• Choosing the right delivery reagent is critical for transfection and gene editing efficiency.See our recommendations in Table 2. For more information on transfection reagents, see/transfection.• For cell line specific transfection conditions using the Lipofectamine™ CRISPRMAX™Transfection Reagent or the Neon™ Transfection System, see the Appendix (page 13).• For best results, perform electroporation and transfection of cells using both TrueCut™Cas9 Proteins and TrueGuide™ Synthetic gRNA.HiFi Cas9 Protein.Table 2. Recommended delivery options for TrueCut™ Cas9 Protein v2 and TrueCut™Guidelines for verification of editing efficiencyVerification of gene editingefficiency• Before proceeding with downstream applications, verify the gene editing efficiency of thecontrol target and select the condition that shows the highest level of editing efficiency forfuture screening experiments.• To estimate the CRISPR-Cas9-mediated editing efficiency in a pooled cell population,use the GeneArt™ Genomic Cleavage Detection Kit (Cat. No. A24372), or performIon Torrent™ next generation sequencing or a Sanger sequencing-based analysis.• While the genomic cleavage detection (GCD) assay provides a rapid method forevaluating the efficiency of indel formation following an editing experiment, nextgeneration sequencing (NGS) of the amplicons from the edited population or Sangersequencing of amplicons cloned into plasmids give a more accurate estimate of thepercent editing efficiency and indel types for knockout and HDR knock-in editing.GeneArt™ Genomic CleavageDetection (GCD) Assay• After transfections, use the GeneArt™ Genomic Cleavage Detection Kit (Cat. No. A24372)to estimate the CRISPR-Cas9-mediated cleavage efficiency in a pooled cell population.• You can design and order target-specific primer sets for the GCD assay through ourTrueDesign Genome Editor, available at /crisprdesign.• To perform the GCD assay for the positive control, you need the primers listed in Table 3.We recommend using Invitrogen™ Custom DNA Value or Standard Oligos, available from/oligos, for target specific primer sets needed for the GCD assay.• You can set up the GCD assay in a 96-well plate format and analyze multiple gRNA-treated samples in parallel on a 2% E-Gel™ 48 agarose gel (48-well).• For more information and detailed protocols, see the GeneArt™ Genomic Cleavage DetectionKit User Guide (Pub. No. MAN0009849), available for download at /GCDManual.Table 3. Target sequences for the positive and negative control (non-targeting) TrueGuide™ Synthetic gRNA sequences.Guidelines for clone isolation and validationAfter you have determined the cleavage efficiency of the pooled cell population, isolate single cell clones for further validation and banking. You can isolate single cell clones from the selected pool using limiting dilution cloning (LDC) in 96-well plates or by single cell sorting using a flow cytometer.Limiting dilution cloning(LDC)• Based on the editing efficiency and estimated cell viability, you can estimate the number of single clones needed to obtain a desired knock-out (KO) clonal cell line. For example, if you desire a homozygous KO with mutations in both copies of a gene and the resulting GeneArt ™ cleavage detection efficiency was 50%, then the probability of having both alleles knocked out in any cell is 25% (0.5 × 0.5 = 0.25).If the probability of an indel leading to frame shift is 2/3, then the chance of having a homozygous KO is ~11% per cell [(0.5 × 0.5) × (0.66 × 0.66) = 0.11].• We recommend performing limiting dilution by targeting 0.8 cells/well, which requires you to resuspend the transfected cells (post-counting) at a density of8 cells/mL in complete growth medium, then transferring 100 µL of this to each well of a 96-well plate. If you plate at least ten 96-well plates in this manner and expect only 20% of cells tosurvive, then the probability of having homozygous KO clones in the 192 surviving cells will be 19–21 cells (192 × 11%).• Note that single cell clone survivability varies by cell type. Some cells that do not like to remain as single cells need to be plated at a low density to get well separated colonies, which will then have to be manually picked for further screening.Example LDC procedureusing 293FT cells1. Wash the transfected cells in each well of the 24-well plate with 500 µL of PBS. Carefully aspirate the PBS and discard.2. Add 500 µL of TrypLE ™ cell dissociation reagent to the cells and incubate for2–5 minutes at 37°C.3. Add 500 µL of complete growth medium to the cells to neutralize the dissociation reagent.Pipette the cells up and down several times to break up the cell aggregates. Make sure that the cells are well separated and are not clumped together. 4. Centrifuge the cells at 300 × g for 5 minutes to pellet.5. Aspirate the supernatant, resuspend the cells in an appropriate volume of pre-warmed(37°C) growth medium, then perform a cell count. 6. Dilute the cells to a density of 8 cells/mL of complete growth medium. Prepare a total ofSequence analysis• For next generation sequencing (NGS) based editing efficiency analysis, you canspecifically amplify the edited region and barcode amplicons by pooling all amplicons in a single tube and performing sequencing using various NGS platforms such as the Ion Torrent ™ Targeted Amplicon-seq Validation (TAV). For more information on NGS analysis, refer to Ion Torrent ™ targeted sequencing solutions at /ionapliseqsolutions .• For Sanger sequencing-based editing efficiency analysis, refer to our application note referenced at /sangercrispr .• Use the SeqScreener Gene Edit Confirmation App on Thermo Fisher ™ Connect todetermine the spectrum and frequency of targeted mutations (see Pub. No. MAN0019454 at . for details).50 mL of cell suspension at this cell density and transfer to a sterile reservoir.Note: You can also perform a serial dilution to get a better estimate of cell density.Using a multichannel pipettor, transfer 100 µL of the cell suspension into each well of 96-well7.tissue culture plates until the desired number of plates is seeded. Make sure to mix the cellsin between seeding the plates to avoid the formation of cell aggregates.Note: In general, we seed ten 96-well plates to achieve a large number of clones. Numberof plates to seed depends on the editing efficiency of pooled cell population and viability ofcells post single cell isolation.Incubate the plates in a 37°C, 5% CO2 incubator.8.Scan the plates for single cell colonies as soon as small aggregates of cells are visible under a9.4X microscope (usually after first week, depending on the growth rate of the cell line).Continue incubating the plates for an additional 2–3 weeks to expand the clonal populations10.for further analysis and characterization.Example single cell sortingprocedure in a 96-well plateusing flow cytometer Single cells can be sorted into a 96-well plate format using a flow cytometer with singlecell sorting capability. After sorting and expanding the single cell clones, analyze andcharacterize the clonal populations using suitable assays.1.Wash the transfected 293FT cells in each well of the 24-well plate with 500 µL of PBS.Carefully aspirate the PBS and discard.Add 500 µL of TrypLE™ cell dissociation reagent and incubate for 2–5 minutes at 37°C.2.Add 500 µL of complete growth medium to the cells to neutralize the dissociation reagent.3.Pipette the cells up and down several times to break up the cell aggregates. Make sure thatthe cells are well separated and are not clumped together.Centrifuge the cells at 300 × g for 5 minutes to pellet.4.5.Aspirate the supernatant, then wash the cell pellet once with 500 μL of PBS.Resuspend 1 × 106 cells in 1 mL of FACS buffer, then add propidium iodide (PI) to the cells at6.a final concentration of 1 µg/mL. Keep the resuspended cells on ice.Filter the cells using suitable filters before analyzing them on a flow cytometer with single7.cell sorting capability.Sort PI-negative cells into a 96-well plate containing 100 μL of complete growth medium. If8.desired, you can use 1X antibiotics with the complete growth medium.Incubate the plates in a 37°C, 5% CO2 incubator.9.Scan the plates for single cell colonies as soon as small aggregates of cells are visible under10.a 4X microscope. Colonies should be large enough to see as soon as 7–14 days (usually afterfirst week, depending on the growth rate of the cell line). You can perform image analysis toensure that the colonies are derived from single cells.11.After image analysis, continue incubating the plates for an additional 2–3 weeks to expandthe clonal populations for further analysis and characterization.Characterize edited clones You can analyze the single cell clones for purity and the desired genotype (homozygous orheterozygous allele) by various molecular biology methods such as genotyping PCR, qPCR,next generation sequencing, or western blotting.Supporting tools At Thermo Fisher Scientific, you can find a wide variety of tools to meet your gene editingand validation needs, including Invitrogen™ LentiArray CRISPR and Silencer™ Select RNAilibraries for screening, primers for targeted amplicon sequencing, antibody collection forknock-out validation, and ORF collections and GeneArt™ gene synthesis service for cDNAexpression clones that can be used for rescue experiment reagents.thermofisher .com/support | thermofisher .com/askaquestion thermofisher .comLimited product warrantyLife Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies’ General Terms and Conditions of Sale found on Life Technologies’ website at /us/en/home/global/terms-and-conditions.html . If you have any questions, please contact Life Technologies at www /support .Manufacturer: Thermo Fisher Scientific Baltics UAB | V. A. Graiciuno 8 | LT-02241 Vilnius, LithuaniaThe information in this guide is subject to change without notice.DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, LIFE TECHNOLOGIES AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Revision history:Pub. No. MAN0017066Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all applicable Limited Use Label Licenses.©2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified .。
四正柏生物科技基因定点突变试剂盒说明书

基因定点突变试剂盒保存条件及效期:−20℃保存,有效期一年。
产品描述:定点突变是一种广泛应用的分析蛋白和DNA 的非常有力的工具。
普通的定点突变试剂盒通常是基于反向PCR 技术,采用高保真耐热DNA 聚合酶和互补的突变引物向目的基因引入单个碱基或多个邻近碱基的突变、缺失、或插入。
当遇到多个位点突变的需求时,利用单点突变试剂盒只能对目标位点逐一引入突变,耗时费力。
Mut Multi Site-directed Mutagenesis(MSDM)Kit 针对每一个目标突变只采用一条突变引物,利用Mut MSDMEnzyme 的耐热DNA 聚合酶活性合成与模板互补的带有引物突变的DNA 链,然后通过其耐热的连接酶活性修复突变DNA 链之间的切刻(nicks),使其连接成为一条与模板互补的突变单链DNA 。
经过多个PCR 循环反应后,带有多个特定突变的DNA 链成倍增长。
PCR 反应结束后,采用DpnI 限制性内切酶消化含有甲基化的双链质粒模板,而带有突变的环形单链质粒则通过转化而在大肠杆菌体内复制,最终得到带有不同突变组合的质粒DNA 。
本试剂盒适用于≤6kb 质粒的多点突变,根据质粒性质不同,最多可引入5个位点的突变、插入或删除(通常以1~3个位点突变为主)。
该方法操作简单快捷,突变阳性率高。
产品组分:产品编号规格MSM5303-1010rxnMut MSDM Enzyme12μl 10x MutMSDM Buffer 100μl MutdNTPs25μl Dpn I (10U/ l)12μl ddH 2O1ml版本号:2016-02-19产品说明书图1.Mut 多点基因定点突变试剂盒原理和操作流程示意图使用方法:1.模板准备:(1)请使用6kb以下的质粒作为模板;如果模板质粒过大,可将所需突变的序列亚克隆到较小的载体中,完成突变后再克隆到目的载体中。
(2)对于非甲基化的质粒(例如从大肠杆菌JM110或SCS110菌株中提取的质粒),可通过转化dam+的大肠杆菌菌株(如DH5α、TOP10、JM109、XL1-Blue等),再抽提获得甲基化的质粒作为PCR反应模板。
α-Estradio_57-91-0_MedBio_参考使用

ICI 204,448 hydrochloride
ICI 204,448 hydrochloride
121264-04-8
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12175
Finasteride acetate
Finasteride acetate
222989-99-3
3、α-Estradiol同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12185
Clomiphene citrate
Clomiphene citrate
50-41-9
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12197
Erteberel (LY500307)
200mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12170
ODM-201
ODM-201
1297538-32-9
50mg
≥98%
体内研究
α-雌二醇(17-α-雌二醇,0.01,0.1,1μg)显着降低小鼠幼崽中央无血管/总视网膜面积的百分比。在暴露于高氧的幼鼠的视网膜中,α-雌二醇(1μg)显着降低出生后第9天,第13天和第17天的丙二醛(MDA)水平。 α-雌二醇(1μg)还减少NADPH-氧化酶阳性细胞的数量,NADPH氧化酶浓度和幼仔视网膜中的活性。在1.0-μgα-雌二醇处理的幼崽中,VEGF视网膜浓度在PND 9上较高,但在PND 14和17上较低.1.1-μgα-雌二醇处理的幼崽的视网膜中的最佳效果在PND上被ICI182780部分逆转。 14和17 [3]。
磷酸特地唑胺产品说明

450.32
Bacterial
Anti-infection
项目 Test
外观 Appearance
纯度 Purity
溶解性 Solubility
保存/复检期 Storage/Recommended Retest Period
检测指标 Specification White to off-white (Solid)
北京索莱宝科技有限公司磷酸特地唑胺产品说明产品编号tcatnumberit0410产品名称tproductname磷酸特地唑胺tedizolidphosphate产品类型tproducttype小分子抑制剂smallmoleculeinhibitorscas
磷酸特地唑胺产品说明
北京索莱宝科技有限公司
产品编号 Cat Number 产品名称 Product Name 产品类型 Product Type
CAS.
分子式 Molecular Formula 分子量 Molecular Wt
靶点 Target
通路 Pathway
IT0410 磷酸特地唑胺 Tedizolid phosphate 小分子抑制剂 Small molecule inhibitors 856867-55-5
Purityห้องสมุดไป่ตู้98%
Soluble in DMSO Powder 4℃ 2 years In solvent -20℃ 1 month
注意:我司生产的小分子抑制剂均为非无菌包装,若用于细胞实验,请提前做好预处理。
印度药典2010

New Features of IP 2010This new edition of the Indian Pharmacopoeia entitled 6th edition (Indian Pharmacopoeia 2010) is published by the Indian Pharmacopoeia Commission (IPC) in accordance with a plan and completed through the untiring efforts of its members, Secretariat and Laboratory over a period of about two years. It supersedes the 2007 edition but any monograph of the earlier edition that does not figure in this edition continues to be official as stipulated in the Second Schedule of the Drugs and Cosmetics Act, 1940.PresentationThe Indian Pharmacopoeia 2010 is presented in three volumes. V olume I contains the Notices, Preface, the Structure of the IPC, Acknowledgements, Introduction, and the General Chapters. V olume II contains the General Notice, General Monographs on Dosage Forms, Monographs on drug substances, dosage forms and pharmaceutical aids (A to M). V olume III contains Monographs on drug substances, dosage forms and pharmaceutical aids (N to Z) followed by Monographs on V accines and Immunosera for Human use, Herbs and Herbal products, Blood and blood-related products, Biotechnology products and V eterinary products.The scope of the Pharmacopoeia has been extended to include products of biotechnology, indigenous herbs and herbal products, veterinary vaccines and additional antiretroviral drugs and formulations, inclusive of commonly used fixed-dose combinations. Standards for new drugs and drugs used under National Health Programmes are added and the drugs as well as their formulations not in use now a days are omitted from this edition. The number of monographs of Excipients, Anticancer drugs, Herbal products and Antiretroviral drugs have been increased in this edition. Monographs of Vaccines and Immunosera are also upgraded in view of development of latest technology in the field. A new chapter on Liposomal products and a monograph of Liposomal Amphotericin B injection is an added advantage in view of latest technology adopted for drug delivery. A chapter on NMR is incorporated in Appendices. The chapter on microbial contamination is also updated to a great extent to harmonise with prevailing international requirements.FormatIn an effort to make the pharmacopoeia more user-friendly, design of the texts of the monographs and of the test methods are kept same. Cross-referencing has been avoided to make each monograph complete in itself thus making it convenient to the analyst.Basis of Pharmacopoeial RequirementsAs in the past, this compendium provides a publicly available statement concerning the quality of a product that can be expected and demonstrated at any time throughout the accepted shelf-life of the article. The standards laid down represent the minimum with which the article must comply and it is inculcate on the manufacturer to ensure that the article is manufactured in accordance with the Good Manufacturing Practices (GMPs). It is essential that sufficiently stringent limits are applied at the time of release of a batch of a drug substance or drug product so that the pharmacopoeial standards are met until its expiry date when stored under the storage conditions specified.It must be noted that a valid interpretation of any requirement of the Pharmacopoeia should be done in the context of the monograph as a whole, the relevant general monograph, where appropriate, the specified tests and methods of analysis including any reference to the relevant General Notices. Familiarity with the General Notices will facilitate the correct application of the requirements.ChangesKeeping in view the essential requirement under the Drugs and Cosmetics Act, 1940 and Rules thereunder in the information on category of a drug, dosage and usual available strengths of dosage forms has been re-kept in this edition. General chemical tests for identification of an article have been almost eliminated and the more specific infrared and ultraviolet spectrophotometric tests have been given emphasis. The concept of relying on published infrared spectra as a basis for identification has been continued.The use of chromatographic methods has been greatly extended to cope with the need for more specificity in assays and in particular, in assessing the nature and extent of impurities in drug substances and drug products. Most of the existing Assays and Related substances tests are upgraded by liquid chromatography method in view to have more specificity and to harmonise with other International Pharmacopoeias.The test for pyrogens involving the use of animals has been virtually eliminated. The test for bacterial endotoxins introduced in the previous edition is now applicable to more items. The test for abnormal toxicity is now confined to certain vaccines.General ChaptersV olume I is devoted mainly to test methods that are applicable to all the articles of the pharmacopoeia and general informationpertaining to the quality requirements of medicinal substances. It also includes reference data such as reference spectra, typical chromatograms etc. The test methods reflect the sophistication of analytical methodology and instrumentation. Analytical methods are, in general, in harmony with those adopted internationally for monitoring the quality of drugs. The steps taken for harmonization have been initiated by the need to cope with the increasing demand for drugs manufactured in the country to meet globally accepted standards.The trend towards controlling the microbial quality of all medicinal products has been recognized and the requirement regarding limits of bacterial contamination even of products for oral administration and topical application so that adequate controls are exercised by manufacturers by the adoption of GMPs has been continued.The chapter on Vaccines: General requirements has been updated. Minor corrections have been made in the appendices entitled Tests on Chicken flocks free from specified pathogens for the production and quality control of vaccines and General provisions: Avian viral vaccines- Tests for extraneous agents in seed lot. The peptide mapping test for Inactivated Hepatitis B Vaccine has been deleted. Wherever appropriate, other corrections have also been incorporated and overall presentation improved.In view of considering the microbiological quality, the whole microbiological general chapter comprising of effectiveness of antimicrobial preservatives, microbial contamination in nonsterile products and microbiological quality of raw material, dosage forms, herbs, processed herbs and herbal products have been extensively revised. For the first time in this chapter the analysis of strain Shigella boydii has been introduced which is possibly not available in other Pharmacopoeias. The addition of this strain Shigella boydii is essential as it is acute dysentry causing strain of tropical region of our country.The chapter on biotechnology derived therapeutic products has been fully revised. Special emphasis has been given on monoclonal antibodies Antisera.MonographsThe General Monographs for dosage forms of active pharmaceutical ingredients (APIs) are grouped together at the beginning of Volume II. They are followed by the monographs for the APIs, pharmaceutical aids and individual dosage forms, all in alphabetical order. Monographs for other articles of a special nature such as vaccines and immunosera for human use, herbs and herbal products, blood and blood related products, biotechnology products and veterinary products are given in separate sections in V olume III.A list of 287 new monographs items not included in the 2007 edition of the Indian Pharmacopoeia and its addendum 2008 but added in this edition is given below:AdmissionsMonographs on drug substances, dosage forms and pharmaceutical aidsAcepromazine MaleateAllantoinAluminium Magnesium SilicateS-Amlodipine BesylateS-Amlodipine TabletsLiposomal Amphotericin B InjectionAnastrozoleAnastrozole TabletsAnhydrous LactoseArtesunateAtazanavir SulphateAtazanavir CapsulesBenzoic Acid SolutionBetamethasone DipropionateBetamethasone CreamBetamethasone LotionBetamethasone OintmentBifonazoleBifonazole CreamBumetanideBumetanide InjectionBumetanide Oral solutionBumetanide TabletsButylparabenCalcium Chloride InjectionCapecitabineCapecitabine TabletsCefamandole NafateCefamandole InjectionCetrimide CreamCetyl PalmitateChlorothiazideChlorothiazide Oral SuspensionChlorothiazide TabletsChymotrypsinCilastatin SodiumClindamycin HydrochlorideClindamycin CapsulesCodeine Phosphate Tablets Cyproterone Acetate Cyproterone Tablets Daunorubicin Hydrochloride Daunorubicin Injection Dexchlorpheniramine Maleate Dexchlorpheniramine Oral Solution Dexchlorpheniramine Tablets Dextropropoxyphene Hydrochloride Dextropropoxyphene Capsules Dextropropoxyphene Napsilate DiacereinDiacerein CapsulesDiazoxideDiazoxide TabletsDicloxacillin SodiumDicloxacillin Capsules Dicloxacillin Oral Suspension Diethanolamine Dihydroergocristine Mesylate Dihydroergotamine Mesylate DimethiconeDisopyramideDisopyramide Capsules Disopyramide Phosphate Capsules Disopyramide Phosphate Sustained-release CapsulesDivalproex Sustained-release Tablets Docetaxel TrihydrateDocetaxel Injection DomperidoneDoxofyllineDoxofylline TabletsEnoxaparin SodiumEnoxaparin Injection Escitalopram Oxalate Escitalopram TabletsEstradiol and Norethisterone Tablets EtodolacEtodolac CapsulesEtodolac TabletsFamotidineFamotidine Tablets FelodipineFelodipine Sustained-release TabletsFenbendazoleFenofibrateFentanylFentanyl CitrateFentanyl InjectionFinasterideFinasteride TabletsFluconazoleFluconazole CapsulesFluconazole TabletsFlucytosineFlucytosine CapsulesFlucytosine Oral SuspensionFlucytosine TabletsFluorescein InjectionFlutamideFlutamide CapsulesFumaric AcidGefitinibGefitinib TabletsGemifloxacin MesylateGemifloxacin TabletsGliclazideGliclazide TabletsGlimepirideGlimepiride TabletsHomatropine MethylbromideHomatropine Methylbromide TabletsHyoscyamine SulphateHyoscyamine InjectionHyoscyamine Oral SolutionHyoscyamine TabletsIbuprofen CreamIbuprofen GelImatinib MesylateImatinib CapsulesIndapamide TabletsIsobutaneIsopropyl MyristateLactuloseLamotrigine Sustained-release TabletsLansoprazoleLansoprazole Sustained-releaseCapsulesLecithinLevosalbutamol SulphateLinezolidLinezolid TabletsLosartan Potassium and AmlodipineTabletsLosartan Potassium andHydrochlorothiazide TabletsMaleic AcidMalic AcidMaltitolLiquid MaltitolMaltodextrinMefloquine HydrochlorideMeloxicam Oral SuspensionMenthol and Benzoin InhalationMetformin Hydrochloride Sustained-release TabletsMethadone LinctusMetronidazole Sterile SuspensionMiconazoleMicrocrystalline Cellulose andCarboxymethylcellulose SodiumMisoprostolMometasone FuroateMometasone Aqueous Nasal SprayMometasone CreamMometasone OintmentMontelukast SodiumMontelukast TabletsMycophenolate MofetilMycophenolate Mofetil CapsulesMyristic AcidNaloxone HydrochlorideNaloxone InjectionNaltrexone HydrochlorideNaltrexone TabletsNaproxenNaproxen Oral SuspensionNaproxen SuppositoriesNaproxen Sustained-release TabletsNaproxen TabletsNeotameOndansetron Orally Disintegrating TabletsOndansetron Oral Solution Pantoprazole SodiumPantoprazole Sustained-release Tablets PerphenazinePerphenazine Tablets Phenoxyethanol Phenylpropanolamine Hydrochloride PhenytoinPhenytoin CapsulesPhenytoin Oral Suspension PimozidePimozide TabletsPiperacillinPiperacillin Intravenous Infusion PoloxamersPolyoxyl 35 Castor OilPolyoxyl 40 Hydrogenated Castor Oil Potassium SorbatePravastatin SodiumPravastatin TabletsPraziquantelPraziquantel TabletsPrednisolone AcetatePregabalinPregabalin Capsules Pregelatinised Starch Progesterone Injectable Suspension Promazine TabletsPropanePropionic AcidPropofolPropofol InjectionProtriptyline Hydrochloride Protriptyline Tablets Pyrimethamine Tablets Quiniodochlor Cream Quiniodochlor Ointment Quiniodochlor and Hydrocortisone CreamQuiniodochlor and Hydrocortisone OintmentRamipril and Hydrochlorothiazide Tablets RibavirinRibavirin Inhalation SolutionSerratiopeptidaseSerratiopeptidase TabletsSildenafil CitrateSildenafil TabletsSimvastatinSimvastatin TabletsSorbitan OleateSucraloseSumatriptanSumatriptan InjectionTelmisartanTelmisartan TabletsTemozolomideTemozolomide CapsulesTerazosin HydrochlorideThiocolchicosideThiocolchicoside CapsulesTicarcillin and Clavulanic AcidInjectionTolazamideTolazamide TabletsTolnaftateTolnaftate CreamTolnaftate GelTolnaftate Topical PowderTolnaftate Topical SolutionTolterodine TartrateTramadol HydrochlorideTramadol CapsulesTrandolaprilTrandolapril TabletsTravoprostTravoprost Eye dropsTributyl CitrateTrichloromonofluoromethaneTriethyl CitrateTrimetazidine HydrochlorideV alproate InjectionV alproic AcidV alproic Acid CapsulesV alproic Acid Oral SolutionV alsartanV alsartan TabletsV alsartan and HydrochlorothiazideTabletsV ancomycin HydrochlorideV ancomycin CapsulesV ancomycin Intravenous InfusionV ancomycin Oral SolutionXanthan GumZoledronic AcidZoledronic Acid InjectionHerbal MonographsAmla Juice PowderArjuna Dry ExtractAshwagandha Dry ExtractBelladonna TinctureBhibhitaki Aqueous ExtractBrahmi ExtractCoconut OilColeus Dry ExtractCoriander OilGarcinia Aqueous ExtractHaridra Dry ExtractHaritaki ExtractHaritaki Aqueous ExtractIpecac TinctureLavangMethiNeemSarpagandha PowderSarpagandha TabletsSunthi ExtractTulasi Dry ExtractV asaka ExtractV eterinary MonographsInfectious Bursal Disease Vaccine ,LiveInfectious Chicken Aneamia V accine,InactivatedInfectious Chicken Aneamia V accine,LiveMarek’s Disease V accine, LiveReo Virus V accine, InactivatedReo Virus V accine, LiveSalmonella V accine, Inactivated Sterile Diluent for Live V accinesMonographs upgraded Monographs on drug substances, dosage forms and pharmaceutical aids AcarboseAciclovirAdrenaline InjectionAlprazolamAlprazolam TabletsAminophyllineAminophylline Injection Aminophylline Tablets Amitriptyline Hydrochloride Amodiaquine TabletsArginineArteetherArtemetherArtemesininAtorvastatin CalciumAzithromycinAzithromycin Oral Suspension Azithromycin TabletsBacitracinBacitracin ZincBenzyl alcoholBromhexine Hydrochloride Bromhexine TabletsBromocriptine Mesylate Bromocriptine Capsules Bromocriptine TabletsBisacodylBisacodyl TabletsBuprenorphine Hydrochloride Benzhexol HydrochlorideCalcium Gluconate TabletsCalcium LevulinateCalcium StearateCaptoprilCitric AcidCitric Acid MonohydrateCellulose Acetate Phthalate Clofazimine Capsules CarbimazoleCarvedilol TabletsCefadroxilCrospovidoneCefuroxime InjectionCefaclor Oral suspensionCefadroxil CapsulesCefotaxime SodiumCefazolin SodiumCefiximeCinnarizineCefaperazone SodiumCefazolin InjectionCimetidine TabletsCephalexinCetostearyl AlcoholCetyl AlcoholCarbimazole TabletsCefadroxil TabletsChlordiazepoxideCisplatinClopidogrel BisulphateClopidogrel TabletsClotrimazole CreamCresol with Soap SolutionCytarabineDanazolDanazol CapsulesDesferrioxamine MesylateDesferrioxamine InjectionDexamethasone Sodium phosphateDextromethorphan HydrobromideDibutyl PhthalateDicyclomine InjectionDigoxinDisodium EdetateDiphenhydramine HydrochlorideErythromycin EstolateEsomeprazole Magnesium TrihydrateEsomeprazole TabletsEthambutol HydrochlorideFerrous GluconateFerrous SulphateDried Ferrous SulphateFexofenadine HydrochlorideFluocinolone AcetonideFluorescein InjectionFolic AcidFrusemideFrusemide InjectionFrusemide TabletsGallamine TriethiodideGallamine InjectionGentamycin SulphateGlibenclamideGlibenclamide TabletsGuaiphenesinHeparin SodiumHeparin InjectionIbuprofenIbuprofen TabletsImipenemImipenem and Cilastatin InjectionIsoxsuprine HydrochlorideKetamine HydrochlorideKetamine InjectionKetonazoleKetonazole TabletsKetoprofenKetoprofen CapsulesLactoseLevamisole TabletsLevocitrizine HydrochlorideLevocetirizine TabletsLevofloxacin TabletsMagnesium StearateMagnesium TrisilicateMannitolMannitol InjectionMebendazoleMebendazole TabletsMegestrol AcetateMercaptopurineMethotrexate InjectionMethylergometrine TabletsMetronidazoleMetronidazole TabletsMetronidazole InjectionMorphine SulphateMosapride Citrate Dihydrate Naphazoline NitrateNebivolol HydrochlorideNebivolol TabletsNefedipine Sustained- release Tablets NiclosamideNiclosamide TabletsNitrazepamNitrazepam TabletsNitrofurazoneNorethisteroneNoscapineNystatinOfloxacinOlanzapineOmeprazoleOndansetron TabletsOxytocinOxytocin InjectionOxytocin Nasal Solution PhenobarbitonePhenobarbitone Sodium ParacetamolParacetamol TabletsDiluted Pentaerythritol Tetranitrate Pentamidine Isethionate Pentamidine InjectionPethidine HydrochloridePethidine Injection Phenolphthalein PhenylbutazonePiracetamPiroxicamPolysorbate 20Polysorbate 80Potassium IodidePovidonePrednisolonePropranolol Hydrochloride Propranolol InjectionPropranolol Tablets Pseudoephedrine Hydrochloride Pyrantel PamoateQuetiapine FumarateQuinine Dihydrochloride Injection Rabeprazole TabletsRiboflavine Sodium PhosphateRitonavir TabletsSalbutamolSalbutamol SulphateSalbutamol InjectionSalmeterol and Fluticasone PropionatePowder for InhalationSecnidazoleSodium AlginateSorbitolSoritol Solution (70 per cent)(Crystallizing)Soritol Solution (70 per cent) (Non-crystallizing)SpironolactoneSpironolactone TabletsSodium FusidateSodium Fusidate CapsulesSodium PropylparabenSodium MethylparabenMethylparabenPropylparabenStearyl AlcoholSucroseSulphacetamide SodiumSulphacetamide Eye dropsTalcTerbutaline TabletsTheophyllineTheophylline TabletsThiamine HydrochlorideThiamine NitrateTenofovir and Emtricitabine TabletsTerbutaline SulphateThiotepaTopiramate TabletsV erapamil HydrochlorideV erapamil TabletsV essopressin InjectionVincristine SulphateVincristine InjectionXylometazoline HydrochlorideWarfarin SodiumWarfarin Sodium ClatharateZidovudine TabletsMonographs on Vaccines for HumanUseDiphtheria and Tetanus V accine(Adsorbed) for Adults andAdolescentsDiphtheria, Tetanus and PertussisV accine (Adsorbed)Diphtheria, Tetanus, Pertussis (WholeCell), Hepatitis B (rDNA) andHaemophilus Type b ConjugateV accine (Adsorbed)Hepatitis B V accine (rDNA)Measles, Mumps and Rubella Vaccine(Live)Tetanus V accine (Adsorbed)Monographs on V accines for V eterinaryUseAvian Infectious Bronchitis V accine,InactivatedAvian Infectious Bronchitis V accine,LiveAvian Spirochaetosis V accineEgg Drop Syndrome’76 (Adenovirus)V accine, InactivatedFoot-and-Mouth Disease Vaccine,InactivatedFowl Cholera V accine, InactivatedFowl Pox V accine, LiveInclusion Body Hepatitis (IBH)V accine, InactivatedInfectious Avian EncephalomyelitisV accine, LiveInfectious Bursal Disease Vaccine,InactivatedInfectious Coryza V accinePeste Des Petits Ruminants V accine,LiveRanikhet Disease Vaccine, InactivatedRanikhet Disease Vaccine, Live(Lentogenic Strain)Ranikhet Disease Vaccine, Live(Mesogenic Strain)Monographs on V eterinary DiagnosticsAvian Mycoplasma AntigenSalmonella Pullorum AntigenChanged Titles of Monographs From ‘Leptospira V eterinary V accine, Inactivated’ to ‘ Canine Leptospirosis V accine, Inactivated’From ‘Avian Pleuropneumonia Live Antigen’ to ‘ Avian Mycoplasma Antigen’From ‘Salmonella Pullorum Coloured Antigen’ to ‘Salmonella Pullorum Antigen’OmissionsAdenineAluminium SulphateAnalginAnalgin TabletsButylated HydroxyanisoleCaramelCyclopropaneDeslanosideDeslanoside Injection Dibutyl PhthalateEmetine HydrochlorideEmetine InjectionEphedrineErythromycin EstolateErythromycin Estolate TabletsFusidic Acid Oral Solution2- Deoxy- D- GlucoseProtamine Zinc Insulin InjectionLanatoside CLanatoside C TabletsLaryngotracheitis Vaccine, LiveMenadioneMethdilazine HydrochlorideMethdilazine TabletsOxyphenbutazoneOxyphenbutazone TabletsPhenindamine TartratePhenindamine TabletsPhenylbutazonePhenylbutazone TabletsPropantheline BromidePropantheline TabletsSodium AurothiomalateSodium Aurothiomalate InjectionSodium CromoglycateSodium Cromoglycate Powder forInhalationSodium Fusidate CapsulesPrepared StoraxSulphadimethoxineSulphadimethoxine TabletsSulphadimidineSulphadimidine SodiumSulphadimidine InjectionSulphadimidine TabletsSulphafurazoleSulphafurazole TabletsSulphaleneSulphaphenazoleSulphaphenazole TabletsSulphobromophthalein SodiumSulphobromophthalein SodiumInjection。
雌孕激素与炔雌醇环丙孕酮治疗子宫出血的疗效比较

Comparing the efficacy of estrogen-progesterone and ethinylestradiol and cyproterone acetate in the treatment of uterine bleeding
YANG Jinmin Department of Obstetrics and Gynecology, Xiangyang Hospital of Traditional Chinese Medicine, Hubei, Xiangyang 441000, China [Abstract] Objective To analyze and compare the application effect of estrogen-progesterone and ethinylestradiol and cyproterone acetate in the treatment of uterine bleeding. Methods A total of 84 patients with uterine bleeding treated in our hospital from September 2019 to September 2020 were retrospectively analyzed. 42 patients treated with estrogenprogesterone were taken as the control group and the other 42 patients treated with ethinylestradiol and cyproterone acetate were taken as the observation group. The improvement of clinical indexes, efficacy and occurrence of adverse reactions (ARs) were compared between the two groups. Results The bleeding control time, complete hemostasis control time and endometrial thickness in the observation group were significantly shorter or thinner than those in the control group (P < 0.05). The hemoglobin content in the observation group was significantly higher than that in the control group (P < 0.05). The effective rate of treatment in the observation group was significantly higher than that in the control group (P < 0.05). The incidence of ARs in the observation group was significantly lower than that in the control group (P < 0.05). Conclusion The treatment of ethinylestradiol and cyproterone acetate for patients with uterine bleeding can effectively shorten the bleeding time of patients, and effectively improve the clinical indexes of patients, with high clinical safety and promotion value. [Key words] Estrogen-progesterone; Ethinylestradiol and cyproterone acetate; Uterine bleeding; Hemostasis time; Efficacy; Adverse reactions
复方环丙孕酮开发报告参考模板

醋酸环丙孕酮及复方制剂开发报告(化药6类)技术中心信息情报部中文名:醋酸环丙氯地孕酮; 环丙氯地孕酮醋酸酯英文名:Cyproterone acetate(CPA)分子式:C24H29ClO4分子量:416.94CAS号:427-51-0结构式:复方制剂:炔雌醇环丙孕酮片商品名:达英-35英文名:Ethinylestradiol and Cyproterone Acetate Tablets成分:其组份为2mg醋酸环丙孕酮(色普龙)和0.035mg乙炔基雌二醇。
复方制剂:复方醋酸环丙孕酮片英文名称:Compound Cyproterone Acetate Tablets成分:每片含醋酸环丙孕酮2mg和炔雌醇0.035mg一、基本情况醋酸环丙孕酮是合成的17-羟孕酮衍生物,是一种强效孕激素,抗雄性激素作用较突出。
其能与睾酮竞争雄激素受体,所产生的环丙孕酮-难激素受体复合物也能进入细胞核中,但不产生雄激素效应,从而可阻断雄激素作用。
其可降低5α-还原酶活性从而抑制睾酮和DHT 的作用。
因其本身为孕激素,故可抑制促性腺激素的分泌,从而减卵巢产生睾酮和雄烯二酮;其还能增强肝酶活性,从而增加睾酮的清除率。
但大剂量使用时可伴有低雌激素、不规则阴道出血和水肿、体重增加、乳房发胀、肾上腺机能不全和性欲缺乏等现象,故目前常使用的中低剂量并与雌激素联用。
德国先灵公司开发生产的达英-35®为第三代口服避孕药,非处方药品,其主要成分为2mg醋酸环丙孕酮和35μg乙炔雌二醇,是目前惟一具有治疗高雄激素血症适应症的口服避孕药。
达英-35®有独特的多环节抗雄激素作用,可显著改善多囊卵巢综合征(PCOS)患者的多毛,痤疮皮疹等症状。
若单独给予醋酸环丙孕酮,可能导致月经周期紊乱,而加入了炔雌醇的复方醋酸环丙孕酮片则可避免这种情况,复方醋酸环丙孕酮片治疗期间,醋酸环丙孕酮能抑制促性腺激素分泌,从而抑制卵巢排卵,并能阻止孕卵着床和增加宫颈黏液稠度,阻止精子的穿透,可以防止妊娠;炔雌醇亦能抑制促性腺激素分泌,从而抑制排卵,两种成分配伍,可增强避孕效果,减少不良反应;此外,该品具有较强的抗雄性激素的作用,更适用于患有痤疮及油脂性皮肤的妇女。
OC的治疗性应用

g →→ 20-35μ g →→ 15μ g
雌激素剂量的减少___
过去30多年来口服避孕药中雌激素含量的趋势
微克
150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0
150
75
50
30 20
1965
1970
1975
1980
1988
时间(年〕
Danazol / MPA
Days of cycle
Months of treatment
林玉梅等在宫颈微波术后让患者连续口
服避孕药2 个月 结果发现:应用口服避孕药可有效预防 宫颈微波治疗术后宫颈子宫内膜异位症 的发生
林玉梅,李瑞兰,陈秀法. 避孕药在预防宫颈微波术后宫颈子宫内膜异位症中的应用[J]. 中国现代应用药学,2006,4(23):340-342
内异症常用药物的作用
No treatment Oral contraceptive 类固醇 GnRH agonist
FSH
LH
FSH
LH
FSH
LH
FSH
LH
Endometrium
Menstrual debris Endometriosis Estradiol Progesterone Progestin Ethynyl estradiol
19-nortestosterone 19去甲睾酮
Trimegestone Drosperinone 屈螺酮
Norethindrone(estranes) 炔诺酮(雌烷) Norethindrone acetate(NETA) 醋酸炔诺酮 norethynodrel 异炔诺酮 Lynestrenol 去氧炔诺酮 ethynodiol diacetate 双醋炔诺酮
炔雌醇环丙孕酮片与雌孕激素联用方案治疗功能性子宫出血的临床疗效

炔雌醇环丙孕酮片与雌孕激素联用方案治疗功能性子宫出血的临床疗效吴杰【期刊名称】《中国卫生标准管理》【年(卷),期】2015(000)016【摘要】Objective To explore the clinical effect of Ethinylestradiol and Cyproterone Acetate Tablets combined with estrogen and progestogen in the treatment of dysfunctional uterine bleeding. Methods 56 patients with dysfunctional uterine bleeding from April 2012 to April 2014 in our hospital were selected into control group(28 cases)and observation group(28 cases)randomly. The patients in the control group received Ethinylestradiol and Cyproterone Acetate Tablets treatment,and estrogen and progestogen were added for observation group,the treatment effects of two groups were compared. Results The total effective rate of observation group was 96.43%,the control group was 85.71%,the difference was statistically significant,P< 0.05. Conclusion The clinical effect of Ethinylestradiol and Cyproterone Acetate Tablets combined with estrogen and progestogen in the treatment of dysfunctional uterine bleeding is remarkable.%目的:探讨炔雌醇环丙孕酮片联合雌孕激素治疗功能性子宫出血的临床效果。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Cyproterone acetate CAS No.:
427-51-0Cat. No.:
HY-13604Product Data Sheet
MWt:
402.91Formula:
C23H27ClO4Purity :>98%
Solubility:
DMSO 80 mg/mL; Water <1 mg/mL y Mechanisms:
Biological Activity:
Cyproterone acetate is an androgen receptor (AR)antagonist with IC50of 71nM as well as a weak
Pathways:Others; Target:Androgen Receptor g g
Cyproterone acetate is an androgen receptor (AR) antagonist with IC50 of 7.1 nM, as well as a weak
progesterone receptor agonist with weak pro-gestational and glucocorticoid activity.
Target: Androgen Receptor Cyproterone acetate clearly shows antagonistic properties, while being a partial agonist also,showing agonism for the AR, with EC50 of 4.0 μM, at relatively high concentrations [1]. In the presence of 10 nM Testosterone, low concentrations of Cyproterone acetate inhibits T-stimulated transcription of 3XHRE-LUC, but at higher concentrations, transcription is stimulated. LH levels in Cyproterone acetate-treated rats do not dip below pretreatment levels, although they does not increase as much in the rats treated with 3.2 mg Cyproterone acetate/kg/day as in those which References:
[1]. Sonneveld, E., et al., Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci, 2005.
83(1): p. 136-48.g yp g y
received 0.2 mg Cyproterone acetate/kg/day [2]. Cyproterone acetate exhibits direct negative eff...[2]. Attardi, B.J., S. Koduri, and S.A. Hild, Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to
suppress LH secretion in the castrate male rat. Mol Cell Endocrinol, 2010. 328(1-2): p. 16-21.[3]. Arafa, N.M., Efficacy of echinacea on the action of cyproterone acetate in male rats. Pak J Biol
Sci, 2010. 13(20): p. 966-76.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
