Darunavir_206361-99-1_CoA_MedChemExpress
高效液相色谱法测定糕点和果汁中纳他霉素的方法学验证
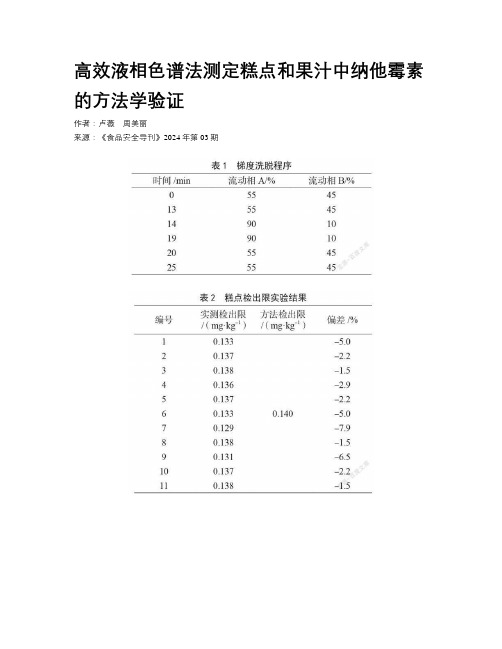
高效液相色谱法测定糕点和果汁中纳他霉素的方法学验证作者:卢薇周美丽来源:《食品安全导刊》2024年第03期摘要:为了验证宝鸡市食品药品检验检测中心是否有能力开展纳他霉素新方法《食品安全国家标准食品中纳他霉素的测定》(GB 5009.286—2022)的检测项目。
本文使用高效液相色谱仪,对糕点和果汁中纳他霉素进行分析,外标法定量,验证了本方法的线性关系、方法检出限、精密度和回收率。
结果表明,纳他霉素在0.5~10.0 μg·mL-1呈现良好的线性关系,方法检出限、精密度和回收率均能达到标准方法要求。
因此,本实验室具备检测纳他霉素的能力。
关键词:纳他霉素;外标法;高效液相色谱仪;方法学验证Methodological Validation of High Performance Liquid Chromatography for the Determination of Natamycin in Pastries and Fruit JuiceLU Wei, ZHOU Meili(Baoji Food and Drug Inspection and Testing Center, Baoji 721000, China)Abstract: In order to verify whether the Baoji Food and Drug Inspection and Testing Center has the ability to carry out the testing project of the new method GB 5009.286—2022 for natamycin. This article uses a high-performance liquid chromatography to analyze natamycin in pastries and fruit juice. The external standard method is used for quantification to verify the linear relationship, detection limit, precision, and recovery rate of this method. The results showed that natamycin exhibited a good linear relationship within the range from 0.5 μg·mL-1 to10 μg·mL-1, and the detection limit,precision, and recovery rate of the method can meet the requirements of the standard method. Therefore, our laboratory has the ability to detect natamycin.Keywords: natamycin; external standard method; high-performance liquid chromatography; methodology validation納他霉素,又名匹马霉素,是一种外观呈近白色或奶油黄色,无臭无味的结晶粉末,它是天然的多烯大环内酯类物质,对大部分的霉菌和酵母菌都具有极强的抑制作用[1]。
SCItop区期刊表

刊名简称刊名全称ATMOS CHEM PHYS ATMOSPHERIC CHEMISTRY AND PHYSICSB AM METEOROL SOC BULLETIN OF THE AMERICAN METEOROLOGICAL SOCIETY CLIM DYNAM CLIMATE DYNAMICSCONTRIB MINERAL PETR CONTRIBUTIONS TO MINERALOGY AND PETROLOGY EARTH PLANET SC LETT EARTH AND PLANETARY SCIENCE LETTERSEARTH-SCI REV EARTH-SCIENCE REVIEWSGEOCHIM COSMOCHIM AC GEOCHIMICA ET COSMOCHIMICA ACTAGEOLOGY GEOLOGYGEOTEXT GEOMEMBRANES GEOTEXTILES AND GEOMEMBRANESJ CLIMATE JOURNAL OF CLIMATEJ PETROL JOURNAL OF PETROLOGYLIMNOL OCEANOGR LIMNOLOGY AND OCEANOGRAPHYNAT GEOSCI Nature GeosciencePALEOCEANOGRAPHY PALEOCEANOGRAPHYPRECAMBRIAN RES PRECAMBRIAN RESEARCHQUATERNARY SCI REV QUATERNARY SCIENCE REVIEWSREV GEOPHYS REVIEWS OF GEOPHYSICSGEOPHYS RES LETT GEOPHYSICAL RESEARCH LETTERSJ ATMOS SCI JOURNAL OF THE ATMOSPHERIC SCIENCESJ GEOPHYS RES JOURNAL OF GEOPHYSICAL RESEARCHJ HYDROL JOURNAL OF HYDROLOGYMON WEATHER REV MONTHLY WEATHER REVIEWANNU REV ASTRON ASTR ANNUAL REVIEW OF ASTRONOMY AND ASTROPHYSICS ASTRON ASTROPHYS REV ASTRONOMY AND ASTROPHYSICS REVIEWASTROPHYS J SUPPL S ASTROPHYSICAL JOURNAL SUPPLEMENT SERIES ASTROPHYS J ASTROPHYSICAL JOURNALACM COMPUT SURV ACM COMPUTING SURVEYSACM T GRAPHIC ACM TRANSACTIONS ON GRAPHICSACS NANO ACS NanoACTA BIOMATER Acta BiomaterialiaACTA MATER ACTA MATERIALIAADV APPL MECH ADVANCES IN APPLIED MECHANICSADV BIOCHEM ENG BIOT ADVANCES IN BIOCHEMICAL ENGINEERING / BIOTECHNOL ADV FUNCT MATER ADVANCED FUNCTIONAL MATERIALSADV MATER ADVANCED MATERIALSANNU REV BIOMED ENG ANNUAL REVIEW OF BIOMEDICAL ENGINEERINGANNU REV MATER RES ANNUAL REVIEW OF MATERIALS RESEARCHAPPL SPECTROSC REV APPLIED SPECTROSCOPY REVIEWSARTIF INTELL ARTIFICIAL INTELLIGENCEBIOMATERIALS BIOMATERIALSBIORESOURCE TECHNOL BIORESOURCE TECHNOLOGYBIOSENS BIOELECTRON BIOSENSORS & BIOELECTRONICSBIOTECHNOL ADV BIOTECHNOLOGY ADVANCESBIOTECHNOL BIOENG BIOTECHNOLOGY AND BIOENGINEERINGBIOTECHNOL BIOFUELS Biotechnology for BiofuelsCARBON CARBONCHEM MATER CHEMISTRY OF MATERIALSCOMPUT INTELL COMPUTATIONAL INTELLIGENCECRIT REV BIOTECHNOL CRITICAL REVIEWS IN BIOTECHNOLOGYCRIT REV FOOD SCI CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION CURR OPIN BIOTECH CURRENT OPINION IN BIOTECHNOLOGY ELECTROCHEM COMMUN ELECTROCHEMISTRY COMMUNICATIONSHUM-COMPUT INTERACT HUMAN-COMPUTER INTERACTIONIEEE J SEL AREA COMM IEEE JOURNAL ON SELECTED AREAS IN COMMUNICATIONS IEEE SIGNAL PROC MAG IEEE SIGNAL PROCESSING MAGAZINEIEEE T EVOLUT COMPUT IEEE TRANSACTIONS ON EVOLUTIONARY COMPUTATION IEEE T IND ELECTRON IEEE TRANSACTIONS ON INDUSTRIAL ELECTRONICS IEEE T NEURAL NETWOR IEEE TRANSACTIONS ON NEURAL NETWORKSIEEE T PATTERN ANAL IEEE TRANSACTIONS ON PATTERN ANALYSIS AND MACHIN IEEE T SOFTWARE ENG IEEE TRANSACTIONS ON SOFTWARE ENGINEERINGINT J COMPUT VISION INTERNATIONAL JOURNAL OF COMPUTER VISIONINT J HYDROGEN ENERG INTERNATIONAL JOURNAL OF HYDROGEN ENERGYINT J NONLIN SCI NUM INTERNATIONAL JOURNAL OF NONLINEAR SCIENCES AND INT J PLASTICITY INTERNATIONAL JOURNAL OF PLASTICITYINT MATER REV INTERNATIONAL MATERIALS REVIEWSJ CATAL JOURNAL OF CATALYSISJ HAZARD MATER JOURNAL OF HAZARDOUS MATERIALSJ MATER CHEM JOURNAL OF MATERIALS CHEMISTRYJ MECH BEHAV BIOMED Journal of the Mechanical Behavior of Biomedical J NEURAL ENG Journal of Neural EngineeringJ POWER SOURCES JOURNAL OF POWER SOURCESJ WEB SEMANT Journal of Web SemanticsLAB CHIP LAB ON A CHIPMACROMOL RAPID COMM MACROMOLECULAR RAPID COMMUNICATIONS MACROMOLECULES MACROMOLECULESMAT SCI ENG R MATERIALS SCIENCE & ENGINEERING R-REPORTS MATER TODAY Materials TodayMED IMAGE ANAL MEDICAL IMAGE ANALYSISMETAB ENG METABOLIC ENGINEERINGMIS QUART MIS QUARTERLYMOL NUTR FOOD RES MOLECULAR NUTRITION & FOOD RESEARCHMRS BULL MRS BULLETINNANO LETT NANO LETTERSNANO TODAY Nano TodayNANOMED-NANOTECHNOL Nanomedicine-Nanotechnology Biology and Medicine NANOTECHNOLOGY NANOTECHNOLOGYNAT BIOTECHNOL NATURE BIOTECHNOLOGYNAT MATER NATURE MATERIALSNAT NANOTECHNOL Nature NanotechnologyORG ELECTRON ORGANIC ELECTRONICSP IEEE PROCEEDINGS OF THE IEEEPLASMONICS PlasmonicsPOLYM REV Polymer ReviewsPROG CRYST GROWTH CH PROGRESS IN CRYSTAL GROWTH AND CHARACTERIZATION PROG ENERG COMBUST PROGRESS IN ENERGY AND COMBUSTION SCIENCEPROG MATER SCI PROGRESS IN MATERIALS SCIENCEPROG PHOTOVOLTAICS PROGRESS IN PHOTOVOLTAICSPROG QUANT ELECTRON PROGRESS IN QUANTUM ELECTRONICSPROG SURF SCI PROGRESS IN SURFACE SCIENCERENEW SUST ENERG REV RENEWABLE & SUSTAINABLE ENERGY REVIEWSSMALL SMALLSOFT MATTER Soft MatterTRENDS BIOTECHNOL TRENDS IN BIOTECHNOLOGYTRENDS FOOD SCI TECH TRENDS IN FOOD SCIENCE & TECHNOLOGYVLDB J VLDB JOURNALAICHE J AICHE JOURNALAPPL MICROBIOL BIOT APPLIED MICROBIOLOGY AND BIOTECHNOLOGYCHEM ENG SCI CHEMICAL ENGINEERING SCIENCEELECTROCHIM ACTA ELECTROCHIMICA ACTAFOOD CHEM FOOD CHEMISTRYIEEE T ANTENN PROPAG IEEE TRANSACTIONS ON ANTENNAS AND PROPAGATION IEEE T AUTOMAT CONTR IEEE TRANSACTIONS ON AUTOMATIC CONTROLIEEE T INFORM THEORY IEEE TRANSACTIONS ON INFORMATION THEORYIEEE T MICROW THEORY IEEE TRANSACTIONS ON MICROWAVE THEORY AND TECHNI IEEE T SIGNAL PROCES IEEE TRANSACTIONS ON SIGNAL PROCESSINGIND ENG CHEM RES INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCHINT J HEAT MASS TRAN INTERNATIONAL JOURNAL OF HEAT AND MASS TRANSFER J AM CERAM SOC JOURNAL OF THE AMERICAN CERAMIC SOCIETYJ ELECTROCHEM SOC JOURNAL OF THE ELECTROCHEMICAL SOCIETYJ MEMBRANE SCI JOURNAL OF MEMBRANE SCIENCEMATER LETT MATERIALS LETTERSSCRIPTA MATER SCRIPTA MATERIALIASENSOR ACTUAT B-CHEM SENSORS AND ACTUATORS B-CHEMICALSURF COAT TECH SURFACE & COATINGS TECHNOLOGYSYNTHETIC MET SYNTHETIC METALSTHIN SOLID FILMS THIN SOLID FILMSJ OPER MANAG JOURNAL OF OPERATIONS MANAGEMENTMANAGE SCI MANAGEMENT SCIENCEOMEGA-INT J MANAGE S OMEGA-INTERNATIONAL JOURNAL OF MANAGEMENT SCIENC PROD OPER MANAG PRODUCTION AND OPERATIONS MANAGEMENTEUR J OPER RES EUROPEAN JOURNAL OF OPERATIONAL RESEARCH ACCOUNTS CHEM RES ACCOUNTS OF CHEMICAL RESEARCHACTA CRYSTALLOGR A ACTA CRYSTALLOGRAPHICA SECTION AALDRICHIM ACTA ALDRICHIMICA ACTAANGEW CHEM INT EDIT ANGEWANDTE CHEMIE-INTERNATIONAL EDITIONANNU REV PHYS CHEM ANNUAL REVIEW OF PHYSICAL CHEMISTRYCATAL REV CATALYSIS REVIEWS-SCIENCE AND ENGINEERINGCHEM REV CHEMICAL REVIEWSCHEM SOC REV CHEMICAL SOCIETY REVIEWSCOORDIN CHEM REV COORDINATION CHEMISTRY REVIEWSENERG ENVIRON SCI Energy & Environmental ScienceINT REV PHYS CHEM INTERNATIONAL REVIEWS IN PHYSICAL CHEMISTRYJ AM CHEM SOC JOURNAL OF THE AMERICAN CHEMICAL SOCIETYJ PHOTOCH PHOTOBIO C JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY C-PHO NAT PROD REP NATURAL PRODUCT REPORTSPROG POLYM SCI PROGRESS IN POLYMER SCIENCESURF SCI REP SURFACE SCIENCE REPORTSTRAC-TREND ANAL CHEM TRAC-TRENDS IN ANALYTICAL CHEMISTRYANAL CHEM ANALYTICAL CHEMISTRYJ ORG CHEM JOURNAL OF ORGANIC CHEMISTRYJ PHYS CHEM B JOURNAL OF PHYSICAL CHEMISTRY BLANGMUIR LANGMUIRAPPL CATAL B-ENVIRON APPLIED CATALYSIS B-ENVIRONMENTALB AM MUS NAT HIST BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTO CRIT REV ENV SCI TEC CRITICAL REVIEWS IN ENVIRONMENTAL SCIENCE AND TE ECOL LETT ECOLOGY LETTERSECOL MONOGR ECOLOGICAL MONOGRAPHSECOLOGY ECOLOGYENVIRON HEALTH PERSP ENVIRONMENTAL HEALTH PERSPECTIVESENVIRON MICROBIOL ENVIRONMENTAL MICROBIOLOGYEVOLUTION EVOLUTIONFRONT ECOL ENVIRON FRONTIERS IN ECOLOGY AND THE ENVIRONMENT GLOBAL CHANGE BIOL GLOBAL CHANGE BIOLOGYGLOBAL ECOL BIOGEOGR GLOBAL ECOLOGY AND BIOGEOGRAPHYISME J ISME JournalATMOS ENVIRON ATMOSPHERIC ENVIRONMENTCHEMOSPHERE CHEMOSPHEREENVIRON SCI TECHNOL ENVIRONMENTAL SCIENCE & TECHNOLOGYWATER RES WATER RESEARCHADV AGRON ADVANCES IN AGRONOMYAGR FOREST METEOROL AGRICULTURAL AND FOREST METEOROLOGYATLA-ALTERN LAB ANIM ATLA-ALTERNATIVES TO LABORATORY ANIMALSEUR J SOIL SCI EUROPEAN JOURNAL OF SOIL SCIENCEFISH FISH FISH AND FISHERIESFISH OCEANOGR FISHERIES OCEANOGRAPHYFISH SHELLFISH IMMUN FISH & SHELLFISH IMMUNOLOGYFOOD BIOPROCESS TECH Food and Bioprocess TechnologyGEODERMA GEODERMAILAR J ILAR JOURNALJ AGR FOOD CHEM JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRYJ ANIM SCI JOURNAL OF ANIMAL SCIENCEJ DAIRY SCI JOURNAL OF DAIRY SCIENCEMOL BREEDING MOLECULAR BREEDINGREV FISH BIOL FISHER REVIEWS IN FISH BIOLOGY AND FISHERIESSOIL BIOL BIOCHEM SOIL BIOLOGY & BIOCHEMISTRYSOIL SCI SOC AM J SOIL SCIENCE SOCIETY OF AMERICA JOURNALTREE PHYSIOL TREE PHYSIOLOGYVET MICROBIOL VETERINARY MICROBIOLOGYVET RES VETERINARY RESEARCHAQUACULTURE AQUACULTURECAN J FISH AQUAT SCI CANADIAN JOURNAL OF FISHERIES AND AQUATIC SCIENC FOREST ECOL MANAG FOREST ECOLOGY AND MANAGEMENTJAVMA-J AM VET MED A JAVMA-JOURNAL OF THE AMERICAN VETERINARY MEDICAL PLANT SOIL PLANT AND SOILAM J BIOETHICS AMERICAN JOURNAL OF BIOETHICSECONOMETRICA ECONOMETRICAJ ECONOMETRICS JOURNAL OF ECONOMETRICSAM J HUM GENET AMERICAN JOURNAL OF HUMAN GENETICSANNU REV BIOCHEM ANNUAL REVIEW OF BIOCHEMISTRYANNU REV BIOPH BIOM ANNUAL REVIEW OF BIOPHYSICS AND BIOMOLECULAR STR ANNU REV BIOPHYS Annual Review of BiophysicsANNU REV CELL DEV BI ANNUAL REVIEW OF CELL AND DEVELOPMENTAL BIOLOGY ANNU REV ECOL EVOL S ANNUAL REVIEW OF ECOLOGY EVOLUTION AND SYSTEMATI ANNU REV ENTOMOL ANNUAL REVIEW OF ENTOMOLOGYANNU REV GENET ANNUAL REVIEW OF GENETICSANNU REV GENOM HUM G ANNUAL REVIEW OF GENOMICS AND HUMAN GENETICS ANNU REV MICROBIOL ANNUAL REVIEW OF MICROBIOLOGYANNU REV PHYTOPATHOL ANNUAL REVIEW OF PHYTOPATHOLOGYANNU REV PLANT BIOL ANNUAL REVIEW OF PLANT BIOLOGYCELL CELLCELL HOST MICROBE Cell Host & MicrobeCELL METAB Cell MetabolismCELL STEM CELL Cell Stem CellCRIT REV BIOCHEM MOL CRITICAL REVIEWS IN BIOCHEMISTRY AND MOLECULAR B CURR BIOL CURRENT BIOLOGYCURR OPIN CELL BIOL CURRENT OPINION IN CELL BIOLOGYCURR OPIN GENET DEV CURRENT OPINION IN GENETICS & DEVELOPMENTCURR OPIN PLANT BIOL CURRENT OPINION IN PLANT BIOLOGYCURR OPIN STRUC BIOL CURRENT OPINION IN STRUCTURAL BIOLOGYDEV CELL DEVELOPMENTAL CELLGENE DEV GENES & DEVELOPMENTGENOME RES GENOME RESEARCHJ CELL BIOL JOURNAL OF CELL BIOLOGYMICROBIOL MOL BIOL R MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWSMOL CELL MOLECULAR CELLMOL SYST BIOL Molecular Systems BiologyNAT CELL BIOL NATURE CELL BIOLOGYNAT CHEM BIOL Nature Chemical BiologyNAT GENET NATURE GENETICSNAT METHODS NATURE METHODSNAT REV GENET NATURE REVIEWS GENETICSNAT REV MICROBIOL NATURE REVIEWS MICROBIOLOGYNAT REV MOL CELL BIO NATURE REVIEWS MOLECULAR CELL BIOLOGYNAT STRUCT MOL BIOL NATURE STRUCTURAL & MOLECULAR BIOLOGYPLANT CELL PLANT CELLPLOS BIOL PLOS BIOLOGYPLOS GENET PLoS GeneticsPLOS PATHOG PLoS PathogensPROG LIPID RES PROGRESS IN LIPID RESEARCHQ REV BIOPHYS QUARTERLY REVIEWS OF BIOPHYSICSTRENDS BIOCHEM SCI TRENDS IN BIOCHEMICAL SCIENCESTRENDS CELL BIOL TRENDS IN CELL BIOLOGYTRENDS ECOL EVOL TRENDS IN ECOLOGY & EVOLUTIONTRENDS GENET TRENDS IN GENETICSTRENDS PLANT SCI TRENDS IN PLANT SCIENCEAPPL ENVIRON MICROB APPLIED AND ENVIRONMENTAL MICROBIOLOGY BIOCHEM J BIOCHEMICAL JOURNALBIOPHYS J BIOPHYSICAL JOURNALDEVELOPMENT DEVELOPMENTEMBO J EMBO JOURNALJ BACTERIOL JOURNAL OF BACTERIOLOGYJ BIOL CHEM JOURNAL OF BIOLOGICAL CHEMISTRYJ MOL BIOL JOURNAL OF MOLECULAR BIOLOGYMOL CELL BIOL MOLECULAR AND CELLULAR BIOLOGYNUCLEIC ACIDS RES NUCLEIC ACIDS RESEARCHPLANT PHYSIOL PLANT PHYSIOLOGYACTA MATH-DJURSHOLM ACTA MATHEMATICAANN MATH ANNALS OF MATHEMATICSANN STAT ANNALS OF STATISTICSB AM MATH SOC BULLETIN OF THE AMERICAN MATHEMATICAL SOCIETY COMMUN PUR APPL MATH COMMUNICATIONS ON PURE AND APPLIED MATHEMATICS FOUND COMPUT MATH FOUNDATIONS OF COMPUTATIONAL MATHEMATICS INVENT MATH INVENTIONES MATHEMATICAEINVERSE PROBL INVERSE PROBLEMSJ AM MATH SOC JOURNAL OF THE AMERICAN MATHEMATICAL SOCIETYJ AM STAT ASSOC JOURNAL OF THE AMERICAN STATISTICAL ASSOCIATION J R STAT SOC B JOURNAL OF THE ROYAL STATISTICAL SOCIETY SERIES MATH MOD METH APPL S MATHEMATICAL MODELS & METHODS IN APPLIED SCIENCE MATH PROGRAM MATHEMATICAL PROGRAMMINGMEM AM MATH SOC MEMOIRS OF THE AMERICAN MATHEMATICAL SOCIETY MULTISCALE MODEL SIM MULTISCALE MODELING & SIMULATIONMULTIVAR BEHAV RES MULTIVARIATE BEHAVIORAL RESEARCHNONLINEAR ANAL-REAL NONLINEAR ANALYSIS-REAL WORLD APPLICATIONSRISK ANAL RISK ANALYSISSIAM REV SIAM REVIEWSTAT SCI STATISTICAL SCIENCESTRUCT EQU MODELING STRUCTURAL EQUATION MODELING-A MULTIDISCIPLINARY FUZZY SET SYST FUZZY SETS AND SYSTEMSJ COMPUT APPL MATH JOURNAL OF COMPUTATIONAL AND APPLIED MATHEMATICS J DIFFER EQUATIONS JOURNAL OF DIFFERENTIAL EQUATIONSJ MATH ANAL APPL JOURNAL OF MATHEMATICAL ANALYSIS AND APPLICATIONNONLINEAR ANAL-THEOR NONLINEAR ANALYSIS-THEORY METHODS & APPLICATIONS SIAM J NUMER ANAL SIAM JOURNAL ON NUMERICAL ANALYSISSIAM J SCI COMPUT SIAM JOURNAL ON SCIENTIFIC COMPUTINGACTA CRYSTALLOGR A ACTA CRYSTALLOGRAPHICA SECTION AADV PHYS ADVANCES IN PHYSICSANNU REV FLUID MECH ANNUAL REVIEW OF FLUID MECHANICSANNU REV NUCL PART S ANNUAL REVIEW OF NUCLEAR AND PARTICLE SCIENCE CRIT REV SOLID STATE CRITICAL REVIEWS IN SOLID STATE AND MATERIALS SC J HIGH ENERGY PHYS JOURNAL OF HIGH ENERGY PHYSICSLASER PHOTONICS REV Laser & Photonics ReviewsLIVING REV RELATIV Living Reviews in RelativityMASS SPECTROM REV MASS SPECTROMETRY REVIEWSNAT PHOTONICS Nature PhotonicsNAT PHYS Nature PhysicsPHYS REP PHYSICS REPORTS-REVIEW SECTION OF PHYSICS LETTER PHYS REV LETT PHYSICAL REVIEW LETTERSPROG NUCL MAG RES SP PROGRESS IN NUCLEAR MAGNETIC RESONANCE SPECTROSC REP PROG PHYS REPORTS ON PROGRESS IN PHYSICSREV MOD PHYS REVIEWS OF MODERN PHYSICSAPPL PHYS LETT APPLIED PHYSICS LETTERSJ CHEM PHYS JOURNAL OF CHEMICAL PHYSICSPHYS REV B PHYSICAL REVIEW BPHYS REV D PHYSICAL REVIEW DADV DRUG DELIVER REV ADVANCED DRUG DELIVERY REVIEWSADV IMMUNOL ADVANCES IN IMMUNOLOGYAM J CLIN NUTR AMERICAN JOURNAL OF CLINICAL NUTRITIONAM J GASTROENTEROL AMERICAN JOURNAL OF GASTROENTEROLOGYAM J PSYCHIAT AMERICAN JOURNAL OF PSYCHIATRYAM J RESP CRIT CARE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CAR AM J TRANSPLANT AMERICAN JOURNAL OF TRANSPLANTATIONANN INTERN MED ANNALS OF INTERNAL MEDICINEANN NEUROL ANNALS OF NEUROLOGYANN RHEUM DIS ANNALS OF THE RHEUMATIC DISEASESANN SURG ANNALS OF SURGERYANNU REV IMMUNOL ANNUAL REVIEW OF IMMUNOLOGYANNU REV MED ANNUAL REVIEW OF MEDICINEANNU REV NEUROSCI ANNUAL REVIEW OF NEUROSCIENCEANNU REV NUTR ANNUAL REVIEW OF NUTRITIONANNU REV PATHOL-MECH Annual Review of Pathology-Mechanisms of Disease ANNU REV PHARMACOL ANNUAL REVIEW OF PHARMACOLOGY AND TOXICOLOGY ANNU REV PHYSIOL ANNUAL REVIEW OF PHYSIOLOGYANNU REV PSYCHOL ANNUAL REVIEW OF PSYCHOLOGYANNU REV PUBL HEALTH ANNUAL REVIEW OF PUBLIC HEALTHARCH GEN PSYCHIAT ARCHIVES OF GENERAL PSYCHIATRYARCH INTERN MED ARCHIVES OF INTERNAL MEDICINEARTERIOSCL THROM VAS ARTERIOSCLEROSIS THROMBOSIS AND VASCULAR BIOLOGY ARTHRITIS RHEUM-US ARTHRITIS AND RHEUMATISMBBA-REV CANCER BIOCHIMICA ET BIOPHYSICA ACTA-REVIEWS ON CANCER BEHAV BRAIN SCI BEHAVIORAL AND BRAIN SCIENCESBIOL PSYCHIAT BIOLOGICAL PSYCHIATRYBLOOD BLOODBLOOD REV BLOOD REVIEWSBRAIN BRAINBRAIN RES REV BRAIN RESEARCH REVIEWSBRIT MED J BRITISH MEDICAL JOURNALCA-CANCER J CLIN CA-A CANCER JOURNAL FOR CLINICIANSCAN MED ASSOC J CANADIAN MEDICAL ASSOCIATION JOURNALCANCER CELL CANCER CELLCANCER METAST REV CANCER AND METASTASIS REVIEWSCANCER RES CANCER RESEARCHCEREB CORTEX CEREBRAL CORTEXCIRC RES CIRCULATION RESEARCHCIRCULATION CIRCULATIONCLIN CANCER RES CLINICAL CANCER RESEARCHCLIN INFECT DIS CLINICAL INFECTIOUS DISEASESCLIN MICROBIOL REV CLINICAL MICROBIOLOGY REVIEWSCLIN PHARMACOL THER CLINICAL PHARMACOLOGY & THERAPEUTICSCRIT CARE MED CRITICAL CARE MEDICINECURR OPIN IMMUNOL CURRENT OPINION IN IMMUNOLOGYCURR OPIN LIPIDOL CURRENT OPINION IN LIPIDOLOGYCURR OPIN NEUROBIOL CURRENT OPINION IN NEUROBIOLOGYCURR OPIN PHARMACOL CURRENT OPINION IN PHARMACOLOGYDIABETES DIABETESDIABETES CARE DIABETES CAREDIABETOLOGIA DIABETOLOGIADRUG DISCOV TODAY DRUG DISCOVERY TODAYDRUG RESIST UPDATE DRUG RESISTANCE UPDATESEMERG INFECT DIS EMERGING INFECTIOUS DISEASESENDOCR REV ENDOCRINE REVIEWSEPIDEMIOL REV EPIDEMIOLOGIC REVIEWSEUR HEART J EUROPEAN HEART JOURNALEUR UROL EUROPEAN UROLOGYFRONT NEUROENDOCRIN FRONTIERS IN NEUROENDOCRINOLOGY GASTROENTEROLOGY GASTROENTEROLOGYGASTROINTEST ENDOSC GASTROINTESTINAL ENDOSCOPYGUT GUTHEPATOLOGY HEPATOLOGYHUM MUTAT HUMAN MUTATIONHUM REPROD UPDATE HUMAN REPRODUCTION UPDATEHYPERTENSION HYPERTENSIONIMMUNITY IMMUNITYIMMUNOL REV IMMUNOLOGICAL REVIEWSJ ALLERGY CLIN IMMUN JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGYJ AM COLL CARDIOL JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGYJ AM SOC NEPHROL JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGYJ AUTOIMMUN JOURNAL OF AUTOIMMUNITYJ BONE MINER RES JOURNAL OF BONE AND MINERAL RESEARCHJ CLIN INVEST JOURNAL OF CLINICAL INVESTIGATIONJ CLIN ONCOL JOURNAL OF CLINICAL ONCOLOGYJ EXP MED JOURNAL OF EXPERIMENTAL MEDICINEJ HEPATOL JOURNAL OF HEPATOLOGYJ NATL CANCER I JOURNAL OF THE NATIONAL CANCER INSTITUTEJ NEUROSCI JOURNAL OF NEUROSCIENCEJ NUCL MED JOURNAL OF NUCLEAR MEDICINEJAMA-J AM MED ASSOC JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION LANCET LANCETLANCET INFECT DIS LANCET INFECTIOUS DISEASESLANCET NEUROL LANCET NEUROLOGYLANCET ONCOL LANCET ONCOLOGYLEUKEMIA LEUKEMIAMED RES REV MEDICINAL RESEARCH REVIEWSMOL ASPECTS MED MOLECULAR ASPECTS OF MEDICINEMOL PSYCHIATR MOLECULAR PSYCHIATRYNAT CLIN PRACT ONCOL Nature Clinical Practice OncologyNAT IMMUNOL NATURE IMMUNOLOGYNAT MED NATURE MEDICINENAT NEUROSCI NATURE NEUROSCIENCENAT REV CANCER NATURE REVIEWS CANCERNAT REV DRUG DISCOV NATURE REVIEWS DRUG DISCOVERYNAT REV IMMUNOL NATURE REVIEWS IMMUNOLOGYNAT REV NEUROSCI NATURE REVIEWS NEUROSCIENCENEUROLOGY NEUROLOGYNEURON NEURONNEUROPSYCHOPHARMACOL NEUROPSYCHOPHARMACOLOGYNEUROSCI BIOBEHAV R NEUROSCIENCE AND BIOBEHAVIORAL REVIEWSNEW ENGL J MED NEW ENGLAND JOURNAL OF MEDICINEOBES REV Obesity ReviewsONCOGENE ONCOGENEPHARMACOL REV PHARMACOLOGICAL REVIEWSPHARMACOL THERAPEUT PHARMACOLOGY & THERAPEUTICSPHYSIOL REV PHYSIOLOGICAL REVIEWSPHYSIOLOGY PHYSIOLOGYPLOS MED PLOS MEDICINEPROG NEUROBIOL PROGRESS IN NEUROBIOLOGYPROG RETIN EYE RES PROGRESS IN RETINAL AND EYE RESEARCHPSYCHOL BULL PSYCHOLOGICAL BULLETINPSYCHOL REV PSYCHOLOGICAL REVIEWREV MED VIROL REVIEWS IN MEDICAL VIROLOGYSCHIZOPHRENIA BULL SCHIZOPHRENIA BULLETINSEMIN CANCER BIOL SEMINARS IN CANCER BIOLOGYSEMIN IMMUNOL SEMINARS IN IMMUNOLOGYSTEM CELLS STEM CELLSSTROKE STROKETHORAX THORAXTRENDS COGN SCI TRENDS IN COGNITIVE SCIENCESTRENDS ENDOCRIN MET TRENDS IN ENDOCRINOLOGY AND METABOLISMTRENDS IMMUNOL TRENDS IN IMMUNOLOGYTRENDS MOL MED TRENDS IN MOLECULAR MEDICINETRENDS NEUROSCI TRENDS IN NEUROSCIENCESTRENDS PHARMACOL SCI TRENDS IN PHARMACOLOGICAL SCIENCESWHO TECH REP SER WHO TECHNICAL REPORT SERIESAM J CARDIOL AMERICAN JOURNAL OF CARDIOLOGYAM J EPIDEMIOL AMERICAN JOURNAL OF EPIDEMIOLOGYAM J PATHOL AMERICAN JOURNAL OF PATHOLOGYAM J PHYSIOL-HEART C AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULA ANTIMICROB AGENTS CH ANTIMICROBIAL AGENTS AND CHEMOTHERAPYBRIT J CANCER BRITISH JOURNAL OF CANCERCANCER-AM CANCER SOC CANCERCHEST CHESTCNS NEUROL DISORD-DR CNS & Neurological Disorders-Drug Targets ENDOCRINOLOGY ENDOCRINOLOGYEUR J NEUROSCI EUROPEAN JOURNAL OF NEUROSCIENCEFREE RADICAL BIO MED FREE RADICAL BIOLOGY AND MEDICINEINFECT IMMUN INFECTION AND IMMUNITYINT J CANCER INTERNATIONAL JOURNAL OF CANCERINT J RADIAT ONCOL INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY BIOL INVEST OPHTH VIS SCI INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCEJ APPL PHYSIOL JOURNAL OF APPLIED PHYSIOLOGYJ CLIN ENDOCR METAB JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM J CLIN MICROBIOL JOURNAL OF CLINICAL MICROBIOLOGYJ COMP NEUROL JOURNAL OF COMPARATIVE NEUROLOGYJ IMMUNOL JOURNAL OF IMMUNOLOGYJ INFECT DIS JOURNAL OF INFECTIOUS DISEASESJ MED CHEM JOURNAL OF MEDICINAL CHEMISTRYJ NEUROCHEM JOURNAL OF NEUROCHEMISTRYJ NEUROPHYSIOL JOURNAL OF NEUROPHYSIOLOGYJ NUTR JOURNAL OF NUTRITIONJ PHARMACOL EXP THER JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPE J PHYSIOL-LONDON JOURNAL OF PHYSIOLOGY-LONDONJ UROLOGY JOURNAL OF UROLOGYJ VIROL JOURNAL OF VIROLOGYKIDNEY INT KIDNEY INTERNATIONALNEUROIMAGE NEUROIMAGENEUROSCIENCE NEUROSCIENCEPEDIATRICS PEDIATRICSRADIOLOGY RADIOLOGYTRANSPLANTATION TRANSPLANTATIONVIROLOGY VIROLOGYNATURE NATUREP NATL ACAD SCI USA PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES SCIENCE SCIENCEANN NY ACAD SCI ANNALS OF THE NEW YORK ACADEMY OF SCIENCESISSN大类名称复分大类分区是否top期刊2009年影响因子1680-7316地学1Y 4.881 0003-0007地学1Y 6.123 0930-7575地学1Y 3.917 0010-7999地学1Y 3.497 0012-821X地学1Y 4.062 0012-8252地学1Y 6.942 0016-7037地学1Y 4.385 0091-7613地学1Y 4.368 0266-1144地学1Y 4.039 0894-8755地学1Y 3.363 0022-3530地学1Y 3.738 0024-3590地学1Y 3.545 1752-0894地学1Y8.108 0883-8305地学1Y 3.644 0301-9268地学1Y 3.574 0277-3791地学1Y 4.245 8755-1209地学1Y8.021 0094-8276地学2Y 3.204 0022-4928地学2Y 2.911 0148-0227地学2Y 3.082 0022-1694地学2Y 2.433 0027-0644地学2Y 2.238 0066-4146地学天文1Y25.640 0935-4956地学天文1Y11.857 0067-0049地学天文1Y12.771 0004-637X地学天文2Y7.364 0360-0300工程技术1Y7.667 0730-0301工程技术1Y 3.619 1936-0851工程技术1Y7.493 1742-7061工程技术1Y 3.975 1359-6454工程技术1Y 3.760 0065-2156工程技术1Y 5.500 0724-6145工程技术1Y 4.165 1616-301X工程技术1Y 6.990 0935-9648工程技术1Y8.379 1523-9829工程技术1Y11.235 1531-7331工程技术1Y7.911 0570-4928工程技术1Y 3.243 0004-3702工程技术1Y 3.036 0142-9612工程技术1Y7.365 0960-8524工程技术1Y 4.253 0956-5663工程技术1Y 5.429 0734-9750工程技术1Y8.250 0006-3592工程技术1Y 3.377 1754-6834工程技术1Y 4.118 0008-6223工程技术1Y 4.5040897-4756工程技术1Y 5.368 0824-7935工程技术1Y 5.378 0738-8551工程技术1Y 3.567 1040-8398工程技术1Y 3.725 0958-1669工程技术1Y7.820 1388-2481工程技术1Y 4.243 0737-0024工程技术1Y 6.190 0733-8716工程技术1Y 3.758 1053-5888工程技术1Y 4.914 1089-778X工程技术1Y 4.589 0278-0046工程技术1Y 4.678 1045-9227工程技术1Y 2.889 0162-8828工程技术1Y 4.378 0098-5589工程技术1Y 3.750 0920-5691工程技术1Y 3.508 0360-3199工程技术1Y 3.945 1565-1339工程技术1Y 5.276 0749-6419工程技术1Y 4.791 0950-6608工程技术1Y 4.857 0021-9517工程技术1Y 5.288 0304-3894工程技术1Y 4.144 0959-9428工程技术1Y 4.795 1751-6161工程技术1Y 3.176 1741-2560工程技术1Y 3.739 0378-7753工程技术1Y 3.792 1570-8268工程技术1Y 3.412 1473-0197工程技术1Y 6.306 1022-1336工程技术1Y 4.263 0024-9297工程技术1Y 4.539 0927-796X工程技术1Y12.217 1369-7021工程技术1Y11.452 1361-8415工程技术1Y 3.093 1096-7176工程技术1Y 4.725 0276-7783工程技术1Y 4.485 1613-4125工程技术1Y 4.356 0883-7694工程技术1Y 6.330 1530-6984工程技术1Y9.991 1748-0132工程技术1Y13.237 1549-9634工程技术1Y 5.440 0957-4484工程技术1Y 3.137 1087-0156工程技术1Y29.495 1476-1122工程技术1Y29.504 1748-3387工程技术1Y26.309 1566-1199工程技术1Y 3.262 0018-9219工程技术1Y 4.878 1557-1955工程技术1Y 3.723 1558-3724工程技术1Y 5.6120960-8974工程技术1Y9.250 0360-1285工程技术1Y11.024 0079-6425工程技术1Y15.769 1062-7995工程技术1Y 4.702 0079-6727工程技术1Y 4.091 0079-6816工程技术1Y7.913 1364-0321工程技术1Y 4.842 1613-6810工程技术1Y 6.171 1744-683X工程技术1Y 4.869 0167-7799工程技术1Y 6.909 0924-2244工程技术1Y 4.051 1066-8888工程技术1Y 4.517 0001-1541工程技术2Y 1.955 0175-7598工程技术2Y 2.896 0009-2509工程技术2Y 2.136 0013-4686工程技术2Y 3.325 0308-8146工程技术2Y 3.146 0018-926X工程技术2Y 2.011 0018-9286工程技术2Y 2.556 0018-9448工程技术2Y 2.357 0018-9480工程技术2Y 2.076 1053-587X工程技术2Y 2.212 0888-5885工程技术2Y 1.758 0017-9310工程技术2Y 1.947 0002-7820工程技术2Y 1.944 0013-4651工程技术2Y 2.241 0376-7388工程技术2Y 3.203 0167-577X工程技术2Y 1.940 1359-6462工程技术2Y 2.949 0925-4005工程技术2Y 3.083 0257-8972工程技术2Y 1.793 0379-6779工程技术2Y 1.901 0040-6090工程技术2Y 1.727 0272-6963管理科学1Y 3.238 0025-1909管理科学1Y 2.227 0305-0483管理科学1Y 3.101 1059-1478管理科学1Y 2.080 0377-2217管理科学2Y 2.093 0001-4842化学1Y18.203 0108-7673化学化学-晶体1Y49.926 0002-5100化学1Y18.688 1433-7851化学1Y11.829 0066-426X化学1Y17.464 0161-4940化学1Y7.765 0009-2665化学1Y35.957 0306-0012化学1Y20.086 0010-8545化学1Y11.2251754-5692化学1Y8.500 0144-235X化学1Y 5.000 0002-7863化学1Y8.580 1389-5567化学1Y7.952 0265-0568化学1Y9.202 0079-6700化学1Y23.753 0167-5729化学1Y13.462 0165-9936化学1Y 6.546 0003-2700化学2Y 5.214 0022-3263化学2Y 4.219 1520-6106化学2Y 3.471 0743-7463化学2Y 3.898 0926-3373环境科学1Y 5.252 0003-0090环境科学1Y 4.133 1064-3389环境科学1Y7.091 1461-023X环境科学1Y10.318 0012-9615环境科学1Y 4.862 0012-9658环境科学1Y 4.411 0091-6765环境科学1Y 6.191 1462-2912环境科学1Y 4.909 0014-3820环境科学1Y 5.429 1540-9295环境科学1Y 6.922 1354-1013环境科学1Y 5.561 1466-822X环境科学1Y 5.913 1751-7362环境科学1Y 6.397 1352-2310环境科学2Y 3.139 0045-6535环境科学2Y 3.253 0013-936X环境科学2Y 4.630 0043-1354环境科学2Y 4.355 0065-2113农林科学1Y 3.800 0168-1923农林科学1Y 3.197 0261-1929农林科学1Y 1.580 1351-0754农林科学1Y 2.131 1467-2960农林科学1Y 4.489 1054-6006农林科学1Y 2.427 1050-4648农林科学1Y 2.892 1935-5130农林科学1Y 2.238 0016-7061农林科学1Y 2.461 1084-2020农林科学1Y 2.806 0021-8561农林科学1Y 2.469 0021-8812农林科学1Y 2.466 0022-0302农林科学1Y 2.463 1380-3743农林科学1Y 2.272 0960-3166农林科学1Y 2.161 0038-0717农林科学1Y 2.978 0361-5995农林科学1Y 2.179 0829-318X农林科学1Y 2.2920378-1135农林科学1Y 2.874 0928-4249农林科学1Y 3.579 0044-8486农林科学2Y 1.925 0706-652X农林科学2Y 1.951 0378-1127农林科学2Y 1.950 0003-1488农林科学2Y 1.714 0032-079X农林科学2Y 2.517 1526-5161社会科学1Y 4.000 0012-9682社会科学1Y 4.000 0304-4076社会科学2Y 1.902 0002-9297生物1Y12.303 0066-4154生物1Y29.875 1056-8700生物1Y18.955 1936-122X生物1Y19.304 1081-0706生物1Y19.571 1543-592X生物1Y8.190 0066-4170生物1Y11.271 0066-4197生物1Y13.235 1527-8204生物1Y11.568 0066-4227生物1Y12.804 0066-4286生物1Y11.212 1543-5008生物1Y23.460 0092-8674生物1Y31.152 1931-3128生物1Y13.021 1550-4131生物1Y17.350 1934-5909生物1Y23.563 1040-9238生物1Y10.216 0960-9822生物1Y10.992 0955-0674生物1Y14.153 0959-437X生物1Y8.987 1369-5266生物1Y10.333 0959-440X生物1Y9.344 1534-5807生物1Y13.363 0890-9369生物1Y12.075 1088-9051生物1Y11.342 0021-9525生物1Y9.575 1092-2172生物1Y12.585 1097-2765生物1Y14.608 1744-4292生物1Y12.125 1465-7392生物1Y19.527 1552-4450生物1Y16.058 1061-4036生物1Y34.284 1548-7091生物1Y16.874 1471-0056生物1Y27.822 1740-1526生物1Y17.644 1471-0072生物1Y42.198 1545-9985生物1Y12.2731040-4651生物1Y9.293 1544-9173生物1Y12.916 1553-7390生物1Y9.532 1553-7366生物1Y8.978 0163-7827生物1Y8.167 0033-5835生物1Y10.200 0968-0004生物1Y11.572 0962-8924生物1Y12.115 0169-5347生物1Y11.564 0168-9525生物1Y8.689 1360-1385生物1Y9.883 0099-2240生物2Y 3.686 0264-6021生物2Y 5.155 0006-3495生物2Y 4.390 0950-1991生物2Y7.194 0261-4189生物2Y8.993 0021-9193生物2Y 3.940 0021-9258生物2Y 5.328 0022-2836生物2Y 3.871 0270-7306生物2Y 6.057 0305-1048生物2Y7.479 0032-0889生物2Y 6.235 0001-5962数学1Y 2.619 0003-486X数学1Y 4.174 0090-5364数学1Y 3.185 0273-0979数学1Y 3.294 0010-3640数学1Y 2.657 1615-3375数学1Y 1.905 0020-9910数学1Y 2.794 0266-5611数学1Y 1.900 0894-0347数学1Y 3.411 0162-1459数学1Y 2.322 1369-7412数学1Y 3.473 0218-2025数学1Y 2.095 0025-5610数学1Y 2.048 0065-9266数学1Y 2.240 1540-3459数学1Y 2.198 0027-3171数学1Y 2.328 1468-1218数学1Y 2.381 0272-4332数学1Y 1.953 0036-1445数学1Y 3.391 0883-4237数学1Y 3.523 1070-5511数学1Y 3.153 0165-0114数学2Y 2.138 0377-0427数学2Y 1.292 0022-0396数学2Y 1.426 0022-247X数学2Y 1.2250362-546X数学2Y 1.487 0036-1429数学2Y 1.840 1064-8275数学2Y 1.595 0108-7673物理物理-晶体1Y49.926 0001-8732物理1Y19.632 0066-4189物理1Y9.353 0163-8998物理1Y11.964 1040-8436物理1Y 5.167 1126-6708物理1Y 6.019 1863-8880物理1Y 5.814 1433-8351物理1Y10.600 0277-7037物理1Y10.623 1749-4885物理1Y22.869 1745-2473物理1Y15.491 0370-1573物理1Y17.752 0031-9007物理1Y7.328 0079-6565物理1Y 6.742 0034-4885物理1Y11.444 0034-6861物理1Y33.145 0003-6951物理2Y 3.554 0021-9606物理2Y 3.093 1098-0121物理2Y 3.475 1550-7998物理2Y 4.922 0169-409X医学1Y11.957 0065-2776医学1Y7.725 0002-9165医学1Y 6.307 0002-9270医学1Y 6.012 0002-953X医学1Y12.522 1073-449X医学1Y10.689 1600-6135医学1Y 6.433 0003-4819医学1Y16.225 0364-5134医学1Y9.317 0003-4967医学1Y8.111 0003-4932医学1Y7.900 0732-0582医学1Y37.902 0066-4219医学1Y9.940 0147-006X医学1Y24.822 0199-9885医学1Y8.783 1553-4006医学1Y13.500 0362-1642医学1Y22.468 0066-4278医学1Y18.170 0066-4308医学1Y22.750 0163-7525医学1Y7.915 0003-990X医学1Y12.257 0003-9926医学1Y9.813 1079-5642医学1Y7.235 0004-3591医学1Y7.332。
Diva Decloaker 10X Pretreatment Reagent 说明书

Intended Use:For In Vitro Diagnostic UseHeat induced antigen retrieval of formalin-fixed paraffin-embedded (FFPE) tissues for immunohistochemistry (IHC) procedures. The clinical interpretation of any staining or its absence should be complimented by morphological studies using proper controls and should be evaluated within the context of the patient's clinical history and other diagnostic tests by a qualified pathologist.Summary & Explanation:Diva Decloaker is a heat retrieval solution that is compatible with virtually all antibodies and eliminates the need for multiple buffers including citrate buffer, EDTA or high pH tris buffers. Antibody titers are doubled and tripled when compared to citrate buffer, pH 6.0. Diva Decloaker incorporates Assure™ tech nology, a color-coded high temperatures pH indicator solution. The end-user is assured by visual inspection that the solution is at the correct dilution and pH. This product is specially formulated for superior pH stability at high temperatures and will help prevent the possibility of losing pH sensitive antigens. Diva Decloaker is non-toxic, non-flammable, odorless and sodium azide and thimerosal free.Known Applications:Immunohistochemistry (formalin-fixed paraffin-embedded tissues) Supplied As:100mlDiva Decloaker, 10X concentrate (DV2004LX)500mlDiva Decloaker, 10X concentrate (DV2004MX)Materials and Reagents (Needed But Not Provided): Microscope slides, positively chargedDesert Chamber* (Drying oven)Positive and negative tissue controlsXylene (Could be substituted with xylene substitute*)Ethanol or reagent alcoholDecloaking Chamber* (Pressure cooker)Deionized or distilled waterWash buffer*(TBS/PBS)Enzyme digestion*Avidin-Biotin Blocking Kit*(Labeled Streptavidin Kits Only) Peroxidase block*Protein block*Primary antibody*Negative control reagents*Detection kits*Detection components*Chromogens*Hematoxylin*Bluing reagent*Mounting medium** Biocare Medical Products: Refer to a Biocare Medical catalog for further information regarding catalog number and ordering information. Certain reagents listed above are based on specific application and detection system used. Storage and Stability:Store at room temperature. Do not use after expiration date printed on vial. If reagents are stored under conditions other than those specified in the package insert, they must be verified by the user. Diluted reagents should be used promptly; any remaining reagent should be stored at room temperature.Protocol Recommendations:1. Deparaffinize tissues and hydrate to water. If necessary, block for endogenous peroxidase and wash in DI water.2. Dilute concentrated Diva Decloaker at a ratio of 1:10 (1 ml Diva to 9 ml of deionized water).3. Place slides into 1X retrieval solution in a slide container (e.g. Coplin Jar, Tissue -Tek™ staining dish or metal slide canister).4. Retrieve sections under pressure using Biocare's Decloaking Chamber. Follow the recommendations on the antibody data sheet and Decloaking Chamber User Manual.5. Check solution for appropriate color change. (See Technical Note #1)6. Gently rinse by gradually adding DI water to the solution, then remove slides and rinse with DI water.Technical Notes:1. Concentrated Diva Decloaker is a bright yellow color. RTU or 1X solution is a pale yellow color. When the solution reaches 80-125°C, the solution turns yellow and indicates that the high temperature solution is at correct pH. Should the pH rise above 7.0, the solution turns a fuschia red color. Should the pH drop too low, thesolution turns a pink color.2. If using Biocare’s Desert Chamber Pro (a programmable turbo-action drying oven), dry sections at 25ºC overnight or at 37ºC for 30-60 minutes and then dry slides at 60ºC for 30 minutes.3. Use positive char ged slides (use Biocare’s Kling-On HIER Slides) and cut tissues at 4-5 microns. Do not use any adhesives in the water bath. Poor fixation and processing of tissues will cause tissue sections to fall off the slides, especially fatty tissues such as breast. Tissues should be fixed a minimum of 6-12 hours.4. Protocol time and temperatures for HIER can vary depending on the Decloaking Chamber model used. Please refer to the relevant Decloaking Chamber manual for appropriate protocol times and temperatures.Limitations:The protocols for a specific application can vary. These include, but are not limited to: fixation, heat-retrieval method, incubation times, tissue section thickness and detection kit used. Due to the superior sensitivity of these unique reagents, the recommended incubation times and titers listed are not applicable to other detection systems, asresults may vary. The data sheet recommendations and protocols are based on exclusive use of Biocare products. Ultimately, it is the responsibility of the investigator to determine optimal conditions. The clinical interpretation of any positive or negative staining should be evaluated within the context of clinical presentation, morphology and other histopathological criteria by a qualified pathologist. The clinical interpretation of any positive or negative staining should be complemented by morphological studies using proper positive and negative internal and external controls as well as other diagnostic tests.Catalog Number: DV2004 LX, MX Description: 100, 500 ml, concentrateQuality Control:Refer to CLSI Quality Standards for Design and Implementation of Immunohistochemistry Assays; Approved Guideline-Second edition (I/LA28-A2). CLSI Wayne, PA, USA (). 2011 Precautions:1. This product is not classified as hazardous. The preservative used in this reagent is Proclin 300 and the concentration is less than 0.25%. Overexposure to Proclin 300 can cause skin and eye irritation and irritation to mucous membranes and upper respiratory tract. The concentration of Proclin 300 in this product does not meet the OSHA criteria for a hazardous substance. Wear disposable gloves when handling reagents.2. Specimens, before and after fixation, and all materials exposed to them should be handled as if capable of transmitting infection and disposed of with proper precautions. Never pipette reagents by mouth and avoid contacting the skin and mucous membranes with reagents and specimens. If reagents or specimens come in contact with sensitive areas, wash with copious amounts of water.3. Microbial contamination of reagents may result in an increase in nonspecific staining.4. Incubation times or temperatures other than those specified may give erroneous results. The user must validate any such change.5. Do not use reagent after the expiration date printed on the vial.6. The SDS is available upon request and is located at /.7. Consult OSHA, federal, state or local regulations for disposal of any toxic substances. Proclin is a trademark of Rohm and Haas Company, or of its subsidiaries or affiliates.Troubleshooting:Follow the antibody specific protocol recommendations according to data sheet provided. If atypical results occur, contact Biocare's Technical Support at 1-800-542-2002.。
生物质谱技术在腺相关病毒(AAV)载体制剂质量控制中的应用

学 报Journal of China Pharmaceutical University 2023,54(6):682 - 694682生物质谱技术在腺相关病毒(AAV)载体制剂质量控制中的应用李孟效,李惠琳*(中山大学药学院,广州 510006)摘 要 腺相关病毒(adeno-associated virus,AAV)是一种基因治疗中常用的病毒载体。
由于其安全性较高且能够靶向多种细胞,在临床前和临床研究中得到了较多的应用。
不过在设计和生产的过程中,AAV载体有着诸多会影响其安全性和疗效的关键质量属性。
生物质谱技术的发展和应用为生物大分子的研究提供了一个便捷的平台,尤其是在蛋白质序列、结构和相互作用方面。
对于AAV载体而言,质谱技术可以实现衣壳蛋白比率、翻译后修饰、血清型、空衣壳比率的测定或表征,从而协助对AAV载体的质量控制。
与现有方法相比,质谱技术具有样品需求量少、分析快速灵敏、适用于完整AAV载体的分析和质量分辨率高的优点,并且可以区分空衣壳、满衣壳和部分包封的衣壳。
未来,通过将更加高效的蛋白质分离技术与质谱技术联用、开发新的信息处理软件平台和新的质谱检测方法,质谱技术有望在AAV载体的设计和生产中发挥更加重要的作用。
关键词腺相关病毒;质谱;质量控制;非变性质谱;电荷检测质谱中图分类号R392 文献标志码 A 文章编号1000 -5048(2023)06 -0682 -13doi:10.11665/j.issn.1000 -5048.2023062901引用本文李孟效,李惠琳.生物质谱技术在腺相关病毒(AAV)载体制剂质量控制中的应用[J].中国药科大学学报,2023,54(6):682–694.Cite this article as:LI Mengxiao,LI Huilin. Application of biological mass spectrometry in quality control of adeno-associated virus carrier preparations[J].J China Pharm Univ,2023,54(6):682–694.Application of biological mass spectrometry in quality control of adeno-asso⁃ciated virus carrier preparationsLI Mengxiao, LI Huilin*School of Pharmaceutical Science, Sun Yat-Sen University, Guangzhou 510006, ChinaAbstract Adeno-associated virus (AAV) is a common viral vector used in gene therapy.Because of its high safe⁃ty and its ability to target a variety of cells, it has been widely used in preclinical and clinical studies.However, during the design and production, AAV vectors have many key quality attributes that affect their safety and efficacy. The development and application of biological mass spectrometry technology provides a convenient platform for the research on biological macromolecules, especially in the aspects of protein sequence, structure and interac⁃tion.For AAV vectors, mass spectrometry can facilitate the determination or characterization of capsid protein ratio, post-translational modification, serotype, and empty capsid ratio, thus assisting in the quality control of AAV pared with the existing methods, mass spectrometry has the advantages of smaller amount of sample size, faster and more sensitive analysis, being more suitable for the analysis of complete AAV vectors with higher mass resolution, and can distinguish empty capsids, full capsids and partial capsids.In the future, mass spectrometry technology is expected to play a more important role in the design and production of AAV vectors through the coupling of more efficient protein separation technology with mass spectrometry, the development of new information processing software platforms and new mass spectrometry detection techniques.Key words adeno-associated virus (AAV); mass spectrometry (MS); quality control; native MS; charge detec⁃tion MS收稿日期2023-06-29 *通信作者Tel:138****4968E-mail:lihlin6@基金项目国家自然科学基金资助项目(No.81872836,No.91953102);广东省自然科学基金资助项目(No.2019A1515011265)第 54 卷第 6 期李孟效,等:生物质谱技术在腺相关病毒(AAV )载体制剂质量控制中的应用This study was supported by the National Natural Science Foundation of China (No.81872836, No.91953102) and the National Nat⁃ural Science Foundation of Guangdong Province (No.2019A1515011265)1 腺相关病毒(AAV)概述基因治疗是指为了治疗目的而修改操纵基因表达或改变活细胞的基因,基因治疗的方式可以划分基因补充和基因编辑,基因补充是指将遗传物质导入需要治疗的靶细胞,基因编辑是指对细胞已有的缺陷基因进行修改和调控,但无论哪种方式都需要依赖特定的递送载体才能完成[1]。
超声误诊无性细胞瘤一例

超声误诊无性细胞瘤一例发布时间:2021-09-24T02:21:37.511Z 来源:《医师在线》2021年23期作者:超声误诊无性细胞瘤一例[导读] 卵巢无性细胞瘤是卵巢恶性生殖细胞瘤贺宇凡,曾思惠通讯作者(广东省妇幼保健院广东广州 511400)【摘要】:卵巢无性细胞瘤是卵巢恶性生殖细胞瘤,来源于尚未分化以前的原始生殖细胞,临床发病率低且缺乏典型早期症状,主要表现为腹痛及腹部肿块。
本文分析总结一例以急腹症就诊行急诊阴超检查的误诊病例报道,以期为无性细胞瘤的超声正确诊断提供经验。
【关键词】无性细胞瘤;卵巢1.临床资料患者,女,24岁,未婚,性生活正常。
因“停经41天,下腹痛4天,加重1天”来我院就诊。
现病史:患者平素月经规则,周期28-30天,经期3天。
2020-6-9无明显诱因出现下腹胀痛,间断发作,疼痛轻微,未予重视。
2020-6-11下腹胀痛较前加重,体位改变时明显,休息后好转,不伴发热、畏寒、寒战、恶心、呕吐等不适,外院就诊HGB:120g/l,腹部超声、泌尿系超声及阑尾区超声均未见明显异常,妇科超声示子宫前方低回声不均质肿块,建议上级医院治疗,现患者为求进一步诊治,我院就诊。
体格检查:体温:36.4℃,脉搏:120次/分,呼吸:20次/分,血压:116/79mmHg。
腹平软,无肌紧张,肠鸣音正常,下腹部轻压痛,无反跳痛。
妇检:阴道少许淡红色分泌物,宫颈举痛(+)及摇摆痛(+),后穹隆穿刺抽出不凝血0.5ml。
子宫前方巨大包块,活动度差,边界触及不清,子宫大小触及不清,有压痛。
实验室检查:尿HCG(+)。
婚育史:未婚,性生活正常。
G0P0.既往史:既往体健,既往检查:7个月前外院体检行妇科超声检查,未见异常。
2020-6-13经阴道超声检查所见:左卵巢大小38mm×23mm,右卵巢大小42mm×34mm,子宫上方见混合性包块,大小132mm×79mm,边界清,似与双卵巢分界清,CDFI:混合性包块边缘及内部见少许彩色血流信号。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
《临床肝胆病杂志》推荐使用的规范医学名词术语

临床肝胆病杂志第40卷第3期2024年3月J Clin Hepatol, Vol.40 No.3, Mar.2024[3]XIA SL, LIU ZM, CAI JR, et al. Liver fibrosis therapy based on biomi⁃metic nanoparticles which deplete activated hepatic stellate cells[J]. J Control Release, 2023, 355: 54-67. DOI: 10.1016/j.jconrel.2023.01.052.[4]LIU YW, DONG YT, WU XJ, et al. The assessment of mesenchymalstem cells therapy in acute on chronic liver failure and chronic liver disease: A systematic review and meta-analysis of randomized con⁃trolled clinical trials[J]. Stem Cell Res Ther, 2022, 13(1): 204. DOI:10.1186/s13287-022-02882-4.[5]ZHANG ZL, SHANG J, YANG QY, et al. Exosomes derived from hu⁃man adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline me⁃tabolism[J]. J Nanobiotechnology, 2023, 21(1): 29. DOI: 10.1186/ s12951-023-01788-4.[6]ZHAO T, SU ZP, LI YC, et al. Chitinase-3 like-protein-1 function andits role in diseases[J]. Signal Transduct Target Ther, 2020, 5(1): 201. DOI: 10.1038/s41392-020-00303-7.[7]YANG H, ZHAO LL, HAN P, et al. Value of serum chitinase-3-likeprotein 1 in predicting the risk of decompensation events in patients with liver cirrhosis[J]. J Clin Hepatol, 2023, 39(7): 1578-1585. DOI:10.3969/j.issn.1001-5256.2023.07.011.杨航, 赵黎莉, 韩萍, 等. 血清壳多糖酶3样蛋白1(CHI3L1)对肝硬化患者发生失代偿事件风险的预测价值[J]. 临床肝胆病杂志, 2023, 39(7): 1578-1585. DOI: 10.3969/j.issn.1001-5256.2023.07.011.[8]MA L, WEI J, ZENG Y, et al. Mesenchymal stem cell-originated exo⁃somal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis[J]. Drug Deliv, 2022, 29(1): 440-453. DOI: 10.1080/10717544.2022.2030428. [9]NISHIMURA N, DE BATTISTA D, MCGIVERN DR, et al. Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis[J]. Proc Natl Acad Sci U S A, 2021, 118(17): e2019633118. DOI: 10.1073/pnas.2019633118.[10]WANG CG, LI SZ, SHI JM, et al. Research progress in differentia⁃tion, identification, and purification methods of human pluripotent stem cells to mesenchymal-like cells in vitro[J]. J Jilin Univ Med Ed, 2023, 49(6): 1655-1661. DOI: 10.13481/j.1671-587X.20230634.王成刚, 李生振, 史嘉敏, 等. 体外人多能干细胞向间充质样细胞分化、鉴定和纯化方法的研究进展[J]. 吉林大学学报(医学版), 2023, 49(6): 1655-1661. DOI: 10.13481/j.1671-587X.20230634.[11]LI TT, WANG ZR, YAO WQ, et al. Stem cell therapies for chronicliver diseases: Progress and challenges[J]. Stem Cells Transl Med, 2022, 11(9): 900-911. DOI: 10.1093/stcltm/szac053.[12]YANG X, LI Q, LIU WT, et al. Mesenchymal stromal cells in hepaticfibrosis/cirrhosis: From pathogenesis to treatment[J]. Cell Mol Im⁃munol, 2023, 20(6): 583-599. DOI: 10.1038/s41423-023-00983-5. [13]ZHAO SX, LIU Y, PU ZH. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apop⁃tosis by activating autophagy in vitro[J]. Drug Des Devel Ther, 2019, 13: 2887-2897. DOI: 10.2147/DDDT.S220190.[14]LEE CG, HARTL D, LEE GR, et al. Role of breast regression protein39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue re⁃sponses and apoptosis[J]. J Exp Med, 2009, 206(5): 1149-1166.DOI: 10.1084/jem.20081271.[15]HIGASHIYAMA M, TOMITA K, SUGIHARA N, et al. Chitinase 3-like 1deficiency ameliorates liver fibrosis by promoting hepatic macro⁃phage apoptosis[J]. Hepatol Res, 2019, 49(11): 1316-1328. DOI:10.1111/hepr.13396.收稿日期:2023-06-09;录用日期:2023-08-17本文编辑:邢翔宇引证本文:LIU PJ, YAO LC, HU X, et al. Effect of human umbilical cord mesenchymal stem cells in treatment of mice with liver fibrosis and its mechanism[J]. J Clin Hepatol, 2024, 40(3): 527-532.刘平箕, 姚黎超, 胡雪, 等. 人脐带间充质干细胞(hUC-MSC)对肝纤维化小鼠模型的治疗作用及其机制分析[J]. 临床肝胆病杂志, 2024, 40(3): 527-532.读者·作者·编者《临床肝胆病杂志》推荐使用的规范医学名词术语有关名词术语应规范统一,以全国自然科学名词审定委员会公布的各学科名词为准。
SCI_Chem 影响因子

Abbreviated JournalTitle(linked to journal information)Total CitesImpact Factor5-YearImpactFactor Immediacy Index综合1Nature 0028-0836********.59738.1599.2432Science0036-807550848931.02733.587 6.6913P Natl Acad Sci Usa 0027-84245349519.73710.583 1.893化学综合1Chem Rev 0009-266511259641.29845.79514.3352Nat Mater 1476-11224634835.74942.3768.4113Nat Nanotechnol 1748-33872192031.1736.011 5.8764Chem Soc Rev 0306-00124764624.89230.1817.9975Prog Mater Sci 0079-6425592123.19422.3337.2176Nat Chem 1755-4330865221.75723.02 5.5327Accounts Chem Res 0001-48424211220.83324.633 5.2958Nano Today 1748-0132294417.68918.1920.7849Annu Rev Mater Res 1531-7331525916.17914.4950.66710Surf Sci Rep 0167-5729411515.33322.2817.7511Adv Mater 0935-96489195214.82913.86 2.55712Mat Sci Eng R 0927-796X 485013.90218.9740.66713Angew Chem Int Edit 1433-785122989413.73413.56 2.95914Annu Rev Phys Chem 0066-426X 700213.36518.121 4.13815Nano Lett 1530-69848843113.02514.132 2.47116Acs Nano 1936-0851*******.06212.524 1.9417Energ Environ Sci 1754-56921284911.65312.462 3.08718Coordin Chem Rev 0010-85452560111.01612.257 2.29419J Am Chem Soc 0002-786343128610.67710.237 2.16420Nat Prod Rep 0265-0568660910.17810.072 2.38521Adv Energy Mater 1614-6832199510.04310.05 2.11822Adv Funct Mater 1616-301X 347589.76510.342 1.81523Med Res Rev 0198-632532979.5839.978 1.81124Npg Asia Mater 1884-40493019.0428.556 2.41425Annu Rev Anal Chem 1936-132711498.612.2830.69626Top Curr Chem 0340-102255078.456 6.205 4.27327Chem Sci 2041-652048458.3148.33 2.77128Chem Mater 0897-4756746518.2387.627 1.12329J Photoch Photobio C 1389-556720268.06911.9520.7530Small 1613-6810181377.8238.084 1.42931Prog Photovoltaics 1062-799545357.7127.023 3.22332J Control Release 0168-3659297557.6338.078 1.13633Annu Rev Chem Biomol 1947-54383317.5127.512 1.04534Int Mater Rev 0950-660822557.487.1491.188RankISSNJCR Data35Chemsuschem1864-563150567.4757.951 1.189 36Prog Solid State Ch0079-678614747.429 3.3380 37Nano Res1998-012430317.3927.8010.979 38Prog Surf Sci0079-681619047.1369.140.273 39Adv Carbohyd Chem Bi0065-23188337.133 5.8460.75 40Green Chem1463-926215554 6.828 6.992 1.269 41Adv Organomet Chem0065-3055841 6.758.9410 42Curr Opin Colloid In1359-02944670 6.6297.0360.884 43J Phys Chem Lett1948-71858575 6.585 6.651 1.301 44Top Organometal Chem1436-60021152 6.384 1.762 45Chem Commun1359-7345122728 6.378 6.226 1.53 46Catal Rev0161-49402529 6.37510.1750.889 47Trac-Trend Anal Chem0165-99367327 6.351 6.7610.92 48Nanoscale2040-33647835 6.233 6.262 1.167 49Adv Colloid Interfac0001-86867115 6.1698.01 1.32 50Org Lett1523-706073440 6.142 5.563 1.572 51Mater Today1369-70213769 6.0718.677 1.977 52Prog Nucl Mag Res Sp0079-65651993 6.022 6.065 1.389 53Crit Rev Solid State1040-8436744 5.9477.3681 54Carbon0008-622332742 5.868 6.35 1.197 55Chem-Eur J0947-653960788 5.831 5.623 1.241 56Appl Catal B-Environ0926-337323011 5.825 6.0310.965 57J Catal0021-951734516 5.787 6.249 1.025 58Wires Comput Mol Sci1759-0876570 5.738 5.738 3.518 59Lab Chip1473-019716485 5.697 6.136 1.256 60Anal Chem0003-270096794 5.695 5.7690.948 61J Med Chem0022-262359227 5.614 5.383 1.225 62Adv Synth Catal1615-415015502 5.535 5.323 1.109 63Curr Opin Solid St M1359-02862508 5.4387.3290.913 64Biosens Bioelectron0956-566322068 5.437 5.389 1.105 65J Chem Theory Comput1549-961811067 5.389 5.936 1.067 66Biomacromolecules1525-779724209 5.371 5.750.721 67Acs Catal2155-54351461 5.265 5.265 1.222 68Adv Catal0360-05641399 5.25 5.2860 69Chemcatchem1867-38802718 5.181 5.3070.941 70Mrs Bull0883-******** 5.024 5.590.569 71Acs Appl Mater Inter1944-82448635 5.008 5.040.683 72Electroanal Chem0070-977837757.50.25 73J Comb Chem1520-47662925 4.933 3.10274Int Rev Phys Chem0144-235X1524 4.92 5.595 2.231 75J Phys Chem C1932-744778595 4.814 5.1520.738 76Pharm Res-Dordr0724-874119035 4.742 5.0460.735 77Cryst Growth Des1528-748322310 4.689 4.8730.869 78Sol Energ Mat Sol C0927-024818447 4.63 5.205 1.215 79J Chromatogr A0021-967363419 4.612 4.5820.71480Inorg Chem0020-166985446 4.593 4.5510.956 81Bioconjugate Chem1043-180213900 4.58 4.7960.768 82Chem-Asian J1861-47286084 4.572 4.488 1.04683J Org Chem0022-326396723 4.564 4.135 1.101 84Anal Chim Acta0003-267039448 4.387 4.3440.684 85Chem Rec1527-89991375 4.377 4.814 1.533 86Int J Plasticity0749-64195276 4.356 4.70.862 87J Chem Inf Model1549-959611250 4.304 4.0670.795 88Langmuir0743-7463106920 4.187 4.4160.793 89Organometallics0276-733339735 4.145 3.653 1.004 90Ultraschall Med0172-46141247 4.116 2.723 1.286 91J Flow Chem2062-249X61 4.091 4.091 1.143 92Curr Med Chem0929-867312773 4.07 4.4710.627 93Struct Bond0081-59931899 4.068 4.24894Mar Drugs1660-33971871 3.978 3.9110.428 95Analyst0003-265416152 3.969 3.9040.785 96Acta Mater1359-645434860 3.941 4.3950.781 97Soft Matter1744-683X15943 3.909 4.35 1.013 98Crystengcomm1466-803312988 3.879 4.0690.863 99Acs Chem Neurosci1948-7193634 3.871 3.9570.729 100Nanotechnology0957-448434133 3.842 3.8380.697 101Org Electron1566-11995856 3.836 4.0210.63 102J Comput Chem0192-865124682 3.835 4.4010.847 103Phys Chem Chem Phys1463-907640969 3.829 3.976 1.052 104Faraday Discuss1359-66405758 3.821 4.148 2.369 105Dalton T1477-922638660 3.806 3.8890.947 106Catal Sci Technol2044-47531079 3.753 3.753 1.024 107Sci Technol Adv Mat1468-69962352 3.752 3.6440.235 108Chembiochem1439-42279616 3.74 3.670.78 109Curr Top Med Chem1568-02664850 3.702 3.8850.655 110Chem Res Toxicol0893-228X10785 3.667 4.0130.807 111Anal Bioanal Chem1618-264221971 3.659 3.7560.727 112Acs Comb Sci2156-8952379 3.636 3.6360.596 113Corros Sci0010-938X18210 3.615 4.0160.757 114J Phys Chem B1520-6106119722 3.607 3.7020.66 115J Am Soc Mass Spectr1044-03058760 3.592 3.5030.925 116J Cheminformatics1758-2946315 3.59 3.6710.314 117Org Biomol Chem1477-052018355 3.568 3.490.952 118Ultrasound Obst Gyn0960-76928490 3.557 3.7080.756 119Colloid Surface B0927-776510312 3.554 3.4170.707 120Int J Hydrogen Energ0360-319933119 3.548 4.0860.584 121Sensor Actuat B-Chem0925-400529909 3.535 3.6680.527 122Dyes Pigments0143-72087664 3.532 3.4330.897 123Expert Opin Ther Pat1354-37761742 3.525 2.5230.6 124Ultrason Sonochem1350-41775008 3.516 3.708 1.234125J Phys Chem Ref Data0047-26894996 3.5 3.7790.391 126Eur J Med Chem0223-523414818 3.499 3.8490.541 127Talanta0039-914026966 3.498 3.7330.531 128Food Hydrocolloid0268-005X6307 3.494 3.5250.953 129Drug Des Dev Ther1177-8881377 3.4860.256 130Carbohyd Polym0144-861718471 3.479 3.9420.665 131Microchim Acta0026-36724743 3.434 2.8830.5 132Appl Catal A-Gen0926-860X27726 3.41 3.910.506 133J Mech Phys Solids0022-509610460 3.406 3.7460.784 134Pure Appl Chem0033-454513333 3.386 3.1150.382 135Micropor Mesopor Mat1387-181115134 3.365 3.4140.869 136J Biol Inorg Chem0949-82574011 3.353 3.3550.713 137Chemphyschem1439-423511279 3.349 3.3880.768 138Eur J Org Chem1434-193X19346 3.344 3.1370.746 139Food Chem0308-814641375 3.334 4.0720.589 140Acs Med Chem Lett1948-58751087 3.311 3.3180.699 141Future Med Chem1756-89191081 3.31 3.2450.933 142J Nat Prod0163-386419898 3.285 3.2670.787 143Electrophoresis0173-083516985 3.261 2.8690.479 144Bioanalysis1757-61801318 3.253 3.0440.723 145J Mass Spectrom1076-51745573 3.214 3.2270.495 146J Inorg Biochem0162-013410014 3.197 3.430.672 147J Mol Catal A-Chem1381-116917999 3.187 3.3190.496 148J Colloid Interf Sci0021-979744929 3.172 3.390.747 149J Anal Atom Spectrom0267-94777362 3.155 2.9530.725 150Sep Purif Rev1542-2119222 3.154 3.5430.3 151J Pharm Sci-Us0022-354917986 3.13 3.3850.6 152Eur J Inorg Chem1434-194816310 3.12 2.9510.728 153Cement Concrete Res0008-884613854 3.112 3.7460.441 154Curr Org Chem1385-27283953 3.039 3.2220.253 155Isr J Chem0021-21481263 3.025 1.9460.478 156Mar Chem0304-420368723 3.3150.466 157Catal Today0920-586123325 2.98 3.4640.515 158Phytomedicine0944-71135244 2.972 3.2580.401 159New J Chem1144-054610014 2.966 2.920.698 160J Pharmaceut Biomed0731-708514648 2.947 2.8530.562 161Photoch Photobio Sci1474-905X4541 2.923 2.810.538 162Catal Commun1566-73679190 2.915 3.3280.535 163Mater Design0261-30699587 2.913 2.8050.703 164J Agr Food Chem0021-856176046 2.906 3.2880.417 165Bioorgan Med Chem0968-089624911 2.903 3.1510.617 166Crit Rev Anal Chem1040-8347979 2.892 3.690.591 167Microchem J0026-265X3428 2.879 2.85 1.048 168Mini-Rev Med Chem1389-55752893 2.865 2.9210.496 169Mol Divers1381-19911289 2.861 3.090.44170Chemmedchem1860-71793723 2.835 3.0750.788 171J Mol Catal B-Enzym1381-11774943 2.823 2.8050.542 172Scripta Mater1359-646219677 2.821 3.1450.534 173Adv Phys Org Chem0065-3160391 2.818 2.609174Electroanal1040-039710646 2.817 2.8620.462 175Fuel Process Technol0378-******** 2.816 3.4930.49 176Tetrahedron0040-402052981 2.803 2.8990.636 177Beilstein J Org Chem1860-53971405 2.801 2.4750.386 178J Phys Chem A1089-563955641 2.771 2.8560.658 179J Ethnopharmacol0378-874121278 2.755 3.3220.519 180Org Process Res Dev1083-61604311 2.739 2.6670.808 181J Supercrit Fluid0896-84466044 2.732 3.1380.472 182Medchemcomm2040-2503690 2.722 2.7220.603 183Adv Inorg Chem0898-******** 2.714 3.3780.25 184J Electroanal Chem1572-665721687 2.672 2.6760.545 185Int J Photoenergy1110-662X945 2.663 2.240.671 186Synlett0936-521417034 2.655 2.4520.604 187Environ Chem1448-25171390 2.652 2.701 1.075 188Opt Mater Express2159-3930593 2.616 2.6220.815 189Anti-Cancer Agent Me1871-52061386 2.610.239 190Top Catal1022-55284896 2.608 2.7080.238 191J Sep Sci1615-93068094 2.591 2.6380.296 192Anal Biochem0003-269739746 2.582 2.9690.558 193Rsc Adv2046-20691816 2.562 2.5670.695 194J Anal Appl Pyrol0165-23705102 2.56 3.0740.447 195Nanoscale Res Lett1931-75733998 2.524 2.7830.2980951-419813285 2.509 2.6110.49 196Rapid Commun Mass Sp196Sci Adv Mater1947-2935553 2.509 2.5610.301 198React Funct Polym1381-51484515 2.505 2.6530.282 199J Chem Technol Biot0268-25756796 2.504 2.4790.573 200Synthesis-Stuttgart0039-788117999 2.5 2.3840.615 201Microsc Microanal1431-92762043 2.495 3.0770.276 202J Chromatogr B1570-023220776 2.487 2.90.319 203Phytochem Analysis0958-03441895 2.48 2.280.292 204Adv Heterocycl Chem0065-2725888 2.478 2.6170.455 205Chem Biol Drug Des1747-02771940 2.469 2.4090.511 206Int J Mol Sci1422-00674706 2.464 2.7320.313 207Ultrasound Med Biol0301-56297839 2.455 2.8440.251 208Gold Bull0017-15571073 2.434 3.1220.04 209Molecules1420-30497552 2.428 2.6790.329 210Plasmonics1557-1955877 2.425 2.9880.525 211J Photoch Photobio A1010-603013061 2.416 2.6910.299 212Tetrahedron Lett0040-403973763 2.397 2.3760.561 213J Alloy Compd0925-838839264 2.39 2.1610.629 214Phys Status Solidi-R1862-62541437 2.388 2.4320.527215Fluid Phase Equilibr0378-******** 2.379 2.3380.544 216Solvent Extr Ion Exc0736-******** 2.375 2.6090.345 217Beilstein J Nanotech2190-4286309 2.374 2.3740.573 218Plant Food Hum Nutr0921-96681569 2.358 2.7620.25 219Acta Pharmacol Sin1671-40835577 2.354 2.5210.596 220Planta Med0032-094311009 2.348 2.4620.304 221Appl Clay Sci0169-13175590 2.342 2.7980.337 222Bioorg Med Chem Lett0960-894X33460 2.338 2.4270.584 222Macromol Mater Eng1438-74922983 2.338 2.3920.509 222Mol Inform1868-1743344 2.338 2.3460.347 225Russ Chem Rev+0036-021X3092 2.299 2.8130.204 226J Chem Thermodyn0021-96145905 2.297 2.2560.735 227Constr Build Mater0950-06187337 2.293 2.8180.391 228Chemometr Intell Lab0169-74394880 2.291 2.4320.253 229Biophys Chem0301-46224822 2.283 2.0940.649 230Arab J Chem1878-5352299 2.2660.343 231J Ginseng Res1226-8453349 2.2590.34 232Materials1996-19441176 2.247 2.3380.222 233Catal Lett1011-372X9373 2.244 2.2610.351 234Theor Chem Acc1432-881X5484 2.233 3.1510.812 235Fitoterapia0367-326X4706 2.231 2.1390.349 236Mater Lett0167-577X23419 2.224 2.3220.489 237Food Addit Contam A1944-00495142 2.22 2.4420.508 238J Biomol Screen1087-05712357 2.207 2.0890.719 239Platin Met Rev0032-1400735 2.194 2.4760.579 240J Nanopart Res1388-07645724 2.175 2.7210.222 241J Mater Sci0022-246131538 2.163 2.10.543 242Adv Quantum Chem0065-3276869 2.161 1.6940.308 242Colloid Polym Sci0303-402X5657 2.161 2.1140.433 244Chem Phys Lett0009-261455163 2.145 2.150.485 244J Ind Eng Chem1226-086X2264 2.145 1.9550.329 246Tetrahedron-Asymmetr0957-416611707 2.115 2.1430.384 247Appl Surf Sci0169-433231193 2.112 2.0990.33 248Synthetic Met0379-677913916 2.109 2.1020.334 249Colloid Surface A0927-775718414 2.108 2.3330.333 249Mat Sci Eng A-Struct0921-509340513 2.108 2.3490.307 251J Anal Toxicol0146-47602660 2.107 1.7580.429 252Solid State Nucl Mag0926-20401202 2.1 2.0570.711 253J Food Compos Anal0889-15753737 2.088 2.7430.19 254J Environ Monitor1464-03254378 2.085 2.1370.322 255Mater Chem Phys0254-058417174 2.072 2.3950.286 256J Pept Sci1075-26171942 2.071 1.8280.434 257Phytother Res0951-418X8059 2.068 2.4380.444 258Nano-Micro Lett2150-5551205 2.057 1.910.35 259Solid State Ionics0167-273820728 2.046 2.5640.26260Carbohyd Res0008-621514176 2.044 2.1780.357 261J Solid State Chem0022-459618166 2.04 2.2950.392 262Curr Org Synth1570-1794673 2.038 2.9140.76 263Ultrasonics0041-624X3651 2.028 2.0540.456 264Smart Mater Struct0964-17267120 2.024 2.3770.289 265Inorg Chem Commun1387-70036416 2.016 1.8810.434 266Appl Organomet Chem0268-26052866 2.011 1.9220.272 267Electrochem Solid St1099-00628883 2.01 2.0260.596 268J Chem Eng Data0021-956815169 2.004 2.1150.295 269Comb Chem High T Scr1386-207314532 1.9750.268 269J Organomet Chem0022-328X217932 1.9920.527 271Thermochim Acta0040-603111408 1.989 2.0460.327 272J Mol Model1610-29403157 1.984 2.3010.378 273J Therm Anal Calorim1388-61509934 1.982 1.7420.243 274Int J Fatigue0142-11235248 1.976 1.9740.352 275J Vib Control1077-54631649 1.966 1.7360.672 276Chem Phys0301-010412935 1.957 2.0590.592 277J Mater Process Tech0924-013618426 1.953 2.1760.332 277Sensors-Basel1424-82207082 1.953 2.3950.321 279Biomed Chromatogr0269-38792861 1.945 1.8150.385 280J Fluorine Chem0022-11394998 1.939 1.9490.465ArticlesCited Half-life Eigenfactor ®Metrics ScoreArticleInfluenc e® Score8699.6 1.5750820.8448329.7 1.3598717.71238018 1.55663 4.8921768.20.2266114.294141 5.20.2278819.481121 3.70.1543615.607390 3.50.178418.8542370.015018.643126 2.40.054018.927207 6.50.108177.90837 3.20.01489 5.62318>10.00.013647.55389.40.008828.821867 5.10.27819 4.24368.40.00872 6.542227 5.50.53637 3.497299.90.01888.1211078 4.40.37491 5.1891191 2.40.20333 4.012473 1.80.05534 3.461368.20.03818 3.07130997.70.83183 2.99465 5.90.01757 3.141169 1.40.00944 3.344569 4.20.12336 3.006377.20.00647 2.73229 1.90.00155 3.27323 3.90.00716 4.441557.70.01045 2.068458 1.40.02121 2.833576 6.70.15215 2.032167.90.00342 3.148457 3.60.07856 2.503112 4.60.01202 1.97501 6.90.05008 1.9062220.00197 2.73916>10.00.003282.792Eigenfactor®Metrics286 2.60.0208 2.0046>10.00.00142 1.13896 2.80.01517 2.301119.20.00397 3.974>10.00.00086 1.894 44940.03848 1.5233>10.00.00076 2.537437.60.01013 2.301 63220.04884 2.393 2150.003483173 4.80.28954 1.5629>10.00.00211 3.009 138 5.80.01695 1.794 1015 1.70.02966 1.597 508.20.01226 2.37 160850.18182 1.40244 5.40.01448 3.213188.30.00431 2.295 107.10.00156 2.34 674 6.40.06437 1.6 1916 4.10.1766 1.469 480 5.40.05059 1.419 284>10.00.04014 1.60356 1.20.00194 1.703 617 3.70.05475 1.603 14797.80.17236 1.533 8907.80.09182 1.35 404 4.40.04405 1.352 239.20.00403 2.705 448 4.10.05929 1.252 507 3.50.04948 1.865 480 5.30.05919 1.416 315 1.30.00585 1.6362>10.00.00057 1.543 255 2.10.01222 1.495 116 6.20.01779 2.376 953 2.30.03563 1.2844>10.00.00016 2.2160 5.10.006940.708137.90.00319 2.303 3283 3.40.34957 1.353 2798.70.02859 1.299 773 3.90.057280.943 493 5.30.04212 1.304 11447.10.090460.92115617.50.124780.98 259 5.70.03346 1.295 372 2.80.02555 1.219 1289>10.00.12482 1.03 7277.10.06585 1.03830 5.80.00343 1.39794 6.80.01439 1.685 303 6.60.019140.892 2119 6.70.21487 1.164 9987.10.059110.76263 3.40.002370.436140.000190.962 475 5.50.03101 1.1898.60.00225 1.225 194 2.50.00680.908 817 6.60.031350.985 68170.09849 1.714 1358 2.50.073 1.334 1194 2.60.032550.749 10720.00309 1.26 1021 4.40.12987 1.194 459 3.20.02416 1.192 2618.90.03446 1.396 1804 3.70.15067 1.296 1418.60.01298 1.609 1709 4.50.08330.842 327 1.30.00320.94181 4.90.00904 1.23 327 4.70.03579 1.258 17750.0131 1.044 2597.10.02119 1.078 877 4.30.068460.99799 1.40.001410.915 449 6.30.028910.786 16157.50.18356 1.081 22860.020170.96535 1.90.00176 1.224 1160 3.80.055440.914 201 6.30.017610.949 426 4.10.025740.772 2120 3.70.074070.708 1016 5.80.054290.757 321 5.50.013530.63690 3.30.006210.726 184 4.80.010070.73123>10.00.00228 1.513 610 3.80.032170.72 842 5.40.057730.829 213 5.40.01270.77439 2.40.001731002 4.80.033670.729 208 4.30.00930.537 5247.60.043570.93 116>10.00.02104 1.812 173>10.00.013330.936 589 5.30.032210.807 108 6.10.009190.931 487 4.70.03951 1.142 76450.047770.779 1666 5.20.091160.854 193 1.80.004620.921 120 2.20.004670.894 3338.70.027140.756 4327.10.027040.625 19520.003610.533 194 6.90.011710.909 2597.30.013360.716 266 6.80.026850.752 87080.070140.869 2187.70.01310.75610 5.60.00062 1.012 4189.20.026590.815 622 5.20.034970.666 1709.90.01712 1.222 178 5.80.009120.836 907.70.00320.65258>10.00.01221 1.371 4877.30.041850.896 187 5.60.009820.662 338 6.50.019280.711 443 6.30.026840.651 20850.01330.806 409 4.50.027060.735 774 3.20.034350.762 15287.90.107180.745 739 5.40.053960.715 227.60.001180.774 105 5.10.007480.647 129 4.90.00780.74475 3.50.002990.639212 3.70.013920.784 201 5.60.009290.599 500 6.30.06138 1.238 >10.00.00042 1.049 290 6.10.019640.625 251 5.60.017330.895 12498.40.075460.68 241 2.20.005560.628 1341 6.70.120620.829 651 6.90.026690.572 224 5.10.010210.672 290 5.30.013220.702 194 1.60.002660.7278>10.00.000620.792 314>10.00.019270.649 32830.001390.381 520 6.30.034550.62153 5.20.004350.904 200 1.30.002460.856 109 3.70.00563143 5.80.013670.825 439 4.20.025470.66 441>10.00.03210.853 17290.80.002020.494 1527.40.009010.741 624 2.40.016810.714 337 6.40.02850.71 173 1.90.002010.614 124 6.30.008010.593 2207.60.010810.602 4708.20.028730.596 221 5.20.00853 1.29 4957.50.036490.714 967.20.003150.52311>10.00.000450.543 184 3.50.006730.588 1064 2.80.017560.666 2437.60.014140.744 257.10.002380.868 105130.022770.574 10130.003550.834 2518.20.017620.63 16669.90.081330.508 1478 4.10.10840.548 14830.008560.8753427.80.015630.587 559.80.002470.6496 1.50.001250.668648.10.002270.561 178 6.20.010850.6 247>10.00.011750.526 202 5.20.013550.701 1484 5.40.069550.546 116 6.40.005740.60572 2.10.00140.58954>10.00.004010.856 351 6.60.011110.57 960 3.90.021660.681 1549.50.006150.597 5790.008180.646 6720.0008353 2.30.00061176 2.60.005320.633 1947.80.016490.569 223 5.60.014960.968 25280.006360.457 1626 5.50.049760.537 1977.10.008730.585 128 5.40.00580.57919>10.00.001080.734 650 3.70.017210.673 9419.10.050830.59113>10.00.00130.598 21090.007690.475 906>10.00.066080.687 350 3.90.005590.396 2247.80.01640.479 1789 5.50.074220.539 419>10.00.014550.491 579 6.80.034280.585 11717.30.084950.73 918.60.003920.471 458.60.00220.655 100 6.20.007370.666 335 5.10.012850.644 964 5.50.038290.59199 5.20.004810.478 302 6.60.012460.48640 2.10.000610.345 392>10.00.021350.719339>10.00.01560.489 5138.90.025150.615 50 4.50.001620.659 1368.50.005580.639 353 5.90.018730.713 495 4.80.011170.321 1147.60.003960.391 156 6.70.022520.706 508 6.50.027410.476 82 5.10.003380.472 410>10.00.020760.421 400>10.00.012770.531 463 4.70.008250.53 728 5.70.012880.253 2367.10.012090.669 180 3.80.004490.45 360>10.00.018270.679 2957.80.035210.653 950 3.40.026580.586 218 5.20.006840.446 2547.50.007820.487。
7. Endotoxin LALTests

Charles River Endosafe
2
Woo Jung BSC Inc.
August 25, 2003
LAL Discoveries by Bang and Levin
Described role of endotoxin in coagulation of Limulus blood
Prepared Endotoxin - responsive lysate from Amoebocytes
Endotoxicity
ENDOTOXIN CAUSES HUMAN TISSUE TO RELEASE INFLAMMATORY MEDIATORS INFLAMMATION INDUCES A VARIETY OF TISSUE DAMAGE SHOCK and MULTIPLE ORGAN DYSFUNCTION MAY OCCUR
Endotoxins and Pyrogens
Pyrogens are fever-inducing agents in humans and animals
include endotoxin, gram + cell debris, fungi
Endotoxins are components from the outer membrane of gram-negative bacteria
Clotting Enzyme
Liquid Coagulogen
M++ pH=7.2
Clotted Coagulin Gel
Summary of Gel Clot Test
Endpoint sought by 180 inversion of sample tube
达格列净联合二甲双胍治疗初诊2_型糖尿病的临床疗效分析

达格列净联合二甲双胍治疗初诊2型糖尿病的临床疗效分析卢宁,刘艳梅,仇颖盐城市第一人民医院内分泌科,江苏盐城224000[摘要]目的探讨达格列净联合二甲双胍治疗初诊2型糖尿病患者的临床疗效及其安全性。
方法选取2021年9月—2022年11月在盐城市第一人民医院内分泌科接受治疗的初诊2型糖尿病患者70例为研究对象,根据系统盲选分组法分成两组,每组35例。
对照组应用二甲双胍治疗,观察组应用达格列净+二甲双胍治疗。
检测并对比两组患者血糖指标、血脂指标、体质指数、不良反应发生情况及疗效。
结果治疗后,观察组血糖及血脂各项指标水平均优于对照组,体质指数低于对照组,差异有统计学意义(P<0.05)。
观察组治疗总有效率高于对照组,差异有统计学意义(P<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
结论达格列净联合二甲双胍对初诊2型糖尿病患者具有良好的治疗作用,有助于改善血糖和血脂、体质量水平,不良反应较少。
[关键词] 达格列净;二甲双胍;初诊2型糖尿病;不良反应;治疗效果[中图分类号] R587.1 [文献标识码] A [文章编号] 1672-4062(2023)03(b)-0091-04 Clinical Analysis of Dapagliflozin Combined with Metformin in the Treat⁃ment of Newly Diagnosed Type 2 Diabetes MellitusLU Ning, LIU Yanmei, QIU YingDepartment of Endocrinology, Yancheng First People´s Hospital, Yancheng, Jiangsu Province, 224000 China[Abstract] Objective To investigate the clinical efficacy and safety of Dapagliflozin combined with metformin in the treatment of newly diagnosed type 2 diabetes patients.Methods Seventy newly diagnosed patients with type 2 diabetes who received treatment in the Endocrinology Department of Yancheng First People´s Hospital from September 2021 to November 2022 were selected as the research objects and divided into two groups with 35 cases in each group accord⁃ing to systematic blind selection grouping. The control group was treated with metformin, and the observation group was treated with dagliazine+metformin. Blood glucose index, blood lipid index, body mass index, occurrence of ad⁃verse reactions and efficacy of the two groups were detected and compared.Results After treatment, the blood glucose and lipid levels of the observation group were better than those of the control group, while the body mass index of the observation group was lower than that of the control group, the difference was statistically significant (P<0.05). The to⁃tal effective rate of the observation group was higher than that of the control group, and the difference was statistically significant (P<0.05). There was no statistically significant difference in the incidence of adverse reactions between the two groups (P>0.05).Conclusion Dapagliflozin combined with metformin has a good therapeutic effect on newly diag⁃nosed type 2 diabetes patients, which can help to improve blood glucose, blood fat and body mass level, and less ad⁃verse reactions.[Key words] Dapagliflozin; Metformin; Newly diagnosed type 2 diabetes mellitus; Adverse reaction; Therapeutic effect目前,老年化问题越发突出,加之国民饮食习惯、膳食结构以及饮食偏好的变化,使得糖尿病患者逐渐增多[1]。
GC测定盐酸普拉克索中三乙胺残留量

WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA

WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
血管外膜细胞钙化及其钙化机制研究

血管外膜细胞钙化及其钙化机制研究谭小青,张旭升,樊小容,黄战军摘要 目的:研究经体外诱导钙化建立大鼠血管外膜细胞钙化模型,检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂方法:原代提取大鼠胸主动脉外膜纤维细胞,取3~6代细胞使用诱导培养基(高糖DMEM +10%胎牛血清+10mmol/L β-甘油磷酸+0.05mmol/L 抗坏血酸+100mmol/L 地塞米松)诱导钙化,诱导时间为3d ㊁6d ㊁9d ㊁12d ㊁15d ,筛选出诱导细胞钙化的最佳时间㊂对细胞采用茜素红S 染色㊁细胞内钙含量测定和碱性磷酸酶(ALP )活性检测,鉴定是否成功构建钙化模型㊂采用实时定量聚合酶链式反应(PT -PCR )检测成骨相关因子骨形态生成蛋白2(BMP2)和核心结合因子α1(Runx2)的mRNA 含量,蛋白免疫印迹法(Western Blot )检测凋亡蛋白Bax ㊁Bcl -2和自噬相关蛋白微血管相关蛋白(LC3)㊁Beclin -1的表达水平,找出血管外膜细胞钙化的潜在机制㊂结果:当诱导钙化时间为15d 时,血管外膜细胞中主要钙化指标胞内钙含量及ALP 活性上调(P <0.05),茜素红S 染色显示钙化组有明显钙盐沉积㊂血管外膜细胞经钙化诱导后,BMP2和Runx2的mRNA 水平上调,Bax 蛋白水平上调,Bcl -2和Beclin -1蛋白水平下调,LC3-Ⅱ/LC3-Ⅰ比值上调(P <0.05)㊂结论:钙化诱导培养基培养血管外膜细胞15d 可成功构建钙化细胞模型,血管外膜细胞钙化可能与细胞向成骨样表型转化有关,血管外膜细胞钙化过程涉及细胞自噬及凋亡调控㊂关键词 血管外膜细胞;钙化;成骨样表型转化;自噬与凋亡;实验研究d o i :10.12102/j.i s s n .1672-1349.2023.18.010 Calcification of Vascular Adventitial Cells and Its MechanismTAN Xiaoqing,ZHANG Xusheng,FAN Xiaorong,HUANG Zhanjun Longgang District People 's Hospital of Shenzhen,Shenzhen 518172,Guangdong,China Corresponding Author ZHANG Xusheng,E -mail:*****************Abstract Objective:To investigate the mananism of calcification of rat vascular adventitial cells,establish the calcification model of rat vascular adventitial cells,and detect the expression changes of osteogenesis -related indicators,apoptosis,and autophagy -related proteins during the calcification process.It aimed to provide more accurate cell models for cardiovascular disease and initially explore the mechanism of calcification.Methods:Rat thoracic aortic adventitial fibroblasts were extracted from the primary generation,and the 3rd to 6th generation cells were used for induction medium(high glucose DMEM +10%fetal bovine serum +10mmol/L β-glycerophosphate +0.05mmol/L ascorbic acid +100mmol/L dexamethasone)to induce calcification,the induction time was 3,6,9,12,and 15d,and the optimal time for inducing cell calcification was selected.The cells were stained with alizarin red S,detected by intracellular calcium content and alkaline phosphatase(ALP)to identify whether the calcification model was successfully constructed.Real -time quantitative reverse transcription polymerase chain reaction(RT -PCR)was used to detect the mRNA levels of osteogenesis -related factors bone morphogenetic protein(BMP2)and runt -related transcription factor 2(Runx2);Western Blot was used to detect the apoptosis proteins Bax,Bcl -2,the autophagy -related proteins LC -3,and Beclin -1expression level;then the potential mechanism of vascular adventitial cell calcification would be revealed.Results:When calcification was induced for 15days,the intracellular calcium content in the adventitial cells of the main calcification indicators and ALP activity were up -regulated(P <0.05).Alizarin red S staining showed obvious calcium deposits in the calcification group.After calcification was induced in adventitial cells,the mRNA levels of BMP2and Runx2up -regulated,the protein levels of Bax up -regulated,the protein levels of Bcl -2and Beclin -1down -regulated,and the ratio of LC3-Ⅱ/LC3-Ⅰdown -regulated(P <0.05).Conclusion:Adventitial cells cultured in the calcification -inducing medium for 15days could successfully construct a calcified cell model.calcification of adventitial cells might be related to the transformation of cells to an osteoblast -like phenotype.The Calcification process of adventitial cells involved autophagy and apoptosis regulation.Keywords adventitial cells;calcification;osteogenic phenotype transformation;autophagy and apoptosis;experimental study血管钙化常见于动脉粥样硬化㊁血脂异常㊁高血压㊁糖尿病㊁慢性肾病及衰老等人群[1],血管钙化引起血管硬度增加㊁顺应性降低,导致心肌缺血㊁心力衰竭㊁血栓形成等,增加脑卒中㊁心脏病㊁动脉粥样硬化斑块破裂等的风险,被认为是影响心血管疾病的重要因素之一[2-4]㊂目前关于血管内膜㊁中膜和心脏瓣膜钙化的关注和研究相对较多㊂临床工作中发现,血管外膜也可发生钙化,然而调查发现,现阶段对血管外膜钙化的作者单位 深圳市龙岗区人民医院(广东深圳518172)通讯作者 张旭升,E -mail :*****************引用信息 谭小青,张旭升,樊小容,等.血管外膜细胞钙化及其钙化机制研究[J ].中西医结合心脑血管病杂志,2023,21(18):3347-3350.关注较少,因此,需要更多的研究来阐明血管钙化的致病机制㊂最初血管钙化被认为是被动和退行性病变,标志着血管老化,但是越来越多研究表明血管钙化是类似于胚胎骨形成的病理生物学过程[5-6]㊂Bostr öm 等[7-8]研究发现,钙化过程中大鼠血管中膜细胞由原有收缩表型转变成为成骨样细胞表型,原有的收缩标志物如平滑肌肌动蛋白α(α-SMA )等表达减少,并表达核心结合因子α1(Runx2)㊁骨形态生成蛋白2(BMP2)等多种成骨样标志物,从而介导骨基质在血管中沉积㊂细胞凋亡与自噬为2种细胞死亡的方式,与血管钙化息息相关,研究表明,血管中膜细胞在细胞凋亡过程中释放凋亡小体,促进细胞钙化,而细胞自噬通过多种机制调控细胞钙化[9-10]㊂本研究对大鼠血管外膜细胞进行体外诱导钙化,建立大鼠血管外膜细胞钙化模型,并检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂1材料与方法1.1试剂胎牛血清(FBS,Gibco),青霉素,链霉素(Gibco,美国),茜素红S溶液,β-甘油磷酸,抗坏血酸,地塞米松(Sigma,美国),抗GAPDH抗体(Bioworld),抗Bcl-2, Bax,Bcelin1和微血管相关蛋白(LC3)抗体(CST),碱性磷酸酶检测试剂盒㊁钙(Ca)检测试剂盒(南京建城生物工程研究所)㊂1.2大鼠血管外膜细胞分离与培养取10只4~6周龄雄性Wistar-Kyoto大鼠(体质量120~180g)胸主动脉分离血管外膜,采用组织黏附法培养㊂使用添加10%胎牛血清的高糖DMEM培养基(Gibco dmem)在37ħ㊁5%二氧化碳条件下培养细胞㊂当细胞增殖至80%~90%融合时,用0.25%胰酶消化传代㊂使用第3代至第6代的细胞进行后续实验㊂1.3体外钙化模型的建立钙化诱导培养基为含10%胎牛血清,10mmol/L β-甘油磷酸钠,0.05mmol/L抗坏血酸和100mmol/L 地塞米松的高糖DMEM培养液㊂将第3代至第6代细胞分为对照组和钙化组,待细胞长至50%融合时,使用钙化诱导培养基培养,每3d更换1次培养基,连续培养15d㊂1.4碱性磷酸酶(ALP)酶活测定细胞钙化诱导后,弃去培养基,1ˑ磷酸缓冲盐溶液(PBS)洗细胞3次,加入裂解液500μL(1%T ritonX-100),冰上裂解40min后,离心,取上清液㊂使用上清液根据试剂盒说明书检测ALP活性及总蛋白含量㊂1.5细胞内钙含量检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,每孔加入500μL0.6mol/L的盐酸4ħ脱钙过夜,取上清,根据钙测试试剂盒说明书检测钙含量㊂将脱钙后的细胞用4ħPBS洗3次,每孔加入500μL NaOH/0.1%SDS裂解细胞,取上清,用二喹啉甲酸法(BCA)测定细胞蛋白含量㊂1.6茜素红S染色细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,加入0.5mL4%多聚甲醛室温固定15min,用双蒸水洗3次,加入1mL0.1%茜素红室温孵育15min,吸去染液,双蒸水洗3次,在倒置显微镜下观察㊂1.7实时定量聚合酶链式反应(RT-PCR)检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,使用TaKaRa MiniBEST Universal RNA Extraction Kit提取总RNA,使用PrimeScrip TM RT reagent Kit将所提取的RNA逆转录合成cDNA,以cDNA为模板,通过SYBR Green I嵌合荧光定量RT-PCR检测BMP-2㊁Runx2和GAPDH的表达量㊂引物序列见表1㊂表1引物序列基因方向序列Runx2正向5'-TGGCTTTGGTTTCAGGTTAGG-3'反向5'-TGGAGATGTTGCTCTGTTCG-3' BMP-2正向5'-TGAGGATTAGCAGGTCTTTGC-3'反向5'-TCTCGTTTGTGGAGTGGATG-3' GAPDH正向5'-GGCTGCCCAGAACATCAT-3'反向5'-CGGACACATTGGGGGTAG-3'1.8蛋白免疫印迹法(Western Blot)检测细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,提取细胞总蛋白㊂使用12%SDS-PAGE胶电泳分离,并转移到聚偏二氟乙烯膜(PVDF)上,封闭后,加入一抗(Bax1ʒ1000,Bcl-21ʒ1000,Beclin11ʒ1000, LC31ʒ1000,GAPDH1:1000)稀释液,4ħ孵育过夜;加入二抗稀释液(1ʒ10000)室温孵育1h后,使用ECL发光试剂盒显影并计算灰度值㊂1.9统计学处理应用SPSS19.0软件进行统计处理,符合正态分布的定量资料以均数ʃ标准差(xʃs)表示,比较采用t检验,以P<0.05为差异有统计学意义㊂2结果2.1大鼠血管外膜细胞可在体外被诱导钙化为验证高磷是否能诱导大鼠血管外膜细胞钙化,使用钙化诱导培养基培养细胞,在不同时间点检测ALP活性和胞内钙含量㊂随着培养时间延长,ALP活性逐渐上升,在培养第12天达到峰值,与对照组比较差异有统计学意义(P<0.05);诱导第3天开始所测得的胞内钙含量与对照组比较升高(P<0.05),ALP 活性和钙含量升高具有时间依赖性㊂详见图1㊁图2㊂诱导15d所测得钙含量最高,因此,后续实验选择的诱导时间为15d㊂对钙化诱导15d的细胞进行茜素红S染色,结果显示,对照组细胞呈长梭形,而钙化组细胞变成菱形㊂茜素红S染色后,钙化组可观察到大量的橘红色钙结节(见图3),而对照组完全没有㊂这也证明大鼠血管外膜细胞可在体外被钙化培养基诱导钙化㊂图1钙化诱导培养基诱导外膜细胞后ALP含量(与0d时比较,*P<0.05)图2钙化诱导培养基诱导外膜细胞后胞内钙含量(与0d时比较,*P<0.05)图3培养15d时细胞经茜素S红染色切片图(ˑ100)2.2血管外膜细胞钙化与细胞向成骨样表型转化有关血管钙化的增加与成骨细胞特异性标志物如BMP2㊁和Runx2的增加有关[11]㊂RT-PCR结果显示,与对照组比较,钙化组的成骨细胞特异性标志物BMP2和Runx2mRNA表达量增加,与对照组比较差异有统计学意义(P<0.05)㊂详见图4㊂图4外膜细胞钙化过程中BMP2和Runx2mRNA表达量(与对照组比较,*P<0.05)2.3血管外膜细胞钙化过程涉及细胞自噬及凋亡调控通过Western Blot检测凋亡和自噬相关蛋白的表达量变化㊂与对照组比较,钙化组促凋亡蛋白Bax表达上调,抑凋亡蛋白Bcl-2表达下调(P<0.05)㊂详见图5㊂钙化组自噬相关蛋白Beclin1表达上调,LC3-Ⅱ/ LC3-Ⅰ比例上调(P<0.05),说明钙化诱导培养后细胞内凋亡水平上调㊁自噬水平升高㊂详见图6㊂图5诱导钙化后促凋亡蛋白及抑凋亡蛋白表达变化图6诱导钙化后凋亡及自噬蛋白Beclin1等表达变化3讨论血管钙化作为心血管疾病病人的并发症之一,其发病率与严重程度逐年增高及加重,是导致心血管疾病病人高死亡率的重要因素㊂血管钙化缺乏有效的治疗药物㊂因此,探究血管钙化发病机制,在分子水平寻找有效的诊断和防治靶点是急需开展的基础研究工作㊂本研究证明,使用10mmol/Lβ-甘油磷酸+0.05 mmol/L抗坏血酸+100mmol/L地塞米松培养外膜细胞即可诱导大鼠血管外膜细胞在体外发生钙化,这是通过茜素红S染色㊁ALP活性检测及胞内钙含量检测结果得以确定的㊂血管钙化过程中,血管中膜细胞向成骨样细胞表型转变并表达相关成骨标志物,从而引起骨基质的沉积,是血管钙化的重要特点及机制[5]㊂本实验所用的血管外膜细胞钙化条件与血管中膜细胞钙化条件一致,说明血管外膜细胞钙化的机制可能与中膜细胞钙化的机制部分一致㊂血管中膜细胞钙化过程中,细胞表达成骨相关的转录因子如Runx2等,进而促进下游表达骨相关蛋白如骨形态发生蛋白BMP2等的表达,从而促使细胞向成骨样细胞主动分化[12-13],本研究也观察到类似的机制㊂通过PT-PCR检测,发现钙化培养基培养大鼠血管外膜细胞15d后,BMP2和Runx2的mRNA表达水平升高㊂本研究通过对钙盐沉积与成骨样细胞表型转变2个维度的探讨,证明血管外膜细胞可在体外被诱导钙化,丰富了血管钙化的分型㊂血管钙化的发生机制复杂,涉及多种信号通路,如细胞自噬和凋亡㊁Wnt/β-catenin信号通路激活㊁内质网应激等均参与调控血管钙化的过程㊂自噬作为一种细胞应激的适应性反应,在维持血管结构与功能中十分关键㊂研究表明,血管钙化过程中自噬水平增高[14-15]㊂在体外实验中,高磷可提高大鼠血管中膜细胞的自噬水平,增加细胞内自噬体数量,从而抑制凋亡与钙化[16]㊂还有研究表明,自噬可通过抑制大鼠血管中膜细胞氧化应激,抑制血管内皮细胞的炎症反应,对三酰甘油等脂代谢进行调控,从而减轻血管钙化[17-18]㊂LC3和Beclin1是2种典型的自噬标志物,Western Blot实验结果表明,用钙化培养基诱导大鼠血管外膜细胞15d,LC3-Ⅱ/LC3-Ⅰ比率升高,Beclin1蛋白水平表达升高,说明细胞内自噬水平升高㊂多项研究表明,细胞凋亡参与促进血管钙化的发生,抑制细胞凋亡和抑制钙化[16-17]㊂在对大鼠的体内研究发现,成纤维细胞生长因子21通过内质网应激调控Caspase-12信号通路来减少血管内中膜细胞凋亡,从而抑制血管钙化[18]㊂另外,提高培养基中的Pi 或Ca2+浓度,可诱导细胞质膜形成并释放基质囊泡(如凋亡小体),从而导致细胞外基质钙化,这种基质钙化可能成为血管钙化的成核位点[19]㊂Bax和Bcl-2是2种典型的凋亡和抑制凋亡蛋白,本实验结果证明,利用钙化培养基对血管外膜细胞诱导钙化过程中,细胞内凋亡水平升高㊂同时细胞内自噬水平也升高,这可能是细胞自我调控以对抗钙化的结果㊂本研究证实血管外膜细胞可在体外被诱导钙化,且外膜钙化过程与骨组织钙化过程类似,为主动可调控的过程㊂血管钙化是一个复杂的过程,涉及细胞凋亡和自噬等调控通路,仍需进一步研究㊂参考文献:[1]梁英权,段亚君,韩际宏.血管钙化分子机制研究进展[J].中国动脉硬化杂志,2020,28(11):921-929.[2]NICOLL R,HENEIN M Y.The predictive value of arterial andvalvular calcification for mortality and cardiovascular events[J].Int J Cardiol Heart Vessel,2014,3:1-5.[3]JOHNSON R C,LEOPOLD J A,LOSCALZO J.Vascularcalcification:pathobiological mechanisms and clinical implications[J].Circulation Research,2006,99(10):1044-1059.[4]YAMADA S,GIACHELLI C M.Vascular calcification in CKD-MBD:roles for phosphate,FGF23,and Klotho[J].Bone,2017,100:87-93.[5]LIN M E,CHEN T M,WALLINGFORD M C,et al.Runx2deletion insmooth muscle cells inhibits vascular osteochondrogenesis andcalcification but not atherosclerotic lesion formation[J].Cardiovascular Research,2016,112(2):606-616.[6]DURHAM A L,SPEER M Y,SCATENA M,et al.Role of smoothmuscle cells in vascular calcification:implications in atherosclerosis andarterial stiffness[J].Cardiovascular Research,2018,114(4):590-600.[7]BOSTRÖM K I,RAJAMANNAN N M,TOWLER D A.The regulationof valvular and vascular sclerosis by osteogenic morphogens[J].Circulation Research,2011,109(5):564-577.[8]SPEER M Y,YANG H Y,BRABB T,et al.Smooth muscle cells giverise to osteochondrogenic precursors and chondrocytes incalcifying arteries[J].Circulation Research,2009,104(6):733-741.[9]PROUDFOOT D,SKEPPER J N,HEGYI L,et al.Apoptosisregulates human vascular calcification in vitro:evidence forinitiation of vascular calcification by apoptotic bodies[J].Circulation Research,2000,87(11):1055-1062.[10]AN S J,BOYD R,ZHU M,et al.NADPH oxidase mediatesangiotensin II-induced endothelin-1expression in vascularadventitial fibroblasts[J].Cardiovascular Research,2007,75(4):702-709.[11]ZEADIN M,BUTCHER M,WERSTUCK G,et al.Effect of leptin onvascular calcification in apolipoprotein E-deficient mice[J].Arterioscler Thromb Vasc Biol,2009,29(12):2069-2075. [12]LEOPOLD J A.Vascular calcification:mechanisms of vascularsmooth muscle cell calcification[J].Trends in CardiovascularMedicine,2015,25(4):267-274.[13]刘聿秀.高尿酸诱导血管钙化的机制研究[D].青岛:青岛大学,2015.[14]LIU Q,LUO Y,ZHAO Y,et al.Nano-hydroxyapatite acceleratesvascular calcification via lysosome impairment and autophagydysfunction in smooth muscle cells[J].Bioact Mater,2022,8:478-493.[15]LIANG J,HUANG J,HE W,et al.β-Hydroxybutyric Inhibits vascularcalcification via autophagy enhancement in models induced byhigh phosphate[J].Front Cardiovasc Med,2021,8:685748. [16]CICERI P,ELLI F,CAPPELLETTI L,et al.A new in vitro model todelay high phosphate-induced vascular calcification progression[J].Mol Cell Biochem,2015,410(1/2):197-206.[17]BYON C H,JAVED A,DAI Q,et al.Oxidative stress inducesvascular calcification through modulation of the osteogenictranscription factor Runx2by AKT signaling[J].The Journal ofBiological Chemistry,2008,283(22):15319-15327.[18]OUIMET M,FRANKLIN V,MAK E,et al.Autophagy regulatescholesterol efflux from macrophage foam cells via lysosomal acidlipase[J].Cell Metabolism,2011,13(6):655-667.[19]REYNOLDS J L,JOANNIDES A J,SKEPPER J N,et al.Humanvascular smooth muscle cells undergo vesicle-mediatedcalcification in response to changes in extracellular calcium andphosphate concentrations:a potential mechanism for acceleratedvascular calcification in ESRD[J].Journal of the AmericanSociety of Nephrology,2004,15(11):2857-2867.(收稿日期:2022-03-30)(本文编辑王雅洁)。
CellTiter Glo Luminescent Cell Viability Assay Protocol

Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·1.Description (1)2.Product Components and Storage Conditions (4)3.Performing the CellTiter-Glo ®Assay (5)A.Reagent Preparation (5)B.Protocol for the Cell Viability Assay (6)C.Protocol for Generating an ATP Standard Curve (optional) (7)4.Appendix (7)A.Overview of the CellTiter-Glo ®Assay..............................................................7B.Additional Considerations..................................................................................8C.References............................................................................................................11D.Related Products. (12)1.DescriptionThe CellTiter-Glo ®Luminescent Cell Viability Assay (a–e)is a homogeneous method to determine the number of viable cells in culture based on quantitation of the ATP present, which signals the presence of metabolically active cells. The CellTiter-Glo ®Assay is designed for use with multiwell-plate formats, making it ideal for automated high-throughput screening (HTS) and cell proliferation and cytotoxicity assays. The homogeneous assay procedure (Figure 1) involves adding a single reagent (CellTiter-Glo ®Reagent) directly to cells cultured in serum-supplemented medium. Cell washing, removal of medium or multiple pipetting steps are not required.The homogeneous “add-mix-measure” format results in cell lysis and generation of a luminescent signal proportional to the amount of ATP present (Figure 2).The amount of ATP is directly proportional to the number of cells present in culture in agreement with previous reports (1). The CellTiter-Glo ®Assay relies on the properties of a proprietary thermostable luciferase (Ultra-Glo™ Recombinant Luciferase), which generates a stable “glow-type” luminescent signal and improves performance across a wide range of assay conditions. The luciferase reaction for this assay is shown in Figure 3. The half-life of the luminescent signal resulting from this reaction is greater than five hours (Figure 4). This extended half-life eliminates the need for reagent injectors and provides flexibility for continuous or batch-mode processing of multiple plates. The unique homogeneous format reduces pipetting errors that may be introduced during the multiple steps required by other ATP-measurement methods.CellTiter-Glo ®Luminescent Cell Viability AssayAll technical literature is available on the Internet at: /protocols/ Please visit the web site to verify that you are using the most current version of this Technical Bulletin. Please contact Promega Technical Services if you have questions on useofthissystem.E-mail:********************Figure 1. Flow diagram showing preparation and use of CellTiter-Glo ®Reagent.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·3170M A 12_0ACellTiter-Glo CellTiter-Glo MixerLuminometer®System Advantages•Homogeneous:“Add-mix-measure” format reduces the number of plate-handling steps to fewer than that required for similar ATP assays.•Fast:Data can be recorded 10 minutes after adding reagent.•Sensitive:Measures cells at numbers below the detection limits of standard colorimetric and fluorometric assays.•Flexible:Can be used with various multiwell formats. Data can be recorded by luminometer or CCD camera or imaging device.•Robust:Luminescent signal is very stable, with a half-life >5 hours,depending on cell type and culture medium used.•Able to Multiplex:Can be used with reporter gene assays or other cell-based assays from Promega (2,3).Figure 3. The luciferase reaction.Mono-oxygenation of luciferin is catalyzed byluciferase in the presence of Mg 2+, ATP and molecular oxygen.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·3171M A 12_0A L u m i n e s c e n c e (R L U )Cells per Well10,00060,00020,00030,00040,00050,0000R² = 0.9990.5 × 1061.0 × 1061.5 × 1062.0 × 1062.5 × 1063.0 × 1063.5 × 1064.0 × 106r² = 0.99020,00010,00030,00040,00050,000r² = 0.9900100200300400HO SN S N O S N S N OCOOH +ATP+O 2Ultra-Glo™ Recombinant Luciferase +AMP+PP i +CO 2+LightBeetle Luciferin OxyluciferinMg 2+0Figure 2. Cell number correlates with luminescent output.A direct relationship exists between luminescence measured with the CellTiter-Glo ®Assay and the number of cells in culture over three orders of magnitude. Serial twofold dilutions of HEK293cells were made in a 96-well plate in DMEM with 10% FBS, and assays wereperformed as described in Section 3.B. Luminescence was recorded 10minutes after reagent addition using a GloMax ®-Multi+ Detection System. Values represent the mean ± S.D. of four replicates for each cell number. The luminescent signal from 50HEK293 cells is greater than three times the background signal from serum-supplemented medium without cells. There is a linear relationship (r 2= 0.99)between the luminescent signal and the number of cells from 0to 50,000 cells per well.Figure 4. Extended luminescent half-life allows high-throughput batchprocessing.Signal stability is shown for three common cell lines. HepG2 and BHK-21cells were grown and assayed in MEM containing 10% FBS, while CHO-K1 cells were grown and assayed in DME/F-12 containing 10% FBS. CHO-K1, BHK-21 and HepG2 cells, at 25,000 cells per well, were added to a 96-well plate. After an equal volume of CellTiter-Glo ®Reagent was added, plates were shaken and luminescence monitored over time with the plates held at 22°C. The half-lives of the luminescent signals for the CHO-K1, BHK-21 and HepG2 cells were approximately 5.4, 5.2 and5.8hours, respectively.2.Product Components and Storage ConditionsProduct Size Cat.#CellTiter-Glo ®Luminescent Cell Viability Assay 10ml G7570Substrate is sufficient for 100 assays at 100µl/assay in 96-well plates or 400 assays at 25µl/assay in 384-well plates. Includes:• 1 × 10mlCellTiter-Glo ®Buffer • 1 vial CellTiter-Glo ®Substrate (lyophilized)Product Size Cat.#CellTiter-Glo ®Luminescent Cell Viability Assay 10 × 10ml G7571Each vial of substrate is sufficient for 100 assays at 100µl/assay in 96-well plates or 400 assays at 25µl/assay in 384-well plates (1,000 to 4,000 total assays). Includes:•10 × 10mlCellTiter-Glo ®Buffer •10 vials CellTiter-Glo ®Substrate (lyophilized)Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·R e l a t i v e L u m i n e s c e n c e (%)Time (minutes)CHO-K101020304050607080901003173M A 12_0AProduct Size Cat.# CellTiter-Glo®Luminescent Cell Viability Assay100ml G7572 Substrate is sufficient for 1,000 assays at 100µl/assay in 96-well plates or 4,000assays at 25µl/assay in 384-well plates. Includes:•1 × 100ml CellTiter-Glo®Buffer• 1 vial CellTiter-Glo®Substrate (lyophilized)Product Size Cat.# CellTiter-Glo®Luminescent Cell Viability Assay10 × 100ml G7573Each vial of substrate is sufficient for 1,000 assays at 100µl/assay in 96-well plates or4,000 assays at 25µl/assay in 384-well plates (10,000to 40,000 total assays). Includes:•10 × 100ml CellTiter-Glo®Buffer•10 vials CellTiter-Glo®Substrate (lyophilized)Storage Conditions:For long-term storage, store the lyophilized CellTiter-Glo®Substrate and CellTiter-Glo®Buffer at –20°C. For frequent use, the CellTiter-Glo®Buffer can be stored at 4°C or room temperature for 48hours without loss of activity. See product label for expiration date information. ReconstitutedCellTiter-Glo®Reagent (Buffer plus Substrate) can be stored at room temperaturefor up to 8hours with <10% loss of activity, at 4°C for 48hours with ~5% lossof activity, at 4°C for 4days with ~20% loss of activity or at –20°C for 21weekswith ~3% loss of activity. The reagent is stable for up to ten freeze-thaw cycles,with less than 10% loss of activity.3.Performing the CellTiter-Glo®AssayMaterials to Be Supplied by the User•opaque-walled multiwell plates adequate for cell culture•multichannel pipette or automated pipetting station for reagent delivery•device (plate shaker) for mixing multiwell plates•luminometer, CCD camera or imaging device capable of reading multiwell plates •optional:ATP for use in generating a standard curve (Section 3.C)3.A.Reagent Preparation1.Thaw the CellTiter-Glo®Buffer, and equilibrate to room temperature priorto use. For convenience the CellTiter-Glo®Buffer may be thawed andstored at room temperature for up to 48hours prior to use.2.Equilibrate the lyophilized CellTiter-Glo®Substrate to room temperatureprior to use.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·3.A.Reagent Preparation (continued)3.Transfer the appropriate volume (10ml for Cat.# G7570 and G7571, or 100mlfor Cat.# G7572 and G7573) of CellTiter-Glo ®Buffer into the amber bottlecontaining CellTiter-Glo ®Substrate to reconstitute the lyophilizedenzyme/substrate mixture. This forms the CellTiter-Glo ®Reagent.4.Mix by gently vortexing, swirling or inverting the contents to obtain ahomogeneous solution. The CellTiter-Glo ®Substrate should go intosolution easily in less than 1minute.3.B.Protocol for the Cell Viability AssayWe recommend that you perform a titration of your particular cells todetermine the optimal number and ensure that you are working within thelinear range of the CellTiter-Glo ®Assay. Figure 2 provides an example of sucha titration of HEK293 cells using 0 to 50,000 cells per well in a 96-well format.1.Prepare opaque-walled multiwell plates with mammalian cells in culturemedium, 100µl per well for 96-well plates or 25µl per well for 384-wellplates.Multiwell plates must be compatible with the luminometer used.2.Prepare control wells containing medium without cells to obtain a value forbackground luminescence.3.Add the test compound to experimental wells, and incubate according toculture protocol.4.Equilibrate the plate and its contents at room temperature forapproximately 30 minutes.5.Add a volume of CellTiter-Glo ®Reagent equal to the volume of cell culturemedium present in each well (e.g., add 100µl of reagent to 100µl of mediumcontaining cells for a 96-well plate, or add 25µl of reagent to 25µl ofmedium containing cells for a 384-well plate).6.Mix contents for 2 minutes on an orbital shaker to induce cell lysis.7.Allow the plate to incubate at room temperature for 10 minutes to stabilizeluminescent signal.Note:Uneven luminescent signal within standard plates can be caused bytemperature gradients, uneven seeding of cells or edge effects in multiwellplates.8.Record luminescence.Note:Instrument settings depend on the manufacturer. An integration timeof 0.25–1 second per well should serve as a guideline.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·3.C.Protocol for Generating an ATP Standard Curve (optional)It is a good practice to generate a standard curve using the same plate onwhich samples are assayed. We recommend ATP disodium salt (Cat.# P1132,Sigma Cat.# A7699 or GE Healthcare Cat.# 27-1006). The ATP standard curveshould be generated immediately prior to adding the CellTiter-Glo®Reagentbecause endogenous ATPase enzymes found in sera may reduce ATP levels.1.Prepare 1µM ATP in culture medium (100µl of 1µM ATP solution contains10–10moles ATP).2.Prepare serial tenfold dilutions of ATP in culture medium (1µM to 10nM;100µl contains 10–10to 10–12moles of ATP).3.Prepare a multiwell plate with varying concentrations of ATP standard in100µl medium (25µl for a 384-well plate).4.Add a volume of CellTiter-Glo®Reagent equal to the volume of ATPstandard present in each well.5.Mix contents for 2 minutes on an orbital shaker.6.Allow the plate to incubate at room temperature for 10 minutes to stabilizethe luminescent signal.7.Record luminescence.4.Appendix4.A.Overview of the CellTiter-Glo®AssayThe assay system uses the properties of a proprietary thermostable luciferase toenable reaction conditions that generate a stable “glow-type” luminescentsignal while simultaneously inhibiting endogenous enzymes released duringcell lysis (e.g., ATPases). Release of ATPases will interfere with accurate ATPmeasurement. Historically, firefly luciferase purified from Photinus pyralis(LucPpy) has been used in reagents for ATP assays (1,4–7). However, it hasonly moderate stability in vitro and is sensitive to its chemical environment,including factors such as pH and detergents, limiting its usefulness fordeveloping a robust homogeneous ATP assay. Promega has successfullydeveloped a stable form of luciferase based on the gene from another firefly,Photuris pennsylvanica(LucPpe2), using an approach to select characteristics thatimprove performance in ATP assays. The unique characteristics of this mutant(LucPpe2m) enabled design of a homogeneous single-reagent-addition approachto perform ATP assays with cultured cells. Properties of the CellTiter-Glo®Reagent overcome the problems caused by factors, such as ATPases, thatinterfere with ATP measurement in cell extracts. The reagent is physicallyrobust and provides a sensitive and stable luminescent output.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·4.A.Overview of the CellTiter-Glo®Assay (continued)Sensitivity and Linearity:The ATP-based detection of cells is more sensitivethan other methods (8–10). In experiments performed by Promega scientists,the luminescent signal from 50HEK293 cells is greater than three standarddeviations above the background signal from serum-supplemented mediumwithout cells. There is a linear relationship (r2= 0.99) between the luminescentsignal and the number of cells from 0 to 50,000 cells per well in the 96-wellformat. The luminescence values in Figure 2 were recorded after 10minutes ofincubation at room temperature to stabilize the luminescent signal as describedin Section3.B. Incubation of the same 96-well plate used in the experimentshown in Figure 2 for 360minutes at room temperature had little effect on therelationship between luminescent signal and number of cells (r2= 0.99).Speed:The homogeneous procedure to measure ATP using the CellTiter-Glo®Assay is quicker than other ATP assay methods that require multiple steps toextract ATP and measure luminescence. The CellTiter-Glo®Assay also is fasterthan other commonly used methods to measure the number of viable cells(such as MTT, alamarBlue®or Calcein-AM) that require prolonged incubationsteps to enable the cells’ metabolic machinery to convert indicator moleculesinto a detectable signal.4.B.Additional ConsiderationsTemperature:The intensity and decay rate of the luminescent signal from theCellTiter-Glo®Assay depends on the luciferase reaction rate. Environmentalfactors that affect the luciferase reaction rate will change the intensity andstability of the luminescent signal. Temperature is one factor that affects therate of this enzymatic assay and thus the light output. For consistent results,equilibrate assay plates to a constant temperature before performing the assay.Transferring eukaryotic cells from 37°C to room temperature has little effect onATP content (5). We have demonstrated that removing cultured cells from a37°C incubator and allowing them to equilibrate to 22°C for 1–2 hours hadlittle effect on ATP content. For batch-mode processing of multiple assayplates, take precautions to ensure complete temperature equilibration. Platesremoved from a 37°C incubator and placed in tall stacks at room temperaturewill require longer equilibration than plates arranged in a single layer.Insufficient equilibration may result in a temperature gradient effect betweenwells in the center and at the edge of the plates. The temperature gradientpattern also may depend on the position of the plate in the stack.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·Chemicals:The chemical environment of the luciferase reaction affects theenzymatic rate and thus luminescence intensity. Differences in luminescenceintensity have been observed using different types of culture media and sera.The presence of phenol red in culture medium should have little impact onluminescence output. Assaying 0.1µM ATP in RPMI medium without phenolred resulted in ~5% increase in luminescence output (in relative light units[RLU]) compared to assays in RPMI containing the standard concentration ofphenol red, whereas assays in RPMI medium containing twice the normalconcentration of phenol red showed a ~2% decrease in luminescence.Solvents for the various test compounds may interfere with the luciferasereaction and thus the light output from the assay. Interference with theluciferase reaction can be detected by assaying a parallel set of control wellscontaining medium without cells. Dimethylsulfoxide (DMSO), commonly usedas a vehicle to solubilize organic chemicals, has been tested at finalconcentrations of up to 2% in the assay and only minimally affects light output.Plate Recommendations:We recommend using standard opaque-walledmultiwell plates suitable for luminescence measurements. Opaque-walledplates with clear bottoms to allow microscopic visualization of cells also maybe used; however, these plates will have diminished signal intensity andgreater cross talk between wells. Opaque white tape may be used to decreaseluminescence loss and cross talk.Cellular ATP Content:Different cell types have different amounts of ATP,and values reported for the ATP level in cells vary considerably (1,4,11–13).Factors that affect the ATP content of cells may affect the relationship betweencell number and luminescence. Anchorage-dependent cells that undergocontact inhibition at high densities may show a change in ATP content per cellat high densities, resulting in a nonlinear relationship between cell numberand luminescence. Factors that affect the cytoplasmic volume or physiology ofcells also will affect ATP content. For example, oxygen depletion is one factorknown to cause a rapid decrease in ATP (1).Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA Toll F ree in USA 800-356-9526·Phone 608-274-4330 ·F ax 608-277-2516 ·4.B.Additional Considerations (continued)Mixing:Optimal assay performance is achieved when the CellTiter-Glo®Reagent is mixed completely with the cultured cells. Suspension cell lines (e.g., Jurkat cells) generally require less mixing to achieve lysis and extract ATP than adherent cells (e.g., L929 cells). Tests were done to evaluate the effect ofshaking the plate after adding the CellTiter-Glo® Reagent. Suspension cellscultured in multiwell plates showed only minor differences in light outputwhether or not the plates were shaken after adding the CellTiter-Glo®Reagent.Adherent cells are more difficult to lyse and show a substantial differencebetween shaken and nonshaken plates.Several additional parameters related to reagent mixing include the force ofdelivery of CellTiter-Glo®Reagent, sample volume and dimensions of the well.All of these factors may affect assay performance. The degree of reagent mixing required may be affected by the method used to add the CellTiter-Glo®Reagent to the assay plates. Automated pipetting devices using a greater or lesser force of fluid delivery may affect the degree of subsequent mixing required.Complete reagent mixing in 96-well plates should be achieved using orbitalplate shaking devices built into many luminometers and the recommended2-minute shaking time. Special electromagnetic shaking devices that use aradius smaller than the well diameter may be required to efficiently mixcontents of 384-well plates. The depth of medium and geometry of themultiwell plates may have an effect on mixing efficiency. We recommend that you take these factors into consideration when performing the assay andempirically determine whether a mixing step is necessary for the individualapplication.LuminometersFor highly sensitive luminometric assays, the luminometer model and settings greatly affect the quality of data obtained. Luminometers from differentmanufacturers will vary in sensitivities and dynamic ranges. We recommend the GloMax®products because these instruments do not require gainadjustments to achieve optimal sensitivity and dynamic range. Additionally, GloMax®instruments are preloaded with Promega protocols for ease of use.If you are not using a GloMax®luminometer, consult the operating manual for your luminometer to determine the optimal settings. The limits should beverified on each instrument before analysis of experimental samples. The assay should be linear in some portion of the detection range of the instrument used.For an individual luminometer there may be different gain settings. Werecommend that you optimize the gain settings.4.C.References1.Crouch, S.P. et al.(1993) The use of ATP bioluminescence as a measure of cellproliferation and cytotoxicity. J. Immunol. Methods160, 81–8.2.Farfan, A.et al.(2004) Multiplexing homogeneous cell-based assays. Cell Notes10, 2–5.3.Riss, T., Moravec, R. and Niles, A. (2005) Selecting cell-based assays for drugdiscovery screening. Cell Notes13, 16–21.4.Kangas, L., Grönroos, M. and Nieminen, A.L. (1984) Bioluminescence of cellular ATP:A new method for evaluating cytotoxic agents in vitro. Med. Biol.62, 338–43.5.Lundin, A. et al.(1986) Estimation of biomass in growing cell lines by adenosinetriphosphate assay.Methods Enzymol. 133, 27–42.6.Sevin, B.U. et al.(1988) Application of an ATP-bioluminescence assay in human tumorchemosensitivity testing. Gynecol. Oncol.31, 191–204.7.Gerhardt, R.T.et al.(1991) Characterization of in vitro chemosensitivity ofperioperative human ovarian malignancies by adenosine triphosphatechemosensitivity assay. Am. J. Obstet. Gynecol. 165, 245–55.8.Petty, R.D. et al.(1995) Comparison of MTT and ATP-based assays for themeasurement of viable cell number. J. Biolumin. Chemilumin.10, 29–34.9.Cree, I.A. et al.(1995) Methotrexate chemosensitivity by ATP luminescence in humanleukemia cell lines and in breast cancer primary cultures: Comparison of the TCA-100assay with a clonogenic assay. AntiCancer Drugs6, 398–404.10.Maehara, Y. et al.(1987) The ATP assay is more sensitive than the succinatedehydrogenase inhibition test for predicting cell viability. Eur. J. Cancer Clin. Oncol.23, 273–6.11.Stanley, P.E. (1986) Extraction of adenosine triphosphate from microbial and somaticcells. Methods Enzymol.133, 14–22.12.Beckers, B. et al.(1986) Application of intracellular ATP determination in lymphocytesfor HLA-typing. J. Biolumin. Chemilumin.1, 47–51.13.Andreotti, P.E. et al.(1995) Chemosensitivity testing of human tumors using amicroplate adenosine triphosphate luminescence assay: Clinical correlation forcisplatin resistance of ovarian carcinoma. Cancer Res. 55, 5276–82.4.D.Related ProductsCell Proliferation ProductsProduct Size Cat.# ApoLive-Glo™ Multiplex Assay10ml G6410 ApoTox-Glo™ Triplex Assay10ml G6320 CellTiter-Fluor™ Cell Viability Assay (fluorescent)10ml G6080 CellTiter-Blue®Cell Viability Assay (resazurin)20ml G8080 CellTiter 96®AQ ueous One SolutionCell Proliferation Assay (MTS, colorimetric)200 assays G3582 CellTiter 96®AQ ueous Non-RadioactiveCell Proliferation Assay (MTS, colorimetric)1,000 assays G5421 CellTiter 96®AQ ueous MTS Reagent Powder1g G1111 CellTiter 96®Non-RadioactiveCell Proliferation Assay (MTT, colorimetric)1,000 assays G4000 Additional sizes available.Cytotoxicity AssaysProduct Size Cat.# CytoTox-Glo™ Cytotoxicity Assay (luminescent)*10ml G9290Mitochondrial ToxGlo™ Assay*10ml G8000 MultiTox-Glo Multiplex Cytotoxicity Assay(luminescent, fluorescent)*10ml G9270 MultiTox-Fluor Multiplex Cytotoxicity Assay(fluorescent)*10ml G9200 CytoTox-Fluor™ Cytotoxicity Assay (fluorescent)*10ml G9260 CytoTox-ONE™ Homogeneous MembraneIntegrity Assay (LDH, fluorometric)*200–800 assays G7890 CytoTox-ONE™ Homogeneous MembraneIntegrity Assay, HTP1,000–4,000 assays G7892 CytoTox 96® Non-Radioactive Cytotoxicity Assay1,000 assays G1780 (LDH, colorimetric)*GSH-Glo™ Glutathione Assay10ml V691150ml V6912 GSH/GSSG-Glo™ Assay10ml V661150ml V6612 *Additional sizes available.LuminometersProduct Size Cat.# GloMax®-Multi+ Detection System with Instinct™ Software:Base Instrument with Shaking 1 each E8032 GloMax®-Multi+ Detection System with Instinct™ Software:Base Instrument with Heating and Shaking 1 each E9032 GloMax®-Multi+ Luminescence Module 1 each E8041Apoptosis ProductsProduct Size Cat.# Caspase-Glo®2 Assay*10ml G0940 Caspase-Glo®6 Assay*10ml G0970 Caspase-Glo®3/7 Assay* 2.5ml G8090 Caspase-Glo®8 Assay* 2.5ml G8200 Caspase-Glo®9 Assay* 2.5ml G8210Apo-ONE®Homogeneous Caspase-3/7 Assay1ml G7792 DeadEnd™ Fluorometric TUNEL System60 reactions G3250 DeadEnd™ Colorimetric TUNEL System20 reactions G7360Anti-ACTIVE®Caspase-3 pAb50µl G7481Anti-PARP p85 Fragment pAb50µl G7341Anti-pS473Akt pAb40µl G7441 Caspase Inhibitor Z-VAD-FMK, 20mM50µl G7231125µl G7232*Additional sizes available.(a)U.S. Pat. Nos. 6,602,677 and 7,241,584, European Pat. No. 1131441, Japanese Pat. Nos. 4537573 and 4520084 and other patents pending(b)U.S. Pat. No. 7,741,067, Japanese Pat. No. 4485470 and other patents pending.(c)U.S. Pat. No. 7,700,310, European Pat. No. 1546374 and other patents pending.(d)U.S. Pat. Nos 7,083,911, 7,452,663 and 7,732,128, European Pat. No. 1383914 and Japanese Pat. Nos. 4125600 and 4275715.(e)The method of recombinant expression of Coleoptera luciferase is covered by U.S. Pat. Nos. 5,583,024, 5,674,713 and 5,700,673.© 2001–2012 Promega Corporation. All Rights Reserved.Anti-ACTIVE, Apo-ONE, Caspase-Glo, CellTiter 96, CellTiter-Blue, CellTiter-Glo, CytoTox 96 and GloMax are registered trademarks of Promega Corporation. ApoTox-Glo, ApoLive-Glo, CellTiter-Fluor, CytoTox-Fluor, CytoTox-Glo, CytoTox-ONE, DeadEnd, GSH-Glo, GSH/GSSG-Glo, Instinct, Mitochondrial ToxGlo and Ultra-Glo are trademarks of Promega Corporation. alamarBlue is a registered trademark of Trek Diagnostic Ssystems, Inc.Products may be covered by pending or issued patents or may have certain limitations. Please visit our Web site for more information.All prices and specifications are subject to change without prior notice.Product claims are subject to change. Please contact Promega Technical Services or access the Promega online catalog for the most up-to-date information on Promega products.。
美洲大蠊抗肝纤维化活性提取物ML-D中粘多糖的含量测定

f r o m P e r i pl a n e t a a me r i c a i r a .M e t h o d s :P h e n o l — v i t i r o l c o l o r i me t y r wa s u s e d f o r d e t e mi r n i n g c o n t e n t o f y c o s a mi n o y c a n ;o r t h o g o n a l
美洲大蠊抗肝纤维化活性提取物M L — D 中粘多糖的含量测定
李夸巧 , 李春艳 , 杨 永寿 , 陈丽娜 , 肖培云
( 大理 学院 药学与 化 学 学院 , 云 南 大理 6 7 1 0 0 0 )
[ 摘要 ]目的 : 建立美洲大蠊抗肝纤维化活性提 取物ML — D中粘 多糖的含量测定方法 。方法 : 采用苯酚一 硫酸法测定粘多糖的含 量, 采用 正交试验设计考察ML — D中粘 多糖 的水解工 艺和 显色条件 , 采用单 因素试验考察加热显色时间、 苯酚和硫酸的用量。结
d e s i g n k( 3 4 ) w a s a d o p t e d t o i n v e s t i g a t e h y d r o l y s i s p r o c e s s o f l g y c o s a mi n o g l y c a n i n ML — D ; s i n g l e f a c t o r e x p e i r m e n t w a s u s e d t o i n v e s t i g a t e
dammarane达玛烷-生物化学与分子生物学

Dactinomycin 放线菌素Ddammarane type 达玛烷型dammarane 达玛烷dampening of charge effects 电荷效应的衰减Dane particle 丹氏粒[乙型肝炎病毒的完整毒粒] dansyl 丹(磺)酰,1-二甲氨基萘-5-磺酰dansyl chloride 丹磺酰氯dansyl method 丹磺酰法dantrolene 硝苯呋海因[肌肉松弛剂]dapsone 氨苯砜dark adaptation 暗适应dark current 暗电流dark field microscope 暗视野显微镜,暗视场显微镜dark field microscopy 暗视野显微术,暗视场显微术dark field 暗视野,暗视场dark reaction 暗反应dark repair 暗修复dark respiration 暗呼吸dark room 暗室,暗房dark seed 需暗种子Darwinian selection 达尔文选择DAS 下游激活部位data accumulation 数据积累data acquisition 数据获取data analysis 数据分析data bank 数据库data base 数据库data handling 数据处理data logger 数据记录器data logging 数据记录data output 数据输出data processing 数据处理data recording 数据记录dative bond 配位键dative covalent bond 配位共价键dauermodification 持续饰变daughter cell 子代细胞daughter chromatid 子染色单体daughter chromosome 子染色体daughter colony 子菌落[由原生菌落续发生长的小菌落] daunomycin 道诺霉素daunorubicin 道诺红菌素day vision 昼视觉,昼视力de novo sequencing 从头测序de novo synthesis 从头合成deactivation 去活化(作用),失活(作用),钝化deacylated tRNA 脱酰tRNAdead time 死时间dead volume 死体积deadenylation 脱腺苷化deadhesion factor 去黏附因子DEAE Sephacel [商]DEAE-葡聚糖纤维素,二乙氨乙基葡聚糖纤维素DEAE-cellulose DEAE-纤维素DEAE-dextran DEAE-葡聚糖dealkylation 脱烷基化deaminase 脱氨酶deamination 脱氨(基)death ligand 死亡配体death phase 死亡期[如见于细胞生长曲线]death point 死点deblocking 去封闭debranching enzyme 脱支酶,支链淀粉酶debris 碎片,残渣decahedron 十面体decamer 十体decamethonium 十烷双胺,癸烷双胺decane 癸烷decanoates 癸酸decantation 倾析decanting 倾析decapacitation 去(获)能decapsidate 脱衣壳decarboxylase 脱羧酶decarboxylation 脱羧(作用)decatenation 解连环{作用}decay accelerating factor 衰变加速因子decay chain 衰变链decay constant 衰变常数decay 原因不明腐败deceleration phase 减速期[如见于细胞生长曲线]dechlorination 脱氯作用deciduous leaf 落叶decline phase [细胞生长曲线的]衰亡期decoagulant 抗凝剂decoding 译码,解码decoginine 德夸菌素decomposer 分解者[可指具有分解动植物残体或其排泄物能力的微生物]decompression 降压,减压decondensation 解凝(聚)decontaminant 净化剂,去污剂decontaminating agent 净化剂,去污剂decontamination 净化,去污decorin 核心蛋白聚糖[一种基质蛋白聚糖,又称为PG-40] decoy receptor 诱杀受体decoy 诱杀物dedifferentiation 去分化,脱分化deep colony 深层菌落deep etching 深度蚀刻deep jet fermentor 深部喷注发酵罐deep refrigeration 深度冷冻deep shaft system 深井系统[如用于污水处理] defasciculation factor 解束因子[取自水蛭,可破坏神经束] defective interfering particle 缺损干扰颗粒,干扰缺损颗粒defective interfering RNA 缺损干扰RNAdefective interfering virus 缺损干扰病毒defective interfering 缺损干扰defective mutant 缺损突变体,缺陷突变型,缺陷突变株defective phage 缺损噬菌体,缺陷噬菌体defective virus 缺损病毒,缺陷病毒defective 缺损的,缺陷的defense peptide 防卫肽defense response 防御反应,防卫反应defense 防御,防卫defensin 防卫素[动物细胞的内源性抗菌肽]deficiency 缺乏,缺损,缺陷deficient 缺少的,缺损的,缺陷的defined medium 确定成分培养基,已知成分培养液defined 确定的defintion 定义defoliating agent 脱叶剂defoliation 脱叶deformylase 去甲酰酶[见于原核细胞,作用于甲酰甲硫氨酸] degasser 脱气装置degassing 脱气,除气degeneracy 简并;简并性,简并度degenerate codon 简并密码子degenerate oligonucleotide 简并寡核苷酸degenerate primer 简并引物degenerate sequence 简并序列degenerate 简并的degeneration 退化,变性degenerin 退化蛋白[与某些感觉神经元的退化有关] deglycosylation 去糖基化degradable polymer 降解性高分子degradation 降解degranulation 脱(颗)粒(作用)degree of acidity 酸度degree of dominance 显性度degree of polymerization 聚合度degron 降解决定子[决定某一蛋白发生降解或部分降解的序列要素] deguelin 鱼藤素dehalogenation 脱卤(作用)dehardening 解除锻炼dehumidifier 除湿器dehydratase 脱水酶dehydrated medium 干燥培养基dehydration 脱水(作用)dehydroepiandrosterone 脱氢表雄酮dehydrogenase 脱氢酶dehydrogenation 脱氢(作用)dehydroluciferin 脱氢萤光素deionization 去离子(作用)deionized water 去离子水deionized 去离子的deionizing 去离子(处理)delayed early transcription (延)迟早期转录[可特指病毒]delayed fluorescence 延迟荧光delayed heat 延迟热delayed hypersensitivity 延迟(型)超敏反应delayed ingeritance 延迟遗传delayed type hypersensitivity 迟发型超敏反应deletant 缺失体deletion mapping 缺失定位,缺失作图deletion mutagenesis 缺失诱变deletion mutant 缺失突变体deletion mutantion 缺失突变deletion 缺失deletional recombination 缺失重组delignification 脱木质化(作用)deliquescence 潮解delivery flask 分液瓶delocalized bond 离域键deltorphin delta啡肽[见于叶泡蛙皮肤,对delta阿片样肽受体的亲和力和选择性很强]demasking 解蔽demethylation 脱甲基化demineralized water 脱矿质水demulsifier 破乳剂demyelination 脱髓鞘denaturant gel 变性凝胶denaturant 变性剂denaturation 变性(作用)denatured DNA 变性DNAdenatured protein 变性蛋白(质)denatured 变性的denaturing gel electrophoresis 变性凝胶电泳denaturing gel 变性凝胶denaturing gradient polyacrylamide gel 变性梯度聚丙烯酰胺凝胶denaturing polyacrylamide gel 变性聚丙烯酰胺凝胶dendrite 树突dendritic cell 树状细胞dendrotoxin 树眼睛蛇毒素dengue virus 登革病毒denitrification 反硝化作用denitrifying bacteria 反硝化细菌denitrogen 排氮densimeter 密度计densitometer 光密度计densitometry 光密度(测定)法density gradient centrifugation 密度梯度离心density gradient electrophoresis 密度梯度电泳density gradient 密度梯度density 密度densovirus 浓核病毒dentritic cell 树枝状细胞Denver system [染色体组型]丹佛体制deoxyadenosine moniphosphate 脱氧腺苷(一磷)酸deoxyadenosine triphosphate 脱氧腺苷三磷酸deoxyadenosine 脱氧腺苷deoxyadenylate 脱氧腺苷酸deoxyadenylic acid 脱氧腺苷酸deoxycholate 脱氧胆酸盐deoxycorticosterone 脱氧皮质酮deoxycytidine monophosphate 脱氧胞苷(一磷)酸deoxycytidine triphosphate 脱氧胞苷三磷酸deoxycytidine 脱氧胞苷deoxycytidylate 脱氧胞苷酸deoxycytidylic acid 脱氧胞苷酸deoxyformycin 脱氧型霉素deoxygenation 脱氧deoxyglucose 脱氧葡萄糖deoxyguanosine monophosphate 脱氧鸟苷(一磷)酸deoxyguanosine triphosphate 脱氧鸟苷三磷酸deoxyguanosine 脱氧鸟苷deoxyguanylate 脱氧鸟苷酸deoxyguanylic acid 脱氧鸟苷酸deoxyhemoglobin 脱氧血红蛋白,去氧血红蛋白deoxyinosine triphosphate 脱氧肌苷三磷酸,脱氧次黄苷三磷酸deoxyinosine 脱氧肌苷,脱氧次黄苷deoxynivalenol 脱氧萎镰菌醇deoxynucleoside methylphosphonate 脱氧核苷膦酸甲酯deoxynucleoside phosphoramidite 脱氧核苷亚磷酰胺deoxynucleoside 脱氧核苷deoxynucleotide 脱氧核苷酸deoxyriboaldolase 脱氧核糖醛缩酶deoxyribomutase 脱氧核糖变位酶deoxyribonuclease 脱氧核糖核酸酶,DNA酶[包括DNA酶I、DNA酶II等] deoxyribonucleic acid 脱氧核糖核酸deoxyribonucleoside diphosphate 脱氧(核糖)核苷二磷酸deoxyribonucleoside hydrogenphosphonate 脱氧(核糖)核苷氢膦酸酯deoxyribonucleoside methylphosphonate 脱氧(核糖)核苷膦酸甲酯deoxyribonucleoside monophosphate 脱氧(核糖)核苷(一磷)酸deoxyribonucleoside phosphoramidite 脱氧(核糖)核苷亚磷酰胺deoxyribonucleoside triphosphate 脱氧(核糖)核苷三磷酸deoxyribonucleoside 脱氧(核糖)核苷deoxyribonucleotide 脱氧(核糖)核苷酸deoxyribose 脱氧核糖deoxysugar 脱氧糖deoxythymidine monophosphate 脱氧胸苷(一磷)酸deoxythymidine triphosphate 脱氧胸苷三磷酸deoxythymidine 脱氧胸腺嘧啶核苷,脱氧胸苷deoxythymidylate 脱氧胸苷酸deoxythymidylic acid 脱氧胸苷酸deoxyuridine monophosphate 脱氧尿苷(一磷)酸deoxyuridine 脱氧尿苷deoxyuridylate 脱氧尿苷酸deoxyuridylic acid 脱氧尿苷酸depactin [肌动蛋白]蚕食蛋白dependovirus 依赖病毒(属)[模式成员是腺伴随病毒] dephosphorylation 去磷酸化(作用)deplasmolysis 质壁分离复原depletion 耗竭,除尽depolarization 去极化,脱极化;解偏振(作用)depollution 去污染depolymerase 解聚酶depolymerization 解聚deposit 沉积;沉积物deposition 沉积(作用);沉积物depressed 脱阻抑的depression 脱阻抑;压抑deprivation 除去,丧失deprived 除去的,丧失的depropagation 负增长反应,逆增长反应deprotection 脱保护deprotonation 去质子化,脱质子化depurination 脱嘌呤(作用)derepressed 脱阻抑的derepression 脱阻抑derivative 衍生物derivatization 衍生化(作用)derivave spectrum 导数光谱dermatan sulfate 硫酸皮肤素dermatogen 表皮原dermatoglyphics 肤纹学dermatonecrotoxin 皮肤坏死毒素dermatophagoides 嗜皮螨属dermenkaphaline 皮脑啡肽dermis 真皮dermorphin 皮啡肽desalting 脱盐desaminase 脱氨酶desaturase 脱饱和酶,脱氢酶desaturation 脱饱和,去饱和descending development 下行展开(法)descending facilitatory system 下行易化系统[用于神经生物学]descending inhibition 下行抑制descending inhibitory system 下行抑制系统desensitization 脱敏(作用)desetope (决定簇)选择位[抗原呈递中,II类主要组织相容性复合体与抗原相互作用的部位] desiccant 干燥剂desiccation 干燥(作用)desiccator cabinet 干燥橱desiccator 干燥器,保干器desmin 结蛋白desmocalmin 桥粒钙调(蛋白)结合蛋白,桥粒钙蛋白desmocollin 桥粒(芯)胶(粘)蛋白[见于桥粒芯的胶粘层,是桥粒芯蛋白的剪接变体] desmoglea 桥粒芯[桥粒的中央核心层]desmoglein 桥粒芯(糖)蛋白[一类跨膜蛋白,见于桥粒芯]desmolase 碳链酶desmoplakin 桥粒斑蛋白desmosine 锁链素[由四个赖氨酸侧链形成的交联体,见于弹性蛋白]desmosome plaque 桥粒斑desmosome 桥粒desmotubule 连丝微管desmoyokin 桥粒联结蛋白[见于桥粒斑]desolvation 去溶剂化desorption ionization 解析电离desorption 解析(作用),脱附(作用)destabilization 去稳定作用destabilizing agent 去稳定剂destaining 脱色destomycin 越霉素desulfurization 脱硫(作用)desynchronization 去同步化detachment 解析(作用),脱附(作用detection limit 检出限detection 检测,检出;观察detector 检测器detention time 滞留时间detention 滞留detergent 去污剂,除垢剂,洗涤剂deterioration (生物)致劣,恶化determinant 决定簇;决定子[用于胚胎学];决定因素determination limit 测定限determination 测定detoxication 解毒detoxification 解毒detritus 碎屑;腐屑detritylation 脱(除)三苯甲基deuteromycetes 半知菌纲deutertion 氘化deutoplasm 滋养层,卵黄质developing solvent 展开剂developing tank 展开槽development folder 显影夹developmental biology 发育生物学developmental genetics 发育遗传学developmental malformation 发育畸形developmental phase 发育期developmental regulation 发育调节,发育调控developmental rhythm 发育节律developmental stage 发育阶段developmentally regulated expression 由发育(所)调节的表达devernalization 脱春化dexamethasone 地塞米松,9-alpha-氟-16-甲基脱氢皮质醇dextran bead 葡聚糖珠dextran sulfate 葡聚糖硫酸酯dextran 葡聚糖,右旋糖酐dextranase 葡聚糖酶dextrin 糊精dextroisomer 右旋异构体dextrorotary 右旋的dextrorotatory 右旋的dextrose 葡聚糖diabetes mellitus 糖尿病diacetoxyscirpenol 蛇形菌素diacylglycerol 二酰甘油diad 二分体,二联体diadric 雄异配(性)的diafiltration 渗滤[溶液通过滤器进行连续循环] diagnostic enzyme 诊断酶diagnostic kit 诊断试剂盒diagnostic procedure 诊断程序,诊断手续diagnostic reagent 诊断试剂diagnostics 诊断学diagonal chromatography 对角线层析diagonal electrophoresis 对角线电泳diagonal 对角线;对角线的diagynic 雌异配(性)的diakinesis 终变期dialkylglycine decarboxylase 二烷基甘氨酸脱羧酶dialkylglycine 二烷基甘氨酸diallel cross 双列杂交[用于进化遗传学] dialysate 透析物,透析液dialysis apparatus 透析仪,透析装置dialysis bag 透析袋dialysis cultivation 透析培养dialysis fermentation 透析发酵dialysis membrane 透析膜dialysis tube 透析袋dialysis tubing 透析袋,透析管dialysis 透析dialyzable 可透析的dialyzate 透析液dialyzator 透析仪diamagnetic compound 抗磁化合物diamagnetism 抗磁性diaminedichloroplatinum 二胺二氯铂diaminobenzidine 二氨基联苯胺diaminopimelate 二氨基庚二酸,二氨基庚二酸盐、酯、根diaminopimelic acid 二氨基庚二酸dianisidine 邻联茴香胺dianthovirus 香石竹病毒组[一组植物病毒,模式成员是香石竹环斑病毒]diapause hormone 滞育激素diapedesis 血细胞渗出[血细胞从血管内渗出]diaphragm 隔膜diarrhea 腹泻diastase 淀粉酶制剂diastereomer 非对映(异构)体diastereotopic 非对映异位的[在分子整体中,碳原子上互为非对映关系的原子、基团或面]diastole 心舒期diatomaceous earth 硅藻土diatrizoate 3,5-双(乙酰氨基)-2,4,6-三碘苯甲酸(盐)diauxie growth curve 双峰生长曲线diazo compound 重氮化合物diazo 重氮基diazoacridine 重氮吖啶diazobenzyloxymethyl paper 重氮苄氧甲基纸,DBM纸diazonorleucine 重氮基正亮氨酸diazophenylthio paper 重氮苯硫醚纸,DPT纸diazotization 重氮化diazouridine 重氮尿苷dibucaine 狄步卡因dicarboxyl cellulose 二羧基纤维素dicarboxylic acid 二羧酸dicarboxylic amino acid 二羧基氨基酸dicentric chromosome 双着丝染色体dichlorodimethylsilane 二氯二甲硅烷dichlorofluorescein 二氯荧光黄dichloromethane 二氯甲烷dichlorovos 敌敌畏dichogamy 雌雄(蕊)异熟dichroism 二色性Dick test 狄克试验[链球菌红斑毒素的皮肤试验] dicotyledons 双子叶植物dicoumarin 双羟香豆素,败坏翘摇素dictyosome (分散)高尔基体dictyostelium 盘基网柄菌属,网柄菌属dicyclohexylcarbodiimide 二环己基碳二亚胺[常用缩合剂] didanosine [商]2',3’-双脱氧肌苷didehydrothymidine 双脱氢胸苷dideoxy sequencing method 双脱氧测序法dideoxyadenosine triphosphate 双脱氧腺苷三磷酸dideoxycytidine triphosphate 双脱氧胞苷三磷酸dideoxycytidine 双脱氧胞苷dideoxyguanosine triphosphate 双脱氧鸟苷三磷酸dideoxyguanosine 双脱氧鸟苷dideoxyinosine 双脱氧肌苷dideoxyribonucleoside triphosphate 双脱氧核苷三磷酸dideoxyribonucleoside 双脱氧核苷dideoxythymidine triphosphate 双脱氧胸苷三磷酸dielectric constant 介电常数dielectric effect 介电效应dielectrometric titration 介电(常数)滴定(法)dielectrometry 介电(常数)滴定(法)dielectrophoresis 介电(电)泳diene 双烯diethyl pyrocarbonate 焦碳酸二乙酯diethyl sulfate 硫酸二乙酯diethylstilbestrol 乙酚,二乙基己烯雌酚difference spectrum 差光谱differential (示)差的,鉴别的;微分;微分的differential analysis 示差分析differential centrifugation 差速离心differential detection 示差检测,鉴别检测differential expression 差异表达differential flotation centrifugation 差速浮式离心differential hybridization 示差杂交(法)differential medium 鉴别培养基differential operation 示差操作differential permeability 差别透性,选择透性differential precipitation 示差沉淀differential refractive index detector 示差折光率检测器differential scattering 差散射differential screening 示差筛选differential sedimentation 差速沉降differential sepctrophotometry 示差分光光度法differential species 区别种differential spectrum (示)差光谱differential staining technique 鉴别染色技术[有时特指染色体显带技术] differential staining 鉴别染色(法)differential type detector 微分型检测仪differentiating solvent 鉴别剂,区分溶剂differentiation antigen 分化抗原differentiation center 分化中心differentiation phase 分化时differentiation 分化diffraction grating 衍射光栅diffraction symmetry 衍射对称性diffraction 衍射diffuser 扩散器;洗料器;[发酵罐]进气装置diffusion chamber 扩散盒,扩散小室diffusion coefficient 扩散系数diffusion controlled reaction 扩散控制(的)反应diffusion controlled termination 扩散控制的终止diffusion 扩散diffusional limitation 扩散限制diffusional resistance 扩散阻力diformazan 二甲digalactosyl diglyceride 双半乳糖甘油二酯digester 消化器,消化罐digestion 消化,(酶切)消化digestive enzyme 消化酶digital control 数字控制digital imaging microscope 数字成像显微镜digital imaging microscopy 数字成像显微术digitalis cardiac glycoside 毛地黄(类)强心苷digitalizer 数字化仪[用于计算机科学]digitonin 毛地黄皂苷digitoxigenin 毛地黄毒苷配基;beta-(丁烯酸内酯)-14-羟甾醇digitoxin 毛地黄毒苷diglyceride 甘油二酯digoxigenin 洋地黄毒苷,地高辛配基digoxin 异羟基洋地黄毒苷原,地高辛dihaploid 双单倍体dihedral angle 二面角,双面角dihydrobiopterin 二氢生物蝶呤dihydrochalcone 双氢查耳酮,二氢查耳酮dihydrofolate reductase 二氢叶酸还原酶dihydrofolate 二氢叶酸dihydrolipoamide dehydrogenase 二氢硫辛酰胺脱氢酶dihydrolipoamide 二氢硫辛酰胺dihydrolipoic acid 二氢硫辛酸dihydroorotase 二氢乳清酸酶dihydroorotate 二氢乳清酸dihydropteridine reductase 二氢蝶啶还原酶dihydropteridine 二氢蝶啶dihydropyridine 二氢吡啶dihydrotestosterone 双氢睾酮dihydrouracil arm 二氢尿嘧啶臂dihydrouracil loop 二氢尿嘧啶环dihydrouracil 二氢尿嘧啶dihydrouridine 二氢尿苷dihydroxyacetone phosphate 二氢丙酮磷酸dihydroxycholecalciferol 二羟胆钙化(固)醇dihydroxyphenylanaline 二羟苯丙氨酸,多巴dihydroxyphenylethylamine 二羟苯基乙胺,羟酪胺,多巴胺diisopropylfluorophosphate 二异丙基氟磷酸dikaryon 双核体diltiazem 硫氮酮diluent 稀释剂,稀释液dilution cloning 稀释克隆法[如用于获得细胞克隆株] dimensional electrophoresis 双向电泳dimer 二聚体dimerization cofactor 二聚化辅因子dimerization 二聚化dimethoxytrityl 二甲氧三苯甲基[在DNA合成中用作羟基保护剂] dimethyl sulfate 硫酸二甲酯dimethyl sulfoxide 二甲基亚砜dimethylallylpyrophosphate 二甲(基)烯丙基焦磷酸dimethylaminoazobenzene 二甲基氨基偶氮苯dimethylformamide 二甲基甲酰胺dimorphism 二态二氢,双态现象dinitro benzene 二硝基苯dinitrochlorobenzene 二硝基氯苯dinitrofluorobenzene 二硝基氟苯dinitrogen 双氮,分子氮dinitrogenase reductase 固氮酶还原酶dinitrogenase 固氮酶dinitrophenol 二硝基苯酚dinitrophenyl 二硝基苯基dinoflagellate 甲藻dinoxanthine 甲藻黄素dinucleotide frequency 二核苷酸频率dinucletide 二核苷酸diodeelectrode 二极管电极dioecism 雌雄异体,雌雄异株diosgenin 薯蓣皂苷配基,薯蓣皂苷元dioxide 二氧化物dioxygen 双氧dioxygenase 双加氧酶dipeptidase 二肽酶dipeptide 二肽diphenylamine blue 二苯胺蓝diphenyloxazole 二苯基唑diphosphate 二磷酸diphosphatidylglycerol 双磷脂酰甘油diphosphoglycerate shunt 二磷酸甘油酸支路diphosphoglycerate 二磷酸甘油酸diphosphoinositide 二磷酸肌醇磷脂,磷脂酰肌醇磷酸diphthamide 白喉酰胺diphthera toxin 白喉毒素dipicolinic acid 2,6-吡啶二羧酸diplobacillus 双杆菌diploblastic 双胚层的diplococcus pneumoniae 肺炎双球菌diplococcus 双球菌diploid cell line 二倍体细胞系diploid 二倍体diploidization 二倍化diploidy 二倍性diplonema 双线期diplotene stage 双线期dipolar aprotic solvent 偶极非质子溶剂dipolar protophilic solvent 偶极亲质子溶剂dipolar protophobic solvent 极疏质子溶剂dipole molecule 偶极分子dipole moment 偶极矩dipole 偶极dipyrromethane 联吡咯甲烷direct cross 正交direct duplication 同向重复direct insertion 同向插入direct repeat 同向重复(序列)direct selection 正选择[使用只有突变体或重组体能生长的条件进行选择] directed cloning 定向克隆directed mutagenesis 定向诱变directed perturbation 定向微扰directed sequencing 定向测序direction selectivity 方向选择性directional cloning 定向克隆disaccharide 二糖disassembly 解装配,分解disc electrophoresis 圆盘电泳disc gel electrophoresis 圆盘凝胶电泳disc membrane 圆盘膜discharge 放电;卸下discoidal cleavage 盘状卵裂discontinuous epitope 非连续表位discontinuous gradient 不连续梯度discontinuous replication 不连续复制discontinuous variation 不连续变异discontinuous zone electrophoresis 不连续区带电泳discrete 分立的,不连续的discriminant analysis 判别分析discrimination 辨别,判别disease association 疾病相关disease resistance 抗病性dish 平皿disinfectant 消毒剂disinfection 消毒disinfestation 灭虫disinhibition 去抑制disintegration 蜕变,衰变;去整合,解整合;分解,破碎disintegrator 粉碎器,粉碎机disjunction 分离disk centrifuge 圆盘(式)离心机[带有成叠的有孔圆盘,常用于溶液的澄清化处理] dislocation 脱位,转位,位错dismutase 岐化酶disome 二体,双体disordered state 无序状态disordered structure 无序结构dispase 分散酶,中性蛋白酶[用于分散组织培养中的动物细胞]dispenser 分液器dispermy 双精人卵dispersant 分散剂disperse medium 分散介质disperse phase 分散相disperse system 分散系统dispersion force 分散力;色散力dispersion spectrum 色散谱dispersion 分散;色散displaced loop 替代环displacement analysis 顶替(分析)法displacement chromatography 顶替层析displacement electrophoresis 顶替电泳displacement loop 替代环,D环[形如英文字母D] displacement reaction 置换反应displacement 顶替,替代,置换disposable glove 一次性手套disposable microcentrifuge tube 一次性(使用的)微量离心管disposable tip 一次性(使用的)吸头disproportionation 岐化(反应)disrotatory 对旋disruption 破裂,破坏dissecting microscope 解剖显微镜dissection 解剖,剖分disseminated intravascular coagulation 弥漫性血管内凝血dissimilation 异化(作用)dissociation constant 解离常数dissociation 解离,离解dissolvability 溶(解)度,(可)溶(解)性dissolvant 溶剂distamycin 偏端霉素distance receptor 距离感受器distant hybirdization 远缘杂交distant hybrid 远缘杂种distorted peak 畸峰diterpene 双萜,二萜dithioerythritol 二硫赤藓糖醇dithiothreitol 二硫苏糖醇divergence 分散[用于神经系统];趋异divergent 趋异进化diverse ion effect 异离子效应diversity gene D基因[为D区编码的基因]diversity region 多变区,D区[免疫球蛋白等分子重链的一个高变区] divinylbenzene 二乙烯苯dizygotic twins 二卵双生,异卵双生DNA adduct DNA加合物DNA amplification in vitro DNA体外扩增DNA amplification polymorphism DNA扩增多态性DNA bending DNA转折,DNA弯曲DNA blotting DNA印迹(法)DNA catenation DNA连环DNA circle DNA环[指环状DNA]DNA cleavage DNA裂解,DNA切割DNA cloning DNA克隆(化)DNA jumping technique DNA跳查技术DNA ladder DNA梯[如大小不同的标准参照物的电泳谱]DNA nicking DNA切口形成DNA pitch DNA螺距DNA sizing DNA大小筛分DNA sizing gene DNA大小决定基因[如见于噬菌体,可决定所包装的DNA量]DNA typing DNA分型DNAase DNA酶DNAase I footprinting DNA酶足迹法docking protein 船坞蛋白,停靠蛋白[内质网上与信号识别颗粒相互作用从而使蛋白质继续翻译的蛋白]docking 停靠dodecahedron 十二面体dodecane 十二烷dodecapeptide motif 十二肽基序dodecyl 十二烷基dolichol 多萜醇,长醇domain assmbly 结构域装配domain deletion 结构域删除domain substitute 结构域置换domain 域,区域,结构域,功能域dominance variance 显性方差dominance 显性;优势(度)dominant acting gene 显性开放基因dominant allele 显性等位基因dominant gene 显性基因dominant hemisphere 优势半球dominant interference 显性干涉dominant lethal 显性致死dominant mutation 显性突变dominant negative mutant 显性失活突变体dominant negative 显性阴性的,显性失活的dominant oncogenic 显性致癌的dominant 显性的,优势的Donnan dialysis 唐南透析Donnan equilibrium 唐南平衡Donnan potential 唐南膜电势donor splicing site 剪接供体donor 供体,给体dopamine 多巴胺dosage compensation 计量补偿(效应)dot blot 斑点印迹,斑点印迹膜dot blotting 点渍法,斑点印迹(法)dot hybridization 斑点杂交dotting 打点,打点杂交double antibody method 双抗体法[免疫测定方法之一种]double balloon catheter 双气囊导管double bar 重棒眼,双棒眼,超棒眼[黑腹果蝇唾液腺染色体的X染色体上16A区段重复三次而出现的特殊表型]double beam mass spectrometer 双束质谱仪double beam spectrophotometer 双光束分光光度计double blind trial 双盲试验double bond migration 双键移位double coupling method 双偶联法,双偶合法double decomposition reaction 复分解double exchange 双交换double fertilization 双受精double focusing mass spectrometer 双聚焦质谱仪double focusing 双聚焦double helix 双螺旋double immunodiffusion 双向免疫扩散,免疫双扩散double innervation 双重神经支配double labeling 双重标记double minute chromosome 双微染色体[所携带基因得到扩增的成对额外小染色体] double recessive 双隐性double resonance 双共振doublet 双联体;双峰doubling time 倍增时间[培养物的生物质翻一番所需的时间]Dower resin Dower树脂[陶氏化学公司离子交换层析介质商品名]down promoter mutation 启动子减效突变downflow fixed bed 下流固定床doxorubicin 阿霉素drift (遗传)漂变drilling mud 钻探泥浆drinking center 饮水中枢drop method 点滴法droplet countercurrent chromatography 液滴反流层析,液滴逆流层析dropping mercury electrode 滴汞电极Drosophila 果蝇属drug susceptibility 药物敏感性drug targeting 药物寻靶,药物导向Duchenne muscular dystrophy Duchenne型肌营养不良,假肥大型肌营养不良duocrinin 促十二指肠液素duplex 双链体;双螺旋;二显性组合duplicon 重复子duramycin 耐久霉素dwarf colony 侏儒型菌落dwarf plant 矮化植物[由遗传因素决定不能长高];矮生植物[由认为措施或特殊环境决定不能长高]dyad symmetry 二重对称dyad 二分体,二联体dye exclusion test 染料排斥试验[用于检查细胞生活力]dynactin 动力蛋白激活蛋白dynamin 发动蛋白dynein arm 动力蛋白臂dynein 动力蛋白dynorphin 强啡肽dysbacteria 菌群失调dysbacteriosis 菌群失调dysentery bacillus 痢疾杆菌dysfunction 功能异常,机能障碍dysregulation 调节异常dystroglycan (肌)营养不良(蛋白)聚糖[与肌)营养不良蛋白相关的蛋白聚糖] dystrophin (肌)营养不良蛋白。
高效液相色谱法测定吲哚美辛控释胶囊的血药浓度和生物利用度

高效液相色谱法测定吲哚美辛控释胶囊的血药浓度和生物利用
度
张丹;曾经泽;边巴仓决
【期刊名称】《色谱》
【年(卷),期】1997(15)6
【摘要】采用ODS柱,甲醇-知磷酸溶液为流动相,260nm为检测波长,建立了测定血浆中吲哚美辛浓度的高效液相色谱法,并测定了吲哚美辛控释胶囊炎痛康的血药浓度。
结果表明,血浆中哚美辛浓度在0.125-5.0mg/L范围内线性关系良好,检测限62.5μg/L,
【总页数】1页(P515)
【作者】张丹;曾经泽;边巴仓决
【作者单位】华西医科大学药学院;华西医科大学药学院
【正文语种】中文
【中图分类】R971.1
【相关文献】
1.高效液相色谱法测定龙胆风湿胶囊中吲哚美辛和吡罗昔康的含量 [J], 符洪;鲁秋红
2.高效液相色谱法测定氨糖美辛肠溶片中吲哚美辛含量 [J], 逯小萌
3.高效液相色谱法测定人血清中阿西美辛和吲哚美辛 [J], 胡玉钦;刘会臣;马锐
4.吲哚美辛缓释胶囊多剂量达稳态时人体药动学与相对生物利用度 [J], 赵语;陈钧;
蒋学华
5.吲哚美辛控释制剂的相对生物利用度 [J], 吴苏澄;姜云平;钟诗龙;曾仁杰;蒋学华因版权原因,仅展示原文概要,查看原文内容请购买。
丹参素钠大孔树脂吸附分离过程的近红外在线监测研究

丹参素钠大孔树脂吸附分离过程的近红外在线监测研究马晋芳;毕昌琼;欧邦露;肖雪;王雪利;葛发欢【期刊名称】《世界科学技术-中医药现代化》【年(卷),期】2018(020)005【摘要】目的:利用近红外光谱技术,以丹参为原料制备目标成分的大孔树脂吸附分离中试过程为例,在线监测其中丹参素钠的含量变化,为后期丹参素大生产过程的在线质量监控提供一种新方法.方法:采用透射模式在线采集大孔树脂吸附分离中试过程中水洗阶段的近红外光谱,同时收集水洗液,用高效液相色谱法测定其中丹参素钠的含量,结合偏最小二乘法建立大孔树脂吸附分离阶段丹参素钠的定量校正模型,并应用于中试生产在线过程中.结果:采用二阶卷积求导的光谱预处理方法,选取833~1 165 nm和1 498~1 890 nm波段,建立丹参素钠定量校正模型,相关系数为0.8473,SECV为0.1776,可对中试过程中的洗脱起点和终点进行准确判断,对洗脱过程中高浓度的丹参素钠可以进行准确定量.结论:建立的定量模型具有良好的应用效果,且高效、快速地用于生产过程中丹参素钠的在线检测和质量控制,为后期丹参相关制剂大生产中的在线监控提供一种新方法.【总页数】8页(P621-628)【作者】马晋芳;毕昌琼;欧邦露;肖雪;王雪利;葛发欢【作者单位】中山大学药学院广州510006;中山大学南沙研究院广州511458;广东省中药超临界流体萃取工程技术研究中心广州510006;贵州景峰注射剂有限公司贵阳550018;贵州景峰注射剂有限公司贵阳550018;广东药科大学广州510006;中山大学南沙研究院广州511458;广东省中药超临界流体萃取工程技术研究中心广州510006;中山大学药学院广州510006;中山大学南沙研究院广州511458;广东省中药超临界流体萃取工程技术研究中心广州510006【正文语种】中文【中图分类】R93【相关文献】1.Bagging-PLS的黄柏中试提取过程在线近红外质量监测研究 [J], 周正;吴志生;史新元;王佳茜;乔延江2.近红外光谱技术在线监测积雪草药材活性成分的大孔树脂分离纯化过程 [J], 刘桦;叶晓岚;杨光;亓云鹏;范国荣3.人参叶总皂苷大孔树脂分离纯化工艺的近红外光谱在线监测模型及其含量测定[J], 刘桦;赵鑫;齐天;亓云鹏;范国荣4.基于近红外光谱技术的白酒酒醅在线监测研究 [J], 周新奇;郑启伟;刘妍;马帅;郭中原;李光;张晓丹5.我国实现了在线近红外光谱分析技术零的突破——英贤仪器NIR-6000在线近红外分析仪荣获BCEIA’2003金奖 [J],因版权原因,仅展示原文概要,查看原文内容请购买。
