Astragalin_DataSheet_MedChemExpress
X-GAL_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :X-GALCatalog No. :HY-15934CAS No. :7240-90-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:BCIGFormula:C14H15BrClNO6Molecular Weight:408.63CAS No. :7240-90-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CCK8检测操作手册 - 细胞技术_MedChemExpress
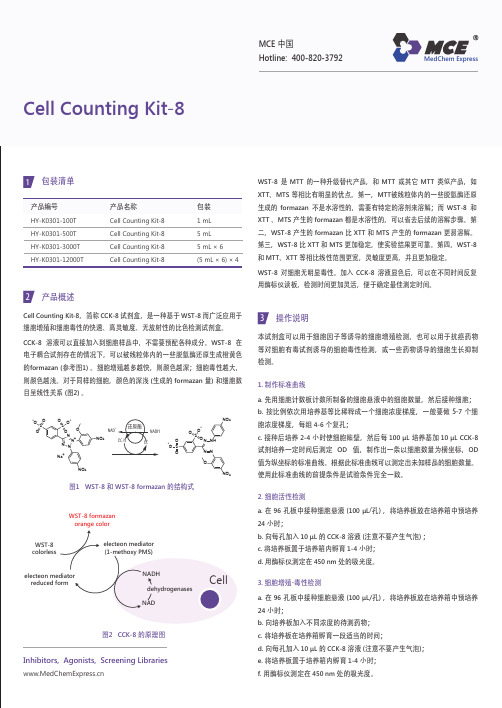
WST-8 是 MTT 的一种升级替代产品,和 MTT 或其它 MTT 类似产品,如 XTT 、MTS 等相比有明显的优点。
第一,MTT 被线粒体内的一些脱氢酶还原生成的 formazan 不是水溶性的,需要有特定的溶剂来溶解;而 WST-8 和 XTT 、MTS 产生的 formazan 都是水溶性的,可以省去后续的溶解步骤。
第二,WST-8 产生的 formazan 比 XTT 和 MTS 产生的 formazan 更易溶解。
第三,WST-8 比 XTT 和 MTS 更加稳定,使实验结果更可靠。
第四,WST-8 和 MTT 、XTT 等相比线性范围更宽,灵敏度更高,并且更加稳定。
WST-8 对细胞无明显毒性。
加入 CCK-8 溶液显色后,可以在不同时间反复用酶标仪读板,检测时间更加灵活,便于确定最佳测定时间。
产品编号HY-K0301-100T HY-K0301-500T HY-K0301-3000T HY-K0301-12000T包装1 mL 5 mL 5 mL × 6(5 mL × 6) × 4产品名称Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-81包装清单产品概述Cell Counting Kit-8,简称 CCK-8 试剂盒,是一种基于 WST-8 而广泛应用于细胞增殖和细胞毒性的快速、高灵敏度、无放射性的比色检测试剂盒。
CCK-8 溶液可以直接加入到细胞样品中,不需要预配各种成分。
WST-8 在电子耦合试剂存在的情况下,可以被线粒体内的一些脱氢酶还原生成橙黄色的formazan (参考图1) 。
细胞增殖越多越快,则颜色越深;细胞毒性越大,则颜色越浅。
对于同样的细胞,颜色的深浅 (生成的 formazan 量) 和细胞数目呈线性关系 (图2) 。
细胞增殖-毒性检测(CCK8试剂盒法)操作步骤_MedChemExpress

WST-8 是 MTT 的一种升级替代产品,和 MTT 或其它 MTT 类似产品,如 XTT 、MTS 等相比有明显的优点。
第一,MTT 被线粒体内的一些脱氢酶还原生成的 formazan 不是水溶性的,需要有特定的溶剂来溶解;而 WST-8 和 XTT 、MTS 产生的 formazan 都是水溶性的,可以省去后续的溶解步骤。
第二,WST-8 产生的 formazan 比 XTT 和 MTS 产生的 formazan 更易溶解。
第三,WST-8 比 XTT 和 MTS 更加稳定,使实验结果更可靠。
第四,WST-8 和 MTT 、XTT 等相比线性范围更宽,灵敏度更高,并且更加稳定。
WST-8 对细胞无明显毒性。
加入 CCK-8 溶液显色后,可以在不同时间反复用酶标仪读板,检测时间更加灵活,便于确定最佳测定时间。
产品编号HY-K0301-100T HY-K0301-500T HY-K0301-3000T HY-K0301-12000T包装1 mL 5 mL 5 mL × 6(5 mL × 6) × 4产品名称Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-81包装清单产品概述Cell Counting Kit-8,简称 CCK-8 试剂盒,是一种基于 WST-8 而广泛应用于细胞增殖和细胞毒性的快速、高灵敏度、无放射性的比色检测试剂盒。
CCK-8 溶液可以直接加入到细胞样品中,不需要预配各种成分。
WST-8 在电子耦合试剂存在的情况下,可以被线粒体内的一些脱氢酶还原生成橙黄色的formazan (参考图1) 。
细胞增殖越多越快,则颜色越深;细胞毒性越大,则颜色越浅。
对于同样的细胞,颜色的深浅 (生成的 formazan 量) 和细胞数目呈线性关系 (图2) 。
α-半乳糖苷酶(α-GAL)活性检测试剂盒说明书 可见分光光度法

α-半乳糖苷酶(α-GAL)活性检测试剂盒说明书可见分光光度法注意:正式测定前务必取2-3个预期差异较大的样本做预测定。
货号:BC2570规格:50T/24S产品内容:提取液:液体50mL×1瓶,4℃保存。
试剂一:粉剂×1瓶,-20℃保存;临用前每瓶加入5mL双蒸水,充分溶解备用;用不完的试剂仍-20℃保存。
试剂二:液体15mL×1瓶,4℃保存。
试剂三:液体80mL×1瓶,4℃保存。
标准液:液体1mL×1支,4℃保存,5μmol/mL对硝基苯酚溶液。
产品说明:α-GAL(EC 3.2.1.22)广泛存在于动物、植物、微生物和培养细胞中,能专一地催化α半乳糖苷键的水解,主要参与棉子糖、水苏糖、蜜二糖和半乳甘露聚糖等半乳糖苷的降解。
α-GAL对于植物种子的萌发至关重要,种子萌发初期,其催化产生的D-半乳糖通过糖酵解途径迅速转化和消耗,为种子的萌发提供最初的能量来源,后期则主要参与细胞壁储藏多糖水解。
α-GAL分解对-硝基苯-α-D-吡喃半乳糖苷生成对-硝基苯酚,后者在400nm有最大吸收峰,通过测定吸光值升高速率来计算α-GAL活性。
试验中所需的仪器和试剂:可见分光光度计、台式离心机、水浴锅、可调式移液器、1mL玻璃比色皿、研钵、冰和蒸馏水。
操作步骤:一、粗酶液提取:1、细菌或培养细胞的处理:收集细菌或细胞到离心管内,离心后弃上清;按照每500万细菌或细胞加入1mL第1页,共3页提取液,超声波破碎细菌或细胞(功率20%,超声3s,间隔10s,重复30次),15000g,4℃,离心20分钟,取上清,置冰上待测。
2、组织的处理:称取约0.1g组织,加入1mL提取液进行冰浴匀浆;15000g,4℃,离心20min,取上清,置冰上待测。
3、标准液的处理:用蒸馏水将标准液稀释至200、100、50、25、12.5、6.25、0nmol/mL.二、测定步骤和加样表(在1.5mLEP管中依次加入下列试剂):试剂名称(μL)测定管对照管标准管试剂一200蒸馏水200试剂二250250样本5050迅速混匀,放入37℃准确水浴30min标准液500试剂三100010001000充分混匀,室温静置2min后,400nm处测定吸光值A。
CCK8中文说明书_MedChemExpress

WST-8 是 MTT 的一种升级替代产品,和 MTT 或其它 MTT 类似产品,如 XTT 、MTS 等相比有明显的优点。
第一,MTT 被线粒体内的一些脱氢酶还原生成的 formazan 不是水溶性的,需要有特定的溶剂来溶解;而 WST-8 和 XTT 、MTS 产生的 formazan 都是水溶性的,可以省去后续的溶解步骤。
第二,WST-8 产生的 formazan 比 XTT 和 MTS 产生的 formazan 更易溶解。
第三,WST-8 比 XTT 和 MTS 更加稳定,使实验结果更可靠。
第四,WST-8 和 MTT 、XTT 等相比线性范围更宽,灵敏度更高,并且更加稳定。
WST-8 对细胞无明显毒性。
加入 CCK-8 溶液显色后,可以在不同时间反复用酶标仪读板,检测时间更加灵活,便于确定最佳测定时间。
产品编号HY-K0301-100T HY-K0301-500T HY-K0301-3000T HY-K0301-12000T包装1 mL 5 mL 5 mL × 6(5 mL × 6) × 4产品名称Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-81包装清单产品概述Cell Counting Kit-8,简称 CCK-8 试剂盒,是一种基于 WST-8 而广泛应用于细胞增殖和细胞毒性的快速、高灵敏度、无放射性的比色检测试剂盒。
CCK-8 溶液可以直接加入到细胞样品中,不需要预配各种成分。
WST-8 在电子耦合试剂存在的情况下,可以被线粒体内的一些脱氢酶还原生成橙黄色的formazan (参考图1) 。
细胞增殖越多越快,则颜色越深;细胞毒性越大,则颜色越浅。
对于同样的细胞,颜色的深浅 (生成的 formazan 量) 和细胞数目呈线性关系 (图2) 。
马传染性贫血病毒cELISA抗体检测试剂盒技术参数

马传染性贫血病毒CELISA抗体检测试剂盒技术参数[作用与用途]本试剂盒采用竞争ELlSA技术,用于检测马血清中马传染性贫血病毒抗体,检测结果可用于马传染性贫血感染的辅助诊断。
【主要成分】______________________________________________________________________________采集待检马的血液,先将全血置37°C条件放置30分钟,后置2~8℃下放置1小时,待血清析出后收集血清。
若血清析出不佳,可将析出液以5000r∕min,离心5分钟,收集上清。
【操作方法】1 .使用前所有试剂应恢复至室温(15~25°C)o试剂应轻轻地旋转或震荡予以混合。
2 .将10倍浓缩洗涤液用双蒸水稀释10倍后备用。
3 .将待检血清样品、阴性对照血清和阳性对照血清与酶标抗原按照1:1 (V/V)混合均匀,推荐以下步骤: 吸取待检血清样品、阴性对照血清和阳性对照血清各60μl,按顺序分别加入60μl的酶标抗原,充分混匀,置37C 孵育30分钟。
4 .取出包被板,将100μl孵育后的样品加入对应的孔中,在37C孵育30分钟。
5 .弃去孔内液体,每孔加入洗涤液250μl,孵育3分钟,弃去洗涤液,洗涤4次,每次拍干。
6 .将底物溶液A和底物溶液B等体积混合均匀,作为底物溶液,现用现配。
向各反应孔中加入底物溶液100μl∕JL,置室温(15-25βC)避光显色5分钟。
7 .加入终止液,50μl∕孔,轻微震荡混合均匀后,在450nm的波长下读数(应在加入终止液后5分钟内完成读数),并记录结果。
8 .计算公式物件I虔_ ______ 阴性对照血清平均OD45θnm 值一待检血清样品OD45Onm 值____押例率二阴性对照血清平均OD450nm值一阳性对照血清平均OD45θnm值【结果判定】1 .试验结果有效的条件是:阴性对照血清的平均OD450nm值>1.0:阳性对照血清的平均OD450nm值<0.20。
Cell Counting Kit-8(CCK-8)细胞活性检测试剂盒使用说明书_MedChemExpress

WST-8 是 MTT 的一种升级替代产品,和 MTT 或其它 MTT 类似产品,如 XTT 、MTS 等相比有明显的优点。
第一,MTT 被线粒体内的一些脱氢酶还原生成的 formazan 不是水溶性的,需要有特定的溶剂来溶解;而 WST-8 和 XTT 、MTS 产生的 formazan 都是水溶性的,可以省去后续的溶解步骤。
第二,WST-8 产生的 formazan 比 XTT 和 MTS 产生的 formazan 更易溶解。
第三,WST-8 比 XTT 和 MTS 更加稳定,使实验结果更可靠。
第四,WST-8 和 MTT 、XTT 等相比线性范围更宽,灵敏度更高,并且更加稳定。
WST-8 对细胞无明显毒性。
加入 CCK-8 溶液显色后,可以在不同时间反复用酶标仪读板,检测时间更加灵活,便于确定最佳测定时间。
产品编号HY-K0301-100T HY-K0301-500T HY-K0301-3000T HY-K0301-12000T包装1 mL 5 mL 5 mL × 6(5 mL × 6) × 4产品名称Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-81包装清单产品概述Cell Counting Kit-8,简称 CCK-8 试剂盒,是一种基于 WST-8 而广泛应用于细胞增殖和细胞毒性的快速、高灵敏度、无放射性的比色检测试剂盒。
CCK-8 溶液可以直接加入到细胞样品中,不需要预配各种成分。
WST-8 在电子耦合试剂存在的情况下,可以被线粒体内的一些脱氢酶还原生成橙黄色的formazan (参考图1) 。
细胞增殖越多越快,则颜色越深;细胞毒性越大,则颜色越浅。
对于同样的细胞,颜色的深浅 (生成的 formazan 量) 和细胞数目呈线性关系 (图2) 。
GAL-021_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:GAL–021 a new intravenous BKCa–channel blocker.In Vitro: GAL–021 is being developed as a novel breathing control modulator to preserve respiratory drive and protect patients from respiratory impairment due to opioids and other modalities. Using inside–out patches in GH3 cells, GAL–021 exertsconcentration–dependent inhibition of single–channel KCa1.1 activity. When evaluated against 12 different cardiac ion channels,inhibition is 35% or less at 30 μM. No significant kinase inhibition is observed at 10 μM. At 30 μM in the radioligand binding assays,interactions (defined as >50% radioligand displacement) are detected at adenosine A1 (65% I), A2A (79% I, IC 50 approximately 5μM),and A3 (93% I; IC 50 approximately 1 μM) receptors, at 5–HT2B receptors (60% I; IC 50 approximately 30 μM)[1].In Vivo: Intravenously administered GAL–021 attenuates opiate–induced respiratory depression in rats and nonhuman primates without affecting morphine analgesia in rats. GAL–021 ventilatory stimulation in rats is attenuated by carotid sinus nervetransection. GAL–021 ventilatory stimulation is attenuated in mice lacking the pore–forming α–subunit of the KCa 1.1 channel [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]GAL–021 is dissolved in DMSO, and final assay concentration of DMSO is 0.1% or less. The effects of GAL–021 (30μM) on a panel of 55 receptors, transporters, and ion channels are evaluated using radioligand binding analyses. Potential kinase inhibition by GAL–021 (10 μM) is assessed using the Kinase HotSpot Screen where activity of 50 kinases is measured in the presence of adenosine triphosphate (10 μM)[1].Animal Administration: GAL–021 is prepared in 0.9% saline.[1]Rat: The effects of GAL–021 on mean arterial pressure (MAP) and heart rate (HR) are evaluated using IV infusions. GAL–021 (0.125 mg /kg/min for 25 min, increasing to 0.20 mg/kg/min for anadditional 25 min IV) and vehicle (0.9% saline, for 50 min) are administered at a constant infusion rate (6 mL/kg/h). All rats receive additional fluid support (50:50 mixture of lactated Ringer's solution and 6% hetastarch in 0.9% saline at 4 mL/kg/min)[1]. For rat andMouse Spirometry section, for rats, tracheal airflow is measured using flow spirometry before and after IV (femoral vein) bolus administration of GAL–021 (0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg) and vehicle (0.9% saline)[1].Mouse: The effects of GAL–021 on ventilation are also evaluated in age–matched male and female adult Slo1+/+ and Slo1–/– mice.Mice are anesthetized using 2 to 2.5% isoflurane in air [1].References:[1]. Golder FJ, et al. Identification and Characterization of GAL–021 as a Novel Breathing Control Modulator. Anesthesiology. 2015 Nov;123(5):1093–104.Product Name:GAL–021Cat. No.:HY-101422CAS No.:1380341-99-0Molecular Formula:C 11H 22N 6O Molecular Weight:254.33Target:Potassium Channel Pathway:Membrane Transporter/Ion Channel Solubility:DMSO: ≥ 30 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
曲妥珠单抗作用机制 - Medchemexpress - MCE中国

Trastuzumab Cat. No.:HY-P9907CAS No.:180288-69-1分⼦式:C₆₄₇₀H₁₀₀₁₂N₁₇₂₆O₂₀₁₃S₄₂分⼦量:145531.5作⽤靶点:EGFR 作⽤通路:JAK/STAT Signaling; Protein Tyrosine Kinase/RTK 储存⽅式:Please store the product under the recommended conditions in the COA.BIOLOGICAL ACTIVITY⽣物活性Trastuzumab 是⼀种⼈源化单克隆抗体,其以⾼亲和⼒与 HER2 选择性结合。
Trastuzumab 已被批准⽤于治疗 HER2 阳性转移性乳腺癌和 HER2 阳性胃癌。
IC₅₀ & TargetHER2[1]体外研究Treatment of HER2-overexpressing breast cancer cell lines with Trastuzumab results in induction of p27KIP1 and theRb-related protein, p130, which in turn significantly reduces the number of cells undergoing S-phase. A number ofother phenotypic changes are observed in vitro as a consequence of Trastuzumab binding to HER2-overexpressingcells. Interaction of Trastuzumab with the human immune system via its human immunoglobulin G1 Fc domain maypotentiate its antitumor activities. in vitro studies demonstrate that Trastuzumab is very effective in mediatingantibody-dependent cell-mediated cytotoxicity against HER2-overexpressing tumor targets [1]. Trastuzumab consistsof two antigen-specific sites that bind to the juxtamembrane portion of the extracellular domain of the HER2 receptor and that prevent the activation of its intracellular tyrosine kinase. Trastuzumab recruits immune effector cells that are responsible for antibody-dependent cytotoxicity [2]. The presence of Trastuzumab IgG significantly increases killing of all breast cancer cell lines. The ADCC activity of PBMCs evoked by Trastuzumab is equally strong againstTrastuzumab-sensitive (SKBR-3) or Trastuzumab-resistant (JIMT-1) breast cancer cells, with dose-dependent celldeath reaching 50–60% killing at an effector/target ratio of 60:1[3].体内研究Trastuzumab treatment of mouse xenograft models results in marked suppression of tumor growth. When given incombination with standard cytotoxic chemotherapeutic agents, Trastuzumab treatment generally results instatistically superior antitumor efficacy compared with either agent given alone [1]. Trastuzumab causes a significantgrowth inhibition of the outgrowth of macroscopic JIMT-1 xenograft tumors in both nude and SCID mice [3].PROTOCOLThe effects of Trastuzumab and Trastuzumab-F(ab′)2 on the growth of JIMT-1, SKBR-3, and BT-474 cells are evaluatedusing the AlamarBlue method. Exponentially growing cells are harvested and plated in single wells of a 96-well flat-bottomed tissue culture plate at defined densities, ranging from 4,500-8,000 cells per well depending on the cell line. After overnight culture, the regular medium is exchanged to medium containing 0, 1, 10, or 100 μg/mL Trastuzumab or Trastuzumab-F(ab′) 2. Cell viability is tested after 72 h of treatment. Fluorescence is detected at an excitation ofCell Assay [3]Product Data SheetInhibitors •Agonists •Screening Libraries544 nm, and emission is detected at 590 nm [3].MCE has not independently confirmed the accuracy of these methods. They are for reference only.AnimalAdministration [3]Trastuzumab and Trastuzumab-F(ab′)2 are given at a dose of 5 and 25 μg/g, respectively, by weekly i.p. injection. The five times greater amount of administered F(ab′)2 is chosen based on the different half-lives of IgG and F(ab′) F(ab′)2.Control mice are treated with weekly i.p. injection of 100 μL physiologic saline (saline). Animals are euthanized by CO2 inhalation [3].MCE has not independently confirmed the accuracy of these methods. They are for reference only.客户使⽤本产品发表的科研⽂献See more customer validations on REFERENCES[1]. Sliwkowski MX, et al. Nonclinical studies addressing the mechanism of action of trastuzumab. Semin Oncol. 1999 Aug;26(4 Suppl 12):60-70.[2]. Hudis CA, et al. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007 Jul 5;357(1):39-51.[3]. Barok M, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007 Jul;6(7):2065-72.McePdfHeightCaution: Product has not been fully validated for medical applications. For research use only.Tel: 400-820-3792; 021-******** Fax: 021-******** E-mail: tech@Master of Small Molecules — 您⾝边的抑制剂⼤师• Nat Commun . 2020 Feb 26;11(1):1049.• Elife . 2019 Jun 7;8. pii: e46983.• Cancer Lett . 2020 Jan 29;475:53-64.• J Exp Clin Cancer Res . 2019 May 22;38(1):214.• Patent . US20190151462A1。
Y-27632_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Y–27632 is an ATP–competitive inhibitor of ROCK–I and ROCK–II , with K i of 220 nM and 300 nM for ROCK–I and ROCK–II , respectively.IC50 & Target: Ki: 220/300 nM (ROCK–I/II)[1]In Vitro: Y–27632 inhibits the ROCK family of kinases 100 times more potently than other kinases including protein kinase C,cAMP–dependent kinase and myosin light chain kinase. Y–27632 prolongs the lag time and delays the appearance of BrdU–labeled cells in a concentration–dependent manner, delays of about 1 and 4 h are noticed in the Swiss 3T3 cells treated with 10 and 100 μM Y–27632, respectively [1]. Y–27632 promotes neuronal differentiation of adipose tissue–derived stem cells (ADSCs). Compared to 1.0and 2.5 μM Y–27632 induced groups, percentages of neuroal–like cells achieved a peak in the 5.0 μM Y–27632 induced group [2].In Vivo: Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of myoclonic jerks when compare with saline group.Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of clonic convulsions when compare with saline group [3].Treatment with Dimethylnitrosamine (DMN) causes a significant decrease in rat body and liver weight (DMN–S group) compared with control animals (S–S group). Oral Y27632 (30 mg/kg) essentially prevents this DMN–induced rat body and liver weight loss (DMN–Y group)[4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Recombinant ROCK–I, ROCK–II, PKN, or citron kinase is expressed in HeLa cells as Myc–tagged proteins by transfection using Lipofectamine, and is precipitated from the cell lysates by the use of 9E10 monoclonal anti–Myc antibodycoupled to G protein–Sepharose. Recovered immunocomplexes are incubated with various concentrations of [32P]ATP and 10 mg of histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 30 min in a total volume of 30 μL of the kinase buffer containing 50 mM HEPES–NaOH, pH 7.4, 10 mM MgCl 2, 5 mM MnCl 2, 0.02% Briji 35, and 2 mM dithiothreitol. PKCa is incubated with 5 μM [32P]ATP and 200 μg/mL histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 10 min in a kinase buffer containing 50 mM Tris–HCl,pH 7.5, 0.5 mM CaCl 2, 5 mM magnesium acetate, 25 μg/mL phosphatidyl serine, 50 ng/mL 12–O–tetradecanoylphorbol–13–acetate and 0.001% leupeptin in a total volume of 30 μL. Incubation is terminated by the addition of 10 μL of 43 Laemmli sample buffer.After boiling for 5 min, the mixture is subjected to SDS–polyacrylamide gel electrophoresis on a 16% gel. The gel is stained withCoomassie Brilliant Blue, and then dried. The bands corresponding to histone type 2 are excised, and the radioactivity is measured [1]. Cell Assay: Y–27632 is dissolved in water and stored [1].[1]HeLa cells are plated at a density of 3×104 cells per 3.5–cm dish. The cells are cultured in DMEM containing 10% FBS in the presence of 10 mM Thymidine for 16 h. After the cells are washed with DMEM containing 10% FBS, they are cultured for an additional 8 h, and then 40 ng/mL of Nocodazole is added. After 11.5 h of theNocodazole treatment, various concentrations of Y–27632 (0–300 μM), Y–30141, or vehicle is added and the cells are incubated for another 30 min [1].Animal Administration: Y–27632 is dissolved in 0.9% NaCl (saline) (Mice)[3].Product Name:Y–27632Cat. No.:HY-10071CAS No.:146986-50-7Molecular Formula:C 14H 21N 3O Molecular Weight:247.34Target:ROCK; ROCK; ROCK Pathway:TGF–beta/Smad; Stem Cell/Wnt; Cell Cycle/DNA Damage Solubility:DMSO: ≥ 32 mg/mLY–27632 is dissolved in saline (final concentration 2%) (Rat)[4].[3][4]Mice[3]Male, inbred Swiss albino mice (2–3 months old) weighing 25–30 g are used. Mice are injected with a sub–convulsive dose of PTZ (35 mg/kg, i.p.) (on Mondays, Wednesdays and Fridays) of each week for a total of 11 injections. After each PTZ injection, mice are observed for 30 min and the occurrence of convulsive activity is recorded. After 30 min, the mice are then injected with either Fasudil (25 mg/kg, i.p.) or Y–27632 (5 mg/kg, i.p.) and returned to their home cages until the next injection. Control mice for Fasudil andY–27632 receives saline.Rat[4]Male Wistar Kind A rats (200–250 g) are used. DMN (1 g/mL) is diluted ten times with saline (final concentration 1%) and 10 mg/kg per day of DMN is injected intraperitoneally (i.p.) on the first 3 days of each week for 4 weeks. Y27632 is given orally once per day at a dose of 30 mg/kg for 4 weeks starting on the day of the first injection of DMN. The dose of 30 mg/kg corrects hypertension in several rat models without toxicity. Twenty rats are randomized into four experimental groups (n=5 in each group) as follows: (1) S–S (injection of saline i.p. and oral administration of saline); (2) S–Y (injection of saline i.p. and oral administration of Y27632); (3) DMN–S (DMN i.p. and oral administration of saline); (4) DMN–Y (DMN i.p. and oral administration of Y27632). The rats are weighed every week. They are sacrificed at the end of the fourth week and the liver is excised. In addition, a blood sample is taken immediately before the rats are sacrificed.References:[1]. Ishizaki T, et al. Pharmacological properties of Y–27632, a specific inhibitor of rho–associated kinases. Mol Pharmacol. 2000 May;57(5):976–83.[2]. Xue ZW, et al. Rho–associated coiled kinase inhibitor Y–27632 promotes neuronal–like differentiation of adult human adipose tissue–derived stem cells.Chin Med J (Engl). 2012 Sep;125(18):3332–5.[3]. Inan S, et al. Antiepileptic effects of two Rho–kinase inhibitors, Y–27632 and fasudil, in mice. Br J Pharmacol. 2008 Sep;155(1):44–51.[4]. Tada S, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine–induced hepatic fibrosis in rats. J Hepatol. 2001 Apr;34(4):529–36.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CCK8数据处理(数据处理,平均值,标准差,抑制率)_MedChemExpress

WST-8 是 MTT 的一种升级替代产品,和 MTT 或其它 MTT 类似产品,如 XTT 、MTS 等相比有明显的优点。
第一,MTT 被线粒体内的一些脱氢酶还原生成的 formazan 不是水溶性的,需要有特定的溶剂来溶解;而 WST-8 和 XTT 、MTS 产生的 formazan 都是水溶性的,可以省去后续的溶解步骤。
第二,WST-8 产生的 formazan 比 XTT 和 MTS 产生的 formazan 更易溶解。
第三,WST-8 比 XTT 和 MTS 更加稳定,使实验结果更可靠。
第四,WST-8 和 MTT 、XTT 等相比线性范围更宽,灵敏度更高,并且更加稳定。
WST-8 对细胞无明显毒性。
加入 CCK-8 溶液显色后,可以在不同时间反复用酶标仪读板,检测时间更加灵活,便于确定最佳测定时间。
产品编号HY-K0301-100T HY-K0301-500T HY-K0301-3000T HY-K0301-12000T包装1 mL 5 mL 5 mL × 6(5 mL × 6) × 4产品名称Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-8Cell Counting Kit-81包装清单产品概述Cell Counting Kit-8,简称 CCK-8 试剂盒,是一种基于 WST-8 而广泛应用于细胞增殖和细胞毒性的快速、高灵敏度、无放射性的比色检测试剂盒。
CCK-8 溶液可以直接加入到细胞样品中,不需要预配各种成分。
WST-8 在电子耦合试剂存在的情况下,可以被线粒体内的一些脱氢酶还原生成橙黄色的formazan (参考图1) 。
细胞增殖越多越快,则颜色越深;细胞毒性越大,则颜色越浅。
对于同样的细胞,颜色的深浅 (生成的 formazan 量) 和细胞数目呈线性关系 (图2) 。
ww人甘丙肽甘丙素(GAL)酶联免疫吸附测定试剂盒使用说明

人甘丙肽/甘丙素(GAL)酶联免疫吸附测定试剂盒使用说明书本试剂盒仅供体外研究使用、不用于临床诊断!预期应用ELISA法定量测定人血清、血浆或其它相关生物液体中GAL含量。
实验原理用纯化的GAL抗体包被微孔板,制成固相载体,往微孔中依次加入标本或标准品、生物素化的GAL抗体、HRP 标记的亲和素,经过彻底洗涤后用底物(TMB)显色。
TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的GAL呈正相关。
用酶标仪在450nm波长下测定吸光度(OD 值),计算样品浓度。
试剂盒组成及试剂配制1、酶标板:一块(96孔)2、标准品(冻干品):2瓶,请临用前15分钟内配制。
每瓶以样品稀释液稀释至0.5ml,盖好后室温静置大约10分钟,同时反复颠倒/搓动以助溶解,其浓度为500pg/ml,然后做系列倍比稀释(注:不要直接在板中进行倍比稀释),分别配制成500pg/ml,250pg/ml,125pg/ml,62.5pg/ml,31.2pg/ml,15.6 pg/ml,7.8pg/ml,样品稀释液直接作为空白孔0pg/ml。
如配制250pg/ml标准品:取0.3ml(不要少于0.3ml)500pg/ml的上述标准品加入含有0.3ml样品稀释液的Eppendorf管中,混匀即可,其余浓度以此类推。
3、样品稀释液:1×20ml。
4、检测稀释液A:1×10ml。
5、检测稀释液B:1×10ml。
6、检测溶液A:1×120/瓶(1:100)。
临用前以检测稀释液A1:100稀释(如:10检测溶液A/990检测稀释液A),充分混匀,稀释前根据预先计算好的每次实验所需的总量配制(100/孔),实际配制时应多配制0.1-0.2ml。
7、检测溶液B:1×120/瓶(1:100)。
临用前以检测稀释液B1:100稀释。
稀释方法同检测溶液A。
8、底物溶液:1×10ml/瓶。
x-α-gal 产品说明

详细信息:X-α-GalX-α-Gal是一种酵母半乳糖苷酶(MEL1)的显色底物,用于在培养基上直接检测GAL4系统的酵母双杂交作用。
通过蓝白颜色筛选,快速和简便地识别阳性的蓝色克隆,无需费时的β-半乳糖苷酶报告基因检测。
X-α-Gal检测酵母MEL1基因的激活,MEL1是GAL4酵母双杂交系统的一个报告基因,编码分泌型的α-半乳糖苷酶。
该酶可水解无色的X-α-Gal底物并最终产生蓝色产物。
表达α-半乳糖苷酶的酵母菌落在含有X-α-Gal的培养基上显蓝色,即阳性的双杂交作用。
用途:(1)α-半乳糖苷酶的显色底物,形成蓝色沉淀。
在培养基上直接检测酵母双杂交相互作用,利用此底物可以省去双杂交体系中的β-半乳糖苷酶溶液和filter-lift assays;(2)用于区分肠杆菌科内的不同菌种以及区分双歧杆菌和乳酸菌;(3)在组织化学中用于酶活性的检测;储存液的配制及使用步骤:A)涂布于预制平板:溶解24mg X-α-gal于6 mL DMF,终浓度为4mg/ mL的溶液。
①涂布200ul(15cm)或者100ul(10cm)X-α-gal储存液于预制平板上;②放于37℃培养箱至液体被吸收(由于DMF挥发性低,最长可放4小时);③将转化细菌或酵母涂于平板上,于37℃或30℃培养直至蓝色菌落出现。
B)直接加入xx中:溶解60mg X-α-gal于3 mL DMF,终浓度为20mg/ mL的溶液。
①将已灭菌琼脂培养基冷却至50-55 ℃;②往已冷却的培养基中直接加入2 ml -10 ml 20 mg/ ml X-α-Gal储存液,混匀,快速倒平板。
外观:白色或灰白色结晶粉末,或者无色结晶;溶解度(1%甲醇溶液):无色,清澈;储存:-15°C避光避潮保存;。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Astragalin (kaempferol–3–O–glucoside) is a flavonoid with anti–inflammatory activity and newly found in persimmon leaves and green tea seeds.
IC50 value:
Target:
in vitro: Astragalin nontoxic at ≤ 20 μM suppressed cellular induction of Toll–like receptor 4 (TLR4) and ROS production enhanced by LPS. Both LPS and H2O2 induced epithelial eotaxin–1 expression, which was blocked by astragalin. LPS activated and induced PLCγ1,PKCβ2, and NADPH oxidase subunits of p22phox and p47phox in epithelial cells and such activation and induction were demoted by astragalin or TLR4 inhibition antagonizing eotaxin–1 induction. H2O2–upregulated phosphorylation of JNK and p38 MAPK was
dampened by adding astragalin to epithelial cells, while this compound enhanced epithelial activation of Akt and ERK. H2O2 and LPS promoted epithelial apoptosis concomitant with nuclear condensation or caspase–3 activation, which was blunted by astragalin [1]. astragalin suppressed the expression of tumor necrosis factor α, interleukin 6, and nitric oxide in a dose–dependent manner in mMECs
[2]. astragalin attenuated the infiltration of inflammatory cells, the activity of myeloperoxidase (MPO) and the expression of tumor necrosis factor–α (TNF–α), interleukin–6 (IL–6) and interleukin–1β (IL–1β) in a dose–dependent manner. Additionally, Western blotting results showed that astragalin efficiently blunt decreased nuclear factor–kappaB (NF–κB) activation by inhibiting the degradation and phosphorylation of IκBα and the nuclear translocation of p65 [3]. Astragalin significantly reduced LPS–induced expression of iNOS,COX–2 and cytokines/chemokines, and production of NO in J774A.1 mouse macrophages. Astragalin inhibited LPSinduced activation of NF–κB as indicated by inhibition of degradation of IκBα, nuclear translocation of NF–κB, and NF–κB dependent gene reporter assay
[4].
in vivo: Mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) (dose range: 5–40 mg/kg). pretreatment with astragalin can improve survival during lethal endotoxemia and attenuate inflammatory responses in a murine model of
lipopolysaccharide–induced acute lung injury [4].
References:
[1]. Cho IH, et al. Astragalin inhibits airway eotaxin–1 induction and epithelial apoptosis through modulating oxidative stress–responsive MAPK signaling.BMC Pulm Med. 2014 Jul 29;14:122.
[2]. Li F, et al. Inhibitory effects of astragalin on lipopolysaccharide–induced inflammatory response in mouse mammary epithelial cells. J Surg Res. 2014 Dec;192(2):573–81.
[3]. Li F, et al. Astragalin suppresses inflammatory responses via down–regulation of NF–κB signaling pathway in lipopolysaccharide–induced mastitis in a murine model. Int Immunopharmacol. 2013 Oct;17(2):478–82.
[4]. Kim MS, et al. Inhibitory effect of astragalin on expression of lipopolysaccharide–induced inflammatory mediators through NF–κB in macrophages. Arch Pharm Res. 2011 Dec;34(12):2101–7.
Product Name:
Astragalin Cat. No.:
HY-N0015CAS No.:
480-10-4Molecular Formula:
C 21H 20O 11Molecular Weight:
448.38Target:
Others Pathway:
Others Solubility:
10 mM in DMSO
[5]. Soromou LW, et al. Astragalin attenuates lipopolysaccharide–induced inflammatory responses by down–regulating NF–κB signaling pathway. Biochem Biophys Res Commun. 2012 Mar 9;419(2):256–61.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
