Irsogladine_DataSheet_MedChemExpress
IRAK_inhibitor_1_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-29-2017Print Date:Jun.-29-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IRAK inhibitor 1Catalog No. :HY-13275CAS No. :1042224-63-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:2–Pyridinamine, 6–imidazo[1,2–a]pyridin–3–yl–N–4–piperidinyl–Formula:C17H19N5Molecular Weight:293.37CAS No. :1042224-63-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
ML324_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:ML324 is a potent JMJD2 demethylase inhibitor with demonstrated antiviral activity.IC50 value: 920 nM(JMJD2E) [1]Target: JMJD2 demethylase inhibitorML324 is a probe molecule that displays submicromolar inhibitory activity toward JMJD2E (in vitro) and possesses excellent in vitro ADME properties. In contrast to previously reported inhibitors of the JMJD proteins, ML324 displays excellent cell permeabilityproviding an opportunity for more extensive cell–based studies of JMJD2 enzymes to be undertaken. In addition, ML324 demonstrates potent anti–viral activity against both herpes simplex virus (HSV) and human cytomegalovirus (hCMV) infection via inhibition viral IE gene expression. ML324 suppresses the formation of HSV plaques, even at high MOI, and blocks HSV–1 reactivation in a mouse ganglia explant model of latently infected mice.PROTOCOL (Extracted from published papers and Only for reference)JMJD2E qHTS FDH Assay [1]:Enzyme and buffer solutions (3 μL) were dispensed into a 1,536–well Greiner black solid–bottom assay plate. The library compounds (23 nL) were transferred using a Kalypsys pintool equipped with 1,536–pin array. The plate was incubated at room temperature (15min), and then a 1 μL aliquot of substrate solution was added to initiate the reaction. The plate was transferred to ViewLux imager where an initial reading using standard UV optics (Ex 340 nm, Em 450 nm) was obtained. The plate was then removed from the reader,incubated for 30 minutes at room temperature, and returned to the reader for a second fluorescence reading. A fully automated robotic screening system (Kalypsys Inc, San Diego, CA) was used to perform the above steps as described previously. Compound plates containing DMSO as a vehicle–only control were included at regular interval throughout the screen to monitor any systematic trend in the assay signal associated with reagent dispenser variation or decreases in enzyme specific activity. For activity calculations,percent values were computed as the difference in fluorescence intensity between last and first time points. The percentage activity was calculated from the median values of the catalyzed, or neutral control, and the uncatalyzed, or 100% inhibited, control,respectively, using in–house software.Inhibition of viral infection in cell culture [1]:Cells were treated with DMSO, LSD1 inhibitor (TCP, tranylcypromine, Sigma P8511), or JMJD2 inhibitorsand infected with HSV–1 or hCMV as described below. cDNA was produced from total RNA and quantitated using an ABI 7900HT (ABI SDS 2.3 Software). Viral yields were determined by titration.References:Product Name:ML324Cat. No.:HY-12725CAS No.:1222800-79-4Molecular Formula:C 21H 23N 3O 2Molecular Weight:349.43Target:Histone Demethylase Pathway:Epigenetics Solubility:DMSO: ≥ 33 mg/mL[1]. Rai G, et al. Discovery of ML324, a JMJD2 demethylase inhibitor with demonstrated antiviral activity.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Emricasan-DataSheet-MedChemExpress
Hale Waihona Puke DescriptionEmricasan is an irreversible pan-caspase inhibitor.
IC₅₀ & Target
Caspase
In Vivo
Emricasan (IDN-6556) is orally active that is retained in the liver for prolonged period of time. TUNEL-positive cells are considerably increased by five-fold in mice fed a HFD and are reduced under Emricasan treatment. In accordance with this observation caspase-3 and -8 are increased in HFD-fed mice by 1.5- and 1.3-fold respectively and are significantly decreased by Emricasan treatment[1].When comparing efficacy by multiple routes of administration, Emricasan is administered i.p., p.o., i.m., or i.v. (0.03-3 mg/kg). Caspase 3-like activities, measured as DEVD-AMC cleavage, dose dependently decreased with a 92.5% reduction after the highest dose of Emricasan (3 mg/kg). Emricasan is initially tested in the α-Fas model of liver injury, marked hepatocellular apoptosis, and peak ALT activities
HA14-1_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HA14–1 is a non–peptidic ligand of a Bcl–2 surface pocket with IC50 of ~9 μM.IC50 Value: 9 μMTarget: Bcl–2in vitro: HA14–1 is a small molecule and nonpeptidic ligand of a Bcl–2 surface pocket with IC50 of approximately 9 μM. HA14–1induces the apoptosis of HL–60 cells in a dose–dependent manner via caspase activation. HA14–1 shows cytotoxic effects on HF1A3,HF4.9 and HF28RA follicular lymphoma B cell lines with LC50 of 4.5 μM, 12.6 μM and 8.1 μM respectively. HA14–1 induces apoptosis of HF1A3, HF4.9 and HF28RA cells via caspase and ROS.in vivo: HA14–1(400 nM) treatment did not have any significant effect on the growth of glioblastoma tumors in immunodeficient mice.But HA14–1 (400 nM) increases the effect of the DNA–damaging agent etoposide (2.5 mg/Kg) on glioblastoma growth in vivo.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [5]IGROV1, OAW42, A2780, and SKOV3 cell lines were established from human ovarian adenocarcinomas. Resistant IGROV1–R10 and OAW42–R cells were obtained by reiterating treatments with increasing concentrations of cisplatin. These cell lines were grown.Exponentially growing cells were exposed for 2 h to 20 μg/mL CDDP in serum–free medium or with HA14–1 dissolved in DMSO in complete medium. For HA14–1 treatment, control cultures received the same dose of DMSO as treated counterparts.References:[1]. Doshi et al (2006) Structure–activity relationship studies of ethyl 2–amino–6–bromo–4–(1–cyano–2–ethoxy–2–oxoethyl)–4H–chromene–3–carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl–2 proteins to overcome drug resistance in cancer. J.Med.Chem. 49 7731[2]. Nyhan MJ, O'Donovan TR, Elzinga B, Crowley LC, O'Sullivan GC, McKenna SL.The BH3 mimetic HA14–1 enhances 5–fluorouracil–induced autophagy and type II cell death in oesophageal cancer cells.Br J Cancer. 2012 Feb 14;106(4):711–8.[3]. Weyland M, Manero F, Paillard A, Grée D, Viault G, Jarnet D, Menei P, Juin P, Chourpa I, Benoit JP, Grée R, Garcion E.Mitochondrial targeting by use of lipid nanocapsules loaded with SV30, an analogue of the small–molecule Bcl–2 inhibitor HA14–1.J Control Release. 2011 Apr 10;151(1):74–82. Epub 2010 Dec 5.[4]. Moon DO, Kim MO, Kang SH, Choi YH, Park SY, Kim GY.HA14–1 sensitizes TNF–alpha–induced apoptosis via inhibition of the NF–kappaB signaling pathway: involvement of reactive oxygen species and JNK.Cancer Lett. 2010 Jun 1;292(1):111–8. Epub 2009 Dec 22.[5]. Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N'diaye M, Denoyelle C, Gauduchon P, Poulain L.Mcl–1 is an important determinant of the apoptotic response to the BH3–mimetic molecule HA14–1 in cisplatin–resistant ovarian carcinoma cells.Mol Cancer Ther. 2009 Nov;8(11):3162–70. Epub 2009 Nov 3.Product Name:HA14–1Cat. No.:HY-12011CAS No.:65673-63-4Molecular Formula:C 17H 17BrN 2O 5Molecular Weight:409.23Target:Bcl–2 Family Pathway:Apoptosis Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Galanthamine_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Galanthamine is a potent acetylcholinesterase (AChE ) inhibitor with IC 50 of 500 nM.IC50 & Target: IC50: 0.5 μM (AChE)[1]In Vitro: Galanthamine inhibits AChE and BChE with IC 50 of 0.5 and 8.5 μM [1]. Galanthamine acts as a positive allosteric modulator (PAM) of human α4β2 AChRs expressed in permanently transfected HEK 293 cells. Galanthamine increases the response of (α4β2)2α5 AChRs to 1 μM ACh by up to 220% with very low concerntration(EC 50=0.25 nM). Only small potentiation (20%) of either α4β2 or (α4β2)2β3 AChRs is detected using FLEXstation assays. Galanthamine at concentrations of 1 μM and above inhibits all three AChR subtypes [2].In Vivo: Acute administration of Galantamine (0.3–3 mg/kg, i.p.) increases IGF2 mRNA levels in the hippocampus, but not in the prefrontal cortex, in time– and dose–dependent manner. Galantamine (3 mg/kg, i.p.) causes a transient increase in fibroblast growth factor 2 mRNA levels and a decrease in brain–derived neurotrophic factor mRNA levels in the hippocampus, while it does not affect the mRNA levels of other neurotrophic/growth factors. The Galantamine–induced increase in the hippocampal IGF2mRNA levels is blocked by Mecamylamine, a nonselective nicotinic acetylcholine (ACh) receptor (nAChR) antagonist, andMethyllycaconitine, a selective α7 nAChR antagonist, but not by Telenzepine, a preferential M1muscarinic ACh receptor antagonist.Moreover, the selective α7 nAChR agonist PHA–543613 increasea the IGF2 mRNA levels, while Donepezil, an acetylcholinesterase inhibitor, does not. Galantamine also increases hippocampal IGF2 protein, which is blocked by Methyllycaconitine [2].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration: Galanthamine is dissolved in saline (0.9 % solution of NaCl) (Mice)[2].[2]Mice [2]Eight–week–old male ddY mice are housed in cages (24 cm×17 cm×12 cm) in each group of five to six animals under controlledenvironmental conditions (22±1°C; 12:12–h light–dark cycle, lights on at 0800 hours, food and water ad libitum) for 1 week before use in the experiments. 453 mice are used in total and in single use for each purpose. The following drugs are used: mecamylamine,methyllycaconitine, oxotremorine, and telenzepine, and Galantamine, Donepezil, and PHA–543613. All drugs are dissolved in saline (0.9 % solution of NaCl). Drugs are administered in a volume of 10 mL/kg intraperitoneally (i.p.) (Galantamine, Donepezil,Mecamylamine, Methyllycaconitine, Oxotremorine) or subcutaneously (s.c.) (PHA–543613, Telenzepine).References:[1]. Melanie–Jayne R. Howes, et al. Acetylcholinesterase inhibitors of natural origin. International Journal of Research in Pharmaceutical and Biomedical Sciences 3(SI 1):67–86.[2]. Kuryatov A, et al. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008 Jul;74(1):132–43.[3]. Kita Y, et al. Galantamine increases hippocampal insulin–like growth factor 2 expression via α7 nicotinic acetylcholine receptors in mice.Product Name:Galanthamine Cat. No.:HY-76299CAS No.:357-70-0Molecular Formula:C 17H 21NO 3Molecular Weight:287.35Target:AChE Pathway:Neuronal Signaling Solubility:DMSO: ≥ 59 mg/mLPsychopharmacology (Berl). 2013 Feb;225(3):543–51.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
HM30181_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HM30181 is a potent and selective inhibitor of P–glycoprotein .In Vitro: HM30181 is shown to be approximately equipotent with the reference Pgp inhibitor tariquidar in inhibiting rhodamine 123efflux from CCRF–CEM T cells (IC 50, tariquidar: 8.2±2.0 nM, HM30181: 13.1±2.3 nM) [1]. HM30181 shows a high selectivity for mP–gp and its potency is 20–50 times higher than that of tariquitar, another third generation P–gp inhibitor [2].In Vivo: PET scans with the Pgp substrate (R)–[11C]verapamil in FVB wild–type mice pretreated i.v. with HM30181 (10 or21 mg/kg) failes to show significant increases in (R)–[11C]verapamil brain uptake compared with vehicle treated animals [1].HM30181 inhibits P–gp mainly in the intestinal endothelium, which can be beneficial because pan–inhibition of P–gp, particularly in the brain, could lead to detrimental adverse events. HM30181 increases the oral bioavailability of co–administered paclitaxel by more than 12 times in rats [2].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration:[1]Mouse: HM30181 mesylate is dissolved in 5% aqueous glucose solution, containing 20 μL 0.01M aq. HCl and injected at a volume of 4 mL/kg. Female FVB wild–type mice, aged 8–12 weeks weighing 24±4 g undergo(R)–[11C]verapamil PET scans without and with i.v. pretreatment with cold HM30181. Animals are assigned to 5 groups (n=4 per group). One group is pretreated with HM30181 vehicle solution (5% aq. glucose solution containing 20 μL 0.01 M aq. HCl) at 60min before start of the PET scan. The other groups are pretreated with either 10 mg/kg HM30181 at 10, 60 or 120 min before PET or with 21 mg/kg HM30181 at 10 min before PET [1].References:[1]. Bauer F, et al. Interaction of HM30181 with P–glycoprotein at the murine blood–brain barrier assessed with positron emission tomography. Eur J Pharmacol. 2012 Dec 5;696(1–3):18–27.[2]. Kim TE, et al. Effects of HM30181, a P–glycoprotein inhibitor, on the pharmacokinetics and pharmacodynamics of loperamide in healthy volunteers. Br J Clin Pharmacol. 2014 Sep;78(3):556–64.Product Name:HM30181Cat. No.:HY-13646CAS No.:849675-66-7Molecular Formula:C 38H 36N 6O 7Molecular Weight:688.73Target:P–glycoprotein Pathway:Membrane Transporter/Ion Channel Solubility:DMSO: 6.6 mg/mL (Need ultrasonic)Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Nelarabine_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :NelarabineCatalog No. :HY-13701CAS No. :121032-29-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:506U78; GW 506U78; NelzarabineFormula:C11H15N5O5Molecular Weight:297.27CAS No. :121032-29-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Veledimex_S_enantiomer_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Veledimex S enantiomer is the S enantiomer of veledimex. Veledimex is an oral activator ligand for a proprietary gene therapy promoter system, and a moderate inhibitor of and substrate for CYP3A4/5.In Vivo: Veledimex generally has moderate to low oral bioavailability after a single oral administration in mice and monkeys (–56%in mice and up to 17.4% in cynomolgus monkeys) with mostly low plasma clearance (1399 and 1170 mL/h per kilogram in mice and monkeys, respectively), high volume of distribution (20271 and 9180 mL/h per kilogram in mice and monkeys, respectively), and long terminal half–lives (–10 hours in mice and –30 hours in monkeys) after intravenous administration [1]. Ad–RTS–mIL–12 +veledimex have demonstrated a dose–related increase in tumor IL–12 mRNA and IL–12 protein expression. Discontinuation of veledimex resulted in a return to baseline IL–12 mRNA and protein expression in numerous syngeneic mouse tumor models.Veledimex crosses the blood–brain–barrier in both naive and orthotopic GL–261 mice with increased brain tissue level of –6 fold observed in tumor bearing vs. normal mice. Ad–RTS–mIL–12 + veledimex demonstrate a dose–related increase in survival without significant adverse events [2].References:[1]. Cai H, et al. Plasma Pharmacokinetics of Veledimex, a Small–Molecule Activator Ligand for a Proprietary Gene Therapy Promoter System, in Healthy Subjects. Clin Pharmacol Drug Dev. 2017 May;6(3):246–257.[2]. John A. Barrett, INTRATUMORAL REGULATED EXPRESSION OF IL–12 AS A GENE THERAPY APPROACH TO TREATMENT OF GLIOMA. Neuro Oncol. 2015Nov; 17(Suppl 5): v113.Product Name:Veledimex (S enantiomer)Cat. No.:HY-16785B CAS No.:1093131-03-3Molecular Formula:C 27H 38N 2O 3Molecular Weight:438.60Target:Interleukin Related; Cytochrome P450Pathway:Immunology/Inflammation; Metabolic Enzyme/Protease Solubility:DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Cilengitide_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Cilengitide is a potent and selective integrin inhibitor for αv β3 and αv β5 receptor, inhibits binding of isolated αv β3 and αv β5 to vitronectin with an IC 50 value of 4 and 79 nM, respectively.IC50 & Target: IC50: 4 and 79 nM (αv β3 and αv β5)[1]In Vitro: Cilengitide (EMD 121974) is the αv β3 and αv β5 integrin receptor antagonist. In cell adhesion studies assessing the human melanoma M21 or UCLA–P3 human lung carcinoma cell lines, Cilengitide inhibits integrin–mediated binding to vitronectin with IC 50s of 0.4 and 0.4 μM [1]. In vitro treatment of Cilengitide, at a concentration greater than 1 μM, shows concentration– andtime–dependent cytotoxic effects. However, lower doses of Cilengitide monotherapy (0.1 and 0.5 μM) does not elicit the effective death of the both U87MG and U251MG cells. Significant cytotoxic effects are observed in the U87MG cells with the addition of 1μM Cilengitide in combination with Belotecan monotherapy at concentration of 6.25 nM. Higher concentrations of Cilengitide (5and 25 μM) does not significantly increase cell death in the U87MG and U251MG compare to a lower concentration of Cilengitide (1 μM)[2].In Vivo: In nude mice bearing M21–L melanoma tumors, Cilengitide dose i.p. at 10, 50, and 250 μg three times per weekdemonstrated inhibition of tumor growth with a reduction in both tumor volume (55%, 75%, and 89%, respectively) and tumor weight (23%, 38%, and 61%, respectively), when compared to controls [2]. In the rat model studied, the systemic pharmacokineticsof i.p. Cilengitide are not affected by ILP with Cilengitide alone or ILP with Cilengitide plus Melphalan, TNF or both. Systemic Cilengitide levels reach around 20 μg/mL (approximately 35 μM) within 10 min of i.p. administration and continued to rise toapproximately 40 μg/mL (approximately 70 μM) in the first hour. Thereafter Cilengitide levels in serum dropped with an elimination half–life of 2.1 hr [3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Cilengitide is supplied as an apyrogenic sterile infusion solution in physiological saline. Cilengitide is diluted in saline to a concentration of 1 mM [2]. [2]The cytotoxicity of the two drugs, Belotecan and Cilengitide, is measured by the Cell Counting Kit–8(CCK–8). U87MG and U251MG cells are seeded in 96 well plates at a density of 4×103 cells per well to allow for adhesion overnight.After this, the cells are treated with Cilengitide at a concentration of 0, 0.1, 0.5, 1, 5 and 25 μM and Belotecan at a concentration of 0,6.25, 12.5, 25, 50 and 100 nM. All possible combinations of concentrations are used to assess the combined therapeutic effect of Cilengitide and Belotecan. After 3 days, 10 μL of the CCK–8 solution is added to each well of the plate, and the plate is incubated for 3 hr in the incubator (37°C; 5% CO 2). The optical density (OD) of the sample plate is measured at 450 nm in a microplate reader.The viability of tumor cells is assessed by calculating the OD ratio of the specific OD in each sample to that of the control. Each experiment is conducted in quadruplicate. The values are averaged and normalized against the controls to generate dose–response curves [2].Animal Administration: Cilengitide is prepared in saline.[2][3]Mice [2]Product Name:Cilengitide Cat. No.:HY-16141CAS No.:188968-51-6Molecular Formula:C 27H 40N 8O 7Molecular Weight:588.66Target:Integrin; Autophagy Pathway:Cytoskeleton; Autophagy Solubility:DMSO: ≥ 44 mg/mL; H 2O: ≥ 32 mg/mLMale Balb/c–nu mice, at 8 weeks of age, are randomly assigned to four groups: control (n=10), Cilengitide (n=10), Belotecan (n=10) and combination (n=10). Cilengitide is administered intraperitoneally at a dose of 20 mg/kg daily and the Belotecan at a dose of 10 mg/kg every 4 days. The optimal dose is calculated. The control group of animals is injected with saline only. A single dose of the drugs comprised a 3–sec infusion in a volume of 3 mL/kg. The drug treatments began 7 days after the implantation of tumor cells for 16 days. Half of the animals are sacrificed 1 month after the implantation of the tumor cells for tumor volume analysis and the rest of the animals are observed for another 2 months to analyze survival. The death of the animals is defined as a weight reduction of over 25% of the initial weight or an unexpected sudden death beforehand.Rat[3]Male inbred Brown Norway rats (250 to 300 g) are injected i.p. with 50 mg/kg Cilengitide or saline 2 hr before and 3 hr after Isolated limb perfusion. The Rats are used to investigate the effects of perfusing various combinations of melphalan, TNF and cilengitide with or without the additional i.p. administration of cilengitide before and after the ILP procedure itself. The i.p. administration pre– and post–ILP is intended to optimally saturate available αVβ3 and αVβ5 integrins. Saline is used as a control in both the i.p. and perfusion settings.References:[1]. Hariharan S, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrinreceptor antagonist, Cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007 Aug;18(8):1400–7.[2]. Kim YH, et al. Combination therapy of cilengitide with belotecan against experimental glioblastoma. Int J Cancer. 2013 Aug 1;133(3):749–56.[3]. Ten Hagen TL, et al. The αVβ3/αVβ5 integrin inhibitor cilengitide augments tumor response to melphalan isolated limb perfusion in a sarcoma model. Int J Cancer. 2012 Nov 13. [Epub ahead of print]Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Irsogladine_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-04-2017Print Date:Sep.-04-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IrsogladineCatalog No. :HY-B0327CAS No. :57381-26-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:DicloguamineFormula:C9H7Cl2N5Molecular Weight:256.09CAS No. :57381-26-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Firategrast-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-11-2018Print Date:Oct.-11-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :FirategrastCatalog No. :HY-14951CAS No. :402567-16-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SB 683699;SB683699;SB-683699Formula:C27H27F2NO6Molecular Weight:499.50CAS No. :402567-16-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AG1024_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AG–1024 (Tyrphostin) inhibits IGF–1R autophosphorylation with IC50 of 7 μM, less potent to IR with IC50 of 57 μM.IC50 value: 7 uM (IGF–1R autophosphorylation); 57 uM (IR) [1]Target: IGF–1R; IRin vitro: AG–1024 blocks the IGF–1 receptor and IR autophosphorylation with IC50 of 7 μM and 57 μM, respectively. AG–1024 also inhibits the receptor tyrosine kinase activity towards exogenous substrates (TKA) with IC50 values of 18 μM and 80 μM, respectively[1]. Human breast cancer cell line MCF–7 exposure to Tyrphostin AG 1024 inhibited proliferation and induced apoptosis in atime–dependent manner, and the degree of growth inhibition for IC20 plus irradiation (4 Gy) was up to 50% compared to the control.Examination of Tyrphostin AG 1024 effects on radiation response demonstrated a marked enhancement in radiosensitivity andamplification of radiation–induced apoptosis [2]. AG–1024 significantly inhibits melanoma cell proliferation with an IC50 of <50 nM in the absence of serum, by blocking MAPK/ERK2 signaling, subsequently rapidly inducing pRb dephosphorylation and activation, and eventually the formation of growth suppressive pRb–E2F complexes [3].in vivo: Administration of AG–1024 at a dose of 30 μg for 10 days significantly inhibits the tumor growth of Ba/F3–p210 xenograft in mice [4].References:[1]. Párrizas M, et al. Specific inhibition of insulin–like growth factor–1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins.Endocrinology. 1997 Apr;138(4):1427–33.[2]. Wen B, et al. Tyrphostin AG 1024 modulates radiosensitivity in human breast cancer cells. Br J Cancer. 2001 Dec 14;85(12):2017–21.[3]. von Willebrand M, et al. The tyrphostin AG1024 accelerates the degradation of phosphorylated forms of retinoblastoma protein (pRb) and restores pRb tumor suppressive function in melanoma cells. Cancer Res. 2003 Mar 15;63(6):1420–9.[4]. Deutsch E, et al. Tyrosine kinase inhibitor AG1024 exerts antileukaemic effects on STI571–resistant Bcr–Abl expressing cells and decreases AKT phosphorylation. Br J Cancer. 2004 Nov 1;91(9):1735–41.Product Name:AG1024Cat. No.:HY-10253CAS No.:65678-07-1Molecular Formula:C 14H 13BrN 2O Molecular Weight:305.17Target:IGF–1R; Autophagy Pathway:Protein Tyrosine Kinase/RTK; Autophagy Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Seletalisib_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Seletalisib (UCB5857) is potent and selective PI3Kδ inhibitor with an IC 50 of 12 nM.IC50 & Target: IC50: 12 nM (PI3Kδ)[1]In Vitro: Seletalisib is a potent, ATP–competitive and highly selective PI3Kδ inhibitor able to block AKT phosphorylation following activation of the BCR in a B–cell line. Seletalisib inhibits N–formyl peptides (fMLP)–stimulated but not phorbol myristate acetate (PMA)–stimulated superoxide release from human neutrophils consistent with a PI3Kδ–specific activity. No indications ofcytotoxicity are observed in PBMCs or other cell types treated with seletalisib. seletalisib blocks human T–cell production of several cytokines from activated T–cells. Seletalisib inhibits T–cell differentiation to Th1, Th2, and Th17 subtypes. Additionally, seletalisib inhibits B–cell proliferation and cytokine release. In human whole blood assays, seletalisib inhibits CD69 expression upon B–cell activation and anti–IgE–mediated basophil degranulation [1].In Vivo: Seletalisib significantly inhibits IL–2 release following TCR stimulation in the rat. The inhibition is observed at all tested doses of seletalisib with almost complete inhibition reached at dose levels ≥1 mg/kg. Seletalisib has potent in vivo effects with an estimated IC 50 value of <10 nM [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Seletalisib is dissolved 1 mM solution in DMSO, and tested in a concentration response (seletalisib), to explore the effects of PI3Kδ–specific inhibition compared with complete inhibition of class I PI3K signaling. In addition, seletalisib is tested in the BioMap BT cell system at concentrations of 1000, 100, 10, and 1 nM. An activity profile is generated based on the effect of the compounds on the levels of cellular readouts, including cytokines, growth factors, adhesion molecules, and proliferation endpoints [1].Animal Administration:[1]Rat: Rats are dosed with seletalisib (0.1–10 mg/kg in 500 μL volume) or vehicle via oral gavage 30 min prior to i.v. administration of anti– CD3 antibody administered in a 200 μL dose volume. The vehicle is methylcellulose or saline for oral and i.v. administration, respectively. Seletalisib levels and IL–2 levels are measured [1].References:[1]. Allen RA, et al. Seletalisib: Characterization of a Novel, Potent, and Selective Inhibitor of PI3Kδ. J Pharmacol Exp Ther. 2017 Apr 25. pii: jpet.116.237347.Product Name:Seletalisib Cat. No.:HY-16754CAS No.:1362850-20-1Molecular Formula:C 23H 14ClF 3N 6O Molecular Weight:482.85Target:PI3K Pathway:PI3K/Akt/mTOR Solubility:DMSO: ≥83.3 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Azilsartan_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AzilsartanCatalog No. :HY-14914CAS No. :147403-03-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:TAK 536; TAK536; TAK⁻536Formula:C25H20N4O5Molecular Weight:456.45CAS No. :147403-03-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
IRAK4-IN-1_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-15-2018Print Date:Jan.-15-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IRAK4-IN-1Catalog No. :HY-101922CAS No. :1820787-94-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C19H23N5OMolecular Weight:337.42CAS No. :1820787-94-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AZD-2461_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AZD2461 is a novel PARP inhibitor, which has the potential to avoid the olaparib resistence mediated by Pgp.IC50 Value:Target: PARPin vitro:in vivo: AZD2461 has an 80–fold increased Mdr1b expression on Olaparib–resistant KB1P tumor T6–28, without inhibition of Pgp.AZD2461 induces loss of 53BP1 expression in mice with KB1P tumors with short–term treatment. Long–term AZD2461 treatment is well tolerated and doubled the median relapse–free survival.Clinical trial: An Open–Label, Dose–Escalation Study of AZD–2461 .Phase 1 cpmpletedPROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]KB1P mice that are backcrossed to a pure FVB/N background. To generate Brca1Δ/Δ;p53Δ/Δ;Mdr1a/b–/– tumors, we crossMdr1a/b–/– mice with KB1P mice to produceK14cre;Brca1F/F;p53F/F;Mdr1a/b–/– (KB1PM) mice. Mdr1a and Mdr1b genotypes are tested by PCR with specific primers (Mdr1a forward: 5′–GTGCATAGACCACCCTCAAGG–3′; Mdr1b forward:5′–AAGCTGTGCATGATTCTGGG–3′) for wild–type (Mdr1a reverse: 5′–GTCATGCACATCAAACCAGCC–3′; Mdr1b reverse:5′–GAGAAACGATGTCCTTCCAG–3′) and deleted alleles (Mdr1a reverse: 5′–GGAGCAAAGCTGCTATTGGC–3′). Orthotopictransplantations into wild–type FVB/N mice, tumor monitoring, and sampling are conducted. For the transplantation of cell lines,500,000 cells in PBS and Matrigel (1:1) are injected in the fourth right mammary fat pad. Starting from 2 weeks after transplantation,tumor size is monitored at least 3 times a week. All treatments are started when tumors reach a size of approximately 200 mm3.Olaparib (50 mg/kg intraperitoneally) and AZD2461 (100 mg/kg per os) are given for 28 consecutive days, unless otherwise indicated.If tumors do not shrink below 50% of the initial volume, treatment is continued for another 28 days; otherwise, a new treatment cycle of 28 days is started when the relapsing tumor reached a size of 100% of the original volume. AZD2461 is diluted in 0.5% w/v hydroxypropyl methylcellulose in deionized water to a concentration of 10 mg/mL.References:[1]. Jaspers JE, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1–mutated mouse mammary tumors. Cancer Discov, 2013, 3(1), 68–81.Product Name:AZD–2461Cat. No.:HY-13536CAS No.:1174043-16-3Molecular Formula:C 22H 22FN 3O 3Molecular Weight:395.43Target:PARP; PARP Pathway:Epigenetics; Cell Cycle/DNA Damage Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
GW274150_DataSheet_MedChemExpress
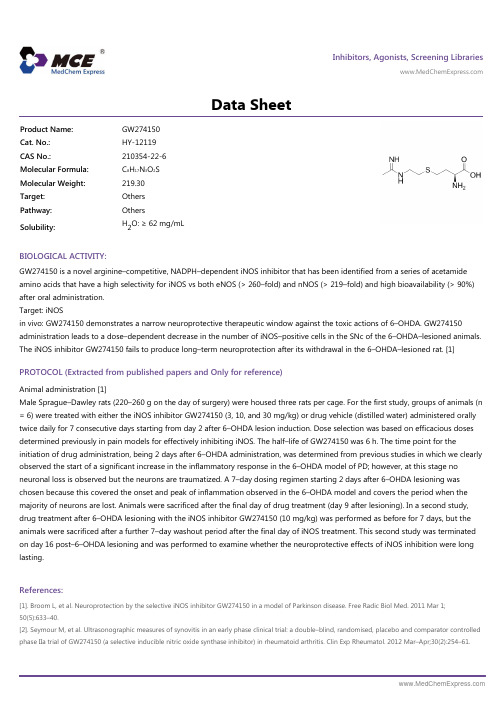
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:GW274150 is a novel arginine–competitive, NADPH–dependent iNOS inhibitor that has been identified from a series of acetamide amino acids that have a high selectivity for iNOS vs both eNOS (> 260–fold) and nNOS (> 219–fold) and high bioavailability (> 90%)after oral administration.Target: iNOSin vivo: GW274150 demonstrates a narrow neuroprotective therapeutic window against the toxic actions of 6–OHDA. GW274150administration leads to a dose–dependent decrease in the number of iNOS–positive cells in the SNc of the 6–OHDA–lesioned animals.The iNOS inhibitor GW274150 fails to produce long–term neuroprotection after its withdrawal in the 6–OHDA–lesioned rat. [1]PROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]Male Sprague–Dawley rats (220–260 g on the day of surgery) were housed three rats per cage. For the first study, groups of animals (n = 6) were treated with either the iNOS inhibitor GW274150 (3, 10, and 30 mg/kg) or drug vehicle (distilled water) administered orally twice daily for 7 consecutive days starting from day 2 after 6–OHDA lesion induction. Dose selection was based on efficacious doses determined previously in pain models for effectively inhibiting iNOS. The half–life of GW274150 was 6 h. The time point for theinitiation of drug administration, being 2 days after 6–OHDA administration, was determined from previous studies in which we clearly observed the start of a significant increase in the inflammatory response in the 6–OHDA model of PD; however, at this stage no neuronal loss is observed but the neurons are traumatized. A 7–day dosing regimen starting 2 days after 6–OHDA lesioning was chosen because this covered the onset and peak of inflammation observed in the 6–OHDA model and covers the period when the majority of neurons are lost. Animals were sacrificed after the final day of drug treatment (day 9 after lesioning). In a second study,drug treatment after 6–OHDA lesioning with the iNOS inhibitor GW274150 (10 mg/kg) was performed as before for 7 days, but the animals were sacrificed after a further 7–day washout period after the final day of iNOS treatment. This second study was terminated on day 16 post–6–OHDA lesioning and was performed to examine whether the neuroprotective effects of iNOS inhibition were long lasting.References:[1]. Broom L, et al. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011 Mar 1;50(5):633–40.[2]. Seymour M, et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double–blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol. 2012 Mar–Apr;30(2):254–61.Product Name:GW274150Cat. No.:HY-12119CAS No.:210354-22-6Molecular Formula:C 8H 17N 3O 2S Molecular Weight:219.30Target:Others Pathway:Others Solubility:H 2O: ≥ 62 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Irsogladine is a PDE4 inhibitor and muscarinic acetylcholine receptor binder.
Target: PDE4; mACHR
Irsogladine treatment (300 and 500 mg/kg/day) resulted in a dose–dependent reduction of angiogenesis in wild–type mice by 21 and 45.3% (P < 0.02, P < 0.001), in tPA–deficient mice by 42.6 and 46% (P < 0.001, P < 0.001), and in uPA–deficient mice by 27.2 and 46%(P < 0.05, p < 0.001), respectively. Irsogladine inhibits bFGF–induced angiogenesis in wild–type, tPA–knockout, and uPA–knockout mice [1]. Irsogladine up–regulates GJIC between PC cells via regulation of the PKA pathway. It also suggests a useful adjuvant of Irsogladine to pancreatic cancer therapy [2]. irsogladine produces the increase of intracellular cAMP content via non–selective inhibition of PDE isozymes, which may be a key mechanism involved in its gastroprotective actions [3].
References:
[1]. Ren, C.J., et al., Irsogladine maleate inhibits angiogenesis in wild–type and plasminogen activator–deficient mice. J Surg Res, 1998. 77(2): p. 126–31.
[2]. Kawasaki, Y., et al., Irsogladine malate up–regulates gap junctional intercellular communication between pancreatic cancer cells via PKA pathway.Pancreas, 2002. 25(4): p. 373–7.
[3]. Kyoi, T., et al., Phosphodiesterase inhibition by a gastroprotective agent irsogladine: preferential blockade of cAMP hydrolysis. Life Sci, 2004. 75(15): p.1833–42.
Product Name:
Irsogladine Cat. No.:
HY-B0327CAS No.:
57381-26-7Molecular Formula:
C 9H 7Cl 2N 5Molecular Weight:
256.09Target:
mAChR; mAChR; Phosphodiesterase (PDE)Pathway:
Neuronal Signaling; GPCR/G Protein; Metabolic Enzyme/Protease Solubility:
DMSO:≥ 2.6 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
