AZD 4017 1024033-43-9 GlpBio
食品冷链品控专业《5. 无公害农产品 叶菜类蔬菜》
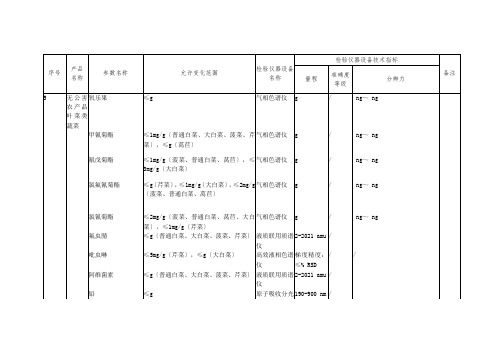
g
/
ng~ ng
氯氟氰菊酯
≤g〔芹菜〕,≤1mg/g〔大白菜〕,≤2mg/g〔菠菜、普通白菜、莴苣〕
气相色谱仪
g
/
ng~ ng
氯氰菊酯
≤2mg/g〔菠菜、普通白菜、莴苣、大白菜〕,≤1mg/g〔芹菜〕
气相色谱仪
g
/
ng~ ng
质谱仪
2-2021 amu
/
吡虫啉
≤5mg/g〔芹菜〕,≤g〔大白菜〕
高效液相色谱仪
梯度精度:≤% RSD
/
/
阿维菌素
≤g〔普通白菜、大白菜、菠菜、芹菜〕
液质联用质谱仪
2-2021 amu
/
铅
≤g
原子吸收分光光度计
190-900 nm
/
镉
≤g
原子吸收分光光度计
190-900 nm
/
序号
产品
名称
参数名称
允许变化范围
检验仪器设备名称
检验仪器设备技术指标
备注
量程
准确度等级
分辨力
5
无公害农产品 叶菜类蔬菜
氧乐果
≤g
气相色谱仪
g
/
ng~ ng
甲氰菊酯
≤1mg/g〔普通白菜、大白菜、菠菜、芹菜〕,≤g〔莴苣〕
气相色谱仪
g
/
ng~ ng
氰戊菊酯
≤1mg/g〔菠菜、普通白菜、莴苣〕,≤3mg/g〔大白菜〕
常用多肽缩合试剂
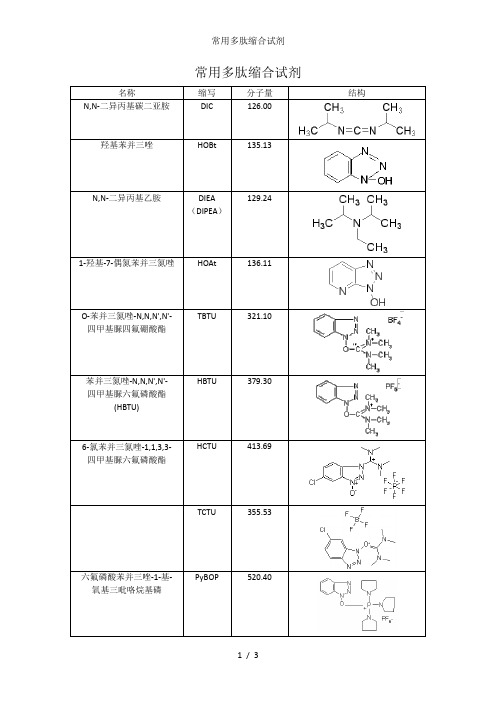
名称缩写分子量结构N,N-二异丙基碳二亚胺DIC 126.00羟基苯并三唑HOBt 135.13129.24N,N-二异丙基乙胺DIEA(DIPEA)1-羟基-7-偶氮苯并三氮唑HOAt 136.11TBTU 321.10O-苯并三氮唑-N,N,N',N'-四甲基脲四氟硼酸酯HBTU 379.30苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸酯(HBTU)HCTU 413.696-氯苯并三氮唑-1,1,3,3-四甲基脲六氟磷酸酯TCTU 355.53PyBOP 520.40六氟磷酸苯并三唑-1-基-氧基三吡咯烷基磷名称缩写分子量结构PyAOP 521.38(3H-1,2,3-三唑并[4,5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐DCC 206.33N,N'-二环己基碳二亚胺4-二甲氨基吡啶DMAP 122.17DBU 152.241,8-二氮杂双环[5.4.0]十一碳-7-烯1,1’-羰基二咪唑CDI 162.15HATU 380.232-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯HOOBt 163.103-羟基-1,2,3-苯并三嗪-4(3H)-酮Cl-HOBt 169.576-氯-1-羟基苯并三氮唑EDC.HCl 191.71-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐TATU 322.1O-(7-氮杂苯并三氮唑)-N,N,N',N'-四甲基脲四氟硼酸盐名称缩写分子量结构O-(1,2-二氢-2-氧-吡啶基)--1,1,3,3-四甲基脲四氟硼酸盐TPTU 297.10O-(N-琥珀酰亚胺基)-N NN'N'-四甲基四氟硼酸脲TSTU 301.10三吡咯烷基溴化鏻六氟磷酸盐PyBrOP 466.20N,N,N',N'-四甲基氯甲脒六氟磷酸盐TCFH 280.583 -二乙氧基磷酰基-1,2,3-苯唑4(3H)-酮DEPBT 299.23O-[(乙氧基羰基)氰基甲胺]-N,N,N',N'-四甲基硫尿四氟硼酸TOTU 328.1苯并三氮唑-1-基氧基三(二甲基氨基)磷鎓六氟磷酸盐BOP(卡特缩合剂)442.50N,N,N',N'-四甲基-O-(3,4-二氢-4-氧代-1,2,3-苯并三嗪-3-基)脲四氟硼酸盐TDBTU 349.09。
鬼笔环肽标记系列产品说明书

鬼笔环肽标记系列产品说明书保存:-20℃干燥、避光保存,有效期一年。
若配制成水溶液,请小量分装保存,避免反复冻融。
注意:本产品为冻干粉形式,使用前请瞬时离心,加适当溶剂溶解后使用。
产品介绍:鬼笔环肽是从致命的伞形毒蕈蘑菇中分离出来的一种毒素。
它是特异性结合于F-肌动蛋白的双环肽(1)。
因此用荧光染料标记的鬼笔环肽可以非常方便的研究F-肌动蛋白的分布。
鬼笔环肽内部,在半胱氨酸和色氨酸之间含有不常见的硫醚桥形成内环结构。
在pH 升高时,该硫醚被裂解,鬼笔环肽失去对肌动蛋白的亲和力。
荧光标记的鬼笔环肽可在纳摩尔水平染色F-肌动蛋白(1-3)。
在各种植物细胞或动物细胞中,标记的鬼笔环肽对大、小细丝具有相似的亲和力,平均每个肌动蛋白亚基结合一个鬼笔环肽分子。
不同于抗体,鬼笔环肽与肌动蛋白的结合亲和力在不同物种间没有显着变化。
非特异性染色可以忽略不计,染色和未染色区域之间的对比度非常大。
鬼笔环肽将单体/聚合物的平衡转向聚合状态,将聚合临界浓度降低至30倍(3,4)。
Phallotoxins 可通过抑制细胞松弛素的解聚,碘化钾和升高的温度,稳定F-肌动蛋白。
因为鬼笔环肽缀合物很小,大约直径12-15埃,分子量<2000道尔顿,多种肌动蛋白结合蛋白,包括肌球蛋白,原肌球蛋白和后肌钙蛋白依然可以和鬼笔环肽标记的肌动蛋白结合。
更重要的是,鬼笔环肽标记的肌动蛋白丝保持功能,标记甘油肌纤维仍然收缩,标记的肌动蛋白丝仍然可以继续移动(5,6)。
而且荧光标记的鬼笔环肽也可用于对细胞中F-肌动蛋白进行定量研究(7,8)。
实验方法:1.储液制备:荧光染料标记的鬼笔环肽:取适量甲醇或无菌水溶解棕色管中冻干的粉末,制备成200U/mL 的储液(300T 规格染料加入1.5mL 的液体)。
荧光标记的标记鬼笔环肽的一个单位(T )的定义是染色一个加载细胞的载玻片所用染料的量。
使用时的推荐稀释比例为1:40-1:200,一个单位相当于200µL 总染色体积中加入1-5µL 200U /mL 储备溶液。
迈勒泰尼生物技术说明书-MACS

.06Miltenyi Biotec B.V. & Co. KGpage 1/4Contents1. Description 1.1 Principle of the MACS® Separation 1.2 Background information 1.3 Applications1.4 Reagent and instrument requirements2. Protocol2.1 Sample preparation 2.2 Magnetic labeling 2.3 Magnetic separation2.4 C ell separation with the autoMACS® Pro Separator3. Example of a separation using CD45 (TIL) MicroBeadsWarningsReagents contain sodium azide. Under acidic conditions sodium azide yields hydrazoic acid, which is extremely toxic. Azide compounds should be diluted with running water before discarding. These precautions are recommended to avoid deposits in plumbing where explosive conditions may develop.1. DescriptionThis product is for research use ponents1 mL CD45 (TIL) MicroBeads, mouse:MicroBeads conjugated to monoclonal anti-mouse CD45 antibodies (isotype: rat IgG2b).CapacityFor 10⁹ total cells, up to 100 separations.Product format CD45 (TIL) MicroBeads are supplied in buffercontaining stabilizer and 0.05% sodium azide.StorageStore protected from light at 2−8 °C. Do not freeze. The expiration date is indicated on the vial label.1.1 Principle of the MACS® SeparationFirst, the CD45+cells are magnetically labeled with CD45 (TIL) MicroBeads. Then, the cell suspension is loaded onto a MACS® Column, which is placed in the magnetic field of a MACS Separator. The magnetically labeled CD45+ cells are retained within the column. The unlabeled cells run through; this cell fraction is thus depleted of CD45+ cells. After removing the column from the magnetic field, the magnetically retained CD45+ cells can be eluted as the positively selected cell fraction. To increase the purity, the positively selected cell fraction containing the CD45+ cells must be separated over a second column.1.2 Background informationCD45 (TIL) MicroBeads, mouse have been developed for theisolation of tumor-infiltrating leukocytes (TILs) from single-cell suspensions of solid mouse tumors. The CD45 antigen is expressed on all cells of hematopoietic origin except erythrocytes and platelets.1.3 Applications●Positive selection of CD45+ leukocytes from solid mouse tumors, e.g., B16F10, 4T1, or CT26.WT.1.4 Reagent and instrument requirements●Buffer: Prepare a solution containing phosphate-buffered saline (PBS), pH 7.2, 0.5% bovine serum albumin (BSA), and 2 mM EDTA by diluting MACS® BSA Stock Solution (# 130-091-376) 1:20 with autoMACS® Rinsing Solution (# 130-091-222). Keep buffer cold (2−8 °C). Degas buffer before use, as air bubbles could block the column. Always use freshly prepared buffer. ▲ Note: EDTA can be replaced by other supplements such as anticoagulant citrate dextrose formula-A (ACD-A) or citrate phosphate dextrose (CPD). BSA can be replaced by other proteins such as mouse serum albumin, mouse serum, or fetal bovine serum (FBS). Buffers or media containing Ca2+ or Mg2+ are not recommended for use.●MACS Columns and MACS Separators: CD45+ cells can be enriched by using MS or LS Columns. Positive selection can also be performed by using the autoMACS Pro or the MultiMACS™ Cell24 Separator.Positive selection MS 10⁷ 2 ×10⁷MiniMACS, OctoMACS, VarioMACS, SuperMACS II LS4 ×10⁷4 ×10⁷5 ×10⁷5 ×10⁷MidiMACS, QuadroMACS, VarioMACS, SuperMACS IIMultiMACS Cell24 Separator PlusautoMACS5 ×10⁷10⁸autoMACS ProMulti-24 Column Block (per column)2 ×10⁷2.5 ×10⁷MultiMACS Cell24 Separator Plus ▲Note: Column adapters are required to insert certain columns into the VarioMACS™ or SuperMACS™ II Separators. For details refer to the respective MACS Separator data sheet. ▲Note: If separating with LS Columns and the MultiMACS Cell24 Separator Plus use the Single-Column Adapter. Refer to the user manual for details.●Tumor Dissociation Kit, mouse (# 130-096-730) for the generation of single-cell suspension from tumor tissues.CD45 (TIL) MicroBeadsmouseOrder no. 130-110-618●gentleMACS™ Dissociator (# 130-093-235), gentleMACS OctoDissociator (# 130-095-937), or gentleMACS Octo Dissociatorwith Heaters (# 130-096-427)●gentleMACS C Tubes (# 130-093-237, # 130-096-334)●(Optional) Fluorochrome-conjugated REA (REAfinity™antibodies: recombinantly engineered, lacking Fcγ-bindingsite) CD45 antibodies for flow cytometric analysis, e.g., CD45-VioBlue®. For more information about antibodies refer to/antibodies.▲Note: Due to expression of Fcγ receptors on tumor-infiltrating leukocytesREA antibodies are recommended.●(Optional) Propidium Iodide Solution (# 130-093-233), DAPIStaining Solution (# 130-111-570), 7-AAD Staining Solution(# 130-111-568), or Viobility™ Fixable Dyes (# 130-109-812,# 130-109-814, # 130-109-816) for flow cytometric exclusion ofdead cells.●(Optional) Dead Cell Removal Kit (# 130-090-101) for thedepletion of dead cells.●(Optional) Pre-Separation Filters (30 µm) (# 130-041-407) toremove cell clumps.●(Optional) MACS SmartStrainers (30 µm) (# 130-098-458) toremove cell clumps.2. Protocol2.1 Sample preparationFor preparation of a single-cell suspension from solid mouse tumors use the Tumor Dissociation Kit, mouse (# 130-096-730) in combination with the gentleMACS™ Dissociators.For details refer to /protocols.▲ Dead cells may bind non-specifically to MACS® MicroBeads. To remove dead cells, we recommend using the Dead Cell Removal Kit (# 130-090-101).2.2 Magnetic labeling▲ Cells can be labeled with MACS MicroBeads using the autolabeling function of the autoMACS® Pro Separator. For more information refer to section 2.4.▲ Work fast, keep cells cold, and use pre-cooled solutions. This will prevent capping of antibodies on the cell surface and non-specific cell labeling.▲ Volumes for magnetic labeling given below are for up to 10⁷ total cells. When working with fewer than 10⁷ cells, use the same volumes as indicated. When working with higher cell numbers, scale up all reagent volumes and total volumes accordingly (e.g. for 2×10⁷ total cells, use twice the volume of all indicated reagent volumes and total volumes).▲ For optimal performance it is important to obtain a single-cell suspension before magnetic labeling. Pass cells through 30 µm nylon mesh (MACS SmartStrainers (30 µm), # 130-098-458). Moisten filter with buffer before use.▲ The recommended incubation temperature is 2–8 °C. Higher temperatures and/or longer incubation times may lead to non-specific cell labeling. Working on ice may require increased incubation times.1. Determine cell number.2. Centrifuge cell suspension at 300×g for 5 minutes. Aspiratesupernatant completely.3. Resuspend cell pellet in 90 µL of buffer per 10⁷ total cells.▲ Note: Always use freshly prepared buffer.4. Add 10 µL of CD45 (TIL) MicroBeads per 10⁷ total cells.5. Mix well and incubate for 15 minutes in the dark in therefrigerator (2−8 °C).6. (Optional) Add staining antibodies according tomanufacturer’s recommendations.7. Add buffer to a final volume of 500 μL for up to 5×10⁷ cells.▲Note: If more cells were used, split the sample onto multiple columns duringmagnetic separation.▲Note: For higher cell numbers, scale up buffer volume accordingly.8.Proceed to magnetic separation (2.3).2.3 Magnetic separation▲ Choose an appropriate MACS Column and MACS Separator according to the number of total cells and the number of CD45+ cells. For details refer to the table in section 1.4.▲ Note: MS Columns are recommended for highest purity of CD45+ cells. LSColumns are recommended for highest recovery of CD45+ cells.▲ For optimal performance it is important to obtain a single-cell suspension before magnetic separation. Pass cells through 30 µm nylon mesh (Pre-Separation Filters (30 µm), # 130-041-407) to remove cell clumps which may clog the column. Moisten filter with buffer before use.▲Always wait until the column reservoir is empty before proceeding to the next step.Magnetic separation with MS or LS Columns1. Place column in the magnetic field of a suitable MACSSeparator. For details refer to the respective MACS Columndata sheet.2. Prepare column by rinsing with the appropriate amount ofbuffer:MS: 500 µL LS: 3 mL3. Apply cell suspension onto the column. Collect flow-throughcontaining unlabeled cells.4. Wash column with the appropriate amount of buffer. Collectunlabeled cells that pass through and combine with theflow-through from step 3.MS: 3×500 µL LS: 2×1 mL▲ Note:Perform washing steps by adding buffer aliquots as soon as the columnreservoir is empty.5. Remove column from the separator and place it on a suitablecollection tube.6. Pipette the appropriate amount of buffer onto the column.Immediately flush out the magnetically labeled cells by firmly pushing the plunger into the column.MS: 1 mL LS: 3 mL7. (Optional) To increase the purity of CD45+cells, the elutedfraction can be enriched over a second MS or LS Column.Repeat the magnetic separation procedure as described in steps 1 to 6 by using a new column.Magnetic separation with the MultiMACS™ Cell24 Separator Refer to the the MultiMACS™ Cell Separator user manual for instructions on how to use the MultiMACS Cell24 Separator.2.4 Cell separation with the autoMACS® Pro Separator▲Refer to the user manual for instructions on how to use the autoMACS® Pro Separator.▲ All buffer temperatures should be ≥10 °C.▲ For appropriate resuspension volumes and cell concentrations, please visit /autolabeling.▲ Place tubes in the following Chill Rack positions:position A = sample, position B = negative fraction,position C = positive fraction.2.4.1 F ully automated cell labeling and separation1. Switch on the instrument for automatic initialization.2. Go to the Reagent menu and select Read Reagent. Scan the2D barcode of each reagent vial with the barcode scanner on the autoMACS® Pro Separator. Place the reagent into the appropriate position on the reagent rack.3. Place sample and collection tubes into the Chill Rack.4. G o to the Separation menu and select the reagent name foreach sample from the Labeling submenu (the correct labeling, separation, and wash protocols will be selected automatically).5. Enter sample volume into the Volume submenu. Press Enter.6. Select Run.2.4.2 M agnetic separation using manual labeling1. Label the sample as described in section2.2 Magnetic labeling.2. Prepare and prime the instrument.3. Apply tube containing the sample and provide tubes forcollecting the labeled and unlabeled cell fractions. Place sample and collection tubes into the Chill Rack.4. For a standard separation choose one the following programs:Positive selection:Posseld2for highest purityorPossels for highest recoveryCollect positive fraction in row C of the tube rack.3. Example of a separation usingCD45 (TIL) MicroBeadsA tumor induced by the B16F10 cell line was dissociated using the gentleMACS™ Octo Dissociator with Heaters in combination with the Tumor Dissociation Kit, mouse. CD45+ TILs were isolated from the single-cell suspension using CD45 (TIL) MicroBeads, an MS Column, and a MiniMACS™ Separator.Cells were fluorescently stained with CD45-PE and Labeling-Check-Reagent-VioBlue® and analyzed by flow cytometry using the MACSQuant® Analyzer. Cell debris and dead cells were excluded from the analysis based on scatter signals and propidium iodide fluorescence.Before separation10³-1110¹10²10³10²10¹CD45-PELabelingCheckReagent-VioBlue-11CD45+ cells10³-1110¹10²10³10²10¹CD45-PELabelingCheckReagent-VioBlue-11Refer to for all data sheets and protocols. Miltenyi Biotec provides technical support worldwide. Visit /local to find your nearest Miltenyi Biotec contact.Legal noticesLimited product warrantyMiltenyi Biotec B.V. & Co. KG and/or its affiliate(s) warrant this product to be free from material defects in workmanship and materials and to conform substantially with Miltenyi Biotec’s published specifications for the product at the time of order, under normal use and conditions in accordance with its applicable documentation, for a period beginning on the date of delivery of the product by Miltenyi Biotec or its authorized distributor and ending on the expiration date of the product’s applicable shelf life stated on the product label, packaging or documentation (as applicable) or, in the absence thereof, ONE (1) YEAR from date of delivery (“Product Warranty”). Miltenyi Biotec’s Product Warranty is provided subject to the warranty terms as set forth in Miltenyi Biotec’s G eneral Terms and Conditions for the Sale of Products and Services available on Miltenyi Biotec’s website at , as in effect at the time of order (“Product Warranty”). Additional terms may apply. BY USE OF THIS PRODUCT, THE CUSTOMER AGREES TO BE BOUND BY THESE TERMS.THE CUSTOMER IS SOLELY RESPONSIBLE FOR DETERMINING IF A PRODUCT IS SUITABLE FOR CUSTOMER’S PARTICULAR PURPOSE AND APPLICATION METHODS.Technical informationThe technical information, data, protocols, and other statements provided by Miltenyi Biotec in this document are based on information, tests, or experience which Miltenyi Biotec believes to be reliable, but the accuracy or completeness of such information is not guaranteed. Such technical information and data are intended for persons with knowledge and technical skills sufficient to assess and apply their own informed judgment to the information. Miltenyi Biotec shall not be liable for any technical or editorial errors or omissions contained herein.All information and specifications are subject to change without prior notice. Please contact Miltenyi Biotec Technical Support or visit for the most up-to-date information on Miltenyi Biotec products.LicensesThis product and/or its use may be covered by one or more pending or issued patents and/or may have certain limitations. Certain uses may be excluded by separate terms and conditions. Please contact your local Miltenyi Biotec representative or visit Miltenyi Biotec’s website at for more information.The purchase of this product conveys to the customer the non-transferable right to use the purchased amount of the product in research conducted by the customer (whether the customer is an academic or for-profit entity). This product may not be further sold. Additional terms and conditions (including the terms of a Limited Use Label License) may apply.CUSTOMER’S USE OF THIS PRODUCT MAY REQUIRE ADDITIONAL LICENSES DEPENDING ON THE SPECIFIC APPLICATION. THE CUSTOMER IS SOLELY RESPONSIBLE FOR DETERMINING FOR ITSELF WHETHER IT HAS ALL APPROPRIATE LICENSES IN PLACE. Miltenyi Biotec provides no warranty that customer’s use of this product does not and will not infringe intellectual property rights owned by a third party. BY USE OF THIS PRODUCT, THE CUSTOMER AGREES TO BE BOUND BY THESE TERMS.TrademarksautoMACS, gentleMACS, MACS, MACSQuant, MidiMACS, the Miltenyi Biotec logo, MiniMACS, MultiMACS, OctoMACS, QuadroMACS, REAfinity, SuperMACS, VarioMACS, Viobility, and VioBlue are registered trademarks or trademarks of Miltenyi Biotec and/or its affiliates in various countries worldwide.Copyright © 2020 Miltenyi Biotec and/or its affiliates. All rights reserved.。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
管理技术-林荣瑞

无菌检查培养基.....................................36 微生物限度检查培养基..................................36 抑菌剂效力检查培养基..................................37 青霉素酶制备培养基...................................38 抗生素检定培养基....................................38 支原检测培养基.....................................39 l 美国药典培养基(USP 标准) ..............................49 l 欧洲药典培养基(EP 标准)...............................40 u 临床检验系列.......................................42 u 军团菌检测培养基系列...................................44 u 菌种保存和样品运输培养基.................................45 u 其它菌检测培养基.....................................45 l 维生素微生物方法检测培养基.. . . . . .... ...................46 l 微生物菌肥检测培养基 .................................46 l 其他培养基 ......................................46 u 原材料系列........................................48 u 微生物学试验配套试剂...................................49 u 植物组织培养基......................................50 u 细菌生化鉴定管 ...... ...............................51 l 大肠杆菌生化鉴定管..................................51 l 沙门氏菌生化鉴定管..................................51 l 志贺氏菌生化鉴定管..................................51 l 大肠杆菌 O157 生化鉴定管 ...............................52 l 单增李斯特氏菌生化鉴定管...............................52 l 副溶血性弧菌生化鉴定管.................................53 l 霍乱弧菌生化鉴定管..................................53 l 蜡样芽孢杆菌生化鉴定管................................54 l 乳酸菌生化鉴定管 GB 标准 ...............................54 l 阪崎杆菌生化鉴定管 GB 标准 ..............................54 u 微生物成套生化鉴定管...................................55 u 培养基添加剂用量表....................................57
北京春达科技有限公司专营进口体外诊断试剂工业原料,透析产品,纯化填料,标准品

北京春达科技有限公司专营进⼝体外诊断试剂⼯业原料,透析产品,纯化填料,标准品SCLPP209碱性磷酸酶3.1.3.1Alkaline phosphatase from Calf intestine-Activity: >30000 U/mlGlycerol solution-Mw: 100,000-Store at -20 °CSCMAD211苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >40 U/mgYellowish amorphous powder-Mw: 140,000-Store at -20 °CSCMDH18C苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Pig heart-Activity: >1250 U/mgprot.White amorphous powder-Mw:140,000-Store at -20 °CSCMDL100苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >60 U/mgWhite amorphous powder-Mw: 80,000-Store at -20 °CSCMUT11C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1500 U/mgWhite amorphous powder-Mw: 40,000-Store at -20 °CSCMUT12C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1000 U/mlAmmonium sulfate suspension-Mw: 40,000-Store at 2-8 °CSCNAL301唾液酸醛缩酶4.1.3.3N-Acetylneuraminic acid aldolase from MicroorganismActivity: >15 U/mg-Yellowish amorphous powderMw: 98,000-Store at -20 °CSCPCO301原⼉茶酸3,4双加氧酶1.13.11.3Protocatechuate 3,4-dioxygenase from Pseudomonas sp.Activity: >3 U/mg-Light brown amorphous powderMw: 700,000-Store at -20 °CSCPEO131过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade IActivity: >250 U/mg-Reddish-brown amorphous powderMw: 40,000-Store at -20 °CSCPEO301过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade I-Activity: >250 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPEO302过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade III-Activity: >110 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPHO12C磷脂酶D Phospholipase D from Streptomyces chromofuscus3.1.4.4Activity: >40 U/mg-Brown amorphous powderMw: 57,000-Store at -20 °CSCPNP301嘌呤核苷磷酸化酶2.4.2.1Purine-nucleoside phosphorylase from MicroorganismActivity: >15 U/mg-White amorphous powderMw: 120,000-Store at -20 °CSCPPC301磷酸烯醇式丙酮酸羧化酶4.1.1.31Phosphoenolpyruvate carboxylase from Corn leavesActivity: >5 U/mg-White amorphous powderMw: 390,000-Store at -20 °CSCPSP101脯氨酸特定的肽链内切酶3.4.21.26Proline specific endopeptidase from Flavobacterium sp.Activity: >5 U/mg-White amorphous powder-Mw: 78,000-Store at -20 °CSCPYK302L丙酮酸激酶2.7.1.40Pyruvate kinase from Rabbit muscle-Activity:>2000 U/mlwhite ammonium sulphate suspension-Mw:237000-Store at 2-8℃SCPYO311丙酮酸氧化酶1.2.3.3Pyruvate oxidase from Microorganism-Activity: >1.5 U/mgYellowish amorphous powder-Mw: 260,000-Store at -20 °CSCSAO341肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 65,000-Store at -20 °CSCSAO351肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 43,000-Store at -20 °CSCSOD302超氧化物歧化酶1.15.1.1Superoxide dismutase from Bovine erythrocyte-Activity: >3000U/mgBluish-green amorphous powder-Mw: 32,000-Store at -20 °CSCUAO201尿酸酶1.7.3.3Uricase from Candida sp.-Activity: >4 U/mg-White amorphous powder-Mw: 120,000-Store at -20 °CSCUAO211尿酸酶1.7.3.3Uricase from Bacillus sp.-Activity:>1.5 U/mg-White amorphous powder-Mw: 150,000-Store at -20 °CSCURH10S脲酶3.5.1.5Urease from Jack bean-Activity: >220 U/mg-White amorphous powder-Mw: 480,000-Store at -20 °CSCURH16C脲素酶GUrease G from Jack bean-Activity: >150 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCURH201脲素酶Urease from Jack bean-Activity: >100 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCXTO212黄嘌呤氧化酶Xanthine oxidase from Microorganism-Activity: >10 U/mgReddish-brown amorphous powder-Mw: 160,000-Store at -20°C1.1.3.222.诊断与研究⽤的辅酶CODES PRODUCTS CAS #SCADP01C 腺苷5'-⼆磷酸单钾盐⼆⽔合物ADP-K.2H2O-Adenosine 5’-diphosphate monopotasium saltdihydrate-C10H14N5O10P2K.2H2O-Mw:501.30-Colorless crystals-Purity:>95 %72696-48-1SCADP22C 腺苷5’-⼆磷酸⼆钠盐ADP-Na2-Adenosine 5’-diphosphate disodium salt-C10H13N5O10P2Na2-Mw:471.20-White powder-Purity:>98 %16178-48-6SCAMP02C 腺苷-5’-磷酸AMP-Adenosine 5’-monophosphoric acid-C10H14N5O7P.H2O-Mw:365.20-White powder-Purity:>98 %18422-05-4SCAMP05C 腺苷5’-磷酸AMP-Na2-Adenosine 5’-monophosphate disodium salt -C10H12N5O7PNa2-Mw:391.18-White powder-Purity:>95%18422-05-4SCATP03C 腺苷5’-三磷酸⼆钠盐3⽔合物ATP-Na2.3H2O-Adenosine 5’-triphosphate disodium salttrihydrate -C10H14N5Na2O13P3.3H2O -Mw:605.19-White powder-Purity:>96 %987-65-5SCBNA207C β-烟酰胺-腺嘌呤⼆核苷酸NAD-β-Nicotinamide-adenine dinucleotide-C21H27N7O14P2-Mw:663.4-White powder-Purity:>98 %53-84-9SCBND210C β-烟酰胺腺嘌呤⼆核苷磷酸,还原型四钠盐NADPH-Na4-β-Nicotinamide-adenine dinucleotide phosphate, reducedtetrasodium salt-C21H26N7O17P3.Na4-Mw:833.40-White powder-Purity:>93 %2646-71-1SCBNH208C β-烟酰胺腺嘌呤⼆核苷酸,还原⼆钠盐NADH-Na2-β-Nicotinamide-adenine dinucleotide, reduceddisodium salt -C21H27N7P2O14Na2-Mw:709.40-White to yellowish powder-Purity:>93 %606-68-8β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐24292-60-2SCBNP209C β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐NADP-Na2-β-Nicotinamide-adenine dinucleotide phosphatedisodium salt-C21H26N7O17P3Na2-Mw:787.40-Yellowishpowder-Purity:>97 %24292-60-2SCCOA11C 辅酶A三锂盐Coenzyme A trilithium salt-C21H33Li3N16O7P3S-Mw:785.40-White powder-Purity:>85 %18439-24-2SCDAD631C ⼆(腺苷-5’-)五磷酸三锂盐Ap5A-Li3-P1,P5 –Di(adenosine -5’-)pentaphosphate trilithiumsalt-C20H26N10O22P5Li3-Mw:934.20-Yellowish powder-Purity:>95 %75522-97-3SCFAD11C 黄素腺嘌呤⼆核苷酸⼆钠盐FAD- Na2-Flavine-adenine dinucleotide disodium salt-C27H31N9O15P2Na2-Mw:829.60-Orange powder-Purity:>93 %146-14-5SCNAL24C N-⼄酰-L-半胱氨酸N-Acetyl-L-cysteine-C5H9NO3S-Mw:163.20-White powder-Purity:>98 %616-91-1SASNAD 硫代辅酶IThio-NAD-C21H27N7O13SP2-Purity:≥ 90%4090-29-3SASNADP 硫代辅酶IIThio-NADP-C21H27N7O16P3S•Na- Purity:≥ 90%19254-05-8SAAldNAD 3-吡啶⼄醛腺嘌呤⼆核苷酸3-Pyridinealdehyde adenine dinucleotide C21H27N6O14P21986-7-7SAAC1023-⼄酰基吡啶腺嘌呤⼆核苷酸磷酸钠(Ac-NADP) APADP-C22H28N6O17P3•Na -Purity:≥ 80%102029-67-4SANAAD 烟酸腺嘌呤⼆核苷酸Nicotinic Acid Adenine Dinucleotide- C21H26N6O15P2104809-30-5SADeNAD 脱氨基NADDeamino Nicotinamide Adenine Dinucleotide-C21H26N6O15P2104809-38-3SAGGNAFB γ-L-⾕氨酰基-4-硝基苯胺Gamma-L-glutamyl-4-nitroanilide,-C11H13N3O5-Purity:≥ 96%7300-59-6SACGGN L-γ-⾕氨酸-(3-甲酸-4硝基苯胺)铵盐L-Glutamic Acid Gamma- (3-Carboxy-4-Nitroanilide),Ammonium Salt-C12H12N3O7P•NH4-Purity:≥ 96%63699-78-5SAPEPCHA 磷酸烯醇丙酮酸单环⼰胺盐PEP-C3H4O6P•C6H13N-Purity :>95%10526-80-4SAPEPK 磷酸烯醇式丙酮酸单钾盐PEP-C3H4O6P•K-Purity :>95%4265-07-03.诊断与研究⽤的底物CODES PRODUCTS CAS #SCAKE05C α–酮戊⼆酸⼆钠盐⼆⽔合物α–Ketoglutaric acid, disodium salt dihydrate-C5H4O5Na2.2H2O-Mw:226.10 -White powder-Purity:>98%305-72-6α–酮戊⼆游离酸328-50-7SCAKE115C α–酮戊⼆游离酸α–Ketoglutaric free acid-C5H4O5-Mw:146.10 -White powder-Purity:>99%328-50-7SCBTC06S-丁酰硫胆碱碘S-Butyrylthiocholine Iodide-C9H20NOSI-Mw:317.23 -White powder-Purity:>97%1866-16-6SCCNP005Gal-G2-α-CNP-C28H51O18Cl-Mw:659.98 -White powder-Purity:>90 %157381-11-8SCCRP59C 磷酸肌酸⼆钠盐四⽔合物Phosphocreatine disodium salt tetrahydrate-C4H8N3O5PNa2.4H2O-Mw:327.15 -White powder-Purity:>97 %19333-65-4SCGGC106C ⽢氨酰⽢氨酸Glycylglycine-C4H8N2O3-Mw:132.12-White powder-Purity:>98 %556-50-3SCGLT100L-⾕氨酸L-Glutamic acid-C5H9N2O4- Mw:147.13-White powder-purity:>99%617-65-2SCGPS15C 葡萄糖-6-磷酸⼆钠盐Glucose-6-phosphate disodium salt-C6H11O9PNa2-Mw:304.20-White powder-Purity:>95 %3671-99-6SCLAC171C DL-乳酸锂盐DL-Lactic acid Lithium salt-C3H5O3Li-Mw:96.01-White powder-Purity:>95%16891-53-5SCLAL29C L-丙氨酸游离酸L-Alanine free acid-C3H7NO2-Mw:89.09-White powder-Purity:>99%56-41-7SCLAP41C L-天门冬氨酸L-Aspartic acid-C4H7NO4-Mw:133.10-White powder-Purity:>99 %56-84-8SCLAP42C L-天门冬氨酸,钠盐⼀⽔合物L-Aspartic acid, sodium salt monohydrate-C4H6NO4Na.H2O-Mw:173.10-White powder-Purity:>98 %323194-76-9SCLAP43C L-天门冬氨酸,镁⼆⽔合物L-Aspartic acid, magnesium salt dihydrate-C8H12N2O8Mg.2H2O-MW:324.50-White powder-Purity:>98 %2068-80-6SCLGC244C L-γ-⾕氨酰-3-羧基-4-硝基苯胺Glupa C-L-γ-Glutamyl-3-carboxy-4-nitranilide-C12H12N3O7NH4-Mw:328.30-Yellow powder-Purity:>99 %63699-78-5SCNAY138C 萘磷酸单钠盐Naphtyl phosphate, monosodium salt-C10H8NaO4P.H2O-Mw:264.15-White powder-Purity:>98%81012-89-7SCPG701C 亚⼄基降-4-硝基苯基-β-D-麦芽庚糖苷pNP-G7-Ethylidene-4-nitrophenyl-D-maltoheptaoside-C50H77NO38-Mw:1300.10-Yellowish powder-Purity:>90%96597-16-9对硝基苯基磷酸酯,⼆钠盐六⽔合物4264-83-9SCPNP264C 对硝基苯基磷酸酯,⼆钠盐六⽔合物PNPP-p-Nitrophenylphosphate, disodium salt hexahydrate-C6H4NO6PNa2.6H2O-Mw :371.10-White to yellow powder-Purity:>98%4264-83-9SCPNS265C p-硝基苯基磷酸酯,⼆tris盐PNPP diTris-p-Nitrophenylphosphate, ditris salt-C14H28N3O12P-Mw:461.40-White powder68189-42-4SCPYN100丙酮酸钠Sodium pyruvate-C3H3NaO3-Mw:110-White powder-Purity:>99%113-24-64.缓冲液Buffers for diagnostic & researchPRODUCTS CAS # N-(2-⼄酰胺基)-2-氨基⼄磺酸ACES-N-(2-Acetamido)-2-aminoethanesulfonic acid7365-82-4N-(2-⼄酰胺基)亚氨基⼆⼄酸ADA-(N-(2-Acetamido)iminodiacetic acid)26239-55-4 2-氨基-2-甲基-1-丙醇AMT -2-amino-2-methyl-1-propanol124-68-5 N,N-双(2-羟⼄基)-2-氨基⼄磺酸BES-N,N-Bis-(2-Hydroxyethyl)-2-Aminoethanesulfonic acid10191-18-1 N,N-双-(2-羟基⼄基)⽢氨酸Bicine-N,N-Bis-(2-Hydroxyethyl)glycine150-25-4 N-环⼰基-3-氨基丙磺酸CAPS-N-Cyclohexyl-3-aminopropanesulfonicacid1135-40-6N-环⼰基-2-羟基-3-氨基丙磺酸CAPSO -N-Cyclohexyl-2-hydroxy-3-aminopropanesulfonic acid73463-39-5。
ADC药物研发现状

Title Originator Highest Dev Status 111In-capromab pendetide Cytogen Corp Launched111In-imciromab pentetate Janssen Biotech Inc Launched131I-chTNT-1/B Peregrine Pharmaceuticals Inc Launched131I-metuximab Fourth Military Medical University PLA Launchedbrentuximab vedotin Seattle Genetics Inc Launchedgemtuzumab Wyeth Research Launchedibritumomab tiuxetan IDEC Pharmaceuticals Corp Launchedtrastuzumab emtansine Genentech Inc LaunchedATL-101, ATLAB Cornell University Phase 3 Clinical inotuzumab ozogamicin Wyeth Research Phase 3 Clinical oportuzumab monatox (intratumoral, head and neck cancer), Viventia University of Zurich Phase 3 ClinicalRIGS CC49Navidea Biopharmaceuticals Inc Phase 3 ClinicalABT-414Abbott Laboratories Phase 2 ClinicalCDX-1401Celldex Therapeutics Inc (pre-merger)Phase 2 Clinical glembatumumab vedotin CuraGen Corp Phase 2 ClinicalLMB-2National Cancer Institute Phase 2 Clinical lorvotuzumab mertansine ImmunoGen Inc Phase 2 Clinical moxetumomab pasudotox National Cancer Institute Phase 2 Clinical oportuzumab monatox (intravesicular, bladder cancer), Viventia University of Zurich Phase 2 ClinicalPSMA-ADC Cytogen Corp Phase 2 ClinicalRG-7593Genentech Inc Phase 2 ClinicalRG-7596Genentech Inc Phase 2 ClinicalSAR-3419ImmunoGen Inc Phase 2 Clinical212-Pb-TCMC-trastuzumab National Cancer Institute Phase 1 ClinicalActimab-A PDL BioPharma Inc Phase 1 ClinicalAGS-16M8F Agensys Inc Phase 1 Clinicalanti-CD3/anti-CD20 bispecific antibody-armed activated T-cells (non-Hodgkin's lymphoma), Wayne State University/Barbara Ann KarmanosCancer Institute Barbara Ann Karmanos Cancer Institute Phase 1 ClinicalASG-5ME Agensys Inc Phase 1 ClinicalBAY-79-4620MorphoSys AG Phase 1 Clinical citatuzumab bogatox Viventia Biotech Inc Phase 1 Clinical doxorubicin-loaded anti-EGFR immunoliposomes (solid tumors), UniversityHospital Basel University Hospital of Basel Phase 1 ClinicalHuM195/rGel (intravenous infusion, AML/CML/meylodisplastic syndrome),Targa Therapeutics Memorial Sloan-Kettering Cancer Center Phase 1 ClinicalIMGN-529ImmunoGen Inc Phase 1 ClinicalIMGN-853ImmunoGen Inc Phase 1 ClinicalIMMU-132Immunomedics Inc Phase 1 Clinical labetuzumab-SN-38Immunomedics Inc Phase 1 ClinicalNHS-IL-12National Cancer Institute Phase 1 ClinicalRG-7450Genentech Inc Phase 1 ClinicalRG-7458Genentech Inc Phase 1 Clinical RG-7598Genentech Inc Phase 1 Clinical RG-7599Genentech Inc Phase 1 Clinical RG-7600Genentech Inc Phase 1 Clinical RG-7636Genentech Inc Phase 1 Clinical SAR-566658ImmunoGen Inc Phase 1 Clinical T-Guard University Medical Center St Radboud Phase 1 Clinical vorsetuzumab mafodotin Seattle Genetics Inc Phase 1 Clinical 131I-catuximab (colorectal cancer), Pacific Meinuoke Fourth Military Medical University PLA Discovery177Lu-tetraxetan-tetulomab (non-Hodgkin's lymphoma), Nordic Nanovector Nordic Nanovector AS Discovery227Th-epratuzumab (hematological cancer), Algeta Algeta ASA Discovery227Th-rituximab (cancer), Algeta Algeta ASA Discovery227Th-trastuzumab (cancer), Algeta Algeta ASA Discovery4s3-0014s3 Bioscience Inc Discovery4s3-0024s3 Bioscience Inc Discovery64Cu-NOTA-ALT-836Altor BioScience Corp DiscoveryAA-A225Actinium Pharmaceuticals Inc Discovery AbGn-107AbGenomics Corp Discovery Actimab-B Fred Hutchinson Cancer Research Center Discovery Actimab-C Actinium Pharmaceuticals Inc Discovery Actimab-P Actinium Pharmaceuticals Inc Discovery adalimumab + anti-Ang2 Zybody (rheumatoid arthritis/inflammatory boweldisease), Zyngenia Zyngenia Inc DiscoveryAGS-15E ADC Agensys Inc DiscoveryAGT-160ArmaGen Technologies Inc DiscoveryAGT-185ArmaGen Technologies Inc DiscoveryAGT-190ArmaGen Technologies Inc Discovery amanitin-trastuzumab conjugate (cancer), Heidelberg Pharma Heidelberg Pharma Holding Ltd Discoveryanti-CD133-immunotoxin conjugates (photochemical internalization, cancer),PCI Biotech PCI Biotech Holding ASA Discoveryanti-ET8R-MC-vc-PAB-MMAE Genentech Inc Discoveryanti-NaPi3b antibody-drug conjugate (cancer), Genentech/Roche Genentech Inc Discovery antibody drug conjugates (cancer), Sanofi Sanofi Discovery antibody-drug conjugates (cancer), ADC Therapeutics ADC Therapeutics Sarl Discovery antibody-drug conjugates (cancer), Seattle Genetics/Oxford BioTherapeutics Seattle Genetics Inc Discovery antibody-drug conjugates (TAP, cancer), Lilly Eli Lilly & Co Discovery antibody-IFN lambda conjugates (cancer), Immunomedics Immunomedics Inc Discovery anticancer therapy (TAP technology), Amgen Amgen Inc DiscoveryAPH-0912Aphios Corp Discovery Aurixin BioIntegrator DiscoveryAZ-05Allozyne Inc Discovery BIOO-1BIOO Therapeutics DiscoveryBIOO-2BIOO Therapeutics Discovery BIOO-3BIOO Therapeutics Discovery BIOO-4BIOO Therapeutics Discovery BIOO-5BIOO Therapeutics Discovery BIOO-6BIOO Therapeutics Discovery BIOO-7BIOO Therapeutics Discovery botulinum toxin B inhibitor (injectable, heteropolymer mAbs, botulism),Immunome Immunome Inc Discovery BT-2111biOasis Technologies Inc Discovery C2-2b-2b Immunomedics Inc Discovery CDX-014CuraGen Corp Discovery chiHEA-125-Ama Heidelberg Pharma Holding Ltd Discovery CK-22-(20)-(20)Immunomedics Inc Discovery complement factor H-derived short consensus repeat-antibody constructs(infection), LysoVac University of Innsbruck Discovery Cymac-001Cytoguide ApS Discovery CYP-Ab Cytune Pharma Discovery D2C7-based immunotoxins (glioma), Duke University Duke University Discovery EGFR modulators (antibody conjugates, PIT, cancer), Aspyrian Aspyrian Therapeutics Inc Discovery engineered cysteine drug conjugates mAbs (cancer), Seattle Genetics Seattle Genetics Inc Discovery epratuzumab-SN-38Immunomedics Inc Discovery ETBs (cancer), Molecular Templates/ Imclone Molecular Templates Inc Discovery Fluorescent-labeled bevacizumab (imaging, ocular disease), Mivenion mivenion Gmbh Discovery gemcitabine + paclitaxel (prodrug, nanomAb, cancer), ImmunePharmaceuticals Immune Pharmaceuticals Corp Discovery Herceptin:Endostatin-P125A University of Miami Discovery hLL1-CL2A-SN-38Immunomedics Inc Discovery hPAM4-CL2A-SN-38Immunomedics Inc Discovery human monoclonal antibody-toxin conjugates (myocardial infarction), Celdara Celdara Medical LLC Discovery HuMax-TF-ADC Genmab A/S Discovery IFNalpha-fused mAbs (HBV infection), Roche Roche Holding AG Discovery IL-13 receptor alpha 2 inhibitors (iv, cancer), Pfizer Pfizer Inc Discovery IMGN-289ImmunoGen Inc Discovery intracellular antibodies (Intraphilin, inflammatory diseases/infectiousdiseases/ophthalmic diseases), Permeon Biologics Permeon Biologics Inc Discovery mapp-66Mapp Biopharmaceutical Inc Discovery MB-2003Mapp Biopharmaceutical Inc Discovery monoclonal antibody-drug conjugates, Chirogenix/ImmunoGen/Celltrion Chirogenix Co Ltd Discovery MP-Ter-ADC MediaPharma Srl Discovery N01-OX2Intellect Neurosciences Inc Discovery PC-91ProCell Therapeutics Inc Discovery ProstaLite PhotoBiotics Ltd Discoveryrecombinant mAb-biocide fusion proteins (oral/Directed Biocide,cryptosporidium infection), ioGenetics ioGenetics Inc Discovery SGN-CD33A Seattle Genetics Inc Discovery SGN-LIV1A Seattle Genetics Inc Discovery SL-101Stemline Therapeutics Inc Discovery SYD-983Synthon Biopharmaceuticals Discovery T01-OX2Intellect Neurosciences Inc Discovery TBL-0306L Transgene Biotek Ltd Discovery TBL-0306M Transgene Biotek Ltd Discovery TBL-0805E Transgene Biotek Ltd Discovery thio-trastuzumab-mpeo-DM1Genentech Inc Discovery trastuzumab-PNU-159682 antibody-drug conjugate (cancer), Genentech Genentech Inc Discovery veltuzumab-IFN alpha 2b conjugate (cancer), IBC/Immunomedics IBC Pharmaceuticals Inc Discovery BIIB-015Biogen Inc Suspended Pretarget technology (gastrointestinal adenocarcinoma), NeoRx Poniard Pharmaceuticals Inc Suspended125I-AnnA1 IgG Sidney Kimmel Cancer Center No Development Reported131I-CC49-SCA Enzon Labs Inc No Development Reported177Lu-capromab pendetide Cytogen Corp No Development Reported90Y-capromab pendetide Cytogen Corp No Development Reported99mTC-BERH2Medac GmbH No Development Reportedanti-CD133-vcMMAF Seattle Genetics Inc No Development Reportedanti-CD22 antibody drug-conjugates, Medarex/BMS Medarex Inc No Development Reportedanti-PSMA antibody-drug conjugates (cancer), Medarex Medarex Inc No Development Reportedantibody-drug conjugates (solid tumors), Daiichi Sankyo Seattle Genetics Inc No Development ReportedAVE-9633ImmunoGen Inc No Development Reportedbectumomab Immunomedics Inc No Development ReportedCA125/MUC16-targeting antibody-drug conjugate (ovarian cancer),Genentech Genentech Inc No Development Reportedcathepsin B-sensitive prodrugs, BMS Bristol-Myers Squibb Pharmaceutical ResearchInstituteNo DevelopmentReportedCC49 humanized radioimmunoconjugates, National Cancer Institute,University of Alabama at Birmingham National Cancer Institute No Development ReportedCD4-BFFI Roche Holding AG No Development ReportedCHB-111ViRexx Medical Corp No Development ReportedCHT-25University College London No Development ReportedCMD-193Wyeth No Development ReportedCNTO-95 immunoconjugates (cancer), Centocor Janssen Biotech Inc No Development Reportedconjugated PEI/anti-CD133 mAb plasmid-based gene therapy (brain tumor),Discovery genomics Discovery Genomics Inc No Development ReportedcT84.66City of Hope No Development Reporteddelta 9-cadherin targeting antibody (gastric cancer), Actinium Helmholtz Zentrum München No Development Reporteddiphtheria toxin, RCT Research Corporation Technologies No Development Reporteddoxorubicin-C225 conjugate (STEALTH), SEQUUS SEQUUS Pharmaceuticals Inc No Development ReportedDTPA-BrE-3University of Colorado System No Development ReportedDXL-625InNexus Biotechnology Inc No Development ReportedG3.519-PAP-S Tanox Inc No Development ReportedhuHMFG1-caspase Antisoma plc No Development ReportedIMGN-007ImmunoGen Inc No Development ReportedIMGN-009ImmunoGen Inc No Development Reportedimmunoconjugate (cancer MN), Bayer Bayer AG No Development ReportedImmuRAID-AFP-99mTc, Immunomedics Immunomedics Inc No Development ReportedIMTOX 22-97A University of Texas Southwestern MedicalCenterNo DevelopmentReportedKSB-201KS Biomedix Holdings plc No Development ReportedLA22-radioimmunoconjugates (cancer), Welson/Peking University Welson Pharmaceuticals Inc No Development Reportedlabetuzumab Immunomedics Inc No Development ReportedLMB-1, NIH National Institutes of Health No Development ReportedLu-177-trastuzumab Tarbiat Modares University No Development ReportedLymphoScan Immunomedics Inc No Development ReportedMDX-11Medarex Inc No Development ReportedMDX-1203Medarex Inc No Development ReportedMDX-1206Medarex Inc No Development Reportedmonoclonal-porphyrins, Quadra Logic QLT Inc No Development Reportedmonoclonals, Quest Quest Biotechnology Inc No Development ReportedNogo receptor modulators, Biogen Idec Yale University No Development ReportedONS-1210Oncobiologics Inc No Development ReportedOP-06 program (cancer), Onco-Pharmakon Onco-Pharmakon Inc No Development Reportedpaclitaxel analogs and immunoconjugates (cancer), Bioxel Bioxel Pharma Inc No Development ReportedPE38-conjugated anti-CD30 immunotoxin, NCI National Cancer Institute No Development Reportedprostate-specific MAb, NIH National Institutes of Health No Development ReportedR-1549The UK Imperial Cancer Research Fund No Development Reportedradiolabeled Tx3.833Beth Israel Deaconess Medical Center No Development Reportedscu-PA-59D8Bristol-Myers Squibb Co No Development ReportedSGN-17/19Seattle Genetics Inc No Development Reportedtaxane-monoclonal antibody conjugates, ImClone ImClone Systems Inc No Development Reportedtranscobalamin (vitamin B12) receptor-targeting mAbTCR23-saporinconjugate (cancer), Kyto Kyto Biopharma Inc No Development Reportedtrastuzumab-autophilic peptide conjugate (breast cancer), InNexusBiotechnology InNexus Biotechnology Inc No Development Reportedtrastuzumab-MC-vc-PAB-MMAF Genentech Inc No Development ReportedTRP-targeted antibody conjugate (Yttrium 90/MX-DTPA), SomantaPharmaceuticals Immunodex Inc No Development Reportedtucotuzumab celmoleukin EMD Lexigen Research Center Corp No Development ReportedVB4-011Viventia Biotech Inc No Development ReportedVB6-011Viventia Biotech Inc No Development ReportedVB6-050Viventia Biotech Inc No Development ReportedXomaZyme-791XOMA Corp No Development Reportednofetumomab Poniard Pharmaceuticals Inc Withdrawn 131I-81C6Duke University Discontinued 131I-ImmuRAIT-HCG, Immunomedics Immunomedics Inc Discontinued B-B4-DC1ImmunoGen Inc Discontinued CC49 radioimmunoconjugates, University of Alabama at Birmingham University of Alabama at Birmingham Discontinued CD5 monoclonals/RIPs, Italfarmaco Italfarmaco SpA Discontinued CVX-045CovX Pharmaceuticals Inc Discontinued CVX-060CovX Pharmaceuticals Inc Discontinued CVX-241CovX Pharmaceuticals Inc Discontinued CVX-343CovX Pharmaceuticals Inc Discontinued doxorubicin-BR96 conjugate, BMS Bristol-Myers Squibb Co Discontinued doxorubicin-CEA conjugate, Immunomedics Immunomedics Inc Discontinued doxorubicin-LL2 conjugate, Immunomedics Immunomedics Inc Discontinued FAP5-DM1Boehringer Ingelheim Corp Discontinued FGFR4-CovX-Body CovX Pharmaceuticals Inc Discontinued HuM-195-Bi-213PDL BioPharma Inc Discontinued human placental growth factor 1-CVX-2000 monoclonal antibody conjugatedtherapeutic (CovX-body, cancer), CovX CovX Pharmaceuticals Inc Discontinued huN901-CC-1065ImmunoGen Inc Discontinued huN901-DC1ImmunoGen Inc Discontinued ImmuRAID-HCG-99mTc, Immunomedics Immunomedics Inc Discontinued ImmuRAIT-CEA-rhenium-188, Immunomedics Immunomedics Inc Discontinued MDX-214Medarex Inc Discontinued MEDI-547MedImmune LLC Discontinued MLN-2704Cornell University Discontinued Oncolym Peregrine Pharmaceuticals Inc Discontinued Oncolysin B Dana-Farber Cancer Institute Inc DiscontinuedOncolysin CD6Dana-Farber Cancer Institute Inc Discontinued Oncolysin M Dana-Farber Cancer Institute Inc Discontinued Oncolysin S Dana-Farber Cancer Institute Inc Discontinued Oncopurge Poniard Pharmaceuticals Inc Discontinued rhenium-188-LL2, Immunomedics Immunomedics Inc Discontinued SMART ABL-364Novartis AG Discontinued targeted ranpirnase conjugates (cancer), Alfacell Tamir Biotechnology Inc DiscontinuedHighest Dev Status。
GLP Bio产品说明书:Nystatin (Fungicidin) (GC10090)

Product Data Sheet Product Name:Nystatin (Fungicidin)Cat. No.:GC10090Chemical PropertiesCas No.1400-61-9化学名(4E,6E,8E,10E,14E,16E,18S,19R,20R,21S,35S)-3-[(2S,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,14,16-hexaene-38-carboxylic acidCanonical SMILES CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O )O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O分子式C47H75NO17分子量926.09溶解度≥ 30.45 mg/mL in DMSO储存条件-20°C, sealed storage, away from moisture and light,unstable in solution, ready to use.General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProtocolCell experiment [1]:Cell lines Oral Candida species and human buccal epithelial cellsPreparation method The solubility of this compound in DMSO is > 30.5 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months.Reacting condition 1 hrApplications The minimal inhibitory concentrations (μg/mL) of Nystatin for C. albicans, C. tropicalis, C. krusei, C. parapsilosis, C. glabrata and C. guilliermondii in RPMI broth were 0.78 ~ 1.56, 1.56 ~ 3.12, 3.12, 1.56 ~ 3.12, 0.78 ~ 1.56 and 0.39 ~ 0.78, respectively. Compared with the control group, Nystatin significantly reduced adhesion of 6 Candida species to buccal epithelial cells. However, the adhesion of C. albicans isolates was least affected by Nystatin treatment, which was significantly different from that of the non-albicans species.Animal experiment [2]:Animal models Aspergillus-infected, neutropenic mice Dosage form2, 4, 6 and 8 mg/kg/day; i.v.Product Data SheetApplications At a dose as low as 2 mg/kg/day, Liposomal Nystatin significantly protected neutropenic mice from Aspergillus-induced death compared to either the no-treatment, the saline or the empty-liposome group. Liposomal Nystatin-treated mice showed no evidence of Aspergillusinfection either at day 5 in all of the treatment groups or at day 52 in the 8 mg/kg/dayliposomal-Nystatin treatment group.Other notesPlease test the solubility of all compounds indoor, and the actual solubility may slightly differwith the theoretical value. This is caused by an experimental system error and it is normal.References:[1]. Ellepola AN, Panagoda GJ, Samaranayake LP. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol Immunol. 1999 Dec;14(6):358-63.[2]. Wallace TL, Paetznick V, Cossum PA, Lopez-Berestein G, Rex JH, Anaissie E. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob Agents Chemother. 1997Oct;41(10):2238-43.BackgroundNystatin (Fungicidin) is a polyene antifungal antibiotic [1].Antifungal antibiotic is a pharmaceutical fungicide used to treat and prevent mycoses.Nystatin is a polyene antifungal antibiotic that is effective against yeast and mycoplasma [1]. In liquid media,Nystatin inhibited C. albicans at concentrations of 5-20 U/ml[2].In a 200 clinical isolates, which comprised of 113 Candida albicans, 54 Candida glabrata, 11 Candida parapsilosis, 11Candida tropicalis and 11 Candida krusei. Nystatin exhibited MIC90 value of 4 mg/L against C. albicans isolates and all non-albicans Candida species tested. The results confirmed C. Albicans was most frequently susceptible andNystatin could be used to treat vulvovaginal candidiasis caused by non-albicans Candida species. Nystatin would be an important choice for women affected by non-albicans Candida species which present higher resistance to the imidazole-based treatments [3].制霉菌素(Fungicidin )是一种多烯类抗真菌抗生素[1]。
各种杂质及价格-

专业承接美国、日本、法国、德国、瑞士、意大利、英国等国家参比制剂一次性进口和代购业务,提供进口(EP、USP、LGC、BP、TLC、TRC、QCC、MC)和国产对照品,有需要的欢迎联系我咨询哈.市振强生物技术劳先生QQ3004867396各种杂质名称及英文名:依鲁替尼(Ibrutinib)杂质6个,palbociclib杂质6个,泰地唑胺杂质tedizolid phosphate 12个达格列净杂质Dapagliflozin 7个,索非布韦杂质sofosbuvir 20个,替卡格雷杂质Ticagrelor 14个,米拉贝隆杂质Mirabegron 10个,TAK 438杂质10个,沃替西汀杂质Vortioxetine 15个,LCZ696杂质6个非布司他杂质Febuxostat 14个泊沙康唑异构体Posaconazole 14个阿普斯特杂质Apremilast 6个阿奇霉素杂质Azithromycin 14个阿考替胺杂质Acotiamide 14个依托考昔杂质Etoricoxib 14个尼达尼布杂质Nintedanib 8个罗库溴铵杂质Rocuronium Bromide 8个恩杂鲁胺杂质Enzalutamide 4个卢帕他定杂质Rupatadine 6个瑞格非尼杂质Regorafenib 20个色瑞替尼杂质Ceritinib 11个依美斯汀杂质Emedastine8个依匹唑派杂质Brexpiprazole6个依帕司他杂质epalrestat 6个乌苯美司杂质Ubenimex 19个福多司坦杂质Fudosteine 6个马来酸匹杉琼杂质Maleic acid Chinese fir, Joan 6个扎鲁司特杂质Zafirlukast 6个贝利司他杂质belinostat 6个奥扎格雷的杂质ozagrel 6个酒石酸伐尼克兰片杂质Varenicline T artrate T ablets 6个莫扎伐普坦杂质Mozavaptan 5个沙芬酰胺杂质Safinamide 4个沃雷生杂质suvorexant 5个依替巴肽杂质Eptifibatide 5个乐伐替尼杂质lenvatinib 8个1.埃索美拉唑杂质esomeprazole impurity2.奥拉西坦杂质oxiracetam3.罗氟司特杂质roflumilast4.阿戈美拉汀杂质Agomelatine5.鲁拉西酮杂质Lurasidone6.莫西沙星杂质moxifloxacin7.阿齐沙坦杂质Azilsartan8.达比加群酯杂质Pradaxa9.利拉利汀杂质Linagliptin10.托法替尼杂质T ofacitinib11.依托考昔杂质12.阿西替尼杂质Axitinib13.维格列汀杂质Vildagliptin14.帕瑞昔布杂质parecoxib15.伊马替尼杂质imatinib16.阿哌沙班杂质Apixaban17.替诺福韦酯杂质T enofovir Disoproxil Fumarate18.普拉格雷杂质Prasugrel19.伊拉地平杂质isradipine20.利托那韦杂质ritonavir21.培美曲塞二钠杂质pemetrexed disodium22.依达拉奉杂质Edaravone23.吉非替尼杂质gefitinib24.替吉奥杂质BCB25.苯达莫司汀杂质Cephalon26.替加环素杂质Tigecycline27.布南色林杂质Blonanserin28.文拉法辛杂质venlafaxine29.替卡格雷杂质30.利伐沙班杂质Rivaroxaban31.伊曲茶碱杂质Istradefylline32.依度沙班杂质Edoxaban33.三氟胸苷杂质Trifluorothymidine34.盐酸阿考替胺杂质acotiamide hydrochloride35.度洛西汀杂质Duloxetine36.泊沙康唑杂质37.泰地唑胺杂质38.沃替西汀杂质39.乐伐替尼杂志40.卡博替尼杂质Cabozantinib41.依鲁替尼杂质42.恩格列净杂质EMpagliflozin43.辛伐他汀杂质simvastatin44.恩杂鲁胺杂质45. 阿苯达唑Albendazole46. 阿达帕林adapalene47. 阿夫唑嗪alfuzosin48. 阿卡地新acadesine49. 阿立哌唑aripiprazole50. 阿莫曲普坦almotriptan51. 阿莫西林amoxicillin52. 阿瑞吡坦Aprepitant53. 阿昔洛韦acyclovir54. 埃罗替尼erlotinib55. 安非他酮bupropion56. 氨苄青霉素ampicillin57. 氨基葡萄糖Glucosamine58. 氨甲环酸tranexamic59. 氨溴索Ambroxol60. 胺碘酮Amiodarone61. 奥氮平olanzapine62. 奥沙利铂Oxaliplatin63. 奥司他韦oseltamivir64. 保胆键素dihydroxydibutylether65. 保特佐米Bortezomib66. 苯达莫司汀Bendamustin67. 比卡鲁胺bicalutamide68. 吡罗昔康piroxicam69. 吡嗪酰胺Pyrazinamide70. 别嘌醇allopurinol71. 波生坦bosentan72. 布洛芬Ibuprofen73. 布美他尼Bumetanide74. 雌甾四烯estratetraenol75. 醋氯芬酸aceclofenac76. 达非那新Darifenacin77. 大黄酸Diacerein78. 地尔硫卓diltiazem79. 地拉罗司deferasirox80. 氨氯地平Amlodipine81. 硝苯地平nifedipine82. 甲氨蝶呤Methotrexate83. 氨基蝶呤Aminopterin84. 丁螺环酮buspirone85. 多奈哌齐Donepezi86. 多立酮Domperidone87. 恩丹西酮ondansetron88. 恩他卡朋entacapone89. 伐昔洛韦valacyclovir90. 泛昔洛韦famciclovir91. 非布索坦Febuxostat92. 非那雄胺inasteride-ep93. 非诺贝特fenofibrate94. 弗斯特罗定fesoterodine95. 伏立康唑Voriconazole96. 氟替卡松丙酸酯fluticasone-propionate97. 氟维司群Fulvestrant98. 格列吡嗪glipizide99. 桂利嗪cinnarizine100. 环苯扎林cyclobenzaprine101. 加巴喷丁gabapentin102. 甲状旁腺激素西那卡塞Cinacalcet 103. 甲状腺素Levothyroxine104. 卡巴拉汀利凡斯的明Rivastigmine RC's 105. 喹硫平Quetiapine106. 奥美拉唑Omeprazole107. 兰索拉唑Lansoprazol108. 雷贝拉唑Rabeprazole109. 泮托拉唑pantoprazol110. 来氟米特leflunomide111. 雷洛昔芬raloxifene112. 雷莫拉宁Ramoplanin113. 雷奈佐利Linezolid114. 利伐沙班Rivaroxaban115. 利培酮Risperidal116. 罗匹尼罗ropinirole117. 阿替洛尔Atenolol118. 比索洛尔Bisoprolol119. 醋丁洛尔Acebutolol120. 美托洛尔metoprolol121. 奈必洛尔nebivolol122. 氯吡格雷Clopidogrel123. 氯雷他定Loratadine124. 霉酚酸mycophenolate125. 美洛昔康meloxicam126. 孟鲁司特montelukast127. 米氮平mirtazapine128. 尼美舒利nimesulide129. 帕罗西汀Paroxetine130. 帕立酮Paliperidone131. 生丁Dipyridamole Dipyridamole 132. 培美曲塞二钠Pemetrexed-disodium 133. 普拉克索Pramipexole134. 喹那普利Quinapril135. 卡托普利captopril136. 赖诺普利Lisinopril137. 雷米普利Ramipril138. 培哚普利Perindopril Imp139. 群多普利Trandolapril140. 伊拉普利Enalapril141. 普瑞巴林pregabalin142. 瑞格列奈Repaglinide143. 塞来西布Celecoxib144. 噻托溴铵Tiotropium bromide 145. 沙丁胺醇salbutamol146. 沙美特罗salmeterol147. 奥美沙坦Olmesartan148. 坎地沙坦Candesartan 149. 罗沙坦Losartan150. 替米沙坦T elmisartan 151. 缬沙坦Valsartan152. 加替沙星gatifloxacin 153. 氟哌酸norfloxacin154. 菲宁达、氧氟沙星Ofloxacin 155. 恩诺沙星enrofloxacin 156. 环丙沙星Ciprofloxacin 157. 莫西沙星moxifloxacin 158. 左氧氟沙星Levofloxacin 159. 舍曲林Sertraline160. 舒马曲坦sumatriptan 161. 双醋瑞因diacerein 162. 双氯芬酸Diclofenac 163. 他达那非T adalafil 164. 阿托伐他汀atorvastatin 165. 洛伐他汀Lovastatin 166. 匹伐他汀pitavastatin 167. 普伐他汀pravastatin 168. 瑞舒伐他汀Rosuvastatin 169. 辛伐他汀Simvastatin170. 坦索罗辛T amsulosin 171. 格列美脲glimepiride172. 吡格列酮pioglitazone 173. 尼扎替丁nizatidine 174. 替卡西林Ticarcillin 175. 酮咯酸氨丁三醇Ketorolac 176. 酮基布洛芬Ketoprofen 177. 头孢氨苄cefalexin178. 头孢克洛cefaclor179. 头孢磺啶cefsulodin 180. 托特罗定tolterodine 181. 拓扑替康topotecan 182. 万古霉素vancomycin 183. 文拉伐辛Venlafaxine 184. 那非Sildenafil185. 西他列汀Sitagliptin 186. 西酞普兰Citalopram 187. 西替利嗪cetirizine 188. 伊立替康Irinotecan 189. 伊马替尼imatinib190. 伊曲康唑Itraconazole 191. 依泽替米贝ezetimibe192. 左乙拉西坦Levetiracetam193. 佐米曲普坦zolmitriptan194. 唑吡坦zolpidem195. 唑尼沙胺Zonisamide依鲁替尼(Ibrutinib)杂质6个,palbociclib杂质6个,泰地唑胺杂质tedizolid phosphate 12个达格列净杂质Dapagliflozin 7个,索非布韦杂质sofosbuvir 20个,替卡格雷杂质Ticagrelor 14个,米拉贝隆杂质Mirabegron 10个,TAK 438杂质10个,沃替西汀杂质Vortioxetine 15个,LCZ696杂质6个非布司他杂质Febuxostat 14个泊沙康唑异构体Posaconazole 14个阿普斯特杂质Apremilast 6个阿奇霉素杂质Azithromycin 14个阿考替胺杂质Acotiamide 14个依托考昔杂质Etoricoxib 14个尼达尼布杂质Nintedanib 8个罗库溴铵杂质Rocuronium Bromide 8个恩杂鲁胺杂质Enzalutamide 4个卢帕他定杂质Rupatadine 6个瑞格非尼杂质Regorafenib 20个色瑞替尼杂质Ceritinib 11个依美斯汀杂质Emedastine8个依匹唑派杂质Brexpiprazole6个依帕司他杂质epalrestat 6个乌苯美司杂质Ubenimex 19个福多司坦杂质Fudosteine 6个马来酸匹杉琼杂质Maleic acid Chinese fir, Joan 6个扎鲁司特杂质Zafirlukast 6个贝利司他杂质belinostat 6个奥扎格雷的杂质ozagrel 6个酒石酸伐尼克兰片杂质Varenicline T artrate T ablets 6个莫扎伐普坦杂质Mozavaptan 5个沙芬酰胺杂质Safinamide 4个沃雷生杂质suvorexant 5个依替巴肽杂质Eptifibatide 5个乐伐替尼杂质lenvatinib 8个。
国家药监局关于批准注册121个医疗器械产品的公告(2021年3月)

国家药监局关于批准注册121个医疗器械产品的公告(2021年3月)文章属性•【制定机关】国家药品监督管理局•【公布日期】2021.04.15•【文号】国家药品监督管理局公告2021年第55号•【施行日期】2021.04.15•【效力等级】部门规范性文件•【时效性】现行有效•【主题分类】药政管理正文国家药品监督管理局公告2021年第55号国家药监局关于批准注册121个医疗器械产品的公告(2021年3月)2021年3月,国家药品监督管理局共批准注册医疗器械产品121个。
其中,境内第三类医疗器械产品77个,进口第三类医疗器械产品18个,进口第二类医疗器械产品24个,港澳台医疗器械产品2个(具体产品见附件)。
特此公告。
附件:2021年3月批准注册医疗器械产品目录国家药监局2021年4月15日附件2021年3月批准注册医疗器械产品目录序号产品名称注册人名称注册证编号境内第三类医疗器械1导引系统先健科技(深圳)有限公司国械注准202130301522一次性使用防针刺精密过滤输液器带针上海宝舜医疗器械有限公司国械注准202131401533导引导管南京沃福曼医疗科技有限公司国械注准20213030154 4血液透析滤过器威海威高血液净化制品有限公司国械注准20213100155 5一次性使用压力延长管深圳市保安医疗用品有限公司国械注准20213030156 6硬脑膜修补片北京博辉瑞进生物科技有限公司国械注准20213130157 7一次性使用单向阀多通连接江苏省华星医疗器械实业有限公国械注准器司202131401588椎间融合器深圳市沃尔德外科医疗器械技术有限公司国械注准202131301599一次性使用精密过滤输液器带针淄博侨森医疗用品股份有限公司国械注准2021314016010椎间融合器武汉德骼拜尔外科植入物有限公司国械注准2021313016111不可吸收带线锚钉北京市富乐科技开发有限公司国械注准20213130162 12一次性使用静脉留置针苏州鑫康道医疗科技有限公司国械注准20213140163 13冠脉球囊扩张导管苏州莱诺医疗器械有限公司国械注准20213030164 14一次性使用输液器带针天津市远东医材有限公司国械注准20213140165 15外周球囊扩张导管北京永益润成科技有限公司国械注准20213030166 16一次性使用体外循环管道常州市康心医疗器械有限公司国械注准20213100167 17钛合金手足锁定接骨板系统创美得医疗器械(天津)有限公司国械注准20213130168 18一次性使用血管内成像导管苏州阿格斯医疗技术有限公司国械注准20213060169 19一次性内镜用注射针诸暨市鹏天医疗器械有限公司国械注准20213140170 20分段控弯导引系统先健科技(深圳)有限公司国械注准20213030171 21一次性使用血液透析管路健帆生物科技集团股份有限公司国械注准2021310017222可折叠人工晶状体天津世纪康泰生物医学工程有限公司国械注准2021316017323乙型肝炎病毒核酸测定试剂上海仁度生物科技有限公司国械注准盒(RNA捕获探针法)2021340017424一次性使用电子输尿管肾盂内窥镜导管北京北方腾达科技发展有限公司国械注准2021306017525新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法)杭州迪安生物技术有限公司国械注准2021340017626儿童手部X射线影像骨龄辅助评估软件杭州依图医疗技术有限公司国械注准2021321017727病人监护仪通用电气医疗系统(中国)有限公司国械注准2021307017828三维腹腔内窥镜山东威高手术机器人有限公司国械注准2021306017929单光子发射计算机断层成像系统滨松光子医疗科技(廊坊)有限公司国械注准2021306018030一次性使用热活检钳诸暨市鹏天医疗器械有限公司国械注准2021301018131一次性使用高频十二指肠乳头切开刀杭州莱恩瑟特医疗技术有限公司国械注准2021301018232生物安全柜苏州安泰空气技术有限公司国械注准2021322018333一次性使用Y型连接阀套装厦门鑫康顺医疗科技有限公司国械注准2021303018434预充式导管冲洗器山东赛克赛斯生物科技有限公司国械注准20213140185 35锚钉系统大博医疗科技股份有限公司国械注准20213130186 36泡沫敷料广州润虹医药科技股份有限公司国械注准2021314018737一次性使用精密过滤避光输液器山东新华安得医疗用品有限公司国械注准2021314018838软性亲水接触镜江苏天眼医药科技股份有限公司国械注准2021316018939一次性使用无菌注射器带针江苏采纳医疗科技有限公司国械注准2021314019040注射用交联透明质酸钠凝胶杭州科腾生物制品有限公司国械注准20213130191 41血液透析浓缩液四川威力生医疗科技有限公司国械注准2021310019242一次性使用防针刺静脉输液针山东威高集团医用高分子制品股份有限公司国械注准2021314019343一次性使用避光输液器河南曙光汇知康生物科技股份有限公司国械注准2021314019444一次性使用精密过滤输液器带针南阳市久康医疗器械有限公司国械注准2021314019545一次性使用无菌自毁式注射器聚民生物科技有限公司国械注准2021314019646夹子装置诸暨市鹏天医疗器械有限公司国械注准2021302019747一次性使用无菌注射针江苏采纳医疗科技有限公司国械注准20213140198 48颅内支撑导管北京久事神康医疗科技有限公司国械注准20213030199 49软性亲水接触镜江苏天眼医药科技股份有限公司国械注准2021316020050一次性使用无菌注射器带针成都市新津事丰医疗器械有限公司国械注准2021314020151一次性使用静脉留置针佳康医用器材(青岛)有限公司国械注准20213140202 52颅内球囊扩张导管依奈德医疗技术(上海)有限公司国械注准2021303020353一次性使用压力延长管山东威高集团医用高分子制品股份有限公司国械注准2021314020454颅内球囊扩张导管浙江归创医疗器械有限公司国械注准2021303020555远端通路导引导管珠海通桥医疗科技有限公司国械注准20213030206 56导管鞘沛嘉医疗科技(苏州)有限公司国械注准20213030207 57一次性使用注射笔用针头甘甘医疗科技江苏有限公司国械注准2021314020858麻醉系统通用电气医疗系统(中国)有限公司国械注准2021308020959肺炎CT影像辅助分诊与评估软件北京推想科技有限公司国械注准2021321021060肺炎CT影像辅助分诊与评估软件杭州深睿博联科技有限公司国械注准2021321021161医用血管造影X射线机东软医疗系统股份有限公司国械注准20213060212 62复合陡脉冲治疗设备上海睿刀医疗科技有限公司国械注准2021309021363放射治疗轮廓勾画软件海创时代(深圳)医疗科技有限公司国械注准2021321021464口腔种植手术导航定位设备北京柏惠维康科技有限公司国械注准20213010215 65超导型磁共振成像系统康达洲际医疗器械有限公司国械注准2021306021666人附睾蛋白4测定试剂盒(化学发光法)深圳市亚辉龙生物科技股份有限公司国械注准2021340021767胃泌素释放肽前体测定试剂盒(化学发光法)深圳市亚辉龙生物科技股份有限公司国械注准2021340021868前列腺酸性磷酸酶测定试剂盒(化学发光免疫分析法)苏州长光华医生物医学工程有限公司国械注准2021340021969神经元特异性烯醇化酶测定试剂盒(化学发光免疫分析法)苏州长光华医生物医学工程有限公司国械注准2021340022070乙型肝炎病毒前S1抗原检迈克生物股份有限公司国械注准测试剂盒(直接化学发光法)2021340022171人JAK2-V617F基因突变检测试剂盒(荧光PCR法)迈杰转化医学研究(苏州)有限公司国械注准2021340022272一次性使用血液透析器广州市恩德氏医疗制品实业有限公司国械注准2021310022373流出道单瓣补片北京佰仁医疗科技股份有限公司国械注准20213130224 74一次性使用静脉留置针威海洁瑞医用制品有限公司国械注准20213140225 75软聚硅酮泡沫敷料江苏诺瓦立医疗用品有限公司国械注准2021314022676幽门螺杆菌23S rRNA基因突变检测试剂盒(PCR-荧光探针法)上海芯超生物科技有限公司国械注准2021340022777新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法)重庆中元汇吉生物技术有限公司国械注准20213400228进口第三类医疗器械78血液透析用中心静脉导管及附件Covidien llc国械注进2021310005779钛夹AESCULAP AG国械注进20213020058 80注射用交联透明质酸钠凝胶Q-Med AB国械注进2021313005981颅内取栓支架Micro Therapeutics,Inc.dba ev3Neurovascular国械注进2021303006082游离前列腺特异性抗原校准品Siemens Healthcare DiagnosticsProducts Limited国械注进2021340007383丙型肝炎病毒(HCV)核酸(RNA)检测试剂盒(实时荧光PCR法)Cepheid AB国械注进2021340007484抗CD20(L26)鼠单克隆抗体试剂(免疫组织化学Ventana Medical Systems, Inc.国械注进20213400075法)85牙科种植体Zimmer Dental, Inc.国械注进20213170076 86空心纤维血液透析器Vital Healthcare Sdn. Bhd.国械注进20213100077 87一次性负压装置及护创敷料Smith & Nephew Medical Ltd国械注进20213140090 88双极器械ERBE Elektromedizin GmbH国械注进2021301009189一次性使用高频圈套器オリンパスメディカルシステムズ株式会社国械注进2021301009290麻醉系统Dr?gerwerk AG & Co. KGaA国械注进20213080093 91X射线计算机体层摄影设备Siemens Healthcare GmbH国械注进20213060094 92关节内窥镜(?)国械注进20213060095 93电子下消化道内窥镜富士フイルム株式会社国械注进20213060096 94植入式给药装置专用针Fresenius Kabi AG国械注进20213140097 95微导管Vascular Solutions LLC国械注进20213030098进口第二类医疗器械96胰岛素样生长因子-1检测试剂盒(电化学发光法)Roche Diagnostics GmbH国械注进2021240006197免疫球蛋白E校准品DiaSys Diagnostic Systems GmbH国械注进2021240006298纤维蛋白原质控品(低值)Instrumentation LaboratoryCompany国械注进2021240006399一次性使用直线型切割吻合器及渐进式钉匣Covidien llc国械注进20212020064100血糖仪OSANG Healthcare Co.,Ltd.国械注进20212220065 101血糖仪OSANG Healthcare Co.,Ltd.国械注进20212220066102内窥镜图像处理及照明装置オリンパスメディカルシステムズ株式会社国械注进20212060067103内镜清洗消毒器STEELCO SPA国械注进20212110068 104电动产床タカラメディカル株式会社国械注进20212180069 105角膜内皮显微镜株式会社コーナン?メディカル国械注进20212160070106肺功能测试系统GANSHORN Medizin ElectronicGmbH国械注进20212070071107牙科低压电动马达株式会社ナカニシ国械注进20212170072108抗β2糖蛋白1结构域1IgG抗体检测试剂盒(化学发光免疫分析法)INOVA Diagnostics, Inc.国械注进20212400078109胱抑素C校准液Siemens Healthcare DiagnosticsInc.国械注进20212400079110降钙素原校准品Ortho-Clinical Diagnostics国械注进20212400080111胰岛素样生长因子-I测定试剂盒(化学发光法)Siemens Healthcare DiagnosticsProducts Limited国械注进20212400081112降钙素原质控品Ortho-Clinical Diagnostics国械注进20212400082113喷砂洁牙机SATELEC A Company ofACTEON Group国械注进20212170083114种植体稳固度检测仪Osstell AB国械注进20212170084 115多导睡眠记录系统SOMNOmedics GmbH国械注进20212070085 116一次性使用超声内窥镜水囊HOYA株式会社豪雅株式会社国械注进20212060086 117多导睡眠记录系统SOMNOmedics GmbH国械注进20212070087 118全自动微生物质谱检测系统ASTA Corporation国械注进20212220088119齿科铸造合金Eisenbacher Dentalwaren EDGmbH国械注进20212170089港澳台医疗器械120软性亲水接触镜望隼科技股份有限公司国械注许20213160003 121软性亲水接触镜星歐光學股份有限公司国械注许20213160004。
百灵威核磁耗材产品

NMR Consumables and Accessories
客服热线:400-666-7788
全球 NMR 耗材 引领 60 年
百灵威
百灵威科技有限公司成立于 1992 年,始终以“为科研和生产提供世界一流的产品和服务”为宗旨,致力于超精细化学品的研发与 制造。经过近二十年的发展,百灵威已具备为化学、分析、生物、材料、物理及药物研发等领域提供近五十万种产品和专业服务 的能力。 百灵威拥有一支强大的具有丰富经验和创新能力的研发团队,在江苏、河北设立的两个研发中心可迅速研发出毫克至数百公斤级 的医药、生化、材料等中间体及特殊高端化学品,并可为客户定制合成各类产品,尤其擅长小分子药物中间体以及催化剂配体的 合成。 百灵威人坚信“发展民族科技”的理念,坚持依靠中国人自己的智慧和力量, 不断建设和发展位于潮白河畔的现代化工业生产基地,发挥百灵威在尖端技术研究、敏捷制造和系统性物流管理等方面的突出优 势,积极地将中国的各种高端化合物推荐给国际同行,为促进中国化学事业发展,推动世界文明与和谐进步而奋斗不息。 百灵威的使命 促进科技和工业发展,造福人类……
管壁厚度(mm) 平均凸度(µm)
包装
0.27
<60>
50只/塑料筒装
0.27
<60>
50只/塑料筒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.60
<60>
50只/塑料筒装
SampleJet®核磁管
AZD3839 1227163-84-9 GlpBio
Peptides, Inhibitors, AgonistsProduct Data SheetProduct Name: AZD3839Cat. No.:GC14130Chemical Name: (S)-3-(2-(difluoromethyl)pyridin-4-yl)-7-fluoro-3-(3-(pyrimidin-5-yl)phenyl)isoindolin-1-imineCHEMICAL PROPERTIESCas No.: 1227163-84-9Molecular Formula: C24H16F3N5Molecular Weight: 431.41Storage: PowderSolubility: Soluble in DMSOChemical Structure:BackgroundIC50: 16.7 nM for BACE1β-Site amyloid precursor protein cleaving enzyme1 (BACE1) is one of the key enzymes involved in the processes of the amyloid precursor protein (APP) and formation of amyloid β peptide (Aβ) species. Because cerebral deposition of Aβ species is possibly critical for the pathogenesis of Alzheimer disease, BACE1 has emerged as a key target for the treatment of this disease. AZD3839 is a potent and selective BACE1 inhibitor.In vitro: AZD3839 concentration-dependently inhibited BACE1 activity in a biochemical fluorescence resonance energy transfer assay, Aβ and sAPPβ release from modified and wild-type human SH-SY5Y cells and mouse N2A cells as well as from guinea pig and mouse primary cortical neurons. Selectivity against BACE2 and cathepsin D was 14 and >1000-fold, respectively [1].In vivo: AZD3839 exhibited dose- and time-dependent lowering of plasma, brain, and cerebrospinal fluid Aβ levels in mouse, guinea pig, and non-human primate. PK/PD analyses of mouse and guinea pig data showed a good correlation between the potency of AZD3839 in primary cortical neurons and in vivo brain effects [1].Clinical trial: Based on the pharmacological profile and its drug like properties, AZD3839 has been progressed into Phase 1 clinicaltrials in man [2].References:[1].Marsell R, Sisask G, Nilsson Y, et al. GSK-3 inhibition by an orally active small molecule increases bone mass in rats. Bone, 2012, 50(3): 619-627.[2].Gilmour PS, O'Shea PJ, Fagura M, et al. Human stem cell osteoblastogenesis mediated by novel glycogen synthase kinase 3 inhibitors induces bone formation and a unique bone turnover biomarker profile in rats. Toxicol Appl Pharmacol, 2013, 272(2): 399-407.[3].Sisask G, Marsell R, Sundgren-Andersson A, et al. Rats treated with AZD2858, a GSK3 inhibitor, heal fractures rapidly without endochondral bone formation. Bone, 2013, 54(1): 126-132.Research Update1. Effect of Hydrofluoric Acid Concentration and Etching Time on Bond Strength to Lithium Disilicate Glass Ceramic. Oper Dent. 2017 Nov/Dec;42(6):606-615. doi: 10.2341/16-215-L. Epub 2017 Jul 14. PMID:28708007AbstractThe aim of this study was to evaluate the influence of different concentrations of hydrofluoric acid (HF) associated with varied etching times on the microshear bond strength (μSBS) of a resin cement to a lithium disilicate glass ceramic. Two hundred seventy-five ceramic blocks (IPS e.max Press [EMX], Ivoclar Vivadent), measuring 8 mm × 3 mm thickness, were randomly distributed into five groups according to the HF concentrations (n=50): 1%, 2.5%, 5%, 7.5%, and 10%.2. Does acid etching morphologically and chemically affect lithium disilicate glass ceramic surfaces? J Appl Biomater Funct Mater. 2017 Jan 26;15(1):e93-e100. doi: 10.5301/jabfm.5000303. PMID:27647389AbstractBACKGROUND: This study evaluated the surface morphology, chemical composition and adhesiveness of lithium disilicate glass ceramic after acid etching with hydrofluoric acid or phosphoric acid.METHODS: Lithium disilicate glass ceramic specimens polished by 600-grit silicon carbide paper were subjected to one or a combination of these surface treatments: airborne particle abrasion with 50-μm alumina (AA), etching with 5% hy drofluoric acid (HF) or 36% phosphoric acid (Phos), and application of silane coupling agent (Si).3. Fatigue failure load of feldspathic ceramic crowns after hydrofluoric acid etching at different concentrations. J Prosthet Dent. 2018 Feb;119(2):278-285. doi: 10.1016/j.prosdent.2017.03.021. Epub 2017 May 26. PMID:28552291AbstractSTATEMENT OF PROBLEM: Hydrofluoric acid etching modifies the cementation surface of ceramic restorations, which is the same surface where failure is initiated. Information regarding the influence of hydrofluoric acid etching on the cyclic loads to failure of ceramic crowns is lacking.PURPOSE: The purpose of this in vitro study was to evaluate the influence of different hydrofluoric acid concentrations on the fatigue failure loads of feldspathic ceramic crowns.。
【CN209499694U】一种便于检查的妇科扩阴器【专利】
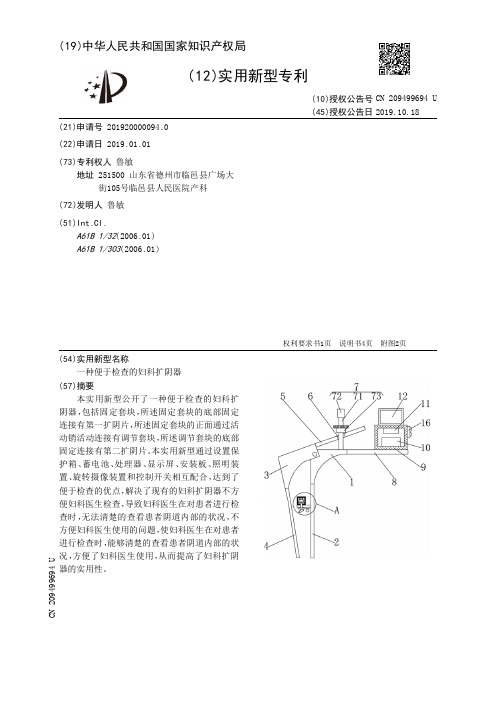
(10)授权公告号 CN 209499694 U (45)授权公告日 2019.10.18
( 54 )实用新型名称 一种便于检查的妇科扩阴器
( 57 )摘要 本实 用新型公 开了一 种便于检查的 妇科 扩
阴器 ,包括固定套块 ,所述固定套块的 底部固 定 连接有第一扩阴片,所述固定套块的正面通过活 动销活动连接有调节套块,所述调节套块的底部 固定连接有第二扩阴片。本实用新型通过设置保 护箱、蓄电 池、处理器、显示屏、安装板、照明装 置 、旋转摄像装置 和控 制开关 相互配合 ,达到了 便于检查的优点,解决了现有的妇科扩阴器不方 便妇科医生检查,导致妇科医生在对患者进行检 查时 ,无法清楚的查看患者阴道内部的状况,不 方便妇科医生使用的问题,使妇科医生在对患者 进行检查时 ,能够清楚的查看患者阴道内部的状 况 ,方便了 妇科医生使 用 ,从而提高了 妇科扩阴 器的实用性。
实用新型内容 [0004] (一)解决的技术问题 [0005] 针对现有技术的不足,本实用新型提供了一种便于检查的妇科扩阴器,具备便于 检查的 优点 ,解决 了现有的 妇科扩阴器不方便 妇科医生检查 ,导致 妇科医生在对患者进行 检查时,无法清楚的查看患者阴道内部的状况,不方便妇科医生使用的问题。 [0006] (二)技术方案 [0007] 为实现上述目的,本实用新型提供如下技术方案: [0008] 一种便于检查的妇科扩阴器,包括固定套块,所述固定套块的底部固定连接有第 一扩阴片 ,所述固定套块的 正面通过活 动销活 动连接有调节套块 ,所述 调节套块的 底部固 定连接有第二扩阴片,所述调节套块的右侧固定连接有下压板,所述下压板的顶部开设有 通槽,所述固定套块的顶部设置有调节装置,所述固定套块的右侧固定连接有固定板,所述 固定板的 顶部固定连接有保 护箱 ,所述保 护箱内壁的 底部固定连接有蓄电 池 ,所述保 护箱 内壁的 顶部固定连接有处理器 ,所述保 护箱的 顶部固定连接有显示屏 ,所述第一扩阴片的 左侧壁固定连接有安装板 ,所述安装板的 底部设置有照明 装置 ,所述安装板的 顶部设置有 旋转摄像装置。 [0009] 优选的,所述调节装置包括螺纹杆,所述螺纹杆的底部与固定套块顶部的右侧固 定连接 ,所述螺纹杆的 顶部贯穿至通槽的 顶部并固定连接有限 位块 ,所述螺纹杆的 表面螺 纹连接有调节螺纹块。 [0010] 优选的,所述照明装置包括灯座,所述灯座的顶部与安装板的底部固定连接,所述 灯座的底部固定连接有照明灯。 [0011] 优选的,所述旋转摄像装置包括箱体,所述箱体的底部与安装板的顶部固定连接, 所述箱体内壁一 侧的 底部开设有环形滑槽 ,所述箱体内壁的 顶部固定连接有微型电 机 ,所
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Data Sheet
Product Name:AZD 4017
Cat. No.:GC31601
Chemical Properties
Cas No.1024033-43-9
Chemical
Name
N/A
Canonical
SMILES
O=C(O)C[C@H]1CN(C2=NC(SCCC)=C(C(NC3CCCCC3)=O)C=C2)CCC1
Formula C22H33N3O3S M.Wt419.58 Solubility Soluble in DMSO Storage Store at -20°C
General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.
Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.
Structure
Background
AZD 4017 is a potent, selective 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) inhibitor, with an IC50 of 7 nM.
Product Data Sheet
AZD 4017 displays excellent selectivity versus the related enzymes 11-βHSD2, 17β-HSD1, 17β-HSD3 (all IC50>30
μM) and shows no measurable activity against the glucocorticoid and mineralocorticoid receptors. Despite having high potency for the human form of 11β-HSD1, AZD 4017 shows much reduced activity across species with the exception of cynomolgous monkey (IC50=0.029 μM). Additionally, as it is believed that adipose is a key target organ, inhibition of 11β-HSD1 activity is measured in isolated human adipocytes from nondiabetic volunteers. AZD 4017 is shown to be a potent inhibitor in this key target tissue (IC50=0.002 μM) in good agreement with the enzyme potency, thus providing some confidence that AZD 4017 is not restricted from adipose tissue by the fact that it was acidic[1].
Since AZD 4017 has lower potency against the mouse enzyme, only a limited number of preclinical pharmacodynamic measurements are performed. Increasing the dose further led to a maximal effect of approximately 70% inhibition at 1500 mg/kg, equivalent to 10×IC50 in the mouse, demonstrating the dose dependent inhibition of 11β-HSD1 by AZD 4017 in this model[1].
[1]. Scott JS, et al. Discovery of a potent, selective, and orally bioavailable acidic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor: discovery of 2-[(3S)-1-[5-(cyclohexylcarbamoyl)-6-propylsulfanylpyridin-2-yl]-3-piperidyl]acetic acid (AZD4017). J Med Chem. 2012 Jun 28;55(12):5951-64.。
