Interactions of quaternary ammonium salt-type gemini surfactants with
复合季铵盐消毒液工作温度

复合季铵盐消毒液工作温度英文回答:The working temperature of compound quaternary ammonium salt disinfectant depends on the specific formulation and purpose of use. Generally, the recommended working temperature for compound quaternary ammonium salt disinfectant is around room temperature, which is typically between 20 to 25 degrees Celsius (68 to 77 degrees Fahrenheit). This temperature range is considered optimal for the disinfection efficacy of the compound quaternary ammonium salt.Compound quaternary ammonium salt disinfectants are commonly used in various settings, such as healthcare facilities, food processing plants, and household cleaning. In healthcare settings, for example, these disinfectants may be used to clean and disinfect surfaces, medical equipment, and instruments. The working temperature is crucial to ensure the effectiveness of the disinfectant inkilling or inactivating microorganisms.In some cases, the working temperature may vary depending on the specific application. For instance, in food processing plants, compound quaternary ammonium salt disinfectants may be used to sanitize equipment and utensils. The working temperature in this case may be higher, around 45 to 50 degrees Celsius (113 to 122 degrees Fahrenheit), to effectively eliminate foodborne pathogens.It is important to follow the manufacturer's instructions and guidelines when using compound quaternary ammonium salt disinfectants. These instructions usually include information on the recommended working temperature for optimal disinfection results. Failure to adhere to the recommended temperature range may compromise the effectiveness of the disinfectant and increase the risk of microbial contamination.中文回答:复合季铵盐消毒液的工作温度取决于具体的配方和使用目的。
甲基丙烯酸缩水甘油酯的合成研究 (1)

甲基丙烯酸缩水甘油酯的合成研究高晓蕾Ξ 卫冬燕(郑州大学化工学院,河南郑州450002)摘 要:在相转移催化剂季铵盐和阻聚剂对苯二酚存在下,采用二步法由甲基丙烯酸(M AA )和环氧氯丙烷(ECH )合成了甲基丙烯酸缩水甘油酯(G M A )。
通过正交实验确定了最佳反应条件:物料配比n (甲基丙烯酸钠)∶n (环氧氯丙烷)=1∶8,反应温度105℃,反应时间4h ,催化剂选用十六烷基三甲基溴化铵。
在该工艺条件下,产品产率为94173%,纯度为96180%。
关键词:甲基丙烯酸;甲基丙烯酸缩水甘油酯;相转移催化剂R esearch on Synthesis of G lycidyl MethacrylateG AO Xiao 2lei ,WEI Dong 2yan(C ollege of Chemical Engineering ,Zhengzhou University ,Zhengzhou 450002,China )Abstract :G lycidyl methacrylate (G M A )is synthesized in tw o steps based on the reaction of methyl methacrylate (M M A )and epichlorhydrin (ECH )in presence of quaternary amm onium salt as phase trans fer catalyst and 1,42dihydroxy 2benzene as poly 2merization inhibitor.The optimum reaction conditions are obtained by orthog onal experimental design :n (s odium methacrylate )∶n (ECH )=1∶8,reaction temperature is 105℃,reaction time is 4h ,and taking hexadecyltrimethylamm onium bromide as phase trans fer catalyst.Under these conditions the yield of product can be reached 94173%with purity of 96180%.K ey w ords :methyl methacrylate ;glycidyl methacrylates ;phase trans fer catalyst 甲基丙烯酸缩水甘油酯(G M A )又称甲基丙烯酸环氧丙酯,是无色透明的液体,密度11074,折光率114480,沸点189℃,熔点-50℃,闪点84℃,色相(APH A )<75,不溶于水,易溶于有机溶剂,对皮肤和粘膜有刺激性,几乎无毒。
有机硅季铵盐—纳米银复合织物抗菌整理剂制备及应用的研究

有机硅季铵盐一纳米银复合织物抗菌整理剂的制备及应用研究摘要有机硅季铵盐类和银系抗菌剂是目前应用最普遍、效果最好的织物抗菌整理剂。
银具有极强的杀菌能力、杀菌耐久性良好、用量小,无毒、无刺激;而有机硅季铵盐不仅赋予织物优良抗菌性,同时还具备良好的吸水性、柔软性、平滑性及回弹性。
这两类抗菌整理剂各具特色,可以互为补充,克服目前纺织品抗菌卫生整理中存在的问题,在纺织行业有较大的应用潜力。
本文在详细分析国内外织物抗菌整理剂特别是有机硅季铵盐及银系抗菌剂的合成、分类、性能及应用等方面最新研究进展的基础上,针对目前国内使用的有机硅季铵盐类抗菌整理剂大多数从国外进口、成本较高,以及银易变色的现状,研制开发了一种新型的有机硅季铵盐ASQA和复合型抗菌整理剂Ag—ASQA。
在100℃用油酸甲酯对氨乙基氨丙基二甲氧基硅烷fDL.602)进行酰胺化反应6h后,再经硫酸二甲酯季铵化反应制备了一种新型的油酰胺乙基二甲基氨丙基硅烷季铵盐(ASQA),并对其结构进行了表征。
用质量分数为1.0%的ASQA溶液整理过的坯布,对大肠杆菌及金黄色葡萄球菌的抑菌率分别为99.55%和99.82%,且具有优良的抗菌耐洗性,洗涤30次后抑菌率仍大于80%。
通过化学还原法以过氧化氢为还原荆、在分散剂聚乙烯吡咯烷酮(PVP)及自制多羧酸聚合物(PMAA)的保护下分别制备了60~90nm和90~150nm的银粉;并通过X.射线衍射仪(XRD)及透射电镜(TEM)观察了银粉的结构及粒径分布;为了避免银变色.影响织物外观,将纳米银粉通过分散剂和粘合剂包覆在纺织纤维上,达到,抗菌持久的良好效果。
抑菌圈实验结果表明:用PVP分散的银粉质量浓度为0.5g·L-1时,对金黄色葡萄球菌的抑菌圈直径为13.1mm,对大肠杆菌的抑菌圈直径为13.7mm。
将抑菌幽试验的培养皿放置6个l月后抑菌环仍然清晰未受细菌感染,具有较好的抗菌耐久性。
为了克服单一抗菌剂的抗菌局限,本文采用无机一有机复配的方式,将有机硅季铵盐ASQA溶液与纳米银粉分散液复配,制备了抗菌整理液Ag-ASQA。
四丁基氟化铵溶解度曲线_概述说明

四丁基氟化铵溶解度曲线概述说明1. 引言1.1 概述本文旨在探讨四丁基氟化铵(Tetra-n-butylammonium fluoride)的溶解度曲线,并对其进行深入分析和讨论。
四丁基氟化铵是一种重要的有机金属试剂,其在化学研究和工业生产中具有广泛的应用。
了解其溶解度特性对于优化催化反应、合成有机材料以及设计高效分离方法等领域至关重要。
1.2 文章结构本文共包括五个部分。
首先,引言部分将介绍文章的背景和目的。
然后,正文部分将详细阐述四丁基氟化铵溶解度的定义、影响因素以及测定方法。
接下来,结果与讨论部分将对实验结果进行分析,并探讨可能的影响因素,并与已有数据进行比较和分析。
最后,结论部分将总结实验结果和主要发现。
1.3 目的本文旨在提供一个全面而准确的关于四丁基氟化铵溶解度曲线的概述,以便研究者能更好地理解该物质在不同条件下的溶解行为。
通过对影响溶解度的因素和测定方法的详细介绍,本文将为相关领域的研究提供理论基础和实验指导,同时也为进一步探索四丁基氟化铵溶液性质的应用提供参考。
最终,通过本文内容的阐述与讨论,希望能够为相关研究领域的发展做出一定的贡献。
2. 正文:2.1 四丁基氟化铵溶解度的定义四丁基氟化铵是一种常用的阳离子表面活性剂,其溶解度是指在给定温度条件下,在水或其他溶剂中能够溶解的最大量四丁基氟化铵。
一般以摩尔浓度或质量浓度表示。
2.2 影响四丁基氟化铵溶解度的因素四丁基氟化铵的溶解度受到多种因素的影响,包括温度、溶剂性质、离子强度等。
以下为主要因素的说明:2.2.1 温度:温度是影响四丁基氟化铵溶解度最重要的因素之一。
通常情况下,随着温度升高,四丁基氟化铵在水中的溶解度会增加。
这是由于温度升高会增加分子间距和分子热运动速率,促进离子势垒突破和分子间相互作用降低,从而增加了四丁基氟化铵分子在水中的扩散速率和相对稳定性。
2.2.2 溶剂性质:不同溶剂对四丁基氟化铵的溶解度有所差异。
一般来说,极性溶剂如水会更好地溶解四丁基氟化铵,而非极性溶剂如石油醚等对其溶解度较低。
相转移

14
Permanganate Oxidation
COMPOUND QUANTITY
1-癸烯 苯 高锰酸钾 三辛酰基甲基氯化铵
乙醚
28g 50mL 125g 5g 100mL
过量的高锰通过添加亚硫酸钠溶液破坏。将反应混合物过滤 以除去的MnO2并用稀HCl酸化。MnOI用100毫升苯洗涤,将其也 用于洗涤滤液中的水相。将合并的苯溶液用100毫升10%NaOH溶 液混合并振荡。含水碱性相用乙醚洗涤,然后用盐酸酸化。加入 100ml 乙醚用来分离羧酸,并在醚溶液干燥(硫酸钠)。乙醚蒸 发留下29g(91%)的壬酸(98%纯度通过GLC测定的)。
这些通过制备气液色谱分离。主要成分(93%)是预期的2-己 基 -1 , 1- 氯代异戊烯,通过 IR 和 NMR 谱,这些用 Weinberg.1 方法 制备的样品的气液色谱保留时间都可以证明。次要组分(7%), 用核磁和质谱鉴定为2-戊基-3-甲基-1,1-氯代异戊烯。这两种产品的 分离的产率分别为60和4%,基于未回收的氯仿。
COMPOUND QUANTITY
氯仿 1-辛烯 NaOH溶液 三辛酰基甲基氯化铵
NaCl
37.5g 75g 150g 5g 20mL
400mL
H2O
用 GLC 分析有机层的 组成,结果表明它含有 59 %的 1- 辛烯, 9.6 %氯仿, 还有 31 个产品。通过一个 12 英 寸 真 空 夹 套 Vigreux 柱蒸馏,得到 28 克馏分产 物 , 沸 点 49℃ ( 0.20.5mm )的,通过 GLC 分 析出包含两种组分。
铜_季铵盐复配木材防腐剂的防腐性能

铜2季铵盐复配木材防腐剂的防腐性能ΞFAN G G Z 方桂珍,任世学(东北林业大学林产化工学院,黑龙江哈尔滨150040)摘 要: 在实验室内采用常规的真空2加压法浸注试件,土壤木块法进行防腐实验,检验了铜2季铵盐类防腐剂(FFJ 21、FFJ 22和FFJ 23)对白腐采绒革盖菌[Coriolus versicolor (L.ex Fr.)Quel.]和褐腐绵腐卧孔菌[Poria placenta (Fr.)Cooke.]的防腐性能,结果表明:在较低的保持量下,都有较好的防腐效果。
与百菌清(可湿性粉剂)、五氯酚钠、三唑酮相比较,它们对白腐菌的防腐性能与百菌清相近,比五氯酚钠和三唑酮好。
关键词: 季铵盐;木材防腐剂中图分类号:S782.33 文献标识码:A 文章编号:025322417(2002)0120071203与铜复配是木材防腐剂发展的一种重要趋势。
一般认为,铜对真菌有较好的抑制作用,再加上它价格适中,对环境柔和,对人畜无害,故被广泛应用于木材防腐剂中。
美国木材保护协会(AWPA )标准中水溶性防腐剂绝大多数都使用了铜,如ACC (酸性铬酸铜)、ACA (氨溶砷酸铜)、ACZA (氨溶砷锌)、CCA 2A (铜铬砷2A )、CCA 2B (铜铬砷2B )、CCA 2C (铜铬砷2C )、ACQ 2A (氨溶季铵铜2A )、ACQ 2B (氨溶季铵铜2B )、ACQ 2D (氨溶季铵铜2D )、CDDC (二甲基二硫代氨基甲酸铜)、CC (柠檬酸铜)、CBA 2A (硼唑铜)等[1]。
关于季铵盐类与其它药剂复合用作防腐剂、防变色剂和防霉剂的研究较多。
如DDAC (二甲基二癸基氯化铵)和IPBC (32碘代222丙炔基甲氨酸丁酯)混合使用具有广泛的杀菌性,并提高了抗流失性;将DDAC 和百菌清复合使用作为防霉剂等[2~3]。
目前,国外研究趋势是将季铵盐与铜盐复配制成ACQ 作水溶性防腐剂[4]。
高效液相色谱法测定延胡索药材中7种异喹啉类生物碱的含量

高效液相色谱法测定延胡索药材中7种异喹啉类生物碱的含量赵新娟;沈梅;石俊敏;韩伟立【摘要】本文建立了同时测定延胡索中巴马汀、小檗碱、去氢紫堇碱、四氢巴马汀、异紫堇球碱、紫堇碱和四氢黄连碱7种主要异喹啉生物碱含量的高效液相色谱方法,并考察了不同来源延胡索中异喹啉生物碱的含量.采用Agilent SB C18柱色谱柱(4.6×250 mm,5μm),流动相为乙腈-0.1%的醋酸水溶液(三乙胺调pH至5.0),梯度洗脱,流速为1.0 mL/min,检测波长为280nm.巴马汀、小檗碱、去氢紫堇碱、四氢巴马汀、异紫堇球碱、紫堇碱和四氢黄连碱在2.0~40.1、2.0~39.5、5.1~101.3、5.0~99.8、2.1 ~41.2、5.0~100.1 μg/mL和2.0 ~39.7μg/mL浓度范围内线性良好,平均加样回收率分别为95.6%、96.1%、96.5%、101.4%、101.9%、97.3%和102.3%,RSD分别为2.77%、2.50%、3.33%、4.18%、2.93%、2.86%和2.60%.不同来源延胡索样品中7种异喹啉类生物碱含量差异较大,研究表明该方法准确、可靠,可用于延胡索原药材质量控制.【期刊名称】《天然产物研究与开发》【年(卷),期】2015(027)012【总页数】5页(P2074-2078)【关键词】高效液相色谱法;异喹啉类生物碱;延胡索;含量测定【作者】赵新娟;沈梅;石俊敏;韩伟立【作者单位】山西省晋中市第一人民医院药剂科,晋中030600;南方医科大学公共卫生与热带医学学院卫生检测中心,广州510515;华南师范大学药物研究院,广州510632;南方医科大学公共卫生与热带医学学院卫生检测中心,广州510515【正文语种】中文【中图分类】R917延胡索为罂粟科紫堇属植物延胡索(Corydalis yanhusuo W.T.Wang)的干燥块茎,其具有活血、利气和止痛的功效。
化学基础英文30阴离子重排_anionic_rearrangements
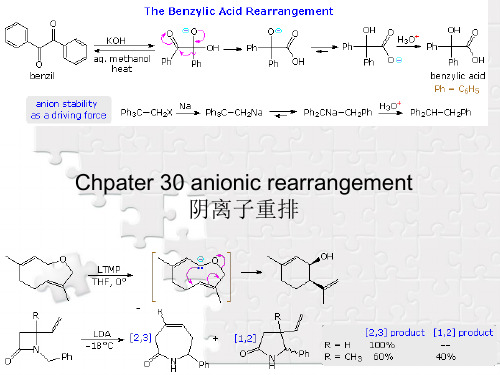
nucleophilic bases often leads to a skeletal rearrangement known as the Favorskii rearrangement. As depicted in the following diagram, this reaction is believed to proceed by way of a cyclopropanone intermediate. Facile conversion of cyclopropanones to hydrates and hemiacetals (relief of angle strain) occurs, and the cyclopropoxide conjugate base undergoes ring opening and solvent protonation.
30-2 Brook Rearrangement
The rearrangement of silicon groups from carbon to oxygen is called the Brook rearrangement. An important driving force for this shift is the increased bond strength of the Si–O bond (110 Kcal/mol) compared with the Si–C bond (76 Kcal/mol). The example given in the following equation is catalyzed by base, and a cyclic transition state is indicated by the high entropy of activation.
生物酶与季铵盐的复合物

生物酶与季铵盐的复合物英文回答:Enzymes are proteins that act as catalysts inbiological reactions. They are highly specific and can speed up chemical reactions by lowering the activation energy required for the reaction to occur. Enzymes can bind to specific molecules called substrates and convert them into products.Quaternary ammonium salts, also known as quats, are organic compounds that contain a positively charged nitrogen atom and four organic groups attached to it. They are widely used as disinfectants, surfactants, and fabric softeners due to their antimicrobial and surface-active properties.The formation of a complex between enzymes and quaternary ammonium salts can have various effects. In some cases, the complex formation can enhance the activity ofthe enzyme, while in others, it can inhibit or even denature the enzyme.One example of a complex between an enzyme and a quaternary ammonium salt is the interaction between cholinesterase and the pesticide paraoxon. Cholinesteraseis an enzyme that breaks down acetylcholine, a neurotransmitter involved in nerve signal transmission. Paraoxon is an organophosphate pesticide that irreversibly inhibits cholinesterase by forming a covalent bond with the enzyme's active site. This complex formation leads to the accumulation of acetylcholine, causing overstimulation of nerve cells and ultimately leading to paralysis or death.Another example is the complex between the enzyme trypsin and the quaternary ammonium salt benzalkonium chloride. Trypsin is a protease enzyme that cleaves peptide bonds in proteins. Benzalkonium chloride, commonly used as a disinfectant, can inhibit trypsin activity by binding to the enzyme's active site and preventing substrate binding. This complex formation can be useful in controlling trypsin activity in certain applications, such as in the productionof protein-based drugs.中文回答:酶是一种在生物反应中起催化剂作用的蛋白质。
聚硅氧烷季铵盐抗菌整理剂的合成及应用[1]
![聚硅氧烷季铵盐抗菌整理剂的合成及应用[1]](https://img.taocdn.com/s3/m/b0d2a1d676eeaeaad1f33031.png)
收稿日期:2002-10-30;修回日期:2003-02-26作者简介:李俊英(1970-),女(汉),宁夏中卫人,讲师,山东大学在职申请硕士学位,联系电话:(0531)8604456。
聚硅氧烷季铵盐抗菌整理剂的合成及应用李俊英1,2,冯圣玉1,李天铎2(11山东大学化工学院,山东 济南 250100;21山东轻工业学院化工系,山东 济南 250100)摘要:介绍了聚硅氧烷季铵盐抗菌整理剂的合成方法及其应用性能。
指出了现存的问题和今后的研究方向。
聚硅氧烷季铵盐的合成主要以DC -5700型季铵盐和氨基硅油、含氢硅油等聚合物为原料,经过平衡聚合、缩聚、季铵化等反应制得。
可用于织物材料的抗菌、除垢、防静电处理,并且能大大降低界面的界面张力。
由于其与被处理材料的表面以化学键结合,因而不易脱落,是一种安全高效的新型阳离子表面活性剂,具有广阔的应用前景。
关键词:阳离子表面活性剂;有机硅季铵盐;抗菌整理剂中图分类号:T Q423112 文献标识码:A 文章编号:1001-1803(2003)04-0249-03 有机硅季铵盐是一类新型阳离子表面活性剂,它具有耐洗、持久的效果,抑菌范围广,能有效地抑制格兰氏阳性菌、格兰氏阴性菌、酵母菌和真菌。
有机硅季铵盐可用于织物亲水整理,赋予织物良好的吸水、吸汗性以及柔软性、平滑性、回弹性和防静电性。
如果纤维被含有季铵盐基团的有机硅处理,污垢的去除很容易,由此可大大提高织物的抗污染性。
它是一种无毒、无害、高效、安全和多功能的柔软抗菌整理剂。
添加在个人护理品如洗发香波中,可配制成干、湿梳阻力小、光泽度好、手感柔软滑爽和成本低的高档香波,这类香波对皮肤及眼睛的刺激性远远小于添加了小分子季铵盐的香波。
有机硅季铵盐在应用时无需添加反应性树脂,不存在抗药性菌出现和织物抗菌失效的问题,日益受到人们的重视[1~5]。
目前国内使用的有机硅季铵盐类抗菌整理剂大多数从国外进口,致使用户使用不便和生产成本升高。
安全守则-英文

Safe Personal Laboratory Habits1. Eye protection must be worn at all times.2. Food/drink is not allowed in laboratories where chemicals are used/ stored.smoking in the laboratory.3. No4. Lab coats must be worn while handling corrosive, toxic, or flammablematerials. Gloves must be worn when necessary, especially when handling corrosives, toxic and dangerously reactive materials.5. Do not work alone.6. Do not mouth pipet.7. If you see a colleague doing something dangerous, point it out to him or her.8. Know where safety equipment (eyewash, shower and extinguisher) islocated.9. Always read MSDS before handling new chemicals.10. Know how to clean up spills of common chemicals and specific chemicalsyou see. Be familiar with the locations and contents of spill carts (See Chapter 11) and how to use it.11. Always wash your hands after handling chemicals and before eating.12. Short skirts, shorts, and open shoes must not be worn.13. Lab coats must not be worn outside laboratories and in public areas.14. Avoid wearing a walkman or other portable music devices while working inthe lab.1. Eye ProtectionAdequate eye protection is required for all individuals in the laboratory. Do not remove your eye protection until you have physically left the lab room. The following types of eye protection are acceptable.•Protective glasses and face shields that cover corrective prescription lenses are commercially available and/or from Chemistry Stores.•Normal prescription eyeglasses, either with or without safety side shields as long as the glasses are shatterproof and cover a large enough area surrounding your eye (this usually means that the frames must be a minimum of 2 inches (5 cm) from top to bottom as well as from side to side). NOTE: check size restrictions with your supervisor/instructor. Use safety glasses with side shields that have been approved by the CSA.•Where exposure to toxic or irritating fumes could be a problem, the best form of eye protection is safety goggles. Safety goggles that will form a tight seal to your face.•Contact lenses can be a hazard and sometimes should not be worn in the lab.Therefore contact lenses wearers have three options in the labs:a) remove the contact lens before entering the lab and wear safety glassesor safety goggles.b) replace the contact lens with prescription glassesc) wear the contact lens into the lab under a pair of safety goggles but youmust inform your supervisor/ instructor about it.• A full-face shield is highly recommended when there is a risk of explosion or splashing, or with combustion and high temperature reactions.2. GlovesDepending on the procedure to be carried out, different types of gloves must be available in the laboratory. The gloves should “fit” the chemical. Asbestos gloves should not be used. If any are found, they should be replaced.•Gloves are made from a variety of materials which vary in their impermeability and wear-resistance.•Disposable gloves are made of PVC, latex, nitrile, and combinations of the aforementioned. These gloves are for general use and have low abrasion resistance.•More resistant, impermeable, reusable gloves are made from butyl rubber, nitrile, or neoprene.•Rubber: good chemical resistance, low abrasion resistance;•Neoprene: almost impermeable to regular solvents, fairly abrasion resistant;•Nitrile: highly resistant, maximum protection from liquids.•Multicomposite gloves are available for special work involving high or low temperatures or special procedures.•For more information on gloves resistance see the glove chart./~mouser/General/labzone/130AL/ndex/ndex2.html3. Lab aprons or lab coatsThe strength and impermeability of aprons depends on the materials used. These materials are also used for gloves, and their characteristics are described in 2.•Aprons should be fire-resistant, chemical-resistant, and easily washed.•Flammable fabrics should be avoided.Lab coats should be made of strong fabric and must be able to be removed quickly in case of accident. They must be long enough to protect the legs. Lab coats exposed to harmful chemicals should not be worn in public areas.4. Footwear•Substantial shoes must be worn and should cover the entire foot.•Open-toed shoes and sandals must not be worn in the laboratory.•Safety shoes or foot guards may be required under certain circumstances (e.g., when moving compressed gas cylinders – foot guards are available in cylinder storage area).•When cleaning up floor spills wear plastic foot covers available on all spill carts.5. RespiratorsRespirators used at the University of British Columbia must provide effective protection against airborne contaminants which may be present. Use of respirators should be considered to control exposure only after engineering and administrative controls have been considered. These types of controls include ventilation (e.g. fume hoods), enclosing the process, substitution of less hazardous products, rescheduling of work procedures, etc. Users are responsible for:1. Obtaining proper certification for respirator use by H.S.&E.2. Using the respirator in accordance with training instructions3. Being properly fit-tested for a respiratorand storing the respiratordisinfecting,4. Cleaning,5. Reporting any respirator malfunction to their supervisorThe following cartridges are available for use with half-mask and full-face respirators. Select the appropriate cartridge according to the chart below. Consult with H.S.&E. for situations not listed. Always ensure that the cartridges used are appropriate for the types of hazardous vapour present.Cartridge Type Colour Examples of Uses Organic vapour and acid gas Yellow Rooftop entry/lab procedures/spills Organic vapour only Black Solvents/PaintsDusts, particulate, and aerosols Purple Toxic dusts/infectiousaerosols/asbestos welding fumes Ammonia/amines Green Ammonia SpillAcid Gas Grey Acid gases/chlorine/sulfur dioxidePERSONNEL MUST BE CERTIFIED BY HS&E PRIOR TO RESPIRATOR USE. When fitting a new respirator, try on several brands and sizes. Different brands will fit slightly differently on your face. Respirator manufacturers usually have small, medium, and large face-pieces available. Adjust the straps so that the respirator fits tightly, but does not dig into your face or leave red marks on your skin. The respirator should feel snug, yet comfortable.1. Remove respirator, cartridges, and filters from plastic bags.Check to see that gasket is in cartridge holder before screwing incartridges. Insert filter into retainer caps and snap onto cartridgeholder or cartridges.2. The cartridge holders are keyed to assure their correctpositioning and maintain the proper balance of the device. Makesure they are properly positioned and seated.3. Place respirator on face with narrow end over nose and bottomunder chin. First attach top headband around crown of headand then bottom around neck. Adjust headbands until a tight butcomfortable fit is obtained.4. TEST FOR TIGHTNESS: Place the palm of the hand or thumbover the valve guard and press lightly. Exhale to cause a slightpressure inside face piece. If no air escapes, respirator isproperly fitted. If air escapes, readjust respirator and test again.There are two simple checks to test the seal. These are calledthe positive and negative pressure fit-checks. These tests mustbe done EVERY TIME the respirator is put on (see overleaf).5. FILTERS: (a) REPLACE when breathing becomes difficult, INSERT new filtersINTO retainer cap and replace cap. Generally the filter discs should be changed after eight hours of dusty exposure. (b) CHEMICAL CARTRIDGES should be replaced when the senses detect ANY abnormal condition, assuming that levels of detection by the senses do not constitute a health hazard.6. MAINTENANCE: The respirator face piece should be cleaned daily to preventskin irritation and for general sanitary purposes. First remove filters and cartridges. Then the face piece may be washed with a hand brush using a good detergent in warm water, rinsing, and air drying in a clean place. Some compounds considered to be suitable for disinfecting are: (1) a hypochlorite solution (50 parts per million of chlorine; immersion time: 2 minutes) (2) an aqueous solution of iodine (50 ppm iodine; immersion time: 2 minutes) (3) a quaternary ammonium solution (200 ppm quaternary ammonium compoundsin water with less than 500 ppm total hardness). RINSE IN CLEAN WARM WATER AND AIR DRY. Inspect respirator daily for worn or faulty parts and replace these at once. Proper parts supplied by the manufacturer must be used.7. For your protection, the DUST FILTERS and CHEMICAL CARTRIDGES mustbe assembled tightly, and changed frequently, according to exposure.8. KEEP RESPIRATOR CLEAN when not in use. Store in containerprovided.a) Put the respirator on and tighten the straps until it feels tight but comfortable.b) Close off the cartridges by covering them gently with the palm of hands, plasticbags, or gloves.c) Breathe in slightly to create a vacuum.d) Hold for 10 seconds.e) If you have a good seal, the face piece should collapse slightly against yourface and stay collapsed. No air should leak into the face piece past the sides, top, or bottom.f) If the face piece doesn’t collapse and stay collapsed, there is an air leak.Check the exhalation valves and try repositioning the respirator on your face and adjusting the head straps. Try the negative pressure check again. If you cannot get a seal after a few attempts, try on another size, make, or model of respirator, and repeat the check until you find a respirator that will pass.a) With the respirator on comfortably, close off the exhaust valve opening bycovering it with the palm of the hand.b) Breathe out slightly to force air into the face piecec) Hold for 10 seconds.d) If you have a good seal, the face piece should bulge out and stay out.e) If the air does leak out, check the inhalation valves, readjust the respirator andtry the check again. Try on another size, make or model if you fail to pass the positive pressure fit-check.1.TOXIC SUBSTANCESAny volatile substances which are dangerous when inhaled must be handled only in an adequately ventilated area or in a fume hood.a) BenzeneBenzene is particularly dangerous since it causes blood diseases.•Avoid using it as a solvent. Chronic poisoning is possible following prolonged inhalation of minute quantities of benzene.•Avoid skin contact.•It is a known carcinogen.b) Carbon tetrachloride and chloroformCarbon tetrachloride and chloroform have specific dangers:•They can be absorbed through the skin.•These substances can eventually cause functional disorders of the kidney and the liver even at low concentrations.•They are suspected carcinogens.c) Cyanides and NitrilesCyanides and Nitriles are some of the most acutely toxic substances known;they react very quickly “in vivo” when they are present in the ambient environment.•Symptoms of poisoning (weakness, difficulty in breathing, nausea) appear as soon as these substances have been absorbed, inhaled, or ingested.•Contact with acid liberates a highly toxic gas. The inhalation of a very minute amount of hydrogen cyanide (HCN) can be fatal.d) PhenolsSolutions of phenols are very dangerous.•Phenols are absorbed rapidly through the skin during contact.•If rapid and complete decontamination is not effected immediately, serious poisoning and even death could occur, depending on the concentration ofthe solvent and the amount of body surface that is contaminated.e) Hydrogen fluorideHydrogen fluoride is extremely corrosive. Due to the absence of immediate pain, penetration can be extensive and lead to serious injury. It can cause severe eye irritation and skin burns.f) Hydrogen sulfideHydrogen sulfide is very toxic. Inhalation causes respiratory paralysis. It can also damage the eyes and mucous membranes.•Small cylinders of it are commercially available for laboratory use.•CAUTION: The gas can be easily synthesized by action of dilute acids on sulfides•Waste gas should be passed through scrubbers before venting.2.DANGEROUS SUBSTANCESa) Perchloric acidPerchloric acid is a strong oxidizing agent capable of reacting violently with reducing agents or organic substances.•Handle it in a specially-constructed fume hood used only for this purpose.This hood should be of the water wash-down type and of non-combustible construction.•Always destroy any organic material with nitric acid before adding perchloric acid•Never mix perchloric acid with sulfuric acid because through dehydration, anhydrous perchloric acid is obtained, which is even more unstable.•Perchlorate esters, when exposed to impact, behave in the same manner as nitroglycerine.b) Organic PeroxidesSome organic peroxides are very unstable and very dangerous. Due to their high sensitivity to heat, friction, impact, sparks, light, and oxidizing and reducing agents, they can cause violent explosions.To minimize the risks of such peroxides, the following precautions must be taken:•Buy only the necessary quantities of peroxides needed.•Use only the minimum amount necessary. Never replace unused peroxide in the original container.•Immediately clean up spilled peroxide.•Reduce the sensitivity of most peroxides to impact and to heat by using them in inert solvents such as aliphatic hydrocarbons.•If a volatile solvent must be used, avoid losses due to evaporation which could increase the peroxide concentration, eventually causing the formation of dangerously explosive crystals upon complete evaporation of the solvent.•Never use a metal spatula to handle peroxides because contamination by metals can lead to the formation of explosive compounds. Use wood, ceramic, or plastic spatulas.•Avoid flames, sources of heat, and direct sunlight.•Avoid friction or impact with solid peroxides. Never use glass containers with ground glass or metal tops. Use only polyethylene bottles with screw tops.•Store peroxides at as low a temperature as possible above the freezing point, so as to minimize the rate of decomposition.•Do not cool liquid peroxides, or those in solution, to temperatures where they could solidify or precipitate because in this form they are extremely sensitive to impact and to heat.3.CARCINOGENSCarcinogens and substances capable of inducing cancer. These substances must be subject to strict guidelines such as those published by the International Agency for Research on Cancer when they are stored, used, and disposed of.•Avoid exposure.•Where exposure is unavoidable, keep it as low as reasonably achievable.•The list of known carcinogens is continually updated. (See next page for some examples of carcinogens).4.MUTAGENS AND TERATOGENSMutagens are substances causing permanent transmissible alterations in genetic information. Teratogens are agents interfering with normal prenatal development causing abnormalities in the fetus. Exposure to mutagens and teratogens should be kept as low as possible. (See following pages for some examples of mutagens and teratogens).CAUTION: This is NOT a complete list of all chemicals having substantial evidence of carcinogenicity. Further, each substance listed here may have additional health hazards.CARCINOGENS MUST BE DISTINCTLY LABELLEDa) KNOWN HUMAN CARCINOGENS•4-Aminobiphenyl (xenylamine, p-phenylaniline)• Arsenic• Arsenic Pentoxide• Arsenic Trichloride• Asbestos• Arsenic Trioxide• Benzene•Benzidine (4,4’-diaminobiphenyl, 4,4’-biphenyldiamine)•Benzo(a)pyrene (3,4-benzpyrene)• Bis(chrloromethyl)ether• 1,4-Butanediol dimethylsulfonate•Calcium arsenate (tricalcium arsenate)•Chloromethyl methyl ether (chloromethyloxymethane)•Chromates (certain insoluble forms such as lead and zinc chromates)•Coal tar pitch volatiles•Cyclophosphamide (N,N-bis (2-chloroethyl) tetrahydro – 2H-1,3,2 –oxazaphosphorin-2-amine-2-oxide)• Lead Arsenate• 2-Napthylamine (2-aminonapthylamine)•N, N-bis (2-chloroethyl)-2- napthylamine• 4-Nitrobiphenyl (p-nitrobiphenyl)• Sodium Arsenate• Sodium Arsenite• Thorium dioxide•Treosulfan (pure product)•Vinyl chloride (chloroethane, chloroethylene)Please Note: These are ALARA substances which means that the contamination concentration of these chemicals must be as low as reasonably achievable.CAUTION: This is NOT a complete list of all chemicals having substantial evidence of carcinogenicity. Further, each substance listed here may have additional health hazards.CARCINOGENS MUST BE DISTINCTLY LABELLED• Acrylamide(propenamide, acrylic amide)•Acrylonitrile (propene nitrile, cyanoethylene, vinyl cyanide)• 1,3-Butadiene (vinylethylene)• Cadmium powder• Cadmium Chloride• Cadmium Sulfate• Beryllium• Carbon tetrachloride(tetrachloromethane)• Chloroform (trichloromethane)•Dimethyl sulfate (sulfuric acid dimethyl ester)•Ethylene dibromide (1,2-dibromoethane), ethylene oxide(1,2 epoxyethane oxirane)• Formaldehyde (methanal,oxomethane)• Hexachlorobutadiene• * Hexamethylphosphoramide (HMPA)(hexamethylphosphoric triamide)• Hydrazine (diamine)• Lead acetate• Lead phosphate• Lead subacetate• Methylhydrazine•Methyl iodide (iodomethane)• Nickel• Nickel carbonate• Nickel carbonyl• Nickel oxide• Nickel hydroxide • Nickel subsulfide• 2-Nitropropane• Phenyl hydrazine• beta-Propiolactone (2-oxetanone, 3-hydroxy-beta-lactone propanoicacid)• Propyleneimine(2 -methylazacyclopropane, or2-methylaziridine)•o-Toluidine (2-methylaniline, or o-aminotoluene)• p-Toluidine (4-aminotoluene)•Vinyl bromide (bromoethylene)•Production of SbO3, AsO3, CdO* HMPA is apparently a particularly nasty carcinogen which is used in several labs throughout the Department of Chemistry. Users should be aware of its extreme toxicity, its ability to be absorbed though the skin, and the dangers of inhalation during distillation procedures. Precautions should include: use restricted to fume hoods, all contaminated vessels labelled “carcinogen”, use of two pairs of gloves, and the transfer of waste directly into the waste solvent containers or a separate correctly labelled vessel. There are at least two alternative solvents, 1,3-Dimethyl-2-imidazolidinone (DMEU) and 1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H) pyrimidinone (DMPU) which are considered safe.CAUTION: This is NOT a complete list of all chemicals having substantial evidence of mutagenicity or teratogenicity. The extent of the hazard to humans associated with exposure to these substances is less clear than it is with carcinogens. However, it is recommended that similar caution should be exercised in handling substances which are mutagenic or teratogenic.• Acetamide• Acridine Orange• Ammonium Chromate• Ammonium Bichromate• Ammonium Dichromate• Anthracene• Antimony Oxide• Beryllium Carbonate• Cobalt Powder• Colchicine• 1,2-Dichloroethane (Ethylene Dichloride)• Formaldehyde• Formamide• Hydroquinone• Indigo Carmine• Lead Diacetate • Mercury• Osmium Tetraoxide• Potassium Chromate• Potassium Permanganate • Pyrogallic Acid• Silver(I) Nitrate• Sodium Azide• Sodium Dichromate• Sodium Nitrate• Sodium Nitrite• Thioacetamide• Toluene•Urethane (Ethyl Carbamate)UNIVERSITY OF BRITISH COLUMBIAPOLICIES AND PROCEDURESU.B.C. POLICY ON HAZARDOUS MATERIALS MANAGEMENT RESPONSIBLE:Vice President Academic & ProvostVice President Administration & FinanceVice President ResearchPURPOSE:As a large teaching and research institution, UBC faces problems that are unique and varied about the acquisition, handling, storage, transportation, and disposal of chemical and biological/human/animal materials and wastes resulting from its teaching, research and operations. This policy has several purposes:•To set out University requirements for proper disposal of hazardous and special wastes•To ensure worker protection•To reduce the amount of dangerous substances used in University activities •To raise awareness and increase knowledge of all members of the University community about problems of handling, storage, transportation and disposal of hazardous materials and waste•To establish good laboratory practices that teach and practise safe handling, storage, transportation and disposal of special wastes•To ensure compliance with applicable legislation.POLICY:All chemical and biological materials considered hazardous unless specifically excluded from Schedule 7 of the Transportation of Dangerous Goods Act. Materials classified as special wastes must be disposed of in a safe manner in compliance with the Special Waste Regulations of the Waste Management Act, and in consultation with the UBC Environment Services Facility. As all of UBC is considered one site, the rules for handling hazardous materials apply equally to small quantities as they do to large quantities. Each member of the University community who uses or has responsibility for hazardous materials must handle, store, transport and dispose of this material in a manner that harms neither the environment nor living beings, and that meets or exceeds legal requirements.Procedures are established for standard methods of handling chemicals, and biological/human/animal materials in all UBC activities. It is the responsibility of the Administrative Heads of Unit, Principal Investigators and Supervisors to ensure that appropriate training is given and documented to all students and staff who come into contact with these materials.Each member who comes into contact with or uses hazardous materials in their study must first become familiar with the hazards associated with the material and the appropriate method for handling, storage, transportation, and disposal. Up-to-date training records are to be maintained.Individual members are expected to conduct themselves and supervise others with the greatest of care, and, if established procedures for the circumstances do not exist, are responsible for seeking guidance from the appropriate source before ordering, handling, sorting, or disposing of materials that could be hazardous to the environment or to living beings. In accordance with Section 122 of the Canadian Environmental Protection Act:“Where a corporation commits an offence under this Act, anyofficer, director or agent of the corporation, who directed,authorized or assented to or acquiesced to or participated in thecommission of the offence is a party to and guilty of the offence,and is liable to punishment provided for the offence, whether or notthe corporation has been prosecuted or convicted.”Consideration should be given to substituting less harmful materials for those that are known to be hazardous at the time of acquisition. Hazardous materials should be purchased in quantities small enough that they do not have to be stored at UBC over long periods.In physical planning for the future research, teaching and operational needs of the University, design elements to address special waste flows should be included to address handling, storage, transportation, emissions, and disposal.PROCEDURES:The number and variety of possibly hazardous materials at UBC are large. Some are created as the result of experimentation. For this reason, the procedures under this policy are meant to provide guidance via illustration and example to individuals at UBC about such areas as chemical, biological, human, and animal materials. For radioisotopes, please see Policy # 11. For pesticides, Please see Policy #12 (http://www.policy.ubc.ca). Individuals unsure about whether a substance (such as paint, oil, pharmaceutical, battery) is hazardous, or about the appropriate steps to take, should contact the UBC expert listed in the procedures below.Laws and regulations governing chemical, human, and biological materials acquisition, handling, storage, and disposalLaws and regulations governing biological materials acquisition, handling, storage transportation and disposal include, but are not limited to:•Canadian Environmental Protection Act•Transportation of Dangerous Goods Act•Provincial Waste Management Act including the Special Waste Regulations and Spill Reporting Regulation•Greater Vancouver Regional District Bylaws, in particular Sewer Use Bylaw # 164 and # 167, Air Quality Management Bylaw # 603 and # 725 and Municipal Solid Waste and Recyclable Material Bylaw # 181 and # 183.•Workers’ Compensation Board Industrial Health and Safety Regulations• WHMIS•Laboratory Biosafety Guidelines for Health Canada•Health Canada, Narcotics/Controlled Products Act for pharmaceuticals •Containment Standards for Veterinary FacilitiesChemical MaterialsThe Chemical Safety Officer develops generic procedures for handling chemicals, which are distributed to all labs. For chemicals unique to a particular laboratory, the principal investigator must develop written procedures, to be vetted by the Health, Safety & Environment Department. Each department or unit using chemical materials must develop or adopt procedures that include:•Acquiring minimum quantities only•Safe and secure storage•Removing out-of-date materials from inventory•Inspection of time sensitive materials•Appropriate labeling consistent with WHMIS requirements•An annual inventory of materials•Training of faculty, staff and students•Proper use of personal protective equipment, emergency spills, and decontamination procedures•Compliance with University (or host institution) procedures for disposalHuman, Animal and Biological MaterialsThe Biosafety Officer develops procedures for handling materials that are used in more than one laboratory. Written procedures are issued to all labs. For materials unique to a particular laboratory, the principle investigator using human, animal, or biological materials must develop written procedures, to be vetted by the biosafety Officer, that deal with regulated medical waste. Regulated medical waste includes, but is not limited to, the following categories:Human and Biological Materials Continued…•Cultures and stocks of infectious agents, and any materials contaminated witha potentially infectious agent, including, culture dishes and devices used totransfer, inoculate and mix cultures•Any human pathological wastes, including waste human blood or blood products generated in medical or research procedures, and other potentially infectious materials, items contaminated with these materials, and any containers that held these potentially infectious materials•Any animal specimens, carcasses or tissues•Any biological material contaminated with an infectious agent• DNA• Vaccines, pharmaceuticals•Wastes from medical or research procedure that were in contact with infectious agents, including slides and cover slips, disposable gloves, and protective equipment.•Sharps: used or new hypodermic needles and syringes (with or without needle attached), scalpels and razor blades. Also, Pasteur pipettes and broken glassware, when contaminated with an infectious agent•Mixed Waste: Biological specimens or material treated with or preserved in chemicals including alcohol or formaldehyde are considered mixed waste (regulated medical waste and hazardous chemical waste)•Bedding for animals•Other regulated medical waste solids must be placed in secure, leak-proof packaging and stored in such a manner that will prevent decomposition or deterioration during storageIt is the responsibility of each generator to set up a work system prior to generating medical wastes. Principal investigators, area supervisors, or other employees generating regulated medical waste materials are responsible for compliance with applicable regulations and disposal program requirements. Consult the Biosafety Officer for more information.Each department or unit using human, animal, or biological materials must develop procedures that include:•Acquiring minimum quantity control•Safe and secure storage•Appropriate labeling and an annual inventory of materials•Training of faculty, staff and students•Proper use of personal protective equipment, emergency, spill and decontamination procedures•Compliance with University (or host institution) procedures for disposal.。
戊二醛化学交联注意事项

戊二醛化学交联注意事项GlutaraldehydeGlutaraldehyde is the most popular bis-aldehyde homobifunctional crosslinker in use today. However, a glance at glutaraldehyde’s structureis not indicative of the complexity of its possible reaction mechanisms. Reactions with proteins and other amine-containing molecules would be expected to proceed through the formation of Schiff bases. Subsequent reduction with sodium cyanoborohydride or another suitable reductant would yield stable secondary amine linkages (Chapter 3, Sections 4.4 and 5.3). This reaction sequence certainly is possible, but other crosslinking reactions also occur. Glutaraldehyde in aqueous solutions can form polymers containing points of unsaturation due to aldol formation ((Figure 5.25) (Chapter 15, Section 2.1) (Hardy et al., 1969, 1976;Monsan et al., 1975). Such α,β-unsaturated glutaraldehyde polymers arehighlyreactive toward nucleophiles, especially primary amines. Reaction with a protein results in alkylation of available amines, forming stable secondary amine link-ages. These glutaraldehyde modified proteins still may react with other amine-containing molecules either through the Schiff base pathway or through addition at other points of unsaturation (Figure 5.26). The proposed reaction mechanism of conjugation using these polymer conjugates may explain the stability ofproteins crosslinked by glutaraldehyde that has not been reduced. Schiff base formation alone would not yield stable crosslinked products without reduction. In addition, a number of other potentialreactions of glutaraldehyde in aqueous solution also can contribute toits stable crosslinking ability. These include reactions involving hemiacetal rings sometimes combined with aldol formation products, which can couple to amine groups without the formation of Schiff base linkages (see Chapter 15, Section 2.1, for an in-depth discussion of these reactions).FIGURE 5.24 Two amine-containing molecules can be crosslinked byformaldehyde through formation of a quaternary ammonium salt with subsequent dehydration to an immonium cation intermediate. This active species then can react with a second amine compound to form stable secondary amine bonds.Crosslinking using glutaraldehyde polymers can be difficult to reproduce and scale up. Since the exact glutaraldehyde state in solution, including its potential polymer size and structure, is difficult todetermine, the exact nature of the conjugates formed by this method may be indeterminable as well. The age of a glutaraldehyde solution is another variable, because the older the solution the more polymer could be formed. Fresh glutaraldehyde often will not yield the same results as aged solutions. Some methods to control the glutaraldehyde activation and coupling process have been successfully carried out for the immobilization of affinity ligands (Chapter 15, Section 2.1); therefore, it also may be possible to use such methods to better control conjugate formation in solution.A third method of using glutaraldehyde in conjugation reactions is through its ability to react rapidly with hydrazide groups. A molecule containing hydrazide functionalities or modified to contain them (Chapter 2,Section 4.5) can be conjugated with another molecule containing either amines or hydrazides. Glutaraldehyde will react with the hydrazide groups to form hydrazone linkages. When two macromolecules are crosslinked in solutions that contain multiple sites of conjugation, the multivalent hydrazone bonds will be strong enough to create a stable conjugate. If a small molecule is involved,however, reduction of the hydrazone with sodium cyanoborohydride is recommended to produce a leak-resistant bond.Glutaraldehyde has been used extensively as a homobifunctional crosslinking reagent, especially for antibody–enzyme conjugations (Avrameas, 1969; Avrameas andTernynck, 1971) and to produce vaccine immunogens (De Filette et al., 2011; Chapter 19, Section 7). To help overcome its tendency to formlarge-molecular-weight polymers upon crosslinking two proteins, a two-step protocol often is employed. In this regard, one protein first is reacted with glutaraldehyde and purified away from excess reagent. The second protein then is added to effect the conjugate formation. See the introduction to this chapter and Chapter 2, Section 1.2 as well as Chapter 20, Section 1.2 for additional information on the use of glutaraldehyde and two-step crosslinking procedures.FIGURE 5.25 Glutaraldehyde in aqueous solution may polymerize at either acid or basic pH.FIGURE 5.26 Glutaraldehyde may react by several routes to form covalent crosslinks withamine-containing molecules.FIGURE 5.27 Epoxide groups are reactive toward sulfhydryls,amines, and hydroxyls.。
离子液体催化二氧化碳合成环状碳酸酯的研究进展

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第9期·3300·化 工 进展离子液体催化二氧化碳合成环状碳酸酯的研究进展杨美1,钟向宏2,陈群3(1中山大学化学与化学工程学院,广东 广州 510275;2中国石油化工股份有限公司茂名分公司研究院,广东茂名525011;3常州大学江苏省精细石油化工重点实验室,江苏 常州 213164)摘要:离子液体作为一种新型绿色环保介质,由于其结构可设计、稳定性高以及催化活性高等优点,使其在CO 2环加成反应的催化方面应用前景广阔。
本文综述了近年来离子液体催化CO 2与环氧化物的环加成反应制备环状碳酸酯的研究进展。
传统离子液体包括咪唑类、吡啶类、季铵盐季盐等离子液体,而功能化离子液体包括羟基功能化、羧基功能化等离子液体。
与传统离子液体相比,功能化离子液体具有更好的催化活性。
无机或有机材料负载的非均相离子液体催化剂报道较多,载体包括SiO 2、氧化石墨烯、聚合物等。
非均相催化剂具备易分离、可在固定反应器中连续反应等优点,更适应工业化生产。
指出了CO 2与环氧化合物反应制备环状碳酸酯过程中出现的催化剂活性低、反应条件苛刻等关键问题,因此寻求高选择性合成环状碳酸酯的环境友好的新型高效催化剂具有重要的学术意义和实用价值。
关键词:二氧化碳;环氧化合物;环状碳酸酯;离子液体中图分类号:O643.3 文献标志码:A 文章编号:1000–6613(2017)09–3300–09 DOI :10.16085/j.issn.1000-6613.2017-0282Recent progress of the synthesis of cyclic carbonates from CO 2 andepoxides catalyzed by ionic liquidsYANG Mei 1,ZHONG Xianghong 2,CHEN Qun 3(1School of Chemistry and Chemical Engineering ,Sun Yat-Sen University ,Guangzhou 510275,Guangdong ,China ;2Research Institute of Maoming Branch Company ,SINOPEC ,Maoming 525011,Guangdong ,China ;3Jiangsu ProvinceKey Lab of Fine Petrochemical Engineering ,Changzhou University ,Changzhou 213164,Jiangsu ,China )Abstract :As a novel environment friendly media ,ionic liquids(ILs) have shown advantages in the catalysis of this cycloaddition reaction due to their structure designability ,high stability ,and high catalytic activity ,etc .This review was mainly focus on the latest progress on the use of ILs as catalysts for the cycloaddition of CO 2 with epoxides. The conventional ILs contained imidazolium ,pyridine ,quaternary ammonium salt ,quaternary phosphonium salts and other ILs. The functionalized ILs included hydroxyl-functionalized ,carboxyl-functionalized and other ILs. Compared with conventional ILs ,functionalized ILs had much better catalytic performance. A series of inorganic or organic support immobilizing ILs catalysts had been developed. Their supports included mesoporous silica ,graphene oxide ,polymers ,etc . The chemical industry has always preferred to use a heterogeneous catalyst due to the ease of separation and the ability to use in a fixed-bed reactor. How to overcome the disadvantages of low activities ,difficulties in separation and tough reaction conditions is a key issue. It is of great academic significance and practical value to pursue the new and efficient catalyst for high selectivity synthesis of cyclic carbonates.Key words :carbon dioxide ;epoxides ;cyclic carbonates ;ionic liquids界面化学。
聚季铵盐化合物

聚季铵盐化合物Quaternary ammonium compounds, also known as quats, are an important class of chemicals used in various industries. These compounds contain a positively charged nitrogen atom linked to four alkyl or aryl groups, giving them antibacterial and antifungal properties. In Chinese, these compounds are known as 聚季铵盐化合物. They are widely used as disinfectants, surfactants, and fabric softeners due to their ability to disrupt cell membranes and inhibit microbial growth.Quats have become increasingly popular in the current global health crisis, as they are effective against a wide range of pathogens, including viruses and bacteria. The use of quaternary ammonium compounds in disinfectants has seen a significant rise in demand, as people strive to maintain clean and hygienic environments to prevent the spread of infectious diseases. In manufacturing processes, quats play a vital role in controlling microbial contamination and ensuring product safety.Despite their efficacy, the use of quats has raised concerns about potential negative impacts on human health and the environment. Studies have shown that prolonged exposure to quaternary ammonium compounds can lead to respiratory issues, skin irritation, and allergic reactions. In addition, the accumulation of these compounds in the environment has been linked to ecological harm, such as toxicity to aquatic organisms and disruption of microbial communities.To address these concerns, researchers are exploring alternative disinfection methods that are less harmful to human health and the environment. One approach is to develop greener quats with improved biodegradability and reduced toxicity. By modifying the chemical structure of quaternary ammonium compounds, scientists aim to create compounds that are effective in disinfection while minimizing the negative impact on living organisms and ecosystems.Another strategy is to promote the responsible use of quaternary ammonium compounds by implementing proper safety measures and disposal practices. This includes providing training on the correct handling of quats, using them in recommended concentrations, andensuring their appropriate disposal to prevent environmental contamination. By raising awareness about the potential risks associated with these compounds, users can make informed decisions and take steps to mitigate any adverse effects.In conclusion, quaternary ammonium compounds, or quats, are valuable chemicals with versatile applications in various industries. While they offer effective disinfection and antimicrobial properties, their widespread use raises concerns about potential health and environmental risks. By adopting sustainable practices and promoting the development of safer alternatives, we can continue to benefit from the valuable properties of quats while minimizing their negative impact on human health and the environment. Let us strive for a balance between efficacy and sustainability in the use of quaternary ammonium compounds to create a safer and healthier world for all.在这个新的时代,消毒、保洁成为大家日常生活的重要一环,为了达到更好的卫生条件,一些消毒性物质的使用不可避免。
食品中矮壮素与缩节胺的形成及其检测方法研究进展

第1期(总第520期) 2021年1月农产品加工Farm Products ProcessingNo.1Jan.文章编号:1671-9646 (2021) 01b-0056-03食品中矮壮素与缩节胺的形成及其检测方法研究进展李雪楠,*袁媛(吉林大学食品科学与工程学院,吉林长春130062)摘要:植物生长调节剂是一种特殊的含有植物激素活性的农药,能够起到改善农作物质量和控制植物生长的作用,近年来植物生长调节剂的毒性及其残留危害吸引了众多研究人员的关注。
其中,矮壮素(C H LM)和缩节胺(MEP)作为常用的2种植物生长调节剂,其使用不当和残留危害所引起的食品安全问题并没有引起足够的重视。
最近的研 究工作将CHLM和MEP确立为大麦麦芽烘烤过程的副产品,而用于研究的大麦属于生态类型,经验证不含任何植物 生长调节剂。
由此可知,CHLM和MEP是由食品中的天然成分经过热加工处理后形成的化合物。
CHLM和MEP作为新 的食品加工污染物已成为食品安全问题的研究热点。
对CHLM、MEP在食品中的形成进行了综述,并对其检测技术 进行总结,以期为CHLM和MEP的研究提供参考。
关键词:缩节胺;矮壮素;形成;检测方法中图分类号:TS207 文献标志码:A doi:10.16693/ki.1671-9646(X).2021.01.049Research Progress on the Formation and Detection Methods ofChlormequat and Mepiquat in FoodUXuenan,TUAN Yuan(College of Food Science and Engineering,Jilin University,Changchun,Jilin 130062,China) Abstract:Plant growth regulators are special pesticides with phytohormonal activity that can be used to improve crop quality and regulate growth. In recent years,the toxicity and residual hazards of plant growth regulators have attracted the attention of many researchers. Chlormequat (CHLM) and mepiquat (MEP) are two plant growth regulators commonly used in agriculture in China. But their toxicity and residual hazards have not received enough attention. Recent research work has established CHLM and MEP as by-products of the barley malt roasting process. The barley used for the study was of the ecological type and had been verified to be free of any quaternary ammonium pesticides. Therefore,CHLM and MEP are compounds formed by thermal processing of natural ingredients in food. As new food processing contaminants,they have become a research hotspot in food safety issues. In this study,the formation of CHLM and MEP in food were reviewed,and their detection methods were summarized,in order to provide reference for the research of CHLM and MEP.Keywords:mepiquat; chlormequat; formation; detection method植物生长调节剂(Plant Growth Regulators)作为 农药在农业生产中被广泛使用,其通过外源物质进 入植物体,从而改善农作物的品质。
新型季铵盐戊二醛消毒液杀菌效果评价

88中国兽医杂志2020年(第56卷)第10期Chinese Journal of Vete/naR Medicine 新型季钱盐戊二醛消毒液杀菌效果评价何淑华1,许琦1,林晓雁1,卢朝成2,傅江南1,和君1(1.暨南大学实验动物管理中心,广东广州510632&2成都中牧生物药业有限公司,四川成都610100)摘要:为了评价一种新型季W盐戊二醛消毒液的杀菌效果,采用喷雾消毒法,比较5种市售常用消毒液(复合过氧乙酸、季W盐戊二醛、微酸性酸、水、)的雾消毒&进一步采试验,比较W盐戊二醛和复合过氧乙酸对大肠埃希菌(E.coil)、金黄色葡萄球菌(S.aureus)$绿脓杆菌(P.aeruginosa)和白色念珠菌(albicans)的杀灭效果。
喷雾消毒,季W盐戊二醛1:100和复合过氧乙酸1:24雾杀灭率达100%;悬量杀菌试验结果显示,季W盐戊二醛1:1000倍稀释对S.aue的杀灭对数值>7,1:500倍稀释对E coiS杀灭对数值>7, 1:100倍稀释对P.aeruginosa的杀灭对数值>7;复合过氧乙酸1:1000倍稀释液对E.coiC、S.aureus$P.aeruginoss的杀灭对数>7;季W盐戊二醛和复合过酸1:500-albcans的杀灭对数值均>6,%型季W盐戊二醛和复合型过氧乙酸消毒液对Ed'S.aureos$P.aeruginDss及albicans均有强的杀灭能力%关键词:季W盐&戊二醛&过酸&芳中图分类号:R187.2文献标志码:A文章编号:0529—6005(2020)10—0088—05Evaluation of Germicidal Efficacy of a New Quaternary AmmoniumSalt Glutaraldehyde DisinfectantHE Shu-hua1,XU Qi1,LIN Xiavpan1,LU Chav-cheng2,FU Jiang-nan1,HE Jun1(1.Institute of LabomtoR Animal Science,Jinan University,Guangzhou510632,China&2Chengdu Zhongmu BiophaemaceuticaeCo,Ltd,Chengdu610100,China) Abstracc:Tv evaluate the germicibal efficacy of a new quaternary ammonium sa/glumraldehyde disiPectant,spray disiPection method wasused tocompaeethespea0disineection e e ctsoeeivedisineectants(compound peeaceticacid,quateenae0ammonium geut-araldehyde,slightly acidic hypochlorous acid,bleach,and ethanol).Suspension quantitative ste/Pzation test was used to observe the germicidal efficacy of quaternaR ammonium sa/glumraldehyde and compound peracetic acid on Escherichia cols(E.coil),Stophylo-coccoe aureus(S.aueus),Pseubomonas aouginosa(P.aouginosa)and Canbila alcans(C.alcans).The results showed that in the spray test,the kil/ng rate of the bacte/a were100%when the1:100dilution of quaternaR ammonium sa/glumraldehyde and /he1:24dieu ion oecompound peeaceicacid weeeused.Suspension quan iaiveseeieiaaion es/eesuesshowed/ha//heki e i ngeog value of the quaternary ammonium salt glutaralUehyde at1:1000dilution to S.aueus was>7,and at1:500dilution to E.coH was >7,at1:100dilution to P.eeroginosa was>7.The kP/ng log value of the compound peracetic acid at1:1000dilution to E.coil, S.aureos,and P.aeruginosa were all>7.The kil/ng log values of C.albicans in the quaternaR ammonium salt glutaraldehyde and thecompound peaaceticacid1:500dieution weaea e>6.A e abovesteaieiaation e e ctsweaeup tostandaad.Thequateanaa0ammoni-um salt glutaralUehyde and the compound peracetic acid have strong kP/ng ability to E.coil,S.aueus,P.aouginosa and C.alcans.Key wordt:quaternaR ammonium salts&glumraldehyde&peracetic acid&germicidal efficacyCoJTespondiiig author:FU Jiang-nan,E-mail:fujiangnanl26@&HE Jun,E-mail:shadow.Oon@收稿日期:2020—05—12基金项目:广东省自然科学基金面上项目(2019A1515011994, 2016A030310085)&广东省科技计划项目(2016A030303013)作者简介:何淑华(1989-),女,本,就读专业为生物技术,E-mail:sausagehe@126-com许琦(1993-),女,本科生,就读专业为动物医学,E-mail:t-qi2017kate@:许琦与何淑华对本文具有同等贡献通讯作者:傅江南,E-mail:fujiangnan126@;和君,E-mai:*******************消毒工作是实验动物管理的关键环节,是实验动物的基础保障。
十六烷基三甲基溴化铵结构导向

十六烷基三甲基溴化铵结构导向1. 引言十六烷基三甲基溴化铵是一种阳离子表面活性剂,具有良好的表面活性和抗静电性能。
它在许多领域中得到广泛应用,如洗涤剂、消毒剂、柔软剂等。
在这篇文章中,我们将探讨十六烷基三甲基溴化铵的结构导向方法。
2. 结构特点十六烷基三甲基溴化铵的分子式为C19H42BrN,其结构特点主要体现在以下几个方面:2.1 碳链长度十六烷基三甲基溴化铵的碳链长度为16,这意味着它具有较长的疏水碳链。
长碳链使得该物质在水中形成胶束结构,从而发挥表面活性剂的作用。
2.2 溴原子取代溴原子作为一个较大的原子,对分子整体的极性和亲水性产生一定影响。
同时,溴原子还带有正电荷,增加了阳离子表面活性剂的亲水性。
2.3 甲基取代十六烷基三甲基溴化铵中的三个甲基基团使得其分子具有较强的亲脂性。
这些甲基基团不仅增加了分子的疏水性,还能提高分子在有机溶剂中的溶解度。
3. 结构导向方法为了合成和优化十六烷基三甲基溴化铵的结构,可以采用以下几种方法:3.1 溴化反应十六烷基三甲基溴化铵可以通过溴代十六烷基三甲胺来合成。
在反应中,通过控制反应物的摩尔比例和反应条件,可以调节产物的结构特征。
例如,增加溴代剂的用量可以增加产物中溴原子的含量,从而提高阳离子表面活性剂的亲水性。
3.2 碳链长度调控为了控制十六烷基三甲基溴化铵的碳链长度,可以选择不同长度的直链醇作为原料进行合成。
较长碳链长度可通过使用较长碳链醇来实现。
3.3 取代位点选择在合成过程中,也可以选择不同的取代位点来引入甲基基团。
这可以通过选择不同的反应条件或催化剂来实现。
不同取代位点上的甲基基团会对分子的疏水性和亲脂性产生影响。
4. 应用领域十六烷基三甲基溴化铵由于其良好的表面活性和抗静电性能,在许多领域中得到广泛应用。
以下是一些主要应用领域:4.1 洗涤剂十六烷基三甲基溴化铵常被用作洗涤剂的成分之一。
它可以有效地降低水表面张力,改善洗涤效果,并具有良好的乳化和分散作用。
不饱和季铵盐的合成及其聚合物在化工及环保领域的应用

[摘 要 ]按不饱 和基 团的类 型对不饱和季铵盐进行 了分类 ,并综述 了其合成方法。介 绍了其 聚合物在含油污水处
理 、抗 菌 、纺织 品 、新 材料等领域 的应 用。 阐述 了在不饱 和季铵盐 的开发及其 聚合物 的应用方 面存 在的问题 。
指 出了今后 的发展方 向 :应 该加大科研投入 ,加快新型不饱和 季铵盐的研发进程 ,特别是在主结构设计 、纯 度
·Hale Waihona Puke 256 · 化 工 环 保 ENVIRONM ENTAL PROTECTION OF CHEMICAL INDUSTRY
20 2 6年 第 36卷第 3期
不饱和季铵盐 的合成及 其聚 合物在 化 工及 环 保 领 域 的应 用
游 娜 ,滕厚 开 ,韩恩 山 ,周立 山
(1.河北 工业 大学 化工学院 ,天津 300130;2.中海油天津化丁研究设计 院 ,天津 300131)
聚 季 铵盐 是 一 类 正 电荷 沿 大 分子 骨 干 分 布 、 电荷 密 度 高 、具 有独 特 性 质 的聚 电解 质 u 。长 范 围的库仑 作用 、水 溶液 中的链 构象 、灵 活性 的长链 结 构 以及 电荷转移 作用 使高分 子季 铵盐 具有诸 多优 点 ,在 日用 化学 品 、污水处 理 、新 材料 、纺织 等行 业 应用 广泛 。
第 3期
游 娜 等.不饱 和季铵盐 的合成及其聚合物在化工及环保领域的应用
·257·
种类 ,可将其 分为丙烯酸型 、丙烯酰胺型 、烯丙 (氧 )基 型 、苯 乙烯 型等类 型 。
根 据 不 饱 和 季 铵盐 中铵 基 取 代 烷 基 的种 类 , 可将其分为二烷基二 甲基铵型 、烷基 二 甲基苄基 型 、吡啶翰盐 型 、烷基 异喹 啉翁盐 型 、氯苄 铵翁 盐 型等类 型 。
不同季铵盐改性剂对蒙脱土性能的影响

赵敏敏女士,在读硕士研究生; 主要研究方向:涂布加工技术 以及造纸湿部化学。
摘 要:分别采用单因素实验和正交实验考察了十二烷基三 甲基溴化铵(DTA B)、十六烷基三甲基溴化铵(C TA B)、十八烷基 三甲基溴化铵(OTA B)和十八烷基二甲基苄基氯化铵(O D B A)四 种改性剂以及改性条件对钠基蒙脱土性能的影响,并利用傅 里叶变换红外光谱(F T I R)、X射线衍射(X R D)以及透射电子 显微镜(T E M)对改性效果进行了对比分析。研究结果表明: 四种改性剂均进入蒙脱土片层间,在一定程度上增大了蒙脱 土的层间距,且O D B A的改性效果最好。通过正交实验得出: 利用ODBA对钠基蒙脱土进行改性,最佳改性条件为反应时间 3h,反应温度80℃,改性剂用量1.2C E C(阳离子交换量),蒙脱 土层间距由1.152nm增加到了1.931nm。 关键词:蒙脱土;改性剂;正交实验
技术进步 Technology
不同季铵盐改性剂对蒙脱土性能的影响
⊙ 赵敏敏 景宜* (南京林业大学江苏省制浆造纸科学与技术重点实验室,南京 210037)
Effect of different quaternary ammonium salt modifiers on the properties of montmorillonite
Abstract: In this paper, single factor experiments and orthogonal experiments were used to investigate the effects of dodecyltrimethyl ammonium bromide (DTAB), cetyltrimethyl ammonium bromide (CTAB), octadecyltrimethylammonium bromide (OTAB) and octadecyl dimethyl benzyl ammonium chloride (ODBA) as well as the modification conditions on the properties of natural montmorillonite. The effects of modification we17年5月 Copyright©博看网 . All Rights Reserved.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Department of Applied Chemistry and Institute of Colloid and Interface Science, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan Received 22 September 2003; accepted 4 March 2004 Available online 10 April 2004
T. Yoshimura et al. / Journal of Colloid and Interface Science 275 (2004) 618–622
619
2.3. Measurements The surface tension of aqueous solutions of surfactants/PSS mixed systems was measured with a Krüss K100 tensiometer by using the Wilhelmy plate technique. Steadystate fluorescence spectra of pyrene in the mixed systems were obtained using a Hitachi 650-10S spectrophotometer in the range 360–400 nm at an excitation wavelength of 335 nm, where the concentration of pyrene was 1 × 10−5 mol dm−3 . Dynamic light scattering was also performed with a DLS-7000, Otsuka Electronics Company, Ltd., spectrophotometer using an argon laser of 488 nm. All solutions were filtered with a 0.2-µm filter of mixed cellulose acetate. All measurements were carried out at 25 ◦ C.
E-mail address: yoshimura@ch.kagu.tus.ac.jp (T. Yoshimura). 0021-9797/$ – see front matter 2004 Elsevier Inc. All rights reserved. doi:10.1016/j.jcis.2004.03.002
Abstract The interactions of cationic gemini surfactants, 1,2-bis(alkyldimethylammonio)ethane dibromide (m-2-m: m is hydrocarbon chain length, m = 10 and 12), and an anionic polymer, sodium poly(styrene sulfonate) (PSS), have been characterized by several techniques such as tensiometry, fluorescence spectroscopy, and dynamic light scattering. The surface tension of gemini surfactant/PSS mixed systems decreases with surfactant concentration, reaching break points, which are taken as critical aggregation concentrations (cac). The surface tension at the cac of mixtures is higher than that of single surfactants, and it is found that at concentrations above the cac, the surfactant molecules are associated with the polymer in the bulk. The 12-2-12/PSS mixed system shows higher surface activity than both 10-2-10/PSS and the monomeric surfactant of dodecyltrimethylammonium bromide/PSS systems. Fluorescence measurements of these mixed systems suggest the formation of a complex with a highly hydrophobic environment in the bulk of the solution. Additionally, dynamic light scattering measurements show that the hydrodynamic diameter of the 12-2-12/PSS mixed system is smaller than that of PSS only at low concentration, indicating interactions between surfactant and polymer. These result from the electrostatic attraction between ammonium and sulfate headgroups as well as the hydrophobic interaction between their hydrocarbon chains. 2004 Elsevier Inc. All rights reserved.
tant/uncharged polymer mixed systems have been reported, there have been few reports about those of gemini surfactant/polymer mixed systems [14,15]. Gemini surfactants, which have two hydrocarbon chains and two hydrophilic groups in a molecule, reportedly possess unique properties, such as better solubilization, lower Krafft temperatures, lower critical micelle concentration (cmc), greater efficiency in lowering the surface tension, and foaming properties than the conventional monomeric surfactants [16–19]. The purpose of this study is to investigate whether any interaction occurs between cationic gemini surfactant and anionic polymer at air/water interface and in solution. Gemini surfactant is used to investigate the effect of chain numbers of these compounds on interactions with anionic polymer. In this work, we investigate the interactions of cationic gemini surfactant, 1,2-bis(alkyldimethylammonio)ethane dibromide (m-2-m, where m is the hydrocarbon chain length, m = 10 and 12), and an anionic polymer, sodium poly(styrene sulfonate) (PSS), by measuring surface tension, fluorescence spectroscopy of pyrene, and dynamic light scattering. In
Journal of Colloid and Interface Science 275 (2004) 618–622 /locate/jcis
Interactions of quaternary ammonium salt-type gemini surfactants with sodium poly(styrene sulfonate)
Keywords: Cationic gemini surfactant; Anionic polyelectrolyte; Interaction; Aggregate; Surface tension; Fluorescence; Hydrodynamic diameter
1. Introduction The study of interactions between surfactants and polymers is an active field of interest in colloid science [1]. The mixtures of surfactants and polymers are used in the fields such as paints and drilling muds, etc. Important information on surfactant and polymer interathe bulk is currently being provided by techniques such as surface tension, neutron and X-ray reflection, ellipsometry, and surface rheology [2]. The mixtures of oppositely charged surfactants and polymers are very sensitive indicators of interaction [3–5]. The interaction between the cationic surfactant alkyltrimethylammonium bromide or chloride and anionic polymer such as polyacrylamide sulfonate, polystyrene sulfonate (PSS), DNA and xanthan has been investigated [6–13]. Although many studies of the properties of the conventional monomeric surfac* Corresponding author. Fax: +81-3-32352214.
