Aprotinin_9087-70-1_DataSheet_MedChemExpress
细胞治疗药典参考目录(Gene and Cell Therapy Products)

1 Chapter Guide: Gene and Cell Therapy ProductsTo return to the Chapter Charts main list, click her e.Universal TestsIdentification•á11ñ USP Reference Standards•á1030ñ Biological Assay Chapters—Overview and Glossary•á1032ñ Design and Development of Biological Assays•á1033ñ Biological Assay Validation•á1034ñ Analysis of Biological Assays•á1044ñ Cryopreservation of Cells•á1102ñ Immunological Test Methods—General Considerations•á1103ñ Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)•á1104ñ Immunological Test Methods—Immunoblot Analysis•á1285ñ Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis•á1285.1ñ Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic ExaminationAssay•á621ñ Chromatography•á1030ñ Biological Assay Chapters—Overview and Glossary•á1032ñ Design and Development of Biological Assays•á1033ñ Biological Assay Validation•á1034ñ Analysis of Biological Assays•á1044ñ Cryopreservation of Cells•á1102ñ Immunological Test Methods—General Considerations•á1103ñ Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)•á1430.1ñ Analytical Methodologies Based on Scattering Phenomena—Static Light Scattering Specific Tests1, 2Biocompatibility•á87ñ Biological Reactivity Tests, In Vitro•á88ñ Biological Reactivity Tests, In Vivo•á1031ñ The Biocompatibility of Materials Used in Drug Containers, Medical Devices, and Implants•á1184ñ Sensitization TestingMicrobial/Sterility Issues•á61ñ Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests•á62ñ Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms•á63ñ Mycoplasma Tests•á71ñ Sterility Tests•á85ñ Bacterial Endotoxins Test•á151ñ Pyrogen Test•á161ñ Medical Devices—Bacterial Endotoxin and Pyrogen Tests•á1050ñ Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin•á1071ñ Rapid Microbial Tests for Release of Sterile Short-Life Products: A Risk-Based Approach•á1085ñ Guidelines on Endotoxins Test•á1113ñ Microbial Characterization, Identification, and Strain Typing•á1116ñ Microbiological Control and Monitoring of Aseptic Processing Environments•á1208ñ Sterility Testing—Validation of Isolator Systems•á1211ñ Sterility Assurance•á1227ñ Validation of Microbial Recovery from Pharmacopeial Articles•á1228ñ Depyrogenation•á1228.1ñ Dry Heat Depyrogenation•á1228.3ñ Depyrogenation by Filtration•á1228.4ñ Depyrogenation by Rinsing•á1228.5ñ Endotoxin Indicators for Depyrogenation•á1229ñ Sterilization of Compendial Articles•á1229.3ñ Monitoring of Bioburden•á1229.4ñ Sterilizing Filtration of Liquids2•á1229.14ñ Sterilization Cycle Development•á1229.15ñ Sterilizing Filtration of Gases•á1229.17ñ Mycoplasma Sterilization•á1229.18ñ Viral Clearance MethodsProduction Issues•á1ñ Injections and Implanted Drug Products (Parenterals)—Product Quality Tests•á90ñ Fetal Bovine Serum—Quality Attributes and Functionality Tests•á92ñ Growth Factors and Cytokines Used in Cell Therapy Manufacturing•á797ñ Pharmaceutical Compounding—Sterile Preparations•á1024ñ Bovine Serum•á1041ñ Biologics•á1043ñ Ancillary Materials for Cell, Gene, and Tissue-Engineered Products•á1044ñ Cryopreservation of Cells•á1046ñ Cell-based Advanced Therapies and Tissue-Based Products•á1047ñ Gene Therapy Products•á1074ñ Excipient Biological Safety Evaluation Guidelines•á1126ñ Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing•á1127ñ Nucleic Acid-Based Techniques—Amplification•á1229.16ñ Prion Inactivation•á1229.17ñ Mycoplasma Sterilization•á1229.18ñ Viral Clearance Methods•á1237ñ Virology Test Methods•á1285ñ Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis•á1285.1ñ Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic Examination Product Issues•á381ñ Elastomeric Components in Injectable Pharmaceutical Product Packaging/Delivery Systems•á382ñ Elastomeric Component Functional Suitability in Parenteral Product Packaging/Delivery Systems•á1046ñ Cell-based Advanced Therapies and Tissue-Based Products•á1086ñ Impurities in Drug Substances and Drug Products•á1121ñ Nomenclature•á1151ñ Pharmaceutical Dosage Forms•á1229.17ñ Mycoplasma SterilizationEquipment•á31ñ Volumetric Apparatus•á41ñ Balances•á1051ñ Cleaning Glass Apparatus•á1228.4ñ Depyrogenation by Rinsing•á1229.13ñ Sterilization-in-Place•á1229.15ñ Sterilizing Filtration of Gases•á1229.16ñ Prion Inactivation•á1251ñ Weighing on an Analytical BalanceCharacterization•á111ñ Design and Analysis of Biological Assays•á507ñ Protein Determination Procedures•á621ñ Chromatography•á785ñ Osmolality and Osmolarity•á787ñ Subvisible Particulate Matter in Therapeutic Protein Injections•á788ñ Particulate Matter in Injections•á791ñ pH•á905ñ Uniformity of Dosage Units•á911ñ Viscosity—Capillary Methods•á912ñ Viscosity—Rotational Methods•á913ñ Viscosity—Rolling Ball Method•á1027ñ Flow Cytometry•á1030ñ Biological Assay Chapters—Overview and Glossary•á1032ñ Design and Development of Biological Assays•á1033ñ Biological Assay Validation•á1034ñ Analysis of Biological Assays•á1044ñ Cryopreservation of Cells•á1046ñ Cell-based Advanced Therapies and Tissue-Based Products3•á1048ñ Quality of Biotechnology Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products•á1049ñ Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products•á1052ñ Biotechnology Derived Articles—Amino Acid Analysis•á1053ñ Capillary Electrophoresis•á1054ñ Biotechnology Derived Articles—Isoelectric Focusing•á1055ñ Biotechnology Derived Articles—Peptide Mapping•á1056ñ Biotechnology Derived Articles—Polyacrylamide Gel Electrophoresis•á1057ñ Biotechnology Derived Articles—Total Protein Assay•á1084ñ Glycoprotein and Glycan Analysis—General Considerations•á1102ñ Immunological Test Methods—General Considerations•á1103ñ Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)•á1104ñ Immunological Test Methods—Immunoblot Analysis•á1126ñ Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing•á1127ñ Nucleic Acid-Based Techniques—Amplification•á1128ñ Nucleic Acid-Based Techniques—Microarray•á1129ñ Nucleic Acid-Based Techniques—Genotyping•á1130ñ Nucleic Acid-Based Techniques—Approaches for Detecting Trace Nucleic Acids (Residual DNA Testing)•á1237ñ Virology Test Methods•á1285ñ Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis•á1285.1ñ Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic Examination•á1430.1ñ Analytical Methodologies Based on Scattering Phenomena—Static Light Scattering•á1776ñ Image Analysis of Pharmaceutical Systems•á1787ñ Measurement of Subvisible Particulate Matter in Therapeutic Protein Injections•á1788ñ Methods for the Determination of Subvisible Particulate Matter•á1788.1ñ Light Obscuration Method for the Determination of Subvisible Particulate Matter•á1788.2ñ Membrane Microscope Method for the Determination of Subvisible Particulate Matter•á1788.3ñ Flow Imaging Method for the Determination of Subvisible Particulate Matter。
富马酸替诺福韦酯杂质 标准品 对照品

富马酸替诺福韦酯是一种化学物质,核苷酸逆转录酶抑制剂,是替诺福韦(PMPA,2)的前药,临床主要用于治疗人类免疫缺陷病毒(HIV)感染。
富马酸替诺福韦酯标准品列表名称结构式替诺福韦单异丙氧碳酸甲基酯Tenofovir Disoproxil RelatedCompound ECAS:211364-69-1替诺福韦酯异丙氧碳酸甲基甲基酯CAS: 1246812-16-7替诺福韦异丙氧碳酸甲基甲氧碳酸甲基酯Mono-POC Methyl TenofovirCAS: 1246812-43-0Tenofovir Impurity Q替诺福韦异丙氧碳酸甲基异丙基酯Tenofovir Disoproxil RelatedCompound GCAS: 1422284-15-8替诺福韦-N-异丙氧羰基异丙氧碳酸甲基酯Tenofovir Impurity ECAS:1244022-56-7替诺福韦酯杂质FTenofovir Impurity F(S)-替诺福韦二异丙氧碳酸甲基酯替诺福韦异丙氧碳酸甲基丙基碳酸甲基酯CAS: 1217542-13-6替诺福韦二聚物单酯CAS:1093279-77-6替诺福韦二聚物Tenofovir Disoproxil DimerCAS:1093279-76-59-丙烯腺嘌呤Tenofovir Disoproxil RelatedCompound BCAS:4121-40-8替诺福韦酯杂质RTenofovir Impurity R替诺福韦异丙氧碳酸甲基乙基碳酸甲基酯替诺福韦二乙酯Tenofovir impurity V替诺福韦单乙酯Tenofovir DisoproxilCarbamateDiethylaminocarboxymethylPOC Tenofovir Fumarate6N-Hydroxymethyl TenofovirDisoproxil1244022-53-4Isopropyl Tenofovir Diisopropyl Tenofovir Fumarate 160616-04-6 (free base)(S)-Tenofovir147127-19-3替诺福韦酯杂质R6N-Bromomethyl TenofovirDisoproxilTenofovir Disoproxil Fumarate Impurity(N6-CH2OH-POC PMPA) Tenofovir Related Compound 2 379270-35-6Tenofovir Related Compound 352364-31-5Tenofovir Related Compound 4851456-00-3Tenofovir Related Compound 51234081-04-9 Tenofovir Related Compound 6 383365-04-6Tenofovir Related Compound 7 79487-89-1Tenofovir monophosphate 206646-04-0Tenofovir Diphosphate166403-66-3Tenofovir Trimer Impurity 1Tenofovir Trimer Impurity 2。
Aprotinin_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Aprotinin is a serine protease inhibitor isolated from bovine lung which inhibits trypsin and chymotrypsin with K i values of 0.06 pM and 9 nM, respectively.IC50 & Target: Ki: 0.06 pM (Trypsin), 9 nM (Chymotrypsin)[1]In Vitro: Aprotinin, a serine protease inhibitor isolated from bovine lung, is a complex protease inhibitor that is an antifibrinolytic,inhibits contact activation, and decreases the inflammatory response to cardiopulmonary bypass [2]. Aprotinin inhibits trypsin (bovine, K i = 0.06 pM), chymotrypsin (bovine, K i = 9 nM), plasmin (human, 0.23 nM)[1]. Aprotinin is also a competitive protein inhibitor of NOS activity. It inhibits NOS–I and NOS–II with K i values of 50 μM and 78 μM, respectively [3]. Aprotinin significantly inhibits fibrinolysis with an IC 50 of 0.16±0.05 μM [4].In Vivo: High dose aprotinin can reduce blood loss and transfusion requirements associated with primary cardiac procedures such as coronary artery bypass graft (CABG) or heart valve replacement surgery [5]. Aprotinin inhibits thrombus formation in adose–dependent manner. Aprotinin at a dose of 1.5 mg kg –1 (bolus) and 3 mg kg –1 h –1 infusion (maintenance infusion) causes a tendency towards a reduction in bleeding time. Aprotinin significantly reduces the bleeding time starting at a dose of 3 mg kg –1bolus plus 6 mg kg –1 h –1 showing a reduction of approximately 84%±2.9%. At the highest dose of 5 mg kg –1 and 10 mg kg –1 h –1,the strongest effects are observed [4]. Aprotinin may affect tumor necrosis factor–alpha (TNF) levels. Soluble TNFRI levels are significantly increased following I/R in the aprotinin treated wild type mice and not detected in all TNFRInull mice [6]. PROTOCOL (Extracted from published papers and Only for reference)Animal Administration:[4][6]Rat: Male Wistar rats (180–220 g) are used in the study. Aprotinin is dissolved in physiological saline.Aprotinin is administered by bolus injection followed by a maintenance infusion. The doses given are 1.5 mg kg –1 and 3 mg kg –1 h –1,3mg kg –1 and 6 mg kg –1 h –1 up to 5 mg kg –1 and 10 mg kg –1 h –1. Plasma concentrations for the two agents are assessed by pharmacokinetic studies in rats [4].Mouse: An intact mouse model of ischemia/reperfusion (30 min–I/60 min–R) is used and left ventricular peak + dP/dt is measured in wild type mice (WT, C57BL/6; n=10), WT mice with aprotinin (4mL/kg; n=10), transgenic mice devoid of the TNFRI (TNFRInull;n=10), and TNFRInull with aprotinin (n=10)[6].References:[1]. Fritz H, et al. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittelforschung. 1983;33(4):479–94.[2]. Levy JH, et al. Efficacy and safety of aprotinin in cardiac surgery. Orthopedics. 2004 Jun;27(6 Suppl):s659–62.[3]. Venturini G, et al. Aprotinin, the first competitive protein inhibitor of NOS activity. Biochem Biophys Res Commun. 1998 Aug 10;249(1):263–5.Product Name:Aprotinin Cat. No.:HY-P0017CAS No.:9087-70-1Molecular Formula:C 284H 432N 84O 79S 7Molecular Weight:6511.44Target:Influenza Virus Pathway:Anti–infection Solubility:H 2O: ≥ 100 mg/mL[4]. Sperzel M, et al. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007 Oct;5(10):2113–8. Epub 2007 Jul 31.[5]. Davis R, et al. Aprotinin. A review of its pharmacology and therapeutic efficacy in reducing blood loss associated withcardiac surgery. Drugs. 1995 Jun; 49(6):954–83.[6]. Sabbagh MJ, et al. Aprotinin exacerbates left ventricular dysfunction after ischemia/reperfusion in mice lacking tumor necrosis factor receptor I. J Cardiovasc Pharmacol. 2008 Oct;52(4):355–62.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Anodyne治疗仪型号120说明书

Anodyne®治療儀和MIRE TM是Anodyne Therapy, LLC 的註册商標 美國專利號5358503。
歐洲專利號0741594© 2013 Anodyne ® Therapy, LLC版權所有IMR-03950EC REP授權歐洲代表Emergo Europe Molenstraat 152513 BH, The Hague The Netherlands電話: (+31) 70 345 570傳真: (+31) 70 346 2990120製造商:Anodyne Therapy, LLC地址: 14105 McCormick Drive Tampa, FL 33626 United States電話: +1-813-342-4432傳真: +1-813-342-4417網址: 北京安耐科技有限公司地址:北京市海淀區巴溝南路35号院京江陽光寫字樓A做136室電話:86-010-********傳真:86-010-********網址: Anodyne®治療儀授權經銷商重要安全信息及說明操作之前請閱讀本說明書Anodyne®單一波長近紅外綫光能治療儀型號120索引Array清潔, 15禁忌症, 4主控箱, 5, 7-8, 16, 19客户服務, 2, 4-5, 6, 8, 11, 17, 22設備處理, 5止痛貼, 4, 18退還政策, 2, 22單人使用, 6搭接帶, 5, 7, 9-10, 12, 15, 19安置治療墊, 4, 9-10, 12-14治療墊受壓, 4, 10-11, 13, 19局部加熱劑, 4, 18故障排除, 17治療頻率, 12-13治療時間, 12-13警告,注意事項, 4-8, 11, 18-19質量保証, 16, 22電綫/治療墊8, 16-19有限保証Anodyne Therapy, LLC(製造商)就Anodyne®治療儀(產品)對直接購買者承諾如下:有限保証對於下述售出的本產品,自購買之日起兩(2)年内,在正常使用情况下,製造商保証其無論是材料上,還是工藝上,均不存在缺陷。
细胞衰老特异性β-半乳糖苷酶检测试剂盒产品说明书(中文版)

细胞衰老特异性β-半乳糖苷酶检测试剂盒产品说明书(中文版)主要用途细胞衰老特异性β-半乳糖苷酶检测试剂是一种旨在以X-Gal为底物,通过细胞内β-半乳糖苷酶在酸性条件下稳定表达,催化生成深蓝色的沉积产物,从而在光学显微镜下观察到蓝色表达的细胞作为细胞复制性衰老(replicative senescence)的分子特征来识别和探测衰老细胞的权威而经典的技术方法。
该技术由大师级科学家精心研制、成功实验证明的。
广泛应用于细胞生物学研究。
其适用于各种人体和动物细胞或活体内(in vivo)检测。
产品即到即用,性能稳定,参数优化,着色敏感,堪称国际上同类产品最佳。
技术背景细胞复制性衰老是细胞控制其生长潜能的保障机制。
衰老细胞停滞在细胞周期的G1期,表现出细胞扁平、胀大、颗粒增多的形态特征,尤其在酸性条件下,细胞内β-半乳糖苷酶活性增加,而成为细胞衰老的生物学标记。
产品内容清理液(Reagent A)毫升固定液(Reagent B)毫升酸性液(Reagent C)毫升稀释液(Reagent D)毫升染色液(Reagent E)毫升产品说明书 1份保存方式保存染色液(Reagent E)在-20℃冰箱里;其余的保存在4℃冰箱里;稀释液(Reagent D)和染色液(Reagent E)用铝箔封裹,避免光照;有效保证6月用户自备2毫升离心管:用于配制染色工作液15毫升锥形离心管:用于配制染色工作液恒温培养箱:用于染色孵育光学显微镜:用于观察染色后的细胞实验步骤操作一、25cm2细胞培养瓶染色实验开始前,将试剂盒里的染色液(Reagent E)从-20℃的冰箱里取出,放进冰槽里等待溶化。
然后移取xx毫升稀释液(Reagent D)到2毫升离心管,加入xx微升染色液(Reagent E),混匀后,放进37℃恒温水槽里预热,标记为染色工作液。
然后进行下列操作:1.小心抽去25cm2细胞培养瓶里的培养液2.小心加入xx毫升清理液(Reagent A),清洗生长中的细胞表面3.小心抽去清理液4.小心加入xx毫升固定液(Reagent B),覆盖整个生长表面5.在室温下孵育5分钟6.小心抽去固定液7.小心加入xx毫升酸性液(Reagent C),清洗细胞表面8.小心抽去酸性液9.重复实验步骤7和8一次10.小心加入xx毫升染色工作液,覆盖整个细胞表面11.放进37℃培养箱,孵育3小时至16小时,或细胞呈现蓝色(注意:避免液体蒸发)12.在光学显微镜下观察和计数:表达衰老特异性β- 半乳糖苷酶的细胞为阳性细胞,呈现蓝色。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
血清Ⅰ型前胶原氨基端前肽、Ⅰ型前胶原羧基端前肽、Ⅰ型胶原羧基端肽在骨转移性癌诊断中的应用

血清Ⅰ型前胶原氨基端前肽、Ⅰ型前胶原羧基端前肽、Ⅰ型胶原羧基端肽在骨转移性癌诊断中的应用王抒;张军宁;乔田奎【摘要】Objective:To study the clinical value of determination of serum procollagen I N-terminal propeptide (PINP), pro-collagen I carboxy-terminal propeptide (PICP) and carboxyterminal telopeptide of type I collagen (ICTP) contents in cancer patients with bone metastases for diagnosis . Methods- Serum PINP, PICP and ICTP contents were measured in 41 patients with bone metastases and 39 controls without bone metastases. The patients with bone metastases consisted of Group A, Group B and Group C. The comparison of bone turnovers among patients with different bone loads were analyzed. Results.- Serum PICP, ICTP contents was significantly greater in patients with bone metastases than without bone metastases(P<0. 01). With the increase of the extent of bone metastases, serum PINP, PICP and ICTP contents elevated. Levels of PICP correlated well to the number of foci(P<0. 01) , Levels of ICTP, PINP correlated to the number of foci(P<0. 05). Levels of PINP correlated well to ICTP. Conclusions: The determinations of serum PINP, PICP and ICTP can reflect the clinical status of metastases size (PICP and ICTP more sensitive); Levels of PICP correlat well to the number of foci.%目的;探讨血清Ⅰ型前胶原氨基端前肽( PINP)、Ⅰ型前胶原羧基端前肤(PICP)、Ⅰ型胶原羧基端肽(ICTP)在骨转移癌诊断中的临床价值.方法:将80例肿瘤患者分为骨转移组41例和无骨转移组(对照组)39例,采用Soloway分级标准,将骨转移组患者再分为A、B、C3个亚组,所有患者空腹抽取静脉血.分析血清PINP、PICP、ICTP与骨转移程度之间的相关性及其彼此之间的相关性.结果:骨转移癌患者血清PINP、PICP、ICTP水平较对照组均升高,其中PICP、ICTP水平与对照组有显著差异(P<0.01);PINP、PICP、ICTP水平在A、B、C3组均依次递增;血清PICP水平与骨转移灶数目相关性良好(P<0.01),ICTP、PINP水平与骨转移灶数目呈一定相关(P<0.05);血清ICTP 水平与PINP水平呈中度相关.结论:检测血清PINP、PICP、ICTP水平都能反映骨转移患者的病情变化,以检测血清PICP、ICTP水平临床价值高;血清PICP水平与骨转移数目的相关性良好.【期刊名称】《中国临床医学》【年(卷),期】2011(018)004【总页数】3页(P456-458)【关键词】Ⅰ型前胶原氨基端前肽(PINP);Ⅰ型前胶原羧基端前肤(PICP);Ⅰ型胶原羧基端肽(ICTP);骨转移;癌【作者】王抒;张军宁;乔田奎【作者单位】复旦大学附属金山医院放疗科,上海200540;苏州大学附属第一医院放疗科,江苏苏州,215006;复旦大学附属金山医院放疗科,上海200540【正文语种】中文【中图分类】R730.4多数恶性肿瘤易发生骨转移且可导致一系列并发症,包括病理骨折、严重骨痛等,影响患者的生活质量和生存期限。
碧云天EDTA抗原修复液(50X)说明书

碧云天生物技术/Beyotime Biotechnology 订货热线:400-1683301或800-8283301 订货e-mail :******************技术咨询:*****************网址:碧云天网站 微信公众号EDTA 抗原修复液(50X)产品编号 产品名称包装 P0085EDTA 抗原修复液(50X)100ml产品简介:碧云天生产的EDTA 抗原修复液(EDTA Antigen Retrieval Solution)是一种常用的抗原修复液,可以用于石蜡切片、冰冻切片等样品使用多聚甲醛、甲醛或其它醛类试剂固定后的抗原修复。
细胞或组织用多聚甲醛、甲醛或其它醛类试剂固定后,会导致蛋白之间的交联(cross-link),从而遮蔽样品的抗原位点,导致免疫染色时染色信号减弱,甚至出现一些假阳性染色结果。
本抗原修复液采用了广泛使用的EDTA ,可以有效去除醛类固定试剂导致的蛋白之间的交联,充分暴露石蜡切片等样品中的抗原表位,从而大大改善免疫染色效果。
通常石蜡切片都需进行抗原修复处理,而冰冻切片可以不进行抗原修复处理。
抗原修复会大大改善石蜡切片的免疫染色效果,但对于冰冻切片的染色效果很多文献资料表明也有显著改善。
特别是当冰冻切片免疫染色效果欠佳时,可以考虑尝试进行抗原修复。
从原理上来看,无论冰冻切片还是细胞爬片等,只要是用多聚甲醛、甲醛或其它醛类试剂固定的样品,进行抗原修复都会有效去除蛋白之间的交联,充分暴露抗原表位,从而大大改善免疫染色效果。
本产品特别适合用于石蜡切片,也可以用于冰冻切片等其它样品。
关于碧云天生产的各种抗原修复液的主要特点和差异可参考我们的相关网页:/support/antigen-retrieval-solution.htm 。
一个包装的本产品可以配制成5000毫升抗原修复液(1X)。
按照每个片子需要10毫升抗原修复液(1X)计算,一个包装的本产品可以用于500个样品。
抑肽酶溶液(Aprotinin)

北京雷根生物技术有限公司 抑肽酶溶液(Aprotinin,2mg/ml)简介:蛋白酶抑制剂(Protease Inhibitor)是指与蛋白酶分子活性中心上的一些基团结合,使蛋白酶活力下降甚至消失但不使蛋白变性的物质。
蛋白酶抑制剂有很多种,包括EDTA 、E-64、NaVO 3、Bestatin 、Leupetin 、Pepstatin A 、Aprotinin 等均可有效抑制蛋白的降解,并维持原有的蛋白间相互作用。
Leagene 抑肽酶溶液(Aprotinin,2mg/ml)主要由抑肽酶Aprotinin 等组成,不含EDTA 。
该抑酶肽溶液是浓缩溶液,其工作浓度一般在1-10μg/ml ,适用于从哺乳动物组织、细胞中提取蛋白质,能够更有效的获得目的蛋白质。
提取出来的蛋白可以用于Western Blot 、免疫共沉淀等试验。
组成:操作步骤(仅供参考):1、 开盖前请低速离心一下,以便将黏附于管壁的液体甩至管底。
2、 使用时,根据裂解液的用量加入抑肽酶溶液(Aprotinin,2mg/ml),使其工作浓度在1-10μg/ml ,多用在3-5μg/ml 。
注意事项:1、 抑肽酶溶液(Aprotinin,2mg/ml)有轻微刺激性,小心操作,同时应避免反复冻融。
2、 为了您的安全和健康,请穿实验服并戴一次性手套操作。
有效期:12个月有效。
相关:编号 名称 PI0036 Storage 抑肽酶溶液(Aprotinin,2mg/ml) 1ml -20℃ 避光 使用说明书 1份编号 名称 DC0032 Masson 三色染色液 PW0082丽春红S 染色液(1×Ponceau S) TC0699 植物总糖和还原糖检测试剂盒(硝基水杨酸法)。
III型前胶原氨基端肽(PIIINP)测定试剂盒 (时间分辨荧光免疫分析法)产品技术要求
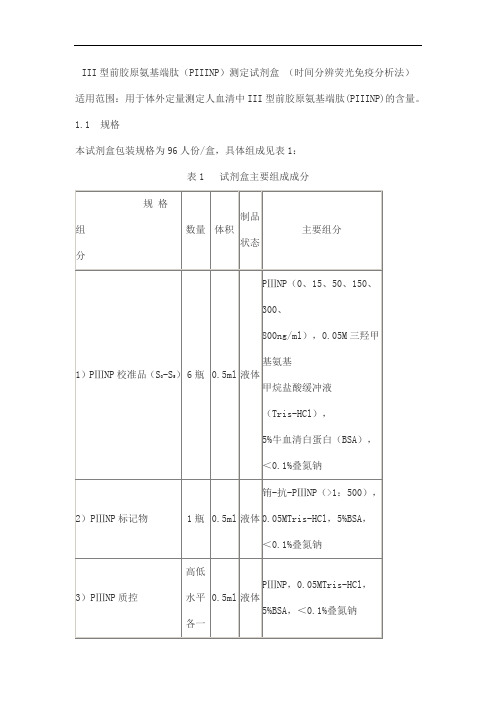
III型前胶原氨基端肽(PIIINP)测定试剂盒(时间分辨荧光免疫分析法)适用范围:用于体外定量测定人血清中III型前胶原氨基端肽(PIIINP)的含量。
1.1 规格
本试剂盒包装规格为96人份/盒,具体组成见表1:
表1 试剂盒主要组成成分
2.1物理性能
液体组分应澄清,无沉淀或絮状物。
各组分装量不少于表1中要求。
2.2准确性
回收率应在85%~115%范围内。
2.3线性
用百分结合率对数(Log-Logit)数学模型拟合,在15~800ng/ml范围内,剂量-反应曲线相关系数(r)应不低于0.9900。
2.4精密度
2.4.1批内精密度(CV%)应不高于15%。
2.4.2批间精密度(CV%)应不高于15%。
2.5空白检测限
试剂盒空白检测限应不大于7ng/ml。
2.6 质控品测定值
每次检测结果均应在允许范围之内。
2.7特异性
与层粘连蛋白(LN)、透明质酸(HA)、IV型胶原(CIV)无显著交叉反应。
表1与其它激素的交叉反应数据
2.8稳定性
试剂盒在2~8℃保存12个月,测定结果应符合上述2.1~2.7要求。
细胞角蛋白(广谱)抗体试剂(免疫组织化学)说明书

1. 供专业人员使用。 2. 该产品中含有叠氮钠 (NaN3),纯品具有高度的化学毒性。产品中叠氮钠的浓度虽然不能被认定为危险
性浓度,但是叠氮钠可以与铅、铜发生化学反应,形成具有爆炸性危险的叠氮化金属物质。处理时需 要用大量清水冲洗,防止管道中形成金属叠氮化物质。 3. 与所有生物来源的产品相同,必须遵循相关操作步骤。 4. 穿戴合适的个人防护装置,避免皮肤和眼睛接触。 5. 请按照当地、地区以及国家的相关法规,处理未使用的溶液。
【主要组成成份】
1. 提供的试剂 即用型单克隆小鼠抗体以液体形式提供,缓冲液中含有稳定蛋白和0.015 mol/L NaN3。 克隆:AE1/AE3 同型:IgG1,kappa 2.免疫原 人体表皮细胞愈伤组织 (1)。 3.特异性 AE1/AE3 包括两种单克隆抗体,采用人愈伤组织细胞角蛋白免疫小鼠的方法得到 (2)。研究显示, AE1/AE3 能够识别大部分人细胞角蛋白,因此可以作为 IHC 中单层和复层上皮来源的判定工具 (1,2,4)。 抗体 AE1 能够与大部分亚组 A 细胞角蛋白的抗原决定簇发生反应,包括 Moll 分型(4) 中的 10、13、14、 15、16 和 19(分子量分别为 56.5、54'、50、50'、48 和 40 kDa),但是不包括编号为 12、17 和 18(分 子量分别为 55、47 和 45 kDa)的细胞角蛋白(4)。抗体 AE3 能够与亚组 B 细胞角蛋白的抗原决定簇发生 反应,包括编号为 1 和 2、3、4、5、6、7 和 8(分子量分别为 65、67、64、59、58、56、54 和 52 kDa) 的细胞角蛋白(5)。
5. Eichner R, Bonitz P, Sun T-T. Classification of epidermal keratins according to their immunoreactivity, isoelectric point and mode of expression. J Cell Biol 1984; 98:1388
华堂宁说明书

修改日期:多格列艾汀片说明书-请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:多格列艾汀片商品名称:华堂宁英文名称:Dorzagliatin Tablets汉语拼音:Duogelie’aiting Pian【成份】活性成份:多格列艾汀化学名称:(2S)-2-[4-(2-氯-苯氧基)-2-氧代-2,5-二氢-1H-吡咯-1-基]-N-{1-[(2R)-2,3-二羟基-丙基]-1H-吡唑-3-基}-4-甲基戊酰胺化学结构式:分子式:C22H27ClN4O5分子量:462.93辅料:甲基丙烯酸-甲基丙烯酸甲酯共聚物,微晶纤维素胶态二氧化硅共处理物,羟丙纤维素,交联羧甲纤维素钠,硬脂酸镁,薄膜包衣预混剂。
本品为浅绿至绿色、双凸面圆形薄膜衣片,一面刻字“H”,另一面刻“75”,除去包衣后显白色或类白色。
【适应症】本品适用于改善成人2型糖尿病患者的血糖控制。
单药:本品单药可配合饮食控制和运动,改善成人2型糖尿病患者的血糖控制。
与盐酸二甲双胍联合使用:在单独使用盐酸二甲双胍血糖控制不佳时,本品可与盐酸二甲双胍联合使用,配合饮食和运动改善成人2型糖尿病患者的血糖控制。
使用限制:本品不适用于治疗1型糖尿病、糖尿病酮症酸中毒或高血糖高渗状态。
【规格】75mg【用法用量】推荐剂量本品推荐剂量为75mg,每日两次,早餐前和晚餐前1小时内任何时间服用。
治疗期间注意遵守用药时间。
如漏药,无需补服。
特殊人群用药:肾功能不全患者肾功能不全患者无需调整剂量。
肝功能损害患者轻度肝功能损害(Child-Pugh A级)患者无需调整剂量。
中度肝功能损害(Child-Pugh B级)患者本品的暴露量增加,尚未在重度肝功能损害(Child-Pugh C级)患者中开展临床研究。
中度和重度肝功能损害(Child-Pugh B和C级,如:中度及以上肝硬化)患者中不推荐使用本品(参见药代动力学)。
CYP3A4诱导剂本品与CYP3A4诱导剂(如苯妥英、利福平和卡马西平)合用应谨慎。
aprotinin配制方法 -回复

aprotinin配制方法-回复如何配制aprotinin?Aprotinin是一种蛋白酶抑制剂,常用于手术过程中减少出血,特别是心脏手术。
在实验室研究中,也常用于保护蛋白质免受降解。
下面将介绍一步一步的aprotinin配制方法。
第一步:准备实验室环境和设备在开始配制aprotinin之前,确保实验室环境干净,并准备好所需的实验设备,包括量筒、容量瓶、pH计、滤器等。
第二步:准备所需试剂和溶液在配制aprotinin之前,需要准备以下试剂和溶液:1. Aprotinin粉末:从供应商购买的Aprotinin粉末通常会标明浓度和保存条件。
2. 缓冲液:aprotinin通常在缓冲液中稀释使用。
常见的缓冲液包括磷酸盐缓冲液、Tris-HCl缓冲液等。
根据实验需求选择合适的缓冲液。
3. 纯水:使用工业纯水或去离子水配制溶液时,确保纯净度。
第三步:计算配制溶液的体积根据实验需求,计算出所需配制的溶液的体积。
通常根据aprotinin 的浓度、试验所需使用的溶液体积等因素确定。
第四步:称量aprotinin粉末使用一个净化的称量纸或电子天平,按照所需浓度称量aprotinin粉末。
根据所需体积和浓度,计算出所需的aprotinin质量。
将称量好的aprotinin粉末置于一个干净的容器中。
第五步:溶解aprotinin粉末将所称量的aprotinin粉末加入到所选择的缓冲液中。
可以用一个玻璃棒或声波浴来辅助搅拌,以确保aprotinin充分溶解。
根据需要,可以调整溶液的pH值以及浓度。
第六步:滤除残留颗粒使用一个0.22微米的滤器,滤除溶液中的残留颗粒。
这可以避免在后续实验中出现颗粒引发的干扰。
第七步:保存和存储将配制好的aprotinin溶液分装到干净的容器中,封闭并标明浓度、配制日期和其他相关信息。
保存在低温(-20C)条件下,避免溶液的冻结和解冻多次。
注意:某些实验室可能还要求在配制过程中添加保存辅助剂(如甘油)以提高aprotinin的稳定性和保存时间。
HPLC法同时测定阿托伐他汀钙片与普罗布考片中2主分含量

中国药房2011年第22卷第41期China Pharmacy 2011V ol.22No.41*在读本科生。
研究方向:临床药学。
E-mail :bckl 888@#通讯作者:教授,硕士。
研究方向:体内药物分析。
电话:0538-*******。
E-mail :*************.cn 阿托伐他汀钙为选择性3-羟基-3-甲基-戊二酰辅酶A (HMG-CoA )还原酶抑制剂,能降低血浆胆固醇和甘油三酯水平。
普罗布考具有抗氧化作用,能够预防动脉粥样硬化的发生。
阿托伐他汀钙与普罗布考联合用药是近几年提出的一种调脂治疗联合方案[1,2]。
目前测定阿托伐他汀钙片剂含量的方法主要有紫外分光光度(UV )法、高效液相色谱(HPLC )法和毛细管区带电泳法[3~5];测定普罗布考片剂含量的方法主要有UV 法和HPLC 法[6,7]。
由于二者在临床联合应用极为广泛,但尚未见同时测定二者血药浓度的有关报道,建立此方法可为临床测定二者的血药浓度提供参考。
由于HPLC 法专一性强、灵敏度高,但采用外标法,易产生操作误差。
为此,笔者选用丹皮酚为内标,建立了同时测定阿托伐他汀钙与普罗布考含量的HPLC 内标法。
结果表明,此法简便、准确、重复性好,而且节省时间、资源,可以为同时测定二者在制剂中的含量或受试体中的血药浓度提供方法依据。
1仪器与试药LC-10AT 型HPLC 仪、SPD-10A 型紫外检测器(日本岛津公司);N 2000双通道色谱工作站、GR-202电子分析天平(日本AND 公司)。
阿托伐他汀钙标准品(批号:100590-200802,按三水合物计含量为95.0%)、普罗布考标准品(批号:100560-200301,供含量测定用)、丹皮酚标准品(批号:110708-200505,供含量测定用)均由中国药品生物制品检定所提供;阿托伐他汀钙片(辉瑞制药有限公司进口分装,批号:138709K ,规格:每片10mg );普罗布考片(承德颈复康药业集团有限公司,批号:100403077,规格:每片0.25g );甲醇为色谱纯,醋酸铵、冰醋酸均为分析纯,水为重蒸水。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name: CAS No.: Cat. No.: MWt: Formula: Purity :
Aprotinin 9087-70-1 HY-P0017 6511.44 C284H432N84O79S7 >98%
Solubility:
>10 mg/mL in H2O
Cautionபைடு நூலகம் Not fully tested. For research purposes only Medchemexpress LLC
m o c . s s e r p x e m e h c d e Am S. Uw ,w 2 5w 8: 8b 0e JW Nm ,o n c o. i s t c s e nr up Jx he t m u oe h mc nd oe Mm D@ 2o 0f n 1i el : t i i a u Sm ,E e v i r D k r a P r e e D 1 1
Mechanisms: Pathways:Others; Target:Others g y Biological Activity: Aprotinin is a 58 Amino Acid peptide protease inhibitor, used clinically to prevent postoperative blood loss and reduce transfusion requirements in those procedures which employ extracorporeal circulation. Target: BPTI Aprotinin is the small protein bovine pancreatic trypsin inhibitor (BPTI), an antifibrinolytic molecule that inhibits trypsin and related proteolyticenzymes, used as medication administered by injection to reduce bleeding during complex surgery, such as heart and liver surgery. Its main effect is the slowing g down of fibrinolysis, y the p process that leads to the breakdown of blood clots. The aim in its use is to decrease the need for blood transfusions during surgery, as well as end-organ damage due to hypotension (low blood pressure) as a result of marked blood loss.... References: [1]. Paul M, et al. Use of Clotted Human Plasma and Aprotinin in Skin Tissue Engineering: A Novel Approach to Engineering Composite Skin on a Porous Scaffold. Tissue Eng Part C Methods. 2015 Jun 30.
