ChemKin使用指南 ChemKin教程
ChemKin使用指南 ChemKin教程

CHEMKIN 使用方法1打开CHEMKIN窗口1)登陆Athena2)在Athena界面上输入athena% add chemkinathena% chemkin3)接下来软件窗口打开图1.Chemkin软件窗口4)可以从Chemkin窗口选取需要应用的运行程序。
可利用的功能和可运用程序描述如下:● Aurara: 完全混合反应模拟器● Creslaf: 通道流体模拟器● Equil: 平衡状态模拟器● Oppdif: 两个对立喷嘴之间的火焰传播● Plug: 化学反应器中的柱塞流模拟● Premix: 稳态的,层流,一维预混合火焰模拟● Senkin: 预测封闭系统中均相气态化学机理的敏感性分析● Shock: 预测产物在入射激波和反射激波后的状态● Spin: 模拟一维旋转反应器● Surftherm: 分析气相和表面化学反应机理中和热力化学和动力学数据在下一个部分我们将描述如何使用Equil应用程序。
其他应用程序可以以相类似的方法使用。
然而,Equil和其它的应用程序有一个本质的区别。
Equil应用程序不利用机理数据,而其它应用程序使用到。
2.如何使用Equil应用程序Equil计算理想气体和溶液混合物的化学平衡状态1)在Chemkin窗口中点击Equil按钮2)窗口如图2所示图2.Equil应用程序窗口3)为了计算平衡状态,需要产生两个输入文件:chem.inp 和gas_equuil.inp。
4)如果你点击气相化学文件的编辑按钮,你可以看到和编辑的化学输入文件如图3所示。
化学输入文件包括元素和组分数据。
图3.化学输入文件5)你可以创建你自己的文件和文件名来取代原有的默认的文件形式。
但是文件是在指定的路径中。
为了生成输入文件,或者使用文本编辑器在Athena和个人电脑上编辑和通过FTP 发送到Athena上。
6)接下来,你需要产生气相平衡输入文件。
当你点击Equil的编辑按钮,你将会见到图4图4.气体平衡应用程序输入文件图4中各个参数含义如下:● REAC 代表反应物;由一个化学符号代表一种反应物和他们在混合物中的摩尔数。
CHEMKIN4.0.1入门指南讲诉

CHEMKIN入门指南《燃烧学》辅助教程上篇基础知识、核心程序、化学平衡(EQUIL)、全混反应(AURORA)如果文中有任何错误,请不吝指出,以便不断改进2004.3第一章CHEMKIN简介本章介绍CHEMKIN的主要功能和求解过程。
第一节安装CHEMKINChemkin最早的版本始于1980,由美国Sandia实验室的Kee RJ等人编写,经过多年的不断发展日趋完善。
后来由Reaction Design公司收购并继续开发,目前最新版为3.7.1。
由于学习和科研需要,我们花费2000$向ReactionDesign公司订购了一套最新版本的CHEMKIN 3.7.1,其中包括20个网络教学许可证,用于《燃烧学》课程的学习。
[安装] 请从ftp://combustion:combustion@166.111.56.202的“CHEMKIN软件”目录内下载安装程序chemkin371_pc_setup.exe,执行安装程序。
安装完后会自动在桌面及开始菜单建立快捷方式。
[运行许可证书] 教学用的CHEMKIN采用网络认证,故电脑必须联网(校内)。
当程序计算(Run)时,系统会提示选择license,选择“Specify license server”,然后next,在下一画面填入“166.111.56.202”即可。
第二节CHEMKIN介绍CHEMKIN是一种非常强大的求解复杂化学反应问题的软件包,常用于对燃烧过程、催化过程、化学气相沉积、等离子体及其他化学反应的模拟。
CHEMKIN包括“核心程序(Core Utilities)”和“应用程序(Application)”两级程序包。
以气相反应、表面反应、传递过程这三个核心软件包为基础,CHEMKIN提供了对12种常见化学过程模拟的软件包及后处理程序。
CHEMKIN的三个核心程序模块:1) 气相动力学(Gas-PhaseKinetics):是所有程序计算的基础,提供气相成分组成、热力学数据、化学反应等信息。
CHEMKIN tutorials2.1-2.3

2.气相燃烧过程2.1 平衡2.1.1绝热火焰温度2.1.1.1项目简述这个用户指南提出了使用气相平衡计算确定绝热火焰温度的氢气/空气系统。
绝热火焰温度是指在某一特定条件下测量的混合气体燃烧可以达到的最高温度。
在一个包含热损失,化学动力学和质量传输局限在内的实际系统中,火焰温度可能低于绝热火焰温度。
2.1.1.2项目设置项目文件可以命名为equilibrium__gas.ckprj。
用于此样品的数据文件可以在samples\equilibrium\gas directory中查询。
该反应堆图包含有一个单一的平衡反应堆。
平衡计算仅需要知道物质种类和它们的热力学数据,而没必要知道参加反应的名单。
对于此示例问题,化学输入的文件只包括3个元素:H,O,N和H2, H, O2, O, OH, HO2, H2O,N2, and H2O2等9种物质。
为了获得准确的火焰温度,产品列表中的基础种类和稳定物种是同等重要的。
对于一般的平衡计算来说,包含一些不重要的物种比忽略一些被证明是重要的物种要好得多。
解决这个问题首先需要涉及C1平衡板。
这个问题类型(恒压和焓),初始温度(300 K)和压力(1 atm)进入反应堆物理性质上的标签。
溶液估温2000 K 用于帮助确保获得该解决方案是点燃的气体,而不是未燃的状态。
对于平衡模拟的时候溶液估温不是必要的,但是当涉及辅助解决方案时可能会用到。
在反应物二级的特异性数据选项卡上输入起始组成数据。
反应物混合规定了包括提供初始摩尔元素及初始能量系统的初始状态。
随着初始温度的增加,补遗集面板用来指定两个额外的模拟。
2.1.1.3项目结果图2-1表示的是这些模拟的平衡温度,它代表H/O为2.0的氢气/空气混合物的绝热火焰温度。
这些温度都在2400以上,因此与对应燃烧气体相符合。
正如人们所料的那样,这些绝热火焰温度随着初始气体温度增长而升高。
图2-1氢气/空气混合的绝热火焰温度2.2利用当量比燃烧过程的很多属性强烈依赖于燃烧混合物的化学计量。
chemkin算例(甲烷+空气)
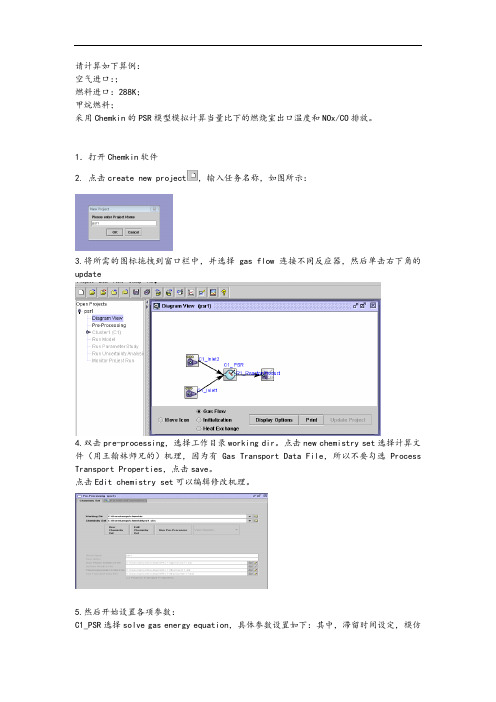
请计算如下算例:空气进口:;燃料进口:288K;甲烷燃料;采用Chemkin的PSR模型模拟计算当量比下的燃烧室出口温度和NOx/CO排放。
1.打开Chemkin软件2. 点击create new project,输入任务名称,如图所示:3.将所需的图标拖拽到窗口栏中,并选择gas flow连接不同反应器,然后单击右下角的update4.双击pre-processing,选择工作目录working dir。
点击new chemistry set选择计算文件(用王翰林师兄的)机理,因为有Gas Transport Data File,所以不要勾选Process Transport Properties,点击save。
点击Edit chemistry set可以编辑修改机理。
5.然后开始设置各项参数:C1_PSR选择solve gas energy equation,具体参数设置如下:其中,滞留时间设定,模仿燃烧室内的滞留时间,温度初设一个估计值,如果温度太低不能点火,再调高。
C1_Inlet1设置为甲烷进口,组分设置完之后点击NormalizeC1_Inlet2设置为空气进口,空气质量通过当量比计算得到,组分设置完之后点击Normalize:(当量比,甲烷1g,空气49g)6.双击Continuations,选择是继续计算还是重新计算,一般选择重新计算,然后选择计算步骤,一般10-20步即可。
然后出现确认参数及设置面板,如果没问题进入下一步7.双击run model点击create input file,点击run model。
运行完之后点击run post processor之后点击process solution data查看生成物以及温度等。
如果没有中间产物证明没有点火,还要回去改点火温度。
具体的组分数据查看工作目录下的文件,用记事本打开。
8.对于数据处理,如果查看nox排放量,则统计NO,NO2,N2O三项数据,最后N2O也要转化为NOX,所以在N2O生成量为它本身摩尔量的2倍。
甲烷燃烧模拟
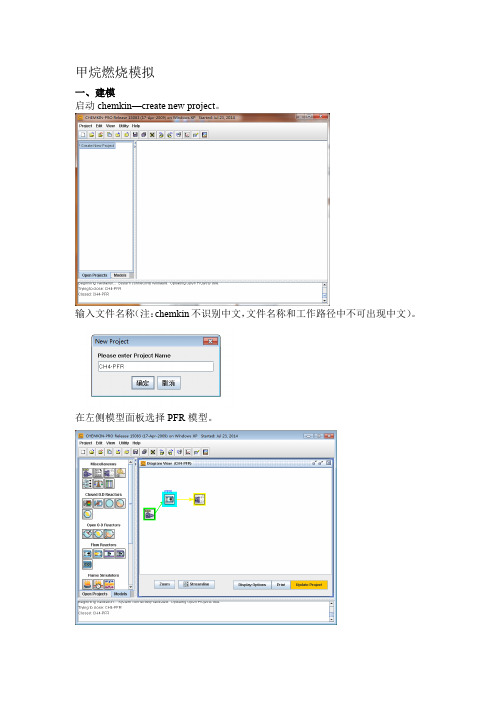
甲烷燃烧模拟一、建模启动chemkin—create new project。
输入文件名称(注:chemkin不识别中文,文件名称和工作路径中不可出现中文)。
在左侧模型面板选择PFR模型。
点击update project二、前处理设置工作路径。
设置反应机理。
第一次设置时,选择new chemistry set,之后可通过edit chemistry set进行修改。
设置模拟所需的气相反应机理gas-phase kinetics file和热力学文件thermodynamics data file。
点击save as进行保存。
点击run pre-processor运行前处理。
前处理完成后,左侧的工作面板被激活。
三、反应器设置1、反应器参数设置2、入口参数与反应物设置3、求解器设置4、输出设置四、计算依次点击Create input file和run model五、后处理点击run post processor,出现后处理面板。
选择需要处理的对象,点击process solution data。
得到后处理控制面板display plot界面可以输出结果曲线,data manager界面可以输出结果数据。
六、多工况计算Chemkin可以在一次性计算多种工况下的结果。
方法一:在界面中,若出现,表示该选项可以变量设置,如temperature中:设置之后,选项会有变化:计算界面为:方法二:点击continuations—setup在面板中设置不同工况下的模型参数。
CHEMKIN II 学生用户手册

How to use Chemkin-II(Summary and condensed instructions for using the Chemkin-II and transport property databases, subroutine libraries, and application codes on the HP workstations)Submitted to:Dr. Thomas FletcherBy:Jeffrey E. DavidsonSeptember 18, 1996Table of ContentsTitle Page (i)Table of Contents (ii)Introduction (1)Introduction to the HP Work Stations (3)The Tool Bar (3)Essential UNIX Commands (5)Other useful commands and tricks in Unix (8)Equilibrium Calculations using Chemkin-II (10)Instructions for using the Chemkin Equilibrium Solver (10)Example Problems for Chemkin Equilibrium Program (13)Laminar, Pre-mixed, One-dimensioned Flame Calculations (14)Setting up a Premixed Flame Problem (14)Example Problems for Premixed Laminar Flame (20)Perfectly Stirred Reactor Calculations (22)Setting up a PSR problem (22)Example Problems for the Perfectly Stirred Reactor (27)Extracting Transport Data from Chemkin (28)Instructions for Extracting Transport Properties (28)Transferring data from the HP workstations to a PC (31)Programing using Chemkin-II (32)Calculations using Global Mechanisms (36)Calculations Using Global Mechanisms (37)Installing the Chemkin Programs into your Directory (38)References (39)Appendix (40)How to use Chemkin-IIIntroductionChemkin-II is a collection of data bases and subroutines written in text files and in FORTRAN code for solving problems involving gas-phase kinetics, equilibrium and transport properties. There are many different types of problems that can be solved using the Chemkin subroutines. Driver programs (programs that direct the subroutines and control the input/output files) have been written for the following types of problems:1. Adiabatic flame temperatures.2. Equilibrium concentrations for a gas mixtures.3. Species mole fractions as a function of time in laminar, steady state, premixed flames when temperature profile in known.4. Flame speed, species mole fractions and temperature profile as a function of time in laminar, steady-state, premixed flames.5. Mole fractions and temperature of product stream from a perfectly stirred reactor.6. Determination of transport properties of gas mixtures as a function of composition, temperature and pressure.This is just a small sampling of the numerous driver programs that have been written. The number and type of computer programs which incorporate Chemkin subroutines are constantly increaseing.To use these programs, the user must modify several input files. As will be explained in detail below, each of the input files is in a Unix-shell file and the user will only need to modify the shell file. In order for the subroutines to work, the user must tell Chemkin about the gas mixture and the gas phase reactions. This is done in the file named mech . The user must name each of the elements and each of the species which are in the gas mixture. The user must also enter the elementary steps of the chemical reactions being considered with their respective Arrhenius rate coefficients for the equation: k = AT β exp ER T ⎛ ⎝ ⎫ ⎭ ⎪ (1)The user must also specify the problem by modifying the inp file. This file generally contains information such as initial temperature of the gas mixture, mole fractions of thereactants, and initial pressure. Depending of the program and the nature of the problem, the user will need to include other keywords which control the program. As these are specific toeach of the programs, they will be discussed separately below. Once the inp and the mech files have been properly modified, the program can be run by executing the shell file.For each of the types of problems discussed here, the application code has already been written. The user must only supply the reaction description and the application input. The application input varies from application to application and each will be discussed individually below. The Chemkin applications have been setup and modified to run the HP workstations called the STATES located in room 308 CB. An introduction to these machines will be discussed before the discussion of the applications.Introduction to the HP Work StationsThe HP work stations are powerful computers which operate much faster than the typical PCs. (Some of these programs would require a few days to run on a PC or MAC). All of the STATES are large enough that multiple users can be logged onto the same state.For example, if you sit down at either the TEXAS or the DALLAS terminal, you will be automatically logged into the TEXAS computer. It is possible to login to UTAH from TEXAS or from a PC or Macintosh via TELNET (a communication software package) or by using the command rlogin state from another workstation, but the graphical interface will not be available.These machines use HP-UNIX for an operating system which incorporates both text and graphical interfaces. When you sit down at one of the terminals, if the screen is blank, move the mouse. The login screen will appear and ask for your user's name and password. After you enter both of these correctly, the screen will be blank except for a border and a tool bar.The Tool BarMail- If you have new or unread E-mail, this button will show a couple of envelopes. Press this button and a listing the mail stored in your mail box will appear. You can use this program to read and send mail.CPU Load- This figure shows the load on that state’s CPU. As the load increases th e area plot increases. Horizontal lines mean that the scale on the original screen had to be reduced. If the computer has more than 3 or 4 horizontal lines it might be wise to find another computer that does not have quite the work load.One, Two, Three, Four, Five, Six- These are multiple screens from which you can access your files. If the first screen (One) gets too cluttered with windows, you can switch back and forth between the other screens with these buttons.Printer- Text files can be sent to the laser printer in the next room by clicking the file in the file manager screen with the middle button on the mouse and then dragging the file to this button. Clicking the left mouse button while the mouse is on the printer button will display the printer status.Text Editor- This button looks like pencil on a piece of paper. When this button is pressed, a window containing the text editor is opened. This editor behaves much like the MS-DOS text editor or Edit on the Macintosh.X-Term- Text user interface. When you press this button, a screen will pop up with a cursor. From this screen, the user can enter commands to copy, delete, rename and run various files. It is similar to a DOS screen on a PC. The commands are discussed below.Style Manager- The attributes (colors, background, etc.) of the graphical interface can be changed using the options under this button.Rename Workspace- Allows you to give names to the One, Two, Three, Four, Five, Six screens discussed above.? - This is the help button which contains a more detailed explanation of the HP work station environment.File Manager- This button (looks like a file cabinet) puts a window on the screen which displays all the files in the current directory. If you double-click on a text file in this window, the text editor will pop up with the contents of the file.Trash- Drag files that you want to erase from your directories on to this button and they will be erased when you logout.Exit - Press this button to logout. The computer will then ask for conformation.Essential UNIX CommandsIn this section, only the most essential UNIX commands will be presented. This presentation is not intended to be detailed but should be sufficient to survive in HP-UNIX. Other commands and further detail can be found in the books found in the computer laboratory. Several of the commands presented here can also be done using the graphical interface although I have found the text interface to have more options and be more useful. The information for the different commands will be described in terms of function and format or will be presented in a tutorial format.To begin, press the X-Term text interface button with the left-hand button on the mouse. If you were logged-on to TEXAS, a screen would pop-up with the prompt:texas$HelpHP-UNIX has an on-line help program which has a very detailed description of the use and options of the UNIX commands. To access the help program type:man command [Enter]Where command would be the name of the command for which you had a question. For example, if you typed:man pwd [Enter]a help screen will pop up and describe the pwd command. To scroll down in help use the space bar and to exit help press q.DirectoriesFiles can be stored in tree-like directories. That is, any directory can have sub-directories. To find out which directory path you are in, type pwd [Enter]. The p rintw orking d irectory command will show you the absolute path name of the working directory. Whenever you start a new text interface, you will automatically be placed in your main directory. The output for the pwd command should be:cheme/usernameAll your files should be kept within this directory or sub-directories of username. To see what files or sub-directories you have in this directory type ls [Enter] for List files. The command ls -l (l is a lowercase L) will provide more information about the files and sub-directories.To make a sub-directory use the mkdir directoryname command. For example at the prompt, type mkdir junk [Enter].Now type ls to see the directory that you created.To move down to that directory, type cd junk [Enter]. The c hange d irectory command can be used to move up and down in the directory tree. To see in which directory you are working, type pwd [Enter]. The result should read:cheme/username/junkNow, to move back to your main directory, use the move-up- one-directory command, cd .. [Enter]. The two periods mean the parent directory or the directory directly above the current directory. Type pwd [Enter] to make sure you are now in your main directory.To remove the junk directory, type rmdir junk [Enter]. In order to remove a directory, the directory must be empty of all files and sub-directories.Note: Every time you call up a text interface screen, it will automatically open to your main directory. In order to access any of your programs or files in other directories, you must first change to the directory in which the files are located.FilesThere are three basic types of files used by Chemkin. Text files can be edited by the text editor. They cannot be executed unless they are a program source code and are first compiled. Shell files are denoted with the .sh extension are similar to .bat file in MS-DOS. They are text files which can be executed and will direct the compilation of FORTRAN codes, direct a sequence of program calls, and direct file input and file output. They can be edited by the text editor and executed by typing:sh shellname.shIf the .sh file is already in the execute attribute (see File...Properties in graphical interface File Manager) then the shell file can be executed by:shellname.shFor the Chemkin programs for which shell files have been written, you will not need to do any programming in FORTRAN but will need to modify the shell files.The third type of file is an non-text executable file. These files are created by compilers and cannot be edited by a simple text editor. Since all the compilation of the FORTRAN source code will be directed by the shell files, you do not need to directly run these programs.There are several commands which are very useful in manipulating files. To make a copy of a file use the cp command. When using the format cp file1 file2, the computer will make a copy of file1 and call it file2. This will not destroy file1, but will destroy any old copies of file2. To copy a file into another directoryuse the format cp file1 path/directoryname. This will place a second copy of file1 into directoryname. To copy all the files in one directory to another use cp *path/directoryname.To move or rename files and directories use the mv command. This has the same format as the cp command except, the original file is destroyed. For example, if you typed mv file1 file2, file2 would be a copy of file1 and file1 would be erased.To erase a file using the text screen, use rm filename. If you use rm -i filename, the computer will request conformation before a file is deleted.Other than using the text editor, text files can be viewed using the more command. (Format more filename). This command will display the contents of a text file to the screen and will pause before scrolling. Because the more command only allows you to scroll down, it is often better to use the text editor to view files.Other useful commands and tricks in UnixTo change your password, type passwd at the prompt.To run a program in the background add an “&” to th e end of the file name. When a program is running in background, you can log-off the system and the program will keep going. To execute the premixed flame shell in the background, you would type:sh premix.sh &If the program is already running but not in the background, press [ctrl]-[z] to pause the program, and type bg at the prompt to put the program in the background.Once a file is running in the background, you can follow its progress by a number of ways. It is often necessary to follow some of the Chemkin programs, because they tend to crash if the initial guesses are far from the true solution. To see if it is still running type jobs -l (lower case L) at the prompt. This will list all the programs currently being executed from that window. To list all the programs currently being executed on that computer type ps -e. (Sometimes this list can be long so to control the screen output use ps -e | more. The | will pipe the output from the ps command through the more command.) Both the ps and the jobs commands will list a number associated each program in execution. This number can be used to stop the execution of the program with the kill command. For example, if you want to kill program number 1, type kill %1.The contents of the output file can be followed as the file is being generated by using the tail command. For example, to follow the output from the premixed flame shell program, type tail -f premix.out. To exit the tail command press [ctrl]-[c]. When shell files are executed, their progress is written to the file .log. To follow the .log file, use tail -f .log.If your program stops running but the output file is empty, look at the files ckout and tpout(in the text editor) and they will tell you if there are any errors in your mech or inp files.If your workstation should freeze, press [shift]-[ctrl]-[reset]. This will reset your computer. These buttons are on the left side of the keyboard. Any files that were not previously saved will probably be lost.To print files, you can either use the text editor or use the lp command. The format is lp filename. This will print two pages per piece of paper. This command and the printing from the text editor will truncate any line which is over 80 columns. To print files that are wider than 80 columns do the following:1. psf -1sc 150 <filename>junk2. lp junkBe careful when you are printing some of the .out files because they can be quite long and can contain a lot of unnecessary information. In most cases it will be better to first erase the unwanted information by using the text editor and then printing the file.Also be careful not to send non-text files to the printer or hundreds of pages could be wasted.From certain STATES or terminals a file cannot be directly printed. If your file does not print after sending it to the printer you will need to rlogin on ALASKA and print from that machine. Do the following on a X-term window of any machine in the room:1. rlogin alaska2. cd to directory where your file is located. (You can use pwd find out in which directory you are located.3. lp filename4. logoutThere are many other commands available in UNIX. Please refer to a manual to learn other commands.Equilibrium Calculations using Chemkin-IIThe Chemkin equilibrium software is easy to use but is not as powerful as other equilibrium programs like the NASA-LEWIS code or the EDCONV code. The latter two programs, will search their data bases to determine what species should be considered in an equilibrium calculation. The Chemkin code requires that the user input all the species which are to be considered. In the example of methane combustion in air, NO and NO2 formation will not be considered unless those two species are entered into the program. CO will not even be considered unless it is input into the program.Instructions for using the Chemkin Equilibrium Solver1. Login to an HP workstation.2. Open the Text Editor.3. Under the File pull-down menu, select Open...4. Open equil.sh (you may need to change directories)5. Scroll down to the line below “cat << EOF > mech”.6. After the word “ELEMENTS” enter the symbols of every elem ent that will be in any species that should be considered in the equilibrium calculation. Isotopes can be considered if their atomic weight follows their symbol in slashes “/”. Lines beginning with an exclamation mark are ignored. Conclude the elements section with the word “END”. Example:ELEMENTSNH! Define DeuteriumD /2.014/OENDAlso Acceptable is:ELEMENTS N H D /2.014/ O END7. After the word “SPECIES”, enter the symbols of every species that should be considered. The format is similar to the elements section. Example:SPECIES H2 O2 H O OH HO2 N2 N NO ENDIf species which are not contained in the thermodynamic data base need to be considered, check out the manual entitled Chemkin-II: A FORTRAN Chemical Kinetics Package for the Analysis of Gas Phase Chemical Kinetics. This manual will provide more detail on extra features of the Chemkin software package. Thermal dynamic data for numerous hydrocarboncombustion species are available in the correct format for the database in Combustion Chemistry, edited by William C. Gardiner or from the NASA-LEWIS equilibrium code data file.8. Scroll past the driver program to the line: “cat << EOF > inp”9. The shell file generates a file named inp that contains the keyword inputs to specify the problem for the equilibrium program.10. The following keyword inputs are allowed in any order:REAC - Specifics the reactants and their molar quantity or mole fraction. Format: REAC H2 2CONH - Constant enthalpy can be used in conjunction with constant volume or pressure, but not with constant temperature, entropy or internal energy.CONP - Constant pressure can be used with constant temperature, volume, enthalpy or entropy, but not with constant internal energy.CONT - Constant temperature can be used with constant pressure, volume or entropy but not with enthalpy, and constant internal energy.CONV - Constant volume can be used with constant temperature, pressure, internal energy, enthalpy or entropy.CONU - Constant internal energy can be used with constant volume only.CONS - Constant entropy can be used with constant temperature, pressure or volume, not with constant internal energy or enthalpy.CONX - Constant mole fractionCHAP - Chapman-Jouguet- cancels all the above specification except REAC. This keyword causes the detonation-wave velocities to be calculated.TEMP - Required input for the starting temperature in K. Format: TEMP 298TEST - Estimate equilibrium temperature in K.Format: TEST 2500PRES - Starting pressure in atmospheres. Format: PRES 1PEST - Estimate of equilibrium pressure in atmospheres.Format: PEST 1.5CNTN- Tells the computer to look for an additional problem after the keyword END.END - End of keyword input.An example of a input file inp to calculate the equilibrium concentrations of a stoichiometric hydrogen flame in air and a fuel rich hydrogen flame is:REAC H2 2REAC O2 1REAC N2 3.76CONHCONPTEMP 300TEST 2000PRES 1CNTNEND/ This is the second problem.REAC H2 3REAC O2 1REAC N2 3.76END11. If you would like to customize the output file name, you must edit the file name on the second to last line in the shell file.Example:Before:make equile; equile < inp > equil.outAfter:make equile; equile < inp > filename12. Under the File pull-down menu of the text editor, select the Save or Save as.. command.13. Open a text interface window by clicking on the X-term button on the tool bar.14. Change the directory to the directory where the Chemkin files are located using the cd command.15. Run the shell program by typing: sh equil.sh16. When the cursor reappears, the program is completed and the output file can be viewed and printed via the text editor.Example Problems for Chemkin Equilibrium Program1. Calculate the adiabatic flame temperature and equilibrium composition of CO in air with equivalence ratios of 0.5, 1,2. (Do not consider NO x formation). (Feed conditions- 300 K, 1 atm). Consider using the CNTN keyword to save some time.2. Repeat problem 1 but consider NO x formation.3. Repeat problem 2, but make the inlet feed contain 10 percent H2.4. Compare the results from problem 3 with either the EDCONV program or the NASA-LEWIS code. If you forgot a significant species in problem 3 as determined by one of the other two programs, repeat problems 3 and 4 again. What are two reasons for using the Chemkin equilibrium program, if one of the other equilibrium codes is available?Laminar, Pre-mixed, One-dimensioned Flame Calculations“Many practical combustors, such as internal combustion engines, rely on pre-mixed flame propagation. Moreover, burner-stabilized laminar premixed flames are very often used to study chemical kinetics in a combustion environment. Such flames are effectively one-dimensional and can be made very steady, thus facilitating detailed experimental measurements of temperature and species profiles. Also, laminar flame speed is often used to characterize the combustion of various fuel-oxidizer combinations. Therefore, the ability to model chemical kinetics and transport processes in these flames in critical to interpreting flame experiments and to understanding the combustion process itself.” (Kee, 1992)The Chemkin premixed flame code is designed to handle a variety of problems. The calculations involve solving systems of non-linear mass balances, energy equations, and transport relations. There are two major divisions in the types of problems that the premixed code will solve. The burner stabilized problem requires a known mass flow rate, and the temperature profile can either be specified or calculated from energy equations. Often it is better to specify the temperature profile if it can be experimentally determined because heat loses from a flame can be difficult to quantify. Problems where the temperature is specified are relatively easy to solve compared to the second type of problem. The second type of problem involves an adiabatic freely propagating flame. From the input, the flame speed, temperature profile, and concentration profile are calculated. This type of problem is very difficult to converge and solving them requires some skill, experience and a lot of patience. The code is currently configured for a burner diameter of 1.0 cm2.Setting up a Premixed Flame Problem1. Login to an HP workstation.2. Open the Text Editor.3. Under the File pull-down menu, select Open...4. Open premix.sh5. Scroll down to the line below “cat << EOF > mech”.6. After the word “ELEMENTS” enter the symbols of every element that will be in any species that should be considered in the equilibrium calculation. Isotopes can be considered if their atomic weight follows their symbol in slashes “/”. Lines beginning with and exclamation mark are ignored. Conclude the elements section with the word “END”. Example:ELEMENTSNH! Define DeuteriumD /2.014/OENDAlso Acceptable is:ELEMENTS N H D /2.014/ O END7. After the word “SPECIES”, enter the symbols of every species that should be considered. The format is similar to the elements section. Example:SPECIES H2 O2 H O OH HO2 N2 N NO ENDIf species which are not contained in the thermodynamic data base need to be considered, check out the manual entitled Chemkin-II: A FORTRAN Chemical Kinetics Package for the Analysis of Gas Phase Chemical Kinetics. This manual will provide more detail on extra features of the Chemkin software package. Thermodynamic data for numerous hydrocarbon combustion species are available in the correct format for the database in Combustion Chemistry, edited by William C. Gardiner. Transport property data may also need to be added to the transport property data base.a. This section must begin with the word REACTIONS. The default units for the Arrhenius rate coefficients are cal/mole for the activation energy and the units of A are in terms of cm, sec, K, and moles. Equation 1 is shown here again: k = AT β exp ER T ⎛ ⎝ ⎫ ⎭ ⎪ (1)These default units of the activation energy can be changed by adding the words CAL/MOLE, KCAL/MOLE, JOULES/MOLE or KELVINS after the word REACTIONS. The default units for A can be changed by adding MOLECULES so that the units of A are in terms of cm, sec, K, and molecules.b. The information of the reaction is fairly free from format but certainconventions must be observed. At the beginning of the line the reaction mechanism must be symbolically written. No more than 3 molecules can be on either side of the equality. All coefficients of reactants must be integers. A plus sign (+) is used to separate the reactants from the reactants and the products from the products. There are two symbols used to separate the reactants from the products. The equals sign (=) is used to represent a reversible reaction. When this symbol is used, the reverse reaction is considered and calculated using the equilibrium constant. When the symbol => is used, the reaction is assumed to be irreversible and the reverse reaction is not considered. Third body reactions can be considered by using thesymbol M as both a reactant and product. The M species does not need to be declared in the SPECIES section. On both sides of the equation, M must be the last species. The defaultthird body efficiency is one, but this can be modified as shown in the example elementary step. The three Arrhenius coefficients (in order of A, , and E) are on the same line as the reaction and must be separated by at least one space. (Multiple spaces or tabs are acceptable for separating the data on a line.) Exclamation marks (!) signify that information following the ! is to be ignored. An example elementary step is:H + O2 + M = HO2 + M 0.361E18 -0.72 0.0H2O/18.6/ H2/2.86/ N2/1.26/ ! 3rd body efficienciesRefer to the manual, Chemkin-II: A FORTRAN Chemical Kinetics Package for the Analysis of Gas Phase Chemical Kinetics for details on handling photo-chemical reactions, pressure-dependent fall-off reactions and Landau-Teller formulation of rate expressions.c. End the mechanism description with the word END.8. Scroll down to the line: “cat << EOF > inp”9. The shell file generates a file named inp that contains the keyword inputs to specify the problem for the premixed laminar flame program. In the Appendix there are several pages directly from the manual and explain all the possible keyword inputs. Some of the most important keywords will be discussed here.BURN - A required keyword for burner-stabilized flame problems.FREE - Required for adiabatic freely propagating flame. (Note: either BURN or FREE must be specified by not both but this can be changed on continuation (CNTN) or restart (RSTR).)TGIV - For burner-stabilized flames, the species equations will be solved using the user-specified temperature profile.ENRG - The temperature profile will be calculated using the energy equations, but the user must enter initial guesses for a temperature profile using the TEMP keyword. (Note: Either TGIV or ENRG must be specified for BURN type problems.)MOLE or MASS - Used to specify that answers and inputs are in either mole or mass fractions.PRES - Pressure of the flame in atmospheres. Format: PRES 1.0.FLRT - Specified flow rate for burner stabilized flames or initial guess for adiabatic freely propagating flames. Units are in g/cm2-sec. Format: FLRT 0.04.REAC - used to specify initial reactant mole or mass fractions.INTM - used to give the computer initial guesses of intermediate species mole fractions.PROD - used to give the computer initial guesses of product species mole fractions。
CHEMKIN-PRO应用培训手册-Applications

CHEMKIN-PROApplicationsMorning Session9:00 –12:00Agenda –Day 2: CHEMKIN-PRO Applications●Overview of CHEMKIN-PRO●Multi-zone IC engine Model●*** BREAK (10:30)***●Reaction Path Analysis●*** LUNCH (12:00)***●Particle Tracking2CHEMKIN Product Feature Comparison3CHEMKIN-PRO New Features●Increase Your Productivity–New 64-bit Windows (Vista and XP) versions allow for more memory use then 32-bit CHEMKIN 4.1–Alert the user immediately regarding machine limitations –New Progress Indicator: know exactly where you are in the calculation process–More intuitive GUI workflow to streamline navigating tasks–Less cumbersome initial guesses4CHEMKIN-PRO New Features●Model Real Fuel Chemistries–Allow reaction mechanisms to include more than one reaction sub-set with different units–Mix-and-match different reaction mechanisms within the same projects file●Simulate more Realistic Engines–Additional heat-release parameters for IC Engine output–Heat release rate provided both in terms of heat-release per time and heat-release per crank angle –Allow use of a non-conventional piston motion in IC Engine models5A Faster CHEMKINFaster on Simple Models●Comparison to Chemkin II (Historical Standard)7Faster on Complex Models8Multi -Zone EngineSimulator10Why is the Multi-Zone Engine Simulator Important?●Understand how CO and HC are formed in different zones of the combustion chamber●Understand how ignition is affected by in-cylinder conditions●Accurately simulate emissionsLower temperaturesnear the walls (COand soot formation)Ignition & HighTemperature in BowlCHEMKIN-PRO Multi-zone Modeling●Homogeneous Charge CompressionIgnition (HCCI) gives gasoline enginesdiesel-like efficiency●Ignition is controlled by kinetics andengine conditions●CFD can not handle the kineticsIgnition11Application:Multi-Zone EngineSimulatorCHEMKIN-PRO Multi-zone Engine Simulator ●Permits the use of detailed combustionchemistry–Ignition timing–Pollutant formation●Addresses in-cylinder temperature and/orcomposition inhomogeneities–Local heat loss–Residual gas or recycled exhaust gas●Facilitates parametric studies13Zone Description●Zones are imaginary regions–Floating and non-contiguous–Total zone volume must equal to cylinder volume●Pressure is uniform inside the cylinder●No mass or heat transfer takes placebetween the zones–Each zone is a closed homogeneous reactor●Zones interact with each other throughpressure work1415●A 10-zone model is used in the simulation:●All zones have the same initial gas composition●A natural gas mechanism (up to C4) is used35251810521112Mass %CoreBoundaryLayerCreviceRegionWall Area %Zone #712223243531221696109876Lower temperaturesnear the walls (COand soot formation) Ignition & HighTemperature in the CoreZone Definitions16●CHEMKIN multi-zone model can be used in thehybrid approach for HCCI engine simulation–Hybrid approach is a two-step simulation process1.A CFD code is used to obtained in-cylinder temperaturedistribution before chemical kinetics becomes important2.The multi-zone model will calculate zone properties usingdetailed chemical kinetics and temperature information from theCFD solution●Zone temperature may be determined in two ways–Constrained with a given temperature vs. time profile–Solved with the energy equation●Transition angle is a model parameter specifyingwhen the energy equation will be used tocompute the zone temperatureHybrid Approach17Wall Heat Transfer●Zones can exchange heat with cylinder wall –Each zone has its own wall heat transfer rate●Zone wall heat transfer rate is computed from zone wall heat transfer coefficient, zonetemperature, and zone wall surface area–Wall heat transfer coefficient is calculated by the Woschni correlationÑThe same set of heat transfer model parameters is appliedto all zones–Zone wall surface area is given as a constant fraction of instantaneous cylinder wall surface areaÑA zone can be made adiabatic by setting its wall surfacearea fraction to zero18User Interface●Reactor properties panel is the same as the single zone model●Zone mass/volume fraction, temperature (profile), initial composition, and wall heat transfer area fraction can be assigned individually● A transition angle (or time) can be specified to turn on the energy equation19●Engine parameters●Fuel–Natural gas/Air, φ= 0.25921Compression ratio 26 cmConnecting rod length 14cmStroke 1000 rpmEngine speed 12.065 cmBore 1600 cm 3Displacement volume Vol %component 91.1CH 40.50.61.41.74.7CON 2n-C 4H 10C 3H 8C 2H 6The 2-bar Boost Case from Aceves et al., SAE 2000-01-032720●Ignition isdetermined by the hottest zone ●Temperatures in cold zones drop quickly due to wall heat loss after the energy equation is turned on –Allow zones to havedifferent heat transfer parameters 600800100012001400160018002000-20-1001020Crank Angle (degree ATDC)T em p er a tu r e(K)single-zone 10-zone aveZone 1Zone 2Zone 3Zone 4Zone 5Zone 6Zone 7Zone 8Zone 9Zone 10use temperature profile solve energy equationθt =-3Using Temperature Profile from CFD21Multi-zone Model●Open Samples problem: ic_engine__multizone.ckprj ●Explore the interface ●Run the example ●Plot temperature resultsHANDS ON22Results from Multi-Zone Engine Simulator ExampleTemperature Distribution in the HCCI EngineIgnitionHANDS ONReaction Path AnalyzerCHEMKIN-PRO’s Reaction Path Analyzer●Key tool for mechanism reduction●Graphically explore reactionmechanism bottlenecks●Identify crucial species andreactions●See the underlying chemistry inyour process●Can be used withall CHEMKIN-PRO reactors●Can be used with sensitivityanalysis24Reaction Path AnalysisInfluence of primary andsecondary radical pathsin the formation of COStart and end speciesRestricting by elementNumber of species drawnVary the point within thesolutionPlots of:Rate of ProductionForward and ReverseComposition25Reaction Path AnalysisLayout optionsLine shape and labelingRelative sizing of linethickness“Side Species”color chartA side species will not bein a diagram, but reactionpaths can be colored bytheir influence26ApplicationReaction Path Analyzer28Reaction Path Analysis Example●Open Samples problem:closed_homogeneous__transient.ckprj●Run Problem●Under Analyze Results select “Analyze Reaction Paths”●Examine how Water is produced in Hydrogen Combustion processHANDS ON29Reaction Path Analysis Example1)Add H2O as a species from the “Species List”2)Select H2 as a Start species and H2O as anEnd species in the “Species Selection”list 3)Remove all Side Species4)Select ignition region on the Temperature plot On “Preferences”tab:1)Select Minimum (relative) ROP drawn 2)Draw line labels all the time 3)Put boxes around the speciesHANDS ONCHEMKIN -PRO ApplicationsAfternoon Session 13:00 –16:00Particle TrackingWhy Particle Chemistry?Have you ever wondered?:●Will my new engine design meet emissionsregulations?●How will different fuels affect particleproduction?●How can I predict particle formation in mymicroelectronics deposition process?Particle Tracking can helpanswer these questions32Ability to Model a Variety of Chemical Interactions●Particle Tracking feature has the ability tosimulate all gas and surface-phase chemicalinteractions, as well as particle-particleinteractionsGas SpeciesSurfaceSpeciesParticle33What is the Particle Tracking?●A utility in CHEMKIN PRO (add-on in 4.1.1)●Enables particle-size distribution tracking●Includes nucleation, coagulation, surface reactions●Reactor models that can be coupled with Particle TrackingBatch ReactorPSRPFRShear FlowPremixOppdif34Industry Applications●Automotive–Soot generation and growth●Propulsion–Aluminum oxide particles●Microelectronics–Silica particles●Materials–Carbon black●Environment–Aerosols35Methodology (1)●Method of Moments–Soot particle simulation by Frenklach and Harris●Evolution of particle size distribution–Moment 0 »total number density–Moment 1 »total mass or total volume–Fractional moments »avg.diameter, surface area 3637●Particle size/class == Number of bulk species in the particle●Mass growth == deposition of certain gas species (such as carbon) on the particle surface●Multiple nucleation pathways ●Coagulation model–Free molecular –Transition –ContinuumMethodology (2)38PTM User Inputs●Gas-phase mechanism: gaseous reactions leading to formation of nucleation precursors ●Surface mechanism: nucleation and gas-particle interactions●Thermodynamic data●Transport data (shear flow, premix, and oppdif)Outputs●Average particle properties–Diameter–Surface area–Volume●Thermodynamic and flow properties of thegaseous mixture39Surface Chemistry Representation (1)●Particle material–Represented by bulk species–Bulk species created in nucleation reaction●Particles composition–Defined by a different surface material andassociated reactions40Surface Chemistry Representation (2)●Nucleation reactions–Initial particle formation from gas-phase species –Define inception particle class and initial surface coverage on the particles–A particle can be formed by multiple nucleationreactions41Surface Chemistry Representation (3)●Chemical processes on particle surface–Surface reactions input●Surface coverage state–Statistical average for all particles–Determines particle reactivity●Particles grow if–the surface reaction results in a net gain of bulk species (and vice versa)4243Example –Surface chemistry input fileMATERIAL SOOT_PARTICLE DISPERSEDENDSITE/PolyC/ SDEN/3.341E-9/H(se) open(se)! se indicates edge or active site. ENDBULK C(B)/1.8/ENDREACTIONS! nucleation from PAH2A4=> 32C(B)+20H(se)+28.72open(se) 9.0E09 0.5 0.0NUCL ! HACA growthH+H(se) => open(se)+H2 4.2E13 0.0 13000.0open(se)+H2 => H(se)+H 3.9E12 0.0 9320.0open(se)+H => H(se) 2.0E13 0.0 0.0 H(se)+OH => H2O+open(se) 1.0E10 0.734 1430.0 H2O+open(se) => OH + H(se) 3.68E8 1.139 17100.0 open(se)+C2H2 => H(se)+2C(B)+H 8.0E7 1.56 3800.0 ! PAH condensationA1+open(se) => H(se)+6C(B)+5H 0.60.0 0.0STICKFORD/open(se) 2.0/DCOL/4.E-8/Inception particle classParticle bulk compositionGrow particle by 2 classes Nucleation reaction Grow particle by 6 classes Indicates a dispersed phase and activates PTMBulk density44Additional Particle Tracking Capabilities in CHEMKIN PRO●Particle Tracking is now available with all of the CHEMKIN Flame reactors–Burner Stabilized Flame –Flame Speed Calculator –Diffusion Flame●Who might be interested in flame + soot capability?–Potential customers working on flame synthesis of particles –Anyone conducting experiments with sooting flames45Typical Particle Tracking ResultsTNucleation RateMean DiameternGrowth RateVolume of ParticlesApplication:Soot Oxidation andGrowth47Application: Soot Formation and Growth●JSR/PFR system developed at MIT (Marr, 1993)●The influence of mass diffusion on gas-phase species is minimized in this configurationHANDS ON48Application: Chemistry Set●Gas-phase: C 2H 4/O 2/N 2(includes formation of PAH precursors)●Surface mechanism (includes nucleation, oxidation and HACA and PAH condensation reactions)HANDS ON491.Open samples project:“\samples45\reactor_network_soot_JSRPFR”2.Pre-process chemistry3.Review various panels4.Run Model5.Post-process Testing PFR results6.Plot various gaseous PAH pre-cursors7.Plot avg particle diameter and number density as afunction of distanceApplication: Soot Formation and Growth HANDSONJSR Ignition (Soot Formation)PFR TransitionSectionPFR TestingSection50Application: Results HANDS ONEnd of CHEMKIN-PRO Applications。
chemkin安装

软件版本:Reaction.Design.Chemkin.v4.1.WiNNT2K-oDDiTy一、安装很简单:1.setup.exe2.复制crack中的reaction.lic文件到安装路径下的Reaction\licenses目录下3.重启电脑XP和Windows 7 32bit(兼容模式)运行都没问题,但是windows 7 64bit不能用。
提示:ERROR: setPlatform(): Did not find a setting for Windows NT (unknown)^6.1解决方法:找到安装目录下:Reaction\chemkin41_pc\data文件夹,修改OSName_Map.properties文件,其中添加:Windows\ NT\ (unknown)^*=WINNT即将文件修改为:###################### Java OSName and OSVersion Strings #################################################################################################### OSName ^ OSVersion = Platform Number####### Any whitespace must be "escaped" with \ character####### * matches any OSVersion####### File is NOT searched in Order Listed Below; Ordering is Random########################################################################### Windows\ NT^4.0=WINNTWindows\ 2000^*=WINNTWindows\ 2003^*=WINNTWindows\ XP^*=WINNTWindows\ NT\ (unknown)^*=WINNTAIX^*=AIXIrix^*=SGIIrix64^*=SGISunOS^*=SUNHP-UX^B.11.00=HP11HP-UX^B.11.11=HP11HP-UX^B.11.20=HP11iHP-UX^B.11.22=HP11iCompaq's\ Digital\ UNIX^*=ALPHAOSF1^*=ALPHALinux^*=LINUX###########################################################################保存后便可打开CHEMKIN软件,注:WIN7是在兼容模式下运行的。
chemkin化学反应机理导入fluent

chemkin化学反应机理导入fluent1.引言在工程领域中,化学反应机理的研究对于模拟和预测各种化学过程起着重要作用。
Fl ue nt作为一种流体力学仿真软件,提供了强大的求解能力和丰富的功能,将化学反应机理导入Fl u en t可以帮助我们更准确地模拟各种复杂的化学反应过程。
2. ch emkin化学反应机理概述C h em ki n是一种广泛应用于化学反应机理研究的软件包,它包含了丰富的化学反应机理模型和反应速率参数。
这些模型和参数可以用来描述和计算各种化学反应的速率、生成物分布等信息。
在导入Fl ue nt之前,我们需要先准备好所需的化学反应机理文件。
3.准备che mkin化学反应机理文件在使用Fl ue nt之前,我们需要将C he mk in化学反应机理文件准备好。
这些文件包括燃料机理文件、氧化剂机理文件、生成物机理文件等。
我们可以通过在C he mk in软件中进行相关操作生成这些文件,并保存为适当的格式(如.ct i格式)。
4.导入che mkin化学反应机理到F l u e n t在F lu en t中导入ch e mk in化学反应机理需要进行以下步骤:4.1启动F l u e n t软件首先,打开F lu en t软件并新建一个工作文件。
4.2打开反应机理界面在Fl ue nt中,点击菜单栏中的“De fi ne”选项,然后选择“M od el s”子菜单,再选择“Ch e mk in”选项。
这样就可以打开反应机理界面。
4.3导入反应机理文件在反应机理界面中,选择“I mp or t”选项,并找到之前准备好的C h em kin化学反应机理文件(.cti格式)。
点击“Op en”按钮导入文件。
4.4定义反应模型在导入反应机理文件后,Fl ue nt会自动识别出其中的反应模型和参数。
我们可以根据需要选择适当的反应模型,并设置相应的参数。
4.5应用反应机理完成反应模型的定义后,将其应用到我们需要进行模拟的物理系统中。
顶好的Chemkin学习资料

大型气相化学反应动力学软件CHEMKIN学习报告作者:范志林1 总体介绍CHEMKIN软件是美国Sandia国家实验室开发的大型气相化学反应动力学软件,可以用来解决带有化学反应的流动问题,是燃烧领域中普遍使用的一个模拟计算工具。
CHEMKIN3.7版本有多种针对不同模型的应用程序,包括AURORA、CRESLAF、EQUIL、OPPDIF、PASR、PLUG、PREMIX、SHOCK、SPIN、SURFTHERM、OVEND、TWAFER 12个应用程序,分别用来模拟充分搅拌反应器(AURORA)、圆柱形或平面形通道内的层流化学反应(CRESLAF)、化学平衡相平衡(EQUIL)、对流扩散火焰(OPPDIF)、部分搅拌反应器(PASR)、柱塞流反应器(PLUG)、一维稳态层流预混火焰(PREMIX)、冲击波化学动力学(SHOCK)、化学气相沉积滞留反应器(SPIN)、气相和表面化学系统的热化学传质及动力学(SURFTHERM)、多晶片低压化学沉淀反应器(OVEND)、用来确定多晶片低压化学沉淀反应中的温度(TWAFER)。
对大多数CHEMKIN应用程序而言,在应用之前需事先准备好三个输入文件:气相输入文件(gas chemistry input file)、表面反应输入文件(surface chemistry input file)和程序应用输入文件(application input file)。
输入文件默认为采用文本文档的形式给出,其中气相输入文件(gas chemistry input file)中指定了反应中元素组成、组分组成、各组分热力学参数、包括基元反应式、Arrhenius系数(pre-exponential factor、temperature exponent和activation energy)的反应机理;而程序应用输入文件(application input file)中则要根据实际情况来指定反应器的几何参数、问题类型、初始条件以及解文件控制参数等等。
chemkin安装

软件版本:Reaction.Design.Chemkin.v4.1.WiNNT2K-oDDiTy一、安装很简单:1.setup.exe2.复制crack中的reaction.lic文件到安装路径下的Reaction\licenses目录下3.重启电脑XP和Windows 7 32bit(兼容模式)运行都没问题,但是windows 7 64bit不能用。
提示:ERROR: setPlatform(): Did not find a setting for Windows NT (unknown)^6.1解决方法:找到安装目录下:Reaction\chemkin41_pc\data文件夹,修改OSName_Map.properties文件,其中添加:Windows\ NT\ (unknown)^*=WINNT即将文件修改为:###################### Java OSName and OSVersion Strings #################################################################################################### OSName ^ OSVersion = Platform Number####### Any whitespace must be "escaped" with \ character####### * matches any OSVersion####### File is NOT searched in Order Listed Below; Ordering is Random########################################################################### Windows\ NT^4.0=WINNTWindows\ 2000^*=WINNTWindows\ 2003^*=WINNTWindows\ XP^*=WINNTWindows\ NT\ (unknown)^*=WINNTAIX^*=AIXIrix^*=SGIIrix64^*=SGISunOS^*=SUNHP-UX^B.11.00=HP11HP-UX^B.11.11=HP11HP-UX^B.11.20=HP11iHP-UX^B.11.22=HP11iCompaq's\ Digital\ UNIX^*=ALPHAOSF1^*=ALPHALinux^*=LINUX###########################################################################保存后便可打开CHEMKIN软件,注:WIN7是在兼容模式下运行的。
CHEMKIN4.0.1入门指南

CHEMKIN入门指南《燃烧学》辅助教程上篇基础知识、核心程序、化学平衡(EQUIL)、全混反应(AURORA)如果文中有任何错误,请不吝指出,以便不断改进2004.3第一章CHEMKIN简介本章介绍CHEMKIN的主要功能和求解过程。
第一节安装CHEMKINChemkin最早的版本始于1980,由美国Sandia实验室的Kee RJ等人编写,经过多年的不断发展日趋完善。
后来由Reaction Design公司收购并继续开发,目前最新版为3.7.1。
由于学习和科研需要,我们花费2000$向ReactionDesign公司订购了一套最新版本的CHEMKIN 3.7.1,其中包括20个网络教学许可证,用于《燃烧学》课程的学习。
[安装] 请从ftp://combustion:combustion@166.111.56.202的“CHEMKIN软件”目录内下载安装程序chemkin371_pc_setup.exe,执行安装程序。
安装完后会自动在桌面及开始菜单建立快捷方式。
[运行许可证书] 教学用的CHEMKIN采用网络认证,故电脑必须联网(校内)。
当程序计算(Run)时,系统会提示选择license,选择“Specify license server”,然后next,在下一画面填入“166.111.56.202”即可。
第二节CHEMKIN介绍CHEMKIN是一种非常强大的求解复杂化学反应问题的软件包,常用于对燃烧过程、催化过程、化学气相沉积、等离子体及其他化学反应的模拟。
CHEMKIN包括“核心程序(Core Utilities)”和“应用程序(Application)”两级程序包。
以气相反应、表面反应、传递过程这三个核心软件包为基础,CHEMKIN提供了对12种常见化学过程模拟的软件包及后处理程序。
CHEMKIN的三个核心程序模块:1) 气相动力学(Gas-PhaseKinetics):是所有程序计算的基础,提供气相成分组成、热力学数据、化学反应等信息。
卓顶精文-最新CHEMKIN入门指南

CHEMKIN 4.0.1入门指南——《燃烧学1》辅助教程文中如有任何错误,敬请指出,以便不断改进;如有任何问题,欢迎提出,共同探讨助教博士生:卢智恒联系方式:热能系系馆办公室201(O)62782108 (H)62779574luzhiheng@2005.3一、CHEMKIN的安装和简介1-1 安装CHEMKINChemkin最早的版本始于1980,由美国Sandia实验室的Kee RJ等人编写,经过多年的不断发展日趋完善。
后来由Reaction Design公司收购并继续开发,目前最新版为4.0.1。
由于学习和科研需要,我们花费12000$向ReactionDesign公司订购了一套最新版本的CHEMKIN 4.0.1,其中包括可供20人同时在线计算的license,用于《燃烧学》课程的学习。
【安装】请登录ftp://combustion:combustion@166.111.56.155 下载相关文件,其中chemkin401_pc_setup.exe为CHEMKIN的安装程序,chemkin.lic为网络认证文件,详细的安装信息可以参看ftp上的“安装说明.txt”文件。
安装完后会自动在桌面及开始菜单建立快捷方式。
【注意】1、本套教学用的CHEMKIN软件采用网络认证的方式,请确保电脑已经联网(校内),否则无法计算。
2、建议采用1024×768的分辨率,否则某些界面将无法完全显示。
1-2 CHEMKIN简介CHEMKIN是一种非常强大的求解复杂化学反应问题的软件包,常用于对燃烧过程、催化过程、化学气相沉积、等离子体及其他化学反应的模拟。
CHEMKIN以气相动力学、表面动力学、传递过程这三个核心软件包为基础,提供了对21种常见化学反应模型及后处理程序。
三个核心程序模块为:1) 气相动力学(Gas-Phase Kinetics):是所有程序计算的基础,包括气相成分组成、气相化学反应与相关的Arrhenius数据等信息。
chemkin层流燃烧速度计算模型介绍

chemkin层流燃烧速度计算模型介绍
层流燃烧速度模型是一维开口模型,需要将入口、燃烧速度模型、出口连接起来。
在模型更新设置中,可以进入预处理、模型物理参数、燃料浓度等参数的设置。
预处理是检查机理文件、热力学文件和传输学文件格式的重要步骤,机理中的错误会在预处理中报错,展示在out文件中。
为了避免软件bug的发生,工作目录等文件夹名称中不要出现中文。
在反应器设置中,最重要的是设置反应器的温度、压力与热损失等参数。
温度、压力与热损失是计算案例的核心工况条件,缺少任何一个设置都会导致案例报错。
层流燃烧速度模型实际是一个类似于“管道”的模型,因此需要设置“管道”内的长度和网格数量、网格节点质量等参数,保证案例计算的收敛。
此外,层流燃烧速度模型的计算还包括燃烧核心参数的计算,如层流燃烧速度、点火延迟、燃烧产物密度和火焰结构等。
同时,还可以进行敏感性分析,如重要基团物质敏感性分析、温度敏感性分析、产热敏感性分析和化学反应
路径等。
机理简化也是其中的一部分,包括机理简化方法介绍、简化目标参数选择和骨架机理优化等。
最后,通过对比参考文献中的实验数据,可以验证仿真计算的可靠性。
也可以计算不同工况条件下点火延迟时间,分析不同温度压力条件对点火延迟的影响。
同时,还可以运用多种简化方法,得到优化的骨架机理。
以上内容仅供参考,如需更多信息,建议查阅Chemkin相关文献或咨询专业化学专家。
chemkin中ford重新定义正向反应时某组分反应级数-概述说明以及解释

chemkin中ford重新定义正向反应时某组分反应级数-概述说明以及解释1.引言1.1 概述概述部分的内容可以描述整篇文章的背景和要解决的问题。
以下是对概述部分的一个可能的编写:在化学动力学研究中,正向反应的定义和测量是非常重要的,因为它们直接影响到反应速率和反应机理的研究。
然而,在某些情况下,传统的Ford反应级数定义可能无法准确地描述某些组分的反应级数。
本文的目的是探讨使用Chemkin软件中的Ford反应重新定义正向反应时,某组分的反应级数的变化。
我们将介绍Ford重新定义正向反应的背景和方法,并分析这一方法对该组分反应级数的影响。
通过对Ford反应的背景和方法进行详细描述,我们希望能够更好地理解正向反应的定义和计算方式,并进一步探讨Ford反应定义对反应级数的影响。
这将有助于我们更准确地研究和理解化学反应的速率和机理。
在本文的结论部分,我们将总结对Ford重新定义正向反应的分析,并提出对今后研究的一些建议。
通过这些分析和总结,我们将对Ford反应的重新定义及其对正向反应的影响有更深入的理解。
综上所述,本文将对Chemkin软件中Ford反应的重新定义正向反应时某组分反应级数进行深入探讨。
我们相信该研究对于进一步理解化学反应的速率和机理将具有重要的意义。
1.2文章结构1.2 文章结构本文将按照以下结构展开讨论Ford如何在Chemkin中重新定义正向反应的方法。
首先,我们将介绍Ford重新定义正向反应的背景,包括其研究意义和现状。
接着,我们将详细阐述Ford重新定义正向反应的方法,并对其进行逐步解析和讨论。
在此过程中,我们将包括对Ford方法的原理和实施步骤的解释,以及对其应用的案例研究和实证分析。
最后,我们将总结Ford重新定义正向反应的影响,并提出对其未来发展的展望。
通过本文的研究,我们旨在揭示Ford重新定义正向反应的重要性和应用潜力,为相关领域的研究和实践提供有益的参考和借鉴。
1.3 目的本文的目的是探讨在Chemkin中,使用Ford方法重新定义正向反应时,对于某组分的反应级数的影响。
chemkin安装

软件版本:Reaction.Design.Chemkin.v4.1.WiNNT2K-oDDiTy一、安装很简单:1.setup.exe2.复制crack中的reaction.lic文件到安装路径下的Reaction\licenses目录下3.重启电脑XP和Windows 7 32bit(兼容模式)运行都没问题,但是windows 7 64bit不能用。
提示:ERROR: setPlatform(): Did not find a setting for Windows NT (unknown)^6.1解决方法:找到安装目录下:Reaction\chemkin41_pc\data文件夹,修改OSName_Map.properties文件,其中添加:Windows\ NT\ (unknown)^*=WINNT即将文件修改为:###################### Java OSName and OSVersion Strings #################################################################################################### OSName ^ OSVersion = Platform Number####### Any whitespace must be "escaped" with \ character####### * matches any OSVersion####### File is NOT searched in Order Listed Below; Ordering is Random########################################################################### Windows\ NT^4.0=WINNTWindows\ 2000^*=WINNTWindows\ 2003^*=WINNTWindows\ XP^*=WINNTWindows\ NT\ (unknown)^*=WINNTAIX^*=AIXIrix^*=SGIIrix64^*=SGISunOS^*=SUNHP-UX^B.11.00=HP11HP-UX^B.11.11=HP11HP-UX^B.11.20=HP11iHP-UX^B.11.22=HP11iCompaq's\ Digital\ UNIX^*=ALPHAOSF1^*=ALPHALinux^*=LINUX###########################################################################保存后便可打开CHEMKIN软件,注:WIN7是在兼容模式下运行的。
ChemKin 操作入门

ChemKin4.0 操作入门安装:运行setup.exe。
然后把carck文件夹中的chemkin.lic文件拷到安装目录下的licenses文件夹中。
一、新算例设置的基本操作1、建立新工程2、设定反应器模型在左侧models面板双击所需反应器模型,如PSR模型,则反应器模型出现在右边的Diagram View面板,下角Update project按钮变成黄色。
当设定好模型后,点击该黄色按钮确定,按键变成灰色,方可进入下一步反应机理设定。
3、设定反应机理在左侧,Open Projects面板,双击Pre-Processing。
进入机理设置界面。
Working Dir 是所有计算结果,包括工程文件的存储位置,自行设定。
点击New Chemistry Set,设定机理文件,必须设定Gas Phase Kinetic File—后缀为.inp;Thermodynamic Data File――后缀为.dat,都通过设定路径来设定。
设好后Save As…,确定,就变成下面的样子,点Run Pre Processor。
运行成功则View Results….变成黑色,Cluster变成黑色,没有跳出任何消息框。
运行成功方可进行计算的初始参数设定。
在cluster中,●properties选求解Gas Energy Equation,●C1表示反应器,在reactor physical properties中设置停留时间、温度、压力、体积、热损失等项。
注意单位。
表面项和传输项设置留待诸位研究设置方法。
在species specific data 中设置反应器中原有的物质组分,要各组分的fraction加起来=1。
这一项我的计算中不需要设置。
注意,此处设置的温度为牛顿迭代计算的初值,默认条件下等于入流温度。
●R1表示入流。
入流可以有多个,比如我的先进再燃就有5个入流,入流再多也应该没有关系,进去就都一样了,看设置参数的方便而定。
CHEMKIN基础介绍

CHEMKIN应用于工程问题
设计方向
改进反应器尺寸的影响 工业过程的参数运行范围 确定过程的可替代性 预测反应过程时间尺度上的可控制性 工况运行条件变化的影响 过程参数扰动的敏感性 反应器的生产能力评估 排放 过程变化对下游的影响 化学机理的发展与简化 实验结果的预测
反应器优化和改进
反应物名字必须包含 在热力学数据中
47780 3626 0 6290 0 0 1073 1073 0 ! ! ! ! ! ! ! ! D-L&W JAM 1986 KLEMM,ET AL DIXON-LEWIS D-L D-L D-L COHEN-WEST.
反应
固定系数: A, B, E
• • • •
CHEMKIN 4.x
基于java的操作界面。 操作界面不再是基于计算模块,而是反应器 模型 全新的后处理功能 项目图形化 全新的参数研究功能 颗粒追踪模块
主要内容
CHEMKIN介绍 CHEMKIN用户界面 CHEMKIN化学设置 CHEMKIN模型 CHEMKIN后处理 CHEMKIN算例
用户可以不考虑特征并明确的规定逆反应速率系数
辅助反应数据: REV/ Arev Brev Erev/
输运数据输入的例子
反应物名
AR AR* C C2 C2O CN2 C2H C2H2 C2H2OH CH2OH
线性
0 0 0 1 1 1 1 1 2 2
输出控制面板
敏感性分析选项
着火延迟定义选项
运行面板
当运行完全, 弹出图 像后处理程序 指定文件名 称
在运行前为反应模型 创建一个“关键词” 输入文件
补充输入, 如元素, 可 以增加到输入文件中
顶好的Chemkin学习资料

大型气相化学反应动力学软件CHEMKIN学习报告作者:范志林1 总体介绍CHEMKIN软件是美国Sandia国家实验室开发的大型气相化学反应动力学软件,可以用来解决带有化学反应的流动问题,是燃烧领域中普遍使用的一个模拟计算工具。
CHEMKIN3.7版本有多种针对不同模型的应用程序,包括AURORA、CRESLAF、EQUIL、OPPDIF、PASR、PLUG、PREMIX、SHOCK、SPIN、SURFTHERM、OVEND、TWAFER 12个应用程序,分别用来模拟充分搅拌反应器(AURORA)、圆柱形或平面形通道内的层流化学反应(CRESLAF)、化学平衡相平衡(EQUIL)、对流扩散火焰(OPPDIF)、部分搅拌反应器(PASR)、柱塞流反应器(PLUG)、一维稳态层流预混火焰(PREMIX)、冲击波化学动力学(SHOCK)、化学气相沉积滞留反应器(SPIN)、气相和表面化学系统的热化学传质及动力学(SURFTHERM)、多晶片低压化学沉淀反应器(OVEND)、用来确定多晶片低压化学沉淀反应中的温度(TWAFER)。
对大多数CHEMKIN应用程序而言,在应用之前需事先准备好三个输入文件:气相输入文件(gas chemistry input file)、表面反应输入文件(surface chemistry input file)和程序应用输入文件(application input file)。
输入文件默认为采用文本文档的形式给出,其中气相输入文件(gas chemistry input file)中指定了反应中元素组成、组分组成、各组分热力学参数、包括基元反应式、Arrhenius系数(pre-exponential factor、temperature exponent和activation energy)的反应机理;而程序应用输入文件(application input file)中则要根据实际情况来指定反应器的几何参数、问题类型、初始条件以及解文件控制参数等等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
CHEMKIN 使用方法
1打开CHEMKIN窗口
1)登陆Athena
2)在Athena界面上输入athena% add chemkin
athena% chemkin
3)接下来软件窗口打开
图1.Chemkin软件窗口
4)可以从Chemkin窗口选取需要应用的运行程序。
可利用的功能和可运用程序描述如下:
● Aurara: 完全混合反应模拟器
● Creslaf: 通道流体模拟器
● Equil: 平衡状态模拟器
● Oppdif: 两个对立喷嘴之间的火焰传播
● Plug: 化学反应器中的柱塞流模拟
● Premix: 稳态的,层流,一维预混合火焰模拟
● Senkin: 预测封闭系统中均相气态化学机理的敏感性分析
● Shock: 预测产物在入射激波和反射激波后的状态
● Spin: 模拟一维旋转反应器
● Surftherm: 分析气相和表面化学反应机理中和热力化学和动力学数据
在下一个部分我们将描述如何使用Equil应用程序。
其他应用程序可以以相类似的方法使用。
然而,Equil和其它的应用程序有一个本质的区别。
Equil应用程序不利用机理数据,而其它应用程序使用到。
2.如何使用Equil应用程序
Equil计算理想气体和溶液混合物的化学平衡状态
1)在Chemkin窗口中点击Equil按钮
2)窗口如图2所示
图2.Equil应用程序窗口
3)为了计算平衡状态,需要产生两个输入文件:chem.inp 和gas_equuil.inp。
4)如果你点击气相化学文件的编辑按钮,你可以看到和编辑的化学输入文件如图3所示。
化学输入文件包括元素和组分数据。
图3.化学输入文件
5)你可以创建你自己的文件和文件名来取代原有的默认的文件形式。
但是文件是在指定的路径中。
为了生成输入文件,或者使用文本编辑器在Athena和个人电脑上编辑和通过FTP 发送到Athena上。
6)接下来,你需要产生气相平衡输入文件。
当你点击Equil的编辑按钮,你将会见到图4
图4.气体平衡应用程序输入文件
图4中各个参数含义如下:
● REAC 代表反应物;由一个化学符号代表一种反应物和他们在混合物中的摩尔数。
图4中
的反应物是氢气和氧气还有氮气。
他们的摩尔数分别是:2,1,和 3.76。
● HP 意思是焓和压力是恒定的反应条件。
其它选项是可以有选择,例如EV等。
● TEMP指定反应起始温度(单位是K)
● TEST是指定反应平衡的温度(单位是K)
● CNTN是用来在结束键后用于不同的初始条件继续计算。
因此,CNTN被使用,就可以得
到另外一个解决途径。
在图4中,反应的起始温度是(键TEMP)的范围是300到400。
● END表示特殊的反应平衡计算的输入的结束
7)当然你也可以产生自己的气相输入文件文件,然后命名。
8)一旦化学和气相成分文件准备以后,你就可以计算化学平衡。
点击运行按钮,程序将会计算化学平衡条件。
9)当计算结束以后,点击平衡输出文件的查看按钮。
你将会见到以下的文本内容:。
