Vardenafil_224785-90-4_DataSheet_MedChemExpress
Naglazyme(galsulfase)(抗生物)商品说明书

Naglazyme® (galsulfase)(Intravenous)Document Number: MH-0084 Last Review Date: 02/01/2022Date of Origin: 11/28/2011Dates Reviewed: 12/2011, 02/2013, 02/2014, 12/2014, 10/2015, 10/2016, 10/2017, 10/2018, 02/2019,02/2020, 02/2021, 02/2022I.Length of AuthorizationCoverage will be provided for 12 months and may be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:•Naglazyme 5 mg vial: 23 vials per 7 daysB.Max Units (per dose and over time) [HCPCS Unit]:•115 billable units every 7 daysIII.Initial Approval Criteria 1Coverage is provided in the following conditions:•Patient is at least 5 years of age; AND•Documented baseline 12-minute walk test (12-MWT), 3-minute stair climb test (3-MSCT), and/or pulmonary function tests (e.g., FEV1, etc.); AND•Documented baseline value for urinary glycosaminoglycan (uGAG); ANDMucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome) † Ф1,4,5•Patient has a definitive diagnosis of MPS VI as confirmed by the following:o Detection of pathogenic mutations in the ARSB gene by molecular genetic testing; ORo Arylsulfatase B (ASB) enzyme activity of <10% of the lower limit of normal in cultured fibroblasts or isolated leukocytes; AND▪Patient has normal enzyme activity of a different sulfatase (excluding patients with Multiple Sulfatase Deficiency [MSD]); AND▪Patient has an elevated urinary glycosaminoglycan (uGAG) level (i.e. dermatan sulfate or chondroitin sulfate) defined as being above the upper limit of normal bythe reference laboratory†FDA-approved indication(s); ‡Compendia recommended indication(s); ФOrphan DrugIV.Renewal Criteria 1,4,5Coverage can be renewed based on the following criteria:•Patient continues to meet indication-specific relevant criteria such as concomitant therapy requirements (not including prerequisite therapy), performance status, etc. identified insection III; AND•Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: anaphylaxis and hypersensitivity reactions, immune-mediated reactions, acute respiratorycomplications associated with administration, acute cardiorespiratory failure, severeinfusion reactions, spinal or cervical cord compression, etc.; AND•Disease response with treatment as defined by improvement or stability from pre-treatment baseline by the following:o Reduction in uGAG levels; AND▪Improvement in or stability of 12-minute walk test compared (12-MWT); OR▪Improvement in or stability of 3-minute stair climb test (3-MSCT); OR▪Improvement in or stability of pulmonary function testing (e.g., FEV1, etc.)V.Dosage/Administration 1Indication DoseMucopolysaccharidosis VI(MPS VI, Maroteaux-Lamy Syndrome) 1 mg/kg administered as an intravenous (IV) infusion oncea weekVI.Billing Code/Availability InformationHCPCS Code:•J1458 – Injection, galsulfase, 1 mg; 1 billable unit = 1 mgNDC:•Naglazyme 5 mg per 5 mL solution; single-use vial: 68135-0020-xxVII.References1.Naglazyme [package insert]. Novato, CA; BioMarin Pharmaceutical Inc.; December 2019.Accessed January 2022.2.Giugliani R, Harmatz P, Wraith JE. Management guidelines for mucopolysaccharidosis VI.Pediatrics. 2007 Aug;120(2):405-18.3.Giugliani R, Federhen A, Rojas MV, et al. Mucopolysaccharidosis I, II, and VI: Brief reviewand guidelines for treatment. Genet Mol Biol. 2010 Oct;33(4):589-604. Epub 2010 Dec 1.4.Vairo F, Federhen A, Baldo G, et al. Diagnostic and treatment strategies inmucopolysaccharidosis VI. Appl Clin Genet. 2015 Oct 30;8:245-55.5.Valaannopoulos V, Nicely H, Harmatz P, et al. Mucopolysaccharidosis VI. Orphanet J RareDis. 2010; 5: 5.6.Harmatz P, Giugliani R, Schwartz I, et al. Enzyme replacement therapy formucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled,multinational study of recombinant human N-acetylgalactosamine 4-sulfatase(recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. JPediatr. 2006 Apr;148(4):533-539.Appendix 1 – Covered Diagnosis CodesICD-10 ICD-10 DescriptionE76.29 Other mucopolysaccharidosesAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National CoverageDetermination (NCD), Local Coverage Determinations (LCDs), and Local Coverage Articles (LCAs) may exist and compliance with these policies is required where applicable. They can be found at: https:///medicare-coverage-database/search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD/LCA): N/AMedicare Part B Administrative Contractor (MAC) JurisdictionsJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
阿昔洛韦杂质列表-标准品
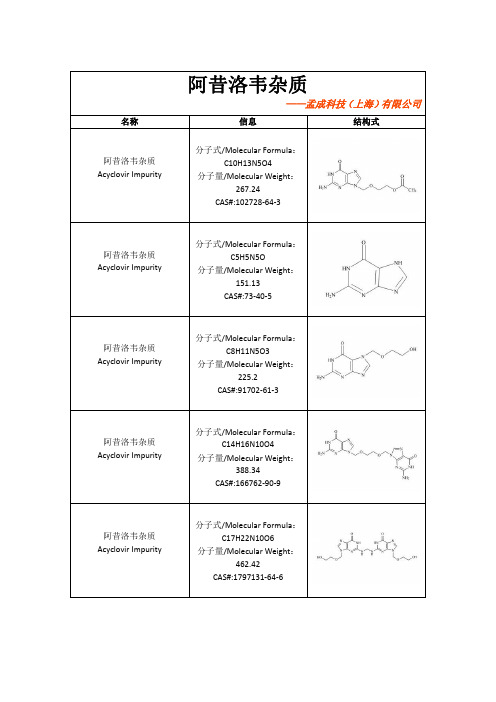
阿昔洛韦杂质——孟成科技(上海)有限公司名称信息结构式阿昔洛韦杂质Acyclovir Impurity分子式/Molecular Formula :C10H13N5O4分子量/Molecular Weight :267.24CAS#:102728-64-3阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C5H5N5O分子量/Molecular Weight :151.13CAS#:73-40-5阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C8H11N5O3分子量/Molecular Weight :225.2CAS#:91702-61-3阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C14H16N10O4分子量/Molecular Weight :388.34CAS#:166762-90-9阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C17H22N10O6分子量/Molecular Weight :462.42CAS#:1797131-64-6阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C12H15N5O5分子量/Molecular Weight :309.28CAS#:91702-60-2阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C7H9N5O2分子量/Molecular Weight :195.18CAS#:23169-33-7阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C8H11N5O3分子量/Molecular Weight :225.21阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C9H13N5O4分子量/Molecular Weight :255.24阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C17H22N10O6分子量/Molecular Weight :462.43阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C9H13N5O4分子量/Molecular Weight :255.24阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C12H15N5O5分子量/Molecular Weight :309.28CAS#:75128-73-3阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C9H9N5O3分子量/Molecular Weight :235.2CAS#:3056-33-5阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C10H13N5O4分子量/Molecular Weight :267.24CAS#:110104-37-5阿昔洛韦杂质Acyclovir Impurity 分子式/Molecular Formula :C14H16N10O4分子量/Molecular Weight :388.34CAS#:1797832-75-7。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
富马酸替诺福韦酯--------印度药典

NOTE - Prepare the solutions immediately before use. Test solution. Dissolve 100 mg of the substance under examination in 50 ml of methanol. Reference solution (a). A 0.2 per cent w/v solution of tenofovir disoproxil jitmarate RS in methanol. Reference solution (b). Dilute 1.0 ml ofreference solution (a) to 100.0 ml with methanol. Reference solution (c). Dissolve 10 mg ofthejUmaric acid in 50 ml of methanol.
TELMISARTAN TABLETS
IP 2010
Solvent mixture. 80 volumes of buffer solution prepared by diluting 5.0 ml of triethylamine to 2000ml with water and 20 volumes of methanol. Test solution. Weigh and powder 20 tablets. Disperse a quantity ofpowder containing about 100 mg ofTelmisartan in 100.0 ml ofsolvent mixture, sonicate for 45 minutes and filter. Reference solution. A 0.0005 per cent w/v solution of telmisartan RS in the solvent mixture.
Amtech Tacky 助焊膏系列安全数据表说明书

Inventec Performance Chemicals USA, LLCSAFETY DATA SHEET (SDS)SECTION 1: PRODUCT AND COMPANY IDENTIFICATIONPRODUCT NAME: Amtech Tacky Paste Flux Series: 200, 400, 500, 600, 4000, SynTECH, WSFC-305L and #61 SYNONYMS:Tacky FluxMANUFACTURER: Inventec Performance Chemicals USA, LLCADDRESS:PO Box 989 Deep River, CT 06417 USAPHONE:860-526-8300FAX:860-526-8243EMERGENCY:Infotrac-(800)535-5035REVISION DATE:December 19, 2014REVISION DATE: 3DOCUMENT NAME:SDS-Tacky Flux-008PRODUCT USE:Bonding solder joints in production and repair of circuit boardsSECTION 2: HAZARDS IDENTIFICATIONCHEMICAL NAME:N/ACHEMICAL FAMILY:MixtureCHEMICAL FORMULA:N/AROUTES OF ENTRY: Inhalation, Ingestion, Skin/Eye ContactGHS:Signal Word: WarningHazard statement(s)H302 Harmful if swallowedH317 May cause an allergic skin reactionH320 Causes eye irritationH335 May cause respiratory irritationPrecautionary statement(s)P102 Keep out of reach of childrenP233 Keep container tightly closedP264 Wash hands thoroughly after handlingP270 Do not eat, drink or smoke when using this productP280 Wear protective gloves/protective clothing/eye protection/face protectionP302+P352 IF ON SKIN: Wash with plenty of soap and waterP305+P351 IF IN EYES: Rinse continuously with water for several minutesP404 Store in a closed containerP501 Dispose of contents/containers in accordance with Federal, State/Provincial, and/or local regulations POTENTIAL HEALTH EFFECTS:EYE CONTACT: May cause moderate irritation. Do not allow material to come in contact with eyes.SKIN CONTACT: May cause moderate skin irritation.INHALATION: May cause irritation to the respiratory tract.INGESTION: Harmful if swallowed. May cause irritation to the mouth, throat, and stomach. May cause abdominal discomfort, nausea, vomiting, and/or diarrhea.CHRONIC: Not established.SECTION 2 NOTES:Inventec Performance Chemicals USA, LLC does not recommend, manufacture, market, or endorse any of its products for human consumption.SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTSIngredient CAS Number Exposure LimitsModified Rosins N/A N/APine Oil Derivatives 8000-41-7 N/AProprietary Ingredients N/A N/AMixed Carboxylic Acids N/A N/ASECTION 3 NOTES:Percentages of individual components are not listed as this information is considered a trade secret.SECTION 4: FIRST AID MEASURESEYES: Flush with plenty of water, contact a physician. If contact lenses can be removed easily, flush eyes without contact lenses. SKIN: Wash affected area with plenty of warm, soapy water. If irritation persists, seek medical attention.INGESTION: Call a physician or Poison Control Center immediately. Do not induce vomiting.INHALATION: Remove to fresh air. If not breathing, seek immediate medical attention.SECTION 5: FIRE-FIGHTING MEASURESEXTINGUISHING MEDIA: Dry chemical, foamSPECIAL FIRE FIGHTING PROCEDURES: Do not use water. Use NIOSH-approved self-contained Breathing Apparatusand full protective clothing if involved in a fire.UNUSUAL FIRE AND EXPLOSION HAZARDS:This product does not present any unusual fire and explosion hazards. SECTION 6: ACCIDENTAL RELEASE MEASURESACCIDENTAL RELEASE MEASURES: If material spills or leaks, collect and place into a properly labeled waste container. Remove traces of tacky flux using cloth rags or paper towels moistened with Isopropyl Alcohol. Follow on-site personal protective equipment recommendations.SECTION 6 NOTES:See Sections 2, 4, and 7 for additional information.SECTION 7: HANDLING AND STORAGEHANDLING/STORAGE: Keep containers tightly closed when not in use. Use care to avoid spills. Avoid inhalation of fumes or dust. Avoid contact with eyes, skin, and clothing.OTHER PRECAUTIONS: Empty containers may retain product residues in vapor, liquid, and/or solid form. All labeled hazard precautions should be observed.WORK HYGIENIC PRACTICES: Cosmetics/Food/Drink/Tobacco should not be consumed or used in work areas. Always wash hands after handling material and before applying or using cosmetics/food/drink/tobacco.SECTION 7 NOTES:For industrial use only.SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONVENTILATION: Provide sufficient mechanical (general and/or local exhaust) ventilation to maintain exposure below TLVs. RESPIRATORY PROTECTION: Use with adequate ventilation.EYE PROTECTION: Use with appropriate safety glasses.SKIN PROTECTION: Protective gloves and clothing should be worn when handling material. Wash hands thoroughly with soap and water upon leaving the work area.SECTION 9: PHYSICAL AND CHEMICAL PROPERTIESAPPEARANCE: Clear, White, or Yellow to Dark Amber gelODOR: Mild odorODOR THRESHOLD: Not establishedpH as SUPPLIED: N/ASECTION 9: PHYSICAL AND CHEMICAL PROPERTIES (continued)MELTING POINT: Not establishedFREEZING POINT: Not establishedINITIAL BOILING POINT: Not establishedBOILING RANGE: Not establishedFLASH POINT: Not establishedEVAPORATION RATE: Not establishedFLAMMABILITY (solid): Not establishedUPPER/LOWER FLAMMABILITY: Not establishedUPPER/LOWER EXPLOSIVE LIMITS:Not establishedVAPOR PRESSURE (mmHg): N/A (°F/°C)VAPOR DENSITY (AIR = 1): N/A (°F/°C)RELATIVE DENSITY: Not establishedSOLUBILITY IN WATER: PartiallyPARTITION COEFFICIENT (n-octanol/water): Not establishedAUTOIGNITION TEMPERATURE: Not establishedDECOMPOSITION TEMPERATURE: Not establishedVISCOSITY: N/A (°F/°C)SECTION 10: STABILITY AND REACTIVITYSTABILITY: StableCONDITIONS TO AVOID (STABILITY): Freezing temperatures. High temperatures. INCOMPATIBILITY (MATERIAL TO AVOID): Strong oxidizing materialsHAZARDOUS DECOMPOSITION/BY-PRODUCTS: Harmful organic fumes and toxic oxide fumes may form at elevatedtemperatures.POSSIBILITY OF HAZARDOUS REACTIONS: Will not occurSECTION 11: TOXICOLOGICAL INFORMATIONACUTE TOXICITY: Not availableSKIN CORRISION/IRRITATION: Not establishedSERIOUS EYE DAMAGE/IRRITATION: Not availableRESPIRATORY OR SKIN SENSITIZATION: Not establishedGERM CELL MUTAGENICITY: Not availableCARCINOGENICITY: Not availableREPRODUCTIVE TOXICITY: Not availableSTOT-SINGLE EXPOSURE: Not availableSTOT-REPEATED EXPOSURE: Not availableASPIRATION HAZARD: Not availableSECTION 12: ECOLOGICAL INFORMATIONTOXICITY: Product not testedPERSISTENCE AND DEGRADIBILITY: Product not testedBIOACCUMULATIVE POTENTIAL: Product not testedMOBILITY IN SOIL: Product not testedOTHER ADVERSE EFFECTS: Product not testedSECTION 13: DISPOSAL CONSIDERATIONSWASTE DISPOSAL METHOD: Scrap and waste solder should be stored in a dry, sealed container for later disposal. Disposal must be in accordance with Federal, State/Provincial, and Local Regulations.SECTION 14: TRANSPORT INFORMATIONTransport in accordance with applicable regulations and requirements.UN Number: Not availableUN Proper Shipping Name: Not availablePackaging Group:Not applicableEnvironmental Hazards:NoneTRANSPORT HAZARD CLASSES:US DOT Hazardous Material Classification: Tacky Flux is not listed as a DOT hazardous materialWater Transportation: Tacky Flux is not listed as a hazardous materialIATA Hazardous Material Classification: Tacky Flux is not listed as IATA hazardous materialSECTION 15: REGULATORY INFORMATIONAll ingredients used to manufacture this product are listed on the EPA TSCA Inventory.U.S. FEDERAL REGULATIONS: Not regulatedSTATE REGULATIONS: Not regulatedINTERNATIONAL REGULATIONS: Not regulatedSECTION 16: OTHER INFORMATIONHMIS Rating: Health=1 Flammability=1 Physical Hazard=0 Personal Protection=X KEY:N/A: Not applicableGHS: Global Harmonized SystemOSHA: Occupational Safety and Health AdministrationACGIH: American Conference of Governmental Industrial HygienistsNTP: National Toxicology ProgramIARC: International Agency for Research on CancerCAS: Chemical Abstract ServiceNIOSH: National Institute for Occupational Safety & HealthSTOT: Specific target organ toxicityTLV: Threshold limit valueUS DOT: United States Department of TransportationDOT: Department of TransportationIATA: International Air Transport AssociationEPA:Environmental Protection AgencyTSCA:Toxic Substance Control ActHMIS:Hazardous Material Identification SystemPREPARATION INFORMATION:This update supersedes all previously released documents.PREPARED BY: Wendy W. GesickAPPROVED BY: Leigh W. GesickDISCLAIMER:The information contained herein is based on data considered to be accurate but does not purport to be all-inclusive and shall be used only as a guide. No warranty is expressed or implied regarding the accuracy of this data and Inventec Performance Chemicals USA, LLC shall not be held liable for any damage resulting from any handling or contact with the above product. Liability is expressly disclaimed for loss or injury arising out of use of this information or the use of any materials designated. This material is not for resale, unauthorized distribution, or personal use.。
抗原恢复缓冲液(100X Tris-EDTA缓冲液,pH 9.0)说明书

Product nameAntigen Retrieval Buffer (100X Tris-EDTA Buffer, pH 9.0)Tested applicationsSuitable for: IHC-P General notes Antigen Retrieval Buffer (100X Tris-EDTA Buffer, pH 9.0) enables target retrieval in formalin-fixed,paraffin-embedded tissue sections in one step. It is optimal for use with primary antibodies thatrequire Tris-EDTA buffer (pH 9.0) pretreatment.This product contains detergent for emulsification of the paraffin.1X Dilution: The 100X stock solution should be diluted 100-fold with distilled water before use.Protocol:Place paraffin-embedded slides in 1x Antigen Retrieval Buffer; cover with a vented plasticwrap, place in microwave and set high power to boil and then set low power to keep itboiling for 10 min. Let the sections cool in the microwave for at least 20min.Wash sections with hot tap water for 1 minute.Wash sections in buffer for 2x3 minutes.Continue with IHC protocolOther kits and reagents for IHCOther antigen retrieval buffers include: Citrate buffer pH 6.0 ab93678, EDTA buffer pH 8.0ab93680, Tris buffer pH 10.0 ab93682, or see the full list of antigen retrieval buffers and enzymes .Find more kits and reagents for antigen retrieval, blocking, signal amplification, visualization,counterstaining, and mounting in the IHC kits and reagents guide .FormLiquid Storage instructionsStore at room temperature.Storage buffer pH: 8.7Constituents: 1.5% 2-butoxyethanol, 5% Sodium EDTA, 5% TrisBuffer 100x concentrated with detergent.Product datasheetAntigen Retrieval Buffer (100X Tris-EDTA Buffer, pH 9.0)ab936843 Abreviews 22 References 3 ImagesOverview PropertiesThe Abpromise guaranteeImmunohistochemistry (Formalin/PFA-fixed paraffin-embedded sections) - Antigen Retriev al Buffer (100X Tris-EDTA Buffer, pH 9.0) (ab93684)Image from M aniati et al., Cell Rep.;30(2):525-540.e7; doi: 10.1016/j.celrep.2019.12.034. Reproduced under the Creative Commons licensehttps:///licenses/by/4.0/Immunohistochemical for RUNX2 on murine omental metastasis Antigen retrieval: Tris-EDTA, pH;9 (ab93684), Blocking: Normal Goat Serum, Primary: Rabbit monoclonal anti-Runx2 [EPR14334] (ab192256), dilution 1:1000, Secondary: HRP anti-rabbitImmunohistochemistry (Formalin/PFA-fixed paraffin-embedded sections) - Antigen Retriev al Buffer (100X Tris-EDTA Buffer, pH 9.0) (ab93684)ab93684 Antigen Retrieval Buffer 100X Tris-EDTA Buffer, pH 9ApplicationsOur Abpromise guarantee covers the use of ab93684 in the following tested applications. The application notes include recommended starting dilutions; optimal dilutions/concentrations should be determined by the end user. Application Abreviews NotesIHC-P Use at an assay dependent dilution.ImagesImmunohistochemistry (Formalin/PFA-fixed paraffin-embedded sections) - Antigen Retriev al Buffer (100X Tris-EDTA Buffer, pH 9.0) (ab93684)Immunohistochemical analysis of paraffin-embedded mouse testis tissue labeling LINE-1 ORF1p with ab216324 at 1/500 dilution, followed by a ready to use Goat Anti-Rabbit IgG H&L (HRP). Cytoplasmic staining on spermatogonia of mouse testis (PMID: 24607009) is observed. Counterstained with hematoxylin. Secondary antibody only control: Used PBS instead of primary antibody, secondary antibody is a ready to use Goat Anti-Rabbit IgG H&L (HRP).Perform heat mediated antigen retrieval using ab93684 (Tris/EDTA buffer, pH 9.0).Please note: A ll products are "FOR RESEA RCH USE ONLY. NOT FOR USE IN DIA GNOSTIC PROCEDURES"Our Abpromise to you: Quality guaranteed and expert technical supportReplacement or refund for products not performing as stated on the datasheetValid for 12 months from date of deliveryResponse to your inquiry within 24 hoursWe provide support in Chinese, English, French, German, Japanese and SpanishExtensive multi-media technical resources to help youWe investigate all quality concerns to ensure our products perform to the highest standardsIf the product does not perform as described on this datasheet, we will offer a refund or replacement. For full details of the Abpromise, please visit https:///abpromise or contact our technical team.Terms and conditionsGuarantee only valid for products bought direct from Abcam or one of our authorized distributors。
Sigma-Aldrich 乙二胺四乙酸安全技术说明书

SIGMA-ALDRICH 化学品安全技术说明书按照GB/T 16483、GB/T 17519编制乙二胺四乙酸SDS 编号Sigma-Aldrich - E9884产品编号Sigma-Aldrich - E9884版本6.5修订日期20.11.2017打印日期17.10.2018最初编制日期27.05.20171. 化学品及企业标识1.1 产品标识产品名称: 乙二胺四乙酸Ethylenediaminetetraacetic acid产品编号: E9884品牌: Sigma-Aldrich化学文摘登记号(CAS No.): 60-00-41.2 安全技术说明书提供者的详情制造商或供应商名称: Sigma-Aldrich (Shanghai) TradingCo. Ltd. (China)41F, K WAH CENTRE1010 HUAI HAI ZHONG ROADSHANGHAI200031 SHANGHAICHINA西格玛奥德里奇(上海)贸易有限公司中国上海市淮海中路1010号嘉华中心41层邮政编码:200031电话号码: +86 86 21 6141-5566传真: +86 86 21 6141-55671.3 应急咨询电话紧急联系电话: +8621-614155601.4 有关的确定了的物质或混合物的用途和建议不适合的用途已确认的各用途: 仅用于研发。
不作为药品、家庭或其它用途。
2. 危险性概述Sigma-Aldrich- 页码 1 8Sigma-Aldrich -页码 2 82.1GHS 危险性类别眼睛刺激 (类别 2A ), H319本部分提及的健康说明(H-)全文请见第16部分。
2.2GHS 标签要素,包括防范说明 象形图信号词 警告危险申明 H319 造成严重眼刺激。
警告申明 预防措施 P264 作业后彻底清洗皮肤。
P280戴防护眼罩/戴防护面具。
事故响应P305 + P351 + P338 如进入眼睛:用水小心冲洗几分钟。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
Crafter’s Choice Moonlight Path 香料油商品说明书
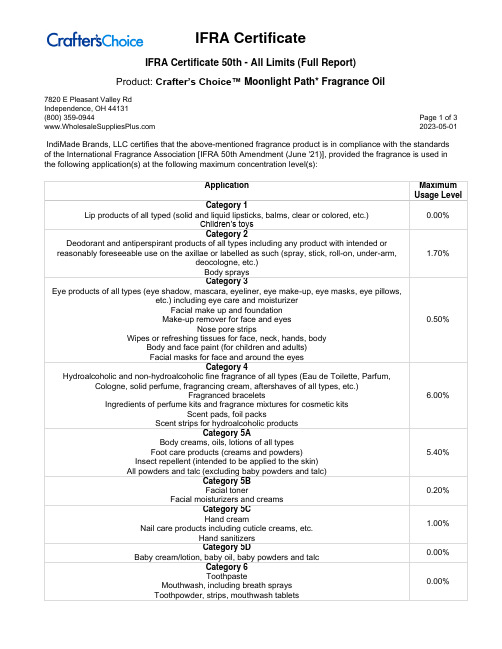
Product: Crafter’s Choice™ Moonlight Path* Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 1 of 3 2023-05-01 IndiMade Brands, LLC certifies that the above-mentioned fragrance product is in compliance with the standards of the International Fragrance Association [IFRA 50th Amendment (June '21)], provided the fragrance is used in the following application(s) at the following maximum concentration level(s):Product: Crafter’s Choice™ Moonlight Path* Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 2 of 3 2023-05-01Product: Crafter’s Choice™ Moonlight Path* Fragrance Oil7820 E Pleasant Valley RdIndependence, OH 44131(800) 359-0944 Page 3 of 3 2023-05-01For all other applications, or use at higher concentration levels, a new evaluation will be required.The IFRA standards regarding use restrictions are based on safety assessments by the Research Institute for Fragrance Materials (RIFM) Expert Panel (REXPAN) and are enforced by the IFRA Scientific Committee. Evaluation of individual fragrance materials is made according to the safety standards contained in the relevant section of the IFRA Code of Practice.It is the ultimate responsibility of the customer to ensure the safety of the final product containing this fragrance, by further testing, if necessary.The above-mentioned fragrance product contains ingredients which are NOT considered GRAS, Generally Regarded as Safe as a Flavor Ingredient.。
芯片质量 Tadiran Batteries 非可重充式锂酯电池信息表说明书

SDS No.- T-36-04 (Revision–M)Lithium Battery Information Sheet1.Section 1: IdentificationProducts Name: Primary (non-rechargeable) Lithium metal Thionyl Chloride (Li/SOCl2) cells and batteries. Cells include the models of TL, TLH, and TLL, 3.6V series. This Battery Information Sheet covers the the above models: 2100, 2134, 2135, 2137, 2150, 2155, 2186, 2200, 2300, 2450, 4902, 4903, 4920, 4930, 4934, 4935, 4937, 4940, 4951, 4955, 4986, 5101, 5104, 5113, 5114, 5134, 5135, 5137, 5151, 5155, 5186, 5233, 5242, 5276, 5293, 5315, 5902, 5903, 5920, 5930, 5934, 5935, 5937, 5940, 5955, 5323, 7902, 7955, 7903, 7920, 7930, 7937, 8902, 8955, 8903, 8920, 8930, and 8937 with all their finishing versions and batteries assembled from them, denoted by “/” followed by letters or digits.Manufacturer Name: Tadiran Batteries Ltd., P. O. Box 1, Kiryat Ekron, Israel 70500.US office address: 2001 Marcus Avenue, Suite 125E, Lake Success, NY 11040 Emergency Telephone No: CHEMTREC: 1-800-424-9300Tel. for information: 1-516-621-4980Tel. for information 972-8-944-45032.The Lithium Thionyl chloride batteries described in this Battery Information Sheet are hermetically sealed units, which are not hazardous when used according to the recommendations of the manufacturer.Under normal condition of use of the batteries, the electrode materials and the liquid electrolyte they contained are non-reactive provided the battery integrity is maintained. Risk of exposure exists only in case of mechanical, electrical or thermal abuse. Thus the batteries should not short circuit, recharge, puncture, incinerate, crush, immerse in water, force discharge, or expose to temperatures above the temperature range of the cell or battery. In these cases there is risk of fire or explosion.Protection from charging:Whenever lithium batteries are not the single power source in a circuit, Whenever lithium batteries are not the single power source in a circuit, the measures recommended by Underwriters Laboratories are relevant. The relevant protection means should be recommended/approved by TADIRAN.* TLV- Threshold Limit Value is personal exposure limits determined by ACGIH (American Council of Governmental Industrial Hygienists).IMPORTANT NOTE: The above levels are not anticipated under normal use conditions.4.Section 4: First aid measuresIn case of battery rupture, explosion, or major leakage, evacuate personnel from contaminated area and provide good ventilation to clear out corrosive fumes, gases or the pungent odor. Seek immediate medical attention.Eyes - First rinse with plenty of water for 15 minutes (remove contact lenses if easily possible), and then seek medical attention.Skin - Remove contaminated clothes and rinse skin with plenty of water or shower for 15 min. Refer to medical attention.Inhalation - Remove to fresh air, rest, and half-upright position, use artificial respiration if needed, and refer to medical attention.Ingestion - rinse mouth, DO NOT induce vomiting, give plenty of water to drink, and refer to medical attention.5.Section 5: Fire - fighting measuresFLASH POINT: NA LOWER (LEL): NA FLAMMABLE LIMIT IN AIR: NA UPPER (LEL): NA EXTINGUISHING MEDIA:1. Lith- X (Class D extinguishing media) is the only effective on fires involving a few lithium batteries. If the cells are directly involved in a fire DO NOT USE: WATER, SAND, CO2, HALON, and DRY POWDER OR SODA ASH EXTINGUISHERS.2. If the fire is in adjacent area and the cells that are either packed in their original containers or unpacked, the fire can be fought based on fueling material, e.g., paper and plastic products. In these cases the use of copious amounts of cold water is effective extinguishing media. Storage area may also employ sprinkler system with cold water.AUTO-IGNITION: NASPECIAL FIRE FIGHTING PROCEDURES: Wear self-contained breathing apparatus to avoid breathing of irritant fumes (NIOSH approved SCBA & full protective equipment). Wear protective clothing and equipment to prevent body contact with electrolyte solution.Fire may be fought, but only from safe fire-fighting distance. Evacuate all persons from immediate area of fire.UNUSUAL EXPLOSION AND FIRE EXPLOSION: Battery may explode when subject to: excessive heat (above 150⁰C), recharged, over-discharged (discharge below 0V), punctured and crushed. During thermal decomposition generation of chlorine (Cl2), hydrogen chloride (HCl), and sulfur dioxide (SO2) can be formed.6.PROCEDURES TO CONTAIN AND CLEAN UP LEAKS OR SPILLS: The material contained within the battery would only be released under abusive conditions.In the event of battery rapture and leakage: contain the spill while wearing proper protective clothing and ventilate the area. Then, cover with sodium carbonate (Na2CO3) or 1:1 mixture of soda ash and slaked lime. Keep away from water, rain, and snow. Placed in approved container (after cooling if necessary) and disposed according to the local regulations. NEUTRALIZING AGENTS: Sodium carbonate (Na2CO3) or 1:1 mixture of soda ash and slaked lime. WASTE DISPOSAL METHOD: Product decomposed by water must be neutralized. if sufficiently diluted, it may be added to waste water if it is sufficiently diluted.PRECAUTIONS IN HANDLING AND STORAGE: avoid short-circuiting, over-charging and heating to high temperatures. Store the batteries in dry and cool area and keep container dry and tightly closed in well-ventilated area. Store cells away from food and drink.OTHER PRECAUTIONS; Never attempt to disassemble, machine, or otherwise modify batteries or injury may result.7.The batteries should not be opened, destroyed or incinerate, since they may leak or rupture and release to the environment the ingredients that they normally contained in the hermetically sealed container.HANDLING- Do not short circuit terminals, or expose to temperatures above the temperature rating of the battery, over charge the battery, forced over-discharge (voltage below 0.0V), throw to fire.Do not crush or puncture the battery, or immerse in liquids.STORAGE- is preferably done in cool (below 30⁰C), dry and ventilated area, which is subject to little temperature change.Do not place the battery near heating equipment, nor expose to direct sunlight for long periods. Elevated temperatures can result in shortened battery life and degrade performance.Keep batteries in original packaging until use and do not jumble them.Do not store batteries in high humidity environment for long periods.OTHER- cells and batteries are not rechargeable batteries and should not be charged. Applying pressure and deforming the battery may lead to disassembly followed by eye skin and throat irritation.Follow manufacturer recommendations regarding maximum recommended current and operating temperature range.GENERAL- The following safety measures are not necessary in normal use. They need only be applied if there is a risk that, in use or handling, the recommendations, as outlined in Section 3, have not been followed.RESPIRATORY PROTECTION: In case of abuse or leak of liquid or fumes, use NIOSH approved Acid Gas Filter Mask or Self-Contained Breathing Apparatus.VENTILATION: In case of abuse, use adequate mechanical ventilation (local exhaust) for battery that vents gas or fumes.PROTECTIVE GLOVES: In case of spill use PVC or Nitrile gloves of 15 mils (0.015 inch) or thicker. EYE PROTECTION: Use ANSI approved chemical worker safety goggles or face shield.OTHER PROTECTIVE EQUIPMENT: In case needed, chemical resistance clothing is recommended along with eye wash station and safety shower should be available meeting ANSI design criteria. WORK HYGIENIC PRACTICES: Use good hygiene practice. Wash hands after use and before drinking, eating or smoking. Launder contaminated cloth before reuse. SUPPLEMENTARY SAFETY AND HEALTH DATA: If the battery is broken or leaked the main hazard is the electrolyte. The electrolyte is mainly solution of Lithium chloride (LiCl), and aluminum chloride (AlCl3) in Thionyl chloride (SOCl2).Fires may be fought but only from safe fire fighting distance, evacuate all persons from immediate area of fire. Prevent heating of the battery, charging the battery, discharge to predetermined limit, do not crush, disassemble, incinerate or short circuit.9.Section 9: Physical and chemical propertiesBoiling point (760 mm Hg) NA, unless individual components exposed Vapor Pressure (mm Hg, 25ºC) NA, unless individual components exposed Vapor Density (air=1) NA, unless individual components exposed Density (gr/cc) > 1 gr/ccVolatile by Volume (%) NAEvaporation Rate (butyl acetate=1) NA, unless individual components exposed Physical State SolidSolubility in Water (% by weight) NA, unless individual components exposed PH NA, unless individual components exposed Appearance Geometric Solid ObjectOdor If leaking, gives off pungent corrosive odor10.Section 10: Stability and reactivitySTABLE OR NOT STABLE StableINCOMPATIBILITY (MATERIAL TO AVOID) Strong mineral acids, water and alkali solutions.HAZARDOUS DECOMPOSITION PRODUCTS 1. Reaction of lithium with water: Hydrogen (H2), Lithium hydroxide (LiOH).2. Thermal decomposition over 150⁰C: Sulfur oxides, (SO2, SO3), Sulfur chlorides (SCl2, S2Cl2), Chlorine (Cl2), Lithium oxide, Li2O3. Electrolyte with water: Hydrogen Chloride (HCl) and SO2DECOMPOSITION TEMPERATURE (⁰F) NAHAZARDOUS POLYMERIZATION: May Occur____ Will Not Occur __X__ CONDITIONS TO AVOID Avoid mechanical abuse and electrical abuse such as short-circuiting, overcharge, over-discharge, (voltage reversal)and heating.THRESHOLD LIMIT VALUE (TLV) AND SOURCE: NAHEALTH HAZARD ACUTE AND CHRONIC: Inhalation, skin contact, eye contact and ingestion are not likely by exposure to sealed battery.Inhalation, skin contact and eye contact are possible when the battery is opened. Exposure to internal contents, the corrosive fumes will be very irritating to skin, eyes and mucous membranes. Overexposure can cause symptoms of non-fibrotic lung injury and membrane irritation.Carcinogenicity- NTP: NoCarcinogenicity- IARC: NoCarcinogenicity- OSHA: NoExplanation of Carcinogenicity- No ingredient of a concentration of 0.1% or greater is listed as a carcinogen or suspected carcinogen.SIGNS AND SYMPTOMS OF OVEREXPOSURE: Exposure to leaking electrolyte from ruptured or leaking battery can cause:For further information refer to section 4.12.Section 12: Ecological information1.When properly used or disposed the battery does not present environmental hazard.2.Cells do not contain mercury, cadmium, lead or other heavy metals.3.Do not let internal components enter marine environment. Avoid release to waterways,wastewater or ground water.13.Section 13: Disposal Considerations4.Dispose in accordance with the applicable regulations in country and state.5.Disposal should be performed by permitted, professional disposal firms knowledgeable inFederal, State or Local requirements of hazardous waste treatment and hazardous waste transportation.6.Incineration should never be performed by battery users, but eventually by trainedprofessional in authorized facility with proper gas and fume treatment.7.Battery recycling should be done in authorized facility.14.Section 14: Transport informationShipping name:UN 3090: Lithium Metal Cells and BatteriesUN 3091: Lithium Metal Cells and Batteries contained in equipment, orLithium Metal Cells and Batteries packed with equipmentShipping information: the cells and batteries have passed successfully the tests defined in “UN Manual of Tests and Criteria”, Section 38.3 (the UN tests). The cells and batteries must be packed in accordance with Packing Instructions / Special Provisions (SP) of the applicable code, e.g., IATA (57th revised edition)/ICAO (Packing Instructions: PI968, PI969 and PI970), IMDG Code (SP188) and ADR (SP188). When required, reference shall be made to UN number 3090 (lithium ion batteries) or UN 3091 when packed with or contained in equipment.Transportation within, to and from the US: are governed by the US DOT CFR 49, Parts 171, 172, 173 and 175. They details the required packaging and labels and transportation mode of cells transported separately or in equipment. The battery cannot be shipped, within, to, and from the US by passenger aircraft. Air shipments of cells can be done only by cargo aircraft.Hazard Classification: Class 9Packing Group: N/ABattery label: Identification and labeling should be in compliance with the applicable regulations. In addition, it should also include cell/battery title, nominal voltage, lot number and warning.15.1.All the cells and batteries are defined as “articles” and thus are exempt from therequirements of the Hazard Communication Standard”.2.The internal component (Thionyl chloride) is hazardous under the criteria of the FederalOHSA Hazard Communication Standard 29 CFR 1920.1200.3.NFPA rating- Lithium batteries are not included in the NFPA material list. Below is the NFPArating for lithium metal. Lithium metal is an internal component, enclosed by hermetically sealed metallic can. Under normal application is not exposed.16.Section 16: Other informationThe information and the recommendations set forth are made in good faith and believed to be accurate at the date of preparation. The present file refers to normal use of the product in question. Tadiran Batteries makes no warranty expressed or implied.Assembly of battery packs:The design and assembly of battery packs require special skills, expertise and experience. Therefore it is not recommended that the end user will attempt to self-assemble battey packs. It is preferable that any battery using lithium cells will be assembled by TADIRAN to ensure proper battery design and construction. A full assembly service is available from TADIRAN who can be contact for further information. If for any reason, this is not possible, TADIRAN can review the pack design in confidential to ensure that the design is safe and capable of meeting the stated performance requirements.。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
Perkadox 16产品数据表说明书

Product Data SheetPerkadox 16Di(4-tert-butylcyclohexyl) peroxydicarbonatePerkadox® 16 is applied as an initiator for the suspension and mass polymerization of vinyl chloride in the temperature range between 40°C and 65°C. Perkadox® 16 can be used alone or in combination with other peroxides, such as 1,1,3,3-Tetramethylbutyl peroxyneodecanoate (Trigonox 423), Cumyl peroxyneodecanoate (Trigonox 99) or Dilauroyl peroxide (Laurox), to increase reactor efficiency.CAS number15520-11-3EINECS/ELINCS No.239-557-1TSCA statuslisted on inventoryMolecular weight398.5SpecificationsAppearance White powderAssay94.0-97.0 %Inorganic + organic hydrolysable chloride≤ 4000 mg/kgCharacteristicsBulk density, 20 °C450-480 kg/m³Density, 20 °C 1.13 g/cm³ApplicationsPerkadox® 16 can be used for the market segments: polymer production, thermoset composites and acrylics with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Perkadox® 16 in chlorobenzene:0.1 hr82°C (180°F)1 hr64°C (147°F)10 hr48°C (118°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa126.39 kJ/moleA7.44E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT40°CEmergency temperature (Tₑ)35°CControl temperature (Tc)30°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria – United Nations,New York and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts max.20°C (please see note below)Note When stored under the recommended storage conditions, Perkadox® 16 willremain within the Nouryon specifications for a period of at least 3 months afterdelivery. The Ts max of 20°C is not to be interpretated as ambient or roomtemperature as this differs per region and season. Perkadox® 16 has a high qualitycomposition and to hold that it should be stored at below 20°C. At temperaturesabove 20°C the decomposition of Perkadox® 16 progresses fast which leads tosignificant loss of quality. If you have questions about this, please contact yourlocal Nouryon account manager for advice.Packaging and transportIn North America Perkadox® 16 is packed in non-returnable cartons containing 25 polyethylene bags of 1 lb net weightor 5 polyethylene bags of 5 lb net weight. In other regions the standard packaging is a cardboard box for 20 kg peroxide. Both packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Perkadox® 16 is classified as Organic peroxide type C; solid, temperature controlled; Division 5. 2; UN 3114.Safety and handlingKeep containers tightly closed. Store and handle Perkadox® 16 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Perkadox® 16. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition products Carbon dioxide, 4-tert-Butyl-cyclohexanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Perkadox® and Trigonox are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2023-1-10© 2023Polymer production Perkadox 16。
CleanGredients 产品指南:更安全的选择成分说明书

Second generation bio-based water soluble polymer which is more sustainably sourced and readily biodegradable.
• Anti-redeposition, anti-encrustation and anti-film forming • Best-in-class readily biodegradable water soluble polymer • Dispersant and anti-scalent with improved calcium, iron and
• Available as aqueous solution or powder
Unique, high-purity hydrotroping acrylic/styrene copolymer.
• Hydrotroping of surfactants into heavy duty liquids • Particulate and oily soil dispersion/suspension • Protective corrosion inhibition • Hypochlorite stable • Calcium binding
• Hard surface cleaning • Vehicle cleaning • Water based alkaline cleaners • Microemulsions • All Purpose Cleaners (APC)
• All Purpose Cleaners (APC) • Hydrotroping • Acidic and alkaline cleaners
Sigma-Aldrich实验室常用生化试剂大促销

缓冲液
产品货号 英文品名 中文品名 优惠价 (R M B ) 目录价 (RMB)
A1542-2.5KG A1542-250G A1542-500G B7901-1KG B7901-500G C3041-100CAP C3041-50CAP C3674-100G C3674-1KG C3674-500G E9508-100ML E9508-10UL E9508-1L E9508-2.5L E9508-500ML E6758-100G E6758-500G H3375-100G H3375-1KG H3375-250G H3375-25G H3375-500G H3375-5KG I0125-100G I0125-10G I0125-1KG I0125-25G I0125-500G I0125-5KG M2933-100G M2933-1KG M2933-25G M2933-500G M1254-100G M1254-1KG M1254-250G M1254-25G M1254-50KG M1254-5KG P5493-1L P5493-4L P4809-100TAB P4809-50TAB
Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose
低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖
Ammonium acetate ~98% Ammonium acetate ~98% Ammonium acetate ~98% Boric acid Boric acid Carbonate-Bicarbonate Buffer Carbonate-Bicarbonate Buffer Citric acid trisodium salt Citric acid trisodium salt Citric acid trisodium salt Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethylenediaminetetraacetic acid >=98.5% Ethylenediaminetetraacetic acid >=98.5% HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) MES hydrate >=99.5% MES hydrate >=99.5% MES hydrate >=99.5% MES hydrate >=99.5% MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) Phosphate buffered saline Phosphate buffered saline Phosphate-Citrate Buffer Phosphate-Citrate Buffer
荧光吸收光谱和发射光谱(6)

Fluorochrome Absorption and Emission SpectraHere we present the absorption and emission spectra of the fluorochromes BD Biosciences Pharmingen conjugates to monoclonal antibodies and other proteins. Four of these fluorochromes, fluorescein isothiocyanate (FITC), R-phycoerythrin (R-PE), BD Cy-Chrome™, and PerCP, can be used with single-laser flow cytometers equipped with an argon-ion laser emitting light at 488 nm for three-color flow cytometric analysis (Fig. 1).BD Biosciences Pharmingen also offers monoclonal antibodies and proteins conjugated to allophycocyanin (APC) and avidin conjugated to Texas Red™. Both of these dyes are useful in experiments where multi-color analysis is desired using flow cytometers with dual laser capabilities (Fig 1). APC can b e excited by a helium-neon (HeNe) laser emitting light at 633 nm, by a krypton laser emitting light at 647 nm, or by a dye laser which can be conveniently tuned to emit light in the 550-650 nm range. (In a dye laser, the lasing medium is a solution of fluo rescent dye excited by a pump laser, usually an ion laser.) Texas Red ™ , available conjugated to avidin, can be excited by an argon-krypton mixed-gas laser at 568 nm, or with a dye laser, where both APC and Texas Red ™ can be used simultaneously. Because many combinations of lasers, detectors, filters and fluorochromes are possible for multi-color analysis, proper precautions need to be taken (i.e.,bandpass filters, dichroic mirrors, longpass filters, etc.) by the operator to ensure each fluorochrome is being detected by only one detector (Fig. 1). In the following descriptions, we give our recommendations for the ideal instrument set-up for use with our reagents.(summarized in Table1) Allophycocyanin (APC) is an accessory photosynthetic pigment found in bluegreen algae. Its molecular weight is approximately 105 kDa. APC has 6 phycocyanobilin chromophores per molecule, which are similar in structure to phycoerythrobilin, the chromophore in R-PE. It has a 650-nm wavelength absorption maximum (Fig. 2) and a 660-nm fluorescence emission maximum (Fig. 2). Using a 660 ± 10 nm BP filter will give optimum detection for this fluorochrome. APC can be used in flow cytometers equipped with dual lasers for multi-color analysis (Fig. 2). It can be excited by laser light between 600-640 nm. For this, we recommend a He-Ne laser at 633 nm, or a tunable dye laser tund between 600-640 nm.BD Cy-Chrome™ is a tandem conjugate system, with an absorption maximum of approximately 650 nm (Fig. 2), which combines R-phycoerythrin and a cyanine dye (MW 1.5 kDa). When excited by 488-nm light, the excited fluorochrome (R-PE) is able to transfer its fluorescent energy to the cyanine molecule, which then fluoresces at a longer wavelength. The resulting fluorescent maximum is approximately 670 nm (Fig. 2). Using a 650-nm longpass filter will give optimum detection for this fluorochrome. The efficiency of the light energy transfer between the two fluorochromes can be seen in Fig. 2F where less than 5% of the absorbed light is lost as fluorescence at 575 nm by R-PE. Compared to other fluorescence energy-transfer systems used in flow cytometry (e.g., RED613™ , EDC, PerCP), BD Cy-Chrome™ is a superior fluorochrome for third color analysis because of its high emission intensity and broad spectrum. As with our R-PE conjugates, an average of one BD Cy-Chrome™ molecule is coupled per antibody or protein. Because of its broad absorption range (Fig. 2), BD Cy-Chrome™ is not recommended for use with du al-laser flow cytometers where excitation by both lasers is possible.Fluorescein isothiocyanate (FITC) is a fluorochrome with a molecular weight of 389 daltons and an absorption maximum at 495 nm (Fig. 2). Its excitation by 488-nm light leads to a fluorescence emission maximum around 520 nm (Fig. 2). Using a 530 ± 15 nm bandpass (BP) filter will give optimum detection for this fluorochrome. The isothiocyanate derivative (FITC) is the most widely used form for conjugation to antibodies and proteins, but other derivatives are available. FITC has a high quantum yield (efficiency of energy transfer from absorption to emission fluorescence) and approximately half of the absorbed photons are emitted as fluorescent light. The number of FITC molecules per conjugate partner (antibody, Avidin, Streptavidin, etc.) is usually in the range of three to five molecules.R-phycoerythrin (R-PE) is an accessory photosynthetic pigment found in red algae. In vivo, it functions to transfer light energy to chlorophyll during photosynthesis. In vitro, it is a 240-kDa protein with 34 phycoerythrobilin fluorochromes per molecule. The large number of fluorochromes per PE molecule makeR-phycoerythrin an ideal pigment for flow cytometry applications. Its absorption maximum is 564 nm (Fi g. 2). When excited by 488-nm light, its fluorescence emission maximum is approximately 575 nm (Fig. 2). For single-laser flow cytometer use, we recommend using a 585 ± 21 nm BP filter for optimal detection (Fig. 1). When performing multi-color analysis with a dual-laser system, a tighter window of detection is required to compensate for the other conjugates being used (e.g.,Texas Red ™ ). For this, we recommend using a 575 ± 13-nm BP filter (Fig.1). Our conjugation chemistry yields an average of one R-PE molecule per antibody orprotein. The emitted light is collected in the fluorescence-2 (FL2) channel.PE-Texas Red™ is a tandem conjugate system which combines R-PE and Texas Red™ and has an absorption maximum of approximately 564 nm. When excited by 488-nm light, the excited fluorochrome (PE) is able to transfer its fluorescent energy to the Texas Red™ molecule, which then fluoresces at a longer wavelength. The resulting fluorescent emission maximum is approximately 615 nm. Special care must be taken when using PE-Texas Red™ conjugates in conjunction with R-PE as there is considerable spectral overlap in the emission profiles of both fluorochromes.Peridinin chlorophyll protein (PerCP) is a component of the photosynthetic apparatus found in the dinoflagellate,Glenodinium . PerCP is a protein complex with a molecular weight of approximately 35 kDa. When excited by light at 488 nm from an argon-ion laser, PerCP has a excitation maximum around 490 nm, with an emission spectrum which peaks at 675 nm. The emitted light is collected in the fluorescence-3 (FL3) channel. Due to its photobleaching characteristics, PerCP conjugates are not recommended for use on stream-in-air flow cytometers.PerCP-Cy5.5 is a tandem conjugate system than combines PerCP with a cyanine d ye (Cy5.5™) and has an absorption maximum of approximately 490 nm. When excited by 488-nm light, the excited fluorochrome (PerCP) is able to transfer its fluorescent energy to the cyanine molecule, which then fluoresces at a longer wavelength. The resulting fluorescent emission maximum is approximately 694 nm. Using a 650 nm longpass filter will give optimum detection for this fluorochrome. The emitted light is collected in the fluorescence-3 (FL3) channel. PerCP-Cy5.5 is recommended for use with stream-in-air flow cytometers. APC-Cy7is a tandem conjugate system that combines APC and a cyanine dye (Cy7™) and has an absorption maximum of approximately 650 nm. When excited by light from a dye or HeNe laser, the excited fluorochrome (APC) is able to transfer its fluorescent energy to the cyanine molecule, which then fluoresces at a longer wavelength. The resulting fluorescent emission maximum is approximately 767 nm. It is recommended that a 750-nm longpass filter be used along with a red-sensitive detector such as the Hammatsu R3896 PMT for this fluorochrome. Special filters are required when using APC-Cy7ª in conjunction with APC. It is recommended that special precautions be taken with PharRed conjugates, and cells stained with them, to protect the fluorochrome from long-term exposure to visible light.Texas Red™ is a sulfonyl chloride derivative of sulforhodamine 101 with a molecular weight of 625 daltons. BD Biosciences Pharmingen offers Texas Red ™ , conjugated to avidin, as a useful second step for multi-color analysis. Because it em its in the long wavelengths of the deep red region (Fig 2), Texas Red ™ has little spectral overlap with FITC. When performing multi-color analysis involving both Texas Red™ and R-PE, BD Biosciences Pharmingen recommends excitation of Texas Red ™ using a d ual-laser flow cytometer equipped with a tunable dye laser to avoid "leaking" into the PE detector. If a krypton laser, emitting light at 568 nm, is used, the laser light will "leak" into the R-PE channel. Texas Red ™ can be used in conjunction with APC for multi-color analysis when both dyes are excited in the 595-605 nm range with a dye laser. Texas Red ™ has an absorption maximum of 596 nm (Fig. 2). Its emission maximum, when excited by 595-600-nm laser light, is 615 nm (Fig. 2). Using a 620 ± 10-nm bandpass filter will give optimum detection for this fluorochrome (Fig. 1).Comparative staining using a monoclonal antibody (RA3-6B2; anti-B220; Cat. No. 557390/553084**) conjugated to different fluorochromes and analyzed on either BD FACSVantage™ (upper panels) or BDFACSCalibur™ (lower panels). The numbers indicate the ratio of the median fluorescence intensity of positive cells to the negative cells (signal to noise ratio). These plots demonstrate how choices in A) fluorochrome-conjugates or B) instrumentation can affect the fluorescence intensity observed for a given population.A. The differences observed between individual fluorochrome-conjugates can be affected by the mAbconjugated. Thus while in the example above the PE-conjugate is brighter than the BD Cy-Chrome™ -conjugate, when analyzed on the BD FACSCalibur™ , for many mAbs the Cy-Chrome ™ -conjugate results in a brighter stain. Contact BD Biosciences Pharmingen Technical Services for more information on specific reagents.B. Similarly different flow cytometers utilize different lasers and different fluorescence filter sets whichcan result in differences in signal to noise ratios when using the same reagent. Note that PEreagents tend to be brighter when used on a BD FACSCalibur™ while APC reagents are brighter on a BD FACSVantage™. Note changes of the signal to noise ratio depending on fluorchrome and instrument used.Enlarge imageFigure 1."Top schematic." A single laser flow cytometer with five parameters of detection. Two detectors detect the light scatter, and three photo-multiplier tubes (PMTs) detect the fluorescent signals. The bandpass filters are set up for optimal detection with BD Biosciences Pharmingen's fluorochromes: FITC, PE, BD Cy-Chrome ™ and Becton Dickinson's PerCP."Bottom schematic." A dual laser flow cytometer with six parameters of detection. Two detectors detect the light scatter, and four PMTs detect the fluorescent signals. The bandpass filters are set up forop timal detection with BD Biosciences Pharmingen's fluorochromes FITC, PE, APC, and Texas Red ™ . The second (orange) laser light is emitted from a tunable dye head using rhodamine 6 G as the fluorescent dye for excitation. Forward light scatter (FSC), side scatter (SSC), FITC, and PE signals are all produced by the primary 488-nm argon-ion laser. APC and Texas Red ™ signals are produced by the second laser (dye head with a 488-nm argon-ion laser).Figure 2. Absorption spectra of Fluorochromes. Individual fluorochrome excitation spectra are found in gray and the corresponding emission spectra in black. Typical band pass filters are given for eachflu orochrome as used on a FACSVantage™ except for BD Cy-Chrome™ and PerCP which are shown for FACSCalibur™ configurations.TABLE 1. Comparison of individual fluorochromes with single and dual laser flow cytometry.*PerCP is highly sensitive to photobleaching and must be used with laser power <150mW++Can only be used with a dye laser#Not recommended (dull)$BD Cy-Chrome and APC cannot be simultaneously used on instruments lacking cross-beam compensation. References:Loken, M.R., 1990. Immunofluorescence Techniques in Flow Cytometry and Sorting, 2nd Ed., Wiley. pp341-353.Parks, D., L. Herzenberg, and L. Herzenberg. 1989. Flow cytometry and fluorescence-activated cell sorting. Fundamental Immunology, Second Edition. William Paul, Ed.Raven Press, Ltd, New York.Zola, H. 1995. Detection of cytokine receptors by flow cytometry. In Current Protocols in Immunology (J. Coligan, A. Kruisbeek, D. Margulies, E. Shevach, W. Strober, eds.) John Wiley and Sons, New York. Unit 6.21.Immunofluorescence and cell sorting. In Current Protocols in Immunology. (J. Coligan, A. Kruisbeek, D. Margulies, E. Shevach, W. Strober, eds) John Wiley and Sons, New York. Unit 5.1 - 5.6.5. Shapiro, H.M. 1988. Practical Flow Cytometry, 2nd Ed. Wiley-Liss, New York.。
ThermoFisher DS-33 Matrix Standard Kit (Dye Set G5

DS-33 Matrix Standard Kit (Dye Set G5) SeqStudio™ Flex, SeqStudio™, 3500, 3730, and 3130 series instruments Catalog Number 4345833Pub. No. 4362884 Rev.GWARNING! Read the Safety Data Sheets (SDSs) andfollow the handling instructions. Wear appropriate protectiveeyewear, clothing, and gloves. Safety Data Sheets (SDSs) areavailable from /support.Product descriptionThe DS-33 Matrix Standard Kit (Dye Set G5) is used to perform spectral calibrations when analyzing DNA fragments labeled with 6‐FAM™, VIC™, NED™, PET™, and LIZ™ dyes. (The LIZ™ dye is usedto label the size standard.) The matrix standard contains five DNA fragments. Each fragment is labeled with a different dye from the dye set.For more information on spectral calibration, see the DNA Fragment Analysis by Capillary Electrophoresis User Guide (Pub. No. 4474504). Contents and storage[1] The kit is stable for 1 year when stored at 2–8°C.Required materials not suppliedGuidelines for use•For more information on the use of matrix standards, see the instrument user guide or getting started guide.•To prepare the matrix standard dilution, combine the appropriate volumes of matrix standard and Hi‑Di™ Formamide (Cat. No. 4311320). Dilution volumes vary depending on theinstrument.•Use the matrix standard within 2 hours of preparation.•Do not add size standard to the matrix standard.•Discard any unused reagent that has been diluted in Hi‑Di™Formamide.Prepare the standard1.Vortex the matrix standard tube for 5–10 seconds to mix, thencentrifuge for 3–5 seconds to bring the mixture to the bottom and eliminate air bubbles.bine the volumes of matrix standard and Hi‑Di™ Formamideappropriate for the instrument. See “Component volumes andwell location for the prepared standard” on page 2.3.Vortex for 5–10 seconds, then centrifuge for 3–5 seconds.4.Dispense the prepared standard into the appropriate wells of areaction plate. See “Component volumes and well location forthe prepared standard” on page 2.5.Cover the plate with adhesive film, then centrifuge for3–5 seconds.6.Denature the DNA fragments:a.Incubate the mixture at 95°C for 5 minutes.b.Incubate the mixture at 4°C, or on ice, for ≥2 minutes.7.Remove the adhesive film, then cover the plate with septa.8.Centrifuge for 3–5 seconds.9.Assemble the plate with the retainer and base, then load on theinstrument.Note: The SeqStudio™ Genetic Analyzer does not require aretainer and base.10.Immediately perform the spectral calibration.For information on setting up the run, see the instrument user guide.For Research Use Only. Not for use in diagnostic procedures.Component volumes and well location for the prepared standardTable 1 SeqStudio ™ Flex Series Genetic AnalyzerTable 2 SeqStudio ™ Genetic AnalyzerTable 3 3500/3500xL Genetic AnalyzerTable 4 3730/3730xl DNA Analyzer[1]For 3730/3730xl Data Collection Software only when running the RCT configuration: Select dye set G5-RCT to perform fragment analysis in applications with a high dynamic range (large peaks with a signal intensity that is much higher than the signal intensity of small peaks).Table 5 3130/3130xl Genetic AnalyzerLimited product warrantyLife Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies' General Terms and Conditions of Sale at /us/en/home/global/terms-and-conditions.html . If you have any questions, please contact Life Technologies at /support .2DS-33 Matrix Standard Kit (Dye Set G5) Product Information SheetThermo Fisher Scientific Baltics UAB | V.A. Graiciuno 8, LT-02241 | Vilnius, LithuaniaFor descriptions of symbols on product labels or product documents, go to /symbols-definition.The information in this guide is subject to change without notice.DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Revision history: Pub. No. 4362884Important Licensing Information: This product may be covered by one or more Limited Use Label Licenses. By use of this product, you accept the terms and conditions of all applicable Limited Use Label Licenses.©2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified./support | /askaquestion31 January 2022。
AZD7545_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD7545Catalog No. :HY-16082CAS No. :252017-04-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD 7545; AZD⁻7545Formula:C19H18ClF3N2O5SMolecular Weight:478.87CAS No. :252017-04-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
阿托伐他汀杂质标准品列表
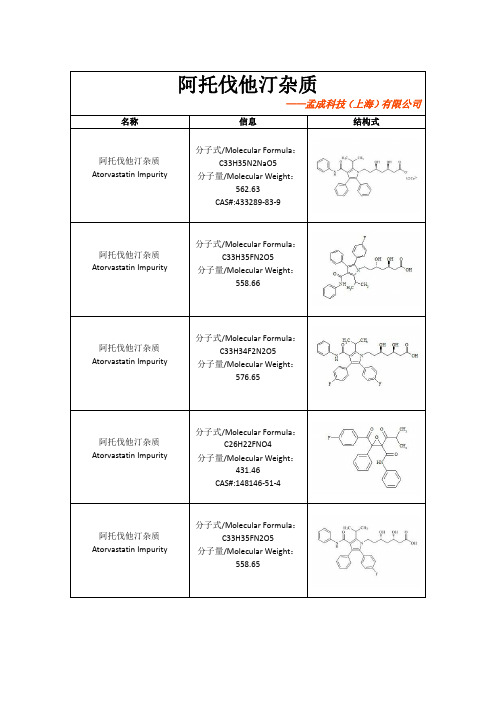
阿托伐他汀杂质——孟成科技(上海)有限公司名称信息结构式阿托伐他汀杂质Atorvastatin Impurity分子式/Molecular Formula :C33H35N2NaO5分子量/Molecular Weight :562.63CAS#:433289-83-9阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C33H35FN2O5分子量/Molecular Weight :558.66阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C33H34F2N2O5分子量/Molecular Weight :576.65阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C26H22FNO4分子量/Molecular Weight :431.46CAS#:148146-51-4阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C33H35FN2O5分子量/Molecular Weight :558.65阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C40H47FN3NaO8分子量/Molecular Weight :739.82CAS#:1371615-56-3阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C34H36FN2NaO5分子量/Molecular Weight :594.65CAS#:887196-29-4阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C33H33FN2O4分子量/Molecular Weight :540.62CAS#:125995-03-1阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C40H47FN2O5分子量/Molecular Weight :654.81CAS#:125971-95-1阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C34H35FN2O5分子量/Molecular Weight :570.67阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C14H27NO4分子量/Molecular Weight :273.37CAS#:947586-93-8阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C38H43FN2O5分子量/Molecular Weight :626.78阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C7H15NO4分子量/Molecular Weight :177.2阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C40H47FN2O8分子量/Molecular Weight :702.83CAS#:1450739-65-7阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C14H23NO4分子量/Molecular Weight :269.34CAS#:1016893-70-1阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C14H23NO4分子量/Molecular Weight :269.34CAS#:1357600-18-0阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula :C27H30FNO6分子量/Molecular Weight :483.54CAS#:1821498-27-4阿托伐他汀杂质Atorvastatin Impurity 分子式/Molecular Formula:C26H23F2NO3分子量/Molecular Weight:435.47CAS#:693793-82-7。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Vardenafil CAS No.:
224785-90-4Cat. No.:
HY-B0442
Product Data Sheet
MWt:
488.60Formula:
C23H32N6O4S Purity :>98%
Solubility:Mechanisms:
Biological Activity:
V d fil i PDE5i hibit d f t ti til d f ti
Pathways:Others; Target:PDE DMSO
Vardenafil is a PDE5inhibitor used for treating erectile dysfunction.
Target: PDE5Vardenafil specifically inhibited the hydrolysis of cGMP by PDE5 with an IC50 of 0.7 nM (6.6 nM). In contrast, the IC50 of vardenafil for PDE1 was 180 nM; for PDE6, 11 nM; for PDE2, PDE3 and PDE4, more than 1000 nM. Relative to PDE5, the ratios of the IC50 for PDE1 were 257 (60), for PDE6 16 (7.4). Vardenafil significantly enhanced the SNP-induced relaxation of human trabecular smooth muscle at 3 nM (10 nM). Vardenafil also significantly potentiated both ACh-induced and transmural electrical stimulation-induced relaxation of trabecular smooth muscle [1]. Both vardenafil doses(10mg or 20mg)significantly enhanced the rates of successful penetration (P <0.0001)and References:
[1]. Saenz de Tejada, I., et al., The phosphodiesterase inhibitory selectivity and the in vitro and in
vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res, 2001. 13(5): p. 282-90.[2]. Goldstein, I., et al., Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of doses(10 mg or 20 mg) significantly enhanced the rates of successful penetration (P 0.0001) and successful intercourse (P < 0.0001) compared with placebo. Vardenafil treatment was effective in increasing intercourse su...
erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose
study. Diabetes Care, 2003. 26(3): p. 777-83.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
