Meropenem trihydrate_119478-56-7_DataSheet_MedChemExpress
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
植物分类检索表-详细编排【范本模板】

植物界分类检索表姓名:班级:学院:植物界分类检索表及种子植物分科检索表一、植物界分类检索表1。
植物体无根、茎、叶的分化;雌性生殖器官由单细胞构成。
2。
无叶绿素3。
细胞中无细胞核的分化..。
.。
.。
.....。
...。
....。
..。
..。
..。
.。
..1)细菌3. 细胞中有细胞核的分化。
..。
.。
.。
.。
...。
.。
.。
..。
.。
.。
....。
..2)真菌2. 有叶绿素..。
.。
...。
.。
.。
...。
...。
...。
.....。
.。
...。
.。
..。
...。
3)藻类植物1. 植物体有根、茎、叶分化(苔藓除外);雌性生殖器官由多细胞构成。
4。
无维管束。
.。
...。
...。
...。
....。
..。
.。
....。
.。
.。
..。
....。
..。
....。
.4)苔藓植物4. 有维管束5. 无种子...。
.。
.。
..。
.。
.。
.。
....。
.。
..。
..。
....。
...。
.。
..5)蕨类植物5. 有种子6. 种子外面无子房包被...。
..。
..。
....。
...。
....。
.。
..。
.。
.。
.。
..6)裸子植物6. 种子外面有子房包被.。
.。
.。
.。
..。
....。
..。
.。
.。
.。
.。
..7)被子植物二、种子植物分科检索表1. 胚珠裸露,不包于子房内;种子裸露,不包于果实内..。
.。
.裸子植物Gymnospermae2. 花无假花被,胚珠无细长的珠被管.3. 叶羽状深裂,集生于常不分枝的树干顶部或块状茎上。
.。
..。
.。
..。
.苏铁科Cycadaceae3。
叶不为羽状深裂,树干多分枝。
4。
叶扇形,具多数2叉状细脉,叶柄长..。
.。
..。
..。
...。
....。
.。
银杏科Ginkgoaceae4. 叶不为扇形,无柄或有短柄。
5. 雌球花发育成球果;种子无肉质假种皮。
6。
雌雄异株,稀同株,雄蕊具4~20个悬垂的花药,苞鳞腹面仅l粒种子。
..。
.南洋杉科 Arauc afiaceae6。
雌雄同株,稀异株;雄蕊具2~9个背腹面排列的花药,种鳞腹面有1至多粒种子。
葎草茎叶石油醚部位化学成分

学报Journal of China Pharmaceutical University2022,53(2):178-184178葎草茎叶石油醚部位化学成分孙彪1,2,敖运林1,2,王德智1,2,王俊雅1,2,叶文才1,2,3,张晓琦1,2,3*(1暨南大学药学院中药及天然药物研究所,广州510632;2暨南大学广东省现代中药工程技术研究中心,广州510632;3国家药品监督管理局中成药质量评价重点实验室,广州510632)摘要研究桑科葎草(Humulus scandens)茎叶石油醚部位化学成分。
采用硅胶、Sephadex LH-20、ODS、制备型高效液相等色谱方法进行分离纯化,从桑科中药葎草(Humulus scandens)茎叶中分离得到15个化合物,应用理化数据和波谱学方法分别鉴定为杨芽黄素(1)、白杨素(2)、5-羟基-3,4',6,7-四甲氧基黄酮(3)、(2S)-5-羟基-7,8-二甲氧基二氢黄酮(4)、欧前胡素(5)、珊瑚菜内酯(6)、4-羟基-3-(3'-甲基-2'-丁烯基)苯甲酸乙酯(7)、对羟基苯丙酸(8)、反式对羟基肉桂酸乙酯(9)、对羟基苯甲醛(10)、anofinic acid(11)、5,6-去氢卡文内酯(12)、大黄素甲醚(13)、齐墩果-12-烯-3,11-二酮(14)、ergosta-4,6,8(14),22-tetraen-3-one(15),以上化合物均为首次从该植物中分离得到。
关键词桑科;葎草;化学成分;黄酮中图分类号R284.1文献标志码A文章编号1000-5048(2022)02-0178-07doi:10.11665/j.issn.1000-5048.20220207引用本文孙彪,敖运林,王德智,等.葎草茎叶石油醚部位化学成分[J].中国药科大学学报,2022,53(2):178–184.Cite this article as:SUN Biao,AO Yunlin,WANG Dezhi,et al.Chemical constituents of petroleum ether extract from the stems and leaves of Humulus scandens[J].J China Pharm Univ,2022,53(2):178–184.Chemical constituents of petroleum ether extract from the stems and leaves of Humulus scandensSUN Biao1,2,AO Yunlin1,2,WANG Dezhi1,2,WANG Junya1,2,YE Wencai1,2,3,ZHANG Xiaoqi1,2,3*1Institute of Traditional Chinese Medicine&Natural Products,College of Pharmacy,Ji'nan University,Guangzhou510632; 2Guangdong Engineering Research Center for Modernization of TCM,Ji'nan University,Guangzhou510632;3NMPA Key Laboratory for Quality Evaluation of TCM,Guangzhou510632,ChinaAbstract To study the chemical constituents of petroleum ether extract from the stems and leaves of Humulus scandens(family of Moraceae),fifteen compounds were isolated from the stems and leaves of H.scandens by silica gel,Sephadex LH-20,ODS,and preparative HPLC chromatography.The structures were identified by physico‑chemical data and spectroscopic method as tectochrysin(1),chrysin(2),5-hydroxy-3,4',6,7-tetramethoxyfla‑vone(3),(2S)-5-hydroxy-7,8-dimethoxyflavanone(4),imperatorin(5),phellopterin(6),ethyl4-hydroxy-3-(3'-methyl-2'-butenyl)benzoate(7),p-hydroxy-phenylpropionic acid(8),ethyl p-hydroxycinnamate(9),p-hydroxy‑benzaldehyde(10),anofinic acid(11),5,6-dehydrokavain(12),physcion(13),olean-12-ene-3,11-dione(14)and ergosta-4,6,8(14),22-tetraen-3-one(15),respectively.All compounds were isolated from this plant for the first time.Key words Moraceae;Humulus scandens;chemical constitutents;flavonesThis study was supported by the National Natural Science Foundation of China(No.U1801287,No.82073712),the Science and Technology Planning Project of Guangdong Province(No.2020B1111110004)and the Science and Technology Planning Project of Guangzhou(No.20212210005)收稿日期2021-08-27*通信作者Tel:************E-mail:tzhxq01@基金项目国家自然科学基金资助项目(No.U1801287,No.82073712);广东省科技计划资助项目(No.2020B1111110004);广州市科技计划资助项目(No.20212210005)第53卷第2期孙彪,等:葎草茎叶石油醚部位化学成分葎草[Humulus scandens.(Lour.)Merr.]为桑科(Moraceae)葎草属植物,为一年生或多年生草本,广泛分布于我国除青海、新疆以外的大部分地区,另外东北亚、北美洲也有分布[1-2]。
欧前胡素对照品的详细参数

欧前胡素
产品自编号:A0011
产品中文名称:欧前胡素
中文别名:欧前胡素
英文名:Imperatorin
英文别名:Ammidin,8-Isopentenyloxypsoralen;9-(3-Methylbut-2-enyloxy)-7H-furo[3,2-g]chromen-7-one CAS登录号:482-44-0
分子式:C16H14O4
分子量:270.27996
分子结构:
外观:白色片状结晶
规格:20mg/支
纯度:≥98%
用途:用于含量测定/鉴定/药理实验等。
提取来源:大风子科(Flacourtiaceae)罗旦梅Flacourtiajangomas(Lour.)Raeusch茎,皮
溶解性:.
熔点:.
旋光度:.
药理药效:欧前胡素具有抗菌、平喘及抗过敏等作用。
贮存条件:4℃冷藏、密封、避光
有效期:2年
注意事项:无
库存情况:
供货能力:大量现货
联系qq:2857290823
生产厂家:成都曼思特生物科技有限公司
1:中药活性单体及对照品定制研发和生产服务,我们的产品均可满足提供从mg级到kg级的不同规格的不同需求,可根据客户需要提供HPLC、MS、NMR等图谱。
2:定制分离中药制剂中的指定成分。
3:药物杂质的分离鉴定等外包服务。
显齿蛇葡萄提取物

显齿蛇葡萄提取物一、植物描述1、植物来源:为葡萄科蛇葡萄属植物显齿蛇葡萄(Ampelopsis grossedentata)的叶,俗称藤茶、藤婆茶、茅岩霉茶。
2、产地生源:湖南、湖北、贵州、云南、广西、广东、江西、福建等海拔400~1300米的山地灌丛中、林中、石上、河边。
二、产品描述1、Cas号:【27200-12-0】2、英文名:Dihydromyricetin3、别名:蛇葡萄素;Ampelopsin4、化学名:(2R,3R)-3,5,7-三羟基-2-(3,4,5-三羟基苯基)苯并二氢吡喃-4-酮(2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-4-one5、成分分类:黄酮类6、分子量:7、分子式:C15H12O88、结构式:9、性状:棕黄色粉末或类白色粉末(98%)10、理化性质:本品为类白色针状结晶(乙醇),mp 245 -246℃。
易溶于热水,热乙醇及丙酮,溶于乙醇、甲醇,微溶于水,极微溶于醋酸乙酯,不溶于氯仿、石油醚。
11、规格:显齿蛇葡萄总黄酮50%-98%(UV)、二氢杨梅素40-98%(HPLC)。
12、药理药效:本品为葡萄属植物显齿蛇葡萄提取物,显齿蛇葡萄中的主要活性成分为黄酮类化合物,此类物质具有清除自由基、抗氧化、抗血栓、抗肿瘤、消炎等多种奇特功效;而二氢杨梅素是显齿蛇葡萄中较为特殊的一种黄酮类化合物,在解除醇中毒、预防酒精肝、脂肪肝、抑制肝细胞恶化、降低肝癌的发病率、抗高血压、抑制体外血小板聚集和体内血栓的形成、降低血脂和血糖水平,提高SOD活性以及保肝护肝等方面具有特殊功效。
(1)抗菌作用:药理实验表明,高纯度二氢杨梅素对枯草芽胞杆菌、金黄色葡萄球菌、沙门菌、大肠埃希菌、产气杆菌、啤酒酵母、黏红酵母、青霉、黑曲霉、黄曲霉、毛霉及根霉均有抑菌作用, 尤其对革兰阳性、革兰阴性球菌或杆菌作用明显。
(2)解热、镇痛、抗炎作用有研究证实二氢杨梅素对小鼠巴豆油性耳廓水肿,大鼠角叉菜胶性、甲醛性足环肿胀及腹腔毛细血管通透性急性、亚急性炎症的渗出过程均有抑制作用;对小鼠酸性扭体反应和热水反应显示有一定的镇痛作用,能提高小鼠的痛阀水平。
美罗培南三水合物试剂产品手册说明书

美罗培南三水合物;MeropenemTrihydrate产品编号:MB1129质量标准:>98%,BR,三水合物包装规格:1 G ;5 G ;25G ;产品形式:白色至微黄色结晶性粉末 基本信息分子式C17H25N3O5S·3H2O 结构式分子量437.52 CAS No.119478-56-7 储存条件 2-8℃,避光防潮密闭干燥 溶解性(25°C) 溶于甲醇、DMF 、DMSO ≥20 mg/mL 、5%磷酸二氢钾溶液、0.1mol/L 氢氧化钠溶液 略溶于水20mM 、0.1mol/L 盐酸溶液不溶于丙酮、乙醇、乙醚注意事项 溶解性是在室温下测定的,如果温度过低,可能会影响其溶解性。
其他说明 为了您的安全和健康,请穿实验服并戴一次性手套操作。
简介:本品为人工合成的人工合成的广谱碳青霉烯类抗生素。
物理性状及指标:外观:……………………白色至微黄色结晶性粉末。
无臭,味苦。
溶解性:……………………溶于甲醇、DMF 、5%磷酸二氢钾溶液、0.1mol/L 氢氧化钠溶液、DMSO ≥20 mg/mL ; …………………………………略溶于水20mM 、0.1mol/L 盐酸溶液;不溶于丙酮、乙醇、乙醚。
用途及描述:科研试剂,广泛应用于分子生物学,药理学等科研方面,严禁用于人体。
美罗培南通过其共价键与参与细胞壁合成的青霉素结合蛋白(PBPs)结合,从而抑制细菌细胞壁的合成,起抗菌作用。
美罗培南对革兰阳性菌、革兰阴性菌均敏感,尤其对革兰阴性菌有很强的抗菌活性。
对约90%肠杆菌属的最小抑菌浓度(MIC)为0.08~0.15mg/L ;90%以上的铜绿假单胞菌菌株对其高度敏感,最小抑菌浓度(MIC)<4mg/L ;全部嗜血菌(包括耐氨苄西林菌株)对其高度敏感,最小抑菌浓度(MIC)为0.06~1mg/L ;淋球菌对美罗培南也高度敏感,其活性强于亚胺培南15倍;表皮葡萄球菌、腐生葡萄球菌和其它凝固酶阴性葡萄球菌对美罗培南敏感;粪肠球菌的大多数菌株对美罗培南高度或中度敏感;美罗培南可抑制几乎全部的脆弱拟杆菌;厌氧菌如消化链球菌属、丙酸杆菌属、放线菌属等也对美罗培南敏感。
美罗培南_美国药典

USP 35Official Monographs / Meropenem3813C U= nominal concentration of mercaptopurine in the essary correction. Each mL of 0.1 N hydrochloric acid is equiva-Sample solution (mg/mL)lent to 12.60 mg of Hg(NH2)Cl.F= relative response factor for each individualimpurity (see Table 2)Acceptance criteriaIndividual impurities: See Table 2. [N OTE—Disregard any Meropenemimpurity peak less than 0.05%.]Table 2Relative Relative AcceptanceRetention Response Criteria,Name Time Factor NMT (%)Didanosine relatedC17H25N3O5S·3H2O437.52compound A a0.54 6.30.31-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 3-[[5-Mercaptopurine 1.00——[(dimethylamino)carbonyl]-3-pyrrolidinyl]thio]-6-(1-hydroxy-Mercaptopurine ethyl)-4-methyl-7-oxo, trihydrate, [4R-disulfide b 2.90 4.40.4[3(3S*,5S*),4α,5β,6β(R*)]]-.Any unspecified—(4R,5S,6S)-3-[[(3S,5S)-5-(Dimethylcarbamoyl)-3-pyrrolidinyl]thio] impurity 1.00.2-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0] Total impurities——0.6hept-2-ene-carboxylic acid, trihydrate [119478-56-7].a Hypoxanthine.Anhydrous383.47 [96036-03-2].b1,2-Di(9H-purin-6-yl)disulfane.» Meropenem contains not less than 98.0 per-v USP35cent and not more than 101.0 percent ofC17H25N3O5S, calculated on the anhydrous basis. ADDITIONAL REQUIREMENTS•P ACKAGING AND S TORAGE: Preserve in well-closed containers.Packaging and storage—Preserve in tight containers. Store •L ABELING: When more than one Dissolution test is given, thethe dry powder at controlled room temperature.labeling states the Dissolution test used only if Test 1 is notLabeling—Where it is intended for use in preparing injectable used.dosage forms, the label states that it is sterile or must be sub-•USP R EFERENCE S TANDARDS〈11〉jected to further processing during the preparation of injectable USP Mercaptopurine RSdosage forms.USP Reference standards 〈11〉—USP Endotoxin RSAmmoniated MercuryUSP Meropenem RSIdentification—Hg(NH2)Cl252.07A: Infrared Absorption 〈197K〉.Mercury amide chloride.Mercury amide chloride [10124-48-8].B: Ultraviolet Absorption 〈197U〉—Solution:30 µg per mL.» Ammoniated Mercury contains not less thanMedium:water.98.0 percent and not more than 100.5 percentSpecific rotation 〈781〉:between −17° and −21°, measured of Hg(NH2)Cl.at 20°.Test solution: 5 mg per mL, in water.Packaging and storage—Preserve in well-closed, light-resis-tant containers.pH 〈791〉: between 4.0 and 6.0, in a solution (1 in 100). Identification—Water, Method Ic 〈921〉:between 11.4% and 13.4%.A: A 0.1-g portion is soluble, with the evolution of ammo-Residue on ignition 〈281〉: not more than 0.1%, igniting at nia, in a cold solution of 1g of sodium thiosulfate in 2 mL of500±50°, instead of at 800±25°. Use a desiccator containing water. When this solution is heated gently, a rust-colored mix-silica gel.ture is formed, from which a red precipitate is obtained on Heavy metals—centrifugation. If the solution is strongly heated, a black mixture Sodium sulfide reagent—Dissolve 5g of sodium sulfide in a forms.mixture of 10 mL of water and 30 mL of glycerin. Preserve in B: When heated with 1N sodium hydroxide, it becomes yel-well-filled, light-resistant bottles, and use within 3 months. low, and ammonia is evolved.Test solution—Transfer 1.0 g of Meropenem to a quartz or C: A solution in warm acetic acid yields with potassium io-porcelain crucible, cover loosely with a lid, and carbonize by dide TS a red precipitate, which is soluble in an excess of the gentle ignition. After cooling, add 2 mL of nitric acid and 5 reagent. The solution yields a white precipitate with silver ni-drops of sulfuric acid, heat cautiously until white fumes evolve, trate TS.and incinerate by ignition at 500° to 600°. Cool, add 2 mL of Residue on ignition 〈281〉: not more than 0.2%.hydrochloric acid, and evaporate on a water bath to dryness.Moisten the residue with 3 drops of hydrochloric acid, add 10 Mercurous compounds—Dissolve 2.5 g in 25 mL of warmmL of hot water, and warm for 2 minutes. Add 1 drop of phe-hydrochloric acid, filter through a tared filtering crucible, washnolphthalein TS, add ammonia TS, dropwise, until the solution with water, and dry at 60° to constant weight: the weight ofdevelops a pale red color, and add 2 mL of 1N acetic acid.the residue does not exceed 5 mg (0.2%).Filter, if necessary, to obtain a clear solution, washing the filter Assay—Mix about 0.25 g of Ammoniated Mercury, accuratelywith 10 mL of water. Transfer the filtrate and the washing to a weighed, with about 10 mL of water. Add 3g of potassium50-mL color-comparison tube, and add water to obtain a vol-iodide, mix occasionally until dissolved, and add about 40 mLume of 50 mL.of water. Add methyl red TS, and titrate with 0.1 N hydrochlo-Standard solution—Evaporate a mixture of 2 mL of nitricric acid VS. Perform a blank determination, and make any nec-acid, 5 drops of sulfuric acid, and 2 mL of hydrochloric acid ona water bath, further evaporate to dryness on a hot sand bath,3814Meropenem / Official Monographs USP 35and moisten the residue with 3 drops of hydrochloric acid. Pro-than 1.5; and the relative standard deviation for replicate injec-ceed as directed for Test solution, beginning with “add 10 mL tions is not more than 2.0%.of hot water,” except add water to obtain a volume of 49 mL.Procedure—Separately inject equal volumes (about 10 µL) of Add 1.0 mL of Standard Lead Solution (see Heavy Metals 〈231〉).the Standard solution and the Test solution into the chromato-Procedure—To the tubes containing the Test solution and the graph, record the chromatograms, using a period of chroma-Standard solution, add 1 drop of Sodium sulfide reagent, mix,tography for the Test solution that is about 3 times the retention and allow to stand for 5 minutes. The color in the tube contain-time of meropenem, and measure the peak responses. Major ing the Test solution is not darker than the color in the tube impurity peaks may be observed at retention times of about containing the Standard solution (0.001%).0.45 and 1.9 in relation to the retention time of meropenem.Calculate the percentage of each impurity in the chromatogram Limit of acetone—obtained from the Test solution by the formula: Internal standard solution—Prepare a solution in dimethyl-formamide containing 0.05 µL of ethyl acetate per mL.(CS/C U)(P)(r i/r S) Standard solution—Transfer about 50 mg of acetone, accu-rately weighed, to a 100-mL volumetric flask, dilute with di-in which CS is the concentration, in mg per mL, of USP Mer-methylformamide to volume, and mix. To 1.0 mL of this solu-openem RS in the Standard solution; CU is the concentration, in tion, add 10.0 mL of the Internal standard solution, and mix.mg per mL, of Meropenem in the Test solution; P is the statedTest solution—Dissolve 100 mg of Meropenem, accurately percentage, calculated on the anhydrous basis, of meropenem weighed, in 0.2 mL of dimethylformamide and 2.0 mL of Inter-in USP Meropenem RS; r i is the peak response of any individual nal standard solution.impurity obtained from the Test solution; and r S is the peak re-sponse of meropenem obtained from the Standard solution. Not Chromatographic system (see Chromatography 〈621〉)—Themore than 0.3% of any of two major impurities is found, calcu-gas chromatograph is equipped with a flame-ionization detectorlated on the anhydrous basis; not more than 0.1% of any other and a 3-mm × 2-m column that contains support S2 and isimpurity is found, calculated on the anhydrous basis; and the maintained at a constant temperature of about 150°. The injec-sum of all such other impurities is not more 0.3%.tion port temperature is maintained at about 170°. Nitrogen isthe carrier gas, with the flow rate adjusted so that the retention Other requirements—Where the label states that Mer-time for acetone is about 3 minutes.openem is sterile, it meets the requirements for Sterility 〈71〉and for Bacterial endotoxins under Meropenem for Injection.Procedure—Separately inject equal volumes (about 2 µL) ofWhere the label states that Meropenem must be subjected to the Standard solution and the Test solution into the chromato-further processing during the preparation of injectable dosage graph, record the chromatograms, and measure the peak re-forms, it meets the requirements for Bacterial endotoxins under sponses for the acetone peak and the internal standard peak.Meropenem for Injection.Calculate the percentage of acetone in the portion of Mer-openem taken by the formula:Assay—Diluted phosphoric acid—Dilute 10 mL of phosphoric acid (W A/5W U)(R U/R S)with water to make 100 mL of solution.Solvent—Transfer 1.0 mL of triethylamine to a 1000-mL volu-in which W A is the weight, in mg, of acetone in the Standard metric flask containing 900 mL of water. Adjust with Dilutedsolution; W U is the quantity, in mg, of Meropenem in the Test phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to vol-solution; and R U and R S are the peak area ratios of acetone to ume, and mix.the internal standard obtained from the Test Solution and theMobile phase—Prepare a mixture of Solvent and methanol Standard solution, respectively. Not more than 0.05% is found.(5:1). Make adjustments if necessary (see System Suitability Chromatographic purity—under Chromatography 〈621〉).Diluted phosphoric acid—Dilute 10 mL of phosphoric acid Standard preparation—Transfer about 25 mg of USP Mer-with water to make 100 mL of solution.openem RS, accurately weighed, to a 50-mL volumetric flask, Solvent—Transfer 1.0 mL of triethylamine to a 1000-mL volu-add Solvent, swirl to dissolve, dilute with Solvent to volume, and metric flask containing 900 mL of water. Adjust with Diluted mix. [NOTE—Immediately after preparation, store this solution in phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to vol- a refrigerator. It may be used for 24 hours.]ume, and mix.Assay preparation—Transfer about 25 mg of Meropenem, ac-Mobile phase—Transfer 1.0 mL of triethylamine to a 1000-mL curately weighed, to a 50-mL volumetric flask, add Solvent, swirl volumetric flask containing 900 mL of water. Adjust with Diluted to dissolve, dilute with Solvent to volume, and mix. Use this phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to vol-solution immediately after preparation.ume, and mix. Mix this solution with 70 mL of acetonitrile.Chromatographic system (see Chromatography 〈621〉)—The Make adjustments if necessary (see System Suitability under liquid chromatograph is equipped with a 300-nm detector and Chromatography 〈621〉). a 4.6-mm × 25-cm column that contains 5-µm packing L1. Ad-Standard solution—Prepare a solution of USP Meropenem RS just the flow rate so that the retention time for meropenem is in Solvent having a known concentration of about 0.025 mg of about 6 to 8 minutes. The flow rate is about 1.5 mL per min-USP Meropenem RS per mL. [NOTE—Immediately after prepara-ute. Chromatograph the Standard preparation, and record the tion, store this solution in a refrigerator and use within 24peak responses as directed for Procedure: the column efficiency hours.]is not less than 2500 theoretical plates; the tailing factor is not Test solution—Dissolve an accurately weighed quantity of more than 1.5; and the relative standard deviation for replicate Meropenem quantitatively in Solvent to obtain a solution having injections is not more than 2.0%.a known concentration of about 5 mg per mL. Use this Test Procedure—Separately inject equal volumes (about 5 µL) of solution immediately.Standard preparation and Assay preparation into the chromato-Chromatographic system (see Chromatography 〈621〉)—The graph, record the chromatograms, and measure the areas for liquid chromatograph is equipped with a 220-nm detector and the major peaks. Calculate the quantity, in mg, of C17H25N3O5S a 4.6-mm × 25-cm column that contains 5-µm packing L1 and in the portion of Meropenem taken by the formula:is maintained at a constant temperature of about 40°. The flowrate is about 1.6 mL per minute, and is adjusted so that the(W S/W U)(P)(r U/r S)retention time of meropenem is between 5 and 7 minutes.Chromatograph the Standard solution, and record the peak re-in which W S is the weight, in mg, of USP Meropenem RS taken sponses as directed for Procedure: the column efficiency is not to prepare the Standard preparation, calculated on the anhy-less than 2500 theoretical plates; the tailing factor is not more drous basis; W U is the weight, in mg, of Meropenem taken toprepare the Assay preparation; P is the stated percentage, calcu-USP 35Official Monographs / Meropenem 3815lated on the anhydrous basis, of meropenem in USP Mer-than 0.8% of the impurity, if any, with a retention time of openem RS; and r U and r S are the meropenem peak responses about 0.45 relative to that of meropenem, is found; and not obtained from the Assay preparation and the Standard prepara-more than 0.6% of the impurity, if any, with a retention time tion, respectively.of about 1.9 relative to that of meropenem, is found.Content of sodium—Potassium chloride solution—Transfer 38.1 g of potassium chloride to a 1000-mL volumetric flask, dissolve in and dilute with water to volume, and mix.Meropenem for InjectionStandard sodium solution—Dissolve 25.42 mg of sodium chloride, previously dried at 105° for 2 hours and accurately » Meropenem for Injection is a sterile dry mixture weighed, quantitatively in water to obtain a solution having a of Meropenem and Sodium Carbonate. It con-concentration of 25.42 µg of sodium chloride per mL. Transfer tains not less than 90.0 percent and not more 5.0 mL of this solution to a 50-mL volumetric flask, add 5.0 mL of Potassium chloride solution , dilute with water to volume, and than 120.0 percent of the labeled amount of mix.meropenem (C 17H 25N 3O 5S).Test solution—Transfer an accurately measured volume of the Packaging and storage—Preserve in tight Containers for stock solution used to prepare Assay preparation 1 or Assay Sterile Solids as described under Injections 〈1〉. Store at con-preparation 2, as appropriate, equivalent to about 25 mg of trolled room temperature.meropenem, to a 200-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL Labeling—It meets the requirements for Labeling under Injec-volumetric flask, add 5.0 mL of Potassium chloride solution , di-tions 〈1〉. Label it to state the quantity, in mg, of sodium (Na) in lute with water to volume, and mix.a given dosage of meropenem.Blank solution—Transfer 5.0 mL of Potassium chloride solution USP Reference standards 〈11〉—to a 50-mL volumetric flask, dilute with water to volume, and USP Endotoxin RS mix.USP Meropenem RSProcedure—Concomitantly determine the absorbances of the Constituted solution—At the time of use, it meets the re-Standard sodium solution and the Test solution at the sodium quirements for Constituted Solutions under Injections 〈1〉.emission line at 589.6 nm with an atomic absorption spectro-Identification—The retention time for the meropenem peak photometer (see Spectrophotometry and Light-Scattering 〈851〉),in the chromatogram of the Assay preparation corresponds to equipped with a sodium hollow-cathode lamp and a single-slot that in the chromatogram of the Standard preparation, as ob-burner, using an air–acetylene flame and the Blank solution as tained in the Assay.the blank. Calculate the quantity, in mg, of sodium (Na) in the constituted Meropenem for Injection by the formula:Bacterial endotoxins 〈85〉—It contains not more than 0.125USP Endotoxin Unit per mg of meropenem.(22.99/58.44)(C )(2000V/vM )(A U /A S )Sterility 〈71〉—It meets the requirements when tested as di-rected for Membrane Filtration under Test for Sterility of the Prod-in which 22.99 and 58.44 are the atomic weight of sodium and uct to be Examined.the molecular weight of sodium chloride, respectively; C is the Uniformity of dosage units 〈905〉: meets the requirements.concentration, in µg per mL, of sodium chloride in the Standard pH 〈791〉: between 7.3 and 8.3, in a solution (1 in 20).sodium solution; V is the volume, in mL, of the stock solution obtained in Assay preparation 1 or Assay preparation 2, as ap-Loss on drying 〈731〉—Dry it in vacuum at 65° for 6 hours: it propriate; v is the volume, in mL, of the portion of the stock loses between 9.0% and 12.0% of its weight.solution taken to prepare the Test solution; M is the total quan-Particulate matter 〈788〉: meets the requirements for small-tity, in mg, of meropenem in the stock solution obtained in volume injections.Assay preparation 1 or Assay preparation 2, as appropriate,Chromatographic purity—based on the result of the Assay; and A U and A S are the ab-Diluted phosphoric acid , Solvent , Mobile phase , and Chromato-sorbances of the Test solution and the Standard sodium solution,graphic system—Proceed as directed in the test for Chromato-respectively: it contains between 80% and 120% of the labeled graphic purity under Meropenem .amount of sodium.Standard solution—Prepare a solution of USP Meropenem RS Assay—in Solvent having a known concentration of about 0.029 mg of Mobile phase—Dilute 15 mL of tetrabutylammonium hydrox-USP Meropenem RS per mL. [NOTE —Immediately after prepara-ide solution (25% in water) with water to 750 mL. Adjust with tion, store this solution in a refrigerator. It may be used for 24dilute phosphoric acid (1 in 10) to a pH of 7.5 ± 0.1. Add 150hours.]mL of acetonitrile and 100 mL of methanol, mix, and degas.Test solution—Transfer an accurately weighed portion of Mer-Make adjustments if necessary (see System Suitability under openem for Injection, equivalent to about 50 mg of mer-Chromatography 〈621〉).openem, based on the labeled amount, to a 10-mL volumetric Standard preparation—Dissolve an accurately weighed por-flask, dilute with Solvent to volume, and mix. Use this Test solu-tion of USP Meropenem RS quantitatively in Mobile phase to tion immediately.obtain a solution having a known concentration of about 0.11Procedure—Proceed as directed in the test for Chromato-mg per mL. This solution contains the equivalent of about 0.1graphic purity under Meropenem . Calculate the percentage of mg of meropenem per mL. [NOTE —Immediately after prepara-each impurity in the portion of Injection taken by the formula:tion, store this solution in a refrigerator and use within 24hours.]10(CP /m )(r i /r S )Assay preparation 1 (where it is represented as being a sin-gle-dose container)—Constitute a container of Meropenem for in which C is the concentration, in mg per mL, of USP Mer-Injection with a volume of water, accurately measured, corre-openem RS in the Standard solution; P is the stated percentage,sponding to the amount of solvent specified in the labeling.calculated on the anhydrous basis, of meropenem in USP Mer-Withdraw all of the withdrawable contents, using a suitable hy-openem RS; m is the amount, in mg, of meropenem in the podermic needle and syringe, and transfer to a 100-mL volu-portion of Injection taken to prepare the Test solution , based on metric flask. Dilute with water to volume, and mix. Dilute an the label claim; r i is the peak response of any individual impu-accurately measured volume of this stock solution quantitatively rity obtained from the Test solution; and r S is the peak response with Mobile phase to obtain a solution having a concentrationof meropenem obtained from the Standard solution . Not more。
沙奎那韦

谢谢观看
联合治疗可以使AIDS合并症或垂危状态的危险性减少53%,死亡率减少72%。这与治疗18个月后AIDS合并症或 死亡率由29.4%降至16.0%是相符的;同样,单纯死亡率由8.6%降至4.1%。在3个治疗组中,平均疗程为11~13个 月,平均随访时间是17个月。
该研究中,所有治疗组CD4细胞基线计数平均为156~176/立方毫米。16周后(DAVG16),沙奎那韦联合ddc 治疗组CD4细胞增加26/立方毫米,血浆病毒载量减少0.6log10RNA拷贝/毫升。16周时,CD4细胞平均值增加47/ 立方毫米。12周时,血浆病毒载量平均值降低0.7log10RNA拷贝/毫升。
与核苷类似物(齐多夫定等)不同,沙奎那韦直接作用于病毒靶酶,不需经代谢激活,对静止细胞也有潜在 作用。在10-10摩尔/升浓度下,沙奎那韦对淋巴母细胞株和单核细胞株以及被实验室病毒株或临床分离的HIV-1 感染的淋巴细胞和单核细胞的起始培养有作用。
实验室细胞培养结果显示,沙奎那韦在与其他逆转录酶抑制剂(包括AZT(齐多夫定)、ddc(扎西他滨)、 ddI(去羟肌苷)进行两联或三联治疗HIV-1感染时,有附加的协同抗病毒作用,但毒性并不增加 。
机体对静注沙奎那韦6、36、72毫克后清除率很高,为1.14升/小时/千克(CV12%),略高于肝血流,并为常 数。体内存留时间平均为7小时。
适应症
沙奎那韦可与其他抗逆转录病毒药物联合使用治疗成人HIV-1感染 。
用法与用量
1
标准剂量
2
剂量调整
3
不良反应
4
禁忌
5
药物相互作用
成人及16岁以上儿童:推荐方案是与核苷类似物联合用药,餐后2小时内服用沙奎那韦600毫克,每天3次。 联合使用的抗逆转录病毒药物的剂量参考处方手册。与其他蛋白酶抑制剂合用时,沙奎那韦应减量(见【药物相 互作用】)。与其他蛋白酶抑制剂一样,强烈推荐按医嘱服药。
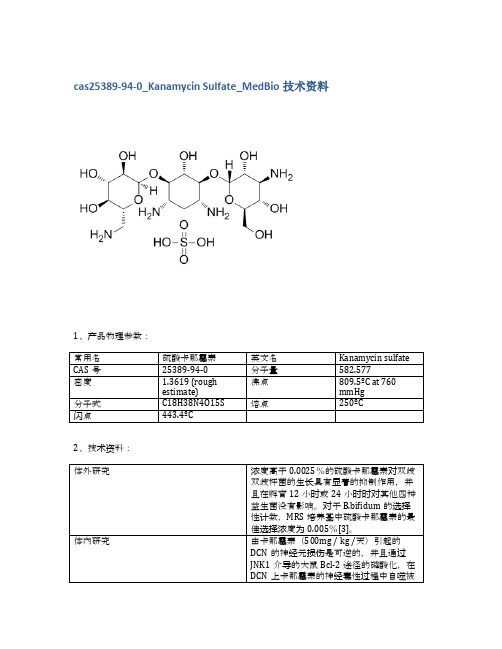
cas25389-94-0_Kanamycin Sulfate_MedBio技术资料
Fialuridine
69123-98-4
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15240
Cephalexin
Cephalexin
15686-71-2
10mM (in 1mL H2O)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15367
155347-36-7
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15228
Meropenem trihydrate
Meropenem trihydrate
119478-56-7
10mM (in 1mL H2O)
≥98%
品牌
货号
中文名称
英文名称
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15280
Pefloxacin Mesylate Dihydrate
Pefloxacin Mesylate Dihydrate
149676-40-4
100mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15350
CAS
包装
纯度
MedBio
MED15291
【2016】信息-头孢丙烯干混悬剂 美国 LUPIN LIMITED
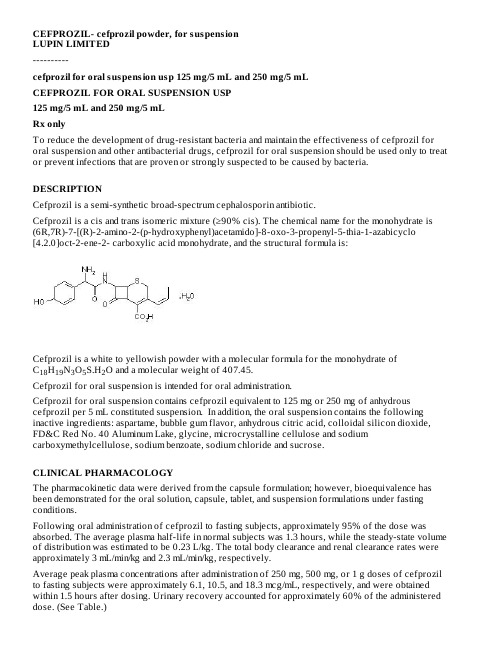
CEFPROZIL- cefprozil powder, for suspensionLUPIN LIMITED----------cefprozil for oral suspension usp 125 mg/5 mL and 250 mg/5 mLCEFPROZIL FOR ORAL SUSPENSION USP125 mg/5 mL and 250 mg/5 mLRx onlyTo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil for oral suspension and other antibacterial drugs, cefprozil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.DESCRIPTIONCefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic.Cefprozil is a cis and trans isomeric mixture (≥90% cis). The chemical name for the monohydrate is (6R,7R)-7-[(R)-2-amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid monohydrate, and the structural formula is:Cefprozil is a white to yellowish powder with a molecular formula for the monohydrate ofC H N O S.H O and a molecular weight of 407.45.1819352Cefprozil for oral suspension is intended for oral administration.Cefprozil for oral suspension contains cefprozil equivalent to 125 mg or 250 mg of anhydrous cefprozil per 5 mL constituted suspension. In addition, the oral suspension contains the following inactive ingredients: aspartame, bubble gum flavor, anhydrous citric acid, colloidal silicon dioxide, FD&C Red No. 40 Aluminum Lake, glycine, microcrystalline cellulose and sodium carboxymethylcellulose, sodium benzoate, sodium chloride and sucrose.CLINICAL PHARMACOLOGYThe pharmacokinetic data were derived from the capsule formulation; however, bioequivalence has been demonstrated for the oral solution, capsule, tablet, and suspension formulations under fasting conditions.Following oral administration of cefprozil to fasting subjects, approximately 95% of the dose was absorbed. The average plasma half-life in normal subjects was 1.3 hours, while the steady-state volume of distribution was estimated to be 0.23 L/kg. The total body clearance and renal clearance rates were approximately 3 mL/min/kg and 2.3 mL/min/kg, respectively.Average peak plasma concentrations after administration of 250 mg, 500 mg, or 1 g doses of cefprozil to fasting subjects were approximately 6.1, 10.5, and 18.3 mcg/mL, respectively, and were obtained within 1.5 hours after dosing. Urinary recovery accounted for approximately 60% of the administered dose. (See Table.)Dosage (mg)Mean Plasma Cefprozil Concentrations (mcg/mL) Peak appx.8 hour Urinary Excretion (%) * 1.5 h 4 h 8 h 250 mg6.1 1.7 0.2 60%500 mg10.5 3.2 0.4 62%1000 mg 18.3 8.4 1.0 54%During the first 4 hour period after drug administration, the average urine concentrations following 250mg, 500 mg, and 1 g doses were approximately 700 mcg/mL, 1000 mcg/mL, and 2900 mcg/mL,respectively.Administration of cefprozil with food did not affect the extent of absorption (AUC) or the peak plasma concentration (C ) of cefprozil. However, there was an increase of 0.25 to 0.75 hours in the time to maximum plasma concentration of cefprozil (T ).The bioavailability of the capsule formulation of cefprozil was not affected when administered 5minutes following an antacid.Plasma protein binding is approximately 36% and is independent of concentration in the range of 2mcg/mL to 20 mcg/mL.There was no evidence of accumulation of cefprozil in the plasma in individuals with normal renal function following multiple oral doses of up to 1000 mg every 8 hours for 10 days.In patients with reduced renal function, the plasma half-life may be prolonged up to 5.2 hours depending on the degree of the renal dysfunction. In patients with complete absence of renal function, the plasma half-life of cefprozil has been shown to be as long as 5.9 hours. The half-life is shortened during hemodialysis. Excretion pathways in patients with markedly impaired renal function have not been determined. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.)In patients with impaired hepatic function, the half-life increases to approximately 2 hours. The magnitude of the changes does not warrant a dosage adjustment for patients with impaired hepatic function.Healthy geriatric volunteers (≥65years old) who received a single 1 g dose of cefprozil had 35% to 60% higher AUC and 40% lower renal clearance values compared with healthy adult volunteers 20 to 40 years of age. The average AUC in young and elderly female subjects was approximately 15% to 20% higher than in young and elderly male subjects. The magnitude of these age- and gender-related changes in the pharmacokinetics of cefprozil is not sufficient to necessitate dosage adjustments.Adequate data on CSF levels of cefprozil are not available.Comparable pharmacokinetic parameters of cefprozil are observed between pediatric patients(6 months to 12 years) and adults following oral administration of selected matched doses. The maximum concentrations are achieved at 1to 2 hours after dosing. The plasma elimination half-life is approximately 1.5 hours. In general, the observed plasma concentrations of cefprozil in pediatric patients at the 7.5, 15, and 30 mg/kg doses are similar to those observed within the same time frame in normal adult subjects at the 250, 500, and 1000 mg doses, respectively. The comparative plasma concentrations of cefprozil in pediatric patients and adult subjects at the equivalent dose level are presented in the table below.Mean (SD) Plasma CefprozilConcentrations (mcg/mL)Population Dose 1 h 2 h 4 h 6 hT (h)children (n=18)7.5 mg/kg 4.70 (1.57) 3.99 (1.24)0.91 (0.30)0.23(0.13)0.94(0.32)adults 250 mg 4.82 4.92 1.70 0.53 1.28*Data represent mean values of 12 healthy volunteers.max max ½a bn=11 n=5 n=9 n=11(n=12)(2.13)(1.13)(0.53)(0.17)(0.34)children (n=19)15 mg/kg 10.86 (2.55)8.47 (2.03) 2.75 (1.07)0.61 (0.27) 1.24(0.43)adults (n=12)500 mg 8.39 (1.95)9.42 (0.98) 3.18 (0.76) 1.00 (0.24) 1.29(0.14)children (n=10)30 mg/kg 16.69 (4.26)17.61 (6.39)8.66 (2.70)-- 2.06(0.21)adults (n=12)1000 mg 11.99 (4.67)16.95 (4.07)8.36 (4.13) 2.79 (1.77) 1.27(0.12)Microbiology:Cefprozil has in vitro activity against a broad range of gram-positive and gram-negative bacteria. The bactericidal action of cefprozil results from inhibition of cell-wall synthesis. Cefprozil has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.Aerobic Gram-Positive Microorganisms:Staphylococcus aureus(including ß-lactamase-producing strains)NOTE: Cefprozil is inactive against methicillin-resistant staphylococci.Streptococcus pneumoniaeStreptococcus pyogenesAerobic Gram-Negative Microorganisms:Haemophilus influenzae(including ß-lactamase-producing strains)Moraxella (Branhamella) catarrhalis(including ß-lactamase-producing strains)The following in vitro data are available; however, their clinical significance is unknown. Cefprozil exhibits in vitro minimum inhibitory concentrations (MICs) of 8 mcg/mL or less against most (≥90%)strains of the following microorganisms; however, the safety and effectiveness of cefprozil in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.Aerobic Gram-Positive Microorganisms:Enterococcus duransEnterococcus faecalisListeria monocytogenesStaphylococcus epidermidisStaphylococcus saprophyticusStaphylococcus warneriStreptococcus agalactiaeStreptococci (Groups C, D, F, and G)viridans group Streptococcia b c d c d dOtitis Media:Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains). (See CLINICAL STUDIES.)NOTE: In the treatment of otitis media due to ß-lactamase producing organisms, cefprozil had bacteriologic eradication rates somewhat lower than those observed with a product containing a specific ß-lactamase inhibitor. In considering the use of cefprozil, lower overall eradication rates should be balanced against the susceptibility patterns of the common microbes in a given geographic area and the increased potential for toxicity with products containing ß-lactamase inhibitors.Acute Sinusitis:Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains).Lower Respiratory Tract:Secondary Bacterial Infection of Acute Bronchitis and Acute Bacterial Exacerbation of Chronic Bronchitis:Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains).Skin And Skin Structure:Uncomplicated Skin and Skin-Structure Infections:Caused by Staphylococcus aureus (including penicillinase producing strains) and Streptococcus pyogenes. Abscesses usually require surgical drainage.To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil for oral suspension and other antibacterial drugs, cefprozil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.CONTRAINDICATIONSCefprozil is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.WARNINGSBEFORE THERAPY WITH CEFPROZIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPROZIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG ß-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPROZIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED. Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefprozil, and may range in severity from mild diarrhea to fatal colitis. Treatment withantibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. PRECAUTIONSGeneral:Prescribing cefprozil for oral suspension in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria.In patients with known or suspected renal impairment (see DOSAGE AND ADMINISTRATION), careful clinical observation and appropriate laboratory studies should be done prior to and during therapy. The total daily dose of cefprozil should be reduced in these patients because high and/or prolonged plasma antibiotic concentrations can occur in such individuals from usual doses. Cephalosporins, including cefprozil, should be given with caution to patients receiving concurrent treatment with potent diuretics since these agents are suspected of adversely affecting renal function. Prolonged use of cefprozil may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.Cefprozil should be prescribed with caution in individuals with a history of gastrointestinal disease particularly colitis.Positive direct Coombs’ tests have been reported during treatment with cephalosporin antibiotics. Information for Patients:Phenylketonurics: Cefprozil for oral suspension contains phenylalanine 28 mg per 5 mL (1 teaspoonful) constituted suspension for both the 125 mg/5 mL and 250 mg/5 mL dosage forms. Patients should be counseled that antibacterial drugs including cefprozil for oral suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefprozil for oral suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefprozil for oral suspension or other antibacterial drugs in the future.Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible. Drug Interactions:Nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporin antibiotics. Concomitant administration of probenecid doubled the AUC for cefprozil.The bioavailability of the capsule formulation of cefprozil was not affected when administered 5minutes following an antacid.Drug/Laboratory Test Interactions:Cephalosporin antibiotics may produce a false positive reaction for glucose in the urine with copper reduction tests (Benedict’s or Fehling’s solution or with Clinitest tablets), but not with enzyme-based tests for glycosuria (e.g., Clinistix ). A false negative reaction may occur in the ferricyanide test for blood glucose. The presence of cefprozil in the blood does not interfere with the assay of plasma or urine creatinine by the alkaline picrate method.Carcinogenesis and Mutagenesis and Impairment of FertilityLong term in vivo studies have not been performed to evaluate the carcinogenic potential of cefprozil.Cefprozil was not found to be mutagenic in either the Ames Salmonella or E. coli WP2 urvA reversion assays or the Chinese hamster ovary cell HGPRT forward gene mutation assay and it did not induce chromosomal abnormalities in Chinese hamster ovary cells or unscheduled DNA synthesis in rathepatocytes in vitro . Chromosomal aberrations were not observed in bone marrow cells from rats dosed orally with over 30 times the highest recommended human dose based upon mg/m .Impairment of fertility was not observed in male or female rats given oral doses of cefprozil up to 18.5times the highest recommended human dose based upon mg/m .Pregnancy:Teratogenic Effects-Pregnancy Category B:Reproduction studies have been performed in rabbits, mice, and rats using oral doses of cefprozil of 0.8, 8.5, and 18.5 times the maximum daily human dose (1000 mg) based upon mg/m , and have revealed no harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women.Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.Labor and DeliveryCefprozil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.Nursing MothersSmall amounts of cefprozil (<0.3% of dose) have been detected in human milk following administration of a single 1 gram dose to lactating women. The average levels over 24 hours ranged from 0.25 to 3.3mcg/mL. Caution should be exercised when Cefprozil for oral suspension is administered to a nursing woman, since the effect of cefprozil on nursing infants is unknown.Pediatric Use(See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION.)The safety and effectiveness of cefprozil in the treatment of otitis media have been established in the age groups 6 months to 12 years. Use of cefprozil for the treatment of otitis media is supported byevidence from adequate and well-controlled studies of cefprozil in pediatric patients. (See CLINICAL STUDIES.)The safety and effectiveness of cefprozil in the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections have been established in the age groups 2 to 12 years. Use of cefprozil for the treatment of these infections is supported by evidence from adequate and well-controlled studies of cefprozil in pediatric patients.The safety and effectiveness of cefprozil in the treatment of acute sinusitis have been established in the age groups 6 months to 12 years. Use of cefprozil in these age groups is supported by evidence from adequate and well-controlled studies of cefprozil in adults.®®222Safety and effectiveness in pediatric patients below the age of 6 months have not been established for the treatment of otitis media or acute sinusitis, or below the age of 2 years for the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections. However, accumulation of other cephalosporin antibiotics in newborn infants (resulting from prolonged drug half-life in this age group) has been reported.Geriatric UseOf the more than 4500 adults treated with cefprozil in clinical studies, 14% were 65 years and older, while 5% were 75 years and older. When geriatric patients received the usual recommended adult doses, their clinical efficacy and safety were comparable to clinical efficacy and safety in nongeriatric adult patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals to the effects of cefprozil cannot be excluded (see CLINICAL PHARMACOLOGY).Cefprozil is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. See DOSAGE AND ADMINISTRATION for dosing recommendations for patients with impaired renal function.ADVERSE REACTIONSThe adverse reactions to cefprozil are similar to those observed with other orally administered cephalosporins. Cefprozil was usually well tolerated in controlled clinical trials. Approximately 2% of patients discontinued cefprozil therapy due to adverse events.The most common adverse effects observed in patients treated with cefprozil are: Gastrointestinal:Diarrhea (2.9%), nausea (3.5%), vomiting (1%), and abdominal pain (1%).Hepatobiliary:Elevations of AST (SGOT) (2%), ALT (SGPT) (2%), alkaline phosphatase (0.2%), and bilirubin values (<0.1%). As with some penicillins and some other cephalosporin antibiotics, cholestatic jaundice has been reported rarely.Hypersensitivity:Rash (0.9%), urticaria (0.1%). Such reactions have been reported more frequently in children than in adults. Signs and symptoms usually occur a few days after initiation of therapy and subside within a few days after cessation of therapy.CNS:Dizziness (1%). Hyperactivity, headache, nervousness, insomnia, confusion, and somnolence have been reported rarely (<1%). All were reversible.Hematopoietic:Decreased leukocyte count (0.2%), eosinophilia (2.3%).Renal:Elevated BUN (0.1%), serum creatinine (0.1%).Other:Diaper rash and superinfection (1.5%), genital pruritus and vaginitis (1.6%).The following adverse events, regardless of established causal relationship to cefprozil, have beenrarely reported during postmarketing surveillance: anaphylaxis, angioedema, colitis (including pseudomembranous colitis), erythema multiforme, fever, serum-sickness like reactions, Stevens-Johnson syndrome, and thrombocytopenia.Cephalosporin Class Paragraph:In addition to the adverse reactions listed above which have been observed in patients treated with cefprozil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:Aplastic anemia, hemolytic anemia, hemorrhage, renal dysfunction, toxic epidermal necrolysis, toxic nephropathy, prolonged prothrombin time, positive Coombs’ test, elevated LDH, pancytopenia,neutropenia, agranulocytosis.Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. (See DOSAGE AND ADMINISTRATION and OVERDOSAGE) If seizures associated with drug therapy occur, the drug should be discontinued.Anticonvulsant therapy can be given if clinically indicated.OVERDOSAGESingle 5000 mg/kg oral doses of cefprozil caused no mortality or signs of toxicity in adult, weanling,or neonatal rats, or adult mice. A single oral dose of 3000 mg/kg caused diarrhea and loss of appetite in cynomolgus monkeys, but no mortality.Cefprozil is eliminated primarily by the kidneys. In case of severe overdosage, especially in patients with compromised renal function, hemodialysis will aid in the removal of cefprozil from the body.DOSAGE AND ADMINISTRATIONCefprozil for oral suspension is administered orally.Population/InfectionDosage (mg) Duration (days)ADULTS (13 years and older)UPPER RESPIRATORY TRACTPharyngitis/Tonsillitis500 q24h 10 Acute Sinusitis250 q12h or 10 (For moderate to severe infections, the higher dose should be used)500 q12h LOWER RESPIRATORY TRACTSecondary Bacterial Infection of Acute Bronchitis and Acute BacterialExacerbation of Chronic Bronchitis500 q12h 10 SKIN AND SKIN STRUCTUREUncomplicated Skin and Skin Structure Infections 250 q12h or10500 q24h or500 q12h CHILDREN (2 years-12 years)UPPER RESPIRATORY TRACTPharyngitis/Tonsillitis7.5 mg/kg q12h 10 SKIN AND SKIN STRUCTUREUncomplicated Skin and Skin Structure Infections20 mg/kg q24h 10 INFANTS & CHILDREN (6 months-12 years)UPPER RESPIRATORY TRACTOtitis Media15 mg/kg q12h 10 (See INDICATIONS AND USAGE and CLINICAL STUDIES)Acute Sinusitis 7.5 mg/kg q12h or 10 a b a b bIn the treatment of infections due to Streptococcus pyogenes, cefprozil for oral suspension should be administered for atleast 10 days.Not to exceed recommended adult doses.(For moderate to severe infections, the higher dose should be used)15 mg/kg q12hRenal Impairment:Cefprozil may be administered to patients with impaired renal function. The following dosage schedule should be used.Creatinine Clearance (mL/min)Dosage (mg) Dosing Interval*30-120 standard standard 0-2950% of standardstandardHepatic Impairment:No dosage adjustment is necessary for patients with impaired hepatic function.HOW SUPPLIEDCefprozil for oral suspension, USP is a pink coloured powder, forming pink coloured suspension with characteristic odour on constitution.Cefprozil For Oral Suspension, USP 125 mg/5 mL is available as follows:50 mL Bottle NDC 68180-401-0175 mL Bottle NDC 68180-401-02100 mL Bottle NDC 68180-401-03Cefprozil For Oral Suspension, USP 250 mg/5 mL is available as follows:50 mL Bottle NDC 68180-402-0175 mL Bottle NDC 68180-402-02100 mL Bottle NDC 68180-402-03All powder formulations for oral suspension contain cefprozil in a bubble-gum flavored mixture.Reconstitution Directions for Oral Suspension:Prepare the suspension at the time of dispensing; for ease in preparation, add water in two portions and shake well after each aliquot.Total Amount of Water Required for ReconstitutionBottle Size Final Concentration 125 mg/5 mL Final Concentration 250 mg/5 mL50 mL 36 mL 36 mL 75 mL 53 mL 53 mL 100 mL70 mL 70 mLStore dry powder at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].Store constituted suspension in refrigerator. Discard after 14 days.Preserve in tight containers.a b Cefprozil is in part removed by hemodialysis; therefore, cefprozil should be administered after the completion of hemodialysis.*The incidence of adverse events in the cefprozil arm was comparable to the incidence of adverse events in the control arm (agent that contained a specific ß-lactamase inhibitor).REFERENCES1. National Committee for Clinical Laboratory Standards. Methods for Dilution AntimicrobialSusceptibility Tests for Bacteria that Grow Aerobically-Third Edition. Approved Standard NCCLS Document M7-A3, Vol.13, No. 25, NCCLS, Villanova, PA, December 1993.2. National Committee for Clinical Laboratory Standards. Methods for Antimicrobial SusceptibilityTesting of Anaerobic Bacteria-Third Edition. Approved Standard NCCLS Document M11-A3, Vol.13, No. 26, NCCLS, Villanova, PA, December 1993.3. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial DiskSusceptibility Tests -Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.®®4. Clintest and Clinistix are registered trademarks of Bayer HealthCare LLC.Manufactured for: Manufactured byLupin Pharmaceuticals, Inc. Lupin LimitedBaltimore, Maryland 21202 Mumbai 400 098United States INDIARevised December 2007 ID#: 213042PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68180-401-01Cefprozil for Oral Suspension USP125 mg/5 mLRx only50 mL (when mixed)NDC 68180-402-01Cefprozil for Oral Suspension USP 250 mg/5 mLRx only50 mL (when mixed)CEFPROZILcefprozil powder, for suspensionProduct InformationProduct T ype HUMAN PRESCRIPTION DRUG Ite m Code (Source)NDC:57297-401 Route of Administration ORALActive Ingredient/Active MoietyIngredient Name Basis of Strength Strength CEFPRO ZIL (UNII: 4W0459ZA4V) (CEFPROZIL ANHYDROUS - UNII:1M698F4H4E)CEFPROZIL ANHYDROUS125 mg in 5 mLInactive IngredientsIngredient Name Strength ANHYDRO US CITRIC ACID (UNII: XF417D3PSL)ASPARTAME (UNII: Z0H242BBR1)FD&C RED NO. 40 (UNII: WZB9127XOA)GLYCINE (UNII: TE7660XO1C)SILICO N DIO XIDE (UNII: ETJ7Z6XBU4)SO DIUM BENZO ATE (UNII: OJ245FE5EU)SUCRO SE (UNII: C151H8M554)CARBO XYMETHYLCELLULO SE SO DIUM (UNII: K679OBS311)CELLULO SE, MICRO CRYSTALLINE (UNII: OP1R32D61U)SO DIUM CHLO RIDE (UNII: 451W47IQ8X)。
多枝柽柳中的酚酸类化学成分

多枝柽柳中的酚酸类化学成分多枝柽柳在宁夏民间常用于治疗类风湿关节炎,为了寻找其中的有效成分,为药用开发提供科学依据,对多枝柽柳的化学成分进行了研究。
利用硅胶柱色谱、凝胶柱色谱和重结晶法对多枝柽柳的化学成分进行分离纯化,化合物的结构依据其理化性质和波谱数据得以鉴定。
从正丁醇层中分离得到10个化合物,分别鉴定为monodecarboxyellagic acid(1),鞣花酸(2),3,3′-二甲基鞣花酸(3),3,3′-二甲基鞣花酸-4-O-β-D-葡萄糖苷(4),3,3′-二甲基鞣花酸-4′-O-α-D-阿拉伯糖苷(5),阿魏酸(6),异阿魏酸(7),咖啡酸(8),4-氧-乙酰基咖啡酸(9),4-甲基-1,2-二苯酚(10)。
除化合物7异阿魏酸外,其余9个化合物均为首次从该植物中分离得到,其中5,9,10为首次从该属植物中分离得到。
标签:多枝柽柳;化学成分;酚酸1 材料熔点测定使用XT4数字显微熔点测定仪(上海精科实业有限公司,温度未校正);紫外光谱测定使用Shimadzu UV-2450型紫外-可见分光光度计(日本岛津制作所);ESI-MS用Thermo TSQ Quantum Access Max质谱仪测定(美国Thermo公司);NMR测定使用Bruker A V ANCE 400型核磁共振波谱仪测定(瑞士Bruker公司)。
柱色谱硅胶(100~200目)和薄层色谱硅胶(GF254)均购于青岛海洋化工厂;葡聚糖凝胶Sephadex LH-20为美国Pharmacia公司产品;所用溶剂均为分析纯,购于天津江天化工技术有限公司;多枝柽柳的细嫩枝条采自宁夏银川,经宁夏药品检验所中药研究室韩文欣主任药师鉴定为柽柳科植物多枝柽柳T. rasissima,标本(No.2011051001)保存于宁夏医科大学药学院中药标本室。
2 提取與分离取自然阴干的多枝柽柳细嫩枝条15 kg,粉碎后用45 L 75%乙醇回流提取3次,每次3 h,合并提取液,减压浓缩得浸膏1 300 g。
马沙骨化醇——精选推荐

马沙骨化醇详细信息
本文详细介绍了马沙骨化醇的产品信息,包括中英文名称、别名、cas号、分子结构等基本信息,以及产品的物化性质、产品用途、产品上下游产品等综合信息,为广大化学品研究、化工产品生产制造从业者提供专业的产品信息。
本文所有信息来源化工字典。
马沙骨化醇
/detail-马沙骨化醇.html 产品介绍:
中文名称:马沙骨化醇
中文别名:玛莎骨化醇
英文名称:Maxacalcitol
英文别名:
CAS:103909-75-7
分子结构式:
EINECS:
分子式:C26H42O4
分子量:418.6093
风险术语:
安全术语:
物化性质:
熔点:
相对密度:1.09g/cm3 溶解性:
用途:
上游原料:
下游产品:。
JP日本药典(药局方)标准品汇总信息-2016-update

头孢曲松钠标准品及杂质对照品
Ceftriaxone sodium
74578-69-1
58
硫酸头孢匹罗标准品及杂质对照品
CefpiromeSulfate
98753-19-6
59
头孢泊肟酯标准品及杂质对照品
CefpodoximeProxetil
87239-81-4
60
头孢米诺钠标准品及杂质对照品
CefminoxSodium
84
法罗培南钠标准品及杂质对照品
FaropenemSodium
122547-49-3
85
非奈西林钾标准品及杂质对照品
PhenethicillinPotassium
132-93-4
86
硫酸新霉素标准品及杂质对照品
FradiomycinSulfate(混合物)
119-04-0,66-86-4, 1405-10-3
33103-22-9,33137-73-4
14
盐酸土霉素标准品及杂质对照品
OxytetracyclineHydrochloride
2058-46-0
15
硫酸卡那霉素标准品及杂质对照品
KanamycinMonosulfate
25389-94-0
16
卡鲁莫南钠标准品及杂质对照品
CarumonamSodium
87
硫酸卡那霉素B标准品及杂质对照品
BekanamycinSulfate
70550-99-1
88
硫酸派来霉素标准品及杂质对照品
PeplomycinSulfate
70384-29-1
89
青霉素钾标准品及杂质对照品
BenzylpenicillinPotassium
MetaPolyzyme DNA free 产品说明书

MAC4LDFpis Rev 04/221Product InformationMetaPolyzyme, DNA freeSuitable for Microbiome researchMAC4LDFSynonym: Multilytic Enzyme Mix Storage Temperature –20 °CProduct DescriptionMetagenomics investigates all DNA that has been isolated directly from given single samples, such as environmental samples or biological organisms.1,2Metagenomics allows for the investigation of microbes that exist in extreme environments, and which have been historically difficult to isolate, culture, andstudy.3 Metagenomics has revealed the existence of novel microbial species.4 Applications ofmetagenomics work include public health dataanalysis,5,6 discovery of novel proteins, enzymes and natural products,7,8 environmental studies,9,10 and agricultural investigations.11,12Microbes are difficult to disrupt because the cell walls may form capsules or resistant spores. DNA can be extracted by using lysing enzymes such as lyticase, chitinase, zymolase, and gluculase to induce partial spheroplast formation. Spheroplasts are subsequently lysed to release DNA.MetaPolyzyme products (Cat. Nos. MAC4L, MAC4LDF) are based on a multi-lytic enzyme mixture, originally developed by Scott Tighe, for use in microbiome and DNA extraction efficiency studies, and formulated for effective lysis of microbiome samples from extreme environments. MetaPolyzyme was originally evaluated and developed in consultation and collaboration with the Association of Biomolecular Resource Facilities (ABRF) Metagenomics and Microbiome ResearchGroup (MMRG; formerly the Metagenomics Research Group, MGRG).13-16Studies of microbial communities have beenenhanced by the use of culture-independent analytical techniques such as 16S rRNA gene sequencing and metagenomics. DNA contamination during sample preparation is a major problem of sequence-based approaches. Extraction reagents free of DNA contaminants are thus essential. MetaPolyzyme, DNA free was developed to address the need for DNA-free reagents, to minimize microbial DNA contamination from reagents. This productundergoes strict quality control testing to ensure the absence of detectable levels of contaminatingmicrobial DNA using 35 cycles PCR amplification of 16S and 18S rDNA using universal primer sets.Precautions and DisclaimerFor R&D use only. Not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.ReagentThe enzymes in MetaPolyzyme, DNA free are:• Mutanolysin • Achromopeptidase • Lyticase • Chitinase • Lysostaphin •LysozymeAll the enzymes are individually tested for theabsence of contaminating DNA using 16S and 18S PCR amplification.Mutanolysin (from Streptomyces globisporus )Mutanolysin is a muralytic enzyme (muramidase) that cleaves the β-N -acetylmuramyl-(1→4)-N -acetylglucosamine linkage of the bacterial cell wall peptidoglycan-polysaccharide, particularly the β(1→4) bond in MurNAc-GlcNAc.17 Mutanolysin particularly acts on many Gram-positive bacteria, where the enzyme’s carboxy -terminal moietiesparticipate in the recognition and binding of unique cell wall structures.MAC4LDFpis Rev 04/22AchromopeptidaseAchromopeptidase (known also as β-lytic protease 18) has potent bacteriolytic activity on many Gram-positive aerobic bacteria 19 with high lytic activity, against bacterial strains with the A1α chemotype (such as Aerococcus viridans ), and the A3αchemotype (such as Staphylococcus epidermidis ) for cell wall peptidoglycan structures. The enzyme has been reported to have particular recognition for Gly-X sites in peptide sequences, and for Gly-Gly and ᴅ-Ala-X sites in peptidoglycans.20Lyticase (from Arthrobacter luteus )Lyticase is useful in digestion of linear glucosepolymers with β(1→3) linkages, of yeast glycan coats and for spheroplast formation, and of the cell wall of active yeast cells.Chitinase (from Streptomyces griseus )Chitinase degrades chitin by enzymatic hydrolysis to N-acetyl-D-glucosamine. Degradation occurs via two consecutive enzyme reactions: •Chitodextrinase-chitinase, apoly(1,4-β-[2-acetamido-2-deoxy-D-glucoside])-glycanohydrolase, removes chitobiose units from chitin.•N-acetylglucosaminidase-chitobiase cleaves the disaccharide to its monomer subunits, N-acetyl-D-glucosamine (NAGA).Lysostaphin (from Staphylococcus staphylolyticus )Lysostaphin is a lytic enzyme with activity against Staphylococcus species, including S. aureus . Lysostaphin has hexosaminidase, amidase, and endopeptidase activities. It cleaves polyglycine crosslinks in the cellular wall of Staphylococcus species, which leads to cell lysis.21,22Lysozyme (from chicken egg white)Lysozyme hydrolyzes β(1→4) linkages betweenN -acetylmuraminic acid and N -acetyl-D-glucosamine residues in peptidoglycan, and betweenN -acetyl-D-glucosamine residues in chitodextrin. Lysozyme lyses the peptidoglycan cell wall of Gram-positive bacteria.23Storage/StabilityThis product ships at cooler temperature conditions. Long-term storage at –20 °C is recommended. Reconstituted solutions of MetaPolyzyme, DNA free may be stored at –20 °C, but long-term solution stability has not been examined.Preparation InstructionsBecause of the great diversity of samples formetagenomics studies, it will be necessary for each researcher to work out particular conditions for optimal sample preparation and treatment. It is recommended to reconstitute MetaPolyzyme, DNA free in sterile PBS buffer, pH 7.5 (no EDTA, calcium or magnesium present in solution). The following is a sample procedure, to be scaled appropriately:1. Add 100 µL of sterile PBS (pH 7.5) to 1 vial ofMetaPolyzyme, DNA free.1.1. Resuspend by gentle agitation or pipetting. 1.2. Set solution aside at 2-8 °C until Step 7. 2. Thoroughly suspend sample in sterile PBS(pH 7.5). 3. Add 200 µL of sample in PBS to a 2 mLpolypropylene microcentrifuge tube. 4. Optional pellet wash:4.1. To sample tube, add 1 mL of PBS (pH 7.5). 4.2. Vortex, centrifuge and remove supernatant. 4.3. Repeat pellet wash two more times ifneeded. 5. Resuspend pelleted sample in 150 µL of PBS(pH 7.5). Vortex thoroughly.6. Optional: if solution will sit for more than 4 hours,sodium azide may be added to 0.02%. 7. Add 20 µL (*) of MetaPolyzyme, DNA free tosample solution. 8. Incubate at 35 °C for 2-24 hours.9. Continue with standard DNA extraction protocol. (*) The optimal volume and concentration of MetaPolyzyme, DNA free may vary in different experiments.References1. Gilbert, J.A., and Dupont, C.L., Ann. Rev. MarineSci., 3, 347-371 (2011). 2. Kang, H.S., and Brady, S.F., J. Am. Chem. Soc.,136(52), 18111-18119 (2014). 3. Ufarté, L. et al., Biotechnol. Adv., 33(8),1845-1854 (2015). 4. Davison, M. et al., Photosynth. Res., 126(1),135-146 (2015). 5. Afshinnekoo, E. et al., Cell Syst., 1(1), 72-87(2015).The life science business of Merck operatesas MilliporeSigma in the U.S. and Canada.Merck and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates.All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.© 2022 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.MAC4LDFpis Rev 04/22 DK,DT,GCY,TJ,RBG,SBC,MAM36.The MetaSUB International Consortium,Microbiome, 4, 24 (2016). [Erratum inMicrobiome, 4, 45 (2016).]7.Trinidade, M. et al., Front. Microbiol., 6, 890(2015).8.Coughlan, L.M. et al., Front. Microbiol., 6, 672(2015).9.Palomo, A. et al., ISME J., 10(11), 2569-2581(2016).10.Pold, G. et al., Appl. Environ. Microbiol., 82(22),6518-6530 (2016).11.Mitra, N. et al., J. Gen. Virol., 97(8), 1771-1784(2016).12.Theuns, S. et al., Infect. Genet. Evol., 43,135-145 (2016).13.Baldwin, D.A. et al., "Life at the Extreme", ABRFMetagenomics Research Group Poster 2015,presented at the ABRF 2015 Conference, St.Louis, MO, USA, March 28-31, 2015.14.Baldwin, D.A. et al., "Implementing NewStandards in Metagenomics and the ExtremeMicrobiome Project", ABRF MetagenomicsResearch Group Poster 2016, presented at theABRF 2016 Conference, Fort Lauderdale, FL, USA, February 20-23, 2016.15.McIntyre, A. et al., "Life at the Extreme: TheABRF Metagenomics Research Group", ABRFMetagenomics Research Group Poster 2017,presented at the ABRF 2017 Conference, SanDiego, CA, March 25-28, 2017.16.Tighe, S. et al., J. Biomol. Tech., 28(1), 31-39(2017).17.Gründling, A., and Schneewind, O., J. Bacteriol.,188(7), 2463-2472 (2006).18.Li, S.L. et al., J. Bacteriol., 172(11), 6506-6511(1990).19.Ezaki, T., and Suzuki, S., J. Clin. Microbiol.,16(5), 844-846 (1982). 20.Li, S. et al., J. Biochem., 124(2), 332-339(1998).21.Browder, H.P. et al., Biochem. Biophys. Res.Commun., 19, 383-389 (1965).22.Robinson, J.M. et al., J. Bacteriol., 137(3),1158-1164 (1979).23.Vocaldo, D.J. et al., Nature, 412(6849), 835-838(2001).NoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose. The information in this document is subject to change without notice and should not be construed as a commitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document.Technical AssistanceVisit the tech service page at/techservice.Standard WarrantyThe applicable warranty for the products listed in this publication may be found at /terms. Contact InformationFor the location of the office nearest you, go to /offices.。
愈创木酚MSDS

储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、酸类、食用化学品分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。
第八部分:接触控制/个体防护
回目录
职业接触限值
中国MAC(mg/m3):
未制定标准
前苏联MAC(mg/m3):
未制定标准
TLVTN:
致敏性:
致突变性:
致畸性:
致癌性:
第十二部分:生态学资料
回目录
生态毒理毒性:
生物降解性:
非生物降解性:
生物富集或生物积累性:
其它有害作用:
无资料。
第十三部分:废弃处置
回目录
废弃物性质:
废弃处置方法:
处置前应参阅国家和地方有关法规。建议用焚烧法处置。
废弃注意事项:
第十四部分:运输信息
回目录
危险货物编号:
本品可燃,具强刺激性。
第四部分:急救措施
回目录
皮肤接触:
立即脱去污染的衣着,用大量流动清水冲洗至少15分钟。就医。
眼睛接触:
立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。
吸入:
脱离现场至空气新鲜处。就医。
食入:
用水漱口,给饮牛奶或蛋清。就医。
第五部分:消防措施
回目录
危险特性:
遇明火、高热可燃。
回目录
参考文献:
填表时间:
填表部门:
数据审核单位:
修改说明:
其他信息:
MSDS修改日期:
无资料
UN编号:
无资料
包装标志:
包装类别:
Z01
包装方法:
无资料。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Meropenem trihydrate CAS No.:
119478-56-7Cat. No.:
HY-13678A
Product Data Sheet
MWt:
437.51Formula:
C17H31N3O8S Purity :>98%
Solubility:
DMSO 77 mg/mL; Water 8 mg/mL;
Mechanisms:
Biological Activity:
Meropenem (trihydrate)is a carbapenem antibiotic which displaying a broad spectrum of
Pathways:Anti-infection; Target:Antibacterial Ethanol <1 mg/mL
Meropenem (trihydrate) is a carbapenem antibiotic, which displaying a broad spectrum of
antibacterial activity.
Target: Antibacterial Meropenem (trihydrate), a new parenteral carbapenem demonstrated increased activity as
compared to imipenem against 336 strains of Neisseria gonorrhoeae, 119 strains of Haemophilus influenzae, and 110 strains of H. Ceftriaxone and ciprofloxacin demonstrated activity superior to that of both carbapenems while the activity of ceftazidime was similar to that of meropenem [1].Meropenem (trihydrate), like imipenem and various experimental penems, may overcome the
resistance problems presented by Class I beta-lactamases [2]. Meropenem (trihydrate) was rapidly
References:
[1]. Slaney, L., et al., In-vitro activity of meropenem against Neisseria gonorrhoeae, Haemophilus influenzae and H. ducreyi from Canada and Kenya. J Antimicrob Chemother, 1989. 24 Suppl A: p.
183-6.p p y []p (y )p y penetrated to the pleural effusion and was retained for a more prolonged time in the pleural effusion than in the blood of patients with accumulated pleural effusion, and it suggested th...
[2]. Yang, Y.J. and D.M. Livermore, Interactions of meropenem with class I chromosomal beta-
lactamases. J Antimicrob Chemother, 1989. 24 Suppl A: p. 207-17.[3]. Makino, J., et al., [Pharmacokinetic study of penetration of meropenem into pleural effusion in
patients with pleurisy]. Jpn J Antibiot, 2002. 55(1): p. 77-88.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
