国外专利EVA--Titre du document
EVA的国内应用案例

EVA的国内应用案例国内应用案例:EVA业绩考核如何驱动企业价值制造 (2)我国企业薪酬鼓舞现状与EVA奖金打算 (16)我国企业EVA应用现状及存在的问题 (31)国内应用案例:某高新技术企业EVA红利银行与虚拟股票期权相结合设计 (42)国内应用案例:EVA在某钢绳公司部门考核中的应用 (50)国内应用案例:某高新技术企业EVA业绩考核设计 (54)国内应用案例:EVA业绩考核如何驱动企业价值制造EV A〔经济增加值〕,是由美国的约尔.思腾恩(Joel Stern)与贝内特.思图尔特(Bennett Stewart)最先正式创立的。
通过多年的进展与完善,EV A差不多扩展成为一个集业绩评判、资本预算、财务决策、奖金打算与价值评估等功能于一体的治理系统。
目前,EV A已在全球数百家企业中应用,许多跨国公司,如可口可乐、西门子、索尼、美国邮政总署、新加坡航空公司等纷纷将EV A引入企业的经营治理并取得了成功。
我国引入EV A只有短短数年的时刻,目前已有部分企业采纳和实施了EV A治理体系,并取得了一定成效。
本文拟通过案例的形式解析EV A的4M价值治理体系在企业中的具体应用。
EV A的4M价值治理体系EV A价值治理包括利润表治理与资产负债表治理两个方面,同时揭示了企业的经营效率和资本的使用效率。
EV A 应用在企业治理系统中,并非简单的财务指标,而是一套完整的价值治理体系。
该体系以〝4M〞理论为基础上,围绕企业的〝价值链〞和〝制度链〞,构建了EV A治理体系的模式。
该体系由业绩考核体系、治理决策模式、企业鼓舞机制和企业经营理念四部分组成。
下文我们将通过案例来解读EV A 4M价值治理体系在企业中的应用。
案例分析1:EV A在企业业绩考核(Measurement)中的应用企业背景:大型多元化国有控股企业A,电气与电子行业应用背景:在20世纪90年代末,A差不多是一个庞大的、多元化的集团企业。
公司一味追求大规模快速增长,旗下部分业绩表现不尽人意。
国内外EVA产品的发展及市场应用概况修订版

国内外E V A产品的发展及市场应用概况集团标准化小组:[VVOPPT-JOPP28-JPPTL98-LOPPNN]国内外EVA产品的发展及市场应用概况2009-8-1110:36:36来源:中国塑料改性技术咨询网乙烯一醋酸乙烯共聚物(EVA)是最主要的乙烯共聚物之一,按共聚物中醋酸乙烯(VA)的含量可分为三大类,即EVA树脂、EVA弹性体及EVA乳液。
与聚乙烯相比,EVA树脂由于在分子链中引人了VA单体,提高了聚合物的支化度,从而降低了结晶度,提高了柔韧性、抗冲击性、填料相容性和热密封性,具有较好的耐环境应力开裂性,良好的光学性能、耐低温性及无毒的特点,因此用途非常广泛。
1生产工艺目前,国内外EVA产品的生产工艺主要有4种:高压法连续本体聚合、中压悬浮聚合、溶液聚合和乳液聚合。
其中,溶液聚合和乳液聚合工艺应用较少。
市场上的EVA树脂大多采用高压法连续本体聚合工艺生产,VA含量(质量分数,以下同)一般为5%-40%。
高压法连续本体聚合工艺通常采用高压釜反应器或管式反应器,工艺原理类似于低密度聚乙烯(LDPE)生产工艺。
管式聚合的典型工艺有巴斯夫管式工艺、Lmhausem/Ruhrchemie管式法工艺、俄罗斯管式法工艺、住友化学管式法工艺和VEBLeuna-Werke管式法工艺等。
管式聚合工艺可生产VA含量小于30%的EVA,管式反应器的单程转化率为25%~35%。
釜式聚合的典型工艺有杜邦、USI等釜式法工艺,可生产VA含量小于40%的EVA,釜式反应器的单程转化率为10%-20%。
2国内外EVA发展概况2.1国外发展概况1960年,美国杜邦公司采用高压法连续本体聚合工艺,首先实现了低VA含量的EVA工业化生产。
随后,UCC,Bayer,Exxon、日本三井、东洋曹达、住友、尤尼卡等30多家公司相继投产EVApEVA作为塑料新品种得到迅速发展。
2003年,全世界EVA生产能力约1700kt以上,其中美国约680kt/a,日本约200kt/a,西欧约450kt/a,亚洲其他国家约300kt/a。
美国专利:包含涂浸润剂和脱浸润剂步骤的玻璃纤维材料制备方法及适用于实施所述方法的设备

《玻璃纤维》2018年 第5期 43专利摘要美国专利:包含涂浸润剂和脱浸润剂步骤的玻璃纤维材料制备方法及适用于实施所述方法的设备公布号:US2018230047 (A1)公布日:2018-08-16申请(专利权)人:LAIR LIQUIDE SA POUR LETUDE ET LEXPLOITATION DES PROCEDES GEORGES CLAUDE[法国]; LAIR LIQUIDE SAPOUR IETUDE ET IEXPLOITATION DES PROCEDES GEORGES CLAUDE[法国]发明人:JARRY LUC[法国]; JOUMANIYOUSSEF[法国]; BEASSE GREGOIRE[法国]摘要:本专利述及一种玻璃纤维材料制备方法和设施,其中经由纺丝、拉丝、涂浸润剂和集束把熔融玻璃转变成纤维的步骤,接着是生产所得玻璃纤维材料的步骤,然后加热使这些材料去除浸润剂。
来自熔炉的烟气被用于以两个步骤预热燃料:第一步,烟气通过热交换来加热空气;第二步,热空气通过热交换来预热燃料,该空气然后用于该玻璃纤维材料的去浸润剂步骤中。
中国专利:一种高模量玻璃纤维组合物以及玻璃纤维公布号: CN108373268 (A)公布日:2018-08-07申请(专利权)人:重庆国际复合材料股份有限公司发明人:韩利雄;刘奇;何建明;郝名扬;张聪;赵世斌;樊正华;张亮;张燕;刘也摘要: 本专利述及一种高模量玻璃纤维组合物以及玻璃纤维。
本专利提供了一种高模量玻璃纤维组合物,包括如下组分:50%~55.9%重量百分比的SiO 2 ;20.5%~28.5%重量百分比的Al 2O 3;72.5%~82.5%重量百分比的SiO 2与Al 2O 3的总含量;Al 2O 3/SiO 2质量分数比值0.38~0.56;0~3.0%重量百分比的B 2O 3;0.2%~1.5%重量百分比的TiO 2;0~2.0%重量百分比的ZnO ;0~2.0%重量百分比的ZrO2;0.1%~0.6%重量百分比的Fe 2O 3;6%~8.1%重量百分比的CaO ;9%~12%重量百分比的MgO ;15.8%~20%重量百分比的CaO与MgO的总含量;MgO/CaO质量分数比值1.2~2.0;0.2%~1.0%重量百分比的Na 2O、K 2O与Li 2O的总含量;上述组分总计100%。
Evaporation device for volatile substances
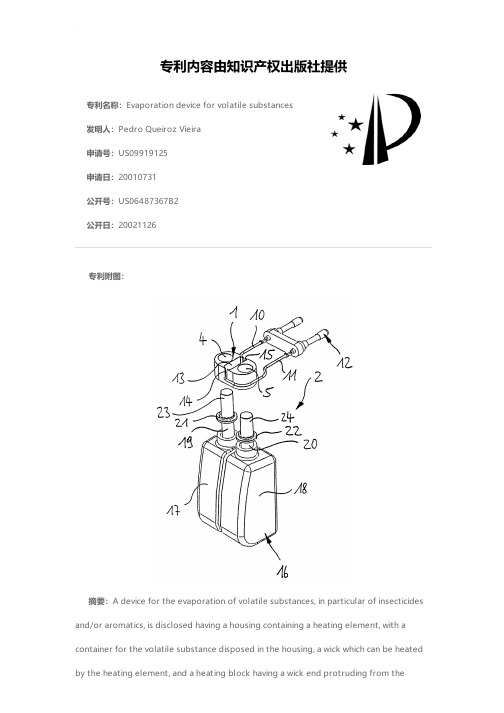
专利名称:Evaporation device for volatile substances发明人:Pedro Queiroz Vieira申请号:US09919125申请日:20010731公开号:US06487367B2公开日:20021126专利内容由知识产权出版社提供专利附图:摘要:A device for the evaporation of volatile substances, in particular of insecticides and/or aromatics, is disclosed having a housing containing a heating element, with acontainer for the volatile substance disposed in the housing, a wick which can be heated by the heating element, and a heating block having a wick end protruding from thecontainer along a wick axis. The improved device includes at least one additional wick opening formed within the heating block; at least one additional container for containing an additional volatile substance for evaporation, and the additional wick opening being operatively associated with the additional container. At least one additional wick is carried in the additional container, having a wick end extending through the additional wick opening for evaporation of the additional volatile substance in the additional container.申请人:C.T.R. CONSULTORIA TECNICA E REPRESENTACOEES LTA.代理机构:McNair Law Firm, P.A.代理人:Cort Flint更多信息请下载全文后查看。
活性物质薄片[发明专利]
![活性物质薄片[发明专利]](https://img.taocdn.com/s3/m/fc14eb6cb9d528ea80c77942.png)
专利名称:活性物质薄片
专利类型:发明专利
发明人:玛丽娜·莫罗兹,兰吉塔·舍戈卡尔,约恩·维德曼申请号:CN201780095141.8
申请日:20170922
公开号:CN111133088A
公开日:
20200508
专利内容由知识产权出版社提供
摘要:本发明涉及一种活性物质薄片及其制备方法。
此外,本发明涉及活性物质薄片用作/用于洗衣护理剂、手洗洗涤剂或餐具洗涤剂,以及从这些制剂中选择的由活性物质薄片组成或包含活性物质薄片的产品。
申请人:西姆莱斯有限公司
地址:德国霍尔茨明登
国籍:DE
代理机构:北京集佳知识产权代理有限公司
更多信息请下载全文后查看。
聚醚类高分子化合物、使用其形成的离子导电性高分子组合物和电化

专利名称:聚醚类高分子化合物、使用其形成的离子导电性高分子组合物和电化学设备
专利类型:发明专利
发明人:西浦圣人,河野通之
申请号:CN02106784.8
申请日:20020308
公开号:CN1380348A
公开日:
20021120
专利内容由知识产权出版社提供
摘要:本发明的目的是提供可提高室温附近离子导电率的聚醚类高分子和使用它的离子导电性高分子组合物和电化学设备。
上述目的是通过使用具有下述特征的聚醚类高分子化合物达到的。
该聚醚类高分子化合物特征在于,具有式(1)所示结构单元和式(2)和/或式(3)所示结构单元,在分子链的各末端具有聚合性官能团和/或非聚合性官能团。
申请人:第一工业制药株式会社
地址:日本京都府
国籍:JP
代理机构:中国国际贸易促进委员会专利商标事务所
代理人:王杰
更多信息请下载全文后查看。
美国专利阅读以及国际专利INID码解释

美国专利阅读以及国际专利INID码解释如何阅读美国专利发明人最不想遇到的状况莫过于花费许多时间与精力将自己新的构想付诸实现后,才发现同样的发明早已有人做过。
避免这种状况的最好办法,就是在研发初期做好信息收集的工作。
根据国际经济暨发展组织(Organization for Economic Cooperation and Development:OECD)的统计结果,有百分之八十以上的科技知识被描述在专利文件中,而大部分被描述在专利中的技术并没有被记载在其它的发行刊物。
因此,发明人绝不可忽视专利信息的重要性。
什么是专利信息?提到专利,一般人会认为专利只是一个保护产品的法律权利。
事实上,除了可以保护产品外,专利也像是产品的使用手册,有文字及图标叙述产品的组件、特征、用途,以及使用方法。
此外,专利信息还包括任何可以从专利局发行的文件中所获得的信息,包括技术资料、市场信息、法律信息、以及公司的任何信息。
如何阅读美国专利?在说明如何收集专利信息前,先告诉各位如何阅读美国专利。
以美国的「发明专利」为例,专利内容常分为四大部份:(一)封面:包括专利的基本资料﹙如专利号码、专利申请日、发明人姓名等﹚和发明摘要;(二)图标:包括描绘发明的图标;(三)说明书的内容部分:包括文字描述;以及(四)申请专利范围:包括请求项(或称权利项)定义专利的保护范围。
(一)封面:封面提供专利的基本资料,在每一笔基本资料旁都印有以 [ ] 框起来的号码,也就是大家熟知的 INID 码(Internationally agreed Numbers for the Identification of Bibliographic Data code;INID code)。
每个 INID 码各代表不同的数据项,例如 [54] 代表发明名称,[76] 代表发明人姓名与户籍地。
由于 INID 码是国际统一的号码,因此各国的专利都是以 [54] 来表示发明名称。
EVA 乙烯 醋酸乙烯共聚物 生产技术配方工艺

EVA 乙烯醋酸乙烯共聚物生产技术配方工艺本站经营各种专利技术,致富项目等,网址: QQ:362303563 电话:专利致富网期待您的光临!并以优质的服务保证您的满意哦!1、专利技术也可以单项或者部分项目购买,单项专利技术,每一项收费50元(网传价)。
购两项赠送一项。
2、这里是技术资料,不是实物产品,请注意哦!3、本店提供定制服务。
把您想要的信息关键词提供给我们如“火锅”“脱发”“美容”........等,我们给您检索出相关的所有专利,价格另行商议。
4、本店所需资料均可网传,专利,不需邮费。
5、一个有一定知识背景的人通过阅读专利说明书就能生产出产品。
我国专利法规定,用于科研目的不属于侵权行为。
通过对专利的查看,能够使查看人快速识别哪些专利信息是有价值的,并加以充分、有效地利用,从而节省宝贵时间和金钱。
她是广大企业和创业者了解技术发展现状,避免重复研究,跟踪技术发展动态,获取免费技术解决方案的最佳途径。
以下技术资料198元/套(含特快专递费)............................................................................. ..............《EVA(乙烯-醋酸乙烯共聚物)生产技术配方工艺》01、吹气球式多色迷彩EVA射出发泡产品的制作方法02、EVA一次注塑成型保暖鞋03、吸震EVA发泡型材配方及其制造方法04、高弹EVA发泡型材配方及其制造方法05、EVA弹性橡胶砖及其制备方法06、辐射交联EVA-PE地板包覆膜及其生产工艺07、一种热固化反应型EVA热熔胶08、臭氧氧化提高EVA/PE共混发泡材料性能的方法09、热收缩双壁管用无卤阻燃乙烯-醋酸乙烯酯共聚物(EVA)热熔胶及其制备方法10、EVA的成型方法及模具结构11、耐老化太阳能电池封装用EVA胶膜及其制备方法12、EVA射出成型中底的制法13、双硬度EVA发泡鞋底之制造方法14、EVA双色双硬度的鞋底倒料发泡制法15、EVA(乙烯-醋酸乙烯共聚物)发泡填充母料的制备方法16、具有EVA内层的单衬板高尔夫球杆把手17、硅烷改性EVA热熔胶组合物的制备18、PVB、EVA胶片的着色方法19、一种以EVA为原料的阻燃喷胶及其制备方法20、EVA橡胶软木脚床及其制作方法21、EVA高分子防水卷材及其制作工艺22、EVA橡胶泡棉在建筑及家居装修材料上的应用23、EVA/植物纤维发泡复合鞋用材料的制备方法24、EVA橡胶泡棉在玩具材料上的应用25、一种EVA双色鞋底制作方法及所用的发泡设备26、一种EVA边角粉碎机27、EVA高发泡塑料保温大棚被的制法28、乙烯――醋酸乙烯酯聚合物EVA可再分散的乳胶粉及其制备方法29、一种将EVA边角料粉碎加工成粉末的方法和系统30、EVA/PE/淀粉复合发泡材料的制备方法31、EVA高保温系列覆盖薄膜及其生产工艺32、EVA发泡鞋中底及其周缘轮廊的制造方法33、EVA射出成型中底的制法34、EVA发泡鞋底的原料混合方法35、除臭性及防臭性EVA组成物及其配方36、EVA树脂成型制品表面纹路印刷方法37、EVA二次发泡成型工艺中的加热方法38、导电聚丙烯-EVA树脂39、一种超疏水EVA涂层及其制备方法40、一步法电加热EVA发泡塑料再生造粒方法及主机41、高弹EVA发泡鞋材专用填充剂的制备方法42、EVA夹网布43、一种工业用EVA橡塑绝热节能材料的制作方法44、一种EVA鞋材及其加工方法45、一种用于制鞋的香味EVA发泡材料及其加工工艺46、止滑耐磨EVA鞋材47、EVA、PE垫体一体发泡成型制造方法48、EVA底材的制程49、水相悬浮法制造氯化乙烯-醋酸乙烯共聚物的工业化生产方法及产品50、乙烯--醋酸乙烯共聚物粉末的制法51、乙烯-醋酸乙烯共聚物的皂化52、一次皂化法制造高皂化度乙烯―醋酸乙烯共聚物53、乙烯-醋酸乙烯共聚物发泡鞋中底制法54、导电性乙烯-醋酸乙烯共聚物塑胶发泡体及其制法55、乙烯-醋酸乙烯共聚物直接发泡一体成型鞋底之制造方法56、水相悬浮法氟化乙烯-醋酸乙烯共聚物制造方法57、乙烯-醋酸乙烯共聚物改性聚乙烯塑料管58、乙烯,醋酸乙烯共聚物尾液废料治理与再生利用方法59、含有烷氧基的乙烯-醋酸乙烯共聚物皂化物及其成形物60、辐照交联制备乙烯-醋酸乙烯共聚物/线性低密度聚乙烯泡沫塑料的方法61、含乙烯-醋酸乙烯共聚物皂化物的树脂组合物及其成形品62、乙烯-醋酸乙烯共聚物组合底换气运动鞋63、纳米氢氧化铝、粘土与乙烯-醋酸乙烯共聚物的阻燃复合材料64、含醋酸乙烯共聚物的涂料组合物65、乙烯-醋酸乙烯共聚物沥青防水卷材的制备方法66、乙烯-醋酸乙烯共聚物注射机67、太阳能电池封装用乙烯―醋酸乙烯共聚物胶膜及制备方法注意事项:1、以上均为专利技术原文(原文什么样,我们提供给您的就什么样).专利文献中包含专利发明人,发明时间,技术原理,工艺流程,配方,图纸,以及实现其产品的生产全过程。
福斯特EVA产品说明书(中文)资料

30
30
软化点Intenerating Point
(Before Laminating)
℃
58
58
密度Density
g/cm3
0.96
96
比热Specific Heat
J/℃、g
2.30
2.30
绝缘电阻Insulating Resistance
MΩ
1.45×106
1.45×106
击穿电压Penetrated Voltage
frsteva胶膜技术参数f406胶膜透光率检验报告testingreportglassevatransmittancegraph玻璃eva透射图谱transmittance玻璃first透射值玻璃jcompetitorwarelengthnmfrstevaf406frsttmeva胶膜和其他胶膜f406耐紫外老化实验紫外老化波长
KV/mm
19
19
抗拉强度Tensile Strength
Mpa
26
26
延伸率ElongationRate
%
420
420
透光率Light Transmittance
(After Laminating)
%
>90.0
>90.0
折射率Refraction Rate
1.491
1.491
吸水率
Water-absorbing Rate(20℃,24h)
N/cm
>30
>30
与TPT剥离强度
Strength of peeling TPT
N/cm
>20
>20
耐紫外老化UV-Resistance
欧洲专利局专利数据库检索与使用

h
24
著录项目信息
查看原文
法律 状态
同族专利信息
h
25
h
26
h
27
同族专利
专利文献大量重复出版的结果,形成了一组组有不同国家出版的内容相同或基本相 同的专利文献。各组专利文献中的每件专利说明书之间,通过一种特殊的媒介--优先权,相互联系在一起
所谓优先权,根据《保护工业产权巴黎公约》的规定,是巴黎联盟各成员国给予本 联盟任意国家的专利申请人的一种优惠权,联盟内某国的专利申请人已在某成员国 第一次正式就一项发明创造申请专利时,申请人有权享有第一次申请的申请日期。
h
11
注意:
入口中空格默认操作符为OR: Publication number Application number Priority Number
h
12
注意
入口中空格默认操作符为AND:
Keyword(s) in title
Keyword(s) in title or abstract
Applicant
h
2
Cou
n t r
Facsimiles from
y
CH 1888, from CH1 onwards
DE 1877, from DE1 onwards
EP 1978
Abstract s fro m
European Classificatio
n
1970 1920
1970 1978
1877, from DE1 onwards
李晓
lixiao@
010-62086776
h
43
h
5
检索方式
快速检索 QUICK SEARCH 高级检索 ADVANCED SEARCH 号码检索 NUMBER SEARCH 分类号检索 CLASSIFICATION SEARCH
EVA简要介绍

EVA简要介绍一、EVA的概念EVA是经济增加值(Economic Value Added)的英文缩写。
从算术角度说,EVA等于税后经营利润减去资本成本,其中包括债务和股本成本,是所有成本被扣除后的剩余收入。
EVA的观念由来已久,但直到1991年才由美国人贝内特·斯图尔特三世(G.Bennett Stewart III)在其著作《价值探寻》(The Quest for Value)首次系统地阐述了EVA的框架。
斯图尔特后来和乔尔·斯特恩(Joel M.Stern)共同创立的思腾思特咨询公司(Stern Stewart & Co.)于是成为EVA管理体系的首倡者和最主要推动者。
该公司把EVA体系的应用归纳为4个M:衡量(Measurement)、管理体系(Management System)、激励(Motivation)、思想观念(Mindset)。
在衡量方面,EVA是衡量任何时期公司业绩的最准确的指标,是把会计利润转化为现实的、真正的经济利润。
虽然只要衡量EVA值就可以使公司更好地专注于经营的业绩。
EVA的真正价值在于,公司可以把它当做是一个综合的财务管理体系,包括指导公司战略和运作的所有政策、程序、方法和指标。
在EVA体系中有一个现金奖励计划,奖金不封顶,企业经理们为股东创造的价值越多,自己获得的奖金就越多,这样对经理们就有较好的激励作用。
而在思想观念方面,EVA为公司不同部门之间、不同经理和员工之间提供了一套共同的语言,因此实施EVA体系将会改革和优化公司的文化和全体员工的思想观念。
而这主要是通过把EVA作为公司所有报告、规划和决策的核心来实现。
二、EVA的核算方法它是指调整后的税后净营业利润(NOPAT)扣除企业现有资产经济价值的机会成本后的余额,用公式可表示为:EVA=NOPAT-C×Kw其中:NOPAT——调整后的税后净营业利润C——全部投入资本的经济价值(包括权益资本和债权资本)Kw——企业加权平均资本成本NOPAT是经调整的税后净营业利润,它不同于会计报表上列示的税后净利润,而是对税后净利润进行一系列的调整后得到的。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Titre du document / Document titleFactors that affect the EVA encapsulant discoloration rate upon accelerated exposureAuteur(s) / Author(s) PERN F. J. (1) ;Affiliation(s) du ou des auteurs / Author(s) Affiliation(s)(1) Measurements and Characterization Branch, Photovoltaics Division, National Renewable Energy Laboratory, 1617 Cole Blvd., Golden, CO 80401,ETATS-UNISRésumé / Abstract Copyright (c) 1996 Elsevier Science B.V. All rights reserved. Several factors that affect the discoloration rate of the ethylene-vinyl acetate (EVA) copolymer encapsulants used in crystalline-Si photovoltaic (PV) modules upon accelerated exposure have been investigated primarily by employing UV-visible spectrophotometry, spectrocolorimetry, and fluorescence analysis. A variety of film samples including the two typical (unprimed) EVA formulations, A9918 and 15295, were studied. The films were laminated, cured, and exposed to either a concentrated 1-kW Xe or an enhanced-UV light source. The results indicate that the extent of EVA discoloration can be affected by factors of two general categories:chemical and physical. Inthe chemical category, thedegradative factors include(1) EVA formulation, (2)presence and concentrationof curing-generated,UV-excitable chromophoresthat depend on the type ofcuring agent used, (3) lossrate of the UV absorber,Cyasorb UV 531TM, (4)curing agent and curingconditions, and (5)photobleaching reactionsdue to diffusion of air intothe laminated films. In thephysical category, thefactors involve (6) UV lightintensity, (7) UV-filteringeffect of glass superstrates,(8) gas permeability ofpolymeric superstrates, (9)film thickness, and (10)lamination−delamination(maybe chemical and/ormechanical effect, too).Photodecomposition of theCyasorb was first verified incyclohexane solutions andthen in Elvax 150TM (EVX)films (the copolymer withoutany additives and curingagent). Cyasorbdecomposition rates incyclohexane solutions areexponentially proportionalto the light intensity, but canbe greatly reduced by afree-radical scavenger,Tinuvin 770TM, andfurthermore by anantioxidant, Naugard P TM.The discoloration rate ofEVA increases withincreasing loss of CyasorbUV 531 and is faster for theEVA A9918 films that havea greater concentration ofUV-excitable chromophoresgenerated from a slowercuring than for the EVA15295 films that are fastcured. In general, the lossrate of the UV absorber andthe rate of discolorationfrom light yellow to brownfollow a sigmoidal pattern. Areasonably good correlationfor changes intransmittance at 420 nm,yellowness index, andfluorescence peak area (orintensity ratio) is obtainedas the extent of EVAdiscoloration progresses.No discoloration wasobserved for the laminatedEVX films that contain nostabilizers andcuring-generatedchromophores. Thediscoloration rate of bothtypes of EVA can be largelyreduced by UV-filteringglass superstrates thatremove UV<320 nm.Photobleaching reactionsare responsible for thenon-discoloration ofunlaminated EVA, thevisually clear perimeteraround the edges oflaminated samples, and theEVA films laminated withgas-permeable polymer filmsuperstrates. Delaminationof EVA films from the topglass superstrate wasobserved after prolongedUV exposure.Revue / Journal TitleSolar energy materials andsolarcells ISSN 0927-0248Source / Source1996, vol. 41-42, pp. 587-615Langue / LanguageAnglaisEditeur / PublisherElsevier, Amsterdam,PAYS-BAS (1992) (Revue)Mots-clés anglais /English KeywordsPhotovoltaic array ;Photovoltaic conversion ;Silicon ; Discoloration ;Irradiance ; Experimentalstudy ; Reactionmechanism ; Stabilization ;INIST-CNRS, Cote INIST :18016, 35400006008917.0098Copyright 2007INIST-CNRS. All rightsreservedToute reproduction oudiffusion même partielle,par quelque procédé ou surtout support que ce soit, nepourra être faite sansl'accord préalable écrit del'INIST-CNRS.No part of these recordsmay be reproduced ofdistributed, in any form orby any means, without theprior written permission ofINIST-CNRS.Nº notice refdoc (ud4) :3119483/pat ents/5447576/description.htmlDescriptionFIELD OF THE INVENTIONThis invention pertains to encapsulants for solar cells. More particularly, this invention pertains to a composition of an EVA encapsulant which minimizes thermal discoloration.BACKGROUND OF THE INVENTIONEncapsulants used to protect solar cells from the environment must possessseveral characteristics. First, the encapsulant must be easily deformable, prior to being cured, so it can conform to the shape of the solar cell forproper sealing without breaking the relatively fragile solar cell. Also, the cured formulation of the encapsulant must be a thermoset compound,i.e. a compound which is resistant to flow when exposed to heat. Because a solar cell is exposed to heat during its normal operation, a compound which readily flows when exposed to heat, i.e. a thermoplastic compound, would not be suitable for use as an encapsulant. Athermoplastic compoundwould likely flow off ofthe solar cell, therebycausing the solar cell tobecome exposed during itsnormal operation.Additionally, theencapsulantmust be adhesive to adhereto the cell and othercomponents duringoperation. Finally, theencapsulant must beoptically transparent.Ethylene-vinyl acetate(EVA) co-polymers areamong the most widely usedencapsulants for themanufacture of solar cells,or solar modules.However, field experienceshows that a gradualdiscoloration developswithin the encapsulant.This discoloration lowersthe power output of themodule by approximately 5%.This discoloration effecthas been primarilynoticed in hotenvironments; however,EVA encapsulants in otherenvironments also tend toeventually showdiscoloration. Thisdiscoloration may becaused by exposure to heat,i.e. thermaldiscoloration, and/or byexposure to light, such asultraviolet light. Thediscoloration appears as ayellowing of the EVAencapsulant, which is anabsorption in the blue endof the spectrum, anddecreases the powerconversion in this range.In the past, someadditives have been usedto attempt to minimizeencapsulant discoloration.One such additive is thecompoundBis-(Tetramethylpiperidinyl sebacate),produced by Ciba-GeigyCorporationof Hawthorne, N.Y. andsold under the trademark"TINUVIN 770". However, aneed still exists tofurther minimize thermaldiscoloration.SUMMARY OF THE INVENTIONThe present invention isdirected to a compositionfor encapsulating asolar cell and minimizingthermal discoloration ofthe encapsulant. Thecomposition includes anethylene-vinyl acetate(EVA) copolymer, a curingagent and one of twoparticularly well-suitedhindred amine lightstabilizers, either0,0-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl)mono-peroxycarbonate orPoly[(4-hydroxy-2,2,6,6-tetramethyl-l-piperidine-ethanol) -co-dimethylsuccinate]. The curingagent may be either2,5-Dimethyl-2,5-Di-t-Butylperoxyhexane; orO,O-t-Butyl-O-(2-ethylhexyl)mono-peroxycarbonate orany other conventionalcuring agent.In an alternativeembodiment of the presentinvention, thecompositionincludes only an EVAcopolymer andO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl) mono-peroxycarbonate,which serves the functionsof both the curing agentand the hindered aminelight stabilizer.In one embodiment of thepresent invention, aprimer may be added to theformulation to serve toenhance adherence of theencapsulant to the solarcell.In another alternativeembodiment of the presentinvention, the selectedhindered amine lightstabilizer is added in anamount in the range of 0.1to 0.5 parts per hundredparts by weight ofethylene-vinyl acetatecopolymer and the curingagent is added in an amountequivalent to 1.5parts per hundred partsethylene-vinyl acetatecopolymer.The present inventionincludes a method ofproducing an encapsulant for asolar cell. This method includes mixing one of the two above-mentioned hindered amine light stabilizers with an ethylene-vinyl acetate copolymer,a curing agent and, optionally, a primer. Then, the method includes applying the mixture to the solar cell and curing the applied mixture to form a thermoset encapsulant for the solar cell. Alternatively, if theselected hindered amine light stabilizer isO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl) mono-peroxycarbonate, the curing agent need not be included.The present invention also includes the product produced by the above method. This product is a cured, transparent encapsulant for a solar celland includes across-linkedethylene-vinyl acetate copolymer and ahindered amine light stabilizer selected from the group consisting of O,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl)mono-peroxycarbonateorPoly[(4-hydroxy-2,2,6,6-tetramethyl-1-piperidine-ethanol) -co-dimethylsuccinate].BRIEF DESCRIPTION OF THEDRAWINGSFIGS. 1 through 6 plot thetime it takes for theoptical transmission todegrade to a particularvalue at a specifiedwavelength.DESCRIPTION OF THEINVENTIONThis invention is directedto a composition forminimizing thediscoloration in anencapsulant for a solarcell. The compositionincludesan ethylene-vinyl acetate(EVA) copolymer, a curingagent and a hinderedamine light stabilizerselected from the groupconsisting ofO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl)mono-peroxycarbonate orPoly[(4-hydroxy-2,2,6,6-tetramethyl-1-piperidine-ethanol) -co-dimethylsuccinate]. Alternatively,if the selected hinderedamine light stabilizeris0,0-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl)mono-peroxycarbonate, thecuring agent need not beincluded. Thiscomposition is cured andthe subsequently formedproduct shows improvedresistance to thermaldiscoloration.Ethylene-vinyl acetatecopolymer, commonly knownin the art as EVA, refersto a whole class ofpolymers (or "resins")which are generally usedtoimprove adhesionproperties of hot-melt andpressure sensitiveadhesives.One particular use of EVAis as an encapsulant for asolar cell.In the present invention,the EVA copolymer used maybe any EVA copolymerknown in the art for thisparticular use.Preferably, the EVAcopolymerhas a vinyl acetatecontent between 25% and35%. One such EVAcopolymerwhich can be used in thepresent invention is soldunder the trademark"ELVAX-150" by DuPont ofWilmington, Del.A formulated version ofthe "ELVAX-150" EVA is"EVA A-9918", whichcontainsthe required curing agentsand stabilizers necessaryfor solar modulefabrication. Inparticular, the EVA-9918formulation which isproduced bySpringborn Laboratoriesof Enfield, Conn. consistsof the followingmixture:______________________________________"phr"______________________________________ELVAX-150 EVA (DuPont)100.0LUPERSOL-101 Peroxide(Atochem)1.5CYASORB UV-531 (Am.Cyanamide)0.3NAUGARD-P (Uniroyal) 0.2TINUVIN-770 (Ciba-Geigy)0.1______________________________________Other suitableformulations are made byRichmond Industries ofRedland Ca.;Bridgestone Tire & Rubberof Tokyo, Japan; andEtimex Gmbh of Germany. Anadditional modificationof this compound,designated A-9918-Pincludes theprimer "Z-6030" at a levelof 0.25 phr. SpringbornLaboratories alsoproduces "EVA 15295" alsoknown as the "fast cure" version, in which the LUPERSOL-101 peroxide curing agent is replaced by LUPERSOL-TBEC.The curing agent used in the present invention may be any curing agentknown in the art. A purpose of the curing agent is to serve tosufficiently cross-link the EVA during the cure cycle. The cured encapsulant should be a thermoset compound so that the integrity of thesolar cell is not breached when exposed to heat. Two curing agents whichhave been used with successful results are2,5-Dimethyl-2,5-Di-t-Bu tylperoxyhexane, which is sold under the trademark "LUPERSOL 101", andO,O-t-Butyl-O-(2-ethylhe xyl) mono-peroxycarbonate, which is sold under the trademark "LUPERSOL TBEC" by Atochem of Buffalo, N.Y.The amount of curing agent used may vary widely depending on theparticularapplication. In experiments disclosed in the examples below, the amount ofcuring agent added was 1.5 parts per hundred parts of the EVA resin"ELVAX-150". The designation "phr" will be used hereinafter toindicateparts per hundred parts ofthe EVA copolymer.One embodiment of theinvention is directed to acomposition forminimizingthermal discoloration inan EVA encapsulant. Thiscomposition includes anEVA copolymer, a curingagent and a discolorationstabilizer (one of thetwo particularlywell-suited hinderedamine light stabilizersdiscussed inmore detail below).According to anotherembodiment of theinvention, a primer isalso added toserve to enhance adherenceof the encapsulant to thesolar cell. Theprimer used in the presentinvention may be anyprimer known in the art.Primers well known in thestate-of-the-art includecompounds in the broadclass of chemistry knownas "organo-silanes".Chemically, this group ofprimers may be representedby the formula (R--O)3--Si--(C3H6)--R'. Primers useful inEVA encapsulant formulashave twochemically functionalparts, R and R'. The first("R") is the part thatreacts with glass, solarcells and other inorganicsurfaces. "R" may bemethyl (CH3--), ethyl (CH3CH2 --), or acetyl (CH3--CO--) The otherfunctional part, R',reacts with the EVApolymer duringthe cure and may be any ofthe following chemicalgroups: methacrylate,acrylate, vinyl, maleate,itaconate, cinnamate orany other chemical groupin which a double bondedcarbon structure isavailable forfree-radicalattack.One primer which may beused isgamma-Methacryloxypropyltrimethoxysilane,which is sold under thetrademark "Z-6030" by DowCorning of Midland Mich.The amount of primer usedmay vary widely dependingon the particularapplication. In variousexperiments withsuccessful results, theamount ofprimer used varied from0.1 to 1.0 phr.The final element of thecomposition of the presentinvention is a hinderedamine light stabilizer(also known as "HALS")selected from the groupconsisting ofO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl)mono-peroxycarbonate andPoly[(4-hydroxy-2,2,6,6-tetramethyl-1-piperidine-ethanol) -co-dimethylsuccinate]. The completechemical characteristicsof these two compounds,along with other testedHALS compounds, aredisclosed on the attachedTable 1. As indicated onTable 1, these two HALScompounds arerespectively sold underthe trademarks"LUPERSOLHA-505" byAtochem and"TINUVIN 622LD" byCiba-Geigy Corporation ofHawthorne, N.Y.As indicated from Table I,the LUPERSOL HA-505 HALSactually representsO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl) mono-peroxycarbonateina fifty percent mixturewith a solvent sold underthe trademark"AROMATIC-100" solvent byShell ChemicalCorporation. The"AROMATIC-100"solvent by Shell ChemicalCorporation consistsprimarily of meta-xylene,para-xylene, ethylbenzene and similarcompounds. It isformulated to be anon-oxygenated highboiling point solvent forcompatibility withacrylicpolymers for automotive topcoats.TABLE 1______________________________________COMMERCIAL HINDERED AMINE LIGHTSTABILIZERS("HALS" Type Stabilizers) Compound Chemistry______________________________________Ciba-Geigy CorporationHawthorne, NY1. TinuvinBis-(Tetramethyl piperidinyl sebacate)770 m.w. = 481, m.p. = 84° C., pKb = 5.0, pKa = 9.0 TMP = 63%2. TinuvinBis-(N-octyloxy-tetramet hyl piperidinyl123 sebacate), liquid, bp. = 367 C. at 760 mm;mw. = 737.2; pKb = 9.6; pKa = 4.2. TMP =41.2% Low basicity, claimed to be synergistic w/ benzotriazole UV absorbers.3. TinuvinPoly(4-hydroxy-tetramethyl piperidino-622D dimethyl succinate), m.w. = (283)n, Mn =>2500, m.p. = 60° C., pKa = 6.5, TMP =53.7%4. ChimassorbComplex polymer ofs-triazine with944FL (tetramethyl-piperidinyl)amino adipatem.w. = (579)n, Mn = >2500,m.p. = 55-70° C.pKa = 9.7, TMP = 52.5%American CyanamidBridgewater, New Jersey1. Cyasorb Oligomer ofN,N'-Bis(tetramethylUV-3346 piperidinyl)1,6-hexanediamine w/dichloromorpholinyl-triazine.M.W = appx. 1600, m.p. =110° C.-130° C.Moderately strong base.Recommended forEVA, and olefinsrequiring extendedweatherability.2. Cyasorb ProprietaryHALS (liquid), mw. = 406.6,UV-3581 b.p. = 212°C./0.7 torr. Moderatelystrongbase, pH = 8.13. CyasorbDodecyl-N-(1,2,2,6,6-pentamethyl-4-UV-3604piperidinyl)-succinimide(liquid),mw. = 420.66, bp. = 220C./0.7 torr; pH = 7.5,TMP = 72.3%4. Cyasorb Proprietaryexperimental HALS(liquid),UV-3668 mw. = 448.67, b.p.= n/a, pH = n/aLow basicityAtochem/Lucidol(previously PennwaltCorporation)Buffalo, New York1. Lupersol(Pentamethyl-4-piperidinyl)-O,O-t-Amyl-HA-505 monoperoxycarbonate, assay: 50%,remainder"Aromatic 100" solvent.Liquid, 32° F.storage required. M.W. =301, m.p. -13° F.TMP = 50.4% Reactive withpolymers;chemically binds HALSgroup.2. Luchem (Tetramethylpiperidinyl)-methylHA-B-phthalimido-oxamide. NOTreactive withHMPA polymer, but haspowerful metaldeactivation properties;m.w. = 392,m.p. = 115° C., TMP =38.7%______________________________________Note:"TMP" indicates theweight percent of thecompound that is theactivetetramethyl piperidinylgroup; the basis if HALSstabilization chemistry.*Material Safety DataSheets (MSDS) areavailable for all thecompoundsinvestigated in thisstudy.Before the utility ofthese two HALS compoundswas discovered, ananalysisof the causes ofencapsulant discolorationwas performed. An initialassumption was made thatthermal exposure, which isan unavoidablecondition for a solar cell,is the dominant stress onthe encapsulationsystem.Although light inducedreactions may alsoaggravate degradation,they aremore difficult toincorporate into testchambers, and much moredifficultto interpret. Regardlessof the relative importancebetween thermaldiscoloration and photodiscoloration, it can besaid that an encapsulantin accordance with thepresent invention reducesoverall discoloration byreducing thediscoloration fromexposure to heat.It was discovered that thethermal discoloration ofEVA is stronglycatalyzed by the presenceof oxygen. Therefore,compounds which interferewith oxygen radicalreactions should improvethe stability of the EVA.Some of the known causes ofdegradation of EVApolymers are shown belowonTable 2.TABLE 2________________________ ________________________ ________________________ __GENERALIZED EVA DEGRADATION PATHWAYS________________________ ________________________ ________________________ __Path 1. Conjugation: (discoloration)##STR1##Path 2.Absorption/Excitation: ##STR2##Path 3. Free-radical Formation:##STR3##Path 4. Reactions with Oxygen (Peroxidation): ##STR4##Path 5. Hydroperoxidation:##STR5##Path 6. Hydroperoxide Scission/Free-Radical Generation:##STR6##Path 7. Norrish Degradation:##STR7##________________________ ________________________ ________________________ __Although Table 2 represents a highly generalized scheme, it does provide some useful guidance. Forexample, the reactions ofall seven paths areinteractive with eachother and may eitherincrease or decrease theirrespective rates, as wellas cause each other torepeat. These reactionsmay, therefore, becompetitive.As stated above, one of thekey discoveries is thatthe thermaldiscoloration of EVA isstrongly catalyzed by thepresence of oxygen.Accordingly, several HALScompounds, which arepowerful antioxidants andfree radical traps, werestudied to determine theireffect ondiscoloration.HALS compounds are a classof compounds, known in theart, which are usedspecifically for thepurpose of stabilizingpolymers. They are usuallytertiary amines, but insome cases may besecondary amines. HALScompoundsare effective compoundsfor interrupting a numberof pathways of thermaldegradation. For example,HALS compounds interruptPath 3 (of Table 2) byradical scavenging, Path 2by excited state quenchingand Path 6 byperoxide decomposition.Additionally, HALScompounds offer thefollowingadvantages:1. HALS compounds do notinterfere with the curechemistry of EVA;2. Very highconcentrations of HALScompounds are possible formaximumlifetime;3. HALS compounds havevery high "molarefficiency" asantioxidants;4. HALS compounds arenon-sacrificial andregenerate themselves in acyclical process, known inliterature as the DenisovCycle, as discussedbelow;5. There may be asynergistic behavior ofcertain UV screeningadditives,in which the UV screen isprotected by the HALScompound; and6. Most HALS compoundshave low volatility andtwo compounds (one for EVA)are available that willchemically graft to thepolymer backbones duringcure.Regarding item 4, HALScompounds, as free radicalscavengers, participatein a cyclic chemicalprocess in which theyregenerate themselves.Themechanism ofstabilization andregeneration is stilldisputed, but ageneral theoretical modelappears to be correct.This theoretical modelindicates that thehindered amine (>N--H)oxides form nitroxylradicals(>N--O.), which in turnreact with polymerfree-radicals (P.) toform an amine-ether,thereby terminating theradical reaction. Theamine-ether then goes onto decomposepolymer-bound peroxyradicals,terminating anotherdegradation reaction andregenerating the nitroxylgroup again. The nitroxylgroup then repeats thisprocess, which is knownas the Denisov cycle (asmodified by Klemchuck)shown as follows in Table3:TABLE 3______________________________________##STR8##______________________________________HALS compounds areavailable as low molecular weight compounds, polymers,and as co-reactive compounds. The source and description of some HALS which were tested is given in Table 1, with some chemical structures being shown in Table 4. Commercially available compounds vary in: (a) molecularweight, (b) base strength (pH), and (c) the ability to graft to thepolymer backbone.TABLE 4________________________ ________________________ ________________________ __CHEMICAL STRUCTURESHINDERED AMINE LIGHT STABILIZERS; - Tinuvin 144##STR9##Tinuvin 770##STR10##Tinuvin 765##STR11##Tinuvin 123##STR12##Tinuvin 622 D##STR13##Chimassorb 944FL##STR14##Lupersol HA-505##STR15##LUCHEM HA-B-HMPA##STR16##________________________ __________________________________________________The molecular weight ofthe HALS compound controlsvolatility, solubilityand diffusion. The rate ofdiffusion frequentlycorrelates to theeffectiveness of theantioxidants, as it mustdiffuse to the chemicalsitewhere it is needed. Thisnormally limits theeffectiveness ofchemicallybound additives; however,some recent work onpolymer bound HALScompoundsshows some improvementover monomeric species,especially in combinationwith phenolic typeantioxidants.The basicity (pH, orcorrectly, pKa) of HALScompounds is now believedtohave a strong influence onstabilization. Stronglybasic HALS compoundsmay have an antagonisticreaction with acidiccomponents in polymerformulations. Lowerbasicity means lessinteraction withprotonatedspecies (acids). Stronglybasic HALS compounds inacidic environments mayprotonate at the aminenitrogen and form inactivequarternary ammoniumsalts, with no stabilizingactivity at all. However,tests confirming thishypothesis have not yetbeen conducted.Only one HALS compoundthat may reactivelycombine with EVA isavailable,manufactured by AtochemCorporation, Buffalo, NewYork. This chemicalcompound is sold under thetrademark "LUPERSOLHA-505", and is shown inTables 1 and 4. Thismaterial likely reactswith EVA during cure togive apolymerically boundstabilizer.The second reactive HALS,sold under the trademark"LUCHEM HA-RiO0", isdesigned to react onlywith maleate modifiedresins and is consequentlyofno use in EVA systems. Itis the basis of a relatedcompound, however,sold under the trademark"HA-B18" which is designedto be compatible withother polyolefins. It maybe of possible benefit inEVA resins.A caution is that theevaluation of HALSstabilizers by certainacceleratedaging devices, e.g. lamps,may underrate theirperformance. This may bedue to an unrealisticallylarge number of radicalsbeing generated whichmay kinetically "swamp"the stabilizer. Foraccuracy, thesestabilizersshould be evaluated byactual field trials. Dueto the differing kineticrates of radicaltermination, a mixture oftwo HALS may even bedesirable.One may function better athigher temperatures, andthe other moreeffectively at lowertemperatures.The method ofencapsulating a solar cellaccording to the presentinventioninvolves first mixing theelements together. Theelements include an EVAcopolymer, a curing agentand an HALS. Alternatively,if the selectedhindered amine lightstabilizer isO,O-t-Amyl-O-(1,2,2,6,6-Pentamethyl-4-piperidinyl) mono-peroxycarbonate,the curing agent need notbe included. These areincorporated byconventional milling andcompounding operations asare other additives.Then, this mixture isapplied to a solar cell ina conventional manner,such as by molding.Finally, the applied。
