Synthesis temperature dependence of morphologies and properties of cobalt oxide and silicon nan
2,4,6-三异丙基苯的合成

Results and Discussion
Phosphoranes 1 and 2 were prepared following procedures developed by Hellwinkel and coworkers,21,22 The synthesis of 1 is outlined in Scheme I ; that for 2 is analogous. The spectrum (Figure 1) of the biphenylylene methyl protons of the unsymmetrical phosphorane 1 in bromobenzene solution at 33" consists of three lines in the ratio of 1 : 1:2: four separate biphenylylene methyl resonances are clearly resolved in nitrobenzene solution. The isopropyl methyl protons consist of a pair of doublets. On warming, this spectrum collapses
(16) R. S. Berry, J. Chem. Phys., 32,933 (1960). (17) 3. I. Musher,J. Amer. Chem. Soc., 94,5662(1972) (18) W. G. Klemperer, J . Amer. Chem. Soc., 94, 6940 (1972); Inorg. Chem., 11,2668(1972). (19) E. L. Muetterties and R. A. Schunn, Quurr. Rec., Chem. SOC., 20,245 (1966). (20) M. RabanandK. Mislow, Top. Stereochem., 1 , 1 (1967). (21) D . Hellwinkel, Chima, 22,488 (1968),and referencescited therein; D. Hellwinkel and H. J. Wilfinger, Tetrahedron Lett., 3423 (1969). (22) M. Schlosser, T. Kadibelban, and G. Steinhoff, Justus Liebigs Ann. Chem., 143,25(1971).
材料物理性能

本课实行点名 7次不到取消考试资格
第1-2次不到考试成绩扣1分/每次 第2-4次不到考试成绩扣1.5分/每次 第5-6次不到考试成绩扣2分/每次
本课无实验。
第二章材料的热学性质
• 主讲:龙毅
解答问题邮箱地址 shallytiger2000@
2.1概论
• 材料主要的热性能参数有哪些? • (1)热容:材料升高一度所需要的热量。 • (2)热膨胀系数:当温度变化1度(单位:K)时, 物质尺寸(或体积)的变化率。其单位是1/K。
1 E i n n 2
• 为了找出与所有晶格振动联系的晶体内能,还需要 考虑在各种频率有多少模式,即在波矢空间里频率 为ω 至ω +dω 包含的模式数,设ρ (ω )是单位频率 内的模式数,那么, ρ (ω )dω 是dω 范围内的模 式数。一摩尔固体晶格振动的内能是:
( q ) E ( ) d E 0 0 ( q ) exp 1 kT
材料的物理性能 第1章前 言
材料科学与工程是关于
材料的成分与结构(composition and structure)、
合成与加工(synthesis and processing)、
性质(proporties)
与服役性能(performance)这四个要素、
以及它们两两之间的互相联系的学科。
本课程中,材料的性能是指“材料性质”。它 是材料科学与工程学科的四个基本要素之一。 所谓的材料性能,是指在给定的外界环境中, 材料受到某种作用时,其状态所发生的变化。 作用于材料上的作用因素通常可以分为应力、 温度、磁场、电场、化学介质、辐照等。 受到这些因素作用时,材料内部会产生一系列 的变化,伴随之产生一些外在表现,也就是所 谓的状态的变化。
耐高温可溶性聚酰亚胺树脂及其复合材料

62%, 层间剪切强度保持率可达 48%, 具有较优异的高温力学性能 . 采用普通模压工艺制备了厚度高达 45 mm
的复合材料制件, 进一步证明这 2 种树脂具有优异的工艺性 .
关键词 聚酰亚胺树脂;可溶性;复合材料;力学性能;热性能
中图分类号 O633
文献标志码 A
聚酰亚胺复合材料作为一类先进的树脂基复合材料,因具有极高的耐温等级、优异的高低温力学 性能和介电性能以及成型工艺上的多样性,在航空航天领域应用广泛[1,2]. 美国 NASA 路易斯研究中心 于 20 世纪 70 年代首先开发出了 PMR(Polymerization of Monomeric Reactants)型聚酰亚胺树脂 PMR-15. PMR-15 的复合材料可在 316 ℃下长期使用,但力学性能与耐热性不能满足更高温度的要求 . 为了进一 步提高材料的使用温度,美国 NASA 及空军材料实验室又开发出 DMBZ-15,PMR-Ⅱ-50V-CAP 和 AFR700 等 PMR 型的聚酰亚胺树脂,其中一些品种可在 371 ℃高温下长期使用[3~8]. 但 PMR 型聚酰亚胺树脂 及其复合材料仍然存在存储期短、所使用的单体存在安全性问题及加工过程中易产生小分子挥发物使 复合材料孔隙率偏高等许多缺点[9~11]. 陈祥宝等[12]合成了 LP-15 树脂,与 PMR-15 相比,LP-15 树脂避免 了高毒性单体的使用,提高了韧性,具有更好的流动性,可以在较低的压力下加工复合材料 .
Fig. 3 Isothermal viscosity profiles of PI⁃1 and PI⁃2
Fig. 4 Processing cycle of polyimide composite
图 4 示出了聚酰亚胺树脂基复合材料的成型工艺 . 首先,将预浸料在烘箱中于 200 ℃处理 3~4 h, 以去除二氧六环溶剂;然后,在室温下装入模具中,在平板硫化机上以 3~5 ℃/min 的速率升温到 350 ℃,并停留 3~5 min,施加 2 MPa 的压力并保持此压力继续升温至 371 ℃固化 2 h,中间有 3~4 次的 泄压除气过程,使复合材料成型固化;最后,保压降温至 200 ℃以下卸压,开模取出复合材料 . 在本文 中,溶剂的去除和封端基的交联反应两个过程完全分开,并且树脂是亚胺化后的低分子量预聚物,固 化过程中无溶剂及水等小分子放出,有利于制备低孔隙率的复合材料 . 2.4 聚酰亚胺树脂低聚物与固化物的热性能
与化学有关英语作文

与化学有关英语作文Title: The Wonders of Chemistry。
Chemistry, often dubbed the central science, permeates every aspect of our lives, from the air we breathe to the food we eat, from the medicines that heal us to the materials that surround us. It is a discipline that delves into the composition, structure, properties, and reactions of matter, offering profound insights into the workings of the universe. In this essay, we will explore the marvels of chemistry, its significance in our daily lives, and its promising future.At its core, chemistry is the study of matter and the transformations it undergoes. From the simplest atoms to complex molecules, everything around us is made up of chemicals. One of the fundamental concepts in chemistry is the periodic table, a systematic arrangement of elements based on their atomic number and chemical properties. Dmitri Mendeleev's ingenious creation of the periodic tablelaid the groundwork for understanding the behavior of elements and predicting their properties, revolutionizing the field of chemistry.Chemistry plays a vital role in addressing global challenges such as climate change, pollution, and sustainable energy. Researchers are developing innovative materials for renewable energy sources like solar panels and fuel cells, aiming to reduce our dependence on fossil fuels and mitigate the impact of climate change. Moreover, chemistry contributes to the development of cleaner technologies and processes, ensuring a more sustainable future for generations to come.Furthermore, chemistry intersects with other scientific disciplines, fostering interdisciplinary collaborations and breakthroughs. For instance, the field of biochemistry explores the chemical processes within and related toliving organisms, elucidating the molecular mechanisms of diseases and paving the way for novel treatments and therapies. Similarly, materials science combines principles from chemistry, physics, and engineering to design andfabricate materials with tailored properties for various applications, ranging from electronics to medicine.In the realm of medicine, chemistry has revolutionized healthcare through the discovery and development of new drugs and therapies. Pharmaceutical chemistry involves the design, synthesis, and testing of compounds to treat diseases and improve human health. From antibiotics that combat bacterial infections to chemotherapy drugs that target cancer cells, the contributions of chemistry to medicine are immeasurable, saving countless lives and alleviating suffering worldwide.Moreover, chemistry enhances our understanding of the natural world, unraveling the mysteries of substances and their interactions. Analytical chemistry, for instance, enables scientists to identify and quantify the composition of substances, whether in environmental samples, food products, or forensic evidence. By employing sophisticated techniques such as chromatography and spectroscopy, analytical chemists unravel complex mixtures and provide valuable insights into their properties and behavior.In addition to its practical applications, chemistry inspires wonder and curiosity, inviting us to explore the unseen realms of the microscopic world. Through experiments and observations, chemists uncover the intricate mechanisms underlying chemical reactions, from the formation of bonds between atoms to the dynamics of complex biochemical processes. The beauty of chemistry lies not only in its practical utility but also in its elegance and complexity, captivating the imagination of scientists and enthusiasts alike.Looking ahead, the future of chemistry is filled with promise and opportunity. Advances in computational chemistry and artificial intelligence are revolutionizing the way we study and predict chemical phenomena, accelerating the discovery of new materials and compounds with tailored properties. Nanotechnology, a burgeoningfield at the intersection of chemistry and engineering, holds immense potential for revolutionizing industries such as electronics, healthcare, and energy.In conclusion, chemistry is a captivating and indispensable science that shapes our world in profound ways. From its fundamental principles to its far-reaching applications, chemistry permeates every facet of our lives, driving innovation, solving complex problems, and enriching our understanding of the natural world. As we continue to unravel the mysteries of matter and molecules, the wonders of chemistry will continue to inspire and empower us to build a brighter future for humanity.。
单晶铋纳米带的制备与生长机制研究

单晶铋纳米带的制备与生长机制研究王彦敏;郝秀红;解辉【摘要】采用溶剂热还原金属铋离子的方法,以硝酸铋为原料,乙二醇为溶剂和还原剂,聚乙烯吡咯唍酮(PVP)为稳定剂制备了单晶铋纳米带.采用X射线衍射(XRD)、高分辨透射电镜(HRTEM)对所得样品的结构和形貌进行表征.结果表明:金属铋纳米带结构完美、表面洁净、内部无缺陷无位错,是一种理想的单晶准一维纳米结构;纳米带宽度为50 nm ~3 m,厚度约为几十nm,长度可以达到几十μm;铋纳米带属于菱面体结构,空间群为R3m (166),JCPDS卡片号为44-1246;纳米带沿着[110]或者[-114]方向生长.【期刊名称】《山东交通学院学报》【年(卷),期】2012(020)004【总页数】5页(P67-70,74)【关键词】铋纳米带;溶剂热法;生长机理;制备【作者】王彦敏;郝秀红;解辉【作者单位】山东交通学院材料科学与工程学院,山东济南250023;山东交通学院材料科学与工程学院,山东济南250023;山东交通学院材料科学与工程学院,山东济南250023【正文语种】中文【中图分类】TG146.17金属铋是一种典型的半金属,又被称为“绿色金属”。
由于具有较小的有效载流子质量、较低的载流子浓度、较长的载流子平均自由程,而表现出特异的电性能[1]。
例如,由于铋具有大的费米波长和载流子平均自由程,在量子传输和有限尺度效应方面,金属铋被广泛的研究[2-4];而由于金属铋具有小的载流子质量和大的平均自由程,在铋单晶、铋薄膜和铋纳米线阵列中具有大的磁致电阻效应[5]。
因此,无论在基础研究还是器件的应用研究方面,金属铋纳米结构一直受到人们的广泛关注。
文献[6]中提出了纳米带的概念,纳米带是研究电子输运现象(Transport Pheonomena)的维度限制(Dimensionality Confined)效应的理想体系[7-8]。
与纳米管以及纳米线状结构相比,纳米带结构完美、表面洁净、内部无缺陷无位错,是一种理想的单晶准一维结构。
大分子单体聚N-乙烯甲酰胺与苯乙烯共聚合成热敏性功能高分子微球

Table 2 Copoiymerization of PNVF macromonomer(M1 )with styrene(M2 )a
Run M(1 mmoi)
1
0 . 10
M(2 mmoi)
"bn
2 . 00
2600
#(cp !m) —
2
0 . 05
5 . 00
4600
3.0
3
0 . 04
4 . 00
Fig. 6 Temperature dependence for opticai transmittance at 500 nm in 1.0 wt% agueous soiution of PSt/PNVF particie ! Heating up;" Cooiing down for Run 2 in Tabie 2; # Heating up;$ Cooiing down for Run 3 in Tabie 2
##
##
CH2 CI 50% K0H,TBP"B
CH2 0CH2 CH2 (S CH2 CH)I H NH
C! 0 H
PNVF macromonomer Fig. 2 Preparation of PNVF macromonomer
1.2.2 热敏性功能高分子微球的合成 合成热 敏性功能高 分 子 微 球( PNVF-St)由 分 散 聚 合 方 法 制备 . 热敏性大分子单体聚 N- 乙烯甲酰胺与疏水 性单体苯乙烯(St)共聚物的制备以 AIBN 为引发 剂,将已知量的大分子单体 PNVF、分散剂 PVP K30 与 St 溶 于 乙 醇 中,反 应 在 氮 气 保 护 下、搅 拌 ( ~ 250 r/min)30 min 脱氧后,以自由基方式进行共 聚合,反应式见图 3 . 1.2.3 聚合物表征 所得聚合物的分子量和分 子量分布用 Perkin-EImer 2/2 GPC 仪测定(凝胶柱: Shocex A802/S-A803/S,紫外线检测仪:Perkin-EImer LC75). 微球粒径大小、分布是在聚合物分散于乙
万青个人简历

6. C. C. Li, Z. F. Du, L. M. Li, H. C. Yu, Q. Wan, and T. H. Wang, "Surface-depletion controlled gas sensing of ZnO nanorods grown at room temperature", Applied Physics Letters. 91, 032101 (2007).
19. Q.Wan, K.Yu, T.H.Wang, C.L.Lin, "Low-field electron emission from tetrapod-like ZnO nanostructures synthesized by rapid evaporation", Applied Physics Letters, 83, 2253 (2003).
21. Q.Wan, T.H.Wang M.Zhu,and C.L.Lin,"Resonant tunneling of Si nanocrystals embedded in Al2O3 matrix prepared by electron-beam co-evaporation",Applied Physics Letters, 81, 538 (2002)
在研课题
2007年,湖南省杰出青年基金项目,负责人(30万)
10级热工信息检索电子资源实习题(1)(2)
10级热工专业信息检索实习题第一部分中文电子资源实习题说明:1.按以下实习报告的格式撰写实习报告;2.检索结果须记录检索的总条目;拷贝已完成检索策略(在检索介面选定检索途径、输入检索词及逻辑组配关系等)的检索介面;将检索结果复制3-5条并整理成题录(题录按教材后面的参考文献的格式写)一、查看本人的借书情况(记录借书册数);借书2本二、图书馆书目查询课题:热工专业方面的书检索途径(1、题名;2、分类TQ174):《热工测量及仪表》 TK31/15.7检索词:热工检索结果:三、检索课题:热工专业的发展前景网站(任选一个搜索引擎):百度 检索途径:网页关键词检索表达式:关键词=热能与动力工程的发展前景检索结果:/view/b85252691eb91a37f1115c10.html热能与动力工程专业将重点围绕国家能源战略,以“新能源、核能、智能电网、常规能源、节能减排”为主线,培养能适应国家能源领域(尤其是电力行业)快速发展要求的高级研究应用型人才。
本专业学生主要学习动力工程及工程热物理的基础理论,学习各种能量转换及有效利用的理论和技术,将在工程设计与科学研究能力、外语与计算机应用能力、分析和解决问题能力、吸收和再创新能力等方面得到全面培养和锻炼。
四、检索课题:热工材料方面的电子书网站:超星数字图书馆检索途径:超星数字图书馆书名检索词:热工材料检索表达式:书名=热工 and 书名=材料检索结果:1.http://sslibbook/fenlei.jsp?sm=adv&username=jxjdztcxy五、检索课题:周健儿2008年在《中国陶瓷》上发表的论文检索网站:CNKI数据库:中国期刊全文数据库检索途径:作者日期来源期刊检索词:周健儿 2008 中国陶瓷检索表达式:作者=周健儿来源期刊=中国陶瓷检索年限:2008检索结果:/KNS/brief/result.aspx?dbPrefix=CJFQ1.陶瓷微滤膜处理含油污水的反冲洗研究INVESTIGATION OF BACK-FLUSHING USED IN OIL IN WATER EMULSION BY CCERAMIC MICRO-FILTRATION MEMBRANE引言陶瓷微滤膜处理含油废水具有操作稳定、出水水质好、占地面积小、扩建方便、正常工作时不消耗化学药剂、不产生新的污泥以及回收油质量比较好等优点,在含油废水处理中已日益显示出极强的竞争力。
高硅MFI分子筛膜的合成与低温脱除膜内有机模板剂_赵淑蘅

Received: September 24, 2015; Revised: November 23, 2015; Publishen on Web: November 24, 2015. *Corresponding author. Email: langlin@; Tel:+86-20-37218289. The project was supported by the National Natural Science Foundation of China (51202245, 51106165) and Natural Science Foundation of Guangdong Province, China (S2013010014896, 10251007006000000). 国家自然科学基金(51202245, 51106165)和广东省自然科学基金(S2013010014896, 10251007006000000)资助项目
低温加氢裂解脱除分子筛膜内有机模板剂的
按照摩尔比为 1TEOS : 0.36TPAOH : 60H2O 的 比例配制合成液,室温下搅拌 5 h,将合成液倒入 以聚四氟乙烯为内衬的不锈钢反应釜中,密封, 150 °C 下晶化 20 h;冷却后取出,并反复经过去离 子水超声清洗、离心分离得到晶种分子筛,置于 105 °C 烘箱中干燥备用;所制备的分子筛晶种大小 均匀,粒径约为 250 nm。 2.2.2 晶种层的制备
采用旋涂法在载体片表面预涂晶种 41。配制一 定浓度的晶种液,并超声 15 min,将载体片固定于 匀胶机上,涂覆适量晶种液,迅速打开旋转按 钮,在较低转速(500 r∙min-1)下停留 6 s,之后在较 高转速(2000 r∙min-1)下停留 20 s,均匀涂覆晶种, 最后置于 60 °C 烘箱中烘干备用。 2.2.3 分子筛膜的制备
韩国2

The oxidation stability of SiC coating deposited on various nuclear grade graphite substrate via chemical vapor reaction.Yootaek Kim1,*, Changsub Jang and Eung-Seon Kim21Department of the Materials Engineering, Kyonggi University, Suwon 443-760, Korea *Corresponding author (Tel: +82-70-4024-9765; E-mail: ytkim@kyonggi.ac.kr) 2Korea Atomic Energy Research Institute, Daejoen 305-353, KoreaAbstractThe dependence of high-temperature oxidation and the thermal shock resistance behavior of ɑ-SiC coated nuclear-grade graphite substrates have been investigated at elevated temperatures. α-SiC was coated on top of the surfaces of three kinds of nuclear-grade graphite substrates by chemical vapor reaction (CVR) under the conditions of a temperature of 1600 o C, a pressure of 1.3 Pa and a pure polysilicone gas atmosphere. Thermal shock and oxidation resistance tests were performed at 1000 o C and 1300 o C, respectively, during which the samples were taken out of a box furnace and directly exposed to air of room temperature. The initial microstructure of the graphite substrate plays an important role in determining the final thermal properties of the SiC coatings in case of using the CVR method.Keywords: SiC, Coating, CVR, Oxidation resistance, Thermal shock resistance, Microstructure. IntroductionCarbon and graphite materials are chemically stable, have low densities and a high strength at elevated temperatures. However, the use of graphite materials has been restricted due to the poor oxidation resistance at elevated temperatures in an oxidizing atmosphere. Oxidation protection for carbon materials has been studied during the past 60 years. In this respect, silicon carbide (SiC) is considered to be the best coating material due to its good mechanical properties, a coefficient of thermal expansion close to that of carbon, and good resistance to oxidation [1,2]. Graphite is also a material used for fuel elements of high-temperature gas-cooled reactors (HTR). For the improvement of the safety of HTR, the oxidation resistance is very important. Some methods have been developed to form SiC coatings on graphite for the fabrication of fuel element matrices of HTR [3,4] but these methods are difficult to apply for the deposition of SiC coatings on graphite balls within the framework of a mass production process.It is necessary to develop a convenient method for the mass production of SiC coatings on graphite [5]. Chemical vapor reaction (CVR) coating, in which molten silicon reacts at the surface of a graphite substrate to form SiC, is one effective way to produce SiC coatings [6]. However, the chemical vapor reaction process often results in a high defect density, and because of oxygen can corrode the substrate through these defects, the oxidation resistance of such coatings is not sufficient at elevatedtemperatures. Another problem is that the coating formation behavior can be quite different when a different carbon material is used [7]. The present study was conducted to investigate the relationship between thermal properties, such as the oxidation and thermal shock resistance, and the microstructure of SiC coatings produced by CVR on top of nuclear-grade graphite substrates.Experimental procedureThree different substrates were used in this experiment, all of them being nuclear-grade graphite provided by Toyo Tanso and SGL Carbon. The properties of the graphite substrates are summarized in Table 1.Table 1. Properties of the three nuclear- grade graphite substrates used in the present study.A B CManufacturer Toyo Tanso SGL CarbonFabrication Isomolded Vibro-molded IsomoldedρBulk (g/cm3) 1.77 1.85 1.81ρCompressive (MPa) 78 75-69 97ρTensile (MPa) 25 22.8-20.3 28Ash Contents < 20 60 11The graphite substrates were machined into bar-shaped specimens with a size of 10×30×5 mm3. The SiC coatings were conducted on the graphite substrates by CVR under the conditions of a temperature of 1600 o C for 80 min and 160 min at a pressure of 1.3 Pa. During the coating process, solid polysilicone was thermally evaporated and reacted with the graphite, thus forming SiC at the surface of the substrates.For the test of the oxidation resistance, specimens both with and without SiC layers were oxidized in air at 1300 o C for 1 hour and, subsequently, were slowly cooled down in a furnace to room temperature. To determine the thermal shock resistance, SiC coated specimens were directly inserted into the furnace preheated to 1000 o C and were kept for 1 hour. Afterwards, the specimens were taken out of the furnace and thus were subject to a thermal shock by direct exposure to air at room temperature. Investigations of the SiC coatings prepared by CVR were carried out by means of X-ray diffraction (XRD), optical microscopy using a camscope, scanning electron microscopy (SEM), and electron probe microanalysis (EPMA).Result and discussionFig. 1 shows the XRD patterns of samples prepared by CVR with different reaction (dwelling) times of (a) 80 min and (b) 160 min at 1600 o C. The XRD analysis confirmed that the coating layer mainly consisted of ɑ-SiC and graphite. This result agrees well with the general tendency of CVRprepared SiC coatings that the coating properties mainly depend on synthesis temperature and reaction time. The higher the synthesis temperature, the more SiC layers were formed and most of the graphite was converted to ɑ-SiC above 1500 o C . An extended reaction time also results in ɑ-SiC enriched coating layers. Because of the slow diffusion rate of Si in graphite, it is necessary to allow for a sufficient reaction time to form a solid ɑ-SiC coating layer [8].The EPMA analysis on the cross-sections of SiC coated specimens prepared by CVR with different reaction times at 1600 o C is shown Fig. 2. The SiC coating layers became thicker and denser on increasing CVR reaction time. The thickness of the SiC coating on top of the graphite substrate is somewhat more than three times larger for a reaction time of 160 min as compared to a deposition time of 80 min.(a) (b)Fig. 1. XRD patterns of the SiC coated specimens prepared by CVR with different reaction times of (a) 80 mim and (b) 160 min at 1600 o C .(a) (b)Fig. 2 EPMA analysis on the cross-sections of SiC coated specimens prepared by CVR with different reaction times of (a) 80 mim and (b) 160 min at 1600 o C .The oxidation resistance of the SiC layers was evaluated by monitoring the surfaces of the specimens after heating them to 1300 o C for 1 hour and then directly exposing to air. It is known that the oxidation resistance of SiC coatings synthesized by CVR is usually insufficient because of the presence of a large number of pinholes, pores, or even cracks [9].I n t e n s i t y2θI n t e n s i t y2θThe upper row of Fig. 3 shows the cross sectional morphologies of three different graphite substrates before CVR coating. Bird’s eye views of the specimens after performing the oxidation resistance test at 1300 o C for 1 hour in an electric furnace are shown in the lower row of Fig. 3. As evident from Fig. 3, the surface morphology of specimen B before coating showed several voids whereas the samples A and C looked relatively clean and dense.Fig. 3. Cross sectional morphologies of three different graphite substrates before CVR coating (upper row) and bird’s eye views of CVR coated specimens after performing the oxidation resistance test at 1300 o C for 1 hour (lower figures). The notations A, B, and C refer to the different samples listed in Table 1.Many white spots appeared at the surface of specimen B after the oxidation test had been performed, as seen in the middle lower frame of Fig. 3. The formation of these white spots is caused by surface cracks of the coating layer, which provide access to the graphite substrate for oxygen molecules. It indicates that the original surface condition, including in particular the defects at the surface of the substrate, may affect the quality of the SiC coating. This means that the formation of carbon dioxide, carbon monoxide, and other residues on top of the graphite substrate after performing the oxidation test may be quite different, depending on the initial substrate conditions. It is concluded that surface morphology control of the substrate is very important to improve the oxidation resistance of SiC coatings formed by the CVR process.The effect of the SiC coatings with respect to thermal shock resistance was evaluated by monitoring the surface of the specimens after exposing them to air at 1000 o C for 1 hour and, subsequently, direct contact to the air at room temperature by taking them out from the furnace to cool down.As shown in Fig.4, the thermal shock resistance behavior of the coated specimens is very similar to their oxidation resistance. Many cracks were found at the surface of specimen B after the thermal shock test. By contrast, only a few cracks or other damages were found at the surfaces of both sample A and C.ABCBefore CVR coatingAfter coating &oxidation testTherefore, it is confirmed that the surface morphology of the substrates before coating is an important factor in determining the thermal properties of the coating layers, such as thermal oxidation and thermal shock resistance. For a successful SiC coating by use of the CVR method, a careful substrate selection is recommended by taking into account the surface condition, as well as other factors such as the manufacturer.ABCBird’s eyes view×50×500×3000Fig. 4. Micrographs and surface morphologies of coated specimens using three different graphite substrates after the thermal shock test that includes keeping the samples at 1000 o C for 1 hour and, subsequently, direct exposure to air at room temperature by taking them out of the furnace. The notations of A, B, and C in the figure refer to the different graphite substrates listed in Table 1.ConclusionBased upon the experiments of synthesizing silicon carbide(SiC) on graphite substrate by CVR process, the thermal property dependence of SiC coating mainly depended on synthetic conditions such as deposition temperature and reaction time. However, in this study, it was concluded that the surface morphology control of the graphite substrate is very important to improve the oxidation resistance and thermal shock resistance of SiC coating layers formed by the CVR process.AcknowledgementThis research was supported by Creative Allied Project (grant number CAP-11-3) funded by the Korea Research Council of Fundamental Science & Technology(KCRF).References[1] J. R. Strife and J. E. Sheehan, Ceram. Bull. 1988;67: 369–374.[2] A. Yamaguchi, Taikabutsu 1983;35: 617-622.[3] Q. S. Zhu, X. L. Qiu, and C. W. Ma, J. Nucl. Mater. 1998;254: 221-225.[4] B. Schroder and Z. Alakan, NEA Workshop on High Temperature Engineering Research Facilities and Experiments, ECN-Pettern, Netherland 1977.[5] C. H. Tang and J. Guan, J. Nucl. Mater. 1995;224: 103-108.[6] D. M. Shuford and G. Prairie, US Patent 4471023, 1984.[7] O. Yamamoto, T. Sasamoto, and M. Inagaki, Carbon 1995;33: 359-365.[8] Q. Zhu, X. Qiu, and C. Ma, Carbon 1999;37: 1475–1484.[9] O. H. Kwon, S. H. Hong, and H. Kim, J. Euro. Cer. Soc., 2003;23: 3119–3124.。
离子液体辅助合成CdMoO_4微球体及其光催化性能

2 种条件下制备的产物均为四方晶相的 CdMoO4 ,但其大小有所差异。以紫外光灯为光源,2 种不同离子液体合成 的 CdMoO4 微球为光催化剂,对有机染料罗 丹 明 B 进 行 了 光 降 解 实 验。研 究 发 现,利 用 双 阳 离 微椭球具有更高的光催化活性,经过 60 min 其光降解率达到了 98. 9% 。
近年来,离子液体作为一种新兴的溶剂因其可 设计性、低熔点、高热稳定性和极其微小的蒸汽压等 突出的特点使其在诸多领域都存在广泛的应用价 值[1]。最近,离子液体在无机材料合成领域的应用 备受关注。Bi2 S3 和 Sb2 S3 纳米棒[2]、CuO 纳米片状
和须 状 结 构[3]、立 方 体 Co3 O4 纳 米 粒 子[4]、叶 片 状 CuO 纳米结构[5]等具有特殊形貌的无机化合物借 助离子液体相继获得,体现了离子液体作为模板剂、 分散剂和表面活性剂的作用。
金属钼酸盐作为一类新兴的光电功能材料和半
收稿日期: 2010-12-30 修改稿日期: 2011-01-07 基金项目: 浙江省自然科学基金项目( Y4080496) ; 浙江省大学生科技创新活动计划项目( 2009R404019) 作者简介: 朱德超( 1990 - ) ,男,浙江义乌人,浙江师范大学学生,师从应桃开教授,从事有机功能材料的研究。电话:
在 本 文 中,我 们 选 用 2 种 不 同 的 离 子 液 体 [Bmim]Cl 和[C4 ( Mim) 2][Br]2 作为辅助剂,得到 了不同形貌的 CdMoO4 微球,并试验了其光催化性 能。结果表明,样品对有机染料罗丹明 B 有很好的
降解作用,并且椭球体比球体具有更好的催化性能。
1 实验部分
的薄 片 叠 加、组 装 形 成 的。 由 图 2 ( c-d ) 可 知, 图) 。实验结果表明,离子液体在反应体系中发挥
水性封闭异氰酸酯乳液的合成及稳定性研究_李阿峰

涂料工业 PAINT & COATINGS INDUSTRY
Vol. 43 No. 2 Feb. 2013
水性封闭异氰酸酯乳液的合成及稳定性研究
李阿峰1 ,樊国栋1* ,李会宁2 ,陈 华1 ,赵 琪1 ( 1. 陕西科技大学教育部轻化工助剂化学 与技术重点实验室,西安 710021; 2. 肇庆千江高新材料科技有限公司,广东肇庆 526238)
取一定量乳液,在磁力搅拌下逐步滴加 2 mol / L 的 NaCl 溶液,出现白色絮状沉淀时停止滴加,计算此时单位质量乳液
探
中和剂制备了水分 散 封 闭 异 氰 酸 酯 ( WBI) 乳 液。通 过 测 试 WBI 水分散液一些性质,如电位、分散系数、粒径、稳定性、贮存
所消耗 的 NaCl 溶 液 的 体 积 ( mL) ,作 为 其 耐 电 解 质 性 能 的 指标。
索
稳定性,离心稳定性等,分析体系稳定性,为改善 WBI 的分散稳 1. 8 DSC 分析
开
定性提供依据,用于指导稳定 PU 水后的解封闭温度。测试温度 40 ~ 180 ℃ ,
发
1 实验部分
N2 流量 40 mL / min,升温速度 5 ℃ / min。
1. 1 主要试剂与仪器
Key Words: waterborne blocked isocyanate; Zeta potential; stability; dimethylol propionic acid; particle size
水性树脂的制备多采用外加乳化剂或自乳化的方式提供 亲水基团,以解决树脂在水相中的分散问题,所制备的水性封 闭型多异 氰 酸 酯 交 联 固 化 剂 可 与 水 性 产 品 形 成 单 组 分 树 脂[1 - 2]。水分散封闭异氰酸酯( WBI) 的合成多是在分子链上
固相合成法制备工程化应用的大应变压电陶瓷材料

第42卷第7期2023年7月硅㊀酸㊀盐㊀通㊀报BULLETIN OF THE CHINESE CERAMIC SOCIETY Vol.42㊀No.7July,2023固相合成法制备工程化应用的大应变压电陶瓷材料李㊀伟,盖学周,汪跃群(中国船舶集团有限公司第七一五研究所,杭州㊀310012)摘要:随着我国科技水平的飞速发展,激光惯性导航系统的精度要求越来越高㊂采用工业级原材料,通过固相合成法制备了铌锑-铌镍-锆钛酸铅(PSN-PNN-PZT)四元系大应变压电陶瓷材料,讨论了不同含量Sr 对PSN-PNN-PZT 压电陶瓷材料介电性能㊁压电性能的影响㊂结果表明:当Sr 含量为1%(摩尔分数)㊁n (Zr)/n (Ti)=43/57(摩尔比)时,PSN-PNN-PZT 组成位于准同型相界附近,压电陶瓷性能较优,获得了一种相对介电常数εT 33/ε0㊁机电耦合系数k p ㊁压电常数d 33㊁介电损耗tan δ㊁居里温度T c 分别为4090㊁0.664㊁686pC /N㊁0.0165及213ħ的大应变压电陶瓷材料;基于该材料配方制备的ϕ24mm ˑϕ5mm ˑ0.4mm 压电陶瓷圆环,在100V 的驱动电压下产生的应变量能达到2.5005μm,较现有的PZT-14(P14)材料提升32.4%,能应用于高精度激光陀螺稳频器中,提高压电陶瓷微位移驱动器的可靠性㊂关键词:压电陶瓷;PSN-PNN-PZT;大应变;介电性能;压电性能;居里温度中图分类号:TM282㊀㊀文献标志码:A ㊀㊀文章编号:1001-1625(2023)07-2597-06Large Strain Piezoelectric Ceramics for Engineering Applications Prepared by Solid-State Synthesis MethodLI Wei ,GAI Xuezhou ,WANG Yuequn(The 715th Research Institute of China Shipbuilding Group Co.,Ltd.,Hangzhou 310012,China)Abstract :With the rapid development of scientific and technological level,the accuracy requirements for laser inertial navigation systems are becoming increasingly high.The quaternary PSN-PNN-PZT piezoelectric ceramics with large strain were prepared by the conventional solid-state synthesis method using industrial grade raw materials.The influence of different content Sr on the dielectric and piezoelectric properties of PSN-PNN-PZT piezoelectric ceramics was discussed.It is found that when the Sr content is 1%(mole fraction)and the molar ratio of n (Zr)/n (Ti)is 43/57,the PSN-PNN-PZT composition locates at morphotropic phase boundary and the piezoelectric ceramics show excellent properties.The large strain piezoelectric ceramics can be achieved,which are as follows:relative dielectric constant εT 33/ε0=4090,electromechanical coupling coefficient k p =0.664,piezoelectric constant d 33=686pC /N,dielectric loss tan δ=0.0165,Curie temperature T c =213ħ.The strain of ϕ24mm ˑϕ5mm ˑ0.4mm piezoelectric ceramic thin rings based on this material formula reaches 2.5005μm at 100V driving voltage,which is 32.4%higher than that of the existing PZT-14(P14)material.This material can be applied in high-precision laser gyro frequency stabilizers to improve the reliability of piezoelectric micro-displacement actuator㊂Key words :piezoelectric ceramics;PSN-PNN-PZT;large strain;dielectric property;piezoelectric property;Curie temperature 收稿日期:2023-03-09;修订日期:2023-05-26作者简介:李㊀伟(1988 ),男,工程师㊂主要从事压电功能材料的研究㊂E-mail:lee2007nuaa@0㊀引㊀言我国电子信息技术与国防工业的高速发展,对激光陀螺的惯性导航系统提出了更高精度的要求㊂在驱动器的应用中,压电陶瓷元件需具有优异的大应变特性,这种大应变特性可以实现在较低的驱动电场强度下产生较大的应变,通常会达到微米级㊂在激光器稳频应用方面,压电陶瓷作为驱动器核心部件得到了广泛应用,激光器谐振腔腔长受温度影响会发生微米级的膨胀或收缩,会导致激光的频率偏移,这对航空航天领域2598㊀陶㊀瓷硅酸盐通报㊀㊀㊀㊀㊀㊀第42卷的应用来说是致命的㊂压电陶瓷微位移驱动器可实现微米级别的位移补偿,从而调整激光器谐振腔腔长以达到激光器稳频效果[1-2]㊂针对此应用,大应变压电陶瓷在性能上应满足高的压电应变系数㊁机电耦合系数及较高的介电常数;同时,为满足实际应用环境需求,大应变压电陶瓷还需要具有高的居里温度和较高的矫顽场㊂目前大应变压电陶瓷体系主要有镧锑锆钛酸铅(PLSZT)㊁铌锌-锆钛酸铅(PZN-PZT)㊁铌镍-锆钛酸铅(PNN-PZT)及铌锌-铌镍-锆钛酸铅(PZN-PNN-PZT)[3]等㊂PNN-PZT压电陶瓷具有优异的压电性能,但居里温度较低,且不太稳定[4-6];而Pb(Sb1/2Nb1/2)O3(PSN)能有效抑制PZT陶瓷的晶粒生长,使PSN-PZT陶瓷体系具有较高的机电耦合系数㊁较好的稳定性和较高的居里温度[7-8]㊂根据PSN和PNN各自的特性,将两者组合在一起与PZT 构成的四元系Pb(Sb1/3Nb2/3)O3-Pb(Ni1/3Nb2/3)O3-Pb(ZrTi)O3(PSN-PNN-PZT)将兼具高机电耦合系数和高压电应变系数的优点,具有较高的研究和应用价值㊂目前大压电应变压电陶瓷材料主要是PZT-14(P14)材料,该材料是在PZT二元系的基础上复合Pb(Zn1/3Nb2/3)O3,然后进行掺杂改性得到的,其相对介电常数εT33/ε0=3400㊃(1ʃ10%),机电耦合系数k p=0.64㊃(1ʃ5%)㊂目前,该材料主要用于航空航天研究院所的军横向压电陀螺仪(年需求量30万件以上,产值800万元以上),以及本单位浮标㊁面元水听器等项目中㊂从客户装配及实际使用的反馈情况看,在器件使用过程中,在相同驱动电压的情况下,丹麦产压电元件的压电应变量要比本单位P14材料的应变量高10%~15%;在近似线性的情况下,要获得相同的应变驱动,丹麦产压电元件所需的驱动电压比P14材料所需的驱动电压要小10%及以上,从而能够有效提高器件的可靠性及优化后端装备,更有利于系统的集成等㊂鉴于此,迫切需要在现有P14材料的基础上进行大应变压电陶瓷新材料的研究,以期进一步提高器件实际使用过程中的应变量㊂本文通过控制Zr/Ti比㊁PSN和PNN含量,研究Sr掺杂对四元系PSN-PNN-PZT压电陶瓷材料介电及压电性能的影响,采用铁电性能测量㊁压电应变测量及居里温度测量等对其进行了表征,并与现有高压电应变P14材料进行了对比㊂1㊀实㊀验1.1㊀样品制备采用工业级纯度的Pb3O4㊁ZrO2㊁TiO2㊁Sb2O3㊁Nb2O5㊁Ni2O3及SrCO3为原料,按照Pb1-x Sr x[(Sb1/3Nb2/3)a (Ni1/3Nb2/3)b(Zr0.43Ti0.57)c]O3(其中a+b+c=1,x=0㊁0.005㊁0.010㊁0.015)准确称料㊂采用传统电子陶瓷制备工艺,在50kg级滚筒球磨机中用纯净水㊁锆球混合球磨40h,烘干后预合成(860ħ,2h);粉碎后搅拌球磨4h,加入质量分数为7%~8%的聚乙烯醇(PVA)溶液作为黏结剂,使用喷雾塔进行喷雾造粒,干压成型为ϕ25mmˑ(2.3~2.8)mm的圆片;在隧道窑炉中进行1250ħ烧结,烧结后采用瓷片机加工成ϕ20mmˑ1mm圆片;超声清洗被银面,采用丝网印刷银电极,网带炉中780ħ烧银,在(135ʃ5)ħ硅油中,2500~ 3000V直流电压下极化15min,保持电压吹冷到温度不高于60ħ㊂1.2㊀性能测试将样品打磨后,采用理学smartlab9kW型X射线衍射仪测试样品的XRD谱,并进行物相分析,通过FEI Quanta250FEG型扫描电子显微镜获得样品断面的晶粒形貌㊂将极化后的样品在室温静置5~7d后,在TH2828S阻抗分析仪上采用电桥法测量元件的谐振频率f r㊁反谐振频率f a,以及相应的电容C T和介电损耗tanδ,参照‘水声实用压电陶瓷元件性能参数的测量与计算方法“(CB/T4314 2013)计算出机电耦合系数k p和相对介电常数εT33/ε0;采用ZJ-2型准静态d33测量仪测试元件的纵向压电常数d33;采用TF Analyzer 2000E型铁电压电分析仪测试元件的矫顽场E c和应变(蝶形回线测量);采用高低温系统测试样品的介电温谱,获得材料的居里温度T c㊂2㊀结果与讨论2.1㊀X射线衍射(XRD)和扫描电子显微镜(SEM)分析图1(a)为不同Sr含量掺杂的样品在1250ħ烧结时的XRD谱㊂由图1(a)可知,在n(Zr)/n(Ti)=第7期李㊀伟等:固相合成法制备工程化应用的大应变压电陶瓷材料2599㊀43/57时,所有的组成样品都获得了纯的钙钛矿结构,无第二相产生,衍射峰强度大,结晶比较完全㊂众所周知,三方相结构在2θ=44ʎ~45ʎ处只有一个峰,在图中标记为(200)R ;四方相结构在2θ=43ʎ~46ʎ处有两个峰,图中分别标记为(002)T 和(200)T ㊂图1(b)为局部放大的XRD 谱,在2θʈ45ʎ处形成很宽的衍射峰,使用Gaussian 函数来分离重叠在一起的(200)峰,如图1(c)所示㊂图中的三个峰意味着样品为三方相和四方相的混合相,为两相共存的相界组成,此组成处具有最佳的压电和介电性能,与准同型相界一致[9-10]㊂图1㊀不同Sr 含量PSN-PNN-PZT 压电陶瓷的XRD 谱和拟合曲线Fig.1㊀XRD patterns and fitting curves of PSN-PNN-PZT piezoelectric ceramics with different Sr content 图2为不同Sr 含量掺杂的样品在1250ħ烧结时的断面SEM 照片㊂由图2可知,在n (Zr)/n (Ti)=43/57时,所有组成样品都具有较致密的断面形貌,没有气孔产生,陶瓷片内部晶粒尺寸大,晶界清晰,材料的致密度较高㊂利用阿基米德排水法测得样品的瓷体密度达到了7.9g /cm 3㊂图2㊀不同Sr 含量PSN-PNN-PZT 压电陶瓷的断面SEM 照片Fig.2㊀SEM images of cross section of PSN-PNN-PZT piezoelectric ceramics with different Sr content 2.2㊀电学性能进行不同Sr 含量掺杂的PSN-PNN-PZT 体系配方实验,n (Zr)/n (Ti)=43/57,1250ħ烧成保温2h 的PSN-PNN-PZT 压电陶瓷介电和压电性能的变化情况如表1及图3所示㊂表1㊀不同Sr 含量PSN-PNN-PZT 压电陶瓷的性能参数Table 1㊀Properties parameters of PSN-PNN-PZT piezoelectric ceramics with different Sr contentSr content tan δεT 33/ε0k p d 33/(pC㊃N -1)E c /(V㊃mm -1)x =00.016137870.650652858x =0.0050.016639860.661673861x =0.0100.016540900.664686878x =0.0150.016039520.6486658712600㊀陶㊀瓷硅酸盐通报㊀㊀㊀㊀㊀㊀第42卷图3为相对介电常数εT33/ε0㊁压电应变系数d33和机电耦合系数k p随Sr掺杂量x的变化㊂由图3可以看出,εT33/ε0㊁d33和k p均随着Sr含量的增加先增大后减小,在x=0.010时,获得实验中的最优介电及压电性能,此时εT33/ε0=4090,k p=0.664,d33=686pC/N,E c=878V/mm㊂这是因为在n(Zr)/n(Ti)=43/57时系统处于准同型相界附近,使抑制晶相结构转变的机械应力松弛,在电场作用下,电畴易于取向,晶格转向更加容易,从而使相界附近的组成具有介电和压电性能的最优值㊂2.3㊀居里温度采用高低温系统测试陶瓷样品的居里温度,图4是不同Sr含量的PSN-PNN-PZT压电陶瓷在1kHz下介电常数随温度的变化曲线㊂从图4中可以看出,随温度升高,介电常数先增大,这是因为样品内部热运动加剧,畴壁运动更容易,热缺陷增多,所以样品的电容增加[11],之后曲线斜率急剧增大,介电常数呈指数形增长,到达最高点后开始下降,最高点对应的温度为样品的居里温度T c㊂未掺杂Sr的PSN-PNN-PZT压电陶瓷样品(x=0)的T c为221ħ,介电常数峰值εmax=32972;Sr元素掺杂后,样品的T c有一定程度降低,在x= 0.010(压电和介电性能最优处)时,样品的T c为213ħ,有最大的介电常数峰值εmax=34579㊂从2.2节可知,添加Sr元素会使体系的压电和介电性能提高,这是由于晶体结构中的晶格畸变增大,会增加畴结构的转向,导致Sr掺杂后的压电陶瓷在高温下更容易发生相转变㊂图3㊀PSN-PNN-PZT压电陶瓷的电学性能随Sr含量的变化Fig.3㊀Change of electrical properties of PSN-PNN-PZT piezoelectric ceramics with Srcontent 图4㊀PSN-PNN-PZT压电陶瓷在1kHz下εT33/ε0随温度的变化曲线Fig.4㊀Temperature dependence ofεT33/ε0of PSN-PNN-PZT piezoelectric ceramics at1kHz2.4㊀压电应变性能2.4.1㊀样品的应变(蝶形回线测量)测量PSN-PNN-PZT(x=0.010)材料ϕ20mmˑ1mm元件在室温3000V/mm电场下的轴向应变,并与P14材料ϕ20mmˑ1mm元件在相同条件下的轴向应变进行对比,如表2㊁图5所示㊂表2㊀PSN-PNN-PZT及P14材料在3000V/mm下应变对比Table2㊀Comparison of strain of PSN-PNN-PZT and P14materials at3000V/mmType of materialTest electricfield/(V㊃mm-1)Average d33positiveside/(pm㊃V-1)Average d33negativeside/(pm㊃V-1)Averaged33/(pm㊃V-1)Strain/%P14PSN-PNN-PZT3000515-5155150.417 581-5705760.432图5为PSN-PNN-PZT及P14材料随着电场强度变化产生的应变量(蝶形回线测量),应变曲线在电场强度E=3000V/mm下表现出典型的蝴蝶状曲线,PSN-PNN-PZT材料具有更高的平均d33和应变极值(S max= 0.432%)㊂图6为在相同测试条件下,两种材料在0~3000V/mm电场下的单级应变曲线,测试条件为25ħ/ 0.1Hz㊂从图6中可以看出PSN-PNN-PZT材料的应变值始终明显高于P14材料㊂第7期李㊀伟等:固相合成法制备工程化应用的大应变压电陶瓷材料2601㊀图5㊀PSN-PNN-PZT 及P14材料在ʃ3000V /mm下的应变曲线Fig.5㊀Strain curves of PSN-PNN-PZT and P14materials at ʃ3000V /mm 图6㊀PSN-PNN-PZT 及P14材料在0~3000V /mm 下的单级应变曲线Fig.6㊀Single-stage strain curves of PSN-PNN-PZT and P14materials at 0~3000V /mm 2.4.2㊀元件在100V 驱动电压下的应变量利用PSN-PNN-PZT(x =0.010)材料进行50kg 中试生产,批量化制备了ϕ24mm ˑϕ5mm ˑ0.4mm 的压电陶瓷圆环元件,测试其在实际使用过程中的应变量,并与P14材料进行比较㊂委托西安618所测试ϕ24mm ˑϕ5mm ˑ0.4mm 元件在100V 驱动电压下产生的应变量,测试设备为微位移测量设备,型号为MDM-CP-01㊂测试数据如表3所示㊂表3㊀ϕ24mm ˑϕ5mm ˑ0.4mm 元件在100V 下的应变量比较Table 3㊀Comparison of strain of ϕ24mm ˑϕ5mm ˑ0.4mm elements at 100VType of materialTest voltage /V Displacement 1/μm Displacement 2/μm Displacement 3/μm Average displacement /μm P14100 1.8835 1.9067 1.8769 1.8890PSN-PNN-PZT 100 2.5226 2.4776 2.5014 2.5005表3中测量结果表明,PSN-PNN-PZT 材料的ϕ24mm ˑϕ5mm ˑ0.4mm 元件在100V 下的应变量为2.5005μm,较现有的P14材料提升32.4%㊂在近似线性的情况下,要获得相同的应变驱动,PSN-PNN-PZT材料所需的驱动电压更小,从而能够有效提高器件的可靠性及优化后端装备,更有利于系统的集成等[12]㊂2.5㊀PSN-PNN-PZT 与P14材料性能对比表4中列出了PSN-PNN-PZT(x =0.010)与P14材料的综合性能对比情况㊂从表4中可以发现PSN-PNN-PZT 材料的压电和介电性能均优于现有的P14材料,而居里温度T c 略低,基于PSN-PNN-PZT 材料配方制备的ϕ24mm ˑϕ5mm ˑ0.4mm 元件在100V 下的应变量较现有的P14材料提升32.4%㊂表4㊀PSN-PNN-PZT 与P14材料综合性能对比Table 4㊀Comparison of comprehensive properties of PSN-PNN-PZT and P14materialsType of material εT 33/ε0k p d 33/(pC㊃N -1)Strain /%T c /ħDisplacement at 100V /μm PSN-PNN-PZT 40900.6646860.432213 2.5005P143400㊃(1ʃ10%)0.64㊃(1ʃ5%)575㊃(1ʃ15%)0.417230 1.88903㊀结㊀论1)采用传统固相烧结法制备Sr 掺杂的PSN-PNN-PZT 压电陶瓷㊂当烧结温度为1250ħ㊁保温时间为2h㊁掺杂量x =0.010时,形成高纯钙钛矿结构,陶瓷中存在三方相与四方相共存的准同型相界,晶粒大小较均匀且晶粒饱满,晶界清晰,压电和介电性能达到最优,此时相对介电常数εT 33/ε0=4090,介电损耗tan δ=0.0165,机电耦合系数k p =0.664,压电常数d 33=686pC /N,矫顽场E c =878V /mm,居里温度T c =213ħ,2602㊀陶㊀瓷硅酸盐通报㊀㊀㊀㊀㊀㊀第42卷应变极值S max=0.432%㊂2)基于PSN-PNN-PZT材料配方制备的ϕ24mmˑϕ5mmˑ0.4mm元件在100V下的应变量较现有的P14材料提升32.4%㊂新材料研制成功后,将提升现有P14压电陶瓷材料实际使用状态下的压电应变量,有效提高器件的可靠性及优化后端装备,提高激光器的稳频效果㊂从用于航空航天研究院所的军横向压电陀螺仪的发展趋势和装备需求来看,市场前景广阔㊂参考文献[1]㊀赵㊀丽,壮㊀凌,张春林,等.压电陶瓷控制系统及其在激光器中的应用[J].压电与声光,2007,29(5):550-552.ZHAO L,ZHUANG L,ZHANG C L,et al.The piezoelectric ceramic controlling system and it s application to laser[J].Piezoelectrics& Acoustooptics,2007,29(5):550-552(in Chinese).[2]㊀纪鈜腾,毛元昊,陈丁博,等.激光陀螺原理㊁现状及展望[J].导航与控制,2022,21(5/6):221-240.JI H T,MAO Y H,CHEN D B,et al.Fundamental principle,current status and prospect of ring laser gyroscope[J].Navigation and Control, 2022,21(5/6):221-240(in Chinese).[3]㊀闫㊀锋,郇正利,王文林.大位移量㊁高弹性模量压电陶瓷材料的制备与研究[J].硅酸盐通报,2015,34(4):1175-1179.YAN F,HUAN Z L,WANG W L.Preparation and investigation of the piezoelectric ceramics material with the large displacement and the high elastic modulus[J].Bulletin of the Chinese Ceramic Society,2015,34(4):1175-1179(in Chinese).[4]㊀CAO R J,LI G R,ZENG J T,et al.The piezoelectric and dielectric properties of0.3Pb(Ni1/3Nb2/3)O3-x PbTiO3-(0.7-x)PbZrO3ferroelectric ceramics near the morphotropic phase boundary[J].Journal of the American Ceramic Society,2010,93(3):737-741. [5]㊀张㊀静,李正权,褚㊀涛,等.低温烧结压电陶瓷驱动器用材料特性研究[J].压电与声光,2022,44(4):502-506.ZHANG J,LI Z Q,CHU T,et al.Study on properties of low temperature sintered piezoelectric ceramic material for actuator[J].Piezoelectrics &Acoustooptics,2022,44(4):502-506(in Chinese).[6]㊀张少坤.PNN-PZT压电陶瓷的结构及其电学性能研究[D].西安:西安工业大学,2022.ZHANG S K.Study on structure and electrical properties of PNN-PZT piezoelectric ceramics[D].Xi an:Xi an Technological University,2022 (in Chinese).[7]㊀张㊀伟,孙清池,马卫兵.制备工艺对铌锑-锆钛酸铅系压电陶瓷性能的影响[J].硅酸盐学报,2009,37(2):238-242.ZHANG W,SUN Q C,MA W B.Influence of fabrication process on properties of lead niobium-stibium zirconate titanate for piezoelectric ceramics[J].Journal of the Chinese Ceramic Society,2009,37(2):238-242(in Chinese).[8]㊀CHEN Z R,LIANG R H,ZHANG C,et al.High-performance and high-thermally stable PSN-PZT piezoelectric ceramics achieved by high-temperature poling[J].Journal of Materials Science&Technology,2022,116:238-245.[9]㊀AHART M,SOMAYAZULU M,COHEN R E,et al.Origin of morphotropic phase boundaries in ferroelectrics[J].Nature,2008,451(7178):545-548.[10]㊀包国翠,李㊀坤,杨㊀光,等.镧掺杂PMN-PT陶瓷在准同型相界处的相组成调控[J].硅酸盐通报,2022,41(10):3647-3657.BAO G C,LI K,YANG G,et al.Phase composition tuning of lanthanum-doped PMN-PT ceramics at morphotropic phase boundary[J].Bulletin of the Chinese Ceramic Society,2022,41(10):3647-3657(in Chinese).[11]㊀EITEL R E,RANDALL C A,SHROUT T R,et al.New high temperature morphotropic phase boundary piezoelectrics based on Bi(Me)O3-PbTiO3ceramics[J].Japanese Journal of Applied Physics,2001,40(10R):5999.[12]㊀王㊀轲,陈效真,杨㊀雨.激光陀螺及其发展[J].导航与控制,2004,3(4):28-31.WANG K,CHEN X Z,YANG Y.Ring laser gyro and its development[J].Navigation and Control,2004,3(4):28-31(in Chinese).。
海报(poster)模板(英文)
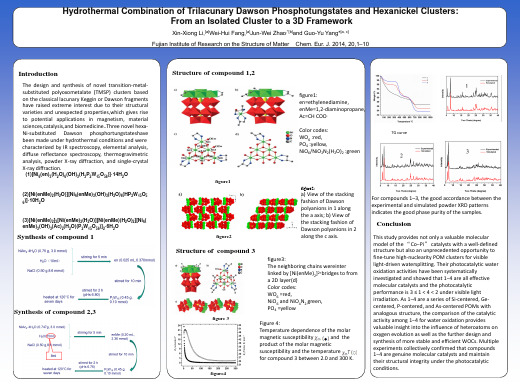
For compounds 1–3, the good accordance between the experimental and simulated powder XRD patterns indicates the good phase purity of the samples.
Conclusion
(1)[Ni6(en)3(H2O)6(OH)3(H3P2W15O56)]· 14H2O
Structure of compound 1,2
figure1: en=ethylenediamine, enMe=1,2-diaminopropane, Ac=CH COOColor codes: WO6 :red, PO4 :yellow, NiO6/NiO2N2(H2O)2 :green
Hydrothermal Combination of Trilacunary Dawson Phosphotungstates and Hexanickel Clusters: From an Isolated Cluster to a 3D Framework
Xin-Xiong Li,[a]Wei-Hui Fang,[a]Jun-Wei Zhao,*[b]and Guo-Yu Yang*[a, c] Fujian Institute of Research on the Structure of Matter Chem. Eur. J. 2014, 20,1–10
This study provides not only a valuable molecular model of the “Co−Pi” catalysts with a well-defined structure but also an unprecedented opportunity to fine-tune high-nuclearity POM clusters for visible light-driven watersplitting. Their photocatalytic water oxidation activities have been systematically investigated and showed that 1−4 are all effective molecular catalysts and the photocatalytic performance is 3 ≤ 1 < 4 < 2 under visible light irradiation. As 1−4 are a series of Si-centered, Gecentered, P-centered, and As-centered POMs with analogous structure, the comparison of the catalytic activity among 1−4 for water oxidation provides valuable insight into the influence of heteroatoms on oxygen evolution as well as the further design and synthesis of more stable and efficient WOCs. Multiple experiments collectively confirmed that compounds 1−4 are genuine molecular catalysts and maintain their structural integrity under the photocatalytic conditions.
Estimation of heat capacities of solid mixed oxides

 5, 166 28 Praha 6, Czech Republic Department of Solid State Engineering, Institute of Chemical Technology, Technicka b  5, 166 28 Praha 6, Czech Republic Department of Physical Chemistry, Institute of Chemical Technology, Technicka c  5, 166 28 Praha 6, Czech Republic Department of Inorganic Chemistry, Institute of Chemical Technology, Technicka Received 30 October 2001; received in revised form 11 February 2002; accepted 11 March 2002
* Corresponding author. Fax: 420-2-2431-0337. E-mail address: jindrich.leitner@vscht.cz (J. Leitner).
0040-6031/02/$ ± see front matter # 2002 Elsevier Science B.V. All rights reserved. PII: S 0 0 4 0 - 6 0 3 1 ( 0 2 ) 0 0 1 7 7 - 6
Thermochimica Acta 395 (2003) 27±46
Estimation of heat capacities of solid mixed oxides
ztuff粉末钢化学成分

ztuff粉末钢化学成分粉末钢是一种特殊的制造材料,其化学成分对于材料的性能具有至关重要的影响。
下面将介绍几种常见的粉末钢化学成分及其相关参考内容。
1. 铁基粉末钢化学成分:铁是粉末钢的主要成分,其化学符号为Fe。
具体的铁基粉末钢化学成分包括碳(C)、硅(Si)、锰(Mn)、硫(S)和磷(P)等元素。
参考内容:- C. Avram, R. Luo, L. Hoglund, et al. Effects of carbon and nitrogen addition on microstructure and mechanical properties of powder metallurgy CrMnNi high entropy alloy. Materials Science and Engineering: A, 2020, 769: 138447.- H.O. Seo, Y.J. Son, S.Y. Shin, et al. Effects of carbon content on microstructure and tensile properties in TRIP-aided sheet steels. Materials Science and Engineering: A, 2014, 612: 276-281.- G.K. Stymanowski, M. Kaczorowski, A. Czyżewski, et al. Microstructure evolution and mechanical properties of a high-strength low-alloy steel as a function of chemical composition. Materials Science and Engineering: A, 2022, 827: 112340.2. 不锈钢粉末钢化学成分:不锈钢是具有耐腐蚀性能的钢种,其化学成分以铬(Cr)和镍(Ni)为主,同时还包含钼(Mo)、铜(Cu)、钛(Ti)和氮(N)等元素。
化学原料 英语

化学原料英语Chemical raw materials are essential components in various industries, including pharmaceuticals, agriculture, manufacturing, and many others. These raw materials play a crucial role in the production of a wide range of products that we use in our daily lives. In this article, we will explore the importance of chemical raw materials and their significance in different industries.One of the key industries that heavily rely on chemical raw materials is the pharmaceutical industry. Chemical raw materials are used in the production of various drugs, medicines, and vaccines. These raw materials are essential in the synthesis of active pharmaceutical ingredients (APIs) that form the basis of many life-saving medications. Without chemical raw materials, the pharmaceutical industry would not be able to develop new drugs and treatments to combat diseases and improve public health.In the agricultural sector, chemical raw materials are used in the production of fertilizers, pesticides, and herbicides. These raw materials help to enhance crop yields, protect plants from pests and diseases, and improve soil quality. Without the use of chemical raw materials, farmers would struggle to meet the growing demand for food and face challenges in ensuring food security for the growing global population.In the manufacturing industry, chemical raw materials are used in the production of a wide range of products, including plastics, textiles, paints, and adhesives. These raw materials serve as building blocks for the manufacturing process, enabling the creation of innovative and high-quality products. Without chemical raw materials, the manufacturing industry would not be able to produce goods efficiently and meet consumer demands for diverse and sustainable products.Furthermore, chemical raw materials are also essential in the production of energy sources such as fuels and lubricants. These raw materials are used in the refining process to produce gasoline, diesel, and other petroleum products that power vehicles and machinery. Chemical raw materials also play a crucial role in the development ofrenewable energy sources such as solar panels, wind turbines, and batteries, helping to reduce dependence on fossil fuels and mitigate the impact of climate change.In conclusion, chemical raw materials are indispensable in various industries and play a vital role in the production of essential products and services that benefit society. From pharmaceuticals to agriculture, manufacturing, and energy production, these raw materials are essential building blocks that drive innovation, economic growth, and sustainable development. As we continue to advance in technology and science, the demand for chemical raw materials will only continue to grow, highlighting their critical importance in shaping the future of industries worldwide.。
N型4H_SiC中载流子密度和霍耳迁移率的模拟及研究_全宏俊

xx , yy , zz 分量 .如取 Z 轴沿 C 轴 , 有 mx =my =mt , mz =ml , p1* 和 q1* 为 Herring Vogt 变换矢
量位移
p1* =pd · M-1
(15)
q1* =q* · M-1
(16)
从方程(5)、(6)可得霍耳迁移率为
μH = Ey (BEx)=-eνd (mxxAx)γx , yy
电离杂质散射和中性杂质散射随温度 T 增加而减弱 , 而声学声子散射 、光学声子散射 、谷间声 子散射和压电极化散射随温度增加而增强 .因此 , 低温时 μH 随温度 T 增加而增加 , 直到 μH 达 最大值 .随后声学声子散射 、光学声子散射和谷间声子散射的作用逐渐显示出来 , 从而 μH 逐 渐减少 .μH 的实验数据也画在图 2 .可见计算结果与实验数据相符 .
及由声学声子光学声子 , 谷间声子散射和压电极化引起的晶格散射 .中性杂质密度可取为 Nn = ND(h)+ND(k)-NA -N .图 1 画出了电子密度 N 与温度倒数 1 T 的函数关系 .实验数据 (40 ~ 500 K)[ 5] 也同时画在图 1 中 .从图可见 , 低温时电子密度随温度增加而增加 , 此结果与实 验数据符合得较好 .当温度超过 500 K 时 , 逐渐趋于饱和值 .这是因为对浅施主和受主能级 , 温 度较低施主和受主的电离随温度增加而增加 , 因此温度 T 增加时电子密度 N 也增加 ;当温度 较高时 , 施主和受主基本上都已电离 , 从而温度 T 增加时电子密度 N 不再继续增加 , 而趋于一 个饱和值 ND(h)+ND(k)-NA , 该结论与 J .Vuillod 在对 3C -SiC 的分析所得结论[ 7] 相似 .图 2 给出了温度范围为 30 K 到 1 000 K 的霍耳迁移率与温度的函数关系 .由图可见 , 当 T 很小时 , μH 随温度增加而增加 .当 T =140 K 时 , 达最大值 592 cm-2 Vs(与实验值 600 cm -2 Vs 相符).
