MK_2206_dihydrochloride_COA_18916_MedChemExpress
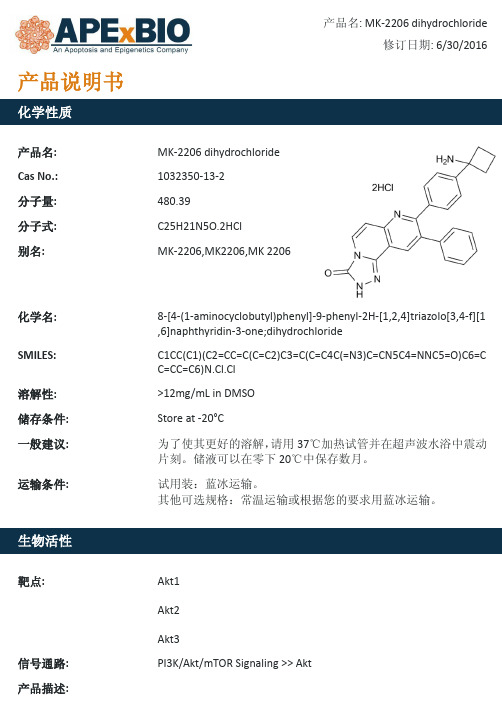
MK-2206 dihydrochloride_Akt123抑制剂_1032350-13-2_Apexbio产品说明书
1. Eren RO, Reverte M, et al. "Mammalian Innate Immune Response to a Leishmania-Resident RNA Virus Increases Macrophage Survival to Promote Parasite Persistence." Cell Host Microbe. 2016 Sep 14;20(3):318-28. PMID:27593513 2. Winter PS, et al. "RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis." Sci Signal. 2014 Dec 23. PMID:25538080 3. Yoshida, S., et al. "Differential Signaling During Macropinocytosis in Response to M-CSF and PMA in Macrophages." Name: Frontiers in Physiology 6.8 (2015). 4. Fu, Xiu-Qiong, and Xue-Gang Sun. "Apigenin attenuates atherogenesis through inducing macrophage apoptosis via inhibition of AKT Ser473 phosphorylation and downregulation of plasminogen activator inhibitor-2." 5. Yoshida, Sei, et al. "Growth factor signaling to mTORC1 by amino acid–laden macropinosomes." The Journal of cell biology 211.1 (2015): 159-172. PMID:26438830 6. Zhang X, Lu X, Akhter S, Georgescu MM, "Legerski RJ. FANCI is a negative regulator of Akt activation. Cell Cycle. 2016 Apr 17;15(8):1134-43." PMID:27097374 7. Choy YY, Fraga M, et al. "The PI3K/Akt pathway is involved in procyanidin-mediated suppression of human colorectal cancer cell growth." Mol Carcinog. 2016 Jan 15. PMID:26774105
二甲基甲氧基苯并二氢吡喃棕榈酸酯化学式

二甲基甲氧基苯并二氢吡喃棕榈酸酯(简称DMDBPPA)是一种化学物质,其化学式为C23H36O5。
它是一种有机化合物,常用作一种新型的阻燃剂和塑料增塑剂。
作为一种广泛应用的化学物质,DMDBPPA具有很多重要的应用价值,同时也引起了人们对其安全性和环境影响的关注。
我们可以从DMDBPPA的结构特点和化学性质入手,来认识这种物质。
DMDBPPA是一种褐色或无色固体,它的结构中包含了苯环、二氢吡喃环和棕榈酸酯基团。
这种结构使得DMDBPPA具有良好的阻燃和增塑效果,同时也决定了它的一些特殊性质,比如溶解度和热稳定性。
理解DMDBPPA的结构特点,有助于我们更好地认识它的应用和安全性。
我们需要深入了解DMDBPPA的应用领域和价值。
作为一种新型的阻燃剂和塑料增塑剂,DMDBPPA被广泛应用于电子产品、建筑材料、汽车零部件等领域。
它可以有效提高材料的阻燃性能和加工性能,提高产品的安全性和可靠性。
然而,由于DMDBPPA是一种化学物质,它的安全性和环境影响也备受关注。
我们需要对DMDBPPA的应用进行全面的评估,以确定其在实际应用中的风险和潜在影响。
我们还需要关注DMDBPPA的安全性和环境影响。
作为一种化学物质,DMDBPPA可能会对人体健康和环境造成潜在的危害,比如毒性、生物富集性和生物降解性等方面。
我们需要对DMDBPPA的毒理学和生态毒理学进行深入研究,以评估其对人体健康和环境的影响。
我们还需要开发更加环保和安全的替代品,以减少DMDBPPA对环境的影响。
二甲基甲氧基苯并二氢吡喃棕榈酸酯(DMDBPPA)作为一种新型的阻燃剂和塑料增塑剂,具有重要的应用价值,但同时也存在一定的安全性和环境影响问题。
我们需要根据其结构特点和化学性质,全面评估其在应用中的风险和潜在影响,并开发更加环保和安全的替代品。
只有这样,我们才能更好地利用DMDBPPA的优势,同时减少其对人体健康和环境的影响。
通过深入分析了解DMDBPPA的结构特点、应用领域和安全性与环境影响的问题,我们对这种化学物质有了更深入全面的认识。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
阿拉丁羟乙基纤维素

阿拉丁羟乙基纤维素
阿拉丁羟乙基纤维素是一种常用的增稠剂和胶凝剂,被广泛应用于食品、药品、化妆品等行业中。
其化学名为羟乙基纤维素,英文名为Hydroxyethyl Cellulose,缩写为HEC。
阿拉丁羟乙
基纤维素的主要特点是水溶性良好,能够在水中形成清澈透明的胶体溶液。
阿拉丁羟乙基纤维素在食品中的应用包括作为增稠剂、稳定剂、乳化剂等。
它可以增加食品的黏性和口感,改善食品的质地,提高乳化性能,增强稳定性。
在药品中,阿拉丁羟乙基纤维素可以用作药片的胶囊剂、乳化剂和混悬剂,能够调整药物的缓释性能、改善口感、增加稳定性。
在化妆品中,阿拉丁羟乙基纤维素常用作凝胶剂和稠化剂,能够改善产品的质地和稠度,增加粘度和黏性,提高使用体验。
总之,阿拉丁羟乙基纤维素在各个行业中都有广泛的应用,能够起到增稠、胶凝、乳化等作用,提高产品的品质和稳定性。
Dyenamo产品目录2014
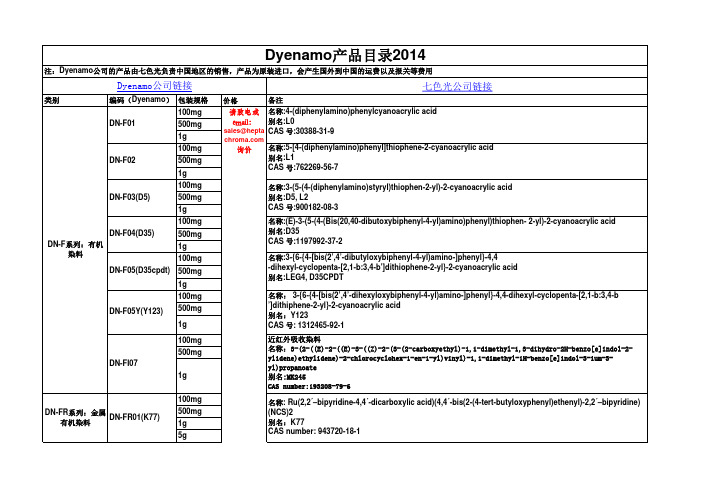
七色光公司链接类别编码(Dyenamo )包装规格价格备注100mg500mg1g100mg500mg1g100mg500mg1g100mg500mg 1g 100mg 500mg1g100mg500mg1g100mg500mg1g100mg500mg 1g5g Dyenamo公司链接DN-FI07近红外吸收染料名称:3-(2-((E)-2-((E)-3-((Z)-2-(3-(2-carboxyethyl)-1,1-dimethyl-1,3-dihydro-2H-benzo[e]indol-2-ylidene)ethylidene)-2-chlorocyclohex-1-en-1-yl)vinyl)-1,1-dimethyl-1H-benzo[e]indol-3-ium-3-yl)propanoate 别名:MK245CAS number:193208-79-6DN-FR 系列:金属有机染料DN-FR01(K77)名称: Ru(2,2´–bipyridine-4,4´-dicarboxylic acid)(4,4´-bis(2-(4-tert-butyloxyphenyl)ethenyl)-2,2´–bipyridine)(NCS)2别名:K77CAS number: 943720-18-1DN-F04(D35)名称:(E)-3-(5-(4-(Bis(20,40-dibutoxybiphenyl-4-yl)amino)phenyl)thiophen- 2-yl)-2-cyanoacrylic acid 别名:D35CAS 号:1197992-37-2DN-F05(D35cpdt)名称:3-{6-{4-[bis(2’,4’-dibutyloxybiphenyl-4-yl)amino-]phenyl}-4,4-dihexyl-cyclopenta-[2,1-b:3,4-b’]dithiophene-2-yl}-2-cyanoacrylic acid 别名:LEG4, D35CPDT DN-F05Y(Y123)名称: 3-{6-{4-[bis(2’,4’-dihexyloxybiphenyl-4-yl)amino-]phenyl}-4,4-dihexyl-cyclopenta-[2,1-b:3,4-b ’]dithiphene-2-yl}-2-cyanoacrylic acid 别名:Y123CAS 号: 1312465-92-1Dyenamo 产品目录2014注:Dyenamo 公司的产品由七色光负责中国地区的销售,产品为原装进口,会产生国外到中国的运费以及报关等费用DN-F 系列:有机染料DN-F01请致电或email:sales@hepta 询价名称:4-(diphenylamino)phenylcyanoacrylic acid 别名:L0CAS 号:30388-31-9DN-F02名称:5-[4-(diphenylamino)phenyl]thiophene-2-cyanoacrylic acid 别名:L1CAS 号:762269-56-7DN-F03(D5)名称:3-(5-(4-(diphenylamino)styryl)thiophen-2-yl)-2-cyanoacrylic acid 别名:D5, L2CAS 号:900182-08-35g10g25g5g10g25g5g10g 25g 5g 10g25g5g10g25g5g10g 25g DN-P0118片/组1微米厚的TiO2电极,含致密层1g 5g10g100mg 500mg1g100mg500mg1g 产品列表不断更新中,更多信息,请访问我们的网站或联系销售 QQ:1007797411, QQ群:50101302大连七色光太阳能科技开发有限公司,Tel0411-********,Fax0411-847963005,*********************, DN-C06化学式:Co(bpy)3(NO3)3名称:Tris-(2,2’-bipyridine)cobalt(II) tri(nitrate)DN-P 系列:钙钛矿型电池组件DN-P02用于制备CH3NH3PbI3的盐。
知母宁分子式

知母宁分子式
知母宁(Momordin)是一种从知母植物(Momordica charantia)中提取的生物活性化合物,具有广泛的药用价值。
它的分子式为C20H24O6,结构中包含一个环氧基和一个双键,使得知母宁具有独特的化学性质。
知母宁的化学性质使其具有良好的抗氧化、抗炎、抗肿瘤、抗菌和抗病毒作用。
在医药领域,知母宁可以用于治疗各种疾病,如高血压、糖尿病、病毒性感冒等。
此外,它还可以作为保健品食用,提高免疫力,预防疾病。
知母宁在医药领域的应用前景十分广阔。
目前,我国科学家正在研究将其开发为新型药物,以治疗癌症、病毒感染等疑难杂症。
此外,知母宁还可以作为农药和兽药,提高农作物的产量和品质,减少病虫害的发生。
知母宁的提取与制备方法是研究的关键环节。
通常采用醇提法、超声波辅助提取法等方法从知母植物中提取知母宁。
提取物经过分离、纯化后,可以得到高纯度的知母宁。
在制备过程中,要注意控制温度、压力等条件,以保证知母宁的生物活性不受破坏。
总之,知母宁作为一种具有广泛药用价值的生物活性化合物,其化学性质、药用价值和制备方法等方面的研究具有重要意义。
随着科学技术的不断发展,知母宁在医药领域的应用前景将更加广泛,为人类健康事业作出更大贡献。
Akt1和2和3抑制剂MK-2206生物活性CAS号1032349-77-1

例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数
(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
参考文献
Phosphatidylinositol 3-Kinase/Akt Signaling Pathway Activates the WNK-OSR1/SPAK-NCC Phosphorylation Cascade in Hyperinsulinemic db/db Mice. Nishida et al. Hypertension. 2012 Sep 4. PMID: 22949526.
生物活性
MK-2206是一种高度选择性的Akt1/2/3抑制剂,IC50分别为8 nM/12 nM/65 nM;对250种其他蛋白激酶没有抑制活性。MK-2206抑制Akt的苏氨酸308位点和丝氨酸 473位点的自身磷酸化作用。另外,MK-2206阻止Akt调节的下游信号分子(包括TSC2, PRAS40,及核糖体S6蛋白)的磷酸化作用。与抑制Ras 突变型细胞系(如 NCI-H358, NCI-H23, NCI-H1299, 和Calu-6)相比,MK-2206 更有效地抑制Ras野生型细胞系(如A431, HCC827, 和NCI-H292)。MK-2206和细胞毒素药剂如erlotinib 和 lapatinib联用作用于肺部NCI-H460肿瘤细胞或者卵巢A2780肿瘤细胞,MK-2206也显示出协同效应。
A novel PKB/Akt inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle. Lai et al. Biochem J. 2012 Jul 13. PMID: 22793019.
2-Deoxy-D-glucose_醣酵解的抑制剂_154-17-6_Apexbio

化学性质
产品名: Cas No.: 分子量: 分子式:
2-Deoxy-D-glucose 154-17-6 164.16 C6H12O5
产品名: 2-Deoxy-D-glucose 修订日期: 6/30/2016
化学名: SMILES:
溶解性: 储存条件: 一般建议:
运输条件:
(4R,5S,6R)-6-(hydroxymethyl)tetrahydro-2H-pyran-2,4,5-triol
引用文献
1. Tian C, Yuan Z, et al. "Inhibition of glycolysis by a novel EGFR/HER2 inhibitor KU004 suppresses the growth of HER2+ cancer." Exp Cell Res. 2017 May 19. pii: S0014-4827(17)30297-5. PMID:28532652
ApexBio Technology
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 :
Others
信号通路:
Hexokinase
产品描述:
2-Deoxy-D-glucose(2DG)(葡萄糖模拟物)是一个竞争性糖酵解的抑制剂[1 - 3]。细胞毒性试 验中对 KIT-阳性细胞株 GIST882 和 GIST430 的 IC50 值分别为 0.5 μM 和 2.5 μM[4]。 糖酵解是 ATP-产生的一个子系统[5]。 如果 2DG/葡萄糖的生理相关比率是 0.8,在包含 25 mmol/L 葡萄糖的 DMEM 中生长的 FaDu
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
百灵威核磁耗材产品

NMR Consumables and Accessories
客服热线:400-666-7788
全球 NMR 耗材 引领 60 年
百灵威
百灵威科技有限公司成立于 1992 年,始终以“为科研和生产提供世界一流的产品和服务”为宗旨,致力于超精细化学品的研发与 制造。经过近二十年的发展,百灵威已具备为化学、分析、生物、材料、物理及药物研发等领域提供近五十万种产品和专业服务 的能力。 百灵威拥有一支强大的具有丰富经验和创新能力的研发团队,在江苏、河北设立的两个研发中心可迅速研发出毫克至数百公斤级 的医药、生化、材料等中间体及特殊高端化学品,并可为客户定制合成各类产品,尤其擅长小分子药物中间体以及催化剂配体的 合成。 百灵威人坚信“发展民族科技”的理念,坚持依靠中国人自己的智慧和力量, 不断建设和发展位于潮白河畔的现代化工业生产基地,发挥百灵威在尖端技术研究、敏捷制造和系统性物流管理等方面的突出优 势,积极地将中国的各种高端化合物推荐给国际同行,为促进中国化学事业发展,推动世界文明与和谐进步而奋斗不息。 百灵威的使命 促进科技和工业发展,造福人类……
管壁厚度(mm) 平均凸度(µm)
包装
0.27
<60>
50只/塑料筒装
0.27
<60>
50只/塑料筒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.60
<60>
50只/塑料筒装
SampleJet®核磁管
化学专业英语词汇常用前后缀和各种物质的缩写

化学专业英语词汇常用前后缀有机化学合成常见缩写Ac Acetyl 乙酰基DMAP 4—dimethylaminopyridine 4—二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis—isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'—dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3。
1]nonane 9-硼二环[3。
3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3—双(二苯基膦基)丙烷BINAP (2R,3S)—2,2’-bis (diphenylphosphino)—1,1’—binaphthyl(2R,3S)—2。
2'—二苯膦-1.1'—联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine 乙二胺Bu n—butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat。
二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐

二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐摘要:一、化合物名称:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐二、化合物结构与性质1.分子式2.分子量3.化学结构4.物理性质三、应用领域1.表面活性剂2.乳化剂3.增稠剂4.其他应用四、安全性与环保1.生态毒性2.生物降解性3.安全使用建议五、结论正文:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐(C24H48NO4S)是一种具有特定化学结构和性质的化合物,广泛应用于多个领域。
一、化合物名称:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐二、化合物结构与性质1.分子式:C24H48NO4S2.分子量:448.7 g/mol3.化学结构:该化合物由二棕榈酰氧乙基羟乙基甲基铵和甲基硫酸盐两部分组成,呈现出一种特殊的化学结构。
4.物理性质:根据环境条件,该化合物可能呈现为固态、液态或气态。
三、应用领域1.表面活性剂:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐具有良好的表面活性和乳化性能,可作为表面活性剂用于清洁剂、洗涤剂等产品中。
2.乳化剂:该化合物在油水乳化体系中具有优良的乳化效果,可用于乳化剂的制备。
3.增稠剂:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐可用作增稠剂,提高体系的粘度。
4.其他应用:该化合物还广泛应用于化工、石油、农业等领域。
四、安全性与环保1.生态毒性:根据现有数据,该化合物对水生生物具有一定的毒性,应避免在环境中大量排放。
2.生物降解性:二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐在自然环境中有一定的生物降解能力,但降解速度可能受到环境条件的影响。
3.安全使用建议:在使用过程中,应遵循相关法规和标准,确保安全、环保。
综上所述,二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐作为一种具有特定化学结构和性质的化合物,在多个领域具有广泛的应用。
二乙氨羟苯甲酰基苯甲酸己酯 原材料

二乙氨羟苯甲酰基苯甲酸己酯原材料【最新版】目录1.二乙氨羟苯甲酰基苯甲酸己酯概述2.二乙氨羟苯甲酰基苯甲酸己酯的用途3.二乙氨羟苯甲酰基苯甲酸己酯在化妆品中的应用4.二乙氨羟苯甲酰基苯甲酸己酯的安全性5.二乙氨羟苯甲酰基苯甲酸己酯的市场前景正文【二乙氨羟苯甲酰基苯甲酸己酯概述】二乙氨羟苯甲酰基苯甲酸己酯(DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE,CAS No.302776-68-7)是一种油溶性化学性防晒剂,其防晒波段在 320-400nm,光稳性佳。
分子量大约在 391 道尔顿,最高含量为 10%。
它与 UVB 防晒剂搭配使用,可以提升产品的 SPF 值,有助于 UVB 的防护。
【二乙氨羟苯甲酰基苯甲酸己酯的用途】二乙氨羟苯甲酰基苯甲酸己酯主要应用于化妆品行业,作为一种较新的油溶性化学性防晒剂,其防晒波段在 320-400nm,光稳性佳,没有Avobenzone 那么容易被分解。
【二乙氨羟苯甲酰基苯甲酸己酯在化妆品中的应用】在化妆品中,二乙氨羟苯甲酰基苯甲酸己酯可以起到防晒作用,帮助消费者抵御紫外线的侵害。
它与生育酚乙酸酯、乳酸等成分配合使用,可以提高化妆品的保湿、抗氧化和美白效果。
【二乙氨羟苯甲酰基苯甲酸己酯的安全性】二乙氨羟苯甲酰基苯甲酸己酯的安全性得到了广泛认可。
它被认为是一种低刺激性的防晒剂,不会对皮肤造成过度刺激。
然而,敏感肌肤的消费者在使用前仍需进行皮肤测试,以确保不会产生过敏反应。
【二乙氨羟苯甲酰基苯甲酸己酯的市场前景】随着消费者对防晒化妆品的需求日益增加,二乙氨羟苯甲酰基苯甲酸己酯作为一款性能优良的防晒剂,市场前景广阔。
二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐

二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐
【最新版】
目录
1.二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的概述
2.二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的用途
3.二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的制备方法
4.二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的储存和运输
5.二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的安全性和注意事项
正文
二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐,是一种有机化合物,分子式为 C21H36NO4S。
它是一种阴离子表面活性剂,具有良好的乳化、分散、润湿性能,广泛应用于日用化工、洗涤剂、化妆品等领域。
二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的用途主要有以下几点:
1.用作洗发水、护发素的主要成分,可以改善头发的梳理性,使头发光滑有弹性。
2.用作沐浴露的主要成分,可以提高浴液的泡沫稳定性,使皮肤清洁更彻底。
3.用于化妆品中,如洗面奶、洁面膏等,可以温和地去除皮肤污垢,保持皮肤水分。
二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐的制备方法通常是通过以
下步骤:
1.将棕榈酰氯与乙醇胺反应,生成二棕榈酰氧乙基羟乙基甲基铵;
2.将二棕榈酰氧乙基羟乙基甲基铵与硫酸反应,生成二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐。
在储存和运输过程中,应注意以下几点:
1.储存于阴凉、通风、干燥的地方,避免阳光直射。
2.运输过程中应避免剧烈震动,保持包装完好。
在使用二棕榈酰氧乙基羟乙基甲基铵甲基硫酸盐时,需注意以下几点:
1.对皮肤和眼睛有刺激性,应避免直接接触。
2.如不慎接触,应立即用大量清水冲洗。
芦荟素对酪氨酸酶活性的抑制作用

目前国内关于芦荟素对酪氨酸酶的作用方面研究较少,该实验以L-多巴为底物,以氢醌为对照,研究芦荟素对酪氨酸酶的活性影响,并为临床上芦荟素或使用含有大量芦荟素的芦荟产品的综合利用提供实验依据。
酶在医药、食品、化工和环保等领域应用广泛。
酪氨酸酶是一种以双铜离子为活性单元的氧化还原性酶,在动物、植物体内广泛存在,对黑色素细胞的合成具有调控作用,是黑色素形成的限速酶[1]。
黑色素细胞是生物体内黑色素的合成主要场所,在黑色素的合成过程中,酪氨酸酶对整个黑色素的合成具有重要催化作用,酪氨酸酶主要存在于黑色素细胞中。
酪氨酸酶的活性升高,则黑色素的形成增加;酪氨酸酶的活性降低,则黑色素的生成减少,因此,酪氨酸酶活性的强弱与黑色素形成的多少存在正相关关系。
临床上酶氨酶活性过强、或者过弱均会导致临床疾病的发生,如黄褐斑、白化病、雀斑、白癜风等异性性色素性皮肤病[2]。
国内外学者常利用抑制酪氨酸酶活性试验,研究筛选治疗皮肤色素沉着症的药物[3]。
该实验采用蘑菇酪氨酸多巴速率氧化法,研究活性单体化合物芦荟素对酪氨酸酶的影响[4]。
1材料与方法1.1试剂与仪器L-多巴胺(L-DOPA),蘑菇酪氨酸酶(美国Sigma公司);芦荟素(中国药品生物制品检定所);氢醌(天津市巴斯夫化工有限公司);其他试剂均为市售分析纯。
UV-1800紫外可见分光光度计(日本岛津);AUY220电子分析天平(日本岛津)。
1.2方法采用pH6.8磷酸缓冲溶液,将L-DOPA、芦荟素、氢醌分别配制成0.1%的溶液,将蘑菇酪氨酸酶配制成100U/mL的溶液。
取上述磷酸缓冲240μL,加入0.1%L-DOPA反应液80μL,蘑菇酪氨酸酶80μL混合均匀,常温静置5min,于分光光度计上在400~600nm扫描。
可见在475nm处有最大吸收峰。
同法分别测定L-DOPA、蘑菇酪氨酸酶均未见有吸收。
采用酪氨酸酶多巴速率氧化法,在pH6.8磷酸缓冲液中,用酪氨酸酶催化多巴变成多巴醌,有肾上腺素红色,利用分光光度计在475nm处的特定吸收测定生成多巴醌时的吸收度:A=A(药+L-DOPA+酶)—A(药+酶)。
他卡西醇分子式

他卡西醇分子式卡西醇(Cortisol)分子式为C21H30O5。
卡西醇是一种重要的类固醇激素,它在人体内起着重要的调节作用。
下面将从卡西醇的生理功能、合成途径和应用领域等方面进行介绍。
一、卡西醇的生理功能卡西醇在人体内具有多种生理功能,主要包括抗炎抗过敏、抑制免疫反应、调节血糖和蛋白质代谢等。
首先,卡西醇能够抑制炎症反应和过敏反应,减轻组织损伤和炎症症状。
其次,卡西醇还能够抑制免疫反应,减少自身免疫性疾病的发生。
此外,卡西醇还能够调节血糖和蛋白质代谢,增加血糖水平,促进肝脏糖原的合成,并抑制蛋白质的合成,以提供足够的能量供给机体。
二、卡西醇的合成途径卡西醇的合成途径主要包括胆固醇合成途径和类固醇激素合成途径。
首先,胆固醇合成途径是卡西醇合成的前提,胆固醇通过一系列酶的作用,最终合成孕酮。
其次,类固醇激素合成途径是卡西醇合成的关键步骤,孕酮通过一系列酶的作用,最终合成卡西醇。
这个过程中,涉及到多个酶的催化作用和共同调节,其中ACTH(促肾上腺皮质激素)是卡西醇合成的主要调节因子。
三、卡西醇的应用领域由于卡西醇具有重要的生理功能,因此在临床上有广泛的应用。
首先,卡西醇常用于治疗肾上腺皮质功能不全症,如原发性肾上腺皮质功能不全和继发性肾上腺皮质功能不全等。
其次,卡西醇还常用于治疗炎症和过敏反应相关的疾病,如风湿性关节炎、过敏性鼻炎等。
此外,卡西醇还可用于治疗某些自身免疫性疾病,如系统性红斑狼疮和类风湿关节炎等。
总结起来,卡西醇是一种重要的类固醇激素,具有多种生理功能。
它通过抗炎抗过敏、抑制免疫反应、调节血糖和蛋白质代谢等途径对人体进行调节。
在临床上,卡西醇常用于肾上腺皮质功能不全症、炎症和过敏反应相关的疾病以及某些自身免疫性疾病的治疗。
通过深入了解卡西醇的生理功能和应用领域,可以更好地认识和理解这一重要的类固醇激素。
