腺病毒介导人内皮抑素基因瘤内注射抑制鼻咽癌生长
广州达博生物溶瘤腺病毒E10A

广州达博生物溶瘤腺病毒E10A重组人内皮抑素蛋白实体瘤的生长和转移依赖于血管生成。
内皮抑素(Endostatin)是胶原XVIII的C末端蛋白水解酶片段,在小鼠肿瘤治疗中是一种有效的内源性血管生成抑制剂。
应用于实体瘤的临床研究,但重组人内皮抑素蛋白难以大规模生产,且日常给药繁琐。
迄今为止,其临床应用一直受到这些问题的阻碍。
内皮抑素,一个20 kDa的C-末端片段的胶原XVIII,已被证明可以阻止内皮细胞的增殖、存活和迁移,部分通过下调促血管生成因子(如Ids、HIF-1a、VEGF-A、bFGF)和上调抗血管生成因子(如血栓反应蛋白-1、2、血管抑制素、激肽原)。
在体内的内源性抗血管生成因子4、5、6,内皮抑素在小鼠和人中具有广泛的抗癌谱和低毒、低耐药性的特点。
内皮抑素是第一个引入人类临床试验的内源性血管生成抑制剂。
然而,重组内皮抑素蛋白的半衰期短、蛋白生产困难和生物活性蛋白的长期贮存,阻碍了其治疗。
此外,抑制肿瘤血管生成是一个长期而慢性的治疗过程。
基因治疗可以通过将人内皮抑素cDNA导入宿主并利用机体作为内源性工厂来产生具有高度生物活性的基因产物来克服这些困难。
腺病毒基因转移表达内皮抑素(Ad-rhEndo,E10A)对小鼠实体瘤模型有很强的全身治疗作用。
E10A 一种复制缺陷型重组人内皮抑素基因腺病毒E10A是一种复制缺陷型重组腺病毒,含有由5型腺病毒(Ad5)构建的野生型人内皮抑素转基因。
临床前研究表明,在肝癌、鼻咽癌和舌癌动物模型中,瘤内注射E10A可显著抑制肿瘤生长,并使血液和肿瘤组织中内皮抑素持续升高。
转染肿瘤细胞后,E10A表达人内皮抑素,抑制血管内皮细胞增殖和肿瘤血管生成,阻断肿瘤血供,从而特异性抑制肿瘤生长,诱导肿瘤细胞凋亡。
临床前和动物模型均证实了E10A的抗肿瘤作用,其治疗头颈部肿瘤的安全性和有效性也在一期和二期研究中得到证实。
注射方式:瘤内注射临床试验最新情况1期临床实体瘤的生长和转移依赖于血管生成。
重组人血管内皮抑制素注射液对中、晚期胃癌的疗效及对血清基质金属蛋白酶-14水平的影响

2. 3 两组远期疗效( 生存分析) 比较 观察组随访 6 ~ 36 个 月,对照组 4 ~ 36 个月,生存分析显示观察组生存明显长于对 照组( χ2 = 11. 26,P = 0. 014 3) 。
3讨论 化疗是中、晚期胃癌常用治疗手段,其治疗方案缺乏一致
性。胃癌在进展过程中表现出明显的蛋白和基因变化。而胃 癌进展过程中,细胞外基质( ECM) 易出现明显变化,当前观察 降解 ECM 重要成分是 MMP〔3〕。MMP-14 是其经典成员,可有 效降解 ECM。MMP-14 作为 MMP 家族重要成员,具有前肽区、 信号肽区、催化区、铰链区和类血红素蛋白结合区 5 个功能区 域,能在细胞表面活化 MMP-2 等重要因子。MMP-14 可 降 解 Ⅰ、Ⅱ、Ⅳ型胶原和纤维连接蛋白,参与包括基膜在内的 ECM 降解过程。而 ECM 和基底膜是肿瘤生长和扩散的屏障,肿瘤 细胞依靠 MMP 降解 ECM,进而促进浸润和转移〔4〕。且有研究 显示 MMP-14 也与肿瘤血管新生有密切关系〔5〕。中、晚期胃癌 时 MMP-14 表达增多,不仅对肿瘤 ECM 降解有重要影响,且对 肿瘤进展及转移有明显促进作用。MMP-14 与血管生成有关, 尤其是与血管内皮生长因子( VEGF) 正向协同作用,使 MMP-14 与血管新生密切联系在一起。恩度为血管生成抑制类新生物 制品,其作用机制是通过抑制形成血管的内皮细胞迁移来达到 抑制肿瘤新生血管生成,阻断了肿瘤细胞营养供给,从而达到 抑制肿瘤增殖或转移目的〔6,7〕。恩度作用机制是多元性的,可 提高肿瘤细胞对化疗药物敏感性。恩度对抑制血管新生可能 有重要作用,对抑制肿瘤 ECM 降解有一定作用,对延缓或阻断 肿瘤进展作用明显〔8,9〕。而恩度可有效降解 MMP-14 表达,可 能与肿瘤对 VEGF 抑制作用有关,进而下调 MMP-14 表达,也表 明恩度作用可能更广泛,对肿瘤抑制通过多方面作用,进而控 制肿瘤生长及转移〔10〕。
重组人血管内皮抑制素靶向治疗联合化疗治疗多种恶性肿瘤临床观察论文

重组人血管内皮抑制素靶向治疗联合化疗治疗多种恶性肿瘤临床观察摘要目的:评价重组人血管内皮抑制素(rh-endostatin,yh-16)联合化疗在多种恶性肿瘤治疗中的有效性和安全性。
方法:2006年10月~2009年3月中晚期恶性肿瘤患者31例接受化疗联合yh-16,恩度15mg/次,加入0.9%生理盐水500ml稀释后,1次/日,连用14天为1个疗程,间歇7天后,重复使用。
研究的终点目标是客观有效率(rr)、生活质量(qol)以及安全性。
结果:31例可评价疗效的患者中,有26例患者可以进行客观疗效、生活质量和安全性评价。
总共完成的周期数为60个周期。
生活质量治疗后有明显提高,治疗后完全缓解(cr)1例,部分缓解(pr)6例,病情稳定(sd)19例。
结论:yh-16与化疗联合,能提高多种恶性肿瘤患者的rr,改善和稳定多种晚期恶性肿瘤患者的生活质量,且安全性较好,是靶向治疗药物联合化疗应用的成功典范,值得临床上推广应用和进一步深入观察。
关键词恩度化疗恶性肿瘤abstract objective:to explore the effectiveness and safety of endostar-combined with chemotherapy in the treatment of cancer.methods:endostar combined with chemotherapy were administrated to 31 advanced cancer cases, who were confirmed by histopathology or cytopathology from october 2006 to march 2009.a total of 15mg endostar solvedin 500ml of normal saline was intravenously admistered from day 1 to day 14,repeated later 7 days.the chemotherapy agents that were not used previously or not cross–resistant were administered simultaneously.the regimen was repeated every 21 days.the terminal point were objective response rate (rr);quality of life (qol) and safety.results:26 patients were totally evaluated for efficacy and side effects after 60 cycles,1 patient achieved cr,6 patients pr,and 19 patient sd.conclusion:the combination of yh-16 with chemotherapy can improve the response rate;efficacy rate and quality of life of patient obviously,this treatment is worthy of clinical generalization and further clinical observation.key wordsendostar chemotherapy cancer资料与方法2006年10月~2009年3月收治中晚期恶性肿瘤患者31例,其中男17例,女14例,中位年龄57.5岁,均有原发肿瘤的病理诊断和(或)胸腔积液的细胞学诊断,且kps评分≥40分。
重组人5型腺病毒注射液局部瘤内注射治疗晚期肝癌的临床研究

.论著•-----------------------中国现代医生2021年4月第59卷第12期重组人5型腺病毒注射液局部瘤内注射治疗晚期肝癌的临床研究陆吉麟1张浩1石伟1崔小红2岳增辉2王永勇2伊萌31.复旦大学附属华山医院普外科,上海200050;2.上海电力医院普外科,上海200031;3.上海三维生物技术有限公司,上海200052[摘要]目的探讨重组人5型腺病毒注射液局部瘤内注射治疗晚期肝癌的临床疗效及安全性。
方法选择2009年1月至2013年1月来自复旦大学附属华山医院的80例晚期肝癌患者,分为对照组(n=40)和观察组(n=40),对照组患者只给予顺铂及5-氟尿嘧啶化疗,观察组患者在化疗基础上联合B超引导下经皮瘤内注射重组人5型腺病毒注射液,连续使用两个周期。
比较两组患者肿瘤客观有效率、肿瘤进展时间及不良反应。
结果观察组的客观有效率为22.50%.,高于对照组的5.00%.,差异有统计学意义(P<0.05)o观察组的平均肿瘤进展时间为(11.93±2.54)周,高于对照组的(8.06±2.21)周,差异有统计学意义(P<0.05)。
局部瘤内注射后48h观察组血液中IFN-7a IL-1«、IL-6及TNF-琢显著高于治疗前,差异有统计学意义(P<0.05)。
两组患者治疗后ALT、AST、ALP、TBIL及AFP指标均有所下降,而观察组下降幅度大于对照组,差异有统计学意义(P<0.05)。
观察组患者不良反应主要为发热、局部反应及流感样症状,均为I/域级。
观察组患者生活质量提高率为55.00%,高于对照组的22.50%,差异有统计学意义(P<0.05)o结论晚期肝癌患者在化疗基础上局部瘤内注射重组人5型腺病毒注射液,可提高客观有效率和患者生存期,且安全性及耐受性好。
[关键词]肝癌;腺病毒;重组人5型腺病毒;基因疗法[中图分类号]R738.6;R730.5[文献标识码]A[文章编号]1673-9701(2021)12-0010-06Clinical research on topical intratumoral injection of recombinant human adenovirus type5in the treatment of advanced liver cancerLU Jilin'ZHANG Hao'SHI Wei'CUI Xiaohong YUE Zenghui2WANG Yong^^ong YI Meng1.DeparLmenL of General Surgery,Huashan HospiLal AffiliaLed Lo Fudan UniversiLy,Shanghai200050,China;2.DeparL-menL of General Surgery,Shanghai ElecLric Power HospiLal,Shanghai200031,China;3.Shanghai Sunway BioLech Co., LTD,Shanghai200052,China[Abstract]Objective To invesLigaLe Lhe clinical efficacy and safeLy of inLraLumoral injecLion of recombinanL human adenovirus Lype5in Lhe LreaLmenL of advanced liver cancer.Methods A LoLal of80paLienLs wiLh advanced liver cancer admiLLed Lo Huashan HospiLal AffiliaLed Lo Fudan UniversiLy from January2009Lo January2013were selecLed,and Lhey were divided inLo Lhe conLrol group(n=40)and Lhe observation group(n=40).The paLienLs in Lhe conLrol group were only LreaLed wiLh cisplaLin and fluorouracil chemoLherapy,while Lhose in Lhe observaLion group were LreaLed wiLh re-combinanL human adenovirus Lype5injecLion on Lhe basis of chemoLherapy combined wiLh B-ulLrasound guided percu-Laneous injecLion for Lwo consecuLive cycles.The objecLive efficacy raLe,Lumor progression Lime and adverse reacLions (ADRs)of Lhe Lwo groups of Lumors were compared.Results The objective efficacy raLe in Lhe observation group was22.50%,which was higher Lhan LhaL of5.00%in Lhe conLrol group,wiLh sLaLisLically significanL difference(P<0.05).TheLumor progression Lime in Lhe observaLion group was(11.93±2.54)weeks,which was higher Lhan LhaL of(8.06±2.21)weeks in Lhe conLrol group,wiLh sLaLisLically significant difference(P<0.05).The blood levels of IFN-酌,IL-1a,IL-6and TNF-琢in Lhe observation group48h afLer Lopical inLraLumoral injecLion were significantly higher Lhan Lhose before injecLion(P<0.05).AfLer LreaLmenL,Lhe index levels of ALT,AST,ALP,TBIL and AFP all decreased in boLh groups,while Lhe decrease in Lhe observaLion group was greaLer Lhan LhaL in Lhe conLrol group,wiLh sLaLisLically significanL difference(P<0.05).The main ADRs in Lhe observaLion group were fever,Lopical reacLions and influenza like sympLoms,all of whichwere grade I/域.The improvement raLe of qualiLy of life in Lhe observation group was55.00%',which was higher Lhan LhaL of22.50%in Lhe conLrol group,wiLh sLaLisLically significant difference(P<0.05).Conclusion Topical inLraLumoral in-jecLion of recombinanL human adenovirus Lype5on Lhe basis of chemoLherapy for paLienLs wiLh advanced liver cancer can improve Lhe objecLive efficacy raLe and survival Lime of paLienLs,wiLh good safeLy and Lolerance.[Key words]Liver cancer;Adenovirus;RecombinanL human adenovirus Lype5;Gene Lherapy10CHINA MODERN DOCTOR Vol.59No.12April2021肝癌具有极高的发病率和死亡率,是严重威胁我国人民健康的恶性疾病之一[1]。
2001年2008年申报的一类治疗用生物制品
至患者体内,携带肿瘤抗原的 DC 会将抗原信息提呈给特异性 T 细胞并
使之活化,从而诱导机体产生大量具有特异性细胞毒性功能的 T 淋巴细
胞,对肿瘤细胞具有特异性杀伤作用。
3
序号
7
受理号
CSLB20020006
制剂剂型
细胞因子诱 导的杀伤细
胞注射剂 (CIK细胞注
射剂)
2001 年~2008 年申报的一类治疗用生物制品
重组人溶菌 酶雾化溶液 重组人溶菌
酶喷雾剂 冻干重组人 促黄体激素 释放激素-绿 脓杆菌外毒 素 A 融合蛋白
已发通知件广东省 申请阶段:临床
已发批件吉林省 申请阶段:临床
重组人溶菌酶是采用基因工程酵母菌经发酵生产的一种生物药品和 长春奇龙生物技术研究
精细化工品,用作人体消炎、抗感染、抗病毒
所
本品是一种低毒高效的抗癌生物导弹,对肺癌、结肠癌、胃癌、胰 吉林亚泰生物药业股份 腺癌、乳腺癌、宫颈癌、卵巢癌等实体恶性肿瘤有疗效,尤其适应 有限公司 于一些放化疗不敏感的腺癌。
CSS01032
殖,从而缩短创面的愈合时间。适用于皮肤烧烫伤创面(浅 Π 度至深Ⅱ 品有限公司
度烧烫伤创面)、残余创面、供皮区 创面及慢性溃疡创面的治疗。
10 CSS00089 抗人白细胞介素
已发批件
银屑病病灶皮肤中的白细胞介素-8 浓度高于正常水平,推测其可能是银 莞宏远逸士生物技术药
-8 单克隆抗体乳 申请阶段:生产 屑病病灶早期形成的因素之一。本品为鼠源性抗人白细胞介素-8 单克 业有限公司加拿大 YES
注册及生产单位
上海华谊生物技术有限 公司
重组人纽表 位肽 12 注射
液 注射用重组 人胸腺素 α
原 注射用重组 人肿瘤坏死 因子-NC
鼻咽癌的靶向治疗和免疫治疗进展

鼻咽癌的靶向治疗和免疫治疗进展鼻咽癌是一种罕见但具有较高恶性程度的头颈部肿瘤,在全球范围内仍然是一种难以治愈的癌症类型。
然而,随着医学科技的不断进步,靶向治疗和免疫治疗在鼻咽癌患者中逐渐显示出显著的治疗效果。
本文将重点介绍鼻咽癌的靶向治疗和免疫治疗的最新进展。
1. 靶向治疗靶向治疗是一种基于分子靶点的治疗方法,通过针对特定的癌症细胞分子靶点,抑制癌症细胞的生长和分裂,从而达到治疗的目的。
在鼻咽癌的靶向治疗中,最为常见的靶向药物是表皮生长因子受体(EGFR)抑制剂和血管内皮生长因子(VEGF)抑制剂。
EGFR抑制剂主要通过阻断鼻咽癌细胞中EGFR的活化,干扰信号传导通路,从而抑制鼻咽癌细胞的生长。
目前,多个EGFR抑制剂已经被批准用于鼻咽癌的治疗,其中包括厄洛替尼、吉西他滨等。
这些药物的应用可以明显改善患者的生存期和治疗效果。
另一类靶向治疗药物是VEGF抑制剂,它通过干扰癌细胞侵袭和血管生成过程,抑制鼻咽癌的生长和转移。
贝伐珠单抗是一种常用的VEGF抑制剂,在鼻咽癌的治疗中已经取得了一定的成果。
2. 免疫治疗免疫治疗是通过调节机体免疫系统,激活免疫细胞对癌细胞进行攻击,从而达到治疗癌症的效果。
在鼻咽癌的免疫治疗中,最为常见的方法是使用免疫检查点抑制剂。
免疫检查点抑制剂特定地抑制体内的调节性T细胞,使活化的免疫细胞对鼻咽癌细胞产生更强的攻击效应。
例如,抗PD-1抗体(例如nivolumab)和抗PD-L1抗体(例如pembrolizumab)是常用的免疫检查点抑制剂,已经显示出在鼻咽癌治疗中的潜力。
此外,疫苗治疗也是鼻咽癌的免疫治疗的一种重要方法。
疫苗通过引入特定的鼻咽癌抗原,激活机体免疫系统对鼻咽癌细胞产生免疫应答。
很多研究已经证明,鼻咽癌疫苗在患者中具有较好的治疗效果和生存期提高等优点。
3. 靶向治疗和免疫治疗的联合应用近年来,越来越多的研究表明,鼻咽癌的靶向治疗和免疫治疗的联合应用具有更好的治疗效果。
重组人血管内皮抑制素注射液治疗恶性肿瘤的作用机制及临床研究进展

有效率为 " 3 5 . 4 %,与对 照组 ( 1 9 . 5 %) 相 比差异有显著性[ 。另
有恩度 +吉西他滨方案治疗晚期 NS C L C 在提 高有 效率 、延长 无疾病进展时间 同时 ,副反应无 明显增加【 l 4 J 。郑 鑫等观察 了 2 O 例晚期 NS C L C 患者采用恩度联合不 同化疗方案 的治疗效果 ,
・
综述 ・
重组人血管 内皮抑制素注射液治疗恶性肿瘤的 作用机制及临床研 究进展
马春 燕 王振 国
有近 1 4 0万患者死于肺癌【 ,其中非小细胞肺癌 ( NS C L C)占
美国癌症协会统计恶性肿瘤位居人类疾病死亡原因之 , 绝 大多数肿 瘤被 确诊 时已失去 手术机会或术后 复发 ,5年生存率 较低 。1 9 7 1年美 国 F o l k ma n t 】 率先提 出了肿瘤生长依赖血管形
同天然 的血管 内皮抑制素相 比,恩度在 N端添加 了 9个氨 基酸序列 ,使得半衰期延长,生物清 f 生和药物稳定性明显提高p J ,
是一 种 具有靶 向性和 非细胞 毒性 等特 点 的广谱 血管 生成 抑制 剂 。其 作用机制 :影 响血管 内皮因子传导通 路和 蛋 白水解酶 的
活性 ;对 内皮细胞迁移 产生影响 ;诱导 内皮 细胞凋亡 ;调节 细 胞 的生长周期 以及 下调 处于生长期 的内皮 细胞的许多基 因或其 表达产物[ 4 1 。 理论 上,抗肿瘤血 管生成可 以 “ 饿死 ”肿瘤 ,但过度抗肿 瘤微血 管生成反而会加 重肿瘤微环境 的低氧 状态 ,导致微血 管
一
、Байду номын сангаас
恩度及 其抗肿瘤血管生成机制
恩度通过抑制血 管内皮细胞切断肿瘤细胞的血液供应并诱
重组人血管内皮抑素联合肿瘤坏死因子治疗乳腺癌的实验性研究
日 I (td) /
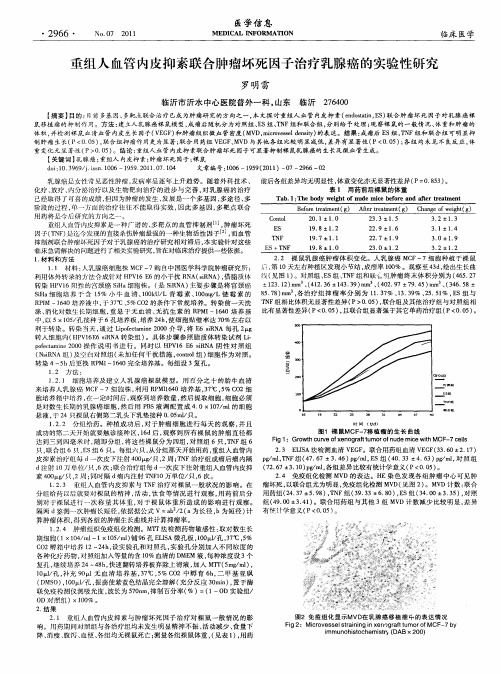
图 1 裸 鼠MCF 7 植 瘤 的 生 长 曲线 一移
F g 1: Gr wt u v f en g a t u i o hc reo x o r lt mor f u e m ie wi CF e l d c tM on h 一7 c l s
2 3 E IA法检测血 清 V F . LS EG 。联合用药组血清 V G (3 6 E F 3 .0±2 1 ) . 7
p/r,N g r T F组 ( 7 6 d 4 . 7±3 4 p r , S组 ( O 3 . 6) r E d 4 . 3±4 6 ) m , 照 组 . 3 p l对 ( 26 3 1 ) gn , 7 .74 .0 p/ ' 各组差异 比较有统计学意义 ( - d P<00 ) .5 。
24 免疫组化检测 MV . D的表 达。H E染 色发现各 组肿瘤 中心 可见肿 瘤坏死 , 以联合组尤为明显 , 免疫组化检测 MV 见 图 2 。MV D( ) D计数 : 联合 用药组 ( 43 5 9 ) T F组 ( 9 3 2 .7± .8 , N 3 . 3±6 8 ),S组 (4 0 .0 E 3 .0±3 3 ) 对照 .5 , 组( 9 0 3 4 ) 4 .0± . 1 。联合用 药组 与其他 3 M D计 数减少 比较 明显 , 组 V 差异 有 统 计 学 意 义 ( 0 0 ) P< . 5 。
26 0 74 0
【 摘要】 目的 : 前 多基 因、 目 多靶点联合治疗 已成为肿瘤研 究的方向之 一, 本文探讨重 组人 血管 内皮抑 素( n o a n E ) e ds t , S 联合肿 瘤坏死 因子对乳腺 癌裸 ti
鼠 移 植 瘤 的 抑 制作 用 。 方 法 : 立 人 乳 腺 癌裸 鼠模 型 , 瘤 后 随 机 分 为 对 照 组 、 s组 、 N 建 成 E T F组 和 联 合 组 , 别 给 予 处 理 ; 察 裸 鼠 的 一般 情 况 、 重 和 肿 瘤 的 分 观 体
腺病毒介导的p53基因治疗肝癌的给药途径研究进展

然而 , 由于肝供体 的获取较难以及移植后肝的复发
转移 , 使得肝移植仅适合于肝 内有少于 3 个可见小 病灶 的病人 ; 巨大的肿块也不能行外 科手术 , 因为 有可能会造成肝衰竭及高危险性的转移 ; 经导管肝 动脉栓塞术 (A E 、 T C . 经皮穿刺肝 内无水 乙醇注射 ) 以及射频治疗 已经被证 明并 不能改善 病人 的中位
维普资讯
国外医学临床放射学分册 Frg ei l c ne Cic aioi l a ie20 a;0( :86 o i M d a Si cs l i R d l c s c 07Jn3 1 5-0 en c e n a l o ga F cl )
反应 为 I Ⅱ度发 热(9 、 战(3 , / 7%)寒 5%)注射部位
疼痛 , 未能检测到最大耐受量嗍 。
重组 人 p 3 病毒的应 用并没有像许多研究 5腺 者想像 的那样安全 。G r a i 指出, e ln 等阎 or 腺病毒的注 射能导致病毒颗粒及转导细胞强烈 的免疫反应 , 释
生存期。最近的体内体外试验均表 明, 因治疗有 基 可能成为肝癌综合治疗的重要组成部分。基因治疗 的关键在于其有效的靶 向性和转染性。试验及临床 证实[腺病毒介导的 p 3 因有很高 的转染率 , 1 】 , 5基 而 且给药途径不 同, 肝癌细胞 中的转染基 因含量也有
差异 , 且其安全性还需进一步的试验和临床研究加 以完善。本文就重组人 p 3 5 腺病毒治疗肝癌的安全
例病人的资料证实 , d p 3 N r /5 A没有插入宿主细 A D 胞的危险 陛。 应用一些有损 D A的肿瘤治疗方法 , N
如放射治疗和化疗 , 不但不增加重组腺病毒的整合 频率 , 相反对放疗 、 化疗有增敏作用四 。实验观察到 把 B 20 腺病毒载体转染后能显著提高 p3基因 B 12 5
重组人血管内皮抑素增强EGFR-TKIs对肺癌的抗瘤作用

重组人血管内皮抑素增强EGFR-TKIs对肺癌的抗瘤作用作者:龙亚辉周辉来源:《中国现代医生》2022年第11期[摘要] 目的探讨重组人血管内皮抑素联合表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKIs)耐药细胞系H1975体外及对裸鼠移植瘤的抗瘤作用。
方法建立H1975肺癌细胞系裸鼠移植瘤模型24只,并随机分为对照组、单一用药组和联合用药组,每组各8只,建立H1975肺癌细胞系裸鼠移植瘤模型,对照组小鼠不做任何处理,单一用药组注射恩度,联合用药组注射厄洛替尼和恩度,2周后处死实验鼠,并将肿瘤取出进行测量,分析不同用药方法对裸鼠移植瘤的抗瘤作用。
结果随着给药时间的延长,对照组小鼠慢慢精神较差,活动量与饮食量减少,卷缩扎堆,毛色灰暗无光泽度。
联合用药组小鼠活动情况与饮食情况基本正常,单一用药组小鼠表现介于两组之间;三组小鼠的肿瘤体积与重量比较,差异有统计学意义(P[关键词] 重组人血管内皮抑素;EGFR-TKIs;耐药;肺癌[中图分类号] R734.2 [文献标识码] A [文章编号] 1673-9701(2022)11-0016-04[Abstract] Objective To investigate the antitumor effect of the epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKIs) in combination with recombinant human vascular endostatin on xenograft tumor in nude mice in vitro using drug-resistant cell line H1975. Methods Twenty four nude mouse xenograft tumor models of the H1975 lung cancer cell line were established and randomly divided into the control group, the monotherapy group and the combination group,with 8 mice in each group. Mice in the control group were given no treatment. The monotherapygroup was injected with endostar, and the combination group was injected with erlotinib and endostar. After 2 weeks, the mice were sacrificed, and the tumors were removed for measurement to analyze the anti-tumor effect of different medication methods on transplanted tumors in nude mice. Results With the prolongation of administration time, the mice in the control group were in poor spirits gradually, with reduced activity and diet, and their hair color was dark without gloss. The activity and diet of mice in the combination group were basically normal, while those in the single group were between the two groups. Statistically significant differences were observed in the tumor volume and weight among the three groups, with the largest in the control group, followed by the monotherapy group, and the smallest in the combination group (P[Key words] Recombinant human vascular endostatin; EGFR-TKIs; Drug resistance; Lung cancer据相关专家统计,到2030年我国每年肺癌的发病人数会达到100万人,患者5年生存率只有10%左右[1],其发病率居全球恶性肿瘤首位,也是我国发病率和死亡率均居第1位的恶性肿瘤。
增殖缺陷型腺病毒介导人内皮抑素基因抗乳腺癌的实验

增殖缺陷型腺病毒介导人内皮抑素基因抗乳腺癌的实验【摘要】目的通过建立裸鼠乳腺癌肿瘤模型,在体内实验中进一步证实携带人内皮抑素基因的增殖缺陷型腺病毒Ad hE的抗肿瘤作用。
方法在BALB/c裸鼠皮下种植人乳腺癌细胞MCF7,建立移植瘤模型。
在瘤体内注射Ad hE治疗,观察肿瘤生长,免疫组化检测肿瘤细胞内皮抑素的表达和计数肿瘤间质中微血管的数量。
结果 Ad hE 具有明显抑制肿瘤细胞生长的作用,瘤体内注射重组腺病毒后4周,Ad hE治疗组肿瘤体积为(225.3±90.2)mm3,明显小于Ad LacZ 病毒对照组(794.9±189.8)mm3和空白对照组(890.7±102.5)mm3。
免疫组化显示,乳腺癌细胞在感染腺病毒后能够有效表达内皮抑素,阳性细胞比率超过60%以上。
Ad hE治疗组瘤组织中血管数量(16.3±7.3)明显少于空白对照组(54.6±17.6)和Ad LacZ病毒对照组(49.8±21.2)。
结论增殖缺陷型腺病毒介导人内皮抑素的表达,具有明显抑制肿瘤细胞生长的作用。
【关键词】腺病毒内皮抑素乳腺癌基因治疗0 引言近年来,抗肿瘤血管生成的基因治疗策略倍受关注[1],已发现的多种具有抑制肿瘤血管生成的活性物质中以血管抑素和内皮抑素最为引人注意[2,3]。
前期我们构建了携带人内皮抑素基因(human endostatin,hE)的增殖缺陷型腺病毒Ad hE,并在体外细胞学实验中证实Ad hE感染乳腺癌细胞株MDA MB231和MCF7后,可介导内皮抑素基因的高效表达,且表达的内皮抑素可明显抑制人脐静脉内皮细胞株ECV304细胞的生长[4]。
在此基础上,本文通过建立裸鼠乳腺癌模型,开展体内实验进一步证实Ad hE的抗肿瘤作用。
1 材料与方法1.1 材料来源携带人内皮抑素基因(Human endostatin, hE)的增殖缺陷型腺病毒Ad hE和携带报告基因LacZ的增殖缺陷型腺病毒Ad LacZ 由我们自己构建并保存,人乳腺癌细胞株MCF7购于美国ATCC细胞库,鼠抗人hE单克隆抗体、鼠抗人CD31单克隆抗体、SP免疫组化试剂盒购自福州迈新生物技术公司。
生长抑素(SST)是在人体内广泛分布的一种激素,表明生长抑素对生成肿瘤血管的多种因子,诸如血管内皮细胞

8AlbiniA,FlorioT,GiunciuglioD,et al.SomatostatincontrolsKaposi′sarcomatumor growth through inhibition ofangiogenesis. FASEB J,1999,13:647-655。
3生长抑素与各调控因子的关系
3.1生长抑素对血管生成的影响
近年来体内、外的研究表明,生长抑素及其类似物是一类有效的抗血管生成剂[12]。Danesi等AgNO3烧灼伤诱导的大鼠角膜血管生成模型,体内研究了生长抑素抗血管生成作用,结果发现眼球表面注射奥曲肽10μg/d,连续6天,角膜新生血管形成明显受到抑制,给予40μg/d,能减少实验诱发的鼠肠系膜新生血管形成。Koizumi等将人直肠神经内分泌癌移植于裸鼠皮下,应用奥曲肽治疗6周,发现奥曲肽能够明显抑制肿瘤生长,治疗组肿瘤组织内的微血管密度较对照组明显减少。Albini等将Kaposi肉瘤细胞皮下注入裸鼠侧腹壁,建立移植肿瘤模型,治疗组皮下注射生长抑素100μg/d,连续20天,结果发现治疗组移植肿瘤的体积较对照组明显减小,行移植肿瘤的组织学检查发现:对照组肿瘤组织内有广泛的新血管生成,而治疗组肿瘤组织内仅有少许的新血管生成。上述的研究显示,生长抑素及其类似物是一类有效的抗肿瘤血管生成剂[13,14]。
3PlonowskiA,SchallyAV,Nagy A,et al. Inhibition ofmetastaticrenal cell carcinomas expressingsomatostatinreceptors by a targetedcytotoxicanalogue ofsomatostatinAN2238. CancerRes,2000,60:2996-3001。
腺病毒介导的nm23-H1对人恶性黑色素瘤A375体外抑制作用的研究

腺病毒介导的nm23-H1对人恶性黑色素瘤A375体外抑制作用的研究李宗河;何学令;刘艳;尹海林【期刊名称】《国外医药(抗生素分册)》【年(卷),期】2011(000)006【摘要】目的观察Ad-GFP-nm23-H1抑制人恶性黑色素瘤(Malignant melanoma,MM)A375细胞生长和转移的作用,为后期nm23-Hl基因和腺病毒载体用于MM及其它肿瘤的基因治疗提供一定的理论和方法.方法采用MTT法检测Ad-GFP-nm23-H1,对A375细胞增殖力的影响,用细胞基质胶黏附实验黏附力和侵袭能力实验来分别检测Ad-GFP-nm23-H1,对A375细胞粘附力和侵袭能力的影响.结果 Ad-GFP-nm23-Hl可以明显抑制A375细胞的增殖、黏附和侵袭能力,其抑制作用呈剂量一效应关系.结论 Ad-GFP-nm23-Hl,对人恶性黑色素瘤A375细胞的生长和转移具有抑制作用.【总页数】7页(P273-278,286)【作者】李宗河;何学令;刘艳;尹海林【作者单位】四川抗菌素工业研究所,成都610052;四川大学实验动物中心,成都610041;四川大学实验动物中心,成都610041;四川大学实验动物中心,成都610041【正文语种】中文【中图分类】R979.1【相关文献】1.腺病毒介导的反义VEGF165对人皮肤恶性黑色素瘤细胞生长的影响 [J], 崔正军;岑瑛;王立夫2.重组腺病毒载体介导人抑瘤素M基因对胰腺癌细胞体外增殖的抑制作用 [J], 胡朝全;孙诚谊;孙连生;王玉芝3.腺病毒介导的nm23-H1对人结直肠癌裸鼠移植作用的实验研究 [J], 王琦;李华玲;何学令;刘艳;尹海林4.Ad-GFP-nm23-H1裸鼠体内抑制人恶性黑色素瘤A375作用的研究 [J], 李宗河;王琦;尹海林5.腺病毒介导M-CSF、IFN-γ基因分别转染黑色素瘤细胞体外生物学特性的研究[J], 雷虹;曹雪涛;于益芝;周正芳;章卫平;郑玲莉因版权原因,仅展示原文概要,查看原文内容请购买。
综述-IL24抗肿瘤研究进展

hIL24抗肿瘤研究进展刘洪福,方哲平,朱昱,郑双温州医学院附属台州医院肝胆外科(浙江省,临海市,317000)【关键词】:IL24;抗肿瘤;肝癌;基因治疗原发性肝癌是我国最常见的恶性肿瘤之一,目前肝癌治疗以外科手术仍是肝癌切除的首选方案。
但是总体治疗效果不佳。
基因治疗的提出已经有很多年。
白细胞介素(interleukin,IL) 是一组由白细胞产生的在细胞间发挥作用的细胞因子,具有重要的免疫学功能。
白介素-24是近年发现的、具有细胞因子样特性的肿瘤抑制基因。
IL -24 基因能抑制肿瘤生长、诱导细胞凋亡。
本文就hIL24抗肿瘤研究进展作一综述1.白细胞介素24白细胞介素24(interleukin24,IL-24)又称黑色素瘤分化相关基因7(melanoma.differentiation associated gene7,mda7),最初于1995 年由美国哥伦比亚大学的Jiang等1通过差示杂交的方法从人干扰素(IFN)-β和瑞香素(mezerein,MEZ)诱导的人类黑色素瘤细胞中获得。
从其结构、染色体定位、碱基序列同源性及细胞因子样特性等方面考虑,MDA7于2002年被归于IL-10家族,正式命名为IL-24。
人IL-24基因是单拷贝基因,与IL-10家族成员IL-10、IL-19、IL-20等共同位于人类第一染色体上,定位于的1q32-41,由7个外显子和6个内含子组成,其mRNA长约2 kb,编码由206个氨基酸组成、相对分子质量约为23.8KD的蛋白质。
含有49个氨基酸的信号肽。
在脾,胸腺和外周血单核细胞(PBMC)等免疫器官中可以检测到IL-24的表达。
除此之外,一些非黑素细胞或造血起源的细胞,在适当的状态下也可以诱导IL-24mRNA一过性的表达。
IL-24表达可以特异性地诱导癌细胞凋亡,抑制原位肿瘤和转移瘤的生长,但对正常细胞没有影响。
研究发现,IL-24在正常黑色素细胞和早期黑色素瘤细胞中表达水平较高,随着肿瘤恶性程度的增加,它的表达逐渐减少,在转移性黑色素瘤细胞中几乎看不到IL-24的表达。
一种重组人内皮抑素腺病毒及其制备方法和用途[发明专利]
![一种重组人内皮抑素腺病毒及其制备方法和用途[发明专利]](https://img.taocdn.com/s3/m/c6a8573b76eeaeaad0f33038.png)
专利名称:一种重组人内皮抑素腺病毒及其制备方法和用途专利类型:发明专利
发明人:魏于全,杨莉
申请号:CN200510021720.7
申请日:20050923
公开号:CN1935999A
公开日:
20070328
专利内容由知识产权出版社提供
摘要:本发明提供了一种将IL-2基因信号肽序列的3’端与人内皮抑素编码序列的5’端连接而成的新的重组人内皮抑素(endostatin)基因。
并用该重组基因构建入腺病毒,将这种重组腺病毒制备成抗肿瘤注射剂。
使用本发明提供的抗肿瘤注射剂,可以在体内抑制肿瘤的生长及转移,并且能使机体持续表达高水平的人内皮抑素并保持完整的生物活性,可以弥补蛋白输注的药物昂贵、体内稳定性差等局限,能降低用药成本、提高患者的生活质量,具有很好的市场前景。
申请人:四川大学,成都恩多施生物工程技术有限公司
地址:610065 四川省成都市一环路南一段24号
国籍:CN
代理机构:成都虹桥专利事务所
代理人:李高峡
更多信息请下载全文后查看。
重组人内皮抑素的基因表达与表征

重组人内皮抑素的基因表达与表征1. 介绍内皮细胞是构成血管壁的重要组成部分,内皮细胞的功能与血管的稳态和功能密切相关。
内皮抑素(EPC)是由内皮细胞产生的一种重要的调节因子,对循环系统和血管功能具有重要影响。
近年来,研究人员对重组人内皮抑素的基因表达和表征进行了深入研究,以期能够更好地探究其在各种疾病中的作用机制并为药物研发提供理论基础。
2. 重组人内皮抑素的基因表达重组人内皮抑素是通过基因工程技术合成的一种生物制剂,其基因表达与正常内皮细胞不同。
在进行重组人内皮抑素的基因表达时,研究人员通常选择适合的宿主细胞,将EPC基因导入宿主细胞的染色体中,并利用细胞内的遗传信息表达EPC蛋白。
这种基因表达方式不仅可以大量制备EPC蛋白进行进一步研究,也为后续的药物生产奠定了基础。
3. 重组人内皮抑素的表征重组人内皮抑素的表征是对其生物学特性和功能进行深入研究的关键环节。
通过对EPC蛋白进行生化、免疫学和生理学等多方面的分析,可以全面评估其在机体内的作用机制和药理特性。
其中,包括EPC蛋白的结构、性质、稳定性、纯度等方面的研究,以及其对各种生物学过程和疾病的影响等内容。
4. 重组人内皮抑素与血管疾病的关系研究发现,重组人内皮抑素在调节血管张力、抑制血管炎症反应、促进内皮细胞再生等方面具有重要作用,对于治疗心血管疾病、高血压、糖尿病等疾病具有重要的临床意义。
深入研究重组人内皮抑素的基因表达与表征,有助于揭示其潜在的药物作用机制,并为相关疾病的治疗提供新的思路和途径。
5. 重组人内皮抑素的临床应用前景重组人内皮抑素作为一种潜在的药物,在心血管疾病、癌症、炎症等疾病的治疗中展现出广阔的应用前景。
通过对其基因表达和表征的深入研究,可以更好地了解其作用机制、药效学特性和生物学功能,从而为其临床应用提供更加可靠的理论支持。
6. 结语重组人内皮抑素的基因表达与表征研究,对于深入理解其生物学特性和药理作用具有重要意义。
通过相关研究,可以为重组人内皮抑素作为潜在药物的开发提供理论支持,促进其在临床上的应用,对于改善人类健康和促进医学进步具有重要意义。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Induction of cell cycle arrest and apoptosis in human nasopharyngeal carcinoma cells by ZD6474,an inhibitor of VEGFR tyrosine kinase with additional activity against EGFR tyrosine kinaseXia Xiao1,Jiangxue Wu1*,Xiaofeng Zhu1,Peng Zhao1,Jinlin Zhou1,Quentin Qiang Liu1,Limin Zheng1,Musheng Zeng1, Ranyi Liu1and Wenlin Huang1,2,3*1State Key Laboratory of Oncology in South China,Cancer Center,Sun Yat-Sen University,Guangzhou510060,People’s Republic of China2Institute of Microbiology,Chinese Academy of Science,Beijing100080,People’s Republic of China3Guangzhou Doublle Bio-product Inc.,Guangzhou516003,People’s Republic of ChinaZD6474is a vascular endothelial growth factor receptor(VEGFR) and epidermal growth factor receptor(EGFR)tyrosine kinase in-hibitor.The present study was undertaken to investigate the direct antiproliferative effect of ZD6474on human nasopharyngeal car-cinoma(NPC)in vitro and the antitumor activity on NPC xeno-grafts in vivo.Results indicated that ZD6474treatment inhibited EGFR phosphorylation and led to a dose-and time-dependent decrease in NPC cell(CNE-1,CNE-2and C666-1)proliferation. Further investigation demonstrated G0/G1cell cycle arrest in all 3cell lines,which was associated with an upregulation of p21and/ or p27,and downregulation of CDK4,CDK6and CDK2.ZD6474 treatment also induced apoptosis in CNE-1and CNE-2cells.The apoptosis mechanisms involved reduction of Bcl-2and/or Bcl-X L, induction of Bak and/or Bax,and activation of caspases-3,-9and/ or-8.The in vivo antitumor activity was evaluated in CNE-2and C666-1xenografted nude mice.Administration of ZD6474(25–100mg/kg/day,once-daily,p.o.)produced a dose-dependent inhi-bition of tumor growth and prolonged survival in both models. This study suggests that ZD6474exerts direct antiproliferative effects on NPC cell lines in vitro by inducing G0/G1arrest and ap-optosis,and potent antitumor effects on NPC xenografts in vivo.It indicates that ZD6474may offer a new and effective treatment for human NPC.'2007Wiley-Liss,Inc.Key words:vascular endothelial growth factor receptor;epidermal growth factor receptor;tyrosine kinase inhibitor;ZD6474;nasopharyn-geal carcinoma;cell cycle;apoptosisReceptor tyrosine kinases(RTKs)are crucial elements in many cell regulatory processes and are altered or overexpressed in human carcinogenesis.A variety of therapeutic agents have been developed to inhibit the activities of RTKs.A recent highlight is the development of quinazoline-derived agents that are selective ATP competitors of RTKs.ZD6474is an orally bioavailable, small molecule,anilinoquinazoline derivative that is selective for VEGFR-2(KDR)and is currently in Phase III clinical develop-ment.ZD6474has an IC50of 0.04l M against the isolated KDR enzyme and blocks VEGF-induced signaling in endothelial cells.1 Consistent with inhibition of VEGF signaling and angiogenesis, once-daily oral dosing of ZD6474produced significant broad-spectrum antitumor activity in a panel of histologically diverse human tumor xenografts in nude mice.2In addition to its antian-giogenic properties,ZD6474inhibits,to a less extent,the activity of epidermal growth factor receptor(EGFR)tyrosine kinase(IC50 50.5l M).3Overexpression of EGFR or its ligands has been reported in many human tumors.4The aberrant EGFR tyrosine ki-nase activity leads to increased tumor cell proliferation,survival, invasiveness and overexpression of proangiogenic factors such as VEGF.5–8Therefore,ZD6474has the potential to inhibit2pivotal pathways in tumor growth by targeting tumor growth indirectly, via inhibition of VEGF-dependent tumor angiogenesis and endo-thelial cell survival,and targeting tumor growth directly,via inhi-bition of EGFR-dependent tumor cell proliferation and survival.9 The ability of ZD6474to inhibit tumor cell proliferation was examined directly in several cancer cell lines(ovarian,colon, gastric and breast)in vitro with IC50values ranging from0.09to 17.7l M.10It seems that the direct antiproliferative effect of ZD6474is highly tissue-and cell-type specific.Thus,elucidation of the mechanisms underlying the direct antitumor effects of ZD6474on particular cancer cells may provide an opportunity to target specific patient subsets who may obtain further benefit from this pharmacological activity.Nasopharyngeal carcinoma(NPC)is a major malignant disease of the head/neck region that is endemic within the young popula-tion of South China and Southeast Asia.The current treatment reg-imens of ionizing radiation combined with cisplatin chemotherapy yields a5-year survival rate of 70%,11,12indicating that new therapies to combat this disease are urgently needed.The purpose of this study is to examine the direct antiprolifera-tive effects of ZD6474,with its additional activity against EGFR tyrosine kinase,on NPC cell growth in vitro and the underlying molecular basis.In vivo,the antitumor effect of ZD6474on NPC cell xenograft models is also evaluated.Material and methodsReagentsZD6474was synthesized according to a patent(number:wo 2,003,039,551)procedure.ZD6474:1H NMR(DMSO d6):8.64(s, 1H),8.07(s,1H),7.74(d,1H),7.53(m,2H),7.32(s,1H),4.09(d, 2H),3.99(s,3H),3.50(d,2H),3.02(t,2H),2.78(s,3H),2.16(m, 1H),2.05(d,2H),1.65(m,2H).ESI-MS m/z475(M11);Anal. Calcd for C22H24N4O2BrF0.5H2O1.8HCl:C,48.2;H,5.0;N, 10.1;Found C,47.8;H,5.1N10.0.Cell linesThree human NPC cell lines were studied:CNE-1(well-differ-entiated NPC cell line,EBV negative),CNE-2(poorly differenti-ated NPC cell line,EBV negative)and C666-1(undifferentiated NPC cell line,EBV positive).13–16C666-1was a kind gift from Dr.Saiwah Tsao(University of Hong Kong,Hong Kong,People’s Republic of China).Cells were cultured in RPMI1640supple-mented with10%fetal bovine serum(Gibco,Paisley,UK),100 units/ml penicillin and100l g/ml streptomycin at37°C in a5%This article contains supplementary material available via the Internet at /jpages/0020-7136/suppmat.Grant sponsor:National Basic Research Program of China(973Pro-gram);Grant number:2004CB518801;Grant sponsor:China Postdoctoral Science Foundation;Grant number:2004046156;Grant sponsor:Hi-tech Research and Development Program of China(863Program);Grant num-ber:2006AA02Z489;Grant sponsor:Guangzhou Science Foundation; Grant number:2004Z3-E4011.Thefirst two authors contributed equally to this work.*Correspondence to:Cancer Center,Sun Yat-Sen University,Guang-zhou510060,PRC.Fax:186-20-8734-3146.E-mail:wl_huang@Received23January2007;Accepted after revision22May2007DOI10.1002/ijc.22955Published online13July2007in Wiley InterScience(www.interscience. ).Int.J.Cancer:121,2095–2104(2007)'2007Wiley-Liss,Inc.Publication of the International Union AgainstCancerCO2humidified atmosphere.Experiments were carried out in the log phase of growth.For experiments on synchronized cultures, cells were synchronized in G0/G1phase by culturing in RPMI 1640without serum for24hr.Cells were then released to reenter the cell cycle by the addition of10%fetal bovine serum,either in the presence or in the absence of ZD6474,and were harvested at the indicated time points.MTT assayCells were seeded in96-well plate at a density of23103–23 104cells/well,allowed to recover for16–24hr and then treated using complete media with or without ZD6474at different con-centrations(0.1–25.6l M).After the indicated incubation time, cell viability was measured by MTT assay.Briefly,20l l of MTT stock solution(5mg/ml in PBS)was added into each well and incubated for4hr in an incubator(37°C,5%CO2).The plate was centrifuged at1,800rpm for5min at4°C and the medium was removed.One hundred microliter of DMSO per well was added to dissolve the formazan crystal,and the absorbance was recorded on a microplate reader at of570/630nm wave length.Cell cycle analysisCell cycle analysis was performed as described before.17 Briefly,bothfloating and adherent cells were harvested after ZD6474treatment for the indicated time courses,washed with ice-cold PBS andfixed overnight with70%ethanol at4°C,fol-lowed by resuspension in500l l of PBS.After addition of10l l RNase(10mg/ml),cells were left for30min at37°C and stained with10l l propidium iodide(1mg/ml).Then,cells were analyzed by a Coulter Epics Eliteflow cytometer(Beckman-Coulter, Miami,FL).Annexin-V/7-AAD binding assayFor Annexin-V/7-AAD binding assay,bothfloating and adher-ent cells were collected after ZD6474treatment for48hr and washed with ice-cold PBS.Cells were resuspended in100l l of binding buffer,and stained with10l l of FITC-Annexin V and 20l l of7-AAD.After incubation on ice in the dark for15min, every sample was diluted with385l l13binding buffer and im-mediately analyzed by a Coulter Epics Altraflow cytometer (Beckman-Coulter).Caspase activity assayCells were plated in175-cm2flasks.After incubation with ZD6474for the desired time,floating and adherent cells were har-vested and combined.Preparation of cell lysates and measurement of caspase-8,caspase-9and caspase-3activity were performed with Caspase-8Assay Kit,Caspase-9Assay Kit and Caspase-3 Cellular Activity Assay Kit(Calbiochem,La Jolla,CA)according to the manufacturer’s instruction.AntibodiesAll of the antibodies,excluding EGFR(Cell Signaling Technol-ogy,Beverly,MA),Bcl-X L and p-BAD(Ser136)(SAT,CA)and GAPDH(Kangcheng,Shanghai,China)were obtained from Santa Cruz(Berkeley,CA).Western blotting analysis and immunoprecipitationCell lysates were prepared by extracting proteins with RIPA lysis buffer containing Protease Inhibitor Cocktail II(state,Lake Placid,NY).Protein estimation was performed using Bradford method.Twenty to40l g total cell protein extracts were resolved by electrophoresis using NuPAGE14–12%Bis-Tris Gel(Invi-trogen,Carlsbad,CA)and transferred to PVDF membrane. SeeBlue1prestained standard(Invitrogen)was used to determine product size.The membrane was blocked with either5%BSA or 5%nonfat milk in Tris-buffered saline and then incubated with primary antibodies for4hr at room temperature followed by washing3times for5min each with TBST.Blots were incubated with horseradish peroxidase-conjugated secondary antibody for1 hr at room temperature.Bands were detected using enhanced chemiluminescence system(Cell Signaling,Danvers,MA)and quantified by densitometry using Bio-rad Quantity One software (Bio-rad,Hercules,CA).For immunoprecipitations,500l g of total protein from cell lysates was incubated with1l g primary antibody for2hr at4°C.Twenty microliter of appropriate protein G Plus-Agarose suspension(Santa Cruz)was added and then incu-bated at4°C on a rocker platform overnight.The precipitates were washed4times with ice-cold PBS buffer,resuspended in43LDS sample buffer(Invitrogen)and resolved using NuPAGE14–12% Bis-Tris Gel followed by immunoblot analysis.Tumor xenograft modelsFemale BALB/c nude mice(6-to8-week-old,weighing>18g) were obtained from Shanghai Slike experimental animals Co. (Shanghai,China;animal experimental license No.SCXKhu2003-0008).Mice were housed in air-filtered laminarflow cabinets with 12-hr light/dark cycles,provided with sterilized food and water ad libitum.After1-week adaptation,mice were injected s.c.in the scapular region with23106CNE-2cells or13107C666-1cells in100l l of serum-free media.When tumors reached a volume of 30–50mm3,mice were randomly allocated into4groups(n56) and received(p.o.)either ZD6474(25–100mg/kg/day)or vehicle (sterilized water),administered once daily at0.1ml/10g body weight.Tumor volume was assessed twice weekly by calipers measurement of tumor diameters and calculated according to the formula:V5L3W2/2(L,length;W,width).After3.5weeks treatment,mice were killed and tumors were resected and weighed.For survival studies(n510),mice were dosed(p.o., once-daily)with either ZD6474(25–100mg/kg/day)or vehicle (sterilized water)for25days,and then the tumors were allowed to grow.Mice either were found dead or were killed when tumors were estimated by palpation to reach10%body weight or individ-ual mice appeared stressed by weight loss,ruffled fur and/or leth-argy.All the animal experiments were conducted in accordance with‘‘Guidelines for the Welfare of Animals in Experimental Neoplasia’’.Statistical analysisResults were evaluated using2-tailed Student’s t test with SPSS 13.0software(SSPS,Chicago,IL),unless otherwise specified. Results of survival were evaluated using Kaplan Meier survival analysis.A p value<0.05was considered statistically significant.ResultsZD6474treatment inhibits the tyrosine phosphorylation of EGFR VEGFR1,VEGFR2and EGFR expression was evaluated in3 NPC cell lines at protein levels.Results showed that both VEGFR1and VEGFR2proteins were undetectable in all the NPC cell lines(data not shown).The relative expression of EGFR in all 3cell lines was as shown in Figure1a.Densitometric analysis showed that the relative abundance of EGFR was1.1,1.23and 0.66in CNE-1,CNE-2and C666-1,respectively,after normaliz-ing with GAPDH.Figure1b showed that ZD6474treatment inhib-ited the tyrosine phosphorylation of EGFR within15min in CNE-1cells.These results were obtained after immunoprecipitation with an anti-EGFR antibody and subsequent Western blotting with an anti-phosphotyrosine antibody of the cell extracts treated with 3l M ZD6474.Similar results were also observed in CNE-2and C666-1(data not shown).Effect of ZD6474on EGFR downstream signaling pathwaysTo further identify the effect of ZD6474on EGFR downstream signaling that might contribute to the observed direct growth inhi-bition,we examined the phosphorylation of several key regulators involved.Figure1c exhibited that the phosphorylation of Akt was significantly inhibited by ZD6474treatment in all3cell lines.2096XIAO ET AL.Consistent with the inhibition of Akt activity,phosphorylation of GSK-3b ,a target of the Akt kinase,was reduced (Fig.1c ).Figure 1d showed that the phosphorylation of ERK1/2was significantly inhibited by ZD6474treatment in CNE-2and C666-1cells.Simi-lar result was also observed in CNE-1(data not shown).Antiproliferative effects of ZD6474on human NPC cell linesWe evaluated the effect of ZD6474on the growth of human NPC cell lines,CNE-1,CNE-2and C666-1using MTT assay.ZD6474treatment caused a significant growth inhibition in NPC cell lines in a dose-and time-dependent manner.After 24hr of incubation,ZD6474had minor effects on cell growth (data not shown).After 48hr of incubation,the IC 50values of CNE-1,CNE-2and C666-1were 3.6,6.2and 23.4l M,respectively (data not shown).Moreover,the corresponding IC 50values,examined after 72hr of incubation,were 2.3,3.6and 4.86l M,respectively (Fig.2).In addition,the result indicated that among the 3NPC cell lines tested,CNE-1is the most sensitive cell line to the antipro-liferative effect of ZD6474.For example,after exposure to 3.2l M ZD6474for 48hr,the proliferation of CNE-1,CNE-2and C666-1decreased to 55.3,69.4and 77.5%,respectively,compared withuntreated cells (data not shown).The corresponding relative growth rates were further reduced to 43.3,52.4and 68.2%after the same concentration of ZD6474treatment for 72hr (Fig.2).ZD6474induces cell cycle arrest in NPC cell linesBased on the results of MTT assay,ZD6474concentrations at 1.5–6l M for CNE-1and CNE-2and at 3–9l M for C666-1,which inhibited the growth of corresponding cell lines by 25–75%over a time period of 72hr,were adopted for further studies.To examine whether the antiproliferative effect of ZD6474on NPC cell lines was partly mediated via specific cell cycle arrest,we investigated the cell cycle phase distribution of ZD6474-treated cells by flow cytometric analysis.The results revealed that ZD6474treatment caused an accumulation of cells in the G0/G1fraction in all the 3cell lines,and significant alterations were observed by 24hr of treatment (Fig.3a ).For example,after expo-sure to ZD6474(6l M in CNE-1and CNE-2;9l M in C666-1)for 24hr,the percentage of G0/G1phase cells increased from (52.366)%to (79.466.9)%,(45.2610.2)%to (59.967.6)%and (54.961.3)%to (58.761.5)%in CNE-1,CNE-2and C666-1cells (Fig.3a ,upper panel;p <0.05),respectively,with aproportionalF IGURE 1–Effect of ZD6474on EGFR signaling pathways.(a )Western blotting analysis of EGFR expression in CNE-1,CNE-2and C666-1.The GAPDH serves as a loading control.(b )ZD6474inhib-its the phosphorylation of EGFR in CNE-1cells.(c )ZD6474inhibits the phosphorylation of Akt and its downstream signaling in all 3cell lines.(d )ZD6474inhibits the phosphorylation of ERK1/2in CNE-2and C666-1.Cells were treated with ZD6474for the indi-cated time periods.Following har-vesting,cells were lysed and proc-essed for immunoprecipitation or Western blotting analysis using antibodies directed against EGFR,p-Try-20,p-Akt1/2/3,p-GSK3b ,p-Bad (ser136),ERK1/2and p-ERK1/2as described in Materials and Methods.2097ANTIPROLIFERATIVE AND ANTITUMORAL EFFECTS OF ZD6474reduction in the S and G2-M phase fractions.Moreover,the G0/G1cell cycle arrest induced by ZD6474exhibited in a dose-de-pendent manner in CNE-1(Fig.3a ,lower panel;p <0.05),CNE-2and C666-1(data not shown).To further dissect the G0/G1cell cycle arrest of ZD6474,C666-1cells were synchronized in G0/G1phase by serum starvation.Then cells reentered into cell cycle by addition of serum,and the cell cycle progression was monitored in the presence or absence of ZD6474.The results showed that untreated cells rapidly exited G0/G1phase and proceeded through S and G2-M phase (Fig.3b ,top).ZD6474-treated cells,however,progressed more slowly (Fig.3b ,bottom).At 16hr,70.7%of the ZD6474-treated cells remained in G0/G1fraction compared with 60.3%of the untreated cells.By 24hr,in contrast to only 53.5%of the untreated cells in G0/G1phase,69%of the ZD6474-treated cells were still delayed in G0/G1phase.Similar results were obtained in CNE-1and CNE-2cells (data not shown).Effect of ZD6474on proteins regulating the G1to S transition in the cell cycleTo investigate the molecular mechanisms involved in the G0/G1cell cycle arrest caused by ZD6474treatment,a number of key molecules regulating cells from the G1to the S phase of the cell cycle were examined.These include cyclins and its catalytic part-ners,the CDKs and inhibitors of CDKs.The results demonstrated that,in CNE-1cells,ZD6474treatment resulted in a significant reduction in CDK4,CDK6and CDK2levels after 24–72hr of incubation (Fig.3c ,upper panel).In CNE-2cells,the same treat-ment resulted in a moderate decrease in CDK6and CDK2levels,with little or no effect on CDK4expression (Fig.3c ,upper panel).In C666-1cells,a detectable decrease in CDK4and CDK6expres-sion was observed (Fig.3c ,upper panel).In addition to the altera-tions in CDKs expression,the inhibitors of CDKs,including the Cip/Kip and INK4family of proteins,were also regulated by ZD6474treatment.As shown in Figure 3c lower panel,treatment with ZD6474resulted in a marked increase in p21and p27levels in CNE-1and CNE-2cells,and these results could be detected as early as 6hr and became apparent after 16hr.However,in C666-1cells,a significant decrease in p21levels and a slight decrease in p27levels were observed (Fig.3c ,lower panel).No effect on p57was detected in all 3cell lines.Furthermore,the results also revealed that,in the 3NPC cell lines we tested,the protein of p16was undetectable and the other INK4family members,p15,p18,p19,were unaltered (data not shown).ZD6474-treated NPC cell lines did not show any detectable changes in the levels of cyclin D1and cyclin E (data not shown).Determination of ZD6474-induced apoptosis in human NPC cell linesTo investigate the fate of ZD6474-treated NPC cell lines,an Annexin-V/7-AAD binding assay was performed (Fig.4a ).The results exhibited an increased percentage of early apoptotic cells (Annexin-V-positive)after ZD6474treatment for 48hr in CNE-2cells (p <0.05).In the same way,ZD6474treatment of CNE-2cells also induced a significant increase in the fraction of late apo-ptotic/necrosis cells (Annexin-V/7-AAD double-positive,p <0.05).For example,after treatment with 6l M ZD6474for 48hr,the percentage of early apoptotic CNE-2cells was (12.261.1)%when compared with (2.561.2)%of the control group (Fig.4a ;p <0.05),and the percentage of late apoptotic/necrosis CNE-2cells was (12.761.8)%in contrast to (0.560.2)%of the control group (Fig.4a ;p <0.05).Similar result was obtained in CNE-1cells (data not shown).No data indicative of apoptosis induced by ZD6474in C666-1cells were obtained.Furthermore,we evaluated the involvement of various caspases including caspase 3(the executioner caspase),caspase 8(the ini-tiator of the extrinsic pathway)and caspase 9(the initiator of the intrinsic pathway)during ZD6474induced apoptosis in NPC cells.As shown in Figure 4b ,ZD6474treatment significantly increased caspase-3and -9activities in CNE-1and CNE-2cells (p <0.05).Additionally,ZD6474treatment also led to caspase-8activation in CNE-1(Fig.4b ;p <0.05).Effect of ZD6474on proteins regulating apoptosisTo elucidate the molecular basis responsible for ZD6474-induced apoptosis,cells were treated with ZD6474from 24to 72hr,and then the expression of Bcl-2family proteins were exam-ined by Western blotting analysis.As shown in Figure 4c ,in CNE-1cells,a marked decrease in Bcl-2and Bcl-X l levels and a moderate increase in the expression of Bak and Bax which are known to heterodimer with Bcl-2or Bcl-X l to favor apoptosis were detected.In CNE-2cells,ZD6474treatment resulted in a moderate increase in Bak and Bax levels,with no effect on Bcl-2and Bcl-X l expressions (Fig.4c ).In addition,Bid,the BH3domain-only protein,which can be cleaved by caspase-8and also translocate to mitochondria to induce cytochrome c release and mitochondrial damage,was found reduced in full length inCNE-1F IGURE 2–Effect of ZD6474on the proliferation of CNE-1,CNE-2and C666-1cells.Cells were treated with ZD6474at doses ranging from 0.1to 25.6l M for 72hr and the cell viability was measured by MTT assay.Data are given as relative growth rates compared with untreated control group.Each point represents mean of 3independent experiments conducted in triplicate;bars,SE.*p <0.05,compared with cells treated with medium alone.2098XIAO ET AL.cells and unaltered in CNE-2cells (Fig.4c ).Furthermore as a result of blocking,the phosphorylation of Akt by ZD6474treat-ment,the levels of phospho-Bad (ser 136)decreased in all 3cell lines (Fig.1c ).ZD6474inhibits the growth of human NPC xenograftsSince the majority of NPC biopsies belong to undifferentiated cell type,CNE-2and C666-1were focused on for in vivo antitu-mor studies.The experiments were performed twice,and the results of representative experiments were presented.As shown in Figure 5a ,once-daily oral administration of ZD6474produced a significant dose-dependent inhibition of tumor growth in both models,compared with the control (p <0.05).The inhibition rates of ZD6474-treated groups were assessed by tumor weight (Fig.5b ;p <0.05).The growth of tumors after ZD6474treatment was significantly slower than that of the control groups (p <0.05).In the CNE-2-exnograft model,the inhibition rates of 100,50and 25mg/kg/day group were 86.4,47.3and 33.5%,respectively,and in the C666-1-exnograft model,the corresponding inhibitionratesF IGURE 3–ZD6474delays G0/G1cell cycle progression in NPC cell lines.(a )FACS analysis of the cell cycle distribution of asynchronized NPC cell lines.Upper panel,NPC cells were treated with the indicated concen-trations of ZD6474for 24hr.Lower panel,CNE-1cells were treated with increasing concentra-tions of ZD6474(1.5–6l M)for 24hr.The total population of cells in each phase of the cell cycle is shown.Column,mean of 3inde-pendent experiments;Bars,SD.*p <0.05,the proportion of cells in G0/G1phase after ZD6474treat-ment versus that after vehicle treat-ment.(b )representative FACS analysis of the cell cycle distribu-tion of synchronized C666-1cells,which were released to reenter the cell cycle either in the absence (top)or in the presence (bottom)of 9l M ZD6474.The experiments were repeated 3times.(c )Western blotting analysis of proteins involved in G0/G1cell cycle arrest.Synchronized NPC cells were treated with ZD6474(4.5l M in CNE-1and CNE-2,and 6l M in C666-1)for the indicated time periods.Following harvesting,cells were lysed and processed for Western blotting analysis using antibodies directed against CDK2,CDK4,CDK6,p21,p27and p57as described in Materials and Methods.[Color figure can be viewed in the online issue,which is available at .]2099ANTIPROLIFERATIVE AND ANTITUMORAL EFFECTS OF ZD6474were 91.5,57.1and 43.6%,respectively.The in vivo antitumor studies also showed that ZD6474treatment was well tolerated with only slight effects on body weight (particularly at dose of <50mg/kg/day)and no adverse effects on clinical condition (even at 100mg/kg/day for 25days)(data not shown).ZD6474treatment prolongs the survival of mice bearing human NPC xenograftsThe long-term outcome of ZD6474treatment in human NPC-xenografted mice was determined by survival rates.The experi-ment was conducted twice,and the results of representative experiment were shown (Fig.5c ).Our data suggested that,in each exnograft model,survival durations significantly prolonged after ZD6474treatment when compared with that of control group (p <0.05,Kaplan-Meier).For CNE-2cell-xenografted mice,the me-dian survival of 100,50,25mg/kg/day and vehicle-treated groups were 6063.2,4662.4,3661.6and 3261.9days,respec-tively.For C666-1cell-xenografted mice,the corresponding median survival was 6865.5,5564.7,4362.4and 3861.6days,respectively (Fig.5c ).DiscussionTumor cell proliferation and angiogenesis are 2crucial pro-cesses for solid tumor growth.18Activation of VEGFR-2is neces-sary and sufficient to VEGF-induced pathological angiogenesis and vascular permeability.Abnormal EGF/EGFR signaling path-way is closely related with tumor cell proliferation and survival.ZD6474is a small molecule tyrosine kinase inhibitor with activityagainst both VEGFR-2and EGFR.1,3By inhibition of EGFR tyro-sine kinase activity,ZD6474could impart a direct inhibitory effect on tumor cell growth and survival.However,this direct in-hibitory action of ZD6474may be highly cell-type specific,and need further investigation to elucidate which subset of tumors may be sensitive to this agent and the involved mechanisms.In this study,we report for the first time that ZD6474exerts direct antiproliferative effect on NPC cells via induction of G0/G1phase arrest and apoptosis in vitro ,and potent antineoplastic effects on CNE-2and C666-1xenograft models in vivo .More importantly,the molecular mechanisms controlling the direct anti-proliferative effect are well defined.There are some papers that have reported the sensitivities of several cancer cell lines to the direct antiproliferative activity of ZD6474in vitro .However,in different papers,different methods were used to measure the sensitivity.To date,a human lung tumor cell line,PC-9,was confirmed to be hypersensitive to the direct antiproliferative action of ZD6474(IC 5050.09l M)because of harboring a 15-bp deletion in the gene encoding the EGFR.Although compared with PC-9,NPC cell lines did not appear to be hypersensitive to the direct antiproliferative effect of ZD6474,we have showed that the proliferation of the 3NPC cell lines with overexpression of EGFR were directly and significantly inhibited in response to low doses of ZD6474.19,20Furthermore,we have found that G0/G1cell cycle arrest con-tributed to the antiproliferative effect of ZD6474.Investigation of the cki-cyclin-cdk machinery exhibited that ZD6474treatment led to a marked increase in both p21and p27expression in CNE-1and CNE-2cells (Fig.3c ,lower panel).Conversely,amoderateF IGURE 3–(C ONTINUED )2100XIAO ET AL.。
