Crizotinib_HNMR_06049_MedChemExpress
酒石酸卡巴拉汀的产品说明书

参考文献:
[1]. Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: a review. Int J Clin Pract. 2009 May;63(5):799-805.
中文别名:
酒石酸卡巴拉汀;(S)-N-乙基-N-甲基氨基甲酸-3-[(1-二甲氨基)乙基]苯酯酒石酸盐;卡巴拉汀或利斯的明酒石酸盐;L-酒石酸卡巴拉汀;2,3',4-三溴二苯醚;N-乙基甲基氨基甲酰氯;Rivastigmine L-Tartrate L-酒石酸卡巴拉汀;酒石酸卡巴拉汀 USP标准品;酒石酸卡巴拉汀Rivastigmine tartrate;酒石酸卡巴拉汀标准品;酒石酸卡巴拉汀氢 EP标准品;酒石酸利凡斯的明;卡巴拉丁;卡巴拉汀酒石酸盐;重酒石酸卡巴拉汀;重酒石酸利凡斯的明;(S)-N-乙基-N-甲基氨基甲酸-3-[(S)-1-(二甲氨基)乙基]苯酯酒石酸盐;氘代重酒石酸利斯的明-d6;酒石酸卡巴拉汀 中文别名:卡巴拉汀或利斯的明酒石酸盐;酒石酸利伐斯的明;酒石酸利瓦斯汀;利斯的明酒石酸盐;卡巴拉汀重酒石酸盐
熔点
123-1250C
分子式
C18H28N2O8
分子量
400.423
闪点
145ºC
精确量
400.18457
PSA
147.84
LogP
0.6371
顶端钠依赖性胆汁酸转运蛋白(ASBT)在肝胆疾病中的作用

顶端钠依赖性胆汁酸转运蛋白(ASBT)在肝胆疾病中的作用谢晓暄,杜丽娜,郭紫云,杨燕国家儿童医学中心,首都医科大学附属北京儿童医院中医科,北京 100045通信作者:杨燕,***************(ORCID: 0000-0003-1070-9614)摘要:顶端钠依赖性胆汁酸转运蛋白(ASBT)是负责胆汁酸肠道重吸收的关键转运体,对维持胆汁酸和胆固醇稳态起重要作用,其表达受到转录因子、核受体和肠道微生物等多种因素的调控。
ASBT的表达和功能异常会导致胆汁酸及胆固醇代谢紊乱,引起多种肝胆相关疾病。
目前,ASBT作为一种治疗靶点已受到广泛关注。
本文阐述了ASBT的生物学特征及表达调控机制,并对ASBT在肝胆疾病中的作用进行了综述,为相关疾病的治疗提供新方向。
关键词:顶端钠依赖性胆汁酸转运蛋白;胆汁酸类;胆汁淤积基金项目:国家自然科学基金(82205184);北京市属医院科研培育计划(PZ2022027)Role of apical sodium-dependent bile acid transporter in hepatobiliary diseasesXIE Xiaoxuan, DU Lina, GUO Ziyun, YANG Yan.(Department of Traditional Chinese Medicine, National Center for Children’s Health, Beijing Children’s Hospital, Capital Medical University, Beijing 100045, China)Corresponding author: YANG yan,***************(ORCID: 0000-0003-1070-9614)Abstract:Apical sodium-dependent bile acid transporter (ASBT) is a key transporter responsible for intestinal reabsorption of bile acid and plays an important role in maintaining bile acid and cholesterol homeostasis, and its expression is regulated by various factors including transcription factors, nuclear receptors, and intestinal microflora. The abnormal expression and function of ASBT can lead to disorders in the metabolism of bile acid and cholesterol, causing a variety of hepatobiliary diseases. At present, ASBT has attracted wide attention as a therapeutic target. This article elaborates on the biological characteristics and expression regulation mechanism of ASBT and reviews the role of ASBT in hepatobiliary diseases, in order to provide a new direction for the treatment of related diseases.Key words:Apical Sodium Dependent Bile Acid Transporter; Bile Acid; CholestasisResearch funding:National Natural Science Foundation of China (82205184);Bejing Municipal Administration of Hospital Incubating Program (PZ2022027)胆汁酸(bile acid,BA)肝肠循环是维持胆酸池稳态的重要调控环节,位于回肠末端的顶端钠依赖性胆汁酸转运蛋白(apical sodium-dependent bile acid transporter,ASBT)是负责BA肠道重吸收的关键转运体。
Cocktail探针药物法评价小檗碱对肝微粒体CYP450酶的抑制作用

Cocktail探针药物法评价小檗碱对肝微粒体CYP450酶的抑制作用目的:研究小檗碱对人肝微粒体CYP活性的影响。
方法:以苯海拉明为内标,建立LC-MS/MS同时测定5种探针药物:咪达唑仑、非那西丁、右美沙芬、甲苯磺丁脲和氯唑沙宗的含量,采用鸡尾酒(cocktail)法探针药物法评价不同浓度小檗碱对混合人肝微粒体CYP不同亚型活性的影响。
结果:与对照组相比,咪达唑仑、非那西丁和甲苯磺丁脲的代谢速率基本没有变化,而氯唑沙宗的代谢速率明显变慢,对于右美沙芬,当小檗碱的质量浓度为50 μg·L-1时,其代谢速率基本没有变化,当小檗碱的质量浓度大于200 μg·L-1时,其代谢速率明显变慢。
结论:小檗碱质量浓度在2 000 μg·L-1以下时对人肝微粒体中CYP3A4,CYP1A2和CYP2C9活性没有明显影响,但对CYP2E1和CYP2D6有明显的浓度依赖性抑制作用。
标签:鸡尾酒法;探针药物;混合人肝微粒体;小檗碱;P450酶抑制细胞色素P450酶(CYP)是肝微粒體混合功能氧化酶系的主要成分,是多种药物在体内代谢的最主要酶系[1]。
药物代谢与其体内浓度和药理活性密切相关,与此同时,药物也会对CYP产生诱导或抑制作用,从而引发药-药物相互作用和不良反应[2]。
复方是中药临床用药常见形式,配伍是中药的特色和优势,研究中药和CYP的关系,不但可以揭示药物的体内代谢过程,而且有助于了解药物间的相互作用与药效之间的内在联系,为组方配伍和临床合理用药提供直接依据[3]。
小檗碱,是中药黄连、黄柏等的主要有效成分,在临床上一直作为抗菌药和清热解毒药,随着研究不断深入,发现其具有抗高血压、抗肿瘤、降血糖等多方面的药理作用[4]。
目前研究表明小檗碱主要由小肠吸收,其口服吸收较差,生物利用度较低[5],有关小檗碱的肝代谢酶表征及代谢产物的研究较清楚,小檗碱主要由CYP1A2和2D6代谢,代谢产物为小檗红碱和去亚甲基小檗碱及其葡萄糖醛酸苷共价结合物[6-7]。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
HPLC_法测定阿瑞匹坦中基因毒性杂质3-氯甲基-1,2,4-三唑啉-5-酮

第52卷第9期 辽 宁 化 工 Vol.52,No. 9 2023年9月 Liaoning Chemical Industry September,2023收稿日期: 2022-09-12HPLC 法测定阿瑞匹坦中基因毒性杂质3-氯甲基-1,2,4-三唑啉-5-酮常月赏,兰公剑*,王阔,陶蕾(南京正大天晴制药有限公司,江苏 南京 210046)摘 要:建立了液相色谱法测定阿瑞匹坦基因毒性杂质3-氯甲基-1,2,4-三唑啉-5-酮的分析方法。
采用安捷伦Poroshell 120系列EC -C18柱为色谱柱,0.1%磷酸溶液为流动相A,乙腈为流动相B,进行线性梯度洗脱,流速为1.0 mL ·min -1,柱温为30 ℃;检测波长为210 nm。
结果表明:溶剂空白及主峰不干扰该杂质的测定;该杂质在限度浓度20%~200%的范围内线性关系良好;该杂质的回收率在99.3%~101.0%范围内,RSD 小于5.0%;对照品溶液及供试液在室温放置18 h 内稳定;重复性和中间精密度RSD 均小于5.0%。
本方法专属性及精密度好,准确度高,可以用于本品中基因毒性杂质3-氯甲 基-1,2,4-三唑啉-5-酮的检测。
关 键 词:阿瑞匹坦;基因毒性杂质;3-氯甲基-1,2,4-三唑啉-5-酮;液相色谱法(HPLC) 中图分类号:TQ460.7 文献标识码: A 文章编号: 1004-0935(2023)09-1399-04阿瑞匹坦与其他止吐药物联合用药,适用于预防高度致吐性抗肿瘤化疗的初次和重复治疗过程中出现的急性和迟发性恶心和呕吐[1-6]。
阿瑞匹坦具有全新的药理作用机制,其作为首个神经激肽-1(NK -1)受体拮抗剂为预防和治疗癌症患者化疗引起的恶心呕吐提供了更多的药物治疗选择[7-9]。
3-氯甲基-1,2,4-三唑啉-5-酮是合成阿瑞匹坦的关键物料,属三唑啉酮类衍生物[10]。
3-氯甲基-1,2, 4-三唑啉-5-酮为单卤代烷烃化合物[11-12],依据ICH M7,该化合物具有基因警示结构。
一甲基澳瑞他汀 e 化学结构-概述说明以及解释

一甲基澳瑞他汀e 化学结构-概述说明以及解释1. 引言1.1 概述一甲基澳瑞他汀(Simvastatin)是一种广泛应用于临床治疗高胆固醇血症和心血管疾病的药物。
它属于被称为他汀类药物的一员,是一种竞争性抑制HMG-CoA还原酶的药物,通过降低胆固醇的合成来达到降低血浆胆固醇的效果。
随着现代生活方式的改变和不良饮食习惯的普遍存在,高胆固醇血症在全球范围内变得越来越普遍。
该疾病不仅与心血管疾病的发展密切相关,还可能导致其他严重的健康问题,如动脉粥样硬化和心肌梗死等。
一甲基澳瑞他汀由黄曲霉属真菌产生,即通过天然发酵法生产得到。
然而,为了提高其药代动力学性质和治疗效果,科学家们通过改进和优化合成方法,合成了合成一甲基澳瑞他汀。
现在,一甲基澳瑞他汀已经成为一种被广泛研究和临床使用的药物。
在本篇文章中,我们将介绍一甲基澳瑞他汀的化学结构、合成方法、性质与用途等方面的内容。
通过深入了解一甲基澳瑞他汀,我们可以更好地理解它在治疗高胆固醇血症和心血管疾病方面的作用机制,并有望对该药物的未来发展提供一定的启示。
在接下来的章节中,我们将详细介绍一甲基澳瑞他汀的化学结构、合成方法以及它在临床上的广泛用途。
最后,我们将总结这篇文章的主要观点,并对一甲基澳瑞他汀的未来进行展望。
通过本文的阅读,读者将能够全面了解一甲基澳瑞他汀,为今后的相关研究和临床实践提供有益的指导与参考。
文章结构部分的内容如下:1.2 文章结构本文主要包含以下几个部分:引言、正文和结论。
引言部分通过概述一甲基澳瑞他汀的化学结构和合成方法,介绍背景知识和研究意义。
此外,本部分还会明确文章的目的,即对一甲基澳瑞他汀进行全面的分析和探讨。
正文部分将围绕一甲基澳瑞他汀展开讨论。
首先,我们将介绍一甲基澳瑞他汀的化学结构,包括其分子式、分子量等信息,并通过图表等形式直观地展示其结构。
其次,我们将详细介绍一甲基澳瑞他汀的合成方法,包括起始原料的选择、反应步骤和条件等。
二甲基吡啶

3,5-二甲基吡啶3,5-二甲基吡啶CAS号:591-22-0英文名称:3,5-Lutidine英文同义词:35L;3,5-LUTIDINE;3,5-DIMETHYLPYRIDINE;3,5-dimethyl-pyridin;pyridine,3,5-dimethyl-;Lutidine,98%;3 5-LUTIDINESTANDARD FOR GC;3,5-LUTIDINE, 98+%;3,5-DIMETHYLPYRIDINE98+%;3,5-Dimetylpyridine;3,5-Lutidine,99%;3,5-LUTIDINE 3,5-DIMETHYLPYRIDINE中文名称:3,5-二甲基吡啶中文同义词:3,5-二甲基吡啶;3,5-卢剔啶;3,5-二甲基氮杂苯;3,5-二甲基啶;3,5-二甲基吡啶,99%CBNumber:CB4412061分子式:C7H9N分子量:107.15MOLFile:591-22-0.mol3,5-二甲基吡啶化学性质熔点: -9 °C沸点: 169-170 °C(lit.)密度: 0.939 g/mL at 25 °C(lit.)蒸气压: 1.5 mm Hg ( 20 °C)折射率: n20/D 1.504(lit.)闪点: 128 °F储存条件: 2-8°C水溶解性: 33 g/L (20 ºC)BRN : 105682CAS 数据库: 591-22-0(CAS DataBase Reference) NIST化学物质信息: Pyridine, 3,5-dimethyl-(591-22-0) EPA化学物质信息: Pyridine, 3,5-dimethyl-(591-22-0)安全信息危险品标志: Xn,F,Xi危险类别码: 10-20/21/22-36/37/38-41安全说明: 16-26-36-36/37危险品运输编号: UN 1993 3/PG 3WGK Germany : 3F : 8Hazard Note : Irritant/FlammableHazardClass : 3PackingGroup : III海关编码: 293339993,5-二甲基吡啶 MSDS3,5-二甲基吡啶3,5-Dimethylpyridine3,5-二甲基吡啶性质、用途与生产工艺用途用作有机合成原料类别易燃液体可燃性危险特性遇明火、高温、强氧化剂可燃; 高温分解有毒氮氧化物气体储运特性包装完整、轻装轻卸; 库房通风、远离明火、高温、与氧化剂分开存放灭火剂泡沫、干粉、二氧化碳、砂土3,5-二甲基吡啶上下游产品信息上游原料下游产品3,5-二甲基-4-氨基吡啶3-氨基-5-甲基吡啶。
Calcipotriol_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-13-2017Print Date:Sep.-13-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CalcipotriolCatalog No. :HY-10001CAS No. :112965-21-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MC 903; CalcipotrieneFormula:C27H40O3Molecular Weight:412.60CAS No. :112965-21-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, protect from light, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
硫辛酸注射液联合胰激肽原酶肠溶片对DPN_的临床疗效及生存质量的影响

DOI:10.16658/ki.1672-4062.2024.01.174硫辛酸注射液联合胰激肽原酶肠溶片对DPN的临床疗效及生存质量的影响王莉,朱海峰濉溪县中医医院内分泌科,安徽淮北235100[摘要]目的探讨硫辛酸注射液联合胰激肽原酶肠溶片对2型糖尿病周围神经病变(Diabetic Peripheral Neu⁃ropathy, DPN)患者的临床疗效、生存质量及安全性的影响。
方法选取2021年2月—2022年4月濉溪县中医医院60名DPN患者作为研究对象。
通过随机数表法分为两组,每组30例。
对照组采用常规治疗,观察组在对照组基础上加用硫辛酸注射液和胰激肽原酶肠溶片治疗。
比较两组患者的神经病变评分、神经电生理指标、生存质量评分、安全性指标和不良反应发生率。
结果治疗后,观察组神经病变评分(6.2±0.9)分低于对照组(7.6±1.1)分,差异有统计学意义(t=5.438,P<0.05);观察组神经电生理指标、生存质量评分、安全性指标均优于对照组,差异有统计学意义(P均<0.05);两组患者不良反应发生率比较,差异无统计学意义(P>0.05)。
结论硫辛酸注射液联合胰激肽原酶肠溶片对DPN患者有良好的临床疗效,能够改善神经功能、改善神经电生理指标、提高生存质量,且安全性高。
[关键词] 硫辛酸注射液;胰激肽原酶肠溶片;2型糖尿病周围神经病变;临床疗效[中图分类号] R587.2 [文献标识码] A [文章编号] 1672-4062(2024)01(a)-0174-05Effect of Lipoic Acid Injection Combined with Pancreatic Kininogenase Enteric-coated Tablets on Clinical Efficacy and Quality of Survival in DPN WANG Li, ZHU HaifengDepartment of Endocrinology, Suixi County Hospital of Traditional Chinese Medicine, Huaibei, Anhui Province, 235100 China[Abstract] Objective To investigate the effects of lipoic acid injection combined with pancreatic kininogenase enteric-coated tablets on the clinical efficacy, quality of survival and safety of patients with type 2 diabetic peripheral neuropathy (DPN). Methods 60 DPN patients admitted to Suixi County Hospital of Traditional Chinese Medicine from February 2021 to April 2022 were selected as the study objects. They were divided into two groups with 30 cases in each group by random number table method. The control group received conventional treatment, and the observation group was treated with lipoic acid injection and pancreatic kininogenase enteric-coated tablets on the basis of control group. Neuropathy score, neuroelectrophysiological index, quality of life score, safety index and incidence of adverse reactions were compared between the two groups. Results After treatment, the neuropathy score of observation group (6.2±0.9) points was lower than that of control group (7.6±1.1) points, and the difference was statistically significant (t= 5.438, P<0.05). Neuroelectrophysiological indexes, quality of survival scores and safety indexes of the observation group were better than those of the control group, and the differences were statistically significant (all P<0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion Li⁃[作者简介]王莉(1982-),女,本科,主治医生,研究方向为糖尿病周围神经病变。
茚地那韦杂质
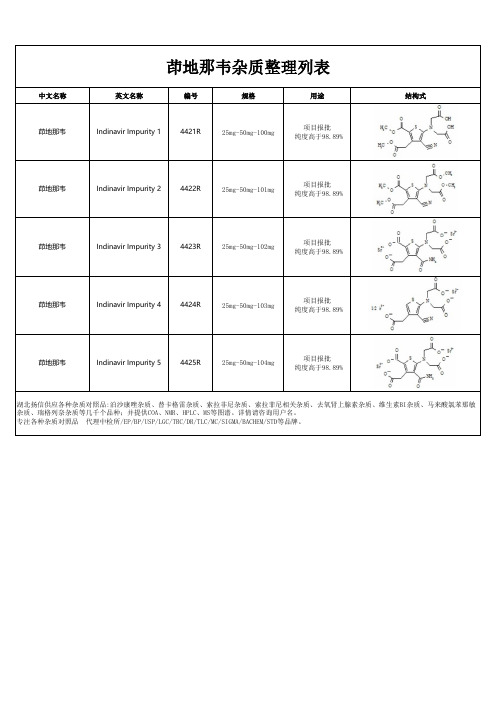
茚地那韦杂质整理列表
中文名称英文名称编号规格用途结构式
茚地那韦Indinavir Impurity 14421R25mg-50mg-100mg项目报批
纯度高于98.89%
茚地那韦Indinavir Impurity 24422R25mg-50mg-101mg项目报批
纯度高于98.89%
茚地那韦Indinavir Impurity 34423R25mg-50mg-102mg项目报批
纯度高于98.89%
茚地那韦Indinavir Impurity 44424R25mg-50mg-103mg项目报批
纯度高于98.89%
茚地那韦Indinavir Impurity 54425R25mg-50mg-104mg项目报批
纯度高于98.89%
湖北扬信供应各种杂质对照品:泊沙康唑杂质、替卡格雷杂质、索拉非尼杂质、索拉菲尼相关杂质、去氧肾上腺素杂质、维生素BI杂质、马来酸氯苯那敏杂质、瑞格列奈杂质等几千个品种;并提供COA、NMR、HPLC、MS等图谱。
详情请咨询用户名。
专注各种杂质对照品 代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌。
新型AChE抑制剂胡椒碱衍生物对阿尔茨海默病小鼠的治疗作用评价

网络出版时间:2023-10-3019:33:31 网络出版地址:https://link.cnki.net/urlid/34.1086.R.20231027.1531.022◇神经精神药理◇新型AChE抑制剂胡椒碱衍生物对阿尔茨海默病小鼠的治疗作用评价孙佳磊,朱仁德,吴 静,曹国敏,刘新华,李 荣,石静波(安徽医科大学药学院,安徽合肥 230032)收稿日期:2023-06-09,修回日期:2023-09-23基金项目:国家自然科学基金资助项目(No21977001);药学创新基金科研项目(NoYXCX202102)作者简介:孙佳磊(1998-),男,硕士生,研究方向:小分子药物的设计、合成及抗炎活性评价,E mail:2971799980@qq.com;石静波(1975-),男,博士,副教授,硕士生导师,研究方向:药物设计与合成/药物化学生物学,通信作者,E mail:sjbo616@126.com;李 荣(1979-),男,博士,教授,博士生导师,研究方向:类风湿关节炎发病机制及药物药理学,通信作者,E mail:lirong@ahmu.edu.cndoi:10.12360/CPB202306026文献标志码:A文章编号:1001-1978(2023)11-2064-06中国图书分类号:R 332;R282 71;R338 64;R345 61;R745 7摘要:目的 研究胡椒碱衍生物4a对乙酰胆碱酯酶的抑制活性及对阿尔茨海默病(Alzheimer′sdisease,AD)小鼠的神经保护的作用。
方法 雄性C57BL/6J小鼠30只,随机分:(i)空白对照组,(ii)模型组,(iii)多奈哌齐(10mg·kg-1,阳性对照)组,(iv)4a低浓度(20mg·kg-1)组,(v)4a高浓度(40mg·kg-1)组。
iii至v组,小鼠给药东莨菪碱(3mg·kg-1)30min后,口服多奈哌齐和4a,连续10d,Morris水迷宫实验观察由东莨菪碱诱导的认知功能障碍小鼠的记忆功能。
20批不同产地桂枝质量研究

20批不同产地桂枝质量研究!于天颖,周劲松,马恩耀,罗文英,吴志坚,党院霞△,孑箭(广州采芝林药业有限公司,广东广州510145)摘要:目的对20批不同产地桂枝的质量进行比较与分析。
方法采用薄层色谱法对桂皮醛及桂枝对照药材进行定性鉴别,同时依照2015年版《中国药典》(一部)“桂枝”项下进行显微、水分、灰分检查及浸出物含量测定。
采用挥发油测定方法测定其含有的挥发油含量,采用高效液相色谱(HPLC)方法测定桂枝中含有的桂皮醛含量。
并结合聚类分析及主成分分析方法对结果进行分析。
结果桂枝显微特征明显,薄层色谱斑点明显,不同产地的桂枝其水分含量范围在8.20%〜10.83%;总灰分含量范围在0.56%〜2.56%;醇溶性浸出物含量范围在6.14%〜9.57%;挥发油含量范围在0.95%〜1.66%;桂皮醛含量范围在1.01%〜3.03%。
结论通过聚类分析得,可将20批不同产地的桂枝药材分为3类,结合主成分分析结果,其中广西平南的桂枝药材质量最好。
关键词:桂枝;桂皮醛;挥发油;聚类分析;主成分分析中图分类号:R285.5文献标志码:A文章编号:1007-2349(2020)10-0057-06桂枝,别名柳桂(Ciirnamomum cassia Presl),是樟科植物肉桂的干燥嫩枝,主产于广西、广东、福建、云南等地⑴。
味辛,温,入肺、心、膀胱经,是我国传统常用中药,药用历史悠久,在抗肿瘤、抗菌以及心脑血管保护、抗病毒等方面具有良好的药理活性。
桂枝除含有桂皮醛、桂皮酸等还含有其他丰富的化学物质⑺。
桂皮醛作为桂枝的基本化学成分,在去热、消痛、抗菌、消炎及其它方面效果明显⑻。
由于桂枝的有效成分桂皮醛为挥发油,在储存过程中有可能因储存环境而损失,导致药效无法达到理想效果。
所以测定其含有的挥发油含量具有极为重要的意义。
杨松等⑼运用高效液相色谱指纹图谱法等方法对不同产地和不同采收季节的桂枝样品,为桂枝质量标准研究打下基础。
益生菌对阿尔茨海默病作用的研究进展

益生菌对阿尔茨海默病作用的研究进展发布时间:2021-12-14T06:08:15.523Z 来源:《中国结合医学杂志》2021年12期作者:宋鑫萍1,2,李盛钰2,金清1[导读] 阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
宋鑫萍1,2,李盛钰2,金清11.延边大学农学院,吉林延吉 1330022.吉林省农业科学院农产品加工研究所,吉林长春 130033摘要:阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
本文综述了近几年来国内外益生菌对阿尔茨海默病的作用进展,以及其预防和治疗阿尔茨海默病的潜在作用机制。
关键词:益生菌;阿尔茨海默病;肠道菌群;机制Recent Progress in Research on Probiotics Effect on Alzheimer’s DiseaseSONG Xinping1,2,LI Shengyu2,JI Qing1*(1.College of Agricultural, Yanbian University, Yanji 133002,China)(2.Institute of Agro-food Technology, Jilin Academy of Agricultural Sciences, Chanchun 130033, China)Abstract:Alzheimer’s disease has become one of the major diseases threatening the life and health of the global elderly. The number of patients is increasing year by year, and the economic cost of nursing is high, which poses a major challenge to the global economy. In recent years, studies have shown that probiotics, as microorganisms beneficial to the health of the host, have a positive impact on the prevention and treatment of Alzheimer’s disease. Its mechanism may be through regulating intestinal flora, affecting the nervous immune system, regulating the neuroactive substances and metabolites, and affecting the occurrence and development of the disease through thegut- brain axis. This paper reviews the progress of probiotics on Alzheimer’s disease at home and abroad in recent years, as well as its potential mechanism of prevention and treatment.Key words:probiotics; Alzheimer’s disease; gut microbiota; mechanism阿尔茨海默病(Alzheimer’s disease, AD),系中枢神经系统退行性疾病,属于老年期痴呆常见类型,临床特征主要包括:记忆力减退、认知功能障碍、行为改变、焦虑和抑郁等。
药物Crizotinib(克唑替尼)合成检索总结报告
药物Crizotinib(克唑替尼)合成检索总结报告
一、Crizotinib(克唑替尼)简介
Crizotinib(克唑替尼)是ATP竞争性的多靶点蛋白激酶抑制剂。
Crizotinib(克唑替尼)分别在ALK、ROS和MET激酶活性异常的肿瘤患者中证实克唑替尼对人体有显著临床疗效,适用于间变性淋巴瘤激酶阳性的局部晚期等。
Crizotinib(克唑替尼)分子结构式如下:
英文名称:Crizotinib
中文名称:克唑替尼
本文主要对Crizotinib(克唑替尼)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Crizotinib(克唑替尼)合成路线
Crizotinib(克唑替尼)19合成路线一:
Crizotinib(克唑替尼)19合成路线二:
三、Crizotinib(克唑替尼)合成检索总结报告(一) Crizotinib(克唑替尼)中间体3的合成方法一
(二) Crizotinib (克唑替尼)中间体3的合成方法二
(三) Crizotinib (克唑替尼)中间体5的合成
(四) Crizotinib(克唑替尼)中间体6的合成方法一。
CAS号877399-52-5_(S)-Crizotinib_MedBio技术资料

1、产品物理参数:
常用名
克唑替尼
英文名
Crizotinib
CAS号
877399-52-5
分子量
450.337
密度
1.5±0.1 g/cm3
沸点
599.2±50.0 °C at 760 mmHg
分子式
C21H22Cl2FN5O
熔点
无资料
闪点
316.2±30.1 °C
2、技术资料:
体外研究
PF-2341066在mIMCD3小鼠或MDCK犬上皮细胞中显示出相似的针对c-Met磷酸化的效力,IC50分别为5nM和20nM。与NIH3T3细胞相比,PF-2341066显示出针对NIH3T3细胞的改善或相似的活性,所述NIH3T3细胞经工程改造以表达c-Met ATP结合位点突变体V1092I或H1094R或P-环突变体M1250T,IC50分别为19nM,2nM和15nM。表达野生型受体,IC50为13 nM。相反,与野生型受体相比,观察到针对经工程改造以表达c-Met活化环突变体Y1230C和Y1235D的细胞的PF-2341066效力的显着变化,IC50分别为127nM和92nM。PF-2341066还有效地阻止了NCI-H69和HOP92细胞中c-Met的磷酸化,IC50分别为13 nM和16 nM,分别表达内源性c-Met变体R988C和T1010I [1]。PF-2341066还有效抑制Karpas299或SU-DHL-1 ALCL细胞中的NPM-ALK磷酸化,IC50为24 nM。PF-2341066有效阻止细胞增殖,这与ALK阳性ALCL细胞中G(1)-S期细胞周期停滞和诱导细胞凋亡有关,IC50为30 nM,而ALK阴性淋巴瘤细胞则不然[2]。此外,PF-2341066可预防与原发肿瘤生长(即增殖和存活)以及转移相关的骨肉瘤行为[3]。
乙酰胆碱酯酶抑制剂

上海应用技术学院研究生课程《高等天然产物化学》试卷2014 / 2015 学年第1 学期课程代码:NX0702013论文题目:乙酰胆碱酯酶抑制剂的研究进展姓名:芮银146061414康满满146061409专业:制药工程学院:化工学院乙酰胆碱酯酶抑制剂的研究进展芮银,陈祎桐,康满满摘要:本文阐述了乙酰胆碱酯酶抑制剂(AChEI)的研究进展,介绍了用于药物治疗的乙酰胆碱酯酶抑制剂的各种来源如植物、微生物等,及其抑制乙酰胆碱的活性物质。
在此基础上,总结了几种现代分析技术,对AChEIs进行筛选,大大加快AD药物资源的开发利用进程。
这些方法主要有基于比色法的Ellman's法及相关的改进方法、薄层显色法、荧光显色法、电喷雾质谱法等。
但是,到目前为止,现代分析技术在AD药物资源中的应用还处在起步阶段。
关键词:乙酰胆碱酯酶抑制剂,筛选方法,薄层显色法,荧光显色法The progress of acetylcholinesteraseinhibitorsRui Yin, Chen Yitong, Kang ManmanAbstract:In this artical, the research elaborates progress of acetylcholinesterase inhibitors (AChEI), and introduces a variety of sources for drug treatment acetylcholinesterase inhibitors such as plants, microorganisms, and its active ingredients. On this basis, the review summarizes several modern analytic techniques such as Ellman's method which based on the colorimetric method, TLC chromogenic method, fluorescent color method, Electrospray ionization mass spectrometry and so on. However, at present, the application of modern analytic techniques in AD drug resources is still in infancy.Key word: Acetylcholinesterase inhibitors, Screening Methods, TLC chromogenic method, Fluorescent color method目录摘要.................................................................................................错误!未定义书签。
瑞舒伐他汀

瑞舒伐他汀[通用名称] rosuvastatin calcium , 瑞舒伐他汀[商品名称] Crest or[化学名称] 双-[(E)-7[4-(4-氟基苯基)-6-异丙基-2-[甲基(甲磺酰基)氨基]-嘧啶-5-基](3R ,5S)-3,5-二羟基庚-6-烯酸]钙盐(2:1),分子式:(C 22H 27FN 3O 6S )2Ca,分子量:1001.15, 结构式为:[简单介绍]:是由日本Shionogi 公司研发的他汀类血脂调节药,2002年11月在荷兰上市。
2003年8月获得美国FDA 批准,目前已在60余个国家上市,在我国已完成临床试验。
本品能强力抑制 HMG-CoA 还原酶,并具有肝细胞作用选择性.降低低密度脂蛋白胆固醇(LDC-C)、升高H DL - C 的作用在已上市的他汀类药物中最优.耐受性与安全性好, 被誉为“超级他汀”近日, AstraZ eneca 公司的瑞舒伐他汀钙获FDA 批准新的适应症, 可用于降低患者脑卒中,心房纤颤 (房 颤 ) 和动脉血管再生的风险。
瑞舒伐他汀钙 200 9 年全球的销售达到了62亿美元,较去年有 32% 的增长, 已经成为了国内外争相进行仿制的对象。
因此,对其生产技术的研究与开发具有重要的经济和社会效益。
[ 理化 性 质] 瑞舒 伐 他汀 是一 个 单对 映 体( 3 R , 5 S ) 羟酸钙盐, 分子中的二羟基庚烯酸部分是 他汀类的共性药效基团, 其余部分区别于其他的他 汀类, 包括一个极性的甲基磺酰胺基团, 它与分子的 低亲脂性相关。
亲水性的化合物较少被动扩散, 不易进入非肝细胞, 但经过器官阴离子的转运过程却极易进入肝细胞。
该化合物的亲水特性可使其避免被细胞色素 P4 50 酶大量代谢 。
瑞舒伐他汀主要作用部位在肝脏, 它选择性地 抑制肝脏中胆固醇合成限速酶 HM G - CoA 还原酶, 使肝脏脂蛋白生成减少, LDL - 胆固醇受体表达 增加, 因此血浆胆固醇水平下降。
国内在研1.1类糖尿病新药

CXHL1000720鲁
CXHL1000719鲁
化药
化药
新药
新药
1.1 2011-05-16 山东轩竹医药
1.1 2011-05-16 科技有限公司
瑞格列汀二甲双胍片(II) CXHL1300534苏 磷酸瑞格列汀 磷酸瑞格列汀片 CXHL0900102苏 CXHL0900103苏
Байду номын сангаас
在研1.1类糖尿病新药申报信息
药品名称
苯甲酸复格列汀
受理号码
CXHL1200845渝
药品 类型 化药 化药 化药 化药
申请 类型 新药 新药 新药 新药
注册 分类
承办日期
国内在研1.1类糖尿病新药
西安万隆制药股份有限公司 王震 2013-10-26
目录
1. 维格列汀---北京诺华制药有限公司 2. 酒石酸艾格列汀----山东绿叶制药有限公司 3. 托西酸贝格列汀----江苏豪森药业股份有限公司 4.沙格列汀片--- 阿斯利康(无锡)贸易有限公司 5.磷酸瑞格列汀----江苏恒瑞医药股份有限公司 6.苯甲酸复格列汀-----重庆复创医药研究有限公司 7.盐酸依格列汀----山东轩竹医药科技有限公司
在研1.1类糖尿病新药申报信息
药品名称 维格列汀 维格列汀片 维格列汀片 维格列汀片 受理号码 JXHL0600218国 CXHS0700083京 CXHL0501674京 CXHL0501675京 药品 类型 化药 化药 化药 化药 化药 化药 化药 申请 类型 进口 新药 新药 新药 新药 新药 新药 注册 分类 1.1 1.1 1.1 1.1 1.1 1.1 1.1 承办 日期 2006-08-17 2007-06-25 北京诺华制药 2005-09-01 2005-09-01 2013-07-30 2013-07-30 2013-07-30 山东绿叶制药 有限公司 有限公司 企业名称
15523666_迷迭香酸对哮喘小鼠氧化性肺损伤的保护作用

$ 材 料 与 方 法
$C$ 材 料 !3!3!试验动物!0""8XDC[ 级雌性 &;B&&S 小鼠购自 广 西 医 科 大 学 实 验 动 物 中 心"动 物 合 格 证
号!D#2*%桂'"8!$?888"# 试 验 前 适 应 性 饲 养:P" 自由饮水 饮 食"饲 养 环 境 温 度 "$ c l! c"相 对 湿 度 $85 "085 # !3!3" 主 要 试 剂 和 药 品 9,D$D,'$1DZ?CQ 检测试 剂 盒 均 购 自 碧 云 天 生 物 技 术 研 究 所*,(; 购自 DEX\@ 公 司*液 态 铝 佐 剂 购 自 >OHM\I 公 司# 肿节风药材购自广 西 玉 林 中 桂 药 材 有 限 公 司"药 材
%!3广西大学动物科学技术学院"南宁 4:8884*"3广西畜牧研究所"南宁 4:888!'
摘要为评价迷迭香酸对哮 喘 小 鼠 模 型 氧 化 性 肺 损 伤 的 保 护 作 用"本 研 究 用 卵 清 蛋 白 %,(;'致 敏$激 发 雌 性 &;B&&S小鼠建立哮喘模型"并用 ,(; 和 Z"," 联合激发小鼠作为氧化肺损伤阳性对照模型#在最后一次滴鼻激 发 "$O 后 "取 支 气 管 肺 泡 灌 洗 液 %&;B['进 行 细 胞 计 数 并 测 定 活 性 氧 %9,D'$超 氧 化 物 歧 化 酶 %D,''和 谷 胱 甘 肽 过 氧化物酶%1DZ?CQ'水平"取左侧肺脏固定做 Z= 染色#结果显示"迷迭香酸可明显减少 &;B[ 中 细 胞 总 数 和 嗜 酸 性粒细胞数目"显著抑制肺组织和 &;B[ 中 9,D的产生"升高 D,' 和 1DZ?CQ水 平"改 善 肺 组 织 病 理 变 化# 本 试 验 结 果 表 明 "迷 迭 香 酸 对 氧 化 肺 损 伤 起 到 明 显 的 保 护 作 用 # 关 键 词 迷 迭 香 酸 *哮 喘 *氧 化 性 肺 损 伤 *抗 氧 化 中 图 分 类 号 9"0434 文 献 标 识 码 ; 文 章 编 号 !+6!?6":+%"8!6'!"?:+48?8+
吡咯里西啶生物碱相关肝窦阻塞综合征的循证临床研究

吡咯里西啶生物碱相关肝窦阻塞综合征的循证临床研究高虹;王吉耀【摘要】在肝病的临床研究中开展循证医学可以给临床医师提供临床决策的依据.服用含吡咯里西啶生物碱(pyrrolizidine alkaloids,PAs)的植物导致的肝窦阻塞综合征(hepatic sinusoidal obstruction syndrome,HSOS)的确诊比较困难,即使进行创伤性检查,也易误诊.在循证医学理念指导下通过开展病因学、诊断和预后的研究,结合实验室检查,确定了含PAs的植物是HSOS的病因之一,且PAs的代谢物吡咯与蛋白质的加合物可以作为PAs导致的HSOS的诊断标志物和预后的指标.这些研究经历提示,必须带着临床问题进行研究,在研究的不同方向进行顶层设计,并在研究过程中贯彻循证医学理念和方法,才能提供可靠的结果.【期刊名称】《胃肠病学和肝病学杂志》【年(卷),期】2018(027)009【总页数】2页(P979-980)【关键词】循证;临床研究;肝病;肝窦阻塞综合征【作者】高虹;王吉耀【作者单位】复旦大学附属中山医院消化科,复旦大学循证医学中心,上海200032;复旦大学附属中山医院消化科,复旦大学循证医学中心,上海200032【正文语种】中文【中图分类】R575肝窦阻塞综合征(hepatic sinusoidal obstruction syndrome, HSOS)曾被称为肝小静脉闭塞症,是由各种原因导致的肝血窦、肝小静脉和小叶间静脉内皮细胞水肿、坏死、脱落进而形成微血栓,引起肝内淤血、肝损伤和门静脉高压的一种肝脏血管性疾病[1]。
服用含吡咯里西啶生物碱(pyrrolizidine alkaloids,PAs)的植物导致的HSOS是我国HSOS最主要的病因和类型[2]。
这种疾病的临床表现为腹胀、肝区疼痛、肝脏肿大、黄疸和腹水等,与Budd-Chiari综合征、肝硬化、其他原因导致的重症肝炎等难以鉴别,容易误诊。
