USP34_POVIDONE
仿制药参比制剂目录(第二十六批)

注射剂
Fresenius Kabi
未进口原研药品
欧盟上市
26-31
注射用高纯度尿促性素
Highly Purified Menotrophin for Injection/Menopur(贺美奇)
75IU FSH+75IU LH
依折麦布10mg/瑞舒伐他汀5mg
片剂
MSD株式会社
未进口原研药品
日本上市
26-10
乙酰唑胺缓释胶囊
Acetazolamide Extended-release Capsules
500mg
胶囊剂(缓释胶囊)
Heritage Pharmaceuticals Inc
国际公认的同种药品
美国橙皮书
26-11
Olanzapine Orally Disintegrating Tablets/Zyprexa
10mg
片剂(口崩片)
日本イーライリリー株式会社
未进口原研药品
日本橙皮书
26-50
奥氮平口崩片
Olanzapine Orodispersible Tablets/Zyprexa Velotab
Eagle Pharmaceuticals Inc
未进口原研药品
美国橙皮书
26-13
酮咯酸氨丁三醇片
Ketorolac Trometamol Tablets/Lixidol
10mg
片剂
Atnahs Pharma Netherlands Bv
未进口原研药品
欧盟上市
26-14
注射用甲磺酸齐拉西酮
usp美国药典等级

usp美国药典等级美国药典级usp calss vi美国药典级(usp)实际上含义:美国药典(usp)是一个非政府组织,通过建立最新的标准来保证药品和其他保健技术的质量,从而支持公共卫生。
该组织与制药和生物技术行业有关。
美国药典规定了质量、纯度、强度和一致性的标准。
这些usp 标准发表在《美国药典》和《国家处方集》(usp nf)中。
usp第四类产品经过一系列的生物试验。
usp第六类化合物必须由具有明确生物相容性历史的成分制成,以满足对渗滤液的严格要求。
动物用来测试材料的毒性。
急性毒性试验:该试验测量试验材料的刺激性,控制其对人体的潜在危害。
毒性由口腔、皮肤和吸入决定。
皮内试验:这种特殊的试验将材料直接注射到正常使用过程中接触到的组织中,不保护皮肤或任何其他身体系统。
这将允许测试团队评估特定组织对材料的响应。
植入试验:植入试验确定植入活体动物时活体组织对材料的反应。
usp六级试验所需的标准植入时间为5天。
如果在5天后没有刺激或毒性的迹象,它将满足试验的植入要求。
温度和时间:用于全身毒性和皮内试验的材料提取物固定在设定的温度和暴露时间,以确保结果符合通用标准。
用三种不同的温度和时间暴露条件处理所有的材料提取物。
前72小时在122°F或50°C下给药,然后在158°F下给药24小时,最后在250°F下给药1小时。
usp第六类塑料试验旨在评价各种塑料材料在体内的生物反应性。
为了测试药物容器,塑料类测试经常在未焊接的塑料树脂和容器上进行。
类塑料测试不是生物相容性测试的替代品,但通常被制造商用来对材料进行分类。
塑料的分类包括三种体内试验。
系统注射试验和皮内试验旨在通过单剂量注射特定提取物来控制对塑料和其他聚合物的全身和局部生物反应。
第三种测试,即植入测试,旨在评估活组织对测试材料的反应。
利用这三个试验和不同提取物的不同排列,完成了六个不同等级的塑料等级试验。
usp定义了六种塑料类别,从i到vi(vi仍然是最严格的)。
依地酸二钠美国药典34版标准

Edetate Disodium(ed' e tate dye soe' dee um).C10H14N2Na2O8·2H2O 372.24Glycine, N,N¢-1,2-ethanediylbis[N-(carboxymethyl)-, disodium salt, dihydrate.Disodium (ethylenedinitrilo)tetraacetate dihydrate [6381-92-6].Anhydrous 336.21 [139-33-3].» Edetate Disodium contains not less than 99.0 percent and not more than 101.0 percent of C10H14N2Na2O8, calculated on the dried basis.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Edetate Disodium RSIdentification—A: Infrared Absorption 197K: undried.B: To 5 mL of water in a test tube add 2 drops of ammonium thiocyanate TS and 2 drops of ferric chloride TS, and mix. To the deep red solution add about 50 mg of Edetate Disodium, and mix: the red color is discharged, leaving a yellowish solution.C: It responds to the flame test for Sodium 191.pH 791: between 4.0 and 6.0, in a solution (1 in 20).Loss on drying 731— Dry it at 150 for 6 hours: it loses not less than 8.7% and not more than 11.4% of its weight.Calc ium—To a solution (1 in 20) add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide. Add 3 N hydrochloric acid dropwise until the solution is just acid, and then add 1 mL of ammonium oxalate TS: no precipitate is formed.Heavy metals, Method II 231: 0.005%.Limit of nitrilotriacetic acid—Mobile phase— Add 10 mL of 1.0 M tetrabutylammonium hydroxide in methanol to 200 mL of water, and adjust with 1 M phosphoric acid to a pH of 7.5 ±0.1. Transfer the solution so obtained to a 1000-mL volumetric flask, add 90 mL of methanol, dilute with water to volume, mix, pass through a filter having a 0.5-µm or finer porosity, and degas.Cupric nitrate solution—Prepare a solution containing about 10 mg of cupric nitrate(Cu(NO3)2) per mL.Standard stock solution— Transfer about 100 mg of nitrilotriacetic acid, accurately weighed, to a 10-mL volumetric flask, add 0.5 mL of ammonium hydroxide, and mix. Dilute with water to volume, and mix.Resolution solution—Transfer 10 mg of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Standard solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Test solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Chromatographic system (see Chromatography 621)— The chromatograph is equipped witha 254-nm detector and a 4.6-mm × 15-cm column that contains packing L7. The flow rate is about2 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.35 for nitrilotriacetic acid, 0.65 for copper, and 1.0 for edetate; and the resolution, R, between nitrilotriacetic acid and copper is not less than 3. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The response of the nitrilotriacetic acid peak obtained from the Test solution does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard solution and the Test solution: not more than 0.1% of nitrilotriacetic acid is found.Assay—Assay preparation— Dissolve about 5 g of Edetate Disodium, accurately weighed, in about 100 mL of water contained in a 250-mL volumetric flask, add water to volume, and mix.Procedure— Place about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours, cooled in a desiccator, and accurately weighed, in a 400-mL beaker, add 10 mL of water, and swirl to form a slurry. Cover the beaker with a watch glass, and without removingthe latter, add 2 mL of 3 N hydrochloric acid from a pipet. Swirl the contents of the beaker, and dissolve the calcium carbonate. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring the solution, preferably with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 0.30 g of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in mg, of C10H14N2Na2O8 in the portion of Edetate Disodium taken by the formula:(336.21/100.09)W(VT /V)in which 336.21 and 100.09 are the molecular weights of edetate disodium and calcium carbonate, respectively; W is the weight, in mg, of calcium carbonate; VT is the volume, in mL, of the Assay preparation; and V is the volume, in mL, of the Assay preparation consumed in the titration.Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Elena Gonikberg, Ph.D.Principal Scientific Liaison1-301-816-8251 (SM32010) Monographs - Small Molecules 3Reference Standards RS Technical Services1-301-816-8129**************USP34–NF29 Page 2663Pharmacopeial Forum: V olume No. 32(4) Page 1070。
usp美国药典结构梳理

vivi2010-10-02USP总目录:修订文件1 New Official Text,修订公告。
勘误表,临时修订声明,修)加快修订过程包括勘误表,临时修订声明(IRAS上刊部分刊出,勘误表,临时修订公告也会在订公告在USP网站上New Official Text PF出前言2front matter药典与处方集增补删减情况,审核人员,辅料收录情况3凡例药典,标题和修订1 2 药典地位和法律认可 3标准复合性专论和通则45 专论组成6 检验规范和检验方法7 测试结果8 术语和定义处方和配药 910 包装存储与标签4通则章节列表)一般检查和含量测定(章节编号小于1000检查和含量分析的一般要求检查和含量分析的仪器,微生物检查,生物检查和含量测定,化学检查和含量测定,物理检查和测定1000一般信息(章节号大于)5食物补充剂通则试剂(试剂,指示剂,溶液等)6.参考表7性状描述和溶解性查询表(按字母顺序)食品补充剂各论(字母顺序)8各论(辅料标准)9NF10 USP各论11术语附件:通则的章节中文目录(使用起来比较方便,直接找对应章节号即可)一、通用试验和检定(1)试验和检定的总要求1 注射剂11 参比标准物(2)试验和检定的装置16 自动分析方法21 测温仪31 容量装置,如容量瓶、移液管、滴定管,各种规格的误差限度41 砝码和天平(3)微生物学试验51 抗菌效力试验55 生物指示剂:耐受性能试验61 微生物限度试验61 非灭菌制品的微生物检查:计数试验62 非灭菌制品的特定菌检查,如大肠杆菌、金葡菌、沙门氏菌等71 无菌试验(4)生物学试验和检定81 抗生素微生物检定85 细菌内毒素试验87 体外生物反应性试验:检查合成橡胶、塑料、高聚物对哺乳类细胞培养的影响88 体内生物反应性试验:检查上述物质对小鼠、兔iv、ip或肌内植入的影响泛酸钙检定91111 生物检定法的设计和分析115 右泛醇检定121 胰岛素检定141 蛋白质——生物适应试验,用缺蛋白饲料大鼠,观察水解蛋白注射液和氨基酸混合物的作用151 热原检查法161 输血、输液器及类似医疗装置的内毒素、热原、无菌检查171 维生素B活性检定12(5)化学试验和检定A 鉴别试验181 有机含氮碱的鉴别191 一般鉴别试验193 四环素类鉴别197 分光光度法鉴别试验201 薄层色谱鉴别试验B 限量试验206 铝211 砷221 氯化物和硫酸盐223 二甲基苯胺226 4-差向脱水四环素231 重金属241 铁251 铅261 汞271 易炭化物试验281 炽灼残渣291 硒C 其他试验和检定中和酸能力301311 藻酸盐检定331 苯丙胺检定341 多剂量容器注射剂中所加防腐剂含量的气相色谱或极谱法测定345 枸橼酸与其盐以及磷酸盐检定351 甾体检定361 巴比妥酸盐检定371 维生素B放射示踪物检定12381 注射剂橡胶塞检查391 肾上腺素检定401 脂肪和固定油检查411 叶酸检定425 抗生素碘量法检定429 微粒大小的光衍射测量431 甲氧基测定441 烟酸或烟酰胺检定451 亚硝酸盐滴定461 氮测定466 普通杂质的薄层色谱法检查467 有机挥发性杂质检查法467 残留溶剂测定471 氧瓶燃烧法481 核黄素检定501 有机含氮碱的盐511 单一甾醇检定521 磺胺类的色谱法检定531 硫胺检定541 滴定法554 α-生育酚检定561 植物来源物品的一般检查项目植物来源物品的各种鉴别项目(植物学部分、显微鉴别、化学鉴别)563 565 植物提取物的一般提取方法和要求571 维生素A检定:化学法、色谱法581 维生素D检定:色谱法、化学法、生物法591 锌测定(6)物理试验和测定601 气雾剂、鼻喷雾剂、计量吸入剂和干粉吸入剂的各项检测611 乙醇含量测定:蒸馏法、气一液色谱法616 固体的疏松密度和叩击密度测定621 色谱法631 色度检查和标准641 溶解的完全性检查643 总有机炭测定645 水导电性测定651 冻凝温度的测定661 药用容器的检测项目要求671 盛装胶囊和片剂容器加盖后对湿气的通透性试验691 棉花吸附性和纤维长度测定695 结晶性检查696 用溶液测热法测定结晶度698 装量检查699 固体密度(粉粒密度测定法)701 崩解试验711 溶出试验721 蒸馏温度范围(馏程)测定724 通过透皮转运系统药物的释放726 电泳727 毛细管电泳730 等离子体光谱化学检查法731 干燥失重炽灼失重733736 质谱法741 熔点范围或温度的测定751 眼膏中的金属颗粒测定755 最低装量检查法761 核磁共振771 眼用软膏的要求776 光学显微镜微粒检查法781 旋光度检查785 渗透压摩尔浓度测定法786 用分析筛测量颗粒大小的分布788 注射液中微粒物质测定法789 眼用溶液中微粒物质测定法791 PH测定法795 非灭菌制剂的药物配制要求797 灭菌制剂的药物配制要求801 极谱法811 粉末细度测定821 放射活性药物823 正电子发射层析X线摄影(PET)所用放射性药物的配制831 折光指数测定841 比重测定846 粉末的比表面积测定851 分光光度法与光散射861 外科缝合线直径检查871 附有针的缝合线检查881 外科缝合线、纺织品与膜片的弹力强度检查891 热分析:温度变化、热解重量分析、易熔杂质分析等905 剂量单位的均匀性检查(含量均匀度、装量差异)911 黏度测定药品含水量的测定921941 结晶型药物的X线衍射分析二、通用资料1010 数据分析方法1015 诊断用放射药的自动合成装置1031 药用容器、医用装置和植入物所用材料的生物相容性检查1035 灭菌用生物指示剂1041 生物制品的批签发1043 细胞、基因和组织工程产品的辅助材料1045 生物技术产品1046 细胞和基因治疗产品1047 生物技术产品的检验法1048 生物技术产品的质量——重组DNA蛋白质产品生产所用细胞表达构成的分析1049 生物技术产品的稳定性试验1050 人或动物来源的细胞系所得生物技术产品的病毒安全性评价1051 玻璃仪器清洗方法1061 颜色的仪器测量1065 离子色谱1072 消毒剂与防腐剂1074 赋形剂生物学安全性评价指导原则1075 复方药物配制质量规范1078 大批量药用赋形剂的生产质量规范1079 储存与运输的质量规范1081 明胶的凝胶强度1086 药品中的杂质来源1087 特性溶出1088 剂型的体外和体内评价1090 体内生物等效性试验指导原则1091 剂型中含有无活性组分的标示1092 溶出试验方法的发展和验证药用滴管11011111 非灭菌药品的微生物特征1111 非灭菌药品的微生物特征检查:药用原料和药物制剂的判定标准1112 非灭菌药品中的水活性测定,即在同一温度时,药品中水的蒸气压与纯水蒸气压之比,它等于药品在密闭系统中产生相对湿度的1%1116 清洁室和其他受控环境的微生物评价1117 微生物实验室的质量规范(GLP)1118 监控装置:时间、温度、湿度1119 近红外分光光度法1120 拉曼(Raman)分光光度法1121 药品命名法1136 药品包装:应用单元1146 口服固体药分装在单疗程剂量容器中的检查方法1150 药物剂型的稳定性1151 药物剂型1160 处方调配的药学计算1171 原料药的位相溶解度分析1174 粉末流动性测定1176 处方天平和容量装置1177 包装质量规范1178 分装质量规范1181 扫描电子显微镜1191 调剂工作中的药品稳定性保持1196 药典协调(指欧洲药典、美国药典、日本药局方三方机构讨论协调的原则和方法)1207 灭菌产品包装:完整性评价1208 灭菌试验:隔离系统的验证1209 灭菌:化学和物理化学的指示剂与积分仪1211 药典收载品种的灭菌和灭菌保证1216 片剂脆性检查1221 茶匙(家用标准为5 ml,可作为病人口服液体药物的量具,误差应小于10%)药品灭菌终点的放行参数12221223 微生物替代方法的验证1225 药典方法的验证1227 在抗菌效力、微生物限度、灭菌等试验中,微生物的恢复验证1230 血液透析用水1231 药用水的制备和要求1241 在制药系统中,水—固体的相互作用1251 用分析天平称量的要求1265 书写药物处方的指导原则三、饮食增补剂2021 营养和饮食增补剂的微生物计数试验2022 营养和饮食增补剂中不允许存在的微生物(如金葡菌、沙门氏菌、大肠杆菌、梭状芽胞杆菌属)检查法2023 非灭菌的营养和饮食增补剂中的微生物特征2030 植物来源物品的增补资料2040 饮食增补剂的崩解和溶出检查2091 饮食增补剂的重(装)量差异检查2750 饮食增补剂的生产条件与质量要求(与药品有别)。
药品 别名 通用名 英文名

序号中文别名/缩写中文通用名英文通用名品牌1 皮维碘聚维酮碘povidone iodine2 枢复来佐米曲普坦zolmitriptan3 枢复宁/枢复灵昂丹司琼注射液zofran/ondansetron injection4 镇痛新喷他佐辛sosegon/pentazocine5 舒喘宁/舒喘灵硫酸沙丁胺醇salbutamol sulfate6 舒喘平沙丁胺醇salbutamol7 安络血肾上腺色腙片carbazochrome8 维生素K1 植物甲萘醌vitamin K1/phytomenadione9 灭滴灵甲硝唑metronidazole10 灭吐灵甲氧氯普安metoclopramide11 速尿/利尿呋塞米furosemide/fursemide12 安洛地平氨氯地平amlodipine13 毓婷左炔诺孕酮lenonorgestrel14 降压0号/降压平片复方利血平氨苯蝶啶片15 氯化钾缓释片potassium chloridesustained-releasetablets 补达秀16 苯磺酸氨氯地平片amlodipine besylate tablets 安内真17 阿莫西林片amoxicillin tablets 阿林新18 病毒唑利巴韦林ribavirin gtanules 新博林/同欣/威利宁先锋4号头孢氨苄cefalexin;keflex;CEX;Cephalexin hydrate先锋5号头孢唑林钠Cefazolin sodium;cefazolin先锋6号头孢拉定Cefradine;cefadroxil;cephradine;Velosef19 头孢克亏颗粒cefixime granules 达力芬20 京必舒新辛伐他汀片simvastatin tablets21 京必妥新左氧氟沙星levofloxacin22 阿莫西林分散片amoxicillin dispersible tablets 再林23 甲苯咪唑片mebendazole tablets 安乐士24 蒙脱石散montmorillonite powder思密达25 盐酸洛哌丁胺胶囊loperamide hydrochloride capsules 易蒙停26 妇炎灵Ⅱ号阴道泡腾片复方氯霉素阴道泡腾片compound chloramphenicol vaginal effervescent tablets27 双氯芬酸纳缓释胶囊diclofenac sodium sustained-release capsules 英太青28 醋酸地塞米松粘贴片dexamethasone acetate adhesive tablets 意可贴29 氯雷他定片loratadine tablets 百为乐30 氯雷他定泡腾片loratadine effervescent tablets 开瑞坦31 复方地塞米松软膏compound acetic acid dexamethasone ointment 皮炎平32 复方酮康唑发用洗剂compound ketoconazole lotion for scalp disorders 康王33 酮康唑ketoconazole 皮康王34 曲咪新乳膏Triamcinolone Acetonide Acetate Miconazole Nitrate Neomycin Sulfate Cream 皮康霜35 硝酸咪康唑miconazole nitrate cream 达克宁36 曲安奈德益康唑乳膏Triamcinolone Acetonide and Econazole Nitrate Cream 派瑞松37 糠酸莫米松乳膏MOMETASONE FUROATE CREAM 艾络松38 枯草杆菌、肠球菌二联活菌颗粒medilac vita/combined bacillussubtilis and enterococcus faecium granules with multivitamines,live 妈咪爱39 多维元素片multivitamim formula with minerals tablets 金施尔康40 维生素AD滴剂vitamin A and D drops 伊可新41 吡诺克辛钠滴眼液pirenoxine sodium eye drops 白内停42 复方硫酸软骨素滴眼液compound chondroitin sulfate eye drops 润洁43 九合维生素丸nine vitamins pills 九维他44 复方铝酸铋片compound bismuth aluminate tablets 胃必治45 地衣芽孢杆菌活菌胶囊Bacillus licheniformis capsule,live 整肠生46 阿苯达唑片albendazole tablets 史克肠虫清47 多潘立酮domperidone 吗丁啉48 枸橼酸铋钾胶囊bismuth potassium citrate 丽珠得乐49 小儿氨酚烷胺颗粒pediatric paracetamol and amantadine hydrochloride granules优卡丹50 无味红霉素依托红霉素Relying on the red enzyme51 三九君必沙琥乙红霉素分散片erythromycin ethylsuccinate dispersible tablets52 三九罗噻嗪头孢曲松钠ceftriaxone sodium53 酚咖片paracetamol and caffeine tablets 加合百服宁54 布洛芬缓释胶囊ibuprofen sustained-release capsules 芬必得55 复方氨酚烷胺胶囊compound paracetamol and amantadine hydrichloride capsules 快克/仁和可立克/感叹号/感康56 复方氨酚葡锌片compound paracetamol and zinc gluconate tables 康必得57 扑热息痛对乙酰氨基酚paracetamol 必理通/泰诺林58 APC 复方对乙酰氨基酚compound paracetamol 散列通59 复方盐酸伪麻黄碱缓释胶囊contact NT/compound pseudiephedrine HCL sustained release capsules 新康泰克60 复方盐酸苯丙醇胺缓释胶囊compound phenylpropanolamine hydrochloride sustained-release capsules 康泰克缓释胶囊61 酚麻美敏片Tylenol cold tablets 泰诺感冒片62 氨酚伪麻美芬片II / 氨麻苯美片 Paracetamol pseadoephedrine hydrochloride and dextromethorphan hydrobromide tablets 白加黑63 阿司匹林乙酰水杨酸acetylsalicylic acid;acetyl salicylic acid;acetylsalicylate;aspirin64 强力霉素盐酸多西环素Doxycycline hydrochloride;Doxycycline Hyclate;doxycycline hcl65 氟哌酸诺氟沙星Norfloxacin;nor;NFX;Noroxin66 氟嗪酸氧氟沙星ofloxacin;levofloxacin;OFLX;OFIX67 黄连素盐酸小檗碱berberine hydrochloride;Berberine;BerberineHydrochloride68 丁胺卡那硫酸阿米卡星amikacin sulfate;Amikacin Sulphate;Amikacin sulphate sterile;Amikacin Sulphate Injectable grade69 曲安缩松曲安奈德益康唑乳膏Triamcinolone Acetonide and Econazole Nitrate Cream70 乐肤液哈西奈德Halcinonide71 呋喃坦啶呋喃妥因AHD;Macrobid72 痢特灵呋喃唑酮furazolidone73 无环鸟苷阿昔洛韦acv;acycloguanosine;Aciclovir;Acyclovir74 安痛定复方氨基比林amidopyrine compound;Antondin75 克罗米芬够橼酸氯米芬Enough clomiphene citrate76 安宫黄体酮醋酸甲羟孕酮Medroxyprogesterone Acetate;medroxyprogesterone;provera;Medroxyprogesterone Acetate USP77 优降糖格列苯脲glibenclamide;glyburide;INN78 降糖灵苯乙双胍phenformin79 D860 甲苯磺丁脲tolbutamide80 乙底粉乙烯雌酚diethylstilbestrol;stillboestrol81 大仑丁苯妥英钠pht;phenytoinum;DPH;phenytoin sodium82 冬眠灵盐酸氯丙嗪chlorpromazine hydrochloride;CPZ;Thorazine;Chlorpromazine hcl83 心痛定硝苯地平Nifedipine;nif;Procardia XL;nifidipine84 心得安盐酸普萘洛尔Propranolol Hydrochloride;propranolol hcl;propranolol85 心律平盐酸普罗帕酮片Propafenone Hydrochloride Tablets86 卡托普利captopril;Capoten;captopriil;diuretics 开博通87 消炎痛吲哚美辛Indo;indomethacin;indocin;indometacin88 他巴唑甲巯咪唑Mercapto-1-methylimidazole;tapazole;methimazole;thiamazole赛治89 利血生利可君片leucogen tablets90 左甲状腺素钠levothyroxine sodium;levothyroxine;levothyrox;Levothyroxine Tablets 雷替斯/优甲乐91 脑复康吡拉西坦piracetam92 维脑路通曲克芦丁troxerutin93 脑益嗪桂利嗪stugeron;mitronal;sapratol;cinnipirine94 ATP 三磷酸腺苷二钠trinosin95 PSS 藻酸双脂钠alginic sodiumdiester96 脑复新盐酸吡硫醇neuroxin;Pyritinol Hydrochloride;Pyritinol HCL;PYRITHIOXINE DIHCL97 脑脉片盐酸苯甲哌丙醇Hydrochloric acid benzene mepiquat chloride propanol98 心脑清软胶囊五福99 银杏叶片Ginkgo Leaf Tablets;Ginkgo leaves tablets;Ginkgo leaf;Ginkgo Biloba Leaves Extract Tablets 依康宁/络欣通100 薯蓣皂苷片Dioscornin Tablets;shuyuzaogan tablet;Dioscin Tablets 维奥欣101 癫健安丙戊酸胺Valproic acid amine102 抗癫灵丙戊酸钠vpa;Sodium Valproate;sodium vedproate;Depakote103 异搏定盐酸维拉帕米Verapamil hydrochloride;VERAPAMIL HYDROCHLORIDES;USP29 Verapamil HCL;VERAPAMIL HCL 盖衡104 棕色合剂复方甘草合剂compound mixture of liquorice;Decloxizine;Compound Glycyrrhizae Mixture;Btown Mixture105 必嗽平盐酸溴己新bromhexine hydrochloride;Bromhexine HCl;bisolvon106 咳必清枸缘酸喷托维林Pentoxyverine Citrate107 胃舒平复方氢氧化铝Gastropine;Compound Aluminium Hydroxide108 小苏打/SB 碳酸氢钠sodium bicarbonate;baking soda;sodium hydrogen carbonate;dicarbonate109 胃复安甲氧氯普胺Metoclopramide;methoxychlorprocainaamide110 果导片酚酞片Phenolphthalein Tablets111 复方苯乙哌啶复方地芬诺酯片Lomotil112 肝太乐葡醛内酯片Glucurolactone Tablets113 盐酸黄铜哌酯Brass piperazine ester hydrochloride 津源灵/洛沃克114 枸缘酸莫沙必利片Mosapride citrate tablets 贝络钠/新络纳115 乙哌立松Eperisone 妙纳/佐宁116 MIX 甲氨蝶呤Methotrexate;MTX117 洛美沙星Lomefloxacin;lml 洛威/奇洛先118 富马酸比索洛尔bisoprolol fumarate;bisoprolol 博苏119 尼索地平Nisoldipine;sular;nisodipine;hisoldipine 尼力120 苯磺酸氨氯地平amlodipine besilate;Amlodipine Besylate;AmlodipineBesylate;Amlodi- pine Besylate 安内真/络活喜121 马来酸氨氯地平Amlodipine Maleate 麦利平122 吲达帕胺indaparnide;Indapamide;triethylamine 寿比山/纳催离123 马来酸依那普利EnalaprilMaleate;Renitec;enalapril 依苏124 脉安定门洞氨酸钾镁片The doorway of ammonia acid potassium magnesium tablets 潘南金125 硫酸氨基葡萄糖胶囊fglucosamine sulfate capsules 维骨力、维固力126 双环醇片Bicyclol 白赛诺127 水飞蓟宾silybin;silymarin;Silibinin 水林佳128 硫普罗宁Tiopronin;tioproni;tiopronine;tipronin 凯西莱129 铝碳酸镁片Hydrotalcite Tablets 达喜130 胶体果胶铋胶囊Aminophylline;Colloidal Bismuth Pectin Capsules 乐普生131 强筋松苯丙氨酯phenprobamate132 甲钴胺mecobalamin;Methylcobalamin;methycobal 弥可保133 洛伐他汀片Lovastatin Tablets;TABELLAELOVASTATINI 俊宁134 甲磺酸倍他司汀片betahistine mesilate tablets 敏使朗135 感冒通氯芬黄敏片Compound Diclofenac Sodium Chlorphenamine Maleate;Compound Diclofenac Sodiu136 解热止痛散阿咖酚散Paracetamol137 钙素母维磷葡钙Weiling vitamins and calcium gluconate138 TMP 甲氧苄啶tmp;Trimethoprim;SMZ-TMP139 紫药水甲紫溶液Gamma Hexachlorocyclohexane;liquor methylis violacei;Methylrosanilinium Chloride Solution140 西地碘含片cydiodine buccal tablets;CydiodineTablets;Erythromycin 华素片141 甲红霉素克拉霉素Carat enzyme 诺邦/甲力/澳扶安142 美他环素methacycline;metacycline;mths;Metacycine 欣静143 氨苄西林Ampicillin安必治/安必先144 阿莫西林克拉维酸钾amoxicillin and clavulanate potassium 君尔清145 司帕沙星Sparfloxacin;SFX;effect of sparfloxacin;arfloxacin 世保扶/利贝尔/森澳欣146 左氧氟沙星LVFX;lev;Levofloxacin;Levaquin 左克/乐朗/特夫比克147 莫西沙星Moxifloxacin;vigamox;MXFX;moxifloxacin hydrochloride 拜复乐148 加替沙星Gatifloxacin;gat 海超149 盐酸富桂利嗪胶囊Rich Cinnarizine Capsules hydrochloride 西比灵150 格列吡嗪Glipizide;glip 瑞易宁/迪沙/美吡达151 辛伐他汀片Simvastatin Tablets;Simvastatin 舒降之152 复方眀片/SMZ 复方磺胺甲恶唑片Compound Sulfamethoxazde Tablets;COSMZ;Compound Sulfamethoxazole Tablets153 舒乐安定艾司唑仑estazolam;Estajolam154 鲁米那苯巴比妥phenobarbitone;barbivis;Luminal;pheno155 速效伤风胶囊氨咖黄敏胶囊paracetamol,caffein,artificial cow-bezoar and chorphenamine maleate capsules156 林可霉素利多卡因凝胶Forest enzyme lidocaine gel 绿药膏157 婴儿素婴儿健脾散158 复方酚咖伪麻胶囊力克舒159 扑感敏酚氨咖敏片160 复方谷氨酰胺肠溶胶囊谷参肠安161 复方氨基酸螯合钙胶囊乐力162 复方利血平氨苯蝶啶片北京0号163 止血敏酚磺乙胺etamsylate164 扑尔敏马来酸氯苯那敏片chlorpheniramine maleate tablets;Chlorphenamine Maleate Tablets;CHLORPHENIRAMINE MALEATE TAB 4MG165 黄药水利凡诺溶液Rivanol solution166 红药水汞溴红溶液Merbromin Solution167 维乐生三维B片Trivitamins B Tablets168 ORS 口服补液盐oral rehydration salts169 GS 葡萄糖注射液glucose injection;dextrose injection;injection glucosi;and glucose injection170 GNS 葡萄糖氯化钠注射液sodium chloride and dextrose injection;glucosesaline;Glucose and Sodium Chloride Injection171 NS 氯化钠注射液sodium chloride injection;and sodium chloride injection;chloride injection;lv hua na zhu she ye172 RFP 利福平rifampicin;RIF;rifampin;RFP173 雷米封/INH 异烟肼isoniazid;INH;isonicotinic acid hydrazide;isoniazide174 DXM 醋酸地塞米松Dexamethasone Acetate;egocort;dexamethason acetate;dexamethason175 PP粉高锰酸钾potassium permanganate;potassium hypermanganate;mineral chameleon;permanganate176 乙醇酒精alcohol;spirit;ethanol;alcoholic177 碘酒碘伏iodopho178 双氧水过氧化氢溶液hydrogen peroxide solution179 炎德平穿心莲片andrographis tablet180 170胃痛片铋镁豆蔻片181 消心痛硝酸异山梨酯片Isosorbide Dinitrate Tablets182 盐酸多虑平盐酸多塞平doxepin hcl183 颅痛定罗痛定184 舒喘灵盐酸沙丁胺醇185 长效舒喘灵硫酸特布他林terbutaline sulfate 博利康尼186 甲氰咪胍西咪替丁cimetidine 泰胃美187 OMZ 奥美拉唑Omeprazole 彼司克/塞洛克188 干酵母食母生Saccharomyces Siccum189 654-2 消旋山莨菪碱Racanisodamine190 消胀片二甲硅油片Dimethicone191 拉米夫定Lamivudine 贺普丁192 卡马西平carbamazepine 得理多193 泮托拉唑钠PantoprazoleSodium 泰美尼克/泮立苏194 潘生丁双嘧达莫片195 双克/双氢克尿噻氢氯噻嗪Hydrochlorothiazide196 速尿呋塞米furosemide197 核黄素维生素B2 vitamin B₂198 胃舒平复方氢氧化铝Compound Aluminium Hydroxide199 滴鼻净盐酸萘甲唑啉滴鼻液200 复方芦丁复方芦丁片Compound Rutin Tablets 明珠欣/丽珠威201 万乃洛韦泛昔洛韦Famciclovir202 竹林胺盐酸酚苄明Phenoxybenzamine hydrochloride203 氨酰心安阿替洛尔atenolol204 安体舒通螺内酯片Spironolactone Tablets205 洁霉素林可霉素Lincomycin206 氯洁霉素克林霉素clindamycin207 普乐安前列康208 强的松醋酸泼尼松prednisone acetate209 维生素K3 亚核酸氢钠甲苯酸Sub nucleic acid hydrogen sodium benzoic acid210 羟氨苄青霉素阿莫西林Amoxicillin211 美多心安酒石酸美托尔倍他乐克212 蒙脱石散Montmorillonite Powder 思密达/必奇213 化痰片羧甲司坦片Carbocisteine Tablets214 复方降压胶囊复方地巴唑氢氯噻嗪胶囊Compound Bendazol and Hydrochlorlthiazide Capsules215 三苯氧胺他莫西芬tamoxifen216 头孢三嗪注射用头孢曲松钠Ceftriaxone Sodium for Injection 菌必治217 罗红霉素erythromycin 压力稀/严迪218 头孢克洛Cefaclor 希刻劳/新达罗219 氯化钾缓释片Potassium Chloride Sustained-release Tablets 补达秀/乐甲220 布洛芬Ibuprofen 芬必得/美林221 病毒灵/AOBO 盐酸吗啉胍片Moroxydine Hydrochloride Tablets222 盐酸氨溴索片ambroxol hydrochloride tablets 沐舒坦223 双氯灭痛双氯芬酸钠diclofenac sodium 扶他林224 维U颠茄铝胶囊斯达舒225 普乐对乙酰氨基酚颗粒paracetamol granules。
USP 通用章节目录

USP29-通用章节指导目录(附录)Guide to General Chapters 通用章节指导目录中此颜色并且带有“***”的为新增内容。
General Requirements for Test and Assays检查与含量分析的一般要求<1>INJECTIONS……2455注射剂<11>USP REFERENCE STANDARDS……2458USP对照品Apparatus for Test and Assays用于检查与含量分析的器具<16>AUTOMATED METHODS OF ANAL YSIS……2491自动化分析方法<21>THERMOMETERS……2497温度计<31>VOLUMETRIC APPARATUS……2497容量器具<41>WEIGHTS AND BALANCES……2499砝码与天平Microbiological Tests 微生物检查法<51>ANTIMICROBIAL EFFECTIVENESS TESTING……2499抗菌剂有效性检查法<55>BIOLOGICAL INDICATORS—RESISTANCE PERFORMANCE TESTS (2501)生物指示剂-耐药性实验<61>MICROBIAL LIMIT TESTS……2503微生物限度检查法<71>STERILITY TESTS……2508无菌检查法Biological tests and assays生物检查法与测定法<81>ANTIBIOTICS—MICROBIAL ASSAYS……2513抗生素-微生物测定<85>BACTERIAL ENDOTOXINS TEST……2521细菌内毒素检查法<87>BIOLOGICAL REACTIVITY TESTS, IN VITRO……2525体外的生物反应性检查法<88>BIOLOGICAL REACTIVITY TESTS, IN VIVO……2526体内的生物反应性检查法<91>CALCIUM PANTOTHENATE ASSAY……2530泛酸钙测定法<111>DESIGN AND ANAL YSIS OF BIOLOGICAL ASSAYS……2531 生物测定法的设计与分析<115>DEXPANTHENOL ASSAY……2543右泛醇(拟胆碱药)测定法<121>INSULIN ASSAYS……2544胰岛素测定法<141>PROTEIN—BIOLOGICAL ADEQUACY TEST……2546蛋白质-生物适应性试验<151>PYROGEN TEST……2546热原检查法<161>TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES (2547)输血输液用具以及相类似的医疗器械<171>VITAMIN B12 ACTIVITY ASSAY……2548维生素B12活性测定法Chemical Tests and assays化学实验与测定法<181>IDENTIFICATION—ORGANIC NITROGENOUS BASES (2549)鉴别-有机氮碱?<191>IDENTIFICATION TESTS—GENERAL……2550鉴别实验-通用<193>IDENTIFICATION—TETRACYCLINES……2551鉴别-四环素<197>SPECTROPHOTOMETRIC IDENTIFICATION TESTS......2552分光光度计鉴别实验<201>THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST.. (2553)薄层色谱鉴别实验Limit Test 限度检查法<206>ALUMINUM……2554铝<211>ARSENIC……2554砷<221>CHLORIDE AND SULFATE……2555氯和硫<223>DIMETHYLANILINE……2555二甲基苯胺<226>4-EPIANHYDRO-TETRACYCLINE……25564-?-四环素<231>HEA VY METALS……2556重金属<241>IRON……2557铁<251>LEAD……2558铅<261>MERCURY……2558汞<271>READIL Y CARBONIZABLE SUBSTANCES TEST……2560易碳化物检查法<281>RESIDUE ON IGNITION……2560灼烧残渣<291>SELENIUM……2560硒Other Tests and Assays 其它检查法与测定法<301>ACID-NEUTRALIZING CAPACITY……2561酸中和容量<311>ALGINATES ASSAY……2562藻酸盐测定法<331>AMPHETAMINE ASSAY……2562苯丙胺测定法<341> ANTIMICROBIAL AGENTS—CONTENT……2563 抗菌剂-含量<345> Assay for Citric Acid/Citrate and Phosphate……2565 柠檬酸/柠檬酸盐和磷酸盐的测定<351>ASSAY FOR STEROIDS……2565类固醇(甾类化合物)测定法<361> BARBITURATE ASSAY……2565 巴比妥类药物测定法<371>COBALAMIN RADIOTRACER ASSAY……2566钴铵素放射性跟踪剂测定法<381>ELASTOMERIC CLOSURES FOR INJECTIONS……2567 注射剂的弹性密封件<391>EPINEPHRINE ASSAY……2567肾上腺测定法<401>FATS AND FIXED OILS……2568脂肪与混合油<411>FOLIC ACID ASSAY……2571叶酸测定法<425>IODOMETRIC ASSAY—ANTIBIOTICS……2572碘量检查法-抗生素<429>LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE (2572)粒子尺寸的光衍射测量<431>METHOXY DETERMINA TION……2575甲氧基测定法<441>NIACIN OR NIACINAMIDE ASSAY……2576烟酰或烟酰胺测定法<451>NITRITE TITRATION……2578亚硝酸盐滴定<461>NITROGEN DETERMINA TION……2578氮测定法<466>ORDINARY IMPURITIES……2579一般杂质<467>ORGANIC VOLATILE IMPURITIES……2580有机的易挥发杂质<471>OXYGEN FLASK COMBUSTION……2590氧瓶燃烧法<481>RIBOFLAVIN ASSAY……2590核黄素测定法<501>SALTS OF ORGANIC NITROGENOUS BASES……2591有机氮盐<511>SINGLE-STEROID ASSAY……2591单一的类固醇测定法<521>SULFONAMIDES……2592磺胺制剂<531>THIAMINE ASSAY……2593硫胺素测定法<541>TITRIMETRY……2593滴定法<551>ALPHA TOCOPHEROL ASSAY……2596α-维生素E测定法<561>ARTICLES OF BOTANICAL ORIGIN……2596植物起源的药品<563>IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN……2603植物药品的鉴别<565>BOTANICAL EXTRACTS……2609植物提取<571>VITAMIN A ASSAY……2611维生素A的测定法<581>VITAMIN D ASSAY……2612维生素D的测定法<591>ZINC DETERMINATION……2616锌的测定法Physical Test and Determinations物理检查与测定法INHALERS, AND DRY POWDER <601>AEROSOLS, NASAL SPRAYS,USP28METERED-DOSEINHALERS……2617气溶胶,鼻用喷雾剂,定量吸入器与干粉吸入器<611>ALCOHOL DETERMINATION……2637乙醇测定法<616>BULK DENSITY AND TAPPED DENSITY……2638堆密度与拍实密度<621>CHROMATOGRAPHY…….2639色谱法<631>COLOR AND ACHROMICITY……2651呈色与消色<641>COMPLETENESS OF SOLUTION……2652完全溶解<643>TOTAL ORGANIC CARBON……2652总有机碳<645>WA TER CONDUCTIVITY……2653水电导率<651>CONGEALING TEMPERA TURE……2654凝点温度<661>CONTAINERS……2655容器<671>CONTAINERS—PERMEATION……2663容器-渗透<691>COTTON……2664棉花<695>CRYSTALLINITY……2665结晶性<696>Crystallinity Determination By Solution Calorimetry……2666 通过溶液量热学测定结晶性<698>DELIVERABLE VOLUME……2667可转移的体积<699>DENSITY OF SOLIDS……2669固体密度<701>DISINTEGRATION……2670崩解时限***<701>Disintegration (Harmonized Chapter, Official April 1,2006)………..2671崩解时限(协调的章节,法定日期,2006.4.1)<711>DISSOLUTION……2673 溶出度***<711>Dissolution (Harmonized Chapter, Official April 1,2006)………..2675 溶出度(协调的章节,法定日期,2006.4.1)<721>DISTILLING RANGE……2682馏程<724>DRUG RELEASE……2682药物释放度***<724>Drug releasee (Harmonized Chapter, Official April 1,2006)………..2690药物释放度(协调的章节,法定日期,2006.4.1)<726>ELECTROPHORESIS……2694电泳<727>CAPILLARY ELECTROPHORESIS……2696毛细管电泳法***<730>Plasma Spectrochemistry….2700 血浆光谱化学<731>LOSS ON DRYING……2704干燥失重<733>LOSS ON IGNITION……2704灼烧失重<736>MASS SPECTROMETRY……2705 质谱<741>MELTING RANGE OR TEMPERATURE……2708熔距或熔点<751>METAL PARTICLES IN OPHTHALMIC OINTMENTS……2709眼用软膏中的金属粒子<755>MINIMUM FILL……2710最低装填量<761>NUCLEAR MAGNETIC RESONANCE……2710核磁共振<771>OPHTHALMIC OINTMENTS……2715眼用软膏<776>OPTICAL MICROSCOPY……2716光学显微镜<781>OPTICAL ROTATION……2718旋光<785>OSMOLALITY AND OSMOLARITY……2718同渗重摩与同渗容摩<786>PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANAL YTICAL SIEVING (2720)通过筛分法估算粒子分布<788>PARTICULATE MATTER IN INJECTIONS……2722注射剂中的颗粒<789>PARTICULATE MATTER IN OPHTHALMIC SOLUTIONS……2729眼用溶液中的颗粒<791>pH (2730)<795>PHARMACEUTICAL COMPOUNDING—NONSTERILE PREPARATIONS (2731)药物混合-非无菌制剂<797>PHARMACEUTICAL COMPOUNDING—STERILE PREPARATIONS (2735)药物混合-无菌制剂<801>POLAROGRAPHY……2752极谱法<811>POWDER FINENESS……2754粉剂细度<821>RADIOACTIVITY……2755放射性<823>RADIOPHARMACEUTICALS FOR POSITRON EMISSION TOMOGRAPHY —COMPOUNDING……2763用于正电子发射断层摄影术的放射性药物<831>REFRACTIVE INDEX……2766折光率<841>SPECIFIC GRA VITY……2766比重<846>SPECIFIC SURFACE AREA……2767 比表面积<851>SPECTROPHOTOMETRY AND LIGHT-SCA TTERING……2770分光光度计与光散射<861>SUTURES—DIAMETER…2775缝线-直径<871>SUTURES—NEEDLE ATTACHMENT……2775缝线-穿孔实验<881>TENSILE STRENGTH…..2776张力<891>THERMAL ANAL YSIS……2776热分析<905>UNIFORMITY OF DOSAGE UNITS……2778制剂单位的含量均匀度<905>UNIFORMITY OF DOSAGE UNITS (Harmonized Chapter, Official April 1,2006)……2780制剂单位的含量均匀度(协调的章节2006.4.1)<911>VISCOSITY……2785粘度<921>WA TER DETERMINA TION……2785水测定法<941>X-RAY DIFFRACTION……2788X光衍射General Information通用信息<1010>ANAL YTICAL DATA—INTERPRETA TION AND TREATMENT (2790)分析数据-解释与处理<1015>AUTOMA TED RADIOCHEMICAL SYNTHESIS APPARATUS (2801)放射性自动合成装置<1031>THE BIOCOMPATIBILITY OF MATERIALS USED IN DRUG CONTAINERS, MEDICAL DEVICES, AND IMPLANTS (2802)用于药物容器、医疗设施和植入剂的材料的生物相容性<1035>BIOLOGICAL INDICATORS FOR STERILIZATION……2811灭菌用生物指示剂<1041>BIOLOGICS……2814生物制剂***<1043>Ancillary Material for Cell, Gene, and Tissue-Engineered Products…….2814 细胞,基因与组织设计产品的辅助材料<1045>BIOTECHNOLOGY-DERIVED ARTICLES……2821生物技术提取产品<1046>CELL AND GENE THERAPY PRODUCTS……2831细胞与基因治疗产品<1047>BIOTECHNOLOGY-DERIVED ARTICLES—TESTS……2858生物技术产品-检查法<1048>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANAL YSIS OF THE EXPRESSION CONSTRUCT IN CELLS USED FOR PRODUCTION OF r-DNA DERIVED PROTEIN PRODUCTS1 (2883)生物产品质量:从蛋白质产品中提取的r-DNA产品在细胞中表达结构的分析<1049>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS1 (2884)生物技术产品的质量:生物技术/生物产品的稳定性实验<1050>VIRAL SAFETY EV ALUA TION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMAL ORIGIN (2887)从人或动物细胞中提取的生物技术产品的病毒安全性评估<1051>CLEANING GLASS APPARATUS……2896玻璃容器的清洗<1061>COLOR—INSTRUMENTAL MEASUREMENT……2896显色-仪器测量***<1065>Ion Chromatography………2898 离子色谱法<1074>EXCIPIENT BIOLOGICAL SAFETY EV ALUA TION GUIDELINES (2900)赋形剂(辅料)生物安全性评估指导<1075>GOOD COMPOUNDING PRACTICES……2903好的混合操作<1078>GOOD MANUFACTURING PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS (2906)批药品赋形剂的生产管理规范***<1079>Good Storage and Shipping Practices……2915 良好的贮存与船运规范<1081>GEL STRENGTH OF GELATIN……2920白凝胶的凝胶强度<1086>IMPURITIES IN OFFICIAL ARTICLES……2920药典物品中的杂质<1087>INTRINSIC DISSOLUTION……2923内部的溶出度<1088>IN VITRO AND IN VIVO EV ALUA TION OF DOSAGE FORMS (2924)体内与体外的剂型的评估<1090>IN VIVO BIOEQUIV ALENCE GUIDANCES……29291体内生物等效性指导<1091>LABELING OF INACTIVE INGREDIENTS……2968非活性成分的标示<1101>MEDICINE DROPPER……2969医用滴管<1111>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE PHARMACEUTICAL PRODUCTS (2969)非无菌药品中的微生物分布<1116>MICROBIOLOGICAL EV ALUA TION OF CLEAN ROOMS AND OTHER CONTROLLED ENVIRONMENTS……2969洁净的房间与其它可控环境的微生物评估<1118>MONITORING DEVICES—TIME, TEMPERATURE, AND HUMIDITY (2976)监控装置-时间、温度与湿度<1119>NEAR-INFRARED SPECTROPHOTOMETRY……2979近红外分光光度测定法***<1120>Raman Spectrophotometry……..2983 Raman分光光度测定法<1121>NOMENCLATURE……2988命名***<1136>Packaging-Unit-of-Use……2989包装-单元使用<1146>PACKAGING PRACTICE—REPACKAGING A SINGLE SOLID ORAL DRUG PRODUCT INTO A UNIT-DOSE CONTAINER……2990 包装操作-将单一固体口服药品产品再包装成单元剂量<1150>PHARMACEUTICAL STABILITY……2994药物稳定性<1151>PHARMACEUTICAL DOSAGE FORMS……2996药物剂型<1160>PHARMACEUTICAL CALCULATIONS IN PRESCRIPTION COMPOUNDING (3006)按处方混合的药物的计算<1171>PHASE-SOLUBILITY ANAL YSIS……3016相溶解分析***<1174>Powder Flow….3017 粉末流动性<1176>PRESCRIPTION BALANCES AND VOLUMETRIC APPARATUS….3020 处方天平与容量器具***<1177>Good Packaging Practices….3021 良好的包装操作***<1178>Good Repackaging Practices….3023 良好的再包装操作<1181>SCANNING ELECTRON MICROSCOPY……3025扫描电子显微镜<1191>STABILITY CONSIDERATIONS IN DISPENSING PRACTICE……3029 分装操作中稳定性考察<1196>PHARMACOPEIAL HARMONIZATION……3031药典的一致性<1207>STERILE PRODUCT PACKAGING—INTEGRITY EV ALUATION (3035)无菌产品包装-完整性评估<1208>STERILITY TESTING—V ALIDATION OF ISOLATOR SYSTEMS (3037)无菌实验-隔离系统的验证<1209>STERILIZATION—CHEMICAL AND PHYSICOCHEMICAL INDICATORS AND INTEGRATORS……3040灭菌-化学与物理化学的指示剂以及二者的综合<1211>STERILIZATION AND STERILITY ASSURANCE OF COMPENDIAL ARTICLES (3041)药典物品中的灭菌与灭菌保证<1216>TABLET FRIABILITY……3046片剂的脆碎度<1221>TEASPOON……3047茶匙<1222>TERMINALL Y STERILIZED PHARMACEUTICAL PRODUCTS—PARAMETRIC RELEASE……3047最终灭菌产品-放行参数<1225>V ALIDATION OF COMPENDIAL METHODS……3050药典方法的验证<1227>V ALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES (3053)从药物中回收微生物的验证<1230>W ATER FOR HEALTH APPLICATIONS……3055健康用水<1231>W ATER FOR PHARMACEUTICAL PURPOSES……3056制药用水<1241>W ATER–SOLID INTERACTIONS IN PHARMACEUTICAL SYSTEMS (3074)在药物系统中水与固体的相互作用<1251>WEIGHING ON AN ANAL YTICAL BALANCE……3076关于分析天平的称重***<1265>Written Prescription Drug Information-Guidelines……….3078 书面的处方药信息-指南Dietary Supplements营养补充剂General Tests and Assays 一般检查法与测定法<2021>MICROBIAL ENUMERATION TESTS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3080)微生物数量实验-营养与食品添加剂<2022>MICROBIOLOGICAL PROCEDURES FOR ABSENCE OF SPECIFIED MICROORGANISMS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3083)不得检出特定微生物的程序-营养与营养补充剂<2023>MICROBIOLOGICAL A TTRIBUTES OF NONSTERILE NUTRITIONAL AND DIETARY SUPPLEMENTS……3087非无菌的营养与食品添加剂中的微生物分布<2040>DISINTEGRATION AND DISSOLUTION OF DIETARY SUPPLEMENTS (3089)食品添加剂的崩解与溶出<2091>WEIGHT VARIATION OF DIETARY SUPPLEMENTS……3092食品添加剂的重量差异<2750>MANUFACTURING PRACTICES FOR DIETARY SUPPLEMENTS (3093)食品添加剂的生产操作。
(仅供参考)聚维酮简介
形成了多 孔的三维 结构,均 匀的粒径
分布
受压力而 产生形变 并保存应
力
遇到水时, 通过毛细 管效应, 水分穿过
孔隙
湿颗粒释 放应力而
膨胀
对周围环 境施加能 量使得片 剂破裂, 从而进一 步吸收水
分
片剂崩解
颗粒良好的流动 性保证了其在片 芯中的均匀分布
更高的生产效 率和稳定的片
重
Polyplasdone膨胀与凝胶化
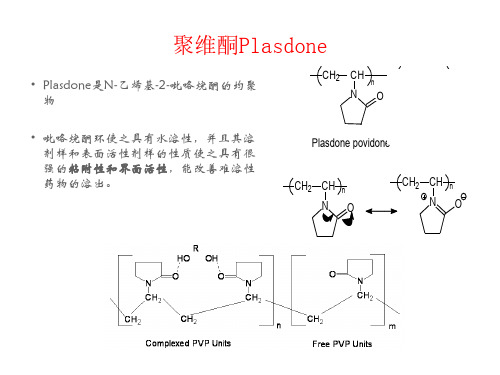
聚维酮Plasdone
• Plasdone是N-乙烯基-2-吡咯烷酮的均聚 物
• 吡咯烷酮环使之具有水溶性,并且其溶 剂样和表面活性剂样的性质使之具有很 强的粘附性和界面活性,能改善难溶性 药物的溶出。
CH2
CH n
NO
CH2
CH m
O CH3
C
O Plasdone povidone
CH2 CH n NO
通常,较快的搅拌/剪切需要较少的水和可适应较高的粘度
Plasdone 在制药领域的主要应用
• 高效粘合剂 • 干法制粒或直接压片粘合剂 • 成膜剂 • 增稠剂 • 混悬剂稳定剂 • 增溶剂:口服溶液剂,软胶囊、 眼用制剂等 • 络合剂:如聚维酮碘 • 某些活性成份的稳定剂 • 制备固体溶液或固体分散剂
过氧化物标准 (PPM)
<30 <400 <50 <400
Polyplasdone特性
湿法制粒,干 法制粒和直接 压片处方中快
速崩解
促进离子型药 物和难溶性药
物的溶出
低用量时提供良 好崩解效果,特 别适合高剂量处
方
良好的可压性, 从而使得片剂具 有较高的硬度和 较低的脆碎度, 适合高速压片机
USP34标准——硫酸软骨素钠

霉菌 、酵母菌 总数 ≤10cug 0 f/; 沙门菌、大肠埃希杆菌 不得 检出。
p H: 本 品 1 液 的p 应 为55 7 。 %溶 H .4 . 5
干 燥 失 重 : 取 本 品 ,在 1 5o 燥4h 0 C干 ,减 失 重 量 不 得 2 。确 保 薄 膜 的两 端 至 少 各 有 05 1 m浸 泡 入 缓 冲 液 .4 . c 0
键 交替 连接 。在 普遍 的糖 胺聚糖 中 ,半 乳糖 胺部 分主要 为 氯 化十六烷基吡啶溶 液 ( 0mgmL),混匀 ,过 滤。取2 3 / 5 4位单硫酸盐 ,较 少为6位 。按干燥 品计算 ,含硫酸软骨素 mL 液检测 ,与00 0mo/ 硫 酸溶液02  ̄ 成 的对照 一 . 滤 .2 l L .5mL l J
包 装 、贮 存 :保 存 于 密 封 容 器 。
电泳 纯 度 : [US (2 ) 电 泳法 ) ( P 76 ]
0 1 l 醋酸钡缓冲 液 (H .)的制备 :取醋酸 钡 . mo/ L p 50
标 签 : 应 标 明 本 品 的 来 源 , 是 提 取 自牛 、 猪 还 是 禽 的 约 2 . ,加 水 溶 解 并稀 释 "9 0mL,用 醋 酸 调 p 50 52 g 4  ̄ 0 H ., 软 骨 组 织 , 或者 是它 们 的 混合 物 。 US P对 照 品 :US P硫 酸 软 骨 素 钠 对 照 品 。 加 水 稀 释 至 1 ,混 匀 。 L 着 色 剂 :01 甲苯 胺 蓝 醋 酸 溶 液 ;取 甲苯 胺 蓝 1g . % ,加
测 。置 1c m的吸收池 内,在4 0l 2 m波长处检测 吸光度 ,以 钠 对 照 品水 溶 液 。 " l
新沸过 的冷 水为对照。吸光度不得大于0 5 .。 3
USP34标准——硫酸软骨素钠

电泳纯度:
[ ( U SP ( 72 6 ) 电泳法 ) 2 的制 备: 取醋酸钡
0 . 1 mo / 醋酸钡缓冲液 ( p H 5 0 l L )
约2 .24 9 . 加水溶解并稀释至9 0 mL , 用 醋酸调pH 至5 0 , 5 0 加水稀释至 I L , 混匀 " 着色剂: 0. 1% 甲苯胺蓝醋酸溶液 ; 取 甲苯胺 蓝1 9, 加 0. 1 mo l/L 西 酸 10 00 mL 使溶解 " 昔 标准 溶液 l : 制 备浓度为3 0 mg mL 的u s P 硫 酸软骨素 / 钠对照 品水溶液 " 标准溶液2 : 取标 雕溶液 1 mL , 加 水稀 释至5 0
mg mL ) , 混匀 , 过滤 " 取2 / 5
mL 滤液检测 , 与0 .0 2 0 mo 几硫酸溶 液0 .2 5 m L 制成的对照 l 液比较, 不得更浓 : 不得过0 .24 % "
重 金 属 : 照 USP ( 2 3 l ) H 法测定 , 不得 过 0 ,00 % " 2
=注意 : 硫 酸软骨素钠具 有很强的引湿性 , 应避 免暴 露 于空气 中, 称 量应迅速 " > 包装 ! 贮存 : 保存于密封容器 " 标签 : 应标 明本品的来源 , 是提取 自牛 ! 猪还是 禽的 软骨组织 , 或者是它们的混合物 " USP 对 照品: U SP 硫酸软份素钠对照 品 " 溶液 的澄 清度 和颜 色 : 取 本 品2 .5 9 , 置5 0 mL 量 瓶 中 , 用 新沸 过 的冷 水 溶解 并稀 释 至刻 度 , 摇匀 后立 即检 测 " 置 1 cm 的吸收池 内, 在4 2 0 nm 波长处检测吸光度 , 以 新沸过 的冷水为对照 " 吸光度不得大于住3 " 5 鉴别 : A . 本 品的红 外光吸收 图谱应 与硫 酸软骨素钠
USP美国药典70种农残检测限度

26
硫丹
Endosulfan (sum of isomers and endosulfan sulfate)
3
27
异狄氏剂
Endrin
0.05
28
乙硫磷
Ethion
2
பைடு நூலகம்29
乙嘧硫磷
Etrimphos
0.05
30
皮蝇硫磷
Fenchlorophos (sum of fenchlorophos and fenchlorophos-oxon)
1.5
36
氟氰戊菊酯
Flucytrinate
0.05
37
氟胺氰菊酯
τ-Fluvalinate(Tau-Fluvalinate (F) )
0.05
38
地虫硫磷
Fonophos
0.05
39
七氯
Heptachlor (sum of heptachlor, cis-heptachlorepoxide and trans-heptachlorepoxide)
3
除虫菊酯
Pyrethrins
65
喹硫磷
Quinalphos
0.05
66
五氯硝基苯
Quintozene (sum of quintozene, pentachloraniline and methyl penthachlorphenyl sulfide)
1
67
八氯二丙醚
S-421
0.02
68
四氯硝基苯
Bromide, inorganic (calculated as bromide ion)
50
7
乙基溴硫磷
高效液相色谱法测定聚维酮碘中的总碘含量

摘要:目的 建立高效液相色谱法测定聚维酮碘中总碘含量的方法。 方法 以 Partisil SAX鄄10(4. 6 mm伊250 mm) 柱为固定相;流动相为 0. 09 mol / L 磷酸二氢钾鄄甲醇(体积比 9 颐1) ,用磷酸调 pH3. 3;流速:1. 0 mL / min;检测波长:223 nm。 结果 碘离子在 3. 505 ~ 17. 52 滋g / mL 范围内,峰面积与浓度呈良好的线性关系( r = 0. 9999,n = 5) 。 平均回收率为 99. 3% ,RSD = 1. 4% 。 结论 本方法简便、准确,可用于 聚维酮碘的质量控制。 关键词:高效液相色谱法;聚维酮碘;碘;测定 中图分类号:R 927. 2摇 文献标识码:A摇 文章编号:1006-8783(2006)01-0048-03
第 1 期摇 胡家炽,等. 高效液相色谱法测定聚维酮碘中的总碘含量
消失,加水稀释至刻度,摇匀。 2. 2摇 含量测定 2. 2. 1摇 色谱条件摇 色谱柱:Partisil SAX—10(4. 6 mm伊 250 mm) ;流动相:0. 09 mol / L 磷酸二氢钾鄄甲醇( 体积 比 9 颐1) ,用磷酸调 pH3. 3;流速:1. 0 mL / min;检测波 长:223 nm。 2. 2. 2摇 测定方法摇 精密量取对照品溶液与供试品溶 液各 10 滋L,分别注入液相色谱仪,记录色谱图,按外标 法以峰面积计算碘离子的含量,即得。 2. 3摇 方法验证 2. 3. 1摇 标准曲线摇 精密量取碘化钾对照品贮备液 5. 00、10. 00、15. 00、20. 00、25. 00 mL,分别 置 于 100 mL 的量瓶中,用水稀释至刻度,摇匀。 各精密量取 10 滋L 注入液相色谱仪,记录色谱图,以碘离子峰面积( A) 为 横坐标,相应的质量浓度( 籽) 为纵坐标作图,求得一元 线性回归方程:籽 = 1. 833 伊10-5 A-0. 0490, r = 0. 9999( n = 5) 。 可见碘离子在 3. 505 ~ 17. 52 滋g / mL 浓度范围 内,其峰面积与浓度呈良好的线性关系。 2. 3. 2摇 重复性试验摇 精密量取碘化钾对照品溶液 10 滋L,连续进样 6 次,结果碘离子峰的平均响应值为 1. 7881伊10-5 ,RSD = 0. 6% 。 2. 3. 3摇 加样回收试验摇 取已测定碘含量的聚维酮碘 约 200 mg,精密称定,置 500 mL 量瓶中,精密加入碘化 钾对照品一定量,按供试品溶液的制备项下,自“ 加水 适量……冶 起同法操作,测定,按加样回收计算回收 率,结果见表 1。 2. 3. 4摇 样品测定摇 按本文方法与药典方法[2] ,分别测 定 3 批样品,结果见表 2。 2. 3. 5摇 溶液稳定性试验摇 测定含量的样品溶液,室温 放置 24 h 后重测,前后结果相差在 依1% 内,表明样品 溶液室温放置 24 h 稳定。
USP34-细菌内毒素《85》

REAGENTS AND TEST SOLUTIONS 试剂和测试溶液Amoebocyte Lysate— A lyophilized product obtained from the lysate of amoebocytes (white blood cells) from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus). This reagent refers only to a product manufactured in accordance with the regulations of the competent authority. [NOTE—Amoebocyte Lysate reacts to some-glucans in addition to endotoxins. Amoebocyte Lysate preparations that do not react to glucans are available: they are prepared by removing the G factor reacting to glucans from Amoebocyte Lysate or by inhibiting the G factor reacting system of Amoebocyte Lysate and may be used for endotoxin testing in the presence of glucans. ] 变形细胞溶解液—由鲎的〔白血细胞〕变形细胞溶解液制得的冻干产品。
该试剂仅指那些按照相关的监管机构的法规要求制得的产品。
[注:变形细胞溶解液除了与内毒素反响外,还会与某些-葡聚糖反响。
可以制成不与-葡聚糖发生反响的变形细胞溶解液制品:可通过从变形细胞溶解液中去除会与葡聚糖反响的G因子或抑制变形细胞溶解液的G因子反响系统来制得该制品,这些制品可用于在存在葡聚糖时进行内毒素检测。
仿制药参比制剂目录(第六十三批)
欧盟上市
63-27
匹伐他汀钙片
Pitavastatin Caical Europe GmbH
未进口原研药品
欧盟上市
63-28
匹伐他汀钙片
Pitavastatin Calcium Tablets
2mg
Kowa Pharmaceutical Europe GmbH
未进口原研药品
增加持证商Angelini Pharma Česká Republika s.r.o.
27-423
左甲状腺素钠片
Levothyroxine Sodium Tablets/Euthyrox;Levothyrox
100μg(以左甲状腺素钠计)
Merck Serono GmbH/Merck Sante/Merck GesellschaftmbH/Merck Healthcare Germany GmbH
100ml:1g(10mg/ml)
B Braunmedical Inc
未进口原研药品
美国橙皮书
63-14
钆特醇注射液
Gadoteridol Injection
/ProHance
279.3mg/mL(1.3965 g/5mL)
Bracco Diagnostics Inc
未进口原研药品
美国橙皮书
63-15
63-241
依折麦布瑞舒伐他汀锌胶囊
Ezetimibe rosuvastatin zinc hard capsule/Cholecomb
20mg/10mg
Proterapia Hungary Ltd
未进口原研药品
欧盟上市
63-251
盐酸氨酮戊酸凝胶
aminolevulinic acid hydrochloride gel/AMELUZ
USP美国药典代码

80%双(3-氰基丙基)-20%3-氰丙基苯聚硅氧烷(百分比参见分子取代度)
Silar 9CP
G9
Methylvinylpolysiloxane.
甲基乙烯基聚硅氧烷
UC W982
G10
Polyamide formed by reacting a C36 dicarboxylic acid with 1,3-di-4-piperidylpropane and piperidine in the respective mole ratios of 1.00:0.90:0.20.
25%苯基-75%甲基聚硅氧烷
DC550
G29
3,3?-Thiodipropionitrile.
3,3’-硫代丙晴
TDPN
G30
Tetraethylene glycol dimethyl ether.
四甘醇二甲基醚
Bis[2(2-Methoxyehoxy)ethy] ether
G31
Nonylphenoxypoly(ethyleneoxy)ethanol (av. ethyleneoxy chain length is 30); Nonoxynol 30.
Dexsil 300GC
G34
Diethylene glycol succinate polyester stabilized with phosphoric acid.
聚乙二醇丁二酸酯,用磷酸固化
DEGS Stabilized
G35
A high molecular weight compound of a polyethylene glycol and a diepoxide that is esterified with nitroterephthalic acid.
USP34标准——硫酸软骨素钠

USP34标准——硫酸软骨素钠
张青
【期刊名称】《食品与药品》
【年(卷),期】2012(014)002
【摘要】软骨素,硫酸氢,钠盐[9082-07-9].rn硫酸软骨素钠是从牛、猪、禽等家畜软骨组织中提取制得的硫酸化线形糖胺聚糖钠盐.主要由N-乙酰半乳糖胺(2-乙酰胺-2-脱氧β-D-吡喃半乳糖)和D-葡糖醛酸的共聚物的硫酸酯钠盐组成,共聚物内己糖通过β-1,4及β-1,3糖苷键交替连接.在普遍的糖胺聚糖中,半乳糖胺部分主要为4-位单硫酸盐,较少为6-位.按干燥品计算,含硫酸软骨素钠90.0%~105.0%.
【总页数】2页(P146-147)
【作者】张青
【作者单位】山东博士伦福瑞达制药有限公司,山东济南250101
【正文语种】中文
【相关文献】
1.高效凝胶色谱法同时测定眼用粘弹剂中透明质酸钠和硫酸软骨素钠的含量 [J], 张莉;赵鹏;何涛;刘文博;王敏珠;蓝瑛瑕
2.硫酸软骨素钠含量检测方法的对比研究 [J], 刘浩;于芳
3.三种方法测定鲨鱼硫酸软骨素钠含量的比较 [J], 焦广飞
P34标准——硫酸软骨素钠 [J], 张青
5.浅谈硫酸软骨素钠及其在保健食品中的质量控制 [J], 刘强
因版权原因,仅展示原文概要,查看原文内容请购买。
USP凡例

目录标题 (2)法定以及法定药品 (2)原子量及化学式 (2)缩写 (2)有效数字及限差 (3)需求的解释 (3)总章 (4)药典论坛报 (4)补充 (4)试剂规程 (4)参考制剂 (4)美国药典对照标准品 (4)单位及效能 (5)成分及步骤 (5)试验及分析 (5)装置 (5)异物及杂质 (5)残留溶剂 (6)操作 (6)实验结果、统计及规程 (8)概述 (8)溶解度 (9)可互换的方法 (9)处方及调剂 (9)保存、包装、贮存及贴标签 (9)器皿 (9)毒品预防包装准则 (10)存储温度和湿度 (11)非特异性条件存储 (11)标签 (11)USP-NF专著包装与存储指导说明 (13)植物体和动物体 (13)度量衡 (13)浓度 (14)通则及要求(以下称通则)和总则中的一般要求以概要的形式为USP 中的标准,试验,分析及其他规范提供了一个总体上的指导方针和解释说明,排除了大量的反复引用的恰当实证。
若没有特殊说明的反例,适用于整篇通则及要求。
通则或总章制订之外,个别专著中的措辞表现优越且有明确的指导方向和意图。
强调这些免责条款的存在,通则和总章在某种程度上利用明确且经过认证的条款,如“除非另有说明”。
在个别专著中,不论是否有陈述性的特例,标准,试验,分析及其他规范的特殊说明是同衍生于通则或总章中的条例是相结合的。
标题该出版物的全称包括补录,是美利坚合众国药典-30修正版。
该名称可以缩写为美国药典-30修正版或USP-30。
USP-30修正版替代所有以往的版本。
USP,不经过进一步鉴定,在使用官方药典期间,指USP 30及相关的附录。
同样的标题,不进一步区别,适用于该项下的出版物及电子文档。
法定以及法定药品原子量及化学式计算相对分子质量以及分析中的因素的原子量,是1997年IUPAC委托原子量及同位素丰度推荐使用的。
化学式,与定义、试验和分析中的不同,目的就是提供信息和计算。
特定专著中的格式是这样的,法定标题之后,部分正文的主要信息,缩写有效数字及限差限度以数字的形式表示,因此,最高限及最低限的范围包括这两个数值及其中间的数字,但是不包括限度外面的数字。
PVP
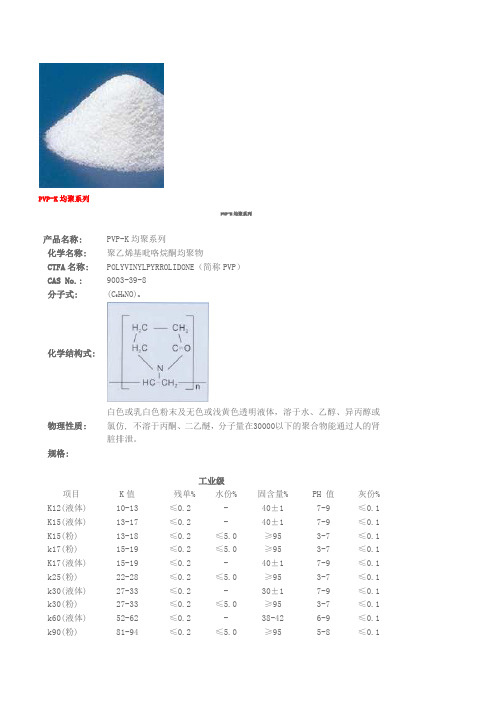
PVP-K均聚系列PVP-K均聚系列产品名称:PVP-K均聚系列化学名称:聚乙烯基吡咯烷酮均聚物CTFA名称:POLYVINYLPYRROLIDONE(简称PVP) CAS No.: 9003-39-8分子式: (C6H9NO)n化学结构式:物理性质:白色或乳白色粉末及无色或浅黄色透明液体,溶于水、乙醇、异丙醇或氯仿, 不溶于丙酮、二乙醚,分子量在30000以下的聚合物能通过人的肾脏排泄。
规格:工业级项目K值残单%水份%固含量%PH 值灰份% K12(液体)10-13≤0.2-40±17-9≤0.1 K15(液体) 13-17≤0.2- 40±17-9≤0.1 K15(粉) 13-18≤0.2≤5.0≥953-7≤0.1 k17(粉) 15-19≤0.2≤5.0≥953-7≤0.1 K17(液体) 15-19 ≤0.2-40±17-9≤0.1 k25(粉) 22-28≤0.2≤5.0≥953-7≤0.1 k30(液体) 27-33≤0.2- 30±17-9≤0.1 k30(粉) 27-33≤0.2≤5.0≥953-7≤0.1 k60(液体)52-62≤0.2-38-426-9≤0.1 k90(粉)81-94≤0.2≤5.0≥955-8≤0.1k90(液体) 81-97≤0.2- 20±16-9≤0.1 K120(液体)110-125≤0.2- 15±17-9≤0.1医药级(CP2005/USP26、USP28 USP30 EP)项目K值残单%水份%灰份%PH值氮含量%醛含量%(以乙醛计)ppm重金属ppm肼ppm过氧化物ppmK15(粉)12-17≤0.1≤5.0≤0.13-711.5-12.8≤500≤10≤1≤40k17(粉) 15-19≤0.1≤5.0≤0.13-711.5-12.8≤500≤10≤1≤40k25(粉) 22-28≤0.1≤5.0≤0.13-711.5-12.8≤500≤10≤1≤40k30(粉) 27-32≤0.1≤5.0≤0.13-711.5-12.8≤500≤10≤1≤40k90(粉)81-94≤0.1≤5.0≤0.15-812.0-12.8≤500≤10≤1≤40应用:化妆品工业:PVPK系列在化妆品工业方向可用作成膜剂,增稠剂,润滑剂及粘合剂,用于喷发剂、摩丝、定发凝胶、定发液;在护肤用品及染发剂、修饰剂、香波、唇膏、除臭剂、防晒剂、牙膏等方面,用作辅助剂。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Pov idone(poe' v i done).(C6H9NO)n2-Pyrrolidinone, 1-ethenyl-, homopolymer;1-Vinyl-2-pyrrolidinone polymer [9003-39-8].DEFINITIONPov idone is a synthetic polymer consisting essentially of linear 1-v inyl-2-pyrrolidinone groups, the degree of polymerization of which results in polymers of v arious molecular weights. The different types of Pov idone are characterized by their v iscosity in aqueous solution, relative to that of water, expressed as a K-value (see Specific Tests, K-value). The K-value of Pov idone hav ing a stated (nominal) K-v alue of 15 or less is NLT 85.0% and NMT 115.0% of the stated v alues. The K-v alue of Pov idone having a stated K-v alue or a stated K-v alue range with an average of more than 15 is NLT 90.0% and NMT 108.0% of the stated value or of the av erage of the stated range.IDENTIFICATION• A. P ROCEDURESample solution: 20 mg/mL of PovidoneAnalysis: To 10 mL of the Sample solution add 20 mL of 1 N hydrochloric acid and 5 mL of potassiumdichromate TS.Acceptance criteria: An orange-yellow precipitate is formed.• B. P ROCEDURESolution A: Dissolv e 75 mg of cobalt nitrate and 300 mg of ammonium thiocyanate in 2 mL of water.Sample solution: 20 mg/mL of PovidoneAnalysis: Combine Solution A and 5 mL of the Sample solution, and render the resulting solution acid by the addition of 3 N hydrochloric acid.Acceptance criteria: A pale blue precipitate is formed.• C. P ROCEDURESample solution: 5 mg/mL of Pov idoneAnalysis: To 5 mL of the Sample solution add a few drops of iodine TS.Acceptance criteria: A deep red color is produced.ASSAY• N ITROGEN D ETERMINA TION, Method II461Sample: 0.1 g of PovidoneAnalysis: Omit the use of hydrogen peroxide; use 5 g of a powdered mixture of potassium sulfate, cupricsulfate, and titanium dioxide (33:1:1) instead of potassium sulfate and cupric sulfate (10:1); heat until a clear, light-green solution is obtained; then heat for an additional 45 min.Acceptance criteria: 11.5%–12.8% on the anhydrous basisIMPURITIESInorganic Impurities• R ESIDUE ON I GNITION281: NMT 0.1%• L EA D251Sample solution: 1.0 g in 25 mL of waterAcceptance criteria: NMT 10 ppmOrganic Impurities• P ROCEDURE 1: L IMIT OF A LDEHYDESSolution A: Transfer 8.3 g of potassium pyrophosphate to a 500-mL v olumetric flask, and dissolv e in 400 mL of water. Adjust, if necessary, with 1 N hydrochloric acid to a pH of 9.0, and dilute with water to volume.Solution B: Transfer a quantity of lyophilized aldehyde dehydrogenase equiv alent to 70 units to a glass vial, anddissolv e in 10.0 mL of water. [NOTE—This solution is stable for 8 h at 4. ]Solution C: Transfer 40 mg of nicotinamide adenine dinucleotide to a glass v ial, and dissolv e in 10.0 mL ofSolution A. [NOTE—This solution is stable for 4 weeks at 4. ]Standard solution: Add 2 mL of water to a glass weighing bottle, and weigh. Add 100 mg (0.13 mL) of freshly distilled acetaldehyde and weigh. Transfer this solution to a 100-mL volumetric flask. Rinse the weighingbottle with sev eral portions of water, transferring each rinsing to the 100-mL v olumetric flask. Dilute thesolution in the 100-mL v olumetric flask with water to v olume. Store at 4 for about 20 h. Pipet 1 mL of this solution into a 100-mL v olumetric flask, and dilute with water to v olume.Sample solution: 20 mg/mL of Povidone in Solution A in a 100-mL v olumetric flask. Insert a stopper into theflask, heat at 60 for 1 h, and cool to room temperature.Blank: WaterSpectrometric conditions(See Spectrophotometry and Light-Scattering 851.)Analytical wav elength: 340 nmCell: 1 cmAnalysisSamples: Standard solution, Sample solution, and BlankPipet 0.5 mL each of the Standard solution, Sample solution, and Blank into separate cells. Add 2.5 mL of Solution A and 0.2 mL of Solution C to each cell. Cov er the cells to exclude oxygen. Mix by inv ersion, andallow to stand for 2–3 min at 22 ± 2. Determine the absorbances of the solutions using the water as the reference. Add 0.05 mL of Solution B to each cell. Cover the cells to exclude oxygen. Mix by inv ersion,and allow to stand for 5 min at 22 ± 2. Determine the absorbances of the solutions, using the Blank asthe reference.Calculate the percentage of aldehydes, expressed as acetaldehyde, in the portion of Povidone taken:Result = 10 × (C/W) × [{(A U2A U1) (A B2A B1)}/{(A S2A S1) (A B2A B1)}] C== concentration of acetaldehyde in the Standardsolution (mg/mL)W== weight of Povidone taken (g)A U2== absorbance of the solution from the Samplesolution, after addition of Solution BA U1== absorbance of the solution from the Samplesolution, before addition of Solution BA B2== absorbance of the solution from the Blank,after addition of Solution BA B1== absorbance of the solution from the Blank,before addition of Solution BA S2== absorbance of the solution from the Standardsolution, after addition of Solution BA S1== absorbance of the solution from the Standardsolution, before addition of Solution BAcceptance criteria: NMT 0.05%• P ROCEDURE 2: L IMIT OF H Y DRA ZINEStandard solution: 9.38 µg/mL of salicylaldazine in tolueneSample solution: Transfer 2.5 g to a 50-mL centrifuge tube, add 25 mL of water, and mix to dissolv e. Add 500µL of a solution (1 in 20) of salicylaldehyde in methanol, swirl, and heat in a water bath at 60 for 15 min.Allow to cool, add 2.0 mL of toluene, insert a stopper in the tube, shake v igorously for 2 min, and centrifuge.Use the clear upper toluene layer in the centrifuge tube as the Sample solution.Chromatographic system(See Chromatography 621, Thin-Layer Chromatography.)Adsorbent: 0.25-mm layer of dimethylsilanized chromatographic silica gel mixtureApplication volume: 10 µLDev eloping solvent system: Methanol and water (2:1)Analytical wav elength: UV 365 nmAnalysisSamples: Standard solution and Sample solutionProceed as directed in the chapter. Allow the spots to dry, and develop the chromatogram with theDeveloping solvent system until the solv ent front has mov ed three-fourths of the length of the plate. Locate the spots on the plate by examination under UV light. Remove the plate from the chamber, mark thesolv ent front, and allow the solv ent to evaporate.Acceptance criteria: Salicylaldazine appears as a fluorescent spot having an R F value of 0.3, and thefluorescence of any salicylaldazine spot from the Sample solution is not more intense than that produced by the spot from the Standard solution: NMT 1 ppm of hydrazine.• P ROCEDURE 3: V INY LPYRROLIDINONEMobile phase: Methanol and water (1:4)System suitability solution: Transfer 10 mg of vinylpyrrolidinone and 500 mg of v inyl acetate to a 100-mLv olumetric flask, and dissolve in and dilute with methanol to volume. Transfer 1.0 mL of this solution to a 100-mL volumetric flask, and dilute with Mobile phase to v olume.Standard stock solution: 5 µg/mL of v inylpyrrolidinone in methanolStandard solution: 0.25 µg/mL from vinylpyrrolidinone Standard stock solution in Mobile phaseSample solution: 25 mg/mL of Povidone in Mobile phaseChromatographic system(See Chromatography 621, System Suitability.)Mode: LCDetector: UV 235 nmColumnGuard: 4.0-mm × 2.5-cm; packing L7Analytical: 4.0-mm × 25-cm; 5-µm packing L7[NOTE—The analysis can also be performed with a 4.0- × 30-mm or a 4.6- × 30-mm guardcolumn containing packing L7 and with a 4.6- × 25-cm analytical column containing 5-µm packing L7. ]Column temperature: 40[NOTE—Adjust the flow rate so that the retention time of vinylpyrrolidinone is about 10 min. ] Injection size: 50 µLSystem suitabilitySamples: System suitability solution and Standard solutionSuitability requirementsResolution: NLT 2.0 between v inylpyrrolidinone and v inyl acetate, System suitability solutionRelative standard deviation: NMT 2.0% of v inylpyrrolidinone, on replicate injections of Standard solution AnalysisSamples: Standard solution and Sample solutionRecord the chromatograms, and measure the responses for the v inylpyrrolidinone peak. [NOTE—Ifnecessary, after each injection of the Sample solution wash the polymeric material ofPovidone from the guard column by passing the Mobile phase through the columnbackwards for 30 min at the same flow rate. ]Calculate the percentage of v inylpyrrolidinone in the sample taken:Result = (r U/r S) × (C S/C U) × 100r U== vinylpyrrolidinone peak response from theSample solutionr S== vinylpyrrolidinone peak response from theStandard solutionC S== concentration of vinylpyrrolidinone in theStandard solution (mg/mL)C U== concentration of Povidone in the Samplesolution (mg/mL)Acceptance criteria: NMT 0.001%SPECIFIC TESTS• P H 791: 3.0–7.0, in a solution (1 in 20)• W A TER D ETERMINA TION, Method I921: NMT 5.0%• K-VA LUESample solution: Weigh a quantity of undried Pov idone equiv alent on the anhydrous basis to the amount specified in the following table.Nominal K-value Quantity(g)18 5.00>18 to 95 1.00>950.10Dissolv e it in 50 mL of water in a 100-mL volumetric flask, and dilute to v olume. Allow to stand for 1 h.AnalysisSample: Sample solutionDetermine the v iscosity of the Sample solution, using a capillary-tube v iscosimeter (see Viscosity 911), at 25 ± 0.2. Calculate the K-v alue of Pov idone:c== weight, on the anhydrous basis, of thespecimen tested in each 100.0 mL of solution(g)z== viscosity of the Sample solution relative tothat of waterAcceptance criteriaK-value of Povidone hav ing a stated (nominal) K-v alue NMT 15: 85.0%–115.0% of the stated v alues K-value of Povidone hav ing a stated K-v alue or a stated K-value range with an av erage of more than 15: 90.0%–108.0% of the stated value or of the av erage of the stated rangeADDITIONAL REQUIREMENTS• P A CKA GING A ND S TORA GE: Preserv e in tight containers.• L A BELING: Label it to state, as part of the official title, the K-value or K-v alue range of the Pov idone. Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Kevin T. Moore, Ph.D.(EXC2010) Monographs - Excipients Senior Scientific Liaison1-301-816-8369USP34–NF29 Page 3981Pharmacopeial Forum: Volume No. 30(4) Page 1292Chromatographic Column—POVIDONEChromatographic columns text is not deriv ed from, and not part of, USP 34 or NF 29.。
