FDAGMP中英文对照标准
中英文对照FDA原料药GMP指南

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP规范指南中英文对照.doc

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
FDA-GMP中英文对照标准版

DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OF RAW MATERIALS BY FDATable of Contents 目录1. INTRODUCTION1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP规范指南中英文对照.doc

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP指南(中英文对照)

Q7a(中英文对照)FDA原料药GMP指南Table ofContents目录1、INTRODUCTION 1、简介1、1 Objective1、1目得1、2Regulatory Applicability 1、2法规得适用性1、3 Scope 1、3范围2、QUALITY MANAGEMENT2、质量管理2、1 Principles 2、1总则2、2质量部门得责任2、2Responsibilities of the Quality Unit(s)2、3生产作业得职责2、3 Responsibility for ProductionActivities2、4内部审计(自检)2、4 InternalAudits (Self Inspection)2、5 Product Quality Review2、5产品质量审核3、PERSONNEL3、人员3、1 PersonnelQualifications 3、人员得资质3、2 Personnel Hygiene 3、2 人员卫生3、3 Consultants 3、3 顾问4、建筑与设施4、BUILDINGS ANDFACILITIES4、1Designand Construction4、1 设计与结构4、2 Utilities4、2 公用设施4、3 Water4、3 水4、4 Containment4、4 限制4、5 Lighting 4、5 照明4、6Sewage and Refuse 4、6 排污与垃圾4、7Sanitation andMaintenance 4、7卫生与保养5、PROCESS EQUIPMENT5、工艺设备5、1DesignandConstruction 5、1 设计与结构5、2Equipment Maintenance and5、2设备保养与清洁Cleaning5、3Calibration 5、3 校验5、4 puterized Systems5、4 计算机控制系统6、DOCUMENTATION AND RECORDS6、文件与记录6、1 Documentation System andSpecifications6、1 文件系统与质量标准6、2EquipmentcleaningandUseRecord6、2 设备得清洁与使用记录6、3 Recordsof Raw Materials,Intermediates,APILabeling andPackaging Materials 6、3 原料、中间体、原料药得标签与包装材料得记录6、4Master Production Instructions (Master Production and ControlRecords)6、4 生产工艺规程(主生产与控制记录)6、5 BatchProduction Records(Batch Production andControlRecords)6、5 批生产记录(批生产与控制记录)6、6Laboratory ControlRecords 6、6 实验室控制记录6、7 Batch Production RecordReview6、7批生产记录审核7、MATERIALSMANAGEMENT7、物料管理7、1GeneralControls 7、1 控制通则7、2Receiptand Quarantine 7、2接收与待验7、3Sampling andTesting of IningProduction Materials7、3 进厂物料得取样与测试7、4 Storage 7、4储存7、5Re-evaluation 7、5复验8、PRODUCTION ANDIN—PROCESS CONTROLS8、生产与过程控制8、1ProductionOperations 8、1 生产操作8、2 Time Limits 8、2 时限8、3 In-process Sampling and Controls8、3 工序取样与控制8、4 BlendingBatches ofIntermediatesor APIs8、4 中间体或原料药得混批8、5 Contamination Control 8、5 污染控制9、PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES9、原料药与中间体得包装与贴签9、1General 9、1 总则9、2Packaging Materials 9、2 包装材料9、3Label Issuance andControl 9、3标签发放与控制9、4Packaging and LabelingOp9、4包装与贴签操作erations10、储存与分发10、STORAGE AND DISTRIBUTION10、1 Warehousing Procedures 10、1 入库程序10、2DistributionProcedures 10、2 分发程序11、LABORATORY CONTROLS 11、实验室控制11、1 General Controls 11、1控制通则11、2中间体与原料药得测试11、2 Testing ofIntermediatesandAPIs11、3 Validation of Analytical11、3 分析方法得验证Procedures11、4CertificatesofAnalysis11、4 分析报告单11、5 原料药得稳定性监测11、5 Stability Monitoringof APIs11、6 Expiryand RetestDating11、6 有效期与复验期11、7Reserve/Retention Samples 11、7 留样12、VALIDATION 12、验证12、1 Validation Policy 12、1 验证方针12、2 Validation Documentation12、2 验证文件12、3Qualification 12、3 确认12、4 工艺验证得方法12、4Approachesto ProcessValidation12、5 Process Validation Program12、5 工艺验证得程序12、6PeriodicReviewof12、6验证系统得定期审核Validated Systems12、7 CleaningValidation 12、7 清洗验证12、8 分析方法得验证12、8 Validation of Analytical Methods13、CHANGECONTROL 13、变更得控制14、REJECTIONANDRE-USEO14、拒收与物料得再利用FMATERIALS14、1 Rejection 14、1 拒收14、2Reprocessing14、2 返工14、3Reworking 14、3 重新加工14、4Recovery of Materialsand14、4 物料与溶剂得回收Solvents14、5Returns14、5 退货15、PLAINTS AND RECALLS 15、投诉与召回16、CONTRACTMANUFACTURERS (INCLUDING LABORATORIES)16、协议生产商(包括实验室)17、AGENTS,BROKERS, TRADERS,DISTRIBUTORS,REPACKERS,AND RELABELLERS17、代理商、经纪人、贸易商、经销商、重新包装者与重新贴签者17、1Applicability 17、1适用性17、2Traceabilityof DistributedAPIs and Intermediates17、2已分发得原料药与中间体得可追溯性17、3QualityManagement 17、3质量管理17、4Repackaging, Relabeling,and Holding of APIsandInterm ediates17、4原料药与中间体得重新包装、重新贴签与待检17、5Stability 17、5稳定性17、6 TransferofInformation 17、6 信息得传达17、7Handling ofplaints andRecalls17、7投诉与召回得处理17、8Handlingof Returns 17、8 退货得处理18、Specific Guidance for APIs Manufactured byCell Culture/Fermentation18、用细胞繁殖/发酵生产得原料药得特殊指南18、1 General 18、1总则18、2Cell Bank Maintenanceand Record Keeping18、2细胞库得维护与记录得保存18、3 CellCulture/Fermentation18、3细胞繁殖/发酵18、4 Harvesting, IsolationandPurification18、4收取、分离与精制18、5Viral Removal/Inactivation steps18、5 病毒得去除/灭活步骤19、APIsfor Use in Clinical Trials19、用于临床研究得原料药19、1General 19、1 总则19、2 Quality 19、2 质量19、3Equipment and Facilities 19、3设备与设施19、4 ControlofRaw Materials 19、4原料得控制19、5Production 19、5 生产19、6Validation 19、6 验证19、7 Changes19、7变更19、8 Laboratory Controls 19、8实验室控制19、9 Documentation 19、9 文件20、Glossary 20、术语Q7a GMP Guidance forAPIs Q7a原料药得GMP指南1、INTRODUCTION1、简介1、1 Objective1、1目得Thisdocument isintendedto provide guidance regarding good manufacturingpractice (GMP) for the manufacturing of active pharmaceuticalingredients (APIs)underan app ropriate systemfor managing qualit y、Itis also intended tohelp ensure that APIsmeet the qualityand puritycharacteristicstha ttheypurport,or arerepresented,to possess、本文件旨在为在合适得质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP规范指南中英文对照.doc

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
GMP中英文单词对照表
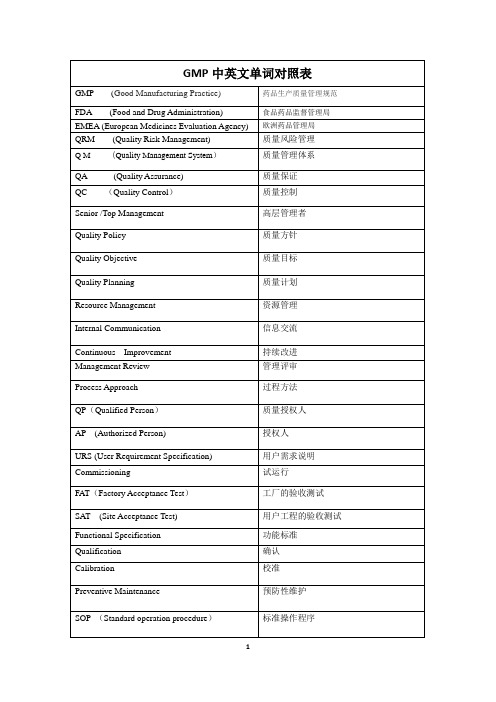
功能标准
Qualification
确认
Calibration
校准
Preventive Maintenance
预防性维护
SOP(Standard operation procedure)
标准操作程序
Retirement Management
退役管理
EquipmentLogbook
Remedial Action
矫正措施
OOS (Out of Specification)
偏差调查和实验室超标结果
PAR(Annual Product review)
产品年度回顾
Risk Identification
风险识别
Collect and Organize Information
收集和组织信息
可编程逻辑控制器
Metrology Confirmation
计量确认
Drinking water
饮用水
Purified water
纯化水
Sterile Purified water
灭菌纯化水
FDS(Functional Design Specification)
功能设计技术说明书
DDS(Detailed Design Specification)
质量目标
Quality Planning
质量计划
Resource Management
资源管理
Internal Communication
信息交流
Continuous Improvement
持续改进
Management Review
FDA-GMP中英文对照标准版

DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OF RAW MATERIALS BY FDATable of Contents 目录1. INTRODUCTION1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
中英文-美国FDA GMP检查

Inspections… 检查 …
• Are FACT finding in nature 是事实性的调查结果
• Require EVIDENCE 需要证据
• Are REGUALTORY in nature 实质上是一种监管 – What is said could end up in court 所说的内容可能最终会在法庭上裁决
• “A drug shall be deemed adulterated if: “一种药品应当被视为掺假药品,如果:
• ... the methods used in, or the facilities or controls used for, its manufacture, processing, packing, or holding do not conform to or are not operated or administered in conformity with current good manufacturing practice ...” …使用于制造、加工、包装或置放的方法或设施、控制装置不符合或 没有遵照在安全性上保证药品符合现行GMP的规定…”
• Complaints 投诉 – Procedures define responsibilities 建立规程明确责任 – Investigation Reports 调查报告
Quality System cont. 质量体系 续
• Deviations (Non-Conformances) / OOS 偏差(不合格项)/ 超规 – SOPs define responsibilities 建立SOP明确责任 – Investigation Reports 调查报告 – Conclusions – Final Disposition 结论-最后处置
Q7aFDA原料药的GMP行业指南(中英文对照版)

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
FDA-GMP中英文对照标准手册

FDA-GMP中英文对照标准手册
简介
本手册介绍了美国食品和药物管理局(cFDA)制定的GMP (Good Manufacturing Practice,良好生产规范)相关标准要求,包
括对药品、保健品、化妆品等品种的生产及质量控制方面的要求。
主要内容
本手册主要包括以下内容:
- GMP的概念及适用范围;
- GMP规范要求:包括生产区域、工艺流程、设备、人力资源
管理、记录和报告等各个方面的要求;
- 质量管理体系要求:包括质量管理手册、质量控制和检测、
审核和审批等各个方面的要求。
目的
本手册旨在帮助企业了解美国cFDA对药品、保健品和化妆品
等品种的GMP相关规定,引导企业建立健全的GMP质量管理体系,增强企业产品的质量稳定性和产品竞争力,提高顾客满意度。
结论
GMP是一项重要的质量管理制度,关系到药品、保健品和化
妆品等产品的制造和市场竞争力。
本手册提供了详细的GMP相关
规定,梳理了药品、保健品和化妆品等品种的生产和质量控制过程,可以帮助企业更好地了解并遵循GMP标准要求,从而提高产品的
质量稳定性和市场竞争力。
q7a(中英文对照)gmpguidanceforapis(fda原料药gmp指南)

Q7a (中英文对照)GMP Guidance for APIs (FDA原料药GMP指南) 1. INTRODUCTION 1. 简介 1.1 Objective 1.1目的 This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
它也着眼于帮助确保原料药符合其旨在达到或表明拥有的质量与纯度要求。
   In this guidance, the term manufacturing is defined to include all operations of receipt of materials, production, packaging, repackaging, labeling, relabeling, quality control, release, storage and distribution of APIs and the related controls. In this guidance, the term should identifies recommendations that, when followed, will ensure compliance with CGMPs. An alternative approach may be used if such approach satisfies the requirements of the applicable statues. For the purposes of this guidance, the terms current good manufacturing practices and good manufacturing practices are equivalent. 本指南中所指的“制造”包括物料接收、生产、包装、重新包装、贴签、重新贴签、质量控制、放行、原料药的储存和分发及其相关控制的所有操作。
美国FDA原料药生产质量管理规范中英文

美国FDA原料药生产质量管理规范中英文美国FDA原料药生产质量管理规范中英文Introduction随着全球经济一体化的加强,越来越多的中国企业涉足国际市场,其中不乏涉足美国市场的API生产企业。
进入美国市场需要遵守美国FDA颁布的诸多规章制度,包括FDA原料药生产质量管理规范。
本文将介绍该规范中英文的要点。
Background美国FDA原料药生产质量管理规范通常被简称为cGMP,是一套针对药品制造和质量控制的可遵守的最低标准。
该规范确保制造药品的每一个阶段都符合FDA的要求,从而保证了药品的安全、有效和质量稳定。
Requirements以下是FDA原料药生产质量管理规范中的主要要求。
以下为英文版。
1. Ensure that manufacturing processes are clearly defined and controlled.2. Establish robust systems for handling deviations andnon-conformances.3. Implement effective quality risk management.4. Ensure that raw materials and components are of appropriate quality.5. Follow appropriate validation procedures for equipment, systems, and processes.6. Establish and maintain a comprehensive quality control system.7. Implement and maintain effective documentation and record-keeping practices.8. Train personnel on GMP principles and procedures.9. Establish and maintain effective measures for preventing contamination and cross-contamination.10. Implement effective procedures for managing recalls and complaints.Translation以下是FDA原料药生产质量管理规范的中文版本。
美国FDA CGMP英汉对照版

美国FDA CGMP英汉对照版Subpart A-General Provisions§211.1 Scopea)The regulations in this part contain theminimum current good manufacturing practice for preparation of drug products for administration to humans or animals.b)The current good manufacturing practiceregulations in this chapter, as they pertain to drug products, and in parts 600 through 680 of this chapter, as they pertain to biological products for human use, shall be considered to supplement, not supersede, the regulations in this part unless the regulations explicitly provide otherwise. In the event it is impossible to comply with applicable regulations both in this part and in other parts of this chapter or in parts 600 through 680 of this chapter, the regulation specifically applicable to the drug product in question shall supersede the regulation in this part.c)Pending consideration of a proposedexemption, published in the Federal Register of September 29, 1978, the requirements in this part shall not be enforced for OTC drug products if the products and all their ingredients are ordinarily marketed and consumed as human foods, and which products may also fall within the legal definition of drugs by virtue of their intended use. Therefore, until further notice, regulations under part 110 of this chapter, and where applicable, parts 113 to 129 of this chapter, shall be applied in determining whether these OTC drug products that are also foods are manufactured, processed, packed, or held under current good manufacturing practice.§211.3 Definitions.The definitions set forth in §210.3 of this chapter apply in this part.A.总则211.1 范围(a)本部分的条例包含人用或兽用药品制备的现行最低限度的药品生产管理规范(GMP)。
FDA,GMP,ICH临床实验专业英语词汇互译

FDA,GMP,ICH临床实验专业英语词汇互译FDA,GMP,ICH临床实验专业英语词汇互译FDA常用词中英对照FDA(food and drug adminisration)美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备,加工,包装和贮存过程中所涉及的设备,生产过程或物品.只有在DMF 持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND, NDA,ANDA时才能参考其内容)holderMF持有者CFR(code of federal regulation)美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health)美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP, NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary)美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICHuality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing for Pharmaceuticals 基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility: An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products 对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including: Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management including Questions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports for Marketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the Pediatric Population小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics)有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status of the guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP: time-to-progression 疾病进展时间SAE: severity Adverse Event 严重不良事件AE: Adverse Event 不良事件-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--SOP: Standard Operating Procedure 标准操作规程CRF: Case Report form 病例报告表DLT: 剂量限制毒性MTD: 最大耐受剂量KPS: Karnofsky Performance Status行为状态评分CR: complete response完全缓解PR: partial response部分缓解SD: 病情稳定PD: progressive disease病情进展CTC: 常用药物毒性标准IEC: independent ethics committee 独立伦理委员会IRB : institutional review board 伦理委员会CRA: 临床研究助理CRO: Contract Research Organization 合同研究组织DFS: Disease Free Survival 无病生存期OS: (Overall Survival) 总生存时间IC: Informed consent 知情同意ADR: Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI rincipal investigator 主要研究者CI: Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP: 医疗器械生产质量管理规范ICF: Informed consent form 知情同意书RCT : randomized controlled trial, 随机对照试验NRCCT: non-randomized concurrent controlled trial, 非随机同期对照试验EBM: evidence-based medicine 循证医学RCD: randomized cross-over disgn 随机交叉对照试验HCT: historial control trial, 历史对照研究RECIST: Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC: Quality Control质量控制UADR: Unexpected Adverse Drug Reaction,非预期药物不良反应-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--GMP英语PIC/S的全称为harmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品.ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBANBritish Approved NameBIRABritish Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPA EDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products) 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)Health Sciences Authority (HSA)HSA's Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相认证同意MRFG Mutual Recognition Facilitation Group MRPMutual Recognition ProcedureNASNew Active SubstanceNCENew Chemical EntityNDANew Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE) 标准运作程序SPC/SmPC Summary of Product Characteristics summary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国) ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--ICH 安全性领域常用专业术语中英文对照表Dead offspring at birth 出生时死亡的子代Degradation 降解 Delay of parturition 分娩延迟Deletion 缺失 Descriptive statistics 描述性统计 Distribution 分布Detection of bacterial mutagen 细菌诱变剂检测 Detection of clastogen 染色体断裂剂检测Determination of metabolites 测定代谢产物 Development of the offspring 子代发育Developmental toxicity 发育毒性 Diminution of the background lawn 背景减少Direct genetic damage 直接遗传损伤DNA adduct DNA加合物 DNA damage DNA损伤DNA repair DNA修复 DNA strand breaks DNA链断裂Dose escalation 剂量递增 Dose dependence 剂量依赖关系 Dose level 剂量水平Dose-limiting toxicity 剂量限制性毒性 Dose-raging studies 剂量范围研究Dose-relatived mutagenicity 剂量相关性诱变性 Dose-related 剂量相关Dose-relatived cytotoxicity 剂量相关性细胞毒性Dose-relatived genotoxic activity 剂量相关性遗传毒性Dose-response curve 剂量-反应曲线 Dosing route 给药途径Duration 周期 Duration of pregnancy 妊娠周期Eaning 断奶 Earlier physical malformation 早期躯体畸形Early embryonic development 早期胚胎发育Early embryonic development to implantation 着床早期的胚胎发育Electro ejaculation 电射精Elimination 清除Embryofetal deaths 胚胎和胎仔死亡 Embryo-fetal development 胚胎-胎仔发育Embryo-fetal toxicity 胚胎-胎仔毒性 Embryonic death 胚胎死亡Embryonic development 胚胎发育 Embryonic period 胚胎期Embryos 胚胎 Embryotoxicity 胚胎毒性Enantiomer 对映异构体End of pregnancy 怀孕终止 Endocytic 内吞噬(胞饮)Endocytic activity 内吞噬活性 Endogenous proteins 内源性蛋白Endogenous components 内源性物质 Endogenous gene 内源性基因Endonuclease 核酸内切酶 Emdpmiclease release from lysosomes 溶酶体释放核酸内切酶End-point 终点Epididymal sperm maturation 附睾精子成熟性 Epitope 抗原决定部位Error prone repair 易错性修复 Escalation 递增Escherichia coli strain 大肠杆菌菌株 Escherichia coli 大肠杆菌Evaluation of test result 试验结果评价Exaggerated pharmacological response 超常增强的药理作用Excretion 排泄(清除) Exposure assessment 接触剂量评价Exposure period 接解期 External metabolizing system 体外代谢系统F1-animals 子一代动物False positive result 假阳性结果Fecundity 多产 Feed-back 反馈 Fertilisation 受精 Fertility 生育力Fertility studies 生育力研究 Fetal abnormalities 胎仔异常Fetal and neonatal parameters 胎仔和仔鼠的生长发育参数Fetal development and growth 肿仔发育和生长 Fetal period 胎仔期 Fetotoxicity 胎仔毒性False negative result 假阴性结果First pass testing 一期试验Fluorescence in situ hybridization(FISH) 原位荧光分子杂交-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--average deviation 平均差Bbar chart 直条图,条图bias 偏性binomial distribution 二项分布biometrics 生物统计学bivariate normal population 双变量正态总体Ccartogram 统计图case fatality rate(or case mortality) 病死率census 普查chi-sguare(X2) test 卡方检验central tendency 集中趋势class interval 组距classification 分组,分类cluster sampling 整群抽样coefficient of correlation 相关系数coefficient of regression 回归系数coefficient of variability(or coefficieut of variation) 变异系数collection of data 收集资料column 列(栏)combinative table 组合表combined standard deviation 合并标准差combined variance(or poolled variance) 合并方差complete survey 全面调查completely correlation 完全相关completely random design 完全随机设计confidence level 可信水平,置信水平confidence limit 可信限,置信限constituent ratio 构成比,结构相对数continuity 连续性control 对照control group 对照组coordinate 坐标correction for continuity 连续性校正correction for grouping 归组校正correction number 校正数correction value 校正值correlation 相关,联系correlation analysis 相关分析correlation coefficient 相关系数critical value 临界值cumulative frequency 累积频率Ddata 资料degree of dispersion 离散程度degree of freedom 自由度degree of variation 变异度dependent variable 应变量design of experiment 实验设计deviation from the mean 离均差diagnose accordance rate 诊断符合率difference with significance 差别不显著difference with significance 差别显著discrete variable 离散变量dispersion tendency 离中趋势distribution 分布,分配-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Eeffective rate 有效率eigenvalue 特征值enumeration data 计数资料equation of linear regression 线性回归方程error 误差error of replication 重复误差estimate value 估计值event 事件experiment design 实验设计experiment error 实验误差experimental group 实验组extreme value 极值Ffatality rate 病死率field survey 现场调查fourfold table 四格表freguency 频数freguency distribution 频数分布GGaussian curve 高斯曲线geometric mean 几何均数grouped data 分组资料Hhistogram 直方图homogeneity of variance 方差齐性homogeneity test of variances 方差齐性检验hypothesis test 假设检验hypothetical universe 假设总体Iincidence rate 发病率incomplete survey 非全面调检indepindent variable 自变量indivedual difference 个体差异infection rate 感染率inferior limit 下限initial data 原始数据inspection of data 检查资料intercept 截距interpolation method 内插法interval estimation 区间估计inverse correlation 负相关Kkurtosis coefficient 峰度系数Llatin sguare design 拉丁方设计least significant difference 最小显著差数least square method 最小平方法,最小乘法leptokurtic distribution 尖峭态分布leptokurtosis 峰态,峭度linear chart 线图linear correlation 直线相关linear regression 直线回归linear regression eguation 直线回归方程link relative 环比logarithmic normal distribution 对数正态分布logarithmic scale 对数尺度lognormal distribution 对数正态分布lower limit 下限Mmatched pair design 配对设计mathematical statistics 数理统计(学) maximum value 极大值mean 均值mean of population 总体均数mean square 均方mean variance 均方,方差measurement data 讲量资料median 中位数medical statistics 医学统计学mesokurtosis 正态峰method of least squares 最小平方法,最小乘法method of grouping 分组法method of percentiles 百分位数法mid-value of class 组中值minimum value 极小值mode 众数moment 动差,矩morbidity 患病率mortality 死亡率Nnatality 出生率natural logarithm 自然对数negative correlation 负相关negative skewness 负偏志no correlation 无相关non-linear correlation 非线性相关non-parametric statistics 非参数统计normal curve 正态曲线normal deviate 正态离差normal distribution 正态分布normal population 正态总体normal probability curve 正态概率曲线normal range 正常范围normal value 正常值normal kurtosis 正态峰normality test 正态性检验nosometry 患病率-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Oobserved unit 观察单位observed value 观察值one-sided test 单测检验one-tailed test 单尾检验order statistic 顺序统计量ordinal number 秩号ordinate 纵坐标Ppairing data 配对资料parameter 参数percent 百分率percentage 百分数,百分率percentage bar chart 百分条图percentile 百分位数pie diagram 园图placebo 安慰剂planning of survey 调查计划point estimation 点估计population 总体,人口population mean 总体均数population rate 总体率population variance 总体方差positive correlation 正相关positive skewness 正偏态prevalence rate 患病率probability 概率,机率probability error 偶然误差proportion 比,比率prospective study 前瞻研究prospective survey 前瞻调查public health statistics 卫生统计学Qquality eontrol 质量控制quartile 四分位数Rrandom 随机random digits 随机数字random numbers table 随机数目表random sample 随机样本random sampling 随机抽样random variable 随机变量randomization 随机化randomized blocks 随机区组,随机单位组randomized blocks analysis of variance 随机单位组方差分析randomized blocks design 随机单位组设计randomness 随机性range 极差,全距range of normal values 正常值范围rank 秩,秩次,等级rank correlation 等级相关rank correlation coefficent 等级相关系数rank-sum test 秩和检验ranked data 等级资料rate 率ratio 比recovery rate 治愈率registration 登记regression 回归regression analysis 回归分析regression coefficient 回归系数regression eguation 回归方程relative number 相对数relative ratio 比较相对数relative ratio with fixed base 定基比remainder error 剩余误差replication 重复retrospective survey 回顾调查Ridit analysis 参照单位分析Ridit value 参照单位值Ssample 样本sample average 样本均数sample size 样本含量sampling 抽样sampling error 抽样误差sampling statistics 样本统计量sampling survay 抽样调查scaller diagram 散点图schedule of survey 调查表semi-logarithmic chart 半对数线图semi-measursement data 半计量资料semi-guartile range 四分位数间距sensitivity 灵敏度sex ratio 性比例sign test 符号检验significance 显著性,意义significance level 显著性水平significance test 显著性检验significant difference 差别显著simple random sampling 单纯随机抽样simple table 简单表size of sample 样本含量skewness 偏态slope 斜率sorting data 整理资料sorting table 整理表sources of variation 变异来源square deviation 方差standard deviation(SD) 标准差standard error (SE) 标准误standard error of estimate 标准估计误差standard error of the mean 均数的标准误standardization 标准化standardized rate 标化率standardized normal distribution 标准正态分布statistic 统计量statistics 统计学statistical induction 统计图statistical inference 统计归纳statistical map 统计推断statistical method 统计地图statistical survey 统计方法statistical table 统计调查statistical test 统计表statistical treatment 统计检验stratified sampling 统计处理stochastic variable 分层抽样sum of cross products of 随机变量deviation from mean 离均差积和sum of ranks 秩和sum of sguares of deviation from mean 离均差平方和superior limit 上限survival rate 生存率symmetry 对称(性)systematic error 系统误差systematic sampling 机械抽样-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Tt-distribution t分布t-test t检验tabulation method 划记法test of normality 正态性检验test of one-sided 单侧检验test of one-tailed 单尾检验test of significance 显著性检验test of two-sided 双侧检验test of two-tailed 双尾检验theoretical frequency 理论频数theoretical number 理论数treatment 处理treatment factor 处理因素treatment of date 数据处理two-factor analysis of variance 双因素方差分析two-sided test 双侧检验two-tailed test 双尾检验type I error 第一类误差type II error 第二类误差typical survey 典型调查Uu test u检验universe 总体,全域ungrouped data 未分组资料upper limit 上限Vvariable 变量variance 方差,均方variance analysis 方差分析variance ratio 方差比variate 变量variation coefficient 变异系数velocity of development 发展速度velocity of increase 增长速度Wweight 权数weighted mean 加权均数Zzero correlation 零相关-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:36:00--世界500强制药企业名称中英对照排名公司名称中文名称总部收入百万美元77 Pfizer 辉瑞美国 45950.092 Johnson & Johnson 强生美国 41862.0114 GlaxoSmithKline 葛兰素史克英国 35050.9193 Novartis 诺华瑞士 24864.0205 Roche Group 罗氏瑞士 23212.9222 Merck 默克美国 22485.9239 Bristol-Myers Squibb 百时美施贵宝美国 20894.0 248 Aventis 安万特法国 20162.4254 Abbott Laboratories 雅培美国 19680.6269 AstraZeneca 阿斯利康英国 18849.0330 Wyeth 惠氏美国 15850.6433 Eli Lilly 礼来大药厂美国 12582.5100 BASF 巴斯夫德国 37757.0125 Dow Chemical 道化学美国 32632.0129 Bayer 拜耳德国 32331.1365 Akzo Nobel 阿克苏诺贝尔荷兰 14770.7。
原料药GMP规范指南中英文对照.doc

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP规范指南中英文对照.doc

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
q7A-GMP中英文对照

Q7a (中英文对照)FDA 原料药GMP 指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System and Specifications6.1 文件系统和质量标准 6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials6.3 原料、中间体、原料药的标签和包装材料的记录 6.4 Master Production Instructions (Master Production and Control Records)6.4 生产工艺规程(主生产和控制记录) 6.5 Batch Production Records (Batch Production and Control Records) 6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 进厂物料的取样与测试7.3 Sampling and Testing of IncomingProduction Materials7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. 生产和过程控制8. PRODUCTION AND IN-PROCESSCONTROLS8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 中间体或原料药的混批8.4 Blending Batches of Intermediates orAPIs8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATION9. 原料药和中间体的包装和贴签 LABELING OF APIs ANDINTERMEDIATES9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试 11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OF14.拒收和物料的再利用 MATERIALS14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs and Intermediates17.2已分发的原料药和中间体的可追溯性 17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and Record Keeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps18.5 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials19. 用于临床研究的原料药 19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20 . 术语Q7a GMP Guidance for APIsQ7a 原料药的GMP 指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and puritycharacteristics that they purport, or arerepresented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP )提供指南。
FDA_GMP_ICH临床实验专业英语词汇互译

FDA常用词中英对照FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备,加工,包装和贮存过程中所涉及的设备,生产过程或物品.只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND, NDA,ANDA时才能参考其内容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary:记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP, NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证) Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2):Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B:Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C:Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D:Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E:Evaluation of Stability Data对稳定性数据的评估处理Q1F:Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A:Text on Validation of Analytical Procedures分析程序的验证Q2B:Validation of Analytical Procedures:Methodology分析程序的验证:方法学Q3A(R):Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R):Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C:Impurities:Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M):Impurities:Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4:Pharmacopoeias药典Q4A:Pharmacopoeial Harmonisation 药典的协调Q4B:Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A:Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B:Quality of Biotechnological Products:Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C:Quality of Biotechnological Products:Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D:Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E:Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6:Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A:Specifications:Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products:Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B:Specifications:Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7:Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A:Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8:Pharmaceutical Development药物研发Q9:Quality Risk Management质量风险管理ICH:Safety-安全S1A:Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B:Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C:Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R):Addendum:Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A:Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B:Genotoxicity:A Standard Battery for Genotoxicity Testing for Pharmaceuticals基因毒性:药物基因毒性检验的标准S3A:Note for Guidance on Toxicokinetics:The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B:Pharmacokinetics:Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4:Single Dose Toxicity Tests单剂量毒性检验S4A:Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A:Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M):Maintenance of the ICH Guideline on Toxicity to Male Fertility:An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6:Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A:Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B:The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8:Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M):Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1:The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A:Clinical Safety Data Management:Definitions and Standards for Expedited Reporting 临床安全数据管理:速报制度的定义和标准E2B(R):Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M):Maintenance of the Clinical Safety Data Management including:Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M):Maintenance of the Clinical Safety Data Management including Questions andAnswers临床安全数据管理的变动,包括问答E2C:Clinical Safety Data Management:Periodic Safety Update Reports for Marketed Drugs 临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C:Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D:Post-Approval Safety Data Management:Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E:Pharmacovigilance Planning药物警戒计划E3:Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4:Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5:Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6:Good Clinical Practice:Consolidated GuidelineGCP:良好的临床规范:统一的指南E7:Studies in Support of Special Populations:Geriatrics对特定族群的支持的研究:老人病学E8:General Considerations for Clinical Trials对临床试验的总的考虑E9:Statistical Principles for Clinical Trials临床试验的统计原则E10:Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11:Clinical Investigation of Medicinal Products in the Pediatric Population小儿科药物的临床调查E12A:Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14:The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1:Medical Terminology医学术语M2:Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3:Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics)有关临床试验的临床前研究的时间安排M4:The Common Technical Document (See CTD section for complete Status of the guidelines) 通用技术文件(见有关CTD章节)M5:Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event 严重不良事件AE:Adverse Event 不良事件SOP:Standard Operating Procedure 标准操作规程CRF:Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics committee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:临床研究助理CRO:Contract Research Organization 合同研究组织DFS:Disease Free Survival 无病生存期OS:(Overall Survival) 总生存时间IC:Informed consent 知情同意ADR:Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator 主要研究者CI:Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP:医疗器械生产质量管理规范ICF:Informed consent form 知情同意书RCT :randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM:evidence-based medicine 循证医学RCD:randomized cross-over disgn 随机交叉对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC:Quality Control质量控制UADR:Unexpected Adverse Drug Reaction,非预期药物不良反应GMP英语PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品.ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM:Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBANBritish Approved NameBIRABritish Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPAEDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products) 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)Health Sciences Authority (HSA)HSA's Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相认证同意MRFG Mutual Recognition Facilitation GroupMRPMutual Recognition ProcedureNASNew Active SubstanceNCENew Chemical EntityNDANew Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE) 标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国) ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典ICH 安全性领域常用专业术语中英文对照表Dead offspring at birth 出生时死亡的子代Degradation 降解Delay of parturition 分娩延迟Deletion 缺失Descriptive statistics 描述性统计Distribution 分布Detection of bacterial mutagen 细菌诱变剂检测Detection of clastogen 染色体断裂剂检测Determination of metabolites 测定代谢产物Development of the offspring 子代发育Developmental toxicity 发育毒性Diminution of the background lawn 背景减少Direct genetic damage 直接遗传损伤DNA adduct DNA加合物DNA damage DNA损伤DNA repair DNA修复DNA strand breaks DNA链断裂Dose escalation 剂量递增Dose dependence 剂量依赖关系Dose level 剂量水平Dose-limiting toxicity 剂量限制性毒性Dose-raging studies 剂量范围研究Dose-relatived mutagenicity 剂量相关性诱变性Dose-related 剂量相关Dose-relatived cytotoxicity 剂量相关性细胞毒性Dose-relatived genotoxic activity 剂量相关性遗传毒性Dose-response curve 剂量-反应曲线Dosing route 给药途径Duration 周期Duration of pregnancy 妊娠周期Eaning 断奶Earlier physical malformation 早期躯体畸形Early embryonic development 早期胚胎发育Early embryonic development to implantation 着床早期的胚胎发育Electro ejaculation 电射精Elimination 清除Embryofetal deaths 胚胎和胎仔死亡Embryo-fetal development 胚胎-胎仔发育Embryo-fetal toxicity 胚胎-胎仔毒性Embryonic death 胚胎死亡Embryonic development 胚胎发育Embryonic period 胚胎期Embryos 胚胎Embryotoxicity 胚胎毒性Enantiomer 对映异构体End of pregnancy 怀孕终止Endocytic 内吞噬(胞饮)Endocytic activity 内吞噬活性Endogenous proteins 内源性蛋白Endogenous components 内源性物质Endogenous gene 内源性基因Endonuclease 核酸内切酶Emdpmiclease release from lysosomes 溶酶体释放核酸内切酶End-point 终点Epididymal sperm maturation 附睾精子成熟性Epitope 抗原决定部位Error prone repair 易错性修复Escalation 递增Escherichia coli strain 大肠杆菌菌株Escherichia coli 大肠杆菌Evaluation of test result 试验结果评价Exaggerated pharmacological response 超常增强的药理作用Excretion 排泄(清除) Exposure assessment 接触剂量评价Exposure period 接解期External metabolizing system 体外代谢系统F1-animals 子一代动物False positive result 假阳性结果Fecundity 多产Feed-back 反馈Fertilisation 受精Fertility 生育力Fertility studies 生育力研究Fetal abnormalities 胎仔异常Fetal and neonatal parameters 胎仔和仔鼠的生长发育参数Fetal development and growth 肿仔发育和生长Fetal period 胎仔期Fetotoxicity 胎仔毒性False negative result 假阴性结果First pass testing 一期试验Fluorescence in situ hybridization(FISH) 原位荧光分子杂交----。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA-GMP中英文对照标准版————————————————————————————————作者:————————————————————————————————日期:DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OF RAW MATERIALS BY FDATable of Contents 目录1. INTRODUCTION1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
