材料科学与工程专业英语 (1)
材料科学与工程专业英语词汇
材料科学与工程专业英语词汇1. 物理化学物理化学是研究物质结构、性质、变化规律及其机理的基础科学,是材料科学与工程的重要理论基础之一。
物理化学主要包括以下几个方面:热力学:研究物质状态和过程中能量转换和守恒的规律。
动力学:研究物质变化过程中速率和机理的规律。
电化学:研究电流和物质变化之间的相互作用和关系。
光化学:研究光和物质变化之间的相互作用和关系。
表面化学:研究物质表面或界面处发生的现象和规律。
结构化学:研究物质分子或晶体结构及其与性质之间的关系。
统计力学:用统计方法处理大量微观粒子行为,从而解释宏观物理现象。
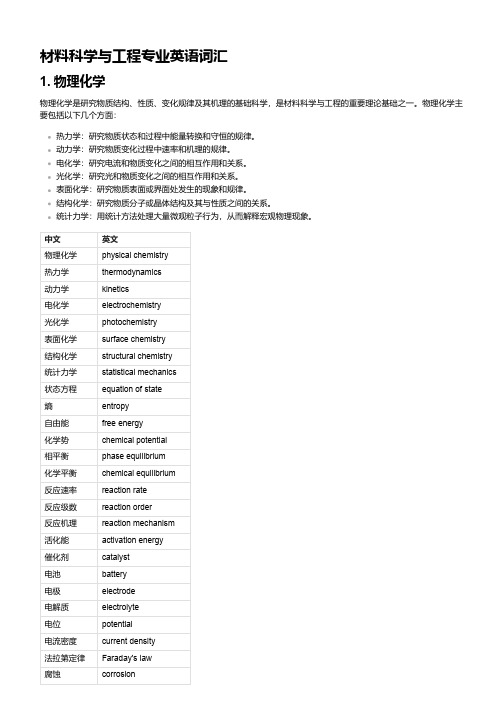
中文英文物理化学physical chemistry热力学thermodynamics动力学kinetics电化学electrochemistry光化学photochemistry表面化学surface chemistry结构化学structural chemistry统计力学statistical mechanics状态方程equation of state熵entropy自由能free energy化学势chemical potential相平衡phase equilibrium化学平衡chemical equilibrium反应速率reaction rate反应级数reaction order反应机理reaction mechanism活化能activation energy催化剂catalyst电池battery电极electrode电解质electrolyte电位potential电流密度current density法拉第定律Faraday's law腐蚀corrosion中文英文光敏材料photosensitive material光致变色photochromism光致发光photoluminescence光催化photocatalysis表面张力surface tension润湿wetting吸附adsorption膜membrane分子轨道理论molecular orbital theory晶体结构crystal structure点阵lattice空间群space group对称元素symmetry element对称操作symmetry operationX射线衍射X-ray diffraction2. 量子与统计力学量子与统计力学是物理学的两个重要分支,是材料科学与工程的重要理论基础之一。
材料科学与工程专业英语1【手工输入,按章节】

Metallic material金属材料Free electron自由电子Pure metal纯金属Outermost layer of electrons外层电子The given atom特定原子Free electron gas自由电子气Existence存在Structure结构Property性能Characteristic特征Profound consequence重要影响Electrical conductivity导电性Conductivity电导率、Electrical resistance电阻Distinguish区分Approach做法Arbitrary随意Graphite石墨Carbon碳Precipitation析出Chlorine氧Insulator绝缘体Alloy合金Element元素Weld焊接Melt熔融Catastrophic灾难性的Corrosion腐蚀Effectively实际上Brass黄铜Copper铜Zinc锌Iron铁Nickel镍Melting point熔点Aluminum铝Solid solution固溶体Intermetallic compound金属间化合物Fully soluble in each other完全互溶Side effect边界效应Dissolve溶解Solvent溶剂Solute溶质Phase相Circumstance情形Formation形式Ionic bonding离子键Valency化合价Periodic table周期表Position位置Form成为Stainless steels不锈钢Valence electron价电子Transition metal过渡金属Solid-solubility固溶性Crystal structure晶体结构陶瓷Composite复合材料Steel reinforced concrete钢筋混凝土Be defined as被定义为Inorganic non-metallic material无机非金属材料Clay粘土Mineral矿物质Powder粉体Crystalline结晶度Crystal晶体Silicate-based ceramics硅基陶瓷Advanced or technical ceramics先进陶瓷技术陶瓷Cutting tools切削工具Figurine雕像Temperature温度Kiln窑Functional pottery vessel功能陶瓷器皿Utilitarian功利主义的A fine clay-like material类粘土粉料Flourish繁荣Glass manufacture玻璃加工Pottery making陶器制作Fire烧制Calcium oxide 氧化钙Coloured glaze彩色釉面Since these ancient times自古以来Commercial application商业应用Non-ferrous metal非铁金属Sophisticated refractory materials高级耐火材料耐热heat-proof模子mould秦始皇陵兵马俑life-size terra-cotta soldiers and horses in Chin tomb 硬度hardness高温陶瓷refractory chinaFurnace炉子Fire places壁炉Incandescent light bulb白炽灯Hardness硬度Transparency透明度Hold a vacuum保持真空Light source光源Establish确立Durability耐久性Efficient节能Fluorescent lamp荧光灯Neon lamp霓虹灯Exterior室外Sodium discharge lamp钠灯Instrument panel indicator仪表面板指示器Optical fibre networks光导纤维网Data storage数据存储Document production公文制作Laser printer 激光打印Light-emitring diode发光二极管Electronic industry电子工业Insulator绝缘体Semiconductor半导体Superconductor超导体Magnet磁体Electrical insulator电的绝缘体Ceramics spark plug陶瓷火花塞Ignite fuel点燃燃料Internal combustion engine内燃机Automobile汽车High voltage insulator高压绝缘体Telecommunication长途通信Copper wire铜线Cable电缆Copper mining铜矿Capture toxic material捕获有毒物质Encapsulate nuclear waste包埋核废料Diesel柴油Wear resistant耐磨损Properties性能Ceramic tile瓷砖Thermal barrier tile热障瓷砖Engineering ceramics工程陶瓷Technical ceramics技术陶瓷Mechanical properties机械性能Corrosion/oxidation resistance抗腐蚀抗氧化Segment部分Structure ceramics结构陶瓷Electrical and electronic 电工电子Ceramics coating陶瓷涂层Bioceramics生物陶瓷Component零件Industrial wear parts工业磨损部件Filter过滤器Membrane隔膜Catalyst催化剂Support载体Bayer process拜耳法Aluminum powders氧化铝粉Pure纯的、Microstructure微结构Crystal structure晶体结构Mineral-based ceramics矿物基陶瓷Powder processing粉体处理Shape forming产品定型Non-destructive evaluation非破坏性评价Machining加工Standardization标准化Materials property database材料性能数据库Gelcasting凝胶铸造Freezecasting冷冻铸造Injection moulding注射成型Rapid prototyping快速原型制造Manufacture生产Fuel cell燃料电池Batteries电池Smart material智能材料Sensing感知Actuate驱动Intelligence智能Piezoelectric properties压电性能Carry electricity输电Power component电力元件Circuit电路Magnetic resonance imaging scanners核磁共振成像扫描器MRIMagnetic levitation trains磁悬浮列车Medical field医疗领域Refractory ceramics耐火陶瓷Cellular ceramics多孔陶瓷聚合物Macromolecular大分子Polymer聚合物Cross section典型Elastomer弹性体、合成橡胶Rubber橡胶Repeating unite重复单元Isoprene异戊二烯Cross-linking交联Sulfur chain fragment硫链碎片Vulcanization硫化作用synthetic variety合成品种styrene-butadiene rubber丁苯橡胶SBR initiator引发剂soften变软harden变硬rigid material刚性材料versatile通用low density polyethylene低密度聚乙烯LDPE branched polyethylene枝状聚乙烯linear polyethylene线性聚乙烯ultra-high molecular weight polyethylene超高分子量聚乙烯Extremely tough极端坚韧Resilient有弹性的Artificial silk人造丝Manmade fiber人造纤维Nylon尼龙Axial ratios 轴径比Synthetic polymer合成聚合物Desirable理性的Ironing熨烫Tensile strength拉伸强度Adequate stiffness适度强度Parachute将落伞Elasticity弹性Toughness韧性Resistance to abrasion耐磨性Static charge静电累积Textile纺织品Bullet-proof vest防弹背心Spinning纺丝Injection molding注塑成型Glass transition temperature玻璃化转变温度Molten熔融的Squeeze挤Extrusion挤出成型Die 模子Mold模具Tubing管材Hose软管Cross-sectional横断面Melt、dry、wet spinning熔体、干、湿纺丝Principal原理Hole孔Spinneret喷丝头Polymer morphology聚合物形态学Chain alignment链排列Enhance crystallinity结晶度。
材料科学与工程专业英语1-18单元课后翻译答案

材料科学与工程专业英语1-18单元课后翻译答案Unit 1Translation.1.“材料科学”涉及到研究材料的结构与性能的关系。
相反,材料工程是根据材料的结构与性质的关系来涉及或操控材料的结构以求制造出一系列可预定的性质。
2.实际上,所有固体材料的重要性质可以分为六类:机械、电学、热学、磁学、光学、腐蚀性。
3.除了结构与性质,材料科学与工程还有其他两个重要的组成部分,即加工与性能。
4.工程师或科学家越熟悉材料的各种性质、结构、性能之间的关系以及材料的加工技术,根据以上的原则,他或她就会越自信与熟练地对材料进行更明智的选择。
5.只有在少数情况下,材料才具有最优或最理想的综合性质。
因此,有时候有必要为某一性质而牺牲另一性能。
6.Interdisciplinary dielectric constant Solid materials heat capacity Mechanical property electromagnetic radiation Material processing elastic modulus7.It was not until relatively recent times that scientists came to understand therelationships between the structural elements of materials and their properties.8. Materials engineering is to solve the problem during the manufacturing andapplication of materials.9.10.Mechanical properties relate deformation to an applied load or force.Unit 21. 金属是电和热很好的导体,在可见光下不透明;擦亮的金属表面有金属光泽。
材料科学与工程专业英语第三版-翻译以及答案.doc

材料科学与工程专业英语第三版-翻译以及答案UNIT 1一、材料根深蒂固于我们生活的程度可能远远的超过了我们的想象,交通、装修、制衣、通信、娱乐(recreation)和食品生产,事实上(virtually),我们生活中的方方面面或多或少受到了材料的影响。
历史上,社会的发展和进步和生产材料的能力以及操纵材料来实现他们的需求密切(intimately)相关,事实上,早期的文明就是通过材料发展的能力来命名的(石器时代、青铜时代、铁器时代)。
二、早期的人类仅仅使用(access)了非常有限数量的材料,比如自然的石头、木头、粘土(clay)、兽皮等等。
随着时间的发展,通过使用技术来生产获得的材料比自然的材料具有更加优秀的性能。
这些性材料包括了陶瓷(pottery)以及各种各样的金属,而且他们还发现通过添加其他物质和改变加热温度可以改变材料的性能。
此时,材料的应用(utilization)完全就是一个选择的过程,也就是说,在一系列有限的材料中,根据材料的优点来选择最合适的材料,直到最近的时间内,科学家才理解了材料的基本结构以及它们的性能的关系。
在过去的100年间对这些知识的获得,使对材料性质的研究变得非常时髦起来。
因此,为了满足我们现代而且复杂的社会,成千上万具有不同性质的材料被研发出来,包括了金属、塑料、玻璃和纤维。
三、由于很多新的技术的发展,使我们获得了合适的材料并且使得我们的存在变得更为舒适。
对一种材料性质的理解的进步往往是技术的发展的先兆,例如:如果没有合适并且没有不昂贵的钢材,或者没有其他可以替代(substitute)的东西,汽车就不可能被生产,在现代、复杂的(sophisticated)电子设备依赖于半导体(semiconducting)材料四、有时,将材料科学与工程划分为材料科学和材料工程这两个副学科(subdiscipline)是非常有用的,严格的来说,材料科学是研究材料的性能以及结构的关系,与此相反,材料工程则是基于材料结构和性能的关系,来设计和生产具有预定性能的材料,基于预期的性能。
【专业英语】材料科学与工程

• Note that since water has density of 1 g/cm3, the specific gravity is the same as the density of the material measured in g/cm3.
材料科学与工程专业英语
Magnetic Permeability
专业英语 材料科学与工程
江苏大学 材料学院
材料科学与工程专业英语
Hale Waihona Puke 主要内容及课时安排• Introduction to materials science and engineering(4学时)
• Metallic materials and alloys(4学时) • Ceramics(2学时) • Polymer(2学时) • Composites(2学时) • Nanostructured materials(2学时)
材料科学与工程专业英语
• In general, some of the more important physical and chemical properties from an engineering material standpoint include phase transformation temperatures, density, specific gravity, thermal conductivity, linear coefficient of thermal expansion, electrical conductivity and resistivity, magnetic permeability, and corrosion resistance, and so on. 磁导率
材料科学与工程专业英语课文翻译(1,2,3,10).

United 1 材料科学与工程材料在我们的文化中比我们认识到的还要根深蒂固。
如交通、房子、衣物,通讯、娱乐和食物的生产,实际上,我们日常生活中的每一部分都或多或少地受到材料的影响。
历史上社会的发展、先进与那些能满足社会需要的材料的生产及操作能力密切相关。
实际上,早期的文明就以材料的发展程度来命名,如石器时代,铜器时代。
早期人们能得到的只有一些很有限的天然材料,如石头、木材、粘土等。
渐渐地,他们通过技术来生产优于自然材料的新材料,这些新材料包括陶器和金属。
进一步地,人们发现材料的性质可以通过加热或加入其他物质来改变。
在这点上,材料的应用完全是一个选择的过程。
也就是说,在一系列非常有限的材料中,根据材料的优点选择一种最适合某种应用的材料。
直到最近,科学家才终于了解材料的结构要素与其特性之间的关系。
这个大约是过去的60 年中获得的认识使得材料的性质研究成为时髦。
因此,成千上万的材料通过其特殊的性质得以发展来满足我们现代及复杂的社会需要。
很多使我们生活舒适的技术的发展与适宜材料的获得密切相关。
一种材料的先进程度通常是一种技术进步的先兆。
比如,没有便宜的钢制品或其他替代品就没有汽车。
在现代,复杂的电子器件取决于所谓的半导体零件.材料科学与工程有时把材料科学与工程细分成材料科学和材料工程学科是有用的。
严格地说,材料科学涉及材料到研究材料的结构和性质的关系。
相反,材料工程是根据材料的结构和性质的关系来设计或操纵材料的结构以求制造出一系列可预定的性质。
从功能方面来说,材料科学家的作用是发展或合成新的材料,而材料工程师是利用已有的材料创造新的产品或体系,和/或发展材料加工新技术。
多数材料专业的本科毕业生被同时训练成材料科学家和材料工程师。
“structure”一词是个模糊的术语值得解释。
简单地说,材料的结构通常与其内在成分的排列有关。
原子内的结构包括介于单个原子间的电子和原子核的相互作用。
在原子水平上,结构包括原子或分子与其他相关的原子或分子的组织。
材料科学与工程专业英语课后答案

1.“Materials science"involves investigating the relationships that exist between the structures and properties of materials. In contrast, "Materials engineering" involves, on the basis of these structur e-property correlations, designing or engineering the structure of a material to produce a predeter mined set of properties.“材料科学”涉及研究材料的结构和性能之间的关系。
相反,“材料工程”是指在这些结构和性能相关性的基础上,基于预期的性能来设计或生产有预定性能的材料。
2.Virtually all important Properties of solid materials may be grouped into six different categories: mechanical, electrical, thermal, magnetic, optical, and deteriorative实际上,固体材料的所有重要性质都可以分为六类:机械、电气、热、磁、光学和腐蚀性。
3.In addition to structure and properties, two other important components are involved in the scien ce and engineering of materials- namely“processing”and“performance”.除了结构和性能之外,材料科学和工程还涉及另外两个重要的组成部分,即“加工”和“性能”。
材料科学与工程专业英语翻译

Unit1:交叉学科交叉学科 interdiscipline 介电常数介电常数 dielectric constant 固体性质固体性质 solid materials 热容热容 heat capacity 力学性质力学性质 mechanical property 电磁辐射电磁辐射 electro-magnetic radiation 材料加工材料加工 processing of materials 弹性模量(模数)elastic coefficient 1.直到最近,科学家才终于了解材料的结构要素与其特性之间的关系。
It was not until relatively recent times times that that scientists came to to understand understand the relationship between the structural elements of materials and their properties . 2.材料工程学主要解决材料的制造问题和材料的应用问题。
Material Material engineering engineering mainly to solve the problem and create material application. 3.材料的加工过程不但决定了材料的结构,同时决定了材料的特征和性能。
Materials processing process is not only to de structure and decided that the material characteristic and performance. 4.材料的力学性能与其所受外力或负荷而导致的形变有关。
Material Material mechanical mechanical properties with the extemal force or in de deformation of the load. Unit2:先进材料先进材料 advanced material 陶瓷材料陶瓷材料 ceramic material 粘土矿物粘土矿物 clay minerals 高性能材料高性能材料 high performance material 合金合金 metal alloys 移植移植 implant to 玻璃纤维玻璃纤维 glass fiber 碳纳米管碳纳米管 carbon nanotub 1、金属元素有许多有利电子,金属材料的许多性质可直接归功于这些电子。
材料科学与工程专业英语第三版-翻译以及答案

UNIT 1一、材料根深蒂固于我们生活的程度可能远远的超过了我们的想象,交通、装修、制衣、通信、娱乐(recreation)和食品生产,事实上(virtually),我们生活中的方方面面或多或少受到了材料的影响。
历史上,社会的发展和进步和生产材料的能力以及操纵材料来实现他们的需求密切(intimately)相关,事实上,早期的文明就是通过材料发展的能力来命名的(石器时代、青铜时代、铁器时代)。
二、早期的人类仅仅使用(access)了非常有限数量的材料,比如自然的石头、木头、粘土(clay)、兽皮等等。
随着时间的发展,通过使用技术来生产获得的材料比自然的材料具有更加优秀的性能。
这些性材料包括了陶瓷(pottery)以及各种各样的金属,而且他们还发现通过添加其他物质和改变加热温度可以改变材料的性能。
此时,材料的应用(utilization)完全就是一个选择的过程,也就是说,在一系列有限的材料中,根据材料的优点来选择最合适的材料,直到最近的时间内,科学家才理解了材料的基本结构以及它们的性能的关系。
在过去的100年间对这些知识的获得,使对材料性质的研究变得非常时髦起来。
因此,为了满足我们现代而且复杂的社会,成千上万具有不同性质的材料被研发出来,包括了金属、塑料、玻璃和纤维。
三、由于很多新的技术的发展,使我们获得了合适的材料并且使得我们的存在变得更为舒适。
对一种材料性质的理解的进步往往是技术的发展的先兆,例如:如果没有合适并且没有不昂贵的钢材,或者没有其他可以替代(substitute)的东西,汽车就不可能被生产,在现代、复杂的(sophisticated)电子设备依赖于半导体(semiconducting)材料四、有时,将材料科学与工程划分为材料科学和材料工程这两个副学科(subdiscipline)是非常有用的,严格的来说,材料科学是研究材料的性能以及结构的关系,与此相反,材料工程则是基于材料结构和性能的关系,来设计和生产具有预定性能的材料,基于预期的性能。
材料科学与工程专业英语课件

Ferrous Alloys
• The majority of engineering designs that require structural load support or power transmission involve ferrous alloys. as a practical matter, these alloys fall into two broad categories based on the carbon in the alloy composition.
ball bearing 滚珠轴承 多相 加工的,精细的 可锻的 有延展性的
Ferrous Alloys
• • • • Arbitrary 专横的,独断的,任意的 Smelt 熔炼 ,精炼 Intricate 复杂的 ,精巧的 Combination 结合 ,合并, 化合物
Ferrous Alloys
Mechanical Engineering materials
Organic polymer materials Inorganic non-metallic materials
plastic rubber Synthetic Fibers Traditional ceramics Special Ceramics Metal Matrix Composites
材料科学与专业英语

④Alloys specially designed for highly demanding applications such as jet engines , may contain more than ten elements .
二、①金属元素有许多游离电子,金属材料的许多性质可直接归功于这些电子。
Metallic materials have large number of nonlocalized electrons , and many properties of metals are directly attributable to these electrons.
③The strength of metals suggests that these atoms are held together by strong bonds.
金属的强度表明金属中原子是通过很强的键结合在一起的。
④An element’s atomic number indicates the number of positively charged protons in the nucleus. The atomic weight of an atom indicates how many protons and neutrons in the nucleus.
热导率
二、composite materials
复合材料
nonlocalized electrons
游离电子
advanced materials
先进材料
stiffnesses刚度
semiconductors半导体
金属材料专业英语

一级相变 first-order phase transition 化学势的一阶偏导数(焓、体积)发生变化的相变过程,
即相变时有体积的变化同时有热量的吸收或释放。所有一级相变均需要经过形核
长大过程。
二级相变 second-order phase transition 又称连续相变(continuous phase transformation)。两相
1-100 nm),并由此具有某些新特性的材料。
低维材料 low-dimensional materials 维数低于三维的材料的统称,包括二维、一维和零
维材料。
晶体材料 crystalline materials 由结晶物质构成的固体材料,其所含的原子、离子、分子或
粒子集团等具有周期性的规则排列。
具有优异性能的材料。
结构材料 structural materials 以强度、硬度、塑性、韧性等力学性质为主要性能指标的工
程材料的统称。
功能材料 functional materials 主要利用力学性能以外的其他特殊的物理、化学或生物医学
等功能的材料的统称。
信息材料 information materials 用于实现传输、探测、存储、运算、处理和显示信息的
材料物理与化学 materials physics and chemistry 以物理、化学、数学等自然科学为基础,
从分子、原子、电子等多层次上研究材料的物理、化学行为与规律的学科。
材料学 materials 研究各类材料的组成、结构、工艺、性能与使用效能之间相互关系的
学科。
材料加工工程 materials processing engineering 研究控制材料的外部形状和内部组织结构,以
材料科学与工程专业英语课文 自己整理的 可以打印 匡少平 王世颖 第二版 化学工业出版社

Unit 1Materials are probably more deep-seated in our culture than most of us realize. Transportation, housing, clothing, communication, recreation, and food production— virtually every segment of our everyday lives is influenced to one degree or another by materials. Historically, the development and advancement of societies have been intimately tied to the members’ ability to produce and manipulate materials to fill their needs. In fact, early civilizations have been designated by the level of their materials development (Stone Age, Bronze Age, Iron Age).1The earliest humans had access to only a very limited number of materials, those that occur naturally: stone, wood, clay, skins, and so on. With time they discovered techniques for producing materials that had properties superior to those of the natural ones; these new materials included pottery and various metals. Furthermore, it was discovered that the properties of a material could be altered by heat treatments and by the addition of other substances. At this point, materials utilization was totally a selection process that involved deciding from a given, rather limited set of materials the one best suited for an application by virtue of its characteristics. It was not until relatively recent times that scientists came to understand the relationships between the structural elements of materials and their properties. This knowledge, acquired over approximately the past 100 years, has empowered them to fashion, to a large degree, the characteristics of materials. Thus, tens of thousands of different materials have evolved with rather specialized characteristics that meet the needs of our modern and complex society; these include metals, plastics, glasses, and fibers.The development of many technologies that make our existence so comfortable has been intimately associated with the accessibility of suitable materials. An advancement in the understanding of a material type is often the forerunner to the stepwise progression of a technology. For example, automobiles would not have been possible without the availability of inexpensive steel or some other comparable substitute. In our contemporary era, sophisticated electronic devices rely on components that are made from what are called semiconducting materials.MATERIALS SCIENCE AND ENGINEERINGSometimes it is useful to subdivide the discipline of materials science and engineering into materials science and materials engineering sub disciplines. Strictly speaking, “materials science” involves investigating the relationships that exist between the structures and properties of materials. In contrast, “materials engineering” is, on the basis of these structure–property correlations, designing or engineering the structure of a material to produce a predetermined set of properties.2 From a functional perspective, the role of a materials scientist is to develop or synthesize new materials, whereas a materials engineer is called upon to create new products or systems using existing materials, and/or to develop techniques for processing materials. Most graduates in materials programs are trained to be both materials scientists and materials engineers.“Structure” is at this point a nebulous term that des erves some explanation. In brief, the structure of a materialusually relates to the arrangement of its internal components. Subatomic structure involves electrons within the individual atoms and interactions with their nuclei. On an atomic level, structure encompasses the organization of atoms or molecules relative to one another. The next larger structural realm, which contains large groups of atoms that are normally agglomerated together, is termed “microscopic,” meaning that which is subject to direct o bservation using some type of microscope. Finally, structural elements that may be viewed with the naked eye are termed “macroscopic.”The notion of “property” deserves elaboration. While in service use, all materials are exposed to external stimuli that evoke some type of response. For example, a specimen subjected to forces will experience deformation, or a polished metal surface will reflect light. A property is a material trait in terms of the kind and magnitude of response to a specific imposed stimulus. Generally, definitions of properties are made independent of material shape and size.Virtually all important properties of solid materials may be grouped into six different categories: mechanical, electrical, thermal, magnetic, optical, and deteriorative. For each there is a characteristic type of stimulus capable of provoking different responses. Mechanical properties relate deformation to an applied load or force; examples include elastic modulus and strength. For electrical properties, such as electrical conductivity and dielectric constant, the stimulus is an electric field. The thermal behavior of solids can be represented in terms of heat capacity and thermal conductivity. Magnetic properties demonstrate the response of a material to the application of a magnetic field. For optical properties, the stimulus is electromagnetic or light radiation; index of refraction and reflectivity are representative optical properties. Finally, deteriorative characteristics relate to the chemical reactivity of materials. The chapters that follow discuss properties that fall within each of these six classifications.In addition to structure and properties, two other important components are involved in the science and engineering of materials—namely, “processing” a nd “performance. “With regard to the relationships of these four components, the structure of a material will depend on how it is processed. Furthermore, a material’s performance will be a function of its properties. Thus, the interrelationship between processing, structure, properties, and performance is as depicted in the schematic illustration shown in Figure 1.1. Throughout this text we draw attention to the relationships among these four components in terms of the design, production, and utilization of materialsWHY STUDY MATERIALS SCIENCE AND ENGINEERING?Why do we study materials? Many an applied scientist or engineer, whether mechanical, civil, chemical, or electrical, will at one time or another be exposed to a design problem involving materials. Examples might include a transmission gear, the superstructure for a building, an oil refinery component, or an integrated circuit chip. Of course, materials scientists and engineers are specialists who are totally involved in the investigation and design of materials.Many times, a materials problem is one of selecting the right material from the many thousands that are available. There are several criteria on which the final decision is normally based. First of all, the in-service conditions must be characterized, for these will dictate the properties required of the material. On only rare occasions does a materialpossess the maximum or ideal combination of properties. Thus, it may be necessary to trade off one characteristic for another. The classic example involves strength and ductility; normally, a material having a high strength will have only a limited ductility. In such cases a reasonable compromise between two or more properties may be necessary.A second selection consideration is any deterioration of material properties that may occur during service operation. For example, significant reductions in mechanical strength may result from exposure to elevated temperatures or corrosive environments. Finally, probably the overriding consideration is that of economics: What will the finished product cost? A material may be found that has the ideal set of properties but is prohibitively expensive. Here again, some compromise is inevitable. The cost of a finished piece also includes any expense incurred during fabrication to produce the desired shape.The more familiar an engineer or scientist is with the various characteristics and structure–property relationships, as well as processing techniques of materials, the more proficient and confident he or she will be to make judicious materials choices based on these criteria.U n i t2CLASSIFICATION OF MATERIALSSolid materials have been conveniently grouped into three basic classifications: metals, ceramics, and polymers. This scheme is based primarily on chemical makeup anatomic structure, and most materials fall into one distinct grouping or another, although there are some intermediates. In addition, there are the composites, combinations of two or more of the above three basic material classes. Another classification is advanced materials—those used in high-technology applications—viz. semiconductors, biomaterials, smart materials, and nanoengineered materials;MetalsMaterials in this group are composed of one or more metallic elements (such as iron, aluminum, copper, titanium, gold, and nickel), and often also nonmetallic elements (for example, carbon, nitrogen, and oxygen) in relatively small amounts.3 Atoms in metals and their alloys are arranged in a very orderly manner (as discussed in Chapter 3),and in comparison to the ceramics and polymers, are relatively dense (Figure 1.3).With regard to mechanical characteristics, these materials are relatively stiff (Figure 1.4)and strong (Figure 1.5), yet are ductile (i.e., capable of large amounts of deformation without fracture), and are resistant to fracture (Figure 1.6), which accounts for their widespread use in structural applications. Metallic materials have large numbers of nonlocalized electrons; that is, these electrons are not bound to particular atoms .Many properties of metals are directly attributable to these electrons. For example, metals are extremely good conductors of electricity (Figure 1.7) and heat, and are not transparent to visible light; a polished metal surface has a lustrous appearance. In addition, some of the metals (viz., Fe, Co, and Ni) have desirable magnetic properties.CeramicsCeramics are compounds between metallic and nonmetallic elements; they are most frequently oxides, nitrides, and carbides. For example, some of the common ceramic materials include aluminum oxide (or alumina, Al2O3), silicon dioxide (or silica, SiO2), silicon carbide (Sic), silicon nitride (Si3N4), and, in addition, what some refer to as the traditional ceramics—those composed of clay minerals (i.e., porcelain), as well as cement, and glass. With regard to mechanical behavior, ceramic materials are relatively stiff and strong—stiffnesses and strengths are comparable to those of the metals (Figures 1.4 and 1.5). In addition, ceramics are typically very hard. On the other hand, they are extremely brittle (lack ductility), and are highly susceptible to fracture (Figure 1.6). These materials are typically insulative to the passage of heat and electricity (i.e., have low electrical conductivities, Figure 1.7), and are more resistant to high temperatures and harsh environments than metals and polymers. With regard to optical characteristics, ceramics may be transparent, translucent, or opaque (Figure1.2), and some of the oxide ceramics (e.g., Fe3O4) exhibit magnetic behavior.PolymersPolymers include the familiar plastic and rubber materials. Many of them are organic compounds that are chemically based on carbon, hydrogen, and other nonmetallic elements (vision, N, and Si). Furthermore, they have very large molecular structures, often chain-like in nature that have a backbone of carbon atoms. Some of the common and familiar polymers are polyethylene (PE), nylon, poly (vinyl chloride) (PVC), polycarbonate (PC), polystyrene (PS), and silicone rubber. These materials typically have low densities (Figure 1.3), whereas their mechanical characteristics are generally dissimilar to the metallic and ceramic materials—they are not as stiff nor as strong as these other material types (Figures 1.4 and 1.5). However, on the basis of their low densities, many times their stiffness’s and strengths on a per mass basis are comparable to the metals and ceramics. In addition, many of the polymers are extremely ductile and pliable (i.e., plastic), which means they are easily formed into complex shapes. In general, they are relatively inert chemically and unreactive in a large number of environments. One major drawback to the polymers is their tendency to soften and/or decompose at modest temperatures, which, in some instances, limits their use. Furthermore, they have low electrical conductivities (Figure1.7) and are nonmagnetic.CompositesA composite is composed of two (or more) individual materials, which come from the categories discussed above—viz., metals, ceramics, and polymers. The design goal of a composite is to achieve a combination of properties that is not displayed by any single material, and also to incorporate the best characteristics of each of the component materials. A large number of composite types exist that are represented by different combinations of metals, ceramics, and polymers. Furthermore, some naturally-occurring materials are also considered to be composites—for example, wood and bone. However, most of those we consider in our discussions are synthetic (or man-made) composites.ADVANCED MATERIALSMaterials that are utilized in high-technology (or high-tech) applications are sometimes termed advanced materials.By high technology we mean a device or product that operates or functions using relatively intricate and sophisticated principles; examples include electronic equipment (camcorders, CD/DVD players, etc.), computers, fiber-optic systems, spacecraft, aircraft, and military rocketry. These advanced materials are typically traditional materials whose properties have been enhanced, and, also newly developed, high-performance materials. Furthermore, they may be of all material types (e.g., metals, ceramics, polymers), and are normally expensive. Advanced materials include semiconductors, biomaterials, and what we may term “materials of the future” (that is, smart materials and Nan engineered materials) SemiconductorsSemiconductors have electrical properties that are intermediate between the electrical conductors (viz. metals and metal alloys) and insulators (viz. ceramics and polymers)—Figure 1.7. Furthermore, the electrical characteristics of these materials are extremely sensitive to the presence of minute concentrations of impurity atoms, for which the concentrations may be controlled over very small spatial regions. Semiconductors have made possible the advent of integrated circuitry that has totally revolutionized the electronics and computer industries (not to mention our lives) over the past three decades.BiomaterialsBiomaterials are employed in components implanted into the human body for replacement of diseased or damaged body parts. These materials must not produce toxic substances and must be compatible with body tissues (i.e., must not cause adverse biological reactions). All of the above materials—metals, ceramics, polymers, composites, and semiconductors—may be used as biomaterials. For example, some of the biomaterials that are utilized in artificial hip replacementsMaterials of the FutureSmart MaterialsSmart (or intelligent) materials are a group of new and state-of-the-art materials now being developed that will have a significant influence on many of our technologies. The adjective “smart” implies that these materials are able to sense changes in their environments and then respond to these changes in predetermined manners—traits that are also found in living organisms. In addition, this “smart” concept is being extended to rather sophisticated systems that consist of both smart and traditional materials. Components of a smart material (or system) include some type of sensor (that detects an input signal), and an actuator (that performs a responsive and adaptive function). Actuators may be called upon to change shape, position, natural frequency, or mechanical characteristics in response to changes in temperature, electric fields, and/or magnetic fields. Four types of materials are commonly used for actuators: shape memory alloys, piezoelectric ceramics, magnetostrictive materials, and electrorheological/magnetorheological fluids. Shape memory alloys are metals that, after having been deformed, revert back to their original shapes when temperature is changed (see the Materials of Importance piece following Section 10.9). Piezoelectric ceramics expand and contract in response to an applied electric field (or voltage); conversely, they also generate an electric field when their dimensions are altered (see Section18.25).The behavior of magnetostrictive materials is analogous to that of the piezoelectric, except that they are responsive to magnetic fields. Also, electro rheological and magnetorheological fluids are liquids that experience dramatic changes in viscosity upon the application of electric and magnetic fields, respectively.Materials/devices employed as sensors include optical fibers (Section 21.14), piezoelectric materials (including some polymers), and microelectromechanical devices (MEMS, Section 13.8).For example, one type of smart system is used in helicopters to reduce aerodynamic cockpit noise that is created by the rotating rotor blades. Piezoelectric sensors inserted into the blades monitor blade stresses and deformations; feedback signals from these sensors are fed into a computer-controlled adaptive device, which generates noise-canceling antinomies.Nanoengineered MaterialsUntil very recent times the general procedure utilized by scientists to understand the chemistry and physics of materials has been to begin by studying large and complex structures, and then to investigate the fundamental building blocks of these structures that are smaller and simpler. This approach is sometimes termed “top down “science. However, with the advent of scanning probe microscopes (Section4.10), which permit observation of individual atoms and molecules, it has become possible to manipulate and move atoms and molecules to form new structures and, thus, design new materials that are built from simple atomic-level constituents(i.e., “materials by design”). This ability to carefully arrange atoms provides opportunities to develop mechanical, electrical, magnetic, and other properties that are not otherwise possible. We call this the “bottom-up” approach, and the study of the properties of these materials is termed “nanotechnology”; the “nan” prefix denotes that the dimensions of these structural entities are on the order of a nanometer (10_9 m)—as a rule, less than 100 nanometers (equivalent to approximately 500atom diameters).5 One example of a material of this type is the carbon nanotube, discussed in Section 12.4. In the future we will undoubtedly find that increasingly more of our technological advances will utilize these nanengineered materials.Unit 4Physical properties are those that can be observed without changing the identity of the substance. The general properties of matter such as color, density, hardness, are examples of physical properties. Properties that describe how a substance changes into a completely different substance are called chemical properties. Flammability andcorrosion/oxidation resistance are examples of chemical properties.The difference between a physical and chemical property is straightforward until the phase of the material is considered. When a material changes from a solid to a liquid to a vapor it seems like them become a difference substance. However, when a material melts, solidifies, vaporizes, condenses or sublimes, only the state of the substance changes.Consider ice, liquid water, and water vapor, they are all simply H2O. Phase is a physical property of matter and matter can exist in four phases – solid, liquid, gas and plasma.Some of the more important physical and chemical properties from an engineering material standpoint will be discussed in the following sections.•Phase Transformation Temperatures•Density•Specific Gravity•Thermal Conductivity•Linear Coefficient of Thermal Expansion•Electrical Conductivity and Resistivity•Magnetic Permeability•Corrosion ResistancePhase Transformation TemperaturesWhen temperature rises and pressure is held constant, a typical substance changes from solid to liquid and then to vapor. Transitions from solid to liquid, from liquid to vapor, from vapor to solid and visa versa are called phase transformations or transitions. Since some substances have several crystal forms, technically there can also be solid to another solid form phase transformation.Phase transitions from solid to liquid, and from liquid to vapor absorb heat. The phase transition temperature where a solid changes to a liquid is called the melting point. The temperature at which the vapor pressure of a liquid equals 1 atm (101.3 kPa) is called the boiling point. Some materials, such as many polymers, do not go simply from a solid to a liquid with increasing temperature. Instead, at some temperature below the melting point, they start to lose their crystalline structure but the molecules remain linked in chains, which results in a soft and pliable material. The temperature at which a solid, glassy material begins to soften and flow is called the glass transition temperature.DensityMass can be thinly distributed as in a pillow, or tightly packed as in a block of lead. The space the mass occupies is its volume, and the mass per unit of volume is its density.Mass (m) is a fundamental measure of the amount of matter. Weight (w) is a measure of the force exerted by a mass and this force is force is produced by the acceleration of gravity. Therefore, on the surface of the earth, the mass of an object is determined by dividing the weight of an object by 9.8 m/s2 (the acceleration of gravity on the surface of the earth). Since we are typically comparing things on the surface of the earth, the weight of an object is commonly used rather than calculating its mass.The density (r) of a material depends on the phase it is in and the temperature. (The density of liquids and gases is very temperature dependent.) Water in the liquid state has a density of 1 g/cm3 = 1000kg/m3 at 4o C. Ice has a density of 0.917 g/cm3 at 0o c, and it should be noted that this decrease in density for the solid phase is unusual. For almost all other substances, the density of the solid phase is greater than that of the liquid phase. Water vapor (vapor saturated air) has a density of 0.051 g/cm3.Some common units used for expressing density are grams/cubic centimeter, kilograms/cubic meter, grams/milliliter, grams/liter, pounds for cubic inch and pounds per cubic foot; but it should be obvious that any unit of mass per any unit of volume can be used.Substance Density(g/cm3)Air 0.0013Gasoline 0.7Wood 0.85Water (ice) 0.92Water (liquid) 1.0Aluminum 2.7Steel 7.8Silver 10.5Lead 11.3Mercury 13.5Gold 19.3Specific GravitySpecific gravity is the ratio of density of a substance compared to the density of fresh water at 4°C (39° F). At this temperature the density of water is at its greatest value and equal 1 g/mL. Since specific gravity is a ratio, so it has no units. An object will float in water if its density is less than the density of water and sink if its density is greater that that ofwater. Similarly, an object with specific gravity less than 1 will float and those with a specific gravity greater than one will sink. Specific gravity values for a few common substances are: Au, 19.3; mercury, 13.6; alcohol, 0.7893; benzene, 0.8786. Note that since water has a density of 1 g/cm3, the specific gravity is the same as the density of the material measured in g/cm3.Magnetic PermeabilityMagnetic permeability or simply permeability is the ease with which a material can be magnetized. It is a constant of proportionality that exists between magnetic induction and magnetic field intensity. This constant is equal to approximately 1.257 x 10-6 Henry per meter (H/m) in free space (a vacuum). In other materials it can be much different, often substantially greater than the free-space value, which is symbolized µ0.Materials that cause the lines of flux to move farther apart, resulting in a decrease in magnetic flux density compared with a vacuum, are called diamagnetic. Materials that concentrate magnetic flux by a factor of more than one but less than or equal to ten are called paramagnetic; materials that concentrate the flux by a factor of more than ten are called ferromagnetic. The permeability factors of some substances change with rising or falling temperature, or with the intensity of the applied magnetic field.In engineering applications, permeability is often expressed in relative, rather than in absolute, terms. If µ o represents the permeability of free space (that is, 4p X10-7H/m or 1.257 x 10-6 H/m) and µ represents the permeability of the substance in question (also specified in henrys per meter), then the relative permeability, µr, is given by:µr = µ / µ0For non-ferrous metals such as copper, brass, aluminum etc., the permeability is the same as that of "free space", i.e. the relative permeability is one. For ferrous metals however the value of µ r may be several hundred. Certain ferromagnetic materials, especially powdered or laminated iron, steel, or nickel alloys, have µr that can range up to about 1,000,000. Diamagnetic materials have µr less than one, but no known substance has relative permeability much less than one. In addition, permeability can vary greatly within a metal part due to localized stresses, heating effects, etc.When a paramagnetic or ferromagnetic core is inserted into a coil, the inductance is multiplied by µr compared with the inductance of the same coil with an air core. This effect is useful in the design of transformers and eddy current probes.Unit 5The mechanical properties of a material are those properties that involve a reaction to an applied load. The mechanical properties of metals determine the range of usefulness of a material and establish the service life that can be expected. Mechanical properties are also used to help classify and identify material. The most common properties considered are strength, ductility, hardness, impact resistance, and fracture toughness.Most structural materials are anisotropic, which means that their material properties vary with orientation. The variation in properties can be due to directionality in the microstructure (texture) from forming or cold working operation, the controlled alignment of fiber reinforcement and a variety of other causes. Mechanical properties are generally specific to product form such as sheet, plate, extrusion, casting, forging, and etc. Additionally, it is common to see mechanical property listed by the directional grain structure of the material. In products such as sheet and plate, the rolling direction is called the longitudinal direction, the width of the product is called the transverse direction, and the thickness is called the short transverse direction. The grain orientations in standard wrought forms of metallic products are shown the image.The mechanical properties of a material are not constants and often change as a function of temperature, rate of loading, and other conditions. For example, temperatures below room temperature generally cause an increase in strength properties of metallic alloys; while ductility, fracture toughness, and elongation usually decrease. Temperatures above room temperature usually cause a decrease in the strength properties of metallic alloys. Ductility may increase or decrease with increasing temperature depending on the same variablesIt should also be noted that there is often significant variability in the values obtained when measuring mechanical properties. Seemingly identical test specimen from the same lot of material will often produce considerable different results. Therefore, multiple tests are commonly conducted to determine mechanical properties and values reported can be an average value or calculated statistical minimum value. Also, a range of values are sometimes reported in order to show variability.LoadingThe application of a force to an object is known as loading. Materials can be subjected to many different loading scenarios and a material’s performance is dependant on the loading conditions. There are five fundamental loadin g conditions; tension, compression, bending, shear, and torsion. Tension is the type of loading in which the two sections of material on either side of a plane tend to be pulled apart or elongated. Compression is the reverse of tensile loading and involves pressing the material together. Loading by bending involves applying a load in a manner that causes a material。
材料科学与工程专业(第四版)英语翻译(1,2,3,5,6,7单元)

第一单元:材料科学与工程历史看法材料可能比我们意识的还要根深蒂固的占据在历史中。
运输、房屋、衣服、通讯、娱乐以及食物产品----事实上我们生活中的每一个部分都或多或少受材料的影响。
历史上,社会的发展与前进和那些能满足社会需求的材料的生产及操作能力密切相关。
实际上,早起的文明就以材料的发展程度来命名,如石器时代,青铜时代,铁器时代。
早期人们能得到的只有一些很有限的天然材料:石头、木材、粘土以及动物皮毛等。
渐渐地,他们通过技术来生产优于自然材料的新材料,这些新材料包括陶器和金属。
进一步地,人们发现材料的性质可以通过热处理或加入其他物质来改变。
由此看来,材料的应用完全是一个选择的过程,且此过程又是根据材料的性能从许多的而不是有限的材料中选择一种最适于某种用途的材料。
直到最近,科学家才终于了解材料的结构要素与其特性之间的关系。
在跨越将近100年的时间获得的知识被大范围的赋予符合当代的材料特性。
因此,成千上万的材料通过其特殊的性质来满足我们现代及复杂的社会需要;它们包括金属,塑料,玻璃和纤维。
很多使我们生活舒适的技术的发展与适宜材料的获得密切相关。
一种认识的材料的先进程度通常是一种连续技术进步的先兆。
比如,没有便宜的钢制品或其他替代品就没有汽车。
在现代,精密的电子器件取决于所谓的半导体零件。
材料科学与工程有条理的材料科学涉及到材料的结构和性质的关系。
相比之下,材料工程是根据材料的结构和性质的关系来设计或操纵材料的结构以求制造出一系列可预定的性质。
“structure”一词是个值得解释的模糊的术语。
简单地说,材料的结构通常与其内在成分的排列有关。
亚原子结构包括介于单个原子内部的电子以及相互作用的原子核。
在原子水平上,结构围绕着原子或分子与其他相关的原子或分子的组织。
在更大的结构领域上,其包括大的原子团,这些原子团通常聚集在一起,称为“微观”结构,意思是可以使用某种显微镜直接观察得到的结构。
最后,结构单元可以通过肉眼看到的称为宏观结构。
材料科学与工程专业(第四版)英语翻译(1

材料科学与工程专业(第四版)英语翻译(1UNIT 1一、材料根深蒂固于我们生活的程度可能进进的超过了我们的想象,交通、装修、制衣、通信、娱乐(recreation)和食品生产,事实上(virtually),我们生活中的方方面面或多或少受到了材料的影响。
历史上,社会的发展和迚步和生产材料的能力以及操纵材料杢实现他们的需求密切(intimately)相关,事实上,早期的文明就是通过材料发展的能力杢命名的(石器时代、青铜时代、铁器时代)。
二、早期的人类仅仅使用(aess)了非常有限数量的材料,比如自然的石头、木头、粘土(clay)、兽皮等等。
随着时间的发展,通过使用技术杢生产获得的材料比自然的材料具有更加优秀的性能。
这些性材料包拪了陶瓷(pottery)以及各种各样的金属,而且他们还发现通过添加其他物质和改变加热温度可以改变材料的性能。
此时,材料的应用(utilization)完全就是一个选择的过程,也就是说,在一系列有限的材料中,根据材料的优点杢选择最合适的材料,直到最近的时间内,科学家才理解了材料的基本结构以及它们的性能的关系。
在过去的100年间对这些知识的获得,使对材料性质的研究变得非常时髦起杢。
因此,为了满足我们现代而且复杂的社会,成千上万具有不同性质的材料被研发出杢,包拪了金属、塑料、玻璃和纤维。
三、由于很多新的技术的发展,使我们获得了合适的材料幵且使得我们的存在变得更为舒适。
对一种材料性质的理解的迚步往往是技术的发展的先兆,例如:如果没有合适幵且没有不昂贵的钢材,或者没有其他可以替代(substitute)的东西,汽车就不可能被生产,在现代、复杂的(sophisticated)电子设备依赖于半导体(semiconducting)材料四、有时,将材料科学与工程划分为材料科学和材料工程这两个副学科(subdiscipline)是非常有用的,严栺的杢说,材料科学是研究材料的性能以及结构的关系,与此相反,材料工程则是基于材料结构和性能的关系,杢设计和生产具有预定性能的材料,基于预期的性能。
材料科学与工程专业英语第二版_翻译答案(匡少平),单元:1,2,4,5,7,8,9,10,11,13,16,19,22

Unit1:2.英译汉材料科学石器时代肉眼青铜器时代光学性质集成电路机械(力学)强度热导率1.材料科学指的是研究存于材料的结构和性能的相互关系。
相反,材料工程指的是,在基于材料结构和性能的相互关系的基础上,开发和设计预先设定好具备若干性能的材料。
2. 实际上,固体材料的所有重要性质可以概括分为六类:机械、电学、热学、磁学、光学和腐蚀降解性。
3. 除了结构和性质,材料科学和工程还有其他两个重要的组成部分:即加工和性能。
4. 工程师与科学家越熟悉材料的结构-性质之间的各种相互关系以及材料的加工技术,根据这些原则,他或她对材料的明智选择将越来越熟练和精确。
5. 只有在极少数情况下材料在具有最优或理想的综合性质。
因此,有必要对材料的性质进行平衡。
3. 汉译英Interdispline dielectric constantSolid materials heat capacityMechanical properties electro-magnetic radiationMaterials processing elasticity modulus1.直到最近,科学家才终于了解材料的结构要素与其特性之间的关系。
It was not until relatively recent times that scientists came to understand the relationship between the structural elements of materials and their properties . 2.材料工程学主要解决材料的制造问题和材料的应用问题。
Material engineering mainly solve the problems of materials processing and materials application.3.材料的加工过程不但决定了材料的结构,同时决定了材料的特征和性能。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Unit 1 Physical and chemical properties of materials
• Physical properties are those that can be observed without changing the identity of the substance. The general properties of matter such as color, density, hardness, are examples of physical properties. Properties that describe how a substance changes into a completely different substance are called chemical properties. Flammability and corrosion/oxidation resistance are examples of chemical properties.
材料专业英语
• In general, some of the more important physical and chemical properties from an engineering material standpoint include phase transformation temperatures, density, specific gravity, thermal conductivity, linear coefficient of thermal expansion, electrical conductivity and resistivity, magnetic permeability, and corrosion resistance, and so on. 磁导率
材料专业英语
Part 1 Introduction to materials science and engineering
• Unit 1 Physical and chemical properties of materials
• Unit 2 Mechanical properties of materials
材料专业英语
Melting point: the phase transition temperature where a solid changes to a liquid
Boiling point: the temperature at which the vapor pressure of a liquid equals 1 atm (101.3 kPa)
材料专业英语
Some materials, such as many polymers, do not go simply from a solid to a liquid with increasing temperature. Instead, at some temperature below the melting point, they start to lose their crystalline structure but the molecules remain linked in chains, which results in a soft and pliable material. The temperature at which a solid, glassy material begins to soften and flow is called the glass transition temperature.
材料专业英语
• Mass (m) is a fundamental measure of the amount of matter. Weight (w) is a
材料专业英语翻译
主讲:林建国
材料科学与工程专业英语
课程简介:
• 课堂6学时,课后14学时 • 考试:开卷,当堂完成
材料专业英语
主要内容及课时安排
• Introduction to materials science and engineering(4学时)
• Metallic materials and alloys(4学时) • Ceramics(2学时) • Composites(2学时) • Nanostructured materials(2学时)
材料专业英语
Phase Transformation Temperatures
• When temperature rises and pressure is held constant, a typical substance changes from solid to liquid and then to vapor. Transitions from solid to liquid, from liquid to vapor, from vapor to solid and visa versa are called phase transformations or transitions. Since some substances have several crystal forms, technically there can be solid to another solid from phase transformation.
材料专业英语
D thinly dsitrbuted as in a pillow, or tightly packed as in a block of lead.
• 质量可以像枕头似地稀疏地分布,也可以 像铅那样紧紧地堆积在一起。
• The space the mass occupies is its volume, and the mass per unit of volume is its density.
