机械专业英语金属热处理
(完整版)机械专业英语词汇【最新完整版】

螺钉 screw 铣削 mill 铣刀 milling cutter 功率 power 工件 workpiece 齿轮加工 gear mechining 齿轮 gear 主运动 main movement 主运动方向 direction of main movement 进给方向 direction of feed 进给运动 feed movement 合成进给运动 resultant movement of feed 合成切削运动 resultant movement of
误差 error 响应 response 定位 allocation 机床夹具 jig 动力学 dynamic 运动学 kinematic 静力学 static 分析力学 analyse mechanics 拉伸 pulling 压缩 hitting
剪切 shear 扭转 twist 弯曲应力 bending stress 强度 intensity 三相交流电 three-phase AC 磁路 magnetic circles 变压器 transformer 异步电动机 asynchronous motor 几何形状 geometrical 精度 precision 正弦形的 sinusoid 交流电路 AC circuit 机械加工余量 machining allowance 变形力 deforming force 变形 deformation 应力 stress 硬度 rigidity 热处理 heat treatment 退火 anneal 正火 normalizing 脱碳 decarburization
mechanical-electrical integration 气压 air pressure pneumatic pressure
机械生产常用专业英语单词
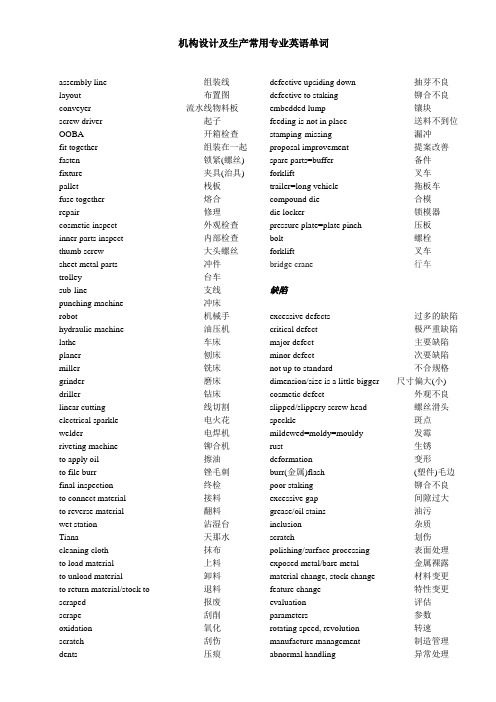
机构设计及生产常用专业英语单词assembly line 组装线layout 布置图conveyer 流水线物料板screw driver 起子OOBA 开箱检查fit together 组装在一起fasten 锁紧(螺丝) fixture 夹具(治具) pallet 栈板fuse together 熔合repair 修理cosmetic inspect 外观检查inner parts inspect 内部检查thumb screw 大头螺丝sheet metal parts 冲件trolley 台车sub-line 支线punching machine 冲床robot 机械手hydraulic machine 油压机lathe 车床planer 刨床miller 铣床grinder 磨床driller 钻床linear cutting 线切割electrical sparkle 电火花welder 电焊机riveting machine 铆合机to apply oil 擦油to file burr 锉毛刺final inspection 终检to connect material 接料to reverse material 翻料wet station 沾湿台Tiana 天那水cleaning cloth 抹布to load material 上料to unload material 卸料to return material/stock to 退料scraped 报废scrape 刮削oxidation 氧化scratch 刮伤dents 压痕defective upsiding down 抽芽不良defective to staking 铆合不良embedded lump 镶块feeding is not in place 送料不到位stamping-missing 漏冲proposal improvement 提案改善spare parts=buffer 备件forklift 叉车trailer=long vehicle 拖板车compound die 合模die locker 锁模器pressure plate=plate pinch 压板bolt 螺栓forklift 叉车bridge crane 行车缺陷excessive defects 过多的缺陷critical defect 极严重缺陷major defect 主要缺陷minor defect 次要缺陷not up to standard 不合规格dimension/size is a little bigger 尺寸偏大(小) cosmetic defect 外观不良slipped/slippery screw head 螺丝滑头speckle 斑点mildewed=moldy=mouldy 发霉rust 生锈deformation 变形burr(金属)flash (塑件)毛边poor staking 铆合不良excessive gap 间隙过大grease/oil stains 油污inclusion 杂质scratch 划伤polishing/surface processing 表面处理exposed metal/bare metal 金属裸露material change, stock change 材料变更feature change 特性变更evaluation 评估parameters 参数rotating speed, revolution 转速manufacture management 制造管理abnormal handling 异常处理production unit 生产单位lots of production 生产批量steel plate 钢板roll material 卷料automation 自动化to stake/staking/riveting 铆合add lubricating oil 加润滑油argon welding 氩焊冲压常词汇vocabulary for stampingstamping/press 冲压punch press/dieing out press 冲床feeder 送料机rack/shelf/stack 料架cylinder 油缸robot 机械手taker 取料机conveyer belt 输送带transmission rack 输送架top stop 上死点bottom stop 下死点one stroke 一行程inch 寸动to continue/cont. 连动to grip(material) 吸料location lump/locating piece/block stop 定位块reset 复位dent 压痕scratch 刮伤deformation 变形filings 铁削to draw holes 抽孔to return delivery to/to send delivery back/to return of abrasion 磨损reverse angle /chamfer 倒角to take apart a die 卸下模具to load a die 装上模具to tight a bolt 拧紧螺栓to looser a bolt 拧松螺栓to move away a die plate 移走模板easily damaged parts 易损件standard parts 标准件breaking/(be)broken/(be)cracked 断裂to lubricate 润滑模具工程常用词汇(common vocabulary for die engineering)die 模具punched hole 冲孔panel board 镶块to cut edges/side cut/side scrap 切边to bending 折弯to pull, to stretch 拉伸Line stretching/line pulling 线拉伸spring 弹簧bolt 螺栓plate 电镀mold 成型steel/rolled steel 钢材wire EDM 线割torch-flame cut 火焰切割stock locater block 定位块under cut=scrap chopper 清角male die 公模female die 母模heat dissipation 热传递rack 上料degrease 脱脂Anodize 阳性处理molding 成型thermocouple 热电偶sand blasting 喷沙grit 砂砾derusting machine 除锈机degate 打浇口concave 凸convex 凹nick 缺口speck 瑕疵检验量测工具用语calibration 校准caliper gauge 卡规cylinder square 圆筒直尺dial snap gauge 卡规inside calipers 内卡钳leveling block 平台monometer 压力计nonius 游标卡尺passimeter 内径仪1protractor 分角器radius 半径ring gauge 环规sine bar 正弦量规snap gauge 卡模square master 直角尺模具钢材alloy tool steel 合金工具钢aluminium alloy 铝合金钢bearing alloy 轴承合金blister steel 浸碳钢carbon tool steel 碳素工具钢forging die steel 锻造模用钢galvanized steel sheet 镀锌铁板hard alloy steel 超硬合金钢hot work die steel 热锻模用钢molybdenum high speed steel 钼系高速钢molybdenum steel 钼钢nickel chromium steel 镍铬钢prehardened steel 顶硬钢silicon steel sheet 矽钢板stainless steel 不锈钢表面处理关连用语age hardening 时效硬化ageing 老化处理air hardening 气体硬化air patenting 空气韧化annealing 退火anode effect 阳极效应anodizing 阳极氧化处理austempering 奥氏体等温淬火austenite 奥斯田体/奥氏体bainite 贝氏体carbide 炭化物coarsening 结晶粒粗大化decarburization 脱碳处理decarburizing 脱碳退火diffusion 扩散diffusion annealing 扩散退火full annealing 完全退火gaseous cyaniding 气体氧化法cementite 渗碳体grain size 结晶粒度hardening 硬化heat treatment 热处理low temperature annealing 低温退火malleablizing 可锻化退火martensite 马氏体/硬化铁炭pearlite 珠光体组织precipitation 析出quench hardening 淬火quenching stress 淬火应力reconditioning 再调质recrystallization 再结晶residual stress 残留应力retained austenite 残留奥氏体segregation 偏析stress relieving annealing 应力消除退火supercooling 过冷surface hardening 表面硬化处理tempering 回火tempering crack 回火裂痕thermal refining 调质处理thermoechanical treatment 加工热处理vacuum hardening 真空淬火vacuum heat treatment 真空热处理vacuum nitriding 真空氮化water quenching 水淬火焊接用语acetylene 乙炔ampere 电流安培angle welding 角焊arc 电弧argon arc welding 氩弧焊接filler rod 焊条fillet weld 填角焊接hand face shield 手握面罩metal electrode insert gas welding MIG熔接nugget 点焊熔核overlaying 堆焊pressure welding 压焊seam 焊缝spark 火花spot welding 点焊接stud arc welding 电弧焊接weld line 焊接纹weld mark 焊接痕welding 焊接welding flux 焊剂2caulking compound 填隙料cell 气孔nozzle 喷嘴模具常用刀具与工作法用语adjustable spanner 活动扳手angle cutter 角铣刀buffing 抛光chamfering machine 倒角机chamfering tool 去角刀具chuck 夹具concave cutter 凹面铣刀convex cutter 凸形铣刀drill stand 钻台edge file 刃用锉刀file 锉刀grinder 砂轮机hatching 剖面线hexagon headed bolt 六角头螺栓hexagon nut 六角螺帽jack 千斤顶nose angle 刀角pinchers 钳子plug 柱塞头polisher 磨光器punch 冲头sand paper 砂纸scraper 刮刀screw driver 螺丝起子scribing 划线spanner 扳手square 直角尺square trowel 直角度T-slot T形槽tool for lathe 车刀tool point angle 刀刃角tool post 刀架tosecan 划线盘trimming 去毛边waffle die flattening 压纹效平wiper 脱模钳wrench 螺旋扳手模具加工方法barrel 滚筒(加工) centering 定中心cutting 切削cylindrical lathe cutting 外圆车削facing 面车削filing 锉刀修润hand finishing 手工修润hemming 卷边加工hobbing 滚齿加工joggling 摇动加工lapping 抛光/研磨修润lathe cutting 车床车削planning 刨削加工polishing 抛亮光reaming 铰孔修润rough machining 粗切削rounding 圆形加工sawing 锯削scaling 清除钢碇缺陷shaping 成形加工skiving 表面研磨slotting 切缝切削taper turning 锥度车削thread cutting 螺纹切削ultrasonic machining 超音波加工up cut milling 逆铣加工学理实验与试验用语air permeability test 透气性试验Brinell hardness 布耐内尔硬度Brinell hardness test 布氏硬度试验Charpy impact test 夏比冲击试验dart drop impact test 落锤冲击试验fatigue test 疲劳试验hot bend test 热弯试验Rockweel hardness test 洛氏硬度试验Rockweel hardness 洛氏威尔硬度scratch hardness 抗刮硬度shore hardness 萧氏硬度tensile impact test 拉伸冲击试验tensile strength 抗拉强度thermal shock test 冷热剧变试验torsion test 扭曲试验Vicat indentation test 维卡针压陷试验3Vickers hardness test 维氏硬度试验warpage test 翘曲试验砂轮用语abrasive 砂轮Al2O3 氧化铝bond 结合buffing wheel 抛光布轮dresser 砂轮整修机endless grinding belt 循环式研磨带finishing allowance 加工余量grain 磨粒grinding disc 研磨盘mesh 网筛目parameter 参数slitting 切缝量vitrified 陶瓷的wheel 旋转机械设计及周边其他用语assembly drawing 装配图auto tool change cycle 自动换刀时间周期beam 横梁bending moment 弯矩bending stress 弯曲应力bottoming 底靠buckling 纵弯曲chamfering 去角斜切channel 凹槽chattering 颤动check point 查核点chip 切屑chip conveyor 排屑输送机coefficient of friction 摩擦系数cooling pipe 冷却管distortion 扭曲变形draft taper 拔模锥度draw out 拉拔fit tolerance 配合公差flexible rigidity 弯曲刚性gas vent 气孔hatching 剖面线heater cooler 加热器冷却装置hook cavity 钩穴inching 寸动lug 凸缘maintenance 维修保固notch effect 切口效果out of roughness 真圆度performance 动作性能pit 坑plane strain 倒角应力repeated load 重覆载荷sand paper 砂纸shift 偏移shrinkage hole 缩孔sinking 凹陷sketch 草图spalling 剥落straightness 直度submarine 深陷式surface roughness 表面粗度tapping 攻螺丝thermocouple 热电耦torsion load 扭转载荷toughness 韧性tracing 描图under cut 凹割图纸常用词汇nozzle 接管喷嘴Orientation 方位lifting lug 吊耳Name plate 铭牌Grinding for smooth transition 打磨圆滑过渡Eradicate 根除Polish 磨光Flange 法兰Overlay 补堆Shell 壳体Reactor 反应器pre-assemble 预组装Catalyst 催化剂Base template 基础模板Solution outlet screen 出口过滤器4。
机械专业英语词汇汇总

机械专业英语词汇(大全)金属切削metal cutting 机床machine tool 金属工艺学technology of metals 刀具cutter 摩擦friction 联结link 传动drive/transmission 轴shaft 弹性elasticity 频率特性frequency characteristic 误差error 响应response 定位allocation 机床夹具jig 动力学dynamic 运动学kinematic 静力学static分析力学analyse mechanics 拉伸pulling 压缩hitting剪切shear 扭转twist 弯曲应力bending stress强度intensity 三相交流电three-phase AC 磁路magnetic circles变压器transformer 异步电动机asynchronous motor几何形状geometrical 精度precision 正弦形的sinusoid交流电路AC circuit 机械加工余量machining allowance变形力deforming force 变形deformation 应力stress硬度rigidity 热处理heat treatment 退火anneal正火normalizing 脱碳decarburization 渗碳carburization电路circuit 半导体元件semiconductor element反馈feedback 发生器generator 直流电源DC electrical source门电路gate circuit 逻辑代数logic algebra 外圆磨削external grinding 内圆磨削internal grinding 平面磨削plane grinding 变速箱gearbox 离合器clutch绞孔fraising 绞刀reamer 螺纹加工thread processing螺钉screw 铣削mill 铣刀milling cutter 功率power 工件workpiece 齿轮加工gear mechining齿轮gear 主运动main movement 主运动方向direction of main movement进给方向direction of feed 进给运动feed movement合成进给运动resultant movement of feed 合成切削运动resultant movement of cutting合成切削运动方向direction of resultant movement of cutting切削深度cutting depth 前刀面rake face刀尖nose of tool 前角rake angle 后角clearance angle 龙门刨削planing 主轴spindle主轴箱headstock 卡盘chuck 加工中心machining center车刀lathe tool 车床lathe 钻削镗削bore车削turning 磨床grinder 基准benchmark钳工locksmith 锻forge 压模stamping焊weld 拉床broaching machine 拉孔broaching装配assembling 铸造found 流体动力学fluid dynamics流体力学fluid mechanics 加工machining 液压hydraulic pressure切线tangent 机电一体化mechanotronics mechanical-electrical integration气压air pressure pneumatic pressure 稳定性stability 介质medium 液压驱动泵fluid clutch液压泵hydraulic pump 阀门valve 失效invalidation强度intensity 载荷load 应力stress安全系数safty factor 可靠性reliability 螺纹thread螺旋helix 键spline 销pin 滚动轴承rolling bearing滑动轴承sliding bearing 弹簧spring制动器arrester brake 十字结联轴节crosshead 联轴器coupling链chain 皮带strap 精加工finish machining粗加工rough machining 变速箱体gearbox casing腐蚀rust 氧化oxidation 磨损wear耐用度durability 随机信号random signal 离散信号discrete signal超声传感器ultrasonic sensor 集成电路integrate circuit挡板orifice plate 残余应力residual stress 套筒sleeve 扭力torsion 冷加工cold machining 电动机electromotor 汽缸cylinder 过盈配合interference fit热加工hotwork 摄像头CCD camera 倒角rounding chamfer优化设计optimal design 工业造型设计industrial moulding design有限元finite element 滚齿hobbing插齿gear shaping 伺服电机actuating motor 铣床milling machine钻床drill machine 镗床boring machine步进电机stepper motor 丝杠screw rod 导轨lead rail组件subassembly 可编程序逻辑控制器Programmable Logic Controller PLC电火花加工electric spark machining 电火花线切割加工electrical discharge wire - cutting 相图phase diagram 热处理heat treatment固态相变solid state phase changes 有色金属nonferrous metal陶瓷ceramics 合成纤维synthetic fibre电化学腐蚀electrochemical corrosion 车架automotive chassis悬架suspension 转向器redirector 变速器speed changer板料冲压sheet metal parts 孔加工spot facing machining车间workshop 工程技术人员engineer 气动夹紧pneuma lock数学模型mathematical model 画法几何descriptive geometry机械制图Mechanical drawing 投影projection视图view 剖视图profile chart 规范件standard component零件图part drawing 装配图assembly drawing尺寸标注size marking 技术要求technical requirements刚度rigidity 内力internal force 位移displacement截面section 疲劳极限fatigue limit 断裂fracture 塑性变形plastic distortion 脆性材料brittleness material刚度准则rigidity criterion 垫圈washer垫片spacer 直齿圆柱齿轮straight toothed spur gear斜齿圆柱齿轮helical-spur gear 直齿锥齿轮straight bevel gear 运动简图kinematic sketch 齿轮齿条pinion and rack 蜗杆蜗轮worm and worm gear 虚约束passive constraint曲柄crank 摇杆racker 凸轮cams共轭曲线conjugate curve 范成法generation method 定义域definitional domain 值域range 导数\\微分differential coefficient求导derivation 定积分definite integral不定积分indefinite integral 曲率curvature偏微分partial differential 毛坯rough游标卡尺slide caliper 千分尺micrometer calipers 攻丝tap 二阶行列式second order determinant 逆矩阵inverse matrix线性方程组linear equations 概率probability随机变量random variable 排列组合permutation and combination气体状态方程equation of state of gas 动能kinetic energy势能potential energy 机械能守恒conservation of mechanical energy动量momentum 桁架truss 轴线axes余子式cofactor 逻辑电路logic circuit 触发器flip-flop脉冲波形pulse shape 数模digital analogy 液压传动机构fluid drive mechanism 机械零件mechanical parts淬火冷却quench 淬火hardening 回火tempering 调质hardening and tempering 磨粒abrasive grain 结合剂bonding agent 砂轮grinding wheel。
(完整版)机械电子类专业英语概要

1机械专业英语词汇Date 约会金属切削metal cuttingServey 调查Liquid Crystal Display LCD机床machine tool金属工艺学technology of metals刀具cutter摩擦friction联结link传动drive/transmission轴shaft axis/'æksis]/弹性elasticity [,elæs'tisəti]频率特性frequency characteristic误差error响应response定位allocation机床夹具jig [dʒiɡ]动力学dynamic运动学kinematic [,kɪnɪ'mætɪk]静力学static分析力学analyse mechanics [mi'kæniks]拉伸pulling压缩hitting剪切shear扭转twist弯曲应力bending stress强度intensity三相交流电three-phase AC磁路magnetic circles变压器transformer异步电动机asynchronous motor[ei'siŋkrənəs]几何形状geometrical精度precision [pri'siʒən]正弦形的sinusoid交流电路AC circuit ['sə:kit]机械加工余量machining allowance变形力deforming force变形deformation应力stress硬度rigidity[ri'dʒidəti]热处理heat treatment退火anneal[ə'ni:l]正火normalizing['nɔ:məlaiziŋ]脱碳decarburization[di,kɑrbjərɪ'zeʃən渗碳carburization电路circuit半导体元件semiconductor element [,semikən'dɔktə]反馈feedback发生器generator直流电源DC electrical source门电路gate circuit逻辑代数logic algebra外圆磨削external grinding内圆磨削internal grinding平面磨削plane grinding变速箱gearbox离合器clutch [klʌtʃ]绞孔fraising绞刀reamer ['riːmə(r)]螺纹加工thread processing螺钉screw铣削mill铣刀milling cutter功率power工件workpiece齿轮加工gear mechining齿轮gear主运动main movement主运动方向direction of main movement进给方向direction of feed进给运动feed movement合成进给运动resultant movement of feed合成切削运动resultant movement ofcutting合成切削运动方向direction of resultant movement of cutting切削深度cutting depth前刀面rake face刀尖nose of tool前角rake angle后角clearance angle龙门刨削planing主轴spindle ['spindl]主轴箱headstock卡盘chuck [tʃʌk]2加工中心machining center车刀lathe tool车床lathe钻削镗削bore车削turning磨床grinder基准benchmark钳工locksmith锻forge压模stamping焊weld拉床broaching machine拉孔broaching装配assembling铸造found流体动力学fluid dynamics流体力学fluid mechanics加工machining液压hydraulic pressure /haɪ'drɔlɪk]/切线tangent ['tændʒənt]机电一体化mechanotronicsmechanical-electrical integration [,inti'ɡreiʃən]气压air pressure pneumatic pressure稳定性stability介质medium液压驱动泵fluid clutch [klʌtʃ]离合器;控制;手;紧急关头液压泵hydraulic pump [pʌmp]阀门valve[vælv]失效invalidation强度intensity载荷load应力stress安全系数safty factor['fæktə]可靠性reliability螺纹thread螺旋helix['hi:liks]键spline[splain]销pin滚动轴承rolling bearing滑动轴承sliding bearing弹簧spring制动器arrester brake十字结联轴节crosshead联轴器coupling链chain皮带strap精加工finish machining粗加工rough machining变速箱体gearbox casing腐蚀rust氧化oxidation磨损wear耐用度durability随机信号random signal离散信号discrete signal超声传感器ultrasonic sensor集成电路integrate circuit挡板orifice plate残余应力residual stress套筒sleeve扭力torsion冷加工cold machining电动机electromotor汽缸cylinder过盈配合interference fit热加工hotwork摄像头CCD camera倒角rounding chamfer优化设计optimal design工业造型设计industrial moulding design 有限元finite element滚齿hobbing插齿gear shaping伺服电机actuating motor铣床milling machine钻床drill machine镗床boring machine步进电机stepper motor丝杠screw rod导轨lead rail组件subassembly可编程序逻辑控制器Programmable Logic Controller PLC电火花加工electric spark machining电火花线切割加工electrical discharge wire - cutting相图phase diagram热处理heat treatment固态相变solid state phase changes有色金属nonferrous metal3陶瓷ceramics合成纤维synthetic fibre电化学腐蚀electrochemical corrosion 车架automotive chassis悬架suspension转向器redirector变速器speed changer板料冲压sheet metal parts孔加工spot facing machining车间workshop工程技术人员engineer气动夹紧pneuma lock数学模型mathematical model画法几何descriptive geometry机械制图Mechanical drawing投影projection视图view剖视图profile chart标准件standard component零件图part drawing装配图assembly drawing尺寸标注size marking技术要求technical requirements刚度rigidity内力internal force位移displacement截面section疲劳极限fatigue limit断裂fracture塑性变形plastic distortion脆性材料brittleness material刚度准则rigidity criterion垫圈washer垫片spacer直齿圆柱齿轮straight toothed spur gear 斜齿圆柱齿轮helical-spur gear直齿锥齿轮straight bevel gear运动简图kinematic sketch齿轮齿条pinion and rack蜗杆蜗轮worm and worm gear虚约束passive constraint曲柄crank摇杆racker凸轮cams共轭曲线conjugate curve范成法generation method定义域definitional domain值域range导数\\微分differential coefficient求导derivation定积分definite integral不定积分indefinite integral曲率curvature偏微分partial differential毛坯rough游标卡尺slide caliper千分尺micrometer calipers攻丝tap二阶行列式second order determinant 逆矩阵inverse matrix线性方程组linear equations概率probability随机变量random variable排列组合permutation and combination 气体状态方程equation of state of gas 动能kinetic energy势能potential energy机械能守恒conservation of mechanical energy动量momentum桁架truss轴线axes余子式cofactor逻辑电路logic circuit触发器flip-flop脉冲波形pulse shape数模digital analogy液压传动机构fluid drive mechanism 机械零件mechanical parts淬火冷却quench淬火hardening回火tempering调质hardening and tempering磨粒abrasive grain结合剂bonding agent砂轮grinding wheel机械零件mechanical parts淬火冷却quench淬火hardening回火tempering4调质hardening and tempering磨粒abrasive grain结合剂bonding agent砂轮grinding wheel机床行业部分英汉对照(1):按英文字母排序3-Jaws indexing spacers 三爪、分割工具头A.T.C.system 加工中心机刀库Aluminum continuous melting & holding fu rnaces 连续溶解保温炉Balancing equipment 平衡设备Bayonet 卡口Bearing fittings 轴承配件Bearing processing equipment 轴承加工机Bearings 轴承Belt drive 带传动Bending machines 弯曲机Blades 刀片Blades,saw 锯片Bolts,screws & nuts 螺栓,螺帽及螺丝Boring heads 搪孔头Boring machines 镗床Cable making tools 造线机Casting,aluminium 铸铝Casting,copper 铸铜Casting,gray iron 铸灰口铁Casting,malleable iron 可锻铸铁Casting,other 其他铸造Casting,steel 铸钢Chain drive 链传动Chain making tools 造链机Chamfer machines 倒角机Chucks 夹盘Clamping/holding systems 夹具/支持系统CNC bending presses 电脑数控弯折机CNC boring machines 电脑数控镗床CNC drilling machines 电脑数控钻床CNC EDM wire-cutting machines 电脑数控电火花线切削机CNC electric discharge machines 电脑数控电火花机CNC engraving machines 电脑数控雕刻机CNC grinding machines 电脑数控磨床CNC lathes 电脑数控车床CNC machine tool fittings 电脑数控机床配件CNC milling machines 电脑数控铣床CNC shearing machines 电脑数控剪切机CNC toolings CNC 刀杆CNC wire-cutting machines 电脑数控线切削机Conveying chains 输送链Coolers 冷却机Coupling 联轴器Crimping tools 卷边工具Cutters 刀具Cutting-off machines 切断机Diamond cutters 钻石刀具Dicing saws 晶圆切割机Die casting dies 压铸冲模Die casting machines 压铸机Dies-progressive 连续冲模Disposable toolholder bits 舍弃式刀头Drawing machines 拔丝机Drilling machines 钻床Drilling machines bench 钻床工作台Drilling machines,high-speed 高速钻床Drilling machines,multi-spindle 多轴钻床Drilling machines,radial 摇臂钻床Drilling machines,vertical 立式钻床drills 钻头Electric discharge machines(EDM) 电火花机Electric power tools 电动刀具Engraving machines 雕刻机Engraving machines,laser 激光雕刻机Etchin g machines 蚀刻机Finishing machines 修整机Fixture 夹具Forging dies 锻模Forging,aluminium 锻铝Forging,cold 冷锻Forging,copper 铜锻Forging,other 其他锻造Forging,steel 钢锻Foundry equipment 铸造设备Gear cutting machines 齿轮切削机5Gears 齿轮Gravity casting machines 重力铸造机Grinde r bench 磨床工作台Grinders,thread 螺纹磨床Grinders,tools & cutters 工具磨床Grinders,ultrasonic 超声波打磨机Grinding machines 磨床Grinding machines,centerless 无心磨床Grinding machines,cylindrical 外圆磨床Grinding machines,universal 万能磨床Grinding tools 磨削工具Grinding wheels 磨轮Hand tools 手工具Hard/soft and free expansion sheet making plant 硬(软)板(片)材及自由发泡板机组Heat preserving furnaces 保温炉Heating treatment funaces 熔热处理炉Honing machines 搪磨机Hydraulic components 液压元件Hydraulic power tools 液压工具Hydraulic power units 液压动力元件Hydra ulic rotary cylinders 液压回转缸Jigs 钻模Lapping machines 精研机Lapping machines,centerless 无心精研机Laser cutting 激光切割Laser cutting for SMT stensil 激光钢板切割机Lathe bench 车床工作台Lathes,automatic 自动车床Lathes,heavy-duty 重型车床Lathes,high-speed 高速车床Lathes,turret 六角车床Lathes,vertical 立式车床Lubricants 润滑液Lubrication Systems 润滑系统Lubricators 注油机Machining centers,general 通用加工中心Machining centers,horizontal 卧式加工中心Machining centers,horizontal & vertical 卧式及立式加工中心Machining centers,vertical 立式加工中心Machining centers,vertical double-column ty pe 立式双柱加工中心Magnetic tools 磁性工具Manifolds 集合管Milling heads 铣头Milling machines 铣床Milling machines,bed type 床身式铣床Milling machines,duplicating 仿形铣床Milling machines,horizontal 卧式铣床Millin g machines,turret vertical 六角立式铣床Milling machines,universal 万能铣床Milling machines,vertical 立式铣床Milling machines,vertical & horizontal 立式及卧式铣床Mold & die components 模具单元Mold changing systems 换模系统Mold core 模芯Mold heaters/chillers 模具加热器/冷却器Mold polishing/texturing 模具打磨/磨纹Mold repair 模具维修Molds 模具Nail making machines 造钉机Oil coolers 油冷却器Overflow cutting machines for aluminium w heels 铝轮冒口切断机P type PVC waterproof rolled sheet making plant P型PVC 高分子防水PCB fine piecing systems 印刷电器板油压冲孔脱料系统Pipe & tube making machines 管筒制造机Planing machines 刨床Planing machines vertical 立式刨床Pneumatic hydraulic clamps 气油压虎钳Pneumatic power tools 气动工具Powder metallurgic forming machines 粉末冶金成型机Presses,cold forging 冷锻冲压机presses,crank 曲柄压力机Presses,eccentric 离心压力机Presses,forging 锻压机Presses,hydraulic 液压冲床Presses,knuckle joint 肘杆式压力机Presses,pneumatic 气动冲床Presses,servo 伺服冲床Presses,transfer 自动压力机Pressing dies 压模Punch formers 冲子研磨器Quick die change systems 速换模系统6Quick mold change systems 快速换模系统Reverberatory furnaces 反射炉Rollers 滚筒Rolling machines 辗压机Rotary tables 转台Sawing machines 锯床Sawing machines,band 带锯床Saws,band 带锯Saws,hack 弓锯Saws,horizontal band 卧式带锯Saws,vertical band 立式带锯shafts 轴Shapers 牛头刨床Shearing machines 剪切机Sheet metal forming machines 金属板成型机Sheet metal working machines 金属板加工机Slotting machines 插床spindles 主轴Stamping parts 冲压机Straightening machines 矫直机Switches & buttons 开关及按钮Tapping machines 攻螺丝机Transmitted chains 传动链Tube bending machines 弯管机Vertical hydraulic broaching machine 立式油压拉床Vises 虎钳Vises,tool-maker 精密平口钳Wheel dressers 砂轮修整器Woven-Cutting machines 织麦激光切割机Wrenches 扳手螺丝词汇的中英文对照六角盖头螺帽HEX CAP NUTS六角锯齿螺帽HEX SERRATED NUTS六角轮缘螺帽HEX FLANGE NUTS 高脚螺帽HEX COUPLING NUTS(HIGH NUT S)圆螺帽ROUND NUTS四角螺帽SQUARE NUTS7HEAVY HEX NUTS不锈钢六角螺帽STAINLESS STEEL HEX NUTS不锈钢尼龙嵌入螺帽STAINLESS STEEL NYLON INSERT LOCK NUTS普通六角螺帽HEX NUTS六角重型螺帽HEAVY HEX NUTS薄型螺帽HEX JAM NUTS尼龙嵌入防松螺帽NYLON INSERT LOCK NUTS机械螺丝用六角螺帽HEX MACHINE SCREW NUT机械工具英语机械工具spanner 扳子(美作:wrench) doub le-ended spanner 双头扳子adjustable spanner, monkey wrench 活扳子,活络扳手box spanner 管钳子(美作:socket wrench) c alipers 卡规pincers, tongs 夹钳shears 剪子hacksaw 钢锯wire cutters 剪线钳multipurpose pliers, universal pliers 万能手钳adjustable pliers 可调手钳punch 冲子drill 钻chuck 卡盘scraper 三角刮刀reamer 扩孔钻calliper gauge 孔径规rivet 铆钉nut 螺母locknut 自锁螺母,防松螺母bolt 螺栓pin, peg, dowel 销钉washer 垫圈staple U形钉oil can 油壶jack 工作服grease gun 注油枪机械加工抛光polishing 安装to assem ble 衬套bushing外贸常用机械英语大全Assembly line 组装线Layout 布置图Conveyer 流水线物料板Rivet table 拉钉机Rivet gun 拉钉枪Screw driver 起子Pneumatic screw driver 气动起子worktable 工作桌OOBA 开箱检查fit together 组装在一起fasten 锁紧(螺丝)fixture 夹具(治具)pallet 栈板barcode 条码barcode scanner 条码扫描器fuse together 熔合fuse machine热熔机8repair修理operator作业员QC 品管supervisor 课长ME 制造工程师MT 制造生技cosmetic inspect 外观检查inner parts inspect 内部检查thumb screw 大头螺丝lbs. inch 镑、英寸EMI gasket 导电条front plate 前板rear plate 后板chassis 基座bezel panel 面板power button 电源按键reset button 重置键Hi-pot test of SPS 高源高压测试Voltage switch of SPS 电源电压接拉键sheet metal parts 冲件plastic parts 塑胶件SOP 制造作业程序material check list 物料检查表work cell 工作间trolley 台车carton 纸箱sub-line 支线left fork 叉车personnel resource department 人力资源部production department生产部门planning department企划部QC Section 品管科stamping factory冲压厂painting factory烤漆厂molding factory成型厂common equipment常用设备uncoiler and straightener整平机punching machine 冲床robot机械手hydraulic machine油压机lathe车床planer |plein|刨床miller 铣床grinder磨床linear cutting线切割electrical sparkle电火花welder电焊机staker=reviting machine铆合机position 职务president董事长general manager总经理special assistant manager特助factory director厂长department director部长deputy manager | =vice manager 副理sectio n supervisor课长deputy section supervisor =vice section supe risor副课长group leader/supervisor组长line supervisor线长assistant manager助理to move, to carry, to handle搬运be put in storage入库pack packing包装to apply oil 擦油to file burr 锉毛刺final inspection终检to connect material接料to reverse material 翻料wet station沾湿台Tiana天那水cleaning cloth抹布to load material上料to unload material卸料to return material/stock to退料scraped |\\'skr?pid|报废scrape ..v.刮;削deficient purchase来料不良manufacture procedure制程deficient manufacturing procedure制程不良oxidation |\\' ksi\\'dei?n|氧化scratch刮伤dents压痕defective upsiding down抽芽不良defective to staking铆合不良embedded lump镶块feeding is not in place送料不到位stamping-missing漏冲production capacity生产力education and training教育与训练9proposal improvement提案改善spare parts=buffer备件forklift叉车trailer=long vehicle拖板车外贸常用机械英语大全(续) compound die合模die locker锁模器pressure plate=plate pinch压板bolt 螺栓administration/general affairs dept总务部automatic screwdriver电动启子thickness gauge厚薄规gauge(or jig)治具power wire电源线buzzle蜂鸣器defective product label不良标签identifying sheet list 标示单location地点present members出席人员subject主题conclusion结论decision items决议事项responsible department负责单位pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/ 审核/承办PCE assembly production schedule sheet PCE 组装厂生产排配表model 机锺work order工令revision版次remark备注production control confirmation生产确认checked by初审approved by核准department部门stock age analysis sheet 库存货龄分析表on-hand inventory现有库存available material良品可使用obsolete material良品已呆滞to be inspected or reworked 待验或重工total 合计cause description原因说明part number/ P/N 料号type形态item/group/class类别quality品质prepared by制表notes说明year-end physical inventory difference analysis sheet 年终盘点差异分析表physical inventory盘点数量physical count quantity帐面数量difference quantity差异量cause analysis原因分析raw materials原料materials物料finished product成品semi-finished product半成品packing materials包材good product/accepted goods/ acceptedparts/good parts良品defective product/non-good parts不良品disposed goods 处理品warehouse/hub仓库on way location在途仓oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓skid/pallet 栈板tox machine自铆机wire EDM线割EDM放电机coil stock卷料sheet stock片料tolerance工差score=groove压线cam block滑块pilot 导正筒trim 剪外边pierce剪内边drag form压锻差pocket for the punch head挂钩槽slug hole 废料孔10feature die公母模expansion dwg展开图radius半径shim(wedge)楔子torch-flame cut火焰切割set screw止付螺丝form block折刀stop pin 定位销round pierce punch=die button圆冲子shape punch=die insert异形子stock locater block定位块under cut=scrap chopper清角active plate活动板baffle plate挡块cover plate盖板male die公模female die母模groove punch压线冲子air-cushion eject-rod气垫顶杆spring-box eject-plate弹簧箱顶板bushing block 衬套insert 入块club car高尔夫球车capability能力parameter参数factor系数phosphate皮膜化成viscosity涂料粘度alkalidipping脱脂main manifold主集流脉bezel斜视规blanking穿落模dejecting顶固模demagnetization去磁;消磁high-speed transmission高速传递heat dissipation热传rack上料degrease脱脂rinse水洗alkaline etch龄咬desmut 剥黑膜D.I. rinse纯水次Chromate铬酸处理Anodize阳性处理seal封孔revision版次part number/P/N料号good products良品scraped products报放心品defective products不良品finished products成品disposed products处理品barcode条码flow chart流程表单assembly组装stamping冲压molding成型spare parts=buffer备品coordinate座标dismantle the die折模auxiliary fuction辅助功能poly-line多义线heater band 加热片thermocouple热电偶sand blasting喷沙grit 砂砾derusting machine除锈机degate打浇口dryer烘干机induction 感应induction light 感应光response=reaction=interaction感应ram连杆edge finder巡边器concave凸convex 凹short 射料不足nick缺口speck瑕??shine亮班splay 银纹gas mark焦痕delamination起鳞cold slug冷块blush 导色gouge沟槽;凿槽satin texture段面咬花witness line证示线patent专利grit 沙砾granule=peuet=grain细粒11grit maker抽粒机cushion缓冲magnalium 镁铝合金magnesium镁金metal plate钣金lathe车mill 锉plane刨grind磨drill 铝boring镗blinster 气泡fillet 镶;嵌边through-hole form通孔形式voller pin formality滚针形式cam driver铡楔shank摸柄crank shaft曲柄轴augular offset角度偏差velocity速度production tempo生产进度现状torque扭矩spline=the multiple keys 花键quenching淬火tempering回火annealing退火carbonization碳化tungsten high speed steel钨高速的moly high speed steel钼高速的organic solvent有机溶剂bracket小磁导liaison 联络单volatile 挥发性resistance电阻ion 离子titrator滴定仪beacon警示灯coolant冷却液crusher破碎机机械类常用英语:生产类PCS Pieces 个(根,块等)PRS Pairs 双(对等)CTN Carton 卡通箱PAL Pallet/skid 栈板PO Purchasing Order 采购订单MO Manufacture Order 生产单D/C Date Code 生产日期码ID/C Identification Code (供应商)识别码SWR Special Work Request 特殊工作需求L/N Lot Number 批号P/N Part Number 料号阀门种类英汉术语对照Air valves 空气阀门Angle Stop valves 角式截止阀Angle Throttle Valves 角式节流阀Angle Type Globe Valves 门角式截止阀Ash valves 排灰阀Aspirating valves 吸(抽)气阀Auxiliary valves 辅助(副)阀Balance valves 平衡阀Bellows valves 波纹管阀Blowdown valves 泄料(放空,排污)阀Brake valves 制动阀Butterfly Type Non-slam Check 蝶式缓冲止回阀Butterfly Valves with Gear Actuator 蜗轮传动蝶阀Buttwelding valves 对焊连接阀Clamp valves 对夹式阀门Cock 二通Combination valves 组合阀CQ Thread Ball Valves CQ螺纹球阀Culvert valves 地下管道阀Deceleration valves 减速阀Diaphragm Valves 隔膜阀Decompression valves 泄压阀Double Disc Flat Gate Valves 双闸板平板闸阀Double Disk Parallel Gate Valves明杆平行式双闸板闸板Double Opening Exhaust Valves 双口排气球12Drainage valves 排水阀Electric Actuated Stop Valves 电动截止阀Electric Actuated Wedge Gate Valves 电动楔式闸阀Electric Double Disk Parallel Gate Valve s 电动平行式双闸板闸板Emergeny Cut-off Valves 紧急切断阀Exhaust valves 排气阀Free Float Type Steam Trap 浮球式疏水阀Flange Ball Valves 法兰球阀Flange Gate Valves 法兰闸阀Flange Globe Valves 法兰截止阀Gauge Valves 仪表阀Hand-operated valves 手动阀Hard Seal Butterfly Valves 金属密封碟阀High Temperature Pressure Power Statio n Gate Valves 高温高压电站闸阀High Temperature Pressure Power Statio n Globe Valves 高温高压电站截止阀Hydraulic relay valves 液压继动阀Lift Check Valves 升降式止回阀Lift Check Valves 升降式止回阀Limit valves 限位阀Lining Ball Valves 衬里球阀Lining Butterfly Valves 衬里碟阀Lining Check Valves 衬里止回阀Lining Cock 衬里二通Lining Globe Valves 衬里截止阀Lining T-Cock Valves 衬里三通旋塞阀Liquid Indicator 液位计LPG Pipe Fitting 液化气管件Magnetic Co-operate Globe Valves 磁耦合截止阀Magnetism Forle Pumps 磁力泵Manual Oil Pumps Valves 手摇油泵(阀) Meter Needle Type Globe Valves 仪表针形截止阀Oblique Stop Valves 直流式截止阀Parallel Slide Valves 浆液阀Pintle valve 针形阀Piping Centrifugal Pumps 管道离心泵Plunger valves 柱塞阀Pressure valve 压力(増压)阀Piping Pumps 管道泵Piping Safety Valves 管道安全阀Plunger Globe Valves 柱塞截止阀Quick Draining Valves 快速排污阀Restrictor Valves 过流阀(或节流阀) Safety Valves 安全阀Screw Pumps 螺杆泵Scum Gate Valves 排渣闸阀S olenoid valves 电磁阀Single Disc Flat Gate Valves 单闸板平板闸阀Single Opening Exhaust Valves 单口排气球Slurry Pumps 泥浆泵Stop Valves 截止阀Strainer 过滤器Submerged Motor Pumps 潜水电泵(排污泵)Swing Check Valves 旋启式止回阀Swing Check Valves 旋启式止回阀Tank Lorry Ball Valves 槽车球阀T-Cock 三通Thin Gate Valves 薄型闸阀Throttle Valves 节流阀Tiny Drag Slow Shut Check Valves 微阻缓闭止回阀Triple (tee) valves 三通阀Two-way valves 二通阀Under Water Pumps 液下泵Vacuum Pumps 水力喷射器(真空泵) Vertical Lift Check Valves 立式止回阀Wafer Check Valves 对夹式止回阀Wafer plate valves 对夹蝶板阀Wafer Type Butterfly Valves with Rubber Itning对夹式衬胶蝶阀Waste Valves 排污箱(阀)Water Seal Gate Valves 水封闸阀Wedge Gate Valves 楔式闸阀Y Type and Cylinder Filters Y型筒型过滤器阀门零部件英汉术语对照Axis Guide 轴套Ball 球、球芯13Ball seat 密封圈Blowdown Sealing Face 启、阀件密封面Body 阀体Bonnet 阀盖Disc 阀瓣Mut 螺母Screw 螺栓Sealing 密封件Spring 弹簧Stem 阀杆Stem Mut 阀杆螺母Stem seal 填料Wedge Disc 闸板阀门规范技术英语术语对照Applicable medium 适用介质Applicable temperature 适用温度Butt Clamp 对夹Chemical analysis 化学成份Connecting format 连接形式Double disc 双闸板Flexible disc 弹性闸板Flange 法兰Hoop 卡箍Inside thread 内螺纹Jacket 夹套Mains 电源Material chemical analysis and mechanical capacity材料化学成份和机械性能materials 材料Materials for main parts 主要零件材料Mechanical capacity 机械性能Max. Discharging Capacity 最大排水量Max. Operating Temperature 最高工作温度Max. Allowable Temperature 最高允许温度Max. Allowable Pressure 最高允许压力Model 型号Name of parts 零件名称nitrogen (N) 氮Nominal bore 公称通径Nominal Pressure 公称压力Nozzle 排气口Outside thread 外螺纹Oxidant 氧化性介质Parallel 平行Piping 管路Piston 活塞Reductant 还原性介质Rising stem 明杆Seal 阀座,密封面Seat testing pressure 压力气密封试验压力Socket 卡套Specifications 性能规范Single disc 单闸板Solid 刚性Strengh testing pressure 强度试验压力Steam , condensate 蒸汽,凝结水Stroke 冲程,行程Water,oil,steam 水,温度,气Wedge 楔式Welding 焊接阀门材质术语英汉对照Atbas metal 镍铬钢Buna-N rubber 丁晴橡胶Casting aluminium brass 铸铝黄铜Casting aluminium bronze 铸铝青铜Ceramic metal 陶瓷金属Chromel alloy 镍铬合金CHR rubber 氯晴橡胶Chrominm-molybdenum-vanadium steel铬钼钒钢Chromium stainless steel 铬不锈钢Chromium-molybdenum steel 铬钼钢Corrugation pad 波形垫Cuprum alloy 铜合金Ductile Cast iron 球墨铸铁Expanded graphite 柔性石墨Fine Steel Casting iron 优质碳素钢Fluorous rubber 氟橡胶Gray Cast iron 灰铸铁Hayne's alloy 钴铬钨合金High tem perature steel 高温钢Monel 蒙乃尔合金Low temperature steel 低温钢Nylon 尼龙塑料Polytetrafluoroethylene(PTEF) 聚四氟乙烯Polythene 聚乙烯Pure aluminium 纯铝Pure cupper 纯铜14Rubber graphite board 橡胶石墨板Spring steel 弹簧钢Stainless acid-resisting steel 不锈耐酸钢Stainless and Graphite 不锈钢/石墨Stainless steel 不锈钢Steel Casting iron 碳素钢铸件Shell Test Pressure 壳体试验压力Service Fluid 工作介质机械类常用英语:钢材类alloy tool steel 合金工具钢 aluminium alloy 铝合金钢bearing alloy 轴承合金 blister steel 浸碳钢bonderized steel sheet 邦德防蚀钢板 carbon tool steel 碳素工具钢clad sheet 被覆板 clod work die steel 冷锻模用钢emery 金钢砂 ferrostatic pressure 钢铁水静压力forging die steel 锻造模用钢 galvanized ste el sheet 镀锌铁板hard alloy steel 超硬合金钢 high speed tool steel 高速度工具钢hot work die steel 热锻模用钢 low alloy tool steel 特殊工具钢low manganese casting steel 低锰铸钢 marg ing steel 马式体高强度热处理钢martrix alloy 马特里斯合金 meehanite cast iron 米汉纳铸钢meehanite metal 米汉纳铁 merchant iron 市售钢材molybdenum high speed steel 钼系高速钢molybdenum steel 钼钢nickel chromium steel 镍铬钢 prehardened steel 顶硬钢silicon steel sheet 矽钢板 stainless steel 不锈钢tin plated steel sheet 镀锡铁板 tough pitch copper 韧铜troostite 吐粒散铁 tungsten steel ?钢vinyl tapped steel sheet 塑胶覆面钢板塑件模具相关英文compre sion molding压缩成型flash mold 溢流式模具plsitive mold 挤压式模具split mold 分割式模具cavity型控母模core模心公模taper锥拔leather cloak仿皮革shiver饰纹flow mark流痕welding mark溶合痕post screw insert螺纹套筒埋值self tapping screw自攻螺丝striper plate脱料板piston 活塞cylinder汽缸套chip细碎物handle mold手持式模具encapsulation molding低压封装成型、射出成型用模具two plate两极式(模具)well type蓄料井insulated runner绝缘浇道方式hot runner热浇道runner plat浇道模块valve gate阀门浇口band heater环带状的电热器spindle 阀针spear head刨尖头slag well冷料井cold slag冷料渣air vent排气道welding line熔合痕eject pin顶出针knock pin顶出销return pin回位销反顶针sleave套筒stripper plate脱料板insert core放置入子runner stripper plate浇道脱料板15guide pin 导销eject rod (bar)(成型机)顶业捧subzero深冷处理three plate三极式模具runner system浇道系统stress crack应力电裂orientation定向sprue gate射料浇口,直浇口nozzle射嘴sprue lock pin料头钩销(拉料杆)slag well冷料井side gate侧浇口edge gate侧缘浇口tab gate搭接浇口film gate薄膜浇口flash gate闸门浇口slit gate 缝隙浇口fan gate扇形浇口dish gate因盘形浇口diaphragm gate隔膜浇口ring gate环形浇口subarine gate潜入式浇口tunnel gate隧道式浇口pin gate针点浇口Runner less无浇道(sprue less)无射料管方式long nozzle 延长喷嘴方式sprue浇口;溶渣__。
机械专业英语词汇【最新完整版】

螺旋helix组件subassembly
键spline可编程序逻辑控制器Programmable
销pinLogic Controller PLC
滚动轴承rolling bearing电火花加工electric spark machining
滑动轴承sliding bearing电火花线切割加工electrical discharge
Casting,copper铸铜
Casting,gray iron铸灰口铁
Casting,malleable iron可锻铸铁
Casting,other其他铸造
Casting,steel铸钢
Chain drive链传动
Chain making tools造链机
Chamfer machines倒角机
Chucks夹盘
Bearings轴承
Belt drive带传动
Bending machines弯曲机
Blades刀片
Blades,saw锯片
Bolts,screws & nuts螺栓,螺帽及螺丝
Boring heads搪孔头
Boring machines镗床
Cable making tools造线机
Casting,aluminium铸铝
误差error平面磨削plane grinding
响应response变速箱gearbox
定位allocation离合器clutch
机床夹具jig绞孔fraising
动力学dynamic绞刀reamer
运动学kinematic螺纹加工thread processing
静力学static螺钉screw
常用化工机械专业英语对照

常用机械专业英语对照Cutting: 切割socket weld 承插焊接fillet weld 角焊 ,填角焊branch connection分支接续fabrication tolerance.制造容差local heat treatment 局部热处理threaded pipe螺纹管seal welding.密封焊接flange joint 凸缘接头undercut 底切feeder 馈电线conduit outlet 电线引出口seal fitting 密封接头 , 密封配件Screw thread lubricant螺纹润滑剂 Seal: 绝缘层weld reinforcement 焊缝补强lock washer 锁紧 [ 止动 , 防松 ]垫圈 electrical panel.配电板 ,配电盘nipple 螺纹接头zinc plated.镀锌的ring joint 环接 , 围缘接合bolt 螺栓control: 控制器National Electrical Code 全国电气规程 master schedule 主要图表 , 综合图表 , 设计任务书 , 主要作业表torque wrench 转矩扳手job site 施工现场flange connection.凸缘联接 Hard hat:安全帽Goggles:护目镜stockpile 贮存packing list 装箱单crate: 柳条箱purchased material list原材料进货单 back-feed 反馈wire coil 线盘,线卷,NPT thread. 美国标准锥管螺纹cable gland 电缆衬垫terminal block 线弧 , 接头排接线盒 , 接线板 , 线夹power drill 机械钻connector. 接线器insulated sleeve绝缘套管wire connector 接线器wire terminal 电线接头motor lead 电动机引出线power wiring 电力布线tender document.提供证件 orifice plate.挡板nut 螺母flange gasket 法兰垫片dimensional inspection 尺寸检验burn through 烧蚀piping system.管道系统reinforcement of weld 加强焊缝fabrication.制造dye penetrant examination染料渗透试验法 magnetic particle examination 磁粉检验 girth weld 环形焊缝cement lined piping 水泥衬里weld joint 焊缝 , 焊接接头 spool drawing 管路图 , 管路详图 spottest 抽查 , 当场测试butt weld 对接焊缝Random Radiography随机射线照相检查radiographic examination 射线照相检查assembly装.配erection 架设examination 试验cable tray.电缆盘rigid steel conduit 钢制电线管power control 功率控制arc welding 电弧焊control cable 控制电缆操纵索normal bend 法向 [ 法线 ]弯管cable glands: 电缆衬垫exfoliation 剥落power receptacle 电力插座grounding conductor 接地导体lighting fixture 照明器材junction box 分线箱 race way电缆管道terminal box 接线盒distribution board 配电盘 , 配电屏receptacle 插座tumble switch.翻转开关 ,拨动式开关cathodic protection system 阴极保护系统 Circuit breaker 断路开关amplifier panel 放大器盘 control console 控制台 electricalmaterial 电气材料 convenience receptacle电.源插座 cableTIG : Tungsten-arc Inert-Gas welding 钨极电弧惰性气体保护焊filler rod 焊条tensile strength 抗张强度shield gas 保护气体shield jig 保护夹具high frequency generator高.频发电机 welding rod 焊条filler metal 焊料 , 焊丝shop fabrication 车间制造field installation 现场安装welding bead 焊道both sides welding.双面焊接residual stress 残余应力electrode 电焊条condensation 冷凝longitudinal. 纵向的horizontal line 水平线circumferential joint 周圈接缝nondestructive examination非.破坏性检验 , qualification: 合格性construction work 施工工程welded joint 焊接缝焊接节点gas cutting.气割arc cutting 电弧切割grind off 磨掉metallic luster 金属光泽Screwed Piping Joints螺丝状的管接头gouging .刨削槽Bending: 挠曲witness test订货人在场的试验 Welding: 焊接Threading: 车缧纹Leveling:校平color identification 彩色识别Alignment :对准 ,定位调整check against 检查 , 核对Fixing :固定Console:控制台cubicle 室,箱audit 审计material certificate.材料合格证vertical panel 竖直面板power distribution panel 配电盘gauge board仪表板beveling 磨斜棱 ,磨斜边local panel 现场配电盘fabrication 加工 ,制造instrument rack 计测器支架tank gauge 液面计 flushing冲洗,填缝 analyzer 分析器piping 管道敷设tubing 敷设管道cable fitting 电缆配件elbow.弯管接头main pipe 主管道bend.弯管弯头solvent 溶剂postweld heat treatment 焊后热处理 jig. 夹具arc gouging 电弧刨削chipping 修琢machining 机械加工bridges.管式桥clamp.夹钳压力容器基本词汇安全阀safety valve安装 Installation鞍式支座saddle support凹面 concave半球形封头Hemispherical heads 棒bars保温支撑 insulation support [wiki] 爆炸 [/wiki] 性 explosive泵pump变径段 reducers标记 stamping标志 marking标志的移植 Transferring marking 波纹板 Corrugating Paper补强管reinforcing nozzle补强圈reinforcing ring不锈钢stainless steel不圆度out-of-roundness材料 materials材料证明书 Certification of material 超声检验 Ultrasonic Examination 衬里 Linings成型 Forming成型封头Formed heads尺寸 Dimensions翅片管finned tubes冲击试验impact test传热面积heat transfer surface磁粉检验Magnetic Particle Examination次要应力secondary stress粗糙度 roughness淬火 Quenching带折边的锥形toriconical弹簧 springs弹簧垫圈spring washer弹性模量modulus of Elasticity挡板 baffle plate低合金钢容器low alloy steel vessels低温容器Low-temperature vessels地震烈度seismic intensity垫板 Backing strip垫片 gasket垫圈 washer碟形封头Dished heads顶丝 jackscrew定距管 spacer定位销 pin dowel定义 Definitions毒性 toxicity镀锌容器Galvanized vessels锻件 Forgings对焊 [wiki] 法兰 [/wiki] welding neck flange耳座 lug[wiki] 阀门 [/wiki] valves法兰 flanges法兰接触面Flange contact facings防冲板 impingement baffle防 [wiki] 腐蚀 [/wiki] 衬里Corrosion resistant linings防火 fire protection防涡流挡板vortex breaker非受压件nonpressure parts非圆形容器Noncircular vessels分析 analysis峰值应力peak stress腐蚀裕量corrosion allowance附加载荷supplementary loading附件 attachments复合板 Clad plate覆层容器clad vessels盖板 Cover plates杆 rods and bars刚性 Stiffness高合金容器high-alloy vessels 工作压力operation pressure固定的fixed管板 tube sheet管壳式换热器shell and tube heat exchanger 管口材料表sheet of material for nozzle管口方位nozzle orientation管箱 channel管子 pipes nozzle tube管嘴 nipple罐桶槽 tank过渡段transition in过渡圆角Knuckles焊后热处理after post weld heat treating焊接工艺welding procedure specification焊接接头welding joints焊接系数welding coefficient厚度 thickness滑动的sliding环向应力hoop stress回火 tempering基本地震加速度basic seismic acceleration 基本分压basic wind pressure计算厚度calculated thickness技术条件form of Specification加强圈stiffening rings夹套容器Jacketed vessels检查孔Inspection openings检验 inspection角焊 Fillet welds接地板earth lug截止阀Stop valves介质特性fluid property金属温度Metal temperature筋板 rib plate径向应力radial stress静(压力 )水头 static head局部 areas局部 local局部焊后热处理Local postweld heat treatment矩形设计rectangular design卡箍 clamp开孔补强reinforcement for openings快开盖Quick-actuating closures拉杆 tie rod裂缝 Cracking流体(介质)fluid螺母 nut螺塞 plug螺栓 bolt螺纹 threaded螺柱 studs名义厚度 nominal thickness铭牌 Nameplates内部构件 Internal structures内衬筒 internal shell盘管 coil tube配件 fittings膨胀节 expansion joint平封头 Flat heads评定 Qualification气孔 Porosity气压试验Pneumatic test钎焊 brazing强度 strength of球形封头 spherically dished曲率 curvature屈服 yielding全容积 total volume缺陷 defects群座 skirt support热处理 thermal treatment热处理 heat treatmen热应力 thermal stress人孔 manholes韧性 ductility容积 volume容器 vessel容器净重empty weight容器类别 vessel classification塞焊 Plug welds设计压力design pressure射线超声检验 Radiographic Examination 渗透检验 Penetrant Examination 石墨graphite试样 Test coupons适用范围Scope手孔 handholes水压试验 hydrostatic test/hydraulic test/hydrotest碳钢 carbon steel搪玻璃容器enameled vessels梯子、平台ladders, platforms填充金属 filler metal 凸面 convex凸缘 socket椭圆封头Ellipsoidal heads外压容器 vessels subjected to external pressure未注尺寸公差tolerance grade not noted 无支撑unstayed系数 factor现场安装Field assembly校核 checking of泄放 Discharge泄压装置 Pressure relieving devices性能 properties许用工作压力 allowable working pressure 许用应力 allowable stress 易然的flammable应力腐蚀stress corrosion应力集中 stress concentration预热 Preheating圆度 roundness圆角和倒角 corners and fillets载荷 Loadings胀接 expanded connections折流板 baffle plate蒸发器Evaporators直边长度length of skirt直径 Diameter制造 fabrication制造厂fabricator制造方法methods of fabrication制造工艺fabrication technology周长 Girth主应力primary stress柱状壳体 Cylindrical shells铸铁容器 cast iron vessels转角半径 knuckle radius 装配 assembling 锥度tapered锥壳 conical shell锥形封头Conical heads资格 qualification纵向接头 Longitudinal joints组对 fitting uppipe closure 管塞 end closure端盖 orifice plate 挡板 exialspacing 轴向间距 manufacturetolerance 制造公差acid passivation 酸洗钝化part drawing 零件图stud bolts 双头螺柱化工设备常用词汇和缩写中英文对照化工设备常用词汇和缩写中英文对照缩写 / 英文 /中文AB Anchor Bolt地脚螺栓Abs Absolute 绝对的Abs Abstract 文摘、摘要A/C Account 帐、帐目AC Alternating Current 交流电Add Addendum 补充、补遗、附录ADL Acceptable Defect Level 允许的缺陷标准Adpt Adapter 连接器、接头AE Absolute Error 绝对误差AET Acoustic Emission Examination 声发射检验AISC American Institute of Steel Construction 美国钢结构学会AISI American Iron and Steel Institute 美国钢铁学会AL Aluminium铝Alk Alkaline 碱的、强碱的ALM Alarm 报警Alt Alternate 交流、改变Amb Ambient 周围的Amt Amount 数量、金额Anh Anhydrous 无水的ANSI American National Standard Institute 美国国家标准学会API American Petroleum Institute 美国石油学会App Apparatus 设备App Appendix 附录、补遗Appl Applied 应用的Appl Applicable 适当的、合适的Approx Approximate 大约、近似Appx Appendix 附录、附件Arrgt Arrangement 布置AS Alloy steel 合金钢Asb Asbestos 石棉ASL Above Sea Level 海拔高度ASM American Society for Metals 美国金属学会Engineers 美国机械工程师学会Assem Assembly 装配ASTM American Society for Testing and Materials 美国材料试验学会Atm Atmosphere 大气atm Atmosphere pressure大气压 Auto Automatic 自动Aux Auxiliary辅助设备、辅助的Avail Available 有效的、可用的Avg Average 平均AW Arc welding 电弧焊AW Automatic Welding 自动焊A.W.G. American Wire Gauge 美国线规AWS(AWI) American WeldingSociety(Institute) 美国焊接学会 BAB Babbitt Metal 巴氏合金Baf Baffle 折流板、缓冲板BB Ball Bearing 滚珠轴承BC Between Centers 中心距、轴间距 BC Bolt circle 螺栓中心圆BD Blow down放空、放料BEDD Basic engineering design data 基础工程设计数据Bet Between 在之间Bev Bevel 斜角、坡口BF Back face 背面、反面BF Blind flange 法兰盖(盲法兰)BHN Brinell hardness number 布氏硬度值BL Battery Limit界区BL Battery Line 界区线B/L Bill of Loading 载荷数据表Bld Blind 盲板Blk Black 黑色Blk Blank 空白BM Bench Mark 基准标志BM Bending Moment 弯矩B/M (BOM) Bill of Material 材料表Bot Bottom 底BP Back Pressure 背压BP Base plate 底板BR Basic Requirements 基本要求 BRG Bearing 轴承BRKT Bracket 支架Brs Brass 黄铜BS Both Side 两边BS British Standard 英国标准BS Balance Sheet 平衡表BTU British Thermal Unit 英国热量单位BV Back View 后视图BV Butterfly Valve碟阀BW Brine Vater 冷冻盐水BW Butt Welding 对焊BWG Birmingham Wire Gauge 伯明翰线规BWRA. British Welding Research Association 英国焊接研究协会C Centigrade(degree) 摄氏度数CA Chemical Analysis 化学分析CA Corrosion Allowance 腐蚀裕量Calc Calculate 计算Cap Capacity 能力、容量CAS Cast Alloy Steel 铸造合金钢Cat Catalyst 触媒、催化剂Catg Catalog 目录、样本C-C(C/C) Center to center 中心距cc carbon copy 复写(纸复制)本 cc cubic centimeter 立方厘米CCW Counter clockwise 反时针方向CD Cold Drawn 冷拉的、冷拔的CE Covered Electrode 焊条Cent Centrifugal 离心的CF Centrifugal Force 离心力CFW Continuous Fillet Weld 连续角焊缝CG Center of Gravity 重心CH Case-Hardening 表面硬化 Ch Chapter 章节Cham Chamfer 倒角、斜角、斜面Chan Channel 通道、沟槽、管箱、槽钢Chk Check 检查CI Cast Iron 铸铁CIF Cost, Insurance and Freight 到岸价格Circ Circumference 圆周、环向CL Class 等级、类别CL Center Line 中心线CL Clearance 间隙CLAS Cast Low Alloy Steel 低合金铸钢CM Center of Mass 质量中心Cnds Condensate冷凝液CO Clean Out 清除Co Company 公司Coef Coefficient 系数Col Column 柱、塔Comb Combination 组合Comp Compare 比较Comp Compound 化合物、复合的Conc Concrete 混凝土Conc Concentration 浓度Cond Conductor 导体Cond Condition 条件Conn Connection 联接、接口Const Constant 常数、恒定的Const Construction 结构Cont Control 控制Cont Contain 包含Cont Content 内容、含量Corp Corporation 公司Corr Corrosion 腐蚀CP Centipoise 厘泊CP Center of Pressure压力中心 Cpl Coupling 管箍 CplgCoupling 联轴节CR Chloroprene Rubber 氯丁橡胶 CS Carbon Steel 碳钢CS Center Section 中心截面CSTG Casting 铸造、铸件Ctr Center 中心CW Cooling Water 冷却水CW Continuous Welding 连续焊Cy Cycle 循环Cyl Cylinder 气缸、圆筒D Density 密度Dbl Double 二倍、双DEDD Detail Engineering Design Data 详细工程设计数据Def Definition定义Deg Degree 度、等级Dept Department 部门Des Design 设计Det Detail 详细Detn Determination 确定、决定Dev Deviation 偏差Dev Device 装置DF Design Formula 设计公式Df Deflection 偏斜Dia Diameter 直径Diag Diagram 图Dim Dimension 尺寸Dir Direction方向Disch Discharge 排出、出口Distr Distribution 分布Div Division 部分、区分 DLDoc Document 文件、资料DP Design Pressure 设计压力DP Differential Pressure 压差、分压Dr Drill 钻孔Dr Drive 驱动DW Dead weight 静重、自重DW Demineralized Water 脱盐水Dwg Drawing 图 E East 东EC Elasticity Coefficient 弹性系数Ecc Eccentric 偏心EF Electric Furnace 电炉Eff Efficiency效率eg exempli gratia 例如EHP Effective Horsepower 有效功率EJ Expansion joint 膨胀节EL Elevation 标高Elb Elbow 弯头Elec Electric 电的Elem Element 元素、元件Ellip Ellipsoidal椭球的、椭圆的Emer、 Emerg Emergency 事故、紧急Encl Enclosure 密封、封闭Engrg、Eng Engineering 工程、设计EP Explosion Proof 防爆Eq Equipment 设备Eq Equation 公式、方程式Eq Equivalent 当量ES Electrostatic 静电EST Estimate 估计ESW Electro-Slag Welding 电渣焊ET Eddy Current Examination 涡流检验etc et cetera (and so on)等等Evap Evaporate 蒸发Ex Example 例如Ex Excess 过剩、超过Exam Examination 检验Exh Exhaust 废气、排气Exp Expansion 膨胀Exptl Experimental 实验的Ext External 外部Ext Extreme 极端的FAO Finish All Over 全部加工FAX Facsimile 传真FB Flat Bar 扁钢FCAW Flux Cored Arc Welding 熔剂芯弧焊(手工焊)FDW Feed Water 给水FF Flat Face 平面F/F Field Fabricated 现场制造Fig Figure 图Fin Finish 加工、完成FL Full Load 满载Flex Flexible 挠性Flg Flange 法兰FOB Free On Board 离岸价格FOC Free Of Charge 免费Forg Forging 锻件FOS Factor Of Safety 安全系数FREQ Frequency 频率FST Forged Steel 锻钢Ft Feet 英尺Ftg Fitting 管件、装配F.V. Full Vacuum 全真空FW Fresh Water 新鲜水FW Field Weld 现场焊接FW Fillet Weld 角焊缝GA General Average 平均值Gal Gallon 加仑Gen General 一般、总的Genr Generator 发电机、发生器 GF Groove Face 槽面Gl Glass 玻璃GL Ground Level 地面标高GMAW Gas Metal Arc Welding 气体保护金属极电弧焊Gnd Ground 接地、地面Govt Government 政府GP General Purpose一般用途、通用 Gr Grade 等级Gr Gravity 重力Grd Ground 地面Grp Group 分组、类Gr- wt Gross weight 总重、毛重HB Brinell Hardness 布氏硬度HC Hydrocarbon 烃类HC High Capacity 大容量HD Head 压头Hex Hexagon 六角HH Hand Hole 手孔Hor Horizontal 水平、卧式hp Horsepower 马力HP High Pressure 高压HR Rockwell Hardness 洛氏硬度HRC Rockwell C Hardness C 级洛氏硬度 HS High Pressure Steam高压蒸汽HS Shore Scleroscope Hardness肖氏硬度HSC High Pressure Condensate 高压蒸汽凝液HT High Temperature 高温HT Heat Treatment 热处理HT Hydrostatic Test 水压试验HV Vickers Hardness 维氏硬度Hvy Heavy 重的、重型的HW Hot Water 热水ICW Inter Cooling Water 中间冷却水ID Inside Diameter 内径IF Interface 交接面Illus Illustration说明、图解IN Inlet 进口in Inch 英寸incl Including包括Ind Indicate 指示Ins Insulation 保温INSP Inspection 检验Instl Installation 安装Int Internal 内部的Int Intermediate 中间的Intmt Intermittent 间歇的、间断的I/O Input/Output 输入 /输出Jt Joint 连接、接头KG Kilogram公斤KW(kw) Kilowatt千瓦LAS Low Alloy Steel 低合金钢lb pound 磅LC Level Control 液位控制器Leng Length 长度LF Female Face 凹面Lg Long 长的LG Level Glass 液位计LH Left Hand 左手Lin Linear 线性的Liq Liquid液体Lj Lap joint搭接LJ Lapped Joint 松套LM Male Face 凸面LMTD Logarithmic Mean Temperature Difference 对数平均温差 LN Liquid Nitrogen 液氮LN Level Normal正常液位Lng Lining衬里Lo Lubrication oil润滑油Lo Low 低LOA Length Over-All 全长总长LOC Location 位置Log Logarithm(to the base 10) 对数(以 10 为底)Long Longitudinal 纵向LP Low Pressure 低压LPG Liquefied Petroleum Gas 液化石油气LT Low Temperature 低温LT Leak Testing 气密试验Ltd Limited有限Ltr Letter 字母、信Lub Lubricate 润滑LW Lap Welding 搭接焊LWN Long Welding Neck 对焊长颈LWS Longitudinal Welded Seam 纵向焊缝 M(m) Meter 米、公尺Mach Machine 机器Maint Maintenance 维修Mat(Mat ’l) Material 材料MAWP Maximum Allowable Working Pressure 最大允许工作压力Max Maximum最大MDMT Min. Design Metallic Temperature 最低设计金属温度Mech Mechanical 机械的Mfd Manufactured 制造的Mfr Manufacturer 制造商MG(mg) Milligram毫克MH Manhole 人孔MI Melt Index熔融指数MIG Metal Inert Gas Arc Welding 熔化极惰性气体保护焊Min Minimum最小MIN(min) Minute分钟MJG Metallic Jacketed Gasket 金属包复垫片Mk Mark标志ml Milliliter毫升mm Millimeter毫米MP Medium Pressure 中压MPC Maximum Permissible Concentration 最大许用浓度MS Medium Pressure Steam 中压蒸汽 MS Medium Steel 中碳钢MSL Mean Sea Level 平均海平面MT Magnetic Particle Examination 磁粉检测Mtd Mounted 安装、装配MTR Material Testing Report 材料试验报告MU Measurement Unit 测量单位MV Mean Value 平均值MW Mineral Wool矿渣棉N North 北NA Not Applicable 不适用的NAT Natural 天然的Natl National 国家的NC America National Coarse Thread 美制粗牙螺纹NDT Nondestructive Testing 无损检验Neg Negative 负NF American National Fine Thread 美国细牙螺纹Nip Nipple 螺纹管接头、短节Nom Nominal 名义Nor Normal 正常NOZ Nozzle 接管NPS American Standard Straight Pipe Thread 美国标准直管螺纹NPSHA Net Positive Suction Head Available 有效汽蚀裕量NPSHR Net Positive Suction Head Required 要求汽蚀裕量NPT American Standard Taper Pipe Thread 美国标准锥管螺纹NT Net Tonnage 净吨数NTP Normal Temperature and Pressure 标准温度和压力NTS Not To Scale 不按比例Num Number 数、编号、号码Obj Object 目标、对象OC Operating Characteristic 操作特性OD Outside Diameter 外径OH Open Hearth 平炉Oper Operating 操作Opp Opposite 对面、相反OR Outside Radius 外半径OR Outside Ring 外环Orien Orientation 方位Ovhd Overhead 高架的、顶部的Oxyg Oxygen 氧 P Page 页P Pressure 压力Par Parallel 平行Para Paragraph节、段PE Polyethylene 聚乙烯PFD Process Flow Diagram 工艺流程图Perform Performance 性能PF Power Factor 功率因素PID Piping & Instruments Diagram 管道和仪表流程图Pl Plate 板Pneum Pneumatic 气、气动PO Purchase Order 订货单Port Portable 便携式、轻便 Posit Positive 正Posit Position 位置ppb Parts per billion 十亿分之几ppm Parts per million 百万分之几Prod Product 产品Proj Project 项目、工程PS Polystyrene 聚苯乙烯psf Pounds per square feet磅/平方英尺 psi Pounds per square inch磅/平方英寸PT Liquid Penetrants Examination 液体渗透检测PTFE Polytetrafluoroethylene 聚四氟乙烯PVA Polyvinyl Acetate 聚醋酸乙烯PVAL Polyvinyl Alcohol聚乙烯醇PVC Polyvinyl Chloride聚氯乙烯PWHT Post Weld Heat Treatment 焊后热处理 QA Quality Assurance 质量保证QC Quality Control 质量控制Qty Quantity 数量Qual Quality 质量R Radius 半径Rad Radial 径向RC Rockwell Hardness 洛氏硬度Recip Reciprocate 往复式Recirc Recirculate 再循环Recom Recommended 建议、推荐Ref Reference 参照、基准Refract Refractory 耐火材料Reg Regulator 调节器Regen Regenerator再生器、再生塔 Reinf Reinforce 加强Rel Relative 相对Rep Report 报告Rep Repeat 重复Reqd Required 要求、需要的REV Revision 修改、版次RF Raise face 突台面RH Relative Humidity 相对湿度RH Right Hand 右手RMS Root Mean Square 均方根 ROT Rotating 旋转rpm revolutions per minute 转/分rps revolutions per second 转/秒RT Radiographic Examination 射线照相检验S South 北SAW Submerged Arc Welding 埋弧焊 Sc Scale 刻度、比例SC Standard Condition 标准状态(温度压力)SCH Schedule 表号、管厚号、进度Sec Second 秒Sec Section 剖面、节、段Seg Segment 节、段 SepSeparator 分离器 SeqSequence次序、顺序 SGSpecific Gravity 比重 SHPShaft Horsepower 轴马力SI Standard International 国际单位制Sig Signal 信号Sld Solid 固体SMAW Shield Metal Arc Welding 手工焊Smls Seamless 无缝的SO Slip on 平焊(法兰)Sol Solution 溶液SP Spare parts备件Sp Special 特殊的、专门的SP Static pressure静压力Spec Specification 说明、规定SpGr Specific Gravity 比重Sq Square 方形、平方SR Stress Relief 消除应力SS Stainless Steel不锈钢 Sta Station 站STD Standard 标准STDWT Standard Weight 标准重量STL Steel 钢STP Standard Temperature and Pressure 标准温度和压力Suc Suction 吸入Suppl Supplement 补充SW Shop Welding 车间焊接SW Spot Weld 点焊SW Socket Welding 承插焊(法兰)SWP Safety Working Pressure安全工作压SYM Symmetry 对称SYS System 系统T Ton 吨TC Tungsten Carbide 碳化钨 Tech Technique 技术TEMA Tubular Exchanger Manufacturers Association 管壳式换热器制造商协会(美国)Temp Temperature 温度Term Terminal 终端、接头Thk Thickness 厚度TIG Tungsten Inert Gas Arc Welding 钨极惰性气体保护焊TL Tangent line 切线Tol Tolerance 公差Tot Total 总Trans Transfer 输送器TW Total Weight 总重TW Tack Welding 定位焊Typ Typical 典型、标准UNC Unified National Coarse Thread 统一标准粗牙螺纹UNF Unified National Fine Thread 统一标准细牙螺纹US Undersize 尺寸过小UT Ultrasonic Examination 超声波探伤UTS Ultimate Tensile Strength 抗拉强度极限Vac Vacuum 真空Vap Vapor 蒸汽Var Variable 变化、变量Vel Velocity 速度Vert Vertical 垂直Vol Volume 体积VT Visual Testing 宏观(目测)检查W Watt 瓦WL Welding Line 焊缝线WL Water Line 水线WPS Welding Procedure Specification 焊接工艺规程WP Working Pressure 工作压力WRC Welding Research Committee 焊接研究委员会(美国)WS Water Supply 供水WT Weight 重量W/V Wind Velocity风速XR X-Ray X 射线Yd Yard 码Yr Year 年external/outide diameter 外径grease 油污。
表面处理、模具等常用机械英语对照

表面处理、热处理关连用语英汉对照age hardening 时效硬化 ageing 老化处理air hardening 气体硬化 air patenting 空气韧化annealing 退火 anode effect 阳极效应anodizing 阳极氧化处理 atomloy treatment 阿托木洛伊表面austempering 奥氏体等温淬火 austenite 奥斯田体/奥氏体bainite 贝氏体 banded structure 条纹状组织barrel plating 滚镀 barrel tumbling 滚筒打光blackening 染黑法 blue shortness 青熟脆性bonderizing 磷酸盐皮膜处理 box annealing 箱型退火box carburizing 封箱渗碳 bright electroplating 辉面电镀bright heat treatment 光辉热处理 bypass heat treatment 旁路热处理carbide 炭化物 carburized case depth 浸碳硬化深层carburizing 渗碳 cementite 炭化铁chemical plating 化学电镀 chemical vapor deposition 化学蒸镀coarsening 结晶粒粗大化 coating 涂布被覆cold shortness 低温脆性 comemtite 渗碳体controlled atmosphere 大气热处理 corner effect 锐角效应creeping discharge 蠕缓放电 decarburization 脱碳处理decarburizing 脱碳退火 depth of hardening 硬化深层diffusion 扩散 diffusion annealing 扩散退火electrolytic hardening 电解淬火 embossing 压花etching 表面蚀刻 ferrite 肥粒铁first stage annealing 第一段退火 flame hardening 火焰硬化flame treatment 火焰处理 full annealing 完全退火gaseous cyaniding 气体氧化法 globular cementite 球状炭化铁grain size 结晶粒度 granolite treatment 磷酸溶液热处理graphitizing 石墨退火 hardenability 硬化性hardenability curve 硬化性曲线 hardening 硬化heat treatment 热处理 hot bath quenching 热浴淬火hot dipping 热浸镀 induction hardening 高周波硬化ion carbonitriding 离子渗碳氮化 ion carburizing 离子渗碳处理ion plating 离子电镀 isothermal annealing 等温退火liquid honing 液体喷砂法 low temperature annealing 低温退火malleablizing 可锻化退火 martempering 麻回火处理martensite 马氏体/硬化铁炭 metallikon 金属喷镀法metallizing 真空涂膜 nitriding 氮化处理nitrocarburizing 软氮化 normalizing 正常化oil quenching 油淬化 overageing 过老化overheating 过热 pearlite 针尖组织phosphating 磷酸盐皮膜处理 physical vapor deposition 物理蒸镀plasma nitriding 离子氮化 pre-annealing 预备退火precipitation 析出 precipitation hardening 析出硬化press quenching 加压硬化 process annealing 制程退火quench ageing 淬火老化 quench hardening 淬火quenching crack 淬火裂痕 quenching distortion 淬火变形quenching stress 淬火应力 reconditioning 再调质recrystallization 再结晶 red shortness 红热脆性residual stress 残留应力 retained austenite 残留奥rust prevention 防蚀 salt bath quenching 盐浴淬火sand blast 喷砂处理 seasoning 时效处理second stage annealing 第二段退火 secular distortion 经年变形segregation 偏析 selective hardening 部分淬火shot blast 喷丸处理 shot peening 珠击法single stage nitriding 等温渗氮 sintering 烧结处理soaking 均热处理 softening 软化退火solution treatment 固溶化热处理 spheroidizing 球状化退火stabilizing treatment 安定化处理 straightening annealing 矫直退火strain ageing 应变老化 stress relieving annealing 应力消除退火subzero treatment 生冷处理 supercooling 过冷surface hardening 表面硬化处理 temper brittleness 回火脆性temper colour 回火颜色 tempering 回火tempering crack 回火裂痕 texture 咬花thermal refining 调质处理 thermoechanical treatment 加工热处理time quenching 时间淬火 transformation 变态tufftride process 软氮化处理 under annealing 不完全退火vacuum carbonitriding 真空渗碳氮化 vacuum carburizing 真空渗碳处理vacuum hardening 真空淬火 vacuum heat treatment 真空热处理vacuum nitriding 真空氮化 water quenching 水淬火wetout 浸润处理模具厂常用之标准零配件英汉对照air vent vale 通气阀 anchor pin 锚梢angular pin 角梢 baffle 调节阻板angular pin 倾斜梢 baffle plate 折流档板ball button 球塞套 ball plunger 定位球塞ball slider 球塞滑块 binder plate 压板blank holder 防皱压板 blanking die 落料冲头bolster 上下模板 bottom board 浇注底板bolster 垫板 bottom plate 下固定板bracket 托架 bumper block 缓冲块buster 堵口 casting ladle 浇注包casting lug 铸耳 cavity 模穴(模仁)cavity retainer plate 模穴托板 center pin 中心梢clamping block 锁定块 coil spring 螺旋弹簧cold punched nut 冷冲螺母 cooling spiral 螺旋冷却栓core 心型 core pin 心型梢cotter 开口梢 cross 十字接头cushion pin 缓冲梢 diaphragm gate 盘形浇口die approach 模头料道 die bed 型底die block 块形模体 die body 铸模座die bush 合模衬套 die button 冲模母模die clamper 夹模器 die fastener 模具固定用零件die holder 母模固定板 die lip 模唇die plate 冲模板 die set 冲压模座direct gate 直接浇口 dog chuck 爪牙夹头dowel 定位梢 dowel hole 导套孔dowel pin 合模梢 dozzle 辅助浇口dowel pin 定位梢 draft 拔模锥度draw bead 张力调整杆 drive bearing 传动轴承ejection pad 顶出衬垫 ejector 脱模器ejector guide pin 顶出导梢 ejector leader busher 顶出导梢衬套ejector pad 顶出垫 ejector pin 顶出梢ejector plate 顶出板 ejector rod 顶出杆ejector sleeve 顶出衬套 ejector valve 顶出阀机械类常用英语:生产类PCS Pieces 个(根,块等) PRS Pairs 双(对等)CTN Carton 卡通箱 PAL Pallet/skid 栈板PO Purchasing Order 采购订单 MO Manufacture Order 生产单D/C Date Code 生产日期码 ID/C Identification Code (供应商)识别码SWR Special Work Request 特殊工作需求L/N Lot Number 批号 P/N Part Number 料号机械专业英语词汇(很全)金属切削 metal cutting 机床 machine tool金属工艺学 technology of metals 刀具 cutter摩擦 friction 联结 link传动 drive/transmission 轴 shaft弹性 elasticity 频率特性 frequency characteristic误差 error 响应 response定位 allocation 机床夹具 jig动力学 dynamic 运动学 kinematic静力学 static 分析力学 analyse mechanics拉伸 pulling 压缩 hitting剪切 shear 扭转 twist弯曲应力 bending stress 强度 intensity三相交流电 three-phase AC 磁路 magnetic circles变压器 transformer 异步电动机 asynchronous motor几何形状 geometrical 精度 precision正弦形的 sinusoid 交流电路 AC circuit机械加工余量 machining allowance 变形力 deforming force变形 deformation 应力 stress硬度 rigidity 热处理 heat treatment退火 anneal 正火 normalizing脱碳 decarburization 渗碳 carburization电路 circuit 半导体元件 semiconductor element反馈 feedback 发生器 generator直流电源 DC electrical source 门电路 gate circuit逻辑代数 logic algebra 外圆磨削 external grinding内圆磨削 internal grinding 平面磨削 plane grinding变速箱 gearbox 离合器 clutch绞孔 fraising 绞刀 reamer螺纹加工 thread processing 螺钉 screw铣削 mill 铣刀 milling cutter功率 power 工件 workpiece齿轮加工 gear mechining 齿轮 gear主运动 main movement 主运动方向 direction of main movement 进给方向 direction of feed 进给运动 feed movement合成进给运动 resultant movement of feed合成切削运动 resultant movement of cutting合成切削运动方向 direction of resultant movement of cutting切削深度 cutting depth 前刀面 rake face刀尖 nose of tool 前角 rake angle后角 clearance angle 龙门刨削 planing主轴 spindle 主轴箱 headstock卡盘 chuck 加工中心 machining center车刀 lathe tool 车床 lathe钻削镗削 bore 车削 turning磨床 grinder 基准 benchmark钳工 locksmith 锻 forge压模 stamping 焊 weld拉床 broaching machine 拉孔 broaching装配 assembling 铸造 found流体动力学 fluid dynamics 流体力学 fluid mechanics加工 machining 液压 hydraulic pressure切线 tangent 机电一体化 mechanotronics mechanical-electrical integration 气压 air pressure pneumatic pressure 稳定性 stability介质 medium 液压驱动泵 fluid clutch液压泵 hydraulic pump 阀门 valve失效 invalidation 强度 intensity载荷 load 应力 stress安全系数 safty factor 可靠性 reliability螺纹 thread 螺旋 helix键 spline 销 pin滚动轴承 rolling bearing 滑动轴承 sliding bearing弹簧 spring 制动器 arrester brake十字结联轴节 crosshead 联轴器 coupling链 chain 皮带 strap精加工 finish machining 粗加工 rough machining变速箱体 gearbox casing 腐蚀 rust氧化 oxidation 磨损 wear耐用度 durability 随机信号 random signal离散信号 discrete signal 超声传感器 ultrasonic sensor集成电路 integrate circuit 挡板 orifice plate残余应力 residual stress 套筒 sleeve扭力 torsion 冷加工 cold machining电动机 electromotor 汽缸 cylinder过盈配合 interference fit 热加工 hotwork摄像头 CCD camera 倒角 rounding chamfer优化设计 optimal design 工业造型设计 industrial moulding design 有限元 finite element 滚齿 hobbing插齿 gear shaping 伺服电机 actuating motor铣床 milling machine 钻床 drill machine镗床 boring machine 步进电机 stepper motor丝杠 screw rod 导轨 lead rail组件 subassembly 可编程序逻辑控制器 Programmable Logic Controller PLC 电火花加工 electric spark machining电火花线切割加工 electrical discharge wire - cutting相图 phase diagram 热处理 heat treatment固态相变 solid state phase changes 有色金属 nonferrous metal陶瓷 ceramics 合成纤维 synthetic fibre电化学腐蚀 electrochemical corrosion 车架 automotive chassis悬架 suspension 转向器 redirector变速器 speed changer 板料冲压 sheet metal parts孔加工 spot facing machining 车间 workshop工程技术人员 engineer 气动夹紧 pneuma lock数学模型 mathematical model 画法几何 descriptive geometry机械制图 Mechanical drawing 投影 projection视图 view 剖视图 profile chart标准件 standard component 零件图 part drawing装配图 assembly drawing 尺寸标注 size marking技术要求 technical requirements 刚度 rigidity内力 internal force 位移 displacement截面 section 疲劳极限 fatigue limit断裂 fracture 塑性变形 plastic distortion脆性材料 brittleness material 刚度准则 rigidity criterion垫圈 washer 垫片 spacer直齿圆柱齿轮 straight toothed spur gear 斜齿圆柱齿轮 helical-spur gear 直齿锥齿轮 straight bevel gear 运动简图 kinematic sketch齿轮齿条 pinion and rack 蜗杆蜗轮 worm and worm gear虚约束 passive constraint 曲柄 crank摇杆 racker 凸轮 cams共轭曲线 conjugate curve 范成法 generation method定义域 definitional domain 值域 range导数\\微分 differential coefficient 求导 derivation定积分 definite integral 不定积分 indefinite integral曲率 curvature 偏微分 partial differential毛坯 rough 游标卡尺 slide caliper千分尺 micrometer calipers 攻丝 tap二阶行列式 second order determinant 逆矩阵 inverse matrix线性方程组 linear equations 概率 probability随机变量 random variable 排列组合 permutation and combination 气体状态方程 equation of state of gas 动能 kinetic energy势能 potential energy 机械能守恒 conservation of mechanical energy 动量 momentum 桁架 truss轴线 axes 余子式 cofactor逻辑电路 logic circuit 触发器 flip-flop脉冲波形 pulse shape 数模 digital analogy液压传动机构 fluid drive mechanism 机械零件 mechanical parts淬火冷却 quench 淬火 hardening回火 tempering 调质 hardening and tempering磨粒 abrasive grain 结合剂 bonding agent砂轮 grinding wheel机械类常用英语:常用加工机械3D coordinate measurement 三次元量床 boring machine 搪孔机cnc milling machine CNC铣床 contouring machine 轮廓锯床copy grinding machine 仿形磨床 copy lathe 仿形车床copy milling machine 仿形铣床 copy shaping machine 仿形刨床cylindrical grinding machine 外圆磨床 die spotting machine 合模机drilling machine 钻孔机 engraving machine 雕刻机engraving E.D.M. 雕模放置加工机 form grinding machine 成形磨床graphite machine 石墨加工机 horizontal boring machine 卧式搪孔机horizontal machine center 卧式加工制造中心internal cylindrical machine 内圆磨床jig boring machine 冶具搪孔机 jig grinding machine 冶具磨床lap machine 研磨机 machine center 加工制造中心multi model miller 靠磨铣床 NC drilling machine NC钻床NC grinding machine NC磨床 NC lathe NC车床NC programming system NC程式制作系统 planer 龙门刨床profile grinding machine 投影磨床 projection grinder 投影磨床radial drilling machine 旋臂钻床 shaper 牛头刨床surface grinder 平面磨床 try machine 试模机turret lathe 转塔车床 universal tool grinding machine 万能工具磨床vertical machine center 立式加工制造中心 wire E.D.M. 线割放电加工机机械类常用英语:钢材类alloy tool steel 合金工具钢 aluminium alloy 铝合金钢bearing alloy 轴承合金 blister steel 浸碳钢bonderized steel sheet 邦德防蚀钢板 carbon tool steel 碳素工具钢clad sheet 被覆板 clod work die steel 冷锻模用钢emery 金钢砂 ferrostatic pressure 钢铁水静压力forging die steel 锻造模用钢 galvanized steel sheet 镀锌铁板hard alloy steel 超硬合金钢 high speed tool steel 高速度工具钢hot work die steel 热锻模用钢 low alloy tool steel 特殊工具钢low manganese casting steel 低锰铸钢 marging steel 马式体高强度热处理钢martrix alloy 马特里斯合金 meehanite cast iron 米汉纳铸钢meehanite metal 米汉纳铁 merchant iron 市售钢材molybdenum high speed steel 钼系高速钢 molybdenum steel 钼钢nickel chromium steel 镍铬钢 prehardened steel 顶硬钢silicon steel sheet 矽钢板 stainless steel 不锈钢tin plated steel sheet 镀锡铁板 ough pitch copper 韧铜troostite 吐粒散铁 tungsten steel 钨钢vinyl tapped steel sheet 塑胶覆面钢板外贸常用机械英语大全Assembly line 组装线 Layout 布置图Conveyer 流水线物料板 Rivet table 拉钉机Rivet gun 拉钉枪 Screw driver 起子Pneumatic screw driver 气动起子 worktable 工作桌OOBA 开箱检查 fit together 组装在一起fasten 锁紧(螺丝) fixture 夹具(治具)pallet 栈板 barcode 条码barcode scanner 条码扫描器 fuse together 熔合fuse machine热熔机 repair修理operator作业员 QC品管supervisor 课长 ME 制造工程师MT 制造生技 cosmetic inspect 外观检查inner parts inspect 内部检查 thumb screw 大头螺丝lbs. inch 镑、英寸 EMI gasket 导电条front plate 前板 rear plate 后板chassis 基座 bezel panel 面板power button 电源按键 reset button 重置键Hi-pot test of SPS 高源高压测试 Voltage switch of SPS 电源电压接拉键sheet metal parts 冲件 plastic parts 塑胶件SOP 制造作业程序 material check list 物料检查表work cell 工作间 trolley 台车carton 纸箱 sub-line 支线left fork 叉车 personnel resource department 人力资源部production department生产部门 planning department企划部QC Section品管科 stamping factory冲压厂painting factory烤漆厂 molding factory成型厂common equipment常用设备 uncoiler and straightener整平机punching machine 冲床 robot机械手hydraulic machine油压机 lathe车床planer |plein|刨床 miller铣床grinder磨床 linear cutting线切割electrical sparkle电火花 welder电焊机staker=reviting machine铆合机 position职务president董事长 general manager总经理special assistant manager特助 factory director厂长department director部长 deputy manager | =vice manager副理section supervisor课长 deputy section supervisor =vice section superisor副课长group leader/supervisor组长 line supervisor线长assistant manager助理 to move, to carry, to handle搬运be put in storage入库 pack packing包装to apply oil擦油 to file burr 锉毛刺final inspection终检 to connect material接料to reverse material 翻料 wet station沾湿台Tiana天那水 cleaning cloth抹布to load material上料 to unload material卸料to return material/stock to退料 scraped报废scrape ..v.刮;削 deficient purchase来料不良manufacture procedure制程 deficient manufacturing procedure制程不良oxidation氧化 scratch刮伤dents压痕 defective upsiding down抽芽不良defective to staking铆合不良 embedded lump镶块feeding is not in place送料不到位 stamping-missing漏冲production capacity生产力 education and training教育与训练proposal improvement提案改善 spare parts=buffer备件forklift叉车 trailer=long vehicle拖板车compound die合模 die locker锁模器pressure plate=plate pinch压板 bolt螺栓administration/general affairs dept总务部 automatic screwdriver电动启子thickness gauge厚薄规 gauge(or jig)治具power wire电源线 buzzle蜂鸣器defective product label不良标签 identifying sheet list标示单location地点 present members出席人员subject主题 conclusion结论decision items决议事项 responsible department负责单位pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/审核/承办PCE assembly production schedule sheet PCE组装厂生产排配表model机锺 work order工令revision版次 remark备注production control confirmation生产确认 checked by初审approved by核准 department部门stock age analysis sheet 库存货龄分析表 on-hand inventory现有库存available material良品可使用 obsolete material良品已呆滞to be inspected or reworked 待验或重工 total合计cause description原因说明 part number/ P/N 料号type形态 item/group/class类别quality品质 prepared by制表notes说明year-end physical inventory difference analysis sheet 年终盘点差异分析表physical inventory盘点数量 physical count quantity帐面数量difference quantity差异量 cause analysis原因分析raw materials原料 materials物料finished product成品 semi-finished product半成品packing materials包材good product/accepted goods/ accepted parts/good parts良品defective product/non-good parts不良品disposed goods处理品 warehouse/hub仓库on way location在途仓 oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓skid/pallet栈板 tox machine自铆机wire EDM线割 EDM放电机coil stock卷料 sheet stock片料tolerance工差 score=groove压线cam block滑块 pilot导正筒trim剪外边 pierce剪内边drag form压锻差 pocket for the punch head挂钩槽slug hole废料孔 feature die公母模expansion dwg展开图 radius半径shim(wedge)楔子 torch-flame cut火焰切割set screw止付螺丝 form block折刀stop pin定位销 round pierce punch=die button圆冲子shape punch=die insert异形子 stock locater block定位块under cut=scrap chopper清角 active plate活动板baffle plate挡块 cover plate盖板male die公模 female die母模groove punch压线冲子 air-cushion eject-rod气垫顶杆spring-box eject-plate弹簧箱顶板 bushing block衬套insert 入块 club car高尔夫球车capability能力 parameter参数factor系数 phosphate皮膜化成viscosity涂料粘度 alkalidipping脱脂main manifold主集流脉 bezel斜视规blanking穿落模 dejecting顶固模demagnetization去磁;消磁 high-speed transmission高速传递heat dissipation热传 rack上料degrease脱脂 rinse水洗alkaline etch龄咬 desmut剥黑膜D.I. rinse纯水次 Chromate铬酸处理Anodize阳性处理 seal封孔revision版次 part number/P/N料号barcode条码 flow chart流程表单assembly组装 stamping冲压molding成型 spare parts=buffer备品coordinate座标 dismantle the die折模auxiliary fuction辅助功能 poly-line多义线heater band 加热片 thermocouple热电偶sand blasting喷沙 grit 砂砾derusting machine除锈机 degate打浇口dryer烘干机 induction感应induction light感应光 response=reaction=interaction感应ram连杆 edge finder巡边器concave凸 convex凹short射料不足 nick缺口speck瑕疵 shine亮班splay 银纹 gas mark焦痕delamination起鳞 cold slug冷块blush 导色 gouge沟槽;凿槽satin texture段面咬花 witness line证示线patent专利 grit沙砾granule=peuet=grain细粒 grit maker抽粒机cushion缓冲 magnalium镁铝合金magnesium镁金 metal plate钣金blinster气泡 fillet镶;嵌边through-hole form通孔形式 voller pin formality滚针形式cam driver铡楔 shank摸柄crank shaft曲柄轴 augular offset角度偏差velocity速度 production tempo生产进度现状torque扭矩 spline=the multiple keys花键quenching淬火 tempering回火annealing退火 carbonization碳化tungsten high speed steel钨高速的 moly high speed steel钼高速的organic solvent有机溶剂 bracket小磁导liaison联络单 volatile挥发性resistance电阻 ion离子titrator滴定仪 beacon警示灯coolant冷却液 crusher破碎机机械工具英语spanner 扳子 (美作:wrench) double-ended spanner 双头扳子adjustable spanner, monkey wrench 活扳子,活络扳手box spanner 管钳子 (美作:socket wrench) calipers 卡规pincers, tongs 夹钳 shears 剪子hacksaw 钢锯 wire cutters 剪线钳multipurpose pliers, universal pliers 万能手钳 adjustable pliers 可调手钳punch 冲子 chuck 卡盘scraper 三角刮刀 reamer 扩孔钻calliper gauge 孔径规 rivet 铆钉nut 螺母 locknut 自锁螺母,防松螺母bolt 螺栓 pin, peg, dowel 销钉staple U形钉 oil can 油壶jack 工作服 grease gun 注油枪抛光 polishing 衬套 bushing半机械化 semi-mechanization; semi-mechanized半自动滚刀磨床 semi-automatic hob grinder半自动化 semi-automation; semi-automatic备件 spare parts 边刨床 side planer变速箱 transmission gear 柄轴 arbor部件 units; assembly parts 插床 slotting machine拆卸 to disassemble 超高速内圆磨床 ultra-high-speed internal grinder。
机械专业术语(英语)大全

表面处理关连用语age hardening 时效硬化ageing 老化处理air hardening 气体硬化air patenting 空气韧化annealing 退火anode effect 阳极效应anodizing 阳极氧化处理atomloy treatment 阿托木洛伊表面austempering 奥氏体等温淬火austenite 奥斯田体/奥氏体bainite 贝氏体banded structure 条纹状组织barrel plating 滚镀barrel tumbling 滚筒打光blackening 染黑法blue shortness 青熟脆性bonderizing 磷酸盐皮膜处理box annealing 箱型退火box carburizing 封箱渗碳bright electroplating 辉面电镀bright heat treatment 光辉热处理bypass heat treatment 旁路热处理carbide 炭化物carburized case depth 浸碳硬化深层carburizing 渗碳cementite 炭化铁chemical plating 化学电镀chemical vapor deposition 化学蒸镀coarsening 结晶粒粗大化coating 涂布被覆cold shortness 低温脆性comemtite 渗碳体controlled atmosphere 大气热处理corner effect 锐角效应creeping discharge 蠕缓放电decarburization 脱碳处理decarburizing 脱碳退火depth of hardening 硬化深层diffusion 扩散diffusion annealing 扩散退火electrolytic hardening 电解淬火embossing 压花etching 表面蚀刻ferrite 肥粒铁first stage annealing 第一段退火flame hardening 火焰硬化flame treatment 火焰处理full annealing 完全退火gaseous cyaniding 气体氧化法globular cementite 球状炭化铁grain size 结晶粒度granolite treatment 磷酸溶液热处理graphitizing 石墨退火hardenability 硬化性hardenability curve 硬化性曲线hardening 硬化heat treatment 热处理hot bath quenching 热浴淬火hot dipping 热浸镀induction hardening 高周波硬化ion carbonitriding 离子渗碳氮化ion carburizing 离子渗碳处理ion plating 离子电镀isothermal annealing 等温退火liquid honing 液体喷砂法low temperature annealing 低温退火malleablizing 可锻化退火martempering 麻回火处理martensite 马氏体/硬化铁炭metallikon 金属喷镀法metallizing 真空涂膜nitriding 氮化处理nitrocarburizing 软氮化normalizing 正常化oil quenching 油淬化overageing 过老化overheating 过热pearlite 针尖组织phosphating 磷酸盐皮膜处理physical vapor deposition 物理蒸镀plasma nitriding 离子氮化pre-annealing 预备退火precipitation 析出precipitation hardening 析出硬化press quenching 加压硬化process annealing 制程退火quench ageing 淬火老化quench hardening 淬火quenching crack 淬火裂痕quenching distortion 淬火变形quenching stress 淬火应力reconditioning 再调质recrystallization 再结晶red shortness 红热脆性residual stress 残留应力retained austenite 残留奥rust prevention 防蚀salt bath quenching 盐浴淬火sand blast 喷砂处理seasoning 时效处理second stage annealing 第二段退火secular distortion 经年变形segregation 偏析selective hardening 部分淬火shot blast 喷丸处理shot peening 珠击法single stage nitriding 等温渗氮sintering 烧结处理soaking 均热处理softening 软化退火solution treatment 固溶化热处理spheroidizing 球状化退火stabilizing treatment 安定化处理straightening annealing 矫直退火strain ageing 应变老化stress relieving annealing 应力消除退火subzero treatment 生冷处理supercooling 过冷surface hardening 表面硬化处理temper brittleness 回火脆性temper colour 回火颜色tempering 回火tempering crack 回火裂痕texture 咬花thermal refining 调质处理thermoechanical treatment 加工热处理time quenching 时间淬火transformation 变态tufftride process 软氮化处理under annealing 不完全退火vacuum carbonitriding 真空渗碳氮化vacuum carburizing 真空渗碳处理vacuum hardening 真空淬火vacuum heat treatment 真空热处理vacuum nitriding 真空氮化water quenching 水淬火wetout 浸润处理焊接用语acetylene 乙炔ampere 电流安培angle welding 角焊arc 电弧argon arc welding 氩弧焊接bare electrode 光熔接条butt welding 对接焊接camber 电弧弯曲cascade 阶叠熔接法clad weld 被覆熔接crator 焊疤excess metal 多余金属filler rod 焊条fillet weld 填角焊接gas shield 气体遮蔽groove welding 起槽熔接hand face shield 手握面罩hard facing 硬表面堆焊jig welding 工模焊接laser beam welding 雷射光焊接metal electrode insert gas welding MIG熔接nugget 点焊熔核overlaying 堆焊peening of welding 珠击熔接法plug welding 塞孔熔接positioned welding 正向熔接pressure welding 压焊propane gas cutting 丙烷气切割pure nickel electrode 纯镍熔接条reinforcement of weld 加强焊接resist 抗蚀护膜root running 背面熔接seam 焊缝seaming 接合seam welding 流缝熔接series seam welding 串联缝熔接skip welding process 跳焊法spark 火花spot welding 点焊接stitch welding 针角焊接stud arc welding 电弧焊接under laying 下部焊层void 焊接空隙weld flow mark 焊接流痕weld flush 焊缝凸起weld line 焊接纹weld mark 焊接痕weld penetration 熔接透入weld zone 焊接区welding 焊接welding bead 焊接泡welding direction 焊接方向welding distortion 焊接变形welding flux 焊剂welding ground 电熔接地welding interval 焊接周期welding stress 熔接应变welding torch 熔接气炬射出成形关联用语activator 活化剂bag moulding 气胎施压成形bonding strength 黏合强度breathing 排气caulking compound 填隙料cell 气孔cold slug 半凝式射出colorant 著色剂color matching 调色color masterbatch 色母料compound 混合料copolymer 共聚合体cull 残料废品cure 凝固化cryptometer 不透明度仪daylight 开隙dry cycle time 空料试车周期时间ductility 延性elastomer 弹性体extruded bead sealing 压出粒涂层法feed 供料filler 充填剂film blowing 薄膜吹制法floating platen 活动模板foaming agent 发泡剂gloss 光泽granule 颗粒料gunk 料斗hot mark 热斑hot stamping 烫印injection nozzle 射出喷嘴injection plunger 射出柱塞injection ram 射出冲柱isomer 同分异构物kneader 混合机leveling agent 匀涂剂lubricant 润滑剂matched die method 配合成形法mould clamping force 锁模力mould release agent 脱模剂nozzle 喷嘴oriented film 取向薄膜parison 吹气成形坏料pellet 粒料plasticizer 可塑剂plunger 压料柱塞porosity 孔隙率post cure 後固化premix 预混料purging 清除reciprocating screw 往复螺杆resilience 回弹性resin injection 树脂射出法rheology 流变学sheet 塑胶片shot 注射shot cycle 射出循环slip agent 光滑剂take out device 取料装置tie bar 拉杆toggle type mould clamping system 肘杆式锁模装置torpedo spreader 鱼雷形分流板transparency 透明性void content 空洞率塑胶原料acrylic 压克力casein 酪素cellulose acetate 醋酸纤维素CA cellulose acetate butyrate 醋酸丁酸纤维素CABcomposite material 复合材料cresol resin 甲酚树脂CFdially phthalate 苯二甲酸二烯丙酯disperse reinforcement 分散性强化复合材料engineering plastics 工程塑胶epoxy resin 环氧树脂EPethyl cellulose 乙基纤维素ethylene vinylacetate copolymer 乙烯-醋酸乙烯EV Aethylene-vinlacetate copolyme 醋酸乙烯共聚物EV Aexpanded polystyrene ?泡聚苯乙烯EPS fiber reinforcement 纤维强化热固性/纤维强化复合材料high density polyethylene 高密度聚乙烯HDPEhigh impact polystyrene 高冲击聚苯乙烯HIPShigh impact polystyrene rigidity 高冲击性聚苯乙烯low density polyethylene 低密度聚乙烯LDPEmelamine resin 三聚氰胺酚醛树脂MF nitrocellulose 硝酸纤维素phenolic resin 酚醛树脂plastic 塑胶polyacrylic acid 聚丙烯酸PAPpolyamide 耐龙PA polybutyleneterephthalate 聚对苯二甲酸丁酯PBTpolycarbonate 聚碳酸酯PC polyethyleneglycol 聚乙二醇PFG polyethyleneoxide 聚氧化乙烯PEO polyethyleneterephthalate 聚乙醇对苯PETP polymetylmethacrylate 聚甲基丙烯酸甲酯PMMApolyoxymethylene 聚缩醛POM polyphenylene oxide 聚硫化亚苯polyphenyleneoxide 聚苯醚PPO polypropylene 聚丙烯PPpolystyrene 聚苯乙烯PS polytetrafluoroethylene 聚四氟乙烯PTFE polytetrafluoroethylene 聚四氟乙烯polythene 聚乙烯PEpolyurethane 聚氨基甲酸酯PU polyvinylacetate 聚醋酸乙烯PV AC polyvinylalcohol 聚乙烯醇PV A polyvinylbutyral 聚乙烯醇缩丁醛PVB polyvinylchloride 聚氯乙烯PVC polyvinylfuoride 聚氟乙烯PVF polyvinylidenechloride 聚偏二氯乙烯PVDC prepolymer 预聚物silicone resin 矽树脂thermoplastic 热塑性thermosetting 热固性thermosetting plastic 塑胶unsaturated polyester 不饱和聚酯树脂成形不良用语aberration 色差atomization ?化bank mark ?料纹bite 咬入blacking hole 涂料孔(铸疵) blacking scab 涂料疤blister 起泡blooming 起霜blow hole 破孔blushing 泛白body wrinkle 侧壁皱纹breaking-in 冒口带肉bubble 膜泡burn mark 糊斑burr 毛边camber 翘曲cell 气泡center buckle 表面中部波皱check 细裂痕checking 龟裂chipping 修整表面缺陷clamp-off 铸件凹痕collapse 塌陷color mottle 色斑corrosion 腐蚀crack 裂痕crazing 碎裂crazing 龟裂deformation 变形edge 切边碎片edge crack 裂边fading 退色filler speak 填充料斑fissure 裂纹flange wrinkle 凸缘起皱flaw 刮伤flow mark 流痕galling 毛边glazing 光滑gloss 光泽grease pits 污斑grinding defect 磨痕haircrack 发裂haze 雾度incrustation 水锈indentation 压痕internal porosity 内部气孔mismatch 偏模mottle 斑点necking 缩颈nick 割痕orange peel 橘皮状表面缺陷overflow 溢流peeling 剥离pit 坑pitting corrosion 点状腐蚀plate mark 模板印痕pock 麻点pock mark 痘斑resin streak 树脂流纹resin wear 树脂脱落riding 凹陷sagging 松垂saponification 皂化scar 疤痕scrap 废料scrap jam 废料阻塞scratch 刮伤/划痕scuffing 深冲表面划伤seam 裂痕shock line 模口挤痕short shot 充填不足shrinkage pool 凹孔sink mark 凹痕skin inclusion 表皮摺叠straightening 矫直streak 条状痕surface check 表面裂痕surface roughening 橘皮状表皮皱摺surging 波动sweat out 冒汗torsion 扭曲warpage 翘曲waviness 波痕webbing 熔塌weld mark 焊痕whitening 白化wrinkle 皱纹模具常用刀具与工作法用语adjustable spanner 活动扳手angle cutter 角铣刀anvil 铁?arbour 心轴backing 衬垫belt sander 带式打磨机buffing 抛光chamfering machine 倒角机chamfering tool 去角刀具chisel 扁錾chuck 夹具compass 两角规concave cutter 凹面铣刀convex cutter 凸形铣刀cross joint 十字接头cutting edge clearance 刃口余隙角drill stand 钻台edge file 刃用锉刀file 锉刀flange joint 凸缘接头grinder 砂轮机hammer 铁锤hand brace 手摇钻hatching 剖面线hexagon headed bolt 六角头螺栓hexagon nut 六角螺帽index head 分度头jack 千斤顶jig 治具kit 工具箱lapping 研磨metal saw 金工锯nose angle 刀角pinchers 钳子pliers 铗钳plug 柱塞头polisher 磨光器protable driller 手提钻孔机punch 冲头sand paper 砂纸scraper 刮刀screw driver 螺丝起子scribing 划线second out file 中纹锉spanner 扳手spline broach 方栓槽拉刀square 直角尺square sleeker 方形镘刀square trowel 直角度stripping 剥离工具T-slot T形槽tool for lathe 车刀tool point angle 刀刃角tool post 刀架tosecan 划线盘trimming 去毛边waffle die flattening 压纹效平wiper 脱模钳wrench 螺旋扳手电脑关联用语3D modeling 三次元模拟access 通路animation 卡通影片application 应用board 基板bug 故障bus 汇流排CAD 电脑辅助设计CAE 电脑辅助工程分析CAM 电脑辅助制造cassette 卡座color display 彩色显示器command 指令communication 通信compact 精简小型computer 电脑copy 复制cursor 游标curve modeling 曲面模拟database 资料库design 设计digitizing 数位化disk 磁碟dot 点eyelet 眼孔floppy 磁碟片format 格式化graphic 圆解hardware 硬体honeycomb 蜂巢interface 界面know how 秘诀laser printer 雷射印表机lay out 布置memory 记忆memory swap 交换记忆microprocessor 微处理器modeling 造型module 模组monitor 萤幕mouse 滑鼠need 需求network 网路new version 新版on line 上线中option 选择PC 个人电脑plotter 绘图机program 程式scanning 扫描simulation 模拟software 软体solid model 实体模型system 系统tape 磁带terminal 终端机texture 构造trim 修边venter 排气风扇word processor 文书处理器各种冲模加工关连用语barreling 滚光加工belling 压凸加工bending 弯曲加工blanking 下料加工bulging 撑压加工burring 冲缘加工cam die bending 凸轮弯曲加工caulking ?合加工coining 压印加工compressing 压缩加工compression bending 押弯曲加工crowning 凸面加工curl bending 卷边弯曲加工curling 卷曲加工cutting 切削加工dinking 切断蕊骨double shearing 叠板裁断drawing 引伸加工drawing with ironing 抽引光滑加工embossing 浮花压制加工extrusion 挤制加工filing 锉削加工fine blanking 精密下料加工finish blanking 光制下料加工finishing 精整加工flanging 凸缘加工folding 折边弯曲加工folding 摺叠加工forming 成形加工impact extrusion 冲击挤压加工indenting 压痕加工ironing 引缩加工knurling 滚花lock seaming 固定接合louvering 百叶窗板加工marking 刻印加工necking 颈缩加工notching 冲口加工parting 分断加工piercing 冲孔加工progressive bending 连续弯曲加工progressive blanking 连续下料加工progressive drawing 连续引伸加工progressive forming 连续成形加工reaming 铰孔加工restriking 二次精冲加工riveting ?接加工roll bending 滚筒弯曲加工roll finishing 滚压加工rolling 压延加工roughing 粗加工scrapless machining 无废料加工seaming 折弯重叠加工shaving 缺口修整加工shearing 切断加工sizing 精压加工/矫正加工slitting 割缝加工spinning 卷边链接staking 链固stamping 锻压加工swaging 挤锻压加工trimming 整缘加工upsetting 锻粗加工wiring 抽线加工冲压机械及周边关连用语back shaft 支撑轴blank determination 胚料展开bottom slide press 下传动式压力机board drop hammer 板落锤brake 煞车buckle 剥砂面camlachie cramp 铸包casting on flat ?合chamotte sand 烧磨砂charging hopper 加料漏斗clearance 间隙closed-die forging 合模锻造clump 夹紧clutch 离合器clutch brake 离合器制动器clutch boss 离合器轮壳clutch lining 离合器覆盖coil car 带卷升降运输机coil cradle 卷材进料装置coil reel stand 钢材卷料架column 圆柱connection screw 连杆调节螺钉core compound 砂心黏结剂counter blow hammer 对击锻锤cradle 送料架crank 曲柄轴crankless 无曲柄式cross crank 横向曲轴cushion 缓冲depression 外缩凹孔dial feed 分度送料die approach 模口角度die assembly 合模die cushion 模具缓冲垫die height 冲压闭合高度die life 模具寿命die opening 母模逃孔die spotting press 调整冲模用压力机double crank press 双曲柄轴冲床draght angle 逃料倾斜角edging 边锻伸embedded core 加装砂心feed length 送料长度feed level 送料高度filling core 埋入砂心filling in 填砂film play 液面花纹fine blanking press 精密下料冲床forging roll 辊锻机finishing slag 炼後熔渣fly wheel 飞轮fly wheel brake 飞轮制动器foot press 脚踏冲床formboard 进模口板frame 床身机架friction 摩擦friction brake 摩擦煞车gap shear 凹口剪床gear 齿轮gib 滑块引导部gripper 夹具gripper feed 夹持进料gripper feeder 夹紧传送装置hammer 槌机hand press 手动冲床hand rack pinion press 手动齿轮齿条式冲床hand screw press 手动螺旋式冲床hopper feed 料斗送料idle stage 空站inching 微调尺寸isothermal forging 恒温锻造key clutch 键槽离合器knockout 脱模装置knuckle mechanic 转向机构land 模具直线刀面部level 水平loader 供料器unloader 卸料机loop controller 闭回路控制器lower die 下模micro inching device 微寸动装置microinching equipment 微动装置motor 马达moving bolster 活动工作台notching press 冲缺口压力机opening 排料逃孔overload protection device 防超载装置pinch roll 导正滚轮pinion 小齿轮pitch 节距pressfit 压入progressive 连续送料pusher feed 推杆式送料pusher feeder 料片押片装置quick die change system 快速换模系统regrinding 再次研磨releasing 松释动作reversed blanking 反转下料robot 机器人roll forming machine 辊轧成形roll forming machine 辊轧成形机roll release 脱辊roller feed 辊式送料roller leveler 辊式矫直机rotary bender 卷弯成形机safety guard 安全保护装置scrap cutter 废料切刀scrap press 废料冲床seamless forging 无缝锻造separate 分离shave 崩砂shear angle 剪角sheet loader 薄板装料机shot 单行程工作shrinkage fit 收缩配合shut height 闭合高度sieve mesh 筛孔sintering of sand 铸砂烧贴slide balancer 滑动平衡器slug hole 逃料孔spin forming machine 旋压成形机spotting 合模stack feeder 堆叠拨送料机stickness 黏模性straight side frame 冲床侧板stretcher leveler 拉伸矫直机strip feeder 料材送料装置stripping pressure 弹出压力stroke 冲程take out device 取料装置toggle press 肘杆式压力机transfer 传送transfer feed 连续自动送料装置turrent punch press 转塔冲床two speed clutch 双速离合器uncoiler 闭卷送料机unloader 卸载机vibration feeder 振动送料机wiring press 嵌线卷边机模板类top plate上托板(顶板)top block上垫脚punch set上模座punch pad上垫板punch holder上夹板stripper pad脱料背板up stripper上脱料板male die公模(凸模)feature die公母模female die母模(凹模)upper plate上模板lower plate下模板die pad下垫板die holder下夹板die set下模座bottom block下垫脚bottom plate下托板(底板) stripping plate内外打(脱料板) outer stripper外脱料板inner stripper内脱料板lower stripper下脱料板五金零件类inner guiding post内导柱inner hexagon screw内六角螺钉dowel pin固定销coil spring弹簧lifter pin顶料销eq-height sleeves=spool等高套筒pin销lifter guide pin浮升导料销guide pin导正销wire spring圆线弹簧outer guiding post外导柱stop screw止付螺丝located pin定位销outer bush外导套模具工程常用词汇die 模具figure file, chart file图档cutting die, blanking die冲裁模progressive die, follow (-on)die连续模compound die复合模punched hole冲孔panel board镶块to cutedges=side cut=side scrap切边to bending折弯to pull, to stretch拉伸Line streching, line pulling线拉伸engraving, to engrave刻印upsiding down edges翻边to stake铆合designing, to design设计design modification设计变化die block模块folded block折弯块sliding block滑块location pin定位销lifting pin顶料销die plate, front board模板padding block垫块stepping bar垫条upper die base上模座lower die base下模座upper supporting blank上承板upper padding plate blank上垫板spare dies模具备品spring 弹簧bolt螺栓document folder文件夹file folder资料夹to put file in order整理资料spare tools location手工备品仓first count初盘人first check初盘复棹人second count 复盘人second check复盘复核人equipment设备waste materials废料work in progress product在制品casing = containerazation装箱quantity of physical invetory second count 复盘点数量quantity of customs count会计师盘,点数量the first page第一联filed by accounting department for reference 会计部存查end-user/using unit(department)使用单位summary of year-end physical inventory bills 年终盘点截止单据汇总表bill name单据名称This sheet and physical inventory list will be sent to accountingdepartment together (Those of NHK will be sent to financialdepartment)本表请与盘点清册一起送会计部-(NHK厂区送财会部)Application status records of year-end physical inventory List andphysical inventory card 年终盘点卡与清册使用-状况明细表blank and waste sheet NO.空白与作废单号plate电镀mold成型material for engineering mold testing工程试模材料not included in physical inventory不列入盘点sample样品incoming material to be inspected进货待验description品名steel/rolled steel钢材material statistics sheet物料统计明细表meeting minutes会议记录meeting type 会别distribution department分发单位location地点chairman主席present members出席人员subject主题conclusion结论decision items决议事项responsible department负责单位pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/审核/承办PCE assembly production schedule sheet PCE组装厂生产排配表model机锺work order工令revision版次remark备注production control confirmation生产确认checked by初审approved by核准department部门stock age analysis sheet库存货龄分析表on-hand inventory现有库存available material良品可使用obsolete material良品已呆滞to be inspected or reworked待验或重工total合计cause description原因说明part number/ P/N 料号type形态item/group/class类别quality品质prepared by制表notes说明year-end physical inventory difference analysis sheet年终盘点差异分析表physical inventory盘点数量physical count quantity帐面数量difference quantity差异量cause analysis原因分析raw materials原料materials物料finished product成品semi-finished product半成品packing materials包材good product/accepted goods/ accepted parts/good parts良品defective product/non-good parts不良品disposed goods处理品warehouse/hub仓库on way location在途仓oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓skid/pallet栈板tox machine自铆机wire EDM线割EDM放电机coil stock卷料sheet stock片料tolerance工差score=groove压线cam block滑块pilot导正筒trim剪外边pierce剪内边drag form压锻差pocket for the punch head挂钩槽slug hole废料孔feature die公母模expansion dwg展开图radius半径shim(wedge)楔子torch-flame cut火焰切割set screw止付螺丝form block折刀stop pin定位销round pierce punch=die button圆冲子shape punch=die insert异形子stock locater block定位块under cut=scrap chopper清角active plate活动板baffle plate挡块cover plate盖板male die公模female die母模groove punch压线冲子air-cushion eject-rod气垫顶杆spring-box eject-plate弹簧箱顶板bushing block衬套insert 入块club car高尔夫球车capability能力parameter参数factor系数phosphate皮膜化成viscosity涂料粘度alkalidipping脱脂main manifold主集流脉bezel斜视规blanking穿落模dejecting顶固模demagnetization去磁;消磁high-speed transmission高速传递heat dissipation热传rack上料degrease脱脂rinse水洗alkaline etch龄咬desmut剥黑膜D.I. rinse纯水次Chromate铬酸处理Anodize阳性处理seal封孔revision版次part number/P/N料号good products良品scraped products报放心品defective products不良品finished products成品disposed products处理品barcode条码flow chart流程表单assembly组装stamping冲压molding成型spare parts=buffer备品coordinate座标dismantle the die折模auxiliary fuction辅助功能poly-line多义线heater band 加热片thermocouple热电偶sand blasting喷沙grit 砂砾derusting machine除锈机degate打浇口dryer烘干机induction感应induction light感应光response=reaction=interaction感应ram连杆edge finder巡边器concave凸convex凹short射料不足nick缺口speck瑕??shine亮班splay 银纹gas mark焦痕delamination起鳞cold slug冷块blush 导色gouge沟槽;凿槽satin texture段面咬花witness line证示线patent专利grit沙砾granule=peuet=grain细粒grit maker抽粒机cushion缓冲magnalium镁铝合金magnesium镁金metal plate钣金lathe车mill锉plane刨grind磨drill铝boring镗blinster气泡fillet镶;嵌边through-hole form通孔形式voller pin formality滚针形式cam driver铡楔shank摸柄crank shaft曲柄轴augular offset角度偏差velocity速度production tempo生产进度现状torque扭矩spline=the multiple keys花键quenching淬火tempering回火annealing退火carbonization碳化alloy合金tungsten high speed steel钨高速的moly high speed steel钼高速的organic solvent有机溶剂bracket小磁导liaison联络单volatile挥发性resistance电阻ion离子titrator滴定仪beacon警示灯coolant冷却液crusher破碎机模具工程类plain die简易模pierce die冲孔模forming die成型模progressive die连续模gang dies复合模shearing die剪边模riveting die铆合模pierce冲孔forming成型(抽凸,冲凸) draw hole抽孔bending折弯trim切边emboss凸点dome凸圆semi-shearing半剪stamp mark冲记号deburr or coin压毛边punch riveting冲压铆合side stretch侧冲压平reel stretch卷圆压平groove压线blanking下料stamp letter冲字(料号) shearing剪断tick-mark nearside正面压印tick-mark farside反面压印冲压名称类extension dwg展开图procedure dwg工程图die structure dwg模具结构图material材质material thickness料片厚度factor系数upward向上downward向下press specification冲床规格die height range适用模高die height闭模高度burr毛边gap间隙weight重量total wt.总重量punch wt.上模重量零件类punch冲头insert入块(嵌入件)deburring punch压毛边冲子groove punch压线冲子stamped punch字模冲子round punch圆冲子special shape punch异形冲子bending block折刀roller滚轴baffle plate挡块located block定位块supporting block for location定位支承块air cushion plate气垫板air-cushion eject-rod气垫顶杆trimming punch切边冲子stiffening rib punch = stinger 加强筋冲子ribbon punch压筋冲子reel-stretch punch卷圆压平冲子guide plate定位板sliding block滑块sliding dowel block滑块固定块active plate活动板lower sliding plate下滑块板upper holder block上压块upper mid plate上中间板spring box弹簧箱spring-box eject-rod弹簧箱顶杆spring-box eject-plate弹簧箱顶板bushing bolck衬套cover plate盖板guide pad导料块塑件&模具相关英文compre sion molding压缩成型flash mold溢流式模具plsitive mold挤压式模具split mold分割式模具cavity型控母模core模心公模taper锥拔leather cloak仿皮革shiver饰纹flow mark流痕welding mark溶合痕post screw insert螺纹套筒埋值self tapping screw自攻螺丝striper plate脱料板piston活塞cylinder汽缸套chip细碎物handle mold手持式模具模具技术用语各种模具常用成形方式accurate die casting 精密压铸powder forming 粉末成形calendaring molding 压延成形powder metal forging 粉末锻造cold chamber die casting 冷式压铸precision forging 精密锻造cold forging 冷锻press forging 冲锻compacting molding 粉末压出成形rocking die forging 摇动锻造compound molding 复合成形rotary forging 回转锻造compression molding 压缩成形rotational molding 离心成形dip mold 浸渍成形rubber molding 橡胶成形encapsulation molding 注入成形sand mold casting 砂模铸造extrusion molding 挤出成形shell casting 壳模铸造foam forming ?泡成形sinter forging 烧结锻造forging roll 轧锻six sides forging 六面锻造gravity casting 重力铸造slush molding 凝塑成形hollow(blow) molding 中空(吹出)成形squeeze casting 高压铸造hot chamber die casting 热室压铸swaging 挤锻hot forging 热锻transfer molding 转送成形injection molding 射出成形warm forging 温锻investment casting 精密铸造matched die method 对模成形法laminating method 被覆淋膜成形low pressure casting 低压铸造lost wax casting 脱蜡铸造matched mould thermal forming 对模热成形模各式模具分类用语bismuth mold 铋铸模landed plunger mold 有肩柱塞式模具burnishing die 挤光模landed positive mold 有肩全压式模具button die 镶入式圆形凹模loading shoe mold 料套式模具center-gated mold 中心浇口式模具loose detail mold 活零件模具chill mold 冷硬用铸模loose mold 活动式模具clod hobbing 冷挤压制模louvering die 百叶窗冲切模composite dies 复合模具manifold die 分歧管模具counter punch 反凸模modular mold 组合式模具double stack mold 双层模具multi-cavity mold 多模穴模具electroformed mold 电铸成形模multi-gate mold 复式浇口模具expander die 扩径模offswt bending die 双折冷弯模具extrusion die 挤出模palletizing die 叠层模family mold 反套制品模具plaster mold 石膏模blank through dies 漏件式落料模porous mold 通气性模具duplicated cavity plate 复板模positive mold 全压式模具fantail die 扇尾形模具pressure die 压紧模fishtail die 鱼尾形模具profile die 轮廓模flash mold 溢料式模具progressive die 顺序模gypsum mold 石膏铸模protable mold 手提式模具hot-runner mold 热流道模具prototype mold 雏形试验模具ingot mold 钢锭模punching die 落料模lancing die 切口模raising(embossing) 压花起伏成形re-entrant mold 倒角式模具sectional die 拼合模runless injection mold 无流道冷料模具sectional die 对合模具segment mold 组合模semi-positive mold 半全压式模具shaper 定型模套single cavity mold 单腔模具solid forging die 整体锻模split forging die 拼合锻模split mold 双并式模具sprueless mold 无注道残料模具squeezing die 挤压模stretch form die 拉伸成形模sweeping mold 平刮铸模swing die 振动模具three plates mold 三片式模具trimming die 切边模unit mold 单元式模具universal mold 通用模具unscrewing mold 退扣式模具yoke type die 轭型模模具厂常用之标准零配件air vent vale 通气阀anchor pin 锚梢angular pin 角梢baffle 调节阻板angular pin 倾斜梢baffle plate 折流档板ball button 球塞套ball plunger 定位球塞ball slider 球塞滑块binder plate 压板blank holder 防皱压板blanking die 落料冲头bolster 上下模板bottom board 浇注底板bolster 垫板bottom plate 下固定板bracket 托架bumper block 缓冲块buster 堵口casting ladle 浇注包casting lug 铸耳cavity 模穴(模仁)cavity retainer plate 模穴托板center pin 中心梢clamping block 锁定块coil spring 螺旋弹簧cold punched nut 冷冲螺母cooling spiral 螺旋冷却栓core 心型core pin 心型梢cotter 开口梢cross 十字接头cushion pin 缓冲梢diaphragm gate 盘形浇口die approach 模头料道die bed 型底die block 块形模体die body 铸模座die bush 合模衬套die button 冲模母模die clamper 夹模器die fastener 模具固定用零件die holder 母模固定板die lip 模唇die plate 冲模板die set 冲压模座direct gate 直接浇口dog chuck 爪牙夹头dowel 定位梢dowel hole 导套孔dowel pin 合模梢dozzle 辅助浇口dowel pin 定位梢draft 拔模锥度draw bead 张力调整杆drive bearing 传动轴承ejection pad 顶出衬垫ejector 脱模器ejector guide pin 顶出导梢ejector leader busher 顶出导梢衬套ejector pad 顶出垫ejector pin 顶出梢ejector plate 顶出板ejector rod 顶出杆ejector sleeve 顶出衬套ejector valve 顶出阀eye bolt 环首螺栓filling core 椿入蕊film gate 薄膜形浇口finger pin 指形梢finish machined plate 角形模板finish machined round plate 圆形模板fixed bolster plate 固定侧模板flanged pin 带凸缘?flash gate 毛边形浇口flask 上箱floating punch 浮动冲头gate 浇口gate land 浇口面gib 凹形拉紧?goose neck 鹅颈管guide bushing 引导衬套guide pin 导梢guide post 引导柱guide plate 导板guide rail 导轨head punch 顶?冲头headless punch 直柄冲头heavily tapered solid 整体模蕊盒hose nippler 管接头impact damper 缓冲器injection ram 压射柱塞inlay busher 嵌入衬套inner plunger 内柱塞inner punch 内冲头insert 嵌件insert pin 嵌件梢king pin 转向梢king pin bush 主梢衬套knockout bar 脱模杵land 合模平坦面land area 合模面leader busher 导梢衬套lifting pin 起模顶?lining 内衬locating center punch 定位中心冲头locating pilot pin 定位导梢locating ring 定位环lock block 压块locking block 定位块locking plate 定位板loose bush 活动衬套making die 打印冲子manifold block 歧管档块master plate 靠模样板match plate 分型板mold base 塑胶模座mold clamp 铸模紧固夹mold platen 模用板moving bolster 换模保持装置moving bolster plate 可动侧模板one piece casting 整体铸件parallel block 平行垫块paring line 分模线parting lock set 合模定位器pass guide 穴型导板peened head punch 镶入式冲头pilot pin 导?pin gate 针尖浇口plate 衬板pre extrusion punch 顶挤冲头punch 冲头puncher 推杆pusher pin 衬套梢rack 机架rapping rod 起模杆re-entrant mold 凹入模retainer pin 嵌件梢retainer plate 托料板return pin 回位梢riding stripper 浮动脱模器ring gate 环型浇口roller 滚筒runner 流道runner ejector set 流道顶出器runner lock pin 流道拉梢screw plug 头塞set screw 固定螺丝shedder 脱模装置shim 分隔片shoe 模座之上下模板shoot 流道shoulderbolt 肩部螺丝skeleton 骨架slag riser 冒渣口slide(slide core) 滑块slip joint 滑配接头spacer block 间隔块spacer ring 间隔环spider 模蕊支架spindle 主轴sprue 注道sprue bushing 注道衬套sprue bushing guide 注道导套sprue lock bushing 注道定位衬套sprue puller 注道拉料?spue line 合模线square key 方键square nut 方螺帽square thread 方螺纹stop collar 限位套stop pin 止动梢stop ring 止动环stopper 定位停止梢straight pin 圆柱?stripper bolt 脱料螺栓stripper bushing 脱模衬套stripper plate 剥料板stroke end block 行程止梢submarine gate 潜入式浇口support pillar 支撑支柱/顶出支柱support pin 支撑梢supporting plate 托板sweep templete 造模刮板tab gate 辅助浇口taper key 推拔键taper pin 拔锥梢/锥形梢teeming 浇注three startscrew 三条螺纹thrust pin 推力销tie bar 拉杵tunnel gate 隧道形浇口vent 通气孔wortle plate 拉丝模板模具常用之工作机械3D coordinate measurement 三次元量床boring machine 搪孔机cnc milling machine CNC铣床contouring machine 轮廓锯床copy grinding machine 仿形磨床copy lathe 仿形车床copy milling machine 仿形铣床copy shaping machine 仿形刨床cylindrical grinding machine 外圆磨床die spotting machine 合模机drilling machine ?孔机engraving machine。
机械专业英语词汇【最新完整版】

流体动力学 fluid dynamics 流体力学 fluid mechanics
加工 machining 液压 hydraulic pressure
切线 tangent 机电一体化 mechanotronics
视图 view 剖视图 profile chart 标准件 standard component 零件图 part drawing 装配图 assembly drawing 尺寸标注 size marking 技术要求 technical requirements
刚度 rigidity 内力 internal force 位移 displacement
套筒 sleeve 扭力 torsion 冷加工 cold machining 电动机 electromotor 汽缸 cylinder 过盈配合 interference fit 热加工 hotwork 摄像头 CCD camera 倒角 rounding chamfer 优化设计 optimal design 工业造型设计 industrial moulding design 有限元 finite element 滚齿 hobbing 插齿 gear shaping 伺服电机 actuating motor 铣床 milling machine 钻床 drill machine 镗床 boring machine 步进电机 stepper motor 丝杠 screw rod 导轨 lead rail 组件 subassembly 可编程序逻辑控制器 Programmable
主轴 spindle 主轴箱 headstock
机械设计制造及其自动化专业英语课后题

机械设计制造及其自动化专业英语课后题第一单元3. Aliuminum铝copper 铜nicke镍titanium 钛structural strength结构强度deep drawing拉伸加工4. 定义defition 力torce 轴axle(roller) 非金属nometal 结构structure 载荷load 用途use(application)性质properties(nature)(character)第二单元4.hardenability硬化性machinability可加工性cold drawn 冷拔steel sheet钢板percent reduction in area断面收缩率endurance limit疲劳极限rolled-steel shapes 轧制钢板corrosion resistance 抗腐蚀性rupture断裂5.低碳钢low-carbon 高强度钢hinger-strengt steel热处理heat treatment屈服强度yield strength弹性模量elastic modulus伸长率percentage elongation韧性toughness内应力internal stresses第三单元4.non-ferrous 非钢的stress-strain curve应力应变曲线yield point屈服点percentage elongation伸长率necking 颈缩sensitivity 灵敏性5.应变硬化strain hardening横截面cross-sectional area断面收缩率reduction in area比例极限limit of proportionality 屈服极限yield limit 延性ductiliy机械性质mechannicalpropertiece用..除..divide…by…第六单元3.tangential notes肤浅的事情flexible manufacturingsystem 柔性制造系统machine instruction机器指令economy of scale规模经济Hardwireyd logic controller硬固线逻辑控制transfer-line运输线,流水线numerically control(NC)数字控制direct numerical control(DNC)直接数字控制computer numerical control(CNC)计算机数字控制4.计算机辅助制造computer-aided manufacturing数控机床手工、半自动化或全自动化manal semiautomatic or full automation 尽管机械制造业一直在持续发展,但知道20世纪50年代才出现又一个重大发展。
机械专业英语词汇(很全)

机械专业英语词汇(很全)金属切削 metal cutting机床 machine tool金属工艺学 technology of metals 刀具 cutter摩擦 friction联结 link传动 drive/transmission轴 shaft弹性 elasticity频率特性 frequency characteristic 误差 error响应 response定位 allocation机床夹具 jig动力学 dynamic运动学 kinematic静力学 static分析力学 analyse mechanics拉伸 pulling压缩 hitting剪切 shear扭转 twist弯曲应力 bending stress强度 intensity三相交流电 three-phase AC磁路 magnetic circles变压器 transformer异步电动机 asynchronous motor几何形状 geometrical精度 precision正弦形的 sinusoid交流电路 AC circuit机械加工余量 machining allowance 变形力 deforming force变形 deformation应力 stress硬度 rigidity热处理 heat treatment退火 anneal正火 normalizing脱碳 decarburization渗碳 carburization电路 circuit半导体元件 semiconductor element 反馈 feedback发生器 generator直流电源 DC electrical source门电路 gate circuit逻辑代数 logic algebra外圆磨削 external grinding内圆磨削 internal grinding平面磨削 plane grinding变速箱 gearbox离合器 clutch绞孔 fraising绞刀 reamer螺纹加工 thread processing螺钉 screw铣削 mill铣刀 milling cutter功率 power工件 workpiece齿轮加工 gear mechining齿轮 gear主运动 main movement主运动方向 direction of main movement 进给方向 direction of feed进给运动 feed movement合成进给运动 resultant movement of feed合成切削运动 resultant movement of cutting合成切削运动方向 direction of resultant movement of cutting 切削深度 cutting depth前刀面 rake face刀尖 nose of tool前角 rake angle机械专业词汇:hardenability 淬透性carbide tool 硬质合金刀具alloy tool steel 合金工具钢alloyed cast iron 合金铸铁carbon steel 碳素钢carbon tool steel 碳素工具钢cast iron 铸铁cast steel 铸钢die material 模具材料high alloy steel 高合金钢high carbon steel 高碳钢low alloy steel 低合金钢low carbon steel 低碳钢shock resistant tool steel 抗冲击工具钢nodular graphite iron 球墨铸铁malleable cast iron 可锻铸铁mottled cast iron 麻口铸铁hardenability curve 淬透性曲线hardening capacity 淬硬性(硬化能力)hardness penetration diagram “U”形曲线hardness profile 硬度分布(硬度梯度)heat treatment procedure 热处理规范heat treatment installation 热处理设备heat treatment furnace 热处理炉heat treatment cycle 热处理工艺周期heat time 加热时间heat system 加热系统heating up time 升温时间heating curve 加热曲线high temperature carburizing 高温渗碳high temperature tempering 高温回火isothermal annealing 等温退火interrupted ageing treatment 分级时效处理local heat treatment 局部热处理overheated structure 过热组织pack carburizing 固体渗碳Oxynitrocarburizing 氧氮碳共渗partial annealing 不完全退火recrystallization temperature 再结晶温度cutting part 切削部分tool angle 刀具角度tool back angle 背前角tool back clearance 背后角tool backlash movement (tool retracting) 退刀tool back wedge angle 背楔角tool base clearance 基后角tool cutting edge plane 切削平面tool cutting edge angle 主偏角tool grinding machine 工具磨床tool geometrical rake 几何前角tool nomal clearance(rake) 法后角(法前角)tool orthogonal clearance(rake, wedge) 后角(前角,楔角)locating device 定位装置locating face 定位面locating pin 定位销(挡料销)locating plate 定位板locating ring 定位圈locating rule 定位尺locating element 定位零件(定位要素)work hardening 加工硬化internal cylindrical grinding machine 内圆磨床internal cylindrical turing 内圆车削internal force 内力internal cylindrical grinding machine with vertica 立式内圆磨床hole scraping (turning,milling, lapping) 刮孔(车孔,铣孔,研孔)hole grinding (slotting,honing, flanging) 磨孔(插孔,珩孔,翻孔)versatile grinding machine 多用磨床versatile lathe 多用车床vertical multi-tool lathe 立式多刀车床precision milling machine 精密铣床spot face 孔口平面drill and countersink 定心钻,中心钻counterbore cutter head 扩孔钻头jig boring machine 坐标镗床jig grinding machine 坐标磨床jig and fixture 夹具fixture of gear cutting machine 齿轮加工机床夹具fixture of milling machine 铣床夹具fixture of grinding machine 磨床夹具fixture of planing machine 刨床夹具fixture of slotting machine 插床夹具vacuum fixture 真空夹具universal fixture (jig) 通用夹具stationary fixture 固定夹具standard fixture (jig) 标准夹具pneumatic fixture (jig) 气动夹具open-side boring and milling machine 悬臂镗铣床magnetic fixture (jig) 磁力夹具locating device (face,element) 定位装置(面,元素)hydraulic fixture (jig) 液压夹具slide gauge 游标卡尺triple action press 三动压力机tow point single action press 双点单动压力机watch press 台式压力机closed type single action crank press 闭式单动(曲柄)压力机one point single action press 单点单动压力机open-back inclinable press 开式双柱可倾压力机four crank press 四曲柄压力机stright side single action double crank press 闭式双点单动双曲柄压力机single piece frame press 整体框架式压力机rocker arm type press 摇臂式压力机top drive sheet metal stamping automatic press 上传动板料冲压自动压力机barrel surface 圆柱形表面antiflowback valve 反流阀reciprocating-screw machine 往复螺旋注塑机single-stage plunger 单级柱塞shot chamber 注射室curing temperature 固化温度shot capacity 注射能力plunger diameter 柱塞直径sectional area of plunger 柱塞面积hydraulic cylinder 液压缸blanking die 冲裁模blanking clearance, die clearance 冲裁间隙blanking force 冲裁力die,stamping and punching die 冲模tonnage of press 压力机吨位shut height of press machine 压力机闭合高度clearance between punch and die 凹凸模间隙tolerance of fit 配合公差shearing force diagram 剪力图peak die load 模具最大负荷center of die, center of load 压力中心clamping force (element, device, piston) 夹紧力(件,装置,活塞)clamp plate (ring) 压板(夹紧环)shearing force (plane) 剪切力(平面)side clearance angle 侧隙角side locating face 侧定位面side-push plate 侧压板shuttle table 移动工作台matrix plate 凹模固定板material removal rate 材料切除率sheet matal 板料blanking die 冲裁模assembling die 复合冲模,装配用模具compound blank and pierce dies 落料冲孔模shaving die 切边模,修边模shankless die 无柄模具scrapless progressive die 无废料连续模return-blank type blanking die 顶出式落料模expanding die 胀形模,扩管模die for special purpose 专用模cavity plate (block) 凹模bend ability 可弯性bend arc 弯曲弧bending angle (line) 弯曲角(线)bending brake (bending machine) 弯板机,拆弯机bending fatigue 弯曲疲劳bending radius 弯曲半径minimum bending radius 最小弯曲半径bending operation 弯曲工序air-bend die 自由弯曲模bending moment diagram 弯矩图blank length of bend 弯曲件展开长度relative bending radius 相对弯曲半径bending equipment for plastics 塑料折弯设备drawing machine 拉拔机drawing numbers 拉伸次数drawing ratio (coefficient,force,speed) 拉伸比(系数,力,速度)foamed (cellular) plastic 泡沫塑料Thermoplastic 热塑性塑料plastic industry 塑料(工业)行业blow molding die for plastics 塑料吹模机standard die components for plastics 塑料模具标准化零部件thermoforming machine for plastics 塑料热成型机foaming mold for plastics 泡沫塑料模型plastic molding press 塑料制品成型压力机other plastics converting machine 其它塑料加工机械compression molding machine 压塑机extruder double-screw for plastics 塑料加工用双螺杆挤压机extruder single-screw for plastics 塑料加工用单螺杆挤压机laminating machine 层压机parting surface 分型面transfer mold 压注模(也称传递模)flash-type mold 溢出式压缩模portable transfer mold 移动式压注模mold for plastics 塑料成型模具(简称塑料模)mold for thermoplastics 热塑性塑料模Draft 脱模斜度transfer mold 传递模injection mold for thermoplastics 热塑性塑料注射模portable transfer mold 移动式传递模fixed transfer mold 固定式传递模insulated runner mold 绝热流道模warm runner mold 温流道模ring gate 环形浇口pin-point gate 点浇口edge gate 侧浇口submarine gate, tunnel gate 潜伏浇口runner plate 流道板spreader 分流锥warm runner plate 温流道板stationary mold, fixed half, fixed die, cover die 定模moving die, movable mold, moving half 动模sprue gating 中心浇口,浇道浇口die-casting die 压力铸造模具(简称压铸模)fixed clamping plate 定模底板moving clamping plate 动模底板support plate, backing plate 支承板movable core 活动型芯baffle 导流块sprue spreader 分流锥(分流器)ejector pin (plate) 推杆(板)ejector pin retaining plate 推杆固定板gate 内浇口air vent 排气槽parting line 分型面feed (gating, runner) system 浇注系统pouring temperature (rate, time) 浇注温度(速度,时间)sprue base (bush, gate, puller) 直浇道窝(浇口套,直接浇口,拉料杆)final forging temperature 终锻温度finishing temperature 终锻温度initial forging temperature 始锻温度flat die hammer 自由锻锤forge furnace 锻炉forge ratio (forge reduction) 锻造比forging crankpress 锻造用曲柄压力机forging die (die steel, drawing) 锻模(锻模钢,锻件图)forging line (load, practice) 锻造生产线(负荷,工艺)forging plane (plant) 锻造面(厂)forging pressure (process, range) 锻造力(工艺,范围)hammer forging die 锤锻模forging heat-treatment 锻件热处理forging temperature interval 锻造温度范围forging tolerance 锻件公差hot forging drawing 热锻件图chip formation 成型切削chip load (force) 切削力drilling and reaming 钻孔和铰孔taper turning 锥度车削external threading 外螺纹车削chuck handle 夹头扳手,夹头钥匙combination chuck 复动夹头shaper and planer 牛头刨床和龙门刨床slotting machine 插床rotary-type bushing 旋转衬(钻)套underlying metal 底层金属perforated electrode 多孔电极electro-chemical machining 电化学加工form electromachining 电加工成形面electric machining 电加工salt bath electrode furnace 电极盐浴炉electrolytic forming machine 电解成形机electrochemical machining 电解加工electrochemical machining tool 电解加工机床electrolytic universal tool and cutter grinder 电解万能工具磨床electrolytic heat treatment 电解液热处理electrohydraulic forming 电液成形electrolytic marking machine 电解刻印机electrolytic surface grinder 电解平面磨spark erosion machining 电火花加工法electrical discharge machining (EDM) 电火花加工electrodischarge cutting machine 电火花切割机electrical discharge machine 电火花加工机床electrical spark-erosion perforation 电火花打孔electrode contact surface 电极接触面electrical discharge forming 电火花成形机laser cutting machine 激光切割机electron beam cutting machine 电子束切割机cavity sinking EDM machines 型腔电火花加工机床travelling-wire EDM machine 线电极电火花加工机床electro-discharge machine tool 电火花加工机床electron beam machining (EBM) 电子束加工electron beam machine tool 电子束加工机床form electromachining 电加工成形面tiny hole spark-erosion grinding machine 电火花小孔磨床spark-erosion cutting with a wire 电火花线切割wire cut electric discharge machine 电火花线切割机encoded transducer 编码传感器compensator 补偿器incremental measuring system 增量测量系统analog control 模拟控制assembly language 汇编语言data processing system 数据处理系统graphic data processing 图形处理linear (circle) interpolator 线形(圆形)插补器DNC--direct numerical control 直接数字控制CNC--computer numerical control 计算机数字控制DPU--Data Processing Unit 数控处理单元DLU--Data Loops Unit 数据循环单元cutter saddle 刀架cylinder saddle 鞍形气缸座a safety loop 保险圈a wire loop 钢丝圈loop a line 环路法连接线路horizontal spindle 轴式vertical spindle 立轴式travelling-column 行程立柱feedback unit 反馈单元machining center 加工中心tool-storage 刀具存贮ball screw 滚珠丝杠tool changer 刀库machine control unit (MCU) 机床控制单元flexible machining system 柔性制造系统disk operating system (DOS) 磁盘操作系统Microsoft dish operating ssytem (MS-DOS) 微软磁盘操作系统program and data files 程序和数据文件internal and external command 内部和外部文件format a diskette 磁盘格式化diskcopy command 磁盘拷贝命令erase (deletion) command 删除命令create (change, remove) directory 建立(改变,移动)目录hard disk drive (HDD) 硬盘驱动器hard (soft) disk 硬(软)盘standard keyboard 标准键盘color display 彩色显示printer operating procedures 打印机操作程序application window 应用程序窗口batch file 批处理文件control (main,system) menu 控制(主,系统)菜单configuration system file 系统配置文件FMS (flexible manufacturing system) 柔性制造系统CNC (computer numerical control) 计算机数字控制revised feed signal 反进给信号default selection mode 默认选择模式MCU (machine control unit) 控制加工单元ACS (adaptive control system) 自动补偿系统CRT (cathode-ray tube) 显像管process planning 制定工艺过程CIM (computer integrated manufacturing) 计算机集成制造vertical stroke 垂直行程system variable 系统变量accurate die casting 精密压铸air bend die 悬空弯曲模具blank through dies 漏件式下了模calendering 压延成形center gated mold 中心浇口式模具cold chamber die casting 冷室压铸compacting molding 粉末压出成形double stack mold 双层模具duplicated cavity plate 复板模encapsulation molding 注入成形expander die 扩径模family mold 成套制品模具fantail die 扇尾形模具fantail die 鱼尾形模具f;ash mold 溢料式模具flash mold 溢料式模具flange bending die 弯缘模具flang trim die 整缘磨具flash trimming die 整缘磨具flat edge teim die 水平整缘磨具foam frming 发泡成形gang die 成排模具gravity casting 重力铸造sand mold casting 砂模铸造sectional die 对合模具shaper 定型模具singe cavity mold 单腔模具squeeze casting die 高压铸造squeezing casting die 挤压模swaging 挤锻tandem die 串列模具shree plates mold 三板式模具transfer molding 传送成形universal mold 通用模具warn foring 温锻air vent valve 通气阀anchir pin 锚梢angular cams 角凸轮angular pin 交梢(倾斜梢)baffle 调节阻板baffle plate 折流档板ball button 球塞套ball plunger 定位球塞binder plate 压板ball silder 球塞滑块binder plate 压板blank holder 防皱压板blanking punch 下料冲头bottom board 浇注底板bottom plate 下固定板bracket 托架casting ladle 浇注包casting lug 铸耳cavity 模穴(模仁/模腔)cavity adaptor plate 固定侧安装板cavityplate 模仁板cavity retainer plate 模穴托板clamping block 锁定块cooling manifold 冷却歧管core adaptor plate 可动侧安装板core plate 心型板core push back spring 心型回位弹簧diaphragm gate 盘形浇口die approach 模头料道die clamper 夹模器die fastener 模具固定用零件die plate 冲模板direct gate 直接浇口draft 拔模锥度draw bead 张力调整杆drive bearing 传动轴承ejector pad 顶出垫ejector plate 顶出板ejector valve 顶出阀calendaring molding 压延成形powder metal forging 粉末锻造rotary forging 回转锻造rotational molding 离心成形shell casting 壳模铸造foam forming 发泡成形hot chamber die casting 热室压铸warm forging 温锻investment casting 精密铸造matched die method 对模成形法laminating method 被覆淋膜成形low pressure casting 低压铸造lost wax casting 脱腊铸造matched mould thermal forming 对模热成形模landed plunger mold 有肩柱塞式模具landed positive mold 有肩全压式模具loading shoe mold 料套式模具center-gated mold 中心浇口式模具loose detail mold 活零件模具manifold die 分歧管模具modular mold 组合式模具multi-cavity mold 多模穴模具multi-gate mold 复式浇口模具palletizing die 叠层模plaster mold 石膏模fishtail die 鱼尾形模具protable mold 手提式模具lancing die 切口模raising(embossing) 压花起伏成形re-entrant mold 倒角式模具single cavity mold 单腔模具three plates mold 三片式模具air vent vale 通气阀anchor pin 锚梢ball slider 球塞滑块cooling spiral 螺旋冷却栓ejection pad 顶出衬垫ejector leader busher 顶出导梢衬套film gate 薄膜形浇口finish machined plate 角形模板finish machined round plate 圆形模板fixed bolster plate 固定侧模板flanged pin 带凸缘销flash gate 毛边形浇口flask 上箱floating punch 浮动冲头gate land 浇口面guide plate 导板guide rail 导轨head punch 顶镦冲头headless punch 直柄沖头heavily tapered solid 整体模蕊盒impact damper 缓冲器injection ram 压射柱塞inlay busher 嵌入衬套knockout bar 脱模杵land 合模平坦面land area 合模面locating center punch 定位中心冲头locating pilot pin 定位导梢locking plate 定位板making die 打印冲子manifold block 歧管档块master plate 靠模样板match plate 分型板mold base 塑胶模座mold clamp 铸模紧固夹mold platen 模用板moving bolster plate 可动侧模板one piece casting 整体铸件parallel block 平行垫块paring line 分模线parting lock set 合模定位器pass guide 穴型导板peened head punch 镶入式冲头pin gate 针尖浇口plate 衬板rack 机架rapping rod 起模杆retainer pin 嵌件梢retainer plate 托料板slag riser 冒渣口spacer block 间隔块spacer ring 间隔环square key 方键square nut 方螺帽square thread 方螺纹stop collar 限位套straight pin 圆柱销stripper plate 剥料板submarine gate 潜入式浇口support pillar 支撑支柱/頂出支柱supporting plate 扥板tab gate 辅助浇口taper key 推拔键taper pin 拔锥梢/锥形梢three start screw 三条螺纹tie bar 拉杵tunnel gate 隧道形浇口wortle plate 拉丝模板3D coordinate measurement 三次元量床boring machine 搪孔机cnc milling machine CNC铣床contouring machine 轮廓锯床copy grinding machine 仿形磨床copy lathe 仿形车床copy milling machine 仿形铣床copy shaping machine 仿形铇床cylindrical grinding machine 外圆磨床die spotting machine 合模机drilling machine 钻孔机engraving machine 雕刻机engraving E.D.M. 雕模放置加工机form grinding machine 成形磨床graphite machine 石墨加工机horizontal boring machine 卧式搪孔机horizontal machine center 臥式加工制造中心internal cylindrical machine 內圆磨床lap machine 研磨机machine center 加工制造中心NC drilling machine NC钻床NC grinding machine NC磨床NC lathe NC车床NC programming system NC程式制作系统planer 龙门铇床profile grinding machine 投影磨床radial drilling machine 旋臂钻床surface grinder 平面磨床try machine 试模机turret lathe 转塔车床universal tool grinding machine 万能工具磨床vertical machine center 立式加工制造中心autocollimator 自动准直机bench comparator 比长仪block gauge 块规calibration 校准caliper gauge 卡规check gauge 校对规clearance gauge 间隙规comparator 比测仪cylinder square 圆筒直尺depth gauge 测深规dial indicator 针盘指示表dial snap gauge 卡规digital micrometer 数位式测微计feeler gauge 测隙规gauge plate 量规定位板height gauge 测高规inside calipers 內卡钳limit gauge 限规morse taper gauge 莫氏锥度量规optical flat 光学平晶optical parallel 光学平行passimeter 內径仪position scale 位置刻度protractor 分角器radius 半径ring gauge 环规sine bar 正弦量规snap gauge 卡模square master 直角尺telescopic gauge 伸缩性量规working gauge 工作量規aluminium alloy 铝合金钢bearing alloy 轴承合金clad sheet 被覆板ferrostatic pressure 钢铁水静压力galvanized steel sheet 镀锌铁板hard alloy steel 超硬合金钢low alloy tool steel 特殊工具钢low manganese casting steel 低锰铸钢marging steel 马式体高強度热处理钢martrix alloy 马特里斯合金meehanite cast iron 米汉纳铸钢meehanite metal 米汉纳铁merchant iron 市售钢材prehardened steel 顶硬钢stainless steel 不銹钢tin plated steel sheet 镀锡铁板vinyl tapped steel sheet 塑胶覆面钢板age hardening 時效硬化ageing 老化处理air hardening 气体硬化air patenting 空气韧化annealing 退火anode effect 阳级效应anodizing 阳级氧化处理atomloy treatment 阿托木洛伊表面austempering 奧氏体等温淬火austenite 奧斯田体/奧氏体bainite 贝氏体banded structure 条纹状组织barrel plating 滾镀barrel tumbling 滾筒打光blackening 染黑法box annealing 箱型退火box carburizing 封箱渗碳bright electroplating 辉面电镀bright heat treatment 光辉热处理bypass heat treatment 旁路热处理carbide 炭化物carburized case depth 浸碳硬化深层carburizing 渗碳chemical plating 化学电镀chemical vapor deposition 化学蒸镀coarsening 結晶粒粗大化coating 涂布被覆controlled atmosphere 大气热处理creeping discharge 蠕缓放电decarburization 脱碳处理decarburizing 脱碳退火depth of hardening 硬化深层diffusion annealing 扩散退火electrolytic hardening 电解淬火first stage annealing 第一段退火flame hardening 火焰硬化flame treatment 火焰处理full annealing 完全退火gaseous cyaniding 气体氧化法globular cementite 球状炭化铁grain size 結晶粒度granolite treatment 磷酸溶液热处理graphitizing 石墨退火hardening 硬化heat treatment 热处理hot bath quenching 热浴淬火induction hardening 高周波硬化ion carbonitriding 离子渗碳氮化ion carburizing 离子渗碳处理ion plating 离子电镀low temperature annealing 低温退火malleablizing 可锻化退火martempering 麻回火处理martensite 马氏体/硬化铁炭metallikon 金属噴镀法metallizing 真空涂膜nitrocarburizing 软氮化normalizing 正常化overageing 过老化overheating 过热pearlite 针尖组织phosphating 磷酸盐皮膜处理physical vapor deposition 物理蒸镀plasma nitriding 离子氮化pre-annealing 预备退火precipitation 析出precipitation hardening 析出硬化process annealing 制程退火quench ageing 淬火老化quench hardening 淬火quenching crack 淬火裂痕recrystallization 再结晶residual stress 残留应力retained austenite 残留奧salt bath quenching 盐浴淬火sand blast 喷砂处理seasoning 時效处理second stage annealing 第二段退火secular distortion 经年变形segregation 偏析selective hardening 部分淬火shot blast 喷丸处理single stage nitriding 等温渗氮soaking 均热处理solution treatment 固溶化热处理stabilizing treatment 安定化处理straightening annealing 矫直退火strain ageing 应变老化stress relieving annealing 应力消除退火subzero treatment 生冷处理surface hardening 表面硬化处理tempering crack 回火裂痕thermal refining 调质处理thermoechanical treatment 加工热处理transformation 变态under annealing 不完全退火vacuum carbonitriding 真空渗碳氮化vacuum carburizing 真空渗碳处理vacuum hardening 真空淬火vacuum heat treatment 真空热处理vacuum nitriding 真空氮化water quenching 水淬火broing machine 搪孔机CMC milling machine CMC铣床wolfrtam (W) 钨vanadium (V) 钒uranium (U) 铀titanium (Ti) 钛tkallium (Ti) 铊tantalum (Ta) 钽stannum (Sn) 锡scandium (Sc) 钪samarium (Sm) 钐driling machine 钻孔机graphite mackine 石墨加工机praner 投影磨床radial driling machine 旋臂钻床bench comparatcr 比长仪feeler guage 测隙规guage plate 量规定位板height guage 测高规morse taper guage 莫氏锥度量规nano 奈米(十亿分之一)plug guage 塞规thickness gauge 厚薄(测隙)规low alloy tool steel 特殊工具钢maraging steel 马式体高强度热处理钢mateix alloy 马特里斯合金meehanite mttal 米汉纳铁perhardened steel 预硬钢ageing treatment 老化处理austepeering 奥氏体等温淬火bright heat treatement 光辉热处理bypass heat treament 旁路热处理diffusion annealing 扩散退火acetylene 乙炔ampere 电流安培angel welding 角焊arc 电弧argon arc welding 氩弧焊接bare electrode 光熔焊接camber 电弧弯曲cascade 階叠熔接法clad weld 被覆熔接crator 焊疤excess metal 多余金属gas shield 气体遮蔽hand face shield 手握面罩hard facing 硬表面堆焊laser beam welding 雷射光焊接metal electrode insert gas welding MIG 熔接overlaying 堆焊propane gas cutting 丙烷气切割seam 焊缝seaming 接合seam welding 流缝熔接series seam welding 串联缝熔接spark 火花stud arc welding 电弧焊接underlaying 下部焊层weld flow mark 焊接流痕weld mark 焊接痕weld penetration 熔接透入welding bead 焊接泡welding interval 焊接周期activator 活化剂adapter ring 接模环bag moulding 气胎施压成形bank 滞料breathing 排气caulking compound 填隙料color masterbatch 色母料color matching 调色colorant 着色剂daylight 开隙elastomer 弹性体extruded bead sealing 压出粒涂层法floating platen 活动模板foaming agent 发泡剂granule 颗粒料hot mark 热斑hot stamping 烫印kiss-roll coater 滚压涂布机kneader 混合机knife coating 刮刀涂布levelling agent 匀涂剂lubricant 润滑剂mandrel 模心型master batch 母料matrix 基料molecular 分子筛mould clamping force 锁模力mould release agent 脱模剂over packing 过充填oxidation 氧化parison 吹气成形坯料pin point gate 针孔形浇口plasticizer 塑化剂plastomer 塑体plating 电镀ram 冲柱ram extruder 活塞式押出机reciprocating screw 往复螺杆restricted gate 限制形浇口sealer 封口机side gate 侧向浇口slip agent 光滑剂snap fit 滑入配合spanishing 凹痕印刷spray up 喷附成形staple fiber 短纤维strain 应变strand 丝束stress cracking 应力龟裂take out device 取料装置toggle type mould clamping system 肘杆式锁模装置torpedo spreader 鱼雷形分流板transparency 透明性under coat 打底突层Steam trace 加热蒸汽管道branch connection 分支接续fabrication tolerance 制造容差threaded pipe 螺纹管seal welding 密封焊接flange joint 凸缘接头seal fitting 密封接头, 密封配件Screw thread lubricant 螺纹润滑剂Seal 绝缘层lock washer 锁紧[止动, 防松]垫圈electrical panel 配电板,配电盘zinc plated 镀锌的National Electrical Code 全国电气规程master schedule 主要图表, 综合图表, 设计任务书, 主要作业表flange connection 凸缘联接Hard hat 安全帽packing list 装箱单crate 柳条箱purchased material list 原材料进货单back-feed 反馈NPT thread 美国标准锥管螺纹cable gland 电缆衬垫terminal block 线弧, 接头排 接线盒, 接线板, 线夹insulated sleeve 绝缘套管wire terminal 电线接头motor lead 电动机引出线orifice plate 挡板flange gasket 法兰垫片dimensional inspection 尺寸检验ABS resin 丙烯轻丁二烯苯乙烯树脂arcylic 压克力acetal copolymer 共聚甲醛acetal resin 聚甲醛树脂acetone resin 丙酮树脂acrylic fiber 丙乙轻纤维acrylic resin 丙烯树脂acrylonitrile-styrene 丙乙轻苯乙烯树脂additional polymer 加聚物adhesive 粘着剂adhesive film 粘着薄膜alkyd resin 醇酸树脂allyl resin 烯丙醇酯树脂amino resin 氨基树脂butadiene 丁二烯butyl acetate 醋酸丁酯carbon fiber 碳纤维catalyst 催化剂cellulose acetate 醋酸纤维素(CA)cellulose acetate butyrate 醋酸丁酸纤维素chlorinated polyethylene 氯化聚乙烯chopped strand 短玻璃丝束crystalline polymer 结晶性聚合物cyclohexanone 环乙酮cyclohexanol 环乙酯dially phthalate 苯二甲酸二烯丙酯dibutyl fumarate 反丁烯二酸二丁酯dioctyl fumarate 反丁烯二酸二辛酯engineering plastics 工程塑胶ethyl acetate 醋酸乙酯ethylene vinylacetate 乙烯-醋酸乙烯共聚物expanded polystyrene 发泡聚苯乙烯fibrillation 原纤化filament 长纤维fluorocarbon resin 氟碳树脂graphite fiber 石墨纤维high impact polystyrene 高冲击聚苯乙烯(HIPS) high impact polystyene 高冲击产聚苯乙烯melamine resin 三聚氰胺酚醛树脂mylar 聚乙烯对苯二酸oxirane 环氧乙烷plastic 塑料polyacetal 聚甲醛polyacetylene 聚乙炔polyacrylic acid 聚丙烯酸(PAP)polyalcohol 多元醇polyamide 耐龙(PA) polybutyleneterphthalate 聚对苯二甲酸丁酯polycarbonate 聚碳酸酯polyethylenterephthalate 聚乙醇对苯(PETP) polymetylmethacrylate 聚甲基丙烯酸甲酯addendum of thread 螺牙冠adjustable spaneer 活动扳手angle cutter 角铣刀approaching face 渐近面arbor 心轴back clearance angle 背隙角back rake 背斜角backing 视垫ball end mill 球端铣刀belt sander 带式打磨机bevel angle 去角bevel lead 倒角blade 刀刃blank 胚料bottoming hand tap 底螺丝攻brazed tool 硬锝刀具centering location 定心ceramic tool 陶瓷刀具chamfer 倒角chamfered corner 去角角隅chamfering machine 倒角机chamfering tool 去角刀具chaser 螺丝钣刀chip breaker 断屑器clamp face 夹持面clearance angle 隙角cold circular saw 常温圆规compass 两角规concave angle 凹角concave cutter 凹面铣刀corner radius 角隅半径cotter diameter 梢径crack 破裂crater 刀痕磨耗crest radius 牙顶圆角半径cutting edge angle 切深角cutting edge inclination 切刃斜角cylinder key broach 键孔拉刀drawing thread 退刀螺蚊end cutting angle 副切深角end gash 刀端深口face 斜面face milling cutter 面铣刀flaking 破损flank 隙面(刀腹)flank wear 隙面磨耗fluted land 槽背gear hob 滚齿hatching 部面线helix angle 螺旋角hexagon headed bolt 六角头螺栓hexagon nut 六角螺帽hook angle 勾角included angle 刀刃角index head 分度头jack 千斤顶lapping 研磨lead 导程machinability 被切削性major cutting edge 主切刃major flank 主隙面margin 刀缘metal saw 金工锯metal slitting saw 锯割铣刀minor flank 副隙面no relief thread 无隙螺纹normal clearance angle 法隙角normal rake 法斜角normal wedge angle 法刀口角nose angle 刀角nut tap 螺帽螺丝攻orthogonal clearance angle 垂直隙角orthogonal rake 正斜角orthogonal wedge angle 垂直刀口角pearn tap 特殊螺丝攻plain milling cutter 平铣刀plain tapered bore 普通推拔孔plug hand tap 塞螺丝攻polot tap 导桿螺丝攻pull head 拉头pulley tap 带轮螺丝攻radial 径向斜面radial thread relief 径向螺牙隙radius end mill 圆角端铣刀radius of circle 圆角rake angle 斜角ratchet broach 刺轮拉刀retrieving head 支持头rifle broach 来福线拉刀sand paper 砂纸scraper 刮刀segment angle 弧角serration broach 细齿拉刀shank 刀柄side clearance angle 侧隙角side rake 侧斜角slab milling cutter 平板铣刀spade drills 铲钻spanner 扳手spline broach 方栓槽拉刀square 直角尺square end mill 方形端铣刀square sleeker 方形镘刀square trowel 直角度straight bore 直孔straight flute 直尺槽straight shank drills 直柄钻头subland drills 多槽钻头super crest tap 高顶螺丝攻tap 螺丝攻taper hand tap 斜螺丝攻taper shank 推拔柄taper tap 推拔螺丝攻tapping torque 攻螺丝扭力target drills 留心钻thread 螺纹thread milling cutter 螺纹铣刀threads per inch 牙数throw away tip 折叠式刀片throw away tool 折叠式刀具tool angles 刀具角tool for lathe 车刀tool point angle 刀刃角tool reference plane 基准面tosecan 画线盘waffle die flattening 压文效平working angles 切削刀barrelling 滚光加工blanking 下料加工cam die bending 凸轮弯曲加工double shearing 叠板裁断drawing 引申加工drawing with ironing 抽引光滑加工fine blanking 精密下料加工finish blacking 光制下料加工flabging 凸缘加工impact extrusion 冲击挤压加工lock seaming 固定接合marking 刻印加工parting 分段加工progressive blanking 连续下料加工progressive drawing 连续引申加工reaming 铰孔加工scrapless machining 无废料加工shaving 缺口修整加工shearing 切断加工staking 铆固stamping 锻压加工accumulator 蓄压器actuator 驱动器adapter 接头back shaft 支撑轴blank determination 胚料展开board drop hanmmer 板落锤brake 煞车camlachie cramp 铸包casting on flat 水平铸造chamotte sand 烧磨砂charging hopper 加料漏斗clutch brake 离合制动器coil car 带卷升降运输机coil cradle 卷材进料装置coil reel stand 钢材卷料架cradle 送料架crank 曲柄轴crankless 无曲柄式cross crank 横向曲轴dial feed 分度送料die assembly 合模double crank press 双曲柄轴冲床film play 液面花纹fine blanking press 精密下料冲床formboard 进模口架frame 床身机架机械专业英语词汇(一)金属切削 metal cutting机床 machine tool金属工艺学 technology of metals 刀具 cutter摩擦 friction联结 link传动 drive/transmission轴 shaft弹性 elasticity频率特性 frequency characteristic 误差 error响应 response定位 allocation机床夹具 jig动力学 dynamic运动学 kinematic静力学 static分析力学 analyse mechanics拉伸 pulling压缩 hitting剪切 shear扭转 twist弯曲应力 bending stress强度 intensity三相交流电 three-phase AC磁路 magnetic circles。
机械专业英语词汇完整版

energy 动量 momentum
桁架 truss 轴线 axes 余子式 cofactor 逻辑电路 logic circuit 触发器 flip-flop 脉冲波形 pulse shape 数模 digital analogy 液压传动机构 fluid drive mechanism 机械零件 mechanical parts 淬火冷却 quench 淬火 hardening 回火 tempering 调质 hardening and tempering 磨粒 abrasive grain 结合剂 bonding agent 砂轮 grinding wheel 机械零件 mechanical parts 淬火冷却 quench 淬火 hardening 回火 tempering
拉床 broaching machine 拉孔 broaching 装配 assembling 铸造 found
流体动力学 fluid dynamics 流体力学 fluid mechanics
加工 machining 液压 hydraulic pressure
切线 tangent 机电一体化 mechanotronics
渗碳 carburization 电路 circuit
半导体元件 semiconductor element 反馈 feedback
发生器 generator 直流电源 DC electrical source
门电路 gate circuit 逻辑代数 logic algebra 外圆磨削 external grinding 内圆磨削 internal grinding 平面磨削 plane grinding
考研机械专业英语词汇

考研机械专业英语词汇考研机械专业英语词汇机械专业主要包括机械设计制造及其自动化、材料成型及控制工程、工业设计、过程装备与控制工程等。
下面是店铺整理的考研机械专业英语词汇,欢迎来参考!陶瓷 ceramics金属切削 metal cutting机床 machine tool金属工艺学 technology of metals刀具 cutter电路 circuit半导体元件 semiconductor element反馈 feedback发生器 generator直流电源 DC electrical source门电路 gate circuit逻辑代数 logic algebra逻辑电路 logic circuit触发器 flip-flop脉冲波形 pulse shape数模 the digital analogy液压传动机构 fluid drive mechanism机械零件 mechanical parts摩擦 friction联结 link传动 drive/transmission轴 shaft弹性 elasticity频率特性 frequency the characteristic误差 error响应 response定位 allocation机床夹具 jig动力学 dynamic运动学 kinematic静力学 static分析力学 analyse mechanics拉伸 pulling压缩 hitting剪切 shear扭转 twist弯曲应力 bending stress强度 intensity三相交流电 three-phase AC磁路 magnetic circles变压器 transformer异步电动机 asynchronous motor 几何形状 geometrical精度 precision正弦形的 sinusoid交流电路 AC circuit机械加工余量 machining allowance 变形力 deforming force变形 deformation应力 the stress硬度 rigidity热处理 heat the treatment退火 anneal正火 normalizing脱碳 decarburization渗碳 carburization淬火冷却 quench淬火 hardening回火 tempering调质 hardening and tempering磨粒 abrasive grain结合剂 bonding agent砂轮 grinding wheel外圆磨削 external grinding内圆磨削 internal grinding平面磨削 plane grinding变速箱 gearbox离合器 clutch绞孔 fraising绞刀 reamer螺纹加工 thread processing螺钉 screw铣削 mill铣刀 milling cutter功率 power工件 the workpiece齿轮加工 gear mechining齿轮 gear主运动 main movement主运动方向 direction of main movement进给方向 direction of feed进给运动 feed movement合成进给运动 resultant movement of feed 合成切削运动 resultant movement of cutting合成切削运动方向 direction of resultant movement of cutting 切削深度 cutting depth前刀面 rake face刀尖 nose of tool前角 rake angle后角 clearance angle龙门刨削 planing主轴 spindle主轴箱 headstock卡盘 chuck加工中心 machining center车刀 the lathe tool车床 lathe钻削镗削 bore车削 turning磨床 grinder基准 benchmark钳工 locksmith锻 forge压模 stamping焊 weld拉床 broaching machine拉孔 the broaching装配 assembling铸造 found流体动力学 fluid dynamics流体力学 fluid mechanics加工 machining液压 hydraulic pressure切线 tangent机电一体化 mechanotronics mechanical-electrical integration 气压 air pressure pneumatic pressure稳定性 stability介质 medium液压驱动泵 fluid clutch液压泵 hydraulic pump阀门 valve失效 invalidation强度 intensity载荷 load应力 stress安全系数 safty factor可靠性 reliability螺纹 thread螺旋 helix键 spline销 pin滚动轴承 rolling bearing滑动轴承 sliding bearing弹簧 spring制动器 arrester brake十字结联轴节 crosshead联轴器 coupling链 chain皮带 strap精加工 finish machining粗加工 rough machining变速箱体 gearbox casing腐蚀 rust氧化 oxidation扩展:AAccrual 累积——在每一次交易期间内,远期外汇交易所分配的升水或折扣直接关系至利益套汇交易,常用英语词汇。
机械专业英语词汇(+音标)

机械专业英语词汇金属切削metal cutting [英] [ˈmetl][美] [ˈmɛtl]机床machine tool [英] [məˈʃi:n tu:l][美] [məˈʃin tul] 金属工艺学technology of metals [tekˈnɔlədʒi] [ˈmetl] 刀具cutter['kʌtɚ]摩擦friction['frikʃən][’frɪkʃən]联结link [liŋk]传动drive/transmission [trænzˈmiʃən]轴shaft [ʃɑ:ft]弹性elasticity[ɪlæ'stɪsɪti:, ,i:læ-][ɪlæ'stɪsɪti, ,ilæ-]频率特性frequency characteristic['fri:kwənsi][ˌkæriktəˈristik]误差error[ˈerə]响应response[ri'spɔns]定位allocation[ˌæləˈkeiʃən]机床夹具jig[dʒɪg]动力学dynamic[dai'næmik]运动学kinematic[,kaini'mætik]静力学static[ˈstætik]分析力学analyse mechanics[ˈænəlaiz] .[mɪˈkænɪks] 拉伸pulling['puliŋ]压缩hitting[hitiŋ]剪切shear[ʃiə]扭转twist[twist]弯曲应力bending stress['bendiŋ] .[stres]强度intensity[inˈtensiti]三相交流电three-phase AC[feiz] 磁路magnetic circles[mæɡˈnetik] .[ˈsə:kl]变压器transformer[trænsˈfɔ:mə]异步电动机asynchronous motor[ei'siŋkrənəs] ['məutə]几何形状geometrical[dʒɪəˈmetrɪkəl]精度precision[priˈsiʒən]正弦形的sinusoid[ˈsainəˌsɔid]交流电路AC circuit [ˈsə:kit]机械加工余量machining allowance [mə'ʃi:niŋ] .[əˈlauəns]变形力deforming force [dɪˈfɔ:miŋ] .[fɔ:s]变形deformation [ˌdi:fɔ:ˈmeɪʃən, ˌdefə-]应力stress [stres]硬度rigidity [rɪˈdʒɪdɪti:]]热处理heat treatment [ˈtri:tmənt]退火anneal [əˈni:l]正火normalizing ['nɔ:məlaiziŋ]脱碳decarburization [di:,kɑ:bjuərai'zeiʃən]渗碳carburization [,kɑ:bjurai'zeiʃən]电路circuit [ˈsə:kit]半导体元件semiconductor element [ˌsemikənˈdʌktə] .[ˈelimənt] 反馈feedback [ˈfi:dbæk]发生器generator [ˈdʒenəreitə]直流电源DC electrical source [i'lektrikəl] .[sɔ:s]门电路gate circuit [ɡeit] [ˈsə:kit]逻辑代数logic algebra [ˈlɔdʒik] .[ˈældʒibrə]外圆磨削external grinding [eksˈtə:nl] ['ɡraindiŋ]内圆磨削internal grinding [inˈtə:nəl] ['ɡraindiŋ]平面磨削plane grinding [plein] ['ɡraindiŋ]变速箱gearbox [ˈɡiəbɔks]离合器clutch [klʌtʃ]绞孔fraising [freiziŋ]绞刀reamer .[ˈri:mə]螺纹加工thread processing [θred] .[prəʊˈsesɪŋ]螺钉screw [skru:]铣削mill [mil]铣刀milling cutter [ˈmiliŋ] .[ˈkʌtə]功率power [ˈpauə]工件workpiece [ˈwɜ:kpi:s]齿轮加工gear machining [ɡiə] [mə'ʃi:niŋ]齿轮gear [ɡiə]主运动main m ovement [mein] .[ˈmu:vmənt]主运动方向direction of main movement [diˈrekʃən]进给方向direction of feed [diˈrekʃən] .[fi:d]进给运动feed movement [fi:d] [ˈmu:vmənt]合成进给运动resultant movement of feed [riˈzʌltənt] [ˈmu:vmənt] [fi:d]合成切削运动resultant movement of cu tting [riˈzʌltənt] [ˈmu:vmənt][ˈkʌtɪŋ]合成切削运动方向direction of resultant movement of cutting [diˈrekʃən] [riˈzʌltənt] [ˈmu:vmənt] [ˈkʌtɪŋ]切削深度cutting depth [ˈkʌtɪŋ] .[depθ]前刀面rake face [reik][ feis]刀尖nose of tool [nəuz] .[tu:l]前角rake angle [reik] .[ˈæŋɡl] 后角clearance angle [ˈkliərəns] .[ˈæŋɡl]龙门刨削planing [plæniŋ]主轴spindle [ˈspɪndl]主轴箱headstock [ˈhedstɔk]卡盘chuck [tʃʌk]加工中心machining center [mə'ʃi:niŋ]车刀lathe tool [leɪð] .[tu:l]车床lathe [leɪð]钻削镗削bore [bɔ:]车削turning [ˈtɜ:nɪŋ]磨床grinder [ˈgraɪndə]基准benchmark [ˈbentʃˌmɑ:k]钳工locksmith [ˈlɔkˌsmɪθ]锻forge [fɔ:dʒ]压模stamping [ˈstæmpiŋ]焊weld [weld]拉床broaching machine ['brəutʃiŋ] .[məˈʃi:n]拉孔broaching['brəutʃiŋ]装配assembling [ə'sembliŋ]铸造found [faund]流体动力学fluid dynamics ['flu(:)id] [dai'næmiks]流体力学fluid mechanics ['flu(:)id] [mi'kæniks]加工machining [mə'ʃi:niŋ]液压hydraulic pressure [hai'drɔ:lik] ['preʃə]切线tangent ['tændʒənt]机电一体化mechanotronics mechanical-electrical integration [,mekənə'trɔniks] [mi'kænikl] [i'lektrikəl] [,inti'ɡreiʃən]气压air pressure pneumatic pressure [nju(:)'mætik] 稳定性stability [stə'biliti]介质medium ['mi:djəm]液压驱动泵fluid clutch ['flu(:)id] [klʌtʃ]液压泵hydraulic pump [hai'drɔ:lik] [pʌmp]阀门valve [vælv]失效invalidation [in'vælideiʃn]强度intensity [in'tensiti]载荷load [ləud]应力stress [stres]安全系数safety factor ['seifti] ['fæktə]可靠性reliability [ri,laiə'biliti]螺纹thread [θred]螺旋helix ['hi:liks]键spline [splain]销pin [pin]滚动轴承rolling bearing ['rəuliŋ] ['bɛəriŋ]滑动轴承sliding bearing ['slaidiŋ] ['bɛəriŋ]弹簧spring [spriŋ]制动器arrester brake [ə'restə] [breik]十字结联轴节crosshead ['krɔshed]联轴器coupling ['kʌpliŋ]链chain [tʃein]皮带strap [stræp]精加工finish machining ['finiʃ] [mə'ʃi:niŋ]粗加工rough machining [rʌf] [mə'ʃi:niŋ] 变速箱体gearbox casing ['ɡiəbɔks] ['keisiŋ]腐蚀rust [rʌst]氧化oxidation [ɔksi'deiʃən]磨损wear [wɛə]耐用度durability [,djuərə'biliti]随机信号random signal ['rændəm] ['siɡnl]离散信号discrete signal [dis'kri:t] ['siɡnl]超声传感器ultrasonic sensor ['ʌltrə'sɔnik] ['sensə]集成电路integrate circuit ['intiɡreit] ['sə:kit]挡板orifice plate ['ɔrifis] [pleit]残余应力residual stress [ri'zidjuəl] [stres]套筒sleeve [sli:v]扭力torsion ['tɔ:ʃən]冷加工cold machining [mə'ʃi:niŋ]电动机electromotor [i,lektrəu'məutə]汽缸cylinder ['silində]过盈配合interference fit [,intə'fiərəns] [fit]热加工hot work [hɔt] [wə:k]摄像头CCD camera ['kæmərə]倒角rounding chamfer ['raundiŋ] ['tʃæmfə]优化设计optimal design ['ɔptiməl] [di'zain]工业造型设计industrial moulding design [in'dʌstriəl] ['məuldiŋ] [di'zain]有限元finite element ['fainait] ['elimənt]滚齿hobbing ['hɔbiŋ]插齿gear shaping [ɡiə] ['ʃeipiŋ]伺服电机actuating motor ['æktjueitiŋ] ['məutə]铣床milling machine ['miliŋ] [mə'ʃi:n]钻床drill machine [dril] [mə'ʃi:n]镗床boring machine ['bɔ:riŋ] [mə'ʃi:n]步进电机stepper motor ['stepə] ['məutə]丝杠screw rod [skru:] [rɔd]导轨lead rail [li:d] [reil]组件subassembly ['sʌbə'sembli]可编程序逻辑控制器Programmable Logic Controller PLC ['prəuɡræməbl] ['lɔdʒik] [kən'trəulə]电火花加工electric spark machining [i'lektrik] [spɑ:k] [mə'ʃi:niŋ] 电火花线切割加工electrical discharge wire –cutting [i'lektrikəl] [dis'tʃɑ:dʒ]相图phase diagram [feiz] ['daiəɡræm]固态相变solid state phase changes [feiz]有色金属nonferrous metal ['nɔn'ferəs] ['metl]陶瓷ceramics [si'ræmiks]合成纤维synthetic fibre [sin'θetic] ['faibə]电化学腐蚀electrochemical corrosion [i,lektrəu'kemikəl] [kə'rəuʒən]车架automotive chassis [ɔ:tə'məutiv] ['ʃæsi]悬架suspension [səs'penʃən]转向器redirector ['ri:di'rektə]变速器speed changer [spi:d] ['tʃeindʒə]板料冲压sheet metal parts [ʃi:t] ['metl] [pɑ:ts]孔加工spot facing machining [spɔt] ['feisiŋ] [mə'ʃi:niŋ]车间workshop ['wə:kʃɔp]工程技术人员engineer [,endʒi'niə]气动夹紧pneuma lock ['nju:mə] [lɔk] 数学模型mathematical model [,mæθi'mætikəl]画法几何descriptive geometry [dis'kriptiv] [dʒi'ɔmitri] 机械制图Mechanical drawing [mi'kænikl] ['drɔ:iŋ]投影projection [prə'dʒekʃən]视图view [vju:]剖视图profile chart ['prəufail] [tʃɑ:t]标准件standard component ['stændəd] [kəm'pəunənt] 零件图part drawing [pɑ:t] ['drɔ:iŋ]装配图assembly drawing [ə'sembli] ['drɔ:iŋ]尺寸标注size marking [saiz] ['mɑ:kiŋ]技术要求technical requirements刚度rigidity [ri'dʒiditi]内力internal force [in'tə:nl] [fɔ:s]位移displacement [dis'pleismənt]截面section ['sekʃən]疲劳极限fatigue limit [fə'ti:ɡ] ['limit]断裂fracture ['fræktʃə]塑性变形plastic distortion ['plæstik, plɑ:stik] [dis'tɔ:ʃən] 脆性材料brittleness material ['britl] [mə'tiəriəl]刚度准则rigidity criterion [ri'dʒiditi] [krai'tiəriən]垫圈washer ['wɔʃə]垫片spacer ['speisə]直齿圆柱齿straight toothed spur gear [tu:ðd] [spə:]斜齿圆柱齿轮helical-spur gear ['helikəl] [spə:][ɡiə]直齿锥齿轮straight bevel gear [streit] ['bevəl] [ɡiə]运动简图kinematic sketch [,kaini'mætik] [sketʃ]齿轮齿条pinion and rack ['pinjən] [ræk]蜗杆蜗轮worm and worm gear [wə:m] [ɡiə]虚约束passive constraint ['pæsiv] [kən'streint]曲柄crank [kræŋk]摇杆racker ['rækə]凸轮cams [kæm]共轭曲线conjugate curve ['kɔndʒuɡit] [kə:v]范成法generation method [,dʒenə'reiʃən] ['meθəd]定义域definitional domain [,defi'niʃən] [dəu'mein]值域range [reindʒ]导数\\微分differential coefficient [,difə'renʃəl][kəui'fiʃənt]求导derivation [deri'veiʃən]定积分definite integral ['definit] ['intiɡrəl]不定积分indefinite integral [in'definit] ['intiɡrəl]曲率curvature ['kə:vətʃə]偏微分partial differential ['pɑ:ʃəl] [,difə'renʃəl]毛坯rough [rʌf]游标卡尺slide caliper [slaid] ['kælipə]千分尺micrometer calipers [mai'krɔmitə] ['kælipəs]攻丝tap [tæp]二阶行列式second order determinant ['sekənd] [' ɔ:də] [di'tə:minənt]逆矩阵inverse matrix ['in'və:s] ['meitriks]线性方程组linear equations ['liniə] [i'kweiʃən]概率probability [,prɔbə'biliti]随机变量random variable ['rændəm] ['vɛəriəbl] 排列组合permutation and combination [,pə:mju(:)'teiʃən][,kɔmbi'neiʃən]气体状态方程equation of state of gas [i'kweiʃən] [steit] [ɡæs]动能kinetic energy [kai'netik] ['enədʒi]势能potential energy [pə'tenʃəl] ['enədʒi]机械能守恒conservation of mechanical energy [,kɔ nsə(:)'veiʃən] [mi'kænikl] ['enədʒi]动量momentum [məu'mentəm]桁架truss [trʌs]轴线axes ['æksi:z]余子式cofactor [kəu'fæktə]逻辑电路logic circuit ['lɔdʒik] ['sə:kit]触发器flip-flop ['flipflɔp]脉冲波形pulse shape [pʌls] [ʃeip]数模digital analogy ['didʒitl] [ə'nælədʒi]液压传动机构fluid drive mechanism ['flu(:)id] [draiv] ['mekənizəm] 机械零件mechanical parts [mi'kænikl] [pɑ:t]淬火冷却quench [kwentʃ]淬火hardening ['hɑ:dəniŋ]回火tempering ['tempəriŋ]调质hardening and tempering ['hɑ:dəniŋ] ['tempəriŋ]磨粒abrasive grain [ə'breisiv] [ɡrein]结合剂bonding agent ['bɔndiŋ] ['eidʒənt]砂轮grinding wheel ['ɡraindiŋ] [wi:l, hw-]机械零件mechanical parts [mi'kænikl] [pɑ:t]淬火冷却quench [kwentʃ]淬火hardening ['hɑ:dəniŋ]回火tempering ['tempəriŋ]调质hardening and tempering ['hɑ:dəniŋ] ['tempəriŋ]磨粒abrasive grain [ə'breisiv] [ɡrein]结合剂bonding agent ['bɔndiŋ] ['eidʒənt]砂轮grinding wheel ['ɡraindiŋ] [wi:l, hw-]机床行业部分英汉对照按英文字母排序3-Jaws indexing spacers[dʒɔ:] ['indeksiŋ] ['speisə]三爪分割工具头A T C system ['sɪstəm]加工中心机刀库Aluminum continuous melting & holding furnaces[ə'lu:mənəm] [kən'tinjuəs] ['meltiŋ] ['həuldiŋ] ['fə:nis] 连续溶解保温炉Balancing equipment ['bælənsiŋ] [i'kwipmənt]平衡设备Bayonet ['beɪənɪt, -,net, ,beɪə'net]卡口Bearing fittings ['bɛəriŋ] ['fitiŋ]轴承配件Bearing processing equipment ['bɛəriŋ] [prəu'sesiŋ][i'kwipmənt]轴承加工机Bearings ['bɛəriŋ]轴承Belt drive [belt] [draɪv]带传动Bending machines ['bendiŋ] [mə'ʃi:n]弯曲机Blades [bleid]刀片Blades,saw [bleid] [sɔ:]锯片Bolts,screws&nuts [bəult][skru:][nʌts]螺栓,螺帽及螺丝Boring heads ['bɔ:riŋ] [hed]搪孔头Boring machines ['bɔ:riŋ] [mə'ʃi:n]镗床Cable making tools ['keibl] ['meikiŋ] [tu:l]造线机Casting,aluminium ['kæstɪŋ] [,ælju:'minjəm]铸铝Casting,copper ['kæstɪŋ] ['kɔpə]铸铜Casting,gray iron ['kæstɪŋ] [ɡrei] ['aiən]铸灰口铁Casting,malleable iron ['kæstɪŋ] ['mæliəbl]可锻铸铁Casting,other ['kæstɪŋ]其他铸造Casting,steel ['kæstɪŋ] [sti:l]铸钢Chain drive [tʃein] [draɪv]链传动Chain making tools [tʃein]造链机Chamfer machines ['tʃæmfə] [mə'ʃi:n]倒角机Chucks [tʃʌk]夹盘Clamping/holding systems ['klæmpiŋ]夹具/支持系统CNC bending presses (CNC=Computerized Numerical Control[kəm'pju:tə] [nju(:)'merikəl] [kən'trol])['bendiŋ] [pres] 电脑数控弯折机CNC boring machines ['bɔ:riŋ] [mə'ʃi:n]电脑数控镗床CNC drilling machines ['driliŋ] [mə'ʃi:n]电脑数控钻床CNC EDMwire-cutting machines(EDM=Electron Discharge Machining [i'lektrɔn] [dis'tʃɑ:dʒ] [mə'ʃi:niŋ]放电加工)[mə'ʃi:n] ['waiə]电脑数控电火花线切削机CNC electric discharge machines [i'lektrik] [dis'tʃɑ:dʒ] [mə'ʃi:n]电脑数控电火花机CNC engraving machines [en'greɪvɪŋ] [mə'ʃi:n]电脑数控雕刻机CNC grinding machines ['ɡraindiŋ] [mə'ʃi:n]电脑数控磨床CNC lathes [leið]电脑数控车床CNC machine tool fittings [mə'ʃi:n] [tu:l] ['fitiŋ]电脑数控机床配件CNC milling machines ['miliŋ] [mə'ʃi:n]电脑数控铣床CNC shearing machines ['ʃiəriŋ] [mə'ʃi:n]电脑数控剪切机CNC toolings CNC ['tu:liŋ]刀杆CNC wire-cutting machines ['waiə] ['kʌtiŋ] [mə'ʃi:n] 电脑数控线切削机Conveying chains [kən'veiiŋ] [tʃein]输送链Coolers ['ku:lə]冷却机Coupling ['kʌpliŋ]联轴器Crimping tools [krimpiŋ] [tu:l]卷边工具Cutters ['kʌtə]刀具Cutting-off machines ['kʌtiŋ] [mə'ʃi:n]切断机Diamond cutters ['daiəmənd] ['kʌtə]钻石刀具Dicing saws ['daisiŋ] [sɔ:]晶圆切割机Die casting dies [dai] ['kɑ:stiŋ]压铸冲模Die casting machines [dai] ['kɑ:stiŋ] [mə'ʃi:n]压铸机Dies-progressive [dai] [prə'ɡresiv]连续冲模Disposable toolholder bits [dis'pəuzəbl] ['tu:l,həuldə] [bit]舍弃式刀头Drawing machines ['drɔ:iŋ] [mə'ʃi:n]拔丝机Drilling machines ['driliŋ] [mə'ʃi:n]钻床Drilling machines bench ['driliŋ] [mə'ʃi:n] [bentʃ]钻床工作台Drilling machines,high-speed ['dri liŋ] [mə'ʃi:n] [hai] [spi:d]高速钻床Drilling machines,multispindle [,m ʌlti] ['spindl]多轴钻床Drilling machines,radial ['driliŋ] [mə'ʃi:n] ['reidjəl]摇臂钻床Drilling machines,vertical ['driliŋ] [mə'ʃi:n] ['və:tikəl] 立式钻床drills [dril]钻头Electric discharge machines(EDM) [i'lektrik] [dis'tʃɑ:dʒ]电火花机Electric power tools [i'lektrik] ['pauə] [tu:l]电动刀具Engraving machines [en'greɪvɪŋ] [mə'ʃi:n]雕刻机Engraving machines,laser [en'greɪvɪŋ] [mə'ʃi:n] ['leizə]激光雕刻机Etching machines ['etʃiŋ] [mə'ʃi:n]蚀刻机Finishing machines ['finiʃiŋ] [mə'ʃi:n]修整机Fixture ['fikstʃə]夹具Forging dies ['fɔ:dʒiŋ] [dai]锻模Forging,aluminium ['fɔ:dʒiŋ] [,ælju:'minjəm]锻铝Forging,cold ['fɔ:dʒiŋ] [kəuld]冷锻Forging,copper ['fɔ:dʒiŋ] ['kɔpə]铜锻Forging,other ['fɔ:dʒiŋ] ['ʌðə]其他锻造Forging,steel ['fɔ:dʒiŋ] [sti:l]钢锻Foundry equipment ['faundri] [i'kwipmənt]铸造设备5 Gear cutting machines [ɡiə] ['kʌtiŋ] [mə'ʃi:n]齿轮切削机Gears [ɡiə]齿轮Gravity casting machines ['ɡræviti] ['kɑ:stiŋ] [mə'ʃi:n]重力铸造机Grinder bench ['ɡraində] [bentʃ]磨床工作台Grinders,thread ['ɡraində] [θred]螺纹磨床Grinders,tools & cutters ['ɡraində] [tu:l] ['kʌtə]工具磨床Grinders,ultrasonic ['ɡraində] ['ʌltrə'sɔnik]超声波打磨机Grinding machines ['ɡraindiŋ] [mə'ʃi:n]磨床Grinding machines,centerless ['ɡraindiŋ] [mə'ʃi:n] ['sentəles]无心磨床Grinding machines,cylindrical [si'lindrik(ə)l]外圆磨床Grinding machines,universal [,ju:ni'və:səl]万能磨床Grinding tools ['ɡraindiŋ] [tu:l]磨削工具Grinding wheels ['ɡraindiŋ] [wi:l, hw-]磨轮Hand tools [hænd] [tu:l]手工具Hard/soft and free expansion sheet making plant[iks'pænʃən] [ʃi:t]硬(软)板(片)材及自由发泡板机组Heat preserving furnaces [pri'zə:v] ['fə:nis]保温炉Heating treatment funaces ['tri:tmənt] ['fə:nis]熔热处理炉Honing machines ['həuniŋ] [mə'ʃi:n]搪磨机Hydraulic components [hai'drɔ:lik][kəm'pəunənt]液压元件Hydraulic power tools [hai'drɔ:lik]液压工具Hydraulic power units [hai'drɔ:lik]液压动力元件Hydraulic rotary cylinders['rəutəri] ['silində]液压回转缸Jigs [dʒiɡ]钻模Lapping machines ['læpiŋ] [mə'ʃi:n]精研机Lapping machines,centerless无心精研机Laser cutting ['leizə] ['kʌtiŋ]激光切割Laser cutting for SMT stencil['stensl, -sil]激光钢板切割机Lathe bench ['leizə] [bentʃ]车床工作台Lathes,automatic ['leizə] [,ɔ:tə'mætik]自动车床Lathes,heavy-duty ['leizə] [hevi:'du:ti:, -'dju:-]重型车床Lathes,high-speed ['leizə] [hai] [spi:d]高速车床Lathes,turret ['leizə] ['tʌrit]六角车床Lathes,vertical ['leizə] ['və:tikəl]立式车床Lubricants ['lu:brikənt]润滑液Lubrication Systems [,lu:bri'keiʃən] ['sɪstəm]润滑系统Lubricators ['lju:brikeitə]注油机Machining centers,general [mə'ʃi:niŋ] ['sentə] ['dʒ enərəl]通用加工中心Machining centers,horizontal [mə'ʃi:niŋ] ['sentə] [,hɔri'zɔntl]卧式加工中心Machining centers,horizontal & vertical [mə'ʃi:niŋ] ['sentə] [,hɔri'zɔntl] ['və:tikəl]卧式及立式加工中心Machining centers,vertical [mə'ʃi:niŋ] ['sentə] ['və:tikəl]立式加工中心Machining centers,vertical double-column type [mə'ʃi:niŋ] ['sentə] ['və:tikəl] ['dʌbl] ['kɔləm] [taip]立式双柱加工中心Magnetic tools [mæɡ'netik] [tu:l]磁性工具Manifolds ['mænifəuld]集合管Milling heads ['miliŋ] [hed]铣头Milling machines ['miliŋ] [mə'ʃi:n]铣床Milling machines,bed type ['miliŋ] [mə'ʃi:n] [taip]床身式铣床Milling machines,dupli cating ['miliŋ] [mə'ʃi:n] ['dju:plikeitiŋ]仿形铣床Milling machines,horizontal ['miliŋ] [mə'ʃi:n] [,hɔri'z ɔntl]卧式铣床Milling machines,turret vertical ['miliŋ] [mə'ʃi:n] ['tʌ rit] ['və:tikəl]六角立式铣床Milling machines,universal ['miliŋ] [mə'ʃi:n] [,ju:ni'və:səl]万能铣床Milling machines,vertical ['miliŋ] [mə'ʃi:n] ['və:tikəl]立式铣床Milling machines,vertical & horizontal ['miliŋ] [mə'ʃ i:n] ['və:tikəl] [,hɔri'zɔntl]立式及卧式铣床Mold & die components [məuld] [dai] [kəm'pəunənt] 模具单元Mold changing systems [məuld] ['tʃeindʒiŋ] ['sɪstəm] 换模系统Mold core [məuld] [kɔ:]模芯Mold heaters/chillers [məuld] ['hi:tə] ['tʃilə]模具加热器/冷却器Mold polishing/texturing [məuld] ['pɔliʃiŋ] ['tekstʃəriŋ]模具打磨/磨纹Mold repair [məuld] [ri'pɛə]模具维修Molds [məuld]模具Nail making machines [neil] [mə'ʃi:n]造钉机Oil coolers [ɔil] ['ku:lə]油冷却器Overflow cutting machines for aluminium wheels ['əuvə'fləu] [mə'ʃi:n] [,ælju:'minjəm] [wi:l, hw-]铝轮冒口切断机P type PVC waterproof rolled sheet making plant 6 [taip] ['wɔ:təpru:f] [rəuld] [ʃi:t] [plɑ:nt] PPVC高分子防水PCB fine piecing systems [fain] [pi:s] ['sɪstəm]( PCB =Printed -circuit Board [bɔ:d]印刷电路板)印刷电器板油压冲孔脱料系统Pipe & tube making machines [paip] [mə'ʃi:n] ['tju:b] [mə'ʃi:n]管筒制造机Planing machines [plæniŋ] [mə'ʃi:n]刨床Planing machines vertical [plæniŋ] [mə'ʃi:n] ['və:tikəl]立式刨床Pneumatic hydraulic clamps [nju(:)'mætik] [hai'dr ɔ:lik] [klæmp]气油压虎钳Pneumatic power tools [nju(:)'mætik] ['pauə] [tu:l]气动工具Powder metallurgic forming machines ['paudə] [,metə'lə:dʒik] ['fɔ:miŋ] [mə'ʃi:n]粉末冶金成型机Presses,cold forging [pres] ['fɔ:dʒiŋ]冷锻冲压机presses,crank [pres] [kræŋk]曲柄压力机Presses,eccentric [pres] [ik'sentrik]离心压力机Presses,forging [pres] ['fɔ:dʒiŋ]锻压机Presses,hydraulic [pres]液压冲床Presses,knuckle joint [pres]肘杆式压力机Presses,pneumatic [pres] [hai'drɔ:lik]气动冲床Presses,servo [pres] ['sə:vəu]伺服冲床Presses,transfer [pres] [træns'fə:]自动压力机Pressing dies [presiŋ] ['daii:z]压模Punch formers [pʌntʃ] ['fɔ:mə]冲子研磨器Quick die change systems [kwik] [dai] [tʃeindʒ] ['sɪ stəm]速换模系统Quick mold change systems [kwik] [məuld] [tʃeindʒ]['sɪstəm]快速换模系统Reverberatory furnaces [ri'və:bərətəri] ['fə:nis]反射炉Rollers ['rəulə]滚筒Rolling machines ['rəuliŋ] [mə'ʃi:n]辗压机Rotary tables ['rəutəri] ['teibl]转台Sawing machines ['sɔ:iŋ] [mə'ʃi:n]锯床Sawing machines,band ['sɔ:iŋ] [mə'ʃi:n] [bænd]带锯床Saws,band [sɔ:] [bænd]带锯Saws,hack [sɔ:] [hæk]弓锯Saws,horizontal band [sɔ:] [,hɔri'zɔntl] [bænd]卧式带锯Saws,vertical band [sɔ:] ['və:tikəl] [bænd]立式带锯shafts [ʃɑ:ft]轴Shapers ['ʃeipə]牛头刨床Shearing machines ['ʃiəriŋ] [mə'ʃi:n]剪切机Sheet metal forming machines [ʃi:t] ['metl] ['fɔ:miŋ] [mə'ʃi:n]金属板成型机Sheet metal working machines [ʃi:t] ['metl] [mə'ʃi:n]金属板加工机Slotting machines ['slɔtiŋ] [mə'ʃi:n]插床spindles ['spindl]主轴Stamping parts ['stæmpiŋ] [pɑ:t]冲压机Straightening machines ['streitniŋ] [mə'ʃi:n]矫直机Switches & buttons [switʃ] ['bʌtn]开关及按钮Tapping machines ['tæpiŋ] [mə'ʃi:n]攻螺丝机Transmitted chains [trænz'mit] [tʃein]传动链Tube bending machines ['tju:b] ['bendiŋ] [mə'ʃi:n]弯管机Vertical hydraulic broaching machine ['və:tikəl] [hai'drɔ:lik] ['brəutʃiŋ] [mə'ʃi:n]立式油压拉床Vises [vais]虎钳Vises,tool-maker [vais][ 'tu:l,meɪkə]精密平口钳Wheel dressers [wi:l, hw-] ['dresə]砂轮修整器Woven-Cutting machines ['wəuvən]织麦激光切割机Wrenches [rentʃ]扳手螺丝词汇的中英文对照六角盖头螺帽HEX CAP NUTS (HEX= hexagon ['heksəɡən] 六角形; 六边形) [kæp] [nʌts]六角锯齿螺帽HEX SERRATED NUTS (HEX= hexagon ['heksəɡən] 六角形; 六边形) [se'reitid [nʌts] 六角轮缘螺帽HEX FLANGE NUTS (HEX= hexagon ['heksəɡən] 六角形; 六边形) [flændʒ] [nʌts]高脚螺帽HEX COUPLING NUTS(HIGH NUTS) (HEX= hexagon['heksəɡən] 六角形; 六边形) ['kʌpliŋ] [nʌts] [hai]圆螺帽ROUND NUTS [raund] [nʌts]四角螺帽SQUARE NUTS HEAVY HEX NUTS [skwɛə] [n ʌts] [ 'hevi] (HEX= hexagon ['heksəɡən] 六角形; 六边形)不锈钢六角螺帽 STAINLESS STEEL HEX NUTS ['steinlis] [sti:l] (HEX= hexagon ['heks əɡən] 六角形; 六边形) [n ʌts] 不锈钢尼龙嵌入螺帽 STAINLESS STEEL NYLON INSERT LOCK NUTS ['steinlis] [sti:l] ['nail ən] [in's ə:t] [l ɔk] [n ʌts] 普通六角螺帽 HEX NUTS (HEX= hexagon ['heks əɡən] 六角形; 六边形) [n ʌts]六角重型螺帽 HEAVY HEX NUTS [ 'hevi]['heks əɡən][n ʌts]薄型螺帽 HEX JAM NUTS ['heks əɡən][d ʒæm] [n ʌts]尼龙嵌入防松螺帽NYLON INSERT LOCK NUTS ['nail ən][in's ə:t] [l ɔk] [n ʌts]机械螺丝用六角螺帽 HEX MACHINE SCREW NUT['heks əɡən] [m ə'ʃi:n] 7 [skru:] [n ʌt]机械工具英语 机械工具 spanner 扳子 (美作:wrench) ['spæn ə] [rent ʃ]double-ended spanner ['spæn ə]双头扳子adjustable spanner, monkey wrench [ə'd ʒʌst əbl] ['spæn ə]['m ʌŋki] [rent ʃ]活扳子,活络扳手 box spanner 管钳子 (美作:socket wrench) [b ɔks] ['spæn ə]['s ɔkit] [rent ʃ]calipers ['kælip ə]卡规pincers, tongs ['pins əz] [t ɔŋz]夹钳shears [ʃi əz]剪子 hacksaw ['hæks ɔ:]钢锯wire cutters ['wai ə] ['k ʌt ə]剪线钳 multipurposepliers,universal pliers ['m ʌlti'p ə:p əs] ['plai ə] [,ju:ni'v ə:s əl] ['plai əz]万能手钳 adjustable pliers [ə'd ʒʌst əbl] ['plai əz]可调手钳punch [p ʌnt ʃ]冲子drill [dril]钻chuck [t ʃʌk]卡盘scraper ['skreip ə]三角刮刀 reamer ['ri:m ə]扩孔钻calliper gauge ['kælip ə] [ɡedʒ]孔径规 rivet ['rivit]铆钉 nut [n ʌt]螺母 locknut ['l ɔkn ʌt]自锁螺母,防松螺母bolt [b əult]螺栓pin, peg, dowel [pin] [pe ɡ] ['dauəl]销钉washer ['w ɔʃə]垫圈 staple ['steipl]U 形钉 oil can 油壶 jack 工作服grease gun [ɡri:s]注油枪机械加工抛光 polishing ['p ɔli ʃiŋ]安装 to assemble [ə'sembl] 衬套 bushing ['bu ʃiŋ] 外贸常用机械英语大全 Assembly line [ə'sembl]组装线 Layout ['lei,aut]布置图 Conveyer [k ən'vei]流水线物料板 Rivet table ['rivit]拉钉机 Rivet gun ['rivit]拉钉枪 Screw driver 起子 Pneumatic screw driver [nju(:)'mætik]气动起子 worktable ['w ə:kteibl]工作桌OOBA 开箱检查fit together [tə'ɡeðə]组装在一起fasten ['fɑ:sn]锁紧(螺丝)ixture ['fikstʃə]夹具(治具)pallet ['pælit]栈板bar code [bɑ:] [kəud]条码bar code scanner [bɑ:] [kəud] ['skænə]条码扫描器fuse together [fju:z] [tə'ɡeðə]熔合fuse machine [fju:z] [mə'ʃi:n]热熔机repair[ri'pɛə]修理operator['ɔpəreitə]作业员QC [=Quality Control ['kwɔliti] [kən'trol]质量控制]品管supervisor ['sju:pəvaizə]课长ME [=Mechanical Engineer机械工程师]制造工程师MT 制造生技cosmetic inspect [kɔz'metik] [in'spekt]外观检查inner parts inspect ['inə] [in'spekt]内部检查thumb screw [θʌm]大头螺丝lbs inch [intʃ]镑、英寸EMI gasket ['ɡæskit]导电条front plate [frʌnt] [pleit]前板rear plate [riə] [pleit]后板chassis ['ʃæsi]基座bezel panel ['bezəl] ['pænl]面板power button ['pauə] ['bʌtn]电源按键reset button ['ri:set] ['bʌtn]重置键Hi-pot test of SPS [test]高源高压测试Voltage switch of SPS ['vəultidʒ] [switʃ]电源电压接拉键sheet metal parts [ʃi:t]冲件plastic parts ['plæstik, plɑ:stik]塑胶件SOP [=Standard Operating Procedure 标准操作程序] 制造作业程序material check list [mə'tiəriəl]物料检查表work cell [wə:k] [sel]工作间trolley ['trɔli]台车carton ['kɑ:tən]纸箱sub-line ['sʌblain]支线left fork [fɔ:k]叉车personnel resource department [,pə:sə'nel] [ri'sɔ:s] [di'pɑ:tmənt]人力资源部production department [prə'dʌkʃən]生产部门planning department ['plænɪŋ] [di'pɑ:tmənt]企划部QC Section ['sekʃən]品管科stamping factory ['stæmpiŋ] ['fæktəri]冲压厂painting factory ['peintiŋ]烤漆厂molding factory ['məuldiŋ] ['fæktəri]成型厂common equipment ['kɔmən] [i'kwipmənt]常用设备uncoiler and straightener [ʌn'kɔilə] ['streitənə]整平机punching machine [pʌntʃiŋ] [mə'ʃi:n]冲床robot ['rəubɔt, 'rɔbət]机械手hydraulic machine [hai'drɔ:lik]油压机lathe [leið]车床planer |plein|刨床miller ['milə]铣床grinder ['ɡraində]磨床linear cutting ['lini ə] ['k ʌtiŋ]线切割electrical sparkle [i'lektrik əl] ['sp ɑ:kl]电火花welder ['weld ə]电焊机staker=reviting machine ['steik ə]铆合机position [p ə'zi ʃən 职务president ['prezid ənt]董事长general manager ['d ʒen ər əl] ['mænid ʒə]总经理special assistant manager ['spe ʃəl] [ə's ɪst ənt] ['mænid ʒə]特助 factory director ['fækt əri] [di'rekt ə, dai'rekt ə]厂长department director[di'p ɑ:tmənt] [di'rektə, dai'rektə] 部长deputy manager | =vice manager 副理 section supervisor 课长 ['depjuti] ['mænid ʒə] ['sju:p əvaiz ə] deputy section supervisor =vice section supe risor['depjuti] ['sek ʃən] ['sju:p əvaiz ə]副课长group leader/supervisor['sju:p əvaiz ə]组长line supervisor['sju:p əvaiz ə]线长assistant manager 助理to move, to carry, to handle ['hændl]搬运be put in storage['st ɔrid ʒ]入库pack packing 包装to apply oil [ə'plai]擦油to file burr [fail] [b ə:]锉毛刺final inspection ['fain əl] [in'spek ʃən]终检to connect material [k ə'nekt] [m ə'ti əri əl]接料to reverse material [ri'v ə:s] [m ə'ti əri əl]翻料wet station ['stei ʃən]沾湿台cleaning cloth ['kli:niŋ] [klɔ(:)θ]抹布 to load material [l əud] [m ə'ti əri əl]上料 to unload material['ʌn'l əud] [m ə'ti əri əl]卸料 to return material/stock to [ri't ə:n] [m ə'ti əri əl] [st ɔk] 退料 scraped [skreipid]报废 scrape [skreip]v 刮;削 deficient purchase[di'fi ʃənt] ['p ə:t ʃəs]来料不良 manufacture procedure[,mænju'fækt ʃə] [pr ə'si:d ʒə]制程 deficient manufacturing procedure[di'fi ʃənt] [,mænju'fækt ʃə] [pr ə'si:d ʒə]制程不良 oxidation [ɔksi'dei ʃən]氧化 scratch[skræt ʃ]刮伤 dents['dents]压痕defective upsiding down[di'fektiv] ['ʌpsaidiŋ]抽芽不良 defective to staking[di'fektiv] ['steikiŋ]铆合不良 embedded lump[em'bedid] [l ʌmp]镶块 feeding is not in place['fi:diŋ] [pleis]送料不到位 stamping-missing ['stæmpiŋ] ['misiŋ]漏冲 production capacity [pr ə'd ʌk ʃən] [k ə'pæsiti]生产力 education and training [,edju(:)'kei ʃən] ['treiniŋ]教育培训 proposal improvement[pr ə'p əuz əl] [im'pru:vm ənt]提案改善 spare parts=buffer['b ʌf ə]备件 forklift['f ɔ:klift]叉车 trailer=long vehicle ['treil ə] ['vi:ikl]拖板车 外贸常用机械英语大全(续) compound die['k ɔmpaund] [dai]合模 die locker [dai] ['l ɔk ə]锁模器。
机械专业课程中英文对照表(自制)

机械制造某专业课程中英文对照表英语 English画法几何及机械制图 Descriptive Geometry & Mechanical Drawing 高等数学 Advanced Mathematics体育 Physical Education普通化学 Chemistry中国革命史 Chinese Revolution HIstory共产主义思想品德 Moral Education大学物理 College Physics军事课 Military Theory物理实验 Physics Experiment中国社会主义建设 Chinese Socialist Construction工程数学 Engineering Mathematics理论力学 Theoretical Mechanics思想品德Moral Education计算机算法语言Computer algorithmic language材料力学 Mechanics of Materials金属工艺学 Metal Technology金工实习 Metalworking Practice哲学 Philosophy机械原理 Mechanics Principle金属材料及热处理 Metal Materials & Heat Treatment公差及技术测量 Technical Measurement with Common Difference电路和电子技术 Electric Circuit & Electronic Technology工业企业管理 Industrial Enterprise Management专业外语 Specialized English机械原理课程设计 Coruse Design of Mechanics Principle马克思主义原理 Marxism Principle机械零件 Mechanical Parts锻压设备液压传动 Forging Press Equipments & Hydraulic Transmisson 塑性成形原理 Principles of Plastic Molding控制工程基础 Control Engineering Foundation测试技术 Testing Technology机械零件课程设计Coruse Design of Mechanical Parts形势与任务 Situation & Policy机械制造工艺学 Technology of Mechanical Manufacture锻造工艺学 Forging Technology冲压工艺学Stamping Technology冲压工艺课程设计Coruse Design of Stamping Technology锻压设备Forging & Stamping Equipments塑料模具设计 Plastic Molding Design生产实习 Production Practice加热炉 Heating Furnace冷锻技术 Cold Forging Technology金属超塑性 Metal Superplasticity锻造课程设计Coruse Design of Forging特种锻造 Special Forging毕业实习与设计 Graduation Practice & Design。
机械工程专业英语单词

Lesson 1 Basic Concepts in M e c h a n i c s机械学的基本概念mechanics n.力学modify v.修改,调解,变更manageable a.可控制管理的incline v.使倾斜ramp n.斜板,斜坡道slope v.使倾斜friction n.摩擦roll v.滚动multiplier n.放大器,乘法器broom n.扫帚convert v.转变化handle n.手柄把sweep v.扫荡描,掠过efficiency n.效率gauge vt.测计量,校验bearing n.轴承ideal mechanical advantage 理想的机械效益neglect vt.忽略Lesson 2 Basic Assumption in Plasticity Theory 塑性理论的基本假设assumption n.假定plasticity n.塑性investigate v.调查,研究deformation n.变形metal forming process 金属成型工艺过程strain rate n.应变速率strength n.强度stress n.应力yield stress 屈服应力flow stress 流动应力tensile stress 拉伸应力compressive stress 压缩应力shear stress 剪切应力geometry n.几何形状elastic a.弹性的springback n.回弹bending n.弯曲,折弯precision forming 精密成型tolerance n.公差continuum n.连续体metallurgical a.冶金学的grain n.晶粒dislocation n.位错uni-,tri-,multi-axial a.单,三,多轴向的anisotropy n.各向异性cylindrical a.圆柱体的cross-section n.横截面platen n.工作台板,模板coincide with 一致,相符validity n.正确有效,合法with ease 轻而易举的,很容易的Lesson 3 Optimization for Finite Element Applications有限元优化的应用optimization n.优化,优选法finite element 有限元iterative a.反复的,迭代的alternative .交替的,可供选择的manual a.手动的,人工的trial-and-error 试凑法bias vt,n.使偏向重、差a desktop platform 计算机桌面平台constraint v,n.强制,约束response n.反响应,灵敏度parameter n.参数parametric a.参数的preprocess vt.预先加工,预处理mesh n,v.网格,啮合capability n.能力,性能,容量loop n.环,回路,循环pose v.提出model n.模型,样品displacement n.位移,排量,替换buckling n.弯翘曲,挠度factor n.因素gradient n.坡梯度,斜率flur n.电,磁,热,光通量,流量multidisciplinary a.多学科的deflection n.偏移转,离,挠曲Lesson 4 Metals 金属toughness n.韧性corrosion n.腐蚀dump v.倾倒,堆放recyle v.反复循环利用copper n.铜aluminum n.铝bronze n.青铜器alloy n.合金wear v.磨损metallic a.含金属制的specification n.操作规程,技术要求,说明书extract vt.提炼,萃取iron n.铁carbon n.碳ferrous a.含铁的ferrous metals 黑色金属lead n.铅zinc n.锌tin n.锡ore n.矿石mineral n,a.矿物的impurity n.杂质,不纯Lesson 5 Metallic and Nonmetallic Materials金属和非金属材料magnesium n.镁nickel n.镍brass n.黄铜luster n.光泽ductility n.延展性,可锻性it is likely that 很可能it is certain to inf.必然,一定density n.密度be distinguished from 与…区分coefficient n.系数in connection with 关于,与…相关结合category n.种类hardness n.硬度elasticity n.弹性beam n.横梁,一束光penetration n.贯穿,渗透abrasion n.磨损耗roll n,v.轧辊,滚,轧mill n.轧钢机,铣床spring n.弹簧permanent a.永久的rupture n.破开裂stamp n.冲压hammer n.锻锤Lesson 6 Plastics and Other Materials塑料和其他材料inorganic acid 无机酸sulphuric acid 硫酸hydrochloric acid 盐酸solvent n.溶剂carbon tetrachloride 四氯化碳rigid a,n.坚硬的,刚性的,刚度mouldmold n.模子,塑模,铸模decoration n.装饰fabricate vt.制造备,生产injection molding 注射模塑法blow molding 吹塑法compression molding 压塑法,模压法extrusion n.挤压vacuum forming 真空模塑法powder metallurgy 粉末冶金constituent n,a.组成的,部分,组元simultaneously adv.同时subsequently adv.随后coherent a.互相凝聚的,协调一致的fusion n.熔化,熔接crystalline n,a.结晶的,晶体的restriction n.限制定,节流blend n,v.混合物,融合press n,v.压力机,压制homogeneous a.均匀的sinter n,vt.烧结物Lesson 7 Die Life and Die Failure 模具的寿命和失效die n.模具,锻冲模,凹模die life 模具寿命die failure 模具损坏deterioration n.变坏,退化,损耗surface finish 表面光洁度breakdown n.破坏,击穿lubrication n.润滑cracking n.裂纹breakage n.断裂mode n.方式,状态,模式thermal fatigue 热疲劳layer n.层abrasive n,a.研磨料的,磨损的impression n.模膛,型腔槽heat checking 热裂纹,龟裂steep n,a.陡坡的,急剧的reversal n.颠倒,相反overload n,v.使过超载initiation n.开初始,发产生discrete a.不连续的,单个的variable n.变量cavity n.模膛,型槽stock n.坯料,原材料impact n,v.冲击,碰撞Lesson 8 Cold Working and Hot Working冷加工和热加工coldhot working 冷热加工forging n.锻造,锻件classification n.分类recrystallization n.再结晶take place 发生strain deformation hardening 应变变形硬化be referred to as 叫做,称为,被认为是warm working 温加工,温锻ultimate a,n.最终的,首要的,极限stress relieving 消除应力处理austenitic a.奥氏体的stainless steel 不锈钢annealing n.退火grain size 晶粒度solid solution 固溶体refinement n.精炼制,细化hazard n.危险,未知数,意外事件inherent a.固有的,先天的,本质的sensitive a.灵敏的,敏感的abnormal a.非正常的critical strain 临界应变Lesson 9 Casting 铸造casting n.铸造件die-casting n.模铸件foundry n.铸造车间pour v.浇注suitability n.适应性pig iron 生铁cupola n.冲天炉,化铁炉erosion n.腐蚀,侵、烧蚀ladle n.铁水包graphite n.石墨solidify v.使凝固disjoin v.拆散,分开ingot n.钢锭destructive a.破坏性的,有害的retard vt,n.使延缓,推迟solvent n,a.溶剂的copper-base alloy 铜基合金Lesson 10 Metal Forming Processes in Manufacturing 制造中的金属成形工艺machine-building 机械制造plastic working 塑性加工billet n 坯料,锻坯blank n.坯料,冲压板坯configuration n.外形,配置,排布stroke n.行程,冲程amortize v.阻尼,缓冲,分期偿还reliability n. 可靠性,安全性drawing n.锻坯拔长,线,管材拉拔deep drawing 深冲压brake forming 压折弯机成型stretch forming 张拉成型military n,a.军队事,人的consumer goods 消费品integrity n.完整性,完全善jet engine 喷气发动机turbine n.涡汽轮机,透平机regarding 考虑到,关于Lesson 11 Forging锻造armor n.铠甲immortalize vt.使不朽灭blacksmith n.锻工mechanical press 机械压力机hydranlic press 液压机anvil n.锤砧,砧座craftsman n.技工handling n.处理,装卸,搬运flexibility n.柔韧性,灵活性drawn out 拔长upset n.镦粗,顶锻closed impression die 闭式模锻rapid-impact blow 快速冲击,猛打vertical a.立式的ram n.锤头,滑块,活动横梁block n.模块draft n.模锻斜度symmetrical a.轴对称的sizing n.整形,校正,定径drop forging 落锻,锤模锻impression die forging 模锻final forging 终锻overheat n.过热furnace n.熔,高炉pyrometer n.高温计Lesson 12 Benefits and Principles of Forging锻造的优点和工作原理metalworking n.金属压力加工knead v.揉搓制refine v.精炼制,细化porosity n.多孔性,疏松orient v.使定取向flow line 流线stress field 应力场manual skill 手工技巧at one’s command 自由使用,支配soundness n.致密性,坚固性,无缺陷attainable a.可达得到的open die forging 自由锻gross n.总共,重大confine vt. 限制,约束convert v.转变换,更换broken up 破断裂,分散microshrinkage n.显微缩孔elimination n.消除,淘汰align v.调整,对中,校直Lesson 13 Welding焊接welding v,n.焊接,熔焊pressurewelding 压力焊spotwelding 点焊buttwelding 对头缝焊fusionwelding 熔焊,熔接fiux-shielded arc welding 熔剂保护电弧焊diversity n.不同多样性fastening v,n.连接件,紧固件shielding n.遮护,屏蔽soldering v,n.软钎焊,低温焊料bismuth n.铋cadmium n.镉rivet n,v.铆钉,铆接braze n,vt.硬钎焊,铜焊oxidation n.氧化flux n.焊接,助溶剂squeeze v.挤压oxy-acetylene n,a.氧乙炔的torch n.焊炬electrode n.电焊极,焊条filler n.填充剂overlap v.搭接,重叠strike v.攻打击,放电Lesson 14 Heat Treatment热处理heat treatment 热处理microstructure n.显微组织low-carbon steel 低碳钢prescribe v.规定,指示microscopic a.显微的,微观的spheroidizing n.球化处理normalizing n.正火,正常化annealing n.退火hardening n.淬火tempering n.回火soaking=holding n.均热,保温retarding media 延缓介质prolonged a.长时间的,持续很久的critical temperature 临界温度globular a.球形状的carbide n.碳化物,硬质合金quenching n.淬火,骤冷removal n.除去,放出Lesson 15 Introduction to Mechanism机构介绍mechanism n.机械,机构,机构学kinematic a.=kinematical 运动的,运动学的kinematic chain 运动链link n.构件,杆件. v.连接结definite a.确定的constrained a.约束的,限定的unconstrained a.无约束的linkage n.连杆组,机构joint n.结接台,铰链. a.连接的,联合的pin n.销钉,铰销revolution v.旋转,转动. n.回转体prismatic a.棱柱形的nonlinear a.非线性的four-bar linkage 四杆机构kinematic chain 运动链prime mover 原动者机,驱动件coupler n.连接件,连杆pivot n.枢轴,轴销,回转副,旋转中心configuration n.外形,构造,结构inversion n.转换,更换slider-crank mechanism 曲柄滑块机构multiloop n.多环链,a.多回路的sketch n.草简,示意图,v.画草图,草拟skeleton diagram 草图,示意图,简图envision v.想象binary a.二双,复的,二元的ternary a.三元的ternary links 三杆组quaternary a.四元的quaternary links 四杆组cam 凸轮cam follower n.凸轮从动件gear n.齿轮sprocket n.链轮belt n.皮,布,钢带pulley n.带轮spherical a.球的,球面的helical a.螺旋的three-dimensional 三维的,空间的intuitively adv.直觉观地synthesis n.合成法,综合kinematician n.运动学研究者家innate a.先天的,固有的Lesson 16 Movement Analysis 运动分析criterion n.判断标准,判据,准则branch n,v.分部,支transmission angle 传动角rock n.摆动, v.摇动oscillate v.摆动,摇动parallelogram n.平行四边形antiparallelogram n.反平行四边形frame n.机架,构架impart v.给予,分给to impart M to N 把M给Ntorque n.力矩,扭矩dynamic a.=dynamical 动力的,动力学的inertia n.惯性物,惯量static a.=statical 静力学的,静的index n.指数,指标friction n.摩擦thumb n.拇指,v.用拇指翻rule of thumb 根据经验和实际所得的做法matrix n.矩阵determinant n.行列式derivative n.导数derivative of M with respect to N M对于N的导数movability n.可动性,易动性parameter n.参数discount v.打折扣,忽视absolute a.绝对的graphical a.图形的,图解的polygon n.多边形theorem n.定理stress n.应力bearing n.轴承centripetal a.向心的Lesson 17 Kinematic Synthesis运动的综合packaging machinery 包装机械lubrication n.润滑specification n.技术要求actuation n.驱动jerk n.震动,冲击axis n.轴线,心,中心线contour n.外形,轮廓线eccentricity n.偏心度,率gear ration 齿轮速,齿数比topologically adv.拓扑学地customary a.通常的,习惯的correlate v.使相关,使发生关系analog n.=analogue 类似物,模拟linear analog 线性模拟second acceleration 二阶加速度higher acceleration 高阶加速度paraphrase v.释义,意译describe v.叙述,描述,作…运动category n.种类,类别deliberation n.慎重考虑province n.省,领域preconceive v.预想,事先想好analog computer 模拟计算机trace v.追踪,描画timing n.定时,计时,配时pitch v.投掷trajectory n.轨迹embed v.嵌入,夹在层间orientation n.定方位,取方向scoop n.勺子,铲斗, v.挖,掘,铲Lesson 18 Cams and Gears凸轮和齿轮cam n.凸轮gear n.齿轮curve n.曲线 a.弯曲的groove n.槽,沟mate v.配合,啮合cylindrical a.圆柱的two-dimensional or planer 两维的或平面的three-dimensional or spatial 三维的或空间的normal n.法线 a.垂直的complement n,a.余角,余的collinear a.共线的lateral a.横向的,侧向的stem n.杆guide n.导向件器,装置,导槽座intermittent a.间断的,不连续的dwell n,v.停止,小停顿inertial a.惯性的,惯量的engage v.啮合rack n.齿条noncircular a.非圆的conjugate a.共轭的,n.共轭值线cycloidal a.摆线的involved a.渐开线的, n.渐开线tolerance n.间隙,公差spur gear 直齿圆柱轮radial a.径向的,沿半径的offset n,vt.偏移,偏心 a.偏心的disk cam 盘形凸轮tangent n.切线 a.相切的,切线的concentric a.同圆的,同心的camshaft n.凸轮轴pitch curve 节线herringbone a.人字形的intersect v.横穿,相交parallel helical gear斜齿轮,平行轴螺旋齿轮crossed helical gear 交错轴螺旋齿轮face gear 端面齿轮spiral bevel gear 螺旋齿圆锥齿轮worm n.蜗杆skew bevel 斜齿圆锥齿轮hypoid gear 准双曲面直角交错轴双曲面齿轮addendum n.齿顶,齿顶高project v.伸出,突出clearance n.间隙dedendum pl. dedendan.齿根,齿根高tooth space 齿间距backlash n.间隙,齿隙Lesson 19 Screws, fasteners and joints螺纹件、紧固件和联接件screw n.螺钉,螺丝, v.旋紧,攻丝fastener n.紧固件joint n.连联接,接合bolt n.螺栓nut n.螺母cap screw 有头螺钉setscrew n.定位固定,调整螺钉rivet n.铆钉, v.用铆钉铆接key n.键weld n,v.焊接,熔焊braze n,v.钎焊,铜焊clip n.夹子, v.夹住,夹紧synonymous a.同意义的monotonous a.单调的taint n.污点,污染 v.弄脏tough a.坚韧的ductile a.可延伸的,有延展性的,韧性的tighten v.上紧,拉紧twist v.扭转jumbo n.大型喷气式客机titanium n.钛close-tolerance 高精密度的tooling n.工具,刀具 v.用刀具切削加工proliferate v.增殖,增殖assembly n.安装,装配,组件tap n.丝锥, v.攻螺丝stud n.双头螺栓resemble v.类似,像thread n.螺纹线,v.车螺纹drill n,v.钻孔hexagon head 六角头fillister n.凹槽flat head 平头hexagon socket head 六角沉头disassemble v.拆开tensile a.拉张力的,受拉的shear n.剪切力 v.剪切断harden v.使硬化washer n.垫圈preload n.预载荷fatigue n.疲劳micrometer n.千分尺,千分表elongation n.拉伸长modulus n.模数,模量wrench n.扳手dial n.刻度盘fractional a.分数的,小数的Lesson 21 Helical, Worm and Bevel Gears斜齿轮、蜗杆蜗轮和锥齿轮helical gear 斜齿轮worm n.蜗杆,螺杆bevel gear 圆锥齿轮helix n,a.pl. helices 或 helixes 螺旋线,螺旋线的right hand 右手,右旋的helicoid .螺旋面,体,螺旋状,纹的wrap v.缠绕unwind v.解开,展开generate v.产生,展成加工engagement n.啮合,接触diagonal n,a.对角线的objectionable a.该反对的,不能采用的spiral n,a. 螺旋线的,卷线的spiral gear 螺旋齿轮mesh n.啮合worm gear 蜗轮pinion n.小齿轮pitch cylinder 节圆柱concave a.中凹的curvature a.曲率screw-like 像螺丝杆的thread n.线状物,螺纹线envelop v.包围,封闭enclose v.包围lead angle 导角cast v.铸造mill v.铣削outboard a,ad.外侧的,向外pronounced a.明确的,显着的stress n.应力tapered a.锥形的positively ad.确定地,强制传动地Pitch-line velocity 节线速度automobile differential 汽车差速器gearing n.齿轮传动装置offset n.偏置,横距hypoid a.准双曲面的hyperboloid n.双曲面,双曲面体Lesson 22 Shafts, Clutches and Brakes轴、离合器和制动器shaft n.轴clutch n.离合器brake n.制动器pulley n.皮,胶带轮flywheel n.飞轮sprocket n.链轮,星轮bending moment 弯曲力矩torsional a.扭转的static a.静力,态的axle n.心轴,轮轴spindle n.心轴,主轴deflection n.偏移,弯曲fillet n.圆角,倒角peening n.喷射加工硬化法shot peening 喷丸硬化stiff a.刚性的inertia n.惯性,惯量slippage n.滑动actuation n.驱动,开动coefficient n.系数statics n.静力学rim n.边缘,轮缘shoe n.闸瓦,制动片块band n.带,条cone n.圆锥miscellaneous a.混合的,杂项的assume v.假设,承担statical a.=static,静态的equilibrium n.平衡reaction n.反应,反力overload-release clutch 超载释放保护离合器magnetic fluid clutch 磁液离合器shift v.变换,使移动lever n.杆,手柄jaw n.颚板,夹爪ratchet n.棘轮circumferentiallyad v.周围地,圆周地mate v.配合,啮合,联接synchronous a.同步的linear drive 线性驱动装置clicking n.‘卡塔’声freewheel v.空转coupling n.联轴器sleeve n.套筒flat n.平面部分 a.平的periphery n.圆周,周边wedge n.楔形物 v.楔入pawl n.棘爪powder n.粉末mixture n.混合物electromagnetic a.电磁的coil n.线圈excitation n.刺激,激励shearing a.剪切的lockup n.锁住Lesson 41 Definition of Robotics and the Robot System manipulator n.操作器,控制器,机械手peripheral a.周围的,外围的idiot n.白痴integrate v.使成为一体,使结合起来hard automation 刚性自动化a host of 许多Lesson 42 Basics of ComputersIexecute v.执行binary a.二进制的read only memory ROM 只读存储器random access memory RAM读写存储器,随机存储器erasable a.可擦去的volatile a.可丢失的Lesson 43 Basics of Computers二harsh a.恶劣的robust a.稳定的configuration n.结构,组态Morse Code 莫尔斯电码suffice v.足够interference n.干扰fluctuation n.脉动,波动expendable a.可消耗的intermediate a.中间的adaptor n.转换器transformer n.变压器rectifier n.整流器capacitor n.电容器Zener diode 齐纳稳压二极管buffer n.缓冲寄存器come across 碰到Baud rate 波特率Lesson 44 Programmable Controllersalbeit conj.虽然light-emitting diodes 发光二极管relay ladder logic 继电器梯形逻辑图archaically a.古体的,旧式的retention n.保留,保持versed a.熟练的,精通的fluidics n.射流sheer a.完全的,绝对的profligate a.浪费的proprietary a.专利的,专有的boolean expression 布尔表达式Lesson 45 CAD/CAMComputed-aided designCAD n.计算机辅助设计Computed-aided manufacturingCAM n.计算机辅助制造automatic factory n.自动化工厂drafting n.制图Computer-aided engineering n.计算机辅助工程management information systems n.管理信息系统graphics terminal n.图像终端a shared data base n.公用数据库three-dimensional a.三维的keyboard n.键盘lightpen=light pen n.光笔magnify v.放大flip v,n.翻转copy v.拷贝a mirror image 镜像symmetrical a.对称的artwork n.印刷线路原图Geometric modeling n.几何模型制造kinematics n.运动学Lesson 48 Flexible Manufacturing Systemsfexible manufacturing systemsFMS 柔性制造系统flexible manufacturing cellesFMC 柔性制造单元automated guided vehicles 自动搬运小车conveyor n.传送装置pallet loading and unloading carts 上下料小车part program 零件程序data base 数据库data processing networks 数据处理网络inspection program 检测程序robot program 自动机程序real-time control data 实时控制数据the Control hierarchy 控制层次real-time fault recovery 实时故障恢复unmanned operation 无人化操作chip removel 排屑module n.模块,组件intelligent node智能节点。
机械专业英语词汇(很全)

机械专业英语词汇(很全)篇一:机械专业英语词汇(很全)金属机械专业英语词汇(很全)切削 metal cutting机床 machine tool金属工艺学 technology of metals刀具 cutter摩擦 friction联结 link传动 drive/transmission轴 shaft弹性 elasticity频率特性 frequency characteristic误差 error响应 response定位 allocation机床夹具 jig动力学 dynamic运动学 kinematic静力学 static分析力学 analyse mechanics拉伸 pulling压缩 hitting剪切 shear扭转 twist弯曲应力 bending stress强度 intensity三相交流电 three-phase AC磁路 magnetic circles变压器 transformer异步电动机 asynchronous motor几何形状 geometrical精度 precision正弦形的 sinusoid交流电路 AC circuit机械加工余量 machining allowance 变形力 deforming force变形 deformation应力 stress硬度 rigidity热处理 heat treatment退火 anneal正火 normalizing脱碳 decarburization渗碳 carburization电路 circuit半导体元件 semiconductor element 反馈 feedback发生器 generator直流电源 DC electrical source门电路 gate circuit逻辑代数 logic algebra外圆磨削 external grinding内圆磨削 internal grinding平面磨削 plane grinding变速箱 gearbox离合器 clutch绞孔 fraising螺纹加工 thread processing螺钉 screw铣削 mill铣刀 milling cutter功率 power工件 workpiece齿轮加工 gear mechining齿轮 gear主运动 main movement主运动方向 direction of main movement进给方向 direction of feed进给运动 feed movement合成进给运动 resultant movement of feed合成切削运动 resultant movement of cutting合成切削运动方向 direction of resultant movement of cutting 切削深度 cutting depth前刀面 rake face刀尖 nose of tool机械专业词汇:hardenability 淬透性carbide tool 硬质合金刀具alloy tool steel 合金工具钢alloyed cast iron 合金铸铁carbon steel 碳素钢carbon tool steel 碳素工具钢cast iron 铸铁cast steel 铸钢die material 模具材料high alloy steel 高合金钢high carbon steel 高碳钢low alloy steel 低合金钢low carbon steel 低碳钢shock resistant tool steel 抗冲击工具钢 nodular graphite iron 球墨铸铁 malleable cast iron 可锻铸铁mottled cast iron 麻口铸铁hardenability curve 淬透性曲线hardening capacity 淬硬性(硬化能力) hardness penetration diagram “U”形曲线 hardness profile 硬度分布(硬度梯度) heat treatment procedure 热处理规范 heat treatment installation 热处理设备 heat treatment furnace 热处理炉 heat treatment cycle 热处理工艺周期 heat time 加热时间篇二:机械专业术语(英语)组装、冲压、喷漆等专业词汇Assembly line组装线 Layout布置图Conveyer流水线物料板 Rivet table拉钉机 Rivet gun拉钉枪 Screw driver起子Electric screw driver电动起子 Pneumatic screw driver气动起子worktable 工作桌 OOBA开箱检查fit together组装在一起 fasten锁紧(螺丝) fixture 夹具(治具) pallet 栈板 barcode条码barcode scanner条码扫描器 fuse together熔合 fuse machine热熔机repair修理 operator作业员 QC品管supervisor 课长 ME制造工程师 MT制造生技cosmetic inspect外观检查 inner parts inspect内部检查 thumb screw 大头螺丝 lbs. inch镑、英寸 EMI gasket导电条front plate前板 rear plate后板 chassis 基座 bezel panel面板power button电源按键 reset button重置键Hi-pot test of SPS高源高压测试 Voltage switch of SPS 电源电压接拉键sheet metal parts 冲件 plastic parts塑胶件 SOP制造作业程序material check list物料检查表 work cell工作间 trolley台车 carton 纸箱 sub-line支线 left fork叉车personnel resource department 人力资源部production department生产部门 planning department企划部 QC Section品管科stamping factory冲压厂 painting factory烤漆厂 molding factory成型厂common equipment常用设备 uncoiler and straightener整平机 punching machine 冲床 robot机械手hydraulic machine油压机 lathe车床 planer | plein #61611;|刨床miller铣床 grinder磨床 driller钻床linear cutting线切割 electrical sparkle电火花 welder电焊机staker=reviting machine铆合机 position职务 president董事长general manager总经理special assistant manager特助 factory director厂长 department director部长deputy manager | =vice manager副理 section supervisor课长deputy section supervisor =vice section superisor副课长group leader/supervisor组长 line supervisor线长 assistant manager助理to move, to carry, to handle搬运 be put in storage入库 pack packing包装 to apply oil擦油 to file burr 锉毛刺 final inspection终检 to connect material接料 to reverse material 翻料 wet station沾湿台 Tiana天那水cleaning cloth抹布 to load material上料 to unload material卸料to return material/stock to退料 scraped | skr aelig;pid|报废scrape ..v.刮;削deficient purchase来料不良 manufacture procedure制程deficient manufacturing procedure制程不良oxidation | ksi dei #61611;n|氧化 scratch刮伤 dents压痕defective upsiding down抽芽不良 defective to staking铆合不良embedded lump镶块feeding is not in place送料不到位 stamping-missing漏冲 production capacity生产力education and training教育与训练 proposal improvement提案改善spare parts=buffer备件 forklift叉车trailer=long vehicle拖板车 compound die合模 die locker锁模器pressure plate=plate pinch压板 bolt螺栓name of a department部门名称administration/general affairs dept总务部automatic screwdriver电动启子 thickness gauge厚薄规gauge(or jig)治具 power wire电源线 buzzle蜂鸣器defective product label不良标签 identifying sheet list标示单screwdriver holder起子插座 pedal踩踏板 stopper阻挡器 flow board流水板hydraulic handjack油压板车 forklift叉车 pallet栈板 glove(s)手套glove(s) with exposed fingers割手套thumb大拇指 forefinger食指midfinger中指 ring finger无名指 little finger小指 band-aid创可贴industrial alcohol工业酒精 alcohol container沾湿台 head of screwdriver起子头 sweeper扫把 mop拖把vaccum cleaner吸尘器 rag 抹布garbage container灰箕 garbage can垃圾箱 garbage bag垃圾袋 chain 链条 jack升降机 production line流水线 chain链条槽magnetizer加磁器 lamp holder灯架 to mop the floor拖地 to clean the floor扫地 to clean a table擦桌子 air pipe 气管packaging tool打包机 packaging打包 missing part漏件 wrong part错件excessive defects过多的缺陷 critical defect极严重缺陷 major defect主要缺陷 minor defect次要缺陷not up to standard不合规格dimension/size is a little bigger尺寸偏大(小)cosmetic defect外观不良slipped screwhead/slippery screw head螺丝滑头slipped screwhead/shippery screw thread滑手 speckle斑点mildewed=moldy=mouldy发霉 rust生锈deformation变形burr(金属)flash(塑件)毛边 poor staking铆合不良 excesssive gap间隙过大 grease/oil stains油污 inclusion杂质painting peel off脏污 shrinking/shrinkage缩水 mixed color杂色scratch划伤poor processing 制程不良 poor incoming part事件不良 fold of pakaging belt打包带折皱 painting make-up补漆 discoloration羿色water spots水渍polishing/surface processing表面处理 exposed metal/bare metal金属裸露 lack of painting烤漆不到位 safety安全 quality品质delivery deadline交货期 cost成本engineering工程 die repair模修enterprise plan = enterprise expansion projects企划 QC品管die worker模工production, to produce生产 equipment设备 to start a press开机stop/switch off a press关机 classification整理 regulation整顿cleanness清扫 conservation清洁 culture教养 qualified products, up-to-grade products良品defective products, not up-to-grade products不良品 waste废料board看板 feeder送料机sliding rack滑料架defective product box不良品箱 die change 换模 to fix a die装模to take apart a die拆模 to repair a die修模 packing material包材basket蝴蝶竺 plastic basket胶筐isolating plate baffle plate; barricade隔板carton box纸箱to pull and stretch拉深to put material in place, to cut material, to input落料to impose lines压线to compress, compressing压缩 character die字模 to feed, feeding 送料 transportation运输(be)qualfied, up to grade合格not up to grade, not qualified不合格 material change, stock change材料变更feature change 特性变更 evaluation评估prepare for, make preparations for 准备parameters参数rotating speed, revolution转速manufacture management制造管理 abnormal handling异常处理production unit生产单位 lots of production生产批量 steel plate钢板roll material卷料manufacture procedure制程 operation procedure作业流程 to revise, modify修订to switch over to, switch---to throw--over switching over切换engineering, project difficulty 工程瓶颈stage die工程模 automation自动化to stake, staking, reviting铆合 add lubricating oil加润滑油 shut die架模shut height of a die架模高度 analog-mode device类模器 die lifter 举模器 argon welding氩焊 vocabulary for stamping 冲压常词汇stamping, press冲压punch press, dieing out press冲床 uncoiler strainghtener整平机feeder送料机rack, shelf, stack料架 cylinder油缸 robot机械手 taker取料机conveyer belt输送带 transmission rack输送架 top stop上死点bottom stop下死点 one stroke一行程 inch寸动to continue, cont.连动 to grip(material)吸料location lump, locating piece, block stop 定位块 reset复位smoothly顺利 dent压痕 scratch刮伤deformation变形 filings铁削to draw holes抽孔 inquiry, search for查寻to stock, storage, in stock库存 receive领取approval examine and verify审核 processing, to process加工delivery, to deliver 交货 to return delivey to.to send delinery backto retrn of goods退货registration登记registration card登记卡 to control管制to put forward and hand in提报 safe stock安全库存acceptance = receive验收 to notice通知application form for purchase请购单 consume, consumption消耗 to fill in填写 abrasion磨损reverse angle = chamfer倒角 character die字模to collect, to gather收集 failure, trouble故障 statistics统计demand and supply需求 career card履历卡to take apart a die卸下模具 to load a die装上模具 to tight a bolt 拧紧螺栓 to looser a bolt拧松螺栓to move away a die plate移走模板 easily damaged parts易损件standard parts标准件breaking.(be)broken,(be)cracked 断裂 to lubricate润滑 common vocabulary for die engineering模具工程常用词汇die 模具figure file, chart file图档cutting die, blanking die冲裁模 progressive die, follow (-on)die 连续模compound die复合模 punched hole冲孔 panel board镶块to cutedges=side cut=side scrap切边 to bending折弯to pull, to stretch拉伸Line streching, line pulling线拉伸 engraving, to engrave刻印upsiding down edges翻边 to stake铆合designing, to design design modification设计变化 die block模块folded block折弯块 sliding block滑块 location pin定位销 lifting pin顶料销die plate, front board模板 padding block垫块 stepping bar垫条upper die base上模座 lower die base下模座upper supporting blank上承板 upper padding plate blank上垫板spare dies模具备品 spring 弹簧 bolt螺栓document folder文件夹to put file in order整理资料 spare tools location手工备品仓 first count初盘人first check初盘复棹人 second count 复盘人 second check复盘复核人equipment设备 waste materials废料work in progress product在制品 casing = containerazation装箱quantity of physical invetory second count 复盘点数量quantity of customs count 会计师盘,点数量 the first page第一联filed by accounting department for reference会计部存查end-user/using unit(department)使用单位summary of year-end physical inventory bills年终盘点截止单据汇总表 bill name单据名称This sheet and physical inventory list will be sent to accounting department together (Those of NHK will be sent to financial department)本表请与盘点清册一起送会计部-(NHK厂区送财会部)Application status records of year-end physical inventory card 年终盘点卡与清册使用-状况明细表 blank and waste sheet NO. 空白与作废单号 plate电镀 mold成型material for engineering mold testing工程试模材料not included in physical inventory不列入盘点sample样品incoming material to be inspected进货待验description品名steel/rolled steel钢材 material statistics sheet 物料统计明细表meeting minutes会议记录 meeting type 会别distribution department分发单位 location地点 chairman主席present members出席人员 subject主题 conclusion结论decision items决议事项responsible department负责单位 pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/审核/承办PCE assembly production schedule PCE组装厂生产排配表 model机锺work order工令 revision版次 remark备注production control confirmation生产确认checked by初审 approved by核准 department部门stock age analysis sheet 库存货龄分析表on-hand inventory现有库存 available material良品可使用 obsolete material良品已呆滞 to be inspected or reworked 待验或重工 total合计 cause description原因说明 part number/ P/N 料号 type形态item/group/class类别 quality品质 prepared by制表 notes说明year-end physical inventory difference analysis sheet 年终盘点差异分析表physical inventory盘点数量 physical count quantity帐面数量difference quantity差异量 raw materials原料 materials物料finished product成品semi-finished product半成品 packing materials包材good product/accepted goods/ accepted parts/good parts良品defective product/non-good parts不良品disposed goods处理品 warehouse/hub仓库 on way location在途仓oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓 skid/pallet栈板 tox machine自铆机wire EDM线割 EDM放电机 coil stock卷料 sheet stock片料 tolerance工差 score=groove压线 cam block滑块 pilot导正筒 trim剪外边 pierce剪内边 drag form压锻差pocket for the punch head挂钩槽 slug hole废料孔expansion dwg展开图 radius半径shim(wedge)楔子torch-flame cut火焰切割 set screw止付螺丝 form block折刀 stop pin定位销round pierce punch=die button圆冲子shape punch=die insert异形子stock locater block定位块 under cut=scrap chopper清角 active plate 活动板 baffle plate挡块 cover plate盖板 male die公模 female die母模 groove punch压线冲子air-cushion eject-rod气垫顶杆 spring-box eject-plate弹簧箱顶板bushing block衬套 insert 入块club car高尔夫球车 capability能力 parameter参数 factor系数phosphate皮膜化成 viscosity涂料粘度 alkalidipping脱脂main manifold主集流脉 bezel斜视规 blanking穿落模 demagnetization 去磁;消磁high-speed transmission高速传递 heat dissipation热传 rack上料degrease脱脂 rinse水洗alkaline etch龄咬 desmut剥黑膜 D.I. rinse纯水次 Chromate铬酸处理Anodize阳性处理 seal封孔 revision版次part number/P/N料号 good products良品scraped products报放心品 defective products不良品 finished products成品 disposed products处理品 barcode条码flow chart流程表单 assembly组装 stamping冲压 molding成型spare parts=buffer备品 coordinate座标dismantle the die折模 auxiliary fuction辅助功能 poly-line多义线heater band 加热片 thermocouple热电偶 grit 砂砾derusting machine除锈机 degate打浇口 dryer烘干机 induction感应 induction light感应光response=reaction=interaction感应ram连杆edge finder巡边器 concave凸 convex凹short射料不足 nick缺口 speck瑕 shine亮班 splay 银纹 gas mark焦痕 delamination起鳞 cold slug冷块 blush 导色gouge沟槽;凿槽satin texture段面咬花 witness line证示线 patent专利 grit沙砾granule=peuet=grain细粒 grit maker抽粒机 cushion缓冲magnalium镁铝合金 magnesium镁金 metal plate钣金 mill锉 plane刨grind磨 drill铝 boring镗 blinster气泡 fillet镶;嵌边through-hole form通孔形式 voller pin formality滚针形式 cam driver 铡楔 shank摸柄crank shaft曲柄轴augular offset角度偏差 velocity速度production tempo生产进度现状 torque扭矩spline=the multiple keys花键 quenching淬火 tempering回火annealing退火 carbonization碳化 alloy合金tungsten high speed steel钨高速的moly high speed steel钼高速的organic solvent有机溶剂 bracket小磁导 liaison联络单 volatile挥发性resistance电阻 ion离子titrator滴定仪篇三:英文版机械专业术语(全)组装、冲压、喷漆等专业词汇Assembly line组装线 Layout布置图Conveyer流水线物料板 Rivet table拉钉机 Rivet gun拉钉枪 Screw driver起子Electric screw driver电动起子 Pneumatic screw driver气动起子worktable 工作桌 OOBA开箱检查fit together组装在一起 fasten锁紧(螺丝) fixture 夹具(治具) pallet 栈板 barcode条码barcode scanner条码扫描器 fuse together熔合 fuse machine热熔机repair修理 operator作业员 QC品管supervisor 课长 ME制造工程师 MT制造生技cosmetic inspect外观检查 inner parts inspect内部检查 thumb screw 大头螺丝 lbs. inch镑、英寸 EMI gasket导电条front plate前板 rear plate后板 chassis 基座 bezel panel面板power button电源按键 reset button重置键 Hi-pot test of SPS高源高压测试 Voltage switch of SPS 电源电压接拉键 sheet metal parts 冲件plastic parts塑胶件 SOP制造作业程序 material check list物料检查表work cell工作间 trolley台车 carton纸箱 sub-line支线left fork叉车 personnel resource department 人力资源部 production department生产部门 planning department企划部 QC Section品管科stamping factory冲压厂 painting factory烤漆厂 molding factory成型厂common equipment常用设备 uncoiler and straightener整平机 punching machine 冲床 robot机械手 hydraulic machine油压机 lathe车床 planer | plein #61611;|刨床 miller铣床 grinder磨床 driller钻床 linearcutting线切割 electrical sparkle电火花 welder电焊机 staker=reviting machine铆合机 position职务 president董事长 general manager总经理special assistant manager特助 factory director厂长 department director部长 deputy manager | =vice manager副理 section supervisor课长deputy section supervisor =vice section superisor副课长 group leader/supervisor组长 line supervisor线长 assistant manager助理 to move, to carry, to handle搬运 be put in storage入库 pack packing包装 to apply oil擦油 to file burr 锉毛刺 final inspection终检 to connect material接料 to reverse material 翻料 wet station沾湿台Tiana天那水 cleaning cloth抹布 to load material上料 to unload material卸料to return material/stock to退料 scraped | skr aelig;pid|报废scrape ..v.刮;削deficient purchase来料不良 manufacture procedure制程deficient manufacturing procedure制程不良oxidation | ksi dei #61611;n|氧化 scratch刮伤 dents压痕defective upsiding down抽芽不良 defective to staking铆合不良embedded lump镶块feeding is not in place送料不到位 stamping-missing漏冲 production capacity生产力education and training与训练 proposal improvement提案改善 spare parts=buffer备件 forklift叉车trailer=long vehicle拖板车 compound die合模 die locker锁模器pressure plate=plate pinch压板 bolt螺栓name of a department部门名称administration/general affairs dept总务部automatic screwdriver电动启子 thickness gauge厚薄规 gauge(or jig)治具power wire电源线 buzzle蜂鸣器defective product label不良标签 identifying sheet list标示单screwdriver holder起子插座 pedal踩踏板 stopper阻挡器 flow board流水板hydraulic handjack油压板车 forklift叉车 pallet栈板 glove(s)手套glove(s) with exposed fingers割手套thumb大拇指 forefinger食指midfinger中指 ring finger无名指 little finger小指 band-aid创可贴iudustrial alcohol工业酒精 alcohol container沾湿台 head of screwdriver起子头 sweeper扫把 mop拖把vaccum cleaner吸尘器 rag 抹布garbage container灰箕 garbage can垃圾箱 garbage bag垃圾袋 chain 链条 jack升降机production line流水线 chain链条槽magnetizer加磁器 lamp holder灯架 to mop the floor拖地 to clean the floor扫地 to clean a table擦桌子 air pipe 气管packaging tool打包机 packaging打包 missing part漏件 wrong part错件excessive defects过多的缺陷 critical defect极严重缺陷 major defect主要缺陷 minor defect次要缺陷not up to standard不合规格dimension/size is a little bigger尺寸偏大(小)cosmetic defect外观不良slipped screwhead/slippery screw head螺丝滑头slipped screwhead/shippery screw thread滑手 speckle斑点mildewed=moldy=mouldy发霉 rust生锈deformation变形burr(金属)flash(塑件)毛边 poor staking铆合不良 excesssive gap间隙过大 grease/oil stains油污 inclusion杂质 painting peel off脏污shrinking/shrinkage缩水 mixed color杂色 scratch划伤poor processing 制程不良 poor incoming part事件不良 fold of pakaging belt打包带折皱 painting make-up补漆 discoloration羿色water spots水渍polishing/surface processing表面处理 exposed metal/bare metal金属裸露 lack of painting烤漆不到位 safety安全 quality品质delivery deadline交货期 cost成本engineering工程 die repair模修enterprise plan = enterprise expansion projects企划 QC品管die worker模工production, to produce生产 equipment设备 to start a press开机stop/switch off a press关机 classification整理 regulation整顿cleanness清扫 conservation清洁 culture教养 qualified products, up-to-grade products良品defective products, not up-to-grade products不良品 waste废料board看板 feeder送料机sliding rack滑料架defective product box不良品箱 die change 换模 to fix a die装模to take apart a die拆模 to repair a die修模 packing material包材basket蝴蝶竺 plastic basket胶筐isolating plate baffle plate; barricade隔板carton box纸箱to pull and stretch拉深to put material in place, to cut material, to input落料to impose lines压线to compress, compressing压缩 character die字模 to feed, feeding 送料 transportation运输(be)qualfied, up to grade合格not up to grade, not qualified不合格 material change, stock change材料变更feature change 特性变更evaluation评估prepare for, make preparations for 准备parameters参数rotating speed, revolution转速manufacture management制造管理 abnormal handling异常处理production unit生产单位 lots of production生产批量 steel plate钢板roll material卷料manufacture procedure制程 operation procedure作业流程 to revise, modify修订to switch over to, switch---to throw--over switching over切换engineering, project difficulty 工程瓶颈stage die工程模 automation自动化to stake, staking, reviting铆合 add lubricating oil加润滑油 shut die架模shut height of a die架模高度 analog-mode device类模器 die lifter 举模器 argon welding氩焊 vocabulary for stamping 冲压常词汇stamping, press冲压punch press, dieing out press冲床 uncoiler strainghtener整平机feeder送料机rack, shelf, stack料架 cylinder油缸 robot机械手 taker取料机conveyer belt输送带 transmission rack输送架 top stop上死点bottom stop下死点 one stroke一行程 inch寸动to continue, cont.连动 to grip(material)吸料location lump, locating piece, block stop 定位块 reset复位smoothly顺利 dent压痕 scratch刮伤deformation变形 filings铁削to draw holes抽孔 inquiry, search for查寻to stock, storage, in stock库存 receive领取approval examine and verify审核 processing, to process加工delivery, to deliver 交货 to return delivey to.to send delinery backto retrn of goods退货 registration登记registration card登记卡 to control管制to put forward and hand in提报 safe stock安全库存acceptance = receive验收 to noticeapplication form for purchase请购单 consume, consumption消耗 to fill in填写 abrasion磨损reverse angle = chamfer倒角 character die字模to collect, to gather收集 failure, trouble故障 statistics统计demand and supply需求 career card履历卡to take apart a die卸下模具 to load a die装上模具 to tight a bolt 拧紧螺栓 to looser a bolt拧松螺栓to move away a die plate移走模板 easily damaged parts易损件standard parts标准件breaking.(be)broken,(be)cracked 断裂 to lubricate润滑common vocabulary for die engineering模具工程常用词汇 die 模具figure file, chart file图档cutting die, blanking die冲裁模 progressive die, follow (-on)die 连续模compound die复合模 punched hole冲孔 panel board镶块to cutedges=side cut=side scrap切边 to bending折弯to pull, to stretch拉伸Line streching, line pulling线拉伸 engraving, to engrave刻印upsiding down edges翻边 to stake铆合designing, to design设计 design modification设计变化 die block模块folded block折弯块 sliding block滑块 location pin定位销 lifting pin顶料销die plate, front board模板 padding block垫块 stepping bar垫条upper die base上模座 lower die base下模座upper supporting blank上承板 upper padding plate blank上垫板spare dies模具备品 spring 弹簧 bolt螺栓document folder文件夹 file folder资料夹to put file in order整理资料spare tools location手工备品仓 first count初盘人first check初盘复棹人 second count 复盘人 second check复盘复核人equipment设备 waste materials废料work in progress product在制品 casing = containerazation装箱quantity of physical invetory second count 复盘点数量quantity of customs count 会计师盘,点数量 the first page第一联filed by accounting department for reference会计部存查end-user/using unit(department)使用单位summary of year-end physical inventory bills年终盘点截止单据汇总表 bill name单据名称This sheet and physical inventory list will be sent to accounting department together (Those of NHK will be sent to financial department)本表请与盘点清册一起送会计部-(NHK厂区送财会部)Application status records of year-end physical inventory List and physical inventory card 年终盘点卡与清册使用-状况明细表 blank and waste sheet NO. 空白与作废单号 plate电镀 mold成型material for engineering mold testing工程试模材料not included in physical inventory不列入盘点sample样品incoming material to be inspected进货待验description品名steel/rolled steel钢材 material statistics sheet 物料统计明细表meeting minutes会议记录 meeting type 会别distribution department分发单位 location地点 chairman主席present members出席人员 subject主题 conclusion结论decision items决议事项responsible department负责单位 pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/审核/承办PCE assembly production schedule sheetPCE组装厂生产排配表 model机锺 work order工令 revision版次 remark 备注production control confirmation生产确认checked by初审 approved by核准 department部门stock age analysis sheet 库存货龄分析表on-hand inventory现有库存 available material良品可使用 obsolete material良品已呆滞 to be inspected or reworked 待验或重工 total合计 cause description原因说明 part number/ P/N 料号 type形态item/group/class类别 quality品质 prepared by制表 notes说明year-end physical inventory difference analysis sheet 年终盘点差异分析表physical inventory盘点数量 physical count quantity帐面数量difference quantity差异量 cause analysis原因分析 raw materials原料materials物料finished product成品semi-finished product半成品 packing materials包材good product/accepted goods/ accepted parts/good parts良品defective product/non-good parts不良品disposed goods处理品 warehouse/hub仓库 on way location在途仓oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓 skid/pallet栈板 tox machine自铆机wire EDM线割 EDM放电机 coil stock卷料 sheet stock片料 tolerance工差 score=groove压线 cam block滑块 pilot导正筒 trim剪外边 pierce剪内边 drag form压锻差pocket for the punch head挂钩槽 slug hole废料孔 feature die公母模 expansion dwg展开图radius半径shim(wedge)楔子torch-flame cut火焰切割 set screw止付螺丝 form block折刀 stop pin定位销round pierce punch=die button圆冲子 shape punch=die insert异形子stock locater block定位块 under cut=scrap chopper清角 active plate 活动板 baffle plate挡块 cover plate盖板 male die公模 female die母模 groove punch压线冲子air-cushion eject-rod气垫顶杆 spring-box eject-plate弹簧箱顶板bushing block衬套 insert 入块club car高尔夫球车 capability能力 parameter参数 factor系数phosphate皮膜化成 viscosity涂料粘度 alkalidipping脱脂main manifold主集流脉 bezel斜视规 blanking穿落模 dejecting顶固模 demagnetization去磁;消磁 high-speed transmission高速传递 heat dissipation热传 rack上料 degrease脱脂 rinse水洗alkaline etch龄咬 desmut剥黑膜 D.I. rinse纯水次 Chromate铬酸处理Anodize阳性处理 seal封孔 revision版次part number/P/N料号 good products良品scraped products报放心品 defective products不良品 finished products成品 disposed products处理品 barcode条码flow chart流程表单 assembly组装 stamping冲压 molding成型spare parts=buffer备品 coordinate座标dismantle the die折模 auxiliary fuction辅助功能 poly-line多义线heater band 加热片 thermocouple热电偶 sand blasting喷沙 grit 砂砾derusting machine除锈机 degate打浇口 dryer烘干机 induction感应induction light感应光response=reaction=interaction感应 ram连杆edge finder巡边器 concave凸 convex凹short射料不足 nick缺口 speck瑕 shine亮班 splay 银纹 gas mark焦痕 delamination起鳞 cold slug冷块 blush 导色gouge沟槽;凿槽satin texture段面咬花 witness line证示线 patent专利 grit沙砾granule=peuet=grain细粒 grit maker抽粒机 cushion缓冲magnalium镁铝合金 magnesium镁金 metal plate钣金 lathe车 mill锉plane刨 grind磨 drill铝 boring镗 blinster气泡 fillet镶;嵌边through-hole form通孔形式 voller pin formality滚针形式 cam driver 铡楔 shank摸柄crank shaft曲柄轴augular offset角度偏差 velocity速度production tempo生产进度现状 torque扭矩spline=the multiple keys花键 quenching淬火 tempering回火annealing退火 carbonization碳化 alloy合金tungsten high speed steel钨高速的moly high speed steel钼高速的organic solvent有机溶剂 bracket小磁导 liaison联络单 volatile挥发性resistance电阻 ion离子titrator滴定仪 beacon警示灯 coolant冷却液。
机械专业术语英文翻译

机械专业英语词汇陶瓷ceramics合成纤维synthetic fibre电化学腐蚀electrochemical corrosion车架automotive chassis悬架suspension转向器redirector变速器speed changer板料冲压sheet metal parts孔加工spot facing machining车间workshop工程技术人员engineer气动夹紧pneuma lock数学模型mathematical model画法几何descriptive geometry机械制图Mechanical drawing投影projection视图view剖视图profile chart标准件standard component零件图part drawing装配图assembly drawing尺寸标注size marking技术要求technical requirements刚度rigidity内力internal force位移displacement截面section疲劳极限fatigue limit断裂fracture塑性变形plastic distortion脆性材料brittleness material刚度准则rigidity criterion垫圈washer垫片spacer直齿圆柱齿轮straight toothed spur gear 斜齿圆柱齿轮helical-spur gear直齿锥齿轮straight bevel gear运动简图kinematic sketch齿轮齿条pinion and rack蜗杆蜗轮worm and worm gear虚约束passive constraint曲柄crank摇杆racker凸轮cams共轭曲线conjugate curve范成法generation method定义域definitional domain值域range导数\\微分differential coefficient求导derivation定积分definite integral不定积分indefinite integral曲率curvature偏微分partial differential毛坯rough游标卡尺slide caliper千分尺micrometer calipers攻丝tap二阶行列式second order determinant逆矩阵inverse matrix线性方程组linear equations概率probability随机变量random variable排列组合permutation and combination气体状态方程equation of state of gas动能kinetic energy势能potential energy机械能守恒conservation of mechanical energy动量momentum桁架truss轴线axes余子式cofactor逻辑电路logic circuit触发器flip-flop脉冲波形pulse shape数模digital analogy液压传动机构fluid drive mechanism机械零件mechanical parts淬火冷却quench淬火hardening回火tempering调质hardening and tempering磨粒abrasive grain结合剂bonding agent砂轮grinding wheel后角clearance angle龙门刨削planing主轴spindle主轴箱headstock卡盘chuck加工中心machining center车刀lathe tool车床lathe钻削镗削bore车削turning磨床grinder基准benchmark钳工locksmith锻forge压模stamping焊weld拉床broaching machine拉孔broaching装配assembling铸造found流体动力学fluid dynamics流体力学fluid mechanics加工machining液压hydraulic pressure切线tangent机电一体化mechanotronics mechanical-electrical integration 气压air pressure pneumatic pressure稳定性stability介质medium液压驱动泵fluid clutch液压泵hydraulic pump阀门valve失效invalidation强度intensity载荷load应力stress安全系数safty factor可靠性reliability螺纹thread螺旋helix键spline销pin滚动轴承rolling bearing滑动轴承sliding bearing弹簧spring制动器arrester brake十字结联轴节crosshead联轴器coupling链chain皮带strap精加工finish machining粗加工rough machining变速箱体gearbox casing腐蚀rust氧化oxidation磨损wear耐用度durability随机信号random signal离散信号discrete signal超声传感器ultrasonic sensor集成电路integrate circuit挡板orifice plate残余应力residual stress套筒sleeve扭力torsion冷加工cold machining电动机electromotor汽缸cylinder过盈配合interference fit热加工hotwork摄像头CCD camera倒角rounding chamfer优化设计optimal design工业造型设计industrial moulding design有限元finite element滚齿hobbing插齿gear shaping伺服电机actuating motor铣床milling machine钻床drill machine镗床boring machine步进电机stepper motor丝杠screw rod导轨lead rail组件subassembly可编程序逻辑控制器Programmable Logic Controller PLC 电火花加工electric spark machining电火花线切割加工electrical discharge wire - cutting相图phase diagram热处理heat treatment固态相变solid state phase changes有色金属nonferrous metal陶瓷ceramics合成纤维synthetic fibre电化学腐蚀electrochemical corrosion车架automotive chassis悬架suspension转向器redirector变速器speed changer板料冲压sheet metal parts孔加工spot facing machining车间workshop工程技术人员engineer气动夹紧pneuma lock数学模型mathematical model画法几何descriptive geometry机械制图Mechanical drawing投影projection视图view剖视图profile chart标准件standard component零件图part drawing装配图assembly drawing尺寸标注size marking技术要求technical requirements刚度rigidity内力internal force位移displacement截面section疲劳极限fatigue limit断裂fracture塑性变形plastic distortion脆性材料brittleness material刚度准则rigidity criterion垫圈washer垫片spacer直齿圆柱齿轮straight toothed spur gear 斜齿圆柱齿轮helical-spur gear直齿锥齿轮straight bevel gear运动简图kinematic sketch齿轮齿条pinion and rack蜗杆蜗轮worm and worm gear虚约束passive constraint曲柄crank摇杆racker凸轮cams共轭曲线conjugate curve范成法generation method定义域definitional domain值域range导数\\微分differential coefficient求导derivation定积分definite integral不定积分indefinite integral曲率curvature偏微分partial differential毛坯rough游标卡尺slide caliper千分尺micrometer calipers攻丝tap二阶行列式second order determinant逆矩阵inverse matrix线性方程组linear equations概率probability随机变量random variable排列组合permutation and combination气体状态方程equation of state of gas动能kinetic energy势能potential energy机械能守恒conservation of mechanical energy 动量momentum桁架truss轴线axes余子式cofactor逻辑电路logic circuit触发器flip-flop脉冲波形pulse shape数模digital analogy液压传动机构fluid drive mechanism机械零件mechanical parts淬火冷却quench淬火hardening回火tempering调质hardening and tempering磨粒abrasive grain结合剂bonding agent砂轮grinding wheelAssembly line 组装线Layout 布置图Conveyer 流水线物料板Rivet table 拉钉机Rivet gun 拉钉枪Screw driver 起子Pneumatic screw driver 气动起子worktable 工作桌OOBA 开箱检查fit together 组装在一起fasten 锁紧(螺丝)fixture 夹具(治具)pallet 栈板barcode 条码barcode scanner 条码扫描器fuse together 熔合fuse machine热熔机repair修理operator作业员QC品管supervisor 课长ME 制造工程师MT 制造生技cosmetic inspect 外观检查inner parts inspect 内部检查thumb screw 大头螺丝lbs. inch 镑、英寸EMI gasket 导电条front plate 前板rear plate 后板chassis 基座bezel panel 面板power button 电源按键reset button 重置键Hi-pot test of SPS 高源高压测试Voltage switch of SPS 电源电压接拉键sheet metal parts 冲件plastic parts 塑胶件SOP 制造作业程序material check list 物料检查表work cell 工作间trolley 台车sub-line 支线left fork 叉车personnel resource department 人力资源部production department生产部门planning department企划部QC Section品管科stamping factory冲压厂painting factory烤漆厂molding factory成型厂common equipment常用设备uncoiler and straightener整平机punching machine 冲床robot机械手hydraulic machine油压机lathe车床planer |plein|刨床miller铣床grinder磨床linear cutting线切割electrical sparkle电火花welder电焊机staker=reviting machine铆合机position职务president董事长general manager总经理special assistant manager特助factory director厂长department director部长deputy manager | =vice manager副理section supervisor课长deputy section supervisor =vice section superisor副课长group leader/supervisor组长line supervisor线长assistant manager助理to move, to carry, to handle搬运be put in storage入库pack packing包装to apply oil擦油to file burr 锉毛刺final inspection终检to connect material接料to reverse material 翻料wet station沾湿台cleaning cloth抹布to load material上料to unload material卸料to return material/stock to退料scraped |\\'skr?pid|报废scrape ..v.刮;削deficient purchase来料不良manufacture procedure制程deficient manufacturing procedure制程不良oxidation |\\' ksi\\'dei?n|氧化scratch刮伤dents压痕defective upsiding down抽芽不良defective to staking铆合不良embedded lump镶块feeding is not in place送料不到位stamping-missing漏冲production capacity生产力education and training教育与训练proposal improvement提案改善spare parts=buffer备件forklift叉车trailer=long vehicle拖板车compound die合模die locker锁模器pressure plate=plate pinch压板bolt螺栓administration/general affairs dept总务部automatic screwdriver电动启子thickness gauge厚薄规gauge(or jig)治具power wire电源线buzzle蜂鸣器defective product label不良标签identifying sheet list标示单location地点present members出席人员subject主题conclusion结论decision items决议事项responsible department负责单位pre-fixed finishing date预定完成日approved by / checked by / prepared by核准/审核/承办PCE assembly production schedule sheet PCE组装厂生产排配表model机锺work order工令revision版次remark备注production control confirmation生产确认checked by初审approved by核准department部门stock age analysis sheet 库存货龄分析表on-hand inventory现有库存available material良品可使用obsolete material良品已呆滞to be inspected or reworked 待验或重工total合计cause description原因说明part number/ P/N 料号type形态item/group/class类别quality品质prepared by制表notes说明year-end physical inventory difference analysis sheet 年终盘点差异分析表physical inventory盘点数量physical count quantity帐面数量difference quantity差异量cause analysis原因分析raw materials原料materials物料finished product成品semi-finished product半成品packing materials包材good product/accepted goods/ accepted parts/good parts良品defective product/non-good parts不良品disposed goods处理品warehouse/hub仓库on way location在途仓oversea location海外仓spare parts physical inventory list备品盘点清单spare molds location模具备品仓skid/pallet栈板tox machine自铆机wire EDM线割EDM放电机coil stock卷料sheet stock片料tolerance工差score=groove压线cam block滑块pilot导正筒trim剪外边pierce剪内边drag form压锻差pocket for the punch head挂钩槽slug hole废料孔feature die公母模expansion dwg展开图radius半径shim(wedge)楔子torch-flame cut火焰切割set screw止付螺丝form block折刀stop pin定位销round pierce punch=die button圆冲子shape punch=die insert异形子stock locater block定位块under cut=scrap chopper清角active plate活动板baffle plate挡块cover plate盖板male die公模female die母模groove punch压线冲子air-cushion eject-rod气垫顶杆spring-box eject-plate弹簧箱顶板bushing block衬套insert 入块club car高尔夫球车capability能力parameter参数factor系数phosphate皮膜化成viscosity涂料粘度alkalidipping脱脂main manifold主集流脉bezel斜视规blanking穿落模dejecting顶固模demagnetization去磁;消磁high-speed transmission高速传递heat dissipation热传rack上料degrease脱脂rinse水洗alkaline etch龄咬desmut剥黑膜D.I. rinse纯水次Chromate铬酸处理Anodize阳性处理seal封孔revision版次part number/P/N料号good products良品scraped products报放心品defective products不良品finished products成品disposed products处理品barcode条码flow chart流程表单assembly组装stamping冲压molding成型spare parts=buffer备品coordinate座标dismantle the die折模auxiliary fuction辅助功能poly-line多义线heater band 加热片thermocouple热电偶sand blasting喷沙grit 砂砾derusting machine除锈机degate打浇口dryer烘干机induction感应induction light感应光response=reaction=interaction感应ram连杆edge finder巡边器concave凸convex凹short射料不足nick缺口speck瑕疵splay 银纹gas mark焦痕delamination起鳞cold slug冷块blush 导色gouge沟槽;凿槽satin texture段面咬花witness line证示线patent专利grit沙砾granule=peuet=grain细粒grit maker抽粒机cushion缓冲magnalium镁铝合金magnesium镁金metal plate钣金lathe车mill锉plane刨grind磨drill铝boring镗blinster气泡fillet镶;嵌边through-hole form通孔形式voller pin formality滚针形式cam driver铡楔shank摸柄crank shaft曲柄轴augular offset角度偏差velocity速度production tempo生产进度现状torque扭矩spline=the multiple keys花键quenching淬火tempering回火annealing退火carbonization碳化tungsten high speed steel钨高速的moly high speed steel钼高速的organic solvent有机溶剂bracket小磁导liaison联络单volatile挥发性ion离子titrator滴定仪beacon警示灯coolant冷却液crusher破碎机阿基米德蜗杆Archimedes worm安全系数safety factor; factor of safety安全载荷safe load凹面、凹度concavity扳手wrench板簧flat leaf spring半圆键woodruff key变形deformation摆杆oscillating bar摆动从动件oscillating follower摆动从动件凸轮机构cam with oscillating follower 摆动导杆机构oscillating guide-bar mechanism 摆线齿轮cycloidal gear摆线齿形cycloidal tooth profile摆线运动规律cycloidal motion摆线针轮cycloidal-pin wheel包角angle of contact保持架cage背对背安装back-to-back arrangement背锥back cone ;normal cone背锥角back angle背锥距back cone distance比例尺scale比热容specific heat capacity闭式链closed kinematic chain闭链机构closed chain mechanism臂部arm变频器frequency converters变频调速frequency control of motor speed变速speed change变速齿轮change gear change wheel变位齿轮modified gear变位系数modification coefficient标准齿轮standard gear标准直齿轮standard spur gear表面质量系数superficial mass factor表面传热系数surface coefficient of heat transfer 表面粗糙度surface roughness并联式组合combination in parallel并联机构parallel mechanism并联组合机构parallel combined mechanism并行工程concurrent engineering并行设计concurred design, CD不平衡相位phase angle of unbalance不平衡imbalance (or unbalance)不平衡量amount of unbalance不完全齿轮机构intermittent gearing波发生器wave generator波数number of waves补偿compensation参数化设计parameterization design, PD残余应力residual stress操纵及控制装置operation control device槽轮Geneva wheel槽轮机构Geneva mechanism ;Maltese cross槽数Geneva numerate槽凸轮groove cam侧隙backlash差动轮系differential gear train差动螺旋机构differential screw mechanism差速器differential常用机构conventional mechanism; mechanism in common use 车床lathe承载量系数bearing capacity factor承载能力bearing capacity成对安装paired mounting尺寸系列dimension series齿槽tooth space齿槽宽spacewidth齿侧间隙backlash齿顶高addendum齿顶圆addendum circle齿根高dedendum齿根圆dedendum circle齿厚tooth thickness齿距circular pitch齿宽face width齿廓tooth profile齿廓曲线tooth curve齿轮gear齿轮变速箱speed-changing gear boxes齿轮齿条机构pinion and rack齿轮插刀pinion cutter; pinion-shaped shaper cutter 齿轮滚刀hob ,hobbing cutter齿轮机构gear齿轮轮坯blank齿轮传动系pinion unit齿轮联轴器gear coupling齿条传动rack gear齿数tooth number齿数比gear ratio齿条rack齿条插刀rack cutter; rack-shaped shaper cutter齿形链、无声链silent chain齿形系数form factor齿式棘轮机构tooth ratchet mechanism插齿机gear shaper重合点coincident points重合度contact ratio冲床punch传动比transmission ratio, speed ratio传动装置gearing; transmission gear传动系统driven system传动角transmission angle传动轴transmission shaft串联式组合combination in series串联式组合机构series combined mechanism串级调速cascade speed control创新innovation creation创新设计creation design垂直载荷、法向载荷normal load唇形橡胶密封lip rubber seal磁流体轴承magnetic fluid bearing从动带轮driven pulley从动件driven link, follower从动件平底宽度width of flat-face从动件停歇follower dwell从动件运动规律follower motion从动轮driven gear粗线bold line粗牙螺纹coarse thread大齿轮gear wheel打包机packer打滑slipping带传动belt driving带轮belt pulley带式制动器band brake单列轴承single row bearing单向推力轴承single-direction thrust bearing单万向联轴节single universal joint单位矢量unit vector当量齿轮equivalent spur gear; virtual gear当量齿数equivalent teeth number; virtual number of teeth当量摩擦系数equivalent coefficient of friction当量载荷equivalent load刀具cutter导数derivative倒角chamfer导热性conduction of heat导程lead导程角lead angle等加等减速运动规律parabolic motion; constant acceleration and deceleration motion 等速运动规律uniform motion; constant velocity motion等径凸轮conjugate yoke radial cam等宽凸轮constant-breadth cam等效构件equivalent link等效力equivalent force等效力矩equivalent moment of force等效量equivalent等效质量equivalent mass等效转动惯量equivalent moment of inertia等效动力学模型dynamically equivalent model底座chassis低副lower pair点划线chain dotted line(疲劳)点蚀pitting垫圈gasket垫片密封gasket seal碟形弹簧belleville spring顶隙bottom clearance定轴轮系ordinary gear train; gear train with fixed axes动力学dynamics动密封kinematical seal动能dynamic energy动力粘度dynamic viscosity动力润滑dynamic lubrication动平衡dynamic balance动平衡机dynamic balancing machine动态特性dynamic characteristics动态分析设计dynamic analysis design动压力dynamic reaction动载荷dynamic load端面transverse plane端面参数transverse parameters端面齿距transverse circular pitch端面齿廓transverse tooth profile端面重合度transverse contact ratio端面模数transverse module端面压力角transverse pressure angle锻造forge对称循环应力symmetry circulating stress对心滚子从动件radial (or in-line ) roller follower对心直动从动件radial (or in-line ) translating follower对心移动从动件radial reciprocating follower对心曲柄滑块机构in-line slider-crank (or crank-slider) mechanism 多列轴承multi-row bearing多楔带poly V-belt多项式运动规律polynomial motion多质量转子rotor with several masses惰轮idle gear额定寿命rating life额定载荷load ratingII 级杆组dyad发生线generating line发生面generating plane法面normal plane法面参数normal parameters法面齿距normal circular pitch法面模数normal module法面压力角normal pressure angle法向齿距normal pitch法向齿廓normal tooth profile法向直廓蜗杆straight sided normal worm法向力normal force反馈式组合feedback combining反向运动学inverse ( or backward) kinematics反转法kinematic inversion反正切Arctan范成法generating cutting仿形法form cutting方案设计、概念设计concept design, CD防振装置shockproof device飞轮flywheel飞轮矩moment of flywheel非标准齿轮nonstandard gear非接触式密封non-contact seal非周期性速度波动aperiodic speed fluctuation非圆齿轮non-circular gear粉末合金powder metallurgy分度线reference line; standard pitch line分度圆reference circle; standard (cutting) pitch circle 分度圆柱导程角lead angle at reference cylinder分度圆柱螺旋角helix angle at reference cylinder分母denominator分子numerator分度圆锥reference cone; standard pitch cone分析法analytical method封闭差动轮系planetary differential复合铰链compound hinge复合式组合compound combining复合轮系compound (or combined) gear train复合平带compound flat belt复合应力combined stress复式螺旋机构Compound screw mechanism复杂机构complex mechanism杆组Assur group干涉interference刚度系数stiffness coefficient刚轮rigid circular spline钢丝软轴wire soft shaft刚体导引机构body guidance mechanism刚性冲击rigid impulse (shock)刚性转子rigid rotor刚性轴承rigid bearing刚性联轴器rigid coupling高度系列height series高速带high speed belt高副higher pair格拉晓夫定理Grashoff`s law根切undercutting公称直径nominal diameter高度系列height series功work工况系数application factor工艺设计technological design工作循环图working cycle diagram工作机构operation mechanism工作载荷external loads工作空间working space工作应力working stress工作阻力effective resistance工作阻力矩effective resistance moment公法线common normal line公共约束general constraint公制齿轮metric gears功率power功能分析设计function analyses design共轭齿廓conjugate profiles共轭凸轮conjugate cam构件link鼓风机blower固定构件fixed link; frame固体润滑剂solid lubricant关节型操作器jointed manipulator惯性力inertia force惯性力矩moment of inertia ,shaking moment 惯性力平衡balance of shaking force惯性力完全平衡full balance of shaking force惯性力部分平衡partial balance of shaking force 惯性主矩resultant moment of inertia惯性主失resultant vector of inertia冠轮crown gear广义机构generation mechanism广义坐标generalized coordinate轨迹生成path generation轨迹发生器path generator滚刀hob滚道raceway滚动体rolling element滚动轴承rolling bearing滚动轴承代号rolling bearing identification code 滚针needle roller滚针轴承needle roller bearing滚子roller滚子轴承roller bearing滚子半径radius of roller滚子从动件roller follower滚子链roller chain滚子链联轴器double roller chain coupling滚珠丝杆ball screw滚柱式单向超越离合器roller clutch过度切割undercutting函数发生器function generator函数生成function generation含油轴承oil bearing耗油量oil consumption耗油量系数oil consumption factor赫兹公式H. Hertz equation合成弯矩resultant bending moment合力resultant force合力矩resultant moment of force黑箱black box横坐标abscissa互换性齿轮interchangeable gears花键spline滑键、导键feather key滑动轴承sliding bearing滑动率sliding ratio滑块slider环面蜗杆toroid helicoids worm环形弹簧annular spring缓冲装置shocks; shock-absorber灰铸铁grey cast iron回程return回转体平衡balance of rotors混合轮系compound gear train积分integrate机电一体化系统设计mechanical-electrical integration system design 机构mechanism机构分析analysis of mechanism机构平衡balance of mechanism机构学mechanism机构运动设计kinematic design of mechanism机构运动简图kinematic sketch of mechanism机构综合synthesis of mechanism机构组成constitution of mechanism机架frame, fixed link机架变换kinematic inversion机器machine机器人robot机器人操作器manipulator机器人学robotics技术过程technique process技术经济评价technical and economic evaluation技术系统technique system机械machinery机械创新设计mechanical creation design, MCD机械系统设计mechanical system design, MSD机械动力分析dynamic analysis of machinery机械动力设计dynamic design of machinery机械动力学dynamics of machinery机械的现代设计modern machine design机械系统mechanical system机械利益mechanical advantage机械平衡balance of machinery机械手manipulator机械设计machine design; mechanical design机械特性mechanical behavior机械调速mechanical speed governors机械效率mechanical efficiency机械原理theory of machines and mechanisms机械运转不均匀系数coefficient of speed fluctuation机械无级变速mechanical stepless speed changes基础机构fundamental mechanism基本额定寿命basic rating life基于实例设计case-based design,CBD基圆base circle基圆半径radius of base circle基圆齿距base pitch基圆压力角pressure angle of base circle基圆柱base cylinder基圆锥base cone急回机构quick-return mechanism急回特性quick-return characteristics急回系数advance-to return-time ratio急回运动quick-return motion棘轮ratchet棘轮机构ratchet mechanism棘爪pawl极限位置extreme (or limiting) position极位夹角crank angle between extreme (or limiting) positions计算机辅助设计computer aided design, CAD计算机辅助制造computer aided manufacturing, CAM计算机集成制造系统computer integrated manufacturing system, CIMS 计算力矩factored moment; calculation moment计算弯矩calculated bending moment加权系数weighting efficient加速度acceleration加速度分析acceleration analysis加速度曲线acceleration diagram尖底从动件knife-edge follower间隙backlash间歇运动机构intermittent motion mechanism 减速比reduction ratio减速齿轮、减速装置reduction gear减速器speed reducer减摩性anti-friction quality渐开螺旋面involute helicoid渐开线involute渐开线齿廓involute profile渐开线齿轮involute gear渐开线发生线generating line of involute渐开线方程involute equation渐开线函数involute function渐开线蜗杆involute worm渐开线压力角pressure angle of involute渐开线花键involute spline简谐运动simple harmonic motion键key键槽keyway交变应力repeated stress交变载荷repeated fluctuating load交叉带传动cross-belt drive交错轴斜齿轮crossed helical gears胶合scoring角加速度angular acceleration角速度angular velocity角速比angular velocity ratio角接触球轴承angular contact ball bearing角接触推力轴承angular contact thrust bearing 角接触向心轴承angular contact radial bearing 角接触轴承angular contact bearing铰链、枢纽hinge校正平面correcting plane接触应力contact stress接触式密封contact seal阶梯轴multi-diameter shaft结构structure结构设计structural design截面section节点pitch point节距circular pitch; pitch of teeth节线pitch line节圆齿厚thickness on pitch circle节圆直径pitch diameter节圆锥pitch cone节圆锥角pitch cone angle解析设计analytical design紧边tight-side紧固件fastener径节diametral pitch径向radial direction径向当量动载荷dynamic equivalent radial load径向当量静载荷static equivalent radial load径向基本额定动载荷basic dynamic radial load rating 径向基本额定静载荷basic static radial load tating径向接触轴承radial contact bearing径向平面radial plane径向游隙radial internal clearance径向载荷radial load径向载荷系数radial load factor径向间隙clearance静力static force静平衡static balance静载荷static load静密封static seal局部自由度passive degree of freedom矩阵matrix矩形螺纹square threaded form锯齿形螺纹buttress thread form矩形牙嵌式离合器square-jaw positive-contact clutch 绝对尺寸系数absolute dimensional factor绝对运动absolute motion绝对速度absolute velocity均衡装置load balancing mechanism抗压强度compression strength开口传动open-belt drive开式链open kinematic chain开链机构open chain mechanism可靠度degree of reliability可靠性reliability可靠性设计reliability design, RD空气弹簧air spring空间机构spatial mechanism空间连杆机构spatial linkage空间凸轮机构spatial cam空间运动副spatial kinematic pair空间运动链spatial kinematic chain空转idle宽度系列width series框图block diagram雷诺方程Reynolds‘s equation离心力centrifugal force离心应力centrifugal stress离合器clutch离心密封centrifugal seal理论廓线pitch curve理论啮合线theoretical line of action隶属度membership力force力多边形force polygon力封闭型凸轮机构force-drive (or force-closed) cam mechanism 力矩moment力平衡equilibrium力偶couple力偶矩moment of couple连杆connecting rod, coupler连杆机构linkage连杆曲线coupler-curve连心线line of centers链chain链传动装置chain gearing链轮sprocket sprocket-wheel sprocket gear chain wheel联组V 带tight-up V belt联轴器coupling shaft coupling两维凸轮two-dimensional cam临界转速critical speed六杆机构six-bar linkage龙门刨床double Haas planer轮坯blank轮系gear train螺杆screw螺距thread pitch螺母screw nut螺旋锥齿轮helical bevel gear螺钉screws螺栓bolts螺纹导程lead螺纹效率screw efficiency螺旋传动power screw螺纹thread (of a screw)螺旋副helical pair螺旋机构screw mechanism螺旋角helix angle螺旋线helix ,helical line绿色设计green design design for environment马耳他机构Geneva wheel Geneva gear马耳他十字Maltese cross脉动无级变速pulsating stepless speed changes脉动循环应力fluctuating circulating stress脉动载荷fluctuating load铆钉rivet迷宫密封labyrinth seal密封seal密封带seal belt密封胶seal gum密封元件potted component密封装置sealing arrangement面对面安装face-to-face arrangement面向产品生命周期设计design for product`s life cycle, DPLC 名义应力、公称应力nominal stress模块化设计modular design, MD模块式传动系统modular system模幅箱morphology box模糊集fuzzy set模糊评价fuzzy evaluation模数module摩擦friction摩擦角friction angle摩擦力friction force摩擦学设计tribology design, TD摩擦阻力frictional resistance摩擦力矩friction moment摩擦系数coefficient of friction摩擦圆friction circle磨损abrasion wear; scratching末端执行器end-effector目标函数objective function耐腐蚀性corrosion resistance耐磨性wear resistance挠性机构mechanism with flexible elements挠性转子flexible rotor内齿轮internal gear内力internal force内圈inner ring能量energy能量指示图viscosity逆时针counterclockwise (or anticlockwise)啮出engaging-out啮合engagement, mesh, gearing啮合点contact points啮合角working pressure angle啮合线line of action啮合线长度length of line of action啮入engaging-in牛头刨床shaper凝固点freezing point; solidifying point扭转应力torsion stress扭矩moment of torque扭簧helical torsion spring诺模图NomogramO 形密封圈密封O ring seal盘形凸轮disk cam盘形转子disk-like rotor抛物线运动parabolic motion疲劳极限fatigue limit疲劳强度fatigue strength偏置式offset偏( 心) 距offset distance偏心率eccentricity ratio偏心质量eccentric mass偏距圆offset circle偏心盘eccentric偏置滚子从动件offset roller follower偏置尖底从动件offset knife-edge follower偏置曲柄滑块机构offset slider-crank mechanism 拼接matching评价与决策evaluation and decision频率frequency平带flat belt平带传动flat belt driving平底从动件flat-face follower平底宽度face width平分线bisector平均应力average stress平均中径mean screw diameter平均速度average velocity平衡balance平衡机balancing machine平衡品质balancing quality平衡平面correcting plane平衡质量balancing mass平衡重counterweight平衡转速balancing speed平面副planar pair, flat pair平面机构planar mechanism平面运动副planar kinematic pair平面连杆机构planar linkage平面凸轮planar cam平面凸轮机构planar cam mechanism平面轴斜齿轮parallel helical gears普通平键parallel key其他常用机构other mechanism in common use起动阶段starting period启动力矩starting torque气动机构pneumatic mechanism奇异位置singular position起始啮合点initial contact , beginning of contact气体轴承gas bearing千斤顶jack嵌入键sunk key强迫振动forced vibration切齿深度depth of cut曲柄crank曲柄存在条件Grashoff`s law曲柄导杆机构crank shaper (guide-bar) mechanism曲柄滑块机构slider-crank (or crank-slider) mechanism 曲柄摇杆机构crank-rocker mechanism曲齿锥齿轮spiral bevel gear曲率curvature曲率半径radius of curvature曲面从动件curved-shoe follower曲线拼接curve matching曲线运动curvilinear motion曲轴crank shaft驱动力driving force驱动力矩driving moment (torque)全齿高whole depth权重集weight sets球ball。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Heat treatment of metalIn industry today there are more than a thousand different metals being used to manufacture products. The modem automobile has more than one hundred different metals used in its construction. An attempt will be made in this passage to give an understanding of the basic classification of metals.Metals were formerly thought to be those elements that had a metallic luster and were good conductor of heat and electricity. Actually, metals are generally defined as those elements whose hydroxides from bases (such as sodium or potassium).the nonmetals* hydroxides from acids (such as sulphur). Metals may exist as pure elements. When two or more metallic elements are combined,they form a mixture called an alloyThe term alloy is used to identify any metallic system. In metallurgy it is a substance, with metallic properties, that is composed of two or more elements, in timately mixed. Of these elements one must be a metal. Plain carbon steel, in the sense, is basically an alloy of iron and carbon. Other elements are present in the form of impurities. However, for commercial purposes, plain carbon steel is not classified as an alloy steel.Alloy maybe further classified as ferrous and nonferrous. Ferrous alloys contain iron. Nonferrous alloys do not contain iron.All commercial varieties of iron and steel are alloys. The ordinary steels are thought of as iron-carbon alloys. However, practically all contain silicon and manganese as well. In addition, there are thousands of recognized alloy steels. Examples are special tool steels, steels for castings, forgings, and rolled shapes. The base metal for all these is iron.Steels arc often called by the principal alloying clement present. Examples arc silicon steel, manganese steel, nickel steel, and tungsten steel. Even nonferrous alloys may contain iron in a small amount, as impurities. Some of the nonferrous alloys are bronze, brass, and monel.Although pure metals solidify at a constant temperature, alloys do not. The first nuclei have a tendency to form at a higher temperature than that at which complete solidification occurs. Each element in an alloy has its own peculiarities relative to temperature. Thus, the change in temperature as solidification progresses causes the solid being formed to change in chemical composition.Many alloying elements dissolve in the base metal in different proportions in liquefied and solidified steels. The proportion of the alloying clement that remainsin solid solutions has a tendency to vary with the temperature and grain structure of the allov that is formed.Nonferrous metals are seldom formed in the pure state. They must be separated from the gangue before the ore can be reduced. Thus, a process known as ore-dressing is performed. Metals and metal compounds arc heavier than the ganguc. They settle to the bottom if such a mixture has been agitated in water. This process is similar to the method used by the early miners who panned for gold. However, refinements have been developed to speed up the accumulation of metal compound of metal compounds by using this principal.The reverberatory furnace is the type most often used in the smelting of nonferrous metals. This furnace is constructed of refractory brick with a steel structure on the outside. The charge is placed in the furnace and heated indirectly by the flame. Slag inducers or fluxes are added to the charge to reduce oxidation.Properties of metalsMetals have properties that distinguish them from other materials. The most important of these properties is strength, or the ability to support weight without bending or breaking. This property combined with toughness, or ability to bend without breaking, is important. Metals also have advantages regarding resistance to corrosion. They arc responsive to heat treatment.Metals can be cast into many shapes and sizes. They can be welded, hardened,and softened. Metals also possess another important property-recycling and reuse. When a particular product is discarded, it can be cut into convenient sections. These sections can be put into a furnace, remelted, and used in another product.The properties of metals may be classified in three categories: chemical properties, mechanical properties, and physical properties. Here we will emphasize the primary mechanical properties of metals. In understanding the related areas of metal working and methods used today, the mechanical properties of metals are of the utmost importance.The hardness of metals varies greatly. Some, like lead, can be indented easily. Others like tungsten carbide, approach diamond hardness. They arc of great value as dies for cutting tools of various types. Heat treatment causes changes in the hardness. Annealed tool steel can readily be machined. Often, this is difficult after it has been hardened and tempered. Annealed brass is comparatively soft. When cold-worked the hardness is greatly increased.A tough metal possesses very high strength. It also has the capability to deform permanently and resist rupture. Toughness enables the metal to survive shock or impact without fracture.The strength of a metal is its ability to resist deformation or rupture. In certain items, a combination of strength and plasticity is desirable. Machine tools are an example.AnnealingThe word anneal has been used before to describe heat-treating processes for softening and regaining ductility in connection with cold working of material. It has a similar meaning when used in connection with the heat treating of allotropic materials. The purpose of full annealing is to decrease hardness, increase ductility, and sometimes improve machinability of highcarbon steels that might otherwise be difficult to cut. The treatment is also used to relieve stresses, refine grain size, and promote uniformity of structure throughout the material.Machinability is not always improved by annealing. The word machinability is used to describe several interrelated factors, including the ability of a material to be cut with a good surface finish. Plain low carbon steel, when fully annealed, are soft and relatively weak, offering litter resistance to cutting, but usually having sufficient ductility and toughness that a cut chip tends to pull and tear the surface from which it is removed, leaving a comparatively poor quality surface, which results in a poor machinablity of many of the higher plain carbon and most of the alloy steels can usually be greatly improved by annealing, as they are often too hard and strong to be easily cut at any but their softest condition.The procedure for annealing hypoeutectoid steel is to heat slowly to approximately 60 "C above the Ac3 line, to soak for a long enough period that the temperature equalizes throughout the material and homogeneous austenite is formed, and then to allow the steel to cool very slowly by cooling it in the furnace or burying it in the maximum ferrite and the coarsest pearlite to place the steel in its softest, most ductile, and least strained condition.NormalizingThe purpose of normalizing is somewhat similar to that of annealing with theExceptions that the steel is not to its softest condition and the pearlite is left rather fine instead of coarse. Refinement of grain size, relief of internalstresses, and improvement of structural uniformity together with recovery of someductility provide high toughness qualities in normalized steel. The process is frequently used for improvement of machinability and for stress relief to reduce distortion that might occur with partial machining or aging.The procedure for normalizing is to austenitize by slowly heating to approximate 80c above the AC3 or Accm3 temperature for hypoeutectoid or hypereutectoid.Steels, respectively; providing soaking time for the formation of austcnitc; and cooling slowly in still air. Note that the steels with more carbon than the eutectoid composition arc heated above the Accm instead of the Ac 13 used for annealing. The purpose of normalizing is to attempt to dissolve all the cementite during austenitization to eliminate, as for as possible, the settling of hard, brittle iron carbide in the grain boundaries. The desired decomposition products are smallgrained, fine pearlite with a minimum of free ferrite and free cementite.SphcroidizingMinimum hardness and maximum ductility of steel can be produced by a process called spheroidizing, which causes the iron carbide to form in small spheres or nodules in a ferrite matrix. In order to start with small grains that spheroidize more readily, the process is usually performed on normalized steel. Several variations of processing are used, but all require the holding of the steel near the Al temperature (usually slightly bclow)for a number of hours to allow the iron carbide to form on its more stable and lower energy state of small, rounded globules.The main need for the process is to improve the machinability quality of high carbon steel and to pretreat hardened steel to help produce greater structural uniformity after quenching because of the lengthy treatment time and therefore rather high cost, sphcroidizing is not performed nearly as annealing or normalizing. Hardening of steelMost of the heat treatment hardening processes for the steel is the based on the production of high percentages of martensite .The first step, therefore, is that Used for most of the other heat-treating processes-treatment to produce austenite.Hypoeutectoid steels are heated to approximately 60t: above the Ac3 temperature and allowed to soak to obtain temperature uniformity and austcnitchomogeneity. Hypereutectoid steels are soaked at about 60'c above the Acl temperature, which leavesSome iron carbide present in the material.The second step involves cooling rapidly in an attempt to avoid pearlite transformation by missing the nose of the I-T curve. The cooling rate is determined by the temperature and the ability of the quenching media to carry heat away from the surface of the material being quenched and by the conduction of heat through the material itself. Table 11 -1 shows some of the commonly used media and the method of application to remove heat, arranged in order of decreasing cooling ability.High temperature gradients contribute to high stresses that cause distortion and cracking, so the quench should only as extreme as is necessary to produce the desired structure. Care must be exercised in quenching that heat is removed uniformly to minimize thermal stresses. For example, along slender bar should be end-quenched, that is, inserted into the quenching medium vertically so that the entire section issubjected to temperature change at one time. If a shape of this kind were to be quenched in a way that caused one side to drop in temperature before the other, change of dimensions would likely cause high stresses producing plastic flow and permanent distortion.Several special types of quench are conducted to minimize quenching stresses and decrease the tendency for distortion and cracking. One of these is called martempering and consists of quenching and austenitized steel in a salt at a temperature above that needed for the start of martensite formation (Ms). The steel being quenched is in this bath until it is of uniform temperature but is removed before there is time for formation of bainite to start. Completion of the cooling in air then caused the same hard martenside that would have formed with quenching from the high temperature, but the high themal or "quench" stresses that are the primary source of cracks and warping will have been eliminated.A similar process performed at a slightly higher temperayure is called austempering. In this case the steel is the formation of bainite. The bainite structure is not as hard as the marten site that could be formed form the same form composition, but in addition n to reducing the thermal shock to which the steel would be subjected under normal hardening procedures, it is unnecessary to perform any further treatment to develop good impact resistance in the high hardness range.TemperingA third step usually required to condition hardened steel for service is tempering, or as it is sometimes referred to, drawing. With the exception of austempered steel, which is frequently used in the as-hardened condition, most steel arc not serviceable "as quenched", the drastic cooling to produce martensite causes the steel to be very hard and to contain both macroscopic and macroscopic internal stresses with the result that the material has little ductility and extreme brittle ness reduction of these faults is accomplished by reheating the steel to some point below the AI (lower transformation )tcmpcraturc. Structural changes caused by tempering of hardened steel are functions of both time and temperature, with temperature being the most important. It should be emphasized that tempering is not a hardening process, but is, instead, the reverse. A tempered steel is one that has been hardened by heat treatment and then stress relieved, softened, and provided with increased ductility by reheating in the tempered or drawing procedure.The magnitude of the structural changes and the change of properties caused by tempering depend upon the temperature to which the steel is reheated. The higher the temperature, the greater the effect, so the choice of temperature will generally depend on willingness to sacrifice hardness and strength to gain ductility and toughness. Reheating to below 100 c has little noticeable effect on hardened plain carbon steel. Between 100'C and 200"C, there is evidence of some structural changes. Above 200"C marked changes in structure and properties appear. Prolonged heating at just under the Al temperature will result in a sphcroidized structure to that produced by the spheroidizing process.In commercial tempering the temperature range of 250'C-425'C is usually avoided because of an unexplained embrittlement, or loss of ductility, that often occurs with steels tempered in this range. Certain alloy steels also develop a "temper brittlcncss" in the temperature range of 425*C-600'C, particularly when cooled slowly from or through this range of temperature. When high temperature tempering is necessary for these steels, they are usually heated to above 600*c and quenched for rapid cooling. Quenches from this temperature, of course, do not cause hardening because austenitization has not been accomplished.As wc know, casting is a mechanical working process that forming a molten material into a desired shape by pouring it into a mold and letting it harden. When metal is not cast in a desired manner, it is formed into special shapes by mechanical working processes. Several factors must be considered when determining whether adesired shape is to be cast or formed by mechanical working. If the shape is very complicated, casting will be necessary to avoid expensive machining of mechanically formed parts. On the other hand, if strength and quality of material are the prime factors in a given part, a cast will be unsatisfactory. For this reason, steel castings arc seldom used in aircraft work.There are there basic methods of metal-working. They are hot working, cold working, and extruding. The process chosen for a particular application depends upon the metal involved and the part required, although in some distances you might employ both hot5-and cold-working methods in making a single part.Almost all steel is hot-working from the ingot into some form from which it is either hot-or cold-worked to the finished shape. When an ingot is stripped from its mold, its surface is solid, but the interior is still molten. The ingot is then placed in s soaking pit, which retards loss of heat, and the molten interior gradually solidifies. After soaking, the temperature is equalized throughout the ingot, which is then reduced to intermediate size by rolling, making it more readily handled.Hot working is the process in which the ingot is deformed mechanically into a desired shape. Hot working is usually performed at an elevated temperature. At high temperature, scaling and oxidation exist. Scaling and oxidation produce undesirable surface finish. Often times, most ferrous metals need to be cold-worked after hot working in order to improve the surface finish.The main principle behind hot working is to cause plastic deformation within the material. The amount of force needed to perform hot working is normally less than that for cold working. As such, the mechanical properties of the material remain unchanged during hot working. The reason that the properties of the materials are unaltered comes from the fact that the deformation is performed above the metal recrystallization temperature. Plastic deformation occurs with metals when deformed at above the recrystallization temperature. Plastic deformation occurs with metals when deformed at above the recrystallization temperature without any strain hardening. As a matter of fact, the metal usually experiences a decrease in yield strength when hot-working. Therefore, it is possible to hot-work the metal without causing any fracture.Hot working has the following advantage:Elimination of porosity.Uniform distribution of impurities.Refinement of coarse or columnar grain-better physical properties.Lesser energy requirement to deform the metal into shape.Disadvantages of hot working Lower dimensional accuracy.Higher total energy required (due to thermal energy to heat the work-piece). Work surface oxidation (scale), poorer surface finish, shooter tool lifeThere are generally two types of hot working process: rolling and forging. Rolling is a process whereby the shape of the hot metal is altered by the action of the rollers which acts to "squeeze" the hot metal into desired shape and thickness. One advantage effect of hot rolling is the fact that there is a grain refinement. Refined grain usually possesses better physical properties.Forging is another hot working method. In forging, the metal is pounded by hammer that or squeezed between a pair of shaped dies. The die acts as a hammer that can "pound" the hot metal into shape. The metal is desired. Forging is done either by pressing or hammering the heated steel until the desired shape is obtained.Complicated sections that cannot be rolled, or sections of which only a small quantity is required, arc usually forged. Forging of steel is a mechanical working of the metal above the critical range to shape the metal as desired. Forging is done either by pressing or hammering the heated steel until the desired shape is obtained.Pressing is used when the parts to be forged are large and heavy, and this process also replace hamming where high-grade steel is required. Since a press is show acting, its force is uniformly transmitted to the exterior to give the best possible structure as well as the exterior to give the best possible structure throughout.Hamming can be used only on relatively small piece. Since hamming transmits its force almost instantly, its effect is limited to a small depth. Thurs, it is necessary to use a very heavy hammer or to subject the part to repeated blows to ensure complete working of the section. If the force applied is too weak to reach the center, the finessed forging surface will be convex or bulged. The advantage of hammering is that the operator has control over the amount of pressure applied and the finishing temperature, and is able to produce parts of the highest grade.This type of forging is usually referred to as smith forging, and it is used extensively where only a small number of parts are needed. Considerable machining and saving when a part is smith forged to approximately the finished shape.。
