低合金钢机械性能
低合金钢铸件标准

低合金钢铸件标准一、概述低合金钢铸件是一种具有优良机械性能和化学成分的合金铸件,广泛应用于机械、建筑、船舶、汽车等领域。
本标准规定了低合金钢铸件的机械性能标准、化学成分标准和表面质量标准等方面的要求。
二、机械性能标准1.抗拉强度:低合金钢铸件应具有一定的抗拉强度,根据不同的使用场合和要求,抗拉强度应符合相关标准的规定。
2.屈服强度:低合金钢铸件应具有一定的屈服强度,以确保铸件在使用过程中不会过早发生塑性变形。
3.伸长率:低合金钢铸件应具有一定的伸长率,以使其在承受冲击载荷时具有较好的塑性变形能力。
4.硬度:低合金钢铸件应具有一定的硬度,以使其在使用过程中具有良好的耐磨性和抗疲劳性能。
5.冲击韧性:低合金钢铸件应具有一定的冲击韧性,以确保在使用过程中能够承受冲击载荷而不发生断裂。
三、化学成分标准1.碳:低合金钢铸件应含有适量的碳元素,以使其具有较好的强度和硬度。
2.硅:低合金钢铸件应含有适量的硅元素,以提高其抗氧化性和耐腐蚀性。
3.锰:低合金钢铸件应含有适量的锰元素,以增强其强度和硬度。
4.磷:低合金钢铸件应控制磷元素的含量,以避免影响其塑性和韧性。
5.硫:低合金钢铸件应控制硫元素的含量,以避免影响其耐腐蚀性和加工性能。
6.铬:低合金钢铸件可含有适量的铬元素,以提高其耐腐蚀性和抗氧化性。
7.钼:低合金钢铸件可含有适量的钼元素,以提高其强度和韧性。
8.其他元素:根据需要,低合金钢铸件可含有适量的其他合金元素,以满足特定的性能要求。
四、表面质量标准1.表面平整度:低合金钢铸件表面应平整,无明显的凸起、凹陷、气孔等缺陷。
2.表面光洁度:低合金钢铸件表面应具有一定的光洁度,以使其在使用过程中具有良好的抗腐蚀性能和外观效果。
3.表面处理:根据需要,低合金钢铸件表面可进行涂层、喷丸等处理,以提高其抗腐蚀性能和使用寿命。
低合金钢标准(一)

低合金钢标准(一)低合金钢标准简介低合金钢是一种具有较低碳含量和比较高含量的合金元素的钢材。
根据不同的标准,低合金钢可以有各种不同的化学成分和机械性能。
本文将介绍一些常见的低合金钢标准。
ASTM A572 标准•ASTM A572 是美国材料与试验协会(American Society for Testing and Materials)发布的一个低合金高强度钢标准。
•该标准适用于各种结构用钢材,包括板材、型钢和焊接材料等。
•ASTM A572 钢材的强度达到了345 MPa至450 MPa,具有良好的可焊性和韧性。
GB/T 1591 标准•GB/T 1591 是中国国家标准(Guo Biao/Ti标准)的代号,是中国低合金高强度结构钢的标准之一。
•标准规定了化学成分、机械性能和技术要求等。
•GB/T 1591 钢材可以分为Q345A、Q345B、Q345C、Q345D和Q345E 等不同牌号,每个牌号的化学成分和强度都有所不同。
EN 10025 标准•EN 10025 是欧洲标准化组织(European Committee for Standardization)发布的一个涵盖多个低合金结构钢的系列标准。
•EN 10025 标准包括多个牌号,例如S275、S355、S420和S460等,每个牌号的化学成分、机械性能和可接受的焊接性都有所不同。
•这些钢材通常用于建筑、桥梁和船舶等领域。
JIS G3106 标准•JIS G3106 是日本工业标准(Japanese Industrial Standards)制定的一个低合金高强度结构钢标准。
•标准涵盖了SM400、SM490和SM570等不同牌号的钢材。
•JIS G3106 钢材具有较高的强度和优异的焊接性能,被广泛应用于建筑、机械和船舶制造等领域。
总结低合金钢是一类重要的结构材料,其标准的制定对于材料的选择、设计和制造具有重要意义。
ASTM A572、GB/T 1591、EN 10025和JISG3106 是常见的低合金钢标准,每个标准都有其特定的适用领域和要求。
低合金钢冷轧窄钢带的金相组织与机械性能研究

低合金钢冷轧窄钢带的金相组织与机械性能研究引言:低合金钢冷轧窄钢带是一种具有广泛用途的材料,在工业生产中被广泛使用。
研究低合金钢冷轧窄钢带的金相组织与机械性能,对于优化材料应用、提高产品质量具有重要意义。
本文将从金相组织和机械性能两个方面对低合金钢冷轧窄钢带进行研究,并分析其相关影响因素。
一、低合金钢冷轧窄钢带的金相组织1.1 低合金钢冷轧窄钢带的组织形态低合金钢冷轧窄钢带的金相组织通常包括铁素体、珠光体和莱氏体。
其中,铁素体是主要组织,珠光体是块状组织,莱氏体是细小的弯曲形态。
1.2 金相组织的形成原因低合金钢冷轧窄钢带的金相组织形成受多种因素影响,包括成分设计、热处理工艺和冷变形处理等。
成分设计可以通过调整合金元素含量实现对金相组织的控制,热处理工艺可以改变材料的相变行为,而冷变形处理则可以促进组织的细化。
1.3 影响金相组织的因素低合金钢冷轧窄钢带的金相组织受多种因素的影响,如碳含量、合金元素含量、残余应力等。
碳含量的增加可以增强钢的硬度和强度,但过高的碳含量可能导致脆性增加。
合金元素的添加可以改变钢的相组成,进而影响其力学性能。
残余应力是冷变形过程中产生的,对材料的力学性能和稳定性具有一定影响。
二、低合金钢冷轧窄钢带的机械性能2.1 机械性能的测试方法低合金钢冷轧窄钢带的机械性能主要包括强度、延伸率和硬度等。
常用的测试方法有拉伸试验、硬度试验和冲击试验等。
拉伸试验可以评估材料的屈服强度、抗拉强度和延伸率等力学性能指标。
硬度试验可以评估材料的硬度,它与材料的强度有一定关系。
冲击试验可以评估材料的吸收冲击能力,用以衡量其韧性。
2.2 机械性能的影响因素低合金钢冷轧窄钢带的机械性能受多种因素影响,例如金相组织、热处理工艺和冷变形工艺等。
金相组织的细化可以提高材料的强度和硬度,而过高的冷变形率可能导致材料的脆性增加。
热处理工艺可以通过热处理参数的选择来改变材料的相组成和晶粒形态,进而影响机械性能。
aisi 8630低合金钢锻件(80k)材料技术要求

以下是AISI 8630低合金钢锻件(80K)的材料技术要求:
1. 化学成分要求:
- 碳含量:0.28-0.33%
- 硅含量:0.15-0.35%
- 锰含量:0.70-0.90%
- 磷含量:不超过0.035%
- 硫含量:不超过0.040%
- 铬含量:0.40-0.60%
- 镍含量:0.40-0.70%
- 钼含量:0.15-0.25%
2. 机械性能要求:
- 屈服强度(抗拉强度):不低于800 MPa
- 抗拉强度:不低于930 MPa
- 延伸率:不低于10%
- 冲击韧性:温度为-20°C时,平均值应不低于54 J
3. 热处理要求:
- 固溶处理(调质):将锻件加热至860-900°C,保温一段时间后迅速冷却至室温。
该过程旨在提高材料的硬度和强度。
- 回火处理:对固溶处理后的锻件进行加热处理,通常在400-600°C范围内进行保温一段时间后冷却。
该过程旨在降低材料的硬度,提高韧性和可加工性。
4. 检测要求:
- 化学成分分析:通过化学分析方法确保材料符合规定的化学成分范围。
- 机械性能测试:进行拉伸试验、冲击试验等,以验证材料的强度、延伸性和韧性等机械性能。
- 尺寸检测:对锻件的尺寸、形状和表面质量进行检查,确保符合要求。
请注意,以上是一般的技术要求,具体的要求可能会根据不同的标准、规范或应用而有所变化。
建议在实际使用前,参考相关标准或与材料供应商进行确认。
q460d钢材料参数

q460d钢材料参数
Q460D钢材料参数
Q460D是一种高强度低合金钢材料,其主要成分包括碳、硅、锰、磷、硫和铬等元素。
其中,碳的含量为0.18%-0.20%;硅的含量为0.20%-0.60%;锰的含量为 1.00%-1.70%;磷的含量为≤0.030%;硫的含量为≤0.025%;铬的含量为≤0.30%。
Q460D钢材的机械性能表现出色,其屈服强度为460MPa,抗拉强度为550-720MPa,伸长率为17%以上,冲击韧性为-20℃≥34J。
同时,Q460D钢材的焊接性能良好,可采用多种焊接方法进行连接。
此外,Q460D钢材的加工性能也较好,可进行冷弯、热弯、剪切等加工处理。
Q460D钢材的应用范围广泛,可用于制造各种机械设备、建筑结构、轨道交通设备等。
在建筑领域中,Q460D钢材常用于制造大跨度桥梁、高层建筑、压力容器等。
在轨道交通领域中,Q460D钢材可用于制造铁路车辆、高速列车等。
需要注意的是,Q460D钢材的使用需要遵循相应的标准和规范。
在使用过程中,应注意防止钢材的腐蚀、疲劳等问题,保持钢材的完好性和安全性。
Q460D钢材是一种优质的高强度低合金钢材料,具有优良的机械性能和加工性能,适用于多种领域的制造和应用。
中低合金钢测试标准

中低合金钢测试标准1.化学成分测试:中低合金钢的化学成分测试是确定其成分是否符合设计要求的重要方法。
常用的测试标准包括GB/T223.3-2024《钢和合金化学分析方法第三部分:钢铁用的化学分析方法第三部分:钢铁用硫酸盐(过氧化碳硫酸氢盐)称量法》和GB/T223.5-2024《钢和合金化学分析方法第五部分:钛合金中碳和硫量的测定》等。
2.机械性能测试:中低合金钢的机械性能测试是评价其强度和韧性的重要手段。
常用的测试标准包括GB/T228.1-2024《金属材料拉伸试验第1部分:室温试验方法》和GB/T229-2024《金属材料室温弯曲试验方法》等。
其中,拉伸试验可以获取抗拉强度、屈服强度、断裂延伸率等指标,而弯曲试验可以评估材料的弯曲性能和韧性。
3.硬度测试:中低合金钢的硬度测试是评价其抗压能力和耐磨性的重要方法。
常用的测试标准包括GB/T230.1-2024《金属材料洛氏硬度试验第1部分:洛氏硬度试验方法》和GB/T4340.1-2024《金属材料显微硬度试验第1部分:试验方法》等。
硬度测试可以通过测定材料的硬度值来评估其抗压能力和耐磨性,常用的硬度指标包括洛氏硬度、显微硬度等。
4.金相组织测试:5.化学腐蚀测试:6.尺寸精度测试:中低合金钢的尺寸精度测试是评价其几何形状和加工精度的重要手段。
常用的测试标准包括GB/T1184-1996《评定金属表面缺陷尺寸和数量》和GB/T1804-2000《一般被动卡紧零件外圆公差》等。
尺寸精度测试可以通过测量材料的几何形状和保证其加工精度,常用的测量指标包括直径、平面度、圆度等。
总之,对于中低合金钢的测试标准包括化学成分测试、机械性能测试、硬度测试、金相组织测试、化学腐蚀测试和尺寸精度测试。
这些测试标准可以保证中低合金钢的质量和性能,从而满足其在不同领域的使用需求。
低合金钢全面分解

低合金钢全面分解低合金钢是一种含有少量合金元素(通常小于5%)的钢材,由于添加了合金元素,低合金钢相比于普通碳钢具有更好的性能和用途。
下面将对低合金钢的牌号、化学成分、性能和用途进行详细介绍。
一、牌号:低合金钢的牌号有很多种,常见的有15CrMo、20CrMo、35CrMo、42CrMo等。
每个牌号代表着不同的化学成分和性能特点。
二、化学成分:低合金钢的化学成分主要由碳、硅、锰、磷、硫、铬、钼等元素组成。
其中,合金元素的含量较低,但它们的添加可以显著改变钢材的性能。
三、性能:1.机械性能:低合金钢具有很高的强度和硬度,通常比普通碳钢更耐用。
同时,低合金钢还具有良好的可塑性和韧性,不易产生断裂。
2.抗蚀性:通过添加合金元素,低合金钢具有更好的抗蚀性能,能够在潮湿、腐蚀性环境下工作,并且不易生锈。
3.焊接性能:低合金钢具有良好的焊接性能,能够通过各种焊接方法进行连接,方便加工和制造。
四、用途:1.低合金钢常用于制造机械零件和构件,如汽车零部件、飞机零件、船舶零件等。
其高强度和硬度可以提供更好的支撑和承载能力。
2.低合金钢也常用于制造工具和刀具,如刀片、冲压模具等。
由于其硬度较高,可以在高负荷和高温环境下保持稳定的性能。
3.低合金钢还常用于制造石油和天然气开采设备,如钻杆、液压缸等。
其良好的抗蚀性和耐磨性能可以适应苛刻的工作环境。
4.另外,低合金钢还可以用于制造建筑材料、电力设备、化工设备等,其高强度和良好的可塑性能够满足各种使用要求。
综上所述,低合金钢是一种性能优异的钢材,具有高强度、耐蚀、耐磨和良好的可塑性等特点,广泛应用于各个领域。
不同的牌号和化学成分可以满足不同的需求,成为现代工业中不可或缺的材料之一。
ZG30Cr06标准

Q/PMJ 平顶山煤矿机械厂企业标准Q/PMJ铸造低合金钢(ZG30Cr06)技术要求2006.6.发布并实施平顶山煤矿机械厂发布言本标准由平顶山煤矿机械厂铸锻分厂提出。
本标准由平顶山煤矿机械厂研究所归口。
本标准由平顶山煤矿机械厂铸锻分厂起草。
平顶山煤矿机械厂研究所、质量管理处、生产处、热处理、一金工分厂参加起草。
本标准主要起草人:宋德宏。
本标准由平顶山煤矿机械厂副总工程师刘建富审核本标准由平顶山煤矿机械厂总工程师周西杰批准ZG30Cr06)技术要求1 使用范围本标准规定了铸造低合金钢(ZG30Cr06)用于液压支架铸件的技术要求。
2.引用标准下列标准所包含的条文通过本标准引用而成为本标准的条文。
GB11352-89 一般工程用铸造碳钢件 GB222-81 钢铁及合金化学分析方法 GB228-76 金属拉力试验法 GB231 金属布氏硬度试验法 3.技术要求 3.1技术要求 3.1.1合金成分低合金铸钢(ZG30Cr06)的化学成分见表1ZG30C06化学成分 表1 注:碳当量CE 按下式计算,此公式已为国际焊接学会和美国ASTM 学会采用。
CE=(C+Mn/6+Cr/5)%, 碳当量CE 一般控制在≤0.61%。
3.1.2 机械性能低合金铸钢(ZG30Cr06)的机械性能见表2,应根据不同的使用要求进行选择。
成分 C Si Mn Cr S 、P 含量 (%)0.24-0.320.20-0.400.50-0.800.50-0.80≤0.04ZG30Cr06的机械性能 表2注:单铸拉力试棒(标准试棒),拉力试棒随铸件同炉浇注,同炉热处理 。
3.1.3其它性能要求低合金铸钢(ZG30Cr06)铸件的技术要求,除机械性能外,其它性能按一般铸件国家通用标准执行。
热处理状态机械性能(≥)备注σbσsδ5αkHBMPa % J淬火 890℃回火 650℃8006901440 230-270 用于强度指标要求高的非焊接用的机械零件(如连接头、十字头等)淬火 890℃ 回火 690℃700 550 1640 230-270 用于综合机械性能要求较高的需焊接的机械零件(如柱窝、柱帽等)正火850℃ 700 550 1440 230-270 具有较高的机械性能,可以作为 ZG35的升级替代产品,用于受力较大的机械零件。
低碳钢特性及成分
mm
抗拉强度бsN/mm2
屈服点бb
N/mm2
伸长率δs%
180C弯曲试验
d=弯心直径
a=试样厚度
冲击试验
温度°C
V型冲击功(纵向),J
不小于
不小于
09MnV
≤16
430-580
295
23
d=2a
20
27
>16-25
275
d=3a
09MnNb
≤16
410-560
295
24
d=2a
20
27
0.015-0.050
—
—
—
0.045
0.045
09Mn2
≤0.12
1.40-1.80
0.20-0.55
—
—
—
—
—
—
0.045
0.045
12Mn
0.09-0.16
1.10-1.50
0.20-0.55
—
—
—
—
—
—
0.045
0.045
18Nb
0.14-0.22
0.40-0.80
0.17-0.37
—
—
0.020-0.050
—
—
—
—
—
0.045
0.045
15MnTi
0.12-0.18
1.20-1.60
0.20-0.55
—
0.12-0.20
—
—
—
—
0.045
0.045
16MnVb
0.12-0.20
1.00-1.40
0.20-0.55
低合金高强度结构钢牌号
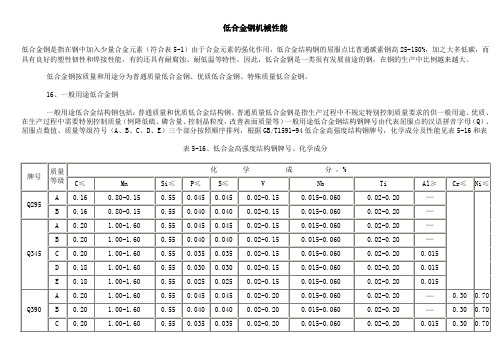
低合金钢机械性能低合金钢是指在钢中加入少量合金元素(符合表5-1)由于合金元素的强化作用,低合金结构钢的屈服点比普通碳素钢高25-150%,加之大多低碳,而具有良好的塑性韧性和焊接性能,有的还具有耐腐蚀、耐低温等特性,因此,低合金钢是一类很有发展前途的钢,在钢的生产中比例越来越大。
低合金钢按质量和用途分为普通质量低合金钢、优质低合金钢、特殊质量低合金钢。
16、一般用途低合金钢一般用途低合金结构钢包括:普通质量和优质低合金结构钢、普通质量低合金钢是指生产过程中不规定特别控制质量要求的供一般用途、优质、在生产过程中需要特别控制质量(例降低硫、磷含量、控制晶粒度,改善表面质量等)一般用途低合金钢结构钢牌号由代表屈服点的汉语拼音字母(Q)、屈服点数值、质量等级符号(A、B、C、D、E)三个部分按照顺序排列,根据GB/T1591-94低合金高强度结构钢牌号,化学成分及性能见表5-16和表表5-16、低合金高强度结构钢牌号、化学成分表5-17、低合金高强度结构钢机械性能在旧标准中低合金结构钢的牌号用两位阿拉伯数字和合金元素符号及数字表示,数字表示平均含碳量(以万分之几计)平均合金含量小于1.5%时,钢号中仅标明元素符号,不标明含量;平均合金含量为1.5-2.49%、2.5-3.49%时,相应写成品2、3……其牌号、化学成分、性能见表5-18和表5-19。
表5-18、一般用途低合金结构钢牌号、化学成分(根据GB1591-881)表5-19、一般用途低合金结构钢机械性能(根据GB1591-88)新旧低合金结构钢标准牌号对照见表5-20表5-20、新旧低合金结构钢标准牌号对照(参考件)一般用途低合金结构钢由于合金元素作用,具有较高强度和韧性,工艺性能较好,生产成本低,应用广泛,大多直接使用,常用于铁路、桥梁、船舶、汽车、压力容器,常用作焊接结构件和机械构件等。
17、其它低合金钢其它低合金钢包括普通质量、优质、特殊质量低合金钢这些钢也都是为某各专用产吕需要生产的专用钢。
低合金钢的防腐蚀和机械性能的研究

Corrosion resistance and mechanicalproperties of low-alloy steels underatmospheric conditionsY.Y.Chen a ,H.J.Tzeng b ,L.I.Wei b ,L.H.Wang c ,J.C.Oung c ,H.C.Shih a,*aDepartment of Materials Science and Engineering,National Tsing Hua University,101Kuang Fu Road,Sec.2,Hsinchu 300,Taiwan,ROC b China Steel Corp.,Kaoshiung,812,Taiwan,ROC c Bldg.52,195-5Chung Hsing Rd.,Section 4,Chutung,Hsinchu 310,Taiwan,ROCReceived 4November 2003;accepted 20April 2004AbstractThe corrosion resistance and mechanical strength of four newly developed low-alloy steels(LAS)were compared with a carbon steel (SS400)and a weathering steel (Acr-Ten A)using alaboratory-accelerated test that involved cyclic wet/dry conditions in a chloride environment(5wt.%NaCl).The new LAS were designated 1605A,1605B,1604A,and 1604B.After72cycles of cyclic corrosion tests,the susceptibility of the steels to corrosion could be listedin the following order based on their weight loss (from high to low):SS400>Acr-TenA >1604B P 1604A >1605B P 1605A.The change in mechanical properties by corrosionwas the least for SS400,Acr-Ten A was second,and effects of corrosion on the mechanicalproperties of the other four low-alloy steels were similar.Finally,the characteristics of the rustlayers on each LAS sample were observed by SEM,and analyzed by FTIR and EPMA.Theresults indicated that most of the rust layers on the test steels were composed of a loose outerrust layer and a dense inner rust layer.The outer rust layer of each steel was composed ofa -FeOOH,c -FeOOH,magnetite (Fe 3O 4),H 2O,and amorphous ferric oxyhydroxide(FeO x (OH)3À2x ,x ¼0–1),while the inner rust layer was composed mainly of Fe 3O 4with alittle a -FeOOH.In addition,it was apparent that the copper and chromium alloying additionswere enriched,respectively,at the rust-layer/substrate interface and in the rust layers.Finally,combining the results of the accelerated tests and the rust layer analysis showed that low-alloy*Corresponding author.Tel.:+886-35-715131;fax:+886-35-710-290.E-mail address:hcshih@.tw (H.C.Shih).0010-938X/$-see front matter Ó2004Elsevier Ltd.All rights reserved.doi:10.1016/j.corsci.2004.04.009/locate/corsciCorrosion Science 47(2005)1001–10211002Y.Y.Chen et al./Corrosion Science47(2005)1001–1021steels,such as1605A and1605B,have better weathering steel properties than Acr-Ten A for use in the humid and salty weather.Ó2004Elsevier Ltd.All rights reserved.Keywords:Atmospheric corrosion;Weathering steel;Accelerated test;Weight loss;Rust layer;FTIR; EPMA1.IntroductionThe most common types of damage that occur to structural steels during their lifetime are fatigue and rusting.Except for stainless steels,which are more costly, almost no uncoated ferrous products can resist the influences of various corrosion factors coming from the atmosphere, e.g.sulfide,chloride,dust,and moisture. However,weathering steel(WS)is a kind of steel that can be maintained for dozens of years at low cost and without coating.It is well known that the enhanced cor-rosion resistance of weathering steel results from the formation of a compact pro-tective,i.e.tightly adherent,rust layer.This protective rust layer will gradually become denser over time and will isolate the steel from various corrosion factors and so reduce the rate of corrosion substantially.In previous studies,the formation of the protective rust layer was shown to be related to the alloying elements(e.g.Cr,P, Cu,Ni,etc.)and to the environmental conditions[1–7].Due to its numerous advantages,WS has potential application in the civil engineering and automobile industries.For example,they are employed in the construction of buildings and bridges and for architectural trim without the necessity of painting,thereby saving appreciable maintenance costs over the life of the structure.Therefore,it makes sense to tailor WS alloys for specific environments and substitute them for the less cor-rosion-resistant common steels wherever applicable.The development of weathering steels dates back to the1930s and they have been used widely since then because of their unique corrosion resistance[8,9].In the 1950s,a WS called Cor-Ten A[8–11]was developed in North America.Its out-standing corrosion resistance is suitable for the North American continental climate, but it does not perform as well in the semi-tropical monsoon climate of an island with high humidity and salinity.The seasonal monsoons bring large amounts of sea salt,and even the higher levels of air pollution can contribute to more aggressive conditions for corrosion.For this reason,it is necessary to develop different types of WS that are suitable locally.Although the real-life exposure test provides the most reliable information on atmospheric corrosion of metals,it is rather time-consuming.However,laboratory accelerated tests produce useful data in a short time and are profitable in assessing the durability of metals in certain specific atmospheres[7].In principle,atmospheric corrosion is primarily ascribed to electrochemical pro-cesses occurring on a metallic surface under wet/dry conditions.Consequently,it is likely that most,if not all,corrosion results from liquid-phase reactions[12].Cyclic wet/dry conditions and ClÀconcentration are important controlling parameters inY.Y.Chen et al./Corrosion Science47(2005)1001–10211003 accelerated tests.According to numerous studies using accelerated tests[6,7,13–15], the rust layers formed on WS are less protective than those on carbon steel(CS) under continuously wet conditions such as when they are buried in soil or totally immersed in water;however,the intermittent wet/dry process facilitates the forma-tion of a protective rust layer in WS that enhances its corrosion resistance.There-fore,a cyclic salt spray and ultraviolet lighting are used to simulate the intermittent wet/dry corrosion environment in chloride-containing atmospheres.Also,the dif-ference between the mechanical and electrochemical properties before and after the accelerated tests are frequently measured.Generally speaking,the corrosion products on the surface of WS are mainly a-FeOOH,c-FeOOH,b-FeOOH,Fe(OH)3,and Fe3O4.These products can coexist partly as crystalline and partly as amorphous structures[6,14].When the steel contacts moisture and corrodes,the initial corrosion product is mainly c-FeOOH, but gradually this transforms to a-FeOOH as a result of the intermittently wet/dry conditions[6,14].The alloying additions in WS result in corrosion products that are denser and more stable than those on CS.Although a-FeOOH and c-FeOOH can also be observed in corrosion products on CS,they are porous in nature since they are not enhanced by the alloying elements,and,in addition,the adhesion is poor between the rust layer and the substrate.Consequently,the corrosive species can readily penetrate the porous rust layers to the substrate where corrosion reactions may proceed endlessly.It is apparent that the rust layers on CS provide little or no protection to the substrate.In this study,we compared the atmospheric corrosion resistance(weathering resistance)of the four low-alloy steels(LAS)that were developed by China Steel Corp.,with two other alloys(SS400and Acr-Ten A)to identify the alloying elements that confer additional corrosion resistance and/or mechanical strength.We also investigated the mechanical properties after these accelerated tests.Finally,we analyzed the morphology,bonding nature,and chemistry of the rust products using SEM,FTIR,and EPMA,respectively.2.Experimental procedures2.1.Test materialsFour LAS,designated1604A,1604B,1605A,and1605B,were compared with the commercial high-phosphorus WS(Acr-Ten A)and CS(SS400).The chemical compositions were analyzed by an inductively coupled plasma-atomic emission spectrometer(ICP-AES)and are listed in Table1.The coiling temperature of1605B was400°C,but the other three steels were coiled at600°C.The test materials were all cut into reduced plate tensile specimens,6.25mm in width,for the accelerated laboratory tests according to ASTM E8M-98[16];specimen dimensions are shown in Fig.1.Fig.2(a)–(e)show the surface microstructures of the four as-received low-alloy steels and Acr-Ten A.They were allfirst mechanically wet ground using a1500 grit SiC paper and then etched in a Nital solution(97%nitric acid+3%alcohol)forTable1Chemical compositions of the test materialsSteel Fe C Si Mn P S Cu Ni Cr Al N Ti 1604A Bal.0.010.008 1.190.060.0080.310.16–0.0330.00280.023 1604B Bal.0.0110.008 1.190.0910.0080.300.16–0.0290.00280.026 1605A Bal.0.091 1.06 1.200.0920.00750.300.160.560.0300.0062–1605B Bal.0.091 1.05 1.200.0910.00750.300.160.560.0300.0062–Acr-Ten A Bal.0.100.440.470.100.0080.310.300.550.020––SS400Bal.0.130.190.810.0150.0080.0630.0490.0210.024––1004 Y.Y.Chenetal. / Corrosion Science 47 (2005) 1001–1021Fig.2.Surface morphology of the as-received low-alloy steels under the scanning electron microscope at a magnification of 1000·:(a)1604A,(b)1604B,(c)1605A,(d)1605B,and (e)Acr-Ten A.Y.Y.Chen et al./Corrosion Science 47(2005)1001–102110051006Y.Y.Chen et al./Corrosion Science47(2005)1001–1021$20s.The microstructures of1604A(Fig.2(a))and1604B(Fig.2(b)),as shown by SEM,are both of pure ferrite;1605A(Fig.2(c))and Acr-Ten A(Fig.2(e))are duplex structures consisting of ferrite and small amounts of pearlite;and1605B(Fig.2(d))is composed of ferrite and bainite.2.2.Alternating wet/dry accelerated corrosion testsPrior to the accelerated cyclic corrosion tests,all the surfaces of the tensile specimens were polished using600-grade emery paper,cleaned ultrasonically in acetone,and then rinsed with distilled water before drying in air.All specimens were put simultaneously into the salt spray tester(Q.U.V.Accelerated Weathering Tester) and were mounted at30°to the horizontal according to the ASTM G50-76speci-fication for simulating natural atmospheric corrosion[17].The accelerated corrosion testing procedure involved cycling between a dry environment and a mist composed of5wt.%($0.86M)NaCl,according to ASTM B117-97[18].Each wet/dry cycle (24h)consisted of a wetting period(8h at35°C,97±1%RH)plus a drying period (16h at60°C,20%RH).The lighting source was an UVB-313tube.The dry/wet ratio was2:1,which was more severe than conditions in a previous study(drying period for18h and wetting period for6h)[19].This was done to determine whether the differences between WS and CS would be more significant than those under the dry/wet ratio of3:1if the time of wetness increased during the24h cycle.On the other hand,the chamber temperature during the drying period was raised from50°C [19]to60°C in order to increase the density of the rust layers[20].NaCl was pro-vided only during the wetting period,and the NaCl concentration in the chamber was controlled to5M.The accelerated corrosion tests lasted for a total of72cycles.2.3.Mechanical properties and weight loss measurementsAfter some pre-determined numbers of testing(e.g.18cycles and45cycles),the tensile specimens were taken out and pulled to failure in a tensile testing machine (Material Testing System810).The strain rate was set at0.1mm/s.The load and elongation were measured continuously by a load cell and electric dial gauge, respectively,the outputs of which were recorded on a computer-controlled X-Y recorder(YEW MODEL3025)until fracture occurred.The stress–strain curves were used to determine the yield strength(YS),the ultimate tensile strength(UTS),and the elongation(%).The results were then plotted versus the number of corrosion cycles to compare the steels tested.In preparation for weight loss measurements after the specimens fractured,cor-rosion products on the specimen surfaces were removed chemically by immersion in a Clark’s solution(100ml HCl,sp gr1.19+20g Sb2O3+50g SnCl2)[21]that was vigorously stirred for$10min at25°C.After corrosion products had been com-pletely removed,the specimens were rinsed with distilled water,dried in air,and then weighed to determine their weight loss.Y.Y.Chen et al./Corrosion Science47(2005)1001–10211007 2.4.Rust products,observation and analysisThroughout the accelerated corrosion tests,the specimens were examined without stripping the rust from the surface.The surface and cross-section of the rust layers were analyzed using various analytical techniques,including scanning electron microscopy(SEM),Fourier transform infrared spectroscopy(FTIR)and electro-probe microanalysis(EPMA).Special case was taken to analyze the interface be-tween the steel substrate and its rust layer,and to understand the distribution of the alloying elements at this interface.3.Results and discussion3.1.Weight lossAlmost all of the rust layers fell offthe tensile specimens as they were being pulled to failure by the MTS810.The remaining rust was removed by immersing the specimens into Clark’s solution and vigorously stirring.The rust-free specimens were then weighed.The results of the relation between weight loss and the test cycles are listed in Fig.3.The weight loss data in this study are expressed as the weight loss per unit surface area.In general,thefinal weight losses are the lowest for1605A and1605B,and the highest for SS400.Therefore,the alloys can be arranged in order of decreasing weight loss,as follows:SS400(pearlite/ferrite)>Acr-Ten A(pearlite/ferrite)>1604B(ferrite)P1604A(ferrite)>1605B(bainite/ferrite)P 1605A(pearlite/ferrite).It is found that the corrosion resistance of steels containing higher chromium and silicon,such as1605A and1605B,need a longer time(>30 Array Fig.3.Relations between the weight loss(mg/cm2)of the steel samples and the test cycles of accelerated cyclic corrosion in a chloride(5wt.%)environment.1008Y.Y.Chen et al./Corrosion Science47(2005)1001–1021cycles)to reveal their corrosion resistance.In other words,the corrosion rate of 1605A and1605B in accelerated tests decreased markedly after$30cycles.This means that,under these severe cyclic wet/dry test conditions,a longer time is nec-essary to form adhesive dense rust layers on high-chromium steels,e.g.1605A, 1605B.A similar phenomenon was observed for Acr-Ten A,but its weathering resistance was less significant than1605A and1605B under this environment.On the other hand,for1604A and1604B(without Cr),the corrosion rates did not decrease with time.This may be explained by the fact that the dense inner rust layer did not appear to adhere tightly to the substrate.The adhesion of rust layers will be dis-cussed later.The lowest weight loss occurred in1604A and1604B at earlier stages of accelerated corrosion(e.g.,<50cycles)is probably because of their pure ferrite microstructure associated with their low carbon content[7,13].Finally,the corrosion (weight loss)rate of the common carbon steel(SS400)containing low chromium did not decrease with an increase in the number of test cycles.The results in Fig.3have been re-plotted in Fig.4using log–log(bi-logarithm) coordinates to reveal a linear relationship between log(weight loss)and log(test cycles)[19].Atmospheric corrosion of WS and CS in the accelerated test followed the well-known bi-logarithmic equation:Log W¼Log XþY Log t or W¼XÂt Yð1Þwhere W is the weight loss(mg/cm2),t is the exposure time(test cycles),and X and Y are constants.Since X is equal to W when time is unityðt¼1Þ,X is considered a measure of the initial corrosion resistance of the metal.On the other hand,Y reflects the change of weight loss with exposure time.Table2gives values of the constants in Eq.(1).From Table2,it can be seen that the Y values for1605A(1.0636)and for 1605B(1.0239)are roughly consistent with the previous observations that the cor-neutralrosion(weight loss)rates for both LASs decrease as time increases.For Array Fig.4.Bi-logarithmic plots of data points(weight loss versus test cycles)from Fig.3.Y.Y.Chen et al./Corrosion Science47(2005)1001–10211009 Table2Linear regression coefficients of Eq.(1)for the steel samples after the cyclic accelerated corrosion tests Coefficient SteelsSS400Acr-Ten A1604A1604B1605A1605B X 1.2599 2.1171 1.3450 1.2264 2.1893 2.6981 Y 1.2709 1.1422 1.1956 1.2209 1.0636 1.0239R20.99860.98360.98640.99420.97730.9784 atmospheric corrosion,the value of Y should not exceed1.However,it is apparent that the Y values here are>1.The constant Y approaches0.5when an ideal diffusion process is the controlling mechanism.Y values>0.5result from an acceleration of the diffusion process when the rust is detached by cracking,erosion,dissolution,etc. Conversely,Y values<0.5arise from a decrease in the diffusion coefficient as the rust layer becomes more and more compact with time.The reason for Y actually being>1 may be that the ratio of wet period/dry period is not optimal.The drying period(16 h)is only twice as long as the wetting period(8h),and a longer drying time would be necessary to allow for the moisture in salty rust layers to become completely dry.In addition,it is possible that the weight loss increases with an increase in the number of test cycles because the presence of thicker rust layers will make it more difficult to dry the surface of the steel.Table2shows the X and Y coefficients for the six steels.Also, the difference between the results shown in Fig.3and Table2for1605A and1605B can be explained by the fact that,for a greater number of test cycles,the protective effect of tightly adhesive rust layers outweighs the negative effect of the rust layers absorbing water.Furthermore,the weathering effect becomes more significant as the number of cycle increases.The values of X and Y obtained from actual exposure tests in the atmosphere can be used to predict the atmospheric corrosion behavior over a longer period of time [22,23].However,there are still some differences between actual atmospheric expo-sure and the laboratory simulation test.In an accelerated corrosion test,the environmental variables are more restricted than those of the actual atmosphere in a real-life situation.X and Y values are mainly influenced by the chloride concentra-tion,temperature,and the wet/dry period ratio in a salt spray test.3.2.Effects of corrosion on the mechanical propertiesAfter the accelerated cyclic corrosion testing,all the tensile specimens were pulled to failure in a MTS810machine to measure their mechanical properties.Figs.5and 6show that the mechanical strength(YS and UTS)of the steels decreases with an increase in the number of test cycles.The decrease in YS and UTS is the smallest for SS400.Interestingly,the YS and UTS for Acr-Ten A decrease quite linearly with an increase of the number of test cycles.On the other hand,the changes of the other four LAS are less regular,but decrease in general.These non-linear reductions in mechanical strength may be due to the negative effect of the surface roughness on the mechanical properties.In Fig.5,it is apparent that the yield strengths of 1604A,1604B,1605A,and 1605B are higher than those of Acr-Ten A and SS400.Furthermore,the YS of 1605A and 1605B are higher than 1604A and 1604B.The strengthening effects are due to the addition of manganese (1604A/B and 1605A/B)and chromium (1605A/B).Manganese will combine with sulfur to form globular MnS and it also strengthens the ferrite in steels by solid–solution strengthening.On the other hand,chromium will combine with carbon in steel to form carbides and also strengthen the steels.Because 1605A and 1605B contain both Cr and Mn,they have higher mechanical strength than the other teststeels.Fig.6.Ultimate tensile strength of the steels as a function of testcycles.Fig.5.Yield strength of the steels as a function of test cycles.1010Y.Y.Chen et al./Corrosion Science 47(2005)1001–1021The changes in mechanical strength,while near linear,are scattered and insuffi-cient data were accumulated to determine linear regression curves,such as S ¼X Ât Y ,that express the variation in strength with the number of test cycles [24],similar to Fig.4.Therefore,we compared the mechanical strength and the weight loss of each steel after 45cycles,as shown in Table 3.It is apparent that the reduction in the mechanical strength,especially of the yield strength,is greater than that of the cross-sectional area.Only the mechanical strength (YS and UTS)ratios for SS400approach the value of remaining cross-section ratio.This may be attributed to the fact that the loosely adhering rust layers on the substrate of SS400provide no local protection,so almost uniform corrosion occurred on the substrate thereby main-taining a smooth surface.The smoother surface of SS400after 45cycles provided less chance of stress concentration than the rougher surfaces developed on the other alloys.The larger decrease in the YS and UTS for the latter alloys may also be due to localized corrosion such as pitting.Fig.7illustrates the relationship between elongation and test period.It shows that elongation decreases with an increase in the number of test cycles,but that decreases are not linear.However,after 20cycles,the elongations of 1605A and 1605B are not changed much.In addition,the ductility of the alloys are roughly 70–82%of their original values.3.3.Analysis of the rust layersThe cross-sections of each steel after 18and after 45cycles of accelerated tests were observed by SEM,as shown in Fig.8(a)–(h).It shows that the rust layers in these test steels all contain voids and/or microcracks,which facilitate the penetration of the chloride solution to the substrate,which,in turn,promotes the corrosionTable 3Comparisons of mechanical strength,weight loss,and remaining cross-section after 45cycles of acceler-ated corrosion testsSteelSS400Acr-Ten A 1604A 1604B 1605A 1605B Weight loss (mg/cm 2)165.4187.3132.4132.8147153.8Thickness loss (l m)a420.8476.6336.9337.9374391.3Remaining cross-section ratio b0.9050.9080.8990.9010.9010.893Yield strength ratio c0.8920.8530.7700.7930.7800.831Ultimate tensilestrength ratio d0.8890.8700.8460.8320.8370.833aThickness loss ¼weight loss/[(upper and lower surfaces)·(iron density)]¼D w =2A q .b Remaining cross-section ratio ¼weight of the specimen (after the rust is stripped off)/weight of the specimen (without corrosion)(assuming the length of a specimen is not changed by corrosion).c Yield strength ratio ¼YS (45cycles)/YS (0cycle)[YS (0cycle)is the mechanical strength of an original (un-corroded)specimen].d Ultimate tensile strength ratio ¼UTS (45cycles)/UTS (0cycle)[UTS (0cycle)is the mechanical strength of an original (un-corroded)specimen].Y.Y.Chen et al./Corrosion Science 47(2005)1001–102110111012Y.Y.Chen et al./Corrosion Science47(2005)1001–1021cycles.Fig.7.Elongation of the steels as a function of test Array Fig.8.Rust layers on the tested steel samples after18and45cycles of the accelerated corrosion tests: (a)SS400(18cycles),(b)SS400(45cycles),(c)Acr-Ten A(18cycles),(d)Acr-Ten A(45cycles),(e)1604A (18cycles),(f)1604A(45cycles),(g)1605A(18cycles),(h)1605A(45cycles).process.Most of the rust layers on the test steels were composed of two parts:the loose outer rust layer and the dense inner rust layer.After 18cycles,the corrosion resistances of 1604A and 1604B are better than that of SS400,because the rust layers are denser and the voids and microcracks are fewer.The rust products on the two LAS (1604A/B)are similar to SS400––in that they can be removed easily from the substrate.The thickness of the rust layers on 1604A and 1604B after 18test cycles are 100–200l m thinner than that on SS400($400l m).On the other hand,the corrosion resistances of 1605A,1605B,and Acr-Ten A are inferior to SS400after 18cycles.This is probably due to the fact that the drying time was not long enough in the cases of the adherent,dense,and deep-colored inner rust layers of the three LAS,thereby exposing the substrates of these steels to humid condition for longer periods of time,and allowing more corrosion.The superior corrosion resistance of SS400at this stage (18cycles)is caused by the gap (separation)between the rust layer and the substrate (Fig.8(a)).This loose rust layer can easily be removed leaving the substrate basically untouched in a dry condition.In addition,it is difficult to transport chloride across the gap between the rust layer and the substrate.For those two reasons,the corrosion weight loss of SS400was lower after longer test cycles (18–45cycles).After the accelerated tests were finished (45cycles),the rust layers of SS400were still easily removed and contained numerous voids.However,although most ofthe Fig.8(continued )Y.Y.Chen et al./Corrosion Science 47(2005)1001–10211013rust layer separated from the substrates of1604A and of1604B,parts of each rust layer did adhere,allowing localized corrosion,which made the substrate rougher than that of SS400.Furthermore,there were deep-colored inner rust layers found at the rust-layer/substrate interfaces of1605A,1605B,and Acr-Ten A.This indicates that the rust layers of these three LASs gradually form the thick(400–500l m)and dense inner rust layers during the later cycles of the test.Experience with the stripping process and SEM observations suggested that the degree of difficulty in removing rust layers is dependent on the amount of chromium in the steels.The rust layers on the low-alloy steels with high-chromium content,such as1605A,1605B, and Acr-Ten A,are more compact and more difficult to remove.The dense,crack-free rust layers on Acr-Ten A and1605A after45cycles are shown in Fig.8(d)and(h),respectively.position and structure of the rustThe composition of the rust layers after18cycles and after45cycles was analyzed using FTIR.The outer rust layers of each steel were quite similar after18and45 cycles,and were composed of a-FeOOH,c-FeOOH,magnetite(Fe3O4),H2O,and amorphous ferric oxyhydroxide(FeO x(OH)3À2x,x¼0–1),as shown in Fig.9(a)and (d).After stripping offthe outer rust layers,the inner rust layers were analyzed, which showed that the main absorption peak for c-FeOOH became weaker(Fig. 9(b)and(e)).For Acr-Ten A,1605A,and1605B,almost no c-FeOOH was found in the surface rust layer(Fig.9(c)).The surface rust layer means that the rust layer just covers above the substrate($50l m thick),and the inner rust layer is just under the outer rust layer.These observations can be explained by acknowledging that the c-FeOOH was transformed into other kinds of corrosion products;for example,much of the c-FeOOH on WS was transformed into a-FeOOH after45cycles of the exposure in the experiment.Misawa et al.proposed that dissolved chromium and phosphorus ions enhance the formation of uniform amorphous ferric oxyhydroxide,which protects the steel substrate,and that this amorphous ferric oxyhydroxide will further trans-form into the more stable and protective structure of a-FeOOH[25,26].However, the presence of ClÀwould facilitate metal dissolution(FefiFe2þ+2eÀ)by depass-ivating the iron surface.The acidic nature of the hydrated Fe2þcation dissociates and therefore lowers the local pH via the following reaction:FeðH2OÞ2þ6!FeðH2OÞ5ðOHÞþþHþð2ÞThis may promote the phase transformation to amorphous ferric oxyhydroxide (FeO x(OH)3À2x,x¼0–1)and a-FeOOH.After45cycles in the cyclic corrosion test, the compact rust layers on the substrate of1605A,1605B,and Acr-Ten A were composed largely of Fe3O4with a little of a-FeOOH(Fig.9(c)).The valence of Fe in Fe3O4is8/3,which indicates that the dense rust layers prevent the diffusion of oxygen and result in the incomplete oxidation of Fe.1014Y.Y.Chen et al./Corrosion Science47(2005)1001–1021。
10crmo910标准

10crmo910标准10CrMo910标准。
10CrMo910是一种低合金钢,通常用于高温高压设备的制造,比如锅炉、换热器等。
它具有良好的耐热性能和抗氧化性能,适用于工作温度高达600℃的环境。
10CrMo910标准是指该钢材的化学成分、机械性能、热处理工艺等方面的标准规定,下面将对其进行详细介绍。
首先,我们来看10CrMo910的化学成分。
根据标准规定,10CrMo910的化学成分应满足一定的要求,其中含有的合金元素如铬、钼等对其耐热性能起着重要作用。
此外,硫、磷等杂质元素的含量也需要受到严格控制,以保证钢材的纯净度和均匀性。
其次,我们需要了解10CrMo910的机械性能。
标准对于钢材的抗拉强度、屈服强度、延伸率、冲击韧性等性能指标都有详细的规定。
这些性能指标直接关系到10CrMo910在高温高压工作环境下的使用安全性和可靠性,因此必须严格符合标准规定。
除了化学成分和机械性能外,热处理工艺也是10CrMo910标准中的重要内容。
钢材经过适当的热处理可以改善其组织结构,提高其耐热性能和强度,标准对于热处理工艺的温度、时间、冷却方式等都有详细规定,制造厂必须严格按照标准要求进行操作。
另外,对于10CrMo910钢材的检测和验收也是标准中不可忽视的部分。
标准规定了对钢材化学成分、机械性能、热处理效果等方面的检测方法和要求,以确保生产出符合标准要求的优质钢材。
总的来说,10CrMo910标准涵盖了钢材的化学成分、机械性能、热处理工艺、检测验收等方面的内容,对于保证10CrMo910钢材在高温高压工作环境下的安全可靠性起着至关重要的作用。
因此,制造厂必须严格按照标准要求进行生产制造,确保生产出符合标准要求的优质10CrMo910钢材,以满足工程和设备的需求。
在实际生产中,制造厂应当建立健全的质量管理体系,加强对原材料、生产工艺、成品的监控和检测,确保产品质量稳定可靠。
同时,加强对操作人员的技术培训和质量意识教育,提高员工的素质和责任心,为生产出优质的10CrMo910钢材提供保障。
低合金钢标准

低合金钢标准低合金钢是一种含有少量合金元素的钢铁材料,通常是指合金元素含量小于5%的钢。
低合金钢具有良好的可焊性、可塑性和机械性能,广泛应用于船舶制造、汽车制造、机械制造等领域。
由于低合金钢的用途广泛,为了保证其质量和性能,制定了一系列的低合金钢标准,以便指导生产和使用。
首先,低合金钢的化学成分是制定标准的重要依据。
合金元素的含量对低合金钢的性能有着重要影响,因此标准中对合金元素的含量范围和允许偏差都有详细规定。
同时,标准还对非金属夹杂物、氧化物等杂质的含量进行了严格限制,以保证低合金钢的纯净度和均匀性。
其次,低合金钢的机械性能也是制定标准的重点内容之一。
标准中对低合金钢的拉伸强度、屈服强度、延伸率、冲击韧性等性能指标都有详细的规定,以确保低合金钢在使用过程中能够满足相应的强度和韧性要求。
此外,标准还对低温、高温等特殊条件下的机械性能进行了特殊规定,以适应不同环境下的使用需求。
另外,低合金钢的热处理和表面质量也是标准所关注的内容之一。
热处理工艺对低合金钢的组织和性能有着重要影响,因此标准中对热处理工艺、温度、时间等参数进行了规定,以保证低合金钢的组织和性能达到标准要求。
同时,标准还对低合金钢的表面质量、缺陷、允许的缺陷尺寸等进行了严格规定,以保证低合金钢在使用过程中不会出现质量问题。
最后,低合金钢的标准化生产和质量控制也是制定标准的重要目的。
标准中对低合金钢的生产工艺、质量控制、检测方法等进行了详细规定,以保证低合金钢的质量稳定和可靠。
同时,标准还对低合金钢的标识、包装、运输等环节进行了规范,以保证低合金钢在流通和使用过程中能够得到有效的管理和保护。
总之,低合金钢标准的制定对于保证低合金钢的质量和性能具有重要意义。
只有严格依照标准要求生产和使用低合金钢,才能够确保其在各个领域的应用效果和安全性。
因此,我们应该充分认识到低合金钢标准的重要性,严格遵守标准要求,共同推动低合金钢产业的健康发展。
低合金钢标准

低合金钢标准
低合金钢是一种具有较低碳含量和适量合金元素的钢材。
它具有较高的强度、硬度和耐磨性,同时又保持了良好的可焊性和可加工性。
为了规范低合金钢的生产和应用,以下是一份低合金钢的标准参考:
1. 材料分类:
低合金钢根据合金元素的不同分类为:Cr-Mo系列、Mn-Mo系列、Cr-Mn-V系列等。
2. 化学成分:
低合金钢化学成分应满足以下标准范围:
碳含量(C):0.10%~0.30%
硅含量(Si):≤0.40%
锰含量(Mn):0.50%~1.60%
硫含量(S):≤0.035%
磷含量(P):≤0.035%
合金元素(如铬、钼、钒等)的含量应符合具体的合金系列要求。
3. 机械性能:
低合金钢的机械性能应满足以下要求:
抗拉强度:≥500MPa
屈服强度:≥320MPa
延伸率:≥22%
冲击韧性:符合规定的冲击试验温度和吸收能量要求。
4. 热处理:
在合适的温度范围内进行热处理(如退火、正火、淬火等),以达到所需的力学性能和组织结构。
5. 外观和质量标准:
低合金钢的外观应光洁无明显缺陷,如折断、裂缝、气泡等。
6. 标志和包装:
低合金钢应在表面或外包装上标示牌号、规格、批次号和生产厂家信息。
请注意,以上是一份低合金钢的标准参考,实际应用时应根据具体需要结合相关标准进行设计和选择。
低合金铸钢机械性能

残余元素,总和≤1%
C
Si
Mn
P,S
Cr
Ni
Mo
Cu
ZG22Mn
0.20—0.25
≤0.50
1.40—1.70
≤0.035
铸件经热处理(880C°正火+680C°回火)后的力学性能
铸件
壁厚范围
屈服强度,
σs/MPa
抗拉强度,
σb/MPa
伸长率,
δ(%)
冲击值AKV,J
硬度,
HBS
<100mm
≥295
≥540
≥18
≥40
160
≤25mm
315—365
注:相关实际力学数据可根据铸件的同炉拉力试棒进行检测获得
铸件
壁厚范围
屈服强度,
σs/MPa
抗拉强度,
σb/MPa
伸长率,
δ(%)
冲击值AKV,J
硬度,
HBS
<100mm
≥295
≥540
≥18
≥40
160
≤25mm
315—365
注:相关实际力学数据可根据铸件的同炉拉力试棒进行检测获得
1.采用精密铸造毛坯,选用材料牌号列表如下
GB/T14408—93
中的牌号
JB/T 6402—19921.采用精密铸造毛坯,选用材料牌号列表如下
GB/T14408—93
中的牌号
化学成分(质量分数),%
残余元素,总和≤1%
C
Si
Mn
P,S
Cr
Ni
Mo
Cu
ZG22Mn
0.20—0.25
≤0.50
1.40—70
普通低合金钢

普通低合金钢普通低合金钢 - 了解它的特性和应用领域引言:普通低合金钢是一种常见的金属材料,在许多工业领域中被广泛应用。
它具有较低的成本和可靠的性能,使得它成为制造业中不可或缺的材料之一。
本文将介绍普通低合金钢的定义、特性以及它在不同领域中的应用。
一、定义:普通低合金钢被视为一种机械性能较差、合金元素含量较低的钢材。
与高合金钢相比,普通低合金钢的合金元素含量通常为2%以下。
这使得它在制造过程中具有低成本和良好的可加工性。
二、特性:1. 机械性能:普通低合金钢具有中等强度和硬度,适用于许多一般工程应用。
它通常具有较好的可塑性和韧性,在受力时能保持稳定的性能。
2. 可焊性:由于其较低的合金元素含量,普通低合金钢具有良好的可焊性。
这使得它在焊接工艺中比较容易操作。
3. 耐腐蚀性:虽然普通低合金钢的耐腐蚀性相对较差,但其经过适当的防腐蚀处理后,仍然可以在一些特定环境中应用。
4. 可加工性:普通低合金钢具有良好的可加工性,可以通过常规的加工方法进行切削、成形和压造。
三、应用领域:由于其成本低廉和性能可靠,普通低合金钢在许多工业领域中得到广泛应用:1. 建筑和基础设施:普通低合金钢常用于建筑结构、桥梁和其他基础设施的制造。
它具有良好的强度和可塑性,可以有效应对自然和人为的力量。
2. 汽车工业:普通低合金钢在汽车制造业中应用广泛。
它可以用于制造车身和底盘零件,提供良好的强度和韧性。
此外,普通低合金钢还可以用于制造引擎和传动系统的部分组件。
3. 机械制造:普通低合金钢在机械制造领域中具有重要的地位。
它可用于制造各种机械设备和部件,如轴承、轴和齿轮等。
普通低合金钢的可加工性使得它成为许多复杂部件的首选材料。
4. 电力行业:普通低合金钢常用于电力行业的输电线杆和电网设备。
它具有良好的强度和耐腐蚀性,在恶劣的环境条件下仍能保持稳定的性能。
5. 航空航天工业:尽管航空航天工业通常更倾向于使用高合金钢,但普通低合金钢在一些次要组件的制造中也有应用。
低合金钢标准

低合金钢标准低合金钢是一种含有较少合金元素的钢铁材料,其含量一般在5%以下。
低合金钢具有良好的可焊性、耐磨性和良好的机械性能,因此在工程结构中得到广泛应用。
为了确保低合金钢的质量和安全性能,各个国家和地区都制定了相应的低合金钢标准,以规范其生产和使用。
低合金钢标准主要包括化学成分、机械性能、热处理要求、检测方法等内容。
首先,化学成分是低合金钢标准中的重要内容之一。
不同的合金元素含量会直接影响到钢材的硬度、强度、韧性等性能,因此在标准中对各种元素的含量范围进行了严格规定。
其次,机械性能也是低合金钢标准中需要重点关注的内容。
标准中通常对低合金钢的拉伸强度、屈服强度、延伸率等机械性能指标进行了详细的规定,以确保材料在使用过程中能够满足相应的要求。
此外,热处理要求也是低合金钢标准中不可忽视的部分。
通过热处理可以改善低合金钢的组织结构,提高其硬度和强度,标准中通常对热处理工艺、温度、时间等方面进行了规定。
最后,检测方法也是低合金钢标准中必不可少的内容。
通过严格的检测方法可以确保低合金钢材料的质量,常见的检测方法包括化学成分分析、金相组织分析、硬度测试、拉伸试验等。
在国际上,低合金钢的标准主要由国际标准化组织(ISO)和国际电工委员会(IEC)制定和管理。
在中国,低合金钢的标准由国家标准化管理委员会负责制定和管理。
各个国家和地区的低合金钢标准可能会有所不同,因此在使用低合金钢材料时,需要根据具体的国家标准进行选择和使用。
总的来说,低合金钢标准是确保低合金钢材料质量和安全性能的重要依据,对于生产和使用低合金钢材料的企业和个人来说,必须严格遵守相应的标准要求,以确保产品质量和安全可靠性。
同时,各个国家和地区也应加强标准的制定和管理,不断完善和提高低合金钢标准,以适应不断发展变化的市场需求和技术要求。
低合金钢与合金钢的区别

低合金钢与合金钢的区别
低合金钢和合金钢在多个方面存在差异:
1. 组成成分:低合金钢的合金元素含量较低,一般在3%以下。
除了碳元素之外,通常只添加一种或几种合金元素,如锰、硅等。
而合金钢的合金元素含量较高,一般在5%以上。
除了碳元素之外,还添加了多种合金元素,如铬、钼、钴、镍等。
2. 机械性能:由于合金元素的加入,合金钢的机械性能要优于低合金钢。
一般来说,合金钢的强度和硬度较高,耐腐蚀性好,耐磨性能也较优秀。
而低合金钢的机械性能一般较为平凡,不耐磨损,但是易于加工和焊接。
3. 用途:由于机械性能表现的不同,低合金钢和合金钢的应用领域也有所不同。
一般来说,低合金钢主要应用于汽车、机器、轮船等机械结构件的制造领域,如车架、前叉等。
而合金钢则主要应用于制造高强度螺栓、制动系统、船舶和航空发动机等高要求的领域。
综上,低合金钢和合金钢在组成成分、机械性能以及用途等方面均存在明显的差异。
请根据具体的使用场景和需求来选择适合的钢材种类。
材料s35c标准

材料s35c标准介绍如下:
S35C是一种碳素钢,主要成分为碳和硅,其化学成分较为单一,属于一种低合金钢。
S35C 钢是一种优质钢材,在工业生产中得到广泛应用,常常用于制造传动零件、机械零件、轴承等。
S35C钢的主要化学成分为:碳(C)0.32%-0.38%、硅(Si)0.15%-0.35%、锰(Mn)0.6%-0.9%、磷(P)0.030%以下、硫(S)0.035%以下。
同时还包含了微量元素如钼(Mo)和铬(Cr),使其具有优良的性能和特点。
S35C钢的热加工性能较好,可进行锻造、淬火和正火处理,具有良好的可焊性、韧性和抗疲劳性能。
S35C钢的力学性能表现如下:
•抗拉强度:440-570 MPa
•屈服强度:305 MPa
•延伸率:20-28%
•冲击韧性:(AKU) 31-39 J/cm2
这些性能决定了S35C钢具有良好的机械性能和综合性能,在各种机械部件和构件的生产中得到广泛应用。
此外,S35C钢还具有优良的加工性能和表面硬度,适合于各种加工方式,如铣削、车削、钻孔等。
在生产和加工过程中,S35C钢还需要根据其特点和性能加以适当的处理和维护。
在热处理过程中,S35C钢需要严格控制加热温度和冷却速度,以保证钢材的硬度和强度。
同时,在使用时,需要对其表面进行适当的保护和维护,以预防生锈和腐蚀,从而保证钢材的正常使用寿命。
总之,S35C钢作为一种低合金钢,具有良好的机械性能和综合性能,在工业制造领域得到广泛应用,并且具有较好的加工性能和表面硬度,是一种常用的优质钢材。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
0.04-0.12
—
—
—
—
—
0.045
0.045
14MnNb
0.12-0.18
0.80-1.20
0.20-0.55
—
—
0.015-0.050
—
—
—
0.045
0.045
16Mn
0.12-0.20
1.20-1.60
0.20-0.55
—
—
—
—
—
—
0.045
0.045
16MnRE
0.12-0.20
0.02-0.20
0.015-0.060
0.02-0.20
—
0.40
0.70
C
0.20
1.00-1.70
0.55
0.035
0.035
0.02-0.20
0.015-0.060
0.02-0.20
0.015
0.40
0.70
D
0.20
1.00-1.70
0.55
0.030
0.030
0.02-0.20
0.015-0.060
0.02-0.20
0.015
0.40
0.70
E
0.20
1.00-1.70
0.55
0.025
0.025
0.02-0.20
0.015-0.060
0.02-0.20
0.015
0.40
0.70
Q460
A
0.20
1.00-1.70
0.55
0.035
0.035
0.02-0.20
0.015-0.060
0.02-0.20
470-630
470-630
470-630
21
21
22
22
22
34
34
34
27
d=2a
d=3a
d=2a
d=3a
d=2a
d=3a
d=2a
d=3a
d=2a
d=3a
Q390
A
B
C
D
E
370
370
370
370
370
370
370
370
370
370
350
350
350
350
350
330
330
330
330
330
490-650
0.015-0.050
—
—
—
0.045
0.045
09Mn2
≤0.12
1.40-1.80
0.20-0.55
—
—
—
—
—
—
0.045
0.045
12Mn
0.09-0.16
1.10-1.50
0.20-0.55
—
—
—
—
—
—
0.045
0.045
18Nb
0.14-0.22
0.40-0.80
0.17-0.37
—
—
0.020-0.050
16、一般用途低合金钢
一般用途低合金结构钢包括:普通质量和优质低合金结构钢、普通质量低合金钢是指生产过程中不规定特别控制质量要求的供一般用途、优质、在生产过程中需要特别控制质量(例降低硫、磷含量、控制晶粒度,改善表面质量等)一般用途低合金钢结构钢牌号由代表屈服点的汉语拼音字母(Q)、屈服点数值、质量等级符号(A、B、C、D、E)三个部分按照顺序排列,根据GB/T1591-94低合金高强度结构钢牌号,化学成分及性能见表5-16和表
表5-16、低合金高强度结构钢牌号、化学成分
牌号
质量等级
化学成分,%
C≤
Mn
Si≤
P≤
S≤
V
Nb
Ti
Al≥
Cr≤
Ni≤
Q295
A
0.16
0.80-0.15
0.55
0.045
0.045
0.02-0.15
0.015-0.060
0.02-0.20
—
B
0.16
0.80-0.15
0.55
0.040
0.040
0.02-0.15
19
d=3a
>25-36
490-640
355
18
d=3a
>36-50
490-640
335
18
d=3a
15MnTi
≤25
530-680
390
20
d=3a
20
27
>25-40
510-660
375
20
d=3a
16MnNb
≤16
530-680
390
20
d=2a
20
27
>16-20
510-660
375
19
d=3a
d=3a
16MnRE
≤16
510-660
345
22
d=2a
20
27
>16-25
490-640
325
21
d=3a
10MnPNbRE
≤10
510-660
390
20
d=2a
20
27
15MnV
≤4
550-700
410
19
d=2a
20
27
>4-16
530-680
390
18
d=3a
>16-25
510-660
375
0.20-0.55
0.04-0.10
0.09-0.16
—
—
—
0.02-0.20
0.045
0.045
15MnVN
0.12-0.20
1.30-1.70
0.20-0.55
0.10-0.20
—
—
—
0.010-0.020
—
0.045
0.045
表5-19、一般用途低合金结构钢机械性能(根据GB1591-88)
牌号
表5-17、低合金高强度结构钢机械性能
牌号
质量等级
屈服点бs,MPa
抗拉强度бb MPa
伸长率δs%
冲击功,AKV,(纵向),J
180°弯曲试验
d=弯心直径;
a=试样厚度(直径)
厚度(直径,边长),mm
+20°C
0°C
-20°C
-40°C
≤16
>16-35
>35-50
>50-100
不小于
钢材厚度(直径),mm
表5-18、一般用途低合金结构钢牌号、化学成分(根据GB1591-881)
牌号
化学成分,%
C
M
Si
V
Ti
Nb
Cu
N
RE
S
P
加入量
不大于
09MnV
≤0.12
0.80-1.20
0.20-0.55
0.04-0.12
—
—
—
—
—
0.045
0.045
09MnNb
≤0.12
0.80-1.20
0.20-0.55
—
—
0.015
0.70
0.70
B
0.20
1.00-1.70
0.55
0.030
0.030
0.02-0.20
0.015-0.060
0.02-0.20
0.015
0.70
0.70
C
0.20
1.00-1.70
0.55
0.025
0.025
0.02-0.20
0.015-0.060
0.02-0.20
0.015
0.70
0.70
14MnVTiRE
≤12
550-700
440
19
d=2a
20
27
>12-20
530-680
410
19
d=3a
15MnV
≤10
590-740
440
19
d=2a
20
28
>10-25
570-720
420
19
d=3a
>25-38
550-700
410
18
d=3a
355
21
d=2a
20
27
>16-25
335
20
d=3a
16Mn
≤16
510-660
345
22
d=2a
20
27
>16-25
490-640
325
21
d=3a
>25-36
470-620
315
21
d=3a
≤36-50
470-620
295
21
d=3a
>50-100方、圆钢
470-620
275
20
0.02-0.20
0.015
Q390
A
0.20
1.00-1.60
0.55
0.045
0.045
0.02-0.20
0.015-0.060
0.02-0.20
—
0.30
0.70
B
0.20
1.00-1.60
