过氧化氢MSDS
过氧化氢(双氧水)

过氧化氢(双氧水)过氧化氢MSDS第一部分:化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide技术说明书编码:559CAS No.:2014分子式:H2O2分子量:50第二部分:危险性概述危险性类别:11(氧化剂),20(腐蚀品)侵入途径:吸入、食入健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
燃爆危险:本品助燃,具强刺激性。
第三部分:成分/组成信息有害物成分含量CAS No.过氧化氢50% 2014第四部分:急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分:消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
灭火剂:水、雾状水、干粉、砂土。
第六部分:泄漏应急处理应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
建议应急处理人员戴自给正压式呼吸器,穿防毒服。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
过氧化氢MSDS

燃烧热(kJ/mol):
无意义
临界温度(c):
无资料
临界压力(MPa):
无资料
辛醇/水分配系数的对数值:
无资料
闪点(c):
无意义
引燃温度(c):
无意义
爆炸上限%(V/V):
无意义
爆炸下限%(V/V):
无意义
溶解性:
溶于水、醇、醚,不溶于苯、石油醚。
主要用途:
用于漂白,用于医药,也用作分析试剂。
其它理化性质:
时最稳定, 在碱性溶液中极易分解, 在遇强光, 特别是短波 射线照射时也能发生分解。当加热到100C以上时,开始急
剧分解。它与许多有机物如糖、 淀粉、醇类、石油产品等形 成爆炸性混合物, 在撞击、受热或电火花作用下能发生爆炸。 过氧化氢与许多无机化合物或杂质接触后会迅速分解而导 致爆炸,放出大量的热量、氧和水蒸气。 大多数重金属(如 铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物 和盐类都是活性催化剂, 尘土、香烟灰、 碳粉、铁锈等也能 加速分解。浓度超过74%的过氧化氢,在具有适当的点火源 或温度的密闭容器中,能产生气相爆炸。 氧气、水。
其它有害作用:
无资料
第十三部分 废弃处置
废弃物性质:
废弃处置方法:
经水稀释后, 发生分解放出氧气, 待充分分解后, 把废液排
入废水系统。
废弃注意事项:
第十四部分 运输信息
危险货物编号:
51001
UN编号:
2015
包装标志:
未制定标准
TLVTN:
ACGIH 1ppm,m3
TLVWN:
未制定标准
监测方法:
四氯化钛分光光度法
工程控制:
生产过程密闭,全面通风。提供安全淋浴和洗眼设备。
过氧化氢MSDS化学品安全技术说明书

浓度超过74%的过氧化氢,在具有适当点火源或温度的密闭容器中,能发生气相爆炸
健康危害
●职业接触限值:PC-TWA 1.5mg/m3
●IDLH:75ppm
●急性毒性:大鼠经口LD50376mg/kg(H20290%);大鼠经皮LD504060mg/kg(H20290%)
措
施
急救
●皮肤接触:立即脱去污染的衣着.用大量流动清水冲洗20~30min。就医
●眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗10~15min。就医
●吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。呼吸、心跳停止,立即进行心肺复苏术。就医
●食入:饮水,禁止催吐。就医
过氧化氢
别名:双氧水
特
别警Leabharlann 示★蒸气或雾对呼吸道有强烈刺激性;眼直接接触液体可致不可逆损伤甚至失明
★与可燃物混合能形成爆炸性混合物
★在限制性空间中加热有爆炸危险
化
学
式
分子式H202结构式HO--OH
危险性类别
过氧化氢(含量≥8%):5.1类氧化剂
危
险
性
燃烧爆炸危险性
本品不燃,可助燃
浓过氧化氢溶液受撞击、高温、光照,易发生爆炸
●蒸气或雾对眼和呼吸道有刺激性
●眼直接接触液体可致灼伤。误服可发生胃扩张,腐蚀性胃炎
理
化
特
性
理化特性
●无色透明液体,有微弱的特殊气味。工业品分为27.5%.35.O%和50.0%三种规格。溶于水
●熔点:-0.43℃
●沸点:150.2℃
●相对密度:l.46
个
体
防
过氧化氢MSDS
For personal use only in study and research; not for commercial use过氧化氢第一部分化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文别称:双氧水CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险:本品助燃,具强刺激性。
第四部分急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
过氧化氢-MSDS

过氧化氢化学品安全技术说明书第一部分:化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文名称2:双氧水技术说明书编码:559CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分:成分/组成信息有害物成分含量CAS No.有害物成分含量CAS No. 过氧化氢35% 7722-84-1第三部分:危险性概述健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
燃爆危险:本品助燃,具强刺激性。
第四部分:急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分:消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
过氧化氢MSDS

过氧化氢第一部分化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文别称:双氧水CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险:本品助燃,具强刺激性。
第四部分急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
过氧化氢MSDS
过氧化氢第一部分化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文别称:双氧水CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险:本品助燃,具强刺激性。
第四部分急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
MSDS危险化学品安全技术说明书——51001--过氧化氢、双氧水

化学品安全技术说明书第一部分化学品及企业标识化学品中文名:过氧化氢;双氧水化学品英文名:hydrogen peroxide企业名称:生产企业地址:邮编: 传真:企业应急电话:电子邮件地址:技术说明书编码:第二部分成分/组成信息√纯品混合物有害物成分浓度CAS No.过氧化氢7722-84-1第三部分危险性概述危险性类别:第5.1类氧化剂侵入途径:吸入、食入健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性,一次大量吸入可引起肺炎或肺水肿。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:无资料。
燃爆危险:助燃。
与可燃物混合会发生爆炸。
在限制性空间中加热有爆炸危险。
第四部分急救措施皮肤接触:立即脱去污染的衣着,用大量流动清水冲洗20~30分钟。
如有不适感,就医。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗10~15分钟。
如有不适感,就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
呼吸、心跳停止,立即进行心肺复苏术。
就医。
食入:饮水,禁止催吐。
如有不适感,就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
过氧化氢——7722-84-1MSDS危险化学品安全技术说明书(16个部分完整版)

化学品安全技术说明书过氧化氢第一部分化学品及企业标识化学品中文名:过氧化氢化学品英文名:hydrogen peroxide供应商名称:供应商地址:供应商电话:邮编:供应商传真:电子邮件地址:产品推荐及限制用途:用于漂白、医药,也用作分析试剂。
第二部分危险性概述紧急情况概述:可引起燃烧或爆炸:强氧化剂,吞咽有害,吸入有害,造成严重的皮肤灼伤和眼损伤。
GHS危险性类别:氧化性液体-类别1;急性毒性-经口-类别4;急性毒性-吸入-类别4;皮肤腐蚀/刺激-类别1A;严重眼损伤/眼刺激-类别1;特异性靶器官毒性-一次接触-类别3(呼吸道刺激);危害水生环境-急性危害-类别3标签要素:象形图:警示词:危险危险信息:H335:可能引起呼吸道刺激H302:吞咽有害H332:吸入有害H271:可引起燃烧或爆炸;强氧化剂H314:造成严重的皮肤灼伤和眼损伤H402:对水生生物有害防范说明:预防措施:P260:不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
P271:只能在室外或通风良好之处使用。
P264:作业后彻底清洗。
P270:使用本产品时不要进食、饮水或吸烟。
P261:避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
P280:戴防护面具。
P210:远离热源/火花/明火/热表面。
禁止吸烟。
P220:避开/贮存处远离服装/可燃材料。
P221:采取一切防范措施,避免与可燃物/混合。
P283:穿防火/阻燃服装。
P273:避免释放到环境中。
—如非其预定用途。
应急响应:P304+P340:如误吸入:将受害人转移到空气新鲜处,保持呼吸舒适的休息姿势。
P312:如感觉不适,呼叫解毒中心或医生。
P301+P310:如误吞咽:立即呼叫解毒中心/医生。
P330:漱口。
P321:具体治疗(见本标签上的)。
P305+P351+P338:如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
P310:立即呼叫解毒中心/医生。
过氧化氢msds
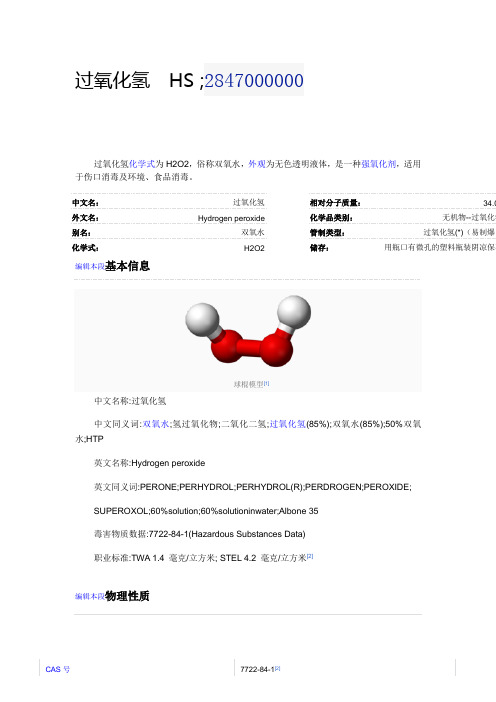
纯过氧化氢
过氧化氢分子为椅型结构,左图为气态时的结构,右图为固态晶体时的结构。
Use water spray to keep fire-exposed containers cool. Substance is noncombustible. Use water with caution and in flooding amounts.
Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Some oxidizers may react explosively with hydrocarbons(fuel). May decompose explosively when heated or involved in a fire. May accelerate burning if involved in a fire.
CAS:
7722-84-1
Section 1 - Chemical Product
MSDS Name:Hydrogen Peroxide 35 wt.% Solution in Water Stabilized P.A. Material Safety Data Sheet
Synonym:Carbamide peroxide; Hydrogen dioxide; Peroxide; Hydroperoxide; Urea peroxide; Hydrogen peroxide 100 volumes
双氧水(过氧化氢)物质特性表(MSDS)

相对蒸汽密度
无资料
分子式
H2O2
分子量
34.01
饱和蒸汽压(kPa)
0.13(15.3℃)
燃烧热(Kj/mol)
临界温度℃
临界压力MPa
闪点℃
燃点℃
火灾危险特性
甲类
最小点火能量(mJ)
爆炸极限%(V/V)
溶解性
溶于水、醇、醚,不溶于苯、石油醚。
其他理化性质
危险性概述
危险性类别
第5.1类氧化剂
有害燃烧产物
氧气、水
灭火方法
消防人员必须穿全身防火防毒服,在上风向灭火。尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:水、雾状水、干粉、砂土。
泄漏应急处理
应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源。防止流入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用大量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。喷雾状水冷却和稀释蒸汽、保护现场人员、把泄漏物稀释成不燃物。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
操作处置与存储
操作注意事项
密闭操作,全面通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(全面罩),穿聚乙烯防毒服,戴氯丁橡胶手套。远离火种、热源,工作场所严禁吸烟。远离易燃、可燃物。防止蒸气泄漏到工作场所空气中。避免与还原剂、活性金属粉末接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。
过氧化氢MSDS化学品安全技术说明书

浓度超过74%的过氧化氢,在具有适当点火源或温度的密闭容器中,能发生气相爆炸
健康危害
●职业接触限值:PC-TWA 1.5mg/m3
●IDLH:75ppm
●急性毒性:大鼠经口LD50376mg/kg(H20290%);大鼠经皮LD504060mg/kg(H20290%)
●蒸气或雾对眼和呼吸道有刺激性
●眼直接接触液体可致灼伤。误服可发生胃扩张,腐蚀性胃炎
理
化
特
性
理化特性
●无色透明液体,有微弱的特殊气味。工业品分为27.5%.35.O%和50.0%三种规格。溶于水
●熔点:-0.43℃
●沸点:150.2℃
●相对密度:l.46
个
体
防
护
●佩戴全防型滤毒罐
●穿封闭式防化服
应
急
过氧化氢
别名:双氧水
特
别
警
示
★蒸气或雾对呼吸道有强烈刺激性;眼直接接触液体可致不可逆损伤甚至失明
★与可燃物混合能形成爆炸性混合物
★在限制性空间中加热有爆炸ቤተ መጻሕፍቲ ባይዱ险
化
学
式
分子式H202结构式HO--OH
危险性类别
过氧化氢(含量≥8%):5.1类氧化剂
危
险
性
燃烧爆炸危险性
本品不燃,可助燃
浓过氧化氢溶液受撞击、高温、光照,易发生爆炸
措
施
急救
●皮肤接触:立即脱去污染的衣着.用大量流动清水冲洗20~30min。就医
●眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗10~15min。就医
●吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。呼吸、心跳停止,立即进行心肺复苏术。就医
过氧化氢MSDS

过氧化氢第一部分化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文别称:双氧水CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险:本品助燃,具强刺激性。
第四部分急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
过氧化氢,MSDS

过氧化氢,MSDS第一部分:化学品名称化学品中文名称: 过氧化氢化学品英文名称: hydrogen peroxide中文名称2: 双氧水英文名称2:技术说明书编码: 559CAS No.: 7722-84-1分子式: HO 22分子量: 34.01第二部分:成分/组成信息有害物成分含量 CAS No.过氧化氢 35% 7722-84-1第三部分:危险性概述危险性类别:侵入途径:健康危害: 吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险: 本品助燃,具强刺激性。
第四部分:急救措施皮肤接触: 脱去污染的衣着,用大量流动清水冲洗。
眼睛接触: 立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入: 迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入: 饮足量温水,催吐。
就医。
第五部分:消防措施危险特性: 爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5,4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到 100?以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74,的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物: 氧气、水。
灭火方法: 消防人员必须穿全身防火防毒服,在上风向灭火。
过氧化氢危险化学品使用安全说明(MSDS)

过氧化氢(一)理化性状与用途无色透明液体,深层时略带淡兰色。
密度;1.44;冰点-0.4℃;爆炸极限:26~100%。
用作氧化剂、漂白剂、杀菌剂、消毒剂、发色剂。
高浓度的过氧化氢可用作火箭动力燃料。
(二)毒性它的毒性主要是由它的活性氧化作用所引起的,如对眼睛、皮肤和黏膜的化学灼伤,以及使普通衣物着火等。
(三)短期暴露的影响由于本品不易挥发,吸入蒸气中毒的可能性很小,且它具有强烈烧灼感,故吞入的可能性很小。
主要是皮肤接触引起烧伤,是局部皮肤和毛发发白(但过一段时间后可复原),产生刺痛、瘙痒。
(四)长期暴露的影响由于量、时间、作用部位不同产生程度不等的化学灼伤。
渗入皮肤角质层后分解产生氧,使表皮起泡,因手掌、指尖及甲床等处角质层较厚,末梢神经丰富,疼痛更为剧烈,难以忍受。
剂量较大、冲洗不及时,可留下永久疤痕。
液滴溅入眼内,可引起结膜炎、虹膜睫状体炎及角膜上皮变性、坏死和浑浊,影响视力或导致完全失明。
(五)火灾与爆炸本品属爆炸性强氧化剂。
它本身是不燃的,但它能与可燃物反应并产生足够的热量而引起着火,又由于它分解所放出的氧能强烈助燃,最终可导致爆炸。
着火时用水扑救,并用水冷却其他容器,若发现高浓度过氧化氢容器排气孔中冒出蒸气,所有人员应迅速撤至安全地方,操作人员均应做到全身防护。
(六)化学反应性在碱溶液中极易分解,在强光,特别是短波射线照射下,也能发生分解。
能与许多有机物如糖、淀粉、醇类、石油产品等形成的混合物是敏感的,在冲击和热量或电火花作用下能发生爆炸,与氧化物混合,存在潜在的危险性。
(七)人身防护皮肤:应使用橡胶和氯丁橡胶手套、天然橡胶高统靴、聚氯乙烯防护服、聚乙烯围裙和袖套以及头巾等。
工作的场所应备有可用的安全淋浴和眼睛冲洗器具。
眼睛;戴护目镜、塑料面具。
(八)急救皮肤接触;应立即用水冲洗,也可以用3%高锰酸钾或2%碳酸钠溶液冲洗。
如皮肤灼伤剧痛不止,应用本巴比妥钠或咖啡,并防止继发性感染。
眼睛接触:应立即用水冲洗15分钟以上,然后就医。
过氧化氢MSDS

For personal use only in study and research; not for commercial use过氧化氢第一部分化学品名称化学品中文名称:过氧化氢化学品英文名称:hydrogen peroxide中文别称:双氧水CAS No.:7722-84-1分子式:H2O2分子量:34.01第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
长期接触本品可致接触性皮炎。
环境危害:燃爆危险:本品助燃,具强刺激性。
第四部分急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
有害燃烧产物:氧气、水。
灭火方法:消防人员必须穿全身防火防毒服,在上风向灭火。
双氧水MSDS安全技术说明
双氧水安全技术说明MSDS标识中文名过氧化氢英文名hydrogen peroxide分子式H2O2危险货物编号51001分子量34.01危险性类别第5.1类氧化剂理化特性熔点(℃)-2(无水)沸点(℃)158(无水)燃烧热/饱和蒸气压(kPa)0.13(15.3℃)相对密度 1.46(无水)(水=1)外观性状无色透明液体,有微弱的特殊气味。
溶解性溶于水、醇、醚,不溶于苯、石油醚。
稳定性不稳定避免接触的条件强光、受热、撞击。
禁忌物易燃或可燃物、强还原剂、铜、铁、铁盐、锌、活性金属粉末等。
燃烧(分解)产物氧气、水燃爆特性燃烧性本品助燃,具强刺激性。
建规火险分级甲类危险特性爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和氧气而引起着火爆炸。
过氧化氢在pH值为3.5~4.5时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如铁、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,能产生气相爆炸。
灭火方法消防人员必须穿全身防火防毒服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
灭火剂:水、雾状水、干粉、砂土。
毒性及健康危害侵入途径吸入、食入、经皮吸收急性毒性LD50:376(H2O290%)大鼠经口(mg/kg)健康危害吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
过氧化氢MSDS

过氧化氢安全技术说明(MSDS)1、物质的理化常数2.对环境的影响:一、健康危害侵入途径:吸入、食入。
健康危害:吸入本品蒸气或雾对呼吸道有强烈刺激性。
眼直接接触液体可致不可逆损伤甚至失明。
口服中毒出现腹痛、胸口痛、呼吸困难、呕吐、一时性运动和感觉障碍、体温升高等。
个别病例出现视力障碍、癫痫样痉挛、轻瘫。
二、毒理学资料及环境行为急性毒性:LD504060mg/kg(大鼠经皮);LC502000mg/m3,4小时(大鼠吸入)致突变性:微生物致突变:鼠伤寒沙门氏菌10μL/皿;大肠杆菌5ppm。
姊妹染色单体交换:仓鼠肺353μmol/L。
致癌性:IARC致癌性评论:动物可疑阳性。
危险特性:爆炸性强氧化剂。
过氧化氢本身不燃,但能与可燃物反应放出大量热量和气氛而引起着火爆炸。
过氧化氢在pH 值为~时最稳定,在碱性溶液中极易分解,在遇强光,特别是短波射线照射时也能发生分解。
当加热到100℃以上时,开始急剧分解。
它与许多有机物如糖、淀粉、醇类、石油产品等形成爆炸性混合物,在撞击、受热或电火花作用下能发生爆炸。
过氧化氢与许多无机化合物或杂质接触后会迅速分解而导致爆炸,放出大量的热量、氧和水蒸气。
大多数重金属(如锨、铜、银、铅、汞、锌、钴、镍、铬、锰等)及其氧化物和盐类都是活性催化剂,尘土、香烟灰、碳粉、铁锈等也能加速分解。
浓度超过74%的过氧化氢,在具有适当的点火源或温度的密闭容器中,会产生气相爆炸。
燃烧(分解)产物:氧气、水。
3.现场应急监测方法:便携式气体检测仪4.实验室监测方法:分光光度法(WS/T132-1999,作业场所空气)电化学法《食品中添加剂的分析方法》,马家骧等译5.环境标准:前苏联(1975)工作环境空气中最大允许浓度m36.应急处理处置方法:一、泄漏应急处理迅速撤离泄漏污染人员至安全区,并进行隔离,严格限制出入。
建议应急处理人员戴自给正压式呼吸器,穿防酸碱工作服。
尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns. Contact with combustible material may cause fire.Corrosive.Light sensitive.
Potential Health Effects
Synonym:
Carbamide peroxide; Hydrogen dioxide; Peroxide; Hydroperoxide; Urea peroxide; Hydrogen peroxide 100 volumes
CAS:
7722-84-1
Section 1 - Chemical Product
过氧化氢HS ;2847000000
过氧化氢化学式为H2O2,俗称双氧水,外观为无色透明液体,是一种强氧化剂,适用于伤口消毒及环境、食品消毒。
中文名:
过氧化氢
外文名:
Hydrogen peroxide
别名:
双氧水
化学式:
H2O2
相对分子质量:
34.01
化学品类别:
无机物--过氧化物
管制类型:
过氧化氢(*)(易制爆)
Eye:
Contact with liquid is corrosive to the eyes and causes severe burns. Contact with the eyes may cause corneal damage.
Skin:
Causes severe skin irritation and possible burns. May cause discoloration, erythema (redness), swelling, and the formation of papules and vesicles (blisters).
MSDS Name:Hydrogen Peroxide 35 wt.% Solution in Water Stabilized P.A. Material Safety Data Sheet
Synonym:Carbamide peroxide; Hydrogen dioxide; Peroxide; Hydroperoxide; Urea peroxide;s
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea. Causes gastrointestinal tract burns. May cause vascular collapse and damage. May cause damage to the red blood cells. May cause difficulty in swallowing, stomach distension, possible cerebral swelling and death. Ingestion may result in irritation of the esophagus, bleeding of the stomach and ulcer formation.
SUPEROXOL;60%solution;60%solutioninwater;Albone 35
毒害物质数据:7722-84-1(Hazardous Substances Data)
职业标准:TWA 1.4毫克/立方米; STEL 4.2毫克/立方米[2]
编辑本段物理性质
CAS号
7722-84-1[2]
Notes to Physician:
Treat symptomatically and supportively. Attempts at evacuating the stomach via emesis induction or gastric lavage should be avoided. In the event of severe distension of the stomach or esophagus due to gas formation, insertion of a gastric tube may be required. To treat corneal damage, careful ophthalmologic evaluation is recommended and the possibility of local corticosteroid therapy should be considered.
熔点(℃)
-33°C[2]
沸点(℃)
108°C[2]
折射率
1.3350[2]
EINECS登录号
231-765-0[2]
密度
1.13 g/mL at 20°C[2]
闪点
107°C
水溶液为无色透明液体,溶于水、醇、乙醚,不溶于石油醚。[3]纯的过氧化氢是一种淡蓝色粘稠状液体。[4]
纯过氧化氢是淡蓝色的粘稠液体,熔点-0.43°C,沸点150.2°C。凝固点时固体密度为1.71g/cm3,密度随温度升高而减小。它的缔合程度比H2O大,所以它的介电常数和沸点比水高。纯过氧化氢比较稳定,若加热到153°C便猛烈的分解为水和氧气。
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Wash mouth out with water. Vomiting may occur spontaneously. If vomiting occurs and the victim is conscious, give water to further dilute the chemical.
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
CAS#
Chemical Name
content
EINECS#
7722-84-1
Hydrogen peroxide
35
231-765-0
Hazard Symbols: O C
Risk Phrases: 34 8
Use water spray to keep fire-exposed containers cool. Substance is noncombustible. Use water with caution and in flooding amounts.
Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Some oxidizers may react explosively with hydrocarbons(fuel). May decompose explosively when heated or involved in a fire. May accelerate burning if involved in a fire.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Chronic:
Prolonged or repeated skin contact may cause dermatitis. Laboratory experiments have resulted in mutagenic effects. Repeated contact may cause corneal damage.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Water runoff can cause environmental damage. Dike and collect water used to fight fire. Strong oxidizer. Contact with other material may cause fire. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
