水的粘度计算表
水的粘度计算表

水的粘度计算表水的黏度表(0~40℃)温度T粘度μPa·s或N·s·m-2温度T粘度μPaN·K ℃K273.16 1.7921 1.7921×10-320.2 293.36 1.00001.000010-3274.16 1.7313 1.7313×10-321 294.16 0.98100.981010-3275.16 1.6728 1.6728×10-322 295.16 0.95790.957910-3276.16 1.6191 1.6191×10-323 296.16 0.93580.935810-3 1.5674×0.9142281.16 1.3860 1.3860×10-328 301.16 0.83600.836010-3282.16 1.3462 1.3462×10-329 302.16 0.81800.818010-3283.16 1.3077 1.3077×10-330 303.16 0.80070.800710-3284.16 1.2713 1.2713×10-331 304.16 0.78400.784010-3285.16 1.2363 1.2363×10-332 305.16 0.76790.767910-3286.16 1.2028 1.2028×10-333 306.16 0.75230.752310-3水的物理性质12.31 988.1 209.30 4.174 64.78 54.94 4.49 67.7 19.932 983.2 251.12 4.178 65.94 46.88 5.11 66.2 31.164 977.8 292.99 4.178 66.76 40.61 5.70 64.3 47.379 971.8 334.94 4.195 67.45 35.65 6.32 62.6 70.136 965.3 376.98 4.208 67.98 31.65 6.95 60.7 101.33 958.4 419.10 4.220 68.04 28.38 7.52 58.8 143.31 951.0 461.34 4.238 68.27 25.89 8.08 56.9 198.64 943.1 503.67 4.250 68.50 23.73 8.64 54.8 270.25 934.8 546.38 4.266 68.50 21.77 9.17 52.8 361.47 926.1 589.08 4.287 68.27 20.10 9.72 50.7 476.24 917.0 632.20 4.312 68.38 18.63 10.3 48.62320.88 840.3 943.70 4.614 64.55 12.46 14.8 33.8 2798.59 827.3 990.18 4.681 63.73 11.97 15.9 31.6 3347.91 813.6 1037.49 4.756 62.80 11.47 16.8 29.1 3977.67 799.0 1085.64 4.844 61.76 10.98 18.1 26.7 4693.75 784.0 1135.04 4.949 60.84 10.59 19.7 24.2 5503.99 767.9 1185.28 5.070 59.96 10.20 21.6 21.9 6417.24 750.7 1236.28 5.229 57.45 9.81 23.7 19.5 7443.29 732.3 1289.95 5.485 55.82 9.42 26.2 17.2 8592.94 712.5 1344.80 5.736 53.96 9.12 29.2 14.7 9877.96 691.1 1402.16 6.071 52.34 8.83 32.9 12.3 11300.3 667.1 1462.03 6.573 50.59 8.53 38.2 10.0F3Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects andthe van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式

水的黏度表(0~40℃)之巴公井开创作水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, sonormally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water;hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式
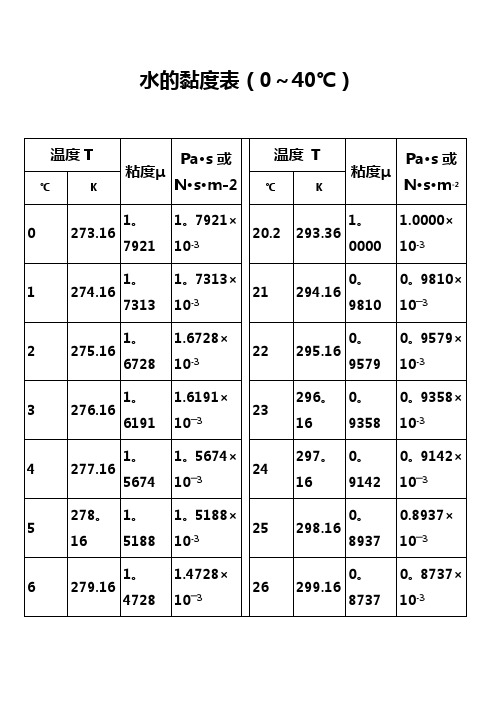
水的黏度表(0~40℃)水的物理性质F3Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them。
As the pressure increases, the volume decreases and the volume of thesevoids reduces,so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534]can be explained by the increased pressure (up to about 150 MPa)causing deformation,so reducing the strength of the hydrogen—bonded network,which is also partially responsible for the viscosity。
This reduction in cohesivity more than compensates for the reduced void volume。
It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558]in water;hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities),the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity,then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength。
水的粘度计算表水的动力粘度计算公式ea

水的黏度表(0~40℃)水的物理性质370 264F3Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist betweenthem. As the pressure increases, the volume decreases and the volume of thesevoids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increasedpressure (up to about 150 MPa) causing deformation, so reducing the strength ofthe hydrogen-bonded network, which is also partially responsible for the viscosity.This reduction in cohesivity more than compensates for the reduced void volume. Itis thus a direct consequence of the balance between hydrogen bonding effects andthe van der Waals dispersion forces [558] in water; hydrogen bonding prevailing atlower temperatures and pressures. At higher pressures (and densities), the balancebetween hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的粘度计算表WTD standardization office【WTD 5AB- WTDK 08- WTD 2C】水的黏度表(0~40℃)水的物理性质350360 109370 264F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence ofthe between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式

水的黏度表(0〜40 C)水的物理性质F3 Viscosity decreases with p ressure(at temp eratures below 33Water's p ressure-viscosity behavior [534] can be explained by the in creased p ressure (up to about 150 MPa) caus ing deformatio n, so reduci ng the stre ngth of the hydroge n-bon ded n etwork, which is also p artially res pon sible for the viscosity. This reduct ion in cohesivity more tha n compen sates for the reduced void volume. It is thus a direct con seque nee of the bala nee betwee n hydroge n bonding effects and the van der Waals dis persion forces [558] in water; hydroge n bonding p revaili ng at lower temp eratures and p ressures. At higher p ressures (and den sities), the bala nee betwee n hydroge n bonding effects and the van der Waals dis persi on forces is tipped in favor of the dis persion forces and the rema ining hydroge n bonds are stron ger dueViscous flow occurs by molecules movi ng through the voids that exist betwee n them. As the p ressure in creases, the volume decreases and the volume ofthese voids reduces, so no rmally in creas ing p ressure in creases the viscosity.|:|k-二 _r 13ireSC去*. i i screr- 丁" \ . / . 一 '气:rJ J:V .; r "舄■ 3 口二K nPV ■■L T 三n 曲 •■ 5 M r丐 町寸 -;J 百* "T N ; 【I bl■呻口 " 口寸津a “ d c i 0 290八rao 800 i wooPressure, MPa g亠C)Co©4— □]J%一MJs」气1□ u古气 a15•” ”〕阳"1■ \■ID% ;:s' ¥口『 屮n◎ 9 r奇* =' f f- ::[丄 备IT记|B - 3 D■i电-'uO丰759勺;】I -一 11 L. Pto the closer p roximity of the con tribut ing oxyge n atoms [655]. Viscosity, the n, in creases with p ressure. The dashed line (opp osite) in dicates the viscosity mi ni ma.1 .a』IflOXThe variati on of viscosity with p ressure and temp erature has bee n used as evide neethat the viscosity is determ ined more by the exte nt of hydroge n bonding rather tha nhydroge n bonding stre ngth.Self-diffusio n is also affected by p ressure where (at low temp eratures) both the tran slatio nal and rotati onal moti on of water ano malously in crease as the p ressure in creases.1血200 oPr^sure ; MPa 75X、” _50^ 山:30°C 20弋5'10X2.2 X51 ---- 护乞fOr,QR 牡m 护,/a"-1- yiy二 h--------- 0c ,宀;:u 占14^ra_ 7^6^-*=0。
水的粘度计算表-水的动力粘度计算公式之欧阳引擎创编

水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressureincreases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures(and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的黏度表0~40℃水的物理性质F3 Viscosity decreases with pressure at temperatures below 33°CViscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior can be explained by the increased pressure up to about 150 MPa causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures and densities, the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms . Viscosity, then, increases with pressure. The dashed line opposite indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where at low temperatures both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的黏度表(0~40℃)水的物理性质F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume.It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式
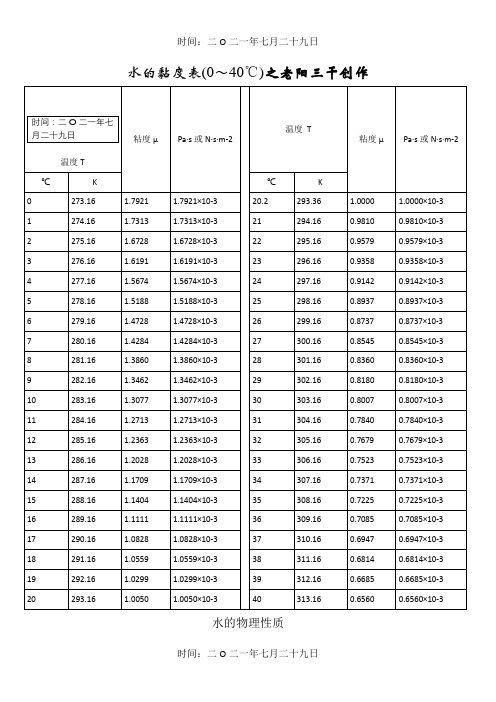
水的黏度表(0~40℃)之老阳三干创作水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, sonormally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water;hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式之欧阳文创编

水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases theviscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waalsdispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima. The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的粘度计算表 Revised as of 23 November 2020水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of thesevoids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式

水的黏度表(0~40℃)之吉白夕凡创作水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, sonormally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water;hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersionforces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表

水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, sonormally increasing pressure increases the viscosity.37021040.9450.51892.4340.31933.73 5.692640.48 6.80Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of thehydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of thebalance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima..The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.如有侵权请联系告知删除,感谢你们的配合!精品。
水的粘度计算表

313.16
0.6560
0.6560×10-3
水的物理性质
温度t/℃
饱和蒸气压p/kPa
密度ρ/kg·m-3
焓
H/kJ·kg-1
比定压热容cp/kJ·kg-1·K-1
导热系数λ/10-2W·m-1·K-1
粘度μ/10-5Pa·s
体积膨胀系数α/10-4K-1
表面张力σ/10-3N·m-1
普兰德数Pr
(at temperatures below33°C)
Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.
290
7443.29
732.3
1289.95
5.485
55.82
9.42
26.2
17.2
0.93
300
8592.94
712.5
1344.80
5.736
53.96
9.12
29.2
14.7
0.97
310
9877.96
691.1
1402.16
6.071
52.34
8.83
32.9
12.3
1.02
320
11300.3
934.8
546.38
4.266
水的粘度计算表

水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度计算表-水的动力粘度计算公式

创作编号:GB8878185555334563BT9125XW创作者:凤呜大王*水的黏度表(0~40℃)水的物理性质F3Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases theviscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.创作编号:GB8878185555334563BT9125XW创作者:凤呜大王*。
