WHO现场主文件编写指南
who验证指南2016年版

1.INTRODUCTION简介1.1Validation is an essential part of good practices including good manufacturing practices (GMP)(4)and good clinical practices(GCP).It is therefore an element of the pharmaceutical quality system.Validation,as a concept,incorporates qualification and should be applied over the life cycle of,e.g.the applicable product,process,system,equipment or utility.验证是包括GMP和GCP在内的良好规范的必要部分。
因此是制药质量体系的组成部分。
验证这个概念包括确认并且应该在产品、工艺、系统、设备或设施的整个生命周期中被使用。
1.2These guidelines cover the general principles of validation and qualification.In addition to the main part,appendices on validation and qualification(e.g.cleaning,computer and computerized systems,equipment,utilities and systems,and analytical methods)are included.这个指南包括验证和确认的一般原则。
除了主体部分外,还包括确认和验证附件(如清洁、计算机和计算机化系统、设备、设施和系统以及分析方法)。
1.3The following principles apply:适用的原则如下:the execution of validation should be in compliance with regulatory expectations;验证的执行应该符合监管预期quality,safety and efficacy must be designed and built into the product;质量、安全和效力应该被设计和构建到产品中quality cannot be inspected or tested into the product;质量不能通过检查或测试赋予产品quality risk management principles should be applied in determining the need,scope and extent of validation;应该应用质量风险管理规则来决定验证的需求、范围和程度ongoing review should take place to ensure that the validated state is maintained and opportunities for continuing improvement are identified.应该进行持续的回顾来确保维持验证状态并识别持续改进的机会1.4The implementation of validation work requires considerable resources such as:验证工作的完成需要大量资源,例如time:generally validation work is subject to rigorous time schedules;时间:一般验证工作需要有一个严密的时间表financial:validation often requires the time of specialized personnel and expensive technology.资金:验证经常需要专业人员和昂贵的技术human:validation requires the collaboration of experts from various disciplines(e.g.a multidisciplinary team,comprising quality assurance, engineering,information technology,manufacturing and other disciplines,as appropriate.).人员:验证需要不同学科专家的协作(例如一个多学科小组,视情况可能包括QA、工程、信息技术、生产以及其他学科人员)范围2.1These guidelines focus mainly on the overall concept of validation and are not intended to be prescriptive in specific validation requirements.This document serves as general guidance only and the principles may be considered useful in its application in the manufacture and control of starting materials and finished pharmaceutical products(FPPs), as well as other areas.Validation of specific processes and systems,for example,in sterile product manufacture,requires much more consideration and a detailed approach that is beyond the scope of this document.这些指南主要关注验证的整体概念,无意规定具体的验证要求。
WHO第技术报告药物生产技术转移指南中英文精编版

W H O第技术报告药物生产技术转移指南中英文公司标准化编码 [QQX96QT-XQQB89Q8-NQQJ6Q8-MQM9N]WHO第961号技术报告附件7 药物生产技术转移指南World Health OrganizationWHO Technical Report Series, No. 961, 2011WHO第961号技术报告附件7 药物生产技术转移指南?Annex 7?附件7WHO guidelines on transfer of technology in pharmaceutical manufacturingWHO药物生产技术转移指南1. Introduction?介绍2. Scope?范围3. Glossary?术语4. Organization and management?组织和管理5. Production: transfer (processing, packaging and cleaning)生产:转移(工艺、包装和清洁)6. Quality control: analytical method transfer质量控制:分析方法转移7. Premises and equipment?厂房设施和设备8. Documentation?文件9. Qualification and validation?确认和验证References?参考文献?1.Introduction?介绍These guiding principles on transfer of technology are intended to serve as a framework which can be applied in a flexible manner rather than as strict rigid guidance. Focus has been placed on the quality aspects, in line with WHO’s mandate.本指南中关于技术转移的原则意在作为一个框架,以不同方式应用,而不是一个需要严格遵守的指南。
WHO《数据完整性指南》-2021(中英文对照版)

WHO《数据完整性指南》-2021(中英文对照版)3月29日,WHO发布了第55 届药物制剂规范专家委员会(ECSPP)技术报告TRS No.1033,其中包含新的《数据完整性指南》,翻译如下,分享给大家!Guideline on data integrity数据完整性指南1. Introduction and background介绍和背景1.1. In recent years, the number ofobservations made regarding the integrity of data, documentation and recordmanagement practices during inspections of good manufacturing practice (GMP) (2),good clinical practice (GCP), good laboratory practice (GLP) and Good Trade andDistribution Practices (GTDP) have been increasing. The possible causes forthis may include近年来,在对良好生产规范(GMP)(2)、良好临床规范(GCP)、良好实验室规范(GLP)和良好贸易和分销规范(GTDP)的检查过程中,对数据完整性、文件和记录管理规范的缺陷数量持续增加。
可能的原因包括:(ⅰ) reliance on inadequate human practices;依赖于不适当的人员操作;(ⅱ)poorly defined procedures;规定糟糕的规程(ⅲ)resource constraints;资源限制(ⅳ) the use of computerized systems that are not capable of meetingregulatory requirements or are inappropriately managed and validated (3, 4);使用不满足法规要求,或管理/验证不当的计算机系统(3,4);(ⅴ) inap propriate and inadequate control of data flow; and不适当和不充分的数据流控制;和(ⅵ)failure to adequately review and manage original data and records.未能充分审核和管理原始数据和记录。
WHO良好的数据和记录管理规范指南中文版

WHO良好的数据和记录管理规范指南中文版良好的数据和记录管理规范旨在确保组织能够有效地管理和保护其数据和记录。
这份草案提供了一套指南,以帮助组织制定和实施数据和记录管理策略,并确保其符合法律、法规和行业标准的要求。
1.引言1.1背景和目的1.2适用范围1.3定义2.数据和记录分类2.1数据分类2.2记录分类2.3敏感数据和记录分类3.数据和记录保护要求3.1访问控制3.2加密3.3备份和恢复3.4存储设备管理3.5数据传输3.6内部控制4.数据和记录保存期限4.1法律和法规要求4.2合同和协议要求4.3内部政策要求5.数据和记录销毁5.1销毁程序5.2销毁方法6.数据和记录审计6.1审计过程6.2审计记录6.3审计结果和建议7.数据和记录追溯7.1追溯流程7.2追溯记录和报告8.数据和记录安全培训8.1培训计划8.2培训内容8.3培训评估9.数据和记录管理责任9.1数据和记录拥有者责任9.2数据和记录管理团队责任9.3内部审计责任10.变更管理10.1变更控制流程10.2变更记录和审核11.风险管理11.1风险评估11.2风险控制措施12.提示和警告12.1数据和记录处理提示12.2数据和记录保护警告13.执行和监控13.1监控措施13.2故障处理13.3改进措施本指南的草案是根据国际数据和记录管理最佳实践编写的,旨在帮助组织确保其数据和记录管理规范符合国际标准。
组织可以根据自身的具体需求对本草案进行调整和完善,并制定符合其业务环境和需求的数据和记录管理策略。
良好的数据和记录管理是组织成功运营和合规的基础。
通过遵循本指南,组织可以建立一个高效和安全的数据和记录管理体系,从而提高运营效率、降低风险,并满足利益相关者的需求和期望。
WHO医院感染预防与控制实用指南(第二版)

WHO医院获得性感染预防控制实用指南(2002年第二版)目录前言第一章医院感染流行病学1.1 医院感染的定义1.2 医院感染部位1.2.1泌尿道感染1.2.2手术部位感染1.2.3医院肺炎1.2.4医院菌血症1.2.5其他医院感染1.3 微生物1.3.1细菌1.3.2病毒1.3.3寄生虫和真菌1.4 宿主和传播第二章感染控制计划2.1 国家或地方计划2.2 医院计划2.2.1感染控制委员会2.2.2感染控制专业人员(感染控制小组)2.2.3感染控制手册2.3 感染控制职责2.3.1医院管理部门的职责2.3.2医生职责2.3.3微生物学家职责2.3.4医院药剂师职责2.3.5护理人员职责2.3.6灭菌中心职责2.3.7饮食服务中心职责2.3.8洗衣房职责2.3.9保洁服务部职责2.3.10检修部门职责2.3.11感染控制小组(医院卫生服务部)职责第三章医院感染监测3.1 目标3.2 战略3.2.1 医院性医院感染监测3.2.2 地区性或全国性医院感染监测3.3 方法3.3.1现患调查3.3.2发病率调查3.3.3计算率3.4 有效的监测组织3.4.1资料收集和分析3.4.2反馈/报告3.4.3预防和评价3.5 评价监测系统3.5.1监测战略评价3.5.2反馈信息评价3.5.3证实/资料质量第四章暴发的处理4.1 识别暴发4.2 调查暴发4.2.1计划调查4.2.2定义病例4.2.3描述暴发4.2.4建议和检测假设4.2.5控制措施和随访4.2.6交流第五章医院感染的预防5.1 危险分层5.2 减少人与人之间的传播5.2.1洗手5.2.2个人卫生5.2.3着装5.2.4口罩5.2.5手套5.2.6安全注射5.3 预防环境传播5.3.1医院环境的清洁5.3.2热水/超热水的使用5.3.3病人器械的消毒5.3.4灭菌第六章常见地方性医院感染流行的预防6.1 泌尿道感染6.2 外科伤口感染(手术部位感染)6.2.1手术室环境6.2.2手术室工作人员6.2.3病人的手术前准备6.2.4预防性抗微生物药物的应用6.2.5外科伤口监测6.3 医院呼吸道感染6.3.1重症监护病房呼吸机相关性肺炎6.3.2内科病房6.3.3外科病房6.3.4 气管切开术的神经科病人6.4 与血管装置相关的感染6.4.1周围血管导管6.4.2中心血管导管6.4.3全植入的中心血管导管第七章病人护理的感染控制预防7.1 预防的实践7.1.1标准(常规)预防7.1.2特殊传播方式的额外预防7.2 抗微生物药物耐药菌第八章医院环境8.1 医院建筑8.1.1建造或改建计划8.1.2建筑分区8.1.3交通流程8.1.4建筑材料8.2 空气8.2.1经空气传播的污染和传染8.2.2通气设备8.2.3手术室8.2.4超净空气8.3 水8.3.1饮用水8.3.2浴水8.3.3药剂(医疗)用水8.3.4微生物学监测8.4 食物8.4.1食物中毒的因素和经食物传播的感染8.4.2食物中毒的因素8.4.3预防食物中毒8.5 废弃物8.5.1定义和分类8.5.2医疗废弃物的处理保存和运输第九章抗微生物药物的使用和抗微生物药物的耐药性9.1 抗微生物药物的合理使用9.1.1 治疗9.1.2 化学药物预防9.2 抗微生物药物的耐药性9.2.1耐甲氧西林金黄色葡萄球菌9.2.2肠球菌9.3 抗生素控制政策9.3.1抗微生物药物应用委员会9.3.2微生物实验室的职责9.3.3监测抗微生物药物的使用第十章预防工作人员感染10.1 暴露于人类免疫缺陷病毒10.2 暴露于乙肝病毒10.3 暴露于丙肝病毒10.4 脑膜炎奈瑟菌感染10.5 分枝结核杆菌10.6 其他感染附录1 推荐阅读丛书附录2 网上资源前言医院感染也称“医院获得性感染”,定义是病人在医院获得的不同于入院病因的感染,这种感染在入院时不存在,也不处于潜伏期,而是发生在医院或其他医疗保健机构内。
who数据完整性指南中文版_图文

WHO数据完整性指南:良好的数据和记录规范红色文字部分为与原草案对比有变化的地方。
1.介绍1.1.世界范围的药品监管系统常常依赖于企业在开发、生产和包装、检测、销售和监控药品方面的知识。
在评估和审核过程中隐含的是监管者和被监管者之间相信注册文件中提交的和用于日常决策的信息是全面、完整和可信的。
因此基于此做出决策的数据应该在完整的同时也要是可追溯至产生数据的人的、清晰易读的、同步产生的、原始的和准确的。
通常这个被称作“ALCOA”。
1.2.这些基础的ALCOA原则和保证数据可靠性的相关良好的规范的期望都不是新的,许多高和中水平的规范性的指南已经存在了。
尽管如此,近几年,在GMP、GCP和GLP检查中出现与良好数据和记录管理规范相关的缺陷项的数量还在增加。
卫生监管机构对数据可靠性的越来越多的关注的原因毋庸置疑是多方面的并包括增加的关于行业选择和适当的现代的控制策略之间的差距的法规意识和关注。
1.3.影响因素包括企业没有实施耐用的系统来约束数据风险、没有改进对数据可靠性的丧失的状况的可检测性、和/或当失效出现时没有调查和找到根本原因。
例如,遵从药品良好规范的企业已经使用计算机化系统几十年但很多没有充分地回顾和管理原始电子记录仅仅是常常回顾和管理不完整和/或不充分的打印出来的资料。
这些缺陷强调了制药行业使历史的控制策略现代化和对当前的经验模式(比如外包和全球化)也对当前使用的技术(比如计算机化系统)应用时髦的质量风险管理和合理的科学原则的需要。
1.4.可能需要开发和强化以确保良好数据管理策略的控制的例子包括但不限于以下方面:1)质量风险管理的方法通过确保管理层的期望和实际过程能力相一致来有效保证患者安全和产品质量及数据有效性。
管理层应该对通过一开始就根据工艺、方法、环境、人员、技术和其他的当前实际的能力设定事实求是的并可实现的期望的方式来实现良好的数据管理负责。
2)工艺的持续监控和由管理层分配必要的资源来确保和根据需要加强基础设施(例如,持续改进工艺和方法;确保建筑、设施、设备和系统的充分设计和维护;确保充足可靠的电和水的供应;提供对人员必要的培训;为确保外包商和供应商充分满足质量标准分配必需资源去监管等)。
世界卫生组织 工厂主文件 起草 指南

Working document QAS/10.378 July 2010 RESTRICTEDWHO GUIDELINES FOR DRAFTING A SITE MASTER FILEDRAFT FOR COMMENTSThe Prequalification Programme currently uses a site master file (SMF). Your feedback would be very much appreciated as to whether this SMF needs to be revised in light of new developments, e.g. by the Pharmaceutical Inspection Co-operation Scheme (PIC/S). Please address any comments on this proposal, by 1 September 2010 to Dr A.J. van Zyl, Head of Inspections, Prequalification Programme, World Health Organization, 1211 Geneva 27, Switzerland, fax: (+41 22) 791 4730 or e-mail: vanzyla@who.int with a copy to gaspardm@who.int and to bonnyw@who.int. During the past few years we have moved more towards an electronic system for sending out our working documents for comment, for convenience and in order to speed up the process. If you do not already receive our documents electronically, please let us have your e-mail address (to bonnyw@who.int) and we will add it to our electronic mailing list.© World Health Organization 2010 All rights reserved. This draft is intended for a restricted audience only, i.e. the individuals and organizations having received this draft. The draft may not be reviewed, abstracted, quoted, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means outside these individuals and organizations (including the organizations' concerned staff and member organizations) without the permission of the World Health Organization. The draft should not be displayed on any web site. Please send any request for permission to: Dr A.J. van Zyl, Head of Inspections, Prequalification Programme, Quality Assurance and Safety: Medicines, Department of Essential Medicines and Pharmaceutical Policies, World Health Organization, CH-1211 Geneva 27, Switzerland. Fax: (41-22) 791 4730; e-mail: vanzyla@who.int. The designations employed and the presentation of the material in this draft do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this draft. However, the printed material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. This draft does not necessarily represent the decisions or the stated policy of the World Health Organization.Working document QAS/10.378 page 2 SCHEDULE FOR THE PROPOSED ADOPTION PROCESS OF DOCUMENT QAS/10.378: Guidelines for drafting a site master fileNeed for possible revision identified by WHO Prequalification May 2010 Programme Draft mailed out for comments Collation of comments received Presentation to the WHO Expert Committee on Specifications for Pharmaceutical Preparations Further action as necessary July-August 2010 September 2010 18-22 October 2010…Working document QAS/10.378 page 3GUIDELINES FOR DRAFTING A SITE MASTER FILE (SMF)A site master file (SMF) for each manufacturing site of a finished pharmaceutical product (FPP) listed in a product dossier, should be submitted on a CD or DVD to the Inspection unit. A SMF for each manufacturing site of active pharmaceutical ingredient (API) and contract research organization (CRO), listed in a product dossier, should be submitted on request from the Inspection unit. A SMF should be succinct and, as far as possible, not exceed 25 A4 pages. A SMF is a document prepared by the manufacturer containing specific and factual good manufacturing practice (GMP) information about the production and/or control of pharmaceutical manufacturing operations carried out at the named site and any closely integrated operations at adjacent and nearby buildings. If only part of a pharmaceutical operation is carried out on the site, the SMF need describe only those operations, e.g. analysis, packaging. Layout of the SMF Front page Table of contents Contents 1. General information1.1 Brief information on the firm (including name and address), relation to other sites, and, in particular, any information relevant to understanding the manufacturing operations. 1.2 1.3 Pharmaceutical manufacturing activities as licensed by the national authority. Any other manufacturing activities carried out on the site.1.4 Name and exact address of the site, including telephone, fax and 24-hour telephone numbers. 1.5 Type of products manufactured on the site and information about any specifically toxic or hazardous substances handled, mentioning the way they are manufactured (in dedicated facilities or on a campaign basis). 1.6 Short description of the site (size, location and immediate environment and other manufacturing activities on the site).Working document QAS/10.378 page 4 1.7 Number of employees engaged in production, quality control, storage and distribution. 1.8 Use of outside scientific, analytical or other technical assistance in relation to manufacture and analysis. 1.9 Short description of the quality management system of the firm responsible for manufacture. 2. Personnel2.1 Organization chart showing the arrangements for quality assurance, including production and quality control. 2.2 Qualifications, experience and responsibilities of key personnel.2.3 Outline of arrangements for basic and in-service training and how records are maintained. 2.4 2.5 3. Health requirements for personnel engaged in production. Personnel hygiene requirements, including clothing. Premises and equipment3.1 Simple plan or description of manufacturing areas with indication of scale (architectural or engineering drawings not required). 3.2 Nature of construction and finishes.3.3 Brief description of ventilation systems. More details should be given for critical areas with potential risks of airborne contamination (schematic drawings of the systems are desirable). Classification of the rooms used for the manufacture of sterile products should be mentioned. 3.4 Special areas for the handling of highly toxic, hazardous, and sensitizing materials. 3.5 Brief description of water systems (schematic drawings of the systems are desirable), including sanitation. 3.6 Description of planned preventive maintenance programmes for premises and of the recording system. Equipment 3.7 Brief description of major equipment used in production and control laboratories (a list of equipment is not required).Working document QAS/10.378 page 5 3.8 Description of planned preventive maintenance programmes for equipment and of the recording system. 3.9 Qualification and calibration, including the recording system. Arrangements for computerized systems validation. Sanitation 3.10 Availability of written specifications and procedures for cleaning manufacturing areas and equipment. 4. Documentation4.1 Arrangements for the preparation, revision, and distribution of necessary documentation for manufacture. 4.2 Any other documentation related to product quality that is not mentioned elsewhere (e.g. microbiological controls on air and water). 5. Production5.1 Brief description of production operations using, wherever possible, flow sheets and charts specifying important parameters. 5.2 Arrangements for the handling of starting materials, packaging materials, and bulk and finished products, including sampling, quarantine, release, and storage. 5.3 5.4 6. Arrangements for the handling of rejected materials and products. Brief description of general policy for process validation. Quality control6.1 Description of the quality control system and of the activities of the quality control department. Procedures for the release of finished products. 7. Contract manufacture and analysis7.1 Description of the way in which the GMP compliance of the contract accepter is assessed. 8. 8.1 8.2 Distribution, complaints and product recall Arrangements and recording system for distribution. Arrangements for the handling of complaints and product recalls.Working document QAS/10.378 page 6 9. 9.1 Self-inspection Short description of the self-inspection system. ***。
WHO组织DMF编制指南

WHO组织DMF编制指南WHO(世界卫生组织)是全球最高级别的公共卫生组织,致力于提高全球人民的健康水平。
WHO通过制定和推动各种指南和政策,帮助各国制定和实施公共卫生策略,以应对各种疾病和健康挑战。
DMF(伤残、功能和健康分级)是一种用于评估和描述人类功能和行动能力的框架,这一概念广泛应用于医疗领域和公共卫生政策制定中。
DMF的编制指南由WHO负责制定,以确保其在全球范围内的一致性和可操作性。
编制DMF指南的目的是提供一个统一的框架,用于评估和描述人类功能和行动能力,从而更好地理解健康和伤残的影响,有助于制定和改进公共卫生政策和服务。
编制DMF指南的过程通常包括以下步骤:1.收集证据:WHO召集一组专家,收集和审查关于人类功能和行动能力的最新研究和证据。
这些证据可以包括生理测量、社会调研、临床研究等。
2.制定概念模型:根据收集到的证据,专家组织整理出一个系统的概念模型,以描述人类功能和行动能力的不同维度和因素。
3.制定指标和分类:基于概念模型,专家组织制定出一套指标和分类,用于评估和描述人类功能和行动能力的不同方面。
这些指标和分类通常包括生理功能、认知功能、社会功能等。
4.草案审查和反馈:专家组制定出初步的DMF指南草案,并征求相关利益相关者以及其他感兴趣方的意见和反馈。
根据收到的反馈,草案进行适当的修订和完善。
5.正式发布和宣传:修订后的DMF指南被正式发布,并通过各种渠道向全球公众、医疗专业人员和政策制定者宣传。
这些渠道包括WHO网站、学术期刊、会议和培训等。
需要注意的是,DMF指南的编制是一个动态的过程,会随着研究进展和社会需求的变化而进行更新和修订。
因此,WHO在制定DMF指南时,通常会考虑将来的发展趋势和需求,以确保指南的持续有效性和适用性。
编制DMF指南对于改善全球公共卫生意义重大。
通过提供一个统一的框架,DMF指南有助于促进全球各国在功能和行动能力评估和描述方面的一致性,提供可比较的数据和信息,为决策者制定和改进公共卫生策略和服务提供科学依据。
WHO报告对技术转移的详细指南(5篇)

WHO报告对技术转移的详细指南(5篇)第一篇:WHO报告对技术转移的详细指南WHO报告对技术转移的详细指南WHO技术报告(No:961,2011年)附件7(第285 – 309页)发布了关于药品生产的技术转移(WHO Guidelines on Transfer of Technology inPharmaceutical Production)。
关于这个主题,到目前为止欧美国家均没有具体指南,仅ISPE有过该主题的一些建议,因此这指南备受业界的重视。
本指南共分九个章节,1.介绍、2.范围、3.术语、4.组织与管理、5.生产转移(工艺、包装和清洁)、6.分析方法转移、7.设备与设备、8.文件、9.确认与验证。
现将一些亮点介始如下:1.技术转移包含文件转移和接受能力转移,从而有效地执行技术转移的关键要素,使得两方和监管部门均满意为宗旨。
2.在第4章的组织与管理中介绍了转移方案的详细清单等内容。
3.在第5章的生产转移中对原料药和辅料的转移信息有非常细的要求清单。
4.在第6章的质量控制的分析方法转移中,包括原材料和包装材料的检验方法,工艺验证和清洁验证的检验方法。
表1是关于《分析方法的设计与接受标准表》,包含了详细的内容。
包含了鉴别、含量、含量均匀度、溶出度、清洁验证(擦洗取样)和微生物的定性与定量、杂质、降解物、残留溶剂等。
对每一个实验方法,你均可在表中找到下列信息:⌝⌝⌝⌝方法转移需要考虑的事项;重复实验的要求清晰的比较因子接受标准(平均值与标准偏差要求)现将表中的一些要求介绍如下:⎫含量测定的方法转移:每个实验室均应需要2人进行实验,三批样品、每批做三个样品,每个实验室需要做2×3×3=18数据。
接收标准是两个实验室的平均值应≤2%;⎫溶出度的方法转移:每个实验室均应需要2人进行实验,一批样品,接收标准是两个实验室的平均值应≤±3%;⎫含量均匀度的方法转移:每个实验室均应6片,一批样品,接收标准是两个实验室的平均值应≤±5%;⎫杂质、降解物、残留溶剂的方法转移:考虑的因子有RRT、定量限、图谱比较、准确度和中间精密度。
who指南制定手册

who指南制定手册《WHO指南制定手册》是世界卫生组织(WHO)发布的一份指导文件,旨在帮助制定各种类型的指南,包括临床实践指南、公共卫生指南和技术指南等。
该手册提供了制定指南的全过程,包括指南的目标和范围、方法学、证据综述、实施和监测等方面的指导。
以下是《WHO指南制定手册》的部分内容:一、前言本手册旨在为制定各种类型的指南提供全面的指导和建议,包括临床实践指南、公共卫生指南和技术指南等。
指南是决策的重要工具,可以帮助决策者、医疗保健提供者和公众了解在预防、诊断和治疗方面的最佳实践和方案。
本手册提供了一步步的方法学,以便为制定高质量的指南提供支持。
二、指南制定过程1. 确定目标和范围:明确指南的目的和适用范围,确定目标受众和相关问题。
2. 建立制定团队:组建一个多学科的团队,包括方法学家、临床医生、公共卫生专家和相关领域的专家。
3. 制定检索策略:根据问题和目标受众,制定检索策略,以获取相关的证据。
4. 收集和筛选证据:收集相关的研究证据,并对证据进行筛选和质量评估。
5. 形成推荐意见:根据证据和专家的意见,形成推荐意见。
6. 编写草案:将推荐意见编写成草案,并进行内部审查和外部咨询。
7. 发布和实施:发布最终指南,并采取措施促进其实施和监测。
三、结论本手册提供了制定高质量指南的全面指导和建议,旨在帮助决策者、医疗保健提供者和公众做出更好的决策。
在制定指南时,需要充分考虑目标受众、证据的获取和评估、推荐意见的形成等方面,以确保指南的质量和可靠性。
通过遵循本手册的建议和方法学,可以制定出高质量的指南,从而为改善卫生服务和促进人群健康做出重要贡献。
WHO组织DMF编制指南

15 January 2007GUIDELINE ONACTIVE PHARMACEUTICAL INGREDIENT MASTER FILE(APIMF) PROCEDURE1(The APIMF procedure guideline does not apply to biological APIs.)TABLE OF CONTENTS1 INTRODUCTION (2)2 SCOPE (2)3 MAIN GUIDELINE TEXT (2)3.1 Content of the APIMF (2)3.2 Use of APIMF Procedure (2)3.3 Content of the PD when the APIMF Procedure is Used (3)3.4 Changes and updates to the APIMF (4)ANNEX 1 - OVERVIEW APIMF CONTENTS (5)ANNEX 2 - TEMPLATE LETTER OF ACCESS (7)ANNEX 3 - PART OF COVERING LETTER (8)ANNEX 4 - LIST OF ABBREVIATIONS (9)ANNEX 5 - GLOSSARY (10)1This guideline is based on the approach described in the document CPMP/QWP/227/02 Rev 2 Consultation draft GUIDELINE ON ACTIVE SUBSTANCE MASTER FILE PROCEDURE (London, 27 April 2005) of the European Medicines Agency1 INTRODUCTIONThe main objective of the APIMF procedure is to allow valuable confidential intellectual property or "know-how" of the Manufacturer of the active pharmaceutical ingredient (API) to be protected, while at the same time allowing the Applicant or holder of Prequalification Dossier (PD) to take full responsibility for the finished pharmaceutical product (FPP) and the quality and quality control of the API. The Prequalification Team thus has access to the complete information that is necessary for an evaluation of the suitability of the use of the API in the FPP.2 SCOPEThis Guideline is intended to assist Applicants/PD holders in the compilation of the API information of their dossiers for a prequalification dossier application (PDA) or a prequalification dossier variation (VPD) of a FPP. It is also intended to help APIMF holders in the compilation of their APIMFs.3 MAIN GUIDELINE TEXT3.1 Content of the APIMFThe overall content of the APIMF should contain detailed scientific information as indicated in Section 2. ACTIVE PHARMACEUTICAL INGREDIENT(s) [API(s)] of the “Guideline on Submission of Documentation for Prequalification of Multi-source (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis”.The scientific information in the APIMF should be physically divided into two separate parts, namely the Open Part (OP) and the Restricted Part (RP). The OP contains the information that the APIMF holder regards as non-confidential to the Applicant/PD holder, whereas the RP contains the information that the APIMF holder regards as confidential, see Annex 1. It is emphasized that the OP is still a confidential document that cannot be submitted by anyone to third parties without the written consent of the APIMF holder. In all cases the OP should contain sufficient information to enable the Applicant/PD holder to take full responsibility for an evaluation of the suitability of the specifications for the API to control the quality of this API for use in the manufacture of a specified FPP.The RP shall contain the remaining information, such as detailed information on the individual steps of the manufacturing method (reaction conditions, temperature, validation and evaluation data of critical steps) and the quality control during the manufacturing method of the API. Information that is relevant to the Applicant/PD holder (see notes 2 and 5 of ANNEX 1 regarding residual solvents and impurities, respectively) should be discussed in the RP but should also be submitted in the OP.In addition to the OP and RP, the APIMF should contain a table of contents, and a separate summary for the OP and the RP. Both summaries should be presented as a Pharmaceutical Quality Information Form (PQIF). The OP and RP should each have a version number. The structure of the version numbers should be unique and follow a logical order. Preferably the following structure is used: Name APIMF holder / Name active pharmaceutical ingredient/ OP or RP/ version number / date in yyyy-mm-dd.3.2 Use of APIMF ProcedureAn APIMF can only be submitted in support of a PDA or VPD. The relationship between the quality of the API and its use in the FPP needs to be justified in this PDA or VPD. Although the APIMF procedure is developed to keep intellectual property of the API confidential, it is also permissible to use the procedure when there is no confidentiality issue between the Applicant/PD holder and the API manufacturer (e.g. when the Applicant/PD holder manufactures the API itself). It is expected that the API manufacturer is also the holder of the APIMF.The APIMF procedure should be used for APIs where a professed standard is declared (namely no monograph exists in the Ph. Eur., Ph. Int. or USP), or where a monograph exists but a manufacturer’sin-house standard is declared.The APIMF procedure can also be used when APIs are included in the Ph. Eur., Ph. Int. or USP.A Drug Master File of an API (active substance, drug substance) assessed by a drug regulatory agency in the International Conference on Harmonization (ICH) regions and associated countries - including among others the EU, Japan and USA - is accepted without further evaluation as a prequalified APIMF on condition that evidence of such regulatory acceptance has been submitted. The holder of the DMF should also declare in writing that there have been no changes to the DMF content and manufacture of batches of API to be supplied for Applicants/PD holders.The APIMF holder should give permission to WHO to assess the data in the APIMF in relation to a specific PDA/VPD, in the form of a 'Letter of Access', see Annex 2. The APIMF holder should submit to the Applicant/PD holder:a copy of the latest version of the OP.a copy of the PQIF on the latest version of the OP.the Letter of Access.In addition, the APIMF holder should submit to WHO:the APIMF accompanied by a covering letter, see Annex 3.the Letter of Access.The APIMF holder should provide the APIMF to WHO only once, independently from the number of the PD holders and the number of PDAs/VPDs. The submission of the relevant documentation by the APIMF holder to WHO must be synchronised to arrive at approximately the same time as the first PDA or the first VPD is received from the FPP manufacturer that references the APIMF.Where the APIMF procedure is used, the Applicant/PD holder should submit the PDA or VPD to WHO together with the Letter of Access.WHO requires that any APIMF updates made in relation to one PD should apply to all. It is the APIMF holder's responsibility to notify the PD holders and WHO about any changes to the OP and/or RP, so that the PD holders can update all affected PDs accordingly and file the appropriate variation(s) to WHO as necessary.3.3 Content of the PD when the APIMF Procedure is UsedThe Applicant/PD holder is responsible for ensuring that he has access to all relevant information concerning the current manufacture of the API.The specifications used by the Applicant/PD holder to control the quality of the API should be laid down unambiguously in the PD. The Applicant/PD holder should quote the OP version number / date in yyyy-mm-dd , or should include a copy of the OP in the PD dossier.The version of the OP in the PD dossier should be the most recent and it should be identical to the OP as supplied by the APIMF holder to WHO as part of the APIMF.The Applicant/PD holder should include all relevant details from the OP in the PQIF of the PD dossier. Issues of the APIMF that are specifically relevant to the FPP under consideration should be highlighted in the PQIF of the PD.In the case of a single supplier and where the APIMF procedure or CEP procedure is used, the specifications of the Applicant/PD holder in the PD dossier should in principle be identical to those of the APIMF holder or the CEP holder. The Applicant/PD holder does however not need to accept redundant specifications, unnecessarily tight specification limits or outdated analytical methods. In cases where the Applicant/PD holder uses a different analytical method than that described in the APIMF, both methods should be validated. Technical specifications relevant for the FPP, which are normally not part of the specifications in the APIMF (e.g. particle size), should be part of the specifications of the Applicant/PD holder.In cases where there is more than one supplier, there should be one single compiled specification that is identical for each supplier. It is acceptable to lay down in the specification more than one acceptance criterion and/or analytical method for a single parameter with the statement “for API from supplier X”(e.g. in case of residual solvents).3.4 Changes and updates to the APIMFAs for FPPs, APIMF holders should keep the content of their APIMFs updated with respect to the actual synthesis / manufacturing process. The quality control methods should be kept in-line with the current regulatory and scientific requirements. APIMF holders shall not modify the contents of their APIMF (e.g. manufacturing process or specifications) and must inform each Applicant/PD holder and WHO when a change in an APIMF requires the filing of a VPD. Before implementation, any change to the APIMF should be reported by every PD holder to WHO by means of an appropriate variation procedure. A covering letter should be provided. In cases where the contents of the APIMF cannot be changed for a certain period of time, the APIMF holder should still provide the aforementioned data to the PD holder and WHO making reference to this reason and requesting a later date of implementation.The APIMF holders' covering letter to WHO should contain the following information (if available): A tabular list summarizing the changes carried out since the first compilation of the APIMF. An overview comparing the old and new content of the APIMF.Information as to whether the change has already been accepted, rejected or withdrawn by another drug regulatory authority in the ICH region and associated countries.The names of the relevant Applicants and PD holders. The new OP and/or RP with each new version number.An updated PQIF,if relevant.A discussion of the potential impact on the quality of the API as a result of the change(s).At the occasion of the 3-yearly renewal of a FPP, PD holders are required to declare that the quality of the product, in respect of the methods of preparation and control, has been regularly updated by variation procedure to take account of technical and scientific progress, and that the product conforms with current WHO/Prequalification quality guidelines. They will also declare that no changes have been made to the product particulars other than those approved by WHO.PD holders should therefore verify with their APIMF holders whether the above declaration can be met in respect to the API particulars. In case changes have not been notified to the PD holder and WHO, the necessary variation procedure should be initiated without delay.ANNEX 1 - OVERVIEW APIMF CONTENTSTable 1 PQIF format Open RestrictedPart PartGeneral information from literature x2.1 Nomenclature x2.2 Properties of API xStructure xX 2.3 Site(s) of manufacture xManufacturer(s) xprocess1) 2)2.4 ManufacturingControl of materials XControl of critical steps and intermediates 3) 4)Process validation and/or Evaluation XManufacturing Process Development XCharacterization xElucidation of Structure and other Characteristics xSelected physicochemical andother relevant properties xImpurities x 5)Control of API x2.5 Specifications xAnalytical procedures xValidation of analytical procedures xBatch analysis xJustification of specification x 6)Reference standards or Materials x2.6 Container Closure System x2.7 Stability xStability summary and conclusion xPost-approval Stability Protocol and Stability xCommitmentStability data x1)Flow chart and textbook-level narrative is regarded as sufficient, if detailed information ispresented in the Restricted Part. However, full validation data on the sterilization process may be requested in the Open Part (in cases where there is no further sterilization of the final product).2)Detailed information.3)In so far as the information is also relevant for the Applicant/PD holder.4)In so far as the information is related to the detailed description of the Manufacturing processand in so far as this information is not relevant for the Applicant/PD holder.5)In so far as the information is related to the detailed description of the Manufacturing processand in so far as the APIMF holder sufficiently justifies that there is no need to control these impurities in the final API.6)In so far as the information is related to the detailed description of the Manufacturing process,control of materials and process validation.ANNEX 2 - TEMPLATE LETTER OF ACCESS[Address of WHO][Date and place]LETTER OF ACCESSNumber of Active Pharmaceutical Ingredient Master File:Manufacturing site: [name and address]Active Pharmaceutical Ingredient Master File holder: [name and address]The aforementioned Active Pharmaceutical Ingredient Master File holder hereby authorizes the [WHO including all PQ Team Members and their experts] to refer to and review the above mentioned Active Pharmaceutical Ingredient Master File in support of the following Prequalification Application(s) or Prequalified Dossier Variation(s) submitted by [name / Prequalification holder / Applicant] on [planned date of submission]:[Name of product and prequalification code number, if known] [Name of Applicant or PD holder] The aforementioned Active Pharmaceutical Ingredient Master File holder commits to ensure batch-to-batch consistency and to inform [name of PD holder/Applicant] and WHO of any change in the OP or RP parts of the Active Pharmaceutical Ingredient Master File.Signature for the Active Pharmaceutical Ingredient Master File holder [Name and address] [Signature]ANNEX 3 - PART OF COVERING LETTERThis Active Pharmaceutical Ingredient Master File is submitted in relation to the PDA / VPD: [Name of product in prequalification procedure][Name of Applicant/PD holder for the application concerned]and describes <changes to> the manufacturing process and specifications of the (or one of the) active pharmaceutical ingredient(s) of this PDA or VPD.[Name active pharmaceutical ingredient]The version number of this Active Pharmaceutical Ingredient Master File isOpen part: version [version number]Restricted part: version [version number]This Active Pharmaceutical Ingredient Master File has previously been submitted for assessment in combination with a PDA / VPD for a pharmaceutical product within the prequalification project: Refer to the prequalification code and name of the FPP and the FPP manufacturer.ANNEX 4 - LIST OF ABBREVIATIONSAPI Active Pharmaceutical IngredientAPIMF Active Pharmaceutical Ingredient Master FileCEP European procedure for a certificate of suitability of monographs of the European pharmacopoeia (here on chemical purity)ICH International Conference on HarmonizationOP Open (Applicants) Part of the APIMFPD Prequalification dossierPDA Prequalification Dossier Application (including line extensions)Ph. Eur. European PharmacopoeiaPh. Int. International Pharmacopoeia of WHOPQIF Pharmaceutical Quality Information FormRP Restricted Part (of APIMF)USP United States PharmacopoeiaVPD Variation to a prequalified dossierWHO World Health Organization20, AVENUE A PPIA –CH-1211G ENEVA 27–S WITZERLAND –T EL CENTRAL +41227912111–F AX CENTRAL +41227913111– WWW.WHO.INTANNEX 5 - GLOSSARYActive pharmaceutical ingredient manufacturerA party involved in the Manufacturing chain of the active pharmaceutical ingredient, including agents, brokers, traders, distributors, repackers or relabellers.Active pharmaceutical ingredient Master File holderThis is the company that has the ultimate responsibility for the Active pharmaceutical ingredient Master File.ApplicantThis is the company requesting prequalification for a pharmaceutical product. Prequalification dossier holderThis is the company that is responsible for the pharmaceutical product on the market Manufacturing chainA clear flow chart or written text explaining the Manufacturing and distribution route of the active pharmaceutical ingredient from the first starting materialQualityAccording to ICH Q6A that is “The suitability of either a drug substance or drug product for its intended use. This term includes such attributes as the identity, strength and purity.” SpecificationAccording to ICH Q6A that is “A list of test, references to analytical procedures, and appropriate acceptance criteria which are numerical limits, ranges or other criteria to which a drug substance or drug product should conform to be considered acceptable for its intended use. Conformance to specifications means that the drug substance and/or drug product, when tested according to the listed analytical procedures will meet the listed acceptance criteria. Specifications are critical quality standards that are proposed and justified by the Manufacturer and approved by regulatory authorities.”。
WHO数据与记录管理规范指南
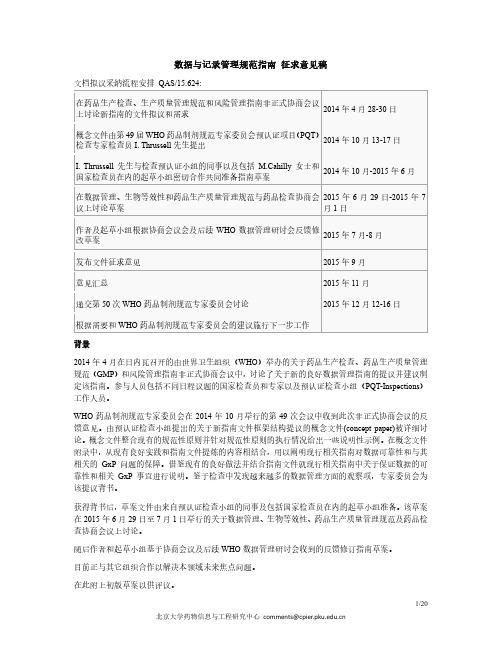
数据与记录管理规范指南征求意见稿文档拟议采纳流程安排QAS/15.624:在药品生产检查、生产质量管理规范和风险管理指南非正式协商会议上讨论新指南的文件拟议和需求2014年4月28-30日概念文件由第49届WHO药品制剂规范专家委员会预认证项目(PQT)检查专家检查员I. Thrussell先生提出2014年10月13-17日I. Thrussell先生与检查预认证小组的同事以及包括M.Cahilly女士和国家检查员在内的起草小组密切合作共同准备指南草案2014年10月-2015年6月在数据管理、生物等效性和药品生产质量管理规范与药品检查协商会议上讨论草案2015年6月29日-2015年7月1日作者及起草小组根据协商会议会及后续WHO数据管理研讨会反馈修改草案2015年7月-8月发布文件征求意见2015年9月意见汇总2015年11月递交第50次WHO药品制剂规范专家委员会讨论2015年12月12-16日根据需要和WHO药品制剂规范专家委员会的建议施行下一步工作背景2014年4月在日内瓦召开的由世界卫生组织(WHO)举办的关于药品生产检查、药品生产质量管理规范(GMP)和风险管理指南非正式协商会议中,讨论了关于新的良好数据管理指南的提议并建议制定该指南。
参与人员包括不同日程议题的国家检查员和专家以及预认证检查小组(PQT-Inspections)工作人员。
WHO药品制剂规范专家委员会在2014年10月举行的第49次会议中收到此次非正式协商会议的反馈意见。
由预认证检查小组提出的关于新指南文件框架结构提议的概念文件(concept paper)被详细讨论。
概念文件整合现有的规范性原则并针对规范性原则的执行情况给出一些说明性示例。
在概念文件附录中,从现有良好实践和指南文件提炼的内容相结合,用以阐明现行相关指南对数据可靠性和与其相关的GxP问题的保障。
借鉴现有的良好做法并结合指南文件就现行相关指南中关于保证数据的可靠性和相关GxP 事宜进行说明。
WHO现场主文件编写指南

© World Health OrganizationWHO Technical Report Series, No.961, 2011附件14Annex 14WHO现场主文件编写指南1WHO guidelines for drafting a site master file11. 介绍Introduction2. 目的Purpose3. 范围Scope4. 现场主文件的内容Content of site master file附件Appendix现场主文件的内容Content of a site master file1. 介绍Introduction1.1 现场主文件是由药品制药商编写的,应当包含下列具体的信息:生产厂区的质量管理方针及活动、在指定的生产厂区实行的对药品生产操作的生产和/或质量控制以及在与其相邻的建筑物内所进行的任何紧密的完整的操作。
如果只有一部分药品生产操作在此厂区内进行,那么在现场主文件中只需要描述这些操作,例如分析、包装等。
The site master file (SMF) is prepared by the pharmaceuticalmanufacturer and should contain specific information about the qualitymanagement policies and activities of the site, the production and/or qualitycontrol of pharmaceutical manufacturing operations carried out at the namedsite and any closely integrated operations at adjacent and nearby buildings.If only part of a pharmaceutical operation is carried out on the site, an SMFneed only describe those operations, e.g. analysis, packaging, etc.1.2 当提交给监管机构时,现场主文件中应当包含对常规监督、有效计划及进行GMP检查有用的、关于制造商与GMP相关的活动的清晰信息。
WHO技术报告系列第908号附录6GMP检查报告指南,2003年译本1

WHO技术报告系列第908号附录6GMP检查报告指南,2003年译本1World Health Organization世界卫生组织WHO Technical Report Series, No. 908, 2003WHO技术报告系列第908号,2003年Annex 6 附录6Guidance on Good Manufacturing Practices (GMP):inspection reportGMP检查报告指南When a site at which pharmaceutical products are manufactured is inspected, the inspector(s) responsible must draw up a report containing the items listed below. Where relevant, the appropriate section of the WHO GMP (Annex 4) is indicated.当药品生产场所进行检查时,检查员有责任草拟包含下文所列项目的报告。
有关内容,在WHO GMP(附录4)部分进行说明A. Manufacturer生产企业(a) Name of inspected manufacturer.被检查的企业名称(b) Address of inspected manufacturer (including telephone, fax, email and 24-hour telephone numbers).被检查企业的地址(包括电话、传真、e-mail和24小时电话号码)(c) Address of manufacturing site if different from that given above.生产场所的地址(如果与上述不一致)(d) Site number (e.g. site master file or number allocated bythe responsible authority).生产场所的注册号(如:生产场所主文件或当地行政机关分配的号码)(e) Manufacturing licence number, if applicable.生产执照号,如果有(f) Activities.业务(g) Pharmaceutical products manufactured.所生产的药品(h) Key personnel.关键人员(i) Key persons met.会见的关键人员B. Inspection details检查细节(a) Date(s) of inspection(s).检查日期(b) Previous inspection date.上次检查日期(c) Type of inspection.检查方式(d) Scope of inspection.检查范围(e) The regulatory authority.行政机关(f) GMP guidelines used for assessing compliance.用于评估符合性的GMP指南(g) For foreign inspections state whether, the national regulatory authority (NRA) of the country where the inspection took place was informed and whether it took part in the inspection.进行海外检查时,是否通知被检查企业所在国的国家行政机构和该国机构是否参与检查。
WHO最新指南(对照阅读)

WHO最新指南(对照阅读)12月4日,世界卫生组织(W H O)于发布《药品生产技术转移指南》(W H O g ui d e l i n e s o n t h e t r a ns f e r of t e c h n o l o g y i np h a r m a c e ut i c a l m an u f ac t u r i n g),提供了药品技术转让期间应考虑的指导原则。
本指南主体部分分为12个章节,内容如下:背景1.简介2.范围3.术语4.尽职调查和差距评估5.组织与管理6.质量管理和质量风险管理7.文件8.设施9.设备和仪器10.确认和验证11.产品生命周期和项目管理原则12.技术转移项目阶段往期推荐(1)药品生产技术转移:概述(2)药品技术转移,40个术语(3)药品技术转移:如何评估组织?(4)药品技术转移:设施设备上有哪些考虑?(5)药品技术转移:确认和验证上有哪些考虑?(5)药品技术转移:项目阶段如何设计?以下是该指南的12章(下)内容:12. 技术转移项目阶段(下)Information on process and finished pharmaceutical product information工艺信息和成品信息Processing, packaging 工艺、包装12.15.Pr o d u c t,p r o c e s s a n d p r o c e d u r e k n o w l e d ge s h o u l d b e a ne s se n t i a l p a r t of t h e t r a n s f e r p r o c e s s f r o m S U t o R U.产品、工艺和程序知识应该是从S U到R U的转移的重要组成部分。
12.16.T h e q u a l i t y t a r g e t p r o d u c t p r o f i l e,c r i t i c a l q u a l i t y a t t r i b u t e s,c r i t i c a l p r o c e ss p a r a m e t e r s,m a t e r i a l a t t r i b u t e s,c o n t r o l s t r a t e g y a nd a n y o t he r i m p a c t i n g e l e m e n t s o n t h e q u a l i t y of t h e p r o d u c t s h o u l d b e a v a i l a b l e.(S e e a l so I C Hg u i d e l i n e s.)质量目标产品概况、关键质量属性、关键工艺参数、物料属性、控制策略、以及对产品质量有任何其它影响的要素,都应可以提供。
who验证指南2016年版

who验证指南2016年版1.INTRODUCTION简介1.1Validation is an essential part of good practices including good manufacturing practices(GMP)(4)and good clinical practices(GCP).It is therefore an element of the pharmaceutical quality system.Validation,as a concept,incorporates qualification and should be applied over the life cycle of, e.g.the applicable product,process,system,equipment or utility.验证是包括GMP和GCP在内的良好规范的必要部分。
因此是制药质量体系的组成部分。
验证这个概念包括确认并且应该在产品、工艺、系统、设备或设施的整个生命周期中被使用。
1.2These guidelines cover the general principles of validation and qualification.In addition to the main part,appendices on validation and qualification(e.g.cleaning,computer and computerized systems,equipment, utilities and systems,and analytical methods)are included.这个指南包括验证和确认的一般原则。
除了主体部分外,还包括确认和验证附件(如清洁、计算机和计算机化系统、设备、设施和系统以及分析方法)。
1.3The following principles apply:适用的原则如下:the execution of validation should be in compliance with regulatory expectations;验证的执行应该符合监管预期quality,safety and efficacy must be designed and built into the product;质量、安全和效力应该被设计和构建到产品中quality cannot be inspected or tested into the product;质量不能通过检查或测试赋予产品quality risk management principles should be applied in determining the need, scope and extent of validation;应该应用质量风险管理规则来决定验证的需求、范围和程度ongoing review should take place to ensure that the validated state is maintained and opportunities for continuing improvement are identified.应该进行持续的回顾来确保维持验证状态并识别持续改进的机会1.4The implementation of validation work requires considerable resources such as:验证工作的完成需要大量资源,例如time:generally validation work is subject to rigorous time schedules;时间:一般验证工作需要有一个严密的时间表financial:validation often requires the time of specialized personnel and expensive technology.资金:验证经常需要专业人员和昂贵的技术human:validation requires the collaboration of experts from various disciplines(e.g.a multidisciplinary team,comprising quality assurance,engineering,information technology,manufacturing and other disciplines,as appropriate.).人员:验证需要不同学科专家的协作(例如一个多学科小组,视情况可能包括QA、工程、信息技术、生产以及其他学科人员)2.SCOPE范围2.1These guidelines focus mainly on the overall concept of validation and are not intended to be prescriptive in specific validation requirements.This document serves as general guidance only and the principles may be considered useful in its application in the manufacture and control of starting materials and finished pharmaceutical products(FPPs),as well as other areas.Validation of specific processes and systems,for example,in sterile product manufacture, requires much more consideration and a detailed approach that is beyond the scope of this document.这些指南主要关注验证的整体概念,无意规定具体的验证要求。
WHO现场主文件编写指南

© World Health OrganizationWHO Technical Report Series, No.961, 2011附件14Annex 14WHO现场主文件编写指南1WHO guidelines for drafting a site master file11. 介绍Introduction2. 目的Purpose3. 范围Scope4. 现场主文件的内容Content of site master file附件Appendix现场主文件的内容Content of a site master file1. 介绍Introduction1.1 现场主文件是由药品制药商编写的,应当包含下列具体的信息:生产厂区的质量管理方针及活动、在指定的生产厂区实行的对药品生产操作的生产和/或质量控制以及在与其相邻的建筑物内所进行的任何紧密的完整的操作。
如果只有一部分药品生产操作在此厂区内进行,那么在现场主文件中只需要描述这些操作,例如分析、包装等。
The site master file (SMF) is prepared by the pharmaceutical manufacturer and should contain specific information about the quality management policies and activities of the site, the production and/or quality control of pharmaceutical manufacturing operations carried out at the named site and any closely integrated operations at adjacent and nearby buildings. If only part of a pharmaceutical operation is carried out on the site, an SMF need only describe those operations, e.g. analysis, packaging, etc.1.2 当提交给监管机构时,现场主文件中应当包含对常规监督、有效计划及进行GMP检查有用的、关于制造商与GMP相关的活动的清晰信息。
药品经营活动场地主文件

药品经营活动场地主文件一、起草必要性一是药品经营活动监管体系基础性数据建设的需要。
新修订药品管理法建立了药品上市许可持有人(MAH)制度和新的药品检查制度,MAH及药品经营活动相关方将可以委托销售、储存和运输,即所谓按药品第三方物流管理。
同一主体的药品经营储存地址将可以跨环节、跨区域分布并实行报告管理。
药品经营活动检查时实施延伸检查、委托检查或联合检查。
在MAH制度下,药品经营场地特别是委托销售储存地址并非仅有生产经营许可管理的地址,且有多个承诺告知方式需监管的地址。
如MAH委托药品销售时,其药品储存不仅可以储存在生产地址设立的仓库,也可以跨行政区委托其他药品批发企业多点储存。
药品批发企业既可以在许可的仓库地址自行储存,也可以跨省、多仓进行委托储存。
二是实行新的药品检查制度,提高监管质效的需要。
新修订药品管理法实施后,已取消原原药品GMP、GSP认证检查制度。
原GMP、GSP认证申报资料,本质上与欧美国家药品生产经营场地主文件相似,只不过在认证申请时才申请报告或更新。
《药品经营监督管理办法和《药品检查管理规定》等征求意见稿,有关检查计划制定(第五十一条)和检查前准备(第十七条)均要求检查组现场检查前需要熟悉被检查场地基本情况和质量体系运行情况。
因此,已实施的《药品生产监督管理办法》实行了生产场地信息管理制度,国家局将出台相关配套管理规定。
三是监督药品经营活动相关企业履行法定义务和质量体系内审的需要。
新修订药品管理法第三十七条明确规定药品上市许可持有人应当建立年度报告制度。
其中药品生产销售情况,包括委托销售情况,相关药品经营企业将配合MAH实施年度报告。
其次,新修订药品管理法第三十五条规定药品上市许可持有人、药品生产企业、药品经营企业委托储存、运输药品的,应当对受托方的质量保证能力和风险管理能力进行评估,与其签订委托协议,约定药品质量责任、操作规程等内容。
委托方应定期对受托方进行质量审核。
而《药品经营质量管理规范》第十七条也明确规定企业应对质量管理体系进行内审。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
© World Health OrganizationWHO Technical Report Series, No.961, 2011附件14Annex 14WHO现场主文件编写指南1WHO guidelines for drafting a site master file11. 介绍Introduction2. 目的Purpose3. 范围Scope4. 现场主文件的内容Content of site master file附件Appendix现场主文件的内容Content of a site master file1. 介绍Introduction1.1 现场主文件是由药品制药商编写的,应当包含下列具体的信息:生产厂区的质量管理方针及活动、在指定的生产厂区实行的对药品生产操作的生产和/或质量控制以及在与其相邻的建筑物内所进行的任何紧密的完整的操作。
如果只有一部分药品生产操作在此厂区内进行,那么在现场主文件中只需要描述这些操作,例如分析、包装等。
The site master file (SMF) is prepared by the pharmaceutical manufacturer and should contain specific information about the quality management policies and activities of the site, the production and/or quality control of pharmaceutical manufacturing operations carried out at the named site and any closely integrated operations at adjacent and nearby buildings. If only part of a pharmaceutical operation is carried out on the site, an SMF need only describe those operations, e.g. analysis, packaging, etc.1.2 当提交给监管机构时,现场主文件中应当包含对常规监督、有效计划及进行GMP检查有用的、关于制造商与GMP相关的活动的清晰信息。
When submitted to a regulatory authority, the SMF should provide clear information on the manufacturer’s good manufacturing practices (GMP)-related activities that can be useful in general supervision and in the efficient planning and undertaking of GMP inspections.1.3 现场主文件中应当包含足够的信息,但加上附件尽量不要超过25~30页。
用简单的计划、轮廓图或示意图来替代叙述性的文字是更可取的。
当用A4纸张打印出时,现场主文件及其附件应当是可读的。
An SMF should contain adequate information but, as far as possible, not exceed 25–30 pages plus appendices. Simple plans, outline drawings or schematic layouts are preferred instead of narratives. The SMF, including appendices, should be readable when printed on A4 paper sheets.1.4 现场主文件应是制造商质量管理体系文件的一部分,且应持续更新。
现场主文件应有版本号、生效期和必须对其进行审核的日期。
应当定期对现场主文件进行回顾以保证其包含了最新的信息和代表了当前所进行的活动。
每个附件可以有单独的生效期,以便进行独立的更新。
The SMF should be a part of documentation belonging to the quality management system of the manufacturer and kept updated accordingly. The SMF should have an edition number, the date it becomes effective and the date by which it has to be reviewed. It should be subject to regular review to ensure that it is up to date and representative of current activities. Each annex can have an individual effective date, allowing for independent updating.2. 目的Purpose这些注释的目的是:在药品制造商编写现场主文件时提供指导,现场主文件在监管机构计划和进行GMP检查时有用的。
The aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an SMF that is useful to the regulatory authority in planning and conducting GMP inspections.3. 范围Scope这些注释适用于现场主文件的编写及内容的确定。
制造商应参照区域性的和/或国家监管要求来确定编写现场主文件是否是强制性的要求。
These explanatory notes apply to the preparation and content of the SMF.Manufacturers should refer to regional and or national regulatory requirements to establish whether it is mandatory for manufacturers of medicinal products to prepare an SMF.这些注释适用于各种制造操作,例如各种药品的生产、包装和贴签、检测、重新贴签和重新包装。
本指南中的概述也可用于指导血液和组织制品和原料药(API)制造商编写现场主文件或相应的文件。
These explanatory notes apply for all kinds of manufacturing operations such as production, packaging and labelling, testing, relabelling and repackaging of all types of medicinal products. The outlines of this guide could also be used in the preparation of an SMF or corresponding document by blood and tissue establishments and manufacturers of active pharmaceutical ingredients (APIs).4. 现场主文件的内容Content of site master file格式参照附件。
Refer to the Appendix for the format to be used.附件Appendix场主文件的内容Content of a site master file1. 制造商基本信息General information on the manufacturer1.1制造商联系信息Contact information on the manufacturer- 制造商名称及官方地址;name and official address of the manufacturer;- 生产厂地名称及街道地址,在生产厂地内的建筑物和生产单元;names and street addresses of the site, buildings and production units located on the site;- 制造商联系信息,包括在产品缺陷或召回时,相关联系人的24小时联系号码;以及contact information of the manufacturer including 24-hour telephone number of the contact personnel in the case of product defects or recalls; and- 生产厂地的识别码,例如全球地位系统(GPS)详细信息、生产厂地或其它地理系统D-U-N-S(数据通用编号系统)编号(由Dun & Bradstreet提供的识别码)。
identification number of the site as e.g. global positioning system (GPS) details, D-U-N-S (Data Universal Numbering System) number (a unique identification number provided by Dun & Bradstreet) of the site or any other geographical location system.1.2生产厂地被授权的药品生产活动Authorized pharmaceutical manufacturing activities of the site- 主管部门颁发的有效的生产许可证的复印件作为附件一;或者当适用时,索引到EudraGMP数据库。
如果主管部门不颁发生产许可证,应当进行声明。
copy of the valid manufacturing authorization issued by the relevant competent authority in Annex 1; or when applicable, reference to the EudraGMP database. If the competent authority does not issue manufacturing authorizations, this should be stated;- 如果没有涵盖在生产许可证范围时,简述由相关主管部门,包括国外监管机构授权的制造、进口、出口、分发和其他活动,授权的剂型/活动,应根据许可的剂型/活动分别叙述;brief description of manufacture, import, export, distribution and other activities asauthorized by the relevant competent authorities including foreign authorities with authorized dosage forms/activities, respectively; where not covered by the manufacturing authorization;- 在超出附件一或EudraGMP数据库范围时,将目前在生产厂地生产的所有产品类型列出(附件二);type of products currently manufactured on-site (list in Annex 2) where not covered by Annex 1 or the EudraGMP database; and- 列出最近5年内接受的所有GMP检查,包括进行检查的监管部门名称/国家和检查日期。
