顺尔宁(Singulair)(孟鲁司特钠颗粒)-推荐下载
顺尔宁(Singulair)(孟鲁司特钠颗粒)

顺尔宁(Singulair)(孟鲁司特钠颗粒)【药品名称】商品名称:顺尔宁(Singulair)通用名称:孟鲁司特钠颗粒英文名称:Montelukast Sodium Oral Granules【成份】本品主要成份为孟鲁司特钠, 其化学名称为:[R-(E)]-1-[[[1-[3-[2-(7-氯-2-喹啉)乙烯基]苯基]-3-[2-(1-羟基-1-甲基乙基)苯基]丙基]硫] 甲基] 环丙烷乙酸钠化学结构式:分子式:C35H35ClNNaO3S分子量: 608.18【适应症】本品适用于1岁以上儿童哮喘的预防和长期治疗,包括预防白天和夜间的哮喘症状,治疗对阿司匹林敏感的哮喘患者以及预防运动诱发的支气管收缩。
本品适用于减...【用法用量】每日一次。
哮喘病人应在睡前服用。
过敏性鼻炎病人可根据自身的情况在需要时间服药。
同时患有哮喘和过敏性鼻炎的病人应每晚用药一次。
1岁至2岁儿童哮喘患者每天一次,每次一袋。
2岁至5岁儿童哮喘患者和/或2岁至5岁过敏性鼻炎患者应每天服用4mg口服颗粒一袋。
口服颗粒的服用本品可直接服用,与一勺室温或冷的软性食物(如苹果酱)混合服用,或溶解于一茶匙室温或冷的婴儿配方奶粉或母乳服用。
在服用时才能打开包装袋。
打开包装袋以后应立即服用全部的剂量(15分钟内)。
与食物、婴儿配方奶粉或母乳混合后的本品不能再贮存至下次继续服用。
本品不应溶解于除婴儿配方奶粉或母乳外的其它液体中服用。
但是服药后可以饮水。
一般建议以哮喘控制指标来评价治疗效果,本品的疗效在用药一天内即出现。
本品可与食物同服或另服。
应建议患者无论在哮喘控制还是恶化阶段都坚持服用。
对肾功能不全患者、轻至中度肝损害的患者及不同性别的患者无需调整剂量。
本品与其它哮喘治疗药物的关系本品可加入患者现有的治疗方案中。
减少合并用药物的剂量:支气管扩张剂单用支气管扩张剂不能有效控制的哮喘患者,可在治疗方案中加入本品,一旦有临床治疗反应(一般出现在首剂用药后),根据患者的耐受情况,可将支气管扩张剂剂量减少。
孟鲁司特钠

35.79
42.66
43.37
46.6
2006~2009年抗哮喘药物前四位销售增长变化表 2006~2009年孟鲁司特钠占国内全身用抗哮喘药物的用药比例
Page 8
立项依据 原料药的合成 剂型设计 质量标准 药理和毒理
Page 9
原料药的合成—合成图
最后要利用柱层析法对孟鲁司特 进行精制,反应步骤复杂冗长, 如对羟基的保护、去保护,不适 用于大规模生产。
孟鲁斯 特钠10 片
研细 溶解并稀释
精密称 文字内容 取适量
臵100mL 容量瓶中
摇匀,滤过
重 复 三 次
取供试品 20uL,进 样,分析
臵10mL容 量瓶中, 作为供试 品溶液
精密量取 续滤液溶 液1.0mL
溶解并稀释
记录色谱 图,计算 RSD值
Page
25
质量标准—影响因素试验
高温(60℃)
高温试验
Page
10
原料药的合成—合成图
这条合成路线被研究的最多,合成工艺相 对成熟一些。用此法制备的孟鲁司特纯度 高,不需气相纯化;避免了使用昂贵和危 险试剂,如正丁基锂、二环己基胺,溶剂 更符合医药工业要求。这种方法虽有明显 优点,但也有不足之处,如手性还原条件 苛刻和总收率还不理想。
Page
11
立项依据 原料药的合成 剂型设计 质量标准 药理和毒理
(4)系统适应性试验
(5)含量测定 (6)回收率
(3)标准曲线和线性范围
(7)方法学考察:专属性、检测限、定量限、精密度、重复性
Page 22
质量标准—制剂含量测定
Hypersi lBDS-C.
进样量 : 20uL
色谱柱(100mm X 4.6mm ,5t xm )
顺尔宁ppt课件

仅供医学药学专业人士阅读
两组患者哮喘急性发作的累积比例 相当1
25
哮喘急性发作的 20 患者比例,% 15
孟鲁司特+氟替卡松(n=747)a 沙美特罗+氟替卡松(n=743)b
10 PHale Waihona Puke NS500
10
20
30
40
50
随机化后的周数
aMontelukast 10 mg + fluticasone 200 µg; bSalmeterol 100 µg + fluticasone 200 µg. Reproduced from British Medical Journal, Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening AP, Haahtela T, Holgate ST, Picado C, Menten J, Dass SB, Leff JA, Polos PG, 327, 891, ©2003, with permission from BMJ Publishing Group Ltd. 1. Bjermer L et al. BMJ. 2003;327:891–895.
80
79.9%
P=NS
80.9%
60
40
20
0
孟鲁司特+ 氟替卡松
沙美特罗+氟替卡松
(n=747)a
(n=743)b
aMontelukast 10 mg + fluticasone 200 µg; bSalmeterol 100 µg + fluticasone 200 µg. Modified-intention-to-treat approach. 20.1% and 19.1% of patients in the 2 groups, respectively, had 1 asthma exacerbation. 1. Bjermer L et al. BMJ. 2003;327:891–895.
顺尔宁(Singulair)(孟鲁司特钠颗粒)

顺尔宁(Singulair)(孟鲁司特钠颗粒)【药品名称】商品名称:顺尔宁(Singulair)通用名称:孟鲁司特钠颗粒英文名称:Montelukast Sodium Oral Granules【成份】本品要紧成份为孟鲁司特钠, 其化学名称为:[R-(E)]-1-[[[1-[3-[2-(7-氯-2-喹啉)乙烯基]苯基]-3-[2-(1-羟基-1-甲基乙基)苯基]丙基]硫] 甲基] 环丙烷乙酸钠化学结构式:分子式:C35H35ClNNaO3S分子量:【适应症】本品适用于1岁以上儿童哮喘的预防和长期医治,包括预防白天和夜间的哮喘病症,医治对阿司匹林灵敏的哮喘患者和预防运动诱发的支气管收缩。
本品适用于减...【用法用量】每日一次。
哮喘病人应在睡前服用。
过敏性鼻炎病人可依照自身的情形在需要时刻服药。
同时患有哮喘和过敏性鼻炎的病人应每晚用药一次。
1岁至2岁儿童哮喘患者天天一次,每次一袋。
2岁至5岁儿童哮喘患者和/或2岁至5岁过敏性鼻炎患者应天天服用4mg口服颗粒一袋。
口服颗粒的服用本品可直接服用,与一勺室温或冷的软性食物(如苹果酱)混合服用,或溶解于一茶匙室温或冷的婴儿配方奶粉或母乳服用。
在服历时才能打开包装袋。
打开包装袋以后应当即服用全数的剂量(15分钟内)。
与食物、婴儿配方奶粉或母乳混合后的本品不能再贮存至下次继续服用。
本品不该溶解于除婴儿配方奶粉或母乳外的其它液体中服用。
可是服药后能够饮水。
一样建议以哮喘操纵指标来评判医治成效,本品的疗效在用药一天内即显现。
本品可与食物同服或另服。
应建议患者不管在哮喘操纵仍是恶化时期都坚持服用。
对肾功能不全患者、轻至中度肝损害的患者及不同性别的患者无需调整剂量。
本品与其它哮喘医治药物的关系本品可加入患者现有的医治方案中。
减少归并用药物的剂量:支气管扩张剂单用支气管扩张剂不能有效操纵的哮喘患者,可在医治方案中加入本品,一旦有临床医治反映(一样出此刻首剂用药后),依照患者的耐受情形,可将支气管扩张剂剂量减少。
孟鲁司特钠颗粒使用说明书

孟鲁司特钠颗粒以下内容仅供参考,请以药品包装盒中的说明书为准。
妊娠:B哺乳:L3孟鲁司特钠颗粒使用说明书【说明书修订日期】核准日期:2007年02月20日修改日期:2007年07月06日修改日期:2009年01月09日修改日期:2010年03月16日修改日期:2011年06月22日修改日期:2012年09月17日修改日期:2012年11月21日修改日期:2013年05月29日【药品名称】孟鲁司特钠颗粒【英文名称】MontelukastSodium Oral Granules【汉语拼音】MenglusitenaKeli【成份】本品主要成份为孟鲁司特钠。
【性状】本品为白色、粗糙的颗粒。
【适应症】本品适用于1岁以上儿童哮喘的预防和长期治疗,包括预防白天和夜间的哮喘症状,治疗对阿司匹林敏感的哮喘患者以及预防运动诱发的支气管收缩。
本品适用于减轻过敏性鼻炎引起的症状(2岁至5岁儿童的季节性过敏性鼻炎和常年性过敏性鼻炎)。
【规格】0.5g:4mg(以孟鲁司特计)。
【用法用量】每日一次。
哮喘病人应在睡前服用。
过敏性鼻炎病人可根据自身的情况在需要时间服药。
同时患有哮喘和过敏性鼻炎的病人应每晚用药一次。
1岁至2岁儿童哮喘患者每天一次,每次一袋。
2岁至5岁儿童哮喘患者和/或2岁至5岁过敏性鼻炎患者应每天服用4mg口服颗粒一袋。
口服颗粒的服用本品可直接服用,与一勺室温或冷的软性食物(如苹果酱)混合服用,或溶解于一茶匙室温或冷的婴儿配方奶粉或母乳服用。
在服用时才能打开包装袋。
打开包装袋以后应立即服用全部的剂量(15分钟内)。
与食物、婴儿配方奶粉或母乳混合后的本品不能再贮存至下次继续服用。
本品不应溶解于除婴儿配方奶粉或母乳外的其它液体中服用。
但是服药后可以饮水。
一般建议以哮喘控制指标来评价治疗效果,本品的疗效在用药一天内即出现。
本品可与食物同服或另服。
应建议患者无论在哮喘控制还是恶化阶段都坚持服用。
对肾功能不全患者、轻至中度肝损害的患者及不同性别的患者无需调整剂量。
孟鲁司特钠片说明书
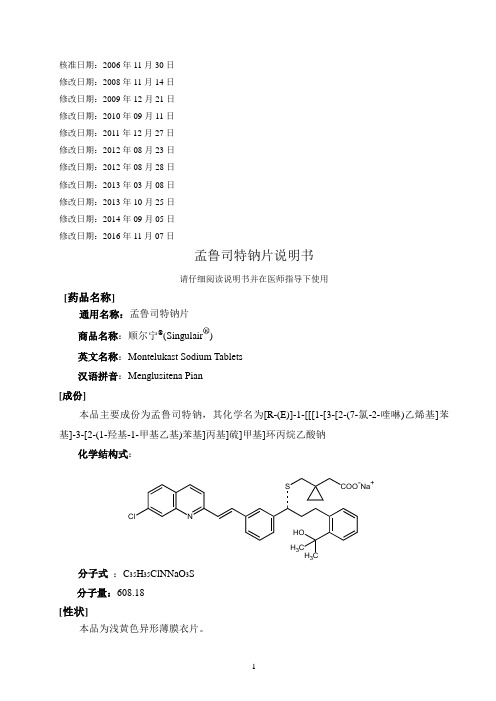
核准日期:2006年11月30日修改日期:2008年11月14日修改日期:2009年12月21日修改日期:2010年09月11日修改日期:2011年12月27日修改日期:2012年08月23日修改日期:2012年08月28日修改日期:2013年03月08日修改日期:2013年10月25日修改日期:2014年09月05日修改日期:2016年11月07日孟鲁司特钠片说明书请仔细阅读说明书并在医师指导下使用[药品名称]通用名称:孟鲁司特钠片商品名称:顺尔宁®(Singulair®)英文名称:Montelukast Sodium Tablets汉语拼音:Menglusitena Pian[成份]本品主要成份为孟鲁司特钠,其化学名为[R-(E)]-1-[[[1-[3-[2-(7-氯-2-喹啉)乙烯基]苯基]-3-[2-(1-羟基-1-甲基乙基)苯基]丙基]硫]甲基]环丙烷乙酸钠化学结构式:N Cl S COO-Na+ H3HOH3C分子式:C35H35ClNNaO3S分子量:608.18[性状]本品为浅黄色异形薄膜衣片。
[适应症]本品适用于15岁及15岁以上成人哮喘的预防和长期治疗,包括预防白天和夜间的哮喘症状,治疗对阿司匹林敏感的哮喘患者以及预防运动诱发的支气管收缩。
本品适用于减轻过敏性鼻炎引起的症状(15岁及15岁以上成人的季节性过敏性鼻炎和常年性过敏性鼻炎)。
[规格]10mg(以孟鲁司特计)。
[用法用量]每日一次,每次一片(10mg)。
哮喘病人应在睡前服用。
过敏性鼻炎病人可根据自身的情况在需要时服药。
同时患有哮喘和过敏性鼻炎的病人应每晚用药一次。
15岁及15岁以上患有哮喘和/或过敏性鼻炎的成人患者每日一次,每次10mg。
一般建议以哮喘控制指标来评价治疗效果,本品的疗效在用药一天内即出现。
本品可与食物同服或另服。
应建议患者无论在哮喘控制还是恶化阶段都坚持服用。
老年患者、肾功能不全患者、轻至中度肝损害的患者及不同性别的患者无需调整剂量。
孟鲁司特-FDA说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SINGULAIR safely and effectively. See full prescribing information for SINGULAIR.SINGULAIR®(montelukast sodium) tablets, chewable tablets, and oral granules Initial U.S. Approval: 1998---------------------------RECENT MAJOR CHANGES --------------------------Warnings and Precautions, Neuropsychiatric Events (5.4) 04/2010 ----------------------------INDICATIONS AND USAGE ---------------------------SINGULAIR® is a leukotriene receptor antagonist indicated for:• Prophylaxis and chronic treatment of asthma in patients12 months of age and older (1.1).• Acute prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older (1.2).• Relief of symptoms of allergic rhinitis (AR): seasonal allergic rhinitis (SAR) in patients 2 years of age and older, and perennial allergic rhinitis (PAR) in patients 6 months of age and older (1.3).-----------------------DOSAGE AND ADMINISTRATION-----------------------Administration (by indications):• Asthma (2.1): Once daily in the evening for patients 12 months and older.• Acute prevention of EIB (2.2): 10 mg tablet at least 2 hours before exercise for patients 15 years of age and older.• Seasonal allergic rhinitis (2.3): Once daily for patients 2 years and older.• Perennial allergic rhinitis (2.3): Once daily for patients 6 months and older.Dosage (by age):• 15 years and older: one 10-mg tablet.• 6 to 14 years: one 5-mg chewable tablet.• 2 to 5 years: one 4-mg chewable tablet or one packet of 4-mg oral granules.• 6 to 23 months: one packet of 4-mg oral granules.Patients with both asthma and allergic rhinitis should take only one dose daily in the evening (2.4). For oral granules: Must administer within 15 minutes after opening the packet (with or without mixing with food) (2.5). ---------------------DOSAGE FORMS AND STRENGTHS --------------------• SINGULAIR 10-mg Film-Coated Tablets• SINGULAIR 5-mg and 4-mg Chewable Tablets• SINGULAIR 4-mg Oral Granules-------------------------------CONTRAINDICATIONS ------------------------------• Hypersensitivity to any component of this product (4).------------------------WARNINGS AND PRECAUTIONS-----------------------• Do not prescribe SINGULAIR to treat an acute asthma attack.• Advise patients to have appropriate rescue medication available(5.1).• Inhaled corticosteroid may be reduced gradually. Do not abruptlysubstitute SINGULAIR for inhaled or oral corticosteroids (5.2).• Patients with known aspirin sensitivity should continue to avoidaspirin or non-steroidal anti-inflammatory agents while takingSINGULAIR (5.3).• Neuropsychiatric events have been reported with SINGULAIR.Instruct patients to be alert for neuropsychiatric events. Evaluatethe risks and benefits of continuing treatment with SINGULAIR ifsuch events occur (5.4 and 6.2).• Systemic eosinophilia, sometimes presenting with clinical featuresof vasculitis consistent with Churg-Strauss syndrome, has beenreported. These events usually, but not always, have beenassociated with the reduction of oral corticosteroid therapy (5.5and 6.2).• Inform patients with phenylketonuria that the 4-mg and 5-mgchewable tablets contain phenylalanine (5.6).------------------------------ADVERSE REACTIONS------------------------------Most common adverse reactions (incidence ≥5% and greater thanplacebo listed in descending order of frequency): upper respiratoryinfection, fever, headache, pharyngitis, cough, abdominal pain,diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis (6.1).To report SUSPECTED ADVERSE REACTIONS, contact MerckSharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877888-4231 or FDA at 1-800-FDA-1088 or /medwatch.See 17 for PATIENT COUNSELING INFORMATION andFDA-approved patient labeling.Revised:12/2010FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE1.1 A sthma1.2 E xercise-InducedBronchoconstriction1.3 A llergicRhinitis2 DOSAGE AND ADMINISTRATION2.1 A sthma2.2 E xercise-Induced Bronchoconstriction (EIB) in Patients 15Years of Age and Older2.3 Allergic Rhinitis2.4 Asthma and Allergic Rhinitis2.5 Instructions for Administration of Oral Granules3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 A cuteAsthma5.2 Concomitant Corticosteroid Use5.3 A spirinSensitivity5.4 N europsychiatricEvents5.5 E osinophilicConditions5.6 P henylketonuria6 ADVERSE REACTIONS6.1 Clinical Trials Experience6.2 P ost-MarketingExperience7 DRUG INTERACTIONS8 USE IN SPECIFIC POPULATIONS8.1 P regnancy8.3 N ursingMothers8.4 P ediatricUse8.5 G eriatricUse8.6 H epaticInsufficiency8.7 R enalInsufficiency10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2 Animal Toxicology and/or Pharmacology14 CLINICAL STUDIES14.1 Asthma14.2 Exercise-Induced Bronchoconstriction (EIB)14.3 Allergic Rhinitis (Seasonal and Perennial)16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION17.1 Information for Patients17.2 FDA-Approved Patient Labeling*Sections or subsections omitted from the full prescribing information are not listed.(Montelukast Sodium) Tablets, Chewable Tablets, and Oral GranulesFULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGE1.1 AsthmaSINGULAIR1 is indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 12 months of age and older.1.2 Exercise-Induced BronchoconstrictionSINGULAIR is indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older.1.3 Allergic RhinitisSINGULAIR is indicated for the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 6 months of age and older.2 DOSAGE AND ADMINISTRATION2.1 AsthmaSINGULAIR should be taken once daily in the evening. The following doses are recommended:For adults and adolescents 15 years of age and older: one 10-mg tablet.For pediatric patients 6 to 14 years of age: one 5-mg chewable tablet.For pediatric patients 2 to 5 years of age: one 4-mg chewable tablet or one packet of 4-mg oral granules.For pediatric patients 12 to 23 months of age: one packet of 4-mg oral granules.Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established.There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion.2.2 Exercise-Induced Bronchoconstriction (EIB) in Patients 15 Years of Age and OlderFor prevention of EIB, a single 10 mg dose of SINGULAIR should be taken at least 2 hours before exercise. An additional dose of SINGULAIR should not be taken within 24 hours of a previous dose. Patients already taking SINGULAIR daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting β-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of SINGULAIR for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.2.3 Allergic RhinitisFor allergic rhinitis, SINGULAIR should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs.The following doses for the treatment of symptoms of seasonal allergic rhinitis are recommended: For adults and adolescents 15 years of age and older: one 10-mg tablet.For pediatric patients 6 to 14 years of age: one 5-mg chewable tablet.For pediatric patients 2 to 5 years of age: one 4-mg chewable tablet or one packet of 4-mg oral granules.Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established.1 Registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.Copyright © 1998-2010 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.All rights reserved(Montelukast Sodium) Tablets, Chewable Tablets, and Oral GranulesThe following doses for the treatment of symptoms of perennial allergic rhinitis are recommended: For adults and adolescents 15 years of age and older: one 10-mg tablet.For pediatric patients 6 to 14 years of age: one 5-mg chewable tablet.For pediatric patients 2 to 5 years of age: one 4-mg chewable tablet or one packet of 4-mg oral granules.For pediatric patients 6 to 23 months of age: one packet of 4-mg oral granules.Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established.2.4 Asthma and Allergic RhinitisPatients with both asthma and allergic rhinitis should take only one SINGULAIR dose daily in the evening.2.5 Instructions for Administration of Oral GranulesSINGULAIR 4-mg oral granules can be administered either directly in the mouth, dissolved in 1 teaspoonful (5 mL) of cold or room temperature baby formula or breast milk, or mixed with a spoonful of cold or room temperature soft foods; based on stability studies, only applesauce, carrots, rice, or ice cream should be used. The packet should not be opened until ready to use. After opening the packet, the full dose (with or without mixing with baby formula, breast milk, or food) must be administered within 15 minutes. If mixed with baby formula, breast milk, or food, SINGULAIR oral granules must not be stored for future use. Discard any unused portion. SINGULAIR oral granules are not intended to be dissolved in any liquid other than baby formula or breast milk for administration. However, liquids may be taken subsequent to administration. SINGULAIR oral granules can be administered without regard to the time of meals.3 DOSAGE FORMS AND STRENGTHS• SINGULAIR 10-mg Film-Coated Tablets are beige, rounded square-shaped tablets, with code MRK 117 on one side and SINGULAIR on the other.• SINGULAIR 5-mg Chewable Tablets are pink, round, bi-convex-shaped tablets, with code MRK 275 on one side and SINGULAIR on the other.• SINGULAIR 4-mg Chewable Tablets are pink, oval, bi-convex-shaped tablets, with code MRK 711 on one side and SINGULAIR on the other.• SINGULAIR 4-mg Oral Granules are white granules with 500 mg net weight, packed in a child-resistant foil packet.4 CONTRAINDICATIONSHypersensitivity to any component of this product.5 WARNINGS AND PRECAUTIONS5.1 Acute AsthmaSINGULAIR is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with SINGULAIR can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled β-agonist.5.2 Concomitant Corticosteroid UseWhile the dose of inhaled corticosteroid may be reduced gradually under medical supervision, SINGULAIR should not be abruptly substituted for inhaled or oral corticosteroids.5.3 Aspirin SensitivityPatients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal antiinflammatory agents while taking SINGULAIR. Although SINGULAIR is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate(Montelukast Sodium) Tablets, Chewable Tablets, and Oral Granulesbronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients [see Clinical Studies (14.1)].5.4 Neuropsychiatric EventsNeuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking SINGULAIR. Post-marketing reports with SINGULAIR use include agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behavior (including suicide), and tremor. The clinical details of some post-marketing reports involving SINGULAIR appear consistent with a drug-induced effect.Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with SINGULAIR if such events occur [see Adverse Reactions (6.2)].5.5 Eosinophilic ConditionsPatients with asthma on therapy with SINGULAIR may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between SINGULAIR and these underlying conditions has not been established [see Adverse Reactions (6.2)].5.6 PhenylketonuriaPhenylketonuric patients should be informed that the 4-mg and 5-mg chewable tablets contain phenylalanine (a component of aspartame), 0.674 and 0.842 mg per 4-mg and 5-mg chewable tablet, respectively.6 ADVERSEREACTIONS6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.Adults and Adolescents 15 Years of Age and Older with AsthmaSINGULAIR has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse experiences reported with SINGULAIR occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo:(Montelukast Sodium) Tablets, Chewable Tablets, and Oral GranulesTABLE 1Adverse Experiences Occurring in ≥1% of Patientswith an Incidence Greater than that in Patients Treated with PlaceboSINGULAIR 10 mg/day(%)(n=1955) Placebo(%) (n=1180)Body As A WholePain, abdominal 2.9 2.5Asthenia/fatigue 1.8 1.2Fever 1.5 0.9TraumaDigestive System Disorders1.0 0.8Dyspepsia 2.1 1.1Pain, dental 1.7 1.0Gastroenteritis, infectiousNervous System/Psychiatric1.5 0.5Headache 18.4 18.1DizzinessRespiratory System Disorders1.9 1.4Influenza 4.2 3.9Cough 2.7 2.4Congestion, nasalSkin/Skin Appendages Disorder1.6 1.3RashLaboratory Adverse Experiences*1.6 1.2ALT increased 2.1 2.0AST increased 1.6 1.2Pyuria 1.0 0.9* Number of patients tested (SINGULAIR and placebo, respectively): ALT and AST, 1935, 1170; pyuria, 1924, 1159.The frequency of less common adverse events was comparable between SINGULAIR and placebo.The safety profile of SINGULAIR, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for SINGULAIR.Cumulatively, 569 patients were treated with SINGULAIR for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change.Pediatric Patients 6 to 14 Years of Age with AsthmaSINGULAIR has been evaluated for safety in 476 pediatric patients 6 to 14 years of age. Cumulatively, 289 pediatric patients were treated with SINGULAIR for at least 6 months, and 241 for one year or longer in clinical trials. The safety profile of SINGULAIR in the 8-week, double-blind, pediatric efficacy trial was generally similar to the adult safety profile. In pediatric patients 6 to 14 years of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: pharyngitis, influenza, fever, sinusitis, nausea, diarrhea, dyspepsia, otitis, viral infection, and laryngitis. The frequency of less common adverse events was comparable between SINGULAIR and placebo. With prolonged treatment, the adverse experience profile did not significantly change.In studies evaluating growth rate, the safety profile in these pediatric patients was consistent with the safety profile previously described for SINGULAIR. In a 56-week, double-blind study evaluating growth rate in pediatric patients 6 to 8 years of age receiving SINGULAIR, the following events not previously observed with the use of SINGULAIR in this age group occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: headache, rhinitis (infective), varicella, gastroenteritis, atopic dermatitis, acute bronchitis, tooth infection, skin infection, and myopia.(Montelukast Sodium) Tablets, Chewable Tablets, and Oral GranulesPediatric Patients 2 to 5 Years of Age with AsthmaSINGULAIR has been evaluated for safety in 573 pediatric patients 2 to 5 years of age in single- and multiple-dose studies. Cumulatively, 426 pediatric patients 2 to 5 years of age were treated with SINGULAIR for at least 3 months, 230 for 6 months or longer, and 63 patients for one year or longer in clinical trials. In pediatric patients 2 to 5 years of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: fever, cough, abdominal pain, diarrhea, headache, rhinorrhea, sinusitis, otitis, influenza, rash, ear pain, gastroenteritis, eczema, urticaria, varicella, pneumonia, dermatitis, and conjunctivitis.Pediatric Patients 6 to 23 Months of Age with AsthmaSafety and effectiveness in pediatric patients younger than 12 months of age with asthma have not been established.SINGULAIR has been evaluated for safety in 175 pediatric patients 6 to 23 months of age. The safety profile of SINGULAIR in a 6-week, double-blind, placebo-controlled clinical study was generally similar to the safety profile in adults and pediatric patients 2 to 14 years of age. In pediatric patients 6 to 23 months of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: upper respiratory infection, wheezing; otitis media; pharyngitis, tonsillitis, cough; and rhinitis. The frequency of less common adverse events was comparable between SINGULAIR and placebo.Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis SINGULAIR has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. SINGULAIR administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with SINGULAIR with a frequency ≥1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving SINGULAIR vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.Pediatric Patients 2 to 14 Years of Age with Seasonal Allergic RhinitisSINGULAIR has been evaluated in 280 pediatric patients 2 to 14 years of age in a 2-week, multicenter, double-blind, placebo-controlled, parallel-group safety study. SINGULAIR administered once daily in the evening had a safety profile similar to that of placebo. In this study, the following events occurred with a frequency ≥2% and at an incidence greater than placebo: headache, otitis media, pharyngitis, and upper respiratory infection.Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis SINGULAIR has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received SINGULAIR in two, 6-week, clinical studies. SINGULAIR administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with SINGULAIR with a frequency ≥1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.Pediatric Patients 6 Months to 14 Years of Age with Perennial Allergic Rhinitis The safety in patients 2 to 14 years of age with perennial allergic rhinitis is supported by the safety in patients 2 to 14 years of age with seasonal allergic rhinitis. The safety in patients 6 to 23 months of age is supported by data from pharmacokinetic and safety and efficacy studies in asthma in this pediatric population and from adult pharmacokinetic studies.6.2 Post-Marketing ExperienceThe following adverse reactions have been identified during post-approval use of SINGULAIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.Blood and lymphatic system disorders: increased bleeding tendency, thrombocytopenia.Immune system disorders: hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration.(Montelukast Sodium) Tablets, Chewable Tablets, and Oral GranulesPsychiatric disorders: agitation including aggressive behavior or hostility, anxiousness, depression, disorientation, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tremor [see Warnings and Precautions (5.4)].Nervous system disorders: drowsiness, paraesthesia/hypoesthesia, seizures.Cardiac disorders: palpitations.Respiratory, thoracic and mediastinal disorders: epistaxis.Gastrointestinal disorders: diarrhea, dyspepsia, nausea, pancreatitis, vomiting.Hepatobiliary disorders: Cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with SINGULAIR. Most of these occurred in combination with other confounding factors, such as use of other medications, or when SINGULAIR was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis.Skin and subcutaneous tissue disorders: angioedema, bruising, erythema nodosum, pruritus, urticaria.Musculoskeletal and connective tissue disorders: arthralgia, myalgia including muscle cramps.General disorders and administration site conditions: edema.Patients with asthma on therapy with SINGULAIR may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients [see Warnings and Precautions (5.5)].7 DRUGINTERACTIONSNo dose adjustment is needed when SINGULAIR is co-administered with theophylline, prednisone, prednisolone, oral contraceptives, terfenadine, digoxin, warfarin, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers [see Clinical Pharmacology (12.3)].8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category B: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, SINGULAIR should be used during pregnancy only if clearly needed.Teratogenic Effect: No teratogenicity was observed in rats and rabbits at doses approximately 100 and 110 times, respectively, the maximum recommended daily oral dose in adults based on AUCs [see Nonclinical Toxicology (13.2)].During worldwide marketing experience, congenital limb defects have been rarely reported in the offspring of women being treated with SINGULAIR during pregnancy. Most of these women were also taking other asthma medications during their pregnancy. A causal relationship between these events and SINGULAIR has not been established.Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., maintains a registry to monitor the pregnancy outcomes of women exposed to SINGULAIR while pregnant. Patients and healthcare providers are encouraged to report any prenatal exposure to SINGULAIR by calling the Pregnancy Registry at 1-800-986-8999.8.3 Nursing MothersStudies in rats have shown that montelukast is excreted in milk. It is not known if montelukast is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SINGULAIR is given to a nursing mother.8.4 Pediatric UseSafety and efficacy of SINGULAIR have been established in adequate and well-controlled studies in pediatric patients with asthma 6 to 14 years of age. Safety and efficacy profiles in this age group are(Montelukast Sodium) Tablets, Chewable Tablets, and Oral Granulessimilar to those seen in adults [see Adverse Reactions (6.1), Clinical Pharmacology, Special Populations (12.3), and Clinical Studies (14.1)].The efficacy of SINGULAIR for the treatment of seasonal allergic rhinitis in pediatric patients 2 to 14 years of age and for the treatment of perennial allergic rhinitis in pediatric patients 6 months to 14 years of age is supported by extrapolation from the demonstrated efficacy in patients 15 years of age and older with allergic rhinitis as well as the assumption that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations.The safety of SINGULAIR 4-mg chewable tablets in pediatric patients 2 to 5 years of age with asthma has been demonstrated by adequate and well-controlled data [see Adverse Reactions (6.1)]. Efficacy of SINGULAIR in this age group is extrapolated from the demonstrated efficacy in patients 6 years of age and older with asthma and is based on similar pharmacokinetic data, as well as the assumption that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations. Efficacy in this age group is supported by exploratory efficacy assessments from a large, well-controlled safety study conducted in patients 2 to 5 years of age.The safety of SINGULAIR 4-mg oral granules in pediatric patients 12 to 23 months of age with asthma has been demonstrated in an analysis of 172 pediatric patients, 124 of whom were treated with SINGULAIR, in a 6-week, double-blind, placebo-controlled study [see Adverse Reactions (6.1)]. Efficacy of SINGULAIR in this age group is extrapolated from the demonstrated efficacy in patients 6 years of age and older with asthma based on similar mean systemic exposure (AUC), and that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations, supported by efficacy data from a safety trial in which efficacy was an exploratory assessment.The safety of SINGULAIR 4-mg and 5-mg chewable tablets in pediatric patients aged 2 to 14 years with allergic rhinitis is supported by data from studies conducted in pediatric patients aged 2 to 14 years with asthma. A safety study in pediatric patients 2 to 14 years of age with seasonal allergic rhinitis demonstrated a similar safety profile [see Adverse Reactions (6.1)]. The safety of SINGULAIR 4-mg oral granules in pediatric patients as young as 6 months of age with perennial allergic rhinitis is supported by extrapolation from safety data obtained from studies conducted in pediatric patients 6 months to 23 months of age with asthma and from pharmacokinetic data comparing systemic exposures in patients 6 months to 23 months of age to systemic exposures in adults.The safety and effectiveness in pediatric patients below the age of 12 months with asthma and 6 months with perennial allergic rhinitis have not been established. The safety and effectiveness in pediatric patients below the age of 15 years with exercise-induced bronchoconstriction have not been established.Growth Rate in Pediatric PatientsA 56-week, multi-center, double-blind, randomized, active- and placebo-controlled parallel group study was conducted to assess the effect of SINGULAIR on growth rate in 360 patients with mild asthma, aged 6 to 8 years. Treatment groups included SINGULAIR 5 mg once daily, placebo, and beclomethasone dipropionate administered as 168 mcg twice daily with a spacer device. For each subject, a growth rate was defined as the slope of a linear regression line fit to the height measurements over 56 weeks. The primary comparison was the difference in growth rates between SINGULAIR and placebo groups. Growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for the SINGULAIR, placebo, and beclomethasone treatment groups were 5.67 (5.46, 5.88), 5.64 (5.42, 5.86), and 4.86 (4.64, 5.08), respectively. The differences in growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for SINGULAIR minus placebo, beclomethasone minus placebo, and SINGULAIR minus beclomethasone treatment groups were 0.03 (-0.26, 0.31), -0.78 (-1.06, -0.49); and 0.81 (0.53, 1.09), respectively. Growth rate (expressed as mean change in height over time) for each treatment group is shown in FIGURE 1.FIGURE 1Change in Height (cm) from Randomization Visit by Scheduled Week(Treatment Group Mean ± Standard Error† of the Mean)。
顺尔宁说明书

顺尔宁说明书【通用名】孟鲁司特钠咀嚼片Montelukast【商品名】顺尔宁Singulair【化学名称】孟鲁司特钠,[R-(E)-1[[[1-[3[2-(7-氯-2-喹啉基)乙烯基]苯基]-3-(1-羟基-1-甲基乙基)苯基]丙基]硫代]甲基]环丙基乙酸,单纳盐]【理化性质】白色粉末,易溶于甲醇、乙醇和水,不溶于乙腈,对光敏感。
【药理作用】本品是一种强效的选择性的白三烯D4(LTD4,cysLT1)受体拮抗剂,是新一代非甾体抗炎药物。
能选择性抑制气道平滑肌中白三烯多肽的活性,并有效预防和抑制白三烯所导致的血管通透性增加、气道嗜酸粒细胞(EOS)浸润及支气管痉挛,能减少气道因变应原刺激引起的细胞和非细胞性炎症物质,能抑制变应原激发的气道高反应。
对二氧化硫、运动和冷空气等刺激及各种变应原如花粉、毛屑等引起的速发相和迟发相炎症反应均有抑制作用。
本品与人类气道中的cysLT受体能高度选择性结合,从而阻断白三烯的病理作用。
已经证明,吸入LTD4可引起较组胺强1000倍的剂量依赖性支气管收缩。
【适应证】本品适用于成人和儿童哮喘的预防和长期治疗,包括预防白天和夜间的哮喘症状,能够改善慢性气道炎症,改善肺功能,控制哮喘症状,减少必需的β2激动剂用量,治疗对阿司匹林敏感的哮喘患者以及预防运动引起的支气管收缩。
【用法与用量】15岁及15岁以上成人剂量为10mg,qd睡前服用。
6~14岁儿童剂量为5mg,qd,睡前服用。
该组患者不需再按年龄调整剂量。
6岁以下患儿的安全有剂量及用法尚未确定。
【不良反应】顺尔宁的一般耐受性良好,不良反应较轻微,通常不需中止治疗。
最常见的不良反应是头痛、偶有腹痛、咳嗽、流感样症状,儿童与成人的发生率相似。
【禁忌症】对本品的任何成分过敏者禁用。
【注意事项】①口服本药不应该用于治疗急性哮喘的发作,应劝告患者准备好必要的急救药品备用。
②虽然合用的吸入皮质类固醇制剂可在医师监督下逐渐减量,但不应骤然使用本品取代吸入或口服皮质类固醇制剂。
孟鲁司特钠参考课件

Page 14
剂型设计—工艺流程
辅料
原料药
混合
过40目
包衣液
压片
包衣
入库
成品寄存
外包装
双铝包衣
检查
外包装材料
消毒
药用包装铝
Page 15
立项依据 剂型设计 药理和毒理
原料药的合成 质量标准
Page 16
质量标准—原料药质量检查
孟鲁司特钠原料药
性状:浅黄色至黄色粉 末,无臭无味见光分解 颜色加深 熔点: 145~148℃,溶 于甲苯、二甲苯、氯仿, 不易溶于甲醇、乙醇, 难溶于乙腈、水。旋光 度+102°
Page 17
质量标准—制剂质量检查(片剂)
鉴别
取本品的细粉 适量(相当于 孟鲁斯特 5mg),加水 10mL,超声 使溶解
孟鲁司特钠片剂
性状:薄膜衣片,除去包衣 后显白色或类白色
滴加5滴高锰 酸钾试液, 紫红色应立 即消失
在色谱图中,供 试品主峰的 保留时间应 与对照品主 峰的保留时 间一致。
Page 18
测定法
(1) 色谱条件 (2)对照品溶液的制备 (3)标准曲线和线性范围
(4)系统适应性试验
(5)含量测定 (6)回收率
(7)方法学考察:专属性、检测限、定量限、精密度、重复性
Page 22
质量标准—制剂含量测定
Hypersi lBDS-C.
进样量 : 20uL
色谱柱(100mm X 4.6mm ,5t xm ) 柱温 27℃, 流速 1.0mL/min 流动相A 磷酸二氢钠缓冲溶液(NaH2PO4H2O 3.9g)加入 1000mL水 使溶解, 用磷酸调节 pH 至 3.7 ) 流动相B 乙腈 梯度洗脱 0~15min, 60%B ,15~20min, 60%B ~ 90%B,20~ 26min, 90%B
孟鲁司特钠ppt课件

质量标准—制剂含量均匀检查中国药典2010版二部附录X E
本品1片,研 细,加流动相 适量再研磨
并用流动相 分次转移至 50mL量瓶中 ,超声15分 钟
摇匀,滤过, 滤液作为供 试品溶液
另精密称 取孟鲁司 特钠对照 品适量
用流动相配成 1mL中含0.2mg 的溶液作为对照 品溶液
照含量测定项 下的方法测定, 应符合规定
Page 16
质量标准—原料药质量检查
孟鲁司特钠原料药
性状:浅黄色至黄色粉 末,无臭无味见光分解 颜色加深 熔点: 145~148℃,溶 于甲苯、二甲苯、氯仿 ,不易溶于甲醇、乙醇 ,难溶于乙腈、水。旋 光度+102°
Page 17
质量标准膜衣片,除去包衣 后显白色或类白色
Page 22
质量标准—制剂含量测定
Hypersi lBDS-C.
进样量 : 20uL
色谱柱(100mm X 4.6mm ,5t xm ) 柱温 27℃, 流速 1.0mL/min 流动相A 磷酸二氢钠缓冲溶液(NaH2PO4H2O 3.9g)加入 1000mL水 使溶解, 用磷酸调节 pH 至 3.7 ) 流动相B 乙腈 梯度洗脱 0~15min, 60%B ,15~20min, 60%B ~ 90%B,20~ 26min, 90%B
检测波长 225 nm
Page 23
质量标准—制剂含量测定
系
精确注入稀释液 20u L 至色谱系统
统
, 记录色谱图。基线平稳, 无可见
适
杂质峰。分别精确注入高、中、低
应
3 个浓度的对照品溶液 5份各 20uL
性
至色谱系统,记录色谱图,进行系统适
试
用性试验。计算每份注入的对照品
【资料】顺尔宁儿科典型病例模版0316汇编

评估后调整治疗 第24 周 第36周
2天3喷
第48 周
用 白三烯受体拮抗剂 孟鲁司特钠 4mg qn
法
用
茶碱
量
抗胆碱药物
停用
其他
治疗体会
半胱氨酰白三烯和对类固醇敏感的介质是哮喘 炎症反应中的两条通道 激素不能阻断半胱氨酰白三烯介导的炎症通路 同时作用哮喘病人气道炎症的两条通路,可以 达到更好的炎症控制及哮喘控制
双通道
The slide represents an artistic rendition. Adapted from Peters-Golden M, Sampson AP J Allergy Clin Immunol 2003;111(1 suppl):S37-S42; Bisgaard H Allergy 2001;56(suppl 66):7-11.
•顺尔宁儿科典型病例模版0316
白三烯受体拮抗剂与皮质激素联合,作 用于炎症反应的双通道
半胱氨酰白三烯 在哮喘炎症中 扮演重要角色
对类固醇敏感的介质 在哮喘炎症中 扮演重要角色
类固醇不能抑制有症状的哮喘病人气道中的半胱氨酰白三烯的形成
孟鲁司特
阻断半胱氨酰 白三烯的作用
吸入激素
阻断对激素 敏感的介质
痰 a) 90
d)
中
70
嗜
50
酸
30
粒
细
胞
20
(%)
10
0 治疗前
治疗后 治疗前
治疗后
孟鲁司特
安慰剂
p=0.026
Pizzichini E, et al . Eur Respir J 1999; 14: 12±18.
白三烯受体拮抗剂可减轻哮喘的气道炎症
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
顺尔宁(Singulair)(孟鲁司特钠颗粒)
【药品名称】
商品名称:顺尔宁(Singulair)
通用名称:孟鲁司特钠颗粒
英文名称:Montelukast Sodium Oral Granules
【成份】
本品主要成份为孟鲁司特钠, 其化学名称为:[R-(E)]-1-[[[1-[3-[2-(7-氯-2-喹啉)乙烯基]苯基]-3-[2-(1-羟基-1-甲基乙基)苯基]丙基]硫] 甲基] 环丙烷乙酸钠
化学结构式:
分子式:C35H35ClNNaO3S
分子量: 608.18
【适应症】
本品适用于1岁以上儿童哮喘的预防和长期治疗,包括预防白天和夜间的哮喘症状,治疗对阿司匹林敏感的哮喘患者以及预防运动诱发的支气管收缩。
本品适用于减...
【用法用量】
每日一次。
哮喘病人应在睡前服用。
过敏性鼻炎病人可根据自身的情况在需要时间服药。
同时患有哮喘和过敏性鼻炎的病人应每晚用药一次。
1岁至2岁儿童哮喘患者每天一次,每次一袋。
2岁至5岁儿童哮喘患者和/或2岁至5岁过敏性鼻炎患者
应每天服用4mg口服颗粒一袋。
口服颗粒的服用
本品可直接服用,与一勺室温或冷的软性食物(如苹果酱)混合服用,或溶解于一茶匙室
温或冷的婴儿配方奶粉或母乳服用。
在服用时才能打开包装袋。
打开包装袋以后应立即服用全部的剂量(15分钟内)。
与食物、婴儿配方奶粉或母乳混合后的本品不能再贮存至下次继续服用。
本品不应溶解于除婴儿配方奶粉或母乳外的其它液体中服用。
但是服药后可以饮水。
一般建议
以哮喘控制指标来评价治疗效果,本品的疗效在用药一天内即出现。
本品可与食物同服或另服。
应建议患者无论在哮喘控制还是恶化阶段都坚持服用。
对肾功能不全患者、轻至中度肝损害的患者及不同性别的患者无需调整剂量。
本品与其它哮喘治疗药物的关系
本品可加入患者现有的治疗方案中。
减少合并用药物的剂量:
支气管扩张剂
单用支气管扩张剂不能有效控制的哮喘患者,可在治疗方案中加入本品,一旦有临床治疗反应(一般出现在首剂用药后),根据患者的耐受情况,可将支气管扩张剂剂量减少。
吸入糖皮质激素
对接受吸入糖皮质激素治疗的哮喘患者加用本品后,可根据患者的耐受情况适当减少糖皮质激素的剂量。
应在医师指导下逐渐减量。
某些患者可逐渐减量直至完全停用吸入糖皮质激素。
但不应当用本品突然替代吸入糖皮质激素或遵医嘱。
【不良反应】
本品一般耐受性良好,不良反应轻微,通常不需要终止治疗。
本品总的不良反应发生率与安慰剂相似。
2至5岁儿童哮喘患者
已在573名2至5岁儿童患者中评价了本品的安全性。
在一项安慰剂对照为期12周的临床研究中,本品治疗组中与药物相关,发生率>1%且比安慰剂组高的唯一不良事件是口渴。
口渴发生率在两组间无显著差异。
累积已有426名2至5岁儿童患者使用本品治疗至少3个月,230名患者治疗6个月或更长时间,63名患者治疗12个月或更长时间。
随着本品治疗时间的延长,不良事件发生的情况无变化。
6个月至2岁儿童哮喘患者
已在大约175名6个月至2岁儿童患者中评价了本品的安全性。
在一项安慰剂对照为期6周的临床研究中,本品治疗组中与药物相关, 发生率>1%且比安慰剂组高的不良事件是腹泻、运动机能亢进、哮喘、湿疹样皮炎和皮疹。
但这些不良反应的发生率在两组间无显著差异。
2至14岁季节性过敏性鼻炎儿童患者
在一项为期2周的安慰剂对照临床研究中,已在280名2至14岁季节性过敏性鼻炎儿童患者中评价了本品的安全性。
每天晚间服用本品一次耐受性良好,不良反应发生率与服用安慰剂组类似。
在这项研究中,本品治疗组的不良反应发生率低于1%,且未发现有与药物相关,发生率高于安慰剂组的不良反应。
临床实践的合并分析
使用有效的自杀行为评估方法对41项安慰剂对照临床研究(35项研究针对15岁及以上患者;6 项研究针对6-14岁儿童患者)进行了合并分析。
在9929例服用本品的患者和7780例服用安慰剂的患者中,一例有自杀意念的患者服用了本品。
任何一组均未出现完成自杀、自杀企图或针对自杀行为的预备行动。
针对46项安慰剂对照临床研究(35项研究针对15岁及以上的患者;11项研究针对3个
月至14岁的儿童患者) 进行了独立的合并分析,评估行为相关性不良事件。
在11,673例服用本品的患者和8827例服用安慰剂的患者中,行为相关性不良事件的发生率分别为2.73%和2.27%;比值比为1.12(95% CI [0.93; 1.36])。
这些合并分析中包含的临床试验没有特定设计自杀率或行为相关性不良事件的检查。
上市后的经验
本品上市使用后有以下不良反应报告:
感染和传染:上呼吸道感染。
血液和淋巴系统紊乱:出血倾向增加。
免疫系统紊乱:包括过敏反应的超敏反应、十分罕见的肝脏嗜酸性粒细胞浸润。
精神系统紊乱:包括攻击性行为或敌对性的兴奋、焦虑、抑郁、方向知觉丧失、注意力不集中、夜梦异常、幻觉、失眠、记忆损伤、精神运动过激(包括易激惹、烦躁不安和震颤)、梦游、自杀的想法和行为(自杀)。
神经系统紊乱:眩晕、嗜睡、感觉异常/触觉减退及十分罕见的癫痫发作。
心脏紊乱:心悸。
呼吸,胸腔和纵隔系统紊乱:鼻衄;肺嗜酸性粒细胞增多症。
胃肠道紊乱:腹泻、消化不良、恶心、呕吐。
肝胆紊乱:ALT和AST升高、十分罕见的肝炎(包括胆汁淤积性,肝细胞和混合型肝损害)。
皮肤和皮下组织紊乱:血管性水肿、挫伤、多形性红斑、结节性红斑、瘙痒、皮疹、荨麻疹。
肌肉骨骼和结缔组织紊乱:关节痛、包括肌肉痉挛的肌痛。
其他紊乱和给药部位情况:衰弱/疲劳,水肿,发热。
【禁忌】
对本品中的任何成份过敏者禁用。
【注意事项】
口服本品治疗急性哮喘发作的疗效尚未确定。
因此,不应用于治疗急性哮喘发作。
应告知
患者准备适当的抢救用药。
虽然在医师的指导下可逐渐减少合并使用的吸入糖皮质激素剂量,但不应用本品突然替代
吸入或口服糖皮质激素。
服用本品的患者有精神神经事件的报道(见不良反应)。
由于其他因素也可能导致这些事件,因此不能确认是否与本品相关。
医生应与患者和/或护理人员探讨这些不良事件。
患者和/
或护理人员应被告知,如果发生这些情况,应通知医生。
接受包括白三烯受体拮抗剂在内的抗哮喘药物治疗的患者,极少病例发生以下一项或多项
情况:嗜酸性粒细胞增多症、血管性皮疹、肺部症状恶化、心脏并发症和/或神经病变(有时诊断为Churg-Strauss综合征——一种全身性嗜酸细胞性血管炎)。
这些情况有时与减
少或停用口服糖皮质激素治疗有关。
虽然这些情况与白三烯受体拮抗剂的因果关系尚未确定,但建议对服用顺尔宁的患者加以注意并作适当的临床监控。
【药物相互作用】
本品可与其它一些常规用于哮喘预防和长期治疗及治疗过敏性鼻炎的药物合用。
在药物相
互作用研究中,推荐剂量的本品不对下列药物产生有临床意义的药代动力学影响:茶碱、
泼尼松、泼尼松龙、口服避孕药(乙炔雌二醇/炔诺酮35/1)、特非那定、地高辛和华法林。
在合并使用苯巴比妥的患者中,孟鲁司特的血浆浓度-时间曲线下面积(AUC)减少大约40%。
但是不推荐调整本品的使用剂量。
体外试验表明孟鲁司特是CYP2C8的抑制剂。
然而,一项关于孟鲁司特和罗格列酮(一种主要通过CYP2C8代谢的典型探测底物)药物相互作用的临床研究数据表明,孟鲁司特在体内对CYP2C8没有抑制作用。
因此认为孟鲁司特不会对通过这种酶代谢的药物(例如:紫杉醇、罗格列酮、瑞格列奈)产生影响。
体外研究表明,孟鲁司特是CYP 2C8、2C9和3A4的底物。
一项涉及孟鲁司特和吉非贝齐(CYP 2C8和2C9的抑制剂)药物间相互作用的临床研究证明,吉非贝齐能使孟鲁司特的全身暴露水平增加4.4倍。
CYP 3A4的强效抑制剂----伊曲康唑,与吉非贝齐和孟鲁司特同时给药后不会进一步增加孟鲁司特的全身暴露水平。
在临床安全性研究中,使用了大于在成人中批准的10 mg剂量(例如连续22周给予成人患者200 mg/天的剂量,以及连续大约1周给予患者最高为900 mg/天的剂量),没有观察到有临床意义的不良事件,基于这样的数据,吉非贝齐对孟鲁司特全身暴露水平的影响被认为是不具有临床意义的。
因此,与吉非贝齐同时给药,无需调整孟鲁司特的剂量。
根据体外数据,孟鲁司特与其他已知的CYP 2C8抑制剂(例如甲氧苄啶)之间预计不会发生有临床意义的药物相互作用。
此外,仅孟鲁司特与伊曲康唑同时给药不会显著增加前者的全身暴露水平。
【贮藏】
密封、避光、室温(15-30°C)保存。
【批准文号】
H20110597。
