Curtius重排有机化学
Curtius重排有机化学
then t-BuOH, reflux, 81% O
CbzN O
HN
O
H
O
N
N
OO
N H
N H
CO2H
H
Syringolin A
C. R. J. Stephenson et.al. Org. Lett., 2010, 12, 3453-3455
2.4 the synthesis of carbamates
3.2 Lossen Rearrangement
Mechanism of Lossen Rearrangement
3.2 Lossen Rearrangement
Recent applicationsLeabharlann OR1OH N
H
CDI MeCN
O
O O
R1 N
CDI: 羰基二咪唑
J. A. stafford. et.al. J. Org. Chem. 1998, 63, 10040
R1 N C O R2 NH2 Isocyanate
O
R1 N H
NHR2
Urea derivative
R2 OH
O
R1 N H
OR2
Carbamate
2.1 Mechanism
Thermal rearrangement
Photochemical rearrangement
注意:由于氮烯存在的证据很难找到,也有人认为 基团的迁移和N2的离去为一协同过程。
Curtius Rearrangment
Contents
1 Introduction 2 CurtiuCsliRckeatorrandgdeTmitelent 3 OtherCNliucckletoopahdidlicTritelearrangement 4 ConcClulsicioknto add Title
有机人名反应-反应机理5

26
Beckmann 重排 反应实例:
(3)
27
用已知试剂及其他常用试剂制备下列化合物并解释所用反应机理,请利用 Beckmann重排
H
N
Br
C
O
??? N
OH
29
N OH
N HO
30
H N OH H
acid heat
HO HN
acid heat
H
31
H N OH H
acid heat
HO HN
O
O
O
54
Claisen 缩合 反应式:
55
机理:
Claisen 缩合
pKa 24.5
pKa 15.9
pKa 10
56
Claisen 缩合
反应实例:
当只有一个a-H时:
pKa 33
57
Claisen 缩合
应用:
O
O
H
O
O
O
Ph O
O O
O
O Ph
Ph O
O O
O O
O
58
Claisen 缩合
Br
Zn
O
OZnBr
RO O
R
OR
R OH O
R
OR 72
Demjanov 重排 反应式:
73
机理:
Demjanov 重排
74
Demjanov 重排 反应实例:
75
76
Diels-Alder反应 反应式:
77
机理:
Diels-Alder反应
78
说明:
Diels-Alder反应
以吸电子与给电子来解释,吸 与给使双键两端出现正负,再 以正负相吸来解释。
库尔提斯重排反应

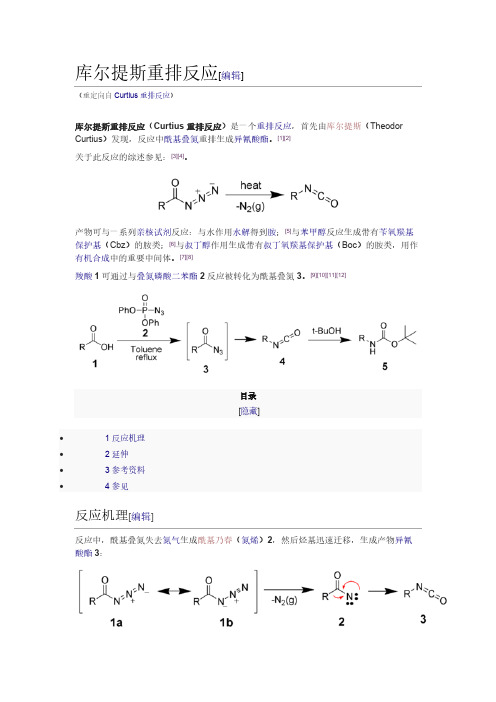
库尔提斯重排反应[编辑](重定向自Curtius重排反应)库尔提斯重排反应(Curtius重排反应)是一个重排反应,首先由库尔提斯(Theodor Curtius)发现,反应中酰基叠氮重排生成异氰酸酯。
[1][2]关于此反应的综述参见:[3][4]。
产物可与一系列亲核试剂反应:与水作用水解得到胺;[5]与苯甲醇反应生成带有苄氧羰基保护基(Cbz)的胺类;[6]与叔丁醇作用生成带有叔丁氧羰基保护基(Boc)的胺类,用作有机合成中的重要中间体。
[7][8]羧酸1可通过与叠氮磷酸二苯酯2反应被转化为酰基叠氮3。
[9][10][11][12]目录[隐藏]∙ 1 反应机理∙ 2 延伸∙ 3 参考资料∙ 4 参见反应机理[编辑]反应中,酰基叠氮失去氮气生成酰基乃春(氮烯)2,然后烃基迅速迁移,生成产物异氰酸酯3:延伸[编辑]在Curtius重排反应的基础上,Darapasky递降反应(A. Darapsky, 1936)以α-氰基酯为原料,通过重排反应生成氨基酸。
[13]参考资料[编辑]1. ^ Curtius, T. Ber.1890, 23, 3023.2. ^ Curtius, T. J. Prakt. Chem.1894, 50, 275.3. ^ Smith, P. A. S. Org. React.1946, 3, 337-449. (Review)4. ^ Scriven, E. F.; Turnbull, K.; Chem. Rev.1988, 88, 297-368. Review5. ^ Kaiser, C.; Weinstock, J. Organic Syntheses, Coll. Vol. 6, p.910 (1988); Vol. 51,p.48 (1971).Article6. ^ Ende, D. J. a.; DeVries, K. M.; Clifford, P. J.; Brenek, S. J. Org. Proc. Res.Dev.1998, 2, 382-392.7. ^ Lebel, H.; Leogane, O.; Org. Lett.2005, 7(19),4107-4110. doi:10.1021/ol051428b8. ^ Shioiri, T.; Yamada, S. Organic Syntheses, Coll. Vol. 7, p.206 (1990); Vol. 62,p.187 (1984).Article9. ^ Shioiri, T.; Ninomiya, K.; Yamada, S. J. Am. Chem. Soc.1972, 94,6203-6205.doi:10.1021/ja00772a05210. ^ Ninomiya, K.; Shioiri, T.; Yamada, S. Tetrahedron1974, 30, 2151-2157.11. ^ Wolff, O.; Waldvogel, S. R. Synthesis2004, 1303-1305.12. ^ Jessup, P. J.; Petty, C. B.; Roos, J.; Overman, L. E. Organic Syntheses, Coll.Vol. 6, p.95 (1988); Vol. 59, p.1 (1979). Article13. ^/reactions/RXN051.htm(重定向自贝克曼重排)贝克曼重排反应(Beckmann rearrangement)是一个由酸催化的重排反应,反应物肟在酸的催化作用下重排为酰胺。
有机重排反应总结

有机化学中重排反应有机化学中重排反应很早就被人们发现,研究并加以利用。
第一次被Wohler发现的,由无机化合物合成有机化合物,从而掀开有机化学神秘面纱的反应—加热氰酸铵而得到尿素,今天也被化学家归入重排反应的范畴。
一般地,在进攻试剂作用或者介质的影响下,有机分子发生原子或原子团的转移和电子云密度重新分布,或者重键位置改变,环的扩大或缩小,碳架发生了改变,等等,这样的反应称为是重排反应。
按照反应的机理,重排反应通常可分为亲核反应、亲电反应、自由基反应和周环反应四大类。
也有按照不同的标准,分成分子内重排和分子间重排,光学活性改变和不改变的重排反应,等等。
一、亲核重排重排反应中以亲核重排为最多,而亲核重排中又以1,2重排为最常见。
(一)亲核1,2重排的一般规律1.亲核1,2重排的三个步骤:离去基团离去,1,2基团迁移,亲核试剂进攻2.发生亲核1,2重排的条件(1)转变成更稳定的正离子(在非环系统中,有时也从较稳定的离子重排成较不稳定的离子)(2)转变成稳定的中性化合物(3)减小基团间的拥挤程度,减小环的张力等立体因素。
(4)进行重排的立体化学条件:带正电荷碳的空p轨道和相邻的C-Z键以及α碳和β碳应共平面或接近共平面(5)重排产物在产物中所占的比例不仅和正电荷的结果有关,而且和反应介质中存在的亲核试剂的亲核能力有关3.迁移基团的迁移能力(1)多由试验方法来确定基团的固有迁移能力(2)与迁移后正离子的稳定性有关(3)邻位协助作用(4)立体因素4.亲核1,2重排的立体化学:(1)迁移基:构象基本保持,没有发现过构型反转,有时有部分消旋(2)迁移终点:取决于离去及离去和迁移基进行迁移的相对时机5.记忆效应:后一次重排好像和第一次重排有关,中间体似乎记住了前一次重排过程(二) 亲核重排主要包括基团向碳正离子迁移,基团向羰基碳原子迁移,基团向碳烯碳原子迁移,基团向缺电子氮原子转移,基团向缺电氧原子的迁移,芳香族亲核重排,下面就这六种迁移作简要介绍:1.基团向碳正离子迁移:(1)Wagner-Meerwein重排:烃基或氢的1,2移位,于是醇重排成烯(2)片那醇重排:邻二醇在酸催化下会重排成醛和酮(3)Demyanov重排,Tiffeneau-Demyanov扩环以及有关反应(4)二烯酮-酚重排:4,4-二取代环己二烯酮经酸处理重排成3,4-二取代酚的反应(5)醛酮同系物的合成:醛或酮和重氮甲烷作用生成高一级的同系物(6)烯丙基重排:烯丙基系统中双键发生位移的反应2.基团向羰基碳原子迁移:(1) Benzil-Benzilic Acid重排:α-二酮经强碱处理会发生重排,生成α-羟基乙酸盐(2) 酸催化下醛酮的重排:在烃基的交换后,醛重排成酮,酮则重排成另一种酮3.基团向碳烯碳原子迁移:(1) Arndt-Eistert合成和Wolff重排:由羧酸经酰卤,重氮酮合成高一级同系物的方法(2) 其他的碳烯重排反应,主要是1,2氢迁移生成烯4.基团向缺电子氮原子转移:(1)Beckmann重排:醛肟或酮肟重排成酰胺(2)Hoffmann重排:氮上无取代基酰胺经溴及碱处理,脱羰生成伯胺(3)Curtius重排:酰基叠氮热分解生成异氰酸酯(4)Schmidt重排:酸、醛和酮在酸催化下和叠氮酸反应,生成胺、酰胺等的反应(5)Lossen重排:异羟肟酸及O-酰基衍生物经类似Hoffmann的重排生成少一个碳的胺(6)Neber重排:肟酮的磺酸酯在乙醇钾处理后水解生成α-氨基酮5.基团向缺电氧原子的迁移:(1)氢过氧化物的重排:氢过氧化物在酸催化下,O-O键断裂,同时烃基从碳原子迁移到氧原子上(2)Baeyer-Villiger重排:酮在酸催化下与过酸作用,在分子中插入氧生成酯的反应6.芳香族亲核重排:(1)芳羟胺重排(Bamberger重排):经硫酸处理重排成氨基酚(2)Sommelet-Hauser重排:苄基季胺盐经氨基钠等强碱处理重排成邻位取代的苄基叔胺二、自由基重排反应1.1,2迁移:比正离子重排反应少得多,主要发生在:(1)某些双自由基的1,2-烷基和氢(2)烯基(迁移的乙烯基若是环的一部分,则发生重排)2.非1,2迁移:多发生1,5迁移3.Barton反应:处于羟基δ位上的甲基氧化成醛基的反应4.Hofmann-loffler-freytag反应:质子化N-卤化胺经热分解或光解形成六氢吡啶等的反应。
有机选论

各种异氰酸酯的非光气合成 异氰酸酯是重要的有机合成中间体,其反应活性高,能与亲核 试剂进行加成,能参加众多的精细有机合成反应,已被广泛应 用于农药和医药等精细有机合成行业和高分子工业。 在Curtius重排里,用亚硫酰氯(SOCl2)作为酰基氯化剂,从而 出现了一些异氰酸酯的非光气合成 例:合成异氰酸苯酯
芳胺在亚硝酸钠和氯化亚锡作用下生成苯肼 ,随后与丙酮酸反应,一锅法制得腙,它与二 苯叠氮磷酸在甲苯中反应,生成酰基叠氮化
物,随后发生Curtius重排,得到异氰酸酯,
最后关环生成三唑啉酮。在制备商品化除草
剂磺酰三唑酮和三唑酮草酯时,利用合成关
键中间体三唑啉酮。
相关文献:
1.绿色的Curtius重排及其新应用的研究 [期刊论文] 陈中元, 严剑峰, 陈玲英 - 《当代化工》 2006年5期 2.三唑啉酮衍生物合成方法研究进展 [期刊论文] 骆焱平- 《化学世界》 2011年9期 3.1-氨基-7-甲氧基-β-咔啉及其1-烷氧羰基氨基衍生物的合成和初 步抗肿瘤活性究 [期刊论文] 徐广宇, 周伊, 左高磊, 蒋勇军 - 《有机化学》 2009年10 期 4.酰基叠氮单体的合成、聚合反应及其聚合物的应用研究 [学位论文] 郑海庭, 2011 - 中国科学技术大学:高分子化学与物 理 5.合成芳香异氰酸酯最佳条件的探讨 [期刊论文] 冯桂荣, 张相平, 张会茹, 史春晖- 《唐山师范学院学报》 2005年5期 6.1-己胺甲酰基-5-氟尿嘧啶的新合成路线 [期刊论文] 古凤才, 史云梅, 王彦广, 王金明, 聂建明 - 《化学工业与 工程》 2001年5期
Curtius重排反应新应用
1.高收率地制备6-氮杂嘌呤衍生物 1,2,4-三嗪类化合物可以对某些肿瘤细胞产生抑制作用, 而6-氮杂嘌呤类衍生物则是制备1,2,4-三嗪类化合物的
有机重排反应详尽总结

有机重排反应详尽总结重排反应(rearrangement reaction)是分子的碳骨架发生重排生成结构异构体的化学反应,是有机反应中的一大类。
重排反应通常涉及取代基由一个原子转移到同一个分子中的另一个原子上的过程,现将有机重排反应进行一个详尽的总结。
(1)Amadori重排反应酸或碱催化下醛糖的N-糖苷(糖胺,glycosylamines)异构化生成1-胺基-1-脱氧酮糖的反应被称为Amadori重排反应。
此反应的底物和产物都称为“Amadori化合物”。
各种Lewis酸都已应用于此反应:CuCl2 , MgCl2 , HgBr2 , CdCl2 , AlCl3 , SnCl4 , etc。
此反应中只需催化量的酸就可以催化胺和醛糖反应,进而重排。
伯胺,仲胺,脂肪胺或芳香胺都可以发生此反应。
糖胺类化合物会发生复杂的美拉德反应(食物在烹饪或储藏过程中糖,胺,氨基酸和蛋白质等进行重排和降解)。
此反应中生成的黑色产物是由于发生了此类非酶褐变反应。
(2)Baker-Venkataraman重排贝克-文卡塔拉曼重排反应(Baker-Venkataraman重排)是2-乙酰氧基苯乙酮衍生物在碱作为催化剂之下生成1,3-二酮的反应。
这个反应常用于制造色酮和黄酮类化合物。
(3)Bamberger RearrangementN-芳基羟胺在强酸水溶液作用下重排为氨基苯酚的反应。
(4)Beckmann Rearrangement贝克曼重排反应(Beckman rearrangement)指醛肟或酮肟在酸催化下生成N-取代酰胺的亲核重排反应,反应中起催化作用的酸常用五氯化磷。
此反应是由德国化学家恩斯特·奥托·贝克曼发现并由此得名。
(5)Brook RearrangementC-Si→O-Si的离子性重排反应,从羟基硅烷到硅醚的转化。
Brook 重排反应的推动力是产物中键能较高的 Si-O 键的生成。
库尔提斯重排反应-推荐下载
在一个研究中[5],研究者使用电脑模拟丙酮肟在贝克曼溶剂中的重排反应,并考虑到了溶 剂分子和取代物的影响。模拟表明,有三个乙酸分子和一个质子(以氧鎓的形式存在)参 与了反应。形成亚胺中间体后(σ 配合物),甲基通过协同反应迁徙到氮上,并推走羟基。 羟基中氧原子受到三个乙酸分子的稳定。接下来,一分子水进攻亲电的碳原子,其中一个 氢原子被一个乙酸接收,生成的中间体为 N-甲基乙酰氨酸,其中氧原子为四配位。最后异 构化形成稳定的产物酰胺。
p.48 (1971).Article 6. ^ Ende, D. J. a.; DeVries, K. M.; Clifford, P. J.; Brenek, S. J. Org. Proc. Res.
Dev. 1998, 2, 382-392. 7. ^ Lebel, H.; Leogane, O.; Org. Lett. 2005, 7(19), 4107-
延伸[编辑]
在 Curtius 重排反应的基础上,Darapasky 递降反应(A. Darapsky, 1936)以 α-氰基酯 为原料,通过重排反应生成氨基酸。[13]
参考资料[编辑]
1. ^ Curtius, T. Ber. 1890, 23, 3023. 2. ^ Curtius, T. J. Prakt. Chem. 1894, 50, 275. 3. ^ Smith, P. A. S. Org. React. 1946, 3, 337-449. (Review) 4. ^ Scriven, E. F.; Turnbull, K.; Chem. Rev. 1988, 88, 297-368. Review 5. ^ Kaiser, C.; Weinstock, J. Organic Syntheses, Coll. Vol. 6, p.910 (1988); Vol. 51,
异氰酸酯中间态的重排反应

强酸(硫酸、聚磷酸、三氯乙酸等)存在下发生分子内重排分别得到胺、腈及酰胺。
反应由卡尔· 弗里德里希· 施密特在1924年发现,一般采用质子酸(如硫酸、多聚磷 酸、三氯乙酸)或路易斯酸催化。如果原料在酸中稳定,则这个反应产率很高, 高于同类型的霍夫曼重排反应、Lossen重排反应及Curtius重排反应。使用的羧酸 可以是一元或二元直链脂肪羧酸、脂环族羧酸或芳香族羧酸。叠氮酸及酰基叠氮 均是易爆且有毒的化合物,使用时需注意安全。
生成异氰酸酯中间态的人名反应
目录
Curtius 反应 Hofmann 重排 Lossen 反应 Schmidt反应
Curtius 重排反应
Curtius重排反应是首先由库尔提斯(Theodor Curtius)于1890年发现,反应中 酰基叠氮在惰性溶剂中加热分解重排生成异氰酸酯。
产物可与一系列亲核试剂反应:与水作用水解得到胺;与苯甲醇反应生成带有
பைடு நூலகம்
苄氧羰基保护基(Cbz)的胺类;与叔丁醇作用生成带有叔丁氧羰基保护基
(Boc)的胺类,用作有机合成中的重要中间体。
Curtius 反应
羧酸可通过与叠氮磷酸二苯酯(DPPA)反应被转化为酰基叠氮加热重排成 异氰酸酯。
反应机理:
Curtius 反应
应用实例:
Hofmann 重排
霍夫曼重排反应又称霍夫曼降解反应(Hofmann降解),由奥古斯特· 威
Hofmann 重排
反应变化:
一些反应物可以用来代替溴,如N-溴代琥珀酰亚胺用1,8-二氮杂二环[5.4.0]十一碳7-烯(DBU)作为生物碱进行反应。
用甲醇钠和甲醇的混合液代替氢氧化钠,可以提高产率。此时中间产物异氰酸酯 会和甲醇反应生成易水解和分离的氨基甲酸甲酯。 参考文献:Org. Synth. 2002, 78, 234
有机化学重排反应,总结

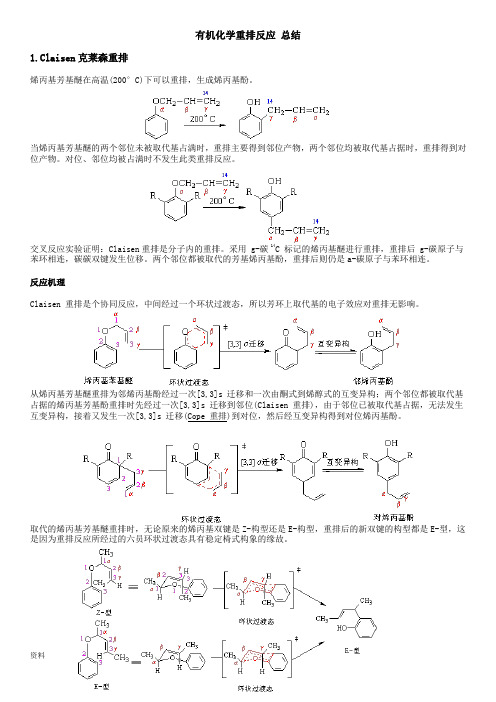
有机化学重排反应,总结有机化学重排反应总结1.Claisen克莱森重排烯丙基芳基醚在高温(200°C)下可以重排,生成烯丙基酚。
当烯丙基芳基醚的两个邻位未被取代基占满时,重排主要得到邻位产物,两个邻位均被取代基占据时,重排得到对位产物。
对位、邻位均被占满时不发生此类重排反应。
交叉反应实验证明:Claisen重排是分子内的重排。
采用g-碳14C 标记的烯丙基醚进行重排,重排后g-碳原子与苯环相连,碳碳双键发生位移。
两个邻位都被取代的芳基烯丙基酚,重排后则仍是a-碳原子与苯环相连。
反应机理Claisen 重排是个协同反应,中间经过一个环状过渡态,所以芳环上取代基的电子效应对重排无影响。
从烯丙基芳基醚重排为邻烯丙基酚经过一次[3,3]s 迁移和一次由酮式到烯醇式的互变异构;两个邻位都被取代基占据的烯丙基芳基酚重排时先经过一次[3,3]s 迁移到邻位(Claisen 重排),由于邻位已被取代基占据,无法发生互变异构,接着又发生一次[3,3]s 迁移(Cope 重排)到对位,然后经互变异构得到对位烯丙基酚。
取代的烯丙基芳基醚重排时,无论原来的烯丙基双键是Z-构型还是E-构型,重排后的新双键的构型都是E-型,这是因为重排反应所经过的六员环状过渡态具有稳定椅式构象的缘故。
反应实例Claisen 重排具有普遍性,在醚类化合物中,如果存在烯丙氧基与碳碳相连的结构,就有可能发生Claisen 重排。
2.Beckmann贝克曼重排肟在酸如硫酸、多聚磷酸以及能产生强酸的五氯化磷、三氯化磷、苯磺酰氯、亚硫酰氯等作用下发生重排,生成相应的取代酰胺,如环己酮肟在硫酸作用下重排生成己内酰胺:反应机理在酸作用下,肟首先发生质子化,然后脱去一分子水,同时与羟基处于反位的基团迁移到缺电子的氮原子上,所形成的碳正离子与水反应得到酰胺。
迁移基团如果是手性碳原子,则在迁移前后其构型不变,例如:反应实例3.Bamberger,E.重排苯基羟胺(N-羟基苯胺)和稀硫酸一起加热发生重排成对-氨基苯酚:在H2SO4-C2H5OH(或CH3OH)中重排生成对-乙氧基(或甲氧基)苯胺:其他芳基羟胺,它的环上的o-p位上未被取代者会起类似的重排。
有机重排反应总结

有机化学中重排反应有机化学中重排反应很早就被人们发现,研究并加以利用。
第一次被Wohler发现的,由无机化合物合成有机化合物,从而掀开有机化学神秘面纱的反应—加热氰酸铵而得到尿素,今天也被化学家归入重排反应的范畴。
一般地,在进攻试剂作用或者介质的影响下,有机分子发生原子或原子团的转移和电子云密度重新分布,或者重键位置改变,环的扩大或缩小,碳架发生了改变,等等,这样的反应称为是重排反应。
按照反应的机理,重排反应通常可分为亲核反应、亲电反应、自由基反应和周环反应四大类。
也有按照不同的标准,分成分子内重排和分子间重排,光学活性改变和不改变的重排反应,等等。
一、亲核重排重排反应中以亲核重排为最多,而亲核重排中又以1,2重排为最常见。
(一)亲核1,2重排的一般规律1.亲核1,2重排的三个步骤:离去基团离去,1,2基团迁移,亲核试剂进攻2.发生亲核1,2重排的条件(1)转变成更稳定的正离子(在非环系统中,有时也从较稳定的离子重排成较不稳定的离子)(2)转变成稳定的中性化合物(3)减小基团间的拥挤程度,减小环的张力等立体因素。
(4)进行重排的立体化学条件:带正电荷碳的空p轨道和相邻的C-Z键以及α碳和β碳应共平面或接近共平面(5)重排产物在产物中所占的比例不仅和正电荷的结果有关,而且和反应介质中存在的亲核试剂的亲核能力有关3.迁移基团的迁移能力(1)多由试验方法来确定基团的固有迁移能力(2)与迁移后正离子的稳定性有关(3)邻位协助作用(4)立体因素4.亲核1,2重排的立体化学:(1)迁移基:构象基本保持,没有发现过构型反转,有时有部分消旋(2)迁移终点:取决于离去及离去和迁移基进行迁移的相对时机5.记忆效应:后一次重排好像和第一次重排有关,中间体似乎记住了前一次重排过程(二) 亲核重排主要包括基团向碳正离子迁移,基团向羰基碳原子迁移,基团向碳烯碳原子迁移,基团向缺电子氮原子转移,基团向缺电氧原子的迁移,芳香族亲核重排,下面就这六种迁移作简要介绍:1.基团向碳正离子迁移:(1)Wagner-Meerwein重排:烃基或氢的1,2移位,于是醇重排成烯(2)片那醇重排:邻二醇在酸催化下会重排成醛和酮(3)Demyanov重排,Tiffeneau-Demyanov扩环以及有关反应(4)二烯酮-酚重排:4,4-二取代环己二烯酮经酸处理重排成3,4-二取代酚的反应(5)醛酮同系物的合成:醛或酮和重氮甲烷作用生成高一级的同系物(6)烯丙基重排:烯丙基系统中双键发生位移的反应2.基团向羰基碳原子迁移:(1) Benzil-Benzilic Acid重排:α-二酮经强碱处理会发生重排,生成α-羟基乙酸盐(2) 酸催化下醛酮的重排:在烃基的交换后,醛重排成酮,酮则重排成另一种酮3.基团向碳烯碳原子迁移:(1) Arndt-Eistert合成和Wolff重排:由羧酸经酰卤,重氮酮合成高一级同系物的方法(2) 其他的碳烯重排反应,主要是1,2氢迁移生成烯4.基团向缺电子氮原子转移:(1)Beckmann重排:醛肟或酮肟重排成酰胺(2)Hoffmann重排:氮上无取代基酰胺经溴及碱处理,脱羰生成伯胺(3)Curtius重排:酰基叠氮热分解生成异氰酸酯(4)Schmidt重排:酸、醛和酮在酸催化下和叠氮酸反应,生成胺、酰胺等的反应(5)Lossen重排:异羟肟酸及O-酰基衍生物经类似Hoffmann的重排生成少一个碳的胺(6)Neber重排:肟酮的磺酸酯在乙醇钾处理后水解生成α-氨基酮5.基团向缺电氧原子的迁移:(1)氢过氧化物的重排:氢过氧化物在酸催化下,O-O键断裂,同时烃基从碳原子迁移到氧原子上(2)Baeyer-Villiger重排:酮在酸催化下与过酸作用,在分子中插入氧生成酯的反应6.芳香族亲核重排:(1)芳羟胺重排(Bamberger重排):经硫酸处理重排成氨基酚(2)Sommelet-Hauser重排:苄基季胺盐经氨基钠等强碱处理重排成邻位取代的苄基叔胺二、自由基重排反应1.1,2迁移:比正离子重排反应少得多,主要发生在:(1)某些双自由基的1,2-烷基和氢(2)烯基(迁移的乙烯基若是环的一部分,则发生重排)2.非1,2迁移:多发生1,5迁移3.Barton反应:处于羟基δ位上的甲基氧化成醛基的反应4.Hofmann-loffler-freytag反应:质子化N-卤化胺经热分解或光解形成六氢吡啶等的反应。
有机化学中重排反应

有机化学中重排反应有机化学中重排反应很早就被人们发现,研究并加以利用。
第一次被Wohler发现的,由无机化合物合成有机化合物,从而掀开有机化学神秘面纱的反应—加热氰酸铵而得到尿素,今天也被化学家归入重排反应的范畴。
一般地,在进攻试剂作用或者介质的影响下,有机分子发生原子或原子团的转移和电子云密度重新分布,或者重键位置改变,环的扩大或缩小,碳架发生了改变,等等,这样的反应称为是重排反应。
按照反应的机理,重排反应通常可分为亲核反应、亲电反应、自由基反应和周环反应四大类。
也有按照不同的标准,分成分子内重排和分子间重排,光学活性改变和不改变的重排反应,等等。
一、亲核重排重排反应中以亲核重排为最多,而亲核重排中又以1,2重排为最常见。
(一)亲核1,2重排的一般规律1.亲核1,2重排的三个步骤:离去基团离去,1,2基团迁移,亲核试剂进攻2.发生亲核1,2重排的条件(1)转变成更稳定的正离子(在非环系统中,有时也从较稳定的离子重排成较不稳定的离子)(2)转变成稳定的中性化合物(3)减小基团间的拥挤程度,减小环的张力等立体因素。
(4)进行重排的立体化学条件:带正电荷碳的空p轨道和相邻的C-Z键以及α碳和β碳应共平面或接近共平面(5)重排产物在产物中所占的比例不仅和正电荷的结果有关,而且和反应介质中存在的亲核试剂的亲核能力有关3.迁移基团的迁移能力(1)多由试验方法来确定基团的固有迁移能力(2)与迁移后正离子的稳定性有关(3)邻位协助作用(4)立体因素4.亲核1,2重排的立体化学:(1)迁移基:构象基本保持,没有发现过构型反转,有时有部分消旋(2)迁移终点:取决于离去及离去和迁移基进行迁移的相对时机5.记忆效应:后一次重排好像和第一次重排有关,中间体似乎记住了前一次重排过程(二) 亲核重排主要包括基团向碳正离子迁移,基团向羰基碳原子迁移,基团向碳烯碳原子迁移,基团向缺电子氮原子转移,基团向缺电氧原子的迁移,芳香族亲核重排,下面就这六种迁移作简要介绍:1.基团向碳正离子迁移:(1)Wagner-Meerwein重排:烃基或氢的1,2移位,于是醇重排成烯(2)片那醇重排:邻二醇在酸催化下会重排成醛和酮(3)Demyanov重排,Tiffeneau-Demyanov扩环以及有关反应(4)二烯酮-酚重排:4,4-二取代环己二烯酮经酸处理重排成3,4-二取代酚的反应(5)醛酮同系物的合成:醛或酮和重氮甲烷作用生成高一级的同系物(6)烯丙基重排:烯丙基系统中双键发生位移的反应2.基团向羰基碳原子迁移:(1) Benzil-Benzilic Acid重排:α-二酮经强碱处理会发生重排,生成α-羟基乙酸盐(2) 酸催化下醛酮的重排:在烃基的交换后,醛重排成酮,酮则重排成另一种酮3.基团向碳烯碳原子迁移:(1) Arndt-Eistert合成和Wolff重排:由羧酸经酰卤,重氮酮合成高一级同系物的方法(2) 其他的碳烯重排反应,主要是1,2氢迁移生成烯4.基团向缺电子氮原子转移:(1)Beckmann重排:醛肟或酮肟重排成酰胺(2)Hoffmann重排:氮上无取代基酰胺经溴及碱处理,脱羰生成伯胺(3)Curtius重排:酰基叠氮热分解生成异氰酸酯(4)Schmidt重排:酸、醛和酮在酸催化下和叠氮酸反应,生成胺、酰胺等的反应(5)Lossen重排:异羟肟酸及O-酰基衍生物经类似Hoffmann的重排生成少一个碳的胺(6)Neber重排:肟酮的磺酸酯在乙醇钾处理后水解生成α-氨基酮5.基团向缺电氧原子的迁移:(1)氢过氧化物的重排:氢过氧化物在酸催化下,O-O键断裂,同时烃基从碳原子迁移到氧原子上(2)Baeyer-Villiger重排:酮在酸催化下与过酸作用,在分子中插入氧生成酯的反应6.芳香族亲核重排:(1)芳羟胺重排(Bamberger重排):经硫酸处理重排成氨基酚(2)Sommelet-Hauser重排:苄基季胺盐经氨基钠等强碱处理重排成邻位取代的苄基叔胺二、自由基重排反应1.1,2迁移:比正离子重排反应少得多,主要发生在:(1)某些双自由基的1,2-烷基和氢(2)烯基(迁移的乙烯基若是环的一部分,则发生重排)2.非1,2迁移:多发生1,5迁移3.Barton反应:处于羟基δ位上的甲基氧化成醛基的反应4三、亲电重排第一步是在亲核试剂作用下,离去基脱离形成富电中心,离去基以氢及金属原子居多;第二步是迁移基团留下一对成键电子,以正离子的形式向富电中心迁移,重排结果是形成新的富电中心。
有机化学重排反应总结

有机化学重排反应总结1、什么叫重排反应?一般地,在进攻试剂作用或者介质的影响下,有机分子发生原子或原子团的转移和电子云密度重新分布,或者重键位置改变,环的扩大或缩小,碳骨架发生了改变等等,这样的反应称为重排反应。
简单的理解:重排反应是指反应中烃基或氢原子或别的取代基从分子中的一个原子迁移到该分子中的另一个原子上的变化。
(指分子内重排)2、重排的分类按反应机理 ,重排反应可分为:基团迁移重排反应和周环反应中的重排。
基团迁移重排反应 即反应物分子中的一个基团在分子范围内从某位置迁移到另一位置的反应。
常见的迁移基团是烃基。
基团迁移重排反应又包括缺电子重排(亲核重排),富电子重排(亲电重排)和自由基重排.。
周环反应中的重排包括电环反应、σ键迁移。
也可按照不同的标准,分成分子内重排和分子间重排,光学活性改变和不改变的重排反应等等。
本讲义把重排分为以下几类:a.从碳原子到碳原子的重排 b.从碳原子到杂原子的重排 c.从杂原子到碳原子的重排 d.其它重排一、从碳原子到碳原子的重排反应1、Wangner-Meerwein 重排(瓦格纳尔—米尔外英重排,简称瓦—米重排)两个相邻原子之间发生的重排叫1,2重排,也叫Wangner-Meerwein 重排。
如:醇或卤代烃在酸催化下进行亲核取代或消除反应时,烯烃进行亲电加成时发生的重排。
例如:a.亲电加成时发生的重排如果反应液中同时存在两种或是两种以上的亲核试剂,则通过中间体碳正离子,能够生成混合加成产物。
R 2C R 3R 1C OHR 4R 5R 2C R 3R 1CR 4R 5R 1CR 2C R 3R4R 5R1CR 2CR 3R 4R 5OH H +(-H O)重排H O(-H +)b.醇进行亲核取代和消除时的重排亲核取代时,除大多数伯醇难以形成正碳离子而按S N 2反应外,仲醇或叔醇反应常伴随着重排产物的产生。
(S N 1)消去时(S N 1):c.卤代烃进行亲核取代和消除时的重排亲核取代按S N 1机理反应时伴随着碳正离子的重排 消去时注意:有碳正离子形成时,就有可能伴随着重排反应 形成C +的方式总结: (a)卤代烃 (AgNO 3醇溶液) (b)含-NH 2,重氮化放氮气(c)-OH ,加 H +(失H 2O),烯烃加H +基团迁移顺序:对迁移顺序的理解:迁移基团的电子云密度越大越容易迁移(但具体情况下,要具体分析)(CH 3)3C-CH 2Cl(CH 3)3C-CH 2Ag (AgNO 3(CH 3)3C-CH 2N 2Cl-N 2(CH 3)3C-CH 2(CH 3)3C-CH 3NH 2NaNO 2△(CH 3)3C-CH 2OH (CH 3)3C-CH 2=CH 2(CH 3)3C-CH 2(CH 3)3C-CH-CH 3H +2H +ClR 3C-R 2CH-RCH 3-CH 3-H->>>>>>OCH 3>反应举例:2、Pinacol (频哪醇)重排(邻二醇重排)当起始物的脱水产物能产生两种不同的正离子时,总是生成更稳定的正碳离子为主,有不同迁移基团时,按迁移的难易程度进行。
Curtius重排

Curtius重排酰基叠氮加热进行1,2-C-N迁移并放出氮气,生成异氰酸酯的反应被称为Curtius重排。
反应中原位生成的异氰酸酯和各种亲核试剂反应,可以得到氨基甲酸酯,脲等各种N-酰基衍生物。
也可以直接水解得到伯胺。
酰氯被转化为酰基叠氮,其加热重排脱去一分子氮气后得到相应的异氰酸酯,异氰酸酯水解或和其他亲核试剂反应得到胺及相应的衍生物。
早期的合成方法都是将酸转变为相应的酰氯,再生成酰基叠氮。
后来Shiori(JACS,1972,94,6203)等人报道了DPPA和羧酸在室温下很温和的生成酰基叠氮,可一锅法合成胺。
若直接用过量的醇或直接用醇做溶剂可得到相应的胺的衍生物。
如用苄醇可一步得到Cbz保护的胺; 用叔丁醇可一步得到Boc保护的胺。
反应机理热重排机理:光照条件下也可以重排,酰基叠氮在光照条件下先生成氮宾,而后重排为异氰酸酯:反应实例相关文献1. Curtius, T. Ber. 1890, 23, 3033-3041. Theodor Curtius (1857-1928) was born in Duisburg, Germany. He studied music before switching to chemistry under Bunsen, Kolbe, and von Baeyer before succeeding Victor Meyer as a Professor of Chemistry at Heidelberg. He discovered diazoacetic ester, hydrazine, pyrazoline derivatives, and many nitrogen-heterocycles. Curtius also sang in concerts and composed music.【Theodor Curtius (1857-1928)生于德国杜伊斯堡。
有机化学重排反应 总结
有机化学重排反应总结1.Claisen克莱森重排烯丙基芳基醚在高温(200°C)下可以重排,生成烯丙基酚。
当烯丙基芳基醚的两个邻位未被取代基占满时,重排主要得到邻位产物,两个邻位均被取代基占据时,重排得到对位产物。
对位、邻位均被占满时不发生此类重排反应。
交叉反应实验证明:Claisen重排是分子内的重排。
采用 g-碳 14C 标记的烯丙基醚进行重排,重排后 g-碳原子与苯环相连,碳碳双键发生位移。
两个邻位都被取代的芳基烯丙基酚,重排后则仍是a-碳原子与苯环相连。
反应机理Claisen 重排是个协同反应,中间经过一个环状过渡态,所以芳环上取代基的电子效应对重排无影响。
从烯丙基芳基醚重排为邻烯丙基酚经过一次[3,3]s 迁移和一次由酮式到烯醇式的互变异构;两个邻位都被取代基占据的烯丙基芳基酚重排时先经过一次[3,3]s 迁移到邻位(Claisen 重排),由于邻位已被取代基占据,无法发生互变异构,接着又发生一次[3,3]s 迁移(Cope 重排)到对位,然后经互变异构得到对位烯丙基酚。
取代的烯丙基芳基醚重排时,无论原来的烯丙基双键是Z-构型还是E-构型,重排后的新双键的构型都是E-型,这是因为重排反应所经过的六员环状过渡态具有稳定椅式构象的缘故。
反应实例Claisen 重排具有普遍性,在醚类化合物中,如果存在烯丙氧基与碳碳相连的结构,就有可能发生Claisen 重排。
2.Beckmann贝克曼重排肟在酸如硫酸、多聚磷酸以及能产生强酸的五氯化磷、三氯化磷、苯磺酰氯、亚硫酰氯等作用下发生重排,生成相应的取代酰胺,如环己酮肟在硫酸作用下重排生成己内酰胺:反应机理在酸作用下,肟首先发生质子化,然后脱去一分子水,同时与羟基处于反位的基团迁移到缺电子的氮原子上,所形成的碳正离子与水反应得到酰胺。
迁移基团如果是手性碳原子,则在迁移前后其构型不变,例如:反应实例3.Bamberger,E.重排苯基羟胺(N-羟基苯胺)和稀硫酸一起加热发生重排成对-氨基苯酚:在H2SO4-C2H5OH(或CH3OH)中重排生成对-乙氧基(或甲氧基)苯胺:其他芳基羟胺,它的环上的o-p位上未被取代者会起类似的重排。
curtius重排反应机理

curtius重排反应机理
Curtius重排反应是一种亲核重排反应,主要涉及羧酸与叠氮化物的反应。
反应过程如下:
1. 羧酸与叠氮化物发生反应,生成酰基叠氮化物。
2. 酰基叠氮化物在惰性溶剂中加热分解,失去氮气,形成异氰酸酯。
3. 异氰酸酯与各种亲核试剂反应,可以得到氨基甲酸酯、脲等各种N-酰基衍生物。
4. 异氰酸酯也可以直接水解,得到伯胺。
反应机理中,叠氮化物首先进攻酰氯的羰基碳原子发生亲核加成,再消去氯离子形成亲核取代产物酰基叠氮化物。
在加热条件下,酰基叠氮化物放出氮气,同时得到异氰酸酯。
异氰酸酯随后与亲核试剂反应,形成相应的衍生物。
Curtius重排反应在有机合成中具有广泛的应用,可以用于制备氨基甲酸酯、脲等化合物,为药物合成和其他有机化学领域提供了一种实用的合成方法。
异氰酸酯中间态的重排反应[研究知识]
![异氰酸酯中间态的重排反应[研究知识]](https://img.taocdn.com/s3/m/73501269b90d6c85ec3ac69f.png)
1
行业倾力
Curtius 反应 Hofmann 重排
Lossen 反应
Schmidt反应
目录
2
行业倾力
Curtius 重排反应
Curtius重排反应是首先由库尔提斯(Theodor Curtius)于1890年发现,反应中 酰基叠氮在惰性溶剂中加热分解重排生成异氰酸酯。
9
行业倾力
反应机理:
Lossen 反应
在重排步骤中,R的迁移和离去基团的离去是协同进行的。当R是手性碳原子时, 重排后其构型保持不变:
10
行业倾力
Lossen 反应
Lossen 反应的两种改进方法: 1. 芳香酸和羟胺在聚磷酸(PPA)存在下加热至150-170℃,生成芳香伯胺:
2. 芳香酸和硝基甲烷在聚磷酸(PPA)存在下加热得芳香伯胺:
产物可与一系列亲核试剂反应:与水作用水解得到胺;与苯甲醇反应生成带有 苄氧羰基保护基(Cbz)的胺类;与叔丁醇作用生成带有叔丁氧羰基保护基 (Boc)的胺类,用作有机合成中的重要中间体。
3
行业倾力
Curtius 反应
羧酸可通过与叠氮磷酸二苯酯(DPPA)反应被转化为酰基叠氮加热重排成 异氰酸酯。
反应机理:
11
行业倾力
Schmidt反应
Schmidt反应是一个有机重排反应,羧酸、醛或酮分别与等摩尔的叠氮酸(HN3)在强 酸(硫酸、聚磷酸、三氯乙酸等)存在下发生分子内重排分别得到胺、腈及酰胺。反 应由卡尔·弗里德里希·施密特在1924年发现,一般采用质子酸(如硫酸、多聚磷酸、 三氯乙酸)或路易斯酸催化。如果原料在酸中稳定,则这个反应产率很高,高于 同类型的霍夫曼重排反应、Lossen重排反应及Curtius重排反应。使用的羧酸可以是 一元或二元直链脂肪羧酸、脂环族羧酸或芳香族羧酸。叠氮酸及酰基叠氮均是易 爆且有毒的化合物,使用时需注意安全。
经典有机化学反应机理大全

O OH H
COOEt
-EtO-
O
H EtOOC
-H+
O
H+
O
-CO2
O
COOEt
H HOOC
22. Diels-Alder反应(共轭二烯与亲二烯体发生环加成得到六元环, 反应具有立体专一性)
MeOOC +
COOMe
H COOMe
MeOOC H
COOMe
H COOMe
反应机理
COOMe
H COOMe
+ Ts
23. Enamine(烯胺)反应(二级胺与具有α-H的醛, 酮发生反应)
O H3C
H +N
O H3O+ H3C
N H3C
Br
N
H3C
反应机理
O
H
CC + NR
H
R
OH CCNR HR
R NR
24. Eschweiler-Clark反应(将伯胺, 仲胺和甲醛及甲酸还原性甲 基化制备叔胺)
=R
EtOH e-
R HH
EtOH
HH
EtOH
-R
R
HH
HH
10. Bouveault-Blanc反应(酯在钠-醇体系中先还原成醛, 再进一步 还原为伯醇)
RCOOR'
Na RCH2OH
EtOH
反应机理
O R OR'
Na
O
EtOH OH
Na
OH EtOH
R OR'
R OR'
R OR'
H R OR'
重要的有机反应机理
1. Arndt-Eistert反应(重氮甲烷与酰氯作用形成 -重氮酮,在Ag离 子催化下酰基碳烯 重排得到烯酮。烯酮水解得到多一个碳的羧酸)
有机化学中的重排反应(上课使用)

C H 3
C H 3
C H 3
C H 3 H
Ph C C O Ts
△
C H 3C H 3
H H 3C C C Ph
C H 3C H 3
苯的迁移速度为甲基的3000倍
⑶.反应实例
H 3 C C H 2 C H 2 B rA g N O 3 H 3 C C H 2 C H 2
B r
H
H 3 C C H C H 3
6. Baeyer—Villger重排(缺电 子氧的重排)
二、 富电子重排(亲电重排)
1. Steven重排 2. 邻二酮重排 3. Sommelet重排 4. Wittig重排 5. Favorski重排
三、 芳环上的重排反应 1. 联苯胺重排 2. Fries重排 3. Claisen重排
多数的有机化学反应是官能团的反应,碳胳一般保持不 变;但在有一些反应中,烃基或别的基团从一个原子迁移 到另一个原子上,碳胳或官能团的位置发生了变化,这类 反应称为分子重排。
NH2 HNO2
+NN -N2
OH +
+CH2
CH2OH +
CH2
而下例是环的扩大:
CH2NH2 HNO2 -N2
+CH2
CH2OH +
CH2
OH +
丙胺与亚硝酸作用的主产物为异丙醇, 说明H-原子也可迁移:
C H 3 C H H C H 2 N H 2 H N O 2 C H 3 C H H C H 2 N 2 +
有机化学中的 重排反应
目录
一、缺电子重排(亲核重排) 1. Wagner—Meerwein重排 (Demyanov重排)
有机化学中的重排反应
氮烯
R NH COOH
异氰酸酯
CO2 + RNH2
• 经过Nitrene的重排 • 酰胺重排生成少一个碳的胺 • Hoffmann重排:RCOOH→RCONH2→RNH2
二元酸的酰亚胺:
O C C O O C NH2 NH
NaOCl
NH2 COOH
COOH
更多例见后
(1) (CH3)3C-CH2CONH2
CH2CH2CO2H H SO + HN3 2 4 CH2CH2CO2H
羧酸可以是直链脂肪族的一元或二元羧酸、脂环酸、芳香 酸等;与Hoffmann重排、Curtius反应相比,本反应胺的 收率较高。
例:
⑷.贝克曼(Beckmann)重排
①定义:醛或酮肟在 酸性条件下,重 排生成酰胺的反 应。(质子酸
基团迁移活泼性顺序如下: 对甲氧基苯基 > 对甲基苯基 > 苯基 > 对溴苯基 > 烷 基>氢
(c) 结构不对称的二醇的重排,可由生 成的碳正离子的稳定性来判断那一个羟基 是离去基团。
R' R R' C C R
OH OH
C CH2OH
H+
_
_
H+
C CHO H 主要
C CH2OH OH
H2O
_
C CH2
0
O OK NH2
H+3O
O OH NH2
-aminoacid
⑵. Curtius (库尔悌斯)重排:
酰基叠氮化合物也发生类似的重排:
R C O
N N N
O C N R +N2
H2O
RNH2 + CO2
curtius重排机理

curtius重排机理The Curtius Rearrangement is an organic reaction which involvesthe isomerization of a substituted acylazide. It is a type of nucleophilic acyl substitution reaction.The reaction is named after Curtius, who first reported it in 1887. The essential feature is the replacement of a nitrene group (−N=N−) derived from the acylazide with an isocyanate group (N=C=O) and the formation of an isocyanate anion. The Curtius Rearrangement is the first step in a number of larger processesand is the second step of the Schmidt reaction, in which thenitrene is first replaced with an isocyanate hydrate and then hydrolyzed to produce an isocyanate anion.In the Curtius Rearrangement, the nitrene reacts with an electrophile, usually an acid transfer agent, such as an acid chloride or anhydride, to form an isocyanate. Product formation is facilitated by heating and a Lewis acid. The reaction typically proceeds with carbon-nitrogen bond cleavage and a rearrangement of atoms. The active species involved in the rearrangement is the acylium ion, which is the two-membered ring structure of an acyl group attached to an electron deficient nitrogen. This ring structure can easily rearrange itself.The reaction is highly stereospecific and regioselective. Stereochemical roles of the reactants and products can be determined. The reaction is advantageous in that all desired products form in one step and that it can be used to introduce a stereocenter into a substituted acyl group.The Curtius Rearrangement is an efficient and useful method for the purpose of ring isotope exchange. By it, a secondary isotope distribution can be installed into a cyclic structure. This makes the Curtius Rearrangement a valuable tool for synthetic chemistry and drug design.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
3.3 Wolff Rearrangement
NHRN12
OR3
Rh2(OAc)4 2H2O, PhH, reflux.
R1 NO
Ar
S
OO
Ar S
CO2R3
K. Kim. et.al. Org. Lett. 2002, 4, 873.
N2 O
Br
OTIPS
HO
OTIPS
C
hν
O Br
Br
Me
DCE, rt; then 80oC
D. A. Evans. et.al. Org. Lett. 2001, 3, 3009-3012
D. ROmo. et.al. J. Am. Chem. Soc. 2003, 125, 6344
3.2 Lossen Rearrangement
W. Lossen (1872)
Lossen Rearrangement
First published in 1881:
Hofmann, A. W. Chem. Ber. 1881, 14, 2725. Shioiri, T. Comp. Org. Syn. 1991, 6, 800‐806. (Review)
MOR or M(OR)2 MOX or NaBrO2
H2O / 0oC then heat
Introduction
亲核重排(缺电子重排)
在缺电子重排反应中,基团Z带着一对电子从原子A迁移到另一个缺 少电子的原子B上,多数为1,2-重排,即基团的迁移在两个相邻原子 间发生:
常见的亲核重排类型
缺电子碳链的重排 Baeyer-Villiger oxidation rearrangement, Backman rearrangement, pinacol rearrangement 等.
3.1 Hofmann Rearrangement
Recent applications
O PhIX2
R NH2
J. W. Keillor et.al. J. Org. Chem. 1997, 62, 7495.
OX I
R N Ph H
1,2-shift
N RC
O
H2O
R NH2
O O PhIX2 S
+
71%
OTIPS
Me
Me
steps
O
O
Me Salvilenone
R. L. Danheiser. et.al. J. Am. Chem. Soc. 1994, 116, 9471.
3.2 Lossen Rearrangement
Mechanism of Lossen Rearrangement
3.2 Lossen Rearrangement
Recent applications
O
R1
OH N
H
CDI MeCN
O
O O
R1 N
CDI: 羰基二咪唑
J. A. stafford. et.al. J. Org. Chem. 1998, 63, 10040
2.4 the synthesis of carbamates
D. H. Appella. et.al. J. Am. Chem. Soc. 2004, 126, 15067-15073
Ph
N Ph 1) TFA, CH2Cl2
O 2) DPPA, Et3N, toluene
3) BnOH
Ot-Bu
2.2 the synthesis of amines
D. L. Boger et.al. J. Am. Chem. Soc. 2006, 128, 15683-15696. Olivier Baudoin et.al. Angew. Chem. Int. Ed. 2009, 48, 179 -182
2.2 the synthesis of amines
Nakahara, S.et.al. J. Am. Chem. Soc. 1993, 115, 10733-10741. F. M. Menger.et.al. Angew. Chem. Int. Ed. 2002, 41, 2581 -2584.
O O
NH
NH NH
1) LiOH, H2O, THF, H2O2 50oC, 12h, 92%
2) DIPEA, acetone, ClCO2Et, 0oC; NaN3, rt, 12h, 75%
O O
NH NH
NH NH
R. L. Funk et.al. Org. Lett., 2006, 8, 3995-3998
碳烯与氮烯的重排 Curtius rearrangement , Schmidt rearangement , Wolff rearrangement, Lossen rearangement , Hofmann rearrangement
Introduction
Julius Wilhelm Theodor Curtius (1957-1928), 德国化 学家,生于德国鲁尔区的Duisburng。先后师从Bensun和 Kolbe 。 1882 年 , 在 Leipzig 大 学 取 得 博 士 学 位 。 1884 年,在慕尼黑大学跟随Baeyer工作。在1890年到1894年 间,发现了酰基叠氮化物的重排反应,并以其名字命名。 除此之外,他还发现了叠氮醋酸乙酯(1883),联氨 (1887),叠氮化合物(1891),吡唑啉衍生物(1891) 四嗪衍生物(1906),多肽(1904)。
then t-BuOH, reflux, 81% O
CbzN O
HN
O
H
O
N
N
Hale Waihona Puke OON HN HCO2H
H
Syringolin A
C. R. J. Stephenson et.al. Org. Lett., 2010, 12, 3453-3455
2.4 the synthesis of carbamates
N2
O
O NuH (1 equiv), PhMe
O
μ W ( 300 W), 1- 30 mi n
n
-N2
n
R
29 examples
R
O Nu
n = 0, 1, 2
Y. Coquerel. et.al. J. Org. Chem. 2009, 74, 415.
H. Yang. et.al. Org. Lett. 2000, 2, 2177.
Carda, M., Marco, J. A. Tetrahedron: Asymmetry, 2002, 13, 1005-1010. M. Lautens. et.al. J. Am. Chem. Soc. 2005, 127, 15028-15029.
2.4 the synthesis of carbamates
M = Na, K, Ba, Ca
X = Cl, Br
LTA or PhI(OCOR)2 or PhI(OH)OTs or
PhIO pH = 1-3 / solvent / H2O
LTA: 四乙酸铅
3.1 Hofmann Rearrangement
Mechanism of Hofmann Rearrangement
55%
Ph N Ph
NH Cbz
1) H2, Pd(OH)2/C MeOH, HCl
2) o-anisaldehyde, NaBH(OAc)3, CH2Cl2
66%
H N Ph
NH OMe
2-Epi-CP-99,994
J. Szymoniak. et.al. Org. Lett., 2008, 10, 2473-2476
2.3 the synthesis of ureas
A Solid-Phase Synthesis of N,N′-Disubstituted Ureas
M. T. Migawa.et.al. Org. Lett., 2000, 2, 3309-3311
H. Lebel, et.al. Org. Lett., 2006, 8, 5717-5720
O R1 C
N
CO2
HNR2 R3 R 4OH
O
R1 N H
NR2R3
O
R1 N H
OR4
P. Dube. et.al. Org. Lett. 2009, 11, 5622.
3.2 Lossen Rearrangement
Recent applications
K. Ohmoto. et.al. Synlett. 2001, 299.
3.3 Wolff Rearrangement
Wolff (1902)
Lossen Rearrangement
3.3 Wolff Rearrangement
Mechanism of Wolff Rearrangement
3.3 Wolff Rearrangement
Recent applications
2.4 the synthesis of carbamates
O H
CbzN O
HO2C steps
Garnes's alde
K.Tomioka. et.al. Org. Lett., 2009, 11, 2007-2009
CbzN
DPPA, Et3N, 4A MS, toluene, rt
BocHN
R1 N C O R2 NH2 Isocyanate
O
R1 N H
NHR2
Urea derivative
