托匹司他 topiroxostat 汤森路透报告 Cortellis报告
托烷司琼市场分析报告

托烷司琼市场分析报告汕头市聚信医药科技有限公司1.概述5-HT3 受体拮抗剂是最近几年发展起来的用于治疗肿瘤病人化疗引起的恶心、呕吐的一种有效药物。
托烷司琼,英文名Tropisetron,被认为是新型5-HT3 受体拮抗剂,为外周神经元和中枢神经系统内5-HT3 受体的高选择性抑制剂,具有外周性和中枢性的双重强效抗吐作用。
在同类药品中,只有托烷司琼的主环结构与5-HT 主环结构完全相同,几乎不与其它受体发生作用,故安全性好,副作用甚少,是选择性更高、亲和力更强的止吐药。
托烷司琼目前在临床主要用于预防和治疗癌症化疗引起的恶心和呕吐以及外科手术后恶心和呕吐。
其常见的不良反应有头痛、头昏、便秘、眩晕、疲劳和胃肠功能紊乱如腹痛和腹泻等;极少数病人可能出现一过性血压改变或过敏反应等。
2 托烷司琼国内形势一路走高托烷司琼是由瑞士Sandoz(三道士)公司研制开发,是继昂丹司琼和格拉司琼之后上市的第三个5-HT3 受体拮抗剂。
1996 年3 月7 日,瑞士两大化学/ 生命科学巨头山德士公司(Sandoz)和汽巴-嘉基公司(Ciba-Geigy)合并为现在的诺华(Novartis)。
托烷司琼带来的利益,自然由诺华接手享有。
1992 年盐酸托烷司琼首次在荷兰上市,接着在英国(1993 年)、西班牙(1994 年)、德国(1994 年)、澳大利亚(1995 年)、法国91996 年)、日本(1998 年)等国家上市。
我国于1993 年进口盐酸托烷司琼,行政保护期已于2001 年9 月到期。
国内司琼类药物市场上,近几年托烷司琼的销售增幅有所波动,但整体性的销售形势还是毋庸置疑的一路走高,市场份额也在不断攀升。
2005 年-2008 年其医院购药推总金额年平均增长率为66.08%,市场份额年平均增长率达到81.85%。
2005 年,对于托烷司琼来说是一个具有转折意义的一年,该年经国家药监局批准生产托烷司琼的新增企业数量也是最多。
汤森路透每月药物快讯-2014年5月

1mg/kg司妥昔单抗(标识号 NCT01024036)。司妥昔单抗组三分之一以上 受试者的肿瘤和症状缓解持续超过18周。安慰剂
组治疗失败中位时间是134天,但治疗组未达到 统计学意义。司妥昔单抗和安慰剂组贫血患者的 血红蛋白分别增加61%(1.5g/dL)和0%。 Janssen公司在201
1.8mcM,在HEK-293细胞中对Ik(v)1.5动作电位 后相的抑制作用增强,而不是对峰值部分。大鼠 心肌梗死模型中,给动物注射钾通道阻滞剂F17727(2
.5和5mg/kgi.v.)或赋形剂。用药后2分钟用心电 图记录心率不齐平均时程,单次使用F-17727后 观察到心律不齐的时程有明显差异(与赋形剂相 比P<0
.05),证实了其预防心律不齐发作的作用。家 兔AF模型报道了相似结果,表明自发AF发作 (F-17727和赋形剂分别为4vs.165s)和诱发AF (分别为82vs
.261s)时程与赋形剂相比明显缩短。这些结果 证实了临床前研究中F-17727作为抗心肌纤维颤 动剂的疗效(Junquero,D.etalExpBiol(Apri
DEMA3和EDEMA4)的结果,以及研究DX88/19的结果,Kalbitor首次获批时DX-88/19仍在 进行之中(标
识号NCT00262080、NCT00457015和 NCT00456508)。对年满12岁儿童患者的疗效 和安全性与对成人群体的相近
2-甲基-3-丁烯腈 /zh/cas-16529-56-9.html
盟获批。单用二甲双胍或与其他药物联用(包括 胰岛素)不能充分控制血糖的患者,以及已经在 分开用卡格列净和二甲双胍的患者,适宜使用本 片剂治疗,每日用药两次。2013年
3月申报了MAA(见2013年3月8日《汤森路透药 物新闻》)。支持其申报的III期计划包括二甲双 胍+卡格列净与二甲双胍+西格列汀或格列美脲的 比较(见2013年1
汤森路透:新药开发率趋于改善

汤森路透:新药开发率趋于改善
平崎诚司
【期刊名称】《生物产业技术》
【年(卷),期】2016(000)001
【摘要】美国大型信息服务企业汤森路透(Thomson Reuters)公司2015年8月发布的报告显示,新药开发率趋于改善。
此前,一般认为新药开发的效率是很低的。
【总页数】2页(P59-60)
【作者】平崎诚司
【作者单位】
【正文语种】中文
【相关文献】
1.汤姆·格罗瑟致路透员工的一封信——汤森路透只会让路透更伟大 [J],
2.78刚汤姆森与157岁路透整合大考金融信息帝国汤森路透 [J], 张刚
3.ABB入选汤森路透“2014全球创新百强企业”率 [J], ;
4.专访汤森路透首席执行官托马斯·格罗瑟——“汤森路透中国不会裁员” [J], 侯隽
5.汤森路透计划裁员12% [J], 央视新闻
因版权原因,仅展示原文概要,查看原文内容请购买。
新药Serlopitant(司洛匹坦)合成检索总结报告
新药Serlopitant(司洛匹坦)合成检索总结报告
一、Serlopitant(司洛匹坦)简介
2020年2月,宣布了Serlopitant(司洛匹坦)的二期临床试验的结果,每日口服抗瘙痒药治疗不明原因慢性瘙痒(CPUO)的安全性和有效性。
Serlopitant(司洛匹坦)被评估为可能与治疗有关的治疗-紧急不良事件的频率为治疗组10.3%,安慰剂组2.6%。
副反应发生率最高的是腹泻(6.9%)、嗜睡(5.2%)、疲劳和头痛(2.6%)。
安慰剂组最常报告的不良事件是胃食管反流病和关节痛(各2.6%)。
两名接受治疗的患者报告了三起被认为与研究药物无关的严重不良事件。
迄今为止,对2 000多人进行了药物治疗,包括接受一年以上治疗的患者。
Serlopitant(司洛匹坦)分子结构式如下:
英文名称:Serlopitant
中文名称:司洛匹坦
本文主要对Serlopitant(司洛匹坦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Serlopitant(司洛匹坦)合成路线
三、Serlopitant(司洛匹坦)合成检索总结报告(一) Serlopitant(司洛匹坦)中间体3的合成
(二) Serlopitant(司洛匹坦)中间体5的合成
(三) Serlopitant(司洛匹坦)中间体6的合成
(四) Serlopitant(司洛匹坦)中间体7的合成
(五) Serlopitant(司洛匹坦)中间体8的合成
(六) Serlopitant(司洛匹坦)中间体9的合成
(七) Serlopitant(司洛匹坦)中间体10的合成方法一。
新药Osilodrostat(奥西卓司他)合成检索总结报告
新药Osilodrostat(奥西卓司他)合成检索总结报告
一、Osilodrostat(奥西卓司他)简介
2020年01月16日欧盟委员会(EC)已批准孤儿药产品Osilodrostat(奥西卓司他)的上市许可,该产品用于治疗成人内源性库欣综合症。
2020年3月6日FDA批准了Osilodrostat(奥西卓司他)用于治疗成人内源性库欣综合症。
Osilodrostat(奥西卓司他)分子结构式如下:
英文名称:Osilodrostat
中文名称:奥西卓司他
本文主要对Osilodrostat(奥西卓司他)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Osilodrostat(奥西卓司他)合成路线
三、Osilodrostat(奥西卓司他)合成检索总结报告(一) Osilodrostat(奥西卓司他)中间体3的合成方法一
(二) Osilodrostat(奥西卓司他)中间体3的合成方法二①Osilodrostat(奥西卓司他)中间体5的合成
(三) Osilodrostat(奥西卓司他)中间体3的合成方法三①Osilodrostat(奥西卓司他)中间体7的合成。
2010 药业新观察(第三季度)

图片版权:iSTOCKPHOTO Movers and Shakers《仿制药发展动态》2010 年7 - 9 月Newport Premium TM收集来自全球仿制药市场的可靠信息,为制造企业在寻找评估新产品项目,以及制定企业发展战略的过程中提供权威的分析数据,帮助企业更快更好地做出决策。
汤森路透借助该数据库提供的战略情报和竞争性分析信息,为美国仿制药行业给出了最新的季度报告。
关于Thomson Reuters API情报解决方案(包括NewportPremium)的更多信息,请访问/newport PHARMA MATTERS | MOVERS AND SHAKERS原创药公司的几种重磅药物就快失去独占期,填补这一空缺推出的新药又很少,让企业的压力继续攀升。
因此,大制药公司在第三季度继续涉足仿制药领域及新兴市场也并不为奇。
Abbott 公司 9 月完成对 Piramal Healthcare 公司在印度制剂业务的收购,一举成为印度头号制药公司,并进入了 Piramal 公司的品牌仿制药大型产品组合。
AstraZeneca 公司与 Aurobindo 公司的交易让 AstraZeneca 公司一箭双雕,同时进入仿制药及新兴市场。
(见第四部分)。
仿制药企业在继续巩固的同时也面临自身的挑战。
世界顶级仿制药公司 Teva 公司在 8 月份宣布以 47.8 亿美元收购 Ratiopharm 公司告罄,让 Teva 公司一举成为欧洲的头号仿制药公司。
Ratiopharm公司在德国市场坐拥第一,此次收购之前 Teva 公司还不是该市场上的王者。
这宗收购还促进了 Teva 公司在加拿大的销售量。
Teva 公司 3 月份在 Ratiopharm 公司的竞标中击败 Pfizer 公司。
仿制药公司还继续追逐专利挑战、地域多样化、产品组合多元化及定位生物仿制药。
现在让我们来看看《简明新药申请 (ANDA)》批准数及 Paragraph IV专利挑战,以及第三季度发生的重大交易。
2016年度全球新药报告中文版(汤森路透)

引言
2016 年共有 44 种新的分子实体和生物制剂上市,其中 一种药物在两个不同的治疗领域分别上市(更多信息参 见表 1 和表 2)。用于增强免疫的免疫调节药物是 2016 年最为活跃的药物种类,共有 9 种新药上市,3 种药物 获得批准,1 种产品线拓展品种。其中包括多种抗病毒 的疫苗产品,如几种新型四价流感疫苗等。从药物开发 管线中不断涌现治疗丙肝的新型药物和联合用药,这一 势头在去年继续保持。与此相对的是,与 2015 年相比, 2016 年新上市的抗肿瘤药物显著减少:仅有 4 种抗肿瘤 新药上市,而 2015 年、2014 年和 2013 年此类新药的 数量分别为 14、10 和 12 种。
概述
2016 年全球批准的新药和生物制剂共 88 种,其中包括 首次上市药物和重要的新产品线拓展产品。与往年相比, 这一数字并不算高。2016 年全球首次上市的新药和新生 物制剂共 44 种,比前一年减少近 10%。新上市药物中, 7 种药物为首创新药(first-in-class),即新作用原理全 球首次通过批准并上市。此外,去年共批准了 23 种新产 品线拓展产品(即新配方、新联合用药和新适应症等)。 本文还讨论了 21 种产品,为 2016 年首次获批,但在 2016 年 12 月 15 日前尚未上市。本次新药报告年度综 述的第一部分将深入报道这些新药的相关信息。
4
科睿唯安白皮书:2016年度新药报告
神经系统药物
2016 年 见 证 了 首 个 3D 打 印 药 物 问 世: 抗 癫 痫 药 物 Spritam ®(左乙拉西坦;Aprecia Pharmaceuticals)。 FDA 批准 Spritam 用于辅助治疗成人及儿童癫痫患者的 癫痫部分性发作、肌阵挛性发作及初级全身性强直阵挛 性发作。Spritam 应用了 Aprecia 的专有 ZipDose ® 技术 平台,该平台应用三维打印(3DP)技术产出一种已知 的抗惊厥药物的多孔性制剂,该制剂在接触少量液体的 情况下即迅速崩解。Aprecia 收购麻省理工学院 3D 打印 技术在药物应用的独家授权。ZipDose 技术能实现高载 量药物的传递:即使一片剂量高达 1000 mg 的药物也能 在唾液中溶解。此外,每片药物均独立包装,极易携带。 药物漏服会影响治疗效果,对于癫痫的治疗来说按时服 药尤其重要,因此利用该技术改善用药依从性,可为治 疗患有该疾病的患者提供极大的便利。
GST-π、TopoⅡ与胃癌生物学行为之间的关系

表 示有 统计 学 意义 。
岁) 。病 理分 化类 型 : 中分化 胃癌 3 高 6例 , 低分 化癌
2 结 果
GS 一 、 p T 丌 To oⅡ在 胃癌组 织 中 的表 达 与 病 理 分 化类 型 、 润深 度 、 巴结转 移情 况 比较 见表 1 浸 淋 。
2 4例 ; 侵犯 黏膜 及 下 层 2例 , 层 1 肌 2例 , 膜 及 浆 浆
关 键 词 :胃分类 号 : 752 R 3.
文 献标 识码 : A
文章 编号 : 09 1420)1 0 1-0 10 —8 9(0 90 - 01 2
了解 胃癌 生物 学行 为 , 对判 断预 后 、 指导 治疗 具
有 重 大意 义 。作 者 应 用 免 疫 组 化 技 术 SP法 , 胃 — 对
用 免 疫 组 化 P法 检 测 GS 一 To oI 6 T 、 p I在 O例 胃癌 组 织 中 的 表 达 , 较 胃癌 病 理 分 化 类 型 、 瘤 浸 润 深 度 、 无 淋 比 肿 有
巴 结 转 移 对 上 述 指 标 表 达 的 影 响 。结 果 GS _ To o T 、 p Ⅱ在 高 、 分 化 组 胃癌 中 的 阳 性 表 达 率 分 别 为 9 . 4 和 中 44
织 中表 达较 低 , 且 不 被 诱 导 剂 诱 导 。然 而 在 胃癌 并 癌变 形 成过 程 中 , S 一 G T 的表 达 会 异 常升 高 。因此 被认 为 是 一 个 典 型 的 癌 前 病 变 标 志 酶 。 常 新 忠 等l 用 自制抗 人 GS 一 的单 抗 通 过免 疫组 织 化学 _ 3 T7 c 方法 , 究 GS 7在 人 类 胃癌 中的 表 达 , 现 其 阳 研 T一 ( 发 性 表 达平 均水 平 明显 高 于正 常 胃黏膜 。这 反映 了在 正常组 织 发展 为 癌 的 过程 中 , T 兀表 达逐 渐 地 增 GS 一 高 。本 研 究结 果显 示 GS 一 高 、 T 丌在 中分 化 的 胃癌 中
托烷司琼调研报告

托烷司琼调研报告
托烷司琼调研报告
报告目的:对托烷司琼进行调研,了解其市场需求、竞争情况和发展前景。
调研方法:本次调研采用问卷调查和文献研究的方法。
调研结果:
市场需求:托烷司琼是一种药物,主要用于治疗焦虑和抑郁症。
根据调查结果显示,抑郁症和焦虑症在社会上普遍存在,很多人对托烷司琼的需求量较大。
竞争情况:目前市场上存在一些竞争对手,主要是其他治疗焦虑和抑郁症的药物。
然而,托烷司琼具有一些特殊的优势,如副作用小、治疗效果好等,这使得它在市场上具有一定的竞争力。
发展前景:随着社会经济的发展和人们健康意识的提升,对心理健康药物的需求将会增加。
托烷司琼具有较好的治疗效果和安全性,因此在未来有望继续保持良好的发展势头。
建议:针对市场需求和竞争情况,建议厂商可以加大市场推广力度,提高产品知名度。
同时,在药物的研发过程中,可以进一步提高其疗效和适应症范围,以满足更多患者的需求。
结论:托烷司琼在市场上具有一定的竞争力和发展潜力。
在未来,随着市场需求的增加,预计托烷司琼的市场份额将进一步扩大。
因此,托烷司琼有着良好的发展前景,并值得厂商持续关注和投资。
抗痛风药市场报告:URAT1、XO等靶点和新型抗炎症

相比传统药物,新型抗炎症药物在疗效和耐受性方面表现出更好的优势,为痛风治 疗提供了新的选择。
新型抗炎症药物研发进展
目前,针对痛风炎症反应的新型药物研发正在不断取得进展。
多种具有抗炎作用的药物已经进入临床试验阶段,包括一些针对特定炎症 通路的单克隆抗体和小分子抑制剂等。
URAT1抑制剂通过抑制尿酸盐转运体1(URAT1)的活性 ,减少尿酸重吸收,从而降低血尿酸水平。该类药物在抗 痛风市场中占据一定份额。
XO抑制剂
XO抑制剂主要抑制黄嘌呤氧化酶(XO)的活性,减少尿 酸生成,用于治疗高尿酸血症和痛风。该类药物市场份额 逐渐增长。
其他靶点药物
除了URAT1和XO抑制剂外,还有其他针对不同靶点的抗 痛风药物,如促进尿酸排泄的药物、抑制尿酸合成的药物 等,这些药物在市场上也占有一定的份额。
。
除了单一靶点的URAT1抑制剂 外,还有针对多个靶点的复合药 物正在研发中,以期提高治疗效
果和降低副作用。
市场前景展望
随着痛风发病率的不断上升, 抗痛风药物市场需求持续增长 。
URAT1靶点药物作为新型抗痛 风药物,具有独特的作用机制 和疗效优势,有望在未来市场 中占据重要地位。
随着更多URAT1抑制剂的成功 研发和上市,将为痛风患者提 供更多有效的治疗选择。
数据来源
政府公开数据、行业报告、企业年报、市场调研数据等。
注
以上内容仅供参考,具体报告内容需根据实际研究和分析进行撰写,以确保准确性和专业性。同时,涉及市 场规模、增长趋势等方面的数据可能需要以图表或具体数值形式呈现,以便更直观地展示市场情况。
02
胃癌组织中检测GSTπ、TopoII及Pgp基因蛋白与术后化疗关系的临床病理研究

胃癌组织中检测GSTπ、TopoII及Pgp基因蛋白与术后化疗关系的临床病理研究陈庆明;张洁民【期刊名称】《中国社区医师(医学专业)》【年(卷),期】2011(013)028【摘要】目的:探索胃癌组织中检测GSTπ、TOPOII及Pgp基因蛋白与术后化疗之间的关系,为临床选择化疗药物提供重要依据.方法:利用免疫组织化学方法对胃癌组织中相关耐药蛋白进行标记,观察和分析胃癌细胞的耐药基因蛋白表达情况.结果:890例胃癌组织中,GSTπ强阳性(+++)824例,阳性(++)52例,弱阳性(+)13例;Pgp强阳性(+++)807例,阳性(++)69例,弱阳性(+)14例;TopoII强阳性(+++)664例,阳性(++)74例,弱阳性(+)58例;GSTπ、TopoII及Pgp基因蛋白表达与胃癌分型的关系,本组病例中主要以中分化和低分化腺癌强阳性表达为主,其他类型以阳性或弱阳为主;GSTπ、TopoII及Pgp基因蛋白表达与术后常规使用化疗药物患者强阳性表达的术后生存时间短,弱阳性表达的时间长;GSTπ、TopoII及Pgp基因蛋白表达与术后不使用或小剂量使用化疗药物患者强阳性表达的术后生存时间短,阳性或弱阳表达的患者术后生存时间略长;通过观察发现,胃癌组织中GSTπ、TopoII及Pgp基因蛋白阳性表达高达90%以上,最低为70%以上的高表达,术后使用化疗药物与不使用化疗药物患者的生存时间的长短无明显差异,胃癌患者术后普遍生存时间短.结论:胃癌患者术前化疗与术后针对性化疗相结合,可减少胃癌细胞的多耐药性的产生,提高化疗效果.【总页数】2页(P240-241)【作者】陈庆明;张洁民【作者单位】733000,甘肃省武威市人民医院;733000,甘肃省武威市人民医院【正文语种】中文【相关文献】1.结直肠癌中错配修复基因表达与临床病理及Pgp、GSTπ、TopoⅡ、Ki-67表达的关系研究2.结直肠癌中错配修复基因表达与临床病理及Pgp、GSTπ、TopoⅡ、Ki-67表达的关系研究3.胃癌组织中多药耐药相关蛋白mRNA的表达及MDR基因产物的检测与其临床病理关系的研究4.胃癌组织中检测GSTπ、TopoII及Pgp基因蛋白与术后化疗关系的临床病理研究5.乳癌组织中GST-Pi,ToPoII和PgP耐药基因的表达因版权原因,仅展示原文概要,查看原文内容请购买。
汤森路透美欧日药物审批趋势解读
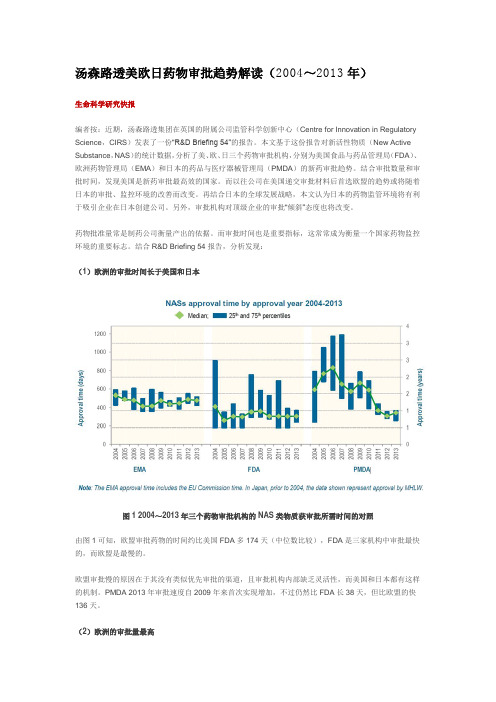
汤森路透美欧日药物审批趋势解读(2004~2013年)生命科学研究快报编者按:近期,汤森路透集团在英国的附属公司监管科学创新中心(Centre for Innovation in Regulatory Science,CIRS)发表了一份“R&D Briefing 54”的报告。
本文基于这份报告对新活性物质(New Active Substance,NAS)的统计数据,分析了美、欧、日三个药物审批机构,分别为美国食品与药品管理局(FDA)、欧洲药物管理局(EMA)和日本的药品与医疗器械管理局(PMDA)的新药审批趋势。
结合审批数量和审批时间,发现美国是新药审批最高效的国家。
而以往公司在美国递交审批材料后首选欧盟的趋势或将随着日本的审批、监控环境的改善而改变。
再结合日本的全球发展战略,本文认为日本的药物监管环境将有利于吸引企业在日本创建公司。
另外,审批机构对顶级企业的审批“倾斜”态度也将改变。
药物批准量常是制药公司衡量产出的依据。
而审批时间也是重要指标,这常常成为衡量一个国家药物监控环境的重要标志。
结合R&D Briefing 54报告,分析发现:(1)欧洲的审批时间长于美国和日本图1 2004~2013年三个药物审批机构的NAS类物质获审批所需时间的对照由图1可知,欧盟审批药物的时间约比美国FDA多174天(中位数比较),FDA是三家机构中审批最快的,而欧盟是最慢的。
欧盟审批慢的原因在于其没有类似优先审批的渠道,且审批机构内部缺乏灵活性,而美国和日本都有这样的机制。
PMDA 2013年审批速度自2009年来首次实现增加,不过仍然比FDA长38天,但比欧盟的快136天。
(2)欧洲的审批量最高2013年,三个机构,包括EMA、FDA 和PMDA审批的NASs数量总和没有2012年的高,美国审批量下降25%,日本下降20%。
尽管EMA审批量比2012年增加了43%,实际上是由于其审批时长较长,把美国之前已经审批的于2013年才完成审批而已。
胃癌TOPO-Ⅱ、GST-π、P-gp表达与临床病理特点的相关性研究

胃癌 T P —I GS 一 、 —P表 达 与 临床 病 理 O O I、 T 兀 Pg 特 点 的相 关性 研 究
蒋正 财, 郑志强 , 张伟 【 摘要】 目的 探讨胃癌 患者 P糖蛋 I(—p、 .  ̄ Pg )谷胱甘肽 s转移酶( T 7 和拓扑异构酶 I(o o I) I GS .) c IT p—I 阳性表达强度与
【】 黄清 水, 腊 根 , 勤 , . 环 瓜 氨 酸肽 1 万 罗忠 等抗 抗 体 对 类 风 湿 性 关 节 炎诊 断 价 值 的 荟萃
ma i a tr J. h u tl y( x od . t df os【 R e maoo O fr ) o c ] g 2 0 ,17 :0 — 1 . 0 2 () 98 4 4 8
应 用 免 疫 组 化 法检 测 16例 胃癌 组 织 中 Pg 、 S 一和 T p I 2 -p G T oo I表
其 临床 病 理 特 点 的相 关性 及 其 临 床 意 义 。方 法
达 , 分 析 其 与 临床 病 理特 征 的 关系 。结 果 男性 患者 T P I、 S 一及 Pg 并 O O I G T7 【 —P表 达 高 于 女 性 , 瘤 大 小 < m 组 肿 5c 高于 ≥5c 组 ; T 7 Pg m GS 一及 .P高 中 分 化 强 于 低 未 分 化 ; T 7 发 灶 在 胃底 贲 门强 于 其 他 部 位 。结 论 c GS .原 c 【 键 词 】 胃肿 瘤 ; ; 疫 组 织 化 学 ;_ 蛋 白; 胱 甘 肽 S 转移 酶 ; 扑 异 构 酶 I 关 癌 免 P糖 谷 : 拓 I d i 03 6 /.s. 7 -8 02 1.5 2 o: .9 9jsn1 10 0 . 00 . 5 1 i 6 0 0 【 图分类号】 R 3. 中 7 52 【 文献 标 志码 】 A 【 章 编 号 】 17 -802 1)50 2 .2 文 6 1 0 (0 00 —550 0 对 于 女性 、 肿 瘤 ≥ 5 m、 化程 度 较 低 患者 化 疗 效 果较 好 , 同时对 于 女 性 、 分 c 但 分化 程 度 低 的 患者 尽 量避 免使 用拓 扑 异 构 酶抑 制 剂 。
ToGA 研究报告

ToGA 研究报告《ToGA研究报告》近年来,癌症一直是困扰全球人类健康的重大问题。
根据世界卫生组织的数据,每年全球约有8百万人死于恶性肿瘤。
为了解决这一严峻挑战,科学家们不断努力寻找新的治疗方法和策略。
而在这个过程中,基因组医学的发展成为了一个重要的突破口。
本报告就是针对ToGA(Trastuzumab for Gastric Cancer)研究的相关进展进行的总结和回顾。
ToGA研究是一项针对胃癌的临床试验,旨在评估特拉莫单抗(Trastuzumab)在HER2阳性进展转移性胃癌患者中的疗效。
HER2(人类表皮生长因子受体2)是一种在某些癌症中过度表达的蛋白质,与肿瘤发生和发展密切相关。
因此,通过针对HER2的药物治疗,可以有效抑制肿瘤的生长和转移。
ToGA研究于2007年开始,并于2010年取得重要的突破性结果。
研究共招募了594位HER2阳性晚期胃癌患者,将其随机分为两组,一组接受特拉莫单抗联合化疗,另一组仅接受化疗。
结果显示,在特拉莫单抗联合化疗组中,患者的总生存期显著延长,且较少出现严重不良事件。
这一研究结果证明了特拉莫单抗在HER2阳性进展转移性胃癌患者中的疗效和安全性,为胃癌治疗提供了新的选择。
ToGA研究的成功不仅在于其对特拉莫单抗的评估,更重要的是它首次证明了靶向HER2的治疗在胃癌中具有显著的临床效果。
这一突破性发现激发了对于胃癌个体化治疗的研究热潮。
随后,几个针对HER2阳性胃癌的临床试验相继展开,其中包括Herceptin Adjuvant Trial in Gastric Cancer(HOT)和HER-2 FISH for Gastric Cancer(HER-FIT)。
HOT试验是针对HER2阳性胃癌患者的辅助治疗临床试验。
该研究结果显示,早期胃癌患者在接受特拉莫单抗辅助治疗后,无病生存期较长,并且接受治疗后的晚期胃癌患者也能获得较好的生存期。
这一研究结果进一步验证了特拉莫单抗在胃癌治疗中的重要地位,并为早期干预提供了新的思路。
全球仿制药、原料药行业发展趋势

全球仿制药、原料药行业发展趋势研究报告全球仿制药、原料药行业发展趋势*李寅张帆汤森路透*通讯作者 E-mail: yin.li@thomsonreuters[ 摘要] 本文通过对汤森路透Thomson Reuters 旗下的一系列药物市场及研发数据库中的药物情报进行挖掘及统计 , 分析了全球及中国的仿制药处方药市场现状及特点 , 并剖析其可能的形成原因 , 最后简要分析市场发展前景及机遇。
[ 关键词] 药物情报仿制药原料药概览1 引言2 全球仿制药市场概览及特点.背景介绍 .仿制药市场宏观特点描述信息对于当前世界的发展变化已经起到了决定性的近年来全球仿制药领域出现了很多变化 , 一些前作用。
尤其是在信息密集型的制药行业 , 由于行业的整所未见的变革日益呈现趋势化发展 , 诸如宏观层面上合以及政策法规、生产研发高度规范化 , 各方向的药物可观察到的 : 发达国家市场增长缓慢 , 发展中国家发情报深刻反映出制药行业的发展特征。
本文从药物情报展迅猛特别是印度与中国的发展步伐逐步加快 ; 竞争的角度 , 剖析了近年来全球仿制药/ 原料药行业发展的特日益激烈 , 生物仿制药逐渐兴起等等。
进一步从数据点及趋势,以及中国相关特色。
层面解读这些趋势不难发现 , 包括处方药和非处方药的全球仿制药市场预计达1 500 亿美元 , 其中欧洲300 .研究方法多亿 , 北美最主要是美国600 多亿 , 东欧、俄罗斯通过对Thomson Reuters 相关数据库及市场调研报告100 多亿 , 中国100 多亿 , 日本相对较少 , 占30 多亿 ,采取数据挖掘的方式 , 借助统计学方法 , 展示了市场发还有其他地区如拉美如图 1 。
这里统计的数字只计算展的特性 , 并结合背景知识进行了讨论。
其中 , 相关仿制药包括处方药及非处方药 , 并不是指整个医药市数据来自Thomson Reuters Newport 仿制药 , Thomson场 , 例如 , 日本整个医药市场潜力包括医疗器械接近Reuters Integrity 早期药物研发 , Cortellis for Competitive 200 亿美元 , 但其仿制药市场包括处方药和非处方药Intelligence 竞争情报 , 立项 , 商务发展 ,IDRAC 政策只有30 多亿。
汤森路透Cortellis 药物研发的综合情报平台介绍

wlccCortellis™ for Competitive Intelligence药物研发的综合情报平台是Thomson Reuters Pharma的升级平台,底层数据与Pharma一致。
为您提供药物研发管线、交易、专利、公司内容及行业最新新闻。
具有多来源信息收集、自带强大分析工具、每天更新。
以报告的方式提供信息一般情况下,我们进行信息调研,最终总会将收集到的所有有用的信息整理为一份调查报告。
信息收集的过程是艰巨而繁琐的,而将信息整理为报告的过程也是十分具有挑战性的工作。
而Cortellis™ for Competitive Intelligence便是直接以报告的方式提供信息。
它将平台下数十个数据库提供的丰富信息整理为八种类型的报告:药物报告,公司报告,专利报告,文献报告、新闻报告、会议报告、交易报告和临床试验报告。
其中每一份报告都由工作人员围绕主题将所有相关信息进行了整理和综合,并附有业界专家撰写的综述和评论。
深入的药物报道通过Cortellis™ for Competitive Intelligence,科研工作者可以了解在研药物和已批准药物从开发、临床试验到上市和销售的各个阶段重要的科研、专利、商业和金融信息。
基于这些信息,研发人员可以对药物的研发过程和现状进行全面的了解,也可以对研发工作的最新进展进行紧密的跟踪,还可以对某个治疗领域的所有药物进行比较和分析。
通过药物报告可以获取以下信息:w w.i n ki n fo.o m.nccw w.i n ki n fo.o m.nwlc c其中“Development Profile”和“SWOT Analysis”是由业界专家根据收集的所有信息撰写的总结和竞争分析。
“Literature Review”是由业界专家根据收集的药物相关文献撰写的文献评论。
“Change History”可对药物的信息更新进行跟踪,详细了解药物不同时段的研发进展情况。
汤森路透:2014年药物市场展望

汤森路透:2014年药物市场展望来源:生命科学研究快报 2014-3-72013年预测的药物的现状2013年1月,Thomson Reuters Cortellis竞争情报研究重点对5类药物进行了观察分析,因这5类药物被认为可以成为药物监管史上的里程碑,预计这5类药物中每类5年内的销售额将超过10亿美元。
目前可知,这5种药物销售达到了预期目标,甚至其中的4种还超出预期。
2013年1月,美国Amarin公司治疗严重甘油三酯的Vascepa投放市场。
2013 年2月份,美国批准Celgene公司用于治疗复发或难治的多发性骨髓瘤药物Pomalyst,并随后很快美国批准投放市场,同样欧盟也批准并投放市场。
2月美国还批准了Roche公司用于治疗HER2阳性转移乳腺癌的Kadcyla,并批准投放市场,当年也获得了日本和欧盟的审批。
2013年3月,Celgene公司研发的治疗牛皮癣的药物Apremilast获批用于治疗牛皮癣关节炎,PDUFA(Prescription Drug User Fee Act;PDUFA date就是一个截止时间,在此时间前FDA必须对报批药物给与审评意见)设定的日期是2014年3月21日。
2013年5月,GlaxoSmithKline公司和Theravance公司的Relovair在美国获批用于治疗COPD(慢性阻塞性肺病),之后在美国投放市场,欧盟批准其用于治疗哮喘以及COPD,日本在几个月后也批准其用于治疗哮喘并投放市场(尽管用于治疗COPD的文件后来被撤退)。
去年这个时段,预计Vascepa的销售额在2016年达到20.7亿美元,2017年将增加至29.63亿美元。
然而,2013年10月,FDA评审委员会反对批准Vascepa 2013年2月的sNDA(疗效补充申请),这将会放宽指标,包括对混合血脂异常的指标的放宽。
2013年年底,FDA取消了Amarin公司申请的针对ANCHOR混合异常血脂试验。
托匹司他用法用量及注意事项

托匹司他(富士痛风降酸药)用法用量及注意事项托匹司他片(商品名:TOPIROXOSTAT),由日本富士药业株式会社首次研发,用于治疗痛风和高尿酸血症,于2013年6月在日本被批准生产销售。
目前托匹司他片尚未在国内上市。
根据目前所能查到的资料,其专利期将至2022-12-3,也就是说,在此之前国内市场都不大可能出现托匹司他的仿制药物。
本品于2004年开始进行临床试验,Ⅰ期及Ⅱ期临床试验由日本富士药业株式会社进行,Ⅲ期由日本三和化学株式会社与日本富士药业株式会社共同进行。
在826例痛风及高尿酸血症患者的给药试验中确定了其有效性及安全性。
其临床实验数据证实了不良反应较小、耐受性好,应用广泛且在中-重度肝肾功能不全的患者中也不需要进行剂量调整。
本品是此类药物中疗效较好的品种药,目前可作为临床上应用最广泛的药物之一。
药理及药物动力学特征:托匹司他片是一种非嘌呤类黄嘌呤氧化酶选择性抑制剂,可选择性、可逆地抑制黄嘌呤氧化还原酶,降低血清尿酸水平,与《美国高尿酸血症痛风诊疗指南》推荐为一线用药的非布司他片属于同一作用机制。
托匹司他属于混合型抑制剂,呈现出基于结构和机制的双重抑制作用。
在结合模式上与非布司他相同,都是结合于酶的同一个疏水空腔,不同的是它又能与酶的钼蝶呤中心形成Mo-O-C共价键,抑制酶与底物结合,从而发挥降尿酸作用。
正是由于托匹司他-XOR复合物分解的半衰期较长,使其表现出长效的降尿酸作用,这是它优于非布司他的地方。
阿木观点:临床数据表明,托匹司他对心血管系统无不良影响(该特点明显优于非布司他),具有更好的安全性。
托匹司他通过抑制黄嘌呤氧化酶的活性阻断嘌呤向尿酸的转化途径,达到降低尿酸生成的作用。
同非布司他和别嘌醇相比,托匹司他能更加精准的找到人们希望抑制的酶,而对其他酶的正常运转几乎不产生影响,从而大大降低了在较长服药时间下的不良事件发生率。
尽管托匹司他-XOR复合物分解的半衰期较长,但半衰期并不是服药周期的唯一决定因素。
欧盟将审查COX-2抑制剂的严重皮肤不良反应

欧盟将审查COX-2抑制剂的严重皮肤不良反应
罗娟(摘)
【期刊名称】《国外药讯》
【年(卷),期】2005(000)009
【摘要】五月份欧盟人用医药产品委员会(CHMP)和环氧化酶-2(COX-2)抑制剂生产商举行进一步听证会,评估该类药物严重的皮肤反应。
在会议上,就AstraZeneca公司生产的降脂药Crestor(rosuvastatin)的首次使用剂量达成一致意见。
【总页数】1页(P38)
【作者】罗娟(摘)
【作者单位】无
【正文语种】中文
【中图分类】R971.1
【相关文献】
1.选择性COX-2抑制剂和非选择性COX-1和COX-2抑制剂在预防全髋关节置换术后异位骨化的Meta分析 [J], 徐步国;薛德挺;王祥华;严世贵
2.COX-2及COX-2抑制剂与肿瘤防治 [J], 刘治坤;陈皇
3.COX-2抑制剂对人舌鳞状细胞癌Tca-8113细胞COX-2和Ang-1基因表达的影响 [J], 王晓彦;武云霞
4.COX-2及COX-2抑制剂对肿瘤防治作用的研究进展 [J], 刘丹丹;江培
5.COX-2抑制剂对大鼠烫伤创面COX-2、VEGF表达的影响 [J], 刘宏;薛晓东;邓津菊;陈应泰;宋一丁;杨荣;国虎
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
topiroxostatTable of ContentsSnapshot...........................................................................................................................................2 Development Profile..........................................................................................................................2 Development Status..........................................................................................................................4 Chemical Structures..........................................................................................................................5 Drug Names......................................................................................................................................6 Clinical Trials..................................................................................................................................6 Deals and Patents............................................................................................................................713 Change History................................................................................................................................. Created:18-Dec-2017topiroxostatSNAPSHOTDEVELOPMENT PROFILESUMMARYFuji Yakuhin, in collaboration with Sanwa Kagaku, has developed and launched topiroxostat (FYX-051; SK-0910; Uriadec; Topiloric), an oral xanthine oxidoreductase inhibitor, which inhibits production of uric acid, as a tablet formulation, for the potential treatment of gout and hyperuricemia [932681], [1068682], [1229008], [1445821]. The product was launched in Japan in September 2013. Sanwa Kagaku launched under the tradename 'Uriadec', and Fuji Yakuhin launched under the name 'Topiloric' [1445821], [1472556].The company is also developing the drug for the potential treatment of diabetic nephropathy and chronic kidney disease [1623871], [1915020]. In March 2015, a phase II trial for diabetic nephropathy was initiated in Japan [1623871]. In June 2012, Fuji Yakuhin was seeking to outlicense the drug outside Japan [1307635]. REGULATORYIn June 2012, an application for approval was filed in Japan for topiroxostat in hyperuricemia and gout [1305082]. In April 2013, the Pharmaceutical Affairs and Food Sanitation Council's First Committee on Drugs was to discuss the application at a meeting on April 26, 2013 [1407389]; at the meeting, the committee recommended approval [1414256]. In June 2013, the drug was approved in Japan. At that time, Sanwa Kagaku Kenkyusho was to launch 'Uriadec' after the NHI price listing, and Fuji Yakuhin was to launch the drug under the name 'Topiloric' [1445821].PREMARKETINGIn March 2017, an open, randomized, parallel trial (UMIN000026741; TARGET-UA) to examine the effect of intensive UA-lowering therapy in patients (expected n=370) with CKD and hyperuricemia was planned to be initiated in Japan in June 2017 [1915020].In November 2016, clinical data from a 24-week, multicenter, open-label, randomized trial (ETUDE; UMIN 000015403), which evaluated the anti-albuminuric effects of topiroxostat in hyperuremic patients with diabetic nephropathy were presented at ASN's Kidney Week 2016 in Chicago, IL. Patients (n = 80) were randomized (1:1) to receive either high or low doses of topiroxostat (160 or 40 mg daily). After 24 weeks of treatment urine albumin-to-creatinine ratio (UACR; primary endpoint) significantly decreased by 122 mg/gCr in the high dose group, while in the low dose group the decrease was by 122 mg/gCr which was not significant. Mild and significant lowering effects on eGFR and systolic and diastolic blood pressure were observed in patients receiving topiroxostat, with steadily reduced serum uric acid levels [1878379].In February 2010, a multicenter, open-label phase III trial (JapicCTI-101068), of topiroxostat, began in patients with hyperuricemia in Japan. At that time, the expected study completion date was March 2012. In December 2011, the study was completed [1151860].In February 2016, a prospective, parallel, randomized, controlled phase II study (UMIN000020939; Excite-UA) was expected to be initiated in Japan, in chronIc heart failure patients complicated with hyperericemia [1736015].In December 2014, a double-blind, placebo-controlled, randomized, parallel assignment, phase II study (NCT02327754; FY1004) called 'UPWARD' was planned to be initiated in Japan to assess the safety and efficacy of the drug on urinary albumin excretion in patients (expected n = 60) with early stage diabetic nephropathy and hyperuricemia with or without gout. At that time, the study was expected to complete in December 2016. In March 2015, the trial was initiated [1623871].By August 2008, data from a phase II trial in patients with hyperuricemia had shown that topiroxostat significantly and dose-dependently lowered uric acid levels compared with placebo [932681].Also in August 2008, data from a completed 12-week study were reported. In the trial, patients received 40 mg bid for the first 2 weeks, followed by 80, 120, or 160 mg of topiroxostat bid for 10 weeks. Results showed that the uric acid levels of patients decreased in a 'close -to-maximum' manner 4 weeks after treatment began. At week 12, the percentage decrease from baseline in serum uric acid levels were 30, 39 and 47% for 80, 120 and 160 mg, respectively. No severe adverse events were reported, and the overall incidence of adverse reactions were similar among different arms [932681].By August 2008, pharmacokinetic studies had shown that topiroxostat was rapidly absorbed, reached Tmax within 1 h, and was eliminated from the blood with a half life of 4 to 7 h [932681].In 2003, a phase I trial began in UK [932681]. By August 2008, safety data from a Japanese phase I study had confirmed the results of the earlier UK phase I trial [932681].OTHER STUDIESIn November 2016, a single arm, interventional, non-randomized, open, uncontrolled trial () was planned to be initiated in Japan in patients (expected n = 36) with hyperuricemic congestive heart failure patients to assess heart function. At that time, the trial was expected to start in January 2017 [1878533]. PRECLINICALIn November 2016, preclinical data were presented at ASN's Kidney Week 2016 in Chicago, IL. In mice fed with febuxostat-high (3 mg/kg) and topiroxostat-high (3 mg/kg) diet, serum creatinine levels, urinary L-type fatty acid binding protein, urinary 15-F2t-isoprostane levels and urinary angiotensinogen were significantly lower when compared with the adenine group [1876279].In November 2015, preclinical data were presented at ASN's Kidney Week 2015 in San Diego, CA. Topiroxostat decreased urinary albumin excretion in db/db mice in dose-dependent manner. Hypertrophy of glomerulus and proximal tubules were attenuated. Plasma XOR inhibitory activity by topiroxostat in db/db mice was more than 12-fold stronger when compared with other XOR inhibitors [1708364].In August 2006, preclinical data were reported. In the in vitro study, metabolism of topiroxostat generated four conjugates in rat, dog, monkey and human. Urinary profile showed that the metabolites were similar among these mammals. The paper also mentioned that topiroxostat reduced serum urate concentration in a dose-dependent manner, and was 30-fold more potent than that of allopurinol in rats [932685]. DEVELOPMENT STATUSCURRENT DEVELOPMENT STATUSHISTORICAL DEVELOPMENT STATUSCHEMICAL STRUCTURESDRUG NAMESCLINICAL TRIALSTrials by Phase and Condition StudiedTotal Trials by Phase and StatusPhase DefinitionsPhase 3 ClinicalIncludes Phase 3, Phase 3b, Phase 3a, Phase 2/3 (where enrolment count is 300 or over)Phase 2 ClinicalIncludes Phase 2, Phase 2a, Phase 2b, Phase 1/2 (where enrolment count is 100 or over), Phase 2/3 (where enrolment count is under 300 or not specified)Phase 1 ClinicalIncludes Phase 1, Phase 1a, Phase 1, Phase 1/2 (where enrolment count is under 100 or not specified), Phase 0DEALS AND PATENTSDEALSDeals by Parent Company Chart Deals by Parent Company TableDeals by Type Chart Deals by Type TablePATENTSPatents by Parent Company ChartPatents by Parent Company TableCHANGE HISTORY SUMMARYCHANGE HISTORY DETAIL。
