拜科奇说明
卡莫齐产品使用说明说明书

Information for the use of Camozzi productsCATALOGUE >Release 8.5APPENDIX >Camozzi products/2.0101A P P E N D I XJust browsing through the pages of our website , you will have the possibility to download GSD files for the configuration of Valve Islands, all relative use and installation manuals and the configuration software of the product codes.Moreover, here you can find all 2D and 3D files in the most commonly used formats.a/2.0102a A P P E N DI XDouble-acting cylinder, fixed cushionsDouble-acting cylinder, cushionedDouble-acting cylinder, adjustable rear cushionDouble-acting cylinder, adjustable front cushionDouble-acting cylinder, through-rod, fixed cushionsDouble-acting cylinder, through-rod,adjustable front and rear cushion Double-acting cylinder, magneticDouble-acting cylinder, magnetic, fixed cushionsDouble-acting cylinder, magnetic, adjustable cushions in both directionsDouble-acting cylinder, magnetic, adjustable rear cushionDouble-acting cylinder, magnetic, adjustable front cushionDouble-acting cylinder, magnetic, through-rod, fixed cushionsDouble-acting cylinder, magnetic, through-rod, adjustable cushions in both directionsDouble-acting cylinder, magnetic, through-rodMagnetic twin rod cylindersMagnetic twin through-rod cylindersDouble-acting rotary cylinderDouble-acting rotary cylinder, magneticSingle-acting rotary cylinderMagnetic tandem cylinder, two stages, fixed cushionsMagnetic tandem cylinder, three stages, fixed cushionsMagnetic tandem cylinder, four stages, fixed cushionsMagnetic multi-position cylinder, fixed cushionsDouble-acting rodless cylinder, magneticCYLINDERSCYLINDERSSingle-acting cylinder, front springSingle-acting cylinder,non cushionedSingle-acting cylinder, through-rodSingle-acting cylinder, through-rod, adjustable cushionSingle-acting cylinder, magneticSingle-acting cylinder, front spring, adjustable rear cushionSingle-acting cylinder, rear spring, magneticSingle-acting cylinder, magnetic, front springSingle-acting cylinder, through-rodSingle-acting cylinder, through-rod, adjustable rear cushionSingle-acting cylinder, front spring, adjustable rear cushionSingle-acting cylinder, through-rod, adjustable rear cushionHydrocheck, regulated rod thrustHydrocheck, regulated rod return Hydrocheck, regulated rod thrust with stop valveHydrocheck, regulated rod return with stop valveHydrocheck, regulated rod thrust with skip valveHydrocheck, regulated rod return with skip valveHydrocheck, regulated rod thrust with skip and stop valveHydrocheck, regulated rod return with skip and stop valve Double-acting magnetic grippersRod lock deviceSOLENOID VALVESDirectly operated solenoid valve, 2/2 NCCD01CD02CD03CD04CD05CD06CD07CD08CD09CD10CD11CD12CD13CD14CD15CD16CD17CD18CD19CD2TCD3TCD4TCDPPCDSSCS03CS04CS05CS06CS07CS08CS09CS10CS11HI01HI02HI03HI04HI05HI06HI07HI08PNZ1RDLKEV01CATALOGUE >Release 8.5APPENDIX >Camozzi products Pneumatic symbols/2.0301A P P E N D I XSymbol TypeSymbol TypeCS13Symbol TypeSymbol TypeSOLENOID VALVESSOLENOID VALVESDirectly operated solenoid valve, 3/2 NCDirectly operated solenoid valve, 3/2 NC, monostable, with manual overrideDirectly operated solenoid valve, 3/2 NODirectly operated solenoid valve, 3/2 NO, monostable, with manual overrideSolenoid valve, 3/2 NC with quick exhaustDirectly operated solenoid valve, 3/2 NC, bistable, with manual overrideDirectly operated solenoid valve, 3/2 NO, bistable, with manual overrideSolenoid valve, 3/2 NC, monostable, with bistable manual overrideSolenoid valve, 3/2, monostable,solenoid pilot with separate air supply and bistable manual override Solenoid valve, 3/2 NO, monostable, with bistable manual overrideSolenoid valve, 3/2, monostable,solenoid pilot with separate air supply and bistable manual override Solenoid valve, 3/2, bistable, with manual override bistabileSolenoid valve, 3/2, bistable,solenoid pilot with separate air supply and bistable manual overrideSolenoid valve, 3/2 NC, monostable, (pneumatic spring) and bistable manual overrideSolenoid valve, 3/2 NO, monostable, (pneumatic spring) and bistable manual overrideSolenoid valve, 5/2, monostable, with bistable manual overrideSolenoid valve, 5/2, monostable,solenoid pilot with separate air supply and bistable manual overrideSolenoid valve, 5/2, monostable,(pneumatic spring) and manual overrideSolenoid valve, 5/2, monostable,(pneumatic spring) and bistable manual overrideSolenoid valve, 5/2, monostable, solenoid pilot with separate air supply, pneumatic spring and bistable manual override Solenoid valve, 5/2, bistable, with bistable manual overrideSolenoid valve, 5/2, bistable, with manual overrideSolenoid valve, 5/2, bistable,solenoid pilot with separate air supply and bistable manual overrideSolenoid valve, 5/3 CC,with bistable manual overrideSolenoid valve, 5/3, solenoid pilot with separate air supply and bistable manual overrideSolenoid valve, 5/3, solenoid pilot with separate air supply and bistable manual override Solenoid valve, 5/3 CO, with manual overrideSolenoid valve, 5/3 CO,with bistable manual overrideSolenoid valve, 5/3 CO,solenoid pilot with separate air supply and bistable manual overrideSolenoid valve, 5/3 CO,solenoid pilot with separate air supply and bistable manual override Solenoid valve, 5/3 CP , with manual overrideSolenoid valve, 5/3 CP ,with bistable manual overrideSolenoid valve, 5/3 CP , solenoid pilot with separate air supply and bistable manual override Solenoid valve, 5/3 CP , solenoid pilot with separate air supply and bistable manual overrideDouble solenoid valve, 3/2 NC,monostable, with bistable manual overrideDouble solenoid valve, 3/2, monostable, solenoid pilot with separate air supply and bistable manual overrideDouble solenoid valve, 3/2 NO,monostable, with bistable manual overrideDouble solenoid valve, 3/2, monostable, solenoid pilot with separate air supply and bistable manual overrideDouble solenoid valve, 3/2 NC, NO,monostable, with bistable manual overrideDouble solenoid valve, 3/2, monostable, solenoid pilot with separate air supply and bistable manual overrideDirectly operated solenoid valve, 3/2,possible universal use, reversed printed ports 1 and 2 on the bodyIndirectly operated solenoid valve, 2/2 NODirectly operated solenoid valve, 2/2 NC, with linked diaphragmIndirectly operated solenoid valve, 2/2 NCPNEUMATICALLY OPERATED VALVES Pneumatically operated valve,3/2, monostable, mechanical spring Pneumatically operated valve, 3/2, bistableEV05EV06EV07EV08EV09EV10EV11EV12EV13EV14EV15EV16EV17EV18EV19EV20EV21EV22EV23EV24EV25EV26EV48EV47EV46EV43EV42EV45EV44EV41EV40EV39EV38EV37EV36EV35EV30EV31EV32EV33EV34APPENDIX >Camozzi productsCATALOGUE >Release 8.5/2.0302aA P P E N D I XEV04EV03EV29EV28CATALOGUE >Release 8.5APPENDIX >Camozzi productsa /2.0303A P P E N D I XVN01VN02VN03VN04VN05VN06VN07VN08VN09VN10VN11VN12VN13VN14VN15VN16VN17VN18VN19VN20VN21VN22VN23VN24VN25Symbol TypeSymbol TypePNEUMATICALLY OPERATED VALVES MECHANICALLY OPERATED VALVES Pneumatically operated valve, 5/2, preferentialPneumatically operated valve, 5/2, bistablePneumatically operated valve, 5/2, monostable, pneumatic spring Pneumatically operated valve, 5/3 CCPneumatically operated valve, 5/3 COPneumatically operated valve, 5/3 CPPneumatically operated double valve, 3/2, monostablePneumatically operated double valve, 3/2, monostablePneumatically operated double valve, 3/2, monostableMECHANICALLY OPERATED VALVES Mechanically operated valve, plunger actuation, 3/2 NC, monostable, mechanical spring Mechanically operated valve, plunger actuation, 3/2, monostable, mechanical springMechanically operated valve, plunger actuation, 3/2 NO, monostable, mechanical spring Mechanically operated valve,lever/roller actuation, 3/2 NC, monostable,mechanical springMechanically operated valve,lever/roller actuation, 3/2, monostable, mechanical springMechanically operated valve,lever/roller actuation, 3/2 NO, monostabile,mechanical springMechanically operated valve, unidirectional lever actuation, 3/2 NC, monostable, mechanical springMechanically operated valve, unidirectional lever actuation,3/2 monostable, mechanical springMechanically operated valve, plunger actuation, 5/2, monostable, mechanical springMechanically operated valve, plunger actuation, 5/2, monostable, mechanical springMechanically operated valve, lever/rolleractuation, 5/2, monostable, mechanical spring Mechanically operated valve, lever/rolleractuation, 5/2, monostable, mechanical spring Mechanically operated valve,unidirectional lever actuation, 5/2, monostable, mechanical spring Mechanically operated sensor valve, 3/2 NO, monostable, mechanical spring Mechanically operated sensor valve, 3/2 NC, monostable, mechanical spring Mechanically operated sensor valve, plunger actuation, 5/2, monostable, mechanical springMechanically operated sensor valve, plunger actuation, 5/2, bistable Valvola a comando meccanico frontale sensibile 5/2, bistabile Mechanically operated sensor valve, lever/roller actuation, 5/2, bistableMANUALLY OPERATED VALVES Manually operated valve, 3/2, bistableManually operated valve, 3/2, bistable, lockable in two positions Manually operated valve, 3/2, bistableManually operated valve, 3/2 NC, monostable, mechanical spring Manually operated valve, 3/2 NO, monostable, mechanical spring Manually operated valve, 3/2, monostable, mechanical spring Manually operated lever valve, 3/2, bistableManually operated lever valve, 3/2, bistableManually operated lever valve, 3/2 NC, monostable, mechanical spring Manually operated lever valve, 3/2, bistableManually operated lever valve, 3/2, monostable, mechanical spring Pedal operated valve, 3/2 NC, monostable, mechanical spring Manually operated valve, 5/2, bistableManually operated valve, 5/2, monostable, mechanical spring Manually operated lever valve, 5/2, bistableManually operated lever valve, 5/2, bistableManually operated lever valve, 5/2, monostable, mechanical spring Pedal operated valve, 5/2, bistablePedal operated valve, 5/2, monostable bistableManually operated lever valve, 5/3 CC, stableManually operated lever valve, 5/3 CC, monostableManually operated lever valve, 5/3 CO, stableManually operated lever valve, 5/3 CO, stableManually operated lever valve, 5/3 CO, monostableManually operated lever valve, JoystikVP06APPENDIX >Camozzi productsCATALOGUE >Release8.5/2.0304aA P P E N D I XFT01FT02FT03FA01FA02FA03FC01PR01PR02PR03PR04PR05PR06LU0FR01FR02AMP1VMP1CATALOGUE >Release 8.5APPENDIX >Technical information about productsSpring loads cylinders/3.0101A P P E N D I XAPPENDIX >Technical information about productsCATALOGUE >Release8.5a /3.0102aA P P E N D IX* F = spring forceFlow and speed cylindersCATALOGUE >Release 8.5APPENDIX >Technical information about products/3.0201A P P E N D IXAPPENDIX >Technical information about productsCATALOGUE >Release8.5/3.0301aA P P E N D I XOutput forces double-acting cylindersThrust sideValues in NewtonTraction sideValues in NewtonTraction sideValues in Newtona /3.03A P P E N D I Xa P E N D I X Table showing air consumption of double-acting cylindersThrust side Values in NL for each 10 mm of stroke Traction side Values in NL for each 10 mm of stroke/3.04A P P E N D I X Traction sideValues in NL for each 10 mm of strokea P E N D I X Dimensioning guide for Shock Absorbers Series SAa /3.05A P P E N D IX Calculation:a P E N D I X = 600 cycles/h ω= 100 cycles/h To ensure the lifetime of the shock absorber, the movement of the impact bodymust be perpendicular to the shock absorbers axial centre.Note : The maximum allowable eccentricity θ≤ 2,5° (0,044 rad).Perpendicularity of the loadωL o a d7. Vacuum switch6. Solenoid valves5. Vacuum generator4. Vacuum hose3. Mounting elements2. Suction pads1. Calculation of the forcesFlowchart for system design Example of Vacuum calculationa /3.07A P P E N D I XComparison:A comparison of the figures for load cases I and II results, in this example, in a maximum value for FTH =1822 N in load case II,and this value is therefore used for further design calculations.a P E N D I X/3.07A P P E N D I Xa P E N DI XCATALOGUE >Release 8.5APPENDIX >Technical information about productsTechnical information about suction padsWhen designing a vacuum circuit and selecting suitable suction pads it is necessary to follow certain calculations toselect each individual component in a correct way.Listed below is a summary of the most common data to take into consideration./3.0801A P P E N D I XAPPENDIX >Technical information about productsCATALOGUE >Release8.5a /3.0802aA P P E N D I X。
伊伐布雷定说明书

核准日期:2015年4月29日盐酸伊伐布雷定片说明书请仔细阅读说明书并在医师指导下使用【药物名称】通用名称:盐酸伊伐布雷定片商品名称:可兰特®/CORLENTOR®英文名称:Ivabradine Hydrochloride Tablets汉语拼音:Yansuan Yifabuleiding Pian【成份】本品活性成份为盐酸伊伐布雷定。
化学名称:3-(3-{[((7S)-3,4-二甲氧基双环[4.2.0]辛-1,3,5-三烯-7-基)甲基]甲氨基}丙基)-1,3,4,5-四氢-7,8-二甲氧基-2H-3-苯并氮杂卓-2-酮,盐酸盐化学结构式:分子式:C27H36N2O5•HCl分子量:505.1【性状】本品为橙色薄膜衣片,5mg片剂为椭圆形,7.5mg片剂为三角形,除去包衣显白色。
【适应症】适用于窦性心律且心率≥75次/分钟、伴有心脏收缩功能障碍的NYHAⅡ~Ⅳ级慢性心力衰竭患者,与标准治疗包括β-受体阻滞剂联合用药,或者用于禁忌或不能耐受β-受体阻滞剂治疗时。
【规格】按C27H36N2O5计:(1)5mg ;(2)7.5mg 。
【用法用量】口服,一日两次,早、晚进餐时服用(见【药代动力学】)。
本品起始治疗仅限于稳定性心力衰竭患者。
建议在有慢性心力衰竭治疗经验的医生指导下使用。
通常推荐的起始剂量为5mg,一日两次。
治疗2周后,如果患者的静息心率持续高于60次/分钟,将剂量增加至7.5mg,一日两次;如果患者的静息心率持续低于50次/分钟或出现与心动过缓有关的症状,例如头晕、疲劳或低血压,应将剂量下调至2.5mg(半片5mg片剂),一日两次;如果患者的心率在50和60次/分钟之间,应维持5mg,一日两次。
治疗期间,如果患者的静息心率持续低于50次/分钟,或者出现与心动过缓有关的症状,应将7.5mg或5mg一日两次的剂量下调至下一个较低的剂量。
如果患者的静息心率持续高于60次/分钟,应将2.5mg或5mg一日两次的剂量上调至上一个较高的剂量。
Ibrance中文说明书

【药物名】Palbociclib【商品名】Ibrance【美国上市时间】乳腺癌,2015年2月【类别】抑制剂【靶点】CDK4,CDK6【分子结构】分子式:C24H29N7O2化学名:6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2yl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one结构式为:分子量:447.54 Da【生产公司】Pfizer 辉瑞公司【购买地】美国【剂型和规格】口服胶囊,规格为:125 mg、100 mg和75 mg。
125mg胶囊:不透明硬明胶胶囊,大小0,在胶囊的焦糖帽上用白墨汁印有“Pfizer”,在浅橙色体上印有“PBC 125”。
100mg胶囊:不透明硬明胶胶囊,大小1,在胶囊的焦糖帽上用白墨汁印有“Pfizer”,在浅橙色胶囊体上印有“PBC 100”。
75mg胶囊:不透明硬明胶胶囊,大小2,在浅橙色胶囊帽上用白墨汁印有“Pfizer”,在浅橙色胶囊体上印有“PBC 75”。
【处方】Palbociclib是一种黄色至橙色粉有pKa为7.4(第二哌嗪氮)和3.9(吡啶氮)。
在或低于pH 4,Palbociclib行为如同高溶解度化合物。
高于pH 4,药物物质溶解度显著减低。
无活性成分:微晶纤维素,一水乳糖,羟基乙酸淀粉钠,胶体二氧化硅,硬脂酸镁,和硬明胶胶囊壳。
浅橙色,浅橙色/焦糖和焦糖不透明胶囊壳含明胶,红色氧化钛,黄色氧化铁,和二氧化钛;和印刷油墨含虫胶,二氧化钛,氢氧化铵,丙二醇和二甲基硅油。
【作用机理】Palbociclib是一种周期蛋白-依赖激酶(CDK)4和6的抑制剂。
周期蛋白D1和CDK4/6是导致细胞增殖信号通路的下游。
在体外,Palbociclib雌激素受体(ER)-阳性乳癌细胞株通过阻断细胞从细胞周期G1进入S期的进展减低细胞增殖。
用palbociclib和抗雌激素的联合处理与单独各个药物处理比较,乳癌细胞株导致视网膜母细胞瘤蛋白(Rb)磷酸化的减低导致减低E2F表达和信号和阻止增加生长。
重组人凝血因子Ⅷ药品安全性及体内药学特性评价

重组人凝血因子Ⅷ药品安全性及体内药学特性评价霍记平;赵志刚【摘要】血友病A是凝血因子Ⅷ缺乏引起的出血性疾病,重组人凝血因子Ⅷ(重组FⅧ)因能大大降低病原体感染风险,被多数指南推荐使用.本文根据中国药品综合评价指南,对我国上市的4种重组FⅧ制剂的药品安全性和体内药学特性从两方面进行比较,以促进临床安全合理用药.这4种产品均可用于A型血友病出血的控制和预防,但4种药品在半衰期方面存在一定差异,在抑制物发生率差异方面尚需在临床实践中进一步验证.临床用药应结合患者个体情况,优先选择半衰期较长的药品,以使患者达到最佳治疗效果.【期刊名称】《药品评价》【年(卷),期】2019(016)010【总页数】5页(P3-6,37)【关键词】血友病A;重组FⅧ;安全性;体内药学特性【作者】霍记平;赵志刚【作者单位】首都医科大学附属北京天坛医院药学部,北京 100070;首都医科大学附属北京天坛医院药学部,北京 100070【正文语种】中文【中图分类】R969.1血友病是一种由凝血因子基因突变引起的X染色体连锁的隐性遗传性出血性疾病,主要分为血友病A(凝血因子Ⅷ缺乏)和血友病B(凝血因子Ⅸ缺乏),分别约占所有血友病患者的80%~85%和15%~20%。
目前我国血友病的患病率为2.73/10万人口。
血友病患者严重出血时如不及时治疗可危及生命[1]。
凝血因子替代治疗是目前血友病有效的治疗措施,替代治疗药物主要包括重组人凝血因子Ⅷ(重组FⅧ)和血源性人凝血因子Ⅷ(血源FⅧ)[1]。
使用不含任何血液成分的基因重组因子能大大降低已知和未知病原体感染风险,多数指南推荐重组FⅧ治疗[2,3]。
由于不同品牌的药品质量差异较大,加上血友病患者需要终生治疗,这就给患者和医生选择药品带来很大压力[4]。
2018年发表在药品评价的《凝血因子Ⅷ药品综合评价》对中国上市的4种血源FⅧ和3种重组FⅧ进行了综合评价。
2018年新型重组FⅧ科跃奇®上市,为使不同重组FⅧ制剂综合评价更加全面,本文拟依据中国药品综合评价指南从药品安全性和体内药学特性两方面对目前国内市售的4种重组FⅧ制剂进行比较,以期为临床合理用药提供参考。
拜科奇产品手册

目录
.1 . . . . . .1 .1.1 .1. .1. .1. .1. . ..1 .. .1 . . . . . . . 10 11
拜科奇的产品特性 ......................................................................................................... 质量保证........................................................................................................................ 产品纯度........................................................................................................................ 耐受性和安全性的临床前证据 ....................................................................................... 临床前免疫原性模型...................................................................................................... 临床前疗效研究............................................................................................................. 拜科奇临床前研究小结 .................................................................................................. 临床研究........................................................................................................................ 对既往接受过治疗的患者(PTPs)的研究.................................................................... 0 药代动力学 .................................................................................................................... 0 疗效............................................................................................................................... 临床免疫原性 ................................................................................................................ 外科手术中的应用 ......................................................................................................... 安全性 ........................................................................................................................... 对既往未接受过治疗(PUPs)或仅接受过最低限度治疗(MTPs)的患者的研究 ....... 拜科奇用于治疗PUPs/MTPs的安全性及疗效 ................................................................ PUPs/MTPs中抑制物的产生率...................................................................................... 重组人凝血因子VIII抑制物的形成.................................................................................. 1 抑制物形成的潜在标志物 .............................................................................................. 1 抑制物形成的发生率...................................................................................................... 抑制物分子 ................................................................................................................... 抑制物的特异性............................................................................................................. 对PTPs的临床免疫原性研究 ......................................................................................... PUPs/MTPs中抑制物发生率的比较 ............................................................................. 抑制物产生的累积风险 .................................................................................................. 0 小结:拜科奇与凝血因子VIII抑制物 .............................................................................. 1 免疫耐受诱导 (ITI) ........................................................................................................ 长期预防........................................................................................................................ 产品亮点总结 ................................................................................................................ 0 参考文献........................................................................................................................
Oxlumo (Lumasiran) 用药指南说明书

UnitedHealthcare ® Community PlanOxlumo ® (Lumasiran)Policy Number : CS2023D0102H Effective Date : December 1, 2023 Instructions for UseTable of Contents Page Application ..................................................................................... 1 Coverage Rationale ....................................................................... 1 Applicable Codes .......................................................................... 2 Background .................................................................................... 2 Clinical Evidence ........................................................................... 3 U.S. Food and Drug Administration ............................................. 3 References ..................................................................................... 3 Policy History/Revision Information ............................................. 4 Instructions for Use ....................................................................... 4 This Medical Benefit Drug Policy does not apply to the states listed below; refer to the state-specific policy/guideline, if noted: StatePolicy/Guideline FloridaRefer to the state's Medicaid clinical policy IndianaOxlumo ® (Lumasiran) (for Indiana Only) KansasNone LouisianaOxlumo ® (Lumasiran) (for Louisiana Only) North CarolinaNone OhioOxlumo ® (Lumasiran) (for Ohio Only)TexasRefer to drug specific criteria found within the Texas Medicaid Provider Procedures ManualOxlumo is proven and medically necessary for the treatment of PH1 when all of the following criteria are met:For initial therapy , all of the following: o Diagnosis of PH1 by, or in consultation with, a specialist (e.g., geneticist, nephrologist, urologist) with expertise in the diagnosis of PH1; ando Confirmation of the PH1 diagnosis based on both of the following: ▪ Metabolic testing demonstrating one of the following:Increased urinary oxalate excretion [e.g., greater than 1 mmol/1.73 m 2 per day (90 mg/1.73 m 2 per day),increased urinary oxalate: creatinine ratio relative to normative values for age]; orIncreased plasma oxalate and glyoxylate concentrations and▪ Genetic testing has confirmed a mutation in the alanine: glyoxylate aminotransferase (AGT or AGXT) geneand o Patient has not received a liver transplant; andCommercial Policy • Oxlumo ® (Lumasiran)o Oxlumo is prescribed by, or in consultation with, a specialist (e.g., geneticist, nephrologist, urologist) with expertise in the treatment of PH1; ando Oxlumo dosing is in accordance with the United States Food and Drug Administration approved labeling; ando Initial authorization will be for no more than 6 monthsFor continuation of therapy, all of the following:o Submission of medical records (e.g., chart notes, laboratory values) documenting a positive clinical response to therapy from pre-treatment baseline (e.g., decreased urinary oxalate concentrations, decreased urinary oxalate:creatinine ratio, decreased plasma oxalate concentrations); ando Patient has not received a liver transplant; ando Oxlumo is prescribed by, or in consultation with, a specialist (e.g., geneticist, nephrologist, urologist) with expertise in the treatment of PH1; ando Oxlumo dosing is in accordance with the United States Food and Drug Administration approved labeling; ando Reauthorization will be for no more than 12 monthsThe following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this policy does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by federal, state, or contractual requirements and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply.HCPCS Code DescriptionJ0224 Injection, lumasiran, 0.5 mgDiagnosis Code DescriptionE72.53 Primary hyperoxaluriaPrimary hyperoxaluria (PH) is a rare inborn error of glyoxylate metabolism characterized by the overproduction of oxalate, which is deposited as calcium oxalate in various organs. In particular, the kidney is a prime target for oxalate deposition, as excessive urinary excretion of oxalate may lead to end-stage renal disease (ESRD). PH is primarily caused by autosomal recessive enzymatic defects in pathways of glyoxylate metabolism that result in enhanced oxalate production. PH type 1 (approximately 80 percent of cases) is due to mutations of hepatic peroxisomal enzyme alanine: glyoxylate aminotransferase (AGT). Glycolate oxidase (GO) is an enzyme responsible for the metabolism of glycolate to glyoxylate and glyoxylate to oxalate. Lumasiran reduces levels of GO enzyme by targeting the hydroxy acid oxidase 1 (HAO1) messenger ribonucleic acid (mRNA) in hepatocytes through RNA interference. Decreased GO enzyme levels reduce the amount of available glyoxylate, a substrate for oxalate production. As the GO enzyme is upstream of the deficient alanine: glyoxylate aminotransferase (AGT) enzyme that causes PH1, the mechanism of action of lumasiran is independent of the underlying AGXT gene mutation. Lumasiran is not expected to be effective in primary hyperoxaluria type 2 (PH2) or type 3 (PH3) because its mechanism of action does not affect the metabolic pathways causing hyperoxaluria in PH2 and PH3.In patients with PH, the increased urinary excretion of oxalate results in an oversaturated urine for calcium oxalate, which leads to urolithiasis and nephrocalcinosis. Recurrent stones and progressive nephrocalcinosis cause renal parenchymal inflammation and fibrosis, and if persistent, may progress to ESRD. As renal function deteriorates, plasma oxalate exceeds 30 micromol/L (the plasma supersaturation threshold for calcium oxalate), because of reduced urinary oxalate excretion. This leads to calcium oxalate deposition into nonrenal tissues including the retina, myocardium, vessel walls, skin, bone, and the central nervous system (systemic oxalosis). Liver transplantation is the only curative intervention for PH type 1 as it corrects the underlying enzymatic defect due to mutations of the AGXT gene.The efficacy of lumasiran was established in a pivotal placebo-controlled and open-label clinical studies (ILLUMINATE-A, ILLUMINATE-B, and a phase 1/2 study) in 77 patients with PH1 (including 56 pediatric patients). Patients ranged in age from 4 months to 61 years at first dose. The median duration of exposure was 9.1 months (range 1.9 to 21.7 months). Overall, 58 patients were treated for at least 6 months, and 18 patients for at least 12 months.A phase 1/2 study evaluated lumasiran at multiple doses in a single blind, randomized, placebo-controlled trial in 20 patients with PH1. Patients were randomized 3:1 to receive lumasiran and all patients received lumasiran in the open-label extension phase. After a median of 7 months on lumasiran, patients experienced a 66% mean reduction of urinary oxalate content from baseline. Among patients receiving 3.0 mg/kg monthly or quarterly doses of lumasiran, 10/12 (83%) achieved urinary oxalate levels within the normal range.ILLUMINATE-A was a randomized, double-blind trial comparing lumasiran and placebo in 39 patients ≥ 6 years of age with PH1 and an eGFR ≥ 30 mL/min/1.73 m2 (ILLUMINATE-A; NCT03681184). Patients received 3 loading doses of 3 mg/kg lumasiran (n = 26) or placebo (n = 13) administered once monthly, followed by quarterly maintenance doses of 3 mg/kg lumasiran or placebo. The primary endpoint from ILLUMINATE A was the percent reduction from baseline in 24-hour urinary oxalate excretion corrected for BSA averaged over months 3 through 6. The LS mean percent change from baseline in 24-hour urinary oxalate in the lumasiran group was -65% (95% CI: -71, -59) compared with -12% (95% CI: -20, -4) in the placebo group, resulting in a between-group LS mean difference of 53% (95% CI: 45, 62; p < 0.0001). By Month 6, 52% (95% CI: 31, 72) of patients treated with lumasiran achieved a normal 24-hour urinary oxalate corrected for BSA (≤ 0.514 mmol/24 hr/1.73 m2) compared to 0% (95% CI: 0, 25) placebo-treated patients (p = 0.001).ILLUMINATE-B was a single-arm study in 18 patients < 6 years of age with PH1 and an eGFR > 45 mL/min/1.73 m2 for patients ≥ 12 months of age or a normal serum creatinine for patients < 12 months of age (ILLUMINATE-B; NCT03905694). The median age was 47 months (range 4 to 74 months). The primary endpoint was the percent reduction from baseline in spot urinary oxalate: creatinine ratio averaged over months 3 through 6. Patients treated with lumasiran achieved a reduction in spot urinary oxalate: creatinine ratio from baseline of 71% (95% CI: 65, 77).The approval of lumasiran for the expanded indication to include lowering of plasma oxalate levels was based on ILLUMINATE-C, a single-arm study in 21 patients with PH1, including patients on hemodialysis. Cohort A included 6 patients who did not require dialysis at the time of study enrollment. Cohort B included 15 patients who were on a stable regimen of hemodialysis. The primary endpoint was the percent change in plasma oxalate from baseline to month 6 for Cohort A and the percent change in pre-dialysis plasma oxalate from baseline to month 6 for Cohort B. The percent change from baseline to month 6 in plasma oxalate levels in Cohort A was a least-squares (LS) mean difference of -33% (95% CI: -82, 15) and in Cohort B it was -42% (95% CI: -51, -34).This section is to be used for informational purposes only. FDA approval alone is not a basis for coverage.Oxlumo (lumasiran) is a HAO1-directed small interfering ribonucleic acid (siRNA) indicated for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary and plasma oxalate levels in pediatric and adult patients.1.Oxlumo [package insert] Cambridge MA, Alnylam Pharmaceuticals, Inc. September 2023.2.Cochat P, Hulton SA, Acquaviva C, et al. Primary Hyperoxaluria Type 1: Indications for Screening and Guidance forDiagnosis and Treatment. Nephrol Dial Transplant 2012; 27:1729.3.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int 2009; 75:1264.4.Niaudet P. Primary Hyperoxaluria. In: UpToDate, Mattoo TK, Kim MS, (Ed), UpToDate, Waltham, MA, 2020.5. A Phase 1/2 Trial of Lumasiran (ALN-GO1), An Investigational RNA Interference (RNAi) Therapeutic, For PrimaryHyperoxaluria Type 1. ESPN Annual Meeting. Antalya, Turkey. 4 October 2018.6. A Study to Evaluate Lumasiran in Children and Adults with Primary Hyperoxaluria Type 1 (ILLUMINATE-A). website: https:///ct2/show/NCT03681184?term=lumasiran&draw=2&rank=4. Accessed December 1, 2020.7. A Study of Lumasiran in Infants and Young Children with Primary Hyperoxaluria Type 1 (ILLUMINATE-B). website: https:///ct2/show/NCT03905694?term=lumasiran&draw=2&rank=2. Accessed December 1, 2020.8. A Study to Evaluate Lumasiran in Patients with Advanced Primary Hyperoxaluria Type 1 (ILLUMINATE-C). website: https:///ct2/show/NCT04152200?term=lumasiran&draw=2&rank=1. Accessed October 11, 2022.Date Summary of Changes12/01/2023 Supporting InformationUpdated References section to reflect the most current informationArchived previous policy version CS2023D0102GThis Medical Benefit Drug Policy provides assistance in interpreting UnitedHealthcare standard benefit plans. When deciding coverage, the federal, state or contractual requirements for benefit plan coverage must be referenced as the terms of the federal, state or contractual requirements for benefit plan coverage may differ from the standard benefit plan. In the event of a conflict, the federal, state or contractual requirements for benefit plan coverage govern. Before using this policy, please check the federal, state or contractual requirements for benefit plan coverage. UnitedHealthcare reserves the right to modify its Policies and Guidelines as necessary. This Medical Benefit Drug Policy is provided for informational purposes. It does not constitute medical advice.UnitedHealthcare may also use tools developed by third parties, such as the InterQual® criteria, to assist us in administering health benefits. The UnitedHealthcare Medical Benefit Drug Policies are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice.。
A型血友病儿童患者的常规预防

本品可用于儿童患者的常规预防,即对既往没有关节损伤的儿童患者常规预防,以降低出血发生频率和降低发生关节 损伤的风险。
本品不适用于血管性血友病。
【规格】 250 IU/瓶 500 IU/瓶 1000 IU/瓶
【用法用量】 配制后仅用于静脉注射 本品首次使用,应在具有治疗 A 型血友病经验的医生的指导下进行。 在标签上,本品均以国际单位(IU)标示重组人凝血因子Ⅷ的效价。 治疗的剂量和持续时间决定于Ⅷ因子缺乏的严重程度,出血的部位和范围以及患者的临床状况。 在大型手术和
1. 在无菌条件下配制本品。 2. 在手中对未开启的药瓶和注射器加温,使其达到舒适温度(切勿超过 37°C 或 99°F)。 3. 将保护盖从药瓶上取下(A)。使用酒精消毒橡胶塞,注意不要手持橡胶塞。 4. 将药瓶放在一个牢固、防滑的表面上。撕下药瓶适配器塑料外罩上的纸质标签 。不要从塑料外罩上取下适配器。
手术类型
小型手术 包括拔牙
治疗所需的Ⅷ因子水平(IU/dL 维持治疗效果的血浆水平所必
或正常水平的%)
须的剂量和给药频率
30~60
15~30IU/kg
每12~24小时重复给药,直到 出血得到控制
2 / 12
大型手术
围手术剂量50IU/kg。术前确定
包括扁桃体切除术、腹股沟疝 切开术、滑膜切除术、全膝关
出血类型
治疗所需的Ⅷ因子水平 维持治疗效果的血浆水平所必须
(IU/dL 或正常水平的%)
的剂量和给药频率
轻度出血
20~40
10~20IU/kg
早期关节积血、小范围的肌肉 或口腔出血
如存在进一步出血的证据,重复给 药。
中度出血
30~60
BEKOMAT 系列蒸汽吸收器产品说明书

Condensate technology | BEKOMAT ® 31U | 32U | 33U | 33U CODuring compressed air generation and processing, the optimum quality for the application should be achieved. It is important to remove contaminants and humidity from the compressed air as these can lead to quality problems, failures or loss of production. Condensate discharge without compressed air lossThe BEKOMAT® drains off condensate withoutloss of compressed air, thus reducing energy costs and CO2emissions. This is made possible by the integrated capacitive sensor,smart electronics for volume-controlled condensate dischargeand the proven pilot-controlled solenoid valve.The BEKOMAT® designed for quick and cost-effective servicing The innovative design of the BEKOMAT® 31U, 32U, 33U and 33U CO models is optimised for easy handling, installation and maintenance. The devices consist of no more than three assemblies joined together with quick-release connectors. Once installed, the control and sensorunit stays in place as only theservice unit (including all wearand pressure parts) needs to beexchanged.This sturdy condensate drain issuitable for both oil-contaminatedand oil-free, aggressivecondensate. ›No loss of compressed air during draining ›Low operating costs›Outstanding reliability›Durable and resistant to dirt›Large valve diameters prevent the formationof emulsions›No delicate mechanical components›Suitable for temperatures up to +70 °C› Easy to install and virtuallymaintenance-free›Versatile connection options›Easy exchange of service unit, even wherespace is confirmed with even in small areas›Servicing requires no installation work›Automated operationand monitoring›Ready for integration into modern systemmonitoring installations›Automatic start of self-cleaning processbased on dirt particle load›Service indicator warns operators in advancewhen the service unit needs to be replacedThe quickest route to efficiency: the BEKOMAT ® with service unitDimensions in mmDepth: 65Dimensions in mm* For more information on climate zones ( | | ) see reverse** Short-term peak volume can only be achieved if the device is correctly installed according to the operating manual. If in doubt, a install venting line.Depth: 73BEKO TECHNOLOGIES Ltd Unit 11-12 Moons Park Burnt Meadow Road North Moons Moat Redditch, B98 9PAPhone + 44 (0) 1527 575778 *************************.uk carbon neutral | DE-077-457728print production78-00073e.g. Northern Europe, Canada, Northern USA, Central Asia e.g. Central and Southern Europe, Central Americae.g. South-East Asian coastal regions, Oceania, Amazon and Congo regionsTemperature range: 1 to + 60 °CClimate – a key factorThe general climate and the ambient temperature are important factors for the formation of condensate in compressed air systems. That is why we quote separate performance data of our BEKOMAT ® models for three climate zones:Like all high-performance devices, the BEKOMAT ® needs to be serviced from time to time. This is done with our service unit containing all the necessary wearing parts. If you require assistance, contact our service technicians, who are also qualified to examine and assess your entire compressed air system for further optimisation.Service unitSubject to technical changes without prior notice. Errors and omissions excepted.Visit us atDo you have questions about the best way of processing your compressed air?We have the answers! We offer efficient solutions for any type of processing chain. Please contact us with all your queries. We would be delighted to tell you more about our condensate treatment, filtration, drying, measuring and process technology, and our comprehensive services.。
忆孟返 Imovane 佐匹克隆片说明书

核准日期:2004年8月27日修改日期:2009年6月17日2009年12月2日2010年1月28日2012年2月28日2013年10月8日2014年11月3日2015年5月21日2016年3月25日2017年5月16日2018年8月8日2020年11月16日佐匹克隆片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:佐匹克隆片商品名称:忆孟返®/Imovane®英文名称:ZOPICLONE TABLETS汉语拼音:ZUOPIKELONG PIAN【成份】活性成份:佐匹克隆化学名称:6-(5-氯吡啶-2-基)-7-[(4-甲基哌嗪-1-基)羰氧基]-5,6-二氢吡咯[3,4-b]吡嗪-5-酮化学结构式:分子式:C 17H 17O 3N 6Cl分子量:388.8【性状】本品为白色,椭圆形,有刻痕的片剂。
【适应症】本品仅限应用在成人以下严重睡眠障碍的短期治疗中:- 短暂性失眠症- 短期失眠症【规格】 7.5mg【用法用量】用法: 口服剂量:• 年龄低于65岁的成年人:每日7.5 mg 。
• 年龄高于65岁的老年人:推荐剂量为每日3.75 mg ,经评估必要时可以增加至7.5mg 。
• 肝脏或呼吸功能损害的患者:推荐剂量为每日3.75mg 。
• 肾脏功能不全的患者:推荐起始剂量为每日3.75mg 。
• 超过65岁的人群及高风险人群的最佳剂量为3.75mg 。
NN N OONN C H 3NClO应该始终在最低有效剂量下开始治疗,每日给药剂量不应超过7.5 mg。
应在晚上临睡前服药,按单次摄入剂量服用,同一晚不得再次服用。
本品不推荐用于18岁以下儿童和青少年。
治疗持续时间:与所有催眠药一样,不建议长期使用佐匹克隆。
治疗持续时间应该尽可能短,从数天到4周,包括减药期。
由于滥用和依赖风险会随治疗持续时间的增加而升高,因此在未对患者状况进行重新评估的情况下,不应在超出最长治疗期后延长治疗时间(见【注意事项】)。
拜科奇

拜科奇(注射用重组人凝血因子VIII)【药品名称】【商品名】拜科奇【通用名】注射用重组人凝血因子VIII【英文名】Recombinant Coagulation FactorVIII for Inje C tion【汉语拼音】ZhuSheYongChongZuRenNingXueYinZi VIII【成份】重组人凝血因子VIII【性状】拜科奇为白色或浅黄色干粉剂。
【适应症】拜科奇用于血浆凝血因子VIII(FVIII)缺乏的甲型血友病治疗。
在纠正或预防出血、急诊或择期手术中,拜科奇起到暂时代替缺失的凝血因子的作用。
拜科奇不含血管性假性血友病因子,故不能用于血管性假性血友病的治疗。
【用法用量】在瓶签上拜科奇用国际单位标示rFVIII(重组人凝血因子VIII)的效价,其效价用一期法测定。
复溶后的药物必须在药物溶解后3小时内注射完毕。
建议使用包装内提供的静脉注射用器具。
一般性治疗方法和疗效评估:下述剂量提供了一般性指导原则。
须强调的是不同患者达到止血所需要的拜科奇剂量各不相同,应视患者的需要、FVIII缺乏的严重程度、出血的严重程度、抗体存在的情况和期望达到的FVIII水平而定。
治疗时监控患者的FVIII尤为重要。
FVIII水平为疗效评估的重要因素。
为达到满意疗效,必要时使用剂量可高于计算值。
如果按公式计算的剂量注射后未达到预期的FVIII水平,或出血未得到控制,应怀疑患者体内是否存在抗体。
通过实验室检查可检测和定量抗体。
存在抗体时,不同患者所需的FVIII剂量差异较大,可根据疗效优化治疗方案。
某些低抗体滴度(<10BU)的患者应用FVIII 制剂成功治疗后,并未产生免疫记忆应答抗体滴度升高。
通过评估FVIII的水平和临床疗效进行适宜治疗。
对FVIII产生记忆应答或具有高滴度抗体的患者,必要时可选择其他治疗药物,如凝血因子IX复合物制剂、抗血友病因子(猪源性)、重组激活凝血因子VIIa或抗抑制剂凝血因子复合物。
Phoenix Quad-T 用户手册说明书

用户手册020-001128-01 Phoenix Quad-T用户手册020-001128-01Phoenix Quad-T注意事项版权和商标版权所有 © 2016 科视 Christie Digital Systems USA, Inc. 保留所有权利。
所有品牌名称和产品名称均为其各自所有者的商标、注册商标或商品名称。
总则尽管为确保准确性做了各种努力,然而,在某些情况下可能会发生本文档中未能反映出来的产品或可用性变更。
科视 Christie 保留随时更改规范的权利,恕不另行通知。
性能规范具有代表性,但可能因不受科视 Christie 控制的情况而所有不同,例如维护工况正常的产品。
性能规范基于印刷时可用的信息。
科视 Christie 不对本资料做任何担保,包括但不限于对特定用途的适用性的暗示担保。
科视 Christie 不对本文包含的错误或与性能或本资料的使用有关的附带损坏或间接损坏承担任何责任。
加拿大生产设施通过了 ISO 9001 和 14001 认证。
保修产品享受科视 Christie 的标准有限保修服务,有关详细信息,请联系您的科视 Christie 经销商或科视 Christie。
除科视 Christie 标准有限保修中指定的其他限制外,在与您的产品相关或适用于您的产品的最大限度内,保修不包括:a)发运(任一方向)期间发生的故障或损坏。
d)搭配使用产品或非科视 Christie 设备,例如配电系统、相机、DVD 播放器等,或者搭配使用产品与任何非科视 Christie 接口设备引起的故障或损坏。
e)使用从未经授权的科视 Christie 灯泡、替换部件或组件经销商(包括但不限于任何通过互联网供应科视 Christie 灯泡、替换部件或组件的经销商(授权经销商证明可以从科视 Christie 获得))购买或获取的任何灯泡、更换部件或组件引起的故障或损坏。
f)滥用、不适当的电源、意外事件、火灾、洪涝、闪电、地震或其他自然灾害引起的故障或损坏。
Quincke Tubo 科学实验用品说明书

3B SCIENTIFIC ® PHYSICS1Tubo di Quincke 1018475Istruzioni per l'uso10/14 TL/UD3214561 Tubo di risonanza con scalae tappo in gomma 2 Vaso di espansione 3 Morsetti orizzontali 4 Tubo di silicone 5 Diapason 6 Martelletto1. Norme di sicurezzaDurante il funzionamento, il tubo di Quincke è pieno d'acqua. Le aperture nel tubo di risonanza e nel vaso diespansione sono chiuse ermeticamente solo dal tubo di silicone. Tutti i componenti sono realizzati in materiale plastico fragile.•Non utilizzare apparecchi elettrici sullo stesso tavolo insieme al tubo di Quincke.• Procedendo con cautela, inserire il tubo di silicone per almeno 10 mm nelle aperture presenti sul tubo di risonanza e sul vaso di espansione.• Evitare sollecitazioni meccaniche eccessive dovute ad es. a colpi e urti.•Non azionare l'apparecchio in presenza di incrinature o altri danni.22. Fornitura1 tubo di risonanza con scala e tappo ingomma1 vaso di espansione 1 tubo di silicone2 morsetti orizzontali1 diapason normale a 1 440 Hz 1 martelletto3. Altri apparecchi necessari1 asta di supporto 1000 mm, ∅ 12 mm 1002936 1 base di supporto a forma di A, 200 mm 1001044 1 manicotto universale 10028304. Dati tecniciAltezza tubo di risonanza: 1 m Diametro tubo di risonanza: 3 cm Altezza vaso di espansione: 24 cm Diametro vaso di espansione:7 cm Massa (senza accessoriné stativo): circa 3,3 kg5. DescrizioneIl tubo di Quincke serve per dimostrare fenomeni di interferenza su onde sonore. L'apparecchio è costituito da un tubo di risonanza con scala, collegato a un vaso di espansione tramite un tubo di silicone. Durante il funzionamento, il tubo di risonanza è collocato in posizione verticale, con il foro inferiore chiuso con un tappo in gomma. Il vaso di espansione è pieno d'acquaSollevando il vaso di espansione, è possibile alzare il livello dell'acqua nel tubo di risonanza e accorciare la colonnina d'aria, come mostra la Fig. 1. La colonnina d'aria viene portata in oscillazione per mezzo di un diapason (in opzione: un altoparlante), colpito con un martelletto. L'onda sonora in uscita dallasorgente sonora situata al di sopra del tuboaperto su un lato si sovrappone all'onda sonorariflessa sulla superficie dell'acqua, dando luogo a interferenza costruttiva o distruttiva. Quando lalunghezza della colonnina d'aria oscillante coincide con multipli dispari di un quarto della lunghezza dell'onda sonora, si producono risonanze udibili.Fig. 1: Funzionamento del tubo di Quincke.6. Messa in funzione•Fissare l’asta di supporto sulla base di modo che l'altezza complessiva della struttura sia pari a circa 105 cm.•Fissare i due morsetti orizzontali presso il terzo superiore e il terzo inferiore dell'asta di supporto.•Sistemare il tubo di risonanza in posizione verticale davanti allo stativo e spingere con delicatezza con la scala in avanti nei due morsetti orizzontali.•A seconda della lunghezza del diapason, fissare il manicotto universale presso l'estremità superiore dell'asta di supporto come da Fig. 2 o Fig. 3.•Fissare il diapason nel manicotto universale come da Fig. 2 o Fig. 3 in modo tale che le due estremità dei rebbi del diapason si vengano a trovare il più vicino possibile all'apertura del tubo di risonanza.3B Scientific GmbH ▪ Rudorffweg 8 ▪ 21031 Amburgo ▪ Germania ▪ Con riserva di modifiche tecniche© Copyright 2014 3B Scientific GmbHFig. 2: Fissaggio di diapason con stelo corto.Fig. 3: Fissaggio di diapason con stelo lungo.•Sistemare il vaso di espansione in prossimità del tubo di risonanza e infilare i due capi del tubo di silicone almeno 10 mm nelle apposite aperture presenti sul tubo di risonanza e sul vaso di espansione.7. Uso• Riempire il vaso di espansione con acqua fino a 4 cm al di sotto del bordo superiore. • Colpire il diapason con il martelletto e sollevare il vaso di espansione (Fig. 1).•Trovare le risonanze aumentando gradualmente l'altezza del vaso di espansione, leggendo sulla scala e annotando ogni volta la lunghezza della colonnina d'aria.•Le lunghezze rilevate della colonnina d'aria oscillante coincidono con multipli dispari n di un quarto della lunghezza dell'onda sonora λ.•Ulteriori risonanze si trovano con λ/4 + n · λ/2.8. NotaEsistono applicazioni gratuite per smartphone (App) che offrono accordatori e generatori di toni. In tali applicazioni, l'altezza del suono e la frequenza vengono visualizzate e riprodotte dall'apparecchio tramite altoparlante. Utilizzando queste applicazioni è parimenti possibile eseguire l'esperimento.L'umidità può danneggiare lo smartphone.9. Conservazione, pulizia, smaltimento• Conservare l'apparecchio in un luogo pulito, asciutto e privo di polvere.• Non impiegare detergenti o soluzioni aggressive per la pulizia.• Per la pulizia utilizzare un panno morbido e umido.•Smaltire l'imballo presso i centri di raccolta e riciclaggio locali.•Non gettare l'apparecchio nei rifiuti domestici. Rispettare le disposizioni vigenti a livello locale.。
Invitrogen EnterokinaseMax 用户手册说明书

EnterokinaseMax™ (EKMax™)iiTable of ContentsTable of Contents (iii)Important Information (iv)Methods (1)Overview (1)EKMax™ Digestion (2)Appendix (6)Recipes (6)Technical Service (7)References (8)iiiImportant InformationShipping/Storage EnterokinaseMax™ and the 10X EKMax™ reaction buffer are shipped on blue iceand should be stored at -20°C.Contents EKMax™ in 50 mM potassium phosphate, pH 8.0, 500 mM NaCl and 50%glycerol, is supplied as follows:Catalog no. E180-01:Item AmountVolume EKMax™250 units, 1 unit/µl 250µl10X EKMax™ Reaction Buffer 500 mM Tris-HCl, pH 8.0 (22°C), 10 mM CaCl2, 1% Tween-20 1.5 mlCatalog no. E180-02:Item AmountVolume EKMax™1000 units, 1 unit/µl 1.0 ml10X EKMax™ Reaction Buffer 500 mM Tris-HCl, pH 8.0 (22°C), 10 mM CaCl2, 1% Tween-20 3 x 1.5 mlAdditional Materials Needed You will need to have the following materials:• 37°C water bath or heat block• Deionized water• 1.5 ml microcentrifuge tubes• SDS-PAGE apparatus and buffers (see page 4 for guidelines) • EK-Away™ Resin and buffers (Invitrogen, Catalog no. R180-01) • 15 ml polypropylene tubes (optional)• Chromatography columns (optional, Catalog no. R640-50) • Rocker or rotatorUnit Definition 1 unit is defined as the amount of EKMax™ that will digest 20 µg of BioEase™Mog1 (multicopy suppressor of GSP1, 1)fusion protein to 90% completionin 20 minutes at 37°C in 50 mM Tris-HCl, pH 8.0, 1 mM CaCl2, and0.1% Tween-20 (1X EKMax™ Buffer).For comparison purposes, 1 Invitrogen unit of EKMax™ = ~190 trypsinogenactivation units. Use this conversion only as a rough estimate of how muchEKMax™ to use. It is important to empirically determine the optimal amount ofEKMax™ to digest your fusion protein (see page 2).Limited Label License No. 62: EKMax™Enterokinase This product is sold under patent license from Genetics Institute, Inc. for Research Use Only. Licenses for commercial manufacture or use may be obtained from Genetics Institute, Inc.ivMethodsOverviewIntroduction Enterokinase is a highly specific serine protease that can be used to digest fusion proteins to release the fusion partner or tag from the desired protein. Theenzyme recognizes the sequence -(Asp)4 Lys and cleaves after the lysine residue.This cleavage sequence is present in many expression vectors available fromInvitrogen (contact Technical Service for more information). Genes cloned intothe multiple cloning site of these vectors express recombinant N-terminal fusionproteins. The native proteins can be released from the N-terminal fusion peptideor protein by digesting with enterokinase.EnterokinaseMax™ (EKMax™) is a specially prepared recombinant enzyme,consisting of the catalytic subunit of the holoenzyme. This subunit is expressedand purified from the yeast, Pichia pastoris, yielding an enzyme with higherspecific activity. This results in more efficient cleavage using less enzyme.Description of Enterokinase Enterokinase (enteropeptidase EC 3.4.21.9) is the physiological activator of trypsinogen and a serine protease that exhibits specificity for the sequence (Asp)4 Lys (Anderson et al., 1977). The bovine holoenzyme is a heterodimer consisting of a 115 kDa structural subunit and a 35 kDa catalytic subunit. The larger subunit acts as a membrane anchor and positions the catalytic subunit on the luminal side of the brush border membrane. The catalytic subunit is homologous to other serine proteases and is inhibited by chemical modification of the serine and histidine active site residues (Grant and Hermon-Taylor, 1977; Light and Liepnieks, 1979; Maroux et al., 1971).Specificity of Enterokinase It has been proposed that the active center of enterokinase possesses a distinctive cationic subsite that binds -(Asp)4. Enterokinase is highly specific and tolerates very few changes to its recognition site. If the ionic charge of the recognition site is preserved, enterokinase will recognize the site, but the rate of hydrolysis of the peptide bond will be reduced (Light and Janska, 1989). The four aspartyl residues act as a signal for enterokinase cleavage. It has been reported that with only three aspartyl residues the rate of hydrolysis is reduced. Two aspartyl residues preceding the lysyl residue are the minimum number of acidic residues needed to maintain specificity (Maroux et al., 1971). Non-specific cleavage by enterokinase may occur in the cases described above, but this is usually alleviated by reducing the amount of enzyme used.Expression of the Recombinant Catalytic Subunit EKMax™ is a clone of the catalytic subunit of enterokinase (LaVallie et al., 1993) expressed in the yeast Pichia pastoris. EKMax™ is secreted into the medium, purified, and migrates at 43 kDa on an SDS-PAGE gel. The calculated molecular weight of the protein is 26.3 kDa, but it contains three sites for asparagine-linked glycosylation. The apparent molecular weight of 43 kDa is consistent with previous observations (LaVallie et al., 1993) and is assumed to be because ofN-linked glycosylation.1EKMax™ DigestionIntroduction You will need to have pure or partially pure fusion protein. This sectiondescribes how to digest the fusion protein with EKMax™ to cleave your proteinfrom the fusion partner. This requires setting up a series of pilot reactions todetermine empirically the best conditions for digestion. The efficiency ofcleavage of EKMax™ will differ with each fusion protein. Also, if you areaccustomed to using EK3 (Biozyme), it is still necessary to test differentconcentrations of EKMax™ to determine the right amount for complete digestion.The table below outlines the steps needed to digest your fusion protein andobtain pure protein.Stage Description1 Obtain purified fusion protein at a concentration of > 0.1 mg/ml2 Dialyze (if necessary) into 1X EKMax™ Buffer3 Set up pilot reactions using different amounts of EKMax™ and digestovernight4 Assay reactions on an SDS-PAGE gel and analyze5 Optimize digestion conditions by adjusting amount of enzyme or thetemperature as needed6 Scale-up digestions to produce more of your native protein7 Purify your native protein away from the fusion partner andEKMax™Additional Materials Needed You will need to have the following materials:• 37°C water bath or heat block• Deionized water• 1.5 ml microcentrifuge tubes• SDS-PAGE apparatus and buffers (see page 4 for guidelines) • EK-Away™ Resin and buffers (Invitrogen, Catalog no. R180-01) • 15 ml snap-cap polypropylene tubes (optional)•Chromatography columns (optional, Catalog no. R640-50)• Rocker or rotatorImportantWe have found that EKMax™ will digest fusion proteins bound to theirrespective affinity columns, releasing the desired protein and leaving the fusionpartner bound to the column. For methods to digest Xpress™ fusions bound toProBond™ and thioredoxin fusion proteins in situ on ThioBond™, contactTechnical Service (see page 7).continued on next page2Obtain Purified Fusion Protein Purify at least 120 µg of your fusion protein using your system of choice following the manufacturer's instructions.• To purify thioredoxin fusion proteins, refer to the ThioBond™ manual included with the ThioBond™ resin or contact Invitrogen for information. • To purify Xpress™ fusion proteins, refer to the ProBond™ Purification System manual or contact Invitrogen for informationNote: Both manuals may be downloaded from .EKMax™ will digest fusion proteins in crude cell lysates. Note however, that you will lose your fusion tag and will have to develop a separate protocol for purification of your protein.Dialysis of the Fusion Protein It may be necessary to dialyze your fusion protein against 1X EKMax™ buffer (see Recipes, page 6) before digesting it with EKMax™. EKMax™ is inhibited by high ionic strength. 250 mM NaCl reduces EKMax™ activity to 75% of normal and 2 M NaCl almost completely inhibits enzyme activity (Barratti et al., 1973). Also, EKMax™ is known to be inhibited by > 2 M urea, > 20 mMβ-mercaptoethanol (β-ME), >0.1% SDS, > 50 mM imidazole, and pH values below 6 and above 9.Use the table below to determine if you need to dialyze your fusion protein prior to digestion with EKMax™.If your purified protein ....Then ......contains > 2 M urea, > 250 mM NaCl,> 20 mM -ME, 0.1% SDS, or > 50 mMimidazoledialyze to remove the inhibitorsis in a buffer where the pH is lowerthan 6 or higher than 9dialyze to adjust the pH to between 6and 9.is free from inhibitors and the pH isbetween 6 and 9do not dialyze. Proceed straight toPreparation of Pilot Reactions, below.Recommendation If you are not sure whether you should dialyze your protein or not, dialyze asmall volume of your protein solution and test both dialyzed and undialyzedsamples in a pilot EKMax™ digestion.continued on next page3Preparation of Pilot Reactions You will need at least 120 µg of your fusion protein for the pilot reactions. The ratio of enzyme to fusion protein to achieve complete digestion may vary depending on the protein expressed. It is very important to use only the minimal amount of EKMax™ necessary to completely digest the fusion protein. Excess EKMax™ may cause non-specific cleavage of your fusion protein in some cases.1. To determine the optimal units of EKMax™ needed for complete digestion,use five different amounts of EKMax™ (4 units, 1 unit, 0.1 unit, 0.01 unit,and 0.001 unit). For 4 units of EKMax™, use 4 µl of undiluted EKMax™. Use 1X EKMax™ buffer to make serial 10-fold dilutions of the enzyme.2. Set up 6 reactions, including a reaction without EKMax™ to control forproteases in your protein solution:Fusion Protein 20 µg10X EKMax™ Buffer 3 µlEKMax™1-4 µlDeionized Water to 29 µl(use 30 µl for the no EKMax™ control)Final Volume 30 µl3. Mix well and incubate at 37°C overnight (~16 hours). If your protein isunstable and degrades at 37°C, try incubation at 22°C, 16°C, or +4°C. It is not necessary to increase the time of digestion to compensate for the decrease in temperature.4. Prepare and load 1-20 µl on an SDS-PAGE gel (see next section). For westernblotting, load ~1 µg of your fusion protein, and for Coomassie-stained gels, load ~10 µg.If you wish to digest your fusion protein in a crude lysate, be sure to dialyze toremove any inhibitors of EKMax™. Set up your pilot reactions as described aboveto determine the amount of EKMax™ needed to digest your fusion protein.SDS-PAGEAnalysisUse an SDS-PAGE gel that will allow you to differentiate between undigested anddigested fusion protein. The type of gel and concentration of acrylamide dependson the size of your fusion protein and the fusion partner. Some fusion partners willbe too small to resolve conveniently (i.e. the Xpress™ tag). You should be able todistinguish the removal of the fusion partner from your protein, either byCoomassie-staining or by using antibody detection methods. Choose the dilutionof EKMax™ that gives you complete digestion of your recombinant fusion protein.Recommendationfor ThioredoxinFusion ProteinsA Tricine gradient gel may be necessary to visualize the 14.6 kDa thioredoxinfusion partner (Schagger and von Jagow, 1987). Alternatively, a western blot usingthe Anti-Thio™ Antibody (Catalog no. R920-25) may be used to detect theaccumulation of the thioredoxin fusion partner and/or subsequent loss of signalfrom the native protein.continued on next page4Recommendation for Xpress™Fusion Proteins Since the Xpress™ peptide is less than 4 kDa, a shift in the size of your protein may be undetectable. A western blot using the Anti-Xpress™ Antibody (Catalog no.R910-25) or the Anti-Xpress™-HRP Antibody (Catalog no. R911-25) may be necessary to visualize the cleavage and removal of the Xpress™ fusion peptide from fusion proteins. Perform a western blot and look for the loss of the signal from your protein. The Xpress™ peptide is so small, it may not transfer well to nitrocellulose or nylon, making it difficult to detect.Optimizing EKMax™ Cleavage In some cases, increasing the calcium chloride concentration to 10 mM or the amount of Tween-20 to 1% in the digestion reaction increases the activity of EKMax™. There is a possibility that EKMax™ may recognize sites that are similar to its recognition site; however, the rate of hydrolysis will be reduced. This is usually alleviated by decreasing the amount of EKMax™.Scale-Up of EKMax™ Reaction After you have optimized the EKMax™ reaction, you may scale up your digestion reaction in a linear manner. You may need a concentrated solution of fusion protein in order to scale up your digestion. Use standard ultrafiltration methods to concentrate your protein solution.Removal of EKMax™After digestion, EKMax™ may be removed by affinity chromatography on soybean trypsin inhibitor (STI) resin. For easy removal of EKMax™, EK-Away™Resin (Catalog no. R180-01) is available from Invitrogen. EK-Away™ Resin consists of soybean trypsin inhibitor immobilized on 4% beaded agarose. For a protocol to use EK-Away™ Resin, refer to the EK-Away™ manual. The manual is available at or contacting Technical Service (see page 7).Removal of Fusion Partners Fusion partners may be removed by the same affinity resin as was used to purify the fusion protein. For protocols to remove the Xpress™ tag or the thioredoxin fusion partner after EKMax™ digestion, contact Technical Service (see page 7). For other fusion proteins, consult the manufacturer of your particular system.5Appendix Recipes10X EKMax™Reaction Buffer 500 mM Tris-HCl, pH 8.010 mM CaCl21% Tween-20 (v/v)1. For 1 liter, dissolve 60.5 g Tris base in 950 ml deionized water.2. Adjust pH to 8.0 with concentrated HCl.3. Add 1.47 g CaCl2-2H2O and 10 ml Tween-20 and mix.4. Adjust the volume to 1 liter. Store at room temperature.6Technical ServiceVisit the Invitrogen website at for:• Technical resources, including manuals, vector maps and sequences,application notes, MSDSs, FAQs, formulations, citations, handbooks, etc.• Complete technical service contact information• Access to the Invitrogen Online Catalog• Additional product information and special offersContact Us For more information or technical assistance, call, write, fax, or email. Additional international offices are listed on our website (). Corporate Headquarters:Invitrogen Corporation1600 Faraday AvenueCarlsbad, CA 92008 USATel:176****7200Tel(TollFree):180****6288Fax:176****6500E-mail: ***************************Japanese Headquarters:Invitrogen JapanLOOP-X Bldg. 6F3-9-15, KaiganMinato-ku, Tokyo 108-0022Tel: 81 3 5730 6509Fax: 81 3 5730 6519E-mail: *********************European Headquarters:Invitrogen LtdInchinnan Business Park3 Fountain DrivePaisley PA4 9RF, UKTel: +44 (0) 141 814 6100Tech Fax: +44 (0) 141 814 6117E-mail: ***********************MSDS MSDSs (Material Safety Data Sheets) are available on our website at/msds.Limited Warranty Invitrogen is committed to providing our customers with high-quality goods and services. Our goal is to ensure that every customer is 100% satisfied with ourproducts and our service. If you should have any questions or concerns about anInvitrogen product or service, contact our Technical Service Representatives.Invitrogen warrants that all of its products will perform according tospecifications stated on the certificate of analysis. The company will replace, freeof charge, any product that does not meet those specifications. This warrantylimits Invitrogen Corporation’s liability only to the cost of the product. Nowarranty is granted for products beyond their listed expiration date. No warrantyis applicable unless all product components are stored in accordance withinstructions. Invitrogen reserves the right to select the method(s) used to analyze aproduct unless Invitrogen agrees to a specified method in writing prior toacceptance of the order.Invitrogen makes every effort to ensure the accuracy of its publications, butrealizes that the occasional typographical or other error is inevitable. ThereforeInvitrogen makes no warranty of any kind regarding the contents of anypublications or documentation. If you discover an error in any of our publications,please report it to our Technical Service Representatives.Invitrogen assumes no responsibility or liability for any special, incidental,indirect or consequential loss or damage whatsoever. The above limitedwarranty is sole and exclusive. No other warranty is made, whether expressedor implied, including any warranty of merchantability or fitness for a particularpurpose.continued on next pageReferencesAnderson, L. E., Walsh, K. A., and Neurath, H. (1977). Bovine Enterokinase. Purification, Specificity, and Some Molecular Properties. Biochemistry 16, 3354-3360.Barratti, J., Maroux, S., and Louvard, D. (1973). Effect of Ionic Strength and Calcium Ions on the Activation of Trypsinogen by Enterokinase. A Modified Test for the Quantitative Evaluation of the Enzyme. Biochem. Biophys. ACTA 321, 632-638.Grant, D. A. W., and Hermon-Taylor, J. (1977). Hydrolysis of Artificial Substrates by Enterokinase and Trypsin and the Development of a Sensitive Specific Assay for Enterokinase in Serum. Biochem J. 155, 243-254.LaVallie, E. R., Rehemtulla, A., Racie, L. A., Diblasio, E. A., Ferenz, C., Grant, K. L., Light, A., and McCoy, J. M. (1993). Cloning and Functional Expression of a cDNA Encoding the Catalytic Subunit of Bovine Enterokinase. J. Biol. Chem. 268, 23311-23317.Light, A., and Janska, H. (1989). Enterokinase (enteropeptidase): Comparative Aspects. TIBS 14, 110-112. Light, A., and Liepnieks, J. J. (1979). The Preparation and Purification of Bovine Enterokinase. J. Biol.Chem. 254, 1677-1683.Maroux, S., Baratti, J., and Desnuelle, P. (1971). Purification and Specificity of Porcine Enterokinase. J.Biol. Chem. 246, 5031-5039.Schagger, H., and von Jagow, G. (1987). Tricine-Sodium dodecyl sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 kDa. Anal. Biochem. 166, 368-379.©1998-2006 Invitrogen Corporation. All rights reserved.For research use only. Not intended for any animal or human therapeutic or diagnostic use.。
注射用盐酸头孢甲肟
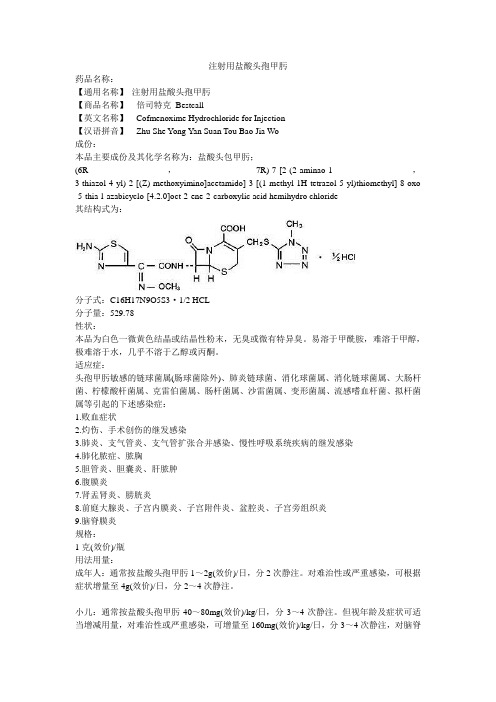
(2)老年者有时出现维生素K缺乏而出血的倾向。
药物相互作用:
0.注意并用(并用时应加以注意)
药理毒理:
1、抗菌作用
(1)对革兰氏阴性和革兰氏阳性的需氧菌及厌氧菌具有广泛的抗菌作用,且其作用是杀菌性的。
(2)对革兰氏阴性菌的抗菌力,以大肠杆菌和肺炎杆菌而论,稍强于头孢替安(CTM),远强于头孢唑啉(CEZ)。此外,对流感杆菌、变形杆菌属、粘质沙雷氏杆菌、枸橼酸杆菌属、肠道菌属的抗菌力也比CTM强,远比CEZ强。另外对似杆菌属也显示有强抗菌力。
1、严重的副作用
1)有时引起休克(不足0.1%),故要仔细观察,若出现感觉不适,口内异常感、喘鸣、眩晕、排便感、耳鸣、出汗等异常症状时,应停止给药,并进行适当处理,
2)偶有急性肾功能不全等严重肾功能障碍(不足0.1%)发生,故要定期检查肾功能,仔细观察,若异常时,要停止给药,并进行适当处理。
3)有时出现粒细胞减少(不足0.1~5%)或无粒细胞症(不足0.1%),另外,其他头孢类抗生素有引起溶血性贫血的报告,出现异常时,应停止给药,并进行适当处理。
对小儿也可考虑上述给药量,将1次用量加于补液内,在30分钟~1小时,进行静脉滴注。
注射液的配制方法:
静注用倍司特克R的助溶剂为无水碳酸钠,在溶解时有二氧化碳产生,故本品装于负压瓶内。
静注用1g时注入约5ml溶解液于瓶内溶解。(静注用1g点滴用的瓶内可注入约100ml的溶解液。)此外,静注时,静注用1g稀释为20ml给药。静脉点滴时不可使用注射用水溶解,因溶解后的溶液不等渗。
(6R,7R)-7-[2-(2-aminao-1,3-thiazol-4-yl)-2-[(Z)-methoxyimino]acetamido]-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-8-oxo-5-thia-l-azabicyclo-[4.2.0]oct-2-ene-2-carboxylic acid hemihydro chloride
VITEK MS PRIME 产品说明书

©2022 bioMérieux, Inc.• BIOMÉRIEUX, the BIOMÉRIEUX logo, CLARION, FLEXPREP, MYLA, PICKME, AND VITEK are used pending and/or registered trademarks belonging to bioMérieux, or one of its subsidiaries, or one of its companies • Patents: ///patents • PRN 059452 Rev01.A
4
URGENT SLIDE PRIORITIZATION
• Prioritize critical samples in one click • Analysis automatically resumes after critical slide completion
5
FLEXIBLE RESULTS REVIEW
AT MULTIPLE BENCHES
• Automatically release high confidence results
• Easily view spot images for quick troubleshooting
6
AUTOMATED FINE-TUNING
• Increase uptime by initiating automated fine-tuning when instrument
is not in use
• Hands-free maintenance to free up technician time
1. Perrot N, Dauwalder O, Paris C, et al. New innovative tool for easy colony picking and sample preparation for MALDI-TOF. Poster presented at: ASM Microbe 2019; June 20-24, 2019; San Francisco, CA.
QUICK-Clone cDNA用户手册说明书

United States/Canada 800.662.2566Asia Pacific+1.650.919.7300Europe+33.(0)1.3904.6880 Japan+81.(0)77.543.6116 Clontech Laboratories, Inc.A T akara Bio Company 1290 T erra Bella Ave. Mountain View, CA 94043 Technical Support (US)E-mail:***************** QUICK-Clone TM cDNA User ManualPT1150-1 (PR752268)Published 25 May 2007QUICK-Clone™ cDNA User ManualT able of ContentsI. Introduction3 II. Applications Discussion4A. Primer Design 4B. Setting up the PCR Reaction 4C. Example of a cDNA Amplification Reaction 5D. Post-Amplification Procedures 6E. Troubleshooting 6 III. References8Notice to PurchaserClontech products are to be used for research purposes only. T hey may not be used for any other purpose, including, but not limited to, use in drugs, in vitro diagnostic purposes, therapeutics, or in humans. Clontech products may not be transferred to third parties, resold, modified for resale, or used to manufacture commercial products or to provide a service to third parties without written approval of Clontech Laboratories, Inc.TaqStart® Antibody and other Hot Start Antibodies are licensed under U.S. Patent No. 5,338,671.U.S. Patent No. 5,436,149 for LA T echnology is owned by T AKARA BIO INCClontech, the Clontech logo and all other trademarks are the property of Clontech Laboratories, Inc. unless noted otherwise. Clontech is a T akara Bio Company. ©2007Clontech Laboratories, Inc. Protocol No. PT1150-1 Version No. PR75 68QUICK-Clone™ cDNA User ManualI. IntroductionCloning of cDNA by PCR is a valuable tool for isolating individual cDNAs. Amplification of cDNA by PCR can often circumvent labor-intensive cDNA library c onstruction a nd s creening p rocedures. P CR c loning m akes u se o f k nown regions of sequence from previously characterized cDNAs or genes to design PCR primers, either unique or degenerate, which are then used to amplify new cDNA segments. PCR-amplified cDNA fragments, generated using degenerate primers based on amino acid sequence data, can also be used to make hybridization probes to screen conventional cDNA or genomic libraries. At Clontech, we have found that amplification of specific gene transcripts from purified double-stranded cDNA yields fewer non-specific amplification products than when performing PCR on single-stranded cDNA. For this reason, we have developed QUICK-Clone™ cDNA, a highly purified form of double-stranded cDNA ready for PCR amplification. Our cDNA is synthesized from our Premium Poly A+ RNA using an oligo(dT) primer and purified to remove interfering RNA and genomic DNA.Human Universal QUICK-Clone cDNA II (Cat.No. 637260) is an optimized mixture of >30 QUICK-Clone cDNAs from normal human tissues. (The exact number may vary based on tissue availability. Please see the Certificate of Analysis for a list of tissues.) It has been specially formulated for the amplification of full-length cDNAs that represent the majority of human genes.Protocol No. PT1150-1 C lontech Laboratories, Inc. Version No. PR75 68QUICK-Clone™ cDNA User ManualII. Applications DiscussionThis section discusses the amplification of QUICK-Clone cDNA and describes several useful post-amplification options.A. Primer DesignPrimer design is the single largest variable in PCR applications and the single most important factor in determining the success or failure of PCR reactions. Always check and recheck your primer design before constructing or ordering primers. Visit / on the web for helpful guidelines on primer design.Length and G-C content: In general, primers should have a Tm ofapproximately 70°C to achieve optimal results in a two-step cycling program with a 68°C annealing/extension step. Therefore, whenever possible, primers should be at least 22 nucleotides (nt) long (25–30-mers are preferred) and should have a G-C content of 45–60%. Furthermore, the 3'-terminal ends of each primer should not be complementary to each other and should possess a low G-C content.B. Setting up the PCR Reaction1. cDNAThe quantity of cDNA template to use in the reaction is related to:(a) the abundance of the target mRNA relative to the entire mRNApopulation used to synthesize the cDNA, and (b) the length of thecDNA required for the 5' primer to recognize its correspondingtemplate. For mRNAs of rare abundance, a larger amount of cDNAtemplate is required. More cDNA may also be required to amplifylong cDNA segments (e.g., greater than 2 kb).2. Primer concentrationFor unique sequence primers of lengths between 25–30 nucleotides,we use a final primer concentration of between 0.2 and 0.5 µM. T heoptimum amount of primers in the PCR reaction depends on theirmolar concentrations and not simply their masses. If the primershave similar lengths and G-C content, optimal amplification willoccur when the molar concentrations of both primers are equal.Unfortunately, there are no general rules for determining the optimalconcentration for degenerate primers, although concentrations aregenerally higher than when unique sequence primers are used.Clontech Laboratories, Inc. Protocol No. PT1150-1 Version No. PR75 68QUICK-Clone™ cDNA User Manual II. Applications Discussion continued3. Choice of thermostable polymeraseFor high-fidelity and efficient amplification of long genesegments (>1 kb), we recommend our Advantage2 PolymeraseMix (Cat. Nos. 639201 & 639202) and an automatic hot start thatreduces nonspecific products. For high yields of products <2kb, we recommend Clontech’s TITANIUM Taq DNA Polymerase(Cat. Nos. 639208 & 639209). T his enzyme is easy to use and doesnot require Mg2+ optimization. T ITANIUM Taq contains our T aqStartAntibody (Cat.No. 639250) which allows hot starts for increasedspecificity and yield in every PCR experiment. See the January 2001and July 2006 issues of Clontechniques for details.4. Amplification of G-C rich regionsIn some instances, amplification of a region with a high G-Ccontent is difficult or impossible using standard PCR. For theseinstances, we recommend our Advantage-GC Kits and PolymeraseMixes. T hese products are optimized to amplify cDNA or genomicsequences with a G-C content as high as 90%. Both the kits and thepolymerase mixes contain GC-Melt, a component that destabilizesthe DNA structure while also making A-T and G-C base pairs equallystable (“isostabilization”; Advantage-GC cDNA PCR Kit (Cat. Nos.639116 & 639115) Advantage-GC cDNA Polymerase Mix (Cat No.639112), and Advantage-GC LA Genomic Polymerase Mix (Cat. No.639153).C. Example of a control cDNA Amplification Reaction using Humanβ-actin cDNA1. cDNA fragment size: 1.1 kb. Distance of 5' primer template fromthe 3' end of the cDNA: 1.8 kb.2. Primers: Unique sequence primers (β-Actin Control Amplimers,(Cat. No. 639001). 5' primer (21-mer), 52 % G-C; 3' primer (33-mer),61% G-C. Final concentration: 0.2 µM each.3. Human Universal QUICK-Clone cDNA: 500 pg.4.Cycle parameters:20–25 cyclesDenature at 94°C for 45 sec.Anneal at 60°C for 45 sec.Extend at 72°C for 2.5 min.1 cycleFinal extension at 72°C for 10 minProtocol No. PT1150-1 C lontech Laboratories, Inc. Version No. PR75 68 5QUICK-Clone™ cDNA User ManualII. Applications Discussion continuedD. Post-Amplification Procedures1. Removal of reaction componentsWe recommend removal of reaction components such as nucleotidesand primers by exclusion chromatography. T his procedure can bedone conveniently using our CHROMA SPIN Columns with gelmatrices stored in T E Buffer. CHROMA SPIN Columns are availablein sizes that remove nucleic acids smaller than 100 or 400 basepairs (CHROMA SPIN + T E-100 Columns, (Cat. Nos. 636072, 636073)CHROMA SPIN + TE-400, (Cat. No. 636076). The PCR product orreaction mixture (40–45 µl) can be loaded directly onto the pre-spun column with no prior manipulations.2. Cloning amplified cDNASeveral procedures exist for subcloning PCR products. Werecommend In-Fusion™2.0 PCR Cloning Kits (Cat. Nos. 639609,639607 & 639608). Cloning using the In-Fusion 2.0 method does notrequire PCR product purification and requires simple extensionsto your PCR primers. In-Fusion is the easy, precise solution formaking expression clones.E. T roubleshootingFor researchers without extensive experience with PCR, we stronglyrecommend the use of β-actin or G3PDH control primers as a positivecontrol in your first few experiments. We offer several amplimersets specific for human β-actin (Cat No. 639001), mouse ß-actin(Cat. No. 639008), and human G3PDH (Cat. No. 639005).Clontech Laboratories, Inc. Protocol No. PT1150-1 6 Version No. PR75 68QUICK-Clone™ cDNA User Manual II. Applications Discussion continued1. Perfect sequence primersWhen mixing perfect sequence primers, inefficient or failedamplification can result from using old reagents, such as olddNTPs or stale reaction buffers. The incidence of this problemcan be reduced by storing aliquots of reagents at –20°C, and byperiodically discarding working stocks. In rare instances, the DNApolymerase may be defective. Ensure that no reagents have beenomitted from the reaction.2. Degenerate primersA number of factors may cause partial or complete failure inexperiments using degenerate primers. Determining whetherthe failure of degenerate primer experiments are due to causesassociated with perfect sequence experiments, or to causesassociated with the degenerate primers, is of utmost importance.To make this determination, you must perform PCR experimentswith perfect sequence control primers such as β-actin. If thesecontrol experiments are successful, the most likely reasons foramplification failures is that at least one of the degenerate primerscontains too many mismatches to allow stable annealing. Loweringthe annealing temperature stepwise by 2–5°C at a time may help.However, you will probably have to construct new primers.In some cases, too many PCR products are observed. T his result isusually due to the presence of unwanted, stable primer templates.Increasing the annealing temperature and/or decreasing theprimer concentrations may resolve this problem. If you still obtainunwanted products, we suggest that you utilize a hot start PCR.For this purpose, we offer T aqStart Antibody that can be added toTaq polymerase. This antibody is also included in our TITANIUMTaq and Advantage 2 Kits. If problems persist, we recommend thatyou construct alternate primers or try nested primers to increasespecificity. However, please note that in some cases, additionalbands may indicate the presence of alternately spliced transcripts(Nakabeppu & Nathans, 1991).Protocol No. PT1150-1 C lontech Laboratories, Inc. Version No. PR75 68 7QUICK-Clone™ cDNA User ManualIII. ReferencesClark, J. M. (1988) Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 16:9677-9686.C ompton, T. (1990) Degenerate Primers for DNA Amplification. PCR Protocols: A Guide to Methods and Applications (Academic Press, San Diego), pp 39-45.Girgis, S. I., et al. (1988) Generation of DNA probes for peptides with highly degenerate codons using mixed primer PCR. Nucleic Acids Res. 16:10371.Lathe, R. (1985) Synthetic oligonucleotide probes deduced from amino acid data: theoretical and practical considerations. J. Mol. Biol. 183:1-12.Lee, C. C., et al. (1988) Generation of cDNA probes directed by amino acid sequence: Cloning of urate oxidase. Science239:1288.M ead, D.A. et al. (1994) Auniversal method for the direct cloning of PCR amplified nucleic acid. Bio/Technology 9:657-663.Nakabeppu, Y. & Nathans, D. (1991) A naturally occurring truncated form of Fos B that inhibits Fos/Jun transcriptional activity. Cell64: 751.Sambrook, J., & Russell, D.W. (2001) Molecular Cloning: A Laboratory Manual, (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY).Schuchman, E. H., et al. (1990) Human Arylsulfatase B: MOPAC Cloning: nucleotide sequence of a full-Length cDNA, and regions of amino acid identity with arylsulfatases A and C. Genomics6 149-158.Clontech Laboratories, Inc. Protocol No. PT1150-1 8 Version No. PR75 68。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
拜科奇说明书
【批准文号】
S2*******
【中文名称】
注射用重组人凝血因子VIII
【产品英文名称】
Recombinant Coagulation FactorVIII for Injection
【生产企业】
Bayer HealthCare AG
【功效主治】
用于血浆凝血因子VⅢ(FVⅢ)缺乏的甲型血友病治疗。
在纠正或预防出血、急诊或择期手术中,本品起到暂时代替缺失的凝血因子的作用。
【化学成分】
重组人凝血因子VIII,加入了蔗糖、甘氨酸组氨酸等。
【药理作用】
人凝血因子VIII是正常血浆的组成成分,在血液凝固过程中起着必不可少的作用。
拜科奇对纠正和预防因因子VIII缺乏而致的严重出血有疗效。
输用每公斤体重1个单位的人凝血因子VIII可使循环血液中的因子VIII水平增加2%到25%。
对血友病患者的临床研究显示因子VIII的生物半衰期在6-14小时之间。
用TNBP和Tween 80进行的有机溶剂清洁剂(SD)灭活病毒处理对人类致病病毒如HIV-1、HBV、HCV等有高度的灭活作用,同时又不影响因子VIII和其他血浆蛋白的活性。
SD处理灭活病毒的效果经用指示病毒如疱疹性口炎病毒,Sindbis病毒和乙脑病毒,以及艾滋病毒(HIV)得到验证。
【药物相互作用】
【不良反应】
罕见严重的不良反应,但有异常情况时,请与医生联系。
极少的情况下可能会出现出血、皮疹和发烧。
【禁忌症】
【产品规格】
250 IU/瓶
【用法用量】
体内FVIII水平升高的百分比可用本品剂量(IU/kg)乘以每公斤体重每个单位的2 %(2%/IU/kg)计算而得。
计算方法依据血浆FVIII和重组AHF 在临床使用的剂量:预计的FVIII升高值(%) = #注射单位
×2%/IU/kg<246>体重(kg)。
【贮藏方法】
本品需冷藏(2-8°C),遮光保存,禁止冷冻。
【注意事项】
使用本品前,如有下列情况,请告知医生:
-刚做过外科手术;
-患有挤压性损伤;
-患有血栓形成性疾病或进行性动脉粥样硬化性疾病;
-患有败血症。
重度出血者须到医院注射本药。
轻度或中度出血者可在家里注射重组人凝血因子VIIa(NovoSeven),应立即向医生或医院汇报重组人凝血因子Ⅶa的使用剂量和效果(如:电
话联系)。
必要时与血液科专家保持密切联系。
如果用药24小时后,出血未得到控制,则不能再继续在家治疗,应到医院就诊。
将本品置于远离儿童的地方。
