标准电极电势表67748
标准电极电势表 (碱)

电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02 Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0) La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88 Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0) H2PO2-+e-=P+2OH--1.82 B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I) Te+2e-=Te2--1.143 P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924 Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691 As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627 Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46 *Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12 O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0) AgCN+e-=Ag+CN--0.017 N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01 Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08 Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108 Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14 Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17 Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247 I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33 Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342 Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358 Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373 O(0)-(-II) O2+2H2O+4e-=4OH-0.401 I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485 *Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490 Mn(VII)-(VI) MnO4-+e-=MnO42-0.558 Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595 Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60 Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607 Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61 Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62 Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66 I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7 Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76 Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761 Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841 *Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95。
标准电极电势表
标准电极电势表标准电极电势可以用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极,溶液中离子有效浓度为1mol/L,气体分压为100kPa,温度为298K,所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注:(s) –固体;(l) –液体;(g) –气体;(aq) –水溶液;(Hg) –汞齐。
E°(V)[注半反应来源1](g) + 4 H2O + 2 e−2 NH2OH(aq) + 2 OH−−3.04 [6]NCs++e−Cs(s) −3.026 [5] (s) + 2 e−Ca(s) + 2 OH−−3.02 [11]Ca(OH)Rb++e−Rb(s) −2.98 [4]K++e−K(s) −2.931 [5] Mg++e−Mg(s) −2.93 [10] Ba2++ 2 e−Ba(s) −2.912 [5]La(OH)(s) + 3 e−La(s) + 3OH−−2.90 [5]Fr++ e−Fr(s) −2.9 [11]E°(V)[注半反应来源1]Sr2++ 2 e−Sr(s) −2.899 [5] (s) + 2 e−Sr(s) + 2 OH−−2.88 [11]Sr(OH)Ca2++ 2 e−Ca(s) −2.868 [5] Eu2++ 2 e−Eu(s) −2.812 [5] Ra2++ 2 e−Ra(s) −2.8 [5] Yb2++ 2 e−Yb(s) −2.76 [11][1] Na++e−Na(s) −2.71 [5][9] Sm2++ 2 e−Sm(s) −2.68 [11][1] No2++ 2 e−No(s) −2.50 [11]E°(V)[注半反应来源1](s) + H2O + 4 e−Hf(s) + 4 OH−−2.50 [11]HfO(OH)Th(OH)(s) + 4 e−Th(s) + 4 OH−−2.48 [11]Md2++ 2 e−Md(s) −2.40 [11] La3++ 3 e−La(s) −2.379 [5]Y3++ 3 e−Y(s) −2.372 [5] Mg2++ 2 e−Mg(s) −2.372 [5] (s) + H2O + 4 e−Zr(s) + 4OH−−2.36 [5]ZrO(OH)Pr3++ 3 e−Pr(s) −2.353 [11] Ce3++ 3 e−Ce(s) −2.336 [11]E°(V)[注半反应来源1]Er3++ 3 e−Er(s) −2.331 [11] Ho3++ 3 e−Ho(s) −2.33 [11]−+ 3 e−Al(s) + 4 OH−−2.33Al(OH)Al(OH)(s) + 3 e−Al(s) + 3OH−−2.31Tb3++ 3 e−Tb(s) −2.28(g) + 2 e−2 H−−2.25HAc3++ 3 e−Ac(s) −2.20Be++e−Be(s) −2.12 [10] Cf2++ 2 e−Cf(s) −2.12 [11]E°(V)[注半反应来源1]Am3++ 3 e−Am(s) −2.048 [11] Cf3++ 3 e−Cf(s) −1.94 [11] Am2++ 2 e−Am(s) −1.9 [11] Be2++ 2 e−Be(s) −1.85Rf4++ 4 e−Rf(s) −1.67 [12]U3++ 3 e−U(s) −1.66 [7]Al3++ 3 e−Al(s) −1.66 [9]Ti2++ 2 e−Ti(s) −1.63 [9]Bk2++ 2 e−Bk(s) −1.6 [11]E°(V)[注半反应来源1]ZrO(s) + 4 H++ 4 e−Zr(s) + 2 H2O −1.553 [5]Hf4++ 4 e−Hf(s) −1.55 [11] Zr4++ 4 e−Zr(s) −1.45 [5]Ti3++ 3 e−Ti(s) −1.37 [13] TiO(s) + 2 H++ 2 e−Ti(s) + H2O −1.31TiO3(s) + 2 H++ 2 e−2 TiO(s) + H2O −1.23−+ 2 e−Zn(s) + 4 OH−−1.199 [14]Zn(OH)Mn2++ 2 e−Mn(s) −1.185 [14]−+ 6 H++ 2 e−Fe(s) + 4HCN(aq) −1.16 [15]Fe(CN)E°(V)[注半反应来源1]V2++ 2 e−V(s) −1.175 [2] Te(s) + 2 e−Te2−−1.143 [2] Nb3++ 3 e−Nb(s) −1.099Sn(s) + 4 H++ 4 e−SnH4(g) −1.07 (s) + 3 e−In(s) + 3 OH−−0.99 [11]In(OH)SiO(s) + 4 H++ 4 e−Si(s) + 2 H2O −0.91(aq) + 3 H++ 3 e−B(s) + 3 H2O −0.89B(OH)Fe(OH)(s) + 2 e−Fe(s) + 2 OH−−0.89 [15]FeO3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 OH−−0.86 [15]E°(V)[注半反应来源1]TiO2++ 2 H++ 4 e−Ti(s) + H2O −0.86O+ 2 e−H2(g) + 2 OH−−0.8277 [5]2 HBi(s) + 3 H++ 3 e−BiH3−0.8 [14] Zn2++ 2 e−Zn(Hg) −0.7628 [5]Zn2++ 2 e−Zn(s) −0.7618 [5] O5(s) + 10 H++ 10 e−2 Ta(s) + 5 H2O −0.75TaCr3++ 3 e−Cr(s) −0.74[Au(CN)]−+e−Au(s) + 2 CN−−0.60Ta3++ 3 e−Ta(s) −0.6E°(V)[注半反应来源1]O + 2 e−Pb(s) + 2 OH−−0.58PbO(s) + H(s) + 2 H++ 2 e−Ti2O3(s) + H2O −0.562 TiOGa3++ 3 e−Ga(s) −0.53U4++e−U3+−0.52 [7] PO2(aq) + H++e−P(白磷[16]) + 2 H2O −0.508 [5]HHPO3(aq) + 2 H++ 2 e−H3PO2(aq) + H2O −0.499 [5]HPO3(aq) + 3 H++ 3 e−P(红磷)[16]+ 3H2O −0.454 [5]Fe2++ 2 e−Fe(s) −0.44 [9](g) + 2 H++ 2 e−HOOCCOOH(aq) −0.432 COE°(V)[注半反应来源1]Cr3++e−Cr2+−0.42Cd2++ 2 e−Cd(s) −0.40 [9] SeO32−+ 4e−+ 3H2O⇌Se + 6OH−−0.37 [17] (s) + 2 H++ 2 e−GeO(s) + H2O −0.37GeOO(s) + H2O + 2 e−2 Cu(s) + 2 OH−−0.360 [5]CuPbSO(s) + 2 e−Pb(s) + SO42−−0.3588 [5]PbSO(s) + 2 e−Pb(Hg) + SO42−−0.3505 [5]Eu3++e−Eu2+−0.35 [7]In3++ 3 e−In(s) −0.34 [2]E°(V)[注半反应来源1]Tl++e−Tl(s) −0.34 [2] Ge(s) + 4 H++ 4 e−GeH4(g) −0.29Co2++ 2 e−Co(s) −0.28 [5] PO4(aq) + 2 H++ 2 e−H3PO3(aq) + H2O −0.276 [5]HV3++e−V2+−0.26 [9]Ni2++ 2 e−Ni(s) −0.25As(s) + 3 H++ 3 e−AsH3(g) −0.23 [2] AgI(s) + e−Ag(s) + I−−0.15224[14] MoO(s) + 4 H++ 4 e−Mo(s) + 2 H2O −0.15E°(V)[注半反应来源1]Si(s) + 4 H++ 4 e−SiH4(g) −0.14Sn2++ 2 e−Sn(s) −0.13(g) + H++e−HO2•(aq) −0.13OPb2++ 2 e−Pb(s) −0.13 [9] WO(s) + 4 H++ 4 e−W(s) + 2 H2O −0.12P(红磷) + 3 H++ 3 e−PH3(g) −0.111 [5] CO(g) + 2 H++ 2 e−HCOOH(aq) −0.11Se(s) + 2 H++ 2 e−H2Se(g) −0.11E°(V)[注半反应来源1]CO(g) + 2 H++ 2 e−CO(g) + H2O −0.11SnO(s) + 2 H++ 2 e−Sn(s) + H2O −0.10 (s) + 2 H++ 2 e−SnO(s) + H2O −0.09SnO(aq) + 6 H++ 6 e−W(s) + 3 H2O −0.09 [2]WOP(白磷) + 3 H++ 3 e−PH3(g) −0.063 [5]Fe3++ 3 e−Fe(s) −0.04 [15] HCOOH(aq) + 2 H++ 2 e−HCHO(aq) + H2O −0.032 H++ 2 e−H2(g)0.00 ≡0 AgBr(s) + e−Ag(s) + Br−+0.07133[14]E°(V)[注半反应来源1]O62−+ 2 e−2 S2O32−+0.08S4FeO4(s) + 8 H++ 8 e−3 Fe(s) + 4 H2O +0.085 [8]N(g) + 2 H2O + 6H++ 6 e−2 NH4OH(aq) +0.092O + 2 e−Hg(l) + 2 OH−+0.0977HgO(s) + H)42++e−Cu(NH3)2++ 2 NH3+0.10 [2]Cu(NH)63++e−Ru(NH3)62++0.10 [7]Ru(NHH4(aq) + 4 H2O + 2 e−2 NH4++ 4 OH−+0.11 [6]NMoO4(aq) + 6 H++ 6 e−Mo(s) + 4 H2O +0.11HGe4++ 4 e−Ge(s) +0.12半反应E ° (V)[注1] 来源C(s ) + 4 H+ + 4 e − CH 4(g ) +0.13 [2] HCHO(aq ) + 2 H+ + 2 e − CH 3OH(aq ) +0.13 S(s ) + 2 H+ + 2 e − H 2S(g ) +0.14 Sn4+ + 2 e − Sn 2+ +0.15 Cu2+ + e − Cu + +0.159 [2] HSO4− + 3 H + + 2 e − SO 2(aq ) + 2 H 2O +0.16 UO22+ + e − UO 2+ +0.163 [7] SO42− + 4 H + + 2 e − SO 2(aq ) + 2 H 2O +0.17 TiO2+ + 2 H + + e − Ti 3+ + H 2O +0.19E°(V)[注半反应来源1]Bi3++ 2e−Bi++0.2SbO++ 2 H++ 3 e−Sb(s) + H2O +0.20AgCl(s) + e−Ag(s) + Cl−+0.22233[14] AsO3(aq) + 3 H++ 3 e−As(s) + 3 H2O +0.24HGeO(s) + 2 H++ 2 e−Ge(s) + H2O +0.26+ 4 H++e−U4++ 2 H2O +0.273 [7]UO+e−2 At-+0.3 [11]AtRe3++ 3 e−Re(s) +0.300Bi3++ 3 e−Bi(s) +0.32E°(V)[注半反应来源1]VO2++ 2 H++e−V3++ H2O +0.34Cu2++ 2 e−Cu(s) +0.340 [2] ]3−+e−[Fe(CN)6]4−+0.36[Fe(CN)Tc2++ 2 e−Tc(s) +0.40 [11](g) + 2 H2O + 4 e−4 OH−(aq) +0.40 [9]OHMoO4+ 6 H++ 3 e−Mo3++ 2 H2O +0.43Ru2++ 2 e−Ru(s) +0.455 [11]Bi++e−Bi(s) +0.50CHOH(aq) + 2 H++ 2 e−CH4(g) + H2O +0.50半反应E ° (V)[注1] 来源SO2(aq ) + 4 H + + 4 e − S(s ) + 2 H 2O +0.50 Cu+ + e − Cu(s ) +0.520 [2] CO(g ) + 2 H+ + 2 e − C(s ) + H 2O +0.52 I3− + 2 e − 3 I − +0.53 [9] I2(s ) + 2 e − 2 I − +0.54 [9][AuI4]− + 3 e − Au(s ) + 4 I − +0.56 H3AsO 4(aq ) + 2 H + + 2 e − H 3AsO 3(aq ) + H 2O +0.56[AuI2]− + e − Au(s ) + 2 I − +0.58 MnO4− + 2 H 2O + 3 e − MnO 2(s ) + 4 OH − +0.59半反应E°(V)[注1]来源Rh++e−Rh(s) +0.600 [11]S2O32 −+ 6 H++ 4 e−2 S(s) + 3 H2O +0.60Fc++ e−Fc(s) +0.641 [18]+ e−Ag +−+0.643 [11]H2MoO4(aq) + 2 H++ 2 e−MoO2(s) + 2 H2O +0.65+ 2 H++ 2 e−+0.6992 [14] O2(g) + 2 H++ 2 e−H2O2(aq) +0.70Tl3++ 3 e−Tl(s) +0.72半反应E ° (V)[注1] 来源PtCl62− + 2 e − PtCl 42− + 2 Cl − +0.726 [7] H2SeO 3(aq ) + 4 H + + 4 e − Se(s ) + 3 H 2O +0.74 Rh3+ + 3 e − Rh(s ) +0.758 [11] PtCl42− + 2 e − Pt(s ) + 4 Cl − +0.758 [7] Fe3+ + e − Fe 2+ +0.77 Ag+ + e − Ag(s ) +0.7996 [5] Hg22+ + 2 e − 2 Hg(l ) +0.80 NO3−(aq ) + 2 H + + e − NO 2(g ) + H 2O +0.80 FeO42− + 5 H 2O + 6 e − Fe 2O 3(s ) + 10 OH − +0.81 [15]半反应E ° (V)[注1] 来源H2(g ) + 2 OH − 2 H 2O + 2 e − +0.828 [19][AuBr4]− + 3 e − Au(s ) + 4 Br − +0.85 Hg2+ + 2 e − Hg(l ) +0.85 MnO4− + H + + e − HMnO 4− +0.90 2 Hg2+ + 2 e − Hg 22+ +0.91 [2] Pd2+ + 2 e − Pd(s ) +0.915 [7][AuCl4]− + 3 e − Au(s ) + 4 Cl − +0.93 MnO2(s ) + 4 H + + e − Mn 3+ + 2 H 2O +0.95[AuBr2]− + e − Au(s ) + 2 Br − +0.96半反应E ° (V)[注1] 来源[HXeO6]3− + 2 H 2O + 2 e − +[HXeO 4]− + 4 OH − +0.99 [20]HNO 2 + H + + e - = NO (g) + H2O +0.996 H6TeO 6(aq ) + 2 H + + 2 e − TeO 2(s ) + 4 H 2O +1.02 [21] Br2(l ) + 2 e − 2 Br − +1.07 Br2(aq ) + 2 e − 2 Br − +1.09 [9] NO 2(g) + H + + e - = HNO 2 +1.093 IO3− + 5 H + + 4 e − HIO(aq ) + 2 H 2O +1.13[AuCl2]− + e − Au(s ) + 2 Cl − +1.15半反应E ° (V)[注1] 来源HSeO4− + 3 H + + 2 e − H 2SeO 3(aq ) + H 2O +1.15 Ir3+ + 3 e − Ir(s ) +1.156 [11] Ag2O(s ) + 2 H + + 2 e − 2 Ag(s ) + H 2O +1.17 ClO3− + 2 H + + e − ClO 2(g ) + H 2O +1.18[HXeO6]3− + 5 H 2O + 8 e − Xe(g ) + 11 OH − +1.18 [20] Pt2+ + 2 e − Pt(s ) +1.188 [7] ClO2(g ) + H + + e − HClO 2(aq ) +1.19 2 IO3− + 12 H + + 10 e − I 2(s ) + 6 H 2O +1.20 ClO4− + 2 H + + 2 e − ClO 3− + H 2O +1.20E°(V)[注半反应来源1](g) + 4 H++ 4 e−2 H2O+1.229 [9]O(s) + 4 H++ 2 e−Mn2++ 2H2O +1.23MnO[HXeO]−+ 3 H2O + 6 e−Xe(g) + 7 OH−+1.24 [20]Tl3++ 2 e−Tl++1.25O72 −+ 14 H++ 6 e−2 Cr3++ 7 H2O +1.33Cr(g) + 2 e−2 Cl−+1.36 [9]Cl(s) + 4 H++e−Co3++ 2 H2O +1.42CoO2 NHOH++ H++ 2 e−N2H5++ 2 H2O +1.42 [6]2 HIO(aq) + 2 H++ 2 e−I2(s) + 2 H2O +1.44半反应E ° (V)[注1] 来源Ce4+ + e − Ce 3+ +1.44 BrO3− + 5 H + + 4 e − HBrO(aq ) + 2 H 2O +1.45 β-PbO2(s ) + 4 H + + 2 e − Pb 2+ + 2 H 2O +1.460 [2] α-PbO2(s ) + 4 H + + 2 e − Pb 2+ + 2 H 2O +1.468 [2] 2 BrO3− + 12 H + + 10 e − Br 2(l ) + 6 H 2O +1.48 2ClO3− + 12 H + + 10 e − Cl 2(g ) + 6 H 2O +1.49 HO2 + H + + e − H 2O 2 +1.495 [11] MnO4− + 8 H + + 5 e − Mn 2+ + 4 H 2O +1.51 HO2• + H + + e − H 2O 2(aq ) +1.51半反应E ° (V)[注1] 来源Au3+ + 3 e − Au(s ) +1.52 NiO2(s ) + 4 H + + 2 e − Ni 2+ + 2 OH − +1.59 2 HClO(aq ) + 2 H+ + 2 e − Cl 2(g ) + 2 H 2O +1.63 Ag2O 3(s ) + 6 H + + 4 e − 2 Ag + + 3 H 2O +1.67 HClO2(aq ) + 2 H + + 2 e − HClO(aq ) + H 2O +1.67 Pb4+ + 2 e − Pb 2+ +1.69 [2] MnO4− + 4 H + + 3 e − MnO 2(s ) + 2 H 2O +1.70 AgO(s ) + 2 H+ + e − Ag + + H 2O +1.77半反应E ° (V)[注1] 来源 H2O 2(aq ) + 2 H + + 2 e − 2 H 2O +1.776 Co3+ + e − Co 2+ +1.82 Au+ + e − Au(s ) +1.83 [2] BrO4− + 2 H + + 2 e − BrO 3− + H 2O +1.85 Ag2+ + e − Ag + +1.98 [2] S2O 82− + 2 e − 2 SO 42− +2.07 O3(g ) + 2 H + + 2 e − O 2(g ) + H 2O +2.075 [7] HMnO4− + 3 H + + 2 e − MnO 2(s ) + 2 H 2O +2.09 XeO3(aq ) + 6 H + + 6 e − Xe(g ) + 3 H 2O +2.12 [20]半反应E ° (V)[注1] 来源H4XeO 6(aq ) + 8 H + + 8 e − Xe(g ) + 6 H 2O +2.18 [20] FeO42− + 3 e − + 8 H + Fe 3+ + 4 H 2O +2.20 [22] XeF2(aq ) + 2 H + + 2 e − Xe(g ) + 2HF(aq ) +2.32 [20] H4XeO 6(aq ) + 2 H + + 2 e − XeO 3(aq ) + H 2O +2.42 [20] F2(g ) + 2 e − 2 F − +2.87 [2][9] F2(g ) + 2 H + + 2 e − 2 HF(aq ) +3.05 [2] Tb4+ + e − Tb 3+ +3.05 [11]Welcome To Download !!!欢迎您的下载,资料仅供参考!。
标准电极电势表

标准电极电势表标准电极电势表,又称为标准还原电极电势表,是用于测量化学反应中电极对的电势的参考表。
它由国际电化学会(IUPAC)制定,用于比较不同电极对的电势大小。
标准电极电势是指在标准状态下,即温度为298K、气体为1标准大气压、物质浓度为1mol/L的情况下,电极对所具有的电势差。
标准电极电势表中,以氢电极为参考电极,将其电势定义为0V,其他电极对的电势相对于氢电极进行了测量。
标准电极电势表是按照电势从高到低排列的。
表中的电势值表示了不同物质的氧化还原能力,具有负值的电极对可以进行自发的氧化还原反应,而具有正值的电极对则不会进行自发的氧化还原反应。
标准电极电势表中常见的电极对包括:1. 氢电极(H^+ / H2):作为参考电极,其电势为0V。
2. 铂电极(Pt / Pt2+):作为惰性电极常常出现在其他电极对中。
3. 银电极(Ag+ / Ag):电势为0.7996V。
4. 铜电极(Cu2+ / Cu):电势为0.337V。
5. 铁电极(Fe2+ / Fe):电势为-0.440V。
6. 锌电极(Zn2+ / Zn):电势为-0.764V。
标准电极电势表可以用于计算化学反应的电动势。
根据标准电极电势表,当一个电极对的电势大于0时,反应是非自发的,需要提供外部电能才能进行。
当一个电极对的电势小于0时,反应是自发的,可以自发地进行。
标准电极电势表对于研究化学反应的方向性和电动势的计算非常重要。
它在化学工业生产、化学分析和电化学领域都有广泛的应用。
通过标准电极电势表,我们可以更好地了解化学反应的本质,并且对于电化学能量转化和储存等方面的研究也起到了重要的参考作用。
标准电极电势表

标准电极电势表1. 引言标准电极电势表是一种常用的参考工具,它用于比较不同化学电池或半电池的电势。
通过测量电池的电势,可以了解到其发生的化学反应过程以及反应的强弱。
标准电极电势表采用了一种标准电极作为参照,以确定其他电极的电势。
2. 标准电极标准电极是指在标准条件下,具有已知电势的电极。
根据国际电化学学会(IUPAC)的定义,标准电极是指与一个摩尔浓度为1的气体(气态物质)或摩尔浓度为1mol/L的溶液(溶解物质)接触的电极。
根据库仑定律,电势与物质浓度之间存在一定的关系。
通过实验测量得到的不同电极的电势与标准氢电极(SHE)的电势之间的差值,被称为标准电势。
标准电极电势表以标准氢电极为参照,给出了一系列常见电极的标准电势值。
3. 标准电势表的组成标准电极电势表由两列构成,分别为电极和标准电势。
电极列列出了不同电极的名称,而标准电势列则列出了对应电极的标准电势值。
常见的标准电极电势表包括了一系列重要的电极,如标准氢电极、标准铜电极、标准锌电极等。
下表为一个常见的标准电极电势表的示例:电极标准电势 (V)标准氢电极0标准锂电极-3.04标准铅电极-0.13标准铜电极+0.34标准银电极+0.80标准铂电极+0.99标准金电极+1.504. 应用与意义标准电极电势表在化学和电化学研究中具有重要的应用和意义。
它可以用于确定电池的电势以及反应的方向和驱动力。
根据标准电极电势表中的电势值,可以将不同电极的电势进行比较,从而判断反应的强弱。
当两个电极之间的标准电势差大于零时,表示反应是自发进行的,当电势差小于零时,则需要外加电势来推动反应。
此外,标准电极电势表还可以用于确定化学反应的电子转移过程。
电子转移是化学反应中常见的一种过程,通过标准电极电势表可以确定电子从高电势一侧转移到低电势一侧的方向。
5. 总结标准电极电势表是一种常用的参考工具,用于测量和比较不同电池或半电池的电势。
它以标准氢电极为参照,给出了一系列常见电极的标准电势值。
标准电极电势表
3十—
Al +3e =Al
AlF36+3e =Al+6F
CIO4+8H十+7e=1/2CI2+4冲0
AaO+61■十+6e—=2As+3HO
HAsO+3H++3e—=As+2H0
CIO4+8H++8e—=CI—+4H2O
HAsO+21■十+2e—=HAsO+2HO
CO+2e=Co
Au十+e—=Au
CcT+e一=C6+(2mol・L—1H2SO)
Au3++3 e—=Au
CQ+2H++2e—=HCOOH
AuCl4+3e=Au+ 4Cl
2+—
Cr+2e=Cr
Au3++2e—=Au+
3+—―2+
Cr+e=Cr
HBO+3H十+3e—=B+ 3f0
8
Cr3++3e—=Cr
HCIO+ H+2e=Cl+H2O
AgOO+2e=2Ag+C2o2
7
CIO2+H++e=HCIO
AgCl+e=Ag+Cl
HCIO+2H++2e一=HCIO+ HO
Ag CO3+2e「=2Ag+ CO2-
HCIQ+3H1+3e=1/2CI2+2H2O
标准电极电势表

标准电极电势表目录[隐藏]电极电势的产生—双电层理论定义公式电极电势内容标准电极电势表[编辑本段]电极电势的产生—双电层理论德国化学家能斯特(H.W.Nernst)提出了双电层理论(electron double lay er theory)解释电极电势的产生的原因。
当金属放入溶液中时,一方面金属晶体中处于热运动的金属离子在极性水分子的作用下,离开金属表面进入溶液。
金属性质越活泼,这种趋势就越大;另一方面溶液中的金属离子,由于受到金属表面电子的吸引,而在金属表面沉积,溶液中金属离子的浓度越大,这种趋势也越大。
在一定浓度的溶液中达到平衡后,在金属和溶液两相界面上形成了一个带相反电荷的双电层(electron double layer),双电层的厚度虽然很小(约为10-8厘米数量级), 但却在金属和溶液之间产生了电势差。
通常人们就把产生在金属和盐溶液之间的双电层间的电势差称为金属的电极电势(electrode potential),并以此描述电极得失电子能力的相对强弱。
电极电势以符号E Mn+/ M表示, 单位为V(伏)。
如锌的电极电势以EZn2+/ Zn 表示, 铜的电极电势以ECu2+/Cu 表示。
电极电势的大小主要取决于电极的本性,并受温度、介质和离子浓度等因素的影响。
[编辑本段]定义标准电极电势是可逆电极在标准状态及平衡态时的电势,也就是标准态时的电极电势.标准电极电势有很大的实用价值,可用来判断氧化剂与还原剂的相对强弱,判断氧化还原反应的进行方向,计算原电池的电动势、反应自由能、平衡常数,计算其他半反应的标准电极电势,等等。
将半反应按电极电势由低到高排序,可以得到标准电极电势表,可十分简明地判断氧还反应的方向.[编辑本段]公式任何温度下标准氢电极的标准电极电势值都为0,但其他电极电势值会受到温度影响。
以Ni/NiO电极为例,它可以用作高温伪参比电极,在0-400°C时的电极电势大致符合以下公式:E°(T)=-0.0003T+0.1414,T为温度[编辑本段]电极电势内容1 在酸性溶液中(298K)电对方程式Eq/VLi(I)-(0) Li++e-=Li -3.0401Cs(I)-(0) Cs++e-=Cs -3.026Rb(I)-(0) Rb++e-=Rb -2.98K(I)-(0) K++e-=K -2.931Ba(II)-(0) Ba2++2e-=Ba -2.912Sr(II)-(0) Sr2++2e-=Sr -2.89Ca(II)-(0) Ca2++2e-=Ca -2.868Na(I)-(0) Na++e-=Na -2.71La(III)-(0) La3++3e-=La -2.379Mg(II)-(0) Mg2++2e-=Mg -2.372Ce(III)-(0) Ce3++3e-=Ce -2.336H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069Th(IV)-(0) Th4++4e-=Th -1.899Be(II)-(0) Be2++2e-=Be -1.847U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724Al(III)-(0) Al3++3e-=Al -1.662Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24Mn(II)-(0) Mn2++2e-=Mn -1.185Cr(II)-(0) Cr2++2e-=Cr -0.913Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644 As(0)-(-III) As+3H++3e-=AsH3 -0.608U(IV)-(III) U4++e-=U3+-0.607Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499 *C(IV)-(III) 2CO2+2H++2e-=H2C2O4 -0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407Cd(II)-(0) Cd2++2e-=Cd -0.4030Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382Tl(I)-(0) Tl++e-=Tl -0.336Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276 Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675Ni (II)-(0) Ni2++2e-=Ni -0.257V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182 Ag(I)-(0) AgI+e-=Ag+I--0.15224Sn(II)-(0) Sn2++2e-=Sn -0.1375Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037H(I)-(0) 2H++2e-=H2 0.0000Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583 S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172 Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233As(III)-(0) HAsO2+3H++3e-=As+2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808 Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560 Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581 Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O2 0.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771Hg(I)-(0) Hg22++2e-=2Hg 0.7973Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os+4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803 Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857 Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86 Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO2 1.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151 Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO2 1.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33 Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913 Au(I)-(0) Au++e-=Au 1.692Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532 在碱性溶液中(298K)电对方程式Eq/VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3 -1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93 S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3 -0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34*Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3 -0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH3 0.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595 Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841*Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95O(0)-(-II) O3+H2O+2e-=O2+2OH- 1.24标准电极电势表半反应E°(V) 来源& -9Zz 9N N2(g) + H+ + e− HN3(aq) -3.09 [6]Li+ + e− Li(s) -3.0401 [5]N2(g) + 4H2O + 2e− 2N H2OH(aq) + 2OH− -3.04 [6] Cs+ + e− Cs(s) -3.026 [5]Rb+ + e− Rb(s) -2.98 [4]K+ + e− K(s) -2.931 [5]Ba2+ + 2e− Ba(s) -2.912 [5]La(OH)3(s) + 3e− La(s) + 3OH− -2.90 [5]Sr2+ + 2e−Sr(s) -2.899 [5]Ca2+ + 2e− Ca(s) -2.868 [5]Eu2+ + 2e− Eu(s) -2.812 [5]Ra2+ + 2e− Ra(s) -2.8 [5]Na+ + e− Na(s) -2.71 [5][9]La3+ + 3e− La(s) -2.379 [5]Y3+ + 3e− Y(s) -2.372 [5]Mg2+ + 2e− Mg(s) -2.372 [5]ZrO(OH)2(s) + H2O + 4e− Zr(s) + 4OH− -2.36 [5]Al(OH)4− + 3e− Al(s) + 4OH− -2.33Al(OH)3(s) + 3e− Al(s) + 3OH− -2.31H2(g) + 2e− 2H− -2.25Ac3+ + 3e− Ac(s) -2.20Be2+ + 2e− Be(s) -1.85U3+ + 3e− U(s) -1.66 [7]Al3+ + 3e− Al(s) -1.66 [9]Ti2+ + 2e− Ti(s) -1.63 [9]ZrO2(s) + 4H+ + 4e− Zr(s) + 2H2O -1.553 [5]Zr4+ + 4e− Zr(s) -1.45 [5]TiO(s) + 2H+ + 2e− Ti(s) + H2O -1.31Ti2O3(s) + 2H+ + 2e− 2T iO(s) + H2O -1.23Ti3+ + 3e− Ti(s) -1.21Te(s) + 2e− Te2− -1.143 [2]V2+ + 2e− V(s) -1.13 [2]Nb3+ + 3e− Nb(s) -1.099Sn(s) + 4H+ + 4e− SnH4(g) -1.07Mn2+ + 2e− Mn(s) -1.029 [9]SiO2(s) + 4H+ + 4e− Si(s) + 2H2O -0.91B(OH)3(aq) + 3H+ + 3e− B(s) + 3H2O -0.89TiO2+ + 2H+ + 4e− Ti(s) + H2O -0.86Bi(s) + 3H+ + 3e− BiH3 -0.8H2H2O + 2e− H2(g) + 2OH− -0.8277 [5]Zn2+ + 2e− Zn(Hg) -0.7628 [5]Zn2+ + 2e− Zn(s) -0.7618 [5]Ta2O5(s) + 10H+ + 10e− 2T a(s) + 5H2O -0.75Cr3+ + 3e− Cr(s) -0.74Au[Au(CN)2]− + e− Au(s) + 2C N− -0.60Ta3+ + 3e− Ta(s) -0.6PbO(s) + H2O + 2e− Pb(s) + 2OH− -0.58Ti2T iO2(s) + 2H+ + 2e− Ti2O3(s) + H2O -0.56Ga3+ + 3e− Ga(s) -0.53U4+ + e− U3+ -0.52 [7]P H3PO2(aq) + H+ + e− P(白磷[10]) + 2H2O -0.508 [5]P H3PO3(aq) + 2H+ + 2e− H3PO2(aq) + H2O -0.499 [5] P H3PO3(aq) + 3H+ + 3e− P(红磷)[10] + 3H2O -0.454 [5] Fe2+ + 2e− Fe(s) -0.44 [9]C2C O2(g) + 2H+ + 2e− HOOCCOOH(aq) -0.43Cr3+ + e− Cr2+ -0.42Cd2+ + 2e− Cd(s) -0.40 [9]GeO2(s) + 2H+ + 2e− GeO(s) + H2O -0.37Cu2O(s) + H2O + 2e− 2C u(s) + 2O H− -0.360 [5]PbSO4(s) + 2e− Pb(s) + SO42− -0.3588 [5]PbSO4(s) + 2e− Pb(Hg) + SO42− -0.3505 [5]Eu3+ + e− Eu2+ -0.35 [7]In3+ + 3e− In(s) 0.34 [2]Tl+ + e− Tl(s) -0.34 [2]Ge(s) + 4H+ + 4e− GeH4(g) -0.29Co2+ + 2e− Co(s) -0.28 [5]P H3PO4(aq) + 2H+ + 2e− H3PO3(aq) + H2O -0.276 [5] V3+ + e− V2+ 0.26 [9]Ni2+ + 2e− Ni(s) -0.25As(s) + 3H+ + 3e− AsH3(g) -0.23 [2]MoO2(s) + 4H+ + 4e− Mo(s) + 2H2O -0.15Si(s) + 4H+ + 4e− SiH4(g) -0.14Sn2+ + 2e− Sn(s) -0.13O2(g) + H+ + e− HO2•(aq) -0.13Pb2+ + 2e− Pb(s) -0.13 [9]WO2(s) + 4H+ + 4e− W(s) + 2H2O -0.12P(红磷) + 3H+ + 3e− PH3(g) -0.111 [5]C CO2(g) + 2H+ + 2e− HCOOH(aq) -0.11Se(s) + 2H+ + 2e− H2Se(g) -0.11C CO2(g) + 2H+ + 2e− CO(g) + H2O -0.11SnO(s) + 2H+ + 2e− Sn(s) + H2O -0.10SnO2(s) + 2H+ + 2e− SnO(s) + H2O -0.09WO3(aq) + 6H+ + 6e− W(s) + 3H2O -0.09 [2]P(白磷) + 3H+ + 3e− PH3(g) -0.063 [5]C HCOOH(aq) + 2H+ + 2e− HCHO(aq) + H2O -0.03 H 2H+ + 2e− H2(g) ≡ 0S4O62− + 2e− 2S2O32− +0.08Fe3O4(s) + 8H+ + 8e− 3F e(s) + 4H2O +0.085 [8]N2(g) + 2H2O + 6H+ + 6e− 2N H4OH(aq) +0.092 HgO(s) + H2O + 2e− H g(l) + 2O H− +0.0977Cu(NH3)42+ + e− Cu(NH3)2+ + 2N H3 +0.10 [2]Ru(NH3)63+ + e− Ru(NH3)62+ +0.10 [7]N2H4(aq) + 4H2O + 2e− 2N H4+ + 4O H− +0.11 [6] Mo H2MoO4(aq) + 6H+ + 6e− Mo(s) + 4H2O +0.11 Ge4+ + 4e− Ge(s) +0.12C(s) + 4H+ + 4e− CH4(g) +0.13 [2]C HCHO(aq) + 2H+ + 2e− CH3OH(aq) +0.13S(s) + 2H+ + 2e− H2S(g) +0.14Sn4+ + 2e− Sn2+ +0.15Cu2+ + e− Cu+ +0.159 [2]S HSO4− + 3H+ + 2e− SO2(aq) + 2H2O +0.16UO22+ + e− UO2+ +0.163 [7]S SO42− + 4H+ + 2e− SO2(aq) + 2H2O +0.17TiO2+ + 2H+ + e− Ti3+ + H2O +0.19Bi3+ + 2e− Bi+ +0.2SbO+ + 2H+ + 3e− Sb(s) + H2O +0.20As H3AsO3(aq) + 3H+ + 3e− As(s) + 3H2O +0.24 GeO(s) + 2H+ + 2e− Ge(s) + H2O +0.26UO2+ + 4H+ + e− U4+ + 2H2O +0.273 [7]Re3+ + 3e− Re(s) +0.300Bi3+ + 3e− Bi(s) +0.32VO2+ + 2H+ + e− V3+ + H2O +0.34Cu2+ + 2e− Cu(s) +0.340 [2]Fe [Fe(CN)6]3− + e− [Fe(CN)6]4− +0.36O2(g) + 2H2O + 4e− 4OH−(aq) +0.40 [9]Mo H2MoO4 + 6H+ + 3e− Mo3+ +2H2O +0.43Bi+ + e− Bi(s) +0.50C CH3OH(aq) + 2H+ + 2e− CH4(g) + H2O +0.50S SO2(aq) + 4H+ + 4e− S(s) + 2H2O +0.50Cu+ + e− Cu(s) +0.520 [2]C CO(g) + 2H+ + 2e− C(s) + H2O +0.52I2(s) + 2e− 2I− +0.54 [9]I3− + 2e− 3I− +0.53 [9]Au [AuI4]− + 3e− Au(s) + 4I− +0.56As H3AsO4(aq) + 2H+ + 2e− H3AsO3(aq) + H2O +0.56 Au [AuI2]− + e− Au(s) + 2I− +0.58MnO4− + 2H2O + 3e− MnO2(s) + 4O H− +0.59S2O32−+ 6H+ + 4e− 2S(s) + 3H2O +0.60Mo H2MoO4(aq) + 2H+ + 2e− MoO2(s) + 2H2O +0.65 O2(g) + 2H+ + 2e− H2O2(aq) +0.70Tl3+ + 3e− Tl(s) +0.72PtCl62− + 2e− PtCl42− + 2C l− +0.726 [7]Se H2SeO3(aq) + 4H+ + 4e− Se(s) + 3H2O +0.74PtCl42− + 2e− Pt(s) + 4C l− +0.758 [7]Fe3+ + e− Fe2+ +0.77Ag+ + e− Ag(s) +0.7996 [5]Hg22+ + 2e− 2H g(l) +0.80N NO3−(aq) + 2H+ + e− NO2(g) + H2O +0.80Au [AuBr4]− + 3e− Au(s) + 4B r− +0.85Hg2+ + 2e− Hg(l) +0.85MnO4− + H+ + e− HMnO4− +0.90Hg 2H g2+ + 2e− Hg22+ +0.91 [2]Pd2+ + 2e− Pd(s) +0.915 [7]Au [AuCl4]− + 3e− Au(s) + 4C l− +0.93MnO2(s) + 4H+ + e− Mn3+ + 2H2O +0.95Au [AuBr2]− + e− Au(s) + 2B r− +0.96Br2(l) + 2e− 2B r− +1.07Br2(aq) + 2e− 2B r− +1.09 [9]I IO3− + 5H+ + 4e− HIO(aq) + 2H2O +1.13Au [AuCl2]− + e− Au(s) + 2C l− +1.15Se HSeO4− + 3H+ + 2e− H2SeO3(aq) + H2O +1.15 Ag2O(s) + 2H+ + 2e− 2A g(s) + H2O +1.17ClO3− + 2H+ + e− ClO2(g) + H2O +1.18Pt2+ + 2e− Pt(s) +1.188 [7]ClO2(g) + H+ + e− HClO2(aq) +1.19I 2I O3− + 12H+ + 10e− I2(s) + 6H2O +1.20ClO4− + 2H+ + 2e− ClO3− + H2O +1.20O2(g) + 4H+ + 4e− 2H2O +1.23 [9]MnO2(s) + 4H+ + 2e− Mn2+ + 2H2O +1.23Tl3+ + 2e− Tl+ +1.25Cl2(g) + 2e− 2C l− +1.36 [9]Cr2O7−−+ 14H+ + 6e− 2C r3+ + 7H2O +1.33CoO2(s) + 4H+ + e− Co3+ + 2H2O +1.42N 2N H3OH+ + H+ + 2e− N2H5+ + 2H2O +1.42 [6]I 2H IO(aq) + 2H+ + 2e− I2(s) + 2H2O +1.44Ce4+ + e− Ce3+ +1.44BrO3− + 5H+ + 4e− HBrO(aq) + 2H2O +1.45PbO β-PbO2(s) + 4H+ + 2e− Pb2+ + 2H2O +1.460 [2] PbO α-PbO2(s) + 4H+ + 2e− Pb2+ + 2H2O +1.468 [2] Br 2B rO3− + 12H+ + 10e− Br2(l) + 6H2O +1.48Cl 2ClO3− + 12H+ + 10e− Cl2(g) + 6H2O +1.49MnO4− + 8H+ + 5e− Mn2+ + 4H2O +1.51O HO2• + H+ + e− H2O2(aq) +1.51Au3+ + 3e− Au(s) +1.52NiO2(s) + 4H+ + 2e− Ni2+ + 2OH− +1.59Cl 2H ClO(aq) + 2H+ + 2e− Cl2(g) + 2H2O +1.63Ag2O3(s) + 6H+ + 4e− 2A g+ + 3H2O +1.67Cl HClO2(aq) + 2H+ + 2e− HClO(aq) + H2O +1.67Pb4+ + 2e− Pb2+ +1.69 [2]MnO4− + 4H+ + 3e− MnO2(s) + 2H2O +1.70O H2O2(aq) + 2H+ + 2e− 2H2O +1.78AgO(s) + 2H+ + e− Ag+ + H2O +1.77Co3+ + e− Co2+ +1.82Au+ + e− Au(s) +1.83 [2]BrO4− + 2H+ + 2e− BrO3− + H2O +1.85Ag2+ + e− Ag+ +1.98 [2]S2O82− + 2e− 2SO42− +2.07O3(g) + 2H+ + 2e− O2(g) + H2O +2.075 [7]Mn HMnO4− + 3H+ + 2e− MnO2(s) + 2H2O +2.09 F2(g) + 2e− 2F− +2.87 [2][9]F2(g) + 2H+ + 2e− 2H F(aq) +3.05 [2]。
最全的标准电极电势(无表格版)

——标准电极电势表—-1 在酸性溶液中(298K)电对方程式E/VLi(I)-(0) Li++e-=Li -3。
0401 Cs(I)-(0)Cs++e-=Cs -3。
026 Rb(I)-(0) Rb++e-=Rb -2。
98 K(I)-(0)K++e-=K -2。
931 Ba(II)-(0)Ba2++2e-=Ba -2。
912 Sr(II)-(0)Sr2++2e-=Sr -2。
89 Ca(II)-(0) Ca2++2e-=Ca -2。
868 Na(I)-(0)Na++e-=Na -2。
71 La(III)-(0) La3++3e-=La -2.379 Mg(II)-(0)Mg2++2e-=Mg -2。
372 Ce(III)-(0) Ce3++3e-=Ce -2。
336 H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069 Th(IV)-(0)Th4++4e-=Th -1.899 Be(II)-(0)Be2++2e-=Be -1。
847 U(III)-(0)U3++3e-=U -1.798 Hf(IV)-(0)HfO2++2H++4e-=Hf+H2O -1。
724 Al(III)-(0)Al3++3e-=Al -1。
662 Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0)ZrO2+4H++4e-=Zr+2H2O -1.553 Si(IV)-(0)[SiF6]2-+4e-=Si+6F--1。
24 Mn(II)-(0)Mn2++2e-=Mn -1.185 Cr(II)-(0)Cr2++2e-=Cr -0。
913 Ti(III)-(II)Ti3++e-=Ti2+-0。
9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0。
8698 *Ti(IV)-(0)TiO2+4H++4e-=Ti+2H2O -0。
标准电极电势表样本
标准电极电势表标准电极电势能够用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极, 溶液中离子有效浓度为1mol/L, 气体分压为100kPa, 温度为298K, 所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注: (s) –固体; (l) –液体; (g) –气体; (aq) –水溶液; (Hg) –汞齐。
半反应E°(V)[注 1]来源Ba++ e−Ba(s) −4.38 [10][1][3]Sr++ e−Sr(s) −4.10 [11][1][3] Ca++ e−Ca(s) −3.8 [11][1][3]Pr3++ e−Pr2+−3.1 [11] N2(g) + H++ e−HN3(aq) −3.09 [6]Li++ e−Li(s) −3.0401 [5]N2(g) + 4 H2O + 2 e−2 NH2OH(aq) + 2 OH−−3.04 [6]Cs++ e−Cs(s) −3.026 [5]半反应E°(V)[注 1]来源Ca(OH)2(s) + 2 e−Ca(s) + 2 OH−−3.02 [11] Rb++ e−Rb(s) −2.98 [4]K++ e−K(s) −2.931 [5] Mg++ e−Mg(s) −2.93 [10] Ba2++ 2 e−Ba(s) −2.912 [5]La(OH)3(s) + 3 e−La(s) + 3OH−−2.90 [5]Fr++ e−Fr(s) −2.9 [11] Sr2++ 2 e−Sr(s) −2.899 [5]Sr(OH)2(s) + 2 e−Sr(s) + 2 OH−−2.88 [11] Ca2++ 2 e−Ca(s) −2.868 [5] Eu2++ 2 e−Eu(s) −2.812 [5] Ra2++ 2 e−Ra(s) −2.8 [5] Yb2++ 2 e−Yb(s) −2.76 [11][1] Na++ e−Na(s) −2.71 [5][9]半反应E°(V)[注 1]来源Sm2++ 2 e−Sm(s) −2.68 [11][1] No2++ 2 e−No(s) −2.50 [11] HfO(OH)2(s) + H2O + 4 e−Hf(s) + 4 OH−−2.50 [11] Th(OH)4(s) + 4 e−Th(s) + 4 OH−−2.48 [11] Md2++ 2 e−Md(s) −2.40 [11] La3++ 3 e−La(s) −2.379 [5]Y3++ 3 e−Y(s) −2.372 [5] Mg2++ 2 e−Mg(s) −2.372 [5] ZrO(OH)2(s) + H2O + 4 e−Zr(s) + 4OH−−2.36 [5]Pr3++ 3 e−Pr(s) −2.353 [11] Ce3++ 3 e−Ce(s) −2.336 [11] Er3++ 3 e−Er(s) −2.331 [11] Ho3++ 3 e−Ho(s) −2.33 [11]Al(OH)4−+ 3 e−Al(s) + 4 OH−−2.33半反应E°(V)[注 1]来源Al(OH)3(s) + 3 e−Al(s) + 3OH−−2.31Tb3++ 3 e−Tb(s) −2.28H2(g) + 2 e−2 H−−2.25Ac3++ 3 e−Ac(s) −2.20Be++ e−Be(s) −2.12 [10] Cf2++ 2 e−Cf(s) −2.12 [11] Am3++ 3 e−Am(s) −2.048 [11] Cf3++ 3 e−Cf(s) −1.94 [11] Am2++ 2 e−Am(s) −1.9 [11] Be2++ 2 e−Be(s) −1.85Rf4++ 4 e−Rf(s) −1.67 [12]U3++ 3 e−U(s) −1.66 [7]Al3++ 3 e−Al(s) −1.66 [9]半反应E°(V)[注 1]来源Ti2++ 2 e−Ti(s) −1.63 [9]Bk2++ 2 e−Bk(s) −1.6 [11] ZrO2(s) + 4 H++ 4 e−Zr(s) + 2 H2O −1.553 [5]Hf4++ 4 e−Hf(s) −1.55 [11]Zr4++ 4 e−Zr(s) −1.45 [5]Ti3++ 3 e−Ti(s) −1.37 [13] TiO(s) + 2 H++ 2 e−Ti(s) + H2O −1.31Ti2O3(s) + 2 H++ 2 e−2 TiO(s) + H2O −1.23Zn(OH)42−+ 2 e−Zn(s) + 4 OH−−1.199 [14] Mn2++ 2 e−Mn(s) −1.185 [14] Fe(CN)64−+ 6 H++ 2 e−Fe(s) + 4HCN(aq) −1.16 [15]V2++ 2 e−V(s) −1.175 [2]Te(s) + 2 e−Te2−−1.143 [2]半反应E°(V)[注 1]来源Nb3++ 3 e−Nb(s) −1.099Sn(s) + 4 H++ 4 e−SnH4(g) −1.07In(OH)3(s) + 3 e−In(s) + 3 OH−−0.99 [11] SiO2(s) + 4 H++ 4 e−Si(s) + 2 H2O −0.91B(OH)3(aq) + 3 H++ 3 e−B(s) + 3 H2O −0.89Fe(OH)2(s) + 2 e−Fe(s) + 2 OH−−0.89 [15] Fe2O3(s) + 3 H2O + 2 e−2Fe(OH)2(s) + 2 OH−−0.86 [15] TiO2++ 2 H++ 4 e−Ti(s) + H2O −0.862 H2O+ 2 e−H2(g) + 2 OH−−0.8277 [5]Bi(s) + 3 H++ 3 e−BiH3−0.8 [14] Zn2++ 2 e−Zn(Hg) −0.7628 [5]Zn2++ 2 e−Zn(s) −0.7618 [5]Ta2O5(s) + 10 H++ 10 e−2 Ta(s) + 5 H2O −0.75半反应E°(V)[注 1]来源Cr3++ 3 e−Cr(s) −0.74[Au(CN)2]−+ e−Au(s) + 2 CN−−0.60Ta3++ 3 e−Ta(s) −0.6PbO(s) + H2O + 2 e−Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H++ 2 e−Ti2O3(s) + H2O −0.56Ga3++ 3 e−Ga(s) −0.53U4++ e−U3+−0.52 [7]H3PO2(aq) + H++ e−P(白磷[16]) + 2 H2O −0.508 [5]H3PO3(aq) + 2 H++ 2 e−H3PO2(aq) + H2O −0.499 [5]H3PO3(aq) + 3 H++ 3 e−P(红磷)[16]+ 3H2O −0.454 [5]Fe2++ 2 e−Fe(s) −0.44 [9]2 CO2(g) + 2 H++ 2 e−HOOCCOOH(aq) −0.43。
最全的标准电极电势(无表格版)

资料范本本资料为word版本,可以直接编辑和打印,感谢您的下载最全的标准电极电势(无表格版)地点:__________________时间:__________________说明:本资料适用于约定双方经过谈判,协商而共同承认,共同遵守的责任与义务,仅供参考,文档可直接下载或修改,不需要的部分可直接删除,使用时请详细阅读内容-- 标准 --标准电极电势表-- 1 在酸性溶液中 (298K)电对方程式E/VLi(I)-(0) Li++e-=Li -3.0401Cs(I)-(0) Cs++e-=Cs -3.026Rb(I)-(0) Rb++e-=Rb -2.98K(I)-(0) K++e-=K -2.931Ba(II)-(0) Ba2++2e-=Ba -2.912Sr(II)-(0) Sr2++2e-=Sr -2.89Ca(II)-(0) Ca2++2e-=Ca -2.868Na(I)-(0) Na++e-=Na -2.71La(III)-(0) La3++3e-=La -2.379Mg(II)-(0) Mg2++2e-=Mg -2.372Ce(III)-(0) Ce3++3e-=Ce -2.336H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069Th(IV)-(0) Th4++4e-=Th -1.899Be(II)-(0) Be2++2e-=Be -1.847U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724Al(III)-(0) Al3++3e-=Al -1.662Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24Cr(II)-(0) Cr2++2e-=Cr -0.913Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750 Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644 As(0)-(-III) As+3H++3e-=AsH3 -0.608U(IV)-(III) U4++e-=U3+-0.607Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C2O4 -0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407Cd(II)-(0) Cd2++2e-=Cd -0.4030Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382Tl(I)-(0) Tl++e-=Tl -0.336P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675Ni (II)-(0) Ni2++2e-=Ni -0.257V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.15224Sn(II)-(0) Sn2++2e-=Sn -0.1375Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037H(I)-(0) 2H++2e-=H2 0.0000Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32- 0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583 S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172 Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808 Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327 C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330 V(IV)-(III) VO2++2H++e-=V3++H2O 0.337Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560 Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581 Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl- 0.68O(0)-(-I) O2+2H++2e-=H2O2 0.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+ 0.771Hg(I)-(0) Hg22++2e-=2Hg 0.7973Ag(I)-(0) Ag++e-=Ag 0.7996N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803 Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857 Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86 Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00 Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02 N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO2 1.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151 Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189 I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO2 1.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33 Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389 Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913 Au(I)-(0) Au++e-=Au 1.692Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532 在碱性溶液中 (298K)电对方程式E/VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88 Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3 -1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH- -0.93 S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59 *Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3 -0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34*Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3 -0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01 Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32- 0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108 Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH- 0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33 Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358 Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36 *Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH3 0.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490 Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH- 0.761Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH- 0.841*Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95O(0)-(-II) O3+H2O+2e-=O2+2OH- 1.24摘自David R.Lide, Handbook of Chemistry and Physics, 8-25-8-30, 78th. edition, 1997-1998* 摘自J.A. Dean Ed,Lange’s Handbook of Chemistry, 13th. edition, 1985** 摘自其他参考书.。
标准电极电势
标准电极电势Standard Electrode Potentials序号 (No.)电极过程 (Electrode process)E ? /V1 Ag ++e ═ Ag0.79962Ag 2++e ═ Ag +1.9803AgBr+e ═ Ag+Br - 0.07134 AgBrO 3+e ═ Ag+BrO 3-0.5465AgCl+e ═ Ag+Cl --0.2226 AgCN+e ═ Ag+CN-0.0177 Ag 2CO 3+2e ═ 2Ag+CO 32-0.4708 Ag 2C 2O 4+2e ═ 2Ag+C 2O 4 2- 0.4659Ag 2CrO 4+2e ═ 2Ag+CrO 42-0.44710-0.779AgF+e ═ Ag+F11 Ag 4[Fe(CN) 6]+4e ═ 4Ag+[Fe(CN)6] 4-0.14812AgI+e ═ Ag+I --0.15213 AgIO 3 +e ═ Ag+IO 3 -0.35414 24+2e ═ 2Ag+MoO 42- 0.457Ag MoO15 [Ag(NH 3)2] ++e ═ Ag+2NH 30.37316 AgNO 2 +e ═ Ag+NO 2-0.56417 Ag 2O+H 2O+2e ═ 2Ag+2OH - 0.34218 2AgO+H 2 O+2e ═ Ag 2O+2OH -0.607192--0.691 Ag 2S+2e ═ 2Ag+S 20 Ag 2S+2H ++2e ═ 2Ag+H 2S -0.036621 AgSCN+e ═ Ag+SCN -0.089522 Ag 2SeO 4+2e ═ 2Ag+SeO 42-0.36323 Ag 2SO 4+2e ═2-0.6542Ag+SO 424 Ag 2WO 4+2e ═ 2Ag+WO 42-0.46625Al 3+3e ═ Al-1.662 26A lF 6 3-+3e ═ Al+6F --2.06927 Al(OH) 3 +3e ═ Al+3OH --2.3128 AlO 2-+2H 2O+3e ═ Al+4OH - -2.3529Am 3++3e ═ Am-2.048 30Am 4+ 3+2.60+e ═ Am31 AmO 2 2+ +3+1.75 +4H +3e ═ Am +2H 2O32As+3H ++3e ═ AsH-0.608333 As+3H 2O+3e ═ AsH 3+3OH --1.37342 3 ++6e ═ 2As+3H 2O0.234 As O +6H + 35═ As+2H 2O0.248HAsO 2+3H +3e 36 AsO 2 -+2H 2O+3e ═ As+4OH --0.6837 H 3AsO 4+2H + +2e ═ HAsO 2+2H 2O0.56038 3- - +4OH --0.71AsO 4 +2H 2O+2e ═ AsO 239- 2--0.75AsS 2 +3e ═ As+2S3-+2e ═ AsS -2-41 Au ++e ═ Au1.69242Au 3++3e ═ Au1.49843Au 3++1.401+2e ═ Au44 AuBr 2 -+e ═ Au+2Br -0.95945 AuBr 4 -+3e ═ Au+4Br -0.85446 AuCl 2- +e ═ Au+2Cl - 1.15 47 AuCl 4 -+3e ═ Au+4Cl -1.00248AuI+e ═ Au+I -0.50 49--0.66 Au(SCN) 4 +3e ═ Au+4SCN50 Au(OH) 3+3H + +3e ═ Au+3H 2O1.4551BF ---1.044 +3e ═ B+4F52 H 2BO 3-+H 2O+3e ═ B+4OH - -1.7953B(OH) 3+7H ++8e ═ BH 4-2-.0481+3H O542++2e ═ Ba-2.912Ba -55-2.99Ba(OH) 2+2e ═ Ba+2OH56Be 2+ +2e ═ Be-1.847 57 Be 2O 3 2-+3H 2O+4e ═ 2Be+6OH --2.6358 Bi ++e ═ Bi 0.5 59Bi 3++3e ═ Bi0.30860 BiCl 4 -+3e ═ Bi+4Cl -0.1661 BiOCl+2H+ +3e ═ Bi+Cl - 20.16+H O62--0.46Bi 2O 3+3H 2O+6e ═ 2Bi+6OH63 Bi 2O 4+4H ++1.593+2e ═ 2BiO +2H 2O 64 Bi 2O 4+H 2O+2e ═ Bi 2O 3+2OH -0.5665 Br 2(水溶液,aq)+2e ═ -1.0872Br66 Br 2(液体 )+2e ═-1.0662Br67 BrO -+H 2O+2e ═ Br -+2OH0.76168 BrO -+6H +-1.4233 +6e ═ Br+3H 2O69 - - -0.61BrO 3 +3H 2O+6e ═ Br+6OH70- +1.4822BrO 3 +12H +10e ═ Br 2+6H 2O71 HBrO+H +-1.331+2e ═ Br+H 2O72 2HBrO+2H + +2e ═ Br1.5742(水溶液 ,aq)+2H 2O73 3+ +2e ═ CH 420.59 CH OH+2H + +H O 74 HCHO+2H0.19+2e ═ CH 3OH75 CH 3COOH+2H ++2e ═ CH 3CHO+H 2O-0.1276 (CN) 2+2H + +2e ═ 2HCN0.37377 (CNS)-0.77 2+2e ═ 2CNS782++2e ═ CO+H 2-0.12CO +2HO79 CO 2+2H ++2e ═ HCOOH-0.199 80Ca 2+ +2e ═ Ca-2.868 81--3.02Ca(OH) 2+2e ═ Ca+2OH82Cd 2+ +2e ═ Cd-0.403 83Cd 2+ +2e ═ Cd(Hg)-0.352842- --1.09Cd(CN) 4 +2e ═ Cd+4CN85CdO+H 2O+2e ═ Cd+2OH --0.783 862--1.17CdS+2e ═ Cd+S 87CdSO 4+2e ═ Cd+SO 4 2--0.246 883+ +3e ═ Ce-2.336 Ce 89Ce 3+ +3e ═ Ce(Hg)-1.437 90 CeO 2+4H +3+1.4 +e ═ Ce +2H 2O91-1.358Cl 2(气体 )+2e ═ 2Cl92- - -0.89ClO +H 2O+2e ═ Cl +2OH93HClO+H + -1.482+2e ═ Cl+H 2O 94 2HClO+2H ++2e ═ Cl 2+2H 2O 1.61195 ClO 2-+2H 2O+4e ═ Cl -+4OH -0.76 96 2ClO 3 -++10e ═ Cl 221.47 +12H+6H O97- + -1.451ClO 3 +6H +6e ═ Cl+3H 2O98 ClO 3-+3H 2O+6e ═ Cl -+6OH -0.62 99-+-1.38ClO 4 +8H +8e ═ Cl+4H 2O100 2ClO 4 -+16H ++14e ═ Cl 2+8H 2O1.39101 Cm3++3e ═ Cm-2.04102Co 2+ +2e ═ Co-0.28Co 3+2+1.808+e ═ Co1033 6 3+ +e ═ [Co(NH 3 6 2+ 0.108 [Co(NH ) ] 2+ ) ] 104 [Co(NH 3)6] +2e ═ Co+6NH 3 -0.43105Co(OH) 2+2e ═ Co+2OH --0.73106 Co(OH) 3+e ═ Co(OH)2+OH -0.17 107Cr 2++2e ═ Cr-0.913 108Cr 3+ 2+-0.407 +e ═ Cr109Cr 3++3e ═ Cr-0.744110 [Cr(CN) 6]3- +e ═ [Cr(CN)6] 4--1.28111--1.48Cr(OH) 3 +3e ═ Cr+3OH112 Cr 2O 7 2-+3+1.232+14H +6e ═ 2Cr +7H 2O113 CrO 2 -+2H 2O+3e ═ Cr+4OH --1.2114 HCrO - ++3e 3+ 1.3504 +7H ═ Cr +4H 2O115 CrO 4 2-2O+3e ═ Cr(OH)3--0.13 +4H++5OH116 Cs +e ═ Cs -2.92117 Cu ++e ═ Cu0.521118Cu 2+ +2e ═ Cu0.3422++0.17 Cu +e ═ Cu119 Cu 2+ +2e ═ Cu(Hg) 0.345120 Cu 2+ +Br -+e ═ CuBr0.66 121Cu 2++Cl -+e ═ CuCl0.57 122Cu 2++I -+e ═ CuI0.86123Cu 2+ +2CN -+e ═ [Cu(CN)2]-1.103--125--0.19 CuCl 2 +e ═ Cu+2Cl126- -0.00 CuI 2 +e ═ Cu+2I127 Cu 2O+H 2O+2e ═ 2Cu+2OH - -0.360 128 Cu(OH) 2+2e ═ Cu+2OH - -0.222129 2Cu(OH)2+2e ═ Cu 2-2-0.080O+2OH +H O1302--0.70 CuS+2e ═ Cu+S131--0.27 CuSCN +e ═ Cu+SCN132Dy 2+ +2e ═ Dy-2.2 1333+ +3e ═ Dy-2.295 Dy134 Er 2+ +2e ═ Er -2.0 135 Er 3+ +3e ═ Er -2.331136 Es 2+ +2e ═ Es-2.23 137Es 3+ +3e ═ Es-1.91 1382+ +2e ═ Eu-2.812 Eu139Eu 3+ +3e ═ Eu-1.991 140 F 2+2H + +2e ═ 2HF 3.053 141 F 2O+2H + +4e ═H 2O+2F -2.153 1422+ +2e ═ Fe-0.447 Fe 143Fe 3+ +3e ═ Fe-0.0373+2+0.68 0.771*Fe +e ═ Fe144 [Fe(CN) 6 ] 3- +e ═ [Fe(CN)6 4-0.358 4-] - 145[Fe(CN) 6] +2e ═ Fe+6CN-1.5 1463-2+-0.4 FeF 6 +e ═ Fe +6F147--0.877Fe(OH) 2 +2e ═ Fe+2OH148Fe(OH) 3+e ═ Fe(OH)2+OH - -0.563 4 + +2e ═ 2+2149 Fe O +8H+4H O1.23 150 Fm 3+ +3e ═ Fm-1.89 151Fr ++e ═ Fr-2.9 152Ga 3+ +3e ═ Ga-0.549153---1.29 H 2GaO 3 +H 2O+3e ═ Ga+4OH154 Gd 3+ +3e ═ Gd -2.279 155Ge 2+ +2e ═ Ge0.24 1564+2+ 0.0Ge +2e ═ Ge 157+═ GeO(棕色 )+H 2O-0.118 GeO 2+2H +2e 158 GeO 2+2H ++2e ═ GeO(黄色 )+H 2O -0.273 159 H 2GeO 3+4H + +4e ═ Ge+3H 2O-0.182 1602H ++2e ═H 20.0000 161H 2 +2e ═ --2.252H-162 2H O+2e ═H-0.82772 2+2OH163 Hf 4+ +4e ═ Hf -1.55 164Hg 2+ +2e ═ Hg0.851 1652++2e ═ 2Hg0.797 Hg 2 1662Hg 2++2e ═ Hg 22+0.920167 Hg 2Br 2+2e ═ 2Hg+2Br - 0.1392 168 HgBr 42- +2e ═ Hg+4Br - 0.21 169 Hg 2Cl 2+2e ═ 2Hg+2Cl -0.2681 170 2HgCl 2 +2e ═ Hg 2Cl 2+2Cl - 0.63 171 Hg 2CrO 4+2e ═ 2Hg+CrO 42-0.54 172Hg 2I 2+2e ═ 2Hg+2I --0.0405 173 Hg 2O+H 2O+2e ═ 2Hg+2OH - 0.123174HgO+H 2O+2e ═ Hg+2OH -0.09771752--0.70HgS(红色 )+2e ═ Hg+S1762--0.67HgS(黑色 )+2e ═ Hg+S177 Hg 2(SCN) 2+2e ═ 2Hg+2SCN -0.22 178 Hg 2SO 4 +2e ═ 2Hg+SO 2-0.6134179 Ho 2++2e ═ Ho-2.1 180Ho 3++3e ═ Ho-2.33181-0.5355I 2+2e ═ 2I182I --0.5363 +2e ═ 3I1832IBr+2e ═I 2+2Br -1.02 184--0.30ICN+2e ═I +CN185 2HIO+2H ++2e ═I1.4392+2H 2O186HIO+H ++2e -0.987═I+H 2O---187 IO +H2O+2e ═I0.485+2OH188- +1.1952IO 3 +12H +10e ═I 2+6H 2 O189- +6H + +6e -1.085IO 3 ═I +3H 2O190IO 3 -+2H 2O+4e ═ IO -+4OH -0.153 ---191 IO +3H 2 O+6e ═I0.26+6OH192 2IO 3-2O+10e ═I 2-0.21+6H+12OH193 H 5IO 6+H ++2e ═ IO -1.6013 +3H 2O194 In++e ═ In-0.14195In 3++3e ═ In- -0.338196-0.99In(OH) 3 +3e ═ In+3OH197 Ir 3++3e ═ Ir1.156198 IrBr 62- +e ═ IrBr 63- 0.99 199IrCl 62- +e ═ IrCl 63-0.867200+-2.931K +e ═K201La 3++3e ═ La-2.379 202 La(OH) 3+3e ═ La+3OH --2.90203Li ++e ═ Li -3.040204 Lr 3++3e ═ Lr-1.96205Lu 3++3e ═ Lu-2.28206 Md2++2e ═ Md -2.40207Md 3++3e ═ Md -1.65208Mg2++2e ═ Mg-2.372 209Mg(OH) 2 +2e ═ Mg+2OH --2.690210 Mn2++2e ═ Mn -1.185211Mn3++3e ═ Mn1.542212 MnO 2+4H +2+1.224+2e ═ Mn +2H 2O213MnO 4 -+4H ++3e ═ MnO 2 +2H 2O1.679- +2+ 2214 MnO 4 +8H +5e ═ Mn1.507+4H O 215 MnO 4- +2H 2O+3e ═M nO 2+4OH - 0.595216 Mn(OH) 2 +2e ═ Mn+2OH --1.562172- Mo3++3e ═ Mo- -0.200218-1.05MoO 4 +4H 2O+6e ═ Mo+8OH219 N 2+2H 2O+6H ++6e ═ 2NH 4OH0.092220 2NH 3OH ++H ++2e ═N 2H 5++2H 2 O1.42 221O+2e ═N -0.762NO+H 22O+2OH2222HNO 2+4H ++4e ═N 221.297O+3H O- + +2e ═ HNO223 NO 3 +3H0.9342+H 2O224 NO 3 -+H 2O+2e ═ NO 2 -+2OH -0.01225 2NO 3 -+2H 2O+2e ═N 2O 4+4OH --0.85226Na ++e ═ Na-2.713 227Nb3++3e ═ Nb -1.099 228 NbO 2+4H ++4e ═ Nb+2H-0.6902O229 Nb 2O 5 +10H ++10e ═ 2Nb+5H-0.6442O230Nd2++2e ═ Nd-2.1231Nd 3+═ Nd-2.323 +3e232Ni 2++2e ═ Ni-0.257233 NiCO 3+2e ═ Ni+CO 3 2- -0.45234Ni(OH) 2 +2e ═ Ni+2OH --0.722 +2+ 2235+2e ═ Ni +2H O 1.678NiO +4H236No 2++2e ═ No -2.50237 No 3++3e ═ No -1.20238Np3++3e ═ Np-1.856 239+-0.962NpO 2+H 2O+H +e ═ Np(OH)3240O 2+4H ++4e ═ 2H O 1.229241 O 2 +2H 2O+4e ═ 4OH - 0.40124232O+2e ═O 2 -1.24O +H2++2OH 243═ Os0.85Os +2e2443- 2+ - 0.4OsCl 6 +e ═ Os +6Cl245 OsO 2+2H 2O+4e ═ Os+4OH - -0.15246 OsO 4 +8H ++8e ═ Os+4H 2O0.8382474++4e ═ OsO 2 21.02OsO +4H +2H O 248 P+3H 2 O+3e ═ PH--0.873(g)+3OH249---1.82H 2 PO 2 +e ═ P+2OH250 H 3PO 3+2H ++2e ═H 3PO 2+H 2O -0.499 251 H 3PO 3+3H + +3e ═ P+3H 2O -0.454 252H 3PO 4+2H + +2e ═H 3PO 3+H 2O --0.276253 4 3-2O+2e ═ HPO 32---1.05 PO +2HPa 3++3e ═ Pa +3OH254 -1.34255 Pa 4++4e ═ Pa -1.49256Pb 2++2e ═ Pb-0.126 2572++2e ═ Pb(Hg)-0.121Pb258--0.284PbBr 2+2e ═ Pb+2Br259--0.268 PbCl 2+2e ═ Pb+2Cl260PbCO 3+2e ═ Pb+CO 3 2--0.506 261--0.344PbF 2+2e ═ Pb+2F262--0.365PbI 2+2e ═ Pb+2I263 PbO+H 2O+2e ═ Pb+2OH --0.580 264PbO+4H ++2e ═ Pb+H0.252O2+22265+2e ═ Pb1.455PbO +4H+2H O266 HPbO 2 -+H 2O+2e ═ Pb+3OH --0.537 267PbO 2+SO 42-+4H ++2e ═ PbSO 4+2H 2O1.6912682--0.359 PbSO 4+2e ═ Pb+SO 4269Pd 2++2e ═ Pd0.9152702--0.6PdBr 4 +2e ═ Pd+4Br271 PdO 2+H 2O+2e ═ PdO+2OH -0.73 272-0.07Pd(OH) 2 +2e ═ Pd+2OH273Pm2++2e ═ Pm-2.202743+═ Pm-2.30P m +3e 275Po 4++4e ═ Po0.76 276 Pr 2++2e ═ Pr -2.0277Pr3++3e ═ Pr-2.353 2782+1.18Pt +2e ═ Pt 279 [PtCl 6] 2- +2e ═ [PtCl 2- +2Cl-0.68 4 ]280-0.14Pt(OH) 2+2e ═ Pt+2OH281 PtO 2+4H ++4e ═ Pt+2HO1.00 2822--0.83PtS+2e ═ Pt+S 283Pu 3++3e ═ Pu-2.0312845+4+1.099Pu+e ═ Pu 285Ra2++2e ═ Ra-2.8286+-2.98Rb +e ═ Rb287Re3++3e ═ Re0.300 288ReO 2 +4H ++4e ═O0.251Re+2H289 ReO 4-+4H + +3e ═ ReO 2+2H 2O0.510290 ReO 4-+4H 2O+7e ═ Re+8OH - -0.584291 Rh 2+ +2e ═ Rh 0.600292 Rh3++3e ═ Rh0.758293Ru 2+ +2e ═ Ru0.455294 RuO 2+4H +2+1.120+2e ═ Ru +2H 2O 295RuO 4+6H ++4e ═ Ru(OH)22++2H 2O1.402962--0.476S+2e ═S297 S+2H + +2e ═H 2S(水溶液 ,aq)0.142298S 2O 6 2-+4H ++2e ═ SO 30.5642H299 2SO 3 2-+3H 2O+4e ═S 2O 3 2-+6OH --0.571300 3 2- 2O+2e ═S 2 4 2- --1.122SO +2H O +4OH301 SO 42- +H 2O+2e ═ SO 32- +2OH --0.93302Sb+3H ++3e ═ SbH-0.5103303 2 3++6e ═2Sb+3HO0.152Sb O +6H304++0.581Sb 2O 5+6H +4e ═ 2SbO+3H 2O305 SbO 3 -+H 2O+2e ═ SbO 2 -+2OH --0.59306Sc 3++3e ═ Sc-2.077 307--2.6 Sc(OH) 3 +3e ═ Sc+3OH3082--0.924+Se+2e ═ Se309-0.399 Se+2H +2e ═H 2Se(水溶液 ,aq)310 H 2 SeO 3+4H + +4e ═ Se+3H 2O -0.74311 SeO 32- +3H 2O+4e ═ Se+6OH --0.366 3122- 2- +2OH -0.05SeO 4 +H 2O+2e ═ SeO 3313++4e ═ SiH 40.102Si+4H(气体 )314 Si+4H 2O+4e ═ SiH 4+4OH --0.733152- --1.24SiF 6 +4e ═ Si+6F316 SiO 2+4H ++4e ═ Si+2HO-0.857 317 2---1.697 SiO 3+3H 2O+4e ═ Si+6OH318Sm 2++2e ═ Sm -2.68319 Sm 3++3e ═ Sm-2.304 320Sn 2++2e ═ Sn-0.1383214+ 2+ 0.151Sn +2e ═ Sn 322 2- --0.19SnCl 4 +2e ═ Sn+4Cl(1mol/LHCl)323 2- - -0.25SnF 6 +4e ═ Sn+6F324 - +3H + +2e 2+0.142Sn(OH) 3 ═ Sn +3H 2O325+-0.117 SnO 2+4H +4e ═ Sn+2HO326 Sn(OH) 6 2- +2e ═ HSnO 2- +3OH - +H 2O-0.93327 Sr 2++2e ═ Sr-2.899 328Sr 2++2e ═ Sr(Hg)-1.793 329--2.88Sr(OH) 2 +2e ═ Sr+2OH330 Ta 3++3e ═ Ta -0.6331Tb 3+ +3e ═ Tb-2.28332Tc 2++2e ═ Tc0.400333 TcO - +8H ++7e ═ Tc+4H 20.4724O334 TcO 4-+2H 2O+3e ═ TcO 2+4OH --0.311 3352--1.143 Te+2e ═ Te 336 Te 4++4e ═ Te 0.568337 Th 4+ +4e ═ Th -1.899 338Ti 2++2e ═ Ti-1.630339Ti3++3e ═ Ti-1.37340 TiO 2+4H +2+ -0.502 +2e ═ Ti +2H 2O341 TiO 2++2H +3+0.1 +e ═ Ti +H 2O342 Tl ++e ═ Tl-0.336 343Tl3++3e ═ Tl0.741344Tl 3++Cl - +2e ═ TlCl1.36 345TlBr+e--0.658═ Tl+Br 346TlCl+e--0.557═ Tl+Cl347TlI+e--0.752═ Tl+I348 Tl 2O 3+3H 2O+4e ═ 2Tl ++6OH -0.02349TlOH+e ═ Tl+OH --0.34350+2e ═ 2Tl+SO 2- -0.436Tl 2SO 44351Tm 2++2e ═ Tm -2.4 352 Tm 3++3e ═ Tm-2.319 353U 3+ +3e ═U-1.798354 UO 2 +4H ++4e ═ U+2HO-1.40355++4H + 4+0.612UO 2 +e ═U +2H 2O356 UO 2++4H + +6e ═ U+2H 2 -1.444 2O 357V 2++2e ═V-1.175358VO 2++2H + 3+0.337+e ═V +H 2O+ +2+359 VO 2+2H+e ═ VO 2 0.991+H O 360+ +3+ 0.668VO 2 +4H +2e ═V +2H 2O361 V 2O 5+10H ++10e ═ 2V+5H 2O-0.242 362W3++3e ═W 0.1 363 WO 3 +6H ++6e ═ W+3H 2 -0.090+2e ═O364 2 5+ 2 -0.031 WO+2H 2WO+H O365 Y 3++3e ═Y -2.372366 Yb 2++2e ═ Yb -2.76367Yb 3++3e ═ Yb-2.19368Zn 2+-0.7618 +2e ═ Zn 369Zn 2++2e ═ Zn(Hg)-0.7628 370 Zn(OH) 2+2e ═ Zn+2OH --1.2493712--1.40ZnS+2e ═ Zn+S3722--0.799ZnSO 4+2e ═ Zn(Hg)+SO 4。
标准电极电势表非常全-nb的电极电势

标准电极电势表非常全-nb的电极电势标准电极电势表非常全 nb 的电极电势在化学的世界里,标准电极电势表是一个极其重要的工具,它就像是一张地图,指引着我们在电化学的领域中探索和前行。
今天,咱们就来深入聊聊这张神秘而又实用的“地图”——标准电极电势表,特别是其中那些令人瞩目的“nb 的电极电势”。
首先,咱们得搞清楚啥是标准电极电势。
简单来说,标准电极电势就是在标准状态下(通常是指温度为 29815K,压强为 100kPa,溶液中各物质的浓度为 1mol/L),某一电极与标准氢电极组成原电池时所测得的电势差。
这个概念听起来可能有点复杂,但其实就是用来衡量一个电极在特定条件下得失电子的能力。
那标准电极电势表有啥用呢?这用处可大了去了!它可以帮助我们判断氧化还原反应进行的方向和限度。
比如说,如果一个氧化还原反应中,氧化剂的标准电极电势大于还原剂的标准电极电势,那么这个反应就能自发进行。
而且,通过标准电极电势表,我们还能计算出电池的电动势,从而了解电池的性能和能量转化效率。
接下来,咱们重点聊聊那些“nb 的电极电势”。
在标准电极电势表中,有一些电极电势的值特别突出,具有重要的意义。
比如说,氟气和氟离子组成的电极,其标准电极电势高达 287V,这意味着氟气具有极强的氧化性,是一种非常强大的氧化剂。
再比如,锂金属和锂离子组成的电极,其标准电极电势为-304V,这表明锂在电池领域有着独特的地位,因为它具有很低的电极电势,能够提供较高的电压。
这些“nb 的电极电势”在实际应用中发挥着关键作用。
以锂为例,由于其低电极电势的特性,锂离子电池在现代电子设备和电动汽车中得到了广泛的应用。
锂离子电池能够提供高能量密度,使得我们的手机、笔记本电脑等设备能够长时间运行,电动汽车也能够行驶更远的距离。
而氟气的强氧化性则在化学合成和工业生产中有重要用途。
它可以用于制备一些难以通过其他方法得到的化合物,提高生产效率和产品质量。
标准电极电势表中的数据并不是一成不变的。
标准电极电势表

标准电极电势表标准电极电势表目录[隐藏]电极电势的产生—双电层理论定义公式电极电势内容标准电极电势表[编辑本段]电极电势的产生—双电层理论德国化学家能斯特(H.W.Nernst)提出了双电层理论(electron double layer th eory)解释电极电势的产生的原因。
当金属放入溶液中时,一方面金属晶体中处于热运动的金属离子在极性水分子的作用下,离开金属表面进入溶液。
金属性质越活泼,这种趋势就越大;另一方面溶液中的金属离子,由于受到金属表面电子的吸引,而在金属表面沉积,溶液中金属离子的浓度越大,这种趋势也越大。
在一定浓度的溶液中达到平衡后,在金属和溶液两相界面上形成了一个带相反电荷的双电层(e lectron double layer),双电层的厚度虽然很小(约为10-8厘米数量级), 但却在金属和溶液之间产生了电势差。
通常人们就把产生在金属和盐溶液之间的双电层间的电势差称为金属的电极电势(electrode potential),并以此描述电极得失电子能力的相对强弱。
电极电势以符号E Mn+/ M表示, 单位为V(伏)。
如锌的电极电势以EZn2+/ Zn 表示, 铜的电极电势以ECu2+/Cu 表示。
电极电势的大小主要取决于电极的本性,并受温度、介质和离子浓度等因素的影响。
[编辑本段]定义标准电极电势是可逆电极在标准状态及平衡态时的电势,也就是标准态时的电极电势.标准电极电势有很大的实用价值,可用来判断氧化剂与还原剂的相对强弱,判断氧化还原反应的进行方向,计算原电池的电动势、反应自由能、平衡常数,计算其他半反应的标准电极电势,等等。
将半反应按电极电势由低到高排序,可以得到标准电极电势表,可十分简明地判断氧还反应的方向.[编辑本段]公式任何温度下标准氢电极的标准电极电势值都为0,但其他电极电势值会受到温度影响。
以Ni/NiO电极为例,它可以用作高温伪参比电极,在0-400°C时的电极电势大致符合以下公式:E°(T)=-0.0003T+0.1414 ,T为温度[编辑本段]电极电势内容1 在酸性溶液中(298K)电对方程式Eq/VLi(I)-(0) Li++e-=Li -3.0401Cs(I)-(0) Cs++e-=Cs -3.026Rb(I)-(0) Rb++e-=Rb -2.98K(I)-(0) K++e-=K -2.931Ba(II)-(0) Ba2++2e-=Ba -2.912Sr(II)-(0) Sr2++2e-=Sr -2.89Ca(II)-(0) Ca2++2e-=Ca -2.868Na(I)-(0) Na++e-=Na -2.71La(III)-(0) La3++3e-=La -2.379Mg(II)-(0) Mg2++2e-=Mg -2.37 2Ce(III)-(0) Ce3++3e-=Ce -2.336H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069Th(IV)-(0) Th4++4e-=Th -1.899Be(II)-(0) Be2++2e-=Be -1.847U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf +H2O -1.724Al(III)-(0) Al3++3e-=Al -1.662Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2 H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24Mn(II)-(0) Mn2++2e-=Mn -1.18 5Cr(II)-(0) Cr2++2e-=Cr -0.913Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2 H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2T a+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2 Nb+5H2O -0.644As(0)-(-III) As+3H++3e-=AsH3-0.608U(IV)-(III) U4++e-=U3+-0.607Ga(III)-(0) Ga3++3e-=Ga -0.54 9P(I)-(0) H3PO2+H++e-=P+2H2 O -0.508P(III)-(I) H3PO3+2H++2e-=H3P O2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C 2O4 -0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.40 7Cd(II)-(0) Cd2++2e-=Cd -0.403 0Se(0)-(-II) Se+2H++2e-=H2Se (aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0. 365Eu(III)-(II) Eu3++e-=Eu2+-0.3 6Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382Tl(I)-(0) Tl++e-=Tl -0.336Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3P O3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675Ni (II)-(0) Ni2++2e-=Ni -0.257V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=G e+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.152 24Sn(II)-(0) Sn2++2e-=Sn -0.137 5Pb(II)-(0) Pb2++2e-=Pb -0.126 2*C(IV)-(II) CO2(g)+2H++2e-=CO +H2O -0.12P(0)-(-III) P(white)+3H++3e-=P H3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037H(I)-(0) 2H++2e-=H2 0.0000Ag(I)-(0) AgBr+e-=Ag+Br-0.07 133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142Sn(IV)-(II) Sn4++2e-=Sn2+0.15 1Sb(III)-(0) Sb2O3+6H++6e-=2Sb +3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583S(VI)-(IV) SO42-+4H++2e-=H2 SO3+H2O 0.172Sb(III)-(0) SbO++2H++3e-=Sb +H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22 233As(III)-(0) HAsO2+3H++3e-=As +2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl -(饱和KCl) 0.26808Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(C N)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=R e+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+Cr O42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3 H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=H AsO2+2H2O 0.560Sb(V)-(III) Sb2O5+6H++4e-=2S bO++3H2O 0.581Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4] 2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O2 0. 695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl -0.755*Se(IV)-(0) H2SeO3+4H++4e-=S e+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771Hg(I)-(0) Hg22++2e-=2Hg 0.797 3Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os +4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2 O4+2H2O 0.803Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N 2O2+2H2O 0.86Hg(II)-(I) 2Hg2++2e-=Hg22+0. 920N(V)-(III) NO3-+3H++2e-=HNO 2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2 O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO 2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4C l- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=T eO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO +2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HN O2 1.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1. 0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl(V)-(IV) ClO3-+2H++e-=ClO 2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=Cl O3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HCl O2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO2 1.277+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e -=2Cr3++7H2O 1.33Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35 827Cl(VII)-(-I) ClO4-+8H++8e-=C l-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2 Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br -+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2 O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl -+3H2O 1.451++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2C l2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2B r2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.54 15Cl(III)-(-I) HClO2+3H++4e-=Cl -+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(a q)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H 2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO +H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2 e-=PbSO4+2H2O 1.6913Au(I)-(0) Au++e-=Au 1.692Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2 O 1.766O(-I)-(-II) H2O2+2H++2e-=2H 2O 1.776Co(III)-(II) Co3++e-=Co2+(2mo l·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H 2O 2.076O(II)-(-II) F2O+2H++4e-=H2O +2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=F e3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532 在碱性溶液中(298K)电对方程式Eq/VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH --3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH --2.99La(III)-(0) La(OH)3+3e-=La+3OH --2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2O H--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=H f+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr +4OH--2.36Al(III)-(0) H2AlO3-+H2O+3e-=A l+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B +4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P +5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si +6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2 PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2O H--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH --1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn +4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH --1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Z n+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr +4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HP O32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn +4NH3 -1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HS nO2-+H2O+3OH--0.9332-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.92 4Sn(II)-(0) HSnO2-+H2O+2e-=S n+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2 O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH --0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691s+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=S b+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=S bO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=R e+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S 2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=T e+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi +6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2 OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3 -0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34*Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2 CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH --0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3 -0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=S eO32-+2OH-0.05Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH -0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2 OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co (NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO +2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO 2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(C N)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=Cl O3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH3 0.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2O H-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(O H)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O +2OH-0.607Br(V)-(-I) BrO3-+3H2O+6e-=B r-+6OH-0.61Cl(V)-(-I) ClO3-+3H2O+6e-=Cl -+6OH-0.62Cl(III)-(I) ClO2-+H2O+2e-=ClO -+2OH-0.66I(VII)-(V) H3IO62-+2e-=IO3-+3 OH-0.7Cl(III)-(-I) ClO2-+2H2O+4e-=C l-+4OH-0.76Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841*Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95O(0)-(-II) O3+H2O+2e-=O2+2O H- 1.24标准电极电势表半反应E°(V) 来源& -9Zz 9N N2(g) + H+ + e− HN3(aq) -3.09 [6]Li+ + e− Li(s) -3.0401 [5]N2(g) + 4 H2O + 2 e− 2 N H2OH(aq) + 2 O H− -3.04 [6]Cs+ + e− Cs(s) -3.026 [5]Rb+ + e− Rb(s) -2.98 [4]K+ + e− K(s) -2.931 [5]Ba2+ + 2 e− Ba(s) -2.912 [5]La(OH)3(s) + 3 e− La(s) + 3OH− -2.9 0 [5]Sr2+ + 2 e− Sr(s) -2.899 [5]Ca2+ + 2 e− Ca(s) -2.868 [5]Eu2+ + 2 e− Eu(s) -2.812 [5]Ra2+ + 2 e− Ra(s) -2.8 [5]Na+ + e− Na(s) -2.71 [5][9]La3+ + 3 e− La(s) -2.379 [5]Y3+ + 3 e− Y(s) -2.372 [5]Mg2+ + 2 e− Mg(s) -2.372 [5]ZrO(OH)2(s) + H2O + 4 e− Zr(s) + 4 OH− -2.36 [5]Al(OH)4− + 3 e− Al(s) + 4 O H− -2.33Al(OH)3(s) + 3 e− Al(s) + 3OH− -2.3 1H2(g) + 2 e− 2 H− -2.25Ac3+ + 3 e− Ac(s) -2.20Be2+ + 2 e− Be(s) -1.85U3+ + 3 e− U(s) -1.66 [7]Al3+ + 3 e− Al(s) -1.66 [9]Ti2+ + 2 e− Ti(s) -1.63 [9]ZrO2(s) + 4 H+ + 4 e− Zr(s) + 2 H2O -1.553 [5]Zr4+ + 4 e− Zr(s) -1.45 [5]TiO(s) + 2 H+ + 2 e− Ti(s) + H2O -1.31Ti2O3(s) + 2 H+ + 2 e− 2 T iO(s) + H2 O -1.23Ti3+ + 3 e− Ti(s) -1.21Te(s) + 2 e− Te2− -1.143 [2]V2+ + 2 e− V(s) -1.13 [2]Nb3+ + 3 e− Nb(s) -1.099Sn(s) + 4 H+ + 4 e− SnH4(g) -1.07Mn2+ + 2 e− Mn(s) -1.029 [9]SiO2(s) + 4 H+ + 4 e− Si(s) + 2 H2O -0.91B(OH)3(aq) + 3 H+ + 3 e− B(s) + 3 H 2O -0.89TiO2+ + 2 H+ + 4 e− Ti(s) + H2O -0.86Bi(s) + 3 H+ + 3 e− BiH3 -0.8H2 H2O + 2 e− H2(g) +2 O H− -0.827 7 [5]Zn2+ + 2 e− Zn(Hg) -0.7628 [5]Zn2+ + 2 e− Zn(s) -0.7618 [5]Ta2O5(s) + 10 H+ + 10 e− 2 T a(s) + 5 H2O -0.75Cr3+ + 3 e− Cr(s) -0.74Au[Au(CN)2]− + e− Au(s) + 2 C N− -0.60Ta3+ + 3 e− Ta(s) -0.6PbO(s) + H2O + 2 e− Pb(s) + 2 O H−-0.58Ti2 T iO2(s) + 2 H+ + 2 e− Ti2O3(s) + H2O -0.56Ga3+ + 3 e− Ga(s) -0.53U4+ + e− U3+ -0.52 [7]P H3PO2(aq) + H+ + e− P(白磷[10]) + 2 H2O -0.508 [5]P H3PO3(aq) + 2 H+ + 2 e− H3PO2(a q) + H2O -0.499 [5]P H3PO3(aq) + 3 H+ + 3 e− P(红磷) [10] + 3H2O -0.454 [5]Fe2+ + 2 e− Fe(s) -0.44 [9]C2 C O2(g) + 2 H+ + 2 e− HOOCCOO H(aq) -0.43Cr3+ + e− Cr2+ -0.42Cd2+ + 2 e− Cd(s) -0.40 [9]GeO2(s) + 2 H+ + 2 e− GeO(s) + H2 O -0.37Cu2O(s) + H2O + 2 e− 2 C u(s) + 2 O H− -0.360 [5]PbSO4(s) + 2 e− Pb(s) + SO42− -0.3 588 [5]PbSO4(s) + 2 e− Pb(Hg) + SO42− -0. 3505 [5]Eu3+ + e− Eu2+ -0.35 [7]In3+ + 3 e− In(s) 0.34 [2]Tl+ + e− Tl(s) -0.34 [2]Ge(s) + 4 H+ + 4 e− GeH4(g) -0.29Co2+ + 2 e− Co(s) -0.28 [5]P H3PO4(aq) + 2 H+ + 2 e− H3PO3(a q) + H2O -0.276 [5]V3+ + e− V2+ 0.26 [9]Ni2+ + 2 e− Ni(s) -0.25As(s) + 3 H+ + 3 e− AsH3(g) -0.23 [2]MoO2(s) + 4 H+ + 4 e− Mo(s) + 2 H2 O -0.15Si(s) + 4 H+ + 4 e− SiH4(g) -0.14Sn2+ + 2 e− Sn(s) -0.13O2(g) + H+ + e− HO2•(aq) -0.13Pb2+ + 2 e− Pb(s) -0.13 [9]WO2(s) + 4 H+ + 4 e− W(s) + 2 H2O -0.12P(红磷) + 3 H+ + 3 e− PH3(g) -0.111[5]C CO2(g) + 2 H+ + 2 e− HCOOH(aq) -0.11Se(s) + 2 H+ + 2 e− H2Se(g) -0.11C CO2(g) + 2 H+ + 2 e− CO(g) + H2 O -0.11SnO(s) + 2 H+ + 2 e− Sn(s) + H2O -0.10SnO2(s) + 2 H+ + 2 e− SnO(s) + H2 O -0.09WO3(aq) + 6 H+ + 6 e− W(s) + 3 H2 O -0.09 [2]P(白磷) + 3 H+ + 3 e− PH3(g) -0.063[5]C HCOOH(aq) + 2 H+ + 2 e− HCHO (aq) + H2O -0.03H 2 H+ + 2 e− H2(g) ≡ 0S4O62− + 2 e− 2 S2O32− +0.08Fe3O4(s) + 8 H+ + 8 e− 3 F e(s) + 4 H 2O +0.085 [8]N2(g) + 2 H2O + 6H+ + 6 e− 2 N H4O H(aq) +0.092HgO(s) + H2O + 2 e− Hg(l) + 2 O H−+0.0977Cu(NH3)42+ + e− Cu(NH3)2+ + 2 N H 3 +0.10 [2]Ru(NH3)63+ + e− Ru(NH3)62+ +0.10 [7]N2H4(aq) + 4 H2O + 2 e− 2 N H4+ + 4 O H− +0.11 [6]Mo H2MoO4(aq) + 6 H+ + 6 e− Mo(s) + 4 H2O +0.11Ge4+ + 4 e− Ge(s) +0.12C(s) + 4 H+ + 4 e− CH4(g) +0.13 [2]C HCHO(aq) + 2 H+ + 2 e− CH3OH(a q) +0.13S(s) + 2 H+ + 2 e− H2S(g) +0.14Sn4+ + 2 e− Sn2+ +0.15Cu2+ + e− Cu+ +0.159 [2]S HSO4− + 3 H+ + 2 e− SO2(aq) + 2 H2O +0.16UO22+ + e− UO2+ +0.163 [7]S SO42− + 4 H+ + 2 e− SO2(aq) + 2 H2O +0.17TiO2+ + 2 H+ + e− Ti3+ + H2O +0.1 9Bi3+ + 2e− Bi+ +0.2SbO+ + 2 H+ + 3 e− Sb(s) + H2O +0.20As H3AsO3(aq) + 3 H+ + 3 e− As(s) + 3 H2O +0.24GeO(s) + 2 H+ + 2 e− Ge(s) + H2O +0.26UO2+ + 4 H+ + e− U4+ + 2 H2O +0. 273 [7]Re3+ + 3 e− Re(s) +0.300Bi3+ + 3 e− Bi(s) +0.32VO2+ + 2 H+ + e− V3+ + H2O +0.34Cu2+ + 2 e− Cu(s) +0.340 [2]Fe [Fe(CN)6]3− + e− [Fe(CN)6]4− +0.36O2(g) + 2 H2O + 4 e− 4 O H−(aq) +0.4 0 [9]Mo H2MoO4 + 6 H+ + 3 e− Mo3+ + 2 H2O +0.43Bi+ + e− Bi(s) +0.50C CH3OH(aq) + 2 H+ + 2 e− CH4(g) + H2O +0.50S SO2(aq) + 4 H+ + 4 e− S(s) + 2 H2 O +0.50Cu+ + e− Cu(s) +0.520 [2]C CO(g) + 2 H+ + 2 e− C(s) + H2O +0.52I2(s) + 2 e− 2 I− +0.54 [9]I3− + 2 e− 3 I− +0.53 [9]Au [AuI4]− + 3 e− Au(s) + 4 I− +0.56As H3AsO4(aq) + 2 H+ + 2 e− H3As O3(aq) + H2O +0.56Au [AuI2]− + e− Au(s) + 2 I− +0.58MnO4− + 2 H2O + 3 e− MnO2(s) + 4 OH− +0.59S2O32 −+ 6 H+ + 4 e− 2 S(s) + 3 H2 O +0.60Mo H2MoO4(aq) + 2 H+ + 2 e− MoO 2(s) + 2 H2O +0.65O2(g) + 2 H+ + 2 e− H2O2(aq) +0.70Tl3+ + 3 e− Tl(s) +0.72PtCl62− + 2 e− PtCl42− + 2 C l− +0.7 26 [7]Se H2SeO3(aq) + 4 H+ + 4 e− Se(s) + 3 H2O +0.74PtCl42− + 2 e− Pt(s) + 4 C l− +0.758 [7]Fe3+ + e− Fe2+ +0.77Ag+ + e− Ag(s) +0.7996 [5]Hg22+ + 2 e− 2 H g(l) +0.80N NO3−(aq) + 2 H+ + e− NO2(g) +H2O +0.80Au [AuBr4]− + 3 e− Au(s) + 4 B r− + 0.85Hg2+ + 2 e− Hg(l) +0.85MnO4− + H+ + e− HMnO4− +0.90Hg 2 H g2+ + 2 e− Hg22+ +0.91 [2]Pd2+ + 2 e− Pd(s) +0.915 [7]Au [AuCl4]− + 3 e− Au(s) + 4 C l− +0.93MnO2(s) + 4 H+ + e− Mn3+ + 2 H2O +0.95Au [AuBr2]− + e− Au(s) + 2 B r− +0.96Br2(l) + 2 e− 2 B r− +1.07Br2(aq) + 2 e− 2 B r− +1.09 [9]I IO3− + 5 H+ + 4 e− HIO(aq) + 2 H2 O +1.13Au [AuCl2]− + e− Au(s) + 2 C l− +1.15Se HSeO4− + 3 H+ + 2 e− H2SeO3(a q) + H2O +1.15Ag2O(s) + 2 H+ + 2 e− 2 A g(s) + H2 O +1.17ClO3− + 2 H+ + e− ClO2(g) + H2O +1.18Pt2+ + 2 e− Pt(s) +1.188 [7]ClO2(g) + H+ + e− HClO2(aq) +1.19I 2 I O3− + 12 H+ + 10 e− I2(s) + 6 H2 O +1.20ClO4− + 2 H+ + 2 e− ClO3− + H2O +1.20O2(g) + 4 H+ + 4 e− 2 H2O +1.23 [9]MnO2(s) + 4 H+ + 2 e− Mn2+ + 2H2 O +1.23Tl3+ + 2 e− Tl+ +1.25Cl2(g) + 2 e− 2 C l− +1.36 [9]Cr2O7− −+ 14 H+ + 6 e− 2 C r3+ + 7 H2O +1.33CoO2(s) + 4 H+ + e− Co3+ + 2 H2O +1.42N 2 N H3OH+ + H+ + 2 e− N2H5+ + 2 H2O +1.42 [6]I 2 H IO(aq) + 2 H+ + 2 e− I2(s) + 2 H 2O +1.44Ce4+ + e− Ce3+ +1.44BrO3− + 5 H+ + 4 e− HBrO(aq) + 2 H 2O +1.45PbO β-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O +1.460 [2]PbO α-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O +1.468 [2]Br 2 B rO3− + 12 H+ + 10 e− Br2(l) + 6 H2O +1.48Cl 2ClO3− + 12 H+ + 10 e− Cl2(g) + 6 H2O +1.49MnO4− + 8 H+ + 5 e− Mn2+ + 4 H2O +1.51O HO2• + H+ + e− H2O2(aq) +1.51Au3+ + 3 e− Au(s) +1.52NiO2(s) + 4 H+ + 2 e− Ni2++ 2 O H−+1.59Cl 2 H ClO(aq) + 2 H+ + 2 e− Cl2(g) + 2 H2O +1.63Ag2O3(s) + 6 H+ + 4 e− 2 A g+ + 3 H2 O +1.67Cl HClO2(aq) + 2 H+ + 2 e− HClO(aq) + H2O +1.67Pb4+ + 2 e− Pb2+ +1.69 [2]MnO4− + 4 H+ + 3 e− MnO2(s) + 2 H 2O +1.70O H2O2(aq) + 2 H+ + 2 e− 2 H2O +1.78AgO(s) + 2 H+ + e− Ag+ + H2O +1.77Co3+ + e− Co2+ +1.82Au+ + e− Au(s) +1.83 [2]BrO4− + 2 H+ + 2 e− BrO3− + H2O +1.85Ag2+ + e− Ag+ +1.98 [2]S2O82− + 2 e− 2 S O42− +2.07O3(g) + 2 H+ + 2 e− O2(g) + H2O + 2.075 [7]Mn HMnO4− + 3 H+ + 2 e− MnO2(s) + 2 H2O +2.09F2(g) + 2 e− 2 F− +2.87 [2][9]F2(g) + 2 H+ + 2 e− 2 H F(aq) +3.05 [2]。
(完整版)标准电极电势表(非常全)

标准电极电势Standard Electrode Potentials下表中所列的标准电极电势(25.0℃,101.325kPa)是相对于标准氢电极电势的值。
标准氢电极电势被规定为零伏特(0.0V)。
序号(No.)电极过程(Electrode process)EÅ/V 1Ag++e═Ag0.79962Ag2++e═Ag+ 1.983AgBr+e═Ag+Br-0.07134AgBrO3+e═Ag+BrO3-0.5465AgCl+e═Ag+Cl-0.2226AgCN+e═Ag+CN--0.0177Ag2CO3+2e═2Ag+CO32-0.478Ag2C2O4+2e═2Ag+C2O42-0.4659Ag2CrO4+2e═2Ag+CrO42-0.44710AgF+e═Ag+F-0.77911Ag4[Fe(CN)6]+4e═4Ag+[Fe(CN)6]4-0.14812AgI+e═Ag+I--0.152 13AgIO3+e═Ag+IO3-0.35414Ag2MoO4+2e═2Ag+MoO42-0.45715[Ag(NH3)2]++e═Ag+2NH30.37316AgNO2+e═Ag+NO2-0.56417Ag2O+H2O+2e═2Ag+2OH-0.342182AgO+H2O+2e═Ag2O+2OH-0.60719Ag2S+2e═2Ag+S2--0.691 20Ag2S+2H++2e═2Ag+H2S-0.0366 21AgSCN+e═Ag+SCN-0.0895 22Ag2SeO4+2e═2Ag+SeO42-0.36323Ag2SO4+2e═2Ag+SO42-0.65424Ag2WO4+2e═2Ag+WO42-0.46625Al3+3e═Al-1.662 26AlF63-+3e═Al+6F--2.069 27Al(OH)3+3e═Al+3OH--2.3128AlO2-+2H2O+3e═Al+4OH--2.3529Am3++3e═Am-2.048 30Am4++e═Am3+ 2.631AmO22++4H++3e═Am3++2H2O 1.7532As+3H++3e═AsH3-0.608 33As+3H2O+3e═AsH3+3OH--1.3734As2O3+6H++6e═2As+3H2O0.23435HAsO2+3H++3e═As+2H2O0.24836AsO2-+2H2O+3e═As+4OH--0.6837H3AsO4+2H++2e═HAsO2+2H2O0.5638AsO43-+2H2O+2e═AsO2-+4OH--0.7139AsS2-+3e═As+2S2--0.7540AsS43-+2e═ AsS2-+2S2--0.641Au++e═Au 1.69242Au3++3e═Au 1.49843Au3++2e═Au+ 1.401 44AuBr2-+e═Au+2Br-0.959 45AuBr4-+3e═Au+4Br-0.854 46AuCl2-+e═Au+2Cl- 1.15 47AuCl4-+3e═Au+4Cl- 1.002 48AuI+e═Au+I-0.5 49Au(SCN)4-+3e═Au+4SCN-0.66 50Au(OH)3+3H++3e═Au+3H2O 1.45 51BF4-+3e═B+4F--1.04 52H2BO3-+H2O+3e═B+4OH--1.79 53B(OH)3+7H++8e═BH4-+3H2O-0.0481 54Ba2++2e═Ba-2.912 55Ba(OH)2+2e═Ba+2OH--2.99 56Be2++2e═Be-1.847 57Be2O32-+3H2O+4e═2Be+6OH--2.63 58Bi++e═Bi0.5 59Bi3++3e═Bi0.308 60BiCl4-+3e═Bi+4Cl-0.16 61BiOCl+2H++3e═Bi+Cl-+H2O0.16 62Bi2O3+3H2O+6e═2Bi+6OH--0.46 63Bi2O4+4H++2e═2BiO++2H2O 1.593 64Bi2O4+H2O+2e═ Bi2O3+2OH-0.56 65Br2(水溶液,aq)+2e═2Br- 1.087 66Br2(液体)+2e═2Br- 1.066 67BrO-+H2O+2e═Br-+2OH0.761 68BrO3-+6H++6e═Br-+3H2O 1.423 69BrO3-+3H2O+6e═Br-+6OH-0.61 702BrO3-+12H++10e═Br2+6H2O 1.482 71HBrO+H++2e═Br-+H2O 1.331 722HBrO+2H++2e═Br2(水溶液,aq)+2H2O 1.574 73CH3OH+2H++2e═CH4+H2O0.59 74HCHO+2H++2e═CH3OH0.19 75CH3COOH+2H++2e═CH3CHO+H2O-0.12 76(CN)2+2H++2e═2HCN0.373 77(CNS)2+2e═2CNS-0.77 78CO2+2H++2e═CO+H2O-0.12 79CO2+2H++2e═HCOOH-0.199 80Ca2++2e═Ca-2.868 81Ca(OH)2+2e═Ca+2OH--3.02 82Cd2++2e═Cd-0.403 83Cd2++2e═Cd(Hg)-0.352 84Cd(CN)42-+2e═Cd+4CN--1.09 85CdO+H2O+2e═Cd+2OH--0.783 86CdS+2e═Cd+S2--1.17 87CdSO4+2e═Cd+SO42--0.246 88Ce3++3e═Ce-2.336 89Ce3++3e═Ce(Hg)-1.437 90CeO2+4H++e═Ce3++2H2O 1.4 91Cl2(气体)+2e═2Cl- 1.35892ClO-+H2O+2e═Cl-+2OH-0.89 93HClO+H++2e═Cl-+H2O 1.482 942HClO+2H++2e═Cl2+2H2O 1.611 95ClO2-+2H2O+4e═Cl-+4OH-0.76 962ClO3-+12H++10e═Cl2+6H2O 1.47 97ClO3-+6H++6e═Cl-+3H2O 1.451 98ClO3-+3H2O+6e═Cl-+6OH-0.62 99ClO4-+8H++8e═Cl-+4H2O 1.38 1002ClO4-+16H++14e═Cl2+8H2O 1.39 101Cm3++3e═Cm-2.04 102Co2++2e═Co-0.28 103[Co(NH3)6]3++e═[Co(NH3)6]2+0.108 104[Co(NH3)6]2++2e═Co+6NH3-0.43 105Co(OH)2+2e═Co+2OH--0.73 106Co(OH)3+e═Co(OH)2+OH-0.17 107Cr2++2e═Cr-0.913 108Cr3++e═Cr2+-0.407 109Cr3++3e═Cr-0.744 110[Cr(CN)6]3-+e═[Cr(CN)6]4--1.28 111Cr(OH)3+3e═Cr+3OH--1.48 112Cr2O72-+14H++6e═2Cr3++7H2O 1.232 113CrO2-+2H2O+3e═Cr+4OH--1.2 114HCrO4-+7H++3e═Cr3++4H2O 1.35 115CrO42-+4H2O+3e═Cr(OH)3+5OH--0.13 116Cs++e═Cs-2.92 117Cu++e═Cu0.521 118Cu2++2e═Cu0.342 119Cu2++2e═Cu(Hg)0.345 120Cu2++Br-+e═CuBr0.66 121Cu2++Cl-+e═CuCl0.57 122Cu2++I-+e═CuI0.86 123Cu2++2CN-+e═[Cu(CN)2]- 1.103 124CuBr2-+e═Cu+2Br-0.05 125CuCl2-+e═Cu+2Cl-0.19 126CuI2-+e═Cu+2I-0 127Cu2O+H2O+2e═2Cu+2OH--0.36 128Cu(OH)2+2e═Cu+2OH--0.222 1292Cu(OH)2+2e═Cu2O+2OH-+H2O-0.08 130CuS+2e═Cu+S2--0.7 131CuSCN+e═Cu+SCN--0.27 132Dy2++2e═Dy-2.2 133Dy3++3e═Dy-2.295 134Er2++2e═Er-2 135Er3++3e═Er-2.331 136Es2++2e═Es-2.23 137Es3++3e═Es-1.91 138Eu2++2e═Eu-2.812 139Eu3++3e═Eu-1.991 140F2+2H++2e═2HF 3.053190IO3-+2H2O+4e═IO-+4OH-0.15 191IO3-+3H2O+6e═I-+6OH-0.26 1922IO3-+6H2O+10e═I2+12OH-0.21 193H5IO6+H++2e═IO3-+3H2O 1.601 194In++e═In-0.14 195In3++3e═In-0.338 196In(OH)3+3e═In+3OH--0.99 197Ir3++3e═Ir 1.156 198IrBr62-+e═ IrBr63-0.99 199IrCl62-+e═IrCl63-0.867 200K++e═K-2.931 201La3++3e═La-2.379 202La(OH)3+3e═La+3OH--2.9 203Li++e═Li-3.04 204Lr3++3e═Lr-1.96 205Lu3++3e═Lu-2.28 206Md2++2e═Md-2.4 207Md3++3e═Md-1.65 208Mg2++2e═Mg-2.372 209Mg(OH)2+2e═Mg+2OH--2.69 210Mn2++2e═Mn-1.185 211Mn3++3e═Mn 1.542 212MnO2+4H++2e═Mn2++2H2O 1.224 213MnO4-+4H++3e═MnO2+2H2O 1.679 214MnO4-+8H++5e═Mn2++4H2O 1.507 215MnO4-+2H2O+3e═MnO2+4OH-0.595 216Mn(OH)2+2e═Mn+2OH--1.56 217Mo3++3e═Mo-0.2 218MoO42-+4H2O+6e═Mo+8OH--1.05 219N2+2H2O+6H++6e═2NH4OH0.092 2202NH3OH++H++2e═N2H5++2H2O 1.42 2212NO+H2O+2e═N2O+2OH-0.76 2222HNO2+4H++4e═N2O+3H2O 1.297 223NO3-+3H++2e═HNO2+H2O0.934 224NO3-+H2O+2e═NO2-+2OH-0.01 2252NO3-+2H2O+2e═N2O4+4OH--0.85 226Na++e═Na-2.713 227Nb3++3e═Nb-1.099 228NbO2+4H++4e═Nb+2H2O-0.69 229Nb2O5+10H++10e═2Nb+5H2O-0.644 230Nd2++2e═Nd-2.1 231Nd3++3e═Nd-2.323 232Ni2++2e═Ni-0.257 233NiCO3+2e═Ni+CO32--0.45 234Ni(OH)2+2e═Ni+2OH--0.72 235NiO2+4H++2e═Ni2++2H2O 1.678 236No2++2e═No-2.5 237No3++3e═No-1.2 238Np3++3e═Np-1.856239NpO2+H2O+H++e═Np(OH)3-0.962 240O2+4H++4e═2H2O 1.229 241O2+2H2O+4e═4OH-0.401 242O3+H2O+2e═O2+2OH- 1.24 243Os2++2e═Os0.85 244OsCl63-+e═Os2++6Cl-0.4 245OsO2+2H2O+4e═Os+4OH--0.15 246OsO4+8H++8e═Os+4H2O0.838 247OsO4+4H++4e═OsO2+2H2O 1.02 248P+3H2O+3e═PH3(g)+3OH--0.87 249H2PO2-+e═P+2OH--1.82 250H3PO3+2H++2e═H3PO2+H2O-0.499 251H3PO3+3H++3e═P+3H2O-0.454 252H3PO4+2H++2e═H3PO3+H2O--0.276 253PO43-+2H2O+2e═HPO32-+3OH--1.05 254Pa3++3e═Pa-1.34 255Pa4++4e═Pa-1.49 256Pb2++2e═Pb-0.126 257Pb2++2e═Pb(Hg)-0.121 258PbBr2+2e═Pb+2Br--0.284 259PbCl2+2e═Pb+2Cl--0.268 260PbCO3+2e═Pb+CO32--0.506 261PbF2+2e═Pb+2F--0.344 262PbI2+2e═Pb+2I--0.365 263PbO+H2O+2e═Pb+2OH--0.58 264PbO+4H++2e═Pb+H2O0.25 265PbO2+4H++2e═Pb2+2H2O 1.455 266HPbO2-+H2O+2e═Pb+3OH--0.537 267PbO2+SO42-+4H++2e═PbSO4+2H2O 1.691 268PbSO4+2e═Pb+SO42--0.359 269Pd2++2e═Pd0.915 270PdBr42-+2e═Pd+4Br-0.6 271PdO2+H2O+2e═PdO+2OH-0.73 272Pd(OH)2+2e═Pd+2OH-0.07 273Pm2++2e═Pm-2.2 274Pm3++3e═Pm-2.3 275Po4++4e═Po0.76 276Pr2++2e═Pr-2 277Pr3++3e═Pr-2.353 278Pt2++2e═Pt 1.18 279[PtCl6]2-+2e═[PtCl4]2-+2Cl-0.68 280Pt(OH)2+2e═Pt+2OH-0.14 281PtO2+4H++4e═Pt+2H2O1 282PtS+2e═Pt+S2--0.83 283Pu3++3e═Pu-2.031 284Pu5++e═Pu4+ 1.099 285Ra2++2e═Ra-2.8 286Rb++e═Rb-2.98 287Re3++3e═Re0.3337Th4++4e═Th-1.899 338Ti2++2e═Ti-1.63 339Ti3++3e═Ti-1.37 340TiO2+4H++2e═Ti2++2H2O-0.502 341TiO2++2H++e═Ti3++H2O0.1 342Tl++e═Tl-0.336 343Tl3++3e═Tl0.741 344Tl3++Cl-+2e═TlCl 1.36 345TlBr+e═Tl+Br--0.658 346TlCl+e═Tl+Cl--0.557 347TlI+e═Tl+I--0.752 348Tl2O3+3H2O+4e═2Tl++6OH-0.02 349TlOH+e═Tl+OH--0.34 350Tl2SO4+2e═2Tl+SO42--0.436 351Tm2++2e═Tm-2.4 352Tm3++3e═Tm-2.319 353U3++3e═U-1.798 354UO2+4H++4e═U+2H2O-1.4 355UO2++4H++e═U4++2H2O0.612 356UO22++4H++6e═U+2H2O-1.444 357V2++2e═V-1.175 358VO2++2H++e═V3++H2O0.337 359VO2++2H++e═VO2++H2O0.991 360VO2++4H++2e═V3++2H2O0.668 361V2O5+10H++10e═2V+5H2O-0.242 362W3++3e═W0.1 363WO3+6H++6e═W+3H2O-0.09 364W2O5+2H++2e═2WO2+H2O-0.031 365Y3++3e═Y-2.372 366Yb2++2e═Yb-2.76 367Yb3++3e═Yb-2.19 368Zn2++2e═Zn-0.7618 369Zn2++2e═Zn(Hg)-0.7628 370Zn(OH)2+2e═Zn+2OH--1.249 371ZnS+2e═Zn+S2--1.4 372ZnSO4+2e═Zn(Hg)+SO42--0.799。
标准电极电势表 (碱)

电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02 Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0) La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88 Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0) H2PO2-+e-=P+2OH--1.82 B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I) Te+2e-=Te2--1.143 P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924 Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691 As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627 Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46 *Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12 O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0) AgCN+e-=Ag+CN--0.017 N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01 Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08 Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108 Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14 Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17 Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247 I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33 Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342 Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358 Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373 O(0)-(-II) O2+2H2O+4e-=4OH-0.401 I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485 *Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490 Mn(VII)-(VI) MnO4-+e-=MnO42-0.558 Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595 Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60 Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607 Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61 Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62 Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66 I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7 Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76 Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761 Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841 *Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95。
标准电极电势表(全)
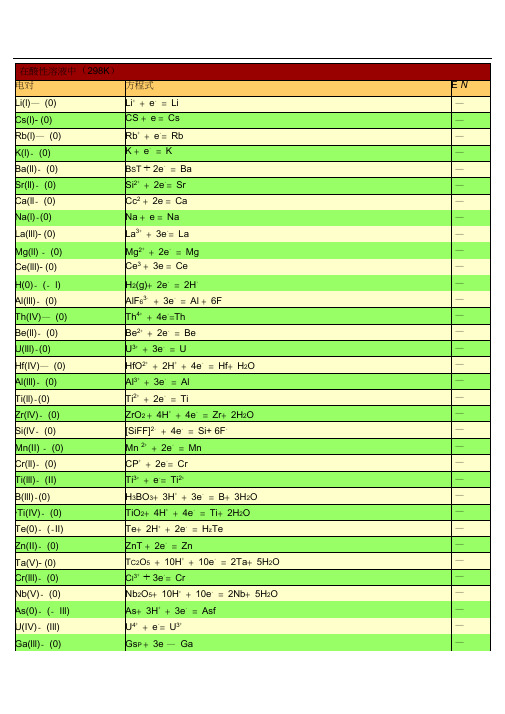
电对
方程式
EN
Li(l)—(0)
Li++e-=Li
—
Cs(l)-(0)
CS+e=Cs
—
Rb(l)—(0)
Rb++e-=Rb
—
K(l)-(0)
K+e-=K
—
Ba(ll)-(0)
BsT+2e-=Ba
—
Sr(ll)-(0)
Si2++2e-=Sr
—
Ca(ll-(0)
Cc2+2e=Ca
TeQ+4H++4e—=Te+2H2O
U(V)—(IV)
UQ++4H++e—=U4++2H2O
**Hg(II)—(I)
2HgC2+2e—=Hg?Cb+2C「
Pt(IV)—(II)
[PtC6]2-+2e—=[PtC4]2-+2C「
O(0)—(—I)
O2+2H+2e=H2O2
Pt(II)—(0)
[PtCl4]2-+2e—=Pt+4C「
—
ln (lll)-(0)
In3++3e-=In
—
Tl(l)-(0)
Tl++e-=Tl
—
Co(ll)- (0)
Cc?+2e=Co
—
P(V)-(lll)
H3PO4+2H++2e-=H3PO3+H2O
—
Pb(ll)-(0)
PbC2+2e-=Pb+2C-
标准电极电势表(酸+碱)

0.761
68
BrO 3-+6H ++6e═ Br -+3H 2O
1.423
69
BrO 3-+3H 2O+6e═ Br -+6OH -
0.61
70
2BrO 3-+12H ++10e═Br 2+6H 2O
1.482
71
HBrO+H ++2e═Br -+H 2O
1.331
72
2HBrO+2H ++2e═Br 2(水溶液 ,aq)+2H 2O
0.465
9
Ag2CrO
4+2e═ 2Ag+CrO
24
0.447
10
AgF+e═ Ag+F -
6]+4e═ 4Ag+[Fe(CN) 6]4-
0.148
12
AgI+e ═ Ag+I -
-0.152
13
AgIO
3+e═ Ag+IO
3
0.354
14
Ag2MoO
4+2e═ 2Ag+MoO
2ClO 3-+12H ++10e═Cl 2+6H 2O
1.47
97
ClO 3-+6H ++6e═ Cl -+3H 2O
1.451
98
ClO 3-+3H 2O+6e═ Cl -+6OH -
0.62
99
ClO 4-+8H ++8e═ Cl -+4H 2O
标准电极电势表67748
5
Na++e-=Na
Sn4++2e-=Sn2+
Nb3++3e-=Nb
Sr++e-=Sr
Ni2++2e-=Ni
Sr2++2e-=Sr
NiO2+4H++2e-=Ni2++2H2O
Sr2++2e-=Sr(Hg)
O2+2H++2e-=H2O2
Te+2H++2e-=H2Te
电极反应
E/V
5
H3PO4+2H++2e-=H3PO3+H2O
I3-+2e-=3I-
Pb2++2e-=Pb
2
H5IO6+H++2e-=IO3-+3H2O
PbBr2+2e-=Pb+2Br-
2HIO+2H++2e-=I2+2H2O
PbCl2+2e-=Pb+2Cl-
5
HIO+H++2e-=I-+H2O
PbF2+2e-=Pb+2F-
BiCl-4+3e-=Bi+4Cl-
Cu2++e-=Cu+
Bi2O4+4H++2e-=2BiO++2H2O
Cu2++2e-=Cu
9
BiO++2H++3e-=Bi+H2O
CuCl+e-=Cu+Cl-
BiOCl+2H++3e-=Bi+Cl-+H2O
3
F2+2H++2e-=2HF
Br2(aq)+2e-=2Br-
0
HClO2+3H++4e-=Cl-+2H2O
AgF+e-=Ag+F-
ClO +2H++e-=ClO2+H2O
AgI+e-=Ag+I-
24
ClO +3H++2e-=HClO2+H2O
Ag2S+2H+2e-=2Ag+H2S
6
ClO +6H++5e-=1/2Cl2+3H2O
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
P(white)+3H++3e-=PH3(g)
Hg2Cl2+2e-=2Hg+2Cl-
08
H3PO2+H++e-=P+2H2O
Hg2I2+2e-=2Hg+2I-
5
H3PO3+2H++2e-=H3PO2+H2O
Hg2SO4+2e-=2Hg+SO
5
H3PO3+3H++3e-=P+3H2O
I2+2e-=2I-
SO +4H++2e-=H2SO3+H2O
N2+2H2O+6H++6e-=2NH4OH
2SO +4H++2e-=S2O +2H2O
3N2+2H++2e-=2NH3(aq)
Sb+3H++3e-=2SbH3
N2O+2H++2e-=N2+H2O
Sb2O3+6H++6e-=2Sb+3H2O
N2O4+2e-=2NO2-
O2+2H2O+2e-=H2O2+2OH-
Cu2+2CN-+e-=[Cu(CN)2]-
O2+2H2O+4e-=4OH-
[Cu(CN)2]-+e-=Cu+2CN-
O3+H2O+2e-=O2+2OH-
Cu2O+H2O+2e-=2Cu+2OH-
HO2-+H2O+2e-=3OH-
电极反应
E/V
电极反应
E/V
Mn(OH)2+2e-=Mn+2OH-
Cl +H2O+2e-=ClO-+2OH-
Mn(OH)3+e-=Mn(OH)2+OH-
Cl +2H2O+4e-=Cl-+4OH-
2NO+H2O+2e-=N2O+2OH-
Cl +H2O+2e-=Cl +2OH-
NO+H2O+e-=NO+2OH-
Cl +3H2O+6e-=Cl-+6OH-
Au3++2e-=Au+
Cr3++e-=Cr2+
H3BO3+3H++3e-=B+3H2O
8
Cr3++3e-=Cr
Ba2++2e-=Ba
Cr2O +14H++6e-=2Cr3++7H2O
Ba2++2e-=Ba(Hg)
HCrO4-+7H++3e-=Cr3++4H2O
Be2++2e-=Be
Cu++e-=Cu
附录八标准电极电势
()
1.在酸性溶液中
电极反应
E/V
电极反应
E/V
Ag++e-=Ag
6
Cd2++2e-=Cd(Hg)
1
Ag2++e-=Ag+
Ce3++3e-=Ce
AgAc+e-=Ag+Ac-
Cl2(g)+2e-=2Cl-
AgBr+e-=Ag+Br-
33
HClO+H++e-=1/2Cl2+H2O
Ag2BrO3+e-=2Ag+BrO3-
0
HClO2+3H++4e-=Cl-+2H2O
AgF+e-=Ag+F-
ClO +2H++e-=ClO2+H2O
AgI+e-=Ag+I-
24
ClO +3H++2e-=HClO2+H2O
Ag2S+2H+2e-=2Ag+H2S
6
ClO +6H++5e-=1/2Cl2+3H2O
AgSCN+e-=Ag+SCN-
Co(OH)3+e-=Co(OH)2+OH-
Ni(OH)2+2e-=Ni+2OH-
Cr +2H2O+3e-=Cr+4OH-
NiO2+2H2O+2e-=Ni(OH)2+2OH-
Cr +4H2O+3e-=Cr(OH)3+5OH-
O2+H2O+2e-=HO2-+OH-
Cr(OH)3+3e-=Cr+3OH-
Th4++4e-=Th
V(OH) +4H++5e-=V+4H2O
Ti2++2e-=Ti
W2O5+2H++2e-=2WO2+H2O
Ti3++e-=Ti2+
WO2+4H++4e-=W+2H2O
TiO2++2H++e-=Ti3++H2O
WO3+6H++6e-=W+3H2O
TiO2+4H++2e-=Ti2++2H2O
La(OH)3+3e-=La+3OH-
Br +3H2O+6e-=Br-+6OH-
Mg(OH)2+2e-=Mg+2OH-
Ca(OH)2+2e-=Ca+2OH-
MnO4-+2H2O+3e-=MnO2+4OH-
Ca(OH)2+2e-=Ca(Hg)+2OH-
Mn +2H2O+2e-=MnO2+4OH-
ClO-+H2O+2e-=Cl-+2OH-
51
ClO +6H++6e-=Cl-+3H2O
Ag2SO4+2e-=2Ag+S
ClO +2H++2e-=ClO3-+H2O
Al3++3e-=Al
AlF +3e-=Al+6F-
ClO +8H++7e-=1/2Cl2+4H2O
As2O3+6H++6e-=2As+3H2O
HAsO2+3H++3e-=As+2H2O
Pd(OH)2+2e-=Pd+2OH-
P+3H2O+3e-=PH3(g)+3OH-
2S +3H2O+4e-=S2 +6OH-
H2PO2-+e-=P+2OH-
S +H2O+2e-=SO +2OH-
HP +2H2O+2e-=H2P +3OH-
Sb +2H2O+3e-=Sb+4OH-
HP +2H2O+3e-=P+5OH-
Sb +H2O+2e-=SbO +2OH-
Sb2O5+6H++4e-=2SbO++3H2O
N2O4+2H++2e-=2HNO2
SbO++2H++3e-=Sb+H2O
N2O4+4H++4e-=2NO+2H2O
Sc3++3e-=Sc
2NO+2H++2e-=N2O+H2O
Se+2H++2e-=H2Se(aq)
HNO2+H++e-=NO+H2O
H2SeO3+4H++4e-=Se+3H2O
P +2H2O+2e-=HP +3OH-
Se +3H2O+4e-=Se+6OH-
PbO+H2O+2e-=Pb+2OH-
Se +H2O+2e-=Se +2OH-
HPbO2-+H2O+2e-=Pb+3OH-
Si +3H2O+4e-=Si+6OH-
PbO2+H2O+2e-=PbO+2OH-
HSnAg2O+H2O+2e-=2Ag+2OH-
[Fe(CN)6 +e-=[Fe(CN)6
2AgO+H2O+2e-=Ag2O+2OH-
Fe(OH)3+e-=Fe(OH)2+OH-
Ag2S+2e-=2Ag+S2-
H2GaO3-+H2O+3e-=Ga+4OH-
H2AlO3-+H2O+3e-=Al+4OH-
2H2O+2e-=H2+2OH-
电极反应
E/V
Te4++4e-=Te
V3++e-=V2+
TeO2+4H++4e-=Te+2H2O
VO2++2H++e-=V3++H2O
Te +8H++7e-=Te+4H2O
VO +2H++e-=VO2++H2O
H6TeO6+2H++2e-=TeO2+4 H2O
V(OH) +2H++e-=VO2++3H2O
5
H3PO4+2H++2e-=H3PO3+H2O
I3-+2e-=3I-
Pb2++2e-=Pb
2
H5IO6+H++2e-=IO3-+3H2O
PbBr2+2e-=Pb+2Br-
2HIO+2H++2e-=I2+2H2O
PbCl2+2e-=Pb+2Cl-
5
HIO+H++2e-=I-+H2O
PbF2+2e-=Pb+2F-
4
2IO +12H++10e-=I2+6H2O
PbI2+2e-=Pb+2I-
IO +6H++6e-=I-+3H2O
PbO2+4H++2e-=Pb2++2H2O
In3++2e-=In+
PbO2+SO +4H++2e-=PbSO4+2H2O
3
In3++3e-=In
2
PbSO4+2e-=Pb+SO
8
Ir3++3e-=Ir
Ba(OH)2+2e-=Ba+2OH-
IO3-+2H2O+4e-=IO-+4OH-
Be2 +3H2O+4e-=2Be+6OH-
IO3-+3H2O+6e-=I-+6OH-
Bi2O3+3H2O+6e-=2Bi+6OH-
Ir2O3+3H2O+6e-=2Ir+6OH-
BrO-+H2O+2e-=Br-+2OH-
ClO +8H++8e-=Cl-+4H2O
H3AsO4+2H++2e-=HAsO2+2H2O
Co2++2e-=Co
Au++e-=Au
Co3++e-=Co2+(2mol·L-1H2SO4)
Au3++3 e-=Au
CO2+2H++2e-=HCOOH
AuCl4-+3e-=Au+4Cl-
Cr2++2e-=Cr
7
As +2H2O+3e-=As+4OH-
Hg2O+H2O+2e-=2Hg+2OH-
As +2H2O+2e-=As +4OH-
HgO+H2O+2e-=Hg+2OH-
7
H2BO +5H2O+8e-=BH4-+8OH-
H3I +2e-=IO3-+3OH-
H2BO +H2O+3e-=B+4OH-
IO-+H2O+2e-=I-+2OH-
BrO +6H++5e-=l/2Br2+3H2O
FeO +8H++3e-=Fe3++4H2O
BrO +6H++6e-=Br-+3H2O
Ga3++3e-=Ga
