毕赤酵母表达操作手册(精译版)
毕赤酵母手册

毕赤酵母表达实验手册作者:Jnuxz 来源:丁香园时间:2007-9-5大肠杆菌表达系统最突出的优点是工艺简单、产量高、周期短、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结构,在大肠杆菌中表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯化,但不利于产物的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增加了成本。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过大肠杆菌表达的,其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调控机制和蛋白质的加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年来,以酵母作为工程菌表达外源蛋白日益引起重视,原因是与大肠杆菌相比,酵母是低等真核生物,除了具有细胞生长快,易于培养,遗传操作简单等原核生物的特点外,又具有真核生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,能有效克服大肠杆菌系统缺乏蛋白翻译后加工、修饰的不足。
因此酵母表达系统受到越来越多的重视和利用。
[1]。
同时与大肠杆菌相比,作为单细胞真核生物的酵母菌具有比较完备的基因表达调控机制和对表达产物的加工修饰能力。
酿酒酵母(Saccharomyces.Cerevisiae)在分子遗传学方面被人们的认识最早,也是最先作为外源基因表达的酵母宿主。
1981年酿酒酵母表达了第一个外源基因----干扰素基因[2],随后又有一系列外源基因在该系统得到表达[3、4、5、6]。
干扰素和胰岛素虽然已经利用酿酒酵母大量生产并被广泛应用,当利用酿酒酵母制备时,实验室的结果很令人鼓舞,但由实验室扩展到工业规模时,其产量迅速下降。
毕赤酵母诱导表达实验流程

毕赤酵母诱导表达实验流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!毕赤酵母(Pichia pastoris)是一种常用于外源蛋白表达的真核生物,具有高表达量、高分泌性和易于操作等优点。
毕赤酵母表达蛋白步骤

毕赤酵母表达蛋白步骤一、引言毕赤酵母(Pichia pastoris)是一种常用的真菌表达系统,被广泛应用于蛋白质的表达和生物技术研究中。
其优势包括高表达水平、易于培养和操作、能够正确折叠复杂蛋白等。
本文将介绍毕赤酵母表达蛋白的步骤。
二、构建表达载体毕赤酵母表达系统的关键是表达载体的构建。
首先,需要选择适合的表达载体,常用的有pPIC6、pPICZα等。
然后,在载体上选择合适的启动子和信号序列,以确保蛋白质能够被正确表达和分泌。
同时,还需要在表达载体上加入选择标记,如His标签、FLAG标签等,以便后续的蛋白质纯化和检测。
三、转化毕赤酵母将构建好的表达载体转化入毕赤酵母中,使其成为表达宿主。
转化方法包括电击转化、化学转化等。
其中,电击转化是常用的方法,通过电击脉冲使毕赤酵母细胞膜发生破裂,使表达载体进入细胞内。
转化后,将细胞培养在选择性培养基上,筛选出带有表达载体的毕赤酵母克隆。
四、表达蛋白经过转化筛选后,得到含有目标蛋白表达载体的毕赤酵母克隆。
接下来,需要将克隆进行培养,在适当的条件下诱导蛋白的表达。
常用的诱导剂包括甲醇、巯基乙醇等,通过加入适量的诱导剂,可以使目标蛋白得到高效表达。
五、蛋白纯化在蛋白表达后,需要进行蛋白纯化,以获得纯度较高的目标蛋白。
常用的纯化方法包括亲和层析、离子交换层析、凝胶过滤层析等。
在选择纯化方法时,需要根据目标蛋白的性质和需求进行合理选择。
同时,可以利用加入的选择标记,如His标签,通过亲和层析纯化进行快速高效的纯化。
六、蛋白鉴定和功能分析蛋白纯化后,需要进行蛋白的鉴定和功能分析。
常用的鉴定方法包括SDS-PAGE、Western blot等,可以确定蛋白的分子量和纯度。
功能分析则可以通过生物学实验来进行,如酶活测定、结合实验等,以验证目标蛋白的功能。
七、应用和展望毕赤酵母表达系统在生物技术和蛋白质研究领域有着广泛的应用。
通过该系统,可以高效表达各种蛋白,包括抗体、酶和重组蛋白等。
GS115毕赤酵母表达菌使用说明

编号
名称
北京华越洋生物 NRR01030 GS115 毕赤酵母表达菌
基 本 信 息 :
名称:GS115 毕赤酵母表达菌
规格:300ul 甘油菌
储 存 温 度 : -‐80℃
发突变为组氨酸野生型的概率一般低于 10-‐8。GS115 毕赤酵母可以在 YPD
培养基中生长,或者在补充有组氨酸的 minimal media 中生长,但是无法
在单独的 minimal media 中生长。GS115 毕赤酵母在做质粒转化的时候,
可 采 用 电 转 化 的 方 式 将 质 粒 转 入 。
基 因 组 :
His4( 基 因 5 是毕赤酵母菌株,是巴斯德毕赤酵母的一种,属于真核细胞。
一般的针对原核生物的抗生素例如卡那和氨苄对酵母是无效的,因此为了
操作说明:
1,本品包含一份甘油菌,使用本甘油菌时可以不用完全融解,在甘油菌表
面蘸取少量涂板或进行液体培养即可。也可以完全融解后使用,但随着冻融次数
的增加,细菌的活力会逐渐下降。
2,为保证菌种纯正,避免其它细菌污染,尽量先划平板,然后再挑单克隆
菌落进行后续操作。
毕赤酵母适宜的生长温度是 28 至 30 度,温度超过 32 度对蛋白的表
达是有害的,并可能导致细胞的死亡。GS115 毕赤酵母是是组氨酸缺陷型
(His4 基因型),如果表达载体上携带有组氨酸基因,可补偿宿主菌的组
氨酸缺陷,因此可以在不含组氨酸的培养基上筛选转化子。这些受体菌自
养。细菌在 30-‐35℃培养箱中培养 24-‐48h,真菌在 23-‐28℃培养箱中培养 24-‐72h
(必要时,可适当延长培养时间)。
毕赤酵母翻译

毕⾚酵母翻译Glycoengineered(糖基化⼯程) 毕⾚ pastoris(毕⾚酵母) 中表达的重组单克隆抗体的纯化⼯艺开发摘要⼀个强健的和可扩展的净化过程被开发快速从 glycoengineered 毕⾚酵母发酵⽣成抗体的纯度⾼、数量充⾜。
蛋⽩ A 亲和层析⽤于捕获从发酵液抗体。
PH 梯度洗脱已应⽤到要防⽌抗体沉淀在低 pH 的蛋⽩ A 列。
从蛋⽩抗体⾊谱法所载⼀些产品的相关杂质,被劈重链、重链和轻链的misassembling。
它也有⼀些处理相关的杂质,包括蛋⽩ A 残留、 endotoxin(内毒素)、宿主细胞 DNA 和蛋⽩质。
Ph 值为 4.5-6.0 的最优氯化钠渐变阳离⼦交换⾊谱法有效去除这些产品和过程相关的杂质。
从 glycoengineered P.酵母抗体是其 heterotetramer 折叠、物理稳定性和约束⼒的亲和性的商业同⾏相媲美。
介绍单克隆抗体(Mab)正迅速成为关键产品的⽣物医药产业。
⼤多数商业治疗性抗体是原封 IgG 分⼦与 IgG1、 IgG2 正在共同的⼦类。
这些 IgGs 由调解抗原和效应器函数之间的联系,在免疫过程中发挥中⼼作⽤。
对⽬标细胞的特异性抗原的 igg 抗体可变域的绑定将抗体依赖的细胞毒作⽤ (ADCC) 及补体依赖性细胞毒性 (CDC) 定向杀害的⽬标单元格。
ADCC 触发由交联体 Fc 域的 IgG 抗体(Fc(Rs),尤其是 Fc (RI 和Fc (制证机对免疫效应细胞。
可能调解 ADCC 效应细胞包括⾃然杀⼿细胞、巨噬细胞和嗜中性粒细胞。
疾病预防控制中⼼是由补充组件 C1q 绑定到 IgG,触发蛋⽩⽔解的级联,以激活补充 fc 功能区启动。
IgG 型抗体有两重链和两条轻链由分⼦内的⼆硫键形成 heterotetramer 在⼀起。
重链是通过共价键固定在天冬酰胺 297 (Asn-297) 低聚糖的糖基化。
⼈免疫球蛋⽩的主要N-聚糖复杂 biantennary (⼆分⽀的) 类型,有 '核⼼' heptasaccharide(七庚糖),GlcNac2Man3GlcNac2,被称为 G0 在我们的⼯作和⽂学。
毕赤酵母表达(pichia pastoris expression )实验手册(3)

毕赤酵母表达(pichia pastoris expression )实验手册(3)液体YPD培养基可常温保存;琼脂YPD平板在4℃可保存几个月。
加入Ze ocin 100ug / ml,成为YPDZ培养基,可以4℃条件下保存1~2周。
2.4 YPDS + Zeocin 培养基(Yeast Extract Peptone Dextrose Medi um):yeast extract 1%peptone 2%dextrose (glucose) 2%sorbitol 1 M+agar 2%+ Zeocin 100 μg/ml不管是液体 YPDS培养基,还是YPDS + Zeocin 培养基,都必须存放4℃条件下,有效期1~2周。
2.5 MGYMinimal Glycerol Medium (最小甘油培养基)(34%YNB;1%甘油;4*10-5%生物素)。
将800ml灭菌水、100ml的 10* YNB母液、2ml的500*B母液和100ml的10*GY母液混匀即可,4℃保存,保存期为2个月。
2.6 MGYHMinimal Glycerol Medium + Histidine (最小甘油培养基 + 0.004%组氨酸)在1000ml的MGY培养基中加入 10ml的100*H母液混匀,4℃保存,保存期为2个月。
2.7 RDRegeneration Dextrose Medium (葡萄糖再生培养基)(含有:1mol/L的山梨醇;2%葡萄糖;1.34%YNB;4*10-5%生物素;0. 005%氨基酸)1. 将186g的山梨醇定容至700ml,高压灭菌;2. 冷却后于45℃水浴;3. 将100ml的10*D、100ml的10*YNB;2ml的500*B;10ml的100*AA等母液和88ml无菌水混匀,预热至45℃后,与步骤2 的山梨醇溶液混合。
4℃保存。
2.8 RDHRegeneration Dextrose Medium + Histidine (葡萄糖再生培养基 + 0.004%组氨酸)在RD培养基配制的第三步中,在加入10ml的100*H母液,同时无菌水的体积减少至78ml即可,其余配制方法与RD相同。
毕赤酵母表达操作手册(精译版)

毕赤酵母多拷贝表达载体试剂盒用于在含多拷贝基因的毕赤酵母菌中表达并分离重组蛋白综述:基本特征:作为真核生物,毕赤酵母具有高等真核表达系统的许多优点:如蛋白加工、折叠、翻译后修饰等。
不仅如此,操作时与E.coli及酿酒酵母同样简单。
它比杆状病毒或哺乳动物组织培养等其它真核表达系统更快捷、简单、廉价,且表达水平更高。
同为酵母,毕赤酵母具有与酿酒酵母相似的分子及遗传操作优点,且它的外源蛋白表达水平是后者的十倍以至百倍。
这些使得毕赤酵母成为非常有用的蛋白表达系统。
与酿酒酵母相似技术:许多技术可以通用:互补转化基因置换基因破坏另外,在酿酒酵母中应用的术语也可用于毕赤酵母。
例如:HIS4基因都编码组氨酸脱氢酶;两者中基因产物有交叉互补;酿酒酵母中的一些野生型基因与毕赤酵母中的突变基因相互补,如HIS4、LEU2、ARG4、TR11、URA3等基因在毕赤酵母中都有各自相互补的突变基因。
毕赤酵母是甲醇营养型酵母:毕赤酵母是甲醇营养型酵母,可利用甲醇作为其唯一碳源。
甲醇代谢的第一步是:醇氧化酶利用氧分子将甲醇氧化为甲醛,还有过氧化氢。
为避免过氧化氢的毒性,甲醛代谢主要在一个特殊的细胞器-过氧化物酶体-里进行,使得有毒的副产物远离细胞其余组分。
由于醇氧化酶与O2的结合率较低,因而毕赤酵母代偿性地产生大量的酶。
而调控产生醇过氧化物酶的启动子也正是驱动外源基因在毕赤酵母中表达的启动子。
两种醇氧化酶蛋白:毕赤酵母中有两个基因编码醇氧化酶-AOX1及AOX2。
细胞中大多数的醇氧化酶是AOX1基因产物。
甲醇可紧密调节、诱导AOX1基因的高水平表达,较典型的是占可溶性蛋白的30%以上。
AOX1基因已被分离,含AOX1启动子的质粒可用来促进编码外源蛋白的目的基因的表达。
AOX2基因与AOX1基因有97%的同源性,但在甲醇中带AOX2基因的菌株比带AOX1基因菌株慢得多,通过这种甲醇利用缓慢表型可分离Muts菌株。
表达:AOX1基因的表达在转录水平受调控。
毕赤酵母表达手册(详细)

毕赤酵母表达(pichia pastoris expression )实验手册2010-07-15 10:54:56| 分类:毕赤酵母| 标签:|字号大中小订阅一.毕赤酵母表达常用溶液及缓冲液的配制二.毕赤酵母表达的培养基配制三.主要试验环节的操作 3.1 酵母菌株的分离纯化 3.2 pPICZαA原核宿主菌TOP10F’的活化培养 3.3毕赤酵母表达的试验方法 3.4 毕赤酵母电转化方法 3.5 Pichia酵母表达直接PCR鉴定重组子的方法 3.6 毕赤酵母基因组提取方法 3.7 Mut+表型重组酵母的诱导表达实验关键词:酵母实验毕赤酵母表达 pichia pastoris expression 毕赤酵母酵母菌株大肠杆菌表达系统最突出的优点是工艺简单、产量高、周期短、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结构,在大肠杆菌中表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯化,但不利于产物的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增加了成本。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过大肠杆菌表达的,其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调控机制和蛋白质的加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年来,以酵母作为工程菌表达外源蛋白日益引起重视,原因是与大肠杆菌相比,酵母是低等真核生物,除了具有细胞生长快,易于培养,遗传操作简单等原核生物的特点外,又具有真核生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,能有效克服大肠杆菌系统缺乏蛋白翻译后加工、修饰的不足。
毕赤酵母表达载体pPICzalpha手册

Recommended Pichia Host Strain
We recommend using the X-33 Pichia strain as the host for expression of recombinant proteins from pPICZα . Other Pichia strains are suitable. The X-33 Pichia strain is available from Invitrogen (see page vii for ordering information) and has the following genotype and phenotype: Genotype: Wild-type Phenotype: Mut+ Continued on next page
Shipping/Storage Reference Sources
The vectors are shipped on wet ice and should be stored at –20°C. The pPICZα A, B, and C vectors may be used with the Original Pichia Expression Kit (Cat. no. K1710-01) and are included in the EasySelect™ Pichia Expression Kit (Cat. no. K1740-01) available from Invitrogen. Additional general information about recombinant protein expression in Pichia pastoris is provided in the manuals for the Original Pichia Expression Kit and the EasySelect™ Pichia Expression Kit. The manuals can be downloaded from our Website () or obtained by calling Technical Support (see page 33). For more information about the Original Pichia Expression Kit or the EasySelect™ Pichia Expression Kit, refer to our Website or contact Technical Support. More detailed information and protocols dealing with Pichia pastoris may also be found in the following general reference (see page vii for ordering information): Higgins, D. R., and Cregg, J. M. (1998) Pichia Protocols. In Methods in Molecular Biology, Vol. 103. (J. M. Walker, ed. Humana Press, Totowaer Manual
毕赤酵母表达手册

Pichia Expression KitVersion M01110225-0043Pichia Expression KitA Manual of Methods for Expression of Recombinant Proteins in Pichia pastorisCatalog no. K1710-01tech_service@iiINDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTThe Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris was developed into an expression system by scientists at Salk Institute Biotechnology/Industry Associates (SIBIA) for high-level expression of recombinant proteins. All patents for Pichia pastoris and licenses for its use as an expression system are owned by Research Corporation Technologies, Inc. Tucson, Arizona. Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial corporations requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license a greement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this Agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.INDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTInvitrogen Corporation (INVITROGEN) grants you a non-exclusive license to use the enclosed Pichia Expression Kit (EXPRESSION KIT) for academic research or for evaluation purposes only. The EXPRESSION KIT is being transferred to you in furtherance of, and reliance on, such license. You may not use the EXPRESSION KIT, or the materials contained therein, for any commercial purpose without a license for such purpose from RESEARCH CORPORATION TECHNOLOGIES, INC., Tucson, Arizona. Commercial purposes include the use in or sale of expressed proteins as a commercial product, or use to facilitate or advance research or development of a commercial product. Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Commercial entities will be contacted by Research Corporation Technologies during the evaluation period regarding the purchase of a commercial license.Access to the EXPRESSION KIT must be limited solely to those officers, employees and students of your institution who need access thereto in order to perform the above-described research or evaluation. You must inform each of such officer, employee and student of the provisions of this Agreement and require them to agree, in writing, to be bound by the provisions of this Agreement. You may not distribute the EXPRESSION KIT to others, even those within your own institution. You may transfer modified, altered or original material from the EXPRESSION KIT to a third party following notification of INVITROGEN such that the recipient can be licensed. You may not assign, sub-license, rent lease or otherwise transfer this License or any of the rights or obligation hereunder, except as expressly permitted.This License is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate automatically if you fail to comply with the terms and conditions of the Agreement. You shall, upon termination of the License, destroy all Pichia Expression Kits in your control, and so notify INVITROGEN in writing.This License Shall be governed in its interpretation and enforcement by the laws of the State of California.Product User Registration CardPlease complete and return the enclosed Product User Registration Card for each Pichia Expression Kit that you purchase. This will serve as a record of your purchase and registration and will allow Invitrogen to provide you with technical support and manual updates. It will also allow Invitrogen to update you on future developments of and improvements to the Pichia Expression Kit. The agreement outlined above becomes effective upon our receipt of your User Registration Card or 10 days following the sale of the Pichia Expression Kit to you. Use of the kit at any time results in immediate obligation to the terms and conditions stated in this Agreement.Technical ServicesInvitrogen provides Technical Services to all of our registered Pichia Expression Kit users. Please contact us if you need assistance with the Pichia Expression Kit.United States Headquarters:Japanese Headquarters European Headquarters:Invitrogen Corporation1600 Faraday AvenueCarlsbad, CA 92008 USATel: 1 760 603 7200Tel (Toll Free): 1 800 955 6288 Fax: 1 760 602 6500E-mail:tech_service@ Invitrogen Japan K.K.Nihonbashi Hama-Cho Park Bldg. 4F2-35-4, Hama-Cho, NihonbashiTel: 81 3 3663 7972Fax: 81 3 3663 8242E-mail: jpinfo@Invitrogen Ltd3 Fountain DriveInchinnan Business ParkPaisley PA4 9RF, UKTel (Free Phone Orders): 0800 269 210Tel (General Enquiries): 0800 5345 5345Fax: +44 (0) 141 814 6287E-mail: eurotech@iiiivTable of ContentsMaterials (vii)Purchaser Notification (x)Product Qualification (xii)Introduction (1)Overview (1)Experimental Outline (3)Recombination and Integration in Pichia (7)Methods (11)Pichia Strains (11)E. coli Strains (13)Selecting a Pichia Expression Vector (14)pHIL-D2 (16)pPIC3.5 (17)pHIL-S1 (18)pPIC9 (19)Signal Sequence Processing (20)Cloning into the Pichia Expression Vectors (21)Transformation into E. coli (26)Preparation of Transforming DNA (27)Growth of Pichia for Spheroplasting (30)Preparation of Spheroplasts (32)Transformation of Pichia (34)Screening for Mut+ and Mut S Transformants (36)PCR Analysis of Pichia Integrants (40)Expression of Recombinant Pichia Strains (42)Analysis by SDS-Polyacrylamide Gel Electrophoresis (45)Optimization of Pichia Protein Expression (47)Scale-up of Expression (49)Protein Purification and Glycosylation (51)Recipes (53)E. coli Media Recipes (53)Pichia Media Recipes (54)Appendix (59)Electroporation of Pichia (59)PEG 1000 Transformation Method for Pichia (60)Lithium Chloride Transformation Method (61)Total DNA Isolation from Pichia (62)Detection of Multiple Integration Events (63)Procedure for Total RNA Isolation from Pichia (64)β-Galactosidase Assay (65)Technical Service (67)References (69)vviMaterialsKit Contents Box 1: Spheroplast Module. Store at room temperature.Reagent Amount ComponentsSOS media 20 ml 1 M Sorbitol0.3X YPD10 mM CaCl2Sterile Water 2 x 125 ml Autoclaved, deionized waterSE 2 x 125 ml 1 M Sorbitol25 mM EDTA, pH 8.0SCE 2 x 125 ml 1 M Sorbitol10 mM Sodium citrate buffer, pH 5.81 mM EDTA1 M Sorbitol2 x 125 ml --CaS 2 x 60 ml 1 M Sorbitol10 mM Tris-HCl, pH 7.5;10 mM CaCl240% PEG 25 ml 40% (w/v) PEG 3350 (Reagent grade) in waterCaT 25 ml 20 mM Tris-HCl, pH 7.520 mM CaCl2Stab Vials: Pichia and E. coli stabs. Store at +4°C.Phenotype(Pichia only)GenotypeStrain Amountstab his4Mut+GS115 1stab arg4 his4 aox1::ARG4 Mut S, Arg+KM71 1GS115 Albumin 1 stab HIS4Mut SGS115 β-Gal 1 stab HIS4Mut+stab F´ {pro AB, lac I q, lac Z∆M15, Tn10 (Tet R)} mcr A,TOP10F´ 1∆(mrr-hsd RMS-mcr BC), φ80lac Z∆M15, ∆lac X74,deo R, rec A1, ara D139, ∆(ara-leu)7697, gal U,gal K, rps L (Str R), end A1, nup G λ-.Box 2: Spheroplast Module. Store at -20°C.ComponentsReagent AmountZymolyase 10 x 20 µl 3 mg/ml Zymolyase in water(100,000 units/g lytic activity)1 M DTT 10 x 1 ml 1 M dithiothreitol in watercontinued on next pageviiKit Contents,continuedVector Box. Store at -20°C.Reagent DescriptionpHIL-D210 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapPIC3.510 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapHIL-S110 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the PHO1 signal sequencepPIC910 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the α-factor signal sequencePrimer Box. Store at -20°C.5´ AOX1 sequencing primer2 µg (312 pmoles), lyophilized5´-GACTGGTTCCAATTGACAAGC-3´3´ AOX1 sequencing primer2 µg (314 pmoles), lyophilized5´-GCAAATGGCATTCTGACATCC-3´α-Factor sequencing primer2 µg (315 pmoles), lyophilized5´-TACTATTGCCAGCATTGCTGC-3´Media The following prepackaged media is included for your convenience. Instructions for use are provided on the package.Media Amount Yield YP Base Medium 2 pouches 2 liters of YP mediumYP Base Agar Medium 2 pouches 2 liters of YP mediumYeast Nitrogen Base 1 pouch 500 ml of 10X YNBFor transformation of Pichia by spheroplasting, the Pichia Spheroplast Module isavailable separately from Invitrogen (see below for ordering information).Product Reactions or Amount Catalog no.Pichia Spheroplast Module 10 spheroplast preparations(50 transformations)K1720-01continued on next pageviiiRequired Equip-ment and Supplies (not provided) • 30°C rotary shaking incubator• Water baths capable of 37°C, 45°C, and 100°C• Centrifuge suitable for 50 ml conical tubes (floor or table-top)• Baffled culture flasks with metal covers (50 ml, 250 ml, 500 ml, 1000 ml, and 3 L)• 50 ml sterile, conical tubes• 6 ml and 15 ml sterile snap-top tubes (Falcon 2059 or similar)• UVSpectrophotometer• Mini agarose gel apparatus and buffers• Polyacrylamide Gel Electrophoresis apparatus and buffers• Media for transformation, growth, screening, and expression (see Recipes, pages 53-58) • 5% SDS solution (10 ml per transformation)• Sterile cheesecloth or gauze• Breaking Buffer (see Recipes, page 58)• Acid-washed glass beads (available from Sigma)• Replica-plating equipment (optional)• BeadBreaker™ (optional)ixPurchaser NotificationIntroduction The Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris wasdeveloped into an expression system by scientists at Salk Institute Biotechnology/ IndustryAssociates (SIBIA) and Phillips Petroleum for high-level expression of recombinantproteins. All patents for Pichia pastoris and licenses for its use as an expression system areowned by Research Corporation Technologies (RCT), Inc., Tucson, Arizona. Forinformation on commercial licenses, please see page x.The Nature of the Invitrogen License Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial entities for any commercial purpose requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license agreement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this license agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.Pichia pastoris Patents Pichia pastoris is covered by one or more of the following U.S. patents and corresponding foreign patents owned and licensed by Research Corporation Technologies:4,683,293 4,808,537 4,812,405 4,818,700 4,837,148 4,855,231 4,857,467 4,879,231 4,882,279 4,885,242 4,895,800 4,929,555 5,002,876 5,004,688 5,032,516 5,122,465 5,135,868 5,166,329Individual Pichia Expression Kit License Agreement Invitrogen Corporation ("Invitrogen") grants you a non-exclusive license to use the enclosed Pichia Expression Kit ("Expression Kit") for academic research or for evaluation purposes only. The Expression Kit is being transferred to you in furtherance of, and reliance on, such license. You may not use the Expression Kit, or the materials contained therein, for any commercial purpose without a license for such purpose from Research Corporation Technologies, Inc., Tucson, Arizona.Definition of Commercial Purpose Commercial purposes include:(a) any use of Expression Products in a Commercial Product(b) any use of Expression Products in the manufacture of a Commercial Product(c) any sale of Expression Products(d) any use of Expression Products or the Expression Kit to facilitate or advanceresearch or development of a Commercial Product(e) any use of Expression Products or the Expression Kit to facilitate or advance anyresearch or development program the results of which will be applied to thedevelopment of Commercial Products"Expression Products" means products expressed with the Expression Kit, or with the use of any vectors or host strains in the Expression Kit. "Commercial Product" means any product intended for sale or commercial use.Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Research Corporation Technologies will contact commercial entities during the evaluation period regarding their desire for a commercial license.continued on next pagexPurchaser Notification, continuedIndividual Responsibilities Access to the Expression Kit must be limited solely to those officers, employees and students of your institution who need access to perform the above-described research or evaluation. You must inform each such officer, employee and student of the provisions of this license agreement and require them to agree, in writing, to be bound by the provisions of this license agreement. You may not distribute neither the Expression Kit nor the vectors or host strains contained in it to others, even to those within your own institution. You may only transfer modified, altered, or original material from the Expression Kit to a third party following written notification of, and written approval from, Invitrogen so that the recipient can be licensed. You may not assign, sub-license, rent, lease or otherwise transfer this license agreement or any of the rights or obligation thereunder, except as expressly permitted by Invitrogen and RCT.Termination of License This license agreement is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate auto-matically if you fail to comply with the terms and conditions of the license agreement. You shall, upon termination of the license agreement, destroy all Pichia Expression Kits in your control, and so notify Invitrogen in writing.This License shall be governed in its interpretation and enforcement by the laws of the State of California.Contact for Commercial Licensing Bennett Cohen, Ph.D.Research Corporation Technologies 101 North Wilmot Road, Suite 600 Tucson, Arizona 85711-3335 Phone: (520) 748-4400Fax: (520)748-0025User Registration Card Please complete and return the enclosed User Registration Card for each PichiaExpression Kit that you purchase. This will serve as a record of your purchase and regis-tration and will allow Invitrogen to provide you with technical support and manualupdates. It will also allow Invitrogen to update you on future developments and improve-ments to the Pichia Expression Kit. The agreement outlined above becomes effectiveupon our receipt of your User Registration Card or 10 days following the sale of thePichia Expression Kit to you. Use of the kit at any time results in immediate obligation tothe terms and conditions stated in this license agreement.xiProduct QualificationIntroduction This section describes the criteria used to qualify the components in the PichiaExpression Kit.Vectors All expression vectors are qualified by restriction enzyme digestion. Restriction digests must demonstrate the correct banding pattern when electrophoresed on an agarose gel.Spheroplast Reagents The spheroplast reagents are qualified by spheroplast preparation of GS115 following the protocol provided in the Pichia Expression Kit manual. At least 70% of the Pichia pastoris cells must form spheroplasts in 30 minutes or less.Pichia Strains The Pichia strains are by demonstrating viability of the culture. Single colonies should arise within 48 hours after streaking on YPD medium from the stabPrimers Sequencing primers are lot tested by automated DNA sequencing experiments.Buffers andSolutionsAll buffers and solutions are extensively tested for sterility.Media All Pichia growth and expression media are qualified by growing the GS115 Pichiastrain.xiiIntroductionOverviewReview Articles The information presented here is designed to give you a concise overview of the Pichia pastoris expression system. It is by no means exhaustive. For further information, pleaseread the articles cited in the text along with recent review articles (Buckholz and Gleeson,1991; Cregg et al., 1993; Sreekrishna et al., 1988; Wegner, 1990). A general review offoreign gene expression in yeast is also available (Romanos et al., 1992).General Characteristics of Pichia pastoris As a eukaryote, Pichia pastoris has many of the advantages of higher eukaryotic expression systems such as protein processing, protein folding, and posttranslational modification, while being as easy to manipulate as E. coli or Saccharomyces cerevisiae. It is faster, easier, and less expensive to use than other eukaryotic expression systems such as baculovirus or mammalian tissue culture, and generally gives higher expression levels. As a yeast, it shares the advantages of molecular and genetic manipulations with Saccharomyces, and has the added advantage of 10- to 100-fold higher heterologous protein expression levels. These features make Pichia very useful as a protein expression system.Similarity to Saccharomyces Many of the techniques developed for Saccharomyces may be applied to Pichia including: • transformation by complementation• genedisruption• genereplacementIn addition, the genetic nomenclature used for Saccharomyces has been applied to Pichia. For example, the HIS4 gene in both Saccharomyces and Pichia encodes histidinol dehydrogenase. There is also cross-complementation between gene products in both Saccharomyces and Pichia. Several wild-type genes from Saccharomyces complement comparable mutant genes in Pichia. Genes such as HIS4, LEU2, ARG4, TRP1, and URA3 all complement their respective mutant genes in Pichia.Pichia pastoris as a Methylotrophic Yeast Pichia pastoris is a methylotrophic yeast, capable of metabolizing methanol as its sole carbon source. The first step in the metabolism of methanol is the oxidation of methanol to formaldehyde using molecular oxygen by the enzyme alcohol oxidase. This reaction generates both formaldehyde and hydrogen peroxide. To avoid hydrogen peroxide toxicity, methanol metabolism takes place within a specialized cell organelle called the peroxisome, which sequesters toxic by-products from the rest of the cell. Alcohol oxidase has a poor affinity for O2, and Pichia pastoris compensates by generating large amounts of the enzyme. The promoter regulating the production of alcohol oxidase drives heterologous protein expression in Pichia.Two Alcohol Oxidase Proteins The AOX1 and AOX2 genes code for alcohol oxidase in Pichia pastoris. The AOX1 gene product accounts for the majority of alcohol oxidase activity in the cell. Expression of the AOX1 gene is tightly regulated and induced by methanol to high levels, typically > 30% ofthe total soluble protein in cells grown with methanol as the carbon source. The AOX1 gene has been isolated and the AOX1 promoter is used to drive expression of the gene of interest (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a). While AOX2 is about 97% homologous to AOX1, growth on methanol is much slower than with AOX1. This slowgrowth allows isolation of Mut S strains (aox1) (Cregg et al., 1989; Koutz et al., 1989).continued on next page1Overview, continuedExpression Expression of the AOX1 gene is controlled at the level of transcription. In methanol-grown cells approximately 5% of the polyA+ RNA is from the AOX1 gene. The regulation of theAOX1 gene is a two step process: a repression/derepression mechanism plus an inductionmechanism (e.g. GAL1 gene in Saccharomyces (Johnston, 1987)). Briefly, growth onglucose represses transcription, even in the presence of the inducer methanol. For thisreason, growth on glycerol is recommended for optimal induction with methanol. Pleasenote that growth on glycerol (derepression) is not sufficient to generate even minute levelsof expression from the AOX1 gene. The inducer, methanol, is necessary for detectablelevels of AOX1 expression (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a).Phenotype of aox1 mutants Loss of the AOX1 gene, and thus a loss of most of the cell's alcohol oxidase activity, results in a strain that is phenotypically Mut S (Methanol utilization slow). This has in the past been referred to as Mut. The Mut S designation has been chosen to accurately describe the phenotype of these mutants. This results in a reduction in the cells' ability to metabolize methanol. The cells, therefore, exhibit poor growth on methanol medium. Mut+ (Methanol utilization plus) refers to the wild type ability of strains to metabolize methanol as the sole carbon source. These two phenotypes are used when evaluating Pichia transformants for integration of your gene (Experimental Outline, page 3).Intracellular and Secretory Protein Expression Heterologous expression in Pichia can be either intracellular or secreted. Secretion requires the presence of a signal sequence on the expressed protein to target it to the secretory pathway. While several different secretion signal sequences have been used successfully, including the native secretion signal present on some heterologous proteins, success has been variable. The secretion signal sequence from the Saccharomyces cerevisiaeα factor prepro peptide has been used most successfully (Cregg et al., 1993; Scorer et al., 1993).The major advantage of expressing heterologous proteins as secreted proteins is that Pichia pastoris secretes very low levels of native proteins. That, combined with the very low amount of protein in the minimal Pichia growth medium, means that the secreted heterologous protein comprises the vast majority of the total protein in the medium and serves as the first step in purification of the protein (Barr et al., 1992). Note: If there are recognized glycosylation sites (Asn-X-Ser/Thr) in your protein's primary sequence, glycosylation may occur at these sites.Posttranslational Modifications In comparison to Saccharomyces cerevisiae, Pichia may have an advantage in the glyco-sylation of secreted proteins because it may not hyperglycosylate. Both Saccharomyces cerevisiae and Pichia pastoris have a majority of N-linked glycosylation of the high-mannose type; however, the length of the oligosaccharide chains added posttranslationally to proteins in Pichia (average 8-14 mannose residues per side chain) is much shorter than those in S. cerevisiae (50-150 mannose residues) (Grinna and Tschopp, 1989; Tschopp et al., 1987b). Very little O-linked glycosylation has been observed in Pichia.In addition, Saccharomyces cerevisiae core oligosaccharides have terminal α1,3 glycan linkages whereas Pichia pastoris does not. It is believed that the α1,3 glycan linkages in glycosylated proteins produced from Saccharomyces cerevisiae are primarily responsible for the hyper-antigenic nature of these proteins making them particularly unsuitable for therapeutic use. Although not proven, this is predicted to be less of a problem for glycoproteins generated in Pichia pastoris, because it may resemble the glycoprotein structure of higher eukaryotes (Cregg et al., 1993).2Experimental OutlineSelection of Vector and Cloning To utilize the strong, highly inducible P AOX1 promoter for expression of your protein, four expression vectors are included in this kit. pHIL-D2 and pPIC3.5 are used for intracellular expression while pHIL-S1 and pPIC9 are used for secreted expression (see pages 14-19 for more information). Before cloning your insert, you must...• decide whether you want intracellular or secreted expression.• analyze your insert for the following restriction sites: Sac I, Stu I, Sal I, Not I, and Bgl II. These sites are recommended for linearizing your construct prior to Pichiatransformation. If your insert has all of these sites, see pages 28-29 for alternate sites.Transformation and IntegrationTwo different phenotypic classes of His+ recombinant strains can be generated: Mut+ and Mut S. Mut S refers to the "Methanol utilization slow" phenotype caused by the loss of alcohol oxidase activity encoded by the AOX1 gene. A strain with a Mut S phenotype has a mutant aox1 locus, but is wild type for AOX2. This results in a slow growth phenotype on methanol medium. Transformation of strain GS115 can yield both classes of transformants, His+ Mut+ and His+Mut S, while KM71 yields only His+ Mut S since the strain itself is Mut S. Both Mut+ and Mut S recombinants are useful to have as one phenotype may favor better expression of your protein than the other. Due to clonal variation, you should test 6-10 recombinants per phenotype. There is no way to predict beforehand which construct or isolate will better express your protein. We strongly recommend that you analyze Pichia recombinants by PCR to confirm integration of your construct (see page 40).Once you have successfully cloned your gene, you will then linearize your plasmid to stimulate recombination when the plasmid is transformed into Pichia. The table below describes the types of recombinants you will get by selective digestion of your plasmid. RestrictionEnzymeIntegration Event GS115 Phenotype KM71 PhenotypeSal I or Stu I Insertion at his4His+ Mut+ His+ Mut SSac I Insertion at 5´AOX1 regionHis+ Mut+ His+ Mut SNot I or Bgl II Replacement atAOX1 locusHis+ Mut SHis+ Mut+His+ Mut S (notrecommended, see page 11)Expression and Scale-up After confirming your Pichia recombinants by PCR, you will test expression of both His+Mut+ and His+ Mut S recombinants. This will involve growing a small culture of each recombinant, inducing with methanol, and taking time points. If looking for intracellular expression, analyze the cell pellet from each time point by SDS polyacrylamide gel electrophoresis (SDS-PAGE). If looking for secreted expression, analyze both the cellpellet and supernatant from each time point. We recommend that you analyze your SDS-PAGE gels by both Coomassie staining and Western blot, if you have an antibody to your protein. We also suggest checking for protein activity by assay, if one is available. Not all proteins express to the level of grams per liter, so it is advisable to check by Western blotor activity assay, and not just by Coomassie staining of SDS-PAGE gels for production of your protein.Choose the Pichia recombinant strain that best expresses your protein and optimizeinduction based on the suggestions on pages 47-48. Once expression is optimized, scale-up your expression protocol to produce more protein.continued on next page3。
裂解酶 毕赤酵母表达

裂解酶毕赤酵母表达裂解酶是一种重要的酶类,能够将高分子物质裂解为较小的分子,被广泛应用于制药、食品、化工等领域。
毕赤酵母是一种常见的酵母菌,因其生长速度快,易培养,被广泛应用于分子生物学研究领域。
本文将围绕着“裂解酶毕赤酵母表达”这一主题,进行详细阐述。
第一步,克隆裂解酶基因首先需要克隆裂解酶基因。
裂解酶基因可以从天然菌株或基因库中获得。
一般情况下,可采用PCR技术或构建文库的方法进行克隆。
PCR克隆是一种常见的将特定基因扩增的方法,需要设计引物。
而文库构建则需要将大量的DNA片段克隆到载体上,获取含有目标基因的克隆体。
第二步,构建表达载体获取裂解酶基因后,需要将其插入到表达载体中,以实现外源基因的表达。
表达载体一般包括启动子、编码区、终止序列等部分。
还需要添加适合裂解酶表达的表达基因启动子和信使RNA聚合酶结合位点。
可采用限制性内切酶、LiGA等方法,将裂解酶基因插入到表达载体中。
第三步,转化毕赤酵母将构建好的表达载体转化到毕赤酵母细胞中。
目前主要有两种转化方法,一种是化学转化,一种是电转化。
化学转化依赖于离子间的相互作用力,可将DNA引入到细胞中。
而电转化则是利用高电压作用于细胞,使其渗透性提高,DNA片段能够进入细胞。
第四步,筛选表达菌株进行转化后,将细胞涂布于含有蔗糖的SD-UF板上进行筛选。
含有蔗糖的培养基只能被表达裂解酶的菌株使用,其他菌株无法生长,通过这种方法可以筛选出表达裂解酶的毕赤酵母菌株。
第五步,表达和纯化裂解酶最后,使用相应的表达条件和纯化方法,纯化表达的裂解酶。
一般情况下,裂解酶是一种外分泌酶,可采用诱导性表达,通过添加诱导因子(如甘露醇)来促进裂解酶的分泌。
常见的纯化方法包括离子交换层析、凝胶过滤层析、亲和层析等。
总之,裂解酶毕赤酵母表达是一个复杂的过程,需要准确的基因克隆、合适的表达载体、科学的转化方法、合适的筛选条件和纯化方法等。
只有在这些步骤都正确执行的情况下,才能够得到表达裂解酶的毕赤酵母菌株,并最终纯化出高质量的裂解酶。
酵母表达系统步骤

酵母表达系统步骤毕赤酵母表达系统步骤(参考Invitrogen公司说明书):一、pPICZαA、B、C质粒以及DH5α菌株的保存1取0.5μl pPICZα A、B、C质粒,热击转化DH5α,在低盐LB (含有25μg/ml Zeocin)的平板上37℃培养过夜。
2挑取转化子,甘油保存。
二、载体构建1将目的基因构建到pPICZα载体上,转化DH5α,用Zeocin筛选转化子。
2提质粒酶切鉴定或PCR鉴定3载体测序测序可用α-Factor引物或5’AOX1引物,3’AOX1引物三、线性化DNA1提取足够量的质粒DNA(一次转化至少需要5-10μg质粒)2 酶切线性化10μg构建好的载体,同时酶切空载体做对照,根据载体选择线性化酶切位点(样品分管酶切),pPICZα载体在5’AOX1区域有三个酶切位点可选择:SacI、PmeI、BstXI3 取1-2μl酶切产物跑电泳,确定是否酶切完全;4 过柱纯化线性化质粒(用50μl EB洗脱);四、线性化DNA的去磷酸化处理线性化质粒43μlCIAP Buffer 5μlCIAP酶2μl四、总体积为50μl的样品37℃ 1h,过柱纯化,用30μl ddH2O 洗脱;五、感受态细胞的制备实验前准备:无抗性YPD平板一个、无抗生素液体YPD培养基,100μg/ml Zeocin YPD 平板和液体、50ml离心管两个、500ml预冷的无菌水、20ml 1M 山梨醇(灭菌预冷的),0.2cm预冷的电击杯;1YPD平板划线培养菌,30℃培养2-3d;250ml三角瓶中,加入5ml YPD,挑取酵母单菌落,30℃培养过夜;3吸取0.5ml菌液,加入至含有200ml新鲜YPD的1L三角瓶中,30℃,225rpm/min培养至OD值1.3-1.5;41500g,4℃离心5min收集菌体;540ml冰预冷的无菌水重悬沉淀;61500g,4℃,5min;730ml无菌水重悬;81500g,4℃,5min;910ml 1M 山梨醇重悬;101500g,4℃,5min;11加入1ml山梨醇,重悬冰上放置,直接做转化,或加入灭菌甘油每管80ul分装,冻存于-80℃(长时间保存会影响转化效率);六、电击转化15-10μg线性化DNA(20μl<)与80ul上述感受态细胞混合,转移至预冷的0.2cm电击杯中(点击条件:电压1.5kV;电容25μF;电阻200Ω,电击时间为4~10msec);2冰上放置5min3电击(按生产厂商提供的适合酵母用的参数)4迅速加入1ml预冷的1M 山梨醇,转移至1.5ml EP管中530℃静置培养1-2h(如果要增加存活率,获得更多的转化克隆,可在30℃静置培养1h后,加入1mlYPD培养基,30℃200rpm培养1h后取部分涂布与不同浓度抗生素的平板)6取50、100、200ul分别涂布于含有Zeocin的YPD平板,30℃培养2-10 d至有菌落出现;7如果要筛选多拷贝转化子,将转化克隆混合在一起,涂布在Zeocin 浓度为500、1000、2000μg/ml的YPD平板,培养2-3d。
毕赤酵母菌种培养手册

毕赤酵母菌种培养手册1. 引言本手册旨在提供毕赤酵母菌种培养的详细步骤和注意事项。
毕赤酵母(Saccharomyces cerevisiae)被广泛应用于食品工业、酿酒业和生物学研究等领域。
通过正确的菌种培养技术,可以确保毕赤酵母的活力和纯度,从而保证实验和应用的可靠性和准确性。
2. 材料和方法2.1 培养基选择适合的培养基是培养毕赤酵母的关键。
常用的培养基包括YPD培养基、SD培养基和SC培养基等。
根据具体实验需求选择合适的培养基配方,并按照相应操作说明制备。
2.2 菌种的制备和传代1. 从冰冻保存的毕赤酵母菌种中取出适量菌种转移到无菌培养基中。
2. 在适当的温度(通常为30°C)下培养菌种至对数生长期。
3. 取适量无菌培养基转移菌种,传代培养。
2.3 菌种培养1. 取适量菌种转移到含有适量无菌培养基的培养瓶中。
2. 控制培养瓶中的菌液浓度,通常为OD600=0.5。
3. 在适当的温度(通常为30°C)下培养菌种至对数生长期或其他实验所需生长期。
2.4 菌种保存菌种的保存有助于长期维持活力和纯度。
常用的保存方法包括冷冻保存和制备冻干菌种等。
3. 结果和讨论通过本手册提供的方法,可以成功培养并维持毕赤酵母菌种的活力和纯度。
在培养过程中,应注意操作的无菌性和培养条件的合适性。
此外,根据具体实验需求,可适当调整菌液的浓度和培养温度等参数。
4. 总结本手册详细介绍了毕赤酵母菌种培养的步骤和注意事项。
正确的菌种培养技术对于保证实验和应用的可靠性和准确性至关重要。
通过遵循本手册的指南和方法,可以有效地培养毕赤酵母菌种,并取得可靠的实验结果。
请注意,本手册仅提供参考,并且在使用过程中应遵守相关的实验室安全操作和法律法规要求。
毕赤酵母发酵手册
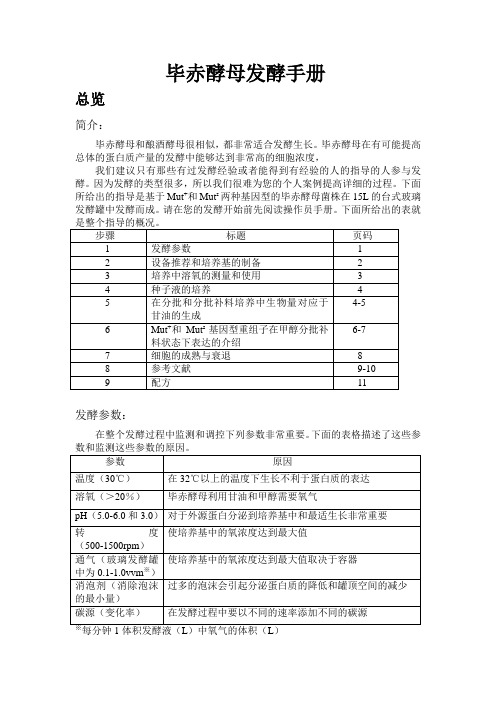
毕赤酵母发酵手册总览简介:毕赤酵母和酿酒酵母很相似,都非常适合发酵生长。
毕赤酵母在有可能提高总体的蛋白质产量的发酵中能够达到非常高的细胞浓度,我们建议只有那些有过发酵经验或者能得到有经验的人的指导的人参与发酵。
因为发酵的类型很多,所以我们很难为您的个人案例提高详细的过程。
下面所给出的指导是基于Mut+和Mut s两种基因型的毕赤酵母菌株在15L的台式玻璃发酵罐中发酵而成。
请在您的发酵开始前先阅读操作员手册。
下面所给出的表就发酵参数:在整个发酵过程中监测和调控下列参数非常重要。
下面的表格描述了这些参设备推荐:下面是所推荐设备的清单:·发酵罐的夹套需要在发酵过程中给酵母菌降温,尤其是在甲醇流加过程中。
你需要一个固定的来源来提供冷却水(5-10℃)。
这可能意味着你需要一个冷冻装置来保持水的冷却。
·一个泡沫探针就像消泡剂一样不可或缺。
·一个氧气的来源——空气(不锈钢的发酵罐需要1-2vvm)或者纯氧(玻璃发酵罐需要0.1-0.3vvm)。
·添加甘油和甲醇的补料泵。
·pH的自动控制。
培养基的准备:你需要准确配置下列溶液:·发酵所需的基本盐类(第11页)·PTM1补充盐类(第11页)·75ml的50%的甘油每升初始发酵液,12ml的PTM1补充盐每升甘油。
·740ml的100%的甲醇每升初始发酵液,12ml的PTM1补充盐每升甲醇。
毕赤酵母生长的测定:在不同的时间点通过测OD600的吸光值和湿细胞的重量来检测毕赤酵母的生长。
培养的代谢速率通过通过观察溶氧浓度对应于有效碳源来测定。
溶氧的测定:简介:溶解氧的浓度时指氧气在培养基中的相关比例,溶氧100%是指培养基中氧达到饱和。
毕赤酵母的生长需要消耗氧气,减少溶解氧的满度。
毕赤酵母在生长时会消耗氧气,减少溶氧的程度。
然而,因为代谢甲醇的最初阶段需要氧气,所以将溶氧浓度维持在一个适当的水平(>20%)来确保毕赤酵母在甲醇上的生长就至关重要。
毕赤酵母表达步骤

毕赤酵母表达系统的构建1.确定转入的目的基因,并设计相应引物,进行PCR反应,获取目的基因片段。
(所需药品-高保真酶,引物,dntp,胶回收试剂盒。
)2.对目的基因及载体Ppic9k进行酶切产生粘性接头并纯化回收。
(所需药品- SalI、StuI、SacI 【用于于GS115产生His+Mut+】;BglII【用于于GS115产生His+Muts】)酶切体系酶切体系50 μL目的DNA 10 μL酶切缓冲液 5 μL限制性内切酶 1 μL超纯水34μL37 ℃酶切 1~4小时。
酶切产物进行琼脂糖凝胶电泳检测分析。
3.将酶切正确的目的片度与质粒相连(所需药品-连接试剂盒SolutionⅠ[宝生物])载体DNA0.5µL目的片段DNA 4.5µL连接试剂盒SolutionⅠ 5 µL充分混匀,置于16 ℃连接4 h或4 ℃连接过夜。
4.转入DH5α扩繁质粒(所需药品- A mp;DH5α;CaCl2)将DNA目的片段和载体的连接产物与200µL感受态细胞混匀,冰浴30min。
45℃热击45-60s,冰浴3min后加入800μL LB 液体培养基37℃震荡培养1h。
(1 )转化后,将转化混合物涂在含50-100ug/ul A mp 的LB平板上,选择A mp 抗性克隆(2)挑取10 个A mp 抗性转化子,接种含150ug/ul A mp 的培养基,37 度振荡培养过夜(3 )提取质粒进行PCR及酶切检测并送交测序。
(pPIC9k 测序时,用α-factor 引物及3’AOX1 测序引物。
将引物重悬于20ul灭菌水中,制成0.1ug/ul 溶液)4 在0.85ml 过夜培养菌液中加入0.15ml 灭菌甘油,以便保存所需克隆,涡旋混匀转入标记好的储存管中。
在液氮或干冰/酒精浴中冷冻后移入-70 度保存。
5 测序证实结构正确后,可准备转化DNA5.毕赤酵母电转化:细胞准备:毕赤酵母感受态制备:(1)取1 mL GS115过夜培养物(OD6006.0~10.0)转接于100 mL YPD液体培养基中28℃-3O℃、250—300 r/min培养至酵母菌的对数生长期(OD600 1.0~1.3)(2)取此菌1 mL分装到1.5 mL EP管中,4℃、10 000 g离心1 min,弃上清液,沉淀用无菌水(4℃预冷)洗涤,同样条件下离心,弃上清液。
BY4742酵母菌使用说明

置会导致菌种衰退;
2、冷冻管开封、冻干粉复溶、菌株恢复培养等操作应在无菌条件下进行;
3、一些菌种经过冷冻干燥保存后,延迟期较长,部分需连续两次继代培养才能
正常生长;
4、苛养菌的培养需采用含特定营养成分的培养基,敬请正确选择,不清楚时来
电询问;
5、某些厌氧菌的培养,自开封到接种完成,均需以无氧气体充填,以保持厌氧 状态;培养过程中亦要保持厌氧状态; 6、某些菌种,如肺炎链球菌、流感嗜血杆菌、淋病奈瑟菌等需要 5-‐10%CO2 促 进生长; 7、如发现冷冻管盖松动、复溶液浑浊等异常情况,应停止使用对应产品。 8、部分菌种有致病性、扩散性,请专业人员在专业环境下有保护性操作。 保 藏 条 件 : -‐20℃保存(复溶液于 2-‐8℃保存) 保 藏 时 间 : 2-‐10 年,应根据菌种状况及时转接
诱 导 方 式 :甲 醇
培 养 方 式 :28℃, 有 氧
保 存 方 式 :30%甘 油 , -‐80℃
操作说明:
1,本品包含一份甘油菌,使用本甘油菌时可以不用完全融解,在甘油菌表
面蘸取少量涂板或进行液体培养即可。也可以完全融解后使用,但随着冻融次数
将得到的菌株的新鲜培养物转接到适宜的固体培养基及液体培养基中(尽量
增大接种量:如用无菌吸管吸取≥50μl 新鲜培养物至固体培养基,边移动边缓
慢释放),适宜温度下培意 事 项 :
1、菌种活化前,将冷冻管保存在低温、清洁、干燥的环境中,长时间室温下放
管中。轻轻振荡,使冻干菌株溶解呈悬浮状。
菌株复壮:
用无菌吸管吸取菌悬液,转移到复溶液滴瓶中。做好标识,在适宜温度下培
养。细菌在 30-‐35℃培养箱中培养 24-‐48h,真菌在 23-‐28℃培养箱中培养 24-‐72h
毕赤酵母诱导表达实验步骤(1)

毕赤酵母诱导表达实验步骤(1)
BMGY的配制(每200 mL)
Yeast extract 2 g
Peptone 4 g
K3PO4(pH 6.0) 20 mL
丙三醇 2 mL
定容至180 mL,121,灭20 min。
使用前再加入
10*YNB 20 mL(10%)(过滤除菌)
生物素0.4 mL(0.02%)(过滤除菌)
加入后,用紫外照射10 min左右再接菌。
BMMY的配制(每200 mL)
Yeast extract 2 g
Peptone 4 g
K3PO4(pH 6.0) 20 mL
定容至180 mL,121,灭20 min。
使用前再加入
10*YNB 20 mL(10%)(过滤除菌)
生物素0.4 mL(0.02%)(过滤除菌)
甲醇0.5%
加入后,用紫外照射10 min左右再接菌。
10*YNB的配制
酵母基础氮源培养基 3.4 g
硫酸铵10 g
双蒸水80 mL
定容至100 mL,过滤除菌(用0.22 μm滤膜过滤)。
毕赤酵母诱导表达
1.配BMGY和BMMY灭菌
2.使用前向BMGY按比例加入生物素、10*YNB,紫外照10 min。
3.接菌于BMGY,250 rpm,28℃摇24 h
4.向BMMY中按比例加入生物素、10*YNB、甲醇,紫外照10
min。
5.将含有菌液的BMGY转移至50 mL离心管,4℃5000 rpm 离心5 min,弃上
清。
6.用BMMY将沉淀菌体重悬,倒回瓶中,250 rpm,28℃,摇3 d。
每24 h补
一次甲醇。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
毕赤酵母多拷贝表达载体试剂盒用于在含多拷贝基因的毕赤酵母菌中表达并分离重组蛋白综述:基本特征:作为真核生物,毕赤酵母具有高等真核表达系统的许多优点:如蛋白加工、折叠、翻译后修饰等。
不仅如此,操作时与E.coli及酿酒酵母同样简单。
它比杆状病毒或哺乳动物组织培养等其它真核表达系统更快捷、简单、廉价,且表达水平更高。
同为酵母,毕赤酵母具有与酿酒酵母相似的分子及遗传操作优点,且它的外源蛋白表达水平是后者的十倍以至百倍。
这些使得毕赤酵母成为非常有用的蛋白表达系统。
与酿酒酵母相似技术:许多技术可以通用:互补转化基因置换基因破坏另外,在酿酒酵母中应用的术语也可用于毕赤酵母。
例如:HIS4基因都编码组氨酸脱氢酶;两者中基因产物有交叉互补;酿酒酵母中的一些野生型基因与毕赤酵母中的突变基因相互补,如HIS4、LEU2、ARG4、TR11、URA3等基因在毕赤酵母中都有各自相互补的突变基因。
毕赤酵母是甲醇营养型酵母:毕赤酵母是甲醇营养型酵母,可利用甲醇作为其唯一碳源。
甲醇代谢的第一步是:醇氧化酶利用氧分子将甲醇氧化为甲醛,还有过氧化氢。
为避免过氧化氢的毒性,甲醛代谢主要在一个特殊的细胞器-过氧化物酶体-里进行,使得有毒的副产物远离细胞其余组分。
由于醇氧化酶与O2的结合率较低,因而毕赤酵母代偿性地产生大量的酶。
而调控产生醇过氧化物酶的启动子也正是驱动外源基因在毕赤酵母中表达的启动子。
两种醇氧化酶蛋白:毕赤酵母中有两个基因编码醇氧化酶-AOX1及AOX2。
细胞中大多数的醇氧化酶是AOX1基因产物。
甲醇可紧密调节、诱导AOX1基因的高水平表达,较典型的是占可溶性蛋白的30%以上。
AOX1基因已被分离,含AOX1启动子的质粒可用来促进编码外源蛋白的目的基因的表达。
AOX2基因与AOX1基因有97%的同源性,但在甲醇中带AOX2基因的菌株比带AOX1基因菌株慢得多,通过这种甲醇利用缓慢表型可分离Muts菌株。
表达:AOX1基因的表达在转录水平受调控。
在甲醇中生长的细胞大约有5%的polyA+ RNA 来自AOX1基因。
AOX1基因调控分两步:抑制/去抑制机制加诱导机制。
简单来说,在含葡萄糖的培养基中,即使加入诱导物甲醇转录仍受抑制。
为此,用甲醇进行优化诱导时,推荐在甘油培养基中培养。
注意即使在甘油中生长(去抑制)时,仍不足以使AOX1基因达到最低水平的表达,诱导物甲醇是AOX1基因可辨表达水平所必需的。
AOX1突变表型:缺失AOX1基因,会丧失大部分的醇氧化酶活性,产生一种表型为Muts的突变株(methanol utilization slow),过去称为Mut,而Muts可更精确地描述突变子的表型。
结果细胞代谢甲醇的能力下降,因而在甲醇培养基中生长缓慢。
Mut+(methanol utilization plus)指利用甲醇为唯一碳源的野生型菌株。
这两种表型用来检测外源基因在毕赤酵母转化子中的整合方式。
蛋白胞内及分泌表达:外源蛋白可在毕赤酵母胞内表达或分泌至胞外。
分泌表达需要蛋白上的信号肽序列,将外源蛋白靶向分泌通路。
几种不同的分泌信号序列已被成功应用,包括几种外源蛋白本身分泌信号序列,利用酿酒酵母α因子前原肽信号序列也获得许多成功。
分泌表达外源蛋白的最大优点是:毕赤酵母只分泌很少的自身蛋白,加上毕赤酵母最小生长培养基中只有少量的蛋白,这意味着分泌的外源蛋白是培养基中蛋白的主要组成成份,也可算作蛋白纯化的第一步。
注意,如果外源蛋白一级结构中有可识别的糖基化位点(Asn-X-Ser/Thr),则这些位点可能发生糖基化。
翻译后修饰:与酿酒酵母相比,毕赤酵母在分泌蛋白的糖基化方面有优势,因为不会使其过糖基化。
酿酒酵母与毕赤酵母大多数为N-连接糖基化高甘露糖型,然而毕赤酵母中蛋白转录后所增加的寡糖链长度(平均每个支链8-14个甘露糖残基)比酿酒酵母中的(50-150个甘露糖残基)短得多。
另外,酿酒酵母核心寡糖有末端α-1,3聚糖连接头,而毕赤酵母则没有。
一般认为酿酒酵母中糖基化蛋白的α-1,3聚糖接头与蛋白的超抗原性有关,使得这些蛋白不适于治疗应用。
虽然未经证明,但这对毕赤酵母产生的糖蛋白不构成问题,因为毕赤酵母表达蛋白与高级真核生物糖蛋白结构相似。
选择载体用于基因多拷贝整合:在某些情况下,毕赤酵母中重组基因多拷贝整合可增加所需蛋白的表达量。
该试剂盒中的三个载体均可用于在体内(pPIC3.5K, pPIC9K)或体外(pAO815)产生并分离多拷贝插入,同时可检测增加重组基因的拷贝数是否增加蛋白表达量。
体内整合可通过高遗传霉素抗性,筛选可能的多拷贝插入;而体外整合可通过连接产生外源基因的串联插入。
pPIC3.5K, pAO815用于胞内表达,而pPIC9K用于分泌表达,所有载体均利用AOX1启动子来诱导高水平表达。
多拷贝插入频率:毕赤酵母His+转化子高拷贝整合事件自发发生的概率为1-10%,体内方法可筛选可能插入多拷贝外源基因的His+转化子,体外方法可通过连接构建多拷贝子。
当选择His+转化子时,它们中插入体外构建结构多聚体的概率很高。
体内多拷贝插入的产生:Ppic3.5k及Ppic9k含有细菌kan基因,赋予毕赤酵母遗传霉素抗性,注意Kan并不赋予毕赤酵母卡那霉素抗性。
遗传霉素抗性水平主要依赖整合的kan基因的数目。
单拷贝Ppic3.5k或Ppic9k整合入毕赤酵母基因组后,赋予毕赤酵母约0.25mg/ml的遗传霉素抗性水平。
任何载体多拷贝整合可增加遗传霉素抗性水平,从0.5mg/ml(1-2拷贝)到4mg/ml(7-12拷贝)。
由于kan基因与表达盒(pAOX1及目的基因)之间有遗传连锁,可从遗传霉素高抗性推断该克隆所包含多拷贝目的基因数。
由于基因的剂量效益,蛋白的表达可能会增加。
因此,kan基因可检测转化子是否含有多拷贝目的基因。
下图显示多拷贝插入及kan基因与表达盒的连锁。
遗传霉素直接选择:在酵母中对遗传霉素抗性进行直接选择并不十分有效,因为新转化的细胞需要时间表达足够量的抗性因子。
由于酵母生长比细菌慢得多,大部分重组酵母在积累足够多的抗性因子以抵抗平板上抗生素之前就已经被杀死了。
最有效的筛选遗传霉素抗性及高抗性克隆的程序需要先对HIS+转化子进行选择,再进行不同水平遗传霉素抗性筛选。
虽然可以用电泳进行直接筛选,但用在遗传霉素筛选之后再进行电泳筛选,获得含高拷贝克隆的机会更大,大约可获得5-9拷贝的克隆,而直接电泳选择只能获得平均为1-3拷贝的克隆。
原生质转化时不能用遗传霉素直接选择。
体外多拷贝插入的产生:下图显示如何产生多表达盒插入载体以转化毕赤酵母。
目的基因插入独个EcoRI位点后,产生的表达盒(pAOX1及目的基因)上下游侧翼分别为独个的BglII及BamHI位点。
含目的基因的pAOX815用BglII及BamHI消化以分离表达盒,表达盒再插入BamHI 位点以产生串联重复表达盒,重复该插入程序可产生一系列含单个HIS4基因及逐渐增加数目表达盒的载体。
用体外形成的多拷贝子转化毕赤酵母增加了多拷贝表达盒重组子出现的频率,可设计包含一特定数目多拷贝插入的毕赤酵母重组子。
转化及整合:可产生两个不同表型的His+重组菌株:质粒DNA线性化位置不同,转化GS115后可产生两种转化子His+Mut+及His+Muts。
KM71只产生His+Muts,因为该菌株为Muts表型。
两种重组子Mut+及Muts都是有用的,因为一个表型可能比另一个表型更有利于蛋白表达。
理想条件下,每一个表型应该检测6-10个重组子。
没有办法预测哪个结构或克隆更利于蛋白表达。
强烈推荐用PCR分析重组子来证实整合情况。
成功将基因构建至AOX1启动子下游后,线性化质粒转化毕赤酵母时激发重组。
下图显示用不同酶消化时产生何种重组子。
限制酶插入事件GS115表型KM71表型SalI或StuI 插入his4 His+Mut+ His+MutsSacI 插入5’AOX1 His+Mut+ His+MutsBglII 取代AOX1 His+Muts His+Muts(不推荐)表达及扩大培养:用PCR证实毕赤酵母重组后,可检测His+Mut+及His+Muts的表达。
小规模培养每个重组子,用甲醇诱导,检测时间点.如果是胞内表达,每个时间点细胞沉淀用SDS-PAGE分析;如果是分泌表达,分析每个时间点的细胞及上清。
如果有蛋白的抗体,推荐既用考马斯亮蓝染色又用western blot分析SDS-PAGE凝胶。
如果可以,建议检测蛋白活性。
因为并不是所有蛋白都能达到g/l的水平,所以建议进行western blot或活性分析,不要仅做SDS-PAGE 考马斯亮蓝染色分析。
如何选择最佳的表达蛋白毕赤酵母菌株及优化诱导见P49-50。
如表达已达最优,大规模表达以产生更多蛋白。
方法毕赤菌株表型:毕赤酵母菌GS115及KM71在组氨酸脱氢酶位点(His4)有突变,因而不能合成组氨酸,所有表达质粒都有HIS4基因可与宿主进行互补,通过不含组氨酸的培养基来选择转化子。
GS115及KM71自发回复突变到His+原养生物机率小于1/108。
KM71的亲本菌在精氨酸琥珀酸裂解酶基因(arg4)有突变,在不含精氨酸的培养基中不能生长。
用野生型ARG4基因破坏AOX1基因后,产生KM71 MutsArg+His-菌株。
GS115及KM71都可在复合培养基如YPD(YEPD)及含组氨酸的最小培养基中生长。
转化之前,GS115及KM71都不能在最小培养基中生长,因为它们是His-。
KM71结构:ARG4基因(约2kb)插入到克隆的野生型AOX1基因的BamHI(AOX1基因15/16密码子)及SalI(AOX1基因227/228密码子)位点。
ARG4取代了AOX1基因16-227密码子。
此结构转化至KM71亲本菌(arg4his4)中,分离Arg+转化子并分析Muts表型。
Arg+转化子遗传分析显示野生型AOX1被aox1::ARG4结构所取代。
重点:用KM71的优点是,不需要在甲醇最小培养基中筛选Mut表型。
所有转化子都是Muts 表型。
第二,AOX1位点没有被完全缺失,理论上可用你的目的结构通过基因取代方法替换aox1::ARG4结构,这样重组菌株的表型是His+MutsArg-,这意味着重组菌株生长时需精氨酸。
不幸的是,仅添加精氨酸并不能完全缓和arg4突变的影响,arg4菌株在含精氨酸的最小培养基中不能很好地生长。
因此不推荐在KM71中通过取代aox1::ARG4结构来获得His +转化子。
菌株表达对照:GS115/His+Muts白蛋白:该菌株为筛选毕赤酵母分泌表达转化子与Muts表型时的对照。
血清白蛋白基因及其自身分泌信号被整合进毕赤酵母AOX1位点。
