联吡啶衍生物芳基钌配合物的合成及其与DNA、蛋白质相互作用
两种三齿多吡啶配体钌化合物与DNA的相互作用研究
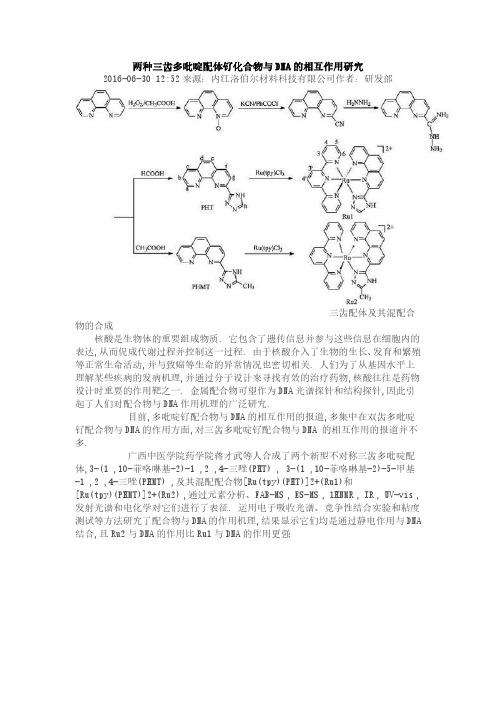
两种三齿多吡啶配体钌化合物与DNA的相互作用研究2016-06-30 12:52来源:内江洛伯尔材料科技有限公司作者:研发部
三齿配体及其混配合物的合成
核酸是生物体的重要组成物质. 它包含了遗传信息并参与这些信息在细胞内的表达,从而促成代谢过程并控制这一过程. 由于核酸介入了生物的生长、发育和繁殖等正常生命活动,并与致癌等生命的异常情况也密切相关. 人们为了从基因水平上理解某些疾病的发病机理,并通过分子设计来寻找有效的治疗药物,核酸往往是药物设计时重要的作用靶之一. 金属配合物可望作为DNA光谱探针和结构探针,因此引起了人们对配合物与DNA作用机理的广泛研究.
目前,多吡啶钌配合物与DNA的相互作用的报道,多集中在双齿多吡啶钌配合物与DNA的作用方面,对三齿多吡啶钌配合物与DNA 的相互作用的报道并不多.
广西中医学院药学院蒋才武等人合成了两个新型不对称三齿多吡啶配体,3-(1 ,10-菲咯啉基-2)-1 ,2 ,4-三唑(PHT) , 3-(1 ,10-菲咯啉基-2)-5-甲基-1 ,2 ,4-三唑(PHMT) ,及其混配配合物[Ru(tpy)(PHT)]2+(Ru1)和
[Ru(tpy)(PHMT)]2+(Ru2) ,通过元素分析、FAB-MS , ES-MS , 1HNMR , IR , UV-vis ,发射光谱和电化学对它们进行了表征. 运用电子吸收光谱、竞争性结合实验和粘度测试等方法研究了配合物与DNA的作用机理,结果显示它们均是通过静电作用与DNA 结合,且Ru2与DNA的作用比Ru1与DNA的作用更强。
多吡啶钌配合物与DNA相互作用的光电化学性能及电化学组装的开题报告

多吡啶钌配合物与DNA相互作用的光电化学性能及
电化学组装的开题报告
研究背景:
DNA和金属配合物的相互作用一直是研究热点之一。
多种金属配合物均已被证明可以与DNA发生相互作用,并且这种相互作用可以导致DNA的结构变化和光学性质的改变。
特别是,钌配合物因其特殊的物理化学性质已经成为DNA相互作用研究的热点之一。
研究内容:
本研究将使用脱氧核糖核酸(DNA)作为底物,并采用电化学组装方法制备了多种多吡啶钌配合物/DNA复合体。
通过XPS,FTIR,CV等表征手段,表征了多吡啶钌配合物和DNA的化学性质和电化学性能。
光电化学性能方面,采用紫外-可见吸收光谱、荧光谱和光电化学技术研究了多吡啶钌配合物和DNA之间的相互作用。
通过光电流和电位分布分析,探讨多吡啶钌配合物/DNA复合物的光电化学性能。
同时,使用荧光共振能量转移(FRET)技术研究了多吡啶钌配合物和DNA复合物中的能量传输机制。
电化学组装方面,采用原子力显微镜和电化学阻抗谱等表征方法,研究多吡啶钌配合物/DNA复合物在电极表面的组装行为。
并通过实验和模拟研究其电导特性和传输行为。
创新意义:
本研究利用多吡啶钌配合物和DNA复合体用于光电化学检测,探索了DNA与金属配合物之间的相互作用。
可以为探索DNA和其他金属配合物之间的相互作用提供参考。
此外,研究结果还可以为光电化学材料的设计和制备提供参考,为制备高效的传感器、光电器件等开发具有现实意义的技术提供了基础数据。
联吡啶钌(Ⅱ)配合物的合成、晶体结构及其在染料敏化太阳能电池中的应用

【u Iiy2(yp] F) H0 () a bandae o ti t no ew t ou o f o pe . o lx2 R bp) ea)e 6・ 2 2 w sot e f r l iz i fh a r lt no m l 1 C mpe i (  ̄ i t v a lao t es i c x
.
为 4 2 m 填 充 0 V,
因子 f 为 04 4光 电转 化 效 率 为 0 2 % 。 r . , 4 . 5 0
关键 词 :吡 啶钌 () 合 物 ;敏化 染料 ; 阳能 电池 ;晶 体 结 构 ;光 电 性 质 Ⅱ配 太 中图 分 类 号 : 6 48 0 1. 1 2 文献 标 识 码 : A 文 章 编 号 :1o 4s 1 o 7m 一7 1 6 o 1 6 ( 0 ) 17 - 2 0
c mp e lo h s b e h r c e z d b l me tla a y i,I , o l x a s a e n c a a tr e y ee n a n lss R H i NMR UV— s L Vi P ,XRD.CV. V.C mp e . I o lx
GUO C i i n W U e Z u— a L Yu HOU — i g 。 L . h Z Yi n M IYi i . Z HEN He Ge G . n ,
( ori t nC e ir s tt Sa e aoao 0 n nC e ir, odn i h ms yI tu , teK yL br r o c o h m t C ao t ni e t t yf sy S ho h mir ad C e cl n i eig N ni n esy N ni 10 3 colfC e s y n hmi gn r , ajn U i rt, aj g2 09 ) o t aE e n g v i n
钌(Ⅱ)多吡啶配合物与DNA相互作用研究

第 1 9卷 第 1 2期 20 0 7年 1 2月
化 学 进
展
Vo . 9 No.2 1 1 1 De ,2 0 e. 0 7
PROGRES N S I CHEMI T s RY
钌 ( 多吡啶配合物 与 D A相互作用研究 * Ⅱ) N
广 泛关注 , 成为 生物 无机化 学的重 要研 究内容之 一。本 文 简要 评述 了钌 ( 多吡啶 配合 物在 D A识别 、 Ⅱ) N 断 裂 及拓扑 异构 酶抑制 方面 的研 究情 况。
关键 词 钌( 多吡 啶配合 物 Ⅱ) D A识别 N D A断裂 拓 扑异构 酶抑制 N
中图分类号 : 64 8 Q 2 文 献标识码 : 0 1 .; 53 A 文章编号 :10 —8x(o 7 1 —840 0 52 1 2 o )2 14 —8
b t e tlc mp e e n ewe n mea o lx s a d DNA a e ev d i tn e i trs n e o n i o a trs ac ed o iio g ne h s rc ie n e s ne e ta d b c me a mp r n e e r h f l fb on ra i t i c e sr .T i rve hg l hss me rc n rg es s i h t de fDNA e o nt n, ce v g n o os meae h mit y h s e iw ih i t o e e tp o rse n t e su iso g rc g io i la a e a d tp io r s
代谢 有着 十分重 要 的 意义 ; 外致 癌 等 生命 的异 常 此 情 况也 与 D A代 谢活 动密切相 关 。研究 核 酸 , 其 N 尤
一种含芴基的钌(Ⅱ)配合物的合成及DNA键合性质

一种含芴基的钌(Ⅱ)配合物的合成及DNA键合性质姚威;吴宝燕;高丽华;王科志【期刊名称】《物理化学学报》【年(卷),期】2007(23)2【摘要】合成了一种新的钌(Ⅱ)配合物[Ru(bpy)2(Hfip)](ClO4)2,其中bpy代表2,2'-联吡啶,Hfip代表2-(9H-芴-2-基)-1H-咪唑-[4,5-f]-[1,10]-邻菲□啉.通过紫外可见光谱、荧光光谱、稳态荧光淬灭、与溴化乙锭的竞争实验、粘度测量和DNA 热变性研究了该配合物与小牛胸腺DNA的键合性质.结果表明,该配合物能嵌入键合DNA,键合常数Kb=8.6×105 L·mol-1(50 mmol·L-1 NaCl).【总页数】5页(P237-241)【作者】姚威;吴宝燕;高丽华;王科志【作者单位】北京师范大学化学系,北京,100875;北京师范大学化学系,北京,100875;北京师范大学化学系,北京,100875;北京工商大学环境与化工学院,北京,100037;北京师范大学化学系,北京,100875【正文语种】中文【中图分类】O646【相关文献】1.一个新型钌(Ⅱ)配合物的合成、表征与DNA的键合及溶剂变色性质 [J], 吴宝燕;高丽华;王科志2.钌(Ⅱ)配合物合成及其与DNA键合方式研究 [J], 黄晓媚;刘云军;何丽新;赵豪杰;梅文杰3.带有位阻基团的钌多吡啶手性配合物与DNA键合及其光断裂DNA性质的研究[J], 蒲小华;杨频4.带有位阻基团的钌多吡啶配合物与DNA键合及其光断裂DNA性质的研究 [J], 蒲小华;陈绘丽;韩高义;杨频5.一种含咔唑基团的混配型钌(Ⅱ)配合物的合成、表征和酸碱性质研究 [J], 凡素华;杨维春;王科志因版权原因,仅展示原文概要,查看原文内容请购买。
钌配合物[Ru(bpy)2(PNT)] 2+的合成、表征及与DNA相互作用研究
![钌配合物[Ru(bpy)2(PNT)] 2+的合成、表征及与DNA相互作用研究](https://img.taocdn.com/s3/m/781c666addccda38376baf4c.png)
20 0 8年 l 2月
高 等 学 校 化 学 学 报
CHEMI CAL J OURNAL OF CHI S NE E UNI VERSTIS I E
No 1 .2
2 9 ~2 0 4 6 5 1
钌 配合 物 [ u b y 构表 征.利用 紫外 一 可见光谱 滴定 、 稳态荧 光光谱 滴定 、 N D A热变性和 黏度实验 研究 了配合物 与
D A的相 互作 用. N
1 实验部 分
1 1 仪器 与试 剂 .
Ee na ai E l t V r L元 素分析仪 , ai 0 MH 核 磁共振 波谱仪 , C me r o V r n50 z a L Q系统 (ingnM T U A) Fni A , S a
基金项 目:国家“ 九七三” 计划( 批准号 : 0 7 B 136 国家 自然科学基金 ( 2 0 C 8 50 )、 批准号 : 07 0 9 0 7 15 、教育部新世纪优秀 2 5 18 ,27 10 ) 人才支持计划( 批准号 :N E -657 8 、 C T0 0 -1 ) 教育部重点项 目( 批准号 :180 ) 0 13 和教育部 留学 回国人员科研启动基金( 批准号 :教外司留 [0 7 2 20 ]4号) 资助. 联系人简介 : 巢 晖, , 男 教授 ,主要从事生物无机化学研究.Ema :csh@ma .yu eu c . i ech l i ss .d .n l
平上 了解 生命现 象 的本质 .近 年来 , 设计合成 小分 子过渡金 属配合 物 , 研究 其与 大分子 D A 的相互 作 N
用受 到了广泛关 注 , 其是钌 (I) 吡啶配 合物 ,因易 于构 造一 个 既 为刚性 又 带手性 的八面 体构 型 , 尤 I多
不同给电子基联吡啶衍生物及配合物的合成、光学性质及应用探索的开题报告

不同给电子基联吡啶衍生物及配合物的合成、光学性质及应用探索的开题报告
本文计划研究不同给电子基的联吡啶衍生物及其配合物的合成、光学性质和应用。
联吡啶是一种重要的杂环芳香化合物,具有良好的光学和电学性质。
通过引入不同的给电子基团可以调节其电荷分布和能带结构,从而影响其光学性质。
而将联吡啶与金属配合可以形成具有新颖光学和电学性质的配合物,具有广泛的应用前景。
具体实验计划包括以下内容:
1. 合成不同给电子基联吡啶衍生物
以联吡啶为基础结构,引入不同的给电子基团,如甲氧基、氨基、芳基基等,通过缩合反应合成目标化合物。
选择不同的反应条件和催化剂,优化反应条件,获得纯度高、收率高的产物。
2. 合成联吡啶金属配合物
将联吡啶与金属离子配位形成配合物,通过改变配合物中金属离子的种类和配位数,调节配合物的光学和电学性质。
选择不同的配合反应条件和配体,优化反应条件,获得纯度高、收率高的产物。
3. 光学性质表征
使用紫外可见吸收光谱、荧光光谱、圆二色性光谱等手段对合成的化合物进行光学性质表征,研究不同给电子基的联吡啶衍生物在吸收、荧光和手性识别等方面的表现,并与文献中报道的结果进行对比分析。
4. 应用探索
通过合成具有不同光学和电学性质的化合物,探索其在有机发光二极管(OLEDs)、有机太阳能电池(OSCs)等方面的应用,研究不同结构的化合物对器件性能的影响。
预期的结果包括成功合成不同给电子基联吡啶衍生物及其配合物,探究其光学和电学性质,及其在OLEDs、OSCs等器件方面的应用。
通过本次研究,将拓展联吡啶在光电领域的应用,为新型化合物的研究和设计提供参考。
钌配合物合成及其光敏切割DNA活性研究的开题报告

钌配合物合成及其光敏切割DNA活性研究的开题报告一、研究背景DNA分子是生命体系的重要组成部分,其结构、功能和稳定性对生命体系的正常发育和生长具有至关重要的作用。
同时,DNA分子又易受到外界的影响和干扰,如辐射、化学药品等,从而导致DNA分子的损伤和突变,从而影响到生命体系的正常功能。
因此,开发新型具有光敏切割DNA活性的钌配合物具有重要的理论意义和应用价值。
通过合成具有光敏切割DNA活性的钌配合物,可以为医学诊疗、生物学研究等领域提供新的工具和方法,为生命科学研究进一步发展做出贡献。
二、研究目的本研究的目的是合成具有光敏切割DNA活性的钌配合物,进一步研究其对DNA分子的切割作用和机理。
三、研究内容和方法研究内容:1. 合成并表征具有光敏切割DNA活性的钌配合物;2. 研究该钌配合物对DNA分子的切割作用和机理;3. 探索该钌配合物在医学诊疗、生物学研究等领域的应用价值。
研究方法:1. 合成并表征钌配合物:通过合成有机合成方法,合成具有光敏切割DNA活性的钌配合物,并通过化学手段对其进行表征;2. 研究DNA分子的切割作用和机理:通过体外实验,研究该钌配合物对DNA分子的切割作用和机理,并利用比色法等方法对实验结果进行定量分析;3. 探索应用价值:通过将该钌配合物应用于医学诊疗、生物学研究等领域,并对其应用效果进行评估,探索其应用价值和未来发展。
四、研究意义1. 增进对DNA分子的认识:通过研究该钌配合物的切割作用和机理,可以增进对DNA分子的认识,为研究DNA分子在生命体系中的作用和机理提供新的视角和方法。
2. 提供新型医学诊疗工具:将具有光敏切割DNA活性的钌配合物应用于医学诊疗领域,可以为医生提供新型诊疗工具,为患者提供更加精准、有效的诊疗方案。
3. 推动生命科学研究进一步发展:通过合成具有光敏切割DNA活性的钌配合物,可以为生命科学研究提供新的工具和方法,推动生命科学研究进一步发展。
五、预期成果1. 合成并表征具有光敏切割DNA活性的钌配合物;2. 研究该钌配合物对DNA分子的切割作用和机理;3. 发表相关研究论文,并将成果应用于医学诊疗、生物学研究等领域。
钌(Ⅱ)基金属配合物的pH传感和DNA键合性质的研究的开题报告

钌(Ⅱ)基金属配合物的pH传感和DNA键合性质
的研究的开题报告
本研究旨在探究钌(Ⅱ)基金属配合物的pH传感和DNA键合性质。
具体来说,主要有以下几个方面:
1. 合成和表征钌(Ⅱ)基金属配合物
本研究将采用化学合成法制备钌(Ⅱ)基金属配合物,并采用FT-IR、UV-Vis、ESI-MS等技术对其进行表征。
通过调节反应条件,可以合成得
到一系列具有不同结构和性质的钌(Ⅱ)基金属配合物。
2. pH传感性质的研究
考虑到钌(Ⅱ)基金属配合物在不同pH值下的电荷状态和结构可能存在变化,因此我们将研究其在不同pH条件下的荧光和吸收光谱变化,以探究其pH传感性质。
同时,我们还将研究其在不同离子强度、温度等条件下的荧光和吸收光谱特性。
3. DNA键合性质的研究
DNA是生命体内重要的分子,钌(Ⅱ)基金属配合物可以与DNA形成稳定的配合物。
本研究将考察钌(Ⅱ)基金属配合物与DNA之间的键
合性质,包括静态结合常数、热力学参数等,并采用凝胶电泳、荧光染
料等技术研究其与DNA的作用机制。
预期成果:本研究将为该金属配合物在生物医学领域中的应用提供
重要的基础研究,为设计和合成更具特异性和选择性的生物传感器和药
物提供有力支撑。
新型联吡啶钌环糊精超分子化合物:合成、性质及其在电致化学发光DNA生物传感器中的应用研究

新型联吡啶钌环糊精超分子化合物:合成、性质及其在电致化学发光DNA生物传感器中的应用研究【摘要】:自然界亿万年的进化创造了生命体,而执行生命功能是生命体中无数个超分子体系。
超分子化学是研究分子间相互作用缔结而形成复杂有序且具有特定功能的分子聚集体的科学。
超分子化学逐渐发展成为一门新兴的分子信息化学,它包括在分子水平和结构特征上的信息存储,以及通过特异性相互作用的分子识别过程,实现在超分子尺寸上的修正、传输和处理。
它是化学和多门学科的交叉领域,它不仅与物理学、材料科学、信息科学、环境科学等相互渗透形成了超分子科学。
而更具有重要理论意义和潜在前景的是在生命科学中的研究和应用。
例如生物体内小分子和大分子之间高度特异的识别在生命过程中的调控,生物体内的信息输送(电子转移、能量传递、物质传输和化学转换)和生物体中受体.底物相互作用等,其基本现象都离不开超分子化学范畴。
环糊精是超分子化学中最重要的主体物质之一,它能与许多有机、无机和生物分子形成主客体包结物,也正是由于这些独特的性质,现在对环糊精的研究已经发展成环糊精超分子化学而被广泛关注。
对它的研究从主客体识别形成包合物的机理已经转移到对其在分析化学、医药制备、环境检测和生物传感器等领域的应用研究。
将功能金属中心与环糊精联接构成的金属环糊精超分子化合物,由于同时具有环糊精的主客体识别特性和功能金属中心(例如,联吡啶钌)的特性,使其更加适合于超分子器件及传感器的设计。
因此,金属环糊精超分子化合物已成为目前超分子化学中的研究热点。
但是目前为止,金属环糊精大多以单核金属中心的形式存在。
多核金属中心的环糊精超分子化合物因为具有多核的电子氧化还原中心,势必在光电子器件、荧光开关及生物分子多标记领域显示更为优越的性质,和更具独特的应用前景。
电致化学发光(ECL),特别是基于Ru(bpy)32+电致化学发光技术己被广泛应用临床的医学检验中,例如,目前临床中免疫、肿瘤标记物等检测均采用联吡啶钌电致化学发光检测技术。
钌多吡啶配合物与DNA相互作用的瞬态发光特性研究的开题报告

钌多吡啶配合物与DNA相互作用的瞬态发光特性研究的开题报告开题报告:一、研究背景DNA是生命体分子的重要组成部分,其二级结构是双链螺旋。
DNA 分子是一条由核苷酸单体组成的螺旋基线,其中的碱基对持续在两条单股里面,通过氢键相互连接。
DNA的结构和性质对人类的生理和疾病研究有着非常重要的作用。
因此,研究DNA与金属配合物的相互作用对于生命科学、药物化学等领域的发展具有重要的意义。
钌多呋啶作为一种重要的金属配合物,具有良好的光物理和化学性质,在生物体系中表现出多种重要的生物和药用性质,因此近年来引起学术界的广泛关注。
钌多呋啶配合物与DNA分子可以通过不同的作用方式相互作用,如插入、静电吸引等方式,对DNA结构和功能造成影响,从而影响生物体的生理和代谢过程。
二、研究目的本研究旨在通过采用瞬态发光技术,研究钌多呋啶配合物与DNA相互作用的动态过程和机理,揭示其对DNA结构的影响和作用机制,为进一步探索其在生物医学领域的应用奠定基础。
三、研究内容和方法1.研究内容:(1)了解钌多呋啶配合物的基本结构和物理化学性质。
(2)研究DNA与钌多呋啶配合物的相互作用特性,探究相互作用机制。
(3)采用瞬态发光技术,研究DNA与钌多呋啶配合物相互作用的瞬态发光特性,探究DNA与钌多呋啶配合物相互作用动态过程。
2.研究方法:(1)合成钌多呋啶配合物;(2)合成DNA溶液,并与钌多呋啶配合物作用;(3)采用紫外吸收光谱、荧光光谱等技术研究DNA与钌多呋啶配合物的相互作用特性;(4)采用瞬态发光技术,对DNA和钌多呋啶配合物样品进行激光闪光瞬态发光特性测量,探究DNA与钌多呋啶配合物相互作用的动态过程。
四、预期成果通过对钌多呋啶配合物与DNA相互作用的瞬态发光特性研究,可揭示其相互作用机制和动态过程,为进一步探索其在生物医学领域的应用奠定基础。
同时,本研究成果有望为DNA及其相互作用的研究提供新的思路和方法。
季铵盐联吡啶-钌(Ⅱ)配合物的合成及其与小牛胸腺DNA的相互作用

( 1 . 广东医学院 药学院 , 广东 东莞 5 2 3 8 0 8 ; 2 . 嘉应学院 化学与环境学院 , 广东 梅州 5 1 4 0 1 5 )
摘
要: 利用紫外一 可 见 吸收 光 谱 、 荧 光光 谱 、 圆 二 色 谱 等 光谱 手段 , 以及 黏 度 测 定 等 流体 力 学 方 法 在 三 羟 甲基 氨
o r e s c e n e e s pe c t r o me t r y,a n d c i r c u l a r di c h r o i s m s pe c t r o me t r y a s we l l a s v i s c os i t y me a s u r e me n t s
s u l t s s h o w t h a t t h e r e e x i s t a c e r t a i n d e g r e e o f a f f i n i t y b e t we e n a s — s y n t h e s i z e d Ru ( I I)c o mp l e x
结果表明 , 合 成 的配 合 物 与 C T — D NA 之 间 存 在 一 定 的亲 和 作 用 , 拟 合 得 到 的结 合 常 数 可 达 1 O ; 与此 同时, 该 配
合物能够稳定 C T — D NA 的 结 构 , 并对 C T — D NA 有 较 好 的立 体 选 择 性 ( 右 旋 异 构 体 优 先 与 DN A结合) . 关键词 : 季铵盐联 吡啶; 钌( I I ) 配合物; 合成 ; 小 牛胸 腺 DN A; 相 互 作 用 中 图分 类 号 : O 6 1 4 .8 2 文献标志码 : A 文章编号 : 1 0 0 8 —1 0 1 1 ( 2 O 1 3 ) 0 4 —0 3 9 4 —0 5
钌、铜吡啶类配合物的制备及与DNA相互作用的研究

钌、铜吡啶类配合物的制备及与DNA相互作用的研究吴莎莎;雷苗;雷春蕾;王小波【摘要】目的研究钌、铜金属配合物对DNA的作用方式和效果,为进一步研究开发新型金属抗癌药物提供基础.方法以钌、铜两种金属分别与2,2-联吡啶及含吡啶四氮环作用,制备了含吡啶的金属配合物[Ru(bpy)3] Cl2及(CuL) (NO3)2{ bpy=2,2'-联吡啶,L=3,6,9,15-四氮杂[9.3.1]十五元双环-1(15),11,13-三烯},通过红外、紫外及质谱等对该两种配合物进行了表征.运用紫外光谱与凝胶电泳手段研究了上述配合物与pBR322 DNA的相互作用.结果表明配合物[Ru(bpy)3]Cl2与DNA主要以静电结合的方式作用,而(CuL) (N03)2则是以嵌插的方式作用,且后者对DNA的切割效果更为显著.结论本论文合成的两种钌、铜配合物与pBR322DNA结合方式不同,这可能与其结构有关系,而切割活性可能与配合物对其结合方式有关.【期刊名称】《湖北科技学院学报(医学版)》【年(卷),期】2018(032)005【总页数】6页(P369-372,375,封2)【关键词】钌配合物;铜配合物;凝胶电泳;DNA切割【作者】吴莎莎;雷苗;雷春蕾;王小波【作者单位】湖北科技学院药学院,湖北咸宁437100;湖北科技学院药学院,湖北咸宁437100;湖北科技学院药学院,湖北咸宁437100;湖北科技学院药学院,湖北咸宁437100【正文语种】中文【中图分类】R943随着生活水平的提高,癌症成为仅次于心血管疾病威胁人类健康的第二大杀手,癌症的发病率和死亡率逐年上升[1]。
当前癌症的三大治疗手段为手术、放疗与化疗。
就化疗而言,目前已经取得实际临床应用并极具潜力的化疗药物当属金属配合物。
其作为药物的研究可追溯到1969年,顺铂首次被报道具有抗肿瘤活性,随之被应用于临床实验,开创了小分子金属配合物药物作为抗癌药研究的新领域[2-3]。
钌多吡啶配合物与DNA作用及抗肿瘤活性

钌多吡啶配合物与DNA作用及抗肿瘤活性徐丽;陈禹;巫佳焕;闻伴康【摘要】应用电子吸收光谱、荧光光谱和粘度测定等方法研究钌多吡啶配合物[Ru(phen)2(Hecip)]2(phen=1,10-邻菲哕啉,Hecip=2(9-乙基-9H-咔唑-3-基)-1H-咪唑并[4,5-f][1,10]菲哕啉)与DNA相互作用.结果表明配合物与DNA键合计量比为2∶1,键合常数超过105 mol-1·L,配合物以插入方式与DNA结合.运用琼脂糖凝胶电泳实验研究配合物诱导pBR322DNA断裂及断裂机理.体外抗肿瘤活性结果表明配合物能有效抑制肿瘤细胞增殖,进一步研究表明配合物可以将细胞周期阻滞在S期.%The interactions of the Ru (Ⅱ) complex,[Ru (phen)2 (Hecip)]2 + (phen =1,10-phenanthroline,Hecip =N-ethyl-4-([1,10]-phenanthroline [5,6-f]imidazol-2-yl)carbazole),with calf thymus DNA (CT DNA) were studied by using absorption spectroscopy,binding stoichiometry,viscosity measurement and photoactivated cleavage.A tight 2:1 complex is formed by the Ru(Ⅱ) polypyridyl complex and CT DNA with a binding const ant exceeding 105 mol-1·L and with a binding mode ofintercalation.Furthermore,the complex exhibits efficient DNA cleavage activity on UV (365 nm) irradiation via a mechanistic pathway involving formation of singlet oxygen as the reactive species.On the other hand,the cytotoxic activity of the complex was tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method.The complex shows prominent anticancer activity against selected tumor cell lines with IC50 values lower than those of cisplatin.Further flow cytometryexperiments show that the cytotoxic Ru(Ⅱ) complex can cause cell cycle arrest in the S phase.【期刊名称】《无机化学学报》【年(卷),期】2013(029)003【总页数】8页(P613-620)【关键词】钌配合物;毒性;DNA键合;细胞周期阻滞【作者】徐丽;陈禹;巫佳焕;闻伴康【作者单位】广东药学院医药化工学院,中山 528458;中山大学生命科学学院有害生物控制与资源利用国家重点实验室,广州 510006;广东药学院医药化工学院,中山528458;广东药学院医药化工学院,中山 528458【正文语种】中文【中图分类】O614.82+10 IntroductionOver the past few decades,Ru(Ⅱ)complexes have been used as probes of DNA structure,artificial nucleases, DNA molecular light switches and DNA photocleavage agents[1-6].Recently,their potential applications as cell imaging and anticancer agents have drawn much attention[7-10].Ruthenium complexes are attractive alternatives to platinum-based anticancer agents and several ruthenium complexes have now been proposed as potential anticancer agents; such complexes can showremarkable anticancer activity and lower general toxicity than platinum compounds[11-12].These studieshave stimulated much interestto investigate the bioactivities of ruthenium(Ⅱ) complexes.The carbazole group has attracted much interest inphotoconductive[13],photorefractive[14],second-order nonlinearoptical[15],solar energy conversion[16],and electroluminescent materials[17]due to its excellent hole-transporting and donor properties.Wang and coworkers[18-19]incorporated carbazole into the RuⅡcomplexes for DNA binding and pH-induced emissionswitching/sensing studies.In our previous work,we demonstrated that ruthenium(Ⅱ)polypyridyl complex,[Ru(phen)2(Hecip)]2+(Scheme 1),co uld display fully reversible pH value controlled “on-off” luminescent signaling[20].To the best of our knowledge,however,little work has been devoted to the antitumor activity of theruthenium complexescontainingcarbazole ligands.It is for this reason that we study the mode of DNA binding,cytotoxicity,cellcycle arrestofcomplex[Ru(phen)2(Hecip)]2+.We demonstrate that the complex can intercalate between the base pairs of DNA.In vitro cytotoxicity assay shows that the complex exhibits cytotoxic potencies higher than that of cisplatin against the four human cancer cell lines screened.Further studyshowsthatthecomplex inhibits cell growth through the induction of S phase cell cycle arrest.1 Experimental1.1 Materials and methodsCalf thymus DNA (CT DNA)was obtained from the Sino-American Biotechnology Company.pBR322 DNA was obtained from Shanghai Sangon Biological Engineering&Services Co.,Ltd.Dimethylsulfoxide(DMSO)was purchased from Sigma-Aldrich.Cell lines of HeLa (human cervical cancer cell line),MCF-7(human breastadenocarcinoma cancercellline),HepG2 (human hepatocellular liver carcinoma cell line)and A549(human lung adenocarcinoma epithelial cell line)were supplied by Center of Experimental Animal Sun Yat-sen University (Guangzhou,China),agarose and ethidium bromide were obtained from Sigma-Aldrich.Doubly distilled water was used to prepare buffers(5 mmol·L-1Tris(hydroxymethylaminomethane)-HCl,50 mmol·L-1NaCl,pH=7.2).A solution of calf thymus DNA in the buffer gave a ratio of UV absorbance at 260 and 280 nm of ca.(1.8~1.9)∶1,indicating that the DNA was sufficiently free of protein[21].The DNA concentration per nucleotide was determined byabsorption spectroscopyusingthe molar absorption coefficient(6 600 mol-1·L·cm-1)at 260 nm[22].UV/Vis spectra were recorded on a Perkin-Elmer Lambda 850 spectrophotometer and emission spectra were recorded on a Perkin-Elmer LS 55 spectrofluorophotometer at room temperature.1.2 DNA binding and photoactivated cleavageAll DNA binding experiments were performed in Tris-HCl buffer at 25℃.The absorption titrations were performed using a fixed concentration(10 μmol·L-1),to which the DNA stock solution was graduallyadded up to the point of saturation.Ru-DNA solutions were incubated for 5 min before the absorption spectra were recorded.The intrinsic binding constant Kbof the Ru(Ⅱ)complex bound to DNA and the binding size s were calculated from the equation reported[23].Thermal denaturation studies were carried out with a Perkin-Elmer Lambda 850 spectrophotometer equipped with a Peltier temperature-controlling programmer(±0.1 ℃).The temperature of the solution was increased from 40 to 95℃ at a rate of 1℃·min-1 and the absorbance at 260 nm was continuously monitored for solutions of CT-DNA(100 μmol·L-1)in the absence and presence of the Ru(Ⅱ)complex(10 μmol·L-1).The data are presented as (A-A0)/(Af-A0)vs temperature,where A,A0,and Afare the observed,the initial,and the final absorbance at 260 nm,respectively. Viscosity measurements were carried out using an Ubbelodhe viscometer maintained a t a constant temperature at(25.0±0.1) ℃ in a thermostatic bath.DNA samples with approximately 200 base pairs in average length were prepared by Sonication[24].Flow time of each sample was measured three times,and an average flow time was calculated.Data were presented as(η/η0)1/3versus binding ratio[25].For the gel electrophoresis experiment,supercoiled pBR322 DNA(0.1μg)was treated with the Ru(Ⅱ)complex in buffer,and the solution was then irradiated at room temperature with a UV lamp (365 nm,10 W).The samples were analyzed by electrophoresis for 1.5 h at 80 V on a0.8%agarose gel in TBE(89 mmol·L-1Tris-borate acid,2 mmol·L-1EDTA,pH=8.3).The gel was stained with 1 μg·mL-1ethidium bromide andphotographed on an Alpha Innotech IS-5500 fluorescence chemiluminescence and visible imaging system.1.3 Continuous variation analysisBinding stoichiometry was obtained forthe complex with CT DNA using the method of continuous variation[26].The concentrations of the complex and DNA were varied,while the sum of the reactant concentrations was kept constant at 50 μmol·L-1(in terms of base pairs for the DNA).In the sample solutions,the molar fraction χ of complex was varied from 0 to 1.0 in 0.1 ratio steps.The fluorescence intensities of these mixtures were measured at 25℃u sing an excitation wavelength of 464 nm.The intensity in fluorescence was plotted versus the molar fraction χ of complex to generate a Job plot.Linear regression analysis of the data was performed in the software of Origin 7.0.1.4 Cell culture and cytotoxicity assay in vitroStandard 3-(4,5-dimethylthiazole)-2,5-diphenyltetraazoliumbromide(MTT)assay procedures[27]were performed using the methodology described in detail previously[28].Four different tumor cell lines were the subjects of this study:HeLa,MCF-7,HepG2 and A549.1.5 Cell cycle analysisCell Cycle analysis was performed as previously described[11].Briefly,HeLa cells were treated with Ru(Ⅱ)complex(10 μmol·L-1)for various time intervals.The cells were harvested and fixed in 2 mL of 70%aqueous ethanol(V/V).After an incubation period of at least 12 h at-20℃,cells were washed with icecold PBS(Phosphate Buffered Saline).The cells wereresuspended in 200 μL staining solution containing PI(10 μg·mL-1)and DNase-free RNase (100 μg·mL-1)and analyzed by a BD FACSCaliburTMcytometer(Becton Dickinson,Heidelberg,Germany).The number of cells analyzed for each sample was 10 000,and the experiments were repeated at least three times under identical conditions.Data were collected by BD CellQuestTMPro software and analyzed by ModFit LT 2.0 software.2 Results and discussion2.1 Electronic absorption titrationIn order to assess the DNA binding behavior of the complex,the absorption titration was carried out.As shown in Fig.1,with the increasing concentration of CT DNA,the electronic absorption spectra of the complex show no shift and little changes in the intensities for the charge transfer band at 464 nm.However,the band of the complex at 263 nm exhibits much more pronounced hypochromism of 29.88%.The large hypochromism observed may support an intercalative mode involving a strong stacking interaction between an aromatic chromophore and the base pairs ofDNA.The intrinsic binding constant,which illustrates the binding strength of the complex with CT DNA,can be obtained by monitoring the changes in absorbance at 263 nm for the complex.The value of K for the complex is(3.57±0.62)×105mol-1·L(s=2.12±0.35 bp(base pair)).The K value is much larger than those of other typical DNA intercalative Ru(Ⅱ)complexes (1.1 ×104~4.8 ×104mol-1·L)[29],and is comparable to that of complexes Δ-[Ru(phen)2(dppz)]2+(3.2×105mol-1·L)and Λ-[Ru(phen)2(dppz)]2+(1.7×105 mol-1·L, dppz=dipyrido-[3,2-a-2′,3′-c]phenazine)[30],but is not as strong as thoseof[Ru(phen)2(dppz)]2+and[Ru(bpy)2(dppz)]2+(>106mol-1·L), which are DNA intercalative Ru(Ⅱ)complexes[31].The result suggests thatthe complex intercalatively binds to DNA,involving a strong stacking interaction between the aromatic chromophore and the base pairs of the DNA.2.2 Luminescence studiesThe complex can luminesce in Tris buffer at room temperature with a maximum appearing at 589 nm.Fig.2 shows the luminescence spectra of the complex in the absence and presence of increasing concentration ofDNA in buffersolution.Upon addition of DNA,the emission intensities increase to 1.46 times of the original intensities. The enhancement of emission intensity is an indication of binding of the complex to the hydrophobic pocket of DNA,and the complex can be protected efficiently by the hydrophobic environment inside the DNA helix.2.3 Continuous variation analysisBinding stoichiometry with CT DNA was also investigated through the luminescence-based Job plot(Fig.3).One major inflection point for the complex is observed at χ=0.64.The data are consistent with a 2∶1 CDNA/Ccomplexbinding mode,suggestive of a specific Ru-CT DNA interaction.2.4 DNA thermal denaturation studiesWhen the temperature in the solution increases,the double-stranded DNA gradually dissociates to single strands[32],and generates a hyperchromic ef fect on the absorption spectra of DNA bases(λmax=260 nm).In order to identify this transition process,the melting temperature Tm,defined as the temperature where half of the total base pairs is unbonded,is usually introduced.According to previous literatures[31,33],the intercalation of natural(or synthesized organics)and metallointercalators generally results in a considerable increase in melting temperature (Tm).The melting curves of CT-DNA in the absence and presence of the complex are presented in Fig.4.Here,The thermal denaturation experiment carried out for DNA in the absence of the Ru(Ⅱ)complex reveals a Tmof(58.7±0.3)℃ under our experimental conditions.The observed melting temperature in the presence of the complex is(76.2±0.1)℃ at a concentration ra tio ofr=0.10(r=CRu/CDNA).The large increase in Tmof the Ru(Ⅱ) complex(the ΔTmis 17.5)is comparable to that observed for classical intercalatorsEB(13 ℃,r=0.10),Δ-[Ru(phen)2(dppz)]2+(16 ℃,r=1),and Λ-[Ru(phen)2(dppz)]2+(5 ℃,r=1)[30].The result also indicate s the intercalative binding mode of[Ru(phen)2(Hecip)]2+to DNA.2.5 Viscosity measurementsTo further verify the interactions between the complex and CT-DNA,viscosity measurements were carried out.Viscosity measurementsprovide the most critical test for intercalative interaction[34].The effect of the Ru(Ⅱ)complex together with EB(ethidiumbromide)and[Ru(bpy)3]2+on the viscosity of CT DNA are shown in Fig.5.EB binds with DNA through the intercalation mode,whereasthe[Ru(bpy)3]2+complex binds with DNA in the electrostaticmode,therefore the[Ru(bpy)3]2+complex exerts essentially no effect on DNA viscosity[35].On increasing the amount of Ru(Ⅱ)complex,the relative viscosity of DNA increases steadily,which is similar to the behaviour of EB.The increased degree of viscosity,which may depend on the complex′s affinity for DNA,follows the order of EB>Ru(Ⅱ)>[Ru(bpy)3]2+.A similar sharp increase in relative viscosity has been observed on the addition of[Ru(bpy)2(dppz)]2+,which was reported to be bound to DNA by intercalation.The results suggest that the Ru(Ⅱ)complex binds to DNA through a classical intercalation model.Such a result is consistent with the foregoing hypothesis.2.6 Photoactivated cleavage of pBR322 DNAFig.6a shows gel electrophoresis separation of pBR322 DNA after incubation with different concentrations of the Ru(Ⅱ)complex upon irradiation at 365 nm for 30 min.No obvious DNA cleavage is observed for control(without complex,or incubation of the plasmid with the Ru(Ⅱ)complex in darkness).As increasing the concentration of the complex,the amount of FormⅠ (supercoiled form)of pBR322 DNA diminishes gradually,whereas that of FormⅡ(circular form)increases.These resultsindicate that scission occurs on one strand(nicked).Fig.6b shows that the DNA cleavage of the plasmid by the complex is not inhibited in the presence of hydroxyl radical(·OH)scavengers such as mannitol[36]and dimethylsulfoxide(DMSO)[37],which indicates that hydroxyl radical is not likely the cleaving agent.In the presence ofsuperoxide dismutase (SOD),a facile superoxide anion radical(O2·-)quencher,the cleavage is obviously improved.The DNA cleavage of the plasmid is inhibited in the presence ofthe singletoxygen (1O2)scavenger histidine and NaN3[38-39],suggesting that1O2is likely the reactive species responsible for the cleavage reaction.Related results of enhancement by SOD and inhibition by singlet oxygen scavengers have been observed by other ruthenium intercalators[40-41].2.7 Cytotoxicity assay in vitroTo explore the antitumor potential of the Ru(Ⅱ)complex,HeLa,MCF-7,HepG-2 and A549 cells were treated with varying concentrations of Ru (Ⅱ)for 48 h,and cell viability was determined by the MTT assay.Cisplatin was used as a positive control.The resulting IC50values for the tested complex are shown in Table 1.Based on the IC50values,the complex has a significant inhibition to the growth and proliferation of all four tumor cells.Interestingly,the antiproliferative effciency of the complex on the MCF-7 cells is much lower than that on the other three cell lines.The result also indicates that the complex is more potent than cisplatin againstallthecancercelllines screened.The cytotoxicity of the complex is higher than the other typical DNA intercalativeRu(Ⅱ)complexes[Ru(phen)2(dppz)](PF6)2[12],[Ru(phen)2(1-PyβC)](PF6)2[11]and[Ru(phen)2(mitatp)]2+[42].Table 1 IC50values for complex against selected cell linesCompoundIC50/(μmol·L-1)HeLa MCF-7 HepG2 A549 Complex 12.5±1.2 33.3±1.8 15.6±0.8 9.4±0.4 Cisplatin 16.5±2.2 35.4±4.4 20.2±2.8 —2.8 Cell cycle arrestThe cell cycle phase distribution of HeLa cells in response to Ru(Ⅱ)treatment was studied by PI(propidium iodide)staining to further elucidate the mechanism of drug activity.Fig.7 shows the time dependenteffectofthe complex on cell-cycle distribution profiles with cells treated withvehicle(1%DMSO)as the control.Cells in G0/G1 phase express a 2 N(diploid)DNA content,whereas cells in G2/M have a 4N(tetraploid)DNA content.DNA content of the cells in S phase is variable (between 2 N and 4 N).Sub-G1 fraction is usually indicative of degraded DNA and identied as a hallmark ofapoptosis[43].Fig.8 shows the representative DNA distribution histograms of HeLa cell in the absence (A)and presence of the complex(B).Fig.8 shows that the treatment of HeLa cells with the complex,a large increase in the percentage of cell(%)at S phase and corresponding reduction at G0/G1 phase is observed,and a concomitant increase in the population of Sub-G1phase cells is also observed,which suggests the antiproliferative mechanism on HeLa is S phase arrest.3 ConclusionsThe DNA-binding of the complex with CT DNA indicates that the complex can intercalate between DNA base pairs.The complex can effectively cleave plasmid DNA upon irradiated at 365 nm for 30 min.The studies of mechanism on photocleavage demonstrate that superoxide anion radical(O2·-)and singlet oxygen (1O2)may play an important role.The data obtained from continuous variation analysis are consistent with a 2∶1 ccomplex/cDNAbinding mode for the complex.The cytotoxicity assay indicates that the complex can inhibitthe proliferation.The flow cytometric analysis shows that treatment of HeLa cell with the complex indicates the induction of S phase arrest.Acknowledgement:Thiswork wassupported bythe Guangdong Pharmaceutical University.References:[1]Greguric I,Aldrich-Wright J R,Collins J G.J.Am.Chem.Soc.,1997,119:3621-3622[2]Nair R B,Teng E S,Kirkland S L,et al.Inorg.Chem.,1998,37:139-141[3]Erkkila K E,Odom D T,Barton J K.Chem.Rev.,1999,99:2777-2796[4]Friedman A E,Chambron J C,Sauvage J P,etal.J.Am.Chem.Soc.,2004,126:8630-8631[5]Zeglis B M,Barton J K.J.Am.Chem.Soc.,2006,128:5654-5655[6]Liu Y,Hammitt R,Lutterman D A,et al.Inorg.Chem.,2007,46:6011-6021[7]Cosgrave L,Devocelle M,Forster R J,et mun.,2010,46:103-105[8]Tan C P,Wu S H,Lai S S,et al.Dalton Trans.,2011,40:8611-8621[9]Tan L F,Shen J L,Liu J,et al.Dalton Trans.,2012,41:4575-4587[10]Liu Y J,Li Z Z,Liang Z H,et al.DNA Cell Biol.,2011,30:839-848[11]Tan C P,Lai S S,Wu S H,et al.J.Med.Chem.,2010,53:7613-7624[12]Schatzschneider U,Niesel J,Ott I,et al.ChemMedChem,2008,3:1104-1109[13]Van Dijken A,Bastiaansen J J A M,Kiggen N M M,etal.J.Am.Chem.Soc.,2004,126:7718-7727[14]Grabowski Z R,Rotkiewicz K.Chem.Rev.,2003,103:3899-4032[15]Zhang Y,Wang L,Wada T,et al.Macromol.Chem.Phys.,1996,197:1877-1888[16]Wagner J,Pielichowski J,Hinsch A,et al.Synth.Met.,2004,146:159-165[17]Xin H,Sun M,Wang K Z,et al.Chem.Phys.Lett.,2004,388:55-57[18]Liu F R,Wang K Z,Bai G Y,et al.Inorg.Chem.,2004,43:1799-1806[19]Lü Y Y,Gao L H,Han M J,et al.Eur.J.Inorg.Chem.,2006,430:430-436[20]Xu L,Liu P X,Liao G L,et al.Aust.J.Chem.,2010,63:1-9[21]Marmur J A.J.Mol.Biol.,1961,3:208-218[22]Reichmann M E,Rice S A,Thomas C A,etal.J.Am.Chem.Soc.,1954,76:3047-3053[23]Wolf A,Shimer Jr G H,Meehan T.Biochemistry,1987,26:6392-6396[24]Chaires J B,Dattagupta N,Crothers D M.Biochemistry,1982,21:3933-3940[25]Cohen G,Eisenberg H.Biopolymers,1969,8:45-55[26]Job P.Ann.Chim.,1928,9:113-203[27]Mosmann T.J.Immunol.Methods.,1983,65:55-63[28]Tan L F,Song F C,Zou X Q,et al.DNA Cell Biol.,2011,30:277-285[29]Pyle A M,Rehmann J P,Meshoyrer R,etal.J.Am.Chem.Soc.,1989,111:3051-3058[30]Han M J,Duan Z M,Wang K Z,et al.J.Phys.Chem.C.,2007,111:16577-16585[31]Friedman A E,Chambron J C,Sauvage J P,etal.J.Am.Chem.Soc.,1990,112:4960-4962[32]Tselepi-Kalouli E,Katsaros N.J.Inorg.Biochem.,1989,37:271-282[33]Liu J G,Zhang Q L,Shi X F,et al.Inorg.Chem.,2001,40:5045-5050[34]Satyanarayana S,Dabrowiak J C,Chaires J B.Biochemistry,1992,31:9319-9324[35]Satyanaryana S,Daborusak J C,Chaires J B.Biochem.,1993,32:2573-2584[36]Cheng C C,Rokita S E,Burrows CJ.Angew.Chem.Int.Ed.Engl.,1993,32:277-278[37]Lesko S A,Lorentzen R J,Ts′o P O.Biochemistry,1980,19:3023-3028[38]Nilsson R,Merkel P B,Kearns D R.Photochem.Photobiol.,1972,16:117-124[39]Patra A K,Nethaji M,Chakravarty A R.J.Inorg.Biochem.,2007,101:233-244[40]Deshpande M S,Kumbhar A A,Kumbhar A S,et al.Bioconjugate Chem.,2009,20:447-459[41]Gao F,Chao H,Ji L N.Chem.Biodivers.,2008,5:1962-1979[42]Yu H J,Chen Y,Yu L,et al.Eur.J.Med.Chem.,2012,55:146-154[43]Karna P,Sharp S M,Yates C,et al.Mol.Cancer.,2009,8:93。
多吡啶钌(Ⅱ)配合物的合成及其与RNA相互作用的光谱学研究

多吡啶钌(Ⅱ)配合物的合成及其与RNA相互作用的光谱学研究徐宏;邓洪;胡红雨;刘建忠;巢晖;刘杰;计亮年【期刊名称】《高等学校化学学报》【年(卷),期】2003(024)001【摘要】RNA是生物的重要组成物质, 它参与蛋白质的生物合成, 在基因的调控中起着重要的作用, 并与一些疾病, 如艾滋病等的诊断与治疗相关联. 与DNA相比, RNA为单链, 其戊糖为核糖, 在碱基组成上没有胸腺嘧啶, 取而代之的是结构十分相近的尿嘧啶.【总页数】3页(P25-27)【作者】徐宏;邓洪;胡红雨;刘建忠;巢晖;刘杰;计亮年【作者单位】中山大学化学与化学工程学院,广州,510275;深圳大学师范学院,深圳,518060;中山大学化学与化学工程学院,广州,510275;中国科学院上海生物化学与细胞生物学研究所,上海,200031;中山大学化学与化学工程学院,广州,510275;中山大学化学与化学工程学院,广州,510275;中山大学化学与化学工程学院,广州,510275;中山大学化学与化学工程学院,广州,510275【正文语种】中文【中图分类】O614.821【相关文献】1.吲哚咪唑基钌(Ⅱ)联吡啶配合物与酵母RNA的相互作用 [J], 杨怀霞;刘艳菊;赵琳;王科志2.三齿多吡啶钴(Ⅲ)、钌(Ⅱ)配合物的合成、表征及与DNA的相互作用 [J], 杨浩3.季铵盐联吡啶-钌(Ⅱ)配合物的合成及其与小牛胸腺DNA的相互作用 [J], 陈嘉曦;郝洪庆;黄大坤;黄丹梅;林海玲;孙静4.双核钌(Ⅱ)多吡啶配合物与酵母RNA的相互作用研究 [J], 蒋尚达;王科志;刘芙蓉;张永安;高丽华5.新型不对称三齿多吡啶配体及其混配钌(II)配合物的合成、表征及与DNA相互作用研究 [J], 蒋才武因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第34 6期2018 6 月无机化 学学报CHINESE JOURNAL OF INORGANIC CHEMISTRYVol.34 No.6 1079-1085联吡啶衍生物芳基钌配合物的合成及其与D N A 、蛋白质相互作用葛超1王红艳1董益利1李季1徐芸1顾秋予1苏志1钱勇P e te r J. S a d le r2刘红科* ,#南京师范大学化学与材料科学学院,南京210023)(2Department of Chemistry ,University of Warwick ,Gibbet Hill Road ,Coventry CV4 7AL ,UK)摘要:以2 种配体 4,4"-dimeth 〇l-2$2"-bipyridi n e (L1)和 4"-methyl-(2,2"-bipyridine)-4-carbaldehyde oxime (L2)$ 分别与芳基钉一聚体[RuCl 2(!6-p-cymene )]2合成了 2 种新型单核配合物[Ru(!6-_p-cymene)(L1)Cl]Cl (1)和[Ru(!6-_p-cymene)(L2)Cl]Cl (2)。
应用元素分析、 ESI-MS 和1H NMR 对配合物的组成和结构进行表征,通过紫外光谱法和荧光光谱法研究了配合物的水解及其与CT-DNA 和血 清蛋白的结合性质,并且进行了细胞毒性研究。
结果表明,在水溶液中配合物1比2在动力学上更稳定("^J'Sh-y i h ^D 'h -1 (2));配合物均通过嵌入作用与双链DNA 结合,但2有较强的结合能力(#b :7.8xl03L .moH (1)、1.86xl04L .moH (2))。
配合物均能 与蛋白质发生相互作用,引起蛋白静态猝灭,但1作用较强(#A :1.04x105 L .mol-1 (1)、8.62x104L .mol-1 (2))。
配合物与蛋白的较强 结合 ,可能是其 性高的 。
关键词:芳基钌;联吡啶;DNA/BSA 结合作用中图分类号:O614.82+1 文献标识码:A 文章编号:1001-4861(2018)06-1079-07DOI : 10.11862/CJIC.2018.148Ru®-Arene Complexes Based on Bipyridyi Derivatives Ligand:Syntheses, Characterization and Interaction with DNA/BSAGE Chao 1 WANG Hong-Yan 1 DONG Yi-Li 1 LI Ji 1 XU Yun 1 GU Qiu-Yu 1SU Zhi 1 QIAN Yong!1 Peter J. Sadler 2 LIU Hong-Ke*'1(School of Chemistry and Materials Science, Nanjing Normal University, Nanjing 21002), China)(^Department of Chemistry, University of Warwick, Gibbet Hill Road, Coventry CV4 1AL, UK)Abstract : Two novel ruthenium-arene complexes of general formula [Ru(!6-p-cymene)(L)Cl]Cl, where L=4,4"-dimethyl-2,2"-bipyridine (1),4"-methyl-(2,2"-bipyridine)-4-carbaldehyde oxime (2),were synthesized. They werecharacterized by elemental analysis, mass spectrometry and 1H NMR spectroscopy. The hydrolytic property and binding activity of complexes with CT-DNA and serum proteins were detected by UV spectroscopy and fluorescence spectroscopy; the cytotoxic assay was also conducted. The results indicated that 1 is more dynamically stable in the aqueous solution than 2. Complexes 1 and 2 bind to DNA double helix structure via intercalation with the binding constants of 7.8x103 L(nol -1 (1) and 1.86x104 Lcnol -1 (2), respectively. These complexes also exhibit strong interaction with protein and cause quiescent quenching of proteins with the binding constants of 1.04x105 L(nol -1 (1) and 8.62x104 Lcnol -1 (2),respectively. Strong binding ability between complexes and protein may be the reason of low cytotoxicity.Keywords : ruthenium-arene; bipyridine; DNA/BSA binding收稿日期:2017-11-06。
收修改稿日期:2018-04-23。
国家自然科学基金重点国际合作项目(No.21420102002),自然科学基金(No.21601088,21771109,21778033),江苏省333工程人才专项基金, 江苏省六大人才高峰和江苏省自然科学资金(No.BE2013716,BK20171472)资助。
*通I联系人。
E -m a il :liuhongke@ ,07250@1080无机化学学报第34卷0引言1965年,美国科学家R osenberg首次发现顺铂能够抑制细胞生长并开展了抗肿瘤实验研究'由于顺铂的抗药性及毒副作用,人们将研究扩展到了过渡金属配合物抗癌方面,已报道了很多新型具有低毒、高效抗癌活性的金属(如铂、钯、铑、铱、锇、钌等元素2配合物,其中钌配合物抗癌成为研究热点/20。
S a d le r等发现双钌配合物对光敏感,具有细胞活成像的潜力[3]。
D y s o n等设计了一系的含配的钌配合物并研究抗癌活性的/40。
合配中,化合物由于化学到系生物,其化合物有,其钌配一有2种方,N;N配位 及C;N配。
的,有N,N- 配体的钌配物化中现,们的抗癌活性人[5!6]。
S heldrick已经报道了一系列具有N,N- 多配的铑和铱配合物[7],们现的细胞毒性并过配、双合的方D N A作用[80。
抗癌配合物的抗性有,含有素的钌配合物细胞发生,为C l- 抑制了配合物的0 100 m m o l.L_1),而细胞质中的C l D浓度低(<25 m m ol. L-1),,的物會巨细胞的D N A作用成D N A,从致癌细胞凋[9]。
D N A抗癌化合物细胞的主靶标之一,核酸和其他分子之间的相互作用生命科学中的本问题[100。
一些疾病的标志性 物D N A靶向药物的相互作用机制涉及的复制、转录、突物的相关等[110。
血清白蛋白一的生物分子,的蛋白质,其具有转运配体如脂肪酸、氨基酸、类固醇、金 属子和药物的能力[12_13]。
药物-白蛋白流中的合能力药物的分布、游度、代谢排泄性质有,同时化疗过程中的药物稳定性毒性[140。
由于牛血清白蛋白人清白蛋白含90J的相序列,性差别,其价格低,许多 文献用牛清白蛋白实验,所以本文选择用牛清白蛋白来研究配合物蛋白的相互作用[150。
研究致配合物抗癌性降低的因素具有特别重要的意义。
过配合物的水解、靶标物如D N A、蛋白等相互作用研究,以让我们全面 了抗癌机理,未来设计高效低毒配合物时避免利因素[100。
本文合成了2单钌联 P比陡衍生物配合物[R u(!6-p-cym ene)(L1)C l]C l (1)和 [R u(r]6-p -cymene) (L2)C l]C l (2) (L1=4,4" -d im e th y l-2,2" -b ip y rid in e,L2=4" -m e th yl-(2,2" -bipyridine)-4-carbald- ehyde oxim e),通过元素分析、E S I-M S和1H N M R进 行了表征。
用M T T法测试了配体及配合物对癌细胞及常细胞的毒性,并用紫外谱法谱法研究了配合物D N A、B S A的作用。
上述果上了配合物抗癌性的素,将为研 究新药物、新靶、新机制。
1实验部分1.1试剂与仪器实验所用溶剂均采用分析纯试剂,根据实验需 ,试用前按照《实验室化学品化手册(五版)&[16] 化。
所有的反应。
4,4"-d im e th y l-2,2"-b ip y rid in e(L1)购自晚 化学品。
根据文献方法[12-13]制备了配4"-m e th yl-(2,2"-bipyridine)-4-carbaldehyde oxim e(L2)。
除 特,所有测试室c iU N T R用B ruker A V A N C E400 spectrometer(德国)测定,C、H、N元素分析用元素分析仪(v a r io E L!,力- 司,)测定,ESI-MS 用LCQ F LE E T谱(科,美国)测定(Source Voltage 4.0 k V,Sheath Gas Flow Rate8a rb,C apillary Voltage70 V,C apillary Temp 275 K,流动相为醇,流速为 200p L*m in-1,进样方式为,品配成甲醇2,紫外分谱为美生的Cary 50 ,谱是用P erkinE lm er Ls-55谱测定。
1.2配合物的合成与结构1.2.1 [R u(!6-jp-cymene)(L1)Cl]Cl (1)的合成第6期葛超等!联吡啶衍生物芳基钌配合物的合成及其与D #A 、蛋白质相互作用1081芳基钌二聚体[R uC l 2(!6-_p-cymene)]2(122.4 mg #0.2 m m ol)、配体(L1)(73.6 m g ,0.4 m m ol )溶于 10 m L甲醇中,在氩气氛围下,338 K 回流12h 。
