BIA_10-2474_LCMS_23515_MedChemExpress
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
自噬研究方法

MDC:取12 mg粉末溶于720 nl DMSO使其浓度为50 mmol/L,分装后-20冰箱保存。
临用前用MEM稀释到终浓度50 umol/L;Rapamycin:用MEM培养基配成终浓度为1 umol/L,现用现配;400ng/ml喹乙醇:称取4 mg喹乙醇,DMSO预溶(体积<0.1%)后加入10 ml MEM培养液至完全溶解,现用现配,避光保存;3-MA:首先用PBS溶解粉末,临用前加热至完全溶解后再加入MEM培养基至终浓度10mmol/L; PI3K抑制剂(3-MA,Wortmannin)可干扰或阻断自噬体的形成用RAPAMYCIN诱导自噬我也查过一部分文献,有用无血清的,也有用,一般培养基的,浓度从25nM到100nM都有,用的是50nM的雷帕霉素,加入一般的培养基中,目的是排除无血清所诱导出来的自噬。
文献说饥饿初期激活的是大分子自噬,在4-6小时活力达到最大,24h后以CMA途径为主Earle's balanced salts solution (EBSS) for 48 hsigma的EBSS,货号E2888,有碳酸氢钠,有酚红的,酚红到不是很必须,只是一个PH指示作用,好看些无血清诱导自噬:EBSS 诱导6个小时就可以了。
EBSS一定可以诱导出来,只是需要说明的是时间点的设置,因为从饥饿诱导开始半个小时就可能开始自噬了,一直到24小时都持续,所以应该设置不同的时间点观察这个作用。
另外一个很大的问题是,饥饿诱导的一个很大的弊端是细胞死亡,这也是我面临的问题,就是在细胞收养的时候蛋白浓度太小了。
24小时就很少了,更不要说48小时和72小时了Hank's诱导,也就是通常所说的饥饿诱导,细胞培养到对数生长期后以Hank's替代常规完全培养基,3h后就可诱导出自噬。
我用Hank's诱导了3h后电镜观察有30%细胞都有自噬这种现象,但不如国外报道的高。
糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析

DOI:10.16658/ki.1672-4062.2023.14.085糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析倪胜南,陈少,陈一鸣泗阳康达医院检验科,江苏宿迁223700[摘要]目的探讨糖尿病患者诊断应用血清C肽联合糖化血红蛋白检测的价值。
方法将2022年1月—2023年1月泗阳康达医院收治的74例疑似糖尿病患者作为研究对象,检测入组患者糖化血红蛋白(glycosylated hemoglobin, HbA1c)以及血清C肽水平,以口服葡萄糖耐量试验(glucose tolerance test check, OGTT)为金标准,统计血清C肽联合糖化血红蛋白检测与单一项目检测的敏感性、特异度和诊断符合率。
结果74例疑似糖尿病患者根据葡萄糖耐量试验结果,确诊患者67例,确诊率为90.54%(67/74);与血清C肽、HbA1c单一检测相比,血清C肽+HbA1c联合检测敏感度更高,差异有统计学意义(P<0.05);血清C肽+HbA1c联合检测的特异度略高于血清C肽、HbA1c单一检测,但差异无统计学意义(P>0.05);联合检测诊断符合率明显高于血清C 肽、HbA1c单项检测,差异有统计学意义(P<0.05)。
结论血清C肽与糖化血红蛋白是临床诊断糖尿病的重要参考指标,二者表达水平的变化有助于检测患者胰岛素分泌功能,评估疾病严重程度,两者联合检验灵敏性与特异度良好,有助于早期明确诊断,临床参考价值较高。
[关键词] 糖尿病;血清C肽;糖化血红蛋白;诊断价值[中图分类号] R446.1 [文献标识码] A [文章编号] 1672-4062(2023)07(b)-0085-04Analysis of the Value of the Diagnostic Application of Combined Serum C-peptide and Glycosylated Hemoglobin Testing in Patients with Diabetes MellitusNI Shengnan, CHEN Shao, CHEN YimingDepartment of Laboratory Medicine, Siyang Kangda Hospital, Suqian, Jiangsu Province, 223700 China[Abstract] Objective To explore the value of applying serum C-peptide combined with glycated hemoglobin test for the diagnosis of diabetic patients. Methods A total of 74 patients with suspected diabetes admitted to Siyang Kangda Hospital from January 2022 to January 2023 were selected as the research objects. The levels of glycosylated hemoglo‐bin (HbA1c) and serum C-peptide were detected. Oral glucose tolerance test (OGTT) was used as the gold standard. The sensitivity, specificity and diagnostic coincidence rate of serum C-peptide combined with glycosylated hemoglo‐bin detection and single item detection were statistically analyzed. Results According to the results of glucose toler‐ance test, 67 patients were diagnosed in 74 patients with suspected diabetes, and the diagnosis rate was 90.54% (67/ 74). Compared with the single detection of serum C-peptide and HbA1c, the sensitivity of combined detection of se‐rum C peptide and HbA1c was higher, and the difference was statistically significant (P<0.05). The specificity of com‐bined detection of serum C-peptide and HbA1c was slightly higher than that of single detection of serum C-peptide and HbA1c, but the difference was no statistically significant (P>0.05). The diagnostic coincidence rate of combined detection was significantly higher than that of single detection of serum C-peptide and HbA1c, and the difference was statistically significant (P<0.05). Conclusion Serum C-peptide and glycosylated hemoglobin are important reference indexes for clinical diagnosis of diabetes mellitus, and changes in the expression levels of the two can help to detect the insulin secretion function of patients and assess the severity of the disease. The sensitivity and specificity of the [作者简介]倪胜南(1991-),女,本科,主管检验师,研究方向为免疫学、分子生物学检验。
反相离子对色谱测定食品中B族维生素的研究进展

反相离子对色谱测定食品中B族维生素的研究进展
张连龙;周华生;成恒嵩
【期刊名称】《现代食品科技》
【年(卷),期】2009(25)7
【摘要】本文综述了近30年来食品中B族维生素,特别是维生素B1、B2、B6及同系物的反相离子对高效液相色谱法.尽管食品种类繁多,只要采取相应的处理方法和选用适合的色谱条件,就能简便、灵敏、快速地测定这类营养物质.
【总页数】4页(P830-833)
【作者】张连龙;周华生;成恒嵩
【作者单位】无锡健特药业有限公司江苏无锡 214091;无锡健特药业有限公司江苏无锡 214091;无锡健特药业有限公司江苏无锡 214091
【正文语种】中文
【中图分类】TS207.3
【相关文献】
1.反相离子对色谱法同时测定饲料中多种水溶性维生素 [J], 戴军;程红专
2.反相离子对色谱法同时测定更年灵胶囊中维生素B1和维生素B6的含量 [J], 陈珊珊;陈永红;孙玉滨;温立义;赵国斌
3.一维反相高效液相色谱法测定保健食品中维生素D2和维生素D3 [J], 刘剑;易路遥;晏亮;吉伟佳;虞雪军
4.反相离子对色谱法测定维乐生片中维生素B_1和B_6的含量 [J], 杨颖;黄俊
5.反相离子对色谱法同时测定维生素咀嚼片中维生素B_1、B_2、B_6和烟酰胺的含量 [J], 柳春芳;朱新和
因版权原因,仅展示原文概要,查看原文内容请购买。
呋喃妥因代谢物(AHD)ELISA检测试剂盒说明书

呋喃妥因代谢物(AHD)ELISA检测试剂盒说明书一、概要用来检测呋喃妥因代谢物的最常用方法是LC-UV,LC-MS和LC-MS/MS。
酶联免疫方法结合了色谱技术,采用AHD衍生物的特异性抗体,具有很高的精确度和灵敏度,较低的操作技术要求和短暂的检测时间,检测样本量大等特点在检测中很好的表现出来。
二、试验原理本试剂盒采用间接竞争ELISA方法,在酶标板微孔条上预包被偶联抗原,样本中的残留物的AHD经衍生化后和微孔条上预包被的偶联抗原竞争抗呋喃妥因代谢物的衍生物抗体,加入酶标二抗后,用TMB底物显色,样本吸光值与其所含残留物AHD代谢物的含量成负相关,与标准曲线比较即可得出样本中呋喃妥因代谢物的残留量。
三、适用范围可定性、定量检测动物组织(鸡、猪、牛、鱼、虾等)、牛奶和蜂蜜中呋喃妥因代谢物的残留量。
四、交叉反应率呋喃妥因代谢物…………………………………………100%呋喃唑酮代谢物…………………………………小于0.1%呋喃它酮代谢物…………………………………小于0.1%硝基糠腙(呋喃西林)代谢物…………………小于0.1%呋喃唑酮………………………………………………小于1%呋喃它酮………………………………………………小于1%呋喃妥因………………………………………………13%呋喃西林…………………………………………小于1%五、使用单位需自备的设备及试剂设备:┅┅微孔板酶标仪450nm/630nm┅┅旋转蒸发仪/氮气吹干装置┅┅均质器┅┅振荡器┅┅涡旋仪┅┅离心机┅┅天平:感量0.01g┅┅刻度移液管:10ml┅┅洗耳球┅┅容量瓶:100ml、1L┅┅玻璃试管:10ml┅┅聚苯乙烯离心管:50ml┅┅微量移液器:单道 20ml~200ml、100ml~1000ml 多道 250ml试剂:┅┅乙酸乙酯(分析纯)┅┅正己烷(或正庚烷)(分析纯)┅┅三水合磷酸氢二钾(分析纯)┅┅甲醇(分析纯)┅┅二水合亚硝基铁氰化钠(Na2Fe(CN)5·NO·2H2O)(分析纯)(供奶样用)┅┅七水合硫酸锌(ZnSO4·7 H2O) (分析纯)(供奶样用)┅┅浓盐酸┅┅氢氧化钠(分析纯)┅┅去离子水六、提供的材料与试剂1、96孔酶标板×1块(包被有偶联抗原)2、标准液×6瓶:(1ml/瓶)0ppb,0.1ppb,0.3ppb,0.9ppb,2.7ppb,8.1ppb3、高浓度标准品:(1ml/瓶) 100ppb4、酶标二抗12ml …………………… 红色帽5、抗体工作液7ml …………………… 绿色帽6、底物液 A 液7ml …………………… 白色帽7、底物液 B 液7ml …………………… 红色帽8、终止液7ml …………………… 黄色帽9、20×浓缩洗涤液40ml……………………… 透明帽10、2×浓缩复溶液 50ml……………………… 蓝色帽11、2-硝基苯甲醛15.1mg…………………… 黑色帽七、溶液的配制配液1: 衍生化试剂向装有2-硝基苯甲醛的试剂瓶中加甲醇溶解定容至10ml(浓度为10mM)。
LCMS检测西他沙星原料中基因毒性杂质的含量

LC-MS检测西他沙星原料中基因毒性杂质的含量石莹1宋雪洁3李浩冬2路显锋2*1药物研究院分析所,扬子江药业集团,泰州2253212药物制剂新技术国家重点实验室,扬子江药业集团,泰州2253213质量管理部,扬子江药业集团,泰州225321摘要建立了LC-MS 法测定西他沙星中基因毒性杂质对甲苯磺酸甲酯和对甲苯磺酸乙酯含量的方法。
方法:采用Agilent Poroshell 120 EC-C18色谱柱;流动相为纯水(0.1%甲酸):甲醇(V/V)=60:40;稀释剂为乙腈(0.1%甲酸):纯水(V/V)=50:10;柱温为40℃;进样体积为5µl;流速为0.4ml/min;采用正离子模式进行扫描。
对甲苯磺酸甲酯测定浓度在0.76ng/ml~15.27ng/ml范围内,线性关系良好;对甲苯磺酸乙酯测定浓度在0.75ng/ml~15.01ng/ml范围内,线性关系良好。
对甲苯磺酸甲酯的定量限为0.0038ng;对甲苯磺酸乙酯的定量限为0.0038ng。
杂质回收率在限度浓度80%、100%和160%三个浓度水平均在90~110%之间,该方法准确度良好。
该方法适用于西他沙星原料中对甲苯磺酸甲酯和对甲苯磺酸乙酯的检测。
西他沙星(sitafloxacin)是日本第一制药有限公司继左氧氟沙星后开发出的一种强力广谱新氟喹诺酮类抗菌剂,该药对革兰氏阳性球菌,革兰氏阴性菌以及厌氧菌的抗菌活性是左氧氟沙星的4~32倍,同时对肺炎球菌DNA 促旋酶和拓扑同功酶有双重抑制作用。
临床表现有极广的抗菌谱,特别是对呼吸道的病菌有极强的抗菌活性。
因西他沙星的一个起始物料为对甲苯磺酸盐,在后续反应中对甲苯磺酸若有残留,可能会与溶剂甲醇、乙醇反应生成具有基因毒性的杂质—对甲苯磺酸甲酯和对甲苯磺酸乙酯,故采用LC-MS法对产品中的对甲苯磺酸甲酯/乙酯进行控制。
1、实验部分1.1仪器与试药Agilent 1200液相色谱仪(美国安捷伦公司);Agilent 6460三重串联四极杆质谱仪(美国安捷伦公司);XP205型电子天平(瑞士梅特勒托利多公司)。
211091464_超高效液相色谱串联质谱法测定乳制品中透明质酸

杜国辉,范维江,陈玉娟,等. 超高效液相色谱串联质谱法测定乳制品中透明质酸[J]. 食品工业科技,2023,44(8):334−340. doi:10.13386/j.issn1002-0306.2022070057DU Guohui, FAN Weijiang, CHEN Yujuan, et al. Determination of Hyaluronic Acid in Dairy Products by Ultra-high Performance Liquid Chromatography-Tandem Mass Spectrometry[J]. Science and Technology of Food Industry, 2023, 44(8): 334−340. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2022070057· 分析检测 ·超高效液相色谱串联质谱法测定乳制品中透明质酸杜国辉1,范维江1,陈玉娟2, *,陈雯雯2,乔莉苹2,王萃玲3(1.山东商业职业技术学院食品工业产业学院,山东济南 250103;2.华熙生物科技股份有限公司,山东济南 250101;3.齐鲁医药学院,山东济南 250103)摘 要:本文建立一种超高效液相色谱-串联质谱(UPLC-MS/MS )测定乳制品中透明质酸含量的分析方法。
样品经透明质酸酶降解,乙腈稀释,PRIME HLB 固相萃取柱净化,Waters BEH Amide (2.1 mm×100 mm ,1.7 μm )酰胺柱分离,以0.2%氨水-乙腈(含0.2%氨水)为流动相梯度洗脱。
采用电喷雾离子源负离子模式扫描,多反应监测模式进行检测,外标法定量。
结果表明:乳制品中透明质酸在0.5~200 mg/kg 添加水平下回收率为91.4%~106.2%,RSD 为2.3%~6.7%。
盐酸非索非那定杂质的LCMS检测及结构鉴定

质,主要杂质为 α,α- 二甲基 -4-[4-[4-( 羟基二苯甲基 )-1- 哌啶基 ]-1- 氧代丁基 ] 苯乙酸,两年留样后含量增加了 1.53%;
其余两个杂质为 α,α- 二甲基 -4-[1- 羟基 -4-[4-( 羟基二苯甲基 )-1- 哌啶基 ] 丁基 ] 苯乙酸甲酯和 α, α- 二甲基 -4-[1- 羟
质峰,保留时间 (tR) 分别为 11.1、12.9 和 13.6 min, 含 量 分 别 为 0.32 %、0.25 % 和 0.06 % ( HPLC 归 一化法 )。该批样品长期留样 ( 20 ℃,相对湿度 65%,24 个月 ) 后仍检测出 3 个杂质峰,其中 tR 为 11.1 和 12.9 min 的两个色谱峰的含量为 1.85% 和 0.22%,是主要的杂质峰;13.6 min 的峰含量 为 0.07%。LC-MS 法也检出 3 个杂质峰 (tR 分别为 2.74、4.90 和 9.47 min)。两种色谱的主杂质峰通过 用 DAD 检测各对应峰的紫外吸收,在两个系统中 主峰及各杂质的对应关系见表 1。
516.3
517.3 498.3 518.4
661.5 720.2
0.15
540.2
0.10
0.05
522.2 558.2
484.2 0.00
m/z
图1
CH3
HO
N
CO2R
HO
N
OH
CH3
O
1: R=H, Mw=501 3: R=CH3, Mw=515 4: R=C4H9, Mw=557
2: Mw=499
CH3 COOCH3
+ H+
CH3
2
Mw=499
[M+H]+ Mw =500
基于毛细管电泳法测定天山堇菜中5种活性成分的含量

适用于了哥王药材的质量控制,测得伞形花内酯质量 浓度为 0.6~24.0 mg/L。Miean 等 [7] 对 62 种食用热带
管柱,永年锐沣色谱器件有限公司,使用 32 Karat 软 件处理数据;KQ-250B 型超声波清洗器,昆山市超
植物进行检测,发现洋葱中含有的黄酮类物质最多, 声仪器有限公司;MilliQ-Gradient 超纯水系统,美国
第3期
阿曼·阿尔斯兰:基于毛细管电泳法测定天山堇菜中5种活性成分的含量
·113·
良好。
2.2.2 线性考察
本方法对 5 种活性成分的线性关系如表 2 所示,
5 种成分均线性关系良好。
2.2.3 重现性考察
从表 3 可以看出,日内重现性实验中,各色谱峰
迁移时间的 RSD 不超过 1.18%,峰面积的 RSD 不超
关键词:天山堇菜;毛细管电泳;活性成分 中图分类号:R284.1 文献标识码:A
Determination of Five Active Components in Viola Tianshanica by Capillary Electro Phoresis Method
Aman·Arslan (xinjiang center for drug Evaluation, Xinjiang Urumqi 830054)
·112·
生物化工
2021 年
已逐步应用于中草药中活性物质的定性、定量分析 中。刘丽霞等 [6] 使用高效液相色谱法对中药了哥王
氢氧化钠、硼砂等,分析纯,购自 Sigma-Aldrich(美国)。 AP/ACETM MDQ 毛 细 管 电 泳 系 统,Beckman,
中伞形花内酯的含量进行了测定,结果表明,该方法 配备有二极管阵列检测器(DAD);熔融石英毛细
吩噻嗪类衍生物

1. Introduction Phenothiazines are important psychotropic compounds, but they also have further biological activities.1–3 For example, phenothiazines have recently been considered as potential drugs in the management of CreutzfeldtJacob disease.4 Metabolism of phenothiazine-based drugs often results in the formation of 7-hydroxylated derivatives or 5-sulfoxides.5–7 Because oxidation of asymmetrically substituted phenothiazines at the S(5) position introduces a new stereogenic center, these 5-sulfoxides are chiral. Although chiral 5-sulfoxide metabolites of the phenothiazine drug thioridazine in human plasma were separated by HPLC,8 to date optically active phenothiazine 5-oxides have not been obtained on preparative scale. Hence, stereoselective methods for the synthesis of optically active phenothiazine-5-oxides would extend the possibilities for investigation of the S -oxide metabolites of phenothiazine-based drugs.
Febuxostat_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-04-2017Print Date:Jul.-04-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :FebuxostatCatalog No. :HY-14268CAS No. :144060-53-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:TEI 6720; TMX 67Formula:C16H16N2O3SMolecular Weight:316.37CAS No. :144060-53-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
大鼠不对称二甲基精氨酸(ADMA)ELISA试剂盒说明书
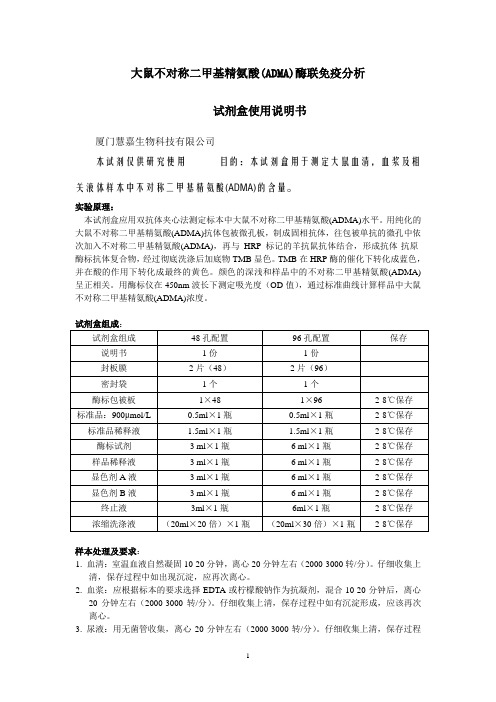
大鼠不对称二甲基精氨酸(ADMA)酶联免疫分析试剂盒使用说明书厦门慧嘉生物科技有限公司本试剂仅供研究使用目的:本试剂盒用于测定大鼠血清,血浆及相关液体样本中不对称二甲基精氨酸(ADMA)的含量。
实验原理:本试剂盒应用双抗体夹心法测定标本中大鼠不对称二甲基精氨酸(ADMA)水平。
用纯化的大鼠不对称二甲基精氨酸(ADMA)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入不对称二甲基精氨酸(ADMA),再与HRP标记的羊抗鼠抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的不对称二甲基精氨酸(ADMA)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中大鼠不对称二甲基精氨酸(ADMA)浓度。
试剂盒组成:样本处理及要求:1. 血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
2. 血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
3. 尿液:用无菌管收集,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
4. 细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4)稀释细胞悬液,细胞浓度达到100万/ml左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
保存过程中如有沉淀形成,应再次离心。
5. 组织标本:切割标本后,称取重量。
葡萄果皮提取物对砷致小鼠小肠毒性的缓解作用

赵丹瑜,仪慧兰. 葡萄果皮提取物对砷致小鼠小肠毒性的缓解作用[J]. 食品工业科技,2024,45(4):305−312. doi:10.13386/j.issn1002-0306.2023040011ZHAO Danyu, YI Huilan. Mitigative Effect of Grape Skin Extract on Arsenic-induced Small Intestinal Toxicity in a Mouse Model[J].Science and Technology of Food Industry, 2024, 45(4): 305−312. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2023040011· 营养与保健 ·葡萄果皮提取物对砷致小鼠小肠毒性的缓解作用赵丹瑜1,2,仪慧兰1,*(1.山西大学生命科学学院,山西太原 030006;2.山西省人民医院消化科,山西太原 030012)摘 要:目的:探究砷摄入对小肠的毒性及葡萄果皮提取物(grape skin extract ,GSE )的干预效应。
方法:模拟人类饮水砷暴露,小鼠饮用浓度10 mg/L 的砷溶液56 d ,建立小鼠砷中毒模型,并用不同浓度GSE 隔天灌胃干预(150 mg/kg bw 和300 mg/kg bw );取小鼠小肠组织,显微镜观察组织形态结构;试剂盒法检测还原型谷胱甘肽(glutathione ,GSH )、丙二醛(malondialdehyde ,MDA )和H 2O 2含量,及总超氧化物歧化酶(total superoxide dismutase ,T-SOD )活性;qRT-PCR 检测紧密连接基因和炎症通路IL-6/JAK2/STAT-3基因表达水平。
结果:砷染毒后,小鼠小肠绒毛变短、排列紊乱,黏膜固有层及黏膜下层大量炎细胞浸润;小肠组织的GSH 含量和T-SOD 活性分别降低17.1%和25.2%,MDA 和H 2O 2含量分别增加68.8%和54.3%(P <0.05);细胞紧密连接基因ZO-1、ZO-2、occludin 、claudin-1和claudin-7显著下调表达(P <0.05);炎症通路IL-6/JAK2/STAT-3基因转录水平显著增高(P <0.05)。
β-淀粉酶(β-AL)活性检测试剂盒说明书(DNS 显色法)__ 微量法UPLC-MS-4172
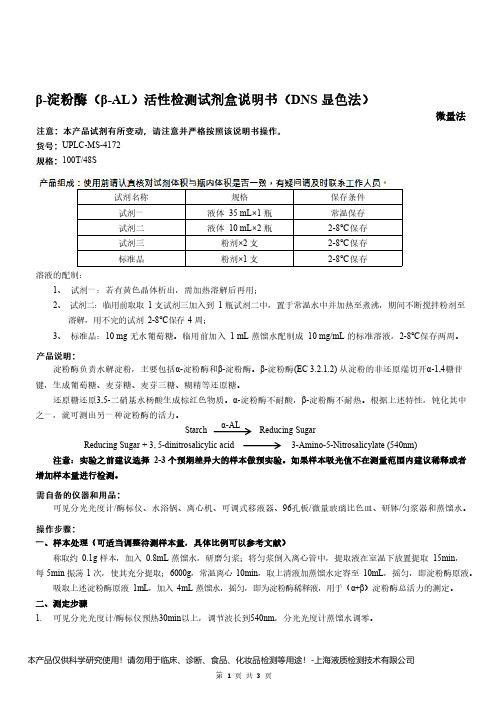
β-淀粉酶(β-AL)活性检测试剂盒说明书(DNS显色法)微量法UPLC-MS-4172100T/48S试剂名称规格保存条件试剂一液体35mL×1瓶常温保存试剂二液体10mL×2瓶2-8℃保存试剂三粉剂×2支2-8℃保存标准品粉剂×1支2-8℃保存溶液的配制:1、试剂一:若有黄色晶体析出,需加热溶解后再用;2、试剂二:临用前取取1支试剂三加入到1瓶试剂二中,置于常温水中并加热至煮沸,期间不断搅拌粉剂至溶解,用不完的试剂2-8℃保存4周;3、标准品:10mg无水葡萄糖。
临用前加入1mL蒸馏水配制成10mg/mL的标准溶液,2-8℃保存两周。
淀粉酶负责水解淀粉,主要包括α-淀粉酶和β-淀粉酶。
β-淀粉酶(EC3.2.1.2)从淀粉的非还原端切开α-1,4糖苷键,生成葡萄糖、麦芽糖、麦芽三糖、糊精等还原糖。
还原糖还原3,5-二硝基水杨酸生成棕红色物质。
α-淀粉酶不耐酸,β-淀粉酶不耐热。
根据上述特性,钝化其中之一,就可测出另一种淀粉酶的活力。
Starchα-AL Reducing SugarReducing Sugar+3,5-dinitrosalicylic acid3-Amino-5-Nitrosalicylate(540nm)注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
可见分光光度计/酶标仪、水浴锅、离心机、可调式移液器、96孔板/微量玻璃比色皿、研钵/匀浆器和蒸馏水。
一、样本处理(可适当调整待测样本量,具体比例可以参考文献)称取约0.1g样本,加入0.8mL蒸馏水,研磨匀浆;将匀浆倒入离心管中,提取液在室温下放置提取15min,每5min振荡1次,使其充分提取;6000g,常温离心10min,取上清液加蒸馏水定容至10mL,摇匀,即淀粉酶原液。
吸取上述淀粉酶原液1mL,加入4mL蒸馏水,摇匀,即为淀粉酶稀释液,用于(α+β)淀粉酶总活力的测定。
