Deoxypseudouridine_DataSheet_MedChemExpress
Idoxuridine_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IdoxuridineCatalog No. :HY-B0307CAS No. :54-42-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:5⁻IUdR; IDU; IdUrd; 5⁻Iodo⁻2′⁻deoxyuridineFormula:C9H11IN2O5Molecular Weight:354.10CAS No. :54-42-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Trigonox B(滴苷但羊水)产品数据表单说明书

Product Data SheetTrigonox BDi-tert-butyl peroxideTrigonox® B is a pure peroxide in liquid form.CAS number110-05-4EINECS/ELINCS No. 203-733-6TSCA statuslisted on inventory Molecular weight 146.2Active oxygen contentperoxide10.94%SpecificationsAppearance Clear liquidAssay≥ 99.0 %ApplicationsTrigonox® B (Di-tert-butyl peroxide) can be used for the market segments: polymer production, polymer crosslinking and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® B in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 164°C (327°F)1 hr at 141°C (286°F)10 hr at 121°C (250°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa153.46 kJ/moleA 4.20E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition may occur with a substance in the packaging as used for transport is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°C (176°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides, a loss of quality will occur over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.40°C (104°F) andTs Min.-30°C (-22°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox® Bwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportIn North America Trigonox® B is packed in non-returnable, five gallon polyethylene containers of 30 lb net weight and steel drums of 100 or 340 lb net weight. In other regions the standard packaging is a 30-liter HDPE can (Nourytainer®) for 20 kg peroxide. Delivery in a 200 l steel drum for 150 kg peroxide is also possible in a number of countries. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® B is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® B in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for detailed information on the safe storage, use and handling of Trigonox® B. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsAcetone, Methane, tert-ButanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox B。
黄嘌呤氧化酶(XOD)活性检测试剂盒说明书

黄嘌呤氧化酶(XOD微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC1095规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液液体110 mL×1瓶4℃保存试剂一液体20mL×1瓶4℃保存试剂二粉剂×1瓶4℃保存溶液的配制:1、试剂二:粉剂置于试剂瓶内EP管中;2、工作液的配制:用时在试剂二中加入9.375 mL试剂一充分溶解,用不完的试剂4℃可保存2周;按需用蒸馏水稀释10倍后备用,现用现配。
产品说明:XOD(EC 1.17.3.2)催化黄嘌呤氧化生成尿酸和超氧阴离子,是活性氧主要来源之一;同时也是核苷酸代谢的关键酶之一。
XOD主要分布于哺乳动物的心、肺、肝脏等组织中,当肝功能受损时,XOD大量释放到血清中,对肝损害的诊断具有特异性的意义。
XOD催化黄嘌呤产生尿酸,尿酸在290nm下有特征吸收峰。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外分光光度计/酶标仪、台式离心机、可调式移液器、微量石英比色皿/96孔UV板、匀浆器/研钵、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1.细菌、细胞或组织样本的制备:细菌或细胞:先收集细菌或细胞到离心管内,离心后弃上清;按照细菌或细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细菌或细胞加入1mL提取液),超声波破碎细菌或细胞(冰浴,功率20%或200W,超声3s,间隔10s,重复30 次);8000g 4℃离心10min,取上清,置冰上待测。
组织:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液),进行冰浴匀浆。
分子生物学词汇(中英文对照表 )

第一页A band|A带A chromosome|A染色体[二倍体染色体组中的正常染色体(不同于B染色体)] A site|[核糖体]A部位ABA|脱落酸abasic site|脱碱基位点,无碱基位点abaxial|远轴的abequose|阿比可糖,beta脱氧岩藻糖aberrant splicing|异常剪接aberration|象差;畸变;失常abiogenesis|自然发生论,无生源论ablastin|抑殖素(抑制微生物细胞分裂或生殖的一种抗体)abnormal distrbution|非正态分布abnormality|异常,失常;畸形,畸变ABO blood group system|ABO血型系统aboriginal mouse|原生鼠abortin|流产素abortion|流产,败育abortive egg|败育卵abortive infection|流产(性)感染abortive transduction|流产(性)转导ABP|肌动蛋白结合蛋白abrin|相思豆毒蛋白abscisic acid|脱落酸abscission|脱落absolute|绝对的absolute configuration|绝对构型absolute counting|绝对测量absolute deviation|绝对偏差absolute error|绝对误差absorbance|吸收,吸光度absorbed dose|吸收剂量absorbent|吸收剂absorptiometer|吸光计absorptiometry|吸光测定法absorption|吸收absorption band|吸收谱带absorption cell|吸收池absorption coefficient|吸收系数absorption spectroscopy|吸收光谱法absorption spectrum|吸收光谱;吸收谱absorptive endocytosis|吸收(型)胞吞(作用) absorptive pinocytosis|吸收(型)胞饮(作用) absorptivity|吸光系数;吸收性abundance|丰度abundant|丰富的,高丰度的abundant mRNAs|高丰度mRNAabzyme|抗体酶acaricidin|杀螨剂accedent variation|偶然变异accelerated flow method|加速流动法accepting arm|[tRNA的]接纳臂acceptor|接纳体,(接)受体acceptor site|接纳位点,接受位点acceptor splicing site|剪接受体acceptor stem|[tRNA的]接纳茎accessible|可及的accessible promoter|可及启动子accessible surface|可及表面accessory|零件,附件;辅助的accessory cell|佐细胞accessory chromosome|副染色体accessory factor|辅助因子accessory nucleus|副核accessory pigment|辅助色素accessory protein|辅助蛋白(质)accommodation|顺应accumulation|积累,累积accuracy|准确度acenaphthene|二氢苊acene|并苯acentric|无着丝粒的acentric fragment|无着丝粒断片acentric ring|无着丝粒环acetal|缩醛acetaldehyde|乙醛acetalresin|缩醛树脂acetamidase|乙酰胺酶acetamide|乙酰胺acetate|乙酸盐acetic acid|乙酸,醋酸acetic acid bacteria|乙酸菌,醋酸菌acetic anhydride|乙酸酐acetification|乙酸化作用,醋化作用acetin|乙酸甘油酯,三乙酰甘油酯acetoacetic acid|乙酰乙酸Acetobacter|醋杆菌属acetogen|产乙酸菌acetogenic bacteria|产乙酸菌acetome body|酮体acetome powder|丙酮制粉[在-30度以下加丙酮制成的蛋白质匀浆物] acetomitrile|乙腈acetone|丙酮acetyl|乙酰基acetyl coenzyme A|乙酰辅酶Aacetylcholine|乙酰胆碱acetylcholine agonist|乙酰胆碱拮抗剂acetylcholine receptor|乙酰胆碱受体acetylcholinesterase|乙酰胆碱酯酶acetylene|乙炔acetylene reduction test|乙炔还原试验[检查生物体的固氮能力] acetylglucosaminidase|乙酰葡糖胺糖苷酶acetylglutamate synthetase|乙酰谷氨酸合成酶acetylsalicylate|乙酰水杨酸;乙酰水杨酸盐、酯、根acetylsalicylic acid|乙酰水杨酸acetylspiramycin|乙酰螺旋霉素AchE|乙酰胆碱酯酶achiral|非手性的acholeplasma|无胆甾原体AchR|乙酰胆碱受体achromatic|消色的;消色差的achromatic color|无色achromatic lens|消色差透镜achromatin|非染色质acid catalysis|酸催化acid fibroblast growth factor|酸性成纤维细胞生长因子acid fuchsin|酸性品红acid glycoprotein|酸性糖蛋白acid hydrolyzed casein|酸水解酪蛋白acid medium|酸性培养基acid mucopolysaccharide|酸性粘多糖acid phosphatase|酸性磷酸酶acid protease|酸性蛋白酶acid solvent|酸性溶剂acidic|酸性的acidic amino acid|酸性氨基酸acidic protein|酸性蛋白质[有时特指非组蛋白]acidic transactivator|酸性反式激活蛋白acidic transcription activator|酸性转录激活蛋白 acidification|酸化(作用)acidifying|酸化(作用)acidolysis|酸解acidophilia|嗜酸性acidophilic bacteria|嗜酸菌acidophilous milk|酸奶aclacinomycin|阿克拉霉素acoelomata|无体腔动物acomitic acid|乌头酸aconitase|顺乌头酸酶aconitate|乌头酸;乌头酸盐、酯、根aconitine|乌头碱aconitum alkaloid|乌头属生物碱ACP|酰基载体蛋白acquired character|获得性状acquired immunity|获得性免疫acridine|吖啶acridine alkaloid|吖啶(类)生物碱acridine dye|吖啶燃料acridine orange|吖啶橙acridine yellow|吖啶黄acriflavine|吖啶黄素acroblast|原顶体acrocentric chromosome|近端着丝染色体acrolein|丙烯醛acrolein polymer|丙烯醛类聚合物acrolein resin|丙烯醛树脂acropetal translocation|向顶运输acrosin|顶体蛋白acrosomal protease|顶体蛋白酶acrosomal reaction|顶体反应acrosome|顶体acrosome reaction|顶体反应acrosomic granule|原顶体acrosyndesis|端部联会acrylamide|丙烯酰胺acrylate|丙烯酸酯、盐acrylic acid|丙烯酸acrylic polymer|丙烯酸(酯)类聚合物acrylic resin|丙烯酸(酯)类树脂acrylketone|丙烯酮acrylonitrile|丙烯腈actidione|放线(菌)酮[即环己酰亚胺]actin|肌动蛋白actin filament|肌动蛋白丝actinin|辅肌动蛋白[分为alfa、beta两种,beta蛋白即加帽蛋白] actinmicrofilament|肌动蛋白微丝actinometer|化学光度计actinomorphy|辐射对称[用于描述植物的花]actinomycetes|放线菌actinomycin D|放线菌素Dactinospectacin|放线壮观素,壮观霉素,奇霉素action|作用action current|动作电流action potential|动作电位action spectrum|动作光谱activated sludge|活性污泥activated support|活化支持体activating group|活化基团activating transcription factor|转录激活因子activation|激活;活化activation analysis|活化分析activation energy|活化能activator|激活物,激活剂,激活蛋白activator protein|激活蛋白active absorption|主动吸收active biomass|活生物质active carbon|活性碳active center|活性中心active chromatin|活性染色质active dry yeast|活性干酵母active dydrogen compounds|活性氢化合物active ester of amino acid|氨基酸的活化酯active hydrogen|活性氢active immunity|主动免疫active oxygen|活性氧active site|活性部位,活性中心active transport|主动转运active uptake|主动吸收activin|活化素[由垂体合成并由睾丸和卵巢分泌的性激素]activity|活性,活度,(放射性)活度actomyosin|肌动球蛋白actophorin|载肌动蛋白[一种肌动蛋白结合蛋白]acute|急性的acute infection|急性感染acute phase|急性期acute phase protein|急性期蛋白,急相蛋白acute phase reaction|急性期反应,急相反应[炎症反应急性期机体的防御反应] acute phase reactive protein|急性期反应蛋白,急相反应蛋白acute phase response|急性期反应,急相反应acute toxicity|急性毒性ACV|无环鸟苷acyclic nucleotide|无环核苷酸acycloguanosine|无环鸟苷,9-(2-羟乙氧甲基)鸟嘌呤acyclovir|无环鸟苷acyl|酰基acyl carrier protein|酰基载体蛋白acyl cation|酰(基)正离子acyl chloride|酰氯acyl CoA|脂酰辅酶Aacyl coenzyem A|脂酰辅酶Aacyl fluoride|酰氟acyl halide|酰卤acylamino acid|酰基氨基酸acylase|酰基转移酶acylating agent|酰化剂acylation|酰化acylazide|酰叠氮acylbromide|酰溴acyloin|偶姻acyltransferase|酰基转移酶adamantanamine|金刚烷胺[曾用作抗病毒剂]adamantane|金刚烷adaptability|适应性adaptation|适应adapter|衔接头;衔接子adapter protein|衔接蛋白质adaptin|衔接蛋白[衔接网格蛋白与其他蛋白的胞质区]adaptive behavior|适应性行为adaptive enzyme|适应酶adaptive molecule|衔接分子adaptive response|适应反应[大肠杆菌中的DNA修复系统]adaptor|衔接头;衔接子adaxial|近轴的addition|加成addition compound|加成化合物addition haploid|附加单倍体addition line|附加系additive|添加物,添加剂additive effect|加性效应additive genetic variance|加性遗传方差additive recombination|插入重组,加插重组[因DNA插入而引起的基因重组] addressin|地址素[选择蛋白(selectin)的寡糖配体,与淋巴细胞归巢有关]adducin|内收蛋白[一种细胞膜骨架蛋白,可与钙调蛋白结合]adduct|加合物,加成化合物adduct ion|加合离子adenine|腺嘌呤adenine arabinoside|啊糖腺苷adenine phosphoribosyltransferase|腺嘌呤磷酸核糖转移酶adenoma|腺瘤adenosine|腺嘌呤核苷,腺苷adenosine deaminase|腺苷脱氨酶adenosine diphoshate|腺苷二磷酸adenosine monophosphate|腺苷(一磷)酸adenosine phosphosulfate|腺苷酰硫酸adenosine triphosphatase|腺苷三磷酸酶adenosine triphosphate|腺苷三磷酸adenovirus|腺病毒adenylate|腺苷酸;腺苷酸盐、酯、根adenylate cyclase|腺苷酸环化酶adenylate energy charge|腺苷酸能荷adenylate kinase|腺苷酸激酶adenylic acid|腺苷酸adenylyl cyclase|腺苷酸环化酶adenylylation|腺苷酰化adherence|粘着,粘附,粘连;贴壁adherent cell|贴壁赴 徽匙牛ㄐ裕┫赴 掣剑ㄐ裕┫赴?/P>adherent culture|贴壁培养adhering junction|粘着连接adhesin|粘附素[如见于大肠杆菌]adhesion|吸附,结合,粘合;粘着,粘附,粘连adhesion factor|粘着因子,粘附因子adhesion molecule|粘着分子,粘附分子adhesion plaque|粘着斑adhesion protein|粘着蛋白,吸附蛋白adhesion receptor|粘着受体adhesion zone|粘着带[如见于细菌壁膜之间]adhesive|粘合剂,胶粘剂adhesive glycoprotein|粘着糖蛋白adipic acid|己二酸,肥酸adipocyte|脂肪细胞adipokinetic hormone|脂动激素[见于昆虫]adipose tissue|脂肪组织adjust|[动]调节,调整;修正adjustable|可调的adjustable miropipettor|可调微量移液管adjustable spanner|活动扳手adjusted retention time|调整保留时间adjusted retention volume|调整保留体积adjuvant|佐剂adjuvant cytokine|佐剂细胞因子adjuvant peptide|佐剂肽adjuvanticity|佐剂(活)性adoptive immunity|过继免疫adoptive transfer|过继转移ADP ribosylation|ADP核糖基化ADP ribosylation factor|ADP核糖基化因子ADP ribosyltransferase|ADP核糖基转移酶adrenal cortical hormone|肾上腺皮质(激)素adrenaline|肾上腺素adrenergic receptor|肾上腺素能受体adrenocepter|肾上腺素受体adrenocorticotropic hormone|促肾上腺皮质(激)素adrenodoxin|肾上腺皮质铁氧还蛋白adriamycin|阿霉素,亚德里亚霉素adsorbent|吸附剂adsorption|吸附adsorption catalysis|吸附催化adsorption center|吸附中心adsorption chromatography|吸附层析adsorption film|吸附膜adsorption isobar|吸附等压线adsorption isotherm|吸附等温线adsorption layer|吸附层adsorption potential|吸附电势adsorption precipitation|吸附沉淀adsorption quantity|吸附量adult diarrhea rotavirus|成人腹泻轮状病毒advanced glycosylation|高级糖基化advanced glycosylation end product|高级糖基化终产物 adventitious|不定的,无定形的adverse effect|反效果,副作用aecidiospore|锈孢子,春孢子aeciospore|锈孢子,春孢子aequorin|水母蛋白,水母素aeration|通气aerator|加气仪,加气装置aerial mycelium|气生菌丝体aerobe|需氧菌[利用分子氧进行呼吸产能并维持正常生长繁殖的细菌] aerobic|需氧的aerobic bacteria|需氧(细)菌aerobic cultivation|需氧培养aerobic glycolysis|有氧酵解aerobic metabolism|有氧代谢aerobic respiration|需氧呼吸aerobic waste treatment|需氧废物处理aerobiosis|需氧生活aerogel|气凝胶aerogen|产气菌aerolysin|气单胞菌溶素Aeromonas|气单胞菌属aerosol|气溶胶aerosol gene delivery|气溶胶基因送递aerospray ionization|气喷射离子化作用aerotaxis|趋氧性[(细胞)随环境中氧浓度梯度进行定向运动]aerotolerant bacteria|耐氧菌[不受氧毒害的厌氧菌]aerotropism|向氧性aesculin|七叶苷,七叶灵aetiology|病原学B cell|B细胞B cell antigen receptor|B细胞抗原受体B cell differentiation factor|B细胞分化因子B cell growth factor|B细胞生长因子B cell proliferation|B细胞增殖B cell receptor|B细胞受体B cell transformation|B细胞转化B chromosome|B染色体[许多生物(如玉米)所具有的异染质染色体] B to Z transition|B-Z转换[B型DNA向Z型DNA转换]Bacillariophyta|硅藻门Bacillus|芽胞杆菌属Bacillus anthracis|炭疽杆菌属Bacillus subtillis|枯草芽胞杆菌bacitracin|杆菌肽back donation|反馈作用back flushing|反吹,反冲洗back mutation|回复突变[突变基因又突变为原由状态]backbone|主链;骨架backbone hydrogen bond|主链氢键backbone wire model|主链金属丝模型[主要反应主链走向的实体模型]backcross|回交backflushing chromatography|反吹层析,反冲层析background|背景,本底background absorption|背景吸收background absorption correction|背景吸收校正background correction|背景校正background gactor|背景因子background genotype|背景基因型[与所研究的表型直接相关的基因以外的全部基因]background hybridization|背景杂交background radiation|背景辐射,本底辐射backmixing|反向混合backside attack|背面进攻backward reaction|逆向反应backwashing|反洗bacmid|杆粒[带有杆状病毒基因组的质粒,可在细菌和昆虫细胞之间穿梭]bacteremia|菌血症bacteria|(复)细菌bacteria rhodopsin|细菌视紫红质bacterial adhesion|细菌粘附bacterial alkaline phosphatase|细菌碱性磷酸酶bacterial artificial chromosome|细菌人工染色体bacterial colony|(细菌)菌落bacterial colony counter|菌落计数器bacterial conjugation|细菌接合bacterial filter|滤菌器bacterial invasion|细菌浸染bacterial motility|细菌运动性bacterial rgodopsin|细菌视紫红质,细菌紫膜质bacterial vaccine|菌苗bacterial virulence|细菌毒力bactericidal reaction|杀(细)菌反应bactericide|杀(细)菌剂bactericidin|杀(细)菌素bactericin|杀(细)菌素bacteriochlorophyll|细菌叶绿素bacteriochlorophyll protein|细菌叶绿素蛋白bacteriocide|杀(细)菌剂bacteriocin|细菌素bacteriocin typing|细菌素分型[利用细菌素对细胞进行分型]bacterioerythrin|菌红素bacteriofluorescein|细菌荧光素bacteriology|细菌学bacteriolysin|溶菌素bacteriolysis|溶菌(作用)bacteriolytic reaction|溶菌反应bacteriophaeophytin|细菌叶褐素bacteriophage|噬菌体bacteriophage arm|噬菌体臂bacteriophage conversion|噬菌体转变bacteriophage head|噬菌体头部bacteriophage surface expression system|噬菌体表面表达系统bacteriophage tail|噬菌体尾部bacteriophage typing|噬菌体分型bacteriophagology|噬菌体学bacteriopurpurin|菌紫素bacteriorhodopsin|细菌视紫红质bacteriosome|细菌小体[昆虫体内一种含有细菌的结构]bacteriostasis|抑菌(作用)bacteriostat|抑菌剂bacteriotoxin|细菌毒素bacteriotropin|亲菌素bacterium|细菌bacteroid|类菌体baculovirus|杆状病毒bag sealer|封边机baking soda|小苏打BAL 31 nuclease|BAL 31核酸酶balance|天平balanced heterokaryon|平衡异核体balanced lethal|平衡致死balanced lethal gene|平衡致死基因balanced linkage|平衡连锁balanced pathogenicity|平衡致病性balanced polymorphism|平衡多态性balanced salt solution|平衡盐溶液balanced solution|平衡溶液balanced translocation|平衡易位balbaini ring|巴尔比亚尼环[由于RNA大量合成而显示特别膨大的胀泡,在多线染色体中形成独特的环]Balbiani chromosome|巴尔比亚尼染色体[具有染色带的多线染色体,1881年首先发现于双翅目摇蚊幼虫]ball mill|球磨ball mill pulverizer|球磨粉碎机ball milling|球磨研磨balloon catheter|气囊导管[可用于基因送递,如将DNA导入血管壁]banana bond|香蕉键band|条带,带[见于电泳、离心等]band broadening|条带加宽band sharpening|条带变细,条带锐化band width|带宽banding pattern|带型banding technique|显带技术,分带技术barbiturate|巴比妥酸盐barium|钡barly strip mosaic virus|大麦条纹花叶病毒barly yellow dwarf virus|大麦黄矮病毒barnase|芽胞杆菌RNA酶[见于解淀粉芽胞杆菌]barophilic baceria|嗜压菌baroreceptor|压力感受器barotaxis|趋压性barotropism|向压性barr body|巴氏小体barrel|桶,圆筒[可用于描述蛋白质立体结构,如beta折叠桶]barrier|屏障,垒barstar|芽胞杆菌RNA酶抑制剂[见于解淀粉芽胞杆菌]basal|基础的,基本的basal body|基粒basal body temperature|基础体温basal component|基本成分,基本组分basal expression|基础表达,基态表达basal granule|基粒basal heat producing rate|基础产热率basal lamina|基膜,基板basal level|基础水平,基态水平basal medium|基本培养基,基础培养基basal medium Eagle|Eagle基本培养基basal metabolic rate|基础代谢率basal metabolism|基础代谢basal promoter element|启动子基本元件basal transcription|基础转录,基态转录basal transcription factor|基础转录因子base|碱基;碱base analog|碱基类似物,类碱基base catalysis|碱基催化base composition|碱基组成base pairing|碱基配对base pairing rules|碱基配对法则,碱基配对规则base peak|基峰base pire|碱基对base ratio|碱基比base stacking|碱基堆积base substitution|碱基置换baseline|基线baseline drift|基线漂移baseline noise|基线噪声basement membrane|基底膜basement membrane link protein|基底膜连接蛋白basic amino acid|碱性氨基酸basic fibroblast growth factor|碱性成纤维细胞生长因子basic fuchsin|碱性品红basic medium|基础培养基basic number of chromosome|染色体基数basic protein|碱性蛋白质basic solvent|碱性溶剂basic taste sensation|基本味觉basidiocarp|担子果basidiomycetes|担子菌basidium|担子basipetal translocation|向基运输basket centrifuge|(吊)篮式离心机basket drier|篮式干燥机basket type evaporator|篮式蒸发器basonuclin|碱(性)核蛋白[见于角质形成细胞,含有多对锌指结构] basophil|嗜碱性细胞basophil degranulation|嗜碱性细胞脱粒basophilia|嗜碱性batch|分批;批,一批batch cultivation|分批培养batch culture|分批培养物batch digestor|分批消化器batch extraction|分批抽提,分批提取batch fermentation|分批发酵,(罐)批发酵batch filtration|分批过滤batch operation|分批操作batch process|分批工艺,分批法batch reactor|间歇反应器,分批反应器batch recycle cultivation|分批再循环培养batch recycle culture|分批再循环培养(物)bathochrome|向红基bathochromic shift|红移bathorhodopsin|红光视紫红质,前光视紫红质batrachotoxin|树蛙毒素[固醇类生物碱,作用于钠通道] baytex|倍硫磷BCG vaccine|卡介苗bead mill|玻珠研磨机bead mill homogenizer|玻珠研磨匀浆机bean sprouts medium|豆芽汁培养基beauvericin|白僵菌素becquerel|贝可(勒尔)bed volume|(柱)床体积bee venom|蜂毒beef broth|牛肉汁beef extract|牛肉膏,牛肉提取物beet yellows virus|甜菜黄化病毒Beggiatoa|贝日阿托菌属[属于硫细菌]behavior|行为;性质,性能behavioral control|行为控制behavioral isolation|行为隔离behavioral thermoregulation|行为性体温调节behenic acid|山yu酸,二十二(烷)酸belt desmosome|带状桥粒belt press|压带机belt press filter|压带(式)滤器bench scale|桌面规模,小试规模benchtop bioprocessing|桌面生物工艺[小试规模]benchtop microcentrifuge|台式微量离心机bend|弯曲;弯管;转折bending|弯曲;转折,回折beneficial element|有益元素bent bond|弯键bent DNA|弯曲DNA,转折DNAbenzene|苯benzhydrylamine resin|二苯甲基胺树脂benzidine|联苯胺benzilate|三苯乙醇酸(或盐或酯)benzimidazole|苯并咪唑benzodiazine|苯并二嗪,酞嗪benzoin|苯偶姻,安息香benzophenanthrene|苯并菲benzopyrene|苯并芘benzoyl|苯甲酰基benzoylglycine|苯甲酰甘氨酸benzyl|苄基benzyladenine|苄基腺嘌呤benzylaminopurine|苄基氨基嘌呤benzylisoquinoline|苄基异喹啉benzylisoquinoline alkaloid|苄基异喹啉(类)生物碱benzylpenicillin|苄基青霉素berberine|小檗碱Bertrand rule|贝特朗法则bestatin|苯丁抑制素[可抑制亮氨酸氨肽酶的一种亮氨酸类似物]C value|C值[单倍基因组DNA的量]C value paradox|C值悖理[物种的C值和它的进化复杂性之间无严格对应关系]C4 dicarboxylic acid cycle|C4二羧酸循环cachectin|恶液质素[即alfa肿瘤坏死因子]cadaverine|尸胺cadherin|钙粘着蛋白[介导依赖(于)钙的细胞间粘着作用的一类跨膜蛋白质,分为E-,N-,P-等若干种,E表示上皮(epithelia),N表示神经(neural),P表示胎盘(placental)] cadmium|镉caerulin|雨蛙肽cage|笼cage compound|笼形化合物cage coordination compound|笼形配合物cage effect|笼效应cage structure|笼形结构[非极性分子周围的水分子所形成的有序结构]calbindin|钙结合蛋白calciferol|麦角钙化(固)醇calcimedin|钙介蛋白[钙调蛋白拮抗剂]calcineurin|钙调磷酸酶[依赖于钙调蛋白的丝氨酸—苏氨酸磷酸酶]calcionin|降钙素calcium binding protein|钙结合蛋白(质)calcium binding site|钙结合部位calcium channel|钙通道calcium chloride|氯化钙calcium influx|钙流入calcium mediatory protein|钙中介蛋白(质)calcium phosphate|磷酸钙calcium phosphate precipitation|磷酸盐沉淀calcium pump|钙泵calcium sensor protein|钙传感蛋白(质)calcium sequestration|集钙(作用)calcyclin|钙(细胞)周边蛋白calcyphosine|钙磷蛋白[是依赖于cAMP的蛋白激酶的磷酸化底物]caldesmon|钙调(蛋白)结合蛋白[主要见于平滑肌,可与钙调蛋白及肌动蛋白结合] calelectrin|钙电蛋白[最初发现于鳗鱼电器官的一种钙结合蛋白]calf intestinal alkaline phosphatase|(小)牛小肠碱性磷酸酶calf serum|小牛血清calf thymus|小牛胸腺calgranulin|钙粒蛋白calibration|校准,标准calibration curve|校正曲线calibration filter|校准滤光片calibration protein|校准蛋白calicheamycin|刺孢霉素[来自刺孢小单胞菌的抗肿瘤抗生素,带有二炔烯官能团] calicivirus|杯状病毒calli|(复)胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callose|胼胝质,愈伤葡聚糖callose synthetase|愈伤葡聚糖合成酶callus|胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callus culture|愈伤组织培养calmodulin|钙调蛋白calnexin|钙联结蛋白[内质网的一种磷酸化的钙结合蛋白]calomel|甘汞calomel electrode|甘汞电极calorie|卡calpactin|依钙(结合)蛋白[全称为“依赖于钙的磷脂及肌动蛋白结合蛋白”]calpain|(需)钙蛋白酶calpain inhibitor|(需)钙蛋白酶抑制剂calpastatin|(需)钙蛋白酶抑制蛋白calphobindin|钙磷脂结合蛋白calphotin|钙感光蛋白[感光细胞的一种钙结合蛋白]calprotectin|(肌)钙网蛋白[骨骼肌肌质网膜上的钙结合蛋白]calretinin|钙(视)网膜蛋白calsequestrin|(肌)集钙蛋白calspectin|钙影蛋白calspermin|钙精蛋白[睾丸的一种钙调蛋白结合蛋白]caltractin|钙牵蛋白[一种与基粒相关的钙结合蛋白]Calvin cycle|卡尔文循环,光合碳还原环calyculin|花萼海绵诱癌素[取自花萼盘皮海绵的磷酸酶抑制剂]calyptra|根冠calyx|花萼cambium|形成层[见于植物]cAMP binding protein|cAMP结合蛋白cAMP receptor protein|cAMP受体蛋白cAMP response element|cAMP效应元件cAMP response element binding protein|cAMP效应元件结合蛋白Campbell model|坎贝尔模型camphane|莰烷camphane derivative|莰烷衍生物camphore|樟脑camptothecin|喜树碱Campylobacter|弯曲菌属Campylobacter fetus|胎儿弯曲菌属Canada balsam|加拿大香脂,枞香脂canaline|副刀豆氨酸canalization|[表型]限渠道化,发育稳态[尽管有遗传因素和环境条件的干扰,表型仍保持正常]canavanine|刀豆氨酸cancer|癌症cancer metastasis|癌症转移cancer suppressor gene|抑癌基因cancer suppressor protein|抑癌基因产物,抑癌蛋白(质)candicidin|杀假丝菌素candida|念珠菌属Candida albicans|白色念珠菌candle jar|烛罐cannabin|大麻苷;大麻碱canonical base|规范碱基canonical molecular orbital|正则分子轨道canonical partition function|正则配分函数canonical sequence|规范序列cantharidin|斑蝥素canthaxanthin|角黄素canyon|峡谷[常用于比喻某些生物大分子的主体结构特征]cap|帽,帽(结构)cap binding protein|帽结合蛋白cap site|加帽位点capacitation|获能[特指镜子在雌性生殖道中停留后获得使卵子受精的能力]capacity|容量capacity factor|容量因子capillarity|毛细现象capillary|毛细管;毛细血管capillary absorption|毛细吸收capillary action|毛细管作用capillary attraction|毛细吸力capillary column|毛细管柱capillary culture|毛细管培养capillary electrode|毛细管电极capillary electrophoresis|毛细管电泳capillary free electrophoresis|毛细管自由流动电泳capillary gas chromatography|毛细管气相层析capillary isoelectric focusing|毛细管等电聚焦capillary isotachophoresis|毛细管等速电泳capillary membrane module|毛细管膜包capillary transfer|毛细管转移[通过毛细管作用进行核酸的印迹转移] capillary tube|毛细管capillary tubing|毛细管capillary zone electrophoresis|毛细管区带电泳capillovirus|毛状病毒组capping|加帽,加帽反应;封闭反应;帽化,成帽capping enzyme|加帽酶capping protein|[肌动蛋白]加帽蛋白caprin|癸酸甘油酯caproin|己酸甘油酯capromycin|卷曲霉素,缠霉素caproyl|己酸基caprylin|辛酸甘油酯capsid|(病毒)衣壳,(病毒)壳体capsid protein|衣壳蛋白capsidation|衣壳化capsomer|(病毒)壳粒capsular polysaccharide|荚膜多糖capsulation|包囊化(作用),胶囊化(作用)capsule|荚膜capsule swelling reaction|荚膜肿胀反应capture|捕捉,俘获capture antigen|捕捉抗原[酶免疫测定中用于捕捉抗体的抗原]capture assay|捕捉试验carbamyl|氨甲酰基carbamyl ornithine|氨甲酰鸟氨酸carbamyl phosphate|氨甲酰磷酸carbamyl phosphate synthetase|氨甲酰磷酸合成酶carbamyl transferase|氨甲酰(基)转移酶carbamylation|氨甲酰化carbanion|碳负离子carbanyl group|羰基carbene|卡宾carbenicillin|羧苄青霉素carbenoid|卡宾体carbocation|碳正离子carbodiimide|碳二亚胺carbohydrate|糖类,碳水化合物carbohydrate fingerprinting|糖指纹分析carbohydrate mapping|糖作图,糖定位carbohydrate sequencing|糖测序carbol fuchsin|石炭酸品红carboline|咔啉,二氮芴carbon assimilation|碳同化carbon balance|碳平衡carbon cycling|碳循环carbon dioxide|二氧化碳carbon dioxide compensation|二氧化碳补偿点carbon dioxide fertilization|二氧化碳施肥carbon dioxide fixation|二氧化碳固定carbon dioxide tension|二氧化碳张力carbon fiber|碳纤维carbon fixation|碳固定carbon isotope|碳同位素carbon isotope analysis|碳同位素分析carbon isotope composition|碳同位素组成carbon monoxide|一氧化碳carbon source|碳源carbonate|碳酸盐,碳酸酯carbonate plant|碳化植物carbonic anhydrase|碳酸酐酶carbonium ion|碳正离子carbonyl|羰基carbonylation|羰基化carboxydismutase|羰基岐化酶,核酮糖二磷酸羧化酶 carboxydotrophic bacteria|一氧化碳营养菌carboxyglutamic acid|羧基谷氨酸carboxyl|羧基carboxyl protease|羧基蛋白酶carboxyl terminal|羧基端carboxyl transferase|羧基转移酶carboxylase|羧化酶carboxylation|羧(基)化carboxylic acid|羧酶carboxymethyl|羧甲基carboxymethyl cellulose|羧甲基纤维素carboxypeptidase|羧肽酶[包括羧肽酶A、B、N等]carcinogen|致癌剂carcinogenesis|致癌,癌的发生carcinogenicity|致癌性carcinoma|癌carcinostatin|制癌菌素cardenolide|强心苷cardiac aglycone|强心苷配基,强心苷元cardiac cycle|心动周期cardiac glycoside|强心苷cardiac receptor|心脏感受器cardiohepatid toxin|心肝毒素[如来自链球菌]cardiolipin|心磷脂cardiotoxin|心脏毒素cardiovascular center|心血管中枢cardiovascular disease|心血管疾病cardiovirus|心病毒属[模式成员是脑心肌炎病毒]carlavirus|香石竹潜病毒组carmine|洋红carminomycin|洋红霉素carmovirus|香石竹斑驳病毒组carnation latent virus|香石竹潜病毒carnation mottle virus|香石竹斑驳病毒carnation ringspot virus|香石竹环斑病毒carnitine|肉碱carnitine acyl transferase|肉碱脂酰转移酶carnosine|肌肽[即beta丙氨酰组氨酸]carotene|胡萝卜素carotene dioxygenase|胡萝卜素双加氧酶carotenoid|类胡萝卜素carotenoprotein|胡萝卜素蛋白carpel|[植物]心皮carrageen|角叉菜,鹿角菜carrageenin|角叉菜胶carrier|载体,运载体,携载体;携带者,带(病)毒者,带菌者 carrier ampholyte|载体两性电解质carrier catalysis|载体催化carrier coprecipitation|载体共沉淀carrier DNA|载体DNAcarrier free|无载体的carrier phage|载体噬菌体carrier precipitation|载体沉淀(作用)carrier state|携带状态carriomycin|腐霉素,开乐霉素cartridge|[萃取柱的]柱体;软片,胶卷;子弹,弹药筒casamino acid|(水解)酪蛋白氨基酸,酪蛋白水解物cascade|串联,级联,级联系统cascade amplification|级联放大cascade chromatography|级联层析cascade fermentation|级联发酵casein|酪蛋白,酪素casein kinase|酪蛋白激酶[分I、II两种]Casparian band|凯氏带[见于植物内表皮细胞]Casparian strip|凯氏带cassette|盒,弹夹[借指DNA序列组件]cassette mutagenesis|盒式诱变casting|铸,灌制CAT box|CAT框[真核生物结构基因上游的顺式作用元件]catabolism|分解代谢catabolite gene activator protein|分解代谢物基因激活蛋白 catabolite repression|分解代谢物阻抑,分解代谢产物阻遏catalase|过氧化氢酶catalytic active site|催化活性位catalytic activity|催化活性catalytic antibody|催化性抗体,具有催化活性的抗体catalytic constant|催化常数[符号Kcat]catalytic core|催化核心catalytic mechanism|催化机理catalytic RNA|催化性RNAcatalytic selectivity|催化选择性catalytic site|催化部位catalytic subunit|催化亚基cataphoresis|阳离子电泳cataract|白内障catechin|儿茶素catechol|儿茶酚,邻苯二酚catecholamine|儿茶酚胺catecholamine hormones|儿茶酚胺类激素catecholaminergic recptor|儿茶酚胺能受体catenane|连环(体),连锁,链条[如DNA连环体];索烃catenating|连环,连接catenation|连环,连锁,成链catenin|连环蛋白[一类细胞骨架蛋白,分alfa/beta/gama三种] catharanthus alkaloid|长春花属生物碱cathepsin|组织蛋白酶[分为A、B、C、D、E…H、L等多种]catheter|导管cathode layer enrichment method|阴极区富集法cathode ray polarograph|阴极射线极谱仪cation acid|阳离子酸cationic acid|阳离子酸cationic catalyst|正离子催化剂cationic detergent|阳离子(型)去污剂cationic initiator|正离子引发剂cationic polymerization|正离子聚合,阳离子聚合 cationic surfactant|阳离子(型)表面活性剂cationization|阳离子化cauliflower mosaic virus|花椰菜花叶病毒caulimovirus|花椰菜花叶病毒组caulobacteria|柄病毒Cavendish laboratory|(英国)卡文迪什实验室caveola|小窝,小凹caveolae|(复)小窝,小凹caveolin|小窝蛋白cavitation|空腔化(作用)cavity|沟槽,模槽,空腔dammarane|达玛烷dammarane type|达玛烷型Dane particle|丹氏粒[乙型肝炎病毒的完整毒粒]dansyl|丹(磺)酰,1-二甲氨基萘-5-磺酰dansyl chloride|丹磺酰氯dansyl method|丹磺酰法dantrolene|硝苯呋海因[肌肉松弛剂]dark current|暗电流dark field|暗视野,暗视场dark field microscope|暗视野显微镜,暗视场显微镜 dark field microscopy|暗视野显微术,暗视场显微术 dark reaction|暗反应dark repair|暗修复dark respiration|暗呼吸dark room|暗室,暗房dark seed|需暗种子data accumulation|数据积累data acquisition|数据获取data analysis|数据分析data bank|数据库data base|数据库data handling|数据处理data logger|数据记录器data logging|数据记录data output|数据输出data processing|数据处理data recording|数据记录dauermodification|持续饰变daughter cell|子代细胞daughter chromatid|子染色单体daughter chromosome|子染色体daughter colony|子菌落[由原生菌落续发生长的小菌落]daunomycin|道诺霉素daunorubicin|道诺红菌素de novo sequencing|从头测序de novo synthesis|从头合成deactivation|去活化(作用),失活(作用),钝化deacylated tRNA|脱酰tRNAdead time|死时间dead volume|死体积deadenylation|脱腺苷化DEAE Sephacel|[商]DEAE-葡聚糖纤维素,二乙氨乙基葡聚糖纤维素 dealkylation|脱烷基化deaminase|脱氨酶deamination|脱氨(基)death phase|死亡期[如见于细胞生长曲线]death point|死点deblocking|去封闭debranching enzyme|脱支酶,支链淀粉酶debris|碎片,残渣decahedron|十面体decane|癸烷decantation|倾析decanting|倾析decapacitation|去(获)能decarboxylase|脱羧酶decarboxylation|脱羧(作用)decay|原因不明腐败decay accelerating factor|衰变加速因子decay constant|衰变常数deceleration phase|减速期[如见于细胞生长曲线]dechlorination|脱氯作用deciduous leaf|落叶decline phase|[细胞生长曲线的]衰亡期decoagulant|抗凝剂decoding|译码,解码decomposer|分解者[可指具有分解动植物残体或其排泄物能力的微生物] decompression|降压,减压decondensation|解凝(聚)decontaminant|净化剂,去污剂decontaminating agent|净化剂,去污剂decontamination|净化,去污decorin|核心蛋白聚糖[一种基质蛋白聚糖,又称为PG-40]dedifferentiation|去分化,脱分化deep colony|深层菌落deep etching|深度蚀刻deep jet fermentor|深部喷注发酵罐deep refrigeration|深度冷冻deep shaft system|深井系统[如用于污水处理]defasciculation factor|解束因子[取自水蛭,可破坏神经束]defective|缺损的,缺陷的defective interfering|缺损干扰defective interfering particle|缺损干扰颗粒,干扰缺损颗粒defective interfering RNA|缺损干扰RNAdefective interfering virus|缺损干扰病毒defective mutant|缺损突变体,缺陷突变型,缺陷突变株defective phage|缺损噬菌体,缺陷噬菌体defective virus|缺损病毒,缺陷病毒defense|防御,防卫defense peptide|防卫肽defense response|防御反应,防卫反应defensin|防卫素[动物细胞的内源性抗菌肽]deficiency|缺乏,缺损,缺陷deficient|缺少的,缺损的,缺陷的defined|确定的defined medium|确定成分培养基,已知成分培养液defintion|定义defoliating agent|脱叶剂defoliation|脱叶deformylase|去甲酰酶[见于原核细胞,作用于甲酰甲硫氨酸]degasser|脱气装置degassing|脱气,除气degeneracy|简并;简并性,简并度degenerate|简并的degenerate codon|简并密码子degenerate oligonucleotide|简并寡核苷酸degenerate primer|简并引物degenerate sequence|简并序列degeneration|退化,变性degenerin|退化蛋白[与某些感觉神经元的退化有关]deglycosylation|去糖基化degradable polymer|降解性高分子degradation|降解degranulation|脱(颗)粒(作用)degree of acidity|酸度degree of dominance|显性度degree of polymerization|聚合度degron|降解决定子[决定某一蛋白发生降解或部分降解的序列要素] deguelin|鱼藤素dehalogenation|脱卤(作用)dehardening|解除锻炼dehumidifier|除湿器dehydratase|脱水酶dehydrated medium|干燥培养基dehydration|脱水(作用)dehydroepiandrosterone|脱氢表雄酮dehydrogenase|脱氢酶dehydrogenation|脱氢(作用)dehydroluciferin|脱氢萤光素deionization|去离子(作用)deionized|去离子的deionized water|去离子水deionizing|去离子(处理)delayed early transcription|(延)迟早期转录[可特指病毒]delayed fluorescence|延迟荧光delayed heat|延迟热delayed hypersensitivity|延迟(型)超敏反应delayed ingeritance|延迟遗传delayed type hypersensitivity|迟发型超敏反应deletant|缺失体deletion|缺失deletion mapping|缺失定位,缺失作图deletion mutagenesis|缺失诱变deletion mutant|缺失突变体deletion mutantion|缺失突变deletional recombination|缺失重组delignification|脱木质化(作用)deliquescence|潮解delivery flask|分液瓶delocalized bond|离域键。
门冬氨酸鸟氨酸杂质总结分享

序号
名称
L-Ornithine
1
L-Aspartate
Impurity 1
门冬氨酸鸟氨酸杂质总结分享
CAS
分子式
规格 用途
1783-96-6
C4H7NO4
10mg 25mg 50mg 100mg
研发申报
结构式
10mg
L-Ornithine
25mg
2
L-Aspartate 60079-mg
Impurity 4
100mg
10mg
L-Ornithine
25mg
5
L-Aspartate 58471-53-7 C8H12N2O7
研发申报
50mg
Impurity 5
100mg
湖北瑞诺医药---专注杂质对照品、标准品: ①多西他赛杂质 ②阿比特龙杂质 ③阿比多尔杂质 ④拉考沙胺杂质 ⑤间苯三酚杂质 q:300⑥罗沙司他杂质 ⑦尼可地尔杂质 ⑧依鲁替尼杂质 ⑨沙丁醇胺杂质 ⑩普拉洛芬杂质 -8058⑪培美曲塞杂质 ⑫奥西替尼杂质 ⑬布瓦西坦杂质 ⑭阿扎胞苷杂质 ⑮达泊西汀杂质 -303 ⑯氨曲南杂质 ......等更多项目品种 并代理:CP/EP/USP/TRC/TLC/MC/LGC/RHINO 等品牌。
研发申报
50mg
Impurity 2
100mg
10mg
L-Ornithine
C5H12N2O2 25mg
3
L-Aspartate 16682-12-5
研发申报
HCl
50mg
Impurity 3
100mg
10mg
L-Ornithine
25mg
4
L-Aspartate 34294-79-6 C5H10N2O
中华医学杂志英文版投稿须知

Instructions for authorsChinese Medical Journal (CMJ) is an international, peer-reviewed general medical journal published in English semimonthly by the Chinese Medical Association and distributed worldwide. Manuscripts are welcome from any part of the world.MANUSCRIPT INFORMATIONManuscript requirementsManuscripts submitted to CMJ should meet the following criteria: the material is original; the writing is clear; the study methods are appropriate; the data are valid; the conclusions are reasonable and supported by the data.Manuscript submissionAuthors are required to submit their manuscripts online at .Previous publication or duplicate submissionManuscripts are considered with the understanding that they have not been published previously and are not under consideration by another publication. Copies of possibly duplicative materials that have been previously published or are being considered elsewhere must be provided at the time of manuscript submission.Previous presentationA complete report following presentation at a meeting or publication of preliminary findings elsewhere (e.g., an abstract) can be considered.CATEGORIES OF ARTICLESCMJ publishes editorial, original article, review article, medical progress, brief report,viewpoint, case report, letter, and many other categories of articles. Topics of interest include all subjects that relate to the practice of medicine and research.EditorialThese are usually commissioned, however, unsolicited editorials are welcome. We are keen to consider editorials or ideas for editorials from authors outside China. Editorials should be up to 2000 words long with no more than 25 references.Original articleManuscripts on epidemiological studies, studies of social medicine, clinical trials, especially large scale randomized controlled trials are welcome. Each manuscript should clearly state an objective or hypothesis, the methods, the main results of the study and the conclusions. The length is limited to 2000–4000words (not including tables, figures, and references).More than 20 references are encouraged to be cited in this kind of articles.Meta analysisOnly results of meta analysis are reported in this kind of article. The length of the article is within 2000–4000 words (not including tables, figures, and references). Medical progressThis kind of article is mainly solicited, but we also consider unsolicited articles. The length of the article is within 2000–4000 words (not including tables, figures, and references).Review articleReview articles include systematic, critical assessments of literature and data sources pertaining to different medical topics, such as cause, diagnosis, prognosis, therapy, or prevention, etc.The length is limited to 2000–4000words (not including tables, figures, and references).Brief reportThese articles are short reports of original studies. They should not exceed 2500 words with no more than 2 tables and/or two illustrations and 15 references.Clinical experienceAuthors of these articles provide their experiences for diagnosis, treatment or prevention of diseases. The length is up to 2500 words with no more than 2 tables and/or two illustrations and 15 references.ViewpointPersonal views are welcome and the length should be 1000–3000 words (not including tables, figures, and references). Authors of this type of articles should sign their real names; no anonymous pieces are published.Case reportAuthors usually describe one to three patients or a single family. The text is limited to no more than 2500 words, and up to 15 references.Clinical solutionsThe articles are evidence-based reviews of topics relevant to practicing physicians. Articles in this series should include the following sections: case report, clinical overview, strategies, clinical difficulties, and author’s personal opinions. The text is limited to 3000 words and a small number of figures and tables. Images for diagnosisAuthors can provide here with typical images of common or uncommon medical conditions. This feature is intended to capture the sense of visual discovery and variety that physicians experience. It is not intended as a vehicle for case reports.LetterLetters to editors discussing a recent CMJ article should be received within 3 months of the article’s publication and should not exceed 500 words of text and 5 references. Letters should also be submitted online.AUTHOR INFORMATIONDesignate a corresponding author and provide a complete address,telephone and fax numbers, and E-mail address.Authorship requirementsEach author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. One or more authors should take responsibility for the integrity of the work as a whole, from inception to published article. Authorship credit should be based on(1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; and (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.Conditions 1, 2, and 3 must all be met.1Group authorshipIf authorship is attributed to a group, all members of the group must meet the full criteria and requirements for authorship as described above. A group must designate at least one individual as corresponding author. Other group members may be listed in an Acknowledgment.Conflicts of interestAuthors should indicate relevant conflicts of interest, including specific financial interests relevant to the subject of their manuscript, in their covering letter. Authors without relevant financial interests in the manuscript should indicate no such interest.EDITORIAL REVIEW AND PUBLICATIONPeer reviewA CMJ editor reviews submitted manuscripts initially. Manuscripts with insufficient priority for publication are rejected promptly. Other manuscripts are sent to expert consultants for peer review. Peer reviewer identities are kept confidential.The manuscript under review is not revealed to anyone other than peer reviewers and editorial staff. We encourage authors to suggest the names of possible reviewers, but we reserve the right of final selection. Rejected manuscriptsRejected manuscripts and reasons for rejection can be found online. EditingAccepted manuscripts are copyedited first by native speakers and then by CMJ editors according to CMJ style and returned to the author for approval. Authors are responsible for all statements made in their work, including changes made by the editor and authorized by the corresponding author.PublicationAuthors are required to pay page fee if their manuscripts are accepted for publication. The publisher will provide the author (s) 2 copies of the journal free of charge.CopyrightThe Chinese Medical Association (CMA) is the owner of all copyrights to any articles published in the journal. Published manuscripts become the permanent property of the Chinese Medical Association and may not be published elsewhere without written permission. Chinese Medical Association keeps the right to use these manuscripts in any form, including print, video, audio, and digital.MANUSCRIPT PREPARATIONManuscripts should be prepared in accordance with the Uniform Requirements for Manuscripts Submitted to Biomedical Journals by the International Committee of Medical Journal Editors (ICMJE) ().Submit the original manuscript online; use 1 side of standard-sized page and 1.5 cm margins.For Chinese authors, submission of a Chinese version of the manuscript (or abstract) is recommended.Use only 10- or 12-point font size.On the title page include the full names and affiliations of all authors. If an author’s affiliation has changed since the work was done, list the new affiliation as well. Titles should be concise and descriptive. The name, address, telephone number, fax number, and E-mail address of the correspondence author should be addressed. Any grant support that requires acknowledgment should be mentioned on this page.Measurements of length, height, weight, and volume should be reported in metric units (meter, kilogram, or liter) or their decimal multiples. Temperatures should be given in degrees Celsius. Blood pressures should be given in millimeters of mercury. All hematological and clinical chemistry measurements should be reported in the metric system in terms of the International System Units (SI).Use nonproprietary names of drugs, devices, and other products, unless the specific trade name of a drug is directly relevant to the discussion.Do not use abbreviations in the title or abstract and limit their use in the text.A covering letter signed by all authors includes(1) information on prior or duplicate publication or submission elsewhere of any part of the study; (2) the statement that the manuscript has been read and approved by all the authors and that the criteria for authorship have been met; (3) the statement on financial or other conflict of interests; and (4) any suggestions such as referring possible unqualified reviewers due to conflict of interests, etc. The corresponding author must sign the acknowledgment statement. Authors should obtain written permission from all individuals named in an acknowledgment.JOURNAL STYLETablesTables should be simple and no duplicate information should appear in the text of the article. Tables should be numbered consecutively, and headed by a concise title. Place explanatory matter in footnotes, not in the heading. Explain in footnotes all non-standard abbreviations that are used in each table. Forfootnotes use the following symbols in this sequence: *, †, ‡, §, ||, ¶, **, ††, ‡‡.FiguresComplete sets of illustrations must be submitted with legends typed on the same page. Only clear photographs are acceptable. All lettering must be legible after reduction to column size. Magnification and staining should be indicated when pertinent. AbbreviationsUse only standard abbreviations. Avoid abbreviations in the title and abstract. The full term for which an abbreviation stands should precede its first use in the text unless it is a standard unit of measurement.Ethical requirementFor experimental investigations of human subjects, state in the Methods section that an appropriate institutional review board approved the project. For those investigators who do not have formal ethics review committees,the principles outlined in the Declaration of Helsinki2 should be followed. For investigations of human subjects, state in the Methods section the manner in which informed consent was obtained from the study participants.Patient descriptions, photographs, and pedigreesInclude a signed statement of informed consent to publish (in print and online) patient descriptions, photographs, and pedigrees from all persons (parents or legal guardians for minors) who can be identified in such written descriptions, photographs, or pedigrees. Such persons should be shown the manuscript before its submission.Permissions required to reproduce or adapt material Acknowledge all text, illustrations, and tables adapted or reproduced from other publications and submit permission from the original publishers(or other copyright owner) to republish in print, online, and licensed versions of CMJ.ReferencesNumber references in the order they appear in the text; do not alphabetize. In text, tables, and legends, identify references with superscript Arabic numerals. When listing references,abbreviate titles of journals according to Medline. Note: List authors and/or editors up to 6; if more than 6, list the first 6 authors followed by et al.Examples of reference style:1. Liu XP, Long DY, Dong JZ, Liu XQ, Fang DP, Hao P, et al. Recurrent atrial tachycardia and atrial fibrillation after circumferential pulmonary vein a blation: What’s the difference? Chin Med J 2005; 118: 1773-1778.2. Xie SZ, Gu MJ, Cheng YP. Inhibitory effect of medroxyprogesterone acetate on angiogenesis induced by malignant neoplasm. Chin J Obstet Gynecol (Chin)* 1998; 33: 113-114.3. Weinstein L, Swartz MN. Pathogenic properties of invading microorganisms. In: Sodeman WA Jr., Sodeman WA, eds. Pathologic physiology: mechanisms of disease. Philadelphia: Saunders; 1974: 457-472.4. Dannenberg AM. Immune mechanisms in the pathogenesis of pulmonary tuberculosis. Rev Infect Dis 1989; 11 Suppl 2: s369-s378.5. Payne DK, Sullivan MD, Massie MJ. Women’s psychological reactions to breast cancer. Semin Oncol 1996; 23(1 Suppl 2): 89-97.6. Ozben T, Nacitarhan S, Tuncer N. Plasma and urine sialic acid in non-insulin dependent diabetes mellitus. Ann Clin Biochem 1995;32 (Pt 3): 303-306.7. Turan I, Wredmark T, Fellander-Tsai L. Arthroscopic ankle arthrodesis in rheumatoid arthritis. Clin Orthop 1995; (320): 110-114.8. Cumulative number of reported cases of severe acute respiratory syndrome (SARS). Geneva: World Health Organization, 2003. (Accessed April 9, 2003 at http://www.who.int/csr/sarscountry/ 2003_04_04/en/.)*: It is especially needed to note “(Chin)” for articles published in Chinese.Authors are responsible for the accuracy and completeness of their references and for correct citation of the text.REPORT OF ORIGINAL DATAAbstractInclude a structured abstract of no more than 300words for original articles, meta analysis, brief report, clinical experience (Background, Methods, Results, Conclusions) and review articles (Objective, Data sources, Study selection, Results, Conclusions); an informative abstract for medical progress, viewpoint, case report, clinical solutions and images for diagnosis.KeywordsThree to 6 words or short phrases should be provided at the top of the abstract page as keywords. Terms from the medical subject heading (MeSH) list of Medline should be used; if suitable MeSH terms are not yet available for recently introduced terms, present terms may be used.IntroductionIntroduction should be short and arresting. State the purpose of the article and summarize the rationale for the study or observation. Give only strictly pertinent references and do not include data or conclusions from the work being reported.MethodsDescribe your selection of the observational or experimental subjects (patients or laboratory animals, including controls) clearly. Identify the age, sex, and other important characteristics of the subjects.Iden tify the methods, apparatus (list the manufacturer’s name and original country in parentheses), and procedures in sufficient detail to allow other workers to reproduce the results. Give references to established methods, including statistical methods; provide references and brief descriptions for methods that have been published but are not well known; describe new or substantially modified methods, give reasons for using them, and evaluate theirlimitations. Identify precisely all drugs and chemicals used, including generic name (s), dose (s), and route (s) of administration.Reports of randomized clinical trials should present information on all major study elements including the protocol (study population, interventions or exposures, outcomes, and the rationale for statistical analysis), assignment of interventions (methods of randomization, concealment of allocation to treatment groups), and the method of masking (blinding). Authors are recommended to refer to the CONSORT Statement 3 for details.ResultsOverall describe the major findings of the study. Present your results in logical sequence in the text, tables and illustrations. Do not repeat in the text all the data in the tables or illustrations; emphasize or summarize only important observations. DiscussionSummarize the major findings. Discuss possible problems with the methods used. Compare your results with previous work. Discuss the clinical and scientific (if any) implications of your findings and their limitations. Suggest further work. Produce a succinct conclusion.MANUSCRIPT CHECKLISTSubmit complete text of your manuscript online (including tables, figures, etc), in addition, domestic authors should submit Chinese version of the complete text or its abstract.Review the sequence: covering letter, title page, key words and abstract, text, acknowledgments, references, tables, legends for illustrations.Check all references for accuracy and completeness. Put references in proper format in numerical order, making sure each is cited in the text.Include written permission from each individual identified as a source for personal communication.Include informed consent forms for identifiable patient descriptions, photographs and pedigrees.Keep copies of everything submitted.Manuscript inquiriesTel:86-10-85158321.Fax:86-10-85158333.Email:***********. cn.REFERENCES1.International Committee of Medical Journal Editors. Uniformrequirements for manuscripts submitted to biomedical journals.(Accessed September 10, 2009 at: )2.World Medical Association. Declaration of Helsinki: Ethicalprinciples for medical research involving human subjects.(Accessed September 6, 2005 at: /e/policy/ pdf/ 17c.pdf)3.Schulz KF, Altman DG, Moher D, for the CONSORT Group.The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. (Accessed October 28, 2010 at: http://www.consort- )。
异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝对比分析

异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝对比分析发表时间:2016-09-24T12:11:20.150Z 来源:《心理医生》2016年13期作者:龚新惠[导读] 与多烯磷脂酰胆碱相比,异甘草酸镁治疗脂肪肝效果更佳,可有效治疗脂肪肝、降低肝功能指标,值得临床推广使用。
(商丘市长征人民医院内科河南商丘 476000) 【摘要】目的:探讨异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝的临床疗效。
方法:选取我院2014年1月至2015年2月确诊患有脂肪肝患者60例,随机分为两组,每组各30例。
观察组采用异甘草酸镁治疗,对照组采用多烯磷脂酰胆碱治疗,对比两组治疗效果及肝功能指标。
结果:观察组治疗总有效率(96.67%)高于对照组(80.00%),差异显著(P<0.05);观察组治疗1、3周后各项肝功能指标均低于对照组,差异显著(P<0.05)。
结论:异甘草酸镁相比多烯磷脂酰胆碱治疗脂肪肝效果更佳,可有效降低肝功能指标,安全性高,值得临床推广。
【关键词】异甘草酸镁;多烯磷脂酰胆碱;脂肪肝【中图分类号】R575 【文献标识码】A 【文章编号】1007-8231(2016)13-0025-02 Comparative analysis of the treatment of fatty liver with magnesium and phosphatidylcholine Gong Xiaolu .Department of internal medicine, Henan people's Hospital, Shangqiu 476000, Shangqiu,China 【Abstract】 Objective To investigate the clinical effect of magnesium and multi - phosphatidylcholine phosphatidylcholine in the treatment of fatty liver. Methods 60 cases of fatty liver were selected from January 2014 to February 2015 in our hospital, were randomly divided into two groups, 30 cases in each group. The observation group was treated with magnesium, and the control group was treated with phosphatidylcholine, and the therapeutic effect and liver function index of the two groups were compared.Results In the observation group the total efficiency (96.67%) was higher than that of the control group (80.00%), and the difference is significant (P < 0.05); the observation group after 1 and 3 weeks of treatment, liver function indexes were lower than the control group, the difference was significant (P < 0.05). Conclusion It is better to treat fatty liver by comparing with magnesium, and it can effectively reduce the liver function index, and has high safety, and is worthy of clinical application.【Key words】 Magnesium; Magnesium; Phosphatidylcholine; Fatty liver 由于脂肪肝发病时无明显特殊症状,导致本病较难发现,因此不能进行及早治疗,从而发展成肝癌、肝硬化等危重病症,严重影响患者身心健康[1]。
盐酸环丙沙星欧洲药典 8.0 (英文版)

Ciprofloxacin hydrochloride EUROPEAN PHARMACOPOEIA8.004/2011:0888corrected 7.4CIPROFLOXACIN HYDROCHLORIDE Ciprofloxacinihydrochloridum C 17H 19ClFN 3O 3,x H 2O M r 367.8(anhydrous)DEFINITION1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid hydrochloride.It containsa variable quantity of water.Content :98.0per cent to 102.0per cent (anhydrous substance).CHARACTERSAppearance :pale yellow,crystalline,slightly hygroscopicpowder.Solubility :soluble in water,slightly soluble in methanol,very slightly soluble in anhydrous ethanol,practically insoluble in acetone,in ethyl acetate and in methylene chloride.IDENTIFICATION A.Infrared absorption spectrophotometry (2.2.24).Comparison :ciprofloxacin hydrochloride CRS .B.0.1g gives reaction (b)of chlorides (2.3.1).TESTSSolution S .Dissolve 0.5g in carbon dioxide-free water R anddilute to 20mL with the same solvent.Appearance of solution .The solution is clear (2.2.1)and not more intensely coloured than reference solution GY 5(2.2.2,Method II ).Dilute 10mL of solution S to 20mL with carbon dioxide-freewater R .pH (2.2.3):3.5to 4.5for solution S.Impurity A .Thin-layer chromatography (2.2.27).Test solution .Dissolve 50mg of the substance to be examinedin water R and dilute to 5mL with the same solvent.Reference solution .Dissolve 10mg of ciprofloxacinimpurity A CRS in a mixture of 0.1mL of dilute ammonia R1and 90mL of water R and dilute to 100mL with water R .Dilute 2mL of the solution to 10mL with water R .Plate :TLC silica gel F 254plate R .Mobile phase :acetonitrile R ,concentrated ammonia R ,methanol R ,methylene chloride R (10:20:40:40V/V/V/V ).Application :5μL.Development :at the bottom of a chromatographic tank,place an evaporating dish containing 50mL of concentratedammonia R .Expose the plate to the ammonia vapour for15min in the closed tank.Withdraw the plate,transfer to a 2nd chromatographic tank and develop over 3/4of the plate.Drying :in air.Detection :examine in ultraviolet light at 254nm.Limit :–impurity A :any spot corresponding to impurity A is notmore intense than the principal spot in the chromatogram obtained with the reference solution (0.2per cent).Related substances .Liquid chromatography (2.2.29).Test solution .Dissolve 25.0mg of the substance to beexamined in the mobile phase and dilute to 50.0mL with themobile phase.Reference solution (a ).Dissolve 25.0mg of ciprofloxacin hydrochloride CRS in the mobile phase and dilute to 50.0mLwith the mobile phase.Reference solution (b).Dissolve 5mg of ciprofloxacin hydrochloride for peak identification CRS (containingimpurities B,C,D and E)in the mobile phase and dilute to 10.0mL with the mobile phase.Reference solution (c).Dilute 1.0mL of the test solution to 50.0mL with the mobile phase.Dilute 1.0mL of this solution to 10.0mL with the mobile phase.Column :–size :l =0.25m,Ø=4.6mm;–stationary phase :base-deactivated octadecylsilyl silica gel forchromatography R (5μm);–temperature :40°C.Mobile phase :mix 13volumes of acetonitrile R and 87volumesof a 2.45g/L solution of phosphoric acid R previously adjustedto pH 3.0with triethylamine R .Flow rate :1.5mL/min.Detection :spectrophotometer at 278nm.Injection :50μL of the test solution and reference solutions (b)and (c).Run time :2.3times the retention time of ciprofloxacin.Identification of impurities :use the chromatogram suppliedwith ciprofloxacin hydrochloride for peak identification CRSand the chromatogram obtained with reference solution (b)to identify the peaks due to impurities B,C,D and E.Relative retention with reference to ciprofloxacin(retention time =about 9min):impurity E =about 0.4;impurity B =about 0.6;impurity C =about 0.7;impurity D =about 1.2.System suitability :reference solution (b):–resolution :minimum 1.3between the peaks due toimpurities B and C.Limits :–correction factors :for the calculation of content,multiplythe peak areas of the following impurities by thecorresponding correction factor:impurity B =0.7;impurity C =0.6;impurity D =1.4;impurity E =6.7;–impurity E :not more than 1.5times the area of the principal peak in the chromatogram obtained with reference solution (c)(0.3per cent);–impurities B,C,D :for each impurity,not more than the area of the principal peak in the chromatogram obtained with reference solution (c)(0.2per cent);–unspecified impurities :for each impurity,not more than 0.5times the area of the principal peak in the chromatogramobtained with reference solution (c)(0.10per cent);–total :not more than 2.5times the area of the principal peak in the chromatogram obtained with reference solution (c)(0.5per cent);–disregard limit :0.25times the area of the principal peak in the chromatogram obtained with reference solution (c)(0.05per cent).Heavy metals (2.4.8):maximum 20ppm.Dissolve 0.25g in water R and dilute to 30mL with the samesolvent.Carry out the prefiltration.The filtrate complieswith test E.Prepare the reference solution using 5mL of lead standard solution (1ppm Pb)R .Water (2.5.12):maximum 6.7per cent,determined on 0.200g.Sulfated ash (2.4.14):maximum 0.1per cent,determined on1.0g in a platinum crucible.1896See the information section on general monographs (cover pages)EUROPEAN PHARMACOPOEIA 8.0CisplatinASSAYLiquid chromatography (2.2.29)as described in the test for related substances with the following modification.Injection :10μL of the test solution and reference solution (a).Calculate the percentage content of C 17H 19ClFN 3O 3.STORAGEIn an airtight container,protected from light.IMPURITIESSpecified impurities :A,B,C,D,E.Other detectable impurities (the following substances would,if present at a sufficient level,be detected by one or other of the tests in the monograph.They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).It is therefore not necessary to identify these impurities for demonstration of compliance.See also 5.10.Control of impurities in substances for pharmaceutical use ):F.A.7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (fluoroquinolonicacid),B.1-cyclopropyl-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid (desfluorocompound),C.7-[(2-aminoethyl)amino]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (ethylenediaminecompound),D.7-chloro-1-cyclopropyl-4-oxo-6-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylicacid,E.1-cyclopropyl-6-fluoro-7-(piperazin-1-yl)quinolin-4(1H )-one (decarboxylatedcompound), F.1-cyclopropyl-6-hydroxy-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid.01/2009:0599corrected 7.0CISPLATINCisplatinumPtCl 2(NH 3)2M r 300.0[15663-27-1]DEFINITIONcis -Diamminedichloroplatinum(II).Content :97.0per cent to 102.0per cent.CHARACTERSAppearance :yellow powder,or yellow or orange-yellow crystals.Solubility :slightly soluble in water,sparingly soluble in dimethylformamide,practically insoluble in ethanol (96per cent).Carry out identification test B,the tests (except that for silver)and the assay protected from light .IDENTIFICATIONFirst identification:A,B .Second identification:B,C .A.Infrared absorption spectrophotometry (2.2.24).Comparison :cisplatin CRS .B.Thin-layer chromatography (2.2.27).Test solution .Dilute 1mL of solution S2(see Tests)to 10mL with dimethylformamide R .Reference solution .Dissolve 10mg of cisplatin CRS in 5mL of dimethylformamide R .Plate :cellulose for chromatography R1as the coating substance.Pretreatment :activate the plate by heating at 150°C for 1h.Mobile phase :acetone R ,dimethylformamide R (10:90V/V ).Application :2μL.Development :over 2/3of the plate.Drying :in air.Detection :spray with a 50g/L solution of stannous chloride R in a mixture of equal volumes of dilute hydrochloric acid R and water R .Examine after 1h.Results :the principal spot in the chromatogram obtained with the test solution is similar in position,colour and size to the principal spot in the chromatogram obtained with the reference solution.C.Add 50mg to 2mL of dilute sodium hydroxide solution R in a glass dish.Evaporate to dryness.Dissolve the residue in a mixture of 0.5mL of nitric acid R and 1.5mL of hydrochloric acid R .Evaporate to dryness.The residue is orange.Dissolve the residue in 0.5mL of water R and add 0.5mL of ammonium chloride solution R .A yellow,crystalline precipitate is formed.General Notices (1)apply to all monographs and other texts1897。
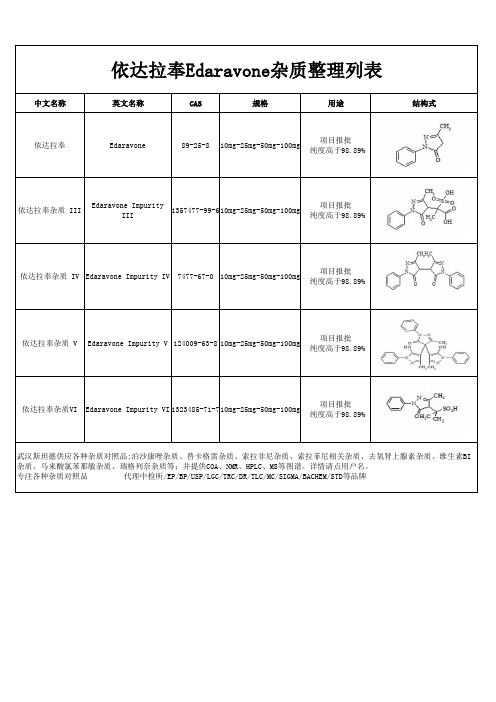
依达拉奉杂质整理列表
杂质、马来酸氯苯那敏杂质、瑞格列奈杂质等;并提供COA、NMR、HPLC、MS等图谱。详情请点用户名。
专注各种杂质对照品
代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌
中文名称 依达拉奉
依达拉奉Edaravone杂质整理列表
英文名称
CAS
规格
用途
结构式
Edaravone
89-25-8
10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
依达拉奉杂质 III
Edaravone Impurity III
1357477-99-6 10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
Hale Waihona Puke 依达拉奉杂质VIEdaravone Impurity VI 1323485-71-710mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
武汉斯坦德供应各种杂质对照品:泊沙康唑杂质、替卡格雷杂质、索拉非尼杂质、索拉菲尼相关杂质、去氧肾上腺素杂质、维生素BI
项目报批 纯度高于98.89%
依达拉奉杂质 IV Edaravone Impurity IV 7477-67-0 10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
依达拉奉杂质 V
Edaravone Impurity V 124009-63-8 10mg-25mg-50mg-100mg
地奥司明相关杂质
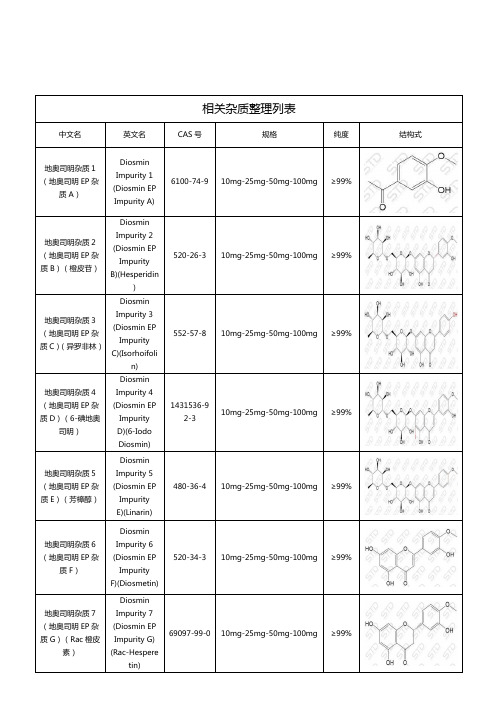
相关杂质整理列表中文名英文名CAS号规格纯度结构式地奥司明杂质1(地奥司明EP杂质A)DiosminImpurity 1(Diosmin EPImpurity A)6100-74-9 10mg-25mg-50mg-100mg ≥99%地奥司明杂质2(地奥司明EP杂质B)(橙皮苷)DiosminImpurity 2(Diosmin EPImpurityB)(Hesperidin)520-26-3 10mg-25mg-50mg-100mg ≥99%地奥司明杂质3(地奥司明EP杂质C)(异罗非林)DiosminImpurity 3(Diosmin EPImpurityC)(Isorhoifolin)552-57-8 10mg-25mg-50mg-100mg ≥99%地奥司明杂质4(地奥司明EP杂质D)(6-碘地奥司明)DiosminImpurity 4(Diosmin EPImpurityD)(6-IodoDiosmin)1431536-92-310mg-25mg-50mg-100mg ≥99%地奥司明杂质5(地奥司明EP杂质E)(芳樟醇)DiosminImpurity 5(Diosmin EPImpurityE)(Linarin)480-36-4 10mg-25mg-50mg-100mg ≥99%地奥司明杂质6(地奥司明EP杂质F)DiosminImpurity 6(Diosmin EPImpurityF)(Diosmetin)520-34-3 10mg-25mg-50mg-100mg ≥99%地奥司明杂质7(地奥司明EP杂质G)(Rac橙皮素)DiosminImpurity 7(Diosmin EPImpurity G)(Rac-Hesperetin)69097-99-0 10mg-25mg-50mg-100mg ≥99%湖北扬信医药科技有限公司经营上万种杂质对照品(优势供应硫酸羟氯喹杂质、硝苯地平杂质、沙丁胺醇杂质、达格列净杂质、厄贝沙坦杂质、阿莫西林克拉维酸钾杂质、利伐沙班杂质、阿托伐他汀钙杂质、西格列汀杂质、利格列汀杂质等),并代理销售中检所、STD、LGC、TLC、EP、USP、TRC等多个品牌产品,提供上万种标准品对照品,真诚为您服务。
依达拉奉右莰醇通过铁死亡-脂质过氧化通路对脑出血大鼠神经保护的作用机制

实验研究依达拉奉右莰醇通过铁死亡-脂质过氧化通路对脑出血大鼠神经保护的作用机制毛权西,李作孝△摘要:目的探讨依达拉奉右莰醇对脑出血大鼠的神经保护作用及血肿周围脑组织脂质过氧化的影响。
方法将128只SD大鼠随机分为假手术组、脑出血组、依达拉奉组和依达拉奉右莰醇组,每组32只。
除假手术组外,其余组大鼠构建急性脑出血模型,依达拉奉组、依达拉奉右莰醇组于造模后分别腹腔注射依达拉奉6mg/kg、依达拉奉右莰醇7.5mg/kg,每12h注射1次,假手术组和脑出血组腹腔注射等量生理盐水。
术后1d、3d、7d和14d按Garcia评分标准进行神经功能评分,HE染色观察血肿周围脑组织病理变化,化学荧光法检测血肿周围脑组织活性氧(ROS)含量,微量酶标法检测血肿周围脑组织还原型谷胱甘肽(GSH)含量,蛋白免疫印迹法检测血肿周围脑组织谷胱甘肽过氧化物酶4(GPX4)、长链脂酰辅酶A合成酶4(ACSL4)和磷脂胆碱酰基转移酶3(LPCAT3)表达。
结果与假手术组比较,脑出血组大鼠神经功能评分降低,血肿周围脑组织出现大量炎性细胞浸润及神经细胞变性,ROS含量、ACSL4和LPCAT3蛋白表达水平升高,GSH含量、GPX4蛋白表达水平降低(P<0.05);与脑出血组比较,依达拉奉组和依达拉奉右莰醇组大鼠神经功能评分升高,血肿周围脑组织病理损伤明显减轻,ROS含量、ACSL4和LPCAT3蛋白表达水平降低,GSH含量、GPX4蛋白表达水平增加(P<0.05);依达拉奉右莰醇组干预效果优于依达拉奉组(P<0.05);除假手术组外,其余各组均在术后3d时变化最明显,术后7d、14d逐渐恢复(P<0.05)。
结论依达拉奉右莰醇可能通过调节脑出血大鼠神经细胞铁死亡相关蛋白的表达,减少脑组织脂质过氧化,抑制神经细胞铁死亡,从而发挥脑保护作用。
关键词:依达拉奉右莰醇;依达拉奉;脑出血;铁死亡;脂质过氧化中图分类号:R743.34文献标志码:A DOI:10.11958/20221777Neuroprotective mechanism of edaravone dexborneol in rats with cerebral hemorrhage throughferroptosis-lipid peroxidation pathwayMAO Quanxi,LI Zuoxiao△Department of Neurology,the Affiliated Hospital of Southwest Medical University,Luzhou646000,China△Corresponding Author E-mail:Abstract:Objective To investigate the neuroprotective effect of edaravone dexborneol on cerebral hemorrhage in rats and the effect of lipid peroxidation on perihematomal brain tissue.Methods A total of128SD rats were randomly divided into the sham-operated group,the cerebral hemorrhage group,the edaravone group and the edaravone dexborneol group, with32rats in each group.The acute cerebral hemorrhage model was constructed in all groups except for the sham-operated group.The edaravone group and edaravone dexamphene group were injected intraperitoneally with6mg/kg of edaravone and edaravone dexamphene7.5mg/kg,one injection every12hours.The sham-operated group and the cerebral hemorrhage group were injected intraperitoneally with equal amounts of saline.The neurological function was scored according to Garcia score at1d,3d,7d,and14d after surgery.Brain tissue around hematoma was stained with HE staining.Chemo fluorescence assay was used to observe pathological changes and reactive oxygen species(ROS)content of brain tissue around hematoma.Micro enzyme labeling assay was used to detect glutathione(GSH)content of brain tissue around hematoma.The expression levels of glutathione peroxidase4(GPX4),long-chain lipid acyl-coenzyme A synthase4(ACSL4) and phospholipid choline acyltransferase3(LPCAT3)in brain tissue around hematoma were detected by protein immunoblotting.Results Compared with the sham-operated group,neurological function scores were decreased in the cerebral hemorrhage group.Massive inflammatory cell infiltration and neuronal degeneration in brain tissue around hematoma were found,and ROS content,ACSL4and LPCAT3protein expression level increased.GSH content and GPX4 protein expression level decreased in the cerebral hemorrhage group(P<0.05).Compared with the cerebral hemorrhage group,neurological function scores were increased,histopathological damage around the hematoma was significantly基金项目:泸州市人民政府-西南医科大学科技战略合作基金项目(2018LZXNYD-ZK17)作者单位:西南医科大学附属医院神经内科(邮编646000)作者简介:毛权西(1990),男,硕士在读,主要从事神经免疫方向研究。
3094-09-5_Doxifluridine技术参数MedBio
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11222
4-[[2-[2-(乙酰基氧基)乙基]-2,3-二氢-4-甲基-1,3-二氧-1H-吡咯并[3,4-c]喹啉-8-基]磺酰基]-吗啉
Ivachtin
745046-84-8
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
MED11299
CPI-1189
CPI-1189
183619-38-7
25mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11254
PRIMA-1
PRIMA-1
5608-24-2
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11254
PRIMA-1
PRIMA-1
5608-24-2
10mM (in 1mL DMSO)≥98%品牌 Nhomakorabea货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11305
GSK'481
GSK481
1622849-58-4
100mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11298
BH3I-1
BH3I-1
300817-68-9
OXOID药敏纸片中英文对照
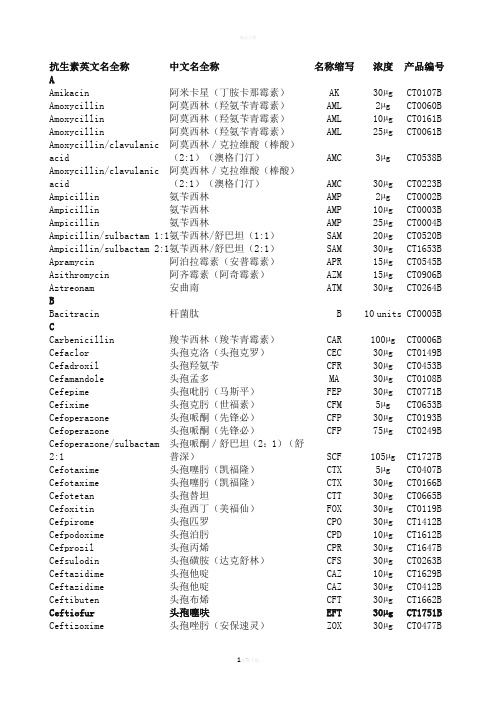
AAmikacin 阿米卡星(丁胺卡那霉素)AK 30µg CT0107B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 2µg CT0060B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 10µg CT0161B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 25µg CT0061BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 3µg CT0538BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 30µg CT0223BAmpicillin 氨苄西林AMP 2µg CT0002B Ampicillin 氨苄西林AMP 10µg CT0003B Ampicillin 氨苄西林AMP 25µg CT0004B Ampicillin/sulbactam 1:1 氨苄西林/舒巴坦(1:1)SAM 20µg CT0520B Ampicillin/sulbactam 2:1 氨苄西林/舒巴坦(2:1)SAM 30µg CT1653B Apramycin 阿泊拉霉素(安普霉素)APR 15µg CT0545B Azithromycin 阿齐霉素(阿奇霉素)AZM 15µg CT0906B Aztreonam 安曲南ATM 30µg CT0264B BBacitracin 杆菌肽 B 10 units CT0005B CCarbenicillin 羧苄西林(羧苄青霉素)CAR 100µg CT0006B Cefaclor 头孢克洛(头孢克罗)CEC 30µg CT0149B Cefadroxil 头孢羟氨苄CFR 30µg CT0453B Cefamandole 头孢孟多MA 30µg CT0108B Cefepime 头孢吡肟(马斯平)FEP 30µg CT0771B Cefixime 头孢克肟(世福素)CFM 5µg CT0653B Cefoperazone 头孢哌酮(先锋必)CFP 30µg CT0193B Cefoperazone 头孢哌酮(先锋必)CFP 75µg CT0249BCefoperazone/sulbactam 2:1 头孢哌酮/舒巴坦(2:1)(舒普深)SCF 105µg CT1727BCefotaxime 头孢噻肟(凯福隆)CTX 5µg CT0407B Cefotaxime 头孢噻肟(凯福隆)CTX 30µg CT0166B Cefotetan 头孢替坦CTT 30µg CT0665B Cefoxitin 头孢西丁(美福仙)FOX 30µg CT0119B Cefpirome 头孢匹罗CPO 30µg CT1412B Cefpodoxime 头孢泊肟CPD 10µg CT1612B Cefprozil 头孢丙烯CPR 30µg CT1647B Cefsulodin 头孢磺胺(达克舒林)CFS 30µg CT0263B Ceftazidime 头孢他啶CAZ 10µg CT1629B Ceftazidime 头孢他啶CAZ 30µg CT0412B Ceftibuten 头孢布烯CFT 30µg CT1662B Ceftiofur 头孢噻呋EFT 30µg CT1751B Ceftizoxime 头孢唑肟(安保速灵)ZOX 30µg CT0477BCeftriaxone 头孢曲松(头孢三嗪)CRO 30µg CT0417B Cefuroxime sodium 头孢呋新钠CXM 5µg CT0406B Cefuroxime sodium 头孢呋新钠CXM 30µg CT0127B Cephalexin 头孢氨苄(头孢力新,先锋IV)CL 30µg CT0007B Cephalothin 头孢噻吩(头孢菌素,先锋I )KF 30µg CT0010B Cephazolin 头孢唑啉(先锋V)KZ 30µg CT0011B Cephradine 头孢拉定(先锋VI)CE 30µg CT0063B Chloramphenicol 氯霉素 C 10µg CT0012B Chloramphenicol 氯霉素 C 30µg CT0013B Chloramphenicol 氯霉素 C 50µg CT0014B Cinoxacin 西诺沙星CIN 100µg CT0162B Ciprofloxacin 环丙沙星(悉复欢)CIP 1µg CT0623B Ciprofloxacin 环丙沙星(悉复欢)CIP 5µg CT0425B Ciprofloxacin 环丙沙星(悉复欢)CIP 10µg CT1615B Clarithromycin 克拉霉素CLR 2µg CT1599B Clarithromycin 克拉霉素CLR 5µg CT1623B Clarithromycin 克拉霉素CLR 15µg CT0693BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 2µg CT0064BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 10µg CT0015BCloxacillin 氯唑西林(邻氯青霉素)OB 5µg CT0016B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 10µg CT0017B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 25µg CT0065B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 50µg CT0664B Compound sulphonamides 磺胺复合物S3 300µg CT0059B 抗生素英文名全称中文名全称名称缩写浓度产品编号DDoxycycline 强力霉素DO 30µg CT0018B EEnrofloxacin 恩诺沙星ENR 5µg CT0639B Ertapenem 厄他培南ETP 10µg CT1761B Erythromycin 红霉素 E 5µg CT0066B Erythromycin 红霉素 E 10µg CT0019B Erythromycin 红霉素 E 15µg CT0020B Erythromycin 红霉素 E 30µg CT0021B抗生素英文名全称中文名全称名称缩写浓度产品编号FFlorfenicol 氟苯尼考FFC 30µg CT1754BFluconazole 氟康唑FCA 25µg CT1806B Flumequine 氟甲喹UB 30µg CT0666B Fosfomycin 磷霉素FOS 50µg CT0183B 抗生素英文名全称中文名全称名称缩写浓度产品编号Framycetin 新霉素B FY 100µg CT0071B Fusidic acid 褐霉素(夫西地酸)FD 5µg CT0493B Fusidic acid 褐霉素(夫西地酸)FD 10µg CT0023B Fusidic acid 褐霉素(夫西地酸)FD 50µg CT1617B GGentamicin 庆大霉素CN 10µg CT0024B Gentamicin 庆大霉素CN 30µg CT0072B Gentamicin 庆大霉素CN 120µg CT0794B Gentamicin 庆大霉素CN 200µg CT0695B IImipenem 亚胺培南(配能)IPM 10µg CT0455B KKanamycin 卡那霉素K 5µg CT0025B Kanamycin 卡那霉素K 30µg CT0026B LLatamoxef 拉氧头孢MOX 30µg CT0302B Levofloxacin 左氧氟沙星(可乐必妥)LEV 1µg CT1586B Levofloxacin 左氧氟沙星(可乐必妥)LEV 5µg CT1587B Lincomycin 林可霉素(洁霉素)MY 2µg CT0027B Lincomycin 林可霉素(洁霉素)MY 10µg CT0123B Lincomycin 林可霉素(洁霉素)MY 15µg CT0028BLincomycin/neomycin 林可霉素(洁霉素)/新霉素LN 75µg CT1757BLincomycin/spectinomycin 林可霉素/壮观霉素LS 109µg CT1758B Linezolid 利奈唑胺LZD 10µg CT1649B Linezolid 利奈唑胺LZD 30µg CT1650B Lomefloxacin 洛美沙星LOM 10µg CT1661B MMecillinam 美西林MEL 10µg CT0096B Mecillinam 美西林MEL 25µg CT0091B Meropenem 美罗培南(美平)MEM 10µg CT0774B Metronidazole 甲硝唑(灭滴灵)MTZ 5µg CT0067B Metronidazole 甲硝唑(灭滴灵)MTZ 50µg CT0466B Mezlocillin 美洛西林MEZ 30µg CT0174B Mezlocillin 美洛西林MEZ 75µg CT0192BMinocycline 米诺环素(二甲胺四环素)MH 30µg CT0030BMoxalactam 拉氧头孢MOX 30µg CT0302B Moxifloxacin 莫西沙星MXF 1µg CT1683B Moxifloxacin 莫西沙星MXF 5µg CT1633BMupirocin 莫匹罗星MUP 5µg CT0522B Mupirocin 莫匹罗星MUP 20µg CT1826B Mupirocin 莫匹罗星MUP 200µg CT0523B NNalidixic acid 萘啶酸NA 30µg CT0031B 抗生素英文名全称中文名全称名称缩写浓度产品编号Neomycin 新霉素N 30µg CT0033BNetilmicin 奈替米星(乙基西梭霉素)NET 10µg CT0424BNetilmicin 奈替米星(乙基西梭霉素)NET 30µg CT0225BNitrofurantoin 呋喃妥因(呋喃妥英) F 50µg CT0069B Nitrofurantoin 呋喃妥因(呋喃妥英) F 100µg CT0034B Nitrofurantoin 呋喃妥因(呋喃妥英) F 200µg CT0035B Nitrofurantoin 呋喃妥因(呋喃妥英) F 300µg CT0036B Norfloxacin 诺氟沙星(氟哌酸)NOR 2µg CT0687B Norfloxacin 诺氟沙星(氟哌酸)NOR 5µg CT0668B Norfloxacin 诺氟沙星(氟哌酸)NOR 10µg CT0434B Novobiocin 新生霉素NV 5µg CT0037B Novobiocin 新生霉素NV 30µg CT0038B Nystatin 制霉菌素NS 100units CT0073B OOfloxacin 氧氟沙星(泰利必妥)OFX 5µg CT0446B Oleandomycin 竹桃霉素OL 15µg CT0039B Oxacillin 苯唑西林OX 1µg CT0159B Oxacillin 苯唑西林OX 5µg CT0040B Oxolinic acid 奥索利酸(恶喹酸)OA 2µg CT0181BOxytetracycline 土霉素(氧四环素,地霉素)OT 30µg CT0041BPPefloxacin 培氟沙星(甲氟哌酸)PEF 5µg CT0661B Penicillin G 青霉素G P 1unit CT0152B Penicillin G 青霉素G P 1.5unit CT0042B Penicillin G 青霉素G P 2units CT0088B Penicillin G 青霉素G P 5units CT0124B Penicllin G 青霉素G P 10units CT0043B Penicillin/novobiocin 青霉素/新生霉素PNV 40 CT1755B Pipemidic acid 吡哌酸PIP 20µg CT0180BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 30µg CT1619BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 75µg CT0261BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 100µg CT0199BPiperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 36µg CT1616B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 40µg CT1628B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 85µg CT0720B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星) TZP 110µg CT0725B Pirlimycin 吡利霉素 PIR 2µg CT1668B Polymyxin B多粘菌素BPB300units CT0044BQQuinupristin/dalfopristin 喹奴普汀/达福普汀 QD 15µg CT1644BR抗生素英文名全称 中文名全称 名称缩写 浓度 产品编号 Rifampicin 利福平 RD 2µg CT0078B Rifampicin 利福平 RD 5µg CT0207B Rifampicin 利福平 RD 30µg CT0104BSSpectinomycin 大观霉素(壮观霉素) SH 10µg CT0046B Spectinomycin 大观霉素(壮观霉素) SH 25µg CT0411B Spectinomycin 大观霉素(壮观霉素) SH 100µg CT0823B Spiramycin 螺旋霉素 SP 100µg CT0232B Streptomycin 链霉素 S 10µg CT0047B Streptomycin链霉素 S 25µg CT0048B Sulbactam/ampicillin 1 : 1舒巴坦/氨苄西林 SAM 20µg CT0520BSulbactam/ampicillin 1 : 2 舒巴坦/氨苄西林(优立新) SAM 30µg CT1653B Sulphafurazole 磺胺异恶唑 SF300µg CT0075B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL25µg CT0051B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL 100µg CT0074B Sulphamethoxazole/trimethoprim 19 : 1 磺胺甲基异恶唑(新诺明)/甲氧苄氨嘧啶 SXT 25µg CT0052B Sulphonamides compound 磺胺复合物 S3 300µg CT0059BT Teicoplanin 替考拉宁(壁霉素) TEC 30µg CT0647B Telithromycin 泰利霉素 TEL 15µg CT1714B Tetracycline 四环素 TE 10µg CT0053B Tetracycline 四环素 TE 30µg CT0054B Ticarcillin 替卡西林(羧噻吩青霉TIC75µgCT0167B素)Ticarcillin/clavulanic acid 7.5 : 1 替卡西林/克拉维酸(7.5:1) TIM 85µg CT0449B Tigecycline 替加环素 TGC 15µg CT1841B Tilmicosin 替米考星 TIL 15µg CT1756B Tobramycin 妥布霉素(托普霉素) TOB 10µg CT0056B Tobramycin 妥布霉素(托普霉素) TOB 30µg CT1618B Trimethoprim 甲氧苄氨嘧啶 W 1.25µg CT0057B Trimethoprim 甲氧苄氨嘧啶 W 2.5µg CT0070B Trimethoprim 甲氧苄氨嘧啶 W 5µg CT0076B Trimethoprim/sulphamethoxazole 1:19 甲氧苄氨嘧啶/磺胺甲基异恶唑(复方新诺明) SXT 25µg CT0052B V Vancomycin 万古霉素(稳可信) VA 5µg CT0188B Vancomycin 万古霉素(稳可信) VA 30µg CT0058B Voriconazole 优立康唑 VOR 1µg CT1807B 注释: CLSI: Clinical and Laboratory Standards Institute 美国临床实验室标准化研究所DIN: Deutsches Institut f ür Normung 德国标准化学会 BSAC: British Society for Antimicrobial Chemotherapy 英国抗生素化疗协会SRGA: Swedish Reference Group for Antibiotics 瑞典抗生素委员会 SFM: Soci ét é Fran çaise de Microbiologie 法国微生物学会注:红色为兽禽用抗生素,绿色为农作物用抗生素,其余为人用抗生素。
5种卤代乙酰胺类消毒副产物对小鼠淋巴瘤细胞Tk基因致突变性研究

括 Tk 位点在 内 的 大 缺 失、易 位、重 组 甚 至 染 色 体
种 DBPs 提出限量控制要求ꎬ我国« 生活饮用水卫生
非整倍性等广泛的遗传学改变ꎮ 欧美国家均已将
标准» 也将 13 种 DBPs 纳入控制范围(包括三卤甲
其列为一组 必 选 的 遗 传 毒 性 测 试ꎬ我 国 亦 将 其 列
烷类、卤乙酸类、卤酸盐、卤乙腈等)ꎮ 近年来随着污
中文名
英文名
分子式
缩写
Chinese name
English name
Molecular formula
Abbrevation
一氯乙酰胺
Chloroacetamide
C2 H4 ClNO
CAcAm
79 ̄07 ̄2
二氯乙酰胺
Dichloroacetamide
C2 H3 Cl2 NO
DCAcAm
683 ̄72 ̄7
摘要: 卤代乙酰胺类(HAcAm)消毒副产物(DBPs)是饮用水中新兴含氮类 DBPs 之一ꎮ 为探究 HAcAms 的致突变性ꎬ选择小鼠
淋巴瘤细胞作为受试细胞ꎬ分别考察了一氯代乙酰胺、二氯代乙酰胺、三氯代乙酰胺、一碘代乙酰胺、二碘代乙酰胺(DIAcAms)
共 5 种 HAcAms 对小鼠淋巴瘤细胞 Tk 基因突变的影响ꎮ 结果表明ꎬ在 0.05 ~ 20 μmolL ̄1 暴露浓度下ꎬ5 种 HacAms 类 DBPs
min 灭活)制成ꎮ L5178Y 细胞液氮下保存ꎬ复苏后
常规开放式悬浮培养于 RPMI ̄10ꎬ在 37 ℃、5% CO 2
条件下培养至稳定的对数增长状态ꎬ细胞密度维持
在 1 × 10 6mL  ̄1 以下ꎬ 使用时传代不应超过 10 代ꎮ
CAS号203849-91-6_MMAD_参考用途MedBio
SAR407899 hydrochloride
SAR407899 hydrochloride
923262-96-8
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11452
ARRY-520 R enantiomer
ARRY-520 R enantiomer
885060-08-2
体内研究
得到的抗体-药物偶联物(ADC)在啮齿动物中显示出完全的肿瘤消退。它们在大鼠中也具有改善的毒理学特征[1]。
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11525
TAI-1
TAI-1
1334921-03-7
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
None
25mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11573
CFSE
CFSE
92557-80-7
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11446
Epothilone B (EPO906, Patupilone)
Epothilone B (EPO906, Patupilone)
10mg
≥98%
品牌
DOXYLAMINE-欧洲药典

Doxylamine hydrogen succinate EUROPEAN PHARMACOPOEIA8.0–symmetry factor :maximum 1.25for the peak due to doxycycline.Limits :–impurity A :not more than the area of the corresponding peak in the chromatogram obtained with reference solution (e)(2.0per cent),–impurity B :not more than the area of the corresponding peak in the chromatogram obtained with reference solution (e)(2.0per cent),–any other impurity :not more than 0.25times the area of the peak due to impurity A in the chromatogram obtained with reference solution (e)(0.5per cent),–disregard limit :0.05times the area of the peak due to impurity A in the chromatogram obtained with reference solution (e)(0.1per cent).Heavy metals (2.4.8):maximum 50ppm.0.5g complies with test C.Prepare the reference solution using 2.5mL of lead standard solution (10ppm Pb)R .Water (2.5.12):3.6per cent to 4.6per cent,determined on 0.200g.Sulfated ash (2.4.14):maximum 0.4per cent,determined on 1.0g.ASSAYLiquid chromatography (2.2.29)as described in the test for related substances with the following modification.Injection :test solution and reference solution (a).Calculate the percentage content of C 22H 24N 2O 8.STORAGEProtected from light.IMPURITIESA.R1=NH 2,R2=R5=H,R3=N(CH 3)2,R4=CH 3:(4S ,4a R ,5S ,5a R ,6S ,12a S )-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (6-epidoxycycline),B.R1=NH 2,R2=H,R3=N(CH 3)2,R4+R5=CH 2:(4S ,4a R ,5S ,5a R ,12a S )-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (metacycline),C.R1=NH 2,R2=N(CH 3)2,R3=R4=H,R5=CH 3:(4R ,4a R ,5S ,5a R ,6R ,12a S )-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epidoxycycline),D.R1=NH 2,R2=N(CH 3)2,R3=R5=H,R4=CH 3:(4R ,4a R ,5S ,5a R ,6S ,12a S )-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide (4-epi-6-epidoxycycline),E.R1=NH 2,R2=H,R3=N(CH 3)2,R4=OH,R5=CH 3:oxytetracycline,F.R1=CH 3,R2=R4=H,R3=N(CH 3)2,R5=CH 3:(4S ,4a R ,5S ,5a R ,6R ,12a S )-2-acetyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-4a,5a,6,12a-tetrahydrotetracene-1,11(4H ,5H )-dione (2-acetyl-2-decarbamoyldoxycycline).01/2013:1589DOXYLAMINE HYDROGENSUCCINATE DoxylaminihydrogenosuccinasC 21H 28N 2O 5M r 388.5[562-10-7]DEFINITIONN,N -Dimethyl-2-[(1RS )-1-phenyl-1-(pyridin-2-yl)ethoxy]ethanamine hydrogen butanedioate.Content :99.0per cent to 101.0per cent (anhydrous substance).CHARACTERSAppearance :white or almost white powder.Solubility :very soluble in water,freely soluble in ethanol (96per cent).It shows polymorphism (5.9).IDENTIFICATIONInfrared absorption spectrophotometry (2.2.24).Comparison :doxylamine hydrogen succinate CRS .If the spectra obtained show differences,dissolve the substance to be examined and the reference substance separately in methanol R ,evaporate to dryness and record new spectra using the residues.TESTSAppearance of solution .The solution is clear (2.2.1)and colourless (2.2.2,Method II ).Dissolve 0.4g in water R and dilute to 20mL with the same solvent.Related substances .Gas chromatography (2.2.28).Test solution .Dissolve 0.650g of the substance to be examined in 20mL of a 10.3g/L solution of hydrochloric acid R .Add 3mL of a 100g/L solution of sodium hydroxide R and extract with 3quantities,each of 25mL,of methylene chloride R .Combine the methylene chloride extracts and filter using hydrophobic phase-separation filter paper.Rinse the filter with 10mL of methylene chloride R and combine the rinsings with the methylene chloride extracts.Evaporate the solvent under reduced pressure at a temperature not exceeding 40°C.Dissolve the residue in 20.0mL of anhydrous ethanol R .Reference solution (a).Dilute 1.0mL of the test solution to 100.0mL with anhydrous ethanol R .Dilute 1.0mL of this solution to 10.0mL with anhydrous ethanol R .Reference solution (b).Dissolve 50mg of doxylamine for system suitability CRS (containing impurity C)in 10mL of a 10.3g/L solution of hydrochloric acid R .Add 1.5mL of a 100g/L solution of sodium hydroxide R and extract with 3quantities,each of 25mL,of methylene chloride R .Combine the methylene chloride extracts and filter using hydrophobic phase-separation filter paper.Rinse the filter with 10mL of methylene chloride R and combine the rinsings with the methylene chloride extracts.Evaporate the solvent under reduced pressure at a temperature not exceeding 40°C.Dissolve the residue in 5.0mL of anhydrous ethanol R .Column :–material :fused silica;–size :l =30m,Ø=0.53mm;2112See the information section on general monographs (cover pages)EUROPEAN PHARMACOPOEIA 8.0Droperidol–stationary phase :poly(dimethyl)(diphenyl)siloxane R (film thickness 1.5μm).Carrier gas :helium for chromatography R .Flow rate :7mL/min.Temperature :Time (min)Temperature(°C)Column0-12160→22012-27220Injection port 250Detector250Detection :flame ionisation.Injection :1μL.Identification of impurities :use the chromatogram supplied with doxylamine for system suitability CRS and thechromatogram obtained with reference solution (b)to identify the peak due to impurity C.Relative retention with reference to doxylamine (retention time =about 12min):impurity C =about 0.96.System suitability :reference solution (b):–resolution :minimum 1.5between the peaks due to impurity C and doxylamine.Limits :–impurity C :not more than 5times the area of the principal peak in the chromatogram obtained with reference solution (a)(0.5per cent);–unspecified impurities :for each impurity,not more than the area of the principal peak in the chromatogram obtained with reference solution (a)(0.10per cent);–total :not more than 7times the area of the principal peak in the chromatogram obtained with reference solution (a)(0.7per cent);–disregard limit :0.5times the area of the principal peak in the chromatogram obtained with reference solution (a)(0.05per cent).Water (2.5.12):maximum 0.5per cent,determined on 2.00g.Sulfated ash (2.4.14):maximum 0.1per cent,determined on 1.0g.ASSAYDissolve 0.150g in 50mL of anhydrous acetic acid R .Titrate with 0.1M perchloric acid ,determining the end-point potentiometrically (2.2.20).1mL of 0.1M perchloric acid is equivalent to 19.43mg of C 21H 28N 2O 5.IMPURITIESSpecified impurities :C.Other detectable impurities (the following substances would,if present at a sufficient level,be detected by one or other of the tests in the monograph.They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).It is therefore not necessary to identify these impurities for demonstration of compliance.See also 5.10.Control of impurities in substances for pharmaceutical use ):A,B,D.A.N,N -dimethyl-2-[(1RS )-1-phenyl-1-(pyridin-4-yl)ethoxy]ethanamine, B.(1RS)-1-phenyl-1-(pyridin-2-yl)ethanol,C.N,N -dimethyl-2-[(1RS )-1-phenyl-1-(pyridin-2-yl)methoxy]ethanamine,D.phenyl(pyridin-2-yl)methanone (2-benzoylpyridine).07/2011:1010DROPERIDOLDroperidolumC 22H 22FN 3O 2M r 379.4[548-73-2]DEFINITION1-[1-[4-(4-Fluorophenyl)-4-oxobutyl]-1,2,3,6-tetrahydropyridin-4-yl]-1,3-dihydro-2H -benzimidazol-2-one.Content :99.0per cent to 101.0per cent (dried substance).CHARACTERSAppearance :white or almost white powder.Solubility :practically insoluble in water,freely soluble in dimethylformamide and in methylene chloride,sparingly soluble in ethanol (96per cent).It shows polymorphism (5.9).IDENTIFICATION First identification:A .Second identification:B,C,D .A.Infrared absorption spectrophotometry (2.2.24).Comparison :droperidol CRS .If the spectra obtained show differences,dissolve the substance to be examined and the reference substance separately in the minimum volume of acetone R ,evaporate to dryness on a water-bath and record new spectra using the residues.B.Thin-layer chromatography (2.2.27).Test solution .Dissolve 30mg of the substance to be examined in the mobile phase and dilute to 10mL with the mobile phase.Reference solution (a).Dissolve 30mg of droperidol CRS in the mobile phase and dilute to 10mL with the mobile phase.General Notices (1)apply to all monographs and other texts2113。
苯乙胺类拟肾上腺素sm

中国药科大学 药物分析教研室
一、适用范围
1) 当含氮药物的pKb>8时,不能采用酸碱滴定,滴 定时需用非水溶剂增强突跃.
2) 常用溶剂: 酸性溶剂:冰醋酸(8<pKb<10)
冰醋酸-醋酐(10<pKb<12) 醋酐(pKb>12) 醋酐可以增加滴定突越,但是要注意在低 温条件下以防止氨基乙酰化。
5mg重酒石酸间羟胺
溶解
红紫色
脂肪伯氨基的专属反应
丙酮不含有甲醛
中国药科大学 药物分析教研室
R1
HN R2 OH R3 R3
苯乙胺类 R1 肾上腺素 异丙肾上腺素
HO HO HO HO HO
Cl
R2 CH3
H H
CH(CH3)2
盐酸多巴胺
克伦特罗
H
C(CH3)3
H
H
HO
麻黄碱
H 2N Cl
CH3
CH3
有机含氮类药物
1。芳香胺类 苯乙胺类 芳氧丙醇胺类 对氨基苯甲酸酯类 酰胺类 2。杂环类药物 吡啶类 巴比妥类 苯并二氮杂卓类 吩噻嗪类 奎啉,莨菪烷
3。生物碱类药物
芳香胺类
芳胺类
O
芳烃胺类
C OR2
H C R1 OH R3 H C N H R2
(1)
R1HN
对氨基苯甲酸酯类:局麻药
例:苯佐卡因, 盐酸普鲁卡因
样品浓度 测定波长 (mg/ml) (nm)
310 310 310 347 310 330
A 不得过0.05 不得大于0.05 不得大于0.20 不得大于0.06 不得大于0.10 不得大于0.47
HCl(9→2000) 2.0 2.0 2.0 1.5 0.24 20
