1-2_2004_jun_a
军用信息技术与软件标准精选(最新)

军用信息技术与软件标准精选(最新)GJZ102A《GJB/Z 102A-2012 军用软件安全性设计指南》GJ117《GJB/T117-1999 军用软件验证和确认计划指南》GJ136Z《GJB/Z 136-2003 Z 通用信息处理平台集成与运行指南》GJ140Z《GJB/Z140-2004 Z 电子对抗装备现场维修数据收集指南》GJ141Z《GJB/Z141-2004 Z 军用软件测试指南》GJ142Z《GJB/Z142-2004 Z 军用软件安全性分析指南》GJ143Z《GJB/Z143-2004 Z 军用智能辅助决策开发工具》GJ156Z《GJB/Z156-2011 Z 军事电子信息系统体系结构设计指南》GJ157Z《GJB/Z157-2011 Z 军用软件安全保证指南》GJZ161《GJB/Z 161-2012 军用软件可靠性评估指南》GJ229A《GJB 229A-2000 军用微波接力通信系统接口》GJ238A《GJB238A-1997 战术调频电台测量方法》GJ318《GJB318A-1997 战术调频电台通用规范》GJ322A《GJB322A-1998 军用计算机通用规范》GJ367A《GJB367A-2001 军用通信设备通用规范》GJ438B《GJB438B-2009 Z 军用软件开发文档通用要求》GJ662《GJB662A-1997 野战数字无线电接力设备的频繁段和容量系列》GJ663A《GJB 663A-2012 军用通信系统安全通用要求》GJ883A《GJB883A-2003 对流层散射通信系统通用规范》GJ915《GJB915A-1997 纤维光学试验方法》GJ1127《GJB 1127A-2003 机载短波单边带通信设备通用规范》GJ1128A《GJB 1128A-2002 机载超波电台通用规范》GJ1142.4《GJB 1142.4A-2004 野战地域通信系统通用要求第:回路群》GJ1142.5《GJB 1142.5A-2004 野战地域通信系统通用要求:单路有线入口》 GJ1145A《GJB1145A-2010 Z 通信和指挥自动化军工产品定型试验规程》GJ1211A《GJB 1211A-2007 野战用户电话交换机通用规范》GJ1268A《GJB1268A-2004 军用软件验收要求》GJ1427A《GJB1427A-1999 光纤总规范》GJ1428A《GJB1428A-1999 光缆总规范》GJ1567.2《GJB1567.2-1992 话音频带调制解调器的差错控制方法 用于带有异步至同步转换DCE的差错校正方法》GJ1659《GJB1659-1993 光纤光缆接头总规范》GJ1783《GJB1783-1993 硬波导组件总规范》GJ1901《GJB 1901A-2003 军用巨型计算机通用规范》GJ2077A《GJB 2077A-2007 短波自适应通信系统自动线路建立规程》GJ2088A《GJB 2088A-2002 压制性雷达干扰机通用规范》GJ2089A《GJB 2089A-2003 通信对抗监测分析接收机通用规范》GJ2090A《GJB 2090A-2003 瞄准式通信干扰机通用规范》GJ2101《GJB 2101A-2002 超短波对空指挥电台通用规范》GJ2242《GJB2242-1994 时统设备通用规范》GJ2434《GJB2434A-2004 军用软件产品评价》GJ2436《GJB2436-1995 天线术语》GJ2454《GJB 2454A-2003 军用光缆填充膏规范》GJ2763《GJB2763-1996 通信设备话音质量等级标准与评测方法》GJ2786A《GJB2786A-2009 Z 军用软件开发通用要求》GJ2824《GJB 2824-1997 军用数据安全要求》GJ2928A《GJB 2928A-2012 战术超短波跳频电台通用规范》GJ2929《GJB2929-1997 战术短波跳频电台通用规范》GJ2991A《GJB2991A-2008 Z B时间码接口终端通用规范》GJ3012A《GJB 3012A-2007 军用无线双工移动通信系统中心台信道机通用规范》GJ3083A《GJB 3083A-2007 军用无线双工移动通信系统移动用户台通用规范》 GJ3084A《GJB 3084A-2007 军用无线双工移动通信系统交换机通用规范》GJ3180《GJB 3180-1998 军用计算机容错要求与测评》GJ3181《GJB3181-1998 军用软件支持环境选用要求》GJ3629《GJB3629-1999 军用通信装备使用手册编写规定》GJ4072A《GJB4072A-2006 军用软件质量监督要求》GJ4195《GJB4195-2001 外设部件互连(PCI)总线》GJ4210.1《GJB4210.1-2001 军用无线电通信设备通用检验验收规程超短波电台》GJ4210.2《GJB4210.2-2001 军用无线电通信设备通用检验验收规程短波电台》 GJ4211《GJB4211-2001 军用通信台站通信电源系统通用要求》GJ4353《GJB4353-2002 关系数据库管理系统功能与性能测试要求》GJ4354《GJB4354-2002 关系数据库管理系统功能通用要求》GJ4371《GJB4371-2002 通信设备诊断知识数据结构》GJ4411《GJB 4411-2002 光缆组件通用规范》GJ4897《GJB 4897-2003 军用计算机病毒防治要求》GJ4951《GJB 4951-2003 军用通信装备维修手册编写规定》GJ4952《GJB 4952-2003 战术互联网通用要求》GJ5000A《GJB5000A-2008 Z 军用软件研制能力成熟度模型》GJ5024《GJB 5024-2003 军用光缆阻水纱规范》GJ5080Z《GJB 5080-2004 Z 军用通信设施雷电防护设计与使用要求》GJ5081Z《GJB 5081-2004 Z 军用ATM层信元传送性能测试通用要求》GJ5082Z《GJB 5082-2004 Z 战术无线通信系统软件通信体系结构》GJ5083Z《GJB 5083-2004 Z 核爆炸冲击波对地面野战通信装备的破坏等级及防护要求》GJ5084Z《GJB 5084-2004 Z 光纤通用数字通道故障检测》GJ5085Z《GJB 5085-2004 Z 军事综合信息网多协议标记交换互通技术要求》 GJ5086Z《GJB 5086-2004 Z 军事综合信息网路由交换设备通用技术要求》GJ5087Z《GJB 5087-2004 Z 指挥自动化网无线接入模式》GJ5088Z《GJB 5088-2004 Z 军用建筑物网络布线系统工程设计通用要求》GJ5095《GJB5095-2002 信息技术安全通用要求》GJ5169《GJB5169-2004 高性能计算机磁盘阵列通用规范》GJ5174《GJB5174-2004 短波/超短波有源测向天线参数系列》GJ5175《GJB5175-2004 合成孔径雷达数据处理系统通用要求》GJ5177《GJB5177-2004 军用通信器材出口包装要求》GJ5185《GJB5185-2004 雷达对抗数字瞬时测频接收机模块规范》GJ5187《GJB5187-2004 有源相控阵通信干扰天线通用规范》GJ5196《GJB5196-2004 短波通信对抗搜索分析接收机通用规范》GJ5234《GJB5234-2004 军用软件验证和确认》GJ5235《GJB5235-2004 军用软件配置管理》GJ5236《GJB5236-2004 军用软件质量度量》GJ5241《GJB5241-2004 概念建模语言IDEF1X的句法和语义》GJ5301《GJB5301-2004 军用无线双工移动通信系统网络管理和控制设备通用规范》GJ5368《GJB 5368-2005 计算机病毒防治系统技术要求》GJ5301《GJB5301-2004 军用无线双工移动通信系统网络管理和控制设备通用规范》GJ5371《GJB 5371.1-2005 信息技术安全评估准则:简介和一般模型》GJ5793《GJB5793-2006 Z 网络安全漏洞扫描分析产品测评方法》GJ5454Z《GJB5454-2005 Z 短波数字化抗干扰系列电台操作显示要求》GJ5455Z《GJB5455-2005 Z 无线综合接入系统通用规范》GJ5462Z《GJB5462-2005 Z 军用通信装备技术说明书编写规定》GJ5463Z《GJB5463-2005 Z 军用通信装备随机文件配发和使用要求》GJ5672《GJB5672-2006 Z 车载式通信对抗测向站通用规范》GJ5716《GJB5716-2006 Z 军用软件开发库、受控库和产品库通用要求》GJ5793《GJB5793-2006 Z 网络安全漏洞扫描分析产品测评方法》GJ5794.1《GJB5794.1-2006 Z 网络入侵检测产品测评方法第1部分:网络型产品》GJ5794.2《GJB5794.2-2006 Z 网络入侵检测产品测评方法第2部分:主机型产品》GJ5806Z《GJB5806-2006 Z 智能密钥卡通用规范》GJ5865K《GJB 5865-2006 K 线性令牌传递多路数据总线有效性测试方法》GJ5866K《GJB 5867-2006 K 航空单芯多模光纤光缆连接器规范》GJ5880K《GJB 5880-2006 K 软件配置管理》GJ5927K《GJB 5927-2007 军用无线双工移动通信系统合路器通用规范》GJ5928K《GJB 5928-2007 军用无线双工移动通信系统信道控制器通用规范》 GJ5930Z《GJB 5930-2007 军用电台配套设备品种系列》GJ5931Z《GJB 5931-2007 军用有中继海底光缆通信系统通用要求》GJ5932Z《GJB 5932-2007 军用搬移式全光中继设备规范》GJ5933Z《GJB5933-2007 Z 军用搬移式同步数字传送网设备规范》GJ5934Z《GJB 5934-2007 军用微波接力通信设备测试接口》GJ5935Z《GJB 5935-2007 军用低速声码器接口通用要求》GJ5942K《GJB 5942-2007 军用光接入网通用要求》GJ5950《GJB 5950-2007 战略卫星通信支线网Ku频段车载站通用规范》GJ5951《GJB 5951-2007 呼入军用电话网主叫标识传送要求》GJ5952《GJB 5952-2007 装甲综合通信车通用要求》GJ5954K《GJB 5954-2007 通信靶场通用试验规程 中长波电台》GJ5955K《GJB 5955-2007 通信靶场通用试验规程 化学电源》GJ5956K《GJB 5956-2007 通信靶场通用试验规程 通信车》GJ5957K《GJB 5957-2007 通信靶场通用试验规程 卫星通信系统地球站》GJ5958K《GJB 5958-2007 战场通信频率管理系统干扰频谱监测仪通用规范》 GJ5959K《GJB 5959-2007 战场通信频率管理系统频率管理终端通用规范》GJ5958K《GJB 5958-2007 战场通信频率管理系统干扰频谱监测仪通用规范》 GJ5959K《GJB 5959-2007 战场通信频率管理系统频率管理终端通用规范》GJ6321Z《GJB6321-2008 Z 军用计算机电磁泄漏干扰器通用规范》GJ6363Z《GJB6363-2008 Z 地空通信干扰设备定型试验规程》GJ6389Z《GJB6389-2008 Z 军用软件评审》GJ6411K《GJB6411-2008 K 光纤通道航空电子环境》GJ6741Z《GJB6741-2009 Z 数字通信干扰效果评定准则》GJ6919Z《GJB6919-2009 Z 导电纤维丝束性能测试评价方法》GJ6921Z《GJB6921-2009 Z 军用软件定性测评大纲编制要求》GJ6922Z《GJB6922-2009 Z 军用软件定性测评报告编制要求》GJ7082Z《GJB7082-2010 Z 卫星通信机载站天线分系统通用规范》GJ7093Z《GJB7093-2010 Z 软件通信体系结构中间件接口》GJ7094Z《GJB7094-2010 Z 软件通信体系结构波形装配与部署要求》GJ7095Z《GJB7095-2010 Z 软件通信系统结构硬件抽象层应用程序接口》GJ7096.1Z《GJB7096.1-2010 Z 软件通信体系结构标准符合性测试方法第1部分:通则》GJ7096.2Z《GJB7096.2-2010 Z 软件通信体系结构标准符合性测试方法第2部分:核心框架》GJ7096.3Z《GJB7096.3-2010 Z 软件通信体系结构标准符合性测试方法第3部分:资源组件》GJ7096.4Z《GJB7096.4-2010 Z 软件通信体系结构标准符合性测试方法第4部分:设备组件》GJ7096.5Z《GJB7096.5-2010 Z 软件通信体系结构标准符合性测试方法第5部分:波形》GJ7151Z《GJB7151-2011 Z 可信计算平台通用要求》GJ7170Z《GJB7170-2011 Z 网络安全管理类产品测评方法》GJ7174Z《GJB7174-2011 Z 抗拒绝服务类产品测评方法》GJ7249Z《GJB7249-2011 Z 信息安全管理体系要求》GJ7250Z《GJB7250-2011 Z 信息安全保障体系框架》GJ7562《GJB 7562-2012 军用计算机信息系统局域网防护通用要求》GJ7563《GJB 7563-2012 无线通信综合测试仪检定规程》GJ7700Z《GJB7700-2012 Z 军用数据库管理系统安全测评要求》H244《HB/Z244-1993 软件支持环境》H7691《HB7691-2001 三十二位计算机指令系统结构》H7692《HB7692-2001 三十二位机载计算机内总线》QJ3126A《QJ3126A-2008 航天软件产品保证要求》QJ3262《QJ 3262-2005 高可靠性实时嵌入式软件设计指南》SJ10367《SJ/T10367-1993 计算机过程控制软件开发规程》SJ10663《SJ/T10663-1995 光纤设备与部件测量方法》SJ10711《SJ/T10711-1996 移动通信设备标准试验条件》SJ10721《SJ/T10721-1996 公用移动通讯系统移动台技术要求和测量方法》 SJ11116《SJ/T11116-1997 光纤预制棒总规范》SJ11118《SJ/T11118-1997 8GHz数字微波通信设备通用技术条件》SJ11193《SJ/T11193-1998 微型数字电子计算机多媒体性能要求》SJ11201《SJ/T11201-1999 2000年符合性测试规范》SJ11228《SJ/T11228-2000 数字集群移动通信系统体制》SJ11229《SJ/T11229-2001 手持式个人信息处理设备中文应用程序接口规范》 SJ11240《SJ11240-2001 信息技术:汉字编码字符集(基本集)12点阵字型》 SJ11241《SJ11241-2001 信息技术:汉字编码字符集(基本集)14点阵字型》 SJ11242.1《SJ11242.1-2001 信息技术:通用多八位编码字符集(Ⅰ区)汉字64点阵字型:宋体》SJ11242.2《SJ11242.2-2001 信息技术:通用多八位编码字符集(Ⅰ区)汉字64点阵字型:黑体》SJ11242.3《SJ11242.3-2001 信息技术:通用多八位编码字符集(Ⅰ区)汉字64点阵字型:楷体》SJ11242.4《SJ11242.4-2001 信息技术:通用多八位编码字符集(Ⅰ区)汉字64点阵字型:仿宋体》SJ11243《SJ/T11243-2001触摸查询一体机通用规范》SJ11262《SJ/T11262-2002 互联网机顶盒通用规范》SJ11270《SJ/T11270-2002 信息技术鼠标器通用规范》SJ11271《SJ/T11271-2002 数字域名规范》SJ11289《SJ/Z11289-2003 面向对象领域工程指南》SJ20657《SJ 20657-1998 特警通信系统通用规范》SJ20682《SJ 20682-1998 Lx波段固态脉冲功率模块通用规范》SJ20686《SJ 20686-1998 中继信令处理模块通用规范》SJ20723《SJ20723-1998 GG6001型脉冲信号光电隔离组件详细规范》SJ20724《SJ20724-1998 GG240型多路高速数据光电隔离组件详细规范》SJ20726《SJ 20726-1999 GPS定时接收设备通用规范》SJ20727《SJ 20727-1999 舰载数字光接收机通用规范》SJ20728《SJ 20728-1999 低频/甚低频发射机通用规范》SJ20771《SJ20771-2000 军用通信系统音质的MOS评价法》SJ20772《SJ20772-2000 军用激光大气通信机规范》SJ20773《SJ20773-2000 野战光缆开口引接系统通用规范》SJ20775《SJ20775-2000 军用磁光盘通用规范》SJ20822《SJ20822-2002 信息技术:软件维护》SJ20823《SJ20823-2002 信息技术:软件生存周期过程配置管理》SJ20839《SJ20839-2002 长波地波传输道计算方法》SJ20840《SJ20840-2002 三军协同无线电通信系统与相关网系互连互通要求》 SJ20841《SJ20841-2002 II型无线双工移动通信系统保密设备接口要求》SJ20849《SJ20849-2002 军用通信网络管理系统通用安全要求》SJ20850《SJ20850-2002 军用UHF移动通信系统数字加密体制》SJ20851《SJ20851-2002 军用数字保密自动电话网密钥管理中心通用规范》 SJ20852《SJ20852-2002 军用通信系统音质MOS评价测试语音数据库》SJ20854《SJ20854-2002 数字视频光纤传输系统通用规范》SJ20855《SJ20855-2002 波分复及光纤通信系统通用规范》SJ20856《SJ20856-2002 高频串行调制解调器互操作性和性能要求》SJ20860《SJ 20860-2003 军用光缆引接设备通用规范》SJ20861《SJ 20861-2003 军用SDHB传送网技术规范》SJ20862《SJ 20862-2003 中长波战术电台通用规范》SJ20863《SJ 20863-2003 数字车内通话器通用规范》SJ20864《SJ 20864-2003 有源抗噪声送受话器组通用规范》SJ20876《SJ 20876-2003 军用测试接收机通用规范》SJ20880《SJ 20880-2003 军用信息传送安全标记》SJ20881《SJ 20881-2003 安全证书管理系统技术要求》SJ20932《SJ 20932-2005 超短波定向机通用规范》WJ431《WJ/Z431-2005 单兵综合作战信息系统安全设计要求》GA216.1《GA 216.1-1999 计算机信息系统安全产品部件:安全功能检测》GA243《GA243-2000 计算机病毒防治产品评级准则》GA715《GA/T 715-2007 公安信息系统应用开发管理规范》GA387《GA/T387-2002 计算机信息系统安全等级保护 网络技术要求》GA388《GA/T388-2002 计算机信息系统安全等级保护 操作系统技术要求》GA389《GA/T389-2002 计算机信息系统安全等级保护 数据库管理系统技术要求》GA390《GA/T390-2002 计算机信息系统安全等级保护 通用技术条件》GA391《GA/T391-2002 计算机信息系统安全等级保护 管理要求》GA609《GA/T 609-2006 互联网信息服务系统 安全保护技术措施 信息代码》 GA610《GA/T 610-2006 互联网信息服务系统 安全保护技术措施 数据格式》 GA611《GA/T 611-2006 互联网信息服务系统 安全保护技术措施技术要求》 GA612《GA/T 612-2006 互联网信息服务系统 安全保护技术措施通讯标准》 GA658《GA 658.1~10-2006 互联网公共上网服务场所信息安全管理系统 信息代码》GA659《GA 659.1~659.9-2006 互联网公共上网服务场所 信息安全管理系统 数据交换格式》GA660《GA 660-2006 互联网公共上网服务场所信息安全管理系统 上网服务场所端功能要求》GA661《GA 661-2006 互联网公共上网服务场所信息安全管理系统 远程通讯端功能要求》GA662《GA 662-2006 互联网公共上网服务场所信息安全管理系统上网服务场所端接口技术要求》GA663《GA 663-2006 互联网公共上网服务场所信息安全管理系统远程通讯端接口技术要求》GA671《GA/T 671-2006 信息安全技术 终端计算机系统安全等级技术要求》 GA672《GA/T 672-2006 信息安全技术 终端计算机系统安全等级评估准则》 GA681《GA/T 681-2007 信息安全技术 网关安全技术要求》GA682《GA/T 682-2006 信息安全技术 路由器安全技术要求》GA683《GA/T 683-2007 信息安全技术 防火墙安全技术要求》GA684《GA/T 684-2007 信息安全技术 交换器安全技术要求》GA685《GA/T 685-2007 信息安全技术 交换器安全评估准则》GA686《GA/T 686-2007 信息安全技术 虚拟专用网安全技术要求》GA687《GA/T 687-2007 信息安全技术 公钥基础设施安全技术要求》GA695《GA/T 695-2007 信息安全技术 网络通讯安全审计数据留存功能要求》 GA696《GA/T 696-2007 信息安全技术 单机防入侵产品安全功能要求》GA697《GA/T 697-2007 信息安全技术 静态网页恢复产品安全功能要求》GA698《GA/T 698-2007 信息安全技术 信息过滤产品安全功能要求》GA699《GA/T 699-2007 信息安全技术 计算机网络入侵报警通讯交换技术要求》GA700《GA/T 700-2007 信息安全技术 计算机网络入侵分级要求》GA708《GA/T 708-2007 信息安全技术 信息系统安全等级保护体系框架》GA709《GA/T 709-2007 信息安全技术 信息系统安全等级保护基本模型》GA710《GA/T 710-2007 信息安全技术 信息系统安全等级保护基本配置》GA711《GA/T 711-2007 信息安全技术 应用软件系统安全等级保护通用技术指南》GA712《GA/T 712-2007 信息安全技术 应用软件系统安全等级保护通用测试指南》GA713《GA/T 713-2007 信息安全技术 信息系统安全管理测评》GA849《GA 849-2009 移动终端病毒防治产品评级准则》GA855《GA/T 855-2009 公安信息网络课件制作规范》GA986《GA/T 986-2012 信息安全技术 反垃圾邮件产品安全技术要求》GA987《GA/T 987-2012 信息安全技术 USB移动储存管理系统安全技术要求》 GA988《GA/T 988-2012 信息安全技术 文件加密产品安全技术要求》。
SAE J 1231-2004

Available from SAE, 400 Commonwealth Drive, Warrendale, PA 150960001.
SAE J475—Screw Threads SAE J476—Dryseal Pipe Threads SAE J512—Automotive Tube Fittings SAE J514—Hydraulic Tube Fittings SAE J846—Coding Systems for Identification of Fluid Conductors and Connectors SAE J1508—Hose Clamp Specifications
Except for nominal sizes and thread specifications, dimensions and tolerances are given in SI units. Tolerance on all dimensions not otherwise limited shall be ±0.25 mm. Angular tolerance on axis of ends on elbows shall be ±2.50 degrees for sizes up to and including 9.52 mm and ±1.50 degrees for sizes larger than 9.52 mm.
3.3 The Oring boss thread dash sizes correspond to the number of sixteenth inch increments in the outside diameter of the tubing with which they are designed to be used.
Scorm1.2与Scorm2004区别

004 年1月30日,高级分布式学习组织发布了2004最新的共享内容对象参考模型(SCORM2004),以前被称为SCORM1.3版。
SCORM2004 版的重心,將完全放在教材的編序上,它的教学规则的设计更加严格,SCORM2004版更进一步整合目前由IMS全球学习联盟所发展的简易编序规范(Simple SequencingSpecification)。
这项规范提供了一个开放格式,可根据学员的表现订定课程进度,将为学员创造个人化的学习机制。
SCORM 1.2与SCORM 2004规范之初步比较:2004年是一个e-Learning标准的重要里程碑,ADL于1月30号终于推出令人期待的SCORM 2004的最新版本规范,SCORM 2004也就是众所周知的SCORM 1.3,就规范内容而言,其中可以归纳出三个最大的不同点,分别是:一、imsmanifest.xml档案中加入sequencing语法:除了原先在SCORM1.2规范中所提供的教材架构及教材实体档案连结等信息外,为了串连各个教材单元,增加了Sequencing的语法,这些语法和SCORM1.2所规范的语法并不冲突,而是以SCORM 1.2之语法为基础再外加于其上,不论SCORM 1.2或SCORM2004规范皆是以XML为实作方式,为了区别SCORM 1.2与SCORM2004中新增的sequencing语法,因此在imsmanfiest.xml档案中以imsss之名称空间作为区别。
二、在学习组件(Sharable Content Object, 简称SCO)中,SCORM 1.2 RTE(Run TimeEnvironment)定义了8个API(Application ProgrammingInterface)做为SCO与平台之间的沟通的管道,在SCORM 1.2 API采用AICC的CMI001规范,但在SCORM 2004API部分改采IEEE P1481.11.2之标准,简而言之API的名称在SCORM 2004规范中,有做部分的更动。
Hypogonadotropic Hypogonadism in Type 2

subnormal testosterone concentrations in these men are associated with a two to three times elevated risk of cardiovascular events and death in two early studies.Short-term studies of tes-tosterone therapy in hypogonadal men with type2diabetes have demonstrated an increase in insulin sensitivity and a decrease in waist circumference.However,the data on the effect of tes-tosterone replacement on glycemic control and cardiovascular risk factors such as cholesterol and C-reactive protein concentrations are inconsistent.As far as sexual function is concerned,testos-terone treatment increases libido but does not improve erectile dysfunction and thus,phospho-diesterase inhibitors may be required.Trials of a longer duration are clearly required to definitively establish the benefits and risks of testosterone replacement in patients with type2diabetes and low testosterone.(J Clin Endocrinol Metab96:2643–2651,2011)S ubnormal free testosterone concentrations in asso-ciation with inappropriately low LH and FSH con-centrations and a normal response to GnRH of LH and FSH in type2diabetes were first described in2004(1). These abnormalities were independent of the duration and severity of hyperglycemia[glycosylated hemoglo-bin(HbA1c)].Magnetic resonance imaging in these hy-pogonadal patients showed no abnormality in brain or the pituitary(1).This association of hypogonadotropic hypogonadism(HH)with type2diabetes has now been confirmed in several studies and is present in25–40% of these men(2–5).In this context,it is important that The Endocrine Society now recommends the measure-ment of testosterone in patients with type2diabetes on a routine basis(6).These observations were recently extended to younger patients with type2diabetes be-tween the ages of18and35yr who had HH at a rate of 33%when the usual normal range for middle age was employed,whereas the rate was58%when age-specific normal range for free testosterone for the young was em-ployed(7).With the advent of more specific liquid chro-matography tandem mass spectrometry assay for measur-ing total testosterone,the reference ranges for total and free testosterone have recently been revised downward. Using this methodology,in our most recent study,we have found that29%of men with type2diabetes have sub-normal free testosterone concentrations,as measured by equilibrium dialysis(8);25%had HH,whereas4%had hypergonadotropic hypogonadism.Type2diabetic men with low testosterone levels have also been found to have a high prevalence of symptoms suggestive of hypogonadism such as fatigability and erectile dysfunction(2).In all of the above studies,total testosterone and free testosterone concentrations wereISSN Print0021-972X ISSN Online1945-7197Printed in U.S.A.Copyright©2011by The Endocrine Societydoi:10.1210/jc.2010-2724Received November19,2010.Accepted July8,2011.Abbreviations:BMD,Bone mineral density;BMI,body mass index;CRP,C-reactive protein; HbA1c,glycosylated hemoglobin;HH,hypogonadotropic hypogonadism;HOMA-IR,ho-meostasis model assessment for insulin resistance;PSA,prostate-specific antigen.J Clin Endocrinol Metab,September2011,96(9):2643–2643inversely related to body mass index(BMI)and age. However,the presence of low testosterone concentra-tion was not entirely dependent upon obesity because 25%of nonobese patients(31%of lean and21%of overweight)also had HH(1).HH is relatively rare in type1diabetes and,therefore,is not a function of di-abetes or hyperglycemia per se(9).Thus,in view of the inverse relationship between BMI and testosterone con-centrations in both type1and type2diabetes,HH is probably related to insulin resistance(1,4,9).Previous studies have shown that hypogonadism is associated with upper abdominal adiposity,insulin resistance,and the metabolic syndrome(10,11).Treatment of systemic insulin resistance by rosiglitazone leads to a modest in-crease in testosterone concentrations in men with type 2diabetes(12),without the restoration of testosterone concentrations to normal.A recent study investigated the prevalence of low tes-tosterone concentrations in a large number of obese and diabetic men(mean age,60yr;range,45–96yr)(13);44% of diabetic and33%of age-matched nondiabetic men had subnormal free testosterone concentrations,respectively. Forty percent of obese men and50%of obese diabetic men had subnormal free testosterone concentrations.Thus, obesity is associated with a high prevalence of hypogo-nadism,and the presence of diabetes adds to that risk. Possible Pathophysiological Mechanisms Underlying HH in Type2DiabetesRole of estradiolBecause testosterone and androstenedione in the male can be converted to estradiol and estrone,respectively, through the action of aromatase in the mesenchymal cells and preadipocytes of adipose tissue,it has been suggested that excessive estrogen secretion due to aromatase activity in the obese may potentially suppress the hypothalamic secretion of GnRH(14).This hypothesis was examined in a recent study that compared the estradiol concentrations in240type2diabetic men with and without HH(8).Total estradiol concentrations were measured by immunoassay, and free estradiol concentrations were calculated using SHBG.Total and free estradiol concentrations in men with HH were significantly lower than in those without HH(8).To confirm these findings,total estradiol concen-trations were measured in a subset of102men by the liquid chromatography tandem mass spectrometry assay,and free estradiol concentrations were measured by equilib-rium dialysis.Estradiol concentrations were25%lower in men with HH.Free estradiol concentrations were directly related to free testosterone concentrations,irrespective of age or BMI.The diminished availability of the substrate, testosterone,may therefore be the major determinant fac-tor of estradiol concentrations in these men.A study in elderly men(European Male Ageing Study)has also found lower estradiol concentrations in hypogonadal men(15). Thus,it appears that the low testosterone concentrations in HH of diabetes,as in aging,are not the consequence of estradiol-dependent suppression of the hypothalamo-hy-pophyseal-gonadal axis.Furthermore,HH in type2dia-betic men with a normal weight is not likely to be associ-ated with increased estradiol concentrations(1).Role of insulin resistanceThe selective deletion of the insulin receptor from neu-rons in mice leads to a reduction in LH concentrations by 60–90%and low testosterone concentrations(16).These animals respond to GnRH challenge by normal or supra-normal release of LH.In addition,these animals had atro-phic seminiferous tubules with markedly impaired or ab-sent spermatogenesis.In addition,it is known that the incubation of hypothalamic neurons with insulin results in the facilitation of secretion of GnRH(17,18).Thus,in-sulin action and insulin responsiveness in the brain are necessary for the maintenance of the functional integrity of the hypothalamo-hypophyseal-gonadal axis.Role of inflammatory mediatorsTNF-␣and IL-1have been shown to suppress hypo-thalamic GnRH and LH secretion in experimental animals and in vitro(19,20).It is therefore relevant that C-reactive protein(CRP)concentrations are markedly increased in hypogonadal type2diabetic men compared with men with type2diabetes and normal testosterone(6.5vs.3.2 mg/liter)(21).These data were confirmed by another study from Australia in which the median CRP concen-tration in type2diabetic patients with low total testos-terone was7.7mg/liter compared with4.5mg/liter in men with normal testosterone(4).Free testosterone concen-trations were inversely related to CRP concentrations(rϭϪ0.27;Pϭ0.02).It is thus possible that inflammatory mediators may contribute to the suppression of the hypo-thalamo-hypophyseal axis and the syndrome of HH in type2diabetes.The presence of inflammation may also contribute to insulin resistance because several inflamma-tion-related mediators,such as suppressor of cytokine sig-naling-3,IB kinase,and c-Jun N-terminal kinase-1in-terfere with insulin signal transduction(22,23)and contribute to insulin resistance.These mediators are also known to be increased in obesity(24).In summary,it is likely that there are several interlinked causative mechanisms underlying HH in men with type2 diabetes.It should also be noted that human chorionic2644Dandona and Dhindsa Hypogonadism in Type2Diabetes J Clin Endocrinol Metab,September2011,96(9):2643–2651gonadotropin-induced testosterone secretion by Leydig cells is inversely related to insulin sensitivity(as measured by hyperinsulinemic euglycemic clamp)among men with varying degrees of glucose tolerance(25).Thus,the lesion resulting in hypogonadism in obesity and type2diabetes may occur at several levels of the hypothalamic-pitu-itary-gonadal axis.However,the absence of an increase in gonadotropin concentrations indicates that the pri-mary defect in type2diabetes and obesity is at the hy-pothalamo-hypophyseal level.What Comes First:Hypogonadism or Type 2Diabetes?Because even young men with type2diabetes and patients with newly discovered type2diabetes have a high prev-alence of HH and obesity is associated with HH,it is pos-sible that HH precedes diabetes.Several epidemiological studies have shown that low testosterone at baseline ap-proximately doubles the odds of development of type2 diabetes(26–28).The data,however,are more consistent with total testosterone than with free testosterone(29). It is possible that low SHBG concentrations may medi-ate a portion of this association.SHBG polymorphisms that lead to lower SHBG concentrations are strongly predictive of the development of type2diabetes, whereas those that lead to higher SHBG concentrations are protective(30,31).Does Hypogonadism Matter?Possible Consequences of Hypogonadism in Type2 DiabetesIt is well accepted that low testosterone concentrations are associated with symptoms such as fatigue,lack of libido, and erectile dysfunction.Recent studies have described pathophysiological effects of subnormal testosterone con-centrations beyond those related to sexual health,as dis-cussed below.Symptoms of sexual dysfunctionCross-sectional studies have found a high prevalence of low libido(64%),erectile dysfunction(74%),and fatigue (63%)in hypogonadal men with type2diabetes(2).How-ever,the presence of these symptoms was similarly high in eugonadal men with type2diabetes as well(48,65,and 57%,respectively).The treatment of erectile dysfunction with phosphodiesterase-5inhibitors such as sildenafil in men with type2diabetes is known to be not as effective as that in nondiabetic subjects(32).Cardiovascular diseaseRecent evidence from longitudinal observational stud-ies shows that low testosterone concentration is prospec-tively associated with an increase in the incidence of car-diovascular ughlin et al.(33)prospectively followed794elderly men(mean age,71yr)for20yr in a community setting.The hazard ratio for men in the lowest quartile of bioavailable testosterone was1.44for all-cause mortality and1.36for cardiovascular mortality.Another prospective study[Osteoporotic Fracture in Men(MrOS) Swedish cohort(34)]that included3014men(mean age, 75yr;mean follow-up,4.5yr)showed a65%increased risk of mortality in men with low free testosterone(Ͻ6.1 ng/dl).Subnormal free testosterone concentrations are as-sociated with a69%increased risk of stroke or transient ischemic attack(35).Many cross-sectional,retrospective, case-control and smaller studies have also demonstrated an association of low testosterone with increased mortal-ity(36–38).However,the relationship between cardio-vascular mortality and low testosterone was not seen in two longitudinal studies(39,40).These studies were done in relatively younger populations(mean ages,52and55 yr)and had much lower mortality rates,which can pos-sibly explain the lack of an association(39,40).A recent study in930men with coronary artery disease reported that a low testosterone at baseline was associated with increased mortality after7yr of follow-up(21vs. 12%)(41).Only one study has looked at the association between subnormal testosterone concentrations and car-diovascular mortality specifically in men with type2di-abetes(42):in153men with type2diabetes and known coronary artery disease,subnormal free testosterone con-centration at baseline increased cardiovascular mortality by three times over2yr.Insulin sensitivityHH in men with type2diabetes is associated with a higher BMI(3–4kg/m2),12%more sc fat mass(measured by dual-energy x-ray absorptiometry),and higher waist-to-hip ratio compared with eugonadal men with type2 diabetes(1,2,43).In one study involving type2diabetic men from the United Kingdom,74%of hypogonadal men were obese compared with54%of eugonadal men(2).As of yet,no study has measured visceral,im,or hepatic fat content in type2diabetic men with and without HH. Many studies have documented that hypogonadism is as-sociated with insulin resistance(reviewed in Refs.44and 45).No study has compared the insulin resistance in type 2diabetic men with subnormal or normal testosterone concentrations.J Clin Endocrinol Metab,September2011,96(9):2643–2645HematocritHypogonadal type2diabetic men have a lower hemat-ocrit than those with normal testosterone concentrations (21).The prevalence of normocytic normochromic ane-mia in such patients is38%compared with3%in those with normal testosterone concentrations.A large study (464men)also found a direct correlation between free testosterone concentrations and hemoglobin in men with type2diabetes and renal insufficiency(46).Testoster-one regulates erythropoiesis(47).However,it has not yet been determined whether the association of anemia with hypogonadism in men with type2diabetes is causal or is secondary to other confounding factors such as inflammation.In these men,hemoglobin is positively related to testosterone but negatively related to CRP concentrations(21).Bone densityHypogonadism is associated with a decrease in bone mineral density(BMD)and an increase in fracture rate (48,49).Furthermore,trabecular bone architecture(mea-sured by high-resolution magnetic resonance imaging)de-teriorates much more in hypogonadal men compared with eugonadal men(50).Hypogonadal men usually have lower estradiol concentrations compared with eugonadal men because testosterone is the substrate for estradiol for-mation by aromatization(15).In epidemiological studies, estradiol concentrations correlate more robustly with BMD than testosterone concentrations in men(51).This is especially true of trabecular bone.However,testoster-one appears to be an independent predictor of cortical bone density(52,53).One study in men with type2dia-betes has shown that free testosterone concentrations are positively associated with BMD in arms and ribs,but not with hip,spine,or total body BMD values(43).Another study has shown a positive relation of lumbar spine BMD with free testosterone concentrations in men with type2 diabetes(54).No study has evaluated the relation between BMD and free estradiol concentrations in these men.It is possible that BMD in men with type2diabetes might relate more strongly to estradiol than to testosterone con-centrations,as has been shown in elderly nondiabetic men. No data are available on the fracture rates of hypogonadal men with type2diabetes.Prostate-specific antigen(PSA)Type2diabetic men have20%lower PSA concentra-tions than nondiabetic men(55).PSA concentrations are lower in hypogonadal than in eugonadal type2diabetic men(0.89vs.1.1ng/ml)(56).It is interesting that the incidence of prostatic carcinoma is lower in men with di-abetes.This is in contrast to the increased incidence of cancer in diabetics in various organs including the co-lon,the kidney,the breast,the endometrium,and the pancreas(57).The diminished incidence of prostate cancer in diabetics may receive a contribution from the high prevalence of HH and low testosterone concentra-tions.However,epidemiological studies do not support a causative role of testosterone in prostate cancer in nondiabetic populations(58).Should Testosterone Be Measured inEvery Patient with Type2Diabetes? Because the frequency of subnormal free testosterone con-centrations in type2diabetes is at least25%,we believe that free testosterone concentration should be measured in every patient with type2diabetes.This is consistent with The Endocrine Society guidelines.The prevalence of hy-pothyroidism is between5and8%in this population,and yet we screen every one for this condition.An Androgen Deficiency in Aging Male(ADAM)questionnaire should be administered in every patient with a low testosterone so that the presence of clinical hypogonadism can be estab-lished.One can argue that if the case for the replacement of testosterone in patients with HH is not proven,as dis-cussed below,is there a case for measuring its concentra-tions in every patient with type2diabetes?We believe that there is because,like hypothyroidism,patients may slide gradually into this clinical state without any overt symp-toms that may be revealed through direct questioning.“Asymptomatic”men may realize that they had been symptomatic only after a trial with testosterone.Such pa-tients may potentially benefit from testosterone replace-ment therapy,as discussed below.Should Men with Type2Diabetes andLow Testosterone Be Replaced with Testosterone?Issues to Be Considered in View of the Above DataThe Endocrine Society recommends that men with low testosterone and symptoms of androgen deficiency be con-sidered for therapy with testosterone(6).The guidelines do not recommend treatment of asymptomatic men with low testosterone.The Institute of Medicine recommends that more short-term studies in selected populations should investigate the benefits and risks of testosterone therapy.Trials in men with type2diabetes and obesity are important in this regard because both are commonly as-sociated with hypogonadism.A few studies on testoster-one replacement in type2diabetic men with low testos-terone have emerged and are described below.2646Dandona and Dhindsa Hypogonadism in Type2Diabetes J Clin Endocrinol Metab,September2011,96(9):2643–2651Insulin resistanceThree studies have shown a decrease in insulin resis-tance after testosterone therapy in hypogonadal men with type2diabetes.Kapoor et al.(59)studied the effects of treatment with im testosterone for3months in24hy-pogonadal type2diabetic men in a placebo-controlled, double-blind,crossover trial.Homeostasis model assess-ment for insulin resistance(HOMA)-IR decreased by1.73 after testosterone therapy compared with placebo.In an-other trial,32men with the metabolic syndrome and newly diagnosed type2diabetes with total testosterone concentration of less than350ng/dl(12nmol/liter)were prescribed diet and exercise(60).Half of them were also given transdermal testosterone for1yr.Testosterone ther-apy resulted in greater improvements in insulin sensitivity (measured by HOMA-IR;Ϫ0.9)compared with diet and exercise alone.A prospective,randomized,double-blind multicenter trial of transdermal testosterone(3g metered-dose2%gel for1yr)therapy in220hypogonadal men with type2diabetes or metabolic syndrome has recently been published[Testosterone Replacement in Hypogo-nadal Men with Either Metabolic Syndrome or Type2 diabetes study(TIMES2)(61)].The primary endpoint of the study was a change in insulin sensitivity,as measured by HOMA-IR.Patients were evaluated every3months.A total of136men in the study had type2diabetes,176men had metabolic syndrome,and92men had both.Testos-terone therapy resulted in a15%(Pϭ0.01)decrease in HOMA-IR at6months and at1yr time-points in men with type2diabetes as well as in those with metabolic syn-drome.One study in lean hypogonadal type2diabetic men with a mean BMI of24kg/m2did not show any change in insulin sensitivity after treatment with low-dose im testosterone(100mg every3wk)for3month(62).This dose is inadequate and may account for the lack of effect. It is,however,possible that the change in insulin sen-sitivity due to testosterone therapy occurs only in obese, and presumably insulin-resistant,men.Thus,it appears that insulin resistance improves with testosterone ther-apy in obese men with type2diabetes.These studies have calculated HOMA-IR to measure insulin resis-tance.This needs to be confirmed by trials that use hy-perinsulinemic-euglycemic clamp methodology.It is also not clear whether the effect is due to a change in body composition or independently of it.Glycemic controlIn three of the above-mentioned studies,glycemic con-trol was also evaluated by measuring HbA1c and fasting glucose.The small study by Kapoor et al.(59)showed a decrease in fasting glucose(28mg/dl)and HbA1c(0.37%) compared with placebo with3months of testosterone re-placement.The trial in men with new onset type2diabetes with transdermal testosterone did show a decrease in HbA1c from7.5to6.3%over a period of1yr(60).This was in conjunction with diet and exercise,but no hypo-glycemic medications.The comparison group in this study was a diet and exercise group.There was a decrease in HbA1c from7.5to7.1%in this group.The mean fasting glucose decreased by34and29mg/dl in the testosterone and diet/exercise groups,respectively(Pϭ0.06for com-parison among groups).However,the larger trial (TIMES2)did not show a clear effect of testosterone re-placement on HbA1c(61).Medication changes were not allowed for the first6months of the study.Patients with type2diabetes showed a trend toward improvement in HbA1c at1yr(Ϫ0.4%;Pϭ0.057)but not at6months (Pϭ0.6).Although no changes were made in patient’s medications for the first6months,the study protocol al-lowed medication changes between6and12months; therefore,no clear conclusions can be made regarding the effect of testosterone therapy on glycemic control from this trial.There were no changes in fasting glucose or in-sulin.Thus,there appears to be a mild decrease in HbA1c with testosterone therapy in men with type2diabetes,but the data are inconsistent and currently testosterone re-placement cannot be recommended for glycemic control. Symptoms and sexual dysfunctionIn the TIMES2trial,there was an improvement in the International Index of Erectile Function score in the tes-tosterone replacement group,mainly due to an increase in sexual desire,but other symptoms did not change.The smaller trial of im testosterone by Kapoor et al.(59)in hypogonadal men with type2diabetes showed an im-provement in symptoms as measured by the ADAM ques-tionnaire.Although there are no specific studies assessing the effect of testosterone replacement on the effectiveness of phosphodiesterase IV inhibitors like sidenafil,studies in hypogonadal nondiabetics do show this benefit(63). Body composition and abdominal adiposity Heufelder et al.(60)showed a decrease in waist cir-cumference of14cm in men with new onset type2diabetes treated for1yr with transdermal testosterone,diet,and exercise.The control group that was prescribed only diet and exercise lost5cm.Kapoor et al.(59)showed a de-crease by1.63cm in waist circumference after im testos-terone treatment.In the TIMES2trial,there was a small but statistically significant decrease in waist circumference (0.8cm)in type2diabetic men treated with testosterone. Significantly,BMI did not change in any of the studies despite the decrease in abdominal girth.J Clin Endocrinol Metab,September2011,96(9):2643–2647Cardiovascular outcomesA recent meta-analysis of testosterone therapy trials ranging from3months to3yr did not show any change in the rates of death,myocardial infarctions,revasculariza-tion procedures,or cardiac arrhythmias compared with placebo/nonintervention groups(64).However,none of these trials was powered to show a difference.Surpris-ingly,a recent trial of testosterone replacement therapy designed to study the effects of testosterone replacement for6months on muscle mass and strength in elderly men (Ͼ65yr old)with limited mobility had to be discontinued prematurely due a higher incidence(22vs.5%)of cardio-vascular-related adverse events in the testosterone treat-ment arm compared with the placebo arm(65).This trial was not included in the previously mentioned meta-anal-ysis.The study population had a high prevalence of chronic conditions,and it is possible that the results could have been due to chance alone.However,other studies in elderly populations have not shown an increase in cardiac events after testosterone replacement(66–68).The TIMES2trial(61)reported that cardiovascular events oc-curred less commonly with testosterone than with placebo (4.6vs.10.7%;Pϭ0.095);however,this effect was short of significance.A recent study presented at the British En-docrine Societies meeting is of interest(69).This study investigated the effect of baseline testosterone concentra-tions and testosterone replacement therapy in hypogo-nadal men with type2diabetes on all-cause mortality.A total of578men with type2diabetes with a mean age of 61yr were followed for5.8Ϯ1.3yr;338men had normal testosterone concentrations at baseline;240were hypogo-nadal,of which58men received testosterone replacement therapy;and72men(12%)died during follow-up.The mortality rate in eugonadal men and untreated hypogo-nadal men was9and20%,respectively.Hypogonadal men treated with testosterone had a mortality rate of 8.6%,significantly lower than that in the untreated hy-pogonadal group.Testosterone replacement in the setting of heart failure has also recently been reported to have beneficial effects on exercise capacity,muscle strength, and HOMA-IR(70).One study showed a decrease of15mg/dl in total cholesterol but no change in low-density lipoprotein cholesterol,high-density lipoprotein cholesterol,or triglycerides after testosterone therapy for3months (59).In the TIMES2trial,men with metabolic syndrome had a15%decline in lipoprotein(a)and a7%decline in total and low-density lipoprotein concentrations.Men with type2diabetes had similar trends,but the results were not significant.There was,however,a6%decline in high-density lipoprotein concentrations in both the metabolic syndrome and type2diabetes groups(61).No changes have been seen in blood pressure after tes-tosterone treatment(59,61).Heufelder et al.showed a decrease in CRP concentra-tions(Ϫ0.5mg/dl)and an increase in adiponectin(0.9g/ml)after testosterone therapy(60).However,CRP, IL-6,resistin,and TNF-␣concentrations did not change after im testosterone replacement for3months in a trial by Kapoor et al.(71).There was also a decrease in adiponec-tin after testosterone therapy.The reasons for the discrep-ancies between studies are not clear but could be related to the differences in study design,route of testosterone ad-ministration,and duration of therapy.Safety issuesThe TIMES2trial(61)did not show an increase in age-adjusted PSA values.PSA concentrations exceeded normal limits in four subjects at12months(three in the testos-terone treatment arm and one in placebo).Mean PSA con-centrations did not change after1yr of therapy in the study by Heufelder et al.(60)either.In this context,it is impor-tant that the replacement of testosterone in hypogonadal patients in general does not lead to an increased risk of prostatic carcinoma,although the trials have been too lim-ited in duration and number of patients(64). ConclusionsHH is found in25%of men with type2diabetes.An additional4%have hypergonadotropic hypogonadism. Low testosterone concentrations in men with type2dia-betes are associated with an increased prevalence of symp-toms of hypogonadism,obesity,very high CRP concen-trations,mild anemia,and decreased BMD.In addition, these men have an elevated risk(two to three times)of cardiovascular events and death in two small studies. Short-term studies of testosterone therapy have demon-strated an increase in libido.In addition,there is an in-crease in insulin sensitivity.Some,but not all studies,have shown an improvement in glycemia,body composition, and cardiovascular risk factors such as cholesterol and CRP concentrations.Trials of a longer duration are clearly required to definitively establish the benefits and risks of testosterone replacement in patients with type2diabetes and HH.AcknowledgmentsAddress all correspondence and requests for reprints to: Paresh Dandona,B.Sc.,M.D.,D.Phil.(Oxon),F.R.C.P.,Di-rector,Diabetes-Endocrinology Center of Western New York,Chief of Endocrinology,State University of New York2648Dandona and Dhindsa Hypogonadism in Type2Diabetes J Clin Endocrinol Metab,September2011,96(9):2643–2651。
handout_1_-_Harvard_APA_referencing_guide
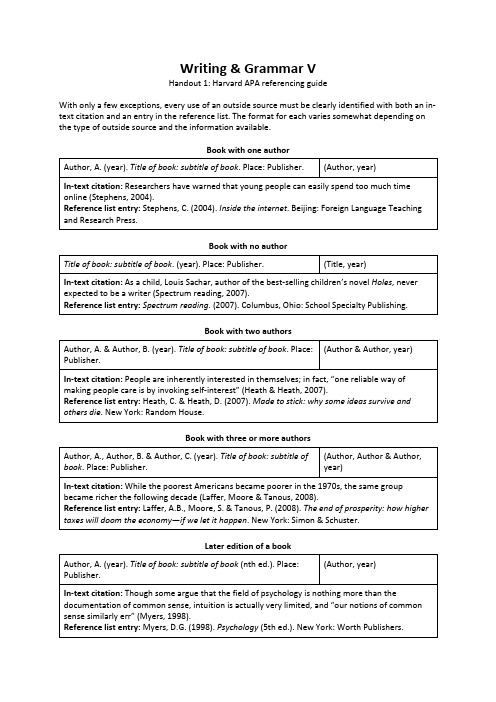
In-text citation: Researchers have warned that young people can easily spend too much time online (Stephens, 2004). Reference list entry: Stephens, C. (2004). Inside the internet. Beijing: Foreign Language Teaching and Research Press. Book with no author Title of book: subtitle of book. (year). Place: Publisher. (Title, year)
In-text citation: While the poorest Americans became poorer in the 1970s, the same group became richer the following decade (Laffer, Moore & Tanous, 2008). Reference list entry: Laffer, A.B., Moore, S. & Tanous, P. (2008). The end of prosperity: how higher taxes will doom the economy—if we let it happen. New York: Simon & Schuster. Later edition of a book Author, A. (year). Title of book: subtitle of book (nth ed.). Place: Publisher. (Author, year)
Journal of the Electrochemical Society 152(4) A653-A657 (200

Preparation and Characterization of Thin Film Li4Ti5O12Electrodes by Magnetron SputteringC.-L.Wang,a,b Y.C.Liao,a,c,z F.C.Hsu,a,b N.H.Tai,b and M.K.Wu a,c,da Materials Science Center,b Department of Materials Science and Engineering,andc Department ofPhysics,National Tsing Hua University,Hsinchu,Taiwand Institute of Physics,Academia Sinica,Nankang,Taipei,TaiwanThis paper reports that spinel-phase Li4Ti5O12thinfilms were successfully grown by radio frequency͑rf͒magnetron sputtering on an Au/Ti/SiO2/Si substrate.In this process,the buffer layer of gold serves as a template for the texture growth of Li4Ti5O12film. The growth temperature affects the microstructure and electrochemical characteristics of the depositedfilms.In our study,the spinel phase of Li4Ti5O12appears at deposition temperatures above500°C.The redox peaks in the cyclic voltammetry of the Li/Li4Ti5O12cell approach the typical value of1.55V as raising the deposition temperature.Moreover,the influences of the surface morphology of thefilm on the capacity were studied.They show that a columnar structure with high porosity was obtained in thefilm deposited above650°C.The columnar grains with good crystallinity of the deposited Li4Ti5O12enhance the capacity of the electrode.In this work,the capacity of53Ah/cm2m can be attained for thefilm with a thickness of230nm deposited at700°C.This study sheds light on the realization of a solid-state thinfilm battery and provides a possible solution of electrical power for a mobile integrated circuit chip.©2005The Electrochemical Society.͓DOI:10.1149/1.1861193͔All rights reserved.Manuscript submitted May17,2004;revised manuscript received September25,2004.Available electronically February10,2005.Solid-state thin-film rechargeable batteries have great advantages over other types of batteries due to theirflexibility,safety,and min-iaturization.There are many potential applications,such as smart cards,complementary metal oxide semiconductor͑CMOS͒-based integrated circuits,and microelectromechanical system͑MEMS͒de-vices.Lithium-transition-metal-oxide thinfilms have long been recognized as good candidates for battery electrode materials. For example,layered-phase LiCoO2,1LiNiO2,2and spinel-phase LiMn2O43with high voltage and stability were successfully used as the positive electrode in lithium ion batteries.Thackeray et al.4pro-posed that the ionic conductor Li4Ti5O12can also be a good elec-trode material for rechargeable lithium ion batteries.This material can be used as the negative electrode in the cell combined with other high voltage materials,such as LiCoO2and LiMn2O4.5,6The theo-retical capacity of Li4Ti5O12is175mAh/g͑60Ah/cm2m͒ac-cording to the following reaction suggested by Ohzuku et al.7͑Li͒8a͑Li1/3,Ti5/3͒16d O4+e−+Li+→͑Li2͒16c͑Li1/3,Ti5/3͒16d O4 Based on this equation,during the insertion process,lithium ions are in the tetrahedral͑8a͒sites and the guest lithium ions move to the octahedral͑16c͒sites,thus the total insertion capacity is determined by the number of free octahedral sites.The merits of adopting spinel Li4Ti5O12include itsflat electrical potential,nearly zero volume change,and excellent reversibility during the insertion/extraction process of Li ions.Li4Ti5O12thinfilm prepared by the sol-gel process for lithium battery electrodes has been reported in the past.8-10The sol-gel growth method is known to be difficult to incorporate into the con-ventional semiconductor process.This article reports the successful growth of spinel Li4Ti5O12thinfilms using a radio frequency͑RF͒magnetron sputtering technique.Through a series of examinations of the crystallinity,surface morphology,and electrochemical properties of the high quality Li4Ti5O12thinfilm,this paper demonstrates the great potential of Li4Ti5O12used as the material of the electrodes of solid-state thin-film batteries.ExperimentalLi4Ti5O12thinfilms were deposited by rf magnetron sputtering from a2in.diameter target onto Au͑100nm͒/ Ti͑10nm͒/SiO2/Si substrate maintained at various temperatures in the range of500-700°C.The substrate was adhered to the surface of the heater by a silver paste,and the temperature was determined bythe thermocouple in the heater.All substrates were cleaned in anorganic solvent͑acetone,methanol,isopropanol͒using a ultrasoniccleaner.The background pressure of the chamber before the heating of the substrate was less than10−5Torr.Aufilm functions as acurrent collector,while the Ti layer is the buffer layer that improves the adhesion between Au and SiO2.These two metal layers were deposited by standard dc magnetron sputtering.The Li4Ti5O12target was prepared by the solid-state reaction of TiO2and Li2CO3pow-ders.The mixed powder was calcined at800°C.Then it was re-ground,cold pelletized,and sintered at950°C in the ambient air.The X-ray diffraction͑XRD͒patterns showed a pure spinel phase with the space group Fd3¯m.Before the deposition,the target was presputtered for about20min.Thefilms were deposited at the pres-sure of30mTorr with the mixed Ar/O2͑3:2͒gas,and the power density was estimated to beϳ4W/cm2.Allfilms have a thickness around230nm.The crystal structure was examined by an X-ray diffractometer͑MAC Science͒employing Cu K␣line.The surface morphologies of Li4Ti5O12films deposited at various temperatureswere observed with a JEOL6500F scanning electron microscope ͑SEM͒.The electrochemical properties of the oxidefilms were measuredin a two-electrode cell at room temperature.The cell uses an oxidefilm as the working electrode combined with a lithium metal foil asthe counter electrode.In the cell,the electrolyte was prepared by adopting1M LiPF6dissolved in a solution of ethylene carbonate ͑EC͒and ethylmethyl carbonate͑EMC͒with the volume ratio of 1:1.All cells were assembled inside the argon-filled glove box.For galvanostatic cycling testing,cells were discharged and charged at the constant current density of10A/cm2between1.0and2.0V. Cyclic voltammetry͑CV͒was performed at a sweep rate of 0.5mV/s for the characterization of thefilm electrode.ResultsTexture and crystallinity of the as-deposited thinfilms.—Figure 1shows the X-ray diffraction͑XRD͒patterns of the Li4Ti5O12thin films grown on the Au/Ti/SiO2/Si substrate at different deposition temperatures.The well-crystallized thinfilm can be obtained at the deposition temperatures above500°C,and it exhibits a texture growth in the͑111͒plane.The texture growth along certain direc-tions is beneficial to the performance of the thin-film electrode.11 These thinfilms are colorless insulators,as expected.As the sub-strate temperature is increased,the crystallinity of thefilms is sub-z E-mail:ycliao@.tw Journal of The Electrochemical Society,152͑4͒A653-A657͑2005͒0013-4651/2005/152͑4͒/A653/5/$7.00©The Electrochemical Society,Inc.A653stantially improved,and it shows a highly preferred orientation along the ͓111͔,which is the major diffusion channel of Li ion.As mentioned earlier,the Au layer functions as the current col-lector for the electrode.Surprisingly,we find that this Au layer en-hances the crystallinity of the as-grown thin films,thus gold acts as a buffer layer between the substrate and the Li 4Ti 5O 12film as well.As shown in Fig.1,the Au layer also exhibits the preferred ͑111͒orientation at the deposition temperature.The preferred orientation of the Au buffer layer provides a better template to grow ͑111͒-oriented Li 4Ti 5O 12films.This statement is proved by comparing the XRD patterns between the Li 4Ti 5O 12films deposited on the sub-strates Au/Ti/SiO 2/Si and SiO 2/Si ͑Fig.2͒.The ͑111͒peak of Li 4Ti 5O 12film grown at 700°C is enhanced by using the Au/Ti/SiO 2/Si substrate,while the amorphous SiO 2layer did not act as a good template to deposit Li 4Ti 5O 12film.The preferred orientation along ͓111͔in the Au layer also plays an important role here.Our experimental results indicate that there is no preferred orientation in the Li 4Ti 5O 12film deposited on a gold foil without a specific texture.This gives further support to the above statement.The lattice constant of Li 4Ti 5O 12film deposited on Au/Ti/SiO 2/Si,calculated from the XRD data,does not show any specific trend with the deposition temperature.The average of cubic lattice constants of the four samples is 8.291Åwith a standard deviationof 0.006Å.This value is only 0.81%less than the bulk value ͑8.358Å͒of Li 4Ti 5O 12.Perhaps it is the strain of gold ͑2a 0=8.158Å,a 0is the cubic lattice constant of gold ͒that leads to this result.To conclude the discussion on the XRD data,the gold layer on the substrate can promote the texture growth of Li 4Ti 5O 12along ͓111͔.The evolvement of surface morphology of Li 4Ti 5O 12film with the deposition temperature is shown in Fig.3.There exists a transi-tion of surface morphology around the growth temperature of 650°C.At the deposition temperature of 600°C,the Li 4Ti 5O 12film is smooth and shows densely packed grains ͑Figs.3a and 4a ͒.Above 650°C,more dispersed island-like grains emerge in the film,as shown in the cross-sectional SEM image of the sample deposited at 700°C ͑Fig.4b ͒,and the film exhibits a rougher surface.The length scale of this porous structure is around 0.1-0.2m.This kind of structure does not exist in the Li 4Ti 5O 12film grown on the SiO 2/Si substrate at the same growth temperature,which is demonstrated in Fig.4c.The formation of these grain structures should be attributed to the presence of islands on the Au layer.These islands,which form preferentially along the ͓111͔plane,serve as the nucleation sites for the depositing Li 4Ti 5O 12materials.Consequently,the preferred ori-ented Li 4Ti 5O 12grains with good crystallinity and the island-like structure appear only on the Au-buffered substrate.This is consistent with our XRD results.Electrochemical measurement of the as-deposited thin films .—Figure 5shows the CVs obtained from the Li 4Ti 5O 12films grown at various substrate temperatures.All cyclic voltammogram measurements were operated in the potential range between 1.0and 2.0V at a scan rate of 0.5mV/s.The measurement results indicate that the primary insertion and extraction potential of Li ion are in a range between 1.5and 1.6V,which have been suggested resulting from the coexistence of the spinel phase and the rock-salt phase during the extraction and insertion processes of Li +ions.12The CV diagrams clearly show that the shape and peak current density of redox peaks depend on the growth temperature.As increasing the deposition temperature,the difference in the peak potential and the width of the redox peak reduce gradually.This reveals the better crystallinity of the film grown at a higher temperature.The value of the potential,which is 1.54and 1.59V in Li insertion and extraction of the film deposited at 700°C respectively,agrees with the typical value of Li 4Ti 5O 12.13This result indicates that the insertion and extraction of lithium ions are easier to accomplish in the film syn-thesized under higher deposition temperature.The observation is consistent with the previous data that the films grown at higher temperature exhibits better crystallinity and preferred orientation.These films provide more reversible channels for Li ions to diffuse in the three-dimensional framework of Li 4Ti 5O 12.14Figure 6shows the discharge behaviors between 1.0and 2.0V of the films deposited at various temperatures at the constant current density of 10A/cm 2.All these as-grown films show the potential plateau around 1.55V,which is the typical redox value of spinel-phase Li 4Ti 5O 12.The discharge capacity for the films deposited at 700°C is about 53Ah/cm 2m,and it is much greater than the films deposited at 600°C.These observations are consistent with the results of cyclic voltammograms,where the peak current density relating to the capacity is enhanced significantly from the deposition temperature of 600to 650°C.The capacity of the deposited thin film increases substantially as the deposition temperature over 650°C.However,the crystallinity does not change drastically above 650°C.This suggests that the crystallinity of the as-grown film is not the sole reason responsible for the large energy capacity.The plot of both ⌬2of ͑111͒diffraction peak and the discharge capacity confirms this suggestion further.In the left axis of Fig.7,it shows that the crystallinity of Li 4Ti 5O 12film improves gradually with rais-ing the growth temperature while there is a significant enhancement of discharge capacity above 650°C,as shown in the right axis.The transitions of electrochemical properties coincide with the transition of surface morphology ͑Fig.3and 4͒.It is apparent that thesurfaceFigure 1.XRD patterns of the Li 4Ti 5O 12films deposited on Au/Ti/SiO 2/Si at various deposition temperatures.It shows that both Li 4Ti 5O 12film and the gold layer have the preferred orientation ͑111͒.Figure 2.XRD patterns of the Li 4Ti 5O 12films on the two substrates Au/Ti/SiO 2/Si and SiO 2/Si grown at 700°C.The film on Au/Ti/SiO 2/Si has a much better crystallinity than the film on SiO 2/Si.A654Journal of The Electrochemical Society ,152͑4͒A653-A657͑2005͒morphology of the film also plays an important role on the capacity.Owing to the finite diffusion length of Li ions in Li 4Ti 5O 12,Li ions cannot fully penetrate into the grains.Those island-like grains that exist in the films deposited at higher temperatures provide much more effective area for the insertion of Li ions.This statement is inagreement with the previous study on the relationship between the charge capability and the particle size of Li 4Ti 5O 12.15In that paper,Kavan et al.showed that the charge capacity is proportional to the surface area of Li 4Ti 5O 12powder before the particle size reaches to few tens of nanometers.Therefore,the high capacity of Li 4Ti 5O 12film deposited on Au/Ti/SiO 2/Si at 700°C results from both the rougher island-like grains and the good crystallinity,and conse-quently,it has sharp redox peaks and a large capacity.ConclusionsSpinel-phase Li 4Ti 5O 12thin films are successfully grown by rf magnetron sputtering on Au/Ti/SiO 2/Si substrate.Thedeposi-Figure 3.SEM images of the surface morphology of Li 4Ti 5O 12thin films deposited on Au/Ti/SiO 2/Si at ͑a ͒600,͑b ͒650,and ͑c ͒700°C.The surface morphology transits to a rougher one at the deposition temperature above650°C.Figure 4.SEM image of the cross-sectional structure of Li 4Ti 5O 12thin films deposited at ͑a ͒600and ͑b ͒700on Au/Ti/SiO 2/Si and ͑c ͒700°C on SiO 2/Si.The film on Au/Ti/SiO 2/Si grown at 700°C has a disperse-like grain structure while the same one deposited at 600°C shows the close-packed grains.This is attributed to the effect of the gold layer because the film grown on SiO 2/Si at 700°C did not have a columnar structure.A655Journal of The Electrochemical Society ,152͑4͒A653-A657͑2005͒tion temperature influences the physical and electrochemical characteristics of the films profoundly.Li 4Ti 5O 12films grown on Au/Ti/SiO 2/Si can possess good crystallinity and proper surface morphology for the application in an electrode of a thin film ing the optimized Li 4Ti 5O 12thin film,the test cell of Li/Li 4Ti 5O 12exhibits sharp redox peaks and a large capacity.The capacity of this film estimated by the discharge curve is 53Ah/cm 2m,and this value is comparable to those elec-trodes prepared by other methods.Both CV diagrams and dis-charge curves show that thin film Li 4Ti 5O 12/Au/Ti/SiO 2/Si depos-ited by sputtering can be used as an excellent negative electrode in a lithium thin-film battery.The results of this study demonstrate the potential for the realization of lithium-based solid-state thin-film batteries.AcknowledgmentsThe authors thank Chen-En Wu for help taking the SEM images.We also thank Phillip Wu for help editing the English writing.This work is supported by the Taiwan National Science Council grant no.NSC91-2112-M-007-056.Figure 5.Cyclic voltammograms of Li 4Ti 5O 12thin films deposited on Au/Ti/SiO 2/Si at various deposition temperatures ͑a ͒600,͑b ͒650,and ͑c ͒700°C in 1M in LiPF 6/EC +EMC at 0.5mV/s.The peak current density is significantly enhanced above 650°C.The potentials of reduction peak ͑Li +insertion ͒and oxidization peak ͑Li +extraction ͒agree with otherstudies.Figure 6.Initial discharge curves of Li 4Ti 5O 12thin films deposited on Au/Ti/SiO 2/Si at various temperatures.The substantial increase of charge capacity indicates that there should be some transition around the deposition temperature of 650°C.The capacity of the film grown at 700°C reaches to nearly 90%of the theoretical value ͑60Ah/cm 2m ͒.Figure 7.The plot of both ⌬2of ͑111͒diffraction peak ͑left axis ͒and the discharge capacity ͑right axis ͒.It indicates that the enhancement of capacity of Li 4Ti 5O 12thin film does not totally result from the improvement of crys-tallinity.Because the discharge curve of the film deposited at 500°C did not have any observable plateau,the discharge capacity of this film is nominally zero in our measurement.A656Journal of The Electrochemical Society ,152͑4͒A653-A657͑2005͒National Tsing Hua University assisted in meeting the publication costs of this article.References1. C.N.Polo da Fonseca,J.Davalos,M.Kleinke,M.C.A.Fantini,and A.Gorenstein,J.Power Sources,81-82,575͑1999͒.2.M.Yoshimura,K.S.Han,and S.Tsurimoto,Solid State Ionics,106,39͑1998͒.3. F.K.Shokoohi,J.M.Tarascon,B.J.Wolkens,D.Guyomard,and C.C.Chang,J.Electrochem.Soc.,137,1845͑1992͒.4. E.Ferg,R.J.Gummow,A.de Kock,and M.M.Thackeray,J.Electrochem.Soc.,141,L147͑1994͒.5.N.Koshiba,K.Takada,M.Nakanishi,K.Chikayama,and Z.Takehara,DenkiKagaku oyobi Kogyo Butsuri Kagaku,62,970͑1994͒.6.G.X.Wang,D.H.Bradhurst,S.X.Dou,and H.K.Liu,J.Power Sources,83,156͑1999͒.7.T.Ohzuku,A.Ueda,and N.Yamamoto,J.Electrochem.Soc.,142,1431͑1995͒.8.Y.H.Rho,K.Kanamura,M.Fujisaki,J.Hamagami,S.Suda,and T.Umegaki,SolidState Ionics,151,151͑2002͒.9.L.Kavan and M.Grätzel,Electrochem.Solid-State Lett.,5,A39͑2002͒.10.Y.H.Rho,K.Kanamura,and T.Umegaki,Chem.Lett.,2001,1322.11.K.-F.Chiu,F.C.Hsu,G.S.Chen,and M.K.Wu,J.Electrochem.Soc.,150,503͑2003͒.12.S.Scharner,W.Weppner,and P.Schmid-Beurmann,J.Electrochem.Soc.,146,857͑1999͒.13. D.Peramunage and K.M.Abraham,J.Electrochem.Soc.,145,2609͑1998͒.14. C.-M.Shen,X.-G.Zhang,Y.-K.Zhou,and H.-L.Li,Mater.Chem.Phys.,78,437͑2002͒.15.L.Kavan,G.Procházka,T.M.Spitler,M.Kalbáč,M.Zakalová,T.Drezen,and M.Grätzel,J.Electrochem.Soc.,150,1000͑2003͒.A657Journal of The Electrochemical Society,152͑4͒A653-A657͑2005͒。
(整理)2004年数二真题及标准答案及解析.

2004年考硕数学(二)真题一. 填空题(本题共6小题,每小题4分,满分24分. 把答案填在题中横线上. )(1)设2(1)()lim1n n xf x nx →∞-=+, 则()f x 的间断点为x = .(2)设函数()y x 由参数方程 333131x t t y t t ⎧=++⎪⎨=-+⎪⎩ 确定, 则曲线()y y x =向上凸的x 取值范围为____..(3)1+∞=⎰_____..(4)设函数(,)z z x y =由方程232x zz ey -=+确定, 则3z zx y∂∂+=∂∂______. (5)微分方程3()20y x dx xdy +-=满足165x y ==的特解为_______. (6)设矩阵210120001A ⎛⎫ ⎪= ⎪ ⎪⎝⎭, 矩阵B 满足2ABA BA E **=+, 其中A *为A 的伴随矩阵, E 是单位矩阵, 则B =______-.二. 选择题(本题共8小题,每小题4分,满分32分. 每小题给出的四个选项中,只有一项符合题目要求, 把所选项前的字母填在题后的括号内. ) (7)把0x +→时的无穷小量2cos xt dt α=⎰, 2x β=⎰, 30t dt γ=⎰排列起来, 使排在后面的是前一个的高阶无穷小, 则正确的排列次序是(A ),,.αβγ (B ),,.αγβ(C ),,.βαγ (D ),,.βγα [](8)设()(1)f x x x =-, 则(A )0x =是()f x 的极值点, 但(0,0)不是曲线()y f x =的拐点. (B )0x =不是()f x 的极值点, 但(0,0)是曲线()y f x =的拐点. (C )0x =是()f x 的极值点, 且(0,0)是曲线()y f x =的拐点. (D )0x =不是()f x 的极值点, (0,0)也不是曲线()y f x =的拐点.[](9)lim ln (1)n n→∞+(A )221ln xdx ⎰. (B )212ln xdx ⎰.(C )212ln(1)x dx +⎰. (D )221ln(1)x dx +⎰ [](10)设函数()f x 连续, 且(0)0f '>, 则存在0δ>, 使得(A )()f x 在(0,)δ内单调增加. (B )()f x 在(,0)δ-内单调减小. (C )对任意的(0,)x δ∈有()(0)f x f >.(D )对任意的(,0)x δ∈-有()(0)f x f >.[](11)微分方程21sin y y x x ''+=++的特解形式可设为(A )2(sin cos )y ax bx c x A x B x *=++++. (B )2(sin cos )y x ax bx c A x B x *=++++. (C )2sin y ax bx c A x *=+++.(D )2cos y ax bx c A x *=+++[](12)设函数()f u 连续, 区域{}22(,)2D x y x y y =+≤, 则()Df xy dxdy ⎰⎰等于(A )11()dx f xy dy -⎰⎰.(B )2002()dy f xy dx ⎰⎰.(C )2sin 200(sin cos )d f r dr πθθθθ⎰⎰.(D )2sin 20(sin cos )d f r rdr πθθθθ⎰⎰[](13)设A 是3阶方阵, 将A 的第1列与第2列交换得B , 再把B 的第2列加到第3列得C , 则满足AQ C =的可逆矩阵Q 为(A )010100101⎛⎫ ⎪ ⎪ ⎪⎝⎭. (B )010101001⎛⎫ ⎪ ⎪ ⎪⎝⎭.(C )010100011⎛⎫ ⎪ ⎪ ⎪⎝⎭. (D )011100001⎛⎫ ⎪⎪ ⎪⎝⎭.[](14)设A ,B 为满足0AB =的任意两个非零矩阵, 则必有(A )A 的列向量组线性相关,B 的行向量组线性相关. (B )A 的列向量组线性相关,B 的列向量组线性相关. (C )A 的行向量组线性相关,B 的行向量组线性相关.(D )A 的行向量组线性相关,B 的列向量组线性相关.[]三. 解答题(本题共9小题,满分94分. 解答应写出文字说明、证明过程或演算步骤. )(15)(本题满分10分)求极限3012cos lim 13x x x x→⎡⎤+⎛⎫-⎢⎥ ⎪⎝⎭⎢⎥⎣⎦.(16)(本题满分10分)设函数()f x 在(,-∞+∞)上有定义, 在区间[0,2]上, 2()(4)f x x x =-, 若对任意的x 都满足()(2)f x k f x =+, 其中k 为常数.(Ⅰ)写出()f x 在[2,0]-上的表达式; (Ⅱ)问k 为何值时, ()f x 在0x =处可导. (17)(本题满分11分) 设2()sin x xf x t dt π+=⎰,(Ⅰ)证明()f x 是以π为周期的周期函数;(Ⅱ)求()f x 的值域.(18)(本题满分12分)曲线2x x e e y -+=与直线0,(0)x x t t ==>及0y =围成一曲边梯形. 该曲边梯形绕x 轴旋转一周得一旋转体, 其体积为()V t , 侧面积为()S t , 在x t =处的底面积为()F t .(Ⅰ)求()()S t V t 的值; (Ⅱ)计算极限()lim()t S t F t →+∞.(19)(本题满分12分)设2e a b e <<<, 证明2224ln ln ()b a b a e ->-. (20)(本题满分11分)某种飞机在机场降落时,为了减小滑行距离,在触地的瞬间,飞机尾部张开减速伞,以增大阻力,使飞机迅速减速并停下来.现有一质量为9000kg 的飞机,着陆时的水平速度为700/km h .经测试,减速伞打开后,飞机所受的总阻力与飞机的速度成正比(比例系数为66.010k =⨯).问从着陆点算起,飞机滑行的最长距离是多少?注 kg 表示千克,/km h 表示千米/小时. (21)(本题满分10分)设22(,)xyz f x y e =-,其中f 具有连续二阶偏导数,求2,,z z z x y x y∂∂∂∂∂∂∂. (22)(本题满分9分) 设有齐次线性方程组1234123412341234(1)0,2(2)220,33(3)30,444(4)0,a x x x x x a x x x x x a x x x x x a x ++++=⎧⎪++++=⎪⎨++++=⎪⎪++++=⎩ 试问a 取何值时, 该方程组有非零解, 并求出其通解.(23)(本题满分9分)设矩阵12314315a -⎛⎫ ⎪-- ⎪ ⎪⎝⎭的特征方程有一个二重根, 求a 的值, 并讨论A 是否可相似对角化.2004年考硕数学(二)真题评注一. 填空题(本题共6小题,每小题4分,满分24分. 把答案填在题中横线上. )(1)设2(1)()lim1n n xf x nx →∞-=+, 则()f x 的间断点为x = 0 .【分析】本题属于确定由极限定义的函数的连续性与间断点.对不同的x ,先用求极限的方法得出()f x 的表达式, 再讨论()f x 的间断点.【详解】显然当0x =时,()0f x =;当0x ≠时, 2221(1)(1)1()lim lim 11n n xn x x n f x nx x x x n →∞→∞--====++, 所以 ()f x 0,01,0x x x=⎧⎪=⎨≠⎪⎩,因为 001lim ()lim(0)x x f x f x→→==∞≠ 故 0x =为()f x 的间断点.(2)设函数()y x 由参数方程 333131x t t y t t ⎧=++⎪⎨=-+⎪⎩ 确定, 则曲线()y y x =向上凸的x 取值范围为1-∞∞(,)(或(-,1]).【分析】判别由参数方程定义的曲线的凹凸性,先用由 ()()x x t y y t =⎧⎨=⎩定义的 223()()()()(())d y y t x t x t y t dx x t ''''''-=' 求出二阶导数,再由 220d ydx< 确定x 的取值范围. 【详解】 22222331213311dydy t t dt dx dx t t t dt--====-+++,222223214113(1)3(1)d y d dy dt tdt dx dx dx t t t '⎛⎫⎛⎫==-⋅= ⎪ ⎪+++⎝⎭⎝⎭, 令220d ydx < ⇒ 0t <.又 331x t t =++ 单调增, 在 0t <时, (,1)x ∈-∞.(0t =时,1x =⇒x ∈(,1]-∞时,曲线凸.)【评注】本题属新题型.已考过的题型有求参数方程所确定的函数的二阶导数, 如1989、1991、1994、2003数二考题,也考过函数的凹凸性.(3)1+∞=⎰2π.【分析】利用变量代换法和形式上的牛顿莱布尼兹公式可得所求的广义积分值. 【详解1】22100sec tan sec tan 2t t dt dt t t πππ+∞⋅==⋅⎰⎰⎰.【详解2】11201101)arcsin 2dt tt π+∞-===⎰⎰⎰.【评注】本题为混合广义积分的基本计算题,主要考查广义积分(或定积分)的换元积分法.(4)设函数(,)z z x y =由方程232x zz ey -=+确定, 则3z zx y∂∂+=∂∂2.【分析】此题可利用复合函数求偏导法、公式法或全微分公式求解. 【详解1】在 232x zz e y -=+ 的两边分别对x ,y 求偏导,z 为,x y 的函数.23(23)x z z z e x x-∂∂=-∂∂,23(3)2x z z ze y y-∂∂=-+∂∂, 从而 2323213x zx zz e x e --∂=∂+,23213x z z y e-∂=∂+ 所以 2323132213x zx zz z e x y e--∂∂++=⋅=∂∂+ 【详解2】令 23(,,)20x zF x y z e y z -=+-=则232x z F e x -∂=⋅∂, 2F y ∂=∂, 23(3)1x z Fe z-∂=--∂2323232322(13)13x z x zx z x z Fz e e x F x e ez----∂∂⋅∂∴=-=-=∂∂-++∂, 232322(13)13x z x z F z y F y e ez--∂∂∂=-=-=∂∂-++∂, 从而 232323313221313x z x zx z z z e x y ee ---⎛⎫∂∂+=+= ⎪∂∂++⎝⎭【详解3】利用全微分公式,得23(23)2x z dz e dx dz dy -=-+2323223x zx z e dx dy e dz --=+-2323(13)22x zx z edz e dx dy --+=+232323221313x z x z x ze dz dx dy e e ---∴=+++ 即 2323213x z x z z e x e--∂=∂+, 23213x z z y e -∂=∂+ 从而 32z zx y∂∂+=∂∂ 【评注】此题属于典型的隐函数求偏导.(5)微分方程3()20y x dx xdy +-=满足165x y ==的特解为315y x =.【分析】此题为一阶线性方程的初值问题.可以利用常数变易法或公式法求出方程的通解,再利用初值条件确定通解中的任意常数而得特解.【详解1】原方程变形为 21122dy y x dx x -=, 先求齐次方程102dy y dx x-= 的通解:12dy dx y x= 积分得 1ln ln ln 2y x c =+y ⇒=设(y c x =,代入方程得211(((22c x c x c x x x '= 从而 321()2c x x '=,积分得 352211()25c x x dx C x C =+=+⎰,于是非齐次方程的通解为53211()55y x C x =+=1615x yC ==⇒=,故所求通解为 315y x =.【详解2】原方程变形为 21122dy y x dx x -=,由一阶线性方程通解公式得1122212dx dx x x y e x e dx C -⎡⎤⎰⎰=+⎢⎥⎣⎦⎰11ln ln 22212x x ex edx C -⎡⎤=+⎢⎥⎣⎦⎰35221125x dx C x C ⎤⎤=+=+⎥⎢⎥⎦⎦⎰6(1)15y C =⇒=,从而所求的解为 315y x =.【评注】此题为求解一阶线性方程的常规题.(6)设矩阵210120001A ⎛⎫ ⎪= ⎪ ⎪⎝⎭, 矩阵B 满足2ABA BA E **=+, 其中A *为A 的伴随矩阵, E 是单位矩阵, 则B =19.【分析】利用伴随矩阵的性质及矩阵乘积的行列式性质求行列式的值.【详解1】 2ABA BA E **=+ 2A B A B A E **⇔-=,(2)A E BA E *⇔-=,21A E B A E *∴-==, 221111010(1)(1)392100001B A E AA *====-⋅---. 【详解2】由1A A A *-=,得 11122ABA BA E AB A A B A A AA **---=+⇒=+2A AB A B A ⇒=+ (2)A A E B A ⇒-= 32AA EB A⇒-= 21192B A A E∴==- 【评注】此题是由矩阵方程及矩阵的运算法则求行列式值的一般题型,考点是伴随矩阵的性质和矩阵乘积的行列式.二. 选择题(本题共8小题,每小题4分,满分32分. 每小题给出的四个选项中,只有一项符合题目要求, 把所选项前的字母填在题后的括号内. ) (7)把0x +→时的无穷小量2cos xt dt α=⎰,2x β=⎰, 30t dt γ=⎰排列起来, 使排在后面的是前一个的高阶无穷小, 则正确的排列次序是(A ),,.αβγ (B ),,.αγβ(C ),,.βαγ (D ),,.βγα[]B【分析】对与变限积分有关的极限问题,一般可利用洛必塔法则实现对变限积分的求导并结合无穷小代换求解.【详解】302000lim limcos x x x t dtt dtγα++→→=⎰⎰30lim x +→=320lim lim 02x x x x++→→===, 即 o ()γα=.又2000tan lim lim x x x βγ++→→=23002tan 22lim lim 01sin 2x x x x x x x ++→→⋅===, 即 o ()βγ=.从而按要求排列的顺序为αγβ、、, 故选(B ).【评注】此题为比较由变限积分定义的无穷小阶的常规题. (8)设()(1)f x x x =-, 则(A )0x =是()f x 的极值点, 但(0,0)不是曲线()y f x =的拐点. (B )0x =不是()f x 的极值点, 但(0,0)是曲线()y f x =的拐点. (C )0x =是()f x 的极值点, 且(0,0)是曲线()y f x =的拐点. (D )0x =不是()f x 的极值点, (0,0)也不是曲线()y f x =的拐点.[]C【分析】求分段函数的极值点与拐点, 按要求只需讨论0x =两方()f x ', ()f x ''的符号.【详解】 ()f x =(1),10(1),01x x x x x x ---<≤⎧⎨-<<⎩,()f x '=12,1012,01x x x x -+-<<⎧⎨-<<⎩,()f x ''=2,102,01x x -<<⎧⎨-<<⎩,从而10x -<<时, ()f x 凹, 10x >>时, ()f x 凸, 于是(0,0)为拐点.又(0)0f =, 01x ≠、时, ()0f x >, 从而0x =为极小值点.所以, 0x =是极值点, (0,0)是曲线()y f x =的拐点, 故选(C ).【评注】此题是判定分段函数的极值点与拐点的常规题目 (9)lim ln (1)n n→∞+(A )221ln xdx ⎰. (B )212ln xdx ⎰.(C )212ln(1)x dx +⎰. (D )221ln (1)x dx +⎰ []B【分析】将原极限变型,使其对应一函数在一区间上的积分和式.作变换后,从四个选项中选出正确的. 【详解】 22lim (1)n n→∞+212lim ln (1)(1)(1)nn nn nn →∞⎡⎤=+++⎢⎥⎣⎦212limln(1)ln(1)(1)n n n n n n →∞⎡⎤=++++++⎢⎥⎣⎦11lim 2ln(1)nn i i n n →∞==+∑ 102ln(1)x dx =+⎰2112ln x t tdt +=⎰212ln xdx =⎰故选(B ).【评注】此题是将无穷和式的极限化为定积分的题型,值得注意的是化为定积分后还必须作一变换,才能化为四选项之一.(10)设函数()f x 连续, 且(0)0f '>, 则存在0δ>, 使得(A )()f x 在(0,)δ内单调增加. (B )()f x 在(,0)δ-内单调减小. (C )对任意的(0,)x δ∈有()(0)f x f >.(D )对任意的(,0)x δ∈-有()(0)f x f >.[]C【分析】可借助于导数的定义及极限的性质讨论函数()f x 在0x =附近的局部性质. 【详解】由导数的定义知 0()(0)(0)lim00x f x f f x →-'=>-,由极限的性质, 0δ∃>, 使x δ<时, 有()(0)0f x f x->即0x δ>>时, ()(0)f x f >, 0x δ-<<时, ()(0)f x f <, 故选(C ).【评注】此题是利用导数的定义和极限的性质讨论抽象函数在某一点附近的性质.(11)微分方程21sin y y x x ''+=++的特解形式可设为(A )2(sin cos )y ax bx c x A x B x *=++++. (B )2(sin cos )y x ax bx c A x B x *=++++. (C )2sin y ax bx c A x *=+++.(D )2cos y ax bx c A x *=+++[]A【分析】利用待定系数法确定二阶常系数线性非齐次方程特解的形式. 【详解】对应齐次方程 0y y ''+= 的特征方程为 210λ+=, 特征根为 i λ=±,对 2021(1)y y x e x ''+=+=+ 而言, 因0不是特征根, 从而其特解形式可设为21y ax bx c *=++对 sin ()ixm y y x I e ''+==, 因i 为特征根, 从而其特解形式可设为2(sin cos )y x A x B x *=+从而 21sin y y x x ''+=++ 的特解形式可设为xy2(sin cos )y ax bx c x A x B x *=++++【评注】这是一道求二阶常系数线性非齐次方程特解的典型题,此题的考点是二阶常系数线性方程解的结构及非齐次方程特解的形式.(12)设函数()f u 连续, 区域{}22(,)2D x y x y y =+≤, 则()Df xy dxdy ⎰⎰等于(A)11()dx f xy dy -⎰⎰. (B )2002()dy f xy dx ⎰⎰.(C )2sin 200(sin cos )d f r dr πθθθθ⎰⎰.(D )2sin 20(sin cos )d f r rdr πθθθθ⎰⎰[]D【分析】将二重积分化为累次积分的方法是:先画出积分区域的示意图,再选择直角坐标系和极坐标系,并在两种坐标系下化为累次积分.【详解】积分区域见图. 在直角坐标系下,20()()Df xy dxdy dy f xy dx =⎰⎰⎰⎰1111()dx f xy dy -=⎰⎰故应排除(A )、(B ). 在极坐标系下, cos sin x r y r θθ=⎧⎨=⎩ ,2sin 20()(sin cos )Df xy dxdy d f r rdr πθθθθ=⎰⎰⎰⎰,故应选(D ).【评注】此题是将二重积分化为累次积分的常规题,关键在于确定累次积分的积分限.(13)设A 是3阶方阵, 将A 的第1列与第2列交换得B , 再把B 的第2列加到第3列得C , 则满足AQ C =的可逆矩阵Q 为(A )010100101⎛⎫ ⎪ ⎪ ⎪⎝⎭. (B )010101001⎛⎫ ⎪ ⎪ ⎪⎝⎭.(C )010100011⎛⎫ ⎪ ⎪ ⎪⎝⎭. (D )011100001⎛⎫ ⎪⎪ ⎪⎝⎭.[]D【分析】根据矩阵的初等变换与初等矩阵之间的关系,对题中给出的行(列)变换通过左(右)乘一相应的初等矩阵来实现.【详解】由题意 010100001B A ⎛⎫ ⎪= ⎪ ⎪⎝⎭, 100011001C B ⎛⎫⎪= ⎪ ⎪⎝⎭,010100100011001001C A ⎛⎫⎛⎫ ⎪⎪∴= ⎪⎪ ⎪⎪⎝⎭⎝⎭011100001A AQ ⎛⎫ ⎪== ⎪ ⎪⎝⎭,从而 011100001Q ⎛⎫⎪= ⎪ ⎪⎝⎭,故选(D ).【评注】此题的考点是初等变换与初等矩阵的关系,抽象矩阵的行列初等变换可通过左、右乘相应的初等矩阵来实现.(14)设A ,B 为满足0AB =的任意两个非零矩阵, 则必有(A )A 的列向量组线性相关,B 的行向量组线性相关. (B )A 的列向量组线性相关,B 的列向量组线性相关. (C )A 的行向量组线性相关,B 的行向量组线性相关.(D )A 的行向量组线性相关,B 的列向量组线性相关.[]A【分析】将A 写成行矩阵, 可讨论A 列向量组的线性相关性.将B 写成列矩阵, 可讨论B 行向量组的线性相关性.【详解】设 (),i j l m A a ⨯=()i j m n B b ⨯=, 记 ()12m A A A A =0AB = ⇒()11121212221212n n m m m mn b b b b b b A A A bb b ⎛⎫ ⎪ ⎪ ⎪⋅⋅⋅ ⎪ ⎪⎝⎭()1111110m m n mn m b A b A b A b A =++++= (1)由于0B ≠, 所以至少有一 0i j b ≠(1,1i m j n ≤≤≤≤), 从而由(1)知, 112210j j i j i m m b A b A b A b A +++++=,于是 12,,,m A A A 线性相关.又记 12m B B B B ⎛⎫⎪ ⎪= ⎪ ⎪ ⎪⎝⎭,则0AB = ⇒11121121222212m m l l l m m a a a B a a a B a a a B ⎛⎫⎛⎫⎪ ⎪ ⎪ ⎪ ⎪ ⎪⋅⋅⋅ ⎪ ⎪ ⎪ ⎪⎝⎭⎝⎭1111221211222211220m m m m l l l m m a B a B a B a B a B a B a B a B a B +++⎛⎫ ⎪+++ ⎪==⎪ ⎪ ⎪+++⎝⎭由于0A ≠,则至少存在一 0i j a ≠(1,1i l j m ≤≤≤≤),使 11220i i i j j im m a B a B a B a B ++++=,从而 12,,,m B B B 线性相关,故应选(A ).【评注】此题的考点是分块矩阵和向量组的线性相关性,此题也可以利用齐次线性方程组的理论求解. 三. 解答题(本题共9小题,满分94分. 解答应写出文字说明、证明过程或演算步骤. )(15)(本题满分10分)求极限3012cos lim 13x x x x→⎡⎤+⎛⎫-⎢⎥ ⎪⎝⎭⎢⎥⎣⎦.【分析】此极限属于型未定式.可利用罗必塔法则,并结合无穷小代换求解. 【详解1】 原式2cos ln 331limx x x ex+⎛⎫ ⎪⎝⎭→-=202cos ln 3lim x x x→+⎛⎫ ⎪⎝⎭=20ln 2cos ln 3lim x x x→+-=() 01sin 2cos lim 2x x x x →⋅-+=()011sin 1lim 22cos 6x x x x →=-⋅=-+【详解2】 原式2cos ln 331limx x x ex+⎛⎫⎪⎝⎭→-=202cos ln 3lim x x x→+⎛⎫ ⎪⎝⎭=20cos 1ln 3lim x x x→-+=(1) 20cos 11lim 36x x x →-==-【评注】此题为求未定式极限的常见题型.在求极限时,要注意将罗必塔法则和无穷小代换结合,以简化运算.(16)(本题满分10分)设函数()f x 在(,-∞+∞)上有定义, 在区间[0,2]上, 2()(4)f x x x =-, 若对任意的x 都满足()(2)f x k f x =+, 其中k 为常数.(Ⅰ)写出()f x 在[2,0]-上的表达式; (Ⅱ)问k 为何值时, ()f x 在0x =处可导.【分析】分段函数在分段点的可导性只能用导数定义讨论. 【详解】(Ⅰ)当20x -≤<,即022x ≤+<时,()(2)f x k f x =+2(2)[(2)4](2)(4)k x x kx x x =++-=++.(Ⅱ)由题设知 (0)0f =.200()(0)(4)(0)lim lim 40x x f x f x x f x x+++→→--'===-- 00()(0)(2)(4)(0)lim lim 80x x f x f kx x x f k x x---→→-++'===-. 令(0)(0)f f -+''=, 得12k =-. 即当12k =-时, ()f x 在0x =处可导. 【评注】此题的考点是用定义讨论分段函数的可导性. (17)(本题满分11分) 设2()sin x xf x t dt π+=⎰,(Ⅰ)证明()f x 是以π为周期的周期函数; (Ⅱ)求()f x 的值域.【分析】利用变量代换讨论变限积分定义的函数的周期性,利用求函数最值的方法讨论函数的值域. 【详解】 (Ⅰ) 32()sin x x f x t dt πππ+++=⎰,设t u π=+, 则有22()sin()sin ()x x xxf x u du u du f x ππππ+++=+==⎰⎰,故()f x 是以π为周期的周期函数.(Ⅱ)因为sin x 在(,)-∞+∞上连续且周期为π, 故只需在[0,]π上讨论其值域. 因为 ()sin()sin cos sin 2f x x x x x π'=+-=-,令()0f x '=, 得14x π=, 234x π=, 且344()s i n 24f t d t πππ==⎰554433443()sin sin sin 24f t dt t dt t dt πππππππ==-=-⎰⎰⎰又 20(0)sin 1f t dt π==⎰, 32()(sin )1f t dt πππ=-=⎰,∴()f x的最小值是2, 故()f x的值域是[2.【评注】此题的讨论分两部分:(1)证明定积分等式,常用的方法是变量代换.(2)求变上限积分的最值, 其方法与一般函数的最值相同.(18)(本题满分12分)曲线2x x e e y -+=与直线0,(0)x x t t ==>及0y =围成一曲边梯形. 该曲边梯形绕x 轴旋转一周得一旋转体, 其体积为()V t , 侧面积为()S t , 在x t =处的底面积为()F t .(Ⅰ)求()()S t V t 的值; (Ⅱ)计算极限()lim()t S t F t →+∞.【分析】用定积分表示旋转体的体积和侧面积,二者及截面积都是t 的函数,然后计算它们之间的关系. 【详解】 (Ⅰ)0()2tS t π=⎰022x x te e π-⎛+= ⎝⎰ 2022x x te e dx π-⎛⎫+= ⎪⎝⎭⎰, 2200()2x x tte e V t y dx dx ππ-⎛⎫+== ⎪⎝⎭⎰⎰, ()2()S t V t ∴=. (Ⅱ)22()2t t x te e F t yππ-=⎛⎫+== ⎪⎝⎭,20222()limlim ()2xxtt t t t e e dx S t F t e e ππ-→+∞→+∞-⎛⎫+⎪⎝⎭=⎛⎫+ ⎪⎝⎭⎰222lim222t t tt t t t e e e e e e ---→+∞⎛⎫+ ⎪⎝⎭=⎛⎫⎛⎫+- ⎪⎪⎝⎭⎝⎭lim 1t tttt e e e e --→+∞+==- 【评注】在 t 固定时,此题属于利用定积分表示旋转体的体积和侧面积的题型,考点是定积分几何应用的公式和罗必塔求与变限积分有关的极限问题.(19)(本题满分12分)设2e a b e <<<, 证明2224ln ln ()b a b a e ->-. 【分析】文字不等式可以借助于函数不等式的证明方法来证明,常用函数不等式的证明方法主要有单调性、极值和最值法等.【详证1】设224()ln x x x e ϕ=-, 则 2ln 4()2x x x e ϕ'=-21l n ()2xx xϕ-''=,所以当x e >时, ()0x ϕ''<, 故()x ϕ'单调减小, 从而当2e x e <<时,22244()()0x e e e ϕϕ''>=-=, 即当2e x e <<时, ()x ϕ单调增加.因此, 当2e a b e <<<时, ()()b a ϕϕ>, 即 222244ln ln b b a a e e ->- 故 2224ln ln ()b a b a e ->-.【详证2】设2224()ln ln ()x x a x a eϕ=---, 则2ln 4()2x x x e ϕ'=-21l n ()2xx xϕ-''=,∴x e >时, ()0x ϕ''<()x ϕ'⇒, 从而当2e x e <<时,22244()()0x e e e ϕϕ''>=-=, 2e x e ⇒<<时, ()x ϕ单调增加.2e a b e ⇒<<<时, ()()0x a ϕϕ>=.令x b =有()0b ϕ>即 2224ln ln ()b a b a e ->-.【详证3】证 对函数2ln x 在[,]a b 上应用拉格朗日定理, 得 222ln ln ln ()b a b a ξξ->-, a b ξ<<.设ln ()t t t ϕ=, 则21ln ()t t tϕ-'=, 当t e >时, ()0t ϕ'<, 所以()t ϕ单调减小, 从而2()()e ϕξϕ>, 即222ln ln 2e e eξξ>=, 故 2224ln ln ()b a b a e->- 【评注】此题是文字不等式的证明题型.由于不能直接利用中值定理证明,所以常用的方法是将文字不等式化为函数不等式,然后借助函数不等式的证明方法加以证明.(20)(本题满分11分)某种飞机在机场降落时,为了减小滑行距离,在触地的瞬间,飞机尾部张开减速伞,以增大阻力,使飞机迅速减速并停下来.现有一质量为9000kg 的飞机,着陆时的水平速度为700/km h .经测试,减速伞打开后,飞机所受的总阻力与飞机的速度成正比(比例系数为66.010k =⨯).问从着陆点算起,飞机滑行的最长距离是多少?注 kg 表示千克,/km h 表示千米/小时.【分析】本题属物理应用.已知加速度或力求运动方程是质点运动学中一类重要的计算,可利用牛顿第二定律,建立微分方程,再求解.【详解1】由题设,飞机的质量9000m kg =,着陆时的水平速度0700/v km h =.从飞机接触跑道开始记时,设t 时刻飞机的滑行距离为()x t ,速度为()v t .根据牛顿第二定律,得 dv mkv dt=-. 又 dv dv dx dv v dt dx dt dx=⋅=, m dx dv k∴=-, 积分得 ()m x t v C k=-+, 由于0(0)v v =,(0)0x =, 故得0m C v k=, 从而 0()(())m x t v v t k =-. 当()0v t →时, 069000700() 1.05()6.010mv x t km k ⨯→==⨯. 所以,飞机滑行的最长距离为1.05km .【详解2】根据牛顿第二定律,得 dv mkv dt=-. 所以 dv k dt v m =-, 两边积分得 k t m v Ce-=, 代入初始条件 00t v v ==, 得0C v =,0()k t m v t v e-∴=, 故飞机滑行的最长距离为 0000() 1.05()k t m mv mv x v t dt e km k k+∞-+∞==-==⎰. 【详解3】根据牛顿第二定律,得 22d x dx m k dt dt=-, 220d x k dx dt m dt+=,其特征方程为 20k r r m +=, 解得10r =, 2k r m=-, 故 12k t m x C C e -=+,由(0)0x =, 2000(0)k t m t t kC dx v e v dt m -====-=,得012mv C C k=-=, 0()(1)k t m mv x t e k-∴=-. 当t →+∞时, 069000700() 1.05()6.010mv x t km k ⨯→==⨯. 所以,飞机滑行的最长距离为1.05km .【评注】此题的考点是由物理问题建立微分方程,并进一步求解.(21)(本题满分10分)设22(,)xyz f x y e =-,其中f 具有连续二阶偏导数,求2,,z z z x y x y ∂∂∂∂∂∂∂. 【分析】利用复合函数求偏导和混合偏导的方法直接计算.【详解】 122xy z x f ye f x∂''=+∂, 122xy z y f xe f y∂''=-+∂, 21112222[(2)]x y x y x y z x f y f x e e f x y e f x y ∂''''''=⋅-+⋅++∂∂2122[(2)]x y x y y e f y f x e''''+⋅-+⋅ 222111222242()(1)xy xy xy xyf x y e f xye f e xy f '''''''=-+-++++. 【评注】此题属求抽象复合函数高阶偏导数的常规题型.(22)(本题满分9分)设有齐次线性方程组1234123412341234(1)0,2(2)220,33(3)30,444(4)0,a x x x x x a x x x x x a x x x x x a x ++++=⎧⎪++++=⎪⎨++++=⎪⎪++++=⎩试问a 取何值时, 该方程组有非零解, 并求出其通解.【分析】此题为求含参数齐次线性方程组的解.由系数行列式为0确定参数的取值,进而求方程组的非零解.【详解1】对方程组的系数矩阵A 作初等行变换, 有11111111222220033333004444400a a a a a B a a a a a a ++⎛⎫⎛⎫ ⎪ ⎪+- ⎪ ⎪→= ⎪ ⎪+- ⎪ ⎪ ⎪ ⎪+-⎝⎭⎝⎭当0a =时, ()14r A =<, 故方程组有非零解, 其同解方程组为12340x x x x +++=.由此得基础解系为1(1,1,0,0)T η=-, 2(1,0,1,0)T η=-, 3(1,0,0,1)T η=-,于是所求方程组的通解为112233x k k k ηηη=++, 其中123,,k k k 为任意常数.当0a ≠时,111110000210021003010301040014001a a B ++⎛⎫⎛⎫ ⎪ ⎪-- ⎪ ⎪→→ ⎪ ⎪-- ⎪ ⎪ ⎪ ⎪--⎝⎭⎝⎭当10a =-时, ()34r A =<, 故方程组也有非零解, 其同解方程组为12131420,30,40,x x x x x x -+=⎧⎪-+=⎨⎪-+=⎩由此得基础解系为(1,2,3,4)Tη=,所以所求方程组的通解为x k η=, 其中k 为任意常数.【详解2】方程组的系数行列式311112222(10)33334444a a A a a a a +⎛⎫ ⎪+ ⎪==+ ⎪+ ⎪ ⎪+⎝⎭. 当0A =, 即0a =或10a =-时, 方程组有非零解.当0a =时, 对系数矩阵A 作初等行变换, 有11111111222200003333000044440000A ⎛⎫⎛⎫ ⎪ ⎪ ⎪ ⎪=→ ⎪ ⎪ ⎪ ⎪ ⎪ ⎪⎝⎭⎝⎭故方程组的同解方程组为 12340x x x x +++=.其基础解系为1(1,1,0,0)T η=-, 2(1,0,1,0)T η=-, 3(1,0,0,1)T η=-,于是所求方程组的通解为112233x k k k ηηη=++, 其中123,,k k k 为任意常数.当10a =-时, 对A 作初等行变换, 有91119111282220100033733001004446400010A --⎛⎫⎛⎫ ⎪ ⎪-- ⎪ ⎪=→ ⎪ ⎪-- ⎪ ⎪ ⎪ ⎪--⎝⎭⎝⎭91110000210021003010301040014001-⎛⎫⎛⎫ ⎪ ⎪-- ⎪ ⎪→→ ⎪ ⎪-- ⎪ ⎪ ⎪ ⎪--⎝⎭⎝⎭故方程组的同解方程组为 2131412,3,4,x x x x x x =⎧⎪=⎨⎪=⎩其基础解系为(1,2,3,4)Tη=,所以所求方程组的通解为x k η=, 其中k 为任意常数【评注】解此题的方法是先根据齐次方程有非零解的条件确定方程组中的参数,再对求得的参数对应的方程组求解.(23)(本题满分9分)设矩阵12314315a -⎛⎫ ⎪-- ⎪ ⎪⎝⎭的特征方程有一个二重根, 求a 的值, 并讨论A 是否可相似对角化. 【分析】由矩阵特征根的定义确定a 的值,由线性无关特征向量的个数与E A λ-秩之间的关系确定A 是否可对角化.【详解】A 的特征多项式为1232201431431515aa λλλλλλλ-----=------- 110100(2)143(2)13315115a a λλλλλλ-=--=--------- 2(2)(8183)a λλλ=--++.若2λ=是特征方程的二重根, 则有22161830a -++=, 解得2a =-.当2a =-时, A 的特征值为2, 2, 6, 矩阵1232123123E A -⎛⎫ ⎪-=- ⎪ ⎪--⎝⎭的秩为1,故2λ=对应的线性无关的特征向量有两个, 从而A 可相似对角化.若2λ=不是特征方程的二重根, 则28183a λλ-++为完全平方,从而18316a +=, 解得23a =-.当23a =-时, A 的特征值为2, 4, 4, 矩阵32321032113E A ⎛⎫ ⎪- ⎪-= ⎪ ⎪-- ⎪⎝⎭的秩为2,故4λ=对应的线性无关的特征向量只有一个, 从而A 不可相似对角化.【评注】此题的考点是由特征根及重数的定义确定a 的值, 对a 的取值讨论对应矩阵的特征根及对应E A λ-的秩, 进而由E A λ-的秩与线性无关特征向量的个数关系确定A 是否可相似对角化.。
Design and test of a high-performance piezoelectric micropump for drug delivery

Keywords: Piezoelectric micropump; PZT actuator; Drug delivery; Cantilever valve; Natural frequency
1. Introduction
Most drugs have a range of concentrations of greatest efficacy in the body, above which they are toxic and below which they have no therapeutic benefit [1]. Conventional drug delivery routes such as oral tablets or injections are not easily able to control the rate of drug delivery or the target area of the drug. Consequently, initial concentration of the drug in the blood peaks above the level of toxicity and then gradually decreases over time to an ineffective level and the patients have to take the drug frequently [2,3]. In order to control drug release better, drug delivery systems (DDS) are necessary. The general advantages of a dominated DDS are the ability for the drug to act directly when needed and not at any fixed time or deliver a localized dosage to reduce the side effects of medication [4,5]. Moreover, with the help of an
人称代词的各种形式

人称代词的各种形式be动词用法be动词的一般现在时有三种形式,即:am,is,are。
1.如果主语是第一人称I(我)时,be动词用am。
如:I am a student.我是一名学生。
I am还可缩写成I'm。
如:I'm David.我是大卫。
2.如果主语是you(你,你们),they(他们,它们,她们)或名词复数(两个以上的人或物)时,be动词必须用are。
如:Are you twelve?你是十二岁吗?Tom and Lily are good friends.汤姆和莉莉是好朋友。
They are at school. 他们在学校。
These are books. 这些是书。
We are students . 我们是学生。
are与主语还可缩写。
如:We are=We're,They are=They're,You are=You're。
而are与not可缩写成aren't。
如:They aren't students.他们不是学生。
但是am与not不能缩写。
3.如果主语是单数名词、不可数名词或单数第三人称代词(he,she,it)时,be动词用is.如:My mother is a teacher.我的妈妈是一名老师。
He is a student.他是一名学生。
She is my friend.她是我的朋友。
It is a dog. 它是一只狗。
is也可与主语缩写,如:He is=He's,My mother is=My mother's等。
但是This is 不可缩写。
而is与not可缩写成isn't。
如:This isn't a book.这不是一本书。
[解题过程]根据以上叙述我们可以把动词的用法以口诀的形式表述出来:我(I)是am,你(you)是are,剩下is留给她(she),他(he),它(it),两个以上都用are。
Fitch:sovereign_ratings_history
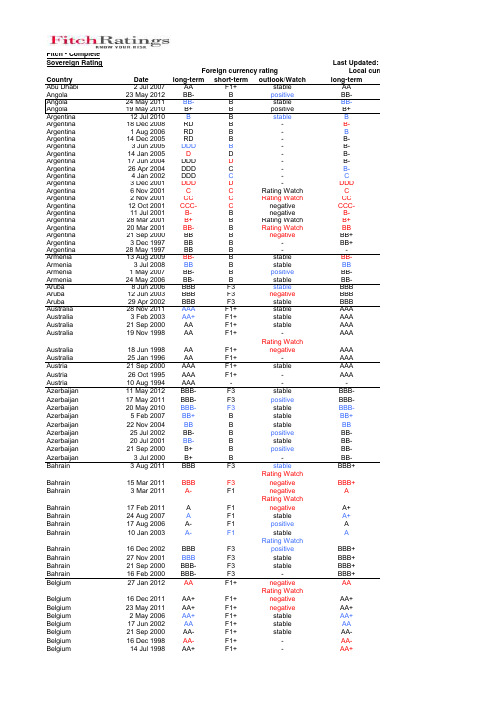
Fitch - Complete Sovereign Rating History Last Updated:Country Datelong-term short-term outlook/Watchlong-termAbu Dhabi 2 Jul 2007AA F1+stable AA Angola 23 May 2012BB-B positiveBB-Angola 24 May 2011BB-B stableBB-Angola 19 May 2010B+B positive B+Argentina 12 Jul 2010B B stable B Argentina 18 Dec 2008RD B -B-Argentina 1 Aug 2006RD B -B Argentina 14 Dec 2005RD B -B-Argentina 3 Jun 2005DDD B -B-Argentina 14 Jan 2005D D -B-Argentina 17 Jun 2004DDD D -B-Argentina 26 Apr 2004DDD C -B-Argentina 4 Jan 2002DDD C -C Argentina 3 Dec 2001DDD D -DDD Argentina 6 Nov 2001C C Rating Watch negative C Argentina 2 Nov 2001CC C Rating Watch negative CC Argentina 12 Oct 2001CCC-C negativeCCC-Argentina 11 Jul 2001B-B negativeB-Argentina 28 Mar 2001B+B Rating Watch negative B+Argentina 20 Mar 2001BB-B Rating Watch negativeBB Argentina 21 Sep 2000BB B negative BB+Argentina 3 Dec 1997BB B -BB+Argentina 28 May 1997BB B --Armenia 13 Aug 2009BB-B stable BB-Armenia 3 Jul 2008BB B stable BB Armenia 1 May 2007BB-B positiveBB-Armenia 24 May 2006BB-B stableBB-Aruba 8 Jun 2006BBB F3stable BBB Aruba 12 Jun 2003BBB F3negative BBB Aruba 29 Apr 2002BBB F3stable BBB Australia 28 Nov 2011AAA F1+stable AAA Australia 3 Feb 2003AA+F1+stable AAA Australia 21 Sep 2000AA F1+stable AAA Australia 19 Nov 1998AA F1+-AAA Australia 18 Jun 1998AA F1+Rating Watch negative AAA Australia 25 Jan 1996AA F1+-AAA Austria 21 Sep 2000AAA F1+stable AAA Austria 26 Oct 1995AAA F1+-AAA Austria 10 Aug 1994AAA ---Azerbaijan 11 May 2012BBB-F3stable BBB-Azerbaijan 17 May 2011BBB-F3positiveBBB-Azerbaijan 20 May 2010BBB-F3stableBBB-Azerbaijan 5 Feb 2007BB+B stable BB+Azerbaijan 22 Nov 2004BB B stable BB Azerbaijan 25 Jul 2002BB-B positiveBB-Azerbaijan 20 Jul 2001BB-B stableBB-Azerbaijan 21 Sep 2000B+B positive BB-Azerbaijan 3 Jul 2000B+B -BB-Bahrain 3 Aug 2011BBB F3stable BBB+Bahrain 15 Mar 2011BBB F3Rating Watch negative BBB+Bahrain 3 Mar 2011A-F1negativeA Bahrain 17 Feb 2011A F1Rating Watch negative A+Bahrain 24 Aug 2007A F1stable A+Bahrain 17 Aug 2006A-F1positiveA Bahrain 10 Jan 2003A-F1stableA Bahrain 16 Dec 2002BBB F3Rating Watch positive BBB+Bahrain 27 Nov 2001BBB F3stable BBB+Bahrain 21 Sep 2000BBB-F3stableBBB+Bahrain 16 Feb 2000BBB-F3-BBB+Belgium 27 Jan 2012AA F1+negativeAA Belgium 16 Dec 2011AA+F1+Rating Watch negative AA+Belgium 23 May 2011AA+F1+negative AA+Belgium 2 May 2006AA+F1+stable AA+Belgium 17 Jun 2002AA F1+stable AA Belgium 21 Sep 2000AA-F1+stableAA-Belgium 16 Dec 1998AA-F1+-AA-Belgium 14 Jul 1998AA+F1+-AA+Belgium 26 Oct 1995AA+F1+-AAA Belgium10 Aug 1994AA+---Foreign currency ratingLocal currenForeign currency rating Local curren Benin25 Jan 2012withdrawn withdrawn withdrawn withdrawnBenin15 Sep 2004B B stable BBermuda26 Jun 2012AA F1+stable AA+Bermuda11 Aug 2006AA+F1+stable AAABermuda21 Sep 2000AA F1+stable AAABermuda26 Oct 1995AA F1+-AAABermuda10 Aug 1994AA---Bolivia 5 Oct 2010B+B stable B+Bolivia8 Sep 2009B B stable BBolivia27 Jul 2007B-B stable B-Bolivia17 Jun 2005B-B negative B-Bolivia17 Mar 2004B-B stable B-Brazil 4 Apr 2011BBB F2stable BBBBrazil28 Jun 2010BBB-F3positive BBB-Brazil29 May 2008BBB-F3stable BBB-Brazil9 May 2007BB+B stable BB+Brazil 5 Feb 2007BB B positive BBBrazil28 Jun 2006BB B stable BBBrazil11 Oct 2005BB-B positive BB-Brazil28 Sep 2004BB-B stable BB-Brazil 6 Nov 2003B+B stable B+Brazil 3 Jun 2003B B positive BBrazil10 Mar 2003B B stable BBrazil21 Oct 2002B B negative BBrazil 1 Aug 2002B+B Rating Watch negative B+Brazil20 Jun 2002B+B negative B+Brazil17 Jul 2001BB-B negative B+Brazil21 Sep 2000BB-B stable B+Brazil19 May 2000BB-B-B+Brazil22 Feb 2000B+B-B+Brazil26 Jan 1999B B-BBrazil 3 Dec 1997B+B-BB-Brazil26 Oct 1995B+B--Brazil 1 Dec 1994B+---Bulgaria13 Dec 2011BBB-F3stable BBBBulgaria24 May 2011BBB-F3positive BBBBulgaria30 Apr 2009BBB-F3negative BBBBulgaria9 Nov 2008BBB-F3stable BBBBulgaria30 Jan 2008BBB F3negative BBB+Bulgaria17 Aug 2005BBB F3stable BBB+Bulgaria 4 Aug 2004BBB-F3stable BBBBulgaria24 Jul 2003BB+B positive BBB-Bulgaria29 Oct 2002BB B positive BB+Bulgaria14 Jan 2002BB-B stable BBBulgaria21 Sep 2000B+B positive BBBulgaria17 Apr 1998B+B-BBCameroon30 May 2012B B stable BCameroon 6 Mar 2007B B stable B-Cameroon12 Jun 2006B B stable CCC Cameroon21 Dec 2005B-B positive CCC Cameroon 4 Nov 2005B-B positive CCC+ Cameroon15 Feb 2005B-B stable CCC+ Cameroon 5 Jul 2004B B Rating Watch Negative BCameroon 4 Sep 2003B B stable BCanada12 Aug 2004AAA F1+stable AAACanada 3 Apr 2001AA+F1+stable AAACanada21 Sep 2000AA F1+stable AAACanada26 Oct 1995AA F1+-AAACanada10 Aug 1994AA---Cape Verde22 Jun 2009B+B stable BB-Cape Verde11 Mar 2008B+B positive BB-Cape Verde15 Aug 2003B+B stable BB-Chile 1 Feb 2011A+F1stable AA-Chile10 Nov 2008A F1stable A+Chile15 May 2007A F1positive A+Chile28 Mar 2005A F1stable A+Chile 2 Feb 2004A-F1positive A+Chile24 Feb 2003A-F1stable A+Chile21 Sep 2000A-F1stable AA-Chile 3 Dec 1997A-F1-AA-Foreign currency rating Local curren Chile25 Nov 1996A-F1-AAChile26 Oct 1995A-F1--Chile30 Aug 1995A----Chile10 Nov 1994BBB+---China12 Apr 2011A+F1stable AA-China 6 Nov 2007A+F1stable AA-China13 Sep 2006A F1positive A+China17 Oct 2005A F1stable A+China13 Oct 2003A-F1positive AChina 6 Dec 2001A-F1stable AChina21 Sep 2000A-F1stable-China22 Dec 1998A-F1--China11 Dec 1997A----Colombia22 Jun 2011BBB-F3Stable BBBColombia21 Oct 2010BB+B positive BBB-Colombia21 Jun 2007BB+B stable BBB-Colombia 5 Jun 2006BB B positive BBB-Colombia 3 May 2004BB B stable BBB-Colombia29 Aug 2002BB B negative BBB-Colombia10 Jan 2002BB B stable BBB-Colombia24 Oct 2000BB+B negative BBBColombia21 Sep 2000BB+B stable BBBColombia17 Mar 2000BB+B-BBBColombia 1 Sep 1999BBB-F3-BBB+Colombia20 Mar 1998BBB F3-A-Colombia10 Aug 1994BBB---Costa Rica 4 Mar 2011BB+B stable BB+Costa Rica 4 Oct 2006BB B stable BB+Costa Rica28 Apr 2003BB B negative BB+Costa Rica21 Sep 2000BB B stable BB+Costa Rica11 May 1998BB B-BB+Croatia21 May 2009BBB-F3negative BBBCroatia 6 Jul 2005BBB-F3stable BBB+Croatia8 Jul 2003BBB-F3positive BBB+Croatia28 Jun 2001BBB-F3stable BBB+Croatia21 Sep 2000BB+B positive BBBCroatia29 Apr 1999BB+B-BBBCroatia17 Jan 1997BBB-F3-A-Cyprus25 Jun 2012BB+B negative BB+Cyprus27 Jan 2012BBB-F3negative BBB-Cyprus16 Dec 2011BBB F3Rating Watch negative BBBCyprus10 Aug 2011BBB F3negative BBBCyprus31 May 2011A-F1negative A-Cyprus12 Jul 2007AA-F1+Rating Watch negative AA-Cyprus12 Jul 2007AA-F1+stable AA-Cyprus 4 Nov 2003A+F1positive AACyprus 1 Feb 2002A+F1stable AACzech Republic13 Dec 2011A+F1stable AA-Czech Republic 4 Jun 2010A+F1positive AA-Czech Republic 4 Mar 2008A+F1stable AA-Czech Republic26 Aug 2005A F1stable A+Czech Republic20 Jun 2003A-F2stable ACzech Republic28 Mar 2001BBB+F2stable ACzech Republic21 Sep 2000BBB+F2stable A+Czech Republic22 Dec 1998BBB+F2-A+Czech Republic24 Nov 1997BBB+F2-AA-Czech Republic26 Oct 1995A-F1--Czech Republic10 Aug 1995A----Denmark10 Nov 2003AAA F1+stable AAADenmark29 Sep 2000AA+F1+stable AAADenmark21 Sep 2000AA+F1+positive AAADenmark26 Oct 1995AA+F1+-AAADenmark10 Aug 1994AA+---Dominican Republic 5 Jan 2011B B positive BDominican Republic25 Sep 2008B B stable BDominican Republic26 Sep 2006B B positive BDominican Republic 5 May 2006B B stable BDominican Republic19 Jul 2005B-B stable BDominican Republic11 May 2005DDD D-BDominican Republic 5 May 2005DDD D-CCC+Foreign currency rating Local curren Dominican Republic21 Apr 2005C C Rating Watch negative CCC+ Dominican Republic30 Jan 2004CCC+C Rating Watch negative CCC+ Dominican Republic24 Oct 2003B B Rating Watch negative BDominican Republic8 Oct 2003B+B Rating Watch negative B+Dominican Republic11 Aug 2003B+B stable B+Ecuador8 Nov 2010B-B stable-Ecuador 4 Sep 2009CCC C stable-Ecuador16 Dec 2008RD D--Ecuador17 Nov 2008CCC C Rating Watch negative-Ecuador30 Oct 2007CCC C stable-Ecuador23 Jan 2007CCC C Rating Watch negative-Ecuador29 Aug 2005B-B negative-Ecuador7 Oct 2004B-B stable-Ecuador26 Sep 2003CCC+C stable-Ecuador24 Mar 2003CCC+C positive-Ecuador8 Nov 2002CCC+C stable-Egypt15 Jun 2012B+B negative B+Egypt30 Dec 2011BB-B negative BBEgypt28 Jun 2011BB B negative BB+Egypt 3 Feb 2011BB B Rating Watch negative BB+Egypt28 Jan 2011BB+B negative BBB-Egypt18 Aug 2008BB+B stable BBB-Egypt18 Jun 2007BB+B positive BBBEgypt15 Dec 2004BB+B stable BBBEgypt 2 Dec 2003BB+B stable BBBEgypt21 Aug 2002BB+B stable BBBEgypt22 Jan 2002BBB-F3negative BBB+Egypt22 Aug 2001BBB-F3stable BBB+Egypt21 Sep 2000BBB-F3stable A-Egypt19 Aug 1997BBB-F3-A-El Salvador24 Jul 2012BB B negative BBEl Salvador29 Jul 2011BB B stable BBEl Salvador18 Jun 2009BB B negative BBEl Salvador13 Oct 2008BB+B negative BB+El Salvador31 Jan 2005BB+B stable BB+El Salvador21 Aug 2002BB+B negative BB+El Salvador16 Aug 2001BB+B stable BB+El Salvador21 Sep 2000BB+B stable BBBEl Salvador8 May 2000BB+B-BBBEl Salvador 5 May 1998BB+B-A-El Salvador23 Sep 1996BB B-A-Estonia 5 Jul 2011A+F1stable A+Estonia19 Jul 2010A F1stable AEstonia30 Mar 2010BBB+F2Rating Watch positive A-Estonia 5 Feb 2010BBB+F2stable A-Estonia8 Apr 2009BBB+F2negative A-Estonia 3 Oct 2008A-F1negative AEstonia31 Jan 2008A F1negative A+Estonia31 Aug 2006A F1stable A+Estonia7 Jul 2004A F1positive A+Estonia22 Oct 2003A-F1positive A+Estonia30 Aug 2001A-F1stable A+Estonia30 Jul 2001BBB+F2Rating Watch positive AEstonia28 Sep 2000BBB+F2stable AEstonia7 Aug 2000BBB F3Rating Watch positive AEstonia11 Sep 1997BBB F3-AFinland21 Sep 2000AAA F1+stable AAAFinland 5 Aug 1998AAA F1+-AAAFinland14 Jul 1998AA+F1+-AA+Finland29 Apr 1997AA+F1+-AAAFinland12 Mar 1996AA F1+-AAAFinland26 Oct 1995AA-F1+-AAAFinland10 Aug 1994AA----France16 Dec 2011AAA F1+negative AAAFrance21 Sep 2000AAA F1+stable AAAFrance26 Oct 1995AAA F1+-AAAFrance10 Aug 1994AAA---Gabon 5 Apr 2012BB-B positive BB-Gabon29 Oct 2007BB-B stable BB-Gambia 6 Jul 2007----Foreign currency rating Local curren Gambia21 Dec 2005CCC C stable CCCGambia26 Jan 2005CCC+C stable CCC+Gambia11 Nov 2002B-B stable B-Georgia15 Dec 2011BB-B stable BB-Georgia 3 Mar 2011B+B positive B+Georgia26 Aug 2009B+B stable B+Georgia7 Apr 2009B+B Rating Watch negative B+Georgia8 Aug 2008B+B negative B+Georgia18 Jul 2007BB-B stable BB-Germany21 Sep 2000AAA F1+stable AAAGermany26 Oct 1995AAA F1+-AAAGermany10 Aug 1994AAA---Ghana24 Sep 2010B+B stable B+Ghana 3 Mar 2009B+B negative B+Ghana7 Feb 2008B+B stable B+Ghana 1 Feb 2006B+B positive B+Ghana17 Mar 2005B+B stable B+Ghana 2 Dec 2003B B positive BGreece17 May 2012CCC C-CCCGreece13 Mar 2012B-B stable B-Greece9 Mar 2012RD C-RDGreece22 Feb 2012C C-CGreece13 Jul 2011CCC C-CCCGreece20 May 2011B+B Rating Watch negative B+Greece14 Jan 2011BB+B negative BB+Greece21 Dec 2010BBB-F2Rating Watch negative BBB-Greece9 Apr 2010BBB-F2negative BBB-Greece8 Dec 2009BBB+F2negative BBB+Greece22 Oct 2009A-F1negative A-Greece12 May 2009A F1negative AGreece20 Oct 2008A F1stable AGreece 5 Mar 2007A F1positive AGreece16 Dec 2004A F1stable AGreece28 Sep 2004A+F1Rating Watch negative A+Greece20 Oct 2003A+F1stable A+Greece23 Oct 2002A F1positive AGreece20 Jun 2001A F1stable AGreece21 Sep 2000A-F1stable A-Greece27 Jul 2000A-F1-A-Greece13 Mar 2000BBB+F2Rating Watch positive A-Greece25 Oct 1999BBB+F2-A-Greece10 Aug 1999BBB F3Rating Watch positive-Greece 4 Jun 1997BBB F3--Greece13 Nov 1995BBB-F3--Guatemala22 Feb 2006BB+B stable BB+Hong Kong26 Nov 2010AA+F1+stable AA+Hong Kong12 Jul 2007AA F1+stable AA+Hong Kong24 Jul 2006AA-F1+positive AA+Hong Kong16 May 2004AA-F1+stable AA+Hong Kong24 Apr 2003AA-F1+negative AA+Hong Kong25 Jun 2001AA-F1+stable AA+Hong Kong21 Sep 2000A+F1+stable AA+Hong Kong14 Nov 1996A+F1+-AA+Hong Kong24 Nov 1995A+F1+--Hong Kong26 Oct 1995A+F1--Hong Kong9 Aug 1995A+---Hong Kong10 Aug 1994AA----Hungary 6 Jan 2012BB+B negative BBB-Hungary11 Nov 2011BBB-F3negative BBBHungary 6 Jun 2011BBB-F3Stable BBBHungary23 Dec 2010BBB-F3negative BBBHungary 2 Mar 2009BBB F3negative BBB+Hungary9 Nov 2008BBB F3stable BBB+Hungary15 Oct 2008BBB+F2negative A-Hungary 5 Nov 2007BBB+F2stable A-Hungary20 Sep 2006BBB+F2negative A-Hungary 6 Dec 2005BBB+F2stable A-Hungary12 Jan 2005A-F2negative AHungary15 Jul 2003A-F2negative A+Hungary30 Nov 2000A-F2stable A+Foreign currency rating Local curren Hungary21 Sep 2000BBB+F2positive AHungary29 Oct 1999BBB+F2-AHungary 2 Jul 1998BBB F3-AHungary24 Jun 1997BBB F3-A-Hungary23 May 1997BBB-F3Rating Watch positive BBB+Hungary25 Apr 1996BBB-F3-BBB+Iceland17 Feb 2012BBB-f3stable BBB+Iceland17 May 2011BB+B stable BBB+Iceland 5 Jan 2010BB+B negative BBB+Iceland23 Dec 2009BBB-F3negative A-Iceland8 Oct 2008BBB-F3Rating Watch negative A-Iceland30 Sep 2008A-F2Rating Watch negative AAIceland 1 Apr 2008A+F1negative AA+Iceland15 Mar 2007A+F1stable AA+Iceland21 Feb 2006AA-F1+negative AAAIceland31 Mar 2003AA-F1+stable AAAIceland15 Feb 2002AA-F1+negative AAAIceland21 Sep 2000AA-F1+stable AAAIceland 3 Feb 2000AA-F1+-AAAIndia18 Jun 2012BBB-F3negative BBB-India14 Jun 2010BBB-F3stable BBB-India15 Jul 2008BBB-F3stable BBB-India 1 Aug 2006BBB-F3stable BBB-India21 Jan 2004BB+B stable BB+India22 Nov 2001BB B stable BB+India31 May 2001BB+B negative BBB-India21 Sep 2000BB+B stable BBB-India8 Mar 2000BB+B-BBB-Indonesia15 Dec 2011BBB-F3stable BBB-Indonesia24 Feb 2011BB+B positive BB+Indonesia25 Jan 2010BB+B stable BB+Indonesia14 Feb 2008BB B stable BBIndonesia28 Jan 2007BB-B positive BB-Indonesia13 Feb 2006BB-B stable BB-Indonesia26 Jan 2005BB-B positive BB-Indonesia 4 Oct 2004B+B positive B+Indonesia20 Nov 2003B+B stable B+Indonesia 1 Aug 2002B B stable BIndonesia30 Nov 2001B-B stable B-Indonesia14 Sep 2001B-B positive B-Indonesia21 Sep 2000B-B stable B-Indonesia19 May 2000B-B-B+Indonesia26 Apr 1999B-B-BB-Indonesia16 Mar 1998B-B Rating Watch negative BB+Indonesia21 Jan 1998B+B Rating Watch negative BBB-Indonesia8 Jan 1998BB-B-BBB-Indonesia22 Dec 1997BB+B-BBB+ Indonesia17 Dec 1997BBB-F3Rating Watch negative AIndonesia 4 Jun 1997BBB-F3-AIran24 Apr 2008----Iran24 Apr 2006B+B stable B+Iran 6 Feb 2006BB-B negative BB-Iran14 Dec 2004BB-B stable BB-Iran9 Dec 2003B+B positive B+Iran10 May 2002B+B stable B+Ireland27 Jan 2012BBB+F2negative BBB+Ireland16 Dec 2011BBB+F2Rating Watch negative BBB+Ireland14 Apr 2011BBB+F2negative BBB+Ireland 1 Apr 2011BBB+F2rating watch negative BBB+Ireland9 Dec 2010BBB+F2stable BBB+Ireland 6 Oct 2010A+F1negative A+Ireland 4 Nov 2009AA-F1+stable AA-Ireland8 Apr 2009AA+F1+negative AA+Ireland 6 Mar 2009AAA F1+Rating Watch negative AAAIreland21 Sep 2000AAA F1+stable AAAIreland16 Dec 1998AAA F1+-AAAIreland14 Jul 1998AA+F1+-AA+Ireland26 Oct 1995AA+F1+-AAAIreland10 Oct 1994AA+---Israel11 Feb 2008A F1stable A+Foreign currency rating Local curren Israel18 Dec 2006A-F1positive AIsrael14 Feb 2005A-F1stable AIsrael16 Dec 2003A-F1stable AIsrael22 Oct 2002A-F1negative AIsrael22 Oct 2001A-F1negative A+Israel21 May 2001A-F1Rating Watch negative A+Israel21 Sep 2000A-F1stable A+Israel 6 May 1999A-F1-A+Israel11 Dec 1995A-F2--Israel24 Nov 1995BBB+F2--Italy27 Jan 2012A-F2negative A-Italy16 Dec 2011A+F1Rating Watch negative A+Italy7 Oct 2011A+F1negative A+Italy19 Oct 2006AA-F1+stable AA-Italy25 May 2006AA F1+Rating Watch negative AAItaly29 Jun 2005AA F1+negative AAItaly17 Jun 2002AA F1+stable AAItaly21 Sep 2000AA-F1+stable AA-Italy14 Jul 1998AA-F1+-AA-Italy26 Oct 1995AA-F1+-AAAItaly23 Feb 1995AA----Italy10 Aug 1994AA---Jamaica16 Feb 2010B-B stable B-Jamaica 3 Feb 2010CCC C Rating Watch positive CCCJamaica 3 Feb 2010RD D-RDJamaica14 Jan 2010CCC C negative CJamaica24 Nov 2009CCC C negative CCCJamaica18 Nov 2008B B negative BJamaica29 Aug 2006B+B+stable B+Japan22 May 2012A+F1+negative A+Japan27 May 2011AA F1+negative AA-Japan9 May 2005AA F1+stable AA-Japan21 Nov 2002AA F1+negative AA-Japan26 Nov 2001AA F1+negative AAJapan 2 Mar 2001AA+F1+negative AA+Japan21 Sep 2000AA+F1+stable AA+Japan29 Jun 2000AA+F1+-AA+Japan21 Sep 1998AA+F1+-AAAJapan 1 Sep 1998AAA F1+Rating Watch negative AAAJapan26 Oct 1995AAA F1+-AAAJapan10 Aug 1994AAA---Kazakhstan21 Nov 2011BBB F3positive BBB+ Kazakhstan20 Dec 2010BBB-F3positive BBB Kazakhstan16 Dec 2009BBB-F3stable BBB Kazakhstan 5 Jun 2009BBB-F3negative BBB Kazakhstan19 Feb 2009BBB-F3Rating Watch negative BBB Kazakhstan9 Nov 2008BBB-F3negative BBB Kazakhstan17 Dec 2007BBB F3negative BBB+ Kazakhstan8 Oct 2007BBB F3stable BBB+ Kazakhstan21 Dec 2006BBB F3positive BBB+ Kazakhstan20 Dec 2005BBB F3stable BBB+ Kazakhstan27 Oct 2004BBB-F3stable BBB Kazakhstan19 Nov 2003BB+B positive BBB-Kazakhstan11 Oct 2002BB+B stable BBB-Kazakhstan12 Jul 2001BB B stable BB+ Kazakhstan21 Sep 2000BB-B stable BB Kazakhstan 5 May 2000BB-B-BB Kazakhstan15 Feb 1999BB-B-BBB-Kazakhstan27 Jan 1998BB B-BBB-Kazakhstan 5 Nov 1996BB-B--Kenya16 Jan 2009B+B stable BB-Kenya30 Jan 2008B+B negative BB-Kenya12 Dec 2007B+B stable BB-Korea7 Nov 2011A+F1positive AAKorea 1 Sep 2009A+F1stable AAKorea9 Nov 2008A+F1negative AAKorea23 Oct 2005A+F1stable AAKorea19 Sep 2005A F1Rating Watch positive AA-Korea27 Jun 2002A F1stable AA-Korea13 May 2002BBB+F2Rating Watch positive AForeign currency rating Local curren Korea21 Sep 2000BBB+F2stable AKorea29 Mar 2000BBB+F2-AKorea24 Jun 1999BBB F2-A-Korea26 Apr 1999BBB-F3Rating Watch positive A-Korea19 Jan 1999BBB-F3-A-Korea22 Dec 1998BB+B Rating Watch positive A-Korea19 Jun 1998BB+B-A-Korea 3 Feb 1998BB+B Rating Watch positive A-Korea21 Jan 1998B-B Rating Watch positive BBB-Korea23 Dec 1997B-B Rating Watch negative BBB-Korea12 Dec 1997BBB-F3Rating Watch negative AKorea11 Dec 1997BBB-F3Rating Watch negative AAKorea26 Nov 1997A F2-AAKorea18 Nov 1997A+F1-AA+Korea27 Jun 1996AA-F1+-AAAKuwait 4 Sep 2008AA F1+stable AAKuwait10 Jun 2002AA-F1+stable AAKuwait24 Aug 2001A+F1positive AAKuwait12 Jun 2001A+F1positive-Kuwait21 Sep 2000A F1positive-Kuwait20 Dec 1995A F1--Latvia13 Dec 2011BBB-F3stable BBBLatvia15 Mar 2011BBB-F3positive BBBLatvia 3 Sep 2010BB+B stable BBB-Latvia8 Apr 2009BB+B negative BBB-Latvia23 Dec 2008BBB-F3negative BBBLatvia12 Nov 2008BBB-F3Rating Watch negative BBBLatvia 3 Oct 2008BBB F3negative BBB+Latvia31 Jan 2008BBB+F2negative A-Latvia17 Aug 2007BBB+F2stable A-Latvia 5 Apr 2007A-F2negative ALatvia26 Aug 2005A-F2stable ALatvia7 Jul 2004A-F2positive ALatvia 4 Nov 2003BBB+F3positive ALatvia21 Jul 2003BBB+F3stable ALatvia17 Jul 2001BBB F3positive ALatvia21 Sep 2000BBB F3stable ALatvia29 Jun 1998BBB F3-ALebanon31 Mar 2010B B stable BLebanon14 Jul 2006B-B stable B-Lebanon18 Nov 2005B-B positive B-Lebanon21 Sep 2001B-B stable B-Lebanon 2 Feb 2001B+B stable B+Lebanon21 Sep 2000BB-B negative BBLebanon30 Jun 1999BB-B-BBLebanon 4 Jun 1998BB-B-BB+Lebanon11 Feb 1998BB B Rating Watch negative BBB-Lebanon26 Feb 1997BB B-BBB-Lesotho31 May 2011BB-B negative BBLesotho27 Apr 2010BB-B stable BBLesotho18 Sep 2006BB-B stable BBLesotho 4 Nov 2005BB-B negative BB+Lesotho30 Nov 2004BB-B stable BB+Lesotho26 Sep 2003B+B positive BBLesotho 2 Sep 2002B+B stable BBLibya13 Apr 2011----Libya13 Apr 2011B B stable BLibya 1 Mar 2011BB B Rating Watch negative BBLibya21 Feb 2011BBB F3Rating Watch negative BBBLibya7 May 2009BBB+F2stable BBB+Lithuania13 Dec 2011BBB F3stable BBB+Lithuania 4 May 2011BBB F3positive BBB+Lithuania8 Mar 2010BBB F3stable BBB+Lithuania8 Apr 2009BBB F3negative BBB+Lithuania22 Dec 2008BBB+F2negative A-Lithuania 3 Oct 2008A-F1negative ALithuania7 Dec 2007A F1negative A+Lithuania23 Oct 2006A F1stable A+Lithuania7 Jul 2004A-F2positive ALithuania28 Jan 2004BBB+F2positive AForeign currency rating Local curren Lithuania 4 Nov 2003BBB F3positive A-Lithuania17 Dec 2002BBB F3stable A-Lithuania28 Feb 2002BBB-F3positive BBB+Lithuania16 May 2001BBB-F3stable BBB+Lithuania21 Sep 2000BB+B stable BBB+Lithuania28 Jan 1997BB+B-BBB+ Luxembourg21 Sep 2000AAA F1+stable AAA Luxembourg26 Oct 1995AAA F1+-AAA Luxembourg10 Aug 1994AAA---Macedonia27 Oct 2010BB+B stable BB+ Macedonia21 May 2009BB+B negative BB+ Macedonia 4 Nov 2008BB+B stable BB+ Macedonia14 Aug 2007BB+B positive BB+ Macedonia13 Jun 2006BB+B stable BB+ Macedonia 1 Nov 2005BB B positive BBMalawi25 Aug 2009----Malawi 6 Mar 2007B-B stable B-Malawi21 Dec 2005CCC C positive CCCMalawi30 Jul 2004CCC+C positive CCC+Malawi20 May 2003CCC+C stable CCC+Malaysia9 Jun 2009A-F2stable AMalaysia 1 Feb 2009A-F2stable A+Malaysia9 Nov 2008A-F2stable A+Malaysia22 Jan 2008A-F2positive A+Malaysia8 Nov 2004A-F2stable A+Malaysia 6 Apr 2004BBB+F2positive AMalaysia7 Aug 2002BBB+F2stable AMalaysia9 Apr 2002BBB F2positive A-Malaysia12 Sep 2001BBB F2stable A-Malaysia21 Sep 2000BBB F2positive A-Malaysia7 Dec 1999BBB F2-A-Malaysia9 Sep 1999BBB-F3Rating Watch positive BBBMalaysia26 Apr 1999BBB-F3-BBBMalaysia9 Sep 1998BB B-BBBMalaysia13 Aug 1998BBB-F3-A-Mali 4 Dec 2009----Mali30 Apr 2004B-B stable B-Malta12 Jul 2007A+F1stable A+Malta 4 Nov 2003A F1positive AA-Malta21 Sep 2000A F1stable AA-Malta23 Jun 1998A F1-AA-Malta20 Jul 1996A F1--Mexico23 Nov 2009BBB F2stable BBB+Mexico10 Nov 2008BBB+F2negative A-Mexico19 Sep 2007BBB+F2stable A-Mexico29 Mar 2007BBB F3positive BBB+Mexico7 Dec 2005BBB F3stable BBB+Mexico15 Jan 2002BBB-F3stable BBBMexico21 Sep 2000BB+B positive BBBMexico 3 May 2000BB+B-BBBMexico11 Apr 2000BB B Rating Watch positive BBB-Mexico15 Sep 1997BB B-BBB-Mexico26 Oct 1995BB B--Mexico30 Aug 1995BB---Moldova17 Dec 2009----Moldova15 Sep 2008B-B stable BMoldova18 Jun 2007B-B positive BMoldova 4 Feb 2003B-B stable BMoldova18 Nov 2002DD D Rating Watch positive CCCMoldova28 Jun 2002DD D stable CCCMoldova26 Jun 2001CC C negative CCCMoldova7 Jun 2001CCC+C negative BMoldova21 Sep 2000B-B stable BMoldova25 Nov 1999B-B-BMoldova30 Jul 1998B B-B+Mongolia23 Nov 2010B+B stable B+Mongolia11 Oct 2009B B stable BMongolia19 Jan 2009B B negative BMongolia10 Dec 2008B+B negative B+Mongolia 3 Jun 2008B+B stable B+Foreign currency rating Local curren Mongolia20 Sep 2007B+B positive B+Mongolia18 Jul 2005B+B stable B+Morocco19 Apr 2007BBB-F3stable BBB Mozambique20 Jul 2012B B positive B+ Mozambique15 Jul 2003B B stable B+Namibia9 Dec 2011BBB-F3stable BBBNamibia13 Dec 2010BBB-F3positive BBBNamibia7 Dec 2005BBB-F3stable BBBNigeria21 Oct 2011BB-B stable BBNigeria22 Oct 2010BB-B negative BBNigeria23 May 2008BB-B stable BBNigeria30 Jan 2006BB-B stable BB-Netherlands21 Sep 2000AAA F1+stable AAA Netherlands26 Oct 1995AAA F1+-AAA Netherlands10 Aug 1994AAA---New Zealand29 Sep 2011AA F1+stable AA+New Zealand15 Jul 2009AA+F1+negative AAANew Zealand18 Aug 2003AA+F1+stable AAANew Zealand27 Mar 2002AA F1+stable AAANorway21 Sep 2000AAA F1+stable AAANorway26 Oct 1995AAA F1+-AAANorway13 Mar 1995AAA---Panama 2 Jun 2011BBB F3Stable BBBPanama23 Mar 2010BBB-F3positive BBB-Panama29 Jan 2008BB+B positive BB+Panama 3 Dec 2003BB+B stable BB+Panama24 Oct 2002BB+B negative BB+Panama 4 Sep 2002BB+B Rating Watch negative BB+Panama21 Sep 2000BB+B stable BB+Panama20 Jul 2000BB+B-BB+Panama14 Feb 2000BB+B Rating Watch positive BB+Panama8 Sep 1998BB+B-BB+Papua New Guinea24 Jan 2010----Papua New Guinea13 Mar 2008B+B stable B+Papua New Guinea9 Jan 2007B B positive B+Papua New Guinea10 Jul 2003B B stable B+Papua New Guinea21 Sep 2000B+B stable BB-Papua New Guinea 1 Jan 1999B+B-BB-Peru10 Nov 2011BBB F2stable BBB+Peru 2 Jun 2010BBB-F3positive BBBPeru 2 Apr 2008BBB-F3stable BBBPeru 6 Mar 2007BB+B positive BBB-Peru31 Aug 2006BB+B stable BBB-Peru 4 Nov 2005BB B positive BB+Peru18 Nov 2004BB B stable BB+Peru 4 Jun 2004BB-B positive BB+Peru22 Oct 2003BB-B stable BB+Peru21 Aug 2002BB-B negative BB+Peru29 Apr 2002BB-B stable BB+Peru18 Apr 2001BB-B negative BB+Peru8 Nov 2000BB B Rating Watch negative BBB-Peru21 Sep 2000BB B stable BBB-Peru14 Oct 1999BB B-BBB-Philippines23 Jun 2011BB+B stable BBB-Philippines13 Feb 2006BB B stable BB+ Philippines11 Jul 2005BB B negative BB+ Philippines26 May 2005BB B stable BB+ Philippines7 Dec 2004BB B negative BB+ Philippines12 Jun 2003BB B stable BB+ Philippines25 Nov 2002BB+B negative BBB-Philippines15 Mar 2001BB+B stable BBB-Philippines17 Jan 2001BB+B Rating Watch negative BBB Philippines21 Sep 2000BB+B stable BBB Philippines8 Jul 1999BB+B-BBBPoland18 Jan 2007A-F2stable APoland23 Mar 2005BBB+F2positive APoland 6 May 2004BBB+F2stable APoland 4 Nov 2003BBB+F2positive A+Poland21 Sep 2000BBB+F2stable A+Poland19 Nov 1998BBB+F2-A+Foreign currency rating Local curren Poland7 Jun 1996BBB F3-A-Poland29 Apr 1996BB+B Rating Watch positive-Poland26 Oct 1995BB+B--Portugal24 Nov 2011BB+B negative BB+Portugal 1 Apr 2011BBB-F3Rating Watch negative BBB-Portugal24 Mar 2011A-F2Rating Watch negative A-Portugal23 Dec 2010A+F1negative A+Portugal24 Mar 2010AA-F1+negative AA-Portugal 3 Sep 2009AA F1+negative AAPortugal 1 May 2007AA F1+stable AAPortugal29 Jun 2005AA F1+negative AAPortugal21 Sep 2000AA F1+stable AAPortugal14 Jul 1998AA F1+-AAPortugal 4 Jun 1998AA F1+-AAAPortugal26 Oct 1995AA-F1+-AAAPortugal10 Aug 1994AA----Ras Al Khaimah23 Jan 2008A F1stable ARomania 4 Jul 2011BBB-F3stable BBBRomania 2 Feb 2010BB+B stable BBB-Romania9 Nov 2008BB+B negative BBB-Romania31 Jan 2008BBB F3negative BBB+Romania31 Aug 2006BBB F3stable BBB+Romania17 Nov 2004BBB-F3stable BBBRomania23 Aug 2004BB B positive BB+Romania18 Dec 2003BB B stable BB+Romania24 Sep 2003BB-B Rating Watch positive BBRomania30 Oct 2002BB-B stable BBRomania14 Jun 2002B+B stable BB-Romania14 Nov 2001B B positive B+Romania16 Nov 2000B B stable BRomania21 Sep 2000B-B stable B-Romania24 Mar 1999B-B-B-Romania23 Dec 1998B B-BB-Romania23 Sep 1998BB-B Rating Watch negative BBB-Romania11 Sep 1997BB-B-BBB-Romania 6 Mar 1996BB-B--Russia16 Jan 2012BBB F3stable BBBRussia8 Sep 2010BBB F3positive BBBRussia22 Jan 2010BBB F3stable BBBRussia 4 Feb 2009BBB F3negative BBBRussia9 Nov 2008BBB+F2negative BBB+Russia25 Jul 2006BBB+F2stable BBB+Russia 3 Aug 2005BBB F3stable BBBRussia18 Nov 2004BBB-F3stable BBB-Russia13 May 2003BB+B stable BB+Russia 2 May 2002BB-B positive BB-Russia 4 Oct 2001B+B stable BRussia17 Jul 2001B B positive B-Russia21 Sep 2000B B stable B-Russia29 Aug 2000B B-B-Russia8 May 2000B-B--Russia14 Feb 2000CCC C Rating Watch positive-Russia27 Aug 1998CCC C--Russia17 Aug 1998B-B Rating Watch negative-Russia30 Jul 1998BB-B--Russia7 Jun 1998BB B Rating Watch negative-Russia 5 Jun 1998BB+B Rating Watch negative-Russia10 Mar 1998BB+B--Russia12 Feb 1998BB+B Rating Watch negative-Russia7 Oct 1996BB+B--Rwanda24 Aug 2010B B stable BRwanda16 Dec 2006B-B positive B-San Marino23 Jul 2012BBB+F2negative-San Marino22 Oct 2009A F1negative-San Marino13 May 2009AA-F1+Rating Watch negative-San Marino10 Jan 2001AA F1+stable-Saudi Arabia9 Jul 2008AA-F1+stable AA-Saudi Arabia31 Jul 2007A+F1positive A+Saudi Arabia17 Aug 2006A+F1stable A+。
1-2_2004_jun_q

23An organisation manufactures and sells a single product. At the budgeted level of output of 2,400 units per week, the unit cost and selling price structure is as follows:£ per unit£ per unitSelling price60Less– variable production cost15– other variable cost15– fixed cost30–––(50)–––Profit 10–––What is the breakeven point (in units per week)?A1,200B1,600C1,800D2,4004 A company manufactures one product which it sells for £40 per unit. The product has a contribution to sales ratio of40%. Monthly total fixed costs are £60,000. At the planned level of activity for next month, the company has a margin of safety of £64,000 expressed in terms of sales value.What is the planned activity level (in units) for next month?A3,100B4,100C5,350D7,7505 A company manufactures and sells two products (X and Y) both of which utilise the same skilled labour. For thecoming period, the supply of skilled labour is limited to 2,000 hours. Data relating to each product are as follows: Product X YSelling price per unit£20£40Variable cost per unit£12£30Skilled labour hours per unit24Maximum demand (units) per period800400In order to maximise profit in the coming period, how many units of each product should the company manufacture and sell?A200 units of X and 400 units of YB400 units of X and 300 units of YC600 units of X and 200 units of YD800 units of X and 100 units of Y3[P.T.O.6An organisation manufactures a single product. The total cost of making 4,000 units is £20,000 and the total cost of making 20,000 units is £40,000. Within this range of activity the total fixed costs remain unchanged.What is the variable cost per unit of the product?A£0·80B£1·20C£1·25D£2·007In a short-term decision-making context, which ONE of the following would be a relevant cost?A Specific development costs already incurred.B The cost of special material which will be purchased.C Depreciation on existing fixed assets.D The original cost of raw materials currently in stock which will be used on the project.8The stock records for one specific stores item for last month show the following information:Date Receipts Issuesunits units14th15013th60015th20022nd250The stock at the beginning of last month consisted of 200 units valued at £5,200.The receipts last month cost £32·50 per unit.Using the FIFO method of valuation, what was the total cost of last month’s issues?A£18,200B£18,300C£18,525D£19,5009The demand for a product is 12,500 units for a three month period. Each unit of product has a purchase price of £15 and ordering costs are £20 per order placed.The annual holding cost of one unit of product is 10% of its purchase price.What is the Economic Order Quantity (to the nearest unit)?A577B816C866D1,155410 A company determines its order quantity for a raw material by using the Economic Order Quantity (EOQ) model.What would be the effects on the EOQ and the total annual holding cost of a decrease in the cost of ordering a batch of raw material?EOQ Total annual holding costA Higher LowerB Higher HigherC Lower HigherD Lower Lower11 A company manufactures two products, X and Y, in a factory divided into two production cost centres, Primary andFinishing. The following budgeted data are available:Cost centre Primary FinishingAllocated and apportioned fixedoverhead costs £96,000£82,500Direct labour minutes per unit:– product X3625– product Y4835Budgeted production is 6,000 units of product X and 7,500 units of product Y.Fixed overhead costs are to be absorbed on a direct labour hour basis.What is the budgeted fixed overhead cost per unit for product Y?A£11B£12C£14D£1512 A company uses an overhead absorption rate of £3·50 per machine hour, based on 32,000 budgeted machine hoursfor the period. During the same period the actual total overhead expenditure amounted to £108,875 and 30,000 machine hours were recorded on actual production.By how much was the total overhead under or over absorbed for the period?A Under absorbed by £3,875B Under absorbed by £7,000C Over absorbed by £3,875D Over absorbed by £7,00013 A company manufactures and sells a single product. For this month the budgeted fixed production overheads are£48,000, budgeted production is 12,000 units and budgeted sales are 11,720 units.The company currently uses absorption costing.If the company used marginal costing principles instead of absorption costing for this month, what would be the effect on the budgeted profit?A£1,120 higherB£1,120 lowerC£3,920 higherD£3,920 lower5[P.T.O.14For which of the following is a profit centre manager normally responsible?A Costs onlyB Revenues onlyC Costs and revenuesD Costs, revenues and investment.The following information relates to questions 15 and 16:The standard direct material cost per unit for a product is calculated as follows:10·5 litres at £2·50 per litreLast month the actual price paid for 12,000 litres of material used was 4% above standard and the direct material usage variance was £1,815 favourable. No stocks of material are held.15What was the adverse direct material price variance for last month?A£1,000B£1,200C£1,212D£1,26016What was the actual production last month (in units)?A1,074B1,119C1,212D1,25817 A company operates a standard marginal costing system. Last month its actual fixed overhead expenditure was 10%above budget resulting in a fixed overhead expenditure variance of £36,000.What was the actual expenditure on fixed overheads last month?A£324,000B£360,000C£396,000D£400,00018Last month a company budgeted to sell 8,000 units at a price of £12·50 per unit.Actual sales last month were 9,000 units giving a total sales revenue of £117,000.What was the sales price variance for last month?A£4,000 favourableB£4,000 adverseC£4,500 favourableD£4,500 adverse619Which department would normally be responsible for completing a standard purchase requisition for goods in a service organisation?A The buying (purchasing) departmentB The department that requires the goodsC The goods inwards departmentD The accounting department staff.20Regression analysis is being used to find the line of best fit (y = a + bx) from eleven pairs of data. The calculations have produced the following information:∑x = 440, ∑y = 330, ∑x2= 17,986, ∑y2= 10,366 and ∑xy = 13,467What is the value of ‘a’ in the equation for the line of best fit (to 2 decimal places)?A0·63B0·69C2·33D5·3321The following information relates to a management consultancy organisation:Salary cost per hour for senior consultants£40Salary cost per hour for junior consultants£25Overhead absorption rate per hour applied to all hours£20The organisation adds 40% to total cost to arrive at the final fee to be charged to a client.Assignment number 789 took 54 hours of a senior consultant’s time and 110 hours of junior consultants’ time.What is the final fee to be charged for Assignment 789?A£6,874B£10,696C£11,466D£12,6427[P.T.O.22T wo products G and H are created from a joint process. G can be sold immediately after split-off. H requires further processing before it is in a saleable condition. There are no opening stocks and no work in progress. The following data are available for last period:£T otal joint production costs384,000Further processing costs (product H)159,600Product Selling price Sales Productionper unit units unitsG£0·84400,000412,000H£1·82200,000228,000Using the physical unit method for apportioning joint production costs, what was the cost value of the closing stock of product H for last period?A£36,400B£37,520C£40,264D£45,18123 A company manufactures and sells a single product. The variable cost of the product is £2·50 per unit and allproduction each month is sold at a price of £3·70 per unit. A potential new customer has offered to buy 6,000 units per month at a price of £2·95 per unit. The company has sufficient spare capacity to produce this quantity. If the new business is accepted, sales to existing customers are expected to fall by two units for every 15 units sold to the new customer.What would be the overall increase in monthly profit which would result from accepting the new business?A£1,740B£2,220C£2,340D£2,70024 A company manufactures four components (L, M, N and P) using the same general purpose machinery. Weeklydemand is 1,500 units of each component but only 24,000 machine hours are available each week. A decision has to be made on which component to buy in from an outside supplier. The following data are available:11L M N P Variable production cost (£ per unit)45403020General purpose machinery hours per unit13151416Purchase price from outside supplier (£ per unit)57555450In order to minimise total cost, which component should be purchased from the outside supplier each week?A Component LB Component MC Component ND Component P89[P.T.O.Section B – ALL FIVE questions are compulsory and MUST be attempted1Duddon Ltd makes a product that has to pass through two manufacturing processes, I and II. All the material is input at the start of process I. No losses occur in process I but there is a normal loss in process II equal to 7% of the input into that process. Losses have no realisable value.Process I is operated only in the first part of every month followed by process II in the second part of the month. All completed production from process I is transferred into process II in the same month. There is no work in progress in process II.Information for last month for each process is as follows:Process IOpening work in progress200 units (40% complete for conversioncosts) valued in total at £16,500Input into the process1,900 units with a material cost of £133,000Conversion costs incurred£93,500Closing work in progress50% complete for conversion costsProcess IIT ransfer from process I1,800 unitsConversion costs incurred£78,4501,650 completed units were transferred to the finished goods warehouse.Required:(a)Calculate for process I:(i)the value of the closing work in progress; and(ii)the total value of the units transferred to process II.(4 marks)(b)Prepare the process II account for last month.(4 marks)(c)Identify TWO main differences between process costing and job costing.(2 marks)(10 marks)102Coledale Ltd manufactures and sells product CC. The company operates a standard marginal costing system.The standard cost card for CC includes the following:£ per unitDirect material20Direct labour (6 hours at £7·50 per hour)45Variable production overheads27–––92–––The budgeted and actual activity levels for the last quarter were as follows:Budget Actualunits unitsSales20,00019,000Production20,00021,000The actual costs incurred last quarter were:£Direct material417,900Direct labour (124,950 hours)949,620Variable production overheads565,740Required:(a)Calculate the total variances for direct material, direct labour and variable production overheads.(3 marks)(b)Provide an appropriate breakdown of the total variance for direct labour calculated in (a).(3 marks)(c)Suggest TWO possible causes for EACH variance calculated in (b).(4 marks)(10 marks)3Braithwaite Ltd manufactures and sells a single product. The following data have been extracted from the current year’s budget:Contribution per unit£8T otal weekly fixed costs£10,000Weekly profit£22,000Contribution to sales ratio40%The company’s production capacity is not being fully utilised in the current year and three possible strategies are under consideration. Each strategy involves reducing the unit selling price on all units sold with a consequential effect on the budgeted volume of sales. Details of each strategy are as follows:Strategy Reduction in unit Expected increase in weeklyselling price sales volume over budget%%A210B518C725The company does not hold stocks of finished goods.Required:(a)Calculate for the current year:(i)the selling price per unit for the product; and(ii)the weekly sales (in units). (3 marks)(b)Determine, with supporting calculations, which one of the three strategies should be adopted by the companyin order to maximise weekly profits.(4 marks)(c)Briefly explain the practical problems that a management accountant might encounter in separating costsinto their fixed and variable components.(3 marks)(10 marks)4Ennerdale Ltd has been asked to quote a price for a one-off contract. The company’s management accountant has asked for your advice on the relevant costs for the contract. The following information is available:MaterialsThe contract requires 3,000 kg of material K, which is a material used regularly by the company in other production.The company has 2,000 kg of material K currently in stock which had been purchased last month for a total cost of £19,600. Since then the price per kilogram for material K has increased by 5%.The contract also requires 200 kg of material L. There are 250 kg of material L in stock which are not required for normal production. This material originally cost a total of £3,125. If not used on this contract, the stock of material L would be sold for £11 per kg.LabourThe contract requires 800 hours of skilled labour. Skilled labour is paid £9·50 per hour. There is a shortage of skilled labour and all the available skilled labour is fully employed in the company in the manufacture of product P. The following information relates to product P:£ per unit£ per unitSelling price100LessSkilled labour38Other variable costs22–––(60)–––40–––Required:(a)Prepare calculations showing the total relevant costs for making a decision about the contract in respect ofthe following cost elements:(i)materials K and L; and(ii)skilled labour. (7 marks)(b)Explain how you would decide which overhead costs would be relevant in the financial appraisal of thecontract.(3 marks)(10 marks)5Langdale Ltd is a small company manufacturing and selling two different products – the Lang and the Dale. Each product passes through two separate production cost centres – a machining department, where all the work is carried out on the same general purpose machinery, and a finishing section. There is a general service cost centre providing facilities for all employees in the factory.The company operates an absorption costing system using budgeted overhead absorption rates. The management accountant has calculated the machine hour absorption rate for the machining department as £3·10 but a direct labour hour absorption rate for the finishing section has yet to be calculated.The following data have been extracted from the budget for the coming year:Product Lang DaleSales (units)6,00019,000Production (units)7,20010,400Direct material cost per unit£52£44Direct labour cost per unit:– machining department (£8 per hour)£72£40– finishing section (£6 per hour)£42£36 Machining department – machine hours per unit53Fixed production overhead costs:£– machining department183,120– finishing section241,320– general service cost centre182,800Number of employees:– machining department14– finishing section32– general service cost centre14Service cost centre costs are reapportioned to production cost centres.Required:(a)Calculate the direct labour hour absorption rate for the finishing section.(5 marks)(b)Calculate the budgeted total cost for one unit of product Dale only, showing each main cost elementseparately.(2 marks)(c)The company is considering a change over to marginal costing. State with reasons, whether the total profitfor the coming year calculated using marginal costing would be higher or lower than the profit calculated using absorption costing. No calculations are required. (3 marks)(10 marks)Formulae SheetEnd of Question Paper。
2004_Ann. N.Y. Acad. Sci_Quantitative PCR Analysis of Mitochondrial DNA Content

304Ann. N.Y. Acad. Sci. 1011: 304–309 (2004). © 2004 New York Academy of Sciences.doi: 10.1196/annals.1293.029Quantitative PCR Analysis of Mitochondrial DNA Content in Patients withMitochondrial DiseaseREN-KUI BAI, CHERNG-LIH PERNG, CHANG-HUNG HSU, AND LEE-JUN C. WONGInstitute for Molecular and Human Genetics, Georgetown University Medical Center, Washington DC 20007, USAA BSTRACT :Molecular diagnosis of mitochondrial DNA disorder is usually focused on point mutations and large deletions. In the absence of detectable mtDNA mutations, abnormal amounts of mtDNA, either depletion or elevation, can be indicative of mitochondrial dysfunction. The amount of mitochondrial DNA (mtDNA), however, varies among individuals of different ages and among different tissues within the same individual. To establish a range of mtDNA levels, we analyzed 300 muscle and 200 blood specimens from patients suspected of having a mitochondrial disorder by real-time quan-titative polymerase chain reaction (PCR) method. Copy numbers were calcu-lated from the standard curve and threshold cycle number using TaqMan probes; 6FAM 5′TTACCGGGCTCTGCCA TCT3′-TAMRA and VIC-5′AGCAA TAACAGGTCTGTGA TG3′-TAMRA for mtDNA and 18S rRNA gene (nDNA), respectively. The copy number ratio of mtDNA to nDNA was used as a measure of mtDNA content in each specimen. The mtDNA content in muscle increases steadily from birth to about 5 years of age; thereafter, it stays about the same. On the contrary, the mtDNA content in blood decreases with age. The amount of mtDNA in skeletal muscle is about 5–20 times higher than that in blood. About 7% of patients had mtDNA levels in muscle below 20% of the mean of the age-matched group, and about 10% of patients had muscle mtDNA levels 2- to 16-fold higher than the mean of the age-matched group.Patients with abnormal levels of mtDNA, either depletion or proliferation, had significant clinical manifestations characteristic of mitochondrial disease in addition to abnormal respiratory enzymes and mitochondrial cytopathies.Cardiomyopathy, lactic acidosis, abnormal brain MRI findings, hypotonia,developmental delay, seizures, and failure to thrive are general clinical pictures of patients with mtDNA depletion. The average age of patients with mtDNA depletion is 4.1 years, compared to 23.6 years in patients with mtDNA prolifer-ation. Mutations in nuclear genes involved in mtDNA synthesis and deoxy-nucleotide pools are probably the cause of mtDNA depletion. Our results demonstrate that real time quantitative PCR is a valuable tool for molecular screening of mitochondrial diseases.K EYWORDS :mtDNA depletion; quantitative analysis of mtDNA; real-time PCR analysis; mitochondrial DNA copy numberAddress for correspondence: Lee-Jun C. Wong, Ph.D., Institute for Molecular and Human Genetics, Georgetown University Medical Center, M4000, 3800 Reservoir Rd., NW, Washington,DC 20007. Voice: 202-784-0760; fax: 202-784-1770.wonglj@305 BAI et al.: PCR ANALYSIS IN MITOCHONDRIAL DISEASEINTRODUCTIONPoint mutations and large deletions in mtDNA account for the molecular defects in a small portion of patients with mitochondrial respiratory deficiency.1–3 Possibly the respiratory defects in some of these patients are caused by a quantitative defi-ciency in mtDNA content rather than specific mutations. Previous studies indicate that mtDNA depletion due to mutations in the thymidine phosphorylase (TP) gene are responsible for the mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) syndrome.4–6 Mutations in nuclear genes that are involved in mtDNA synthesis or maintenance of deoxynucleotide pools may affect the biogenesis of mitochondria and therefore affect the mtDNA content. On the other hand, defective mitochondria are often proliferated. Thus, mtDNA amplification could be a compen-satory mechanism in response to inefficient mitochondrial respiratory function. Here, we report the use of a real-time quantitative PCR assay to determine the mtDNA content in muscle and blood. Quantitative alterations in mtDNA may have implications in molecular defects of nuclear or mitochondrial genes.MATERIAL AND METHODSSpecimens and DNA IsolationPatients were referred to the Molecular Genetics Laboratory, Institute for Molec-ular and Human Genetics at Georgetown University Medical Center for molecular diagnosis of mitochondrial disorders. Total DNA was isolated from 300 muscle specimens using proteinase K digestion followed by standard phenol/chloroform extraction and ethanol precipitation.7 Blood DNA was extracted by a salting-out method8 from peripheral blood lymphocytes from 200 patients.Primers and ProbesThe primers for RT Q-PCR analysis of mtDNA are mtF3212 (5′CACCCAAGAACAGGGTTTGT3′) and mtR3319 (5′TGGCCA TGGGTA TGTT-GTTAA3′), those for the nuclear DNA (nDNA), 18S rRNA gene are 18S1546F (5′TAGAGGGACAAGTGGCGTTC3′) and 18S1650R (5′CGCTGAGCCAGTCA-GTGT3′).9 The TaqMan probes; 6FAM-5′TTACCGGGCTCTGCCA TCT3′-TAMRA and VIC-5′AGCAA TAACAGGTCTGTGA TG3′-TAMRA, were labeled at the 5′ end with a fluorescent reporter, 6FAM and VIC, for the mtDNA and the nDNA 18S rRNA gene, respectively, whereas the 3′ ends were labeled with a quencher TAMRA. The 10 µL PCR reaction contains 1× TaqMan Universal PCR Master Mix (ABI P/N 4304437), 500nM of each primer, 200nM of TaqMan probe, and 0.2–2 ng of total genomic DNA extract. PCR conditions are 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s of denaturation at 95°C and 60 s of annealing/extension at 60°C. Real-time quantitative analysis was performed on the Sequence Detector System ABI-Prism 7700.9306ANNALS NEW YORK ACADEMY OF SCIENCESFIGURE1.Real-time PCR analysis. Standard curve of the mtDNA and the 18S rRNA gene. The cloned PCR products from both the mtDNA and the 18S rDNA were serially diluted for real-time PCR. The copy number in each dilution was calculated from the actual DNA concentration. The threshold cycle number (C T) was plotted against the copy number of the DNA template at the start of PCR. DNA samples of unknown copy numbers were analyzed using the same conditions. The copy number of mtDNA and nDNA was calculated from the C T number and by use of the standard curve.307BAI et al .: PCR ANALYSIS IN MITOCHONDRIAL DISEASE Standard CurveStandard DNA solutions for the mitochondrial genome and the nuclear 18S rRNA gene (nDNA) were generated from PCR products cloned in a vector of pCR2.1-TOPO. Serial dilutions were made and RT Q-PCR reactions were performed as just described to construct the standard curve from the C T values and the number of copies of the standard plasmid DNA (F IG . 1A).Determination of mtDNA/nDNA Ratio as a Measure of mtDNA Content F IGURE 1B illustrates a sample run. The copy number of the mtDNA and the nDNA is calculated using the threshold cycle number (C T ) and intrapolating from the standard curve. The ratio of the copy number of mtDNA to the copy number of nDNA is the measurement of mtDNA content. F IGURE 1B shows four specimens with mtDNA/nDNA ratio from 0.32 to 239.RESULTSThe ratio of mtDNA/nDNA in muscle varies from 0.1 to 1700, and in blood varies from 0.05 to 23. Evidently, some patients had markedly elevated mtDNA and some had severely depleted mtDNA, particularly in muscle. The average mtDNA content was calculated for each age group by excluding the samples in the highest and lowest 10%. The results show that the mtDNA content increases from birth to about 5 years of age and remains at about the same level after that (F IG . 2A). On the contrary, the mtDNA content in blood decreases with age (F IG . 2B). The amount of mtDNA in skeletal muscle is about 5–20 times higher than that in blood. About 7% of patients TABLE parison between the two groups of patients with mtDNA depletion and mtDNA proliferationmtDNA proliferation (>twofold of mean)mtDNA depletion (<30% of mean)Total number 29/30027/300Average age 23.6 years4.1 years Male/female 12/1711/16<10-year-old 15 (9/15 >3.6-fold)24Major clinical manifestationMuscle weaknessExercise intoleranceFatigabilityOphthalmoplegiaLactic acidosisSeizureHypotoniaDevelopmental delayAbnormal histochemistryAbnormal respiratory enzyme Cardiomyopathy Low plasma carnitine Failure to thrive Abnormal MRI Lactic acidosis Seizure Hypotonia Developmental delay Abnormal histochemistry Abnormal respiratory enzymeOphthalmoplegia Elevated CSF protein308ANNALS NEW YORK ACADEMY OF SCIENCES had mtDNA levels in muscle below 20% of the age-matched mean, and about 10%of patients had muscle mtDNA levels 2- to 16-fold higher than that of the age-matched mean. Patients with an abnormally high or low level of mtDNA shared some significant clinical features of mitochondrial disease as well as abnormal res-piratory enzymes and mitochondrial cytopathies (T ABLE 1). Cardiomyopathy, lactic acidosis, abnormal brain MRI, hypotonia, developmental delay, seizure, and failure to thrive are general clinical manifestations that seem to be associated with young patients (<10) with mtDNA depletion (<30% of age-matched mean). However,muscle weakness, exercise intolerance, ophthalmoplegia, and lactic acidosis seem to be the predominant clinical symptoms of patients with elevated mtDNA content. The average age, 4.1 and 23.6 years for patients with mtDNA depletion and mtDNA pro-liferation, respectively, is significantly different (T ABLE1).FIGURE 2.mtDNA content in muscle and blood of various age groups of patients mea-sured by mtDNA/18Sr DNA copy number ratios. (A ) Muscle specimens; (B ) blood specimens.309 BAI et al.: PCR ANALYSIS IN MITOCHONDRIAL DISEASEDISCUSSION AND CONCLUSIONMost patients with severe mtDNA depletion are young children, with an average age of 4.1 years. We sequenced the TP gene of these patients and found mutations in only 1 patient (data not shown) who was 20 years old. Although mutations in the TP gene are the main cause of mtDNA depletion in adult patients with MNGIE, TP gene mutations do not seem to be the reason for mtDNA depletion in children <10 based on these results. It is very likely that mutations in other nuclear genes involved in mtDNA synthesis or deoxynucleotide pools, such as polymerase gamma, DNA heli-case, nucleotide translocase, or thymidine kinase, are responsible for the mtDNA depletion in these patients. The patients who had mtDNA proliferation were much older, and their muscle biopsies showed evidence of ragged-red fibers. The increase in mtDNA content in these cases correlates with mitochondrial proliferation and this is probably a compensatory mechanism for defective mitochondria. Knowing the mtDNA content in muscle tissue will facilitate the search for the molecular defects responsible for the mitochondrial disease.REFERENCES1.L IANG, M.H. & L.-J.C. W ONG. 1998. Yield of mtDNA mutation analysis in 2000patients. Am. J. Med. Genet. 77: 385–400.2.W ONG, L.-J.C. 2001. Recognition of mitochondrial DNA deletion syndrome with non-neuromuscular multisystemic manifestation. Genet. Med. 3: 399–404.3.W ONG, L.-J.C., M.-H. L IANG, H. K WON, et al. 2002. Comprehensive scanning of thewhole mitochondrial genome for mutations. Clin. Chem. 48: 1901–1912.4.N ISHINO, I., A. S PINAZZOLA & M. H IRANO. 1999. Thymidine phosphorylase gene muta-tions in MNGIE, a human mitochondrial disorder. Science 283: 689–692.5.N ISHINO, I., A. S PINAZZOLA, A. P APADIMITRIOU, et al. 2000. Mitochondrial neurogas-trointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann. Neurol. 47: 792–800.6.H IRANO, M., R. M ARTI, C. F ERREIRO-B ARROS, et al. 2001. Defects of intergenomiccommunication: autosomal disorders that cause multiple deletions and depletion of mitochondrial DNA. Semin. Cell Dev. Biol. 12: 417–427.7.W ONG, L.-J.C. & C. L AM. 1997. Alternative, noninvasive tissues for quantitativescreening of mutant mitochondrial DNA. Clin. Chem. 43: 1241–1243.8.L AHIRI, D. & J. N URNBERGER, J R. 1991. A rapid non-enzymatic method for the prepara-tion of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 19: 5444.9.W ONG, L.-J.C. & R. B AI. 2002. Real-time quantitative PCR analysis of mitochodnrialDNA in patients with mitochondrial disease. Am. J. Hum. Genet. Suppl. 71: 501.。
SAMPLE ADMINISTRATIVE PROCESSING RESUME (2) (1)(1)

Name (Please use Characters and Pinyin):Date of Birth:Place of Birth (Please use Characters and Pinyin):Current Passport Number:Preferred Mailing Address (Please use Chinese):Home Address(Please use Chinese):E-mail:Cell Phone:Home Phone:Work Phone:Purpose of Travel (1-2 sentences explaining your reason for going to the United States):Short Itinerary: (list your Itinerary in order and your expected date of arrival)Beijing-Chicago-New York-BeijingDate of Arrival to USA: 25-Jun-2011US Contact:Name:Relationship:Company:Address:Phone Number:E-mail:Accompanying TravelersName (Please use Characters and Pinyin):DOB:1Relationship (Please use Characters and Pinyin):EDUCATION:(List with most recent at the top. Include dates, field of study, degree earned, school name and location)Sep. 2001 to Jun. 2004: PH.D, Chemistry, Peking University, Beijing, China.Sep. 1998 TO Jun. 2001: M.S., Chemistry, Peking University, Beijing, China.Sep. 1994 TO Jul. 1998: B.S., Chemistry, Peking University, Beijing, China.PROFESSIONAL EXPERIENCE:(Please list all work experience starting with the most recent job and listing backwards. Please include dates of employment, position title, company name, location, and 1-2 sentences on your job duties.)Jun. 2010 to Present: Head of Research and Development, China Chemical Company, Beijing, ChinaManaged the research and development team in a large chemical company. Research is primarily focused on polymer research.Sep. 2006 to Jun. 2010: Chemical Engineer, China Chemical Company, Beijing, ChinaWorked developing new polymers and chemicals for a large chemical company.Jul. 2004 to Sep. 2006: Associate Professor, Qingdao University, Qingdao, ChinaTaught graduate level college courses including Organic Chemistry, Catalysts, Chemical Analysis. Conducted research on chemical interaction with organic metals.Publications –please list all publications, if anyPrevious US Visa:(List type of visa and date issued for all previous US visas)J2, 29-JUN-2006Passport Numbers:2(Please list all passports you have held, include the passport number, passport type, and expiration date.) P8888888, Private passport, June 2014G8888888, Service Passport, July 2011Previous Travel:(Please list all travel in last 10 years with dates, place of travel, and reason for travel.)July 2009, England, TourismFeb. 2009, Japan, Chemistry ConferenceJune 2008, Singapore, Chemical Conference3。
一种水基防锈剂的制备研究

第50卷第1期2021年1月辽宁化工Liaoning Chemicdl IndustryVol.50,No.1Janudry,2021—种水基防锈剂的制备研究张宏军(新东北电气集团高压开关有限公司,辽宁沈阳110027)摘要:防锈剂保护技术是一种广泛采用的金属防腐蚀技术,试验以山梨醇、三乙醇胺、苯甲酸钠、硼酸、碳酸钠为主要原料制备水基环保防锈剂。
试验主要考察反应温度、原料用量配比、防锈剂稀释质量分数对防锈效果的影响.同时采用盐雾试验与车间自然环境下测试防锈实际效果:本制备工艺简单,成本较低,所制备防锈剂效果良好.使用方便。
关键词:水基防锈剂;环保;金属腐蚀中图分类号:TQ05096文献标识码:A文章编号:1004-0935(2021)01-0012-03金属在储运、生产过程中,很难不与空气中的氧、湿气或其他腐蚀性介质接触,这些物质在金属表面发生电化学腐蚀而生锈,要防止锈蚀就得阻止以上物质与金属接触山。
一旦发生锈蚀,不但对金属材料的性能产生影响,甚至会造成材料的报废⑵。
据不完全统计,全世界每年因生锈而报废的金属材料有几千万吨,因腐蚀造成的经济损失高达上千亿人民币【2】。
因此,金属材料的防腐蚀研究具有重要意义。
金属防锈剂分为水基型和油基型⑸。
油基型防锈性能虽好,但成本高,而且后期处理比较困难。
水基防锈液使用方便易去除,且价格低廉、防腐效果好,目前应用比较广泛。
水基防锈液多数为含磷酸盐或亚硝酸盐型,但亚硝酸盐可转化成致癌物,出于环境保护与人体健康考虑,其使用和排放均受到了严格限制【"I。
近几年,环保型水基防锈剂已成为国内外的研究热点。
本文以山梨醇、三乙醇胺、苯甲酸钠、硼酸、碳酸钠为主要原料制备一种水基防锈剂,并在新东北电气集团电镀车间试用。
1实验研究1.1实验原料和设备实验的主要原料和设备见表1和表2。
表1原料与试剂试剂名称规格厂家山梨醇分析纯大连通用化工有限公司三乙醇胺分析纯无锡百川化工有限公司苯甲酸钠分析纯江苏灵谷化工有限公司硼酸分析纯天津市同鑫化工有限公司碳酸钠分析纯大连通用化工有限公司实验用水为过滤纯净水。
Span80_Tween80液体石蜡AM_H_2O反相微乳液体系

第56卷 第2期 化 工 学 报 Vol 156 No 12 2005年2月 Journal of Chemical Industry and Engineering (China ) February 2005研究简报Span 802Tween 80/液体石蜡/AM 2H 2O反相微乳液体系王风贺,夏明珠,雷 武,魏运洋,王风云(南京理工大学工业化学研究所,江苏南京210094)关键词:微乳液;电导率;Span 80;Tween 80;丙烯酰胺中图分类号:O 648114 文献标识码:A文章编号:0438-1157(2005)02-0368-04Inverse microemulsion system ofSp an 802Tween 80/liquid paraffin/acrylamide 2H 2OWANG Fenghe ,XI A Mingzhu ,LEI Wu ,WEI Y unyang ,WANG Fengyun(I ndust rial Chemist ry I nstitute ,N anj ing Universit y of Science and Technology ,N anj ing 210094,J iangsu ,China )Abstract :The reverse microemulsion system of Span 802Tween 80/liquid paraffin/acrylamide 2H 2O was p repared ,and t he effect s of n 2butanol ,NaCl ,NaAc were st udied wit h t he conductivity met hod.The stability of reverse microemulsion was reflected ,and t he information of it s dynamic process was obtained by measuring it s state changes continuously t hrough testing it s elect ronic co nductivity by using electrochemist ry.The result s showed t hat t he Span 802Tween 80/liquid paraffin/acrylamide 2H 2O system was most stable when t he HLB value app roached 715,and it s stability was enhanced when 510%(mass )NaCl or 10%(mass )NaAc was added into t his system.Key words :microemulsion ;conductivity ;Span 80;Tween 80;acrylamide 2004-04-12收到初稿,2004-08-02收到修改稿.联系人:王风云.第一作者:王风贺(1976—),男,博士研究生.引 言反相微乳液是将两种互不相溶的油相和水相在表面活性剂和助表面活性剂的作用下形成的各向同性的、热力学稳定的、透明或半透明、粒径在1~100nm 范围的W/O 型的分散体系.自从1943年Hoar 和Schulman [1]首先报道微乳液以来,关于微乳液的研究和应用就一直是人们研究的热点.反相微乳液法是近年来发展的一种制备纳米材料的新方法,由于其热力学稳定性高,粒径细小、均匀等特点,被广泛用来进行催化剂、半导体、超导体、磁性材料等的制备,特别是近年来兴起的药物的微胶囊化、无机纳米材料、水溶性高分子的制备和提高石油采收率等方面的应用[2~11].但是文献中多采用苯系衍生物为油相,乳化剂也多采用琥珀酸二异辛脂磺酸钠(AO T ),难于实现工业化生产. Received date :2004-04-12.Corresponding author :Prof.WAN G Fengyun.E -mail :wangfywater @yahoo 1com 1cn本文通过连续测定体系电导率的变化,制备了Span 802Tween 80/液体石蜡/AM 2H 2O 体系的反相微乳液,并研究正丁醇、NaCl 和NaAc 对体系的影响,为下一步反相微乳液聚合制备聚丙烯酰胺奠定了基础.1 实验部分111 药品与仪器药品:液体石蜡,Span 80,Tween 80,丙烯酰胺(AM ),正丁醇,氯化钠(NaCl )和乙酸钠(NaAc)均为分析纯;蒸馏水,自制.仪器:磁力搅拌器(7821型),电导仪(DDS2 11型),微量进样器(100μl),超级恒温装置. 112 实验[12]将一定质量比(HLB值)的Span80和Tween80溶解在液体石蜡中,形成油相;按一定浓度将AM溶解在蒸馏水中,形成水相[14].在100ml的烧杯中加入50ml的油相,置于磁力搅拌器中,恒温,搅拌.用微量进样器加入水相,形成反相(W/O型)微乳液.观察体系的导电情况,待体系的电导率稳定后,记录电导率[13,14].2 结果与讨论211 H LB值对反相微乳液体系的影响反相微乳液的稳定性主要依赖于吸附在油相表面上的非离子乳化剂分子的空间位阻效应.用于反相微乳液聚合的乳化剂按照亲水2亲油平衡(HLB)值的原则,一般应选择HLB=3~6.文献表明,将2种乳化剂复配使用的效果要比使用单一乳化剂好[15].本文根据不同Span80和Tween80的质量比配制具有不同HLB值的乳化体系,并研究了HLB值对微乳液形成时电导率的影响,见图1.Fig11 Influence of HLB value on reversemicroemulsion system(Because t here are more data examined,and some data are overlaped,so it is shown as t hicker line in Fig11.A similar phenomenon is shown in Figs12~41) HLB:◆15;■1319;▲1017;×917;●715;+413 由图1可知,随着HLB值的增大,体系增溶同样的水相时,电导率相应增大.当HLB值为715时,体系电导率的变化较小,而且体系的稳定性较好,有利于后续反相微乳液聚合稳定的进行.因此,本文以HLB值为715的微乳液体系作为下一步的研究对象.212 正丁醇对反相微乳液体系的影响图2为正丁醇对微乳液体系的影响.Fig12 Influence of n2butanol onreverse microemulsion systemn2butanol/(mass):◆0;■215;▲510;×10由图2可以看出,随着正丁醇含量的增大,体系的电导率的变化逐渐变小.因为中、长链的醇(如丁醇),也可视为助乳化剂.在2种乳化剂分子交替吸附于微乳液滴的表面上,丁醇的加入相当于在非离子型乳化剂之间又 入了另一种乳化剂分子,因此可以增强微乳液体系的稳定性[16].10% (质量)的正丁醇的电导率几乎没有变化,此时所形成的微乳液体系非常稳定,而且所增溶的水相也比较高,对后续的微乳液聚合而言,可以提高聚合物的固含量.213 电解质对体系的影响Holtzscherer等[17]的研究结果表明,具有盐析效应的电解质可以增加微乳液体系的稳定性.因此本文对NaCl、NaAc在微乳液形成过程中的作用进行了初步探讨.试验结果见图3、图4.Fig13 Influence of NaCl on reversemicroemulsion systemNaCl/%(mass):◆0;■215;▲510;×10由图3、图4可知,对Span802Tween80/液体石蜡/AM2H2O反相微乳液体系而言,加入・963・ 第2期 王风贺等:Span802Tween80/液体石蜡/AM2H2O反相微乳液体系Fig14 Influence of NaAc on reversemicroemulsion systemNaAC/%(mass):◆0;■215;▲510;×10510%(质量)NaCl或10%(质量)NaAc可以较好地增强体系的稳定性,说明具有盐析效应的电解质的存在可以使微乳液滴进一步细化,有助于微乳液体系的形成和稳定.此时应该是研究改性丙烯酰胺反相微乳液聚合比较合适的体系.3 结 论对Span802Tween80/液体石蜡/AM2H2O 体系:(1)当HLB值为715时,体系电导率的变化较小,体系稳定性较好,有利于后续反相微乳液聚合的研究;(2)丁醇的加入可以增强微乳液体系的稳定性,随着正丁醇含量的增大,体系的电导率的变化逐渐变小,10%(质量)的正丁醇所形成的微乳液体系非常稳定,而且所增溶的水相也比较高,对后续的微乳液聚合而言,可以提高聚合物的固含量;(3)加入510%(质量)NaCl或10%(质量)NaAc可以较好地增强体系的稳定性,说明具有盐析效应的电解质的存在可以使微乳液滴进一步细化,有助于微乳液体系的形成和稳定.符 号 说 明W———溶水量,μlσ———电导率,μS・cm-1References[1] Shao Qinghui(邵庆辉),Gu Guobang(古国榜),ZhangLijuan(章莉娟),Shen Peikang(沈培康).Presentsituation of research and application of microemulsionsystem and prospectives.J iangsu Chemical I ndust ry(江苏化工),2002,30(1):18—23[2] Zhang Qian(张乾),Fan Xiaodong(范晓东).Study oninverse microemulsion system of acrylamide preparation,polymerization and characterization.Chemical I ndust ry andEngineering(化学工业与工程),2001,18(12):316—322[3] Wang Min(王敏),Wang Yujun(王玉军),Zhu Shenlin(朱慎林).Composition and stability of W/O microemulsionfor preparation of BaSO4nanoparticles.J ournal ofChemical I ndust ry and Engi neeri ng(China)(化工学报),2003,54(10):1450—1454[4] Zhang Liyang(张立央),Yao Shanjing(姚善泾),GuanY ixin(关怡新).Diffusion and cut2off characteristics ofSA/CS2CaCl2/PMCG microcapsules.J ournal of ChemicalI ndust ry and Engineering(China)(化工学报),2004,55(1):74—80[5] G ong Liangfa(龚良发),Guo Lin(郭林),Li Huanjun(李欢军).The preparation and application of oxidenanoparticles.Chemical Engineer(化学工程师),1999,71(2):27—30[6] Pan Qingyi(潘庆谊),Xu Jiaqiang(徐甲强),LiuHongmin(刘宏民),An Chunxian(安春仙),Jia Na(贾娜).Preparation,microstructure and gas sensingproperties of nanosized SnO2materials made bymicroemulsions.J ournal of I norganic M aterials(无机材料学报),1999,14(1):83—89[7] Motte L,Petit C,Boulanger L,Mahrt J.Synt hesis ofcadmium in sit u in cadmium bis(et hyl2hexyl)sulfosccinatereverse micelle:polydispersity and photochemical reaction.L angmui r,1992,8:1049—1053[8] Mark S,J ulian E,Sean D.Formation of BaSO4nanoparticles in microemulsions wit h polymerized surfactantshells.L angmui r,2002,18:5023—5026[9] Arriagada F J,Osseo2Asare K.Synt hesis of nanosize silicain a nonionic water2in2oil microemulsion:effect s of t hewater/surfactant molar ratio and ammonia concentration.J1Colloi d I nter1S ci1,1999,211:210—220[10] Debuigne F,Cuisenaire J,J eunieau L,Masereel B,NagyJ B.Synt hesis of nimesulide nanoparticles in t hemicroemulsion epikuron/isopropyl myristate/water/n2but snol(or isopropanol).J1Colloi d I nter1S ci1,2001,243(1):90—101[11] Petit C,Pileni M P.Synt hesis of cadmium sulfude i n sit u inreverse micelles and in hydrocarbon Gels.J1Phys1Chem1,1988,92:2282—2286[12] Cheng Guoxiang(成国祥),Shen Feng(沈锋),YaoKangde(姚康德),Sun Pingchuan(孙平川).The studyon t he properties of reverse micellar microreactor and t hepreparation of ZnS nanoparticles.Functional M ateri al(功能材料),1998,29(2):183—187[13] Cheng Guoxiang(成国祥),Zhang Renbo(张仁柏),WanY izao(万怡灶),Shen Feng(沈锋),Yao Kangde(姚康德).Properties of reverse micell microreators andpreparation of Fe3O4nanoparticles.Ordnance M ateri al・73・化 工 学 报 第56卷 S cience and Engineeri ng (兵器材料科学与工程),1998,21(6):27—30[14] Ha Runhua (哈润华),Hou Sijian (侯斯健),Li Fuping(粟付平),Wang Desong (王!松).Structure ofmicroemulsion and t he inverse microemulsion polymerization of acrylamide.A cta Pol y merica S inica (高分子学报),1995,1:10—19[15] Lin Hao (林浩),Gao Jiancun (高建村),Demluti (地拉木提).Development on inverse emulsion polymerization ofacrylamide.J ournal of X inj iang Pet roleum I nstit ute (新疆石油学院学报),2001,13(1):58—60[16] Kahlweit M.How to prepare microemulsions at prescribedtemperature ,oil and brine.J 1Phys 1Chem 1,1995,99(4):1281—1284[17] Holtzscherer C ,Candau F.Salt effect on solutions ofnonionic surfactant s and it s influence on t he stability of polymerized microemulsions.J 1Colloi d I nter 1Sci 1,1988,125(1):97—110信息与交流《化工进展》2005年第2期目次进展与述评苯甲醛清洁生产工艺技术研究进展朱 宪 王 彬 张 彰 王 倩 蒋 超………………………………………………化肥工业在循环经济中持续发展孙先良……………………………………………………………………………………………模板法制备空心聚合物微囊和纳米囊的研究进展巨晓洁 褚良银 陈文梅 王海东 张 杰………………………………乙醇电催化氧化反应动力学分析与研究进展章冬云 马紫峰 原鲜霞…………………………………………………………磁流变液材料的研究进展和应用前景浦鸿汀 蒋峰景……………………………………………………………………………纳米/微米复合材料气相制备技术述评杨 毅 刘宏英 李凤生 姜 炜………………………………………………………反胶团在超临界二氧化碳体系中的研究进展肖观秀 吕惠生 张敏华…………………………………………………………小波分析及其在化工信号分析处理中的应用及展望马丽萍 石炎福 余华瑞…………………………………………………高固液比生物催化体系的研究进展黄振龙 陈英文 沈树宝……………………………………………………………………细乳液聚合研究进展郭睿威 戚桂村 金启明 张建华…………………………………………………………………………新己烯生产技术评述徐泽辉 常 慧 黄亚茹 房鼎业…………………………………………………………………………茂金属催化剂负载化及负载机理的研究进展郝小明 刘 伟 代振宇 周 涵 景振华 汪燮卿…………………………研究开发基于WO 3表面改性TiO 2的制备及光催化性能研究王知彩 昝树财……………………………………………………………纳米SiO 2/Al 复合粒子的制备刘耀鹏 杨 毅……………………………………………………………………………………酒精生产过程用能状况的诊断和调优陶忠华 顾兆林 李 云 刘宗宽………………………………………………………水热合成制备超细氢氧化镁阻燃剂任鹏飞 陈建铭 宋云华 陈建峰…………………………………………………………应用技术流化床在生物物料热力干燥中的应用李建国 赵丽娟 潘永康 周 明………………………………………………………石油工业苯中含氮化合物的类型分析董荣芬 董忠杰 田松柏 王建伟 吴王锁……………………………………………HPL C 法测定豆粕中大豆异黄酮的含量王 松 丁 立 周荣琪………………………………………………………………气相色谱法测定维生素D 2中残留有机溶剂孙英北 邓 利 谭天伟……………………………………………………………专栏(石化科技与管理)TS 21/H 2O 2催化氧化体系在超临界CO 2中应用的研究刘郁东 郜 亮 朱 斌 林 民……………………………………磁稳定床反应器用于22乙基蒽醌加氢生产过氧化氢研究陈西波 孟 祥 慕旭宏 刘会洲…………………………………SIS 热塑性嵌段共聚物的生产方法及应用邓彦波……………………………………………………………………………………丁醇、辛醇生产技术与市场需求预测沈佩芝 任 诚……………………………………………………………………………化纤装置空调控制系统的研究与对策李 磊……………………………………………………………………………・173・ 第2期 王风贺等:Span 802Tween 80/液体石蜡/AM 2H 2O 反相微乳液体系。
2410中文手册
0.1uF
SGND
C1009 C1010 0.1uF VCC 0.1uF nCTS0 nRXD0 nRTS0 nTXD0
18 7 3 19 9 16 8 17
SGND C1011 0.1uF C VCC
GND VV+ VCC R2IN R1IN T2OUT T1OUT MAX3223
FORCEON #FORCEOFF #INVALID EN R2OUT R1OUT T2IN T1IN
SGND
B
TOUCH_PANEL1 4 X2 Q302 Q303 XMON 2SK1483 SGND
Y2
YMON SGND R301 4.7K VDD5V SGND 2SK1483
74LVCC4245
3
A
Title
A
LCD AND TOUCH PANEL
Size A4 Date: File: 1 2 3 Number
CON304
2 1
VCC C VDD5V LCD_FRM LCD_LOAD LCD_CP LCD_DISP VDD5V R300 4.7k VDD25V LD7 LD6 LD5 LD4 LD3 LD2 LD1 LD0 nXPON Q300 Q301 nYPON 2SJ197 TSYP SGND 2 CON302 TSYP X_LEFT Y_UPPER 1 R303 4.7K C301 1nF SGND Y_LOWER X_RIGHT AIN5 TSXP R302 4.7K 2SJ197 CON301 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 TSXP C300 1nF AIN7
B
1
B
A Title
Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched

Carbohydrate Polymers 87 (2012) 667–675Contents lists available at SciVerse ScienceDirectCarbohydratePolymersj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /c a r b p olSeparation,structure characterization,conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix AstragaliJun-Yi Yin a ,b ,1,Ben Chung-Lap Chan a ,1,Hua Yu a ,Iris Yuen-Kam Lau a ,Xiao-Qiang Han a ,Sau-Wan Cheng a ,Chun-Kwok Wong a ,d ,Clara Bik-San Lau a ,Ming-Yong Xie b ,∗∗,Kwok-Pui Fung a ,Ping-Chung Leung a ,Quan-Bin Han a ,c ,∗aState Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK),Institute of Chinese Medicine,The Chinese University of Hong Kong,Shatin,NT,Hong Kong,China bState Key Laboratory of Food Science and Technology,Nanchang University,Nanchang 330047,China cSchool of Chinese Medicine,Hong Kong Baptist University,Hong Kong,China dDepartment of Chemical Pathology,The Chinese University of Hong Kong,Prince of Wales Hospital,Shatin,NT,Hong Kong,Chinaa r t i c l ei n f oArticle history:Received 30June 2011Received in revised form 3August 2011Accepted 17August 2011Available online 24 August 2011Keywords:Radix Astragali PolysaccharideStructure character Morphology featureImmunomodulating effecta b s t r a c tA water soluble polysaccharide (RAP)was isolated and purified from Radix Astragali and its structure was elucidated by monosaccharide composition,partial acid hydrolysis and methylation analysis,and further supported by FT-IR,GC–MS and 1H and 13C NMR spectra,SEM and AFM microscopy.Its aver-age molecular weight was 1334kDa.It was composed of Rha,Ara,Glc,Gal and GalA in a molar ratio of 0.03:1.00:0.27:0.36:0.30.The backbone consisted of 1,2,4-linked Rha p ,␣-1,4-linked Glc p ,␣-1,4-linked GalA p 6Me,-1,3,6-linked Gal p ,with branched at O -4of the 1,2,4-linked Rha p and O -3or O -4of -1,3,6-linked Gal p .The side chains mainly consisted of ␣-T-Ara f and ␣-1,5-linked Ara f with O -3as branching points,having trace Glc and Gal.The terminal residues were T-linked Ara f ,T-linked Glc p and T-linked Gal p .Morphology analysis showed that RAP took random coil feature.RAP exhibited significant immunomodu-lating effects by stimulating the proliferation of human peripheral blood mononuclear cells and enhancing its interleukin production.© 2011 Elsevier Ltd. All rights reserved.1.IntroductionRadix Astragali (Astragalus )is the dried root of Astragalus membranaceus (Fisch.)Bunge and Astragalus mongholicus Bunge (Fabaceae).It has been used in the treatment of various renal dis-eases in Traditional Chinese Medicine for over 2000years.Modern researches showed that Radix Astragali possesses a variety of activ-ities,including immunomodulating (Bedir,Pugh,Calis,Pasco,&Khan,2000),anti-hyperglycemic (Chan,Lam,Leung,Che,&Fung,2009),anti-inflammation (Choi et al.,2007),anti-oxidation (Yu,Bao,Wei,&An,2005),antiviral activities (Zhu et al.,2009),etc.Besides saponins and isoflavonoids,polysaccharides are believed as the principle active constituents of Radix Astragali (Chu,Qi,Li,Gao,&Li,2010),which could activate the proliferation and cytokine production of mouse B cells and macrophages (Shao et al.,2004),and showed immunomodulating effects on Peyer’s patch∗Corresponding author.Tel.:+852********.∗∗Corresponding author.Tel.:+8679183969009.E-mail addresses:myxie@ (M.-Y.Xie),simonhan@.hk (Q.-B.Han).1Equal contribution.immunocompetent cells (Kiyohara et al.,2010).The crude polysac-charide could stimulate macrophages to express iNOS gene through the activation of NF-B/Rel (Lee &Jeon,2005).It could also amelio-rate the digestive and absorptive function and regulate amino acid metabolism to beneficially increase the entry of dietary amino acid into the systemic circulation (Yin et al.,2009).An acid polysaccha-ride from Radix Astragali showed significant reticuloendothelial system-potentiating activity (Shimizu,Tomoda,Kanari,&Gonda,1991).Scientists made efforts in the structure characterization of the polysaccharides isolated from Radix Astragali (Kiyohara et al.,2010;Shao et al.,2004;Wang,Shan,Wang,&Hu,2006)and only sev-eral glucans (Fang &Wagner,1988;Li &Zhang,2009)were well characterized.As for heteroglycans,nothing but monosaccharide composition and molecular weight was reported (Yan et al.,2010;Zhang et al.,2011).Therefore,their structures deserve further study.Herein we report the isolation and purification of a water-soluble hyperbranched heteroglycan (coded RAP)from Radix Astragali.Its structure was characterized by a combination of chem-ical and instrumental analysis of monosaccharide compositions,methylation,partial acid hydrolysis,FT-IR,ESI-MS,GC–MS and0144-8617/$–see front matter © 2011 Elsevier Ltd. All rights reserved.doi:10.1016/j.carbpol.2011.08.045668J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–675NMR.Its morphology feature was further analyzed by scanning electron microscopy(SEM)and atomic force microscopy(AFM). RAP exhibited immunomodulating effects on human peripheral blood mononuclear cells.2.Materials and methods2.1.MaterialThe roots of A.membranaceus were purchased from herbal store in Hong Kong and identified by Dr.Chun-Feng Qiao.The voucher specimens are deposited at the Institute of Chinese Medicine,the Chinese University of Hong Kong,with voucher specimen number 2010-3268.Hiload26/60Superdex-200prep grad was purchased from Pharmacia Co.(Uppsala,Sweden).The dextran standards(T-2000,T-270,T-80,T-50,T-25and T-12with molecular masses of2,000,000,270,000,80,000,50,000and12,000respectively) and monosaccharide standards of d-mannose(Man),l-rhamnose (Rha),d-galactose(Gal),d-arabinose(Ara),and d-glucose(Glc) were obtained from Merck Co.(Darmstadt,Germany).Ultra-pure water was produced by a Milli-Q water purification system(Milli-pore,Bedford,MA,USA).Lipopolysaccharide(LPS)was purchased from Sigma(St.Louis,USA).All chemical reagents were of analytical grade.2.2.Extraction and purification of polysaccharideThe air-dried Radix Astragali(100g)was cut into pieces and extracted twice with boiling water(2×1.2L)for1h.The solution wasfiltered and concentrated under reduced pressure.The solu-tion was precipitated with four volumes of absolute ethanol for 12h.The precipitate was resolved again in water and deproteined using Sevag method(Staub,1965)forfive times.Then the solution was dialyzed against distilled water for72h.Finally,the retentate was lyophilized with Virtis Freeze Dryer(The VirTis Company,New York,USA)to yield crude polysaccharide(RACP,1.67g).RACP was dissolved in distilled water(4mg/mL),filtered through a0.45m membrane and separated by the Buchi Puri-fier system(BUCHI Labortechnik AG,Switzerland)coupled with a Hiload26/60Superdex-200(2.6cm×60cm)column,eluted with water at aflow rate of2mL/min.Fractions were collected every 3min and checked using phenol-sulfuric acid under UV detection at490nm.Gel permeation chromatography(GPC)was used to test the homogeneity of the purified polysaccharide.Total carbohydrate was determined using the phenol-sulfuric acid colorimetric method(Dubois,Gilles,Hamilton,Rebers,& Smith,1956).Uronic acid contents were determined accord-ing to Blumenkrantz and Asboe-Hansen’s method(Blumenkr& Asboehan,1973).Protein was estimated by photometric assay using bovine serum albumin as the standard(Bradford,1976). Specific rotation was recorded with a Perkin-Elmer241M digital polarimeter.2.3.Homogeneity and molecular weightThe homogeneity and molecular weight of the purified polysac-charide was determined by GPC.It was analyzed on a Waters UPLC system(Waters,Milford,MA)equipped with a Waters Ultrahydrogel TM1000column(7.8mm×300mm),a Waters ELS Detector,controlled with a Binary Solvent Manager system.The ELS Detector conditions were as follows:drift tube temperature (75◦C),nebulizer temperature(48◦C),gain(300◦C),gas pressure (45Psi).Dextran standards with different molecular weight were used to calibrate the column and establish a standard curve.2.4.Monosaccharide composition analysisRAP was hydrolyzed with2M trifluoroacetic acid(TFA)at120◦C for2h in a sealed test tube.The acid was removed under reduced pressure by repeated evaporation with methanol,and then the hydrolysate was converted into alditol acetates(Chen,Xie,Nie,Li,& Wang,2008;Jones&Albersheim,1972).Shimadzu GC/MS-QP2010 equipment(Nishinokyo Kuwabaracho,Kyoto,Japan)was used for the identification and quantification of monosaccharides.2.5.Methylation and GC–MS analysis2.5.1.Reduction of polysaccharideThe reduction of the uronic acid was conducted following a pro-cedure as described in the literature(Taylor&Conrad,1972)with slight modifications.RAP(20mg)was added into water and treated with1-cyclohexyl-3-(2-morpholinoethyl)-carbodimide methyl-p-toluenesulfonate(CMC)forfive times,until the reduction of uronic acid completed.The polysaccharide after reduction(RAP-R)was subjected to monosaccharide composition and methylation analy-sis.2.5.2.Methylation and GC–MS analysisMethylation analysis of polysaccharide(RAP and RAP-R)was conducted according to the reported methods(Ciucanu&Kerek, 1984;Guo,Cui,Wang,&Christopher Young,2008)with some mod-ifications.The dried polysaccharide was dissolved in anhydrous dimethyl sulphoxide.Dry sodium hydroxide(30mg)was added, and the mixture was stirred for3h at room temperature.Methyl iodide was added into the mixture.The reaction was stopped by adding water.The methylated polysaccharides were then extracted with chloroform followed by washing with distilled water for three times.They were acetylated with acetic anhydride to obtain par-tially methylated alditol acetates(PMAA).GC/MS analysis of PMAA was performed on a DB-5ms capillary column(0.25m×0.25m×30m)using a temperature program-ming of140◦C(3min)to250◦C(40min)at2◦C/min.Helium was used as the carrier gas.The components were identified by a combi-nation of the main fragments in their mass spectra and relative GC retention times,comparing with the literature(Wang,He,&Huang, 2007).2.6.Partial acid hydrolysisRAP(50.5mg)was treated with0.05M TFA(10mL)at100◦C for1h.The product was concentrated by evaporation of TFA with methanol,and then dialyzed against distilled water(5×500mL, molecular weight3500Da cut off).The solution outside of the dial-ysis bag(RAP-P-L)was collected for monosaccharide analysis and ESI-MS.The solution in the dialysis bag(RAP-P-H)was concen-trated and lyophilized.RAP-P-H’s homogeneity was confirmed by GPC.It was further applied to monosaccharide compositions and methylation analysis.2.7.FT-IR analysisInfrared spectra were recorded with a Thermo Nicolet5700 infrared spectrometer(Madison,WI,USA),using KBr disks method.2.8.NMR spectroscopyRAP was treated with deuterium by lyophilizing with D2O for three times.The deuterium-exchanged RAP(25mg)was put in a5-mm NMR tube and dissolved in0.5mL of99.9%D2O.NMR-spectra were recorded with a Brucker AM-600NMR(Karlsrhue,Germany).J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–6756692.9.Molecular morphology analysisPolysaccharide powder was placed on the sample stage,and coated with a thin layer of gold in a MODEL IB-3ion coater(Eiko Corp.,Mito City,Japan).Then it was examined in a QUANTA200F scanning electron microscope(FEI Company,Holland).The sample was viewed at an accelerating voltage of30kV.The atomic force microscopy in this study was manufactured by Shanghai AJ Nano-Science Development Limited(Shanghai,China) and operated in the tapping mode.RAP was diluted to thefinal concentration of5g/mL in distilled water.5L of solutions was dropped onto freshly cleaved mica and allowed to stand in air before imaging.The quoted spring constant was between5.5and 25N m−1.2.10.Immunomodulatory activities of RAP on human peripheral blood mononuclear cells(PBMC)Immunomodulatory activities of RAP were determined by the capacity of the compounds to influence the cytokine production by human PBMC.PBMCs were obtained from the buffy coat collected from Hong Kong Red Cross by density gradient separation.Buffy coat was diluted1:1with phosphate buffer saline(PBS)and this was overlaid on Ficoll Paque Plus(Amersham Biosciences).Cells were washed with PRMI and plated in96-well plates at105cells/well. Serial dilutions of RAP from10to10,000ng/mL were added to the wells.The plates were maintained in a37◦C incubator for24h. The immunomodulatory effects on PBMC of RAP were compared with a well known mitogen lipopolysaccharide(LPS)from Gram negative bacteria.The cell free supernatants were then assayed for GM-CSF(granulocyte-macrophage colony-stimulating factor), IFN(interferon)-␥,IL(interleukin)-1,IL-2,IL-4,IL-10IL-12and TNF(tumor necrosis factor)-␣production by commercially avail-able human cytokine ELISA kits(BD OptEIA,USA)according to the manufacturer instruction with detection limits ranged from3.1to 7.8pg/mL.3.Results and discussion3.1.Isolation and purification of RAPRAP was purified from the crude polysaccharide RACP through a Hiload26/60Superdex-200column.It presented a single and symmetrical peak in GPC(gel-permeation chromatography)exam-ination on an Ultrahydrogel TM1000column(Fig.1).The average molecular weight was1334kDa with reference to Dextran T-series standard samples of known molecular weight.The total sugar con-tent was determined to be76.5%using the phenol-sulfuric acid method.It had a high specific rotation of[␣]D20+125.8(0.54,H2O) and weak UV absorption at280nm which was consistent with its low protein content(only0.72%).The uronic acid content was56.7% using colorimetric method.3.2.Monosaccharide compositions analysisAfter complete hydrolysis of RAP by2M TFA,its monosaccha-ride composition was determined using GC–MS.As demonstrated in Table1,RAP contained Rha,Ara,Glc and Gal.Reduction of RAP with CMC-NaBH4gave the carboxyl-reduced derivative RAP-R and further analysis of RAP and RAP-R both indicated the presence of GalA.The molar ratio of Rha,Ara,Glc,Gal and GalA of RAP was 0.03:1.00:0.27:0.36:0.30,in which the ration of GalA was calculated by the increase of Gal content in RAP-R.Table1Monosaccharide composition of RAP,RAP-R(RAP after reduction),RAP-P-H,RAP-P-L,and RAP-P-L-NH.RAP,after partially hydrolysis followed by dialysis,gave two parts:RAP-P-H(the part in the dialysis bag)and RAP-P-L(the part outside of the dialysis bag).RAP-P-L-NH meant direct examination on the monosac-charides in RAP-P-L before complete hydrolysis.The results were obtained ona Shimadzu GC/MS-QP2010series coupled with a DB-5ms capillary column(0.25m×0.25m×30m).Samples Mw(kDa)Monosaccharide composition(molar,%)Rha Ara Glc GalRAP13340.03(1.8) 1.00(60.2)0.27(16.3)0.36(21.7) RAP-R n.d.0.01(0.6) 1.00(55.6)0.13(7.2)0.66(36.7) RAP-P-H12150.28(5.4) 1.00(19.2) 1.84(35.3) 2.00(38.4) RAP-P-L n.d.n.d. 1.00(89.3)0.10(8.9)0.02(1.8) RAP-P-L-NH n.d.n.d. 1.00(100.0)n.d.n.d.n.d.not determined.3.3.Methylation analysisRAP and RAP-R were methylated and analyzed by GC–MS in order to elucidate the linkages(Table2).Compared with GalA’s content given in monosaccharide composition analysis,the carboxyl-reduced RAP-R showed a significant increase of1,4-linked Gal p,suggesting the GalA in RAP was1,4-linked.Similarly,it could be suggested that RAP was mainly composed of T-linked Ara f,1,5-linked Ara f,1,4-linked Glc p,1,4-linked GalA p,1,3-linked Glc p and 1,3,6-linked Gal p.The terminals consisted of Ara f(20.6%),Glc p (2.0%)and Gal p(5.2%),indicating RAP was significantly branched and the side chains were terminated by the Ara residues.The ratio of T-,1,5-and1,3,5-linked Ara f(50.9:41.7:7.4)sug-gested that the Ara side chains contained a central core of1,5-linked Ara f residues.The high proportion of T-linked Ara f residues sug-gested that some terminal Ara residues existed in the Ara side chains,and others were attached to the highly branched Gal side chains or connected to the back bone directly(Ros,Schols,& Voragen,1996;Sun,Cui,Tang,&Gu,2010).The proportion of terminal,1,4-,1,3-,1,6-,1,2,4-,1,4,6-and 1,3,6-linked Gal p was9.3:8.7:16.1:8.1:5.0:11.1:41.6.The low pro-portion of terminal residues of Gal(9.3%)indicated that a part of the Gal side chains were terminated by the Ara residues.The Rha residues were exclusively1,2,4-linked.Glc residues were mainly terminal,1,4-linked units,with a small amount of1,3,4-linkedTable2GC–MS analysis for methylation of RAP,RAP-R and RAP-P-H on a DB-5ms capillary column(0.25m×0.25m×30m).PMAA a Molar ratios b Linkages cRAP RAP-R RAP-P-H2,3,5-Me3-Ara13.420.6 4.2T-2,3-Me2-Ara10.919.89.01,5-2-Me-Ara 2.0 4.3–1,3,5-3-Me-Rha 3.9 5.8 3.91,2,4-2,3,4,6-Me4-Glc 4.4 2.0 4.2T-2,3,6-Me3-Glc24.315.330.11,4-2,6-Me2-Glc 1.9 1.7 3.91,3,4-2,3,4,6-Me4-Gal 3.6 5.27.5T-2,3,6-Me3-Gal 3.414.4 3.31,4-2,4,6-Me3-Gal 6.3 2.014.21,3-2,3,4-Me3-Gal 3.2 1.8 6.91,6-3,6-Me2-Gal 1.90.8–1,2,4-2,3-Me2-Gal 4.4 2.1 5.11,4,6-2,4-Me2-Gal16.3 4.17.51,3,6-–,not determined.a The sugar type was confirmed both with the literature and mass spectrum anal-ysis.b Molar ratios were given as percentage of total ion count(TIC).c The pyranosyl or furanosyl forms of glycosyl residues was confirmed with13C NMR.670J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–675Fig.1.GPC chromatogram of RAP on an Ultrahydrogel TM1000column(7.8mm×300mm),mobile phase:water,at aflow rate of0.3mL/min,the ELS Detector conditions were:drift tube temperature(75◦C),nebulizer temperature(48◦C),gain(300◦C),gas pressure(45Psi).Fig.2.The IR spectra of RAP(A)and RAP-P-H(B,partially hydrolyzed RAP),recorded in KBr tablet at the absorbance mode from4000to400cm−1(mid infrared region)at a resolution of4cm−1.J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–675671Fig.3.The1H NMR(600.1MHz)spectrum of RAP that was measured in a5-mm NMR tube with0.5mL of99.9%D2O.(A)At27◦C;(B)at50◦C.Fig.4.The13C NMR(151.0MHz)spectra of RAP(A)and RAP-P-H(B,partially hydrolyzed RAP)that were measured in a5-mm NMR tube with0.5mL of99.9%D2O.672J.-Y.Yin et al./Carbohydrate Polymers 87 (2012) 667–675Fig.5.SEM images of RAP at 1000×(A)and 3000×(B).The molecular morphology of RAP was first coated with a thin layer of gold,then observed using SEM at an accelerating voltage of 30kV.units.RAP contained two types of intra-chain linkages for galac-tose,a 1,4-linked that is common in type I arabinogalactan,and a 1,3,6-linked in type II arabinogalactan,suggesting that RAP contain different types of Gal branches.3.4.Partial acid analysisPartial degradation of polysaccharide by acid hydrolysis is based on the fact that some glycosidic linkages are tolerable to acid.To determine more structural features,RAP (50.5mg)was partially hydrolyzed with 0.1M TFA to give two parts,RAP-P-H (in the dialysis bag)and RAP-P-L (outside of the dialysis bag).Direct exam-ination on the monosaccharides in RAP-P-L found only Ara,while after complete hydrolysis it gave a small amount of Glc (9.0%)and Gal (2.1%)in addition to Ara (88.8%)(Table 1).It was suggested that RAP probably contained terminal residues of Ara in the branch areas.There was no GalA found in RAP-P-L in HPLC examination (Honda et al.,1989),which confirmed that GalA was located intheFig.6.AFM image of RAP.RAP was dissolved in distilled water at the concentration of 5g/mL.5L of solution was dropped onto freshly cleaved mica and allowed to stand in air before imaging.backbone of RAP.These results suggested that GalA and Rha existed in the backbone and the neutral sugars were located in the side chains.ESI-MS of RAP-P-L presented sodium cationized pseu-domolecular ions at m /z 1159.9[Ara 6(Glc/Gal)2+Na]+,997.3[Ara 6(Glc/Gal)+Na]+,702.0[Ara 5+Na]+,569.1[Ara 4+Na]+,407.1[Ara 3+Na]+and 305.1[Ara 2+Na]+.RAP-P-L seemed a mixture of monosaccharide (terminal Ara residues)and oligosaccharide (containing [Ara-Ara]linkages).The degraded polysaccharide RAP-P-H (30mg)was mainly com-posed of Gal and Glc,with small amounts of Rha and Ara (Table 1).Its average molecular weight was 1215kDa.The methylation analy-sis (Table 2)showed that the removal of most Ara f residues resulted in an increase of the ratio of 1,6-linked Gal p and significant increase for 1,3-linked Gal p .Therefore it could be deduced that Ara residues were attached to 1,6-linked and mainly 1,3-linked Gal residues.RAP-P-H gave no 1,3,5-linked Ara f ,suggesting that 1,3,5-linked Ara f should be in the branch.A great amount of 1,4-linked Glc p residues still detected in RAP-P-H indicated that 1,4-linked Glc p residues were located in the backbone of RAP.3.5.FT-IR spectra analysisThe IR spectrum of RAP (Fig.2A)showed a strong band at 3404cm −1which was attributed to the hydroxyl stretching vibra-tion of the polysaccharide.The band at 2935cm −1was due to C–H stretching vibration.Bands at 1743and 1618cm −1indicated the ester carbonyl (COOR)groups and carboxylated ion groups (COO −)(Gnanasambandam &Proctor,2000).The FT-IR spectrum of RAP showed a strong absorbance at 1021,1100and 1142cm −1attributed to the stretching vibrations of ␣-pyranose ring of the glucosyl residue.Moreover,the characteristic absorptions at 830and 916cm −1indicated that both ␣-and -configurations existed.These observations confirmed that the RAP was a polysaccharide containing uronic acid.The IR spectrum of RAP-P-H (Fig.2B)showed the same charac-teristic absorption with RAP which indicated RAP-P-H retained the backbone of RAP.3.6.NMR analysisSignals in the 1H and 13C NMR spectra of RAP were assigned as much as possible,according to monosaccharide compositions analysis,methylation results and literature values (Bock,Pedersen,&Pedersen,1984;Chandra,Ghosh,Ojha,&Islam,2009;Polle,J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–675673Fig.7.Cytokines production(GM-CSF,IFN-␥,IL-1,IL-2,IL-4,IL-10IL-12and TNF-␣)of human blood mononuclear cells(PBMC)with the addition of RAP or LPS from2to 10,000ng/mL.Each bar represents the mean±SEM of duplicates(n=7).Ovodova,Shashkov,&Ovodov,2002;Sun et al.,2010;Xu,Dong, Qiu,Cong,&Ding,2010).The1H NMR spectrum(Fig.3A)showed signals in the anomeric region.Due to the existence of H2O in the sample or HDO from D2O,there was large signal atı4.815ppm which disturbed the analysis of RAP.Therefore,another1H NMR spectrum was obtained at50◦C(Fig.3B).From methylation analysis,1,4-linked Glc p was the main resides in RAP.So the signal atı5.096ppm was assigned to␣-1,4-linked Glc p.The signal atı 5.254ppm was assigned to␣-1,5-linked Ara f,and the signal atı5.162ppm was originated from␣-1,3,5-linked Ara f.The signal atı4.968ppm was assigned to␣-1,4-linked GalA p6Me,which meant some of1,4-linked GalA p was present as methyl ester.The signal atı4.922could be assigned to␣-1,4-linked GalA p.The signals atı4.693and4.653ppm were assigned to-1,3,6-linked Gal p.The signal atı4.469ppm was assigned to-1,3-linked Gal p and corresponded with-1,3-linked Gal p anomeric carbon resonance atı104.43ppm in the HSQC.The proton signals nearbyı2.083,2.131and2.186ppm could be assigned from the–CH3of the O-acetyl groups.It suggested that RAP contained kinds of O-acetyl groups at the different positions of the sugar residues or different chemical environments.Signal atı1.260ppm was identified to be H-6from methyl group of the Rha residues.The overlapped signals in the range ofı3.355–4.410ppm were assigned to protons H-2to H-5(or H-6)of the glycosidic ring.The anomeric signals in the13C NMR spectrum of RAP (Fig.4A)were assigned partly according to correlations in the HSQC spectrum.The signal atı108.66ppm correlated to H-1 (ı108.66/5.096ppm)of T-Ara f.The signal atı110.48ppm cou-pled to H-1(ı110.48/5.254ppm)of1,5-linked Ara f.The signal atı108.13ppm corresponded to1,3,5-linked Ara f,which was confirmed by its absence in the13C NMR spectrum of RAP-P-H (Fig.4B).The low-field chemical shifts indicated the Ara residues were in furnanose form and adopted␣-anomeric configuration(Xu et al.,2010).Methylation analysis results showed that1,3-linked674J.-Y.Yin et al./Carbohydrate Polymers87 (2012) 667–675Gal p residues increased significantly after partial acid hydrolysis of pared with the13C NMR of RAP,signal atı104.43ppm became much stronger in RAP-P-H.So the signal atı104.43ppm (ı104.43/4.469ppm from HSQC)was assigned to1,3-linked Gal p. The signal atı101.37ppm was assigned to C-1of1,4-linked GalA p. The signals atı101.60and100.18ppm were assigned to C-1 of1,3,6-linked Gal p and␣-1,4-linked GalA p6Me.And the signal atı100.61ppm was assigned to C-1of1,4-linked Glc p.The sig-nal atı62.36ppm was assigned to C-5of a terminal Ara,while the stronger signal atı61.68ppm was attributed to the C-6of 1,4-linked Glc p.The signal atı18.06ppm could be assigned to the methyl carbon of Rha.The signal atı54.15ppm could be assigned to methyl ester groups of RAP.The presence of methyl esterified GalA p was also supported by the signals atı54.15/3.814ppm in HSQC spectrum (Bushneva,Ovodova,Shashkov,&Ovodov,2002).In the lowfield, typical signals for the C-6carboxyl group of GalA were observed at ı176.33and172.06ppm,which confirmed the presence of free and esterified carboxyl groups of GalA.RAP and RAP-R after hydrolysis were acetylated,and no methylated monosaccharide was detected by GC–MS(Samuelsen et al.,1999).It was confirmed that the1,4-linked GalAp was present as1,4-linked GalAp6Me.3.7.Molecular morphology of RAPIts molecular morphology was further investigated by scanning electron microscopy(SEM)and atomic force microscopy(AFM).The SEM micrograph of RAP was shown in Fig.5.RAP appeared as loose flaky and curly aggregation.The observed irregular microstructure demonstrated that RAP was a type of amorphous solid.The molecular morphology of RAP was further investigated by single molecular AFM.The topographical image was shown in Fig.6. The results showed that there were many spherical lumps within the height of3–70nm while the height of a single polysaccharide chain is generally0.1–1nm,which suggested molecular aggrega-tion happened somehow.There might be a repulsive force between the polysaccharide and the mica causing aggregation(Chen et al., 2009)because both RAP and the mica a type of aluminum sili-cate were negative.The side chains might be another reason for the aggregation(Sletmoen,Maurstad,Sikorski,Paulsen,&Stokke, 2003).3.8.Immunomodulatory activities on human peripheral blood mononuclear cells(PBMC)RAP has been investigated for its in vitro effect on the cytokine profile(GM-CSF,IFN-␥,IL-1,IL-2,IL-4,IL-10,IL-12and TNF-␣) of unstimulated human PBMC compared with LPS.No significant productions of cytokines were detected in drug free negative con-trol.When RAP was added to RPMI for24h incubations,potent stimulatory effects on the production of two pro-inflammatory cytokines IL-1and TNF-␣from PBMC were observed from200to 10,000ng/mL(Fig.7).These cytokines are important in mediating the immune response against bacterial infections.Dose dependent stimulation of IL-10,IL-12and GM-CSF productions from PBMC were also observed with RAP addition but the stimulatory activ-ities was not weaker than LPS.The source of these5cytokines is mainly from monocytes and these results suggested that RAP is an activator of monocytes and further studies are required to inves-tigate its mechanism of action such as the involvement of toll like receptors.For T cell producing cytokines(IL-2,IL-4and IFN-␥),RAP did not produce any significant effects on PBMC(Fig.7).4.ConclusionA water soluble polysaccharide(RAP),with the average molec-ular weight1334kDa,was isolated from Radix Astragali.It was composed of Rha,Ara,Glc,Gal and GalA in a molar ratio of 0.03:1.00:0.27:0.36:0.30.The structure was characterized by par-tial acid hydrolysis,methylation analysis,FT-IR,GC–MS and1H and13C NMR analysis.The backbone of RAP mainly consisted of 1,2,4-linked Rha p,␣-1,4-linked Glc p,␣-1,4-linked GalA p6Me,-1,3,6-linked Gal p.It had branches at O-4of the1,2,4-linked Rha p and O-3or O-6of-1,3,6-linked Gal p.The side chains mainly consisted of␣-T-Ara f and␣-1,5-linked Ara f possessing O-3as branching points,with trace Glc and Gal.The terminal residues were T-linked Ara f,T-linked Glc p and T-linked Gal p.Morphol-ogy analysis using SEM and AFM showed that RAP took random coil feature.This hyperbranched heteroglycan exhibited significant immunomodulating effects by stimulating the cytokines produc-tion mainly from monocytes in a dose dependent manner. AcknowledgementThis research is funded by the Innovation and Technology Fund (ITS/311/09and InP/108/10)of the Government of the Hong Kong Special Administrative Region.ReferencesBedir,E.,Pugh,N.,Calis,I.,Pasco,D.S.,&Khan,I.A.(2000).Immunostimulatory effects of cycloartane-type triterpene glycosides from Astragalus species.Biological& Pharmaceutical Bulletin,23(7),834–837.Blumenkr,N.,&Asboehan,G.(1973).New method for quantitative determination of uronic acids.Analytical Biochemistry,54(2),484–489.Bock,K.,Pedersen,C.,&Pedersen,H.(1984).Carbon-13nuclear magnetic resonance data for oligosaccharides.Advances in Carbohydrate Chemistry and Biochemistry, 42,193–225.Bradford,M.M.(1976).A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding.Analytical Biochemistry,72(1–2), 248–254.Bushneva,O.A.,Ovodova,R.G.,Shashkov,A.S.,&Ovodov,Y.S.(2002).Structural studies on hairy region of pectic polysaccharide from campion Silene vulgaris (Oberna behen).Carbohydrate Polymers,49(4),471–478.Chan,J.Y.W.,Lam,F.C.,Leung,P.C.,Che,C.T.,&Fung,K.P.(2009).Antihyper-glycemic and antioxidative effects of a herbal formulation of Radix Astragali Radix Codonopsis and Cortex Lycii in a mouse model of type2diabetes mellitus.Phytotherapy Research,23(5),658–665.Chandra,K.,Ghosh,K.,Ojha,A.K.,&Islam,S.S.(2009).Chemical analysis of a polysaccharide of unripe(green)tomato(Lycopersicon esculentum).Carbohy-drate Research,344(16),2188–2194.Chen,Y.,Xie,M.Y.,Nie,S.P.,Li,C.,&Wang,Y.X.(2008).Purification,composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum.Food Chemistry,107(1),231–241.Chen,H.X.,Wang,Z.S.,Qu,Z.S.,Fu,L.L.,Dong,P.,&Zhang,X.(2009).Physicochem-ical characterization and antioxidant activity of a polysaccharide isolated from oolong tea.European Food Research and Technology,229(4),629–635.Choi,S.I.,Heo,T.R.,Min,B.H.,Cui,J.H.,Choi,B.H.,&Park,S.R.(2007).Allevi-ation of osteoarthritis by calycosin-7-O-beta-d-glucopyranoside(CG)isolated from Astragali Radix(AR)in rabbit osteoarthritis(OA)model.Osteoarthritis and Cartilage,15(9),1086–1092.Chu,C.,Qi,L.W.,Li,B.,Gao,W.,&Li,P.(2010).Radix Astragali(Astragalus):Latest advancements and trends in chemistry,analysis,pharmacology and pharma-cokinetics.Current Organic Chemistry,14(16),1792–1807.Ciucanu,I.,&Kerek,F.(1984).A simple and rapid method for the permethylation of carbohydrates.Carbohydrate Research,131(2),209–217.Dubois,M.,Gilles,K.A.,Hamilton,J.K.,Rebers,P.A.,&Smith,F.(1956).Colorimetric method for determination of sugars and related substances.Analytical Chemistry, 28(3),350–356.Fang,J.N.,&Wagner,H.(1988).Chemical structure of a glucan from Astragalus mongholicus.Acta Chimica Sinica,46(11),1101–1104.Gnanasambandam,R.,&Proctor,A.(2000).Determination of pectin degree of ester-ification by diffuse reflectance Fourier transform infrared spectroscopy.Food Chemistry,68(3),327–332.Guo,Q.,Cui,S.W.,Wang,Q.,&Christopher Young,J.(2008).Fractionation and physic-ochemical characterization of psyllium gum.Carbohydrate Polymers,73(1), 35–43.Honda,S.,Akao,E.,Suzuki,S.,Okuda,M.,Kakehi,K.,&Nakamura,J.(1989).High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive1-phenyl-3-methyl5-pyrazolone derivatives.Analytical Biochemistry,180(2),351–357.。
da1_2004_dec_a

7C
FIFO means that the stock is taken to include:
all of the delivery on 28 Nov 300 kg at £60 = £18,000
part of the delivery on 19 Nov 130 kg at £52 = £6,760
Thus the correct operating profit is : £325,800 (as reported) + £85,000 (exceptional item) + £42,000 (prior period adjustment) = £452,800.
6B The depreciation charge in the first year on both the reducing balance and straight line bases is £22,000. Using the reducing balance basis, the charge in subsequent years is calculated on the net book value. In the second year, this leads to a charge of £16,500, or a reduction of £5,500. This increases the reported profit.
The restatement of stock is an example of a correction of a fundamental error, which is one of two reasons for treating an item as a prior period adjustment (the other being a change in accounting policy). As the opening stock was overstated, the resulting adjustment is a charge. However the charge should be a prior period adjustment and should therefore be removed from the calculation of the current period’s operating profit. This will increase the operating profit by £42,000.
一种实用的软件测频算法
率.分析了二次插值法用于计算正弦曲线的过零点时刻的理论误差;构造了该测频方案的详细实
现方法,并分析了该测频方案的整体测量误差。试验测试结果表明:谊算法可以消除谐波、直流分
量的影响,计的频率值离散小、精度高。
关键词:频率测量:二次插值法:离散傅里叶变换
中图分类号:TM 935.1
文献标识码:A
文章编号:l006—6047(2007)12—0036—03
仅当t=0时才使用式(9)计算,由图3知:在t=0 的小区域内,计箅相对误差近似为O;当£≠0时,使 用式(8)计算,正弦信号的零点时问最大计算相对误 差不大于±0,018%。
3测频算法的实现
对式(1)所示的交流电压,每周采样Ⅳ点,每周 期调整一次采样间隔,采样及计算过程见图4。
再中断赢开始丽碗捌团噩圈
2个相邻同方向变化的过零点之间的时间.计算其周期和频率。继而推出交流量本身的周期和额
率。以正弦曲线为例,从理论上证明了离散傅里叶变换计算输出的向量实部或虚部值是按以时间
为自变量的正弦函数形式变化.且其变化频率等于正弦曲线自身变化频率:使用二次插值法计算
正弦曲线的过零点时刻,2个相郐的同方向变化的过零点间的时间即是信号变化周期。从而计算其频
[10]周航慈,饶运涛.单片机程序设计基础[M]北京:北京航空航 天大学出版社,L997.
(责任编辑:康鲁豫)
洼:输人交流电压的基波频率为50 Hz;乩,也、皿.地分别指直 流分量,二、三、五扶谐波:H:.45%指在基渡上迭加45% 基波幅值的二次谐渡.苴他类推。
裹2有谐波输入的测频误差分布统计
1址.2 EⅡDr distribmi帅of measu地d雠quency
for i“put wlth hamonic comp0丌ent
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
(ii) Value of transfer of 1,800 units to Process II = 1,600 x (70 + 50) + (120 x 50) + 16,500 = £214,500
Conversion EU 120 1,600 150
–––––– 1,870 –––––– £93,500 –––––––– 1,870 = £50
22 A
Joint costs apportioned to product H:
£
(228 ÷ 640) x 384,000
136,800
Further processing costs
159,600 –––––––-
Total cost of H production (228,000 units)
£ 949,620
937,125
945,000
Variance (£) Rate 12,495 A Efficiency 7,875 F
(c) Rate: – Higher graded workers paid at a higher rate. – Higher than expected wage settlement for the company.
Efficiency: – The higher graded workers being more skilled took less than the standard time. – Highly motivated workers.
Planned activity level
£16
3,750 units
1,600 units –––––––––– 5,350 units ––––––––––
5D CPU Contribution per hour Ranking
X
Y
£8
£10
£4
£2·50
1st
2nd
800 units of product X uses 1,600 hours and in the remaining 400 hours, 100 units of product Y can be manufactured.
–
Started and finished units last month
1,600
Closing work in progress
300
––––––
Work done last month
1,900
––––––
Cost per EU
£133,000 –––––––––
1,900
= £70
(i) Value of closing work in progress = (300 x 70) + (150 x 50) = £28,500
Adverse price variance (0·04 x 2·50 x 12,000) = £1,200
12,000 litres at £2·50 per litre Add Favourable usage variance
Standard cost of actual production
19 B 20 C
b=
11 x 13,467 – (440 x 330) ––––––––––––––––––––––––
(11 x 17,986) – (440)2
£ 112,500 117,000 –––––––-
4,500 favourable –––––––-
= 0·6917
a = (330 ÷ 11) – 0·6917 (440 ÷ 11) = 2·33
9D EOQ = (2 x 20 x (4 x 12,500) = 1,155 0 ⋅10 x 15
10 D 11 D
Total direct labour hours:
Primary (6,000 x 36 ÷ 60) + (7,500 x 48 ÷ 60)
9,600
Finishing (6,000 x 25 ÷ 60) + (7,500 x 35 ÷ 60)
6 C £(40,000 – 20,000) ÷ (20,000 – 4,000) units = £1·25 per unit
7B
8 A Closing stock (units) = 200 + 600 – 150 – 200 – 250 = 200 Issues = £5,200 + (600 – 200) x £32·50 = £18,200
2 (a)
Direct material Actual quantity at actual price
Standard quantity for actual production at standard price Direct labour Actual hours at actual rate
Units
126
24
1,650 –––––– 1,800 ––––––
£
–
4,200
288,750 –––––––– 292,950 ––––––––
(c) – –
In job costing each job is costed separately whereas in process costing it is the process itself which is costed. The total cost of the process is then averaged over all the units of production.
Workings
214,500 + 78,450 Cost per unit = –––––––––––––––––––– = £175
(0·93 x 1,800)
Valuations:
Abnormal loss
= 24 x 175 = £4,200
Finished production = 1,650 x 175 = £288,750
949,620 945,000
565,740 567,000
Total variance
£ 2,100 F
4,620 A
1,260 F
19
(b) Actual hours at actual rate
Actual hours at standard rate
Standard hours for actual production at standard rate
1,740 ––––––-
24 B
L
M
Additional cost of buying in one unit (£)
12
15
Machine hours per unit
13
15
Additional cost of buying in per machine hour (£)
14
13
Ranking for buying in
2nd
1st
Buy in component M.
N
P
24
30
14
16
16
15
4th
3rd
25 D
18
Section B
1 (a) Cost per equivalent unit (EU) calculations for Process I:
Materials
EU
Completion of opening work in progress
21 C
Salary costs (54 x 40) + (110 x 25) Overhead cost (164 x 20)
Total cost Mark-up (40% on total cost)
Final fee
£ 4,910 3,280 –––––––8,190 3,276 –––––––11,466 –––––––-
£
Total contribution from new business (6,000 x 0·45) 2,700
Less Lost contribution from existing business
2 x (6,000 ÷ 15) x 1·20
(960) ––––––-
Overall increase in contribution and profit
6,875
Absorption rates:
Primary (96,000 ÷ 9,600) £10 per hour
Finishing (82,500 ÷ 6,875) £12 per hour
Fixed cost per unit (Y): (48 ÷ 60) x 10 + (35 ÷ 60) x 12 = £15
Answers
Part 1 Examination – Paper 1.2 Financial Information for Management
June 2004 Answers
Section A
1A 2D 3C 4C 5D 6C 7B 8A 9D 10 D 11 D 12 A 13 B 14 C 15 B 16 C 17 C 18 C 19 B 20 C 21 C 22 A 23 A 24 B 25 D
1A
2D
3 C Contribution per unit (CPU) £(60 – 15 – 5) Total fixed cost £(30 x 2,400) Breakeven point (72,000 ÷ 40)
