GSK-923295_SDS_MedChemExpress
进口药品注册标准目录2

甘氨酸 固体脂肪36型 固体脂肪38型 共聚维酮 H 黄凡士林 滑石粉
糊精
药品生产企业使用药用辅料调查情况汇总表
镇江市环宇药用辅料厂 海盐六和淀粉化工有限公司 嘉兴市白浪淀粉制品有限公司 华北制药康欣有限公司 安徽山河药用辅料有限公司 河南精忠威尔药业有限公司 J 甲基丙烯酸-丙烯酸乙酯 Rohm GmbH & Co.KG (德国进口) 共聚物水分散体 甲基丙烯酸共聚物A型 Rohm GmbH & Co.KG (德国进口) 聚维酮K30 湖州展望药业有限公司 BASF Corporation (美国进口) 安徽山河药用辅料有限公司 博爱新开源制药有限公司 海南南杭药业有限公司 聚山梨酯80(吐温80) 南京威尔化工有限公司 上海申宇医药化工有限公司 龙游聚兴粮油医药化工有限公司 北京市海淀会友精细化工厂 南京威尔化工有限公司 辽阳奥克纳米材料有限公司 北京海淀会友精细化工厂 上海浦东高南化工厂 南京威尔化工有限公司 International Specialty Products Inc. ( 美 国进口) 连云港华瑞化工有限责任公司 INTERNATIONAL SPECIALTY PRODUCTS INC.(美国进口) 苏卫药准字(94)第3022-2号 浙卫药准字(1998)第220902号 浙卫药准字(1996)第220901号 冀卫药准字(1995)第000057号 皖药准字(2002)第F0001号 豫药准字F20040002号 进口药品注册证号:H20060013 进口药品注册证号:H20060593 浙药准字F20060062号 进口药品注册证号:H20040430 皖药准字F20060001 (95)卫药准字F-01号 琼药准字F20040001 苏卫药准字(2001)第423201号 沪卫药准字(1995)第186001号 浙卫药准字(1996)第184101号 京卫药准字(95)第023号 苏卫药准字(2001)第423301号 辽药准字F(2004)第900033号 京卫药准字(95)第008号 沪卫药准字(1995)第105004号 苏卫药准字(2001)第423601号 进口药品注册证号:H20030492 苏卫药准字(1983)第308001号 进口药品注册证号:H20030278 第 3 页 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 进口药用辅料注册标准 JX20020020 进口药品注册标准 JX20020322 《中国药典》2005年版二部 进口药品注册标准 JX20030277 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 《中国药典》2005年版二部 进口药品注册标准 JX20020325 《中国药典》2005年版二部 进口药品注册标准 JX19990242
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
高效液相色谱法测定柑橘皮中柠檬苦素

高效液相色谱法测定柑橘皮中柠檬苦素
徐旭耀;黄秋儿;黄惠玲
【期刊名称】《理化检验-化学分册》
【年(卷),期】2012(048)006
【摘要】提出了高效液相色谱法测定柑橘皮中柠檬苦素含量的方法。
样品用体积
分数为70%乙醇溶液于50C超声提取75min。
以Sinochrom ODS-BP
(250min×4.6mm,10μm)为分离柱,以乙腈-水(45+55)溶液作为流动相,用紫外检测器在波长210nm处进行测定。
柠檬苦素的质量浓度在12.0-
384mg·L^-1范围内与其峰面积呈线性关系,方法的平均回收率为99.9%,相
对标准偏差(n=5)为1.8%。
【总页数】4页(P696-698,704)
【作者】徐旭耀;黄秋儿;黄惠玲
【作者单位】湛江师范学院化学科学与技术学院,湛江524048;湛江师范学院化学
科学与技术学院,湛江524048;湛江师范学院化学科学与技术学院,湛江524048【正文语种】中文
【中图分类】O657
【相关文献】
1.高效液相色谱法测定柑橘中的柠檬苦素类似物 [J], 刘亮;戚向阳;董绪燕;李蕙蕙;
刘传菊
2.响应面法优化柑橘皮柠檬苦素提取工艺 [J], 王菁;郑俊霞;蒲彪
3.高效液相色谱法测定柑橘皮中新橙皮苷 [J], 徐旭耀;苏欣;汪秋安
4.柑橘皮中柠檬苦素的提取工艺 [J], 叶沙滨
5.高效液相色谱法测定柑橘汁中的柠檬苦素和柚皮苷 [J], 陈静;高彦祥;吴伟莉;李绍振
因版权原因,仅展示原文概要,查看原文内容请购买。
UDS溶剂组分设计及其脱除黏胶纤维废气中CS2效果

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2018年第37卷第1期·292·化 工 进展UDS 溶剂组分设计及其脱除黏胶纤维废气中CS 2效果霍珍珍,沈本贤,陈曦,孙辉,詹国雄,于惠波(华东理工大学石油加工研究所,化学工程联合国家重点实验室,上海 200237)摘要:为满足黏胶纤维废气脱硫净化需求,设计开发复配型溶剂并进行优化。
基于密度泛函理论,利用AIM 拓扑分析和RDG 分析研究活性组分与CS 2的相互作用机理,通过分子模拟计算不同活性组分与CS 2分子间相互作用,根据黏胶纤维废气组成特点,设计优化UDS 溶剂,进一步在常压实验装置上考察其脱除黏胶纤维废气中CS 2和H 2S 效果。
分子模拟计算结果表明,哌嗪(PZ )、环丁砜(SUL )、聚乙二醇二甲醚(NHD )、二甲基亚砜(DMSO )4种活性组分与CS 2分子间均为弱相互作用,相互作用的强度顺序为PZ >SUL >NHD >DMSO 。
选取与CS 2具有较强结合能的活性组分作为提高CS 2溶解性能的溶剂组分。
脱硫实验结果表明,在常压、吸收温度50℃、气液比500条件下,优化的UDS-F 溶剂可将模拟黏胶纤维废气中CS 2含量由400mg/m 3脱除至79mg/m 3,脱除率较原UDS-A 溶剂高出19个百分点,较甲基二乙醇胺高出65个百分点,表现出优异的脱除性能。
同时,其再生贫液在循环使用过程中依旧能够保持较好的H 2S 和CS 2净化效果。
对其抗发泡性能和抗腐蚀性能进行考察,UDS-F 溶剂表现出良好的抗发泡性能和抗腐蚀性能。
关键词:UDS 溶剂;黏胶纤维废气;吸收净化;二硫化碳;硫化氢中图分类号:X783.4 文献标志码:A 文章编号:1000–6613(2018)01–0292–09 DOI :10.16085/j.issn.1000-6613.2017-0748Compositional design of UDS and its application on removal of CS 2 fromviscose fiber waste gasHUO Zhenzhen ,SHEN Benxian ,CHEN Xi ,SUN Hui ,ZHAN Guoxiong ,YU Huibo(Research Institute of Petroleum Processing ,State Key Laboratory of Chemical Engineering ,East China University ofScience and Technology ,Shanghai 200237,China )Abstract :To meet the desulfurization requirement for the waste gas from viscose fiber production ,the desulfurization solvent UDS was developed. Based on density functional theory ,the interaction mechanism of the active components and CS 2 was studied by using AIM (atoms-in-molecules )topological analysis and RDG (reduced density gradient )analysis. The energy for the interactions between active components and CS 2 was calculated by molecular simulation. Based on the composition of viscose fiber waste gas ,the UDS solvent was designed and optimized. The efficiency for removing CS 2 and H 2S from viscose fiber waste gas was studied in an atmospheric pressure experimental device. Molecular simulation results showed that the interactions between four active components and CS 2 were weak intermolecular forces ,and the intensity ranked in the order of :PZ >SUL >NHD >DMSO.The compounds that have strong interaction with CS 2 can be considered as the promisingsolvent components to improve the solubility of CS 2 in UDS-F. From the experimental results of the atmospheric absorption ,the content of CS 2 was reduced from 400mg/m 3 in raw material to 79mg/m 3 in(SKL-ChE-16C01)项目。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
活性表面增强拉曼光谱纳米基底及其在食品检测中的应用

活性表面增强拉曼光谱纳米基底及其在食品检测中的应用作者:欧已铭,吴酉芝,吴昊宇来源:《现代食品》 2019年第10期◎ 欧已铭1,吴酉芝1,吴昊宇2(1.上海中侨职业技术学院,上海?201500;2.凯惠药业(上海)有限公司,上海?201499)Ou Yiming1, Wu Youzhi1, Wu Haoyu2(1.Shanghai Zhongqiao College, Shanghai?201500, China;2.Kaihui Pharmaceutical Limited Company, Shanghai?201499, China)摘?要:表面增强拉曼光谱是一种快速无损的分析方法,活性纳米粒子基底的构建是提高其灵敏度的关键。
本文介绍了4种活性纳米粒子基底,可满足不同食品分析的需要。
关键词:表面增强拉曼光谱;纳米基底;食品检测Abstract:Surface enhanced Raman spectroscopy is a fast and non-destructive method and one way to improve its sensitivity is to synthesis active nano substrates. In this paper, four kinds of active nanoparticle substrates are introduced, which can apply to analyze different contaminants in food.Key words:Surface enhanced Raman spectroscopy; Nano substrate; Food analysis中图分类号:O657.37表面增强拉曼光谱技术(Surface enhanced raman spectroscopy or scattering,SERS)迄今已有40多年的发展历史。
茶多酚对人脂肪来源间充质干细胞成骨分化的影响

茶多酚对人脂肪来源间充质干细胞成骨分化的影响王华1,齐玉成-杨云芳-赵艺洋2,王慧1,陈培1,杨旭芳1(1.牡丹江医学院,黑龙江牡丹江157011;2.南方医科大学第一临床医学院,广东广州510515)摘要:目的探讨茶多酚(Epigallocatechin-3-gallate,EGCG)对人脂肪间充质干细胞(human adipose-derived mesenchy^-mal stem cells,hADSCs)成骨分化的影响。
方法利用胶原酶消化法和贴壁筛选法从人脂肪组织中分离、培养及扩增hADSCs,倒置显微镜下观察各代hADSCs的形态学特点;利用流式细胞术检测各代hADSCs免疫学表型;取P3代细胞进行成骨诱导分化,实验分三组,即未诱导组、常规成骨诱导组与EGCG组(常规成骨诱导+5^mol/L EGCG),14d后,镜下观察细胞形态学改变及碱性磷酸酶(ALP)染色。
结果体外分离、培养的hADSCs形态均一;流式细胞术结果显示hADSCs具备间充质干细胞的免疫学表型,即CD44、CD73、CD105阳性;成骨诱导14d后部分细胞由长梭形变成多角形,细胞呈现聚集趋势;ALP染色显示EGCG组呈强阳性。
结论成功的从脂肪组织中分离培养出了hADSCs,EGCG能加强其成骨分化能力,这将为骨质疏松症的临床药物开发提供新的思路,亦为组织工程骨的构建提供丰富可靠的种子细胞来源。
关键词:EGCG;人脂肪来源间充质干细胞;成骨分化中图分类号:R595.2文献标识码:A文章编号:1001-7550(2021)01-0001-04Effect of EGCG on osteogenic differentiation of human adipose-derived mesenchymal stem cellsWANG Hua et al(Mudanjiang Medical University,Mudanjiang157011,China)Abstract:Objective To explore the effect of tea polyphenol EGCG on the osteogenic differentiation of human adipose-derived mesenchymal stem cells(hADSCs) .Methods To isolate,culture and amplify hADSCs from human adipose tissue by collagenase digestion and adherent screening methods, the morphological characteristics of each passage of hADSCs were observed under an inverted microscope.The immunophenotype of each generation of hADSCs was detected by flow-cytometry.P3passage cells were taken for osteogenic induction and differentiation,and were divided into three groups:non-induced group, conventional osteogenic induction group and EGCG group(conventional osteogenic induction with+5Rmol/L EGCG).After14days,morphological changes and alkaline phosphatase(ALP)staining were observed under the microscope.Results The morphology of hADSCs isolated and cultured in vitro was uni-form.The results of flow cytometry showed that hADSCs had the immunophenotype of mesenchymal stem cells,such as CD44,CD73and CD105.After14days of osteogenic induction,some cells changed from long spindle shape to polygonal shape,and the cells showed aggregation trend.ALP staining showed strong positive in EGCG group.Conclusion hADSCs have been successfully isolated and cultured from adipose tissue.EGCG can enhance the osteogenic differentiation ability of hADSCs,which will provide a new idea for the clinical drug development of osteoporosis and provide an abundant and reliable source of seed cells for the construction of tissue-engineered bone.Key words:EGCG;human adipose-derived mesenchymal stem cells;osteogenic differentiation随着人口老龄化,骨质疏松症已成为影响人们生活质量的主要因素之一⑷。
医药中常用有机溶剂分类和残留限度

医药中常用有机溶剂分类与残留限度医药中常用有机溶剂分类与残留限度药品的残留溶剂无治疗作用并可能对人体的健康和环境造成危害,本文对国际协调大会〔ICH〕制订的指导原如此与各国执行情况作了较为详尽的介绍。
药品的残留溶剂,又称有机挥发性杂质,是指在活性药物成分、辅料和药品生产过程中使用和产生的有机挥发性化学物质。
药品还可被来自包装、运输、仓储中的有机溶剂污染。
药品生产商有责任确保终产品中的任何一种残留溶剂对人体无害。
各国药监部门曾使用不同的药品残留溶剂指导原如此,为此国际组织展开了协调工作。
经相关程序讨论和审查后,国际协调大会的指导原如此于1997年7月17日获得通过,被推荐至国际协调大会〔ICH〕的指导委员会采用。
该指导原如此要求,如果某个药品的生产或纯化过程可导致溶剂残留,就应对这个药品进展检测,并且只检测生产过程或纯化中使用或产生的那种溶剂。
根据使用量的多少,可采用累加的方法计算药品中残留溶剂的量。
如果累加量低于或等于指导原如此中的推荐量,如此该药品无需进展残留溶剂检测;如果累加量高于推荐量,如此必须对该药品进展残留溶剂检测。
该指导原如此适用于颁布以后上市的所有剂型和给药途径,但不适用于在临床研究阶段使用的潜在新药和新辅料,也不适用于已上市的现有药物。
在某些情况如短期〔小于30天〕或局部应用下,视具体情况,溶剂的高残留量也可承受。
按照毒性大小和对环境的危害程度,该指导原如此将溶剂分成三类〔所列举的溶剂并不完全,应对合成和生产过程所有可能的残留溶剂进展评估〕:第一类溶剂是指可以致癌并被强烈怀疑对人和环境有害的溶剂。
在可能的情况下,应防止使用这类溶剂。
如果在生产治疗价值较大的药品时不可防止地使用了这类溶剂,除非能证明其合理性,残留量必须控制在规定的X围内,如:苯〔2ppm〕、四氯化碳〔4ppm〕、1,2-二氯乙烷〔5ppm〕、1,1-二氯乙烷〔8ppm〕、1,1,1-三氯乙烷〔1500ppm〕。
第二类溶剂是指无基因毒性但有动物致癌性的溶剂。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
GC测定盐酸普拉克索中三乙胺残留量

气相色谱法测定药用辅料聚西托醇1000中的残留杂质

学报Journal of China Pharmaceutical University2022,53(3):293-299293气相色谱法测定药用辅料聚西托醇1000中的残留杂质李浩宇1,2,唐宝强1,2,何东升1,2*,涂家生1,2**(1中国药科大学药学院药用辅料及仿创药物研发评价中心,南京210009;2国家药品监督管理局药物制剂及辅料研究与评价重点实验室,南京210009)摘要建立气相色谱法测定聚西托醇1000中残留的环氧乙烷、1,4-二氧六环、乙二醇、二甘醇和三甘醇等杂质,为聚西托醇1000生产质量控制提供参考。
采用DB-1色谱柱检测环氧乙烷和1,4-二氧六环,顶空进样,进样口温度150℃,检测器温度250℃,顶空平衡温度70℃,平衡时间45min。
采用VF-17MS色谱柱检测乙二醇、二甘醇和三甘醇,液体进样,进样口温度270℃,检测器温度290℃。
实验结果显示,环氧乙烷和1,4-二氧六环在各加样量范围内线性良好(r>0.999),精密度RSD小于8.0%,平均回收率分别为90.6%和101.2%;乙二醇、二甘醇和三甘醇在3~60μg/mL内线性关系良好(r> 0.999),精密度RSD小于3.0%,回收率均在96%~103%。
本研究所建立的方法具有良好的专属性、线性、精密度和回收率,能够有效检测聚西托醇1000中多组分极微量杂质。
关键词聚西托醇1000;杂质;气相色谱法;环氧乙烷;1,4-二氧六环;乙二醇;二甘醇;三甘醇中图分类号R944;R917文献标志码A文章编号1000-5048(2022)03-0293-07doi:10.11665/j.issn.1000-5048.20220306引用本文李浩宇,唐宝强,何东升,等.气相色谱法测定药用辅料聚西托醇1000中的残留杂质[J].中国药科大学学报,2022,53(3):293–299.Cite this article as:LI Haoyu,TANG Baoqiang,HE Dongsheng,et al.Determination of residual impurities in pharmaceutical excipient ceto⁃macrogol1000by gas chromatography[J].J China Pharm Univ,2022,53(3):293–299.Determination of residual impurities in pharmaceutical excipient cetomacro⁃gol1000by gas chromatographyLI Haoyu1,2,TANG Baoqiang1,2,HE Dongsheng1,2*,TU Jiasheng1,2**1Center for Research Development and Evaluation of Pharmaceutical Excipients and Generic Drugs,School of Pharmacy,China Pharmaceutical University,Nanjing210009;2National Medical Products Administration(NMPA)Key Laboratory for Research and Evaluation of Pharmaceutical Preparations and Excipients,Nanjing210009,ChinaAbstract For the quality control of cetomacrogol1000,a gas chromatographic method for the determination of residual impurities in cetomacrogol1000,such as ethylene oxide,1,4-dioxane,ethylene glycol,diethylene glycol and triethylene glycol,was established and validated.The DB-1column with headspace injection was used to detect ethylene oxide and1,4-dioxane with the inlet temperature of150°C,the FID temperature of250°C,the headspace equilibration temperature of70°C and the equilibration time of45min.The VF-17MS column with liquid injection was used to detect ethylene glycol,diethylene glycol and triethylene glycol with the inlet tempera⁃ture of270°C,and the FID temperature of290°C.The results showed that ethylene oxide and1,4-dioxane havea good linearity within their specified addition amount ranges(r>0.999),with the RSD of precision of below8.0%and the average recovery rates of90.6%and101.2%;and that ethylene glycol,diethylene glycol and triethylene glycol also have a good linearity between3‒60μg/mL(r>0.999),with the RSD of precision of below3.0%,and the recovery rates of96%~103%.The method established in this study has good specificity,收稿日期2022-01-06通信作者*Tel:025-********E-mail:dongshenghe@**Tel:025-********E-mail:jiashengtu@基金项目国家“重大新药创制”科技重大专项资助项目(No.2017ZX09101001-006-002);国家药典委员会药品标准制修订研究课题2020Y047学报Journal of China Pharmaceutical University2022,53(3):293-299第53卷linearity,precision and recovery rate,which can effectively detect the multi-component and trace impurities. Key words cetomacrogol1000;impurity;gas chromatography;ethylene oxide;1,4-dioxane;ethylene glycol;diethylene glycol;triethylene glycolThis study was supported by China National Key Hi-Tech Innovation Project for the R&D of Novel Drugs(No.2017ZX09101001-006-002)and the Drug Standard Establishment and Revision Project of Chinese Pharmacopoeia Commission(No.2020Y047)聚西托醇1000(又称聚乙二醇十六烷基醚),是一种常见的药用辅料,化学式为C m H2m+1(OCH2CH2)n OH(m=16,n~20),本品由十六醇与一定数量的环氧乙烷通过缩合反应制得,产品中聚乙二醇的重复单元在2~20之间,当n=20时即为聚西托醇1000(商品名Brij-58)[1]。
活性氧在乳香挥发油抑制结肠癌细胞增殖中的作用

活性氧在乳香挥发油抑制结肠癌细胞增殖中的作用发表时间:2015-10-14T16:42:06.120Z 来源:《河南中医》2015年5月供稿作者:郝雪微1 阮莹2 刘欣宇1 顾园竹1 李金桐1[导读] 1哈尔滨医科大学大庆校区医学检验与技术学院2哈尔滨医科大学药学院黑龙江大庆所有实验结果均是以3次独立实验数据分析所得。
采用SPSS13.0统计学软件进行t检验及方差分析。
郝雪微1 阮莹2 刘欣宇1 顾园竹1 李金桐1(1哈尔滨医科大学大庆校区医学检验与技术学院黑龙江大庆 163000)(2哈尔滨医科大学药学院黑龙江大庆 163319)【摘要】目的:研究活性氧在乳香挥发油抑制结肠癌细胞增殖中的作用。
方法:用MTT法检测乳香挥发油对结肠癌细胞SW480的抑制率,用流式细胞术检测细胞内ROS含量及细胞的凋亡率。
结果:乳香挥发油对结肠癌细胞有抑制作用,同时使细胞内ROS含量增加,凋亡率增加,加入GSH后可以减少ROS含量,减少凋亡率。
结论:乳香挥发油可以抑制结肠癌细胞的增殖及诱导凋亡,GSH可以通过减少活性氧含量减少细胞凋亡。
【关键词】乳香挥发油;人结肠癌细胞SW480;活性氧;凋亡【中图分类号】R34 【文献标识码】B 【文章编号】1003-5028(2015)5-0235-021 实验材料药品与试剂:乳香挥发油为课题组之前制备[2],成分大多为单萜、倍半萜、醇及酯类等,其中含量最高的成分为乙酸辛酯、橙花叔醇异丁酯、3,7,11-三甲基-1,6,10-十二碳三烯-3-醇甲酸酯及β-榄香烯,含量分别为34.660%、18.290%、9.612%和5.614%。
左旋谷胱甘肽(L-Glutathione, GSH)购于Sigma公司。
MTT,活性氧(Reactive Oxygen Species, ROS)检测试剂盒、Annexin V-FITC细胞凋亡检测试剂盒购于碧云天生物技术公司。
细胞及培养:人结肠癌细胞SW480细胞为本室保存, 接种在含10%胎牛血清、2mmol/L谷氨酰胺、100U/ml青霉素和100μg /ml链霉素的RPMI-1640培养液中,在37℃,5%的C02环境下培养。
盐酸二甲双胍缓释片的工艺及质量研究
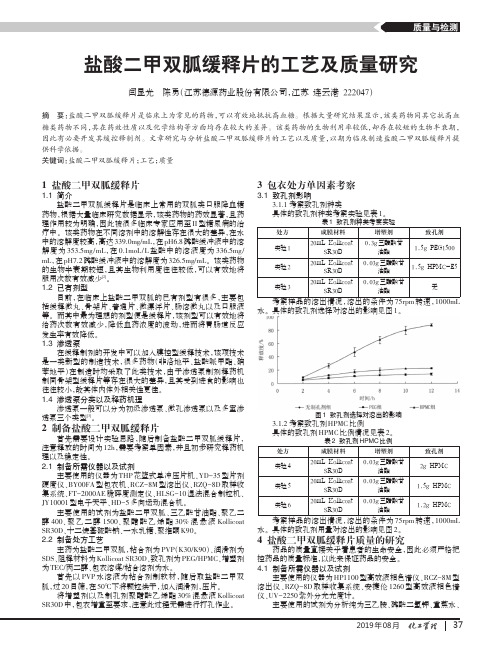
2019年08月盐酸二甲双胍缓释片的工艺及质量研究闫显光陈勇(江苏德源药业股份有限公司,江苏连云港222047)摘要:盐酸二甲双胍缓释片是临床上为常见的药物,可以有效地抵抗高血糖。
根据大量研究结果显示,该类药物同其它抗高血糖类药物不同,其在药效性质以及化学结构等方面均存在较大的差异。
该类药物的生物利用率较低,却存在较短的生物半衰期,因此有必要开发其缓控释制剂。
文章研究与分析盐酸二甲双胍缓释片的工艺以及质量,以期为临床制造盐酸二甲双胍缓释片提供科学依据。
关键词:盐酸二甲双胍缓释片;工艺;质量1盐酸二甲双胍缓释片1.1简介盐酸二甲双胍缓释片是临床上常用的双胍类口服降血糖药物,根据大量临床研究数据显示,该类药物的药效显著,且药理作用较为明确,因此被很多临床专家应用至II 型糖尿病的治疗中。
该类药物在不同溶剂中的溶解性存在很大的差异,在水中的溶解度较高,高达339.0mg/mL ,在pH6.8磷酸缓冲液中的溶解度为353.5mg/mL ,在0.1moL/L 盐酸中的溶液度为336.5mg/mL ,在pH7.2磷酸缓冲液中的溶解度为326.5mg/mL 。
该类药物的生物半衰期较短,且其生物利用度往往较低,可以有效地将服用次数有效减少[2]。
1.2已有剂型目前,在临床上盐酸二甲双胍的已有剂型有很多,主要包括缓释微丸、骨架片、普通片、微漂浮片、肠溶微丸以及口服液等。
而其中最为理想的剂型便是缓释片,该剂型可以有效地将给药次数有效减少,降低血药浓度的波动,进而将胃肠道反应发生率有效降低。
1.3渗透泵在缓释制剂的开发中可以加入膜控型缓释技术,该项技术是一类新型的制造技术,很多药物(非洛地平、盐酸哌甲酯、硝苯地平)在制造时均采取了此类技术,由于渗透泵制剂释药机制同骨架型缓释片等存在很大的差异,且其受到进食的影响也往往较小,故其体内体外相关性更佳。
1.4渗透泵分类以及释药机理渗透泵一般可以分为初级渗透泵、微孔渗透泵以及多室渗透泵三个类型[3]。
谷胱甘肽S-转移酶(GST)活性检测试剂盒说明书微量法

谷胱甘肽S-转移酶(GST)活性检测试剂盒说明书微量法谷胱甘肽S-转移酶(GST)活性检测试剂盒说明书微量法注意:正式测定前务必取2-3个预期差异较大的样本做预测定。
货号:BC0355规格:100T/96S产品内容:试剂一:液体100mL×1瓶,4℃保存。
试剂二:液体22mL×1瓶,4℃保存。
试剂三:粉剂×1瓶,4℃保存。
临用前加2mL蒸馏水溶解。
产品说明:GST是一种具有多种生理功能的蛋白质家族,主要存在于细胞质内。
GST是体内解毒酶系统的重要组成部分,主要催化各种化学物质及其代谢产物与GSH的巯基共价结合,使亲电化合物变为亲水物质,易于从胆汁或尿液中排泄,达到将体内各种潜在或具备毒性的物质降解并排出体外的目的。
因此,GST在保护细胞免受亲电子化合物的损伤中发挥着重要的生物学功能。
此外,因为GST具有GSH-Px活性,亦称为non-Se GSH-Px,具有修复氧化破坏的大分子如DNA、蛋白质等的功能。
注意,GST催化的反应减少GSH含量,但是不增加GSSG含量。
GST催化GSH与CDNB结合,其结合产物的光吸收峰波长为340nm;通过测定340nm波长处吸光度上升速率,即可计算出GST 活性。
自备仪器和用品:低温离心机、水浴锅、可调节移液器、紫外-可见分光光度计/酶标仪、微量石英比色皿/96孔UV板和蒸馏水。
操作步骤:一、粗酶液提取:1.组织:按照组织质量(g):试剂一体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL试剂一)进行冰浴匀浆。
8000g,4℃离心10min,取上清置冰上待测。
2.细菌、真菌:按照细胞数量(104个):试剂一体积(mL)为500~1000:1的比例(建议500万细胞加入1mL试剂一),冰浴超声波破碎细胞(功率300w,超声3秒,间隔7秒,总时间3min);然后8000g,4℃,离心10min,取上清置于冰上待测。
3.血清等液体:直接测定。
GSK3326595-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-08-2018Print Date:Oct.-08-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :GSK3326595Catalog No. :HY-101563CAS No. :1616392-22-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Skin corrosion ⁄irritation (Category 2),H315Serious eye damage ⁄eye irritation (Category 2A),H319Specific target organ toxicity, single exposure; Respiratory tract irritation (Category 3),H3352.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H315 Causes skin irritationH319 Causes serious eye irritationH335 May cause respiratory irritationPrecautionary statement(s)P261 Avoid breathing dust ⁄fume ⁄gas ⁄mist ⁄vapours ⁄spray.P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.P302+352 IF ON SKIN: wash with plenty of soap and water.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:GSK-3326595;GSK 3326595Formula:C24H32N6O3Molecular Weight:452.55CAS No. :1616392-22-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
2013年6月20日发布最新影响因子IF_完整版

Total Cites Impact5-Year ImmediacyArticles (linked to journal informationFactor Impact Index1439CA-CANCER J CLIN0007-923513722153.45988.5527.0425 6399NEW ENGL J MED0028-479324560551.65850.80712.667360 7457REV MOD PHYS0034-68613572044.98251.882 6.47846 1675CHEM REV0009-266511259641.29845.79514.335176 6266NAT REV GENET1471-00562335841.06336.4 6.31470 5693LANCET0140-673616692239.0636.4279.556313 6278NATURE0028-0836********.59738.1599.243869 6269NAT REV MOL CELL BIO1471-00723134137.16244.026 5.98565 668ANNU REV IMMUNOL0732-05821596336.55643.7428.42928 6247NAT MATER1476-11224634835.74942.3768.411141 6240NAT GENET1061-40368118335.20934.52 5.511225 6260NAT REV CANCER1474-175X326283539.361 6.33369 277ADV PHYS0001-8732484934.29431.1670.8577 6267NAT REV IMMUNOL1474-173********.12935.851 4.83165 6263NAT REV DRUG DISCOV1474-177********.07833.2058.65143 6232NAT BIOTECHNOL1087-01563872832.43832.1827.08792 1565CELL0092-867417876231.95734.366 6.499415 6272NAT REV NEUROSCI1471-003X2693831.67335.888 5.06562 6250NAT NANOTECHNOL1748-33872192031.1736.011 5.876121 7624SCIENCE0036-807550848931.02733.587 6.691832 6948PHYSIOL REV0031-93332253730.17438.707 2.53839 5608JAMA-J AM MED ASSOC0098-748412150429.97829.2738.504232 652ANNU REV BIOCHEM0066-41541942027.68131.964 4.31232 6252NAT PHOTONICS1749-48851399327.25431.5677.492120 7140PROG POLYM SCI0079-67001445726.38331.706 4.60756 3430IEEE ENG MED BIOL0739-5175291326.3038.0190 6245NAT IMMUNOL1529-29083246526.19925.005 4.961128 677ANNU REV PATHOL-MECH1553-4006232225.79419.915 6.38918 1599CELL STEM CELL1934-59091259325.31527.361 4.336122 5696LANCET ONCOL1470-20451700525.11721.856 6.536153 1678CHEM SOC REV0306-00124764624.89230.1817.997390 1500CANCER CELL1535-61082220024.75527.059 4.465114 5695LANCET NEUROL1474-44221516023.91722.959 4.47289 682ANNU REV PLANT BIOL1543-50081343823.65429.248 3.18527 6249NAT METHODS1548-70911924123.56523.231 4.404151 651ANNU REV ASTRON ASTR0066-4146824023.33334.261 2.21414 7125PROG MATER SCI0079-6425592123.19422.3337.21723 6912PHYS REP0370-157********.92922.99 6.69843 6248NAT MED1078-89565735022.86426.441 5.763186 6268NAT REV MICROBIOL1740-152********.4923.227 4.02969 6854PHARMACOL REV0031-69971033122.34522.114 2.33336 5764LIVING REV RELATIV1433-8351141722.33316.417 2.81811 6234NAT CHEM1755-4330865221.75723.02 5.532126 678ANNU REV PHARMACOL0362-1642720521.54321.6447.26926 15ACCOUNTS CHEM RES0001-48424211220.83324.633 5.295207 6233NAT CELL BIOL1465-73923222120.76120.691 3.97132 674ANNU REV NEUROSCI0147-006X1263820.61431.028 3.11526 5694LANCET INFECT DIS1473-30991047119.96618.095780 3581IMMUNITY1074-76133254619.79520.722 3.713164 680ANNU REV PHYSIOL0066-4278791419.54718.952 4.90521 6253NAT PHYS1745-24731800419.35219.367 6.854137 1098BEHAV BRAIN SCI0140-525X640218.57123.173 2.286144480J CLIN ONCOL0732-183X12867918.03817.255 4.836597 655ANNU REV CELL DEV BI1081-0706886617.98319.8060.78628 2458ECOL LETT1461-023X1753317.94918.495 1.403154 6221NANO TODAY1748-0132294417.68918.1920.78437 666ANNU REV GENET0066-4197677217.43621.7890.31232 1843CLIN MICROBIOL REV0893-85121205517.31319.065 2.21428 1422BRIT MED J1756-183********.21515.887.756328 6045MICROBIOL MOL BIOL R1092-2172907916.41717.718 1.51729 931ASTROPHYS J SUPPL S0067-00492476416.23814.437 3.074162 671ANNU REV MATER RES1531-7331525916.17914.4950.66718 8125TRENDS COGN SCI1364-66131571716.00816.845 4.05654 6356NEURON0896-62736952615.76616.403 2.603348 7191PSYCHOL BULL0033-29093081415.57519.676 2.31638 6271NAT REV NEUROL1759-4758229515.51813.994 1.65452 8126TRENDS ECOL EVOL0169-53472427915.38917.112 2.97476 7907SURF SCI REP0167-5729411515.33322.2817.758 6126MOL CELL1097-27654781815.2814.902 2.818296 683ANNU REV PSYCHOL0066-43081063515.26526.624 4.81822 7023PLOS MED1549-16761482015.25316.426 3.235115 6251NAT NEUROSCI1097-62564251915.25116.412 2.882228 1770CIRCULATION0009-732215228115.20215.385 2.98592 658ANNU REV CONDEN MA P1947-545462415.13915.139 5.13315 7122PROG ENERG COMBUST0360-1285476715.08917.778330 6262NAT REV CLIN ONCOL1759-4774276815.03114.931 2.660 6168MOL PSYCHIATR1359-41841268614.89713.985 3.879116 2532ENDOCR REV0163-769X1316614.87322.16 3.526 262ADV MATER0935-96489195214.82913.86 2.557867 458AM J PSYCHIAT0002-953X4273014.72114.396 2.574108 1583CELL METAB1550-41311243214.61917.551 3.25148 672ANNU REV MED0066-4219504314.60311.681 4.48635 389ALZHEIMERS DEMENT1552-5260290714.4838.672 2.2162 6236NAT CLIM CHANGE1758-678X84414.47214.5 2.65120 3122GENOME RES1088-90512885614.39714.104 3.416238 670ANNU REV MAR SCI1941-1405111414.36818.196 4.47621 5617JNCI-J NATL CANCER I0027-88743742414.33614.794 3.677124 95ACTA CRYSTALLOGR D0907-44491245314.1037.540.604182 2674EUR HEART J0195-668X3262914.09711.991 3.451273 4214J AM COLL CARDIOL0735-10977302114.08613.71 3.34427 597ANN INTERN MED0003-48194690513.97616.26 4.011176 7425REV GEOPHYS8755-1209646113.90613.333 2.0825 5849MAT SCI ENG R0927-796X485013.90218.9740.6676 5765LIVING REV SOL PHYS1614-496149813.833 1.8336 815ARCH GEN PSYCHIAT0003-990X3741213.77214.466 2.488125 529ANGEW CHEM INT EDIT1433-785122989413.73413.56 2.9592227 662ANNU REV ENTOMOL0066-4170877713.58914.047 3.09122 8134TRENDS NEUROSCI0166-22361788913.58214.466 2.31573 679ANNU REV PHYS CHEM0066-426X700213.36518.121 4.13829 7330REP PROG PHYS0034-48851022313.23214.937 3.55267 2892FEMS MICROBIOL REV0168-6445792613.23112.987 3.48152 4682J EXP MED0022-10076508813.21414.102 2.616190 8121TRENDS BIOCHEM SCI0968-00041564213.07612.005 2.40664 6219NANO LETT1530-69848843113.02514.132 2.4711078 6235NAT CHEM BIOL1552-44501081912.94815.6 3.715130 673ANNU REV MICROBIOL0066-4227844912.915.214 1.0825241ADV DRUG DELIVER REV0169-409X2156312.88815.431 1.488162 2296DEV CELL1534-58072030612.86114.091 2.174201 3076GASTROENTEROLOGY0016-50856040812.82112.835 3.424276 4471J CLIN INVEST0021-97389426412.81214.689 2.586394 7020PLOS BIOL1545-78852290812.6913.447 2.151152 654ANNU REV BIOPHYS1936-122X152912.6316.591 2.34626 1580CELL HOST MICROBE1931-3128610812.60913.567 2.943122 664ANNU REV FLUID MECH0066-4189675012.612.933 3.91323 3092GENE DEV0890-93695762712.44412.741 2.149249 657ANNU REV CLIN PSYCHO1548-5943204312.42214.073 1.44418 6241NAT GEOSCI1752-0894745112.36712.905 2.446148 7943SYST BIOL1063-51571141112.16913.316 2.80873 3590IMMUNOL REV0105-28961263412.15511.892 3.028106 55ACS NANO1936-0851*******.06212.524 1.941191 4200J ALLERGY CLIN IMMUN0091-67493607412.04710.108 2.752327 1010AUTOPHAGY1554-8627666112.0428.503 1.982114 3296HEPATOLOGY0270-91395012712.00311.4 2.169419 6275NAT STRUCT MOL BIOL1545-99932429911.90212.307 2.576184 7236Q REV BIOPHYS0033-5835227711.87512.163 1.2512 1761CIRC RES0009-73304644811.86111.028 2.465275 8137TRENDS PLANT SCI1360-138********.80811.675 2.1182 8124TRENDS CELL BIOL0962-89241103911.72112.095 2.32474 2544ENERG ENVIRON SCI1754-56921284911.65312.462 3.087473 2174CURR OPIN CELL BIOL0955-06741386611.4112.034 1.291110 6171MOL SYST BIOL1744-4292633511.3412.392 1.87757 215ADV APPL MECH0065-215696011.3337.1430.65 422AM J HUM GENET0002-92973468711.20212.512 2.188223 608ANN NEUROL0364-51343225611.19311.047 1.994173 462AM J RESP CRIT CARE1073-449X4971211.04110.919 2.705264 6264NAT REV ENDOCRINOL1759-5029202111.02511.479 1.85964 2070COORDIN CHEM REV0010-85452560111.01612.257 2.294136 653ANNU REV BIOMED ENG1523-9829315010.94614.5660.47419 4418J CELL BIOL0021-95257297410.82210.367 1.657283 7622SCI TRANSL MED1946-6234557410.75710.481 3.694219 3233GUT0017-57493071910.7329.988 3.821190 4213J AM CHEM SOC0002-786343128610.67710.237 2.1643099 1597CELL RES1001-0602702610.52610.216 2.84899 2789EUR UROL0302-28381708810.4768.083 4.043211 6265NAT REV GASTRO HEPAT1759-5045155210.4269.715 1.13666 6261NAT REV CARDIOL1759-5002167210.410.155 1.64751 661ANNU REV ECOL EVOL S1543-592X1490910.37516.8310.04821 6112MOL ASPECTS MED0098-2997301610.37510.821 1.46239 6115MOL BIOL EVOL0737-40383216810.35311.221 1.561294 3119GENOME BIOL1474-75961747810.2888.959 1.468154 1194BIOL REV1464-7931632610.25610.949 1.81153 7124PROG LIPID RES0163-7827389310.2510.69 1.58324 681ANNU REV PHYTOPATHOL0066-4286567610.22912.3130.69623 6255NAT PROD REP0265-0568660910.17810.072 2.38565 5735LEUKEMIA0887-69241825910.1648.692 2.401242 1505CANCER DISCOV2159-827463910.14310.143 2.97271 244ADV ENERGY MATER1614-6832199510.04310.05 2.118169 6237NAT COMMUN2041-1723732210.01510.02 2.096612 1360BRAIN0006-8950411899.91510.87 2.261280 4812J HEPATOL0168-8278222819.8588.897 2.863362516EMBO J0261-4189759509.8229.602 2.553333 7197PSYCHOL REV0033-295X213749.79711.342 1.22236 7141PROG QUANT ELECTRON0079-67277459.7868.29011 8129TRENDS GENET0168-9525107989.7729.325 2.10169 249ADV FUNCT MATER1616-301X347589.76510.342 1.815569 6273NAT REV RHEUMATOL1759-479019219.7459.318 1.44665 6692P NATL ACAD SCI USA0027-84245349519.73710.583 1.8933801 141ACTA NEUROPATHOL0001-6322116649.7348.562 2.565124 8122TRENDS BIOTECHNOL0167-779998339.669.289 1.61673 2132CSH PERSPECT BIOL1943-026439219.6310.3670.884155 1255BIOTECHNOL ADV0734-975066999.59911.85 1.248133 5984MED RES REV0198-632532979.5839.978 1.81137 8133TRENDS MOL MED1471-491459459.57110.142 1.48776 667ANNU REV GENOM HUM G1527-820423279.514.1250.620 921ASTRON ASTROPHYS REV0935-********.513.1947 2145CURR BIOL0960-9822431919.49410.445 2.307371 8131TRENDS IMMUNOL1471-490678509.4869.715 2.05178 2175CURR OPIN CHEM BIOL1367-593188699.4719.2560.98776 6377NEUROSCI BIOBEHAV R0149-7634129689.449.924 2.129147 7143PROG RETIN EYE RES1350-946235449.43910.188 1.57633 1828CLIN INFECT DIS1058-4838455569.3748.98 2.231523 2625EPIDEMIOL REV0193-936X29139.26919.051 3.07114 2571ENTERP INF SYST-UK1751-75755799.256 4.771 2.68222 6977PLANT CELL1040-4651390679.25110.125 1.526325 8136TRENDS PHARMACOL SCI0165-6147104179.2510.158 2.2774 1189BIOL PSYCHIAT0006-3223378409.2479.773 2.131251 6750PART FIBRE TOXICOL1743-897714719.1780.45544 676ANNU REV NUTR0199-988544909.15810.1880.920 2403DRUG RESIST UPDATE1368-764619659.1149.8130.8723 627ANN RHEUM DIS0003-4967270209.1118.351 2.308325 1278BLOOD0006-49711436709.069.338 2.1061251 6457NPG ASIA MATER1884-40493019.0428.556 2.41429 7129PROG NEUROBIOL0301-0082107149.03510.322 1.3275 1095BBA-REV CANCER0304-419X33349.03310.118 1.7560 4237J AM SOC NEPHROL1046-6673301328.9878.477 2.051195 8348WORLD PSYCHIATRY1723-861711938.974 6.413 2.42921 4133ISME J1751-736261298.9518.927 2.264208 8127TRENDS ENDOCRIN MET1043-276056138.9018.248 1.40574 3380HUM REPROD UPDATE1355-478656858.8479.512 1.88443 660ANNU REV EARTH PL SC0084-659746538.8339.861 1.51927 2246CYTOKINE GROWTH F R1359-610145878.8317.9190.58834 2186CURR OPIN IMMUNOL0952-791586898.7718.826 1.45284 2207CURR OPIN STRUC BIOL0959-440X101168.7389.02 1.45293 7728SLEEP MED REV1087-079229768.6818.26 1.94335 6371NEUROPSYCHOPHARMACOL0893-133X198828.6787.796 1.802248 1520CANCER RES0008-54721435228.658.576 1.539625 650ANNU REV ANAL CHEM1936-132711498.612.2830.69623 7022PLOS GENET1553-7404222128.5179.44 1.204691 7590SCHIZOPHRENIA BULL0586-7614116068.4868.934 1.699136 8058TOP CURR CHEM0340-102255078.456 6.205 4.27355 2202CURR OPIN PLANT BIOL1369-526694618.4559.221 1.22996 8132TRENDS MICROBIOL0966-842X82268.4348.09 2.15372 8038THORAX0040-6376177868.3767.808 2.371159 1577CELL DEATH DIFFER1350-9047145518.3718.395 2.2791901676CHEM SCI2041-652048458.3148.33 2.771458 6474NUCLEIC ACIDS RES0305-10481187158.2788.055 2.2051425 7656SEMIN LIVER DIS0272-808735158.274 6.8240.64937 6352NEUROLOGY0028-3878744088.2498.397 1.816527 1663CHEM MATER0897-4756746518.2387.627 1.123576 2190CURR OPIN MICROBIOL1369-527476958.238.104 1.06199 4330J AUTOIMMUN0896-841141688.145 6.075 1.30698 7026PLOS PATHOG1553-7374197438.1368.917 1.306627 2460ECOL MONOGR0012-961584478.0858.1290.84626 5271J PHOTOCH PHOTOBIO C1389-556720268.06911.9520.7516 3031FRONT NEUROENDOCRIN0091-302228427.98510.876 1.79329 5714LASER PHOTONICS REV1863-888015497.9768.779 2.44243 1000AUTOIMMUN REV1568-997250027.975 6.069 1.656163 6257NAT PROTOC1754-2189169227.9611.743 1.362174 6270NAT REV NEPHROL1759-506113547.9437.409 1.20663 6918PHYS REV LETT0031-90073621857.9437.435 2.176**** ****KIDNEY INT0085-2538380947.916 6.968 2.603234 2311DIABETES0012-1797461587.8958.611 1.542347 2172CURR OPIN BIOTECH0958-166995017.869.006 1.328131 6230NANOTOXICOLOGY1743-539013877.8447.758 1.24374 1374BRAIN STRUCT FUNCT1863-265312597.837 6.821 1.38255 1794CLIN CANCER RES1078-0432657047.8377.827 1.814665 7730SMALL1613-6810181377.8238.084 1.429457 1372BRAIN RES REV0165-017389037.8188.7860 8028THERANOSTICS1838-76404287.8067.806 1.98993 2517EMBO MOL MED1757-467612907.7959.39 1.698106 7054POLYM REV1558-372411557.79410.021 1.45511 6855PHARMACOL THERAPEUT0163-7258106557.7938.736 1.343108 1516CANCER METAST REV0167-765950167.78710.0880.85368 2312DIABETES CARE0149-5992490257.7357.555 2.234427 5846MASS SPECTROM REV0277-703739447.7359.924228 5716LASER PHYS LETT1612-201141167.714 4.9740.703158 7138PROG PHOTOVOLTAICS1062-799545357.7127.023 3.223112 7845STEM CELLS1066-5099182537.7018.368 1.297269 3374HUM MOL GENET0964-6906360417.6927.541 1.554478 7614SCI SIGNAL1937-914551687.6487.603 1.478182 4543J CONTROL RELEASE0168-3659297557.6338.078 1.136501 3024FRONT ECOL ENVIRON1540-929543167.61510.061 1.2560 7446REV MED VIROL1052-927617587.6157.024 1.35728 1204BIOMATERIALS0142-9612697927.6048.496 1.597898 5231J PATHOL0022-3417144687.585 6.928 2.376173 415AM J GASTROENTEROL0002-9270275217.5537.019 2.074188 4970J MAMMARY GLAND BIOL1083-302121207.524 5.838 1.225 656ANNU REV CHEM BIOMOL1947-54383317.5127.512 1.04522 7235Q REV BIOL0033-577033127.57.9040.1119 4037INT MATER REV0950-660822557.487.149 1.18816 876ARTHRITIS RHEUM-US0004-3591452007.4777.63 1.659411 1698CHEMSUSCHEM1864-563150567.4757.951 1.189286 2183CURR OPIN GENET DEV0959-437X81787.478.209 1.53880 7647SEMIN CANCER BIOL1044-579X42997.4367.107 2.22653 6140MOL ECOL RESOUR1755-098X47367.432 4.150.848132 7145PROG SOLID STATE CH0079-678614747.429 3.33806 675ANNU REV NUCL PART S0163-899821167.48.7770.73719 3210GONDWANA RES1342-937X43947.396 6.171 2.1341576220NANO RES1998-012430317.3927.8010.97996 615ANN ONCOL0923-7534228057.384 6.473 1.214541 6552ONCOGENE0950-9232605267.3577.18 2.218458 2436EARTH-SCI REV0012-825262047.3398.759 1.04981 2193CURR OPIN NEUROBIOL0959-4388114297.3358.165 1.111135 5064J MOL CELL BIOL1674-27886517.3088.271 1.75737 5298J PINEAL RES0742-309849567.304 5.451 1.10992 2593ENVIRON HEALTH PERSP0091-6765301117.267.522 1.56268 258ADV IMMUNOL0065-277623257.2568.020.87131 6132MOL CELL PROTEOMICS1535-9476142017.2518.051 1.532310 7206PSYCHOTHER PSYCHOSOM0033-319027617.23 5.825 1.26926 3201GLOBAL ECOL BIOGEOGR1466-822X52767.2237.284 1.935108 695ANTIOXID REDOX SIGN1523-0864118627.1897.548 1.991222 2518EMBO REP1469-221X105747.1897.396 1.68799 1796CLIN CHEM0009-9147265827.1497.192 2.737152 7146PROG SURF SCI0079-681619047.1369.140.27311 223ADV CARBOHYD CHEM BI0065-23188337.133 5.8460.754 2812EXERC IMMUNOL REV1077-55524387.053 5.2560.1258 6172MOL THER1525-0016127597.041 6.457 1.676219 6190MUCOSAL IMMUNOL1933-021*******.119 1.65767 3826INT J EPIDEMIOL0300-577114451 6.9827.001 3.281128 4205J AM ACAD CHILD PSY0890-856716470 6.977.148 2.0196 6223NANOMED-NANOTECHNOL1549-96343097 6.937.647 1.263160 2228CURR TOP DEV BIOL0070-21532246 6.912 6.4670.76242 6685P IEEE0018-921918840 6.9117.6940.697195 3200GLOBAL CHANGE BIOL1354-101318398 6.917.819 1.3297 6534OCEANOGR MAR BIOL0078-32182142 6.9099.8790.56 5139J NEUROSCI0270-6474160915 6.9087.8690.9781668 3362HUM BRAIN MAPP1065-947113379 6.8787.032 1.451226 3398HYPERTENSION0194-911X32323 6.873 6.984 1.588342 6518OBES REV1467-78815154 6.877.021 1.436101 6008METAB ENG1096-71762855 6.859 6.696 1.34772 1864CLIN PHARMACOL THER0009-923614263 6.846 6.349 1.995200 1627CEREB CORTEX1047-321121409 6.8287.463 2.05258 3224GREEN CHEM1463-926215554 6.828 6.992 1.269449 6929PHYS TODAY0031-92283738 6.762 5.263 1.51437 275ADV ORGANOMET CHEM0065-3055841 6.758.94103 6949PHYSIOLOGY1548-92132500 6.758.388 1.13829 6407NEW PHYTOL0028-646X26842 6.736 6.888 1.373378 929ASTROPHYS J0004-637X191940 6.733 5.945 2.0473075 1764CIRC-CARDIOVASC GENE1942-325X1641 6.728 6.398 1.17182 6921PHYS REV X2160-3308414 6.711 6.737 2.22571 6158MOL ONCOL1574-78911193 6.701 6.3790.91760 1768CIRC-HEART FAIL1941-32892007 6.684 6.67 1.41993 1309BMC MED1741-70152691 6.679 6.413 1.275109 1820CLIN GASTROENTEROL H1542-35658295 6.648 6.108 1.458179 6561ONCOTARGET1949-25531450 6.636 6.636 1.316114 2177CURR OPIN COLLOID IN1359-02944670 6.6297.0360.88443 2862EXPERT REV MOL MED1462-39941734 6.6230.78919 1417BRIT J PSYCHIAT0007-125021472 6.6067.112 1.872117 1019B AM METEOROL SOC0003-000711821 6.5917.704 2.48778 5280J PHYS CHEM LETT1948-71858575 6.585 6.651 1.301632 6902PHYS LIFE REV1571-0645659 6.583 6.699.45511 6990PLANT J0960-741232497 6.5827.113 1.5333456997PLANT PHYSIOL0032-088962407 6.5557.084 1.313469 5605JACC-CARDIOVASC INTE1936-87983378 6.552 6.834 1.603126 2396DRUG DISCOV TODAY1359-64468855 6.551 6.89 1.177158 271ADV MICROB PHYSIOL0065-29111006 6.545 6.267 1.25 1766CIRC-CARDIOVASC INTE1941-76401534 6.543 6.239 1.23797 1292BMC BIOL1741-70072631 6.531 6.384 1.69559 405AM J CLIN NUTR0002-916548233 6.5047.196 1.2325 2325DIABETOLOGIA0012-186X24906 6.487 6.772 1.749339 6150MOL INTERV1534-03841243 6.4818.0310 1490CAN MED ASSOC J0820-394611869 6.4657.53 1.766124 4906J INTERN MED0954-******** 6.455 5.913 1.609110 4465J CLIN ENDOCR METAB0021-972X68170 6.43 6.568 1.087809 6203MUTAT RES-REV MUTAT1383-57422736 6.4268.202122 6368NEUROPSYCHOL REV1040-73081611 6.427.5260.86229 353AIDS0269-937023034 6.407 6.131 1.679265 8059TOP ORGANOMETAL CHEM1436-60021152 6.384 1.76221 1643CHEM COMMUN1359-7345122728 6.378 6.226 1.533173 1557CATAL REV0161-49402529 6.37510.1750.8899 2780EUR RESPIR J0903-193625962 6.355 6.32 1.82317 222ADV CANCER RES0065-230X1890 6.351 5.5120.57121 8074TRAC-TREND ANAL CHEM0165-99367327 6.351 6.7610.92138 930ASTROPHYS J LETT2041-820545993 6.345 1.687670 7279RADIOLOGY0033-841945413 6.339 6.7380.832381 873ARTERIOSCL THROM VAS1079-564231851 6.338 6.986 1.097370 636ANN SURG0003-493236761 6.3298.264 1.15301 6973PLANT BIOTECHNOL J1467-76443136 6.279 5.813 1.04101 6139MOL ECOL0962-108330411 6.275 6.792 1.369445 2110CRIT REV TOXICOL1040-84442955 6.253 5.9720.94335 6339NEUROIMAGE1053-811961770 6.2527.063 1.2911222 2595ENVIRON INT0160-41209059 6.248 6.1220.935199 5339J PSYCHIATR NEUROSCI1180-48822253 6.242 6.473 1.64734 6226NANOSCALE2040-33647835 6.233 6.262 1.1671015 7886STUD MYCOL0166-06161237 6.231 6.813 4.1676 6869PHILOS T R SOC B0962-843626581 6.237.298 3.522289 2310DEVELOPMENT0950-199151191 6.208 6.888 1.258434 7648SEMIN CELL DEV BIOL1084-95215613 6.202 6.51 1.034116 3764INT J CANCER0020-713644529 6.198 5.474 1.39738 4915J INVEST DERMATOL0022-202X23976 6.193 6.065 1.898264 471AM J TRANSPLANT1600-613516088 6.192 6.014 1.506348 6318NEURO-ONCOLOGY1522-85173845 6.18 5.9470.694157 231ADV COLLOID INTERFAC0001-86867115 6.1698.01 1.3250 6320NEUROBIOL AGING0197-458015479 6.166 6.098 1.583537 5604JACC-CARDIOVASC IMAG1936-878X2977 6.164 6.703 1.717113 2080CORTEX0010-94525265 6.161 5.042 2.745106 7864STROKE0039-249952524 6.158 6.831 1.086567 1640CHEM BIOL1074-55219695 6.157 6.097 1.486144 6621ORG LETT1523-706073440 6.142 5.563 1.5721608 4378J BONE MINER RES0884-043122111 6.128 6.227 1.101257 6165MOL PLANT1674-20522346 6.126 5.77 1.585118 2094CRIT CARE MED0090-349332733 6.124 6.401 2.614363 2371DIVERS DISTRIB1366-95164336 6.122 5.7430.935107 1863CLIN PHARMACOKINET0312-******** 6.109 5.4860.98154 8313WHO TECH REP SER0512-******** 6.1 3.2560.35714 8039THROMB HAEMOSTASIS0340-624517392 6.094 4.782 1.3462465510J THROMB HAEMOST1538-793314645 6.081 6.176 1.17265 5876MATER TODAY1369-70213769 6.0718.677 1.97744 1578CELL DEATH DIS2041-48891481 6.044 6.0440.565191 4548J COSMOL ASTROPART P1475-751613656 6.036 5.295 2.387555 1523CANCER TREAT REV0305-73724233 6.024 6.246 1.458107 7131PROG NUCL MAG RES SP0079-65651993 6.022 6.065 1.38918 1285BLOOD REV0268-960X17706 6.6620.76939 7877STRUCTURE0969-212612441 5.994 6.081 1.466191 2519EMERG INFECT DIS1080-604021567 5.993 6.312 1.689244 322AGEING RES REV1568-16371965 5.953 6.549 1.43941 7706SIAM REV0036-14455051 5.9528.4140.40922 1763CIRC-ARRHYTHMIA ELEC1941-31492173 5.947 6.434 1.175143 2108CRIT REV SOLID STATE1040-8436744 5.9477.368110 1543CARDIOVASC RES0008-636320689 5.94 6.113 1.689222 3241HAEMATOL-HEMATOL J0390-607811392 5.935 6.002 1.454251 7654SEMIN IMMUNOL1044-53233190 5.9267.250.89146 6031METHODS ECOL EVOL2041-210X822 5.92460.854123 203ADDICT BIOL1355-62151977 5.914 5.322 1.01193 1081BASIC RES CARDIOL0300-84283292 5.904 5.362 1.16772 6390NEUROTHERAPEUTICS1933-72131923 5.904 5.720.90563 381ALLERGY0105-453812173 5.883 5.983 1.58193 4422J CELL SCI0021-953340261 5.877 6.375 1.032535 1391BREAST CANCER RES1465-542X7547 5.872 6.2640.82172 1528CARBON0008-622332742 5.868 6.35 1.197674 2915FISH FISH1467-29601846 5.8557.326 1.53826 1699CHEST0012-369244596 5.854 6.42 3.394307 4871J INFECT DIS0022-189942783 5.848 5.914 1.556493 2189CURR OPIN LIPIDOL0957-96723620 5.839 5.7380.92264 6966PIGM CELL MELANOMA R1755-14713385 5.839 5.434 1.39768 1685CHEM-EUR J0947-653960788 5.831 5.623 1.2411916 717APPL CATAL B-ENVIRON0926-337323011 5.825 6.0310.965480 4486J CLIN PSYCHIAT0160-668917639 5.812 5.6390.726157 1765CIRC-CARDIOVASC IMAG1941-96511650 5.795 6.579 1.17984 5924MAYO CLIN PROC0025-61969444 5.79 5.638 1.44109 4413J CATAL0021-951734516 5.787 6.249 1.025284 5164J NUCL MED0161-550521262 5.774 6.4020.934243 2597ENVIRON MICROBIOL1462-291213228 5.756 5.943 1.455242 2626EPIDEMIOLOGY1044-39838979 5.738 6.241 2.12588 8328WIRES COMPUT MOL SCI1759-0876570 5.738 5.738 3.51856 2539ENDOSCOPY0013-726X8401 5.735 5.355 1.514144 4048INT REV IMMUNOL0883-******* 5.733 4.140.44829 324AGING CELL1474-97184134 5.705 6.415 1.485130 2885FASEB J0892-663839540 5.704 6.222 1.13462 1895COCHRANE DB SYST REV1469-493X34230 5.703 6.5120.728977 5027J MED GENET0022-259311563 5.703 5.793 1.353116 5687LAB CHIP1473-019716485 5.697 6.136 1.256617 510ANAL CHEM0003-270096794 5.695 5.7690.9481479 6697P ROY SOC B-BIOL SCI0962-845237539 5.683 5.832 1.224624 8330WIRES NANOMED NANOBI1939-5116983 5.681 6.34 1.08547 1767CIRC-CARDIOVASC QUAL1941-77051551 5.658 5.647 1.43100 7369RETROVIROLOGY1742-46903084 5.657 4.857 1.505107 6713PAIN0304-395929370 5.644 6.125 1.264269 3917INT J NEUROPSYCHOPH1461-14574003 5.641 5.0920.805123 1530CARCINOGENESIS0143-333421223 5.635 5.5570.9553106383NEUROSCIENTIST1073-85843259 5.633 6.417 1.35645 7327RENEW SUST ENERG REV1364-032110347 5.627 6.5770.803584 6321NEUROBIOL DIS0969-996111288 5.624 5.482 1.493296 5925MBIO2150-75111260 5.621 5.621 1.536151 4817J HIGH ENERGY PHYS1126-670852898 5.618 4.712 2.6581861 1589CELL MOL LIFE SCI1420-682X17974 5.615 6.359 1.072290 5022J MED CHEM0022-262359227 5.614 5.383 1.225890 1362BRAIN BEHAV IMMUN0889-15916137 5.612 5.698 1.082147 6124MOL CANCER THER1535-716314654 5.599 5.72 1.201259 1873CLIN REV ALLERG IMMU1080-05491685 5.59 3.955 1.30662 7194PSYCHOL MED0033-291716680 5.587 6.1480.913230 2098CRIT REV BIOCHEM MOL1040-92382729 5.5788.032 1.23330 6583OPHTHALMOLOGY0161-642026007 5.563 5.777 1.065354 3107GENET MED1098-36004119 5.56 5.035 1.554121 1259BIOTECHNOL BIOFUELS1754-6834903 5.552 6.4590.35288 2401DRUG METAB REV0360-25322342 5.538 5.843 1.2516 291ADV SYNTH CATAL1615-415015502 5.535 5.323 1.109404 5793MACROMOLECULES0024-929797921 5.521 5.209 1.0941069 6176MON NOT R ASTRON SOC0035-871196083 5.521 5.009 1.7942574 4853J IMMUNOL0022-1767129339 5.52 5.6730.951289 7791SPACE SCI REV0038-63087134 5.519 5.1060.921114 8135TRENDS PARASITOL1471-49224819 5.513 4.878 1.11470 943ATMOS CHEM PHYS1680-731622919 5.51 5.556 1.176723 3765INT J CARDIOL0167-527312371 5.509 4.125 1.681326 2133CSH PERSPECT MED2157-1422211 5.5 5.5 1.099142 6511NUTR RES REV0954-******** 5.5 5.2020.11817 3323HIPPOCAMPUS1050-96318046 5.492 5.484 1.603184 2798EUROSURVEILLANCE1560-79175236 5.491 1.34188 3492IEEE T FUZZY SYST1063-67066237 5.484 4.8850.58193 6155MOL NEUROBIOL0893-******** 5.471 5.5350.782101 6289NEOPLASIA1522-80025715 5.47 5.077 1.208120 4096INVEST RADIOL0020-99965353 5.46 4.444 1.392102 1090BBA-GENE REGUL MECH1874-93994518 5.456 4.338 1.217129 2201CURR OPIN PHARMACOL1471-48925198 5.443 6.4470.765102 50ACS CHEM BIOL1554-89293417 5.442 5.478 1.387230 2206CURR OPIN SOLID ST M1359-02862508 5.4387.3290.91323 1248BIOSENS BIOELECTRON0956-566322068 5.437 5.389 1.105448 4592J ECOL0022-047713938 5.431 6.199**** ****BIOESSAYS0265-92478695 5.423 4.814 1.291134 4448J CHILD PSYCHOL PSYC0021-963012841 5.422 6.2350.976127 2194CURR OPIN NEUROL1350-75404449 5.416 5.0350.80497 4427J CEREBR BLOOD F MET0271-678X14671 5.398 5.66 1.093194 4439J CHEM THEORY COMPUT1549-961811067 5.389 5.936 1.067507 7655SEMIN IMMUNOPATHOL1863-22971214 5.379 5.668 1.6958 6128MOL CELL BIOL0270-730667211 5.372 5.745 1.036415 1200BIOMACROMOLECULES1525-779724209 5.371 5.750.721480 4261J ANTIMICROB CHEMOTH0305-745320364 5.338 4.686 1.257428 4466J CLIN EPIDEMIOL0895-435615399 5.332 5.120.711149 5284J PHYS G NUCL PARTIC0954-******** 5.326 2.812 1.37184 1165BIOINFORMATICS1367-480351753 5.323 6.9110.729728 3052FUNGAL DIVERS1560-27451665 5.319 4.2 1.73653 6993PLANT MOL BIOL REP0735-******** 5.319 4.020.293157 2152CURR GENE THER1566-52321362 5.318 4.3220.6141 4224J AM MED DIR ASSOC1525-86102480 5.302 3.912 1.3431781398BRIEF BIOINFORM1467-54633073 5.2987.51 1.27658 426AM J KIDNEY DIS0272-638619101 5.294 5.417 1.724199 2813EXERC SPORT SCI REV0091-63312207 5.283 4.841127 5781MABS-AUSTIN1942-0862765 5.275 4.8240.78565 3012FREE RADICAL BIO MED0891-584931156 5.271 5.969 1.033450 49ACS CATAL2155-54351461 5.265 5.265 1.222315 2533ENDOCR-RELAT CANCER1351-00884611 5.261 5.1720.73494 6224NANOMEDICINE-UK1743-58892713 5.26 6.2360.746114 4068INTENS CARE MED0342-464214780 5.258 5.036 1.074215 2611ENVIRON SCI TECHNOL0013-936X92385 5.257 5.8650.7921608 4364J BIOMED NANOTECHNOL1550-70332198 5.256 4.366 1.491108 6110MODERN PATHOL0893-395210184 5.253 5.058 1.371175 224ADV CATAL0360-05641399 5.25 5.28602 226ADV CHEM PHYS0065-23852306 5.25 4.250.71746 1066B WORLD HEALTH ORGAN0042-968611151 5.25 6.076 1.04295 2708EUR J HEART FAIL1388-98425824 5.247 4.738 1.469147 2770EUR PHYS J C1434-604411003 5.247 3.603 2.222406 1576CELL CYCLE1538-410115668 5.243 4.806 1.079444 4679J EXP BOT0022-095724541 5.242 5.5420.903493 7814SPORTS MED0112-16427069 5.237 5.7550.64164 3202GLOBAL ENVIRON CHANG0959-37804587 5.236 6.9010.88285 7047POLYM CHEM-UK1759-99543328 5.231 5.231 1.305397 3018FRONT AGING NEUROSCI1663-4365392 5.224 5.1690.72733 3933INT J OBESITY0307-056518052 5.221 5.522 1.174213 852ARCH TOXICOL0340-57614824 5.215 3.990.94149 3376HUM MUTAT1059-779411082 5.213 5.761 1.754224 3077GASTROINTEST ENDOSC0016-510718497 5.21 5.276 2.32297 1524CANCER-AM CANCER SOC0008-543X62757 5.201 5.693 1.108674 2205CURR OPIN RHEUMATOL1040-87113701 5.191 4.256 1.04589 1688CHEMCATCHEM1867-38802718 5.181 5.3070.941255 2315DIABETES OBES METAB1462-89024587 5.181 4.415 1.491173 2463ECOLOGY0012-965850217 5.175 6.3720.583283 3497IEEE T IND ELECTRON0278-004617404 5.165 5.0780.943470 528ANESTHESIOLOGY0003-302222547 5.163 5.302 1.959219 5065J MOL CELL CARDIOL0022-282811674 5.148 5.034 1.478232 7200PSYCHONEUROENDOCRINO0306-45309603 5.137 5.926 1.091198 6978PLANT CELL ENVIRON0140-779114009 5.135 5.861 1.639158 6122MOL CANCER1476-45984995 5.134 5.0970.3686 6849PHARMACOGENOMICS J1470-269X2084 5.134 4.455 1.12365 2444ECOGRAPHY0906-******** 5.124 5.7910.925120 2179CURR OPIN DRUG DISC1367-67331825 5.121 4.1080 3657INFLAMM BOWEL DIS1078-09988279 5.119 5.233 1.161261 6801PEDIATRICS0031-400559035 5.119 5.930.951687 2702EUR J EPIDEMIOL0393-******** 5.118 4.1160.49489 2729EUR J NUCL MED MOL I1619-707010881 5.114 4.821 1.202198 4805J HEART LUNG TRANSPL1053-24987139 5.112 3.6270.975159 5104J NATL COMPR CANC NE1540-14051890 5.1120.522115 7318REMOTE SENS ENVIRON0034-425724105 5.103 6.1440.987388 7724SLEEP0161-810514444 5.1 6.175 1.068162 2099CRIT REV BIOTECHNOL0738-******** 5.095 5.9660.95221 604ANN MED0785-38903665 5.094 4.640.76397 74ACTA BIOMATER1742-706110169 5.093 5.3780.903453 1575CELL COMMUN SIGNAL1478-811X520 5.0930.20539 7499RNA1355-838211854 5.088 5.43 1.01201920ASTRON ASTROPHYS0004-6361101305 5.084 4.422 1.4511892 1404BRIT J CANCER0007-092038414 5.082 5.2060.827561 5569J VIROL0022-538X93028 5.076 4.893 1.2581559 1831CLIN J AM SOC NEPHRO1555-90418449 5.068 5.445 1.1240 1415BRIT J PHARMACOL0007-118828119 5.067 4.898 1.288539 3198GLIA0894-149110371 5.066 5.406 1.38166 2105CRIT REV MICROBIOL1040-841X1335 5.065 5.615 1.09521 2675EUR HEART J SUPPL1520-765X977 5.065 1.817 1.910 2681EUR J CANCER0959-804921316 5.061 5.2570.991343 208ADV AGRON0065-21132753 5.06 5.5120.45824 5336J PROTEOME RES1535-389317943 5.056 5.2230.882544 3916INT J NEURAL SYST0129-0657992 5.054 3.6460.45735 1975COMPR REV FOOD SCI F1541-43371046 5.053 4.7920.26730 3273HEART RHYTHM1547-52717449 5.045 4.719 2.089237 1775CLADISTICS0748-30073315 5.043 6.183 1.86730 7737SOC COGN AFFECT NEUR1749-50161980 5.042 6.78 1.347101 3173GEOSCI MODEL DEV1991-959X626 5.03 4.50.84691 6186MRS BULL0883-******** 5.024 5.590.569116 3269HEART1355-603713857 5.014 4.769 1.667243 48ACS APPL MATER INTER1944-82448635 5.008 5.040.683953 2491ELECTROANAL CHEM0070-977837757.50.254 216ADV APPL MICROBIOL0065-21641233 4.974 4.7840.33321 4045INT REV CEL MOL BIO1937-6448825 4.973 4.9440.40849 2713EUR J IMMUNOL0014-298020974 4.97 4.7680.739322 663ANNU REV ENV RESOUR1543-59381402 4.9687.25018 923ASTRON J0004-625634668 4.965 4.571 1.162334 6154MOL MICROBIOL0950-382X37172 4.961 5.099 1.123359 2351DIS MODEL MECH1754-84031212 4.959 5.0090.98988 1407BRIT J HAEMATOL0007-104820668 4.942 4.7260.891266 4497J COMB CHEM1520-47662925 4.933 3.1020 5789MACROMOL RAPID COMM1022-133612103 4.929 4.6180.916261 5129J NEUROL NEUROSUR PS0022-305023923 4.924 5.144 1.69187 2628EPIGENETICS-US1559-22942289 4.92 4.8810.773150 4050INT REV PHYS CHEM0144-235X1524 4.92 5.595 2.23113 1119BEST PRACT RES CL EN1521-690X2576 4.912 5.1810.25866 1091BBA-MOL BASIS DIS0925-44396277 4.91 4.932 1.248202 5451J STAT SOFTW1548-76602629 4.91 5.9070.75377 5356J R SOC INTERFACE1742-56895016 4.907 5.1650.806320 4206J AM ACAD DERMATOL0190-962219760 4.906 4.5150.896280 1518CANCER PREV RES1940-62073114 4.891 5.126 1.401142 2187CURR OPIN INFECT DIS0951-******** 4.87 4.610.83987 2846EXPERT OPIN DRUG DEL1742-52472829 4.869 5.1330.495103 469AM J SURG PATHOL0147-518516422 4.868 4.8570.798223 7021PLOS COMPUT BIOL1553-735811758 4.867 5.9390.824507 6837PFLUG ARCH EUR J PHY0031-67689307 4.866 3.9280.853129 2810EVOLUTION0014-382030585 4.864 5.4020.939313 4347J BIOGEOGR0305-027010682 4.863 4.979 1.399183 3042FUNCT ECOL0269-84639681 4.861 5.3930.882153 2231CURR TOP MICROBIOL0070-217X4958 4.86 4.744 5.05319 1875CLIN SCI0143-52218069 4.859 4.843 1.2110 174ACTA PSYCHIAT SCAND0001-690X11048 4.857 4.625198 386ALTERN MED REV1089-51591288 4.857 5.0340.56 250ADV GENET0065-26601198 4.85 4.102010 4253J ANIM ECOL0021-879012354 4.841 5.1660.881134。
谷胱甘肽 S-转移酶(GST)活性检测试剂盒说明书__ 紫外分光光度法UPLC-MS-4496
谷胱甘肽S-转移酶(GST)活性检测试剂盒说明书紫外分光光度法货号:UPLC-MS-4496规格:50T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系工作人员。
试剂名称规格保存条件试剂一液体50mL×1瓶4℃保存试剂二液体45mL×1瓶4℃保存试剂三粉剂×1瓶4℃保存溶液配制:试剂三:临用前加5mL蒸馏水溶解。
产品说明:谷胱甘肽S-转移酶(glutathione S-transferase,GST)是一种具有多种生理功能的蛋白质家族,主要存在于细胞质内。
GST是体内解毒酶系统的重要组成部分,主要催化各种化学物质及其代谢产物与GSH的巯基共价结合,使亲电化合物变为亲水物质,易于从胆汁或尿液中排泄,达到将体内各种潜在或具备毒性的物质降解并排出体外的目的。
因此,GST在保护细胞免受亲电子化合物的损伤中发挥着重要的生物学功能。
此外,因为GST具有GSH-Px活性,亦称为non-Se GSH-Px,具有修复氧化破坏的大分子如DNA、蛋白质等的功能。
注意,GST催化的反应减少GSH含量,但是不增加GSSG含量。
GST催化GSH与CDNB结合,其结合产物的光吸收峰波长为340nm;通过测定340nm波长处吸光度上升速率,即可计算出GST活性。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外-可见分光光度计、低温离心机、水浴锅、可调节移液器、研钵/匀浆器、1mL石英比色皿和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1.组织:按照组织质量(g):试剂一体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL试剂一)进行冰浴匀浆。
8000g,4℃离心10min,取上清置冰上待测。
2.细菌、真菌:按照细胞数量(104个):试剂一体积(mL)为500~1000:1的比例(建议500万细胞加入1mL试剂一),冰浴超声波破碎细胞(功率300w,超声3秒,间隔7秒,总时间3min);然后8000g,4℃,离心10min,取上清置于冰上待测。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :GSK-923295Catalog No. :HY-10299CAS No. :1088965-37-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:GSK 923295; GSK923295Formula:C32H38ClN5O4Molecular Weight:592.13CAS No. :1088965-37-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
