Low-Temperature Solution Processed Tin Oxide Efficient Perovskite
PCR常见问题分析及对策

PCR常见问题分析及对策PCR常见问题分析及对策(无扩增产物、非特异性扩增、拖尾、假阳性) 问题1:无扩增产物现象:正对照有条带,而样品则无原因:1.模板:含有抑制物,含量低2.Buffer对样品不合适3.引物设计不当或者发生降解4.反应条件:退火温度太高,延伸时间太短对策:1.纯化模板或者使用试剂盒提取模板DNA或加大模板的用量2.更换Buffer或调整浓度3.重新设计引物(避免链间二聚体和链内二级结构)或者换一管新引物4.降低退火温度、延长延伸时间问题2:非特异性扩增现象:条带与预计的大小不一致或者非特异性扩增带原因:1.引物特异性差2.模板或引物浓度过高3.酶量过多4.Mg2+浓度偏高5.退火温度偏低现非特异条带而另一来源的酶则不出现,酶量过多有时也会出现非特异性扩增。
其对策有:①必要时重新设计引物。
②减低酶量或调换另一来源的酶。
③降低引物量,适当增加模板量,减少循环次数。
④适当提高退火温度或采用二温度点法(93℃变性,65℃左右退火与延伸)。
出现片状拖带或涂抹带PCR扩增有时出现涂抹带或片状带或地毯样带。
其原因往往由于酶量过多或酶的质量差,dNTP浓度过高,Mg2+浓度过高,退火温度过低,循环次数过多引起。
其对策有:①减少酶量,或调换另一来源的酶。
②减少dNTP的浓度。
③适当降低Mg2+浓度。
④增加模板量,减少循环次数。
克隆PCR产物的最优条件是什么?最佳插入片段:载体比需实验确定。
1:1(插入片段:载体)常为最佳比,摩尔数比1:8或8:1也行。
应测定比值范围。
连接用5ul 2X连接液, 50ng质粒DNA,1Weiss单位的T4连接酶,插入片段共10ul。
室温保温1小时,或4oC过夜。
在这2种温度下,缺T-凸出端的载体会自连,产生蓝斑。
室温保温1小时能满足大多数克隆要求,为提高连接效率,需4oC过夜。
PCR产物是否需要用凝胶纯化?如凝胶分析扩增产物只有一条带,不需要用凝胶纯化。
如可见其他杂带,可能是积累了大量引物的二聚体。
汽车零部件测试简介

汽车零部件测试简介--广电计量检测股份有限公司汽车是一个由数以万计零部件组成的机电混合复杂系统,GRGT能帮助汽车整车厂及零部件厂商快速提升零部件性能,满足您对产品品质和安全的高要求,服务涵盖汽车零部件的环境可靠性测试、电学性能测试、功能测试、EMC测试、材料测试、绿色环保测试及化学法规符合性服务项目。
[1] 测试范围汽车是一个由数以万计零部件组成的机电混合复杂系统,GRGT能帮助汽车整车厂及零部件厂商快速提升零部件性能,满足您对产品品质和安全的高要求,服务涵盖汽车零部件的环境可靠性测试、电学性能测试、功能测试、EMC测试、材料测试、绿色环保测试及化学法规符合性服务项目。
环境可靠性测试高温储存(可带表面红外加热)High Temperature Test(can with IR)低温储存Low Temperature Test湿热交变(可带表面红外加热)Hemperature & Humidity Test (canwith IR)凝露测试Condensation Test低气压测试Low Pressure Test温度冲击测试Thermal Shock Test防尘防水测试Dust & Water Resistant Test盐雾测试Salt Spray Test耐气体腐蚀gas Corrosion Resistant Test耐化学试剂Chemical Resistant Test振动测试(随机、正弦、扫频等)Vibration Test (Random/Sine /Sweep)机械冲击测试Mechanical Shock Test碰撞测试Bump Test跌落测试Drop Test三综合测试(温湿度+振动)Compositive Environment Test(Temperature & Humidity &Vibration) 高加速测试HALT & HASS插拔力检测Operation Force Test刚度测试Rigidity Test按键动作耐久测试Button Operation Durability插拔耐久测试Connection DurabilityCD机动作耐久测试CD Player Operation Durability电学性能测试电源特性测试Resistance to Power Supply Voltages电源缓升缓降测试Resistance to Slow Decrease andIncrease Power of Supply Voltages电压特性测试Re-initialization Test电压异常测试Resistance to Non Usual Power SupplyVoltages线路(短路至地/电源)短路测试Resistance to Ground and PositiveSupply Voltages Short Circuit 感性负载电源连接测试Resistance of InductiveLoad Connected Circuits电源微中断测试Resistance to Power Supply Micro-interruptions(UPDATED)启动测试Resistance to Starting Profile电源电压特性测试Power Supply Voltage CharacteristicsTest浮地测试Floating Ground Test感应噪声抗扰度Induction Noise Resistance Test抛负载测试Load Dump Test过压测试Overvoltage Test点火脉冲测试Ignition Pulse Test反极性测试Reversed Polarity Test工作电压Working Voltage工作电流Working Current绝缘电阻测试Insulation Resistance Test耐电强度测试Electric Strength Test温升测试Temperature Rise Test功能测试按客户要求进行功能测试Function Test as Requirement ofClientEMC测试RE辐射骚扰Radiated Emission CE传导骚扰Conducted Emission BCI大电流注入Bulk CurrentInjectionRI电波暗室法辐射抗扰度RI 瞬态传导骚扰Transient,Conducted Emissions瞬态传导抗扰度ConductedImmunity静电放电抗扰度ESD脉冲波测试Resistance to on Board Power SystemVoltage Ripples高电压注入抗扰度测试Immunity to InjectionHigh Voltage脉冲抗扰度测试Resistance to Impulsive Transient音频磁场辐射抗扰度测试Immunity to AudioFrequency Magnetic Field手持式收发机抗扰度测试Resistance to HandyTransmitters脉冲波抗扰度测试ImmunityAgainst High Frequency Surge (Burst Wave Form)高周波抗扰度测试ImmunityAgainst High Frequency Surge (Impulse Wave Form) 低周波测试LowFrequency Surge Resistance(Induced Load Surge)汽车电子暗室场均匀性Anechoic ChamberField Validation汽车电子暗室有效性CISPR1391CD RadioDisturbance Characteristics CISPR1391CD 屏蔽室屏蔽效能Shielding Room Shieding Effective暗室电压驻波比VSWR磁场骚扰测试Measurement of Magnetic FieldRadiated Emissions宽带辐射抗扰度测试Broadband AntennaNearby Test辐射天线靠近抗扰度Radio EquipmentAntenna Nearby Test移动手机天线靠近测试Mobile PhoneAntenna Nearby Test材料测试物理性能测试尺寸测量Dimension Measurement密度Density硬度Hardness拉伸性能Tensile Property冲击性能Impact Property弯曲测试Bend Test熔融指数Melt Index维卡软化点VEKA Softening Point低温脆化Low Temperature Brittleness抗化学试剂测试Chemical Resistant附着力Adhesion Test涂镀层厚度Thickness of Coating And Film铅笔硬度Pencil Hardness色差Chromatism光泽度Gloss耐刮擦测试Scratch Resistant Test耐摩擦测试Rub Resistant Test耐化学试剂Chemical Resistant Test落锤冲击(常温/低温)Drop Ball Impact Test(Room/Low Temperature)落砂磨耗Sand Abrasion Test碎石冲击测试Gravel Resistant Test盐雾测试Salt Spray TestⅡ材料老化测试温湿度测试Temperature & Humidity Test(or with IR)耐水老化Water Resistant Test氙弧灯老化Xenon Arc Aging碳弧灯老化Carbon Arc Aging紫外灯老化Uv Arc Aging卤素灯老化Halogen Lamp AgingⅢ材料分析金属化学成分分析Chemical Composition Analysis forMetal金相测试Metallographic Test·显微断裂分析Microstructure and Fracture Analysis·非金属夹杂物分析Non-metallic Inclusion Analysis·晶粒度Average Grain Size·定量金相学Quantitative Metallography·镀层厚度Thickness of Film & Coating Measure·宏观检测Macroscopical Examination·电路板的镀层厚度Coating Thickness of PCB Board·电路板焊脚质量Welding Quality of Feet金属机械性能测试Metal Material Mechanics Test ·抗压试验Compression Test·剪切试验Shear Test·弯曲试验Bending Test·压扁试验Flattening Test·扩口试验Flaring Test·卷边试验Flange Test·冲击试验Impact Test ·杯突试验Cupping Test硬度测试Hardness Test·洛氏硬度Rockwell Hardness·布氏硬度Brinell Hardness·维氏硬度Vickers Hardness·显微维氏硬度Micro Vickers Hardness·里氏硬度Leeb Hardness高分子材料成分分析ChemicalComposition Analysis for PolymerⅣ材料燃烧测试燃烧测试Flame Resistant汽车内饰材料的燃烧性能测试Flammability Test ofAutomotive Interior Materials绿色环保测试及化学法规符合性服务Ⅰ挥发性有机化合物测试Volatile Organic Compound Test气味测试Odor Test雾化测试Fogging Test甲醛测试Formaldehyde Test汽车材料VOC测试Volatile Organic Compound Test of AutoMaterials汽车部件VOC测试Volatile Organic Compound Test of Auto Parts车内挥发性有机物和醛酮类物质测试Volatile Organic Compounds and CarbonylCompounds Test in Cabin of VehiclesⅡ有害物质测试HazardousSubstances Test报废车辆指令(ELV指令)End-of Life Vehicle Test汽车禁用物质测试Test for Prohibited Substances on Automobiles Reach高度关注物质(SVHC)测试Substances of Very High Concern (SVHC)Test 汽车涂料检测Automobile Coatings TestⅢ其他测试OtherTest石棉、多环芳烃、邻苯二甲酸酯、偶氮染料、重金属等Asbestos, PAHs,Phthalates, AZO Dye, Heavy Metals Test etc。
Bright light-emitting diodes based on organometal 基于有机金属的发光二极管

halide perovskites. We demonstrate electroluminescence in the
near-infrared, green and red by tuning the halide compositions
in the perovskite. In our infrared device, a thin 15 nm layer of
current densities. This demonstration of effective perovskite
electroluminescence offers scope for developing this unique class of materials into efficient and colour-tunable light emitters for low-cost display, lighting and optical communication
PSS/CH3NH3PbBr3/F8/Ca/Ag structure, we achieved a luminance of 364 cd m−2 at a current density of 123 mA cm−2, giving external and internal quantum efficiencies of 0.1% and 0.4%, respectively. We show, using photoluminescence
伊士达高Tg、低CTE、多功能填充环氧树脂和酚醛固化层压板和预浸料IT-180ABS IT-180A

IT-180ABS/IT-180ATCHigh Tg, Low CTE, Multifunctional Filled Epoxy Resin and Phenolic-Cured Laminate & PrepregIT-180A is an advanced high Tg (175℃ by DSC) multifunctional filled epoxy with low CTE, high thermal reliability and CAF resistance. It’s design for high layer PCB and can pass 260℃ Lead free assembly and sequential lamination process.Key Features =============================== Advanced High Tg Resin TechnologyIndustrial standard material with high Tg (175℃ by DSC) multifunctional filled epoxy resin and excellent thermal reliability.Lead-Free Assembly CompatibleRoHS compliant and suitable for high thermal reliability needs, and Lead free assemblies with a maximum reflow temperature of 260℃. Friendly Processing and CAF ResistanceFriendly PCB process like high Tg FR4. Users can short the learning curve when using this material.CAF ResistanceLow thermal expansion coefficient (CTE) helps to excellent thermal reliability and CAF resistance providing long-term reliability for industrial boards and automobile application.Available in Variety of ConstructionsAvailable in a various of constructions, copper weights and glass styles, including standard(HTE), RTF and VLP copper foil. ApplicationsMultilayer and High Layer PCB AutomobileBackplanesServers and Networking TelecommunicationsData StorageHeavy Copper ApplicationIndustrial ApprovalUL 94 V-0IPC-4101C Spec / 99/ 101/ 126 RoHS CompliantGlobal AvailabilityArea Address Contact e-mail TELTaiwan 22,Kung Yen 1st Rd. Ping Chen Industry Zone. Ping Chen,Taoyuan, Taiwan, R.O.C.Sales: *************.twTechnician: *****************.tw886-3-4152345 #3168886-3-4152345 #5300East China Chun Hui Rd., Xishan Economic Development Zone,Wuxi City, Jiangsu Province, ChinaSales : ****************Technician: *********************86-510-8223-5888 #516886-510-8223-5888 #3000South China168, Dongfang Road, Nanfang Industrial Park, BeiceVillage, Humen Town, Dongguan City, Guangdong Province, China Sales: ***********.cnTechnician : ***************.cn86-769-88623268 #32086-769-88623268 #550Japan No.2, Huafang Rd, Yonghe Economic Zone, Economic andTechnological Development Zone, Guangzhou,Guangdong Province, ChinaSales: ****************.cnTechnician : *****************.tw86-20-6286-8088 #8027886-3-4152345 #5388USA Tapco Circuit Supply1225 Greenbriar Drive, Suite AAddison, IL 60101, USASales: *******************************Technician : ********************************1-614-937-52051-310-699-8028Europe ITEQ Europe,Via L. Pergher, 16 38121 Trento, Italy Sales: ********************Technician : *********************39-0461-82052639-0461-820526REV 06-12ITEQ Laminate/ Prepreg : IT-180ATC / IT-180ABS IPC-4101C Spec / 99 / 101 / 126LAMINATE( IT-180ATC)Thickness<0.50 mm[0.0197 in] Thickness≧0.50 mm[0.0197 in] Units T est MethodPropertyTypical Value Spec Typical Value SpecMetric(English)IPC-TM-650(or as noted)Peel Strength, minimumA. Low profile copper foil and very low profile copperfoil - all copper weights > 17µm [0.669 mil]B. Standard profile copper foil1.After Thermal Stress2.At 125°C [257 F]3.After Process Solutions 0.88 (5.0)1.23 (7.0)1.05 (6.0)1.05 (6.0)0.70 (4.00)0.80 (4.57)0.70 (4.00)0.55 (3.14)0.88 (5.0)1.40 (8.0)1.23 (7.0)1.23 (7.0)0.70 (4.00)1.05 (6.00)0.70 (4.00)0.80 (4.57)N/mm(lb/inch)2.4.82.4.8.22.4.8.3Volume Resistivity, minimumA. C-96/35/90B. After moisture resistanceC. At elevated temperature E-24/125 3.0x1010--5.0x1010106--103--3.0x10101.0x1010--104103MΩ-cm 2.5.17.1Surface Resistivity, minimumA. C-96/35/90B. After moisture resistanceC. At elevated temperature E-24/125 3.0x1010--4.0x1010104--103--3.0x10104.0x1010--104103MΩ 2.5.17.1Moisture Absorption, maximum -- -- 0.12 0.8 % 2.6.2.1 Dielectric Breakdown, minimum -- -- 60 40 kV 2.5.6 Permittivity (Dk, 50% resin content)(Laminate & Laminated Prepreg)A. 1MHzB. 1GHzC. 2GHzD. 5GHzE. 10GHz 4.44.44.24.14.05.44.44.44.34.14.15.4 --2.5.5.92.5.5.13Loss Tangent (Df, 50% resin content) (Laminate & Laminated Prepreg)A. 1MHzB. 1GHzC. 2GHzD. 5GHzE. 10GHz 0.0150.0150.0150.0160.0170.0350.0140.0150.0150.0160.0160.035 --2.5.5.92.5.5.13Flexural Strength, minimumA. Length directionB. Cross direction ----------------500-530(72,500-76,850)410-440(59,450-63,800)415(60,190)345(50,140)N/mm2(lb/in2)2.4.4Arc Resistance, minimum 125 60 125 60 s 2.5.1 Thermal Stress 10 s at 288°C [550.4F],minimumA. UnetchedB. Etched PassPassPass VisualPass VisualPassPassPass VisualPass VisualRating 2.4.13.1Electric Strength, minimum(Laminate & Laminated Prepreg)45 30 -- -- kV/mm 2.5.6.2 Flammability,(Laminate & Laminated Prepreg)V-0 V-0 V-0 V-0 Rating UL94 Glass Transition Temperature(DSC) 175 170 minimum 175 170 minimum ˚C 2.4.25Decomposition Temperature-- -- 345 340 minimum ˚C2.4.24.6 (5% wt loss)X/Y Axis CTE (40℃ to 125℃) -- -- 10-13 -- PPM/˚C 2.4.24 Z-Axis CTEA. Alpha 1B. Alpha 2C. 50 to 260 Degrees C ------------452102.760 maximum300 maximum3.0 maximumPPM/˚CPPM/˚C%2.4.24Thermal ResistanceA. T260B. T288 -------->60>3030 minimum15 minimumMinutesMinutes2.4.24.1CAF Resistance -- -- Pass AABUS Pass/Fail 2.6.25The above data and fabrication guide provide designers and PCB shop for their reference. We believe that these information are accurate, however, the data may vary depend on the test methods and specification used. The actual sales of the product should be according to specification in the agreement between ITEQ and its customer. ITEQ reserves the right to revise its data at any time without notice and maintain the best information available to users.REV 06-12。
锡的冶炼流程详解

锡的冶炼流程详解Extracting and smelting tin is a complex process that requires a careful balance of various factors to ensure the final product meets the desired quality standards. Tin ore is typically mined from underground or open-pit mines and then transported to a processing facility for further refinement. The first step in the process is crushing and grinding the ore to reduce it to a fine powder, which increases the surface area for chemical reactions to take place.将锡提炼和冶炼是一个复杂的过程,需要仔细平衡各种因素,以确保最终产品符合期望的质量标准。
锡矿通常是从地下或露天矿井中开采出来,然后运送到加工设施进行进一步精炼。
过程的第一步是将矿石粉碎和研磨,将其减小到细粉末,这增加了化学反应发生的表面积。
Once the ore has been crushed and ground, it is then subjected to a process called flotation, where chemicals are added to the ore to separate the tin from other minerals. This process relies on the differences in the physical and chemical properties of the minerals to selectively separate the tin from the gangue. The tin concentrate isthen dried and transported to a smelting facility where it is further processed to extract the pure tin metal.一旦矿石被粉碎和研磨,它就会接受一种称为浮选的过程,在这个过程中,化学品被添加到矿石中,将锡与其他矿物分离开来。
Solution-processed, high-performance light-emitting diodes based on quantum dots
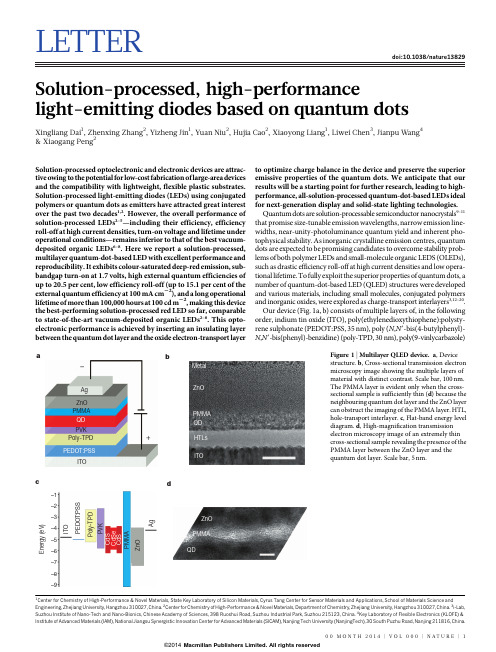
Institute of Advanced Materials (IAM), National Jiangsu Synergistic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211816, China.
Quantum dots are solution-processable semiconductor nanocrystals9–11 that promise size-tunable emission wavelengths, narrow emission linewidths, near-unity-photoluminance quantum yield and inherent photophysical stability. As inorganic crystalline emission centres, quantum dots are expected to be promising candidates to overcome stability problems of both polymer LEDs and small-molecule organic LEDS (OLEDs), such as drastic efficiency roll-off at high current densities and low operational lifetime. To fully exploit the superior properties of quantum dots, a number of quantum-dot-based LED (QLED) structures were developed and various materials, including small molecules, conjugated polymers and inorganic oxides, were explored as charge-transport interlayers3,12–20.
一种简单的溶胶-凝胶法制备二氧化锆介质层

一种简单的溶胶-凝胶法制备二氧化锆介质层李国安;杜惊雷;Leander Schulz【摘要】在电子产业中,能够进行低压操作以及能够在非常薄的薄层上进行器件设计的材料备受关注.并且,这些材料需要使用方法简单、快速、可靠.作者提出一种简单且快速可靠的沉积高k二氧化锆介电层的方法.整个过程从原来常规过程的140分钟缩短到10分钟.这种改进的方法在操作流程方面有显著的提升.这种溶胶凝胶法制备的样品介电层厚度远小于与之前报道的样品,其厚度约为原方法制备样品的25%.在优化操作过程与降低介电层厚度的同时,二氧化锆的介电性质并没有被影响.作者进一步研究了样品的介电特性,在90K到300K之间介电性质的变化小于10%.【期刊名称】《四川大学学报(自然科学版)》【年(卷),期】2016(053)004【总页数】4页(P843-846)【关键词】二氧化锆;溶胶-凝胶法;电介质【作者】李国安;杜惊雷;Leander Schulz【作者单位】四川大学物理科学与技术学院,成都610065;四川大学物理科学与技术学院,成都610065;四川大学物理科学与技术学院,成都610065【正文语种】中文【中图分类】O64现代电子学中,特别是触摸屏和未来的透明显示屏领域,需要大量使用透明材料,这些技术是建立在较高的可见光传输系数或者特别薄的薄膜厚度基础上的.典型的应用如有源矩阵显示[1],柔性电子学[2,3],忆阻器[4]和传感器[5],这些技术通常需要用到透明的氧化半导体,如锌氧化锡、铟的氧化物,用以制备晶体管[3,5-9].为了能够获得较低的操作电压,高k 电介质作为透明薄膜材料(但是不仅仅是这些)被广泛使用[11-13].而二氧化锆是一种常见的高k 电介质.已经有多种方法能够成功制备二氧化锆绝缘层,如物理气相沉积法[15],溅射法[16],原子层沉积[17],分子束沉积[18]和电子束蒸镀[19]等方法.尽管这些方法有很多优点,但是他们都很难应用于工业化的大规模量产.溶液法[13]能够实现低成本、高产量,简单快速直接应用[8].本文中,我们提出一种改良后的溶胶凝胶法制备二氧化锆薄膜,这种方法更加简单,且极大的缩短了制备时间.这种新型的溶胶-凝胶法制备二氧化锆的过程只需要10分钟左右,且不会影响到二氧化锆的电介质特性,较之前长达140分钟的过程,改善显著[8,9].与此同时,二氧化锆薄膜样品在厚度足够薄的情况下却介电性质不变,不仅如此,我们还研究了这些样品的温度特性:在90k-300k的情况下,薄膜的介电性能变化小于10%.为了研究电介质层的性质,我们采用了一种金属-电介质-金属的结构,电介质层位于铂(底层电极)和铝(顶层电极)之间.所有的样品都在硅片基底上生长,硅片先依次浸没在丙酮、甲醇、异丙酮中各超声5分钟,用氮气吹干然后再电热板上用150℃温度烘干.在清洁步骤过后,利用电子束蒸发沉积厚度为5nm钛和20nm的铂.钛层作为黏附层.顶部的铝电极面积约为50*50μm2,利用掩模遮挡,通过阻蒸直接蒸镀在已制备的二氧化锆层上,厚度约为50nm.制备介电二氧化锆薄膜的过程最近刚刚发表[8-10],现在我们进一步地讨论相关细节:首先,制备前驱体溶液,将579mg氯化锆和963mg异丙醇锆(Sigma-Aldrich购买)溶解于10ml 2-甲氧基乙醇.为了能够完全溶解氯化锆和异丙醇锆粉末,将前驱体溶液密封振荡12小时以上.然后,待溶液稳定后,制备成为介电层,若干周内均可使用.对于参考样品而言,介电层制备方式如下[8,9]:首先,用0.25μm PTFE滤头过滤前驱体溶液,然后将溶液在硅基底上进行旋涂一分钟,2000rpm.在样品的边角处,利用甲醇处理后的棉签将旋涂的薄膜移走,使部分底部电极暴露出来.将刚刚沉积的前体薄膜置于手套箱中100℃的电热板上,退火10分钟.随后在置于空气中的电热板上500℃退火一个小时(烧结).整个过程重复一次,才能制备双层二氧化锆.因此,之前的制作方法整个工艺过程大概是140分钟.对于全新的优化制程而言,整个过程只需要10分钟:省略之前报道的退火步骤,缩短烧结步骤到10分钟,此样品被称之为“十分钟样品”.为了进一步研究介电层的厚度相关问题,我们使用与前驱体溶液相同溶剂,对前驱体溶液进行1:1比例稀释,那么溶液浓度将会变为原来的一半,稀释后的溶液用于制备样品.相比于传统的沉积介质膜两次的方法,我们只需要沉积一次,因此将制备过程又缩短了五分钟.此样品在后文中被称作“薄层样品”.通常利用泄漏电流以及电容频率特性来表征介电层.“十分钟样品”的泄漏电流如图1a所示,在整个电压区间内,对于50*50μm2的样品,泄漏电流均低于500pA.样品单位面积电容与外加直流电压在不同频率下的关系如图1b所示.图1b 中同样给出了使用传统方法制备的样品的单位面积电容,对比图b两组数据可以发现,二者的单位面积电容几乎一致,因此证明了改良后的制备过程同样能够得到质量较好的样品.平行板间电容与介电常数关系式为:其中,C, ε0, εr, A,d分别为电容,真空介电常数,材料介电常数,极板面积以及金属-绝缘体-金属结构中间的厚度.利用原子力显微镜,观测到样品绝缘层总厚度均为107±8 nm,与制备过程无关.从低频1kHz到1MHz范围内,十分钟样品单位面积电容在200 nF/cm2至220 nF/cm2 范围内变化,从而可以得到εr的值在24.2 ± 2.0 至26.6 ± 2.0之间,传统样品εr值为25,与之相符[14].从而说明改良后的溶胶凝胶法能够显著而有效的缩短制备过程.从旋涂前驱体薄膜,到烧结和介电层退火以及氧化,整个过程能够在15分钟内完成.而且,退火过程也不会影响到薄膜形态,最多会导致溶液的蒸发.我们推测是因为在100°C下进行的退火,并不足以诱发制备绝缘层的化学反应,或者是由于没有充足的反应需要的氧气.我们相信之前报道的退火过程对于薄膜制备过程并没有提升,甚至有可能降低样品介电性能.我们进一步分析了十分钟样品的介电性能与温度之间的关系.图2为不同温度下单位面积电容.对比图2a与2b中的电容数据,不难发现,对于不同的频率,电容的变化率仅仅在5 % - 10 %.图2c与2d进一步分析不同温度下的单位面积电容以及同一温度,不同频率下的电容:可知,在f = 100 kHz条件下,温度从300K降到90K时,电容减少量小于5%,且与外加电压无关(图2c).由于电容与外加电压无关,我们进一步给出了当V= 0 V时,在不同频率下的单位面积电容曲线.ZrO2薄膜单位面积电容随温度的升高而增加,该现象源于在较高温度下可以获得更高的热能从而激活更多的载流子:虽然ZrO2是绝缘的,但是文章中使用的制作工艺使得生长的非晶薄膜中在导带和价带附近形成了不同的陷阱态.在较高温度时,陷阱态中的载流子将被激活而贡献于电容.因此,较高温度下将有更多的载流子贡献于电容,与探测频率无关,因而电容随温度升高而增加的现象也与探测频率无关.事实上,在100kHz及其以下的探测频率时,单位面积电容几乎一致,只有探测频率在1MHz时单位面积电容才会有较为明显的增加,但也仅约为5%. 改变温度的过程中,探测台的探针会从样品中移除,因此,接触效应对该现象的影响也可以排除. 最后一步中,我们降低了薄膜的厚度(薄层样品).图3a展示了此薄膜的泄漏电流.此薄膜仍然是绝缘性的,因此漏源电流为典型的μA级或者更高.需要指出,由于薄膜厚度的变化,此处的测试所用最高电压为2V.图3b中展示了该薄膜在不同频率下的单位面积电容,电容值在800-1000 nF/cm2之间.考虑到此时的薄膜厚度仅为25nm,单位面积电容增加至4倍也不足为奇:仅有一层沉积膜以及使用的前驱体溶液的浓度仅为之前的一半.显然,电容与薄膜的厚度成反比(公式1).另外还需说明,在交流应用中,较厚的薄膜(十分钟样品)更加适用,因为该薄膜的单位面积电容受频率影响更小.我们提供了一种非常简单的优化的溶胶凝胶方法,可以在10分钟或者更短的时间内沉积绝缘ZrO2薄膜.相比于其他方法,除了加工时间短之外,此方法还拥有许多其他优势,例如原子层沉积技术需要非常昂贵的设备,而本方法需要的设备则比较廉价易得.该方法所需加工时间仅为10分钟,大大缩短了之前报道的制作ZrO2薄膜的加工时间,因此可能迅速实现工业化和商业化.【相关文献】[1] Ryu M K, Lee S Y, et al. AMOLED driven by solution-processed oxide semiconductor TFT[J]. SID Symp:Digest of Technical Papers, 2009, 40: 188.[2] Kim Y H, Heo J S, Park S K, et al. Flexible metal-oxide devices made by room-temperature photochemical activation of sol-gel films[J]. Nature, 2012, 489: 128.[3] Nomura K, Takagi A, Hirano M, et al. Amorphous oxide semiconductors for high-performance flexible thin-film transistors[J]. Jpn J Appl Phys, 2006, 45: 4303.[4] Murali S, Rajachidambaram J S, Han S Y, et al. Resistive switching in zinc-tin-oxide[J]. Solid-State Electron, 2013, 79: 248.[5] Dutta S, Dodabalapur A. Zinc tin oxide thin film transistor sensor[J]. Sensors and Actuators B: Chemical, 2009, S34: 50.[6] Lee C G, Dodabalapur A. Solution-processed zinc-tin oxide thin-film transistors with low interfacial trap density and improved performance[J]. Appl Phys Lett, 2010, 96: 243501.[7] Lee C G, Cobb B, Dodabalapur A. Band transport and mobility edge in amorphous solution-processed zinc tin oxide thin-film transistors[J]. ApplPhys Lett, 2010, 97: 203505.[8] Lee C G, Dodabalapur A. Solution-processed ZTO TFTs with recessed gate and low operating voltage[J]. IEEE Electron Device Letters, 2010, 31: 1410.[9] Schulz L, Yun E J. A Dodabalapur, Effects of contact resistance on the evaluation ofcharge carrier mobilities and transport parameters in amorphous zinc tin oxide thin-film transistors[J]. Appl Phys A, 2014, 115: 1103.[10] Seon J B, Lee S, Kim J M. Spin-Coated CDS Thin Films for n-Channel Thin Film Transistors[J]. Chem Mater, 2009, 21: 604.[11] Wong H, Iwai H. On the scaling issues and high-κ replacement of ultrathin gate dielectrics for nanoscale MOS transistors[J]. Microelectron Eng, 2006, 83: 1867.[12] Robertson J. High dielectric constant oxides[J]. Eur Phys J Appl Phys, 2004, 28: 265.[13] Aoki Y, Kunitake T. Solution-based Fabrication of high-κ gate dielectrics for next-generation metal-oxide semiconductor transistors[J]. Adv Mater, 2004, 16: 118.[14] Harrop P J, Wanklyn J N. The dielectric constant of zirconia[J]. Br J Appl Phys, 1967, 18: 739-742.[15] Kim E T, Yoon S G. Characterization of zirconium dioxide film formed by plasma enhanced metal-organic chemical vapour deposition[J]. Thin Solid Films, 1993, 227: 7. [16] Thaveedeetrakul A, Boonamnuayvitaya V, Witit-anun N. Apatite deposition on ZrO2 thin films by DC unbalanced magnetron sputtering[J]. Advances in Materials Physics and Chemistry, 2012, 2: 45.[17] Kuklia K, Forsgrenc K, Harsta A, et al. Atomic layer deposition of zirconium oxide from zirconium tetraiodide, water and hydrogen peroxide[J]. J Cryst Growth, 2001, 231: 262.[18] Freese A, Grube M, Mikolajick T, et al. Molecular beam deposited zirconium dioxide as a high-κ dielectric for future GaN based power devices[J]. J Vac Sci and Technol B, 2013, 31: 03C111.[19] Kalkur T S, Lu Y C. Electrical characteristics of ZrO2-based metal-insulator-semiconductor structures on p-Si[J]. Thin Solid Films, 1992, 207: 193.。
极性色谱柱的区别

2. Toluene
4
3. Ethylbenzene
4. p-Xylene
5
5. m-Xylene
3
6. o-Xylene
1
3
6
2
10
11
12 min
0
5
10
15 min
2
HP-INNOWax temperature range: 40°C to 260°C;
0.53 mm id columns: 40°C to 240°C
Oven: 60°C (1 min) to 250°C at 10°C/min Injection: Split (40:1), 0.5 µl, inlet at 250°C Detector: FID 275°C
不同蜜炙方法对红芪中毛蕊异黄酮和芒柄花素含量的影响

不同蜜炙方法对红芪中毛蕊异黄酮和芒柄花素含量的影响目的比較不同蜜炙方法對红芪中毛蕊异黄酮和芒柄花素含量的影响,为其蜜炙最佳工艺的建立提供依据。
方法采用传统炒制、烘制、微波制的方法蜜炙红芪;采用Agilent HC-C18色谱柱(4.6 mm×250 mm,5 ?m);流动相为乙腈-0.01%磷酸水溶液,梯度洗脱(0~12 min,30%~33%乙腈;12~13 min,31%~40%乙腈,13~25 min,40%乙腈),流速1.0 mL/min,检测波长248 nm,柱温30 ℃,进样量10 ?L。
结果红芪不同蜜炙品中毛蕊异黄酮和芒柄花素含量存在差异。
红芪烘制炙品中毛蕊异黄酮和芒柄花素含量最高,分别为7.911 6、49.699 6 ?g/g;红芪传统炙品中二者含量最低,分别为4.776 7、37.291 0 ?g/g;红芪生品和红芪微波炙品中二者含量相当,分别为5.080 2、42.798 9 ?g/g和3.983 9、42.314 5 ?g/g。
结论不同蜜炙方法对红芪中毛蕊异黄酮和芒柄花素含量有一定影响,烘制炙法是红芪蜜炙的最优方法。
Abstract:Objective To compare the effects of different methods of honey sunburn on the contents of calycosin and formononetin in Hedysari Radix;To provide the basis for the establishment of the optimum processing technology. Methods By frying (traditional method),baking,and microwave methods put Hedysari Radix under honey sunburn. Agilent HC-C18 column (4.6 mm × 250 mm,5 ?m)was used;the mobile phase was acetonitrile-0.01% phosphoric acid solution,gradient elution (0–12 min,30%–33% acetonitrile;12–13 min,31%–40% acetonitrile;13–25 min,40% acetonitrile)with velocity of 1.0 mL/min;detection wavelength was 248 nm;the column temperature was 30 ℃;sample volume was 10 ?L. Results There was statistical significance in the contents of calycosin and formononetin of different methods of honey sunburn for Hedysari Radix. Among them,the contents of calycosin and formononetin in Hedysari Radix processed by honey roast were the highest,7.911 6 and 49.699 6 ?g/g,respectively;the contents of calycosin and formononetin in Hedysari Radix processed by traditional method were the lowest,4.776 7 and 37.291 0 ?g/g,respectively;the contents of calycosin and formononetin in raw Hedysari Radix and Hedysari Radix by honey microwave method were the same,5.080 2,42.798 9 ?g/g,and 3.983 9,42.314 5 ?g/g,respectively. Conclusion Different honey sunburn methods for the contents of calycosin and formononetin in Hedysari Radix have certain effects,and honey roast method is the optimum method.Key words:Hedysari Radix;honey sunburn;calycosin;formononetin 基金项目:国家自然科学基金(81460611);教育部科學技术研究重点项目(212186);甘肃省自然科学研究基金计划(1010RJZA212、145RJZA076);甘肃省高等学校基本科研业务费专项资金(2013-2);甘肃省中医药管理局科研立项项目(GZK-2015-57);兰州市科技计划项目(2014-1-188)红芪为豆科植物多序岩黄芪Hedysarum polybotrys Hand.-Mazz.的干燥根,具有补气升阳、固表止汗、利水消肿、生津养血、行滞通痹、托毒排脓、敛疮生肌功效。
低温稳定化处理箱 英语

低温稳定化处理箱英语Low-Temperature Stabilization Treatment Chamber.The low-temperature stabilization treatment chamber is an essential piece of equipment in various industries, including biotechnology, pharmaceuticals, and materials science. It provides a controlled environment where samples can be exposed to low temperatures for prolonged periods, ensuring stability and preservation of their properties.Working Principles.The chamber works by maintaining a constant low temperature using refrigeration systems, heaters, and temperature sensors. The refrigeration system cools the chamber, while the heaters are used to maintain the desired temperature. Temperature sensors monitor the internal temperature and adjust the refrigeration and heating systems accordingly.Applications.1. Biotechnology: In biotechnology, low-temperature stabilization chambers are used to preserve cells, tissues, and other biological samples. By maintaining a low temperature, the chambers slow down biological processes, such as decay and metabolism, ensuring the integrity of the samples.2. Pharmaceuticals: Pharmaceutical companies rely on low-temperature chambers to store drugs and vaccines. These chambers ensure that the drugs retain their potency and effectiveness by preventing degradation at higher temperatures.3. Materials Science: In materials science, low-temperature chambers are used to study the properties of materials at low temperatures. This helps researchers understand how materials behave in extreme conditions, such as those found in space or deep-sea environments.Advantages.Temperature Control: The chambers provide precise temperature control, ensuring consistent conditions for experiments and samples.Stability: By maintaining a constant low temperature, the chambers ensure stability and prevent sample degradation.Versatility: These chambers can be used in various industries and applications, making them highly versatile.Ease of Use: Modern chambers are equipped with user-friendly interfaces and advanced controls, making them easy to operate.Disadvantages.High Cost: Low-temperature stabilization chambers can be expensive, especially for small businesses and research institutions.Energy Consumption: Maintaining a constant low temperature requires a significant amount of energy, leading to increased operational costs.Maintenance Requirements: Regular maintenance is necessary to ensure the chambers operate efficiently and maintain temperature stability.Conclusion.Low-temperature stabilization treatment chambers play a crucial role in various industries, providing a controlled environment for sample preservation and research. While they have several advantages, such as precise temperature control and stability, they also have some disadvantages, such as high cost and energy consumption. However, with their widespread use in biotechnology, pharmaceuticals, and materials science, these chambers have become essential tools for researchers and industry professionals.Future developments in low-temperature stabilization chambers could focus on improving energy efficiency,reducing operational costs, and enhancing temperature control accuracy. With ongoing research and advancements in refrigeration and temperature control technologies, we can expect these chambers to become more efficient, cost-effective, and reliable in the coming years.。
isosinensetin 裂解规律

isosinensetin 裂解规律
isosinensetin是一种取自檀香科植物的化合物。
它具有多种生物活性,例如抗菌、
抗氧化、抗炎等,因此近年来备受研究关注。
在研究这种化合物时,其裂解规律也备受研
究人员关注。
以下是isosinensetin裂解规律的中文解释。
1. 规律性:isosinensetin的裂解呈现一定的规律性,有助于我们理解这种化合物的分解过程。
2. 温和条件:研究发现,isosinensetin的裂解反应需要温和的条件,一般在50-70℃的温度下反应。
这说明,在实际应用中应该控制裂解反应的温度,以充分利用这种化合物
的活性。
3. 环路开启:在裂解反应中,isosinensetin的苯并环路会首先发生开启反应,形成一个烯丙基羧酸。
4. 稳定结构:烯丙基羧酸在反应中形成的中间体非常不稳定,因此需要在反应过程
中立即参与下一步反应,以稳定其结构。
5. 羰基还原:在下一步反应中,烯丙基羧酸经过还原反应,形成一个羟基烯醇酮。
6. 分解产物:最终产物是一种含有羟基和醛基的化合物,其结构与起始物质差异较大。
这种化合物对于生物体内某些过程具有活性。
总之,isosinensetin的裂解规律是复杂的,需要在温和条件下进行反应,且中间体
非常不稳定。
通过探究其裂解规律,可以更好地理解这种化合物的作用机制,为其进一步
的研究提供依据。
橡胶管低温曲挠试验不合格的

橡胶管低温曲挠试验不合格的英文回答,The rubber hose low temperature flex test did not meet the required standards. This is a cause forconcern as it could potentially affect the performance and safety of the product. It is important to investigate the reasons behind the failure and take necessary steps torectify the issue.One possible reason for the failure could be thequality of the rubber material used in the hose. It is essential to ensure that the rubber material is capable of withstanding low temperatures without losing itsflexibility. Conducting thorough material testing and analysis can help in identifying any deficiencies in the rubber material.Another factor to consider is the design andconstruction of the hose. It is possible that the hose may not have been designed to withstand low temperature flexing. Reviewing the design specifications and making necessarymodifications to improve its performance in low temperature conditions is crucial.Furthermore, the testing procedures and conditions should also be scrutinized. It is important to verify that the testing equipment and procedures are in accordance with the industry standards and accurately simulate real-world low temperature conditions. Any discrepancies in thetesting process should be addressed to ensure reliable and consistent results.In conclusion, the non-compliance of the rubber hose in the low temperature flex test requires a comprehensive investigation into the material, design, and testing procedures. Addressing these factors will be crucial in ensuring that the rubber hose meets the required standards and performs optimally in low temperature conditions.中文回答,橡胶管低温曲挠试验不合格,可能影响产品的性能和安全,需要对失败的原因进行调查,并采取必要的措施来解决问题。
低熔点磷酸盐玻璃制作工艺流程

低熔点磷酸盐玻璃制作工艺流程英文回答:To make low-melting-point phosphate glass, several steps are involved in the manufacturing process. I will explain each step in detail.1. Raw materials selection: The first step is to select the appropriate raw materials. Phosphate glass typically consists of a mixture of phosphorus pentoxide (P2O5) and metal oxides, such as sodium oxide (Na2O) or lead oxide (PbO). The selection of raw materials depends on the desired properties of the glass.2. Batch preparation: Once the raw materials are selected, they need to be mixed in specific proportions to form a batch. The batch is usually prepared by weighing the required amounts of each raw material and mixing them thoroughly. For example, if we want to make a sodium phosphate glass, we would mix sodium oxide and phosphoruspentoxide in the desired ratio.3. Melting: The prepared batch is then heated in a furnace to melt the mixture. The temperature and duration of the melting process depend on the composition of the glass and the desired properties. During melting, the batch forms a molten glass that is homogeneous and free from any impurities.4. Molding: Once the glass is melted, it needs to be shaped into the desired form. This can be done through various molding techniques, such as pouring the molten glass into molds or using glass blowing techniques. For example, if we want to make a glass bottle, we would pour the molten glass into a bottle-shaped mold and let it cool and solidify.5. Annealing: After the glass is molded, it undergoes an annealing process to relieve any internal stresses and improve its mechanical strength. Annealing involves slowly cooling the glass to room temperature to prevent cracking or breaking. This step is crucial to ensure the stabilityand durability of the glass product.6. Finishing: Once the glass is annealed and cooled, it can undergo additional finishing processes, such as polishing or etching, to enhance its appearance or add decorative elements. These processes are optional and depend on the specific requirements of the glass product.中文回答:制作低熔点磷酸盐玻璃的工艺流程包括以下几个步骤。
溶剂对钙钛矿吸光层成膜质量及其光电性能的影响研究

溶剂对钙钛矿吸光层成膜质量及其光电性能的影响研究王亚丽;侯丽新;王佳;刘贤豪【摘要】本文通过优选两步法制备钙钛矿吸光层中的溶剂,研究了溶剂对钙钛矿吸光层薄膜形貌、晶型结构及光电性能的影响.采用紫外-可见吸收光谱分析了溶剂对PbI2成膜的影响;采用SEM和XRD分别测试了钙钛矿吸光层的薄膜形貌和晶型结构;在标准光照下,测试了钙钛矿太阳能电池的光电性能.结果表明:二甲基亚砜(DMSO)比N,N-二甲基甲酰胺(DMF)与PbI2有更强的结合作用,利用S=0与pb2+的紧密结合作用,形成稳定的DMSO-PbI2中间体,抑制PbI2的快速结晶,形成均匀无定形PbI2薄膜,显著提高了钙钛矿吸光层制备时反应速率、改善钙钛矿层吸光层薄膜形貌和晶型结构,进而提高了钙钛矿太阳能电池光电性能,转换效率达15.1%.【期刊名称】《信息记录材料》【年(卷),期】2017(018)005【总页数】4页(P6-9)【关键词】钙钛矿太阳能电池;溶剂;抑制结晶;光电性能【作者】王亚丽;侯丽新;王佳;刘贤豪【作者单位】中国乐凯集团有限公司河北保定 071000;中国乐凯集团有限公司河北保定 071000;河北大学物理科学与技术学院河北保定 071002;中国乐凯集团有限公司河北保定 071000;中国乐凯集团有限公司河北保定 071000【正文语种】中文【中图分类】TQ1322PbI2的快速结晶,形成均匀无定形PbI2薄膜,显著提高了钙钛矿吸光层制备时反应速率、改善钙钛矿层吸光层薄膜形貌和晶型结构,进而提高了钙钛矿太阳能电池光电性能,转换效率达15.1%。
在各种新型的太阳电池材料中,层状结构钙钛矿型有机卤化铅材料(如CH3NH3PbI3)[1-5]由于具有直接带隙、高吸光系数、高载流子迁移率等独特的光电性能,受到了众多科研人员的关注,被广泛用作薄膜太阳电池的吸光层材料[6-9]。
钙钛矿太阳电池的构造通常采用体相异质结结构、平面异质结结构和无空穴输运材料异质结结构等。
洛阳“PEP”2024年小学六年级下册F卷英语第三单元期末试卷

洛阳“PEP”2024年小学六年级下册英语第三单元期末试卷考试时间:90分钟(总分:120)A卷考试人:_________题号一二三总分得分一、选择题(共计20题,共40分)1、What do you call a person who studies history?A. SociologistB. PsychologistC. HistorianD. Archaeologist2、What do we call a collection of stories?A. AnthologyB. NovelC. SeriesD. Collection3、What is the opposite of ‘full’?A. EmptyB. PackedC. CompleteD. Whole4、选择题:What instrument is used to measure temperature?A. BarometerB. ThermometerC. SpeedometerD. Altimeter5、选择题:What do we call the process of turning solid into liquid?A. FreezingB. MeltingC. BoilingD. EvaporatingWhat is the color of a typical grape?A. RedB. GreenC. PurpleD. All of the above7、What do we use to cut paper?A. KnifeB. ScissorsC. RulerD. Tape8、What is the capital city of Gabon?A. LibrevilleB. Port-GentilC. FrancevilleD. Moanda9、选择题:What is the capital of the Netherlands?A. AmsterdamB. RotterdamC. The HagueD. Utrecht10、Which planet is known for its Great Red Spot?A. MarsB. JupiterC. SaturnD. Neptune11、选择题:What do we call a young frog?A. TadpoleB. ChickC. CalfD. Kitten12、What do you call a person who takes care of trees?A. GardenerB. ArboristC. FarmerD. LandscaperWhat do we call a sweet dessert made with fruit and cream?A. PavlovaB. Eton MessC. Fruit SaladD. All of the above14、What do you call a young fox?A. CubB. KitC. PupD. Leveret15、选择题:What is the name of the insect that makes silk?A. AntB. ButterflyC. SilkwormD. Bee16、选择题:What color are school buses?A. BlueB. GreenC. YellowD. Red17、选择题:What is the name of the famous river in South America?A. AmazonB. OrinocoC. ParanáD. All of the above18、What is the capital of the United States?A. New YorkB. Washington, D.C.C. Los AngelesD. Chicago19、How many legs does a spider have?A. 6B. 8C. 10D. 12What do we call the measurement of how much space an object occupies?A. MassB. VolumeC. WeightD. Density二、听力题(共计20题,共40分)1、听力题:The ______ is home to many species.2、听力题:My friend has a new ____ (bicycle) that is red.3、听力题:He is reading a ___. (story)4、听力题:I like to _____ (bake) cookies.5、听力题:She loves to _______ (travel) overseas.6、听力题:A catalyst lowers the ______ energy of a reaction.7、听力题:I enjoy ________ in the summer.8、听力题:The __________ can influence human activities and settlements.9、听力题:My friend is a ______. He enjoys writing reviews.10、听力题:The girl loves to ________.11、听力题:The cake is _______ (decorated) with fresh fruit.12、听力题:A _______ is a solution that does not conduct electricity.A ______ is a region of flat land with higher elevation.14、听力题:The _____ (seashell) is pretty.15、听力题:They are friends since _____ (childhood/adulthood).16、听力题:The _____ (car/bike) is red.17、听力题:The cake is very ________.18、听力题:The ______ is a chart that shows all the elements.19、听力题:I see a __ in the sky. (plane)20、听力题:I like to _______ (watch) the stars at night.三、填空题(共计20题,共10分)1、填空题:The __________ (历史的反思) fosters growth.2、填空题:When two or more substances are mixed together, they form a _______. (混合物)3、填空题:The owl is a _______ (神秘的) night hunter.4、填空题:The _____ (拼图) has many pieces to fit together.5、填空题:My favorite fruit is _______ (桃子).6、填空题:I love to go ______ (滑雪) in the mountains.My brother loves to __________ (去公园) on weekends.8、community service project) addresses local challenges. 填空题:The ____9、填空题:I love to ______ (写) letters to my friends.10、填空题:A ________ (湖泊) can be a tourist attraction.11、填空题:The _______ (浣熊) washes its food.12、填空题:I love to cuddle with my ________ (玩具名称).13、填空题:The ________ was a key figure in the establishment of democracy.14、小龙虾) scuttles across the riverbed. 填空题:The ____15、填空题:I love to listen to stories from my grandparents about their ________ (年轻时代).16、填空题:My mom loves to _______ (动词) to relax. 她觉得这个很 _______ (形容词).17、填空题:I enjoy ______ (与朋友一起) playing board games.18、填空题:I like to play with my toy ________ (玩具名称) in the sunshine.19、填空题:This girl, ______ (这个女孩), loves to read fairy tales.20、填空题:The butterfly's wings are like ______ (彩虹).。
低温管保藏法流程

低温管保藏法流程英文文档内容:Low-Temperature Tube Storage Method FlowThe low-temperature tube storage method is a preservation technique that involves storing samples in test tubes at sub-freezing temperatures.This method is widely used in various fields such as biology, medicine, and food preservation.The flowchart below outlines the steps involved in the low-temperature tube storage method:1.Sample Collection: Collect the sample to be preserved.This could be biological specimens, blood, sperm, or other materials.2.Sample Transfer: Transfer the collected sample into a suitable test tube.Ensure that the test tube is clean and sterile.3.Add Cryoprotectant: Add a cryoprotectant to the test tube.The cryoprotectant helps to protect the sample from damage during the freezing mon cryoprotectants include dimethyl sulfoxide (DMSO), glycerol, and ethylene glycol.4.Mixing: Thoroughly mix the sample and the cryoprotectant.This ensures that the sample is evenly preserved and that the cryoprotectant does not separate from the sample.5.Cooling: Place the test tube containing the sample and cryoprotectant in a cooling device such as a controlled-rate freezer or acryogenic tank.The temperature should be reduced gradually to prevent sample damage.6.Storage: Once the sample has reached the desired storage temperature, usually below -150°C, it can be stored in a cryogenic tank for long-term preservation.Ensure that the tank is properly insulated and maintained to maintain low temperatures.beling: Properly label the test tube with relevant information such as the sample type, collection date, and any other necessary details for identification purposes.8.Inventory Management: Maintain an accurate inventory of the stored samples, including their location, storage dates, and any other relevant information.9.Retrieval: When needed, retrieve the sample from storage.Allow the test tube to warm up to room temperature gradually to prevent sample damage.10.Thawing: Once the test tube reaches room temperature, transfer the sample to a suitable container and thaw it completely.Discard any ice plugs or solidified cryoprotectant.11.Further Processing: The thawed sample can now be used for further processing, such as testing, experimentation, or other applications as needed.It is important to follow proper safety protocols when handlingcryogenic materials and to use appropriate equipment to ensure the success of the low-temperature tube storage method.中文文档内容:低温管保藏法流程低温管保藏法是一种将样本保存在冷冻温度下的保存技术。
热处理专业术语中英文对照

90。cinder inclusion夹渣
91. lattice晶格
92。abrasion/abrasive/rub/wear/wearing resistance(property)耐磨性
93. spectrum analysis光谱分析
94. heat/thermal treatment热处理
bright electroplating辉面电镀
bright heat treatment光辉热处理
bypass heat treatment旁路热处理
carbide炭化物
carburized case depth浸碳硬化深层
carburizing渗碳
cementite炭化铁
chemical plating化学电镀
subzero treatment生冷处理
supercooling过冷
surface hardening表面硬化处理
temper brittleness回火脆性
temper colour回火颜色
tempering回火
tempering crack回火裂痕
texture咬花
thermal refining调质处理
72. induction hardening感应淬火
73。impedance matching感应淬火
74。hardening and tempering调质
75. crack裂纹
76。shrinkage缩孔,疏松
77。forging锻(件)
78。casting铸(件)
79. rolling轧(件)
64。creep蠕变
65。deflection挠度
66。elongation延伸率
接触氧化法英文

接触氧化法英文Introduction:The contact oxidation method is an important wastewater treatment technology that has been widely used in various industries such as chemical engineering, textile, food processing and so on. In this article, we will discuss the principle, advantages and disadvantages, as well as the application scope of contact oxidation method.Principle:Contact oxidation method refers to the use of aerobic microorganisms to degrade organic pollutants in wastewater. The process mainly consists of two stages: the first stage is the adsorption and decomposition of organic pollutants on the surface of the carrier, in which the organic matter is converted into CO2 and H2O under the action of oxygen and bacteria; the second stage is the final purification and release of wastewater, which is achieved through the settling of activated sludge.Advantages and Disadvantages:Advantages:1. High treatment efficiency: contact oxidation method has a higher treatment efficiency compared to other biological treatment technologies, which can effectively remove the biochemical oxygen demand (BOD) and chemical oxygen demand (COD) in wastewater.2. Low investment and operating costs: the equipment required for contact oxidation method is simple and easy to maintain, and the energy consumption is low, which can save a lot ofinvestment and operating costs.3. Wide adaptability: contact oxidation method can adapt to various types of wastewater, including high concentration, low temperature, and low pH wastewater.Disadvantages:1. Limited treatment capacity: the treatment capacity of contact oxidation method is relatively low, which is not suitable for large-scale wastewater treatment.2. High sensitivity to shock: the contact oxidation method is sensitive to the shock of organic pollutants, and the sudden increase of organic pollutants may cause a decrease in the treatment efficiency.3. Impact on the environment: the sludge generated during contact oxidation may cause secondary pollution to the environment if not treated properly.Application Scope:Contact oxidation method is widely used in various industries such as chemical engineering, textile, food processing, and municipal wastewater treatment. It is particularly suitable for the treatment of small and medium-sized enterprises' wastewater, as well as the purification of urban sewage and the reconstruction of old sewage treatment plants.Conclusion:The contact oxidation method is an important technology for wastewater treatment, which has the advantages of high treatment efficiency, low investment and operating costs, and wide adaptability. However, it also has its weaknesses, such as limited treatment capacity and high sensitivity to shock. In practice, it should be used in combination with other wastewater treatment methods to achieve better results.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
SEE PROFILE
572
Jing Wang University of Southampton
148 PUBLICATIONS 2,875 CITATIONS
SEE PROFILE
Lei Hongwei Huazhong Agricultural University
The user has requested enhancement of the downloaded file.
Communication /JACS
Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells
O
making it not the ultimate ETL material. For example, the electron mobility of TiO2 is not high enough. Zhou et al. showed that Y-doping can increase the electron mobility and electrical conductivity of TiO2 and therefore improve the efficiencies for perovskite cells.7 However, doping may not be able to completely overcome the intrinsic low electron mobility issue. Moreover, Snaith et al. reported that perovskite solar cells using mesoporous TiO2 are sensitive to ultraviolet (UV) illumination.24 There exist other transparent metal oxides, such as ZnO, In2O3, and SnO2, that exhibit similar or even better electrical and optical properties as compared to TiO2. Especially, these oxides exhibit a much higher electron mobility than TiO2.25 Recently, Liu et al. reported that a planar perovskite solar cell using a low-temperature solution-processed nanoparticle (ZnO) ETL achieved a high PCE of 15.7%.26 The results suggest that metal oxides other than TiO2 can be good ETL materials for high-efficiency perovskite solar cells. SnO2 is a metal oxide that has not only a much higher electron mobility but also a wider band gap than TiO2.25,27 Because ETLs absorb photons with energies higher than the band gap but do not contribute to photocurrents, such absorptions cause only a small current loss. Therefore, SnO2 should lead to a smaller ETL-induced current loss than TiO2. For ultra-high-efficiency cells, every potential energy loss should be eliminated. Moreover, SnO2, with a wider band gap, is more stable than TiO2 under UV illumination.25 Fluorine-doped SnO2 (FTO) is a robust transparent conducting electrode that has been widely used in the thin- fi lm solar cell industry. Gelled SnO2 nanoparticles have been used as ETLs for polymer-based solar cells.28 Dye-sensitized solar cells using high-temperature prepared mesoporous SnO2 particles coated with TiO2 and MgO have achieved high efficiencies.27 However, there is no report on efficient perovskite solar cells using SnO2 as both ETLs and antireflection films. Here, we report on low-cost and low-temperature solutionprocessed SnO2 as an ETL material for achieving highly efficient planar perovskite solar cells. The best-performing planar cell using a SnO2 ETL has achieved PCEs of 17.21% and 14.82% when measured under reverse and forward voltage scans, respectively. The perovskite solar cells using SnO2 ETLs
31 PUBLICATIONS 691 CITATIONS
SEE PROFILE
Yanfa Yan University of Toledo
427 PUBLICATIONS 9,236 CITATIONS
SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Article in Journal of the American Chemical Society · May 2015
DOI: 10.1021/jacs.5b01994 · Source: PubMed
CITATIONS
READS
157
12 authors, including: Liangbin Xiong Hubei Engineering University
†
Key Laboratory of Artificial Micro- and Nano-structures of Ministry of Education of China, School of Physics and Technology, Wuhan University, Wuhan 430072, People’s Republic of China ‡ Department of Physics and Astronomy and Wright Center for Photovoltaics Innovation and Commercialization, The University of Toledo, Toledo, Ohio 43606, United States
See discussions, stats, and author profiles for this publication at: https:///publication/277087577
Low-Temperature Solution Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar...
S Supporting Information *
ABSTRACT: Lead halide perovskite solar cells with the high efficiencies typically use high-temperature processed TiO2 as the electron transporting layers (ETLs). Here, we demonstrate that low-temperature solution-processed nanocrystalline SnO2 can be an excellent alternative ETL material for efficient perovskite solar cells. Our bestperforming planar cell using such a SnO2 ETL has achieved an average efficiency of 16.02%, obtained from efficiencies measured from both reverse and forward voltage scans. The outstanding performance of SnO2 ETLs is attributed to the excellent properties of nanocrystalline SnO2 films, such as good antireflection, suitable band edge positions, and high electron mobility. The simple lowtemperature process is compatible with the roll-to-roll manufacturing of low-cost perovskite solar cells on flexible substrates. rganic−inorganic lead halide perovskite solar cells have attracted enormous attention in recent years. The power conversion efficiency (PCE) of perovskite solar cells has rapidly increased from 3.8% to 20.1% (certified) in just 6 years.1−10 Such a rapid increase in efficiency is largely attributed to the superior photovoltaic properties of lead halide perovskites, such as the extremely high optical absorption coefficient and very long carrier lifetime.11−14 High-efficiency perovskite solar cells typically use electron transporting layers (ETLs)/hole blocking layers and hole transporting layers (HTLs)/electron blocking layers to separate and collect photogenerated charge carriers produced in perovskite absorbers. These layers are critical for achieving high-efficiency cells because they prevent severe carrier recombination at interfaces, which may dictate the opencircuit voltages (Voc’s) and fill factors (FFs) of solar cells. Perovskite solar cells without ETLs and/or HTLs have exhibited lower efficiencies as compared to the cells with ETLs and HTLs.15,16 The electrical and optical properties of ETLs and HTLs can significantly affect the performance of perovskite solar cells. Perovskite solar cells use either regular or inverted architectures.17−23 So far, the record efficiency cells have the regular architecture, in which light enters from the ETL and compact TiO2 is used as the ETL material. Though the record efficiency cells use TiO2 ETLs, the optical and electronic properties of TiO2 still exhibit some shortfalls,
