_S_-Gossypol_acetic_acid_LCMS_15682_MedChemExpress
ACCUSPIN系统-Histopaque 1077产品说明书

Technical BulletinACCUSPIN™ System – Histopaque ® 1077Catalog Numbers A6929, A7054, and A0561Product DescriptionACCUSPIN System-Histopaque -1077products are intended for use in the isolation of lymphocytes and other mononuclear cells. The separation medium, Histopaque-1077, is a sterile-filtered, endotoxin tested solution of polysucrose and sodium diatrizoate, adjusted to a density of 1.077 g/mL. The ACCUSPIN tube is specially designed with two chambers separated by a porous high density polyethylene barrier (frit).Separation of lymphocytes and other mononuclear cells from whole blood and bone marrow using density gradientseparation media is based on a published method.1 Histopaque-1077 is suitable for human lymphocyte antigen (HLA) typing 2 and as the initial isolation step prior toenumeration of T, B, and ‘null’ lymphocytes.3 It may also be employed in the preparation of pure lymphocyte suspensions for cell culture and cytotoxicity assays.4ACCUSPIN System-Histopaque-1077 products consist of radiation sterilized polypropylene tubes fitted with a highdensity polyethylene frit and aseptically filled with Histopaque-1077.Histopaque-1077 is a sterile-filtered solution of polysucrose, 57 g/L, and sodium diatrizoate, 90 g/L.Density: 1.076–1.078 g/mL Endotoxin: 0.3 EU/mL pH: 8.8–9.0ACCUSPIN System-Histopaque-1077Catalog No. A692940 × 3 mLEach tube contains 3 mL ofHistopaque 1077-1 and will separate 3-6 mL of anticoagulated blood Catalog No. A7054 12 × 15 mLCatalog No. A0561100 × 15 mLEach tube contains 15 mL ofHistopaque 1077-1 and will separate 15-30 mL of anticoagulated bloodReagents and Equipment Required but Not ProvidedCentrifuge (swinging bucket rotor)capable of generating 100 to 1,000 g Centrifuge tubes for washing mononuclear cellsIsotonic phosphate buffered saline solution or appropriate cell culture mediumPrecautions and DisclaimerFor R&D use only. Not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.Preparation InstructionsSpecimen Collection - Collect blood in preservative-free anticoagulant (EDTA or heparin) or use defibrinated blood. For best results, blood should be processed within 2 hours.On occasion, it may be necessary to dilute the blood sample 3 to 5-fold, depending on absolute cell numbers. A similar volume of prediluted blood may be used or the blood sample may be diluted directly in upper chamber of the ACCUSPIN tube (seeProcedure, step 3). This is appropriate for specimens with hematocrits above normal.Storage/StabilityStore the products at 2–8 C.Histopaque-1077 has an expiration period of 3 years. Reagent label bears expiration date.ProcedureAnticoagulated blood can be added to the top chamber of the tube without risk of mixing with the Histopaque-1077 in the lowerchamber under the frit. On centrifugation the whole blood migrates through the frit to contact with the Histopaque-1077. The elements of greater density displace a volume of Histopaque-1077 above the frit giving a clear separation of the bloodcomponents. The erythrocytes aggregate and the granulocytes become slightly hypertonic, increasing their sedimentation rate, resulting in pelleting at the bottom of the ACCUSPIN Tube. Lymphocytes and other mononuclear cells, e.g., monocytes, remain at the plasma/Histopaque-1077 interface. This dense band of mononuclear cells may be collected by pouring off the contents of the upper chamber or by means of a pipette. Erythrocyte contamination is avoided due to the barrier between the chambers.Most extraneous platelets are removed by low speed centrifugation during the washing steps.1. Bring desired number of tubes to roomtemperature. If Histopaque-1077 isabove the frit prior to use, centrifuge at 1,000 g for 30 seconds at room temperature.Note: Failure to bring ACCUSPIN System-Histopaque-1077 to room temperature may cause limited recovery of mononuclear cells. 2. Label tube(s).3. Freely pour the blood sample into theupper chamber of each ACCUSPIN System-Histopaque-1077 tube.a. Use 3–6 mL of whole blood withACCUSPIN System-Histopaque-1077 tubes, Catalog No. A6929. b. Use 15–30 mL of whole blood withACCUSPIN System-Histopaque-1077 tubes, Catalog Nos. A7054 or A0561. Note: Use of volumes of prediluted or whole blood other than those recommended may result in decreased recovery.4. Centrifuge at 1,000 g for 10 minutes atroom temperature or centrifuge at 800 g for 15 minutes at roomtemperature. Centrifugation at lower temperatures, such as 4 C, may result in cell clumping and poor recovery.Note: If platelet contamination is a concern, add the mononuclear cells to a 4-20% sucrose gradient that has been layered over Histopaque-1077.Centrifuge at 1,000 × g for 10 minutes at room temperature. The platelets will pellet at the bottom, while themononuclear cells will migrate to the Histopaque-1077 layer.5. After centrifugation, carefully aspiratethe plasma layer with a Pasteur pipette to within 0.5 cm of the opaque interface containing mononuclear cells. Properly dispose of the plasma layer.Note: Failure to remove the excesssupernatant may result in contamination of the mononuclear band with plasma proteins.6. Carefully transfer the opaque interfacewith a Pasteur pipette into a clean conical centrifuge tube.Note: Removal of Histopaque-1077 with the mononuclear band increasesgranulocyte contamination from residual granulocytes, which may remain at the mononuclear interface.7. Wash the cells by adding 10 mL ofisotonic phosphate buffered saline solution or appropriate cell culture medium and mix by gently drawing in and out of a Pasteur pipette. 8. Centrifuge at 250 g for 10 minutes. 9. Aspirate the supernatant and discard. 10. Resuspend cell pellet with 5 mL ofisotonic phosphate buffered saline solution or appropriate cell culture medium and mix by gently drawing in and out of a Pasteur pipette.11. Centrifuge at 250 g for 10 minutes. 12. Repeat steps 9, 10, and 11, discardsupernatant and resuspend cell pellet in 0.5 mL of isotonic phosphate buffered saline solution or appropriate cell culture medium. Erythrocytes and granulocytes should pellet to the bottom of the ACCUSPIN tube. Mononuclear cells should band at the interface between the Histopaque-1077 and the plasma. If observed results vary from expected results, please contact Sigma-Aldrich Technical Service for assistance.References1. Boyum, A., Separation of leukocytesfrom blood and bone marrow. Scand. J. Clin. Lab. Invest ., 21 (Suppl 97), 77 (1968).2. Amos, D.B., and Pool, P., “HLA typing” inManual of Clinical Immunology, Rose, N.R., and Friedman, H., eds., American Society for Microbiology, (Washington, DC: 1976) pp. 797-804.3. Winchester, R.J., and Ross, G., “Methodsfor enumerating lymphocyte populations” in Manual of Clinical Immunology, Rose, N.R., and Friedman, H. eds., American Society for Microbiology, (Washington, DC: 1976) pp. 64-76.4. Thorsby, E., and Bratlie, A., “A rapidmethod for preparation of pure lymphocyte suspensions.”Histocompatibility Testing, Terasaki, P.I., ed., 665-666 (1970).The life science business of Merck operates as MilliporeSigma in the U.S. and Canada.Merck, Sigma-Aldrich, ACCUSPIN, and Histopaque are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.© 2022 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.NoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose.The information in this document is subject to change without notice and should not be construed as a commitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document.Contact InformationFor the location of the office nearest you, go to /offices .Technical ServiceVisit the tech service page on our web site at /techservice .Standard WarrantyThe applicable warranty for the products listed in this publication may be found at /terms .A0561 Technical Bulletin Rev 06/2022。
SAS-1
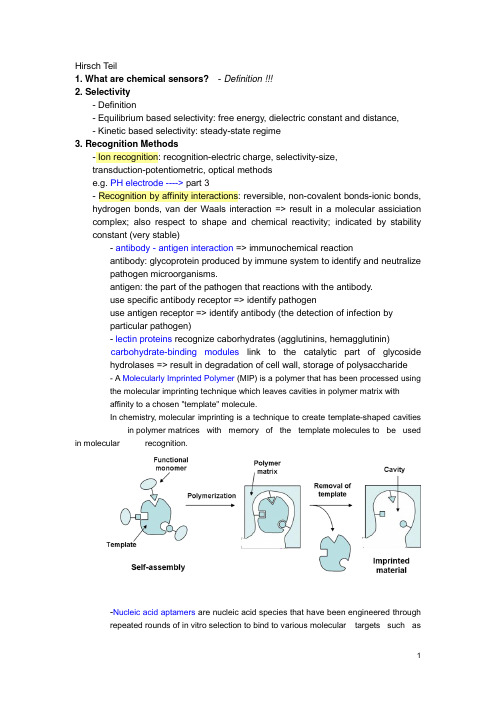
Hirsch Teil1. What are chemical sensors?- Definition !!!2. Selectivity- Definition- Equilibrium based selectivity: free energy, dielectric constant and distance,- Kinetic based selectivity: steady-state regime3. Recognition Methods- Ion recognition: recognition-electric charge, selectivity-size,transduction-potentiometric, optical methodse.g. PH electrode ----> part 3- Recognition by affinity interactions: reversible, non-covalent bonds-ionic bonds, hydrogen bonds, van der Waals interaction => result in a molecular assiciation complex; also respect to shape and chemical reactivity; indicated by stability constant (very stable)- antibody - antigen interaction => immunochemical reactionantibody: glycoprotein produced by immune system to identify and neutralizepathogen microorganisms.antigen: the part of the pathogen that reactions with the antibody.use specific antibody receptor => identify pathogenuse antigen receptor => identify antibody (the detection of infection byparticular pathogen)- lectin proteins recognize caborhydrates (agglutinins, hemagglutinin)carbohydrate-binding modules link to the catalytic part of glycosidehydrolases => result in degradation of cell wall, storage of polysaccharide- A Molecularly Imprinted Polymer (MIP) is a polymer that has been processed usingthe molecular imprinting technique which leaves cavities in polymer matrix withaffinity to a chosen "template" molecule.In chemistry, molecular imprinting is a technique to create template-shaped cavities in polymer matrices with memory of the template molecules to be used in molecular recognition.-Nucleic acid aptamers are nucleic acid species that have been engineered throughrepeated rounds of in vitro selection to bind to various molecular targets such assmall molecules, proteins, nucleic acids, and even cells, tissues and organisms.Aptamers are useful in biotechnological and therapeutic a pplications as they offer molecular recognition properties that rival that of the commonly used bimolecular antibodies.- Recognition by nucleic acids: hydrogen bonds between two distinct pairs of nucleobases => two complementary nucleic acids form a double strand association complex => called hybridizationnucleic acid sensors: short single strand NA as receptor to recognize a particular NA sequence in the analyte NA => detection of genetic anomalies and pathogen mircoorganism- Recognition by enzyme: dynamic processEnzyme: protein compound that function as catalysts in chemical reaction occurring in living system.- Recognition by cells and tissues: advantages of enzyme incorporated in biological materials => in their natural environmentsee part 3, Wegener - Recognition by gases and vapors: based on sorption at solid material => surface-adsorption, inner-absorption; purely physical phenomenon or chemical reaction.4. Transduction MetohdosChemical transduction: monitoring the change of chemical composition of the sensing element in response to the recognition process. => change in concentration/amount is measured => detect primary product -> secondary product or coreagent -> labeling productLABEL can be a simple molecular species or nanoparticals that can be detected by available physiochemical methods => enzyme, fluorescent dyes, luminescent dyes, electroactive compoundsPhysical transduction: a specific physical property of the sensing element that is affected by its interaction with the analyte is monitored. => mass, reflective index, dielectric properties, electrical resistivity => LABEL-FREE- Thermometric transductionRecognition of the analyte leads to change in temperature => only catalytical processes generate sufficient heat to the measurement => application: combustible gases react with O2 at the surface of a catalyst.- Transduction based on mechanical effectsRecognition leads to change in mass of the sensing element => monitored by mass tranducer based on quartz crystal microbalance (QCM)----------------------------------------------------------------------------------------------------------------- QCM, correct name: Thickness shear modePiezoelectric effect:generation of electrical charges on the surface of a solid by strain, pressure or torsion (mechanical deformation of solid) =>electricity resulting from pressureI nverse piezoelectric effect:application of charges to surfaces of piezoelectricsolid generates mechanical deformation (elongation, contraction, torsion)QCM is based on Inverse piezoelectric effect!# AT cut => 35`15`=> minimum temperature coefficient at 50~70 CIt makes the AT-cut well suited to applications requiring high degree of frequency stability over wide temperature ranges.## Electrodes are applied on both sides, and AC voltage applied.DC cannot flow across the crystal because it consists of an insulator material;however the crystal somewhat behaves as capacitor and allow an AC current to f low along the left-hand loop.AC voltage applied => leads to shear oscillation of crystal => when the voltage frequency matches the intrinsic vibration frequency of the crystal => the vibration amplitude is at maximal => the resonant => resonant frequency (f0) => depend on crystal thickness (e.g. d q= 330 um, f0= 5MHZ), density and elasticity of piezoelectric material### AT-cut resonator: thickness: ~0.2 mm, diameter of the active area: 5~20 mm #### Deposition of a homogenous mass film (a rigid overlay)Sauerbrey equation:Cf indicate sensitivity of QCMcondition of this equation: rigid deposited mass; △m<2% of crystal mass;operated in vacuum or in gaseous atmopphereIn liquid: the liquid breaks the vibration by friction => lessen f0Thickness of the layer must be greater than the wave decay lengththat is of 250 nm of 5 MHz resonator at water. ----> part 2!!!##### QCM in practice => see p.41----------------------------------------------------------------------------------------------------------------- - Resistive and capacitive transductionRecognition leads to changes in the electrical property of this materialResistive transduction: gases interact with MOS => change in electrical resistivity Capactive transduction => dielectric constant- Electrochemical transductionsee part 2, Matysik - Optical transductionOptical transduction can be based on light emission or light absorption, also by physical quantity (reflective index) and light scattering.5. Sensor Configuration and Fabrication- Lateral flow assayA typical test strip consists of the following components:1. Sample pad – an absorbent pad onto which the test sample is applied2. Conjugate pad –this contains antibodies specific to the target analyte;conjugated to coloured particles (e.g. gold nanoparticles)3. Reaction membrane –typically a hydrophobic nitrocellulose or celluloseacetate membrane onto which anti-target analyte antibodies are immobilized in a line across the membrane as a capture zone or test line, and a control zonecontaining antibodies specific for the conjugate antibodies.4. Wicking pad –a further absorbent pad designed to draw the sample acrossthe reaction membrane by capillary action and collect it.Double antibody sandwich assays: the sample migrates from the sample pad through the conjugate pad where any target analyte present will bind to the c onjugate.=> The sample then continues to migrate across the membrane until it reaches the test line where the target or conjugate complex will bind to the immobilized antibodies producing a visible line on the membrane. => The sample then migrates further along the strip until it reaches the control line, where excess conjugate will bind and producea second visible line on the membrane.This control line indicates that the sample has migrated across the membrane as intended. Two clear lines on the membrane is a positive result. A single line in the control zone is a negative result. Double antibody sandwich assays are most suitable for larger analytes, such as bacterial pathogens and viruses, with multiple antigenic sites. 6. Methods and Material in Sensor Preparation- Immobilization at solid surface => integration of a transducer with the receptor Physical adsorption at a solid supportNon covalent immobilization at solid surface => hydrophobic interaction, hydrogen bonding, electrostatic attraction; monolayer; no restrict access; not stable; Langmuir isotherm -> equilibrium interactionSupport material: silica, cellulose acetate, PVCCovalent bonding to the solid supportCovalent conjugation => stable, covalent bond, time consuming, expensiveCommon reactive group: -OH, -NH2, -C=O, -SH- Carboxylic acid with DCC- Glutaraldehyde reacts with the a.a. of lysine in protein => widely used Support: porous material => high specific area, high density of immobilized compounds => hydrogel: immobilized by entrapment/covalent corsslink - Natural polymers: Cellulose, Dextran- Synthetic polymers: Polystyrene- Active polymers: Epoxide (without preliminary activation) -->DNA array !!!- Inactive Polymers: Vicinal hydroxyls actived by CNBr- Inorganic support: Silica, AL2O3, TiO2 => stable at extreme PH- Metal support: noble metals, thiols on golds --> self assembled monolayers!Affinity reaction: avidin-biotin !!!Thin molecular layers: one or several molecular layers in solid support - Self-assembly of amphiphilic compounds: preparation of liposome andmicelles; liposome can be used of entrapment of molecular- Bilipid layer membranes: Langmuir-Blodgett technique- Layer by Layer assembly- Sol-Gel chemistry methods: silica gel => -O-Si-O-- Hydrogels: Xerogel, aerogel- Conducting polymers: Polyacetylene, polyaniline --> gas senor based on CP (----> part 3 !!!); also as entrapment matrix for biological receptors- Mesoporous materials: porous materials with pore (diameter: 2-50 nm,close to protein) => enzyme immobilization by entrapment (crosslinking withglutaraldehyde)- Deposition of polymers onto solid surfaces: dip coating, drop coating, spin coating ----> part 2 !!!Perm-selective memberanes: Nafin ----> Clark oxygen electrode Support-free crosslinkingEntrapment in a polymer networkEncapsulation7. Microfabrication Methodes- Spot Arraying: Contact-based & Noncontact-based; DNA microarray !!!!!Pros & Cons- Thick-film Technology: screen-printing technique (5-50 um thick layer)- Thin-film Technology: Photolithography (2 um)- Softlithography ----> experiment !!!!- Microcontact printing ----> experiment !!!!8. Optical Sensors- Electromagnetic RadiationOptical sensor => interaction of electromagnetic radiation with sensor layer - frequency; wavelength; photon energy (definition)- Structure: integration with wavelength-selection (optical filters) device and light sources (lasers), light detectors (phototransistors)- Optical Waveguides- Optical FibersOptical fibers' structuretotal internal reflection => evanescent wave- Spectrochemical Transduction MethodsSpectrochemical method analysis => light absorption or emission by sample => optical label performs absorption or emission (organic dye or metal complexes) - Light absorption: absorbance => concentration; sensitivity => thickness, absorpyivity, absorptivity => wavelength- Diffuse reflectance spectrometry: refelctance => concentration; suitable forsolid in near IR- Luminescence: Fluorescence spectromerty => fluorophore (label, organic dye or metal complexes, luminescent nanparticle ); steady-statefluorescence measurement, Time-resolved fluormetry; fluorescencequenching; resonance energy transfer (FRET); chemical- andbioluminescence => luminol; electrochemicaluminescence; Ramanspetrometry- Surface Plasmon Resonance Spectroscopy (SPR)。
金丝桃苷检测

迪信泰检测平台
金丝桃苷检测
金丝桃苷(Hyperoside),又称为槲皮素-3-O-β-D-吡喃半乳糖苷,是一种黄酮醇苷类化合物,广泛存在于各种植物体内,如金丝桃科、蔷薇科、桔梗科等果实与全草中。
具有抗炎、解痉、利尿、止咳、降压、降低胆固醇、蛋白同化、局部和中枢镇疼以及对心、脑血管的保护作用等多种生理活性,是一种重要的天然产物。
迪信泰检测平台采用高效液相色谱(HPLC)法,结合蒸发光散射检测器(ELSD)或DAD 检测器,可高效、精准的检测样本中金丝桃苷的含量变化。
此外,迪信泰检测平台还提供其他多种植物黄酮检测服务,以满足您的不同需求。
HPLC测定金丝桃苷样本要求:
1. 请确保样本量大于0.2g或者0.2mL。
周期:2~3周。
项目结束后迪信泰检测平台将会提供详细中英文双语技术报告,报告包括:
1. 实验步骤(中英文)。
2. 相关质谱参数(中英文)。
3. 质谱图片。
4. 原始数据。
5. 金丝桃苷含量信息。
迪信泰检测平台可根据需求定制其他物质测定方案,具体可免费咨询技术支持。
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
CydXisasubunitof...

copper centers(Cu A and Cu B)and two hemes—low-spin heme a and high-spin heme a3.Despite many years of research,the individual absolute absorption spectra of the two hemes in the Soret band(420–460nm)have not yet been resolved because they overlap strongly. There is but a single classical work of Vanneste[1]reporting the absolute individual spectra of the reduced hemes a and a3.We revisited the problem with new approaches as summarized below.(1)Calcium binding to mitochondrial COX induces a small red shift of the absorption spectrum of heme a.Treating the calcium-induced difference spectrum as thefirst derivative(differential)of the ab-sorption spectrum of the reduced heme a,it is possible to reconstruct the line shape of the parent absolute spectrum of a2+by integration. The Soret band absolute spectrum of the reduced heme a obtained in this way differs strongly form that in ref.[1].It is fairly symmetric and can be easily approximated by two10nm Gaussians with widely split maxima at442and451nm.In contrast to Vanneste,no evidence for the~428nm shoulder is observed for heme a2+.(2)The overall Soret band of the reduced COX reveals at least5 more Gaussians that are not affected by Ca2+.Two of them at436 and443nm can be attributed to electronic B0transitions in heme a3, and two more can represent their vibronic satellites.(3)A theoretical dipole–dipole interaction model was developed [2]for calculation of absorption and CD spectra.The model allows to optimize parameters of the B x,y electronic transitions in the hemes a and a3to obtain bestfit to the experimental spectra.The optimized parameters agree with the characteristics of the reconstructed spectra of hemes a and a3.References[1]W.H.Vanneste,The stoichiometry and absorption spectra ofcomponents a and a-3in cytochrome c oxidase,Biochemistry,5 (1966)838–48.[2]A.V.Dyuba,A.M.Arutyunyan,T.V.Vygodina,N.V.Azarkina,A.V.Kalinovich,Y.A.Sharonov,and A.A.Konstantinov,Circular dichroism of cytochrome c oxidase,Metallomics,3(2011),417–432.doi:10.1016/j.bbabio.2014.05.171S9.P8Flavodiiron enzymes as oxygen and/or nitric oxide reductases Vera Gonçalves a,b,João B.Vicente b,c,Liliana Pinto a,Célia V.Romão a, Carlos Frazão a,Paolo Sarti d,e,f,Alessandro Giuffrèf,Miguel Teixeira a a Instituto de Tecnologia Química e Biológica António Xavier,Universidade Nova de Lisboa,Av.da República,2781–901Oeiras,Portugalb Metabolism and Genetics Group,Institute for Medicines and Pharmaceutical Sciences(iMed.UL),Faculty of Pharmacy,University of Lisbon,Av.Prof.Gama Pinto,1649–003Lisboa,Portugalc Department of Biochemistry and Human Biology,Faculty of Pharmacy, University of Lisbon,Av.Prof.Gama Pinto,1649-003Lisboa,Portugald Department of Biochemical Sciences,Sapienza University of Rome,Piazzale Aldo Moro5,I-00185Rome,Italye Fondazione Cenci Bolognetti—Istituto Pasteur,Italyf Institute of Biology,Molecular Medicine and Nanobiotechnology,National Research Council of Italy(CNR),ItalyE-mail:**************.ptThe Flavodiiron proteins(FDPs)are present in all life domains, from unicellular microbes to higher eukaryotes.FDPs reduce oxygen to water and/or nitrous oxide to nitrous oxide,actively contributing to combat the toxicity of O2or NO.The catalytic ability of FDPs is comparable to that of bonafide heme–copper/iron O2/NO transmem-brane reductases.FDPs are multi-modular water soluble enzymes, exhibiting a two-domain catalytic core,whose the minimal functional unit is a‘head-to-tail’homodimer,each monomer being built by a beta-lactamase domain harbouring a diiron catalytic site,and a short-chainflavodoxin,binding FMN[1–3].Despite extensive data collected on FDPs,the molecular determi-nants defining their substrate selectivity remain unclear.To clarify this issue,two FDPs with known and opposite substrate preferences were analysed and compared:the O2-reducing FDP from the eukaryote Entamoeba histolytica(EhFdp1)and the NO reductase FlRd from Escherichia coli.While the metal ligands are strictly conserved in these two enzymes,differences near the active site were observed.Single and double mutants of the EhFdp1were produced by replacing the residues in these positions with their equivalent in the E.coli FlRd.The biochemical and biophysical features of the EhFdp1WT and mutants were studied by potentiometric-coupled spectroscopic methods(UV–visible and EPR spectroscopies).The O2/NO reactivity was analysed by amperometric methods and stopped-flow absorption spectroscopy.The reactivity of the mutants towards O2was negatively affected, while their reactivity with NO was enhanced.These observations suggest that the residues mutated have a role in defining the substrate selectivity and reaction mechanism.References[1]C.Frazao,G.Silva,C.M.Gomes,P.Matias,R.Coelho,L.Sieker,S.Macedo,M.Y.Liu,S.Oliveira,M.Teixeira,A.V.Xavier,C.Rodrigues-Pousada,M.A.Carrondo,J.Le Gall,Structure of a dioxygen reduction enzyme from Desulfovibrio gigas,Nature Structural Biology,7(2000)1041–1045.[2]J.B.Vicente,M.A.Carrondo,M.Teixeira,C.Frazão,FlavodiironProteins:Nitric Oxide and/or Oxygen Reductases,in:Encyclopedia of Inorganic and Bioinorganic Chemistry,(2011).[3]V.L.Gonçalves,J.B.Vicente,L.M.Saraiva,M.Teixeira,FlavodiironProteins and their role in cyanobacteria,in: C.Obinger,G.A.Peschek(Eds.)Bioenergetic Processes of Cyanobacteria,Springer Verlag,(2011),pp.631–656.doi:10.1016/j.bbabio.2014.05.172S9.P9CydX is a subunit of Escherichia coli cytochrome bd terminal oxidase and essential for assembly and stability of the di-heme active siteJo Hoeser a,Gerfried Gehmann a,Robert B.Gennis b,Thorsten Friedrich ca Institut für Biochemie/Uni Freiburg,Germanyb Department of Biochemistry,University of Illinois at Urbana Champaign, USAc Albert-Ludwigs-Universitat Freiburg,GermanyE-mail:*****************.uni-freiburg.deThe cytochrome bd ubiquinol oxidase is part of many prokaryotic respiratory chains.It catalyzes the oxidation of ubiquinol to ubiqui-none while reducing molecular oxygen to water.The reaction is coupled to the vectorial transfer of1H+/e−across the membrane, contributing to the proton motive force essential for energy consum-ing processes.The presence of this terminal oxidase is known to be related to the virulence of several human pathogens,making it a very attractive drug target.The three heme groups of the oxidase are presumably located in subunit CydA.Heme b558is involved in ubiquinol oxidation,while the reduction of molecular oxygen is catalyzed by a di-nuclear heme center containing hemes b595and d [1].A severe change in Escherichia coli phenotype was noticed when a 111nt gene,denoted as cydX and located at the5′end of the cyd operon,was deleted.This small gene codes for a single transmem-brane helix obviously needed for the activity of the oxidase[2].WeAbstracts e98overproduced the terminal oxidase with and without the cydX gene product.The resulting enzyme was purified by chromatographic steps and the cofactors were spectroscopically characterized.We demon-strated that CydX tightly binds to the CydAB complex and is co-purified.The identity of CydX was determined by mass spectrometry. Additionally,the di-heme active site was only detectable in the variant containing CydX.Thus,CydX is the third subunit of the E.coli bd oxidase and is essential for the assembly and stability of the di-heme site[3].References[1]V.B.Borisov,R.B.Gennis,J.Hemp,M.I.Verkhovsky,The cytochromebd respiratory oxygen reductases,Biochim.Biophys.Acta.1807 (2011)1398–1413./10.1016/j.bbabio.2011.06.016.[2]C.E.VanOrsdel,S.Bhatt,R.J.Allen,E.P.Brenner,J.J.Hobson,A.Jamil,et al.,The Escherichia coli CydX protein is a member of the CydAB cytochrome bd oxidase complex and is required for cytochrome bd oxidase activity,J.Bacteriol.195(2013)3640–3650./10.1128/JB.00324-13.[3]J.Hoeser,S.Hong,G.Gehmann,R.B.Gennis,T.Friedrich,SubunitCydX of Escherichia coli cytochrome bd ubiquinol oxidase is essential for assembly and stability of the di-heme active site,FEBS Lett.(2014)./10.1016/j.febslet.2014.03.036.doi:10.1016/j.bbabio.2014.05.173S9.P10Characterization of the two cbb3-type cytochrome c oxidase isoforms from Pseudomonas stutzeri ZoBellMartin Kohlstaedt a,Hao Xie a,Sabine Buschmann a,Anja Resemann b, Julian nger c,Hartmut Michel ca MPI of Biophysics,Germanyb Bruker Daltonik GmbH,Germanyc Max-Planck-Institute of Biophysics,Department of Molecular Membrane Biology,GermanyE-mail:*****************************.deCytochrome c oxidases(CcOs)are the terminal enzymes of the respiratory chain and are members of the heme-copper oxidase superfamily(HCO).CcOs catalyze the reduction of molecular O2to water and couple this exergonic reaction with transmembrane proton pared to family A and B CcOs,the cbb3-type CcOs which represent the C-family,feature a distinctly different subunit composition,a reduced proton pumping stoichiometry and higher catalytic activity at low oxygen concentrations[1][2].The genome of Pseudomonas stutzeri ZoBell contains two independent cbb3-operons, encoding Cbb3-1(CcoNOP)and Cbb3-2(CcoNOQP).We generated variants with a focus on ccoQ whose function is unknown.The purified variants and the wildtype Cbb3were analyzed using UV–vis spec-troscopy,BN-and SDS-PAGE,O2reductase activity(ORA)and immunoblotting with an antibody specific for CcoQ.We found that the deletion of ccoQ has an influence on a b-type heme in the binuclear center,and that both the stability and the ORA are decreased without ccoQ compared to the WT.The O2affinity(OA)of Cbb3was spec-trophotometrically determined with oxygenated leghemoglobin as an O2delivery system.The determined Km values for the recombinant Cbb3-1are similar to previously published data[2].The Km value of rec.Cbb3-2is about2-fold higher than the value of rec.Cbb3-1.In addition,the OA and ORA of different variants introduced into the O2-cavity of rec.Cbb3-1show significant differences compared to the WT. In the structure of Cbb3,an additional transmembraneαhelix was detected but so far not assigned to any protein[3].We sequenced and identified the polypeptide chain using a customized MALDI-Tandem-MS-based setup and found a putative protein.The amino acid sequence of this proteinfits the electron density of the unknown helix and we are currently investigating the functional relevance of this protein.References[1]RS.Pitcher,NJ.Watmough The bacterial cytochrome cbb3oxidaseBiochim Biophys Acta,1655(2004),pp.388–399[2]O.Preisig,R.Zufferey,L.Thöny-Meyer,C.A.Appleby,H.HenneckeA high-affinity cbb3-type cytochrome oxidase terminates thesymbiosis-specific respiratory chain of Bradyrhizobium japonicum J.Bacteriol,178(1996),pp.1532–1538[3]S.Buschmann,E.Warkentin,H.Xie,nger,U.Ermler,H.MichelThe structure of cbb3cytochrome oxidase provides insights into proton pumping Science,329(2010),pp.327–330.doi:10.1016/j.bbabio.2014.05.174S9.P11Expression of terminal oxidases under nutrient-limited conditions in Shewanella oneidensis MR-1Sébastien Le Laz a,Arlette Kpebe b,Marielle Bauzan c,Sabrina Lignon d, Marc Rousset a,Myriam Brugna aa BIP,CNRS,Marseille,Franceb BIP,CNRS/AMU,Francec CNRS,Aix-Marseille Université,Unitéde fermentation,FR3479,IMM, Franced CNRS,Aix-Marseille Université,Plate-forme Protéomique,FR3479,IMM, MaP IBiSA,FranceE-mail:***************.frShewanella species are facultative anaerobic bacteria renowned for their remarkable respiratory versatility that allows them to use,in addition to O2,a broad spectrum of compounds as electron acceptors. In the aerobic respiratory chain,terminal oxidases catalyze the last electron transfer step by reducing molecular oxygen to water.The genome of Shewanella oneidensis MR-1encodes for three terminal oxidases:a bd-type quinol oxidase and two heme-copper oxidases, a A-type cytochrome c oxidase(Cox)and a cbb3-type oxidase.In a previous study,we investigate the role of these terminal oxidases under aerobic and microaerobic conditions in rich medium using a biochemical approach[1].Our results revealed the particularity of the aerobic respiratory pathway in S.oneidensis since the cbb3-type oxidase was the predominant oxidase under aerobic conditions while the bd-type and the cbb3-type oxidases were involved in respira-tion at low-O2tensions.Against all expectation,the low-affinity Cox oxidase had no physiological significance in our experimental conditions.Do these data reflect a functional loss of Cox resulting from evolutionary mechanisms as suggested by Zhou et al.[2]?Is Cox expressed under specific conditions like the aa3oxidase in Pseudo-monas aeruginosa,maximally expressed under starvation conditions [3]?To address these questions,we investigated the expression pattern of the terminal oxidases under nutrient-limited conditions and different dissolved O2tensions by measuring oxidase activities coupled to mass-spectrometry analysis.In addition to the notable modulation of the expression of the bd-type and cbb3-type oxidases in the different tested conditions,we detected Cox oxidase under carbon-starvation conditions.This constitutes thefirst report of a condition under which the A-type oxidase is expressed in S.oneidensis. We suggest that Cox may be crucial for energy conservation in carbon-limited environments and we propose that Cox may be a component of a general protective response against oxidative stress allowing S.oneidensis to thrive under highly aerobic habitats.Abstracts e99。
茶多酚对人脂肪来源间充质干细胞成骨分化的影响

茶多酚对人脂肪来源间充质干细胞成骨分化的影响王华1,齐玉成-杨云芳-赵艺洋2,王慧1,陈培1,杨旭芳1(1.牡丹江医学院,黑龙江牡丹江157011;2.南方医科大学第一临床医学院,广东广州510515)摘要:目的探讨茶多酚(Epigallocatechin-3-gallate,EGCG)对人脂肪间充质干细胞(human adipose-derived mesenchy^-mal stem cells,hADSCs)成骨分化的影响。
方法利用胶原酶消化法和贴壁筛选法从人脂肪组织中分离、培养及扩增hADSCs,倒置显微镜下观察各代hADSCs的形态学特点;利用流式细胞术检测各代hADSCs免疫学表型;取P3代细胞进行成骨诱导分化,实验分三组,即未诱导组、常规成骨诱导组与EGCG组(常规成骨诱导+5^mol/L EGCG),14d后,镜下观察细胞形态学改变及碱性磷酸酶(ALP)染色。
结果体外分离、培养的hADSCs形态均一;流式细胞术结果显示hADSCs具备间充质干细胞的免疫学表型,即CD44、CD73、CD105阳性;成骨诱导14d后部分细胞由长梭形变成多角形,细胞呈现聚集趋势;ALP染色显示EGCG组呈强阳性。
结论成功的从脂肪组织中分离培养出了hADSCs,EGCG能加强其成骨分化能力,这将为骨质疏松症的临床药物开发提供新的思路,亦为组织工程骨的构建提供丰富可靠的种子细胞来源。
关键词:EGCG;人脂肪来源间充质干细胞;成骨分化中图分类号:R595.2文献标识码:A文章编号:1001-7550(2021)01-0001-04Effect of EGCG on osteogenic differentiation of human adipose-derived mesenchymal stem cellsWANG Hua et al(Mudanjiang Medical University,Mudanjiang157011,China)Abstract:Objective To explore the effect of tea polyphenol EGCG on the osteogenic differentiation of human adipose-derived mesenchymal stem cells(hADSCs) .Methods To isolate,culture and amplify hADSCs from human adipose tissue by collagenase digestion and adherent screening methods, the morphological characteristics of each passage of hADSCs were observed under an inverted microscope.The immunophenotype of each generation of hADSCs was detected by flow-cytometry.P3passage cells were taken for osteogenic induction and differentiation,and were divided into three groups:non-induced group, conventional osteogenic induction group and EGCG group(conventional osteogenic induction with+5Rmol/L EGCG).After14days,morphological changes and alkaline phosphatase(ALP)staining were observed under the microscope.Results The morphology of hADSCs isolated and cultured in vitro was uni-form.The results of flow cytometry showed that hADSCs had the immunophenotype of mesenchymal stem cells,such as CD44,CD73and CD105.After14days of osteogenic induction,some cells changed from long spindle shape to polygonal shape,and the cells showed aggregation trend.ALP staining showed strong positive in EGCG group.Conclusion hADSCs have been successfully isolated and cultured from adipose tissue.EGCG can enhance the osteogenic differentiation ability of hADSCs,which will provide a new idea for the clinical drug development of osteoporosis and provide an abundant and reliable source of seed cells for the construction of tissue-engineered bone.Key words:EGCG;human adipose-derived mesenchymal stem cells;osteogenic differentiation随着人口老龄化,骨质疏松症已成为影响人们生活质量的主要因素之一⑷。
marked manuscript

Quality evaluation of Flos Lonicerae through a simultaneous determination of seven saponins by HPLC with ELSDXing-Yun Chai1, Song-Lin Li2, Ping Li1*1Key Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China2Institute of Nanjing Military Command for Drug Control, Nanjing, 210002, People’s Republic of China*Corresponding author: Ping LiKey Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing 210009, People’s Republic of China.E-mail address: lipingli@Tel.: +86-25-8324-2299; 8539-1244; 135********Fax: +86-25-8532-2747AbstractA new HPLC coupled with evaporative light scattering detection (ELSD) method has been developed for the simultaneous quantitative determination of seven major saponins, namely macranthoidinB (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)in Flos Lonicerae, a commonly used traditional Chinese medicine (TCM) herb.Simultaneous separation of these seven saponins was achieved on a C18 analytical column with a mixed mobile phase consisting of acetonitrile(A)-water(B)(29:71 v/v) acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min on linear gradient, and then keep isocratic elution with 54%B from 25 to 30min.The drift tube temperature of ELSD was set at 106℃, and with the nitrogen flow-rate of 2.6 l/min. All calibration curves showed good linear regression (r2 0.9922) within test ranges. This method showed good reproducibility for the quantification of these seven saponins in Flos Lonicerae with intra- and inter-day variations of less than 3.0% and 6.0% respectively. The validated method was successfully applied to quantify seven saponins in five sources of Flos Lonicerae, which provides a new basis of overall assessment on quality of Flos Lonicerae.Keywords: HPLC-ELSD; Flos Lonicerae; Saponins; Quantification1. IntroductionFlos Lonicerae (Jinyinhua in Chinese), the dried buds of several species of the genus Lonicera (Caprifoliaceae), is a commonly used traditional Chinese medicine (TCM) herb. It has been used for centuries in TCM practice for the treatment of sores, carbuncles, furuncles, swelling and affections caused by exopathogenic wind-heat or epidemic febrile diseases at the early stage [1]. Though four species of Lonicera are documented as the sources of Flos Lonicerae in China Pharmacopeia (2000 edition), i.e. L. japonica, L. hypoglauca,L. daystyla and L. confusa, other species such as L. similes and L. macranthoides have also been used on the same purpose in some local areas in China [2]. So it is an important issue to comprehensively evaluate the different sources of Flos Lonicerae, so as to ensure the clinical efficacy of this Chinese herbal drug.Chemical and pharmacological investigations on Flos Lonicerae resulted in discovering several kinds of bioactive components, i.e. chlorogenic acid and its analogues, flavonoids, iridoid glucosides and triterpenoid saponins [3]. Previously, chlorogenic acid has been used as the chemical marker for the quality evaluation of Flos Lonicerae,owing to its antipyretic and antibiotic property as well as its high content in the herb. But this compound is not a characteristic component of Flos Lonicerae, as it has also been used as the chemical marker for other Chinese herbal drugs such as Flos Chrysanthemi and so on[4-5]. Moreover, chlorogenic acid alone could not be responsible for the overall pharmacological activities of Flos Lonicerae[6].On the other hand, many studies revealed that triterpenoidal saponins of Flos Lonicerae possess protection effects on hepatic injury caused by Acetaminophen, Cd, and CCl4, and conspicuous depressant effects on swelling of ear croton oil [7-11]. Therefore, saponins should also be considered as one of the markers for quality control of Flos Lonicerae. Consequently, determinations of all types of components such as chlorogenic acid, flavonoids, iridoid glucosides and triterpenoidal saponins in Flos Lonicerae could be a better strategy for the comprehensive quality evaluation of Flos Lonicerae.Recently an HPLC-ELSD method has been established in our laboratory for qualitative and quantitative determination of iridoid glucosides in Flos Lonicerae [12]. But no method was reported for the determination of triterpenoidal saponins in Flos Lonicera. As a series studies on the comprehensive evaluation of Flos Lonicera, we report here, for the first time, the development of an HPLC-ELSD method for simultaneous determination of seven triterpenoidal saponins in the Chinese herbal drug Flos Lonicerae, i.e.macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) (Fig. 1).2. Experimental2.1. Samples, chemicals and reagentsFive samples of Lonicera species,L. japonica from Mi county, HeNan province (LJ1999-07), L. hypoglauca from Jiujang county, JiangXi province (LH2001-06), L. similes from Fei county, ShanDong province (LS2001-07), L. confuse from Xupu county, HuNan province (LC2001-07), and L. macranthoides from Longhu county, HuNan province (LM2000-06) respectively, were collected in China. All samples were authenticated by Dr. Ping Li, professor of department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. The voucher specimens were deposited in the department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. Seven saponin reference compounds: macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) were isolated previously from the dried buds of L. confusa by repeated silica gel, sephadex LH-20 and Rp-18 silica gel column chromatography, their structures were elucidated by comparison of their spectral data (UV, IR, MS, 1H- NMR and 13C-NMR) with references [13-15]. The purity of these saponins were determined to be more than 98% by normalization of the peak areas detected by HPLC with ELSD, and showed very stable in methanol solution.HPLC-grade acetonitrile from Merck (Darmstadt, Germany), the deionized water from Robust (Guangzhou, China), were purchased. The other solvents, purchased from Nanjing Chemical Factory (Nanjing, China) were of analytical grade.2.2. Apparatus and chromatographic conditionsAglient1100 series HPLC apparatus was used. Chromatography was carried out on an Aglient Zorbax SB-C18 column(250 4.6mm, 5.0µm)at a column temperature of 25℃.A Rheodyne 7125i sampling valve (Cotati, USA) equipped with a sample loop of 20µl was used for sample injection. The analog signal from Alltech ELSD 2000 (Alltech, Deerfield, IL, USA)was transmitted to a HP Chemstation for processing through an Agilent 35900E (Agilent Technologies, USA).The optimum resolution was obtained by using a linear gradient elution. The mobile phase was composed of acetonitrile(A) and water(B) which acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min in linear gradient, and back to the isocratic elution of 54%B from 25 to 30 min.The drift tube temperature for ELSD was set at 106℃and the nitrogen flow-rate was of 2.6 l/min. The chromatographic peaks were identified by comparing their retention time with that of each reference compound tried under the same chromatographic conditions with a series of mobile phases. In addition, spiking samples with the reference compounds further confirmed the identities of the peaks.2.3. Calibration curvesMethanol stock solutions containing seven analytes were prepared and diluted to appropriate concentration for the construction of calibration curves. Six concentrationof the seven analytes’ solution were injected in triplicate, and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analyte. The results were demonstrated in Table1.2.4. Limits of detection and quantificationMethanol stock solution containing seven reference compounds were diluted to a series of appropriate concentrations with methanol, and an aliquot of the diluted solutions were injected into HPLC for analysis.The limits of detection (LOD) and quantification (LOQ) under the present chromatographic conditions were determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. LOD and LOQ for each compound were shown in Table1.2.5. Precision and accuracyIntra- and inter-day variations were chosen to determine the precision of the developed assay. Approximate 2.0g of the pulverized samples of L. macranthoides were weighted, extracted and analyzed as described in 2.6 Sample preparation section. For intra-day variability test, the samples were analyzed in triplicate for three times within one day, while for inter-day variability test, the samples were examined in triplicate for consecutive three days. Variations were expressed by the relative standard deviations. The results were given in Table 2.Recovery test was used to evaluate the accuracy of this method. Accurate amounts of seven saponins were added to approximate 1.0g of L. macranthoides,and then extracted and analyzed as described in 2.6 Sample preparation section. The average recoveries were counted by the formula: recovery (%) = (amount found –original amount)/ amount spiked ×100%, and RSD (%) = (SD/mean) ×100%. The results were given in Table 3.2.6. Sample preparationSamples of Flos Lonicerae were dried at 50℃until constant weight. Approximate 2.0g of the pulverized samples, accurately weighed, was extracted with 60% ethanol in a flask for 4h. The ethanol was evaporated to dryness with a rotary evaporator. Residue was dissolved in water, followed by defatting with 60ml of petroleum ether for 2 times, and then the water solution was evaporated, residue was dissolved with methanol into a 25ml flask. One ml of the methanol solution was drawn and transferred to a 5ml flask, diluted to the mark with methanol. The resultant solution was at last filtrated through a 0.45µm syringe filter (Type Millex-HA, Millipore, USA) and 20µl of the filtrate was injected to HPLC system. The contents of the analytes were determined from the corresponding calibration curves.3. Results and discussionsThe temperature of drift tube and the gas flow-rate are two most important adjustable parameters for ELSD, they play a prominent role to an analyte response. In ourprevious work [12], the temperature of drift tube was optimized at 90°C for the determination of iridoids. As the polarity of saponins are higher than that of iridoids, more water was used in the mobile phase for the separation of saponins, therefore the temperature for saponins determination was optimized systematically from 95°C to 110°C, the flow-rate from 2.2 to 3.0 l/min. Dipsacoside B was selected as the testing saponin for optimizing ELSD conditions, as it was contained in all samples. Eventually, the drift tube temperature of 106℃and a gas flow of 2.6 l/min were optimized to detect the analytes. And these two exact experimental parameters should be strictly controlled in the analytical procedure [16].All calibration curves showed good linear regression (r2 0.9922) within test ranges. Validation studies of this method proved that this assay has good reproducibility. As shown in Table 2, the overall intra- and inter-day variations are less than 6% for all seven analytes. As demonstrated in Table 3, the developed analytical method has good accuracy with the overall recovery of high than 96% for the analytes concerned. The limit of detection (S/N=3) and the limit of quantification (S/N=10) are less than 0.26μg and 0.88μg respectively (Table1), indicating that this HPLC-ELSD method is precise, accurate and se nsitive enough for the quantitative evaluation of major non- chromaphoric saponins in Flos Lonicerae.It has been reported that there are two major types of saponins in Flos Lonicerae, i.e. saponins with hederagenin as aglycone and saponins with oleanolic acid as the aglycone [17]. But hederagenin type saponins of the herb were reported to have distinct activities of liver protection and anti-inflammatory [7-11]. So we adoptedseven hederagenin type saponins as representative markers to establish a quality control method.The newly established HPLC-ELSD method was applied to analyze seven analytes in five plant sources of Flos Lonicerae, i.e. L. japonica,L. hypoglauca,L. confusa,L. similes and L. macranthoides(Table 4). It was found that there were remarkable differences of seven saponins contents between different plant sources of Flos Lonicerae. All seven saponins analyzed could be detected in L. confusa and L. hypoglauca, while only dipsacoside B was detected in L. japonica. Among all seven saponins interested, only dipsacoside B was found in all five plant species of Flos Lonicerae analyzed, and this compound was determined as the major saponin with content of 53.7 mg/g in L. hypoglauca. On the other hand, macranthoidin B was found to be the major saponin with the content higher than 41.0mg/g in L. macranthoides,L. confusa, and L. similis, while the contents of other analytes were much lower.In our previous study [12], overall HPLC profiles of iridoid glucosides was used to qualitatively and quantitatively distinguish different origins of Flos Lonicerae. As shown in Fig.2, the chromatogram profiles of L. confusa, L. japonica and L. similes seem to be similar, resulting in the difficulty of clarifying the origins of Flos Lonicerae solely by HPLC profiles of saponins, in addition to the clear difference of the HPLC profiles of saponins from L. macranthoides and L. hypoglauca.Therefore, in addition to the conventional morphological and histological identification methods, the contents and the HPLC profiles of saponins and iridoids could also be used as accessory chemical evidence toclarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.4. ConclusionsThis is the first report on validation of an analytical method for qualification and quantification of saponins in Flos Lonicerae. This newly established HPLC-ELSD method can be used to simultaneously quantify seven saponins, i.e. macranthoidin B, macranthoidin A, dipsacoside B, hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester, macranthoside B, macranthoside A, and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside in Flos Lonicerae. Together with the HPLC profiles of iridoids, the HPLC-ELSD profiles of saponins could also be used as an accessory chemical evidence to clarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.AcknowledgementsThis project is financially supported by Fund for Distinguished Chinese Young Scholars of the National Science Foundation of China (30325046) and the National High Tech Program(2003AA2Z2010).[1]Ministry of Public Health of the People’s Republic of China, Pharmacopoeia ofthe People’s Republic of China, V ol.1, 2000, p. 177.[2]W. Shi, R.B. Shi, Y.R. Lu, Chin. Pharm. J., 34(1999) 724.[3]J.B. Xing, P. Li, D.L. Wen, Chin. Med. Mater., 26(2001) 457.[4]Y.Q. Zhang, L.C. Xu, L.P. Wang, J. Chin. Med. Mater., 21(1996) 204.[5] D. Zhang, Z.W. Li, Y. Jiang, J. Pharm. Anal., 16(1996) 83.[6]T.Z. Wang, Y.M. Li, Huaxiyaoxue Zazhi, 15(2000) 292.[7]J.ZH. Shi, G.T. Liu. Acta Pharm. Sin., 30(1995) 311.[8]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 209.[9]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 213.[10]J.ZH. Shi, L. Wan, X.F. Chen.ZhongYao YaoLi Yu LinChuang, 6 (1990) 33.[11]J. Liu, L. Xia, X.F. Chen. Acta Pharmacol. Sin., 9 (1988) 395[12]H.J. Li, P. Li, W.C. Ye, J. Chromatogr. A 1008(2003) 167-72.[13]Q. Mao, D. Cao, X.SH. Jia. Acta Pharm. Sin., 28(1993) 273.[14]H. Kizu, S. Hirabayashi, M. Suzuki, et al. Chem. Pharm. Bull., 33(1985) 3473.[15]S. Saito, S. Sumita, N. Tamura, et al. Chem Pharm Bull., 38(1990) 411.[16]Alltech ELSD 2000 Operating Manual, Alltech, 2001, p. 16. In Chinese.[17]J.B. Xing, P. Li, Chin. Med. Mater., 22(1999) 366.Fig. 1 Chemical structures of seven saponins from Lonicera confusa macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)Fig. 2Representative HPLC chromatograms of mixed standards and methanol extracts of Flos Lonicerae.Column: Agilent Zorbax SB-C18 column(250 4.6mm, 5.0µm), temperature of 25℃; Detector: ELSD, drift tube temperature 106℃, nitrogen flow-rate 2.6 l/min.A: Mixed standards, B: L. confusa, C: L. japonica, D: L. macranthoides, E: L. hypoglauca, F: L. similes.Table 1 Calibration curves for seven saponinsAnalytes Calibration curve ar2Test range(μg)LOD(μg)LOQ(μg)1 y=6711.9x-377.6 0.9940 0.56–22.01 0.26 0.882 y=7812.6x-411.9 0.9922 0.54–21.63 0.26 0.843 y=6798.5x-299.0 0.9958 0.46–18.42 0.22 0.724 y=12805x-487.9 0.9961 0.38–15.66 0.10 0.345 y=4143.8x-88.62 0.9989 0.42–16.82 0.18 0.246 y=3946.8x-94.4 0.9977 0.40–16.02 0.16 0.207 y=4287.8x-95.2 0.9982 0.42–16.46 0.12 0.22a y: Peak area; x: concentration (mg/ml)Table 2 Reproducibility of the assayAnalyteIntra-day variability Inter-day variability Content (mg/g) Mean RSD (%) Content (mg/g) Mean RSD (%)1 46.1646.2846.2246.22 0.1346.2245.3647.4226.33 2.232 5.385.385.165.31 2.405.285.345.045.22 3.043 4.374.304.184.28 2.244.284.464.024.255.204 nd1)-- -- nd -- --5 1.761.801.821.79 1.701.801.681.841.77 4.706 1.281.241.221.252.451.241.341.201.26 5.727 tr2)-- -- tr -- -- 1): not detected; 2): trace. RSD (%) = (SD/Mean) ×100%Table 3 Recovery of the seven analytesAnalyteOriginal(mg) Spiked(mg)Found(mg)Recovery(%)Mean(%)RSD(%)1 23.0823.1423.1119.7122.8628.1042.7346.1351.0199.7100.699.399.8 0.722.692.672.582.082.913.164.735.515.7698.197.6100.698.8 1.632.172.152.091.732.182.623.884.404.6598.8103.297.799.9 2.94nd1)1.011.050.980.981.101.0297.0104.8104.1102.0 4.250.880.900.910.700.871.081.561.752.0197.197.7101.898.9 2.660.640.620.610.450.610.751.081.211.3397.796.796.096.8 0.97tr2)1.021.101.081.031.111.07100.9102.799.1100.9 1.81): not detected; 2): trace.a Recovery (%) = (Amount found –Original amount)/ Amount spiked ×100%, RSD (%) = (SD/Mean) ×100%Table 4 Contents of seven saponins in Lonicera spp.Content (mg/g)1 2 3 4 5 6 7 L. confusa45.65±0.32 5.13±0.08 4.45±0.11tr1) 2.04±0.04tr 1.81±0.03 L. japonica nd2)nd 3.44±0.09nd nd nd nd L. macranthoides46.22±0.06 5.31±0.13 4.28±0.10 tr 1.79±0.03 1.25±0.03 tr L. hypoglauca11.17±0.07 nq3)53.78±1.18nd 1.72±0.02 2.23±0.06 2.52±0.04 L. similes41.22±0.25 4.57±0.07 3.79±0.09nd 1.75±0.02tr nd 1): trace; 2): not detected.. 3) not quantified owing to the suspicious purity of the peak.。
微生物代谢产物在肠-脑轴中作用机制研究进展

与 SCFA 一样,BA 也可 以 作 为 信 号 分 子 激 活 法 尼 醇 X 受体(FXR)、G 蛋白偶联胆汁酸受体5(TGR5)、孕 烷 X 受 体 (PXR)及维生素 D 受体(VDR)等。 通 过 激 活 这 些 受 体,BA 控制葡萄糖稳态、脂 质 代 谢 和 能 量 消 耗 等,对 宿 主 新 陈 代 谢 有显著影响。微生物群功能的变化 可 以 改 变 BA 池 的 组 成, 并 改 变 其 整 体 信 号 传 导 能 力 。 [27] 已 经 在 人 类 和 啮 齿 类 动 物 的大脑中检测到 BA,并且它们的受体和转运蛋白在 CNS的 细 胞 中 表 达 。 [28-29] 这 表 明 BA 可 能 在 CNS 中 起 信 号 传 导 作 用。虽然目前对这种信号传导潜能的了解有限,但在 小 鼠 中 发现 FXR 缺失扰乱了多种神经递质系统,并改变 了 情 感、认
SCFA 可以通过刺 激 肠 内 分 泌 细 胞 释 放 肠 道 激 素 和 肽 类来间接调节 GBA。SCFA 还可通过刺激 胰 高 血 糖 素 样 肽1(GLP-1)、肽 YY(PYY)和瘦素等厌食激素的分泌来调 节 摄 食 行 为 。 [11,17-19] 这 些 食 欲 激 素 除 了 可 以 作 用 于 大 脑 中 的 受 体,还可 以 作 用 于 迷 走 神 经。GOSWAMI等 研 [20] 究 证 明 了 迷走神经在肠道微生物控制食欲中的作用,其中 SCFA 的 厌 食效应在迷走 神 经 切 断 的 小 鼠 中 明 显 降 低。SCFA 也 可 以 通过中枢机制参与食欲调节。 肠 源 性 乙 酸 盐 可 以 穿 过 血-脑 脊液屏障,通过改变神经肽的表达对下丘脑控制食欲 有 直 接 影 响 。 [21]
Calcipotriol_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-13-2017Print Date:Sep.-13-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CalcipotriolCatalog No. :HY-10001CAS No. :112965-21-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MC 903; CalcipotrieneFormula:C27H40O3Molecular Weight:412.60CAS No. :112965-21-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, protect from light, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
LCMS检测西他沙星原料中基因毒性杂质的含量

LC-MS检测西他沙星原料中基因毒性杂质的含量石莹1宋雪洁3李浩冬2路显锋2*1药物研究院分析所,扬子江药业集团,泰州2253212药物制剂新技术国家重点实验室,扬子江药业集团,泰州2253213质量管理部,扬子江药业集团,泰州225321摘要建立了LC-MS 法测定西他沙星中基因毒性杂质对甲苯磺酸甲酯和对甲苯磺酸乙酯含量的方法。
方法:采用Agilent Poroshell 120 EC-C18色谱柱;流动相为纯水(0.1%甲酸):甲醇(V/V)=60:40;稀释剂为乙腈(0.1%甲酸):纯水(V/V)=50:10;柱温为40℃;进样体积为5µl;流速为0.4ml/min;采用正离子模式进行扫描。
对甲苯磺酸甲酯测定浓度在0.76ng/ml~15.27ng/ml范围内,线性关系良好;对甲苯磺酸乙酯测定浓度在0.75ng/ml~15.01ng/ml范围内,线性关系良好。
对甲苯磺酸甲酯的定量限为0.0038ng;对甲苯磺酸乙酯的定量限为0.0038ng。
杂质回收率在限度浓度80%、100%和160%三个浓度水平均在90~110%之间,该方法准确度良好。
该方法适用于西他沙星原料中对甲苯磺酸甲酯和对甲苯磺酸乙酯的检测。
西他沙星(sitafloxacin)是日本第一制药有限公司继左氧氟沙星后开发出的一种强力广谱新氟喹诺酮类抗菌剂,该药对革兰氏阳性球菌,革兰氏阴性菌以及厌氧菌的抗菌活性是左氧氟沙星的4~32倍,同时对肺炎球菌DNA 促旋酶和拓扑同功酶有双重抑制作用。
临床表现有极广的抗菌谱,特别是对呼吸道的病菌有极强的抗菌活性。
因西他沙星的一个起始物料为对甲苯磺酸盐,在后续反应中对甲苯磺酸若有残留,可能会与溶剂甲醇、乙醇反应生成具有基因毒性的杂质—对甲苯磺酸甲酯和对甲苯磺酸乙酯,故采用LC-MS法对产品中的对甲苯磺酸甲酯/乙酯进行控制。
1、实验部分1.1仪器与试药Agilent 1200液相色谱仪(美国安捷伦公司);Agilent 6460三重串联四极杆质谱仪(美国安捷伦公司);XP205型电子天平(瑞士梅特勒托利多公司)。
毕赤酵母表达手册

Pichia Expression KitVersion M01110225-0043Pichia Expression KitA Manual of Methods for Expression of Recombinant Proteins in Pichia pastorisCatalog no. K1710-01tech_service@iiINDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTThe Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris was developed into an expression system by scientists at Salk Institute Biotechnology/Industry Associates (SIBIA) for high-level expression of recombinant proteins. All patents for Pichia pastoris and licenses for its use as an expression system are owned by Research Corporation Technologies, Inc. Tucson, Arizona. Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial corporations requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license a greement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this Agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.INDIVIDUAL PICHIA EXPRESSION KIT LICENSE AGREEMENTInvitrogen Corporation (INVITROGEN) grants you a non-exclusive license to use the enclosed Pichia Expression Kit (EXPRESSION KIT) for academic research or for evaluation purposes only. The EXPRESSION KIT is being transferred to you in furtherance of, and reliance on, such license. You may not use the EXPRESSION KIT, or the materials contained therein, for any commercial purpose without a license for such purpose from RESEARCH CORPORATION TECHNOLOGIES, INC., Tucson, Arizona. Commercial purposes include the use in or sale of expressed proteins as a commercial product, or use to facilitate or advance research or development of a commercial product. Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Commercial entities will be contacted by Research Corporation Technologies during the evaluation period regarding the purchase of a commercial license.Access to the EXPRESSION KIT must be limited solely to those officers, employees and students of your institution who need access thereto in order to perform the above-described research or evaluation. You must inform each of such officer, employee and student of the provisions of this Agreement and require them to agree, in writing, to be bound by the provisions of this Agreement. You may not distribute the EXPRESSION KIT to others, even those within your own institution. You may transfer modified, altered or original material from the EXPRESSION KIT to a third party following notification of INVITROGEN such that the recipient can be licensed. You may not assign, sub-license, rent lease or otherwise transfer this License or any of the rights or obligation hereunder, except as expressly permitted.This License is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate automatically if you fail to comply with the terms and conditions of the Agreement. You shall, upon termination of the License, destroy all Pichia Expression Kits in your control, and so notify INVITROGEN in writing.This License Shall be governed in its interpretation and enforcement by the laws of the State of California.Product User Registration CardPlease complete and return the enclosed Product User Registration Card for each Pichia Expression Kit that you purchase. This will serve as a record of your purchase and registration and will allow Invitrogen to provide you with technical support and manual updates. It will also allow Invitrogen to update you on future developments of and improvements to the Pichia Expression Kit. The agreement outlined above becomes effective upon our receipt of your User Registration Card or 10 days following the sale of the Pichia Expression Kit to you. Use of the kit at any time results in immediate obligation to the terms and conditions stated in this Agreement.Technical ServicesInvitrogen provides Technical Services to all of our registered Pichia Expression Kit users. Please contact us if you need assistance with the Pichia Expression Kit.United States Headquarters:Japanese Headquarters European Headquarters:Invitrogen Corporation1600 Faraday AvenueCarlsbad, CA 92008 USATel: 1 760 603 7200Tel (Toll Free): 1 800 955 6288 Fax: 1 760 602 6500E-mail:tech_service@ Invitrogen Japan K.K.Nihonbashi Hama-Cho Park Bldg. 4F2-35-4, Hama-Cho, NihonbashiTel: 81 3 3663 7972Fax: 81 3 3663 8242E-mail: jpinfo@Invitrogen Ltd3 Fountain DriveInchinnan Business ParkPaisley PA4 9RF, UKTel (Free Phone Orders): 0800 269 210Tel (General Enquiries): 0800 5345 5345Fax: +44 (0) 141 814 6287E-mail: eurotech@iiiivTable of ContentsMaterials (vii)Purchaser Notification (x)Product Qualification (xii)Introduction (1)Overview (1)Experimental Outline (3)Recombination and Integration in Pichia (7)Methods (11)Pichia Strains (11)E. coli Strains (13)Selecting a Pichia Expression Vector (14)pHIL-D2 (16)pPIC3.5 (17)pHIL-S1 (18)pPIC9 (19)Signal Sequence Processing (20)Cloning into the Pichia Expression Vectors (21)Transformation into E. coli (26)Preparation of Transforming DNA (27)Growth of Pichia for Spheroplasting (30)Preparation of Spheroplasts (32)Transformation of Pichia (34)Screening for Mut+ and Mut S Transformants (36)PCR Analysis of Pichia Integrants (40)Expression of Recombinant Pichia Strains (42)Analysis by SDS-Polyacrylamide Gel Electrophoresis (45)Optimization of Pichia Protein Expression (47)Scale-up of Expression (49)Protein Purification and Glycosylation (51)Recipes (53)E. coli Media Recipes (53)Pichia Media Recipes (54)Appendix (59)Electroporation of Pichia (59)PEG 1000 Transformation Method for Pichia (60)Lithium Chloride Transformation Method (61)Total DNA Isolation from Pichia (62)Detection of Multiple Integration Events (63)Procedure for Total RNA Isolation from Pichia (64)β-Galactosidase Assay (65)Technical Service (67)References (69)vviMaterialsKit Contents Box 1: Spheroplast Module. Store at room temperature.Reagent Amount ComponentsSOS media 20 ml 1 M Sorbitol0.3X YPD10 mM CaCl2Sterile Water 2 x 125 ml Autoclaved, deionized waterSE 2 x 125 ml 1 M Sorbitol25 mM EDTA, pH 8.0SCE 2 x 125 ml 1 M Sorbitol10 mM Sodium citrate buffer, pH 5.81 mM EDTA1 M Sorbitol2 x 125 ml --CaS 2 x 60 ml 1 M Sorbitol10 mM Tris-HCl, pH 7.5;10 mM CaCl240% PEG 25 ml 40% (w/v) PEG 3350 (Reagent grade) in waterCaT 25 ml 20 mM Tris-HCl, pH 7.520 mM CaCl2Stab Vials: Pichia and E. coli stabs. Store at +4°C.Phenotype(Pichia only)GenotypeStrain Amountstab his4Mut+GS115 1stab arg4 his4 aox1::ARG4 Mut S, Arg+KM71 1GS115 Albumin 1 stab HIS4Mut SGS115 β-Gal 1 stab HIS4Mut+stab F´ {pro AB, lac I q, lac Z∆M15, Tn10 (Tet R)} mcr A,TOP10F´ 1∆(mrr-hsd RMS-mcr BC), φ80lac Z∆M15, ∆lac X74,deo R, rec A1, ara D139, ∆(ara-leu)7697, gal U,gal K, rps L (Str R), end A1, nup G λ-.Box 2: Spheroplast Module. Store at -20°C.ComponentsReagent AmountZymolyase 10 x 20 µl 3 mg/ml Zymolyase in water(100,000 units/g lytic activity)1 M DTT 10 x 1 ml 1 M dithiothreitol in watercontinued on next pageviiKit Contents,continuedVector Box. Store at -20°C.Reagent DescriptionpHIL-D210 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapPIC3.510 µg, lyophilized in TE, pH 8.0Vector for intracellular expression in PichiapHIL-S110 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the PHO1 signal sequencepPIC910 µg, lyophilized in TE, pH 8.0 Vector for secreted expression in Pichia. Uses the α-factor signal sequencePrimer Box. Store at -20°C.5´ AOX1 sequencing primer2 µg (312 pmoles), lyophilized5´-GACTGGTTCCAATTGACAAGC-3´3´ AOX1 sequencing primer2 µg (314 pmoles), lyophilized5´-GCAAATGGCATTCTGACATCC-3´α-Factor sequencing primer2 µg (315 pmoles), lyophilized5´-TACTATTGCCAGCATTGCTGC-3´Media The following prepackaged media is included for your convenience. Instructions for use are provided on the package.Media Amount Yield YP Base Medium 2 pouches 2 liters of YP mediumYP Base Agar Medium 2 pouches 2 liters of YP mediumYeast Nitrogen Base 1 pouch 500 ml of 10X YNBFor transformation of Pichia by spheroplasting, the Pichia Spheroplast Module isavailable separately from Invitrogen (see below for ordering information).Product Reactions or Amount Catalog no.Pichia Spheroplast Module 10 spheroplast preparations(50 transformations)K1720-01continued on next pageviiiRequired Equip-ment and Supplies (not provided) • 30°C rotary shaking incubator• Water baths capable of 37°C, 45°C, and 100°C• Centrifuge suitable for 50 ml conical tubes (floor or table-top)• Baffled culture flasks with metal covers (50 ml, 250 ml, 500 ml, 1000 ml, and 3 L)• 50 ml sterile, conical tubes• 6 ml and 15 ml sterile snap-top tubes (Falcon 2059 or similar)• UVSpectrophotometer• Mini agarose gel apparatus and buffers• Polyacrylamide Gel Electrophoresis apparatus and buffers• Media for transformation, growth, screening, and expression (see Recipes, pages 53-58) • 5% SDS solution (10 ml per transformation)• Sterile cheesecloth or gauze• Breaking Buffer (see Recipes, page 58)• Acid-washed glass beads (available from Sigma)• Replica-plating equipment (optional)• BeadBreaker™ (optional)ixPurchaser NotificationIntroduction The Pichia Expression Kit is based on the yeast Pichia pastoris. Pichia pastoris wasdeveloped into an expression system by scientists at Salk Institute Biotechnology/ IndustryAssociates (SIBIA) and Phillips Petroleum for high-level expression of recombinantproteins. All patents for Pichia pastoris and licenses for its use as an expression system areowned by Research Corporation Technologies (RCT), Inc., Tucson, Arizona. Forinformation on commercial licenses, please see page x.The Nature of the Invitrogen License Invitrogen has an exclusive license to sell the Pichia Expression Kit to scientists for research purposes only, under the terms described below. Use of Pichia pastoris by commercial entities for any commercial purpose requires the user to obtain a commercial license as detailed below. Before using the Pichia Expression Kit, please read the following license agreement. If you do not agree to be bound by its terms, contact Invitrogen within 10 days for authorization to return the unused Pichia Expression Kit and to receive a full credit. If you do agree to the terms of this license agreement, please complete the User Registration Card and return it to Invitrogen before using the kit.Pichia pastoris Patents Pichia pastoris is covered by one or more of the following U.S. patents and corresponding foreign patents owned and licensed by Research Corporation Technologies:4,683,293 4,808,537 4,812,405 4,818,700 4,837,148 4,855,231 4,857,467 4,879,231 4,882,279 4,885,242 4,895,800 4,929,555 5,002,876 5,004,688 5,032,516 5,122,465 5,135,868 5,166,329Individual Pichia Expression Kit License Agreement Invitrogen Corporation ("Invitrogen") grants you a non-exclusive license to use the enclosed Pichia Expression Kit ("Expression Kit") for academic research or for evaluation purposes only. The Expression Kit is being transferred to you in furtherance of, and reliance on, such license. You may not use the Expression Kit, or the materials contained therein, for any commercial purpose without a license for such purpose from Research Corporation Technologies, Inc., Tucson, Arizona.Definition of Commercial Purpose Commercial purposes include:(a) any use of Expression Products in a Commercial Product(b) any use of Expression Products in the manufacture of a Commercial Product(c) any sale of Expression Products(d) any use of Expression Products or the Expression Kit to facilitate or advanceresearch or development of a Commercial Product(e) any use of Expression Products or the Expression Kit to facilitate or advance anyresearch or development program the results of which will be applied to thedevelopment of Commercial Products"Expression Products" means products expressed with the Expression Kit, or with the use of any vectors or host strains in the Expression Kit. "Commercial Product" means any product intended for sale or commercial use.Commercial entities may conduct their evaluation for one year at which time this license automatically terminates. Research Corporation Technologies will contact commercial entities during the evaluation period regarding their desire for a commercial license.continued on next pagexPurchaser Notification, continuedIndividual Responsibilities Access to the Expression Kit must be limited solely to those officers, employees and students of your institution who need access to perform the above-described research or evaluation. You must inform each such officer, employee and student of the provisions of this license agreement and require them to agree, in writing, to be bound by the provisions of this license agreement. You may not distribute neither the Expression Kit nor the vectors or host strains contained in it to others, even to those within your own institution. You may only transfer modified, altered, or original material from the Expression Kit to a third party following written notification of, and written approval from, Invitrogen so that the recipient can be licensed. You may not assign, sub-license, rent, lease or otherwise transfer this license agreement or any of the rights or obligation thereunder, except as expressly permitted by Invitrogen and RCT.Termination of License This license agreement is effective until terminated. You may terminate it at any time by destroying all Pichia expression products in your control. It will also terminate auto-matically if you fail to comply with the terms and conditions of the license agreement. You shall, upon termination of the license agreement, destroy all Pichia Expression Kits in your control, and so notify Invitrogen in writing.This License shall be governed in its interpretation and enforcement by the laws of the State of California.Contact for Commercial Licensing Bennett Cohen, Ph.D.Research Corporation Technologies 101 North Wilmot Road, Suite 600 Tucson, Arizona 85711-3335 Phone: (520) 748-4400Fax: (520)748-0025User Registration Card Please complete and return the enclosed User Registration Card for each PichiaExpression Kit that you purchase. This will serve as a record of your purchase and regis-tration and will allow Invitrogen to provide you with technical support and manualupdates. It will also allow Invitrogen to update you on future developments and improve-ments to the Pichia Expression Kit. The agreement outlined above becomes effectiveupon our receipt of your User Registration Card or 10 days following the sale of thePichia Expression Kit to you. Use of the kit at any time results in immediate obligation tothe terms and conditions stated in this license agreement.xiProduct QualificationIntroduction This section describes the criteria used to qualify the components in the PichiaExpression Kit.Vectors All expression vectors are qualified by restriction enzyme digestion. Restriction digests must demonstrate the correct banding pattern when electrophoresed on an agarose gel.Spheroplast Reagents The spheroplast reagents are qualified by spheroplast preparation of GS115 following the protocol provided in the Pichia Expression Kit manual. At least 70% of the Pichia pastoris cells must form spheroplasts in 30 minutes or less.Pichia Strains The Pichia strains are by demonstrating viability of the culture. Single colonies should arise within 48 hours after streaking on YPD medium from the stabPrimers Sequencing primers are lot tested by automated DNA sequencing experiments.Buffers andSolutionsAll buffers and solutions are extensively tested for sterility.Media All Pichia growth and expression media are qualified by growing the GS115 Pichiastrain.xiiIntroductionOverviewReview Articles The information presented here is designed to give you a concise overview of the Pichia pastoris expression system. It is by no means exhaustive. For further information, pleaseread the articles cited in the text along with recent review articles (Buckholz and Gleeson,1991; Cregg et al., 1993; Sreekrishna et al., 1988; Wegner, 1990). A general review offoreign gene expression in yeast is also available (Romanos et al., 1992).General Characteristics of Pichia pastoris As a eukaryote, Pichia pastoris has many of the advantages of higher eukaryotic expression systems such as protein processing, protein folding, and posttranslational modification, while being as easy to manipulate as E. coli or Saccharomyces cerevisiae. It is faster, easier, and less expensive to use than other eukaryotic expression systems such as baculovirus or mammalian tissue culture, and generally gives higher expression levels. As a yeast, it shares the advantages of molecular and genetic manipulations with Saccharomyces, and has the added advantage of 10- to 100-fold higher heterologous protein expression levels. These features make Pichia very useful as a protein expression system.Similarity to Saccharomyces Many of the techniques developed for Saccharomyces may be applied to Pichia including: • transformation by complementation• genedisruption• genereplacementIn addition, the genetic nomenclature used for Saccharomyces has been applied to Pichia. For example, the HIS4 gene in both Saccharomyces and Pichia encodes histidinol dehydrogenase. There is also cross-complementation between gene products in both Saccharomyces and Pichia. Several wild-type genes from Saccharomyces complement comparable mutant genes in Pichia. Genes such as HIS4, LEU2, ARG4, TRP1, and URA3 all complement their respective mutant genes in Pichia.Pichia pastoris as a Methylotrophic Yeast Pichia pastoris is a methylotrophic yeast, capable of metabolizing methanol as its sole carbon source. The first step in the metabolism of methanol is the oxidation of methanol to formaldehyde using molecular oxygen by the enzyme alcohol oxidase. This reaction generates both formaldehyde and hydrogen peroxide. To avoid hydrogen peroxide toxicity, methanol metabolism takes place within a specialized cell organelle called the peroxisome, which sequesters toxic by-products from the rest of the cell. Alcohol oxidase has a poor affinity for O2, and Pichia pastoris compensates by generating large amounts of the enzyme. The promoter regulating the production of alcohol oxidase drives heterologous protein expression in Pichia.Two Alcohol Oxidase Proteins The AOX1 and AOX2 genes code for alcohol oxidase in Pichia pastoris. The AOX1 gene product accounts for the majority of alcohol oxidase activity in the cell. Expression of the AOX1 gene is tightly regulated and induced by methanol to high levels, typically > 30% ofthe total soluble protein in cells grown with methanol as the carbon source. The AOX1 gene has been isolated and the AOX1 promoter is used to drive expression of the gene of interest (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a). While AOX2 is about 97% homologous to AOX1, growth on methanol is much slower than with AOX1. This slowgrowth allows isolation of Mut S strains (aox1) (Cregg et al., 1989; Koutz et al., 1989).continued on next page1Overview, continuedExpression Expression of the AOX1 gene is controlled at the level of transcription. In methanol-grown cells approximately 5% of the polyA+ RNA is from the AOX1 gene. The regulation of theAOX1 gene is a two step process: a repression/derepression mechanism plus an inductionmechanism (e.g. GAL1 gene in Saccharomyces (Johnston, 1987)). Briefly, growth onglucose represses transcription, even in the presence of the inducer methanol. For thisreason, growth on glycerol is recommended for optimal induction with methanol. Pleasenote that growth on glycerol (derepression) is not sufficient to generate even minute levelsof expression from the AOX1 gene. The inducer, methanol, is necessary for detectablelevels of AOX1 expression (Ellis et al., 1985; Koutz et al., 1989; Tschopp et al., 1987a).Phenotype of aox1 mutants Loss of the AOX1 gene, and thus a loss of most of the cell's alcohol oxidase activity, results in a strain that is phenotypically Mut S (Methanol utilization slow). This has in the past been referred to as Mut. The Mut S designation has been chosen to accurately describe the phenotype of these mutants. This results in a reduction in the cells' ability to metabolize methanol. The cells, therefore, exhibit poor growth on methanol medium. Mut+ (Methanol utilization plus) refers to the wild type ability of strains to metabolize methanol as the sole carbon source. These two phenotypes are used when evaluating Pichia transformants for integration of your gene (Experimental Outline, page 3).Intracellular and Secretory Protein Expression Heterologous expression in Pichia can be either intracellular or secreted. Secretion requires the presence of a signal sequence on the expressed protein to target it to the secretory pathway. While several different secretion signal sequences have been used successfully, including the native secretion signal present on some heterologous proteins, success has been variable. The secretion signal sequence from the Saccharomyces cerevisiaeα factor prepro peptide has been used most successfully (Cregg et al., 1993; Scorer et al., 1993).The major advantage of expressing heterologous proteins as secreted proteins is that Pichia pastoris secretes very low levels of native proteins. That, combined with the very low amount of protein in the minimal Pichia growth medium, means that the secreted heterologous protein comprises the vast majority of the total protein in the medium and serves as the first step in purification of the protein (Barr et al., 1992). Note: If there are recognized glycosylation sites (Asn-X-Ser/Thr) in your protein's primary sequence, glycosylation may occur at these sites.Posttranslational Modifications In comparison to Saccharomyces cerevisiae, Pichia may have an advantage in the glyco-sylation of secreted proteins because it may not hyperglycosylate. Both Saccharomyces cerevisiae and Pichia pastoris have a majority of N-linked glycosylation of the high-mannose type; however, the length of the oligosaccharide chains added posttranslationally to proteins in Pichia (average 8-14 mannose residues per side chain) is much shorter than those in S. cerevisiae (50-150 mannose residues) (Grinna and Tschopp, 1989; Tschopp et al., 1987b). Very little O-linked glycosylation has been observed in Pichia.In addition, Saccharomyces cerevisiae core oligosaccharides have terminal α1,3 glycan linkages whereas Pichia pastoris does not. It is believed that the α1,3 glycan linkages in glycosylated proteins produced from Saccharomyces cerevisiae are primarily responsible for the hyper-antigenic nature of these proteins making them particularly unsuitable for therapeutic use. Although not proven, this is predicted to be less of a problem for glycoproteins generated in Pichia pastoris, because it may resemble the glycoprotein structure of higher eukaryotes (Cregg et al., 1993).2Experimental OutlineSelection of Vector and Cloning To utilize the strong, highly inducible P AOX1 promoter for expression of your protein, four expression vectors are included in this kit. pHIL-D2 and pPIC3.5 are used for intracellular expression while pHIL-S1 and pPIC9 are used for secreted expression (see pages 14-19 for more information). Before cloning your insert, you must...• decide whether you want intracellular or secreted expression.• analyze your insert for the following restriction sites: Sac I, Stu I, Sal I, Not I, and Bgl II. These sites are recommended for linearizing your construct prior to Pichiatransformation. If your insert has all of these sites, see pages 28-29 for alternate sites.Transformation and IntegrationTwo different phenotypic classes of His+ recombinant strains can be generated: Mut+ and Mut S. Mut S refers to the "Methanol utilization slow" phenotype caused by the loss of alcohol oxidase activity encoded by the AOX1 gene. A strain with a Mut S phenotype has a mutant aox1 locus, but is wild type for AOX2. This results in a slow growth phenotype on methanol medium. Transformation of strain GS115 can yield both classes of transformants, His+ Mut+ and His+Mut S, while KM71 yields only His+ Mut S since the strain itself is Mut S. Both Mut+ and Mut S recombinants are useful to have as one phenotype may favor better expression of your protein than the other. Due to clonal variation, you should test 6-10 recombinants per phenotype. There is no way to predict beforehand which construct or isolate will better express your protein. We strongly recommend that you analyze Pichia recombinants by PCR to confirm integration of your construct (see page 40).Once you have successfully cloned your gene, you will then linearize your plasmid to stimulate recombination when the plasmid is transformed into Pichia. The table below describes the types of recombinants you will get by selective digestion of your plasmid. RestrictionEnzymeIntegration Event GS115 Phenotype KM71 PhenotypeSal I or Stu I Insertion at his4His+ Mut+ His+ Mut SSac I Insertion at 5´AOX1 regionHis+ Mut+ His+ Mut SNot I or Bgl II Replacement atAOX1 locusHis+ Mut SHis+ Mut+His+ Mut S (notrecommended, see page 11)Expression and Scale-up After confirming your Pichia recombinants by PCR, you will test expression of both His+Mut+ and His+ Mut S recombinants. This will involve growing a small culture of each recombinant, inducing with methanol, and taking time points. If looking for intracellular expression, analyze the cell pellet from each time point by SDS polyacrylamide gel electrophoresis (SDS-PAGE). If looking for secreted expression, analyze both the cellpellet and supernatant from each time point. We recommend that you analyze your SDS-PAGE gels by both Coomassie staining and Western blot, if you have an antibody to your protein. We also suggest checking for protein activity by assay, if one is available. Not all proteins express to the level of grams per liter, so it is advisable to check by Western blotor activity assay, and not just by Coomassie staining of SDS-PAGE gels for production of your protein.Choose the Pichia recombinant strain that best expresses your protein and optimizeinduction based on the suggestions on pages 47-48. Once expression is optimized, scale-up your expression protocol to produce more protein.continued on next page3。
罗氏(英文版)-TUNEL-细胞凋亡原位检测试剂盒-POD

In Situ Cell Death Detection Kit, POD
y Version 14
Content version: July 2012
Kit for immunohistochemical detection and quantification of apoptosis (programmed cell death) at single cell level, based on labeling of DNA strand breaks (TUNEL technology): Analysis by light microscopy.
Cat. No. 11 684 817 910
Store the kit at Ϫ15 to Ϫ25°C
1 Kit (50 tests)
1. 1.1 1. 1.1 1.2 2. 2.1 2.2 3. 3.1 3.2
Preface Table of contents Preface .............................................................................................................................2 Table of contents ..................................................................................................................................... 2 Kit contents ................................................................................................................................................ 3 Introduction .....................................................................................................................5 Product overview ..................................................................................................................................... 5 Background information ....................................................................................................................... 8 Procedures and required materials ...........................................................................9 Flow chart .................................................................................................................................................10 Preparation of sample material ........................................................................................................10 3.2.1 Adherent cells, cell smears and cytospin preparations ..............................................11 3.2.2 Tissue sections ...........................................................................................................................11 3.2.2.1 Treatment of paraffin-embedded tissue ............................................................11 3.2.2.2 Treatment of cryopreserved tissue ......................................................................12 Labeling protocol ...................................................................................................................................13 3.3.1 Before you begin .......................................................................................................................13 3.3.2 Labeling protocol for adherent cells, cell smears, cytospin preparations and tissues ........................................................................................................14 3.3.3 Labeling protocol for difficult tissue ..................................................................................15 Signal conversion ..................................................................................................................................16 Appendix ....................................................................................................................... 17 Troubleshooting .....................................................................................................................................17 References ...............................................................................................................................................20 Ordering guide .......................................................................................................................................21
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
索莱宝原果胶含量检测试剂盒说明书(微量法)

原果胶含量检测试剂盒说明书微量法货号:BC3685规格:100T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液一液体100 mL×1瓶(自备)4℃保存提取液二液体50 mL×1瓶4℃保存提取液三液体70 mL×1瓶4℃保存试剂一液体30 mL×1瓶(自备)4℃保存试剂二液体3 mL×1瓶4℃保存试剂三液体5 mL×1瓶4℃保存标准品粉剂×1支4℃保存溶液的配制:1、提取液一:80%乙醇,自备。
即将80 mL 无水乙醇和20 mL 蒸馏水混合。
2、试剂一:浓硫酸30 mL ,自备。
3、标准品:10 mg 半乳糖醛酸,临用前加入0.943 mL 提取液三,配成50μmol/mL 的标准液。
产品说明:果胶是植物细胞壁主要组成成分之一,分为水溶性果胶和不溶性果胶,不溶性果胶为原果胶。
原果胶是不溶于水的物质,但可在酸、碱、盐等化学试剂及酶的作用下,加水分解转变成水溶性果胶,在食品、纺织、印染、烟草、冶金等领域具有较广泛的应用。
原果胶在碱性条件下水解为可溶性果胶,并进一步转化为半乳糖醛酸,产物在强酸中与咔唑缩合生成紫红色化合物,在530nm 处有特征吸收峰。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、台式离心机、水浴锅、微量玻璃比色皿/96孔板、可调式移液枪、研钵/匀浆器、丙酮、浓硫酸、无水乙醇和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)取约0.1g 样本,加入1mL 提取液一,室温快速匀浆,95℃水浴20min ,冷却至室温,4000g 25℃离心10min ,弃上清。
沉淀加入1.5mL 提取液一和丙酮交替各洗2遍(涡旋振荡2min 左右,4000g 25℃离心10min ,弃上清即可),沉淀即为粗细胞壁,加入1mL 提取液二(去除淀粉)浸泡15小时,4000g 25℃离心10min ,弃上清,加入1mL 提取液三,充分匀浆。
2006-Arch. Environ.Contam.Toxicol-The Effect of Paraquat on Hepatic EROD Activity, Liver, and Gonada

The Effect ofHepatic EROD Activity,Liver,and Gonadal Histology in Males and Females of Nile Tilapia,Oreochromis niloticus,Exposed at Different TemperaturesA.Figueiredo-Fernandes,1A.Fontaínhas-Fernandes ,1,2E.Rocha,3,4M.A.Reis-Henriques 3,41UTAD-Universidade de Trµs-os-Montes e Alto Douro,Apartado 1013,5000-911,Vila Real,Portugal2CETAV-Centro de Estudos Tecnológicos,do Ambiente e da Vida,Apartado 1013,5000-911,Vila Real,Portugal 3ICBAS-Instituto de CiÞncias BiomØdicas de Abel Salazar,Largo Professor Abel,Salazar 2,4099-003,Porto,Portugal 4CIIMAR-Centro Interdisciplinar de Investigażo Marinha e Ambiental,Ruados Bragas,289,4050-123,Porto,PortugalReceived:25August 2005/Accepted:12March 2006Abstract.The activity of fish monooxygenases hasbeen used as a monitoring tool to evaluate contamination by cytochrome P450inducing agents.In this study ethoxyresorufin O -deeth-ylase (EROD)activity was analyzed in males and females of Nile tilapia exposed to a low concentration of paraquat (PQ)at 17°C and 27°C.PQ-treated fish showed a high hepato-somatic index,except females acclimated at 17°C.No differences were found for the gonado-somatic index (GSI)between males.However,PQ-treated females showed high GSI values (6.46€1.75)when compared with the control group (2.30€0.26)maintained at 27°C.Males and females exposed to PQ showed higher microsomal protein values than the control group (9.46€0.22vs. 6.20€0.18at 17°C;9.51€0.35vs.4.70€0.19mg of protein at 27°C,in PQ-treated and control groups,respectively).The EROD activity was high in females exposed to PQ when compared with the control group at 17°and 27°C.The liver histology showed that PQ also caused hepatic alterations of parenchyma,like vacuolization,necrosis,and an increase of macrophages aggregates and eosinophilic granular cells.Females exposed to PQ showed a greater increase of late-vitellogenic (22.2€3.2)and mature (12.1€2.0)percentage of oocytes than the control group (9.9€3.0and 8.0€4.3,respectively),and a lower percentage of primary oocytes (8.0€3.3)at 27°C.In short,this work has advanced new knowledge on the influence of gender in biotransformation activity and the reproductive activity of Nile tilapia exposed to a low concentration of paraquat,and demonstrated that their effects could be observed at different temperatures.substances that lead to the deterioration of water quality and adversely affect fish and human health.Pesticides can be transferred through phytoplankton to fish and ultimately to humans.Paraquat (PQ)is a non-selective,contact broad-spectrum,and post-emergent herbicide widely used in agricultural practices,like weed control (Lajmanovich et al.1998).PQ causes alterations in fish (Tortorelli et al.1990;Figueiredo-Fernandes et al.2006),but information about the effect of this herbicide on hepatic biochemical and histological parameters is still scarce.The study of these parameters in fish liver can be a helpful tool in monitoring the exposure of fish to contami-nants in laboratory and field studies (Biagianty-Risbourg 1997;Handy et al .2002).The liver plays a primary role in the metabolism of xenobiotic compounds with biochemical alterations occurring in some toxic conditions (van Dyk et al.2006),and it is a detoxification organ essential for the excretion of toxic substances in fish (Hinton and LaurØn 1990).A complex multienzyme family commonly referred to as the biotransformation system accomplishes the biotransformation of xenobiotics in fish.This system metabolizes both endoge-nous and foreign compounds through a series of reactions that can be described as two steps of reactions:phase I,which often produces reactive or toxic metabolites,and phase II,where larger groups are conjugated to the oxygenated xenobiotic into polar,water-soluble,and less-reactive end-products that can be excreted from the organism (van der Oost et al.2003).The cytochrome P450(CYP)monooxygenase system comprises a family of enzymes that catalyze phase I monooxygenation reactions.Several toxic pollutants,such as polycyclic aromatic hydrocarbons (PAHs),polychlorinated biphenyls (PCBs),and pesticides are known to markedly induce cytochrome P4501A isoforms (CYP1A)in mammalian and fish (Haasch et al.1993;Parkinson 1996).The induction of the CYP1A system has become an important tool for monitoring environmental exposure of fish to organic compounds (Hodson et al .1991)and has been extensively used as a biomarker of aquatic contamination by different pollutants in freshwater and marineCorrespondence to:A.Fontaínhas-Fernandes;email:fontain@utad.ptArch.Environ.Contam.Toxicol.51,626–632(2006)environments(Narbonne et al.1991;Parente et al.2004). Fishes are useful experimental models to evaluate the health of aquatic ecosystems and biochemical changes have been used as biomarkers of environmental pollution.Nile tilapia(Ore-ochromis niloticus)is a good model for toxicological experi-ments because it has high growth rates,adapts easily to commercial diets,is resistant to diseases and injury from handling practices,reproduces well in captivity,and has a good tolerance to a wide variety of husbandry conditions (Fontainhas-Fernandes1998).The aim of this study was to investigate the effects of paraquat on the hepatic EROD activity of males and females of Nile tilapia acclimated at two temperatures(17°and27°C);in which the gonadal maturation is inactive and promoted, respectively.The analytical determination was complemented with the observation of liver and gonadal histology. Material and MethodsFish and Experimental DesignAdult males(97.6€0.98g of mean body weight and17.5€0.19cm of length)and females(76.5€1.49g and15.9€0.19cm)of O.niloticus used in this study originated from stocks of fish reared and maintained at the University of Trµs-os-Montes and Alto Douro.The experiment was carried out in semi-static systems,under controlled photoperiods (12D:12L)and constant filtration.Fishes were distributed in12rect-angular glass tanks with100L water for45days.Four tilapias of both sexes were randomly allocated per tank,distributed in4groups(in triplicate)of tanks with a water flow rate of5L min)1.Two groups were exposed to0.5mg L)1of paraquat(PQ)(1,1¢-dimethyl-4,4¢bipyridium dichloride,Sigma M-2254)and acclimated at two temperatures(17°and27°C).The other two groups were maintained in similar conditions without the pollutant.Supplemental aeration was provided in tanks to maintain dissolved oxygen near saturation.Other water quality vari-ables were maintained at acceptable levels by filtration.Fish were fed daily to visual satiation with a diet previously tested in tilapia(Fon-taínhas-Fernandes et al.1999).The selected test concentration of PQ was25%of the LC50(20 mg.L)1),using the Probit analysis program based on Finney(1971). This preliminary experiment showed that1.0mg.L)1of PQ caused extensive areas of hepatocytic necrosis.Then,a low concentration of PQ(0.5mg.L)1)was chosen to assure that the hepatocytes showed no necrosis.The experiment described meets the terms for the European Union Council Guidelines(86/609/EU).Both control and experimental tanks were submitted to water renovation every two days.Ten males and10 females of each group were anaesthetized with2-phenoxiethanol (Sigma,Barcelona,Spain)(1ml L)1water),measured,and weighed. Fish were killed rapidly by decapitation.Livers were removed, weighed,and frozen in liquid nitrogen and stored at)80°C until they were assayed.Biochemical AnalysisLivers were homogenized in ice-cold buffer(50mM Tris-HCl,pH 7.4,containing0.15M KCl).Microsomes were prepared in resus-pension buffer(50mM Tris-HCl,1mM NaEDTA,pH7.4,1mM dithiothreitol,20%(v/v)glycerol),and were obtained by centrifuga-tion of the10,000g supernatant at20,000g for90min.The obtained pellet was resuspended,washed in resuspension buffer,and spun down at20,000rpm for120min(Fent and Bucheli1994).Micro-somes resuspended in EDTA-free resuspension buffer were stored at )80°C until use.The EROD activity was measured according to Pacheco and Santos(1998)at22°C.The results were expressed aspmol/min/mg of protein.The protein content was determinedaccording to Bradford(1976)with bovine serum albumin as standard.All chemicals used in the enzymatic activity were of analytical purityand were obtained from Sigma Chemical Co.Gonad and Liver HistologyGonads from males and females were also quickly dissected and fixedin BouinÕs fixative,dehydrated,and embedded in paraffin.Histolog-ical sections(5-l m-thick)of gonad tissue were made and stained withhaematoxylin-eosin,mounted on glass slides,and examined by lightmicroscopy(LM).The oocytes were classified according to theircellular aspect(Reis-Henriques1997).In each histological section,the oocytes were counted to determine the ripeness of each fish usingthe described classification.The liver was also immersed in BouinÕsfixative during24h,at room temperature,and sliced into a fewsections of identical thickness(4mm),performing a cascade of ale-atory samplings.This outline assured equal sampling probabilities toall the zones of the hepatic tissue(Gundersen1986).Five slides foreach fish were processed for LM.Statistical AnalysisMeans€standard deviation(SD)were calculated for each experi-mental group.Statistical differences between exposed groups andrespective control group were analysed using ANOVA and multiplecomparison by Student-Newman-Keuls test,at a5%significant level.All tests were performed using the software STATISTICA,version6.0(StatSoft Inc,2001).ResultsBiological ParametersFigure1shows the hepato-somatic index(HSI)and gonado-somatic index(GSI)of males and females exposed to PQ at two temperatures(17°and27°C).Tilapias exposed to PQ showed higher HSI values than the reference group at both temperatures,except when the females were maintained at 17°C.No differences were found for GSI between males at both temperatures,while females exposed to PQ showed higher GSI values than the control group at27°C,corre-sponding to a maturation phase of the ovary.Males showed higher total protein(TP)values when com-pared with females at both temperatures(Fig.2).The treat-ment with PQ caused an increase on TP levels in males at17°and27°C,while no effect was found in females at17°C. However,females exposed to PQ showed higher TP values at 27°C than the females maintained at17°C.In contrast,no effect of temperature on TP levels was observed in males.The exposure to PQ caused a significant increase in microsomal protein(MP)values in males and females at both tempera-tures(Fig.2),the differences being more expressive between females.Liver and Gonads Changes in O.niloticus Exposed to Paraquat627EROD ActivityThe ethoxyresorufin O-deethylase activity was markedly higherin tilapia exposed to PQ than in the control group at17°and27°C (Fig.3).No difference was observed between males and females of the control group.Females exposed to PQ showed an EROD activity higher at27°C than PQ-treated females main-tained at17°C.Similar results were observed in males.The EROD activity was high in PQ-treated females at17°and27°C when compared with males at the same temperatures. HistologyControl fish liver exhibited a normal architecture and the he-patocytes presented a homogeneous cytoplasm and a large spherical nucleus.The hepatic parenchyma was characterized by a general moderate eosinophilia,and the venous element of pancreatic area showed continuous endothelium without rup-ture.The hepatic parenchyma of fish exposed to PQ (Fig.4B,C)showed lower eosinophilia and an increase of cytoplasmatic vacuolization.The necrotic cells appeared in the periphery of vascular regions and the membrane of hepato-cytes revealed rapture.It was possible to observe the appear-ance of macrophage aggregates.Additionally,tilapias exposed to PQ showed more eosinophilic granular cells in the interface of the pancreatic areas with the hepatic parenchyma.Histological sections of ovaries revealed that all groups presented oocytes in different stages of development(Fig.5). The percentage of oocyte stages in the sections from females exposed to paraquat at17°and27°C are presented in Table1. Females of the control group showed a high percentage of primary oocytes(45€5.7and46.9€3.8,at17°and27°C, respectively),which reveals an early stage of gonadal devel-opment.In contrast,females exposed to PQ showed a high percentage of late vitellogenic(17.4€1.0and22.2€3.2,at 17°and27°C,respectively)and mature oocytes(13.3€3.4 and12.1€2.0,at17°and27°C,respectively),which was associated with a more developed stage.The testicular sections of males are shown in Figure6.The qualitative analysis of the histological slides revealed that the males exposed to PQ at17°and27°C showed a general tes-ticular eosinophilia,a higher lumen on the spermatic ductules, and a lower visibility of spermatozoa.DiscussionThe cytochrome P450-dependent monooxygenase induction measured as EROD activity is a sensitive indicator of exposure to pollutants in fish(Stegeman et al.1997).In this context,theFig.1.Hepato-somatic(HSI)and gonado-somatic(GSI)indices inNile tilapia O.niloticus exposed to paraquat at17°and27°C.Lower case letters represent significant differences between treatments within each temperature,and uppercase letters represents significant differences between males and females Fig.2.Liver total and microsomal protein in Nile tilapia O.niloticus exposed to paraquat at17°and27°C.Lower-case letters represent sig-nificant differences between treatments within each temperature. Asterisk represents significant differences between temperatures within each level of treatment,while uppercase letters represent significant differences between males and females within each temperature and level of treatment628Figueiredo-Fernandes et al.activity of EROD has been used as a sensitive catalytic probe to determine the inductive response of the cytochrome P450 system in fish(Goksøyr and Fçrlin1992).Several studies have demonstrated an increase in hepatic EROD activity in fish exposed to different pollutants,like PAHs,PCBs,and TCDDs (Van der Weiden et al.1992;Gadagbui et al.1996;Pacheco and Santos1998).Induced activities of hepatic EROD were also found in liver microsomes of tilapia O.niloticus caught in polluted areas(Bainy et al.1999;Parente et al.2004).How-ever,information about gender differences and influence of temperature on EROD activity in fish exposed to pollutants is scarce.The results of the present study indicated increasing levelsof MP and EROD activity in males and females exposed to PQ.The strongest induction of EROD activity was observed at 27°C(343%for males and502%for females when compared with the control group).Our data indicated that tilapia is more responsive to PQ at27°C,on account of the higher induction response upon treatment with this pollutant.These results suggest that the high EROD activity can be due to the increase of metabolic activity at high temperature that leads to an in-crease in the biotransformation capacity of pollutants.Tem-perature and other factors,such as physiological health and the nutritional status of the organism,can also modulate EROD expression(Lange et al.1998;Whyte et al.2000).Sex differences have been seen in EROD activity with females displaying greater activity than females.However,these re-sults are controversial,because SolØet al.(2002)observed that EROD activity in males was usually higher than in females of Cyprinis carpio caught in polluted rivers.The biological parameters are sometimes indicative of tox-icant effects(Mayer et al.1992).The HSI were higher in males and females of treated tilapia.On the contrary, kerman et al.(2003)found that rainbow trout Oncorhynchm mykiss injected with PQ showed a decrease in the HSI after9weeks, which might be due to lower glycogen lower however,histo-logical results were not shown.The qualitative liver histology showed an increase in the vacuolated hepatocyte size in tilapia exposed to PQ,which may be due to the high content of lipids.Huuskonen and Lindstrçm-Seppa(1995)and Stephensen et al.(2000)reported that the high HSI found in the perch(Perca fluviatilis)and sculpin (Myoxocephalus scorpius)can be indicative of increased activity of xenobiotic biotransformation enzymes.Slooff et al. (1983)also suggested a positive relationship between the rel-ative liver weight and the xenobiotic-metabolizing enzymes of fish from polluted waters.The present study showed a general increase of HSI,TP,and MP values,which can be indicative of metabolic modifications induced by paraquat depending on sex and temperature of exposure.The liver histology showed that PQ also caused some hepatic alterations of parenchyma,like vacuolization,necrosis,and an increase of macrophage aggregates and eosinophilic granular cells.These alterations are often associated with a degenerative-necrotic condition (Myers et al.1987).Tilapias exposed to paraquat also showed a regular appearance of macrophages aggregates.The increase of the density of hepatic macrophage aggregates can reflect an important liver lesion and is associated with a reduction of the liver biotransformation capacity,because a considerable por-tion of hepatic parenchyma may be occupied by these aggre-gates(Pacheco and Santos2002).However,tilapias exposed to PQ showed a regular appearance of hepatic macrophages aggregates and they do not appear to display a reduction but even an increase in the biotransformation pathway. Research in recent years has demonstrated that some com-pounds can act as endocrine disrupters,namely methoxychlor, DDT,and dieldrin(Welch et al.1969;Soto et al.1994;Gray et al.1999).These pollutants were banned in most countries because of their bioaccumulative properties and their negative impact on fish reproduction.Identically,the carbamates, propamocarb,and piricarb,as well as the regulator daminozib, also had potentiating effects in the estrogen receptor transac-tivation and on account of this they have been reported as possessing endocrine-disrupting properties(Andersen et al. 2002).In fact,widespread xenobiotics are known to act as antiestrogenic compounds.However,a consensus has not yet been reached in relation to the proposed mechanism of action. Considering the distribution of oocyte growth patterns pre-sented in histological observation,our results reveal that the hepatic EROD activity increase was followed by high GSI values,as well as by an increase in vitelogenesis and matu-ration,mainly at27°C.The PQ treatment caused an increase of the percentage of late-vitellogenic and mature oocytes when compared with the control group.A reduction of the propor-tion of primary oocytes was also observed.This gonadal development is in part correlated with an increase in hepaticFig.3.Ethoxyresorufin O-deethylase(EROD)activity in Nile tilapiaO.niloticus liver exposed to paraquat at17°and27°C.Lowercaseletters represent significant differences between treatments withineach temperature.Asterisk represents significant differences betweentemperatures within each level of treatment,while uppercase lettersrepresent significant differences between males and femalesLiver and Gonads Changes in O.niloticus Exposed to Paraquat629metabolic activity,raising the protein and lipids contents.No differences were found for GSI and histological testicular analysis between males of control and exposed tilapia.How-ever,males exposed to PQ showed characteristics that can alter the reproductive activity,reducing the number of mature cells.In conclusion,the cytochrome P450-dependent monooxy-genase measured as EROD activity increased in tilapias ex-posed to the PQ,the effect being more expressive at 27°C.Gender differences were also observed.The histological analysis of liver also showed that PQ induces specific altera-Fig.4.Photomicrographs of Nile tilapia O.niloticus liver tissue (control group)acclimated at 27°C (A ).h,hepatocytes;v,venous element;pa,pancreatic area that corresponds to the acini of exocrine pancreas.B ,C :Sections of fish exposed to paraquat maintained at 17°C (B )and 27°C (C ),revealing some rupture of the hepatocytic membrane (arrowheads),adiscontinuity in the neighbourhood of the pancreatic areas (whitearrows),and granulate eosinophilic cells (gc)at 17°C.The endothelium of the central venous element shows some ruptures (black arrow)and more macrophages aggregates (m)could be seen at 27°C.H&E,bars =50l mFig.5.Photomicrograph of Nile tilapia O.niloticus ovary sections:control group at 17°C (A )and 27°C (B ),and fish exposed to paraquat at 17°C (C )and 27°C (D ).The control group shows primary oocytes (po),pre-vitellogenic oocytes (pv),oocytes in endogenousvitellogenesis (ev),and a larger stroma portion (s).C and D show oocytes in exogenous vitellogenesis (exv).H&E,bars =0.3mm630Figueiredo-Fernandes et al .tions and may exert a toxic effect.The females showed an increase in vitelogenesis and maturation when exposed to PQ at hightemperatures.These results suggest that there are dif-ferences between males and females in the biotransformation activities caused by PQ,as well as in the reproductive activity.Referenceskerman G,Amcoff P,Tjärnlund U,Fogelberg K,Torrissen O,BalkL (2003)Paraquat and menadione exposure of rainbow trout (Oncorhynchus mykiss )-Studies of effects on the penthose-phosphate shunt and thiamine levels in liver and kidney.Chem Biol Int 142:269–283Andersen HR,Vinggaard AM,Rasmussen TH,Gjermandsen MI,Bonefeld-Jorgensen EC (2002)Effects of currently used pesti-cides in assays for estrogenicity,androgenicity,and aromatase activity in vitro .Toxicol Appl Pharmacol 179:1–12Bainy ACD,Woodin BR,Stegeman JJ (1999)Elevated levels ofmultiple cytochrom P450forms in tilapia from Billings reservoir-S¼o Paulo,Brazil.Aquat Toxicol 44:289–305Biagianti-Risbourg S (1997)Les perturbations (ultra)structurales dufoie des poissons utilisØes comme biomarqueurs de la qualitØsanitaire des milieux aquatiques.In:Lagadic L,Caquet T,Amiard JC,Ramade F (eds),Utilisation de biomarqueurs en Øcotoxicol-ogie:Aspects fondamentaux.Masson Publications,pp.355–391Bradford MM (1976)A rapid and sensitive method for the quantifi-cation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem 72:248–254Fent K,Bucheli TD (1994)Inhibition of hepatic microssomalmonooxygenase system by organotins in vitro in freshwater fish.Aquat Toxicol 28:107–126Figueiredo-Fernandes A,Fontaínhas-Fernandes A,Peixoto F,RochaE,Reis-Henriques HA (2006)Effect of I paraquat on oxidativestress enzymes in tilapia Oreochromis niloticus at two levels of temperature.Pestic Biochem Physiol 85:97–103Finney DJ (1971)Probit analysis Cambridge University Press,NewYork,668pFontaínhas-Fernandes A (1998)Tilapia Production.In:Reis-Henri-ques MA (ed)Aquaculture Handbook,pp.135–150Fontaínhas-Fernandes AA,Gomes E,Reis-Henriques MA,Coimbra J(1999)Replacement of fish meal by plant proteins in the diet of Nile tilapia:Digestibility and growth performance.Aquae Intern 7:57–67Gadagbui BKM,Addy M,Goksøyr A (1996)Species characteristicsof hepatic biotransformation enzymes in two tropical freshwater teleosts,tilapia (Oreochromis niloticus )and mudfish (Clarias anguillaris ).Comp Biochem Physiol 114C:201–211Goksøyr A,Fçrlin L (1992)The cytochrome P450system in fish,aquatic toxicology,and environmental monitoring.Aquat Toxicol 22:287–311Gray LE,Ostby J,Cooper RL,Kelce WR (1999)The estrogenie andantiandrogenic pesticide methoxychlor alters the reproductive tract and behaviour without affecting pituitary size or LH and prolactin secretion in male rats.Toxicol Ind Health 15:37–47Gundersen HG (1986)Stereology of arbitrary particles.A review ofunbiased number and size estimators and the presentation of some new ones,in memory of William R.Thompson.J Microsc 143:3–45Haasch ML,Prince R,Wejksnora PJ,Cooper KR,Lech JJ (1993)Caged and wild fish:induction of hepatic cytochrome-P450(CYP1A1)as an environmental biomonitor.Environ Toxicol Chem 12:885–895Handy RD,Runnalls T,Russell PM (2002)Histopathologic biomar-kers in three sticklebacks,Gasterosteus aculeatus ,from several rivers in Southern England that meet the freshwater fisheries directive.Ecotoxicology 11:467–479Hinton DE,Lauren DJ (1990)Liver structural alterations accompa-nying chronic toxicjty in fishes:potential biomarkers of exposure.Table 1.Percentage of oocyte stages in Nile tilapia O.niloticus females exposed to paraquat at 17°and 27°C17°C27°C Oocytes classes C P C P Primary45.0€5.736.6€2.0(*)46.9€3.834.5€5.7(**)Pre-vitellogenic 15.0€3.015.7€4.0(*)14.3€3.9(a)16.4€2.9(b**)Early-vitellogenic 22.6€3.4(a)17.0€2.0(b*)21.0€3.314.7€2.5(**)Late-vitellogenic 9.2€2.917.4€1.0(*)9.9€3.0(a)22.2€3.2(b**)Mature8.2€2.613.3€3.4(*)8.0€3.3(a)121€2.0(b**)C,control group;P,exposed to paraquat.Lowercase letters represent significant differences between treatments within each oocyte stage.*Significant differences between temperatures within each level of treatment.All significant differences are expressed with p <0.005.Fig.6.Photomicrograph of Nile tilapia O.niloticus testicularsections of the control group (left)and fish exposed to paraquat (right)at 27°C,H&E,bars =100l mLiver and Gonads Changes in O.niloticus Exposed to Paraquat 631In:McCarthy JF,Shugart LR,(eds)Biomarkers of environmental contamination.Lewis,Boca Raton,pp17–57Hodson PV,Klopper-Sams PJ,Munkttrick KR,Lockhart WL,Metner DA,Luxo PI,Smith IR,Gagnon MM,Servos M,Payne JF(1991) Protocols for measuring mixed function oxygenases of fish liver.Canadian Technical Report of Fisheries and Aquatic Sciences 1829,p.51Huuskonen S,Lindstrom-Seppa P(1995)Hepatic cytochrome P4501A and other biotransformation activities in perch(Perca fluviatilis):the effects of unbleached pulp mill effluents.Aquat Toxicol31:27–41Lajmanovich RC,Izaguirre MF,Casco VH(1998)Paraquat tolerance and lteration of internal gill structure of Scinax nasica tadpoles (Anura:Hylidae).Arch Environ Contam Toxicol34:364–369 Lange U,Saborowski R,Siebers D,Buchholz F,Karbe L(1998)tem-perature as a key factor determining the regional variability of the xenobiotic-inducible ethoxyresorufin-O-deethylase activity in the liver of dab(Limanda limanda).Can J Fish Aquat Sci55:328–338 Mayer FL,Versteeg DG,McKee MJ,Folmar LC,Graney RL,McCume DC,Rattner BA(1992)Metabolic products as biomarkers.In: Hugget RJ,Kimerly RA,Mehrle PM,Bergman HL,(eds)Bio-markers:biochemical,physiological and histological markers of anthropogenic stress.Lewis Publishers,Chelsea,USA,pp5–86 Myers MS,Rhodes LD,McCain BB(1987)Pathologic anatomy and patterns of occurrence of hepatic neoplasms,putative preneo-plastic lesions,and other iodiopathic hepatic conditions in English sole(Parophrys vetulus)from Puget Sound,Washington.J Natl Cancer Inst78:333–363Narbonne JF,Garrigues P,Ribera D,Raoux C,Mathieu A,Lemaire P, Salaun JP,Lafaurie M(1991)Mixed-function oxygenase en-zymes as tools for pollution monitoring:field studies on the French coast of the Mediterranean p Biochem Physiol C 100:37–42Pacheco M,Santos MA(1998)Induction of liver EROD and eryth-rocytic nuclear abnormalities by cyclophosphamide and PAHs in Anguilla anguilla L.Ecotoxicol Environ Safe40:71–76 Pacheco M,Santos MA(2002)Biotransformation,genotoxic,and histopathological effects of environmental contaminants in Europeen eel(Anguilla anguilla L.).Ecotoxicol Environ Safe 53:331–347Parente TE,De-Oliveira AC,Silva IB,Araujo FG,Paumgartten FJ (2004)Induced alkoxyresorufin-O-dealkylases in tilapias (Oreochromius niloticus)from Gandu river,Rio de Janeiro, Brazil.Chemosphere54:1613–1618Parkinson A(1996)Biotransformation of xenobiotics.In:Klaassen CD,Amdur MO,Doull J,(eds)Casarett and DoullÕs toxicology.The basic science of poisons.McGraw-Hill,New York,pp113–186Reis-Henriques MA(1997)Regulażo hormonal da fase vitelogØnica nos peixes.Rev Port Zoot IV(II):115–125.Slooff W,van Kreijl CF,Baars AJ(1983)Relative liver weights and xenobiotic-metabolizing enzymes of fish from polluted surface waters in the Netherlands.Aquat Toxicol4:1–14SolØM,BarcelóD,Porte C(2002)Seasonal variation of plasmatic and hepatic vitellogenin and EROD activity in carp,Cyprinus carpio,in relation to sewage treatment plants.Aquat Toxicol 60:233–248Soto AM,Chung KL,Sonnenschein C(1994)The pesticides endosulfan,toxaphene,and dieldrin have estrogenic effects on human estrogen-sensitive cells.Environ Health Perspect 102:380–383Stegeman JJ,Woodin BR,Singh H,Oleksiak MF,Celander M(1997) Cytochromes P450(CYP)in tropical fishes:catalytic activities, expression of multiple CYP proteins and high levels of micro-somal P450in liver of fishes from p Biochem Physiol C116:61–75Stephensen E,Svavarsson J,Starve J,Ericson G,Adolfson-Erici M, Fçrlin L(2000)Biochemical indicators of pollution exposure in shorthorn sculpin(Myoxocephalus scorpius),caught in four har-bours on the south-west coast of Iceland.Aquat Toxicol48:431–442Tortorelli MC,Hernandez DA,Rey-Vazquez G,Saibiµn A(1990) Effects of paraquat on mortality and cardiorespiratory function of catfish fry Plecostomus commersoni.Arch Environ Contam Toxicol19:523–529Van der Oost R,Beyer J,Vermeulen NPE(2003)Fish bioaccumu-lation and biomarkers in environmental risk assessment:A re-view.Environ Toxicol Pharmacol13:57–149Van der Weiden MEJ,Van der Kolk J,Bleumink R,Seinen W,van der Berg M(1992)Concurrence of P4501A1induction and toxic effects alter administration of a low dose of2,3,7,8-tetrachlo-rodibenzo-p-dioxin(TCDD)in the rainbow trout(Oncorhynchus mykiss).Aquat Toxicol24:123–142van Dyk JC,Pieterse GM,van Vuren JHJ(2006)Histological changes in the liver of Oreochromis mossambicus(Cichlidae) after exposure to cadmium and zinc.Ecotoxicol Environ Safe (in press)Welch RM,Levin W,Conney A(1969)Estrogenic action of DDT and its analogs.Toxicol Appl Pharmacol14:358–367Whyte JJ,Jung RE,Schmitt CJ,Tilltitt DE(2000)Ethoxyresofurin O-deethylase(EROD)activity in fish as a biomarker of chemical exposure.Crit Rev Toxicol30:347–570632Figueiredo-Fernandes et al.。
阿魏酰低聚糖分子量

阿魏酰低聚糖分子量
(实用版)
目录
1.阿魏酰低聚糖的概述
2.阿魏酰低聚糖的分子量
3.阿魏酰低聚糖分子量的测定方法
4.阿魏酰低聚糖分子量的应用
正文
一、阿魏酰低聚糖的概述
阿魏酰低聚糖(Aristolochic oligosaccharides)是一类具有广泛生物活性的天然产物,其化学结构独特且复杂。
阿魏酰低聚糖广泛存在于植物中,尤其是在马兜铃科植物中,具有多种生物学活性,如抗肿瘤、抗病毒、抗炎、免疫调节等。
二、阿魏酰低聚糖的分子量
阿魏酰低聚糖的分子量是指由阿魏酰糖分子通过糖苷键连接而成的
低聚糖分子的相对分子质量。
阿魏酰低聚糖的分子量不同,其生物活性也可能存在差异。
一般来说,分子量较大的阿魏酰低聚糖具有更强的生物活性。
三、阿魏酰低聚糖分子量的测定方法
阿魏酰低聚糖分子量的测定方法通常采用质谱法,如高效液相色谱 - 质谱法(HPLC-MS)和气相色谱 - 质谱法(GC-MS)。
这些方法能够准确测定阿魏酰低聚糖的分子量,并为研究其生物活性提供重要信息。
四、阿魏酰低聚糖分子量的应用
阿魏酰低聚糖分子量的研究在药物开发、生物活性筛选、药物代谢等
方面具有重要意义。
通过测定阿魏酰低聚糖的分子量,可以筛选出具有特定生物活性的低聚糖,并为药物开发提供候选化合物。
此外,阿魏酰低聚糖分子量的研究还有助于揭示其药效物质基础和作用机制。
总之,阿魏酰低聚糖分子量研究对于深入了解这类天然产物的生物活性、药物开发以及作用机制具有重要意义。
