Dirithromycin_DataSheet_MedChemExpress
Oxfendazole_53716-50-0_DataSheet_MedChemExpress

Product Name:Oxfendazole CAS No.:53716-50-0Cat.No.:HY-B0291Product Data SheetCat. No.:HY B0291MWt:315.35Formula:C15H13N3O3S Purity :>98%DMSO 10mg/mL;Water <1mg/mLSolubility:Mechanisms:Biological Activity:O f d l i th lf id f f f b d l hi h i b d t b i id lPathways:Anti-infection; Target:Antiparasitic DMSO 10 mg/mL; Water 1 mg/mL Oxfendazole is the sulfoxide form of fenbendazole which is a broad spectrum benzimidazoleanthelmintic.Target: Antiparasitic Oxfendazole is the sulfoxide form of fenbendazole, a broad spectrum benzimidazole anthelmintic. Its main use is for protecting livestock against roundworm, strongyles and pinworms.[1]. Pigs in the treated group received Oxfendazole orally at 30 mg/kg dose. At five days post-treatment, animals were sacrificed and the clinical efficacy of the Oxfendazole treatment was established following the currently available WAAVP guidelines for a controlled efficacy test. None of the animals involved in this experiment showed any adverse events during the study Oxfendazole treatment given as a References:[1]. /lifescience/phar/OXFENDAZOLE.htm [2]. Alvarez, L., et al., Efficacy of a single high oxfendazole dose against gastrointestinal nematodesin naturally infected pigs. Veterinary parasitology, 2013. 194(1): p. 70-74.this experiment showed any adverse events during the study. Oxfendazole treatment given as a single 30 mg/kg oral dose showed a 100% efficacy against all the nematode parasites present in the three experiments. In conclusion, under the current experimental c...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p re s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
盐酸度洛西汀欧洲药典

Duloxetine Hydrochloride(doo lox' e teen hye'' droe klor' ide).C18H19NOS ·HCl 333.882-Thiophenepropanamine, N-methyl--(1-naphthalenyloxy)-, hydrochloride, (S)-; (+)-(S)-N-Methyl--(1-naphthyloxy)-2-thiophenepropylamine hydrochloride[136434-34-9].DEFINITIONDuloxetine Hydrochloride contains NLT 97.0% and NMT 102.0% of C18H19NOS ·HCl, calculated on the dried basis.IDENTIFICATIONChange to read:• A. Infrared Absorption 197K (ERR 1-Jul-2012)• B. The retention time of the major peak in the Sample solution correspondsto that of the duloxetine S-isomer from the System suitability solution in the test for Limit of Duloxetine Related Compound A.Change to read:• C. Identification Tests —General, Chloride 191Sample solution: 5 mg/mL in methanolAcceptance criteria: Meets the requirements (ERR 1-Jul-2012)ASSAY• ProcedureProtect solutions of duloxetine from light.Buffer: 2.9 g/L of phosphoric acid in water. Adjust with sodium hydroxide solution to a pH of 2.5. To each L of this solution add 10.3 g of sodium 1-hexanesulfonate monohydrate, and dissolve.Mobile phase: Acetonitrile, n-propanol, and Buffer (13:17:70)Diluent: Acetonitrile and water (25:75)ClickSystem suitability solution: 0.2 mg/mL of USP Duloxetine Hydrochloride RS in Mobile phase. Heat the solution to at least 40for a minimum of 1 h.[Note —The resulting solution contains duloxetine impurity B, duloxetine impurity C, duloxetine impurity D, duloxetine impurity E, and duloxetine related compound F. ] Standard solution: 0.1 mg/mL of USP Duloxetine Hydrochloride RS in DiluentSample solution: 0.1 mg/mL of Duloxetine Hydrochloride in DiluentChromatographic system(See Chromatography 621, System Suitability .)Mode: LCDetector: UV 230 nmColumn: 4.6-mm × 15-cm; 3.5-µm packing L7Column temperature: 40 ± 3Flow rate: 1 mL/minInjection size: 10 µLRun time: 2 times the retention time of duloxetineSystem suitabilitySample: System suitability solution[Note —See Table 1 for relative retention times. ]Suitability requirements Resolution: NLT 1.5 between duloxetine and duloxetine relatedcompound F peaksTailing factor: NMT 1.5 for the duloxetine peakRelative standard deviation: NMT 1.0% for the duloxetine peakAnalysisSamples: Standard solution and Sample solutionCalculate the percentage of duloxetine hydrochloride (C18H19NOS ·HCl) in the portion of sample taken:Result = (rU/rS) × (CS/CU) × 100Acceptance criteria: 97.0%–102.0% on the dried basisIMPURITIES• Heavy Metals, Method II 231: NMT 10 ppm• Residue On Ignition281: NMT 0.2% rU =peak response from the SamplesolutionrS =peak response from the StandardsolutionCS =concentration of USP DuloxetineHydrochloride RS in the Standardsolution (mg/mL)CU =concentration of DuloxetineHydrochloride in the Sample solution(mg/mL)• Organic ImpuritiesProtect solutions of duloxetine from light.Buffer, Mobile phase, Diluent, and System suitability solution: Proceed as directed in the Assay.Sensitivity solution: 0.2 µg/mL of USP Duloxetine Hydrochloride RS in DiluentSample solution: 0.2 mg/mL of Duloxetine Hydrochloride in DiluentChromatographic system: Proceed as directed in the AssayRun time: 2.4 times the retention time of duloxetineSystem suitabilitySamples: System suitability solution and Sensitivity solution[Note —See Table 1 for relative retention times. ]Suitability requirementsResolution: NLT 1.5 between duloxetine impurity C and duloxetineimpurity D; NLT 1.5 between duloxetine and duloxetine relatedcompound F, System suitability solutionTailing factor: NMT 1.5 for the duloxetine peak, System suitabilitysolutionRelative standard deviation: NMT 1.0% for the duloxetine peak, System suitability solutionSignal-to-noise ratio: NLT 20 for the duloxetine peak, Sensitivity solution AnalysisSample: Sample solutionCalculate the percentage of any individual impurity in the portion ofDuloxetine Hydrochloride taken:Result = (rU/rT) × (1/F) × 100Acceptance criteria: See Table 1. Table 1 rU =peak response of each impurity fromthe Sample solutionrT =sum of the responses of all the peaksfrom the Sample solutionF =relative response factor (see Table 1)Name Relative Retention TmeRelative Response Factor AcceptanceCriteriaNMT (%)Duloxetine impurity B a ,g 0.150.36—Duloxetine impurity C b ,g0.43 1.0—Duloxetine impurity D c ,g0.48 1.8—Duloxetine impurity E d ,g0.74 1.0—Duloxetine 1.0——• Limit of Duloxetine Related Compound AMobile phase: Hexane and isopropyl alcohol (83:17). To 1 L of this mixture add 2 mL of diethylamine.System suitability solution: 0.1 mg/mL each of USP DuloxetineHydrochloride RS and USP Duloxetine Related Compound A RS inMobile phase. Sonication may be used to aid in dissolution.Sensitivity solution: 0.1 µg/mL of USP Duloxetine Hydrochloride RS in Mobile phaseSample solution: 0.1 mg/mL of Duloxetine Hydrochloride in Mobile phase.Sonication may be used to aid in dissolution.Chromatographic system (See Chromatography 621, System Suitability .)Mode: LCDetector: UV 230 nmColumn: 4.6-mm × 25-cm; 5-µm packing L40Column temperature: 40Flow rate: 1 mL/minInjection size: 10 µLRun time: 2 times the retention time of duloxetineSystem suitabilitySamples: Sensitivity solution and System suitability solution[Note —The relative retention times for duloxetine and duloxetine related compound A are 1.0 and 1.3, respectively. ]Suitability requirementsResolution: NLT 3.5 between duloxetine and duloxetine relatedcompound A, System suitability solutionTailing: Between 0.8 and 1.5 each for duloxetine and duloxetine related compound A peaks, System suitability solutionRelative standard deviation: NMT 5.0% for the duloxetine peak, System suitability solutionSignal-to-noise ratio: NLT 3, Sensitivity solutionAnalysisDuloxetine related compound F e 1.1 1.00.5Duloxetine impurity G f ,g 1.40.51—Any individual unspecified impurity — 1.00.1Total impurities——0.6a 3-(Methylamino)-1-(thiophen-2-yl)propan-1-ol.b 4-[3-(Methylamino)-1-(thiophen-2-yl)propyl]naphthalen-1-ol.c Naphthalen-1-ol.d 1-(3-(Methylamino)-1-(thiophen-2-yl)propyl)naphthalen-2-ol.e (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-3-yl)propan-1-amine.f 1-Fluoronaphthalene.g Controlled at Any individual unspecified impurity level.Sample: Sample solutionCalculate the percentage of duloxetine related compound A in the portion of Duloxetine Hydrochloride taken:Result = (rU/rT) × 100Acceptance criteria: NMT 0.5%SPECIFIC TESTS• Loss On Drying 731: Dry at 105 for 3 h: it loses NMT 0.5% of its weight. ADDITIONAL REQUIREMENTS• Packaging And Storage: Protect from light. Store at room temperature. • USP Reference Standards 11:USP Duloxetine Hydrochloride RSUSP Duloxetine Related Compound A RS(R)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine hydrochloride.C18H19NOS ·HCl 333.88Auxiliary Information — Please check for your question in the FAQs before contactingUSP. USP36–NF31 Page 3354Pharmacopeial Forum: Volume No. 37(4)Chromatographic Column —DULOXETINE HYDROCHLORIDEChromatographic columns text is not derived from, and not part of, USP 36 or NF 31.rU =peak response for duloxetine relatedcompound A from the SamplesolutionrT =sum of the responses of duloxetineand duloxetine related compound Apeaks from the Sample solutionTopic/Question Contact Expert CommitteeMonograph Heather R. Joyce, Ph.D. Associate Scientific Liaison1-301-918-8442(SM42010) Monographs - SmallMolecules 4Reference Standards RS Technical Services1-301-816-8129rstech@。
西他沙星杂质

5
2524S
西他沙星
Sitafloxacin Impurity 4
N/A
STD
6
2525S
西他沙星
Sitafloxacin Impurity 5
N/A
STD
7
2526S
西他沙星
Sitafloxacin Impurity 6
N/A
STD
等各种西他沙星杂质
序号 货号
中文名称
1
252S
西他沙星
名称
西他沙星杂质 湖北扬信医药科技有限公司
CAS
品牌
Sitafloxacin
163253-37-0
STD
结构式
2
2521S
西他沙星
Sitafloxacin Impurity 1
127254-11-9
STD
3
2522S
西他沙星
Sitafloxacin Impurity 2
127199-他沙星
Sitafloxacin Impurity 3
N/A
STD
扬信医药代理各品种杂质对照品:阿伐那非、色瑞替尼、沙芬酰胺、舒更葡糖、索非那新、西地那非、西他沙星、西酞普兰、硝呋太尔、辛伐他汀 、 阿 比 特 龙 等 杂 质 ; 并 提 供 COA 、 NMR 、 HPLC 、 MS 等 结 构 确 认 图 谱 。 专 业 < 杂 质 对 照 品 > 解 决 方 案 , 代 理 中 检 所 /EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACGEM/STD等品牌。
Maraviroc_376348-65-1_DataSheet_MedChemExpress
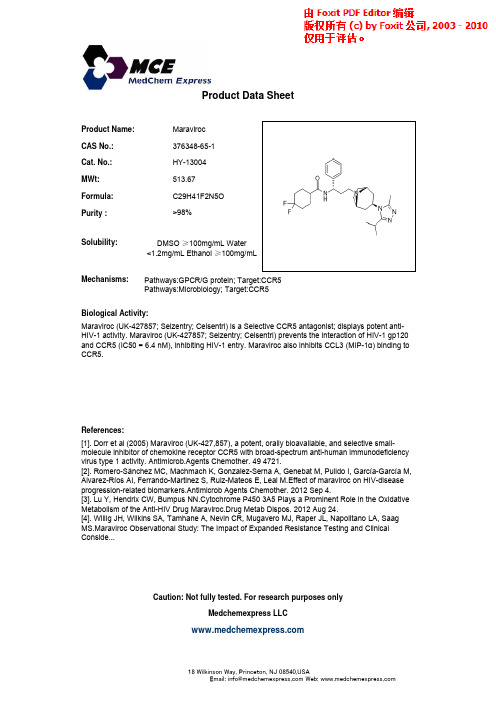
Mechanisms:
Pathways:GPCR/G protein; Target:CCR5 Pathways:Microbiology; Target:CCR5
g y Biological Activity: Maraviroc (UK-427857; Selzentry; Celsentri) is a Selective CCR5 antagonist; displays potent antiHIV-1 activity. Maraviroc (UK-427857; Selzentry; Celsentri) prevents the interaction of HIV-1 gp120 and CCR5 (IC50 = 6.4 nM), inhibiting HIV-1 entry. Maraviroc also inhibits CCL3 (MIP-1α) binding to CCR5.
References: [1]. Dorr et al (2005) Maraviroc (UK-427,857), a potent, orally bioavailable, and selective smallmolecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob.Agents Chemother. 49 4721. [2]. [ ] Romero-Sánchez MC, Machmach K, Gonzalez-Serna A, Genebat M, Pulido I, García-García M, Alvarez-Ríos AI, Ferrando-Martinez S, Ruiz-Mateos E, Leal M.Effect of maraviroc on HIV-disease progression-related biomarkers.Antimicrob Agents Chemother. 2012 Sep 4. [3]. Lu Y, Hendrix CW, Bumpus NN.Cytochrome P450 3A5 Plays a Prominent Role in the Oxidative Metabolism of the Anti-HIV Drug Maraviroc.Drug Metab Dispos. 2012 Aug 24. [4]. Willig JH, Wilkins SA, Tamhane A, Nevin CR, Mugavero MJ, Raper JL, Napolitano LA, Saag MS.Maraviroc Observational Study: The Impact of Expanded Resistance Testing and Clinical Conside...
近五年药学英文文献

6.Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: a systematic review and meta-analysis
7.Dissemination of VIM-2 pr233 at tertiary care hospitals in Egypt.
8.Development of Clinical Pharmacy Key Performance Indicators for Hospital Pharmacists Using a Modified Delphi Approach
近五年Pubmed英文文献总结
1.Determinants of non-adherence to antiretroviral therapy in adult hospitalized patients, Northwest Ethiopia
2.Prognostic factors for surgically managed patients with stage II non-small cell lung cancer.
9.Achieving target voriconazole concentrations more accurately in children and adolescents.
10.Effectiveness of pharmaceutical care at discharge in the emergency department: study protocol of a randomized controlled trial.
琥珀酸美托洛尔缓释片联合曲美他嗪在冠心病心绞痛治疗中的应用效果

DOI:10.16662/ki.1674-0742.2023.14.120琥珀酸美托洛尔缓释片联合曲美他嗪在冠心病心绞痛治疗中的应用效果杜慧锋吉林省人民医院心血管内科,吉林长春130021[摘要]目的分析对冠心病心绞痛患者使用琥珀酸美托洛尔缓释片+曲美他嗪的临床效果。
方法回顾性分析2020年11月—2021年11月吉林省人民医院心血管内科接受治疗的156例冠心病心绞痛患者的临床资料,以治疗方式不同将其分成对照组(n=78,单独使用琥珀酸美托洛尔缓释片治疗)和观察组(n=78,在对照组基础上使用曲美他嗪治疗),对比两组临床疗效、西雅图心绞痛量表(SAQ)评分及用药期间不良反应发生情况。
结果观察组治疗有效率为98.72%高于对照组的89.74%,差异有统计学意义(χ2=4.244,P<0.05)。
治疗后,观察组PL、AS、AF、TS以及DP评分结果均高于对照组,差异有统计学意义(P<0.05)。
观察组不良反应发生率为2.56%低于对照组的14.10%,差异有统计学意义(P<0.05)。
结论对冠心病心绞痛患者应用琥珀酸美托洛尔缓释片+曲美他嗪治疗的临床疗效显著且安全性高。
[关键词]冠心病;心绞痛;琥珀酸美托洛尔缓释片;曲美他嗪[中图分类号]R4 [文献标识码]A [文章编号]1674-0742(2023)05(b)-0120-05Effect of Metoprolol Succinate Extended-release Tablets Combined with Trimetazidine in the Treatment of Angina Pectoris in Coronary Artery Dis⁃easeDU HuifengDepartment of Cardiovascular Medicine, Jilin Provincial People's Hospital, Changchun, Jilin Province, 130021 China [Abstract] Objective To analyze the clinical effect of using metoprolol succinate extended-release tablets+trimetazi‐dine in patients with angina pectoris of coronary artery disease. Methods Retrospective analysis of clinical data of 156 patients with coronary heart disease and angina pectoris treated in the Cardiovascular Department of Jilin Provincial People's Hospital from November 2020 to November 2021, they were divided into the control group (n=78, treated with metoprolol succinate sustained-release tablets alone) and the observation group (n=78, treated with trimetazidine on the basis of the control group) according to different treatment methods. Compared the clinical efficacy, Seattle An‐gina Scale (SAQ) scores, and incidence of adverse reactions during medication between two groups. Results The effec‐tive rate of treatment in the observation group was 98.72%, which was higher than 89.74% in the control group, the difference was statistically significant (χ2=4.244, P<0.05). After treatment, the scores of PL, AS, AF, TS, and DP in the observation group were higher than those in the control group, the difference was statistically significant (P<0.05). The incidence of adverse reactions in the observation group was 2.56% lower than 14.10% in the control group, the difference was statistically significant (P<0.05). Conclusion The clinical efficacy and safety of metoprolol succinate extended-release tablets+trimetazidine in patients with angina pectoris of coronary artery disease is significant.[Key words] Coronary heart disease; Angina pectoris; Metoprolol succinate extended-release tablets; Trimetazidine[作者简介] 杜慧锋(1978-),男,硕士,副主任医师,研究方向为心血管内科。
OXOID药敏纸片中英文对照
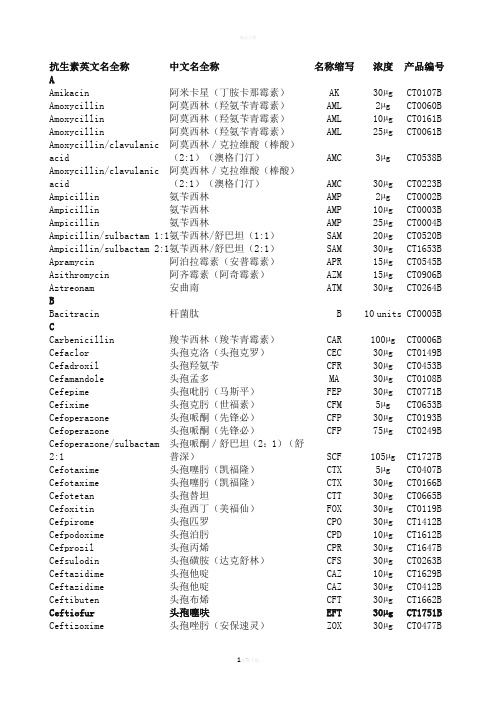
AAmikacin 阿米卡星(丁胺卡那霉素)AK 30µg CT0107B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 2µg CT0060B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 10µg CT0161B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 25µg CT0061BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 3µg CT0538BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 30µg CT0223BAmpicillin 氨苄西林AMP 2µg CT0002B Ampicillin 氨苄西林AMP 10µg CT0003B Ampicillin 氨苄西林AMP 25µg CT0004B Ampicillin/sulbactam 1:1 氨苄西林/舒巴坦(1:1)SAM 20µg CT0520B Ampicillin/sulbactam 2:1 氨苄西林/舒巴坦(2:1)SAM 30µg CT1653B Apramycin 阿泊拉霉素(安普霉素)APR 15µg CT0545B Azithromycin 阿齐霉素(阿奇霉素)AZM 15µg CT0906B Aztreonam 安曲南ATM 30µg CT0264B BBacitracin 杆菌肽 B 10 units CT0005B CCarbenicillin 羧苄西林(羧苄青霉素)CAR 100µg CT0006B Cefaclor 头孢克洛(头孢克罗)CEC 30µg CT0149B Cefadroxil 头孢羟氨苄CFR 30µg CT0453B Cefamandole 头孢孟多MA 30µg CT0108B Cefepime 头孢吡肟(马斯平)FEP 30µg CT0771B Cefixime 头孢克肟(世福素)CFM 5µg CT0653B Cefoperazone 头孢哌酮(先锋必)CFP 30µg CT0193B Cefoperazone 头孢哌酮(先锋必)CFP 75µg CT0249BCefoperazone/sulbactam 2:1 头孢哌酮/舒巴坦(2:1)(舒普深)SCF 105µg CT1727BCefotaxime 头孢噻肟(凯福隆)CTX 5µg CT0407B Cefotaxime 头孢噻肟(凯福隆)CTX 30µg CT0166B Cefotetan 头孢替坦CTT 30µg CT0665B Cefoxitin 头孢西丁(美福仙)FOX 30µg CT0119B Cefpirome 头孢匹罗CPO 30µg CT1412B Cefpodoxime 头孢泊肟CPD 10µg CT1612B Cefprozil 头孢丙烯CPR 30µg CT1647B Cefsulodin 头孢磺胺(达克舒林)CFS 30µg CT0263B Ceftazidime 头孢他啶CAZ 10µg CT1629B Ceftazidime 头孢他啶CAZ 30µg CT0412B Ceftibuten 头孢布烯CFT 30µg CT1662B Ceftiofur 头孢噻呋EFT 30µg CT1751B Ceftizoxime 头孢唑肟(安保速灵)ZOX 30µg CT0477BCeftriaxone 头孢曲松(头孢三嗪)CRO 30µg CT0417B Cefuroxime sodium 头孢呋新钠CXM 5µg CT0406B Cefuroxime sodium 头孢呋新钠CXM 30µg CT0127B Cephalexin 头孢氨苄(头孢力新,先锋IV)CL 30µg CT0007B Cephalothin 头孢噻吩(头孢菌素,先锋I )KF 30µg CT0010B Cephazolin 头孢唑啉(先锋V)KZ 30µg CT0011B Cephradine 头孢拉定(先锋VI)CE 30µg CT0063B Chloramphenicol 氯霉素 C 10µg CT0012B Chloramphenicol 氯霉素 C 30µg CT0013B Chloramphenicol 氯霉素 C 50µg CT0014B Cinoxacin 西诺沙星CIN 100µg CT0162B Ciprofloxacin 环丙沙星(悉复欢)CIP 1µg CT0623B Ciprofloxacin 环丙沙星(悉复欢)CIP 5µg CT0425B Ciprofloxacin 环丙沙星(悉复欢)CIP 10µg CT1615B Clarithromycin 克拉霉素CLR 2µg CT1599B Clarithromycin 克拉霉素CLR 5µg CT1623B Clarithromycin 克拉霉素CLR 15µg CT0693BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 2µg CT0064BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 10µg CT0015BCloxacillin 氯唑西林(邻氯青霉素)OB 5µg CT0016B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 10µg CT0017B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 25µg CT0065B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 50µg CT0664B Compound sulphonamides 磺胺复合物S3 300µg CT0059B 抗生素英文名全称中文名全称名称缩写浓度产品编号DDoxycycline 强力霉素DO 30µg CT0018B EEnrofloxacin 恩诺沙星ENR 5µg CT0639B Ertapenem 厄他培南ETP 10µg CT1761B Erythromycin 红霉素 E 5µg CT0066B Erythromycin 红霉素 E 10µg CT0019B Erythromycin 红霉素 E 15µg CT0020B Erythromycin 红霉素 E 30µg CT0021B抗生素英文名全称中文名全称名称缩写浓度产品编号FFlorfenicol 氟苯尼考FFC 30µg CT1754BFluconazole 氟康唑FCA 25µg CT1806B Flumequine 氟甲喹UB 30µg CT0666B Fosfomycin 磷霉素FOS 50µg CT0183B 抗生素英文名全称中文名全称名称缩写浓度产品编号Framycetin 新霉素B FY 100µg CT0071B Fusidic acid 褐霉素(夫西地酸)FD 5µg CT0493B Fusidic acid 褐霉素(夫西地酸)FD 10µg CT0023B Fusidic acid 褐霉素(夫西地酸)FD 50µg CT1617B GGentamicin 庆大霉素CN 10µg CT0024B Gentamicin 庆大霉素CN 30µg CT0072B Gentamicin 庆大霉素CN 120µg CT0794B Gentamicin 庆大霉素CN 200µg CT0695B IImipenem 亚胺培南(配能)IPM 10µg CT0455B KKanamycin 卡那霉素K 5µg CT0025B Kanamycin 卡那霉素K 30µg CT0026B LLatamoxef 拉氧头孢MOX 30µg CT0302B Levofloxacin 左氧氟沙星(可乐必妥)LEV 1µg CT1586B Levofloxacin 左氧氟沙星(可乐必妥)LEV 5µg CT1587B Lincomycin 林可霉素(洁霉素)MY 2µg CT0027B Lincomycin 林可霉素(洁霉素)MY 10µg CT0123B Lincomycin 林可霉素(洁霉素)MY 15µg CT0028BLincomycin/neomycin 林可霉素(洁霉素)/新霉素LN 75µg CT1757BLincomycin/spectinomycin 林可霉素/壮观霉素LS 109µg CT1758B Linezolid 利奈唑胺LZD 10µg CT1649B Linezolid 利奈唑胺LZD 30µg CT1650B Lomefloxacin 洛美沙星LOM 10µg CT1661B MMecillinam 美西林MEL 10µg CT0096B Mecillinam 美西林MEL 25µg CT0091B Meropenem 美罗培南(美平)MEM 10µg CT0774B Metronidazole 甲硝唑(灭滴灵)MTZ 5µg CT0067B Metronidazole 甲硝唑(灭滴灵)MTZ 50µg CT0466B Mezlocillin 美洛西林MEZ 30µg CT0174B Mezlocillin 美洛西林MEZ 75µg CT0192BMinocycline 米诺环素(二甲胺四环素)MH 30µg CT0030BMoxalactam 拉氧头孢MOX 30µg CT0302B Moxifloxacin 莫西沙星MXF 1µg CT1683B Moxifloxacin 莫西沙星MXF 5µg CT1633BMupirocin 莫匹罗星MUP 5µg CT0522B Mupirocin 莫匹罗星MUP 20µg CT1826B Mupirocin 莫匹罗星MUP 200µg CT0523B NNalidixic acid 萘啶酸NA 30µg CT0031B 抗生素英文名全称中文名全称名称缩写浓度产品编号Neomycin 新霉素N 30µg CT0033BNetilmicin 奈替米星(乙基西梭霉素)NET 10µg CT0424BNetilmicin 奈替米星(乙基西梭霉素)NET 30µg CT0225BNitrofurantoin 呋喃妥因(呋喃妥英) F 50µg CT0069B Nitrofurantoin 呋喃妥因(呋喃妥英) F 100µg CT0034B Nitrofurantoin 呋喃妥因(呋喃妥英) F 200µg CT0035B Nitrofurantoin 呋喃妥因(呋喃妥英) F 300µg CT0036B Norfloxacin 诺氟沙星(氟哌酸)NOR 2µg CT0687B Norfloxacin 诺氟沙星(氟哌酸)NOR 5µg CT0668B Norfloxacin 诺氟沙星(氟哌酸)NOR 10µg CT0434B Novobiocin 新生霉素NV 5µg CT0037B Novobiocin 新生霉素NV 30µg CT0038B Nystatin 制霉菌素NS 100units CT0073B OOfloxacin 氧氟沙星(泰利必妥)OFX 5µg CT0446B Oleandomycin 竹桃霉素OL 15µg CT0039B Oxacillin 苯唑西林OX 1µg CT0159B Oxacillin 苯唑西林OX 5µg CT0040B Oxolinic acid 奥索利酸(恶喹酸)OA 2µg CT0181BOxytetracycline 土霉素(氧四环素,地霉素)OT 30µg CT0041BPPefloxacin 培氟沙星(甲氟哌酸)PEF 5µg CT0661B Penicillin G 青霉素G P 1unit CT0152B Penicillin G 青霉素G P 1.5unit CT0042B Penicillin G 青霉素G P 2units CT0088B Penicillin G 青霉素G P 5units CT0124B Penicllin G 青霉素G P 10units CT0043B Penicillin/novobiocin 青霉素/新生霉素PNV 40 CT1755B Pipemidic acid 吡哌酸PIP 20µg CT0180BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 30µg CT1619BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 75µg CT0261BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 100µg CT0199BPiperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 36µg CT1616B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 40µg CT1628B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 85µg CT0720B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星) TZP 110µg CT0725B Pirlimycin 吡利霉素 PIR 2µg CT1668B Polymyxin B多粘菌素BPB300units CT0044BQQuinupristin/dalfopristin 喹奴普汀/达福普汀 QD 15µg CT1644BR抗生素英文名全称 中文名全称 名称缩写 浓度 产品编号 Rifampicin 利福平 RD 2µg CT0078B Rifampicin 利福平 RD 5µg CT0207B Rifampicin 利福平 RD 30µg CT0104BSSpectinomycin 大观霉素(壮观霉素) SH 10µg CT0046B Spectinomycin 大观霉素(壮观霉素) SH 25µg CT0411B Spectinomycin 大观霉素(壮观霉素) SH 100µg CT0823B Spiramycin 螺旋霉素 SP 100µg CT0232B Streptomycin 链霉素 S 10µg CT0047B Streptomycin链霉素 S 25µg CT0048B Sulbactam/ampicillin 1 : 1舒巴坦/氨苄西林 SAM 20µg CT0520BSulbactam/ampicillin 1 : 2 舒巴坦/氨苄西林(优立新) SAM 30µg CT1653B Sulphafurazole 磺胺异恶唑 SF300µg CT0075B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL25µg CT0051B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL 100µg CT0074B Sulphamethoxazole/trimethoprim 19 : 1 磺胺甲基异恶唑(新诺明)/甲氧苄氨嘧啶 SXT 25µg CT0052B Sulphonamides compound 磺胺复合物 S3 300µg CT0059BT Teicoplanin 替考拉宁(壁霉素) TEC 30µg CT0647B Telithromycin 泰利霉素 TEL 15µg CT1714B Tetracycline 四环素 TE 10µg CT0053B Tetracycline 四环素 TE 30µg CT0054B Ticarcillin 替卡西林(羧噻吩青霉TIC75µgCT0167B素)Ticarcillin/clavulanic acid 7.5 : 1 替卡西林/克拉维酸(7.5:1) TIM 85µg CT0449B Tigecycline 替加环素 TGC 15µg CT1841B Tilmicosin 替米考星 TIL 15µg CT1756B Tobramycin 妥布霉素(托普霉素) TOB 10µg CT0056B Tobramycin 妥布霉素(托普霉素) TOB 30µg CT1618B Trimethoprim 甲氧苄氨嘧啶 W 1.25µg CT0057B Trimethoprim 甲氧苄氨嘧啶 W 2.5µg CT0070B Trimethoprim 甲氧苄氨嘧啶 W 5µg CT0076B Trimethoprim/sulphamethoxazole 1:19 甲氧苄氨嘧啶/磺胺甲基异恶唑(复方新诺明) SXT 25µg CT0052B V Vancomycin 万古霉素(稳可信) VA 5µg CT0188B Vancomycin 万古霉素(稳可信) VA 30µg CT0058B Voriconazole 优立康唑 VOR 1µg CT1807B 注释: CLSI: Clinical and Laboratory Standards Institute 美国临床实验室标准化研究所DIN: Deutsches Institut f ür Normung 德国标准化学会 BSAC: British Society for Antimicrobial Chemotherapy 英国抗生素化疗协会SRGA: Swedish Reference Group for Antibiotics 瑞典抗生素委员会 SFM: Soci ét é Fran çaise de Microbiologie 法国微生物学会注:红色为兽禽用抗生素,绿色为农作物用抗生素,其余为人用抗生素。
Erythrosin_B_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-22-2018Print Date:Jan.-22-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Erythrosin BCatalog No. :HY-D0259CAS No. :16423-68-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Chronic aquatic toxicity (Category 4), H4132.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H413 May cause long lasting harmful effects to aquatic life.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 + P330 IF SWALLOWED: Call a POISON CENTER ⁄doctor if you feel unwell.Rinse mouth.P501 Dispose of contents ⁄ container to an approved waste disposal plant.H413 May cause long lasting harmful effects to aquatic life.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Erythrosin extra bluishFormula:C20H6I4Na2O5Molecular Weight:879.86CAS No. :16423-68-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Pink to red (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Y-33075-dihydrochloride-DataSheet-MedChemExpress
Y-33075 dihydrochloridedye embedding, rats is euthanized and the eyes is enucleated for preparation of retinal flat-mounts. The posterioreyecup is then separated from the vitreous body and postfixed with 4% paraformaldehyde solution in phosphatebuffer for around 1 hour at room temperature. Fluorescence micrographs of the labeled cells is imported using afluorescence microscope connected to a computer. Labeled cells is counted using image analysis software. As anormal group, the subsequent procedure for retrograde labeling with 4-Di-10ASP is performed without graftingsciatic nerve and administering the test drug. Statistical analysis is performed using logarithmically transformedvalues due to differences in variance among the groups. The statistical significance of differences between the normaland saline groups and the saline and Y-33075 groups is examined by t-test (onesided) and William’s test (one-sided).Findings of p < 0.05 is considered significant[2].MCE has not independently confirmed the accuracy of these methods. They are for reference only.CUSTOMER VALIDATION• Science. 2017 Dec 1;358(6367). pii: eaan4368.• Cell. 2018 Jul 26;174(3):636-648.e18.• Neurotox Res. 2013 Apr;23(3):238-48.• Patent. US20170349879A1.See more customer validations on REFERENCES[1]. Hideki Tokushige, et al. Effects of Topical Administration of Y-39983, a Selective Rho-Associated Protein Kinase Inhibitor, on Ocular Tissues in Rabbits and Monkeys Invest. Ophthalmol. Vis. Sci. July 2007 vol. 48no. 7 3216-3222[2]. Tokushige H, et al. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res. 2011 Oct;36(10):964-70.[3]. Watabe H, et al. Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries.Jpn J Ophthalmol. 2011 Jul;55(4):411-7. Epub 2011 Jun 11.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Icariin_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Icariin(Ieariline) is a major constituent of flavonoids from the Chinese medicinal herb Epimedium brevicornum; exhibits multiple biological properties, including anti–inflammatory, neuroregulatory and neuroprotective activities.IC50 value:Target:in vitro: Icariin significantly protected pulmonary function and attenuated CS–induced inflammatory response by decreasing inflammatory cells and production of TNF–α, IL–8 and MMP–9 in both the serum and BALF of CS–exposed mice and decreasing production of TNF–α and IL–8 in the supernatant of CSE–exposed A549 cells [1]. 4 μM or 20 μM Icariin treatment significantlyinhibited the cholesterol ester (CE)/total cholesterol (TC) and oxLDL–mediated foam cell formation (P < 0.05). The binding of oxLDL to LPS–activated macrophages was also significantly hindered by Icariin (P < 0.05). Furthermore, Icariin down–regulated the expression of CD36 in LPS–activated macrophages in a dose–dependent manner and CD36 over–expression restored the inhibitory effect of Icariin on foam cell formation [2].in vivo: icariin treatment leads to alleviated inflammatory infiltration and reduced blood–brain barrier leakage (BBB) of the paracellular tracer (FITC–dextran) in EAE. Mice that received icariin–treated T cells also displayed lower EAE scores and better clinical recovery from EAE. Icariin administration suppresses the frequencies of Th1 and Th17 cells in the splenocytes and lymph node cells. Icariin–treated mice also show lower frequency of Th17 cells in CNS mononuclear cells [3]. Icariin was suspended in carboxymethylcellulose and given orally to APP/PS1 mice. Following an oral treatment of 10 days, Icariin significantly attenuated Aβ deposition, microglial activation and TGF–β1 immunoreactivity at amyloid plaques in cortex and hippocampus of transgenic mice 5 months of age, and restored impaired nesting ability [4].References:[1]. Li L, et al. Icariin ameliorates cigarette smoke induced inflammatory responses via suppression of NF–κB and modulation of GR in vivo and in vitro. PLoS One. 2014 Aug 4;9(8):e102345.[2]. Yang H, et al. Icariin Inhibits Foam Cell Formation By Down–Regulating the Expression of CD36 and Up–Regulating the Expression of SR–BI. J Cell Biochem. 2014 Nov 11.[3]. Shen R, et al. A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis.Int Immunopharmacol. 2014 Dec 17;24(2):224–231.[4]. Zhang ZY, et al. Icariin ameliorates neuropathological changes, TGF–β1 accumulation and behavioral deficits in a mouse model of cerebral amyloidosis.PLoS One. 2014 Aug 7;9(8):e104616.Product Name:Icariin Cat. No.:HY-N0014CAS No.:489-32-7Molecular Formula:C 33H 40O 15Molecular Weight:676.66Target:IKK; Autophagy Pathway:NF–κB; Autophagy Solubility:DMSO: ≥ 34 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Probenecid_57-66-9_DataSheet_MedChemExpress

Product Name:Probenecid CAS No.:57-66-9Cat No :Product Data SheetCat. No.:HY-B0545MWt:285.36Formula:C13H19NO4S Purity :>98%DMSO 57mg/mL;Water <1mg/mL Solubility:Mechanisms:Biological Activity:Pathways:Others; Target:Others DMSO 57 mg/mL; Water <1 mg/mLProbenecid inhibits the renal excretion of organic anions and reduces tubular reabsorption of urate.Target: Others Probenecid is able to prevent the efflux of calcium-sensitive fluorescent dyes during studies of cellular calcium mobilization. Probenecid (2.5 mM) is found to block the export of Fura-2 from1321N1 astrocytoma cells, and does not change the basal calcium concentration or the muscarinic calcium response [1]. Probenecid is able to interact with organic anion transporters (OAT). 0.1 mM Probenecid efficiently inhibits ATP-dependent active vesicular N-ethylmaleimide glutathione (NEM-GS) uptake by Human Multidrug Resistance Proteins 1 (MRP1) and MRP2. In isolated Sf9 cell membranes Probenecid stimulates ATPase activity of MRP2with approximate KACT of 250M References:[1]. McDonough, P.M. and D.C. Button, Measurement of cytoplasmic calcium concentration in cell suspensions: correction for extracellular Fura-2 through use of Mn2+ and probenecid. Cell Calcium,1989. 10(3): p. 171-80.[2]B k E t l I t ti f th h ltid i t t i MRP1d MRP2ith membranes, Probenecid stimulates ATPase activity of MRP2 with approximate KACT of 250 μM,but inhibits ATPase activity of MRP1 [2]. Probenecid is an inhibitor of the hTAS2R16, hTAS2R38,and h...[2]. Bakos, E., et al., Interactions of the human multidrug resistance proteins MRP1 and MRP2 withorganic anions. Mol Pharmacol, 2000. 57(4): p. 760-8.[3]. Greene, T.A., et al., Probenecid inhibits the human bitter taste receptor TAS2R16 andsuppresses bitter perception of salicin. PLoS One, 2011. 6(5): p. e20123.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
8 利多卡因

10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于 98%
有 各 Kou 类 q: 2 标 8 准 5 品 3 对 1 照 1 品 7 和 7 杂 5 质 2
57333-95-6
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于 98%
曲克芦丁 杂质 1
Troxerutin Impurity 1
6980-20-7
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于 98%
阿法替尼 杂质 23
Afatinib Impurity 23 Methanol
138071-82-6
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于 98%
项目报批 纯度高于 98%
项目报批 纯度高于 98%
依维莫司 杂质 8
Everolimus Impurity 8 (211-97) (Everolimus EP
Impurity C
908340-97-6
2121530-37-6
10mg 25mg 50mg 100mg 更大规格请咨询
氨基葡萄 糖rochloride Impurity 16
576-44-3
10mg 25mg 50mg 100mg 更大规格请咨询
伽多布特 罗单体
Gadobutrol monomer
利多卡因、伦伐替尼、他卡西托、曲克芦丁、阿法替尼、氨基葡萄
糖盐酸盐、伽多布特罗、依维莫司类杂质列表
中文名称
英文名称
利多卡因 杂质 9
Lidocaine Impurity 9 (Lidocaine EP Impurity I)
Dirithromycin_62013-04-1_DataSheet_MedChemExpress

Product Name:Dirithromycin CAS No.:62013-04-1Cat. No.:HY-B0643Product Data SheetMWt:835.07Formula:C42H78N2O14Purity :>98%Solubility:DMSO 10 mg/mL; Water <1y Mechanisms:Biological Activity:Dirithromycin(LY 237216)is a macrolide glycopeptide antibiotic by binding to the 50S subunit of thePathways:Anti-infection; Target:Antibacterial gmg/mL; Ethanol 100 mg/mLDirithromycin(LY 237216) is a macrolide glycopeptide antibiotic by binding to the 50S subunit of the70S bacterial ribosome to inhibit the translocation of peptides.Target: Antibacterial Dirithromycin is a new macrolide with a spectrum and degree of in vitro antimicrobial activity similar to that of erythromycin. Compared with erythromycin, dirithromycin has a long elimination half-life enabling once-daily administration, and it also achieves a greater cellular:extracellular concentration ratio and higher concentration in some tissues. Multicentre double-blind clinical trials have shown dirithromycin to be similar in efficacy to erythromycin in the treatment of uncomplicated bacterial infections of the respiratory tract and of skin and soft tissues [1]. Dirithromycin offers some attractive References:[1]. Brogden, R.N. and D.H. Peters, Dirithromycin. A review of its antimicrobial activity,pharmacokinetic properties and therapeutic efficacy. Drugs, 1994. 48(4): p. 599-616.[2]. Wintermeyer, S.M., S.M. Abdel-Rahman, and M.C. Nahata, Dirithromycin: a new macrolide. Ann p y []ypharmacokinetic properties. The long elimination half-life of dirithromycin all...Pharmacother, 1996. 30(10): p. 1141-9.[3]. Sides, G.D., et al., Pharmacokinetics of dirithromycin. J Antimicrob Chemother, 1993. 31 SupplC: p. 65-75.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Voreloxin Hydrochloride_175519-16-1_DataSheet_MedChemExpress

Product Name:Voreloxin Hydrochloride CAS No.:175519-16-1Cat. No.:HY-16518Product Data SheetMWt:437.90Formula:C18H20ClN5O4S Purity :>98%Solubility:DMSOMechanisms:Biological Activity:Voreloxin Hcl(SNS-595;AG 7352)is a small molecule and a naphthyridine analogue withPathways:Cell Cycle/DNA Damage; Target:Topoisomerase Voreloxin Hcl(SNS 595; AG 7352) is a small molecule and a naphthyridine analogue withantineoplastic activity; inhibitor of Topo II.IC50 Value:Target: Topoisomerase II Vosaroxin intercalates into DNA in a site-specific manner and blocks the re-ligation process carried out by topoisomerase II during DNA replication. As a result, inhibition of DNA replication, RNA and protein synthesis occurs, followed by cell cycle arrest at G2 phase and induced p53-independent apoptosis. This agent shows a favorable toxicity profile in several aspects: it does not generate reactive oxygen species, as do anthracyclines, reducing the risk of cardiotoxicity; it is not a P-References:[1]. Hoch U, Lynch J, Sato Y et al. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009Jun;64(1):53-65.glycoprotein (P-gp) substrate, and thereby evades the common mechanism for multidrug resistance;and it has limited distribution to normal tissues and a more chemically stable molecular structure....;()[2]. Hawtin RE, Stockett DE, Byl JA et al. Voreloxin is an anticancer quinolone derivative thatintercalates DNA and poisons topoisomerase II. PLoS One. 2010 Apr 15;5(4):e10186.[3]. Lancet JE, Ravandi F, Ricklis RM et al. A phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia. 2011 Dec;25(12):1808-14.[4]. Krug LM, Crawford J, Ettinger DS et al. Phase II multicenter trial of voreloxin as second-line therapy in chemotherapy-sensitive or refractory small cell lung cancer. J Thorac Oncol. 2011Feb;6(2):384-6.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[5]. Advani RH, Hurwitz HI, Gordon MS et al. Voreloxin, a first-in-class...18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Dirithromycin(LY 237216) is a macrolide glycopeptide antibiotic by binding to the 50S subunit of the 70S bacterial ribosome to inhibit the translocation of peptides.
Target: Antibacterial
Dirithromycin is a new macrolide with a spectrum and degree of in vitro antimicrobial activity similar to that of erythromycin.
Compared with erythromycin, dirithromycin has a long elimination half–life enabling once–daily administration, and it also achieves a greater cellular:extracellular concentration ratio and higher concentration in some tissues. Multicentre double–blind clinical trials have shown dirithromycin to be similar in efficacy to erythromycin in the treatment of uncomplicated bacterial infections of the respiratory tract and of skin and soft tissues [1]. Dirithromycin offers some attractive pharmacokinetic properties. The long elimination half–life of dirithromycin allows once–daily dosing and higher and more prolonged tissue concentrations than are achievable with erythromycin.The spectrum of activity, adverse effect profile, clinical efficacy, and bacteriologic eradication rate of dirithromycin may be similar to those of erythromycin [2, 3].
References:
[1]. Brogden, R.N. and D.H. Peters, Dirithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs, 1994.48(4): p. 599–616.
[2]. Wintermeyer, S.M., S.M. Abdel–Rahman, and M.C. Nahata, Dirithromycin: a new macrolide. Ann Pharmacother, 1996. 30(10): p. 1141–9.
[3]. Sides, G.D., et al., Pharmacokinetics of dirithromycin. J Antimicrob Chemother, 1993. 31 Suppl C: p. 65–75.
Product Name:
Dirithromycin Cat. No.:
HY-B0643CAS No.:
62013-04-1Molecular Formula:
C 42H 78N 2O 14Molecular Weight:
835.07Target:
Bacterial Pathway:
Anti–infection Solubility:
10 mM in DMSO
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
