p0103 protein-how our body use it and how much we need 蛋白质-机体的利用与需要量
Nature探寻NLRP3炎症小体激活之谜
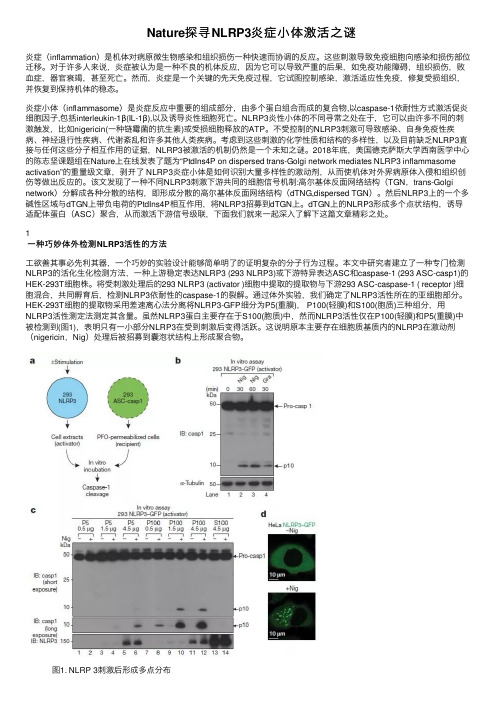
Nature探寻NLRP3炎症⼩体激活之谜炎症(inflammation)是机体对病原微⽣物感染和组织损伤⼀种快速⽽协调的反应。
这些刺激导致免疫细胞向感染和损伤部位迁移。
对于许多⼈来说,炎症被认为是⼀种不良的机体反应,因为它可以导致严重的后果,如免疫功能障碍,组织损伤,败⾎症,器官衰竭,甚⾄死亡。
然⽽,炎症是⼀个关键的先天免疫过程,它试图控制感染,激活适应性免疫,修复受损组织,并恢复到保持机体的稳态。
炎症⼩体(inflammasome)是炎症反应中重要的组成部分,由多个蛋⽩组合⽽成的复合物,以caspase-1依耐性⽅式激活促炎细胞因⼦,包括interleukin-1β(IL-1β),以及诱导炎性细胞死亡。
NLRP3炎性⼩体的不同寻常之处在于,它可以由许多不同的刺激触发,⽐如nigericin(⼀种链霉菌的抗⽣素)或受损细胞释放的ATP。
不受控制的NLRP3刺激可导致感染、⾃⾝免疫性疾病、神经退⾏性疾病、代谢紊乱和许多其他⼈类疾病。
考虑到这些刺激的化学性质和结构的多样性,以及⽬前缺乏NLRP3直接与任何这些分⼦相互作⽤的证据,NLRP3被激活的机制仍然是⼀个未知之谜。
2018年底,美国德克萨斯⼤学西南医学中⼼的陈志坚课题组在Nature上在线发表了题为“PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation”的重量级⽂章,剥开了 NLRP3炎症⼩体是如何识别⼤量多样性的激动剂,从⽽使机体对外界病原体⼊侵和组织创伤等做出反应的。
该⽂发现了⼀种不同NLRP3刺激下游共同的细胞信号机制:⾼尔基体反⾯⽹络结构(TGN,trans-Golgi network)分解成各种分散的结构,即形成分散的⾼尔基体反⾯⽹络结构(dTNG,dispersed TGN)。
然后NLRP3上的⼀个多碱性区域与dTGN上带负电荷的PtdIns4P相互作⽤,将NLRP3招募到dTGN上。
EMSA,凝胶阻滞实验技术文献,以及很好protocol,troubleshooting

Aromatic Hydrocarbon Receptor(AhR)⅐AhR NuclearTranslocator-and p53-mediated Induction of theMurine Multidrug Resistance mdr1Gene by3-Methylcholanthrene and Benzo(a)pyrene in Hepatoma Cells*Received for publication,September18,2000,and in revised form,November10,2000Published,JBC Papers in Press,November28,2000,DOI10.1074/jbc.M008495200Marie-Claude Mathieu,Isabelle Lapierre,Karine Brault‡,and Martine Raymond§From the Institut de Recherches Cliniques de Montre´al,Montre´al,Que´bec H2W1R7,CanadaThe mouse multidrug resistance gene family consists of three genes(mdr1,mdr2,and mdr3)encoding P-gly-coprotein.We show that the expression of mdr1is in-creased at the transcriptional level upon treatment of the hepatoma cell line Hepa-1c1c7with the polycyclic aromatic hydrocarbon3-methylcholanthrene(3-MC). This increase is not observed in the aromatic hydrocar-bon receptor(AhR)-defective TAOc1BP r c1and the AhR nuclear translocator(Arnt)-defective BP r c1variants, demonstrating that the induction of mdr1by3-MC re-quires AhR⅐Arnt.We show that the mdr1promoter (؊1165to؉84)is able to activate the expression of a reporter gene in response to3-MC in Hepa-1c1c7but not in BP r c1cells.Deletion analysis indicated that the re-gion from؊245to؊141contains cis-acting sequences mediating the induction,including a potential p53bind-ing sequence.3-MC treatment of the cells increased the levels of p53and induced p53binding to the mdr1pro-moter in an AhR⅐Arnt-dependent manner.Mutations in the p53binding site abrogated induction of mdr1by 3-MC,indicating that p53binding to the mdr1promoter is essential for the induction.Benzo(a)pyrene,a polycy-clic aromatic hydrocarbon and AhR ligand,which,like 3-MC,is oxidized by metabolizing enzymes regulated by AhR⅐Arnt,also activated p53and induced mdr1tran-scription.2,3,7,8-Tetrachlorodibenzo-p-dioxin,an AhR ligand resistant to metabolic breakdown,had no effect. These results indicate that the transcriptional induc-tion of mdr1by3-MC and benzo(a)pyrene is directly mediated by p53but that the metabolic activation of these compounds into reactive species is necessary to trigger p53activation.The ability of the anticancer drug and potent genotoxic agent daunorubicin to induce mdr1independently of AhR⅐Arnt further supports the proposition that mdr1is transcriptionally up-regulated by p53in response to DNA damage.Multidrug resistance(MDR)1is characterized by cross-resis-tance of the cells to a large number of structurally and func-tionally unrelated cytotoxic agents used in chemotherapy.In cultured cells,MDR is frequently caused by the overexpression of P-glycoprotein(Pgp),an integral membrane protein belong-ing to the ATP-binding cassette superfamily of transporters and which functions as an energy-dependent efflux pump of cytotoxic drugs(1,2).Pgp is encoded by a small family of genes with two members in humans(MDR1and MDR2/MDR3)and three in rodents(mdr1/mdr1b,mdr2,and mdr3/mdr1a)(1,2). Only one human gene(MDR1)and two rodent genes(mdr1/ mdr1b and mdr3/mdr1a)can confer MDR upon overexpression in drug-sensitive cells(1,2).The different mdr genes and Pgp isoforms are expressed in a tissue-specific manner(1,2).In the mouse,mdr1is expressed mostly in the adrenal cortex,kidney,and pregnant uterus, mdr2in the liver at the canalicular face,and mdr3in the intestine and to a lesser extent in the heart,liver,lung,and capillaries of the brain(3).Pgps are localized on the apical membrane of epithelial cells lining luminal spaces,suggesting that they function in normal tissues as transporters of toxic substances and/or specific endogenous cellular products(4). Knockout mice experiments have demonstrated a role for the mdr3gene in the maintenance of the blood-brain barrier and drug elimination and for the mdr2gene in the transport of phospholipids in the bile(5,6).No physiological function has been attributed to the mouse mdr1gene so far,since knockout mdr1(Ϫ/Ϫ)mice display no obvious physiological abnormali-ties(7).However,different experimental evidence indicates that Pgp encoded by mdr1can serve in the transport of steroids(8).A number of factors have been found to modulate the level of mdr gene expression in the liver.For example,high levels of MDR1RNA have been found in human hepatocarcinomas,and overexpression of the mdr1isoforms has also been observed in rodent liver during cholestasis,during regeneration following partial hepatectomy,during chemically induced hepatocarcino-genesis,and following administration of various natural and synthetic xenobiotics(1,2).In particular,it has been shown that expression of the rat mdr1b gene is increased in liver cells in response to treatment with various polycyclic aromatic hy-*This work was supported by a research grant from the Cancer Research Society Inc.(to M.R).The costs of publication of this article were defrayed in part by the payment of page charges.This article must therefore be hereby marked“advertisement”in accordance with18 U.S.C.Section1734solely to indicate this fact.‡Supported by a studentship from the Medical Research Council ofCanada.Present address:Dept.of Biological Sciences,Bio-Mega Re-search Division,Boehringer Ingelheim(Canada)Ltd.,Laval,Que´bec H7S2G5,Canada.§Supported by a scholarship from Le Fonds de la recherche en sante´du Que´bec.To whom correspondence should be addressed:Institut de recherches cliniques de Montre´al,110Pine Ave.W.,Montre´al,Que´bec H2W1R7,Canada.Tel.:514-987-5770;Fax:514-987-5764;E-mail: raymonm@ircm.qc.ca.1The abbreviations used are:MDR,multidrug resistance;Pgp,P-glycoprotein;3-MC,3-methylcholanthrene;B(a)P,benzo(a)pyrene; TCDD,2,3,7,8-tetrachlorodibenzo-p-dioxin;DN,daunorubicin;CAT, chloramphenicol acetyl transferase;AhR,aromatic hydrocarbon recep-tor;Arnt,AhR nuclear translocator;EMSA,electrophoretic mobility shift assay;DME,drug metabolizing enzymes;PAH polycyclic aromatic hydrocarbon;XRE,xenobiotic response element;bp,base pair(s);kb, kilobase pair(s).T HE J OURNAL OF B IOLOGICAL C HEMISTRY Vol.276,No.7,Issue of February16,pp.4819–4827,2001©2001by The American Society for Biochemistry and Molecular Biology,Inc.Printed in U.S.A.This paper is available on line at 4819 at ZHEJIANG UNIVERSITY, on November 21, Downloaded fromdrocarbon(PAH)compounds,including3-methylcholanthrene (3-MC),and that this increased expression occurs at the tran-scriptional level(9–11).However,the precise molecular mech-anisms involved in mdr1b regulation in response to3-MC are still unknown.PAHs are carcinogenic compounds arising from the incom-plete combustion of organic matter and are widespread in the environment,including tobacco smoke and tar.PAHs such as 3-MC and benzo(a)pyrene(B(a)P)as well as halogenated aro-matic hydrocarbons such as2,3,7,8-tetrachlorodibenzo-p-di-oxin(TCDD)are specific inducers of genes coding for drug-metabolizing enzymes(DME),including cyp1a1and cyp1a2, that code for cytochromes P450involved in metabolic oxidation (12).PAHs and TCDD bind in the cytoplasm to the aromatic hydrocarbon receptor(AhR),a member of the bHLH-PAS(basic helix-loop-helix Per-Arnt-Sim)family of transcription factors (12,13).The ligand-bound AhR translocates to the nucleus, where it binds as a heterodimer with the AhR nuclear trans-locator(Arnt;another bHLH-PAS protein)to specific cis-acting regulatory DNA sequences located in the promoter of its tar-gets(known as AH-,dioxin-,or xenobiotic-responsive elements (or AHRE,DRE,or XRE,respectively))to enhance their tran-scription(12,13).Given that mdr1b expression is increased in liver cells in response to treatment with various PAHs,it was postulated that mdr1b may be under the control of the AhR(9). However,studies failing to show mdr1induction in the liver of mice treated with TCDD,one of the most potent agonists of the AhR,suggested that mdr1expression was not regulated by AhR(14).The involvement of AhR in the regulation of mdr1 has so far remained controversial.The mouse hepatoma cell lines Hepa-1c1c7(wild type), TAOc1BP r c1(AhR-defective),and BP r c1(Arnt-defective)con-stitute a powerful experimental system to investigate the tran-scriptional regulation of different AhR⅐Arnt targets in response to xenobiotics(12).The two mutant cell lines were derived as B(a)P-resistant variants of Hepa-1c1c7and were identified based on their inability to induce aryl hydrocarbon hydroxylase activity in response to TCDD treatment(15).TAOc1BP r c1cells have a decreased level of AhR(ϳ10%of wild-type cells)and therefore decreased induction of the cyp1a1promoter and lower aryl hydrocarbon hydroxylase activity in response to TCDD and other AhR ligands(15–18).BP r c1cells have a nor-mal cytosolic AhR,which fails to accumulate in the nucleus because of a defective Arnt(15).They have virtually no basal or inducible levels of cyp1a1expression and aryl hydrocarbon hydroxylase activity(15–17).In the present report,we have used this panel of cell lines to investigate the transcriptional regulation of the murine mdr1 gene by3-MC and other xenobiotic compounds.Our results demonstrate that mdr1is transcriptionally induced by3-MC and B(a)P and that this induction is mediated by p53but also requires AhR⅐Arnt.A model for the AhR⅐Arnt-and p53-medi-ated transactivation of mdr1in response to genotoxic stress is proposed.EXPERIMENTAL PROCEDURESCell Culture—Wild-type Hepa-1c1c7and Hepa1–6,AhR-defective TAOc1BP r c1,and Arnt-defective BP r c1cells were obtained from the American Type Culture Collection(ATCC;Manassas,VA)and main-tained in culture under the conditions recommended by the ATCC. Chinese hamster ovary LR73cell lines stably transfected with plasmid constructs carrying full-length cDNAs for the mouse mdr1,mdr2,or mdr3genes(LR73mdr1,LR73mdr2,and LR73mdr3,respectively;a gift from Dr.Philippe Gros,McGill University,Montre´al,Canada)were grown as described elsewhere(19,20).For inductions,cells atϳ50% confluence were exposed to different concentrations of xenobiotics for various periods of time(the exact conditions for each experiment are indicated in the figure legends).3-MC,B(a)P,and daunorubicin were obtained from Sigma,and TCDD was obtained from the Centre d’expertise en analyze environnementale du Que´bec(Laval,Canada).Stock solutions of3-MC(5m M)and B(a)P(25m M)were prepared in Me2SO,and the stock solutions of daunorubicin(1mg/ml)were pre-pared in water.TCDD was obtained in n-nonane at a concentration of 50g/ml and was stored at room temperature.Stock solutions of3-MC, B(a)P,and daunorubicin were stored atϪ80°C.RNA Preparation—Total RNA was prepared from3-MC-treated and untreated hepatocytes as well as from the LR73mdr1,LR73mdr2,and LR73mdr3cell lines by homogenizing the cells in a solution containing guanidium hydrochloride(6M)followed by sequential ethanol precipi-tation,as described previously(21).RNase Protection Assay—The plasmid constructed to detect the mdr1 RNA consisted of a165-bp Bam HI fragment isolated from the mdr1 cDNA(positions1926–2090relative to the ATG initiation codon(22)), blunt-ended with T4DNA polymerase,and cloned into plasmid pGEM-7Z(Promega,Madison,WI)at the Sma I site,giving plasmid pmdr1-G7.This plasmid was linearized with Eco RI and used as a template to synthesize an antisense mdr1probe using SP6RNA polym-erase(Amersham Pharmacia Biotech).The pKX10–3Z plasmid consist-ing of an Xba I–Kpn I mouse-actin cDNA fragment(positions724–969 in the-actin cDNA)cloned into pGEM-3Z at the Xba I and Kpn I sites (kindly provided by Dr.Rashmi Kothary,Institut du cancer de Mon-tre´al,Montre´al,Canada)was used to generate a control actin probe. pKX10–3Z was linearized with Xba I and used to synthesize an anti-sense actin RNA probe with T7RNA polymerase.The riboprobes were synthesized in the presence of[␣-32P]UTP,and the RNase protection assay was performed according to standard protocols(23).Nuclear Run-on Transcription Assay—The run-on experiment was performed essentially as described by Fisher et al.(24).Nuclei wereisolated from Hepa-1c1c7cells treated with Me2SO or with3-MC(5M) for48h and were used to label nascent RNAs with[␣-32P]UTP.Plas-mids pVT101-U/mdr1,carrying the full-length mouse mdr1cDNA(25); pmP1450–3Ј,carrying a1.2-kb Pst I cDNA fragment overlapping part of the mouse cyp1a1cDNA(26)(obtained from the ATCC);and pKX10–3Z were linearized with Stu I,Bam HI,and Xba I,respectively.The linear-ized plasmids were denatured,immobilized in duplicate onto a nylon membrane,and hybridized with the[␣-32P]UTP-labeled RNAs for48h at65°C.The membranes were washed and exposed for7days with two intensifying screens.Slot Blot Analyses—Slot blotting was performed as previously de-scribed(21).RNA samples(10g)were denatured in7ϫSSC-7.5% formaldehyde for15min at65°C and applied to a nylon membrane (Zeta-Probe).Detection of specific RNAs was performed by hybridiza-tion at65°C in0.5M NaPO4,pH7.2,1m M EDTA,7%SDS,1%bovine serum albumin,and100g/ml salmon sperm DNA with32P-labeled DNA probes.The mdr1probe was a4.2-kb Sph I–Eco RI fragment over-lapping the full-length mouse mdr1cDNA,isolated from plasmid pGEM7/mdr1(a gift from Dr.Philippe Gros,McGill University,Mon-tre´al);the cyp1a1probe was a 1.2-kb Pst I fragment isolated from plasmid pmP1450–3Ј;and the actin probe was a245-bp Xba I–Kpn I fragment isolated from pKX10–3Z.The membranes were washed twiceat65°C with a solution containing40m M NaPO4,pH7.2,5%SDS,1 m M EDTA,0.5%bovine serum albumin and twice with a solutioncontaining40m M NaPO4,pH7.2,5%SDS,and1m M EDTA before autoradiography.Chloramphenicol Acetyl Transferase(CAT)Expression Plasmids—Plasmid pMcat5.9consists of a482-bp DNA fragment containing the dioxin-responsive elements of the cyp1a1gene cloned upstream of the mouse mammary tumor virus promoter and the CAT gene(24)(kindly provided by Dr.Allan Okey,University of Toronto).Plasmids pmdr1, p-452,p-245,p-141,and p-93(previously referred to as pSacICAT, pExo6CAT,pExo2CAT,pExo1CAT,and pAluCAT,respectively)have been described elsewhere(27).The mdr1promoter sequence in these constructs ends at positionϩ84with respect to the transcription start site(27).To produce the p53mutant constructs,pM1and pM2,plasmid pSBM13was used.This plasmid consists of a1.2-kb Sac I–Hin dIII mdr1 promoter fragment(positionsϪ1165toϩ84)cloned into M13mp18. Single-stranded DNA was prepared from pSBM13and used as a tem-plate to perform site-directed mutagenesis of the p53binding site,using the mutant oligonucleotides M15Ј-TACCTGAA T AC A TAAAGACA and M25Ј-CGTAAAGA T AA A TCTATGTA(the base changes are shown in boldface type).The resulting M1and M2mdr1promoter fragments were then excised from pSBM13with Sac I and Hin dIII,blunt-ended with T4DNA polymerase,and cloned into plasmid pCAT at the Hin dIII site also blunt-ended with T4DNA polymerase,yielding plasmids pM1 and pM2.The presence of the mutations in the resulting constructs was confirmed by DNA sequencing.Transient Transfections and CAT Assays—Cells were plated at aInduction of the Mouse mdr1Gene by PAHs4820at ZHEJIANG UNIVERSITY, on November 21, Downloaded fromdensity of 8ϫ105/60-mm plate and transfected on the following day with 10g of plasmid DNA,using a standard calcium phosphate pre-cipitation method (28).After incubation with the DNA precipitate for 16h,the cells were washed twice with phosphate-buffered saline and supplied with fresh medium containing the different xenobiotics.After 48h,the cells were collected.Cell extracts were prepared,and protein concentrations were determined by the Bradford method (29).CAT activities were assayed by standard protocols as described previously,using 2g of proteins (27).Preparation of Nuclear Extracts—Nuclear extracts were prepared according to Schreiber et al .(30),with some modifications.Cells were harvested in cold phosphate-buffered saline,0.6m M EDTA and col-lected by centrifugation.The cell pellets were resuspended in 400l of ice-cold buffer A (10m M Tris,pH 8.0,10m M KCl,0.1m M EDTA,0.1m M EGTA,1m M dithiothreitol)containing 0.5m M phenylmethylsulfonyl fluoride,10g/ml aprotinin,1g/ml pepstatin,and 5g/ml leupeptin and swelled on ice for 15min.Subsequently,25l of 10%Nonidet P-40were added,and the tubes were vortexed vigorously.The nuclear pellets were collected by centrifugation and resuspended in 100l of cold buffer C (20m M Tris,pH 8.0,400m M NaCl,1m M EDTA,1m M EGTA,1m M dithiothreitol)in the presence of protease inhibitors.The suspen-sions were shaken vigorously at 4°C for 1h and centrifuged for 15min at 4°C,and the supernatants were frozen in aliquots at Ϫ80°C.Proteinconcentrations were determined by the Bradford method (29).ElectrophoreticMobility Shift Assay—Oligonucleotides overlapping the potential p53binding site in the mdr1promoter (5Ј-GAACACGTA-AAGACAAGTCTAT)and the p53consensus sequence in the p21waf1/cip1promoter (5Ј-GAACATGTCCCAACATGTTGAG)(31)were end-labeled with ␥-32P using T4polynucleotide kinase and annealed to their respec-tive in a M M 2.5m M dithiothreitol,4%Ficoll,1g of poly(dI-dC),and 20,000cpm of radiolabeled probe.The binding reactions were carried out at room temperature for 15min.Where needed,1g of the monoclonal anti-p53antibody pAb421(32)(Calbiochem)or of the polyclonal anti-Jun or anti-Skn-1antibodies (Santa Cruz Biotechnology,Inc.,Santa Cruz,CA)was added,and the incubation was continued for an additional 15min.The complexes were separated on 5%nondenaturing polyacrylamide gels in 1ϫTBE (90m M Tris,65m M boric acid,2.5m M EDTA,pH 8.0)at 200V.The gels were exposed to XAR films (Eastman Kodak Co.)for 16h with two intensifying screens at Ϫ80°C.Western Blotting—Total proteins from 3-MC-or Me 2SO-treated Hepa-1c1c7and BP r c1cells were extracted in ice-cold buffer (10m M Tris-HCl,pH 8.0,150m M NaCl,1m M EDTA,1%Nonidet P-40,and 1%sodium deoxycholate)containing 10g/ml leupeptin,10g/ml aproti-nin,1M sodium orthovanadate,and 1m M phenylmethylsulfonyl flu-oride.Total proteins (75g/sample)or nuclear extracts (30g/sample)were separated by SDS-polyacrylamide gel electrophoresis on a 10%acrylamide gel,transferred to a nitrocellulose membrane,and analyzed with the monoclonal anti-p53antibody pAb421(32)(Calbiochem)at a concentration of 5g/ml.Immune complexes were revealed by incuba-tion with a goat anti-mouse IgG antibody coupled to alkaline phospha-tase (Bio-Rad)and developed with 5-bromo-4-chloro-3-indolylphosphate p -toluidine salt and nitro blue tetrazolium chloride substrates as rec-ommended by the manufacturer (Life Technologies,Inc.).RESULTSTranscriptional Induction of the Mouse mdr1Gene by 3-MC in Hepatoma Cells—We have used an RNase protection assay to study the expression of mdr1in the hepatoma cell line Hepa-1c1c7upon exposure to 3-MC (Fig.1).An mdr1-specific riboprobe was prepared by cloning into pGEM7-Zf a mouse mdr1cDNA fragment overlapping the linker region of the protein,this domain displaying the lowest sequence homology among the three mouse mdr cDNAs (21).When tested with RNA prepared from LR73stable transfectants expressing each of the three mouse mdr cDNAs,the mdr1riboprobe was found to recognize the mdr1RNA but not the mdr2or mdr3RNA,thus confirming its specificity (Fig.1,top right ).The mdr1probe was then used with RNA from Hepa-1c1c7cells treated or not with 3-MC (Fig.1,top left ).This experiment showed that the amount of mdr1RNA detected is very low in untreated cells but is strongly increased in 3-MC-treated cells,demonstrating that expression of the mouse mdr1gene is induced by 3-MCtreatment.The use of an actin probe confirmed that equal quantities of RNA were used in the assay (Fig.1,bottom ).A similar experiment performed with mdr2-and mdr3-specific riboprobes showed that the expression of these genes is not induced under such conditions,demonstrating that the induc-tion of mdr1expression by 3-MC is isoform-specific (data not shown).A nuclear run-on experiment was performed to determine whether mdr1induction by 3-MC occurs at the transcriptional level (Fig.2).In addition to the mouse mdr1cDNA,cDNAs for the mouse cyp1a1gene (known to be transcriptionally regu-lated by 3-MC (12))and for the actin gene were also included as positive and negative controls,respectively.The data in Fig.2show that 3-MC induces an increase in the rate of mdr1mRNA synthesis,indicating that 3-MC acts at the transcriptional level to induce mdr1gene expression in Hepa-1c1c7cells.AhR ⅐Arnt-dependent Induction of mdr1Expression by 3-MC—To determine whether the increase in mdr1expression in response to 3-MC exposure is AhR ⅐Arnt-mediated,we ana-lyzed the mdr1RNA levels upon 3-MC treatment in two wild-type hepatoma cell lines Hepa-1c1c7and Hepa 1–6and in two variant cell lines derived from Hepa-1c1c7,TAOc1BP r c1(AhR-defective)and BP r c1(Arnt-defective)(15)(Fig.3).As controls,we also analyzed the level of cyp1a1and actin expression under the same conditions (Fig.3,middle and right ,respectively).This experiment showed that mdr1is expressed at low levels in the four cell lines in the absence of 3-MC induction (Fig.3,left panel ).Upon 3-MC treatment,the expression of mdr1is in-duced in the two wild-type hepatoma cell lines (by ϳ5-fold),this induction being completely abrogated in the AhR-defective or in the Arnt-defective variants (Fig.3,left panel ).The actin control probe confirmed that equal amounts of RNA had been applied to the membrane (Fig.3,right panel ).These data clearly demonstrate that the induction of mdr1in response to 3-MC requires an intact AhR ⅐Arnt complex,like cyp1a1(Fig.3,middle )(12).The Mouse mdr1Promoter Confers 3-MC-regulated Expres-sion in an AhR ⅐Arnt-dependent Manner—To determine if reg-ulatory sequences responsible for mdr1induction by 3-MC are present in the promoter region of the gene,plasmid pmdr1,consisting of a 1.2-kb Sac I–Hin dIII DNA fragment overlapping the mdr1promoter region (positions Ϫ1165to ϩ84with respect to the transcription start site (27))fused to the CAT reporter gene,was analyzed in transient transfection experiments.Plasmid pMcat5.9,which consists of a 482-bp fragment derived from the cyp1a1promoter fused to the mouse mammary tumorF IG .1.Increased mdr1expression in Hepa-1c1c7upon 3-MC treatment.The expression of mdr1was analyzed by RNase protection assay.Total RNAs (45g)from Hepa-1c1c7cells treated with 5M 3-MC (ϩMC )or with Me 2SO (ϪMC )for 56h and from the control cell lines LR73/mdr1,LR73/mdr2,and LR73/mdr3were analyzed with an mdr1riboprobe,which protects a 169-nt fragment within the mdr1transcript,or with a -actin riboprobe,which protects a 245-nt actin transcript fragment.Autoradiography was for 15h with two intensify-ing screens (mdr1)or for 5h without intensifying screens (actin ).Induction of the Mouse mdr1Gene by PAHs4821at ZHEJIANG UNIVERSITY, on November 21, 2012 Downloaded fromvirus promoter and to the CAT gene (24),as well as the empty pCAT vector were also included as positive and negative con-trols,respectively.The three plasmids were transiently trans-fected into Hepa-1c1c7and BP r c1cells.The cells were treated with 3-MC or with Me 2SO for 48h,and the cellular extracts were prepared and assayed for CAT activity.This experiment showed that the mdr1promoter is transcriptionally active in Hepa-1c1c7cells and BP r c1cells,since it can drive the expres-sion of the CAT gene in both cell lines,albeit at low levels (Fig.4).This result is consistent with the basal level of expression of mdr1detected by slot blot analysis in these cells (Fig.3).3-MC treatment of the Hepa-1c1c7cells transfected with pmdr1re-sulted in a 10-fold induction in CAT activity as compared with untreated cells,reaching levels of CAT activity similar to those detected in the Hepa-1c1c7pMcat5.9transfectants upon 3-MC treatment.However,this induction was completely abrogated in BP r c1cells (Fig.4),consistent with the lack of mdr1induc-tion at the RNA level observed in the slot blot assay (Fig.3).Similar results were obtained upon transfection in TAOc1BP r c1cells (data not shown).These results,showing that the mdr1promoter is able to activate the expression of the reporter gene in response to 3-MC in Hepa-1c1c7but not in BP r c1and TAOc1BP r c1cells,demonstrate that (i)the mdr1promoter is able to confer 3-MC-mediated transcriptional acti-vation;(ii)this activation requires a functional AhR ⅐Arnt com-plex;and (iii)the sequences mediating this induction are lo-cated between positions Ϫ1165and ϩ84in the mdr1promoter.Two Putative XREs Located in the mdr1Promoter Are Dis-pensable for the Induction of mdr1by 3-MC—The AhR ⅐Arnt transcriptional complex binds to a specific DNA sequence,5Ј-(A/T)NGCGTG,known as an XRE to activate transcription (12).XREs render heterologous promoters responsive to xeno-biotics and function in a position-and orientation-independent manner (33,34).Examination of the mdr1promoter sequence indicated the presence of two potential XREs in an inverted orientation in the distal portion of the promoter at positionsϪ1129and Ϫ620(5Ј-CACGCAT and 5Ј-CACGCAA,respective-ly).To identify the cis -acting sequences responsible for the induction of mdr1by 3-MC and to investigate the possible involvement of these putative XREs,we analyzed the tran-scriptional activity of a series of mdr1promoter 5Ј-deletion CAT constructs after transient transfection into Hepa-1c1c7and treatment of the resulting transfectants with 3-MC (Fig.5A ).3-MC treatment of Hepa-1c1c7cells transfected with plas-mids p-452or p-245resulted in a level of CAT induction similar to that observed in cells transfected with plasmid pmdr1car-rying the full-length promoter,indicating that sequences lo-cated within positions Ϫ1165to Ϫ245are dispensable for the induction of mdr1by 3-MC,including the two putative XREs as well as a potential AP-1binding site (5Ј-TGACTCA;positions Ϫ265to Ϫ255(35))(Fig.5,B and C ).However,further deletion of a 104-bp region down to position Ϫ141(p Ϫ141)was found to greatly diminish the induction of CAT activity by 3-MC (Fig.5,B and C ),demonstrating that sequences important for the induction are located between positions Ϫ245and Ϫ141.CAT activity in the absence of 3-MC was reduced in the p Ϫ141transfectants when compared with the p Ϫ245transfectants,indicating that sequences between positions Ϫ245and Ϫ141are also involved in the basal transcriptional activity of the mdr1promoter in hepatoma cells.Finally,we found that alowF IG .2.Nuclear run-on experiment.Nuclei were isolated from Hepa-1c1c7cells treated with 5M 3-MC (ϩMC )or with Me 2SO (ϪMC )for 48h.Nascent RNAs were radiolabeled with [␣-32P]UTP and used to probe duplicate nylon membranes on which denatured cDNAs for mdr1,cyp1a1,and actin had been immobilized.The membranes were washed and exposed for 7days with two intensifyingscreens.F IG .3.AhR ⅐Arnt-dependent induction of mdr1expression by 3-MC.Total RNAs (10g)from wild-type Hepa-1c1c7and Hepa 1–6,AhR-defective TAOc1BP r c1,and Arnt-defective BP r c1cells treated (ϩMC )or not treated (ϪMC )with 3-MC at 5M for 56h were applied onto a nylon membrane.The membrane was hybridized sequentially with an mdr1(left ),a cyp1a1(middle ),and a -actin (right )probe.Autoradiography was for 18h (mdr1and cyp1a1)or for 2h (actin)with two intensifyingscreens.F IG .4.AhR ⅐Arnt-dependent induction of the mdr1promoter by 3-MC.Plasmids pCAT (no promoter),pmdr1(mdr1promoter from position Ϫ1165to ϩ84),and pMcat5.9(pMcat;482-bp fragment from the cyp1a1promoter fused to the mouse mammary tumor virus pro-moter)were transiently transfected into Hepa-1c1c7and BP r c1cells by the calcium phosphate method.The cells were then treated with 3-MC (5M )or Me 2SO for 48h.Total cellular extracts were prepared,and equal quantities of proteins (2g)were assayed for CAT activity.A ,autoradiogram of a representative CAT assay,showing the activity of plasmids pCAT,pmdr1and pMcat in Hepa-1c1c7and BP r c1cells treated (ϩ)or not treated (Ϫ)with 3-MC (MC ).The position of the [14C]chloramphenicol (CM )and of its acetylated products (AcCM )is indicated on the left .B ,quantitative analysis of CAT activities.The percentage of conversion of [14C]chloramphenicol to its acetylated de-rivatives was quantitated by liquid scintillation counting.Open bars ,ϪMC ;filled bars ,ϩMC .The results presented are the averages of three independent transfections performed in duplicate.S.D.values are rep-resented by the bars .Induction of the Mouse mdr1Gene by PAHs4822 at ZHEJIANG UNIVERSITY, on November 21, 2012 Downloaded from。
APOBEC3蛋白与HPV及宫颈癌的研究进展

复旦学报(医学版)Fudan Univ J Med Sci2021Mar.,48(2)APOBEC3蛋白与HPV及宫颈癌的研究进展魏智1(综述)赵洪波1张涛2冯璇1杜琰1△(审校)(1复旦大学附属妇产科医院临床流行病学研究室上海200011;2香港中文大学医学院妇产科系香港999077)【摘要】宫颈癌是严重威胁女性健康的恶性肿瘤之一,已知人乳头瘤病毒(human papilloma virus,HPV)感染是宫颈癌的主要病因,但具体机制尚不明确。
人载脂蛋白B mRNA编辑酶催化多肽(apolipoprotein B mRNA-editing enzyme catalytic polypeptide,APOBEC)家族是一组能够编辑DNA或RNA序列的胞苷脱氨酶。
APOBEC3成员是固有免疫系统的重要成员,在抗病毒感染防御过程中扮演重要角色。
APOBEC3与多种肿瘤的发生发展密切相关,可能与HPV感染以及宫颈癌的关系尤为密切。
APOBEC3B可能通过“协助”HPV病毒使抑癌蛋白失活及促进HPV病毒癌蛋白的突变,从而促进癌症的发生。
本文就APOBEC3与HPV清除、突变和宫颈癌发生方面的研究进行综述。
【关键词】载脂蛋白B mRNA编辑催化多肽3(APOBEC3);宫颈癌;人乳头瘤病毒(HPV)【中图分类号】R711.74【文献标志码】B doi:10.3969/j.issn.1672-8467.2021.02.020Research progress on the relationship between APOBEC3andHPV and cervical cancerWEI Zhi1,ZHAO Hong-bo1,ZHANG Tao2,FENG Xuan1,DU Yan1△(1Department of Clinical Epidemiology,Obstetrics and Gynecology Hospital,Fudan University,Shanghai200011,China;2Department of Obstetrics and Gynecology,Faculty of Medicine,the Chinese University of Hong Kong,Hong Kong999077,China)【Abstract】Cervical cancer is one of the malignant tumors that seriously threaten women’s health.It is established that infection of human papilloma virus(HPV)is the main cause of cervical cancer,while the exact mechanism remains unclear.The human apolipoprotein B mRNA editing enzyme catalyzed polypeptide(APOBEC)family is a group of cytidine deaminases that can edit DNA or RNA sequences.Recent studies have found that APOBEC3s are important members of the innate immune system,which play an important role in the defense process of anti-viral infection and are also closely related to the occurrence and development of a variety of tumors.APOBEC3B may be especially closely associated with HPV infection and cervical cancer.This paper reviews the relationships of APOBEC3with HPV clearance,mutation,and occurrence of cervical cancer.【Key words】apolipoprotein B mRNA-editing enzyme catalytic polypeptide3(APOBEC3);cervical cancer;human papilloma virus(HPV)*This work was supported by Shanghai Talent Development Fund(2017017).人载脂蛋白B mRNA编辑酶催化多肽3(apolipoprotein B mRNA-editing enzyme catalytic polypeptide3,APOBEC3)家族的胞苷脱氨酶通过编辑病毒基因组,在病毒感染的先天免疫应答中起上海市人才发展基金(2017017)△Corresponding author E-mail:*******************网络首发时间:2021-03-1517∶18∶16网络首发地址:https:///kcms/detail/31.1885.R.20210312.1446.038.html271复旦学报(医学版)2021年3月,48(2)着重要作用[1]。
间充质干细胞来源外泌体通过NLRP3通路缓解糖尿病肾病的作用及机制演示稿件

contents
目录
• 绪论 • 间充质干细胞来源外泌体概述 • NLRP3通路在糖尿病肾病中的作用 • 间充质干细胞来源外泌体通过NLRP3通
路缓解糖尿病肾病的作用 • 结论与展望
01
CATALOGUE
NLRP3通路在糖尿病肾病中的作用
NLRP3通路的概述
01
NLRP3是一种在炎症和免疫反应中起重要作用的蛋白复合 物,属于NOD样受体家族。
02
NLRP3通路是一种炎症反应通路,在识别和应对感染、组 织损伤等危险信号时发挥作用。
03
NLRP3通路在炎症和免疫反应中起着关键作用,参与多种 疾病的发生和发展。
NLRP3通路作为炎症反应中的关键信号通路,与糖尿病肾病的发生发展密 切相关。
国内外研究者正致力于探究间充质干细胞来源外泌体通过NLRP3通路在糖 尿病肾病治疗中的作用及机制。
研究目的与内容
本研究旨在探究间充质干细胞来源外 泌体对糖尿病肾病的治疗作用,并探 讨其通过NLRP3通路发挥作用的分子 机制。
间充质干细胞来源外泌体对NLRP3通路的影响
抑制NLRP3炎症小体的激活
间充质干细胞来源外泌体通过抑制NLRP3炎症小体的激活,从而降低炎症反应,减轻糖尿病肾病的症 状。
调节NLRP3通路的信号转导
间充质干细胞来源外泌体能够调节NLRP3通路的信号转导,影响炎症因子的表达,发挥对糖尿病肾病 的保护作用。
的外泌体。
间充质干细胞来源外泌体的生物学功能
要点一
总结词
要点二
详细描述
间充质干细胞来源外泌体具有多种生物学功能,包括免疫 调节、组织修复、抗炎等。
无机砷调节小鼠胚胎干细胞中含硒蛋白质的表达

无机 砷是 公认 的人类 致癌 物 , 能 使皮肤 、 膀胱、 肝 脏 和其他 组织 患肿 瘤 的风险增 加 。它具有 与 R Os相 关 的遗 传毒 性 , 尽管 i As 致 癌 的分子 机制仍 然 了解甚 少 。硒 是人 体必须 的微 量元 素并具 有抗 癌作 用 , 研究 认为 它能够 整合 到含 硒 蛋 白质 发 挥 抗 氧化 和解 毒 作
( S e l k ) , 内质 网相关 硒蛋 白包 括 1 5 一 S e p 、 S e 1 K、 S e l M、 S e l N、 S e l S 、 S e l T, 其 他 种 类 的 硒 蛋 白如 S e l H、 S e l I 、 S e l O、 S e l P 、 S e l V、 S e l w 序列同源性很小 , 但 有 着 非 常广 泛 的功能 , 硒 蛋 白 Tr l和 G p x 2的启动 子 区有 抗 氧化 反应 的序列 。砷 能 够 诱 导 氧化 应 激 并 引起 转 录 因子 Nr f 2的表 达 增 加 。 已有 报 道 显 示 砷 能 够 通 过 Nr f 2激活 ARE使 类 角 质 细 胞 中 T r l的水 平 升 高 。 仍 需进 一步研 究砷 暴露 后 硒 蛋 白的表 达 来 阐 明砷 与 硒 的交 互作用 。 i AS暴 露如 何 调节 胚 胎 干 细 胞 系 C GR 8干细 胞 中硒 蛋 白的 表达 , 补 充硒 对 i AS暴 露 后硒 蛋 白表 达 的影 响 , 为 深入 了解 砷 、 硒 之 间相 互作 用 的分 子 机 制
实验操作

免疫沉淀(Immunoprecipitation IP )美国芝加哥大学分子肿瘤实验室提供一、准备试剂:IP裂解液配制方法(100毫升体积):50mM Tris-HCl pH 7.5 1M 5ml100mM NaCl 5M 2ml0.5% NP-40 (10% stock) 10ml0.3mM NaVO3 5.52mg50mM Na F 210mg20mM Na Pyrphosphate 892mg1mM PMSF 17.42mg加水至总体积达到100 ml二、实验步骤:(本方法适用于从细胞培养来源的蛋白质)1.将细胞培养液移去,加入裂解液-蛋白酶抑制剂混合液(IP-PI buffer)(T25培养瓶中加入1毫升,T75中加入3毫升),充分裂解后,将混合液转入微量离心管中。
2.冰上放置20分钟,偶尔轻微震荡。
3.4℃下,微量离心机最高速离心5分钟。
将上清液转移至另一微量离心管中。
4.加入30ul protein G- Sepharose beads(与IP-PI buffer 1:1混合)至样品中,4℃下充分混合震荡反应1小时。
5.微量离心机最高速离心1分钟,将上清转移至另一微量离心管中。
6.加入抗需要沉淀的蛋白质的抗体(2-5ug/ 样品),4℃下充分混合震荡反应1小时。
7.加入30ul protein G- Sepharose beads,4℃下充分混合震荡反应1小时。
8.微量离心机最高速离心1分钟,将上清吸弃,收集beads沉淀。
9.沉淀中加入2ul 2-巯基乙醇,煮沸10分钟,离心后取上清,行SDS-PAGE胶电泳及Wester-blotting 检测。
Western Blot(NC膜)重庆医科大学感染病分子生物学实验室一、SDS-PAGE胶电泳1. 75%酒精擦洗洁净玻片及加样梳,组装制胶槽;2. 配制分离胶:1)根据分子量选择分离胶的浓度;2)依次加入ddH2O、30%丙烯酰胺(普通滤纸过滤,避光保存)、1.5MTris-Cl(pH8.8)、10%SDS、10%过硫酸胺、TEMED,充分混匀,将混合液加至双层玻片之间;注意:1、TEMED是促凝剂,加了以后应迅速灌胶;2、分离胶的高度为插上加样梳后梳子下缘下1cm;3)以ddH2O封闭,室温放置1h以上;(可以加大促凝剂和催化剂的量,这样可以在短时间内完成分离胶配制;4)当水和分离胶有了可见的明显界限,倒掉上层水,以滤纸吸尽多余的水分(或将制胶器倾斜用加样枪吸去水即可);注意:手法轻柔一些,不可损伤分离胶;3. 配制积层胶:1)浓度均为5%,只需选择体积,宁多勿少;2)配制方法同上,灌至平低玻片上缘,插上加样梳,小心不能产生气泡;3)室温放置1h以上;(可以加大促凝剂和催化剂的量,节省时间)注意:1、BioRad的两块板需要分离胶7ml,积层胶3ml;2、此时Tris-Cl为1.0M(pH6.8);3、加样梳不能做平行移动,只能上下移动;4. 样品处理:1)细菌和细胞离心所得沉淀以PBS(也可以直接加入上样buffer)重悬,体积根据细菌量和细胞数调节;2)以等倍体积2×蛋白上样缓冲液混匀;3)沸水煮3-5分钟;注意:时间不宜过长,尤其是Marker;(Marker参看说明书要求,MBI的比较好,Prome ga的较差)4)离心,取上清加样;5. 电泳:1)电泳装置组装完毕后,加入甘氨酸电泳缓冲液,拔出加样梳;2)依次加样;(Marker最好选用预染型,这样在电泳时就可方便的判断目的蛋白的位置);3)积层胶电压宜小,90-120V,分离胶可增至120-180V。
吸入胰岛素通过NLRP3炎症小体改善散发性阿尔茨海默病小鼠的认知功能

网络出版时间:2023-10-3018:20:45 网络出版地址:https://link.cnki.net/urlid/34.1086.R.20231027.1531.020吸入胰岛素通过NLRP3炎症小体改善散发性阿尔茨海默病小鼠的认知功能陶舒琪1,2,范雯媛1,2,郑昊宁1,2,王 通3,陈燕春2,4,周 进5,丁小娣1,周风华1,2,郭章玉1,2(1.潍坊医学院基础医学院临床病理系,2.山东省神经疾病与再生修复重点实验室,山东潍坊 261053;3.潍坊市人民医院神经外科,山东潍坊 261000;潍坊医学院4.基础医学院组织学与胚胎学教研室、5.药学院,山东潍坊 261053)收稿日期:2023-04-20,修回日期:2023-07-18基金项目:山东省自然科学基金资助项目(NoZR2019BH060,ZR2020MH149);山东省高等学校青创科技支持计划(No2019KJK004);潍坊市科技发展计划项目(No2021YX063)作者简介:陶舒琪(2000-),女,硕士生,研究方向:神经退行性疾病,E mail:tsq17861526959@163.com;郭章玉(1986-),女,博士,讲师,研究方向:神经退行性疾病,通信作者,E mail:guozhy@wfmc.edu.cn;周风华(1977-),女,博士,教授,研究方向:神经退行性疾病,通信作者,E mail:zhoufh@wfmc.edu.cndoi:10.12360/CPB202303043文献标志码:A文章编号:1001-1978(2023)11-2050-06中国图书分类号:R 332;R338 64;R364 5;R745 7;R977 15摘要:目的 探究吸入胰岛素对散发性阿尔茨海默病(spo radicAlzheimer’sdisease,sAD)小鼠认知功能的影响及机制。
方法 采用链脲佐菌素(streptozotocin,STZ)侧脑室注射法构建散发性AD模型。
英语国际会议PPT课件
Materials and Methods
Patients
Materials and Methods
Cytokine assessment by ELISA
Western blot
Cell isolation and culture
Statistical analysis
7
Methods
IFN-c-induced protein of 10-kDa (IP-10)/CXCL10
we studied the effects of α-toxin on Th1- and Th2related chemokines in macrophages from patients with AD and psoriasis where the intrinsic abnormal and different chemokines production profile is well defined.
13
Figure 3 Punch biopsies (3 mm) from healthy individuals were left either unstimulated (A) or stimulated with a-toxin (100 ng/ ml) (B) or IFN-c (100 ng/ml) (C) for 24 h at 37C. 5-lm paraffin sections were stained for CXCL10 along with appropriate isotype as well as CD68.
16
Low effect of a-toxin on CXCL10 induction (Th1-related chemokine) in macrophages from patients with AD
含t细胞免疫球蛋白和粘蛋白结构域的蛋白质3

含t细胞免疫球蛋白和粘蛋白结构域的蛋白质3下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!含T细胞免疫球蛋白和粘蛋白结构域的蛋白质3引言在生物学领域,蛋白质3(Protein 3)是一种备受关注的蛋白质,因其含有T细胞免疫球蛋白和粘蛋白结构域而备受研究者们的关注。
n-3多不饱和脂肪酸对小鼠血液外泌体中miRNA的调节和抑制肥胖作用

n-3多不饱和脂肪酸对小鼠血液外泌体中miRNA的调节和抑制肥胖作用吴聪,葛科立,路宗博,郑征,张金玉,薛美兰,葛银林*(青岛大学基础医学院,山东青岛 266021)摘 要:目的:研究体内n-3多不饱和脂肪酸(n-3 polyunsaturated fatty acids,n-3 PUFAs)含量的增加对小鼠体质量和血液中外泌体miRNAs表达的影响,探讨n-3 PUFAs通过外泌体抑制肥胖的作用机制。
方法:利用能自发生成n-3 PUFAs的fat-1转基因小鼠和同窝野生型小鼠(对照),通过高脂饮食(high-fat diet,HFD)建立肥胖动物实验模型,测定小鼠体质量。
提取小鼠血浆中的外泌体并鉴定;分离外泌体内的RNA,构建文库并进行miRNA高通量测序。
根据测序结果通过生物信息学方法分析找到其调控的靶基因和相关联的通路,发现miRNA-靶基因互作关系。
验证miRNA与肥胖的关联度以及在肥胖中所发挥的作用。
结果:fat-1转基因小鼠体质量明显低于野生型;外泌体提取鉴定成功;miRNA高通量测序结果显示,不同小鼠组间进行对比时,差异表达显著(P<0.05且差异倍数(fold change,FC)≠1)的miRNA有46 个;生物信息学分析发现6 个重要miRNA(mmu-miR-665-3p、mmu-miR-122-5p、mmu-miR-122-3p、mmu-miR-194-5p、mmu-miR-34c-5p、mmu-miR-223-3p)落在脂肪酸代谢通路以及内吞通路关键位置,功能与脂质代谢和肥胖相关,所对应的靶基因分别为Fads1、Elovl2、Elov6、Hadha、Scad1、Scad2、Hsd17b12、Acot2、Acot4和Arf6、H2-T-ps、Arrb1、Ist1、H2-T10、Wwp1、Snx4、IL2rb、Mvb12b、Rab11、fip3、Kif5a、Nedd4l。
结论:n-3 PUFAs含量的增加能够有效降低小鼠的体质量,抑制肥胖。
英文文献课件

RPM1-INTERACTING PROTEIN4-------RIN4
Plant Cell
Protein Disorder Prediction System(PrDOS)
circular dichroism(CD)spectrosccopy
H+-pumping activity was detected by a decrease of acridine orange absorbance at 495nm
split luciferase complementation assay:分裂荧光素 酶互补分析法
AHA1--NLUC RIN4---CLUC
RLU:相对光单位
cor-mutant reduce virulence upon surface inocultio
RIN4 ADP
the ROS burst analyzed with a luminometer
缺失体<互补型<野生型<过表达型
抗病性微观结果(菌量)应为: 缺失体>互补型>野生型>过表达型
寻找与R基因互作的物质A
(1)体外实验:酵母双杂交实验。
(2)体内试验:例如 1 )双分子荧光互补实验。 2)抗体检测(磷酸化抗体、乙酰化抗体等)。
缺失突变与过表物质A
理论结果: 1 缺失突变:拟南芥抗病性能减弱。 2 过表达突变:拟南芥抗病性能增强。
即抗病结果为: 缺失型<WT<过表达型
syringe infiltrated which is unable to deliver effectors and elicits strong PT
pRIN4 suppress the ROS burst
中图版生物必修三 1.4.2细胞免疫与体液免疫 同步测试

2019-2019学年中图版生物必修三 1.4.2细胞免疫与体液免疫同步测试一、单项选择题1.糖尿病患者可以用尿糖试纸化验自己的尿糖情况.尿糖试纸中含有葡萄糖氧化酶 ,它可以催化葡萄糖形成葡萄糖酸和过氧化氢 ,过氧化氢可以将试纸中一种无色化合物氧化成有色化合物.试纸颜色的变化情况就可以显示尿中葡萄糖含量的上下.以下为上述作用机理的表达式:以下有关此表达式的表达 ,不正确的选项是〔〕A. a在适宜的温度中才能更好地发挥作用B. b为葡萄糖酸C. c为题干中所述的无色化合物D. e 为有色化合物2.某校利用节假日组织学生义务为糖尿病患者检测尿液 ,看其是否康复.以下表达不正确的选项是〔〕①所用试剂为质量浓度为0.1g/mL的NaOH溶液和0.01g/mL的CuSO4溶液②向尿液中先参加NaOH溶液 ,摇荡均匀后 ,再参加CuSO4溶液③向尿液中参加试剂后 ,水浴加热 ,观察是否有紫色反响④为了使结果更可信 ,应取等量的含有蔗糖的蒸馏水溶液为对照组.A. ①②③B. ②③④C. ①②④D. ①②③④3.以下有关艾滋病的说法中 ,正确的选项是〔〕A. 与艾滋病患者拥抱会传染艾滋病B. HIV在离开人体后能存活很长时间 ,危害极大C. HIV主要攻击人体内的T细胞 ,最终使人丧失一切免疫功能D. HIV主要由DNA ,RNA和蛋白质构成 ,但没有核糖体4.以下是与人体生命活动调节相关的表达 ,其中正确的选项是〔〕A. 激素在体液的运输下到达各组织细胞内并发挥调节作用B. 组织液是组织细胞进行正常生命活动和代谢的主要场所C. HIV在人体内环境中不能增殖 ,但能破坏人体体液免疫过程D. 内环境成分影响细胞代谢 ,但细胞代谢不影响内环境成分5.糖尿病患者可以用尿糖试纸化验自己的尿糖情况.尿糖试纸中含有葡萄糖氧化酶 ,它可以催化葡萄糖形成葡萄糖酸和过氧化氢 ,过氧化氢可以将试纸中一种无色化合物氧化成为有色化合物.试纸颜色的变化情况就可以显示尿中葡萄糖含量的上下.如图为上述作用机理的表达式 ,有关表达错误的选项是〔〕A. 表达式中 ,a的根本组成单位最可能为氨基酸B. 表达式中 ,b为葡萄糖酸C. 表达式中 ,c为无色化合物 D. 表达式中 ,e为有色化合物6.艾滋病已被全世界广泛关注.以下有关艾滋病的说法中 ,错误的选项是〔〕A. HIV侵入人体 ,主要攻击T淋巴细胞 ,破坏人体的免疫功能B. 艾滋病主要通过性接触、血液和母婴三种途径传播C. 与艾滋病人握手和共进晚餐不会感染艾滋病病毒HIVD. 艾滋病是一种传染病 ,也是一种遗传病7.糖尿病在现代社会中的发病率越高.检测人体尿液中是否含有葡萄糖 ,可选用的试剂是〔〕A. 斐林试剂B. 苏丹Ⅲ染液 C. 龙胆紫溶液 D. 碘液8.以下关于艾滋病的表达中 ,不正确的选项是〔〕A. 艾滋病是一种免疫缺陷病 B. 艾滋病的病原体是一种病毒C. 艾滋病主要通过性行为、吸毒、输血等途径感染D. 与艾滋病患者的任何接触都可能染病9.艾滋病已成为威胁人类健康的一大杀手.以下有关艾滋病的说法正确的选项是〔〕A. HIV主要攻击人体内的T细胞 ,使人几乎丧失一切免疫功能B. HIV主要攻击人体内的B细胞C. HIV主要由DNA ,RNA和蛋白质构成 ,但没有核糖体D. HIV可以离开细胞单独生存10.关于HIV的表达 ,正确的选项是〔〕A. HIV在活细胞外能大量增殖 B. HIV仅含有核糖体这一种细胞器C. HIV主要攻击B细胞 ,使人体无法产生抗体D. 艾滋病患者的血液中可以检出HIV这种病毒11.以下关于艾滋病病毒〔HIV〕的表达中 ,正确的选项是〔〕A. HIV只能感染辅助性T淋巴细胞B. HIV可以经蚊虫叮咬而传播C. 人体感染HIV后 ,一般短时间内就会发病D. 志愿者在接种HIV试验疫苗后 ,体内出现相应抗体12.“世界艾滋病日〞会议在我国天津市举行 ,会议的主题为:行动起来 ,遏制艾滋。
含patatin样磷脂酶域蛋白3

含patatin样磷脂酶域蛋白3
patatin样磷脂酶域蛋白3(PATL3)是一种重要的蛋白质,在细胞内起着关键的调控作用。
它主要存在于细胞质和内质网中,并参与多种生物过程的调节。
PATL3在细胞内具有多种功能。
首先,它参与脂质代谢调控。
研究表明,PATL3能够催化脂质的降解和合成,从而影响细胞内脂质的水平和组成。
其次,PATL3还参与蛋白质合成和降解的调控。
它能够与其他蛋白质结合,形成复合物,从而促进或抑制蛋白质的合成和降解过程。
此外,PATL3还参与细胞的信号传导和细胞骨架的重构等生物过程。
PATL3的功能调控机制非常复杂。
研究发现,PATL3的活性受到多种因素的调控,如细胞环境、信号通路和其他蛋白质的调控等。
此外,研究还发现,PATL3的突变可能导致一些疾病的发生和发展,如肝脏疾病和肥胖等。
因此,对PATL3的研究具有重要的理论和实际意义。
尽管我们对PATL3的功能和调控机制有了一定的了解,但仍有很多问题有待进一步研究。
例如,PATL3在细胞内的定位和转运机制是如何调控的?PATL3与其他蛋白质的相互作用有哪些?PATL3对细胞的功能调控如何影响疾病的发生和发展?这些问题的研究将有助于更深入地理解PATL3的功能和调控机制,为疾病的预防和治疗提供新的思路和方法。
总的来说,PATL3作为一种重要的蛋白质,在细胞内具有多种功能和调控机制。
对其功能和调控机制的研究将有助于更深入地了解细胞生物学的基本原理,并为疾病的治疗和预防提供新的思路和方法。
我们相信,在未来的研究中,PATL3将会揭示更多的秘密,为人类健康和疾病的治疗带来新的希望。
健身宝对衰老模型小鼠血清SOD和MDA的影响

健身宝对衰老模型小鼠血清SOD和MDA的影响郝军;赵健雄【期刊名称】《兰州大学学报(医学版)》【年(卷),期】2004(030)001【摘要】目的研究健身宝补脾益肾、延缓衰老的作用机制.方法将60只小鼠随机分为6组,除正常对照组外均每日注射对D一半乳糖造成衰老模型,同时给予不同剂量的健身宝和金匮肾气丸.6周后检测小鼠血清中SOD活性、MDA含量.结果衰老模型组与正常对照组比较,小鼠血清中SOD活性显著降低,MDA含量显著升高(P<0.01);而健身宝大、中剂量组与正常对照组相比较,小鼠血清中SOD活性显著升高,MDA含量显著降低,与衰老模型组相比较有显著性差异(P<0.05).结论健身宝明显提升D一半乳糖拟衰小鼠血清中SOD活性,降低MDA含量,可从调节自由基代谢和提高抗氧化能力方面发挥延缓衰老的作用.【总页数】3页(P8-10)【作者】郝军;赵健雄【作者单位】730000,兰州医学院中西医结合研究所;730000,兰州医学院中西医结合研究所【正文语种】中文【中图分类】R318【相关文献】1.七味都气丸对D-半乳糖衰老模型小鼠血清和皮肤SOD、MDA的影响 [J], 张丹丹;柴恕;柴昕2.逍遥散对衰老模型小鼠血清中SOD、MDA含量影响的实验研究 [J], 庞俊伟;翟春涛3.TBWDS水煎剂对亚急性衰老模型小鼠血清SOD、MDA和T-AOC的影响 [J], 曹瑞珍;白靓;于丽丽;狄建军;张树军4.火龙果对衰老模型小鼠血清和肝脏中GSH-Px、SOD活性和MDA含量的影响[J], 郑守欢;韦玉娜;黎瑜霜;魏海帆;李朝敢5.火麻仁油对D-半乳糖致亚急性衰老模型小鼠血清NO、SOD、GSH-Px、MDA 的影响 [J], 曹俊岭;李祖伦;陈建武;李华利因版权原因,仅展示原文概要,查看原文内容请购买。
激活素A对糖尿病肾脏病小鼠肾脏β-catenin信号通路调控的实验研究

激活素A对糖尿病肾脏病小鼠肾脏β-catenin信号通路调控的实验研究李泽璇;刘高虹;高艺文;任小军【期刊名称】《中国中西医结合肾病杂志》【年(卷),期】2024(25)4【摘要】目的:观察靶向调控激活素A(ACT A)下游β-连环蛋白(β-catenin)/T细胞因子(TCF)与β-catenin/叉头框转录因子-O(Foxo)信号的竞争抑制对糖尿病肾脏病(DKD)小鼠肾脏纤维化的影响。
方法:30只小鼠随机将其分为对照组(A组)、糖尿病组(B组)、ACT A处理组(C组)、ACT A+iCRT3处理组(D组)和ACTA+iCRT3+AS1842856处理组(E组)。
留取各组小鼠血、尿和肾组织样本,分别检测常规生化指标及肾脏病理改变,采用免疫组织化学法观察小鼠肾脏Ⅰ型胶原蛋白(CollagenⅠ)、N-钙黏蛋白(N-Cadherin)和波形蛋白(Vimentin)的表达。
结果:与A组小鼠相比,B组小鼠肾脏指数(KW/BW)、尿微量白蛋白/肌酐比值(UACR)明显升高,肾组织Collagen I、N-cadherin和Vimentin的表达均增加;与B组小鼠比较,使用ACT A处理的C组小鼠的KW/BW、UACR及肾组织Collagen I、N-Cadherin和Vimentin的表达进一步上调;与C组相比,使用TCF阻断剂处理的D组小鼠KW/BW、UACR及肾组织Collagen I、N-Cadherin和Vimentin的表达下调;与D组相比,使用TCF和Foxo双重阻断剂处理的E组小鼠上述指标有所上调。
结论:ACT A能够通过增强β-catenin/TCF的信号活化,促进糖尿病肾脏病小鼠肾脏纤维化进程。
【总页数】5页(P300-303)【作者】李泽璇;刘高虹;高艺文;任小军【作者单位】山西医科大学第三医院(山西白求恩医院)肾内科;山西省人民医院肾内科【正文语种】中文【中图分类】R73【相关文献】1.CD36在糖尿病肾病患者肾组织中的表达及调控W nt/β-catenin信号通路对人肾小管上皮细胞增殖凋亡的实验研究2.槲皮素调控白细胞介素-6/信号转导及转录激活蛋白3信号通路在结肠炎性相关结肠癌小鼠模型的机制研究3.卡格列净通过Sirt1/Claudin-1/β-catenin/Snail信号通路对糖尿病肾病模型小鼠肾脏的保护作用4.小鼠AAN激活Wnt/β-Catenin信号通路及诱导肾脏纤维化的观察性研究5.加味大黄附子汤调节Wnt/β-catenin信号通路保护慢性肾脏病小鼠认知功能的机制因版权原因,仅展示原文概要,查看原文内容请购买。
灵芝孢子油与深海鱼油联合应用对小鼠学习记忆及海马神经元表达NOS的影响

灵芝孢子油与深海鱼油联合应用对小鼠学习记忆及海马神经元表达NOS的影响陈穗君;曾园山;张惠君;张伟;丁英;钟志强【期刊名称】《解剖学研究》【年(卷),期】2007(29)1【摘要】目的探讨灵芝孢子油与深海鱼油联合应用对小鼠学习记忆及海马神经元表达NOS的影响。
方法将受孕小鼠随机分为4组,自受孕第1天起分别胃饲生理盐水、灵芝孢子油、深海鱼油和灵芝孢子油加深海鱼油。
在母鼠分娩21d后改为胃饲其幼鼠,然后将出生后45d的幼鼠处死。
处死前进行Morris水迷宫行为学测试,处死后应用酶组织化学法检测海马神经元NOS的表达。
结果在Morris水迷宫检测中,灵芝孢子油组、深海鱼油组和灵芝孢子油加深海鱼油组小鼠的逃逸潜伏期明显缩短。
灵芝孢子油组和灵芝孢子油加深海鱼油组的小鼠平台象限游泳距离有增加。
灵芝孢子油加深海鱼油组的小鼠大脑海马NOS阳性神经元与其它组的小鼠比较有显著性增加。
结论灵芝孢子油和深海鱼油联合应用能够促进小鼠学习记忆能力及其大脑海马神经元表达NOS。
【总页数】5页(P7-11)【关键词】灵芝孢子油;深海鱼油;海马;Morris水迷宫;学习记忆;一氧化氮合酶【作者】陈穗君;曾园山;张惠君;张伟;丁英;钟志强【作者单位】中山大学中山医学院组织胚胎学教研室神经科学研究室;中山大学中山医学院解剖学教研室【正文语种】中文【中图分类】R651.2【相关文献】1.激光氧液对癫痫持续状态大鼠学习记忆及海马神经元突触素表达的影响 [J], 耿丽娟;王实;王娟;王宁;袁宝强;樊秋萍2.宽叶缬草对血管性痴呆小鼠学习记忆及海马区神经元病理学改变的影响 [J], 严洁;潘庆敏;刘伟3.唑尼沙胺对癫痫小鼠学习记忆及海马神经元的影响 [J], 王丽辉;杨花芳;郑华城;陈芳;杜雅坤4.虫草素改善脑缺血小鼠学习记忆及对海马神经元数量的影响 [J], 蔡昭林;李楚华;王晓琦;蒋中娇;郑月;李海航;肖鹏5.灵芝孢子油对铝中毒小鼠学习记忆及海马超微结构的影响 [J], 沈志勇;郭家松;钟志强;李振林;李新顺;秦建强因版权原因,仅展示原文概要,查看原文内容请购买。
多发性骨髓瘤患者超氧化物歧化酶和脂质过氧化物的改变及其意义

多发性骨髓瘤患者超氧化物歧化酶和脂质过氧化物的改变及其意义张梅;邵文斌【期刊名称】《西安医科大学学报》【年(卷),期】1995(016)001【摘要】用邻苯三酚自氧化法和改良硫代巴比妥酸(TBA)荧光微量法测定了多发性骨髓瘤(MM)患者初发期、缓解期和复发期红细胞超氧化物歧化酶(SOD)活性和血浆脂质过氧化物(LPO)含量。
结果表明:MM初发和复发病例红细胞SOD活性明显降低,血浆中LPO含量明显增高,较正常对照均有明显差异。
缓解期患者的SOD活性和LPO含量大致恢复正常水平。
MM红细胞SOD活力与血浆LPO含量呈负相关(r=-0.425,P<0.05)),与血红蛋白浓度呈正相关(r=0.722,P<0.001),与骨髓中浆细胞百分数呈负相关(r=-0.526,P<0.05)。
以上结果提示自由基损伤作用参与了MM的发生、发展和转归过程,且与病情的严重程度有关。
【总页数】3页(P60-62)【作者】张梅;邵文斌【作者单位】不详;不详【正文语种】中文【中图分类】R733.302【相关文献】1.急性有机磷农药中毒患者血清脂质过氧化物超氧化物歧化酶的变化及意义 [J], 单红卫;林军;杨兴易;郭昌星;景炳文2.慢性肾功能衰竭患者脂质过氧化物、超氧化物歧化酶的变化及其临床意义的探讨[J], 王丽萍;杜建;吴开木;魏仲南3.脑血管病急性期患者血清脂质过氧化物、谷胱甘肽过氧化物酶和超氧化物歧化酶的检测及其临床意义的研究 [J], 戚其学;徐楠;吴哲4.鼻咽癌患者血清中超氧化物歧化酶和脂质过氧化物及肿瘤坏死因子-α水平测定和意义 [J], 姚俊;罗国庆;张月飞;崔德威;杨利荣5.冠心病患者红细胞ATP酶、超氧化物歧化酶、钠、钾、钙、镁离子和血浆脂质过氧化物的变化及其意义 [J], 卓孝福;林孟戈;郭永建因版权原因,仅展示原文概要,查看原文内容请购买。
活性初乳素对吸臭氧小鼠的抗衰老作用

活性初乳素对吸臭氧小鼠的抗衰老作用
汪岱迪;孟群;金卫红;曹苹
【期刊名称】《药学与临床研究》
【年(卷),期】1997(000)004
【摘要】小鼠吸臭氧以后,使机体的助氧化-抗氧化系统平衡失调,引起了体温、体重和体力下降,免疫器官萎缩与自由基代谢有关的酶(MAO-B、MDA)活性改变,产生了一系列衰老现象,服用了活性初乳素以后,明显改善上述症状,达到抗衰老目的。
【总页数】3页(P12-14)
【作者】汪岱迪;孟群;金卫红;曹苹
【作者单位】[1]江苏省药品检验所;[2]江苏省药品检验所南京 210008;[3]南京210008;[4]南京 210008
【正文语种】中文
【中图分类】R151
【相关文献】
1.复合维生素对D-半乳糖小鼠衰老模型的抗衰老作用 [J], 王燕;陶钧
2.活性初乳素对吸O3小鼠的抗衰老作用 [J], 汪岱迪;孟群;金卫红
3.活性初乳素对小鼠的抗衰老作用 [J], 程光宇;唐梓进;吴京燕
4.夏黑葡萄花青素在D-半乳糖致衰老小鼠大脑、肾脏和脾脏组织中的抗衰老作用[J], 杨琛擘;连秀仪;申培红;王兵亚;刘文涛;李倩
5.巴马火麻仁油、蛋白粉和木脂素酰胺类提取物对老年小鼠的抗衰老作用研究 [J], 蔡霈;付珣;邓安刚;詹雪晶;蔡光明;李顺祥
因版权原因,仅展示原文概要,查看原文内容请购买。
P物质和降钙素基因相关肽在大鼠垂体前叶神经纤维内的共存

P物质和降钙素基因相关肽在大鼠垂体前叶神经纤维内的共存董玉书;刘惠玲;张萍;鞠躬
【期刊名称】《神经解剖学杂志》
【年(卷),期】2005(21)3
【摘要】为观察P物质(SP)和降钙素基因相关肽(CGRP)在大鼠垂体前叶神经纤维内的共存,用种属特异性抗体(兔抗-SP多克隆抗体和小鼠抗-CGRP单克隆抗体)进行免疫荧光双标记,激光共聚焦扫描显微镜观察进行研究。
结果显示:所有的CGRP 免疫阳性神经纤维都是SP免疫阳性,同时所有的SP免疫阳性神经纤维也都是CGRP免疫阳性;CGRP免疫阳性神经纤维的分布和形态与SP免疫阳性神经纤维相一致。
结论:P物质和降钙素基因相关肽在大鼠垂体前叶神经纤维内完全共存。
【总页数】3页(P238-240)
【关键词】垂体前叶;神经肽P物质;降钙素基因相关肽;神经纤维;大鼠;递质共存【作者】董玉书;刘惠玲;张萍;鞠躬
【作者单位】第四军医大学全军神经科学研究所
【正文语种】中文
【中图分类】Q422
【相关文献】
1.烫伤大鼠垂体前叶P物质和降钙素基因相关肽神经的变化 [J], 胡大海;陈璧;王波涛;马丹
2.大鼠垂体前叶内神经纤维对LH分泌的兴奋性调节作用 [J], 刘玲;高立志;鞠躬
3.大鼠垂体前叶中一些免疫内分泌物质的共存 [J],
4.羊垂体前叶降钙素基因相关肽(CGRP)与P物质(SP)免疫反应神经纤维 [J], 沈霞芬;贾维真;卿素珠;陈谊;刘惠玲;林英华;鞠躬
5.大鼠生后垂体前叶P物质免疫反应阳性神经纤维的发育变化 [J], 卢春蓉;孟繁东;刘惠玲;邱建勇;鞠躬
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Protein
-How our body use it and how much we need
Digestion & absorption of protein
胰蛋白酶trypsin 肠蛋白酶ereptase 胃酸g astric acid 胃蛋白酶p epsin 多肽
少量氨基酸
寡肽、三肽、
二肽、氨基酸
小肠细胞表面肽酶peptidase
氨基酸
Amino acid pool
氨基酸池
存在于人体各组织、器官和体液中的游离氨基酸(free amino acids) 的统称每天全身的蛋白质约有3%被更新
Metabolism of amino acid pool
氨基酸
池
食物
代谢转变成尿素、氨、尿酸、肌酐
由尿液排出组织蛋白
体内含氮
化合物
转化为糖
原/脂肪
Nitrogen balance
氮平衡摄入氮排出氮
尿
氮粪氮皮肤等B=I-(U+F+S)
B:氮平衡I:摄入氮
U:尿氮
F:粪氮
S:皮肤等氮损失
摄入氮排出氮
摄入氮
排出
氮
><正氮平衡负氮平衡
=
零氮平衡
摄入
氮
排出
氮
B=0
B>0B<0
Reference daily intake of protein
成人:
•0.8g/(kg·day)
•中国1.16g/(kg·day)
•10%~12%膳食总能量
儿童:
12%~14%膳食总能量12%-14% 10%-12%
Excessive protein intake is harmful
增加肾脏负担
加快钙流失,易
发生骨质疏松
可能增加脂肪、
胆固醇摄入蛋白质摄入过多
Balance it !。
