大气复习题 部分汉化版
大气复习资(含答案)

大气复习资料一、概念解释(1)Globe warming: Global warming is the increase in the average measured temperature of the Earth's near-surface air and oceanssince the mid-twentieth century, and its projectedcontinuation.(2) Temperature inversions:A temperature inversions is a thin layer of the atmosphere where the decrease in temperature withheight is much less than normal (or in extreme cases, thetemperature increases with height).(3)ESP: Electrostatic precipitator, which is like a gravity setter or centrifugal separator, but electrostatic force drives theparticles to the wall.(4)HEPA: High Efficiency Particulate Air(5)Lean bum :Lean burn refers to the use of lean mixtures in an internal combustion engine .The air-fuel can be as high as65:1 ,so the mixture has considerably less fuel in comparisonto the stoichiometric combustion ratio (14.7 for petrol , forexample ).(6)Plume rise: the plume rising a distance △h above the top of the stack before leveling out.(7)Wet scrubber: A device that collects particles by contacting the dirty gas stream with liquid drops.(8) Photochemical smog:(9)Thermal NO: Thermal NO x refers to NO x formed through high temperature oxidation of the diatomic nitrogen found incombustion air.(10)A/F ratio: Air to fuel ratio for auto engines.(11)PM2.5:particle with the aerodynamic diameter less than 2.5 um,which is also called Respirable Particles.(12)Alternative fuel: Several other fuels except of conventional gasoline and diesel, which have been used for many years in slighutly modified automobile engines, for reasons of cost and availability.(13)VOCs: Volatile organic compounds are those organic liquids or solids whose room temperature vapor pressure are greater than about 0.01 psia(=0.0007atm) and whose atmospheric boiling points are up to about 500℉, which means most organic compounds with less than 12 carbon atoms.(1)SCR and SNCR:6NO+4NH3→5N2+6H2O, 4NO+4NH3+O2→4N2+6H2O2NO2+4NH3→3N2+6H2O.These reactions can be carried out over a variety of catalysts, the temperature is between 1600℉and 1800℉, once the temperature increases, the dominant reaction isNH3+O2→NO+1.5H2O, the catalytic processes are called SCR, andthe higher-temperature ones, without catalysts, call SNCR.(2)Aerodynamic diameter: Airborne particles have irregular shapes, and their aerodynamic behavior is expressed in terms of thediameter of an idealized spherical particle known asAerodynamic diameter.(3)Primary Particles: Particles found in the atmosphere in the form in which they were emitted, for examples, NO,CO,SO2(4)Point Sources: small number of large sources that emit larger amounts per source, at higher elevations (power plants,smelters, cement plants, etc.) called point sources二、Answer following questions(1)Which are the main constituents for the ground level ozoneformation?Ozone is formed when the following constituents are present. Nitrogen oxides, Volatile Organic, Compounds, Sunlight, High temperature(>18 ℃)NO+VOC+O2+Sunlight→NO2+O3(2)Please list five major types of wet scrubbers.Plate Scrubber (板式)Packed Scrubber (填料式)Preformed Spray Scrubber (喷雾式)Gas-Atomized Spray Scrubber (气体雾化)Centrifugal Scrubber (离心式)Impingement-Entrainment Scrubber(冲击夹带式)Mechanically Aided Scrubber (机械辅助式)Moving Bed Scrubber (移动床式)(3)How to control VOCs pollution by prevention? Two examples.Substitution(代替),Replacing gasoline as a motor fuel with compressed natural gas or propane is a form of substitution.Process Modification(过程修改),Replacing gasoline-powered vehicles with electric-powered vehicles is a form of process modification.Leakage(渗漏) Control,Storing large amounts of gasoline in floating roof tanks.(4)Please introduce the process of forced-oxidation Limestone WetScrubbing briefly?(5)What are the most different points between SCR and SNCR?(6)Can TWC be applied in the treatment of diesel exhaust (=emissionof diesel engine)? Why?·The characteristic of emission of diesel engine: Ample PM and Excessive O2, Lower temperature;·The difficulty in the reaction of solid-gas-solid.Key: The mixture process of fuel and air for gas engines is distinguished from that for diesel engines, hence the character of one type engine is different from the other one: there are mainly five gases(NOX , HC, CO and O2, CO2) in the exhaust of gas motors, while there areample O2and four other gases mentioned above in the tailpipe emissionof diesel engine. And the presence of abundant O2would inhibit the performance of TWC.Moreover, the contact and reaction of solid-solid-gas resulting in the difficulties for catalysts to oxidation the particulate in the exhaust of diesel engine, while the contact and reaction of solid-gas-gas occur in the exhaust of gasoline engine and the latter reaction is easier.(7)What kinds of indoor air pollutants are mostly concerned bypublic?a)Randomb)Combustion by-products1.CO, CO2, SO2,Formaldehyde,Hydrocarbons,NOx2.Particulates, polyaromatic hydrocarbonsc)Cigarettes d)Volatile organic compoundsf) Biological contaminants(8)List the technology strategy for the control of particles.(9)Give names of three typical kinds of combustion reactors. Whichhas lowest operator temperature among those reactors?1.Direct flame incineration2.Thermal incineration3.Catalytic incineration (has lowest operator temperature)(10)What are the major development problems of Forced-oxidationlimestone wet scrubbing?(11)What are primary air pollutants and secondary air pollutants?Any example?primary air pollutants are directly from the sources, for examples, NO, CO, SO2.The secondary air pollutants are from the primary pollutants, such as NO2, NO3, fine particles.NO+CH+O2+sunlight → NO2+O3(12)Basic strategy of control for particulate pollutants (three aspects )?Impaction 碰撞 Interception 截留 Diffusion 扩散By forcing the individual particles to contact each other,By contacting them with drops of water,By preventing the emission of gaseous Pollutants.(13)How to reduce the formation of NOx in flue gas by modifyingthe combustion processes?p459(14)Please explain the formation of acid rain?Sulfur oxides and nitrogen oxidesNO NO2 HNO3nitric acid SO2H2SO4sulfuric acid(15)What are basic principles of electrostatic Precipitators (sixactivities)?·Ionization - Charging of particles·Migration - Transporting the charged particles to the collecting surfaces·Collection - Precipitation of the charged particles onto the collecting surfaces·Charge dissipation - Neutralizing the charged particles on the collecting surfaces·Particle dislodging - Removing the particles from the collecting surface to the hopper·Particle removal - Conveying the particles from the hopper to a disposal point三、Calculation1) Estimate the concentration of carbon monoxide at the downwind edge of a city. The city may be considered to consist of three parallel strips, located perpendicular to the wind. For all of the strips the wind velocity u equals 3 m/s . The properties of each of the strips are described in the following table,solution :uHb c += c 1=0+(100*5000)/(3*400)=416.7 μg/m 3 uHqL c +=1C c 2=416.7+(500*2000)/(3*500)=1083.4μg/m 3 uHqL c +=2C c 3=1083.4+(100*5000)/(3*400)=1500.1μg/m 32) An ESP is designed to treat 540,000 acfm (actual cubic feet per minute ) with 99% efficiency . Assuming an effective drift velocity of 0.12 m/s , calculate the required plate area and the number of plates. The plate size is 5.4m tall by 3m long (A =-Q×㏑(1-η)/ωp , acfm =1ft 3/min, 1m=3.28ft, 1m 2=1550 in 2=10.7639 ft 2). solution1ft 3/min=(1/3.28)3m 3/min=0.0283 m 3/min540000acfm=540000*0.028315282 m 3/min=254.7 m 3/sA= -(254.7/0.12)ln(1-0.99)=9774.47m 2N=9774.47/514.3=6043) We wish to treat an airstream containing 0.005mol fraction (0.5%, 5000ppm ) toluene, moving at a flow rate 2240m 3/h at 0℃ and 1 atm , so as to remove 99% of the toluene by water absorption. Estimate the required water flow rate. Here Henry’s law constant is 10000 atm .Solution :4)A power plant flue gas contains 1000ppm of SO 2 and is emitted at a rate of 224m 3/s at 546K and 1 atm . A Forced-oxidation limestone wet scrubbing system is to be used to achieve 90% removal of the SO 2. Calculate the amount of CaSO 4·2H 2Ocontained in the final solid product in t/d.Solution : RTPV n = =(101325*224*1000*10-6)/(8.315*546)=4.999mol/s SO 2~ CaSO 4·2H 2O so n (c a so 4)=n (so 2)=4.999mol/s*90%=4.4991mol/s m=4.4991mol/s *172g/mol *3600*24s/d *10-6 t/d=66.86 t/d5)A power plant emits 36 kg/h of SO 2 at height H=120m and the wind speed is 2 m/s. Dispersion Coefficients: σy =40m and σz =30m, Estimate the ground-level concentration of SO 2 from this source at a distance 1km directly downwind?()⎪⎭⎪⎬⎫⎪⎩⎪⎨⎧⎥⎥⎦⎤⎢⎢⎣⎡⎥⎦⎤⎢⎣⎡+-+⎥⎥⎦⎤⎢⎢⎣⎡⎥⎦⎤⎢⎣⎡--⎥⎥⎦⎤⎢⎢⎣⎡⎥⎥⎦⎤⎢⎢⎣⎡-=22221exp 21exp 21exp 2,,z z y z y H z H z y u Q z y x C σσσπσσ Solution :u=2m/s , H=120m, Q=36kg/h=10g/s , y=0 , z=H=120 , x=1000m⎭⎬⎫⎩⎨⎧+⎥⎦⎤⎢⎣⎡⎥⎦⎤⎢⎣⎡+-=130********exp 30*40*3.14*10*210C =0.406)A power plant flue gas contains 1000×10-6 (1000ppm )of NOx ,and is emitted at a rate of 89.6m 3/s at 546K and 1 atm. The NOx is 90%mol NO, balance NO 2. A selective catalytic reduction system is to be used to remove the NOx. Calculate the minimum of ammonia needed in kg/h.Solution: 4NO+4NH 3+O 2→4N 2+6H 2O 2O+4NH 3+O 2→3N 2+6H 2OPV=nRT n=(101325*89.6*1000*10-6)/(8.315*546)=2.0mol/sn (no)=0.9*2mol/s=1.8mol/s so n (no2)=2.0-1.8=0.2mol/sn (NH3)= n (no)+2* n (no2)=1.8+0.4=2.2mol/sm=2.2mol/s * 17 g/mol =37.4g/s=134.64kg/h7)The efficiency of an ESP is 98%. The efficiency of the ESP drops to 93% as a result of flow rate changes. Calculate the ratio of flow rates for the above situation. Use appropriate assumptionSolution: A =-Q×㏑(1-η)/ωp SO Q=-A*ωp/㏑(1-η)Assume the plate area A and the effective drive velocity ωp are unchanged.ThenQ1/Q2=(-A1*ωp/㏑(1-η1)/ (-A2*ωp/㏑(1-η2) =㏑(1-η2)/ ㏑(1-η1)=㏑(1-0.93)/ ㏑(1-0.98)=0.68。
大气单元测试卷

大气单元测试卷一、选择题(每题2分,共20分)1. 大气层中,对地面起保温作用的是哪一层?A. 对流层B. 平流层C. 臭氧层D. 电离层2. 以下哪种气体不是大气中的主要组成成分?A. 氮气B. 氧气C. 二氧化碳D. 氦气3. 影响气候的主要因素不包括以下哪项?A. 纬度B. 地形C. 人类活动D. 月球引力4. 以下哪种现象不是大气污染的表现?A. 酸雨B. 温室效应C. 臭氧层空洞D. 潮汐现象5. 大气压力与以下哪个因素无关?A. 海拔高度B. 温度C. 湿度D. 风力大小6. 以下哪种天气现象与气压有关?A. 彩虹B. 雷电C. 龙卷风D. 极光7. 大气中的水汽主要来源于?A. 海洋B. 河流C. 湖泊D. 植物蒸腾8. 以下哪种现象不属于大气中的气象现象?A. 风B. 雨C. 雪D. 地震9. 大气层中的平流层是臭氧层所在的位置,其主要作用是什么?A. 吸收紫外线B. 反射红外线C. 产生温室效应D. 维持大气压力10. 以下哪种天气现象是大气中水汽凝结形成的?A. 雾B. 霜C. 露D. 所有选项二、填空题(每空1分,共10分)11. 大气层可以分为_______、_______、_______等主要层次。
12. 地球的大气层中,_______层是天气变化的主要场所。
13. 温室效应主要是由于大气中_______气体的增加造成的。
14. 酸雨的形成与大气中_______含量的增加有关。
15. 气象学中,风向是指风从_______方向吹来。
三、简答题(每题5分,共20分)16. 请简述大气中水循环的过程。
17. 什么是气候类型?请列举至少三种不同的气候类型。
18. 请解释什么是气象现象,并给出两个例子。
19. 请简述大气污染对人类和环境可能造成的影响。
四、计算题(每题5分,共10分)20. 如果一个地区的年平均降水量为1000毫米,该地区的年平均蒸发量为800毫米,请计算该地区的年平均降水盈余量。
大气考试复习

15. 下列哪种气体不属于温室气体 D
A. CO2 B. N2O C.CH4 D. N2 16. 下列哪种除尘器属于机械除尘器 A
A. 重力沉降室 B. 袋式除尘器 C. 电除尘器 D. 文丘里洗涤器
17. 下列哪项不属于全球性大气污染的主要三大问题 D
A. 温室效应 B. 臭氧层破坏 C. 酸雨 D. 重金属污染
D 暖层
(标准状态下气体密度为 1.293kg/m3)
B 1230Pa
D 1856Pa
D 动力学当量直径
为宜。
……………答……………题……………不
A ≤10μm
C >10μm
30.标准状态下的温度和压力值分别是 A
A 273K,1atm
C 0K,1atm
B ≤100μm
D 介于 10μm~100μm
13. 电除尘装置发生电晕闭塞现象的主要原因是 D A 烟尘的电阻率小于 104Ω·cm B 烟尘的电阻率大于 1011Ω·cm C 烟气温度太高或者太低 D 烟气含尘浓度太高 14. 对于高温、高湿烟气的烟尘治理工艺,在选择设备时拟采用 D
A. 旋风除尘器 B. 袋式除尘器 C. 静电除尘器 D. 湿式除尘器
18.下列不属于气溶胶状态的污染物为 D
A 粉尘 B 烟
19.下列哪项不属于煤的工业分析成分 C
A 水分
C 飞灰 D 二氧化硫
B 灰分 C 颗粒物 D 固定碳
20. 大气中的水蒸气主要集中在哪一层 A
A 对流层
B 平流层 C 中间层
21.某旋风除尘器的进口气体流速为 17m/s,如果该除尘器的阻力系数为 8.5,
A. 烟气温度
C. 云量
40、以下不是温室气体的是 C
最新大气复习资料含答案

大气复习资料含答案大气复习资料一、概念解释(1)Globe warming:Global warming is the increase in the average measured temperature of the Earth's near-surfaceair and oceans since the mid-twentieth century, and itsprojected continuation.(2) Temperature inversions:A temperature inversions is a thin layer of the atmosphere where the decrease in temperaturewith height is much less than normal (or in extreme cases,the temperature increases with height).(3)ESP: Electrostatic precipitator, which is like a gravity setter or centrifugal separator, but electrostatic forcedrives the particles to the wall.(4)HEPA: High Efficiency Particulate Air(5)Lean bum :Lean burn refers to the use of lean mixtures in an internal combustion engine .The air-fuel can be as highas 65:1 ,so the mixture has considerably less fuel incomparison to the stoichiometric combustion ratio (14.7 forpetrol , for example ).(6)Plume rise: the plume rising a distance △h above the top of the stack before leveling out.(7)Wet scrubber: A device that collects particles by contacting the dirty gas stream with liquid drops.(8) Photochemical smog:(9)Thermal NO: Thermal NO x refers to NO x formed through high temperature oxidation of the diatomic nitrogen found incombustion air.(10)A/F ratio: Air to fuel ratio for auto engines.(11)PM2.5:particle with the aerodynamic diameter less than2.5 um, which is also called Respirable Particles.(12)Alternative fuel: Several other fuels except of conventional gasoline and diesel, which have been used formany years in slighutly modified automobile engines, forreasons of cost and availability.(13)VOCs: Volatile organic compounds are those organic liquids or solids whose room temperature vapor pressure aregreater than about 0.01 psia(=0.0007atm) and whoseatmospheric boiling points are up to about 500℉, whichmeans most organic compounds with less than 12 carbon atoms.(1)SCR and SNCR:6NO+4NH3→5N2+6H2O, 4NO+4NH3+O2→4N2+6H2O2NO2+4NH3→3N2+6H2O.These reactions can be carried out over a variety of catalysts, the temperature is between 1600℉and 1800℉, once the temperature increases, the dominant reaction isNH3+O2→NO+1.5H2O, the catalytic processes are called SCR,and the higher-temperature ones, without catalysts, callSNCR.(2)Aerodynamic diameter: Airborne particles have irregular shapes, and their aerodynamic behavior is expressed interms of the diameter of an idealized spherical particleknown as Aerodynamic diameter.(3)Primary Particles: Particles found in the atmosphere in the form in which they were emitted, for examples, NO,CO,SO2(4)Point Sources: small number of large sources that emit larger amounts per source, at higher elevations (powerplants, smelters, cement plants, etc.) called point sources 二、Answer following questions(1)Which are the main constituents for the ground level ozone formation?Ozone is formed when the following constituents are present. Nitrogen oxides, Volatile Organic, Compounds, Sunlight, High temperature(>18 ℃)NO+VOC+O2+Sunlight→NO2+O3(2)Please list five major types of wet scrubbers.Plate Scrubber (板式)Packed Scrubber (填料式)Preformed Spray Scrubber(喷雾式)Gas-Atomized Spray Scrubber (气体雾化)Centrifugal Scrubber (离心式)Impingement-Entrainment Scrubber(冲击夹带式)Mechanically Aided Scrubber (机械辅助式)Moving Bed Scrubber (移动床式)(3)How to control VOCs pollution by prevention? Two examples. Substitution(代替),Replacing gasoline as a motor fuel with compressed natural gas or propane is a form of substitution. Process Modification(过程修改),Replacing gasoline-powered vehicles with electric-powered vehicles is a form of process modification.Leakage(渗漏)Control,Storing large amounts of gasoline in floating roof tanks.(4)Please introduce the process of forced-oxidation LimestoneWetScrubbing briefly?(5)What are the most different points between SCR and SNCR?(6)Can TWC be applied in the treatment of diesel exhaust(=emissionof diesel engine)? Why?·The characteristic of emission of diesel engine: Ample PM and Excessive O2, Lower temperature;·The difficulty in the reaction of solid-gas-solid.Key: The mixture process of fuel and air for gas engines is distinguished from that for diesel engines, hence the character of one type engine is different from the other one: there are mainlyfive gases (NOX , HC, CO and O2, CO2) in the exhaust of gas motors,while there are ample O2and four other gases mentioned above in thetailpipe emission of diesel engine. And the presence of abundant O2 would inhibit the performance of TWC.Moreover, the contact and reaction of solid-solid-gas resulting in the difficulties for catalysts to oxidation the particulate in the exhaust of diesel engine, while the contact and reaction of solid-gas-gas occur in the exhaust of gasoline engine and the latter reaction is easier.(7)What kinds of indoor air pollutants are mostly concernedbypublic?a)Randomb)Combustion by-products1.CO, CO2, SO2,Formaldehyde,Hydrocarbons,NOx2.Particulates, polyaromatic hydrocarbonsc)Cigarettes d)Volatile organic compoundsf) Biological contaminants(8)List the technology strategy for the control of particles.(9)Give names of three typical kinds of combustion reactors.Whichhas lowest operator temperature among those reactors?1.Direct flame incineration2.Thermal incineration3.Catalytic incineration (has lowest operator temperature)(10)What are the major development problems of Forced-oxidation limestone wet scrubbing?(11)What are primary air pollutants and secondary airpollutants?Any example?primary air pollutants are directly from the sources, for examples, NO, CO, SO2.The secondary air pollutants are from the primary pollutants, such as NO2, NO3, fine particles.NO+CH+O2+sunlight → NO2+O3 (12)Basic strategy of control for particulate pollutants(threeaspects )?Impaction 碰撞 Interception 截留 Diffusion 扩散By forcing the individual particles to contact each other,By contacting them with drops of water,By preventing the emission of gaseous Pollutants.(13)How to reduce the formation of NOx in flue gas bymodifying the combustion processes?p459(14)Please explain the formation of acid rain?Sulfur oxides and nitrogen oxidesNO NO2 HNO3nitric acid SO2H2SO4sulfuric acid(15)What are basic principles of electrostatic Precipitators(sixactivities)?·Ionization - Charging of particles·Migration - Transporting the charged particles to the collecting surfaces·Collection - Precipitation of the charged particles onto thecollecting surfaces·Charge dissipation - Neutralizing the charged particles on the collecting surfaces·Particle dislodging - Removing the particles from the collecting surface to the hopper·Particle removal - Conveying the particles from the hopper to a disposal point三、Calculation1) Estimate the concentration of carbon monoxide at the downwind edge of a city. The city may be considered to consist of three parallel strips, located perpendicular to the wind. For all of the strips the wind velocity u equals 3 m/s . The properties of each of the strips are described in the following table,solution :uHb c += c 1=0+(100*5000)/(3*400)=416.7 μg/m 3 uHqL c +=1C c 2=416.7+(500*2000)/(3*500)=1083.4μg/m 3 uHqL c +=2C c 3=1083.4+(100*5000)/(3*400)=1500.1μg/m 32) An ESP is designed to treat 540,000 acfm (actual cubic feet per minute ) with 99% efficiency . Assuming an effective drift velocity of 0.12 m/s , calculate therequired plate area and the number of plates. The plate size is 5.4m tall by 3m long (A =-Q×㏑(1-η)/ωp , acfm =1ft 3/min, 1m=3.28ft, 1m 2=1550 in 2=10.7639 ft 2).solution1ft 3/min=(1/3.28)3m 3/min=0.0283 m 3/min540000acfm=540000*0.028315282 m 3/min=254.7 m 3/sA= -(254.7/0.12)ln(1-0.99)=9774.47m 2N=9774.47/514.3=6043) We wish to treat an airstream containing 0.005mol fraction (0.5%, 5000ppm )toluene, moving at a flow rate 2240m 3/h at 0℃ and 1 atm , so as to remove 99% of the toluene by water absorption. Estimate the required water flow rate. Here Henry’s law constant is 10000 atm .Solution :4)A power plant flue gas contains 1000ppm of SO 2 and is emitted at a rate of 224m 3/s at 546K and 1 atm . A Forced-oxidation limestone wet scrubbing system is to be used to achieve 90% removal of the SO 2. Calculate the amount of CaSO 4·2H 2O contained in the final solid product in t/d.Solution : RTPV n = =(101325*224*1000*10-6)/(8.315*546)=4.999mol/s SO 2~ CaSO 4·2H 2O so n (c a so 4)=n (so 2)=4.999mol/s*90%=4.4991mol/sm=4.4991mol/s *172g/mol *3600*24s/d *10-6 t/d=66.86 t/d5)A power plant emits 36 kg/h of SO 2 at height H=120m and the wind speed is 2 m/s. Dispersion Coefficients: σy =40m and σz =30m, Estimate the ground-level concentration of SO 2 from this source at a distance 1km directly downwind?()⎪⎭⎪⎬⎫⎪⎩⎪⎨⎧⎥⎥⎦⎤⎢⎢⎣⎡⎥⎦⎤⎢⎣⎡+-+⎥⎥⎦⎤⎢⎢⎣⎡⎥⎦⎤⎢⎣⎡--⎥⎥⎦⎤⎢⎢⎣⎡⎥⎥⎦⎤⎢⎢⎣⎡-=22221exp 21exp 21exp 2,,z z y z y H z H z y u Q z y x C σσσπσσ Solution :u=2m/s , H=120m, Q=36kg/h=10g/s , y=0 , z=H=120 , x=1000m⎭⎬⎫⎩⎨⎧+⎥⎦⎤⎢⎣⎡⎥⎦⎤⎢⎣⎡+-=130********exp 30*40*3.14*10*210C =0.406)A power plant flue gas contains 1000×10-6 (1000ppm )of NOx ,and isemitted at a rate of 89.6m3/s at 546K and 1 atm. The NOx is 90%mol NO, balance NO2. A selective catalytic reduction system is to be used to remove the NOx. Calculate the minimum of ammonia needed in kg/h.Solution: 4NO+4NH3+O2→4N2+6H2O 2O+4NH3+O2→3N2+6H2OPV=nRT n=(101325*89.6*1000*10-6)/(8.315*546)=2.0mol/sn(no)=0.9*2mol/s=1.8mol/s so n(no2)=2.0-1.8=0.2mol/sn(NH3)= n(no)+2* n(no2)=1.8+0.4=2.2mol/sm=2.2mol/s * 17 g/mol =37.4g/s=134.64kg/h7)The efficiency of an ESP is 98%. The efficiency of the ESP drops to 93% as a result of flow rate changes. Calculate the ratio of flow rates for the above situation. Use appropriate assumptionSolution: A =-Q×㏑(1-η)/ωp SO Q=-A*ωp/㏑(1-η)Assume the plate area A and the effective drive velocity ωp are unchanged.ThenQ1/Q2=(-A1*ωp/㏑(1-η1)/ (-A2*ωp/㏑(1-η2)=㏑(1-η2)/ ㏑(1-η1)=㏑(1-0.93)/ ㏑(1-0.98)=0.68。
大气相关考试题及答案高中

大气相关考试题及答案高中一、选择题1. 大气层中,最接近地面的层是什么?A. 平流层B. 对流层C. 臭氧层D. 电离层答案:B2. 以下哪项不是大气中的主要气体成分?A. 氮气B. 氧气C. 二氧化碳D. 氦气答案:D3. 温室效应的主要原因是什么?A. 大气中的水蒸气增多B. 森林砍伐C. 大气中二氧化碳含量增加D. 工业污染答案:C4. 以下哪种现象不属于大气污染?A. 雾霾B. 酸雨C. 臭氧层破坏D. 植物生长答案:D5. 大气压力的单位是什么?A. 帕斯卡(Pa)B. 牛顿(N)C. 米(m)D. 千克(kg)答案:A二、填空题6. 大气层中,温度随高度增加而升高的层是_________。
答案:平流层7. 风的形成是由于_________和_________的作用。
答案:气压差;科里奥利力8. 地球表面温度的日变化规律是白天_________,夜晚_________。
答案:升高;降低9. 气象学中,降水量是指在一定时间内,单位面积上_________的总量。
答案:降水水体10. 云的形成是由于大气中的水蒸气_________。
答案:凝结三、简答题11. 请简述大气层的垂直分层及其特点。
答案:大气层主要分为对流层、平流层和高层大气。
对流层是大气的最底层,温度随高度增加而降低,天气现象主要发生在这一层。
平流层位于对流层之上,温度随高度增加而升高,臭氧层就在这一层。
高层大气包括中间层、热层和外层,温度随高度增加而变化,主要研究电离层等现象。
12. 什么是厄尔尼诺现象?它对全球气候有什么影响?答案:厄尔尼诺现象是指赤道太平洋中部和东部海域的海水温度异常升高的现象。
它会导致全球气候模式的改变,如极端天气事件的增加,部分地区降水增多,而其他地区则可能遭受干旱。
四、论述题13. 论述大气污染对人类健康和环境的影响。
答案:大气污染对人类健康的影响主要表现在呼吸系统疾病、心血管疾病的增加,以及对儿童生长发育的影响。
大气环境科学考试试题

大气环境科学考试试题1. 什么是大气?大气是地球周围的气体层,由氮气、氧气、氩气等组成。
大气对地球的生物活动和气候起着至关重要的作用。
2. 请简要说明大气层的结构和组成。
大气层主要分为对流层、平流层、中间层、热层和外层。
其主要气体成分有氮气占78%、氧气占21%、氩气、二氧化碳、水蒸气等。
3. 大气层中的臭氧和温室气体分别起着什么作用?臭氧层可以阻挡紫外线,保护地球生物。
温室气体能够吸收地球表面辐射而使得地球表面温度升高。
4. 空气中的污染物有哪些?请简要说明其来源和危害。
空气污染物主要包括废气、废水、固体废物和噪声。
工厂、汽车、焚烧等都是污染源,空气污染对人体健康和环境产生严重危害。
5. 大气环境科学的意义是什么?大气环境科学通过研究大气的结构和成分,来了解大气对生物和气候的影响,从而保护环境,维护生态平衡。
6. 大气环境科学中的观测技术有哪些?大气环境科学中的观测技术包括气象雷达、卫星遥感、大气探测器等多种现代高科技手段,能够准确监测大气中的污染物浓度以及气候变化情况。
7. 请简要介绍大气环境科学的研究范围。
大气环境科学研究大气中的气体成分、污染物、温室气体以及气候变化等多方面内容,涉及大气物理、气象、环境科学等多个学科领域。
8. 大气环境科学的发展历程是怎样的?大气环境科学的发展经历了气象学、空气质量监测、气候变化研究等多个阶段,为人类认识大气环境提供了重要的科学依据。
9. 大气环境科学在生活中的应用有哪些?大气环境科学在气象预报、环境保护、气候变化应对等方面都有着广泛应用,对人类的生活和发展产生着积极的影响。
总结:大气环境科学是一门重要的学科,通过对大气层结构、成分和环境影响的研究,能够有效保护地球生态平衡,维护人类健康和环境可持续发展。
希望广大学生能够认真学习大气环境科学知识,为保护环境、改善空气质量贡献自己的力量。
大气环境考试试题

大气环境考试试题一、选择题1. 下面哪种气体是大气中最主要的成分?A. 氮气B. 氧气C. 二氧化碳D. 氩气2. 大气污染主要来源于哪些方面?A. 工业排放B. 机动车尾气C. 生活垃圾焚烧D. 以上都是3. 大气环境中超标的主要污染物为以下哪种?A. PM2.5B. 二氧化硫C. 一氧化碳D. 氯气4. 大气环境中的酸雨主要是由哪些物质引起的?A. 二氧化碳B. 一氧化氮和二氧化氮C. 二氧化硫和氮氧化物D. 铅和镍5. 大气中主要的温室气体是什么?A. 氯氟碳化合物B. 甲烷C. 二氧化碳D. 氧气二、简答题1. 什么是光化学烟雾?它对大气环境有何影响?2. 解释一下大气逆温和大气温层的概念。
3. 请简要介绍一下大气中的臭氧层及其重要性。
4. 什么是酸雨?它对环境有哪些危害?5. 简述一下大气污染的治理措施。
三、论述题请结合你对大气环境的了解,谈谈大气环境污染对人类生活和自然生态系统的影响,以及未来应对大气环境挑战的措施。
四、计算题假设一座城市的PM2.5浓度为80μg/m³,空气质量等级为重度污染,若一名成年人在该城市呼吸24小时,则其总吸入的PM2.5质量为多少?五、应用题你现在是当地环境保护部门的工作人员,请针对近期大气环境污染问题,结合实际情况制定一份针对性的环境保护措施和宣传教育方案,并阐述实施这些方案的作用和影响。
以上为大气环境考试试题,请根据题目要求进行作答。
祝你顺利通过考试!。
大气专题复习(习题)概要

解析:b地位于赤道附近,常年受赤道低气压的控制,盛行上升气流,多 对流雨,故选A。
5.此时正值(B )
A.华北地区冬小麦返青
B.好望角附近正值风浪最高季节
C.澳大利亚东南部小麦播种正忙碌 D.阿斯旺水电站的发电量进入低谷期
解析:读图可知,赤道低气压带全部位于赤道以北,据此可判断太阳直射北半
球,此时为北半球夏季。好望角位于非洲最南端,属于地中海气候,北半球夏季
大气专题
一、选择题
(2011·晋中模拟)下图为“某地一年中气温日变化的分布图”,读图,回答 1~2题。
1.该地4月份气温日较差最大不超过( C )
A.11℃
B.9℃
C.7℃
D.5℃
解析:直接读图可知,4月份气温最高在30~31℃之间、最低在24~25℃之
间,气温日较差在5~7℃之间即最大不超过7℃。
C.图示月份为1月
D.图示月份为7月
16.判断甲地气候类型可能是( D ) A.亚热带季风气候 C.温带大陆性气候
B.温带海洋性气候 D.地中海气候
(2011届浙江省嘉兴市高三学科基础测试)下图是陆地与海上风速随高度变化 图。读图回答17-18题。
17.相同高度的风塔其发电能力 (A ) A.海上高于陆地 B.陆地高于海上 C.海上陆上相同 D.无法判定
(2011·银川模拟)雾是近地面大气层中大量微小水滴或冰晶组成的悬浮体。 2010年入冬以来,华北地区遭遇多次大雾天气,有些地区的能见度甚至只 有50米左右。气象台也多次发布大雾黄色预警。据此完成13~14题。 13.深秋、初冬时节是该地大雾的多发期,这其中的道理是( D ) A.昼夜温差减小,水汽易凝结,但风力微弱,水汽不易扩散 B.昼夜温差减小,水汽不易凝结,直接悬浮于大气中 C.昼夜温差较大,水汽不易凝结,直接附着在地面上 D.昼夜温差较大,水汽易凝结,干燥空气又提供了足够的扬尘颗粒
地球上的大气复习题
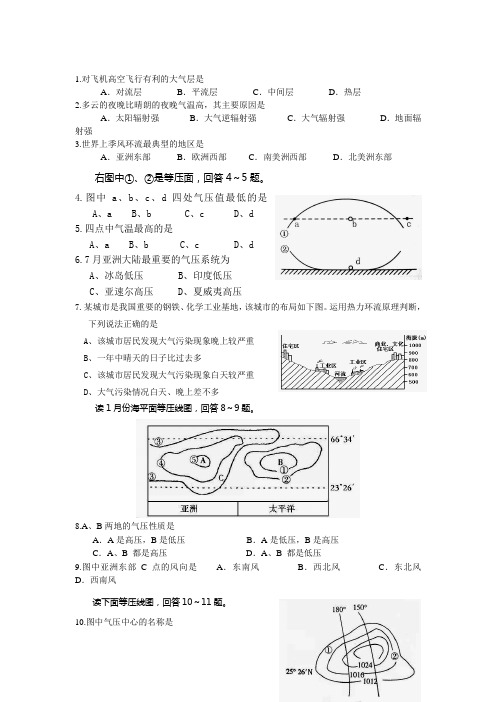
1.对飞机高空飞行有利的大气层是A.对流层B.平流层C.中间层D.热层2.多云的夜晚比晴朗的夜晚气温高,其主要原因是A.太阳辐射强B.大气逆辐射强C.大气辐射强D.地面辐射强3.世界上季风环流最典型的地区是A.亚洲东部B.欧洲西部C.南美洲西部D.北美洲东部右图中①、②是等压面,回答4~5题。
4.图中a、b、c、d四处气压值最低的是A、aB、bC、cD、d5.四点中气温最高的是A、aB、bC、cD、d6.7月亚洲大陆最重要的气压系统为A、冰岛低压B、印度低压C、亚速尔高压D、夏威夷高压7.某城市是我国重要的钢铁、化学工业基地,该城市的布局如下图。
运用热力环流原理判断,下列说法正确的是A、该城市居民发现大气污染现象晚上较严重B、一年中晴天的日子比过去多C、该城市居民发现大气污染现象白天较严重D、大气污染情况白天、晚上差不多读1月份海平面等压线图,回答8~9题。
8.A、B两地的气压性质是A.A是高压,B是低压B.A是低压,B是高压C.A、B 都是高压D.A、B 都是低压9.图中亚洲东部C点的风向是A.东南风B.西北风C.东北风D.西南风读下面等压线图,回答10~11题。
10.图中气压中心的名称是B.亚速尔高压D.印度低压11.当该地气压强盛时,下列说法正确的是A.此时我国长江正处于枯水期B.此时南极洲气候正好有利于科学考察C.此时一艘海轮经过马六甲海峡后向波斯湾航行正好是顺水D.此时印度进入多雨季节12.对飞机高空飞行有利的大气层是A.对流层B.平流层C.中间层D.热层13.多云的夜晚比晴朗的夜晚气温高,其主要原因是A.太阳辐射强B.大气逆辐射强C.大气辐射强D.地面辐射强14.世界上季风环流最典型的地区是A.亚洲东部B.欧洲西部C.南美洲西部D.北美洲东部图瓦卢是南太平洋一个岛国,然而由于海平面的上升,再过50年,这个拥有9个小岛的国家将在世界地图中消失。
据此回答3~4题。
15.下列因素促使全球海平面上升的是:①大量砍伐森林②大量燃烧矿石燃料③海水体积膨胀④陆地外流区人海河水大增A.①B.①②C.①②③D.①②③④16.下列措施能减缓海平面上升的是:A.调整生产结构,加大对能源的使用量B.提高能源的利用率,加强国际合作C.提高农业在国民经济中的地位,减少对工业的依赖性D.分散城市人口,减少城市中二氧化碳排放量读七月某台风移动路径图,回答17-18问题。
大气复习资料含答案精品文档7页

大气复习资料一、概念解释(1)Globe warming: Global warming is the increase in the average measured temperature of the Earth's near-surface air and oceanssince the mid-twentieth century, and its projectedcontinuation.(2) Temperature inversions:A temperature inversions is a thin layer of the atmosphere where the decrease in temperature withheight is much less than normal (or in extreme cases, thetemperature increases with height).(3)ESP: Electrostatic precipitator, which is like a gravity setter or centrifugal separator, but electrostatic force drives theparticles to the wall.(4)HEPA: High Efficiency Particulate Air(5)Lean bum :Lean burn refers to the use of lean mixtures in an internal combustion engine .The air-fuel can be as high as65:1 ,so the mixture has considerably less fuel in comparisonto the stoichiometric combustion ratio (14.7 for petrol , forexample ).(6)Plume rise: the plume rising a distance △h above the top of the stack before leveling out.(7)Wet scrubber: A device that collects particles by contacting the dirty gas stream with liquid drops.(8) Photochemical smog:(9)Thermal NO: Thermal NO x refers to NO x formed through high temperature oxidation of the diatomic nitrogen found incombustion air.(10)A/F ratio: Air to fuel ratio for auto engines.(11)PM2.5:particle with the aerodynamic diameter less than 2.5 um,which is also called Respirable Particles.(12)Alternative fuel: Several other fuels except of conventional gasoline and diesel, which have been used for many years in slighutly modified automobile engines, for reasons of cost and availability.(13)VOCs: Volatile organic compounds are those organic liquids or solids whose room temperature vapor pressure are greater than about 0.01 psia(=0.0007atm) and whose atmospheric boiling points are up to about 500℉, which means most organic compounds with less than 12 carbon atoms.(1)SCR and SNCR:6NO+4NH3→5N2+6H2O, 4NO+4NH3+O2→4N2+6H2O2NO2+4NH3→3N2+6H2O.These reactions can be carried out over a variety of catalysts, the temperature is between 1600℉and 1800℉, once the temperature increases, the dominant reaction isNH3+O2→NO+1.5H2O, the catalytic processes are called SCR, andthe higher-temperature ones, without catalysts, call SNCR.(2)Aerodynamic diameter: Airborne particles have irregular shapes, and their aerodynamic behavior is expressed in terms of thediameter of an idealized spherical particle known asAerodynamic diameter.(3)Primary Particles: Particles found in the atmosphere in the form in which they were emitted, for examples, NO,CO,SO2(4)Point Sources: small number of large sources that emit larger amounts per source, at higher elevations (power plants,smelters, cement plants, etc.) called point sources二、Answer following questions(1)Which are the main constituents for the ground level ozoneformation?Ozone is formed when the following constituents are present. Nitrogen oxides, Volatile Organic, Compounds, Sunlight, High temperature(>18 ℃)NO+VOC+O2+Sunlight→NO2+O3(2)Please list five major types of wet scrubbers.Plate Scrubber (板式)Packed Scrubber (填料式)Preformed Spray Scrubber (喷雾式)Gas-Atomized Spray Scrubber (气体雾化)Centrifugal Scrubber (离心式)Impingement-Entrainment Scrubber(冲击夹带式)Mechanically Aided Scrubber (机械辅助式)Moving Bed Scrubber (移动床式)(3)How to control VOCs pollution by prevention? Two examples.Substitution(代替),Replacing gasoline as a motor fuel with compressed natural gas or propane is a form of substitution.Process Modification(过程修改),Replacing gasoline-powered vehicles with electric-powered vehicles is a form of process modification.Leakage(渗漏) Control,Storing large amounts of gasoline in floating roof tanks.(4)Please introduce the process of forced-oxidation Limestone WetScrubbing briefly?(5)What are the most different points between SCR and SNCR?(6)Can TWC be applied in the treatment of diesel exhaust (=emissionof diesel engine)? Why?·The characteristic of emission of diesel engine: Ample PM and Excessive O2, Lower temperature;·The difficulty in the reaction of solid-gas-solid.Key: The mixture process of fuel and air for gas engines is distinguished from that for diesel engines, hence the character of one type engine is different from the other one: there are mainly five gases(NOX , HC, CO and O2, CO2) in the exhaust of gas motors, while there areample O2and four other gases mentioned above in the tailpipe emissionof diesel engine. And the presence of abundant Owould inhibit the2performance of TWC.Moreover, the contact and reaction of solid-solid-gas resulting in the difficulties for catalysts to oxidation the particulate in the exhaust of diesel engine, while the contact and reaction of solid-gas-gas occur in the exhaust of gasoline engine and the latter reaction is easier.(7)What kinds of indoor air pollutants are mostly concerned bypublic?a)Randomb)Combustion by-products1.CO, CO2, SO2,Formaldehyde,Hydrocarbons,NOx2.Particulates, polyaromatic hydrocarbonsc)Cigarettes d)Volatile organic compoundsf) Biological contaminants(8)List the technology strategy for the control of particles.(9)Give names of three typical kinds of combustion reactors. Whichhas lowest operator temperature among those reactors?1.Direct flame incineration2.Thermal incineration3.Catalytic incineration (has lowest operator temperature)(10)What are the major development problems of Forced-oxidationlimestone wet scrubbing?(11)What are primary air pollutants and secondary air pollutants?Any example?primary air pollutants are directly from the sources, for examples, NO, CO, SO2.The secondary air pollutants are from the primary pollutants, such as NO2, NO3, fine particles.NO+CH+O2+sunlight → NO2+O3(12)Basic strategy of control for particulate pollutants (threeaspects )?Impaction 碰撞 Interception 截留 Diffusion 扩散By forcing the individual particles to contact each other,By contacting them with drops of water,By preventing the emission of gaseous Pollutants.(13)How to reduce the formation of NOx in flue gas by modifyingthe combustion processes?p459(14)Please explain the formation of acid rain?Sulfur oxides and nitrogen oxidesNO NO2 HNO3nitric acid SO2H2SO4sulfuric acid(15)What are basic principles of electrostatic Precipitators (sixactivities)?·Ionization - Charging of particles·Migration - Transporting the charged particles to the collecting surfaces·Collection - Precipitation of the charged particles onto the collecting surfaces·Charge dissipation - Neutralizing the charged particles on the collecting surfaces·Particle dislodging - Removing the particles from the collecting surface to the hopper·Particle removal - Conveying the particles from the hopper to a disposal point三、Calculation1) Estimate the concentration of carbon monoxide at the downwind edge of a city. The city may be considered to consist of three parallel strips, located perpendicular to the wind. For all of the strips the wind velocity u equals 3 m/s. The properties of each of the strips are described in the following table,solution :uHb c += c 1=0+(100*5000)/(3*400)=416.7 μg/m 3 uHqL c +=1C c 2=416.7+(500*2000)/(3*500)=1083.4μg/m 3 uHqL c +=2C c 3=1083.4+(100*5000)/(3*400)=1500.1μg/m 3 2) An ESP is designed to treat 540,000 acfm (actual cubic feet per minute ) with 99% efficiency . Assuming an effective drift velocity of 0.12 m/s , calculate the required plate area and the number of plates. The plate size is 5.4m tall by 3m long (A =-Q×㏑(1-η)/ωp , acfm =1ft 3/min, 1m=3.28ft, 1m 2=1550 in 2=10.7639 ft 2). solution1ft 3/min=(1/3.28)3m 3/min=0.0283 m 3/min540000acfm=540000*0.028315282 m 3/min=254.7 m 3/sA= -(254.7/0.12)ln(1-0.99)=9774.47m 2N=9774.47/514.3=6043) We wish to treat an airstream containing 0.005mol fraction (0.5%, 5000ppm ) toluene, moving at a flow rate 2240m 3/h at 0℃ and 1 atm , so as to remove 99% of the toluene by water absorption. Estimate the required water flow rate. Here Henr y’s law constant is 10000 atm .Solution :4)A power plant flue gas contains 1000ppm of SO 2 and is emitted at a rate of 224m 3/s at 546K and 1 atm . A Forced-oxidation limestone wet scrubbing system is to be used to achieve 90% removal of the SO 2. Calculate the amount of CaSO 4·2H 2O contained in the final solid product in t/d.Solution : RT PV n = =(101325*224*1000*10-6)/(8.315*546)=4.999mol/s SO 2~ CaSO 4·2H 2O so n (c a so 4)=n (so 2)=4.999mol/s*90%=4.4991mol/sm=4.4991mol/s *172g/mol *3600*24s/d *10-6 t/d=66.86 t/d5)A power plant emits 36 kg/h of SO 2 at height H=120m and the wind speed is 2 m/s. Dispersion Coefficients: σy =40m and σz =30m, Estimate the ground-level concentration of SO 2 from this source at a distance 1km directly downwind? Solution :u=2m/s , H=120m, Q=36kg/h=10g/s , y=0 , z=H=120 , x=1000m ⎭⎬⎫⎩⎨⎧+⎥⎦⎤⎢⎣⎡⎥⎦⎤⎢⎣⎡+-=130********exp 30*40*3.14*10*210C =0.40 6)A power plant flue gas contains 1000×10-6 (1000ppm )of NOx ,and is emittedat a rate of 89.6m3/s at 546K and 1 atm. The NOx is 90%mol NO, balance NO2. A selective catalytic reduction system is to be used to remove the NOx. Calculate the minimum of ammonia needed in kg/h.Solution: 4NO+4NH3+O2→4N2+6H2O 2O+4NH3+O2→3N2+6H2OPV=nRT n=(101325*89.6*1000*10-6)/(8.315*546)=2.0mol/sn(no)=0.9*2mol/s=1.8mol/s so n(no2)=2.0-1.8=0.2mol/sn(NH3)= n(no)+2* n(no2)=1.8+0.4=2.2mol/sm=2.2mol/s * 17 g/mol =37.4g/s=134.64kg/h7)The efficiency of an ESP is 98%. The efficiency of the ESP drops to 93% as a result of flow rate changes. Calculate the ratio of flow rates for the above situation. Use appropriate assumptionSolution: A =-Q×㏑(1-η)/ωp SO Q=-A*ωp/㏑(1-η)Assume the plate area A and the effective drive velocity ωp are unchanged.ThenQ1/Q2=(-A1*ωp/㏑(1-η1)/ (-A2*ωp/㏑(1-η2)=㏑(1-η2)/ ㏑(1-η1)=㏑(1-0.93)/ ㏑(1-0.98)=0.68。
大气全章练习试题目

2010~2014年高考真题备选题库第二章地球上的大气第一讲大气的受热过程与大气运动(2014.山东高考)下图为我国某区域冬季某日8时至次日8时的降雪量和积雪深度分布图,该时段该区域风向主要为偏东风,云量分布差异不明显。
完成4~5题。
4.造成该区域东西部积雪深度差异的主要因素是()A.降雪量B.温度C.光照D.地形5.图中M地积雪深度低于周围地区,该地可能是()A.农田B.林地C.城区D.乡村解析:4.B 5.C 第4题,该区域位于长江三角洲,其东西部均为平原地形;冬季降雪量东西部地区相差不大;纬度相同,光照条件也相差不大;图示西部地区距海较远,受海洋影响较小,气温低于东部地区,故积雪量大、积雪深度大。
第5题,图中M地积雪深度低于周围地区,说明该地温度高于周围地区,最可能是城区。
解析:选B本题以大气受热过程示意图为背景,考查大气受热过程及影响因素,意在考查考生调动和运用知识的能力。
臭氧层破坏会导致大气吸收紫外线能力减弱,①会减少;二氧化碳浓度降低,会使大气吸收地面辐射能力减弱,②会减少;可吸入颗粒物增加,大气反射等削弱太阳辐射能力增加,③地面吸收太阳辐射会减少;出现雾霾天气,会增强大气逆辐射作用,④在夜间会增加。
(2011全国高考)一般情况下,空气的密度与气温、空气中的水汽含量呈负相关。
下图示意北半球中纬某区域的地形和8时气温状况剖面。
高空自西向东的气流速度约20千米/时。
据此完成12~14。
12.此时甲、乙、丙三地的大气垂直状况相比较()A.甲地比乙地稳定B.乙地对流最旺盛C.乙地比丙地稳定D.丙地最稳定解析:垂直方向上,温差越大,大气对流运动越强烈,大气越稳定。
图中甲、乙、丙三地由地面距离高空2 500m高空范围内,垂直温差最小的是丙地,故丙地大气最稳定。
答案:D13.正午前后()A.甲地气温上升最快B.乙地可能出现强对流天气C.丙地刮起东北风D.甲地出现强劲的偏南风解析:本题考查天气系统知识,意在考查考生的知识迁移能力。
大气科学考试试题

大气科学考试试题一、选择题(每题 3 分,共 30 分)1、大气的主要成分中,体积占比最大的是()A 氮气B 氧气C 二氧化碳D 稀有气体2、以下哪种气体属于温室气体()A 氧气B 氮气C 甲烷D 氦气3、大气的垂直分层中,对流层的主要特点是()A 气温随高度升高而升高B 天气现象复杂多变C 气流平稳D 臭氧含量丰富4、形成风的直接原因是()A 水平气压梯度力B 地转偏向力C 摩擦力D 惯性离心力5、全球气候变暖主要是由于()A 大气中二氧化碳浓度增加B 太阳活动增强C 火山活动频繁D 森林面积增加6、冷锋过境时,通常会出现的天气现象是()A 升温、降雨B 降温、降雨C 升温、晴天D 降温、晴天7、大气环流的主要动力是()A 海陆热力差异B 太阳辐射能C 地球自转D 地形差异8、以下哪种云属于高云()A 积云B 卷云C 层云D 雨层云9、厄尔尼诺现象发生时,赤道附近太平洋东部海域的水温会()A 升高B 降低C 不变D 先升后降10、影响大气能见度的主要因素是()A 气温B 气压C 水汽含量D 颗粒物浓度二、填空题(每题 3 分,共 30 分)1、大气中的水汽主要来源于_____。
2、平流层中气温随高度升高而升高,主要是因为其中含有大量的_____。
3、台风是一种强烈发展的_____。
4、天气预报中的“高压”和“低压”,指的是_____的分布状况。
5、大气对太阳辐射的削弱作用主要有_____、_____和_____。
6、气候的形成因素主要有_____、_____、_____、_____和_____。
7、赤道低气压带控制下的地区,气候特点是_____。
8、我国冬季盛行_____风,夏季盛行_____风。
9、城市热岛效应是指城市气温_____周边郊区气温的现象。
10、大气中的臭氧层能够吸收_____,保护地球上的生物。
三、简答题(每题 10 分,共 20 分)1、简述热力环流的形成过程。
答:由于地面冷热不均,首先会出现受热地区空气膨胀上升,近地面形成低气压,而高空形成高气压;相对地,冷却地区空气收缩下沉,近地面形成高气压,高空形成低气压。
地球上的大气部分经典试题

地球上的大气部分经典试题(总10页)-CAL-FENGHAI.-(YICAI)-Company One1-CAL-本页仅作为文档封面,使用请直接删除地球上的大气部分经典试题读地球上较大尺度大气活动中心的气流运动示意图,完成1~2题。
1.若甲、乙两地均位于30°N附近,下列说法正确的是( )A.此时,我国东部盛行西北风B.甲、乙均是由热力原因形成C.甲地温和湿润D.乙地炎热干燥2.若乙地的气温年较差小于甲地,最可以确定的原因是( )A.甲地太阳辐射强于乙地B.甲地纬度位置低于乙地C.甲地海拔高于乙地D.甲地的热容量小于乙地解析:第1题,北纬30°附近为副热带高气压带,呈现图示状况时说明其被割裂,应为夏季,我国盛行偏南风;此时,甲地为低压,由热力原因所致;乙地受高压控制,炎热干燥。
第2题,从图中信息无法确定甲、乙的经纬度位置,因而无法判断选项A、B、C 的正误,热容量小则气温变化大,甲地气温年较差大则其热容量小。
答案: 1.D 2.D每年随着干、湿季节的变化,东非高原上数以百万计的野生食草动物就会组成一支壮观的大军,浩浩荡荡地在坦桑尼亚的塞伦盖蒂国家公园和肯尼亚的马萨伊马拉国家自然保护区间来回迁徙。
下图是食草野生动物迁徙路线。
据此完成3~4题。
3.当①地有充足的水源和青草时,当地受()A.西风带控制B.副热带高气压带控制C.信风带控制D.赤道低气压带控制4.野生食草动物从②地迁徙到③地的时间可能是()A.12月至次年5月B.7月底至8月中下旬C.10月底至11月底D.1月中上旬至3月底解析:第3题,读图可知该地位于赤道以南区域,受赤道低气压带控制降水多。
第4题,7月底至8月中下旬②受信风控制降水少,野生食草动物迁往③地。
答案: 3.D 4.B下图中①②③④分别为二分二至日气压带、风带分布示意图的一部分,读图回答5~6题。
5.处于同一日的一组是()A.①②B.②③C.③④D.①④6.如果图中风带和气压带皆影响大陆西岸,那么①②③④图中都有可能形成的气候类型是()A.温带海洋性气候B.地中海气候C.热带沙漠气候D.热带草原气候解析:第5题,①为西南风,为北半球西风带,气压带为副极地低气压带,处于偏北(北极圈以北)位置,因而可推知,①为夏至日;同理,根据风向和气压带可推知,②为夏至日,③为冬至日,④为二分日。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2.EGR isExhaust Gas Recycle.(EGR:废气再循环技术)
3.ESPs isElectrostatic Precipitators.(ESPs:静电除尘器)
4.ODP isozone depletion potential.(ODP:臭氧消耗潜能)
29.The fine particles in the atmosphere are largelysecondary particles, formed in theatmosphere fromgaseous precursors. Most of the coarser particles in the atmosphere areprimary particles, which enter the atmosphere asparticles.(大气中细颗粒主要是次级粒子,形成于大气中的gaseous precursors,大气中大部分的粗颗粒是初级粒子,他们作为粒子进入大气层)
5.VOCs isvolatile organic compounds.(VOCs:挥发性有机化合物)
6.Some of the most important air pollutions aresecondary pollutants, formed in the atmosphere fromprimary pollutant precursors.(一些最重要的空气污染都是二次污染,他们是由主要的初期污染物质在大气中形成的)
8.Air pollution: is the presence of man-made harmful materials in the air, in quantities large enough to produce harmful effects.(空气污染:指空气中存在的有害的人造材料,在数量足够大时造成的有害影响)
10.The overall air pollution problem takes the following form:emission→transport, dilution, and modification in the atmosphere→effects on people, property, and the environment.(大体上大气污染问题的构成如下:排放→在大气中逸散、稀释、反应→对人体,财产,环境造成影响)
27.These small particles behave quite differently from the particles with which we are familiar, like sand and gravel. Their highsurface area per unit massmakes them adhere to one another if they are brought into contact.(这些小颗粒的性质完全不同于我们说熟悉的像沙子,砾石一类的颗粒。他们有很高的比表面积,这是他们相接触时很容易黏在一起)
28.Because particles of air pollution interest are rarely present in the air or in a gas stream as a uniform particle size set, we normally have to deal withthe distributionof particle size.(由于interest空气污染颗粒在空气或气流中很少有相同的大小,我们通常要处理粒度的分布)
(3)Use a downstream pollution control device.(采用下游污染控制策略)
17.“3T” aretime, temperature and turbulence.(时间,温度,燃料与空气混合)
18.If one is face with an air pollution problem, the alternatives for alleviating it areimproved dispersion,process change, ordownstream control devices.(减轻污染的方案是改善排放途径,工艺改进,或采用下游污染控制策略)
7.Before 1960, our principal concern about air pollution effects was withproperty damage. Since 1960, we have been concerned primarily withhuman health.(1960之前,我们的资金主要用于关注空气污染带来的财产损失,1960之后,我们主要关注的是人类的健康。)
19.In current U.S. law and practice the first choice isprocess change; the second isa downstream control device; and only if thet the applicable standard mayimproved dispersionbe used.(目前在美国法律和实践第一选择的是改进工艺,第二是采用下游污染控制策略,如果这两者都不能达到适用的标准就可能会改善排放途径)
11.The basic problems in ambient monitoring and source testing arethe collection of a representative sampleandthe correct analysis of that sample. The collection of a representative sample is the harder part.(环境监测和污染原因测试的基本问题是有代表性样本的搜集和这些本的正确分析。)
25.The particles of air pollution interest are mostly in the size range0.1to 10μ.(interest空气污染颗粒直径范围为0.1to 10μ)
26.Particles smaller than about 2μ are rarely produced by mechanical means; they are primarily produced by condensation or chemical reaction ofgases or vapors.(直径小于2μ的颗粒很少是机械方法产生的,他们主要是由气体和蒸汽的凝结或化学反应产生的)
21.In most calculations, thepenetrationis a more convenient measure of control equipment performance than is the controlefficiency.(大量计算结果表明,penetration在控制设备性能方面比控制效率是一种更方便的措施)
22.Most air pollutants are the direct and indirect result ofcombustionorprocesses using combustion.(大部分污染物是充分燃烧或非充分燃烧直接或间接的产物)
23.All pollution control devices that teat gases with even trace amounts of SO2must be protected againstacid dew point corrosion.(所有选用优质的二氧化硫气体的污染控制设备,即使量很少也必须得到保护,以防酸露点腐蚀)
30.Gravity settling chambers,cyclones, andESPswork by driving the particles to a solid wall where they form agglomerates that can be collected.(重力沉降室,旋风除尘器,静电除尘器的工作原理就是把颗粒drive to adriver,使他们凝聚在一起能够被收集)
31.Filtersandscrubbersdivided the flow. They have different design equations from wall collection devices and from each other.(过滤器和洗涤器)
12.The two meteorological parameters of greatest interest to air pollution engineers arethe atmosphere stabilityandthe wind speed.(大气污染工程师最感兴趣的两个气象因素是大气的稳定性和风速)
(1)Improve dispersion: tall stacks, intermittent control schemes, relocate the plant;(改善排放途径:高烟囱,间歇控制计划,搬迁工厂)
(2)Reduce emission by process change, pollution prevention;(改进工艺以减少排放;污染防治)
13.In general,stable atmospheresandlow wind speedslead to the highest ground-level pollution concentration.(大体上讲,稳定的大气和低风速导致了最高级别的地面污染浓度)
14.Pollutant concentration models are based on knownemission ratesandmeteorology.(污染物浓度模型是基于众所周知的排放速率和气象)
