4195-4197 Paclitaxel
317种化学物质IDLH(立即威胁生命和健康浓度)

序号
污染物中文名称
污染物英文名祢
IDLH
浓度'
PPm
Ippm换算mg/m3系数b
(20C)
IDLH浓度’
mg/m3
(20C)
26
苯
benzene
3 000
3. 25
9 800
27
过氧化(二)苯甲酰
benzoyl peroxide
7 000
28
氯化节
benzyl chloride
10
5. 26
8. 72
22000
104
二缩水甘油醯
diglycidyl ether
25
5.41
MO
105
二异丁基甲酮
diisobutyl ketone
2 000
5. 92
12 000
106
二异丙胺
diisopropylamine
1 000
4, 21
4 200
107
二甲基乙酰胺
dimethyl acetamide
acrylonitrile, vinyl cyanide
500
2. 21
1 100
9
艾氏剂
aldrin
100
10
烯丙醇
allyl alcohol
150
2. 42
36。
11
烯丙基氯
allyl chloride
300
3. 18
95()
12
缩水甘油烯丙酰
allyl glycidyl ether
270
4. 75
500
82
十硼烷,十硼氢
decaborane
美国药典 紫杉醇 Paclitaxel
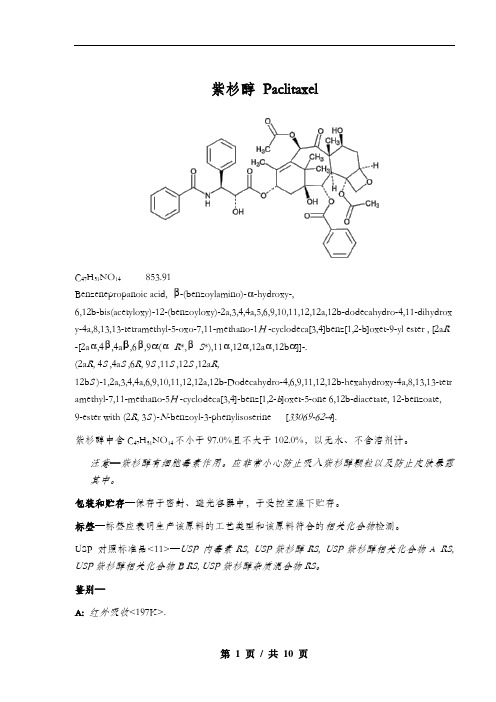
紫杉醇PaclitaxelC47H51NO14853.91Benzenepropanoic acid, -(benzoylamino)--hydroxy-,6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydrox y-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H -cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester , [2a R -[2a,4,4a,6,9(R*,S*),11,12,12a,12b]]-.(2a R, 4S ,4a S ,6R, 9S ,11S ,12S ,12a R,12b S )-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetr amethyl-7,11-methano-5H -cyclodeca[3,4]-benz[1,2-b]oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R, 3S )-N-benzoyl-3-phenylisoserine [33069-62-4].紫杉醇中含C47H51NO14不小于97.0%且不大于102.0%,以无水、不含溶剂计。
注意—紫杉醇有细胞毒素作用。
应非常小心防止吸入紫杉醇颗粒以及防止皮肤暴露其中。
包装和贮存—保存于密封、避光容器中,于受控室温下贮存。
标签—标签应表明生产该原料的工艺类型和该原料符合的相关化合物检测。
USP 对照标准品<11>—USP 内毒素RS, USP紫杉醇RS, USP紫杉醇相关化合物A RS, USP紫杉醇相关化合物B RS, USP紫杉醇杂质混合物RS。
吡啶化学性质

ACS;PVC Coated Bottles;Aluminum Bottles;CHROMASOLV Plus;Chromatography Reagents&;HPLC &;HPLC Plus Grade Solvents (CHROMASOLV);HPLC/UHPLC Solvents(CHROMASOLV);UHPLC Solvents (CHROMASOLV);ACS Grade Solvents;Carbon Steel Cans with NPT Threads;Semi-Bulk Solvents;分析标准品;精细化学品Mol110-86-1.mol文件:吡啶性质熔点-42 °C沸点96-98 °C(lit.)密度0.983 g/mL at 20 °C蒸气密度 2.72 (vs air)蒸气压23.8 mm Hg ( 25 °C)折射率n20/D 1.509(lit.)FEMA 2966闪点68 °F储存条件Store at RT.水溶解性Miscible凝固点-42℃Merck 14,7970BRN 103233稳定性Stable. Flammable. Incompatible with strong oxidizing agents, strong acids.CAS 数据库110-86-1(CAS DataBase Reference)NIST化学物质信息Pyridine(110-86-1)EPA化学物质信息Pyridine(110-86-1)吡啶用途与合成方法概述吡啶(分子式C6H5N)含有一个氮杂原子的六元杂环化合物,即苯分子中的一个-CH=被氮取代而生成的化合物,与苯类似,具有相同的电子结构,仍有芳香性,故又称氮苯和氮杂苯,在常温下是一种无色有特殊气味的液体,熔点-41.6℃,沸点115.2℃,与水形成共沸混合物,沸点92~93℃。
Paclitaxel (Taxol)_抗肿瘤药_33069-62-4_Apexbio

生物活性
靶点 :
Cell Cycle/Checkpoint
信号通路:
Microtubule/Tubulin
产品描述:
紫杉醇(Paclitaxel)是一种新型抗肿瘤剂,是由国家癌症研究所在数千种植物提取物和天然 产物中筛选具有抗肿瘤活性的产物时发现的。尽管紫杉醇作为有丝分裂的抑制剂而起作用, 如长春花生物碱,但紫杉醇促进微管蛋白的聚合,而不是诱导微管的去组装,其抑制微管的
实验操作
细胞实验: 细胞系 溶解方法 反应时间 应用
人类动脉内皮细胞(haEC)
动物实验: 动物模型 剂量 注意事项
雌性 CB17 SCID 小鼠
静脉注射,12.5 mg/kg
请于室内测试所有化合物的溶解度。虽然化合物的实际溶解度可 能与其理论值略有不同,但仍处于实验系统误差的允许范围内。
参考文献: [1] Axel D I, Kunert W, G?ggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation, 1997, 96(2): 636-645. [2] Kunstfeld R, Wickenhauser G, Michaelis U, et al. Paclitaxel encapsulated in cationic liposomes diminishes tumor angiogenesis and melanoma growth in a “humanized” SCID mouse model. Journal of investigative dermatology, 2003, 120(3): 476-482.
恩拉霉素制剂化学品安全技术说明书

版本3.0修订日期:2023/03/20SDS编号:24581-00020前次修订日期: 2022/10/01最初编制日期: 2014/10/221. 化学品及企业标识产品名称: Enramycin Formulation制造商或供应商信息制造商或供应商名称: MSD地址: 第485號荊抬道普陀區 - 上海 - 中國200331电话号码: +1-908-740-4000应急咨询电话: 86-571-87268110电子邮件地址: **********************推荐用途和限制用途推荐用途: 兽用产品限制用途:不适用2. 危险性概述紧急情况概述外观与性状: 粉末颜色: 淡棕气味: 特征的对水生生物毒性极大。
对水生生物有毒并具有长期持续影响。
GHS危险性类别急性(短期)水生危害: 类别 1长期水生危害: 类别 2GHS标签要素象形图:信号词: 警告危险性说明: H400对水生生物毒性极大。
H411对水生生物有毒并具有长期持续影响。
版本3.0修订日期:2023/03/20SDS编号:24581-00020前次修订日期: 2022/10/01最初编制日期: 2014/10/22防范说明:预防措施:P273避免释放到环境中。
事故响应:P391收集溢出物。
废弃处置:P501将内装物/容器送到批准的废物处理厂处理。
物理和化学危险根据现有信息无需进行分类。
健康危害根据现有信息无需进行分类。
环境危害对水生生物毒性极大。
对水生生物有毒并具有长期持续影响。
GHS未包括的其他危害粉尘与眼睛接触会导致机械性刺激。
与粉尘接触会引起机械性刺激或皮肤干燥。
如果发生散布,可能会形成可爆炸的粉尘和空气混合物。
3. 成分/组成信息物质/混合物: 混合物组分化学品名称化学文摘登记号(CAS No.)浓度或浓度范围 (% w/w) 滑石14807-96-6>=90 - <=100恩拉霉素B34304-21-7>=2.5 - <104. 急救措施一般的建议: 出事故或感觉不适时,立即就医。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
nevirapine质量标准

nevirapine,中文名为奈韦拉平,是一种抗逆转录病毒药物,主要用于治疗HIV感染。
以下是nevirapine的质量标准:
1. 含量:按照无水物计算,奈韦拉平的含量应在%~%之间。
2. 有关物质:奈韦拉平的有关物质包括杂质Ⅰ、杂质Ⅱ和杂质Ⅲ。
在色谱
条件下,这些杂质的峰面积不得超过对照溶液主峰面积的2倍(%),其他单个杂
质峰面积不得超过对照溶液的主峰面积(%),各杂质峰面积的和不得超过对照溶
液主峰面积的6倍(%)。
3. 残留溶剂:如果使用了有机溶剂进行合成或精制,应检查奈韦拉平中的
残留溶剂。
根据不同的有机溶剂,其残留量有不同的限制。
4. 外观:奈韦拉平应为白色至淡黄色结晶性粉末。
5. 鉴别:采用红外光谱、紫外光谱或核磁共振等方法对奈韦拉平进行鉴别。
6. 纯度:通过高效液相色谱法或其他适当的方法对奈韦拉平进行纯度检查,确保其纯度符合要求。
7. 溶液的澄清度与颜色:将奈韦拉平溶解在适当的溶剂中,检查其澄清度
和颜色,以确保符合规定。
8. 酸碱度:检查奈韦拉平的酸碱度,以确保其符合规定。
9. 干燥失重:如果奈韦拉平中含有结晶水或其他形式的溶剂,应进行干燥
失重检查,以确保其符合规定。
10. 微生物限度:对奈韦拉平进行微生物限度检查,以确保其微生物含量符合规定。
总之,为了确保奈韦拉平的质量和安全性,必须对其进行全面的质量标准检测。
这些标准包括含量、有关物质、残留溶剂、外观、鉴别、纯度、溶液的澄清度与颜色、酸碱度、干燥失重和微生物限度等。
Ia医药生化试剂

浓度
纯度
型号
规格
500ml/瓶 5g/瓶
单位
瓶 瓶 瓶 瓶 瓶 支 支 瓶 瓶 瓶 瓶 瓶 箱 瓶 合 瓶 瓶 瓶 甁 瓶 支 支 瓶 支 瓶 瓶 瓶 支 克拉玛尔 Sigma
品牌/厂家
科伦制药有限公司
解剖
组胚
分析纯 生化试剂 生化试剂 生化试剂 生化试剂 60~80目 分析纯 分析纯 医用 进口分装 进口分装
北京义翘神舟生物
Ia101
北京义翘神舟生物
Ia102
北京义翘神舟生物
Ia103 Ia104 Ia105 Ia106 Ia107 Ia108 Ia109 Ia110 Ia111 Ia112 Ia113 Ia114 Ia115 Ia116 Ia117 Ia118 Ia119 Ia120 Ia121 Ia122
浓度
纯度
型号
规格
100克/瓶
单位
瓶 瓶 瓶 支 瓶 瓶 瓶 瓶
品牌/厂家
whatman分装 北京索莱宝科技有限公司 上海原叶 福州迈新生物技术开发有限公 司 吉诺生物医药 西陇化工股份有限公司 国药集团化学试剂有限公司 赛齐(上海)生物工程有限公 司 GIBCO 汕头市西陇化工厂有限公司 北京艾德莱生物科技有限公司 上海生工 天根生化科技有限公司 厂家:上海生工 天根生化科技有限公司 索莱宝 福州迈新生物技术开发有限公 司 厂家:上海生工 NEB 上海生工 中天美行 天津光复 汕头市西陇化工厂有限公司 福州迈新生物技术开发有限公 司 北京索莱宝科技有限公司 NEB公司
浓度
纯度
分析纯 AR 分析纯 AR 分析纯 分析纯 生化试剂 化学纯
型号
规格
10ml/支 100ml/瓶 250ml/瓶 25毫升/瓶 100ml/瓶 100毫升/瓶 100毫升/瓶
酒石酸匹莫范色林 质量标准

酒石酸匹莫范色林质量标准
摘要:
一、酒石酸匹莫范色林的基本信息
二、酒石酸匹莫范色林的适应症
三、酒石酸匹莫范色林与其他药物的比较
四、酒石酸匹莫范色林的剂量与用法
五、酒石酸匹莫范色林的质量标准
正文:
酒石酸匹莫范色林(Pimavanserin tartrate)是一种新型药物,其化学式为C25H34FN3O2。
这款药物的主要剂型有片剂(10mg、17mg)和胶囊(34mg),以匹莫范色林计。
它被推荐用于治疗与帕金森病精神病相关的幻觉和妄想。
酒石酸匹莫范色林是首个获FDA批准用于治疗帕金森病精神症状的药物,被誉为突破性疗法。
相较于其他药物如喹硫平和氯氮平,酒石酸匹莫范色林的耐受性和安全性更佳,不良反应较少。
值得一提的是,它对多巴胺无作用,因此在治疗过程中更为安全。
关于酒石酸匹莫范色林的剂量,推荐剂量为每天34mg,可以一次性口服两片17mg的片剂。
值得注意的是,该药物无需滴定调整,方便患者服用。
在质量标准方面,酒石酸匹莫范色林的生产和检验均遵循严格的标准,确保产品的纯度和有效性。
为确保患者的安全和疗效,使用时需遵循医生建议,并按照规定的剂量和用法进行用药。
总之,酒石酸匹莫范色林作为一种治疗帕金森病精神病症状的药物,具有较高的安全性和耐受性,为广大患者带来了福音。
在使用过程中,患者只需遵循医生的建议,按照规定的剂量进行用药,便可获得良好的疗效。
然而,为确保用药安全,患者在用药过程中需密切关注任何不良反应,并随时与医生保持沟通。
立他司特lifitegrast

2.作用机制
lifitegrast 是一种新型小分子T 细胞抑制剂。通过模拟细胞黏附分子( intercellu-laradhesion molecule,ICAM-1) 与淋巴细胞功能相关抗原( lymphocyte function-associated antigen 1,LFA-1) 相连部位的结构,从而有效阻断两者的结合,进而抑制 T 细胞介导的炎症反应。Murphy 等选取了被 诊断患有干燥性角结膜炎的犬进行药物临床有效性的实验,在 1% lifitegrast 溶液、tid 使用28 d 后发 现,犬眼部的干燥症状显著改善。在结膜活检的组织学评价中也证实,与研究基线样本相比,犬眼周 的 T 细胞炎症明显减少。体外试验结果表明,lifitegrast 还可有效地抑制T 细胞的活化、细胞因子的 释放和免疫突触的形成。研究人员准备了 lifitegrast 溶液、环孢素、超抗原金黄色葡萄球菌肠毒素 B( staphylococcal enter-otoxin B,SEB) 进行实验以有 SEB 的人类单核细胞作为阳性对照,无 SEB 的为阴性对照。结果发现lifitegrast 溶液可抑制 IL-1,IL-2,IL-4,IL-10,IL-17,INF-γ,TNF-α, MIP-1α 等细胞因子的释放。
立他司特lifitegrast
立他司特(Lifitegrast)是一种新的细胞间黏附因子的抑制剂,可以通过阻断细胞间黏附分子1和整合素蛋白淋巴细胞功能相关抗原-1之间的结合起效。2016年7月,美国食品药品管理局(FDA)正 式批准了5%立他司特滴眼剂(商品名XiidraTM)的申请。立他司特是FDA批准的第一个可以改善和治 疗干眼症症状的新药,其他类似药物只有环孢素,在不久的将来其临床应用会更加广泛。业界对其商 业前景十分看好,预计年销售额将突破10亿美元,同时将成为艾尔健干眼病药物Restasis(品牌名 :丽眼达,通用名:cyclosporine,环孢霉素)的强有力竞争对手。目前国内还没有申报公司,以 下就几个方面对该产品做综述。
2010.4.1 REACH ANNEX XVII (限制物偾鍐 修 订

歐盟法規1907/2006/EC化學物質之註冊、評估、許可和限制(REACH)附錄
17(ANNEX XVII)之修訂
本文內容主要是修訂2008年12月18日歐洲國會及協會法規1907/2006/EC化學物質之註冊、評估、許可和限制(REACH)附錄17的規定,設立歐洲化學總署以修改指令1999/45/EC,以及廢除協會法規793/93/EEC、法規1488/94/EC、指令76/769/EEC、指令91/155/EEC、93/67/EC、93/105/EC和2000/21/EC,特別是第131條文的相關內容。
附錄17危險物質、混合物與成品,其製造與置於市場之限制
用於膠捲、相紙和印版的照相塗層;非裝飾性硬六價鉻鍍層的抗霧劑,或受
航空用液壓油;
向大眾銷售,除非包裝符合下列要求:
—患有哮喘,濕疹或皮膚問題,應避免接觸,—本產品不應在通風不良的條件下使用,除
—此產品不能在通風不良的條件下使用。
—此產品不能用於地毯的鋪設。
益利素勒产品型号

≤ 60 0
≤ 60 2
≤ 25 1
≤ 60 0
≤ 60 2
≤ 25 1
≤ 60 0
≤ 60 2 25 1
≤ 60 0
≤ 60 2
≤ 25 1
≤ 60 0
≤ 60 2
≤ 25 1
≤ 60 0
≤ 60 2
≤ 25 1
≤ 60 0
≤ 60 0
≤ 60 0
≤ 60 2
≤ 25 1
≤ 60
≥ 175°C 190°C
≥ 175°C 180°C
≥ 230°C 265°C
≥ 200°C 210°C
≥ 200°C 200°C
≥ 265°C 325°C
≥ 200°C 260°C
≥ 200°C 250°C
≥ 300°C 360°C
≥ 200°C 230°C
≥ 200°C 220°C
点击查看 耐热稳定 性图表
电 工数值
380°C
≥ 350°C ≥ 400°C
410°C
450°C
≥ 220°C
≥ 220°C ≥ 240°C
250°C
250°C
300°C
≥ 220°C ≥ 240°C
240°C
≥ 240°C
300°C
产品代号
P155
PN155
P155p
P180
EБайду номын сангаас80
A200
AI210
I220
ML240
低电压连续 性-以
220 V/µm 180 V/µm
220 V/µm 180 V/µm
220 V/µm 180 V/µm
JXHL0900401_棕榈酸帕利哌酮注射液进口标准

国家食品药品监督管理局进口药品注册标准标准号:JX20100263棕榈酸帕利哌酮注射液Zonglvsuan Palipaitong ZhusheyePaliperidone Palmitate Injection本品含棕榈酸帕利哌酮按帕利哌酮(C23H27FN4O4)计算,应为标示量90.0%~110.0%。
【性状】本品为白色至灰白色的混悬液。
【鉴别】(1)取本品1支,摇匀,取1滴至溴化钾片的表面。
照红外分光光度法(中国药典2010年版二部附录IV C)测定,其红外光吸收图谱在3200-2600cm-1,1800-1500cm-1和1200-750cm-1范围内应与对照品的红外光吸收图谱一致。
(2)在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品溶液主峰的保留时间一致。
【检查】重混悬性与抽针试验取本品3支,在10秒钟内振摇30次,振摇幅度约为25cm,振摇后溶液应均匀,不得检出可见异物或团块物。
用23号针头(蓝色针座)的注射器抽取,应顺利通过,不得阻塞。
pH值应为6.5~7.5(中国药典2010年版二部附录VI H)。
粒度分布取本品1支,加水稀释至250ml(1.5ml规格稀释至500ml),混匀后测试。
应用Malvern Mastersizer 2000 激光粒度仪,红光检测,泵速为1250转/分,颗粒折射率为1.56,颗粒吸收率为0.01,遮光度在6.8%-7.2%之间稳定1分钟后测试,测试时间为30秒。
d(0.1)应为0.3-0.6μm,d(0.5)应为0.9-1.4μm,d(0.9)应为2.0-4.4μm。
有关物质照含量测定项下的色谱条件,精密量取含量测定项下供试品溶液和对照品溶液各10μl注入液相色谱仪,记录色谱图。
供试品溶液色谱图中如有杂质峰,加校对因子校正后,按外标法以对照品溶液中主峰面积计算各杂质的含量,单个杂质峰面积不得过0.2%,各杂质的总和不得过0.4%。
单个杂质含量计算公式如下:式中G 为注射液的密度,1.037g/mlq s为供试品称样量,gP r为对照品纯度F r为棕榈酸帕利哌酮与帕利哌酮的换算因子1.56RRF为校正因子,杂质R130696为0.933(相对保留时间为0.70),其他杂质均为1.0 r i为供试品溶液中单个杂质色谱峰的峰面积Q th为标示含量,100mg/mlr r为对照品溶液色谱图中主峰的峰面积q r为对照品溶液中对照品的称样量,mg释放度取本品,照溶出度测定法(美国药典32版<711> 第二法),以0.489%聚山梨醇酯20的0.001mol/L盐酸溶液900ml为释放介质,介质温度为25 ︒C±0.5︒C,转速为每分钟50 转,依法操作,向每个溶出杯中加入相当于0.5ml±0.025ml的均匀混悬液样品【加样方式:取本品足够支数,摇匀,预先混合,量取0.5ml±0.025ml,置样品杯(规格为高14mm,内径14mm,壁厚1mm)中,精密称定,使样品杯悬于释放介质正上方,接近溶出杯的边沿,在桨转动时将样品投入;或将预先混合注射液约0.5ml±0.025ml,置1ml规格的带针头注射器内,精密称定,当桨转动时,将上述混悬液加入每个溶出杯中,精密称定带有针头的空注射器】。
帕利哌酮相关杂质
0
10mg-25mg-50mg-100mg
>99%
OH
丁丁kfl
0
帕利哌酮杂质10(帕利哌酮USP
RC D)
Paliperidone Impurity 10 (Paliperidon e USP RC D)
761460-08
6
10mg-25mg-50mg-100mg
>99%
CfY■■
1
10mg-25mg-50mg-100mg
>99%
1
口
人:
帕利哌酮杂质40
Paliperidone Impurity 40
152542-03
5
10mg-25mg-50mg-100mg
>99%
7
0
相关杂质整理列表
中文名
英文名
CAS号
规格
纯度
结构式
帕利哌酮杂质1(5-氟异构体)
Paliperidone Impurity1 (5-Fluoro Isomer)
1346598-3
4-2
10mg-25mg-50mg-100mg
>99%
□
c
帕利哌酮杂质2(9-酮帕利y 2
>99%
如
HCI
八〜
J/
p
A一
帕利哌酮杂质24
Paliperidone Impurity 24
N/A
10mg-25mg-50mg-100mg
>99%
Hr
:i
J
帕利哌酮杂质25
Paliperidone Impurity 25
N/A
10mg-25mg-50mg-100mg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
USP 35Official Monographs / Paclitaxel 4195Water, Method Ic 〈921〉: not more than 4.0%.Residue on ignition 〈281〉: not more than 0.2%.PaclitaxelHeavy metals, Method II 〈231〉: 0.002%.Related compounds—TEST 1 (FOR MATERIAL LABELED AS ISOLATED FROM NATURAL SOURCES)—If the material complies with this test, the labeling indicates that it meets USP Related compounds Test 1.Diluent—Prepare as directed in the Assay.Solution A—Prepare filtered and degassed acetonitrile.Solution B—Prepare filtered and degassed water.Mobile phase—Use variable mixtures of Solution A and Solu-C 47H 51NO 14853.91tion B as directed for Chromatographic system. Make adjust-Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, 6,12b-ments if necessary (see System Suitability under Chromatography bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,〈621〉).12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-System suitability solution—Dissolve accurately weighed quan-oxo-7,11-methano-1H -cyclodeca[3,4]benz[1,2-b]oxet-9-yl es-tities of USP Paclitaxel Related Compound A RS and USPter, [2a R -[2a α,4β,4a β,6β,9α(αR *,βS *),11α,12α,12a α,12b α]]-.Paclitaxel Related Compound B RS in methanol to obtain a so-(2a R,4S ,4a S ,6R,9S ,11S ,12S ,12a R,12b S )-1,2a,3,4,4a,6,9,10,11,12,lution having known concentrations of about 10 µg of each per 12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask,13-tetramethyl-7,11-methano-5H -cyclodeca[3,4]-benz[1,2-b ]dilute with Diluent to volume, and mix.oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S )-Standard solution—Dissolve, with the aid of sonication, an N -benzoyl-3-phenylisoserine [33069-62-4].accurately weighed quantity of USP Paclitaxel RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to » Paclitaxel contains not less than 97.0 percent obtain a solution having a known concentration of about 5 µg and not more than 102.0 percent of C 47H 51NO 14,per mL.calculated on the anhydrous, solvent-free basis.Test solution—Use the Assay preparation.Caution—Paclitaxel is cytotoxic. Great care Chromatographic system (see Chromatography 〈621〉)—The should be taken to prevent inhaling particles of liquid chromatograph is equipped with a 227-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L43.Paclitaxel and exposing the skin to it.The flow rate is about 2.6 mL per minute. The column temper-Packaging and storage—Preserve in tight, light-resistant ature is maintained at 30°. The chromatograph is programmed containers, and store at controlled room temperature.as follows.Labeling—The labeling indicates the type of process used to produce the material and the Related compounds test with Time Solution A Solution B which the material complies.(minutes)(%)(%)Elution0–353565isocraticUSP Reference standards 〈11〉—35–6035→8065→20linear gradient USP Endotoxin RS 60–7080→3520→65linear gradient USP Paclitaxel RSUSP Paclitaxel Related Compound A RS70–803565isocraticCephalomannine.Chromatograph the System suitability solution, and record the USP Paclitaxel Related Compound B RSpeak responses as directed for Procedure: the relative retention 10-Deacetyl-7-epipaclitaxel.times are about 0.78 for paclitaxel related compound A and USP Paclitaxel Impurity Mixture RS0.86 for paclitaxel related compound B (relative to the retention Mixture of paclitaxel and the following related compounds:time for paclitaxel obtained from the Test solution ); and the propyl analog, cephalomannine, sec -butyl analog, n -butyl resolution, R, between paclitaxel related compound A and analog, benzyl analog, baccatin VI, pentyl analog, and 7-paclitaxel related compound B is not less than 1.0. Chromato-epipaclitaxel.graph the Standard solution, and record the peak responses as Identification—directed for Procedure: the relative standard deviation for repli-A: Infrared Absorption 〈197K 〉.cate injections is not more than 2.0%.B: The retention time of the major peak in the chromato-Procedure—Inject a volume (about 15 µL) of the Test solution gram of the Assay preparation corresponds to that in the chro-into the chromatograph, record the chromatogram, and meas-matogram of the Standard preparation, as obtained in the Assay.ure the areas for the major peaks. Calculate the percentage of Specific rotation 〈781S 〉: between −49.0° and −55.0° at 20°,each impurity in the portion of Paclitaxel taken by the formula:calculated on the anhydrous, solvent-free basis.100(Fr i /r U )Test solution: 10 mg per mL, in methanol.Microbial enumeration tests 〈61〉 and Tests for specified in which F is the relative response factor for each impurity peak microorganisms 〈62〉—The total aerobic microbial count does (see Table 1 for values); r i is the peak area for each individual not exceed 100 cfu per g. It meets the requirements of the impurity; and r U is the peak area for paclitaxel.tests for the absence of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella species, and Escherichia coli.Bacterial endotoxins 〈85〉—It contains not more than 0.4USP Endotoxin Unit per mg of paclitaxel.4196Paclitaxel / Official Monographs USP 35Table 1standard deviation for replicate injections is not more than2.0%.Relative RelativeProcedure—Separately inject equal volumes (about 15 µL) of Retention Response Limitthe Diluent and the Test solution into the chromatograph, record Time Factor (F)Name(%)the chromatograms, and measure the areas for all the peaks.0.24 1.29Baccatin III0.2Disregard any peaks due to the Diluent. Calculate the percent-0.53 1.0010-Deacetylpaclitaxel0.5age of each impurity in the portion of Paclitaxel taken by the0.57 1.007-Xylosylpaclitaxel0.2formula:0.78 1.26Cephalomannine (paclitaxel a11related compound A)100(Fr i/r s)0.78 1.262′′,3′′-Dihydrocephaloman-a21in which F is the relative response factor for each impurity (see nineTable 2 for values); r i is the peak area for each impurity ob-0.86 1.0010-Deacetyl-7-epipaclitaxel0.5tained from the Test solution; and r s is the sum of the areas of (paclitaxel related com-all the peaks obtained from the Test solution.pound B)1.10 1.00Benzyl analog3b12Table 21.10 1.003′′,4′′-Dehydropaclitaxel C b22Relative Relative1.40 1.007-Epicephalomannine0.3Retention Response Limit1.85 1.007-Epipaclitaxel0.5Time Factor (F)Name(%) 1Resolution may be incomplete for these peaks, depending upon the relative0.11 1.2410-Deacetylbaccatin III0.1 amounts present; the sum of a1 and a2 is not more than 0.5%.0.20 1.29Baccatin III0.22Resolution may be incomplete for these peaks, depending upon the relativeamounts present; the sum of b1 and b2 is not more than 0.5%.0.42 1.39Photodegradant20.13The following chemical name is assigned to the related compound, benzyl0.47 1.0010-Deacetylpaclitaxel0.5 analog:Baccatin III 13-ester with (2R,3S)-2-hydroxy-3-phenyl-3-(2-phenyl-0.80 1.002-Debenzoylpaclitaxel-2-0.7 acetylamino)propanoic acid.pentenoateIn addition to not exceeding the limits for paclitaxel related 0.921 1.00Oxetane ring opened, ace-x1 impurities in Table 1, not more than 0.1% of any other single tyl and benzoyl migrated2impurity is found; and not more than 2.0% of total impurities is 0.921 1.0010-Acetoacetylpaclitaxel x2 found. 0.941 1.0010-Deacetyl-7-epipaclitaxel x3 TEST2(FOR MATERIAL LABELED AS PRODUCED BY A SEMISYNTHETIC PRO-(paclitaxel related com-CESS)—If the material complies with this test, the labeling indi-pound B)cates that it meets USP Related compounds Test 2. 1.37 1.007-Epipaclitaxel0.4 Diluent—Use acetonitrile. 1.45 1.0010,13-Bissidechainpaclitax-0.5 Solution A—Use a filtered and degassed mixture of water and el2acetonitrile (3:2). 1.54 1.007-Acetylpaclitaxel0.6 Solution B—Use filtered and degassed acetonitrile. 1.80 1.7513-Tes-baccatin III0.1 Mobile phase—Use variable mixtures of Solution A and Solu- 2.14 1.007-Tes-paclitaxel0.3 tion B as directed for Chromatographic system. Make adjust-1Resolution may be incomplete for these peaks, depending upon the relative ments if necessary (see System Suitability under Chromatography amounts present; the sum of x1, x2, and x3 is not more than 0.4%.〈621〉).2The following chemical names are assigned to the related compounds System suitability solution—Dissolve accurately weighed quan-Photodegradant; Oxetane ring opened, acetyl and benzoyl migrated; and tities of USP Paclitaxel RS and USP Paclitaxel Related Compound10,13-Bissidechainpaclitaxel:B RS in Diluent, shaking and sonicating if necessary, to obtain a Photodegradantsolution having known concentrations of about 0.96 mg and(1R,2R,4S,5S,7R,10S,11R,12S,13S,15S,16S)-2,10-diacetyloxy-5,13-dihydroxy-0.008 mg per mL, respectively.4,16,17,17-tetramethyl-8-oxa-3-oxo-12-phenylcarbonyloxypentacyclo[11.3.1.01,11.04,11.07,10]heptadec-15-ylTest solution—Transfer about 10 mg of Paclitaxel, accurately(2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoate weighed, to a 10-mL volumetric flask, dissolve in and diluteOxetane ring opened, acetyl and benzoyl migratedwith Diluent to volume, shaking and sonicating if necessary, and(1S,2S,3R,4S,5S,7S,8S,10R,13S)-5,10-diacetyloxy-1,2,4,7-tetrahydroxy-mix.8,12,15,15-tetramethyl-9-oxo-4-(phenylcarbonyloxymethyl)tricyclo[9.3.1.03,8] Chromatographic system (see Chromatography 〈621〉)—Thepentadec-11-en-13-ylliquid chromatograph is equipped with a 227-nm detector and(2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylamino)propanoatea 4.6-mm × 15-cm column that contains 3-µm packing L1. The10,13-Bissidechainpaclitaxelflow rate is about 1.2 mL per minute. The column temperatureBaccatin III 13-ester with (2R,3S)-2-hydroxy-3-phenyl-3-(phenylcarbonylami-is maintained at 35°. The chromatograph is programmed asno)propanoic acid, 10-ester with (2S,3S)-2-hydroxy-3-phenyl-3-(phenyl-follows.carbonylamino)propanoic acidIn addition to not exceeding the limits for paclitaxel related Time Solution A Solution Bimpurities in Table 2, not more than 0.1% of any other single (minutes)(%)(%)Elutionimpurity is found; and not more than 2.0% of total impurities is 0–201000isocraticfound.20–60100→100→90linear gradientTEST3(FOR MATERIAL LABELED AS PRODUCED BY A PLANT CELL FERMEN-60–6210→10090→0linear gradientTATION PROCESS)—If the material complies with this test, the la-62–701000isocratic beling indicates that it meets USP Related compounds Test 3.Solution A—Prepare a filtered and degassed mixture of water Chromatograph the System suitability solution, and record theand acetonitrile (3:2).peak responses as directed for Procedure: the relative retentiontimes are about 0.94 for paclitaxel related compound B and 1.0Solution B—Prepare filtered and degassed acetonitrile.for paclitaxel; the resolution, R, between paclitaxel related com-Mobile phase—Use variable mixtures of Solution A and Solu-pound B and paclitaxel is not less than 1.2; and the relative tion B as directed for Chromatographic system. Make adjust-USP 35Official Monographs / Paclitaxel4197ments if necessary (see System Suitability under Chromatography ure the areas for all the peaks. Calculate the percentage of each 〈621〉).impurity in the portion of Paclitaxel taken by the formula: System suitability solution—Dissolve USP Paclitaxel Impurity100(r i/r U)Mixture RS in acetonitrile, sonicating if necessary, to obtain asolution having a known concentration of about 1 mg per mL.in which r i is the response of each individual impurity; and r U is Standard solution—Dissolve an accurately weighed quantity the sum of the areas of all the peaks obtained from the Testof USP Paclitaxel RS in acetonitrile, sonicating if necessary, to solution. In addition to not exceeding the limits for paclitaxel obtain a solution having a known concentration of about 1 mg related impurities in Table 3, not more than 0.1% of any other per mL.single impurity is found; and not more than 2.0% of total im-Test solution—Transfer about 10 mg of Paclitaxel, accurately purities is found.weighed, to a 10-mL volumetric flask. Dissolve in and dilute Assay—with acetonitrile to volume, sonicating if necessary, and mix.Diluent—Prepare a mixture of methanol and acetic acid Chromatographic system (see Chromatography 〈621〉)—The(200:1).liquid chromatograph is equipped with a 227-nm detector andMobile phase—Prepare a filtered and degassed mixture ofa 4.6-mm × 15-cm column that contains 3-µm packing L1. Thewater and acetonitrile (11:9). Make adjustments if necessary flow rate is about 1.2 mL per minute. The chromatograph is(see System Suitability under Chromatography 〈621〉). programmed as follows.Standard preparation—Dissolve, using sonication if necessary,an accurately weighed quantity of USP Paclitaxel RS in Diluent, Time Solution A Solution B and dilute quantitatively, and stepwise if necessary, with Diluent (minutes)(%)(%)Elution to obtain a solution having a known concentration of about 1 0–281000isocratic mg per mL.28–33100→980→2linear gradient Assay preparation—Transfer about 10 mg of Paclitaxel, accu-33–5898→102→90linear gradient rately weighed, to a 10-mL volumetric flask. Dissolve in Diluent, 58–601090isocratic using sonication if necessary, dilute with Diluent to volume, andmix.60–6310→10090→0linear gradient63–701000isocratic Chromatographic system (see Chromatography 〈621〉)—Theliquid chromatograph is equipped with a 227-nm detector and Chromatograph the System suitability solution, and record the a 4.6-mm × 25-cm column that contains 5-µm packing L43. peak responses as directed for Procedure: the resolution, R, be-The flow rate is about 1.5 mL per minute. Chromatograph the tween paclitaxel and benzyl analog is not less than 1.8. Chro-Standard preparation, and record the peak responses as directed matograph the Standard solution, and record the peak re-for Procedure: the tailing factor is between 0.7 and 1.3; and the sponses as directed for Procedure: the relative standard deviation relative standard deviation for replicate injections is not more for replicate injections is not more than 2.0%. [NOTE—For the than 1.5%.purpose of peak identification, the approximate relative reten-Procedure—Separately inject equal volumes (about 10 µL) of tion times are given in Table 3. The relative retention times are the Standard preparation and the Assay preparation into the measured versus Paclitaxel.]chromatograph, record the chromatograms, and measure theareas for the major peaks. Calculate the quantity, in mg, ofTable 3C47H51NO14 in the portion of Paclitaxel taken by the formula:Relative10C(rU/r S)Retention LimitName Time(%)in which C is the concentration, in mg per mL, of USPPropyl analog10.540.2Paclitaxel RS in the Standard preparation; and rU and r S are the Cephalomannine0.760.5peak responses for paclitaxel obtained from the Assay prepara-(Paclitaxel related tion and the Standard preparation, respectively.compound A)sec-Butyl analog20.810.2n-Butyl analog30.890.1Benzyl analog 1.100.4Paclitaxel InjectionBaccatin VI 1.230.2Pentyl analog4 1.310.2» Paclitaxel Injection is a sterile, stabilized solu-7-Epipaclitaxel 1.510.4tion of Paclitaxel, suitable for dilution for intrave-1The following chemical name is assigned to the related compound Propyl nous administration. It contains not less than analog:Baccatin III 13-ester with (2R,3S)-3-butanoylamino-2-hydroxy-3-90.0 percent and not more than 110.0 percent phenylpropanoic acid.of the labeled amount of paclitaxel (C47H51NO14). 2The following chemical name is assigned to the related compound sec-Butylanalog:Baccatin III 13-ester with (2R,3S)-2-hydroxy-3-(2-methylbutanoy-Packaging and storage—Preserve in single-dose or multiple-lamino)-3-phenylpropanoic acid.dose containers, preferably of Type I glass, at controlled room 3The following chemical name is assigned to the related compound n-Butyl temperature.analog:Baccatin III 13-ester with (2S,3S)-2-hydroxy-3-(pentanoylamino)-3-Labeling—Label it to indicate that it is to be diluted with a phenylpropanoic acid.suitable parenteral vehicle prior to intravenous infusion.4The following chemical name is assigned to the related compound Pentylanalog:Baccatin III 13-ester with (2R,3S)-3-(hexanoylamino)-2-hydroxy-3-USP Reference standards 〈11〉—phenylpropanoic P Endotoxin RSUSP Paclitaxel RSProcedure—Inject a volume (about 12 µL) of the Test solution USP Paclitaxel Related Compound B RSinto the chromatograph, record the chromatogram, and meas-10-Deacetyl-7-epipaclitaxel.Identification—A: The retention time of the major peak in the chromato-gram of the Test solution corresponds to that in the chromato-。
