Storage Temperature and Humidity Test
高温储存测试规范(中英文)

德信诚培训网高温储存测试规范High Temperature Storage Test Procedure1.0 PURPOSE (目的):1.1The high temperature storage test is designed to determine the effects on the product, while package in it's shipping container, due to high temperatures. This is an accelerated test to uncover weaknesses in components, assemblies, and processes, which may appear in the field during normal shipping situations.1.1 高温储存试验是用来判定高温对产品包装后在运输过程中的影响,这是一个加速性测试,用来显露出任何组成零件,在正常运输中可能造成的损坏。
1.22.0 SCOPE (范围):2.1This test describes the minimum temperature storage performance test required for all products.2.1对所有产品都须经过此测试。
3.0 SPECIFICATIONS (规格):3.1Class 1 product - Switching Power supply and Linear Power Supply 3.1 第一级- Switch和Linear电源供应器 ;Temperature - +85 ℃ C 90% RH温度:+85℃湿度90%更多免费资料下载请进:好好学习社区。
OV08865- 规格书
产品承认书SPECIFICATIONS品名(Module Name):P8V10S客户型号(Cust.P/N):M812 8M AF 后置摄像头产品承认书共21 页(These specifications are composed of 21 pages, including this title page.)Revise History1. ScopeThis approval sheet contains the general information of P8V10S QSXGA CMOS camera module designed for NINGBO SUNNY OPOTECH CO., LTD. It contains the key features of the module as well as the information for the quality inspection and reliability test purposes.2. Features• automatic black level calibration (ABLC)• programmable controls for frame rate, mirror and flip,cropping, and windowing• static defective pixel canceling• supports output formats: 10-bit RAW RGB (MIPI)• supports horizontal and vertical subsampling• supports images sizes: 3264x2448, 3264x1836,2816x1584, 1632x1224, 1408x792• supports 2x2 binning, re-sampling filter• standard serial SCCB interface• up to 4-lane MIPI serial output interface• embedded 1536 bytes one-time programmable(OTP) memory for part identification, etc. • two on-chip phase lock loops (PLLs)• programmable I/O drive capability• built-in temperature sensorApplications• cellular phones• digital still cameras (DSC)• digital video camcorders (DVC)• PC multimedia2.1 General Description2.1.1 Camera Module Specification2.1.2 Camera Lens Specification2.1.3 Auto-Focus Specification2.2 Camera Module Sensor Electrical Specification 2.2.1 Absolute Maximum Ratings2.2.2 Functional Temperature2.2.3 timing characteristics2.2.4 DC characteristics2.3 VCM Driver Specification■ Absolute Maximum ratings■ Recommended Operating condition■ Electrical Specification2.4 VCM (Voice Coil Motor)Specification3. Camera Module Configuration Specification3. 1 Schematic3.2 Lens Specification3.3 VCM Specification3.4 PCB Layout4. Camera Module PIN Description5. Reliability Test6. Packaging6.1. Packaging Process1. Every module is placed into a tray until all empty slots of a tray are filled.2. Each tray uses an anti-static bag to prevent the module from moisture by partially socking out the air from the stack.3. A stack has 10 trays.4. Insert three stacks into a outside box. Then attach the label onto the outside box.6.2. Labeling6.2.1 External box label 6.2.2 internal case label6.3. Sketch Map of Packing Process7. Precautions7.1. Storage and Operating ConditionsTo keep the product and packaging material in good condition, care must be taken to control temperature and humidity in the storage area.Recommended conditions:Ambient temperature: 0~+40℃Humidity: 30~70%RHNo rapid change on temperature and humidity.The products listed in this catalog are not designed for use under the following conditions. Storage and/or usage under following conditions is prohibited.1). Exposure to corrosive gas such as chlorine, hydrogen sulfide, ammonia, sulfur dioxide, nitrogen oxide, etc.2). Exposure to direct sunlight.3). Exposure to dust.4). Exposure to excessive moisture or wet locations.5). Exposure to salt water or sea breezes.6). Exposure to strong static electricity or electromagnetic waves.7.2. Transportation and Handling1). Minimize any mechanical vibration or shock and avoid dropping of the product during transportation or dropping the product that contains the substrate.2). Since the application of static electricity or over voltage may cause defect in the product or deterioration of its reliability, caution must be taken against exposure to any static electricity generated by electrified items such as workbenches, soldering irons, tools, carrying containers, etc.3). Caution shall be taken to avoid overstress to the product.V1.9Add 12M requirementKeJun 20130227V.2.0- Modify PSNR specification greater than 8M- Modify lens shading specification greater than 8M - Modify resolution specification in 8M- Modify color saturation specification greater than 5MJenny Ma Wood Yu20130604V1.7 -Add low light (10 Lux) test item , 25dB at 10 Lux of PSNR for more than 2M camera- Adjust Saturation specificationKeJun 20120709V1.8Delete gray scale requirementKeJun 20121226V1.5Add color shading specification KeJun 20110615V1.6Add camera spec on 8MKeJun 20120203V1.3Add 5M specificationKeJun 20100827V1.4remove AWB in horizontal light KeJun 20110104V1.1Modify PSNR specification greater than 1.3MKeJun 20090812V1.2Add View Angle test spec and modify resolution spec in 3MKeJun 20100612Camera Test SpecificationVersion NODiscriptionAuthor Time V1.0Camera test specificationKeJun 20090525T C LC o n fi de nt ia l。
AMS-可靠性实验标准中英文版

Low Temperature Storage Test 低温储存实验
Temperature Changing Test 温度交变实验
High Temperature&Humidity Storage Test 恒定湿热储存实验
Normal Temperature Life Test 常温耐久实验
2
℃±2℃,保持不少于3小时。
判断:1、样品应连续工作,画面、声音、按钮、遥控功能应正常;
2、样品外表应无锈蚀、霉斑、镀涂层剥落、塑料件起泡、开裂、变形、港注物溢出等
现象;样品丝印文字符号、标志应清晰,结构件与控制件应完整,无机械损伤。
实验数量:≥3台
环境实验 之 常温耐久实验
目的:确定产品在长时间使用时的稳定性 测试条件:实验环境温度
目的:确定产品在高温的仓库储存或运输的条件下,应符合规定要求 测试条件:温度 -20℃±2℃ 测试方法:1、已包装、不通电样品放入实验箱内;
2、箱温按10~15℃/h.的平均速率下降至-20±2℃; 3、样品上述测试条件下保持8h; 4、实验完毕后,箱温按10~15℃/h.的速率上升至25℃±2℃,保持不少于2小时 。 判断:1、样品应连续工作,画面、声音、按钮、遥控功能应正常; 2、样品外表应无锈蚀、霉斑、镀涂层剥落、塑料件起泡、开裂、变形、港注物溢出等 现象;样品丝印文字符号、标志应清晰,结构件与控制件应完整,无机械损伤。 实验数量:≥3台
额定电压 ±10% 工作状态 DVB节目连续播放,1/3额定功率播放,EQ设为正常 测试方法:1、无包装样品放入老化实验房; 2、NPA&PP样品连续工作168小时;MP样品连续工作48小时; 3、每天记录检查。 判断:1、样品应连续工作,画面、声音、按钮、遥控功能应正常; 2、样品外表应无锈蚀、霉斑、镀涂层剥落、塑料件起泡、开裂、变形、港注物溢出等 现象;样品丝印文字符号、标志应清晰; 3、搖控器功能檢查应正常; 4、内部结构检查应正常; 5、PCB外觀檢查应正常。 实验数量:≥3台
Highly Accelerated Temperature and Humidity Stress Test (HAST)JESD22-A110E

The information included in JEDEC standards and publications represents a sound approach to product specification and application, principally from the solid state device manufacturer
by the JEDEC legal counsel.
JEDEC standards and publications are designed to serve the public interest through eliminating misunderstandings between manufacturers and purchasers, facilitating interchangeability and improvement of products, and assisting the purchaser in selecting and obtaining with minimum delay the proper product for use by those other than JEDEC members, whether the standard is to
be used either domestically or internationally.
JEDEC standards and publications are adopted without regard to whether or not their adoption may involve patents or articles, materials, or processes. By such action JEDEC does not assume any liability to any patent owner, nor does it assume any obligation whatever to parties adopting
杭州天丰电源股份有限公司聚合物锂离子电池产品规格书说明书

杭州天丰电源股份有限公司Hangzhou Skyrich Power Co.,Ltd.客户名称Customer:No.Q/TFDYJ03252-2016Edition No. 1.0聚合物锂离子电池产品规格书Polymer Lithium-ion BatteryProduct Specification(常规电池General battery)型号Model:401020杭州天丰电源股份有限公司Hangzhou Skyrich power Co.,Ltd.厂址:浙江省杭州市拱墅区临半路118号邮编:310022 Address:Linban road No.118, GongShu Hangzhou Zhejiang China, Zip:310022Tel:(0571)88368618Fax:(0571)88368922编制:陈武凤审核:韩改格批准:金明钢Prepared by:Chen Wufeng Checked by: HanGaige Approve by: Jin Minggang第 1 页共 10 页1. 适用范围、严正申明:本产品规格书适用于杭州天丰电源股份有限公司生产的二次聚合物锂离子电池主要性能指标的描述。
用户请务必严格按照规格书中的测试或使用方法进行测试或使用,如对表中的测试项目或测试方法有异议,请与供应方协商解决。
本规格书列举的注意事项请用户在首次使用前务必仔细阅读,在电池使用过程中采取可靠的防护措施。
客户如需要在超出本文件规定的环境或条件下使用电池,必须事先与杭州天丰电源股份有限公司联系,取得书面认可。
对于未经书面认可的电池超出本文件规定的环境或条件下使用造成的所有意外事故或损失,杭州天丰电源股份有限公司声明拒绝承担任何责任。
对于2串以上的电池组合,建议采用平衡充电器充电,以保证电池性能稳定与安全使用。
This product specification describes Tianfeng polymer lithium-ion battery. Please using the test methods that recommend in this specification. If you have any opinions or advices about the test items and methods, please contact us. Please read the cautions recommended in the specifications first, take the credibility measure of the cell’s using.If the cells should be using at the environment that not preferred in this document, please connect with our first and get our authorization. For the reason of stable Performance and better safety, battery pack with more than 2 cells connected in serial way should be charged with a balance charger.It is claimed that we should have no any responsibility with the contingency and loss due to the cells’ wrong usage (not preferred in the product specification).2.产品类型、型号和外形尺寸Product Type, Model and Dimension:2.1产品类型Type:二次聚合物锂离子电池Polymer lithium-ion battery2.2产品型号Model:401020(转镍)(Cell adding Ni Tabs)2.3电芯外形尺寸 Dimension( Max. T*W*L)mm:4.2×11.3×21.53. 产品规格Specification:项目Item 特征值Parameter备注Remark标称容量Nominal Capacity 50mAh±5%25℃,0.2C5A放电容量0.2C5A discharge,25℃标称电压Nominal V oltage 3.7V25℃,0.2C5A放电平均电压Average V oltage at 0.2C5A discharge标准充电电流Standard Charge Current 0.2C5A工作温度:0~45℃Working temperature:0~45℃快速充电电流Max Charge Current 1.0C5A工作温度:0~45℃Working temperature:0~45℃充电截止电压Charge cut-off V oltage4.20±0.05V CC/CV放电电流Discharge Current Cont.0.2C5A~Max:2.0C5A Workingtemperature:-20~60℃放电截止电压Discharge cut-off V oltage2.75V出厂电压Cell V oltage 3.76~3.9V Whenleavefactory标称内阻Impedance ≤400mΩ半充电态,AC 1KHz,25±5℃AC 1KHz after 50% charge,25℃重量WeightApprox2g≤1个月≤1month-10~45℃≤3个月≤3month 0~30℃贮存温度StorageTemperature≤6个月≤6month0~25℃贮存湿度Storage humidity 65±20% RH长期贮存温度:20±5℃(推荐)Best 20±5℃ for long-time storage4. 常规性能General Performance:标准充电定义:在环境温度20±5℃条件下,对电池以0.2C5A恒流充电至4.2V,然后以4.2V恒压充电至充电电流小于等于0.05C5A。
0805共模电感规格书

A
B
Epoxy
C
Terminations Wires
①
④
(0.4)
②
(0.45)
(0.4)
③
(0.45)
Equivalent circuit
①
④
②
③
No Polarity
A : 2.0 ± 0.2 B : 1.2 ± 0.2 C : 1.2 ± 0.2
Drawn by Checked by Approved by
(1) Product name (2) Shapes and dimensions (3) Shielding Type (4) Impedance【 at 100MHz】
900:90Ω (5) Number of Line
2P:2-Line (6) Taping Type
3. Shapes and Dimensions [Dimensions in mm]
Measurement terminal
①
④
②
③
PRODUCT SPECIFICATION
5.Reliability Test
6/10 SPEC. NO.
T-0602-001T
Operating temperature : -25 to +85℃
Storage temp and humidity : 20~25℃ ,60%RH max.
1000 100
CMF2012F-400-2P-T
Common Mode Differential Mode
Insulation Resistance (MΩ)Min.
10 10 10 10 10 10 10 10 10 10 10 10 10 10 10 10
德国富奇公司-气候试验箱

Dimensions 尺寸
Test space 试验空间
Weight 重量
From: hkcd/march2004 Jp/april2004
580 mm wide 宽 450 mm deep 深 750 mm high 高
approx. 425 kg net
Cabinet (required space) 外形尺寸(所需空间)
最大允许环境温度+55 °C。
polished stainless steel - grade 1.4301
Ausdruck: 2004-6-11 Stand Technik: 04-6-11
5
VC 4018 – detailed text
试验空间
Shelf 试验样品架
Door 门 Entry ports 引线孔 Refrigeration unit 制冷机组
• low-noise (silent) refrigeration unit 低噪音(静音)制冷机组
• air-cooled 风冷式机组
• continuously variable power adjustment by electronic monitoring and control system 由电子监控系统连续调节功率
870 mm wide 宽 1280 mm deep 深 1775 mm high 高
Ausdruck: 2004-6-11 Stand Technik: 04-6-11
4
VC 4018 – detailed text
Sound pressure level 声压级
EMC Test (electromagneti compatibility) EMC 测试(电磁兼容性) Interference immunity 抗干扰 Electrical connection 电源连接
RFE-18650NE2100中英文规格书(三元)

充电温度范围 Charging Temperature 13.使用温度范围 Operating Temperature Range 放电温度范围 Discharging Temperature 存储温度范围 Storage Temperature 14.存储寿命 Storage Life 15.外观 Appearance 内阻 Internal Resistance 16.PTC 最大保护值 Max Time to Trip
0.5C Cycle Life Curve
-10 ℃ 0℃ 25 ℃ 55 ℃
4.0 3.8 3.6
60 40 20 0 0 50 100 150 200 250 300 Cycle Num 350 400 450
3.4 3.2 3.0 2.8 2.6 2.4 0 10 20 30 40 50 60 70 Capacity , % 80 90 100
山东润峰集团新能源科技有限公司 Shandong RealForce Enterprises Co., Ltd.
DOC №: SPEC-TC1-009 REV: A/0 PAGE: 1 of 11
钢壳圆柱锂离子可充电电池
Steel Shell Cylindrical Rechargeable Battery
产品规格书
Specification
型号 / Model: 18650N E 18650NE 标称容量 / Nominal Capacity: 2100mAh 日期 / Date: 2011-02-10
制定 Prepared by
审核 Checked by
批准 Approved by
本公司保留对此规格书的修改权不做另行通知 Product Specifications are subjected to change at any time with RealForce reserving all rights and privileges
JEDEC标准族

JEDEC工业标准环境应力试验[JDa1]JESD22-A100-B Cycled Temperature-Humidity-Bias Life Test 上电温湿度循环寿命试验, (Revision of JESD22-A100-A) April 2000 [Text-jd001][JDa2]JESD22-A101-B Steady State Temperature Humidity Bias Life Test 上电温湿度稳态寿命试验, (Revision of JESD22-A101-A) April 1997 [Text-jd002][JDa3]JESD22-A102-C Accelerated Moisture Resistance -Unbiased Autoclave高加速蒸煮试验, (Revision of JESD22-A102-B) December 2000 [Text-jd003][JDa4]JESD22-A103-A Test Method A103-A High Temperature Storage Life高温储存寿命试验, (Revision of Test Method A103 Previously Published in JESD22-B) July 1989 [Text-jd004] [JDa5]JESD22-A103-B High Temperature Storage Life高温储存寿命试验, (Revision of JESD22-A103-A) August 2001 [Text-jd005][JDa6]JESD22-A104-B Temperature Cycling温度循环, (Revision of JESD22-A104-A) July 2000 (参见更新版本A104C) [Text-jd006][JDa7]EIA/JESD22-A105-B Test Method A105-B Power and Temperature Cycling上电和温度循环, (Revision of Test Method A105-A) February 1996 [Text-jd007][JDa8]JESD22-A106-A Test Method A106-A Thermal Shock热冲击, (Revision of Test Method A106-Previously Published in JESD22-B) April 1995 [Text-jd008][JDa9]JESD22-A107-A Salt Atmosphere盐雾试验, (Revision of Test Method A107-Previously Published in JESD22-B) December 1989 [Text-jd009][JDa10]JESD22-A108-B Temperature, Bias, and Operating Life高温环境条件下的工作寿命试验, (Revision of JESD22-A108-A) December 2000[JDa11]JESD22-A110-B Test Method A110-B Highly-Accelerated Temperature and Humidity Stress Test (HAST)高加速寿命试验, (Revision of Test Method A110-A) February 1999[Text-jd010][JDa12]JESD22-A113-B Preconditioning of Nonhermetic Surface Mount Devices Prior to Reliability Testing非密封表贴器件在可靠性试验以前的预处理, (Revision of Test Method A113-A)March 1999 [Text-jd011][JDa13]JESD22-A118 Accelerated Moisture Resistance - Unbiased HAST不上电的高加速湿气渗透试验, December 2000 [Text-jd012][JDa14]JESD22-B106-B Test Method B106-B Resistance to Soldering Temperature for Through-Hole Mounted Devices插接器件的抗焊接温度试验, (Revision of Test MethodB106-A) February 1999 [Text-jd013][JDa15]EIA/JESD47 Stress-Test-Driven Qualification of Integrated Circuits集成电路施加应力的产品验收试验, July 1995 [Text-jd031][JDa16]JESD22-A104C Temperature Cycling, (Revision of JESD22-A104-B) May 2005 [Text-jd040]电应力和电测试试验[JDb1]JESD22-A114-B Electrostatic Discharge (ESD) Sensitivity Testing Human Body Model (HBM)人体模型条件下的静电放电敏感度试验, (Revision of JESD22-A114-A) June 2000 [Text-jd014][JDb2]EIA/JESD22-A115-A Electrostatic Discharge (ESD) Sensitivity Testing Machine Model (MM)机器模型条件下的静电放电敏感度试验, (Revision of EIA/JESD22-A115) October1997 [Text-jd015][JDb3]JESD22-A117 Electrically Erasable Programmable ROM (EEPROM) Program/Erase Endurance and Data Retention Test EEPROM的擦涂和数据保存试验, January 2000[Text-jd016][JDb4]EIA/JESD78 IC Latch-Up Test集成电路器件闩锁试验, March 1997 [Text-jd017][JDb5]JESD22-C101-A Field-Induced Charged-Device Model Test Method forElectrostatic-Discharge-Withstand Thresholds of Microelectronic Components微电子器件在电荷感应模型条件下的抗静电放电试验, (Revision of JESD22-C101) June2000 [Text-jd018]机械应力试验[JDc1]JESD-22-B103-A Test Method B103-A Vibration, Variable Frequency振动和扫频试验(Revision of Test Method B103 Previously Published in JESD22-B) July 1989 [Text-jd019] [JDc2]JESD22-B104-A Test Method B104-A Mechanical Shock机械冲击(Revision of Test Method B104, Previously Published in JEDEC Standard No.22-B) September 1990[Text-jd020][JDc3]EIA/JESD22-B116 Wire Bond Shear Test Method焊线邦定的剪切试验方法, July 1998 [Text-jd021][JDc4]JESD22-B117 BGA Ball Shear BGA焊球的剪切试验, July 2000 [Text-jd022][JDc5]JESD22B113 Board Level Cyclic Bend Test Method for Interconnect Reliability Characterization of Components for Handheld Electronic Products, March 2006 [Text-jd038] [JDc6]JESD22-B111 Board Level Drop Test Method of Components for Handheld Electronic Products, July 2003 [Text-jd039]综合试验与测试[JDd1]JEDEC Standard No.22-A109 Test Method A109 Hermeticity密封性试验, July 1988 [Text-jd023][JDd2]JESD22-A120 Test Method for the Measurement of Moisture Diffusivity and Water Solubility in Organic Materials Used in Integrated Circuits集成电路器件中使用的有机材料水分扩散和水溶性测定试验方法, June 2001 [Text-jd024][JDd3]JESD22-B100-A Physical Dimensions物理尺寸的测量, (Revision of Test Method B100-Previously Published in JESD22-B) April 1990 [Text-jd025][JDd4]JESD22-B101 Test Method B101 External Visual外观检查, (Previously published in JESD22-B) September 1987 [Text-jd026][JDd5]EIA/JESD22-B102-C Solderability Test Method可焊性试验方法, September 1998 [Text-jd027][JDd6]EIA/JESD22-B105-B Test Method B105-B Lead Integrity器件管脚的完整性试验, (Revision of Test Method B105-A) January 1999 [Text-jd028][JDd7]EIA/JESD22-B107-A Test Method B107-A Marking Permanency图标的耐久性试验, (Revision of Test Method B107-Previously Published in JESD22-B) September 1995[Text-jd029][JDd8]JESD22-B108 Coplanarity Test for Surface-Mount Semiconductor Devices表贴半导体器件的共面性试验, November 1991 [Text-jd030]其它[JDe1]JEP113-B Symbol and Labels for Moisture-Sensitive Devices湿度敏感器件的符号和标识, (Revision of JEP113-A) May 1999 [Text-jd032][JDe2]EIA/JEP122 Failure Mechanisms and Models for Silicon Semiconductors Devices硅半导体器件的失效机理和模型, February 1996 [Text-jd033][JDe3]IPC/JEDEC J-STD-020A Moisture/Reflow Sensitivity Classification for Nonhermetic Solid State Surface Mount Devices针对非密封表贴半导体器件的湿度/回流焊敏感度分类和级别, April 1999 [Text-jd034][JDe4]IPC/JEDEC J-STD-033 Standard for Handling, Packing, Shipping and Use of Moisture/Reflow Sensitive Surface Mount Devices湿度/回流焊敏感标贴器件的处理、包装、运输和使用的标准, May 1999 [Text-jd035][JDe5]EIA/JEP103-A Suggested Product-Documentation Classifications and Disclaimers, (Revision of JEP103) July 1996 [Text-jd036][JDe6]IPC/JEDEC J-STD-020D.1 Moisture/Reflow Sensitivity Classification for Nonhermetic Solid State Surface Mount Devices针对非密封表贴半导体器件的湿度/回流焊敏感度分类和级别, Supersedes IPC/JEDEC J-STD-020D August 2007, March 2008 [Text-jd037]。
Storage test

QUALITY ENGINEERING DIVISION1. High temperature testTest condition:Climatic chamber set: +60℃, relative humidity 40%The unit under test shall be retained in its original gift box –in a non operation state and shall be placed in a climatic test chamber, which conforms to the temperature conditions as specified in the above table, for 24 hours. The unit is taken out of the climatic test chamber and immediately checked for any appearance and structural damage. The unit is then left at normal room temperature for 4 hours, after which time functional and electrical parameters are then checked.Before the test, the function of the products is:Function check:2. Low temperature testTest condition:Climatic chamber set: -25℃The unit under test shall be retained in its original gift box –in a non operation state and shall be placed in a climatic test chamber, which conforms to the temperature conditions as specified in the above table, for 24 hours. The unit is taken out of the climatic test chamber and immediately checked for any appearance and structural damage. The unit is then left at normal room temperature for 4 hours, after which time functional and electrical parameters are then checked.Before the test, the function of the products is:Function check:3. High relative humidity testClimatic chamber set: +60℃, relative humidity 90%The unit under test shall be retained in its original gift box –in a non operation state and shall be placed in a climatic test chamber, which conforms to the temperature conditions as specified in the above table, for 24 hours. The unit is taken out of the climatic test chamber and immediately checked for any appearance and structural damage. The unit is then left at normal room temperature for 4 hours, after which time functional and electrical parameters are then checked.Before the test, the function of the products is: PassedFunction check:Remarks:Disposition Accepted Waived Rejected Tested by: Checked by: Authorisation:。
USP40版1117 良好微生物实验室规范

Ljungqvist B, Reinmüller B. Interaction between air movements and the dispersion of contaminants: clean zones with unidir-ectional air flow. J Parenter Sci Technol. 1993; 47(2):60–69.Ljungqvist B, Reinmüller B. Airborne viable particles and total number of airborne particles: comparative studies of active air sampling. PDA J Sci Technol. 2000; 54:112–116.Maruyama M, Matsuoka T, Deguchi M, Akers J. The application of robotics to aseptic surface monitoring. Pharm Technol. 2007; 32(7):40–44.Process simulation testing for sterile bulk pharmaceutical chemicals. PDA Technical Report No. 28. J Parenter Sci Technol. 1998; 52 S3.Reinmüller B. Dispersion and risk assessment of airborne contaminants in pharmaceutical cleanrooms. Building Serv Eng Bull (Sweden). 2001; Bulletin No. 56.Stewart SL, Grinshpun SA, Willeke K, Terzieva S, Ulevicius V, Donnelly J. Effect of impact stress on microbial recovery on an agar surface. Appl Environ Micro. 1995; 61:1232–1239.Whyte W. Reduction of microbial dispersion by clothing. J Parenter Sci Technol. 1985; 39(1):51–60.á1117ñ MICROBIOLOGICAL BEST LABORATORY PRACTICESINTRODUCTIONGood laboratory practices in a microbiology laboratory consist of activities that depend on several principles: aseptic techni-que, control of media, control of test strains, operation and control of equipment, diligent recording and evaluation of data, and training of the laboratory staff. Because of the inherent risk of variability in microbiology data, reliability and reproducibili-ty are dependent on the use of accepted methods and adherence to good laboratory practices.MEDIA PREPARATION AND QUALITY CONTROLMedia PreparationCulture media are the basis for most microbiological tests. Safeguarding the quality of the media is therefore critical to the success of the microbiology laboratory. Media preparation, proper storage, and quality control testing can ensure a consistent supply of high-quality media.It is important to choose the correct media or components in making media based on the use of accepted sources or refer-ences for formulas. The manufacturer's formula and instructions for preparation routinely accompany dehydrated media and ready-made media. Because different media types may have different preparation requirements (e.g., heating, additives, and pH adjustment), it is important to follow these instructions to ensure preparation of acceptable media quality. A certificate of analysis describing expiration dating and recommended storage conditions accompanies ready-made media, as well as the quality control organisms used in growth-promotion and selectivity testing of that media.Water is the universal diluent for microbiological media. Purified Water is most often used for media preparation, but in cer-tain cases the use of deionized or distilled water may be appropriate. Water of lesser quality should not be used for microbio-logical media preparation. The volume of the water used should be recorded.Consistent preparation of media requires accurate weighing of dehydrated media or media constituents. A calibrated bal-ance with the appropriate weight range for the ingredients should be used (See Weighing on an Analytical Balance á1251ñ). Clean weighing containers and tools (such as spatulas) should be used to prevent foreign substances from entering the formu-lation. The weight of the components should be recorded.Dehydrated media should be thoroughly dissolved in water before dispensing and sterilization. If heating is necessary to help dissolve the media, care should be taken not to overheat media, because all culture media, to a greater or lesser extent, are heat-sensitive. Equipment used in the preparation of media should be appropriate to allow for controlled heating, constant agitation, and mixing of the media. Darkening of media (Maillard-type reaction or nonenzymatic browning) is a general indi-cation of overheating. When adding required supplements to media, adequate mixing of the medium after adding the supple-ment should be performed.Preparation of media in poorly cleaned glassware can allow inhibitory substances to enter the media. Inhibitory substances can come from detergent residue after cleaning glassware or from prior materials used in the glassware. Be sure that the clean-ing process removes debris and foreign matter, and that the detergent is thoroughly rinsed out with Purified Water. See Clean-ing Glass Apparatus á1051ñ for additional guidance.Sterilization of media should be performed within the parameters provided by the manufacturer or validated by the user. Commercially prepared media should provide documentation of the sterilization method used. Autoclaving by moist heat is the preferred sterilization technique, except in instances when boiling is required in order to avoid deterioration of heat-labilecomponents of the media. Sterilization by filtration may also be appropriate for some formulations.USP 40General Information / á1117ñ Microbiological Best Laboratory Practices1443The effects of the sterilization method and conditions on the media should be validated by sterility and growth-promotion testing of the media. In addition, if sterilized by moist heat, the autoclave cycle should be validated to ensure proper heat dis-tribution for selected loads and volumes. Typically, manufacturers recommend using an autoclave cycle of 121° for 15 minutes using a validated autoclave. These conditions apply to time at temperature of the media. As container size and the load config-uration of the autoclave will influence the rate of heating, longer cycles may be required for larger loads. However, the sterili-zation time will be dependent on the media volume and autoclave load. Sterilization cycles in which the autoclave is slow to come up to temperature may result in overheating of the media. Therefore, care must be taken to validate a sterilization cycle,balancing the need for sterile media against the tendency of the media to degrade under excessive heating. Storage of the media in the autoclave after the liquid cycle is completed is not recommended after cooling, as it may damage the media.Improper heating or sterilizing conditions—for commercially prepared or internally prepared media—may result in a difference in color change, loss of clarity, altered gel strength, or pH drift from the manufacturer's recommended range, as well as re-duced growth-promotion activity and/or selectivity.The pH of each batch of medium should be confirmed after it has cooled to room temperature (20°–25°) by aseptically withdrawing a sample for testing. Refrigerated purchased media should be allowed to warm up to ambient room temperature if it is to be checked for pH confirmation. A flat pH probe is recommended for agar surfaces, and an immersion probe is rec-ommended for liquids. See pH á791ñ for guidance with pH measurement and instrument calibration. The pH of media should be in a range of ±0.2 of the value indicated by the manufacturer, unless a wider range is acceptable by the validated method.Prepared media should be checked by appropriate inspection of plates and tubes for the following:•Cracked containers or lids•Unequal filling of containers•Dehydration resulting in cracks or dimpled surfaces on solid medium•Hemolysis•Excessive darkening or color change•Crystal formation from possible freezing•Excessive number of bubbles•Microbial contamination•Status of redox indicators (if appropriate)•Lot number and expiration date checked and recorded•Sterility of the media•Cleanliness of plates (lid should not stick to dish)Media StorageIt is prudent to consider how the manufacturer or supplier transports and stores media before distribution to the end user.Manufacturers of media should use transport and storage conditions that minimize the loss of moisture, control the tempera-ture, prevent microbial contamination, and provide mechanical protection to the prepared media.Media should be labeled properly with batch or lot numbers, preparation and expiration dates, and media identification.Media should be stored according to the manufacturer's instructions. Media prepared in house should be stored under valida-ted conditions. Do not store agar at or below 0°, as freezing could damage the gel structure. Protect stored media from expo-sure to light and excessive temperature. Before prolonged storage, agar plates should be placed into a sealed package or con-tainer to retard moisture loss.Remelting of an original container of solid media should be performed only once to avoid media whose quality is compro-mised by overheating or potential contamination. It is recommended that remelting be performed in a heated water bath or by using free-flowing steam. The use of microwave ovens and heating plates is common, but care should be taken to avoid damaging media by overheating and to avoid the potential injury to laboratory personnel from glass breakage and burns. The molten agar medium should be held in a monitored water bath at a temperature of 45° to 50° for not more than 8 hours.Caution should be taken when pouring the media from a container immersed in a water bath to prevent water from the bath commingling with the poured sterile media. Wiping the exterior of the container dry before pouring may be advisable.Disposal of used cultured media (as well as expired media) should follow local biological hazard safety procedures.Quality Control TestingAlthough growth media can be prepared in a laboratory from individual components, many laboratories, for ease of use, use dehydrated media or purchase commercially prepared media in plastic plates or glass containers. Manufacturers of media at-tempt to standardize raw materials from biological sources, but must constantly deal with unavoidable differences in raw ma-terials obtained from natural sources, and therefore, lot-to-lot variability of media must be considered. In addition, the per-formance of media prepared in a laboratory or by a manufacturer is highly dependent on preparation and storage conditions.Improper media preparation can cause unsatisfactory conditions for microbial growth or recovery and unreliable results.Therefore, quality control tests should be performed on all prepared media, including media associated with swabs or media in strips and other nontraditional formats. Tests routinely performed on in-house prepared media should include pH, growthpromotion, inhibition, and indicative properties (as appropriate), and periodic stability checks to confirm the expiration dating.1444 á1117ñ Microbiological Best Laboratory Practices / General Information USP 40When in-house prepared microbiological media are properly prepared and sterilized using a validated method, the growth-promotion testing may be limited to each incoming lot of dehydrated media, unless otherwise instructed by the relevant com-pendial method. If the media preparation procedure was not validated, then every batch of media should be subjected to growth-promotion testing. Test organisms may be selected from the appropriate compendial test chapter. In addition, micro-organisms used in growth-promotion testing may be based on the manufacturer's recommendation for a particular medium, or may include representative environmental isolates (but these latter are not to be construed as compendial requirements). Expiration dates on media should have supporting growth-promotion testing to indicate that the performance of the media still meets acceptance criteria up to and including the expiration date. The length of shelf life of a batch of media will depend on the stability of the ingredients and formulation under specified conditions, as well as the type of container and closure. When a batch of media does not meet the requirements of growth-promotion testing, an investigation should be initiated to identify the cause. This investigation should include a corrective action plan to prevent the recurrence of the problem. Any batch of media that fails growth-promotion testing is unsuitable for use. [NOTE—Failed growth-promotion test results may not be used to negate positive test results.]Some reagents are used for diagnostic purposes to help support identification of microbial organisms, e.g., Gram stain and oxidase test reagents. These may have attributes that can be quality control tested similar to microbiological media. Select the correct quality control standard microorganisms, following the manufacturer's instructions, and perform the testing before un-known sample diagnostic testing. All relevant diagnostic reagents should be subjected to incoming quality confirmation before use.Special care should be taken with media that is used in sterility tests (see Sterility Tests á71ñ for requirements) and in environ-mental monitoring studies. Media used for environmental monitoring of critical areas should preferably be double-wrapped and terminally sterilized. If terminal sterilization is not performed, media should be subjected to 100% pre-incubation and in-spection before use within a critical area. [N OTE—Growth-promotion testing for this media must be performed after the pre-incubation stage.] This will prevent extraneous contamination from being carried into controlled environments and will pre-vent false-positive results. A raised agar level for surface contact plates should be verified.MAINTENANCE OF MICROBIOLOGICAL CULTURESBiological specimens can be the most delicate standards to handle because their viability and characteristics are dependent on adequate handling and storage. Standardizing the handling and storage of cultures by the user laboratory should be done in a way that will minimize the opportunity for contamination or alteration of growth characteristics. The careful and consis-tent treatment of stock cultures is critically important to the consistency of microbiological test results. Cultures for use in com-pendial tests should be acquired from a national culture collection or a qualified secondary supplier. They can be acquired fro-zen, freeze-dried, on slants, or in ready-to-use forms. Confirmation of the purity of the culture and the identity of the culture should be performed before its use in quality control testing. Ready-to-use cultures should be subjected to incoming testing for purity and identity before use. The confirmation of identity for commonly used laboratory strains should ideally be done at the level of genus and species.Preparation and resuscitation of cultures should follow the instructions of the supplier or a validated, established method. The “Seed-Lot” technique is recommended for storage of stock cultures.The original sample from the national culture collection or a qualified secondary supplier is resuscitated and grown in an appropriate medium. Aliquots of this stock culture (the first transfer or passage) are suspended in a cryoprotective medium, transferred to vials, and frozen at –30° or below, until use. If stored at –70°, or in lyophilized form, strains may be kept indefi-nitely. These frozen stocks can then be used to inoculate monthly or weekly working cultures. Once opened, do not refreeze unused cell suspensions after culturing a working suspension. The unused portion should be discarded to minimize the risk of loss of viability and contamination of the stock.The number of transfers of working control cultures should be tracked to prevent excessive subculturing that increases the risk of phenotypic alteration or mutation. The number of transfers allowable for specific compendial tests may be specified in that test. One passage is defined as the transfer of organisms from a viable culture to a fresh medium with growth of the mi-croorganisms. Any form of subculturing is considered to be a transfer/passage.LABORATORY EQUIPMENTMost equipment (incubators, water baths, and autoclaves) is subject to standard validation practices of incoming qualifica-tion, operational qualification, and performance qualification. Additionally, periodic calibration (generally annually) is com-monly required. New equipment, critical to the operation of the laboratory, should be qualified according to a protocol ap-proved by the quality assurance unit (QAU). In addition, regular cleaning and sanitization of equipment such as incubators, refrigerators, and water baths should be performed to minimize the potential for contamination in the laboratory. Door seals of incubators and refrigerators should be cleaned and checked for state of repair.Instruments (pH meters and spectrophotometers) used in a microbiology laboratory should be calibrated on a regular schedule and tested to verify performance on a routine basis. The frequency of calibration and performance verification willvary based on the type of instrument and the importance of that equipment to the generation of data in the laboratory. USP 40General Information / á1117ñ Microbiological Best Laboratory Practices1445Equipment that is difficult to sanitize (such as refrigerators and incubators) should be dedicated to aseptic operations (such as storage of media for testing and incubation of sterility test samples) and live culture operations to minimize the potential for inadvertent contamination of the tests.Autoclaves are central to the operation of the laboratory and must have proper validation in place to demonstrate adequate sterilization for a variety of operations. Autoclave resources must be available (and validated) to sterilize waste media (if per-formed in that laboratory) as well as the media prepared in that laboratory. The choice of one or several autoclaves is not driv-en by a need to separate aseptic and live operations (everything in the properly maintained autoclave is sterile after the cycle)but rather driven by resource considerations (see below).LABORATORY LAYOUT AND OPERATIONSLaboratory layout and design should carefully consider the requirements of good microbiological practices and laboratory safety. It is essential that cross-contamination of microbial cultures be minimized to the greatest extent possible, and it is also important that microbiological samples be handled in an environment that makes contamination highly unlikely.In general, a laboratory should be divided into clean or aseptic areas and live culture areas. Areas in which environmental or sterile product samples are handled and incubated should be maintained completely free of live cultures, if possible. If com-plete separation of live and clean culture zones cannot be accomplished, then other barriers and aseptic practices should be employed to reduce the likelihood of accidental contamination. These barriers include protective clothing, sanitization and dis-infection procedures, and biological safety cabinets designated for clean or aseptic operations only. Procedures for handling spills or mishaps with live cultures should be in place, and all relevant technical personnel should be trained regarding thesemethods.Some samples will demonstrate microbial growth and require further laboratory analysis to identify the contaminants. Whengrowth is detected, the sample should be taken from the clean section of the laboratory to the live culture section without undue delay. Subculturing, staining, microbial identification, or other investigational operations should be undertaken in thelive culture section of the laboratory. If possible, any sample found to contain growing colonies should not be opened in theclean zone of the laboratory. Careful segregation of contaminated samples and materials will reduce false-positive results.Staff engaged in sampling activities should not enter or work in the live culture handling section of a laboratory unless spe-cial precautions are taken, including wearing protective clothing and gloves and careful sanitizing of hands upon exiting. Ideal-ly, staff assigned to sampling activities, particularly those in support of aseptic processing, should not work in the vicinity of live culture laboratory operations.It is important to consider that microbial contamination of samples, which leads to false-positive results, is always possible unless careful aseptic precautions are taken. Facilities should be designed so that raw material and excipient sampling can be done under controlled conditions, including proper gowning and sterilized sampling equipment. It may not always be possible to sample utility systems, such as water systems, under full aseptic conditions; however, it should be noted that when samples are not taken aseptically, their reliability is inevitably compromised.Environmental sampling methods should require minimal aseptic handling in loading and unloading sampling instruments.Whenever possible, sampling equipment should be loaded with its microbiological recovery media in the environment that is to be sampled.All testing in laboratories used for critical testing procedures, such as sterility testing of final dosage forms, bulk product,seed cultures for biological production, or cell cultures used in biological production, should be performed under controlled conditions. Isolator technology is also appropriate for critical, sterile microbiological testing. Isolators have been shown to have lower levels of environmental contamination than manned clean rooms, and therefore, are generally less likely to produce false-positive results. Proper validation of isolators is critical both to ensure environmental integrity and to prevent the possibili-ty of false-negative results as a result of chemical disinfection of materials brought into or used within isolators (see Sterility Testing—Validation of Isolator Systems á1208ñ).SAMPLE HANDLINGViable microorganisms in most microbiology samples, particularly water, environmental monitoring and bioburden samples,are sensitive to handling and storage conditions. Critical parameters in these conditions include product (or sample) composi-tion, container composition, time of storage, and temperature of storage. Therefore, it is important to minimize the amount of time between the sampling event and the initiation of testing and to control, as much as possible, the conditions of storage. If the sample is to be transported to a distant location for testing, then the conditions of transport (time, temperature, etc.)should be qualified as suitable for that test and sample. Guidance for water testing in this regard can be found in Water for Pharmaceutical Purposes á1231ñ. Product mixing before sampling may need to be evaluated and applied in order to ensure mi-crobial dispersement and representation in the sample aliquot.All microbiological samples should be taken using aseptic techniques, including those taken in support of nonsterile prod-ucts. If possible, all microbiological samples should be taken under full aseptic conditions in specialized sampling areas. The areas should be as close to the point of use as possible to minimize contamination during transit.Samples submitted to the microbiology laboratory should be accompanied by documentation detailing source of the sam-ple, date the sample was taken, date of sample submission, person or department responsible for the submission, and any1446 á1117ñ Microbiological Best Laboratory Practices / General Information USP 40potentially hazardous materials associated with the sample. The testing department should acknowledge receipt of the sample and reconcile the identity and number of samples as part of this sample documentation.MICROBIOLOGICAL MEDIA INCUBATION TIMESIncubation times for microbiological tests of less than 3 days' duration should be expressed in hours: e.g., “Incubate at 30°to 35° for 18 to 72 hours”. Tests longer than 72 hours' duration should be expressed in days: e.g., “Incubate at 30° to 35° for 3 to 5 days”. For incubation times expressed in hours, incubate for the minimum specified time, and exercise good microbio-logical judgment when exceeding the incubation time. For incubation times expressed in days, incubations started in the morning or afternoon should generally be concluded at that same time of day.TRAINING OF PERSONNELEach person engaged in each phase of pharmaceutical manufacture should have the education, training, and experience to do his or her job. The demands of microbiological testing require that the core educational background of the staff, supervi-sors, and managers be in microbiology or a closely related biological science. They should be assigned responsibilities in keep-ing with their level of skill and experience.A coherent system of standard operating procedures (SOPs) is necessary to run the microbiology laboratory. These proce-dures serve two purposes in a training program. Firstly, these SOPs describe the methodology that the microbiologist will fol-low to obtain accurate and reproducible results, and so serve as the basis for training. Secondly, by tracking the procedures in which a particular microbiologist has demonstrated proficiency, the procedure number or title also serves to identify what training the microbiologist has received specific to his or her job function.Training curricula should be established for each laboratory staff member specific to his or her job function. He or she should not independently conduct a microbial test until qualified to run the test. Training records should be current, documenting the microbiologist's training in the current revision to the particular SOP.Periodic performance assessment is a wise investment in data quality. This performance testing should provide evidence of competency in core activities of the microbiology laboratory such as hygiene, plating, aseptic technique, documentation, and others as suggested by the microbiologist's job function.Microbiologists with supervisory or managerial responsibilities should have appropriate education and in-house training in supervisory skills, laboratory safety, scheduling, budgeting, investigational skills, technical report writing, relevant SOPs, and other critical aspects of the company's processes as suggested in their role of directing a laboratory function.Competency may be demonstrated by specific course work, relevant experience, and routinely engaging in relevant con-tinuing education. Achieving certification through an accredited body is also a desirable credential. Further, it is expected that laboratory supervisors and managers have a demonstrated level of competence in microbiology at least as high as those they supervise. Expertise in microbiology can be achieved by a variety of routes in addition to academic course work and accredita-tion. Each company is expected to evaluate the credentials of those responsible for designing, implementing, and operating the microbiology program. Companies can thus ensure that those responsible for the program understand the basic principles of microbiology, can interpret guidelines and regulations based on good science, and have access to individuals with theoreti-cal and practical knowledge in microbiology to provide assistance in areas in which the persons responsible for the program may not have adequate knowledge and understanding. It should be noted that microbiology is a scientifically based discipline that deals with biological principles substantially different from those of analytical chemistry and engineering disciplines. Many times it is difficult for individuals without specific microbiological training to make the transition.LABORATORY RESOURCESThe laboratory management is responsible for ensuring that the laboratory has sufficient resources to meet the existing test-ing requirements. This requires some proficiency in budget management and in determining appropriate measures of labora-tory performance. A measure of laboratory performance is the number of investigations performed on tests conducted by the laboratory, but this measure alone is not sufficient. In addition to tracking investigations, the period of time between sample submission and initiation of testing should be tracked, as well as the period of time between end of test and report release (or test closure). Significant delays in these measures are also indications of an under-resourced laboratory staff.The laboratory management should have sufficient budget to meet testing requirements. Particular measures of budgetary requirements will be specific to the given laboratory, but budgetary considerations related directly to the need of the laborato-ry for sufficient resources must be addressed to ensure reliable testing results.DOCUMENTATIONDocumentation should be sufficient to demonstrate that the testing was performed in a laboratory and by methods that were under control. This includes, but is not limited to, documentation of the following:•Microbiologist training and verification of proficiencyUSP 40General Information / á1117ñ Microbiological Best Laboratory Practices1447。
充电器测试标准

CONTENTS1.Apply Scope(适用范围) (2)2.Quote Criterion(引用标准) (2)3.Electrical characteristic (电气特性) (2)4.Input Characteristics(输入特性) (2)4.1Rated Input Voltage(额定输入电压) (2)4.2Input Voltage Range (输入电压范围) (2)4.3Input Frequency (输入频率) (2)4.4Input Frequency Range ( 输入频率范围) (3)4.5Input AC Current(AC输入电流) (3)4.6Inrush Current ( 峰值输入电流) (3)4.7Efficiency(效率) (3)5.Output Characteristics(输出特性) (3)5.1Rated Output Voltage(输出额定电压) (3)5.2Output Voltage (输出电压) (3)5.3Rated Output Current (额定输出电流) (3)5.4Rated Power ( 额定功率) (3)5.5LED Indicate Function (LED指示功能) (3)5.6Charger output Voltage/Current characteristics(充电器输出电压/电流特性图) (4)5.7Ripple and Noise ( 输出纹波、噪音) (4)5.8Current Ripple and Noise ( 输出电流纹波、噪音) (4)5.9Turn On Delay Time(启动延时) (4)5.10Turn-Off Delay (关断时延) (4)5.11Overshoot(过冲) (4)5.12Counter current(电流倒灌) (5)5.13Protection(保护) (5)5.13.1Over Voltage Protection(过压保护) (5)5.13.2 Over Current Protection(过流保护) (5)5.13.3 Short Circuit Protection(短路保护) (5)6.Reliability Items ( 信赖性项目) (5)6.1Electrostatic discharge (静电) (5)6.2Hi-Pot Test (高压测试) (5)6.3Insulation Resistance (绝缘电阻) (5)6.4Leakage Current ( 泄漏电流) (5)6.5Temperature Rise (温升) (6)6.6Continuous Working (连续工作时间) (6)6.7Mean Time Between Failure (平均无故障时间) (6)6.8EMI Standards (EMI标准) (6)7.Environmental Requirement (环境要求) (6)7.1Operating Temperature ( 工作温度) (6)7.2Storage Temperature (储藏温度) (6)7.3Operating Humidity (工作湿度) (6)7.4Storage Humidity (储藏湿度) (6)8.Mechanical Requirement (机械要求) (6)8.1Dimension(尺寸) (6)8.2Weight(重量) (6)8.3Input plug type(输入插头类型) (6)8.4USB Plug Type(USB接口类型) (6)8.5USB Plug Test(USB接口测试) (7)8.6Drop Test (跌落试验) (7)8.7Vibration Test Requirement (振动试验) (7)8.8Plug in and out Test (插拔实验) (7)8.9Salty Fog Test for Metal part (五金件盐雾实验) (7)9.Mechanical Characteristics (机械性能) (7)9.1Appearance (外观) (7)9.2Case/Resin materials (外壳材质) (8)10.Environmental Performances(环境性能) (8)10.1Operating at the lower temperature (低温工作实验) (8)10.2Operating at the high temperature(高温工作实验) (8)10.3Storage at the lower temperature (低温存储) (8)10.4Storage at the higher temperature (高温存储) (8)10.5Operating at the invariable temperature and invariable humidity (恒温恒湿工作) (8)1. Apply Scope(适用范围)This specification shall be applied to USB charger ET860 RUS ENG RoverPC S7 ROVER Titan 本规格适用于ET860 RUS ENG RoverPC S7 ROVER Titan充电器。
苹果DRT测试项目之环境测试
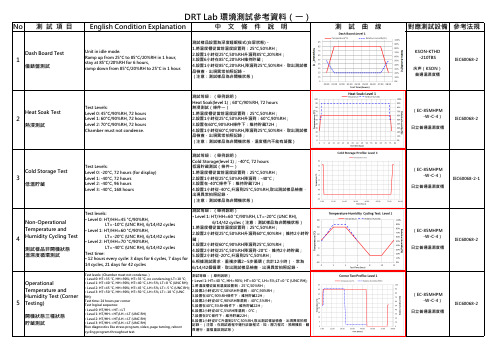
1.將溫度櫃從當前溫度設置到:25°C,50%RH; 2.設置1小時從25°C,50%RH升溫到85°C,85%RH; 3.設置500小時在85°C,85%RH維持貯藏; 4.設置1小時從85°C,85%RH,降溫到25°C,50%RH,取出測試樣 品檢查,出現異常拍照記錄。
这个测试主要针对模组等级测试. 测试时运行模组程序, 确认模组是否符合规格.
2.檢查側面斜率控制器,設定是否正確,該等級設為14°C/分鐘;
• Level 1 for board or component: -40 °C (LT) to 85°C
3.預熱預冷結束後,運行過程說明:每一小時為一個測試週期,其中高 溫和低溫分別駐留23分鐘,以最小斜率上升或下降,高低溫差100°C,
庆声(KSON) 普通溫濕度櫃
IEC60068-2
(EC-85MHPM -W-C-4)
日立普通溫濕度櫃
IEC60068-2
(EC-85MHPM -W-C-4)
日立普通溫濕度櫃
IEC60068-2-1
(EC-85MHPM -W-C-4)
日立普通溫濕度櫃
IEC60068-2
(EC-85MHPM -W-C-4)
• Level 1: HT=40 °C, HH=90%; HT=40 °C, LH=5%;LT=0 °C (UNC RH);
• Level 2: HT=45 °C, HH=90%; HT=45 °C, LH=5%; LT=-5 °C (UNC RH); 1.將溫度櫃從當前溫度設置到:25°C,50%RH;
8
Salt Mist 鹽霧測試
Per IEC 68-2-52, severity (2):
根据IEC 68-2-52, 等级(2)
高温储存测试规范(中英文)

高温储存测试规范High Temperature Storage Test Procedure1.0 PURPOSE (目的):1.1The high temperature storage test is designed to determine the effects on the product, while package in it's shipping container, due to high temperatures. This is an accelerated test to uncover weaknesses in components, assemblies, and processes, which may appear in the field during normal shipping situations.1.1 高温储存试验是用来判定高温对产品包装后在运输过程中的影响,这是一个加速性测试,用来显露出任何组成零件,在正常运输中可能造成的损坏。
1.22.0 SCOPE (范围):2.1This test describes the minimum temperature storage performance test required for all products.2.1对所有产品都须经过此测试。
3.0 SPECIFICATIONS (规格):3.1Class 1 product - Switching Power supply and Linear Power Supply 3.1 第一级- Switch和Linear电源供应器 ;Temperature - +85 ℃ C 90% RH温度:+85℃湿度90%Temperature Gradient - +/- 20 ℃/hr.温度变化率 + / - 20℃/hr3.2Class 2 Products - Charger Base, Headset and Data Transfer box3.2 第二级- Charger Base , Headset and Data transfer box ; Temperature - +80 ℃ 90% RH温度:+80℃湿度90%Temperature Gradient - +/- 20 ℃/hr.温度变化率 + / - 20℃/hrNOTE: When this specification conflicts which product specification the product specification take precedence in all respects.备注: 当这份规范与产品规格书所规定的规格有所抵触时, 以产品规格书所规定的规格为准.4.0 TEST EQUIPMENT (测试设备):4.1Environmental chamber capable of sustaining constant temperatures of 85 deg C. and of sustaining constant humidity of between 20% to 90% RH. The chamber must also have the capabilities to record both temperature and humidity vs. time.4.1恒温恒湿机须有能力提供 85℃且湿度为 20% 到 90% 之间,还须有能力记录温度、湿度与时间的关系。
连接器行业专业英语高频词句

连接器行业专业英语高频词句(中英对照)Connecter 连接器Cable 导线Wire-to-wire 线至线Wire-to-board 线至板Mini-universal 小型通用Circular plastic connectors 塑料密封环连接器DL (dual-leaf) contact sockets 双叶式触点插座Drawn spring DIP contact socket 弹簧触点插座Zig-zag package sockets 锯齿形封装插座Positive lock connectors 确立锁定连接器Coax mix 同轴混合Shielded circular DIN connectors 屏蔽式圆型DIN连接器Memory card connectors 记忆装置插结连接器SIMM sockets 单列记忆模件插座Low profile 低高度CIS (commercial interconnection system) 商用连接系统Hinge connectors 铰连连接器Card-edge connectors 边缘插接连接器Euro card connectors 欧式插接连接器Modular interconnection system 组合连接系统interconnection system 互连系统RF connectors 射频连接器Twin-Ax connectors 双轴互连系统Low profile coaxial tap 低高度型有源同轴电缆线器4 position data connectors 4位数据连接器Insulation and wire support 绝缘体及导线套Ring-Tongue terminals 环形舌端子Wire size range 线径范围Slotted-Tongue terminals 叉式舌片端子Butt splices 对头接头Dentented Ring-Tongue terminals 卡爪环舌型端子Fits tab thickness 配合接片厚度Accepts terminals 配合端子Crimp-type tabs 压接式接片Free-Hanging and Panel-Mount Applications 自由悬挂及面板装嵌适用Cable clamp 电缆钳位夹PC Board vertical pin headers 垂直接插式接插头组件PC Board Right-Angle pin and socket headers 水平接插式接插头组件Panel-Mount connectors 面板装嵌连接器Insulation Displacement plugs 绝缘位移连接插头Dielectric withstanding voltage 介质承受电压Free-Hanging type cap housings 自由悬挂式套座壳体Semiautomatic crimping machine 半自动压接机Power supply 供给功率Air pir pressure 气压Panel mount type cap housings 面板装嵌套座壳体Post header for printed circuit board 印刷底板用柱头Mass termination assemblies 集体端接组件Post header assemblies 接插柱头组件Polarized right angle post header assembly 有极向性水平接插柱头组件Friction lock 锁尖装置Sigle leaf connectors 单叶片连接器CPC (circular plastic connectors) 塑料密封环型连接器Crimp Snap-In contacts 压接式套入触点Socket with straight solder tails 直立焊脚插座Retention feature solder tails 弯曲焊脚Sockets with retention feature solder tails 面板附定曲脚插座2High Pressure Tin (HPT) sockets 镀锡高压芯片Positive lock retention feature 彻底锁定装置Plug-Grounding Indents 插头外罩接地凹位Solder Cup connector 焊杯触点连接器Straight Exit RFI/EMI shield for Jacketed cable 直线引出RFI/EMI屏蔽外壳外套导线束适用Backshell assembly 后部外壳组件Board-Mount connectors with threaded inserts and board locks 底板安装连接器附内丝牙及板扣Board-Mount Receptacles 底板安装插座Non-Polarized Receptacles 无定位型插座Handset plug assembly 电话线插头组件Line cord plug assembly 听筒插头组件射频同轴连接器RF coaxial connector1.按尺寸分类 Connector types by size●标准连接器standard connector●小型连接器miniature connector●微型连接器sub-miniature connector●超小型连接器micro-miniature connector2. 结构和安装类型 Connector configurations & mounting styles2.1接电缆的连接器 Cabled connector●直式 straight●弯式 right angle (直角)●穿墙式 bulkhead n. 隔壁,防水壁,分壁2.2不接电缆的连接器 Uncabled connector●PC板安装 PC mount●表面贴装 surface mount●边缘安装(偏腿)end launch●法兰安装 flange mount3. 联接机构 Coupling mechanism●螺纹联接 threaded connection(e.g. SMA)●卡口式联接 bayonet coupling(e.g. BNC)●推入式联接 snap-on coupling(e.g. MMCX)●快速锁紧联接 quick-lock coupling(e.g. QMA)●滑入式联接 slide-on coupling (e.g. BMA)4. 组成部件 Parts●壳体 body/shell/housing●绝缘子 insulator●夹头绝缘子 clamp top insulator●中心接触件 center contacta.插针 male/pinb.插孔 female/jack●中心导体 center conductor●内导体 inner conductor●外导体 outer conductor●插座/插口 jack-panel jack 面板插口/插座-bulkhead jack 穿墙插座4●插头 receptacle5. 材料 Material●黄铜 copper●铍青铜 beryllium copper●锡磷青铜 phosphor bronze●聚四氟乙烯 PTFE polytetrafluoroethylene 特氟纶teflon ●不锈钢 stainless steel●合金 alloy●实心聚乙烯 solid PE●泡沫聚乙烯 foam PE /cellular polyethylene●白铜 white bronze●硅橡胶 silicon rubber●裸铜 BC=bare copper●易切削黄铜free cutting brass●稀土磁铁rare earth magnet●铝箔 aluminium foil●基材 base material●FR-4板材 FR-4 plate●硅脂 silicon grease●润滑硅脂 lubricating silicone grease6. 电镀 Plating/finishing●镀金 gold plated●镀镍 nickel plated●钝化 passivated●镀三元合金 SUCO plating●镍磷合金(无磁镍)镀层 SUCOPRO●铜,锡,锌镀层 copper-tin-zinc plating●滚镀 barrel plating●合金电镀 alloy plating7. 电气特性 Electrical characteristics●阻抗(欧姆) impedance (ohms)a.特性阻抗 characteristic impedanceb.标称阻抗 nominal impedance●频率范围 frequency range●工作频率 operating frequency●工作电压 working volt●额定电压 voltage rating●介质耐压 DWV=dielectric withstanding voltage (如:最大/小有效值为a伏 e.g. aVrms.max/min)●海平面耐电压:proof voltage at sea level●射频泄露 RF leakage●绝缘电阻 insulation resistance●插入损耗 insertion loss●电压驻波比 VSWR6●额定热电流 rated thermal current●介电常数 dielectric constant●击穿电压 breakdown voltage●额定电流 current rating●电容 capacitance●电感 inductance●电晕损耗 corona loss●熄灭电压 extinction voltage●屏蔽效应 shielding efficiency●低损耗 low loss8. 机械特性Mechanical characteristics●耐久性(次数)durability/mating life(cycles)●电缆保持力 cable retention force●啮合力 engagement force●分离力 separation force/ disengagement●轴向力 axial force ( insertion & withdrawal 插入&抽出力) ●扭矩 torque●径向扭矩力 radial torque●弯曲力矩 bending moment●弯曲半径 bending radius●抗张强度 tensile strength●机械应力 mechanical stress●机械互换性:mechanical compatibility●中心接触件固定性 center contact captivation●制动 cathers mit9. 环境性能及试验Environmental characteristics & test●工作温度范围 operating temperature range●贮存温度 storage temperature●密封,透气/不透气 sealing,hermetic/non-hermetic●防潮 moistureproof/ dampproof●耐湿 humidity resistance●防水 waterproof●耐高温 high temperature resistance●耐热性 heat resistance●抗腐蚀性 corrrosion resistance●阻燃 flame resistant●抗风化,耐风雨,不受气候影响的 weather proof●热冲击 thermal shock●冲击 shock●振动 vibration●稳态湿热 damp heat, steady state●恒温 costant temperature●大气压(力),气压 barometric pressure●热处理,低温退火 annealing8●盐雾试验 salt mist/spray test●高压绝缘试验,闪点试验,火花试验 flash test●破坏性试验 destructive test●气密性试验 air tightness test●放电试验 discharge test10. 附件 Accessory●线夹 crimp ferrule●垫片 gasket●热缩管 heat shrink tubing/ heat-shrinkable tubing ●护线套 barrel crimp●衬套 bushing/braid clamp●卡环 lock ring/ snap ring●卡圈/弹簧卡环/压紧圈/挡圈 retainer ring●卡圈 collar●限位环 spacing ring/ stop collar●防晃圈 anti-rock ring●弹性接触件 resilient contact●锁销 lockpin●锁紧垫圈 lock washer●弹簧/性垫圈 spring washer●密封圈 seal ring●开口垫圈 slotted washer●防尘帽 dustproof cover/ dust cap●螺母 nut/screw nut●螺纹套筒 threaded sleeve●滚花螺母 knurled nut●直纹滚花 straight knurls●网纹滚花 crossed knurls●防松螺母/自锁螺母 locknut●锁紧螺母,止动螺母,压紧螺母 jam nut●紧固螺母 clamp nut●螺丝钉screw●法兰盘/凸缘盘 flange plate●橡胶圈 gum ring●纯胶管,软管 gum rubber tubing●连接套 barrel splice / joint sleeve●波导管 waveguide●弹簧夹 spring finger●弹簧片 spring lamination●紧固件 fixture/fastener●紧固螺栓 fastening bolt/ binding bolt●密封塞子 sealing plug●密封盖 sealing cover●匹配针 matched pin10●两芯电源插座 2-Core Socket●无源元件 passive component11. 工装夹具 Work fixture●夹具 clamping yoke●冲压工具 stamping tool●去毛刺工具 deburr tool●螺丝刀 screwdriver●平口螺丝刀 slotted screwdrier●十字花螺丝刀 phillips screwdrier●铆合机 staker / riveting machine ●翻边器/卷边器 hemmer/fold back tool ●钳子/镊子 pliers●螺丝钳/夹钳 clamp●电缆钳 cable nipper●剪钳,老虎钳 cutting nipper●尖嘴钳 sharp-nose pliers●钢丝剪钳 wire nipper●锉刀 file●扳手 wrench●钢锯 hacksaw●铆钉钳 rivet clipper●铆钉机 rivet driver●整平器,剔除器 flush trimmer●修边模 flush trim die●卡接槽口 clamping Slot●卡接工具 clamping cool●卡线钳 clamping pincers●力矩扳手 torque spanner●拧上 screw on●拧下 screw off射频同轴电缆及组件RF coaxial cable & cable assemblies1. 电缆类型 Cable types●超柔电缆 super-flexible cable●柔软电缆 flexible cable●半柔电缆 semi-flexible cable●半刚电缆 semi-rigid cable●跳/馈线 jumper/feeder cable●波纹电缆 corrugated cable2. 电缆组件类型 Cable assembly types●半刚电缆组件 semi-rigid cable assembly●半柔电缆组件 semi-flexible cable assembly●柔性电缆组件 flexible cable assembly●低损耗、稳相电缆组件 low loss & stable phase cable assembly12●毫米波电缆组件 millimeter wave cable assembly3.电缆设计Cable design3.1外导体设计 Design of outer conductor●单屏蔽 single screen●双屏蔽 double screen●绕包带和屏蔽层 tape and screen●金属管(半刚)tube(semi-rigid)●波纹 corrugated3.2 内导体设计 Design of inner conductor●实芯线 solid wire●绞合线 stranded wire●金属管 tube●螺纹管 screwed tube3.3 电缆结构 Cable construction●内导体 inner conductor●介质 dielectric●屏蔽层 shielding layer 编织层 braid layer/ply●护套 jacket3.4 电缆材料●镀锡铜 TC=tinned copper●铜包钢 BCCS=bare copper clad steel●镀银铜线 SC=silver covered copper●镀锡铜线 tinned copper●铜包钢线 CCS=copper clad steel●镀银铜包钢线 SCCS=silver covered copper clad steel●铜包铝线 BCCAl=bare copper clad aluminum●镀锡铜编织层 tinned copper braid●裸导线/无镀层导体 plain conductor●裸线, 裸导线 naked wire4. 电缆的装接方式 Cable attachment method4.1 柔软电缆 Flexible cable●压接式 crimp●夹持式 clamp4.2 半刚电缆 Semi-rigid cable●焊接式 solder5. 其它 Others●电缆旋转(挠动)cable rotation (nutation)●电缆拉伸 cable pulling●电缆弯曲 cable bending●电缆扭转 cable torsion/twisting●屏蔽线 shielded wire●双绞线 twisted pair●屏蔽双绞电缆 STP=shielded twisted pair●扁形软电缆 flat flexible cable●扁形双芯软线 flat twin flexible cord14●绕包带式 tape wrapping type●挤出型 extruded type●包带节距 tapping pitch射频同轴转接器RF coaxial adapter1. 转接器类型 Adapter types●系列间转接器:between-series adapter●系列内转接器:in-series adapter焊接用词汇●焊接槽 solder bucket●焊接柱 solder spill●焊片 solder gasket●焊锡圈 soldering ring●焊接孔solder eye●浸锡 tin soaked●焊锡丝 tin wire●漏焊 missing welding●虚焊 incomplete weld / weld defect/cold solder joint ●硬钎焊 solder brazing●焊割两用气焊枪 welding and cutting torch●焊接夹具 welding fixture●焊剂/焊料 welding fluid/ flux●焊接应力 welding stress●焊锡坑 solder cup●焊接涂料 welding paste●焊接部分 welding portion●无焊缝的 weldless●烙铁 soldering iron●烙铁架 iron stand●脱焊 loose weld●元器件表面焊接整洁,焊点圆滑 component surface is clearlywelded with smooth welding point●元器件无虚焊,漏焊,错贴 no cold solder joint, solder skipsand wrong welding of components●solder bath method 焊槽法技术要求&其它 Techinical requirments & others●机械和电气基准面 mechanical and electrical referenceplane●符合RoHS要求 RoHS compliant (e.g.this part complies withRoHS)●括号中尺寸为参考尺寸dimensions with()denote referencedimensions●铣槽和涨口/收口 slotted & flared/closed●去除毛刺 remove burrs/ deburr16●除锈 rust cleaning/removing●点铆 stamp●扩口 enlarge kerfs●毛边flash●编织层翻边 wrap the braid over the braid clamp(衬套)●外观,外形 appearance/outline●界面防水等级 interface waterproof level●标记内容:商标 型号 批次号 labels: trade mark,type, batchnumber●未注明公差按… non-specified tolerances according...●…要求为非磁性材料 …required to have non-magneticmaterial●导电环氧树脂回填 conductive epoxy back fill●外观检查:镀层表面无斑点和痕迹,界面无金属碎屑 Visualinspection: plating surface must be without spot and cinch marks; interface must be clean of metal chip●外观良好 good appearance●产品承认书 product acknowledgement●喷锡,黑油,无铅工艺 tin sprayed,black oil,lead free●无杂质 free of foreign matter●整涂覆 overall braid●现场安装field installation●两体结构two-body structure●尾部微带 rear strip●中心接触件弹性支撑center contact with elasticity support缩写和首字母缩写词 Abbreviations & acronyms:●连接器 CONN=connector●接触件 CONT=contact●超小D型连接器 D-sub CONN=D-subminiature connector●阴连接器/外壳 HSG=housing●印制电路板 PCB=print circuit board●产品型号,零件号 P/N=part number●订购单 P/O=purchasing order●数量 QTY=quantity●图纸 DWG=drawing●组件 ASS’Y=assembly●日期代码 D/C=date code●序号,编号 S/N=serial number●不良品,不合格品 NG=no good●不适用 N/A=not applicable●不可用,无法使用 N.A.=not available●直角 R/A=right angle●检验水平 IL=inspection level●可接受的 AC=acceptable18●可接受质量水平 AQL=acceptable quality level●退货,拒收 REJ=rejection●物料单,材料(零件)清单 BOM=bill of materials●无源交调 PIM=passive intermodulation●三阶交调 IM3= Intermodulation 3-tone●印制电路板PCB= printed circuit board●技术更改指令 ECO=Engineering Change Order●工程更改请求 ECR= Engineering Change Request●工艺更改通知单 PCN=Process Change Notice●制程能力/生产过程能力指数 CPK=capability of productionindex●过程工艺持续改进 CPI=continuous process improvement●大批量生成 MP=mass production●操作指南,使用说明书 OI=operating instruction●生产制造 MFG=manufacturing●作业指导书,制作规程 MI=manufacturing instruction●生产控制 PC=production control●质量保证 QA=quality assurance●质量控制 QC=quality control●统计过程管控 SPC=statistical process control●十亿分之一 PPB=part per billion●百万分之一 PPM=part per million●尽快 ASAP=as soon as possible●供参考 FYI=for your information●美国线规 AWG=American wire gauge(e.g. AWG23,意思就是23号线)●平均线规 AWG=Average wire gauge●部门 Dept.=department●有 W/=with●没有 W/O=without**瓦楞纸箱 corrugated carton/slotted carton20。
Lab reliability 可靠性测试

Purpose of HTST
高温仓测试的目的
To know the durability of Semiconductor package when exposed under the high temperature for long time. 知道半导体器件长期暴露在高温下的能力
16
知道目前EMC和L/F之间的差别
24
Pressure Cooker Test
知道焊接后半导体元件的可使用性
It simulates delivery from assembly house to customers plant and soldering on PCB.
模拟从封装厂到客户处的运输及在线路板上的焊 接
3
Procedure of Precon Test 预处理的程序
10
Temperature Cycle Test
温度周期测试
150 C Air
-65 C Air
T/C Chamber
温度周期变化测试箱
Test Conditions 测试条件 1) Temp : +150 / -65 deg.C
温度: +150/-65摄氏度 2) Time : 15 min/ zone
DELAMINATION CHIP CRACK
PKG CRACK
PKG CRACK
6
Defects after Precon Test
预处理后的失效
Die Top
Delamination
芯片顶部的分层
Die
Crack
芯片裂缝
Package Crack
封装面裂缝
7
II. Reliability Test 可靠性测试
WHO第961号技术报告附录9:时间和温度敏感的药品的贮运指南技术补充之温控存贮区的确...

WHO第961号技术报告附录9:时间和温度敏感的药品的贮运指南技术补充之温控存贮区的确...Qualification of temperature-controlled storage areasTechnical supplement toWHO Technical Report Series, No. 961, 2011Annex 9: Model guidance for the storage and transport of time andtemperature–sensitive pharmaceutical productsAugust 2014WHO第961号技术报告附录9:时间和温度敏感的药品的贮运指南技术补充之温控存贮区的确认(小编按:有兴趣的会员朋友们可来信索取完整中英文PDF)World Health Organization 2014WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 7914857;e-mail:******************).Requestsforpermission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: *******************).The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or areaor of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the WorldHealth Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication.Acknowledgments 致谢(略)Abbreviations 缩写CAPA Corrective and Preventive Action纠正预防措施(procedures)EDLM Electronic Data Logging Monitor 电子数据记录监测仪IATA International Air Transport Authority 国际空运局IQ Installation Qualification 安装确认OQ Operational Qualification 运行确认PDA Parenteral Drug Association 注射剂协会PQ Performance Qualification 性能确认SMS Short Message Service 短信服务SOP Standard Operating Procedure 标准操作规程TTSPP Time and Temperature-Sensitive时间和温度敏感的药品Pharmaceutical ProductUPS Uninterrupted Power Supply 不间断电源Glossary 术语(略)1. Introduction 概述This technical supplement has been written to amplify the recommendations given in clause 4.7 of WHO Technical Report Series No. 961, 2011, Annex 9: Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products[4]. It covers the three stages of qualification needed to release a temperature-controlled storage area for routine use: installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ). Related topics are covered in the following Technical Supplements:本技术补充文件是为了细化WHO第961号技术报告附录9时间和温度敏感的药品的贮运指南技术补充第4.7条里给出的建议。
不同药典温度描述

CP 中国药典阴凉处系指不超过20°C;凉暗处系指避光并不超过20'C;冷处系指2~10'C;常温系指10~30'C。
除另有规定外,贮藏项下未规定贮藏温度的一般系指常温。
EP欧洲药典Temperature. Where an analytical procedure describes temperature without a figure, the general terms used have the following meaning:温度:如分析程序中所规定的温度无具体描述,通常使用的术语的含义如下所示:–in a deep-freeze: below − 15 °C;低温冷冻:− 15°C以下–in a refrigerator: 2 °C to 8 °C;冷藏柜中2-8°C–cold or cool: 8 °C to 15 °C;冷却:8-15°C–room temperature: 15 °C to 25 °C.室温:15 °C to 25 °CUSP美国药典10.30. Storage Temperature and Humidity10.30.贮藏的温度和湿度Specific directions are stated in some monographs with respect to the temperatures and humidity at which official articles shall be stored and distributed (including the shipment of articles to the consumer) when stability data indicate that storage and distribution at a lower or a higher temperature and a higher humidity produce undesirable results. Such directions apply except where the label on an article states a different storage temperature on the basis of stability studies of that particular formulation. Where no specific storage directions or limitations are provided in the individual monograph, but the label of an article states a storage temperature that is based on stability studies of that particular formulation, such labeled storage directions apply.(See also Pharmaceutical Stability 〈1150〉.) The conditions are defined by the following terms.当稳定性数据表明药品在一个较低或较高的温度和高湿度的条件下贮藏与分发会造成不良的后果,在一些各论中对于药典药品的贮藏和分发(包括发到客户的装运)的温度和湿度有具体说明。
4x4轻触规格书

1.General specification 基本说明1.1Scope 范围This specification covers the requirements for single key switches which have no keytop(TACT SWITCHES:MECHANICAL CONTACT ). 此规范含盖单推柄和无推柄的轻触开关要求。
11.2Operating Temperature Range 使用温度范围-20 to 70 ℃(normal humidity, normal press).正常湿度,标准压力1.3Storage Temperature Rang 保存温度范围:-20 to 80 ℃(normal humidity, normal press).1.4Test Conditions 测试条件Tests and measurements shall be made in the following standard clnditions unless otherwise specified:测试和计量按下列标准条件除非特殊说明Normal temperature (temperature 5 to 35℃) 标准温度Normal humidity (relative humidity 45 to 85%) 正常湿气Normal pressure (pressure860 to 1060 mbars ) 标准压力In case any question arises from the judgement made, tests shall be conducted in the following conditions:Temperature (20±2℃) 温度Relative humidity (65±5%) 相对湿度Pressure (860 to 1060 mbars) 压力2. APPEARNCE, STYLE, AND DIMENSIONS 外形,类型和尺寸2.1Appearance 外形There shall be no defects that affect the serviceability of the product 外形必须无缺陷才不影响产品适用性2.2 Style and Dimensions 类型和尺寸Shall conform to the assembly drawings. 符合装配图3. TYPE OF ACTUATION 动作类型Tactile feedback 轻触返回4. CONTACT ARRANGEMENT 1 poles 1 throws 接触形式1接点1 回路(Details of contact arrangement are given in the assembly drawings).细接点形式在装配图中5.MAXOMUM RA TINGS最大额定值DC 12 V 50 mA2/9 TACT SWITCH SPECIFICATION( 轻 触 开 关 说 明 书 )6.2Mechanical 机械特性Item项目Test Condition试验条件Requirements规格6.2.1. Actuating Force 动作力 Placing the switch such that the direction of switch operationis vertical and then gradually increasing the load applied to thecenter of the stem, the maximum load required for the stem tocome to a stop shall be measured.开关的动作方向为垂直放置开关向推柄中心逐渐增加负荷直到推柄停止时所测量的最大负荷260±30 gf6.2.2.Travel 行程Switch from free position to push the handlemoves to exert force to stop the high-margin开关从自由位置到推柄施加动作力后停止的高差值0.25±0 .1 mm6.2.3. Return Force 返回力The sample switch is installed such that the direction of switchoperation is vertical and, upon depression of the stem in itscenter the whole travel distance ,the force of the stem to returnto its free position shall be measured.开关的动作方向垂直放置开关,在已有行程的推柄中心向上减小压力,推柄回到自由位置时所测量到的力70±30gf min6.2.4.Stop Strength 静止强度Placing the switch such that the direction of switch operationis vertical, a static load of3 kgf shall be applied in thedirection of stem operation for a period of 60 seconds.开关的动作方向为垂直放置开关,在推柄动作方向施加 3 KG的静负荷,60秒时间There shall be no signof damagemechanically andelectrically无机械的和电气的损伤迹象6.2.5.Stem Strength 推柄的强度Placing the switch such that the direction of switch operationis vertical, the maximum force to withstand a pull appliedoppsite to the direction of stem operation shall be measured.开关的动作方向为垂直放置开关从推柄动作方向反方向施加拉力所测量到的最大承受力3 kgf4/9TACT SWITCH SPECIFICATION( 轻 触 开 关 说 明 书 )TACTING SWITCH SPECIFICATION7.1 Other precautions 其他注意事项(1)Following the soldering process,do not try to clean the switch with a solvent or thelike .进行焊接过程中,不可以用溶剂或类似品清洗开关(2)Safeguard the switch assembly against flux penetration from its topside.防止助焊剂从开关的顶端渗入。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1.1 STORAGE TEMPERATURE AND HUMIDITY TEST
1.1.1 Test Owner
Mark Tracy mark.tracy@
1.1.2 Purpose
The purpose of this test is to ensure there are no adverse electrical, mechanical, or cosmetic effects due to sustained storage and
shipping temperature extremes.
1.1.3 Test Philosophy
To determine the system’s resistance to sustained temperature
extremes using an environmental test chamber such as a
Thermotron to simulate non-climate controlled storage.
1.1.4 Specification
2 systems - Configuration determined by the HP Mechanical
Engineer per the storage and humidity profile graph below
Duration of Test: 168 hours (7 days)
1.1.5 Test Procedure
⏹Pre-testing of the units shall test for electrical and
mechanical functionality, and cosmetic appearance
⏹Run Meatgrinder or equivalent factory type diagnostics for a
period of 2 hours on the systems before and after testing
⏹The flatness of the system to a tabletop shall be measured
before and after testing
⏹The units under test shall be not be operational during the
test
⏹The LCD Panel shall be closed for the duration of the test
1.1.6 Test Parameters
⏹-20° C (-4° F) to +65° C (149°F)
⏹Relative Humidity range between 0 and 95%
⏹Information is for a single 24 hr cycle
⏹Standard test is 7 cycles = 168 hours
⏹All temperatures are Dry Bulb values
1.1.7 Pass/Fail Criteria
⏹Pass–
☐The unit shall power up and function as designed.
☐No errors observed running Meatgrinder or equivalent diagnostics.
☐The LCD panel shall not be effected in anyway
☐All buttons, latches, display hinges, doors, or mechanical mechanisms shall function as designed.
☐The cosmetic appearance of the system shall not change such as color, icons, or labels
☐The system flatness shall not change and the notebook shall not wobble or warp when placed on a flat surface such as a
table
☐All cosmetic gaps and parting lines shall not exceed 10% of the original value prior to the test
⏹Fail–
☐Any system assembly or functional anomalies or functional errors.
☐Any deviation from the above Pass criteria.。
