Marbofloxacin_SDS_MedChemExpress
雷帕霉素-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RapamycinCatalog No. :HY-10219CAS No. :53123-88-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Flammable liquids (Category 4), H2272.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word WarningHazard statement(s)H227 Combustible liquid.Precautionary statement(s)P210 Keep away from heat ⁄sparks ⁄open flames ⁄hot surfaces. - No smoking.P280 Wear protective gloves ⁄ protective clothing ⁄ eye protection ⁄ face protection.P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.P403 + P235 Store in a well-ventilated place. Keep cool.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SirolimusFormula:C51H79NO13Molecular Weight:914.17CAS No. :53123-88-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 years* The compound is unstable in solutions, freshly prepared is recommended.Shipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
莫博赛替尼化学结构

莫博赛替尼化学结构
莫博赛替尼是一种抗癌药物,其化学名称为N-(4-((3-(2-amino-4-pyrimidinyl)-2-pyridinyl)oxy)phenyl)-4-(4-methyl-2-thiazolyl)-2-pyrimidinamine。
其化学结构可以描述为一种含有嘧啶和吡啶环的有机化合物。
具体来说,它包括一个苯基环和两个嘧啶环,其中一个嘧啶环连接着一个吡啶环。
此外,它还含有一个氨基和一个硫代咪唑基团。
这些结构特征赋予了莫博赛替尼其特定的生物活性和抗癌作用。
从结构角度来看,莫博赛替尼的苯基环和嘧啶环可能与靶标蛋白发生相互作用,从而影响癌细胞的生长和增殖。
其嘧啶环的存在也表明了其可能与DNA或RNA发生相互作用,从而影响癌细胞的遗传物质合成和复制。
此外,硫代咪唑基团的存在可能赋予了莫博赛替尼一些特殊的化学性质,使其在生物体内表现出特定的药理学效应。
总的来说,莫博赛替尼的化学结构为其在抗癌治疗中的作用提供了重要的基础,对于研究其药理学效应和与靶标蛋白的相互作用具有重要意义。
通过深入了解其化学结构,可以更好地理解莫博赛替尼在抗癌治疗中的作用机制,为其临床应用提供理论支持。
莫博赛替尼化学结构

莫博赛替尼化学结构全文共四篇示例,供读者参考第一篇示例:莫博赛替尼(Imatinib,商品名格列卫)是一种靶向治疗白血病的药物,也被用于治疗一些其他类型的癌症。
它是通过靶向特定的蛋白质,从而阻断癌细胞的生长和扩散。
莫博赛替尼的化学结构是一种非常复杂的有机分子,其分子式为C29H31N7O,分子量为493.604克/摩尔。
莫博赛替尼的化学结构包含一个核心环结构,由苯环和嘧啶环连接而成。
苯环是由六个碳原子和六个氢原子组成的环状结构,而嘧啶环是由一个氮原子和四个碳原子组成的环状结构。
这两个环通过一个氮原子相连,形成了一种特殊的结构,这种结构对于莫博赛替尼的药效非常重要。
莫博赛替尼在体内主要靶向作用于一种叫做BCR-ABL融合蛋白的蛋白质。
这种融合蛋白质是一种异常的蛋白质,由Bcr(一种信号传导蛋白)和Abl(一种酪氨酸激酶)两种蛋白质的部分结构融合而成。
这种异常蛋白质的产生是由于某些白血病细胞的染色体发生了特定的基因突变,导致Bcr和Abl两种基因相互融合。
而莫博赛替尼正是针对这种异常的蛋白质进行靶向治疗的。
莫博赛替尼的化学结构设计和药效机制是经过长期的研究和开发的。
科学家们通过不断的实验和研究,逐渐了解了白血病细胞的基因突变以及融合蛋白质的结构和功能特点。
通过对这些信息的深入研究,他们最终设计出了莫博赛替尼这种能够精准干预癌细胞生长的药物。
莫博赛替尼在临床应用中被证实具有显著的疗效,尤其是对于慢性髓细胞白血病(CML)和胃肠间质瘤(GIST)等罕见白血病和恶性肿瘤的治疗效果非常显著。
通过连续服用莫博赛替尼,患者的白血细胞数量可以得到控制,进而延缓疾病的进展并提高生存率。
莫博赛替尼的治疗效果和安全性已经得到了许多临床试验和长期随访的验证,被广泛应用于临床治疗。
莫博赛替尼是一种非常重要的抗癌药物,通过靶向作用于异常蛋白质来抑制癌细胞的生长和扩散。
其化学结构复杂且精确,对于白血病和其他类型的癌症具有显著的治疗效果。
佐匹克隆快速检验

佐匹克隆快速检验赵海雨;阚旭升【摘要】利用电喷雾液质联用法快速检验血中佐匹克隆。
少量血液样品采用乙腈沉淀蛋白后直接取上清液进样,使用 Allure PFP Propyl(5μm,100mm×2.1mm)柱,流动相:乙腈:10mmol/L 乙酸铵-0.1%甲酸缓冲液=70:30进行分离,经电喷雾离子源正离子化后通过三重四级杆质谱多反应监测(MRM)对佐匹克隆(m/z 389.3/345.4和 m/z 389.3/245.2)进行定性分析。
被害人血中检出佐匹克隆成分。
液质联用法检验血中佐匹克隆快速、准确、简单,可用于案件中检测血中佐匹克隆成分。
%Zopiclone, one kind of new generation of sedative-hypnotic of the cyclopyrrolone compounds, has different chemical structures and different pharmacological function from that of the first and second generations of the hypnagogue like barbitals and benzodiazepines. Recently, in a robbery case, the suspects utilized the zopiclone to anesthetize the victims. In our laboratory, the blood sample of the victim was treated with acetonitrile to precipitate the proteins and the supernatant was separated through an Allure PFP Propyl column (5μm,100mm×2.1mm) with the mobile phase containing acetonitrile (diluted to 70% by the buffer of 10mmol/L ammonium acetate mixed with 0.1% formic acid). The AB Sciex 4000Qtrap mass spectrometer equipped with an electro-spraying ionization source was used to detect two ion combinations of m/z 389.3/345.4 and m/z 389.3/245.2 by multiple reaction monitoring (MRM) for qualitative analysis of zopiclone. Finally, zopiclone was detected in the blood of the victims.【期刊名称】《刑事技术》【年(卷),期】2015(000)002【总页数】2页(P164-165)【关键词】法医毒物分析;LC/MS/MS;快速检验;佐匹克隆【作者】赵海雨;阚旭升【作者单位】辽宁省刑事科学技术研究所,沈阳 110032;辽宁省刑事科学技术研究所,沈阳 110032【正文语种】中文【中图分类】DF794.1DOΙ: 10.16467/j.1008-3650.2015.02.021佐匹克隆(zopiclone)是新一代环吡咯酮类镇静催眠药,纯品佐匹克隆是白色至淡黄色结晶或结晶性粉末,无臭,味苦,易溶于二甲亚砜或氯仿,较易溶于醋酸,较难溶于甲醇、丙酮或乙腈,极难溶于乙醚或异丙醇,几乎不溶于水,熔点178℃。
玛巴洛沙韦片说明书
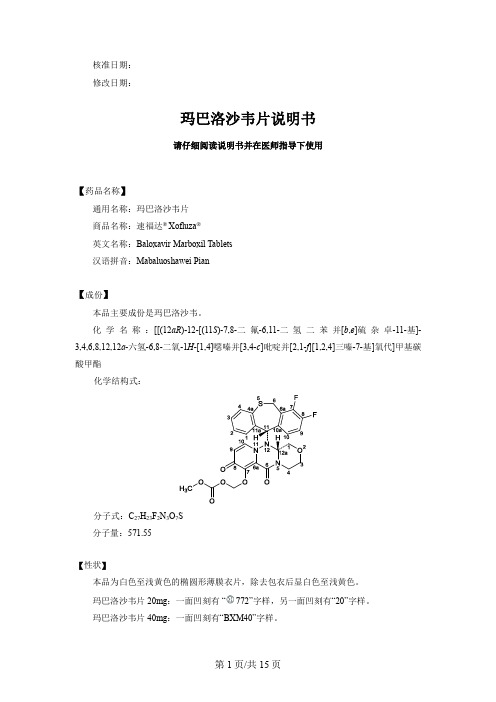
核准日期:修改日期:玛巴洛沙韦片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:玛巴洛沙韦片商品名称:速福达® Xofluza®英文名称:Baloxavir Marboxil Tablets汉语拼音:Mabaluoshawei Pian【成份】本品主要成份是玛巴洛沙韦。
化学名称:[[(12aR)-12-[(11S)-7,8-二氟-6,11-二氢二苯并[b,e]硫杂卓-11-基]-3,4,6,8,12,12a-六氢-6,8-二氧-1H-[1,4]噁嗪并[3,4-c]吡啶并[2,1-f][1,2,4]三嗪-7-基]氧代]甲基碳酸甲酯化学结构式:分子式:C27H23F2N3O7S分子量:571.55【性状】本品为白色至浅黄色的椭圆形薄膜衣片,除去包衣后显白色至浅黄色。
玛巴洛沙韦片20mg:一面凹刻有 “772”字样,另一面凹刻有“20”字样。
玛巴洛沙韦片40mg:一面凹刻有“BXM40”字样。
【适应症】本品适用于12周岁及以上单纯性甲型和乙型流感患者,包括既往健康的患者以及存在流感并发症高风险的患者。
【规格】(1) 20mg;(2)40mg【用法用量】在症状出现后48小时内单次服用本品,可与或不与食物同服(参见【药代动力学】)。
应避免本品与乳制品、钙强化饮料、含高价阳离子的泻药、抗酸药或口服补充剂(如,钙、铁、镁、硒或锌)同时服用。
本品适用于成人和青少年(≥12岁),基于体重的给药方案如表1所示:表1 基于体重的给药方案剂量调整:不建议降低本品的剂量。
肾功能损害尚未在肾功能损害患者中研究本品的安全性与有效性。
在肌酐清除率(CrCl)≥50 mL/min的患者中,群体药代动力学分析未发现肾功能对巴洛沙韦的药代动力学产生有临床意义的影响。
尚未评价重度肾损害对玛巴洛沙韦或其活性代谢物巴洛沙韦的药代动力学的影响。
肝功能损害无需调整轻度(Child-Pugh A级)至中度(Child-Pugh B级)肝功能损害患者的用药剂量(参见【药代动力学】)。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
恩美曲妥单抗化学式

恩美曲妥单抗化学式
恩美曲妥单抗化学式:C43H58N10O13
恩美曲妥单抗(Trastuzumab)的化学式为C43H58N10O13,它是一种人源化的IgG1κ单克隆抗体,用于治疗HER2阳性的乳腺癌和胃癌等恶性肿瘤。
恩美曲妥单抗是一种单克隆抗体药物,由人类IgG1κ Fc 区域和人源化的单克隆抗体结合片段组成。
它的化学式为C43H58N10O13,分子量为约1100 kDa。
恩美曲妥单抗的结构中包含了两个重链和两个轻链,每个链都由多个氨基酸组成。
恩美曲妥单抗是一种人源化的单克隆抗体药物,它可以与HER2受体结合,抑制HER2受体的信号转导,从而减少癌细胞的增殖和转移。
恩美曲妥单抗主要用于治疗HER2阳性的乳腺癌和胃癌等恶性肿瘤。
恶性胸膜间皮瘤靶向治疗的研究进展

恶性胸膜间皮瘤靶向治疗的研究进展傅芬;张扬;沈红【期刊名称】《中国肺癌杂志》【年(卷),期】2024(27)5【摘要】恶性胸膜间皮瘤(malignant pleural mesothelioma, MPM)是侵袭性极强的罕见胸膜表面恶性肿瘤,危险因素包括吸入石棉、遗传因素、基因突变等。
现有的化疗、抗血管生成治疗、免疫治疗的效果均不佳,患者的生存期极短。
亟需寻找治疗MPM的潜在靶点,目前发现有基因突变靶点如BRCA1相关蛋白1(BRCA associated protein1, BAP1)和细胞周期蛋白依赖性激酶抑制剂2A(cyclin-dependent kinase 2A, CDKN2A)等;表观遗传靶点如组蛋白赖氨酸去甲基酶4A[lysine (K)-specific demethylase 4A, KDM4A]和赖氨酸特异性去甲基酶1(lysine-specific demethylase1, LSD1)等;信号蛋白靶点如葡萄糖调节蛋白78(glucose-regulated protein 78, GRP78)及信号转导和转录激活因子3(signal transducer and activator of transcription 3, STAT3)等。
迄今为止,可查询的临床试验有组蛋白甲基转移酶抑制剂Tazemetostat、多聚ADP-核糖聚合酶[poly (ADP-ribose) polymerase, PARP]抑制剂Rucaparib和细胞周期蛋白依赖性激酶4/6(cyclin-dependent kinases 4 and 6, CDK4/6)抑制剂Abemaciclib的II期临床试验,以及靶向间皮素的嵌合抗原受体T细胞免疫疗法(chimeric antigen receptor T-cell immunotherapy, CAR-T)细胞胸腔注射、TEA结构域家族成员(TEA domain family member, TEAD)抑制剂VT3989和IK-930的I期临床试验,显示出一定的临床疗效。
玛巴洛沙韦 结构式

玛巴洛沙韦结构式全文共四篇示例,供读者参考第一篇示例:玛巴洛沙韦(Mavlabosavir,又称Pimodivir)是一种广谱的抗流感病毒药物,属于口服的新型RNA干扰剂。
其化学结构式为C23H26FN7O2,分子量为449.499 g/mol。
玛巴洛沙韦如今已被证实对包括甲型和乙型流感病毒在内的多种流感病毒具有很强的抑制作用。
它采用一种独特的机制来阻断病毒的生长和复制,从而有效地减轻病毒感染的症状。
玛巴洛沙韦的化学结构体现了其抗流感病毒的作用机制。
该药物是一种强效的非核苷类反转录酶抑制剂,通过干扰流感病毒的RNA复制而达到抑制病毒生长的效果。
其结构中包含有一个异氮杂环和多个氟原子,这些特殊的结构单元赋予了玛巴洛沙韦出色的抗病毒活性。
从结构上看,玛巴洛沙韦的核心结构是一个含氮杂环的环戊烯酮(cyclopentenone),在环戊烯的位置上连接有一个含氟的芳香族环。
这种结构形式为玛巴洛沙韦提供了非常强的疏水性,使其能够在病毒颗粒内部精准地与反转录酶结合,进而阻断病毒的复制与传播。
在化学结构中还包含有一个异噻唑环和多个取代基。
异噻唑环是一种含硫杂环结构,能够增强分子的稳定性和生物活性。
这些取代基对分子的生物活性及亲合力也起到了关键的作用,通过特定的相互作用与病毒RNA或蛋白结合,最终实现抗病毒作用。
玛巴洛沙韦的化学结构具有独特的设计,使其在与流感病毒的特定靶标相互作用时能够表现出卓越的活性。
通过对病毒复制过程的干扰,玛巴洛沙韦有效地抑制了病毒的生长,为治疗流感提供了一种新的选择。
随着对其药效和安全性的进一步研究,玛巴洛沙韦有望成为未来治疗流感的重要药物之一。
【参考资料来源于维基百科等网站】。
第二篇示例:玛巴洛沙韦(Mavrolashavi)是一种抗病毒药物,广泛用于治疗各种病毒性疾病。
它主要用于治疗艾滋病毒感染和流感等病毒感染。
玛巴洛沙韦的结构式为C44H46F2N6O8,化学式为C44H46F2N6O8,分子量为810.871。
甲磺酸瑞波西汀对照标化记录

名称
配制批号
氨试液
酚酞指示液
醋酸盐缓冲液(pH3.5)
标准铅贮备液
硫代乙酰胺试液
标准铅溶液取用量
ml
试验结果
乙管中显出的颜色甲管颜色
结论
符合规定()不符合规定()
检验人:检验日期:
对照品标化记录(5/5)
品名
甲磺酸瑞波西汀
批号
4.色谱纯度(附液相图谱)
仪器型号及编号
高效液相色谱仪,型号:编号:
对照品标化记录(1/5)
产品名称
甲磺酸瑞波西汀
批号
规格
收样日期
数量
检验日期
检品来源
有效期至
检验依据
国家食品药品监督管理局标准(试行)YBH02392008
甲磺酸瑞波西汀对照品质量标准
一、性状:
1.本品为_____________________。【应为白色或类白色结晶性粉末】
检验人:检验日期:
2.熔点
(应>1.5)
面积%=(应≥99.5%)
结论
符合规定()不符合规定()
检验人:检验日期:
复核人
复核日期
品名
甲磺酸瑞波西汀
批号
3.红外鉴别:取本品及甲磺酸瑞波西汀对照品适量,分别按SOP-QTY 004《红外分光光度法》测定。【红外光谱(KBr压片法)应与对照品谱图一致】(附红外图谱)
甲磺酸瑞波西汀对照品来源:批号:含量:
仪器型号及编号
傅立叶变换红外光谱仪,型号:编号:
电热鼓风干燥箱,型号:编号:
试验温度、湿度
品名
甲磺酸瑞波西汀
批号
二、鉴别:
1.理化鉴别
仪器型号及编号
硫培非格司亭注射液详细说明书与重点

硫培非格司亭注射液【药品名称】英文名称: Mecapegfilgrastim Injection【成分】活性成份:硫培非格司亭(由重组人粒细胞刺激因子与20 kD的聚乙二醇交联反应并经纯化得到);分子量39 kD。
辅料:醋酸钠、醋酸、聚山梨酯80、山梨醇等。
【性状】无色澄明液体。
【适应症】本品适用于成年非髓性恶性肿瘤患者在接受容易引起发热性中性粒细胞减少症的骨髓抑制性抗癌药物治疗时,降低以发热性中性粒细胞减少症为表现的感染发生率。
本品不用于造血干细胞移植的外周血祖细胞的动员。
【规格】0.6ml:6mg【用法用量】本品在每个化疗周期抗肿瘤药物给药结束后48小时皮下注射1次。
推荐使用剂量为一次注射固定剂量6 mg。
本品也可按患者体重,以100 μg/kg进行个体化治疗。
请勿在使用细胞毒性化疗药物前14天到化疗后24小时内给予本品。
注射前,应当检查本品溶液是否澄清透明,如果有悬浮物质产生或变色,不得继续使用。
【不良反应】以下几项严重不良反应见【注意事项】:脾破裂、急性呼吸窘迫综合征、严重变态反应、镰状细胞危象、肾小球肾炎、白细胞增多症、毛细血管漏综合征和对肿瘤恶性细胞生长的潜在刺激效应。
根据本品临床试验结果,本品主要不良反应如下:1.肌肉骨骼系统:疼痛较常见,主要是肌肉关节或全身疼痛等,严重程度多为轻度,多数可自行缓解;2.消化系统:恶心、呕吐、腹部不适、食欲差等;3.实验室检查:可见到丙氨酸氨基转移酶、门冬氨酸氨基转移酶升高4.其他一般症状:偶见乏力、头部不适、发热等;5.过敏反应:本品在临床试验中未观察到对本品过敏病例,但基于目前对生物制剂的科学认识,治疗性蛋白质大分子药品均有引起过敏反应的潜在风险。
6.免疫原性:与所有治疗性蛋白一样,聚乙二醇重组人粒细胞刺激因子(PEG-rhG-CSF)具有潜在的免疫原性。
本品临床试验中抗药抗体发生率为0.56%。
本品在临床试验中试验组发生率>2%的不良反应信息见【临床试验】部分。
中药剂学:ma秋水仙碱

中药剂学:ma秋水仙碱中药剂学:ma秋水仙碱[中文名称] ma秋水仙碱[英文名称] Colchicine[别名] 无[化学名称] Acetamide,N-(5,6,7,9-tetrahydro-1,2,3,10-tetrametho_y-9-o_obenzo [α]heptalen-7-yl)-, (S)-[分子式] C22H25NO6;[分子量] 399.43[物理性质] 苍黄色鳞状结晶或粉末, 熔点142~150__176;,暴光变暗。
苍黄色针状结晶(醋酸乙酯), 熔点157__176;。
[α]27D-427__176;(c=1.27),[α]27D-121__176;,(C=0.9,氯仿)。
1g溶于20ml 水、220ml乙醚及100ml苯。
易溶于乙醇或氯仿。
几乎不溶于石油醚。
IRνma_ cm-1:3450,3230,2940,1670,1610,1590,1560,1490,1470,1390,1350,1320,1280,1250,1190, 1180,1140, 1090,1040,1020,990,920,900,850;UVλMeOHma_nm:244;NMR(CDCI3)δ:2.0,2.4,3.7,4.6,6.6,6.9,7.4[1]。
[成分分类] 生物碱类[药理作用] 本品是细胞有丝分裂毒的一个典型代表, 能抑制癌细胞的增长。
临床用以治疗癌症, 特别对乳腺癌有一定疗效, 对皮肤癌、白血病和何杰金氏病等亦有一定作用。
对痛风急性发作有特异性作用, 12~24小时内减轻炎症状并迅速止痛。
长期应用可减少发作次数。
还有雌激素样活性, 能延长大鼠动情期和动情后期, 而缩短间情期和动情前期[11]。
毒性较大, 能引起恶心、食欲减退、腹胀, 严重者出现肠麻痹和便秘, 四肢酸痛较为多见。
尚可引起白细胞和血小板减少, 但严重骨髓抑制较少见。
[毒性][不良反应][用途] 抗肿瘤, 毒性[成分来源] 百合科植物秋水仙 Colchicum autumnale L., 百合 Lilium brownii F. E. Brown var. colchesteri Wils. 鳞茎[2], 老鸦瓣 Tulipa cdulis Bak.[标签:内容]。
富马酸奥赛利定 化学式

富马酸奥赛利定化学式
富马酸奥赛利定,化学式C18H23NO3,是一种常用的非处方药物。
它属于非甾体抗炎药(NSAIDs)的一类,广泛用于缓解疼痛和减轻炎症。
富马酸奥赛利定是一种白色结晶性粉末,溶于水和乙醇。
它的化学结构中含有苯环和吡咯环,这些结构赋予了它抗炎和镇痛的特性。
富马酸奥赛利定主要通过抑制前列腺素的合成来发挥其药理作用。
前列腺素是一种导致炎症和疼痛的化学物质,而富马酸奥赛利定能够阻断前列腺素的产生,从而减轻炎症和疼痛症状。
富马酸奥赛利定常用于缓解关节炎、肌肉疼痛、头痛和牙痛等症状。
它可以通过口服或外用的方式进行使用,具有快速和持久的镇痛效果。
然而,使用富马酸奥赛利定也需要注意一些事项。
长期或过量使用可能会导致胃肠道问题,如胃溃疡和出血。
因此,在使用富马酸奥赛利定时,应按照医生的建议使用适当剂量,并避免与其他药物同时使用,尤其是其他NSAIDs类药物。
富马酸奥赛利定是一种常用的非处方药物,具有抗炎和镇痛的作用。
它通过抑制前列腺素的合成来缓解炎症和疼痛症状。
然而,使用时需要注意剂量和与其他药物的相互作用,以避免不良反应的发生。
高效液相色谱法测定阿立哌唑片剂的含量

高效液相色谱法测定阿立哌唑片剂的含量
程颖;濮存海;吴爱兰;王波
【期刊名称】《药学实践杂志》
【年(卷),期】2005(23)5
【摘要】目的:采用高效液相法测定阿立哌唑片中阿立哌唑的含量.方法:用Beckman C18柱,以磷酸水溶液(0.1mL磷酸,加水500mL,加三乙胺调节pH至3.0,摇匀)-乙腈(46:54)为流动相,用紫外检测器于254nm波长处检测.结果:线性范围为2.088μg/mL~66.816μg/mL(r=0.999 99).平均回收率为99.39%.结论:本法具有操作简便,分析快速准确、干扰小等优点.
【总页数】3页(P297-299)
【作者】程颖;濮存海;吴爱兰;王波
【作者单位】江苏省药品检验所,江苏,南京,210008;南京中山制药厂,江苏,南
京,210012;南京中山制药厂,江苏,南京,210012;南京中山制药厂,江苏,南京,210012【正文语种】中文
【中图分类】R917
【相关文献】
1.高效液相色谱法测定硝酸异山梨酯及其片剂的含量与含量均匀度 [J], 张秀玲
2.反相高效液相色谱法测定神经酸片剂中神经酸含量 [J], 吴乐艳;侯雯清;孙孔春;杨璨瑜;舒纹;沈报春
3.反相高效液相色谱法测定神经酸片剂中神经酸含量 [J], 吴乐艳;侯雯清;孙孔春;杨璨瑜;舒纹;沈报春
4.高效液相色谱法测定微胶囊化片剂型保健食品中VK2的含量 [J], 陈晓嘉;周芳梅;罗小宝;吴家玲;叶帆;冯志强
5.高效液相色谱法测定伊格列净片剂的含量 [J], 黄佳雯;梁佳威;颜亮;郑枫
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :MarbofloxacinCatalog No. :HY-B0126CAS No. :115550-35-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C17H19FN4O4Molecular Weight:362.36CAS No. :115550-35-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
