SB756050_HNMR_25435_MedChemExpress
美国FDA批准一种用于阿尔茨海默病早期诊断的检测仪

美国FDA批准一种用于阿尔茨海默病早期诊断的检测仪夏训明(编译)
【期刊名称】《广东药科大学学报》
【年(卷),期】2022(38)3
【摘要】美国FDA于2022年5月4日批准Fujirebio诊断公司(Fujirebio Diagnostics,Inc.)研发的一种名为“The Lumipulse G β-Amyloid Ratio(1-42/1-40)test”的体外诊断检测仪,用于检测与阿尔茨海默病(Alzheimer’s Disease)相关的淀粉样斑块(amyloid plaques),可有效改进阿尔茨海默病的早期诊断。
【总页数】1页(P48-48)
【作者】夏训明(编译)
【作者单位】不详
【正文语种】中文
【中图分类】R74
【相关文献】
1.美国FDA批准一种试剂用于辅助诊断白血病和淋巴瘤
2.美国FDA批准一种试剂用于辅助诊断白血病和淋巴瘤
3.美国FDA批准一种试剂用于检测急性淋巴细胞白血病及多发性骨髓瘤微小残留病
4.美国FDA批准一种检测试剂盒用于检测耐甲氧西林金黄色葡萄球菌(MRSA)
5.美国FDA批准Aduhelm(aducanumab)用于治疗阿尔茨海默病
因版权原因,仅展示原文概要,查看原文内容请购买。
Bedaquiline fumarate_845533-86-0_DataSheet_MedChemExpress
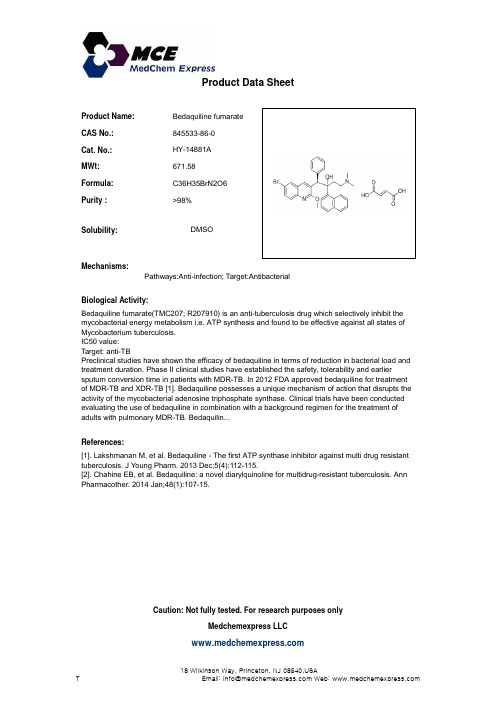
Product Name:Bedaquiline fumarate CAS No.:845533-86-0Cat. No.:HY-14881A Product Data SheetMWt:671.58Formula:C36H35BrN2O6Purity :>98%Solubility:DMSOMechanisms:Biological Activity:Bedaquiline fumarate(TMC207; R207910) is an anti-tuberculosis drug which selectively inhibit the Pathways:Anti-infection; Target:Antibacterial q (;)g y mycobacterial energy metabolism i.e. ATP synthesis and found to be effective against all states ofMycobacterium tuberculosis.IC50 value:Target: anti-TB Preclinical studies have shown the efficacy of bedaquiline in terms of reduction in bacterial load and treatment duration. Phase II clinical studies have established the safety, tolerability and earliersputum conversion time in patients with MDR-TB. In 2012 FDA approved bedaquiline for treatment of MDR-TB and XDR-TB [1]. Bedaquiline possesses a unique mechanism of action that disrupts the ti it f th b t i l d i t i h h t th Cli i l t i l h b d t d References:[1]. Lakshmanan M, et al. Bedaquiline - The first ATP synthase inhibitor against multi drug resistanttuberculosis. J Young Pharm. 2013 Dec;5(4):112-115.[2]Chahine EB et al Bedaquiline:a novel diarylquinoline for multidrug-resistant tuberculosis Ann activity of the mycobacterial adenosine triphosphate synthase. Clinical trials have been conducted evaluating the use of bedaquiline in combination with a background regimen for the treatment of adults with pulmonary MDR-TB. Bedaquilin...[2]. Chahine EB, et al. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. AnnPharmacother. 2014 Jan;48(1):107-15.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AT E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
光度法测定依达拉奉注射液中抗氧剂焦亚硫酸钠的含量

文章编号:1004-3918(2010)11-1403-02光度法测定依达拉奉注射液中抗氧剂焦亚硫酸钠的含量梁艳利,张慧丽,吴拥军(郑州大学化学系,郑州450001)摘要:为建立测定依达拉奉注射液中抗氧剂焦亚硫酸钠的质量控制方法,利用二氧化硫能使酸性品红溶液褪色的性质,将供试液在室温放置25min ,采用比色法在549nm 波长处测定吸光度,用标准曲线法测定含量.结果显示无水亚硫酸钠在4.4~26.4μg /mL 范围内,吸光度的倒数与浓度呈良好的线性关系,复相关系数R 2>0.99,平均加样回收率为94.9%,RSD 为3.0%.该法操作方便,结果准确可靠、可用于依达拉奉注射液中焦亚硫酸钠含量的测定.关键词:依达拉奉注射液;焦亚硫酸钠;比色法;含量测定中图分类号:O 657文献标识码:A依达拉奉(MC-971)是一种脑保护剂(自由基清除剂),其注射液中的抗氧剂焦亚硫酸钠氧化后的残留物亚硫酸盐可能会对人体造成损害,应对其加入量进行控制,以提高产品的安全性指标.亚硫酸盐在溶液中分解释放出的二氧化硫能使酸性品红溶液褪色,据此原理并参考复方氨基酸注射液(18AA-Ⅰ)[1]中焦亚硫酸钠的检查方法,建立了依达拉奉注射液中焦亚硫酸钠的含量测定方法.以前的相关质量标准中未对其进行质量控制,《中国药典》作为药用辅料收载了其含量测定方法[2];有关依达拉奉注射液中焦亚硫酸钠的比色法含量测定方法国内外未见报道,本实验建立的含量测定方法可对抗氧化剂的加入量进行质量控制,完善质量标准.1仪器与试剂722s 可见分光光度仪(上海精密科学仪器有限公司),电子天平AL104(梅特勒托利多仪器(上海)有限公司)),依达拉奉注射液(河南某药业有限公司,批号20090817),酸性品红(天津市科密欧化学试剂开发中心),无水亚硫酸钠(上海硫酸厂),乙二胺四乙酸二钠(EDTA)(中国医药(集团)上海化学试剂公司),冰乙酸(天津市恒兴化学试剂制造有限公司),无水乙酸钠(天津市风船化学试剂科技有限公司),所用试剂均为分析纯.2方法和结果2.1酸性品红溶液的制备精密称取酸性品红0.085g ,加硫酸0.25mL ,加水溶解定容到1000mL (7d 内使用).2.2醋酸盐缓冲液的制备称取醋酸钠136.1g ,EDTA 0.4g ,加入冰醋酸57mL ,加水溶解定容到1000mL .2.3亚硫酸钠对照溶液的制备精密称取无水亚硫酸钠0.110g ,用4×10-4g /mL 的EDTA 溶液溶解定容到250mL (临用新制).2.4标准曲线的建立精密量取酸性品红溶液5mL ,共6份,分别置50mL 量瓶中,各加缓冲液约40mL ,分别精密加对照品溶液0.5,1.0,1.5,2.0,2.5,3.0mL ;用缓冲液稀释至刻度,摇匀,室温放置25min ,以缓冲溶液做空白,在549nm 波长处测定吸光度.以亚硫酸钠的浓度为横坐标,吸光度的倒数为纵坐标,做标准曲线并进行线性回归,得到回归方程Y =9.7271X +0.7723,R 2=0.9987.多次试验的结果表明:亚硫酸钠在4.4~26.4μg /mL 范围内,吸收稿日期:2010-09-26作者简介:梁艳利(1986-),女,河南洛阳人,硕士研究生,主要研究方向为药物分析通信作者:吴拥军(1968-),男,河南郑州人,教授,博士,主要研究方向为药物分析.第28卷第11期2010年11月河南科学HENAN SCIENCE Vol.28No.11Nov.2010第28卷第11期河南科学光度的倒数与亚硫酸的浓度呈良好的线性关系.2.5重复性试验取同一批供试品,同法测定.分别测定6次亚硫酸钠含量,求得相对标准偏差(RSD )为2.2%.2.6加样回收率试验精密称取无水亚硫酸钠对照品0.0661,0.1109,0.1553g ,分别置于250mL 的容量瓶中,用质量分数为0.04%的EDTA 溶液溶解并稀释至刻度,摇匀,作为对照品溶液.精密量取酸性品红溶液5mL ,共9份,分别置50mL 容量瓶中,各加醋酸盐缓冲液约40mL ,依次分别精密加对照品溶液1.5mL 和已知含量的供试品溶液1.5mL ,并用醋酸盐缓冲液稀释至刻度,摇匀,照含量测定项下操作,求得平均回收率为94.9%,RSD 为3.0%(n =9).2.7焦亚硫酸钠的含量测定精密量取供试品溶液3mL ,同法测定,以对照品溶液吸光度的倒数与其浓度拟合标准曲线.根据标准曲线计算并将结果与0.754相乘,即得样品中焦亚硫酸钠的含量[1-3].此批次供试品的6次测定结果依次为:0.1849,0.1732,0.1822,0.1854,0.1804,0.1801mg /mL ;平均值为0.1810;RSD 2.3%.3讨论1)文献[4]的反应条件为28℃水浴15min ,吴小曼等[1]认为反应温度并不重要,关键是对照品溶液与供试品溶液同时平行操作即可.为便于操作,本研究将反应条件改为室温放置25min .2)文献[1]认为吸光度的对数与浓度的对数呈良好的线性关系,本实验经过比较后认为,按亚硫酸盐的浓度与吸光度的倒数拟合得到的标准曲线,其相关系数要优于双对数方式.3)文献[5]采用碱性品红-甲醛比色法测定亚硫酸盐,对照品为亚硫酸氢钠.考虑到对照品的稳定性影响,本文选用相对较稳定的无水亚硫酸钠作为对照品,其纯度可达97%以上.4)参阅的相关文献中,均采用缓冲溶液做比色法的空白,考虑到依达拉奉注射液处方中,其他物质对吸光度可能造成的影响,本研究对比了缓冲溶液空白和成相应比例的依达拉奉和缓冲溶液混合液的空白,结果相差不大,为简单起见,本文仍采用缓冲溶液做空白对照.参考文献:[1]吴小曼,纪宇.比色法测定复发氨基酸注射液(18AA-I )中焦亚硫酸钠的含量[J ].中国药品标准,2007,8(5):38-40.[2]中国药典委员会.中国药典:二部[S ].2005年版.北京:中国医药科技出版社,2005:914.[3]中国药典委员会.国家药品标准:新药转正标准(第27册)[S ].北京:人民卫生出版社,2003(7):102-103.[4]姚克荣,徐连连.影响复方氨基酸注射液中焦亚硫酸钠含量测定的因素[J ].中国生化药物杂志,1997,18(3):132-133.[5]谭仕轩.比色法测定复方氨基酸注射液中亚硫酸盐的含量[J ].氨基酸和生物资源,2005,27(3):63-65.Determination of Antioxygen Sodium Pyrosulfite in Edaravone Injectionby the Spectrophotometric MethodLiang Yanli ,Zhang Huili ,Wu Yongjun(Department of Chemistry ,Zhengzhou University ,Zhengzhou 450001,China )Abstract :To establish a spectrophotometric method for the assay of antioxidant sodium pyrosulfite in edaravone injection.Sulfur dioxide released from sodium pyrosulfite can fade fuchsin acid solution.The sample solution was determined at the wavelength of 549nm at room temperature for 25minutes.The amount of sodium pyrosulfite in sample was calculated by reference to a calibration curve obtained ,under the same condition ,for known amount of sodium sulfite.The results of experiments prove that s odium sulfite showed good linearity ,R 2was above 0.99,the linear range was from 4.4μg /mL to 26.4μg /mL.The average recovery was 94.4%with RSD 3.0%.The method is simple ,rapid and accurate ,suitable for the determination of sodium pyrosulfite in injection.Key words :edaravone injection ;sodium pyrosulfite ;colorimetric method ;determination 1404--。
气相色谱法测定那格列奈原药中二氯甲烷和吡啶残留量

气相色谱法测定那格列奈原药中二氯甲烷和吡啶残留量刘振莲【摘要】建立气相色谱法测定那格列奈中二氯甲烷和吡啶的残留量.采用6%氰丙基苯基-94%二甲基聚硅氧烷为固定液的毛细管柱,以氮气为载气,FID检测,直接进样,测定残留溶剂的含量.二氯甲烷质量浓度在0.75~36.0 μg/mL范围内线性关系良好,相关系数r=0.999 9,回收率为97.45%,最低检测限为0.24 μg/mL;吡啶质量浓度在0.27~12.30 μg/mL范围内线性关系良好,相关系数r=0.999 6,回收率为93.49%,最低检测限为0.08 μg/mL.【期刊名称】《淮海工学院学报(自然科学版)》【年(卷),期】2013(022)001【总页数】3页(P35-37)【关键词】那格列奈;二氯甲烷;吡啶;气相色谱法【作者】刘振莲【作者单位】江苏正大天晴药业股份有限公司连云港研究院,江苏连云港222062【正文语种】中文【中图分类】R9170 引言那格列奈即Nateglinide(商品名Starsis),化学名为 N-(反-4-异丙基环己羰基)-D-苯丙氨酸,是新一代具有氨基酸结构的降糖药物[1-3]。
其能有效控制餐时血糖,效果优于磺脉类降糖药,并且能降低低血糖的发生率。
据美国糖尿病学会会议上公布的临床资料显示,在用于Ⅱ型糖尿病治疗时,该药与同样具有刺激胰岛素分泌作用的其他产品相比,具有作用效果好、起效快、作用时间短和给药灵活等优点。
药品中的残留溶剂是指在合成原料药、辅料或制剂生产过程中使用或产生的挥发性有机化学物质,它们在实际生产中未能被完全清除。
近年来,药品中残存的有机溶剂的毒性和致癌作用日益引起各方面的重视[4-8]。
由于在那格列奈原药合成过程中使用了有机溶剂二氯甲烷和吡啶,因此在成品中可能会有一定量的残留。
按《中华人民共和国药典2005年版》(附录Ⅷ)规定,该2种有机溶剂均有残留限量,因此对那格列奈原药中二氯甲烷和吡啶的残留量检测具有一定意义。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
氟达拉滨杂质全套最新列表

中文名
磷酸氟达拉滨杂质1(磷酸氟 达拉滨EP杂质A)
CAS号 62314-92-5
用途
新药研发申报注册/ 鉴别、检查、含量测定
等 181+7164+1670
结构式
磷酸氟达拉滨杂质2(磷酸氟 达拉滨EP杂质B)
3373-53-3
新药研发申报注册/ 鉴别、检查、含量测定
等
磷酸氟达拉滨杂质3(磷酸氟 达拉滨EP杂质C)
7561-54-8
新药研发申报注册/ 鉴别、检查、含量测定
等
磷酸氟达拉滨杂质10(磷酸 氟达拉滨EP杂质J)
N/A
新药研发申报注册/ 鉴别、检查、含量测定
等
磷酸氟达拉滨杂质11 磷酸氟达拉滨杂质12 磷酸氟达拉滨杂质13 磷酸氟达拉滨杂质14 磷酸氟达拉滨杂质15 磷酸氟达拉滨杂质16 磷酸氟达拉滨杂质17 磷酸氟达拉滨杂质18 磷酸氟达拉滨杂质19 磷酸氟达拉滨杂质20 磷酸氟达拉滨杂质21 磷酸氟达拉滨杂质22
新药研发申报注册/ 鉴别、检查、含量测定
等
266360-74-1
新药研发申报注册/ 鉴别、检查、含量测定
等
新药研发申报注册/
N/A
鉴别、检查、含量测定
等
102783-36-8
新药研发申报注册/ 鉴别、检查、含量测定
等
新药研发申报注册/
N/A
鉴别、检查、含量测定
等
磷酸氟达拉滨杂质35
新药研发申报注册/ 2734853-80-4 鉴别、检查、含量测定
等
新药研发申报注册/
N/A
鉴别、检查、含量测定
等
新药研发申报注册/
N/A
鉴别、检查、含量测定
等
血管外膜细胞钙化及其钙化机制研究

血管外膜细胞钙化及其钙化机制研究谭小青,张旭升,樊小容,黄战军摘要 目的:研究经体外诱导钙化建立大鼠血管外膜细胞钙化模型,检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂方法:原代提取大鼠胸主动脉外膜纤维细胞,取3~6代细胞使用诱导培养基(高糖DMEM +10%胎牛血清+10mmol/L β-甘油磷酸+0.05mmol/L 抗坏血酸+100mmol/L 地塞米松)诱导钙化,诱导时间为3d ㊁6d ㊁9d ㊁12d ㊁15d ,筛选出诱导细胞钙化的最佳时间㊂对细胞采用茜素红S 染色㊁细胞内钙含量测定和碱性磷酸酶(ALP )活性检测,鉴定是否成功构建钙化模型㊂采用实时定量聚合酶链式反应(PT -PCR )检测成骨相关因子骨形态生成蛋白2(BMP2)和核心结合因子α1(Runx2)的mRNA 含量,蛋白免疫印迹法(Western Blot )检测凋亡蛋白Bax ㊁Bcl -2和自噬相关蛋白微血管相关蛋白(LC3)㊁Beclin -1的表达水平,找出血管外膜细胞钙化的潜在机制㊂结果:当诱导钙化时间为15d 时,血管外膜细胞中主要钙化指标胞内钙含量及ALP 活性上调(P <0.05),茜素红S 染色显示钙化组有明显钙盐沉积㊂血管外膜细胞经钙化诱导后,BMP2和Runx2的mRNA 水平上调,Bax 蛋白水平上调,Bcl -2和Beclin -1蛋白水平下调,LC3-Ⅱ/LC3-Ⅰ比值上调(P <0.05)㊂结论:钙化诱导培养基培养血管外膜细胞15d 可成功构建钙化细胞模型,血管外膜细胞钙化可能与细胞向成骨样表型转化有关,血管外膜细胞钙化过程涉及细胞自噬及凋亡调控㊂关键词 血管外膜细胞;钙化;成骨样表型转化;自噬与凋亡;实验研究d o i :10.12102/j.i s s n .1672-1349.2023.18.010 Calcification of Vascular Adventitial Cells and Its MechanismTAN Xiaoqing,ZHANG Xusheng,FAN Xiaorong,HUANG Zhanjun Longgang District People 's Hospital of Shenzhen,Shenzhen 518172,Guangdong,China Corresponding Author ZHANG Xusheng,E -mail:*****************Abstract Objective:To investigate the mananism of calcification of rat vascular adventitial cells,establish the calcification model of rat vascular adventitial cells,and detect the expression changes of osteogenesis -related indicators,apoptosis,and autophagy -related proteins during the calcification process.It aimed to provide more accurate cell models for cardiovascular disease and initially explore the mechanism of calcification.Methods:Rat thoracic aortic adventitial fibroblasts were extracted from the primary generation,and the 3rd to 6th generation cells were used for induction medium(high glucose DMEM +10%fetal bovine serum +10mmol/L β-glycerophosphate +0.05mmol/L ascorbic acid +100mmol/L dexamethasone)to induce calcification,the induction time was 3,6,9,12,and 15d,and the optimal time for inducing cell calcification was selected.The cells were stained with alizarin red S,detected by intracellular calcium content and alkaline phosphatase(ALP)to identify whether the calcification model was successfully constructed.Real -time quantitative reverse transcription polymerase chain reaction(RT -PCR)was used to detect the mRNA levels of osteogenesis -related factors bone morphogenetic protein(BMP2)and runt -related transcription factor 2(Runx2);Western Blot was used to detect the apoptosis proteins Bax,Bcl -2,the autophagy -related proteins LC -3,and Beclin -1expression level;then the potential mechanism of vascular adventitial cell calcification would be revealed.Results:When calcification was induced for 15days,the intracellular calcium content in the adventitial cells of the main calcification indicators and ALP activity were up -regulated(P <0.05).Alizarin red S staining showed obvious calcium deposits in the calcification group.After calcification was induced in adventitial cells,the mRNA levels of BMP2and Runx2up -regulated,the protein levels of Bax up -regulated,the protein levels of Bcl -2and Beclin -1down -regulated,and the ratio of LC3-Ⅱ/LC3-Ⅰdown -regulated(P <0.05).Conclusion:Adventitial cells cultured in the calcification -inducing medium for 15days could successfully construct a calcified cell model.calcification of adventitial cells might be related to the transformation of cells to an osteoblast -like phenotype.The Calcification process of adventitial cells involved autophagy and apoptosis regulation.Keywords adventitial cells;calcification;osteogenic phenotype transformation;autophagy and apoptosis;experimental study血管钙化常见于动脉粥样硬化㊁血脂异常㊁高血压㊁糖尿病㊁慢性肾病及衰老等人群[1],血管钙化引起血管硬度增加㊁顺应性降低,导致心肌缺血㊁心力衰竭㊁血栓形成等,增加脑卒中㊁心脏病㊁动脉粥样硬化斑块破裂等的风险,被认为是影响心血管疾病的重要因素之一[2-4]㊂目前关于血管内膜㊁中膜和心脏瓣膜钙化的关注和研究相对较多㊂临床工作中发现,血管外膜也可发生钙化,然而调查发现,现阶段对血管外膜钙化的作者单位 深圳市龙岗区人民医院(广东深圳518172)通讯作者 张旭升,E -mail :*****************引用信息 谭小青,张旭升,樊小容,等.血管外膜细胞钙化及其钙化机制研究[J ].中西医结合心脑血管病杂志,2023,21(18):3347-3350.关注较少,因此,需要更多的研究来阐明血管钙化的致病机制㊂最初血管钙化被认为是被动和退行性病变,标志着血管老化,但是越来越多研究表明血管钙化是类似于胚胎骨形成的病理生物学过程[5-6]㊂Bostr öm 等[7-8]研究发现,钙化过程中大鼠血管中膜细胞由原有收缩表型转变成为成骨样细胞表型,原有的收缩标志物如平滑肌肌动蛋白α(α-SMA )等表达减少,并表达核心结合因子α1(Runx2)㊁骨形态生成蛋白2(BMP2)等多种成骨样标志物,从而介导骨基质在血管中沉积㊂细胞凋亡与自噬为2种细胞死亡的方式,与血管钙化息息相关,研究表明,血管中膜细胞在细胞凋亡过程中释放凋亡小体,促进细胞钙化,而细胞自噬通过多种机制调控细胞钙化[9-10]㊂本研究对大鼠血管外膜细胞进行体外诱导钙化,建立大鼠血管外膜细胞钙化模型,并检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂1材料与方法1.1试剂胎牛血清(FBS,Gibco),青霉素,链霉素(Gibco,美国),茜素红S溶液,β-甘油磷酸,抗坏血酸,地塞米松(Sigma,美国),抗GAPDH抗体(Bioworld),抗Bcl-2, Bax,Bcelin1和微血管相关蛋白(LC3)抗体(CST),碱性磷酸酶检测试剂盒㊁钙(Ca)检测试剂盒(南京建城生物工程研究所)㊂1.2大鼠血管外膜细胞分离与培养取10只4~6周龄雄性Wistar-Kyoto大鼠(体质量120~180g)胸主动脉分离血管外膜,采用组织黏附法培养㊂使用添加10%胎牛血清的高糖DMEM培养基(Gibco dmem)在37ħ㊁5%二氧化碳条件下培养细胞㊂当细胞增殖至80%~90%融合时,用0.25%胰酶消化传代㊂使用第3代至第6代的细胞进行后续实验㊂1.3体外钙化模型的建立钙化诱导培养基为含10%胎牛血清,10mmol/L β-甘油磷酸钠,0.05mmol/L抗坏血酸和100mmol/L 地塞米松的高糖DMEM培养液㊂将第3代至第6代细胞分为对照组和钙化组,待细胞长至50%融合时,使用钙化诱导培养基培养,每3d更换1次培养基,连续培养15d㊂1.4碱性磷酸酶(ALP)酶活测定细胞钙化诱导后,弃去培养基,1ˑ磷酸缓冲盐溶液(PBS)洗细胞3次,加入裂解液500μL(1%T ritonX-100),冰上裂解40min后,离心,取上清液㊂使用上清液根据试剂盒说明书检测ALP活性及总蛋白含量㊂1.5细胞内钙含量检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,每孔加入500μL0.6mol/L的盐酸4ħ脱钙过夜,取上清,根据钙测试试剂盒说明书检测钙含量㊂将脱钙后的细胞用4ħPBS洗3次,每孔加入500μL NaOH/0.1%SDS裂解细胞,取上清,用二喹啉甲酸法(BCA)测定细胞蛋白含量㊂1.6茜素红S染色细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,加入0.5mL4%多聚甲醛室温固定15min,用双蒸水洗3次,加入1mL0.1%茜素红室温孵育15min,吸去染液,双蒸水洗3次,在倒置显微镜下观察㊂1.7实时定量聚合酶链式反应(RT-PCR)检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,使用TaKaRa MiniBEST Universal RNA Extraction Kit提取总RNA,使用PrimeScrip TM RT reagent Kit将所提取的RNA逆转录合成cDNA,以cDNA为模板,通过SYBR Green I嵌合荧光定量RT-PCR检测BMP-2㊁Runx2和GAPDH的表达量㊂引物序列见表1㊂表1引物序列基因方向序列Runx2正向5'-TGGCTTTGGTTTCAGGTTAGG-3'反向5'-TGGAGATGTTGCTCTGTTCG-3' BMP-2正向5'-TGAGGATTAGCAGGTCTTTGC-3'反向5'-TCTCGTTTGTGGAGTGGATG-3' GAPDH正向5'-GGCTGCCCAGAACATCAT-3'反向5'-CGGACACATTGGGGGTAG-3'1.8蛋白免疫印迹法(Western Blot)检测细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,提取细胞总蛋白㊂使用12%SDS-PAGE胶电泳分离,并转移到聚偏二氟乙烯膜(PVDF)上,封闭后,加入一抗(Bax1ʒ1000,Bcl-21ʒ1000,Beclin11ʒ1000, LC31ʒ1000,GAPDH1:1000)稀释液,4ħ孵育过夜;加入二抗稀释液(1ʒ10000)室温孵育1h后,使用ECL发光试剂盒显影并计算灰度值㊂1.9统计学处理应用SPSS19.0软件进行统计处理,符合正态分布的定量资料以均数ʃ标准差(xʃs)表示,比较采用t检验,以P<0.05为差异有统计学意义㊂2结果2.1大鼠血管外膜细胞可在体外被诱导钙化为验证高磷是否能诱导大鼠血管外膜细胞钙化,使用钙化诱导培养基培养细胞,在不同时间点检测ALP活性和胞内钙含量㊂随着培养时间延长,ALP活性逐渐上升,在培养第12天达到峰值,与对照组比较差异有统计学意义(P<0.05);诱导第3天开始所测得的胞内钙含量与对照组比较升高(P<0.05),ALP 活性和钙含量升高具有时间依赖性㊂详见图1㊁图2㊂诱导15d所测得钙含量最高,因此,后续实验选择的诱导时间为15d㊂对钙化诱导15d的细胞进行茜素红S染色,结果显示,对照组细胞呈长梭形,而钙化组细胞变成菱形㊂茜素红S染色后,钙化组可观察到大量的橘红色钙结节(见图3),而对照组完全没有㊂这也证明大鼠血管外膜细胞可在体外被钙化培养基诱导钙化㊂图1钙化诱导培养基诱导外膜细胞后ALP含量(与0d时比较,*P<0.05)图2钙化诱导培养基诱导外膜细胞后胞内钙含量(与0d时比较,*P<0.05)图3培养15d时细胞经茜素S红染色切片图(ˑ100)2.2血管外膜细胞钙化与细胞向成骨样表型转化有关血管钙化的增加与成骨细胞特异性标志物如BMP2㊁和Runx2的增加有关[11]㊂RT-PCR结果显示,与对照组比较,钙化组的成骨细胞特异性标志物BMP2和Runx2mRNA表达量增加,与对照组比较差异有统计学意义(P<0.05)㊂详见图4㊂图4外膜细胞钙化过程中BMP2和Runx2mRNA表达量(与对照组比较,*P<0.05)2.3血管外膜细胞钙化过程涉及细胞自噬及凋亡调控通过Western Blot检测凋亡和自噬相关蛋白的表达量变化㊂与对照组比较,钙化组促凋亡蛋白Bax表达上调,抑凋亡蛋白Bcl-2表达下调(P<0.05)㊂详见图5㊂钙化组自噬相关蛋白Beclin1表达上调,LC3-Ⅱ/ LC3-Ⅰ比例上调(P<0.05),说明钙化诱导培养后细胞内凋亡水平上调㊁自噬水平升高㊂详见图6㊂图5诱导钙化后促凋亡蛋白及抑凋亡蛋白表达变化图6诱导钙化后凋亡及自噬蛋白Beclin1等表达变化3讨论血管钙化作为心血管疾病病人的并发症之一,其发病率与严重程度逐年增高及加重,是导致心血管疾病病人高死亡率的重要因素㊂血管钙化缺乏有效的治疗药物㊂因此,探究血管钙化发病机制,在分子水平寻找有效的诊断和防治靶点是急需开展的基础研究工作㊂本研究证明,使用10mmol/Lβ-甘油磷酸+0.05 mmol/L抗坏血酸+100mmol/L地塞米松培养外膜细胞即可诱导大鼠血管外膜细胞在体外发生钙化,这是通过茜素红S染色㊁ALP活性检测及胞内钙含量检测结果得以确定的㊂血管钙化过程中,血管中膜细胞向成骨样细胞表型转变并表达相关成骨标志物,从而引起骨基质的沉积,是血管钙化的重要特点及机制[5]㊂本实验所用的血管外膜细胞钙化条件与血管中膜细胞钙化条件一致,说明血管外膜细胞钙化的机制可能与中膜细胞钙化的机制部分一致㊂血管中膜细胞钙化过程中,细胞表达成骨相关的转录因子如Runx2等,进而促进下游表达骨相关蛋白如骨形态发生蛋白BMP2等的表达,从而促使细胞向成骨样细胞主动分化[12-13],本研究也观察到类似的机制㊂通过PT-PCR检测,发现钙化培养基培养大鼠血管外膜细胞15d后,BMP2和Runx2的mRNA表达水平升高㊂本研究通过对钙盐沉积与成骨样细胞表型转变2个维度的探讨,证明血管外膜细胞可在体外被诱导钙化,丰富了血管钙化的分型㊂血管钙化的发生机制复杂,涉及多种信号通路,如细胞自噬和凋亡㊁Wnt/β-catenin信号通路激活㊁内质网应激等均参与调控血管钙化的过程㊂自噬作为一种细胞应激的适应性反应,在维持血管结构与功能中十分关键㊂研究表明,血管钙化过程中自噬水平增高[14-15]㊂在体外实验中,高磷可提高大鼠血管中膜细胞的自噬水平,增加细胞内自噬体数量,从而抑制凋亡与钙化[16]㊂还有研究表明,自噬可通过抑制大鼠血管中膜细胞氧化应激,抑制血管内皮细胞的炎症反应,对三酰甘油等脂代谢进行调控,从而减轻血管钙化[17-18]㊂LC3和Beclin1是2种典型的自噬标志物,Western Blot实验结果表明,用钙化培养基诱导大鼠血管外膜细胞15d,LC3-Ⅱ/LC3-Ⅰ比率升高,Beclin1蛋白水平表达升高,说明细胞内自噬水平升高㊂多项研究表明,细胞凋亡参与促进血管钙化的发生,抑制细胞凋亡和抑制钙化[16-17]㊂在对大鼠的体内研究发现,成纤维细胞生长因子21通过内质网应激调控Caspase-12信号通路来减少血管内中膜细胞凋亡,从而抑制血管钙化[18]㊂另外,提高培养基中的Pi 或Ca2+浓度,可诱导细胞质膜形成并释放基质囊泡(如凋亡小体),从而导致细胞外基质钙化,这种基质钙化可能成为血管钙化的成核位点[19]㊂Bax和Bcl-2是2种典型的凋亡和抑制凋亡蛋白,本实验结果证明,利用钙化培养基对血管外膜细胞诱导钙化过程中,细胞内凋亡水平升高㊂同时细胞内自噬水平也升高,这可能是细胞自我调控以对抗钙化的结果㊂本研究证实血管外膜细胞可在体外被诱导钙化,且外膜钙化过程与骨组织钙化过程类似,为主动可调控的过程㊂血管钙化是一个复杂的过程,涉及细胞凋亡和自噬等调控通路,仍需进一步研究㊂参考文献:[1]梁英权,段亚君,韩际宏.血管钙化分子机制研究进展[J].中国动脉硬化杂志,2020,28(11):921-929.[2]NICOLL R,HENEIN M Y.The predictive value of arterial andvalvular calcification for mortality and cardiovascular events[J].Int J Cardiol Heart Vessel,2014,3:1-5.[3]JOHNSON R C,LEOPOLD J A,LOSCALZO J.Vascularcalcification:pathobiological mechanisms and clinical implications[J].Circulation Research,2006,99(10):1044-1059.[4]YAMADA S,GIACHELLI C M.Vascular calcification in CKD-MBD:roles for phosphate,FGF23,and Klotho[J].Bone,2017,100:87-93.[5]LIN M E,CHEN T M,WALLINGFORD M C,et al.Runx2deletion insmooth muscle cells inhibits vascular osteochondrogenesis andcalcification but not atherosclerotic lesion formation[J].Cardiovascular Research,2016,112(2):606-616.[6]DURHAM A L,SPEER M Y,SCATENA M,et al.Role of smoothmuscle cells in vascular calcification:implications in atherosclerosis andarterial stiffness[J].Cardiovascular Research,2018,114(4):590-600.[7]BOSTRÖM K I,RAJAMANNAN N M,TOWLER D A.The regulationof valvular and vascular sclerosis by osteogenic morphogens[J].Circulation Research,2011,109(5):564-577.[8]SPEER M Y,YANG H Y,BRABB T,et al.Smooth muscle cells giverise to osteochondrogenic precursors and chondrocytes incalcifying arteries[J].Circulation Research,2009,104(6):733-741.[9]PROUDFOOT D,SKEPPER J N,HEGYI L,et al.Apoptosisregulates human vascular calcification in vitro:evidence forinitiation of vascular calcification by apoptotic bodies[J].Circulation Research,2000,87(11):1055-1062.[10]AN S J,BOYD R,ZHU M,et al.NADPH oxidase mediatesangiotensin II-induced endothelin-1expression in vascularadventitial fibroblasts[J].Cardiovascular Research,2007,75(4):702-709.[11]ZEADIN M,BUTCHER M,WERSTUCK G,et al.Effect of leptin onvascular calcification in apolipoprotein E-deficient mice[J].Arterioscler Thromb Vasc Biol,2009,29(12):2069-2075. [12]LEOPOLD J A.Vascular calcification:mechanisms of vascularsmooth muscle cell calcification[J].Trends in CardiovascularMedicine,2015,25(4):267-274.[13]刘聿秀.高尿酸诱导血管钙化的机制研究[D].青岛:青岛大学,2015.[14]LIU Q,LUO Y,ZHAO Y,et al.Nano-hydroxyapatite acceleratesvascular calcification via lysosome impairment and autophagydysfunction in smooth muscle cells[J].Bioact Mater,2022,8:478-493.[15]LIANG J,HUANG J,HE W,et al.β-Hydroxybutyric Inhibits vascularcalcification via autophagy enhancement in models induced byhigh phosphate[J].Front Cardiovasc Med,2021,8:685748. [16]CICERI P,ELLI F,CAPPELLETTI L,et al.A new in vitro model todelay high phosphate-induced vascular calcification progression[J].Mol Cell Biochem,2015,410(1/2):197-206.[17]BYON C H,JAVED A,DAI Q,et al.Oxidative stress inducesvascular calcification through modulation of the osteogenictranscription factor Runx2by AKT signaling[J].The Journal ofBiological Chemistry,2008,283(22):15319-15327.[18]OUIMET M,FRANKLIN V,MAK E,et al.Autophagy regulatescholesterol efflux from macrophage foam cells via lysosomal acidlipase[J].Cell Metabolism,2011,13(6):655-667.[19]REYNOLDS J L,JOANNIDES A J,SKEPPER J N,et al.Humanvascular smooth muscle cells undergo vesicle-mediatedcalcification in response to changes in extracellular calcium andphosphate concentrations:a potential mechanism for acceleratedvascular calcification in ESRD[J].Journal of the AmericanSociety of Nephrology,2004,15(11):2857-2867.(收稿日期:2022-03-30)(本文编辑王雅洁)。
一类含哌啶酮的单羰基姜黄素类化合物在制备抗炎药物中的应用[发明专利]
![一类含哌啶酮的单羰基姜黄素类化合物在制备抗炎药物中的应用[发明专利]](https://img.taocdn.com/s3/m/333cdc450029bd64793e2cd0.png)
专利名称:一类含哌啶酮的单羰基姜黄素类化合物在制备抗炎药物中的应用
专利类型:发明专利
发明人:梁广,吴建章,姜鑫,张亚利,陈高帜,王哲
申请号:CN201310132910.0
申请日:20130402
公开号:CN103181922A
公开日:
20130703
专利内容由知识产权出版社提供
摘要:本发明属药物化学领域,具体涉及特定的含哌啶酮的单羰基姜黄素类化合物在制备抗炎药物及与炎症相关疾病的治疗药物中的应用,这些姜黄素化合物能够抑制炎症因子IL-6的表达和释放,也能明显逆转炎症调控相关的IκB的降解,抑制炎症信号通路ERK和JNK的磷酸化,体内实验能明显提高LPS诱导小鼠致死的生存率。
申请人:温州医学院
地址:325035 浙江省温州市茶山高教园区温州医学院
国籍:CN
更多信息请下载全文后查看。
Febuxostat_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-04-2017Print Date:Jul.-04-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :FebuxostatCatalog No. :HY-14268CAS No. :144060-53-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:TEI 6720; TMX 67Formula:C16H16N2O3SMolecular Weight:316.37CAS No. :144060-53-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
