(完整版)基因毒性杂质的评估与控制
【医药】如何控制基因毒性杂质

01、何为基因毒性杂质基因毒性杂质(或遗传毒性杂质,Genotoxic Impurity,GTI)是指能直接或间接损害DNA,引起DNA突变、染色体断裂、DNA重组及DNA 复制过程中共价键结合或插入,导致基因突变或癌症的物质(如卤代烷烃、烷基磺酸酯类等)。
潜在基因毒性杂质(Potential Genotoxic Impurity ,PGI)结构中含有与基因毒性杂质反应活性相似的基团(如肼类、环氧化合物、N-亚硝胺类等),通常也作为基因毒性杂质来评估。
基因毒性杂质主要来源于原料药合成过程中的起始物料、中间体、试剂和反应副产物。
此外,药物在合成、储存或者制剂过程中也可能会降解产生基因毒性杂质。
除此之外,有些药物通过激活正常细胞而产生基因毒性物质导致突变,如化疗药物顺铂等。
02、何为基因毒性杂质“警示结构”由于杂质结构的多样性,一般很难进行归类,因此,在缺乏安全性数据支持的情况下,法规和指导原则采用“警示结构”用来区分普通杂质和基因毒性杂质。
所谓“警示结构”,是指杂质中的特殊基团可能与遗传物质发生化学反应,诱导基因突变或者染色体断裂,因此具有潜在的致癌风险。
对于含有警示结构的杂质,应当进行(Q)SAR预测和体内外遗传毒性和致癌性研究,或者将杂质水平控制在毒理学关注阈值(TTC)之下。
但是含有警示结构并不能说明该杂质一定具有遗传毒性,而确认有遗传毒性的物质也不一定会产生致癌作用。
杂质自身性质和结构特点会对其毒性产生抑制或调节作用。
警示结构的重要性在于它提示了可能存在的遗传毒性和致癌性,为进一步的杂质安全性评价与控制指明方向。
(关于基因毒杂质警示结构的详细信息可参考欧盟发布的警示结构《Development ofstructure alerts for the in vivo micronucleus assay in rodents》)。
03、基因毒性杂质严格控制的必要性基因毒性杂质最主要的特点是在极低浓度时即可造成人体遗传物质的损伤,导致基因突变并促使肿瘤发生。
基因毒性杂质的评估与控制

6. Istituto Superiore di Sanita (Italy) 7. Prous Institute (Spain) 8. Swedish Toxicology Science Research Center(Sweden)
1.临床使用剂量增加 2.用药时长显著增加 3.病情严重或危及生命的病患状态下采用较高可接 受摄入量,变为不那么严重的病患情况后,原有的 杂质可接受摄入量不再适当 4.使用新的给药途径 5.扩大使用患者群包括孕妇和(或)小儿
◆新的杂质被确知属于第一类或第二类诱变性杂
质
THREE
基因毒性杂质的评估
一、评估内容
五、杂质残留风险评估考量
基毒杂质清除因子计算表
代码
GTI
到API 步骤
3 2 1
反应活 性
1-100 X X
溶解性
1-10(100) X X
挥发性
1-10 X X
电离性
5 X X
消除因 子/步
XXX
总消除因 子
清除 原理
Xx,xxx,xxx
参考文献: Teasdale A, Elder D, Chang S-J, et al. Risk assessment of genotoxic impurities in new chemical entities: strategies to demonstrate control[J]. Org Process Res Dev, 2013, 17: 221-230.
FDA
2008 年签发《原料药和成品药中遗传 毒性和致癌性杂质,推荐方法》
内容和EMA指南基本一致,主要包括: ◆ 原料药和制剂中的基因毒性杂质生 成的预防办法
基因毒性杂质-全面信息资料

基因毒性杂质的来源
1 环境污染
工业排放、废水、废气等 对环境的污染会导致基因 毒性杂质的增加。
2 食物
食物中的农药残留、添加 剂以及食品加工过程中产 生的致癌物质都是来源之 一。
3 药物
某些药物和化学药品具有 基因毒性作用。
基因毒性杂质的检测பைடு நூலகம்法
Ames试验
一种常见的基因毒性检测方法, 通过检测细菌的突变来判断样 本的基因毒性。
基因毒性杂质的监管与控制
1
立法与标准制定
国家和国际机构制定标准,以确保基因
检测与监测
2
毒性杂质的合理监管。
建立监测体系,对食品、环境和药品中
的基因毒性杂质进行定期检测。
3
信息公开与教育
提高公众对基因毒性杂质的认知,加强 相关知识的宣传和教育。
基因毒性杂质防范和应对的策略和建 议
1 环保意识
更加重视环境保护,减少毒性杂质的排放和环境污染。
基因毒性杂质-全面信息 资料
欢迎来到基因毒性杂质全面信息资料的世界,通过本次演示,您将全面了解 基因毒性杂质的定义、分类以及对人体健康的影响。
毒性杂质的定义和分类
毒性杂质指的是那些可以对生物体细胞的遗传物质DNA产生损害的化学物质。它们可以根据其毒性的性质和 机制进行分类。
常见的基因毒性杂质及其危害
2 选择健康食品
选购来自可靠供应商的食品,避免食用过多的加工食品。
3 合理用药
按照医生的指导合理用药,避免滥用药物。
细胞培养法
将样本与细胞培养在一起,观 察是否对细胞产生损害。
基因表达分析
通过检测基因在样本中的表达, 判断是否存在基因毒性。
基因毒性杂质对人体健康的影响
基因毒杂质控制策略案例

基因毒杂质控制策略案例基因毒杂质(Genotoxic Impurities)控制策略是药物开发和制造过程中的重要环节,旨在确保药物产品中基因毒性杂质的控制和限制。
以下是一个基因毒杂质控制策略的案例示例:
1. 风险评估:首先,对药物候选化合物进行综合的基因毒性风险评估。
评估包括利用体外基因毒性测试(如Ames 试验)和计算毒性预测模型,对化合物进行筛选和分类。
2. 导入限值:基于风险评估结果,制定适当的基因毒杂质导入限值。
此限值应与国际指南(如ICH M7指南)和适用的监管要求相一致。
3. 合成和纯化策略:在药物合成和纯化过程中,采取特定的操作条件和工艺控制,包括选择合成路线、溶剂使用、温度控制、反应时间和条件等,以最小化基因毒杂质的产生和残留。
4. 检测和分析:开发和验证适当的分析方法,用于检测和定量基因毒杂质的存在。
常见的分析技术包括高效液相色谱(HPLC)、质谱法(如LC-MS/MS)、核磁共振(NMR)
等。
5. 清洁验证:使用适当的清洁验证方法和程序,确保生产设备和工艺的清洁性,在不同批次之间避免交叉污染和残留。
6. 临床监控:在临床阶段,对药物进行基因毒杂质的监控和评估,以确保在实际使用中的毒性风险得到控制。
这只是一个基本的基因毒杂质控制策略案例,具体策略会因药物特性、制造过程和监管要求等因素而有所不同。
在实际应用中,需要根据具体情况制定并执行适合的控制策略,并与相关的监管机构保持合作与沟通。
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT P OTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group andhas been subject to consultation by the regulatory parties, in accordance with theICH Process. At Step 4 of the Process the final draft is recommended for adoptionto the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史Code 文件代码History 历史Date 日期6 February 2013 M7 Approval by the Steering Committee under Step 2and release for public consultation.第2阶段由筹委会批准,公开征求意见5 June 2014M7 Approval by the Steering Committee under Step 4and recommendation for adoption to the three ICHregulatory bodies.第4阶段由筹委会批准,推荐ICH三方药监局采用Current Step 4 version 现行版本第4阶段23 June 2014M7 Corrigendum to fix typographical errors andreplace word “degradants” with “degradationproducts” throughout the document.修正输入错误,将全文中“degradants”替换成“degradation products”.Legal Notice: This document is protected by copyright and may b e used, reproduced, incorporated into other works, adapted, modified, translated or distributed undera public license provided that ICH's copyright in the document is acknowledged atall times. In case of any adaption, modification or translation of the document,reasonable steps must be taken to clearly label, demarcate or otherwise identifythat changes were made to or based on the original document. Any impression thatthe adaption, modification or translation of the original document is endorsed orsponsored by the ICH must be avoided.The document is provided "as is" without warranty of any kind. In no event shallthe ICH or the authors of the original document be liable for any claim, damagesor other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meetingon 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHTABLE OF CONTENTS目录1. INTRODUCTION概述2. SCOPE OF GUIDELINE指南范围3. GENERAL PRINCIPLES通用原则4. CONSIDERATIONS FOR MARKETED PRODUCTS上市产品应考虑的问题批准后原料药化学、生产和质量变更4.1 Post-Approval Changes to the DrugSubstance Chemistry, Manufacturing, andControls4.2 Post-Approval Changes to the Drug批准后制剂的化学、生产和质量变更Product Chemistry, Manufacturing, andControls上市产品临床使用变更4.3 Changes to the Clinical Use ofMarketed Products4.4 Other Considerations for Marketed上市产品其它应考虑问题Products原料药和制剂杂质评估5. DRUG SUBSTANCE A ND DRUG PRODUCTIMPURITY ASSESSMENT5.1 Synthetic Impurities 合成杂质5.2 Degradation Products 降解产物5.3 Considerations for ClinicalDevelopment临床研发要考虑的问题6. HAZARD ASSESSMENT ELEMENTS危害性评估要素7. RISK CHARACTERIZATION风险特征7.1 TTC-based Acceptable Intakes 根据TTC制订可接受摄入量7.2 Acceptable Intakes Based on Compound-Specific Risk Assessments 根据化合物特定风险评估制订的可接受摄入量7.2.1 Mutagenic Impurities with Positive Carcinogenicity Data (Class 1 in Table 1)致癌数据有利的诱变性杂质(表1中的第1类)7.2.2 Mutagenic Impurities with Evidencefor a Practical Threshold具有实用阈值证据的诱变性杂质7.3 Acceptable Intakes in Relation to LTLExposure与LTL暴露相关的可接受摄入量7.3.1 Clinical Development临床研发7.3.2 Marketed Products已上市产品7.4 Acceptable Intakes for MultipleMutagenic Impurities多个诱变性杂质的可接受摄入量7.5 Exceptions and Flexibility inApproaches方法例外情况和弹性8. CONTROL控制8.1 Control of Process Related Impurities 工艺相关杂质的控制8.2 Considerations for Control Approaches 控制方法要考虑的问题8.3 Considerations for Periodic Testing定期检查要考虑的问题8.4 Control of Degradation Products降解产物的控制8.5 Lifecycle Management生命周期管理临床研发要考虑的问题8.6 Considerations for ClinicalDevelopment9. DOCUMENTATION文件记录9.1 Clinical Trial Applications临床试验应用9.2 Common Technical Document (Marketing通用技术文件(上市申报)Application)NOTES注解GLOSSARY术语REFERENCES参考文献APPENDICES附录ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesisor subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2):Impurities in New D rug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide apractical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2)(Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of HumanClinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
(完整版)基因毒性杂质的评估与控制

杂质 来源
降解杂质
加速试验、长期试验、光降 解、强制降解试验
环境污染
三、基因毒性杂质识别
工艺杂质
实际杂质
潜在杂质
API中实际观察到>ICH Q3A 报告限(0.05%)工艺杂质
1)合成API过程:起始物料,中间体,化学试剂 2)风险评估可能带入API中的,存在于起始物料,中间体中已识别 的杂质,以及合理机理预测产生的副产物(对于工艺早期杂质携带 入API的风险可忽略,但要提供基于风险论证的表明哪步后应该评估 杂质的潜在突变性) 3)对后工艺引入的起始物料,评价起始物料合成最后一步的潜在基 因毒性杂质
2018年
华海药业生产的缬沙坦原料药中含有微量基因毒性杂质N,N-二甲 基亚硝胺(NDMA),缬沙坦及其相关制剂从欧洲、美国和中国市 场被召回。
2018年
印度Torrent制药生产的缬沙坦片剂中也检测出含有NDMA,该公司 也从美国市场上自愿召回了14批相关药品。
二、相关概念
何为基因毒性杂质?
基因毒性杂质,也称遗传毒性杂质,通常指较低水平可直接造成DNA损伤,进而导致DNA突变, 因此可能引发癌症的DNA反应性物质。
EMA
2006年颁布《基因毒性杂质限度指南》
2010年发布《遗传毒性杂质限度指导 原则问答》 ◆为限制新活性物质中的基因毒性杂 质提供了解决问题的框架和具体方案。
◆新药必须进行基因毒性杂质分析
◆对于现有药品,不强制进行基因毒 性杂质分析评估
◆对已上市产品进行化学合成变更或 仿制药上市前,需对合成路线、过程 控制、杂质概括评价并与现有产品对 比,以确定未引入新的或更高水平的 基因毒性杂质
降解杂质
长 期 稳 定 性 试 验 , API 中 观 察 到>ICH Q3A报告限降解产物
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
基因毒性杂质控制相关文件及指南介绍
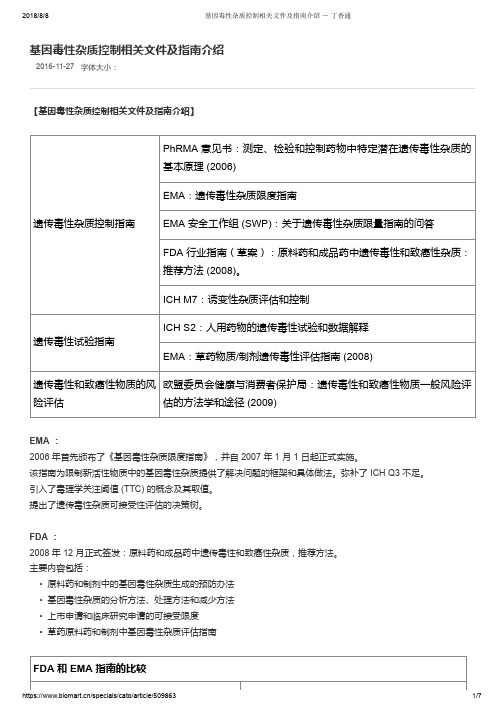
2016-11-27字体大小:基因毒性杂质控制相关文件及指南介绍【基因毒性杂质控制相关文件及指南介绍】遗传毒性杂质控制指南PhRMA 意见书:测定、检验和控制药物中特定潜在遗传毒性杂质的基本原理 (2006)EMA:遗传毒性杂质限度指南EMA 安全工作组 (SWP):关于遗传毒性杂质限量指南的问答FDA 行业指南(草案):原料药和成品药中遗传毒性和致癌性杂质:推荐方法 (2008)。
ICH M7:诱变性杂质评估和控制遗传毒性试验指南ICH S2:人用药物的遗传毒性试验和数据解释EMA:草药物质/制剂遗传毒性评估指南 (2008)遗传毒性和致癌性物质的风险评估欧盟委员会健康与消费者保护局:遗传毒性和致癌性物质一般风险评估的方法学和途径 (2009)EMA :2006 年首先颁布了《基因毒性杂质限度指南》,并自 2007 年 1 月 1 日起正式实施。
该指南为限制新活性物质中的基因毒性杂质提供了解决问题的框架和具体做法。
弥补了 ICH Q3 不足。
引入了毒理学关注阈值 (TTC) 的概念及其取值。
提出了遗传毒性杂质可接受性评估的决策树。
FDA :2008 年 12 月正式签发:原料药和成品药中遗传毒性和致癌性杂质,推荐方法。
主要内容包括:• 原料药和制剂中的基因毒性杂质生成的预防办法• 基因毒性杂质的分析方法、处理方法和减少方法• 上市申请和临床研究申请的可接受限度• 草药原料药和制剂中基因毒性杂质评估指南FDA 和 EMA 指南的比较相似点不同点推荐的鉴定和认证潜在遗传性杂质的方法相同 推荐的处理遗传毒性和致癌性杂质的方法相同FDA 指南包含致癌性杂质TTC 设定为 1.5 μg/天指南允许的 14 天内用药的 TTC 水平为 120 μg , 而非仅针对单次用药临床试验中短期暴露的 TTC 更高FDA 指南不允许根据现售药品的短期暴露情况而 提高 TTC ICH M7【基因毒性杂质的控制策略】具有阳性致癌数据的诱变杂质(第1类)---计算可接受摄入量( AI ): • M7 Addendum 中列出的 15 种化合物中有 10 个为该计算方法计算 • Carcinogenicity Potency Database (CPDB )中列明了 1574 种致癌物质的 TD50 值毒理学关注门槛---TTC 法(第 2/3 类): • ICHM7 主要讨论的方法,主要针对第 2/3 类基因毒性杂质,比如低级磺酸酯类等。
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
基因杂质的评估和控制 ICH M7

(细菌诱变呈阳性*,无啮齿动物 TTC)
rodents》。或进入 The Carcinogenic Potency
致癌数据
Database (CPDB),里面有 1547 种致癌物质的列
3
警示结构,与原料药结构无关,无 控制不高于可接受限度(适当的
诱变数据
TTC)或检测细菌诱变含量:
表,结构式,CAS 号,作用部位,TTC 值等一系
基因杂质的评估和控制 ICH M7
1 概述
1 概述
ICH Q3A(R2) 新原料药中的杂质 ICH Q3B(R2) 新制剂中的杂质
关于主要杂质定性和控制的指南
ICH M3(R2) 药物人用临床试验和上市许可中的非临床安全性研究
EMA《遗传毒性杂质限度指导原则》
ICH M7 为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制 目的:提供使用框架,以应用于这些诱变杂质的鉴别、分类、定性和控制,对潜在致癌风险进行了控制。 强调:在建立诱变性杂质水平时考虑安全性和质量风险管理两方面 人用条件下,给出对原料药或制剂中残留或可能残留的诱变性杂质评估和控制的建议
➢ 如果有足够后续试验,可由单独的体外试验结果,可以对它的体内关联性进行评估。
➢ 若缺乏这些信息时,体外基因毒性物质经常被考虑为假定的体内诱变剂和致癌剂。
----摘自EMA《毒性杂质限度
指南》
✓ 为何重点研究基因毒性杂质? 基毒杂质特点:很低浓度时即可造成人体遗传物质的损伤,进而导致基因突变并可能促使肿瘤发生。
补充
1 概述
✓ 何为基因毒性杂质、诱变杂质(Genotoxic Impurities,Mutagenic Impurities)?
基因毒性杂质(或遗传毒性杂质,Genotoxic Impurity ,GTI)是指化合物本身直接或间接损伤细胞DNA,产生基因突变或体内
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
ICHM7(step4)基因毒性杂质评估和控制◆中英

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
基因毒性杂质的控制

在某些特定情况下,TTC值高于1.5 μ g/day 也是可以接受 的,比如药品的短期接触,即治疗某些生命预期在5年以下 的某些严重疾病,或者这种杂质是一种已知物质,人类在其 他方式上对它的摄入会更高(比如在食品上)。有些基因毒 性杂质同时也是一种体内代谢产物,对它的评价也要以对代 谢产物的接受性为基础。 基因杂质的浓度限度以ppm为单位,公式如下: Concentration limit (ppm) = 1.5 μ g / daily dose
——基因毒性杂质磺酸盐的风险评估
临床研究发现甲磺酸酯的DNA 烷基化作用会导致诱 变效应 ,其中甲磺酸甲酯和甲磺酸乙酯已有这方面 报导,因此有理由怀疑其它低分子量磺酸(如对甲 苯磺酸)的烷基酯可能也存在着类似的毒性影响。 尽管无数据表明这些酯对人的毒性影响,然后依然 有上述基因毒性物质以杂质的形式存在于含磺酸酯 类药物活性成分的药品中的潜在风险。 EMEA/44714/2008
不能进行阀值鉴定的基因毒性杂质的可接受剂量
评价应该包括药学的和毒理学的评价。一般来说, 如果避免毒性是不可能的,那么药学的评价措施 应该以ALARP的 ( as low as reasonably practicable)控制水平为指导原则。
含有多个基因毒性杂质的评估
EMEA CHMP 结构不同的——单个杂质的限度应该小于1.5ug/day 结构相似的——杂质总和的限度应该小于1.5ug/day FDA(和EMEA类似) 单个杂质造成的癌症风险机率应该小于10-5 有相同作用机制的结构相似的杂质,其含量总和应该 参考1.5ug/day的限量进行评估。
——基因毒性杂质卤代烃的风险评估
有数据表明氯乙烷、氯甲烷为基因毒性杂质, 因此有理由怀疑其他低分子卤代烃类也有类 似的作用。在生产中应该对其进行相应的控 制。
ICH M7(step4)基因毒性杂质评估和控制◆中英剖析

ASSESSMENT AND CONTROL OF DNA REACTIVE(MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TOLIMIT POTENTIAL CARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制M7Current Step 4 versiondated 23 June 2014This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.M7Document History 文件历史The document is provided "as is" without warranty of any kind. In no event shall the ICH or the authors of the original document be liable for any claim, damages or other liability arising from the use of the document.The above-mentioned permissions do not apply to content supplied by third parties. Therefore, for documents where the copyright vests in a third party, permission for reproduction must be obtained from this copyright holder.ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制ICH Harmonised Tripartite GuidelineICH三方协调指南Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 5 June 2014, this Guideline is recommended for adoption to the three regulatory parties to ICHASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIALCARCINOGENIC RISK为限制潜在致癌风险而对药物中DNA活性(诱变性)杂质进行的评估和控制1. INTRODUCTION概述The synthesis of drug substances involves the use of reactive chemicals, reagents, solvents, catalysts, and other processing aids. As a result of chemical synthesis or subsequent degradation, impurities reside in all drug substances and associated drug products. While ICH Q3A(R2): Impurities in New Drug Substances and Q3B(R2): Impurities in New Drug Products (Ref. 1, 2) provides guidance for qualification and control for the majority of the impurities, limited guidance is provided for those impurities that are DNA reactive. The purpose of this guideline is to provide a practical framework that is applicable to the identification, categorization, qualification, and control of these mutagenic impurities to limit potential carcinogenic risk. This guideline is intended to complement ICH Q3A(R2), Q3B(R2) (Note 1), and ICH M3(R2): Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorizations for Pharmaceuticals (Ref. 3).原料药合成牵涉到使用活性化学物质、试剂、溶剂、催化剂和其它工艺助剂,导致在所有原料药及其制剂中会残留有化学合成或其降解产物、杂质。
基因毒性杂质控制

可接受风险的摄入量
可接受其风险的摄入量一般通用的被定义为 Threshold of Toxicological Concern (TTC) 。具 体含义为:一个“1.5ug/day”的TTC值,即相当于 每天摄入1.5ug的基因毒性杂质,被认为对于大多数 药品来说是可以接受的风险(一生中致癌的风险小 于100000分之1)。按照这个阀值,可以根据预期的 每日摄入量计算出活性药物中可接受的杂质水平。 在特定的条件下一些基因毒性杂质也可以有较高的 阈值。如接触时间比较短等,这个需要根据实际情 况再进行推算。
药学评价
应该根据现有的配方选择和生产技术,提供 生产方法的合理性。申请人应该指明涉及到 的所有具有基因毒性或有致癌性的化学物质, 如所用试剂、中间体、副产品等。更进一步, 在药物活性物质中没有出现的基因毒性反应 物和有基因毒性结构(alerting structure) 的物质,都应该被考虑。实际生产中尽量避 免使用该类物质。
药学评价
如果在合成路线、起始物料方面没有更好选择,则需要提供 一个正当的理由。就是物质中能引起基因毒性和致癌性的结 构部分在化学合成是不可避免的。 假如基因毒性杂质被认为是不可避免的,那么应该采取技术 手段尽可能的减少基因毒性杂质在产品中的含量,使其符合 安全的需要或使其降低到一个合理的水平。对于活性中间体、 反应物、以及其它化合物的化学稳定性都应该进行评估。 应该有合理的分析方法去检测和量化这些杂质的残留量。
基因毒性杂质的风险
按照目前的法规来说,(体内)基因毒性物 质在任何摄入量水平上对DNA都有潜在的破坏 性,这种破坏可能导致肿瘤的产生。因此, 对于基因毒性致癌物,不能说“不存在明显 的阀值,或是任何的摄入水平都具有致癌的 风险”。
