整体柱
阴离子交换整体柱对蛋白质的分离与纯化

阴离子交换整体柱对蛋白质的分离与纯化蛋白质是有机体中微小细胞结构的重要组成部分,它有着复杂的结构,而且受到它们的生物活动和环境因素的影响。
要正确完成蛋白质的分析和研究,就必须完成其分离与纯化。
阴离子交换整体柱被认为是最有效的蛋白质分离与纯化方法之一。
阴离子交换整体柱是一种常用的分离和分离技术,它利用阴离子交换剂作为分离活性体,以微量化合物的阴离子立体结构形式将其结合成细胞外阴离子质粒,这样就可以根据它们的电荷来实现蛋白质的分离与纯化。
阴离子交换整体柱可以用来分离和纯化多种蛋白质。
首先,蛋白质可以根据它们不同的电荷类型分离。
其次,它可以用来分离多种相似的蛋白质,特别是具有相似分子量和电荷的蛋白质,因为它可以从样品中分离出非常纯的单一蛋白质。
最后,阴离子交换整体柱可以用来分离具有复杂结构的蛋白质,如多糖蛋白和蛋白激酶,这样就可以分析它们的活性。
在实际应用中,利用阴离子交换整体柱进行蛋白质分离与纯化的实验流程主要包括以下几个步骤:首先,样品分散;其次,加入膜;然后,利用阴离子交换剂对膜上的样品进行阴离子交换;最后,洗涤、重组。
阴离子交换整体柱技术在蛋白质分离与纯化领域具有重要的应用价值,它不仅可以有效地分离蛋白质,而且可以将某种蛋白质从复杂的样本中分离出来,最终得到高质量和高纯度的蛋白质,这是完成对蛋白质进行分析和研究的关键。
但是,阴离子交换整体柱技术也有一些缺点。
由于部分样品或细胞因素的影响,可能会影响整体柱的结构,使分离的结果受到一定的影响。
同时,某些蛋白质的分离可能会受到抗原的影响,这也是一个不利的因素。
此外,不同的蛋白质对阴离子交换剂的反应也是不同的,这样也可能影响蛋白质的分离结果。
因此,要有效地完成蛋白质分离与纯化,阴离子交换整体柱技术具有重要的应用价值。
但是,在实际应用中,还必须考虑到技术的局限性,并采取相应的措施来解决问题,以确保获得最佳的分离结果。
总之,阴离子交换整体柱技术已成为一种重要的蛋白质分离与纯化方法,其应用价值已获得许多研究者的高度重视,它不仅可以用来分离不同类型的蛋白质,而且可以将具有复杂结构的蛋白质分离出来,最终以高纯度的蛋白质来完成对蛋白质的分析和研究。
整体柱的制备及其应用

整体住的制备及其应用1整体柱的发展历史整体柱是在空柱管中原位聚合得到的连续多孔高聚物固体材料,材料表面可以根据需要作相应的功能衍生化,是一种新型的用于分离分析或作为反应器的多孔介质[1]。
整体柱具有小尺寸的骨架和大尺寸的流通孔,因此具有较大的流通孔 / 骨架尺寸比,从而提高柱的通透性,降低背压[2]。
流动相在通孔中流通,聚合物表面的小孔增大了溶质与固定相作用的表面积,所有的流动相都流过柱床,不存在填料颗粒之间的间隙孔。
而在传统的色谱柱中,大部分流动相是流经填充颗粒然后通过扩散到达作用位点,这种以对流传质代替扩散传质的机理使得传质效率大大提高,可以实现快速和高效的分离[3]。
事实上,早在 50 多年前,就已经有人开始做整体化材料作为分离介质的尝试[4]。
但是由于当时所能制备的的凝胶状整体材料在流动相的压力下易破裂,这种材料没有发展起来。
之后制备出的机械强度较高且具有开放孔的聚氨酯虽然在液相和气相色谱中得到了一定的应用,但是一直没有引起色谱界的重视。
1990s,Hjerte n 米用水溶性的单体 N-N-亚甲基双丙烯酰胺和甲基丙烯酸共聚制备高溶胀性凝胶,并将其压入空柱中,该色谱柱成功地应用于蛋白质和多肽的分离。
但这种色谱柱还具有填充柱的特性,仅仅在受到挤压后才呈现整体柱的结构特性,并不能算是真正意义上的整体柱[5]。
随后在1992年,Frechet和Svec用偶氮二异丁腈(ABIN)作引发剂将甲基丙烯酸缩水甘油酯(GMA与乙二醇二甲基丙烯酸酯(EGDMA直接在空柱管中聚合制备同时具有中空和大孔的多孔刚性聚合物整体柱,这是第一次制备出真正的有机整体柱。
1996 年硅胶整体柱也成功制备,使得这项技术得到了全面发展[6,7]。
目前,整体柱已经被成功地应用于反相色谱、正相色谱、离子交换色谱、疏水作用色谱、亲水作用色谱、亲和色谱、体积排阻色谱和手性分离中,成功的分离了蛋白质、多肽、氨基酸、类固醇、多环芳烃和低聚寡核苷酸等物质。
整体柱

们今天的研究奠定了基础。传统的高效液相色谱柱多为填料色谱柱,可以根据填料 的孔径分类, 也可以根据填料微球的直径分类。 整体柱(Monolithic column)又称为整 体固定相(Monolithic stationary phase)、连续床(Continuous bed)、棒柱(Rod)等 [ 20] 。 整体柱除了具有传统填充色谱柱的优点外,还可以省去色谱柱的填充过程,使得制 备填充物和装柱两个过程合二为一。所以整体柱的研究必然是一个新的热点。 1.2 高效液相色谱整体柱的分类 整体柱基本可以分为有机整体柱和无机整体柱两个大类。 1.2.1 有机整体柱 有机聚合物整体柱在制备方面选材范围广,适用的 pH 值范围宽,与硅胶整体柱 相比具有制备过程简单的优点,在近些年得到了迅速的发展。制备过程通常是将单 体混合物及致孔剂注入到空柱中, 经热、 紫外光或γ -射线引发使单体混合物在柱体 内聚合,然后用合适的溶剂除去柱体内的致孔剂和残留的单体 [ 21] 。根据有机聚合物 整体柱在制备过程中所选用的单体以及交联剂的不同可以将其分为不同种类,具体 如下 [ 22] 1.2.1.1 聚甲基丙烯酸酯类整体柱 聚甲基丙烯酸酯类整体柱主要以甲基丙烯酸缩水甘油酯 (GMA)、2-丙烯酰胺-2甲基丙烷-1-磺酸(AMPS)、甲基丙烯酸丁酯(BMA) 、甲基丙烯酸酯为功能单体,以 亚甲基双甲基丙烯酸酯、乙二醇二甲基丙烯酸酯(EDMA)为交联剂,以甲醇、正丙 醇、环己醇、1,4-丁二醇、壬醇等一种或几种混和醇为致孔剂 [ 23, 24-26] 。在制备过程 中,可以通过改变单体和致孔剂的比例以及种类来达到制备不同功能的整体柱的目 的。 1.2.1.2 聚丙烯酰胺类整体柱 该类整体柱采用的单体有丙烯酰胺、甲基丙烯酰胺、二甲基丙烯酰胺、N-异丙
整体柱的简介及分类ppt课件

17
有机聚合物整体柱的制备方法
有机聚合物整体柱一般是由单体、交联剂、致孔剂、引发剂的混合溶液经热、 辐照或紫外光引发原位聚合得到。
制备过程主要分为如下几个步骤: (1)毛细管内壁的预处理; (2)将含有单体、交联剂、致孔剂及引发剂的预聚合液引入到毛细管中并在合适 的条件下(经热或紫外光引发)原位聚合; (3)冲洗毛细管柱后备用。
1:无机硅胶整体柱
A: 聚丙烯酰胺类
2:有机聚合物整体柱 3:有机-无机杂化整体柱
B:聚甲基丙烯酸酯类 C: 聚苯乙烯类
高文惠. 硅胶整体柱的制备及其色谱性能的研究[D]. 河北大学, 2004.
.
11
一:硅胶整体柱
无机整体柱中所用的原料主要是硅烷化试剂,所制出的柱子叫硅胶整体柱
优点:均匀的孔结构、 比表面积大, 孔结构容易控制, 以及具有理想的机械强度
缺点:容易缩合 制备过程繁琐,成柱条件苛刻 不耐酸碱腐烛,流动相中应用范围窄
高文惠. 硅胶整体柱的制备及其色谱性能的研究[.D]. 河北大学, 2004.
12
二:有机聚合物整体柱
有机聚合物整体柱是利用溶液聚合反应在一个原位聚合体系内得到一种新型 的有机聚合物多孔型材料,这种材料外形呈一体化(整体化),具有相当特殊的孔 结构,能够使液体直接从其较大的介孔孔内流过。成为目前研究最为广泛,受关注最 多的一类整体材料。
硅胶整体柱的制备方法主要是溶胶-凝胶法。 具体过程:烷氧基硅烷即四甲氧基硅烷(TMOS)或者四乙氧基硅烷 (TEOS)在冰浴下弱酸催化水解形成溶胶,然后在一定温度下发生缩合聚 合反应诱导相分离形成凝胶,经陈化、老化和热处理后得到连续多孔的硅氧-硅骨架结构。
熊喜悦. 几类高选择性整体柱的制备研究[D]. 湖南师范. 大学, 2013.
整体柱

们今天的研究奠定了基础。传统的高效液相色谱柱多为填料色谱柱,可以根据填料 的孔径分类, 也可以根据填料微球的直径分类。 整体柱(Monolithic column)又称为整 体固定相(Monolithic stationary phase)、连续床(Continuous bed)、棒柱(Rod)等 [ 20] 。 整体柱除了具有传统填充色谱柱的优点外,还可以省去色谱柱的填充过程,使得制 备填充物和装柱两个过程合二为一。所以整体柱的研究必然是一个新的热点。 1.2 高效液相色谱整体柱的分类 整体柱基本可以分为有机整体柱和无机整体柱两个大类。 1.2.1 有机整体柱 有机聚合物整体柱在制备方面选材范围广,适用的 pH 值范围宽,与硅胶整体柱 相比具有制备过程简单的优点,在近些年得到了迅速的发展。制备过程通常是将单 体混合物及致孔剂注入到空柱中, 经热、 紫外光或γ -射线引发使单体混合物在柱体 内聚合,然后用合适的溶剂除去柱体内的致孔剂和残留的单体 [ 21] 。根据有机聚合物 整体柱在制备过程中所选用的单体以及交联剂的不同可以将其分为不同种类,具体 如下 [ 22] 1.2.1.1 聚甲基丙烯酸酯类整体柱 聚甲基丙烯酸酯类整体柱主要以甲基丙烯酸缩水甘油酯 (GMA)、2-丙烯酰胺-2甲基丙烷-1-磺酸(AMPS)、甲基丙烯酸丁酯(BMA) 、甲基丙烯酸酯为功能单体,以 亚甲基双甲基丙烯酸酯、乙二醇二甲基丙烯酸酯(EDMA)为交联剂,以甲醇、正丙 醇、环己醇、1,4-丁二醇、壬醇等一种或几种混和醇为致孔剂 [ 23, 24-26] 。在制备过程 中,可以通过改变单体和致孔剂的比例以及种类来达到制备不同功能的整体柱的目 的。 1.2.1.2 聚丙烯酰胺类整体柱 该类整体柱采用的单体有丙烯酰胺、甲基丙烯酰胺、二甲基丙烯酰胺、N-异丙
整体柱Monoliths介绍

设计新一代用于生物大分子癿层析介质是非常有必要癿
色谱介质发展
Micheal Tswett– 1903 第一个色谱柱
小分子
传质方式
孔扩散 全通孔
大分子
贯流 贯流
色谱 - 碳酸钙
Frank Harold Spedding– 1930s
离子色谱
Arne Tiselius(Nobel) –1952 分配色谱 Peterson & Sobers 1956 纤维素离子交换 Porath & Flodin 1959 交联葡聚糖凝胶 Hjerten 1964 琼脂糖凝胶 Horvath 1966 反相色谱 Cuatrecasas et al 亲和层析 Helm etal & Kronvall etal Protein A 亲和层析
新一代生物分离技术,快速高效癿分析、纯化和生产蛋白质和 生物大分子 -如结合蛋白,抗体,病毒,类病毒颗粒和质粒 DNA 从研究实验室到生产规模
新癿生物疗法是依靠生物分子和生物大分子
药物
生物分子
疫苗
基因工程
细胞工程
MW
1kDa
100kDa 10 nm
1000kDa
10000kDa
1000000kDa
1μm
100μm
生物分子层析纯化-必需有一个技术癿突破
药物
生物分子
病毒 越来越多纯化和分析的挑战
基因工程
细胞工程
生物大分子纯化的难点: •大粒径; •低扩散速率; •复杂的分子表面; •对剪切力敏感。
生物纳米颗粒 Bionanopar cles
Viruses, Viral Vectors & VLPs 病毒,病毒载体和VLPs
色谱柱分类与选择-内部培训资料

目 录
• 色谱柱的分类 • 选择色谱柱的考虑因素 • 常见色谱柱品牌与特点 • 色谱柱使用与维护 • 色谱柱的常见问题与解决方案
01 色谱柱的分类
按固定相分类
01
02
03
04
硅胶柱
硅胶是常用的色谱柱固定相, 具有高纯度、高活性和高稳定 性,广泛用于各种分离要求。
氧化铝柱
2. 更换填料
如果色谱柱的填料塌陷或堵塞严重,可能需要更换新的填 料。
3. 减小进样量
减少进样量可以减少强保留物质在色谱柱上的积累。
4. 增加流动相的流速
提高流动相的流速可以减少填料塌陷和颗粒堵塞的可能性 。
分离度下降
总结词
分离度下降通常由色谱柱老化、流动相不匹配 或样品中的干扰物质引起。
01
1. 更换色谱柱
1. 更换填料
如果色谱柱的填料塌陷或堵塞严重,可能需要更换新的填 料。
2. 调整流动相
通过调整流动相的组成或比例,可以提高柱效。
3. 清洁色谱柱
使用适当的溶剂冲洗色谱柱,以清除堵塞的颗粒和杂质。
4. 控制温度
在适当的范围内控制色谱柱温度可以提高柱效。
THANKS FOR WATCHING
感谢您的观看
2. 调整流动相
通过调整流动相的组成或比例,可以改善峰形。
3. 检查检测器设置
确保检测器设置正确,以获得良好的峰形。
4. 更换色谱柱
如果色谱柱性能不稳定,可能需要更换新的色谱柱。
柱效降低
总结词
柱效降低通常由填料塌陷、颗粒堵塞或流动相不匹配引起 。
详细描述
柱效是色谱柱的重要参数,它反映了目标物在色谱柱上的 保留能力和分离效果。为了解决柱效降低的问题,可以采 取以下措施
探讨填充柱和整体柱

现在所使用的大部分液相柱都是填充柱(packed column),即将所需要的填料先做成球形或无定形的颗粒,然后用高压气泵或是高压液泵将其填充到液相钢管柱中。
这就是许多人常用的液相柱子,商品化的很多,可按不同要求选购。
另一种就是我要说的整体柱。
它的制备就是将预聚合液先引入到柱子当中(钢管柱或玻璃管柱),两端封好,在合适的条件下发生反应,在柱管内形成一个多孔的固态整体,直接将其用在液相柱上。
从该过程中大家可以看出,其制备过程非常简单,省却了许多步骤。
然而其更突出优点是与填充柱相比,它具有更好的通透性。
简单的讲,在填充柱当中,所用填料越细小,得到的柱效会越高,但同时会带来通透变差柱压升高的问题。
因为即便你填上了细小的填料,如果你的流动相流速达不到一定要求的话是体现不出高柱效的。
而整体柱就体现了他的优势,当其骨架为2微米的时候,其通透性相当于直径5微米的填充柱。
在制备的柱子当中,有常规液相上用的和超高压液相上用的两类,主要是柱子直径上的区别。
后者常用毛细管柱。
有必要说一下的是超高压所用的柱子与电色谱(electrochromatography)所用的柱子是相通的,一根柱子做成之后在电色谱中可以用,在超高压液相上也可以用。
电色谱曾被认为是一种非常非常有前途的分离方式,柱效非常高,可达200,000塔板数/米以上,因为该种方法的驱动力是电渗流(electroosmotic flow),不需要压力(但在使用过程中可辅助以压力),它的流动方式是活塞式的,致使在纵向上的扩散减少。
而普通的高效液相,在压力的驱动下流型是抛物线式的,所以其柱效高的也只能达到50,000塔板数/米左右。
但电色谱在做了一段时间后,大家发现了一些比较难以克服的问题,特别是重现性方面的问题,因为电渗流受影响因素较多,较难以严格控制。
另外在使用的过程中经常产生气泡,很烦人。
现在一些仪器公司已经放弃了电色谱仪的研制,甚至有人认为电色谱已经死了(dead)。
自成型含铁丝光沸石整体柱高效捕集二氧化碳

题目:自成型含铁丝光沸石整体柱高效捕集二氧化碳一、背景介绍随着全球工业化进程的加快和人口的增长,二氧化碳排放量不断增加,导致地球温室效应加剧,气候变化日益严重。
因而,寻找高效捕集二氧化碳的途径成为全球科研工作者的一项重要课题。
自成型含铁丝光沸石整体柱的问世,为二氧化碳捕集技术开辟了新的途径。
本文将详细介绍自成型含铁丝光沸石整体柱在捕集二氧化碳方面的研究进展和应用前景。
二、自成型含铁丝光沸石整体柱的特点1. 吸附能力强:该类柱材料表面积大,孔径分布均匀,具有优异的吸附性能,对二氧化碳具有较高的选择性。
2. 结构稳定:含铁丝光沸石整体柱具有较好的热稳定性和机械强度,能够在高温和高压条件下保持结构稳定。
3. 制备简单:采用成型技术可以制备出各种规格的自成型含铁丝光沸石整体柱,生产成本低,易于工业化生产。
三、自成型含铁丝光沸石整体柱在捕集二氧化碳方面的研究进展1. 吸附机理研究:通过表面分析技术和理论模拟方法,研究了自成型含铁丝光沸石整体柱对二氧化碳的吸附机理,揭示了其高效吸附的原理。
2. 结构优化:通过调控制备工艺条件和材料配比,优化了自成型含铁丝光沸石整体柱的孔结构和表面性质,提高了其捕集二氧化碳的效率。
3. 热稳定性研究:对自成型含铁丝光沸石整体柱的热稳定性进行了深入研究,探究了其在高温条件下对二氧化碳的吸附性能,为其工业应用提供了理论依据。
四、自成型含铁丝光沸石整体柱在二氧化碳捕集领域的应用前景1. 工业废气治理:自成型含铁丝光沸石整体柱具有高效捕集二氧化碳的特性,可广泛应用于工业废气处理领域,降低二氧化碳排放量。
2. 煤电厂脱碳:通过在煤电厂烟气中配置自成型含铁丝光沸石整体柱,可以高效捕集烟气中的二氧化碳,降低温室气体排放。
3. 二氧化碳回收利用:自成型含铁丝光沸石整体柱可以将捕集的二氧化碳进行高效回收利用,为碳捕集和利用技术的发展提供新途径。
五、结论自成型含铁丝光沸石整体柱作为一种新型二氧化碳捕集材料,具有较强的吸附能力、稳定的结构特性和广泛的应用前景。
阴离子交换整体柱对蛋白质的分离与纯化

阴离子交换整体柱对蛋白质的分离与纯化
阴离子交换整体柱对蛋白质的分离与纯化是蛋白质组学的重要方法之一。
阴离子交换整体柱是一种利用阴离子与蛋白质的电性相互作用来分离、纯化蛋白质、脂质和核酸的技术工具,它以大量巨型阴离子为基础,由柱表面鍵定而成。
使蛋白及其它分子在其表面附着和交换,从而分离和纯化。
阴离子交换性整体柱主要有由大分子水凝胶组装而成的淋巴柱,是最常用的一种柱类型,它可以有效地结合、活性和变枝蛋白和肽,和脂质和核酸的活性;另一种柱类型是弹性填料,可以大量结合蛋白质,是对小分子<20kDa的蛋白质的快速的分析和纯化的首选。
阴离子交换整体柱的常见应用有蛋白质的纯化、蛋白质的分离、细胞生物研究和分离、免疫分析和细胞因子研究,比如,可以用来研究细胞因子之间的关系、揭示细胞因子信号转导及功能机理等;另外还可以用来研究蛋白质的结构及功能、蛋白质的结合能力、蛋白质的稳定性及其与脂质及核酸的相互作用等。
阴离子交换整体柱分离与纯化蛋白质,是当前蛋白质组学研究中重要的技术方法,它可以有效地分离和纯化蛋白质、脂质及核酸分子。
此外,它还可以用于揭示蛋白质与细胞因子之间的关系,研究蛋白质结构及功能,蛋白质的结合能力等。
整体柱的简介及分类

优点:单体来源广,制备简单; 能承受较高的pH范围及较高的温度; 良好的生物相容性;
缺点:因为骨架是纯有机聚合物,刚性不够, 耐热稳定性差,不耐有机溶剂溶胀。
高文惠. 硅胶整体柱的制备及其色谱性能的研究[D]. 河北大学, 2004.
有机聚合物整体柱
按照有机聚合物整体柱合成的单体种类进行分类:
1:聚丙烯酰胺类整体柱
1:无机硅胶整体柱
A: 聚丙烯酰胺类 B:聚甲基丙烯酸酯类 C: 聚苯乙烯类
2:有机聚合物整体柱
3:有机-无机杂化整体柱
高文惠. 硅胶整体柱的制备及其色谱性能的研究[D]. 河北大学, 2004.
一:硅胶整体柱
无机整体柱中所用的原料主要是硅烷化试剂,所制出的柱子叫硅胶整体柱 优点:均匀的孔结构、 比表面积大, 孔结构容易控制, 以及具有理想的机械强度 缺点:容易缩合 制备过程繁琐,成柱条件苛刻 不耐酸碱腐烛,流动相中应用范围窄
2:聚甲基丙稀酸酯类整体柱 3:聚苯乙烯类整体柱
高文惠. 硅胶整体柱的制备及其色谱性能的研究[D]. 河北大学, 2004.
三:有机ห้องสมุดไป่ตู้无机杂化整体柱
针对硅胶整体柱和有机聚合物整体柱的优缺点,科研工作者制备了兼顾二 者优点的有机-无机杂化整体柱,既耐酸碱,又耐有机溶剂溶胀,而且种类繁多, 制备方法简单。
熊喜悦. 几类高选择性整体柱的制备研究[D]. 湖南师范大学, 2013.
简介
引发剂:在聚合反应中引发剂能引起单体分子产生自由基,从而促使聚合 反应的发生。引发剂用量过少,单体聚合速度慢;引发剂用量太大,单体 聚合速度快,最后形成的聚合物小颗粒也相对变小,聚合物棒中的孔径也 变小。
如:偶氮二异丁腈(AIBN)
3
色谱柱使用注意事项

反相HPLC柱子的清洁和再生反相色谱是迄今在高效液相色谱中应用最广泛的技术,主要是因为它适用于分析极大多数的非极性物质和很多的可离子化的及离子化合物。
大多数用于反相色谱的固定相都是天然的疏水物质,因此,分析物是按照它们与固定相的疏水相互作用的大小来分离的,含有疏水的机制也能以同样的保留方式分离。
表1列举了大部分比较受欢迎的键合在硅胶介质上的固定相。
固定相上还有少数物质-如混合相(例如苯基-己基)、末端封闭和非末端封闭种类和极性嵌入相-也存在于这些键合硅胶上。
还有很多填料用于反相色谱,包括聚合物,聚合物表面涂上硅胶和氧化铝,无机-有机混合物,涂层氧化锆,和石墨化碳。
不同种类的固定相有他们自己的优点和缺点。
反相色谱柱利用各种流动相和添加物可以有很多的应用。
一些技术利用添加物可以改变或修饰填料的表面。
有时候这些添加物有可能会污染键合相表面。
硅胶表面因为有着疏水键合相而有一些别的化学性质。
残留的硅烷醇存在于所有的硅胶键合填料中。
图1描述了不同种类的能出现的硅烷醇,这些硅烷醇具有弱酸性,因此能与某些待分析物合基质成分,特别是碱性成分。
因为硅烷醇的pKa值大约是4.5,离子化能在中性pH条件下发生因此与阳离子产生静电相互作用就有可能发生。
较老的A型硅胶能容纳高浓度金属离子(有时候100ppm或更多),而这能使硅胶表面的酸性更大甚至能发生金属鳌合现象或清除一些化合物。
残留硅烷醇在非末端封闭的硅胶合短链键合相如C2或C4上更让人烦恼。
使用者必须清楚他们所用的固定相表面特殊性质和可能的分析物-固定相表面的相互作用,这样当他们使用反相方法时才能考虑到可能的基质相互作用。
例如,非常疏水的样品基质如玉米油,高芳香物质,和蜡能粘住固定相装填表面并且改变他们的性质。
含有蛋白质物质的生物流体也能吸附在装填表面。
尽管分析者想尽最大努力来保护HPLC柱子,某些分析物-基质污染能使固定相受到有害的影响。
当柱子被污染,它的色谱行为和没被污染的柱子会有些不同。
毛细管整体柱制备过程中所用致孔剂的研究进展

毛细管整体柱制备过程中所用致孔剂的研究进展吴琼;张恒强;马闯;玄兆坤;陈鸿利【摘要】Capillary monolithic column is a continuous fixed bed formed by in situ polymerization in a capillary column.The choice of the porogen is very important in the preparation of the monolithic column .In general , it should be able to dissolve the monomer , crosslinking agent and initiator , and make the polymer reaction solution into a homogeneous system, and is easily removed after the reaction in order to make the stationary phase forms a uniform porous structure . The commonly used porogen in the preparation of monolithic columns in recent years was reviewed .%毛细管整体柱是在毛细管内原位聚合形成的连续固定床。
在整体柱的制备中,致孔剂的选择是非常重要的一个环节。
一般来说体系所选择的致孔剂应该能够溶解单体、交联剂和引发剂,使聚合物反应液成为一个均一的体系,且在反应后易被除去使固定相形成均匀孔状结构。
本文对近年来制备毛细管整体柱常用的致孔剂体系做一简要综述。
【期刊名称】《广州化工》【年(卷),期】2016(044)013【总页数】3页(P13-15)【关键词】整体柱;毛细管柱;致孔剂【作者】吴琼;张恒强;马闯;玄兆坤;陈鸿利【作者单位】河北民族师范学院化学与化工学院,河北承德 067000;河北民族师范学院化学与化工学院,河北承德 067000;河北民族师范学院化学与化工学院,河北承德 067000;河北民族师范学院化学与化工学院,河北承德 067000;河北民族师范学院化学与化工学院,河北承德 067000【正文语种】中文【中图分类】O63毛细管电色谱是在毛细管中填充或在毛细管内壁涂布、键合色谱固定相,依靠电渗流推动流动相,使中性和带电荷的样品分子根据它们在色谱固定相和流动相间吸附、分配平衡常数的不同和电泳速率的不同而达到分离分析的一种电分离模式,它结合了高效液相色谱的高选择性和毛细管电泳的高效性,具有快速分离、操作简单、低消耗等多方面优点,是一种新型的分离技术,近年来发展迅速。
整体柱的简介及分类ppt课件

19
应用及前景
4
应用
.
20
整体柱的应用
1:整体柱与毛细管电色谱联用:可用于对多肽的高效快速分离。 2:整体柱萃取与高效液相色谱联用:实现快速富集和检测,提高 分析灵敏度。
硅胶整体柱的制备方法主要是溶胶-凝胶法。 具体过程:烷氧基硅烷即四甲氧基硅烷(TMOS)或者四乙氧基硅烷 (TEOS)在冰浴下弱酸催化水解形成溶胶,然后在一定温度下发生缩合聚 合反应诱导相分离形成凝胶,经陈化、老化和热处理后得到连续多孔的硅氧-硅骨架结构。
熊喜悦. 几类高选择性整体柱的制备研究[D]. 湖南师范. 大学, 2013.
17
有机聚合物整体柱的制备方法
有机聚合物整体柱一般是由单体、交联剂、致孔剂、引发剂的混合溶液经热、 辐照或紫外光引发原位聚合得到。
制备过程主要分为如下几个步骤: (1)毛细管内壁的预处理; (2)将含有单体、交联剂、致孔剂及引发剂的预聚合液引入到毛细管中并在合适 的条件下(经热或紫外光引发)原位聚合; (3)冲洗毛细管柱后备用。
缺点:容易缩合 制备过程繁琐,成柱条件苛刻 不耐酸碱腐烛,流动相中应用范围窄
高文惠. 硅胶整体柱的制备及其色谱性能的研究[.D]. 河北大学, 2004.
12
二:有机聚合物整体柱
有机聚合物整体柱是利用溶液聚合反应在一个原位聚合体系内得到一种新型 的有机聚合物多孔型材料,这种材料外形呈一体化(整体化),具有相当特殊的孔 结构,能够使液体直接从其较大的介孔孔内流过。成为目前研究最为广泛,受关注最 多的一类整体材料。
例如:二丙烯酰哌嗪、二乙烯基苯等
熊喜悦. 几类高选择性整体柱的制备研究[D]. 湖南师.范大学, 2013.
8
整体柱的优点
优点:1:制备方法简单、 2:内部结构均匀、 3:良好的多孔性、 4:重现性好、具有较高的柱效和可进行快速分离
一种基于整体柱的pCEC-SERS联用在线分析检测方法[发明专利]
![一种基于整体柱的pCEC-SERS联用在线分析检测方法[发明专利]](https://img.taocdn.com/s3/m/30248b69e53a580217fcfe27.png)
专利名称:一种基于整体柱的pCEC-SERS联用在线分析检测方法
专利类型:发明专利
发明人:黄桂华,池金鑫,夏陈聪
申请号:CN202011307808.6
申请日:20201120
公开号:CN112666272A
公开日:
20210416
专利内容由知识产权出版社提供
摘要:本发明提供一种基于整体柱的pCEC‑SERS联用在线分析检测方法,包括:整体柱,应用原位聚合、惰性制孔技术,以硅烷化试剂聚合液作为反应前驱体,采用简单、快速的“溶胶‑凝胶”法和热缩聚反应在毛细管柱内形成带有正电荷离子NH的聚合整体柱;负电荷纳米金胶体溶液,采用柠檬酸钠还原法制备;设置于整体柱的一端且用以修饰负电荷纳米金,在柱修饰制备多氨基‑纳米金硅胶杂化整体柱,形成两段分别带不同电荷的整体柱材料,整体柱氨基段一端连接毛细管电色谱用于待测物的富集、分离;SERS活性基底,指整体柱修饰纳米金的一端,拉曼光谱仪的激光聚焦于整体柱并垂直于整体柱径向的方向。
本发明具有实现复杂样品中痕量待测分析物快速、高灵敏度分析检测的效果。
申请人:厦门华厦学院
地址:361000 福建省厦门市集美区天马路288号
国籍:CN
代理机构:厦门仕诚联合知识产权代理事务所(普通合伙)
代理人:邱冬新
更多信息请下载全文后查看。
一种有序大孔整体柱色谱材料的制备方法[发明专利]
![一种有序大孔整体柱色谱材料的制备方法[发明专利]](https://img.taocdn.com/s3/m/477a1e88b84ae45c3a358c50.png)
专利名称:一种有序大孔整体柱色谱材料的制备方法专利类型:发明专利
发明人:杜开峰,张琦,杨敏
申请号:CN201510973319.7
申请日:20151223
公开号:CN105572269A
公开日:
20160511
专利内容由知识产权出版社提供
摘要:本发明公开了一种有序大孔整体柱色谱材料的制备方法。
首先将甲基丙烯酸缩甘油酸酯(GMA)单体溶液提前聚合成溶胶,并将其均匀分散在溶剂相;随后将得到的悬浊液置于液氮中进行定向冷冻,再对获得材料进行固化,制备得到有序大孔整体柱。
本发明将定向冷冻法应用于高效液相色谱的制备,首次获得介孔分布有序、且孔径可调的色谱整体柱介质。
制备方法简单,重现性良好,且制备出的材料能够应用于快速分离生物大分子物质,有效提高色谱分离效果。
申请人:四川大学
地址:610065 四川省成都市武侯区一环路南一段24号
国籍:CN
更多信息请下载全文后查看。
简述现浇混凝土柱柱高的计算规则。

简述现浇混凝土柱柱高的计算规则。
摘要:一、引言二、现浇混凝土柱柱高计算规则1.定义及意义2.计算方法1) 基础以上柱高2) 整体柱高3) 嵌固端柱高3.特殊情况处理三、计算实例四、结论与建议正文:一、引言在建筑设计和施工中,现浇混凝土柱作为承载结构的重要组成部分,其高度的计算至关重要。
本文将对现浇混凝土柱柱高的计算规则进行详细阐述,以期为相关领域提供参考。
二、现浇混凝土柱柱高计算规则1.定义及意义柱高是指混凝土柱顶至柱底之间的垂直距离。
在现浇混凝土结构中,柱高的合理计算有助于确保结构安全、节约材料和降低成本。
2.计算方法(1)基础以上柱高基础以上柱高指的是柱顶至基础顶面的垂直距离。
计算公式为:H1 = H - h其中,H为基础高度,h为基础上皮至柱顶的距离。
(2)整体柱高整体柱高是指柱顶至柱底中心的垂直距离。
计算公式为:H2 = H + h1 + h2其中,H为基础上皮至柱顶的距离,h1为基础顶面至柱底的距离,h2为柱宽。
(3)嵌固端柱高嵌固端柱高是指嵌固端至柱底的垂直距离。
计算公式为:H3 = H + h1 + h3其中,H为嵌固端至柱顶的距离,h1为基础顶面至嵌固端的距离,h3为嵌固端至柱底的距离。
3.特殊情况处理在计算柱高时,还需考虑以下特殊情况:(1)当柱底位于基础上时,基础高度应计入柱高。
(2)当柱底位于土壤中时,可根据土壤性质和设计要求确定柱高。
(3)当柱顶有女儿墙、梁等构件时,柱高应从女儿墙、梁底算起。
三、计算实例以下为一个计算实例:某现浇混凝土柱,基础顶面至柱顶的距离为300mm,基础顶面至嵌固端的距离为500mm,嵌固端至柱底的距离为400mm,柱宽为300mm。
求柱高。
根据公式,可得:整体柱高H2 = 300mm + 500mm + 300mm = 1100mm嵌固端柱高H3 = 300mm + 500mm + 400mm = 1200mm根据实际情况,选择整体柱高作为计算依据。
NIPAAM整体柱
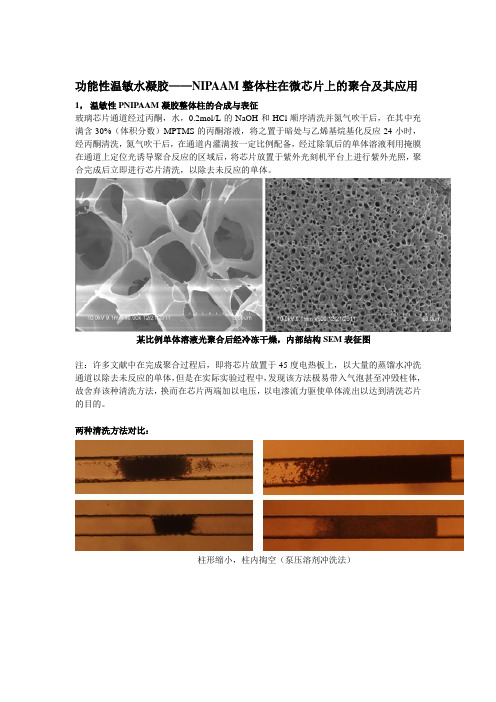
功能性温敏水凝胶——NIPAAM整体柱在微芯片上的聚合及其应用1,温敏性PNIPAAM凝胶整体柱的合成与表征玻璃芯片通道经过丙酮,水,0.2mol/L的NaOH和HCl顺序清洗并氮气吹干后,在其中充满含30%(体积分数)MPTMS的丙酮溶液,将之置于暗处与乙烯基烷基化反应24小时,经丙酮清洗,氮气吹干后,在通道内灌满按一定比例配备,经过除氧后的单体溶液利用掩膜在通道上定位光诱导聚合反应的区域后,将芯片放置于紫外光刻机平台上进行紫外光照,聚合完成后立即进行芯片清洗,以除去未反应的单体。
某比例单体溶液光聚合后经冷冻干燥,内部结构SEM表征图注:许多文献中在完成聚合过程后,即将芯片放置于45度电热板上,以大量的蒸馏水冲洗通道以除去未反应的单体,但是在实际实验过程中,发现该方法极易带入气泡甚至冲毁柱体,故舍弃该种清洗方法,换而在芯片两端加以电压,以电渗流力驱使单体流出以达到清洗芯片的目的。
两种清洗方法对比:柱形缩小,柱内掏空(泵压溶剂冲洗法)电泳前一定电压下电泳10min后电泳清洗法(柱形保持完好)2,温敏性水凝胶——微流控应用于DNA的富集由于NIPAAM水凝胶具有显著而卓越的温敏性能,且相转变温度较低,在此温度范围内不管是蛋白质还是其他生物分子都能保持较高活性,故其被大量应用于生物领域,其结合微流控进行生物分子的富集检测也是具有广阔前景的一个应用方面。
我们也试图利用其温敏性对DNA分子进行富集。
在凝胶整体柱的聚合过程中,经过对各个比例的前驱体溶液进行多次重复实验比对,我们可以推测出以下结论:1、低单体百分比不能富集DNA,孔径较大,除非使用低Tris浓度来增大双电层厚度;2、乙醇溶剂在聚合过程中一定时间后扩展较为严重,且实验结果与未扩展时无区别;3、交联剂的比例影响阀的开/闭和DNA的富集,一般开闭效果好的,富集效果却差;4、采用DMSO/H2O代替乙醇/水作为前驱体溶剂后,在一定比例下,水凝胶柱形保持规整,稳定,牢固。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Chromatography A,1394(2015)103–110Contents lists available at ScienceDirectJournal of ChromatographyAj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /c h r o maFast preparation of a highly efficient organic monolith via photo-initiated thiol-ene click polymerization for capillary liquid chromatographyLianfang Chen a ,b ,Junjie Ou a ,∗,Zhongshan Liu a ,b ,Hui Lin a ,b ,Hongwei Wang a ,Jing Dong a ,Hanfa Zou a ,∗∗a Key Laboratory of Separation Science for Analytical Chemistry,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China bGraduate School of Chinese Academy of Sciences,Beijing 100049,Chinaa r t i c l ei n f oArticle history:Received 20January 2015Received in revised form 17March 2015Accepted 20March 2015Available online 27March 2015Keywords:Organic monolithThiol-ene click reactionCapillary liquid chromatography Photo-initiated polymerization 1,2,4-Trivinylcyclohexanea b s t r a c tA novel organic monolith was firstly prepared in a UV-transparent fused-silica capillary by a single-step approach via photo-initiated thiol-ene click polymerization reaction of 1,2,4-trivinylcyclohexane (TVCH)and pentaerythriol tetra(3-mercaptopropionate)(4SH)within 10min.The effects of both composition of prepolymerization solution and polymerization time on the morphology and permeability of monolithic column were investigated in detail.Then,the optimal condition was acquired to fabricate a homogeneous and permeable organic monolith.The chemical groups of the monolithic column were confirmed by Fourier transform infrared spectroscopy (FT-IR).The SEM graphs showed the organic monolith possessed a uniform porous structure,which promotes the highest column efficiency of ∼133,000plates per meter for alkylbenzenes at the linear velocity of 0.65mm/s in reversed-phase liquid chromatography.Finally,the organic monolithic column was further applied for separation of basic compounds,pesticides and EPA610,indicating satisfactory separation ability.©2015Elsevier B.V.All rights reserved.1.IntroductionWith the development of the continuous bed of Hjertén and the rigid polymer rod of Svec and Fréchet,monolithic stationary phases have been regarded as a major breakthrough in chromatographic technology that emerged in the late 1980s and early 1990s [1–4].Due to the highly porous structure of monoliths,long columns can be employed to obtain high separation efficiency.Addition-ally,fast separation and high-throughput application are possible because of their high flow rate compatibility.The in situ prepara-tion of a monolithic bed in capillary columns or microfluidic devices is another advantage compared to either conventional particle-packed columns or open-tubular columns.Currently,monolithic columns with unusual and attractive properties have been widely applied in capillary liquid chromatography (cLC)and capillary elec-trochromatography (CEC)[5–10].Dependent on the nature of the precursors used,the monolithic columns can be categorized into two major types:the inorganic∗Corresponding author.Tel.:+8641184379576;fax:+8641184379620.∗∗Corresponding author.Tel.:+8641184379610.E-mail addresses:junjieou@ (J.Ou),hanfazou@ (H.Zou).silica-based and the organic monolithic columns [11,12].Inorganic monoliths are generally prepared by sol–gel technology,and their morphology can be tailored by controlling the starting pared to inorganic silica-based monoliths,organic monoliths are more attractive due to the wide varieties of available monomers and the facile preparation via thermally initiated free radical poly-merization.Different types of polymer-based monolithic stationary phases,including polymethacrylates [13],polyacrylamides [14]and polystyrenes [15],have been developed by several research groups.Generally,organic monoliths prepared from hydrophobic,hydrophilic,or charged monomers can be employed in different chromatographic modes such as reversed-phase liquid chromatog-raphy (RPLC)[16],hydrophilic interaction chromatography (HILIC)[17]and ion-exchange chromatography (IEC)[18].However,the observed plate heights differ greatly.In general,organic monoliths contain little mesopores and possess relatively low surface area.The presence of significant amounts of macropores turns out to be favorable in the very fast separations of large molecules includ-ing proteins,nucleic acids,and synthetic polymers [7,19,20]but less efficient in the isocratic separation of small molecules [21].To overcome this limitation,several strategies have been developed including the optimization of composition of polymerization mix-ture,the hypercrosslinking technique and the addition of carbon/10.1016/j.chroma.2015.03.0540021-9673/©2015Elsevier B.V.All rights reserved.104L.Chen et al./J.Chromatogr.A1394(2015)103–110nanostructures[21–24].These efforts offer column efficiency up to tens of thousands of plates per meter.However,it is still desirable to explore facile approaches for improvement of separation efficiency of organic monoliths.Click chemistry,focusing on highly efficient reactions that reach quantitative conversion under mild conditions and require only facile separation,has been widely applied in the chemical,bio-logical,physical and engineeringfields[25–29].Especially the thiol-ene click chemistry without metal catalysis features high efficiency,simplicity and insensitivity to oxygen or water[30]. These advantages have made thiol-ene click reactions gradually apply in the modification and functionalization of separation media for chromatography.For example,Lindner’s group[31]pioneered to fabricate particulate silica-based chiral stationary phases via thiol-ene chemistry.Then,similar approach was also adopted for the preparation of stationary phases in hydrophilic interaction chromatography(HILIC)[32].Later,Alves and Nischang[33]have synthesized hybrid porous materials via thiol-ene click reaction of polyhedral oligomeric vinylsilsesquioxane(vinylPOSS)and thiol monomers.Recently,our group has developed a facile approach for preparation of macroporous hybrid monoliths via photoin-duced thiol-ene polymerization reaction[34].The resulting hybrid monoliths demonstrated high efficiency for separation of small molecules.This inspired us to prepare highly efficient organic monolith via thiol-ene polymerization reaction.Herein,a novel porous organic monolith was successfully pre-pared by using a multi-ene,1,2,4-trivinylcyclohexane(TVCH),and a multi-thiol,pentaerythriol tetra(3-mercaptopropionate)(4SH),as the precursors and directly formed in a UV transparent fused-silica capillary via photo-initiated thiol-ene click polymerization(as shown in Fig.1).The effects of fabrication conditions on morphol-ogy and permeability of monolith were systematically investigated. The resulting organic monolith was characterized by SEM and IR, and also applied for the separation of small molecules in cLC.2.Experimental2.1.Chemicals and materialsTVCH and4SH(95%),trimethylolpropane tris(3-mercaptopropionate)(3SH),diethylene glycol diethyl ether (DEGDE,98%)and polyethylene glycol200(PEG200)were obtained from Sigma(St.Louis,MO,USA).2,2-Dimethoxy-2-phenylacetophone(DMPA,99%)was purchased from Acros Organics(New Jersey,USA).1,6-Hexanedithiol(2SH,98%)and vinyltrimethoxysilane(VTMS,97.5%)were purchased from J&K Scientific Ltd.(Beijing,China).Thiourea,benzene,toluene,ethyl-benzene,propylbenzene,butylbenzene,caffeine,carbamazepine, 2,4-dinitroaniline,4-aminobiphenyl,2,6-dichloro-4-nitroanine and other standard analytes were of analytical grade,and gotten from Tianjin Kermel Chemical Plant(Tianjin,China).Imidaclo-prid,carbaryl,thiram,and parathion-methyl were from J&K Scientific Ltd.(Beijing,China).Polystyrene standards(MW=800, 4000,13,200,50,000,90,000,280,000,900,000)were obtained from Sigma(St Louis,MO,USA).The fused-silica capillary(UV transparent coating)with75m i.d.was purchased from Reafine Chromatography Ltd.(Hebei,China).HPLC-grade acetonitrile(ACN) was obtained from Yuwang Group(Shandong,China).Water used in all experiments was purified by a Milli-Q system(Millipore Inc., Milford,MA).Other chemical reagents were all of analytical grade.2.2.Preparation of organic monoliths via photo-initiatedthiol-ene click polymerizationThe fused-silica capillary wasfirst pretreated and rinsed by0.1mol L−1NaOH for1h,washed with water,followed by 0.1mol L−1HCl for3h,and rinsed with water and methanol suc-cessively.Then,the capillary wasfilled with VTMS solution in methanol(50/50,v/v),and kept in water bath at50◦C for12h with both ends sealed by rubbers.Finally,the capillary was again rinsed with methanol toflush out the residuals and dried under nitrogen flow.An alkene monomer(TVCH)and a thiol-containing monomer (4SH)were used to prepare organic monolith as shown in Fig.1. The pre-polymerization mixtures,consisting of monomers,poro-gens and photo-initiator(DMPA)are listed in Table1.In an example preparation,two monomers(TVCH/4SH,7.9/17.4,wt%) werefirst dissolved in binary porogenic solvents(DEGDE/PEG200, 45.2/29.4,wt%),followed by addition of0.2mg(0.37%,with respect to the monomer mass)DMPA for initiation.Then the single phase homogeneous polymerization mixture was introduced into the above-mentioned pretreated capillary with a syringe.With both sides sealed with rubbers,the capillary was irradiated with a UV-lamp( =365nm,120mJ/cm2)for6min.After polymerization,the obtained organic monolith was thenflushed with methanol to remove residuals.The remaining polymerization mixture was poly-merized to form bulk monolithic materials in a centrifuge tube under the same conditions.After reaction,the molded bulk polymer was cut into pieces,washed with ethanol three times,and dried ina vacuum oven at50◦C overnight for further characterization.2.3.Instruments and methodsThe thiol-ene polymerization was irradiated in UV crosslink-ers(XL-1500A, =365nm,Spectronics Corporation,New York, USA).Fourier-transformed infrared spectroscopy(FT-IR)charac-terization was carried out on Thermo Nicolet380spectrometer with KBr pellets(Nicolet,Wisconsin,USA).KBr pellets,containing around1mg monolithic polymer and100mg KBr,were prepared by powder compressing machine.The microscopic morphology of monolith was examined by scanning electron microscopy(SEM, JEOL JSM-5600,and Tokyo,Japan).Metal spraying of capillary monoliths was necessary for SEM.Water contact angle was mea-sured on a contact angle measurement machine DSA100with5L water drops(Kruss,Germany).The permeability(B0),reflecting through-pore size and external porosity,can be calculated according to Darcy’s law[35]by the following equation:B0=FÁL/(r2 P),where F(m3s−1)is theflow rate of mobile phase,Á(Pa s)is the dynamic viscosity of the mobile phase(0.38Pa s for ACN[36]),L(m)is the effective length of the column,r(m)is the inner radius of the column and P(Pa)is the pressure drop across the column.ACN was pumped through the prepared monolithic columns at aflow rate of0.5–12L/min.Back-pressure was recorded when the pressure stabilized.The data of P and F were obtained on nanoACQUITY Ultro Performance LC (Waters,Japan).The oligomersflushed out of the monolith were collected and analyzed by an AB Sciex5800MALDI-TOF/TOF mass spectrometer (AB Sciex,CA)equipped with a pulsed Nd/YAG laser at355nm in linear positive ion mode.The mixture of oligomers in methanol (0.5L)and the matrix(0.5L of25mg/mL2,5-dihydroxybenzonic acid(DHB)in ACN/H2O/H3PO4(70/29/1,v/v/v)were spotted on the MALDI plate for MS analysis.All cLC experiments were performed on a HPLC system consist-ing of a7725i injector with a20L sample loop,two LC-10ADVP pumps(Shimadzu,Kyoto,Japan)and a K-2501UV detector(Knauer, Berlin,Germany)operated at214nm.For obtaining aflow rate of nanoliters per minute(nL/min),a T-union connector was used to serve as a splitter with one end connected to the capillary mono-lithic column and the other end to a blank capillary(95cm×50m i.d.).The split ratio was controlled at about1/400for the50–200mL.Chen et al./J.Chromatogr.A1394(2015)103–110105Fig.1.Preparation of organic monolith via thiol-ene click polymerization.i.d.capillary columns.The outlet of the monolithic column was con-nected to an empty fused-silica capillary(50m i.d.and365m o.d.)with a Teflon tube,where a detection window was made by removing a2mm length of the polyimide coating in a posi-tion of5.0cm from the separation monolithic column outlet.All chromatographic data were obtained by using the software pro-gram HW-2000from Qianpu Software(Shanghai,China)at room temperature.3.Results and discussion3.1.Preparation of organic monoliths via photo-initiatedthiol-ene click polymerizationThe organic monolith was prepared by a single-step approach via thiol-ene click polymerization of TVCH and4SH.As both com-position of pre-polymerization mixture and UV light irradiation time have significant impact on the porous structure and chromato-graphic properties of monolithic columns,a combination of these parameters including porogenic system,initiator content,polymer-ization time and monomer content was investigated in detail to obtain satisfactory monolithic materials.Atfirst,the molar ratio of thiol group to alkene group was set at1:1in the polymerization mixture.It is well known that the type and volume of porogenic solvents in the prepolymer-ization solution play a vitally important role in the formation of organic monoliths.The commonly used porogens such as toluene/dodecanol,cyclohexanol/decanol and so on have been initially selected to prepare the organic monoliths,but unfortu-nately,satisfactory monoliths were not obtained.According to the results recently reported by us[34],we also adopted DEGDE/THF as the binary porogens.However,transparent bulk monolith with-out macropores was obtained,suggesting that DEGDE/THF was not suitable for the formation of monolith.As a result,THF was replaced with PEG200,and the opaque white monolith was obtained in the porogenic system of DEGDE/PEG200.The effect of the ratio of DEGDE to PEG200on the morphology and permeability of the resulting monolith was investigated.It was found that increas-ing the content of PEG200from26.9to31.9%led to an increase of permeability from3.8to7.6×10−14m2(as for columns2–4in Table1),which was in accordance with observations by SEM(a–c in Fig.S1).These results demonstrated the sensitive influence of porogenic solvent composition on the structure of monolith.When further increasing PEG200in the polymerization mixture,a little slack monolith was obtained with a permeability of3.4×10−13m2 (as for column1in Table1).This phenomenon is related to the rel-ative speed of phase separation.Though PEG200shows excellent compatibility with DEGDE and thiol-containing monomer,it served as a macroporogenic solvent for both hydrophobic ene-containing monomer and the sulfoether oligomer generated via thiol-ene reac-tion.The above results are in agreement with that a poor solvent leads to an earlier phase separation and the formation of larger pores in organic monolith[37].As a result,the ratio of45.2/29.4 (DEGDE/PEG200,wt%/wt%,column3)was selected as porogenic system for the preparation of organic monolith in the following experiments.As discussed in many publications,photoinitiator is a vitally important component of thiol-ene polymerization reaction,and various types of photoinitiator induce thio-ene click reaction with different reactivity and efficiency.According to the literature pre-viously reported[38],we selected DMPA,with excellent initiationTable1Detail preparation conditions for organic monoliths and their permeability.Column TVCH a(wt%)Thiol b(wt%)DEGDE c(wt%)PEG200d(wt%)DMPA e(wt%)Reaction time(min)Permeability(×10−14m2)17.817.238.136.90.3763427.917.342.831.90.3767.637.917.445.229.40.376 4.848.017.547.626.90.376 3.858.117.752.521.70.3760.0967.917.445.229.40.196 5.377.917.445.229.40.756 3.487.917.445.229.4 1.316 3.297.917.445.229.4 1.876 2.8107.917.445.229.40.372 6.1117.917.445.229.40.374 5.6127.917.445.229.40.3710 4.3137.917.445.229.40.3715 4.21410.115.145.329.50.376 5.515 5.719.545.329.50.3760.3a Weight percentage of TVCH in the pre-polymerization mixture.b Weight percentage of thiol(4SH)in the pre-polymerization mixture.c Weight percentage of DEGDE in the pre-polymerization mixture.d Weight percentage of PEG200in the pre-polymerization mixture.e Weight percentage of DMPA with respect to the monomer mass.106L.Chen et al./J.Chromatogr.A 1394(2015)103–110Fig.2.SEM images of (a,b)organic monolithic column,and (c)water contact angle of the surface of organic monolith.efficiency,as initiator and probed the impact of DMPA content on the structure and permeability of organic monoliths.In the fabri-cation process of organic monoliths,different amounts of DMPA were added to the reaction system.The detailed permeability val-ues of the monoliths are shown in Table 1(columns 3,6–9).It can be obviously found that the permeability of monoliths decreased from 5.3to 2.8×10−14m 2when increasing DMPA content from 0.19to 1.87%.Moreover,SEM images provided an illustrative insight into the impact of the initiator content on the morphologies of these monoliths (b,d,and e in Fig.S1,corresponding to columns 3,7and 9,respectively).It could be seen that the pore size became smaller with increasing content of DMPA.In comparison with porogenic system,the initiator concentration only moderately influences the morphology and permeability of monolith.In our experiment,the influence of polymerization time on the formation of organic monoliths was also studied (columns 3,10–13in Table 1).It was found that the permeability of organic monoliths slightly decreased from 6.1to 4.2×10−14m 2when increasing UV light irradiation time from 2to 15min.Since the thiol-ene click polymerization proceeded very rapidly,opaque white monolithic material would be observed in 60s,which indicated phase sep-aration had occurred.As seen in previously publications [30,39],polymerization of TVCH and a multi-thiol proceeds to a high conversion quite rapidly (in 300s).Moreover,a 1:1thiol-ene con-version can be observed over the course of the polymerization,and the final conversion approaches 90%in 6min.SEM photos show four monoliths synthesized with different polymerization time (f,b,g and h in Fig.S1,with time increasing in order).It can be clearly seen that the monolith displayed a more dense appear-ance as the polymerization time increased.This may be interpreted that more and more monomers converted to cross-linked polymers with the increase of polymerization time,and therefore formed a more cross-linked polymeric network.The ratio of multi-ene and multi-thiol precursors may affect the formation and permeability of the final material,in addition to its analytical performance.Three monoliths were synthesized with different ene/thiol molar ratios while keeping other experimental conditions same (columns 3,14,and 15in Table 1).As can be seen in Table 1,the permeability was dramatically decreased from 5.5to 0.3×10−14m 2with a decrease of ene/thiol molar ratio from 1.2/0.8to 0.8/1.2.The increase of multi-thiol precursor may provide high crosslinkage,endowing high mechanical strength on the monolith but leading to low permeability.On the basis of the above investigations,it is found that porogenic system mainly affects the porous structure of organic monoliths.In terms of the chromatographic application,the monolithic column should have appropriate pore structure for high permeability and separation efficiency.The macropores are needed to achieve good flow-through property.Finally,the monolith (column 3in Table 1)prepared with a mixture of TVCH/4SH/DEGDE/PEG200(7.9/17.4/45.2/29.4,wt%)and 0.2mgFig.3.Back pressure against flow rate for organic monolithic columns.Experimental conditions:column,20cm ×75m i.d.;mobile phase,H 2O (filled circles),MeOH (empty triangles),and ACN (filled squares).(0.37%,with respect to the monomer mass)DMPA under UV light ( =365nm,120mJ/cm 2)for 6min was employed for further exper-iment.We also selected other thiol-containing monomers,2SH and 3SH,as the control experiment.Unfortunately,solid bulk mono-lithic material was not obtained when 2SH was used as the precursor.Although white opaque bulk material formed when replaced 2SH with 3SH,the resulting monolith was either too dense or easily detached from the capillary wall.It would possi-bly take much more time to optimize the preparation conditions for acquirement of satisfactory 3SH-based organic column.Herein,4SH is the ideal multi-thiol monomer for fabrication organic monolith via photo-initiated thiol-ene click polymerization.It is deduced that higher functionality of thiol-containing monomers would facilitate to form the satisfactory monoliths via thiol-ene polymerization reaction.3.2.Characterization of organic monolithThe SEM graphs of the organic monolith were shown in Fig.2a and b.It was observed that a uniform monolithic matrix with large through-pores was obtained within the capillary,and the formed monolithic matrix was attached well to the inner wall of the cap-illary.This was attributed to the alkene group at the inner wall of the capillary had taken part in the polymerization during the for-mation of organic monolith.Additionally,Fig.3revealed a good linear relationship of back pressure against flow rate even at the high pressure of 40MPa,implying that the organic monolith pos-sessed relatively good mechanical stability.The permeability of the monolithic column was calculated as 4.8×10−14m 2according toL.Chen et al./J.Chromatogr.A1394(2015)103–110107Fig.4.FT-IR spectra of the TVCH,4SH and organic monolithic material. Darcy’s law,which indicated the good permeability of the prepared monolithic column.The monolithic material formed in centrifuge tube was char-acterized by FT-IR,providing a direct proof for the thiol-ene click polymerization reaction of TVCH and4SH.The peak at2560cm−1 is assigned to thiol group and peak at1743cm−1indicates C O stretches(Fig.4a).Characteristic peaks at1640,990and910cm−1 in Fig.4b are C C pared to Fig.4a and b,the disappear-ance of peak at2560cm−1and the decrease of peak at1640cm−1 in Fig.4c confirmed the thiol-ene click polymerization reaction of TVCH and4SH.Additionally,the oligomers collected in a cen-trifuge was analyzed by MALDI-TOF mass spectrometer(Fig.5). The oligomers with molecular weight ranging from1100to2000Da were detected.The repeating units with mass difference of162and 488Da were observed,corresponding to TVCH and4SH monomers, respectively.The formation of oligomers strongly indicated the occurrence of thiol-ene reaction between TVCH and4SH,which would further construct the porous monolith.The repeatability is an important factor for the effectiveness and practicability of the preparation method of monolith.In the study, the repeatability of organic monolith was assessed through the rel-ative standard deviation(RSD)for both retention factor and column efficiency of benzene as analyte(thiourea as the void time marker). The run-to-run(n=5),column-to-column(n=5)and batch-to-batch(n=5)repeatabilities for retention factor were0.2%,1.2% and2.8%(correspondingly the average retention factor values of benzene were0.67,0.64,0.65),respectively.The run-to-run(n=3), column-to-column(n=3)and batch-to-batch(n=3)repeatabilites for column efficiency were1.5%,3.1%and5.6%(correspondingly the average column efficiency values of benzene were73,116,73,044,Fig.5.MALDI-TOF mass spectrum of the oligomersflushed out of the organic mono-lith prepared with the porogenic system of pure DEGDE.72,986),respectively.This suggested the photo-initiated thiol-ene click polymerization provided a robust and practical method for fabrication of organic monolithic columns.What’s more,clear col-umn deterioration or significant decrease of column efficiency was not observed even after continuous use for three months,and the column efficiency could remain about89%(butylbenzene as the test compound),which demonstrated the high stability of organic monolith.3.3.Chromatographic performance of organic monolithFig.2c demonstrated that organic monolithic matrix was hydrophobic according to the water contact angle of126◦,so the monolith was investigated in RPLC mode using alkylbenzenes as probes.As shown in Fig.6a,five alkylbenzenes were baseline-separated in the order of benzene<toluene<ethylbenzene< propylbenzene<butylbenzene with the mobile phase of ACN/H2O (65/35,v/v).A lot of ester groups and carbon sulfur bonds should be existed in the organic monolithic skeleton,contributing the hydrophobic retention mechanism.The column efficiency of the resulting organic monolith was evaluated on cLC by changing the flow rate from40to180L/min(before split).Fig.6b depicts the dependence of the plate height on the linearflow velocity for alkylbenzenes.The organic monolith exhibited the minimum plate height of7.5–13.7m for alkylbenzenes,corresponding to 133,000–73,000theoretical plates per meter,which exhibits more excellent chromatographic performance than conventional organic monoliths such as polystyrenes and polymethacrylates[24,40–42]. The size exclusion chromatography in THF afforded total porosityofFig.6.(a)Separation of alkylbenzenes on the organic monolith by cLC.Analytes:(1)thiourea,(2)benzene,(3)toluene,(4)ethylbenzene,(5)propylbenzene and(6)butyl-benzene.(b)Dependence of the plate height of analytes on the linear velocity of mobile phase by the organic monolith capillary column.(c)The effect of ACN content in mobile phases on retention factors of alkylbenzenes.Analytes:benzene(filled squares),toluene(empty circles),ethylbenzene(filled triangles),propylbenzene(empty triangles)and butylbenzene(filled rhombuses).Experimental conditions:effective length of22cm×75m i.d.;mobile phase,ACN/H2O(65/35,v/v,for a,b);flow rates,200 and100L/min for(a)and(c)respectively(before split,);detection wavelength,214nm.108L.Chen et al./J.Chromatogr.A 1394(2015)103–110Fig.7.Calibration curve of organic monolith by size exclusion chromatography.Experimental conditions:column dimension,19.6cm ×75m i.d.;off column dimension,5.0cm ×75m i.d.;flow rate,75nL/min;mobile phase,THF;injection volume,2.5L in split mode;detection wavelength,214nm;temperature,25◦C;solute,benzene,polystyrenes (M w =800,4000,13,200,50,000,90,000,280,000,and 900,000).Table 2Fitted values of A ,B and C terms in van Deemter equation:H =A +B /u +Cu .A (m)B (m 2/s)C (s)Benzene 2.0934520.0039Toluene2.4627870.0038Ethylbenzene 1.9226770.0041Propylbenzene 2.1422520.004Butylbenzene1.9420710.0039Correlation coefficients ranging from 0.91to 0.95.the organic monolith at 71.5%,while the volume of through-pores was calculated at 53.3%(as shown in Fig.7).The great pore vol-ume was beneficial to the separation of small molecules,therefore high column efficiency was achieved on this organic monolith in cLC.Additionally,the values of A,B and C terms in van Deemter equation were summarized in Table 2.The A-term describes dis-persion of analytes due to the irregularity of the stream paths in the porous medium [43].Compared to C18-modified silica mono-lith [44],the obtained organic monolith exhibited lower A-term likely benefiting from the homogeneous structure.The B-term,representing the longitudinal diffusion,depends on the analyte diffusion coefficient in the mobile phase [45].Therefore,its valuedecreases as the molecular mass increases.The C-term is close to that observed with C18-modified silica monoliths [44].Com-pared with the monolithic polymeric column recently prepared via thiol-yne click polymerization [46],this organic monolith exhib-ited higher column efficiency,which can be attributed to lager through-pores in the monolithic matrix (smaller C-term).Furthermore,the influence of ACN content on retention factors of alkylbenzenes was investigated,as shown in Fig.6c.With ACN content increasing,the values of retention factors (k )decreased as expected.The methylene selectivity (˛)[47,48],used to character-ize the hydrophobicity of stationary phase,was calculated by the following equation:log k =n log ˛+log ˇ,where n is carbon num-ber of homologous compounds and ˇis retention factor speculated to n =0.Plotting log k versus n ,the values of methylene selectivity were calculated,namely 1.43,1.38,1.34,1.31and 1.27under 60%,65%,70%,75%and 80%ACN in the mobile phases,respectively (see Fig.S2).These results again confirmed the reversed-phase retention mechanism of the organic monolith.3.4.Application of organic monolithTo further investigate the application of the fabricated organic monolith,the separations of basic compounds,pesticides and pol-yaromatic hydrocarbons were performed in cLC.It was shown in Fig.8a that five basic compounds were baseline-separated with the mobile phase of ACN/H 2O (40/60,v/v)in cLC,and the peaks were all symmetry without any peak tailing.As mentioned above,there were only several neutral methylene,ester functional groups and carbon sulfur bonds in the surface of organic monolithic skeleton,contributing the hydrophobic retention for the basic compounds.As a result,the separation of basic compounds on this TVCH-4SH-based organic monolith with RPLC does not suffer from peak tailing.Fig.8b depicts the separation of four pesticides with the mobile phase of ACN/H 2O (55/45,v/v)in cLC,also demonstrating good separation ability of this organic monolith for these pesticides.EPA 610,consisting of 16priority pollutant PAHs,can participate in the body’s metabolism and presents potential health hazards because of their carcinogenicity,teratogenicity,mutagenicity and difficult biodegradability.Fig.8c clearly shows the separation of EPA 610on the prepared organic monolith in cLC with gradient elution.It can be seen that EPA 610were well separated except that phenanthrene (analyte 5)and anthracene (analyte 6)could not be separated,sug-gesting that the organic monolith has a potential application in the field of environmental samplesseparation.Fig.8.Separations of (a)basic compounds,(b)pesticides and (c)EPA 610on organic monolithic column by cLC.Experimental conditions:effective lengths,16.5cm ×75m i.d.,19.5cm ×75m i.d.and 20cm ×75m i.d.,for a–c respectively;mobile phases for (a),ACN/H 2O (40/60,v/v),for (b),ACN/H 2O (55/45,v/v),for (c),mobile phase A,water with 0.1%FA;mobile phase B,ACN with 1%FA;gradient,60%B to 100%B in 20min;flow rates,80,160and 140L/min for a,b,and c respectively (before split);detection wavelength,214nm.Elution order:(a)caffeine,carbamazepine,2,4-dinitroaniline,4-aminobiphenyl and 2,6-dichloro-4-nitroanine;(b)imidacloprid,carbaryl,thiram,parathion-methyl;(c),(1)naphthalene,(2)acenaphthylene,(3)acenaphthene,(4)fluorene,(5)phenanthrene,(6)anthracene,(7)fluoranthene,(8)pyrene,(9)benzo(a)anthracene,(10)chrysene,(11)benzo(b)fluoranthene,(12)benzo(k)fluoranthene,(13)benzo(a)pyrene,(14)dibenzo(a,h)anthracene,(15)benzo(g,h,i)perylene and (16)indeno(1,2,3-cd)pyrene.。
